User login
STOP using synthetic mesh for routine repair of pelvic organ prolapse
Have you read these recent articles in OBG Management about the surgical use of mesh? Click here to access the list.
CASE: Stage 2 prolapse to the hymenal ring
A 54-year-old Para 3 woman presents with stage 2 prolapse to the hymenal ring. The prolapse predominantly involves the anterior vagina, with her cervix prolapsing to 2 cm within the hymenal ring. The patient is bothered by the bulge and stress urinary leakage. She does not want to use a pessary and prefers to have definitive surgical correction, including hysterectomy.
The first surgeon she consulted recommended a vaginal hysterectomy, anterior colporrhaphy, anterior synthetic mesh vaginal colpopexy, and synthetic midurethral sling. The patient was concerned about mesh placement for prolapse and the sling after seeing ads on the Internet about vaginal mesh. She presents for a second opinion about surgical alternatives.
Stop routinely offering synthetic vaginal mesh for prolapse
Advantages to the use of synthetic vaginal mesh include improved subjective and objective cure rates for prolapse (especially for the anterior compartment) and fewer repeat surgeries for recurrent prolapse. However, disadvantages include:
- mesh exposure and extrusion through the vaginal epithelium
- overall higher reoperation for mesh-related complications and de novo stress urinary incontinence.1
Synthetic vaginal mesh should be reserved for special situations. Currently, experts agree that synthetic vaginal mesh is appropriate in cases of recurrent prolapse, advanced-stage prolapse, collagen deficiency, or in cases with relative contraindications to longer endoscopic or abdominal surgery, such as medical comorbidities or adhesions.2,3 Other indications for synthetic vaginal mesh include vaginal hysteropexy procedures.
Mesh is likely not necessary in:
- primary repairs
- prolapse < POPQ (pelvic organ prolapse quantification system) stage 2
- posterior prolapse
- patients with chronic pelvic pain.
Start offering, learning, and mastering native tissue repairs
For the patient in the opening case, who has symptomatic stage 2 uterovaginal prolapse and stress urinary incontinence, surgery, including a transvaginal hysterectomy, anterior colporrhaphy, uterosacral ligament suspension, and synthetic midurethral sling, is a reasonable alternative and has a high subjective and objective cure rate—81.2% rate for anterior prolapse and 98.3% rate for apical prolapse. Better outcomes are noted with stage 2 compared with stage 3 prolapse (92.4% vs 66.8%, respectively).4
Final note
If you are offering selective transvaginal synthetic mesh for prolapse repairs:
- Undergo training specific to each device.
- Track your outcomes—including objective, subjective, quality of life, and reoperation for complications and recurrence.
- Enroll in the national pelvic floor disorders registry, which is scheduled to debut in Fall 2013.
We want to hear from you! Tell us what you think.
1. Schmid C, Maher C, Feiner B, Baessler K, Glazener C. 2012 Cochrane Review: Surgical management of pelvic organ prolapse. Int Urogynecol J. 2012;23(suppl 2).-
2. Davila GW, Baessler K, Cosson M, Cardozo L. Selection of patients in whom vaginal graft use may be appropriate. Consensus of the 2nd IUGA Grafts Roundtable: optimizing safety and appropriateness of graft use in transvaginal pelvic reconstructive surgery. Int Urogynecol J. 2012;23(suppl 1):S7-S14.
3. Committee on Gynecologic Practice. American College of Obstetricians and Gynecologists American Urogynecologic Society. Committee Opinion No. 513: Vaginal placement of synthetic mesh for pelvic organ prolapse. Obstet Gynecol. 2011;118(6):1459-1464.
4. Margulies RU, Rogers MA, Morgan DM. Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis. Am J Obstet Gynecol. 2010;202(2):124-134.
Have you read these recent articles in OBG Management about the surgical use of mesh? Click here to access the list.
CASE: Stage 2 prolapse to the hymenal ring
A 54-year-old Para 3 woman presents with stage 2 prolapse to the hymenal ring. The prolapse predominantly involves the anterior vagina, with her cervix prolapsing to 2 cm within the hymenal ring. The patient is bothered by the bulge and stress urinary leakage. She does not want to use a pessary and prefers to have definitive surgical correction, including hysterectomy.
The first surgeon she consulted recommended a vaginal hysterectomy, anterior colporrhaphy, anterior synthetic mesh vaginal colpopexy, and synthetic midurethral sling. The patient was concerned about mesh placement for prolapse and the sling after seeing ads on the Internet about vaginal mesh. She presents for a second opinion about surgical alternatives.
Stop routinely offering synthetic vaginal mesh for prolapse
Advantages to the use of synthetic vaginal mesh include improved subjective and objective cure rates for prolapse (especially for the anterior compartment) and fewer repeat surgeries for recurrent prolapse. However, disadvantages include:
- mesh exposure and extrusion through the vaginal epithelium
- overall higher reoperation for mesh-related complications and de novo stress urinary incontinence.1
Synthetic vaginal mesh should be reserved for special situations. Currently, experts agree that synthetic vaginal mesh is appropriate in cases of recurrent prolapse, advanced-stage prolapse, collagen deficiency, or in cases with relative contraindications to longer endoscopic or abdominal surgery, such as medical comorbidities or adhesions.2,3 Other indications for synthetic vaginal mesh include vaginal hysteropexy procedures.
Mesh is likely not necessary in:
- primary repairs
- prolapse < POPQ (pelvic organ prolapse quantification system) stage 2
- posterior prolapse
- patients with chronic pelvic pain.
Start offering, learning, and mastering native tissue repairs
For the patient in the opening case, who has symptomatic stage 2 uterovaginal prolapse and stress urinary incontinence, surgery, including a transvaginal hysterectomy, anterior colporrhaphy, uterosacral ligament suspension, and synthetic midurethral sling, is a reasonable alternative and has a high subjective and objective cure rate—81.2% rate for anterior prolapse and 98.3% rate for apical prolapse. Better outcomes are noted with stage 2 compared with stage 3 prolapse (92.4% vs 66.8%, respectively).4
Final note
If you are offering selective transvaginal synthetic mesh for prolapse repairs:
- Undergo training specific to each device.
- Track your outcomes—including objective, subjective, quality of life, and reoperation for complications and recurrence.
- Enroll in the national pelvic floor disorders registry, which is scheduled to debut in Fall 2013.
We want to hear from you! Tell us what you think.
Have you read these recent articles in OBG Management about the surgical use of mesh? Click here to access the list.
CASE: Stage 2 prolapse to the hymenal ring
A 54-year-old Para 3 woman presents with stage 2 prolapse to the hymenal ring. The prolapse predominantly involves the anterior vagina, with her cervix prolapsing to 2 cm within the hymenal ring. The patient is bothered by the bulge and stress urinary leakage. She does not want to use a pessary and prefers to have definitive surgical correction, including hysterectomy.
The first surgeon she consulted recommended a vaginal hysterectomy, anterior colporrhaphy, anterior synthetic mesh vaginal colpopexy, and synthetic midurethral sling. The patient was concerned about mesh placement for prolapse and the sling after seeing ads on the Internet about vaginal mesh. She presents for a second opinion about surgical alternatives.
Stop routinely offering synthetic vaginal mesh for prolapse
Advantages to the use of synthetic vaginal mesh include improved subjective and objective cure rates for prolapse (especially for the anterior compartment) and fewer repeat surgeries for recurrent prolapse. However, disadvantages include:
- mesh exposure and extrusion through the vaginal epithelium
- overall higher reoperation for mesh-related complications and de novo stress urinary incontinence.1
Synthetic vaginal mesh should be reserved for special situations. Currently, experts agree that synthetic vaginal mesh is appropriate in cases of recurrent prolapse, advanced-stage prolapse, collagen deficiency, or in cases with relative contraindications to longer endoscopic or abdominal surgery, such as medical comorbidities or adhesions.2,3 Other indications for synthetic vaginal mesh include vaginal hysteropexy procedures.
Mesh is likely not necessary in:
- primary repairs
- prolapse < POPQ (pelvic organ prolapse quantification system) stage 2
- posterior prolapse
- patients with chronic pelvic pain.
Start offering, learning, and mastering native tissue repairs
For the patient in the opening case, who has symptomatic stage 2 uterovaginal prolapse and stress urinary incontinence, surgery, including a transvaginal hysterectomy, anterior colporrhaphy, uterosacral ligament suspension, and synthetic midurethral sling, is a reasonable alternative and has a high subjective and objective cure rate—81.2% rate for anterior prolapse and 98.3% rate for apical prolapse. Better outcomes are noted with stage 2 compared with stage 3 prolapse (92.4% vs 66.8%, respectively).4
Final note
If you are offering selective transvaginal synthetic mesh for prolapse repairs:
- Undergo training specific to each device.
- Track your outcomes—including objective, subjective, quality of life, and reoperation for complications and recurrence.
- Enroll in the national pelvic floor disorders registry, which is scheduled to debut in Fall 2013.
We want to hear from you! Tell us what you think.
1. Schmid C, Maher C, Feiner B, Baessler K, Glazener C. 2012 Cochrane Review: Surgical management of pelvic organ prolapse. Int Urogynecol J. 2012;23(suppl 2).-
2. Davila GW, Baessler K, Cosson M, Cardozo L. Selection of patients in whom vaginal graft use may be appropriate. Consensus of the 2nd IUGA Grafts Roundtable: optimizing safety and appropriateness of graft use in transvaginal pelvic reconstructive surgery. Int Urogynecol J. 2012;23(suppl 1):S7-S14.
3. Committee on Gynecologic Practice. American College of Obstetricians and Gynecologists American Urogynecologic Society. Committee Opinion No. 513: Vaginal placement of synthetic mesh for pelvic organ prolapse. Obstet Gynecol. 2011;118(6):1459-1464.
4. Margulies RU, Rogers MA, Morgan DM. Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis. Am J Obstet Gynecol. 2010;202(2):124-134.
1. Schmid C, Maher C, Feiner B, Baessler K, Glazener C. 2012 Cochrane Review: Surgical management of pelvic organ prolapse. Int Urogynecol J. 2012;23(suppl 2).-
2. Davila GW, Baessler K, Cosson M, Cardozo L. Selection of patients in whom vaginal graft use may be appropriate. Consensus of the 2nd IUGA Grafts Roundtable: optimizing safety and appropriateness of graft use in transvaginal pelvic reconstructive surgery. Int Urogynecol J. 2012;23(suppl 1):S7-S14.
3. Committee on Gynecologic Practice. American College of Obstetricians and Gynecologists American Urogynecologic Society. Committee Opinion No. 513: Vaginal placement of synthetic mesh for pelvic organ prolapse. Obstet Gynecol. 2011;118(6):1459-1464.
4. Margulies RU, Rogers MA, Morgan DM. Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis. Am J Obstet Gynecol. 2010;202(2):124-134.
Native tissue is superior to vaginal mesh for prolapse repair, two studies report
Have you read recent articles in OBG Management about the surgical use of mesh?
Click here to access the list.
- Michele Jonsson Funk, PhD, and colleagues from University of North Carolina (UNC) at Chapel Hill concluded that using vaginal mesh versus native tissue for anterior prolapse repair is associated with 5-year increased risk of any repeat surgery, especially surgery for mesh removal.1
- Shunaha Kim-Fine, MD, and colleagues from Mayo Clinic, Rochester, Minnesota, believe that traditional native tissue repair is the best procedure for most women undergoing vaginal POP repair.2
UNC study details
Investigators from the Gillings School of Global Public Health at UNC studied health-care claims from 2005 to 2010. They identified women who, after undergoing anterior wall prolapse repair, experienced repeat surgery for recurrent prolapse or mesh removal. Of the initial 27,809 anterior prolapse surgeries, 6,871 (24.7%) included the use of vaginal mesh.1
5-year risk of repeat surgery. The authors determined that1:
- the 5-year cumulative risk of any repeat surgery was significantly higher with the use of vaginal mesh than with the use of native tissue (15.2% vs 9.8%, respectively; P .0001 with a risk of mesh revision or removal>
- the 5-year risk for recurrent prolapse surgery between both groups was comparable (10.4% vs 9.3%, P = .70).
Mayo Clinic study details
Researchers from Female Pelvic Medicine and Reconstructive Surgery, Division of Gynecologic Surgery at Mayo Clinic reviewed the literature and compared vaginal native tissue repair with vaginal mesh–augmented repair of pelvic organ prolapse. Their report was published online ahead of print on January 17, 2013, in Current Bladder Dysfunction Reports.
The authors discuss POP; the procedures available to treat symptomatic POP; the Public Heath Notifications issued in 2008 and 2011 from U.S. Food and Drug Administration (FDA) regarding the use of transvaginal mesh in POP repair; and success, failure, and complication rates from both techniques.2
“Given the lack of robust and long-term data in these relatively new procedures for [mesh-augmentation] repair, we agree with the caution and prudence communication in the recent FDA warning,” state the authors.2 However, a caveat is offered that native tissue repair must utilize best principles of surgical technique and incorporate a multicompartment repair to achieve optimal outcome. The authors strongly advise that appropriate surgical technique, obtained only through adequate surgical training, can be improved for both repair procedures.2
Risks and complications from mesh. Mesh introduces unique risks related to the mesh itself, including mesh erosion, and complications, including new onset pain and dyspareunia following mesh-augmented repair. Complications are possibly related to the intrinsic properties of the mesh, (ie, shrinkage); to the patient (ie, scarring); or to the operative technique (ie, the placement/location of the mesh and increased tension on the mesh). The authors conclude that additional studies are needed, given the lack of robust and long-term data on mesh-augmentation repair of POP.2
“The evidence thus far has not shown that the benefits of mesh outweigh the added risks in vaginal prolapse repairs,” write the authors.2 Therefore, although patient-centered success rates for both techniques of POP repair are equivalent, the authors conclude: “there does not appear to be a clear advantage of mesh augmentation repair over native tissue in terms of anatomic success.”2
To access the Jonsson Funk abstract, click here.
To access the Kim-Fine abstract, click here.
We want to hear from you! Tell us what you think.
1. Jonsson Funk M, Visco AG, Weidner AC, Pate V, Wu JM. Long-term outcomes of vaginal mesh versus native tissue repair for anterior vaginal wall prolapse [published online ahead of print February 12, 2013]. Int Urogynecol J. doi:10.1007/s00192-013-2043-9.
2. Kim-Fine S, Occhino JA, Gebhart JB. Vaginal prolapse repair—Native tissue repair versus mesh augmentation: Newer isn’t always better [published online ahead of print January 17, 2013]. Curr Bladder Dysfunct Rep. 2013;8(1):25-31doi:10.1007/s11884-012-0170-7.
More NEWS FOR YOUR PRACTICE…
appropriate for?
Have you read recent articles in OBG Management about the surgical use of mesh?
Click here to access the list.
- Michele Jonsson Funk, PhD, and colleagues from University of North Carolina (UNC) at Chapel Hill concluded that using vaginal mesh versus native tissue for anterior prolapse repair is associated with 5-year increased risk of any repeat surgery, especially surgery for mesh removal.1
- Shunaha Kim-Fine, MD, and colleagues from Mayo Clinic, Rochester, Minnesota, believe that traditional native tissue repair is the best procedure for most women undergoing vaginal POP repair.2
UNC study details
Investigators from the Gillings School of Global Public Health at UNC studied health-care claims from 2005 to 2010. They identified women who, after undergoing anterior wall prolapse repair, experienced repeat surgery for recurrent prolapse or mesh removal. Of the initial 27,809 anterior prolapse surgeries, 6,871 (24.7%) included the use of vaginal mesh.1
5-year risk of repeat surgery. The authors determined that1:
- the 5-year cumulative risk of any repeat surgery was significantly higher with the use of vaginal mesh than with the use of native tissue (15.2% vs 9.8%, respectively; P .0001 with a risk of mesh revision or removal>
- the 5-year risk for recurrent prolapse surgery between both groups was comparable (10.4% vs 9.3%, P = .70).
Mayo Clinic study details
Researchers from Female Pelvic Medicine and Reconstructive Surgery, Division of Gynecologic Surgery at Mayo Clinic reviewed the literature and compared vaginal native tissue repair with vaginal mesh–augmented repair of pelvic organ prolapse. Their report was published online ahead of print on January 17, 2013, in Current Bladder Dysfunction Reports.
The authors discuss POP; the procedures available to treat symptomatic POP; the Public Heath Notifications issued in 2008 and 2011 from U.S. Food and Drug Administration (FDA) regarding the use of transvaginal mesh in POP repair; and success, failure, and complication rates from both techniques.2
“Given the lack of robust and long-term data in these relatively new procedures for [mesh-augmentation] repair, we agree with the caution and prudence communication in the recent FDA warning,” state the authors.2 However, a caveat is offered that native tissue repair must utilize best principles of surgical technique and incorporate a multicompartment repair to achieve optimal outcome. The authors strongly advise that appropriate surgical technique, obtained only through adequate surgical training, can be improved for both repair procedures.2
Risks and complications from mesh. Mesh introduces unique risks related to the mesh itself, including mesh erosion, and complications, including new onset pain and dyspareunia following mesh-augmented repair. Complications are possibly related to the intrinsic properties of the mesh, (ie, shrinkage); to the patient (ie, scarring); or to the operative technique (ie, the placement/location of the mesh and increased tension on the mesh). The authors conclude that additional studies are needed, given the lack of robust and long-term data on mesh-augmentation repair of POP.2
“The evidence thus far has not shown that the benefits of mesh outweigh the added risks in vaginal prolapse repairs,” write the authors.2 Therefore, although patient-centered success rates for both techniques of POP repair are equivalent, the authors conclude: “there does not appear to be a clear advantage of mesh augmentation repair over native tissue in terms of anatomic success.”2
To access the Jonsson Funk abstract, click here.
To access the Kim-Fine abstract, click here.
We want to hear from you! Tell us what you think.
Have you read recent articles in OBG Management about the surgical use of mesh?
Click here to access the list.
- Michele Jonsson Funk, PhD, and colleagues from University of North Carolina (UNC) at Chapel Hill concluded that using vaginal mesh versus native tissue for anterior prolapse repair is associated with 5-year increased risk of any repeat surgery, especially surgery for mesh removal.1
- Shunaha Kim-Fine, MD, and colleagues from Mayo Clinic, Rochester, Minnesota, believe that traditional native tissue repair is the best procedure for most women undergoing vaginal POP repair.2
UNC study details
Investigators from the Gillings School of Global Public Health at UNC studied health-care claims from 2005 to 2010. They identified women who, after undergoing anterior wall prolapse repair, experienced repeat surgery for recurrent prolapse or mesh removal. Of the initial 27,809 anterior prolapse surgeries, 6,871 (24.7%) included the use of vaginal mesh.1
5-year risk of repeat surgery. The authors determined that1:
- the 5-year cumulative risk of any repeat surgery was significantly higher with the use of vaginal mesh than with the use of native tissue (15.2% vs 9.8%, respectively; P .0001 with a risk of mesh revision or removal>
- the 5-year risk for recurrent prolapse surgery between both groups was comparable (10.4% vs 9.3%, P = .70).
Mayo Clinic study details
Researchers from Female Pelvic Medicine and Reconstructive Surgery, Division of Gynecologic Surgery at Mayo Clinic reviewed the literature and compared vaginal native tissue repair with vaginal mesh–augmented repair of pelvic organ prolapse. Their report was published online ahead of print on January 17, 2013, in Current Bladder Dysfunction Reports.
The authors discuss POP; the procedures available to treat symptomatic POP; the Public Heath Notifications issued in 2008 and 2011 from U.S. Food and Drug Administration (FDA) regarding the use of transvaginal mesh in POP repair; and success, failure, and complication rates from both techniques.2
“Given the lack of robust and long-term data in these relatively new procedures for [mesh-augmentation] repair, we agree with the caution and prudence communication in the recent FDA warning,” state the authors.2 However, a caveat is offered that native tissue repair must utilize best principles of surgical technique and incorporate a multicompartment repair to achieve optimal outcome. The authors strongly advise that appropriate surgical technique, obtained only through adequate surgical training, can be improved for both repair procedures.2
Risks and complications from mesh. Mesh introduces unique risks related to the mesh itself, including mesh erosion, and complications, including new onset pain and dyspareunia following mesh-augmented repair. Complications are possibly related to the intrinsic properties of the mesh, (ie, shrinkage); to the patient (ie, scarring); or to the operative technique (ie, the placement/location of the mesh and increased tension on the mesh). The authors conclude that additional studies are needed, given the lack of robust and long-term data on mesh-augmentation repair of POP.2
“The evidence thus far has not shown that the benefits of mesh outweigh the added risks in vaginal prolapse repairs,” write the authors.2 Therefore, although patient-centered success rates for both techniques of POP repair are equivalent, the authors conclude: “there does not appear to be a clear advantage of mesh augmentation repair over native tissue in terms of anatomic success.”2
To access the Jonsson Funk abstract, click here.
To access the Kim-Fine abstract, click here.
We want to hear from you! Tell us what you think.
1. Jonsson Funk M, Visco AG, Weidner AC, Pate V, Wu JM. Long-term outcomes of vaginal mesh versus native tissue repair for anterior vaginal wall prolapse [published online ahead of print February 12, 2013]. Int Urogynecol J. doi:10.1007/s00192-013-2043-9.
2. Kim-Fine S, Occhino JA, Gebhart JB. Vaginal prolapse repair—Native tissue repair versus mesh augmentation: Newer isn’t always better [published online ahead of print January 17, 2013]. Curr Bladder Dysfunct Rep. 2013;8(1):25-31doi:10.1007/s11884-012-0170-7.
More NEWS FOR YOUR PRACTICE…
appropriate for?
1. Jonsson Funk M, Visco AG, Weidner AC, Pate V, Wu JM. Long-term outcomes of vaginal mesh versus native tissue repair for anterior vaginal wall prolapse [published online ahead of print February 12, 2013]. Int Urogynecol J. doi:10.1007/s00192-013-2043-9.
2. Kim-Fine S, Occhino JA, Gebhart JB. Vaginal prolapse repair—Native tissue repair versus mesh augmentation: Newer isn’t always better [published online ahead of print January 17, 2013]. Curr Bladder Dysfunct Rep. 2013;8(1):25-31doi:10.1007/s11884-012-0170-7.
More NEWS FOR YOUR PRACTICE…
appropriate for?
When is her pelvic pressure and bulge due to Pouch of Douglas hernia?
CASE: Pelvic organ prolapse or Pouch of Douglas hernia?
A 42-year-old G3P2 woman is referred to you by her primary care provider for pelvic organ prolapse. Her medical history reveals that she has been bothered by a sense of pelvic pressure and bulge progressing over several years, and she has noticed that her symptoms are particularly worse during and after bowel movements. She reports some improved bowel evacuation with external splinting of her perineum. Upon closer questioning, the patient reports a history of chronic constipation since childhood associated with straining and a sense of incomplete emptying. She reports spending up to 30 minutes three to four times per day on the commode to completely empty her bowels.
Physical examination reveals an overweight woman with a soft, nontender abdomen remarkable for laparoscopic incision scars from a previous tubal ligation. Inspection of the external genitalia at rest is normal. Cough stress test is negative. At maximum Valsalva, however, there is significant perineal ballooning present.
Speculum examination demonstrates grade 1 uterine prolapse, grade 1 cystocele, and grade 2 rectocele. There is no evidence of pelvic floor tension myalgia. She has weak pelvic muscle strength. Visualization of the anus at maximum Valsalva reveals there is some asymmetric rectal prolapse of the anterior rectal wall. Digital rectal exam is unremarkable.
Are these patient’s symptoms due to pelvic organ prolapse or Pouch of Douglas hernia?
Pelvic organ prolapse: A common problem
Pelvic organ prolapse has an estimated prevalence of 55% in women aged 50 to 59 years.1 More than 200,000 pelvic organ prolapse surgeries are performed annually in the United States.2 Typically, patients report:
- vaginal bulge causing discomfort
- pelvic pressure or heaviness, or
- rubbing of the vaginal bulge on undergarments.
In more advanced pelvic organ prolapse, patients may report voiding dysfunction or stool trapping that requires manual splinting of the prolapse to assist in bladder and bowel evacuation.
Pouch of Douglas hernia: A lesser-known
(recognized) phenomenon
Similar to pelvic organ prolapse, Pouch of Douglas hernia also can present with symptoms of:
- pelvic pressure
- vague perineal aching
- defecatory dysfunction.
The phenomenon has been variably referred to in the literature as enterocele, descending perineum syndrome, peritoneocele, or Pouch of Douglas hernia. The concept was first introduced in 19663 and describes descent of the entire pelvic floor and small bowel through a hernia in the Pouch of Douglas (FIGURE 1).
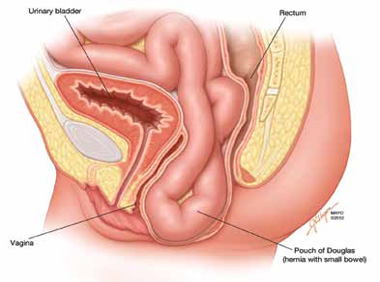
FIGURE 1: Pouch of Douglas hernia. The pelvic floor and small bowel descend into the Pouch of Douglas.
How does it occur? The pathophysiology is thought to be related to excessive abdominal straining in individuals with chronic constipation. This results in diminished pelvic floor muscle tone. Eventually, the whole pelvic floor descends, becoming funnel shaped due to stretching of the puborectalis muscle. Thus, stool is expelled by force, mostly through forces on the anterior rectal wall (which tends to prolapse after stool evacuation, with accompanied mucus secretion, soreness, and irritation).
Clinical pearl: Given the rectal wall prolapse that occurs after stool evacuation in Pouch of Douglas hernia, some patients will describe a rectal lump that bleeds after a bowel movement. The sensation of the rectal lump from the anterior rectal wall prolapse causes further straining.
Your patient reports pelvic pressure and bulge.
How do you proceed?
Physical examination
Look for perineal ballooning. Physical examination should start with inspection of the external genitalia. This inspection will identify any pelvic organ prolapse at or beyond the introitus. However, a Pouch of Douglas hernia will be missed if the patient is not examined during Valsalva or maximal strain. This maneuver will demonstrate the classic finding of perineal ballooning and is crucial to a final diagnosis of Pouch of Douglas hernia. Normally, the perineum will descend 1 cm to 2 cm during maximal strain; in Pouch of Douglas hernias, the perineum can descend up to 4 cm to 8 cm.4
Clinical pearl: It should be noted that, often, patients will not have a great deal of vaginal prolapse accompanying the perineal ballooning. In our opinion, this finding distinguishes Pouch of Douglas hernia from a vaginal vault prolapse caused by an enterocele.
Is rectal prolapse present? Beyond perineal ballooning, the presence of rectal prolapse should be evaluated. A rectocele of some degree is usually present. Asymmetric rectal prolapse affecting the anterior aspect of the rectal wall is consistent with a Pouch of Douglas hernia. This anatomic finding should be distinguished from true circumferential rectal prolapse, which remains in the differential diagnosis.
Basing the diagnosis of Pouch of Douglas hernia on physical examination alone can be difficult. Therefore, imaging studies are essential for accurate diagnosis.
Imaging investigations
Several imaging modalities can be used to diagnose such disorders of the pelvic floor as Pouch of Douglas hernia. These include:
- dynamic colpocystoproctography5
- defecography with oral barium6
- dynamic pelvic magnetic resonance imaging (MRI).7
In our experience, dynamic pelvic MRI has a high accuracy rate for diagnosing Pouch of Douglas hernia. FIGURE 2 illustrates the large Pouch of Douglas hernia filled with loops of small bowel. Perineal descent of the anorectal junction more than 3 cm below the pubococcygeal line during maximal straining is a diagnostic finding on imaging.7
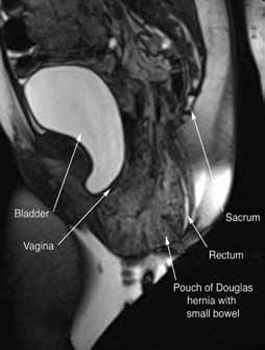
FIGURE 2: MRI
Sagittal MRI during maximal Valsalva straining, demonstrating Pouch of Douglas hernia filled with small bowel.
What are your patient’s treatment options?
Reduce straining during bowel movements. The primary goal of treatment for Pouch of Douglas hernia should be relief of bothersome symptoms. Therefore, further damage can be prevented by eliminating straining during defecation. This can be accomplished with a bowel regimen that combines an irritant suppository (glycerin or bisacodyl) with a fiber supplement (the latter to increase bulk of the stool). Oral laxatives have limited use as many patients have lax anal sphincters and liquid stool could cause fecal incontinence.
Pelvic floor strengthening. The importance of pelvic floor physical therapy should be stressed. Patients can benefit from the use of modalities such as biofeedback to learn appropriate pelvic floor muscle relaxation techniques during defecation.8 While there is limited published evidence supporting the use of pelvic floor physical therapy, our anecdotal experience suggests that patients can gain considerable benefit with such conservative therapy.
Surgical therapy
Surgical repair of Pouch of Douglas hernia requires obliteration of the deep cul-de-sac (to prevent the small bowel from filling this space) and simultaneous pelvic floor reconstruction of the vaginal apex and any other compartments that are prolapsing (if pelvic organ prolapse is present). In our experience, these patients typically have derived greatest benefit from an abdominal approach. This usually can be accomplished with a sacrocolpopexy (if vaginal vault prolapse exists) with a Moschowitz or Halban procedure,9 uterosacral ligament plication, or a modified sacrocolpopexy with mesh augmentation to the sidewalls of the pelvis.10 There are currently no studies supporting one particular approach over another, but the most important feature of a surgical intervention is obliteration of the cul-de-sac (FIGURES 3, 4, and 5).
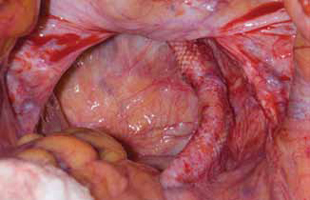
FIGURE 3: Open cul-de-sac. Open cul-de-sac after a prior abdominal sacrocolpopexy in a patient with a Pouch of Douglas hernia.
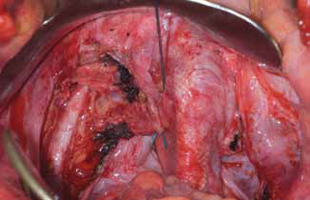
FIGURE 4: Obliterated cul-de-sac. Obliteration of the cul-de-sac with uterosacral ligament plication. Care is taken to prevent obstruction of the rectum at this level.
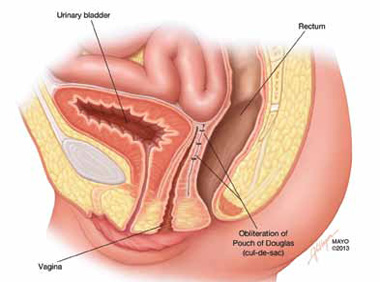
FIGURE 5: Cul-de-sac obliteration. Schematic diagram of obliteration of the cul-de-sac with uterosacral ligament plication sutures.
Final takeaways
Pouch of Douglas hernia is an important but often unrecognized cause of pelvic pressure and defecatory dysfunction. Perineal ballooning during maximal straining is highly suggestive of the diagnosis, with final diagnosis confirmed with various functional imaging studies of the pelvic floor. Management should include both conservative and surgical interventions to alleviate and prevent recurrence of symptoms.
ACKNOWLEDGMENT. The authors would like to thank Mr. John Hagen, Medical Illustrator, Mayo Clinic, for producing the illustrations in Figures 1 and 5.
We want to hear from you! Tell us what you think.
Urinary incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (Update, December 2012)
When and how to place an autologous rectus fascia
pubovaginal sling
Mickey Karram, MD, and Dani Zoorob, MD (Surgical Techniques, November 2012)
Pelvic floor dysfunction
Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (Update, October 2012)
Step by step: Obliterating the vaginal canal to correct pelvic organ prolapse
Mickey Karram, MD, and Janelle Evans, MD (Surgical Techniques, February 2012)
1. Samuelsson EC, Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299-305.
2. Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States 1979-1997. Am J Obstet Gynecol. 2003;188(1):108-115.
3. Parks AG, Porter NH, Hardcastle J. The syndrome of the descending perineum. Proc R Soc Med. 1966;59(6):477-482.
4. Hardcastle JD. The descending perineum syndrome. Practitioner. 1969;203(217):612-619.
5. Maglinte DD, Bartram CI, Hale DA, et al. Functional imaging of the pelvic floor. Radiology. 2011;258(1):23-39.
6. Roos JE, Weishaupt D, Wildermuth S, Willmann JK, Marincek B, Hilfiker PR. Experience of 4 years with open MR defecography: pictorial review of anorectal anatomy and disease. Radiographics. 2002;22(4):817-832.
7. Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98(2):399-411.
8. Harewood GC, Coulie B, Camilleri M, Rath-Harvey D, Pemberton JH. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999;94(1):126-130.
9. Moschcowitz AV. The pathogenesis anatomy and cure of prolapse of the rectum. Surg Gyncol Obstetrics. 1912;15:7-21.
10. Gosselink MJ, van Dam JH, Huisman WM, Ginai AZ, Schouten WR. Treatment of enterocele by obliteration of the pelvic inlet. Dis Colon Rectum. 1999;42(7):940-944.
CASE: Pelvic organ prolapse or Pouch of Douglas hernia?
A 42-year-old G3P2 woman is referred to you by her primary care provider for pelvic organ prolapse. Her medical history reveals that she has been bothered by a sense of pelvic pressure and bulge progressing over several years, and she has noticed that her symptoms are particularly worse during and after bowel movements. She reports some improved bowel evacuation with external splinting of her perineum. Upon closer questioning, the patient reports a history of chronic constipation since childhood associated with straining and a sense of incomplete emptying. She reports spending up to 30 minutes three to four times per day on the commode to completely empty her bowels.
Physical examination reveals an overweight woman with a soft, nontender abdomen remarkable for laparoscopic incision scars from a previous tubal ligation. Inspection of the external genitalia at rest is normal. Cough stress test is negative. At maximum Valsalva, however, there is significant perineal ballooning present.
Speculum examination demonstrates grade 1 uterine prolapse, grade 1 cystocele, and grade 2 rectocele. There is no evidence of pelvic floor tension myalgia. She has weak pelvic muscle strength. Visualization of the anus at maximum Valsalva reveals there is some asymmetric rectal prolapse of the anterior rectal wall. Digital rectal exam is unremarkable.
Are these patient’s symptoms due to pelvic organ prolapse or Pouch of Douglas hernia?
Pelvic organ prolapse: A common problem
Pelvic organ prolapse has an estimated prevalence of 55% in women aged 50 to 59 years.1 More than 200,000 pelvic organ prolapse surgeries are performed annually in the United States.2 Typically, patients report:
- vaginal bulge causing discomfort
- pelvic pressure or heaviness, or
- rubbing of the vaginal bulge on undergarments.
In more advanced pelvic organ prolapse, patients may report voiding dysfunction or stool trapping that requires manual splinting of the prolapse to assist in bladder and bowel evacuation.
Pouch of Douglas hernia: A lesser-known
(recognized) phenomenon
Similar to pelvic organ prolapse, Pouch of Douglas hernia also can present with symptoms of:
- pelvic pressure
- vague perineal aching
- defecatory dysfunction.
The phenomenon has been variably referred to in the literature as enterocele, descending perineum syndrome, peritoneocele, or Pouch of Douglas hernia. The concept was first introduced in 19663 and describes descent of the entire pelvic floor and small bowel through a hernia in the Pouch of Douglas (FIGURE 1).

