User login
Oral Microbes Tied to Pancreatic Cancer Risk
Could oral microbiome profiling help spot people at risk for pancreatic cancer?
It may be possible, according to a recent analysis published in JAMA Oncology.
Researchers found that , which could be a step toward earlier detection of the deadly malignancy.
“We identified 27 individual bacterial and fungal species significantly associated with pancreatic cancer development,” said Jiyoung Ahn, PhD, of NYU Grossman School of Medicine in New York City.
“If validated, oral microbiome profiling could serve as a noninvasive biomarker to identify individuals at elevated risk who might benefit from enhanced surveillance,” Ahn told GI & Hepatology News by email.
Rates of pancreatic cancer are on the rise. But detecting the disease before it becomes unresectable has remained an elusive goal, and the US Preventive Services Task Force discourages screening of asymptomatic adults.
For their study, Ahn and her colleagues analyzed data from 122,000 participants who provided oral wash samples as part of two cohort studies conducted in the US. The researchers used whole-genome shotgun sequencing and internal transcribed spacer sequencing to identity the bacterial and fungal species in the samples, respectively.
Over a median follow-up of nearly 9 years, 445 people developed pancreatic cancer and were matched with 445 who did not. Three oral bacterial periodontal pathogens — Porphyromonas gingivalis (odds ratio [OR], 1.27), Eubacterium nodatum (OR, 1.42), and Parvimonas micra (OR, 1.36) — as well as the fungal genus Candida were all linked to significantly increased odds of developing pancreatic cancer.
In a bacteriome-wide scan, the researchers pinpointed another 20 oral bacteria associated with pancreatic cancer — eight with a decreased risk and 13 with an increased risk for the disease.
The researchers also calculated a microbial risk score, which was the weighted sum of the relative abundance of bacterial and fungal species. In a meta-analysis of data from the two cohorts, the microbial risk score derived from 23 bacterial species and four fungal species, including various Candida species, was associated with pancreatic cancer (multivariate OR per 1-SD increase in the score, 3.44; 95% CI, 2.63-4.51).
“The oral microbiota holds promise as a biomarker to identify individuals at high risk of pancreatic cancer, potentially enabling personalized pancreatic cancer prevention,” Ahn and her colleagues concluded.
But Gil Welch, MD, of Brigham and Women’s Hospital in Boston, who has written about screening for decades, isn’t so sure.
Given the “impressive volume of information” included in the analysis, “it is not surprising that the investigators are able to create a microbial risk score (based on 27 species of bacteria and fungi) that is highly related to pancreatic cancer,” Welch said. “The authors are careful to emphasize these are associations, not causal relationships.”
But even if the relationship were causal, finding more people with the malignancy can also have downsides, said Welch.
In a study out last year, Welch and colleagues found that while the incidence of pancreatic cancer among young Americans has been rising, mortality rates in this demographic haven’t budged, suggesting a potential for overdiagnosis.
“Screening for pancreatic cancer has never been shown to reduce pancreatic cancer mortality,” Welch told GI & Hepatology News. “Why screen large swaths of the population simply to enumerate ‘risk factors’ for an unproven benefit that, at best, could help only a few? Meanwhile, the burdens for everyone else are real: the mental and financial strains of ‘high risk’ labels, false alarms, and endless follow-ups. It’s a recipe to make us all worried sick — and poorer.”
Ahn reported having no disclosures. Welch reported receiving royalties from three books including “Should I be tested for cancer?”
A version of this article appeared on Medscape.com.
Could oral microbiome profiling help spot people at risk for pancreatic cancer?
It may be possible, according to a recent analysis published in JAMA Oncology.
Researchers found that , which could be a step toward earlier detection of the deadly malignancy.
“We identified 27 individual bacterial and fungal species significantly associated with pancreatic cancer development,” said Jiyoung Ahn, PhD, of NYU Grossman School of Medicine in New York City.
“If validated, oral microbiome profiling could serve as a noninvasive biomarker to identify individuals at elevated risk who might benefit from enhanced surveillance,” Ahn told GI & Hepatology News by email.
Rates of pancreatic cancer are on the rise. But detecting the disease before it becomes unresectable has remained an elusive goal, and the US Preventive Services Task Force discourages screening of asymptomatic adults.
For their study, Ahn and her colleagues analyzed data from 122,000 participants who provided oral wash samples as part of two cohort studies conducted in the US. The researchers used whole-genome shotgun sequencing and internal transcribed spacer sequencing to identity the bacterial and fungal species in the samples, respectively.
Over a median follow-up of nearly 9 years, 445 people developed pancreatic cancer and were matched with 445 who did not. Three oral bacterial periodontal pathogens — Porphyromonas gingivalis (odds ratio [OR], 1.27), Eubacterium nodatum (OR, 1.42), and Parvimonas micra (OR, 1.36) — as well as the fungal genus Candida were all linked to significantly increased odds of developing pancreatic cancer.
In a bacteriome-wide scan, the researchers pinpointed another 20 oral bacteria associated with pancreatic cancer — eight with a decreased risk and 13 with an increased risk for the disease.
The researchers also calculated a microbial risk score, which was the weighted sum of the relative abundance of bacterial and fungal species. In a meta-analysis of data from the two cohorts, the microbial risk score derived from 23 bacterial species and four fungal species, including various Candida species, was associated with pancreatic cancer (multivariate OR per 1-SD increase in the score, 3.44; 95% CI, 2.63-4.51).
“The oral microbiota holds promise as a biomarker to identify individuals at high risk of pancreatic cancer, potentially enabling personalized pancreatic cancer prevention,” Ahn and her colleagues concluded.
But Gil Welch, MD, of Brigham and Women’s Hospital in Boston, who has written about screening for decades, isn’t so sure.
Given the “impressive volume of information” included in the analysis, “it is not surprising that the investigators are able to create a microbial risk score (based on 27 species of bacteria and fungi) that is highly related to pancreatic cancer,” Welch said. “The authors are careful to emphasize these are associations, not causal relationships.”
But even if the relationship were causal, finding more people with the malignancy can also have downsides, said Welch.
In a study out last year, Welch and colleagues found that while the incidence of pancreatic cancer among young Americans has been rising, mortality rates in this demographic haven’t budged, suggesting a potential for overdiagnosis.
“Screening for pancreatic cancer has never been shown to reduce pancreatic cancer mortality,” Welch told GI & Hepatology News. “Why screen large swaths of the population simply to enumerate ‘risk factors’ for an unproven benefit that, at best, could help only a few? Meanwhile, the burdens for everyone else are real: the mental and financial strains of ‘high risk’ labels, false alarms, and endless follow-ups. It’s a recipe to make us all worried sick — and poorer.”
Ahn reported having no disclosures. Welch reported receiving royalties from three books including “Should I be tested for cancer?”
A version of this article appeared on Medscape.com.
Could oral microbiome profiling help spot people at risk for pancreatic cancer?
It may be possible, according to a recent analysis published in JAMA Oncology.
Researchers found that , which could be a step toward earlier detection of the deadly malignancy.
“We identified 27 individual bacterial and fungal species significantly associated with pancreatic cancer development,” said Jiyoung Ahn, PhD, of NYU Grossman School of Medicine in New York City.
“If validated, oral microbiome profiling could serve as a noninvasive biomarker to identify individuals at elevated risk who might benefit from enhanced surveillance,” Ahn told GI & Hepatology News by email.
Rates of pancreatic cancer are on the rise. But detecting the disease before it becomes unresectable has remained an elusive goal, and the US Preventive Services Task Force discourages screening of asymptomatic adults.
For their study, Ahn and her colleagues analyzed data from 122,000 participants who provided oral wash samples as part of two cohort studies conducted in the US. The researchers used whole-genome shotgun sequencing and internal transcribed spacer sequencing to identity the bacterial and fungal species in the samples, respectively.
Over a median follow-up of nearly 9 years, 445 people developed pancreatic cancer and were matched with 445 who did not. Three oral bacterial periodontal pathogens — Porphyromonas gingivalis (odds ratio [OR], 1.27), Eubacterium nodatum (OR, 1.42), and Parvimonas micra (OR, 1.36) — as well as the fungal genus Candida were all linked to significantly increased odds of developing pancreatic cancer.
In a bacteriome-wide scan, the researchers pinpointed another 20 oral bacteria associated with pancreatic cancer — eight with a decreased risk and 13 with an increased risk for the disease.
The researchers also calculated a microbial risk score, which was the weighted sum of the relative abundance of bacterial and fungal species. In a meta-analysis of data from the two cohorts, the microbial risk score derived from 23 bacterial species and four fungal species, including various Candida species, was associated with pancreatic cancer (multivariate OR per 1-SD increase in the score, 3.44; 95% CI, 2.63-4.51).
“The oral microbiota holds promise as a biomarker to identify individuals at high risk of pancreatic cancer, potentially enabling personalized pancreatic cancer prevention,” Ahn and her colleagues concluded.
But Gil Welch, MD, of Brigham and Women’s Hospital in Boston, who has written about screening for decades, isn’t so sure.
Given the “impressive volume of information” included in the analysis, “it is not surprising that the investigators are able to create a microbial risk score (based on 27 species of bacteria and fungi) that is highly related to pancreatic cancer,” Welch said. “The authors are careful to emphasize these are associations, not causal relationships.”
But even if the relationship were causal, finding more people with the malignancy can also have downsides, said Welch.
In a study out last year, Welch and colleagues found that while the incidence of pancreatic cancer among young Americans has been rising, mortality rates in this demographic haven’t budged, suggesting a potential for overdiagnosis.
“Screening for pancreatic cancer has never been shown to reduce pancreatic cancer mortality,” Welch told GI & Hepatology News. “Why screen large swaths of the population simply to enumerate ‘risk factors’ for an unproven benefit that, at best, could help only a few? Meanwhile, the burdens for everyone else are real: the mental and financial strains of ‘high risk’ labels, false alarms, and endless follow-ups. It’s a recipe to make us all worried sick — and poorer.”
Ahn reported having no disclosures. Welch reported receiving royalties from three books including “Should I be tested for cancer?”
A version of this article appeared on Medscape.com.
Getting Ahead of Gastrointestinal Cancer
Early-onset gastrointestinal (GI) cancers are climbing among those younger than 50 years, in the US and globally. Although colorectal cancer accounts for approximately half of such cases, rates are also increasing for gastric, esophageal, pancreatic, and several rarer GI malignancies.
Because most in this age group are not included in screening protocols and may present with vague symptoms, diagnosis and treatment is frequently delayed. According to experts in the field, counteracting this trend requires establishing a lower threshold for evaluation, attention to modifiable risk factors, and embracing emerging noninvasive diagnostic tools.
Diagnostic Dilemmas
“Colorectal cancer in particular is often diagnosed later in life,” said Nicholas DeVito, MD, assistant professor at Duke University Medical Center, Durham, North Carolina, and a specialist in GI malignancies. “When the patient is too young for routine screening colonoscopy (< 45 years), they aren’t screened at all, they do not have alarming symptoms, or their symptoms are overlooked.” Other increasingly common GI cancers in young people (esophageal, gastric, pancreatic) lack routine screening guidelines due to limited evidence, he added.
Symptoms such as nausea, weight loss, upset stomach, and abdominal pain are often nonspecific and have many other potential causes, so GI cancers may not be high on the list of possible diagnoses in patients younger than 50 years, said DeVito.
“Insurance coverage, socioeconomic status, appointment availability, and awareness of symptoms and screening methods are all barriers to diagnosis as well, which affect the diagnostic timeline of many cancers,” he added.“While there are multiple factors that contribute to a cancer diagnosis, it seems that obesity, a Western diet, a sedentary lifestyle are all major contributors to the rise in early GI cancers,” DeVito told GI & Hepatology News. “There is no blame or judgement to go around as cancer can happen to anyone at any time, with none of these factors present,” he emphasized.
When counseling patients about GI cancer risk, DeVito recommends keeping advice simple and specific. In general, they should restrict red meat to once a week, emphasize fresh fruits and vegetables, cap alcohol to ≤ 1 serving per day, and limit ultraprocessed foods (e.g., packaged snacks, preprepared meals, and sugary beverages).
Exercise is another pillar. “Find an activity you enjoy and work toward 30 minutes of aerobic exercise three times a week,” he advised. He also encourages finding opportunities to incorporate physical activity in daily lives, such as using a standing desk at work, while keeping patients’ socioeconomic constraints in mind.
Evidence around GI cancer prevention interventions is still evolving. However, a randomized phase 3 trial presented at American Society of Clinical Oncology’s 2025 meeting found significant improvement in disease-free survival among adults with resected stage III or high-risk stage II colon cancer (median age, 61 years) who reported higher intake of anti-inflammatory foods and greater exercise than a comparator group.
“In general, clinicians should be aware of the risk factors, make referrals to physical therapy, weight-loss specialists, endocrinologists, and nutritionists when appropriate, and be consistent and clear with patients about recommendations and what’s achievable,” DeVito said. “Meeting patients where they are can help make incremental progress, as these interventions take time and patience, and we should be understanding of that.”
Identifying at-risk younger adults goes beyond discussing family history and obesity to include diet, exercise, and daily lifestyle, he added.
“Symptoms of potential GI cancer need to be taken seriously in all patients, and there should be a lower threshold in 2025 to get a colonoscopy, endoscopy, or CT scan than in previous years given all that we know today. We then need to establish through clinical studies who needs screening tests and who doesn’t, and what interventions work best to reduce risk.”
Vigilance in the Absence of Screening
“Most GI cancers, unfortunately, can grow a fair amount before symptoms arise, so many patients present with symptoms only when a tumor has grown enough to affect organ function,” said Miguel Burch, MD, chief of minimally invasive and GI surgery at Cedars-Sinai Medical Center, Los Angeles.
Early screening improves outcomes in gastric cancer, Burch noted, and survival benefits are reflected in several East Asian countries that offer gastric cancer screening starting at age 40. In one study from Korea, a single upper endoscopy was associated with an approximate 40% reduction in gastric cancer mortality compared with no screening.
, Burch emphasized. The impact is wide-ranging, contributing to increased morbidity and mortality in younger adults often in their most productive years, leading to lost wages and emotional strains upon patients and their families.Routine endoscopic or imaging screening is not typically performed in the US, and newer blood-based tests such as circulating tumor DNA are not yet sensitive enough to reliably detect very early-stage disease. Nonetheless, there is evidence that noninvasive biomarkers could soon help expand GI cancer screening.
