User login
Sublingual film well tolerated for Parkinson ‘off’ episodes
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.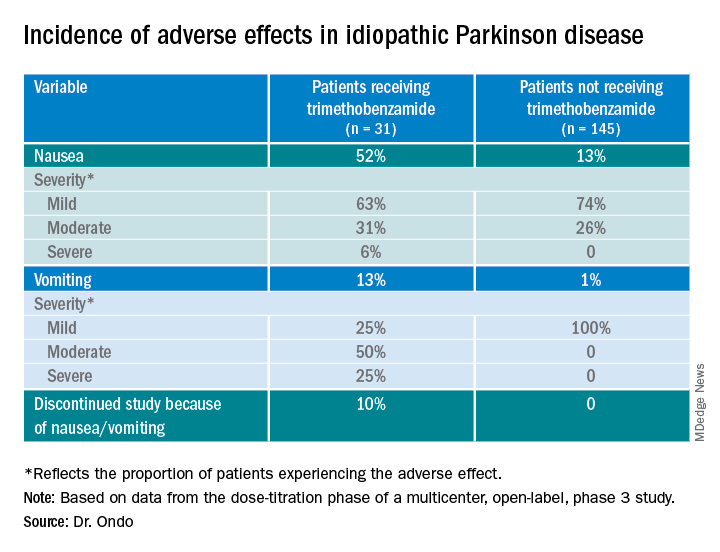
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Survey identifies clinicians’ unease with genetic testing
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
FROM MDS VIRTUAL CONGRESS 2021
Toward ‘superhuman cognition’: The future of brain-computer interfaces
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
Nonmotor symptoms common in Parkinson’s
The hallmark of Parkinson’s disease is the accompanying motor symptoms, but the condition can bring other challenges. Among those are nonmotor symptoms, including depression, dementia, and even psychosis.
The culprit is Lewy bodies, which are also responsible for Lewy body dementia. “What we call Lewy body dementia and Parkinson’s disease are caused by the same pathological process – the formation of Lewy bodies in the brain,” Leslie Citrome, MD, MPH, said in an interview. Dr. Citrome discussed some of the psychiatric comorbidities associated with Parkinson’s disease at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In fact, the association goes both ways. “Many people with Parkinson’s disease develop a dementia. Many people with Lewy body dementia develop motor symptoms that look just like Parkinson’s disease,” said Dr. Citrome, professor of psychiatry and behavioral sciences at New York Medical College, Valhalla, and president of the American Society for Clinical Psychopharmacology.
The motor symptoms of Parkinson’s disease are generally attributable to loss of striatal dopaminergic neurons, while nonmotor symptoms can be traced to loss of neurons in nondopaminergic regions. Nonmotor symptoms – often including sleep disorders, depression, cognitive changes, and psychosis – may occur before motor symptoms. Other problems may include autonomic dysfunction, such as constipation, sexual dysfunction, sweating, or urinary retention.
Patients might not be aware that nonmotor symptoms can occur with Parkinson’s disease and may not even consider mentioning mood changes or hallucinations to their neurologist. Family members may also be unaware.
Sleep problems are common in Parkinson’s disease, including rapid eye-movement sleep behavior disorders, vivid dreams, restless legs syndrome, insomnia, and daytime somnolence. Dopamine agonists may also cause unintended sleep.
Depression is extremely common, affecting up to 90% of Parkinson’s disease patients, and this may be related to dopaminergic losses. Antidepressant medications can worsen Parkinson’s disease symptoms: Tricyclic antidepressants increase risk of adverse events from anticholinergic drugs. Selective serotonin reuptake inhibitors (SSRIs) can exacerbate tremor and may increase risk of serotonin syndrome when combined with MAO‐B inhibitors.
Dr. Citrome was not aware of any antidepressant drugs that have been tested specifically in Parkinson’s disease patients, though “I’d be surprised if there wasn’t,” he said during the Q&A session. “There’s no one perfect antidepressant for people with depression associated with Parkinson’s disease. I would make sure to select one that they would tolerate and be willing to take and that doesn’t interfere with their treatment of their movement disorder, and (I would make sure) that there’s no drug-drug interaction,” he said.
This can include reduced working memory, learning, and planning, and generally does not manifest until at least 1 year after motor symptoms have begun. Rivastigmine is Food and Drug Administration–approved for treatment of cognitive impairment in Parkinson’s disease.
As many as 60% of Parkinson’s disease patients suffer from psychosis at some point, often visual hallucinations or delusions, which can include beliefs of spousal infidelity.
Many clinicians prescribe quetiapine off label, but there are not compelling data to support that it reduces intensity and frequency of hallucinations and delusions, according to Dr. Citrome. However, it is relatively easy to prescribe, requiring no preauthorizations, it is inexpensive, and it may improve sleep.
The FDA approved pimavanserin in 2016 for hallucinations and delusions in Parkinson’s disease, and it doesn’t worsen motor symptoms, Dr. Citrome said. That’s because pimavanserin is a highly selective antagonist of the 5-HT2A receptor, with no effect on dopaminergic, histaminergic, adrenergic, or muscarinic receptors.
The drug improves positive symptoms beginning at days 29 and 43, compared with placebo. An analysis by Dr. Citrome’s group found a number needed to treat (NNT) of 7 to gain a benefit over placebo if the metric is a ≥ 30% reduction in baseline symptom score. The drug had an NNT of 9 to achieve a ≥ 50% reduction, and an NNT of 5 to achieve a score of much improved or very much improved on the Clinical Global Impression–Improvement (CGI-I) scale. In general, an NNT less than 10 suggests that a drug is clinically useful.
In contrast, the number needed to harm (NNH) represents the number of patients who would need to receive a therapy to add one adverse event, compared with placebo. A number greater than 10 indicates that the therapy may be tolerable.
Using various measures, the NNH was well over 10 for pimavanserin. With respect to somnolence, the NNH over placebo was 138, and for a weight gain of 7% or more, the NNH was 594.
Overall, the study found that 4 patients would need to be treated to achieve a benefit over placebo with respect to a ≥ 3–point improvement in the Scale of Positive Symptoms–Parkinson’s Disease (SAPS-PD), while 21 would need to receive the drug to lead to one additional discontinuation because of an adverse event, compared to placebo.
When researchers compared pimavanserin to off-label use of quetiapine, olanzapine, and clozapine, they found a Cohen’s d value of 0.50, which was better than quetiapine and olanzapine, but lower than for clozapine. However, there is no requirement of blood monitoring, and clozapine can potentially worsen motor symptoms.
Dr. Citrome’s presentation should be a reminder to neurologists that psychiatric disorders are an important patient concern, said Henry A. Nasrallah, MD, professor of psychiatry, neurology, and neuroscience at the University of Cincinnati, who moderated the session.
“I think this serves as a model to recognize that many neurological disorders actually present with numerous psychiatric disorders,” Dr. Nasrallah said during the meeting, presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
Dr. Citrome has consulted for AbbVie, Acadia, Alkermes, Allergan, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer-Ingelheim, Cadent Therapeutics, Eisai, Impel, Intra-Cellular, Janssen, Karuna, Lundbeck, Lyndra, MedAvante-ProPhase, Merck, Neurocrine, Noven, Otsuka, Ovid, Relmada, Sage, Sunovion, and Teva. He has been a speaker for most of those companies, and he holds stock in Bristol Myers Squibb, Eli Lilly, J&J, Merck, and Pfizer.
Dr. Nasrallah has consulted for Acadia, Alkermes, Allergan, Boehringer-Ingelheim, Indivior, Intra-Cellular, Janssen, Neurocrine, Otsuka, Sunovion, and Teva. He has served on a speakers bureau for most of those companies, in addition to that of Noven.
The hallmark of Parkinson’s disease is the accompanying motor symptoms, but the condition can bring other challenges. Among those are nonmotor symptoms, including depression, dementia, and even psychosis.
The culprit is Lewy bodies, which are also responsible for Lewy body dementia. “What we call Lewy body dementia and Parkinson’s disease are caused by the same pathological process – the formation of Lewy bodies in the brain,” Leslie Citrome, MD, MPH, said in an interview. Dr. Citrome discussed some of the psychiatric comorbidities associated with Parkinson’s disease at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In fact, the association goes both ways. “Many people with Parkinson’s disease develop a dementia. Many people with Lewy body dementia develop motor symptoms that look just like Parkinson’s disease,” said Dr. Citrome, professor of psychiatry and behavioral sciences at New York Medical College, Valhalla, and president of the American Society for Clinical Psychopharmacology.
The motor symptoms of Parkinson’s disease are generally attributable to loss of striatal dopaminergic neurons, while nonmotor symptoms can be traced to loss of neurons in nondopaminergic regions. Nonmotor symptoms – often including sleep disorders, depression, cognitive changes, and psychosis – may occur before motor symptoms. Other problems may include autonomic dysfunction, such as constipation, sexual dysfunction, sweating, or urinary retention.
Patients might not be aware that nonmotor symptoms can occur with Parkinson’s disease and may not even consider mentioning mood changes or hallucinations to their neurologist. Family members may also be unaware.
Sleep problems are common in Parkinson’s disease, including rapid eye-movement sleep behavior disorders, vivid dreams, restless legs syndrome, insomnia, and daytime somnolence. Dopamine agonists may also cause unintended sleep.
Depression is extremely common, affecting up to 90% of Parkinson’s disease patients, and this may be related to dopaminergic losses. Antidepressant medications can worsen Parkinson’s disease symptoms: Tricyclic antidepressants increase risk of adverse events from anticholinergic drugs. Selective serotonin reuptake inhibitors (SSRIs) can exacerbate tremor and may increase risk of serotonin syndrome when combined with MAO‐B inhibitors.
