User login
‘They’re out to get me!’: Evaluating rational fears and bizarre delusions in paranoia
Even among healthy individuals, feelings of paranoia are not unusual. In modern psychiatry, we consider paranoia to be a pattern of unfounded thinking, centered on the fearful experience of perceived victimization or threat of intentional harm. This means that a patient with paranoia is, by nature, difficult to engage in treatment. A patient might perceive the clinician as attempting to mislead or manipulate him. A therapeutic alliance could require patience on the part of the clinician, creativity,1 and abandoning attempts at rational “therapeutic” persuasion. The severity of symptoms determines the approach.
In this article, we review the nature of paranoia and the continuum of syndromes to which it is a central feature, as well as treatment approaches.
Categorization and etiology
Until recently, clinicians considered “paranoid” to be a subtype of schizophrenia (Box2-7); in DSM-5 the limited diagnostic stability and reliability of the categorization rendered the distinction obsolete.8 There are several levels of severity of paranoia; this thought process can present in simple variations of normal fears and concerns or in severe forms, with highly organized delusional systems.
The etiology of paranoia is not clear. Over the years, it has been attributed to defense mechanisms of the ego, habitual fears from repetitive exposure, or irregular activity of the amygdala. It is possible that various types of paranoia could have different causes. Functional MRIs indicate that the amygdala is involved in anxiety and threat perception in both primates and humans.9
Rational fear vs paranoia
Under the right circumstances, anyone could sense that he (she) is being threatened. Such feelings are normal in occupied countries and nations at war, and are not pathologic in such contexts. Anxiety about potential danger and harassment under truly oppressive circumstances might be biologically ingrained and have value for survival. It is important to employ cultural sensitivity when distinguishing pathological and nonpathological paranoia because some immigrant populations might have increased prevalence rates but without a true mental illness.10
Perhaps the key to separating realistic fear from paranoia is the recognition of whether the environment is truly safe or hostile; sometimes this is not initially evident to the clinician. The first author (J.A.W.) experienced this when discovering that a patient who was thought to be paranoid was indeed being stalked by another patient.
Rapid social change makes sweeping explanations about the range of threats experienced by any one person of limited value. Persons living with serious and persistent mental illness experience stigma—harassment, abuse, disgrace—and, similar to victims of repeated sexual abuse and other violence, are not necessarily unreasonable in their inner experience of omnipresent threat. In addition, advances in surveillance technology, as well as the media proliferation of depictions of vulnerability and threat, can plant generalized doubt of historically trusted individuals and systems. Under conditions of severe social discrimination or life under a totalitarian regime, constant fear for safety and worry about the intentions of others is reasonable. We must remember that during the Cold War many people in Eastern Europe had legitimate concerns that their phones were tapped. There are still many places in the world where the fear of government or of one’s neighbors exists.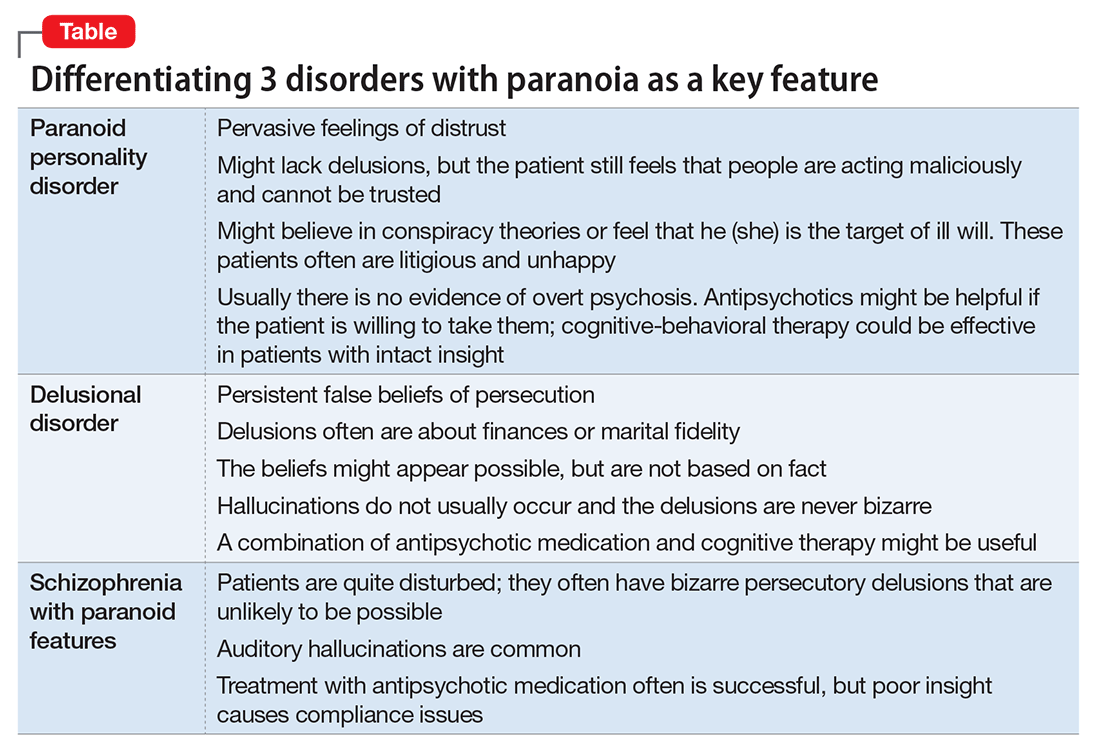
- paranoid personality disorder
- delusional disorder
- paranoia in schizophrenia (Table).
Paranoid personality disorder
The nature of any personality disorder is a long-standing psychological and behavioral pattern that differs significantly from the expectations of one’s culture. Such beliefs and behaviors typically are pervasive across most aspects of the individual’s interactions, and these enduring patterns of personality usually are evident by adolescence or young adulthood. Paranoid personality disorder is marked by pervasive distrust of others. Typical features include:
- suspicion about other people’s motives
- sensitivity to criticism
- keeping grudges against alleged offenders.8
The patient must have 4 of the following symptoms to confirm the diagnosis:
- suspicion of others and their motives
- reluctance to confide in others, due to lack of trust
- recurrent doubts about the fidelity of a significant other
- preoccupation with doubt regarding trusting others
- seeing threatening meanings behind benign remarks or events
- perception of attacks upon one’s character or reputation
- bears persistent grudges.8
Individuals with paranoid personality disorder tend to lead maladaptive lifestyles and might present as irritable, unpleasant, and emotionally guarded. Paranoid personality disorder is not a form of delusion, but is a pattern of habitual distrust of others.
The disorder generally is expressed verbally, and is seldom accompanied by hallucinations or unpredictable behavior. Distrust of others might result in social isolation and litigious behavior.8 Alternately, a patient with this disorder might not present for treatment until later in life after the loss of significant supporting factors, such as the death of parents or loss of steady employment. Examination of these older individuals is likely to reveal long-standing suspiciousness and distrust that previously was hidden by family members. For example, a 68-year-old woman might present saying that she can’t trust her daughter, but her recently deceased spouse would not let her discuss the topic outside of the home.
The etiology of paranoid personality disorder is unknown. Family studies suggest a possible a genetic connection to paranoia in schizophrenia.12 Others hypothesize that this dysfunction of personality might originate in early feelings of anxiety and low self-esteem, learned from a controlling, cruel, or sadistic parent; the patient then expects others to reject him (her) as the parent did.13,14 Such individuals might develop deep-seated distrust of others as a defense mechanism. Under stress, such as during a medical illness, patients could develop brief psychoses. Antipsychotic treatment might be useful in some cases of paranoid personality disorder, but should be limited.
Delusional disorder
Delusional disorder is a unique form of psychosis. Patients with delusional disorder might appear rational—as long as they are in independent roles—and their general functioning could go unnoticed. This could change when the delusions predominate their thoughts, or their delusional behavior is unacceptable in a structured environment. Such individuals often suffer from a highly specific delusion fixed on 1 topic. These delusions generally are the only psychotic feature. The most common theme is that of persecution. For example, a person firmly believes he is being followed by foreign agents or by a religious organization, which is blatantly untrue. Another common theme is infidelity.
Paranoia in delusional disorder is about something that is not actually occurring, but could.3 In other words, the delusion is not necessarily bizarre. The patient may have no evidence or could invent “evidence,” yet remain completely resistant to any logical argument against his belief system. In many situations, individuals with delusional disorder function normally in society, until the delusion becomes severe enough to prompt clinical attention.
Paranoia in schizophrenia
In patients with schizophrenia with paranoia, the typical symptoms of disorganization and disturbed affect are less prominent. The condition develops in young adulthood, but could start at any age. Its course typically is chronic and requires psychiatric treatment; the patient may require hospital care.
Although patients with delusional disorder and those with schizophrenia both have delusions, the delusions of the latter typically are bizarre and unlikely to be possible. For example, the patient might believe that her body has been replaced with the inner workings of an alien being or a robot. The paranoid delusions of persons with delusional disorder are much more mundane and could be plausible. Karl Jaspers, a clinician and researcher in the early 20th century, separated delusional disorder from paranoid schizophrenia by noting that the former could be “understandable, even if untrue” while the latter was “not within the realm of understandability.”5
A patient with schizophrenia with paranoid delusions usually experiences auditory hallucinations, such as voices threatening persecution or harm. When predominant, patients could be aroused by these fears and can be dangerous to others.2,4,5
Other presentations of paranoia
Paranoia can occur in affective disorders as well.13 Although the cause is only now being understood, clinicians have put forth theories for many years. A depressed person might suffer from excessive guilt and feel that he deserves to be persecuted, while a manic patient might think she is being persecuted for her greatness. In the past, response to electroconvulsive therapy was used to distinguish affective paranoia from other types.2
Paranoia in organic states
Substance use. Psychostimulants, which are known for their motor activity and arousal enhancing properties, as well as the potential for abuse and other negative consequences, could lead to acute paranoid states in susceptible individuals.15-17 In addition, tetrahydrocannabinol, the active chemical in Cannabis, can cause acute psychotic symptoms, such as paranoia,18,19 in a dose-dependent manner. A growing body of evidence suggests that a combination of Cannabis use with a genetic predisposition to psychosis may put some individuals at high risk of decompensation.19 Of growing concern is the evidence that synthetic cannabinoids, which are among the most commonly used new psychoactive substances, could be associated with psychosis, including paranoia.20
Dementia. Persons with dementia often are paranoid. In geriatric patients with dementia, a delusion of thievery is common. When a person has misplaced objects and can’t remember where, the “default” cognition is that someone has taken them. This confabulation may progress to a persistent paranoia and can be draining on caregivers.
Treating paranoia
A patient with paranoia usually has poor insight and cannot be reasoned with. Such individuals are quick to incorporate others into their delusional theories and easily develop notions of conspiracy. In acute psychosis, when the patient presents with fixed beliefs that are not amenable to reality orientation, and poses a threat to his well-being or that of others, alleviating underlying fear and anxiety is the first priority. Swift pharmacologic measures are required to decrease the patient’s underlying anxiety or anger, before you can try to earn his trust.
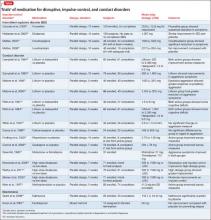
Psychopharmacologic interventions should be specific to the diagnosis. Antipsychotic medications generally will help decrease most paranoia, but affective syndromes usually require lithium or divalproex for best results.14,21
Develop a therapeutic relationship. The clinician must approach the patient in a practical and straightforward manner, and should not expect a quick therapeutic alliance. Transference and countertransference develop easily in the context of paranoia. Focus on behaviors that are problematic for the patient or the milieu, such as to ensure a safe environment. The patient needs to be aware of how he could come across to others. Clear feedback about behavior, such as “I cannot really listen to you when you’re yelling,” may be effective. It might be unwise to confront delusional paranoia in an agitated patient. Honesty and respect must continue in all communications to build trust. During assessment of a paranoid individual, evaluate the level of dangerousness. Ask your patient if he feels like acting on his beliefs or harming the people that are the targets of his paranoia.
As the patient begins to manage his anxiety and fear, you can develop a therapeutic alliance. The goals of treatment need be those of the patient—such as staying out of the hospital, or behaving in a manner that is required for employment. Over time, work toward growing the patient’s capacity for social interaction and productive activity. Insight might be elusive; however, some patients with paranoia can learn to take a detached view of their thoughts and emotions, and consider them impermanent events of the mind that make their lives difficult. Practice good judgment when aiming for recovery in a patient who does not have insight. For example, a patient can recognize that although there could be a microchip in his brain, he feels better when he takes medication.
In the case of paranoid personality disorder, treatment, as with most personality disorders, can be difficult. The patient might be unlikely to accept help and could distrust caregivers. Cognitive-behavioral therapy could be useful, if the patient can be engaged in the therapeutic process. Although it might be difficult to obtain enhanced insight, the patient could accept logical explanations for situations that provoke distrust. As long as anxiety and anger can be kept under control, the individual might learn the value of adopting the lessons of therapy. Pharmacological treatments are aimed at reducing the anxiety and anger experienced by the paranoid individual. Antipsychotics may be useful for short periods or during a crisis.14,21
The clinician must remain calm and reassuring when approaching an individual with paranoia, and not react to the projection of paranoid feelings from the patient. Respect for the patient can be conveyed without agreeing with delusions or bizarre thinking. The clinician must keep agreements and appointments with the client to prevent the erosion of trust. Paranoid conditions might respond slowly to pharmacological treatment, therefore establishing a consistent therapeutic relationship is essential.
1. Frank C. Delirium, consent to treatment, and Shakespeare. A geriatric experience. Can Fam Physician. 1999;45:875-876.
2. Hamilton M. Fish’s schizophrenia. Bristol, United Kingdom: John Wright and Sons; 1962.
3. Munro A. Delusional disorder. New York, NY: Cambridge University Press; 2000.
4. Kahlbaum K. Die gruppierung de psychischen krankheiten. Danzig, Germany: Verlag von A. W. Kafemann; 1853.
5. Kraepelin E. Manic depressive insanity and paranoia. Barclay RM, trans. New York, NY: Arno Press; 1976.
6. Bleuler E. Dementia praecox or the group of schizophrenias. Ainkia J, trans. New York, NY: International University Press; 1950.
7. Mayer W. Uber paraphrene psychosen. Zeitschrift fur die gesamte. Neurology und Psychiatrie. 1921;71:187-206.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Pinkham AE, Liu P, Lu H, et al. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172(8):784-792.
10. Sen P, Chowdhury AN. Culture, ethnicity and paranoia. Curr Psychiatry Rep. 2006;8(3):174-178.
11. Szasz TS. The manufacture of madness: a comparative study of the inquisition and the mental health movement. New York, NY: Harper and Row; 1970.
12. Schanda H, Berner P, Gabriel E, et al. The genetics of delusional psychosis. Schizophr Bull. 1983;9(4):563-570.
13. Levy B, Tsoy E, Brodt T, et al. Stigma, social anxiety and illness severity in bipolar disorder: implications for treatment. Ann Clin Psychiatry. 2015;27(1):55-64.
14. Benjamin LS. Interpersonal diagnosis and treatment of personality disorders. New York, NY: Gilford Press; 1993.
15. Busardo FP, Kyriakou C, Cipilloni L, et al. From clinical application to cognitive enhancement. Curr Neuropharmacol. 2015;13(2):281-295.
16. McKetin R, Gardner J, Baker AL, et al. Correlates of transient versus persistent psychotic symptoms among dependent methylamphetamine users. Psychiatry Res. 2016;238:166-171.
17. Djamshidian A. The neurobehavioral sequelae of psychostimulant abuse. Int Rev Neurobiol. 2015;120:161-177.
18. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401.
19. Bui QM, Simpson S, Nordstrom K. Psychiatric and medical management of marijuana intoxication in the emergency department. West J Emerg Med. 2015;16(3):414-417.
20. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234-243.
21. Lake CR. Hypothesis: grandiosity and guilt cause paranoia; paranoid schizophrenia is a psychotic mood disorder: a review. Schizophr Bull. 2008;34(6):1151-1162.
Even among healthy individuals, feelings of paranoia are not unusual. In modern psychiatry, we consider paranoia to be a pattern of unfounded thinking, centered on the fearful experience of perceived victimization or threat of intentional harm. This means that a patient with paranoia is, by nature, difficult to engage in treatment. A patient might perceive the clinician as attempting to mislead or manipulate him. A therapeutic alliance could require patience on the part of the clinician, creativity,1 and abandoning attempts at rational “therapeutic” persuasion. The severity of symptoms determines the approach.
In this article, we review the nature of paranoia and the continuum of syndromes to which it is a central feature, as well as treatment approaches.
Categorization and etiology
Until recently, clinicians considered “paranoid” to be a subtype of schizophrenia (Box2-7); in DSM-5 the limited diagnostic stability and reliability of the categorization rendered the distinction obsolete.8 There are several levels of severity of paranoia; this thought process can present in simple variations of normal fears and concerns or in severe forms, with highly organized delusional systems.
The etiology of paranoia is not clear. Over the years, it has been attributed to defense mechanisms of the ego, habitual fears from repetitive exposure, or irregular activity of the amygdala. It is possible that various types of paranoia could have different causes. Functional MRIs indicate that the amygdala is involved in anxiety and threat perception in both primates and humans.9
Rational fear vs paranoia
Under the right circumstances, anyone could sense that he (she) is being threatened. Such feelings are normal in occupied countries and nations at war, and are not pathologic in such contexts. Anxiety about potential danger and harassment under truly oppressive circumstances might be biologically ingrained and have value for survival. It is important to employ cultural sensitivity when distinguishing pathological and nonpathological paranoia because some immigrant populations might have increased prevalence rates but without a true mental illness.10
Perhaps the key to separating realistic fear from paranoia is the recognition of whether the environment is truly safe or hostile; sometimes this is not initially evident to the clinician. The first author (J.A.W.) experienced this when discovering that a patient who was thought to be paranoid was indeed being stalked by another patient.
Rapid social change makes sweeping explanations about the range of threats experienced by any one person of limited value. Persons living with serious and persistent mental illness experience stigma—harassment, abuse, disgrace—and, similar to victims of repeated sexual abuse and other violence, are not necessarily unreasonable in their inner experience of omnipresent threat. In addition, advances in surveillance technology, as well as the media proliferation of depictions of vulnerability and threat, can plant generalized doubt of historically trusted individuals and systems. Under conditions of severe social discrimination or life under a totalitarian regime, constant fear for safety and worry about the intentions of others is reasonable. We must remember that during the Cold War many people in Eastern Europe had legitimate concerns that their phones were tapped. There are still many places in the world where the fear of government or of one’s neighbors exists.
- paranoid personality disorder
- delusional disorder
- paranoia in schizophrenia (Table).
Paranoid personality disorder
The nature of any personality disorder is a long-standing psychological and behavioral pattern that differs significantly from the expectations of one’s culture. Such beliefs and behaviors typically are pervasive across most aspects of the individual’s interactions, and these enduring patterns of personality usually are evident by adolescence or young adulthood. Paranoid personality disorder is marked by pervasive distrust of others. Typical features include:
- suspicion about other people’s motives
- sensitivity to criticism
- keeping grudges against alleged offenders.8
The patient must have 4 of the following symptoms to confirm the diagnosis:
- suspicion of others and their motives
- reluctance to confide in others, due to lack of trust
- recurrent doubts about the fidelity of a significant other
- preoccupation with doubt regarding trusting others
- seeing threatening meanings behind benign remarks or events
- perception of attacks upon one’s character or reputation
- bears persistent grudges.8
Individuals with paranoid personality disorder tend to lead maladaptive lifestyles and might present as irritable, unpleasant, and emotionally guarded. Paranoid personality disorder is not a form of delusion, but is a pattern of habitual distrust of others.
The disorder generally is expressed verbally, and is seldom accompanied by hallucinations or unpredictable behavior. Distrust of others might result in social isolation and litigious behavior.8 Alternately, a patient with this disorder might not present for treatment until later in life after the loss of significant supporting factors, such as the death of parents or loss of steady employment. Examination of these older individuals is likely to reveal long-standing suspiciousness and distrust that previously was hidden by family members. For example, a 68-year-old woman might present saying that she can’t trust her daughter, but her recently deceased spouse would not let her discuss the topic outside of the home.
The etiology of paranoid personality disorder is unknown. Family studies suggest a possible a genetic connection to paranoia in schizophrenia.12 Others hypothesize that this dysfunction of personality might originate in early feelings of anxiety and low self-esteem, learned from a controlling, cruel, or sadistic parent; the patient then expects others to reject him (her) as the parent did.13,14 Such individuals might develop deep-seated distrust of others as a defense mechanism. Under stress, such as during a medical illness, patients could develop brief psychoses. Antipsychotic treatment might be useful in some cases of paranoid personality disorder, but should be limited.
Delusional disorder
Delusional disorder is a unique form of psychosis. Patients with delusional disorder might appear rational—as long as they are in independent roles—and their general functioning could go unnoticed. This could change when the delusions predominate their thoughts, or their delusional behavior is unacceptable in a structured environment. Such individuals often suffer from a highly specific delusion fixed on 1 topic. These delusions generally are the only psychotic feature. The most common theme is that of persecution. For example, a person firmly believes he is being followed by foreign agents or by a religious organization, which is blatantly untrue. Another common theme is infidelity.
Paranoia in delusional disorder is about something that is not actually occurring, but could.3 In other words, the delusion is not necessarily bizarre. The patient may have no evidence or could invent “evidence,” yet remain completely resistant to any logical argument against his belief system. In many situations, individuals with delusional disorder function normally in society, until the delusion becomes severe enough to prompt clinical attention.
Paranoia in schizophrenia
In patients with schizophrenia with paranoia, the typical symptoms of disorganization and disturbed affect are less prominent. The condition develops in young adulthood, but could start at any age. Its course typically is chronic and requires psychiatric treatment; the patient may require hospital care.
Although patients with delusional disorder and those with schizophrenia both have delusions, the delusions of the latter typically are bizarre and unlikely to be possible. For example, the patient might believe that her body has been replaced with the inner workings of an alien being or a robot. The paranoid delusions of persons with delusional disorder are much more mundane and could be plausible. Karl Jaspers, a clinician and researcher in the early 20th century, separated delusional disorder from paranoid schizophrenia by noting that the former could be “understandable, even if untrue” while the latter was “not within the realm of understandability.”5
A patient with schizophrenia with paranoid delusions usually experiences auditory hallucinations, such as voices threatening persecution or harm. When predominant, patients could be aroused by these fears and can be dangerous to others.2,4,5
Other presentations of paranoia
Paranoia can occur in affective disorders as well.13 Although the cause is only now being understood, clinicians have put forth theories for many years. A depressed person might suffer from excessive guilt and feel that he deserves to be persecuted, while a manic patient might think she is being persecuted for her greatness. In the past, response to electroconvulsive therapy was used to distinguish affective paranoia from other types.2
Paranoia in organic states
Substance use. Psychostimulants, which are known for their motor activity and arousal enhancing properties, as well as the potential for abuse and other negative consequences, could lead to acute paranoid states in susceptible individuals.15-17 In addition, tetrahydrocannabinol, the active chemical in Cannabis, can cause acute psychotic symptoms, such as paranoia,18,19 in a dose-dependent manner. A growing body of evidence suggests that a combination of Cannabis use with a genetic predisposition to psychosis may put some individuals at high risk of decompensation.19 Of growing concern is the evidence that synthetic cannabinoids, which are among the most commonly used new psychoactive substances, could be associated with psychosis, including paranoia.20
Dementia. Persons with dementia often are paranoid. In geriatric patients with dementia, a delusion of thievery is common. When a person has misplaced objects and can’t remember where, the “default” cognition is that someone has taken them. This confabulation may progress to a persistent paranoia and can be draining on caregivers.