In a study published in JAMA Surgery, Sui and colleagues tested a 10-microRNA signature assay (Destinex) for early detection of gastric cancer and reported robust identification rates above 95%.
“In recent years, the liquid biopsy has gained momentum with the hope of augmenting cancer detection from peripheral blood, even indicating potential as a screening test for healthy populations,” wrote Max R. Coffey, MD, and Vivian E. Strong, MD, both of the Memorial Sloan Kettering Cancer Center in New York City, in an accompanying editorial.
“Early detection is absolutely critical; when gastric cancer is found early, outcomes are dramatically better,” Strong told GI & Hepatology News. Subtle symptoms — reflux, persistent GI discomfort, or unexplained weight loss — should never be ignored, she added.
Early detection should also focus on additional risk factors such as prior Helicobacter pylori infection, smoking, and family history.
“Anyone with a personal or family history of H pylori should have very careful follow-up, and if one household member tests positive, all should be checked,” Strong said. “Just as importantly, if one or more family members have had stomach cancer, that should be discussed with a healthcare provider, as it may warrant higher-level surveillance and genetic testing.”
Individuals concerned about increased risk for GI cancer should proactively ask their doctors whether they might benefit from testing or surveillance, Strong added.
“Lifestyle changes, timely medical evaluation, and tailored surveillance all play a vital role in prevention.”
DeVito disclosed clinical trial funding from the Gateway foundation, Xilio, Phanes, Astellas, GSK, as well as consulting fees/advisory board participation for Guardant, Agenus, and Xilio. Strong disclosed speaking honoraria for Merck and Astra Zeneca.
The study by Sui and colleagues was supported by the National Cancer Institute, National Institutes of Health, as well as by a grant from the American Gastroenterological Association Robert & Sally Funderburg Research Award in Gastric Cancer, and the Stupid Strong Foundation.
Burch had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Early-onset gastrointestinal (GI) cancers are climbing among those younger than 50 years, in the US and globally. Although colorectal cancer accounts for approximately half of such cases, rates are also increasing for gastric, esophageal, pancreatic, and several rarer GI malignancies.
Because most in this age group are not included in screening protocols and may present with vague symptoms, diagnosis and treatment is frequently delayed. According to experts in the field, counteracting this trend requires establishing a lower threshold for evaluation, attention to modifiable risk factors, and embracing emerging noninvasive diagnostic tools.
Diagnostic Dilemmas
“Colorectal cancer in particular is often diagnosed later in life,” said Nicholas DeVito, MD, assistant professor at Duke University Medical Center, Durham, North Carolina, and a specialist in GI malignancies. “When the patient is too young for routine screening colonoscopy (< 45 years), they aren’t screened at all, they do not have alarming symptoms, or their symptoms are overlooked.” Other increasingly common GI cancers in young people (esophageal, gastric, pancreatic) lack routine screening guidelines due to limited evidence, he added.
Symptoms such as nausea, weight loss, upset stomach, and abdominal pain are often nonspecific and have many other potential causes, so GI cancers may not be high on the list of possible diagnoses in patients younger than 50 years, said DeVito.
“Insurance coverage, socioeconomic status, appointment availability, and awareness of symptoms and screening methods are all barriers to diagnosis as well, which affect the diagnostic timeline of many cancers,” he added.“While there are multiple factors that contribute to a cancer diagnosis, it seems that obesity, a Western diet, a sedentary lifestyle are all major contributors to the rise in early GI cancers,” DeVito told GI & Hepatology News. “There is no blame or judgement to go around as cancer can happen to anyone at any time, with none of these factors present,” he emphasized.
When counseling patients about GI cancer risk, DeVito recommends keeping advice simple and specific. In general, they should restrict red meat to once a week, emphasize fresh fruits and vegetables, cap alcohol to ≤ 1 serving per day, and limit ultraprocessed foods (e.g., packaged snacks, preprepared meals, and sugary beverages).
Exercise is another pillar. “Find an activity you enjoy and work toward 30 minutes of aerobic exercise three times a week,” he advised. He also encourages finding opportunities to incorporate physical activity in daily lives, such as using a standing desk at work, while keeping patients’ socioeconomic constraints in mind.
Evidence around GI cancer prevention interventions is still evolving. However, a randomized phase 3 trial presented at American Society of Clinical Oncology’s 2025 meeting found significant improvement in disease-free survival among adults with resected stage III or high-risk stage II colon cancer (median age, 61 years) who reported higher intake of anti-inflammatory foods and greater exercise than a comparator group.
“In general, clinicians should be aware of the risk factors, make referrals to physical therapy, weight-loss specialists, endocrinologists, and nutritionists when appropriate, and be consistent and clear with patients about recommendations and what’s achievable,” DeVito said. “Meeting patients where they are can help make incremental progress, as these interventions take time and patience, and we should be understanding of that.”
Identifying at-risk younger adults goes beyond discussing family history and obesity to include diet, exercise, and daily lifestyle, he added.
“Symptoms of potential GI cancer need to be taken seriously in all patients, and there should be a lower threshold in 2025 to get a colonoscopy, endoscopy, or CT scan than in previous years given all that we know today. We then need to establish through clinical studies who needs screening tests and who doesn’t, and what interventions work best to reduce risk.”
Vigilance in the Absence of Screening
“Most GI cancers, unfortunately, can grow a fair amount before symptoms arise, so many patients present with symptoms only when a tumor has grown enough to affect organ function,” said Miguel Burch, MD, chief of minimally invasive and GI surgery at Cedars-Sinai Medical Center, Los Angeles.
Early screening improves outcomes in gastric cancer, Burch noted, and survival benefits are reflected in several East Asian countries that offer gastric cancer screening starting at age 40. In one study from Korea, a single upper endoscopy was associated with an approximate 40% reduction in gastric cancer mortality compared with no screening.
, Burch emphasized. The impact is wide-ranging, contributing to increased morbidity and mortality in younger adults often in their most productive years, leading to lost wages and emotional strains upon patients and their families.Routine endoscopic or imaging screening is not typically performed in the US, and newer blood-based tests such as circulating tumor DNA are not yet sensitive enough to reliably detect very early-stage disease. Nonetheless, there is evidence that noninvasive biomarkers could soon help expand GI cancer screening.
In a study published in JAMA Surgery, Sui and colleagues tested a 10-microRNA signature assay (Destinex) for early detection of gastric cancer and reported robust identification rates above 95%.
“In recent years, the liquid biopsy has gained momentum with the hope of augmenting cancer detection from peripheral blood, even indicating potential as a screening test for healthy populations,” wrote Max R. Coffey, MD, and Vivian E. Strong, MD, both of the Memorial Sloan Kettering Cancer Center in New York City, in an accompanying editorial.
“Early detection is absolutely critical; when gastric cancer is found early, outcomes are dramatically better,” Strong told GI & Hepatology News. Subtle symptoms — reflux, persistent GI discomfort, or unexplained weight loss — should never be ignored, she added.
Early detection should also focus on additional risk factors such as prior Helicobacter pylori infection, smoking, and family history.
“Anyone with a personal or family history of H pylori should have very careful follow-up, and if one household member tests positive, all should be checked,” Strong said. “Just as importantly, if one or more family members have had stomach cancer, that should be discussed with a healthcare provider, as it may warrant higher-level surveillance and genetic testing.”
Individuals concerned about increased risk for GI cancer should proactively ask their doctors whether they might benefit from testing or surveillance, Strong added.
“Lifestyle changes, timely medical evaluation, and tailored surveillance all play a vital role in prevention.”
DeVito disclosed clinical trial funding from the Gateway foundation, Xilio, Phanes, Astellas, GSK, as well as consulting fees/advisory board participation for Guardant, Agenus, and Xilio. Strong disclosed speaking honoraria for Merck and Astra Zeneca.
The study by Sui and colleagues was supported by the National Cancer Institute, National Institutes of Health, as well as by a grant from the American Gastroenterological Association Robert & Sally Funderburg Research Award in Gastric Cancer, and the Stupid Strong Foundation.
Burch had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Early-onset gastrointestinal (GI) cancers are climbing among those younger than 50 years, in the US and globally. Although colorectal cancer accounts for approximately half of such cases, rates are also increasing for gastric, esophageal, pancreatic, and several rarer GI malignancies.
Because most in this age group are not included in screening protocols and may present with vague symptoms, diagnosis and treatment is frequently delayed. According to experts in the field, counteracting this trend requires establishing a lower threshold for evaluation, attention to modifiable risk factors, and embracing emerging noninvasive diagnostic tools.
Diagnostic Dilemmas
“Colorectal cancer in particular is often diagnosed later in life,” said Nicholas DeVito, MD, assistant professor at Duke University Medical Center, Durham, North Carolina, and a specialist in GI malignancies. “When the patient is too young for routine screening colonoscopy (< 45 years), they aren’t screened at all, they do not have alarming symptoms, or their symptoms are overlooked.” Other increasingly common GI cancers in young people (esophageal, gastric, pancreatic) lack routine screening guidelines due to limited evidence, he added.
Symptoms such as nausea, weight loss, upset stomach, and abdominal pain are often nonspecific and have many other potential causes, so GI cancers may not be high on the list of possible diagnoses in patients younger than 50 years, said DeVito.
“Insurance coverage, socioeconomic status, appointment availability, and awareness of symptoms and screening methods are all barriers to diagnosis as well, which affect the diagnostic timeline of many cancers,” he added.“While there are multiple factors that contribute to a cancer diagnosis, it seems that obesity, a Western diet, a sedentary lifestyle are all major contributors to the rise in early GI cancers,” DeVito told GI & Hepatology News. “There is no blame or judgement to go around as cancer can happen to anyone at any time, with none of these factors present,” he emphasized.
When counseling patients about GI cancer risk, DeVito recommends keeping advice simple and specific. In general, they should restrict red meat to once a week, emphasize fresh fruits and vegetables, cap alcohol to ≤ 1 serving per day, and limit ultraprocessed foods (e.g., packaged snacks, preprepared meals, and sugary beverages).
Exercise is another pillar. “Find an activity you enjoy and work toward 30 minutes of aerobic exercise three times a week,” he advised. He also encourages finding opportunities to incorporate physical activity in daily lives, such as using a standing desk at work, while keeping patients’ socioeconomic constraints in mind.
Evidence around GI cancer prevention interventions is still evolving. However, a randomized phase 3 trial presented at American Society of Clinical Oncology’s 2025 meeting found significant improvement in disease-free survival among adults with resected stage III or high-risk stage II colon cancer (median age, 61 years) who reported higher intake of anti-inflammatory foods and greater exercise than a comparator group.
“In general, clinicians should be aware of the risk factors, make referrals to physical therapy, weight-loss specialists, endocrinologists, and nutritionists when appropriate, and be consistent and clear with patients about recommendations and what’s achievable,” DeVito said. “Meeting patients where they are can help make incremental progress, as these interventions take time and patience, and we should be understanding of that.”
Identifying at-risk younger adults goes beyond discussing family history and obesity to include diet, exercise, and daily lifestyle, he added.
“Symptoms of potential GI cancer need to be taken seriously in all patients, and there should be a lower threshold in 2025 to get a colonoscopy, endoscopy, or CT scan than in previous years given all that we know today. We then need to establish through clinical studies who needs screening tests and who doesn’t, and what interventions work best to reduce risk.”
Vigilance in the Absence of Screening
“Most GI cancers, unfortunately, can grow a fair amount before symptoms arise, so many patients present with symptoms only when a tumor has grown enough to affect organ function,” said Miguel Burch, MD, chief of minimally invasive and GI surgery at Cedars-Sinai Medical Center, Los Angeles.
Early screening improves outcomes in gastric cancer, Burch noted, and survival benefits are reflected in several East Asian countries that offer gastric cancer screening starting at age 40. In one study from Korea, a single upper endoscopy was associated with an approximate 40% reduction in gastric cancer mortality compared with no screening.
, Burch emphasized. The impact is wide-ranging, contributing to increased morbidity and mortality in younger adults often in their most productive years, leading to lost wages and emotional strains upon patients and their families.Routine endoscopic or imaging screening is not typically performed in the US, and newer blood-based tests such as circulating tumor DNA are not yet sensitive enough to reliably detect very early-stage disease. Nonetheless, there is evidence that noninvasive biomarkers could soon help expand GI cancer screening.
In a study published in JAMA Surgery, Sui and colleagues tested a 10-microRNA signature assay (Destinex) for early detection of gastric cancer and reported robust identification rates above 95%.
“In recent years, the liquid biopsy has gained momentum with the hope of augmenting cancer detection from peripheral blood, even indicating potential as a screening test for healthy populations,” wrote Max R. Coffey, MD, and Vivian E. Strong, MD, both of the Memorial Sloan Kettering Cancer Center in New York City, in an accompanying editorial.
“Early detection is absolutely critical; when gastric cancer is found early, outcomes are dramatically better,” Strong told GI & Hepatology News. Subtle symptoms — reflux, persistent GI discomfort, or unexplained weight loss — should never be ignored, she added.
Early detection should also focus on additional risk factors such as prior Helicobacter pylori infection, smoking, and family history.
“Anyone with a personal or family history of H pylori should have very careful follow-up, and if one household member tests positive, all should be checked,” Strong said. “Just as importantly, if one or more family members have had stomach cancer, that should be discussed with a healthcare provider, as it may warrant higher-level surveillance and genetic testing.”
Individuals concerned about increased risk for GI cancer should proactively ask their doctors whether they might benefit from testing or surveillance, Strong added.
“Lifestyle changes, timely medical evaluation, and tailored surveillance all play a vital role in prevention.”
DeVito disclosed clinical trial funding from the Gateway foundation, Xilio, Phanes, Astellas, GSK, as well as consulting fees/advisory board participation for Guardant, Agenus, and Xilio. Strong disclosed speaking honoraria for Merck and Astra Zeneca.
The study by Sui and colleagues was supported by the National Cancer Institute, National Institutes of Health, as well as by a grant from the American Gastroenterological Association Robert & Sally Funderburg Research Award in Gastric Cancer, and the Stupid Strong Foundation.
Burch had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Large Language Models Cut Time, Cost of Guideline Development
, according to a pilot study from the American Gastroenterological Association (AGA).
