User login
Fluorescein, 10% dextrose topped other media for visualizing ureteral patency
DENVER – Ureteral jets were best visualized during cystoscopy with the help of 10% dextrose or sodium fluorescein, instead of phenazopyridine or normal saline, findings from a multicenter, randomized controlled trial suggested.
User satisfaction also was significantly higher with 10% dextrose and fluorescein, compared with the other interventions, Luis Espaillat-Rijo, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
Average total cystoscopy time, time to first jet visualization, and rates of postoperative urinary tract infections were similar among groups, noted Dr. Espaillat-Rijo, who conducted the research at the Cleveland Clinic Florida in Weston.
Historically, surgeons used intravenous indigo carmine to evaluate ureteral patency during intraoperative cystoscopy, but the Food and Drug Administration announced a shortage in June 2014 that has not resolved.
Hypothesizing that not all alternatives confer equivalent visibility, the researchers randomly assigned 174 female cystoscopy patients aged 18 years or older to one of four interventions: intravenous sodium fluorescein, preoperative oral phenazopyridine (Pyridium), 10% dextrose solution, or normal saline (control).
The researchers excluded patients with kidney disease or a history of surgery for renal or ureteral obstruction. The primary outcome was visibility of the urine jet, which surgeons described as clearly visible, somewhat visible, or not at all visible. Surgeons also were asked how satisfied or confident they were in assessing ureteral patency with the test media, Dr. Espaillat-Rijo said.
The study groups were demographically similar. Fluorescein and 10% dextrose resulted in the highest visibility, with more than 80% of surgeons describing the ureteral jets as “clearly visible” with these modalities, compared with about 60% of cases in which phenazopyridine or saline was used (P = .001, for differences among groups).
Similarly, more than 80% of surgeons reported being very or somewhat satisfied with 10% dextrose and fluorescein, while about 60% of surgeons said they were very or somewhat satisfied with phenazopyridine and saline (P less than .001).
“It was interesting that phenazopyridine was not more visible than control saline,” Dr. Espaillat-Rijo said. “Surgeons also noted that it was harder to evaluate the uroepithelium when it was tinged with Pyridium.”
Use of these interventions did not add time to the procedure, he noted. Total cystoscopy time averaged 4.5 minutes and was similar among groups. Average time to detection of the first jet also was similar among all four interventions, ranging from 1.8 to 2.5 minutes.
None of the patients had allergic reactions or adverse events considered related to an intervention. The rates of urinary tract infection ranged from approximately 22% to 30% and were similar among groups. Three ureteral obstructions were correctly identified and released intraoperatively, and none was identified postoperatively. One patient had an episode of acute urinary retention 4 days after surgery that led to bladder rupture.
“Because we had no postoperative ureteral injuries, we were unable to detect the sensitivity or specificity for each method,” Dr. Espaillat-Rijo said. “Another limitation is that we did not have the power to find a difference in our secondary outcomes, and indigo carmine was not used as a control.”
The Cleveland Clinic Florida sponsored the study. The researchers reported having no relevant financial disclosures.
DENVER – Ureteral jets were best visualized during cystoscopy with the help of 10% dextrose or sodium fluorescein, instead of phenazopyridine or normal saline, findings from a multicenter, randomized controlled trial suggested.
User satisfaction also was significantly higher with 10% dextrose and fluorescein, compared with the other interventions, Luis Espaillat-Rijo, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
Average total cystoscopy time, time to first jet visualization, and rates of postoperative urinary tract infections were similar among groups, noted Dr. Espaillat-Rijo, who conducted the research at the Cleveland Clinic Florida in Weston.
Historically, surgeons used intravenous indigo carmine to evaluate ureteral patency during intraoperative cystoscopy, but the Food and Drug Administration announced a shortage in June 2014 that has not resolved.
Hypothesizing that not all alternatives confer equivalent visibility, the researchers randomly assigned 174 female cystoscopy patients aged 18 years or older to one of four interventions: intravenous sodium fluorescein, preoperative oral phenazopyridine (Pyridium), 10% dextrose solution, or normal saline (control).
The researchers excluded patients with kidney disease or a history of surgery for renal or ureteral obstruction. The primary outcome was visibility of the urine jet, which surgeons described as clearly visible, somewhat visible, or not at all visible. Surgeons also were asked how satisfied or confident they were in assessing ureteral patency with the test media, Dr. Espaillat-Rijo said.
The study groups were demographically similar. Fluorescein and 10% dextrose resulted in the highest visibility, with more than 80% of surgeons describing the ureteral jets as “clearly visible” with these modalities, compared with about 60% of cases in which phenazopyridine or saline was used (P = .001, for differences among groups).
Similarly, more than 80% of surgeons reported being very or somewhat satisfied with 10% dextrose and fluorescein, while about 60% of surgeons said they were very or somewhat satisfied with phenazopyridine and saline (P less than .001).
“It was interesting that phenazopyridine was not more visible than control saline,” Dr. Espaillat-Rijo said. “Surgeons also noted that it was harder to evaluate the uroepithelium when it was tinged with Pyridium.”
Use of these interventions did not add time to the procedure, he noted. Total cystoscopy time averaged 4.5 minutes and was similar among groups. Average time to detection of the first jet also was similar among all four interventions, ranging from 1.8 to 2.5 minutes.
None of the patients had allergic reactions or adverse events considered related to an intervention. The rates of urinary tract infection ranged from approximately 22% to 30% and were similar among groups. Three ureteral obstructions were correctly identified and released intraoperatively, and none was identified postoperatively. One patient had an episode of acute urinary retention 4 days after surgery that led to bladder rupture.
“Because we had no postoperative ureteral injuries, we were unable to detect the sensitivity or specificity for each method,” Dr. Espaillat-Rijo said. “Another limitation is that we did not have the power to find a difference in our secondary outcomes, and indigo carmine was not used as a control.”
The Cleveland Clinic Florida sponsored the study. The researchers reported having no relevant financial disclosures.
DENVER – Ureteral jets were best visualized during cystoscopy with the help of 10% dextrose or sodium fluorescein, instead of phenazopyridine or normal saline, findings from a multicenter, randomized controlled trial suggested.
User satisfaction also was significantly higher with 10% dextrose and fluorescein, compared with the other interventions, Luis Espaillat-Rijo, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
Average total cystoscopy time, time to first jet visualization, and rates of postoperative urinary tract infections were similar among groups, noted Dr. Espaillat-Rijo, who conducted the research at the Cleveland Clinic Florida in Weston.
Historically, surgeons used intravenous indigo carmine to evaluate ureteral patency during intraoperative cystoscopy, but the Food and Drug Administration announced a shortage in June 2014 that has not resolved.
Hypothesizing that not all alternatives confer equivalent visibility, the researchers randomly assigned 174 female cystoscopy patients aged 18 years or older to one of four interventions: intravenous sodium fluorescein, preoperative oral phenazopyridine (Pyridium), 10% dextrose solution, or normal saline (control).
The researchers excluded patients with kidney disease or a history of surgery for renal or ureteral obstruction. The primary outcome was visibility of the urine jet, which surgeons described as clearly visible, somewhat visible, or not at all visible. Surgeons also were asked how satisfied or confident they were in assessing ureteral patency with the test media, Dr. Espaillat-Rijo said.
The study groups were demographically similar. Fluorescein and 10% dextrose resulted in the highest visibility, with more than 80% of surgeons describing the ureteral jets as “clearly visible” with these modalities, compared with about 60% of cases in which phenazopyridine or saline was used (P = .001, for differences among groups).
Similarly, more than 80% of surgeons reported being very or somewhat satisfied with 10% dextrose and fluorescein, while about 60% of surgeons said they were very or somewhat satisfied with phenazopyridine and saline (P less than .001).
“It was interesting that phenazopyridine was not more visible than control saline,” Dr. Espaillat-Rijo said. “Surgeons also noted that it was harder to evaluate the uroepithelium when it was tinged with Pyridium.”
Use of these interventions did not add time to the procedure, he noted. Total cystoscopy time averaged 4.5 minutes and was similar among groups. Average time to detection of the first jet also was similar among all four interventions, ranging from 1.8 to 2.5 minutes.
