User login
Have you tried a progestin for your patient’s pelvic pain?
The date of the changeover to the 10th revision of International Classification of Diseases (ICD-10-CM) codes is incorrectly stated in the November 2011 Reimbursement Adviser, page 51. The date should be October 1, 2013.
To read the corrected version of this article, Click here
—The Editors
CASE
Your patient is a 26-year-old G0 woman who has a long history of progressively worsening dysmenorrhea, pelvic pain, and dyspareunia. In the recent past, she was treated with nonsteroidal anti-inflammatory drugs, a cyclic estrogen-progestin contraceptive, and a continuous estrogen-progestin contraceptive—in that order, and without appreciable relief of the pain.
Recently, the woman underwent laparoscopy, which demonstrated Stage-II endometriosis, which was ablated.
What would you prescribe for her postoperatively to alleviate symptoms?
Endometriosis will be diagnosed in approximately 8% of women of reproductive age.1 Pelvic pain, dysmenorrhea, and deep dyspareunia are common symptoms of endometriosis that interfere with quality of life.
Endometriosis is a chronic disease best managed by developing a life-long treatment plan. Following laparoscopic diagnosis and treatment, many experts strongly recommend postoperative hormone-suppressive therapy to reduce the risk that severe pelvic pain will recur, requiring re-operation.
Options for postoperative hormonal treatment of endometriosis include:
- an estrogen–progestin contraceptive
- a progestin (norethindrone acetate [NEA]; depot medroxyprogesterone acetate [DMPA]; oral medroxyprogesterone acetate; the levonorgestrel-releasing intrauterine system [LNG-IUS; Mirena]; and the progestin-releasing implant [Implanon])
- a gonadotropin-releasing hormone (GnRH) agonist (depot leuprolide [Depot Lupron]; nafarelin nasal spray [Synarel]).
CASE Continued
Considering that both cyclic and continuous estrogen-progestin contraceptives have already failed to provide adequate pain relief for your patient, you know that you should offer an alternative to her. Taking into account that progestins are significantly less costly than a GnRH agonist, a progestin formulation might, for her, be considered a first-line postoperative treatment of symptoms of endometriosis.
Options when considering a progestin
Norethindrone acetate
This agent is available in a single formulation: a 5-mg tablet; however, dosages ranging from 2.5 mg/d (half of a tablet) to 15 mg/d have been reported to be effective for relieving pain caused by endometriosis.
What is it? NEA is an androgenic progestin that suppresses luteinizing hormone and follicle-stimulating hormone, thus reducing production of ovarian estrogen. In the absence of ovarian estrogen, endometriosis lesions atrophy. In addition, NEA binds to, and stimulates, endometrial progestin and androgen receptors, resulting in decidualization and atrophy of both eutopic and ectopic endometrial tissue.
Importantly, NEA does not appear to cause bone loss, a phenomenon that is common with agents such as the GnRH agonists or DPMA.2-4
The research record. One randomized study, two pilot studies, and one large observational study have reported that NEA is effective for pelvic pain caused by endometriosis.
In the randomized trial, 90 women who had moderate or severe pelvic pain and rectovaginal endometriosis, and who remained symptomatic after conservative surgery, were randomized to receive NEA, 2.5 mg/d, or a low-dose estrogen-progestin contraceptive (ethinyl estradiol, 10 μg, plus cyproterone acetate, 3 mg) daily for 12 months.5 Both treatment groups reported significant and similar decreases in dysmenorrhea, deep dyspareunia, non-menstrual pain and dyschezia.
In a small pilot study, 40 women who had pelvic pain and colorectal endometriosis were treated with NEA 2.5 mg/d for 12 months. The drug produced significant improvement in dysmenorrhea, pelvic pain, deep dyspareunia, dyschezia, and cyclic rectal bleeding.6
In another pilot study, women who had pelvic pain and rectovaginal endometriosis were treated with either an aromatase inhibitor (letrozole, 2.5 mg/d) plus NEA (2.5 mg/d) or NEA (2.5 mg/d) alone for 6 months. Both treatments resulted in a significant improvement in pelvic pain and deep dyspareunia. Improvement in pain scores was greater with letrozole plus NEA; patients were more satisfied with NEA monotherapy than with the combined letrozole-NEA treatment, however, because the former was associated with fewer side effects.7
In a large (n=194) observational study of the postoperative use of NEA in young women with pelvic pain and endometriosis, NEA at dosages as high as 15 mg/d significantly diminished pelvic pain and self-reported menstrual bleeding. All subjects were started on a dosage of 5 mg/d, which was increased in 2.5-mg increments every 2 weeks to achieve the goals of amenorrhea and a lessening of pelvic pain; the maximum dosage administered was 15 mg/d. Mean duration of NEA use was 13 months; 75% of subjects took the maximum prescribed dosage of 15 mg at some point during treatment. The most commonly reported side effects were weight gain (16% of women); acne (10%); mood lability (9%); and vasomotor symptoms (8%).8
In summary. NEA is effective for treating pelvic pain caused by endometriosis at dosages from 2.5 mg/d to 15 mg/d. An important goal of treatment is a decrease in pain symptoms and amenorrhea; a dosage of 2.5 mg is often insufficient to reliably achieve both of those objectives.
In my practice I begin therapy at a dosage of 5 mg/d; the drug is effective for most patients at that dosage. If 5 mg/d does not reduce pain, I increase the dosage by 2.5 mg (half of a tablet) daily every 4 weeks, to a maximum dosage of 10 mg/d (two tablets). If that dosage is ineffective, I usually discontinue NEA and switch to a GnRH agonist.
Depot medroxyprogesterone acetate; oral medroxy-progesterone acetate
DMPA is available in two FDA-approved formulations:
- a 150-mg dose given by intramuscular injection every 3 months
- a 104-mg dose given by subcutaneous injection every 3 months.
Research. The results of two large clinical trials, comprising a total of more than 550 subjects, showed that DMPA (104 mg, SC, every 3 months) and depot leuprolide (11.25 mg, IM, every 3 months or 3.75 mg, monthly) were each equally effective in relieving dysmenorrhea, dyspareunia, pelvic pain, pelvic tenderness, and pelvic induration in women who had endometriosis.9,10
DMPA was associated with a greater rate of episodes of irregular bleeding than depot leuprolide; conversely, depot leuprolide was associated with greater loss of bone density and a higher incidence of vasomotor symptoms. Weight gain was in the range of 0.6 kg in both groups.
Of note, DPMA is much less expensive than depot leuprolide.
Another study showed that increasing the dosage of DMPA did not improve efficacy over the standard dosage11: DMPA, 150 mg IM, monthly, and DMPA, 150 mg IM, every 3 months produced similar relief of pelvic pain.
Oral medroxyprogesterone acetate, prescribed at high dosages, is also effective for pelvic pain caused by endometriosis. In a pilot study (n=21), oral MPA, 50 mg/d for 4 months, alleviated dysmenorrhea, dyspareunia, pelvic pain, dyschezia, and pelvic tenderness and decreased pelvic nodularity. Sixty percent of subjects reported weight gain— 1.5 kg, on average.12
Progestin-releasing devices: Mirena and Implanon
Many pilot studies have reported that the levonorgestrel-releasing intrauterine system (LNG-IUS) is effective for pelvic pain caused by endometriosis.13-17 For example:
Research. In a small clinical trial, 30 women who had pelvic pain and endometriosis were randomized to receive an LNG-IUS (Mirena) or DMPA, 150 mg IM, every 3 months for 3 years.13 Both therapies were effective at reducing pelvic pain.
At the conclusion of the study, more women opted to retain the LNG-IUS (87%) than to continue DMPA injection (47%). Bone density was maintained in women who had the LNG-IUS placed but slightly diminished in women receiving DMPA.
In a pilot study of an etonogestrel releasing implant (Implanon), 41 women who had pelvic pain and endometriosis were randomized to receive the implant or DMPA, 150 mg IM, every 3 months for 1 year.18 Both therapies were similarly effective at reducing pelvic pain.
Notably, irregular uterine bleeding is a common problem when the etonogestrel-releasing implant is used to treat endometriosis. Achieving amenorrhea or oligomenorrhea is an important goal for women who suffer from pelvic pain caused by endometriosis.
My recommendation
Most ObGyns see patients who are suffering from difficult-to-treat pelvic pain caused by endometriosis. Many of these patients have not had a trial of a progestin, such as NEA, DMPA, or the LNG-IUS that I use in my practice.
Progestins are, as I’ve described, effective for pelvic pain. They are also relatively inexpensive and have a side-effect profile that most patients find acceptable. I recommend that you try a progestin for your patients who have refractory pelvic pain.
What is your preferred hormone treatment for women with unrelieved pelvic pain from endometriosis?
1. Missmer SA, Hankinson S, Spiegelman D, et al. The incidence of laparoscopically confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160(8):784-796.
2. Abdalla HI, Hart DM, Lindsay R, Leggate I, Hooke A. Prevention of bone mineral loss in postmenopausal women by norethisterone. Obstet Gynecol. 1985;66(6):789-792.
3. Riss BJ, Lehmann HJ, Christiansen C. Norethisterone acetate in combination with estrogen: effects on the skeleton and other organs. Am J Obstet Gynecol. 2002;187(4):1101-1116.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-back Study Group. Obstet Gynecol. 1998;91(1):16-24.
5. Vercellini P, Pietropauolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
6. Ferrero S, Camerini G, Ragni N, Venturini PL, Biscaldi E, Remorgida V. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25(1):94-100.
7. Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod. 2009;24(12):3033-3341.
8. Kaser DJ, Missmer SA, Berry KF, Laufer MR. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms [published online ahead of print December 9 2011]. J Pediatr Adolesc Gynecol. doi:10.1016/j.jpag.2011.09.013.
9. Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis associated pain. Fertil Steril. 2006;85(2):314-325.
10. Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod. 2006;21(1):248-256.
11. Cheewadhanaraks S, Peeyananjarassri K, Choksuchat C, Dhanaworavibul K, Choobun T, Bunyapipat S. Interval of injections of intramuscular depot medroxyprogesterone acetate in the long-term treatment of endometriosis-associated pain: a randomized clinical trial. Gynecol Obstet Invest. 2009;68(2):116-121.
12. Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 Pt 1):323-327.
13. Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomized controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273-279.
14. Lockhat FB, Emembolu JO, Konje JC. The efficacy side-effects and continuation rates in women with symptomatic endometriosis undergoing treatment with an intrauterine administered progestogen (levonorgestrel): a 3 year follow-up. Hum Reprod. 2005;20(3):789-793.
15. Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005;20(7):1993-1998.
16. Vercellini P, Aimi G, Panazza S, De Giorgi O, Pesole A, Crosignani PG. A levonorgestrel-releasing intrauterine system for the treatment of dysmenorrhea associated with endometriosis: a pilot study. Fertil Steril. 1999;72(3):505-508.
17. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
18. Walch K, Unfried G, Huber J, Kurz C, van Trotsenburg M, Pernicka E, Wenzl R. Implanon versus medroxyprogesterone acetate: effects on pain scores in patients with symptomatic endometriosis—a pilot study. Contraception. 2009;79(1):29-34.
The date of the changeover to the 10th revision of International Classification of Diseases (ICD-10-CM) codes is incorrectly stated in the November 2011 Reimbursement Adviser, page 51. The date should be October 1, 2013.
To read the corrected version of this article, Click here
—The Editors
CASE
Your patient is a 26-year-old G0 woman who has a long history of progressively worsening dysmenorrhea, pelvic pain, and dyspareunia. In the recent past, she was treated with nonsteroidal anti-inflammatory drugs, a cyclic estrogen-progestin contraceptive, and a continuous estrogen-progestin contraceptive—in that order, and without appreciable relief of the pain.
Recently, the woman underwent laparoscopy, which demonstrated Stage-II endometriosis, which was ablated.
What would you prescribe for her postoperatively to alleviate symptoms?
Endometriosis will be diagnosed in approximately 8% of women of reproductive age.1 Pelvic pain, dysmenorrhea, and deep dyspareunia are common symptoms of endometriosis that interfere with quality of life.
Endometriosis is a chronic disease best managed by developing a life-long treatment plan. Following laparoscopic diagnosis and treatment, many experts strongly recommend postoperative hormone-suppressive therapy to reduce the risk that severe pelvic pain will recur, requiring re-operation.
Options for postoperative hormonal treatment of endometriosis include:
- an estrogen–progestin contraceptive
- a progestin (norethindrone acetate [NEA]; depot medroxyprogesterone acetate [DMPA]; oral medroxyprogesterone acetate; the levonorgestrel-releasing intrauterine system [LNG-IUS; Mirena]; and the progestin-releasing implant [Implanon])
- a gonadotropin-releasing hormone (GnRH) agonist (depot leuprolide [Depot Lupron]; nafarelin nasal spray [Synarel]).
CASE Continued
Considering that both cyclic and continuous estrogen-progestin contraceptives have already failed to provide adequate pain relief for your patient, you know that you should offer an alternative to her. Taking into account that progestins are significantly less costly than a GnRH agonist, a progestin formulation might, for her, be considered a first-line postoperative treatment of symptoms of endometriosis.
Options when considering a progestin
Norethindrone acetate
This agent is available in a single formulation: a 5-mg tablet; however, dosages ranging from 2.5 mg/d (half of a tablet) to 15 mg/d have been reported to be effective for relieving pain caused by endometriosis.
What is it? NEA is an androgenic progestin that suppresses luteinizing hormone and follicle-stimulating hormone, thus reducing production of ovarian estrogen. In the absence of ovarian estrogen, endometriosis lesions atrophy. In addition, NEA binds to, and stimulates, endometrial progestin and androgen receptors, resulting in decidualization and atrophy of both eutopic and ectopic endometrial tissue.
Importantly, NEA does not appear to cause bone loss, a phenomenon that is common with agents such as the GnRH agonists or DPMA.2-4
The research record. One randomized study, two pilot studies, and one large observational study have reported that NEA is effective for pelvic pain caused by endometriosis.
In the randomized trial, 90 women who had moderate or severe pelvic pain and rectovaginal endometriosis, and who remained symptomatic after conservative surgery, were randomized to receive NEA, 2.5 mg/d, or a low-dose estrogen-progestin contraceptive (ethinyl estradiol, 10 μg, plus cyproterone acetate, 3 mg) daily for 12 months.5 Both treatment groups reported significant and similar decreases in dysmenorrhea, deep dyspareunia, non-menstrual pain and dyschezia.
In a small pilot study, 40 women who had pelvic pain and colorectal endometriosis were treated with NEA 2.5 mg/d for 12 months. The drug produced significant improvement in dysmenorrhea, pelvic pain, deep dyspareunia, dyschezia, and cyclic rectal bleeding.6
In another pilot study, women who had pelvic pain and rectovaginal endometriosis were treated with either an aromatase inhibitor (letrozole, 2.5 mg/d) plus NEA (2.5 mg/d) or NEA (2.5 mg/d) alone for 6 months. Both treatments resulted in a significant improvement in pelvic pain and deep dyspareunia. Improvement in pain scores was greater with letrozole plus NEA; patients were more satisfied with NEA monotherapy than with the combined letrozole-NEA treatment, however, because the former was associated with fewer side effects.7
In a large (n=194) observational study of the postoperative use of NEA in young women with pelvic pain and endometriosis, NEA at dosages as high as 15 mg/d significantly diminished pelvic pain and self-reported menstrual bleeding. All subjects were started on a dosage of 5 mg/d, which was increased in 2.5-mg increments every 2 weeks to achieve the goals of amenorrhea and a lessening of pelvic pain; the maximum dosage administered was 15 mg/d. Mean duration of NEA use was 13 months; 75% of subjects took the maximum prescribed dosage of 15 mg at some point during treatment. The most commonly reported side effects were weight gain (16% of women); acne (10%); mood lability (9%); and vasomotor symptoms (8%).8
In summary. NEA is effective for treating pelvic pain caused by endometriosis at dosages from 2.5 mg/d to 15 mg/d. An important goal of treatment is a decrease in pain symptoms and amenorrhea; a dosage of 2.5 mg is often insufficient to reliably achieve both of those objectives.
In my practice I begin therapy at a dosage of 5 mg/d; the drug is effective for most patients at that dosage. If 5 mg/d does not reduce pain, I increase the dosage by 2.5 mg (half of a tablet) daily every 4 weeks, to a maximum dosage of 10 mg/d (two tablets). If that dosage is ineffective, I usually discontinue NEA and switch to a GnRH agonist.
Depot medroxyprogesterone acetate; oral medroxy-progesterone acetate
DMPA is available in two FDA-approved formulations:
- a 150-mg dose given by intramuscular injection every 3 months
- a 104-mg dose given by subcutaneous injection every 3 months.
Research. The results of two large clinical trials, comprising a total of more than 550 subjects, showed that DMPA (104 mg, SC, every 3 months) and depot leuprolide (11.25 mg, IM, every 3 months or 3.75 mg, monthly) were each equally effective in relieving dysmenorrhea, dyspareunia, pelvic pain, pelvic tenderness, and pelvic induration in women who had endometriosis.9,10
DMPA was associated with a greater rate of episodes of irregular bleeding than depot leuprolide; conversely, depot leuprolide was associated with greater loss of bone density and a higher incidence of vasomotor symptoms. Weight gain was in the range of 0.6 kg in both groups.
Of note, DPMA is much less expensive than depot leuprolide.
Another study showed that increasing the dosage of DMPA did not improve efficacy over the standard dosage11: DMPA, 150 mg IM, monthly, and DMPA, 150 mg IM, every 3 months produced similar relief of pelvic pain.
Oral medroxyprogesterone acetate, prescribed at high dosages, is also effective for pelvic pain caused by endometriosis. In a pilot study (n=21), oral MPA, 50 mg/d for 4 months, alleviated dysmenorrhea, dyspareunia, pelvic pain, dyschezia, and pelvic tenderness and decreased pelvic nodularity. Sixty percent of subjects reported weight gain— 1.5 kg, on average.12
Progestin-releasing devices: Mirena and Implanon
Many pilot studies have reported that the levonorgestrel-releasing intrauterine system (LNG-IUS) is effective for pelvic pain caused by endometriosis.13-17 For example:
Research. In a small clinical trial, 30 women who had pelvic pain and endometriosis were randomized to receive an LNG-IUS (Mirena) or DMPA, 150 mg IM, every 3 months for 3 years.13 Both therapies were effective at reducing pelvic pain.
At the conclusion of the study, more women opted to retain the LNG-IUS (87%) than to continue DMPA injection (47%). Bone density was maintained in women who had the LNG-IUS placed but slightly diminished in women receiving DMPA.
In a pilot study of an etonogestrel releasing implant (Implanon), 41 women who had pelvic pain and endometriosis were randomized to receive the implant or DMPA, 150 mg IM, every 3 months for 1 year.18 Both therapies were similarly effective at reducing pelvic pain.
Notably, irregular uterine bleeding is a common problem when the etonogestrel-releasing implant is used to treat endometriosis. Achieving amenorrhea or oligomenorrhea is an important goal for women who suffer from pelvic pain caused by endometriosis.
My recommendation
Most ObGyns see patients who are suffering from difficult-to-treat pelvic pain caused by endometriosis. Many of these patients have not had a trial of a progestin, such as NEA, DMPA, or the LNG-IUS that I use in my practice.
Progestins are, as I’ve described, effective for pelvic pain. They are also relatively inexpensive and have a side-effect profile that most patients find acceptable. I recommend that you try a progestin for your patients who have refractory pelvic pain.
What is your preferred hormone treatment for women with unrelieved pelvic pain from endometriosis?
The date of the changeover to the 10th revision of International Classification of Diseases (ICD-10-CM) codes is incorrectly stated in the November 2011 Reimbursement Adviser, page 51. The date should be October 1, 2013.
To read the corrected version of this article, Click here
—The Editors
CASE
Your patient is a 26-year-old G0 woman who has a long history of progressively worsening dysmenorrhea, pelvic pain, and dyspareunia. In the recent past, she was treated with nonsteroidal anti-inflammatory drugs, a cyclic estrogen-progestin contraceptive, and a continuous estrogen-progestin contraceptive—in that order, and without appreciable relief of the pain.
Recently, the woman underwent laparoscopy, which demonstrated Stage-II endometriosis, which was ablated.
What would you prescribe for her postoperatively to alleviate symptoms?
Endometriosis will be diagnosed in approximately 8% of women of reproductive age.1 Pelvic pain, dysmenorrhea, and deep dyspareunia are common symptoms of endometriosis that interfere with quality of life.
Endometriosis is a chronic disease best managed by developing a life-long treatment plan. Following laparoscopic diagnosis and treatment, many experts strongly recommend postoperative hormone-suppressive therapy to reduce the risk that severe pelvic pain will recur, requiring re-operation.
Options for postoperative hormonal treatment of endometriosis include:
- an estrogen–progestin contraceptive
- a progestin (norethindrone acetate [NEA]; depot medroxyprogesterone acetate [DMPA]; oral medroxyprogesterone acetate; the levonorgestrel-releasing intrauterine system [LNG-IUS; Mirena]; and the progestin-releasing implant [Implanon])
- a gonadotropin-releasing hormone (GnRH) agonist (depot leuprolide [Depot Lupron]; nafarelin nasal spray [Synarel]).
CASE Continued
Considering that both cyclic and continuous estrogen-progestin contraceptives have already failed to provide adequate pain relief for your patient, you know that you should offer an alternative to her. Taking into account that progestins are significantly less costly than a GnRH agonist, a progestin formulation might, for her, be considered a first-line postoperative treatment of symptoms of endometriosis.
Options when considering a progestin
Norethindrone acetate
This agent is available in a single formulation: a 5-mg tablet; however, dosages ranging from 2.5 mg/d (half of a tablet) to 15 mg/d have been reported to be effective for relieving pain caused by endometriosis.
What is it? NEA is an androgenic progestin that suppresses luteinizing hormone and follicle-stimulating hormone, thus reducing production of ovarian estrogen. In the absence of ovarian estrogen, endometriosis lesions atrophy. In addition, NEA binds to, and stimulates, endometrial progestin and androgen receptors, resulting in decidualization and atrophy of both eutopic and ectopic endometrial tissue.
Importantly, NEA does not appear to cause bone loss, a phenomenon that is common with agents such as the GnRH agonists or DPMA.2-4
The research record. One randomized study, two pilot studies, and one large observational study have reported that NEA is effective for pelvic pain caused by endometriosis.
In the randomized trial, 90 women who had moderate or severe pelvic pain and rectovaginal endometriosis, and who remained symptomatic after conservative surgery, were randomized to receive NEA, 2.5 mg/d, or a low-dose estrogen-progestin contraceptive (ethinyl estradiol, 10 μg, plus cyproterone acetate, 3 mg) daily for 12 months.5 Both treatment groups reported significant and similar decreases in dysmenorrhea, deep dyspareunia, non-menstrual pain and dyschezia.
In a small pilot study, 40 women who had pelvic pain and colorectal endometriosis were treated with NEA 2.5 mg/d for 12 months. The drug produced significant improvement in dysmenorrhea, pelvic pain, deep dyspareunia, dyschezia, and cyclic rectal bleeding.6
In another pilot study, women who had pelvic pain and rectovaginal endometriosis were treated with either an aromatase inhibitor (letrozole, 2.5 mg/d) plus NEA (2.5 mg/d) or NEA (2.5 mg/d) alone for 6 months. Both treatments resulted in a significant improvement in pelvic pain and deep dyspareunia. Improvement in pain scores was greater with letrozole plus NEA; patients were more satisfied with NEA monotherapy than with the combined letrozole-NEA treatment, however, because the former was associated with fewer side effects.7
In a large (n=194) observational study of the postoperative use of NEA in young women with pelvic pain and endometriosis, NEA at dosages as high as 15 mg/d significantly diminished pelvic pain and self-reported menstrual bleeding. All subjects were started on a dosage of 5 mg/d, which was increased in 2.5-mg increments every 2 weeks to achieve the goals of amenorrhea and a lessening of pelvic pain; the maximum dosage administered was 15 mg/d. Mean duration of NEA use was 13 months; 75% of subjects took the maximum prescribed dosage of 15 mg at some point during treatment. The most commonly reported side effects were weight gain (16% of women); acne (10%); mood lability (9%); and vasomotor symptoms (8%).8
In summary. NEA is effective for treating pelvic pain caused by endometriosis at dosages from 2.5 mg/d to 15 mg/d. An important goal of treatment is a decrease in pain symptoms and amenorrhea; a dosage of 2.5 mg is often insufficient to reliably achieve both of those objectives.
In my practice I begin therapy at a dosage of 5 mg/d; the drug is effective for most patients at that dosage. If 5 mg/d does not reduce pain, I increase the dosage by 2.5 mg (half of a tablet) daily every 4 weeks, to a maximum dosage of 10 mg/d (two tablets). If that dosage is ineffective, I usually discontinue NEA and switch to a GnRH agonist.
Depot medroxyprogesterone acetate; oral medroxy-progesterone acetate
DMPA is available in two FDA-approved formulations:
- a 150-mg dose given by intramuscular injection every 3 months
- a 104-mg dose given by subcutaneous injection every 3 months.
Research. The results of two large clinical trials, comprising a total of more than 550 subjects, showed that DMPA (104 mg, SC, every 3 months) and depot leuprolide (11.25 mg, IM, every 3 months or 3.75 mg, monthly) were each equally effective in relieving dysmenorrhea, dyspareunia, pelvic pain, pelvic tenderness, and pelvic induration in women who had endometriosis.9,10
DMPA was associated with a greater rate of episodes of irregular bleeding than depot leuprolide; conversely, depot leuprolide was associated with greater loss of bone density and a higher incidence of vasomotor symptoms. Weight gain was in the range of 0.6 kg in both groups.
Of note, DPMA is much less expensive than depot leuprolide.
Another study showed that increasing the dosage of DMPA did not improve efficacy over the standard dosage11: DMPA, 150 mg IM, monthly, and DMPA, 150 mg IM, every 3 months produced similar relief of pelvic pain.
Oral medroxyprogesterone acetate, prescribed at high dosages, is also effective for pelvic pain caused by endometriosis. In a pilot study (n=21), oral MPA, 50 mg/d for 4 months, alleviated dysmenorrhea, dyspareunia, pelvic pain, dyschezia, and pelvic tenderness and decreased pelvic nodularity. Sixty percent of subjects reported weight gain— 1.5 kg, on average.12
Progestin-releasing devices: Mirena and Implanon
Many pilot studies have reported that the levonorgestrel-releasing intrauterine system (LNG-IUS) is effective for pelvic pain caused by endometriosis.13-17 For example:
Research. In a small clinical trial, 30 women who had pelvic pain and endometriosis were randomized to receive an LNG-IUS (Mirena) or DMPA, 150 mg IM, every 3 months for 3 years.13 Both therapies were effective at reducing pelvic pain.
At the conclusion of the study, more women opted to retain the LNG-IUS (87%) than to continue DMPA injection (47%). Bone density was maintained in women who had the LNG-IUS placed but slightly diminished in women receiving DMPA.
In a pilot study of an etonogestrel releasing implant (Implanon), 41 women who had pelvic pain and endometriosis were randomized to receive the implant or DMPA, 150 mg IM, every 3 months for 1 year.18 Both therapies were similarly effective at reducing pelvic pain.
Notably, irregular uterine bleeding is a common problem when the etonogestrel-releasing implant is used to treat endometriosis. Achieving amenorrhea or oligomenorrhea is an important goal for women who suffer from pelvic pain caused by endometriosis.
My recommendation
Most ObGyns see patients who are suffering from difficult-to-treat pelvic pain caused by endometriosis. Many of these patients have not had a trial of a progestin, such as NEA, DMPA, or the LNG-IUS that I use in my practice.
Progestins are, as I’ve described, effective for pelvic pain. They are also relatively inexpensive and have a side-effect profile that most patients find acceptable. I recommend that you try a progestin for your patients who have refractory pelvic pain.
What is your preferred hormone treatment for women with unrelieved pelvic pain from endometriosis?
1. Missmer SA, Hankinson S, Spiegelman D, et al. The incidence of laparoscopically confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160(8):784-796.
2. Abdalla HI, Hart DM, Lindsay R, Leggate I, Hooke A. Prevention of bone mineral loss in postmenopausal women by norethisterone. Obstet Gynecol. 1985;66(6):789-792.
3. Riss BJ, Lehmann HJ, Christiansen C. Norethisterone acetate in combination with estrogen: effects on the skeleton and other organs. Am J Obstet Gynecol. 2002;187(4):1101-1116.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-back Study Group. Obstet Gynecol. 1998;91(1):16-24.
5. Vercellini P, Pietropauolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
6. Ferrero S, Camerini G, Ragni N, Venturini PL, Biscaldi E, Remorgida V. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25(1):94-100.
7. Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod. 2009;24(12):3033-3341.
8. Kaser DJ, Missmer SA, Berry KF, Laufer MR. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms [published online ahead of print December 9 2011]. J Pediatr Adolesc Gynecol. doi:10.1016/j.jpag.2011.09.013.
9. Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis associated pain. Fertil Steril. 2006;85(2):314-325.
10. Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod. 2006;21(1):248-256.
11. Cheewadhanaraks S, Peeyananjarassri K, Choksuchat C, Dhanaworavibul K, Choobun T, Bunyapipat S. Interval of injections of intramuscular depot medroxyprogesterone acetate in the long-term treatment of endometriosis-associated pain: a randomized clinical trial. Gynecol Obstet Invest. 2009;68(2):116-121.
12. Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 Pt 1):323-327.
13. Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomized controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273-279.
14. Lockhat FB, Emembolu JO, Konje JC. The efficacy side-effects and continuation rates in women with symptomatic endometriosis undergoing treatment with an intrauterine administered progestogen (levonorgestrel): a 3 year follow-up. Hum Reprod. 2005;20(3):789-793.
15. Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005;20(7):1993-1998.
16. Vercellini P, Aimi G, Panazza S, De Giorgi O, Pesole A, Crosignani PG. A levonorgestrel-releasing intrauterine system for the treatment of dysmenorrhea associated with endometriosis: a pilot study. Fertil Steril. 1999;72(3):505-508.
17. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
18. Walch K, Unfried G, Huber J, Kurz C, van Trotsenburg M, Pernicka E, Wenzl R. Implanon versus medroxyprogesterone acetate: effects on pain scores in patients with symptomatic endometriosis—a pilot study. Contraception. 2009;79(1):29-34.
1. Missmer SA, Hankinson S, Spiegelman D, et al. The incidence of laparoscopically confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160(8):784-796.
2. Abdalla HI, Hart DM, Lindsay R, Leggate I, Hooke A. Prevention of bone mineral loss in postmenopausal women by norethisterone. Obstet Gynecol. 1985;66(6):789-792.
3. Riss BJ, Lehmann HJ, Christiansen C. Norethisterone acetate in combination with estrogen: effects on the skeleton and other organs. Am J Obstet Gynecol. 2002;187(4):1101-1116.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-back Study Group. Obstet Gynecol. 1998;91(1):16-24.
5. Vercellini P, Pietropauolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
6. Ferrero S, Camerini G, Ragni N, Venturini PL, Biscaldi E, Remorgida V. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25(1):94-100.
7. Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod. 2009;24(12):3033-3341.
8. Kaser DJ, Missmer SA, Berry KF, Laufer MR. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms [published online ahead of print December 9 2011]. J Pediatr Adolesc Gynecol. doi:10.1016/j.jpag.2011.09.013.
9. Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis associated pain. Fertil Steril. 2006;85(2):314-325.
10. Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod. 2006;21(1):248-256.
11. Cheewadhanaraks S, Peeyananjarassri K, Choksuchat C, Dhanaworavibul K, Choobun T, Bunyapipat S. Interval of injections of intramuscular depot medroxyprogesterone acetate in the long-term treatment of endometriosis-associated pain: a randomized clinical trial. Gynecol Obstet Invest. 2009;68(2):116-121.
12. Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 Pt 1):323-327.
13. Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomized controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273-279.
14. Lockhat FB, Emembolu JO, Konje JC. The efficacy side-effects and continuation rates in women with symptomatic endometriosis undergoing treatment with an intrauterine administered progestogen (levonorgestrel): a 3 year follow-up. Hum Reprod. 2005;20(3):789-793.
15. Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005;20(7):1993-1998.
16. Vercellini P, Aimi G, Panazza S, De Giorgi O, Pesole A, Crosignani PG. A levonorgestrel-releasing intrauterine system for the treatment of dysmenorrhea associated with endometriosis: a pilot study. Fertil Steril. 1999;72(3):505-508.
17. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
18. Walch K, Unfried G, Huber J, Kurz C, van Trotsenburg M, Pernicka E, Wenzl R. Implanon versus medroxyprogesterone acetate: effects on pain scores in patients with symptomatic endometriosis—a pilot study. Contraception. 2009;79(1):29-34.
UPDATE ON PELVIC FLOOR DYSFUNCTION
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.
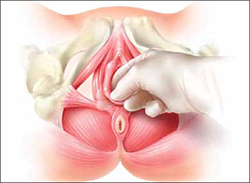
FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.
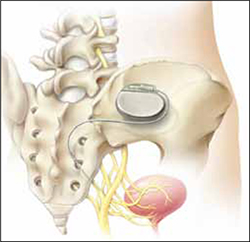
FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.

FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.

FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.

FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.

FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.
UPDATE: PELVIC FLOOR DYSFUNCTION
When a woman has advanced prolapse of the anterior vaginal wall, it is highly likely that she has apical prolapse as well. Consider a study by Rooney and associates that determined that clinically significant vault prolapse is present in most women who have anterior vaginal prolapse of stage II or higher.1 For that reason, suspension of the vaginal apex should be considered whenever surgical treatment of anterior wall defects is planned.
Sacrocolpopexy involves suspension of the vaginal vault from the anterior longitudinal ligament of the sacrum, using Y-shaped mesh to augment native tissue (FIGURE).2 It is an effective, durable treatment for vaginal apical prolapse. With a success rate approaching 93%, this procedure has become the gold standard for repair of vault prolapse. Among its advantages are maximization of vaginal depth and preservation of a normal vaginal axis.
Sacrocolpopexy preserves the vaginal axis
With the vaginal vault suspended from the anterior longitudinal
ligament of the sacrum, the normal vaginal axis is preserved
and vaginal depth is maximized.
Sacrocolpopexy can be performed via the abdominal, laparoscopic, or robotic-assisted approach (TABLE 1). Minimally invasive techniques are attractive because they involve faster recovery than abdominal sacrocolpopexy does. Minimally invasive techniques have also advanced to the point that they are both effective and durable. However, these advantages must be weighed against the effort required to learn the techniques, as well as their higher cost.
TABLE 1
How the 3 approaches to sacrocolpopexy compare
| Approach | Advantages and disadvantages |
|---|---|
| Abdominal | Shortest operative time No significant Trendelenburg position required Highest estimated blood loss Longest length of stay Low rate of complications Longest postoperative recovery Well-established long-term durability |
| Laparoscopic | Longer operative time Moderate Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique least similar to abdominal procedure Low rate of complications Shorter postoperative recovery Long-term durability less firmly established |
| Robotic-assisted | Longest operative time Steep Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique resembles that of abdominal approach Low rate of complications Shorter postoperative recovery Long-term durability appears to be good |
In this article, we highlight:
- a comparison of the laparoscopic and abdominal approaches to sacrocolpopexy
- an investigation of the learning curve associated with robotic-assisted sacrocolpopexy
- a study exploring the durability of robotic-assisted repair
- an estimate of the costs associated with each route of operation.
Laparoscopic vs abdominal sacrocolpopexy—how do they compare?
Paraiso MF, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol. 2005;192(5):1752–1758.
When surgeons at the Cleveland Clinic performed a retrospective cohort study to compare laparoscopic and abdominal sacrocolpopexy, they found significantly longer operative time with the laparoscopic route, with an average difference of 51 minutes (P < .0001). However, the laparoscopic approach was associated with lower blood loss (although there was no difference between groups in hematocrit on postoperative day 1); shorter hospital stay (average of 1.8 days versus 4 days [P < .001]); and comparable rates of intraoperative and postoperative complications.
Details of the trial
Paraiso and colleagues reviewed the medical charts of 56 consecutive patients who had undergone laparoscopic sacrocolpopexy, comparing them with the charts of 61 consecutive patients who had undergone the procedure using the abdominal approach. The operations had been performed between 1998 and 2003 for treatment of posthysterectomy vaginal prolapse.
The groups underwent similar rates of concurrent procedures. The laparotomy group had a significantly higher number of Burch procedures (P = .007), and the laparoscopic group had a significantly higher rate of adhesiolysis (P = .002).
Among the complications noted— which occurred at comparable rates between groups—were cystotomy, enterotomy, need for transfusion, deep-vein thrombosis, ileus, small bowel obstruction, wound infection, ventral hernia, mesh erosion, and recurrent prolapse. One laparoscopic case was converted to laparotomy because of excessive bleeding during the rectopexy portion of the operation.
Laparoscopy may have taken longer than this trial suggests
This study is one of very few well-designed trials comparing laparoscopic sacrocolpopexy to the historical gold standard of abdominal sacrocolpopexy for vault prolapse.
Twenty-eight percent of laparoscopic procedures in this study used tacking devices in lieu of suturing. Had suturing been performed universally, an even greater difference in surgical time may have been observed.
There may also be differences between groups in the durability of the two types of repair, an outcome not included in this particular study.
The laparoscopic approach offers a shorter hospital stay with no increase in intraoperative or postoperative complications, compared with abdominal sacrocolpopexy. However, it entails a significantly longer operative time than the abdominal approach does.
How steep is the learning curve for robotic-assisted sacrocolpopexy?
Akl MN, Long JB, Giles DL, et al. Robotic-assisted sacrocolpopexy: technique and learning curve. Surg Endosc. 2009;23(10):2390–2394.
Akl and coworkers reviewed the medical records of all patients who had undergone robotic-assisted sacrocolpopexy at the Mayo Clinics in Arizona and Florida between 2004 and 2007. All operations were performed by the same four urogynecologists, with an average operative time of 197.9 minutes (standard deviation, ± 66.8 minutes). However, after the first 10 cases, the operative time decreased by 64.3 minutes—a decline of 25.4% (P < .01; 95% confidence interval [CI], 16.1–112.4 minutes).
Details of the trial
Researchers collected baseline information on participants’ age, stage of prolapse, and concomitant procedures. They also gathered data on average operative time, estimated blood loss, intraoperative and postoperative complications, conversion to laparotomy, and length of hospitalization.
Of 80 women who had advanced pelvic organ prolapse (stage III/IV) who underwent robotic-assisted sacrocolpopexy, 88% underwent concomitant robotic and vaginal procedures, including robotic supracervical hysterectomy, Burch procedure, paravaginal repair, lysis of adhesions, bilateral salpingooophorectomy, vaginal cystocele or rectocele repair, and placement of a midurethral sling.
Estimated blood loss for the robotic-assisted approach ranged from 25 mL to 300 mL, with a mean loss of 96.8 mL. Average length of hospitalization was 2.6 days. Four cases (5%) were converted to laparotomy because of limited exposure and one intraoperative bladder injury. Other intraoperative complications included small-bowel injury during trocar placement and one ureteral injury. Postoperative complications included one case of ileus and five (6%) vaginal mesh erosions. Three patients developed recurrent prolapse and underwent subsequent correction.
Learning curve could have been measured more precisely
The authors did not specifically measure the learning curve for robotic-assisted sacrocolpopexy, as they took into account the concomitant procedures. For this reason, the decrease in operative time observed after 10 cases may not accurately reflect an improvement in the performance of sacrocolpopexy.
Akl and colleagues consider this detail to be a strength of the study because most women who undergo prolapse surgery have concomitant procedures. However, recording the length of time it took to perform the sacrocolpopexy portion of the procedure would have been more accurate.
The average length of stay approached that of the abdominal route. Length of stay may decline as a surgeon gains experience with the robotic-assisted approach.
Robotic-assisted sacrocolpopexy has a steep learning curve with respect to technique and surgical time.
Does robotic-assisted sacrocolpopexy provide durable support?
Elliott DS, Krambeck AE, Chow GK. Long-term results of robotic assisted laparoscopic sacrocolpopexy for the treatment of high grade vaginal vault prolapse. J Urol. 2006;176(2):655–659.
Among the few recent series reporting long-term outcomes after robotic-assisted sacrocolpopexy is this observational study from the Mayo Clinic. It involved 30 women who underwent the operation for the treatment of Baden Walker grade 4/4 posthysterectomy vaginal vault prolapse. The authors concluded that advanced prolapse can be treated with robotic-assisted sacrocolpopexy with long-term success and minimal complications.
Details of the trial
Of 30 women in this trial, 52% underwent an anti-incontinence procedure at the time of sacrocolpopexy. Women who had multiple vaginal defects or a history of abdominal surgery were excluded from the study.
Average operative time was 3.1 hours (range, 2.15–4.75 hours) in the early phase of development of operative technique (described in the manuscript) but diminished over time to an average of 2.5 hours.
Twenty-nine patients were discharged from the hospital after an overnight stay. Very few immediate postoperative complications were observed. Two patients experienced mild port-site infections that required outpatient treatment, and one patient had persistent vaginal bleeding from the incision made during the anti-incontinence procedure.
Most patients were followed for at least 1 year
The mean follow-up in this study was 24 months (range, 16–39 months). During this period, 21 women were followed for a full year. Long-term observation revealed that the repair of vault prolapse remained successful in 19 of these women.
One patient experienced recurrent prolapse 7 months after surgery. Another developed a rectocele 9 months after sacrocolpopexy. Vaginal mesh erosions occurred in two patients within 6 months after the procedure; both patients were treated with outpatient resection of the exposed mesh, with no recurrence of the prolapse.
Although a larger sample size and longer follow-up would be ideal, this study demonstrates a low rate of recurrent prolapse 1 year after the procedure.
Robotic sacrocolpopexy appears to provide long-term durability for the treatment of advanced vaginal vault prolapse.
Depending on where you practice, you may have as many as three options: abdominal, laparoscopic, or robotic-assisted. Here are basic questions you should address when choosing one:
- How familiar are you with the technique? if the answer is “not much,” you can anticipate that the cost and time required to perform it will be significantly higher.
- Are the appropriate instruments and surgical team available?
- Does the patient have comorbidities? Consider, for example, the fact that she may not be able to tolerate a steep Trendelenberg position—required for the robotic-assisted approach—if she has severe cardiac or pulmonary disease. However, if she has a risk of poor wound healing, a large abdominal incision may not be advisable and postoperative immobility can be risky. if she is obese, laparoscopic or robotic port placement is challenging, but visualization and retraction will be easier. The need for anticoagulation is another consideration, as it will affect estimated blood loss and the choice of an incision, among other things.
- Let’s not forget the patient. Given the pros and cons, what approach does she prefer?
How much do laparoscopic, abdominal, and robotic-assisted sacrocolpopexy cost?
Judd JP, Siddiqui NY, Barnett JC, et al. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim Invasive Gynecol. 2010;17: 493–499.
This cost-minimization analysis concluded that robotic-assisted sacrocolpopexy incurs the highest hospital charges but is reimbursed by Medicare at a rate similar to reimbursement for the abdominal and laparoscopic routes (TABLE 2).
TABLE 2
Cost of sacrocolpopexy is significant—especially using the robotic approach
| Approach | Cost of a procedure | Operative time, min (range) |
|---|---|---|
| Robotic-assisted | $8,508 | 328 (130–383) |
| Laparoscopic | $7,353 | 269 (97–334) |
| Abdominal | $5,792 | 170 (110–286) |
| Source: Judd JP, Siddiqui NY, Barnett JC, Visco AG, Havrilesky LJ, Wu JM. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim invasive Gynecol. 2010;17(4):493–499. | ||
The analysis accounted for realistic practices, such as the inclusion of concurrent hysterectomy and other procedures.
Details of the trial
Surgeons from Duke University developed a decision-analysis model in which a hypothetical group of women with advanced vaginal prolapse could choose between one of the three routes of sacrocolpopexy: abdominal, laparoscopic, or robotic-assisted. Researchers postulated two different scenarios:
- the hospital had ownership of a robotic system
- the hospital invested in the initial purchase and maintenance of such a system.
Researchers reviewed the literature to formulate their estimates of operative time, rate of conversion to laparotomy, rate of transfusion, and length of hospital stay. In addition, the costs of initial anesthesia setup, professional fees, per-minute intraoperative fees, and postanesthesia care were applied to each approach. Operating room costs per minute and the cost of disposable items such as drapes, gowns, gloves, and single-use instruments were added. For the robotic approach, the costs of reusable instruments were distributed across 10 operations. Reusable instruments for laparoscopic and abdominal surgery were assumed to incur no additional investment. Last, postoperative care—including laboratory tests, pharmacy usage, and the need for a hospital room—were individualized for each route of surgery and applied to the cost.
Costs were estimated in 2008 US dollars, based on procedure costs incurred at Duke University Medical Center.
Physician reimbursement data were obtained from Medicare reimbursement rates for anesthesia and from surgeon Current Procedural Terminology (CPT) codes specific to each procedure.
Quality-of-life assessments were not measured. Nor was the cost to society of the postoperative loss of productivity and wages for each surgical route. Had these losses been recognized, the authors observed, the cost of robotic surgery may have been lower.
The cost of robotic surgery was equivalent to the cost of laparoscopy in only two instances:
- when the operative time of robotic surgery was reduced to 149 minutes
- when the cost of robotic disposable items was less than $2,132 (reduced from a baseline cost of $3,293).
Robotic sacrocolpopexy is costly. this is an important consideration when implementing new technology. cost-saving scenarios are useful to maximize patient benefit and minimize financial burden.
We want to hear from you! Tell us what you think.
When a woman has advanced prolapse of the anterior vaginal wall, it is highly likely that she has apical prolapse as well. Consider a study by Rooney and associates that determined that clinically significant vault prolapse is present in most women who have anterior vaginal prolapse of stage II or higher.1 For that reason, suspension of the vaginal apex should be considered whenever surgical treatment of anterior wall defects is planned.
Sacrocolpopexy involves suspension of the vaginal vault from the anterior longitudinal ligament of the sacrum, using Y-shaped mesh to augment native tissue (FIGURE).2 It is an effective, durable treatment for vaginal apical prolapse. With a success rate approaching 93%, this procedure has become the gold standard for repair of vault prolapse. Among its advantages are maximization of vaginal depth and preservation of a normal vaginal axis.
Sacrocolpopexy preserves the vaginal axis

