User login
Internal Carotid Artery Dissection After Indirect Blunt Cervical Trauma in an Ice Hockey Goaltender
Take-Home Points
- ICA dissections may occur from direct or indirect trauma.
- Symptoms can be mild, including a persistent headache.
- High clinical suspicion is required for diagnosis when symptoms are mild.
- Neuroimaging is required for definitive diagnosis.
- Conservative management with serial imaging can yield successful outcomes.
Cervical artery dissection (CAD) is an uncommon but potentially life-threatening condition that accounts for a high proportion of ischemic strokes in patients under the age of 45 years.1-4 The extracranial internal carotid arteries (ICAs) and vertebral arteries are most commonly involved; dissections can occur after either direct trauma to the neck, or indirect trauma resulting in acute hyperextension or hyperflexion.4-7 ICA dissection can be difficult to diagnose because of the varying symptomatology. Clinical presentation depends on stenosis location, degree of luminal narrowing, and presence or absence of ischemic stroke. Neurologic symptoms may be delayed, and misdiagnosis of an isolated soft-tissue contusion, whiplash, can be made in the setting of indirect cervical trauma.
Although this entity is well described in the literature,2,3,5,8 there are few reported cases of injuries sustained during high-intensity athletic competition. In this case report, we describe the symptoms, physical examination findings, diagnostic imaging results, and treatment of a young male athlete who presented with delayed-onset symptoms of ICA dissection resulting from indirect cervical trauma sustained during an ice hockey game. We discuss the importance of a high level of clinical suspicion in the diagnosis of neck injuries sustained during athletic competition, as well as the need for early vascular imaging for diagnosis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right-handed 32-year-old professional hockey goaltender. Four days before diagnosis, his goaltending mask and attached neck-protector were inadvertently lifted by another player’s stick just as a puck traveling at high speed struck him in the neck, to the right of the larynx, causing acute neck hyperextension. He immediately experienced discomfort and fell to the ice, saying he was “dizzy and light-headed.” Play was stopped, and medical personnel attended to him. His symptoms resolved, and he resumed play without any notable deficits. The next day, he noted discomfort at the impact site, but no additional symptoms, and received a presumptive diagnosis of cervical soft-tissue contusion. Continuing to participate in hockey that day, he did not develop any symptoms other than superficial cervical discomfort. However, the next morning, he presented complaining of severe right frontotemporal headache, which had persisted overnight. Orthopedic examination revealed palpable tenderness over the anterior cervical musculature, including the sternocleidomastoid and strap muscles. There was no appreciable hematoma in the contused area. Cervical range of motion was otherwise preserved. Cervical spine examination, including dermatomal and myotomal examination, was normal, as was cranial nerve examination. However, given the headache intensity and the recency of the injury, the potential for vascular or neurologic injury was considered. A neurology consultation was obtained, and arrangements were made for advanced cross-sectional imaging.
On further evaluation, the patient denied loss of consciousness, seizure, vomiting, amnesia, visual disturbance, language or cognitive impairment, balance or coordination difficulties, or any appreciable face or limb weakness. Review of systems was otherwise negative. Detailed neurologic examination did not reveal any cranial nerve deficits, and pupils were 3 mm, equal, and normally responsive to light and accommodation. Muscular tone and strength were symmetric and full in the upper and lower extremities. Gait, coordination, and response to vibration and temperature sensation were all preserved.
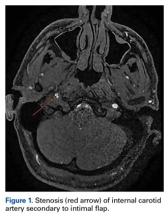
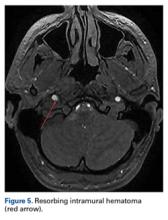
Magnetic resonance imaging of the head and neck was normal, but magnetic resonance angiography (MRA) of the neck showed a 1-cm-long region of the ICA, before piercing the petrous bone, with evidence of dissection.
Given the normal neurologic examination, and no evidence of brain infarction or other neurovascular complications, the acute ICA dissection was managed with antiplatelet therapy using aspirin (325 mg/d). In addition, the patient was advised to refrain from strenuous physical activity and to present to the hospital immediately if symptoms worsened or any neurologic impairment developed. Follow-up and repeat MRA were planned to monitor healing progression.
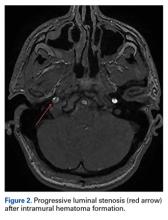
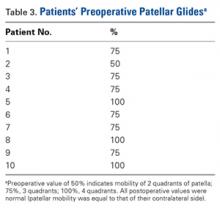
Two weeks after injury, the patient returned for follow-up. His headache and neck pain had resolved. Physical examination findings were unchanged, and there were no notable neurologic deficits. Repeat MRA findings were essentially unchanged, except for slightly increased luminal stenosis, exceeding 50% (Figure 2), attributable to intramural hematoma formation.
At 6-week follow-up, the patient had no clinical symptoms and no recurrence of headaches.
Discussion
In cases of direct (blunt) or indirect cervical trauma, CAD should be considered, as it carries a risk of potentially debilitating ischemic stroke in otherwise healthy young patients. Fortunately, CAD is rare; its annual incidence is 1 in 100,000, occurring in 0.08% to 1.2% of blunt trauma cases.9
As symptoms of ICA dissection can vary depending on stenosis severity, diagnosis can be challenging. The classically associated triad of symptoms includes unilateral head, facial, or neck pain accompanied by partial Horner syndrome with progression to cerebral or retinal ischemia. However, these symptoms occur in less than a third of patients with ICA dissection.2 Neck pain may occur secondary to blunt cervical trauma, consistent with a cervical soft-tissue contusion; however, it may have more severe implications and should be carefully monitored, particularly if accompanied by additional symptoms, such as headache. Headaches, which are present in 44% to 69% of patients, are often unilateral and constant. Either headache or neck pain in isolation is relatively uncommon, occurring in <10% of cases,2 though retrospective reviews of delayed-onset ICA dissection found atypical headache or neck pain in 100% of patients,11 indicating that persistent symptoms should be further evaluated.
More commonly, patients present with neurologic symptoms, particularly Horner syndrome, which is caused by the disruption of the sympathetic nerve fibers adjacent to the ICA, resulting in ipsilateral ptosis and miosis. In addition, patients may present with cranial nerve palsies, most commonly involving cranial nerve XII (the hypoglossal nerve), resulting in tongue weakness and abnormal taste. These and other neurologic findings associated with retinal or cerebral ischemia should raise clinical suspicion for the injury and prompt computed tomography or MRA evaluation.
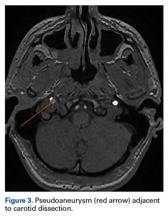
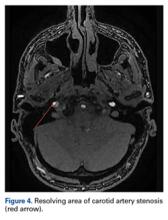
MRA has largely replaced conventional angiography for the diagnosis of CAD. As MRA is noninvasive, it allows for improved visualization of luminal narrowing and for evaluation of the arterial wall and intramural hematoma.2 Because of the potential for devastating sequelae with missed or delayed diagnosis, several authors have become proponents of early aggressive screening for detection of these injuries.9 Postdiagnostic treatment depends on the presence of neurologic symptoms. Management is directed toward limiting neurologic deficits; anticoagulant or antiplatelet agents are used to prevent thromboembolic events. A randomized controlled trial and other studies have failed to find any appreciable difference in subsequent rates of stroke or associated complications with use of either class of medication.8,12 Conventionally, treatment is continued for 3 to 6 months, depending on clinical resolution. Endovascular or surgical intervention typically is reserved for extreme luminal narrowing, conditions that are preventing anticoagulation, an expanding area of dissection with a persistent pseudoaneurysm, and cases of failed medical management with subsequent ischemic stroke.2The literature includes several case reports involving indirect trauma in recreational athletes. First, a 31-year-old woman sustained an ICA dissection secondary to a head injury that occurred during a soccer match; she presented with headache, altered sense of taste, and objective findings of ptosis and miosis consistent with Horner syndrome.13 Second, a 39-year-old man had an ICA dissection after a snowboarding fall that caused neck hyperextension; he presented with periocular headache, ptosis, and miosis.6 Third, 3 people who participated in CrossFit training sustained ICA dissection.7 They presented with varying degrees of neurologic symptoms: ptosis and miosis; right-side upper extremity ataxia; and visual distortion and receptive aphasia. Our patient’s ICA dissection resulted from indirect trauma that caused sudden hyperextension and lateral flexion in response to contact from a hockey puck. However, his case is unique in that symptoms onset was delayed, and there were no associated neurologic findings on clinical presentation. His case should raise awareness of this potential diagnosis, even in the absence of overt neurologic findings. In addition, the patient’s return to sport at 8 weeks was facilitated by full clinical resolution of symptoms and thorough radiographic documentation of improved intramural narrowing. Finally, to our knowledge this is the first report of this injury in a professional athlete.
Conclusion
We have reported the case of a 32-year-old professional hockey goaltender who presented with isolated, persistent, worsening headache of delayed onset after ICA dissection. The ICA dissection resulted from indirect trauma, with reaction to a puck causing acute hyperextension and rotational injury. To our knowledge, this is the first report of a case of ICA dissection in an athlete, lacking neurologic examination findings that could aid in the diagnosis. The index of suspicion for CAD should be high after direct or indirect cervical trauma when patients present with unilateral neck pain or headache, even in the absence of neurologic findings, as stroke is a catastrophic but preventable complication.
Am J Orthop. 2017;46(3):E139-E143. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mohan IV. Current optimal assessment and management of carotid and vertebral spontaneous and traumatic dissection. Angiology. 2014;65(4):274-283.
2. Patel RR, Adam R, Maldjian C, Lincoln CM, Yuen A, Arneja A. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012;20(3):145-152.
3. Biller J, Sacco RL, Albuquerque FC, et al; American Heart Association Stroke Council. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(10):3155-3174.
4. Fukunaga N, Hanaoka M, Sato K. Asymptomatic common carotid artery dissection caused by blunt injury. Emerg Med J. 2011;28(1):50.
5. Chen J, Zhou X, Li C, Cheung BM. Risk of stroke due to spontaneous cervical artery dissection. Intern Med. 2013;52(19):2237-2240.
6. Kalantzis G, Georgalas I, Chang BY, Ong C, El-Hindy N. An unusual case of traumatic internal carotid artery dissection during snowboarding. J Sports Sci Med. 2014;13(2):451-453.
7. Lu A, Shen P, Lee P, et al. CrossFit-related cervical internal carotid artery dissection. Emerg Radiol. 2015;22(4):449-452.
8. CADISS Trial Investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
9. van Wessem KJ, Meijer JM, Leenen LP, van der Worp HB, Moll FL, de Borst GJ. Blunt traumatic carotid artery dissection still a pitfall? The rationale for aggressive screening. Eur J Trauma Emerg Surg. 2011;37(2):147-154.
10. Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28(1):57-63.
11. Thomas LC, Rivett DA, Attia JR, Levi C. Risk factors and clinical presentation of cervical arterial dissection: preliminary results of a prospective case-control study. J Orthop Sports Phys Ther. 2015;45(7):503-511.
12. Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010;(10):CD000255.
13. Creavin ST, Rice CM, Pollentine A, Cowburn P. Carotid artery dissection presenting with isolated headache and Horner syndrome after minor head injury. Am J Emerg Med. 2012;30(9):2103.e5-e7.
Take-Home Points
- ICA dissections may occur from direct or indirect trauma.
- Symptoms can be mild, including a persistent headache.
- High clinical suspicion is required for diagnosis when symptoms are mild.
- Neuroimaging is required for definitive diagnosis.
- Conservative management with serial imaging can yield successful outcomes.
Cervical artery dissection (CAD) is an uncommon but potentially life-threatening condition that accounts for a high proportion of ischemic strokes in patients under the age of 45 years.1-4 The extracranial internal carotid arteries (ICAs) and vertebral arteries are most commonly involved; dissections can occur after either direct trauma to the neck, or indirect trauma resulting in acute hyperextension or hyperflexion.4-7 ICA dissection can be difficult to diagnose because of the varying symptomatology. Clinical presentation depends on stenosis location, degree of luminal narrowing, and presence or absence of ischemic stroke. Neurologic symptoms may be delayed, and misdiagnosis of an isolated soft-tissue contusion, whiplash, can be made in the setting of indirect cervical trauma.
Although this entity is well described in the literature,2,3,5,8 there are few reported cases of injuries sustained during high-intensity athletic competition. In this case report, we describe the symptoms, physical examination findings, diagnostic imaging results, and treatment of a young male athlete who presented with delayed-onset symptoms of ICA dissection resulting from indirect cervical trauma sustained during an ice hockey game. We discuss the importance of a high level of clinical suspicion in the diagnosis of neck injuries sustained during athletic competition, as well as the need for early vascular imaging for diagnosis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right-handed 32-year-old professional hockey goaltender. Four days before diagnosis, his goaltending mask and attached neck-protector were inadvertently lifted by another player’s stick just as a puck traveling at high speed struck him in the neck, to the right of the larynx, causing acute neck hyperextension. He immediately experienced discomfort and fell to the ice, saying he was “dizzy and light-headed.” Play was stopped, and medical personnel attended to him. His symptoms resolved, and he resumed play without any notable deficits. The next day, he noted discomfort at the impact site, but no additional symptoms, and received a presumptive diagnosis of cervical soft-tissue contusion. Continuing to participate in hockey that day, he did not develop any symptoms other than superficial cervical discomfort. However, the next morning, he presented complaining of severe right frontotemporal headache, which had persisted overnight. Orthopedic examination revealed palpable tenderness over the anterior cervical musculature, including the sternocleidomastoid and strap muscles. There was no appreciable hematoma in the contused area. Cervical range of motion was otherwise preserved. Cervical spine examination, including dermatomal and myotomal examination, was normal, as was cranial nerve examination. However, given the headache intensity and the recency of the injury, the potential for vascular or neurologic injury was considered. A neurology consultation was obtained, and arrangements were made for advanced cross-sectional imaging.
On further evaluation, the patient denied loss of consciousness, seizure, vomiting, amnesia, visual disturbance, language or cognitive impairment, balance or coordination difficulties, or any appreciable face or limb weakness. Review of systems was otherwise negative. Detailed neurologic examination did not reveal any cranial nerve deficits, and pupils were 3 mm, equal, and normally responsive to light and accommodation. Muscular tone and strength were symmetric and full in the upper and lower extremities. Gait, coordination, and response to vibration and temperature sensation were all preserved.
Magnetic resonance imaging of the head and neck was normal, but magnetic resonance angiography (MRA) of the neck showed a 1-cm-long region of the ICA, before piercing the petrous bone, with evidence of dissection.
Given the normal neurologic examination, and no evidence of brain infarction or other neurovascular complications, the acute ICA dissection was managed with antiplatelet therapy using aspirin (325 mg/d). In addition, the patient was advised to refrain from strenuous physical activity and to present to the hospital immediately if symptoms worsened or any neurologic impairment developed. Follow-up and repeat MRA were planned to monitor healing progression.
Two weeks after injury, the patient returned for follow-up. His headache and neck pain had resolved. Physical examination findings were unchanged, and there were no notable neurologic deficits. Repeat MRA findings were essentially unchanged, except for slightly increased luminal stenosis, exceeding 50% (Figure 2), attributable to intramural hematoma formation.
At 6-week follow-up, the patient had no clinical symptoms and no recurrence of headaches.
Discussion
In cases of direct (blunt) or indirect cervical trauma, CAD should be considered, as it carries a risk of potentially debilitating ischemic stroke in otherwise healthy young patients. Fortunately, CAD is rare; its annual incidence is 1 in 100,000, occurring in 0.08% to 1.2% of blunt trauma cases.9
As symptoms of ICA dissection can vary depending on stenosis severity, diagnosis can be challenging. The classically associated triad of symptoms includes unilateral head, facial, or neck pain accompanied by partial Horner syndrome with progression to cerebral or retinal ischemia. However, these symptoms occur in less than a third of patients with ICA dissection.2 Neck pain may occur secondary to blunt cervical trauma, consistent with a cervical soft-tissue contusion; however, it may have more severe implications and should be carefully monitored, particularly if accompanied by additional symptoms, such as headache. Headaches, which are present in 44% to 69% of patients, are often unilateral and constant. Either headache or neck pain in isolation is relatively uncommon, occurring in <10% of cases,2 though retrospective reviews of delayed-onset ICA dissection found atypical headache or neck pain in 100% of patients,11 indicating that persistent symptoms should be further evaluated.
More commonly, patients present with neurologic symptoms, particularly Horner syndrome, which is caused by the disruption of the sympathetic nerve fibers adjacent to the ICA, resulting in ipsilateral ptosis and miosis. In addition, patients may present with cranial nerve palsies, most commonly involving cranial nerve XII (the hypoglossal nerve), resulting in tongue weakness and abnormal taste. These and other neurologic findings associated with retinal or cerebral ischemia should raise clinical suspicion for the injury and prompt computed tomography or MRA evaluation.
MRA has largely replaced conventional angiography for the diagnosis of CAD. As MRA is noninvasive, it allows for improved visualization of luminal narrowing and for evaluation of the arterial wall and intramural hematoma.2 Because of the potential for devastating sequelae with missed or delayed diagnosis, several authors have become proponents of early aggressive screening for detection of these injuries.9 Postdiagnostic treatment depends on the presence of neurologic symptoms. Management is directed toward limiting neurologic deficits; anticoagulant or antiplatelet agents are used to prevent thromboembolic events. A randomized controlled trial and other studies have failed to find any appreciable difference in subsequent rates of stroke or associated complications with use of either class of medication.8,12 Conventionally, treatment is continued for 3 to 6 months, depending on clinical resolution. Endovascular or surgical intervention typically is reserved for extreme luminal narrowing, conditions that are preventing anticoagulation, an expanding area of dissection with a persistent pseudoaneurysm, and cases of failed medical management with subsequent ischemic stroke.2The literature includes several case reports involving indirect trauma in recreational athletes. First, a 31-year-old woman sustained an ICA dissection secondary to a head injury that occurred during a soccer match; she presented with headache, altered sense of taste, and objective findings of ptosis and miosis consistent with Horner syndrome.13 Second, a 39-year-old man had an ICA dissection after a snowboarding fall that caused neck hyperextension; he presented with periocular headache, ptosis, and miosis.6 Third, 3 people who participated in CrossFit training sustained ICA dissection.7 They presented with varying degrees of neurologic symptoms: ptosis and miosis; right-side upper extremity ataxia; and visual distortion and receptive aphasia. Our patient’s ICA dissection resulted from indirect trauma that caused sudden hyperextension and lateral flexion in response to contact from a hockey puck. However, his case is unique in that symptoms onset was delayed, and there were no associated neurologic findings on clinical presentation. His case should raise awareness of this potential diagnosis, even in the absence of overt neurologic findings. In addition, the patient’s return to sport at 8 weeks was facilitated by full clinical resolution of symptoms and thorough radiographic documentation of improved intramural narrowing. Finally, to our knowledge this is the first report of this injury in a professional athlete.