Treating paranoia
A patient with paranoia usually has poor insight and cannot be reasoned with. Such individuals are quick to incorporate others into their delusional theories and easily develop notions of conspiracy. In acute psychosis, when the patient presents with fixed beliefs that are not amenable to reality orientation, and poses a threat to his well-being or that of others, alleviating underlying fear and anxiety is the first priority. Swift pharmacologic measures are required to decrease the patient’s underlying anxiety or anger, before you can try to earn his trust.
Psychopharmacologic interventions should be specific to the diagnosis. Antipsychotic medications generally will help decrease most paranoia, but affective syndromes usually require lithium or divalproex for best results.14,21
Develop a therapeutic relationship. The clinician must approach the patient in a practical and straightforward manner, and should not expect a quick therapeutic alliance. Transference and countertransference develop easily in the context of paranoia. Focus on behaviors that are problematic for the patient or the milieu, such as to ensure a safe environment. The patient needs to be aware of how he could come across to others. Clear feedback about behavior, such as “I cannot really listen to you when you’re yelling,” may be effective. It might be unwise to confront delusional paranoia in an agitated patient. Honesty and respect must continue in all communications to build trust. During assessment of a paranoid individual, evaluate the level of dangerousness. Ask your patient if he feels like acting on his beliefs or harming the people that are the targets of his paranoia.
As the patient begins to manage his anxiety and fear, you can develop a therapeutic alliance. The goals of treatment need be those of the patient—such as staying out of the hospital, or behaving in a manner that is required for employment. Over time, work toward growing the patient’s capacity for social interaction and productive activity. Insight might be elusive; however, some patients with paranoia can learn to take a detached view of their thoughts and emotions, and consider them impermanent events of the mind that make their lives difficult. Practice good judgment when aiming for recovery in a patient who does not have insight. For example, a patient can recognize that although there could be a microchip in his brain, he feels better when he takes medication.
In the case of paranoid personality disorder, treatment, as with most personality disorders, can be difficult. The patient might be unlikely to accept help and could distrust caregivers. Cognitive-behavioral therapy could be useful, if the patient can be engaged in the therapeutic process. Although it might be difficult to obtain enhanced insight, the patient could accept logical explanations for situations that provoke distrust. As long as anxiety and anger can be kept under control, the individual might learn the value of adopting the lessons of therapy. Pharmacological treatments are aimed at reducing the anxiety and anger experienced by the paranoid individual. Antipsychotics may be useful for short periods or during a crisis.14,21
The clinician must remain calm and reassuring when approaching an individual with paranoia, and not react to the projection of paranoid feelings from the patient. Respect for the patient can be conveyed without agreeing with delusions or bizarre thinking. The clinician must keep agreements and appointments with the client to prevent the erosion of trust. Paranoid conditions might respond slowly to pharmacological treatment, therefore establishing a consistent therapeutic relationship is essential.
Even among healthy individuals, feelings of paranoia are not unusual. In modern psychiatry, we consider paranoia to be a pattern of unfounded thinking, centered on the fearful experience of perceived victimization or threat of intentional harm. This means that a patient with paranoia is, by nature, difficult to engage in treatment. A patient might perceive the clinician as attempting to mislead or manipulate him. A therapeutic alliance could require patience on the part of the clinician, creativity,1 and abandoning attempts at rational “therapeutic” persuasion. The severity of symptoms determines the approach.
In this article, we review the nature of paranoia and the continuum of syndromes to which it is a central feature, as well as treatment approaches.
Categorization and etiology
Until recently, clinicians considered “paranoid” to be a subtype of schizophrenia (Box2-7); in DSM-5 the limited diagnostic stability and reliability of the categorization rendered the distinction obsolete.8 There are several levels of severity of paranoia; this thought process can present in simple variations of normal fears and concerns or in severe forms, with highly organized delusional systems.
The etiology of paranoia is not clear. Over the years, it has been attributed to defense mechanisms of the ego, habitual fears from repetitive exposure, or irregular activity of the amygdala. It is possible that various types of paranoia could have different causes. Functional MRIs indicate that the amygdala is involved in anxiety and threat perception in both primates and humans.9
Rational fear vs paranoia
Under the right circumstances, anyone could sense that he (she) is being threatened. Such feelings are normal in occupied countries and nations at war, and are not pathologic in such contexts. Anxiety about potential danger and harassment under truly oppressive circumstances might be biologically ingrained and have value for survival. It is important to employ cultural sensitivity when distinguishing pathological and nonpathological paranoia because some immigrant populations might have increased prevalence rates but without a true mental illness.10
Perhaps the key to separating realistic fear from paranoia is the recognition of whether the environment is truly safe or hostile; sometimes this is not initially evident to the clinician. The first author (J.A.W.) experienced this when discovering that a patient who was thought to be paranoid was indeed being stalked by another patient.
Rapid social change makes sweeping explanations about the range of threats experienced by any one person of limited value. Persons living with serious and persistent mental illness experience stigma—harassment, abuse, disgrace—and, similar to victims of repeated sexual abuse and other violence, are not necessarily unreasonable in their inner experience of omnipresent threat. In addition, advances in surveillance technology, as well as the media proliferation of depictions of vulnerability and threat, can plant generalized doubt of historically trusted individuals and systems. Under conditions of severe social discrimination or life under a totalitarian regime, constant fear for safety and worry about the intentions of others is reasonable. We must remember that during the Cold War many people in Eastern Europe had legitimate concerns that their phones were tapped. There are still many places in the world where the fear of government or of one’s neighbors exists.
- paranoid personality disorder
- delusional disorder
- paranoia in schizophrenia (Table).
Paranoid personality disorder
The nature of any personality disorder is a long-standing psychological and behavioral pattern that differs significantly from the expectations of one’s culture. Such beliefs and behaviors typically are pervasive across most aspects of the individual’s interactions, and these enduring patterns of personality usually are evident by adolescence or young adulthood. Paranoid personality disorder is marked by pervasive distrust of others. Typical features include:
- suspicion about other people’s motives
- sensitivity to criticism
- keeping grudges against alleged offenders.8
The patient must have 4 of the following symptoms to confirm the diagnosis:
- suspicion of others and their motives
- reluctance to confide in others, due to lack of trust
- recurrent doubts about the fidelity of a significant other
- preoccupation with doubt regarding trusting others
- seeing threatening meanings behind benign remarks or events
- perception of attacks upon one’s character or reputation
- bears persistent grudges.8
Individuals with paranoid personality disorder tend to lead maladaptive lifestyles and might present as irritable, unpleasant, and emotionally guarded. Paranoid personality disorder is not a form of delusion, but is a pattern of habitual distrust of others.
The disorder generally is expressed verbally, and is seldom accompanied by hallucinations or unpredictable behavior. Distrust of others might result in social isolation and litigious behavior.8 Alternately, a patient with this disorder might not present for treatment until later in life after the loss of significant supporting factors, such as the death of parents or loss of steady employment. Examination of these older individuals is likely to reveal long-standing suspiciousness and distrust that previously was hidden by family members. For example, a 68-year-old woman might present saying that she can’t trust her daughter, but her recently deceased spouse would not let her discuss the topic outside of the home.
The etiology of paranoid personality disorder is unknown. Family studies suggest a possible a genetic connection to paranoia in schizophrenia.12 Others hypothesize that this dysfunction of personality might originate in early feelings of anxiety and low self-esteem, learned from a controlling, cruel, or sadistic parent; the patient then expects others to reject him (her) as the parent did.13,14 Such individuals might develop deep-seated distrust of others as a defense mechanism. Under stress, such as during a medical illness, patients could develop brief psychoses. Antipsychotic treatment might be useful in some cases of paranoid personality disorder, but should be limited.
Delusional disorder
Delusional disorder is a unique form of psychosis. Patients with delusional disorder might appear rational—as long as they are in independent roles—and their general functioning could go unnoticed. This could change when the delusions predominate their thoughts, or their delusional behavior is unacceptable in a structured environment. Such individuals often suffer from a highly specific delusion fixed on 1 topic. These delusions generally are the only psychotic feature. The most common theme is that of persecution. For example, a person firmly believes he is being followed by foreign agents or by a religious organization, which is blatantly untrue. Another common theme is infidelity.
Paranoia in delusional disorder is about something that is not actually occurring, but could.3 In other words, the delusion is not necessarily bizarre. The patient may have no evidence or could invent “evidence,” yet remain completely resistant to any logical argument against his belief system. In many situations, individuals with delusional disorder function normally in society, until the delusion becomes severe enough to prompt clinical attention.
Paranoia in schizophrenia
In patients with schizophrenia with paranoia, the typical symptoms of disorganization and disturbed affect are less prominent. The condition develops in young adulthood, but could start at any age. Its course typically is chronic and requires psychiatric treatment; the patient may require hospital care.
Although patients with delusional disorder and those with schizophrenia both have delusions, the delusions of the latter typically are bizarre and unlikely to be possible. For example, the patient might believe that her body has been replaced with the inner workings of an alien being or a robot. The paranoid delusions of persons with delusional disorder are much more mundane and could be plausible. Karl Jaspers, a clinician and researcher in the early 20th century, separated delusional disorder from paranoid schizophrenia by noting that the former could be “understandable, even if untrue” while the latter was “not within the realm of understandability.”5
A patient with schizophrenia with paranoid delusions usually experiences auditory hallucinations, such as voices threatening persecution or harm. When predominant, patients could be aroused by these fears and can be dangerous to others.2,4,5
Other presentations of paranoia
Paranoia can occur in affective disorders as well.13 Although the cause is only now being understood, clinicians have put forth theories for many years. A depressed person might suffer from excessive guilt and feel that he deserves to be persecuted, while a manic patient might think she is being persecuted for her greatness. In the past, response to electroconvulsive therapy was used to distinguish affective paranoia from other types.2
Paranoia in organic states
Substance use. Psychostimulants, which are known for their motor activity and arousal enhancing properties, as well as the potential for abuse and other negative consequences, could lead to acute paranoid states in susceptible individuals.15-17 In addition, tetrahydrocannabinol, the active chemical in Cannabis, can cause acute psychotic symptoms, such as paranoia,18,19 in a dose-dependent manner. A growing body of evidence suggests that a combination of Cannabis use with a genetic predisposition to psychosis may put some individuals at high risk of decompensation.19 Of growing concern is the evidence that synthetic cannabinoids, which are among the most commonly used new psychoactive substances, could be associated with psychosis, including paranoia.20
Dementia. Persons with dementia often are paranoid. In geriatric patients with dementia, a delusion of thievery is common. When a person has misplaced objects and can’t remember where, the “default” cognition is that someone has taken them. This confabulation may progress to a persistent paranoia and can be draining on caregivers.
Treating paranoia
A patient with paranoia usually has poor insight and cannot be reasoned with. Such individuals are quick to incorporate others into their delusional theories and easily develop notions of conspiracy. In acute psychosis, when the patient presents with fixed beliefs that are not amenable to reality orientation, and poses a threat to his well-being or that of others, alleviating underlying fear and anxiety is the first priority. Swift pharmacologic measures are required to decrease the patient’s underlying anxiety or anger, before you can try to earn his trust.
Psychopharmacologic interventions should be specific to the diagnosis. Antipsychotic medications generally will help decrease most paranoia, but affective syndromes usually require lithium or divalproex for best results.14,21
Develop a therapeutic relationship. The clinician must approach the patient in a practical and straightforward manner, and should not expect a quick therapeutic alliance. Transference and countertransference develop easily in the context of paranoia. Focus on behaviors that are problematic for the patient or the milieu, such as to ensure a safe environment. The patient needs to be aware of how he could come across to others. Clear feedback about behavior, such as “I cannot really listen to you when you’re yelling,” may be effective. It might be unwise to confront delusional paranoia in an agitated patient. Honesty and respect must continue in all communications to build trust. During assessment of a paranoid individual, evaluate the level of dangerousness. Ask your patient if he feels like acting on his beliefs or harming the people that are the targets of his paranoia.
As the patient begins to manage his anxiety and fear, you can develop a therapeutic alliance. The goals of treatment need be those of the patient—such as staying out of the hospital, or behaving in a manner that is required for employment. Over time, work toward growing the patient’s capacity for social interaction and productive activity. Insight might be elusive; however, some patients with paranoia can learn to take a detached view of their thoughts and emotions, and consider them impermanent events of the mind that make their lives difficult. Practice good judgment when aiming for recovery in a patient who does not have insight. For example, a patient can recognize that although there could be a microchip in his brain, he feels better when he takes medication.
In the case of paranoid personality disorder, treatment, as with most personality disorders, can be difficult. The patient might be unlikely to accept help and could distrust caregivers. Cognitive-behavioral therapy could be useful, if the patient can be engaged in the therapeutic process. Although it might be difficult to obtain enhanced insight, the patient could accept logical explanations for situations that provoke distrust. As long as anxiety and anger can be kept under control, the individual might learn the value of adopting the lessons of therapy. Pharmacological treatments are aimed at reducing the anxiety and anger experienced by the paranoid individual. Antipsychotics may be useful for short periods or during a crisis.14,21
The clinician must remain calm and reassuring when approaching an individual with paranoia, and not react to the projection of paranoid feelings from the patient. Respect for the patient can be conveyed without agreeing with delusions or bizarre thinking. The clinician must keep agreements and appointments with the client to prevent the erosion of trust. Paranoid conditions might respond slowly to pharmacological treatment, therefore establishing a consistent therapeutic relationship is essential.
1. Frank C. Delirium, consent to treatment, and Shakespeare. A geriatric experience. Can Fam Physician. 1999;45:875-876.
2. Hamilton M. Fish’s schizophrenia. Bristol, United Kingdom: John Wright and Sons; 1962.
3. Munro A. Delusional disorder. New York, NY: Cambridge University Press; 2000.
4. Kahlbaum K. Die gruppierung de psychischen krankheiten. Danzig, Germany: Verlag von A. W. Kafemann; 1853.
5. Kraepelin E. Manic depressive insanity and paranoia. Barclay RM, trans. New York, NY: Arno Press; 1976.
6. Bleuler E. Dementia praecox or the group of schizophrenias. Ainkia J, trans. New York, NY: International University Press; 1950.
7. Mayer W. Uber paraphrene psychosen. Zeitschrift fur die gesamte. Neurology und Psychiatrie. 1921;71:187-206.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Pinkham AE, Liu P, Lu H, et al. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172(8):784-792.
10. Sen P, Chowdhury AN. Culture, ethnicity and paranoia. Curr Psychiatry Rep. 2006;8(3):174-178.
11. Szasz TS. The manufacture of madness: a comparative study of the inquisition and the mental health movement. New York, NY: Harper and Row; 1970.
12. Schanda H, Berner P, Gabriel E, et al. The genetics of delusional psychosis. Schizophr Bull. 1983;9(4):563-570.
13. Levy B, Tsoy E, Brodt T, et al. Stigma, social anxiety and illness severity in bipolar disorder: implications for treatment. Ann Clin Psychiatry. 2015;27(1):55-64.
14. Benjamin LS. Interpersonal diagnosis and treatment of personality disorders. New York, NY: Gilford Press; 1993.
15. Busardo FP, Kyriakou C, Cipilloni L, et al. From clinical application to cognitive enhancement. Curr Neuropharmacol. 2015;13(2):281-295.
16. McKetin R, Gardner J, Baker AL, et al. Correlates of transient versus persistent psychotic symptoms among dependent methylamphetamine users. Psychiatry Res. 2016;238:166-171.
17. Djamshidian A. The neurobehavioral sequelae of psychostimulant abuse. Int Rev Neurobiol. 2015;120:161-177.
18. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401.
19. Bui QM, Simpson S, Nordstrom K. Psychiatric and medical management of marijuana intoxication in the emergency department. West J Emerg Med. 2015;16(3):414-417.
20. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234-243.
21. Lake CR. Hypothesis: grandiosity and guilt cause paranoia; paranoid schizophrenia is a psychotic mood disorder: a review. Schizophr Bull. 2008;34(6):1151-1162.
1. Frank C. Delirium, consent to treatment, and Shakespeare. A geriatric experience. Can Fam Physician. 1999;45:875-876.
2. Hamilton M. Fish’s schizophrenia. Bristol, United Kingdom: John Wright and Sons; 1962.
3. Munro A. Delusional disorder. New York, NY: Cambridge University Press; 2000.
4. Kahlbaum K. Die gruppierung de psychischen krankheiten. Danzig, Germany: Verlag von A. W. Kafemann; 1853.
5. Kraepelin E. Manic depressive insanity and paranoia. Barclay RM, trans. New York, NY: Arno Press; 1976.
6. Bleuler E. Dementia praecox or the group of schizophrenias. Ainkia J, trans. New York, NY: International University Press; 1950.
7. Mayer W. Uber paraphrene psychosen. Zeitschrift fur die gesamte. Neurology und Psychiatrie. 1921;71:187-206.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Pinkham AE, Liu P, Lu H, et al. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172(8):784-792.
10. Sen P, Chowdhury AN. Culture, ethnicity and paranoia. Curr Psychiatry Rep. 2006;8(3):174-178.
11. Szasz TS. The manufacture of madness: a comparative study of the inquisition and the mental health movement. New York, NY: Harper and Row; 1970.
12. Schanda H, Berner P, Gabriel E, et al. The genetics of delusional psychosis. Schizophr Bull. 1983;9(4):563-570.
13. Levy B, Tsoy E, Brodt T, et al. Stigma, social anxiety and illness severity in bipolar disorder: implications for treatment. Ann Clin Psychiatry. 2015;27(1):55-64.
14. Benjamin LS. Interpersonal diagnosis and treatment of personality disorders. New York, NY: Gilford Press; 1993.
15. Busardo FP, Kyriakou C, Cipilloni L, et al. From clinical application to cognitive enhancement. Curr Neuropharmacol. 2015;13(2):281-295.
16. McKetin R, Gardner J, Baker AL, et al. Correlates of transient versus persistent psychotic symptoms among dependent methylamphetamine users. Psychiatry Res. 2016;238:166-171.
17. Djamshidian A. The neurobehavioral sequelae of psychostimulant abuse. Int Rev Neurobiol. 2015;120:161-177.
18. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401.
19. Bui QM, Simpson S, Nordstrom K. Psychiatric and medical management of marijuana intoxication in the emergency department. West J Emerg Med. 2015;16(3):414-417.
20. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234-243.
21. Lake CR. Hypothesis: grandiosity and guilt cause paranoia; paranoid schizophrenia is a psychotic mood disorder: a review. Schizophr Bull. 2008;34(6):1151-1162.
Managing borderline personality disorder
What do >700 letters to a mass murderer tell us about the people who wrote them?
Little is known about people who write to criminals incarcerated for a violent crime. However, existence of Web sites such as WriteAPrisoner.com, Meet-An-Inmate.com, and PrisonPenPals.com suggests some appetite among the public for corresponding with the incarcerated. Writers of letters might be drawn to the “bad boy” image of prisoners. Furthermore, much has been written of the willingness of some battered women to remain in an abusive domestic relationship, leading them to correspond with their abusers even after those abusers are incarcerated.1,2
To our knowledge, no examination of letters written to a mass murderer has been published. Therefore, we categorized and analyzed 784 letters sent to a high-profile male mass murderer whose crime was committed during the past decade. Here is a description of the study and what we found, as well as discussion of how our findings might offer utility in a psychiatric practice.
Goals of the study
We hypothesized that a large percentage of those letters could be classified as “Romantic,” given the lay perception that it is women who write to mass murderers. We also sought to evaluate follow-up letters sent by these writers to test the assumption that their individual goals would be constant over time.
We performed this study in the hope that the research could assist psychiatric practitioners in treating patients who seek to associate with a violent person (see “Treatment considerations,”). We thought it might be helpful for practitioners to get a better understanding of the nature of people who write to a violent offender or express a desire to do so.
Methods of study
Two authors (R.S.J. and D.P.G.) evaluated 819 letters that had been written by non-incarcerated, non-family adults to 1 mass murderer. The initial letter and follow-up letters written by each unique writer (n = 333) were categorized as follows:
• state or country from which the letter was sent
• age
• sex
• number of letters sent by each writer
• whether a photograph was enclosed
• whether additional items were enclosed (eg, gifts, drawings)
• whether the letter was rejected by prison authorities
• the writer’s purpose.
The study was approved by the institutional review board of Baylor College of Medicine.
Letters were assigned to 1 of 5 categories:
Acquaintance letters sought ongoing correspondence relationship with the murderer. They focused largely on conveying information about the writer.
Show of support letters also sought an ongoing correspondence relationship with the murderer, but instead focused on him, not the writer.
Romance letters used words that conveyed romantic or non-platonic affection.
Spiritual letters gave advice to the murderer with a religious tone.
Words of wisdom letters offered advice but lacked a religious tone.
Given the nonstandardized nature of categorization and the lack of a formal questionnaire, we were unable to perform an exploratory factor analysis on our categorizations. Inter-rater reliability of letter categorization was 0.79.
Results: Writer profiles, purpose for writing
In all, we reviewed 819 letters:
• Thirty-five letters were excluded because they were written by family members, children, or other prisoners
• Of the remaining 784 letters, there were 333 unique writers
• Two-hundred sixty letters were written by women, 61 by men; 2 were co-written by both sexes; sex could not be determined for 10.
Women were more likely than men to write a letter (P = .014) and to write ≥3 letters (P = .001). The age of the writer was determined for 117 (35.1%) letters; mean age was 27.8 (± 8.9) years (range, 18 to 59 years).
The purpose of the letters differed by sex (P < .001) but not by the writer’s age (P = .058). Women were more likely than men to write letters categorized as “Acquaintance,” “Romance,” and “Show of support”; in contrast, men were more likely than women to write a letter categorized as “Spiritual” (Table 1). Approximately 95% of letters were handwritten. Letters averaged 3 pages (range, 1 to 16 pages).
Two-hundred sixteen writers wrote a single letter; 53 wrote 2 letters; 18 wrote 3 letters; 11 wrote 4 letters; 30 wrote 5 to 10 letters; and 9 wrote 11 to 43 letters. The purpose of follow-up letters was associated with the age of the writer (P < .001) and with the writer’s sex (P < .001). Women were more likely to write “Show of support” and “Romance” follow-up letters; men were more likely to write “Spiritual” follow-up letters (Table 2).
Results suggested that the purpose of the initial letter was a reasonable predictor of the purpose of follow-up letters (P < .001) (Table 3). The murderer never responded to any letters. Letters were most often written from his state of incarceration; next, from contiguous states; then, from non-contiguous states; and, last, from international locations (P < .001).
Of the initial letters from writers who wrote ≥10, 60% were categorized as “Acquaintance” and 20% as “Romance.” The writer who wrote the most letters (43) moved during the course of her letter-writing to live in the same state as the murderer; she stated in her letters that she did so to be closer to him and to be able to attend his court hearings. Four other writers, each of whom wrote >5 letters, stated that they had traveled to the murderer’s state of incarceration to attend some of his hearings in person.
Composite examples of more common categories of letters
Names and other pertinent identifying information have been changed.
Acquaintance. Hi, Steve. I’ve been following your case and just wanted to write you so that maybe we could be friends or keep in touch since you’re probably pretty bored. I’m a 27-year-old college student studying marketing and working at Applebee’s as a waitress (for now) until I can land my dream job. I’ve enclosed a picture of me and my dachshund along with a photo of my favorite beach in the world. Write me back if you want. Jenny.
Show of support. Steve: I’ve been really worried about you since first seeing you on TV. You look different lately and I hope they’re treating you OK and feeding you decent food. In case they’re not, I’ve enclosed a little something to buy yourself a treat. Just know that there are many of us that care about you and are really pulling for you to be strong in this tough situation you’re in. Yours truly, Karen.