None of the patients had allergic reactions or adverse events considered related to an intervention. The rates of urinary tract infection ranged from approximately 22% to 30% and were similar among groups. Three ureteral obstructions were correctly identified and released intraoperatively, and none was identified postoperatively. One patient had an episode of acute urinary retention 4 days after surgery that led to bladder rupture.
“Because we had no postoperative ureteral injuries, we were unable to detect the sensitivity or specificity for each method,” Dr. Espaillat-Rijo said. “Another limitation is that we did not have the power to find a difference in our secondary outcomes, and indigo carmine was not used as a control.”
The Cleveland Clinic Florida sponsored the study. The researchers reported having no relevant financial disclosures.
Key clinical point: Major finding: Visibility of the ureteral jet was significantly greater with 10% dextrose and oral phenazopyridine than with intravenous fluorescein or saline (P = .001).
Data source: A multicenter, randomized controlled trial of 174 women undergoing intraoperative cystoscopy.
Disclosures: The Cleveland Clinic Florida sponsored the study. The researchers reported having no relevant financial disclosures.
Laparoscopic sacrocolpopexy offers advantages over abdominal route
BOSTON – Laparoscopic sacrocolpopexy offers some distinct advantages over the abdominal route for treatment of pelvic organ prolapse, including reduced intraoperative blood loss and shorter hospital stays, according to findings from a new research review.
“We wanted to compare the efficiency and safety of abdominal sacral colpopexy and laparoscopic sacral colpopexy for the treatment of pelvic organ collapse,” Juan Liu, MD, of Guangzhou Medical University in China said at the annual Minimally Invasive Surgery Week, held by the Society of Laparoendoscopic Surgeons.
Analyses directly comparing the safety and effectiveness of the two surgical routes are low in number, Dr. Liu added.
The researchers looked at published articles, written in English or Chinese, that were either retrospective analyses or randomized controlled trial studies examining laparoscopic sacrocolpopexy (LSC) or abdominal sacrocolpopexy (ASC), with follow-up times of at least 30 days.
Studies that investigated robot-assisted sacrocolpopexy were excluded, as well as studies for which there were no specific feature data or for which the full text of the study was inaccessible. Of 1,807 articles identified, 10 studies containing 3,816 cases were included for the analysis.
The studies were used to compare laparoscopic and abdominal sacrocolpopexy on the following criteria: operating time; blood loss; hospital length of stay; intraoperative complications such as urinary, bladder, and rectal injury; and postoperative complications such as infection, intestinal obstruction, mesh exposure, new urinary incontinence, and dyspareunia. Weighted mean difference was calculated to account for the different sample sizes across the studies.
The weighted mean difference in intraoperative blood loss in the laparoscopic cohort, compared with the abdominal cohort, was –100.68 mL (P less than .01). Hospital length of stay was also significantly reduced in the laparoscopic cohort, with a weighted mean difference of –1.77 days (P less than .01). The odds ratio for gastrointestinal complications was 0.30 for the laparoscopic route, compared with the abdominal route (P less than .01).
Additionally, pulmonary complications and blood transfusions were also found to be reduced with laparoscopic sacrocolpopexy, compared with abdominal sacrocolpopexy, with an odds ratio of 0.59 (P = .02) and 0.47 (P = .03), respectively.
But the review found little difference in other areas. The weighted mean difference for operating time in the laparoscopic cohort was 0.06 minutes, compared with the abdominal cohort, which was not statistically significant (P= .84). And there was not a statistically significant difference between the two surgical approaches in urinary complications (OR, 0.41; P = .11), cardiovascular complications (OR, 0.31; P = .49), or mesh exposure (OR, 1.60, P = .18).
No funding source for this study was disclosed. Dr. Liu reported having no relevant financial disclosures.
BOSTON – Laparoscopic sacrocolpopexy offers some distinct advantages over the abdominal route for treatment of pelvic organ prolapse, including reduced intraoperative blood loss and shorter hospital stays, according to findings from a new research review.
“We wanted to compare the efficiency and safety of abdominal sacral colpopexy and laparoscopic sacral colpopexy for the treatment of pelvic organ collapse,” Juan Liu, MD, of Guangzhou Medical University in China said at the annual Minimally Invasive Surgery Week, held by the Society of Laparoendoscopic Surgeons.
Analyses directly comparing the safety and effectiveness of the two surgical routes are low in number, Dr. Liu added.
The researchers looked at published articles, written in English or Chinese, that were either retrospective analyses or randomized controlled trial studies examining laparoscopic sacrocolpopexy (LSC) or abdominal sacrocolpopexy (ASC), with follow-up times of at least 30 days.
Studies that investigated robot-assisted sacrocolpopexy were excluded, as well as studies for which there were no specific feature data or for which the full text of the study was inaccessible. Of 1,807 articles identified, 10 studies containing 3,816 cases were included for the analysis.
The studies were used to compare laparoscopic and abdominal sacrocolpopexy on the following criteria: operating time; blood loss; hospital length of stay; intraoperative complications such as urinary, bladder, and rectal injury; and postoperative complications such as infection, intestinal obstruction, mesh exposure, new urinary incontinence, and dyspareunia. Weighted mean difference was calculated to account for the different sample sizes across the studies.
The weighted mean difference in intraoperative blood loss in the laparoscopic cohort, compared with the abdominal cohort, was –100.68 mL (P less than .01). Hospital length of stay was also significantly reduced in the laparoscopic cohort, with a weighted mean difference of –1.77 days (P less than .01). The odds ratio for gastrointestinal complications was 0.30 for the laparoscopic route, compared with the abdominal route (P less than .01).
Additionally, pulmonary complications and blood transfusions were also found to be reduced with laparoscopic sacrocolpopexy, compared with abdominal sacrocolpopexy, with an odds ratio of 0.59 (P = .02) and 0.47 (P = .03), respectively.
But the review found little difference in other areas. The weighted mean difference for operating time in the laparoscopic cohort was 0.06 minutes, compared with the abdominal cohort, which was not statistically significant (P= .84). And there was not a statistically significant difference between the two surgical approaches in urinary complications (OR, 0.41; P = .11), cardiovascular complications (OR, 0.31; P = .49), or mesh exposure (OR, 1.60, P = .18).
No funding source for this study was disclosed. Dr. Liu reported having no relevant financial disclosures.
BOSTON – Laparoscopic sacrocolpopexy offers some distinct advantages over the abdominal route for treatment of pelvic organ prolapse, including reduced intraoperative blood loss and shorter hospital stays, according to findings from a new research review.
“We wanted to compare the efficiency and safety of abdominal sacral colpopexy and laparoscopic sacral colpopexy for the treatment of pelvic organ collapse,” Juan Liu, MD, of Guangzhou Medical University in China said at the annual Minimally Invasive Surgery Week, held by the Society of Laparoendoscopic Surgeons.
Analyses directly comparing the safety and effectiveness of the two surgical routes are low in number, Dr. Liu added.
The researchers looked at published articles, written in English or Chinese, that were either retrospective analyses or randomized controlled trial studies examining laparoscopic sacrocolpopexy (LSC) or abdominal sacrocolpopexy (ASC), with follow-up times of at least 30 days.
Studies that investigated robot-assisted sacrocolpopexy were excluded, as well as studies for which there were no specific feature data or for which the full text of the study was inaccessible. Of 1,807 articles identified, 10 studies containing 3,816 cases were included for the analysis.
The studies were used to compare laparoscopic and abdominal sacrocolpopexy on the following criteria: operating time; blood loss; hospital length of stay; intraoperative complications such as urinary, bladder, and rectal injury; and postoperative complications such as infection, intestinal obstruction, mesh exposure, new urinary incontinence, and dyspareunia. Weighted mean difference was calculated to account for the different sample sizes across the studies.
The weighted mean difference in intraoperative blood loss in the laparoscopic cohort, compared with the abdominal cohort, was –100.68 mL (P less than .01). Hospital length of stay was also significantly reduced in the laparoscopic cohort, with a weighted mean difference of –1.77 days (P less than .01). The odds ratio for gastrointestinal complications was 0.30 for the laparoscopic route, compared with the abdominal route (P less than .01).