Faster, cheaper study screening could allow societies to update clinical recommendations more frequently, improving alignment with the latest evidence, lead author Sunny Chung, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Each guideline typically requires 5 to 15 systematic reviews, making the process time-consuming (averaging more than 60 weeks) and costly (more than $140,000),” the investigators wrote in Gastroenterology . “One of the most critical yet time-consuming steps in systematic reviews is title and abstract screening. LLMs have the potential to make this step more efficient.”
To test this approach, the investigators developed, validated, and applied a dual-model LLM screening pipeline with human-in-the-loop oversight, focusing on randomized controlled trials in AGA guidelines.
The system was built using the 2021 guideline on moderate-to-severe Crohn’s disease, targeting biologic therapies for induction and maintenance of remission.
Using chain-of-thought prompting and structured inclusion criteria based on the PICO framework, the investigators deployed GPT-4o (OpenAI) and Gemini-1.5-Pro (Google DeepMind) as independent screeners, each assessing titles and abstracts according to standardized logic encoded in JavaScript Object Notation. This approach mimicked a traditional double-reviewer system.
After initial testing, the pipeline was validated in a 2025 update of the same guideline, this time spanning 6 focused clinical questions on advanced therapies and immunomodulators. Results were compared against manual screening by 2 experienced human reviewers, with total screening time documented.
The system was then tested across 4 additional guideline topics: fecal microbiota transplantation (FMT) for irritable bowel syndrome and Clostridioides difficile, gastroparesis, and hepatocellular carcinoma. A final test applied the system to a forthcoming guideline on complications of acute pancreatitis.
Across all topics, the dual-LLM system achieved 100% sensitivity in identifying randomized controlled trials (RCTs). For the 2025 update of the AGA guideline on Crohn’s disease, the models flagged 418 of 4,377 abstracts for inclusion, captur-ing all 25 relevant RCTs in just 48 minutes. Manual screening of the same dataset previously took almost 13 hours.
Comparable accuracy and time savings were observed for the other topics.
The pipeline correctly flagged all 13 RCTs in 4,820 studies on FMT for irritable bowel syndrome, and all 16 RCTs in 5,587 studies on FMT for Clostridioides difficile, requiring 27 and 66 minutes, respectively. Similarly, the system captured all 11 RCTs in 3,919 hepatocellular carcinoma abstracts and all 18 RCTs in 1,578 studies on gastroparesis, completing each task in under 65 minutes. Early testing on the upcoming guideline for pancreatitis yielded similar results.
Cost analysis underscored the efficiency of this approach. At an estimated $175–200 per hour for expert screeners, traditional abstract screening would cost around $2,500 per review, versus approximately $100 for the LLM approach—a 96% reduction.
The investigators cautioned that human oversight remains necessary to verify the relevance of studies flagged by the models. While the system’s sensitivity was consistent, it also selected articles that were ultimately excluded by expert reviewers. Broader validation will be required to assess performance across non-RCT study designs, such as observational or case-control studies, they added.
“As medical literature continues to expand, the integration of artificial intelligence into evidence synthesis processes will become increasingly vital,” Dr. Chung and colleagues wrote. “With further refinement and broader validation, this LLM-based pipeline has the potential to revolutionize evidence synthesis and set a new standard for guideline development.”
This study was funded by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. The investigators reported no conflicts of interest.
Ethan Goh, MD, executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, described the AGA pilot as both timely and promising.
“I’m certainly bullish about the use case,” he said in an interview. “Their study design and application is also robust, so I would congratulate them.”
Goh, a general editor for BMJ Digital Health & AI, predicted “huge potential” in the strategy for both clinicians and the general population, who benefit from the most up-to-date guidelines possible.
“I believe that using AI can represent a much faster, more cost effective, efficient way of gathering all these information sources,” he said.
Still, humans will need to be involved in the process.
“[This AI-driven approach] will always need some degree of expert oversight and judgement,” Goh said.
Speaking more broadly about automating study aggregation, Goh said AI may still struggle to determine which studies are most clinically relevant.
“When we use [AI models] to pull out medical references, anecdotally, I don’t think they’re always getting the best ones all the time, or even necessarily the right ones,” he said.
And as AI models grow more impressive, these shortcomings become less apparent, potentially lulling humans into overconfidence.
“Humans are humans,” Goh said. “We get lazy over time. That will be one of the challenges. As the systems get increasingly good, humans start to defer more and more of their judgment to them and say, ‘All right, AI, you’re doing good. Just do 100% automation.’ And then [people] start fact checking or reviewing even less.”
AI could also undermine automated reviews in another way: AI-generated publications that appear genuine, but aren’t, may creep into the dataset.
Despite these concerns, Goh concluded on an optimistic note.
“I think that there are huge ways to use AI, tools, not to replace, but to augment and support human judgment,” he said.
Ethan Goh, MD, is senior research engineer and executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, at Stanford (Calif.) University. He declared no conflicts of interest.
Ethan Goh, MD, executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, described the AGA pilot as both timely and promising.
“I’m certainly bullish about the use case,” he said in an interview. “Their study design and application is also robust, so I would congratulate them.”
Goh, a general editor for BMJ Digital Health & AI, predicted “huge potential” in the strategy for both clinicians and the general population, who benefit from the most up-to-date guidelines possible.
“I believe that using AI can represent a much faster, more cost effective, efficient way of gathering all these information sources,” he said.
Still, humans will need to be involved in the process.
“[This AI-driven approach] will always need some degree of expert oversight and judgement,” Goh said.
Speaking more broadly about automating study aggregation, Goh said AI may still struggle to determine which studies are most clinically relevant.
“When we use [AI models] to pull out medical references, anecdotally, I don’t think they’re always getting the best ones all the time, or even necessarily the right ones,” he said.
And as AI models grow more impressive, these shortcomings become less apparent, potentially lulling humans into overconfidence.
“Humans are humans,” Goh said. “We get lazy over time. That will be one of the challenges. As the systems get increasingly good, humans start to defer more and more of their judgment to them and say, ‘All right, AI, you’re doing good. Just do 100% automation.’ And then [people] start fact checking or reviewing even less.”
AI could also undermine automated reviews in another way: AI-generated publications that appear genuine, but aren’t, may creep into the dataset.
Despite these concerns, Goh concluded on an optimistic note.
“I think that there are huge ways to use AI, tools, not to replace, but to augment and support human judgment,” he said.
Ethan Goh, MD, is senior research engineer and executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, at Stanford (Calif.) University. He declared no conflicts of interest.
Ethan Goh, MD, executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, described the AGA pilot as both timely and promising.
“I’m certainly bullish about the use case,” he said in an interview. “Their study design and application is also robust, so I would congratulate them.”
Goh, a general editor for BMJ Digital Health & AI, predicted “huge potential” in the strategy for both clinicians and the general population, who benefit from the most up-to-date guidelines possible.
“I believe that using AI can represent a much faster, more cost effective, efficient way of gathering all these information sources,” he said.
Still, humans will need to be involved in the process.
“[This AI-driven approach] will always need some degree of expert oversight and judgement,” Goh said.
Speaking more broadly about automating study aggregation, Goh said AI may still struggle to determine which studies are most clinically relevant.
“When we use [AI models] to pull out medical references, anecdotally, I don’t think they’re always getting the best ones all the time, or even necessarily the right ones,” he said.
And as AI models grow more impressive, these shortcomings become less apparent, potentially lulling humans into overconfidence.
“Humans are humans,” Goh said. “We get lazy over time. That will be one of the challenges. As the systems get increasingly good, humans start to defer more and more of their judgment to them and say, ‘All right, AI, you’re doing good. Just do 100% automation.’ And then [people] start fact checking or reviewing even less.”
AI could also undermine automated reviews in another way: AI-generated publications that appear genuine, but aren’t, may creep into the dataset.
Despite these concerns, Goh concluded on an optimistic note.
“I think that there are huge ways to use AI, tools, not to replace, but to augment and support human judgment,” he said.
Ethan Goh, MD, is senior research engineer and executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, at Stanford (Calif.) University. He declared no conflicts of interest.
, according to a pilot study from the American Gastroenterological Association (AGA).
Faster, cheaper study screening could allow societies to update clinical recommendations more frequently, improving alignment with the latest evidence, lead author Sunny Chung, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Each guideline typically requires 5 to 15 systematic reviews, making the process time-consuming (averaging more than 60 weeks) and costly (more than $140,000),” the investigators wrote in Gastroenterology . “One of the most critical yet time-consuming steps in systematic reviews is title and abstract screening. LLMs have the potential to make this step more efficient.”
To test this approach, the investigators developed, validated, and applied a dual-model LLM screening pipeline with human-in-the-loop oversight, focusing on randomized controlled trials in AGA guidelines.
The system was built using the 2021 guideline on moderate-to-severe Crohn’s disease, targeting biologic therapies for induction and maintenance of remission.
Using chain-of-thought prompting and structured inclusion criteria based on the PICO framework, the investigators deployed GPT-4o (OpenAI) and Gemini-1.5-Pro (Google DeepMind) as independent screeners, each assessing titles and abstracts according to standardized logic encoded in JavaScript Object Notation. This approach mimicked a traditional double-reviewer system.
After initial testing, the pipeline was validated in a 2025 update of the same guideline, this time spanning 6 focused clinical questions on advanced therapies and immunomodulators. Results were compared against manual screening by 2 experienced human reviewers, with total screening time documented.
The system was then tested across 4 additional guideline topics: fecal microbiota transplantation (FMT) for irritable bowel syndrome and Clostridioides difficile, gastroparesis, and hepatocellular carcinoma. A final test applied the system to a forthcoming guideline on complications of acute pancreatitis.
Across all topics, the dual-LLM system achieved 100% sensitivity in identifying randomized controlled trials (RCTs). For the 2025 update of the AGA guideline on Crohn’s disease, the models flagged 418 of 4,377 abstracts for inclusion, captur-ing all 25 relevant RCTs in just 48 minutes. Manual screening of the same dataset previously took almost 13 hours.
Comparable accuracy and time savings were observed for the other topics.
The pipeline correctly flagged all 13 RCTs in 4,820 studies on FMT for irritable bowel syndrome, and all 16 RCTs in 5,587 studies on FMT for Clostridioides difficile, requiring 27 and 66 minutes, respectively. Similarly, the system captured all 11 RCTs in 3,919 hepatocellular carcinoma abstracts and all 18 RCTs in 1,578 studies on gastroparesis, completing each task in under 65 minutes. Early testing on the upcoming guideline for pancreatitis yielded similar results.
Cost analysis underscored the efficiency of this approach. At an estimated $175–200 per hour for expert screeners, traditional abstract screening would cost around $2,500 per review, versus approximately $100 for the LLM approach—a 96% reduction.
The investigators cautioned that human oversight remains necessary to verify the relevance of studies flagged by the models. While the system’s sensitivity was consistent, it also selected articles that were ultimately excluded by expert reviewers. Broader validation will be required to assess performance across non-RCT study designs, such as observational or case-control studies, they added.
“As medical literature continues to expand, the integration of artificial intelligence into evidence synthesis processes will become increasingly vital,” Dr. Chung and colleagues wrote. “With further refinement and broader validation, this LLM-based pipeline has the potential to revolutionize evidence synthesis and set a new standard for guideline development.”
This study was funded by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. The investigators reported no conflicts of interest.
, according to a pilot study from the American Gastroenterological Association (AGA).
Faster, cheaper study screening could allow societies to update clinical recommendations more frequently, improving alignment with the latest evidence, lead author Sunny Chung, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Each guideline typically requires 5 to 15 systematic reviews, making the process time-consuming (averaging more than 60 weeks) and costly (more than $140,000),” the investigators wrote in Gastroenterology . “One of the most critical yet time-consuming steps in systematic reviews is title and abstract screening. LLMs have the potential to make this step more efficient.”
To test this approach, the investigators developed, validated, and applied a dual-model LLM screening pipeline with human-in-the-loop oversight, focusing on randomized controlled trials in AGA guidelines.
The system was built using the 2021 guideline on moderate-to-severe Crohn’s disease, targeting biologic therapies for induction and maintenance of remission.
Using chain-of-thought prompting and structured inclusion criteria based on the PICO framework, the investigators deployed GPT-4o (OpenAI) and Gemini-1.5-Pro (Google DeepMind) as independent screeners, each assessing titles and abstracts according to standardized logic encoded in JavaScript Object Notation. This approach mimicked a traditional double-reviewer system.
After initial testing, the pipeline was validated in a 2025 update of the same guideline, this time spanning 6 focused clinical questions on advanced therapies and immunomodulators. Results were compared against manual screening by 2 experienced human reviewers, with total screening time documented.
The system was then tested across 4 additional guideline topics: fecal microbiota transplantation (FMT) for irritable bowel syndrome and Clostridioides difficile, gastroparesis, and hepatocellular carcinoma. A final test applied the system to a forthcoming guideline on complications of acute pancreatitis.
Across all topics, the dual-LLM system achieved 100% sensitivity in identifying randomized controlled trials (RCTs). For the 2025 update of the AGA guideline on Crohn’s disease, the models flagged 418 of 4,377 abstracts for inclusion, captur-ing all 25 relevant RCTs in just 48 minutes. Manual screening of the same dataset previously took almost 13 hours.
Comparable accuracy and time savings were observed for the other topics.
The pipeline correctly flagged all 13 RCTs in 4,820 studies on FMT for irritable bowel syndrome, and all 16 RCTs in 5,587 studies on FMT for Clostridioides difficile, requiring 27 and 66 minutes, respectively. Similarly, the system captured all 11 RCTs in 3,919 hepatocellular carcinoma abstracts and all 18 RCTs in 1,578 studies on gastroparesis, completing each task in under 65 minutes. Early testing on the upcoming guideline for pancreatitis yielded similar results.
Cost analysis underscored the efficiency of this approach. At an estimated $175–200 per hour for expert screeners, traditional abstract screening would cost around $2,500 per review, versus approximately $100 for the LLM approach—a 96% reduction.
The investigators cautioned that human oversight remains necessary to verify the relevance of studies flagged by the models. While the system’s sensitivity was consistent, it also selected articles that were ultimately excluded by expert reviewers. Broader validation will be required to assess performance across non-RCT study designs, such as observational or case-control studies, they added.