Romance. Dearest Steven: My mind has been filled with thoughts of you and of us since I last saw you in my dreams! Be strong, because you are going to beat this once they understand that you are not responsible for what happened! Don’t you see, sweetie, the system failed you, and now you’re caught up in something that you will soon overcome. When I think of the day that you get released, and how we’ll be able to settle down somewhere together, it gets me incredibly excited. You and I are meant to be together, because I understand you and can help you get better. I love you, Steven! Please write me back so that I know we’re on the same page about our plans for the future. Love, ♥ Your sweetie, Rachel.
Spiritual. Dear Child of God: The Lord has a plan for you. I know that things right now might be confusing, and you’re in a black place, but He is there right beside you. If you need some reading materials to give you comfort, just let me know and I can get a Bible to you along with some other books to give you solace and strengthen your walk with Him. God forgives you and he loves you so much! Much love in Christ, Mary.
Discussion
Given that the mass murderer in this study was a young man, it is not surprising that 78% of writers of initial letters were women. However, it is interesting that, among women’s initial letters, 44% were “Acquaintance” letters and only 15% were categorized as “Romance.”
Given the severity of the murderer’s crime, it is remarkable that he received only 1 “Hate mail” letter.
Initial “Spiritual” letters were more likely to be followed by letters of the same category than any other category; “Romance” letters were a close second. This demonstrates the consistent efforts of writers in these 2 categories. Highly persistent writers (≥10 letters) were most likely to fall into “Acquaintance” and “Romance” categories. The persistence of these writers is remarkable, in view of the fact that none of their letters were answered. We hypothesize that the killer did not reply because he had no interest in correspondence.
Similarities to stalking. Given that 9 writers wrote >10 letters each and 2 wrote >20 each, elements of their behavior are not unlike what is seen in stalkers.3 Consistent with the stalking literature and Mullen et al4 stalker typology, many writers in this study appeared to seek intimacy with the perpetrator through “Romance” or “Show of support” letters, and might be akin to Mullen’s so-called intimacy-seeking stalker. Such stalkers’ behavior arises out of loneliness, with a strong desire for a relationship with the target; a significant percentage of such stalkers suffer a delusional disorder.
Mullen’s so-called incompetent suitor stalker is similar to the intimacy-seeking type but, instead, has an interest in a short-term relationship and is far less persistent in his (her) stalking behavior4; this type might apply to the writers in this study who wrote >1 but <10 letters.
Two additional observations also are notable when trying to characterize people who write letters: (1) A high percentage of people who stalk a celebrity suffer a psychotic disorder5,6; (2) 4 letter-writers traveled, and 1 relocated, to the murderer’s state of incarceration to attend his hearings and be closer to him.
This study has limitations:
• categorization of letters is inherently subjective and the categories themselves were created by the researchers
• the nature and categorization of such letters might vary considerably with the age and sex of the violent criminal; our findings in this case are not generalizable.
Last, researchers who plan to study writers of letters to incarcerated criminals should consider sending a personality test and other questionnaires to those writers to understand this population better.
Treatment considerations
Psychiatrists treating patients who seek a romantic attachment with a violent person should consider psychotherapy as a means of treating possible character pathology. The desire for romance with a violent criminal was greater among repeat writers (20%) than in initial letters (15%), suggesting that people who have a strong inclination to associate with a violent person might benefit from exploring romantic feelings in therapy. Specifically, therapists would be wise to explore with such patients the possibility that they experienced violence or verbal abuse in childhood or adulthood.
To the extent that evidence of prior abuse exists, a diagnosis of posttraumatic stress disorder (PTSD) might be appropriate; specialized therapy for men and women with a history of abuse might be indicated. It is important to provide validation for patients who are victims when they describe their abuse, and to stress that they did nothing to provoke the violence. Furthermore, investigation of why the patient feels drawn romantically toward a violent criminal is helpful, as well as an examination of how such behavior is self-defeating.
There might be value in having patients keep a journal in lieu of actually sending letters; there is evidence that “journaling” can reduce substance use recidivism.7 This work can be performed in conjunction with group or individual psychotherapy that addresses any history of abuse and subsequent PTSD.
Many patients are reluctant to discuss their romantic feelings toward a violent criminal until the psychiatrist has established a strong doctor−patient relationship. Last, clinicians should not hesitate to refer these patients to a therapist who specializes in domestic violence.
Related Resource
• Marazziti D, Falaschi V, Lombardi A, et al. Stalking: a neurobiological perspective. Riv Psichiatr. 2015;50(1):12-18.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mouradian VE. Women’s stay-leave decisions in relationships involving intimate partner violence. Wellesley, MA: Wellesley Centers for Women Publications; 2004:3,4.
2. Bell KM, Naugle AE. Understanding stay/leave decisions in violent relationships: a behavior analytic approach. Behav Soc Issues. 2005;14(1):21-46.
3. Westrup D, Fremouw WJ. Stalking behavior: a literature review and suggested functional analytic assessment technology. Aggression and Violent Behavior. 1998;3: 255-274.
4. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
5. West SG, Friedman SH. These boots are made for stalking: characteristics of female stalkers. Psychiatry (Edgmont). 2008;5(8):37-42.
6. Nadkarni R, Grubin D. Stalking: why do people do it? BMJ. 2000;320(7248):1486-1487.
7. Proctor SL, Hoffmann NG, Allison S. The effectiveness of interactive journaling in reducing recidivism among substance-dependent jail inmates. Int J Offender Ther Comp Criminol. 2012;56(2):317-332.
Little is known about people who write to criminals incarcerated for a violent crime. However, existence of Web sites such as WriteAPrisoner.com, Meet-An-Inmate.com, and PrisonPenPals.com suggests some appetite among the public for corresponding with the incarcerated. Writers of letters might be drawn to the “bad boy” image of prisoners. Furthermore, much has been written of the willingness of some battered women to remain in an abusive domestic relationship, leading them to correspond with their abusers even after those abusers are incarcerated.1,2
To our knowledge, no examination of letters written to a mass murderer has been published. Therefore, we categorized and analyzed 784 letters sent to a high-profile male mass murderer whose crime was committed during the past decade. Here is a description of the study and what we found, as well as discussion of how our findings might offer utility in a psychiatric practice.
Goals of the study
We hypothesized that a large percentage of those letters could be classified as “Romantic,” given the lay perception that it is women who write to mass murderers. We also sought to evaluate follow-up letters sent by these writers to test the assumption that their individual goals would be constant over time.
We performed this study in the hope that the research could assist psychiatric practitioners in treating patients who seek to associate with a violent person (see “Treatment considerations,”). We thought it might be helpful for practitioners to get a better understanding of the nature of people who write to a violent offender or express a desire to do so.
Methods of study
Two authors (R.S.J. and D.P.G.) evaluated 819 letters that had been written by non-incarcerated, non-family adults to 1 mass murderer. The initial letter and follow-up letters written by each unique writer (n = 333) were categorized as follows:
• state or country from which the letter was sent
• age
• sex
• number of letters sent by each writer
• whether a photograph was enclosed
• whether additional items were enclosed (eg, gifts, drawings)
• whether the letter was rejected by prison authorities
• the writer’s purpose.
The study was approved by the institutional review board of Baylor College of Medicine.
Letters were assigned to 1 of 5 categories:
Acquaintance letters sought ongoing correspondence relationship with the murderer. They focused largely on conveying information about the writer.
Show of support letters also sought an ongoing correspondence relationship with the murderer, but instead focused on him, not the writer.
Romance letters used words that conveyed romantic or non-platonic affection.
Spiritual letters gave advice to the murderer with a religious tone.
Words of wisdom letters offered advice but lacked a religious tone.
Given the nonstandardized nature of categorization and the lack of a formal questionnaire, we were unable to perform an exploratory factor analysis on our categorizations. Inter-rater reliability of letter categorization was 0.79.
Results: Writer profiles, purpose for writing
In all, we reviewed 819 letters:
• Thirty-five letters were excluded because they were written by family members, children, or other prisoners
• Of the remaining 784 letters, there were 333 unique writers
• Two-hundred sixty letters were written by women, 61 by men; 2 were co-written by both sexes; sex could not be determined for 10.
Women were more likely than men to write a letter (P = .014) and to write ≥3 letters (P = .001). The age of the writer was determined for 117 (35.1%) letters; mean age was 27.8 (± 8.9) years (range, 18 to 59 years).
The purpose of the letters differed by sex (P < .001) but not by the writer’s age (P = .058). Women were more likely than men to write letters categorized as “Acquaintance,” “Romance,” and “Show of support”; in contrast, men were more likely than women to write a letter categorized as “Spiritual” (Table 1). Approximately 95% of letters were handwritten. Letters averaged 3 pages (range, 1 to 16 pages).
Two-hundred sixteen writers wrote a single letter; 53 wrote 2 letters; 18 wrote 3 letters; 11 wrote 4 letters; 30 wrote 5 to 10 letters; and 9 wrote 11 to 43 letters. The purpose of follow-up letters was associated with the age of the writer (P < .001) and with the writer’s sex (P < .001). Women were more likely to write “Show of support” and “Romance” follow-up letters; men were more likely to write “Spiritual” follow-up letters (Table 2).
Results suggested that the purpose of the initial letter was a reasonable predictor of the purpose of follow-up letters (P < .001) (Table 3). The murderer never responded to any letters. Letters were most often written from his state of incarceration; next, from contiguous states; then, from non-contiguous states; and, last, from international locations (P < .001).
Of the initial letters from writers who wrote ≥10, 60% were categorized as “Acquaintance” and 20% as “Romance.” The writer who wrote the most letters (43) moved during the course of her letter-writing to live in the same state as the murderer; she stated in her letters that she did so to be closer to him and to be able to attend his court hearings. Four other writers, each of whom wrote >5 letters, stated that they had traveled to the murderer’s state of incarceration to attend some of his hearings in person.
Composite examples of more common categories of letters
Names and other pertinent identifying information have been changed.
Acquaintance. Hi, Steve. I’ve been following your case and just wanted to write you so that maybe we could be friends or keep in touch since you’re probably pretty bored. I’m a 27-year-old college student studying marketing and working at Applebee’s as a waitress (for now) until I can land my dream job. I’ve enclosed a picture of me and my dachshund along with a photo of my favorite beach in the world. Write me back if you want. Jenny.
Show of support. Steve: I’ve been really worried about you since first seeing you on TV. You look different lately and I hope they’re treating you OK and feeding you decent food. In case they’re not, I’ve enclosed a little something to buy yourself a treat. Just know that there are many of us that care about you and are really pulling for you to be strong in this tough situation you’re in. Yours truly, Karen.
Romance. Dearest Steven: My mind has been filled with thoughts of you and of us since I last saw you in my dreams! Be strong, because you are going to beat this once they understand that you are not responsible for what happened! Don’t you see, sweetie, the system failed you, and now you’re caught up in something that you will soon overcome. When I think of the day that you get released, and how we’ll be able to settle down somewhere together, it gets me incredibly excited. You and I are meant to be together, because I understand you and can help you get better. I love you, Steven! Please write me back so that I know we’re on the same page about our plans for the future. Love, ♥ Your sweetie, Rachel.
Spiritual. Dear Child of God: The Lord has a plan for you. I know that things right now might be confusing, and you’re in a black place, but He is there right beside you. If you need some reading materials to give you comfort, just let me know and I can get a Bible to you along with some other books to give you solace and strengthen your walk with Him. God forgives you and he loves you so much! Much love in Christ, Mary.
Discussion
Given that the mass murderer in this study was a young man, it is not surprising that 78% of writers of initial letters were women. However, it is interesting that, among women’s initial letters, 44% were “Acquaintance” letters and only 15% were categorized as “Romance.”
Given the severity of the murderer’s crime, it is remarkable that he received only 1 “Hate mail” letter.
Initial “Spiritual” letters were more likely to be followed by letters of the same category than any other category; “Romance” letters were a close second. This demonstrates the consistent efforts of writers in these 2 categories. Highly persistent writers (≥10 letters) were most likely to fall into “Acquaintance” and “Romance” categories. The persistence of these writers is remarkable, in view of the fact that none of their letters were answered. We hypothesize that the killer did not reply because he had no interest in correspondence.
Similarities to stalking. Given that 9 writers wrote >10 letters each and 2 wrote >20 each, elements of their behavior are not unlike what is seen in stalkers.3 Consistent with the stalking literature and Mullen et al4 stalker typology, many writers in this study appeared to seek intimacy with the perpetrator through “Romance” or “Show of support” letters, and might be akin to Mullen’s so-called intimacy-seeking stalker. Such stalkers’ behavior arises out of loneliness, with a strong desire for a relationship with the target; a significant percentage of such stalkers suffer a delusional disorder.
Mullen’s so-called incompetent suitor stalker is similar to the intimacy-seeking type but, instead, has an interest in a short-term relationship and is far less persistent in his (her) stalking behavior4; this type might apply to the writers in this study who wrote >1 but <10 letters.
Two additional observations also are notable when trying to characterize people who write letters: (1) A high percentage of people who stalk a celebrity suffer a psychotic disorder5,6; (2) 4 letter-writers traveled, and 1 relocated, to the murderer’s state of incarceration to attend his hearings and be closer to him.
This study has limitations:
• categorization of letters is inherently subjective and the categories themselves were created by the researchers
• the nature and categorization of such letters might vary considerably with the age and sex of the violent criminal; our findings in this case are not generalizable.
Last, researchers who plan to study writers of letters to incarcerated criminals should consider sending a personality test and other questionnaires to those writers to understand this population better.
Treatment considerations
Psychiatrists treating patients who seek a romantic attachment with a violent person should consider psychotherapy as a means of treating possible character pathology. The desire for romance with a violent criminal was greater among repeat writers (20%) than in initial letters (15%), suggesting that people who have a strong inclination to associate with a violent person might benefit from exploring romantic feelings in therapy. Specifically, therapists would be wise to explore with such patients the possibility that they experienced violence or verbal abuse in childhood or adulthood.
To the extent that evidence of prior abuse exists, a diagnosis of posttraumatic stress disorder (PTSD) might be appropriate; specialized therapy for men and women with a history of abuse might be indicated. It is important to provide validation for patients who are victims when they describe their abuse, and to stress that they did nothing to provoke the violence. Furthermore, investigation of why the patient feels drawn romantically toward a violent criminal is helpful, as well as an examination of how such behavior is self-defeating.
There might be value in having patients keep a journal in lieu of actually sending letters; there is evidence that “journaling” can reduce substance use recidivism.7 This work can be performed in conjunction with group or individual psychotherapy that addresses any history of abuse and subsequent PTSD.
Many patients are reluctant to discuss their romantic feelings toward a violent criminal until the psychiatrist has established a strong doctor−patient relationship. Last, clinicians should not hesitate to refer these patients to a therapist who specializes in domestic violence.
Related Resource
• Marazziti D, Falaschi V, Lombardi A, et al. Stalking: a neurobiological perspective. Riv Psichiatr. 2015;50(1):12-18.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Little is known about people who write to criminals incarcerated for a violent crime. However, existence of Web sites such as WriteAPrisoner.com, Meet-An-Inmate.com, and PrisonPenPals.com suggests some appetite among the public for corresponding with the incarcerated. Writers of letters might be drawn to the “bad boy” image of prisoners. Furthermore, much has been written of the willingness of some battered women to remain in an abusive domestic relationship, leading them to correspond with their abusers even after those abusers are incarcerated.1,2
To our knowledge, no examination of letters written to a mass murderer has been published. Therefore, we categorized and analyzed 784 letters sent to a high-profile male mass murderer whose crime was committed during the past decade. Here is a description of the study and what we found, as well as discussion of how our findings might offer utility in a psychiatric practice.
Goals of the study
We hypothesized that a large percentage of those letters could be classified as “Romantic,” given the lay perception that it is women who write to mass murderers. We also sought to evaluate follow-up letters sent by these writers to test the assumption that their individual goals would be constant over time.
We performed this study in the hope that the research could assist psychiatric practitioners in treating patients who seek to associate with a violent person (see “Treatment considerations,”). We thought it might be helpful for practitioners to get a better understanding of the nature of people who write to a violent offender or express a desire to do so.
Methods of study
Two authors (R.S.J. and D.P.G.) evaluated 819 letters that had been written by non-incarcerated, non-family adults to 1 mass murderer. The initial letter and follow-up letters written by each unique writer (n = 333) were categorized as follows:
• state or country from which the letter was sent
• age
• sex
• number of letters sent by each writer
• whether a photograph was enclosed
• whether additional items were enclosed (eg, gifts, drawings)
• whether the letter was rejected by prison authorities
• the writer’s purpose.
The study was approved by the institutional review board of Baylor College of Medicine.
Letters were assigned to 1 of 5 categories:
Acquaintance letters sought ongoing correspondence relationship with the murderer. They focused largely on conveying information about the writer.
Show of support letters also sought an ongoing correspondence relationship with the murderer, but instead focused on him, not the writer.
Romance letters used words that conveyed romantic or non-platonic affection.
Spiritual letters gave advice to the murderer with a religious tone.
Words of wisdom letters offered advice but lacked a religious tone.
Given the nonstandardized nature of categorization and the lack of a formal questionnaire, we were unable to perform an exploratory factor analysis on our categorizations. Inter-rater reliability of letter categorization was 0.79.
Results: Writer profiles, purpose for writing
In all, we reviewed 819 letters:
• Thirty-five letters were excluded because they were written by family members, children, or other prisoners
• Of the remaining 784 letters, there were 333 unique writers
• Two-hundred sixty letters were written by women, 61 by men; 2 were co-written by both sexes; sex could not be determined for 10.
Women were more likely than men to write a letter (P = .014) and to write ≥3 letters (P = .001). The age of the writer was determined for 117 (35.1%) letters; mean age was 27.8 (± 8.9) years (range, 18 to 59 years).
The purpose of the letters differed by sex (P < .001) but not by the writer’s age (P = .058). Women were more likely than men to write letters categorized as “Acquaintance,” “Romance,” and “Show of support”; in contrast, men were more likely than women to write a letter categorized as “Spiritual” (Table 1). Approximately 95% of letters were handwritten. Letters averaged 3 pages (range, 1 to 16 pages).
Two-hundred sixteen writers wrote a single letter; 53 wrote 2 letters; 18 wrote 3 letters; 11 wrote 4 letters; 30 wrote 5 to 10 letters; and 9 wrote 11 to 43 letters. The purpose of follow-up letters was associated with the age of the writer (P < .001) and with the writer’s sex (P < .001). Women were more likely to write “Show of support” and “Romance” follow-up letters; men were more likely to write “Spiritual” follow-up letters (Table 2).
Results suggested that the purpose of the initial letter was a reasonable predictor of the purpose of follow-up letters (P < .001) (Table 3). The murderer never responded to any letters. Letters were most often written from his state of incarceration; next, from contiguous states; then, from non-contiguous states; and, last, from international locations (P < .001).
Of the initial letters from writers who wrote ≥10, 60% were categorized as “Acquaintance” and 20% as “Romance.” The writer who wrote the most letters (43) moved during the course of her letter-writing to live in the same state as the murderer; she stated in her letters that she did so to be closer to him and to be able to attend his court hearings. Four other writers, each of whom wrote >5 letters, stated that they had traveled to the murderer’s state of incarceration to attend some of his hearings in person.
Composite examples of more common categories of letters
Names and other pertinent identifying information have been changed.
Acquaintance. Hi, Steve. I’ve been following your case and just wanted to write you so that maybe we could be friends or keep in touch since you’re probably pretty bored. I’m a 27-year-old college student studying marketing and working at Applebee’s as a waitress (for now) until I can land my dream job. I’ve enclosed a picture of me and my dachshund along with a photo of my favorite beach in the world. Write me back if you want. Jenny.
Show of support. Steve: I’ve been really worried about you since first seeing you on TV. You look different lately and I hope they’re treating you OK and feeding you decent food. In case they’re not, I’ve enclosed a little something to buy yourself a treat. Just know that there are many of us that care about you and are really pulling for you to be strong in this tough situation you’re in. Yours truly, Karen.
Romance. Dearest Steven: My mind has been filled with thoughts of you and of us since I last saw you in my dreams! Be strong, because you are going to beat this once they understand that you are not responsible for what happened! Don’t you see, sweetie, the system failed you, and now you’re caught up in something that you will soon overcome. When I think of the day that you get released, and how we’ll be able to settle down somewhere together, it gets me incredibly excited. You and I are meant to be together, because I understand you and can help you get better. I love you, Steven! Please write me back so that I know we’re on the same page about our plans for the future. Love, ♥ Your sweetie, Rachel.
Spiritual. Dear Child of God: The Lord has a plan for you. I know that things right now might be confusing, and you’re in a black place, but He is there right beside you. If you need some reading materials to give you comfort, just let me know and I can get a Bible to you along with some other books to give you solace and strengthen your walk with Him. God forgives you and he loves you so much! Much love in Christ, Mary.
Discussion
Given that the mass murderer in this study was a young man, it is not surprising that 78% of writers of initial letters were women. However, it is interesting that, among women’s initial letters, 44% were “Acquaintance” letters and only 15% were categorized as “Romance.”
Given the severity of the murderer’s crime, it is remarkable that he received only 1 “Hate mail” letter.
Initial “Spiritual” letters were more likely to be followed by letters of the same category than any other category; “Romance” letters were a close second. This demonstrates the consistent efforts of writers in these 2 categories. Highly persistent writers (≥10 letters) were most likely to fall into “Acquaintance” and “Romance” categories. The persistence of these writers is remarkable, in view of the fact that none of their letters were answered. We hypothesize that the killer did not reply because he had no interest in correspondence.
Similarities to stalking. Given that 9 writers wrote >10 letters each and 2 wrote >20 each, elements of their behavior are not unlike what is seen in stalkers.3 Consistent with the stalking literature and Mullen et al4 stalker typology, many writers in this study appeared to seek intimacy with the perpetrator through “Romance” or “Show of support” letters, and might be akin to Mullen’s so-called intimacy-seeking stalker. Such stalkers’ behavior arises out of loneliness, with a strong desire for a relationship with the target; a significant percentage of such stalkers suffer a delusional disorder.
Mullen’s so-called incompetent suitor stalker is similar to the intimacy-seeking type but, instead, has an interest in a short-term relationship and is far less persistent in his (her) stalking behavior4; this type might apply to the writers in this study who wrote >1 but <10 letters.
Two additional observations also are notable when trying to characterize people who write letters: (1) A high percentage of people who stalk a celebrity suffer a psychotic disorder5,6; (2) 4 letter-writers traveled, and 1 relocated, to the murderer’s state of incarceration to attend his hearings and be closer to him.
This study has limitations:
• categorization of letters is inherently subjective and the categories themselves were created by the researchers
• the nature and categorization of such letters might vary considerably with the age and sex of the violent criminal; our findings in this case are not generalizable.
Last, researchers who plan to study writers of letters to incarcerated criminals should consider sending a personality test and other questionnaires to those writers to understand this population better.
Treatment considerations
Psychiatrists treating patients who seek a romantic attachment with a violent person should consider psychotherapy as a means of treating possible character pathology. The desire for romance with a violent criminal was greater among repeat writers (20%) than in initial letters (15%), suggesting that people who have a strong inclination to associate with a violent person might benefit from exploring romantic feelings in therapy. Specifically, therapists would be wise to explore with such patients the possibility that they experienced violence or verbal abuse in childhood or adulthood.
To the extent that evidence of prior abuse exists, a diagnosis of posttraumatic stress disorder (PTSD) might be appropriate; specialized therapy for men and women with a history of abuse might be indicated. It is important to provide validation for patients who are victims when they describe their abuse, and to stress that they did nothing to provoke the violence. Furthermore, investigation of why the patient feels drawn romantically toward a violent criminal is helpful, as well as an examination of how such behavior is self-defeating.