FIGURE 1: Pouch of Douglas hernia. The pelvic floor and small bowel descend into the Pouch of Douglas.
How does it occur? The pathophysiology is thought to be related to excessive abdominal straining in individuals with chronic constipation. This results in diminished pelvic floor muscle tone. Eventually, the whole pelvic floor descends, becoming funnel shaped due to stretching of the puborectalis muscle. Thus, stool is expelled by force, mostly through forces on the anterior rectal wall (which tends to prolapse after stool evacuation, with accompanied mucus secretion, soreness, and irritation).
Clinical pearl: Given the rectal wall prolapse that occurs after stool evacuation in Pouch of Douglas hernia, some patients will describe a rectal lump that bleeds after a bowel movement. The sensation of the rectal lump from the anterior rectal wall prolapse causes further straining.
Your patient reports pelvic pressure and bulge.
How do you proceed?
Physical examination
Look for perineal ballooning. Physical examination should start with inspection of the external genitalia. This inspection will identify any pelvic organ prolapse at or beyond the introitus. However, a Pouch of Douglas hernia will be missed if the patient is not examined during Valsalva or maximal strain. This maneuver will demonstrate the classic finding of perineal ballooning and is crucial to a final diagnosis of Pouch of Douglas hernia. Normally, the perineum will descend 1 cm to 2 cm during maximal strain; in Pouch of Douglas hernias, the perineum can descend up to 4 cm to 8 cm.4
Clinical pearl: It should be noted that, often, patients will not have a great deal of vaginal prolapse accompanying the perineal ballooning. In our opinion, this finding distinguishes Pouch of Douglas hernia from a vaginal vault prolapse caused by an enterocele.
Is rectal prolapse present? Beyond perineal ballooning, the presence of rectal prolapse should be evaluated. A rectocele of some degree is usually present. Asymmetric rectal prolapse affecting the anterior aspect of the rectal wall is consistent with a Pouch of Douglas hernia. This anatomic finding should be distinguished from true circumferential rectal prolapse, which remains in the differential diagnosis.
Basing the diagnosis of Pouch of Douglas hernia on physical examination alone can be difficult. Therefore, imaging studies are essential for accurate diagnosis.
Imaging investigations
Several imaging modalities can be used to diagnose such disorders of the pelvic floor as Pouch of Douglas hernia. These include:
- dynamic colpocystoproctography5
- defecography with oral barium6
- dynamic pelvic magnetic resonance imaging (MRI).7
In our experience, dynamic pelvic MRI has a high accuracy rate for diagnosing Pouch of Douglas hernia. FIGURE 2 illustrates the large Pouch of Douglas hernia filled with loops of small bowel. Perineal descent of the anorectal junction more than 3 cm below the pubococcygeal line during maximal straining is a diagnostic finding on imaging.7

FIGURE 2: MRI
Sagittal MRI during maximal Valsalva straining, demonstrating Pouch of Douglas hernia filled with small bowel.
What are your patient’s treatment options?
Reduce straining during bowel movements. The primary goal of treatment for Pouch of Douglas hernia should be relief of bothersome symptoms. Therefore, further damage can be prevented by eliminating straining during defecation. This can be accomplished with a bowel regimen that combines an irritant suppository (glycerin or bisacodyl) with a fiber supplement (the latter to increase bulk of the stool). Oral laxatives have limited use as many patients have lax anal sphincters and liquid stool could cause fecal incontinence.
Pelvic floor strengthening. The importance of pelvic floor physical therapy should be stressed. Patients can benefit from the use of modalities such as biofeedback to learn appropriate pelvic floor muscle relaxation techniques during defecation.8 While there is limited published evidence supporting the use of pelvic floor physical therapy, our anecdotal experience suggests that patients can gain considerable benefit with such conservative therapy.
Surgical therapy
Surgical repair of Pouch of Douglas hernia requires obliteration of the deep cul-de-sac (to prevent the small bowel from filling this space) and simultaneous pelvic floor reconstruction of the vaginal apex and any other compartments that are prolapsing (if pelvic organ prolapse is present). In our experience, these patients typically have derived greatest benefit from an abdominal approach. This usually can be accomplished with a sacrocolpopexy (if vaginal vault prolapse exists) with a Moschowitz or Halban procedure,9 uterosacral ligament plication, or a modified sacrocolpopexy with mesh augmentation to the sidewalls of the pelvis.10 There are currently no studies supporting one particular approach over another, but the most important feature of a surgical intervention is obliteration of the cul-de-sac (FIGURES 3, 4, and 5).

FIGURE 3: Open cul-de-sac. Open cul-de-sac after a prior abdominal sacrocolpopexy in a patient with a Pouch of Douglas hernia.

FIGURE 4: Obliterated cul-de-sac. Obliteration of the cul-de-sac with uterosacral ligament plication. Care is taken to prevent obstruction of the rectum at this level.

FIGURE 5: Cul-de-sac obliteration. Schematic diagram of obliteration of the cul-de-sac with uterosacral ligament plication sutures.
Final takeaways
Pouch of Douglas hernia is an important but often unrecognized cause of pelvic pressure and defecatory dysfunction. Perineal ballooning during maximal straining is highly suggestive of the diagnosis, with final diagnosis confirmed with various functional imaging studies of the pelvic floor. Management should include both conservative and surgical interventions to alleviate and prevent recurrence of symptoms.
ACKNOWLEDGMENT. The authors would like to thank Mr. John Hagen, Medical Illustrator, Mayo Clinic, for producing the illustrations in Figures 1 and 5.
We want to hear from you! Tell us what you think.
Urinary incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (Update, December 2012)
When and how to place an autologous rectus fascia
pubovaginal sling
Mickey Karram, MD, and Dani Zoorob, MD (Surgical Techniques, November 2012)
Pelvic floor dysfunction
Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (Update, October 2012)
Step by step: Obliterating the vaginal canal to correct pelvic organ prolapse
Mickey Karram, MD, and Janelle Evans, MD (Surgical Techniques, February 2012)
CASE: Pelvic organ prolapse or Pouch of Douglas hernia?
A 42-year-old G3P2 woman is referred to you by her primary care provider for pelvic organ prolapse. Her medical history reveals that she has been bothered by a sense of pelvic pressure and bulge progressing over several years, and she has noticed that her symptoms are particularly worse during and after bowel movements. She reports some improved bowel evacuation with external splinting of her perineum. Upon closer questioning, the patient reports a history of chronic constipation since childhood associated with straining and a sense of incomplete emptying. She reports spending up to 30 minutes three to four times per day on the commode to completely empty her bowels.
Physical examination reveals an overweight woman with a soft, nontender abdomen remarkable for laparoscopic incision scars from a previous tubal ligation. Inspection of the external genitalia at rest is normal. Cough stress test is negative. At maximum Valsalva, however, there is significant perineal ballooning present.
Speculum examination demonstrates grade 1 uterine prolapse, grade 1 cystocele, and grade 2 rectocele. There is no evidence of pelvic floor tension myalgia. She has weak pelvic muscle strength. Visualization of the anus at maximum Valsalva reveals there is some asymmetric rectal prolapse of the anterior rectal wall. Digital rectal exam is unremarkable.
Are these patient’s symptoms due to pelvic organ prolapse or Pouch of Douglas hernia?
Pelvic organ prolapse: A common problem
Pelvic organ prolapse has an estimated prevalence of 55% in women aged 50 to 59 years.1 More than 200,000 pelvic organ prolapse surgeries are performed annually in the United States.2 Typically, patients report:
- vaginal bulge causing discomfort
- pelvic pressure or heaviness, or
- rubbing of the vaginal bulge on undergarments.
In more advanced pelvic organ prolapse, patients may report voiding dysfunction or stool trapping that requires manual splinting of the prolapse to assist in bladder and bowel evacuation.
Pouch of Douglas hernia: A lesser-known
(recognized) phenomenon
Similar to pelvic organ prolapse, Pouch of Douglas hernia also can present with symptoms of:
- pelvic pressure
- vague perineal aching
- defecatory dysfunction.
The phenomenon has been variably referred to in the literature as enterocele, descending perineum syndrome, peritoneocele, or Pouch of Douglas hernia. The concept was first introduced in 19663 and describes descent of the entire pelvic floor and small bowel through a hernia in the Pouch of Douglas (FIGURE 1).

FIGURE 1: Pouch of Douglas hernia. The pelvic floor and small bowel descend into the Pouch of Douglas.
How does it occur? The pathophysiology is thought to be related to excessive abdominal straining in individuals with chronic constipation. This results in diminished pelvic floor muscle tone. Eventually, the whole pelvic floor descends, becoming funnel shaped due to stretching of the puborectalis muscle. Thus, stool is expelled by force, mostly through forces on the anterior rectal wall (which tends to prolapse after stool evacuation, with accompanied mucus secretion, soreness, and irritation).
Clinical pearl: Given the rectal wall prolapse that occurs after stool evacuation in Pouch of Douglas hernia, some patients will describe a rectal lump that bleeds after a bowel movement. The sensation of the rectal lump from the anterior rectal wall prolapse causes further straining.
Your patient reports pelvic pressure and bulge.
How do you proceed?
Physical examination
Look for perineal ballooning. Physical examination should start with inspection of the external genitalia. This inspection will identify any pelvic organ prolapse at or beyond the introitus. However, a Pouch of Douglas hernia will be missed if the patient is not examined during Valsalva or maximal strain. This maneuver will demonstrate the classic finding of perineal ballooning and is crucial to a final diagnosis of Pouch of Douglas hernia. Normally, the perineum will descend 1 cm to 2 cm during maximal strain; in Pouch of Douglas hernias, the perineum can descend up to 4 cm to 8 cm.4
Clinical pearl: It should be noted that, often, patients will not have a great deal of vaginal prolapse accompanying the perineal ballooning. In our opinion, this finding distinguishes Pouch of Douglas hernia from a vaginal vault prolapse caused by an enterocele.
Is rectal prolapse present? Beyond perineal ballooning, the presence of rectal prolapse should be evaluated. A rectocele of some degree is usually present. Asymmetric rectal prolapse affecting the anterior aspect of the rectal wall is consistent with a Pouch of Douglas hernia. This anatomic finding should be distinguished from true circumferential rectal prolapse, which remains in the differential diagnosis.
Basing the diagnosis of Pouch of Douglas hernia on physical examination alone can be difficult. Therefore, imaging studies are essential for accurate diagnosis.
Imaging investigations
Several imaging modalities can be used to diagnose such disorders of the pelvic floor as Pouch of Douglas hernia. These include:
- dynamic colpocystoproctography5
- defecography with oral barium6
- dynamic pelvic magnetic resonance imaging (MRI).7
In our experience, dynamic pelvic MRI has a high accuracy rate for diagnosing Pouch of Douglas hernia. FIGURE 2 illustrates the large Pouch of Douglas hernia filled with loops of small bowel. Perineal descent of the anorectal junction more than 3 cm below the pubococcygeal line during maximal straining is a diagnostic finding on imaging.7

FIGURE 2: MRI
Sagittal MRI during maximal Valsalva straining, demonstrating Pouch of Douglas hernia filled with small bowel.
What are your patient’s treatment options?
Reduce straining during bowel movements. The primary goal of treatment for Pouch of Douglas hernia should be relief of bothersome symptoms. Therefore, further damage can be prevented by eliminating straining during defecation. This can be accomplished with a bowel regimen that combines an irritant suppository (glycerin or bisacodyl) with a fiber supplement (the latter to increase bulk of the stool). Oral laxatives have limited use as many patients have lax anal sphincters and liquid stool could cause fecal incontinence.
Pelvic floor strengthening. The importance of pelvic floor physical therapy should be stressed. Patients can benefit from the use of modalities such as biofeedback to learn appropriate pelvic floor muscle relaxation techniques during defecation.8 While there is limited published evidence supporting the use of pelvic floor physical therapy, our anecdotal experience suggests that patients can gain considerable benefit with such conservative therapy.
Surgical therapy
Surgical repair of Pouch of Douglas hernia requires obliteration of the deep cul-de-sac (to prevent the small bowel from filling this space) and simultaneous pelvic floor reconstruction of the vaginal apex and any other compartments that are prolapsing (if pelvic organ prolapse is present). In our experience, these patients typically have derived greatest benefit from an abdominal approach. This usually can be accomplished with a sacrocolpopexy (if vaginal vault prolapse exists) with a Moschowitz or Halban procedure,9 uterosacral ligament plication, or a modified sacrocolpopexy with mesh augmentation to the sidewalls of the pelvis.10 There are currently no studies supporting one particular approach over another, but the most important feature of a surgical intervention is obliteration of the cul-de-sac (FIGURES 3, 4, and 5).