“As medical literature continues to expand, the integration of artificial intelligence into evidence synthesis processes will become increasingly vital,” Dr. Chung and colleagues wrote. “With further refinement and broader validation, this LLM-based pipeline has the potential to revolutionize evidence synthesis and set a new standard for guideline development.”
This study was funded by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. The investigators reported no conflicts of interest.
FROM GASTROENTEROLOGY
Forceps Assistance Improves Outcomes in Difficult ERCP Cannulations
The results emerged from the small, single-center SOCCER trial of 152 patients recruited from March 2022 to October 2024 and are published in The American Journal of Gastroenterology.
Both groups had a slightly higher number of female participants, and the mean ages of the participants were 61.9 years in the forceps group and 68.3 years in the no forceps group.
First author Steven M. Hadley Jr, an MD candidate at Northwestern Feinberg School of Medicine in Chicago, and colleagues reported that forceps assistance in difficult cannulations yielded significantly higher success rates than no forceps assistance (100% vs 83.9%; P < .001).
The investigators noted that difficult cannulations during ERCP have a frequency of 42%. Cannulation failure is associated with increased morbidity — including longer hospitalization, increased ICU admissions, readmissions, and increased financial cost — as well as mortality rates of up to 10%.
SOCCER defined difficult cannulation as a papilla in or on the rim of a diverticulum, five or more attempts, attempts lasting 5 or more minutes, or two or more unintended pancreatic duct wire passages. Other features were redundant tissue overlaying the papilla or a type 2, 3, or 4 papilla.
The study found forceps assistance also had a nonstatistically significant lower rate of difficult cannulations than no forceps (57.1% vs 69.1%; P = .132). The rate of post-ERCP pancreatitis (PEP) was similarly low in both groups: 5.7% with forceps vs 3.7% without forceps (P = .705). The no forceps group had significantly more cannulation attempts after randomization than the forceps group (14 vs 8.3; P = .026).
Patients who crossed over to forceps assistance all had successful cannulations.
The technique has long been used to overcome cannulation difficulties, said Timothy B. Gardner, MD, MS, a gastroenterologist at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire, and a coauthor of the study. “It was particularly effective for cannulations with redundant tissue limiting access to the papilla,” Gardner told GI & Hepatology News. “We decided to design a randomized trial to determine the extent to which this technique worked. We believed our study would answer an important question that would hopefully lead to an improvement in endoscopy practice.”
While a few case reports and video demos had described the technique, no trials had assessed its effectiveness, Hadley added. “We found the technique to be effective based on our experience, but it was exciting to see that a rigorously designed randomized trial proved that it is indeed a very effective technique to facilitate cannulation.”
Hadley noted the technique does not increase PEP incidence, unlike the commonly used precut sphincterotomy and the double-wire method for difficult cannulations. “As a result, the forceps-assisted technique may be an effective first-line option and may reduce the need for additional, more invasive procedures including surgery and repeat ERCP to obtain the therapeutic intent of the original ERCP.”
The paper outlines the technique’s methodology, he added, “so we believe endoscopists who read the manuscript will be able to start implementing the technique into their practice.”
Commenting on the paper but not involved in it, Christopher J. DiMaio, MD, regional director of Endoscopy for Northwell Health Physician Partners Gastroenterology and a gastroenterologist in Greenlawn, New York, called it potentially helpful but aimed at a niche group of expert practitioners. “The technique appears safe and very effective, which is the number one concern, and I would definitely keep it in my back pocket,” he said. “I expect it will be used more commonly now because of this study.”
He added that although expert endoscopists are familiar with the approach, they use more time-tested and sometimes more aggressive maneuvers to cope with difficult cannulations. “But this is a simple technique using a device that should be available to most high-volume endoscopists.”
DiMaio also noted that he would have liked to see an actual decrease in PEP incidence in the intervention group.
Looking ahead, Hadley said it would be interesting to compare the effectiveness of the double-wire technique against forceps-assisted cannulation in a randomized context. “A study we’re already looking into is seeing whether physician experience with the technique impacts outcomes.”
This study was supported by the American College of Gastroenterology. The authors and DiMaio reported having no relevant competing interests.
A version of this article first appeared on Medscape.com.
The results emerged from the small, single-center SOCCER trial of 152 patients recruited from March 2022 to October 2024 and are published in The American Journal of Gastroenterology.
Both groups had a slightly higher number of female participants, and the mean ages of the participants were 61.9 years in the forceps group and 68.3 years in the no forceps group.
First author Steven M. Hadley Jr, an MD candidate at Northwestern Feinberg School of Medicine in Chicago, and colleagues reported that forceps assistance in difficult cannulations yielded significantly higher success rates than no forceps assistance (100% vs 83.9%; P < .001).
The investigators noted that difficult cannulations during ERCP have a frequency of 42%. Cannulation failure is associated with increased morbidity — including longer hospitalization, increased ICU admissions, readmissions, and increased financial cost — as well as mortality rates of up to 10%.
SOCCER defined difficult cannulation as a papilla in or on the rim of a diverticulum, five or more attempts, attempts lasting 5 or more minutes, or two or more unintended pancreatic duct wire passages. Other features were redundant tissue overlaying the papilla or a type 2, 3, or 4 papilla.
The study found forceps assistance also had a nonstatistically significant lower rate of difficult cannulations than no forceps (57.1% vs 69.1%; P = .132). The rate of post-ERCP pancreatitis (PEP) was similarly low in both groups: 5.7% with forceps vs 3.7% without forceps (P = .705). The no forceps group had significantly more cannulation attempts after randomization than the forceps group (14 vs 8.3; P = .026).
Patients who crossed over to forceps assistance all had successful cannulations.
The technique has long been used to overcome cannulation difficulties, said Timothy B. Gardner, MD, MS, a gastroenterologist at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire, and a coauthor of the study. “It was particularly effective for cannulations with redundant tissue limiting access to the papilla,” Gardner told GI & Hepatology News. “We decided to design a randomized trial to determine the extent to which this technique worked. We believed our study would answer an important question that would hopefully lead to an improvement in endoscopy practice.”
While a few case reports and video demos had described the technique, no trials had assessed its effectiveness, Hadley added. “We found the technique to be effective based on our experience, but it was exciting to see that a rigorously designed randomized trial proved that it is indeed a very effective technique to facilitate cannulation.”
Hadley noted the technique does not increase PEP incidence, unlike the commonly used precut sphincterotomy and the double-wire method for difficult cannulations. “As a result, the forceps-assisted technique may be an effective first-line option and may reduce the need for additional, more invasive procedures including surgery and repeat ERCP to obtain the therapeutic intent of the original ERCP.”
The paper outlines the technique’s methodology, he added, “so we believe endoscopists who read the manuscript will be able to start implementing the technique into their practice.”
Commenting on the paper but not involved in it, Christopher J. DiMaio, MD, regional director of Endoscopy for Northwell Health Physician Partners Gastroenterology and a gastroenterologist in Greenlawn, New York, called it potentially helpful but aimed at a niche group of expert practitioners. “The technique appears safe and very effective, which is the number one concern, and I would definitely keep it in my back pocket,” he said. “I expect it will be used more commonly now because of this study.”
He added that although expert endoscopists are familiar with the approach, they use more time-tested and sometimes more aggressive maneuvers to cope with difficult cannulations. “But this is a simple technique using a device that should be available to most high-volume endoscopists.”
DiMaio also noted that he would have liked to see an actual decrease in PEP incidence in the intervention group.
Looking ahead, Hadley said it would be interesting to compare the effectiveness of the double-wire technique against forceps-assisted cannulation in a randomized context. “A study we’re already looking into is seeing whether physician experience with the technique impacts outcomes.”
This study was supported by the American College of Gastroenterology. The authors and DiMaio reported having no relevant competing interests.
A version of this article first appeared on Medscape.com.
The results emerged from the small, single-center SOCCER trial of 152 patients recruited from March 2022 to October 2024 and are published in The American Journal of Gastroenterology.
Both groups had a slightly higher number of female participants, and the mean ages of the participants were 61.9 years in the forceps group and 68.3 years in the no forceps group.
First author Steven M. Hadley Jr, an MD candidate at Northwestern Feinberg School of Medicine in Chicago, and colleagues reported that forceps assistance in difficult cannulations yielded significantly higher success rates than no forceps assistance (100% vs 83.9%; P < .001).
The investigators noted that difficult cannulations during ERCP have a frequency of 42%. Cannulation failure is associated with increased morbidity — including longer hospitalization, increased ICU admissions, readmissions, and increased financial cost — as well as mortality rates of up to 10%.
SOCCER defined difficult cannulation as a papilla in or on the rim of a diverticulum, five or more attempts, attempts lasting 5 or more minutes, or two or more unintended pancreatic duct wire passages. Other features were redundant tissue overlaying the papilla or a type 2, 3, or 4 papilla.
The study found forceps assistance also had a nonstatistically significant lower rate of difficult cannulations than no forceps (57.1% vs 69.1%; P = .132). The rate of post-ERCP pancreatitis (PEP) was similarly low in both groups: 5.7% with forceps vs 3.7% without forceps (P = .705). The no forceps group had significantly more cannulation attempts after randomization than the forceps group (14 vs 8.3; P = .026).
Patients who crossed over to forceps assistance all had successful cannulations.
The technique has long been used to overcome cannulation difficulties, said Timothy B. Gardner, MD, MS, a gastroenterologist at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire, and a coauthor of the study. “It was particularly effective for cannulations with redundant tissue limiting access to the papilla,” Gardner told GI & Hepatology News. “We decided to design a randomized trial to determine the extent to which this technique worked. We believed our study would answer an important question that would hopefully lead to an improvement in endoscopy practice.”
While a few case reports and video demos had described the technique, no trials had assessed its effectiveness, Hadley added. “We found the technique to be effective based on our experience, but it was exciting to see that a rigorously designed randomized trial proved that it is indeed a very effective technique to facilitate cannulation.”
Hadley noted the technique does not increase PEP incidence, unlike the commonly used precut sphincterotomy and the double-wire method for difficult cannulations. “As a result, the forceps-assisted technique may be an effective first-line option and may reduce the need for additional, more invasive procedures including surgery and repeat ERCP to obtain the therapeutic intent of the original ERCP.”
The paper outlines the technique’s methodology, he added, “so we believe endoscopists who read the manuscript will be able to start implementing the technique into their practice.”
Commenting on the paper but not involved in it, Christopher J. DiMaio, MD, regional director of Endoscopy for Northwell Health Physician Partners Gastroenterology and a gastroenterologist in Greenlawn, New York, called it potentially helpful but aimed at a niche group of expert practitioners. “The technique appears safe and very effective, which is the number one concern, and I would definitely keep it in my back pocket,” he said. “I expect it will be used more commonly now because of this study.”
He added that although expert endoscopists are familiar with the approach, they use more time-tested and sometimes more aggressive maneuvers to cope with difficult cannulations. “But this is a simple technique using a device that should be available to most high-volume endoscopists.”
DiMaio also noted that he would have liked to see an actual decrease in PEP incidence in the intervention group.
Looking ahead, Hadley said it would be interesting to compare the effectiveness of the double-wire technique against forceps-assisted cannulation in a randomized context. “A study we’re already looking into is seeing whether physician experience with the technique impacts outcomes.”
This study was supported by the American College of Gastroenterology. The authors and DiMaio reported having no relevant competing interests.
A version of this article first appeared on Medscape.com.
Analysis of the Frequency of level 1 OncoKB Genomic Alterations in Veterans With Various Solid Organ Malignancies
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Successful Targeted Therapy with Alectinib in ALK-Positive Metastatic Pancreatic Cancer
Background
Pancreatic cancer has one of the highest mortality rates due to its typical late-stage diagnosis and subsequent limited surgical options. However, recent advances in molecular profiling offer hope for targeted therapies. We present a case of locally advanced pancreatic adenocarcinoma which progressed despite surgery and chemotherapy yet showed a positive respond to Alectinib.
Case Description
A 79-year-old male with medical history of tobacco use and ulcerative colitis presented to the clinic with 15lb unintentional weight loss over the past few months in 04/2021. Computed tomography (CT) showed dilated common bile duct due to 2.2 x 1.9 x 1.7 cm mass with no metastatic disease. Biopsy was consistent with pancreatic adenocarcinoma and patient completed 6 cycles of dose-reduced neoadjuvant gemcitabine and paclitaxel in late 2021 due to his severe neuropathy and ECOG. Subsequent CT and PET-CT showed stable disease prior to undergoing pylorus-sparing pancreatoduodenectomy and cholecystectomy with portal vein resection in 05/2022 with surgical pathology grading yPT4N2cM0. The follow- up PET scan in 09/2022 revealed new pulmonary and liver metastases, along with increased uptake in the pancreatic region, suggesting recurrent disease. Next generation sequencing (NGS) identified an ELM4-ALK chromosomal rearrangement on the surgical pathology. Given the patient’s cancer progression and concerns about chemotherapy tolerance, Alectinib, a second-generation ALK inhibitor more commonly used in lung cancer, was considered as a treatment option. Patient began Alectinib 10/2022 with no significant side effects and PET scan on 03/2023 and 06/2023 showing resolution of his lung nodules and liver lesions. Patient remained on Alectinib until he transitioned to hospice after an ischemic stroke in 03/2024.
Discussion
Pancreatic cancer urgently requires novel therapies as about 25% of patients harbor actionable molecular alterations that have led to the success of targeted therapies. ALK fusion genes are identified in multiple cancers, but the prevalence is only 0.16% in pancreatic ductal adenocarcinoma. Alectinib provided an extended progression free survival compared with standard chemotherapy in our patient. ALK inhibitors may demonstrate a remarkable response in metastatic pancreatic cancer even in poor candidates for standard chemotherapy highlighting the emphasis of NGS and targeted therapy options for pancreatic cancer to improve survival.
Background
Pancreatic cancer has one of the highest mortality rates due to its typical late-stage diagnosis and subsequent limited surgical options. However, recent advances in molecular profiling offer hope for targeted therapies. We present a case of locally advanced pancreatic adenocarcinoma which progressed despite surgery and chemotherapy yet showed a positive respond to Alectinib.