Dr. Citrome was not aware of any antidepressant drugs that have been tested specifically in Parkinson’s disease patients, though “I’d be surprised if there wasn’t,” he said during the Q&A session. “There’s no one perfect antidepressant for people with depression associated with Parkinson’s disease. I would make sure to select one that they would tolerate and be willing to take and that doesn’t interfere with their treatment of their movement disorder, and (I would make sure) that there’s no drug-drug interaction,” he said.
This can include reduced working memory, learning, and planning, and generally does not manifest until at least 1 year after motor symptoms have begun. Rivastigmine is Food and Drug Administration–approved for treatment of cognitive impairment in Parkinson’s disease.
As many as 60% of Parkinson’s disease patients suffer from psychosis at some point, often visual hallucinations or delusions, which can include beliefs of spousal infidelity.
Many clinicians prescribe quetiapine off label, but there are not compelling data to support that it reduces intensity and frequency of hallucinations and delusions, according to Dr. Citrome. However, it is relatively easy to prescribe, requiring no preauthorizations, it is inexpensive, and it may improve sleep.
The FDA approved pimavanserin in 2016 for hallucinations and delusions in Parkinson’s disease, and it doesn’t worsen motor symptoms, Dr. Citrome said. That’s because pimavanserin is a highly selective antagonist of the 5-HT2A receptor, with no effect on dopaminergic, histaminergic, adrenergic, or muscarinic receptors.
The drug improves positive symptoms beginning at days 29 and 43, compared with placebo. An analysis by Dr. Citrome’s group found a number needed to treat (NNT) of 7 to gain a benefit over placebo if the metric is a ≥ 30% reduction in baseline symptom score. The drug had an NNT of 9 to achieve a ≥ 50% reduction, and an NNT of 5 to achieve a score of much improved or very much improved on the Clinical Global Impression–Improvement (CGI-I) scale. In general, an NNT less than 10 suggests that a drug is clinically useful.
In contrast, the number needed to harm (NNH) represents the number of patients who would need to receive a therapy to add one adverse event, compared with placebo. A number greater than 10 indicates that the therapy may be tolerable.
Using various measures, the NNH was well over 10 for pimavanserin. With respect to somnolence, the NNH over placebo was 138, and for a weight gain of 7% or more, the NNH was 594.
Overall, the study found that 4 patients would need to be treated to achieve a benefit over placebo with respect to a ≥ 3–point improvement in the Scale of Positive Symptoms–Parkinson’s Disease (SAPS-PD), while 21 would need to receive the drug to lead to one additional discontinuation because of an adverse event, compared to placebo.
When researchers compared pimavanserin to off-label use of quetiapine, olanzapine, and clozapine, they found a Cohen’s d value of 0.50, which was better than quetiapine and olanzapine, but lower than for clozapine. However, there is no requirement of blood monitoring, and clozapine can potentially worsen motor symptoms.
Dr. Citrome’s presentation should be a reminder to neurologists that psychiatric disorders are an important patient concern, said Henry A. Nasrallah, MD, professor of psychiatry, neurology, and neuroscience at the University of Cincinnati, who moderated the session.
“I think this serves as a model to recognize that many neurological disorders actually present with numerous psychiatric disorders,” Dr. Nasrallah said during the meeting, presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
Dr. Citrome has consulted for AbbVie, Acadia, Alkermes, Allergan, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer-Ingelheim, Cadent Therapeutics, Eisai, Impel, Intra-Cellular, Janssen, Karuna, Lundbeck, Lyndra, MedAvante-ProPhase, Merck, Neurocrine, Noven, Otsuka, Ovid, Relmada, Sage, Sunovion, and Teva. He has been a speaker for most of those companies, and he holds stock in Bristol Myers Squibb, Eli Lilly, J&J, Merck, and Pfizer.
Dr. Nasrallah has consulted for Acadia, Alkermes, Allergan, Boehringer-Ingelheim, Indivior, Intra-Cellular, Janssen, Neurocrine, Otsuka, Sunovion, and Teva. He has served on a speakers bureau for most of those companies, in addition to that of Noven.
The hallmark of Parkinson’s disease is the accompanying motor symptoms, but the condition can bring other challenges. Among those are nonmotor symptoms, including depression, dementia, and even psychosis.
The culprit is Lewy bodies, which are also responsible for Lewy body dementia. “What we call Lewy body dementia and Parkinson’s disease are caused by the same pathological process – the formation of Lewy bodies in the brain,” Leslie Citrome, MD, MPH, said in an interview. Dr. Citrome discussed some of the psychiatric comorbidities associated with Parkinson’s disease at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
In fact, the association goes both ways. “Many people with Parkinson’s disease develop a dementia. Many people with Lewy body dementia develop motor symptoms that look just like Parkinson’s disease,” said Dr. Citrome, professor of psychiatry and behavioral sciences at New York Medical College, Valhalla, and president of the American Society for Clinical Psychopharmacology.
The motor symptoms of Parkinson’s disease are generally attributable to loss of striatal dopaminergic neurons, while nonmotor symptoms can be traced to loss of neurons in nondopaminergic regions. Nonmotor symptoms – often including sleep disorders, depression, cognitive changes, and psychosis – may occur before motor symptoms. Other problems may include autonomic dysfunction, such as constipation, sexual dysfunction, sweating, or urinary retention.
Patients might not be aware that nonmotor symptoms can occur with Parkinson’s disease and may not even consider mentioning mood changes or hallucinations to their neurologist. Family members may also be unaware.
Sleep problems are common in Parkinson’s disease, including rapid eye-movement sleep behavior disorders, vivid dreams, restless legs syndrome, insomnia, and daytime somnolence. Dopamine agonists may also cause unintended sleep.
Depression is extremely common, affecting up to 90% of Parkinson’s disease patients, and this may be related to dopaminergic losses. Antidepressant medications can worsen Parkinson’s disease symptoms: Tricyclic antidepressants increase risk of adverse events from anticholinergic drugs. Selective serotonin reuptake inhibitors (SSRIs) can exacerbate tremor and may increase risk of serotonin syndrome when combined with MAO‐B inhibitors.
Dr. Citrome was not aware of any antidepressant drugs that have been tested specifically in Parkinson’s disease patients, though “I’d be surprised if there wasn’t,” he said during the Q&A session. “There’s no one perfect antidepressant for people with depression associated with Parkinson’s disease. I would make sure to select one that they would tolerate and be willing to take and that doesn’t interfere with their treatment of their movement disorder, and (I would make sure) that there’s no drug-drug interaction,” he said.
This can include reduced working memory, learning, and planning, and generally does not manifest until at least 1 year after motor symptoms have begun. Rivastigmine is Food and Drug Administration–approved for treatment of cognitive impairment in Parkinson’s disease.
As many as 60% of Parkinson’s disease patients suffer from psychosis at some point, often visual hallucinations or delusions, which can include beliefs of spousal infidelity.
Many clinicians prescribe quetiapine off label, but there are not compelling data to support that it reduces intensity and frequency of hallucinations and delusions, according to Dr. Citrome. However, it is relatively easy to prescribe, requiring no preauthorizations, it is inexpensive, and it may improve sleep.
The FDA approved pimavanserin in 2016 for hallucinations and delusions in Parkinson’s disease, and it doesn’t worsen motor symptoms, Dr. Citrome said. That’s because pimavanserin is a highly selective antagonist of the 5-HT2A receptor, with no effect on dopaminergic, histaminergic, adrenergic, or muscarinic receptors.
The drug improves positive symptoms beginning at days 29 and 43, compared with placebo. An analysis by Dr. Citrome’s group found a number needed to treat (NNT) of 7 to gain a benefit over placebo if the metric is a ≥ 30% reduction in baseline symptom score. The drug had an NNT of 9 to achieve a ≥ 50% reduction, and an NNT of 5 to achieve a score of much improved or very much improved on the Clinical Global Impression–Improvement (CGI-I) scale. In general, an NNT less than 10 suggests that a drug is clinically useful.
In contrast, the number needed to harm (NNH) represents the number of patients who would need to receive a therapy to add one adverse event, compared with placebo. A number greater than 10 indicates that the therapy may be tolerable.
Using various measures, the NNH was well over 10 for pimavanserin. With respect to somnolence, the NNH over placebo was 138, and for a weight gain of 7% or more, the NNH was 594.
Overall, the study found that 4 patients would need to be treated to achieve a benefit over placebo with respect to a ≥ 3–point improvement in the Scale of Positive Symptoms–Parkinson’s Disease (SAPS-PD), while 21 would need to receive the drug to lead to one additional discontinuation because of an adverse event, compared to placebo.
When researchers compared pimavanserin to off-label use of quetiapine, olanzapine, and clozapine, they found a Cohen’s d value of 0.50, which was better than quetiapine and olanzapine, but lower than for clozapine. However, there is no requirement of blood monitoring, and clozapine can potentially worsen motor symptoms.
Dr. Citrome’s presentation should be a reminder to neurologists that psychiatric disorders are an important patient concern, said Henry A. Nasrallah, MD, professor of psychiatry, neurology, and neuroscience at the University of Cincinnati, who moderated the session.