FIGURE 3: Open cul-de-sac. Open cul-de-sac after a prior abdominal sacrocolpopexy in a patient with a Pouch of Douglas hernia.

FIGURE 4: Obliterated cul-de-sac. Obliteration of the cul-de-sac with uterosacral ligament plication. Care is taken to prevent obstruction of the rectum at this level.

FIGURE 5: Cul-de-sac obliteration. Schematic diagram of obliteration of the cul-de-sac with uterosacral ligament plication sutures.
Final takeaways
Pouch of Douglas hernia is an important but often unrecognized cause of pelvic pressure and defecatory dysfunction. Perineal ballooning during maximal straining is highly suggestive of the diagnosis, with final diagnosis confirmed with various functional imaging studies of the pelvic floor. Management should include both conservative and surgical interventions to alleviate and prevent recurrence of symptoms.
ACKNOWLEDGMENT. The authors would like to thank Mr. John Hagen, Medical Illustrator, Mayo Clinic, for producing the illustrations in Figures 1 and 5.
We want to hear from you! Tell us what you think.
Urinary incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (Update, December 2012)
When and how to place an autologous rectus fascia
pubovaginal sling
Mickey Karram, MD, and Dani Zoorob, MD (Surgical Techniques, November 2012)
Pelvic floor dysfunction
Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (Update, October 2012)
Step by step: Obliterating the vaginal canal to correct pelvic organ prolapse
Mickey Karram, MD, and Janelle Evans, MD (Surgical Techniques, February 2012)
1. Samuelsson EC, Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299-305.
2. Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States 1979-1997. Am J Obstet Gynecol. 2003;188(1):108-115.
3. Parks AG, Porter NH, Hardcastle J. The syndrome of the descending perineum. Proc R Soc Med. 1966;59(6):477-482.
4. Hardcastle JD. The descending perineum syndrome. Practitioner. 1969;203(217):612-619.
5. Maglinte DD, Bartram CI, Hale DA, et al. Functional imaging of the pelvic floor. Radiology. 2011;258(1):23-39.
6. Roos JE, Weishaupt D, Wildermuth S, Willmann JK, Marincek B, Hilfiker PR. Experience of 4 years with open MR defecography: pictorial review of anorectal anatomy and disease. Radiographics. 2002;22(4):817-832.
7. Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98(2):399-411.
8. Harewood GC, Coulie B, Camilleri M, Rath-Harvey D, Pemberton JH. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999;94(1):126-130.
9. Moschcowitz AV. The pathogenesis anatomy and cure of prolapse of the rectum. Surg Gyncol Obstetrics. 1912;15:7-21.
10. Gosselink MJ, van Dam JH, Huisman WM, Ginai AZ, Schouten WR. Treatment of enterocele by obliteration of the pelvic inlet. Dis Colon Rectum. 1999;42(7):940-944.
1. Samuelsson EC, Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299-305.
2. Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States 1979-1997. Am J Obstet Gynecol. 2003;188(1):108-115.
3. Parks AG, Porter NH, Hardcastle J. The syndrome of the descending perineum. Proc R Soc Med. 1966;59(6):477-482.
4. Hardcastle JD. The descending perineum syndrome. Practitioner. 1969;203(217):612-619.
5. Maglinte DD, Bartram CI, Hale DA, et al. Functional imaging of the pelvic floor. Radiology. 2011;258(1):23-39.
6. Roos JE, Weishaupt D, Wildermuth S, Willmann JK, Marincek B, Hilfiker PR. Experience of 4 years with open MR defecography: pictorial review of anorectal anatomy and disease. Radiographics. 2002;22(4):817-832.
7. Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98(2):399-411.
8. Harewood GC, Coulie B, Camilleri M, Rath-Harvey D, Pemberton JH. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999;94(1):126-130.
9. Moschcowitz AV. The pathogenesis anatomy and cure of prolapse of the rectum. Surg Gyncol Obstetrics. 1912;15:7-21.
10. Gosselink MJ, van Dam JH, Huisman WM, Ginai AZ, Schouten WR. Treatment of enterocele by obliteration of the pelvic inlet. Dis Colon Rectum. 1999;42(7):940-944.
IN THIS ARTICLE
Clinical pearls at physical exam
Treatment options
Have you tried these innovative alternatives to antibiotics for UTI prevention?
The authors report no financial relationships relevant to this article.
CASE: Recurrent UTI and antibiotic resistance
A 53-year-old postmenopausal woman with a history of culture-proven recurrent Escherichia coli urinary tract infections (UTIs) presents to the clinic with symptoms of UTI. She was previously treated with a postcoital regimen of trimethoprim/sulfamethoxazole, based on sensitivities identified by culture. A past work-up of her upper and lower urinary tract was negative. You send a catheterized specimen for culture; again, E. coli is identified as the pathogen but proves resistant to her current antibiotic regimen.
What treatment alternatives, aside from antibiotics, are available for this patient—and how might they affect resistance?
Increased antibiotic usage has led to greater bacterial resistance, which is perpetuated by clonal spread. Resistant strains of E. coli have been found in household members, suggesting host-host transmission as a mechanism for dissemination. Alternative treatments that reduce the use of antibiotics may minimize bacterial resistance and increase the efficacy of treatment. In the TABLE , we summarize alternative approaches to the treatment of recurrent UTI. We also describe a strategy to alleviate symptoms.
Alternatives to antibiotics in the treatment and prevention of recurrent UTI
| Category | Type | Examples and doses, if recommended |
|---|---|---|
| Vaginal estrogen | Conjugated estrogen cream Estradiol
| Premarin cream, 0.5–2 g vaginally twice weekly
|
| Nutritive agents | Cranberry juice Cranberry tablets Cystopurin Lactobacilli Blueberry products | Not recommended 1 tablet (300 to 400 mg, depending on manufacturer) twice daily Not recommended Vivag, EcoVag, 1 capsule daily by vagina for 5 days, then once weekly for 10 weeks Not recommended |
| Anti-infective drugs | Methenamine hippurate Methenamine mandelate Methylene blue | Urex or Hiprex, 1 g orally twice daily Mandelamine, 1 g orally 4 times daily Future therapy |
| Urinary acidifiers | Vitamin C/ascorbic acid | 1–3 g orally 3–4 times daily |
| Herbal remedies | Uva ursi Forskolin | Not recommended for long-term use Not recommended |
| Behavioral changes | Adequate hydration Postcoital voiding |
Vaginal estrogen is the only proven alternative to antibiotics for postmenopausal women
A lack of estrogen is a risk factor for UTI and is associated with atrophic mucosa, leading to decreased colonization with lactobacilli, increased vaginal pH, and E. coli colonization.
A randomized, double-blind, placebo-controlled trial of intravaginal estriol cream versus placebo in 93 postmenopausal women found a significant decrease in the rate of UTI among women who used the cream.1 After 8 months of follow-up, the incidence of UTI was 0.5 vs 5.9 episodes per patient-year (P <.001). Interestingly, all pretreatment cultures were negative for lactobacilli. One month after treatment, 61% of women in the estriol group were culture-positive for lactobacilli, compared with 0% of the placebo group.1
A 2008 Cochrane review of nine studies concluded that vaginal estrogen reduces the number of UTIs in postmenopausal women, with variation based on the type of estrogen and duration of use.2
Adverse effects are mild
Twenty-eight percent of the estriol group in the randomized trial described above withdrew from treatment, with 20% citing local side effects, including vaginal irritation, burning, or itching—all of which were mild and self-limited.1 Other possible adverse effects include breast tenderness, vaginal bleeding or spotting, and discharge.2
Clinical recommendations
Given the efficacy of this therapy, we recommend topical estrogen for postmenopausal patients with recurrent UTIs.
Cranberry juice may reduce UTI, but many patients withdraw
from treatment
Cranberries belong to the Vaccinium species, which contains all flavonoids, including anthocyanins and proanthocyanidins. It was previously thought that the acidification of urine produced an antibacterial effect, but several trials have documented no change in urine levels of hippuric acid when cranberry products are given, with no acidification of the urine.3 Current theory suggests that cranberries prevent bacteria from adhering to the uroepithelial cells of the walls of the bladder, by blocking expression of E. coli’s adhesion molecule, P. fimbriae, so that bacteria are unable to penetrate the mucosal surface.4,5 The major benefit of cranberry products over antibiotic prophylaxis is that they do not have the potential for resistance.4
A 2008 Cochrane review concluded that cranberry juice may reduce symptomatic UTIs, particularly among young, sexually active women—but there is a high rate of withdrawal from treatment.6 The optimal method of administration and dose remain unclear. In contrast, two recent randomized, controlled trials—published after the Cochrane review—found no difference in the rate of recurrent UTI in premenopausal women.7,8 Adverse effects in these two trials included constipation, heartburn, loose stools, vaginal itching and dryness, and migraines. Of note, there was no statistical difference in side effects between the cranberry and placebo groups.7
Vaccinium tablets may be protective in older women
Cranberry extracts of 500 mg to 1,000 mg daily have been compared with antimicrobial prophylaxis in two randomized, double-blind, controlled trials. The trials demonstrated mixed benefits. Trimethoprim/sulfamethoxazole was associated with a lower rate of UTI in younger women, compared with cranberry extracts alone (P=.02), while cranberry extracts were slightly more effective than trimethoprim alone in older women.9,10 Cranberry tablets were not associated with bacterial resistance, were cheaper, and were viewed as a more natural option. The interventions were equally well tolerated.
Overall efficacy of cranberry tablets is unclear. Side effects, albeit mild, included gastrointestinal disturbances, vaginal complaints, and rash or urticaria. There was no significant difference in the rate of adverse effects between antimicrobial treatment and cranberry tablets.9
Cystopurin has not been studied
Cystopurin is an over-the-counter (OTC) tablet containing cranberry extract and potassium citrate that is taken three times daily (3 g/dose) for 2 days. Interestingly, although a proposed mechanism for the efficacy of vitamin C and cranberry juice has been a reduction of pH, potassium citrate is an alkalizing agent that is reported to relieve burning and reduce urinary urgency and frequency. No studies have assessed this medication in the treatment of a UTI or its symptoms.
Clinical recommendations
The evidence is mixed on the use of cranberry products to reduce recurrent UTI. However, given the limited side effects associated with these products, we offer cranberry tablets to patients who have recurrent UTIs who are interested in a more natural alternative.
We generally do not recommend cranberry juice because the added fluid volume tends to exacerbate frequency and urgency symptoms.
Lactobacilli suppositories may benefit
postmenopausal women
Lactobacilli are fastidious gram-positive rods and are usually the dominant component of the vaginal flora.11 They prevent colonization and infection by more virulent bacteria by competing for adhesion receptors and nutrients as well as producing antimicrobial substances such as hydrogen peroxide and lactic acid. A decrease in lactobacilli leaves the urinary tract susceptible to infectious organisms that may colonize the vaginal mucosa and increase the risk of recurrent UTI.12-14
A 2008 review of randomized, controlled trials of oral lactobacilli and UTI was inconclusive, due to inconsistent dosing strategies and small sample sizes.15 A 2011 randomized, double-blind, placebo-controlled phase 2 trial of Lactin-V, a lactobacilli vaginal suppository, found that it reduced the rate of recurrent UTI. Lactin-V contains a hydrogen-peroxide–producing Lactobacillus crispatus developed as a probiotic that was determined to be safe and tolerable as a vaginal suppository in a phase 1 trial.16 The phase 2 trial enrolled 100 young premenopausal women with a history of recurrent UTI who took either Lactin-V or placebo daily for 5 days, then weekly for 10 weeks. Women in the Lactin-V group who had high levels of L. crispatus colonization experienced a significant reduction in the rate of UTI (15% vs 27% in the placebo group), but the effect did not reach statistical significance.14
Little difference in adverse effects
Adverse effects were reported among 56% of patients who received Lactin-V versus 50% of those given placebo. The most common of these were vaginal discharge, itching, and moderate abdominal discomfort.14 Although lactobacillus can potentially promote UTI, this phenomenon is rare.11
Regrettably, Lactin-V is not currently available in the United States. However, there are other lactobacilli vaginal suppositories on the market ( TABLE ). Given the low risk associated with their use, they should be considered as an alternative for patients who cannot or will not use estrogen.
Clinical recommendations
Probiotics such as lactobacilli are categorized as “dietary supplements”; as such, they are not regulated by the US Food and Drug Administration. We recommend the use of lactobacilli suppositories in postmenopausal women who have a contraindication to (or prefer to avoid) vaginal estrogen.
Skip blueberry products for now
Like cranberries, blueberries belong to the Vaccinium species and are thought to interfere with bacterial adhesion to the walls of the bladder. One in vitro trial suggests that blueberries also have antiproliferation effects, although no clinical studies have been performed to date to further investigate safety or efficacy.4 Consequently, we do not recommend use of these products.
Methenamine salts may benefit some populations
These anti-infective agents, including methenamine hippurate and methenamine mandelate, often are used to prevent UTI. They are found in combination OTC medications, such as Prosed DS and Urelle. Methenamine salts are bacteriostatic to all urinary tract pathogens due to their production of formaldehyde.3,16
Although methenamine produces varying concentrations of formaldehyde, depending on the acidity of the urine, there is no evidence that acidified urine enhances methenamine’s effects.16
Advantages of methenamine include the fact that it produces no changes in gut flora, poses no risk for antimicrobial resistance, and has low toxicity. It also is low in cost.17
Methenamine is contraindicated in patients with renal insufficiency or severe hepatic disease.
Adverse reactions are generally mild and include gastrointestinal disturbances, skin rashes, dysuria, and microscopic hematuria.16
Methenamine hippurate
A 2007 Cochrane review, deemed up to date in 2010, analyzed 13 randomized, controlled trials involving 2,032 participants. Subgroup analyses suggested that methenamine hippurate may be of some benefit to patients without renal tract abnormalities; these patients experienced significantly reduced symptoms after short-term treatment of 1 week or less.
Patients with spinal injury do not appear to benefit from treatment, according to a randomized, double-blind, placebo-controlled trial by Lee and colleagues.18
Methenamine mandelate
This agent is commonly used to prevent recurrent UTI, although there is a paucity of randomized, controlled trials to support its use. One such trial in patients with neurogenic bladder found that methenamine mandelate with acidification was superior to placebo (P <.02) for preventing UTI.19 Beyond this population, however, it’s difficult to assess methenamine’s efficacy in the prevention of recurrent UTI.
Methylene blue may be useful in the elderly
Methylene blue is a light-activated compound described as “photodynamic antimicrobial chemotherapy” (PACT). Once illuminated, it becomes a bactericide, causing excitation of electrons followed by one of two reactions:
- reduction oxidation
- formation of a labile singlet oxygen and then oxidation.
This makes resistance unlikely.
In an in vitro study, methylene blue was as effective as levofloxacin against Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus mirabilis, Enterococcus, and Staphylococcus aureus when illuminated.
Potential benefits of this mode of treatment include local exposure and no drug interactions.
We suggest that methylene blue be placed and illuminated with a special catheter that would target UTI in the elderly population, among whom 52% of UTIs are associated with use of catheters.20 In vivo effects, adverse effects, and cost are not known, which limits current applicability of this compound.
Vitamin C may reduce UTI in pregnancy
The proposed mechanism for the efficacy of vitamin C for the treatment of UTI is the acidification of urine, which is believed to reduce the proliferation of bacteria. However, several studies have shown that vitamin C, at various doses, does not reliably reduce urine pH.15,21,22 Nonetheless, ascorbic acid was tested for its effect on UTI prevention during pregnancy.
In a single-blind trial, 110 pregnant women were divided into two groups (55 in each group):
- One group received ferrous sulfate (200 mg), folic acid (5 mg), and vitamin C (100 mg) daily for 3 months
- The other received ferrous sulfate (200 mg) and folic acid (5 mg) daily for 3 months.
Urine was cultured monthly. The incidence of UTI was significantly lower in the group receiving vitamin C (12.7%), compared with the control group (29.1%) (P=.03; odds ratio [OR], 0.35).23
Uva ursi may have a prophylactic effect
Uva ursi (UVA-E) is one of the most commonly used herbal supplements for treatment of UTI. The crude extract from Bearberry or Arctostaphylos uva-ursi has been shown to act as an antimicrobial by decreasing bacterial adherence.24 In addition, investigators have found the extract to have diuretic and anti-inflammatory properties.25,26 However, few studies have explored its efficacy. One randomized, controlled trial that included 57 women (30 allocated to UVA-E and 27 to placebo) found a statistically significant reduction in the rate of recurrence at 1 year among women taking UVA-E, compared with those who did not.27
Note that the women in this trial were given UVA-E for 1 month only. Long-term use of this herb has not been studied and may cause liver damage.
In a mouse model, forskolin reduced urinary-tract E. coli
This herb is derived from Indian coleus (Coleus forskohlii), a member of the mint family. It has been used primarily for its antiasthmatic, spasmolytic, and antihypertensive effects. It is believed to activate adenylate cyclase, increasing intracellular cyclic AMP (cAMP) concentrations and activating a number of key enzymatic pathways.28
The findings of a recent observational study have spurred the use of forskolin in the treatment of recurrent UTI.29 In the study, conducted on mice, when forskolin was injected into the bladder, intracellular E. coli decreased.
It is theorized that the incorporation of bacteria into intracellular vesicles of the bladder prevents exposure to antibiotics. When combined with an antibiotic, forskolin may increase bacterial elimination and thus lower the risk of recurrent infection. However, no randomized trials have evaluated the efficacy of this treatment.
Patients who are already taking antihypertensive medications should be cautious when using this herb, as it may lead to a drop in blood pressure.
Behavior changes are risk-free
One of the natural mechanisms that promotes bacterial elimination and prevents bacterial growth is urination. A recent review article on the subject found several contradictory studies on the effect of fluid intake on the risk of UTI.30 Although there is no definitive evidence that susceptibility to UTI is linked to fluid intake, adequate hydration may reduce the risk of recurrent infection.
Similarly, voiding shortly after sexual intercourse may prevent UTI. One case-control study found a modest protective effect in patients who voided after intercourse.31
Clinical recommendations
Given the low risk of these measures, it seems reasonable to recommend postcoital voiding and increased fluid intake to prevent recurrent UTI.
A focus on symptoms
Phenazopyridine, the chemical found in numerous OTC medications, such as Pyridium, AZO, and Uristat, was discovered by Swiss chemist Bernhard Joos in the 1950s. Its mechanism of action is still unclear, but approximately 65% of the oral dose is excreted by the kidneys, where it has a direct topical analgesic effect.32,33
Clinical recommendations
Patients should be warned that phenazopyridine will lead to orange urine discoloration.
The medication is generally well tolerated but should be used with caution in patients with acute renal failure, hemolytic anemia, or methemoglobinemia, as it may exacerbate these conditions.
Last words
Recurrent UTIs are common and impose a significant financial burden on our healthcare system. Although there are several antibiotic treatment options and dosing regimens available, increasing antibiotic resistance has made management of recurrent UTIs more difficult. Effective alternative treatments that reduce the reliance on antibiotics may minimize bacterial resistance and decrease the financial burden of this common condition.
CASE: Resolved
To reduce vaginal E. coli, the patient is started on vaginal estrogen cream. She is also advised to purchase cranberry tablets to help prevent future infections. Last, she is counseled about behavioral changes she can make and prescribed a short course of antibiotics to treat her culture-proven infection.
We want to hear from you! Tell us what you think.
URINARY PROBLEMS?
CLICK HERE to access 9 articles about treating urinary incontinence and urinary tract infections, published in OBG MANAGEMENTin 2012.
1. Raz R, Stamm W. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753-756.
2. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;2:CD005131.-
3. Mayrer AR, Andriole VT. Urinary tract antiseptics. Med Clin North Am. 1982;66(1):199-208.
4. Jepson RG, Craig JC. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol Nutr Food Res. 2007;51(6):738-745.
5. Salvatore S, Salvatore S, Cattoni E, et al. Urinary tract infections in women. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):131-136.
6. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;1:CD001321.-
7. Stapleton AE, Dziura J, Hooton TM, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87(2):143-150.
8. Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52(1):23-30.
9. Beerepoot MAJ, Reit G, Nys S, et al. Cranberries vs antibiotics to prevent urinary tract infections. Arch Intern Med. 2011;171(14):1270-1278.
10. McMurdo MET, Argo I, Phillips G, Daly F, Davey P. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J Antimicrob Chemother. 2009;63(2):389-395.
11. Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clinical Therapeutics. 2008;30(3):453-468.
12. Miller JL, Krieger JN. Urinary tract infections: cranberry juice underwear, and probiotics in the 21st century. Urol Clin N Am. 2002;29(3):695-699.
13. Osset J, Bartolome R, Garcia E, et al. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183(3):485-491.
14. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212-1217.
15. Castello T, Girona L, Gomez MR, Mena Mur A, Garcia L. The possible value of ascorbic acid as a prophylactic agent for urinary tract infection. Spinal Cord. 1996;34(10):592-593.
16. Gleckman R, Alvarez S, Joubert DW, Matthews SJ. Drug therapy reviews: methenamine mandelate and methenamine hippurate. Am J Hosp Pharm. 1979;36(11):1509-1512.
17. Vainrub B, Musher DM. Lack of effect of methenamine in suppression of or prophylaxis against, chronic urinary tract infection. Antimicrob Agents Chemother. 1977;12(5):625-629.
18. Lee BB, Simpson JM, Craig JC, Bhuta T. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;4:CD003265.-
19. Kevorkian CG, Merritt JL, Ilstrup DM. Methenamine mandelate with acidification: an effective urinary antiseptic in patients with neurogenic bladder. Mayo Clin Proc. 1984;59(8):523-529.
20. Wainwright M, Stanforth A, Jones R, Loughran C, Meegan K. Photoantimicrobials as a potential local approach to geriatric UTIs. Lett Appl Microbiol. 2010;50(5):486-492.
21. Bannwart C, Hagmaier V, Straumann E, Hofer H, Buillemier JP, Trutishauser G. Modification of urinary pH through ascorbic acid. Helv Chir Acta. 1981;48(3-4):425-428.
22. Hetey SK, Kleinberg ML, Parker WD, Johnson EW. Effect of ascorbic acid on urine pH in patients with injured spinal cords. Am J Hosp Pharm. 1980;37(2):235-237.
23. Ochoa-Brust GJ, Fernandez AR, Villanueva-Ruiz GJ, et al. Daily intake of 100 mg ascorbic acid as urinary tract infection prophylactic agent during pregnancy. Acta Obstet Gynecol Scand. 2007;86(7):783-787.
24. Turi M, Turi E, Kotjalg S, Mikelsaar M. Influence of aqueous extracts of medicinal plants on surface hydrophobicity of Escherichia coli strains of different origin. APMIS. 1997;105(12):956-962.
25. Beaux D, Fleurentin J, Mortier F. Effect of extracts of Orthosiphon stamineus Benth Hieracium pilosellaL., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phytother Res. 1999;13(3):222-225.
26. Kubo M, Ito M, Nakata H, Matsuda H. Pharmacological studies on leaf of Arctostaphylos uva-ursi (L.) Spreng. I. Combined effect of 50% methanolic extract from Arctostaphylos uva-ursi (L.) Spreng. (bearberry leaf) and prednisolone on immuno-inflammation [article in Japanese]. Yakugaku Zasshi. 1990;110(1):59-67.
27. Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res. 1993;53(4):441-443.
28. Christenson JT, Thulesius D, Nazzal MM. The effect of forskolin on blood flow platelet metabolism, aggregation and ATP release. Vasa. 1995;24(1):56-61.
29. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13(5):625-630.
30. Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(Suppl 2):S52-58.
31. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43(4):329-337.
32. Thomas BH, Whitehouse LW, Solomonraj G, Paul CJ. Excretion of phenazopyridine and its metabolites in the urine of humans rats, mice, and guinea pigs. J Pharm Sci. 1990;79(4):321-325.
33. Aizawa N, Wyndaele JJ. Effects of phenazopyridine on rat bladder primary afferent activity and comparison with lidocaine and acetaminophen. Neurourol Urodyn. 2010;29(8):1445-1550.
The authors report no financial relationships relevant to this article.
CASE: Recurrent UTI and antibiotic resistance
A 53-year-old postmenopausal woman with a history of culture-proven recurrent Escherichia coli urinary tract infections (UTIs) presents to the clinic with symptoms of UTI. She was previously treated with a postcoital regimen of trimethoprim/sulfamethoxazole, based on sensitivities identified by culture. A past work-up of her upper and lower urinary tract was negative. You send a catheterized specimen for culture; again, E. coli is identified as the pathogen but proves resistant to her current antibiotic regimen.
What treatment alternatives, aside from antibiotics, are available for this patient—and how might they affect resistance?
Increased antibiotic usage has led to greater bacterial resistance, which is perpetuated by clonal spread. Resistant strains of E. coli have been found in household members, suggesting host-host transmission as a mechanism for dissemination. Alternative treatments that reduce the use of antibiotics may minimize bacterial resistance and increase the efficacy of treatment. In the TABLE , we summarize alternative approaches to the treatment of recurrent UTI. We also describe a strategy to alleviate symptoms.
Alternatives to antibiotics in the treatment and prevention of recurrent UTI
| Category | Type | Examples and doses, if recommended |
|---|---|---|
| Vaginal estrogen | Conjugated estrogen cream Estradiol
| Premarin cream, 0.5–2 g vaginally twice weekly
|
| Nutritive agents | Cranberry juice Cranberry tablets Cystopurin Lactobacilli Blueberry products | Not recommended 1 tablet (300 to 400 mg, depending on manufacturer) twice daily Not recommended Vivag, EcoVag, 1 capsule daily by vagina for 5 days, then once weekly for 10 weeks Not recommended |
| Anti-infective drugs | Methenamine hippurate Methenamine mandelate Methylene blue | Urex or Hiprex, 1 g orally twice daily Mandelamine, 1 g orally 4 times daily Future therapy |
| Urinary acidifiers | Vitamin C/ascorbic acid | 1–3 g orally 3–4 times daily |
| Herbal remedies | Uva ursi Forskolin | Not recommended for long-term use Not recommended |
| Behavioral changes | Adequate hydration Postcoital voiding |
Vaginal estrogen is the only proven alternative to antibiotics for postmenopausal women
A lack of estrogen is a risk factor for UTI and is associated with atrophic mucosa, leading to decreased colonization with lactobacilli, increased vaginal pH, and E. coli colonization.
A randomized, double-blind, placebo-controlled trial of intravaginal estriol cream versus placebo in 93 postmenopausal women found a significant decrease in the rate of UTI among women who used the cream.1 After 8 months of follow-up, the incidence of UTI was 0.5 vs 5.9 episodes per patient-year (P <.001). Interestingly, all pretreatment cultures were negative for lactobacilli. One month after treatment, 61% of women in the estriol group were culture-positive for lactobacilli, compared with 0% of the placebo group.1
A 2008 Cochrane review of nine studies concluded that vaginal estrogen reduces the number of UTIs in postmenopausal women, with variation based on the type of estrogen and duration of use.2
Adverse effects are mild
Twenty-eight percent of the estriol group in the randomized trial described above withdrew from treatment, with 20% citing local side effects, including vaginal irritation, burning, or itching—all of which were mild and self-limited.1 Other possible adverse effects include breast tenderness, vaginal bleeding or spotting, and discharge.2
Clinical recommendations
Given the efficacy of this therapy, we recommend topical estrogen for postmenopausal patients with recurrent UTIs.
Cranberry juice may reduce UTI, but many patients withdraw
from treatment
Cranberries belong to the Vaccinium species, which contains all flavonoids, including anthocyanins and proanthocyanidins. It was previously thought that the acidification of urine produced an antibacterial effect, but several trials have documented no change in urine levels of hippuric acid when cranberry products are given, with no acidification of the urine.3 Current theory suggests that cranberries prevent bacteria from adhering to the uroepithelial cells of the walls of the bladder, by blocking expression of E. coli’s adhesion molecule, P. fimbriae, so that bacteria are unable to penetrate the mucosal surface.4,5 The major benefit of cranberry products over antibiotic prophylaxis is that they do not have the potential for resistance.4
A 2008 Cochrane review concluded that cranberry juice may reduce symptomatic UTIs, particularly among young, sexually active women—but there is a high rate of withdrawal from treatment.6 The optimal method of administration and dose remain unclear. In contrast, two recent randomized, controlled trials—published after the Cochrane review—found no difference in the rate of recurrent UTI in premenopausal women.7,8 Adverse effects in these two trials included constipation, heartburn, loose stools, vaginal itching and dryness, and migraines. Of note, there was no statistical difference in side effects between the cranberry and placebo groups.7
Vaccinium tablets may be protective in older women
Cranberry extracts of 500 mg to 1,000 mg daily have been compared with antimicrobial prophylaxis in two randomized, double-blind, controlled trials. The trials demonstrated mixed benefits. Trimethoprim/sulfamethoxazole was associated with a lower rate of UTI in younger women, compared with cranberry extracts alone (P=.02), while cranberry extracts were slightly more effective than trimethoprim alone in older women.9,10 Cranberry tablets were not associated with bacterial resistance, were cheaper, and were viewed as a more natural option. The interventions were equally well tolerated.
Overall efficacy of cranberry tablets is unclear. Side effects, albeit mild, included gastrointestinal disturbances, vaginal complaints, and rash or urticaria. There was no significant difference in the rate of adverse effects between antimicrobial treatment and cranberry tablets.9
Cystopurin has not been studied
Cystopurin is an over-the-counter (OTC) tablet containing cranberry extract and potassium citrate that is taken three times daily (3 g/dose) for 2 days. Interestingly, although a proposed mechanism for the efficacy of vitamin C and cranberry juice has been a reduction of pH, potassium citrate is an alkalizing agent that is reported to relieve burning and reduce urinary urgency and frequency. No studies have assessed this medication in the treatment of a UTI or its symptoms.
Clinical recommendations
The evidence is mixed on the use of cranberry products to reduce recurrent UTI. However, given the limited side effects associated with these products, we offer cranberry tablets to patients who have recurrent UTIs who are interested in a more natural alternative.
We generally do not recommend cranberry juice because the added fluid volume tends to exacerbate frequency and urgency symptoms.