There might be value in having patients keep a journal in lieu of actually sending letters; there is evidence that “journaling” can reduce substance use recidivism.7 This work can be performed in conjunction with group or individual psychotherapy that addresses any history of abuse and subsequent PTSD.
Many patients are reluctant to discuss their romantic feelings toward a violent criminal until the psychiatrist has established a strong doctor−patient relationship. Last, clinicians should not hesitate to refer these patients to a therapist who specializes in domestic violence.
Related Resource
• Marazziti D, Falaschi V, Lombardi A, et al. Stalking: a neurobiological perspective. Riv Psichiatr. 2015;50(1):12-18.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mouradian VE. Women’s stay-leave decisions in relationships involving intimate partner violence. Wellesley, MA: Wellesley Centers for Women Publications; 2004:3,4.
2. Bell KM, Naugle AE. Understanding stay/leave decisions in violent relationships: a behavior analytic approach. Behav Soc Issues. 2005;14(1):21-46.
3. Westrup D, Fremouw WJ. Stalking behavior: a literature review and suggested functional analytic assessment technology. Aggression and Violent Behavior. 1998;3: 255-274.
4. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
5. West SG, Friedman SH. These boots are made for stalking: characteristics of female stalkers. Psychiatry (Edgmont). 2008;5(8):37-42.
6. Nadkarni R, Grubin D. Stalking: why do people do it? BMJ. 2000;320(7248):1486-1487.
7. Proctor SL, Hoffmann NG, Allison S. The effectiveness of interactive journaling in reducing recidivism among substance-dependent jail inmates. Int J Offender Ther Comp Criminol. 2012;56(2):317-332.
1. Mouradian VE. Women’s stay-leave decisions in relationships involving intimate partner violence. Wellesley, MA: Wellesley Centers for Women Publications; 2004:3,4.
2. Bell KM, Naugle AE. Understanding stay/leave decisions in violent relationships: a behavior analytic approach. Behav Soc Issues. 2005;14(1):21-46.
3. Westrup D, Fremouw WJ. Stalking behavior: a literature review and suggested functional analytic assessment technology. Aggression and Violent Behavior. 1998;3: 255-274.
4. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
5. West SG, Friedman SH. These boots are made for stalking: characteristics of female stalkers. Psychiatry (Edgmont). 2008;5(8):37-42.
6. Nadkarni R, Grubin D. Stalking: why do people do it? BMJ. 2000;320(7248):1486-1487.
7. Proctor SL, Hoffmann NG, Allison S. The effectiveness of interactive journaling in reducing recidivism among substance-dependent jail inmates. Int J Offender Ther Comp Criminol. 2012;56(2):317-332.
‘Acting out’ or pathological?
Choosing a treatment for disruptive, impulse-control, and conduct disorders
Chronic disruptive and impulsive behaviors are significant concerns for psychiatric clinicians because of their persistence and potential legal ramifications. To date, few studies have assessed treatment options for pyromania, oppositional defiant disorder (ODD), intermittent explosive disorder (IED), kleptomania, and conduct disorder (CD).
This article reviews the literature on the treatment of these disorders, focusing primarily on randomized, controlled studies. Because of the lack of clinical studies for these disorders, however, case studies and open trials are mentioned for reference. Summaries of supported medication and psychological interventions are provided for each disorder.
Categorizing impulse-control disorders
The DSM-5 created a new chapter on disruptive, impulse control, and conduct disorders that brought together disorders previously classified as disorders usually first diagnosed in infancy, childhood, or adolescence (ODD, CD) and impulse-control disorders not elsewhere classified. These disorders are unified by the presence of difficult, disruptive, aggressive, or antisocial behavior. Disruptive, aggressive, or antisocial behavior usually is a multifaceted behavior, often associated with physical or verbal injury to self, others, or objects or with violating the rights of others. These behaviors can appear in several forms and can be defensive, premeditated, or impulsive.
Despite a high prevalence in the general population1 and in psychiatric cohorts,2 disruptive and impulse-control disorders have been relatively understudied. Controlled trials of treatments do not exist for many impulse-control disorders, and there are no FDA-approved medications for any of these disorders.
Oppositional defiant disorder
Irritability, anger, defiance, and temper are specific descriptors of ODD. ODD seems to be a developmental antecedent for some youth with CD, suggesting that these disorders could reflect different stages of a spectrum of disruptive behavior. Transient oppositional behavior is common among children and adolescents, but ODD occurs in 1% to 11% of youth.3 The disorder is more prevalent among boys before puberty and has an equal sex prevalence in young people after puberty.
Regrettably, most ODD research has included patients with comorbidities, most commonly attention-deficit/hyperactivity disorder (ADHD). Because of this limitation, the drugs and programs discussed below are drawn from meta-analyses and review articles.
Pharmacotherapy. No medications have been FDA-approved for ODD. Studies assessing ODD have employed a variety of methodologies, not all of which are double-blind. The meta-analyses and reviews cited in this section include both randomized and open trials, and should be interpreted as such.
Stimulants are commonly used to treat ODD because of a high comorbidity rate with ADHD, and these drugs have improved ODD symptoms in randomized trials.4 Methylphenidate and d-amphetamine have shown some efficacy in trials of ODD and CD.5-7 These medications are most commonly used when ODD is complicated by ADHD symptoms.
Antipsychotics also have been used to treat ODD, with the largest body of research suggesting that risperidone has some efficacy. Risperidone usually is considered a second- or third-line option because it has been associated with adverse effects in children and adolescents and requires caution in younger populations, despite its potential efficacy.4,8-10
Alpha-2 agonists—clonidine and guanfacine—have shown some efficacy in treating ODD but have not been studied extensively. Studies of clonidine, however, often have grouped ODD, CD, and ADHD, which limits our understanding of this medication for ODD alone.4,5,11
Atomoxetine has been studied for ODD, but its efficacy is limited, with different meta-analyses finding distinct results regarding efficacy. One explanation for these disparate findings is that improvements in oppositional symptoms may be secondary to improvement in ADHD symptoms.7,12-14
Psychological treatments. As noted for pharmacotherapy, this section provides general information on empirically studied therapies. A series of meta-analyses have been included for further review, but are not isolated to randomized, controlled studies.
Individual therapy has shown consistent improvements in ODD. Examples include behavior modification therapy and parent-child interaction therapy. These sessions emphasize skills to manage outbursts and erratic emotionality. Emotion regulation and behavior and social skills training have shown significant reductions in target measures. Some of these programs incorporate both patient and parent components.15-17
Family/teacher training programs such as “Helping the Noncompliant Child” and the “Triple P” have yielded significant improvements. These programs focus on ways to manage the child’s oppositional behavior at home and in the classroom, as well as strategies to limit positive reinforcement for problem behaviors.17-20
Group programs have shown some efficacy with ODD. These programs cover a wide number of needs and intents. Examples include the “Incredible Years” program and the Community Parent Education Program. Research has found that these programs show some efficacy as preemptive measures to reduce the rate of ODD among adolescents.
Conclusions. A number of treatment options for ODD have shown some efficacy. However, many of these options have only been studied in patients with comorbid ADHD, which limits current knowledge about ODD as a distinct disorder.
Intermittent explosive disorder
IED is defined by recurrent, significant outbursts of aggression, often leading to assaultive acts against people or property, which are disproportionate to outside stressors and are not better explained by another psychiatric diagnosis. Research suggests IED is common, with 6.3% of a community sample meeting criteria for lifetime IED.21
IED symptoms tend to start in adolescence and appear to be chronic.21,22 People with IED regard their behavior as distressing and problematic.22 Outbursts generally are short-lived (usually <30 minutes) and frequent (multiple times a month22). Legal and occupational difficulties are common.22
Pharmacotherapy. Data on drug treatment for IED comes for a small set of double-blind studies (Table). Although pharmacotherapies have been studied for treating aggression, impulsivity, and violent behavior, only 5 controlled studies are specific to IED.
A double-blind, randomized, placebo-controlled trial of fluoxetine in 100 participants with IED found that fluoxetine produced a sustained reduction in aggression and irritability as early as the second week of treatment. Full or partial remission of impulsive aggressive behaviors occurred in 46% of fluoxetine-treated subjects. These findings have been supported by studies assessing other samples of aggressive patients, but not specifically IED.23,24 Another treatment study found that oxcarbazepine produced significant improvements in IED symptom severity, specifically on impulsive aggression.25
In a randomized, double-blind, placebo-controlled study, 96 participants with Cluster B personality disorders, 116 with IED, and 34 with posttraumatic stress disorder were assigned to divalproex sodium or placebo for 12 weeks. Using an intent-to-treat analysis, divalproex had no significant influence on aggression in patients with IED.26 Similarly, a study assessing levetiracetam for IED did not show any improvements to measures of impulsive aggression.27
Psychological treatments. The only available study on psychological treatments for IED found that patients receiving active cognitive-behavioral therapy (CBT) or group therapy showed significant improvements compared with waitlist controls. These improvements spanned several target symptoms of IED.28
Conclusions. Although there is a paucity of treatment studies for IED, fluoxetine may be an effective treatment based on available studies, and oxcarbazepine has shown some preliminary efficacy. CBT also has shown some initial efficacy in reducing symptom severity in IED.
Conduct disorder
The essential feature of CD is a repetitive and persistent pattern of behavior in which the basic rights of others or social norms are violated.3 These behaviors can entail:
• aggressive conduct that causes or threatens harm to others or to animals
• nonaggressive behavior resulting in property damage
• deceitfulness or theft
• serious violation of rules.
Prevalence among the general population is 2% to 10%. The disorder is more common among boys than girls.3
Pharmacotherapy. No medication is FDA-approved to treat CD. Fifteen controlled studies have examined medications in patients with CD (Table), although a number of these included a high rate of comorbid ADHD.
To date, 7 studies have shown efficacy with lithium for patients with CD.29-35 A number of trials assessing lithium also included a treatment condition with haloperidol, which showed significant improvement.29,30,33,34 Both lithium and haloperidol were associated with select deficits on cognitive tests, suggesting that there may be risks associated with these medications.
Preliminary double-blind results have indicated that methylphenidate, risperidone, quetiapine, molindone, thioridazine, and carbamazepine might be effective options for treating CD.36-43 The evidence for these medications is limited and additional studies are needed to replicate initial findings.
Three studies of divalproex sodium have shown some efficacy in randomized studies comparing high and low dosages of the drug.40-42 Because these studies did not include a placebo, additional studies are necessary to corroborate these findings.
Psychological treatments. Several forms of behavioral, family-based, and school-based therapies have been found effective in randomized trials. Specifically, behavioral therapy and parental skills training have shown consistent benefits for patients and their families. As with ODD, parental training programs for CD focus on parents’ skill acquisition to help manage outbursts and aggressive behavior. These treatments often follow a similar course to those used for other externalizing and disruptive disorders.44-46
Conclusions. Based on evidence, psychotherapy and some pharmacotherapies (eg, lithium) could be considered first-line treatment options for CD. Psychotherapy programs have shown efficacy in reducing aggression in high-risk groups.44 Lithium or antipsychotics could be useful for patients who do not respond sufficiently to psychotherapy. The risk of cognitive deficits with lithium and antipsychotics should be weighed against potential benefits of these medications.33,34
Kleptomania
Kleptomania is characterized by repetitive, poorly controlled stealing of items that are not needed for personal use. Kleptomania often begins in late adolescence or early adulthood.47 The course of the illness generally is chronic, with waxing and waning symptoms. Women are twice as likely as men to suffer from kleptomania.48 People with kleptomania frequently hoard, discard, or return stolen items.47
Most people with kleptomania try unsuccessfully to stop stealing, which often leads to feelings of shame and guilt.48 Many (64% to 87%) have been arrested because of their stealing behavior47; a smaller percentage (15% to 23%) have been incarcerated.48 Suicide attempts are common among these patients.49
Pharmacotherapy. There has been only 1 randomized, placebo-controlled study of pharmacotherapy for kleptomania (Table). An 8-week, double-blind, placebo-controlled trial was conducted to evaluate the safety and efficacy of oral naltrexone, 50 to 150 mg/d, in 25 patients with kleptomania. Those taking naltrexone had a significantly greater reduction in total score than those taking placebo on the Yale-Brown Obsessive Compulsive Scale Modified for Kleptomania; in stealing urges; and in stealing behavior. The mean effective dosage of naltrexone was 116.7 (± 44.4) mg/d.50
Naltrexone was well tolerated, with minimal nausea, and did not cause elevation of liver enzymes.
There is one available open-label study with a double-blind discontinuation phase assessing the efficacy of escitalopram for kleptomania. Continuation of escitalopram during the blinded discontinuation phase did produce lower relapse rates.51
Psychological treatments. There are no controlled studies of psychological treatments for kleptomania. Case reports suggest that cognitive and behavioral therapies might be effective:
• A young man who underwent 7 sessions of covert sensitization, combined with exposure and response prevention, over a 4-month period was able to reduce his stealing frequency.52
• In another case, a young woman underwent 5 weekly sessions when she was instructed to practice covert sensitization whenever she had an urge to steal. She remained in remission for 14 months with only a single lapse in behavior and with no reported urges to steal.53
• In 2 patients, imaginal desensitization in fourteen 15-minutes sessions over 5 days resulted in complete remission of symptoms for a 2-year period.54
Conclusions. The single controlled study of naltrexone for kleptomania suggests that naltrexone might be a beneficial treatment for this disorder. No controlled trials of psychosocial interventions have been reported. The current psychological research is based primarily on case reports.
This state of affairs likely is because of (1) the low prevalence of kleptomania and (2) clinical difficulties in treating patients involved in illegal activities. Nevertheless, there is a need for systematic studies of treating this disorder; such studies could involve collaboration across multiple treatment centers because of the disorder’s low prevalence.
Pyromania
Pyromania is characterized by (1) deliberate and purposeful fire setting on >1 occasion; (2) tension or affective arousal before the act; (3) fascination with, interest in, curiosity about, or attraction to fire and its situational contexts; and (4) pleasure, gratification, or relief when setting fires or when witnessing or participating in their aftermath.3
Although pyromania is thought to be a disorder primarily affecting men, recent research suggests that the sex ratio is equal among adults and may be slightly higher among adolescent females. Mean age of onset usually is late adolescence. Pyromania appears to be chronic if untreated.55
Urges to set fires are common and the fire setting is almost always pleasurable. Severe distress follows the fire setting, and persons with pyromania report significant functional impairment. High rates of co-occurring psychiatric disorders (depression, substance use disorders, other impulse-control disorders) are common among persons with pyromania.55
Pharmacotherapy. There are no randomized, controlled clinical trials examining pharmacotherapy for treating pyromania. There are no FDA-approved medications for pyromania.
In case reports, medications that have shown benefit in treating pyromania include topiramate, escitalopram, sertraline, fluoxetine, lithium, and a combination of olanzapine and sodium valproate. An equal number of medications have shown no benefit: fluoxetine, valproic acid, lithium, sertraline, olanzapine, escitalopram, citalopram, and clonazepam. A case report of an 18-year-old man with pyromania described successfully using a combination of topiramate with 3 weeks of daily CBT to achieve significant symptom improvement.56,57
Pyromania is a largely unrecognized disorder that causes significant psychological, social, and legal repercussions. Because few persons with pyromania volunteer information regarding fire-setting, it is important that clinicians recognize the disorder and screen patients appropriately. Various treatments have been helpful in case studies, but more research on the etiology and treatment of the disorder is needed.56,57
Conclusions based on the literature
In disruptive, impulse-control, and conduct disorders, the systematic study of treatment efficacy and tolerability is in its infancy. With few controlled studies published, it is not possible to make treatment recommendations with confidence. There are no FDA-approved drugs for treating any of these disorders.
Nonetheless, specific psychotherapies and drug therapies offer promising options, but often are based on small studies, often in patient populations with prominent comorbidities, and have not been replicated by independent investigators. For all of these disorders, issues such as which psychotherapy or medication to use and the ideal duration of treatment cannot be sufficiently addressed with the available data.
In conjunction with emerging epidemiological data supporting a relatively high prevalence of disruptive, impulse-control, and conduct disorders, the small amount of data regarding effective treatments highlights the clinical need for additional research.
Bottom Line
Empirically supported treatment options for impulse-control disorders currently are limited, because only select disorders have been studied across multiple trials. New research is needed to confirm possible treatment options and identify effective psychotherapeutic and pharmacological treatment alternatives.
Related Resources
• Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W. W. Norton & Company; 2008.
• Grant JE, Kim SW. Stop me because I can’t stop myself: taking control of impulsive behavior. New York, NY: McGraw- Hill; 2003.
• American Academy of Child and Adolescent Psychiatry. Conduct disorder resource center. http://www.aacap.org/AACAP/FamiliesandYouth/ResourceCenters/ConductDisorderResourceCenter/Home.aspx.
Drug Brand Names
Atomoxetine • Strattera Methylphenidate • Ritalin
Carbamazepine • Tegretol Molindone • Moban
Citalopram • Celexa Naltrexone • ReVia
Clonazepam • Klonopin Olanzapine • Zyprexa
Clonidine • Catapres Oxcarbazepine • Trileptal
D-amphetamine • Dexedrine Quetiapine • Seroquel
Divalproex sodium • Depakote Risperidone • Risperdal
Escitalopram • Lexapro Sertraline • Zoloft
Fluoxetine • Prozac Sodium valproate • Depacon
Guanfacine • Intuniv Thioridazine • Mellaril
Haloperidol • Haldol Topiramate • Topamax
Levetiracetam • Keppra Valproic acid • Depakote
Lithium • Eskalith, Lithobid
Disclosures
Dr. Grant receives grant or research support from Brainsway, Forest Pharmaceuticals, and Roche Pharmaceuticals. Mr. Leppink reports no financial relationship with any company whose products are mentioned in this article or with competing products.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Turgay A. Psychopharmacological treatment of oppositional defiant disorder. CNS Drugs. 2009;23(1):1-17.
5. Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry. 2010;18(6):556-559.
6. Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: a review of the past 10 years, part II. J Am Acad Child Adolesc Psychiatry. 2002; 41(11):1275-1293.
7. Connor DF, Steeber J, McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr. 2010;31(5):427-440.
8. Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53(1):47-60.e1.
9. Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behavior disorders in children and youths. Cochrane Database Syst Rev. 2012;9:CD008559.
10. Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53(9):948-959.e1.
12. Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):130-137.
13. Bangs ME, Hazell P, Danckaerts M, et al; Atomoxetine ADHD/ODD Study Group. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314-e320.
14. Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology (Berl). 2007;190(1):31-41.
15. Miller NV, Haas SM, Waschbusch DA, et al. Behavior therapy and callous-unemotional traits: effects of a pilot study examining modified behavioral contingencies on child behavior. Behav Ther. 2014;45(5):606-618.
16. Hamilton SS, Armando J. Oppositional defiant disorder. Am Fam Physician. 2008;78(7):861-866.
17. Steiner H, Remsing L; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
18. Winther J, Carlsson A, Vance A. A pilot study of a school-based prevention and early intervention program to reduce oppositional defiant disorder/conduct disorder. Early Interv Psychiatry. 2014;8(2):181-189.
19. Plueck J, Eichelberger I, Hautmann C, et al. Effectiveness of a teacher-based indicated prevention program for preschool children with externalizing problem behavior [published online April 22, 2014]. Prev Sci. doi: 10.1007/s11121-014- 0487-x.
20. Dretzke J, Frew E, Davenport C, et al. The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children. Health Tech Assess. 2005;9(50):iii, ix-x, 1-233.
21. Coccaro EF, Schmidt CA, Samuels JF, et al. Lifetime and 1-month prevalence rates of intermittent explosive disorder in a community sample. J Clin Psychiatry. 2004;65(6):820-824.
22. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry. 1998;59(4):203-210; quiz 211.
23. Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70(5):653-662.
24. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577-588.
25. Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2005;25(6):575-579.
26. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
27. Mattes JA. Levetiracetam in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(2):310-315.
28. McCloskey MS, Noblett KL, Deffenbacher JL, et al. Cognitive-behavioral therapy for intermittent explosive disorder: a pilot randomized clinical trial. J Consult Clin Psychol. 2008;76(5):876-886.
29. Campbell M, Small AM, Green WH, et al. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41(7):650-656.
30. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
31. Malone RP, Simpson GM. Psychopharmacology: use of placebos in clinical trials involving children and adolescents. Psychiatr Serv. 1998;49(11):1413-1414, 1417.
32. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
33. Platt JE, Campbell M, Green WH, et al. Effects of lithium carbonate and haloperidol on cognition in aggressive hospitalized school-age children. J Clin Psychopharmacol. 1981;1(1):8-13.
34. Platt JE, Campbell M, Green WH, et al. Cognitive effects of lithium carbonate and haloperidol in treatment-resistant aggressive children. Arch Gen Psychiatry. 1984;41(7):657-662.
35. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154(4):554-555.
36. Cueva JE, Overall JE, Small AM, et al. Carbamazepine in aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1996;35(4):480-490.
37. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(4):509-516.
38. Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18(2):140-156.
39. Greenhill LL, Solomon M, Pleak R, et al. Molindone hydrochloride treatment of hospitalized children with conduct disorder. J Clin Psychiatry. 1985;46(8 pt 2):20-25.
40. Khanzode LA, Saxena K, Kraemer H, et al. Efficacy profiles of psychopharmacology: divalproex sodium in conduct disorder. Child Psychiatry Hum Dev. 2006;37(1):55-64.
41. Padhy R, Saxena K, Remsing L, et al. Symptomatic response to divalproex in subtypes of conduct disorder. Child Psychiatry Hum Dev. 2011;42(5):584-593.
42. Steiner H, Petersen ML, Saxena K, et al. Divalproex sodium for the treatment of conduct disorder: a randomized controlled clinical trial. J Clin Psychiatry. 2003;64(10):1183-1191.
43. Klein RG, Abikoff H, Klass E, et al. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073-1080.
44. Heneggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. J Marital Fam Ther. 2012;38(1):30-58.
45. Lochman JE, Powell NP, Boxmeyer CL, et al. Cognitive-behavioral therapy for externalizing disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20(2):305-318.
46. Furlong M, McGilloway S, Bywater T, et al. Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev. 2012;2:CD008225.
47. McElroy SL, Pope HG Jr, Hudson JI, et al. Kleptomania: a report of 20 cases. Am J Psychiatry. 1991;148(5):652-657.
48. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients with kleptomania. Compr Psychiatry. 2002;43(5):378-384.
49. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
50. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7): 600-606.
51. Koran LM, Aboujaoude EN, Gamel NN. Escitalopram treatment of kleptomania: an open-label trial followed by double-blind discontinuation. J Clin Psychiatry. 2007;68(3):422-427.
52. Guidry LS. Use of a covert punishing contingency in compulsive stealing. J Behav Therapy Exp Psychiatry. 1975;6(2):169.
53. Gauthier J, Pellerin D. Management of compulsive shoplifting through covert sensitization. J Behav Therapy Exp Psychiatry. 1982;13(1):73-75.
54. McConaghy N, Blaszczynski A. Imaginal desensitization: a cost-effective treatment in two shop-lifters and a binge-eater resistant to previous therapy. Aus N Z J Psychiatry. 1988;22(1):78-82.
55. Grant JE, Won Kim S. Clinical characteristics and psychiatric comorbidity of pyromania. J Clin Psychiatry. 2007;68(11):1717-1722.
56. Grant JE, Odlaug B. Assessment and treatment of pyromania. In: Oxford handbook of impulse control disorders. Grant JE, Potenza MN, eds. Oxford, United Kingdom: Oxford University Press; 2012:353-359.
57. Dell’Osso B, Altamura AC, Allen A, et al. Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):464-475.
Chronic disruptive and impulsive behaviors are significant concerns for psychiatric clinicians because of their persistence and potential legal ramifications. To date, few studies have assessed treatment options for pyromania, oppositional defiant disorder (ODD), intermittent explosive disorder (IED), kleptomania, and conduct disorder (CD).