“I think this serves as a model to recognize that many neurological disorders actually present with numerous psychiatric disorders,” Dr. Nasrallah said during the meeting, presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
Dr. Citrome has consulted for AbbVie, Acadia, Alkermes, Allergan, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer-Ingelheim, Cadent Therapeutics, Eisai, Impel, Intra-Cellular, Janssen, Karuna, Lundbeck, Lyndra, MedAvante-ProPhase, Merck, Neurocrine, Noven, Otsuka, Ovid, Relmada, Sage, Sunovion, and Teva. He has been a speaker for most of those companies, and he holds stock in Bristol Myers Squibb, Eli Lilly, J&J, Merck, and Pfizer.
Dr. Nasrallah has consulted for Acadia, Alkermes, Allergan, Boehringer-Ingelheim, Indivior, Intra-Cellular, Janssen, Neurocrine, Otsuka, Sunovion, and Teva. He has served on a speakers bureau for most of those companies, in addition to that of Noven.
FROM FOCUS ON NEUROPSYCHIATRY 2021
Anxiety, inactivity linked to cognitive impairment in Parkinson’s
Parkinson’s disease patients who develop anxiety early in their disease are at risk for reduced physical activity, which promotes further anxiety and cognitive decline, data from nearly 500 individuals show.
Anxiety occurs in 20%-60% of Parkinson’s disease (PD) patients but often goes undiagnosed, wrote Jacob D. Jones, PhD, of California State University, San Bernardino, and colleagues.
“Anxiety can attenuate motivation to engage in physical activity leading to more anxiety and other negative cognitive outcomes,” although physical activity has been shown to improve cognitive function in PD patients, they said. However, physical activity as a mediator between anxiety and cognitive function in PD has not been well studied, they noted.
In a study published in Mental Health and Physical Activity Participants were followed for up to 5 years and completed neuropsychological tests, tests of motor severity, and self-reports on anxiety and physical activity. Anxiety was assessed using the State-Trait Anxiety Inventory-Trait (STAI-T) subscale. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE). Motor severity was assessed using the Unified Parkinson’s Disease Rating Scale-Part III (UPDRS). The average age of the participants was 61 years, 65% were men, and 96% were White.
Using a direct-effect model, the researchers found that individuals whose anxiety increased during the study period also showed signs of cognitive decline. A significant between-person effect showed that individuals who were generally more anxious also scored lower on cognitive tests over the 5-year study period.
In a mediation model computed with structural equation modeling, physical activity mediated the link between anxiety and cognition, most notably household activity.
“There was a significant within-person association between anxiety and household activities, meaning that individuals who became more anxious over the 5-year study also became less active in the home,” reported Dr. Jones and colleagues.
However, no significant indirect effect was noted regarding the between-person findings of the impact of physical activity on anxiety and cognitive decline. Although more severe anxiety was associated with less activity, cognitive performance was not associated with either type of physical activity.
The presence of a within-person effect “suggests that reductions in physical activity, specifically within the first 5 years of disease onset, may be detrimental to mental health,” the researchers emphasized. Given that the study population was newly diagnosed with PD “it is likely the within-person terms are more sensitive to changes in anxiety, physical activity, and cognition that are more directly the result of the PD process, as opposed to lifestyle/preexisting traits,” they said.
The study findings were limited by several factors, including the use of self-reports to measure physical activity, and the lack of granular information about the details of physical activity, the researchers noted. Another limitation was the inclusion of only newly diagnosed PD patients, which might limit generalizability.
“Future research is warranted to understand if other modes, intensities, or complexities of physical activity impact individuals with PD in a different manner in relation to cognition,” they said.
Dr. Jones and colleagues had no disclosures. The PPMI is supported by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including numerous pharmaceutical companies.
Parkinson’s disease patients who develop anxiety early in their disease are at risk for reduced physical activity, which promotes further anxiety and cognitive decline, data from nearly 500 individuals show.
Anxiety occurs in 20%-60% of Parkinson’s disease (PD) patients but often goes undiagnosed, wrote Jacob D. Jones, PhD, of California State University, San Bernardino, and colleagues.
“Anxiety can attenuate motivation to engage in physical activity leading to more anxiety and other negative cognitive outcomes,” although physical activity has been shown to improve cognitive function in PD patients, they said. However, physical activity as a mediator between anxiety and cognitive function in PD has not been well studied, they noted.
In a study published in Mental Health and Physical Activity Participants were followed for up to 5 years and completed neuropsychological tests, tests of motor severity, and self-reports on anxiety and physical activity. Anxiety was assessed using the State-Trait Anxiety Inventory-Trait (STAI-T) subscale. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE). Motor severity was assessed using the Unified Parkinson’s Disease Rating Scale-Part III (UPDRS). The average age of the participants was 61 years, 65% were men, and 96% were White.
Using a direct-effect model, the researchers found that individuals whose anxiety increased during the study period also showed signs of cognitive decline. A significant between-person effect showed that individuals who were generally more anxious also scored lower on cognitive tests over the 5-year study period.
In a mediation model computed with structural equation modeling, physical activity mediated the link between anxiety and cognition, most notably household activity.
“There was a significant within-person association between anxiety and household activities, meaning that individuals who became more anxious over the 5-year study also became less active in the home,” reported Dr. Jones and colleagues.
However, no significant indirect effect was noted regarding the between-person findings of the impact of physical activity on anxiety and cognitive decline. Although more severe anxiety was associated with less activity, cognitive performance was not associated with either type of physical activity.
The presence of a within-person effect “suggests that reductions in physical activity, specifically within the first 5 years of disease onset, may be detrimental to mental health,” the researchers emphasized. Given that the study population was newly diagnosed with PD “it is likely the within-person terms are more sensitive to changes in anxiety, physical activity, and cognition that are more directly the result of the PD process, as opposed to lifestyle/preexisting traits,” they said.
The study findings were limited by several factors, including the use of self-reports to measure physical activity, and the lack of granular information about the details of physical activity, the researchers noted. Another limitation was the inclusion of only newly diagnosed PD patients, which might limit generalizability.
“Future research is warranted to understand if other modes, intensities, or complexities of physical activity impact individuals with PD in a different manner in relation to cognition,” they said.
Dr. Jones and colleagues had no disclosures. The PPMI is supported by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including numerous pharmaceutical companies.
Parkinson’s disease patients who develop anxiety early in their disease are at risk for reduced physical activity, which promotes further anxiety and cognitive decline, data from nearly 500 individuals show.
Anxiety occurs in 20%-60% of Parkinson’s disease (PD) patients but often goes undiagnosed, wrote Jacob D. Jones, PhD, of California State University, San Bernardino, and colleagues.
“Anxiety can attenuate motivation to engage in physical activity leading to more anxiety and other negative cognitive outcomes,” although physical activity has been shown to improve cognitive function in PD patients, they said. However, physical activity as a mediator between anxiety and cognitive function in PD has not been well studied, they noted.
In a study published in Mental Health and Physical Activity Participants were followed for up to 5 years and completed neuropsychological tests, tests of motor severity, and self-reports on anxiety and physical activity. Anxiety was assessed using the State-Trait Anxiety Inventory-Trait (STAI-T) subscale. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE). Motor severity was assessed using the Unified Parkinson’s Disease Rating Scale-Part III (UPDRS). The average age of the participants was 61 years, 65% were men, and 96% were White.
Using a direct-effect model, the researchers found that individuals whose anxiety increased during the study period also showed signs of cognitive decline. A significant between-person effect showed that individuals who were generally more anxious also scored lower on cognitive tests over the 5-year study period.
In a mediation model computed with structural equation modeling, physical activity mediated the link between anxiety and cognition, most notably household activity.
“There was a significant within-person association between anxiety and household activities, meaning that individuals who became more anxious over the 5-year study also became less active in the home,” reported Dr. Jones and colleagues.
However, no significant indirect effect was noted regarding the between-person findings of the impact of physical activity on anxiety and cognitive decline. Although more severe anxiety was associated with less activity, cognitive performance was not associated with either type of physical activity.
The presence of a within-person effect “suggests that reductions in physical activity, specifically within the first 5 years of disease onset, may be detrimental to mental health,” the researchers emphasized. Given that the study population was newly diagnosed with PD “it is likely the within-person terms are more sensitive to changes in anxiety, physical activity, and cognition that are more directly the result of the PD process, as opposed to lifestyle/preexisting traits,” they said.
The study findings were limited by several factors, including the use of self-reports to measure physical activity, and the lack of granular information about the details of physical activity, the researchers noted. Another limitation was the inclusion of only newly diagnosed PD patients, which might limit generalizability.
“Future research is warranted to understand if other modes, intensities, or complexities of physical activity impact individuals with PD in a different manner in relation to cognition,” they said.
Dr. Jones and colleagues had no disclosures. The PPMI is supported by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including numerous pharmaceutical companies.
FROM MENTAL HEALTH AND PHYSICAL ACTIVITY
Psychotic features among older adults tied to Parkinson’s
Adults aged 65 years and older who develop psychotic manifestations are significantly more likely than those without such manifestations to develop prodromal Parkinson’s disease, data from 925 individuals suggest.
“The presence of perceptual abnormalities and/or delusional ideation among community-dwelling elderly individuals is more widespread than considered in the past,” wrote Ioanna Pachi, MD, of National and Kapodistrian University of Athens Medical School and colleagues. However, those psychoses and their potential impact on prodromal Parkinson’s disease (PD) have not been well studied in community-dwelling populations, they noted in the study, published in Parkinsonism and Related Disorders.
In the study, Dr. Pachi and colleagues reviewed data from 914 participants in the Hellenic Longitudinal Investigation of Aging and Diet study (HELIAD), a cross-sectional, population-based cohort study of older adults in Greece. The average age of the participants was 76 years, and 41% were men. Participants had no delusional features at baseline; delusional features were assessed using the Neuropsychiatric Inventory scale and the Columbia University Scale for Psychopathology in Alzheimer’s disease. The researchers calculated the probability of prodromal PD (pPD) for each participant based on the 2019 International Parkinson and Movement Disorders Society research criteria for prodromal PD.