With the vaginal vault suspended from the anterior longitudinal
ligament of the sacrum, the normal vaginal axis is preserved
and vaginal depth is maximized.
Sacrocolpopexy can be performed via the abdominal, laparoscopic, or robotic-assisted approach (TABLE 1). Minimally invasive techniques are attractive because they involve faster recovery than abdominal sacrocolpopexy does. Minimally invasive techniques have also advanced to the point that they are both effective and durable. However, these advantages must be weighed against the effort required to learn the techniques, as well as their higher cost.
TABLE 1
How the 3 approaches to sacrocolpopexy compare
| Approach | Advantages and disadvantages |
|---|---|
| Abdominal | Shortest operative time No significant Trendelenburg position required Highest estimated blood loss Longest length of stay Low rate of complications Longest postoperative recovery Well-established long-term durability |
| Laparoscopic | Longer operative time Moderate Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique least similar to abdominal procedure Low rate of complications Shorter postoperative recovery Long-term durability less firmly established |
| Robotic-assisted | Longest operative time Steep Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique resembles that of abdominal approach Low rate of complications Shorter postoperative recovery Long-term durability appears to be good |
In this article, we highlight:
- a comparison of the laparoscopic and abdominal approaches to sacrocolpopexy
- an investigation of the learning curve associated with robotic-assisted sacrocolpopexy
- a study exploring the durability of robotic-assisted repair
- an estimate of the costs associated with each route of operation.
Laparoscopic vs abdominal sacrocolpopexy—how do they compare?
Paraiso MF, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol. 2005;192(5):1752–1758.
When surgeons at the Cleveland Clinic performed a retrospective cohort study to compare laparoscopic and abdominal sacrocolpopexy, they found significantly longer operative time with the laparoscopic route, with an average difference of 51 minutes (P < .0001). However, the laparoscopic approach was associated with lower blood loss (although there was no difference between groups in hematocrit on postoperative day 1); shorter hospital stay (average of 1.8 days versus 4 days [P < .001]); and comparable rates of intraoperative and postoperative complications.
Details of the trial
Paraiso and colleagues reviewed the medical charts of 56 consecutive patients who had undergone laparoscopic sacrocolpopexy, comparing them with the charts of 61 consecutive patients who had undergone the procedure using the abdominal approach. The operations had been performed between 1998 and 2003 for treatment of posthysterectomy vaginal prolapse.
The groups underwent similar rates of concurrent procedures. The laparotomy group had a significantly higher number of Burch procedures (P = .007), and the laparoscopic group had a significantly higher rate of adhesiolysis (P = .002).
Among the complications noted— which occurred at comparable rates between groups—were cystotomy, enterotomy, need for transfusion, deep-vein thrombosis, ileus, small bowel obstruction, wound infection, ventral hernia, mesh erosion, and recurrent prolapse. One laparoscopic case was converted to laparotomy because of excessive bleeding during the rectopexy portion of the operation.
Laparoscopy may have taken longer than this trial suggests
This study is one of very few well-designed trials comparing laparoscopic sacrocolpopexy to the historical gold standard of abdominal sacrocolpopexy for vault prolapse.
Twenty-eight percent of laparoscopic procedures in this study used tacking devices in lieu of suturing. Had suturing been performed universally, an even greater difference in surgical time may have been observed.
There may also be differences between groups in the durability of the two types of repair, an outcome not included in this particular study.
The laparoscopic approach offers a shorter hospital stay with no increase in intraoperative or postoperative complications, compared with abdominal sacrocolpopexy. However, it entails a significantly longer operative time than the abdominal approach does.
How steep is the learning curve for robotic-assisted sacrocolpopexy?
Akl MN, Long JB, Giles DL, et al. Robotic-assisted sacrocolpopexy: technique and learning curve. Surg Endosc. 2009;23(10):2390–2394.
Akl and coworkers reviewed the medical records of all patients who had undergone robotic-assisted sacrocolpopexy at the Mayo Clinics in Arizona and Florida between 2004 and 2007. All operations were performed by the same four urogynecologists, with an average operative time of 197.9 minutes (standard deviation, ± 66.8 minutes). However, after the first 10 cases, the operative time decreased by 64.3 minutes—a decline of 25.4% (P < .01; 95% confidence interval [CI], 16.1–112.4 minutes).
Details of the trial
Researchers collected baseline information on participants’ age, stage of prolapse, and concomitant procedures. They also gathered data on average operative time, estimated blood loss, intraoperative and postoperative complications, conversion to laparotomy, and length of hospitalization.
Of 80 women who had advanced pelvic organ prolapse (stage III/IV) who underwent robotic-assisted sacrocolpopexy, 88% underwent concomitant robotic and vaginal procedures, including robotic supracervical hysterectomy, Burch procedure, paravaginal repair, lysis of adhesions, bilateral salpingooophorectomy, vaginal cystocele or rectocele repair, and placement of a midurethral sling.
Estimated blood loss for the robotic-assisted approach ranged from 25 mL to 300 mL, with a mean loss of 96.8 mL. Average length of hospitalization was 2.6 days. Four cases (5%) were converted to laparotomy because of limited exposure and one intraoperative bladder injury. Other intraoperative complications included small-bowel injury during trocar placement and one ureteral injury. Postoperative complications included one case of ileus and five (6%) vaginal mesh erosions. Three patients developed recurrent prolapse and underwent subsequent correction.
Learning curve could have been measured more precisely
The authors did not specifically measure the learning curve for robotic-assisted sacrocolpopexy, as they took into account the concomitant procedures. For this reason, the decrease in operative time observed after 10 cases may not accurately reflect an improvement in the performance of sacrocolpopexy.
Akl and colleagues consider this detail to be a strength of the study because most women who undergo prolapse surgery have concomitant procedures. However, recording the length of time it took to perform the sacrocolpopexy portion of the procedure would have been more accurate.
The average length of stay approached that of the abdominal route. Length of stay may decline as a surgeon gains experience with the robotic-assisted approach.
Robotic-assisted sacrocolpopexy has a steep learning curve with respect to technique and surgical time.
Does robotic-assisted sacrocolpopexy provide durable support?
Elliott DS, Krambeck AE, Chow GK. Long-term results of robotic assisted laparoscopic sacrocolpopexy for the treatment of high grade vaginal vault prolapse. J Urol. 2006;176(2):655–659.
Among the few recent series reporting long-term outcomes after robotic-assisted sacrocolpopexy is this observational study from the Mayo Clinic. It involved 30 women who underwent the operation for the treatment of Baden Walker grade 4/4 posthysterectomy vaginal vault prolapse. The authors concluded that advanced prolapse can be treated with robotic-assisted sacrocolpopexy with long-term success and minimal complications.
Details of the trial
Of 30 women in this trial, 52% underwent an anti-incontinence procedure at the time of sacrocolpopexy. Women who had multiple vaginal defects or a history of abdominal surgery were excluded from the study.
Average operative time was 3.1 hours (range, 2.15–4.75 hours) in the early phase of development of operative technique (described in the manuscript) but diminished over time to an average of 2.5 hours.
Twenty-nine patients were discharged from the hospital after an overnight stay. Very few immediate postoperative complications were observed. Two patients experienced mild port-site infections that required outpatient treatment, and one patient had persistent vaginal bleeding from the incision made during the anti-incontinence procedure.
Most patients were followed for at least 1 year
The mean follow-up in this study was 24 months (range, 16–39 months). During this period, 21 women were followed for a full year. Long-term observation revealed that the repair of vault prolapse remained successful in 19 of these women.
One patient experienced recurrent prolapse 7 months after surgery. Another developed a rectocele 9 months after sacrocolpopexy. Vaginal mesh erosions occurred in two patients within 6 months after the procedure; both patients were treated with outpatient resection of the exposed mesh, with no recurrence of the prolapse.
Although a larger sample size and longer follow-up would be ideal, this study demonstrates a low rate of recurrent prolapse 1 year after the procedure.
Robotic sacrocolpopexy appears to provide long-term durability for the treatment of advanced vaginal vault prolapse.
Depending on where you practice, you may have as many as three options: abdominal, laparoscopic, or robotic-assisted. Here are basic questions you should address when choosing one:
- How familiar are you with the technique? if the answer is “not much,” you can anticipate that the cost and time required to perform it will be significantly higher.
- Are the appropriate instruments and surgical team available?
- Does the patient have comorbidities? Consider, for example, the fact that she may not be able to tolerate a steep Trendelenberg position—required for the robotic-assisted approach—if she has severe cardiac or pulmonary disease. However, if she has a risk of poor wound healing, a large abdominal incision may not be advisable and postoperative immobility can be risky. if she is obese, laparoscopic or robotic port placement is challenging, but visualization and retraction will be easier. The need for anticoagulation is another consideration, as it will affect estimated blood loss and the choice of an incision, among other things.
- Let’s not forget the patient. Given the pros and cons, what approach does she prefer?
How much do laparoscopic, abdominal, and robotic-assisted sacrocolpopexy cost?
Judd JP, Siddiqui NY, Barnett JC, et al. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim Invasive Gynecol. 2010;17: 493–499.
This cost-minimization analysis concluded that robotic-assisted sacrocolpopexy incurs the highest hospital charges but is reimbursed by Medicare at a rate similar to reimbursement for the abdominal and laparoscopic routes (TABLE 2).
TABLE 2
Cost of sacrocolpopexy is significant—especially using the robotic approach
| Approach | Cost of a procedure | Operative time, min (range) |
|---|---|---|
| Robotic-assisted | $8,508 | 328 (130–383) |
| Laparoscopic | $7,353 | 269 (97–334) |
| Abdominal | $5,792 | 170 (110–286) |
| Source: Judd JP, Siddiqui NY, Barnett JC, Visco AG, Havrilesky LJ, Wu JM. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim invasive Gynecol. 2010;17(4):493–499. | ||
The analysis accounted for realistic practices, such as the inclusion of concurrent hysterectomy and other procedures.
Details of the trial
Surgeons from Duke University developed a decision-analysis model in which a hypothetical group of women with advanced vaginal prolapse could choose between one of the three routes of sacrocolpopexy: abdominal, laparoscopic, or robotic-assisted. Researchers postulated two different scenarios:
- the hospital had ownership of a robotic system
- the hospital invested in the initial purchase and maintenance of such a system.
Researchers reviewed the literature to formulate their estimates of operative time, rate of conversion to laparotomy, rate of transfusion, and length of hospital stay. In addition, the costs of initial anesthesia setup, professional fees, per-minute intraoperative fees, and postanesthesia care were applied to each approach. Operating room costs per minute and the cost of disposable items such as drapes, gowns, gloves, and single-use instruments were added. For the robotic approach, the costs of reusable instruments were distributed across 10 operations. Reusable instruments for laparoscopic and abdominal surgery were assumed to incur no additional investment. Last, postoperative care—including laboratory tests, pharmacy usage, and the need for a hospital room—were individualized for each route of surgery and applied to the cost.
Costs were estimated in 2008 US dollars, based on procedure costs incurred at Duke University Medical Center.
Physician reimbursement data were obtained from Medicare reimbursement rates for anesthesia and from surgeon Current Procedural Terminology (CPT) codes specific to each procedure.
Quality-of-life assessments were not measured. Nor was the cost to society of the postoperative loss of productivity and wages for each surgical route. Had these losses been recognized, the authors observed, the cost of robotic surgery may have been lower.
The cost of robotic surgery was equivalent to the cost of laparoscopy in only two instances:
- when the operative time of robotic surgery was reduced to 149 minutes
- when the cost of robotic disposable items was less than $2,132 (reduced from a baseline cost of $3,293).
Robotic sacrocolpopexy is costly. this is an important consideration when implementing new technology. cost-saving scenarios are useful to maximize patient benefit and minimize financial burden.
We want to hear from you! Tell us what you think.
When a woman has advanced prolapse of the anterior vaginal wall, it is highly likely that she has apical prolapse as well. Consider a study by Rooney and associates that determined that clinically significant vault prolapse is present in most women who have anterior vaginal prolapse of stage II or higher.1 For that reason, suspension of the vaginal apex should be considered whenever surgical treatment of anterior wall defects is planned.
Sacrocolpopexy involves suspension of the vaginal vault from the anterior longitudinal ligament of the sacrum, using Y-shaped mesh to augment native tissue (FIGURE).2 It is an effective, durable treatment for vaginal apical prolapse. With a success rate approaching 93%, this procedure has become the gold standard for repair of vault prolapse. Among its advantages are maximization of vaginal depth and preservation of a normal vaginal axis.
Sacrocolpopexy preserves the vaginal axis