This article reviews the literature on the treatment of these disorders, focusing primarily on randomized, controlled studies. Because of the lack of clinical studies for these disorders, however, case studies and open trials are mentioned for reference. Summaries of supported medication and psychological interventions are provided for each disorder.
Categorizing impulse-control disorders
The DSM-5 created a new chapter on disruptive, impulse control, and conduct disorders that brought together disorders previously classified as disorders usually first diagnosed in infancy, childhood, or adolescence (ODD, CD) and impulse-control disorders not elsewhere classified. These disorders are unified by the presence of difficult, disruptive, aggressive, or antisocial behavior. Disruptive, aggressive, or antisocial behavior usually is a multifaceted behavior, often associated with physical or verbal injury to self, others, or objects or with violating the rights of others. These behaviors can appear in several forms and can be defensive, premeditated, or impulsive.
Despite a high prevalence in the general population1 and in psychiatric cohorts,2 disruptive and impulse-control disorders have been relatively understudied. Controlled trials of treatments do not exist for many impulse-control disorders, and there are no FDA-approved medications for any of these disorders.
Oppositional defiant disorder
Irritability, anger, defiance, and temper are specific descriptors of ODD. ODD seems to be a developmental antecedent for some youth with CD, suggesting that these disorders could reflect different stages of a spectrum of disruptive behavior. Transient oppositional behavior is common among children and adolescents, but ODD occurs in 1% to 11% of youth.3 The disorder is more prevalent among boys before puberty and has an equal sex prevalence in young people after puberty.
Regrettably, most ODD research has included patients with comorbidities, most commonly attention-deficit/hyperactivity disorder (ADHD). Because of this limitation, the drugs and programs discussed below are drawn from meta-analyses and review articles.
Pharmacotherapy. No medications have been FDA-approved for ODD. Studies assessing ODD have employed a variety of methodologies, not all of which are double-blind. The meta-analyses and reviews cited in this section include both randomized and open trials, and should be interpreted as such.
Stimulants are commonly used to treat ODD because of a high comorbidity rate with ADHD, and these drugs have improved ODD symptoms in randomized trials.4 Methylphenidate and d-amphetamine have shown some efficacy in trials of ODD and CD.5-7 These medications are most commonly used when ODD is complicated by ADHD symptoms.
Antipsychotics also have been used to treat ODD, with the largest body of research suggesting that risperidone has some efficacy. Risperidone usually is considered a second- or third-line option because it has been associated with adverse effects in children and adolescents and requires caution in younger populations, despite its potential efficacy.4,8-10
Alpha-2 agonists—clonidine and guanfacine—have shown some efficacy in treating ODD but have not been studied extensively. Studies of clonidine, however, often have grouped ODD, CD, and ADHD, which limits our understanding of this medication for ODD alone.4,5,11
Atomoxetine has been studied for ODD, but its efficacy is limited, with different meta-analyses finding distinct results regarding efficacy. One explanation for these disparate findings is that improvements in oppositional symptoms may be secondary to improvement in ADHD symptoms.7,12-14
Psychological treatments. As noted for pharmacotherapy, this section provides general information on empirically studied therapies. A series of meta-analyses have been included for further review, but are not isolated to randomized, controlled studies.
Individual therapy has shown consistent improvements in ODD. Examples include behavior modification therapy and parent-child interaction therapy. These sessions emphasize skills to manage outbursts and erratic emotionality. Emotion regulation and behavior and social skills training have shown significant reductions in target measures. Some of these programs incorporate both patient and parent components.15-17
Family/teacher training programs such as “Helping the Noncompliant Child” and the “Triple P” have yielded significant improvements. These programs focus on ways to manage the child’s oppositional behavior at home and in the classroom, as well as strategies to limit positive reinforcement for problem behaviors.17-20
Group programs have shown some efficacy with ODD. These programs cover a wide number of needs and intents. Examples include the “Incredible Years” program and the Community Parent Education Program. Research has found that these programs show some efficacy as preemptive measures to reduce the rate of ODD among adolescents.
Conclusions. A number of treatment options for ODD have shown some efficacy. However, many of these options have only been studied in patients with comorbid ADHD, which limits current knowledge about ODD as a distinct disorder.
Intermittent explosive disorder
IED is defined by recurrent, significant outbursts of aggression, often leading to assaultive acts against people or property, which are disproportionate to outside stressors and are not better explained by another psychiatric diagnosis. Research suggests IED is common, with 6.3% of a community sample meeting criteria for lifetime IED.21
IED symptoms tend to start in adolescence and appear to be chronic.21,22 People with IED regard their behavior as distressing and problematic.22 Outbursts generally are short-lived (usually <30 minutes) and frequent (multiple times a month22). Legal and occupational difficulties are common.22
Pharmacotherapy. Data on drug treatment for IED comes for a small set of double-blind studies (Table). Although pharmacotherapies have been studied for treating aggression, impulsivity, and violent behavior, only 5 controlled studies are specific to IED.
A double-blind, randomized, placebo-controlled trial of fluoxetine in 100 participants with IED found that fluoxetine produced a sustained reduction in aggression and irritability as early as the second week of treatment. Full or partial remission of impulsive aggressive behaviors occurred in 46% of fluoxetine-treated subjects. These findings have been supported by studies assessing other samples of aggressive patients, but not specifically IED.23,24 Another treatment study found that oxcarbazepine produced significant improvements in IED symptom severity, specifically on impulsive aggression.25
In a randomized, double-blind, placebo-controlled study, 96 participants with Cluster B personality disorders, 116 with IED, and 34 with posttraumatic stress disorder were assigned to divalproex sodium or placebo for 12 weeks. Using an intent-to-treat analysis, divalproex had no significant influence on aggression in patients with IED.26 Similarly, a study assessing levetiracetam for IED did not show any improvements to measures of impulsive aggression.27
Psychological treatments. The only available study on psychological treatments for IED found that patients receiving active cognitive-behavioral therapy (CBT) or group therapy showed significant improvements compared with waitlist controls. These improvements spanned several target symptoms of IED.28
Conclusions. Although there is a paucity of treatment studies for IED, fluoxetine may be an effective treatment based on available studies, and oxcarbazepine has shown some preliminary efficacy. CBT also has shown some initial efficacy in reducing symptom severity in IED.
Conduct disorder
The essential feature of CD is a repetitive and persistent pattern of behavior in which the basic rights of others or social norms are violated.3 These behaviors can entail:
• aggressive conduct that causes or threatens harm to others or to animals
• nonaggressive behavior resulting in property damage
• deceitfulness or theft
• serious violation of rules.
Prevalence among the general population is 2% to 10%. The disorder is more common among boys than girls.3
Pharmacotherapy. No medication is FDA-approved to treat CD. Fifteen controlled studies have examined medications in patients with CD (Table), although a number of these included a high rate of comorbid ADHD.
To date, 7 studies have shown efficacy with lithium for patients with CD.29-35 A number of trials assessing lithium also included a treatment condition with haloperidol, which showed significant improvement.29,30,33,34 Both lithium and haloperidol were associated with select deficits on cognitive tests, suggesting that there may be risks associated with these medications.
Preliminary double-blind results have indicated that methylphenidate, risperidone, quetiapine, molindone, thioridazine, and carbamazepine might be effective options for treating CD.36-43 The evidence for these medications is limited and additional studies are needed to replicate initial findings.
Three studies of divalproex sodium have shown some efficacy in randomized studies comparing high and low dosages of the drug.40-42 Because these studies did not include a placebo, additional studies are necessary to corroborate these findings.
Psychological treatments. Several forms of behavioral, family-based, and school-based therapies have been found effective in randomized trials. Specifically, behavioral therapy and parental skills training have shown consistent benefits for patients and their families. As with ODD, parental training programs for CD focus on parents’ skill acquisition to help manage outbursts and aggressive behavior. These treatments often follow a similar course to those used for other externalizing and disruptive disorders.44-46
Conclusions. Based on evidence, psychotherapy and some pharmacotherapies (eg, lithium) could be considered first-line treatment options for CD. Psychotherapy programs have shown efficacy in reducing aggression in high-risk groups.44 Lithium or antipsychotics could be useful for patients who do not respond sufficiently to psychotherapy. The risk of cognitive deficits with lithium and antipsychotics should be weighed against potential benefits of these medications.33,34
Kleptomania
Kleptomania is characterized by repetitive, poorly controlled stealing of items that are not needed for personal use. Kleptomania often begins in late adolescence or early adulthood.47 The course of the illness generally is chronic, with waxing and waning symptoms. Women are twice as likely as men to suffer from kleptomania.48 People with kleptomania frequently hoard, discard, or return stolen items.47
Most people with kleptomania try unsuccessfully to stop stealing, which often leads to feelings of shame and guilt.48 Many (64% to 87%) have been arrested because of their stealing behavior47; a smaller percentage (15% to 23%) have been incarcerated.48 Suicide attempts are common among these patients.49
Pharmacotherapy. There has been only 1 randomized, placebo-controlled study of pharmacotherapy for kleptomania (Table). An 8-week, double-blind, placebo-controlled trial was conducted to evaluate the safety and efficacy of oral naltrexone, 50 to 150 mg/d, in 25 patients with kleptomania. Those taking naltrexone had a significantly greater reduction in total score than those taking placebo on the Yale-Brown Obsessive Compulsive Scale Modified for Kleptomania; in stealing urges; and in stealing behavior. The mean effective dosage of naltrexone was 116.7 (± 44.4) mg/d.50
Naltrexone was well tolerated, with minimal nausea, and did not cause elevation of liver enzymes.
There is one available open-label study with a double-blind discontinuation phase assessing the efficacy of escitalopram for kleptomania. Continuation of escitalopram during the blinded discontinuation phase did produce lower relapse rates.51
Psychological treatments. There are no controlled studies of psychological treatments for kleptomania. Case reports suggest that cognitive and behavioral therapies might be effective:
• A young man who underwent 7 sessions of covert sensitization, combined with exposure and response prevention, over a 4-month period was able to reduce his stealing frequency.52
• In another case, a young woman underwent 5 weekly sessions when she was instructed to practice covert sensitization whenever she had an urge to steal. She remained in remission for 14 months with only a single lapse in behavior and with no reported urges to steal.53
• In 2 patients, imaginal desensitization in fourteen 15-minutes sessions over 5 days resulted in complete remission of symptoms for a 2-year period.54
Conclusions. The single controlled study of naltrexone for kleptomania suggests that naltrexone might be a beneficial treatment for this disorder. No controlled trials of psychosocial interventions have been reported. The current psychological research is based primarily on case reports.
This state of affairs likely is because of (1) the low prevalence of kleptomania and (2) clinical difficulties in treating patients involved in illegal activities. Nevertheless, there is a need for systematic studies of treating this disorder; such studies could involve collaboration across multiple treatment centers because of the disorder’s low prevalence.
Pyromania
Pyromania is characterized by (1) deliberate and purposeful fire setting on >1 occasion; (2) tension or affective arousal before the act; (3) fascination with, interest in, curiosity about, or attraction to fire and its situational contexts; and (4) pleasure, gratification, or relief when setting fires or when witnessing or participating in their aftermath.3
Although pyromania is thought to be a disorder primarily affecting men, recent research suggests that the sex ratio is equal among adults and may be slightly higher among adolescent females. Mean age of onset usually is late adolescence. Pyromania appears to be chronic if untreated.55
Urges to set fires are common and the fire setting is almost always pleasurable. Severe distress follows the fire setting, and persons with pyromania report significant functional impairment. High rates of co-occurring psychiatric disorders (depression, substance use disorders, other impulse-control disorders) are common among persons with pyromania.55
Pharmacotherapy. There are no randomized, controlled clinical trials examining pharmacotherapy for treating pyromania. There are no FDA-approved medications for pyromania.
In case reports, medications that have shown benefit in treating pyromania include topiramate, escitalopram, sertraline, fluoxetine, lithium, and a combination of olanzapine and sodium valproate. An equal number of medications have shown no benefit: fluoxetine, valproic acid, lithium, sertraline, olanzapine, escitalopram, citalopram, and clonazepam. A case report of an 18-year-old man with pyromania described successfully using a combination of topiramate with 3 weeks of daily CBT to achieve significant symptom improvement.56,57
Pyromania is a largely unrecognized disorder that causes significant psychological, social, and legal repercussions. Because few persons with pyromania volunteer information regarding fire-setting, it is important that clinicians recognize the disorder and screen patients appropriately. Various treatments have been helpful in case studies, but more research on the etiology and treatment of the disorder is needed.56,57
Conclusions based on the literature
In disruptive, impulse-control, and conduct disorders, the systematic study of treatment efficacy and tolerability is in its infancy. With few controlled studies published, it is not possible to make treatment recommendations with confidence. There are no FDA-approved drugs for treating any of these disorders.
Nonetheless, specific psychotherapies and drug therapies offer promising options, but often are based on small studies, often in patient populations with prominent comorbidities, and have not been replicated by independent investigators. For all of these disorders, issues such as which psychotherapy or medication to use and the ideal duration of treatment cannot be sufficiently addressed with the available data.
In conjunction with emerging epidemiological data supporting a relatively high prevalence of disruptive, impulse-control, and conduct disorders, the small amount of data regarding effective treatments highlights the clinical need for additional research.
Bottom Line
Empirically supported treatment options for impulse-control disorders currently are limited, because only select disorders have been studied across multiple trials. New research is needed to confirm possible treatment options and identify effective psychotherapeutic and pharmacological treatment alternatives.
Related Resources
• Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W. W. Norton & Company; 2008.
• Grant JE, Kim SW. Stop me because I can’t stop myself: taking control of impulsive behavior. New York, NY: McGraw- Hill; 2003.
• American Academy of Child and Adolescent Psychiatry. Conduct disorder resource center. http://www.aacap.org/AACAP/FamiliesandYouth/ResourceCenters/ConductDisorderResourceCenter/Home.aspx.
Drug Brand Names
Atomoxetine • Strattera Methylphenidate • Ritalin
Carbamazepine • Tegretol Molindone • Moban
Citalopram • Celexa Naltrexone • ReVia
Clonazepam • Klonopin Olanzapine • Zyprexa
Clonidine • Catapres Oxcarbazepine • Trileptal
D-amphetamine • Dexedrine Quetiapine • Seroquel
Divalproex sodium • Depakote Risperidone • Risperdal
Escitalopram • Lexapro Sertraline • Zoloft
Fluoxetine • Prozac Sodium valproate • Depacon
Guanfacine • Intuniv Thioridazine • Mellaril
Haloperidol • Haldol Topiramate • Topamax
Levetiracetam • Keppra Valproic acid • Depakote
Lithium • Eskalith, Lithobid
Disclosures
Dr. Grant receives grant or research support from Brainsway, Forest Pharmaceuticals, and Roche Pharmaceuticals. Mr. Leppink reports no financial relationship with any company whose products are mentioned in this article or with competing products.
Chronic disruptive and impulsive behaviors are significant concerns for psychiatric clinicians because of their persistence and potential legal ramifications. To date, few studies have assessed treatment options for pyromania, oppositional defiant disorder (ODD), intermittent explosive disorder (IED), kleptomania, and conduct disorder (CD).
This article reviews the literature on the treatment of these disorders, focusing primarily on randomized, controlled studies. Because of the lack of clinical studies for these disorders, however, case studies and open trials are mentioned for reference. Summaries of supported medication and psychological interventions are provided for each disorder.
Categorizing impulse-control disorders
The DSM-5 created a new chapter on disruptive, impulse control, and conduct disorders that brought together disorders previously classified as disorders usually first diagnosed in infancy, childhood, or adolescence (ODD, CD) and impulse-control disorders not elsewhere classified. These disorders are unified by the presence of difficult, disruptive, aggressive, or antisocial behavior. Disruptive, aggressive, or antisocial behavior usually is a multifaceted behavior, often associated with physical or verbal injury to self, others, or objects or with violating the rights of others. These behaviors can appear in several forms and can be defensive, premeditated, or impulsive.
Despite a high prevalence in the general population1 and in psychiatric cohorts,2 disruptive and impulse-control disorders have been relatively understudied. Controlled trials of treatments do not exist for many impulse-control disorders, and there are no FDA-approved medications for any of these disorders.
Oppositional defiant disorder
Irritability, anger, defiance, and temper are specific descriptors of ODD. ODD seems to be a developmental antecedent for some youth with CD, suggesting that these disorders could reflect different stages of a spectrum of disruptive behavior. Transient oppositional behavior is common among children and adolescents, but ODD occurs in 1% to 11% of youth.3 The disorder is more prevalent among boys before puberty and has an equal sex prevalence in young people after puberty.
Regrettably, most ODD research has included patients with comorbidities, most commonly attention-deficit/hyperactivity disorder (ADHD). Because of this limitation, the drugs and programs discussed below are drawn from meta-analyses and review articles.
Pharmacotherapy. No medications have been FDA-approved for ODD. Studies assessing ODD have employed a variety of methodologies, not all of which are double-blind. The meta-analyses and reviews cited in this section include both randomized and open trials, and should be interpreted as such.
Stimulants are commonly used to treat ODD because of a high comorbidity rate with ADHD, and these drugs have improved ODD symptoms in randomized trials.4 Methylphenidate and d-amphetamine have shown some efficacy in trials of ODD and CD.5-7 These medications are most commonly used when ODD is complicated by ADHD symptoms.
Antipsychotics also have been used to treat ODD, with the largest body of research suggesting that risperidone has some efficacy. Risperidone usually is considered a second- or third-line option because it has been associated with adverse effects in children and adolescents and requires caution in younger populations, despite its potential efficacy.4,8-10
Alpha-2 agonists—clonidine and guanfacine—have shown some efficacy in treating ODD but have not been studied extensively. Studies of clonidine, however, often have grouped ODD, CD, and ADHD, which limits our understanding of this medication for ODD alone.4,5,11
Atomoxetine has been studied for ODD, but its efficacy is limited, with different meta-analyses finding distinct results regarding efficacy. One explanation for these disparate findings is that improvements in oppositional symptoms may be secondary to improvement in ADHD symptoms.7,12-14
Psychological treatments. As noted for pharmacotherapy, this section provides general information on empirically studied therapies. A series of meta-analyses have been included for further review, but are not isolated to randomized, controlled studies.
Individual therapy has shown consistent improvements in ODD. Examples include behavior modification therapy and parent-child interaction therapy. These sessions emphasize skills to manage outbursts and erratic emotionality. Emotion regulation and behavior and social skills training have shown significant reductions in target measures. Some of these programs incorporate both patient and parent components.15-17
Family/teacher training programs such as “Helping the Noncompliant Child” and the “Triple P” have yielded significant improvements. These programs focus on ways to manage the child’s oppositional behavior at home and in the classroom, as well as strategies to limit positive reinforcement for problem behaviors.17-20
Group programs have shown some efficacy with ODD. These programs cover a wide number of needs and intents. Examples include the “Incredible Years” program and the Community Parent Education Program. Research has found that these programs show some efficacy as preemptive measures to reduce the rate of ODD among adolescents.
Conclusions. A number of treatment options for ODD have shown some efficacy. However, many of these options have only been studied in patients with comorbid ADHD, which limits current knowledge about ODD as a distinct disorder.
Intermittent explosive disorder
IED is defined by recurrent, significant outbursts of aggression, often leading to assaultive acts against people or property, which are disproportionate to outside stressors and are not better explained by another psychiatric diagnosis. Research suggests IED is common, with 6.3% of a community sample meeting criteria for lifetime IED.21
IED symptoms tend to start in adolescence and appear to be chronic.21,22 People with IED regard their behavior as distressing and problematic.22 Outbursts generally are short-lived (usually <30 minutes) and frequent (multiple times a month22). Legal and occupational difficulties are common.22
Pharmacotherapy. Data on drug treatment for IED comes for a small set of double-blind studies (Table). Although pharmacotherapies have been studied for treating aggression, impulsivity, and violent behavior, only 5 controlled studies are specific to IED.
A double-blind, randomized, placebo-controlled trial of fluoxetine in 100 participants with IED found that fluoxetine produced a sustained reduction in aggression and irritability as early as the second week of treatment. Full or partial remission of impulsive aggressive behaviors occurred in 46% of fluoxetine-treated subjects. These findings have been supported by studies assessing other samples of aggressive patients, but not specifically IED.23,24 Another treatment study found that oxcarbazepine produced significant improvements in IED symptom severity, specifically on impulsive aggression.25
In a randomized, double-blind, placebo-controlled study, 96 participants with Cluster B personality disorders, 116 with IED, and 34 with posttraumatic stress disorder were assigned to divalproex sodium or placebo for 12 weeks. Using an intent-to-treat analysis, divalproex had no significant influence on aggression in patients with IED.26 Similarly, a study assessing levetiracetam for IED did not show any improvements to measures of impulsive aggression.27
Psychological treatments. The only available study on psychological treatments for IED found that patients receiving active cognitive-behavioral therapy (CBT) or group therapy showed significant improvements compared with waitlist controls. These improvements spanned several target symptoms of IED.28
Conclusions. Although there is a paucity of treatment studies for IED, fluoxetine may be an effective treatment based on available studies, and oxcarbazepine has shown some preliminary efficacy. CBT also has shown some initial efficacy in reducing symptom severity in IED.
Conduct disorder
The essential feature of CD is a repetitive and persistent pattern of behavior in which the basic rights of others or social norms are violated.3 These behaviors can entail:
• aggressive conduct that causes or threatens harm to others or to animals
• nonaggressive behavior resulting in property damage
• deceitfulness or theft
• serious violation of rules.
Prevalence among the general population is 2% to 10%. The disorder is more common among boys than girls.3
Pharmacotherapy. No medication is FDA-approved to treat CD. Fifteen controlled studies have examined medications in patients with CD (Table), although a number of these included a high rate of comorbid ADHD.
To date, 7 studies have shown efficacy with lithium for patients with CD.29-35 A number of trials assessing lithium also included a treatment condition with haloperidol, which showed significant improvement.29,30,33,34 Both lithium and haloperidol were associated with select deficits on cognitive tests, suggesting that there may be risks associated with these medications.
Preliminary double-blind results have indicated that methylphenidate, risperidone, quetiapine, molindone, thioridazine, and carbamazepine might be effective options for treating CD.36-43 The evidence for these medications is limited and additional studies are needed to replicate initial findings.
Three studies of divalproex sodium have shown some efficacy in randomized studies comparing high and low dosages of the drug.40-42 Because these studies did not include a placebo, additional studies are necessary to corroborate these findings.
Psychological treatments. Several forms of behavioral, family-based, and school-based therapies have been found effective in randomized trials. Specifically, behavioral therapy and parental skills training have shown consistent benefits for patients and their families. As with ODD, parental training programs for CD focus on parents’ skill acquisition to help manage outbursts and aggressive behavior. These treatments often follow a similar course to those used for other externalizing and disruptive disorders.44-46
Conclusions. Based on evidence, psychotherapy and some pharmacotherapies (eg, lithium) could be considered first-line treatment options for CD. Psychotherapy programs have shown efficacy in reducing aggression in high-risk groups.44 Lithium or antipsychotics could be useful for patients who do not respond sufficiently to psychotherapy. The risk of cognitive deficits with lithium and antipsychotics should be weighed against potential benefits of these medications.33,34
Kleptomania
Kleptomania is characterized by repetitive, poorly controlled stealing of items that are not needed for personal use. Kleptomania often begins in late adolescence or early adulthood.47 The course of the illness generally is chronic, with waxing and waning symptoms. Women are twice as likely as men to suffer from kleptomania.48 People with kleptomania frequently hoard, discard, or return stolen items.47
Most people with kleptomania try unsuccessfully to stop stealing, which often leads to feelings of shame and guilt.48 Many (64% to 87%) have been arrested because of their stealing behavior47; a smaller percentage (15% to 23%) have been incarcerated.48 Suicide attempts are common among these patients.49
Pharmacotherapy. There has been only 1 randomized, placebo-controlled study of pharmacotherapy for kleptomania (Table). An 8-week, double-blind, placebo-controlled trial was conducted to evaluate the safety and efficacy of oral naltrexone, 50 to 150 mg/d, in 25 patients with kleptomania. Those taking naltrexone had a significantly greater reduction in total score than those taking placebo on the Yale-Brown Obsessive Compulsive Scale Modified for Kleptomania; in stealing urges; and in stealing behavior. The mean effective dosage of naltrexone was 116.7 (± 44.4) mg/d.50
Naltrexone was well tolerated, with minimal nausea, and did not cause elevation of liver enzymes.