Lactobacilli suppositories may benefit
postmenopausal women
Lactobacilli are fastidious gram-positive rods and are usually the dominant component of the vaginal flora.11 They prevent colonization and infection by more virulent bacteria by competing for adhesion receptors and nutrients as well as producing antimicrobial substances such as hydrogen peroxide and lactic acid. A decrease in lactobacilli leaves the urinary tract susceptible to infectious organisms that may colonize the vaginal mucosa and increase the risk of recurrent UTI.12-14
A 2008 review of randomized, controlled trials of oral lactobacilli and UTI was inconclusive, due to inconsistent dosing strategies and small sample sizes.15 A 2011 randomized, double-blind, placebo-controlled phase 2 trial of Lactin-V, a lactobacilli vaginal suppository, found that it reduced the rate of recurrent UTI. Lactin-V contains a hydrogen-peroxide–producing Lactobacillus crispatus developed as a probiotic that was determined to be safe and tolerable as a vaginal suppository in a phase 1 trial.16 The phase 2 trial enrolled 100 young premenopausal women with a history of recurrent UTI who took either Lactin-V or placebo daily for 5 days, then weekly for 10 weeks. Women in the Lactin-V group who had high levels of L. crispatus colonization experienced a significant reduction in the rate of UTI (15% vs 27% in the placebo group), but the effect did not reach statistical significance.14
Little difference in adverse effects
Adverse effects were reported among 56% of patients who received Lactin-V versus 50% of those given placebo. The most common of these were vaginal discharge, itching, and moderate abdominal discomfort.14 Although lactobacillus can potentially promote UTI, this phenomenon is rare.11
Regrettably, Lactin-V is not currently available in the United States. However, there are other lactobacilli vaginal suppositories on the market ( TABLE ). Given the low risk associated with their use, they should be considered as an alternative for patients who cannot or will not use estrogen.
Clinical recommendations
Probiotics such as lactobacilli are categorized as “dietary supplements”; as such, they are not regulated by the US Food and Drug Administration. We recommend the use of lactobacilli suppositories in postmenopausal women who have a contraindication to (or prefer to avoid) vaginal estrogen.
Skip blueberry products for now
Like cranberries, blueberries belong to the Vaccinium species and are thought to interfere with bacterial adhesion to the walls of the bladder. One in vitro trial suggests that blueberries also have antiproliferation effects, although no clinical studies have been performed to date to further investigate safety or efficacy.4 Consequently, we do not recommend use of these products.
Methenamine salts may benefit some populations
These anti-infective agents, including methenamine hippurate and methenamine mandelate, often are used to prevent UTI. They are found in combination OTC medications, such as Prosed DS and Urelle. Methenamine salts are bacteriostatic to all urinary tract pathogens due to their production of formaldehyde.3,16
Although methenamine produces varying concentrations of formaldehyde, depending on the acidity of the urine, there is no evidence that acidified urine enhances methenamine’s effects.16
Advantages of methenamine include the fact that it produces no changes in gut flora, poses no risk for antimicrobial resistance, and has low toxicity. It also is low in cost.17
Methenamine is contraindicated in patients with renal insufficiency or severe hepatic disease.
Adverse reactions are generally mild and include gastrointestinal disturbances, skin rashes, dysuria, and microscopic hematuria.16
Methenamine hippurate
A 2007 Cochrane review, deemed up to date in 2010, analyzed 13 randomized, controlled trials involving 2,032 participants. Subgroup analyses suggested that methenamine hippurate may be of some benefit to patients without renal tract abnormalities; these patients experienced significantly reduced symptoms after short-term treatment of 1 week or less.
Patients with spinal injury do not appear to benefit from treatment, according to a randomized, double-blind, placebo-controlled trial by Lee and colleagues.18
Methenamine mandelate
This agent is commonly used to prevent recurrent UTI, although there is a paucity of randomized, controlled trials to support its use. One such trial in patients with neurogenic bladder found that methenamine mandelate with acidification was superior to placebo (P <.02) for preventing UTI.19 Beyond this population, however, it’s difficult to assess methenamine’s efficacy in the prevention of recurrent UTI.
Methylene blue may be useful in the elderly
Methylene blue is a light-activated compound described as “photodynamic antimicrobial chemotherapy” (PACT). Once illuminated, it becomes a bactericide, causing excitation of electrons followed by one of two reactions:
- reduction oxidation
- formation of a labile singlet oxygen and then oxidation.
This makes resistance unlikely.
In an in vitro study, methylene blue was as effective as levofloxacin against Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus mirabilis, Enterococcus, and Staphylococcus aureus when illuminated.
Potential benefits of this mode of treatment include local exposure and no drug interactions.
We suggest that methylene blue be placed and illuminated with a special catheter that would target UTI in the elderly population, among whom 52% of UTIs are associated with use of catheters.20 In vivo effects, adverse effects, and cost are not known, which limits current applicability of this compound.
Vitamin C may reduce UTI in pregnancy
The proposed mechanism for the efficacy of vitamin C for the treatment of UTI is the acidification of urine, which is believed to reduce the proliferation of bacteria. However, several studies have shown that vitamin C, at various doses, does not reliably reduce urine pH.15,21,22 Nonetheless, ascorbic acid was tested for its effect on UTI prevention during pregnancy.
In a single-blind trial, 110 pregnant women were divided into two groups (55 in each group):
- One group received ferrous sulfate (200 mg), folic acid (5 mg), and vitamin C (100 mg) daily for 3 months
- The other received ferrous sulfate (200 mg) and folic acid (5 mg) daily for 3 months.
Urine was cultured monthly. The incidence of UTI was significantly lower in the group receiving vitamin C (12.7%), compared with the control group (29.1%) (P=.03; odds ratio [OR], 0.35).23
Uva ursi may have a prophylactic effect
Uva ursi (UVA-E) is one of the most commonly used herbal supplements for treatment of UTI. The crude extract from Bearberry or Arctostaphylos uva-ursi has been shown to act as an antimicrobial by decreasing bacterial adherence.24 In addition, investigators have found the extract to have diuretic and anti-inflammatory properties.25,26 However, few studies have explored its efficacy. One randomized, controlled trial that included 57 women (30 allocated to UVA-E and 27 to placebo) found a statistically significant reduction in the rate of recurrence at 1 year among women taking UVA-E, compared with those who did not.27
Note that the women in this trial were given UVA-E for 1 month only. Long-term use of this herb has not been studied and may cause liver damage.
In a mouse model, forskolin reduced urinary-tract E. coli
This herb is derived from Indian coleus (Coleus forskohlii), a member of the mint family. It has been used primarily for its antiasthmatic, spasmolytic, and antihypertensive effects. It is believed to activate adenylate cyclase, increasing intracellular cyclic AMP (cAMP) concentrations and activating a number of key enzymatic pathways.28
The findings of a recent observational study have spurred the use of forskolin in the treatment of recurrent UTI.29 In the study, conducted on mice, when forskolin was injected into the bladder, intracellular E. coli decreased.
It is theorized that the incorporation of bacteria into intracellular vesicles of the bladder prevents exposure to antibiotics. When combined with an antibiotic, forskolin may increase bacterial elimination and thus lower the risk of recurrent infection. However, no randomized trials have evaluated the efficacy of this treatment.
Patients who are already taking antihypertensive medications should be cautious when using this herb, as it may lead to a drop in blood pressure.
Behavior changes are risk-free
One of the natural mechanisms that promotes bacterial elimination and prevents bacterial growth is urination. A recent review article on the subject found several contradictory studies on the effect of fluid intake on the risk of UTI.30 Although there is no definitive evidence that susceptibility to UTI is linked to fluid intake, adequate hydration may reduce the risk of recurrent infection.
Similarly, voiding shortly after sexual intercourse may prevent UTI. One case-control study found a modest protective effect in patients who voided after intercourse.31
Clinical recommendations
Given the low risk of these measures, it seems reasonable to recommend postcoital voiding and increased fluid intake to prevent recurrent UTI.
A focus on symptoms
Phenazopyridine, the chemical found in numerous OTC medications, such as Pyridium, AZO, and Uristat, was discovered by Swiss chemist Bernhard Joos in the 1950s. Its mechanism of action is still unclear, but approximately 65% of the oral dose is excreted by the kidneys, where it has a direct topical analgesic effect.32,33
Clinical recommendations
Patients should be warned that phenazopyridine will lead to orange urine discoloration.
The medication is generally well tolerated but should be used with caution in patients with acute renal failure, hemolytic anemia, or methemoglobinemia, as it may exacerbate these conditions.
Last words
Recurrent UTIs are common and impose a significant financial burden on our healthcare system. Although there are several antibiotic treatment options and dosing regimens available, increasing antibiotic resistance has made management of recurrent UTIs more difficult. Effective alternative treatments that reduce the reliance on antibiotics may minimize bacterial resistance and decrease the financial burden of this common condition.
CASE: Resolved
To reduce vaginal E. coli, the patient is started on vaginal estrogen cream. She is also advised to purchase cranberry tablets to help prevent future infections. Last, she is counseled about behavioral changes she can make and prescribed a short course of antibiotics to treat her culture-proven infection.
We want to hear from you! Tell us what you think.
URINARY PROBLEMS?
CLICK HERE to access 9 articles about treating urinary incontinence and urinary tract infections, published in OBG MANAGEMENTin 2012.
The authors report no financial relationships relevant to this article.
CASE: Recurrent UTI and antibiotic resistance
A 53-year-old postmenopausal woman with a history of culture-proven recurrent Escherichia coli urinary tract infections (UTIs) presents to the clinic with symptoms of UTI. She was previously treated with a postcoital regimen of trimethoprim/sulfamethoxazole, based on sensitivities identified by culture. A past work-up of her upper and lower urinary tract was negative. You send a catheterized specimen for culture; again, E. coli is identified as the pathogen but proves resistant to her current antibiotic regimen.
What treatment alternatives, aside from antibiotics, are available for this patient—and how might they affect resistance?
Increased antibiotic usage has led to greater bacterial resistance, which is perpetuated by clonal spread. Resistant strains of E. coli have been found in household members, suggesting host-host transmission as a mechanism for dissemination. Alternative treatments that reduce the use of antibiotics may minimize bacterial resistance and increase the efficacy of treatment. In the TABLE , we summarize alternative approaches to the treatment of recurrent UTI. We also describe a strategy to alleviate symptoms.
Alternatives to antibiotics in the treatment and prevention of recurrent UTI
| Category | Type | Examples and doses, if recommended |
|---|---|---|
| Vaginal estrogen | Conjugated estrogen cream Estradiol
| Premarin cream, 0.5–2 g vaginally twice weekly
|
| Nutritive agents | Cranberry juice Cranberry tablets Cystopurin Lactobacilli Blueberry products | Not recommended 1 tablet (300 to 400 mg, depending on manufacturer) twice daily Not recommended Vivag, EcoVag, 1 capsule daily by vagina for 5 days, then once weekly for 10 weeks Not recommended |
| Anti-infective drugs | Methenamine hippurate Methenamine mandelate Methylene blue | Urex or Hiprex, 1 g orally twice daily Mandelamine, 1 g orally 4 times daily Future therapy |
| Urinary acidifiers | Vitamin C/ascorbic acid | 1–3 g orally 3–4 times daily |
| Herbal remedies | Uva ursi Forskolin | Not recommended for long-term use Not recommended |
| Behavioral changes | Adequate hydration Postcoital voiding |
Vaginal estrogen is the only proven alternative to antibiotics for postmenopausal women
A lack of estrogen is a risk factor for UTI and is associated with atrophic mucosa, leading to decreased colonization with lactobacilli, increased vaginal pH, and E. coli colonization.
A randomized, double-blind, placebo-controlled trial of intravaginal estriol cream versus placebo in 93 postmenopausal women found a significant decrease in the rate of UTI among women who used the cream.1 After 8 months of follow-up, the incidence of UTI was 0.5 vs 5.9 episodes per patient-year (P <.001). Interestingly, all pretreatment cultures were negative for lactobacilli. One month after treatment, 61% of women in the estriol group were culture-positive for lactobacilli, compared with 0% of the placebo group.1
A 2008 Cochrane review of nine studies concluded that vaginal estrogen reduces the number of UTIs in postmenopausal women, with variation based on the type of estrogen and duration of use.2
Adverse effects are mild
Twenty-eight percent of the estriol group in the randomized trial described above withdrew from treatment, with 20% citing local side effects, including vaginal irritation, burning, or itching—all of which were mild and self-limited.1 Other possible adverse effects include breast tenderness, vaginal bleeding or spotting, and discharge.2
Clinical recommendations
Given the efficacy of this therapy, we recommend topical estrogen for postmenopausal patients with recurrent UTIs.
Cranberry juice may reduce UTI, but many patients withdraw
from treatment
Cranberries belong to the Vaccinium species, which contains all flavonoids, including anthocyanins and proanthocyanidins. It was previously thought that the acidification of urine produced an antibacterial effect, but several trials have documented no change in urine levels of hippuric acid when cranberry products are given, with no acidification of the urine.3 Current theory suggests that cranberries prevent bacteria from adhering to the uroepithelial cells of the walls of the bladder, by blocking expression of E. coli’s adhesion molecule, P. fimbriae, so that bacteria are unable to penetrate the mucosal surface.4,5 The major benefit of cranberry products over antibiotic prophylaxis is that they do not have the potential for resistance.4
A 2008 Cochrane review concluded that cranberry juice may reduce symptomatic UTIs, particularly among young, sexually active women—but there is a high rate of withdrawal from treatment.6 The optimal method of administration and dose remain unclear. In contrast, two recent randomized, controlled trials—published after the Cochrane review—found no difference in the rate of recurrent UTI in premenopausal women.7,8 Adverse effects in these two trials included constipation, heartburn, loose stools, vaginal itching and dryness, and migraines. Of note, there was no statistical difference in side effects between the cranberry and placebo groups.7
Vaccinium tablets may be protective in older women
Cranberry extracts of 500 mg to 1,000 mg daily have been compared with antimicrobial prophylaxis in two randomized, double-blind, controlled trials. The trials demonstrated mixed benefits. Trimethoprim/sulfamethoxazole was associated with a lower rate of UTI in younger women, compared with cranberry extracts alone (P=.02), while cranberry extracts were slightly more effective than trimethoprim alone in older women.9,10 Cranberry tablets were not associated with bacterial resistance, were cheaper, and were viewed as a more natural option. The interventions were equally well tolerated.
Overall efficacy of cranberry tablets is unclear. Side effects, albeit mild, included gastrointestinal disturbances, vaginal complaints, and rash or urticaria. There was no significant difference in the rate of adverse effects between antimicrobial treatment and cranberry tablets.9
Cystopurin has not been studied
Cystopurin is an over-the-counter (OTC) tablet containing cranberry extract and potassium citrate that is taken three times daily (3 g/dose) for 2 days. Interestingly, although a proposed mechanism for the efficacy of vitamin C and cranberry juice has been a reduction of pH, potassium citrate is an alkalizing agent that is reported to relieve burning and reduce urinary urgency and frequency. No studies have assessed this medication in the treatment of a UTI or its symptoms.
Clinical recommendations
The evidence is mixed on the use of cranberry products to reduce recurrent UTI. However, given the limited side effects associated with these products, we offer cranberry tablets to patients who have recurrent UTIs who are interested in a more natural alternative.
We generally do not recommend cranberry juice because the added fluid volume tends to exacerbate frequency and urgency symptoms.