With the vaginal vault suspended from the anterior longitudinal
ligament of the sacrum, the normal vaginal axis is preserved
and vaginal depth is maximized.
Sacrocolpopexy can be performed via the abdominal, laparoscopic, or robotic-assisted approach (TABLE 1). Minimally invasive techniques are attractive because they involve faster recovery than abdominal sacrocolpopexy does. Minimally invasive techniques have also advanced to the point that they are both effective and durable. However, these advantages must be weighed against the effort required to learn the techniques, as well as their higher cost.
TABLE 1
How the 3 approaches to sacrocolpopexy compare
| Approach | Advantages and disadvantages |
|---|---|
| Abdominal | Shortest operative time No significant Trendelenburg position required Highest estimated blood loss Longest length of stay Low rate of complications Longest postoperative recovery Well-established long-term durability |
| Laparoscopic | Longer operative time Moderate Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique least similar to abdominal procedure Low rate of complications Shorter postoperative recovery Long-term durability less firmly established |
| Robotic-assisted | Longest operative time Steep Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique resembles that of abdominal approach Low rate of complications Shorter postoperative recovery Long-term durability appears to be good |
In this article, we highlight:
- a comparison of the laparoscopic and abdominal approaches to sacrocolpopexy
- an investigation of the learning curve associated with robotic-assisted sacrocolpopexy
- a study exploring the durability of robotic-assisted repair
- an estimate of the costs associated with each route of operation.
Laparoscopic vs abdominal sacrocolpopexy—how do they compare?
Paraiso MF, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol. 2005;192(5):1752–1758.
When surgeons at the Cleveland Clinic performed a retrospective cohort study to compare laparoscopic and abdominal sacrocolpopexy, they found significantly longer operative time with the laparoscopic route, with an average difference of 51 minutes (P < .0001). However, the laparoscopic approach was associated with lower blood loss (although there was no difference between groups in hematocrit on postoperative day 1); shorter hospital stay (average of 1.8 days versus 4 days [P < .001]); and comparable rates of intraoperative and postoperative complications.
Details of the trial
Paraiso and colleagues reviewed the medical charts of 56 consecutive patients who had undergone laparoscopic sacrocolpopexy, comparing them with the charts of 61 consecutive patients who had undergone the procedure using the abdominal approach. The operations had been performed between 1998 and 2003 for treatment of posthysterectomy vaginal prolapse.
The groups underwent similar rates of concurrent procedures. The laparotomy group had a significantly higher number of Burch procedures (P = .007), and the laparoscopic group had a significantly higher rate of adhesiolysis (P = .002).
Among the complications noted— which occurred at comparable rates between groups—were cystotomy, enterotomy, need for transfusion, deep-vein thrombosis, ileus, small bowel obstruction, wound infection, ventral hernia, mesh erosion, and recurrent prolapse. One laparoscopic case was converted to laparotomy because of excessive bleeding during the rectopexy portion of the operation.
Laparoscopy may have taken longer than this trial suggests
This study is one of very few well-designed trials comparing laparoscopic sacrocolpopexy to the historical gold standard of abdominal sacrocolpopexy for vault prolapse.
Twenty-eight percent of laparoscopic procedures in this study used tacking devices in lieu of suturing. Had suturing been performed universally, an even greater difference in surgical time may have been observed.
There may also be differences between groups in the durability of the two types of repair, an outcome not included in this particular study.
The laparoscopic approach offers a shorter hospital stay with no increase in intraoperative or postoperative complications, compared with abdominal sacrocolpopexy. However, it entails a significantly longer operative time than the abdominal approach does.
How steep is the learning curve for robotic-assisted sacrocolpopexy?
Akl MN, Long JB, Giles DL, et al. Robotic-assisted sacrocolpopexy: technique and learning curve. Surg Endosc. 2009;23(10):2390–2394.
Akl and coworkers reviewed the medical records of all patients who had undergone robotic-assisted sacrocolpopexy at the Mayo Clinics in Arizona and Florida between 2004 and 2007. All operations were performed by the same four urogynecologists, with an average operative time of 197.9 minutes (standard deviation, ± 66.8 minutes). However, after the first 10 cases, the operative time decreased by 64.3 minutes—a decline of 25.4% (P < .01; 95% confidence interval [CI], 16.1–112.4 minutes).
Details of the trial
Researchers collected baseline information on participants’ age, stage of prolapse, and concomitant procedures. They also gathered data on average operative time, estimated blood loss, intraoperative and postoperative complications, conversion to laparotomy, and length of hospitalization.
Of 80 women who had advanced pelvic organ prolapse (stage III/IV) who underwent robotic-assisted sacrocolpopexy, 88% underwent concomitant robotic and vaginal procedures, including robotic supracervical hysterectomy, Burch procedure, paravaginal repair, lysis of adhesions, bilateral salpingooophorectomy, vaginal cystocele or rectocele repair, and placement of a midurethral sling.
Estimated blood loss for the robotic-assisted approach ranged from 25 mL to 300 mL, with a mean loss of 96.8 mL. Average length of hospitalization was 2.6 days. Four cases (5%) were converted to laparotomy because of limited exposure and one intraoperative bladder injury. Other intraoperative complications included small-bowel injury during trocar placement and one ureteral injury. Postoperative complications included one case of ileus and five (6%) vaginal mesh erosions. Three patients developed recurrent prolapse and underwent subsequent correction.
Learning curve could have been measured more precisely
The authors did not specifically measure the learning curve for robotic-assisted sacrocolpopexy, as they took into account the concomitant procedures. For this reason, the decrease in operative time observed after 10 cases may not accurately reflect an improvement in the performance of sacrocolpopexy.
Akl and colleagues consider this detail to be a strength of the study because most women who undergo prolapse surgery have concomitant procedures. However, recording the length of time it took to perform the sacrocolpopexy portion of the procedure would have been more accurate.
The average length of stay approached that of the abdominal route. Length of stay may decline as a surgeon gains experience with the robotic-assisted approach.
Robotic-assisted sacrocolpopexy has a steep learning curve with respect to technique and surgical time.
Does robotic-assisted sacrocolpopexy provide durable support?
Elliott DS, Krambeck AE, Chow GK. Long-term results of robotic assisted laparoscopic sacrocolpopexy for the treatment of high grade vaginal vault prolapse. J Urol. 2006;176(2):655–659.
Among the few recent series reporting long-term outcomes after robotic-assisted sacrocolpopexy is this observational study from the Mayo Clinic. It involved 30 women who underwent the operation for the treatment of Baden Walker grade 4/4 posthysterectomy vaginal vault prolapse. The authors concluded that advanced prolapse can be treated with robotic-assisted sacrocolpopexy with long-term success and minimal complications.
Details of the trial
Of 30 women in this trial, 52% underwent an anti-incontinence procedure at the time of sacrocolpopexy. Women who had multiple vaginal defects or a history of abdominal surgery were excluded from the study.
Average operative time was 3.1 hours (range, 2.15–4.75 hours) in the early phase of development of operative technique (described in the manuscript) but diminished over time to an average of 2.5 hours.
Twenty-nine patients were discharged from the hospital after an overnight stay. Very few immediate postoperative complications were observed. Two patients experienced mild port-site infections that required outpatient treatment, and one patient had persistent vaginal bleeding from the incision made during the anti-incontinence procedure.
Most patients were followed for at least 1 year
The mean follow-up in this study was 24 months (range, 16–39 months). During this period, 21 women were followed for a full year. Long-term observation revealed that the repair of vault prolapse remained successful in 19 of these women.
One patient experienced recurrent prolapse 7 months after surgery. Another developed a rectocele 9 months after sacrocolpopexy. Vaginal mesh erosions occurred in two patients within 6 months after the procedure; both patients were treated with outpatient resection of the exposed mesh, with no recurrence of the prolapse.
Although a larger sample size and longer follow-up would be ideal, this study demonstrates a low rate of recurrent prolapse 1 year after the procedure.
Robotic sacrocolpopexy appears to provide long-term durability for the treatment of advanced vaginal vault prolapse.
Depending on where you practice, you may have as many as three options: abdominal, laparoscopic, or robotic-assisted. Here are basic questions you should address when choosing one:
- How familiar are you with the technique? if the answer is “not much,” you can anticipate that the cost and time required to perform it will be significantly higher.
- Are the appropriate instruments and surgical team available?
- Does the patient have comorbidities? Consider, for example, the fact that she may not be able to tolerate a steep Trendelenberg position—required for the robotic-assisted approach—if she has severe cardiac or pulmonary disease. However, if she has a risk of poor wound healing, a large abdominal incision may not be advisable and postoperative immobility can be risky. if she is obese, laparoscopic or robotic port placement is challenging, but visualization and retraction will be easier. The need for anticoagulation is another consideration, as it will affect estimated blood loss and the choice of an incision, among other things.
- Let’s not forget the patient. Given the pros and cons, what approach does she prefer?
How much do laparoscopic, abdominal, and robotic-assisted sacrocolpopexy cost?
Judd JP, Siddiqui NY, Barnett JC, et al. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim Invasive Gynecol. 2010;17: 493–499.
This cost-minimization analysis concluded that robotic-assisted sacrocolpopexy incurs the highest hospital charges but is reimbursed by Medicare at a rate similar to reimbursement for the abdominal and laparoscopic routes (TABLE 2).
TABLE 2
Cost of sacrocolpopexy is significant—especially using the robotic approach
| Approach | Cost of a procedure | Operative time, min (range) |
|---|---|---|
| Robotic-assisted | $8,508 | 328 (130–383) |
| Laparoscopic | $7,353 | 269 (97–334) |
| Abdominal | $5,792 | 170 (110–286) |
| Source: Judd JP, Siddiqui NY, Barnett JC, Visco AG, Havrilesky LJ, Wu JM. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim invasive Gynecol. 2010;17(4):493–499. | ||
The analysis accounted for realistic practices, such as the inclusion of concurrent hysterectomy and other procedures.
Details of the trial
Surgeons from Duke University developed a decision-analysis model in which a hypothetical group of women with advanced vaginal prolapse could choose between one of the three routes of sacrocolpopexy: abdominal, laparoscopic, or robotic-assisted. Researchers postulated two different scenarios:
- the hospital had ownership of a robotic system
- the hospital invested in the initial purchase and maintenance of such a system.
Researchers reviewed the literature to formulate their estimates of operative time, rate of conversion to laparotomy, rate of transfusion, and length of hospital stay. In addition, the costs of initial anesthesia setup, professional fees, per-minute intraoperative fees, and postanesthesia care were applied to each approach. Operating room costs per minute and the cost of disposable items such as drapes, gowns, gloves, and single-use instruments were added. For the robotic approach, the costs of reusable instruments were distributed across 10 operations. Reusable instruments for laparoscopic and abdominal surgery were assumed to incur no additional investment. Last, postoperative care—including laboratory tests, pharmacy usage, and the need for a hospital room—were individualized for each route of surgery and applied to the cost.
Costs were estimated in 2008 US dollars, based on procedure costs incurred at Duke University Medical Center.
Physician reimbursement data were obtained from Medicare reimbursement rates for anesthesia and from surgeon Current Procedural Terminology (CPT) codes specific to each procedure.
Quality-of-life assessments were not measured. Nor was the cost to society of the postoperative loss of productivity and wages for each surgical route. Had these losses been recognized, the authors observed, the cost of robotic surgery may have been lower.
The cost of robotic surgery was equivalent to the cost of laparoscopy in only two instances:
- when the operative time of robotic surgery was reduced to 149 minutes
- when the cost of robotic disposable items was less than $2,132 (reduced from a baseline cost of $3,293).
Robotic sacrocolpopexy is costly. this is an important consideration when implementing new technology. cost-saving scenarios are useful to maximize patient benefit and minimize financial burden.
We want to hear from you! Tell us what you think.
Pessary and pelvic floor exercises for incontinence—are two better than one?
Because we will all be seeing more patients with stress urinary incontinence and other urogynecologic issues, it is critical that we keep abreast of the treatment options available—and their relative effectiveness.1
In this exploration of nonsurgical approaches to stress incontinence, Richter and colleagues started with the premise that a combination of instructed pelvic floor exercises and an incontinence pessary would be better than either treatment alone. They (very appropriately) designated the following as primary outcome measures:
- patient-reported improvement
- symptoms of stress incontinence
- patient satisfaction, as measured using validated instruments.
As reported above, combination therapy did not prove to be superior to single-modality intervention. And although behavioral therapy was superior to a pessary at 3 months, by 12 months the modalities were roughly equivalent, and only about half of patients were still using the prescribed therapy: pessary (45%) or pelvic floor exercises (57%).
This is not a real-world study
Most women who have stress incontinence and who select nonsurgical therapy choose only one option—pelvic floor exercises (if very motivated), a vaginal pessary or other device (if not so motivated), or another conservative option such as radiofrequency therapy (if even less motivated). In this study, women enrolled in behavioral therapy paid four visits (at roughly 2-week intervals) to approved “interventionists,” who instructed them in the technique for pelvic floor exercise and explained other skills and strategies to prevent urge and stress incontinence.
Many women find it difficult to attend the four to eight physiotherapy sessions that are necessary for behavioral intervention and are unwilling to devote 1 year to a therapy that they don’t find effective early on. (Physiotherapy is effective but requires a motivated patient.) Other women dislike inserting a vaginal device on a regular basis. 2
What’s more, very safe minimally invasive slings are available that offer more definitive therapy to patients who have stress incontinence. That said, a sling procedure should not be undertaken lightly. Patient selection should be based on preoperative testing, including an assessment of urethral function, for the transobturator sling.3 A retropubic sling requires a greater degree of expertise to tension appropriately but is suitable for a wider range of severity, including intrinsic sphincteric deficiency. The role of single-incision slings is unclear.
Bottom line: individualize care
The authors’ concluding statements are right on the money: “Individualization of care should continue to be the cornerstone of our approach to [stress incontinent] patients.” These women have several effective options available. We should help them make an educated choice based on symptom severity, lifestyle, and willingness to enroll in self-help intervention versus surgical therapy.
Many patients seek to avoid surgery, either because they believe that their stress incontinence is not severe enough to warrant it, or because they are unwilling to take the 6 to 8 weeks of relative inactivity required for the sling to settle in.
In the absence of approved pharmacotherapy for stress incontinence, I tell patients that they 1) can expect their symptoms to become worse over time and 2) should designate a period of time for a trial of conservative therapy—usually, 3 months. If their condition has not improved to their satisfaction over that period, I recommend that they identify a 6-week window during which they can avoid the gym and the golf course, as well as sexual activity, to allow for unstressed healing from a sling procedure.—G. WILLY DAVILA, MD
1. Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in US women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278-1283.
2. Davila GW, Bernier F. Multimodality pelvic physiotherapy treatment of urinary incontinence in adult women. Int Urogynecol J Pelvic Floor Dysfunct. 1995;6(4):187-194.
3. Guerette N, Bena J, Davila GW. Transobturator slings for stress incontinence: using urodynamic parameters to predict outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):97-102.
Because we will all be seeing more patients with stress urinary incontinence and other urogynecologic issues, it is critical that we keep abreast of the treatment options available—and their relative effectiveness.1
In this exploration of nonsurgical approaches to stress incontinence, Richter and colleagues started with the premise that a combination of instructed pelvic floor exercises and an incontinence pessary would be better than either treatment alone. They (very appropriately) designated the following as primary outcome measures:
- patient-reported improvement
- symptoms of stress incontinence
- patient satisfaction, as measured using validated instruments.
As reported above, combination therapy did not prove to be superior to single-modality intervention. And although behavioral therapy was superior to a pessary at 3 months, by 12 months the modalities were roughly equivalent, and only about half of patients were still using the prescribed therapy: pessary (45%) or pelvic floor exercises (57%).
This is not a real-world study
Most women who have stress incontinence and who select nonsurgical therapy choose only one option—pelvic floor exercises (if very motivated), a vaginal pessary or other device (if not so motivated), or another conservative option such as radiofrequency therapy (if even less motivated). In this study, women enrolled in behavioral therapy paid four visits (at roughly 2-week intervals) to approved “interventionists,” who instructed them in the technique for pelvic floor exercise and explained other skills and strategies to prevent urge and stress incontinence.
Many women find it difficult to attend the four to eight physiotherapy sessions that are necessary for behavioral intervention and are unwilling to devote 1 year to a therapy that they don’t find effective early on. (Physiotherapy is effective but requires a motivated patient.) Other women dislike inserting a vaginal device on a regular basis. 2
What’s more, very safe minimally invasive slings are available that offer more definitive therapy to patients who have stress incontinence. That said, a sling procedure should not be undertaken lightly. Patient selection should be based on preoperative testing, including an assessment of urethral function, for the transobturator sling.3 A retropubic sling requires a greater degree of expertise to tension appropriately but is suitable for a wider range of severity, including intrinsic sphincteric deficiency. The role of single-incision slings is unclear.
Bottom line: individualize care
The authors’ concluding statements are right on the money: “Individualization of care should continue to be the cornerstone of our approach to [stress incontinent] patients.” These women have several effective options available. We should help them make an educated choice based on symptom severity, lifestyle, and willingness to enroll in self-help intervention versus surgical therapy.
Many patients seek to avoid surgery, either because they believe that their stress incontinence is not severe enough to warrant it, or because they are unwilling to take the 6 to 8 weeks of relative inactivity required for the sling to settle in.
In the absence of approved pharmacotherapy for stress incontinence, I tell patients that they 1) can expect their symptoms to become worse over time and 2) should designate a period of time for a trial of conservative therapy—usually, 3 months. If their condition has not improved to their satisfaction over that period, I recommend that they identify a 6-week window during which they can avoid the gym and the golf course, as well as sexual activity, to allow for unstressed healing from a sling procedure.—G. WILLY DAVILA, MD
Because we will all be seeing more patients with stress urinary incontinence and other urogynecologic issues, it is critical that we keep abreast of the treatment options available—and their relative effectiveness.1
In this exploration of nonsurgical approaches to stress incontinence, Richter and colleagues started with the premise that a combination of instructed pelvic floor exercises and an incontinence pessary would be better than either treatment alone. They (very appropriately) designated the following as primary outcome measures:
- patient-reported improvement
- symptoms of stress incontinence
- patient satisfaction, as measured using validated instruments.
As reported above, combination therapy did not prove to be superior to single-modality intervention. And although behavioral therapy was superior to a pessary at 3 months, by 12 months the modalities were roughly equivalent, and only about half of patients were still using the prescribed therapy: pessary (45%) or pelvic floor exercises (57%).
This is not a real-world study
Most women who have stress incontinence and who select nonsurgical therapy choose only one option—pelvic floor exercises (if very motivated), a vaginal pessary or other device (if not so motivated), or another conservative option such as radiofrequency therapy (if even less motivated). In this study, women enrolled in behavioral therapy paid four visits (at roughly 2-week intervals) to approved “interventionists,” who instructed them in the technique for pelvic floor exercise and explained other skills and strategies to prevent urge and stress incontinence.
Many women find it difficult to attend the four to eight physiotherapy sessions that are necessary for behavioral intervention and are unwilling to devote 1 year to a therapy that they don’t find effective early on. (Physiotherapy is effective but requires a motivated patient.) Other women dislike inserting a vaginal device on a regular basis. 2
What’s more, very safe minimally invasive slings are available that offer more definitive therapy to patients who have stress incontinence. That said, a sling procedure should not be undertaken lightly. Patient selection should be based on preoperative testing, including an assessment of urethral function, for the transobturator sling.3 A retropubic sling requires a greater degree of expertise to tension appropriately but is suitable for a wider range of severity, including intrinsic sphincteric deficiency. The role of single-incision slings is unclear.
Bottom line: individualize care
The authors’ concluding statements are right on the money: “Individualization of care should continue to be the cornerstone of our approach to [stress incontinent] patients.” These women have several effective options available. We should help them make an educated choice based on symptom severity, lifestyle, and willingness to enroll in self-help intervention versus surgical therapy.
Many patients seek to avoid surgery, either because they believe that their stress incontinence is not severe enough to warrant it, or because they are unwilling to take the 6 to 8 weeks of relative inactivity required for the sling to settle in.
In the absence of approved pharmacotherapy for stress incontinence, I tell patients that they 1) can expect their symptoms to become worse over time and 2) should designate a period of time for a trial of conservative therapy—usually, 3 months. If their condition has not improved to their satisfaction over that period, I recommend that they identify a 6-week window during which they can avoid the gym and the golf course, as well as sexual activity, to allow for unstressed healing from a sling procedure.—G. WILLY DAVILA, MD
1. Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in US women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278-1283.
2. Davila GW, Bernier F. Multimodality pelvic physiotherapy treatment of urinary incontinence in adult women. Int Urogynecol J Pelvic Floor Dysfunct. 1995;6(4):187-194.
3. Guerette N, Bena J, Davila GW. Transobturator slings for stress incontinence: using urodynamic parameters to predict outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):97-102.
1. Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in US women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278-1283.
2. Davila GW, Bernier F. Multimodality pelvic physiotherapy treatment of urinary incontinence in adult women. Int Urogynecol J Pelvic Floor Dysfunct. 1995;6(4):187-194.
3. Guerette N, Bena J, Davila GW. Transobturator slings for stress incontinence: using urodynamic parameters to predict outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):97-102.
PELVIC FLOOR DYSFUNCTION
The authors report no financial relationships relevant to this article.
Overactive bladder (OAB)—urinary urgency, with or without incontinence, usually with frequency and nocturia1—is a common problem among women who seek care from an ObGyn. In fact, the condition is estimated to carry a health-care cost in excess of $12 billion annually in the United States.2
A recent community-based survey in Norway estimated the prevalence of urinary incontinence there to be 27% in women between the ages of 65 and 69 years and 35% to 40% in those 80 years or older.3 A population-based study in the United States suggested an even higher rate of urinary incontinence here: greater than 50% in women 60 years or older, with 1) urge urinary incontinence (UUI) predominating4 and 2) the prevalence particularly high among older women who are homebound or who live in a long-term care facility.5
OAB can undermine quality of life in several ways: social isolation, anxiety, poor sleep, higher risk of fracture after a fall,6 reduced ability to function, and poor self-perception. Despite these harmful effects, many women delay seeking care for OAB because they are embarrassed to talk about it with their physician.
Treatment by generalists is feasible—but there is a catch
It’s possible to treat most patients with OAB without referral to a specialist. Two common concerns, however, may set up a roadblock to successful management: the adverse effects associated with some agents and suboptimal control of symptoms.
In this Update, we review recent findings about 1) the potential that anticholinergic therapy has for impairing cognitive function in the older population of women and 2) the important role that concomitant behavioral therapy plays in the long-term success of, and patients’ satisfaction with, treatment of OAB.
Behavioral therapy for OAB: Is it worth all the effort?
Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370–374.
The authors of this article followed a randomized clinical trial of older women that compared behavioral and drug therapy for OAB. In the trial, biofeedback-assisted behavioral training (comprising anorectal biofeedback, urge strategies, pelvic muscle biofeedback, and practitioner-directed review with optimization) was compared with treatment with oxybutynin, between 2.5 and 15 mg/day. Both biofeedback-assisted behavioral therapy and the drug regimen were found effective, although neither treatment provided an entirely satisfactory result for all patients. (For a brief description of what constitutes behavioral treatment, see “6 tenets of behavioral therapy for urge urinary incontinence.”)
Second phase of the trial. To determine if treatment satisfaction could be enhanced, the investigators performed a modified crossover study to determine whether combination therapy—biofeedback-assisted behavioral training plus oxybutynin—added any benefit over treatment with behavioral therapy or drug therapy alone. Eligibility was determined by age (55 years or older), demonstrated UUI for at least 3 months, and incomplete dryness or incomplete satisfaction with the outcome of 8 weeks of single-intervention treatment (with either treatment) during the initial phase of the trial.
This subgroup was offered an additional 8 weeks of combination therapy. The primary outcome measure was a reduction in the frequency of episodes of incontinence episodes as recorded by subjects in a bladder diary.
Of 197 women who participated in the original randomized clinical trial, 35—27 who completed drug therapy and 8 who completed behavioral treatment—elected to receive combination therapy. Those 35 subjects did not differ in any of the multiple baseline variables; mean age was 69.3 years (standard deviation [SD], ±7.9 years).
Among subjects originally assigned to behavioral therapy alone, overall reduction in incontinence increased from a mean of 57.5% to a mean of 88.5% after combined therapy (P=.034). Subjects originally assigned to drug therapy alone demonstrated an improvement from 72.7% reduction in incontinence to a mean 84.3% overall reduction with combined therapy (P=.001).
These data suggest that combined therapy can be more effective than behavioral therapy or drug therapy alone. The impact of this study is limited, however, by the relatively low percentage (12.7%) of patients who had received behavioral therapy and chose to add drug therapy, compared with the 41.5% who moved from drug therapy alone to add behavioral therapy.
Furthermore, subjects were self-selected: They chose to continue with an additional 8 weeks of therapy after their initial suboptimal outcome. It is possible that some subjects who were neither totally continent nor completely satisfied with initial therapy chose not to continue with the crossover segment of the trial because it posed too great a burden or because they were discouraged with the initial degree of improvement.
Generalizing these results to all older women with UUI is difficult. The authors point out, however, that, in practice, patients may be more likely than not to choose combination therapy in the hope of shortening the duration of medical therapy. Although it isn’t known whether providing combination therapy from the outset would have yielded better outcomes than either single therapy did, the authors hypothesize that initial combination therapy may result in greater improvement because patients have a high level of motivation and expectation of improvement at the beginning of treatment.
Importance of this article. The investigators demonstrated that a combination of behavioral and drug therapies can provide increased effectiveness in patients for whom each treatment alone led to suboptimal satisfaction. Furthermore, by targeting women older than 55 years, the investigators were able to demonstrate this effectiveness in a group for whom pelvic-floor training may be more difficult than it is for younger women.
It will be interesting to see if future research will 1) validate these findings and 2) determine whether combined therapy can reduce the duration of drug therapy in this older population through behavioral modification and pelvic floor reeducation.
Fluid management
This first-step therapy can involve providing a handout to the patient that details techniques she can use to monitor and control her fluid intake in a manner that addresses her problem. Among such steps:
- avoiding caffeine and artificial sweeteners
- tracking her diet to identify any other bladder irritants
- limiting fluids before times she is more likely to be incontinent—during a long drive, for example, or, in the case of nocturia, after the evening meal.
Scheduled voiding
With scheduled, or prompted, voiding, the patient empties her bladder at a set interval—usually, every 1.5 to 2 hours. If nocturia, or the more severe enuresis, is a problem, the patient can be prompted by an alarm clock or (if she is institutionalized) by nursing staff. Combining scheduled voiding with fluid management principles helps the patient avoid reaching a bladder volume at which an episode of incontinence becomes more likely.
Bladder training
This is a modification of scheduled voiding that attempts to establish a normal voiding interval in patients who have significant frequency but a small voided volume. It imposes a regimented voiding schedule that gradually (over 7 to 10 days) extends the duration between voids.
Pelvic floor-muscle exercises
The focus here is on using pelvic-floor muscles to prevent incontinence. The muscles are strengthened by having the patient perform Kegel exercises (named for Arnold H. Kegel, MD, who, in 1948, recognized the role of pelvic floor-muscle rehabilitation in the treatment of incontinence). The exercises involve simultaneous 1) contraction of the pelvic and periurethral musculature and 2) relaxation of other muscles, including abdominal muscles, which can increase pressure on the bladder.
Once the patient learns to perform Kegel exercises, she can use them to suppress urgency: Instead of hurrying to the bathroom when urgency arises, she is encouraged to sit down, relax, and contract the pelvic-floor muscles repeatedly until the urge to void diminishes. Once it does, the patient proceeds to the toilet to void normally.
Pelvic exam
By self-exam, the patient can identify and familiarize herself with her purposeful contractions of the pelvic-floor musculature and thereby strengthen those muscles with effective exercise.
Biofeedback
Direct feedback about contractions of the pelvic-floor muscles—by a display of data on a gauge or computer monitor, gathered using an intravaginal or anorectal sensor or probe—allows a patient who is exercising those muscles to better target her efforts and maximize their effectiveness.
Combining behavioral therapy and an anticholinergic medication for urge urinary incontinence may yield a superior result after either modality alone has been disappointing by the patient’s account of success.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