There is one available open-label study with a double-blind discontinuation phase assessing the efficacy of escitalopram for kleptomania. Continuation of escitalopram during the blinded discontinuation phase did produce lower relapse rates.51
Psychological treatments. There are no controlled studies of psychological treatments for kleptomania. Case reports suggest that cognitive and behavioral therapies might be effective:
• A young man who underwent 7 sessions of covert sensitization, combined with exposure and response prevention, over a 4-month period was able to reduce his stealing frequency.52
• In another case, a young woman underwent 5 weekly sessions when she was instructed to practice covert sensitization whenever she had an urge to steal. She remained in remission for 14 months with only a single lapse in behavior and with no reported urges to steal.53
• In 2 patients, imaginal desensitization in fourteen 15-minutes sessions over 5 days resulted in complete remission of symptoms for a 2-year period.54
Conclusions. The single controlled study of naltrexone for kleptomania suggests that naltrexone might be a beneficial treatment for this disorder. No controlled trials of psychosocial interventions have been reported. The current psychological research is based primarily on case reports.
This state of affairs likely is because of (1) the low prevalence of kleptomania and (2) clinical difficulties in treating patients involved in illegal activities. Nevertheless, there is a need for systematic studies of treating this disorder; such studies could involve collaboration across multiple treatment centers because of the disorder’s low prevalence.
Pyromania
Pyromania is characterized by (1) deliberate and purposeful fire setting on >1 occasion; (2) tension or affective arousal before the act; (3) fascination with, interest in, curiosity about, or attraction to fire and its situational contexts; and (4) pleasure, gratification, or relief when setting fires or when witnessing or participating in their aftermath.3
Although pyromania is thought to be a disorder primarily affecting men, recent research suggests that the sex ratio is equal among adults and may be slightly higher among adolescent females. Mean age of onset usually is late adolescence. Pyromania appears to be chronic if untreated.55
Urges to set fires are common and the fire setting is almost always pleasurable. Severe distress follows the fire setting, and persons with pyromania report significant functional impairment. High rates of co-occurring psychiatric disorders (depression, substance use disorders, other impulse-control disorders) are common among persons with pyromania.55
Pharmacotherapy. There are no randomized, controlled clinical trials examining pharmacotherapy for treating pyromania. There are no FDA-approved medications for pyromania.
In case reports, medications that have shown benefit in treating pyromania include topiramate, escitalopram, sertraline, fluoxetine, lithium, and a combination of olanzapine and sodium valproate. An equal number of medications have shown no benefit: fluoxetine, valproic acid, lithium, sertraline, olanzapine, escitalopram, citalopram, and clonazepam. A case report of an 18-year-old man with pyromania described successfully using a combination of topiramate with 3 weeks of daily CBT to achieve significant symptom improvement.56,57
Pyromania is a largely unrecognized disorder that causes significant psychological, social, and legal repercussions. Because few persons with pyromania volunteer information regarding fire-setting, it is important that clinicians recognize the disorder and screen patients appropriately. Various treatments have been helpful in case studies, but more research on the etiology and treatment of the disorder is needed.56,57
Conclusions based on the literature
In disruptive, impulse-control, and conduct disorders, the systematic study of treatment efficacy and tolerability is in its infancy. With few controlled studies published, it is not possible to make treatment recommendations with confidence. There are no FDA-approved drugs for treating any of these disorders.
Nonetheless, specific psychotherapies and drug therapies offer promising options, but often are based on small studies, often in patient populations with prominent comorbidities, and have not been replicated by independent investigators. For all of these disorders, issues such as which psychotherapy or medication to use and the ideal duration of treatment cannot be sufficiently addressed with the available data.
In conjunction with emerging epidemiological data supporting a relatively high prevalence of disruptive, impulse-control, and conduct disorders, the small amount of data regarding effective treatments highlights the clinical need for additional research.
Bottom Line
Empirically supported treatment options for impulse-control disorders currently are limited, because only select disorders have been studied across multiple trials. New research is needed to confirm possible treatment options and identify effective psychotherapeutic and pharmacological treatment alternatives.
Related Resources
• Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W. W. Norton & Company; 2008.
• Grant JE, Kim SW. Stop me because I can’t stop myself: taking control of impulsive behavior. New York, NY: McGraw- Hill; 2003.
• American Academy of Child and Adolescent Psychiatry. Conduct disorder resource center. http://www.aacap.org/AACAP/FamiliesandYouth/ResourceCenters/ConductDisorderResourceCenter/Home.aspx.
Drug Brand Names
Atomoxetine • Strattera Methylphenidate • Ritalin
Carbamazepine • Tegretol Molindone • Moban
Citalopram • Celexa Naltrexone • ReVia
Clonazepam • Klonopin Olanzapine • Zyprexa
Clonidine • Catapres Oxcarbazepine • Trileptal
D-amphetamine • Dexedrine Quetiapine • Seroquel
Divalproex sodium • Depakote Risperidone • Risperdal
Escitalopram • Lexapro Sertraline • Zoloft
Fluoxetine • Prozac Sodium valproate • Depacon
Guanfacine • Intuniv Thioridazine • Mellaril
Haloperidol • Haldol Topiramate • Topamax
Levetiracetam • Keppra Valproic acid • Depakote
Lithium • Eskalith, Lithobid
Disclosures
Dr. Grant receives grant or research support from Brainsway, Forest Pharmaceuticals, and Roche Pharmaceuticals. Mr. Leppink reports no financial relationship with any company whose products are mentioned in this article or with competing products.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Turgay A. Psychopharmacological treatment of oppositional defiant disorder. CNS Drugs. 2009;23(1):1-17.
5. Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry. 2010;18(6):556-559.
6. Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: a review of the past 10 years, part II. J Am Acad Child Adolesc Psychiatry. 2002; 41(11):1275-1293.
7. Connor DF, Steeber J, McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr. 2010;31(5):427-440.
8. Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53(1):47-60.e1.
9. Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behavior disorders in children and youths. Cochrane Database Syst Rev. 2012;9:CD008559.
10. Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53(9):948-959.e1.
12. Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):130-137.
13. Bangs ME, Hazell P, Danckaerts M, et al; Atomoxetine ADHD/ODD Study Group. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314-e320.
14. Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology (Berl). 2007;190(1):31-41.
15. Miller NV, Haas SM, Waschbusch DA, et al. Behavior therapy and callous-unemotional traits: effects of a pilot study examining modified behavioral contingencies on child behavior. Behav Ther. 2014;45(5):606-618.
16. Hamilton SS, Armando J. Oppositional defiant disorder. Am Fam Physician. 2008;78(7):861-866.
17. Steiner H, Remsing L; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
18. Winther J, Carlsson A, Vance A. A pilot study of a school-based prevention and early intervention program to reduce oppositional defiant disorder/conduct disorder. Early Interv Psychiatry. 2014;8(2):181-189.
19. Plueck J, Eichelberger I, Hautmann C, et al. Effectiveness of a teacher-based indicated prevention program for preschool children with externalizing problem behavior [published online April 22, 2014]. Prev Sci. doi: 10.1007/s11121-014- 0487-x.
20. Dretzke J, Frew E, Davenport C, et al. The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children. Health Tech Assess. 2005;9(50):iii, ix-x, 1-233.
21. Coccaro EF, Schmidt CA, Samuels JF, et al. Lifetime and 1-month prevalence rates of intermittent explosive disorder in a community sample. J Clin Psychiatry. 2004;65(6):820-824.
22. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry. 1998;59(4):203-210; quiz 211.
23. Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70(5):653-662.
24. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577-588.
25. Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2005;25(6):575-579.
26. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
27. Mattes JA. Levetiracetam in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(2):310-315.
28. McCloskey MS, Noblett KL, Deffenbacher JL, et al. Cognitive-behavioral therapy for intermittent explosive disorder: a pilot randomized clinical trial. J Consult Clin Psychol. 2008;76(5):876-886.
29. Campbell M, Small AM, Green WH, et al. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41(7):650-656.
30. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
31. Malone RP, Simpson GM. Psychopharmacology: use of placebos in clinical trials involving children and adolescents. Psychiatr Serv. 1998;49(11):1413-1414, 1417.
32. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
33. Platt JE, Campbell M, Green WH, et al. Effects of lithium carbonate and haloperidol on cognition in aggressive hospitalized school-age children. J Clin Psychopharmacol. 1981;1(1):8-13.
34. Platt JE, Campbell M, Green WH, et al. Cognitive effects of lithium carbonate and haloperidol in treatment-resistant aggressive children. Arch Gen Psychiatry. 1984;41(7):657-662.
35. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154(4):554-555.
36. Cueva JE, Overall JE, Small AM, et al. Carbamazepine in aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1996;35(4):480-490.
37. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(4):509-516.
38. Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18(2):140-156.
39. Greenhill LL, Solomon M, Pleak R, et al. Molindone hydrochloride treatment of hospitalized children with conduct disorder. J Clin Psychiatry. 1985;46(8 pt 2):20-25.
40. Khanzode LA, Saxena K, Kraemer H, et al. Efficacy profiles of psychopharmacology: divalproex sodium in conduct disorder. Child Psychiatry Hum Dev. 2006;37(1):55-64.
41. Padhy R, Saxena K, Remsing L, et al. Symptomatic response to divalproex in subtypes of conduct disorder. Child Psychiatry Hum Dev. 2011;42(5):584-593.
42. Steiner H, Petersen ML, Saxena K, et al. Divalproex sodium for the treatment of conduct disorder: a randomized controlled clinical trial. J Clin Psychiatry. 2003;64(10):1183-1191.
43. Klein RG, Abikoff H, Klass E, et al. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073-1080.
44. Heneggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. J Marital Fam Ther. 2012;38(1):30-58.
45. Lochman JE, Powell NP, Boxmeyer CL, et al. Cognitive-behavioral therapy for externalizing disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20(2):305-318.
46. Furlong M, McGilloway S, Bywater T, et al. Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev. 2012;2:CD008225.
47. McElroy SL, Pope HG Jr, Hudson JI, et al. Kleptomania: a report of 20 cases. Am J Psychiatry. 1991;148(5):652-657.
48. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients with kleptomania. Compr Psychiatry. 2002;43(5):378-384.
49. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
50. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7): 600-606.
51. Koran LM, Aboujaoude EN, Gamel NN. Escitalopram treatment of kleptomania: an open-label trial followed by double-blind discontinuation. J Clin Psychiatry. 2007;68(3):422-427.
52. Guidry LS. Use of a covert punishing contingency in compulsive stealing. J Behav Therapy Exp Psychiatry. 1975;6(2):169.
53. Gauthier J, Pellerin D. Management of compulsive shoplifting through covert sensitization. J Behav Therapy Exp Psychiatry. 1982;13(1):73-75.
54. McConaghy N, Blaszczynski A. Imaginal desensitization: a cost-effective treatment in two shop-lifters and a binge-eater resistant to previous therapy. Aus N Z J Psychiatry. 1988;22(1):78-82.
55. Grant JE, Won Kim S. Clinical characteristics and psychiatric comorbidity of pyromania. J Clin Psychiatry. 2007;68(11):1717-1722.
56. Grant JE, Odlaug B. Assessment and treatment of pyromania. In: Oxford handbook of impulse control disorders. Grant JE, Potenza MN, eds. Oxford, United Kingdom: Oxford University Press; 2012:353-359.
57. Dell’Osso B, Altamura AC, Allen A, et al. Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):464-475.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Turgay A. Psychopharmacological treatment of oppositional defiant disorder. CNS Drugs. 2009;23(1):1-17.
5. Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry. 2010;18(6):556-559.
6. Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: a review of the past 10 years, part II. J Am Acad Child Adolesc Psychiatry. 2002; 41(11):1275-1293.
7. Connor DF, Steeber J, McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr. 2010;31(5):427-440.
8. Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53(1):47-60.e1.
9. Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behavior disorders in children and youths. Cochrane Database Syst Rev. 2012;9:CD008559.
10. Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53(9):948-959.e1.
12. Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):130-137.
13. Bangs ME, Hazell P, Danckaerts M, et al; Atomoxetine ADHD/ODD Study Group. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314-e320.
14. Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology (Berl). 2007;190(1):31-41.
15. Miller NV, Haas SM, Waschbusch DA, et al. Behavior therapy and callous-unemotional traits: effects of a pilot study examining modified behavioral contingencies on child behavior. Behav Ther. 2014;45(5):606-618.
16. Hamilton SS, Armando J. Oppositional defiant disorder. Am Fam Physician. 2008;78(7):861-866.
17. Steiner H, Remsing L; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
18. Winther J, Carlsson A, Vance A. A pilot study of a school-based prevention and early intervention program to reduce oppositional defiant disorder/conduct disorder. Early Interv Psychiatry. 2014;8(2):181-189.
19. Plueck J, Eichelberger I, Hautmann C, et al. Effectiveness of a teacher-based indicated prevention program for preschool children with externalizing problem behavior [published online April 22, 2014]. Prev Sci. doi: 10.1007/s11121-014- 0487-x.
20. Dretzke J, Frew E, Davenport C, et al. The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children. Health Tech Assess. 2005;9(50):iii, ix-x, 1-233.
21. Coccaro EF, Schmidt CA, Samuels JF, et al. Lifetime and 1-month prevalence rates of intermittent explosive disorder in a community sample. J Clin Psychiatry. 2004;65(6):820-824.
22. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry. 1998;59(4):203-210; quiz 211.
23. Coccaro EF, Lee RJ, Kavoussi RJ. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psychiatry. 2009;70(5):653-662.
24. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577-588.
25. Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2005;25(6):575-579.
26. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
27. Mattes JA. Levetiracetam in patients with impulsive aggression: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(2):310-315.
28. McCloskey MS, Noblett KL, Deffenbacher JL, et al. Cognitive-behavioral therapy for intermittent explosive disorder: a pilot randomized clinical trial. J Consult Clin Psychol. 2008;76(5):876-886.
29. Campbell M, Small AM, Green WH, et al. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41(7):650-656.
30. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
31. Malone RP, Simpson GM. Psychopharmacology: use of placebos in clinical trials involving children and adolescents. Psychiatr Serv. 1998;49(11):1413-1414, 1417.
32. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
33. Platt JE, Campbell M, Green WH, et al. Effects of lithium carbonate and haloperidol on cognition in aggressive hospitalized school-age children. J Clin Psychopharmacol. 1981;1(1):8-13.
34. Platt JE, Campbell M, Green WH, et al. Cognitive effects of lithium carbonate and haloperidol in treatment-resistant aggressive children. Arch Gen Psychiatry. 1984;41(7):657-662.
35. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154(4):554-555.
36. Cueva JE, Overall JE, Small AM, et al. Carbamazepine in aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1996;35(4):480-490.
37. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(4):509-516.
38. Connor DF, McLaughlin TJ, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol. 2008;18(2):140-156.
39. Greenhill LL, Solomon M, Pleak R, et al. Molindone hydrochloride treatment of hospitalized children with conduct disorder. J Clin Psychiatry. 1985;46(8 pt 2):20-25.
40. Khanzode LA, Saxena K, Kraemer H, et al. Efficacy profiles of psychopharmacology: divalproex sodium in conduct disorder. Child Psychiatry Hum Dev. 2006;37(1):55-64.
41. Padhy R, Saxena K, Remsing L, et al. Symptomatic response to divalproex in subtypes of conduct disorder. Child Psychiatry Hum Dev. 2011;42(5):584-593.
42. Steiner H, Petersen ML, Saxena K, et al. Divalproex sodium for the treatment of conduct disorder: a randomized controlled clinical trial. J Clin Psychiatry. 2003;64(10):1183-1191.
43. Klein RG, Abikoff H, Klass E, et al. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073-1080.
44. Heneggeler SW, Sheidow AJ. Empirically supported family-based treatments for conduct disorder and delinquency in adolescents. J Marital Fam Ther. 2012;38(1):30-58.
45. Lochman JE, Powell NP, Boxmeyer CL, et al. Cognitive-behavioral therapy for externalizing disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20(2):305-318.
46. Furlong M, McGilloway S, Bywater T, et al. Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev. 2012;2:CD008225.
47. McElroy SL, Pope HG Jr, Hudson JI, et al. Kleptomania: a report of 20 cases. Am J Psychiatry. 1991;148(5):652-657.
48. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients with kleptomania. Compr Psychiatry. 2002;43(5):378-384.
49. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
50. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7): 600-606.
51. Koran LM, Aboujaoude EN, Gamel NN. Escitalopram treatment of kleptomania: an open-label trial followed by double-blind discontinuation. J Clin Psychiatry. 2007;68(3):422-427.
52. Guidry LS. Use of a covert punishing contingency in compulsive stealing. J Behav Therapy Exp Psychiatry. 1975;6(2):169.
53. Gauthier J, Pellerin D. Management of compulsive shoplifting through covert sensitization. J Behav Therapy Exp Psychiatry. 1982;13(1):73-75.
54. McConaghy N, Blaszczynski A. Imaginal desensitization: a cost-effective treatment in two shop-lifters and a binge-eater resistant to previous therapy. Aus N Z J Psychiatry. 1988;22(1):78-82.
55. Grant JE, Won Kim S. Clinical characteristics and psychiatric comorbidity of pyromania. J Clin Psychiatry. 2007;68(11):1717-1722.
56. Grant JE, Odlaug B. Assessment and treatment of pyromania. In: Oxford handbook of impulse control disorders. Grant JE, Potenza MN, eds. Oxford, United Kingdom: Oxford University Press; 2012:353-359.
57. Dell’Osso B, Altamura AC, Allen A, et al. Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):464-475.
BPD and the broader landscape of neuropsychiatric illness
Dr. Henry A. Nasrallah’s recent Editorial on borderline personality disorder (BPD) (Current Psychiatry, From the Editor, April 2014, p. 19-20, 32 [http://bit.ly/1e8yAwE]) describes BPD as a heritable brain disease. I have been arguing this point for many years, often finding support from my colleague, Hagop Akiskal, MD, and opposition from my psychoanalytic colleagues.
In recent papers1,2 on brain changes in BPD and the connection between BPD and bipolar disorders, I wrote that there often is a heritable aspect to the condition. There are exceptions to such heritability, as in the setting of a horrific environment (eg, father-daughter incest, parental brutality), where the same symptoms seen in BPD develop primarily from post-natal influences. Dr. Akiskal and I were discussing this a long time back, before MRI. Now I feel vindicated, with generous help from someone of Dr. Nasrallah’s prestige and influence.
There also is electrophysiological (including evoked potential) evidence for neural pathology in BPD, as well as data derived from single photon emission CT scanning. The burgeoning literature on MRI and functional MRI studies of BPD is in good agreement about the brain changes most relevant to BPD and that are found with regularity in this condition.
Particularly when BPD is diagnosed in people (usually women) who do not have a history of neglect, sexual molestation, parental humiliation or cruelty, or head injury, what else is there, if not genetically predisposed alterations in the frontolimbic structures (and maybe the periaqueductal gray) that underlie the so-called “personality disorder,” and, not surprisingly, bipolar disorders, especially bipolar II disorder, which often is the other side of the coin as BPD, and amenable to the same combination of medication and psychotherapy?
Michael H. Stone, MD
Professor of Clinical Psychiatry Columbia College of Physicians and Surgeons
New York, New York
----------------------------------------------------------------------------------------------------------
As a psychiatrist/psychoanalyst who works with BPD patients, I read Dr. Nasrallah’s April 2014 Editorial with great interest and enthusiasm. Over the past 10 years, I have been impressed with the number of patients with BPD whose nonverbal learning disorders and auditory and visual processing disorders have gone undiagnosed. Recently, I lectured on this topic to the staff of a school for children with a range of neuropsychiatric disorders; the staff found my observations about such comorbidity consistent with their observations. These dysfunctions, or neurological variations—unknown to the parent and the child—interfere with early object-relation formation, attachment capacity, and learning. Neuropsychiatry and psychological development are, in fact, part of the same system.
An example: For 12 years, I have been treating a patient who has auditory processing and working memory problems, meaning that she could not process the connections among different ideas. This difficulty frustrated her parents, who, in their frustration, criticized her for not paying attention. She was labeled “bad” and assumed the role of the “black sheep” in her family. Although she was intelligent, she was often wrong in her judgments and choices, and easily frustrated. In therapy, as I realized what part of her problem was, I changed my technique.
When my patient asked me to tell her the sequence of understandings that we had just put together, I invited her to take my pad and write down her sense of it. As she described each part of that sequence to me, we would discuss it and I would remind her of lost fragments. Gradually, she learned to put ideas together; however, I also watched her struggle to hold these ideas in working memory and to use them.
Over time, she has improved and is more functional. After several years of disability, she returned to work, although she still struggles interpersonally.
With many of such patients, I have had to modify traditional techniques of psychotherapy. I am fascinated by, and enjoy, such intensive psychotherapy. I am also amazed to see the impact of previously unknown neuropathologic variations on development. The more I learn about the impact of neuropsychiatry on psychological development, the more I can help my patients.
Howard Wishnie, MD
Cambridge, Massachusetts
Dr. Nasrallah responds
I appreciate Dr. Stone’s kind words and concurrence with my thinking about BPD. It would have been appropriate to include discussion of neurophysiological findings in my Editorial, but I opted to use my limited space to focus on structural and functional neuroimaging and genetics.
Henry A. Nasrallah, MD
Professor and Chairman Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
1. Stone MH. The spectrum of borderline personality disorder: a neurophysiological view. Neuropsychiatric Electrophysiology. In press.
2. Stone MH. A new look at borderline personality disorder and related disorders: hyper-activity in the limbic system and lower centers. Psychodyn Psychiatry. 2013;41(3):437-466.
Dr. Henry A. Nasrallah’s recent Editorial on borderline personality disorder (BPD) (Current Psychiatry, From the Editor, April 2014, p. 19-20, 32 [http://bit.ly/1e8yAwE]) describes BPD as a heritable brain disease. I have been arguing this point for many years, often finding support from my colleague, Hagop Akiskal, MD, and opposition from my psychoanalytic colleagues.
In recent papers1,2 on brain changes in BPD and the connection between BPD and bipolar disorders, I wrote that there often is a heritable aspect to the condition. There are exceptions to such heritability, as in the setting of a horrific environment (eg, father-daughter incest, parental brutality), where the same symptoms seen in BPD develop primarily from post-natal influences. Dr. Akiskal and I were discussing this a long time back, before MRI. Now I feel vindicated, with generous help from someone of Dr. Nasrallah’s prestige and influence.
There also is electrophysiological (including evoked potential) evidence for neural pathology in BPD, as well as data derived from single photon emission CT scanning. The burgeoning literature on MRI and functional MRI studies of BPD is in good agreement about the brain changes most relevant to BPD and that are found with regularity in this condition.
Particularly when BPD is diagnosed in people (usually women) who do not have a history of neglect, sexual molestation, parental humiliation or cruelty, or head injury, what else is there, if not genetically predisposed alterations in the frontolimbic structures (and maybe the periaqueductal gray) that underlie the so-called “personality disorder,” and, not surprisingly, bipolar disorders, especially bipolar II disorder, which often is the other side of the coin as BPD, and amenable to the same combination of medication and psychotherapy?