Additionally, pulmonary complications and blood transfusions were also found to be reduced with laparoscopic sacrocolpopexy, compared with abdominal sacrocolpopexy, with an odds ratio of 0.59 (P = .02) and 0.47 (P = .03), respectively.
But the review found little difference in other areas. The weighted mean difference for operating time in the laparoscopic cohort was 0.06 minutes, compared with the abdominal cohort, which was not statistically significant (P= .84). And there was not a statistically significant difference between the two surgical approaches in urinary complications (OR, 0.41; P = .11), cardiovascular complications (OR, 0.31; P = .49), or mesh exposure (OR, 1.60, P = .18).
No funding source for this study was disclosed. Dr. Liu reported having no relevant financial disclosures.
AT MINIMALLY INVASIVE SURGERY WEEK
Key clinical point:
Major finding: The weighted mean difference in intraoperative blood loss in the laparoscopic cohort, compared with the abdominal cohort, was –100.68 mL (P less than .01).
Data source: Retrospective review of 10 studies involving 3,816 sacrocolpopexy cases.
Disclosures: Dr. Liu reported having no relevant financial disclosures.
Botox for overactive bladder led to low rate of catheterization
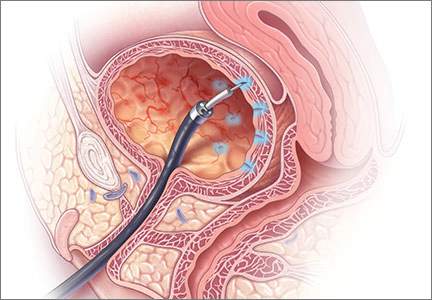
DENVER – Less than 2% of onabotulinumtoxinA injections for idiopathic detrusor overactivity resulted in clean intermittent catheterization, a substantially lower rate than previously found, Juzar Jamnagerwalla, MD, reported at Pelvic Floor Disorders Week.
These included two cases of acute urinary retention and one case in which a patient complained of problems voiding and had a postvoid residual urine volume (PVR) of 353 mL, said Dr. Jamnagerwalla of Cedars-Sinai, Los Angeles. Taken together, the findings suggest that postprocedural PVR can usually be managed safely by observation alone, which may reassure patients who are considering treatment options for overactive bladder, he added.
But these “strict” criteria contrast with real-world practice, in which patients with postprocedural PVR often are observed without CIC unless they have subjective complaints or other contraindications, Dr. Jamnagerwalla said. The discrepancy is especially relevant because patients with overactive bladder who decline onabotulinumtoxinA often cite the risk of CIC as the reason, he added.
To better understand CIC rates at Cedars-Sinai, Dr. Jamnagerwalla and his colleagues reviewed 27 months of records from patients with idiopathic detrusor overactivity who received injections of 100 U of onabotulinumtoxinA given by female pelvic medicine and reconstructive surgery physicians. The patients were followed up immediately and 2 weeks later, but underwent CIC only if they could not void or had PVRs above 350 mL and subjective voiding complaints.
In all, 99 patients received a total of 187 injections, of which only 3 (1.6%) led to urinary retention requiring CIC. The median postprocedure PVR was 117 mL. About three-quarters of patients had PVRs less than 200 mL, 29 (16%) had PVRs between 200 mL and 350 mL, and 13 (7%) had PVRs greater than 350 mL.
Age, body mass index, and preprocedure PVR did not predict urinary retention in the univariate analysis, Dr. Jamnagerwalla said at the meeting, which was sponsored by the American Urogynecologic Society.
The results support the practice of observing patients with elevated PVRs after Botox, as long as they do not develop obstructive symptoms, he advised.
“While it remains important to counsel patients on the risk of urinary retention after Botox injection for idiopathic detrusor overactivity, patients can be reassured that the true rate of retention requiring CIC is relatively low,” he said.
Dr. Jamnagerwalla reported no funding sources and reported having no financial disclosures. Two coauthors reported ties to Boston Scientific, Astora, and Allergan.
DENVER – Less than 2% of onabotulinumtoxinA injections for idiopathic detrusor overactivity resulted in clean intermittent catheterization, a substantially lower rate than previously found, Juzar Jamnagerwalla, MD, reported at Pelvic Floor Disorders Week.
These included two cases of acute urinary retention and one case in which a patient complained of problems voiding and had a postvoid residual urine volume (PVR) of 353 mL, said Dr. Jamnagerwalla of Cedars-Sinai, Los Angeles. Taken together, the findings suggest that postprocedural PVR can usually be managed safely by observation alone, which may reassure patients who are considering treatment options for overactive bladder, he added.
But these “strict” criteria contrast with real-world practice, in which patients with postprocedural PVR often are observed without CIC unless they have subjective complaints or other contraindications, Dr. Jamnagerwalla said. The discrepancy is especially relevant because patients with overactive bladder who decline onabotulinumtoxinA often cite the risk of CIC as the reason, he added.
To better understand CIC rates at Cedars-Sinai, Dr. Jamnagerwalla and his colleagues reviewed 27 months of records from patients with idiopathic detrusor overactivity who received injections of 100 U of onabotulinumtoxinA given by female pelvic medicine and reconstructive surgery physicians. The patients were followed up immediately and 2 weeks later, but underwent CIC only if they could not void or had PVRs above 350 mL and subjective voiding complaints.
In all, 99 patients received a total of 187 injections, of which only 3 (1.6%) led to urinary retention requiring CIC. The median postprocedure PVR was 117 mL. About three-quarters of patients had PVRs less than 200 mL, 29 (16%) had PVRs between 200 mL and 350 mL, and 13 (7%) had PVRs greater than 350 mL.
Age, body mass index, and preprocedure PVR did not predict urinary retention in the univariate analysis, Dr. Jamnagerwalla said at the meeting, which was sponsored by the American Urogynecologic Society.
The results support the practice of observing patients with elevated PVRs after Botox, as long as they do not develop obstructive symptoms, he advised.
“While it remains important to counsel patients on the risk of urinary retention after Botox injection for idiopathic detrusor overactivity, patients can be reassured that the true rate of retention requiring CIC is relatively low,” he said.
Dr. Jamnagerwalla reported no funding sources and reported having no financial disclosures. Two coauthors reported ties to Boston Scientific, Astora, and Allergan.
DENVER – Less than 2% of onabotulinumtoxinA injections for idiopathic detrusor overactivity resulted in clean intermittent catheterization, a substantially lower rate than previously found, Juzar Jamnagerwalla, MD, reported at Pelvic Floor Disorders Week.
These included two cases of acute urinary retention and one case in which a patient complained of problems voiding and had a postvoid residual urine volume (PVR) of 353 mL, said Dr. Jamnagerwalla of Cedars-Sinai, Los Angeles. Taken together, the findings suggest that postprocedural PVR can usually be managed safely by observation alone, which may reassure patients who are considering treatment options for overactive bladder, he added.
But these “strict” criteria contrast with real-world practice, in which patients with postprocedural PVR often are observed without CIC unless they have subjective complaints or other contraindications, Dr. Jamnagerwalla said. The discrepancy is especially relevant because patients with overactive bladder who decline onabotulinumtoxinA often cite the risk of CIC as the reason, he added.
To better understand CIC rates at Cedars-Sinai, Dr. Jamnagerwalla and his colleagues reviewed 27 months of records from patients with idiopathic detrusor overactivity who received injections of 100 U of onabotulinumtoxinA given by female pelvic medicine and reconstructive surgery physicians. The patients were followed up immediately and 2 weeks later, but underwent CIC only if they could not void or had PVRs above 350 mL and subjective voiding complaints.
In all, 99 patients received a total of 187 injections, of which only 3 (1.6%) led to urinary retention requiring CIC. The median postprocedure PVR was 117 mL. About three-quarters of patients had PVRs less than 200 mL, 29 (16%) had PVRs between 200 mL and 350 mL, and 13 (7%) had PVRs greater than 350 mL.
Age, body mass index, and preprocedure PVR did not predict urinary retention in the univariate analysis, Dr. Jamnagerwalla said at the meeting, which was sponsored by the American Urogynecologic Society.
The results support the practice of observing patients with elevated PVRs after Botox, as long as they do not develop obstructive symptoms, he advised.
“While it remains important to counsel patients on the risk of urinary retention after Botox injection for idiopathic detrusor overactivity, patients can be reassured that the true rate of retention requiring CIC is relatively low,” he said.