Case Description
A 79-year-old male with medical history of tobacco use and ulcerative colitis presented to the clinic with 15lb unintentional weight loss over the past few months in 04/2021. Computed tomography (CT) showed dilated common bile duct due to 2.2 x 1.9 x 1.7 cm mass with no metastatic disease. Biopsy was consistent with pancreatic adenocarcinoma and patient completed 6 cycles of dose-reduced neoadjuvant gemcitabine and paclitaxel in late 2021 due to his severe neuropathy and ECOG. Subsequent CT and PET-CT showed stable disease prior to undergoing pylorus-sparing pancreatoduodenectomy and cholecystectomy with portal vein resection in 05/2022 with surgical pathology grading yPT4N2cM0. The follow- up PET scan in 09/2022 revealed new pulmonary and liver metastases, along with increased uptake in the pancreatic region, suggesting recurrent disease. Next generation sequencing (NGS) identified an ELM4-ALK chromosomal rearrangement on the surgical pathology. Given the patient’s cancer progression and concerns about chemotherapy tolerance, Alectinib, a second-generation ALK inhibitor more commonly used in lung cancer, was considered as a treatment option. Patient began Alectinib 10/2022 with no significant side effects and PET scan on 03/2023 and 06/2023 showing resolution of his lung nodules and liver lesions. Patient remained on Alectinib until he transitioned to hospice after an ischemic stroke in 03/2024.
Discussion
Pancreatic cancer urgently requires novel therapies as about 25% of patients harbor actionable molecular alterations that have led to the success of targeted therapies. ALK fusion genes are identified in multiple cancers, but the prevalence is only 0.16% in pancreatic ductal adenocarcinoma. Alectinib provided an extended progression free survival compared with standard chemotherapy in our patient. ALK inhibitors may demonstrate a remarkable response in metastatic pancreatic cancer even in poor candidates for standard chemotherapy highlighting the emphasis of NGS and targeted therapy options for pancreatic cancer to improve survival.
Background
Pancreatic cancer has one of the highest mortality rates due to its typical late-stage diagnosis and subsequent limited surgical options. However, recent advances in molecular profiling offer hope for targeted therapies. We present a case of locally advanced pancreatic adenocarcinoma which progressed despite surgery and chemotherapy yet showed a positive respond to Alectinib.
Case Description
A 79-year-old male with medical history of tobacco use and ulcerative colitis presented to the clinic with 15lb unintentional weight loss over the past few months in 04/2021. Computed tomography (CT) showed dilated common bile duct due to 2.2 x 1.9 x 1.7 cm mass with no metastatic disease. Biopsy was consistent with pancreatic adenocarcinoma and patient completed 6 cycles of dose-reduced neoadjuvant gemcitabine and paclitaxel in late 2021 due to his severe neuropathy and ECOG. Subsequent CT and PET-CT showed stable disease prior to undergoing pylorus-sparing pancreatoduodenectomy and cholecystectomy with portal vein resection in 05/2022 with surgical pathology grading yPT4N2cM0. The follow- up PET scan in 09/2022 revealed new pulmonary and liver metastases, along with increased uptake in the pancreatic region, suggesting recurrent disease. Next generation sequencing (NGS) identified an ELM4-ALK chromosomal rearrangement on the surgical pathology. Given the patient’s cancer progression and concerns about chemotherapy tolerance, Alectinib, a second-generation ALK inhibitor more commonly used in lung cancer, was considered as a treatment option. Patient began Alectinib 10/2022 with no significant side effects and PET scan on 03/2023 and 06/2023 showing resolution of his lung nodules and liver lesions. Patient remained on Alectinib until he transitioned to hospice after an ischemic stroke in 03/2024.
Discussion
Pancreatic cancer urgently requires novel therapies as about 25% of patients harbor actionable molecular alterations that have led to the success of targeted therapies. ALK fusion genes are identified in multiple cancers, but the prevalence is only 0.16% in pancreatic ductal adenocarcinoma. Alectinib provided an extended progression free survival compared with standard chemotherapy in our patient. ALK inhibitors may demonstrate a remarkable response in metastatic pancreatic cancer even in poor candidates for standard chemotherapy highlighting the emphasis of NGS and targeted therapy options for pancreatic cancer to improve survival.
Journal Highlights: May-July 2025
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Most GI Service Chiefs Support POCUS Training, But Uptake Is Slow
, according to a national survey.
Low POCUS uptake may be explained by substantial barriers to implementation, including lack of trained instructors, necessary equipment, and support staff, lead author Keerthi Thallapureddy, MD, of the University of Texas Health San Antonio, and colleagues, reported.
“POCUS is being increasingly used by gastroenterologists due to its portability and real-time diagnostic ability,” the investigators wrote in Gastro Hep Advances, but “there is limited understanding of how gastroenterologists use POCUS.”
To learn more, the investigators conducted a nationwide survey of the VA healthcare system. Separate questionnaires were sent to chiefs of staff (n = 130) and GI service chiefs (n = 117), yielding response rates of 100% and 79%, respectively.
Respondents represented a wide distribution of geographic regions and institutional complexity levels, with 80% of GI groups based at high-complexity centers and 92% in urban locations. A minority (8%) reported the presence of a liver transplant program.
Data collection focused on the prevalence of POCUS use, types of clinical applications, institutional policies and training processes, and perceived or actual barriers to wider adoption. Barriers were sorted into three categories: training, equipment, and infrastructure.
Of the 93 GI service chiefs who participated in the survey, 44% reported that at least 1 gastroenterologist at their facility currently uses POCUS. Most common procedural uses were paracentesis (23%) and liver biopsy (13%), while ascites assessment (19%) and biliary visualization (7%) were the most common diagnostic uses.
Among the same respondents, 69% said they would support sending clinicians to a POCUS training course, and 37% said their teams had expressed an active interest in pursuing such training. Only 17% of facilities had a formal process in place to obtain POCUS training, and an equal proportion had implemented a facility-wide policy to guide its use.
Barriers to implementation were widespread and often multifactorial.
Most challenges related to training: 48% of sites reported a lack of trained providers, 28% cited insufficient funding for training, 24% noted a lack of training opportunities, and 14% reported difficulty securing travel funds.
Equipment limitations were also common, with 41% of sites lacking ultrasound machines and 27% lacking funding to purchase them.
Institutional infrastructure posed further hurdles. Nearly a quarter of GI chiefs (23%) reported lacking a clinician champion to lead implementation, while others cited a lack of support staff, simulation space, privileging criteria, image archiving capabilities, or standardized reporting forms.
“Our findings on current POCUS use, training, barriers, and infrastructure can guide expansion of POCUS use and training among GI groups,” Dr. Thallapureddy and colleagues wrote, noting that early efforts to expand access to GI-specific POCUS training are already underway.
They cited growing interest from national organizations such as the American Gastroenterological Association and the American Association for the Study of Liver Diseases, the latter of which piloted training workshops at the 2024 Liver Meeting. Similarly, the International Bowel Ultrasound Group now offers a 3-part certification program in intestinal ultrasound and is developing additional online and interactive modules to improve training accessibility.
The study was supported by the US Department of Veterans Affairs, Quality Enhancement Research Initiative Partnered Evaluation Initiative Grant, and the VA National Center for Patient Safety. The investigators reported no conflicts of interest.
, according to a national survey.
Low POCUS uptake may be explained by substantial barriers to implementation, including lack of trained instructors, necessary equipment, and support staff, lead author Keerthi Thallapureddy, MD, of the University of Texas Health San Antonio, and colleagues, reported.
“POCUS is being increasingly used by gastroenterologists due to its portability and real-time diagnostic ability,” the investigators wrote in Gastro Hep Advances, but “there is limited understanding of how gastroenterologists use POCUS.”
To learn more, the investigators conducted a nationwide survey of the VA healthcare system. Separate questionnaires were sent to chiefs of staff (n = 130) and GI service chiefs (n = 117), yielding response rates of 100% and 79%, respectively.
Respondents represented a wide distribution of geographic regions and institutional complexity levels, with 80% of GI groups based at high-complexity centers and 92% in urban locations. A minority (8%) reported the presence of a liver transplant program.
Data collection focused on the prevalence of POCUS use, types of clinical applications, institutional policies and training processes, and perceived or actual barriers to wider adoption. Barriers were sorted into three categories: training, equipment, and infrastructure.
Of the 93 GI service chiefs who participated in the survey, 44% reported that at least 1 gastroenterologist at their facility currently uses POCUS. Most common procedural uses were paracentesis (23%) and liver biopsy (13%), while ascites assessment (19%) and biliary visualization (7%) were the most common diagnostic uses.
Among the same respondents, 69% said they would support sending clinicians to a POCUS training course, and 37% said their teams had expressed an active interest in pursuing such training. Only 17% of facilities had a formal process in place to obtain POCUS training, and an equal proportion had implemented a facility-wide policy to guide its use.
Barriers to implementation were widespread and often multifactorial.
Most challenges related to training: 48% of sites reported a lack of trained providers, 28% cited insufficient funding for training, 24% noted a lack of training opportunities, and 14% reported difficulty securing travel funds.
Equipment limitations were also common, with 41% of sites lacking ultrasound machines and 27% lacking funding to purchase them.
Institutional infrastructure posed further hurdles. Nearly a quarter of GI chiefs (23%) reported lacking a clinician champion to lead implementation, while others cited a lack of support staff, simulation space, privileging criteria, image archiving capabilities, or standardized reporting forms.
“Our findings on current POCUS use, training, barriers, and infrastructure can guide expansion of POCUS use and training among GI groups,” Dr. Thallapureddy and colleagues wrote, noting that early efforts to expand access to GI-specific POCUS training are already underway.
They cited growing interest from national organizations such as the American Gastroenterological Association and the American Association for the Study of Liver Diseases, the latter of which piloted training workshops at the 2024 Liver Meeting. Similarly, the International Bowel Ultrasound Group now offers a 3-part certification program in intestinal ultrasound and is developing additional online and interactive modules to improve training accessibility.
The study was supported by the US Department of Veterans Affairs, Quality Enhancement Research Initiative Partnered Evaluation Initiative Grant, and the VA National Center for Patient Safety. The investigators reported no conflicts of interest.
, according to a national survey.
Low POCUS uptake may be explained by substantial barriers to implementation, including lack of trained instructors, necessary equipment, and support staff, lead author Keerthi Thallapureddy, MD, of the University of Texas Health San Antonio, and colleagues, reported.
“POCUS is being increasingly used by gastroenterologists due to its portability and real-time diagnostic ability,” the investigators wrote in Gastro Hep Advances, but “there is limited understanding of how gastroenterologists use POCUS.”
To learn more, the investigators conducted a nationwide survey of the VA healthcare system. Separate questionnaires were sent to chiefs of staff (n = 130) and GI service chiefs (n = 117), yielding response rates of 100% and 79%, respectively.
Respondents represented a wide distribution of geographic regions and institutional complexity levels, with 80% of GI groups based at high-complexity centers and 92% in urban locations. A minority (8%) reported the presence of a liver transplant program.
Data collection focused on the prevalence of POCUS use, types of clinical applications, institutional policies and training processes, and perceived or actual barriers to wider adoption. Barriers were sorted into three categories: training, equipment, and infrastructure.
Of the 93 GI service chiefs who participated in the survey, 44% reported that at least 1 gastroenterologist at their facility currently uses POCUS. Most common procedural uses were paracentesis (23%) and liver biopsy (13%), while ascites assessment (19%) and biliary visualization (7%) were the most common diagnostic uses.
Among the same respondents, 69% said they would support sending clinicians to a POCUS training course, and 37% said their teams had expressed an active interest in pursuing such training. Only 17% of facilities had a formal process in place to obtain POCUS training, and an equal proportion had implemented a facility-wide policy to guide its use.
Barriers to implementation were widespread and often multifactorial.
Most challenges related to training: 48% of sites reported a lack of trained providers, 28% cited insufficient funding for training, 24% noted a lack of training opportunities, and 14% reported difficulty securing travel funds.
Equipment limitations were also common, with 41% of sites lacking ultrasound machines and 27% lacking funding to purchase them.
Institutional infrastructure posed further hurdles. Nearly a quarter of GI chiefs (23%) reported lacking a clinician champion to lead implementation, while others cited a lack of support staff, simulation space, privileging criteria, image archiving capabilities, or standardized reporting forms.
“Our findings on current POCUS use, training, barriers, and infrastructure can guide expansion of POCUS use and training among GI groups,” Dr. Thallapureddy and colleagues wrote, noting that early efforts to expand access to GI-specific POCUS training are already underway.
They cited growing interest from national organizations such as the American Gastroenterological Association and the American Association for the Study of Liver Diseases, the latter of which piloted training workshops at the 2024 Liver Meeting. Similarly, the International Bowel Ultrasound Group now offers a 3-part certification program in intestinal ultrasound and is developing additional online and interactive modules to improve training accessibility.
The study was supported by the US Department of Veterans Affairs, Quality Enhancement Research Initiative Partnered Evaluation Initiative Grant, and the VA National Center for Patient Safety. The investigators reported no conflicts of interest.
FROM GASTRO HEP ADVANCES
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.