Lactobacilli suppositories may benefit
postmenopausal women
Lactobacilli are fastidious gram-positive rods and are usually the dominant component of the vaginal flora.11 They prevent colonization and infection by more virulent bacteria by competing for adhesion receptors and nutrients as well as producing antimicrobial substances such as hydrogen peroxide and lactic acid. A decrease in lactobacilli leaves the urinary tract susceptible to infectious organisms that may colonize the vaginal mucosa and increase the risk of recurrent UTI.12-14
A 2008 review of randomized, controlled trials of oral lactobacilli and UTI was inconclusive, due to inconsistent dosing strategies and small sample sizes.15 A 2011 randomized, double-blind, placebo-controlled phase 2 trial of Lactin-V, a lactobacilli vaginal suppository, found that it reduced the rate of recurrent UTI. Lactin-V contains a hydrogen-peroxide–producing Lactobacillus crispatus developed as a probiotic that was determined to be safe and tolerable as a vaginal suppository in a phase 1 trial.16 The phase 2 trial enrolled 100 young premenopausal women with a history of recurrent UTI who took either Lactin-V or placebo daily for 5 days, then weekly for 10 weeks. Women in the Lactin-V group who had high levels of L. crispatus colonization experienced a significant reduction in the rate of UTI (15% vs 27% in the placebo group), but the effect did not reach statistical significance.14
Little difference in adverse effects
Adverse effects were reported among 56% of patients who received Lactin-V versus 50% of those given placebo. The most common of these were vaginal discharge, itching, and moderate abdominal discomfort.14 Although lactobacillus can potentially promote UTI, this phenomenon is rare.11
Regrettably, Lactin-V is not currently available in the United States. However, there are other lactobacilli vaginal suppositories on the market ( TABLE ). Given the low risk associated with their use, they should be considered as an alternative for patients who cannot or will not use estrogen.
Clinical recommendations
Probiotics such as lactobacilli are categorized as “dietary supplements”; as such, they are not regulated by the US Food and Drug Administration. We recommend the use of lactobacilli suppositories in postmenopausal women who have a contraindication to (or prefer to avoid) vaginal estrogen.
Skip blueberry products for now
Like cranberries, blueberries belong to the Vaccinium species and are thought to interfere with bacterial adhesion to the walls of the bladder. One in vitro trial suggests that blueberries also have antiproliferation effects, although no clinical studies have been performed to date to further investigate safety or efficacy.4 Consequently, we do not recommend use of these products.
Methenamine salts may benefit some populations
These anti-infective agents, including methenamine hippurate and methenamine mandelate, often are used to prevent UTI. They are found in combination OTC medications, such as Prosed DS and Urelle. Methenamine salts are bacteriostatic to all urinary tract pathogens due to their production of formaldehyde.3,16
Although methenamine produces varying concentrations of formaldehyde, depending on the acidity of the urine, there is no evidence that acidified urine enhances methenamine’s effects.16
Advantages of methenamine include the fact that it produces no changes in gut flora, poses no risk for antimicrobial resistance, and has low toxicity. It also is low in cost.17
Methenamine is contraindicated in patients with renal insufficiency or severe hepatic disease.
Adverse reactions are generally mild and include gastrointestinal disturbances, skin rashes, dysuria, and microscopic hematuria.16
Methenamine hippurate
A 2007 Cochrane review, deemed up to date in 2010, analyzed 13 randomized, controlled trials involving 2,032 participants. Subgroup analyses suggested that methenamine hippurate may be of some benefit to patients without renal tract abnormalities; these patients experienced significantly reduced symptoms after short-term treatment of 1 week or less.
Patients with spinal injury do not appear to benefit from treatment, according to a randomized, double-blind, placebo-controlled trial by Lee and colleagues.18
Methenamine mandelate
This agent is commonly used to prevent recurrent UTI, although there is a paucity of randomized, controlled trials to support its use. One such trial in patients with neurogenic bladder found that methenamine mandelate with acidification was superior to placebo (P <.02) for preventing UTI.19 Beyond this population, however, it’s difficult to assess methenamine’s efficacy in the prevention of recurrent UTI.
Methylene blue may be useful in the elderly
Methylene blue is a light-activated compound described as “photodynamic antimicrobial chemotherapy” (PACT). Once illuminated, it becomes a bactericide, causing excitation of electrons followed by one of two reactions:
- reduction oxidation
- formation of a labile singlet oxygen and then oxidation.
This makes resistance unlikely.
In an in vitro study, methylene blue was as effective as levofloxacin against Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus mirabilis, Enterococcus, and Staphylococcus aureus when illuminated.
Potential benefits of this mode of treatment include local exposure and no drug interactions.
We suggest that methylene blue be placed and illuminated with a special catheter that would target UTI in the elderly population, among whom 52% of UTIs are associated with use of catheters.20 In vivo effects, adverse effects, and cost are not known, which limits current applicability of this compound.
Vitamin C may reduce UTI in pregnancy
The proposed mechanism for the efficacy of vitamin C for the treatment of UTI is the acidification of urine, which is believed to reduce the proliferation of bacteria. However, several studies have shown that vitamin C, at various doses, does not reliably reduce urine pH.15,21,22 Nonetheless, ascorbic acid was tested for its effect on UTI prevention during pregnancy.
In a single-blind trial, 110 pregnant women were divided into two groups (55 in each group):
- One group received ferrous sulfate (200 mg), folic acid (5 mg), and vitamin C (100 mg) daily for 3 months
- The other received ferrous sulfate (200 mg) and folic acid (5 mg) daily for 3 months.
Urine was cultured monthly. The incidence of UTI was significantly lower in the group receiving vitamin C (12.7%), compared with the control group (29.1%) (P=.03; odds ratio [OR], 0.35).23
Uva ursi may have a prophylactic effect
Uva ursi (UVA-E) is one of the most commonly used herbal supplements for treatment of UTI. The crude extract from Bearberry or Arctostaphylos uva-ursi has been shown to act as an antimicrobial by decreasing bacterial adherence.24 In addition, investigators have found the extract to have diuretic and anti-inflammatory properties.25,26 However, few studies have explored its efficacy. One randomized, controlled trial that included 57 women (30 allocated to UVA-E and 27 to placebo) found a statistically significant reduction in the rate of recurrence at 1 year among women taking UVA-E, compared with those who did not.27
Note that the women in this trial were given UVA-E for 1 month only. Long-term use of this herb has not been studied and may cause liver damage.
In a mouse model, forskolin reduced urinary-tract E. coli
This herb is derived from Indian coleus (Coleus forskohlii), a member of the mint family. It has been used primarily for its antiasthmatic, spasmolytic, and antihypertensive effects. It is believed to activate adenylate cyclase, increasing intracellular cyclic AMP (cAMP) concentrations and activating a number of key enzymatic pathways.28
The findings of a recent observational study have spurred the use of forskolin in the treatment of recurrent UTI.29 In the study, conducted on mice, when forskolin was injected into the bladder, intracellular E. coli decreased.
It is theorized that the incorporation of bacteria into intracellular vesicles of the bladder prevents exposure to antibiotics. When combined with an antibiotic, forskolin may increase bacterial elimination and thus lower the risk of recurrent infection. However, no randomized trials have evaluated the efficacy of this treatment.
Patients who are already taking antihypertensive medications should be cautious when using this herb, as it may lead to a drop in blood pressure.
Behavior changes are risk-free
One of the natural mechanisms that promotes bacterial elimination and prevents bacterial growth is urination. A recent review article on the subject found several contradictory studies on the effect of fluid intake on the risk of UTI.30 Although there is no definitive evidence that susceptibility to UTI is linked to fluid intake, adequate hydration may reduce the risk of recurrent infection.
Similarly, voiding shortly after sexual intercourse may prevent UTI. One case-control study found a modest protective effect in patients who voided after intercourse.31
Clinical recommendations
Given the low risk of these measures, it seems reasonable to recommend postcoital voiding and increased fluid intake to prevent recurrent UTI.
A focus on symptoms
Phenazopyridine, the chemical found in numerous OTC medications, such as Pyridium, AZO, and Uristat, was discovered by Swiss chemist Bernhard Joos in the 1950s. Its mechanism of action is still unclear, but approximately 65% of the oral dose is excreted by the kidneys, where it has a direct topical analgesic effect.32,33
Clinical recommendations
Patients should be warned that phenazopyridine will lead to orange urine discoloration.
The medication is generally well tolerated but should be used with caution in patients with acute renal failure, hemolytic anemia, or methemoglobinemia, as it may exacerbate these conditions.
Last words
Recurrent UTIs are common and impose a significant financial burden on our healthcare system. Although there are several antibiotic treatment options and dosing regimens available, increasing antibiotic resistance has made management of recurrent UTIs more difficult. Effective alternative treatments that reduce the reliance on antibiotics may minimize bacterial resistance and decrease the financial burden of this common condition.
CASE: Resolved
To reduce vaginal E. coli, the patient is started on vaginal estrogen cream. She is also advised to purchase cranberry tablets to help prevent future infections. Last, she is counseled about behavioral changes she can make and prescribed a short course of antibiotics to treat her culture-proven infection.
We want to hear from you! Tell us what you think.
URINARY PROBLEMS?
CLICK HERE to access 9 articles about treating urinary incontinence and urinary tract infections, published in OBG MANAGEMENTin 2012.
1. Raz R, Stamm W. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753-756.
2. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;2:CD005131.-
3. Mayrer AR, Andriole VT. Urinary tract antiseptics. Med Clin North Am. 1982;66(1):199-208.
4. Jepson RG, Craig JC. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol Nutr Food Res. 2007;51(6):738-745.
5. Salvatore S, Salvatore S, Cattoni E, et al. Urinary tract infections in women. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):131-136.
6. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;1:CD001321.-
7. Stapleton AE, Dziura J, Hooton TM, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87(2):143-150.
8. Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52(1):23-30.
9. Beerepoot MAJ, Reit G, Nys S, et al. Cranberries vs antibiotics to prevent urinary tract infections. Arch Intern Med. 2011;171(14):1270-1278.
10. McMurdo MET, Argo I, Phillips G, Daly F, Davey P. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J Antimicrob Chemother. 2009;63(2):389-395.
11. Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clinical Therapeutics. 2008;30(3):453-468.
12. Miller JL, Krieger JN. Urinary tract infections: cranberry juice underwear, and probiotics in the 21st century. Urol Clin N Am. 2002;29(3):695-699.
13. Osset J, Bartolome R, Garcia E, et al. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183(3):485-491.
14. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212-1217.
15. Castello T, Girona L, Gomez MR, Mena Mur A, Garcia L. The possible value of ascorbic acid as a prophylactic agent for urinary tract infection. Spinal Cord. 1996;34(10):592-593.
16. Gleckman R, Alvarez S, Joubert DW, Matthews SJ. Drug therapy reviews: methenamine mandelate and methenamine hippurate. Am J Hosp Pharm. 1979;36(11):1509-1512.
17. Vainrub B, Musher DM. Lack of effect of methenamine in suppression of or prophylaxis against, chronic urinary tract infection. Antimicrob Agents Chemother. 1977;12(5):625-629.
18. Lee BB, Simpson JM, Craig JC, Bhuta T. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;4:CD003265.-
19. Kevorkian CG, Merritt JL, Ilstrup DM. Methenamine mandelate with acidification: an effective urinary antiseptic in patients with neurogenic bladder. Mayo Clin Proc. 1984;59(8):523-529.
20. Wainwright M, Stanforth A, Jones R, Loughran C, Meegan K. Photoantimicrobials as a potential local approach to geriatric UTIs. Lett Appl Microbiol. 2010;50(5):486-492.
21. Bannwart C, Hagmaier V, Straumann E, Hofer H, Buillemier JP, Trutishauser G. Modification of urinary pH through ascorbic acid. Helv Chir Acta. 1981;48(3-4):425-428.
22. Hetey SK, Kleinberg ML, Parker WD, Johnson EW. Effect of ascorbic acid on urine pH in patients with injured spinal cords. Am J Hosp Pharm. 1980;37(2):235-237.
23. Ochoa-Brust GJ, Fernandez AR, Villanueva-Ruiz GJ, et al. Daily intake of 100 mg ascorbic acid as urinary tract infection prophylactic agent during pregnancy. Acta Obstet Gynecol Scand. 2007;86(7):783-787.
24. Turi M, Turi E, Kotjalg S, Mikelsaar M. Influence of aqueous extracts of medicinal plants on surface hydrophobicity of Escherichia coli strains of different origin. APMIS. 1997;105(12):956-962.
25. Beaux D, Fleurentin J, Mortier F. Effect of extracts of Orthosiphon stamineus Benth Hieracium pilosellaL., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phytother Res. 1999;13(3):222-225.
26. Kubo M, Ito M, Nakata H, Matsuda H. Pharmacological studies on leaf of Arctostaphylos uva-ursi (L.) Spreng. I. Combined effect of 50% methanolic extract from Arctostaphylos uva-ursi (L.) Spreng. (bearberry leaf) and prednisolone on immuno-inflammation [article in Japanese]. Yakugaku Zasshi. 1990;110(1):59-67.
27. Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res. 1993;53(4):441-443.
28. Christenson JT, Thulesius D, Nazzal MM. The effect of forskolin on blood flow platelet metabolism, aggregation and ATP release. Vasa. 1995;24(1):56-61.
29. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13(5):625-630.
30. Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(Suppl 2):S52-58.
31. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43(4):329-337.
32. Thomas BH, Whitehouse LW, Solomonraj G, Paul CJ. Excretion of phenazopyridine and its metabolites in the urine of humans rats, mice, and guinea pigs. J Pharm Sci. 1990;79(4):321-325.
33. Aizawa N, Wyndaele JJ. Effects of phenazopyridine on rat bladder primary afferent activity and comparison with lidocaine and acetaminophen. Neurourol Urodyn. 2010;29(8):1445-1550.
1. Raz R, Stamm W. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753-756.
2. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;2:CD005131.-
3. Mayrer AR, Andriole VT. Urinary tract antiseptics. Med Clin North Am. 1982;66(1):199-208.
4. Jepson RG, Craig JC. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol Nutr Food Res. 2007;51(6):738-745.
5. Salvatore S, Salvatore S, Cattoni E, et al. Urinary tract infections in women. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):131-136.
6. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;1:CD001321.-
7. Stapleton AE, Dziura J, Hooton TM, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87(2):143-150.
8. Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52(1):23-30.
9. Beerepoot MAJ, Reit G, Nys S, et al. Cranberries vs antibiotics to prevent urinary tract infections. Arch Intern Med. 2011;171(14):1270-1278.
10. McMurdo MET, Argo I, Phillips G, Daly F, Davey P. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J Antimicrob Chemother. 2009;63(2):389-395.
11. Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clinical Therapeutics. 2008;30(3):453-468.
12. Miller JL, Krieger JN. Urinary tract infections: cranberry juice underwear, and probiotics in the 21st century. Urol Clin N Am. 2002;29(3):695-699.
13. Osset J, Bartolome R, Garcia E, et al. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183(3):485-491.
14. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212-1217.
15. Castello T, Girona L, Gomez MR, Mena Mur A, Garcia L. The possible value of ascorbic acid as a prophylactic agent for urinary tract infection. Spinal Cord. 1996;34(10):592-593.
16. Gleckman R, Alvarez S, Joubert DW, Matthews SJ. Drug therapy reviews: methenamine mandelate and methenamine hippurate. Am J Hosp Pharm. 1979;36(11):1509-1512.
17. Vainrub B, Musher DM. Lack of effect of methenamine in suppression of or prophylaxis against, chronic urinary tract infection. Antimicrob Agents Chemother. 1977;12(5):625-629.
18. Lee BB, Simpson JM, Craig JC, Bhuta T. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;4:CD003265.-
19. Kevorkian CG, Merritt JL, Ilstrup DM. Methenamine mandelate with acidification: an effective urinary antiseptic in patients with neurogenic bladder. Mayo Clin Proc. 1984;59(8):523-529.
20. Wainwright M, Stanforth A, Jones R, Loughran C, Meegan K. Photoantimicrobials as a potential local approach to geriatric UTIs. Lett Appl Microbiol. 2010;50(5):486-492.
21. Bannwart C, Hagmaier V, Straumann E, Hofer H, Buillemier JP, Trutishauser G. Modification of urinary pH through ascorbic acid. Helv Chir Acta. 1981;48(3-4):425-428.
22. Hetey SK, Kleinberg ML, Parker WD, Johnson EW. Effect of ascorbic acid on urine pH in patients with injured spinal cords. Am J Hosp Pharm. 1980;37(2):235-237.
23. Ochoa-Brust GJ, Fernandez AR, Villanueva-Ruiz GJ, et al. Daily intake of 100 mg ascorbic acid as urinary tract infection prophylactic agent during pregnancy. Acta Obstet Gynecol Scand. 2007;86(7):783-787.
24. Turi M, Turi E, Kotjalg S, Mikelsaar M. Influence of aqueous extracts of medicinal plants on surface hydrophobicity of Escherichia coli strains of different origin. APMIS. 1997;105(12):956-962.
25. Beaux D, Fleurentin J, Mortier F. Effect of extracts of Orthosiphon stamineus Benth Hieracium pilosellaL., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phytother Res. 1999;13(3):222-225.
26. Kubo M, Ito M, Nakata H, Matsuda H. Pharmacological studies on leaf of Arctostaphylos uva-ursi (L.) Spreng. I. Combined effect of 50% methanolic extract from Arctostaphylos uva-ursi (L.) Spreng. (bearberry leaf) and prednisolone on immuno-inflammation [article in Japanese]. Yakugaku Zasshi. 1990;110(1):59-67.
27. Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res. 1993;53(4):441-443.
28. Christenson JT, Thulesius D, Nazzal MM. The effect of forskolin on blood flow platelet metabolism, aggregation and ATP release. Vasa. 1995;24(1):56-61.
29. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13(5):625-630.
30. Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(Suppl 2):S52-58.
31. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43(4):329-337.
32. Thomas BH, Whitehouse LW, Solomonraj G, Paul CJ. Excretion of phenazopyridine and its metabolites in the urine of humans rats, mice, and guinea pigs. J Pharm Sci. 1990;79(4):321-325.
33. Aizawa N, Wyndaele JJ. Effects of phenazopyridine on rat bladder primary afferent activity and comparison with lidocaine and acetaminophen. Neurourol Urodyn. 2010;29(8):1445-1550.
An app to help your patient with chronic pelvic pain
In this series, I review what I call prescription apps—apps that you might consider recommending to your patient to enhance her medical care. Many patients are already looking at medical apps and want to hear your opinion. Often, the free apps I recommend to patients are downloaded before they leave my office. When recommending apps, their cost (not necessarily a measure of quality or utility) and platform (device that the app has been designed for) should be taken into account. It is helpful to know whether the app you are recommending is supported by your patient’s smartphone.
Chronic pelvic pain: multifactorial
Chronic pelvic pain, like most chronic pain conditions, is multifactorial in nature. It is not surprising then that most women with chronic pelvic pain do best with a multidisciplinary management approach that addresses both physical and emotional well-being, including the mind-body aspect of chronic pain (how mood and emotions affect pain), exercise, pacing of activities, attention to sleep hygiene, and the role of dysfunctional eating patterns. However, a patient’s access to formal mind-body programs or even a pain psychologist can be hard to come by for a variety of reasons.

An app that tracks pain and treatment
WebMD Pain Coach is a mobile mind-body program and pain coach all rolled into one. While specifically designed for nongynecologic pain conditions (fibromyalgia, migraine, back pain), the app works just as well for pelvic pain. Pain conditions, such as pelvic pain, that are not preloaded into the app are easy to add.1,2
WebMD Pain Coach provides a way for the user to journal as well as track her pain scores, pain triggers, mood, sleep, diet, and response to therapies. It can provide a snapshot, yearly for instance, of tracked pain levels and is preloaded with goals that a user can customize easily. The app also is loaded with excellent pain management tips, videos, and slide shows. There are more than 300 patient-focused articles from the archives of WebMD and other sources that have been reviewed by experts. Progress and notes can be converted into a PDF for use at home or with a health-care provider—a very helpful tool as it can be hard to arrange the many domains of food, rest, exercise, mood, treatments, and pain scores in an organized fashion.1,2
Pros: With a multidisciplinary approach to managing chronic pain, it can be very helpful for patients to track their daily activity, pain triggers, pain levels, and tried therapies. The app provides an opportunity to learn more about the mind–body connection, which is a core component of effective pain management. This app also has excellent medical information and useful strategies for managing chronic pain. It’s easy to use as a source of information, a journal, and a pocket coach.
Cons: This is a free app for iPhone, iTouch, and the iPad—but currently only available for Apple products.
Verdict: This is a great tool on many levels. It would be useful for someone who just wants to track their pain and triggers, but also helpful for the patient who wants to obtain more control and learn more about managing pain. This app would be complementary for someone already engaged in mind–body work, but also be useful for someone who does not have access to those services.
- Why (and how) you should encourage your patients’ search for health information on the Web (December 2011)
- Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception? (Guest Editorial, October 2012)
- To blog or not to blog? What’s the answer for you and your practice? (August 2011)
- For better or, maybe worse, patients are judging your care online (March 2011)
- Twitter 101 for ObGyns: Pearls, pitfalls, and potential (September 2010)
We want to hear from you! Tell us what you think.
1. WebMD. WebMD Pain Coach: A Better Day Starts Here. http://www.webmd.com/webmdpaincoachapp. Accessed January 17, 2013.
2. WebMD. WebMD Pain Coach. iTunes Preview. Apple, Inc. https://itunes.apple.com/us/app/webmd-pain-coach/id536303342?mt=8. Released September 17, 2012. Accessed January 17, 2013.
In this series, I review what I call prescription apps—apps that you might consider recommending to your patient to enhance her medical care. Many patients are already looking at medical apps and want to hear your opinion. Often, the free apps I recommend to patients are downloaded before they leave my office. When recommending apps, their cost (not necessarily a measure of quality or utility) and platform (device that the app has been designed for) should be taken into account. It is helpful to know whether the app you are recommending is supported by your patient’s smartphone.
Chronic pelvic pain: multifactorial
Chronic pelvic pain, like most chronic pain conditions, is multifactorial in nature. It is not surprising then that most women with chronic pelvic pain do best with a multidisciplinary management approach that addresses both physical and emotional well-being, including the mind-body aspect of chronic pain (how mood and emotions affect pain), exercise, pacing of activities, attention to sleep hygiene, and the role of dysfunctional eating patterns. However, a patient’s access to formal mind-body programs or even a pain psychologist can be hard to come by for a variety of reasons.

An app that tracks pain and treatment
WebMD Pain Coach is a mobile mind-body program and pain coach all rolled into one. While specifically designed for nongynecologic pain conditions (fibromyalgia, migraine, back pain), the app works just as well for pelvic pain. Pain conditions, such as pelvic pain, that are not preloaded into the app are easy to add.1,2
WebMD Pain Coach provides a way for the user to journal as well as track her pain scores, pain triggers, mood, sleep, diet, and response to therapies. It can provide a snapshot, yearly for instance, of tracked pain levels and is preloaded with goals that a user can customize easily. The app also is loaded with excellent pain management tips, videos, and slide shows. There are more than 300 patient-focused articles from the archives of WebMD and other sources that have been reviewed by experts. Progress and notes can be converted into a PDF for use at home or with a health-care provider—a very helpful tool as it can be hard to arrange the many domains of food, rest, exercise, mood, treatments, and pain scores in an organized fashion.1,2
Pros: With a multidisciplinary approach to managing chronic pain, it can be very helpful for patients to track their daily activity, pain triggers, pain levels, and tried therapies. The app provides an opportunity to learn more about the mind–body connection, which is a core component of effective pain management. This app also has excellent medical information and useful strategies for managing chronic pain. It’s easy to use as a source of information, a journal, and a pocket coach.
Cons: This is a free app for iPhone, iTouch, and the iPad—but currently only available for Apple products.
Verdict: This is a great tool on many levels. It would be useful for someone who just wants to track their pain and triggers, but also helpful for the patient who wants to obtain more control and learn more about managing pain. This app would be complementary for someone already engaged in mind–body work, but also be useful for someone who does not have access to those services.
- Why (and how) you should encourage your patients’ search for health information on the Web (December 2011)
- Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception? (Guest Editorial, October 2012)
- To blog or not to blog? What’s the answer for you and your practice? (August 2011)
- For better or, maybe worse, patients are judging your care online (March 2011)
- Twitter 101 for ObGyns: Pearls, pitfalls, and potential (September 2010)
We want to hear from you! Tell us what you think.
In this series, I review what I call prescription apps—apps that you might consider recommending to your patient to enhance her medical care. Many patients are already looking at medical apps and want to hear your opinion. Often, the free apps I recommend to patients are downloaded before they leave my office. When recommending apps, their cost (not necessarily a measure of quality or utility) and platform (device that the app has been designed for) should be taken into account. It is helpful to know whether the app you are recommending is supported by your patient’s smartphone.
Chronic pelvic pain: multifactorial
Chronic pelvic pain, like most chronic pain conditions, is multifactorial in nature. It is not surprising then that most women with chronic pelvic pain do best with a multidisciplinary management approach that addresses both physical and emotional well-being, including the mind-body aspect of chronic pain (how mood and emotions affect pain), exercise, pacing of activities, attention to sleep hygiene, and the role of dysfunctional eating patterns. However, a patient’s access to formal mind-body programs or even a pain psychologist can be hard to come by for a variety of reasons.