Dr. Jamnagerwalla reported no funding sources and reported having no financial disclosures. Two coauthors reported ties to Boston Scientific, Astora, and Allergan.
Key clinical point:
Major finding: Of 187 injections, 1.6% of led to acute urinary retention requiring clean intermittent catheterization.
Data source: A single-center, retrospective study of 99 patients receiving 187 onabotulinumtoxinA injections.
Disclosures: Dr. Jamnagerwalla and his colleagues reported no funding sources and had no disclosures.
Incontinence trial finds small advantage for Botox over sacral neuromodulation
OnabotulinumtoxinA decreased daily episodes of urinary incontinence by a small amount, compared with sacral neuromodulation, but did not appear to impact several quality of life measures and raised the rates of urinary tract infection and self-catheterization, according to findings from a comparative effectiveness study.
In an open-label randomized trial directly comparing the two approaches for refractory urgency incontinence, onabotulinumtoxinA showed a statistically significant advantage over sacral neuromodulation, but whether this translates into a clinically significant difference is unclear.
“Overall, these findings make it uncertain whether onabotulinumtoxinA provides a clinically important net benefit, compared with sacral neuromodulation,” said Cindy L. Amundsen, MD, of Duke University, Durham N.C., and her associates.
Noting that a recent systematic review of the literature found insufficient evidence to recommend one of these treatments over the other, the investigators performed their study at nine medical centers participating in the National Institutes of Health’s Pelvic Floor Disorder Network. Study participants included 386 women who had a minimum of six urgency incontinence episodes per day and whose symptoms persisted despite treatment with at least one behavioral or physical therapy intervention and at least two medical therapies. They were followed up at 6 months.
In the intention-to-treat analysis, the 190 women who received a single injection of onabotulinumtoxinA showed a mean reduction of 3.9 daily episodes of urinary incontinence, compared with a reduction of 3.3 episodes for the 174 women who underwent sacral neuromodulation. The onabotulinumtoxinA group also showed slightly greater improvement on the Overactive Bladder Short Form score for symptom bother and on the Overactive Bladder Satisfaction of Treatment questionnaire, Dr. Amundsen and her associates reported (JAMA. 2016;316[13]:1366-1374).
However, there were no significant differences between the two study groups in measures of convenience, adverse effects, treatment preference, or other quality of life factors. And onabotulinumtoxinA was associated with a higher rate of urinary tract infection (35% vs. 11%) and of intermittent self-catheterization (8% vs. 0% at 1 month).
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health. Dr. Amundsen reported having no relevant financial disclosures; two of her associates reported ties to Pfizer, Medtronic (maker of the InterStim sacral neuromodulation device), Allergan (maker of Botox), and Axonics.
OnabotulinumtoxinA decreased daily episodes of urinary incontinence by a small amount, compared with sacral neuromodulation, but did not appear to impact several quality of life measures and raised the rates of urinary tract infection and self-catheterization, according to findings from a comparative effectiveness study.
In an open-label randomized trial directly comparing the two approaches for refractory urgency incontinence, onabotulinumtoxinA showed a statistically significant advantage over sacral neuromodulation, but whether this translates into a clinically significant difference is unclear.
“Overall, these findings make it uncertain whether onabotulinumtoxinA provides a clinically important net benefit, compared with sacral neuromodulation,” said Cindy L. Amundsen, MD, of Duke University, Durham N.C., and her associates.
Noting that a recent systematic review of the literature found insufficient evidence to recommend one of these treatments over the other, the investigators performed their study at nine medical centers participating in the National Institutes of Health’s Pelvic Floor Disorder Network. Study participants included 386 women who had a minimum of six urgency incontinence episodes per day and whose symptoms persisted despite treatment with at least one behavioral or physical therapy intervention and at least two medical therapies. They were followed up at 6 months.
In the intention-to-treat analysis, the 190 women who received a single injection of onabotulinumtoxinA showed a mean reduction of 3.9 daily episodes of urinary incontinence, compared with a reduction of 3.3 episodes for the 174 women who underwent sacral neuromodulation. The onabotulinumtoxinA group also showed slightly greater improvement on the Overactive Bladder Short Form score for symptom bother and on the Overactive Bladder Satisfaction of Treatment questionnaire, Dr. Amundsen and her associates reported (JAMA. 2016;316[13]:1366-1374).
However, there were no significant differences between the two study groups in measures of convenience, adverse effects, treatment preference, or other quality of life factors. And onabotulinumtoxinA was associated with a higher rate of urinary tract infection (35% vs. 11%) and of intermittent self-catheterization (8% vs. 0% at 1 month).
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health. Dr. Amundsen reported having no relevant financial disclosures; two of her associates reported ties to Pfizer, Medtronic (maker of the InterStim sacral neuromodulation device), Allergan (maker of Botox), and Axonics.
OnabotulinumtoxinA decreased daily episodes of urinary incontinence by a small amount, compared with sacral neuromodulation, but did not appear to impact several quality of life measures and raised the rates of urinary tract infection and self-catheterization, according to findings from a comparative effectiveness study.
In an open-label randomized trial directly comparing the two approaches for refractory urgency incontinence, onabotulinumtoxinA showed a statistically significant advantage over sacral neuromodulation, but whether this translates into a clinically significant difference is unclear.
“Overall, these findings make it uncertain whether onabotulinumtoxinA provides a clinically important net benefit, compared with sacral neuromodulation,” said Cindy L. Amundsen, MD, of Duke University, Durham N.C., and her associates.
Noting that a recent systematic review of the literature found insufficient evidence to recommend one of these treatments over the other, the investigators performed their study at nine medical centers participating in the National Institutes of Health’s Pelvic Floor Disorder Network. Study participants included 386 women who had a minimum of six urgency incontinence episodes per day and whose symptoms persisted despite treatment with at least one behavioral or physical therapy intervention and at least two medical therapies. They were followed up at 6 months.
In the intention-to-treat analysis, the 190 women who received a single injection of onabotulinumtoxinA showed a mean reduction of 3.9 daily episodes of urinary incontinence, compared with a reduction of 3.3 episodes for the 174 women who underwent sacral neuromodulation. The onabotulinumtoxinA group also showed slightly greater improvement on the Overactive Bladder Short Form score for symptom bother and on the Overactive Bladder Satisfaction of Treatment questionnaire, Dr. Amundsen and her associates reported (JAMA. 2016;316[13]:1366-1374).
However, there were no significant differences between the two study groups in measures of convenience, adverse effects, treatment preference, or other quality of life factors. And onabotulinumtoxinA was associated with a higher rate of urinary tract infection (35% vs. 11%) and of intermittent self-catheterization (8% vs. 0% at 1 month).
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health. Dr. Amundsen reported having no relevant financial disclosures; two of her associates reported ties to Pfizer, Medtronic (maker of the InterStim sacral neuromodulation device), Allergan (maker of Botox), and Axonics.
Key clinical point:
Major finding: The 190 women who received a single injection of onabotulinumtoxinA showed a mean reduction of 3.9 daily episodes of urinary incontinence, compared with a reduction of 3.3 episodes for the 174 women who underwent sacral neuromodulation.
Data source: A multicenter open-label randomized trial involving 386 women followed for 6 months.
Disclosures: This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health. Dr. Amundsen reported having no relevant financial disclosures; two of her associates reported ties to Pfizer, Medtronic (maker of the InterStim sacral neuromodulation device), Allergan (maker of Botox), and Axonics.
Absorbable suture performs well in sacrocolpopexy with mesh
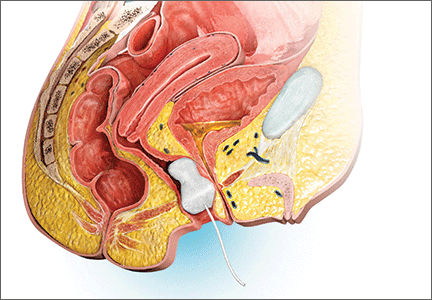
DENVER – Using absorbable polydioxanone suture during laparoscopic sacrocolpopexy was associated with a mesh erosion rate of just 1.6%, according to a single-center, 1-year prospective study of 64 patients.