Does oxybutynin for UUI further erode cognition in elderly women who are cognitively impaired?
Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56:862–870.
Although anticholinergic therapy is modestly effective against UUI in nursing home residents, past studies have suggested that such treatment can impair, or further impair, cognition in this population—a concern that may lead to underuse. This double-blinded, randomized, placebo-controlled trial compared short-term oral extended-release oxybutynin with placebo.
Consequently, the authors sought to determine the cognitive effect, safety, and tolerability of 5 mg/day oral extended-release oxybutynin (the most commonly prescribed dosage) in cognitively impaired older nursing home residents who have UUI.
Subjects were eligible if they:
- were 65 years or older
- had UUI
- lived in a nursing home longer than 3 months
- had cognitive impairment.
Women already being treated for urinary incontinence, those who had an indwelling Foley catheter or urinary retention, and those who were bed-bound or incommunicative were excluded.
Fifty women, mean age 88.6 years (SD, ±6.2), from 12 nursing home facilities, agreed to participate. They were further stratified based on the score of a Mini-Mental State Exam (MMSE): 13 had severe cognitive impairment (MMSE score, 5–10) and 37 had mild or moderate impairment (score, 11–23).
Subjects were randomized to 4 weeks’ treatment with either 5 mg/day oral extended-release oxybutynin or one placebo tablet daily. A nurse practitioner who was blinded to randomization collected all data. The Confusion Assessment Method (CAM) algorithm, MMSE, and Severe Impairment Battery (SIB) were used to assess cognitive decline. The Brief Agitation Rating Scale (BARS) assessed agitation.
No baseline differences were noted with regard to: age; demographic, functional, and neuropsychiatric characteristics; clinical factors predisposing to delirium; and serum anticholinergic activity. Adherence was similar in the treatment (97%) and placebo (97.4%) groups.
Finding: Cognitive impairment. Treatment and placebo groups in the baseline mild-or-moderate stratum (by MMSE) showed equivalent mean changes in CAM scores at all time points. Because of the small sample size, however, CAM score equivalence could not be definitively determined for the groups in the severe impairment stratum. Evaluation of mean MMSE and BARS scores showed no significant changes between groups.
Finding: Tolerability. Excellent tolerability was noted in the treatment group: 96% of subjects completed the trial (compared with 92% of the placebo group). No difference in the rate of adverse events was noted between treatment and placebo groups; of adverse events recorded, 90% were judged “mild” by the investigators. Constipation and dry mouth were most common.
Finding: Falls. More than half—54%—of subjects in both groups experienced at least one fall during the trial or during the preceding or following 3 months. Despite this, no difference in the rate of falls between the treatment and placebo groups was noted. Furthermore, regression analysis revealed no treatment or period effect on falls per month across the time of observation.
Conclusions. Treatment with 5 mg/day oral extended-release oxybutynin in older patients with some cognitive impairment is well tolerated, the study’s findings suggest, with minimal risk of further cognitive decline or delirium over the short term. The potential that long-term therapy has to harm cognitive function remains, however; data on long-term treatment are needed to illuminate that area.
The authors also address the importance of dosing, especially over time, and discuss the lower potential of newer-generation anticholinergics to produce cognitive impairment.
A limited number of articles in the medical literature address anticholinergics in an older population, specifically, and only a few of those evaluated the effects of the drugs on cognitive function. By investigating patients who had an existing cognitive impairment, the authors of this article were able to target a cohort at risk of further cognitive impairment from medication use—thereby giving further weight to their findings of no significant effect.
Main strengths and limitations of the study. The investigators used validated, standardized cognitive tests that were administered by a uniform blinded evaluator in a randomized, controlled trial. The study was limited, however, because patients were evaluated only over a relatively short period (1 month) and because the efficacy of therapy was not addressed.
Further studies of anticholinergic medications, using the same rigorous scientific approach that these investigators applied, are needed to address 1) the long-term efficacy of oxybutynin and similar agents and 2) the cognitive effects of long-term treatment in this older population.
Further impairment is unlikely over the short term when a cognitively impaired nursing home patient who has urge urinary incontinence is treated with 5 mg/day oral extended-release oxybutynin.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116-126.
2. Hu TW, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123-1128.
3. Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000;53:1150-1157.
4. Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537-542.
5. Fantl JA, Newman DK, Colling J, et al. Managing Acute and Chronic Urinary Incontinence. Clinical Practice Guideline. Quick Reference Guide for Clinicians, No. 2, 1996 Update. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research. AHCPR Pub. No. 96-0686. January 1996. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat6.chapter.32554. Accessed September 11, 2009.
6. Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721-725.
The authors report no financial relationships relevant to this article.
Overactive bladder (OAB)—urinary urgency, with or without incontinence, usually with frequency and nocturia1—is a common problem among women who seek care from an ObGyn. In fact, the condition is estimated to carry a health-care cost in excess of $12 billion annually in the United States.2
A recent community-based survey in Norway estimated the prevalence of urinary incontinence there to be 27% in women between the ages of 65 and 69 years and 35% to 40% in those 80 years or older.3 A population-based study in the United States suggested an even higher rate of urinary incontinence here: greater than 50% in women 60 years or older, with 1) urge urinary incontinence (UUI) predominating4 and 2) the prevalence particularly high among older women who are homebound or who live in a long-term care facility.5
OAB can undermine quality of life in several ways: social isolation, anxiety, poor sleep, higher risk of fracture after a fall,6 reduced ability to function, and poor self-perception. Despite these harmful effects, many women delay seeking care for OAB because they are embarrassed to talk about it with their physician.
Treatment by generalists is feasible—but there is a catch
It’s possible to treat most patients with OAB without referral to a specialist. Two common concerns, however, may set up a roadblock to successful management: the adverse effects associated with some agents and suboptimal control of symptoms.
In this Update, we review recent findings about 1) the potential that anticholinergic therapy has for impairing cognitive function in the older population of women and 2) the important role that concomitant behavioral therapy plays in the long-term success of, and patients’ satisfaction with, treatment of OAB.
Behavioral therapy for OAB: Is it worth all the effort?
Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370–374.
The authors of this article followed a randomized clinical trial of older women that compared behavioral and drug therapy for OAB. In the trial, biofeedback-assisted behavioral training (comprising anorectal biofeedback, urge strategies, pelvic muscle biofeedback, and practitioner-directed review with optimization) was compared with treatment with oxybutynin, between 2.5 and 15 mg/day. Both biofeedback-assisted behavioral therapy and the drug regimen were found effective, although neither treatment provided an entirely satisfactory result for all patients. (For a brief description of what constitutes behavioral treatment, see “6 tenets of behavioral therapy for urge urinary incontinence.”)
Second phase of the trial. To determine if treatment satisfaction could be enhanced, the investigators performed a modified crossover study to determine whether combination therapy—biofeedback-assisted behavioral training plus oxybutynin—added any benefit over treatment with behavioral therapy or drug therapy alone. Eligibility was determined by age (55 years or older), demonstrated UUI for at least 3 months, and incomplete dryness or incomplete satisfaction with the outcome of 8 weeks of single-intervention treatment (with either treatment) during the initial phase of the trial.
This subgroup was offered an additional 8 weeks of combination therapy. The primary outcome measure was a reduction in the frequency of episodes of incontinence episodes as recorded by subjects in a bladder diary.
Of 197 women who participated in the original randomized clinical trial, 35—27 who completed drug therapy and 8 who completed behavioral treatment—elected to receive combination therapy. Those 35 subjects did not differ in any of the multiple baseline variables; mean age was 69.3 years (standard deviation [SD], ±7.9 years).
Among subjects originally assigned to behavioral therapy alone, overall reduction in incontinence increased from a mean of 57.5% to a mean of 88.5% after combined therapy (P=.034). Subjects originally assigned to drug therapy alone demonstrated an improvement from 72.7% reduction in incontinence to a mean 84.3% overall reduction with combined therapy (P=.001).
These data suggest that combined therapy can be more effective than behavioral therapy or drug therapy alone. The impact of this study is limited, however, by the relatively low percentage (12.7%) of patients who had received behavioral therapy and chose to add drug therapy, compared with the 41.5% who moved from drug therapy alone to add behavioral therapy.
Furthermore, subjects were self-selected: They chose to continue with an additional 8 weeks of therapy after their initial suboptimal outcome. It is possible that some subjects who were neither totally continent nor completely satisfied with initial therapy chose not to continue with the crossover segment of the trial because it posed too great a burden or because they were discouraged with the initial degree of improvement.
Generalizing these results to all older women with UUI is difficult. The authors point out, however, that, in practice, patients may be more likely than not to choose combination therapy in the hope of shortening the duration of medical therapy. Although it isn’t known whether providing combination therapy from the outset would have yielded better outcomes than either single therapy did, the authors hypothesize that initial combination therapy may result in greater improvement because patients have a high level of motivation and expectation of improvement at the beginning of treatment.
Importance of this article. The investigators demonstrated that a combination of behavioral and drug therapies can provide increased effectiveness in patients for whom each treatment alone led to suboptimal satisfaction. Furthermore, by targeting women older than 55 years, the investigators were able to demonstrate this effectiveness in a group for whom pelvic-floor training may be more difficult than it is for younger women.
It will be interesting to see if future research will 1) validate these findings and 2) determine whether combined therapy can reduce the duration of drug therapy in this older population through behavioral modification and pelvic floor reeducation.
Fluid management
This first-step therapy can involve providing a handout to the patient that details techniques she can use to monitor and control her fluid intake in a manner that addresses her problem. Among such steps:
- avoiding caffeine and artificial sweeteners
- tracking her diet to identify any other bladder irritants
- limiting fluids before times she is more likely to be incontinent—during a long drive, for example, or, in the case of nocturia, after the evening meal.
Scheduled voiding
With scheduled, or prompted, voiding, the patient empties her bladder at a set interval—usually, every 1.5 to 2 hours. If nocturia, or the more severe enuresis, is a problem, the patient can be prompted by an alarm clock or (if she is institutionalized) by nursing staff. Combining scheduled voiding with fluid management principles helps the patient avoid reaching a bladder volume at which an episode of incontinence becomes more likely.
Bladder training
This is a modification of scheduled voiding that attempts to establish a normal voiding interval in patients who have significant frequency but a small voided volume. It imposes a regimented voiding schedule that gradually (over 7 to 10 days) extends the duration between voids.
Pelvic floor-muscle exercises
The focus here is on using pelvic-floor muscles to prevent incontinence. The muscles are strengthened by having the patient perform Kegel exercises (named for Arnold H. Kegel, MD, who, in 1948, recognized the role of pelvic floor-muscle rehabilitation in the treatment of incontinence). The exercises involve simultaneous 1) contraction of the pelvic and periurethral musculature and 2) relaxation of other muscles, including abdominal muscles, which can increase pressure on the bladder.
Once the patient learns to perform Kegel exercises, she can use them to suppress urgency: Instead of hurrying to the bathroom when urgency arises, she is encouraged to sit down, relax, and contract the pelvic-floor muscles repeatedly until the urge to void diminishes. Once it does, the patient proceeds to the toilet to void normally.
Pelvic exam
By self-exam, the patient can identify and familiarize herself with her purposeful contractions of the pelvic-floor musculature and thereby strengthen those muscles with effective exercise.
Biofeedback
Direct feedback about contractions of the pelvic-floor muscles—by a display of data on a gauge or computer monitor, gathered using an intravaginal or anorectal sensor or probe—allows a patient who is exercising those muscles to better target her efforts and maximize their effectiveness.
Combining behavioral therapy and an anticholinergic medication for urge urinary incontinence may yield a superior result after either modality alone has been disappointing by the patient’s account of success.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
Does oxybutynin for UUI further erode cognition in elderly women who are cognitively impaired?
Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56:862–870.
Although anticholinergic therapy is modestly effective against UUI in nursing home residents, past studies have suggested that such treatment can impair, or further impair, cognition in this population—a concern that may lead to underuse. This double-blinded, randomized, placebo-controlled trial compared short-term oral extended-release oxybutynin with placebo.
Consequently, the authors sought to determine the cognitive effect, safety, and tolerability of 5 mg/day oral extended-release oxybutynin (the most commonly prescribed dosage) in cognitively impaired older nursing home residents who have UUI.
Subjects were eligible if they:
- were 65 years or older
- had UUI
- lived in a nursing home longer than 3 months
- had cognitive impairment.
Women already being treated for urinary incontinence, those who had an indwelling Foley catheter or urinary retention, and those who were bed-bound or incommunicative were excluded.
Fifty women, mean age 88.6 years (SD, ±6.2), from 12 nursing home facilities, agreed to participate. They were further stratified based on the score of a Mini-Mental State Exam (MMSE): 13 had severe cognitive impairment (MMSE score, 5–10) and 37 had mild or moderate impairment (score, 11–23).
Subjects were randomized to 4 weeks’ treatment with either 5 mg/day oral extended-release oxybutynin or one placebo tablet daily. A nurse practitioner who was blinded to randomization collected all data. The Confusion Assessment Method (CAM) algorithm, MMSE, and Severe Impairment Battery (SIB) were used to assess cognitive decline. The Brief Agitation Rating Scale (BARS) assessed agitation.
No baseline differences were noted with regard to: age; demographic, functional, and neuropsychiatric characteristics; clinical factors predisposing to delirium; and serum anticholinergic activity. Adherence was similar in the treatment (97%) and placebo (97.4%) groups.
Finding: Cognitive impairment. Treatment and placebo groups in the baseline mild-or-moderate stratum (by MMSE) showed equivalent mean changes in CAM scores at all time points. Because of the small sample size, however, CAM score equivalence could not be definitively determined for the groups in the severe impairment stratum. Evaluation of mean MMSE and BARS scores showed no significant changes between groups.
Finding: Tolerability. Excellent tolerability was noted in the treatment group: 96% of subjects completed the trial (compared with 92% of the placebo group). No difference in the rate of adverse events was noted between treatment and placebo groups; of adverse events recorded, 90% were judged “mild” by the investigators. Constipation and dry mouth were most common.
Finding: Falls. More than half—54%—of subjects in both groups experienced at least one fall during the trial or during the preceding or following 3 months. Despite this, no difference in the rate of falls between the treatment and placebo groups was noted. Furthermore, regression analysis revealed no treatment or period effect on falls per month across the time of observation.
Conclusions. Treatment with 5 mg/day oral extended-release oxybutynin in older patients with some cognitive impairment is well tolerated, the study’s findings suggest, with minimal risk of further cognitive decline or delirium over the short term. The potential that long-term therapy has to harm cognitive function remains, however; data on long-term treatment are needed to illuminate that area.
The authors also address the importance of dosing, especially over time, and discuss the lower potential of newer-generation anticholinergics to produce cognitive impairment.
A limited number of articles in the medical literature address anticholinergics in an older population, specifically, and only a few of those evaluated the effects of the drugs on cognitive function. By investigating patients who had an existing cognitive impairment, the authors of this article were able to target a cohort at risk of further cognitive impairment from medication use—thereby giving further weight to their findings of no significant effect.
Main strengths and limitations of the study. The investigators used validated, standardized cognitive tests that were administered by a uniform blinded evaluator in a randomized, controlled trial. The study was limited, however, because patients were evaluated only over a relatively short period (1 month) and because the efficacy of therapy was not addressed.
Further studies of anticholinergic medications, using the same rigorous scientific approach that these investigators applied, are needed to address 1) the long-term efficacy of oxybutynin and similar agents and 2) the cognitive effects of long-term treatment in this older population.
Further impairment is unlikely over the short term when a cognitively impaired nursing home patient who has urge urinary incontinence is treated with 5 mg/day oral extended-release oxybutynin.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
The authors report no financial relationships relevant to this article.
Overactive bladder (OAB)—urinary urgency, with or without incontinence, usually with frequency and nocturia1—is a common problem among women who seek care from an ObGyn. In fact, the condition is estimated to carry a health-care cost in excess of $12 billion annually in the United States.2
A recent community-based survey in Norway estimated the prevalence of urinary incontinence there to be 27% in women between the ages of 65 and 69 years and 35% to 40% in those 80 years or older.3 A population-based study in the United States suggested an even higher rate of urinary incontinence here: greater than 50% in women 60 years or older, with 1) urge urinary incontinence (UUI) predominating4 and 2) the prevalence particularly high among older women who are homebound or who live in a long-term care facility.5
OAB can undermine quality of life in several ways: social isolation, anxiety, poor sleep, higher risk of fracture after a fall,6 reduced ability to function, and poor self-perception. Despite these harmful effects, many women delay seeking care for OAB because they are embarrassed to talk about it with their physician.
Treatment by generalists is feasible—but there is a catch
It’s possible to treat most patients with OAB without referral to a specialist. Two common concerns, however, may set up a roadblock to successful management: the adverse effects associated with some agents and suboptimal control of symptoms.
In this Update, we review recent findings about 1) the potential that anticholinergic therapy has for impairing cognitive function in the older population of women and 2) the important role that concomitant behavioral therapy plays in the long-term success of, and patients’ satisfaction with, treatment of OAB.
Behavioral therapy for OAB: Is it worth all the effort?
Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370–374.
The authors of this article followed a randomized clinical trial of older women that compared behavioral and drug therapy for OAB. In the trial, biofeedback-assisted behavioral training (comprising anorectal biofeedback, urge strategies, pelvic muscle biofeedback, and practitioner-directed review with optimization) was compared with treatment with oxybutynin, between 2.5 and 15 mg/day. Both biofeedback-assisted behavioral therapy and the drug regimen were found effective, although neither treatment provided an entirely satisfactory result for all patients. (For a brief description of what constitutes behavioral treatment, see “6 tenets of behavioral therapy for urge urinary incontinence.”)
Second phase of the trial. To determine if treatment satisfaction could be enhanced, the investigators performed a modified crossover study to determine whether combination therapy—biofeedback-assisted behavioral training plus oxybutynin—added any benefit over treatment with behavioral therapy or drug therapy alone. Eligibility was determined by age (55 years or older), demonstrated UUI for at least 3 months, and incomplete dryness or incomplete satisfaction with the outcome of 8 weeks of single-intervention treatment (with either treatment) during the initial phase of the trial.
This subgroup was offered an additional 8 weeks of combination therapy. The primary outcome measure was a reduction in the frequency of episodes of incontinence episodes as recorded by subjects in a bladder diary.
Of 197 women who participated in the original randomized clinical trial, 35—27 who completed drug therapy and 8 who completed behavioral treatment—elected to receive combination therapy. Those 35 subjects did not differ in any of the multiple baseline variables; mean age was 69.3 years (standard deviation [SD], ±7.9 years).
Among subjects originally assigned to behavioral therapy alone, overall reduction in incontinence increased from a mean of 57.5% to a mean of 88.5% after combined therapy (P=.034). Subjects originally assigned to drug therapy alone demonstrated an improvement from 72.7% reduction in incontinence to a mean 84.3% overall reduction with combined therapy (P=.001).
These data suggest that combined therapy can be more effective than behavioral therapy or drug therapy alone. The impact of this study is limited, however, by the relatively low percentage (12.7%) of patients who had received behavioral therapy and chose to add drug therapy, compared with the 41.5% who moved from drug therapy alone to add behavioral therapy.
Furthermore, subjects were self-selected: They chose to continue with an additional 8 weeks of therapy after their initial suboptimal outcome. It is possible that some subjects who were neither totally continent nor completely satisfied with initial therapy chose not to continue with the crossover segment of the trial because it posed too great a burden or because they were discouraged with the initial degree of improvement.
Generalizing these results to all older women with UUI is difficult. The authors point out, however, that, in practice, patients may be more likely than not to choose combination therapy in the hope of shortening the duration of medical therapy. Although it isn’t known whether providing combination therapy from the outset would have yielded better outcomes than either single therapy did, the authors hypothesize that initial combination therapy may result in greater improvement because patients have a high level of motivation and expectation of improvement at the beginning of treatment.
Importance of this article. The investigators demonstrated that a combination of behavioral and drug therapies can provide increased effectiveness in patients for whom each treatment alone led to suboptimal satisfaction. Furthermore, by targeting women older than 55 years, the investigators were able to demonstrate this effectiveness in a group for whom pelvic-floor training may be more difficult than it is for younger women.
It will be interesting to see if future research will 1) validate these findings and 2) determine whether combined therapy can reduce the duration of drug therapy in this older population through behavioral modification and pelvic floor reeducation.
Fluid management
This first-step therapy can involve providing a handout to the patient that details techniques she can use to monitor and control her fluid intake in a manner that addresses her problem. Among such steps:
- avoiding caffeine and artificial sweeteners
- tracking her diet to identify any other bladder irritants
- limiting fluids before times she is more likely to be incontinent—during a long drive, for example, or, in the case of nocturia, after the evening meal.
Scheduled voiding
With scheduled, or prompted, voiding, the patient empties her bladder at a set interval—usually, every 1.5 to 2 hours. If nocturia, or the more severe enuresis, is a problem, the patient can be prompted by an alarm clock or (if she is institutionalized) by nursing staff. Combining scheduled voiding with fluid management principles helps the patient avoid reaching a bladder volume at which an episode of incontinence becomes more likely.
Bladder training
This is a modification of scheduled voiding that attempts to establish a normal voiding interval in patients who have significant frequency but a small voided volume. It imposes a regimented voiding schedule that gradually (over 7 to 10 days) extends the duration between voids.
Pelvic floor-muscle exercises
The focus here is on using pelvic-floor muscles to prevent incontinence. The muscles are strengthened by having the patient perform Kegel exercises (named for Arnold H. Kegel, MD, who, in 1948, recognized the role of pelvic floor-muscle rehabilitation in the treatment of incontinence). The exercises involve simultaneous 1) contraction of the pelvic and periurethral musculature and 2) relaxation of other muscles, including abdominal muscles, which can increase pressure on the bladder.
Once the patient learns to perform Kegel exercises, she can use them to suppress urgency: Instead of hurrying to the bathroom when urgency arises, she is encouraged to sit down, relax, and contract the pelvic-floor muscles repeatedly until the urge to void diminishes. Once it does, the patient proceeds to the toilet to void normally.
Pelvic exam
By self-exam, the patient can identify and familiarize herself with her purposeful contractions of the pelvic-floor musculature and thereby strengthen those muscles with effective exercise.
Biofeedback
Direct feedback about contractions of the pelvic-floor muscles—by a display of data on a gauge or computer monitor, gathered using an intravaginal or anorectal sensor or probe—allows a patient who is exercising those muscles to better target her efforts and maximize their effectiveness.
Combining behavioral therapy and an anticholinergic medication for urge urinary incontinence may yield a superior result after either modality alone has been disappointing by the patient’s account of success.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
Does oxybutynin for UUI further erode cognition in elderly women who are cognitively impaired?
Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56:862–870.
Although anticholinergic therapy is modestly effective against UUI in nursing home residents, past studies have suggested that such treatment can impair, or further impair, cognition in this population—a concern that may lead to underuse. This double-blinded, randomized, placebo-controlled trial compared short-term oral extended-release oxybutynin with placebo.
Consequently, the authors sought to determine the cognitive effect, safety, and tolerability of 5 mg/day oral extended-release oxybutynin (the most commonly prescribed dosage) in cognitively impaired older nursing home residents who have UUI.
Subjects were eligible if they:
- were 65 years or older
- had UUI
- lived in a nursing home longer than 3 months
- had cognitive impairment.
Women already being treated for urinary incontinence, those who had an indwelling Foley catheter or urinary retention, and those who were bed-bound or incommunicative were excluded.
Fifty women, mean age 88.6 years (SD, ±6.2), from 12 nursing home facilities, agreed to participate. They were further stratified based on the score of a Mini-Mental State Exam (MMSE): 13 had severe cognitive impairment (MMSE score, 5–10) and 37 had mild or moderate impairment (score, 11–23).
Subjects were randomized to 4 weeks’ treatment with either 5 mg/day oral extended-release oxybutynin or one placebo tablet daily. A nurse practitioner who was blinded to randomization collected all data. The Confusion Assessment Method (CAM) algorithm, MMSE, and Severe Impairment Battery (SIB) were used to assess cognitive decline. The Brief Agitation Rating Scale (BARS) assessed agitation.
No baseline differences were noted with regard to: age; demographic, functional, and neuropsychiatric characteristics; clinical factors predisposing to delirium; and serum anticholinergic activity. Adherence was similar in the treatment (97%) and placebo (97.4%) groups.
Finding: Cognitive impairment. Treatment and placebo groups in the baseline mild-or-moderate stratum (by MMSE) showed equivalent mean changes in CAM scores at all time points. Because of the small sample size, however, CAM score equivalence could not be definitively determined for the groups in the severe impairment stratum. Evaluation of mean MMSE and BARS scores showed no significant changes between groups.
Finding: Tolerability. Excellent tolerability was noted in the treatment group: 96% of subjects completed the trial (compared with 92% of the placebo group). No difference in the rate of adverse events was noted between treatment and placebo groups; of adverse events recorded, 90% were judged “mild” by the investigators. Constipation and dry mouth were most common.
Finding: Falls. More than half—54%—of subjects in both groups experienced at least one fall during the trial or during the preceding or following 3 months. Despite this, no difference in the rate of falls between the treatment and placebo groups was noted. Furthermore, regression analysis revealed no treatment or period effect on falls per month across the time of observation.
Conclusions. Treatment with 5 mg/day oral extended-release oxybutynin in older patients with some cognitive impairment is well tolerated, the study’s findings suggest, with minimal risk of further cognitive decline or delirium over the short term. The potential that long-term therapy has to harm cognitive function remains, however; data on long-term treatment are needed to illuminate that area.
The authors also address the importance of dosing, especially over time, and discuss the lower potential of newer-generation anticholinergics to produce cognitive impairment.
A limited number of articles in the medical literature address anticholinergics in an older population, specifically, and only a few of those evaluated the effects of the drugs on cognitive function. By investigating patients who had an existing cognitive impairment, the authors of this article were able to target a cohort at risk of further cognitive impairment from medication use—thereby giving further weight to their findings of no significant effect.
Main strengths and limitations of the study. The investigators used validated, standardized cognitive tests that were administered by a uniform blinded evaluator in a randomized, controlled trial. The study was limited, however, because patients were evaluated only over a relatively short period (1 month) and because the efficacy of therapy was not addressed.
Further studies of anticholinergic medications, using the same rigorous scientific approach that these investigators applied, are needed to address 1) the long-term efficacy of oxybutynin and similar agents and 2) the cognitive effects of long-term treatment in this older population.
Further impairment is unlikely over the short term when a cognitively impaired nursing home patient who has urge urinary incontinence is treated with 5 mg/day oral extended-release oxybutynin.—JOHN P. JUDD, MD, AND CINDY L. AMUNDSEN, MD
1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116-126.
2. Hu TW, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123-1128.
3. Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000;53:1150-1157.
4. Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537-542.
5. Fantl JA, Newman DK, Colling J, et al. Managing Acute and Chronic Urinary Incontinence. Clinical Practice Guideline. Quick Reference Guide for Clinicians, No. 2, 1996 Update. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research. AHCPR Pub. No. 96-0686. January 1996. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat6.chapter.32554. Accessed September 11, 2009.
6. Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721-725.
1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116-126.
2. Hu TW, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123-1128.
3. Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000;53:1150-1157.
4. Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537-542.
5. Fantl JA, Newman DK, Colling J, et al. Managing Acute and Chronic Urinary Incontinence. Clinical Practice Guideline. Quick Reference Guide for Clinicians, No. 2, 1996 Update. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research. AHCPR Pub. No. 96-0686. January 1996. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat6.chapter.32554. Accessed September 11, 2009.
6. Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721-725.
Voices of experience weigh in: Do electronic medical records make for a better practice?
MODERATOR