An app that tracks pain and treatment
WebMD Pain Coach is a mobile mind-body program and pain coach all rolled into one. While specifically designed for nongynecologic pain conditions (fibromyalgia, migraine, back pain), the app works just as well for pelvic pain. Pain conditions, such as pelvic pain, that are not preloaded into the app are easy to add.1,2
WebMD Pain Coach provides a way for the user to journal as well as track her pain scores, pain triggers, mood, sleep, diet, and response to therapies. It can provide a snapshot, yearly for instance, of tracked pain levels and is preloaded with goals that a user can customize easily. The app also is loaded with excellent pain management tips, videos, and slide shows. There are more than 300 patient-focused articles from the archives of WebMD and other sources that have been reviewed by experts. Progress and notes can be converted into a PDF for use at home or with a health-care provider—a very helpful tool as it can be hard to arrange the many domains of food, rest, exercise, mood, treatments, and pain scores in an organized fashion.1,2
Pros: With a multidisciplinary approach to managing chronic pain, it can be very helpful for patients to track their daily activity, pain triggers, pain levels, and tried therapies. The app provides an opportunity to learn more about the mind–body connection, which is a core component of effective pain management. This app also has excellent medical information and useful strategies for managing chronic pain. It’s easy to use as a source of information, a journal, and a pocket coach.
Cons: This is a free app for iPhone, iTouch, and the iPad—but currently only available for Apple products.
Verdict: This is a great tool on many levels. It would be useful for someone who just wants to track their pain and triggers, but also helpful for the patient who wants to obtain more control and learn more about managing pain. This app would be complementary for someone already engaged in mind–body work, but also be useful for someone who does not have access to those services.
- Why (and how) you should encourage your patients’ search for health information on the Web (December 2011)
- Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception? (Guest Editorial, October 2012)
- To blog or not to blog? What’s the answer for you and your practice? (August 2011)
- For better or, maybe worse, patients are judging your care online (March 2011)
- Twitter 101 for ObGyns: Pearls, pitfalls, and potential (September 2010)
We want to hear from you! Tell us what you think.
1. WebMD. WebMD Pain Coach: A Better Day Starts Here. http://www.webmd.com/webmdpaincoachapp. Accessed January 17, 2013.
2. WebMD. WebMD Pain Coach. iTunes Preview. Apple, Inc. https://itunes.apple.com/us/app/webmd-pain-coach/id536303342?mt=8. Released September 17, 2012. Accessed January 17, 2013.
1. WebMD. WebMD Pain Coach: A Better Day Starts Here. http://www.webmd.com/webmdpaincoachapp. Accessed January 17, 2013.
2. WebMD. WebMD Pain Coach. iTunes Preview. Apple, Inc. https://itunes.apple.com/us/app/webmd-pain-coach/id536303342?mt=8. Released September 17, 2012. Accessed January 17, 2013.
When and how to place an autologous rectus fascia pubovaginal sling
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.
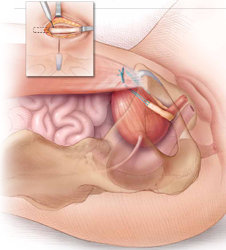
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).
FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).
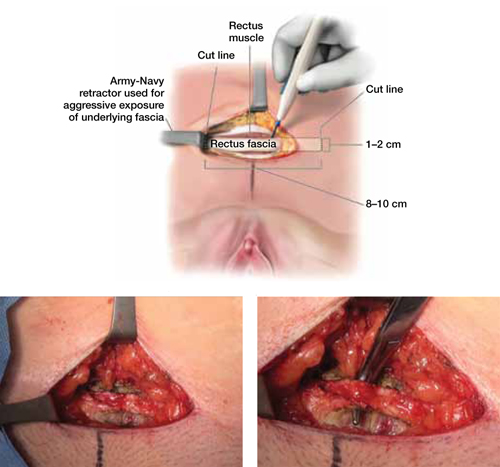
FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).
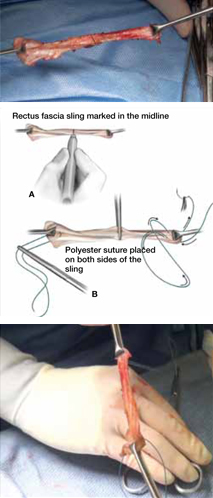
FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).
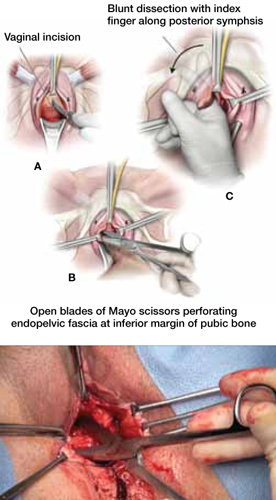
FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.
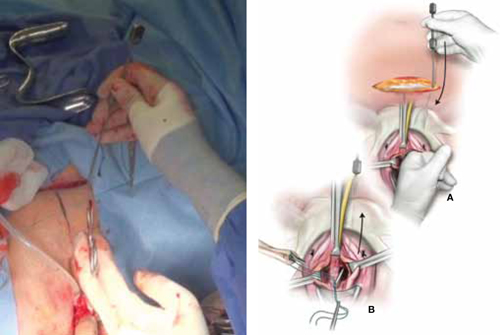
FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).
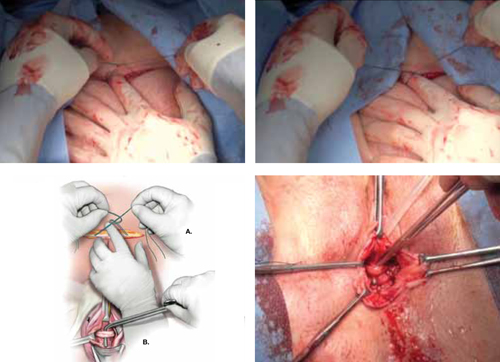
FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.

The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).

FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).

FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).

FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).

FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.

FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).

FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.

The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).

FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).

FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).

FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).

FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.

FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).

FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Novel strategy to prevent recurrent UTI in premenopausal women
Related article Update on Pelvic Floor Dysfunction (October 2012)
Related article Update on Pelvic Floor Dysfunction (October 2012)
Related article Update on Pelvic Floor Dysfunction (October 2012)
UPDATE: PELVIC FLOOR DYSFUNCTION
10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Megan O. Schimpf, MD (June 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Urinary tract infections (UTIs) are prevalent among women, afflicting as many as 60% of women during their lifetime.1 Symptoms include urgency, frequency, and dysuria. Although the diagnosis can be made on the basis of symptoms alone in many cases, urinalysis and urine cultures often are helpful in confirming it.2 The differential diagnosis includes infectious or atrophic vaginitis, urethritis from a sexually transmitted infection, urethral diverticulum, painful bladder syndrome, urinary tract calculi, and urinary tract neoplasms. Common risk factors for UTIs are listed in TABLE 1.3
TABLE 1
Risk factors for urinary tract infection in women
Premenopausal women •History of urinary tract infection (UTI) Postmenopausal women |
| SOURCE: Adapted from ACOG3 |
Recurrent UTIs are defined as three infections in 12 months or two infections in 6 months. In this Update, we explore strategies to prevent recurrent UTIs in three groups of women:
- sexually active premenopausal women
- postmenopausal women
- women undergoing pelvic surgery.
In the process, we summarize the results of five trials that explore treatment modalities such as prophylactic antibiotics, vaginal estrogen therapy, cranberry supplementation, and probiotics (TABLE 2).
TABLE 2
Summary of therapeutic strategies for prevention of recurrent urinary tract infections
| Strategy | Dose | Advantages | Disadvantages |
|---|---|---|---|
| Prophylactic antibiotics | Trimethoprim-sulfamethoxazole (Bactrim): 1 double-strength tablet* OR Nitrofurantoin: 50 or 100 mg Either drug can be given daily for 6 months or as one dose postcoitally | Highly effective Inexpensive | Potential for future microbial resistance Caution with nitrofurantoin, particularly in older patients or women who have renal insufficiency In pregnancy, nitrofurantoin is better studied |
| Vaginal estrogen** | Conjugated estrogens (0.625 mg conjugated estrogens/1 g cream [Premarin]). Give 0.5–2.0 g cream twice weekly. Estradiol (100 μg estradiol/1 g cream [Estrace]). Give 1–4 g cream | Highly effective in postmenopausal women, who can be difficult to treat Few true contraindications | Can be expensive Compliance may be an issue |
| Cranberry supplement | Dosing varies among products. Unsweetened natural cranberry juice or cranberry tablets, 1–3 times daily. | Generally well-tolerated Few side effects or contraindications | Can be expensive Compliance may be an issue May not be as effective in postmenopausal patients |
| Probiotics | Dosing varies among products and local availability | Few side effects or contraindications | Limited data Can be expensive |
| * Consider trimethoprim (100 mg) alone if the patient has an allergy to sulfa. ** Creams are preferred to the vaginal ring or tablets because they can be applied to periurethral tissues | |||
Postcoital antibiotic prophylaxis prevents some cases of recurrent UTI
Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157(3):935–939.
UTIs typically involve fecal flora that colonize the vagina and perineum, most commonly Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis. These pathogens ascend to the bladder via the urethra. Sexual intercourse is thought to facilitate this process, and recurrent UTIs in premenopausal women are often postcoital in temporal pattern.
When fecal flora ascend via the urethra from the vagina and perineum to the bladder, the bladder mucosa and urethra may become inflamed, leading to urinary tract infection. The most commonly involved pathogens are Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis.Daily antibiotic prophylaxis for 6 to 12 months has proved to be effective in the prevention of recurrent UTIs, reducing the risk of recurrence by 95%, compared with placebo.4
In this trial by Melekos and colleagues, sexually active premenopausal women who had a history of three or more documented UTIs in the preceding 12 months were randomly assigned to:
- oral ciprofloxacin, one dose daily, or
- oral ciprofloxacin, one dose immediately after intercourse.
A total of 135 patients (65 in the daily group and 70 in the postcoital group) were followed for 12 months. The regimens were equally effective at preventing UTIs. The mean number of UTIs in 12 months decreased significantly in both groups—from 3.74 to 0.031 in the daily group and from 3.67 to 0.043 in the postcoital group.
The best antibiotic? Nitrofurantoin or trimethoprim-sulfamethoxazole
This randomized, controlled trial was rigorous and well-executed and included only healthy premenopausal women. However, given the emergence of antibiotic resistance since this trial was conducted, ciprofloxacin is not an ideal antibiotic for prophylaxis.
Both the American Urological Association and the Infectious Disease Society of America recommend that fluoroquinolones be avoided, if possible, in the treatment of uncomplicated UTIs.5 A better therapeutic choice would be nitrofurantoin or trimethoprim-sulfamethoxazole.
Postcoital antibiotic prophylaxis is an effective strategy for the prevention of UTIs associated with sexual intercourse in premenopausal women. Although the optimal duration of such a regimen was not addressed in this study, it would be appropriate to revisit the need for prophylaxis after 1 year.
Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175–178.
Urinary tract catheterization and urogynecologic surgery are associated with an increased risk for UTI. The risk of UTI following a midurethral sling procedure, in particular, ranges from 4.1% to 33.6% in the literature.6,7 To further explore the risk of UTI after placement of a midurethral sling, Dieter and colleagues followed 138 women who had undergone the procedure with and without concomitant pelvic surgery. The primary outcome was treatment of UTI within the first 3 weeks postoperatively.
Catheterization increased the risk of UTI
Fifty-eight percent of women required placement of a catheter postoperatively—either an indwelling Foley or intermittent self-catheterization. The duration of catheterization ranged from 1 to 14 days, with a mean of 4 days. The incidence of UTI was significantly higher in the group that was catheterized postoperatively, compared with the group that was not (30.0% vs 5.2%), and catheterization remained an independent risk factor for UTI after adjusting for other confounding factors.
Data may not be applicable to other types of surgery
This large retrospective cohort study of a well-characterized population was based on consistent postoperative data related to catheterization and UTI treatment. Because the study focused on patients who had undergone placement of a midurethral sling, its findings may not be applicable to women undergoing other types of pelvic surgery, including general gynecologic procedures. However, given the significant difference in the rate of UTI between the two groups, the increased risk of UTI may be at least partially attributable to short-term postoperative catheterization rather than urinary tract instrumentation during the procedure.
The risk of UTI is increased with short-term catheterization following placement of a midurethral sling. There may be a role for antibiotic prophylaxis in the setting of short-term postoperative catheterization; however, a prospective, randomized, placebo-controlled study is needed to determine whether the rate of UTI would be reduced.
Vaginal estrogen prevents recurrent UTIs among postmenopausal women
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–756.
The tissues of the vagina, urethra, bladder, and pelvic floor musculature all express estrogen receptors.8 In postmenopausal women, the effects of decreased estrogen on the urinary tract include a rise in the vaginal pH level and decreased colonization with Lactobacillus. These effects predispose this population to an increased risk for UTI.3 The literature does not support the use of oral estrogen replacement as a therapy for recurrent UTI; however, data suggest that vaginal estrogen replacement may be helpful.9
Raz and Stamm conducted their randomized trial of 93 postmenopausal women with a history of recurrent UTIs to elucidate the effects of vaginal estrogen on the risk of UTI. Fifty women were randomly assigned to treatment with intravaginal estriol cream (0.5 mg nightly for 2 weeks, followed by 0.5 mg twice weekly for 8 months), and 43 women were randomly assigned to placebo (equivalent regimen). Compared with the placebo group, the women treated with estriol experienced a significantly reduced risk of UTI (0.5 vs 5.9 infections per patient-year), increased lactobacilli on vaginal cultures (61% vs 0%), decreased vaginal pH, and a lower rate of colonization with Enterobacteriaceae species.
Although this rigorous double-blind, randomized, placebo-controlled trial was published 20 years ago, its findings remain significant—and have been corroborated in other studies.9
Pros and cons of vaginal estrogen replacement
Raz and Stamm utilized vaginal estriol; the preparations used most commonly today are conjugated estrogens (Premarin) and estradiol (Estrace). Vaginal estrogen formulations can be expensive. Compliance also can wane over time. This study, in particular, showed a discontinuation rate of 28%; mild local reactions were the reason. Although the women who discontinued treatment in this study were included in the final analysis, no subanalysis of these patients was published.
Despite these challenges, local estrogen replacement is generally well-tolerated and, with infrequent dosing (twice weekly), has few contraindications. In fact, local estrogen replacement is one of the most highly effective regimens for UTI prevention among postmenopausal women, who can otherwise be difficult to treat for recurrent UTIs.
Vaginal estrogen is an effective therapy for the prevention of UTIs in postmenopausal women.
Cranberry supplementation may prevent UTIs,
but products vary widely
Stothers L. A randomized trial to evaluate effectiveness and cost-effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9(3):1558–1562.
Cranberries have been used for many years in various formulations to prevent UTI, but no definitive mechanism has been established. In theory, cranberries keep bacteria from adhering to the urothelium.10 In vitro studies have revealed that Escherichia coli is prevented from adhering to uroepithelial cells by two components of cranberry—fructose and proanthocyanidins.10
In this trial of 150 sexually active women (ages 21–72 years) who had experienced at least two UTIs in the past calendar year, Stothers randomly assigned participants to one of three arms for 12 months:
- placebo tablets and cranberry juice (n = 50)
- cranberry tablets and placebo juice (n = 50)
- placebo tablets and placebo juice (n = 50).
Tablets were taken twice daily, and juice was consumed three times daily. All cranberry juice was organic, unsweetened, and unfiltered and taken in 250-mL servings; cranberry tablets were 1:30 parts concentrated cranberry juice.
The risk of UTI during treatment was reduced significantly in the groups taking a cranberry formulation, compared with placebo. Twenty percent of patients consuming cranberry juice experienced a UTI during treatment, compared with 18% of those taking a cranberry tablet and 32% of those in the placebo group (P<.05). In this study, the annual cost of prophylaxis with cranberry juice was $1,400 per woman, and it was $624 per woman for the cranberry tablets. Compliance was lowest among women consuming cranberry juice, decreasing at times to less than 80%.
Findings are difficult to extrapolate
This randomized, double-blind study demonstrated a significant reduction in the rate of UTI with cranberry supplementation, compared with placebo, among women with a mean age of 40 to 44 years. However, because cranberry preparations, juice, and tablets are not regulated as to the amount and bioavailability of the active ingredient, it is difficult to compare one to another and extrapolate to a particular type of preparation.
This study does highlight the higher rate of noncompliance and cost with cranberry juice, although it was as effective at reducing UTIs as cranberry tablets.
Cranberry supplementation reduced the risk of UTIs in sexually active women; placebo did not. Cranberry use may be an alternative to postcoital antibiotic prophylaxis; a randomized comparison of these therapies is needed.
Can nonhormonal therapy alter vaginal flora?
Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217.
Probiotics have been used recently in attempts to prevent recurrent UTI, albeit with very little evidence in the literature. Their effectiveness is plausible due to promotion of healthy vaginal flora.
This study by Stapleton and colleagues enrolled premenopausal women (ages 18–40) with a history of one UTI within the past calendar year and a current, active, uncomplicated UTI. Ninety-nine percent of participants were sexually active. All women were treated with a standard antibiotic regimen for UTI. Seven to 10 days later, participants were randomly assigned to:
- Lactobacillus crispatus vaginal suppository [Lactin-V (Osel)], daily for 5 days and then weekly for 10 weeks (n = 50), or
- placebo (same regimen) (n = 50).
The risk of UTI was 15% among women in the probiotic group, compared with 27% in the placebo group—but this difference was only statistically significant for women who had a higher level of Lactobacillus crispatus vaginal colonization in the treatment group.
Vaginal probiotic formulations may be hard to obtain
The use of probiotics to prevent recurrent UTIs is new and innovative. However, vaginal probiotic formulations are not widely available, and most commercially available oral probiotic formulations are marketed for digestive health—an area where the effects have been studied widely.
In this study, the mean age was 21 years. Given that hypoestrogenization is associated with decreased vaginal colonization with Lactobacillus, an interesting area of future study would be the use of probiotics in postmenopausal women.
Continued investigation of probiotics is warranted, as this approach could help in the treatment of women who have intolerance to antibiotics and is generally considered safe and well-tolerated.
Intravaginal probiotic prophylaxis may reduce the risk of recurrent UTIs. However, further studies are needed to confirm early enthusiasm and delineate ideal populations.
We want to hear from you! Tell us what you think.
1. Foxman B, Barolow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515.
2. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701-2710.
3. ACOG Practice Bulletin #91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785-794.
4. Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259-268.
5. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Disease Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-120.
6. Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8):955-961.
7. Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175-178.
8. Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn. 2011;30(5):754-757.
9. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.-
10. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321.-
10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Megan O. Schimpf, MD (June 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Urinary tract infections (UTIs) are prevalent among women, afflicting as many as 60% of women during their lifetime.1 Symptoms include urgency, frequency, and dysuria. Although the diagnosis can be made on the basis of symptoms alone in many cases, urinalysis and urine cultures often are helpful in confirming it.2 The differential diagnosis includes infectious or atrophic vaginitis, urethritis from a sexually transmitted infection, urethral diverticulum, painful bladder syndrome, urinary tract calculi, and urinary tract neoplasms. Common risk factors for UTIs are listed in TABLE 1.3
TABLE 1
Risk factors for urinary tract infection in women
Premenopausal women •History of urinary tract infection (UTI) Postmenopausal women |
| SOURCE: Adapted from ACOG3 |
Recurrent UTIs are defined as three infections in 12 months or two infections in 6 months. In this Update, we explore strategies to prevent recurrent UTIs in three groups of women:
- sexually active premenopausal women
- postmenopausal women
- women undergoing pelvic surgery.
In the process, we summarize the results of five trials that explore treatment modalities such as prophylactic antibiotics, vaginal estrogen therapy, cranberry supplementation, and probiotics (TABLE 2).
TABLE 2
Summary of therapeutic strategies for prevention of recurrent urinary tract infections
| Strategy | Dose | Advantages | Disadvantages |
|---|---|---|---|
| Prophylactic antibiotics | Trimethoprim-sulfamethoxazole (Bactrim): 1 double-strength tablet* OR Nitrofurantoin: 50 or 100 mg Either drug can be given daily for 6 months or as one dose postcoitally | Highly effective Inexpensive | Potential for future microbial resistance Caution with nitrofurantoin, particularly in older patients or women who have renal insufficiency In pregnancy, nitrofurantoin is better studied |
| Vaginal estrogen** | Conjugated estrogens (0.625 mg conjugated estrogens/1 g cream [Premarin]). Give 0.5–2.0 g cream twice weekly. Estradiol (100 μg estradiol/1 g cream [Estrace]). Give 1–4 g cream | Highly effective in postmenopausal women, who can be difficult to treat Few true contraindications | Can be expensive Compliance may be an issue |
| Cranberry supplement | Dosing varies among products. Unsweetened natural cranberry juice or cranberry tablets, 1–3 times daily. | Generally well-tolerated Few side effects or contraindications | Can be expensive Compliance may be an issue May not be as effective in postmenopausal patients |
| Probiotics | Dosing varies among products and local availability | Few side effects or contraindications | Limited data Can be expensive |
| * Consider trimethoprim (100 mg) alone if the patient has an allergy to sulfa. ** Creams are preferred to the vaginal ring or tablets because they can be applied to periurethral tissues | |||
Postcoital antibiotic prophylaxis prevents some cases of recurrent UTI
Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157(3):935–939.
UTIs typically involve fecal flora that colonize the vagina and perineum, most commonly Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis. These pathogens ascend to the bladder via the urethra. Sexual intercourse is thought to facilitate this process, and recurrent UTIs in premenopausal women are often postcoital in temporal pattern.