Michael H. Stone, MD
Professor of Clinical Psychiatry Columbia College of Physicians and Surgeons
New York, New York
----------------------------------------------------------------------------------------------------------
As a psychiatrist/psychoanalyst who works with BPD patients, I read Dr. Nasrallah’s April 2014 Editorial with great interest and enthusiasm. Over the past 10 years, I have been impressed with the number of patients with BPD whose nonverbal learning disorders and auditory and visual processing disorders have gone undiagnosed. Recently, I lectured on this topic to the staff of a school for children with a range of neuropsychiatric disorders; the staff found my observations about such comorbidity consistent with their observations. These dysfunctions, or neurological variations—unknown to the parent and the child—interfere with early object-relation formation, attachment capacity, and learning. Neuropsychiatry and psychological development are, in fact, part of the same system.
An example: For 12 years, I have been treating a patient who has auditory processing and working memory problems, meaning that she could not process the connections among different ideas. This difficulty frustrated her parents, who, in their frustration, criticized her for not paying attention. She was labeled “bad” and assumed the role of the “black sheep” in her family. Although she was intelligent, she was often wrong in her judgments and choices, and easily frustrated. In therapy, as I realized what part of her problem was, I changed my technique.
When my patient asked me to tell her the sequence of understandings that we had just put together, I invited her to take my pad and write down her sense of it. As she described each part of that sequence to me, we would discuss it and I would remind her of lost fragments. Gradually, she learned to put ideas together; however, I also watched her struggle to hold these ideas in working memory and to use them.
Over time, she has improved and is more functional. After several years of disability, she returned to work, although she still struggles interpersonally.
With many of such patients, I have had to modify traditional techniques of psychotherapy. I am fascinated by, and enjoy, such intensive psychotherapy. I am also amazed to see the impact of previously unknown neuropathologic variations on development. The more I learn about the impact of neuropsychiatry on psychological development, the more I can help my patients.
Howard Wishnie, MD
Cambridge, Massachusetts
Dr. Nasrallah responds
I appreciate Dr. Stone’s kind words and concurrence with my thinking about BPD. It would have been appropriate to include discussion of neurophysiological findings in my Editorial, but I opted to use my limited space to focus on structural and functional neuroimaging and genetics.
Henry A. Nasrallah, MD
Professor and Chairman Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
Dr. Henry A. Nasrallah’s recent Editorial on borderline personality disorder (BPD) (Current Psychiatry, From the Editor, April 2014, p. 19-20, 32 [http://bit.ly/1e8yAwE]) describes BPD as a heritable brain disease. I have been arguing this point for many years, often finding support from my colleague, Hagop Akiskal, MD, and opposition from my psychoanalytic colleagues.
In recent papers1,2 on brain changes in BPD and the connection between BPD and bipolar disorders, I wrote that there often is a heritable aspect to the condition. There are exceptions to such heritability, as in the setting of a horrific environment (eg, father-daughter incest, parental brutality), where the same symptoms seen in BPD develop primarily from post-natal influences. Dr. Akiskal and I were discussing this a long time back, before MRI. Now I feel vindicated, with generous help from someone of Dr. Nasrallah’s prestige and influence.
There also is electrophysiological (including evoked potential) evidence for neural pathology in BPD, as well as data derived from single photon emission CT scanning. The burgeoning literature on MRI and functional MRI studies of BPD is in good agreement about the brain changes most relevant to BPD and that are found with regularity in this condition.
Particularly when BPD is diagnosed in people (usually women) who do not have a history of neglect, sexual molestation, parental humiliation or cruelty, or head injury, what else is there, if not genetically predisposed alterations in the frontolimbic structures (and maybe the periaqueductal gray) that underlie the so-called “personality disorder,” and, not surprisingly, bipolar disorders, especially bipolar II disorder, which often is the other side of the coin as BPD, and amenable to the same combination of medication and psychotherapy?
Michael H. Stone, MD
Professor of Clinical Psychiatry Columbia College of Physicians and Surgeons
New York, New York
----------------------------------------------------------------------------------------------------------
As a psychiatrist/psychoanalyst who works with BPD patients, I read Dr. Nasrallah’s April 2014 Editorial with great interest and enthusiasm. Over the past 10 years, I have been impressed with the number of patients with BPD whose nonverbal learning disorders and auditory and visual processing disorders have gone undiagnosed. Recently, I lectured on this topic to the staff of a school for children with a range of neuropsychiatric disorders; the staff found my observations about such comorbidity consistent with their observations. These dysfunctions, or neurological variations—unknown to the parent and the child—interfere with early object-relation formation, attachment capacity, and learning. Neuropsychiatry and psychological development are, in fact, part of the same system.
An example: For 12 years, I have been treating a patient who has auditory processing and working memory problems, meaning that she could not process the connections among different ideas. This difficulty frustrated her parents, who, in their frustration, criticized her for not paying attention. She was labeled “bad” and assumed the role of the “black sheep” in her family. Although she was intelligent, she was often wrong in her judgments and choices, and easily frustrated. In therapy, as I realized what part of her problem was, I changed my technique.
When my patient asked me to tell her the sequence of understandings that we had just put together, I invited her to take my pad and write down her sense of it. As she described each part of that sequence to me, we would discuss it and I would remind her of lost fragments. Gradually, she learned to put ideas together; however, I also watched her struggle to hold these ideas in working memory and to use them.
Over time, she has improved and is more functional. After several years of disability, she returned to work, although she still struggles interpersonally.
With many of such patients, I have had to modify traditional techniques of psychotherapy. I am fascinated by, and enjoy, such intensive psychotherapy. I am also amazed to see the impact of previously unknown neuropathologic variations on development. The more I learn about the impact of neuropsychiatry on psychological development, the more I can help my patients.
Howard Wishnie, MD
Cambridge, Massachusetts
Dr. Nasrallah responds
I appreciate Dr. Stone’s kind words and concurrence with my thinking about BPD. It would have been appropriate to include discussion of neurophysiological findings in my Editorial, but I opted to use my limited space to focus on structural and functional neuroimaging and genetics.
Henry A. Nasrallah, MD
Professor and Chairman Department of Neurology & Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
1. Stone MH. The spectrum of borderline personality disorder: a neurophysiological view. Neuropsychiatric Electrophysiology. In press.
2. Stone MH. A new look at borderline personality disorder and related disorders: hyper-activity in the limbic system and lower centers. Psychodyn Psychiatry. 2013;41(3):437-466.
1. Stone MH. The spectrum of borderline personality disorder: a neurophysiological view. Neuropsychiatric Electrophysiology. In press.
2. Stone MH. A new look at borderline personality disorder and related disorders: hyper-activity in the limbic system and lower centers. Psychodyn Psychiatry. 2013;41(3):437-466.
Deaf and self-signing
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
Should you use an anticonvulsant to treat impulsivity and aggression?
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
Borderline personality disorder is a heritable brain disease
The prevailing view among many psychiatrists and mental health professionals is that borderline personality disorder (BPD) is a “psychological” condition. BPD often is conceptualized as a behavioral consequence of childhood trauma; treatment approaches have emphasized intensive psychotherapeutic modalities, less so biologic interventions. You might not be aware that a large body of research over the past decade provides strong evidence that BPD is a neurobiological illness—a finding that would drastically alter how the disorder should be conceptualized and managed.
Neuropathology underpins the personality disorder
Foremost, BPD must be regarded as a serious, disabling brain disorder, not simply an aberration of personality. In DSM-5, symptoms of BPD are listed as: feelings of abandonment; unstable and intense interpersonal relationships; unstable sense of self; impulsivity; suicidal or self-mutilating behavior; affective instability (dysphoria, irritability, anxiety); chronic feelings of emptiness; intense anger episodes; and transient paranoid or dissociative symptoms. Clearly, these clusters of psychopathological and behavioral symptoms reflect a pervasive brain disorder associated with abnormal neurobiology and neural circuitry that might, at times, stubbornly defy therapeutic intervention.
No wonder that 42 published studies report that, compared with healthy controls, people who have BPD display extensive cortical and subcortical abnormalities in brain structure and function.1 These anomalous patterns have been detected across all 4 available neuroimaging techniques.
Magnetic resonance imaging. MRI studies have revealed the following abnormalities in BPD:
• hypoplasia of the hippocampus, caudate, and dorsolateral prefrontal cortex
• variations in the CA1 region of the hippocampus and subiculum
• smaller-than-normal orbitofrontal cortex (by 24%, compared with healthy controls) and the mid-temporal and left cingulate gyrii (by 26%)
• larger-than-normal volume of the right inferior parietal cortex and the right parahippocampal gyrus
• loss of gray matter in the frontal, temporal, and parietal cortices
• an enlarged third cerebral ventricle
• in women, reduced size of the medial temporal lobe and amygdala
• in men, a decreased concentration of gray matter in the anterior cingulate
• reversal of normal right-greater-than-left asymmetry of the orbitofrontal cortex gray matter, reflecting loss of gray matter on the right side
• a lower concentration of gray matter in the rostral/subgenual anterior cingulate cortex
• a smaller frontal lobe.
In an analysis of MRI studies,2 correlation was found between structural brain abnormalities and specific symptoms of BPD, such as impulsivity, suicidality, and aggression. These findings might someday guide personalized interventions—for example, using neurostimulation techniques such as repetitive transcranial magnetic stimulation and deep brain stimulation—to modulate the activity of a given region of the brain (depending on which symptom is most prominent or disabling).
Magnetic resonance spectroscopy. In BPD, MRS studies reveal:
• compared with controls, a higher glutamate level in the anterior cingulate cortex
• reduced levels of N-acetyl aspartate (NAA; found in neurons) and creatinine in the left amygdala
• a reduction (on average, 19%) in the NAA concentration in the dorsolateral prefrontal cortex.
Functional magnetic resonance imaging. From fMRI studies, there is evidence in BPD of:
• greater activation of the amygdala and prolonged return to baseline
• increased functional connectivity in the left frontopolar cortex and left insula
• decreased connectivity in the left cuneus and left inferior parietal and the right middle temporal lobes
• marked frontal hypometabolism
• hypermetabolism in the motor cortex, medial and anterior cingulate, and occipital and temporal poles
• lower connectivity between the amygdala during a neutral stimulus
• higher connectivity between the amygdala during fear stimulus
• higher connectivity between the amygdala during fear stimulus
• deactivation of the opioid system in the left nucleus accumbens, hypothalamus, and hippocampus
• hyperactivation of the left medial prefrontal cortex during social exclusion
• more mistakes made in differentiating an emotional and a neutral facial expression.
Diffusion tensor imaging. DTI white-matter integrity studies of BPD show:
• a bilateral decrease in fractional anisotropy (FA) in frontal, uncinated, and occipitalfrontal fasciculi
• a decrease in FA in the genu and rostrum of the corpus callosum
• a decrease in inter-hemispheric connectivity between right and left anterior cigulate cortices.
Genetic Studies
There is substantial scientific evidence that BPD is highly heritable—a finding that suggests that brain abnormalities of this disorder are a consequence of genes involved in brain development (similar to what is known about schizophrenia, bipolar disorder, and autism).
A systematic review of the heritability of BPD examined 59 published studies that were categorized into 12 family studies, 18 twin studies, 24 association studies, and 5 gene-environment interaction studies.3 The authors concluded that BPD has a strong genetic component, although there also is evidence of gene-environment (G.E) interactions (ie, how nature and nurture influence each other).
The G.E interaction model appears to be consistent with the theory that expression of plasticity genes is modified by childhood experiences and environment, such as physical or sexual abuse. Some studies have found evidence of hypermethylation in BPD, which can exert epigenetic effects. Childhood abuse might, therefore, disrupt certain neuroplasticity genes, culminating in morphological, neurochemical, metabolic, and white-matter aberrations—leading to pathological behavioral patterns identified as BPD.
The neuropsychiatric basis of BPD must guide treatment
There is no such thing as a purely psychological disorder: Invariably, it is an abnormality of brain circuits that disrupts normal development of emotions, thought, behavior, and social cognition. BPD is an exemplar of such neuropsychiatric illness, and treatment should support psychotherapeutic approaches to mend the mind at the same time it moves aggressively to repair the brain.
1. McKenzie CE, Nasrallah HA. Neuroimaging abnormalities in borderline personality disorder: MRI, MRS, fMRI and DTI findings. Poster presented at: 52nd Annual Meeting of the American College of Neuropsychopharmacology; December 8-12, 2013; Hollywood, FL.
2. McKenzie CE, Nasrallah HA. Clinical symptoms of borderline personality disorder are associated with cortical and subcortical abnormalities on brain magnetic resonance imaging (MRI). Poster presented at: 26th Annual Meeting of the U.S. Psychiatric and Mental Health Congress; September 31-October 3, 2013; Las Vegas, NV.
3. Amad A, Ramoz N, Thomas P, et al. Genetics of borderline personality disorder: systematic review and proposal of an integrative model. Neurosci Biobehav Rev. 2014;40C:6-19.
The prevailing view among many psychiatrists and mental health professionals is that borderline personality disorder (BPD) is a “psychological” condition. BPD often is conceptualized as a behavioral consequence of childhood trauma; treatment approaches have emphasized intensive psychotherapeutic modalities, less so biologic interventions. You might not be aware that a large body of research over the past decade provides strong evidence that BPD is a neurobiological illness—a finding that would drastically alter how the disorder should be conceptualized and managed.
Neuropathology underpins the personality disorder
Foremost, BPD must be regarded as a serious, disabling brain disorder, not simply an aberration of personality. In DSM-5, symptoms of BPD are listed as: feelings of abandonment; unstable and intense interpersonal relationships; unstable sense of self; impulsivity; suicidal or self-mutilating behavior; affective instability (dysphoria, irritability, anxiety); chronic feelings of emptiness; intense anger episodes; and transient paranoid or dissociative symptoms. Clearly, these clusters of psychopathological and behavioral symptoms reflect a pervasive brain disorder associated with abnormal neurobiology and neural circuitry that might, at times, stubbornly defy therapeutic intervention.
No wonder that 42 published studies report that, compared with healthy controls, people who have BPD display extensive cortical and subcortical abnormalities in brain structure and function.1 These anomalous patterns have been detected across all 4 available neuroimaging techniques.
Magnetic resonance imaging. MRI studies have revealed the following abnormalities in BPD:
• hypoplasia of the hippocampus, caudate, and dorsolateral prefrontal cortex
• variations in the CA1 region of the hippocampus and subiculum
• smaller-than-normal orbitofrontal cortex (by 24%, compared with healthy controls) and the mid-temporal and left cingulate gyrii (by 26%)
• larger-than-normal volume of the right inferior parietal cortex and the right parahippocampal gyrus
• loss of gray matter in the frontal, temporal, and parietal cortices
• an enlarged third cerebral ventricle
• in women, reduced size of the medial temporal lobe and amygdala
• in men, a decreased concentration of gray matter in the anterior cingulate
• reversal of normal right-greater-than-left asymmetry of the orbitofrontal cortex gray matter, reflecting loss of gray matter on the right side
• a lower concentration of gray matter in the rostral/subgenual anterior cingulate cortex
• a smaller frontal lobe.
In an analysis of MRI studies,2 correlation was found between structural brain abnormalities and specific symptoms of BPD, such as impulsivity, suicidality, and aggression. These findings might someday guide personalized interventions—for example, using neurostimulation techniques such as repetitive transcranial magnetic stimulation and deep brain stimulation—to modulate the activity of a given region of the brain (depending on which symptom is most prominent or disabling).
Magnetic resonance spectroscopy. In BPD, MRS studies reveal:
• compared with controls, a higher glutamate level in the anterior cingulate cortex
• reduced levels of N-acetyl aspartate (NAA; found in neurons) and creatinine in the left amygdala
• a reduction (on average, 19%) in the NAA concentration in the dorsolateral prefrontal cortex.
Functional magnetic resonance imaging. From fMRI studies, there is evidence in BPD of:
• greater activation of the amygdala and prolonged return to baseline
• increased functional connectivity in the left frontopolar cortex and left insula
• decreased connectivity in the left cuneus and left inferior parietal and the right middle temporal lobes
• marked frontal hypometabolism
• hypermetabolism in the motor cortex, medial and anterior cingulate, and occipital and temporal poles
• lower connectivity between the amygdala during a neutral stimulus
• higher connectivity between the amygdala during fear stimulus
• higher connectivity between the amygdala during fear stimulus
• deactivation of the opioid system in the left nucleus accumbens, hypothalamus, and hippocampus
• hyperactivation of the left medial prefrontal cortex during social exclusion
• more mistakes made in differentiating an emotional and a neutral facial expression.
Diffusion tensor imaging. DTI white-matter integrity studies of BPD show:
• a bilateral decrease in fractional anisotropy (FA) in frontal, uncinated, and occipitalfrontal fasciculi
• a decrease in FA in the genu and rostrum of the corpus callosum
• a decrease in inter-hemispheric connectivity between right and left anterior cigulate cortices.
Genetic Studies
There is substantial scientific evidence that BPD is highly heritable—a finding that suggests that brain abnormalities of this disorder are a consequence of genes involved in brain development (similar to what is known about schizophrenia, bipolar disorder, and autism).
A systematic review of the heritability of BPD examined 59 published studies that were categorized into 12 family studies, 18 twin studies, 24 association studies, and 5 gene-environment interaction studies.3 The authors concluded that BPD has a strong genetic component, although there also is evidence of gene-environment (G.E) interactions (ie, how nature and nurture influence each other).
The G.E interaction model appears to be consistent with the theory that expression of plasticity genes is modified by childhood experiences and environment, such as physical or sexual abuse. Some studies have found evidence of hypermethylation in BPD, which can exert epigenetic effects. Childhood abuse might, therefore, disrupt certain neuroplasticity genes, culminating in morphological, neurochemical, metabolic, and white-matter aberrations—leading to pathological behavioral patterns identified as BPD.
The neuropsychiatric basis of BPD must guide treatment
There is no such thing as a purely psychological disorder: Invariably, it is an abnormality of brain circuits that disrupts normal development of emotions, thought, behavior, and social cognition. BPD is an exemplar of such neuropsychiatric illness, and treatment should support psychotherapeutic approaches to mend the mind at the same time it moves aggressively to repair the brain.
The prevailing view among many psychiatrists and mental health professionals is that borderline personality disorder (BPD) is a “psychological” condition. BPD often is conceptualized as a behavioral consequence of childhood trauma; treatment approaches have emphasized intensive psychotherapeutic modalities, less so biologic interventions. You might not be aware that a large body of research over the past decade provides strong evidence that BPD is a neurobiological illness—a finding that would drastically alter how the disorder should be conceptualized and managed.
Neuropathology underpins the personality disorder
Foremost, BPD must be regarded as a serious, disabling brain disorder, not simply an aberration of personality. In DSM-5, symptoms of BPD are listed as: feelings of abandonment; unstable and intense interpersonal relationships; unstable sense of self; impulsivity; suicidal or self-mutilating behavior; affective instability (dysphoria, irritability, anxiety); chronic feelings of emptiness; intense anger episodes; and transient paranoid or dissociative symptoms. Clearly, these clusters of psychopathological and behavioral symptoms reflect a pervasive brain disorder associated with abnormal neurobiology and neural circuitry that might, at times, stubbornly defy therapeutic intervention.
No wonder that 42 published studies report that, compared with healthy controls, people who have BPD display extensive cortical and subcortical abnormalities in brain structure and function.1 These anomalous patterns have been detected across all 4 available neuroimaging techniques.
Magnetic resonance imaging. MRI studies have revealed the following abnormalities in BPD:
• hypoplasia of the hippocampus, caudate, and dorsolateral prefrontal cortex
• variations in the CA1 region of the hippocampus and subiculum
• smaller-than-normal orbitofrontal cortex (by 24%, compared with healthy controls) and the mid-temporal and left cingulate gyrii (by 26%)
• larger-than-normal volume of the right inferior parietal cortex and the right parahippocampal gyrus
• loss of gray matter in the frontal, temporal, and parietal cortices
• an enlarged third cerebral ventricle
• in women, reduced size of the medial temporal lobe and amygdala
• in men, a decreased concentration of gray matter in the anterior cingulate
• reversal of normal right-greater-than-left asymmetry of the orbitofrontal cortex gray matter, reflecting loss of gray matter on the right side
• a lower concentration of gray matter in the rostral/subgenual anterior cingulate cortex
• a smaller frontal lobe.
In an analysis of MRI studies,2 correlation was found between structural brain abnormalities and specific symptoms of BPD, such as impulsivity, suicidality, and aggression. These findings might someday guide personalized interventions—for example, using neurostimulation techniques such as repetitive transcranial magnetic stimulation and deep brain stimulation—to modulate the activity of a given region of the brain (depending on which symptom is most prominent or disabling).
Magnetic resonance spectroscopy. In BPD, MRS studies reveal:
• compared with controls, a higher glutamate level in the anterior cingulate cortex
• reduced levels of N-acetyl aspartate (NAA; found in neurons) and creatinine in the left amygdala
• a reduction (on average, 19%) in the NAA concentration in the dorsolateral prefrontal cortex.
Functional magnetic resonance imaging. From fMRI studies, there is evidence in BPD of:
• greater activation of the amygdala and prolonged return to baseline
• increased functional connectivity in the left frontopolar cortex and left insula
• decreased connectivity in the left cuneus and left inferior parietal and the right middle temporal lobes
• marked frontal hypometabolism
• hypermetabolism in the motor cortex, medial and anterior cingulate, and occipital and temporal poles
• lower connectivity between the amygdala during a neutral stimulus
• higher connectivity between the amygdala during fear stimulus
• higher connectivity between the amygdala during fear stimulus
• deactivation of the opioid system in the left nucleus accumbens, hypothalamus, and hippocampus
• hyperactivation of the left medial prefrontal cortex during social exclusion
• more mistakes made in differentiating an emotional and a neutral facial expression.
Diffusion tensor imaging. DTI white-matter integrity studies of BPD show:
• a bilateral decrease in fractional anisotropy (FA) in frontal, uncinated, and occipitalfrontal fasciculi
• a decrease in FA in the genu and rostrum of the corpus callosum
• a decrease in inter-hemispheric connectivity between right and left anterior cigulate cortices.
Genetic Studies
There is substantial scientific evidence that BPD is highly heritable—a finding that suggests that brain abnormalities of this disorder are a consequence of genes involved in brain development (similar to what is known about schizophrenia, bipolar disorder, and autism).
A systematic review of the heritability of BPD examined 59 published studies that were categorized into 12 family studies, 18 twin studies, 24 association studies, and 5 gene-environment interaction studies.3 The authors concluded that BPD has a strong genetic component, although there also is evidence of gene-environment (G.E) interactions (ie, how nature and nurture influence each other).
The G.E interaction model appears to be consistent with the theory that expression of plasticity genes is modified by childhood experiences and environment, such as physical or sexual abuse. Some studies have found evidence of hypermethylation in BPD, which can exert epigenetic effects. Childhood abuse might, therefore, disrupt certain neuroplasticity genes, culminating in morphological, neurochemical, metabolic, and white-matter aberrations—leading to pathological behavioral patterns identified as BPD.
The neuropsychiatric basis of BPD must guide treatment
There is no such thing as a purely psychological disorder: Invariably, it is an abnormality of brain circuits that disrupts normal development of emotions, thought, behavior, and social cognition. BPD is an exemplar of such neuropsychiatric illness, and treatment should support psychotherapeutic approaches to mend the mind at the same time it moves aggressively to repair the brain.
1. McKenzie CE, Nasrallah HA. Neuroimaging abnormalities in borderline personality disorder: MRI, MRS, fMRI and DTI findings. Poster presented at: 52nd Annual Meeting of the American College of Neuropsychopharmacology; December 8-12, 2013; Hollywood, FL.