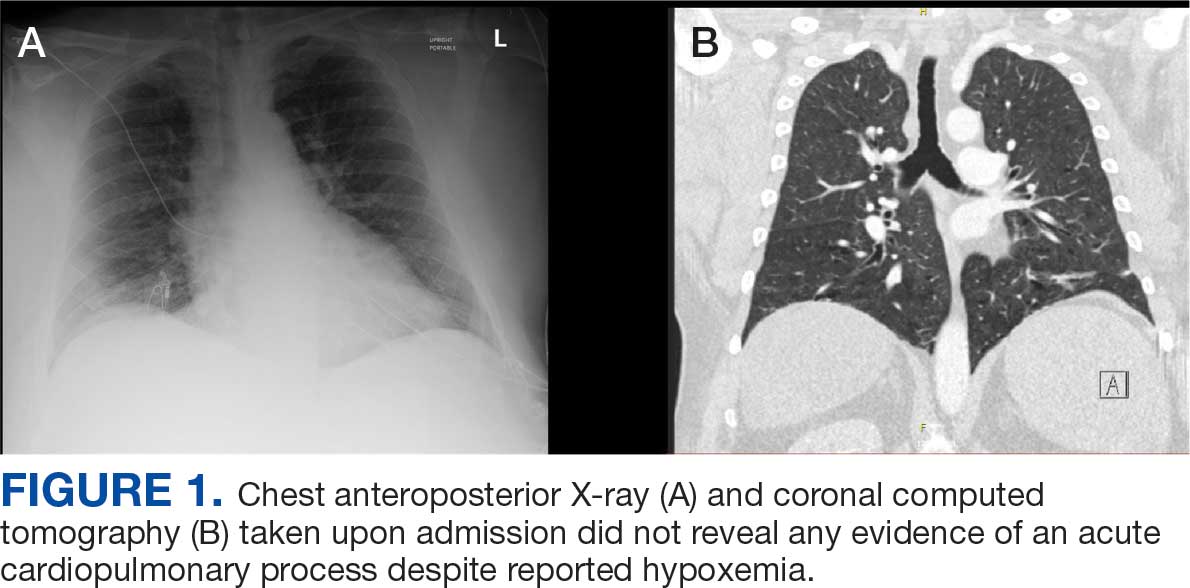
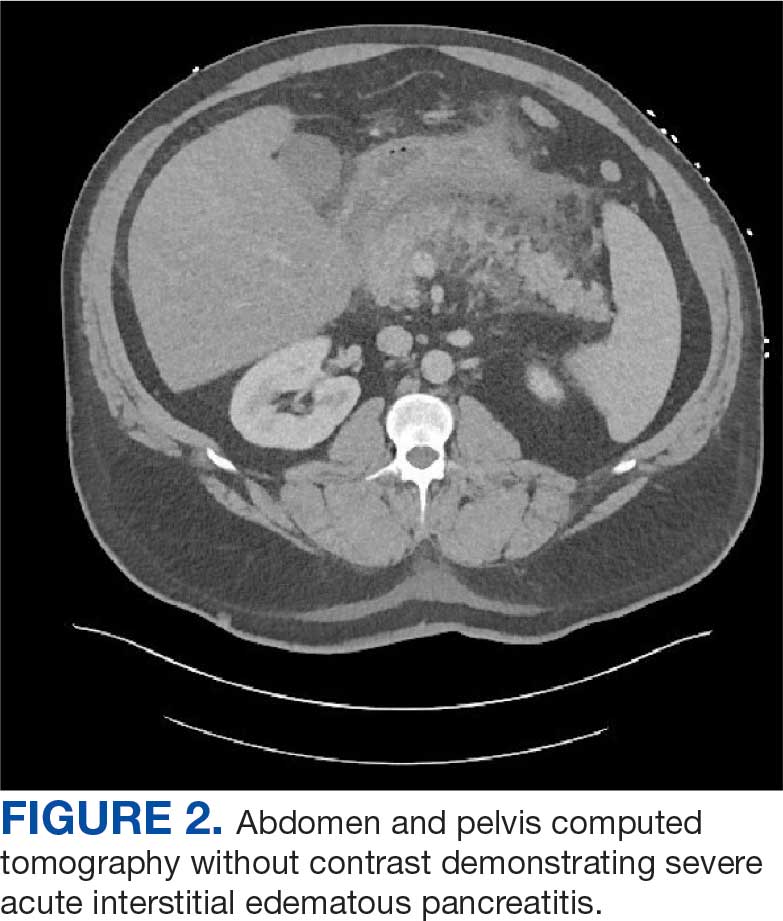
The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).
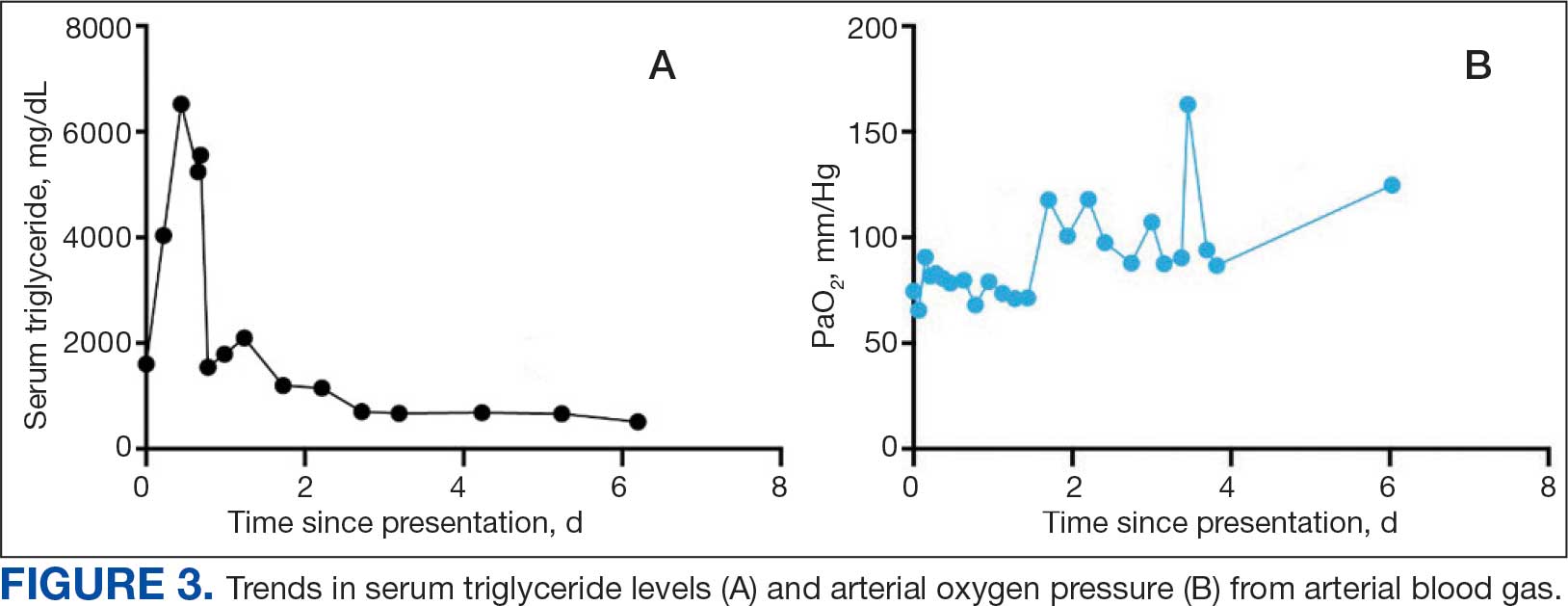
Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1
Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
An Approach to Exocrine Pancreatic Insufficiency: Considerations in Diagnosis and Treatment
Exocrine pancreatic insufficiency (EPI) is a recognized condition in patients with underlying pancreatic disease. However, it is a disease state that requires a meticulous approach to diagnose, as misdiagnosis can lead to inappropriate testing and unnecessary treatment.
EPI has been defined as “a near total decline in the quantity and/or activity of endogenous pancreatic enzymes to a level that is inadequate to maintain normal digestive capacity leading to steatorrhea.”1 It can lead to complications including malnutrition, micronutrient deficiencies, metabolic bone disease and have significant impact on quality of life. In this article,
EPI Diagnosis
EPI results from ineffective or insufficient pancreatic digestive enzyme secretion. In 2021, a group of experts from the American Gastroenterological Association (AGA) and PancreasFest met and proposed a new mechanistic definition of EPI. This suggests that EPI is the failure of sufficient pancreatic enzymes to effectively reach the intestine in order to allow for optimal digestion of ingested nutrients, leading to downstream macronutrient and micronutrient deficiencies with symptoms of maldigestion including post-prandial abdominal pain, bloating, steatorrhea, loose stools, or weight loss.2
A more pragmatic definition by Khan, et al in 2022 utilized a staging system to distinguish exocrine pancreatic dysfunction (EDP) from EPI. As such EPD occurs when there is a decline in pancreatic function without impaired digestive capacity, while EPI requires digestive capacity impairment leading to objective steatorrhea (coefficient of fat absorption <93 %).3Differential Diagnosis: There are many factors that can impact normal digestion. In approaching EPI, symptoms are often the most common reason to test for disease state in the appropriate clinical context. There can be pancreatic causes of EPI and non-pancreatic (secondary) causes of EPI (see Figure 1), though the latter can be challenging to detect.
The most common parenchymal etiologies for EPI include chronic pancreatitis, recurrent acute pancreatitis, cystic fibrosis, pancreatic cancer or prior pancreatic resections. Non-pancreatic conditions that impact synchronous mixing of endogenous pancreatic enzymes with meals (i.e., Roux-en-Y gastric bypass, short bowel syndrome, delayed gastric emptying), mucosal barriers causing decrease endogenous pancreatic stimulation despite intact parenchyma, such as celiac disease, foregut Crohn’s disease, intraluminal inactivation of pancreatic enzymes (Zollinger-Ellison syndrome), and bile salts de-conjugation with small intestinal bacterial overgrowth (SIBO) can predispose to EPI.4-6 The true prevalence of EPI is difficult to ascertain due to a variety of factors including challenges in diagnosis and misdiagnosis.
Some of the major challenges in the diagnosis and treatment of EPI is that the symptoms of EPI overlap with many other GI conditions including celiac disease, diabetes mellitus, SIBO, irritable bowel syndrome (IBS), bile acid diarrhea, and other functional GI syndromes. These non-pancreatic conditions can also be associated with falsely low FE-1. Hence, ordering FE-1 should be employed with caution when the pretest probability is low. Patients with EPI will generally have a significant response to pancreatic enzyme replacement therapy (PERT) if it is adequately dosed and a lack of response should prompt consideration of an alternative diagnosis. A framework to factors which contribute to EPI is outlined in Figure 2.
Symptoms Screening and Signs: Pancreatic enzymes output estimation is the most reliable indicator for pancreatic digestive capacity. However, EPI diagnosis requires a combination of symptoms screening, stool-based (indirect pancreatic function) testing or direct pancreatic function testing (PFT).
Although symptoms might not correlate with objective disease state, in screening for symptoms of steatorrhea or maldigestion, it is important to ask specific questions regarding bloating, abdominal pain, stool frequency, consistency, and quality. Screening questions should be specific and include question such as, “Is there oil in the toilet bowl or is the stool greasy/shiny?”, “Is the stool sticky and difficult to flush or wipe?”, “Is there malodorous flatus?” If patients screen positive for EPI symptoms and there is a high pre-test probability of EPI such as the presence of severe chronic pancreatitis or significant pancreatic resection (> 90% loss of pancreatic parenchyma), then cautious trial of PERT and assessment for treatment response can be considered without additional stool-based testing. However, this practice end points are unclear and mainly based on subjective response.
Patients with EPI are at increased risk for malnutrition and micronutrient deficiencies. While not required for the diagnosis, low levels of fat-soluble vitamins (vitamin AEDK) or other minerals (zinc, selenium, magnesium, phosphorus) can suggest issues with malabsorption. Once the diagnosis of EPI is made, micronutrient screening should occur annually.
Stool Based Testing: The gold standard clinical test for steatorrhea is measuring coefficient of fat absorption (CFA). With a normal range of 93% fat absorption, the test is performed on a 72-hours fecal fat collection kit. To ensure accurate results, a patient must adhere to a diet with a minimum of 100 grams of fat per day in the three days leading up to the test and during the duration of the test. Patients must also abstain from taking PERT during the duration of the test. This can be incredibly challenging for someone with underlying steatorrhea but can reliably distinguish between EPD and EPI.
A more commonly used stool test is fecal elastase (FE-1). While easier to perform, the test often results in many false positives and false negatives. FE-1 is an ELISA based test, which measures the concentration of the specific isoform CELA3 (chymotrypsin-like elastase family) in the stool sample. The test must be run on a solid stool sample as soft or liquid stool will dilute down elastase concentration falsely. One test advantage is that a patient can continue PERT if needed. FE-1 test measures the concentration of patients’ elastase and PERT is porcine derived. As such, there is no interaction between porcine lipase and human elastase in stool. FE-1 sensitivity and specificity are high for severe disease (<100 mcg/g) if the test is performed properly on patients with a high pretest probability. However, the sensitivity and specificity are poor in mild to moderate pancreatic disease and in the absence of known pancreatic disease.7
Our suggested approach to utilizing FE-1 test is to reserve it for patients with known severe chronic pancreatitis or prior pancreatic surgery in patients with symptoms. In patients without pancreatic disease who are at low risk of EPI, a positive FE-1 can lead to misdiagnosis, further diagnostic testing, and unnecessary treatment. Currently, there is no stool-based test that is accurate, reproducible, and reliable.
Direct Pancreatic Function testing: Secretin stimulated PFT is highly reliable in measuring ductal function with bicarbonate concentration. However, it cannot reliably estimate acinar function as both do not decline at the same rate, unless in severe pancreatic disease. A much more robust test should include cholecystokinin analog to measure pancreatic enzymes concentration. This test involves endoscopy, administration of secretin, and/or a cholecystokinin analog, and subsequent measurement of bicarbonate and digestive enzymes in the pancreatic juice. This test is not routinely offered as it is invasive, cumbersome, and difficult to repeat for reassessment of pancreatic function over time.8
Treatment
The primary goal of treatment is to improve symptoms and nutritional status of the patient. EPI treatment consists of PERT and nutritional counseling. In the United States, there are multiple FDA approved PERT preparations, which include Creon, Zenpep, Pancreaze, Pertzye, Viokase, and Relizorb. While dosing is dependent on lipase concentration, all PERT (aside from Relizorb) preparations have a combination of lipase, proteases, and amylase. All but Viokace and Relizorb are enteric-coated formulations.9
In patients with an inadequate response to enteric coated PERT, non-enteric coated PERT can be added as it may provide a more immediate effect than enteric coated formulations, specially if concern about rapid gut transit with inadequate mixing is raised. If a non-enteric formations is used, acid suppression should be added to prevent inactivation of the PERT. Relizorb is a cartridge system which delivers lipase directly to tube feeds. This cartridge is only utilized in patients receiving enteral nutrition and allows for treatment of EPI even when patients are unable to tolerate oral feeding.
PERT dosing is intended to at least compensate for 10% of the physiologically secreted amount of endogenous lipase after a normal meal (approximately 30,000 IU). Hence, dosing is primarily weight-based. In symptomatic adults, PERT dose of 500-1000 units/kg/meal and half of the amount with snacks is appropriate. Although higher doses of 1500-2000 units/kg/meal may be needed when there is significant steatorrhea, weight loss, or micronutrient deficiencies, PERT doses exceeding 2500 units/kg/meal are not recommended and warrant further investigation.10
Proper counseling is important to ensure compliance with pancreatin preparations. PERT will generally be effective in improving steatorrhea, weight loss, bowel movement frequency, and reversal of nutritional deficiencies, but it does not reliably help symptoms of bloating or abdominal pain. If a patient’s steatorrhea does not respond to PERT, then alternative diagnoses such as SIBO, or diarrhea-predominant IBS should be considered.