Conclusion
We have reported the case of a 32-year-old professional hockey goaltender who presented with isolated, persistent, worsening headache of delayed onset after ICA dissection. The ICA dissection resulted from indirect trauma, with reaction to a puck causing acute hyperextension and rotational injury. To our knowledge, this is the first report of a case of ICA dissection in an athlete, lacking neurologic examination findings that could aid in the diagnosis. The index of suspicion for CAD should be high after direct or indirect cervical trauma when patients present with unilateral neck pain or headache, even in the absence of neurologic findings, as stroke is a catastrophic but preventable complication.
Am J Orthop. 2017;46(3):E139-E143. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- ICA dissections may occur from direct or indirect trauma.
- Symptoms can be mild, including a persistent headache.
- High clinical suspicion is required for diagnosis when symptoms are mild.
- Neuroimaging is required for definitive diagnosis.
- Conservative management with serial imaging can yield successful outcomes.
Cervical artery dissection (CAD) is an uncommon but potentially life-threatening condition that accounts for a high proportion of ischemic strokes in patients under the age of 45 years.1-4 The extracranial internal carotid arteries (ICAs) and vertebral arteries are most commonly involved; dissections can occur after either direct trauma to the neck, or indirect trauma resulting in acute hyperextension or hyperflexion.4-7 ICA dissection can be difficult to diagnose because of the varying symptomatology. Clinical presentation depends on stenosis location, degree of luminal narrowing, and presence or absence of ischemic stroke. Neurologic symptoms may be delayed, and misdiagnosis of an isolated soft-tissue contusion, whiplash, can be made in the setting of indirect cervical trauma.
Although this entity is well described in the literature,2,3,5,8 there are few reported cases of injuries sustained during high-intensity athletic competition. In this case report, we describe the symptoms, physical examination findings, diagnostic imaging results, and treatment of a young male athlete who presented with delayed-onset symptoms of ICA dissection resulting from indirect cervical trauma sustained during an ice hockey game. We discuss the importance of a high level of clinical suspicion in the diagnosis of neck injuries sustained during athletic competition, as well as the need for early vascular imaging for diagnosis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right-handed 32-year-old professional hockey goaltender. Four days before diagnosis, his goaltending mask and attached neck-protector were inadvertently lifted by another player’s stick just as a puck traveling at high speed struck him in the neck, to the right of the larynx, causing acute neck hyperextension. He immediately experienced discomfort and fell to the ice, saying he was “dizzy and light-headed.” Play was stopped, and medical personnel attended to him. His symptoms resolved, and he resumed play without any notable deficits. The next day, he noted discomfort at the impact site, but no additional symptoms, and received a presumptive diagnosis of cervical soft-tissue contusion. Continuing to participate in hockey that day, he did not develop any symptoms other than superficial cervical discomfort. However, the next morning, he presented complaining of severe right frontotemporal headache, which had persisted overnight. Orthopedic examination revealed palpable tenderness over the anterior cervical musculature, including the sternocleidomastoid and strap muscles. There was no appreciable hematoma in the contused area. Cervical range of motion was otherwise preserved. Cervical spine examination, including dermatomal and myotomal examination, was normal, as was cranial nerve examination. However, given the headache intensity and the recency of the injury, the potential for vascular or neurologic injury was considered. A neurology consultation was obtained, and arrangements were made for advanced cross-sectional imaging.
On further evaluation, the patient denied loss of consciousness, seizure, vomiting, amnesia, visual disturbance, language or cognitive impairment, balance or coordination difficulties, or any appreciable face or limb weakness. Review of systems was otherwise negative. Detailed neurologic examination did not reveal any cranial nerve deficits, and pupils were 3 mm, equal, and normally responsive to light and accommodation. Muscular tone and strength were symmetric and full in the upper and lower extremities. Gait, coordination, and response to vibration and temperature sensation were all preserved.
Magnetic resonance imaging of the head and neck was normal, but magnetic resonance angiography (MRA) of the neck showed a 1-cm-long region of the ICA, before piercing the petrous bone, with evidence of dissection.
Given the normal neurologic examination, and no evidence of brain infarction or other neurovascular complications, the acute ICA dissection was managed with antiplatelet therapy using aspirin (325 mg/d). In addition, the patient was advised to refrain from strenuous physical activity and to present to the hospital immediately if symptoms worsened or any neurologic impairment developed. Follow-up and repeat MRA were planned to monitor healing progression.
Two weeks after injury, the patient returned for follow-up. His headache and neck pain had resolved. Physical examination findings were unchanged, and there were no notable neurologic deficits. Repeat MRA findings were essentially unchanged, except for slightly increased luminal stenosis, exceeding 50% (Figure 2), attributable to intramural hematoma formation.
At 6-week follow-up, the patient had no clinical symptoms and no recurrence of headaches.
Discussion
In cases of direct (blunt) or indirect cervical trauma, CAD should be considered, as it carries a risk of potentially debilitating ischemic stroke in otherwise healthy young patients. Fortunately, CAD is rare; its annual incidence is 1 in 100,000, occurring in 0.08% to 1.2% of blunt trauma cases.9
As symptoms of ICA dissection can vary depending on stenosis severity, diagnosis can be challenging. The classically associated triad of symptoms includes unilateral head, facial, or neck pain accompanied by partial Horner syndrome with progression to cerebral or retinal ischemia. However, these symptoms occur in less than a third of patients with ICA dissection.2 Neck pain may occur secondary to blunt cervical trauma, consistent with a cervical soft-tissue contusion; however, it may have more severe implications and should be carefully monitored, particularly if accompanied by additional symptoms, such as headache. Headaches, which are present in 44% to 69% of patients, are often unilateral and constant. Either headache or neck pain in isolation is relatively uncommon, occurring in <10% of cases,2 though retrospective reviews of delayed-onset ICA dissection found atypical headache or neck pain in 100% of patients,11 indicating that persistent symptoms should be further evaluated.
More commonly, patients present with neurologic symptoms, particularly Horner syndrome, which is caused by the disruption of the sympathetic nerve fibers adjacent to the ICA, resulting in ipsilateral ptosis and miosis. In addition, patients may present with cranial nerve palsies, most commonly involving cranial nerve XII (the hypoglossal nerve), resulting in tongue weakness and abnormal taste. These and other neurologic findings associated with retinal or cerebral ischemia should raise clinical suspicion for the injury and prompt computed tomography or MRA evaluation.
MRA has largely replaced conventional angiography for the diagnosis of CAD. As MRA is noninvasive, it allows for improved visualization of luminal narrowing and for evaluation of the arterial wall and intramural hematoma.2 Because of the potential for devastating sequelae with missed or delayed diagnosis, several authors have become proponents of early aggressive screening for detection of these injuries.9 Postdiagnostic treatment depends on the presence of neurologic symptoms. Management is directed toward limiting neurologic deficits; anticoagulant or antiplatelet agents are used to prevent thromboembolic events. A randomized controlled trial and other studies have failed to find any appreciable difference in subsequent rates of stroke or associated complications with use of either class of medication.8,12 Conventionally, treatment is continued for 3 to 6 months, depending on clinical resolution. Endovascular or surgical intervention typically is reserved for extreme luminal narrowing, conditions that are preventing anticoagulation, an expanding area of dissection with a persistent pseudoaneurysm, and cases of failed medical management with subsequent ischemic stroke.2The literature includes several case reports involving indirect trauma in recreational athletes. First, a 31-year-old woman sustained an ICA dissection secondary to a head injury that occurred during a soccer match; she presented with headache, altered sense of taste, and objective findings of ptosis and miosis consistent with Horner syndrome.13 Second, a 39-year-old man had an ICA dissection after a snowboarding fall that caused neck hyperextension; he presented with periocular headache, ptosis, and miosis.6 Third, 3 people who participated in CrossFit training sustained ICA dissection.7 They presented with varying degrees of neurologic symptoms: ptosis and miosis; right-side upper extremity ataxia; and visual distortion and receptive aphasia. Our patient’s ICA dissection resulted from indirect trauma that caused sudden hyperextension and lateral flexion in response to contact from a hockey puck. However, his case is unique in that symptoms onset was delayed, and there were no associated neurologic findings on clinical presentation. His case should raise awareness of this potential diagnosis, even in the absence of overt neurologic findings. In addition, the patient’s return to sport at 8 weeks was facilitated by full clinical resolution of symptoms and thorough radiographic documentation of improved intramural narrowing. Finally, to our knowledge this is the first report of this injury in a professional athlete.
Conclusion
We have reported the case of a 32-year-old professional hockey goaltender who presented with isolated, persistent, worsening headache of delayed onset after ICA dissection. The ICA dissection resulted from indirect trauma, with reaction to a puck causing acute hyperextension and rotational injury. To our knowledge, this is the first report of a case of ICA dissection in an athlete, lacking neurologic examination findings that could aid in the diagnosis. The index of suspicion for CAD should be high after direct or indirect cervical trauma when patients present with unilateral neck pain or headache, even in the absence of neurologic findings, as stroke is a catastrophic but preventable complication.
Am J Orthop. 2017;46(3):E139-E143. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mohan IV. Current optimal assessment and management of carotid and vertebral spontaneous and traumatic dissection. Angiology. 2014;65(4):274-283.
2. Patel RR, Adam R, Maldjian C, Lincoln CM, Yuen A, Arneja A. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012;20(3):145-152.
3. Biller J, Sacco RL, Albuquerque FC, et al; American Heart Association Stroke Council. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(10):3155-3174.
4. Fukunaga N, Hanaoka M, Sato K. Asymptomatic common carotid artery dissection caused by blunt injury. Emerg Med J. 2011;28(1):50.
5. Chen J, Zhou X, Li C, Cheung BM. Risk of stroke due to spontaneous cervical artery dissection. Intern Med. 2013;52(19):2237-2240.
6. Kalantzis G, Georgalas I, Chang BY, Ong C, El-Hindy N. An unusual case of traumatic internal carotid artery dissection during snowboarding. J Sports Sci Med. 2014;13(2):451-453.
7. Lu A, Shen P, Lee P, et al. CrossFit-related cervical internal carotid artery dissection. Emerg Radiol. 2015;22(4):449-452.
8. CADISS Trial Investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
9. van Wessem KJ, Meijer JM, Leenen LP, van der Worp HB, Moll FL, de Borst GJ. Blunt traumatic carotid artery dissection still a pitfall? The rationale for aggressive screening. Eur J Trauma Emerg Surg. 2011;37(2):147-154.
10. Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28(1):57-63.
11. Thomas LC, Rivett DA, Attia JR, Levi C. Risk factors and clinical presentation of cervical arterial dissection: preliminary results of a prospective case-control study. J Orthop Sports Phys Ther. 2015;45(7):503-511.
12. Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010;(10):CD000255.
13. Creavin ST, Rice CM, Pollentine A, Cowburn P. Carotid artery dissection presenting with isolated headache and Horner syndrome after minor head injury. Am J Emerg Med. 2012;30(9):2103.e5-e7.
1. Mohan IV. Current optimal assessment and management of carotid and vertebral spontaneous and traumatic dissection. Angiology. 2014;65(4):274-283.
2. Patel RR, Adam R, Maldjian C, Lincoln CM, Yuen A, Arneja A. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012;20(3):145-152.
3. Biller J, Sacco RL, Albuquerque FC, et al; American Heart Association Stroke Council. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(10):3155-3174.
4. Fukunaga N, Hanaoka M, Sato K. Asymptomatic common carotid artery dissection caused by blunt injury. Emerg Med J. 2011;28(1):50.
5. Chen J, Zhou X, Li C, Cheung BM. Risk of stroke due to spontaneous cervical artery dissection. Intern Med. 2013;52(19):2237-2240.
6. Kalantzis G, Georgalas I, Chang BY, Ong C, El-Hindy N. An unusual case of traumatic internal carotid artery dissection during snowboarding. J Sports Sci Med. 2014;13(2):451-453.
7. Lu A, Shen P, Lee P, et al. CrossFit-related cervical internal carotid artery dissection. Emerg Radiol. 2015;22(4):449-452.
8. CADISS Trial Investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
9. van Wessem KJ, Meijer JM, Leenen LP, van der Worp HB, Moll FL, de Borst GJ. Blunt traumatic carotid artery dissection still a pitfall? The rationale for aggressive screening. Eur J Trauma Emerg Surg. 2011;37(2):147-154.
10. Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28(1):57-63.
11. Thomas LC, Rivett DA, Attia JR, Levi C. Risk factors and clinical presentation of cervical arterial dissection: preliminary results of a prospective case-control study. J Orthop Sports Phys Ther. 2015;45(7):503-511.
12. Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010;(10):CD000255.
13. Creavin ST, Rice CM, Pollentine A, Cowburn P. Carotid artery dissection presenting with isolated headache and Horner syndrome after minor head injury. Am J Emerg Med. 2012;30(9):2103.e5-e7.
Encapsulated Fat Necrosis Lesion Caused by Morel-Lavallée Lesion in a Professional Ice Hockey Player
Take-Home Points
- ML lesions usually occur with high-energy injuries and have been reported in wrestlers, football players, and other athlete populations.
- Encapsulated fat necrosis lesions are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions.
- Encapsulated fat necrosis lesions are rare; only 65 have been reported.
- Encapsulated fat necrosis lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.
- Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
What would become known as the Morel-Lavallée (ML) lesion was first reported in 1853 by French physician Maurice Morel-Lavallée. He described a proximal thigh soft-tissue injury that resulted in a hemolymphatic collection between superficial fascial planes. Deforming forces of pressure and shear result in an internal degloving injury in which subcutaneous tissue is stripped from the fascia and replaced with a hematoma or, less commonly, necrotic fat.1-4 The injury can take several weeks to heal. Up to one-third of such injuries are initially missed because of the initial ecchymosis covering the injured area.5
ML lesions usually occur with high-energy injuries and have been reported in wrestlers,6 football players,7-9 and other athlete populations. ML lesions usually occur about the knee, the site of the sheer mechanism in these athletes’ sports. Tejwani and colleagues9 reported on 24 National Football League (NFL) players (27 knees). These elite athletes typically were able to return to practice and game play long before complete resolution of their lesions.
Nodular cystic fat necrosis was first described by Przyjemski and Schuster10 in 1977. The terms encapsulated fat necrosis lesions and mobile encapsulated lipomas11 were introduced later. Clinically, these entities usually present as lesions on the lower limbs of young men and middle-aged women and can range in size from 1 mm to 35 mm. Most of these lesions are mobile.11 They are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions. Trauma accounts for the usual occurrence in the lower extremities, though only 40% of patients recall a precipitating event.12 Histologically, these lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.13In this article, we report the case of a professional ice hockey player who presented with an ML lesion of the hip and then developed a symptomatic encapsulated fat necrosis lesion that required surgical removal. To our knowledge, this is the first reported case of an encapsulated fat necrosis lesion caused by an ML lesion in an athlete. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
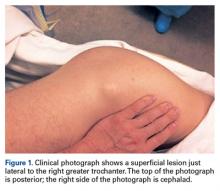
A 21-year-old professional hockey player presented with a history of pain from a mass on his right hip. He first noticed the lesion, just lateral to the greater trochanter, about 3 years earlier. The mass appeared after he sustained a shearing-type injury to the lateral aspect of the hip. At the time, there was significant swelling along the lateral aspect, with ecchymosis that resolved over 2 months. The mass, diagnosed as an ML lesion, resolved with nonoperative treatment. However, in the area where the swelling had occurred, a hard mobile mass remained. At times, this mass became painful when direct pressure was applied, as when he hit the boards while playing hockey, or when he lay on his right side or used a roller in the training room. He rated the pain as a 4 on a 1-to-10 scale and said the mass was mobile and had not changed in size or consistency.
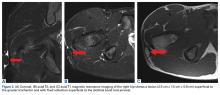
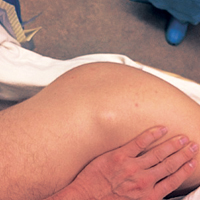
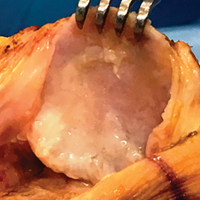
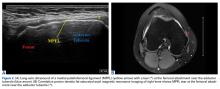
Physical examination revealed a palpable mass over the lateral aspect of the hip, over the greater trochanter. The mass, about 3 cm in diameter (Figure 1), was mobile in a subcutaneous pocket, consistent with an old ML lesion.
Options discussed with the patient included use of ice, activity modification, and use of protective padded equipment. As the patient had tried these treatments before and was still intermittently having pain with direct pressure, he asked for surgical removal of the mass.
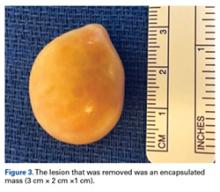
For the surgery, the patient was positioned in the lateral decubitus position with his right hip facing up. The right hip and thigh were prepared and draped in sterile fashion. An incision 4 cm in length was made directly over the mass, along the lateral aspect of the hip, over the greater trochanter. The incision was taken through skin and subcutaneous tissue down to the deep fascia. The fascia was incised longitudinally in line with the overlying skin incision. As soon as the incision was made through the fascia, the mass was easily seen. The 3-cm × 2-cm × 1-cm mass was free, not attached to any underlying soft tissue (Figure 3).