G. William Bates, MD, MBA
Vanderbilt University Medical Center, Nashville, Tenn
PANELISTS
Have introduced EMR to their practice

B. David Hall, MD, FACOG
Rowan OB/GYN Associates, Salisbury, NC

Don Shuwarger, MD, FACOG
Forest Women’s Center, Forest, Va
PANELISTS
Have not introduced EMR

Frank O. Page, MD, FACOG
Henderson Walton Women’s Center, Birmingham, Ala

Mark A. VanMeter
Group Practice Manager, Columbus Obstetricians– Gynecologists, Inc., Columbus, Ohio
Are your colleagues in private practice who have made the transition to a system of electronic medical records (EMR) satisfied with their decision and experience? Yes and, on some points, less than yes.
For practices that—perhaps, like yours—haven’t made the leap, the question is: What’s holding them back?
In this concluding installment of a two-part article on EMR, a panel of three ObGyns and one ObGyn practice administrator talk with Moderator G. William Bates, MD, MBA, about, in the case of two practices, the work of bringing EMR into their offices. Two other panelists describe their practices’ calculated reluctance to discard paper processes right now.
Why have you and your partners adopted EMR?
Shuwarger: Our practice quickly identified the direct and indirect benefits of bringing technology to bear on our processes. Paper records were often illegible, misplaced, or being used by another staff member. We recognized that to meet our internal goals for growth, increasing patient safety, and streamlining processes, we would have to adopt an EMR solution that met those needs.
Hall: Our practice was drowning in paperwork. An exam room was recently converted to hold more charts, and two warehouses held our overflow. Employees were constantly searching for records, and telephone messages were delayed for hours or days until the chart could be reviewed. Notoriously bad handwriting and incomplete documentation hampered good communication and good medical care. Transcription costs were out of control. Forms helped but added to ongoing costs and storage problems.
What efficiency gains have you achieved?
Shuwarger: Forest Women’s Center is able to see more patients in the day because our ObGyn-specific EMR system has a “Patient Portal” that enables patients to enter all their history and complaint-specific information in advance of a visit. Another efficiency is the time gained by never searching for lost or misplaced charts. We also like the ability to access our records 24-7-365.
Hall: The patient’s chart is readily available. Hours of searching have been eliminated, and patients’ questions, lab reports, and prescription refills can be managed with very few steps. The physician can record recommendations and treatment plans, which the staff relays to the patient. Records take about the same time to finish, but they are much more complete and legible, with dramatic gains in safety for the patient and improved liability protection for the physician.
Which features provide the greatest value?
Shuwarger: The patient portal that I mentioned is a great time saver for us. We were amazed at the acceptance and rapid adoption. Even our octogenarians love it. Universal access to data is of incalculable value. One of our physicians loves to go home early, have dinner, and then review his charts from home. EMR improves my recordkeeping, makes encounter documentation more complete, and helps me avoid medication errors. Our billing staff loves the thorough documentation when it is time to file or appeal claims.
Hall: Immediate access to a clear, legible, and complete patient record provides a solid foundation for our medical decision making.
How have your patients reacted to your conversion from paper to EMR?
Shuwarger: At the beginning, there were people who resisted the patient portal, but when they saw for themselves how it enhances the visit experience and helps their physician address their needs, they became vocal proponents.
Hall: Our patients are impressed with our knowledge of their history, with the fact that reports are immediately available, and with how responsive our staff is to their needs. Rather than creating a barrier to communication, TabletPCs allow them to see images of their own procedures, illustrations, treatment outlines, and even education videos. Flow sheets help mark their progress or encourage them to better adherence. Many seem pleased that their medical records are so cutting-edge. Their confidence in our medical skills appears enhanced.
Has your vendor met expectations?
Shuwarger: No—our vendor exceeded our expectations. We had experience with technology vendors before—“We’ll overpromise and underdeliver” was their mantra! With our EMR vendor, however, our preparation was outstanding, the training was thorough, and implementation went better than any we had experienced. Our uptime has exceeded expectations. Enhancements have been well thought out.
And customer support was good at first but now is even better.
Hall: The program is extremely powerful, with an excellent architecture, but its flexibility is also its main limitation. Recently, core clinical content for primary care medicine has been added, but specialty content remains severely limited. Value-added vendors have developed—at additional cost—excellent form-editing tools and specialty forms, and a vigorous users’ community is generous in sharing forms and workflows. But untold hours were required to develop clinical and office workflows, document templates, and just to discover all the options in the system. The learning curve was huge, and further automation requires the skills of a computer programmer.
Our EMR and practice management systems are interfaced but not integrated—even though the same vendor developed them. The problem is that the interface requires several translation programs and multiple servers to implement. Our dependence on our network engineering firm to maintain our bank of servers and interfaces is worrisome— and costly.
Training on our system was inadequate. The basics of the system were covered but, beyond that, we are just now able to shift into second gear. Much of the system’s potential remains untapped.
What is your approximate return on investment?
Shuwarger: We’ve grown receipts by 20%, year over year, since going with our ObGyn-specific EMR system. The rise in revenue is related directly to increased productivity, a reduction in lost charges, and improved collection from third-party payers because we can provide better documentation. At the least, our EMR system has returned $3 for every $1 spent, not counting intangibles.
Hall: Charge capture is much more complete and accurate, with readily available codes and guidelines. The greatest savings are in chart transcription, management, and storage.
Ongoing maintenance and upgrade costs, including hardware and networking software, have gone far beyond our initial investment, however. Problems with training and initial workflow design have slowed our return on investment. But we’re making progress in that direction.
- Streamlined history-taking and complaint-reporting may mean greater productivity in a practice—and a resulting ability to see more patients in a day
- A so-called patient portal gives patients easier access to providers and the varied resources and services of a practice, which boosts satisfaction
- Caveat emptor! Shop carefully when selecting a system vendor—the experiences of practices from installation through system maintenance range very widely
- Interconnectivity between an EMR system and other databases is not a given
- For a large, multisite practice, the cost of hardware alone may have a chilling effect on implementing an EMR system
- All physicians in a practice must buy into an EMR system that’s being put into place—and a range of ages, attitudes, and practice patterns may be a cause for disagreement on how the system is to be best used
- There is concern among some that the federal government may shape the future of EMR by mandating that all systems in private practices interface with hospitals, insurers, and other providers.
Are features lacking that would bring greater efficiency?
Shuwarger: Our labor suite wants data from our ACOG obstetric record to flow into its system to avoid the need to reenter data manually. And our practice’s physicians want the labor and delivery summary to populate our EMR. These issues of interconnection will be worked out as CCHIT certification (see “EMR certifying body arises from the private sector,” page 62) brings disparate systems into proximity.
Hall: Physicians aren’t computer programmers. We practice medicine, not EMR system development, and we are rarely on top of the “best practices” in practice workflow. Many of us who work with EMR may wish to customize a system to the way we practice, but that is not the best way to proceed. A robust and comprehensive specialty-specific set of clinical content that can be loaded as a unit and easily updated is going to provide far greater efficiency than an infinitely customizable basic program.
I look forward to being able to integrate our private medical record with a central data repository, in which interactions with other specialists and medical centers—not the faulty memory of patients—provide a more accurate background and reduce costly duplication of our increasingly stretched medical resources.
In 2004, President George W. Bush set a goal: nationwide adoption of EMR—to include all medical practices—within a decade. Subsequently, the US Department of Health and Human Services (HHS) established the Office of the National Coordinator for Health Information Technology and the American Health Information Community. The sweeping goal of these bodies? Better health care by application of information technology and creation of standards for certifying EMR systems that provide core functionality.
In response, three private-sector health information management groups jointly formed the Certification Commission for Healthcare Information Technology (CCHIT; www.cchit.org). In 2005, this independent private-sector entity entered into a contract with HHS, to, in the commission’s words, “develop and evaluate certification criteria and create a voluntary inspection process for healthcare information technology” in three areas:
- Ambulatory EMR for offices
- Inpatient EMR for hospitals and health systems
- The network components through which EMR share information.
The work of CCHIT is ongoing; the commission provides voluntary certification of EMR systems, publishes a list of certified EMR systems, provides consultative services to providers and payers through its Web site, and even offers a bank of resources for patients on the intricacies and legalities of medical-record-keeping.
Why haven’t you and your partners adopted EMR?
Page: We recently converted to a new practice management software system, and we want to have all systems working properly and efficiently before implementing an EMR system. All options and processes must be reviewed before we implement EMR for the practice. These options include voice-activation software integrated with the EMR, practice process changes, and practice workflow adaptation.
VanMeter: For our independent practice, with five locations, the initial cost of hardware and software is clearly an early concern. With a rapidly changing hardware environment, once a decision is made, the technology that was proposed may be obsolete before being implemented. Then the continuing cost of hardware and software upgrades—read: “the newest gadget”—and maintenance is also a major budgetary item that we need to consider.
As with most medical practices, our organizational structure is flat. If we were to implement a client-server application, we’d need a systems administrator—and that again increases the cost to the practice. Then we’re faced with the question of how we best utilize this person. Or do we outsource this function? And outsourcing then raises a concern of timely responsiveness to major system problems that may extend downtime, prohibiting the use of your EMR system.
Today, telecommunication costs have plummeted, so the costs of a T-1 line [for high-volume Internet access] and high-speed Internet service are not as onerous as they once were. But a major expense will be to retrofit all our offices (wiring, etc.) to adapt to an electronic environment.
Overall, this is a young industry. I compare it to what we saw with video-tape technology in the 1970s: You had to choose between Beta and VHS formats. Once you made that decision, you paid a premium for the early technology.
Similarly, no one knows which EMR system will prevail over time. The early players are paying for the cost of startup and research and development. As time goes on, we all know that costs should fall—significantly.
Another concern that we have is the long-term viability of the software vendor. Until recently, most applications were developed by small independent firms. Their product was a proprietary one—for which only they have the code and only they could manipulate. If that vendor goes out of business, we’d be left to find a new system, and incur all those implementation costs again.
I think we’ll see a major consolidation of vendors over the next several years— one that leaves only premier vendors with superior products in the market.
As a final concern, and perhaps most important, the role of the federal government weighs heavily on our minds. We believe that, very soon, Washington will mandate EMR and how they are to be accomplished. We also believe that the feds will require integration of medical practice EMR systems with the systems of hospitals, third-party payers, and other medical providers. Our belief is that money may become available—like the funding recently authorized for hospitals to subsidize software and maintenance costs—that will defray the cost of implementing an EMR system in our practice. When this comes to pass, we don’t want to have to reinvent the wheel.
What economic barriers does EMR present?
Page: The economic barrier is really not capital expense but the perception that, for a significant period, EMR will require additional time from the medical staff, which reduces the number of patients seen by a physician and, therefore, affects compensation.”
VanMeter: It seems that, when you purchase an EMR system, you have to comply with the way it works. The tail wags the dog. More flexibility in how a system works at the level of the individual provider would make it more economical in terms of productivity.
What features are lacking that causes you to delay adoption?
Page: Successful voice activation and complete handwriting functionality from laptop to chart.
Are there political barriers to adoption?
Page: EMR represents change, and this is always difficult for larger physician groups. Some physicians are still hesitant to make the transition to an EMR from a paper chart, even when the benefit of EMR is proven. Others are hesitant because they are not acclimated to using a computer in the setting of a patient visit.
VanMeter: First, and foremost, the buy-in of all physicians in a group is needed. In my group of 16 physicians and two nurse practitioners, this is tough—especially when age ranges from 31 to 67 years (four in their 60s and close to retirement). Finding consensus on a system will be difficult for that reason alone.
Second, for physicians who are in the twilight of their career, there’s hesitancy to spend a large sum on a new system that, for them, is going to have a relatively short life span.
Third, and last, I am concerned about up-coding. Although an EMR system may allow you to document a level-4 or level-5 service, is that truly necessary for the patient’s problem? With a yeast infection, for example, is a level-4 or level-5 service appropriate, even if the documentation supports it?
Did this roundtable—or the descriptive article on EMR in the July 2007 issue of OBG Management—leave you with questions on what electronic medical records can do for your practice? Write to the Editors at [email protected] and tell us what you still need to know. Your question may become part of upcoming coverage of the topic in these pages.
Dr. Bates is founder and chief executive officer of digiChart, Inc., an electronic medical records system for ObGyn practices.
Dr. Shuwarger is a current user of digiChart’s electronic medical records system for ObGyns. He pays for his service and received no consideration for this article from digiChart.
Dr. Hall, Dr. Page, and Mr. VanMeter report no financial relationships relevant to this article.
MODERATOR

G. William Bates, MD, MBA
Vanderbilt University Medical Center, Nashville, Tenn
PANELISTS
Have introduced EMR to their practice

B. David Hall, MD, FACOG
Rowan OB/GYN Associates, Salisbury, NC

Don Shuwarger, MD, FACOG
Forest Women’s Center, Forest, Va
PANELISTS
Have not introduced EMR

Frank O. Page, MD, FACOG
Henderson Walton Women’s Center, Birmingham, Ala

Mark A. VanMeter
Group Practice Manager, Columbus Obstetricians– Gynecologists, Inc., Columbus, Ohio
Are your colleagues in private practice who have made the transition to a system of electronic medical records (EMR) satisfied with their decision and experience? Yes and, on some points, less than yes.
For practices that—perhaps, like yours—haven’t made the leap, the question is: What’s holding them back?
In this concluding installment of a two-part article on EMR, a panel of three ObGyns and one ObGyn practice administrator talk with Moderator G. William Bates, MD, MBA, about, in the case of two practices, the work of bringing EMR into their offices. Two other panelists describe their practices’ calculated reluctance to discard paper processes right now.
Why have you and your partners adopted EMR?
Shuwarger: Our practice quickly identified the direct and indirect benefits of bringing technology to bear on our processes. Paper records were often illegible, misplaced, or being used by another staff member. We recognized that to meet our internal goals for growth, increasing patient safety, and streamlining processes, we would have to adopt an EMR solution that met those needs.
Hall: Our practice was drowning in paperwork. An exam room was recently converted to hold more charts, and two warehouses held our overflow. Employees were constantly searching for records, and telephone messages were delayed for hours or days until the chart could be reviewed. Notoriously bad handwriting and incomplete documentation hampered good communication and good medical care. Transcription costs were out of control. Forms helped but added to ongoing costs and storage problems.
What efficiency gains have you achieved?
Shuwarger: Forest Women’s Center is able to see more patients in the day because our ObGyn-specific EMR system has a “Patient Portal” that enables patients to enter all their history and complaint-specific information in advance of a visit. Another efficiency is the time gained by never searching for lost or misplaced charts. We also like the ability to access our records 24-7-365.
Hall: The patient’s chart is readily available. Hours of searching have been eliminated, and patients’ questions, lab reports, and prescription refills can be managed with very few steps. The physician can record recommendations and treatment plans, which the staff relays to the patient. Records take about the same time to finish, but they are much more complete and legible, with dramatic gains in safety for the patient and improved liability protection for the physician.
Which features provide the greatest value?
Shuwarger: The patient portal that I mentioned is a great time saver for us. We were amazed at the acceptance and rapid adoption. Even our octogenarians love it. Universal access to data is of incalculable value. One of our physicians loves to go home early, have dinner, and then review his charts from home. EMR improves my recordkeeping, makes encounter documentation more complete, and helps me avoid medication errors. Our billing staff loves the thorough documentation when it is time to file or appeal claims.
Hall: Immediate access to a clear, legible, and complete patient record provides a solid foundation for our medical decision making.
How have your patients reacted to your conversion from paper to EMR?
Shuwarger: At the beginning, there were people who resisted the patient portal, but when they saw for themselves how it enhances the visit experience and helps their physician address their needs, they became vocal proponents.
Hall: Our patients are impressed with our knowledge of their history, with the fact that reports are immediately available, and with how responsive our staff is to their needs. Rather than creating a barrier to communication, TabletPCs allow them to see images of their own procedures, illustrations, treatment outlines, and even education videos. Flow sheets help mark their progress or encourage them to better adherence. Many seem pleased that their medical records are so cutting-edge. Their confidence in our medical skills appears enhanced.
Has your vendor met expectations?
Shuwarger: No—our vendor exceeded our expectations. We had experience with technology vendors before—“We’ll overpromise and underdeliver” was their mantra! With our EMR vendor, however, our preparation was outstanding, the training was thorough, and implementation went better than any we had experienced. Our uptime has exceeded expectations. Enhancements have been well thought out.
And customer support was good at first but now is even better.
Hall: The program is extremely powerful, with an excellent architecture, but its flexibility is also its main limitation. Recently, core clinical content for primary care medicine has been added, but specialty content remains severely limited. Value-added vendors have developed—at additional cost—excellent form-editing tools and specialty forms, and a vigorous users’ community is generous in sharing forms and workflows. But untold hours were required to develop clinical and office workflows, document templates, and just to discover all the options in the system. The learning curve was huge, and further automation requires the skills of a computer programmer.
Our EMR and practice management systems are interfaced but not integrated—even though the same vendor developed them. The problem is that the interface requires several translation programs and multiple servers to implement. Our dependence on our network engineering firm to maintain our bank of servers and interfaces is worrisome— and costly.
Training on our system was inadequate. The basics of the system were covered but, beyond that, we are just now able to shift into second gear. Much of the system’s potential remains untapped.
What is your approximate return on investment?
Shuwarger: We’ve grown receipts by 20%, year over year, since going with our ObGyn-specific EMR system. The rise in revenue is related directly to increased productivity, a reduction in lost charges, and improved collection from third-party payers because we can provide better documentation. At the least, our EMR system has returned $3 for every $1 spent, not counting intangibles.
Hall: Charge capture is much more complete and accurate, with readily available codes and guidelines. The greatest savings are in chart transcription, management, and storage.
Ongoing maintenance and upgrade costs, including hardware and networking software, have gone far beyond our initial investment, however. Problems with training and initial workflow design have slowed our return on investment. But we’re making progress in that direction.
- Streamlined history-taking and complaint-reporting may mean greater productivity in a practice—and a resulting ability to see more patients in a day
- A so-called patient portal gives patients easier access to providers and the varied resources and services of a practice, which boosts satisfaction
- Caveat emptor! Shop carefully when selecting a system vendor—the experiences of practices from installation through system maintenance range very widely
- Interconnectivity between an EMR system and other databases is not a given
- For a large, multisite practice, the cost of hardware alone may have a chilling effect on implementing an EMR system
- All physicians in a practice must buy into an EMR system that’s being put into place—and a range of ages, attitudes, and practice patterns may be a cause for disagreement on how the system is to be best used
- There is concern among some that the federal government may shape the future of EMR by mandating that all systems in private practices interface with hospitals, insurers, and other providers.
Are features lacking that would bring greater efficiency?
Shuwarger: Our labor suite wants data from our ACOG obstetric record to flow into its system to avoid the need to reenter data manually. And our practice’s physicians want the labor and delivery summary to populate our EMR. These issues of interconnection will be worked out as CCHIT certification (see “EMR certifying body arises from the private sector,” page 62) brings disparate systems into proximity.
Hall: Physicians aren’t computer programmers. We practice medicine, not EMR system development, and we are rarely on top of the “best practices” in practice workflow. Many of us who work with EMR may wish to customize a system to the way we practice, but that is not the best way to proceed. A robust and comprehensive specialty-specific set of clinical content that can be loaded as a unit and easily updated is going to provide far greater efficiency than an infinitely customizable basic program.
I look forward to being able to integrate our private medical record with a central data repository, in which interactions with other specialists and medical centers—not the faulty memory of patients—provide a more accurate background and reduce costly duplication of our increasingly stretched medical resources.
In 2004, President George W. Bush set a goal: nationwide adoption of EMR—to include all medical practices—within a decade. Subsequently, the US Department of Health and Human Services (HHS) established the Office of the National Coordinator for Health Information Technology and the American Health Information Community. The sweeping goal of these bodies? Better health care by application of information technology and creation of standards for certifying EMR systems that provide core functionality.
In response, three private-sector health information management groups jointly formed the Certification Commission for Healthcare Information Technology (CCHIT; www.cchit.org). In 2005, this independent private-sector entity entered into a contract with HHS, to, in the commission’s words, “develop and evaluate certification criteria and create a voluntary inspection process for healthcare information technology” in three areas:
- Ambulatory EMR for offices
- Inpatient EMR for hospitals and health systems
- The network components through which EMR share information.
The work of CCHIT is ongoing; the commission provides voluntary certification of EMR systems, publishes a list of certified EMR systems, provides consultative services to providers and payers through its Web site, and even offers a bank of resources for patients on the intricacies and legalities of medical-record-keeping.
Why haven’t you and your partners adopted EMR?
Page: We recently converted to a new practice management software system, and we want to have all systems working properly and efficiently before implementing an EMR system. All options and processes must be reviewed before we implement EMR for the practice. These options include voice-activation software integrated with the EMR, practice process changes, and practice workflow adaptation.
VanMeter: For our independent practice, with five locations, the initial cost of hardware and software is clearly an early concern. With a rapidly changing hardware environment, once a decision is made, the technology that was proposed may be obsolete before being implemented. Then the continuing cost of hardware and software upgrades—read: “the newest gadget”—and maintenance is also a major budgetary item that we need to consider.
As with most medical practices, our organizational structure is flat. If we were to implement a client-server application, we’d need a systems administrator—and that again increases the cost to the practice. Then we’re faced with the question of how we best utilize this person. Or do we outsource this function? And outsourcing then raises a concern of timely responsiveness to major system problems that may extend downtime, prohibiting the use of your EMR system.
Today, telecommunication costs have plummeted, so the costs of a T-1 line [for high-volume Internet access] and high-speed Internet service are not as onerous as they once were. But a major expense will be to retrofit all our offices (wiring, etc.) to adapt to an electronic environment.
Overall, this is a young industry. I compare it to what we saw with video-tape technology in the 1970s: You had to choose between Beta and VHS formats. Once you made that decision, you paid a premium for the early technology.
Similarly, no one knows which EMR system will prevail over time. The early players are paying for the cost of startup and research and development. As time goes on, we all know that costs should fall—significantly.
Another concern that we have is the long-term viability of the software vendor. Until recently, most applications were developed by small independent firms. Their product was a proprietary one—for which only they have the code and only they could manipulate. If that vendor goes out of business, we’d be left to find a new system, and incur all those implementation costs again.
I think we’ll see a major consolidation of vendors over the next several years— one that leaves only premier vendors with superior products in the market.
As a final concern, and perhaps most important, the role of the federal government weighs heavily on our minds. We believe that, very soon, Washington will mandate EMR and how they are to be accomplished. We also believe that the feds will require integration of medical practice EMR systems with the systems of hospitals, third-party payers, and other medical providers. Our belief is that money may become available—like the funding recently authorized for hospitals to subsidize software and maintenance costs—that will defray the cost of implementing an EMR system in our practice. When this comes to pass, we don’t want to have to reinvent the wheel.
What economic barriers does EMR present?
Page: The economic barrier is really not capital expense but the perception that, for a significant period, EMR will require additional time from the medical staff, which reduces the number of patients seen by a physician and, therefore, affects compensation.”
VanMeter: It seems that, when you purchase an EMR system, you have to comply with the way it works. The tail wags the dog. More flexibility in how a system works at the level of the individual provider would make it more economical in terms of productivity.
What features are lacking that causes you to delay adoption?
Page: Successful voice activation and complete handwriting functionality from laptop to chart.
Are there political barriers to adoption?
Page: EMR represents change, and this is always difficult for larger physician groups. Some physicians are still hesitant to make the transition to an EMR from a paper chart, even when the benefit of EMR is proven. Others are hesitant because they are not acclimated to using a computer in the setting of a patient visit.
VanMeter: First, and foremost, the buy-in of all physicians in a group is needed. In my group of 16 physicians and two nurse practitioners, this is tough—especially when age ranges from 31 to 67 years (four in their 60s and close to retirement). Finding consensus on a system will be difficult for that reason alone.
Second, for physicians who are in the twilight of their career, there’s hesitancy to spend a large sum on a new system that, for them, is going to have a relatively short life span.
Third, and last, I am concerned about up-coding. Although an EMR system may allow you to document a level-4 or level-5 service, is that truly necessary for the patient’s problem? With a yeast infection, for example, is a level-4 or level-5 service appropriate, even if the documentation supports it?
Did this roundtable—or the descriptive article on EMR in the July 2007 issue of OBG Management—leave you with questions on what electronic medical records can do for your practice? Write to the Editors at [email protected] and tell us what you still need to know. Your question may become part of upcoming coverage of the topic in these pages.
MODERATOR

G. William Bates, MD, MBA
Vanderbilt University Medical Center, Nashville, Tenn
PANELISTS
Have introduced EMR to their practice

B. David Hall, MD, FACOG
Rowan OB/GYN Associates, Salisbury, NC

Don Shuwarger, MD, FACOG
Forest Women’s Center, Forest, Va
PANELISTS
Have not introduced EMR