PERT must be taken with meals. There are studies that support split dosing as a more effective way of absorbing fat.11 If PERT is ineffective or minimally effective, review of appropriate dosing and timing of PERT to a meal is recommended. Addition of acid suppression may be required to improve treatment efficacy, especially in patients with abnormal intestinal motility or prior pancreatic surgery as PERT is effective at a pH of 4.5. Cost, pill burden, and persistence of certain symptoms may impact adherence to PERT and thus pre-treatment counseling and close follow-up after initiation is important. This aids in assessment of patients’ response to therapy, ensure appropriate PERT administration, and identifying any barriers to therapy adherence.
Nutritional management of EPI consists of an assessment of nutritional status, diet, and lifestyle. An important component of nutritional management is the assessment of micronutrient deficiencies. Patients with a confirmed diagnosis of EPI should be screened for the following micronutrients annually: Vitamins (A, E, D, K, B12), folate, zinc, selenium, magnesium, and iron. Patients with chronic pancreatitis and EPI should also be screened for metabolic bone disease once every two years and for diabetes mellitus annually.4, 12
Conclusion
EPI is a challenging diagnosis as many symptoms overlap with other GI conditions. Pancreas exocrine function is rich with significant reserve to allow for proper digestive capacity, yet EPI occurs when an individual’s pancreatic digestive enzymes are insufficient to meet their nutritional needs. In patients with high likelihood of having EPI, such as those with pre-existing pancreatic disease, diagnosing EPI combines clinical evidence based on subjective symptoms and stool-based testing to support a disease state.
Appropriate dosing and timing of PERT is critical to improve nutritional outcomes and improve certain symptoms of EPI. Failure of PERT requires evaluating for proper dosing/timing, and consideration of additional or alternative diagnosis. EPI morbidity can lead to significant impact on patients’ quality of life, but with counseling, proper PERT use, nutritional consequences can be mediated, and quality of life can improve.
Dr. Hernandez-Barco is based in the Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts. Dr. Ashkar is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Hernandez-Barco disclosed consulting for AMGEN and served as a scientific advisor for Nestle Health Science. She had project-related funding support or conflicts of interest to disclose. Dr. Ashkar disclosed consulting for AMGEN. He had no project-related funding support or conflicts of interest to disclose.
References
1. Othman MO, et al. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018 Feb. doi: 10.1111/ijcp.13066.
2. Whitcomb DC, et al. AGA-PancreasFest Joint Symposium on Exocrine Pancreatitic Insufficiency. Gastro Hep Adv. 2022 Nov. doi: 10.1016/j.gastha.2022.11.008.
3. Khan A, et al. Staging Exocrine Pancreatic Dysfunction. Pancreatology. 2022 Jan. doi: 10.1016/j.pan.2021.11.005.
4. Whitcomb DC, et al. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatitis insufficiency: Expert Review. Gastroenterology. 2023 Nov. doi: 10.1053/j.gastro.2023.07.007.
5. Kunovský L, et al. Causes of Exocrine Pancreatic Insufficiency Other than Chronic Pancreatitis. J Clin Med. 2021 Dec. doi: 10.3390/jcm10245779.
6. Singh VK, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017 Oct. doi: 10.3748/wjg.v23.i39.7059.
7. Lankisch PG, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mid to moderate exocrine pancreatic insufficiency. Gut. 1998 Apr. doi: 10.1136/gut.42.4.551.
8. Gardner TB, et al. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020 Mar. doi: 10.14309/ajg.0000000000000535.
9. Lewis DM, et al. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08184-w.
10. Borowitz DS, et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995 Nov. doi: 10.1016/s0022-3476(95)70153-2.
11. Domínguez-Muñoz JE, et al. Effect of the Administration Schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005 Apr. doi: 10.1111/j.1365-2036.2005.02390.x.
12. Hart PA and Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol. 2020 Jan. doi: 10.14309/ajg.0000000000000421.
Exocrine pancreatic insufficiency (EPI) is a recognized condition in patients with underlying pancreatic disease. However, it is a disease state that requires a meticulous approach to diagnose, as misdiagnosis can lead to inappropriate testing and unnecessary treatment.
EPI has been defined as “a near total decline in the quantity and/or activity of endogenous pancreatic enzymes to a level that is inadequate to maintain normal digestive capacity leading to steatorrhea.”1 It can lead to complications including malnutrition, micronutrient deficiencies, metabolic bone disease and have significant impact on quality of life. In this article,
EPI Diagnosis
EPI results from ineffective or insufficient pancreatic digestive enzyme secretion. In 2021, a group of experts from the American Gastroenterological Association (AGA) and PancreasFest met and proposed a new mechanistic definition of EPI. This suggests that EPI is the failure of sufficient pancreatic enzymes to effectively reach the intestine in order to allow for optimal digestion of ingested nutrients, leading to downstream macronutrient and micronutrient deficiencies with symptoms of maldigestion including post-prandial abdominal pain, bloating, steatorrhea, loose stools, or weight loss.2
A more pragmatic definition by Khan, et al in 2022 utilized a staging system to distinguish exocrine pancreatic dysfunction (EDP) from EPI. As such EPD occurs when there is a decline in pancreatic function without impaired digestive capacity, while EPI requires digestive capacity impairment leading to objective steatorrhea (coefficient of fat absorption <93 %).3Differential Diagnosis: There are many factors that can impact normal digestion. In approaching EPI, symptoms are often the most common reason to test for disease state in the appropriate clinical context. There can be pancreatic causes of EPI and non-pancreatic (secondary) causes of EPI (see Figure 1), though the latter can be challenging to detect.
The most common parenchymal etiologies for EPI include chronic pancreatitis, recurrent acute pancreatitis, cystic fibrosis, pancreatic cancer or prior pancreatic resections. Non-pancreatic conditions that impact synchronous mixing of endogenous pancreatic enzymes with meals (i.e., Roux-en-Y gastric bypass, short bowel syndrome, delayed gastric emptying), mucosal barriers causing decrease endogenous pancreatic stimulation despite intact parenchyma, such as celiac disease, foregut Crohn’s disease, intraluminal inactivation of pancreatic enzymes (Zollinger-Ellison syndrome), and bile salts de-conjugation with small intestinal bacterial overgrowth (SIBO) can predispose to EPI.4-6 The true prevalence of EPI is difficult to ascertain due to a variety of factors including challenges in diagnosis and misdiagnosis.
Some of the major challenges in the diagnosis and treatment of EPI is that the symptoms of EPI overlap with many other GI conditions including celiac disease, diabetes mellitus, SIBO, irritable bowel syndrome (IBS), bile acid diarrhea, and other functional GI syndromes. These non-pancreatic conditions can also be associated with falsely low FE-1. Hence, ordering FE-1 should be employed with caution when the pretest probability is low. Patients with EPI will generally have a significant response to pancreatic enzyme replacement therapy (PERT) if it is adequately dosed and a lack of response should prompt consideration of an alternative diagnosis. A framework to factors which contribute to EPI is outlined in Figure 2.
Symptoms Screening and Signs: Pancreatic enzymes output estimation is the most reliable indicator for pancreatic digestive capacity. However, EPI diagnosis requires a combination of symptoms screening, stool-based (indirect pancreatic function) testing or direct pancreatic function testing (PFT).
Although symptoms might not correlate with objective disease state, in screening for symptoms of steatorrhea or maldigestion, it is important to ask specific questions regarding bloating, abdominal pain, stool frequency, consistency, and quality. Screening questions should be specific and include question such as, “Is there oil in the toilet bowl or is the stool greasy/shiny?”, “Is the stool sticky and difficult to flush or wipe?”, “Is there malodorous flatus?” If patients screen positive for EPI symptoms and there is a high pre-test probability of EPI such as the presence of severe chronic pancreatitis or significant pancreatic resection (> 90% loss of pancreatic parenchyma), then cautious trial of PERT and assessment for treatment response can be considered without additional stool-based testing. However, this practice end points are unclear and mainly based on subjective response.
Patients with EPI are at increased risk for malnutrition and micronutrient deficiencies. While not required for the diagnosis, low levels of fat-soluble vitamins (vitamin AEDK) or other minerals (zinc, selenium, magnesium, phosphorus) can suggest issues with malabsorption. Once the diagnosis of EPI is made, micronutrient screening should occur annually.
Stool Based Testing: The gold standard clinical test for steatorrhea is measuring coefficient of fat absorption (CFA). With a normal range of 93% fat absorption, the test is performed on a 72-hours fecal fat collection kit. To ensure accurate results, a patient must adhere to a diet with a minimum of 100 grams of fat per day in the three days leading up to the test and during the duration of the test. Patients must also abstain from taking PERT during the duration of the test. This can be incredibly challenging for someone with underlying steatorrhea but can reliably distinguish between EPD and EPI.
A more commonly used stool test is fecal elastase (FE-1). While easier to perform, the test often results in many false positives and false negatives. FE-1 is an ELISA based test, which measures the concentration of the specific isoform CELA3 (chymotrypsin-like elastase family) in the stool sample. The test must be run on a solid stool sample as soft or liquid stool will dilute down elastase concentration falsely. One test advantage is that a patient can continue PERT if needed. FE-1 test measures the concentration of patients’ elastase and PERT is porcine derived. As such, there is no interaction between porcine lipase and human elastase in stool. FE-1 sensitivity and specificity are high for severe disease (<100 mcg/g) if the test is performed properly on patients with a high pretest probability. However, the sensitivity and specificity are poor in mild to moderate pancreatic disease and in the absence of known pancreatic disease.7
Our suggested approach to utilizing FE-1 test is to reserve it for patients with known severe chronic pancreatitis or prior pancreatic surgery in patients with symptoms. In patients without pancreatic disease who are at low risk of EPI, a positive FE-1 can lead to misdiagnosis, further diagnostic testing, and unnecessary treatment. Currently, there is no stool-based test that is accurate, reproducible, and reliable.
Direct Pancreatic Function testing: Secretin stimulated PFT is highly reliable in measuring ductal function with bicarbonate concentration. However, it cannot reliably estimate acinar function as both do not decline at the same rate, unless in severe pancreatic disease. A much more robust test should include cholecystokinin analog to measure pancreatic enzymes concentration. This test involves endoscopy, administration of secretin, and/or a cholecystokinin analog, and subsequent measurement of bicarbonate and digestive enzymes in the pancreatic juice. This test is not routinely offered as it is invasive, cumbersome, and difficult to repeat for reassessment of pancreatic function over time.8
Treatment
The primary goal of treatment is to improve symptoms and nutritional status of the patient. EPI treatment consists of PERT and nutritional counseling. In the United States, there are multiple FDA approved PERT preparations, which include Creon, Zenpep, Pancreaze, Pertzye, Viokase, and Relizorb. While dosing is dependent on lipase concentration, all PERT (aside from Relizorb) preparations have a combination of lipase, proteases, and amylase. All but Viokace and Relizorb are enteric-coated formulations.9
In patients with an inadequate response to enteric coated PERT, non-enteric coated PERT can be added as it may provide a more immediate effect than enteric coated formulations, specially if concern about rapid gut transit with inadequate mixing is raised. If a non-enteric formations is used, acid suppression should be added to prevent inactivation of the PERT. Relizorb is a cartridge system which delivers lipase directly to tube feeds. This cartridge is only utilized in patients receiving enteral nutrition and allows for treatment of EPI even when patients are unable to tolerate oral feeding.
PERT dosing is intended to at least compensate for 10% of the physiologically secreted amount of endogenous lipase after a normal meal (approximately 30,000 IU). Hence, dosing is primarily weight-based. In symptomatic adults, PERT dose of 500-1000 units/kg/meal and half of the amount with snacks is appropriate. Although higher doses of 1500-2000 units/kg/meal may be needed when there is significant steatorrhea, weight loss, or micronutrient deficiencies, PERT doses exceeding 2500 units/kg/meal are not recommended and warrant further investigation.10
Proper counseling is important to ensure compliance with pancreatin preparations. PERT will generally be effective in improving steatorrhea, weight loss, bowel movement frequency, and reversal of nutritional deficiencies, but it does not reliably help symptoms of bloating or abdominal pain. If a patient’s steatorrhea does not respond to PERT, then alternative diagnoses such as SIBO, or diarrhea-predominant IBS should be considered.
PERT must be taken with meals. There are studies that support split dosing as a more effective way of absorbing fat.11 If PERT is ineffective or minimally effective, review of appropriate dosing and timing of PERT to a meal is recommended. Addition of acid suppression may be required to improve treatment efficacy, especially in patients with abnormal intestinal motility or prior pancreatic surgery as PERT is effective at a pH of 4.5. Cost, pill burden, and persistence of certain symptoms may impact adherence to PERT and thus pre-treatment counseling and close follow-up after initiation is important. This aids in assessment of patients’ response to therapy, ensure appropriate PERT administration, and identifying any barriers to therapy adherence.
Nutritional management of EPI consists of an assessment of nutritional status, diet, and lifestyle. An important component of nutritional management is the assessment of micronutrient deficiencies. Patients with a confirmed diagnosis of EPI should be screened for the following micronutrients annually: Vitamins (A, E, D, K, B12), folate, zinc, selenium, magnesium, and iron. Patients with chronic pancreatitis and EPI should also be screened for metabolic bone disease once every two years and for diabetes mellitus annually.4, 12
Conclusion
EPI is a challenging diagnosis as many symptoms overlap with other GI conditions. Pancreas exocrine function is rich with significant reserve to allow for proper digestive capacity, yet EPI occurs when an individual’s pancreatic digestive enzymes are insufficient to meet their nutritional needs. In patients with high likelihood of having EPI, such as those with pre-existing pancreatic disease, diagnosing EPI combines clinical evidence based on subjective symptoms and stool-based testing to support a disease state.
Appropriate dosing and timing of PERT is critical to improve nutritional outcomes and improve certain symptoms of EPI. Failure of PERT requires evaluating for proper dosing/timing, and consideration of additional or alternative diagnosis. EPI morbidity can lead to significant impact on patients’ quality of life, but with counseling, proper PERT use, nutritional consequences can be mediated, and quality of life can improve.