That is substantially less than typical erosion rates of about 5% when permanent suture is used, Danielle Taylor, DO, of Akron (Ohio ) General Medical Center said at Pelvic Floor Disorders Week sponsored by the American Urogynecologic Society.
The researchers observed no anatomic failures or suture extrusions, and patients reported significant postoperative improvements on several validated measures of quality of life.
“Larger samples and longer follow-up may be needed,” said Dr. Taylor. “But our study suggests that permanent, nondissolving suture material may not be necessary for sacrocolpopexy.”
Sacrocolpopexy with mesh usually involves using nonabsorbable suture to attach its anterior and posterior arms to the vaginal mucosa. Instead, Dr. Taylor and colleagues used 90-day delayed absorbable 2.0 V-Loc (Covidien) suture during laparoscopic sacrocolpopexy for patients with baseline Pelvic Organ Prolapse Quantification (POP-Q) scores of at least 2 and symptomatic uterovaginal prolapse.
Two permanent Gore-Tex sutures were also placed at the apex of the cervix in each of the 64 patients, said Dr. Taylor, a urogynecology fellow at the University of Massachusetts, Worcester, who worked on the study as a resident at the Cleveland Clinic Akron General, in Ohio. She and her colleagues rechecked patients at postoperative weeks 2 and 6, and at months 6 and 12. They lost two patients to follow-up, both after week 2.
At baseline, 37 patients (58%) were in stage II pelvic organ prolapse, 27% were in stage III, and 14% were in stage IV. At 6 months after surgery, 85% had no detectable prolapse, 8% had stage I, and 6% had stage II. At 1 year, 82% remained in pelvic organ prolapse stage 0 and the rest were in stage I or II. All stage II patients remained asymptomatic, Dr. Taylor said.
At baseline, the median value for POP-Q point C was -3 (range, –8 to +6). At 6 months and 1 year later, the median value had improved to –8, and patients ranged between –10 and –8.
Quality of life surveys of 54 patients reflected these outcomes, Dr. Taylor said. A year after surgery, average scores on the Pelvic Floor Distress Index (PFDI) dropped by 67 points, from 103 to 35 (P less than .0001). Likewise, average scores on the Pelvic Floor Impact Questionnaire (PFIQ) dropped by 29 points (P less than .0001), and scores on the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ) indicated a significant decrease in the effects of pelvic organ prolapse on sexual functioning (P = .008).
In addition to a single case of mesh erosion, one patient developed postoperative ileus and one experienced small bowel obstruction, both of which resolved, Dr. Taylor reported. The researchers aim to continue the study with longer follow-up intervals and detailed analyses of postoperative pain.
Dr. Taylor reported no funding sources and had no disclosures. One coauthor disclosed ties to Coloplast Corp.
DENVER – Using absorbable polydioxanone suture during laparoscopic sacrocolpopexy was associated with a mesh erosion rate of just 1.6%, according to a single-center, 1-year prospective study of 64 patients.
That is substantially less than typical erosion rates of about 5% when permanent suture is used, Danielle Taylor, DO, of Akron (Ohio ) General Medical Center said at Pelvic Floor Disorders Week sponsored by the American Urogynecologic Society.
The researchers observed no anatomic failures or suture extrusions, and patients reported significant postoperative improvements on several validated measures of quality of life.
“Larger samples and longer follow-up may be needed,” said Dr. Taylor. “But our study suggests that permanent, nondissolving suture material may not be necessary for sacrocolpopexy.”
Sacrocolpopexy with mesh usually involves using nonabsorbable suture to attach its anterior and posterior arms to the vaginal mucosa. Instead, Dr. Taylor and colleagues used 90-day delayed absorbable 2.0 V-Loc (Covidien) suture during laparoscopic sacrocolpopexy for patients with baseline Pelvic Organ Prolapse Quantification (POP-Q) scores of at least 2 and symptomatic uterovaginal prolapse.
Two permanent Gore-Tex sutures were also placed at the apex of the cervix in each of the 64 patients, said Dr. Taylor, a urogynecology fellow at the University of Massachusetts, Worcester, who worked on the study as a resident at the Cleveland Clinic Akron General, in Ohio. She and her colleagues rechecked patients at postoperative weeks 2 and 6, and at months 6 and 12. They lost two patients to follow-up, both after week 2.
At baseline, 37 patients (58%) were in stage II pelvic organ prolapse, 27% were in stage III, and 14% were in stage IV. At 6 months after surgery, 85% had no detectable prolapse, 8% had stage I, and 6% had stage II. At 1 year, 82% remained in pelvic organ prolapse stage 0 and the rest were in stage I or II. All stage II patients remained asymptomatic, Dr. Taylor said.
At baseline, the median value for POP-Q point C was -3 (range, –8 to +6). At 6 months and 1 year later, the median value had improved to –8, and patients ranged between –10 and –8.
Quality of life surveys of 54 patients reflected these outcomes, Dr. Taylor said. A year after surgery, average scores on the Pelvic Floor Distress Index (PFDI) dropped by 67 points, from 103 to 35 (P less than .0001). Likewise, average scores on the Pelvic Floor Impact Questionnaire (PFIQ) dropped by 29 points (P less than .0001), and scores on the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ) indicated a significant decrease in the effects of pelvic organ prolapse on sexual functioning (P = .008).
In addition to a single case of mesh erosion, one patient developed postoperative ileus and one experienced small bowel obstruction, both of which resolved, Dr. Taylor reported. The researchers aim to continue the study with longer follow-up intervals and detailed analyses of postoperative pain.
Dr. Taylor reported no funding sources and had no disclosures. One coauthor disclosed ties to Coloplast Corp.
DENVER – Using absorbable polydioxanone suture during laparoscopic sacrocolpopexy was associated with a mesh erosion rate of just 1.6%, according to a single-center, 1-year prospective study of 64 patients.
That is substantially less than typical erosion rates of about 5% when permanent suture is used, Danielle Taylor, DO, of Akron (Ohio ) General Medical Center said at Pelvic Floor Disorders Week sponsored by the American Urogynecologic Society.
The researchers observed no anatomic failures or suture extrusions, and patients reported significant postoperative improvements on several validated measures of quality of life.
“Larger samples and longer follow-up may be needed,” said Dr. Taylor. “But our study suggests that permanent, nondissolving suture material may not be necessary for sacrocolpopexy.”
Sacrocolpopexy with mesh usually involves using nonabsorbable suture to attach its anterior and posterior arms to the vaginal mucosa. Instead, Dr. Taylor and colleagues used 90-day delayed absorbable 2.0 V-Loc (Covidien) suture during laparoscopic sacrocolpopexy for patients with baseline Pelvic Organ Prolapse Quantification (POP-Q) scores of at least 2 and symptomatic uterovaginal prolapse.
Two permanent Gore-Tex sutures were also placed at the apex of the cervix in each of the 64 patients, said Dr. Taylor, a urogynecology fellow at the University of Massachusetts, Worcester, who worked on the study as a resident at the Cleveland Clinic Akron General, in Ohio. She and her colleagues rechecked patients at postoperative weeks 2 and 6, and at months 6 and 12. They lost two patients to follow-up, both after week 2.
At baseline, 37 patients (58%) were in stage II pelvic organ prolapse, 27% were in stage III, and 14% were in stage IV. At 6 months after surgery, 85% had no detectable prolapse, 8% had stage I, and 6% had stage II. At 1 year, 82% remained in pelvic organ prolapse stage 0 and the rest were in stage I or II. All stage II patients remained asymptomatic, Dr. Taylor said.
At baseline, the median value for POP-Q point C was -3 (range, –8 to +6). At 6 months and 1 year later, the median value had improved to –8, and patients ranged between –10 and –8.
Quality of life surveys of 54 patients reflected these outcomes, Dr. Taylor said. A year after surgery, average scores on the Pelvic Floor Distress Index (PFDI) dropped by 67 points, from 103 to 35 (P less than .0001). Likewise, average scores on the Pelvic Floor Impact Questionnaire (PFIQ) dropped by 29 points (P less than .0001), and scores on the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ) indicated a significant decrease in the effects of pelvic organ prolapse on sexual functioning (P = .008).