Discussion
We have described a case of symptomatic encapsulated fat necrosis lesion caused by an ML lesion in a professional hockey player. The ML lesion had resolved with nonoperative treatment (compression), but a subcutaneous pocket remained at the lesion site. Given the patient’s lesion site and occupation as a hockey player, pain with direct pressure on this lesion was a concern.
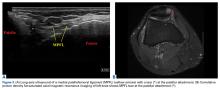
Long-standing ML lesions have 3 common patterns on MRI.14 A central region, encapsulated partially or completely by a peripheral ring of fibrous tissue or hemosiderin, shows signal properties consistent with a seroma, a homogeneous hemorrhagic collection, or a heterogeneous hemorrhagic collection. In our patient’s case, MRI was used to characterize the mobile mass for operative planning. Although thin strands or lobules of fat have been found within ML lesions, this case was the first to demonstrate a sequestered mass of necrotic fat.
Most football players who develop ML lesions on their knees do not wear kneepads.7-9 Of the 24 NFL players in the study by Tejwani and colleagues,9 52% were successfully treated with compression wrap, cryotherapy, and motion exercises. The rest, however, were treated with aspiration, and 11% underwent doxycycline sclerodesis for recurrent fluid collection. After treatment, all of their players were able to return to football. Their outcomes are consistent with that of our patient, who was treated with compression wrap and returned to hockey without any other intervention.
After our patient’s ML lesion resolved, he developed an encapsulated fat necrosis lesion from the disruption of the blood supply in the subcutaneous pocket. Encapsulated fat necrosis lesions are rare; only 65 have been reported.13,15 Clinically, these lesions are single or multiple pale-yellow encapsulated nodes.13 Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
The literature includes 1 report of an adolescent football player who developed multiple encapsulated fat necrosis lesions 4 months after landing on another player’s cleats.15 The patient, who was having pain with direct pressure during squatting and kneeling, elected to have the lesions surgically removed. These lesions are rare and usually asymptomatic,11 but our patient had his lesion surgically removed to address the pain induced by the direct impacts that came with playing professional hockey. Surgical removal is the treatment for symptomatic encapsulated fat necrosis lesions. Other than 1 case of recurrence after excision,16 these lesions have an excellent prognosis.
Conclusion
Our patient, a professional hockey player, underwent successful surgical removal of a symptomatic encapsulated fat necrosis lesion that had developed from an ML lesion.
Am J Orthop. 2017;46(3):E144-E147. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Aguiar RO, Viegas FC, Fernandez RY, Trudell D, Haghighi P, Resnick D. The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography. AJR Am J Roentgenol. 2007;188(4):W355-W358.
2. Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma. 1997;42(6):1046-1051.
3. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
4. Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin North Am. 2005;13(4):775-782.
5. Dye SF, Campagna-Pinto D, Dye CC, Shifflett S, Eiman T. Soft-tissue anatomy anterior to the human patella. J Bone Joint Surg Am. 2003;85(6):1012-1017.
6. Northam MC, Gaskin CM. Presumed prepatellar fibrosis in collegiate wrestlers: imaging findings and clinical correlation. Skeletal Radiol. 2015;44(2):271-277.
7. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Concealed degloving injury (the Morel-Lavallée lesion) in childhood sports: a case report. J Bone Joint Surg Am. 2011;93(24):e148.
8. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-Lavallée lesion in a professional American football player. Am J Orthop. 2010;39(3):144-147.
9. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
10. Przyjemski CJ, Schuster SR. Nodular-cystic fat necrosis. J Pediatr. 1977;91(4):605-607.
11. Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis—a clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27(1):19-23.
12. Santos-Juanes J, Coto P, Galache C, Sánchez del Rio J, Soto de Delás J. Encapsulated fat necrosis: a form of traumatic panniculitis. J Eur Acad Dermatol Venereol. 2007;21(3):405-406.
13. Sempau L, Sambucetty PS, Garcia JL, Sixto BG, Morán AG, Prieto MA. Mobile encapsulated lipoma. Int J Dermatol. 2012;51(4):448-450.
14. Mellado JM, Pérez del Palomar L, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289-1294.
15. Sole JS, Wisniewski SJ, Dahm DL, Bond J, Smith J. Posttraumatic fat necrosis presenting as prepatellar loose bodies in an adolescent football player. PM R. 2014;6(8):749-752.
16. Felipo F, Vaquero M, del Agua C. Pseudotumoral encapsulated fat necrosis with diffuse pseudomembranous degeneration. J Cutan Pathol. 2004;31(8):565-567.
Take-Home Points
- ML lesions usually occur with high-energy injuries and have been reported in wrestlers, football players, and other athlete populations.
- Encapsulated fat necrosis lesions are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions.
- Encapsulated fat necrosis lesions are rare; only 65 have been reported.
- Encapsulated fat necrosis lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.
- Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
What would become known as the Morel-Lavallée (ML) lesion was first reported in 1853 by French physician Maurice Morel-Lavallée. He described a proximal thigh soft-tissue injury that resulted in a hemolymphatic collection between superficial fascial planes. Deforming forces of pressure and shear result in an internal degloving injury in which subcutaneous tissue is stripped from the fascia and replaced with a hematoma or, less commonly, necrotic fat.1-4 The injury can take several weeks to heal. Up to one-third of such injuries are initially missed because of the initial ecchymosis covering the injured area.5
ML lesions usually occur with high-energy injuries and have been reported in wrestlers,6 football players,7-9 and other athlete populations. ML lesions usually occur about the knee, the site of the sheer mechanism in these athletes’ sports. Tejwani and colleagues9 reported on 24 National Football League (NFL) players (27 knees). These elite athletes typically were able to return to practice and game play long before complete resolution of their lesions.
Nodular cystic fat necrosis was first described by Przyjemski and Schuster10 in 1977. The terms encapsulated fat necrosis lesions and mobile encapsulated lipomas11 were introduced later. Clinically, these entities usually present as lesions on the lower limbs of young men and middle-aged women and can range in size from 1 mm to 35 mm. Most of these lesions are mobile.11 They are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions. Trauma accounts for the usual occurrence in the lower extremities, though only 40% of patients recall a precipitating event.12 Histologically, these lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.13In this article, we report the case of a professional ice hockey player who presented with an ML lesion of the hip and then developed a symptomatic encapsulated fat necrosis lesion that required surgical removal. To our knowledge, this is the first reported case of an encapsulated fat necrosis lesion caused by an ML lesion in an athlete. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 21-year-old professional hockey player presented with a history of pain from a mass on his right hip. He first noticed the lesion, just lateral to the greater trochanter, about 3 years earlier. The mass appeared after he sustained a shearing-type injury to the lateral aspect of the hip. At the time, there was significant swelling along the lateral aspect, with ecchymosis that resolved over 2 months. The mass, diagnosed as an ML lesion, resolved with nonoperative treatment. However, in the area where the swelling had occurred, a hard mobile mass remained. At times, this mass became painful when direct pressure was applied, as when he hit the boards while playing hockey, or when he lay on his right side or used a roller in the training room. He rated the pain as a 4 on a 1-to-10 scale and said the mass was mobile and had not changed in size or consistency.
Physical examination revealed a palpable mass over the lateral aspect of the hip, over the greater trochanter. The mass, about 3 cm in diameter (Figure 1), was mobile in a subcutaneous pocket, consistent with an old ML lesion.
Options discussed with the patient included use of ice, activity modification, and use of protective padded equipment. As the patient had tried these treatments before and was still intermittently having pain with direct pressure, he asked for surgical removal of the mass.
For the surgery, the patient was positioned in the lateral decubitus position with his right hip facing up. The right hip and thigh were prepared and draped in sterile fashion. An incision 4 cm in length was made directly over the mass, along the lateral aspect of the hip, over the greater trochanter. The incision was taken through skin and subcutaneous tissue down to the deep fascia. The fascia was incised longitudinally in line with the overlying skin incision. As soon as the incision was made through the fascia, the mass was easily seen. The 3-cm × 2-cm × 1-cm mass was free, not attached to any underlying soft tissue (Figure 3).
Discussion
We have described a case of symptomatic encapsulated fat necrosis lesion caused by an ML lesion in a professional hockey player. The ML lesion had resolved with nonoperative treatment (compression), but a subcutaneous pocket remained at the lesion site. Given the patient’s lesion site and occupation as a hockey player, pain with direct pressure on this lesion was a concern.
Long-standing ML lesions have 3 common patterns on MRI.14 A central region, encapsulated partially or completely by a peripheral ring of fibrous tissue or hemosiderin, shows signal properties consistent with a seroma, a homogeneous hemorrhagic collection, or a heterogeneous hemorrhagic collection. In our patient’s case, MRI was used to characterize the mobile mass for operative planning. Although thin strands or lobules of fat have been found within ML lesions, this case was the first to demonstrate a sequestered mass of necrotic fat.
Most football players who develop ML lesions on their knees do not wear kneepads.7-9 Of the 24 NFL players in the study by Tejwani and colleagues,9 52% were successfully treated with compression wrap, cryotherapy, and motion exercises. The rest, however, were treated with aspiration, and 11% underwent doxycycline sclerodesis for recurrent fluid collection. After treatment, all of their players were able to return to football. Their outcomes are consistent with that of our patient, who was treated with compression wrap and returned to hockey without any other intervention.
After our patient’s ML lesion resolved, he developed an encapsulated fat necrosis lesion from the disruption of the blood supply in the subcutaneous pocket. Encapsulated fat necrosis lesions are rare; only 65 have been reported.13,15 Clinically, these lesions are single or multiple pale-yellow encapsulated nodes.13 Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
The literature includes 1 report of an adolescent football player who developed multiple encapsulated fat necrosis lesions 4 months after landing on another player’s cleats.15 The patient, who was having pain with direct pressure during squatting and kneeling, elected to have the lesions surgically removed. These lesions are rare and usually asymptomatic,11 but our patient had his lesion surgically removed to address the pain induced by the direct impacts that came with playing professional hockey. Surgical removal is the treatment for symptomatic encapsulated fat necrosis lesions. Other than 1 case of recurrence after excision,16 these lesions have an excellent prognosis.
Conclusion
Our patient, a professional hockey player, underwent successful surgical removal of a symptomatic encapsulated fat necrosis lesion that had developed from an ML lesion.
Am J Orthop. 2017;46(3):E144-E147. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- ML lesions usually occur with high-energy injuries and have been reported in wrestlers, football players, and other athlete populations.
- Encapsulated fat necrosis lesions are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions.
- Encapsulated fat necrosis lesions are rare; only 65 have been reported.
- Encapsulated fat necrosis lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.
- Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
What would become known as the Morel-Lavallée (ML) lesion was first reported in 1853 by French physician Maurice Morel-Lavallée. He described a proximal thigh soft-tissue injury that resulted in a hemolymphatic collection between superficial fascial planes. Deforming forces of pressure and shear result in an internal degloving injury in which subcutaneous tissue is stripped from the fascia and replaced with a hematoma or, less commonly, necrotic fat.1-4 The injury can take several weeks to heal. Up to one-third of such injuries are initially missed because of the initial ecchymosis covering the injured area.5
ML lesions usually occur with high-energy injuries and have been reported in wrestlers,6 football players,7-9 and other athlete populations. ML lesions usually occur about the knee, the site of the sheer mechanism in these athletes’ sports. Tejwani and colleagues9 reported on 24 National Football League (NFL) players (27 knees). These elite athletes typically were able to return to practice and game play long before complete resolution of their lesions.
Nodular cystic fat necrosis was first described by Przyjemski and Schuster10 in 1977. The terms encapsulated fat necrosis lesions and mobile encapsulated lipomas11 were introduced later. Clinically, these entities usually present as lesions on the lower limbs of young men and middle-aged women and can range in size from 1 mm to 35 mm. Most of these lesions are mobile.11 They are usually attributable to trauma and disruption of the blood supply in the subcutaneous area, which occurs with ML lesions. Trauma accounts for the usual occurrence in the lower extremities, though only 40% of patients recall a precipitating event.12 Histologically, these lesions are characterized by massive fat necrosis encapsulated by fibrous tissue.13In this article, we report the case of a professional ice hockey player who presented with an ML lesion of the hip and then developed a symptomatic encapsulated fat necrosis lesion that required surgical removal. To our knowledge, this is the first reported case of an encapsulated fat necrosis lesion caused by an ML lesion in an athlete. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 21-year-old professional hockey player presented with a history of pain from a mass on his right hip. He first noticed the lesion, just lateral to the greater trochanter, about 3 years earlier. The mass appeared after he sustained a shearing-type injury to the lateral aspect of the hip. At the time, there was significant swelling along the lateral aspect, with ecchymosis that resolved over 2 months. The mass, diagnosed as an ML lesion, resolved with nonoperative treatment. However, in the area where the swelling had occurred, a hard mobile mass remained. At times, this mass became painful when direct pressure was applied, as when he hit the boards while playing hockey, or when he lay on his right side or used a roller in the training room. He rated the pain as a 4 on a 1-to-10 scale and said the mass was mobile and had not changed in size or consistency.
Physical examination revealed a palpable mass over the lateral aspect of the hip, over the greater trochanter. The mass, about 3 cm in diameter (Figure 1), was mobile in a subcutaneous pocket, consistent with an old ML lesion.
Options discussed with the patient included use of ice, activity modification, and use of protective padded equipment. As the patient had tried these treatments before and was still intermittently having pain with direct pressure, he asked for surgical removal of the mass.
For the surgery, the patient was positioned in the lateral decubitus position with his right hip facing up. The right hip and thigh were prepared and draped in sterile fashion. An incision 4 cm in length was made directly over the mass, along the lateral aspect of the hip, over the greater trochanter. The incision was taken through skin and subcutaneous tissue down to the deep fascia. The fascia was incised longitudinally in line with the overlying skin incision. As soon as the incision was made through the fascia, the mass was easily seen. The 3-cm × 2-cm × 1-cm mass was free, not attached to any underlying soft tissue (Figure 3).
Discussion
We have described a case of symptomatic encapsulated fat necrosis lesion caused by an ML lesion in a professional hockey player. The ML lesion had resolved with nonoperative treatment (compression), but a subcutaneous pocket remained at the lesion site. Given the patient’s lesion site and occupation as a hockey player, pain with direct pressure on this lesion was a concern.
Long-standing ML lesions have 3 common patterns on MRI.14 A central region, encapsulated partially or completely by a peripheral ring of fibrous tissue or hemosiderin, shows signal properties consistent with a seroma, a homogeneous hemorrhagic collection, or a heterogeneous hemorrhagic collection. In our patient’s case, MRI was used to characterize the mobile mass for operative planning. Although thin strands or lobules of fat have been found within ML lesions, this case was the first to demonstrate a sequestered mass of necrotic fat.
Most football players who develop ML lesions on their knees do not wear kneepads.7-9 Of the 24 NFL players in the study by Tejwani and colleagues,9 52% were successfully treated with compression wrap, cryotherapy, and motion exercises. The rest, however, were treated with aspiration, and 11% underwent doxycycline sclerodesis for recurrent fluid collection. After treatment, all of their players were able to return to football. Their outcomes are consistent with that of our patient, who was treated with compression wrap and returned to hockey without any other intervention.
After our patient’s ML lesion resolved, he developed an encapsulated fat necrosis lesion from the disruption of the blood supply in the subcutaneous pocket. Encapsulated fat necrosis lesions are rare; only 65 have been reported.13,15 Clinically, these lesions are single or multiple pale-yellow encapsulated nodes.13 Most are small and asymptomatic; however, in some cases, athletes can develop symptoms from frequent impacts to the region where the lesions are located.
The literature includes 1 report of an adolescent football player who developed multiple encapsulated fat necrosis lesions 4 months after landing on another player’s cleats.15 The patient, who was having pain with direct pressure during squatting and kneeling, elected to have the lesions surgically removed. These lesions are rare and usually asymptomatic,11 but our patient had his lesion surgically removed to address the pain induced by the direct impacts that came with playing professional hockey. Surgical removal is the treatment for symptomatic encapsulated fat necrosis lesions. Other than 1 case of recurrence after excision,16 these lesions have an excellent prognosis.
Conclusion
Our patient, a professional hockey player, underwent successful surgical removal of a symptomatic encapsulated fat necrosis lesion that had developed from an ML lesion.
Am J Orthop. 2017;46(3):E144-E147. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Aguiar RO, Viegas FC, Fernandez RY, Trudell D, Haghighi P, Resnick D. The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography. AJR Am J Roentgenol. 2007;188(4):W355-W358.
2. Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma. 1997;42(6):1046-1051.
3. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
4. Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin North Am. 2005;13(4):775-782.
5. Dye SF, Campagna-Pinto D, Dye CC, Shifflett S, Eiman T. Soft-tissue anatomy anterior to the human patella. J Bone Joint Surg Am. 2003;85(6):1012-1017.
6. Northam MC, Gaskin CM. Presumed prepatellar fibrosis in collegiate wrestlers: imaging findings and clinical correlation. Skeletal Radiol. 2015;44(2):271-277.
7. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Concealed degloving injury (the Morel-Lavallée lesion) in childhood sports: a case report. J Bone Joint Surg Am. 2011;93(24):e148.
8. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-Lavallée lesion in a professional American football player. Am J Orthop. 2010;39(3):144-147.
9. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
10. Przyjemski CJ, Schuster SR. Nodular-cystic fat necrosis. J Pediatr. 1977;91(4):605-607.
11. Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis—a clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27(1):19-23.
12. Santos-Juanes J, Coto P, Galache C, Sánchez del Rio J, Soto de Delás J. Encapsulated fat necrosis: a form of traumatic panniculitis. J Eur Acad Dermatol Venereol. 2007;21(3):405-406.