When fecal flora ascend via the urethra from the vagina and perineum to the bladder, the bladder mucosa and urethra may become inflamed, leading to urinary tract infection. The most commonly involved pathogens are Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis.Daily antibiotic prophylaxis for 6 to 12 months has proved to be effective in the prevention of recurrent UTIs, reducing the risk of recurrence by 95%, compared with placebo.4
In this trial by Melekos and colleagues, sexually active premenopausal women who had a history of three or more documented UTIs in the preceding 12 months were randomly assigned to:
- oral ciprofloxacin, one dose daily, or
- oral ciprofloxacin, one dose immediately after intercourse.
A total of 135 patients (65 in the daily group and 70 in the postcoital group) were followed for 12 months. The regimens were equally effective at preventing UTIs. The mean number of UTIs in 12 months decreased significantly in both groups—from 3.74 to 0.031 in the daily group and from 3.67 to 0.043 in the postcoital group.
The best antibiotic? Nitrofurantoin or trimethoprim-sulfamethoxazole
This randomized, controlled trial was rigorous and well-executed and included only healthy premenopausal women. However, given the emergence of antibiotic resistance since this trial was conducted, ciprofloxacin is not an ideal antibiotic for prophylaxis.
Both the American Urological Association and the Infectious Disease Society of America recommend that fluoroquinolones be avoided, if possible, in the treatment of uncomplicated UTIs.5 A better therapeutic choice would be nitrofurantoin or trimethoprim-sulfamethoxazole.
Postcoital antibiotic prophylaxis is an effective strategy for the prevention of UTIs associated with sexual intercourse in premenopausal women. Although the optimal duration of such a regimen was not addressed in this study, it would be appropriate to revisit the need for prophylaxis after 1 year.
Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175–178.
Urinary tract catheterization and urogynecologic surgery are associated with an increased risk for UTI. The risk of UTI following a midurethral sling procedure, in particular, ranges from 4.1% to 33.6% in the literature.6,7 To further explore the risk of UTI after placement of a midurethral sling, Dieter and colleagues followed 138 women who had undergone the procedure with and without concomitant pelvic surgery. The primary outcome was treatment of UTI within the first 3 weeks postoperatively.
Catheterization increased the risk of UTI
Fifty-eight percent of women required placement of a catheter postoperatively—either an indwelling Foley or intermittent self-catheterization. The duration of catheterization ranged from 1 to 14 days, with a mean of 4 days. The incidence of UTI was significantly higher in the group that was catheterized postoperatively, compared with the group that was not (30.0% vs 5.2%), and catheterization remained an independent risk factor for UTI after adjusting for other confounding factors.
Data may not be applicable to other types of surgery
This large retrospective cohort study of a well-characterized population was based on consistent postoperative data related to catheterization and UTI treatment. Because the study focused on patients who had undergone placement of a midurethral sling, its findings may not be applicable to women undergoing other types of pelvic surgery, including general gynecologic procedures. However, given the significant difference in the rate of UTI between the two groups, the increased risk of UTI may be at least partially attributable to short-term postoperative catheterization rather than urinary tract instrumentation during the procedure.
The risk of UTI is increased with short-term catheterization following placement of a midurethral sling. There may be a role for antibiotic prophylaxis in the setting of short-term postoperative catheterization; however, a prospective, randomized, placebo-controlled study is needed to determine whether the rate of UTI would be reduced.
Vaginal estrogen prevents recurrent UTIs among postmenopausal women
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–756.
The tissues of the vagina, urethra, bladder, and pelvic floor musculature all express estrogen receptors.8 In postmenopausal women, the effects of decreased estrogen on the urinary tract include a rise in the vaginal pH level and decreased colonization with Lactobacillus. These effects predispose this population to an increased risk for UTI.3 The literature does not support the use of oral estrogen replacement as a therapy for recurrent UTI; however, data suggest that vaginal estrogen replacement may be helpful.9
Raz and Stamm conducted their randomized trial of 93 postmenopausal women with a history of recurrent UTIs to elucidate the effects of vaginal estrogen on the risk of UTI. Fifty women were randomly assigned to treatment with intravaginal estriol cream (0.5 mg nightly for 2 weeks, followed by 0.5 mg twice weekly for 8 months), and 43 women were randomly assigned to placebo (equivalent regimen). Compared with the placebo group, the women treated with estriol experienced a significantly reduced risk of UTI (0.5 vs 5.9 infections per patient-year), increased lactobacilli on vaginal cultures (61% vs 0%), decreased vaginal pH, and a lower rate of colonization with Enterobacteriaceae species.
Although this rigorous double-blind, randomized, placebo-controlled trial was published 20 years ago, its findings remain significant—and have been corroborated in other studies.9
Pros and cons of vaginal estrogen replacement
Raz and Stamm utilized vaginal estriol; the preparations used most commonly today are conjugated estrogens (Premarin) and estradiol (Estrace). Vaginal estrogen formulations can be expensive. Compliance also can wane over time. This study, in particular, showed a discontinuation rate of 28%; mild local reactions were the reason. Although the women who discontinued treatment in this study were included in the final analysis, no subanalysis of these patients was published.
Despite these challenges, local estrogen replacement is generally well-tolerated and, with infrequent dosing (twice weekly), has few contraindications. In fact, local estrogen replacement is one of the most highly effective regimens for UTI prevention among postmenopausal women, who can otherwise be difficult to treat for recurrent UTIs.
Vaginal estrogen is an effective therapy for the prevention of UTIs in postmenopausal women.
Cranberry supplementation may prevent UTIs,
but products vary widely
Stothers L. A randomized trial to evaluate effectiveness and cost-effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9(3):1558–1562.
Cranberries have been used for many years in various formulations to prevent UTI, but no definitive mechanism has been established. In theory, cranberries keep bacteria from adhering to the urothelium.10 In vitro studies have revealed that Escherichia coli is prevented from adhering to uroepithelial cells by two components of cranberry—fructose and proanthocyanidins.10
In this trial of 150 sexually active women (ages 21–72 years) who had experienced at least two UTIs in the past calendar year, Stothers randomly assigned participants to one of three arms for 12 months:
- placebo tablets and cranberry juice (n = 50)
- cranberry tablets and placebo juice (n = 50)
- placebo tablets and placebo juice (n = 50).
Tablets were taken twice daily, and juice was consumed three times daily. All cranberry juice was organic, unsweetened, and unfiltered and taken in 250-mL servings; cranberry tablets were 1:30 parts concentrated cranberry juice.
The risk of UTI during treatment was reduced significantly in the groups taking a cranberry formulation, compared with placebo. Twenty percent of patients consuming cranberry juice experienced a UTI during treatment, compared with 18% of those taking a cranberry tablet and 32% of those in the placebo group (P<.05). In this study, the annual cost of prophylaxis with cranberry juice was $1,400 per woman, and it was $624 per woman for the cranberry tablets. Compliance was lowest among women consuming cranberry juice, decreasing at times to less than 80%.
Findings are difficult to extrapolate
This randomized, double-blind study demonstrated a significant reduction in the rate of UTI with cranberry supplementation, compared with placebo, among women with a mean age of 40 to 44 years. However, because cranberry preparations, juice, and tablets are not regulated as to the amount and bioavailability of the active ingredient, it is difficult to compare one to another and extrapolate to a particular type of preparation.
This study does highlight the higher rate of noncompliance and cost with cranberry juice, although it was as effective at reducing UTIs as cranberry tablets.
Cranberry supplementation reduced the risk of UTIs in sexually active women; placebo did not. Cranberry use may be an alternative to postcoital antibiotic prophylaxis; a randomized comparison of these therapies is needed.
Can nonhormonal therapy alter vaginal flora?
Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217.
Probiotics have been used recently in attempts to prevent recurrent UTI, albeit with very little evidence in the literature. Their effectiveness is plausible due to promotion of healthy vaginal flora.
This study by Stapleton and colleagues enrolled premenopausal women (ages 18–40) with a history of one UTI within the past calendar year and a current, active, uncomplicated UTI. Ninety-nine percent of participants were sexually active. All women were treated with a standard antibiotic regimen for UTI. Seven to 10 days later, participants were randomly assigned to:
- Lactobacillus crispatus vaginal suppository [Lactin-V (Osel)], daily for 5 days and then weekly for 10 weeks (n = 50), or
- placebo (same regimen) (n = 50).
The risk of UTI was 15% among women in the probiotic group, compared with 27% in the placebo group—but this difference was only statistically significant for women who had a higher level of Lactobacillus crispatus vaginal colonization in the treatment group.
Vaginal probiotic formulations may be hard to obtain
The use of probiotics to prevent recurrent UTIs is new and innovative. However, vaginal probiotic formulations are not widely available, and most commercially available oral probiotic formulations are marketed for digestive health—an area where the effects have been studied widely.
In this study, the mean age was 21 years. Given that hypoestrogenization is associated with decreased vaginal colonization with Lactobacillus, an interesting area of future study would be the use of probiotics in postmenopausal women.
Continued investigation of probiotics is warranted, as this approach could help in the treatment of women who have intolerance to antibiotics and is generally considered safe and well-tolerated.
Intravaginal probiotic prophylaxis may reduce the risk of recurrent UTIs. However, further studies are needed to confirm early enthusiasm and delineate ideal populations.
We want to hear from you! Tell us what you think.
10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Megan O. Schimpf, MD (June 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Urinary tract infections (UTIs) are prevalent among women, afflicting as many as 60% of women during their lifetime.1 Symptoms include urgency, frequency, and dysuria. Although the diagnosis can be made on the basis of symptoms alone in many cases, urinalysis and urine cultures often are helpful in confirming it.2 The differential diagnosis includes infectious or atrophic vaginitis, urethritis from a sexually transmitted infection, urethral diverticulum, painful bladder syndrome, urinary tract calculi, and urinary tract neoplasms. Common risk factors for UTIs are listed in TABLE 1.3
TABLE 1
Risk factors for urinary tract infection in women
Premenopausal women •History of urinary tract infection (UTI) Postmenopausal women |
| SOURCE: Adapted from ACOG3 |
Recurrent UTIs are defined as three infections in 12 months or two infections in 6 months. In this Update, we explore strategies to prevent recurrent UTIs in three groups of women:
- sexually active premenopausal women
- postmenopausal women
- women undergoing pelvic surgery.
In the process, we summarize the results of five trials that explore treatment modalities such as prophylactic antibiotics, vaginal estrogen therapy, cranberry supplementation, and probiotics (TABLE 2).
TABLE 2
Summary of therapeutic strategies for prevention of recurrent urinary tract infections
| Strategy | Dose | Advantages | Disadvantages |
|---|---|---|---|
| Prophylactic antibiotics | Trimethoprim-sulfamethoxazole (Bactrim): 1 double-strength tablet* OR Nitrofurantoin: 50 or 100 mg Either drug can be given daily for 6 months or as one dose postcoitally | Highly effective Inexpensive | Potential for future microbial resistance Caution with nitrofurantoin, particularly in older patients or women who have renal insufficiency In pregnancy, nitrofurantoin is better studied |
| Vaginal estrogen** | Conjugated estrogens (0.625 mg conjugated estrogens/1 g cream [Premarin]). Give 0.5–2.0 g cream twice weekly. Estradiol (100 μg estradiol/1 g cream [Estrace]). Give 1–4 g cream | Highly effective in postmenopausal women, who can be difficult to treat Few true contraindications | Can be expensive Compliance may be an issue |
| Cranberry supplement | Dosing varies among products. Unsweetened natural cranberry juice or cranberry tablets, 1–3 times daily. | Generally well-tolerated Few side effects or contraindications | Can be expensive Compliance may be an issue May not be as effective in postmenopausal patients |
| Probiotics | Dosing varies among products and local availability | Few side effects or contraindications | Limited data Can be expensive |
| * Consider trimethoprim (100 mg) alone if the patient has an allergy to sulfa. ** Creams are preferred to the vaginal ring or tablets because they can be applied to periurethral tissues | |||
Postcoital antibiotic prophylaxis prevents some cases of recurrent UTI
Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157(3):935–939.
UTIs typically involve fecal flora that colonize the vagina and perineum, most commonly Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis. These pathogens ascend to the bladder via the urethra. Sexual intercourse is thought to facilitate this process, and recurrent UTIs in premenopausal women are often postcoital in temporal pattern.