Frank O. Page, MD, FACOG
Henderson Walton Women’s Center, Birmingham, Ala

Mark A. VanMeter
Group Practice Manager, Columbus Obstetricians– Gynecologists, Inc., Columbus, Ohio
Are your colleagues in private practice who have made the transition to a system of electronic medical records (EMR) satisfied with their decision and experience? Yes and, on some points, less than yes.
For practices that—perhaps, like yours—haven’t made the leap, the question is: What’s holding them back?
In this concluding installment of a two-part article on EMR, a panel of three ObGyns and one ObGyn practice administrator talk with Moderator G. William Bates, MD, MBA, about, in the case of two practices, the work of bringing EMR into their offices. Two other panelists describe their practices’ calculated reluctance to discard paper processes right now.
Why have you and your partners adopted EMR?
Shuwarger: Our practice quickly identified the direct and indirect benefits of bringing technology to bear on our processes. Paper records were often illegible, misplaced, or being used by another staff member. We recognized that to meet our internal goals for growth, increasing patient safety, and streamlining processes, we would have to adopt an EMR solution that met those needs.
Hall: Our practice was drowning in paperwork. An exam room was recently converted to hold more charts, and two warehouses held our overflow. Employees were constantly searching for records, and telephone messages were delayed for hours or days until the chart could be reviewed. Notoriously bad handwriting and incomplete documentation hampered good communication and good medical care. Transcription costs were out of control. Forms helped but added to ongoing costs and storage problems.
What efficiency gains have you achieved?
Shuwarger: Forest Women’s Center is able to see more patients in the day because our ObGyn-specific EMR system has a “Patient Portal” that enables patients to enter all their history and complaint-specific information in advance of a visit. Another efficiency is the time gained by never searching for lost or misplaced charts. We also like the ability to access our records 24-7-365.
Hall: The patient’s chart is readily available. Hours of searching have been eliminated, and patients’ questions, lab reports, and prescription refills can be managed with very few steps. The physician can record recommendations and treatment plans, which the staff relays to the patient. Records take about the same time to finish, but they are much more complete and legible, with dramatic gains in safety for the patient and improved liability protection for the physician.
Which features provide the greatest value?
Shuwarger: The patient portal that I mentioned is a great time saver for us. We were amazed at the acceptance and rapid adoption. Even our octogenarians love it. Universal access to data is of incalculable value. One of our physicians loves to go home early, have dinner, and then review his charts from home. EMR improves my recordkeeping, makes encounter documentation more complete, and helps me avoid medication errors. Our billing staff loves the thorough documentation when it is time to file or appeal claims.
Hall: Immediate access to a clear, legible, and complete patient record provides a solid foundation for our medical decision making.
How have your patients reacted to your conversion from paper to EMR?
Shuwarger: At the beginning, there were people who resisted the patient portal, but when they saw for themselves how it enhances the visit experience and helps their physician address their needs, they became vocal proponents.
Hall: Our patients are impressed with our knowledge of their history, with the fact that reports are immediately available, and with how responsive our staff is to their needs. Rather than creating a barrier to communication, TabletPCs allow them to see images of their own procedures, illustrations, treatment outlines, and even education videos. Flow sheets help mark their progress or encourage them to better adherence. Many seem pleased that their medical records are so cutting-edge. Their confidence in our medical skills appears enhanced.
Has your vendor met expectations?
Shuwarger: No—our vendor exceeded our expectations. We had experience with technology vendors before—“We’ll overpromise and underdeliver” was their mantra! With our EMR vendor, however, our preparation was outstanding, the training was thorough, and implementation went better than any we had experienced. Our uptime has exceeded expectations. Enhancements have been well thought out.
And customer support was good at first but now is even better.
Hall: The program is extremely powerful, with an excellent architecture, but its flexibility is also its main limitation. Recently, core clinical content for primary care medicine has been added, but specialty content remains severely limited. Value-added vendors have developed—at additional cost—excellent form-editing tools and specialty forms, and a vigorous users’ community is generous in sharing forms and workflows. But untold hours were required to develop clinical and office workflows, document templates, and just to discover all the options in the system. The learning curve was huge, and further automation requires the skills of a computer programmer.
Our EMR and practice management systems are interfaced but not integrated—even though the same vendor developed them. The problem is that the interface requires several translation programs and multiple servers to implement. Our dependence on our network engineering firm to maintain our bank of servers and interfaces is worrisome— and costly.
Training on our system was inadequate. The basics of the system were covered but, beyond that, we are just now able to shift into second gear. Much of the system’s potential remains untapped.
What is your approximate return on investment?
Shuwarger: We’ve grown receipts by 20%, year over year, since going with our ObGyn-specific EMR system. The rise in revenue is related directly to increased productivity, a reduction in lost charges, and improved collection from third-party payers because we can provide better documentation. At the least, our EMR system has returned $3 for every $1 spent, not counting intangibles.
Hall: Charge capture is much more complete and accurate, with readily available codes and guidelines. The greatest savings are in chart transcription, management, and storage.
Ongoing maintenance and upgrade costs, including hardware and networking software, have gone far beyond our initial investment, however. Problems with training and initial workflow design have slowed our return on investment. But we’re making progress in that direction.
- Streamlined history-taking and complaint-reporting may mean greater productivity in a practice—and a resulting ability to see more patients in a day
- A so-called patient portal gives patients easier access to providers and the varied resources and services of a practice, which boosts satisfaction
- Caveat emptor! Shop carefully when selecting a system vendor—the experiences of practices from installation through system maintenance range very widely
- Interconnectivity between an EMR system and other databases is not a given
- For a large, multisite practice, the cost of hardware alone may have a chilling effect on implementing an EMR system
- All physicians in a practice must buy into an EMR system that’s being put into place—and a range of ages, attitudes, and practice patterns may be a cause for disagreement on how the system is to be best used
- There is concern among some that the federal government may shape the future of EMR by mandating that all systems in private practices interface with hospitals, insurers, and other providers.
Are features lacking that would bring greater efficiency?
Shuwarger: Our labor suite wants data from our ACOG obstetric record to flow into its system to avoid the need to reenter data manually. And our practice’s physicians want the labor and delivery summary to populate our EMR. These issues of interconnection will be worked out as CCHIT certification (see “EMR certifying body arises from the private sector,” page 62) brings disparate systems into proximity.
Hall: Physicians aren’t computer programmers. We practice medicine, not EMR system development, and we are rarely on top of the “best practices” in practice workflow. Many of us who work with EMR may wish to customize a system to the way we practice, but that is not the best way to proceed. A robust and comprehensive specialty-specific set of clinical content that can be loaded as a unit and easily updated is going to provide far greater efficiency than an infinitely customizable basic program.
I look forward to being able to integrate our private medical record with a central data repository, in which interactions with other specialists and medical centers—not the faulty memory of patients—provide a more accurate background and reduce costly duplication of our increasingly stretched medical resources.
In 2004, President George W. Bush set a goal: nationwide adoption of EMR—to include all medical practices—within a decade. Subsequently, the US Department of Health and Human Services (HHS) established the Office of the National Coordinator for Health Information Technology and the American Health Information Community. The sweeping goal of these bodies? Better health care by application of information technology and creation of standards for certifying EMR systems that provide core functionality.
In response, three private-sector health information management groups jointly formed the Certification Commission for Healthcare Information Technology (CCHIT; www.cchit.org). In 2005, this independent private-sector entity entered into a contract with HHS, to, in the commission’s words, “develop and evaluate certification criteria and create a voluntary inspection process for healthcare information technology” in three areas:
- Ambulatory EMR for offices
- Inpatient EMR for hospitals and health systems
- The network components through which EMR share information.
The work of CCHIT is ongoing; the commission provides voluntary certification of EMR systems, publishes a list of certified EMR systems, provides consultative services to providers and payers through its Web site, and even offers a bank of resources for patients on the intricacies and legalities of medical-record-keeping.
Why haven’t you and your partners adopted EMR?
Page: We recently converted to a new practice management software system, and we want to have all systems working properly and efficiently before implementing an EMR system. All options and processes must be reviewed before we implement EMR for the practice. These options include voice-activation software integrated with the EMR, practice process changes, and practice workflow adaptation.
VanMeter: For our independent practice, with five locations, the initial cost of hardware and software is clearly an early concern. With a rapidly changing hardware environment, once a decision is made, the technology that was proposed may be obsolete before being implemented. Then the continuing cost of hardware and software upgrades—read: “the newest gadget”—and maintenance is also a major budgetary item that we need to consider.
As with most medical practices, our organizational structure is flat. If we were to implement a client-server application, we’d need a systems administrator—and that again increases the cost to the practice. Then we’re faced with the question of how we best utilize this person. Or do we outsource this function? And outsourcing then raises a concern of timely responsiveness to major system problems that may extend downtime, prohibiting the use of your EMR system.
Today, telecommunication costs have plummeted, so the costs of a T-1 line [for high-volume Internet access] and high-speed Internet service are not as onerous as they once were. But a major expense will be to retrofit all our offices (wiring, etc.) to adapt to an electronic environment.
Overall, this is a young industry. I compare it to what we saw with video-tape technology in the 1970s: You had to choose between Beta and VHS formats. Once you made that decision, you paid a premium for the early technology.
Similarly, no one knows which EMR system will prevail over time. The early players are paying for the cost of startup and research and development. As time goes on, we all know that costs should fall—significantly.
Another concern that we have is the long-term viability of the software vendor. Until recently, most applications were developed by small independent firms. Their product was a proprietary one—for which only they have the code and only they could manipulate. If that vendor goes out of business, we’d be left to find a new system, and incur all those implementation costs again.
I think we’ll see a major consolidation of vendors over the next several years— one that leaves only premier vendors with superior products in the market.
As a final concern, and perhaps most important, the role of the federal government weighs heavily on our minds. We believe that, very soon, Washington will mandate EMR and how they are to be accomplished. We also believe that the feds will require integration of medical practice EMR systems with the systems of hospitals, third-party payers, and other medical providers. Our belief is that money may become available—like the funding recently authorized for hospitals to subsidize software and maintenance costs—that will defray the cost of implementing an EMR system in our practice. When this comes to pass, we don’t want to have to reinvent the wheel.
What economic barriers does EMR present?
Page: The economic barrier is really not capital expense but the perception that, for a significant period, EMR will require additional time from the medical staff, which reduces the number of patients seen by a physician and, therefore, affects compensation.”
VanMeter: It seems that, when you purchase an EMR system, you have to comply with the way it works. The tail wags the dog. More flexibility in how a system works at the level of the individual provider would make it more economical in terms of productivity.
What features are lacking that causes you to delay adoption?
Page: Successful voice activation and complete handwriting functionality from laptop to chart.
Are there political barriers to adoption?
Page: EMR represents change, and this is always difficult for larger physician groups. Some physicians are still hesitant to make the transition to an EMR from a paper chart, even when the benefit of EMR is proven. Others are hesitant because they are not acclimated to using a computer in the setting of a patient visit.
VanMeter: First, and foremost, the buy-in of all physicians in a group is needed. In my group of 16 physicians and two nurse practitioners, this is tough—especially when age ranges from 31 to 67 years (four in their 60s and close to retirement). Finding consensus on a system will be difficult for that reason alone.
Second, for physicians who are in the twilight of their career, there’s hesitancy to spend a large sum on a new system that, for them, is going to have a relatively short life span.
Third, and last, I am concerned about up-coding. Although an EMR system may allow you to document a level-4 or level-5 service, is that truly necessary for the patient’s problem? With a yeast infection, for example, is a level-4 or level-5 service appropriate, even if the documentation supports it?
Did this roundtable—or the descriptive article on EMR in the July 2007 issue of OBG Management—leave you with questions on what electronic medical records can do for your practice? Write to the Editors at [email protected] and tell us what you still need to know. Your question may become part of upcoming coverage of the topic in these pages.
Dr. Bates is founder and chief executive officer of digiChart, Inc., an electronic medical records system for ObGyn practices.
Dr. Shuwarger is a current user of digiChart’s electronic medical records system for ObGyns. He pays for his service and received no consideration for this article from digiChart.
Dr. Hall, Dr. Page, and Mr. VanMeter report no financial relationships relevant to this article.
Dr. Bates is founder and chief executive officer of digiChart, Inc., an electronic medical records system for ObGyn practices.
Dr. Shuwarger is a current user of digiChart’s electronic medical records system for ObGyns. He pays for his service and received no consideration for this article from digiChart.
Dr. Hall, Dr. Page, and Mr. VanMeter report no financial relationships relevant to this article.
Repair of a constricted or shortened vagina: What works?
To watch a demonstration of the surgical treatment of vaginal stenosis using bilateral groin flaps and a demonstration of the takedown of iatrogenic vaginal constriction, visit the Video Library.
Patients who want to be sexually active but suffer iatrogenic vaginal constriction or shortening, or both, are a surgical challenge. Their condition may require any of a variety of nonsurgical and surgical procedures to restore the ability to have gratifying sexual intercourse, and they may need considerable preoperative and postoperative counseling and management.
What is the basis of this problem?
The cause of vaginal shortening or constriction is most often surgical. Rarely is systemic disease or a localized condition, such as urogenital atrophy, responsible.
Prolapse procedures. Most procedures that result in vaginal shortening or constriction are ones performed to correct pelvic organ prolapse (POP), notably:
- posterior colpoperineorrhaphy with levatorplasty
- hysterectomy, whether abdominal or vaginal, during which too much of the upper vagina is taken with the cervix
- anterior and posterior colporrhaphy in which vaginal plication and trimming are performed overzealously.
Radiation therapy to the pelvis can result in vaginal shortening, constriction, and obliteration.
How do you avoid creating these problems?
Techniques to avoid vaginal shortening and constriction during vaginal reconstructive surgery include appropriate use of levatorplasty during posterior colpoperineorrhaphy. Although levatorplasty is, at times, the only way to decrease the size of a large vaginal hiatus, it should be used only in the distal third of the vagina. Levatorplasty above this area often creates vaginal constriction that results in postoperative dyspareunia.
Also, avoid 1) overzealous trimming during anterior and posterior colporrhaphy and 2) removing too much vagina at vaginal or abdominal hysterectomy.
Last, it is important that a patient who has undergone vaginal reconstructive surgery have a vaginal exam within 2 weeks after surgery. This will ensure that the vaginal incisions do not fuse, thus creating vaginal scarring, closure, and constriction.
How is correction approached?
Various modifications of a McIndoe procedure have been described for vaginal agenesis, but surgical correction of iatrogenic vaginal shortening or constriction is not well described; few case series exist in the literature. Consensus is lacking on what the minimal length of a vagina must be to preserve normal sexual function, and no standard exists in regards to either normal vaginal caliber or the relationship of the perineum and perineal body to the distal posterior vagina.
That being said, we have recognized the following correlates of a successful return to sexual function after surgery for vaginal constriction or shortening:
- The vagina should, at minimum, be 7 cm long to have the potential for normal function
- The vaginal opening should easily admit two fingers during examination
- The relationship of the posterior vagina and the perineum should be a perpendicular one, in which a built-up perineum attaches to the posterior vagina at the posterior fourchette at a 90° angle
- There should be no buildup of perineal skin above and beyond the posterior fourchette.
Is surgery the first intervention?
No. The patient should first undergo an attempt at nonsurgical management. This usually involves:
- vaginal estrogen cream in a postmenopausal patient
- appropriate utilization of a vaginal dilator.
What are options for surgery?
If nonsurgical management of vaginal constriction or shortening is unsuccessful or unsatisfactory, next choose an operation based on the needs of the individual patient. Some procedures involve placement of a skin graft or, possibly, other biologic material in the vagina to close over defects after constriction has been taken down or the vagina has been appropriately opened up. (It is fortunate that the vagina heals well by secondary intention; often, simply taking down the constriction and allowing the vagina to heal by secondary intention is successful.)
It is important to cut through the constriction and completely separate the tissue during the takedown of vaginal constriction. At this point, you need to decide whether to allow the separated vagina to heal by secondary intention or to cover the defect with a skin graft or other biologic material.
Whichever course you choose, keep the vagina open during the immediate postoperative period. Doing so may require placement of a vaginal stent, numerous postoperative exams, use of a vaginal dilator, or a combination of these measures.
When constriction rings are present in the face of ample vaginal length, you can perform a Z-plasty, in which the lines of a letter Z are incised transversely or longitudinally across the constricted region and the two flaps that have been created from the Z are transposed. This maneuver releases constriction well.
When constriction extends distally, the procedure used is, basically, a reverse perineoplasty: Cut the constriction band longitudinally, undermine the vagina, and then sew it back transversely. This relieves the distal band.
In a severe case of vaginal constriction, thigh flaps that are left on their vascular pedicle can be brought into the vagina to fill the gap created by cutting through the constriction. Initial incisions are made laterally in the vagina (unilaterally or bilaterally, depending on the degree of constriction) and extended to the perineum/vulva. Measurements are made to determine the length and width of flap(s) needed. The flaps are then mobilized, rotated into the defect(s), and sutured into place. This technique significantly increases the diameter of the vagina and can add length, if needed.
What about correcting shortening?
An iatrogenically shortened vagina presents the most challenging of cases. The vagina must be opened up at the cuff; ideally, this produces adequate length without having to enter the peritoneum.
Dr. Karram is a course director and Dr. Gebhart is on the faculty of the 10th Annual Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS), to be held December 6-8, 2007, in Las Vegas (www.pags-cme.org).
To watch a demonstration of the surgical treatment of vaginal stenosis using bilateral groin flaps and a demonstration of the takedown of iatrogenic vaginal constriction, visit the Video Library.
Patients who want to be sexually active but suffer iatrogenic vaginal constriction or shortening, or both, are a surgical challenge. Their condition may require any of a variety of nonsurgical and surgical procedures to restore the ability to have gratifying sexual intercourse, and they may need considerable preoperative and postoperative counseling and management.
What is the basis of this problem?
The cause of vaginal shortening or constriction is most often surgical. Rarely is systemic disease or a localized condition, such as urogenital atrophy, responsible.
Prolapse procedures. Most procedures that result in vaginal shortening or constriction are ones performed to correct pelvic organ prolapse (POP), notably:
- posterior colpoperineorrhaphy with levatorplasty
- hysterectomy, whether abdominal or vaginal, during which too much of the upper vagina is taken with the cervix
- anterior and posterior colporrhaphy in which vaginal plication and trimming are performed overzealously.
Radiation therapy to the pelvis can result in vaginal shortening, constriction, and obliteration.
How do you avoid creating these problems?
Techniques to avoid vaginal shortening and constriction during vaginal reconstructive surgery include appropriate use of levatorplasty during posterior colpoperineorrhaphy. Although levatorplasty is, at times, the only way to decrease the size of a large vaginal hiatus, it should be used only in the distal third of the vagina. Levatorplasty above this area often creates vaginal constriction that results in postoperative dyspareunia.
Also, avoid 1) overzealous trimming during anterior and posterior colporrhaphy and 2) removing too much vagina at vaginal or abdominal hysterectomy.
Last, it is important that a patient who has undergone vaginal reconstructive surgery have a vaginal exam within 2 weeks after surgery. This will ensure that the vaginal incisions do not fuse, thus creating vaginal scarring, closure, and constriction.
How is correction approached?
Various modifications of a McIndoe procedure have been described for vaginal agenesis, but surgical correction of iatrogenic vaginal shortening or constriction is not well described; few case series exist in the literature. Consensus is lacking on what the minimal length of a vagina must be to preserve normal sexual function, and no standard exists in regards to either normal vaginal caliber or the relationship of the perineum and perineal body to the distal posterior vagina.
That being said, we have recognized the following correlates of a successful return to sexual function after surgery for vaginal constriction or shortening:
- The vagina should, at minimum, be 7 cm long to have the potential for normal function
- The vaginal opening should easily admit two fingers during examination
- The relationship of the posterior vagina and the perineum should be a perpendicular one, in which a built-up perineum attaches to the posterior vagina at the posterior fourchette at a 90° angle
- There should be no buildup of perineal skin above and beyond the posterior fourchette.
Is surgery the first intervention?
No. The patient should first undergo an attempt at nonsurgical management. This usually involves:
- vaginal estrogen cream in a postmenopausal patient
- appropriate utilization of a vaginal dilator.
What are options for surgery?
If nonsurgical management of vaginal constriction or shortening is unsuccessful or unsatisfactory, next choose an operation based on the needs of the individual patient. Some procedures involve placement of a skin graft or, possibly, other biologic material in the vagina to close over defects after constriction has been taken down or the vagina has been appropriately opened up. (It is fortunate that the vagina heals well by secondary intention; often, simply taking down the constriction and allowing the vagina to heal by secondary intention is successful.)
It is important to cut through the constriction and completely separate the tissue during the takedown of vaginal constriction. At this point, you need to decide whether to allow the separated vagina to heal by secondary intention or to cover the defect with a skin graft or other biologic material.
Whichever course you choose, keep the vagina open during the immediate postoperative period. Doing so may require placement of a vaginal stent, numerous postoperative exams, use of a vaginal dilator, or a combination of these measures.
When constriction rings are present in the face of ample vaginal length, you can perform a Z-plasty, in which the lines of a letter Z are incised transversely or longitudinally across the constricted region and the two flaps that have been created from the Z are transposed. This maneuver releases constriction well.
When constriction extends distally, the procedure used is, basically, a reverse perineoplasty: Cut the constriction band longitudinally, undermine the vagina, and then sew it back transversely. This relieves the distal band.
In a severe case of vaginal constriction, thigh flaps that are left on their vascular pedicle can be brought into the vagina to fill the gap created by cutting through the constriction. Initial incisions are made laterally in the vagina (unilaterally or bilaterally, depending on the degree of constriction) and extended to the perineum/vulva. Measurements are made to determine the length and width of flap(s) needed. The flaps are then mobilized, rotated into the defect(s), and sutured into place. This technique significantly increases the diameter of the vagina and can add length, if needed.
What about correcting shortening?
An iatrogenically shortened vagina presents the most challenging of cases. The vagina must be opened up at the cuff; ideally, this produces adequate length without having to enter the peritoneum.
To watch a demonstration of the surgical treatment of vaginal stenosis using bilateral groin flaps and a demonstration of the takedown of iatrogenic vaginal constriction, visit the Video Library.
Patients who want to be sexually active but suffer iatrogenic vaginal constriction or shortening, or both, are a surgical challenge. Their condition may require any of a variety of nonsurgical and surgical procedures to restore the ability to have gratifying sexual intercourse, and they may need considerable preoperative and postoperative counseling and management.
What is the basis of this problem?
The cause of vaginal shortening or constriction is most often surgical. Rarely is systemic disease or a localized condition, such as urogenital atrophy, responsible.
Prolapse procedures. Most procedures that result in vaginal shortening or constriction are ones performed to correct pelvic organ prolapse (POP), notably:
- posterior colpoperineorrhaphy with levatorplasty
- hysterectomy, whether abdominal or vaginal, during which too much of the upper vagina is taken with the cervix
- anterior and posterior colporrhaphy in which vaginal plication and trimming are performed overzealously.
Radiation therapy to the pelvis can result in vaginal shortening, constriction, and obliteration.
How do you avoid creating these problems?
Techniques to avoid vaginal shortening and constriction during vaginal reconstructive surgery include appropriate use of levatorplasty during posterior colpoperineorrhaphy. Although levatorplasty is, at times, the only way to decrease the size of a large vaginal hiatus, it should be used only in the distal third of the vagina. Levatorplasty above this area often creates vaginal constriction that results in postoperative dyspareunia.
Also, avoid 1) overzealous trimming during anterior and posterior colporrhaphy and 2) removing too much vagina at vaginal or abdominal hysterectomy.
Last, it is important that a patient who has undergone vaginal reconstructive surgery have a vaginal exam within 2 weeks after surgery. This will ensure that the vaginal incisions do not fuse, thus creating vaginal scarring, closure, and constriction.
How is correction approached?
Various modifications of a McIndoe procedure have been described for vaginal agenesis, but surgical correction of iatrogenic vaginal shortening or constriction is not well described; few case series exist in the literature. Consensus is lacking on what the minimal length of a vagina must be to preserve normal sexual function, and no standard exists in regards to either normal vaginal caliber or the relationship of the perineum and perineal body to the distal posterior vagina.
That being said, we have recognized the following correlates of a successful return to sexual function after surgery for vaginal constriction or shortening:
- The vagina should, at minimum, be 7 cm long to have the potential for normal function
- The vaginal opening should easily admit two fingers during examination
- The relationship of the posterior vagina and the perineum should be a perpendicular one, in which a built-up perineum attaches to the posterior vagina at the posterior fourchette at a 90° angle
- There should be no buildup of perineal skin above and beyond the posterior fourchette.
Is surgery the first intervention?
No. The patient should first undergo an attempt at nonsurgical management. This usually involves:
- vaginal estrogen cream in a postmenopausal patient
- appropriate utilization of a vaginal dilator.
What are options for surgery?
If nonsurgical management of vaginal constriction or shortening is unsuccessful or unsatisfactory, next choose an operation based on the needs of the individual patient. Some procedures involve placement of a skin graft or, possibly, other biologic material in the vagina to close over defects after constriction has been taken down or the vagina has been appropriately opened up. (It is fortunate that the vagina heals well by secondary intention; often, simply taking down the constriction and allowing the vagina to heal by secondary intention is successful.)
It is important to cut through the constriction and completely separate the tissue during the takedown of vaginal constriction. At this point, you need to decide whether to allow the separated vagina to heal by secondary intention or to cover the defect with a skin graft or other biologic material.
Whichever course you choose, keep the vagina open during the immediate postoperative period. Doing so may require placement of a vaginal stent, numerous postoperative exams, use of a vaginal dilator, or a combination of these measures.
When constriction rings are present in the face of ample vaginal length, you can perform a Z-plasty, in which the lines of a letter Z are incised transversely or longitudinally across the constricted region and the two flaps that have been created from the Z are transposed. This maneuver releases constriction well.
When constriction extends distally, the procedure used is, basically, a reverse perineoplasty: Cut the constriction band longitudinally, undermine the vagina, and then sew it back transversely. This relieves the distal band.