13. Sempau L, Sambucetty PS, Garcia JL, Sixto BG, Morán AG, Prieto MA. Mobile encapsulated lipoma. Int J Dermatol. 2012;51(4):448-450.
14. Mellado JM, Pérez del Palomar L, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289-1294.
15. Sole JS, Wisniewski SJ, Dahm DL, Bond J, Smith J. Posttraumatic fat necrosis presenting as prepatellar loose bodies in an adolescent football player. PM R. 2014;6(8):749-752.
16. Felipo F, Vaquero M, del Agua C. Pseudotumoral encapsulated fat necrosis with diffuse pseudomembranous degeneration. J Cutan Pathol. 2004;31(8):565-567.
1. Aguiar RO, Viegas FC, Fernandez RY, Trudell D, Haghighi P, Resnick D. The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography. AJR Am J Roentgenol. 2007;188(4):W355-W358.
2. Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma. 1997;42(6):1046-1051.
3. Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89(5):853-855.
4. Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin North Am. 2005;13(4):775-782.
5. Dye SF, Campagna-Pinto D, Dye CC, Shifflett S, Eiman T. Soft-tissue anatomy anterior to the human patella. J Bone Joint Surg Am. 2003;85(6):1012-1017.
6. Northam MC, Gaskin CM. Presumed prepatellar fibrosis in collegiate wrestlers: imaging findings and clinical correlation. Skeletal Radiol. 2015;44(2):271-277.
7. Anakwenze OA, Trivedi V, Goodman AM, Ganley TJ. Concealed degloving injury (the Morel-Lavallée lesion) in childhood sports: a case report. J Bone Joint Surg Am. 2011;93(24):e148.
8. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-Lavallée lesion in a professional American football player. Am J Orthop. 2010;39(3):144-147.
9. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the National Football League. Am J Sports Med. 2007;35(7):1162-1167.
10. Przyjemski CJ, Schuster SR. Nodular-cystic fat necrosis. J Pediatr. 1977;91(4):605-607.
11. Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis—a clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27(1):19-23.
12. Santos-Juanes J, Coto P, Galache C, Sánchez del Rio J, Soto de Delás J. Encapsulated fat necrosis: a form of traumatic panniculitis. J Eur Acad Dermatol Venereol. 2007;21(3):405-406.
13. Sempau L, Sambucetty PS, Garcia JL, Sixto BG, Morán AG, Prieto MA. Mobile encapsulated lipoma. Int J Dermatol. 2012;51(4):448-450.
14. Mellado JM, Pérez del Palomar L, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182(5):1289-1294.
15. Sole JS, Wisniewski SJ, Dahm DL, Bond J, Smith J. Posttraumatic fat necrosis presenting as prepatellar loose bodies in an adolescent football player. PM R. 2014;6(8):749-752.
16. Felipo F, Vaquero M, del Agua C. Pseudotumoral encapsulated fat necrosis with diffuse pseudomembranous degeneration. J Cutan Pathol. 2004;31(8):565-567.
Joint-Preserving Osteotomies for Isolated Patellofemoral Osteoarthritis: Alternatives to Arthroplasty
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
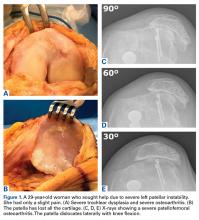
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
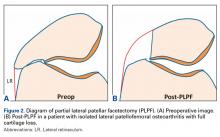
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
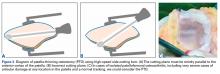
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
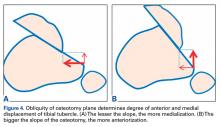
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
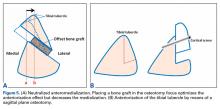
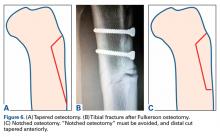
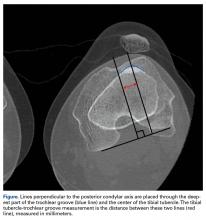
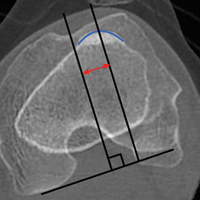
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
Measuring Malalignment on Imaging in the Treatment of Patellofemoral Instability
Take-Home Points
- Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG.
- TT-TG distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.
- TT-TG distance criteria should serve as a guide, rather than a rigid threshold, in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
- Factors such as knee flexion angle, imaging modality, and landmarks used for the measurements should be considered when using TT-TG distance as an indication for surgery.
- There has been significant variability in reported TT-TG measurements. A surgeon using this measurement should understand how it is obtained because many technical factors are involved.
Assessment of malalignment is an important factor in determining surgical treatment options for patellar instability. Although soft-tissue reconstruction of the medial soft-tissue stabilizers is often performed to address patellar instability, bony malalignment may increase stress on the medial soft tissues; therefore, it must be adequately identified and addressed.
Bony malalignment, which is often thought of as lateralization of the tibial tubercle (TT), can be influenced by tibiofemoral alignment, external tibial torsion, and femoral anteversion.
Clinically, coronal alignment can be assessed with a measurement such as quadriceps (Q) angle, but this has been reported to have low interrater reliability and high variability in the reported optimal conditions and positions in which the measurement should be made.1-3An anatomically lateralized TT pulls the extensor mechanism laterally with respect to the trochlear groove (TG), and this can accentuate problems related to patellofemoral instability. A recent biomechanical study found that increased TT lateralization significantly increased lateral patellar translation and tilt in the setting of medial patellofemoral ligament (MPFL) deficiency.4 Although MPFL reconstruction restored patellar kinematics and contact mechanics, this restoration did not occur when the TT was lateralized more than 10 mm relative to its normal position.
Realigning the extensor mechanism by moving the TT medially decreases the lateralizing forces on the patella and the stress on the soft-tissue restraints. This raises the issues of when to correct a lateralized TT and how to identify and measure malalignment.
Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG. Originally described on radiographs and subsequently on computed tomography (CT) and magnetic resonance imaging (MRI) scans, distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.5,6However, there has been significant variability in reported TT-TG measurements. Studies have found that TT-TG distance is 3.8 mm larger on CT scans than on MRI scans.7 Furthermore, factors such as knee flexion angle at time of imaging have been found to reduce TT-TG distance.1 More recently, patient size and TT-TG ratios relative to patellar and trochlear width were identified as important factors in assessing TT-TG distance.8 Therefore, TT-TG distance measurements should serve as a guide rather than a rigid threshold in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
What You Need to Know About Measuring Patellofemoral Malalignment
TT-TG distance can guide decisions about performing a medializing TT osteotomy for patellar instability because the measurement can aid in assessing bony malalignment caused by an anatomically lateralized tubercle. TT-TG distance can be used to determine when and how far to move the tubercle in TT osteotomy.
Background
A normal TT-TG value is approximately 10 mm. The measurement originally used bony landmarks, including the deepest part of the bony TG and the anterior-most part of the TT, as described by Goutallier and colleagues.9 In their original study, Dejour and colleagues5 found that patients with recurrent symptoms of patellar instability had TT-TG distances >20 mm.
Increased TT-TG distance has been shown to correlate with patellar position, including increased lateral shift and lateral tilt of the patella. In a study using dynamic CT scans of patients with recurrent patellar instability, we found that TT-TG distance increased with knee extension, and that this increase correlated with the lateral shift and lateral tilt of the patella.10An excessively lateralized TT can be corrected with a medializing osteotomy that reduces TT-TG distance to within the normal range. TT surgery can be performed with flat osteotomy, as described by Elmslie and Trillat,11 or with oblique osteotomy, as described by Fulkerson,6 to obtain concomitant anteriorization. In a computational study, Elias and colleagues12 found that medializing TT osteotomy not only reduced TT-TG distance but led to correction of lateral patellar tilt and displacement. Patellofemoral contact forces have also shown to be reduced with anteromedialization.6Although reported outcomes of TT osteotomy have been excellent for patients with patellar instability, the procedure has higher risks and longer rehabilitation relative to a soft-tissue procedure alone. Reported risks associated with TT osteotomy include fracture, nonunion, delayed union, painful screws, and deep vein thrombosis.6,10,13,14Understanding the limitations of and variability in radiographic assessments of TT and TG positions can help when deciding whether to perform TT osteotomy for patellar instability.
Discussion
When considering TT osteotomy for patellar instability, some surgeons use a TT-TG distance of more than 15 mm or 20 mm as a threshold for performing medialization. The variability is based on the multiple patient and imaging factors that can influence TT-TG distance measurement.
Several TG and TT landmarks have been used to measure TT-TG distance. The deepest part of the TG, based on bony anatomy, was used originally, but the cartilaginous landmark at the deepest part of the cartilaginous TG has also been described.15 Similarly, on the TT, the original description of TT-TG distance, by Goutallier and colleagues,9 involved the anterior-most part of the TT on CT scan, though the central part of the TT has also been described.15 We found a 4.2-mm difference in TT-TG distance with use of different landmarks (central tubercle, anterior tubercle) within the same study population.16 Therefore, within a practice, the distance used as an indication for TT osteotomy should be measured consistently.
Knee flexion angle at the time of imaging can also affect measurement of TT-TG distance. Several authors have reported smaller TT-TG distance with increased knee flexion angle.10,16,17 In a study of patients with symptomatic patellar instability, we found that TT-TG distance decreases by an estimated 1 mm for every 4.4° of knee flexion >0°.10 In measurements of TT-TG distance, the sagittal view can be used to assess knee flexion angle because positioning protocols and patient comfort at the time of imaging may produce variable knee flexion angles.
Given the variability that occurs in TT-TG distance with knee flexion angles, some surgeons use TT–posterior cruciate ligament (PCL) distance as another measurement of TT lateralization.18 This measurement is made with both tibial landmarks, from the TT to the medial border of the PCL insertion on the tibia, and theoretically eliminates knee flexion angle as a measurement factor. Seitlinger and colleagues18 found that values >24 mm were associated with symptoms of patellar instability. More study is needed to determine the precise indications for TT osteotomy with use of this measurement.
In addition to patient positioning during knee imaging, patient size should be considered when TT-TG distance is used for malalignment measurement. Camp and colleagues8 discussed the importance of “individualizing” TT-TG distance on the basis of patient size and bony structure. They reported that the ratio of TT-TG distance to trochlear width or patellar width more effectively predicted recurrent patellar instability than TT-TG distance alone.
Measurement of TT-TG distance is valuable in planning surgical treatment for patellar instability because it quantifies a component of malalignment and aids in deciding whether to perform TT osteotomy. However, this distance should be understood in the context of many measurement factors to allow for an individualized procedure that addresses the specific contributors to patellar instability in each patient.
Am J Orthop. 2017;46(3):148-151. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. France L, Nester C. Effect of errors in the identification of anatomical landmarks on the accuracy of Q angle values. Clin Biomech (Bristol, Avon). 2001;16(8):710-713.
2. Greene CC, Edwards TB, Wade MR, Carson EW. Reliability of the quadriceps angle measurement. Am J Knee Surg. 2001;14(2):97-103.
3. Smith TO, Hunt NJ, Donell ST. The reliability and validity of the Q-angle: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1068-1079.
4. Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med. 2015;43(9):2198-2207.
5. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
6. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176-181.
7. Camp CL, Stuart MJ, Krych AJ, et al. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med. 2013;41(8):1835-1840.
8. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
9. Goutallier D, Bernageau J, Lecudonnec B. [The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
10. Tanaka MJ, Elias JJ, Williams AA, Carrino JA, Cosgarea AJ. Correlation between changes in tibial tuberosity-trochlear groove distance and patellar position during active knee extension on dynamic kinematic computed tomography imaging. Arthroscopy. 2015;31(9):1748-1755.
11. Trillat A, Dejour H, Couette A. [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Motur. 1964;50(6):813-824.
12. Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2350-2356.
13. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
14. Wilcox JJ, Snow BJ, Aoki SK, Hung M, Burks RT. Does landmark selection affect the reliability of tibial tubercle-trochlear groove measurements using MRI? Clin Orthop Relat Res. 2012;470(8):2253-2260.
15. Schoettle PB, Zanetti M, Seifert B, Pfirrmann CWA, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13(1):26-31.
16. Williams AA, Tanaka MJ, Elias JJ, et al. Measuring tibial tuberosity-trochlear groove distance on CT: Where to begin? Presented at the American Academy of Orthopaedic Surgeons Annual Meeting, New Orleans, LA, March 11-15, 2014.
17. Dietrich TJ, Betz M, Pfirrmann CWA, Koch PP, Fucentese SF. End-stage extension of the knee and its influence on tibial tuberosity-trochlear groove distance (TTTG) in asymptomatic volunteers. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):214-218.
18. Seitlinger G, Scheurecker G, Hogler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
Take-Home Points
- Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG.
- TT-TG distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.
- TT-TG distance criteria should serve as a guide, rather than a rigid threshold, in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
- Factors such as knee flexion angle, imaging modality, and landmarks used for the measurements should be considered when using TT-TG distance as an indication for surgery.
- There has been significant variability in reported TT-TG measurements. A surgeon using this measurement should understand how it is obtained because many technical factors are involved.
Assessment of malalignment is an important factor in determining surgical treatment options for patellar instability. Although soft-tissue reconstruction of the medial soft-tissue stabilizers is often performed to address patellar instability, bony malalignment may increase stress on the medial soft tissues; therefore, it must be adequately identified and addressed.
Bony malalignment, which is often thought of as lateralization of the tibial tubercle (TT), can be influenced by tibiofemoral alignment, external tibial torsion, and femoral anteversion.
Clinically, coronal alignment can be assessed with a measurement such as quadriceps (Q) angle, but this has been reported to have low interrater reliability and high variability in the reported optimal conditions and positions in which the measurement should be made.1-3An anatomically lateralized TT pulls the extensor mechanism laterally with respect to the trochlear groove (TG), and this can accentuate problems related to patellofemoral instability. A recent biomechanical study found that increased TT lateralization significantly increased lateral patellar translation and tilt in the setting of medial patellofemoral ligament (MPFL) deficiency.4 Although MPFL reconstruction restored patellar kinematics and contact mechanics, this restoration did not occur when the TT was lateralized more than 10 mm relative to its normal position.
Realigning the extensor mechanism by moving the TT medially decreases the lateralizing forces on the patella and the stress on the soft-tissue restraints. This raises the issues of when to correct a lateralized TT and how to identify and measure malalignment.
Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG. Originally described on radiographs and subsequently on computed tomography (CT) and magnetic resonance imaging (MRI) scans, distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.5,6However, there has been significant variability in reported TT-TG measurements. Studies have found that TT-TG distance is 3.8 mm larger on CT scans than on MRI scans.7 Furthermore, factors such as knee flexion angle at time of imaging have been found to reduce TT-TG distance.1 More recently, patient size and TT-TG ratios relative to patellar and trochlear width were identified as important factors in assessing TT-TG distance.8 Therefore, TT-TG distance measurements should serve as a guide rather than a rigid threshold in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
What You Need to Know About Measuring Patellofemoral Malalignment
TT-TG distance can guide decisions about performing a medializing TT osteotomy for patellar instability because the measurement can aid in assessing bony malalignment caused by an anatomically lateralized tubercle. TT-TG distance can be used to determine when and how far to move the tubercle in TT osteotomy.
Background
A normal TT-TG value is approximately 10 mm. The measurement originally used bony landmarks, including the deepest part of the bony TG and the anterior-most part of the TT, as described by Goutallier and colleagues.9 In their original study, Dejour and colleagues5 found that patients with recurrent symptoms of patellar instability had TT-TG distances >20 mm.
Increased TT-TG distance has been shown to correlate with patellar position, including increased lateral shift and lateral tilt of the patella. In a study using dynamic CT scans of patients with recurrent patellar instability, we found that TT-TG distance increased with knee extension, and that this increase correlated with the lateral shift and lateral tilt of the patella.10An excessively lateralized TT can be corrected with a medializing osteotomy that reduces TT-TG distance to within the normal range. TT surgery can be performed with flat osteotomy, as described by Elmslie and Trillat,11 or with oblique osteotomy, as described by Fulkerson,6 to obtain concomitant anteriorization. In a computational study, Elias and colleagues12 found that medializing TT osteotomy not only reduced TT-TG distance but led to correction of lateral patellar tilt and displacement. Patellofemoral contact forces have also shown to be reduced with anteromedialization.6Although reported outcomes of TT osteotomy have been excellent for patients with patellar instability, the procedure has higher risks and longer rehabilitation relative to a soft-tissue procedure alone. Reported risks associated with TT osteotomy include fracture, nonunion, delayed union, painful screws, and deep vein thrombosis.6,10,13,14Understanding the limitations of and variability in radiographic assessments of TT and TG positions can help when deciding whether to perform TT osteotomy for patellar instability.
Discussion
When considering TT osteotomy for patellar instability, some surgeons use a TT-TG distance of more than 15 mm or 20 mm as a threshold for performing medialization. The variability is based on the multiple patient and imaging factors that can influence TT-TG distance measurement.
Several TG and TT landmarks have been used to measure TT-TG distance. The deepest part of the TG, based on bony anatomy, was used originally, but the cartilaginous landmark at the deepest part of the cartilaginous TG has also been described.15 Similarly, on the TT, the original description of TT-TG distance, by Goutallier and colleagues,9 involved the anterior-most part of the TT on CT scan, though the central part of the TT has also been described.15 We found a 4.2-mm difference in TT-TG distance with use of different landmarks (central tubercle, anterior tubercle) within the same study population.16 Therefore, within a practice, the distance used as an indication for TT osteotomy should be measured consistently.
Knee flexion angle at the time of imaging can also affect measurement of TT-TG distance. Several authors have reported smaller TT-TG distance with increased knee flexion angle.10,16,17 In a study of patients with symptomatic patellar instability, we found that TT-TG distance decreases by an estimated 1 mm for every 4.4° of knee flexion >0°.10 In measurements of TT-TG distance, the sagittal view can be used to assess knee flexion angle because positioning protocols and patient comfort at the time of imaging may produce variable knee flexion angles.