When fecal flora ascend via the urethra from the vagina and perineum to the bladder, the bladder mucosa and urethra may become inflamed, leading to urinary tract infection. The most commonly involved pathogens are Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumonia, and Proteus mirabilis.Daily antibiotic prophylaxis for 6 to 12 months has proved to be effective in the prevention of recurrent UTIs, reducing the risk of recurrence by 95%, compared with placebo.4
In this trial by Melekos and colleagues, sexually active premenopausal women who had a history of three or more documented UTIs in the preceding 12 months were randomly assigned to:
- oral ciprofloxacin, one dose daily, or
- oral ciprofloxacin, one dose immediately after intercourse.
A total of 135 patients (65 in the daily group and 70 in the postcoital group) were followed for 12 months. The regimens were equally effective at preventing UTIs. The mean number of UTIs in 12 months decreased significantly in both groups—from 3.74 to 0.031 in the daily group and from 3.67 to 0.043 in the postcoital group.
The best antibiotic? Nitrofurantoin or trimethoprim-sulfamethoxazole
This randomized, controlled trial was rigorous and well-executed and included only healthy premenopausal women. However, given the emergence of antibiotic resistance since this trial was conducted, ciprofloxacin is not an ideal antibiotic for prophylaxis.
Both the American Urological Association and the Infectious Disease Society of America recommend that fluoroquinolones be avoided, if possible, in the treatment of uncomplicated UTIs.5 A better therapeutic choice would be nitrofurantoin or trimethoprim-sulfamethoxazole.
Postcoital antibiotic prophylaxis is an effective strategy for the prevention of UTIs associated with sexual intercourse in premenopausal women. Although the optimal duration of such a regimen was not addressed in this study, it would be appropriate to revisit the need for prophylaxis after 1 year.
Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175–178.
Urinary tract catheterization and urogynecologic surgery are associated with an increased risk for UTI. The risk of UTI following a midurethral sling procedure, in particular, ranges from 4.1% to 33.6% in the literature.6,7 To further explore the risk of UTI after placement of a midurethral sling, Dieter and colleagues followed 138 women who had undergone the procedure with and without concomitant pelvic surgery. The primary outcome was treatment of UTI within the first 3 weeks postoperatively.
Catheterization increased the risk of UTI
Fifty-eight percent of women required placement of a catheter postoperatively—either an indwelling Foley or intermittent self-catheterization. The duration of catheterization ranged from 1 to 14 days, with a mean of 4 days. The incidence of UTI was significantly higher in the group that was catheterized postoperatively, compared with the group that was not (30.0% vs 5.2%), and catheterization remained an independent risk factor for UTI after adjusting for other confounding factors.
Data may not be applicable to other types of surgery
This large retrospective cohort study of a well-characterized population was based on consistent postoperative data related to catheterization and UTI treatment. Because the study focused on patients who had undergone placement of a midurethral sling, its findings may not be applicable to women undergoing other types of pelvic surgery, including general gynecologic procedures. However, given the significant difference in the rate of UTI between the two groups, the increased risk of UTI may be at least partially attributable to short-term postoperative catheterization rather than urinary tract instrumentation during the procedure.
The risk of UTI is increased with short-term catheterization following placement of a midurethral sling. There may be a role for antibiotic prophylaxis in the setting of short-term postoperative catheterization; however, a prospective, randomized, placebo-controlled study is needed to determine whether the rate of UTI would be reduced.
Vaginal estrogen prevents recurrent UTIs among postmenopausal women
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–756.
The tissues of the vagina, urethra, bladder, and pelvic floor musculature all express estrogen receptors.8 In postmenopausal women, the effects of decreased estrogen on the urinary tract include a rise in the vaginal pH level and decreased colonization with Lactobacillus. These effects predispose this population to an increased risk for UTI.3 The literature does not support the use of oral estrogen replacement as a therapy for recurrent UTI; however, data suggest that vaginal estrogen replacement may be helpful.9
Raz and Stamm conducted their randomized trial of 93 postmenopausal women with a history of recurrent UTIs to elucidate the effects of vaginal estrogen on the risk of UTI. Fifty women were randomly assigned to treatment with intravaginal estriol cream (0.5 mg nightly for 2 weeks, followed by 0.5 mg twice weekly for 8 months), and 43 women were randomly assigned to placebo (equivalent regimen). Compared with the placebo group, the women treated with estriol experienced a significantly reduced risk of UTI (0.5 vs 5.9 infections per patient-year), increased lactobacilli on vaginal cultures (61% vs 0%), decreased vaginal pH, and a lower rate of colonization with Enterobacteriaceae species.
Although this rigorous double-blind, randomized, placebo-controlled trial was published 20 years ago, its findings remain significant—and have been corroborated in other studies.9
Pros and cons of vaginal estrogen replacement
Raz and Stamm utilized vaginal estriol; the preparations used most commonly today are conjugated estrogens (Premarin) and estradiol (Estrace). Vaginal estrogen formulations can be expensive. Compliance also can wane over time. This study, in particular, showed a discontinuation rate of 28%; mild local reactions were the reason. Although the women who discontinued treatment in this study were included in the final analysis, no subanalysis of these patients was published.
Despite these challenges, local estrogen replacement is generally well-tolerated and, with infrequent dosing (twice weekly), has few contraindications. In fact, local estrogen replacement is one of the most highly effective regimens for UTI prevention among postmenopausal women, who can otherwise be difficult to treat for recurrent UTIs.
Vaginal estrogen is an effective therapy for the prevention of UTIs in postmenopausal women.
Cranberry supplementation may prevent UTIs,
but products vary widely
Stothers L. A randomized trial to evaluate effectiveness and cost-effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9(3):1558–1562.
Cranberries have been used for many years in various formulations to prevent UTI, but no definitive mechanism has been established. In theory, cranberries keep bacteria from adhering to the urothelium.10 In vitro studies have revealed that Escherichia coli is prevented from adhering to uroepithelial cells by two components of cranberry—fructose and proanthocyanidins.10
In this trial of 150 sexually active women (ages 21–72 years) who had experienced at least two UTIs in the past calendar year, Stothers randomly assigned participants to one of three arms for 12 months:
- placebo tablets and cranberry juice (n = 50)
- cranberry tablets and placebo juice (n = 50)
- placebo tablets and placebo juice (n = 50).
Tablets were taken twice daily, and juice was consumed three times daily. All cranberry juice was organic, unsweetened, and unfiltered and taken in 250-mL servings; cranberry tablets were 1:30 parts concentrated cranberry juice.
The risk of UTI during treatment was reduced significantly in the groups taking a cranberry formulation, compared with placebo. Twenty percent of patients consuming cranberry juice experienced a UTI during treatment, compared with 18% of those taking a cranberry tablet and 32% of those in the placebo group (P<.05). In this study, the annual cost of prophylaxis with cranberry juice was $1,400 per woman, and it was $624 per woman for the cranberry tablets. Compliance was lowest among women consuming cranberry juice, decreasing at times to less than 80%.
Findings are difficult to extrapolate
This randomized, double-blind study demonstrated a significant reduction in the rate of UTI with cranberry supplementation, compared with placebo, among women with a mean age of 40 to 44 years. However, because cranberry preparations, juice, and tablets are not regulated as to the amount and bioavailability of the active ingredient, it is difficult to compare one to another and extrapolate to a particular type of preparation.
This study does highlight the higher rate of noncompliance and cost with cranberry juice, although it was as effective at reducing UTIs as cranberry tablets.
Cranberry supplementation reduced the risk of UTIs in sexually active women; placebo did not. Cranberry use may be an alternative to postcoital antibiotic prophylaxis; a randomized comparison of these therapies is needed.
Can nonhormonal therapy alter vaginal flora?
Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217.
Probiotics have been used recently in attempts to prevent recurrent UTI, albeit with very little evidence in the literature. Their effectiveness is plausible due to promotion of healthy vaginal flora.
This study by Stapleton and colleagues enrolled premenopausal women (ages 18–40) with a history of one UTI within the past calendar year and a current, active, uncomplicated UTI. Ninety-nine percent of participants were sexually active. All women were treated with a standard antibiotic regimen for UTI. Seven to 10 days later, participants were randomly assigned to:
- Lactobacillus crispatus vaginal suppository [Lactin-V (Osel)], daily for 5 days and then weekly for 10 weeks (n = 50), or
- placebo (same regimen) (n = 50).
The risk of UTI was 15% among women in the probiotic group, compared with 27% in the placebo group—but this difference was only statistically significant for women who had a higher level of Lactobacillus crispatus vaginal colonization in the treatment group.
Vaginal probiotic formulations may be hard to obtain
The use of probiotics to prevent recurrent UTIs is new and innovative. However, vaginal probiotic formulations are not widely available, and most commercially available oral probiotic formulations are marketed for digestive health—an area where the effects have been studied widely.
In this study, the mean age was 21 years. Given that hypoestrogenization is associated with decreased vaginal colonization with Lactobacillus, an interesting area of future study would be the use of probiotics in postmenopausal women.
Continued investigation of probiotics is warranted, as this approach could help in the treatment of women who have intolerance to antibiotics and is generally considered safe and well-tolerated.
Intravaginal probiotic prophylaxis may reduce the risk of recurrent UTIs. However, further studies are needed to confirm early enthusiasm and delineate ideal populations.
We want to hear from you! Tell us what you think.
1. Foxman B, Barolow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515.
2. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701-2710.
3. ACOG Practice Bulletin #91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785-794.
4. Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259-268.
5. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Disease Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-120.
6. Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8):955-961.
7. Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175-178.
8. Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn. 2011;30(5):754-757.
9. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.-
10. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321.-
1. Foxman B, Barolow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515.
2. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701-2710.
3. ACOG Practice Bulletin #91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785-794.
4. Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259-268.
5. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Disease Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-120.
6. Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8):955-961.
7. Dieter AA, Amundsen CL, Visco AG, Siddiqui NY. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18(3):175-178.
8. Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn. 2011;30(5):754-757.
9. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;(2):CD005131.-
10. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321.-
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Stop performing median episiotomy!
Robert L. Barbieri, MD (Editorial, April 2012)
Episiotomy is an incision into the perineal body that is made during the second stage of labor to expedite delivery. Despite guidelines recommending restrictions on its use, episiotomy is still performed in more than 25% of vaginal deliveries in the United States. Suggested benefits include a shortened second stage of labor, the substitution of a straight surgical incision for a ragged spontaneous tear, and a reduced incidence of severe perineal injury and resultant pelvic floor dysfunction. Few data support these assertions, however.
Episiotomy is no OASIS
Absolute indications for episiotomy have yet to be established. Although there is general agreement that episiotomy may be indicated in select circumstances (such as to expedite delivery in the setting of nonreassuring fetal testing in the second stage of labor, shoulder dystocia, or at the time of operative vaginal delivery), routine use is discouraged.1,2 Besides the lack of data showing its benefit, episiotomy is associated with several potential complications, including increased blood loss, fetal injury, and localized pain. In contrast to the stated goal of reducing perineal trauma, episiotomy is associated with an increased incidence of third- or fourth-degree perineal lacerations,3,4 referred to in this study as obstetric anal sphincter injuries (OASIS).
Third- or fourth-degree tears are identified clinically at the time of vaginal delivery in 0.6% to 9% of patients.4 Studies using two-dimensional endoanal ultrasonography suggest that the true incidence of rectal injury is probably closer to 11%.5 Such injuries are associated with an increased risk of subsequent urinary or fecal incontinence (or both) and pelvic organ prolapse.
If episiotomy is indicated, how should it be performed?
There are two main types of episiotomy: median (favored in the United States) and mediolateral episiotomy. Complications—especially OASIS—are more common with median episiotomy,3,6,7 which involves a vertical midline incision from the posterior fourchette toward the rectum. Mediolateral episiotomy (favored in Europe), refers to an incision performed at a 45° angle from the posterior fourchette. OASIS is more common after median episiotomy, compared with the mediolateral approach.4,6 What is not yet clear is whether mediolateral episiotomy actually protects against OASIS.
Details of the study
de Vogel and colleagues evaluated the frequency of OASIS in women at high risk—specifically, those undergoing operative vaginal delivery; they also sought to determine whether mediolateral episiotomy is protective against OASIS. To this end, they performed a retrospective analysis of 2,861 consecutive women who delivered a singleton liveborn infant at term by vacuum, forceps, or both, from 2001–2009. Women were identified through the Netherlands Perinatal Registry, a voluntary reporting national database that includes approximately 96% of the 190,000 births that occur after 16 weeks’ gestation each year in the Netherlands. Exclusion criteria included multiple gestation, breech presentation, and use of median episiotomy.
The overall frequency of OASIS was 5.7% (162 cases among 2,861 deliveries). After logistic-regression modeling, a number of variables were significantly associated with OASIS, all of which have been identified previously: forceps delivery, occiput posterior position, primiparity, and epidural anesthesia. Women with a mediolateral episiotomy were at a significantly lower risk for OASIS, compared with women without mediolateral episiotomy (3.5% vs 15.6%, respectively; P<.001). Further analysis suggested that 8.6 mediolateral episiotomies would be needed to prevent one OASIS during vacuum extraction, whereas 5.2 procedures would be necessary to prevent one OASIS during forceps delivery. de Vogel and colleagues concluded that mediolateral episiotomy should be performed during all operative vaginal deliveries to reduce the incidence of OASIS.
Although this study included a large sample from a well-established and validated dataset (collected prospectively), it was, by design, retrospective. There was no standardization of when or how to cut the mediolateral episiotomy. However, many of these uncontrolled variables (such as cutting an episiotomy that is more median than mediolateral or cutting an episiotomy only in women who appear to be at imminent risk of sustaining a perineal laceration) would increase—not decrease—the risk of severe perineal injury. This fact suggests that the protective effect of mediolateral episiotomy may be even more dramatic than the sixfold protection reported in this study.
This study focused on women who underwent operative vaginal delivery. It remains controversial whether mediolateral episiotomy is protective in women who have a spontaneous (noninstrumental) vaginal delivery.
The study also lacks follow-up data on how many women with OASIS went on to develop fecal or urinary incontinence or pelvic organ prolapse. However, a third- or fourth-degree perineal laceration is serious enough that it can serve as an acceptable primary outcome measure even in the absence of long-term functional data.
In this study, use of median episiotomy was an exclusion, mostly likely because it is rarely performed in Europe.
While the battle over “to cut or not to cut” continues to rage, one fact is clear: median episiotomy should be abandoned. If you are going to perform episiotomy, make it mediolateral. According to this report, accoucheurs should consider cutting a mediolateral episiotomy for perineal protection each time they perform operative vaginal delivery.
ERROL R. NORWITZ, MD, PHD
After reading this article, and Dr. Barbieri’s April Editorial, we want to know if these articles have changed your practice. If you were faced with a difficult vaginal delivery, would you use a median or mediolateral episiotomy incision? Why?
We want to hear from you! Tell us what you think.
1. Hartmann K, Viswanathan M, Palmieri R, Gartlehner G, Thorp J, Lohr KN. Outcomes of routine episiotomy: a systematic review. JAMA. 2005;293(17):2141-2148.
2. Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;(1):CD000081.-
3. Helwig JT, Thorp JM, Jr, Bowes WA, Jr. Does midline episiotomy increase the risk of third- and fourth-degree lacerations in operative vaginal deliveries? Obstet Gynecol. 1993;82(2):276-279.
4. Dudding TC, Vaizey C, Kamm MA. Obstetric anal sphincter injury: incidence, risk factors, and management. Ann Surg. 2008;247(2):224-237.
5. Williams AB, Bartram CI, Halligan S, Spencer JA, Nicholls RJ, Kmiot WA. Anal sphincter damage after vaginal delivery using three-dimensional endosonography. Obstet Gynecol. 2001;97(5 Pt 1):770-775.
6. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87(5):408-412.
7. Kudish B, Blackwell S, McNeeley SG, et al. Operative vaginal delivery and midline episiotomy: a bad combination for the perineum. Am J Obstet Gynecol. 2006;195(3):749-754.
Stop performing median episiotomy!
Robert L. Barbieri, MD (Editorial, April 2012)
Episiotomy is an incision into the perineal body that is made during the second stage of labor to expedite delivery. Despite guidelines recommending restrictions on its use, episiotomy is still performed in more than 25% of vaginal deliveries in the United States. Suggested benefits include a shortened second stage of labor, the substitution of a straight surgical incision for a ragged spontaneous tear, and a reduced incidence of severe perineal injury and resultant pelvic floor dysfunction. Few data support these assertions, however.
Episiotomy is no OASIS
Absolute indications for episiotomy have yet to be established. Although there is general agreement that episiotomy may be indicated in select circumstances (such as to expedite delivery in the setting of nonreassuring fetal testing in the second stage of labor, shoulder dystocia, or at the time of operative vaginal delivery), routine use is discouraged.1,2 Besides the lack of data showing its benefit, episiotomy is associated with several potential complications, including increased blood loss, fetal injury, and localized pain. In contrast to the stated goal of reducing perineal trauma, episiotomy is associated with an increased incidence of third- or fourth-degree perineal lacerations,3,4 referred to in this study as obstetric anal sphincter injuries (OASIS).
Third- or fourth-degree tears are identified clinically at the time of vaginal delivery in 0.6% to 9% of patients.4 Studies using two-dimensional endoanal ultrasonography suggest that the true incidence of rectal injury is probably closer to 11%.5 Such injuries are associated with an increased risk of subsequent urinary or fecal incontinence (or both) and pelvic organ prolapse.
If episiotomy is indicated, how should it be performed?
There are two main types of episiotomy: median (favored in the United States) and mediolateral episiotomy. Complications—especially OASIS—are more common with median episiotomy,3,6,7 which involves a vertical midline incision from the posterior fourchette toward the rectum. Mediolateral episiotomy (favored in Europe), refers to an incision performed at a 45° angle from the posterior fourchette. OASIS is more common after median episiotomy, compared with the mediolateral approach.4,6 What is not yet clear is whether mediolateral episiotomy actually protects against OASIS.
Details of the study
de Vogel and colleagues evaluated the frequency of OASIS in women at high risk—specifically, those undergoing operative vaginal delivery; they also sought to determine whether mediolateral episiotomy is protective against OASIS. To this end, they performed a retrospective analysis of 2,861 consecutive women who delivered a singleton liveborn infant at term by vacuum, forceps, or both, from 2001–2009. Women were identified through the Netherlands Perinatal Registry, a voluntary reporting national database that includes approximately 96% of the 190,000 births that occur after 16 weeks’ gestation each year in the Netherlands. Exclusion criteria included multiple gestation, breech presentation, and use of median episiotomy.
The overall frequency of OASIS was 5.7% (162 cases among 2,861 deliveries). After logistic-regression modeling, a number of variables were significantly associated with OASIS, all of which have been identified previously: forceps delivery, occiput posterior position, primiparity, and epidural anesthesia. Women with a mediolateral episiotomy were at a significantly lower risk for OASIS, compared with women without mediolateral episiotomy (3.5% vs 15.6%, respectively; P<.001). Further analysis suggested that 8.6 mediolateral episiotomies would be needed to prevent one OASIS during vacuum extraction, whereas 5.2 procedures would be necessary to prevent one OASIS during forceps delivery. de Vogel and colleagues concluded that mediolateral episiotomy should be performed during all operative vaginal deliveries to reduce the incidence of OASIS.
Although this study included a large sample from a well-established and validated dataset (collected prospectively), it was, by design, retrospective. There was no standardization of when or how to cut the mediolateral episiotomy. However, many of these uncontrolled variables (such as cutting an episiotomy that is more median than mediolateral or cutting an episiotomy only in women who appear to be at imminent risk of sustaining a perineal laceration) would increase—not decrease—the risk of severe perineal injury. This fact suggests that the protective effect of mediolateral episiotomy may be even more dramatic than the sixfold protection reported in this study.
This study focused on women who underwent operative vaginal delivery. It remains controversial whether mediolateral episiotomy is protective in women who have a spontaneous (noninstrumental) vaginal delivery.
The study also lacks follow-up data on how many women with OASIS went on to develop fecal or urinary incontinence or pelvic organ prolapse. However, a third- or fourth-degree perineal laceration is serious enough that it can serve as an acceptable primary outcome measure even in the absence of long-term functional data.
In this study, use of median episiotomy was an exclusion, mostly likely because it is rarely performed in Europe.
While the battle over “to cut or not to cut” continues to rage, one fact is clear: median episiotomy should be abandoned. If you are going to perform episiotomy, make it mediolateral. According to this report, accoucheurs should consider cutting a mediolateral episiotomy for perineal protection each time they perform operative vaginal delivery.
ERROL R. NORWITZ, MD, PHD
After reading this article, and Dr. Barbieri’s April Editorial, we want to know if these articles have changed your practice. If you were faced with a difficult vaginal delivery, would you use a median or mediolateral episiotomy incision? Why?
We want to hear from you! Tell us what you think.
Stop performing median episiotomy!
Robert L. Barbieri, MD (Editorial, April 2012)
Episiotomy is an incision into the perineal body that is made during the second stage of labor to expedite delivery. Despite guidelines recommending restrictions on its use, episiotomy is still performed in more than 25% of vaginal deliveries in the United States. Suggested benefits include a shortened second stage of labor, the substitution of a straight surgical incision for a ragged spontaneous tear, and a reduced incidence of severe perineal injury and resultant pelvic floor dysfunction. Few data support these assertions, however.
Episiotomy is no OASIS
Absolute indications for episiotomy have yet to be established. Although there is general agreement that episiotomy may be indicated in select circumstances (such as to expedite delivery in the setting of nonreassuring fetal testing in the second stage of labor, shoulder dystocia, or at the time of operative vaginal delivery), routine use is discouraged.1,2 Besides the lack of data showing its benefit, episiotomy is associated with several potential complications, including increased blood loss, fetal injury, and localized pain. In contrast to the stated goal of reducing perineal trauma, episiotomy is associated with an increased incidence of third- or fourth-degree perineal lacerations,3,4 referred to in this study as obstetric anal sphincter injuries (OASIS).
Third- or fourth-degree tears are identified clinically at the time of vaginal delivery in 0.6% to 9% of patients.4 Studies using two-dimensional endoanal ultrasonography suggest that the true incidence of rectal injury is probably closer to 11%.5 Such injuries are associated with an increased risk of subsequent urinary or fecal incontinence (or both) and pelvic organ prolapse.
If episiotomy is indicated, how should it be performed?
There are two main types of episiotomy: median (favored in the United States) and mediolateral episiotomy. Complications—especially OASIS—are more common with median episiotomy,3,6,7 which involves a vertical midline incision from the posterior fourchette toward the rectum. Mediolateral episiotomy (favored in Europe), refers to an incision performed at a 45° angle from the posterior fourchette. OASIS is more common after median episiotomy, compared with the mediolateral approach.4,6 What is not yet clear is whether mediolateral episiotomy actually protects against OASIS.
Details of the study
de Vogel and colleagues evaluated the frequency of OASIS in women at high risk—specifically, those undergoing operative vaginal delivery; they also sought to determine whether mediolateral episiotomy is protective against OASIS. To this end, they performed a retrospective analysis of 2,861 consecutive women who delivered a singleton liveborn infant at term by vacuum, forceps, or both, from 2001–2009. Women were identified through the Netherlands Perinatal Registry, a voluntary reporting national database that includes approximately 96% of the 190,000 births that occur after 16 weeks’ gestation each year in the Netherlands. Exclusion criteria included multiple gestation, breech presentation, and use of median episiotomy.
The overall frequency of OASIS was 5.7% (162 cases among 2,861 deliveries). After logistic-regression modeling, a number of variables were significantly associated with OASIS, all of which have been identified previously: forceps delivery, occiput posterior position, primiparity, and epidural anesthesia. Women with a mediolateral episiotomy were at a significantly lower risk for OASIS, compared with women without mediolateral episiotomy (3.5% vs 15.6%, respectively; P<.001). Further analysis suggested that 8.6 mediolateral episiotomies would be needed to prevent one OASIS during vacuum extraction, whereas 5.2 procedures would be necessary to prevent one OASIS during forceps delivery. de Vogel and colleagues concluded that mediolateral episiotomy should be performed during all operative vaginal deliveries to reduce the incidence of OASIS.
Although this study included a large sample from a well-established and validated dataset (collected prospectively), it was, by design, retrospective. There was no standardization of when or how to cut the mediolateral episiotomy. However, many of these uncontrolled variables (such as cutting an episiotomy that is more median than mediolateral or cutting an episiotomy only in women who appear to be at imminent risk of sustaining a perineal laceration) would increase—not decrease—the risk of severe perineal injury. This fact suggests that the protective effect of mediolateral episiotomy may be even more dramatic than the sixfold protection reported in this study.
This study focused on women who underwent operative vaginal delivery. It remains controversial whether mediolateral episiotomy is protective in women who have a spontaneous (noninstrumental) vaginal delivery.
The study also lacks follow-up data on how many women with OASIS went on to develop fecal or urinary incontinence or pelvic organ prolapse. However, a third- or fourth-degree perineal laceration is serious enough that it can serve as an acceptable primary outcome measure even in the absence of long-term functional data.
In this study, use of median episiotomy was an exclusion, mostly likely because it is rarely performed in Europe.
While the battle over “to cut or not to cut” continues to rage, one fact is clear: median episiotomy should be abandoned. If you are going to perform episiotomy, make it mediolateral. According to this report, accoucheurs should consider cutting a mediolateral episiotomy for perineal protection each time they perform operative vaginal delivery.
ERROL R. NORWITZ, MD, PHD
After reading this article, and Dr. Barbieri’s April Editorial, we want to know if these articles have changed your practice. If you were faced with a difficult vaginal delivery, would you use a median or mediolateral episiotomy incision? Why?
We want to hear from you! Tell us what you think.
1. Hartmann K, Viswanathan M, Palmieri R, Gartlehner G, Thorp J, Lohr KN. Outcomes of routine episiotomy: a systematic review. JAMA. 2005;293(17):2141-2148.
2. Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;(1):CD000081.-
3. Helwig JT, Thorp JM, Jr, Bowes WA, Jr. Does midline episiotomy increase the risk of third- and fourth-degree lacerations in operative vaginal deliveries? Obstet Gynecol. 1993;82(2):276-279.
4. Dudding TC, Vaizey C, Kamm MA. Obstetric anal sphincter injury: incidence, risk factors, and management. Ann Surg. 2008;247(2):224-237.
5. Williams AB, Bartram CI, Halligan S, Spencer JA, Nicholls RJ, Kmiot WA. Anal sphincter damage after vaginal delivery using three-dimensional endosonography. Obstet Gynecol. 2001;97(5 Pt 1):770-775.
6. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87(5):408-412.
7. Kudish B, Blackwell S, McNeeley SG, et al. Operative vaginal delivery and midline episiotomy: a bad combination for the perineum. Am J Obstet Gynecol. 2006;195(3):749-754.
1. Hartmann K, Viswanathan M, Palmieri R, Gartlehner G, Thorp J, Lohr KN. Outcomes of routine episiotomy: a systematic review. JAMA. 2005;293(17):2141-2148.
2. Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;(1):CD000081.-
3. Helwig JT, Thorp JM, Jr, Bowes WA, Jr. Does midline episiotomy increase the risk of third- and fourth-degree lacerations in operative vaginal deliveries? Obstet Gynecol. 1993;82(2):276-279.
4. Dudding TC, Vaizey C, Kamm MA. Obstetric anal sphincter injury: incidence, risk factors, and management. Ann Surg. 2008;247(2):224-237.
5. Williams AB, Bartram CI, Halligan S, Spencer JA, Nicholls RJ, Kmiot WA. Anal sphincter damage after vaginal delivery using three-dimensional endosonography. Obstet Gynecol. 2001;97(5 Pt 1):770-775.
6. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87(5):408-412.
7. Kudish B, Blackwell S, McNeeley SG, et al. Operative vaginal delivery and midline episiotomy: a bad combination for the perineum. Am J Obstet Gynecol. 2006;195(3):749-754.
In women who have stress incontinence and intrinsic sphincter deficiency, which midurethral sling produces the best long-term results?
- Which sling for which SUI patient?
Mark D. Walters, MD, and Anne M. Weber, MD (Surgical Techniques, May 2012)
![]()
These videos were selected by Dr. Walters and presented courtesy of the International Academy of Pelvic Surgery (IAPS)
When ISD is present, the urethra cannot coaptate and loses its ability to maintain a watertight seal. Women who have this condition often are severely incontinent, leaking urine at low volumes and pressures and with minimal exertion.
In this randomized trial, Schierlitz and colleagues hypothesized that TOT would produce higher objective and subjective failure rates than the TVT. This was confirmed by 6-month data published in 2008.
Details of the trial
Women who had SUI were included in the trial if they had ISD based on urodynamic findings (i.e., maximum urethral closure pressure ≤20 cm H2O or Valsalva leak-point pressure ≤60 cm H2O, or both) and were randomly assigned to TVT or TOT. The primary endpoint was symptomatic SUI (confirmed by repeat urodynamic testing) that required a second procedure upon patient request.
Participants were followed for 3 years. If a patient reported symptoms, urodynamic testing was repeated. In addition, the patient was offered another surgery, usually involving placement of a TVT sling.
Schierlitz and colleagues concluded that, if TVT were used in all patients, repeat surgery would be avoided in one in every six patients. The risk of repeat surgery was 15 times greater for TOT, compared with the TVT sling. The median time to failure was 15.6 months for the TOT sling, compared with 43.7 months for the TVT.
Of the 16 patients who underwent repeat surgery, 56% were cured, 25% reported minimal leakage, and 19% remained unchanged.
Quality-of-life scores were similar between groups at the 6-month follow-up.
Why did the TVT outperform the TOT in this population?
Investigators theorized that there is a difference in sling axis, with the TVT placed at a more acute angle than the TOT sling. In addition, the location of the TOT sling is more distal than that of the TVT, based on ultrasonographic imaging. As a result, more effective urethral kinking and support are likely with the TVT sling, improving continence rates.
Strengths and limitations of the trial
The randomization of participants and long-term follow-up bolster the trial’s credibility.
Weaknesses include unblinded participation and postoperative surgical assessment.
Although the sample size was underpowered, there was a significant difference in the primary outcome between the two groups.
Long-term success is more likely with placement of a TVT sling in women who have SUI with ISD.
Urodynamic assessment still serves an important role in the diagnosis of ISD, and aids in preoperative planning.
LADIN A. YURTERI-KAPLAN, MD, AND AMY J. PARK, MD
We want to hear from you! Tell us what you think.
- Which sling for which SUI patient?
Mark D. Walters, MD, and Anne M. Weber, MD (Surgical Techniques, May 2012)
![]()
These videos were selected by Dr. Walters and presented courtesy of the International Academy of Pelvic Surgery (IAPS)
When ISD is present, the urethra cannot coaptate and loses its ability to maintain a watertight seal. Women who have this condition often are severely incontinent, leaking urine at low volumes and pressures and with minimal exertion.
In this randomized trial, Schierlitz and colleagues hypothesized that TOT would produce higher objective and subjective failure rates than the TVT. This was confirmed by 6-month data published in 2008.
Details of the trial
Women who had SUI were included in the trial if they had ISD based on urodynamic findings (i.e., maximum urethral closure pressure ≤20 cm H2O or Valsalva leak-point pressure ≤60 cm H2O, or both) and were randomly assigned to TVT or TOT. The primary endpoint was symptomatic SUI (confirmed by repeat urodynamic testing) that required a second procedure upon patient request.
Participants were followed for 3 years. If a patient reported symptoms, urodynamic testing was repeated. In addition, the patient was offered another surgery, usually involving placement of a TVT sling.
Schierlitz and colleagues concluded that, if TVT were used in all patients, repeat surgery would be avoided in one in every six patients. The risk of repeat surgery was 15 times greater for TOT, compared with the TVT sling. The median time to failure was 15.6 months for the TOT sling, compared with 43.7 months for the TVT.
Of the 16 patients who underwent repeat surgery, 56% were cured, 25% reported minimal leakage, and 19% remained unchanged.
Quality-of-life scores were similar between groups at the 6-month follow-up.
Why did the TVT outperform the TOT in this population?
Investigators theorized that there is a difference in sling axis, with the TVT placed at a more acute angle than the TOT sling. In addition, the location of the TOT sling is more distal than that of the TVT, based on ultrasonographic imaging. As a result, more effective urethral kinking and support are likely with the TVT sling, improving continence rates.
Strengths and limitations of the trial
The randomization of participants and long-term follow-up bolster the trial’s credibility.
Weaknesses include unblinded participation and postoperative surgical assessment.
Although the sample size was underpowered, there was a significant difference in the primary outcome between the two groups.
Long-term success is more likely with placement of a TVT sling in women who have SUI with ISD.
Urodynamic assessment still serves an important role in the diagnosis of ISD, and aids in preoperative planning.
LADIN A. YURTERI-KAPLAN, MD, AND AMY J. PARK, MD
We want to hear from you! Tell us what you think.
- Which sling for which SUI patient?
Mark D. Walters, MD, and Anne M. Weber, MD (Surgical Techniques, May 2012)
![]()
These videos were selected by Dr. Walters and presented courtesy of the International Academy of Pelvic Surgery (IAPS)
When ISD is present, the urethra cannot coaptate and loses its ability to maintain a watertight seal. Women who have this condition often are severely incontinent, leaking urine at low volumes and pressures and with minimal exertion.
In this randomized trial, Schierlitz and colleagues hypothesized that TOT would produce higher objective and subjective failure rates than the TVT. This was confirmed by 6-month data published in 2008.
Details of the trial
Women who had SUI were included in the trial if they had ISD based on urodynamic findings (i.e., maximum urethral closure pressure ≤20 cm H2O or Valsalva leak-point pressure ≤60 cm H2O, or both) and were randomly assigned to TVT or TOT. The primary endpoint was symptomatic SUI (confirmed by repeat urodynamic testing) that required a second procedure upon patient request.
Participants were followed for 3 years. If a patient reported symptoms, urodynamic testing was repeated. In addition, the patient was offered another surgery, usually involving placement of a TVT sling.
Schierlitz and colleagues concluded that, if TVT were used in all patients, repeat surgery would be avoided in one in every six patients. The risk of repeat surgery was 15 times greater for TOT, compared with the TVT sling. The median time to failure was 15.6 months for the TOT sling, compared with 43.7 months for the TVT.
Of the 16 patients who underwent repeat surgery, 56% were cured, 25% reported minimal leakage, and 19% remained unchanged.
Quality-of-life scores were similar between groups at the 6-month follow-up.
Why did the TVT outperform the TOT in this population?
Investigators theorized that there is a difference in sling axis, with the TVT placed at a more acute angle than the TOT sling. In addition, the location of the TOT sling is more distal than that of the TVT, based on ultrasonographic imaging. As a result, more effective urethral kinking and support are likely with the TVT sling, improving continence rates.
Strengths and limitations of the trial
The randomization of participants and long-term follow-up bolster the trial’s credibility.
Weaknesses include unblinded participation and postoperative surgical assessment.
Although the sample size was underpowered, there was a significant difference in the primary outcome between the two groups.
Long-term success is more likely with placement of a TVT sling in women who have SUI with ISD.
Urodynamic assessment still serves an important role in the diagnosis of ISD, and aids in preoperative planning.
LADIN A. YURTERI-KAPLAN, MD, AND AMY J. PARK, MD
We want to hear from you! Tell us what you think.