In addition to a single case of mesh erosion, one patient developed postoperative ileus and one experienced small bowel obstruction, both of which resolved, Dr. Taylor reported. The researchers aim to continue the study with longer follow-up intervals and detailed analyses of postoperative pain.
Dr. Taylor reported no funding sources and had no disclosures. One coauthor disclosed ties to Coloplast Corp.
Key clinical point:
Major finding: When 90-day delayed absorbable polydioxanone suture was used, the mesh erosion rate was 1.6%. There were no anatomic failures or cases of suture extrusion.
Data source: A single-center prospective case series of 64 patients.
Disclosures: Dr. Taylor reported having no financial disclosures. One coauthor reported ties to Coloplast Corp.
How do you break the ice with patients to ask about their sexual health?
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
In this Article
- Conversation icebreakers
- The sexual status examination
- When to refer
Stand up for research benefiting our patients, and more
Focus on decreasing unintended pregnanciesI found the letters in response to Dr. Barbieri’s Editorial on inadequate contraception to be much overwrought. Dr. Will’s suggestion to have “automatic contraception … for all reproductive-age women including ‘children’ who are … menstruating” is excessive. Shouldn’t parents have the final decision making in their minor children’s health care?
An anonymous clinician ex-presses frustration with a Catholichealth care system for not allowing prescription of contraceptives, which does actually stay true to the religious beliefs of the institution, and proposes decreased reimbursements to these facilities across the board as a form of financial punishment for these practices. Not only would that be illegal and unconstitutional but it also demonstrates a lack of understanding of our First Amendment protections.
Overall, these letters and Dr. Barbieri’s response show a very narrow understanding of the issues involved. I think we can and should be focused on decreasing unintended pregnancies while also respecting the rights of all without resorting to Draconian and totalitarian solutions.
Myles Dotto, MD
Oradell, New Jersey
Dr. Barbieri respondsI share Dr. Dotto’s concern that government mandates regarding health care are potentially very dangerous. It is better for communities of clinicians and patients to develop optimal approaches to health care, without government interference.
“THE CRUSHING OF INNOVATION FOR TREATING FEMALE PELVIC FLOOR DISORDERS: A STORY OF ‘LEAD OR BE LED’”ANDREW CASSIDENTI, MD (GUEST EDITORIAL; APRIL 2016)Stand up for research benefiting our patientsI salute Dr. Cassidenti’s courage to call surgeons and the respective professional organizations to step up to defend the research and expose inappropriate expert testimony. We should be ashamed to be scattered like dogs because of fear and lack of courage to be advocates for what is in the best interest of our patients. Please continue the campaign to encourage physicians and surgeons to stand up.
Cleve Waters, MD
Chattanooga, Tennessee
Caving to class action litigation is a mistakeIn his Guest Editorial Dr. Cassidenti clarifies the importance of looking forward regarding mesh devices for pelvic organ prolapse (POP) treatment. As an advocate for women with POP and Founder/Executive Director of the Association for Pelvic Organ Prolapse Support—a US-based 501(c)(3)advocacy agency with global arms focused on generating awareness of POP and providing guidance and support to women navigating POP treatment—I found Endo International’s decision to close its Astora Women’s Health division extremely unsettling.
The nature of medicine is to continually advance, and that includes learning from experience and recognizing paths to evolution. Caving to class action litigation is a mistake. Research findings frequently indicate that up to half of the female population will experience POP and/or comorbid conditions.1 It is imperative that health care, industry, research, academia, policy, and advocacy agencies continue to shine a light on this much needed field in women’s health.
Sherrie Palm
Milwaukee, Wisconsin
Reference
- Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. 2013;24(11):1783–1790.
Avoidance: the greatest tool to address shoulder dystociaAlthough avoiding endometrial injury at cesarean delivery, including the possibility of later pathologic implantation, can be attained with vaginal delivery, vaginal birth at all cost leads to a dangerous situation. The emergency environment of shoulder dystocia is not a preferable or safer stratagem.
It is granted that shoulder dystocia will happen at some point but avoidance, by employing cesarean delivery when it is indicated, is the greatest tool for addressing this very dangerous problem.
J. Michael Arnold, MD
Oconto Falls, Wisconsin
Another suggestion for shoulder dystociaMy senior partner taught me a technique that works well, although I do not know its name. After suprapubic and McRoberts maneuvers fail and the shoulders do not deliver with gentle downward guidance in one direction, I rotate the head 180° and try again. Usually this works. I have taught this technique to several midwives, and they swear by it.
Annette Fineberg, MD
Davis, California
Dr. Barbieri respondsI thank Drs. Arnold and Fineberg for sharing their perspective and experience with our readers. Dr. Arnold notes that recommending cesarean delivery in high-risk situations such as cases in which the mother has diabetes and the fetus is macrosomic would surely reduce the frequency of shoulder dystocia. I respect Dr. Fineberg’s recommendation, based on extensive clinical experience, that by rotating the fetal head the shoulder dystocia may be resolved. My concern with this technique is that the torque transmitted to the neck might cause fetal damage. I think that rotating the shoulders (Rubin or Wood maneuver) would be less likely to result in fetal injury.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Focus on decreasing unintended pregnanciesI found the letters in response to Dr. Barbieri’s Editorial on inadequate contraception to be much overwrought. Dr. Will’s suggestion to have “automatic contraception … for all reproductive-age women including ‘children’ who are … menstruating” is excessive. Shouldn’t parents have the final decision making in their minor children’s health care?
An anonymous clinician ex-presses frustration with a Catholichealth care system for not allowing prescription of contraceptives, which does actually stay true to the religious beliefs of the institution, and proposes decreased reimbursements to these facilities across the board as a form of financial punishment for these practices. Not only would that be illegal and unconstitutional but it also demonstrates a lack of understanding of our First Amendment protections.
Overall, these letters and Dr. Barbieri’s response show a very narrow understanding of the issues involved. I think we can and should be focused on decreasing unintended pregnancies while also respecting the rights of all without resorting to Draconian and totalitarian solutions.
Myles Dotto, MD
Oradell, New Jersey
Dr. Barbieri respondsI share Dr. Dotto’s concern that government mandates regarding health care are potentially very dangerous. It is better for communities of clinicians and patients to develop optimal approaches to health care, without government interference.
“THE CRUSHING OF INNOVATION FOR TREATING FEMALE PELVIC FLOOR DISORDERS: A STORY OF ‘LEAD OR BE LED’”ANDREW CASSIDENTI, MD (GUEST EDITORIAL; APRIL 2016)Stand up for research benefiting our patientsI salute Dr. Cassidenti’s courage to call surgeons and the respective professional organizations to step up to defend the research and expose inappropriate expert testimony. We should be ashamed to be scattered like dogs because of fear and lack of courage to be advocates for what is in the best interest of our patients. Please continue the campaign to encourage physicians and surgeons to stand up.
Cleve Waters, MD
Chattanooga, Tennessee
Caving to class action litigation is a mistakeIn his Guest Editorial Dr. Cassidenti clarifies the importance of looking forward regarding mesh devices for pelvic organ prolapse (POP) treatment. As an advocate for women with POP and Founder/Executive Director of the Association for Pelvic Organ Prolapse Support—a US-based 501(c)(3)advocacy agency with global arms focused on generating awareness of POP and providing guidance and support to women navigating POP treatment—I found Endo International’s decision to close its Astora Women’s Health division extremely unsettling.
The nature of medicine is to continually advance, and that includes learning from experience and recognizing paths to evolution. Caving to class action litigation is a mistake. Research findings frequently indicate that up to half of the female population will experience POP and/or comorbid conditions.1 It is imperative that health care, industry, research, academia, policy, and advocacy agencies continue to shine a light on this much needed field in women’s health.
Sherrie Palm
Milwaukee, Wisconsin
Reference
- Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. 2013;24(11):1783–1790.
Avoidance: the greatest tool to address shoulder dystociaAlthough avoiding endometrial injury at cesarean delivery, including the possibility of later pathologic implantation, can be attained with vaginal delivery, vaginal birth at all cost leads to a dangerous situation. The emergency environment of shoulder dystocia is not a preferable or safer stratagem.
It is granted that shoulder dystocia will happen at some point but avoidance, by employing cesarean delivery when it is indicated, is the greatest tool for addressing this very dangerous problem.