Given the variability that occurs in TT-TG distance with knee flexion angles, some surgeons use TT–posterior cruciate ligament (PCL) distance as another measurement of TT lateralization.18 This measurement is made with both tibial landmarks, from the TT to the medial border of the PCL insertion on the tibia, and theoretically eliminates knee flexion angle as a measurement factor. Seitlinger and colleagues18 found that values >24 mm were associated with symptoms of patellar instability. More study is needed to determine the precise indications for TT osteotomy with use of this measurement.
In addition to patient positioning during knee imaging, patient size should be considered when TT-TG distance is used for malalignment measurement. Camp and colleagues8 discussed the importance of “individualizing” TT-TG distance on the basis of patient size and bony structure. They reported that the ratio of TT-TG distance to trochlear width or patellar width more effectively predicted recurrent patellar instability than TT-TG distance alone.
Measurement of TT-TG distance is valuable in planning surgical treatment for patellar instability because it quantifies a component of malalignment and aids in deciding whether to perform TT osteotomy. However, this distance should be understood in the context of many measurement factors to allow for an individualized procedure that addresses the specific contributors to patellar instability in each patient.
Am J Orthop. 2017;46(3):148-151. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG.
- TT-TG distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.
- TT-TG distance criteria should serve as a guide, rather than a rigid threshold, in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
- Factors such as knee flexion angle, imaging modality, and landmarks used for the measurements should be considered when using TT-TG distance as an indication for surgery.
- There has been significant variability in reported TT-TG measurements. A surgeon using this measurement should understand how it is obtained because many technical factors are involved.
Assessment of malalignment is an important factor in determining surgical treatment options for patellar instability. Although soft-tissue reconstruction of the medial soft-tissue stabilizers is often performed to address patellar instability, bony malalignment may increase stress on the medial soft tissues; therefore, it must be adequately identified and addressed.
Bony malalignment, which is often thought of as lateralization of the tibial tubercle (TT), can be influenced by tibiofemoral alignment, external tibial torsion, and femoral anteversion.
Clinically, coronal alignment can be assessed with a measurement such as quadriceps (Q) angle, but this has been reported to have low interrater reliability and high variability in the reported optimal conditions and positions in which the measurement should be made.1-3An anatomically lateralized TT pulls the extensor mechanism laterally with respect to the trochlear groove (TG), and this can accentuate problems related to patellofemoral instability. A recent biomechanical study found that increased TT lateralization significantly increased lateral patellar translation and tilt in the setting of medial patellofemoral ligament (MPFL) deficiency.4 Although MPFL reconstruction restored patellar kinematics and contact mechanics, this restoration did not occur when the TT was lateralized more than 10 mm relative to its normal position.
Realigning the extensor mechanism by moving the TT medially decreases the lateralizing forces on the patella and the stress on the soft-tissue restraints. This raises the issues of when to correct a lateralized TT and how to identify and measure malalignment.
Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG. Originally described on radiographs and subsequently on computed tomography (CT) and magnetic resonance imaging (MRI) scans, distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.5,6However, there has been significant variability in reported TT-TG measurements. Studies have found that TT-TG distance is 3.8 mm larger on CT scans than on MRI scans.7 Furthermore, factors such as knee flexion angle at time of imaging have been found to reduce TT-TG distance.1 More recently, patient size and TT-TG ratios relative to patellar and trochlear width were identified as important factors in assessing TT-TG distance.8 Therefore, TT-TG distance measurements should serve as a guide rather than a rigid threshold in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
What You Need to Know About Measuring Patellofemoral Malalignment
TT-TG distance can guide decisions about performing a medializing TT osteotomy for patellar instability because the measurement can aid in assessing bony malalignment caused by an anatomically lateralized tubercle. TT-TG distance can be used to determine when and how far to move the tubercle in TT osteotomy.
Background
A normal TT-TG value is approximately 10 mm. The measurement originally used bony landmarks, including the deepest part of the bony TG and the anterior-most part of the TT, as described by Goutallier and colleagues.9 In their original study, Dejour and colleagues5 found that patients with recurrent symptoms of patellar instability had TT-TG distances >20 mm.
Increased TT-TG distance has been shown to correlate with patellar position, including increased lateral shift and lateral tilt of the patella. In a study using dynamic CT scans of patients with recurrent patellar instability, we found that TT-TG distance increased with knee extension, and that this increase correlated with the lateral shift and lateral tilt of the patella.10An excessively lateralized TT can be corrected with a medializing osteotomy that reduces TT-TG distance to within the normal range. TT surgery can be performed with flat osteotomy, as described by Elmslie and Trillat,11 or with oblique osteotomy, as described by Fulkerson,6 to obtain concomitant anteriorization. In a computational study, Elias and colleagues12 found that medializing TT osteotomy not only reduced TT-TG distance but led to correction of lateral patellar tilt and displacement. Patellofemoral contact forces have also shown to be reduced with anteromedialization.6Although reported outcomes of TT osteotomy have been excellent for patients with patellar instability, the procedure has higher risks and longer rehabilitation relative to a soft-tissue procedure alone. Reported risks associated with TT osteotomy include fracture, nonunion, delayed union, painful screws, and deep vein thrombosis.6,10,13,14Understanding the limitations of and variability in radiographic assessments of TT and TG positions can help when deciding whether to perform TT osteotomy for patellar instability.
Discussion
When considering TT osteotomy for patellar instability, some surgeons use a TT-TG distance of more than 15 mm or 20 mm as a threshold for performing medialization. The variability is based on the multiple patient and imaging factors that can influence TT-TG distance measurement.
Several TG and TT landmarks have been used to measure TT-TG distance. The deepest part of the TG, based on bony anatomy, was used originally, but the cartilaginous landmark at the deepest part of the cartilaginous TG has also been described.15 Similarly, on the TT, the original description of TT-TG distance, by Goutallier and colleagues,9 involved the anterior-most part of the TT on CT scan, though the central part of the TT has also been described.15 We found a 4.2-mm difference in TT-TG distance with use of different landmarks (central tubercle, anterior tubercle) within the same study population.16 Therefore, within a practice, the distance used as an indication for TT osteotomy should be measured consistently.
Knee flexion angle at the time of imaging can also affect measurement of TT-TG distance. Several authors have reported smaller TT-TG distance with increased knee flexion angle.10,16,17 In a study of patients with symptomatic patellar instability, we found that TT-TG distance decreases by an estimated 1 mm for every 4.4° of knee flexion >0°.10 In measurements of TT-TG distance, the sagittal view can be used to assess knee flexion angle because positioning protocols and patient comfort at the time of imaging may produce variable knee flexion angles.
Given the variability that occurs in TT-TG distance with knee flexion angles, some surgeons use TT–posterior cruciate ligament (PCL) distance as another measurement of TT lateralization.18 This measurement is made with both tibial landmarks, from the TT to the medial border of the PCL insertion on the tibia, and theoretically eliminates knee flexion angle as a measurement factor. Seitlinger and colleagues18 found that values >24 mm were associated with symptoms of patellar instability. More study is needed to determine the precise indications for TT osteotomy with use of this measurement.
In addition to patient positioning during knee imaging, patient size should be considered when TT-TG distance is used for malalignment measurement. Camp and colleagues8 discussed the importance of “individualizing” TT-TG distance on the basis of patient size and bony structure. They reported that the ratio of TT-TG distance to trochlear width or patellar width more effectively predicted recurrent patellar instability than TT-TG distance alone.
Measurement of TT-TG distance is valuable in planning surgical treatment for patellar instability because it quantifies a component of malalignment and aids in deciding whether to perform TT osteotomy. However, this distance should be understood in the context of many measurement factors to allow for an individualized procedure that addresses the specific contributors to patellar instability in each patient.
Am J Orthop. 2017;46(3):148-151. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. France L, Nester C. Effect of errors in the identification of anatomical landmarks on the accuracy of Q angle values. Clin Biomech (Bristol, Avon). 2001;16(8):710-713.
2. Greene CC, Edwards TB, Wade MR, Carson EW. Reliability of the quadriceps angle measurement. Am J Knee Surg. 2001;14(2):97-103.
3. Smith TO, Hunt NJ, Donell ST. The reliability and validity of the Q-angle: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1068-1079.
4. Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med. 2015;43(9):2198-2207.
5. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
6. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176-181.
7. Camp CL, Stuart MJ, Krych AJ, et al. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med. 2013;41(8):1835-1840.
8. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
9. Goutallier D, Bernageau J, Lecudonnec B. [The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
10. Tanaka MJ, Elias JJ, Williams AA, Carrino JA, Cosgarea AJ. Correlation between changes in tibial tuberosity-trochlear groove distance and patellar position during active knee extension on dynamic kinematic computed tomography imaging. Arthroscopy. 2015;31(9):1748-1755.
11. Trillat A, Dejour H, Couette A. [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Motur. 1964;50(6):813-824.
12. Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2350-2356.
13. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
14. Wilcox JJ, Snow BJ, Aoki SK, Hung M, Burks RT. Does landmark selection affect the reliability of tibial tubercle-trochlear groove measurements using MRI? Clin Orthop Relat Res. 2012;470(8):2253-2260.
15. Schoettle PB, Zanetti M, Seifert B, Pfirrmann CWA, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13(1):26-31.
16. Williams AA, Tanaka MJ, Elias JJ, et al. Measuring tibial tuberosity-trochlear groove distance on CT: Where to begin? Presented at the American Academy of Orthopaedic Surgeons Annual Meeting, New Orleans, LA, March 11-15, 2014.
17. Dietrich TJ, Betz M, Pfirrmann CWA, Koch PP, Fucentese SF. End-stage extension of the knee and its influence on tibial tuberosity-trochlear groove distance (TTTG) in asymptomatic volunteers. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):214-218.
18. Seitlinger G, Scheurecker G, Hogler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
1. France L, Nester C. Effect of errors in the identification of anatomical landmarks on the accuracy of Q angle values. Clin Biomech (Bristol, Avon). 2001;16(8):710-713.
2. Greene CC, Edwards TB, Wade MR, Carson EW. Reliability of the quadriceps angle measurement. Am J Knee Surg. 2001;14(2):97-103.
3. Smith TO, Hunt NJ, Donell ST. The reliability and validity of the Q-angle: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1068-1079.
4. Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med. 2015;43(9):2198-2207.
5. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
6. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176-181.
7. Camp CL, Stuart MJ, Krych AJ, et al. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med. 2013;41(8):1835-1840.
8. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
9. Goutallier D, Bernageau J, Lecudonnec B. [The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
10. Tanaka MJ, Elias JJ, Williams AA, Carrino JA, Cosgarea AJ. Correlation between changes in tibial tuberosity-trochlear groove distance and patellar position during active knee extension on dynamic kinematic computed tomography imaging. Arthroscopy. 2015;31(9):1748-1755.
11. Trillat A, Dejour H, Couette A. [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Motur. 1964;50(6):813-824.
12. Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2350-2356.
13. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
14. Wilcox JJ, Snow BJ, Aoki SK, Hung M, Burks RT. Does landmark selection affect the reliability of tibial tubercle-trochlear groove measurements using MRI? Clin Orthop Relat Res. 2012;470(8):2253-2260.
15. Schoettle PB, Zanetti M, Seifert B, Pfirrmann CWA, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13(1):26-31.
16. Williams AA, Tanaka MJ, Elias JJ, et al. Measuring tibial tuberosity-trochlear groove distance on CT: Where to begin? Presented at the American Academy of Orthopaedic Surgeons Annual Meeting, New Orleans, LA, March 11-15, 2014.
17. Dietrich TJ, Betz M, Pfirrmann CWA, Koch PP, Fucentese SF. End-stage extension of the knee and its influence on tibial tuberosity-trochlear groove distance (TTTG) in asymptomatic volunteers. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):214-218.
18. Seitlinger G, Scheurecker G, Hogler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
Ultrasound-Guided Percutaneous Repair of Medial Patellofemoral Ligament: Surgical Technique and Outcomes
Take-Home Points
- Use ultrasound to identify integrity and location of MPFL tear.
- Anatomic repair allows native tissue to reintegrate into bone.
- Repairs done early can prevent complications of recurrent instability.
- Repair maintains biological and proprioceptive qualities of tissue.
- 10Ultrasound-guided percutaneous repair is quick and effective.
The medial patellofemoral ligament (MPFL) is the primary passive restraint to lateral patellar excursion1-5 and helps control patellar tilt and rotation.6,7 More than 90% of lateral patellar dislocations cause the MPFL to rupture, and roughly 90% of these detachments involve the femoral insertion.4 Ensuing patellar instability often results from MPFL insufficiency. It has been suggested that re-creating the anatomy and functionality of this ligament is of utmost importance in restoring normal patellar biomechanics.1-5,7,8
Anatomical risk factors for recurrent patellar instability include patella alta, increased tibial tuberosity-trochlear groove (TT-TG) distance, trochlear dysplasia, and torsional abnormalities.1-4,6 A medial reefing technique with a lateral tissue release traditionally was used to restore proper kinematics, but was shown to have associated postoperative issues.9
Methods
Patient Demographics
Dr. Hirahara developed this technique in 2013 and performed it 11 times between 2013 and 2016. Of the 11 patients, 1 was excluded from our retrospective analysis because of trochlear dysplasia, now considered a relative contraindication. Of the remaining 10 patients, 5 (50%) had the repair performed on the right knee. Eight patients (80%) were female. Mean (SD) age was 17.21 (3.53) years. One patient had concurrent femur- and patella-side detachments; otherwise, 6 (60%) of 10 repairs were performed exclusively at the patella. We grade patellar instability according to amount of glide based on patellar width and quadrants. Normal lateral displacement was usually 1 to 2 quadrants of lateral glide relative to the contralateral side. Before surgery, 6 (60%) of the 10 patients presented with lateral glide of 3 quadrants, and 3 (30%) presented with lateral glide of 4 quadrants. All had patellar instability apprehension on physical examination.
Surgical Indications
Before surgery, MPFL integrity is determined by ultrasound evaluation. Repair is considered if the MPFL has a femur- or patella-side tear and is of adequate quantity and quality, and if there are minimal or no arthritic changes (Table 2).
Surgical Technique
The patient is brought to the operating room and placed supine. Patellar stability of the affected knee is assessed and compared with that of the contralateral side with patellar glide. The knee is prepared and draped in usual sterile fashion. With the knee flexed at 90º, a tourniquet is inflated. Diagnostic arthroscopy is performed with standard anteromedial and anterolateral portals, and, if necessary, arthroscopic procedures are performed.
Femoral Attachment Repair
With the leg in extension, ultrasound is used to identify the tear at the femoral attachment (watch part 1 of the video). A spinal needle is placed at the femoral insertion, typically just anterior and distal to the adductor tubercle (Figure 4).10
Patellar Attachment Repair
With the leg in extension, ultrasound is used to identify where the MPFL is detached from the patella (watch part 2 of the video). A spinal needle is placed at the detachment site (Figure 5). A scalpel is used to make a 1-cm incision down to the patella.
In this description, we showcase knotless and knotted techniques for each repair site. Either method is appropriate for the 2 repair sites. Owing to the superficial nature of the attachment sites—they may have very little fat, particularly at the patella—knot stacks are more prominent, can be felt after surgery, and have the potential to irritate surrounding tissues. Therefore, we prefer knotless fixation for both sites.
Rehabilitation
Rehabilitation after MPFL repair is much like rehabilitation after quadriceps tendon repair. The patient is locked in a brace in full extension when up and moving. Early weight-bearing and minimal use of assistive devices (crutches) are allowed because, when the leg is in full extension, there is no tension at the repair sites. Rehabilitation begins within 1 week, and normal daily function is quickly attained. The protocol emphasizes pain-free motion and suitable patellar mobility, and allows the immobilizing brace to be unlocked for exercise and sitting. During the first 4 weeks, quadriceps activation is limited; progression to full ROM occurs by 4 to 6 weeks. During the strengthening phase, loading the knee in early flexion should be avoided. Return to heavy lifting, physical activity, and sports is delayed until after 6 months in order to allow the construct to mature and integrate. Once the patient has satisfied all the strength, ROM, and functional outcome measurements, a brace is no longer required during sports and normal activity.
Results
Mean tourniquet time for each procedure, which includes diagnostic arthroscopy and ultrasound-guided percutaneous repair, was 26.9 minutes.
Discussion
Conservative management typically is recommended for acute patellar dislocations. In the event of failed conservative management or chronic patellar instability, surgical intervention is indicated. Studies have found that conservative management has recurrent-dislocation rates of 35% at 3-year follow-up and 73% at 6-year follow-up, and recurrent dislocations significantly increase patients’ risk of developing chondral and bony damage.13 MPFL repair is designed to restore proper patellar tracking and kinematics while maintaining the anatomical tissue. Lateral patellar dislocations often cause the MPFL to rupture; tears are reported in more than 90% of incidents.4 The significant rate indicates that, even after a single patellar dislocation, the MPFL should be evaluated. The MPFL contributes 50% to 60% of the medial stabilizing force during patellar tracking1,7,14 and is the primary restraint to lateral patellar excursion and excessive patellar tilt and rotation.1-5 Its absence plays a key role in recurrent lateral patellar instability. With this structure being so important, proper identification and intervention are vital. Studies have established that redislocation rates are significantly higher for nonoperatively (vs operatively) treated primary patellar dislocations.13 Simple and accurate percutaneous repair of the MPFL should be performed early to avoid the long-term complications of recurrent instability that could damage the cartilage and bone of the patella and trochlea.