Dr. Hernandez-Barco is based in the Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts. Dr. Ashkar is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Hernandez-Barco disclosed consulting for AMGEN and served as a scientific advisor for Nestle Health Science. She had project-related funding support or conflicts of interest to disclose. Dr. Ashkar disclosed consulting for AMGEN. He had no project-related funding support or conflicts of interest to disclose.
References
1. Othman MO, et al. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018 Feb. doi: 10.1111/ijcp.13066.
2. Whitcomb DC, et al. AGA-PancreasFest Joint Symposium on Exocrine Pancreatitic Insufficiency. Gastro Hep Adv. 2022 Nov. doi: 10.1016/j.gastha.2022.11.008.
3. Khan A, et al. Staging Exocrine Pancreatic Dysfunction. Pancreatology. 2022 Jan. doi: 10.1016/j.pan.2021.11.005.
4. Whitcomb DC, et al. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatitis insufficiency: Expert Review. Gastroenterology. 2023 Nov. doi: 10.1053/j.gastro.2023.07.007.
5. Kunovský L, et al. Causes of Exocrine Pancreatic Insufficiency Other than Chronic Pancreatitis. J Clin Med. 2021 Dec. doi: 10.3390/jcm10245779.
6. Singh VK, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017 Oct. doi: 10.3748/wjg.v23.i39.7059.
7. Lankisch PG, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mid to moderate exocrine pancreatic insufficiency. Gut. 1998 Apr. doi: 10.1136/gut.42.4.551.
8. Gardner TB, et al. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020 Mar. doi: 10.14309/ajg.0000000000000535.
9. Lewis DM, et al. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08184-w.
10. Borowitz DS, et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995 Nov. doi: 10.1016/s0022-3476(95)70153-2.
11. Domínguez-Muñoz JE, et al. Effect of the Administration Schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005 Apr. doi: 10.1111/j.1365-2036.2005.02390.x.
12. Hart PA and Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol. 2020 Jan. doi: 10.14309/ajg.0000000000000421.
Exocrine pancreatic insufficiency (EPI) is a recognized condition in patients with underlying pancreatic disease. However, it is a disease state that requires a meticulous approach to diagnose, as misdiagnosis can lead to inappropriate testing and unnecessary treatment.
EPI has been defined as “a near total decline in the quantity and/or activity of endogenous pancreatic enzymes to a level that is inadequate to maintain normal digestive capacity leading to steatorrhea.”1 It can lead to complications including malnutrition, micronutrient deficiencies, metabolic bone disease and have significant impact on quality of life. In this article,
EPI Diagnosis
EPI results from ineffective or insufficient pancreatic digestive enzyme secretion. In 2021, a group of experts from the American Gastroenterological Association (AGA) and PancreasFest met and proposed a new mechanistic definition of EPI. This suggests that EPI is the failure of sufficient pancreatic enzymes to effectively reach the intestine in order to allow for optimal digestion of ingested nutrients, leading to downstream macronutrient and micronutrient deficiencies with symptoms of maldigestion including post-prandial abdominal pain, bloating, steatorrhea, loose stools, or weight loss.2
A more pragmatic definition by Khan, et al in 2022 utilized a staging system to distinguish exocrine pancreatic dysfunction (EDP) from EPI. As such EPD occurs when there is a decline in pancreatic function without impaired digestive capacity, while EPI requires digestive capacity impairment leading to objective steatorrhea (coefficient of fat absorption <93 %).3Differential Diagnosis: There are many factors that can impact normal digestion. In approaching EPI, symptoms are often the most common reason to test for disease state in the appropriate clinical context. There can be pancreatic causes of EPI and non-pancreatic (secondary) causes of EPI (see Figure 1), though the latter can be challenging to detect.
The most common parenchymal etiologies for EPI include chronic pancreatitis, recurrent acute pancreatitis, cystic fibrosis, pancreatic cancer or prior pancreatic resections. Non-pancreatic conditions that impact synchronous mixing of endogenous pancreatic enzymes with meals (i.e., Roux-en-Y gastric bypass, short bowel syndrome, delayed gastric emptying), mucosal barriers causing decrease endogenous pancreatic stimulation despite intact parenchyma, such as celiac disease, foregut Crohn’s disease, intraluminal inactivation of pancreatic enzymes (Zollinger-Ellison syndrome), and bile salts de-conjugation with small intestinal bacterial overgrowth (SIBO) can predispose to EPI.4-6 The true prevalence of EPI is difficult to ascertain due to a variety of factors including challenges in diagnosis and misdiagnosis.
Some of the major challenges in the diagnosis and treatment of EPI is that the symptoms of EPI overlap with many other GI conditions including celiac disease, diabetes mellitus, SIBO, irritable bowel syndrome (IBS), bile acid diarrhea, and other functional GI syndromes. These non-pancreatic conditions can also be associated with falsely low FE-1. Hence, ordering FE-1 should be employed with caution when the pretest probability is low. Patients with EPI will generally have a significant response to pancreatic enzyme replacement therapy (PERT) if it is adequately dosed and a lack of response should prompt consideration of an alternative diagnosis. A framework to factors which contribute to EPI is outlined in Figure 2.
Symptoms Screening and Signs: Pancreatic enzymes output estimation is the most reliable indicator for pancreatic digestive capacity. However, EPI diagnosis requires a combination of symptoms screening, stool-based (indirect pancreatic function) testing or direct pancreatic function testing (PFT).
Although symptoms might not correlate with objective disease state, in screening for symptoms of steatorrhea or maldigestion, it is important to ask specific questions regarding bloating, abdominal pain, stool frequency, consistency, and quality. Screening questions should be specific and include question such as, “Is there oil in the toilet bowl or is the stool greasy/shiny?”, “Is the stool sticky and difficult to flush or wipe?”, “Is there malodorous flatus?” If patients screen positive for EPI symptoms and there is a high pre-test probability of EPI such as the presence of severe chronic pancreatitis or significant pancreatic resection (> 90% loss of pancreatic parenchyma), then cautious trial of PERT and assessment for treatment response can be considered without additional stool-based testing. However, this practice end points are unclear and mainly based on subjective response.
Patients with EPI are at increased risk for malnutrition and micronutrient deficiencies. While not required for the diagnosis, low levels of fat-soluble vitamins (vitamin AEDK) or other minerals (zinc, selenium, magnesium, phosphorus) can suggest issues with malabsorption. Once the diagnosis of EPI is made, micronutrient screening should occur annually.
Stool Based Testing: The gold standard clinical test for steatorrhea is measuring coefficient of fat absorption (CFA). With a normal range of 93% fat absorption, the test is performed on a 72-hours fecal fat collection kit. To ensure accurate results, a patient must adhere to a diet with a minimum of 100 grams of fat per day in the three days leading up to the test and during the duration of the test. Patients must also abstain from taking PERT during the duration of the test. This can be incredibly challenging for someone with underlying steatorrhea but can reliably distinguish between EPD and EPI.
A more commonly used stool test is fecal elastase (FE-1). While easier to perform, the test often results in many false positives and false negatives. FE-1 is an ELISA based test, which measures the concentration of the specific isoform CELA3 (chymotrypsin-like elastase family) in the stool sample. The test must be run on a solid stool sample as soft or liquid stool will dilute down elastase concentration falsely. One test advantage is that a patient can continue PERT if needed. FE-1 test measures the concentration of patients’ elastase and PERT is porcine derived. As such, there is no interaction between porcine lipase and human elastase in stool. FE-1 sensitivity and specificity are high for severe disease (<100 mcg/g) if the test is performed properly on patients with a high pretest probability. However, the sensitivity and specificity are poor in mild to moderate pancreatic disease and in the absence of known pancreatic disease.7
Our suggested approach to utilizing FE-1 test is to reserve it for patients with known severe chronic pancreatitis or prior pancreatic surgery in patients with symptoms. In patients without pancreatic disease who are at low risk of EPI, a positive FE-1 can lead to misdiagnosis, further diagnostic testing, and unnecessary treatment. Currently, there is no stool-based test that is accurate, reproducible, and reliable.
Direct Pancreatic Function testing: Secretin stimulated PFT is highly reliable in measuring ductal function with bicarbonate concentration. However, it cannot reliably estimate acinar function as both do not decline at the same rate, unless in severe pancreatic disease. A much more robust test should include cholecystokinin analog to measure pancreatic enzymes concentration. This test involves endoscopy, administration of secretin, and/or a cholecystokinin analog, and subsequent measurement of bicarbonate and digestive enzymes in the pancreatic juice. This test is not routinely offered as it is invasive, cumbersome, and difficult to repeat for reassessment of pancreatic function over time.8
Treatment
The primary goal of treatment is to improve symptoms and nutritional status of the patient. EPI treatment consists of PERT and nutritional counseling. In the United States, there are multiple FDA approved PERT preparations, which include Creon, Zenpep, Pancreaze, Pertzye, Viokase, and Relizorb. While dosing is dependent on lipase concentration, all PERT (aside from Relizorb) preparations have a combination of lipase, proteases, and amylase. All but Viokace and Relizorb are enteric-coated formulations.9
In patients with an inadequate response to enteric coated PERT, non-enteric coated PERT can be added as it may provide a more immediate effect than enteric coated formulations, specially if concern about rapid gut transit with inadequate mixing is raised. If a non-enteric formations is used, acid suppression should be added to prevent inactivation of the PERT. Relizorb is a cartridge system which delivers lipase directly to tube feeds. This cartridge is only utilized in patients receiving enteral nutrition and allows for treatment of EPI even when patients are unable to tolerate oral feeding.
PERT dosing is intended to at least compensate for 10% of the physiologically secreted amount of endogenous lipase after a normal meal (approximately 30,000 IU). Hence, dosing is primarily weight-based. In symptomatic adults, PERT dose of 500-1000 units/kg/meal and half of the amount with snacks is appropriate. Although higher doses of 1500-2000 units/kg/meal may be needed when there is significant steatorrhea, weight loss, or micronutrient deficiencies, PERT doses exceeding 2500 units/kg/meal are not recommended and warrant further investigation.10
Proper counseling is important to ensure compliance with pancreatin preparations. PERT will generally be effective in improving steatorrhea, weight loss, bowel movement frequency, and reversal of nutritional deficiencies, but it does not reliably help symptoms of bloating or abdominal pain. If a patient’s steatorrhea does not respond to PERT, then alternative diagnoses such as SIBO, or diarrhea-predominant IBS should be considered.
PERT must be taken with meals. There are studies that support split dosing as a more effective way of absorbing fat.11 If PERT is ineffective or minimally effective, review of appropriate dosing and timing of PERT to a meal is recommended. Addition of acid suppression may be required to improve treatment efficacy, especially in patients with abnormal intestinal motility or prior pancreatic surgery as PERT is effective at a pH of 4.5. Cost, pill burden, and persistence of certain symptoms may impact adherence to PERT and thus pre-treatment counseling and close follow-up after initiation is important. This aids in assessment of patients’ response to therapy, ensure appropriate PERT administration, and identifying any barriers to therapy adherence.
Nutritional management of EPI consists of an assessment of nutritional status, diet, and lifestyle. An important component of nutritional management is the assessment of micronutrient deficiencies. Patients with a confirmed diagnosis of EPI should be screened for the following micronutrients annually: Vitamins (A, E, D, K, B12), folate, zinc, selenium, magnesium, and iron. Patients with chronic pancreatitis and EPI should also be screened for metabolic bone disease once every two years and for diabetes mellitus annually.4, 12
Conclusion
EPI is a challenging diagnosis as many symptoms overlap with other GI conditions. Pancreas exocrine function is rich with significant reserve to allow for proper digestive capacity, yet EPI occurs when an individual’s pancreatic digestive enzymes are insufficient to meet their nutritional needs. In patients with high likelihood of having EPI, such as those with pre-existing pancreatic disease, diagnosing EPI combines clinical evidence based on subjective symptoms and stool-based testing to support a disease state.
Appropriate dosing and timing of PERT is critical to improve nutritional outcomes and improve certain symptoms of EPI. Failure of PERT requires evaluating for proper dosing/timing, and consideration of additional or alternative diagnosis. EPI morbidity can lead to significant impact on patients’ quality of life, but with counseling, proper PERT use, nutritional consequences can be mediated, and quality of life can improve.
Dr. Hernandez-Barco is based in the Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts. Dr. Ashkar is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Hernandez-Barco disclosed consulting for AMGEN and served as a scientific advisor for Nestle Health Science. She had project-related funding support or conflicts of interest to disclose. Dr. Ashkar disclosed consulting for AMGEN. He had no project-related funding support or conflicts of interest to disclose.
References
1. Othman MO, et al. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018 Feb. doi: 10.1111/ijcp.13066.
2. Whitcomb DC, et al. AGA-PancreasFest Joint Symposium on Exocrine Pancreatitic Insufficiency. Gastro Hep Adv. 2022 Nov. doi: 10.1016/j.gastha.2022.11.008.
3. Khan A, et al. Staging Exocrine Pancreatic Dysfunction. Pancreatology. 2022 Jan. doi: 10.1016/j.pan.2021.11.005.
4. Whitcomb DC, et al. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatitis insufficiency: Expert Review. Gastroenterology. 2023 Nov. doi: 10.1053/j.gastro.2023.07.007.
5. Kunovský L, et al. Causes of Exocrine Pancreatic Insufficiency Other than Chronic Pancreatitis. J Clin Med. 2021 Dec. doi: 10.3390/jcm10245779.
6. Singh VK, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017 Oct. doi: 10.3748/wjg.v23.i39.7059.
7. Lankisch PG, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mid to moderate exocrine pancreatic insufficiency. Gut. 1998 Apr. doi: 10.1136/gut.42.4.551.
8. Gardner TB, et al. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020 Mar. doi: 10.14309/ajg.0000000000000535.
9. Lewis DM, et al. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08184-w.
10. Borowitz DS, et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995 Nov. doi: 10.1016/s0022-3476(95)70153-2.
11. Domínguez-Muñoz JE, et al. Effect of the Administration Schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005 Apr. doi: 10.1111/j.1365-2036.2005.02390.x.
12. Hart PA and Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol. 2020 Jan. doi: 10.14309/ajg.0000000000000421.