Over a 3-year follow-up period, 20 participants developed psychotic manifestations and were 1.3 times more likely to have pPD, compared with those without psychoses (P = .006). Those with new-onset psychotic features were categorized together as the NPSY group, regardless of symptom severity or frequency; those with no symptoms at either baseline or during follow-up were categorized as unaffected (UPSY). Most of the NPSY participants showed isolated delusional features, although some expressed hallucinations. Most symptoms were mild.
New-onset psychosis was associated with a fivefold increased risk of both subthreshold parkinsonism and depression (adjusted odds ratios, 4.5 and 5.0, respectively) and with a threefold increased risk of constipation (aOR 2.6). Other factors, including nonsmoking, global cognitive deficit, and anxiety were not significantly associated with new-onset psychotic symptoms after adjusting for confounding factors.
Although the mechanism behind the association remains unclear,
The study findings were limited by several factors, including the administration of neuropsychiatric questionnaires by nonpsychiatrists, and lack of detailed psychiatric history, including complete information on medication use, the researchers noted. The small size of the NPSY group also prevented evaluation of the potential associations between pPD and different modalities of hallucinations, they said.
However, the results were strengthened by the overall large and population-based sample size, and the comprehensive evaluation of psychotic features, they wrote. More follow-up evaluations in the HELIAD cohort are planned to further explore the underlying mechanism of the association between late-life psychosis and pPD.
“Provided that these results are confirmed in other community cohorts of elderly subjects, psychotic features may be added to the list of manifestations of pPD,” they concluded.
The study was supported in part by grants from the Alzheimer’s Association, ARISTEIA, and the ESPA-EU program Excellence Grant. It was cofunded by the European Social Fund and Greek National resources, the Ministry for Health and Social Solidarity, Greece, and the Greek State Scholarships Foundation. Dr. Pachi had no disclosures.
Adults aged 65 years and older who develop psychotic manifestations are significantly more likely than those without such manifestations to develop prodromal Parkinson’s disease, data from 925 individuals suggest.
“The presence of perceptual abnormalities and/or delusional ideation among community-dwelling elderly individuals is more widespread than considered in the past,” wrote Ioanna Pachi, MD, of National and Kapodistrian University of Athens Medical School and colleagues. However, those psychoses and their potential impact on prodromal Parkinson’s disease (PD) have not been well studied in community-dwelling populations, they noted in the study, published in Parkinsonism and Related Disorders.
In the study, Dr. Pachi and colleagues reviewed data from 914 participants in the Hellenic Longitudinal Investigation of Aging and Diet study (HELIAD), a cross-sectional, population-based cohort study of older adults in Greece. The average age of the participants was 76 years, and 41% were men. Participants had no delusional features at baseline; delusional features were assessed using the Neuropsychiatric Inventory scale and the Columbia University Scale for Psychopathology in Alzheimer’s disease. The researchers calculated the probability of prodromal PD (pPD) for each participant based on the 2019 International Parkinson and Movement Disorders Society research criteria for prodromal PD.
Over a 3-year follow-up period, 20 participants developed psychotic manifestations and were 1.3 times more likely to have pPD, compared with those without psychoses (P = .006). Those with new-onset psychotic features were categorized together as the NPSY group, regardless of symptom severity or frequency; those with no symptoms at either baseline or during follow-up were categorized as unaffected (UPSY). Most of the NPSY participants showed isolated delusional features, although some expressed hallucinations. Most symptoms were mild.
New-onset psychosis was associated with a fivefold increased risk of both subthreshold parkinsonism and depression (adjusted odds ratios, 4.5 and 5.0, respectively) and with a threefold increased risk of constipation (aOR 2.6). Other factors, including nonsmoking, global cognitive deficit, and anxiety were not significantly associated with new-onset psychotic symptoms after adjusting for confounding factors.
Although the mechanism behind the association remains unclear,
The study findings were limited by several factors, including the administration of neuropsychiatric questionnaires by nonpsychiatrists, and lack of detailed psychiatric history, including complete information on medication use, the researchers noted. The small size of the NPSY group also prevented evaluation of the potential associations between pPD and different modalities of hallucinations, they said.
However, the results were strengthened by the overall large and population-based sample size, and the comprehensive evaluation of psychotic features, they wrote. More follow-up evaluations in the HELIAD cohort are planned to further explore the underlying mechanism of the association between late-life psychosis and pPD.
“Provided that these results are confirmed in other community cohorts of elderly subjects, psychotic features may be added to the list of manifestations of pPD,” they concluded.
The study was supported in part by grants from the Alzheimer’s Association, ARISTEIA, and the ESPA-EU program Excellence Grant. It was cofunded by the European Social Fund and Greek National resources, the Ministry for Health and Social Solidarity, Greece, and the Greek State Scholarships Foundation. Dr. Pachi had no disclosures.
Adults aged 65 years and older who develop psychotic manifestations are significantly more likely than those without such manifestations to develop prodromal Parkinson’s disease, data from 925 individuals suggest.
“The presence of perceptual abnormalities and/or delusional ideation among community-dwelling elderly individuals is more widespread than considered in the past,” wrote Ioanna Pachi, MD, of National and Kapodistrian University of Athens Medical School and colleagues. However, those psychoses and their potential impact on prodromal Parkinson’s disease (PD) have not been well studied in community-dwelling populations, they noted in the study, published in Parkinsonism and Related Disorders.
In the study, Dr. Pachi and colleagues reviewed data from 914 participants in the Hellenic Longitudinal Investigation of Aging and Diet study (HELIAD), a cross-sectional, population-based cohort study of older adults in Greece. The average age of the participants was 76 years, and 41% were men. Participants had no delusional features at baseline; delusional features were assessed using the Neuropsychiatric Inventory scale and the Columbia University Scale for Psychopathology in Alzheimer’s disease. The researchers calculated the probability of prodromal PD (pPD) for each participant based on the 2019 International Parkinson and Movement Disorders Society research criteria for prodromal PD.
Over a 3-year follow-up period, 20 participants developed psychotic manifestations and were 1.3 times more likely to have pPD, compared with those without psychoses (P = .006). Those with new-onset psychotic features were categorized together as the NPSY group, regardless of symptom severity or frequency; those with no symptoms at either baseline or during follow-up were categorized as unaffected (UPSY). Most of the NPSY participants showed isolated delusional features, although some expressed hallucinations. Most symptoms were mild.
New-onset psychosis was associated with a fivefold increased risk of both subthreshold parkinsonism and depression (adjusted odds ratios, 4.5 and 5.0, respectively) and with a threefold increased risk of constipation (aOR 2.6). Other factors, including nonsmoking, global cognitive deficit, and anxiety were not significantly associated with new-onset psychotic symptoms after adjusting for confounding factors.
Although the mechanism behind the association remains unclear,
The study findings were limited by several factors, including the administration of neuropsychiatric questionnaires by nonpsychiatrists, and lack of detailed psychiatric history, including complete information on medication use, the researchers noted. The small size of the NPSY group also prevented evaluation of the potential associations between pPD and different modalities of hallucinations, they said.
However, the results were strengthened by the overall large and population-based sample size, and the comprehensive evaluation of psychotic features, they wrote. More follow-up evaluations in the HELIAD cohort are planned to further explore the underlying mechanism of the association between late-life psychosis and pPD.
“Provided that these results are confirmed in other community cohorts of elderly subjects, psychotic features may be added to the list of manifestations of pPD,” they concluded.
The study was supported in part by grants from the Alzheimer’s Association, ARISTEIA, and the ESPA-EU program Excellence Grant. It was cofunded by the European Social Fund and Greek National resources, the Ministry for Health and Social Solidarity, Greece, and the Greek State Scholarships Foundation. Dr. Pachi had no disclosures.
FROM PARKINSONISM AND RELATED DISORDERS
Increased risk of hospitalization and death with Parkinson’s drug
, according to a new study.
A retrospective cohort study of elderly patients with Parkinson’s disease who were in long-term care facilities found that the use of pimavanserin (Nuplazid) was associated with an increased risk of 30-day hospitalization and mortality for up to a year.
“Given that a previous study showed typical and atypical antipsychotics more than doubled mortality risk in patients with Parkinson’s disease, we aimed to assess the risk of hospitalization and death associated with pimavanserin,” wrote lead author Y. Joseph Hwang, MD, Johns Hopkins University, Baltimore, and colleagues in the paper. “These findings, in a large real-world cohort within long-term care facilities, may help to inform decisions regarding its risk-benefit balance among patients with Parkinson’s disease.”
The findings were published online Aug. 13 in Neurology.
The researchers enrolled 2,186 patients with Parkinson’s disease aged 65 years and older in Medicare-certified long-term care facilities who also had a pimavanserin prescription and 18,212 nonusers of pimavanserin between Nov. 1, 2015, and December 31, 2018. Patients in the pimavanserin group used the drug over the course of the entire study period. Hospitalization and mortality were calculated from the date of pimavanserin prescription. Propensity score–based inverse probability of treatment weighting (IPTW) was used to balance the two groups on 24 baseline characteristics such as age, sex, and comorbidities.
Pimavanserin use was associated with a 24% higher risk of 30-day hospitalization (adjusted hazard ratio, 1.24; 95% confidence interval, 1.06-1.43). However, “the association did not reach statistical significance in a smaller subcohort of propensity score-matched users and nonusers,” Dr. Hwang and colleagues wrote.