The primary advantage of this technique is its novel use of musculoskeletal ultrasound to accurately identify anatomy and pathology and the placement of anatomical repairs. Accurate preoperative and intraoperative assessment of MPFL anatomy is vital to the success of a procedure. Descriptions of MPFL anatomy suggest discrepancies in the exact locations of the femoral and patellar attachments.2,5,7,10,12,15,16 Tanaka5 noted that, even within paired knees, there was “marked variability” in the MPFL insertions. McCarthy and colleagues10 contended the femoral attachment of the MPFL is just anterior and distal to the adductor tubercle, the landmark addressed in this technique. Steensen and colleagues16 described this attachment site as being statistically the “single most important point affecting isometry” of the MPFL. Sallay and colleagues4 asserted that an overwhelming majority of MPFL tears (87%) occur at the adductor tubercle. The variable distribution of tear locations and the importance of re-creating patient anatomy further highlight the need for individualized treatment, which is afforded by ultrasound. Fluoroscopy has been inadequate in identifying MPFL anatomy; this modality is difficult, cumbersome, inaccurate, and inconsistent.11,12 Conversely, ultrasound provides real-time visualization of anatomy and allows for precise identification of MPFL attachments and accurate placement of suture anchors for repair during surgery (Figures 3, 4).
For femur-side and patella-side tears, repairs can and should be performed. For midsubstance tears, however, repair is not feasible, and reconstruction is appropriate. MPFL repair is superior to reconstruction in several ways. Repair is a simple percutaneous procedure that had a mean tourniquet time of 26.9 minutes in this study. For tissue that is quantitatively and qualitatively adequate, repair allows the structure to reintegrate into bone without total reconstruction. In the event of multiple tears, the percutaneous procedure allows for repair of each attachment. As the MPFL sits between the second and third tissue layers of the medial knee, reconstruction can be difficult and invasive and require establishment of a between-layers plane, which can disrupt adjacent tissue.4,7,17 Repair also maintains native tissue and its neurovascular and proprioceptive properties.
Reconstruction of the MPFL has become the gold-standard treatment for recurrent lateral patellar instability but has limitations and complications.3,7,12,17 Reconstruction techniques use either surface anatomy palpation (requiring large incisions) or fluoroscopy to identify tunnel placement locations, and accurate placement has often been difficult and inconsistent. Our repair technique has several advantages over reconstruction. It does not burn any bridges; it allows for subsequent reconstruction. It does not require a graft and, using small suture anchors instead of large sockets and anchors, involves less bone loss. It also allows for early repair of tears—patients can return to activities, sports, and work quicker—and avoids the risk of chondral and bony damage with recurrent dislocations. According to our review of the MPFL repairs performed by Dr. Hirahara starting in 2013, the procedure is quick and successful and has outstanding outcomes.
Another treatment option for recurrent lateral patellar instability combines reefing of the medial patellofemoral tissues with a lateral release. This combination has had several postoperative complications and is no longer indicated.9 TT transfer and trochleoplasty procedures have been developed to address different aspects of patellar instability, increased TT-TG distance, and dysplastic trochlea (Table 2). Both types of procedures are highly invasive and difficult to perform, requiring technical expertise. They are best used when warranted by the anatomy, but this is uncommon. The technique we have presented allows for easy and reliable repair of dislocations in the absence of associated pathology that would require larger, more complex surgery. The ease of use and accuracy of musculoskeletal ultrasound make this technique superior to others.
Conclusion
The MPFL is a vital static stabilizer of the patella and as such should be evaluated in the setting of patellar injury. The novel preoperative and intraoperative use of musculoskeletal ultrasound described in this article allows for easy real-time identification of the MPFL and simple and accurate percutaneous repair of torn structures. Nonoperative treatments of acute patellar dislocations have higher rates of recurrent dislocations, which put patella and trochlea at risk for bony and chondral damage. Given appropriate tear location and tissue quality, repairs should be considered early and before reconstruction. To our knowledge, a reliable, easily reproducible MPFL repair was not described until now. We have reported on use of such a technique and on its promising patient outcomes, which should be considered when addressing MPFL injuries.
Am J Orthop. 2017;46(3):152-157. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
2. Nomura E, Inoue M, Osada N. Anatomical analysis of the medial patellofemoral ligament of the knee, especially the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):510-515.
3. Petri M, Ettinger M, Stuebig T, et al. Current concepts for patellar dislocation. Arch Trauma Res. 2015;4(3):e29301.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Tanaka MJ. Variability in the patellar attachment of the medial patellofemoral ligament. Arthroscopy. 2016;32(8):1667-1670.
6. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336.
7. Philippot R, Chouteau J, Wegrzyn J, Testa R, Fessy MH, Moyen B. Medial patellofemoral ligament anatomy: implications for its surgical reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):475-479.
8. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
9. Song GY, Hong L, Zhang H, Zhang J, Li Y, Feng H. Iatrogenic medial patellar instability following lateral retinacular release of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2825-2830.
10. McCarthy M, Ridley TJ, Bollier M, Wolf B, Albright J, Amendola A. Femoral tunnel placement in medial patellofemoral ligament reconstruction. Iowa Orthop J. 2013;33:58-63.
11. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297.
12. Barnett AJ, Howells NR, Burston BJ, Ansari A, Clark D, Eldridge JD. Radiographic landmarks for tunnel placement in reconstruction of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2380-2384.
13. Regalado G, Lintula H, Kokki H, Kröger H, Väätäinen U, Eskelinen M. Six-year outcome after non-surgical versus surgical treatment of acute primary patellar dislocation in adolescents: a prospective randomized trial. Knee Surg Sports Traumatol Arthrosc. 2016;24(1):6-11.
14. Sandmeier RH, Burks RT, Bachus KN, Billings A. The effect of reconstruction of the medial patellofemoral ligament on patellar tracking. Am J Sports Med. 2000;28(3):345-349.
15. Baldwin JL. The anatomy of the medial patellofemoral ligament. Am J Sports Med. 2009;37(12):2355-2361.
16. Steensen RN, Dopirak RM, McDonald WG 3rd. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513.
17. Godin JA, Karas V, Visgauss JD, Garrett WE. Medial patellofemoral ligament reconstruction using a femoral loop button fixation technique. Arthrosc Tech. 2015;4(5):e601-e607.
Take-Home Points
- Use ultrasound to identify integrity and location of MPFL tear.
- Anatomic repair allows native tissue to reintegrate into bone.
- Repairs done early can prevent complications of recurrent instability.
- Repair maintains biological and proprioceptive qualities of tissue.
- 10Ultrasound-guided percutaneous repair is quick and effective.
The medial patellofemoral ligament (MPFL) is the primary passive restraint to lateral patellar excursion1-5 and helps control patellar tilt and rotation.6,7 More than 90% of lateral patellar dislocations cause the MPFL to rupture, and roughly 90% of these detachments involve the femoral insertion.4 Ensuing patellar instability often results from MPFL insufficiency. It has been suggested that re-creating the anatomy and functionality of this ligament is of utmost importance in restoring normal patellar biomechanics.1-5,7,8
Anatomical risk factors for recurrent patellar instability include patella alta, increased tibial tuberosity-trochlear groove (TT-TG) distance, trochlear dysplasia, and torsional abnormalities.1-4,6 A medial reefing technique with a lateral tissue release traditionally was used to restore proper kinematics, but was shown to have associated postoperative issues.9
Methods
Patient Demographics
Dr. Hirahara developed this technique in 2013 and performed it 11 times between 2013 and 2016. Of the 11 patients, 1 was excluded from our retrospective analysis because of trochlear dysplasia, now considered a relative contraindication. Of the remaining 10 patients, 5 (50%) had the repair performed on the right knee. Eight patients (80%) were female. Mean (SD) age was 17.21 (3.53) years. One patient had concurrent femur- and patella-side detachments; otherwise, 6 (60%) of 10 repairs were performed exclusively at the patella. We grade patellar instability according to amount of glide based on patellar width and quadrants. Normal lateral displacement was usually 1 to 2 quadrants of lateral glide relative to the contralateral side. Before surgery, 6 (60%) of the 10 patients presented with lateral glide of 3 quadrants, and 3 (30%) presented with lateral glide of 4 quadrants. All had patellar instability apprehension on physical examination.
Surgical Indications
Before surgery, MPFL integrity is determined by ultrasound evaluation. Repair is considered if the MPFL has a femur- or patella-side tear and is of adequate quantity and quality, and if there are minimal or no arthritic changes (Table 2).
Surgical Technique
The patient is brought to the operating room and placed supine. Patellar stability of the affected knee is assessed and compared with that of the contralateral side with patellar glide. The knee is prepared and draped in usual sterile fashion. With the knee flexed at 90º, a tourniquet is inflated. Diagnostic arthroscopy is performed with standard anteromedial and anterolateral portals, and, if necessary, arthroscopic procedures are performed.
Femoral Attachment Repair
With the leg in extension, ultrasound is used to identify the tear at the femoral attachment (watch part 1 of the video). A spinal needle is placed at the femoral insertion, typically just anterior and distal to the adductor tubercle (Figure 4).10
Patellar Attachment Repair
With the leg in extension, ultrasound is used to identify where the MPFL is detached from the patella (watch part 2 of the video). A spinal needle is placed at the detachment site (Figure 5). A scalpel is used to make a 1-cm incision down to the patella.
In this description, we showcase knotless and knotted techniques for each repair site. Either method is appropriate for the 2 repair sites. Owing to the superficial nature of the attachment sites—they may have very little fat, particularly at the patella—knot stacks are more prominent, can be felt after surgery, and have the potential to irritate surrounding tissues. Therefore, we prefer knotless fixation for both sites.
Rehabilitation
Rehabilitation after MPFL repair is much like rehabilitation after quadriceps tendon repair. The patient is locked in a brace in full extension when up and moving. Early weight-bearing and minimal use of assistive devices (crutches) are allowed because, when the leg is in full extension, there is no tension at the repair sites. Rehabilitation begins within 1 week, and normal daily function is quickly attained. The protocol emphasizes pain-free motion and suitable patellar mobility, and allows the immobilizing brace to be unlocked for exercise and sitting. During the first 4 weeks, quadriceps activation is limited; progression to full ROM occurs by 4 to 6 weeks. During the strengthening phase, loading the knee in early flexion should be avoided. Return to heavy lifting, physical activity, and sports is delayed until after 6 months in order to allow the construct to mature and integrate. Once the patient has satisfied all the strength, ROM, and functional outcome measurements, a brace is no longer required during sports and normal activity.
Results
Mean tourniquet time for each procedure, which includes diagnostic arthroscopy and ultrasound-guided percutaneous repair, was 26.9 minutes.
Discussion
Conservative management typically is recommended for acute patellar dislocations. In the event of failed conservative management or chronic patellar instability, surgical intervention is indicated. Studies have found that conservative management has recurrent-dislocation rates of 35% at 3-year follow-up and 73% at 6-year follow-up, and recurrent dislocations significantly increase patients’ risk of developing chondral and bony damage.13 MPFL repair is designed to restore proper patellar tracking and kinematics while maintaining the anatomical tissue. Lateral patellar dislocations often cause the MPFL to rupture; tears are reported in more than 90% of incidents.4 The significant rate indicates that, even after a single patellar dislocation, the MPFL should be evaluated. The MPFL contributes 50% to 60% of the medial stabilizing force during patellar tracking1,7,14 and is the primary restraint to lateral patellar excursion and excessive patellar tilt and rotation.1-5 Its absence plays a key role in recurrent lateral patellar instability. With this structure being so important, proper identification and intervention are vital. Studies have established that redislocation rates are significantly higher for nonoperatively (vs operatively) treated primary patellar dislocations.13 Simple and accurate percutaneous repair of the MPFL should be performed early to avoid the long-term complications of recurrent instability that could damage the cartilage and bone of the patella and trochlea.
The primary advantage of this technique is its novel use of musculoskeletal ultrasound to accurately identify anatomy and pathology and the placement of anatomical repairs. Accurate preoperative and intraoperative assessment of MPFL anatomy is vital to the success of a procedure. Descriptions of MPFL anatomy suggest discrepancies in the exact locations of the femoral and patellar attachments.2,5,7,10,12,15,16 Tanaka5 noted that, even within paired knees, there was “marked variability” in the MPFL insertions. McCarthy and colleagues10 contended the femoral attachment of the MPFL is just anterior and distal to the adductor tubercle, the landmark addressed in this technique. Steensen and colleagues16 described this attachment site as being statistically the “single most important point affecting isometry” of the MPFL. Sallay and colleagues4 asserted that an overwhelming majority of MPFL tears (87%) occur at the adductor tubercle. The variable distribution of tear locations and the importance of re-creating patient anatomy further highlight the need for individualized treatment, which is afforded by ultrasound. Fluoroscopy has been inadequate in identifying MPFL anatomy; this modality is difficult, cumbersome, inaccurate, and inconsistent.11,12 Conversely, ultrasound provides real-time visualization of anatomy and allows for precise identification of MPFL attachments and accurate placement of suture anchors for repair during surgery (Figures 3, 4).
For femur-side and patella-side tears, repairs can and should be performed. For midsubstance tears, however, repair is not feasible, and reconstruction is appropriate. MPFL repair is superior to reconstruction in several ways. Repair is a simple percutaneous procedure that had a mean tourniquet time of 26.9 minutes in this study. For tissue that is quantitatively and qualitatively adequate, repair allows the structure to reintegrate into bone without total reconstruction. In the event of multiple tears, the percutaneous procedure allows for repair of each attachment. As the MPFL sits between the second and third tissue layers of the medial knee, reconstruction can be difficult and invasive and require establishment of a between-layers plane, which can disrupt adjacent tissue.4,7,17 Repair also maintains native tissue and its neurovascular and proprioceptive properties.
Reconstruction of the MPFL has become the gold-standard treatment for recurrent lateral patellar instability but has limitations and complications.3,7,12,17 Reconstruction techniques use either surface anatomy palpation (requiring large incisions) or fluoroscopy to identify tunnel placement locations, and accurate placement has often been difficult and inconsistent. Our repair technique has several advantages over reconstruction. It does not burn any bridges; it allows for subsequent reconstruction. It does not require a graft and, using small suture anchors instead of large sockets and anchors, involves less bone loss. It also allows for early repair of tears—patients can return to activities, sports, and work quicker—and avoids the risk of chondral and bony damage with recurrent dislocations. According to our review of the MPFL repairs performed by Dr. Hirahara starting in 2013, the procedure is quick and successful and has outstanding outcomes.
Another treatment option for recurrent lateral patellar instability combines reefing of the medial patellofemoral tissues with a lateral release. This combination has had several postoperative complications and is no longer indicated.9 TT transfer and trochleoplasty procedures have been developed to address different aspects of patellar instability, increased TT-TG distance, and dysplastic trochlea (Table 2). Both types of procedures are highly invasive and difficult to perform, requiring technical expertise. They are best used when warranted by the anatomy, but this is uncommon. The technique we have presented allows for easy and reliable repair of dislocations in the absence of associated pathology that would require larger, more complex surgery. The ease of use and accuracy of musculoskeletal ultrasound make this technique superior to others.
Conclusion
The MPFL is a vital static stabilizer of the patella and as such should be evaluated in the setting of patellar injury. The novel preoperative and intraoperative use of musculoskeletal ultrasound described in this article allows for easy real-time identification of the MPFL and simple and accurate percutaneous repair of torn structures. Nonoperative treatments of acute patellar dislocations have higher rates of recurrent dislocations, which put patella and trochlea at risk for bony and chondral damage. Given appropriate tear location and tissue quality, repairs should be considered early and before reconstruction. To our knowledge, a reliable, easily reproducible MPFL repair was not described until now. We have reported on use of such a technique and on its promising patient outcomes, which should be considered when addressing MPFL injuries.
Am J Orthop. 2017;46(3):152-157. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Use ultrasound to identify integrity and location of MPFL tear.
- Anatomic repair allows native tissue to reintegrate into bone.
- Repairs done early can prevent complications of recurrent instability.
- Repair maintains biological and proprioceptive qualities of tissue.
- 10Ultrasound-guided percutaneous repair is quick and effective.
The medial patellofemoral ligament (MPFL) is the primary passive restraint to lateral patellar excursion1-5 and helps control patellar tilt and rotation.6,7 More than 90% of lateral patellar dislocations cause the MPFL to rupture, and roughly 90% of these detachments involve the femoral insertion.4 Ensuing patellar instability often results from MPFL insufficiency. It has been suggested that re-creating the anatomy and functionality of this ligament is of utmost importance in restoring normal patellar biomechanics.1-5,7,8
Anatomical risk factors for recurrent patellar instability include patella alta, increased tibial tuberosity-trochlear groove (TT-TG) distance, trochlear dysplasia, and torsional abnormalities.1-4,6 A medial reefing technique with a lateral tissue release traditionally was used to restore proper kinematics, but was shown to have associated postoperative issues.9
Methods
Patient Demographics
Dr. Hirahara developed this technique in 2013 and performed it 11 times between 2013 and 2016. Of the 11 patients, 1 was excluded from our retrospective analysis because of trochlear dysplasia, now considered a relative contraindication. Of the remaining 10 patients, 5 (50%) had the repair performed on the right knee. Eight patients (80%) were female. Mean (SD) age was 17.21 (3.53) years. One patient had concurrent femur- and patella-side detachments; otherwise, 6 (60%) of 10 repairs were performed exclusively at the patella. We grade patellar instability according to amount of glide based on patellar width and quadrants. Normal lateral displacement was usually 1 to 2 quadrants of lateral glide relative to the contralateral side. Before surgery, 6 (60%) of the 10 patients presented with lateral glide of 3 quadrants, and 3 (30%) presented with lateral glide of 4 quadrants. All had patellar instability apprehension on physical examination.
Surgical Indications
Before surgery, MPFL integrity is determined by ultrasound evaluation. Repair is considered if the MPFL has a femur- or patella-side tear and is of adequate quantity and quality, and if there are minimal or no arthritic changes (Table 2).
Surgical Technique
The patient is brought to the operating room and placed supine. Patellar stability of the affected knee is assessed and compared with that of the contralateral side with patellar glide. The knee is prepared and draped in usual sterile fashion. With the knee flexed at 90º, a tourniquet is inflated. Diagnostic arthroscopy is performed with standard anteromedial and anterolateral portals, and, if necessary, arthroscopic procedures are performed.