J. Michael Arnold, MD
Oconto Falls, Wisconsin
Another suggestion for shoulder dystociaMy senior partner taught me a technique that works well, although I do not know its name. After suprapubic and McRoberts maneuvers fail and the shoulders do not deliver with gentle downward guidance in one direction, I rotate the head 180° and try again. Usually this works. I have taught this technique to several midwives, and they swear by it.
Annette Fineberg, MD
Davis, California
Dr. Barbieri respondsI thank Drs. Arnold and Fineberg for sharing their perspective and experience with our readers. Dr. Arnold notes that recommending cesarean delivery in high-risk situations such as cases in which the mother has diabetes and the fetus is macrosomic would surely reduce the frequency of shoulder dystocia. I respect Dr. Fineberg’s recommendation, based on extensive clinical experience, that by rotating the fetal head the shoulder dystocia may be resolved. My concern with this technique is that the torque transmitted to the neck might cause fetal damage. I think that rotating the shoulders (Rubin or Wood maneuver) would be less likely to result in fetal injury.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Focus on decreasing unintended pregnanciesI found the letters in response to Dr. Barbieri’s Editorial on inadequate contraception to be much overwrought. Dr. Will’s suggestion to have “automatic contraception … for all reproductive-age women including ‘children’ who are … menstruating” is excessive. Shouldn’t parents have the final decision making in their minor children’s health care?
An anonymous clinician ex-presses frustration with a Catholichealth care system for not allowing prescription of contraceptives, which does actually stay true to the religious beliefs of the institution, and proposes decreased reimbursements to these facilities across the board as a form of financial punishment for these practices. Not only would that be illegal and unconstitutional but it also demonstrates a lack of understanding of our First Amendment protections.
Overall, these letters and Dr. Barbieri’s response show a very narrow understanding of the issues involved. I think we can and should be focused on decreasing unintended pregnancies while also respecting the rights of all without resorting to Draconian and totalitarian solutions.
Myles Dotto, MD
Oradell, New Jersey
Dr. Barbieri respondsI share Dr. Dotto’s concern that government mandates regarding health care are potentially very dangerous. It is better for communities of clinicians and patients to develop optimal approaches to health care, without government interference.
“THE CRUSHING OF INNOVATION FOR TREATING FEMALE PELVIC FLOOR DISORDERS: A STORY OF ‘LEAD OR BE LED’”ANDREW CASSIDENTI, MD (GUEST EDITORIAL; APRIL 2016)Stand up for research benefiting our patientsI salute Dr. Cassidenti’s courage to call surgeons and the respective professional organizations to step up to defend the research and expose inappropriate expert testimony. We should be ashamed to be scattered like dogs because of fear and lack of courage to be advocates for what is in the best interest of our patients. Please continue the campaign to encourage physicians and surgeons to stand up.
Cleve Waters, MD
Chattanooga, Tennessee
Caving to class action litigation is a mistakeIn his Guest Editorial Dr. Cassidenti clarifies the importance of looking forward regarding mesh devices for pelvic organ prolapse (POP) treatment. As an advocate for women with POP and Founder/Executive Director of the Association for Pelvic Organ Prolapse Support—a US-based 501(c)(3)advocacy agency with global arms focused on generating awareness of POP and providing guidance and support to women navigating POP treatment—I found Endo International’s decision to close its Astora Women’s Health division extremely unsettling.
The nature of medicine is to continually advance, and that includes learning from experience and recognizing paths to evolution. Caving to class action litigation is a mistake. Research findings frequently indicate that up to half of the female population will experience POP and/or comorbid conditions.1 It is imperative that health care, industry, research, academia, policy, and advocacy agencies continue to shine a light on this much needed field in women’s health.
Sherrie Palm
Milwaukee, Wisconsin
Reference
- Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. 2013;24(11):1783–1790.
Avoidance: the greatest tool to address shoulder dystociaAlthough avoiding endometrial injury at cesarean delivery, including the possibility of later pathologic implantation, can be attained with vaginal delivery, vaginal birth at all cost leads to a dangerous situation. The emergency environment of shoulder dystocia is not a preferable or safer stratagem.
It is granted that shoulder dystocia will happen at some point but avoidance, by employing cesarean delivery when it is indicated, is the greatest tool for addressing this very dangerous problem.
J. Michael Arnold, MD
Oconto Falls, Wisconsin
Another suggestion for shoulder dystociaMy senior partner taught me a technique that works well, although I do not know its name. After suprapubic and McRoberts maneuvers fail and the shoulders do not deliver with gentle downward guidance in one direction, I rotate the head 180° and try again. Usually this works. I have taught this technique to several midwives, and they swear by it.
Annette Fineberg, MD
Davis, California
Dr. Barbieri respondsI thank Drs. Arnold and Fineberg for sharing their perspective and experience with our readers. Dr. Arnold notes that recommending cesarean delivery in high-risk situations such as cases in which the mother has diabetes and the fetus is macrosomic would surely reduce the frequency of shoulder dystocia. I respect Dr. Fineberg’s recommendation, based on extensive clinical experience, that by rotating the fetal head the shoulder dystocia may be resolved. My concern with this technique is that the torque transmitted to the neck might cause fetal damage. I think that rotating the shoulders (Rubin or Wood maneuver) would be less likely to result in fetal injury.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The latest treatments for urinary and fecal incontinence: Which hold water?
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
In this Article
- New OTC option for SUI
- Second-line OAB treatments
- Promising vaginal insert for fecal incontinence
2015 Update on pelvic floor dysfunction: Bladder pain syndrome
Interstitial cystitis (IC) is a debilitating disease that presents with a constellation of symptoms, including pain, urinary urgency, frequency, nocturia, and small voided volumes in the absence of other identifiable etiologies.1 The overall prevalence of IC among US women is between 2.7% and 6.5%—affecting approximately 3.3 to 7.9 million women2—and it results in substantial costs1,3 and impairments in health-related quality of life.4 Unfortunately, there is a lack of consensus on the pathophysiology and etiology of this prevalent and costly disorder. Thus, therapies are often empiric, with limited evidence and variable levels of improvement.5
There has been no clear evidence that bladder inflammation (cystitis) is involved in the etiology or pathophysiology of the condition. As a result, there has been a movement to rename it “bladder pain syndrome.” Current literature refers to the spectrum of symptoms as interstitial cystitis/bladder pain syndrome (IC/BPS).
Currently, the American Urological Association (AUA) defines IC/BPS as an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than 6 weeks’ duration, in the absence of infection or other identifiable causes.6 This is still a broad, clinical diagnosis that has significant overlap with other pain syndromes but allows for treatment to begin after a relatively short symptomatic period.7 Because gynecologists are frequently the main care providers for women, understanding the diagnosis and treatment options for IC/BPS is important to avoid delayed treatment in a difficult to diagnose population.
Recently, the AUA published an amendment to their 2011 management guidelines to provide direction to clinicians and patients regarding how to recognize IC/BPS, conduct valid diagnostic testing, and approach treatment with the goals of maximizing symptom control and patient quality of life.7
In this article, we review the AUA diagnostic and treatment algorithms and the results of recently published randomized trials comparing the efficacy of various treatment modalities for IC/BPS, including pentosoan polysulfate sodium (PPS; Elmiron, Janssen Pharmaceuticals, Titusville, New Jersey) and botulinum toxin (Botox, Allergan, Irvine, California) with hydrodistension.
- Anger JT, Zabihi N, Clemens JQ, Payne CK, Saigal CS, Rodriguez LV. Treatment choice, duration, and cost in patients with interstitial cystitis and painful bladder syndrome. Int Urogynecol J. 2011;22(4):395–400.
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–544.
- Payne CK, Joyce GF, Wise M, Clemens JQ; Urologic Diseases in America Project. Interstitial cystitis and painful bladder syndrome. J Urol. 2007;177(6):2042–2049.
- Nickel JC, Payne CK, Forrest J, Parsons CL, Wan GJ, Xiao X. The relationship among symptoms, sleep disturbances and quality of life in patients with interstitial cystitis. J Urol. 2009;181(6):2555–2561.
- Giannantoni A, Bini V, Dmochowski R, et al. Contemporary management of the painful bladder: a systematic review. Eur Urol. 2012;61(1):29–53.