Pimavanserin use was also linked to higher mortality at:
- 90 days (aHR, 1.20; 95% CI, 1.02-1.41).
- 180 days (aHR, 1.28; 95% CI, 1.13-1.45).
- 365 days (aHR, 1.56; 95% CI, 1.42-1.72).
No associations were found between pimavanserin use and 90-day hospitalization (aHR, 1.10; 95% CI, 0.99-1.24) nor with 30-day mortality (aHR, 0.76; 95% CI, 0.56-1.03).
Important considerations
“This study raises three important points to consider for any practicing neurology provider: 1) how to address and interpret risks associated with pimavanserin use in this patient population 2) utility of pimavanserin 3) interpretation of data showing increased mortality in patients being treated for Parkinson’s disease psychosis,” wrote Farwa Ali, MBBS, of the Mayo Clinic, Rochester, Minn., in an accompanying editorial published in Neurology.
Hallucinations and delusions are highly prevalent in Parkinson’s disease; as many as 60% of patients will develop psychosis over the course of their illness. Pimavanserin is a selective serotonin inverse agonist which targets 5-HT2A serotonin receptors in the brain, decreasing their activity in order to attenuate hallucinations and delusions.
“Pimavanserin has been approved by the FDA [Food and Drug Administration] for Parkinson’s disease psychosis, but its safety has been called into question based on previous reports of increased mortality risk, compared with a rather modest benefit seen in a 6-week clinical trial, the duration of which limits determination of long-term safety,” wrote Dr. Ali.
Pimavanserin carries a boxed warning that elderly patients with dementia may be at an increased risk of death. After its approval in 2016, the U.S. FDA later reviewed 893 deaths in association with pimavanserin during the postmarketing surveillance period – “an unexpected number in a new drug,” Dr. Hwang and colleagues noted. “It [the FDA] noted that most reports occurred in a population with high underlying death rates and did not signal any additional risk beyond the current warning for all antipsychotics, which could have resulted in annual mortality rates of up to 60%.”
As the first cohort study to examine hospitalization and death between pimavanserin users and nonusers, “the study confirms previous concerns regarding safety of pimavanserin and more importantly brings to attention the importance of carefully considering risks and benefits of pharmacotherapy in Parkinson’s disease psychosis, clear communication with patients and families, and close observation to ensure safety,” wrote Dr. Ali.
The study limitations include its observational design, which subjected the findings to residual confounding.
“While we developed models to maximize the strength of causal inference, our comparison group was pimavanserin nonusers and the very reason for prescription of pimavanserin could have predisposed its users to the outcomes of hospitalization and death, introducing confounding by indication,” Dr. Hwang and colleagues wrote in the paper.
Additionally, “while robust analyses were conducted to ensure pimavanserin users and nonusers were comparable, Dr. Hwang et al. did find that pimavanserin users were more likely to concomitantly use other antipsychotic drugs which has been demonstrated as increasing the mortality risk,” Dr. Ali pointed out.
Since patients living in long-term care facilities may have a higher risk of mortality because of more severe or later-stage Parkinson’s disease, the study results “may not be generalizable to community-dwelling PD patients,” Dr. Ali wrote. “These factors are important to consider while making individual management decisions.”
Dr. Hwang and Dr. Ali disclosed no relevant financial relationships. The study authors reported no targeted funding.
, according to a new study.
A retrospective cohort study of elderly patients with Parkinson’s disease who were in long-term care facilities found that the use of pimavanserin (Nuplazid) was associated with an increased risk of 30-day hospitalization and mortality for up to a year.
“Given that a previous study showed typical and atypical antipsychotics more than doubled mortality risk in patients with Parkinson’s disease, we aimed to assess the risk of hospitalization and death associated with pimavanserin,” wrote lead author Y. Joseph Hwang, MD, Johns Hopkins University, Baltimore, and colleagues in the paper. “These findings, in a large real-world cohort within long-term care facilities, may help to inform decisions regarding its risk-benefit balance among patients with Parkinson’s disease.”
The findings were published online Aug. 13 in Neurology.
The researchers enrolled 2,186 patients with Parkinson’s disease aged 65 years and older in Medicare-certified long-term care facilities who also had a pimavanserin prescription and 18,212 nonusers of pimavanserin between Nov. 1, 2015, and December 31, 2018. Patients in the pimavanserin group used the drug over the course of the entire study period. Hospitalization and mortality were calculated from the date of pimavanserin prescription. Propensity score–based inverse probability of treatment weighting (IPTW) was used to balance the two groups on 24 baseline characteristics such as age, sex, and comorbidities.
Pimavanserin use was associated with a 24% higher risk of 30-day hospitalization (adjusted hazard ratio, 1.24; 95% confidence interval, 1.06-1.43). However, “the association did not reach statistical significance in a smaller subcohort of propensity score-matched users and nonusers,” Dr. Hwang and colleagues wrote.
Pimavanserin use was also linked to higher mortality at:
- 90 days (aHR, 1.20; 95% CI, 1.02-1.41).
- 180 days (aHR, 1.28; 95% CI, 1.13-1.45).
- 365 days (aHR, 1.56; 95% CI, 1.42-1.72).
No associations were found between pimavanserin use and 90-day hospitalization (aHR, 1.10; 95% CI, 0.99-1.24) nor with 30-day mortality (aHR, 0.76; 95% CI, 0.56-1.03).
Important considerations
“This study raises three important points to consider for any practicing neurology provider: 1) how to address and interpret risks associated with pimavanserin use in this patient population 2) utility of pimavanserin 3) interpretation of data showing increased mortality in patients being treated for Parkinson’s disease psychosis,” wrote Farwa Ali, MBBS, of the Mayo Clinic, Rochester, Minn., in an accompanying editorial published in Neurology.
Hallucinations and delusions are highly prevalent in Parkinson’s disease; as many as 60% of patients will develop psychosis over the course of their illness. Pimavanserin is a selective serotonin inverse agonist which targets 5-HT2A serotonin receptors in the brain, decreasing their activity in order to attenuate hallucinations and delusions.
“Pimavanserin has been approved by the FDA [Food and Drug Administration] for Parkinson’s disease psychosis, but its safety has been called into question based on previous reports of increased mortality risk, compared with a rather modest benefit seen in a 6-week clinical trial, the duration of which limits determination of long-term safety,” wrote Dr. Ali.
Pimavanserin carries a boxed warning that elderly patients with dementia may be at an increased risk of death. After its approval in 2016, the U.S. FDA later reviewed 893 deaths in association with pimavanserin during the postmarketing surveillance period – “an unexpected number in a new drug,” Dr. Hwang and colleagues noted. “It [the FDA] noted that most reports occurred in a population with high underlying death rates and did not signal any additional risk beyond the current warning for all antipsychotics, which could have resulted in annual mortality rates of up to 60%.”
As the first cohort study to examine hospitalization and death between pimavanserin users and nonusers, “the study confirms previous concerns regarding safety of pimavanserin and more importantly brings to attention the importance of carefully considering risks and benefits of pharmacotherapy in Parkinson’s disease psychosis, clear communication with patients and families, and close observation to ensure safety,” wrote Dr. Ali.
The study limitations include its observational design, which subjected the findings to residual confounding.
“While we developed models to maximize the strength of causal inference, our comparison group was pimavanserin nonusers and the very reason for prescription of pimavanserin could have predisposed its users to the outcomes of hospitalization and death, introducing confounding by indication,” Dr. Hwang and colleagues wrote in the paper.
Additionally, “while robust analyses were conducted to ensure pimavanserin users and nonusers were comparable, Dr. Hwang et al. did find that pimavanserin users were more likely to concomitantly use other antipsychotic drugs which has been demonstrated as increasing the mortality risk,” Dr. Ali pointed out.
Since patients living in long-term care facilities may have a higher risk of mortality because of more severe or later-stage Parkinson’s disease, the study results “may not be generalizable to community-dwelling PD patients,” Dr. Ali wrote. “These factors are important to consider while making individual management decisions.”
Dr. Hwang and Dr. Ali disclosed no relevant financial relationships. The study authors reported no targeted funding.
, according to a new study.
A retrospective cohort study of elderly patients with Parkinson’s disease who were in long-term care facilities found that the use of pimavanserin (Nuplazid) was associated with an increased risk of 30-day hospitalization and mortality for up to a year.
“Given that a previous study showed typical and atypical antipsychotics more than doubled mortality risk in patients with Parkinson’s disease, we aimed to assess the risk of hospitalization and death associated with pimavanserin,” wrote lead author Y. Joseph Hwang, MD, Johns Hopkins University, Baltimore, and colleagues in the paper. “These findings, in a large real-world cohort within long-term care facilities, may help to inform decisions regarding its risk-benefit balance among patients with Parkinson’s disease.”
The findings were published online Aug. 13 in Neurology.
The researchers enrolled 2,186 patients with Parkinson’s disease aged 65 years and older in Medicare-certified long-term care facilities who also had a pimavanserin prescription and 18,212 nonusers of pimavanserin between Nov. 1, 2015, and December 31, 2018. Patients in the pimavanserin group used the drug over the course of the entire study period. Hospitalization and mortality were calculated from the date of pimavanserin prescription. Propensity score–based inverse probability of treatment weighting (IPTW) was used to balance the two groups on 24 baseline characteristics such as age, sex, and comorbidities.
Pimavanserin use was associated with a 24% higher risk of 30-day hospitalization (adjusted hazard ratio, 1.24; 95% confidence interval, 1.06-1.43). However, “the association did not reach statistical significance in a smaller subcohort of propensity score-matched users and nonusers,” Dr. Hwang and colleagues wrote.