Femoral Attachment Repair
With the leg in extension, ultrasound is used to identify the tear at the femoral attachment (watch part 1 of the video). A spinal needle is placed at the femoral insertion, typically just anterior and distal to the adductor tubercle (Figure 4).10
Patellar Attachment Repair
With the leg in extension, ultrasound is used to identify where the MPFL is detached from the patella (watch part 2 of the video). A spinal needle is placed at the detachment site (Figure 5). A scalpel is used to make a 1-cm incision down to the patella.
In this description, we showcase knotless and knotted techniques for each repair site. Either method is appropriate for the 2 repair sites. Owing to the superficial nature of the attachment sites—they may have very little fat, particularly at the patella—knot stacks are more prominent, can be felt after surgery, and have the potential to irritate surrounding tissues. Therefore, we prefer knotless fixation for both sites.
Rehabilitation
Rehabilitation after MPFL repair is much like rehabilitation after quadriceps tendon repair. The patient is locked in a brace in full extension when up and moving. Early weight-bearing and minimal use of assistive devices (crutches) are allowed because, when the leg is in full extension, there is no tension at the repair sites. Rehabilitation begins within 1 week, and normal daily function is quickly attained. The protocol emphasizes pain-free motion and suitable patellar mobility, and allows the immobilizing brace to be unlocked for exercise and sitting. During the first 4 weeks, quadriceps activation is limited; progression to full ROM occurs by 4 to 6 weeks. During the strengthening phase, loading the knee in early flexion should be avoided. Return to heavy lifting, physical activity, and sports is delayed until after 6 months in order to allow the construct to mature and integrate. Once the patient has satisfied all the strength, ROM, and functional outcome measurements, a brace is no longer required during sports and normal activity.
Results
Mean tourniquet time for each procedure, which includes diagnostic arthroscopy and ultrasound-guided percutaneous repair, was 26.9 minutes.
Discussion
Conservative management typically is recommended for acute patellar dislocations. In the event of failed conservative management or chronic patellar instability, surgical intervention is indicated. Studies have found that conservative management has recurrent-dislocation rates of 35% at 3-year follow-up and 73% at 6-year follow-up, and recurrent dislocations significantly increase patients’ risk of developing chondral and bony damage.13 MPFL repair is designed to restore proper patellar tracking and kinematics while maintaining the anatomical tissue. Lateral patellar dislocations often cause the MPFL to rupture; tears are reported in more than 90% of incidents.4 The significant rate indicates that, even after a single patellar dislocation, the MPFL should be evaluated. The MPFL contributes 50% to 60% of the medial stabilizing force during patellar tracking1,7,14 and is the primary restraint to lateral patellar excursion and excessive patellar tilt and rotation.1-5 Its absence plays a key role in recurrent lateral patellar instability. With this structure being so important, proper identification and intervention are vital. Studies have established that redislocation rates are significantly higher for nonoperatively (vs operatively) treated primary patellar dislocations.13 Simple and accurate percutaneous repair of the MPFL should be performed early to avoid the long-term complications of recurrent instability that could damage the cartilage and bone of the patella and trochlea.
The primary advantage of this technique is its novel use of musculoskeletal ultrasound to accurately identify anatomy and pathology and the placement of anatomical repairs. Accurate preoperative and intraoperative assessment of MPFL anatomy is vital to the success of a procedure. Descriptions of MPFL anatomy suggest discrepancies in the exact locations of the femoral and patellar attachments.2,5,7,10,12,15,16 Tanaka5 noted that, even within paired knees, there was “marked variability” in the MPFL insertions. McCarthy and colleagues10 contended the femoral attachment of the MPFL is just anterior and distal to the adductor tubercle, the landmark addressed in this technique. Steensen and colleagues16 described this attachment site as being statistically the “single most important point affecting isometry” of the MPFL. Sallay and colleagues4 asserted that an overwhelming majority of MPFL tears (87%) occur at the adductor tubercle. The variable distribution of tear locations and the importance of re-creating patient anatomy further highlight the need for individualized treatment, which is afforded by ultrasound. Fluoroscopy has been inadequate in identifying MPFL anatomy; this modality is difficult, cumbersome, inaccurate, and inconsistent.11,12 Conversely, ultrasound provides real-time visualization of anatomy and allows for precise identification of MPFL attachments and accurate placement of suture anchors for repair during surgery (Figures 3, 4).
For femur-side and patella-side tears, repairs can and should be performed. For midsubstance tears, however, repair is not feasible, and reconstruction is appropriate. MPFL repair is superior to reconstruction in several ways. Repair is a simple percutaneous procedure that had a mean tourniquet time of 26.9 minutes in this study. For tissue that is quantitatively and qualitatively adequate, repair allows the structure to reintegrate into bone without total reconstruction. In the event of multiple tears, the percutaneous procedure allows for repair of each attachment. As the MPFL sits between the second and third tissue layers of the medial knee, reconstruction can be difficult and invasive and require establishment of a between-layers plane, which can disrupt adjacent tissue.4,7,17 Repair also maintains native tissue and its neurovascular and proprioceptive properties.
Reconstruction of the MPFL has become the gold-standard treatment for recurrent lateral patellar instability but has limitations and complications.3,7,12,17 Reconstruction techniques use either surface anatomy palpation (requiring large incisions) or fluoroscopy to identify tunnel placement locations, and accurate placement has often been difficult and inconsistent. Our repair technique has several advantages over reconstruction. It does not burn any bridges; it allows for subsequent reconstruction. It does not require a graft and, using small suture anchors instead of large sockets and anchors, involves less bone loss. It also allows for early repair of tears—patients can return to activities, sports, and work quicker—and avoids the risk of chondral and bony damage with recurrent dislocations. According to our review of the MPFL repairs performed by Dr. Hirahara starting in 2013, the procedure is quick and successful and has outstanding outcomes.
Another treatment option for recurrent lateral patellar instability combines reefing of the medial patellofemoral tissues with a lateral release. This combination has had several postoperative complications and is no longer indicated.9 TT transfer and trochleoplasty procedures have been developed to address different aspects of patellar instability, increased TT-TG distance, and dysplastic trochlea (Table 2). Both types of procedures are highly invasive and difficult to perform, requiring technical expertise. They are best used when warranted by the anatomy, but this is uncommon. The technique we have presented allows for easy and reliable repair of dislocations in the absence of associated pathology that would require larger, more complex surgery. The ease of use and accuracy of musculoskeletal ultrasound make this technique superior to others.
Conclusion
The MPFL is a vital static stabilizer of the patella and as such should be evaluated in the setting of patellar injury. The novel preoperative and intraoperative use of musculoskeletal ultrasound described in this article allows for easy real-time identification of the MPFL and simple and accurate percutaneous repair of torn structures. Nonoperative treatments of acute patellar dislocations have higher rates of recurrent dislocations, which put patella and trochlea at risk for bony and chondral damage. Given appropriate tear location and tissue quality, repairs should be considered early and before reconstruction. To our knowledge, a reliable, easily reproducible MPFL repair was not described until now. We have reported on use of such a technique and on its promising patient outcomes, which should be considered when addressing MPFL injuries.
Am J Orthop. 2017;46(3):152-157. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
2. Nomura E, Inoue M, Osada N. Anatomical analysis of the medial patellofemoral ligament of the knee, especially the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):510-515.
3. Petri M, Ettinger M, Stuebig T, et al. Current concepts for patellar dislocation. Arch Trauma Res. 2015;4(3):e29301.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Tanaka MJ. Variability in the patellar attachment of the medial patellofemoral ligament. Arthroscopy. 2016;32(8):1667-1670.
6. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336.
7. Philippot R, Chouteau J, Wegrzyn J, Testa R, Fessy MH, Moyen B. Medial patellofemoral ligament anatomy: implications for its surgical reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):475-479.
8. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
9. Song GY, Hong L, Zhang H, Zhang J, Li Y, Feng H. Iatrogenic medial patellar instability following lateral retinacular release of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2825-2830.
10. McCarthy M, Ridley TJ, Bollier M, Wolf B, Albright J, Amendola A. Femoral tunnel placement in medial patellofemoral ligament reconstruction. Iowa Orthop J. 2013;33:58-63.
11. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297.
12. Barnett AJ, Howells NR, Burston BJ, Ansari A, Clark D, Eldridge JD. Radiographic landmarks for tunnel placement in reconstruction of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2380-2384.
13. Regalado G, Lintula H, Kokki H, Kröger H, Väätäinen U, Eskelinen M. Six-year outcome after non-surgical versus surgical treatment of acute primary patellar dislocation in adolescents: a prospective randomized trial. Knee Surg Sports Traumatol Arthrosc. 2016;24(1):6-11.
14. Sandmeier RH, Burks RT, Bachus KN, Billings A. The effect of reconstruction of the medial patellofemoral ligament on patellar tracking. Am J Sports Med. 2000;28(3):345-349.
15. Baldwin JL. The anatomy of the medial patellofemoral ligament. Am J Sports Med. 2009;37(12):2355-2361.
16. Steensen RN, Dopirak RM, McDonald WG 3rd. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513.
17. Godin JA, Karas V, Visgauss JD, Garrett WE. Medial patellofemoral ligament reconstruction using a femoral loop button fixation technique. Arthrosc Tech. 2015;4(5):e601-e607.
1. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
2. Nomura E, Inoue M, Osada N. Anatomical analysis of the medial patellofemoral ligament of the knee, especially the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):510-515.
3. Petri M, Ettinger M, Stuebig T, et al. Current concepts for patellar dislocation. Arch Trauma Res. 2015;4(3):e29301.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Tanaka MJ. Variability in the patellar attachment of the medial patellofemoral ligament. Arthroscopy. 2016;32(8):1667-1670.
6. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336.
7. Philippot R, Chouteau J, Wegrzyn J, Testa R, Fessy MH, Moyen B. Medial patellofemoral ligament anatomy: implications for its surgical reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):475-479.
8. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
9. Song GY, Hong L, Zhang H, Zhang J, Li Y, Feng H. Iatrogenic medial patellar instability following lateral retinacular release of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2825-2830.
10. McCarthy M, Ridley TJ, Bollier M, Wolf B, Albright J, Amendola A. Femoral tunnel placement in medial patellofemoral ligament reconstruction. Iowa Orthop J. 2013;33:58-63.
11. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297.
12. Barnett AJ, Howells NR, Burston BJ, Ansari A, Clark D, Eldridge JD. Radiographic landmarks for tunnel placement in reconstruction of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2380-2384.
13. Regalado G, Lintula H, Kokki H, Kröger H, Väätäinen U, Eskelinen M. Six-year outcome after non-surgical versus surgical treatment of acute primary patellar dislocation in adolescents: a prospective randomized trial. Knee Surg Sports Traumatol Arthrosc. 2016;24(1):6-11.
14. Sandmeier RH, Burks RT, Bachus KN, Billings A. The effect of reconstruction of the medial patellofemoral ligament on patellar tracking. Am J Sports Med. 2000;28(3):345-349.
15. Baldwin JL. The anatomy of the medial patellofemoral ligament. Am J Sports Med. 2009;37(12):2355-2361.
16. Steensen RN, Dopirak RM, McDonald WG 3rd. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513.
17. Godin JA, Karas V, Visgauss JD, Garrett WE. Medial patellofemoral ligament reconstruction using a femoral loop button fixation technique. Arthrosc Tech. 2015;4(5):e601-e607.
In-Office Diagnostic Needle Arthroscopy
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
Editorial Board Biographies
Matthew J. Matava, MD
Associate Editor for Professional Sports
Dr. Matava is a professor of Orthopedic Surgery and Physical Therapy, Chief of the Sports Medicine Service, and the Head Team Physician for the varsity athletic program at Washington University in St. Louis. He is also a team physician for the National Hockey League’s St. Louis Blues. Formerly, he was the Head Team Physician for the St. Louis Rams, and was President of the National Football League Physicians Society (NFLPS) from 2013-2015. Dr. Matava earned his Medical Degree from the University of Missouri-Kansas City. He completed his internship and orthopedic surgery residency at Emory University in Atlanta, GA, followed by a fellowship in sports medicine and arthroscopic surgery at the Cincinnati Sports Medicine and Orthopedic Center. He is the recipient of several research awards from Emory University, is a member of the Alpha Omega Medical Honor Society, and received the Palma Chironis Award for Excellence in Teaching from the Washington University Department of Orthopedic Surgery in 2012. Dr. Matava has been listed as a “Best Doctor in America” since 2005, and was recently hailed by Orthopedics This Week as one of the top 28 sports knee surgeons in the nation.
Jeffrey Sawyer, MD
Associate Editor for Pediatrics
Dr. Sawyer is a professor of Orthopaedic Surgery and the Pediatric Orthopaedic Fellowship Director at the University of Tennessee-Campbell Clinic. He also serves as a reviewer/editor for the Journal of Pediatric Orthopaedics and Orthopedic Clinics of North America. He graduated from the University of Rochester School of Medicine and completed his residency at the University of Pennsylvania, prior to completing his Pediatric Orthopaedic Fellowship at the University of Tennessee-Campbell Clinic. Dr. Sawyer has held numerous leadership positions in the Pediatric Orthopaedic Society of North America (POSNA). He also was a POSNA Traveling Fellow and won the POSNA Special Achievement Award for his work on the Pediatric Orthopaedic Workforce. He is a national authority on pediatric orthopedic trauma, and is on the Executive Committee of the Children’s Spine Foundation.
Brian K. Vickaryous, MD
Associate Editor for Trauma
Dr. Vickaryous is a specialist in orthopedic traumatology at the Florida Hospital Orthopedic Institute in Orlando, Florida, and has an additional subspecialty board certification in sports medicine. He attended the University of Miami, Florida through the combined degree Medical
Honors Program and completed his residency at the William Beaumont Army Medical Center/Texas Tech University of the Health Sciences. Dr. Vickaryous has also deployed overseas as Commander of the Trauma Unit, the 8th Forward Surgical Team, in Iraq in support of Operation Iraqi Freedom. He currently is a member of the American Academy of Orthopaedic
Surgeons (AAOS) and the Orthopaedic Trauma Association (OTA).
Michael B. Gerhardt, MD
Associate Editor for Sports Medicine
Dr. Gerhardt is a sports medicine specialist at the Kerlan-Jobe Institute and Santa Monica Orthopaedic Group in Los Angeles, CA. He also serves as faculty in the Department of Orthopaedic Surgery at Cedars-Sinai Medical Center. Dr. Gerhardt earned his undergraduate degree from UC San Diego and graduated medical school with honors from the Medical College of Pennsylvania. He received the Leonard Marmur Award for excellence in research and education during his orthopedic residency at the University of Southern California, prior to completing a Sports Medicine Fellowship in 2003. He received further training in hip arthroscopy at the Nashville Orthopaedic Sports Medicine and Orthopaedic Clinic, and maintains a leadership role in the area of sports medicine and hip preservation on a national and international level. Currently, he serves as Team Physician for the US Soccer Men’s National Team, the Los Angeles Galaxy, and Pepperdine University.
Matthew J. Matava, MD
Associate Editor for Professional Sports
Dr. Matava is a professor of Orthopedic Surgery and Physical Therapy, Chief of the Sports Medicine Service, and the Head Team Physician for the varsity athletic program at Washington University in St. Louis. He is also a team physician for the National Hockey League’s St. Louis Blues. Formerly, he was the Head Team Physician for the St. Louis Rams, and was President of the National Football League Physicians Society (NFLPS) from 2013-2015. Dr. Matava earned his Medical Degree from the University of Missouri-Kansas City. He completed his internship and orthopedic surgery residency at Emory University in Atlanta, GA, followed by a fellowship in sports medicine and arthroscopic surgery at the Cincinnati Sports Medicine and Orthopedic Center. He is the recipient of several research awards from Emory University, is a member of the Alpha Omega Medical Honor Society, and received the Palma Chironis Award for Excellence in Teaching from the Washington University Department of Orthopedic Surgery in 2012. Dr. Matava has been listed as a “Best Doctor in America” since 2005, and was recently hailed by Orthopedics This Week as one of the top 28 sports knee surgeons in the nation.
Jeffrey Sawyer, MD
Associate Editor for Pediatrics
Dr. Sawyer is a professor of Orthopaedic Surgery and the Pediatric Orthopaedic Fellowship Director at the University of Tennessee-Campbell Clinic. He also serves as a reviewer/editor for the Journal of Pediatric Orthopaedics and Orthopedic Clinics of North America. He graduated from the University of Rochester School of Medicine and completed his residency at the University of Pennsylvania, prior to completing his Pediatric Orthopaedic Fellowship at the University of Tennessee-Campbell Clinic. Dr. Sawyer has held numerous leadership positions in the Pediatric Orthopaedic Society of North America (POSNA). He also was a POSNA Traveling Fellow and won the POSNA Special Achievement Award for his work on the Pediatric Orthopaedic Workforce. He is a national authority on pediatric orthopedic trauma, and is on the Executive Committee of the Children’s Spine Foundation.
Brian K. Vickaryous, MD
Associate Editor for Trauma
Dr. Vickaryous is a specialist in orthopedic traumatology at the Florida Hospital Orthopedic Institute in Orlando, Florida, and has an additional subspecialty board certification in sports medicine. He attended the University of Miami, Florida through the combined degree Medical
Honors Program and completed his residency at the William Beaumont Army Medical Center/Texas Tech University of the Health Sciences. Dr. Vickaryous has also deployed overseas as Commander of the Trauma Unit, the 8th Forward Surgical Team, in Iraq in support of Operation Iraqi Freedom. He currently is a member of the American Academy of Orthopaedic
Surgeons (AAOS) and the Orthopaedic Trauma Association (OTA).