- Hanno P, Dmochowski R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol Urodyn. 2009;28(4):274–286.
- Hanno PM, Erickson D, Moldwin R, Faraday MM; American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–1553.
- Hanno PM, Burks DA, Clemens JQ, et al; Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162–2170.
- Boudry G, Labat JJ, Riant T, et al. Validation of voiding diary for stratification of bladder pain syndrome according to the presence/absence of cystoscopic abnormalities: a two-centre prospective study. BJU Int. 2013;112(2):E164−168.
- O’Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarish-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(5A suppl):58–63.
- Nickel JC, Herschom S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo-controlled study. J Urol. 2015;193(3):857–862.
- Nickel JC, Barkin J, Forrest J, et al; Elmiron Study Group. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65(4):654–658.
- Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial. Contemp Clin Trials, 2012;33(1):184–196.
Interstitial cystitis (IC) is a debilitating disease that presents with a constellation of symptoms, including pain, urinary urgency, frequency, nocturia, and small voided volumes in the absence of other identifiable etiologies.1 The overall prevalence of IC among US women is between 2.7% and 6.5%—affecting approximately 3.3 to 7.9 million women2—and it results in substantial costs1,3 and impairments in health-related quality of life.4 Unfortunately, there is a lack of consensus on the pathophysiology and etiology of this prevalent and costly disorder. Thus, therapies are often empiric, with limited evidence and variable levels of improvement.5
There has been no clear evidence that bladder inflammation (cystitis) is involved in the etiology or pathophysiology of the condition. As a result, there has been a movement to rename it “bladder pain syndrome.” Current literature refers to the spectrum of symptoms as interstitial cystitis/bladder pain syndrome (IC/BPS).
Currently, the American Urological Association (AUA) defines IC/BPS as an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than 6 weeks’ duration, in the absence of infection or other identifiable causes.6 This is still a broad, clinical diagnosis that has significant overlap with other pain syndromes but allows for treatment to begin after a relatively short symptomatic period.7 Because gynecologists are frequently the main care providers for women, understanding the diagnosis and treatment options for IC/BPS is important to avoid delayed treatment in a difficult to diagnose population.
Recently, the AUA published an amendment to their 2011 management guidelines to provide direction to clinicians and patients regarding how to recognize IC/BPS, conduct valid diagnostic testing, and approach treatment with the goals of maximizing symptom control and patient quality of life.7
In this article, we review the AUA diagnostic and treatment algorithms and the results of recently published randomized trials comparing the efficacy of various treatment modalities for IC/BPS, including pentosoan polysulfate sodium (PPS; Elmiron, Janssen Pharmaceuticals, Titusville, New Jersey) and botulinum toxin (Botox, Allergan, Irvine, California) with hydrodistension.
Interstitial cystitis (IC) is a debilitating disease that presents with a constellation of symptoms, including pain, urinary urgency, frequency, nocturia, and small voided volumes in the absence of other identifiable etiologies.1 The overall prevalence of IC among US women is between 2.7% and 6.5%—affecting approximately 3.3 to 7.9 million women2—and it results in substantial costs1,3 and impairments in health-related quality of life.4 Unfortunately, there is a lack of consensus on the pathophysiology and etiology of this prevalent and costly disorder. Thus, therapies are often empiric, with limited evidence and variable levels of improvement.5
There has been no clear evidence that bladder inflammation (cystitis) is involved in the etiology or pathophysiology of the condition. As a result, there has been a movement to rename it “bladder pain syndrome.” Current literature refers to the spectrum of symptoms as interstitial cystitis/bladder pain syndrome (IC/BPS).
Currently, the American Urological Association (AUA) defines IC/BPS as an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than 6 weeks’ duration, in the absence of infection or other identifiable causes.6 This is still a broad, clinical diagnosis that has significant overlap with other pain syndromes but allows for treatment to begin after a relatively short symptomatic period.7 Because gynecologists are frequently the main care providers for women, understanding the diagnosis and treatment options for IC/BPS is important to avoid delayed treatment in a difficult to diagnose population.
Recently, the AUA published an amendment to their 2011 management guidelines to provide direction to clinicians and patients regarding how to recognize IC/BPS, conduct valid diagnostic testing, and approach treatment with the goals of maximizing symptom control and patient quality of life.7
In this article, we review the AUA diagnostic and treatment algorithms and the results of recently published randomized trials comparing the efficacy of various treatment modalities for IC/BPS, including pentosoan polysulfate sodium (PPS; Elmiron, Janssen Pharmaceuticals, Titusville, New Jersey) and botulinum toxin (Botox, Allergan, Irvine, California) with hydrodistension.
- Anger JT, Zabihi N, Clemens JQ, Payne CK, Saigal CS, Rodriguez LV. Treatment choice, duration, and cost in patients with interstitial cystitis and painful bladder syndrome. Int Urogynecol J. 2011;22(4):395–400.
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–544.
- Payne CK, Joyce GF, Wise M, Clemens JQ; Urologic Diseases in America Project. Interstitial cystitis and painful bladder syndrome. J Urol. 2007;177(6):2042–2049.
- Nickel JC, Payne CK, Forrest J, Parsons CL, Wan GJ, Xiao X. The relationship among symptoms, sleep disturbances and quality of life in patients with interstitial cystitis. J Urol. 2009;181(6):2555–2561.
- Giannantoni A, Bini V, Dmochowski R, et al. Contemporary management of the painful bladder: a systematic review. Eur Urol. 2012;61(1):29–53.
- Hanno P, Dmochowski R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol Urodyn. 2009;28(4):274–286.
- Hanno PM, Erickson D, Moldwin R, Faraday MM; American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–1553.
- Hanno PM, Burks DA, Clemens JQ, et al; Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162–2170.
- Boudry G, Labat JJ, Riant T, et al. Validation of voiding diary for stratification of bladder pain syndrome according to the presence/absence of cystoscopic abnormalities: a two-centre prospective study. BJU Int. 2013;112(2):E164−168.
- O’Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarish-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(5A suppl):58–63.
- Nickel JC, Herschom S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo-controlled study. J Urol. 2015;193(3):857–862.
- Nickel JC, Barkin J, Forrest J, et al; Elmiron Study Group. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65(4):654–658.
- Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial. Contemp Clin Trials, 2012;33(1):184–196.
- Anger JT, Zabihi N, Clemens JQ, Payne CK, Saigal CS, Rodriguez LV. Treatment choice, duration, and cost in patients with interstitial cystitis and painful bladder syndrome. Int Urogynecol J. 2011;22(4):395–400.
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–544.
- Payne CK, Joyce GF, Wise M, Clemens JQ; Urologic Diseases in America Project. Interstitial cystitis and painful bladder syndrome. J Urol. 2007;177(6):2042–2049.
- Nickel JC, Payne CK, Forrest J, Parsons CL, Wan GJ, Xiao X. The relationship among symptoms, sleep disturbances and quality of life in patients with interstitial cystitis. J Urol. 2009;181(6):2555–2561.
- Giannantoni A, Bini V, Dmochowski R, et al. Contemporary management of the painful bladder: a systematic review. Eur Urol. 2012;61(1):29–53.
- Hanno P, Dmochowski R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol Urodyn. 2009;28(4):274–286.
- Hanno PM, Erickson D, Moldwin R, Faraday MM; American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–1553.
- Hanno PM, Burks DA, Clemens JQ, et al; Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162–2170.
- Boudry G, Labat JJ, Riant T, et al. Validation of voiding diary for stratification of bladder pain syndrome according to the presence/absence of cystoscopic abnormalities: a two-centre prospective study. BJU Int. 2013;112(2):E164−168.
- O’Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarish-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(5A suppl):58–63.
- Nickel JC, Herschom S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo-controlled study. J Urol. 2015;193(3):857–862.
- Nickel JC, Barkin J, Forrest J, et al; Elmiron Study Group. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65(4):654–658.
- Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial. Contemp Clin Trials, 2012;33(1):184–196.
In this Article
- AUA diagnosis guidelines
- Treatment algorithm
- A new FDA-approved oral treatment option
Pubovaginal sling for stress urinary incontinence using autologous fascia lata
For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
This video is brought to you by ![]()