Pimavanserin use was also linked to higher mortality at:
- 90 days (aHR, 1.20; 95% CI, 1.02-1.41).
- 180 days (aHR, 1.28; 95% CI, 1.13-1.45).
- 365 days (aHR, 1.56; 95% CI, 1.42-1.72).
No associations were found between pimavanserin use and 90-day hospitalization (aHR, 1.10; 95% CI, 0.99-1.24) nor with 30-day mortality (aHR, 0.76; 95% CI, 0.56-1.03).
Important considerations
“This study raises three important points to consider for any practicing neurology provider: 1) how to address and interpret risks associated with pimavanserin use in this patient population 2) utility of pimavanserin 3) interpretation of data showing increased mortality in patients being treated for Parkinson’s disease psychosis,” wrote Farwa Ali, MBBS, of the Mayo Clinic, Rochester, Minn., in an accompanying editorial published in Neurology.
Hallucinations and delusions are highly prevalent in Parkinson’s disease; as many as 60% of patients will develop psychosis over the course of their illness. Pimavanserin is a selective serotonin inverse agonist which targets 5-HT2A serotonin receptors in the brain, decreasing their activity in order to attenuate hallucinations and delusions.
“Pimavanserin has been approved by the FDA [Food and Drug Administration] for Parkinson’s disease psychosis, but its safety has been called into question based on previous reports of increased mortality risk, compared with a rather modest benefit seen in a 6-week clinical trial, the duration of which limits determination of long-term safety,” wrote Dr. Ali.
Pimavanserin carries a boxed warning that elderly patients with dementia may be at an increased risk of death. After its approval in 2016, the U.S. FDA later reviewed 893 deaths in association with pimavanserin during the postmarketing surveillance period – “an unexpected number in a new drug,” Dr. Hwang and colleagues noted. “It [the FDA] noted that most reports occurred in a population with high underlying death rates and did not signal any additional risk beyond the current warning for all antipsychotics, which could have resulted in annual mortality rates of up to 60%.”
As the first cohort study to examine hospitalization and death between pimavanserin users and nonusers, “the study confirms previous concerns regarding safety of pimavanserin and more importantly brings to attention the importance of carefully considering risks and benefits of pharmacotherapy in Parkinson’s disease psychosis, clear communication with patients and families, and close observation to ensure safety,” wrote Dr. Ali.
The study limitations include its observational design, which subjected the findings to residual confounding.
“While we developed models to maximize the strength of causal inference, our comparison group was pimavanserin nonusers and the very reason for prescription of pimavanserin could have predisposed its users to the outcomes of hospitalization and death, introducing confounding by indication,” Dr. Hwang and colleagues wrote in the paper.
Additionally, “while robust analyses were conducted to ensure pimavanserin users and nonusers were comparable, Dr. Hwang et al. did find that pimavanserin users were more likely to concomitantly use other antipsychotic drugs which has been demonstrated as increasing the mortality risk,” Dr. Ali pointed out.
Since patients living in long-term care facilities may have a higher risk of mortality because of more severe or later-stage Parkinson’s disease, the study results “may not be generalizable to community-dwelling PD patients,” Dr. Ali wrote. “These factors are important to consider while making individual management decisions.”
Dr. Hwang and Dr. Ali disclosed no relevant financial relationships. The study authors reported no targeted funding.
FROM NEUROLOGY
Dance training ‘drastically’ reduces Parkinson’s progression, eases symptoms
Over 3 years, weekly participation in dance training classes “drastically” reduced the expected decline in motor function and significantly improved speech, tremors, balance, and stiffness, the researchers reported.
Dance training also appeared to have benefits regarding cognition, hallucinations, depression, and anxiety.
“These findings strongly suggest the benefits of dance for people with PD as a supplement to a normal treatment regimen,” the investigators noted.
Although the mechanism of benefit is unclear, dance training may help “train neural network nodes that helps either strengthen networks damaged or builds neural road maps that pass the damage,” study investigator Joseph DeSouza, PhD, principal investigator and associate professor, department of psychology, York University, Toronto, said in an interview.
The study was published online July 7, 2021, in Brain Sciences.
Multiple benefits
PD is a neurodegenerative disease associated with progression of motor dysfunction within the first 5 years of diagnosis. The annual rate of motor decline, as determined with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), is between 5.2 and 8.9 points.
Prior studies that assessed various styles of dance by patients with PD showed beneficial effects regarding gait speed, balance, locomotion, and aspects of quality of life.
To investigate further, DeSouza and coauthor Karolina Bearss, a PhD candidate at York University, followed 16 patients with mild to moderate PD who participated in a weekly dance class at Canada’s National Ballet School and Trinity St. Paul’s church.
Dance for Parkinson’s Disease, which is an established dance curriculum, involves aerobic and anaerobic movements. The protocol begins with a seated warm-up, followed by barre work, and ends with moving across the floor. All participants learn choreography for an upcoming performance.
In the study, 16 patients with PD who did not participant in the dance classes served as control patients.
Over 3 years, the daily rate of motor decline, as indicated by scores on part III of the MDS-UPDRS, was zero among the dancers (slope = 0.000146), indicating no motor impairment, whereas among the nondancers, the motor decline during follow-up was as expected (P < .01), the researchers reported.
In modeling the data, the researchers determined that after completing 1,000 days of dance training, dancers will have a motor score of 19.07, compared with a score of 28.27 for nondancers.
“Our data further showed that training in dance will slow the rate of PD motor impairment progression, as measured by the UPDRS III, by close to 3 points annually in comparison to our PD subjects who did not train,” the researchers reported.
Dance training also had a beneficial effect on motor or nonmotor aspects of daily living and on motor complications, for which there was no significant decline among the PD dancers.
“For those with Parkinson’s disease, even when it’s mild, motor impairment can impact their daily functioning – how they feel about themselves. Many of these motor symptoms lead to isolation because once they get extreme, these people don’t want to go out,” Dr. DeSouza said in a news release.
“These motor symptoms lead to further psychological issues, depression, social isolation and eventually the symptoms do get worse over time. Our study shows that training with dance and music can slow this down and improve their daily living and daily function,” he added.
‘Great potential’
Reached for comment, Demian Kogutek, PhD, director of music therapy, University of Evansville (Indiana), said that these preliminary findings from a longitudinal study are “promising.”
“I believe that dance therapy has a great potential for PD. The longitudinal aspect of this study undoubtedly adds to the current literature. Although it is a standardized assessment, it is somewhat subjective,” Dr. Kogutek said in an interview.
Going forward, Dr. Kogutek said he’d like to see other objective outcomes measured, such as objective assessments of balance, gait, hand strength, and dexterity.
Also weighing in on the results, Karen Lee, PhD, president and CEO of Parkinson Canada, said her organization is “encouraged by these preliminary findings as exercise and healthy activities are important for people with Parkinson’s. This study is part of a growing body of research that explores the link between the impact of activities and both motor and nonmotor symptoms of Parkinson’s.
“This research adds to growing evidence about the importance of exercise as part of the management of Parkinson’s, and we encourage people living with Parkinson’s to incorporate exercise as part of their approach to managing their health,” Dr. Lee said in an interview.
Funding for the project is provided in part by a National Science and Engineering Research Council Discovery Grant and by donations from the Irpinia Club of Toronto and others. Dr. Dr. DeSouza, Ms. Bearss, Dr. Kogutek, and Dr. Lee disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over 3 years, weekly participation in dance training classes “drastically” reduced the expected decline in motor function and significantly improved speech, tremors, balance, and stiffness, the researchers reported.
Dance training also appeared to have benefits regarding cognition, hallucinations, depression, and anxiety.
“These findings strongly suggest the benefits of dance for people with PD as a supplement to a normal treatment regimen,” the investigators noted.
Although the mechanism of benefit is unclear, dance training may help “train neural network nodes that helps either strengthen networks damaged or builds neural road maps that pass the damage,” study investigator Joseph DeSouza, PhD, principal investigator and associate professor, department of psychology, York University, Toronto, said in an interview.
The study was published online July 7, 2021, in Brain Sciences.
Multiple benefits
PD is a neurodegenerative disease associated with progression of motor dysfunction within the first 5 years of diagnosis. The annual rate of motor decline, as determined with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), is between 5.2 and 8.9 points.
Prior studies that assessed various styles of dance by patients with PD showed beneficial effects regarding gait speed, balance, locomotion, and aspects of quality of life.
To investigate further, DeSouza and coauthor Karolina Bearss, a PhD candidate at York University, followed 16 patients with mild to moderate PD who participated in a weekly dance class at Canada’s National Ballet School and Trinity St. Paul’s church.
Dance for Parkinson’s Disease, which is an established dance curriculum, involves aerobic and anaerobic movements. The protocol begins with a seated warm-up, followed by barre work, and ends with moving across the floor. All participants learn choreography for an upcoming performance.
In the study, 16 patients with PD who did not participant in the dance classes served as control patients.
Over 3 years, the daily rate of motor decline, as indicated by scores on part III of the MDS-UPDRS, was zero among the dancers (slope = 0.000146), indicating no motor impairment, whereas among the nondancers, the motor decline during follow-up was as expected (P < .01), the researchers reported.
In modeling the data, the researchers determined that after completing 1,000 days of dance training, dancers will have a motor score of 19.07, compared with a score of 28.27 for nondancers.