In a severe case of vaginal constriction, thigh flaps that are left on their vascular pedicle can be brought into the vagina to fill the gap created by cutting through the constriction. Initial incisions are made laterally in the vagina (unilaterally or bilaterally, depending on the degree of constriction) and extended to the perineum/vulva. Measurements are made to determine the length and width of flap(s) needed. The flaps are then mobilized, rotated into the defect(s), and sutured into place. This technique significantly increases the diameter of the vagina and can add length, if needed.
What about correcting shortening?
An iatrogenically shortened vagina presents the most challenging of cases. The vagina must be opened up at the cuff; ideally, this produces adequate length without having to enter the peritoneum.
Dr. Karram is a course director and Dr. Gebhart is on the faculty of the 10th Annual Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS), to be held December 6-8, 2007, in Las Vegas (www.pags-cme.org).
Dr. Karram is a course director and Dr. Gebhart is on the faculty of the 10th Annual Pelvic Anatomy and Gynecologic Surgery Symposium (PAGS), to be held December 6-8, 2007, in Las Vegas (www.pags-cme.org).
Treating the range of lower-tract symptoms in prolapse
To watch a demonstration of how pelvic organ prolapse is repaired, visit the Video Library.
Lower urinary tract symptoms are common in women who have pelvic organ prolapse (POP). For some, these symptoms resolve or improve after surgery for prolapse; for others, symptoms remain unchanged or become worse. These clinical pearls can help you decide how to counsel, evaluate, and treat patients who have POP and coexisting lower-tract symptoms.
How are POP and lower-tract symptoms related?
Lower-tract symptoms that result from, or coexist with, POP include urinary incontinence (stress, urge, mixed), irritative symptoms (frequency, urgency, nocturia), and difficulty voiding (hesitancy, weak or intermittent stream). Prolapse can produce lower-tract symptoms by:
- causing urethral obstruction
- dissipating the effects of abdominal pressure during Valsalva voiding, which makes voiding more difficult
- masking sphincteric incontinence.
Is prolapse causing symptoms or masking stress incontinence?
Some clues to answering this question can be obtained from the history:
- If the patient says that she voids better when the prolapse is reduced, prolapse is probably causing urethral obstruction
- If the patient says that she experienced stress incontinence previously but that it has subsided and she now only has difficulty voiding, she probably has occult stress incontinence and, possibly, urethral obstruction.
How is treatment established for lower-tract symptoms?
Prolapse is graded by any of several classifications that are based on the severity and extent of the condition.
Mild degrees of prolapse rarely, if ever, cause urethral obstruction or mask stress incontinence; you can manage lower-tract symptoms in these patients as if they did not have prolapse. Stress incontinence, which is common among these women, can be corrected either in isolation or in conjunction with repair of the prolapse, if such repair is indicated.
More advanced degrees of prolapse, defined as prolapse that extends to or beyond the hymen, are commonly associated with urethral obstruction or occult sphincteric incontinence, or both. This makes it important to diagnose these conditions (by means described earlier) before you intervene surgically to repair the prolapse.
Can surgery for POP affect lower-tract symptoms?
Paradoxically, surgical treatment of prolapse can treat lower-tract symptoms successfully in some patients but cause them in others. How can this be?
- Surgery works when prolapse has caused obstruction and the obstruction is relieved when you resupport the pelvic floor
- Surgery can cause symptoms when occult stress incontinence goes unrecognized and is unmasked after repair of prolapse without concomitant anti-incontinence surgery
- De novo irritative symptoms and OAB can arise secondary to placement of a sling.
Treat all prolapse surgery patients with prophylactic anti-incontinence surgery?
Some experts recommend that practice. But anti-incontinence surgery carries its own risk of complications, so we believe that the need for anti-incontinence surgery should be individualized—based on symptoms, anatomy, the results of diagnostic testing, and the patient’s quality-of-life priorities. Of course, when there is pre-existing or occult stress incontinence, you should routinely consider concomitant anti-incontinence surgery.
When is POP surgery effective for OAB symptoms?
The literature is scant on this question. We believe that, in patients who have an advanced degree of prolapse (especially when urethral obstruction has been documented), symptoms of OAB subside most of the time after effective prolapse surgery.
We do not recommend surgery for mild degrees of prolapse or when there is no pre-existing obstruction.
Drs. Blaivas and Karram co-chair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007, in Las Vegas (www.urogyn-cme.org).
To watch a demonstration of how pelvic organ prolapse is repaired, visit the Video Library.
Lower urinary tract symptoms are common in women who have pelvic organ prolapse (POP). For some, these symptoms resolve or improve after surgery for prolapse; for others, symptoms remain unchanged or become worse. These clinical pearls can help you decide how to counsel, evaluate, and treat patients who have POP and coexisting lower-tract symptoms.
How are POP and lower-tract symptoms related?
Lower-tract symptoms that result from, or coexist with, POP include urinary incontinence (stress, urge, mixed), irritative symptoms (frequency, urgency, nocturia), and difficulty voiding (hesitancy, weak or intermittent stream). Prolapse can produce lower-tract symptoms by:
- causing urethral obstruction
- dissipating the effects of abdominal pressure during Valsalva voiding, which makes voiding more difficult
- masking sphincteric incontinence.
Is prolapse causing symptoms or masking stress incontinence?
Some clues to answering this question can be obtained from the history:
- If the patient says that she voids better when the prolapse is reduced, prolapse is probably causing urethral obstruction
- If the patient says that she experienced stress incontinence previously but that it has subsided and she now only has difficulty voiding, she probably has occult stress incontinence and, possibly, urethral obstruction.
How is treatment established for lower-tract symptoms?
Prolapse is graded by any of several classifications that are based on the severity and extent of the condition.
Mild degrees of prolapse rarely, if ever, cause urethral obstruction or mask stress incontinence; you can manage lower-tract symptoms in these patients as if they did not have prolapse. Stress incontinence, which is common among these women, can be corrected either in isolation or in conjunction with repair of the prolapse, if such repair is indicated.
More advanced degrees of prolapse, defined as prolapse that extends to or beyond the hymen, are commonly associated with urethral obstruction or occult sphincteric incontinence, or both. This makes it important to diagnose these conditions (by means described earlier) before you intervene surgically to repair the prolapse.
Can surgery for POP affect lower-tract symptoms?
Paradoxically, surgical treatment of prolapse can treat lower-tract symptoms successfully in some patients but cause them in others. How can this be?
- Surgery works when prolapse has caused obstruction and the obstruction is relieved when you resupport the pelvic floor
- Surgery can cause symptoms when occult stress incontinence goes unrecognized and is unmasked after repair of prolapse without concomitant anti-incontinence surgery
- De novo irritative symptoms and OAB can arise secondary to placement of a sling.
Treat all prolapse surgery patients with prophylactic anti-incontinence surgery?
Some experts recommend that practice. But anti-incontinence surgery carries its own risk of complications, so we believe that the need for anti-incontinence surgery should be individualized—based on symptoms, anatomy, the results of diagnostic testing, and the patient’s quality-of-life priorities. Of course, when there is pre-existing or occult stress incontinence, you should routinely consider concomitant anti-incontinence surgery.
When is POP surgery effective for OAB symptoms?
The literature is scant on this question. We believe that, in patients who have an advanced degree of prolapse (especially when urethral obstruction has been documented), symptoms of OAB subside most of the time after effective prolapse surgery.
We do not recommend surgery for mild degrees of prolapse or when there is no pre-existing obstruction.
To watch a demonstration of how pelvic organ prolapse is repaired, visit the Video Library.
Lower urinary tract symptoms are common in women who have pelvic organ prolapse (POP). For some, these symptoms resolve or improve after surgery for prolapse; for others, symptoms remain unchanged or become worse. These clinical pearls can help you decide how to counsel, evaluate, and treat patients who have POP and coexisting lower-tract symptoms.
How are POP and lower-tract symptoms related?
Lower-tract symptoms that result from, or coexist with, POP include urinary incontinence (stress, urge, mixed), irritative symptoms (frequency, urgency, nocturia), and difficulty voiding (hesitancy, weak or intermittent stream). Prolapse can produce lower-tract symptoms by:
- causing urethral obstruction
- dissipating the effects of abdominal pressure during Valsalva voiding, which makes voiding more difficult
- masking sphincteric incontinence.
Is prolapse causing symptoms or masking stress incontinence?
Some clues to answering this question can be obtained from the history:
- If the patient says that she voids better when the prolapse is reduced, prolapse is probably causing urethral obstruction
- If the patient says that she experienced stress incontinence previously but that it has subsided and she now only has difficulty voiding, she probably has occult stress incontinence and, possibly, urethral obstruction.
How is treatment established for lower-tract symptoms?
Prolapse is graded by any of several classifications that are based on the severity and extent of the condition.
Mild degrees of prolapse rarely, if ever, cause urethral obstruction or mask stress incontinence; you can manage lower-tract symptoms in these patients as if they did not have prolapse. Stress incontinence, which is common among these women, can be corrected either in isolation or in conjunction with repair of the prolapse, if such repair is indicated.
More advanced degrees of prolapse, defined as prolapse that extends to or beyond the hymen, are commonly associated with urethral obstruction or occult sphincteric incontinence, or both. This makes it important to diagnose these conditions (by means described earlier) before you intervene surgically to repair the prolapse.
Can surgery for POP affect lower-tract symptoms?
Paradoxically, surgical treatment of prolapse can treat lower-tract symptoms successfully in some patients but cause them in others. How can this be?
- Surgery works when prolapse has caused obstruction and the obstruction is relieved when you resupport the pelvic floor
- Surgery can cause symptoms when occult stress incontinence goes unrecognized and is unmasked after repair of prolapse without concomitant anti-incontinence surgery
- De novo irritative symptoms and OAB can arise secondary to placement of a sling.
Treat all prolapse surgery patients with prophylactic anti-incontinence surgery?
Some experts recommend that practice. But anti-incontinence surgery carries its own risk of complications, so we believe that the need for anti-incontinence surgery should be individualized—based on symptoms, anatomy, the results of diagnostic testing, and the patient’s quality-of-life priorities. Of course, when there is pre-existing or occult stress incontinence, you should routinely consider concomitant anti-incontinence surgery.
When is POP surgery effective for OAB symptoms?
The literature is scant on this question. We believe that, in patients who have an advanced degree of prolapse (especially when urethral obstruction has been documented), symptoms of OAB subside most of the time after effective prolapse surgery.
We do not recommend surgery for mild degrees of prolapse or when there is no pre-existing obstruction.
Drs. Blaivas and Karram co-chair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007, in Las Vegas (www.urogyn-cme.org).
Drs. Blaivas and Karram co-chair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007, in Las Vegas (www.urogyn-cme.org).
Managing troublesome urethral diverticula
Urethral diverticula are often overlooked as a source of recurrent urinary tract infection, voiding dysfunction, dyspareunia, and chronic pelvic pain. Here, in brief, is how to diagnose and manage this condition, including a look at surgical options.
What are the common complaints?
Urethral diverticula present in myriad ways—most often, as recurrent urinary tract infection, overactive bladder, stress urinary incontinence, and pelvic pain. Other common presenting symptoms include voiding dysfunction, a painful or palpable mass, and postvoid dribbling.
What can be done routinely during a pelvic exam to make the Dx?
Become accustomed to massaging the anterior vaginal wall underneath the urethra. Any discharge or excretion of fluid that you observe from the external urethral meatus as you massage is pathognomonic for urethral diverticulum. In addition, palpate the anterior vaginal wall for paraurethral masses. Sometimes, a diverticulum is ballotable but not palpable.
Which test is best?
Imaging has been used in different ways, with variable success.
- Most diverticula are well visualized by voiding cystourethrography or magnetic resonance imaging (MRI); we view these as complementary techniques, in fact, because some diverticula are visualized only by one modality or the other. MRI provides a superior examination for surgical planning because it defines urethral and diverticular anatomy most clearly
- Ultrasonography has been used with some success
- Positive-pressure urethrography, using a Tratner or double balloon catheter, is difficult to perform and uncomfortable for the patient.
What is the role of urethroscopy?
We find urethroscopy very helpful. One caveat: Inability to visualize a diverticulum or its opening does not, by any means, exclude a urethral diverticulum.
How should you manage a urethral diverticulum?
- Urinary tract infection should be treated with a culture-specific antibiotic; in some cases, the patient will become asymptomatic afterwards
- Overactive bladder symptoms can be treated with an anticholinergic
- In most cases, surgery proves necessary
- When you identify a urethral diverticulum during pregnancy, manage the patient conservatively during the antenatal period
- A patient who has an asymptomatic urethral diverticulum can be managed expectantly, but perform a pelvic exam periodically.
When is surgery appropriate? By what method?
Several observations are useful:
- Hardness or induration of the diverticular mass is extremely rare; such a finding should prompt surgical excision because it may signal cancer
- Marsupialization has been demonstrated to be successful for very distal and small urethral diverticula
- Most diverticula at the level of the midurethra and proximal urethra require some form of excision, broadly classified as partial ablation or complete excision
- Placement of a suburethral sling is controversial, but some experts believe that, to prevent stress incontinence, this intervention should be undertaken simultaneously with any other surgical treatment for diverticula of the proximal urethra
- Sometimes a Martius fat pad must be brought into the field to avoid devascularization and breakdown of the repair. When a suburethral sling is necessary, we routinely place a Martius flap between the urethra and the sling.
Drs. Karram and Blaivas cochair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007 in Las Vegas (www.urogyn-cme.org).
Urethral diverticula are often overlooked as a source of recurrent urinary tract infection, voiding dysfunction, dyspareunia, and chronic pelvic pain. Here, in brief, is how to diagnose and manage this condition, including a look at surgical options.
What are the common complaints?
Urethral diverticula present in myriad ways—most often, as recurrent urinary tract infection, overactive bladder, stress urinary incontinence, and pelvic pain. Other common presenting symptoms include voiding dysfunction, a painful or palpable mass, and postvoid dribbling.
What can be done routinely during a pelvic exam to make the Dx?
Become accustomed to massaging the anterior vaginal wall underneath the urethra. Any discharge or excretion of fluid that you observe from the external urethral meatus as you massage is pathognomonic for urethral diverticulum. In addition, palpate the anterior vaginal wall for paraurethral masses. Sometimes, a diverticulum is ballotable but not palpable.
Which test is best?
Imaging has been used in different ways, with variable success.
- Most diverticula are well visualized by voiding cystourethrography or magnetic resonance imaging (MRI); we view these as complementary techniques, in fact, because some diverticula are visualized only by one modality or the other. MRI provides a superior examination for surgical planning because it defines urethral and diverticular anatomy most clearly
- Ultrasonography has been used with some success
- Positive-pressure urethrography, using a Tratner or double balloon catheter, is difficult to perform and uncomfortable for the patient.
What is the role of urethroscopy?
We find urethroscopy very helpful. One caveat: Inability to visualize a diverticulum or its opening does not, by any means, exclude a urethral diverticulum.
How should you manage a urethral diverticulum?
- Urinary tract infection should be treated with a culture-specific antibiotic; in some cases, the patient will become asymptomatic afterwards
- Overactive bladder symptoms can be treated with an anticholinergic
- In most cases, surgery proves necessary
- When you identify a urethral diverticulum during pregnancy, manage the patient conservatively during the antenatal period
- A patient who has an asymptomatic urethral diverticulum can be managed expectantly, but perform a pelvic exam periodically.
When is surgery appropriate? By what method?
Several observations are useful:
- Hardness or induration of the diverticular mass is extremely rare; such a finding should prompt surgical excision because it may signal cancer
- Marsupialization has been demonstrated to be successful for very distal and small urethral diverticula
- Most diverticula at the level of the midurethra and proximal urethra require some form of excision, broadly classified as partial ablation or complete excision
- Placement of a suburethral sling is controversial, but some experts believe that, to prevent stress incontinence, this intervention should be undertaken simultaneously with any other surgical treatment for diverticula of the proximal urethra
- Sometimes a Martius fat pad must be brought into the field to avoid devascularization and breakdown of the repair. When a suburethral sling is necessary, we routinely place a Martius flap between the urethra and the sling.
Urethral diverticula are often overlooked as a source of recurrent urinary tract infection, voiding dysfunction, dyspareunia, and chronic pelvic pain. Here, in brief, is how to diagnose and manage this condition, including a look at surgical options.
What are the common complaints?
Urethral diverticula present in myriad ways—most often, as recurrent urinary tract infection, overactive bladder, stress urinary incontinence, and pelvic pain. Other common presenting symptoms include voiding dysfunction, a painful or palpable mass, and postvoid dribbling.
What can be done routinely during a pelvic exam to make the Dx?
Become accustomed to massaging the anterior vaginal wall underneath the urethra. Any discharge or excretion of fluid that you observe from the external urethral meatus as you massage is pathognomonic for urethral diverticulum. In addition, palpate the anterior vaginal wall for paraurethral masses. Sometimes, a diverticulum is ballotable but not palpable.
Which test is best?
Imaging has been used in different ways, with variable success.
- Most diverticula are well visualized by voiding cystourethrography or magnetic resonance imaging (MRI); we view these as complementary techniques, in fact, because some diverticula are visualized only by one modality or the other. MRI provides a superior examination for surgical planning because it defines urethral and diverticular anatomy most clearly
- Ultrasonography has been used with some success
- Positive-pressure urethrography, using a Tratner or double balloon catheter, is difficult to perform and uncomfortable for the patient.
What is the role of urethroscopy?
We find urethroscopy very helpful. One caveat: Inability to visualize a diverticulum or its opening does not, by any means, exclude a urethral diverticulum.
How should you manage a urethral diverticulum?
- Urinary tract infection should be treated with a culture-specific antibiotic; in some cases, the patient will become asymptomatic afterwards
- Overactive bladder symptoms can be treated with an anticholinergic
- In most cases, surgery proves necessary
- When you identify a urethral diverticulum during pregnancy, manage the patient conservatively during the antenatal period
- A patient who has an asymptomatic urethral diverticulum can be managed expectantly, but perform a pelvic exam periodically.
When is surgery appropriate? By what method?
Several observations are useful:
- Hardness or induration of the diverticular mass is extremely rare; such a finding should prompt surgical excision because it may signal cancer
- Marsupialization has been demonstrated to be successful for very distal and small urethral diverticula
- Most diverticula at the level of the midurethra and proximal urethra require some form of excision, broadly classified as partial ablation or complete excision
- Placement of a suburethral sling is controversial, but some experts believe that, to prevent stress incontinence, this intervention should be undertaken simultaneously with any other surgical treatment for diverticula of the proximal urethra
- Sometimes a Martius fat pad must be brought into the field to avoid devascularization and breakdown of the repair. When a suburethral sling is necessary, we routinely place a Martius flap between the urethra and the sling.
Drs. Karram and Blaivas cochair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007 in Las Vegas (www.urogyn-cme.org).
Drs. Karram and Blaivas cochair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007 in Las Vegas (www.urogyn-cme.org).
How to work up and treat voiding dysfunction after surgery for stress incontinence
Watch a demonstration of the surgical takedown of anti-incontinence procedures.
Voiding dysfunction—either difficulty voiding or urinary retention—after surgery for stress incontinence distresses the patient and challenges the surgeon. Here is our systematic approach to evaluating and managing such cases.
What does the operative note say?
Determine exactly what operation the patient underwent and whether appropriate steps were taken during surgery to evaluate the lower urinary tract. Remember: There are well over 30 different synthetic midurethral slings on the market; a variety of biologic materials are used for slings; and conventional suspension procedures are still being performed. Sling composition and surgical technique are the major determinants of subsequent treatment, so it is imperative to obtain the operative note.
Is intermittent self-catheterization an option?
If the patient has an indwelling catheter—of any type—remove it whenever possible and teach her intermittent self-catheterization.
Are symptoms consistent with expected outcome?
In the case of a patient who had a large cystocele repair in conjunction with an anti-incontinence procedure, for example, it is common for some form of retention or voiding dysfunction to be present for 2 weeks or longer. On the other hand, if a patient had a synthetic midurethral sling but no other procedure, it is highly unlikely, during a normal postoperative course, that she would be in retention 2 weeks after the procedure—unless the sling was placed too tightly.
Is there actual (or impending) lower-tract injury? Foreign body penetration?
Good endoscopic evaluation, with visualization of the urethra, of the vesical neck and anterolateral walls of the bladder, will answer these questions.
What is the condition of the pelvic floor?
Make certain that the patient has the ability to appropriately relax the pelvic floor when she attempts to void.
Is urethral dilatation or medication an option?
We believe that urethral dilatation is contraindicated because it might cause urethral erosion of the sling. It is also generally ineffective.
No pharmaceutical agent hastens the return of voiding. Cholinergic agents such as bethanechol are ineffective and cause considerable discomfort. Some experts recommend empiric diazepam (Valium) for patients who are unable to relax sufficiently.
Will intervention succeed?
Ultimately, you and the patient must agree on whether urethrolysis is to be performed or whether the suburethral sling or tape should be cut. Undertake a detailed discussion with her about the potential for, first, persistent voiding dysfunction and, second, recurrent stress incontinence. Cutting a synthetic, allograft, xenograft, or autologous sling will almost always result in resumption of normal voiding, provided the sling is appropriately detached from the urethra and there were no preoperative voiding symptoms. With synthetic, allograft, and xenograft slings, stress incontinence recurs in at least 50% of patients over time. With an autologous sling, the recurrence rate of stress incontinence is less than 10%.
Is it time to operate?
When urinary retention after a synthetic sling procedure is believed to be caused by obstruction, consider surgery within a few weeks. For a patient in retention who has an autologous, allograft, or xenograft sling, it is best to wait approximately 3 months before operating.
Be aware of the risk of failure!
Takedowns of Burch and Marshall-Marchetti operations are much more technically challenging, and yield a much lower success rate, than takedowns of sling procedures. No matter what the prior operation, there is a risk of recurrent sphincteric incontinence.
Drs. Karram and Blaivas cochair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007 in Las Vegas (www.urogyn-cme.org).
Watch a demonstration of the surgical takedown of anti-incontinence procedures.
Voiding dysfunction—either difficulty voiding or urinary retention—after surgery for stress incontinence distresses the patient and challenges the surgeon. Here is our systematic approach to evaluating and managing such cases.
What does the operative note say?
Determine exactly what operation the patient underwent and whether appropriate steps were taken during surgery to evaluate the lower urinary tract. Remember: There are well over 30 different synthetic midurethral slings on the market; a variety of biologic materials are used for slings; and conventional suspension procedures are still being performed. Sling composition and surgical technique are the major determinants of subsequent treatment, so it is imperative to obtain the operative note.
Is intermittent self-catheterization an option?
If the patient has an indwelling catheter—of any type—remove it whenever possible and teach her intermittent self-catheterization.
Are symptoms consistent with expected outcome?
In the case of a patient who had a large cystocele repair in conjunction with an anti-incontinence procedure, for example, it is common for some form of retention or voiding dysfunction to be present for 2 weeks or longer. On the other hand, if a patient had a synthetic midurethral sling but no other procedure, it is highly unlikely, during a normal postoperative course, that she would be in retention 2 weeks after the procedure—unless the sling was placed too tightly.
Is there actual (or impending) lower-tract injury? Foreign body penetration?
Good endoscopic evaluation, with visualization of the urethra, of the vesical neck and anterolateral walls of the bladder, will answer these questions.
What is the condition of the pelvic floor?
Make certain that the patient has the ability to appropriately relax the pelvic floor when she attempts to void.
Is urethral dilatation or medication an option?
We believe that urethral dilatation is contraindicated because it might cause urethral erosion of the sling. It is also generally ineffective.
No pharmaceutical agent hastens the return of voiding. Cholinergic agents such as bethanechol are ineffective and cause considerable discomfort. Some experts recommend empiric diazepam (Valium) for patients who are unable to relax sufficiently.
Will intervention succeed?
Ultimately, you and the patient must agree on whether urethrolysis is to be performed or whether the suburethral sling or tape should be cut. Undertake a detailed discussion with her about the potential for, first, persistent voiding dysfunction and, second, recurrent stress incontinence. Cutting a synthetic, allograft, xenograft, or autologous sling will almost always result in resumption of normal voiding, provided the sling is appropriately detached from the urethra and there were no preoperative voiding symptoms. With synthetic, allograft, and xenograft slings, stress incontinence recurs in at least 50% of patients over time. With an autologous sling, the recurrence rate of stress incontinence is less than 10%.
Is it time to operate?
When urinary retention after a synthetic sling procedure is believed to be caused by obstruction, consider surgery within a few weeks. For a patient in retention who has an autologous, allograft, or xenograft sling, it is best to wait approximately 3 months before operating.
Be aware of the risk of failure!
Takedowns of Burch and Marshall-Marchetti operations are much more technically challenging, and yield a much lower success rate, than takedowns of sling procedures. No matter what the prior operation, there is a risk of recurrent sphincteric incontinence.
Watch a demonstration of the surgical takedown of anti-incontinence procedures.
Voiding dysfunction—either difficulty voiding or urinary retention—after surgery for stress incontinence distresses the patient and challenges the surgeon. Here is our systematic approach to evaluating and managing such cases.
What does the operative note say?
Determine exactly what operation the patient underwent and whether appropriate steps were taken during surgery to evaluate the lower urinary tract. Remember: There are well over 30 different synthetic midurethral slings on the market; a variety of biologic materials are used for slings; and conventional suspension procedures are still being performed. Sling composition and surgical technique are the major determinants of subsequent treatment, so it is imperative to obtain the operative note.
Is intermittent self-catheterization an option?
If the patient has an indwelling catheter—of any type—remove it whenever possible and teach her intermittent self-catheterization.
Are symptoms consistent with expected outcome?
In the case of a patient who had a large cystocele repair in conjunction with an anti-incontinence procedure, for example, it is common for some form of retention or voiding dysfunction to be present for 2 weeks or longer. On the other hand, if a patient had a synthetic midurethral sling but no other procedure, it is highly unlikely, during a normal postoperative course, that she would be in retention 2 weeks after the procedure—unless the sling was placed too tightly.
Is there actual (or impending) lower-tract injury? Foreign body penetration?
Good endoscopic evaluation, with visualization of the urethra, of the vesical neck and anterolateral walls of the bladder, will answer these questions.
What is the condition of the pelvic floor?
Make certain that the patient has the ability to appropriately relax the pelvic floor when she attempts to void.
Is urethral dilatation or medication an option?
We believe that urethral dilatation is contraindicated because it might cause urethral erosion of the sling. It is also generally ineffective.
No pharmaceutical agent hastens the return of voiding. Cholinergic agents such as bethanechol are ineffective and cause considerable discomfort. Some experts recommend empiric diazepam (Valium) for patients who are unable to relax sufficiently.
Will intervention succeed?
Ultimately, you and the patient must agree on whether urethrolysis is to be performed or whether the suburethral sling or tape should be cut. Undertake a detailed discussion with her about the potential for, first, persistent voiding dysfunction and, second, recurrent stress incontinence. Cutting a synthetic, allograft, xenograft, or autologous sling will almost always result in resumption of normal voiding, provided the sling is appropriately detached from the urethra and there were no preoperative voiding symptoms. With synthetic, allograft, and xenograft slings, stress incontinence recurs in at least 50% of patients over time. With an autologous sling, the recurrence rate of stress incontinence is less than 10%.
Is it time to operate?
When urinary retention after a synthetic sling procedure is believed to be caused by obstruction, consider surgery within a few weeks. For a patient in retention who has an autologous, allograft, or xenograft sling, it is best to wait approximately 3 months before operating.
Be aware of the risk of failure!
Takedowns of Burch and Marshall-Marchetti operations are much more technically challenging, and yield a much lower success rate, than takedowns of sling procedures. No matter what the prior operation, there is a risk of recurrent sphincteric incontinence.
Drs. Karram and Blaivas cochair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007 in Las Vegas (www.urogyn-cme.org).
Drs. Karram and Blaivas cochair the 6th Annual International Symposium on Female Urology & Urogynecology, to be held April 26–28, 2007 in Las Vegas (www.urogyn-cme.org).