Michael B. Gerhardt, MD
Associate Editor for Sports Medicine
Dr. Gerhardt is a sports medicine specialist at the Kerlan-Jobe Institute and Santa Monica Orthopaedic Group in Los Angeles, CA. He also serves as faculty in the Department of Orthopaedic Surgery at Cedars-Sinai Medical Center. Dr. Gerhardt earned his undergraduate degree from UC San Diego and graduated medical school with honors from the Medical College of Pennsylvania. He received the Leonard Marmur Award for excellence in research and education during his orthopedic residency at the University of Southern California, prior to completing a Sports Medicine Fellowship in 2003. He received further training in hip arthroscopy at the Nashville Orthopaedic Sports Medicine and Orthopaedic Clinic, and maintains a leadership role in the area of sports medicine and hip preservation on a national and international level. Currently, he serves as Team Physician for the US Soccer Men’s National Team, the Los Angeles Galaxy, and Pepperdine University.
Matthew J. Matava, MD
Associate Editor for Professional Sports
Dr. Matava is a professor of Orthopedic Surgery and Physical Therapy, Chief of the Sports Medicine Service, and the Head Team Physician for the varsity athletic program at Washington University in St. Louis. He is also a team physician for the National Hockey League’s St. Louis Blues. Formerly, he was the Head Team Physician for the St. Louis Rams, and was President of the National Football League Physicians Society (NFLPS) from 2013-2015. Dr. Matava earned his Medical Degree from the University of Missouri-Kansas City. He completed his internship and orthopedic surgery residency at Emory University in Atlanta, GA, followed by a fellowship in sports medicine and arthroscopic surgery at the Cincinnati Sports Medicine and Orthopedic Center. He is the recipient of several research awards from Emory University, is a member of the Alpha Omega Medical Honor Society, and received the Palma Chironis Award for Excellence in Teaching from the Washington University Department of Orthopedic Surgery in 2012. Dr. Matava has been listed as a “Best Doctor in America” since 2005, and was recently hailed by Orthopedics This Week as one of the top 28 sports knee surgeons in the nation.
Jeffrey Sawyer, MD
Associate Editor for Pediatrics
Dr. Sawyer is a professor of Orthopaedic Surgery and the Pediatric Orthopaedic Fellowship Director at the University of Tennessee-Campbell Clinic. He also serves as a reviewer/editor for the Journal of Pediatric Orthopaedics and Orthopedic Clinics of North America. He graduated from the University of Rochester School of Medicine and completed his residency at the University of Pennsylvania, prior to completing his Pediatric Orthopaedic Fellowship at the University of Tennessee-Campbell Clinic. Dr. Sawyer has held numerous leadership positions in the Pediatric Orthopaedic Society of North America (POSNA). He also was a POSNA Traveling Fellow and won the POSNA Special Achievement Award for his work on the Pediatric Orthopaedic Workforce. He is a national authority on pediatric orthopedic trauma, and is on the Executive Committee of the Children’s Spine Foundation.
Brian K. Vickaryous, MD
Associate Editor for Trauma
Dr. Vickaryous is a specialist in orthopedic traumatology at the Florida Hospital Orthopedic Institute in Orlando, Florida, and has an additional subspecialty board certification in sports medicine. He attended the University of Miami, Florida through the combined degree Medical
Honors Program and completed his residency at the William Beaumont Army Medical Center/Texas Tech University of the Health Sciences. Dr. Vickaryous has also deployed overseas as Commander of the Trauma Unit, the 8th Forward Surgical Team, in Iraq in support of Operation Iraqi Freedom. He currently is a member of the American Academy of Orthopaedic
Surgeons (AAOS) and the Orthopaedic Trauma Association (OTA).
Michael B. Gerhardt, MD
Associate Editor for Sports Medicine
Dr. Gerhardt is a sports medicine specialist at the Kerlan-Jobe Institute and Santa Monica Orthopaedic Group in Los Angeles, CA. He also serves as faculty in the Department of Orthopaedic Surgery at Cedars-Sinai Medical Center. Dr. Gerhardt earned his undergraduate degree from UC San Diego and graduated medical school with honors from the Medical College of Pennsylvania. He received the Leonard Marmur Award for excellence in research and education during his orthopedic residency at the University of Southern California, prior to completing a Sports Medicine Fellowship in 2003. He received further training in hip arthroscopy at the Nashville Orthopaedic Sports Medicine and Orthopaedic Clinic, and maintains a leadership role in the area of sports medicine and hip preservation on a national and international level. Currently, he serves as Team Physician for the US Soccer Men’s National Team, the Los Angeles Galaxy, and Pepperdine University.
Presentation of the 2016 Resident Writer’s Award
Darla Conrad (left), Senior Director, North America Education Solutions, Johnson & Johnson Medical Devices, presents Kalpit N. Shah, MD (right) with his plaque for the second-place Resident Writer’s Award, and Christopher Rice, MD (center) with his plaque for the third-place Resident Writer’s Award at the 2017 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) in San Diego.
Winners of the 2016 Resident Writer’s Award
First-Place Award
An Original Study
Clinical Outcomes of Anatomical Total Shoulder Arthroplasty in a Young, Active Population
Dr. Kusnezov is a senior resident, completing his orthopedic surgery residency training, at the Texas Tech University Health Sciences Center/William Beaumont Army Medical Center joint military-civilian program in El Paso, Texas. Prior to residency, he completed both his undergraduate education and medical school at the University of California, Los Angeles, graduating Summa Cum Laude and AOA, respectively. Dr. Kusnezov is currently engaged in a multitude of ongoing projects with over 50 peer-reviewed publications to date. His research interests include trauma and limb salvage, complex total joint reconstruction, and interdisciplinary system improvement.
Second-Place Award
An Original Study
Patient-Reported Outcome Measures: How Do Digital Tablets Stack Up to Paper Forms? A Randomized, Controlled Study
Dr. Shah is currently in his third year of orthopedic surgery residency training at Brown University in Providence, Rhode Island. Prior to residency, he completed undergraduate education at the University of California, Berkeley, and medical school at the University of California, Irvine. He hopes to pursue a hand and upper extremity fellowship after residency. His research interests include upper extremity trauma and surgical complications, as well as technology and its implications on orthopedic surgery.
Third-Place Award
An Original Study
Treating Tibia Fractures With Far Cortical Locking Implants
Dr. Rice is an orthopedic surgery resident at the University of Wisconsin, Madison. He received his medical degree from the University of Utah and attended Brigham Young University for his undergraduate studies. He has a special interest in disorders of the hand and upper extremity trauma.
Darla Conrad (left), Senior Director, North America Education Solutions, Johnson & Johnson Medical Devices, presents Kalpit N. Shah, MD (right) with his plaque for the second-place Resident Writer’s Award, and Christopher Rice, MD (center) with his plaque for the third-place Resident Writer’s Award at the 2017 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) in San Diego.
Winners of the 2016 Resident Writer’s Award
First-Place Award
An Original Study
Clinical Outcomes of Anatomical Total Shoulder Arthroplasty in a Young, Active Population
Dr. Kusnezov is a senior resident, completing his orthopedic surgery residency training, at the Texas Tech University Health Sciences Center/William Beaumont Army Medical Center joint military-civilian program in El Paso, Texas. Prior to residency, he completed both his undergraduate education and medical school at the University of California, Los Angeles, graduating Summa Cum Laude and AOA, respectively. Dr. Kusnezov is currently engaged in a multitude of ongoing projects with over 50 peer-reviewed publications to date. His research interests include trauma and limb salvage, complex total joint reconstruction, and interdisciplinary system improvement.
Second-Place Award
An Original Study
Patient-Reported Outcome Measures: How Do Digital Tablets Stack Up to Paper Forms? A Randomized, Controlled Study
Dr. Shah is currently in his third year of orthopedic surgery residency training at Brown University in Providence, Rhode Island. Prior to residency, he completed undergraduate education at the University of California, Berkeley, and medical school at the University of California, Irvine. He hopes to pursue a hand and upper extremity fellowship after residency. His research interests include upper extremity trauma and surgical complications, as well as technology and its implications on orthopedic surgery.
Third-Place Award
An Original Study
Treating Tibia Fractures With Far Cortical Locking Implants
Dr. Rice is an orthopedic surgery resident at the University of Wisconsin, Madison. He received his medical degree from the University of Utah and attended Brigham Young University for his undergraduate studies. He has a special interest in disorders of the hand and upper extremity trauma.
Darla Conrad (left), Senior Director, North America Education Solutions, Johnson & Johnson Medical Devices, presents Kalpit N. Shah, MD (right) with his plaque for the second-place Resident Writer’s Award, and Christopher Rice, MD (center) with his plaque for the third-place Resident Writer’s Award at the 2017 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) in San Diego.
Winners of the 2016 Resident Writer’s Award
First-Place Award
An Original Study
Clinical Outcomes of Anatomical Total Shoulder Arthroplasty in a Young, Active Population
Dr. Kusnezov is a senior resident, completing his orthopedic surgery residency training, at the Texas Tech University Health Sciences Center/William Beaumont Army Medical Center joint military-civilian program in El Paso, Texas. Prior to residency, he completed both his undergraduate education and medical school at the University of California, Los Angeles, graduating Summa Cum Laude and AOA, respectively. Dr. Kusnezov is currently engaged in a multitude of ongoing projects with over 50 peer-reviewed publications to date. His research interests include trauma and limb salvage, complex total joint reconstruction, and interdisciplinary system improvement.
Second-Place Award
An Original Study
Patient-Reported Outcome Measures: How Do Digital Tablets Stack Up to Paper Forms? A Randomized, Controlled Study
Dr. Shah is currently in his third year of orthopedic surgery residency training at Brown University in Providence, Rhode Island. Prior to residency, he completed undergraduate education at the University of California, Berkeley, and medical school at the University of California, Irvine. He hopes to pursue a hand and upper extremity fellowship after residency. His research interests include upper extremity trauma and surgical complications, as well as technology and its implications on orthopedic surgery.
Third-Place Award
An Original Study
Treating Tibia Fractures With Far Cortical Locking Implants
Dr. Rice is an orthopedic surgery resident at the University of Wisconsin, Madison. He received his medical degree from the University of Utah and attended Brigham Young University for his undergraduate studies. He has a special interest in disorders of the hand and upper extremity trauma.
Medial Patellofemoral Ligament Repair
Video, Part 1. Femoral Attachment
1. Ultrasound is used to identify femoral and patellar attachments of medial patellofemoral ligament (MPFL).
2. MPFL is followed from patella to its attachment near adductor tubercle.
3. In-plane ultrasound guidance is used to place needle anterior and distal to tubercle.
4. Percutaneous incision is made down to needle tip. Spear is placed at needle tip for anatomical placement of socket.
5. Socket is drilled.
6. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed.
7. Leading edge of torn MPFL is identified.
8. Suture passer (Labral FastPass Scorpion; Arthrex) is used to pass sutures through leading edge of torn MPFL to create horizontal mattress.
9. Sutures are tied.
10. Repair is complete.
Video, Part 2. Patellar Attachment
1. Ultrasound is used to scan patella to identify ideal or exact location of tear. In-plane ultrasound guidance is used to place spinal needle at desired socket location.
2. After spinal needle is positioned, in-line percutaneous incision is made, and needle is palpated at patella.
3. Spear is then placed at spinal needle tip for anatomical positioning of socket.
4. Socket is drilled.
5. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed in socket.
6. Leading edge of torn medial patellofemoral ligament (MPFL) is identified.
7. Suture passer (Labral Past Pass Scorpion; Arthrex) is used to pass suture from anchor in horizontal mattress fashion through leading edge of torn MPFL.
8. Wire with loop (FiberSnare; Arthrex) is used as part of knotless technology to pull suture back through anchor to create knotless fixation.
9. Suture is pulled for appropriate tensioning of tissue.
10. Ultrasound is used to visualize construct to confirm that MPFL tissue abuts anchor and that repair is complete.
Video, Part 1. Femoral Attachment
1. Ultrasound is used to identify femoral and patellar attachments of medial patellofemoral ligament (MPFL).
2. MPFL is followed from patella to its attachment near adductor tubercle.
3. In-plane ultrasound guidance is used to place needle anterior and distal to tubercle.
4. Percutaneous incision is made down to needle tip. Spear is placed at needle tip for anatomical placement of socket.
5. Socket is drilled.
6. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed.
7. Leading edge of torn MPFL is identified.
8. Suture passer (Labral FastPass Scorpion; Arthrex) is used to pass sutures through leading edge of torn MPFL to create horizontal mattress.
9. Sutures are tied.
10. Repair is complete.
Video, Part 2. Patellar Attachment
1. Ultrasound is used to scan patella to identify ideal or exact location of tear. In-plane ultrasound guidance is used to place spinal needle at desired socket location.
2. After spinal needle is positioned, in-line percutaneous incision is made, and needle is palpated at patella.
3. Spear is then placed at spinal needle tip for anatomical positioning of socket.
4. Socket is drilled.
5. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed in socket.
6. Leading edge of torn medial patellofemoral ligament (MPFL) is identified.
7. Suture passer (Labral Past Pass Scorpion; Arthrex) is used to pass suture from anchor in horizontal mattress fashion through leading edge of torn MPFL.
8. Wire with loop (FiberSnare; Arthrex) is used as part of knotless technology to pull suture back through anchor to create knotless fixation.
9. Suture is pulled for appropriate tensioning of tissue.
10. Ultrasound is used to visualize construct to confirm that MPFL tissue abuts anchor and that repair is complete.
Video, Part 1. Femoral Attachment
1. Ultrasound is used to identify femoral and patellar attachments of medial patellofemoral ligament (MPFL).
2. MPFL is followed from patella to its attachment near adductor tubercle.
3. In-plane ultrasound guidance is used to place needle anterior and distal to tubercle.
4. Percutaneous incision is made down to needle tip. Spear is placed at needle tip for anatomical placement of socket.
5. Socket is drilled.
6. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed.
7. Leading edge of torn MPFL is identified.
8. Suture passer (Labral FastPass Scorpion; Arthrex) is used to pass sutures through leading edge of torn MPFL to create horizontal mattress.
9. Sutures are tied.
10. Repair is complete.
Video, Part 2. Patellar Attachment
1. Ultrasound is used to scan patella to identify ideal or exact location of tear. In-plane ultrasound guidance is used to place spinal needle at desired socket location.
2. After spinal needle is positioned, in-line percutaneous incision is made, and needle is palpated at patella.
3. Spear is then placed at spinal needle tip for anatomical positioning of socket.
4. Socket is drilled.
5. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed in socket.
6. Leading edge of torn medial patellofemoral ligament (MPFL) is identified.
7. Suture passer (Labral Past Pass Scorpion; Arthrex) is used to pass suture from anchor in horizontal mattress fashion through leading edge of torn MPFL.
8. Wire with loop (FiberSnare; Arthrex) is used as part of knotless technology to pull suture back through anchor to create knotless fixation.
9. Suture is pulled for appropriate tensioning of tissue.
10. Ultrasound is used to visualize construct to confirm that MPFL tissue abuts anchor and that repair is complete.
Arthritis Is On the Rise—But There Are Ways to Help Reduce the Effects
Arthritis aches and pains are not a normal part of aging. Nonetheless, approximately 54 million American adults who took the CDC’s National Health Survey said their doctor had diagnosed them with arthritis. That’s about 1 in 4 US adults, the majority of whom are of working age.
Related: Taking Steps to Reduce Arthritis Pain
Arthritis can make it hard to lift a cup, let alone a bag of groceries or a heavy briefcase. The percentage of adults with arthritis who have activity limitations grew from 35.9% in 2002 to 42.8% in 2014, a 20% increase independent of the aging of the population.
Research has shown that engaging in physical activity can reduce arthritis symptoms by up to 40%. But one third of adults with arthritis say they don’t engage in physical activity during leisure time. And, while they also can reduce their symptoms by participating in disease management education programs, only 1 in 10 has taken part in such programs.
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
“It’s extremely important for primary care providers to encourage their patients with arthritis to be physically active,” says CDC epidemiologist Kamil Barbour, PhD. But Barbour adds that it’s just as important to motivate patients to attend education programs. The CDC says adults with arthritis are significantly more likely to attend an education program when a health care provider has recommended it.
Arthritis aches and pains are not a normal part of aging. Nonetheless, approximately 54 million American adults who took the CDC’s National Health Survey said their doctor had diagnosed them with arthritis. That’s about 1 in 4 US adults, the majority of whom are of working age.
Related: Taking Steps to Reduce Arthritis Pain
Arthritis can make it hard to lift a cup, let alone a bag of groceries or a heavy briefcase. The percentage of adults with arthritis who have activity limitations grew from 35.9% in 2002 to 42.8% in 2014, a 20% increase independent of the aging of the population.
Research has shown that engaging in physical activity can reduce arthritis symptoms by up to 40%. But one third of adults with arthritis say they don’t engage in physical activity during leisure time. And, while they also can reduce their symptoms by participating in disease management education programs, only 1 in 10 has taken part in such programs.
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
“It’s extremely important for primary care providers to encourage their patients with arthritis to be physically active,” says CDC epidemiologist Kamil Barbour, PhD. But Barbour adds that it’s just as important to motivate patients to attend education programs. The CDC says adults with arthritis are significantly more likely to attend an education program when a health care provider has recommended it.
Arthritis aches and pains are not a normal part of aging. Nonetheless, approximately 54 million American adults who took the CDC’s National Health Survey said their doctor had diagnosed them with arthritis. That’s about 1 in 4 US adults, the majority of whom are of working age.
Related: Taking Steps to Reduce Arthritis Pain
Arthritis can make it hard to lift a cup, let alone a bag of groceries or a heavy briefcase. The percentage of adults with arthritis who have activity limitations grew from 35.9% in 2002 to 42.8% in 2014, a 20% increase independent of the aging of the population.
Research has shown that engaging in physical activity can reduce arthritis symptoms by up to 40%. But one third of adults with arthritis say they don’t engage in physical activity during leisure time. And, while they also can reduce their symptoms by participating in disease management education programs, only 1 in 10 has taken part in such programs.
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
“It’s extremely important for primary care providers to encourage their patients with arthritis to be physically active,” says CDC epidemiologist Kamil Barbour, PhD. But Barbour adds that it’s just as important to motivate patients to attend education programs. The CDC says adults with arthritis are significantly more likely to attend an education program when a health care provider has recommended it.