“Our data further showed that training in dance will slow the rate of PD motor impairment progression, as measured by the UPDRS III, by close to 3 points annually in comparison to our PD subjects who did not train,” the researchers reported.
Dance training also had a beneficial effect on motor or nonmotor aspects of daily living and on motor complications, for which there was no significant decline among the PD dancers.
“For those with Parkinson’s disease, even when it’s mild, motor impairment can impact their daily functioning – how they feel about themselves. Many of these motor symptoms lead to isolation because once they get extreme, these people don’t want to go out,” Dr. DeSouza said in a news release.
“These motor symptoms lead to further psychological issues, depression, social isolation and eventually the symptoms do get worse over time. Our study shows that training with dance and music can slow this down and improve their daily living and daily function,” he added.
‘Great potential’
Reached for comment, Demian Kogutek, PhD, director of music therapy, University of Evansville (Indiana), said that these preliminary findings from a longitudinal study are “promising.”
“I believe that dance therapy has a great potential for PD. The longitudinal aspect of this study undoubtedly adds to the current literature. Although it is a standardized assessment, it is somewhat subjective,” Dr. Kogutek said in an interview.
Going forward, Dr. Kogutek said he’d like to see other objective outcomes measured, such as objective assessments of balance, gait, hand strength, and dexterity.
Also weighing in on the results, Karen Lee, PhD, president and CEO of Parkinson Canada, said her organization is “encouraged by these preliminary findings as exercise and healthy activities are important for people with Parkinson’s. This study is part of a growing body of research that explores the link between the impact of activities and both motor and nonmotor symptoms of Parkinson’s.
“This research adds to growing evidence about the importance of exercise as part of the management of Parkinson’s, and we encourage people living with Parkinson’s to incorporate exercise as part of their approach to managing their health,” Dr. Lee said in an interview.
Funding for the project is provided in part by a National Science and Engineering Research Council Discovery Grant and by donations from the Irpinia Club of Toronto and others. Dr. Dr. DeSouza, Ms. Bearss, Dr. Kogutek, and Dr. Lee disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over 3 years, weekly participation in dance training classes “drastically” reduced the expected decline in motor function and significantly improved speech, tremors, balance, and stiffness, the researchers reported.
Dance training also appeared to have benefits regarding cognition, hallucinations, depression, and anxiety.
“These findings strongly suggest the benefits of dance for people with PD as a supplement to a normal treatment regimen,” the investigators noted.
Although the mechanism of benefit is unclear, dance training may help “train neural network nodes that helps either strengthen networks damaged or builds neural road maps that pass the damage,” study investigator Joseph DeSouza, PhD, principal investigator and associate professor, department of psychology, York University, Toronto, said in an interview.
The study was published online July 7, 2021, in Brain Sciences.
Multiple benefits
PD is a neurodegenerative disease associated with progression of motor dysfunction within the first 5 years of diagnosis. The annual rate of motor decline, as determined with the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), is between 5.2 and 8.9 points.
Prior studies that assessed various styles of dance by patients with PD showed beneficial effects regarding gait speed, balance, locomotion, and aspects of quality of life.
To investigate further, DeSouza and coauthor Karolina Bearss, a PhD candidate at York University, followed 16 patients with mild to moderate PD who participated in a weekly dance class at Canada’s National Ballet School and Trinity St. Paul’s church.
Dance for Parkinson’s Disease, which is an established dance curriculum, involves aerobic and anaerobic movements. The protocol begins with a seated warm-up, followed by barre work, and ends with moving across the floor. All participants learn choreography for an upcoming performance.
In the study, 16 patients with PD who did not participant in the dance classes served as control patients.
Over 3 years, the daily rate of motor decline, as indicated by scores on part III of the MDS-UPDRS, was zero among the dancers (slope = 0.000146), indicating no motor impairment, whereas among the nondancers, the motor decline during follow-up was as expected (P < .01), the researchers reported.
In modeling the data, the researchers determined that after completing 1,000 days of dance training, dancers will have a motor score of 19.07, compared with a score of 28.27 for nondancers.
“Our data further showed that training in dance will slow the rate of PD motor impairment progression, as measured by the UPDRS III, by close to 3 points annually in comparison to our PD subjects who did not train,” the researchers reported.
Dance training also had a beneficial effect on motor or nonmotor aspects of daily living and on motor complications, for which there was no significant decline among the PD dancers.
“For those with Parkinson’s disease, even when it’s mild, motor impairment can impact their daily functioning – how they feel about themselves. Many of these motor symptoms lead to isolation because once they get extreme, these people don’t want to go out,” Dr. DeSouza said in a news release.
“These motor symptoms lead to further psychological issues, depression, social isolation and eventually the symptoms do get worse over time. Our study shows that training with dance and music can slow this down and improve their daily living and daily function,” he added.
‘Great potential’
Reached for comment, Demian Kogutek, PhD, director of music therapy, University of Evansville (Indiana), said that these preliminary findings from a longitudinal study are “promising.”
“I believe that dance therapy has a great potential for PD. The longitudinal aspect of this study undoubtedly adds to the current literature. Although it is a standardized assessment, it is somewhat subjective,” Dr. Kogutek said in an interview.
Going forward, Dr. Kogutek said he’d like to see other objective outcomes measured, such as objective assessments of balance, gait, hand strength, and dexterity.
Also weighing in on the results, Karen Lee, PhD, president and CEO of Parkinson Canada, said her organization is “encouraged by these preliminary findings as exercise and healthy activities are important for people with Parkinson’s. This study is part of a growing body of research that explores the link between the impact of activities and both motor and nonmotor symptoms of Parkinson’s.
“This research adds to growing evidence about the importance of exercise as part of the management of Parkinson’s, and we encourage people living with Parkinson’s to incorporate exercise as part of their approach to managing their health,” Dr. Lee said in an interview.
Funding for the project is provided in part by a National Science and Engineering Research Council Discovery Grant and by donations from the Irpinia Club of Toronto and others. Dr. Dr. DeSouza, Ms. Bearss, Dr. Kogutek, and Dr. Lee disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Excessive drooling is a sign of greater dysfunction in patients with Parkinson’s disease
, new research shows. “Sialorrhea is not just a cosmetic problem,” study investigator Francesca Morgante, MD, associate professor of neurology, St. George’s University, London, told this news organization.
“We need to understand the relationship between sialorrhea and these speech and swallowing disturbances and whether treatment for sialorrhea improves that,” Dr. Morgante added.
The findings were presented at the 2021 Congress of the European Academy of Neurology.
Underrecognized symptom
Sialorrhea is an underrecognized nonmotor symptom that can affect up to 70% of patients with Parkinson’s disease, said co-investigator Ioana Cociasu, PhD, postdoctoral research fellow, Neurosciences Research Center, St. George’s University. The impact on quality of life increases with disease severity, she said.
The current study included 101 consecutive patients attending an advanced Parkinson’s disease disorders clinic. Researchers collected demographic data that included information on gender, age, age at Parkinson’s disease onset, and disease duration. They also gathered data on motor symptoms by assessing total levodopa equivalent daily dose (LEDD) and LEDD dopamine agonists. They also assessed results on the Unified Parkinson’s Disease Rating Scale (UPDRS) part III and the Hoehn and Yahr scale for on- and off-medication states.
Nonmotor functioning was assessed using the Non-Motor Symptoms Scale (NMSS) and Scales for Outcomes in Parkinson’s disease–autonomic dysfunction (SCOPA-AUT) questionnaire. Among patients with Parkinson’s disease, autonomic dysfunction can precede motor impairment and can involve orthostatic and postprandial hypotension, among other symptoms, the investigators noted.
Health status and quality of life were assessed using the Parkinson’s disease questionnaire–39 items (PDQ-39). The Radboud Oral Motor Inventory for PD (ROMP) was used to measure orofacial symptoms. ROMP is a self-administered questionnaire that evaluates speech, swallowing disturbances, and drooling of saliva. The Montreal Cognitive Assessment test was also used.
Investigators compared participants with sialorrhea to those without sialorrhea, described as droolers and nondroolers. Droolers were defined as those scoring higher than 1 on the UPDRS-II item 6. This signified slight but definite presence of saliva in the mouth and/or the possibility of nighttime drooling.
Greater impairment
Among the participants, 65 (64.4%) were classified as droolers, and 36 (35.6%) as nondroolers.
Patients with both Parkinson’s disease and sialorrhea were significantly more impaired in terms of motor functioning than those without sialorrhea. In these patients, the UPDRS-III was more severe in both the off- (P = .03) and on-states (P = .002), and they had less improvement with the levodopa challenge test (P = .007).
Droolers were also more severely affected by nonmotor problems. They had more severe speech dysfunction (P < .0001) and swallowing dysfunction (P < .05), and they had higher scores on the NMSS (P = .0008) and SCOPA-AUT (P = .003) and poorer quality-of-life scores on the PDQ-39 (P = .049).
To evaluate respiratory tract infections, the researchers used electronic health records. About 15.4% of the study population had had a documented respiratory infection since they were diagnosed with Parkinson’s disease.
Upper and lower respiratory tract infections were more frequent among droolers than nondroolers (P = .05).
“Infections might arise from swallowing disturbances leading to aspiration and drooling,” Dr. Morgante noted.
The drooling did not appear to affect cognition or sleep in these patients.
Treatment options?
Following the study presentation, session co-chair Philippe G. Damier, MD, PhD, professor of neurology, University Hospital, Nantes, France, asked about the best treatment for sialorrhea for these patients.
In general, those with milder disease might try chewing gum to improve swallowing; patients with more severe cases may benefit from botulinum toxin injections, said Dr. Cociasu. The treatment choice, she added, “very much depends on the severity of the sialorrhea.”
Botulinum toxin therapy involves injections into the salivary gland to reduce saliva production. It is typically administered about every 4 months.
The second session co-chair, Elena Moro, MD, PhD, director of the Movement Disorders Unit at Grenoble Alpes University, France, pointed out that chewing gum may be a swallowing hazard for patients with PD and severe dementia.
Asked by Dr. Moro whether patients with higher scores on balance and posture were more likely to have sialorrhea, Dr. Cociasu said she and her colleagues are currently looking into this.
Dr. Morgante said that the current study did not examine the effect of treatment on speech disorders associated with sialorrhea. “We are running another study now to understand the effect of treatment of sialorrhea on these features,” she said.
Dr. Morgante and Dr. Cociasu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. “Sialorrhea is not just a cosmetic problem,” study investigator Francesca Morgante, MD, associate professor of neurology, St. George’s University, London, told this news organization.
“We need to understand the relationship between sialorrhea and these speech and swallowing disturbances and whether treatment for sialorrhea improves that,” Dr. Morgante added.
The findings were presented at the 2021 Congress of the European Academy of Neurology.
Underrecognized symptom
Sialorrhea is an underrecognized nonmotor symptom that can affect up to 70% of patients with Parkinson’s disease, said co-investigator Ioana Cociasu, PhD, postdoctoral research fellow, Neurosciences Research Center, St. George’s University. The impact on quality of life increases with disease severity, she said.
The current study included 101 consecutive patients attending an advanced Parkinson’s disease disorders clinic. Researchers collected demographic data that included information on gender, age, age at Parkinson’s disease onset, and disease duration. They also gathered data on motor symptoms by assessing total levodopa equivalent daily dose (LEDD) and LEDD dopamine agonists. They also assessed results on the Unified Parkinson’s Disease Rating Scale (UPDRS) part III and the Hoehn and Yahr scale for on- and off-medication states.
Nonmotor functioning was assessed using the Non-Motor Symptoms Scale (NMSS) and Scales for Outcomes in Parkinson’s disease–autonomic dysfunction (SCOPA-AUT) questionnaire. Among patients with Parkinson’s disease, autonomic dysfunction can precede motor impairment and can involve orthostatic and postprandial hypotension, among other symptoms, the investigators noted.
Health status and quality of life were assessed using the Parkinson’s disease questionnaire–39 items (PDQ-39). The Radboud Oral Motor Inventory for PD (ROMP) was used to measure orofacial symptoms. ROMP is a self-administered questionnaire that evaluates speech, swallowing disturbances, and drooling of saliva. The Montreal Cognitive Assessment test was also used.
Investigators compared participants with sialorrhea to those without sialorrhea, described as droolers and nondroolers. Droolers were defined as those scoring higher than 1 on the UPDRS-II item 6. This signified slight but definite presence of saliva in the mouth and/or the possibility of nighttime drooling.
Greater impairment
Among the participants, 65 (64.4%) were classified as droolers, and 36 (35.6%) as nondroolers.
Patients with both Parkinson’s disease and sialorrhea were significantly more impaired in terms of motor functioning than those without sialorrhea. In these patients, the UPDRS-III was more severe in both the off- (P = .03) and on-states (P = .002), and they had less improvement with the levodopa challenge test (P = .007).
Droolers were also more severely affected by nonmotor problems. They had more severe speech dysfunction (P < .0001) and swallowing dysfunction (P < .05), and they had higher scores on the NMSS (P = .0008) and SCOPA-AUT (P = .003) and poorer quality-of-life scores on the PDQ-39 (P = .049).
To evaluate respiratory tract infections, the researchers used electronic health records. About 15.4% of the study population had had a documented respiratory infection since they were diagnosed with Parkinson’s disease.
Upper and lower respiratory tract infections were more frequent among droolers than nondroolers (P = .05).
“Infections might arise from swallowing disturbances leading to aspiration and drooling,” Dr. Morgante noted.
The drooling did not appear to affect cognition or sleep in these patients.
Treatment options?
Following the study presentation, session co-chair Philippe G. Damier, MD, PhD, professor of neurology, University Hospital, Nantes, France, asked about the best treatment for sialorrhea for these patients.
In general, those with milder disease might try chewing gum to improve swallowing; patients with more severe cases may benefit from botulinum toxin injections, said Dr. Cociasu. The treatment choice, she added, “very much depends on the severity of the sialorrhea.”
Botulinum toxin therapy involves injections into the salivary gland to reduce saliva production. It is typically administered about every 4 months.
The second session co-chair, Elena Moro, MD, PhD, director of the Movement Disorders Unit at Grenoble Alpes University, France, pointed out that chewing gum may be a swallowing hazard for patients with PD and severe dementia.
Asked by Dr. Moro whether patients with higher scores on balance and posture were more likely to have sialorrhea, Dr. Cociasu said she and her colleagues are currently looking into this.
Dr. Morgante said that the current study did not examine the effect of treatment on speech disorders associated with sialorrhea. “We are running another study now to understand the effect of treatment of sialorrhea on these features,” she said.
Dr. Morgante and Dr. Cociasu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. “Sialorrhea is not just a cosmetic problem,” study investigator Francesca Morgante, MD, associate professor of neurology, St. George’s University, London, told this news organization.
“We need to understand the relationship between sialorrhea and these speech and swallowing disturbances and whether treatment for sialorrhea improves that,” Dr. Morgante added.
The findings were presented at the 2021 Congress of the European Academy of Neurology.
Underrecognized symptom
Sialorrhea is an underrecognized nonmotor symptom that can affect up to 70% of patients with Parkinson’s disease, said co-investigator Ioana Cociasu, PhD, postdoctoral research fellow, Neurosciences Research Center, St. George’s University. The impact on quality of life increases with disease severity, she said.
The current study included 101 consecutive patients attending an advanced Parkinson’s disease disorders clinic. Researchers collected demographic data that included information on gender, age, age at Parkinson’s disease onset, and disease duration. They also gathered data on motor symptoms by assessing total levodopa equivalent daily dose (LEDD) and LEDD dopamine agonists. They also assessed results on the Unified Parkinson’s Disease Rating Scale (UPDRS) part III and the Hoehn and Yahr scale for on- and off-medication states.
Nonmotor functioning was assessed using the Non-Motor Symptoms Scale (NMSS) and Scales for Outcomes in Parkinson’s disease–autonomic dysfunction (SCOPA-AUT) questionnaire. Among patients with Parkinson’s disease, autonomic dysfunction can precede motor impairment and can involve orthostatic and postprandial hypotension, among other symptoms, the investigators noted.
Health status and quality of life were assessed using the Parkinson’s disease questionnaire–39 items (PDQ-39). The Radboud Oral Motor Inventory for PD (ROMP) was used to measure orofacial symptoms. ROMP is a self-administered questionnaire that evaluates speech, swallowing disturbances, and drooling of saliva. The Montreal Cognitive Assessment test was also used.
Investigators compared participants with sialorrhea to those without sialorrhea, described as droolers and nondroolers. Droolers were defined as those scoring higher than 1 on the UPDRS-II item 6. This signified slight but definite presence of saliva in the mouth and/or the possibility of nighttime drooling.
Greater impairment
Among the participants, 65 (64.4%) were classified as droolers, and 36 (35.6%) as nondroolers.
Patients with both Parkinson’s disease and sialorrhea were significantly more impaired in terms of motor functioning than those without sialorrhea. In these patients, the UPDRS-III was more severe in both the off- (P = .03) and on-states (P = .002), and they had less improvement with the levodopa challenge test (P = .007).
Droolers were also more severely affected by nonmotor problems. They had more severe speech dysfunction (P < .0001) and swallowing dysfunction (P < .05), and they had higher scores on the NMSS (P = .0008) and SCOPA-AUT (P = .003) and poorer quality-of-life scores on the PDQ-39 (P = .049).
To evaluate respiratory tract infections, the researchers used electronic health records. About 15.4% of the study population had had a documented respiratory infection since they were diagnosed with Parkinson’s disease.
Upper and lower respiratory tract infections were more frequent among droolers than nondroolers (P = .05).
“Infections might arise from swallowing disturbances leading to aspiration and drooling,” Dr. Morgante noted.
The drooling did not appear to affect cognition or sleep in these patients.
Treatment options?
Following the study presentation, session co-chair Philippe G. Damier, MD, PhD, professor of neurology, University Hospital, Nantes, France, asked about the best treatment for sialorrhea for these patients.
In general, those with milder disease might try chewing gum to improve swallowing; patients with more severe cases may benefit from botulinum toxin injections, said Dr. Cociasu. The treatment choice, she added, “very much depends on the severity of the sialorrhea.”
Botulinum toxin therapy involves injections into the salivary gland to reduce saliva production. It is typically administered about every 4 months.
The second session co-chair, Elena Moro, MD, PhD, director of the Movement Disorders Unit at Grenoble Alpes University, France, pointed out that chewing gum may be a swallowing hazard for patients with PD and severe dementia.
Asked by Dr. Moro whether patients with higher scores on balance and posture were more likely to have sialorrhea, Dr. Cociasu said she and her colleagues are currently looking into this.
Dr. Morgante said that the current study did not examine the effect of treatment on speech disorders associated with sialorrhea. “We are running another study now to understand the effect of treatment of sialorrhea on these features,” she said.
Dr. Morgante and Dr. Cociasu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From EAN 2021
Depression remains common among dystonia patients
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
FROM NEUROSCIENCE AND BIOBEHAVIORAL REVIEWS