2. McKenzie CE, Nasrallah HA. Clinical symptoms of borderline personality disorder are associated with cortical and subcortical abnormalities on brain magnetic resonance imaging (MRI). Poster presented at: 26th Annual Meeting of the U.S. Psychiatric and Mental Health Congress; September 31-October 3, 2013; Las Vegas, NV.
3. Amad A, Ramoz N, Thomas P, et al. Genetics of borderline personality disorder: systematic review and proposal of an integrative model. Neurosci Biobehav Rev. 2014;40C:6-19.
1. McKenzie CE, Nasrallah HA. Neuroimaging abnormalities in borderline personality disorder: MRI, MRS, fMRI and DTI findings. Poster presented at: 52nd Annual Meeting of the American College of Neuropsychopharmacology; December 8-12, 2013; Hollywood, FL.
2. McKenzie CE, Nasrallah HA. Clinical symptoms of borderline personality disorder are associated with cortical and subcortical abnormalities on brain magnetic resonance imaging (MRI). Poster presented at: 26th Annual Meeting of the U.S. Psychiatric and Mental Health Congress; September 31-October 3, 2013; Las Vegas, NV.
3. Amad A, Ramoz N, Thomas P, et al. Genetics of borderline personality disorder: systematic review and proposal of an integrative model. Neurosci Biobehav Rev. 2014;40C:6-19.
Bipolar disorder or something else?
CASE: Unclear diagnosis
Police find Ms. S, age 31, extremely intoxicated and drinking alcohol in her car in a city park parking lot. In the emergency room, she becomes increasingly somnolent and clinicians intubate her trachea to protect her airway. Lab testing shows she has elevated acetaminophen and lithium serum levels, and she is transferred to our hospital for further management after being started on N-acetylcysteine to treat acetaminophen toxicity. Her “ex-fiancé,” the father of her 2 children, saw her earlier the day of the episode and says she was distraught, intoxicated, and had several empty pill bottles in her purse.
In our hospital, Ms. S’ lithium level increases from 2.3 mEq/L to a peak of 5.32 mEq/L, and she undergoes hemodialysis. On hospital day 2, her serum lithium level is trending downward. After Ms. S is able to breathe spontaneously, her trachea is extubated and her hemodialysis line is removed. A psychiatric consultation is obtained, but she is unable to provide a coherent history and the treating clinicians believe she has delirium caused by multiple factors.
On hospital day 3, Ms. S’ delirium clears enough for her to engage in an interview, and she is transferred to our inpatient psychiatry ward for further monitoring and stabilization.
She reports that she was diagnosed with bipolar disorder (BD) at age 12, when she faced multiple psychosocial stressors, including physical abuse by her mother’s boyfriend. She took several psychotropics—although she cannot remember which ones—until age 14, when she stopped all medications until the year before her current hospitalization. Although throughout adolescence and adulthood Ms. S experienced chronic irritability, anxiety, impulsive behavior, poor self-esteem, abusive relationships, self-cutting, and depressed mood, she maintains that she felt worse when she was taking psychotropics and doubts the BD diagnosis. She attributes her longstanding mood issues to low self-worth, a “codependent nature,” and a tendency to gravitate toward abusive relationships. Although she admits to experimenting with several illicit drugs during adolescence, she denies more recent substance use and states she drinks alcohol only once every few months.
The authors’ observations
BD is underdiagnosed in several patient populations, such as individuals previously diagnosed with MDD.1-3 Misdiagnosis can have severe implications, including delay in receiving treatment with effective medications (eg, mood stabilizers) or use of agents that can induce mania or rapid-cycling, such as antidepressants. Perhaps in response to this concern, in recent years clinicians increasingly have diagnosed BD in adolescents and adults. An analysis of a national database of physician practices found a 40-fold increase in office visits for BD among youth and a near doubling among adults from 1994 to 2003.4
Although underdiagnosis of BD remains important, some researchers have suggested that overdiagnosis may be more prevalent and equally harmful. In a study of 180 patients being treated for depression in a family care clinic, there was a 21.6% initial underdiagnosis rate among those eventually found to have BD.1 However, among 43 patients with a prior BD diagnosis, the diagnosis was not confirmed in 33%.1 In a study of 700 psychiatric outpatients in Rhode Island, only 43% of 145 patients who reported a prior BD diagnosis had that diagnosis confirmed.5 Three times as many patients were overdiagnosed with BD as underdiagnosed.
Are there characteristics common to individuals incorrectly diagnosed with BD? In a study that compared patients who had been mistakenly diagnosed with BD with those who had not been diagnosed with BD, the overdiagnosis group was significantly more likely to be diagnosed with a personality disorder, in particular borderline or antisocial personality disorder.6 Only lifetime and current BPD, current posttraumatic stress disorder (PTSD), and lifetime impulse control disorders were independently associated with BD overdiagnosis. The odds ratio for overdiagnosis of BD in patients found to have BPD was 3.7.
EVALUATION: Rethink the diagnosis
In the last few months, Ms. S had complained to her primary care provider (PCP) of worsening anxiety and depressed mood. She was the victim of ongoing physical and emotional abuse by her ex-fiancé and was concerned that she may lose custody of her 2 sons. Approximately 8 months before admission, Ms. S’ PCP prescribed lithium, 450 mg, 3 times a day, for “mood stabilization” and depression because she’d already been diagnosed with BD. This was the first mood stabilizer she’d taken since she was 14. She also was taking unknown doses of hydrocodone/acetaminophen, cyclobenzaprine, and tramadol for pain and temazepam for insomnia. Ms. S continued to suffer from labile and depressed mood, and fought with her ex-fiancé and legal authorities to maintain custody of her 2 children until she was found in the park.
Throughout her hospitalization she denies that she attempted suicide that day, and maintains that this incident was caused by unintentional mismanagement of her medications. Although she continues to have a sense of low self-worth, she denies feeling depressed; in contrast, she says she feels like she has a “new lease on life.” During several interviews she cannot provide a history of any prolonged (ie, several days) episodes of elevated mood, increased goal-directed behavior, decreased need for sleep, tangential thought, pressured speech, or other symptoms that suggest hypomania or mania. She does not endorse prolonged periods of neurovegetative symptoms that would indicate a major depressive episode.
We feel that Ms. S’ symptoms of affective dysregulation, impulsivity, and interpersonal dysfunction are consistent with BPD, and we determine that she meets 6 of the 9 DSM-IV-TR diagnostic features of BPD (≥5 are required for a BPD diagnosis) (Table 1).7 Ms. S describes efforts to avoid abandonment, unstable and intense interpersonal relationships, marked and persistent unstable self-image, recurrent suicidal and self-mutilating behavior, affective instability, and chronic feelings of emptiness. She is discharged to follow up with a psychotherapist and family practitioner. She is not continued on any psychotropic medications.
The authors’ observations
Although it can be difficult to accurately diagnose psychiatric illness during a brief inpatient hospitalization, several clinicians who cared for Ms. S felt that her presentation was more consistent with BPD than BD. Her case is an example of the potential harm of incorrectly diagnosing personality-disordered patients with BD. Ms. S is impulsive and used lithium—a medication that is the standard of care for BD—in an overdose, which lead to a costly and dangerous hospitalization marked by a difficult tracheal intubation and hemodialysis.
Table 1
DSM-IV-TR diagnostic criteria for borderline personality disorder
| A pervasive pattern of instability of interpersonal relationships, self-image, and affects, and marked impulsivity, as indicated by ≥5 of the following: |
|
| Source: Reference 7 |
Distinguishing BD and BPD
There is considerable overlap in symptoms of BD and BPD. Although the episodic nature of BD is well differentiated from the more chronic course of BPD, many hypomania and mania symptoms are similar to those of BPD (Table 2).7 For example, patients with BD or BPD may exhibit impulsive behavior and labile moods. Substance use, risky and self-destructive behaviors, and inflammatory interpersonal relationships can occur in both disorders. Some researchers have suggested that pathophysiologically, BPD may fall on a spectrum of bipolar illness, and have proposed a clinical entity they call bipolar type IV or ultra-rapid cycling BD.2,8,9 There may be more co-occurrence of BD with BPD than would be expected by chance10; 1 review of BPD studies found the rate of comorbid BD ranged from 5.6% to 19%.11 However, because of differences in several factors—including phenomenology, family prevalence, longitudinal course, and medication response—some researchers have concluded that evidence does not support categorizing BPD as part of a bipolar spectrum.10-14 Nonetheless, BPD and other personality disorders often co-occur with axis I disorders, including MDD, BD, or PTSD.
Some research has suggested that the increasing availability and marketing campaigns of medications to treat BD may promote diagnosis of the disorder.15 Zimmerman15 hypothesizes that physicians may be more likely to diagnose a condition that responds to medication (ie, BD) than one that is less responsive (ie, BPD). Financial compensation for treating axis I disorders is significantly better than for treating personality disorders.16 The inpatient setting confers barriers to accurately diagnosing personality disorders, including limits on the amount of time that clinicians can spend with patients or ability to communicate with sources of collateral information. A patient’s observed personality and behaviors while hospitalized may not accurately reflect his or her personality and behaviors in that patient’s “natural” environment.
Several diagnostic strategies can help distinguish BPD from BD. For BD to be the primary diagnosis, a patient must have had a hypomanic or manic episode. Sustained episodes of elation or extreme irritability without evident stressors suggest BD rather than BPD.10 According to Gunderson et al,10 “repeated angry outbursts, suicide attempts, or acts of deliberate self harm that are reactive to interpersonal stress and reflect extreme rejection sensitivity are axiomatic of borderline personality disorder.” In a review of clinical practice, Gunderson17 found that hypersensitivity to rejection and fearful preoccupation with expected abandonment are the most distinctive characteristics of BPD patients. He suggested that clinicians can establish the diagnosis by asking patients directly if they believe the criteria for BPD characterize them, which also can help a patient to accept the diagnosis.
Finally, during a short hospitalization, it can be helpful to obtain collateral information from the patient’s friends and family or further characterize the time course of symptoms and diagnostic features in the patient’s natural environment. Clinicians who are reluctant to diagnose BPD in an inpatient setting could suggest the presence of borderline traits or discuss the possibility of the BPD diagnosis in documentation (eg, in the assessment or formulation). Doing so would avoid a premature BPD diagnosis and allow outpatient providers to confirm or rule out personality disorder diagnoses over time. It is important to screen patients with BPD for co-occurring axis I disorders, including BD, MDD, PTSD, and substance abuse.
A false-positive BD diagnosis in patients with BPD has serious treatment implications. Antipsychotics, antidepressants, and anticonvulsants have been used to target BPD symptoms such as affective dysregulation, impulsivity, and cognitive/perceptual abnormalities, but no medications are FDA-approved for treating BPD. American Psychiatric Association guidelines recommend symptom-based pharmacologic strategies for BPD,18 although some researchers believe that these recommendations are out-of-date and not evidence-based.17,19 Some evidence suggests pharmacotherapy can have modest short-term benefits on specific BPD symptoms, but no data suggest that medication can reduce the severity of BPD or lead to remission.19-23 Just 1 randomized controlled trial (N = 17) has examined lithium for BPD and found no effect on mood.11,24
Misdiagnosis of BD in the context of BPD may create unrealistic expectations regarding the potential efficacy of medications for relieving symptoms. Patients may be diverted from potentially helpful psychotherapeutic treatments—such as DBT or mentalization therapy—which evidence suggests can effectively reduce symptoms, the need for additional treatments, and self-harm or suicidal behaviors.10,17,19 Evidence from long-term longitudinal studies suggests that psychosocial or psychotherapeutic treatment may protect against suicide in BPD patients.25
Table 2
DSM-IV-TR diagnostic criteria for a manic episode
|
The DSM-IV-TR diagnostic criteria for a hypomanic episode are similar to criteria for a manic episode, except:
|
| Source: Reference 7 |
Related Resources
- National Education Alliance Borderline Personality Disorder. www.borderlinepersonalitydisorder.com.
- Hoffman PD, Steiner-Grossman P. Borderline personality disorder: meeting the challenges to successful treatment. Philadelphia, PA: Haworth Press; 2008.
Drug Brand Names
- Cyclobenzaprine • Flexeril
- Hydrocodone/acetaminophen • Lorcet, Vicodin, others
- Lithium • Eskalith, Lithobid
- Temazepam • Restoril
- Tramadol • Ultram
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18(4):233-239.
2. Ghaemi SN, Ko JY, Goodwin FK. “Cade’s disease” and beyond: Misdiagnosis antidepressant use, and a proposed definition for bipolar spectrum disorder. Can J Psychiatry. 2002;47(2):125-134.
3. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv. 2001;52(1):51-55.
4. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
5. Zimmerman M, Ruggero CJ, Chelminski I, et al. Is bipolar disorder overdiagnosed? J Clin Psychiatry. 2008;69(6):935-940.
6. Zimmerman M, Ruggero CJ, Chelminski I, et al. Psychiatric diagnoses in patients previously overdiagnosed with bipolar disorder. J Clin Psychiatry. 2010;71(1):26-31.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Akiskal HS. The bipolar spectrum-the shaping of a new paradigm in psychiatry. Curr Psychiatry Rep. 2002;4(1):1-3.
9. Akiskal HS, Pinto O. The evolving bipolar spectrum. Prototypes I II, III, and IV. Psychiatr Clin North Am. 1999;22(3):517-534, vii.
10. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
11. Paris J, Gunderson J, Weinberg I. The interface between borderline personality disorder and bipolar spectrum disorders. Compr Psychiatry. 2007;48(2):145-154.
12. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-39.
13. Paris J. Borderline or bipolar? Distinguishing borderline personality disorder from bipolar spectrum disorders. Harv Rev Psychiatry. 2004;12(3):140-145.
14. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
15. Zimmerman M. Problems diagnosing bipolar disorder in clinical practice. Expert Rev Neurother. 2010;10(7):1019-1021.
16. Stone MH. Relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1126-1128.
17. Gunderson JG. Clinical practice. Borderline personality disorder. N Engl J Med. 2011;364(21):2037-2042.
18. American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Washington D.C.: American Psychiatric Association; 2001.
19. Paris J. The treatment of borderline personality disorder: implications of research on diagnosis etiology, and outcome. Annu Rev Clin Psychol. 2009;5:277-290.
20. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653.-
21. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
22. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174.
23. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
24. Links PS, Steiner M, Boiago I, et al. Lithium therapy for borderline patients: preliminary findings. J Pers Disord. 1990;4(2):173-181.
25. Goodman M, Roiff T, Oakes AH, et al. Suicidal risk and management in borderline personality disorder. Curr Psychiatry Rep. 2012;14(1):79-85.
CASE: Unclear diagnosis
Police find Ms. S, age 31, extremely intoxicated and drinking alcohol in her car in a city park parking lot. In the emergency room, she becomes increasingly somnolent and clinicians intubate her trachea to protect her airway. Lab testing shows she has elevated acetaminophen and lithium serum levels, and she is transferred to our hospital for further management after being started on N-acetylcysteine to treat acetaminophen toxicity. Her “ex-fiancé,” the father of her 2 children, saw her earlier the day of the episode and says she was distraught, intoxicated, and had several empty pill bottles in her purse.
In our hospital, Ms. S’ lithium level increases from 2.3 mEq/L to a peak of 5.32 mEq/L, and she undergoes hemodialysis. On hospital day 2, her serum lithium level is trending downward. After Ms. S is able to breathe spontaneously, her trachea is extubated and her hemodialysis line is removed. A psychiatric consultation is obtained, but she is unable to provide a coherent history and the treating clinicians believe she has delirium caused by multiple factors.
On hospital day 3, Ms. S’ delirium clears enough for her to engage in an interview, and she is transferred to our inpatient psychiatry ward for further monitoring and stabilization.
She reports that she was diagnosed with bipolar disorder (BD) at age 12, when she faced multiple psychosocial stressors, including physical abuse by her mother’s boyfriend. She took several psychotropics—although she cannot remember which ones—until age 14, when she stopped all medications until the year before her current hospitalization. Although throughout adolescence and adulthood Ms. S experienced chronic irritability, anxiety, impulsive behavior, poor self-esteem, abusive relationships, self-cutting, and depressed mood, she maintains that she felt worse when she was taking psychotropics and doubts the BD diagnosis. She attributes her longstanding mood issues to low self-worth, a “codependent nature,” and a tendency to gravitate toward abusive relationships. Although she admits to experimenting with several illicit drugs during adolescence, she denies more recent substance use and states she drinks alcohol only once every few months.
The authors’ observations
BD is underdiagnosed in several patient populations, such as individuals previously diagnosed with MDD.1-3 Misdiagnosis can have severe implications, including delay in receiving treatment with effective medications (eg, mood stabilizers) or use of agents that can induce mania or rapid-cycling, such as antidepressants. Perhaps in response to this concern, in recent years clinicians increasingly have diagnosed BD in adolescents and adults. An analysis of a national database of physician practices found a 40-fold increase in office visits for BD among youth and a near doubling among adults from 1994 to 2003.4
Although underdiagnosis of BD remains important, some researchers have suggested that overdiagnosis may be more prevalent and equally harmful. In a study of 180 patients being treated for depression in a family care clinic, there was a 21.6% initial underdiagnosis rate among those eventually found to have BD.1 However, among 43 patients with a prior BD diagnosis, the diagnosis was not confirmed in 33%.1 In a study of 700 psychiatric outpatients in Rhode Island, only 43% of 145 patients who reported a prior BD diagnosis had that diagnosis confirmed.5 Three times as many patients were overdiagnosed with BD as underdiagnosed.
Are there characteristics common to individuals incorrectly diagnosed with BD? In a study that compared patients who had been mistakenly diagnosed with BD with those who had not been diagnosed with BD, the overdiagnosis group was significantly more likely to be diagnosed with a personality disorder, in particular borderline or antisocial personality disorder.6 Only lifetime and current BPD, current posttraumatic stress disorder (PTSD), and lifetime impulse control disorders were independently associated with BD overdiagnosis. The odds ratio for overdiagnosis of BD in patients found to have BPD was 3.7.
EVALUATION: Rethink the diagnosis
In the last few months, Ms. S had complained to her primary care provider (PCP) of worsening anxiety and depressed mood. She was the victim of ongoing physical and emotional abuse by her ex-fiancé and was concerned that she may lose custody of her 2 sons. Approximately 8 months before admission, Ms. S’ PCP prescribed lithium, 450 mg, 3 times a day, for “mood stabilization” and depression because she’d already been diagnosed with BD. This was the first mood stabilizer she’d taken since she was 14. She also was taking unknown doses of hydrocodone/acetaminophen, cyclobenzaprine, and tramadol for pain and temazepam for insomnia. Ms. S continued to suffer from labile and depressed mood, and fought with her ex-fiancé and legal authorities to maintain custody of her 2 children until she was found in the park.
Throughout her hospitalization she denies that she attempted suicide that day, and maintains that this incident was caused by unintentional mismanagement of her medications. Although she continues to have a sense of low self-worth, she denies feeling depressed; in contrast, she says she feels like she has a “new lease on life.” During several interviews she cannot provide a history of any prolonged (ie, several days) episodes of elevated mood, increased goal-directed behavior, decreased need for sleep, tangential thought, pressured speech, or other symptoms that suggest hypomania or mania. She does not endorse prolonged periods of neurovegetative symptoms that would indicate a major depressive episode.
We feel that Ms. S’ symptoms of affective dysregulation, impulsivity, and interpersonal dysfunction are consistent with BPD, and we determine that she meets 6 of the 9 DSM-IV-TR diagnostic features of BPD (≥5 are required for a BPD diagnosis) (Table 1).7 Ms. S describes efforts to avoid abandonment, unstable and intense interpersonal relationships, marked and persistent unstable self-image, recurrent suicidal and self-mutilating behavior, affective instability, and chronic feelings of emptiness. She is discharged to follow up with a psychotherapist and family practitioner. She is not continued on any psychotropic medications.
The authors’ observations
Although it can be difficult to accurately diagnose psychiatric illness during a brief inpatient hospitalization, several clinicians who cared for Ms. S felt that her presentation was more consistent with BPD than BD. Her case is an example of the potential harm of incorrectly diagnosing personality-disordered patients with BD. Ms. S is impulsive and used lithium—a medication that is the standard of care for BD—in an overdose, which lead to a costly and dangerous hospitalization marked by a difficult tracheal intubation and hemodialysis.
Table 1
DSM-IV-TR diagnostic criteria for borderline personality disorder
| A pervasive pattern of instability of interpersonal relationships, self-image, and affects, and marked impulsivity, as indicated by ≥5 of the following: |
|
| Source: Reference 7 |
Distinguishing BD and BPD
There is considerable overlap in symptoms of BD and BPD. Although the episodic nature of BD is well differentiated from the more chronic course of BPD, many hypomania and mania symptoms are similar to those of BPD (Table 2).7 For example, patients with BD or BPD may exhibit impulsive behavior and labile moods. Substance use, risky and self-destructive behaviors, and inflammatory interpersonal relationships can occur in both disorders. Some researchers have suggested that pathophysiologically, BPD may fall on a spectrum of bipolar illness, and have proposed a clinical entity they call bipolar type IV or ultra-rapid cycling BD.2,8,9 There may be more co-occurrence of BD with BPD than would be expected by chance10; 1 review of BPD studies found the rate of comorbid BD ranged from 5.6% to 19%.11 However, because of differences in several factors—including phenomenology, family prevalence, longitudinal course, and medication response—some researchers have concluded that evidence does not support categorizing BPD as part of a bipolar spectrum.10-14 Nonetheless, BPD and other personality disorders often co-occur with axis I disorders, including MDD, BD, or PTSD.
Some research has suggested that the increasing availability and marketing campaigns of medications to treat BD may promote diagnosis of the disorder.15 Zimmerman15 hypothesizes that physicians may be more likely to diagnose a condition that responds to medication (ie, BD) than one that is less responsive (ie, BPD). Financial compensation for treating axis I disorders is significantly better than for treating personality disorders.16 The inpatient setting confers barriers to accurately diagnosing personality disorders, including limits on the amount of time that clinicians can spend with patients or ability to communicate with sources of collateral information. A patient’s observed personality and behaviors while hospitalized may not accurately reflect his or her personality and behaviors in that patient’s “natural” environment.
Several diagnostic strategies can help distinguish BPD from BD. For BD to be the primary diagnosis, a patient must have had a hypomanic or manic episode. Sustained episodes of elation or extreme irritability without evident stressors suggest BD rather than BPD.10 According to Gunderson et al,10 “repeated angry outbursts, suicide attempts, or acts of deliberate self harm that are reactive to interpersonal stress and reflect extreme rejection sensitivity are axiomatic of borderline personality disorder.” In a review of clinical practice, Gunderson17 found that hypersensitivity to rejection and fearful preoccupation with expected abandonment are the most distinctive characteristics of BPD patients. He suggested that clinicians can establish the diagnosis by asking patients directly if they believe the criteria for BPD characterize them, which also can help a patient to accept the diagnosis.
Finally, during a short hospitalization, it can be helpful to obtain collateral information from the patient’s friends and family or further characterize the time course of symptoms and diagnostic features in the patient’s natural environment. Clinicians who are reluctant to diagnose BPD in an inpatient setting could suggest the presence of borderline traits or discuss the possibility of the BPD diagnosis in documentation (eg, in the assessment or formulation). Doing so would avoid a premature BPD diagnosis and allow outpatient providers to confirm or rule out personality disorder diagnoses over time. It is important to screen patients with BPD for co-occurring axis I disorders, including BD, MDD, PTSD, and substance abuse.
A false-positive BD diagnosis in patients with BPD has serious treatment implications. Antipsychotics, antidepressants, and anticonvulsants have been used to target BPD symptoms such as affective dysregulation, impulsivity, and cognitive/perceptual abnormalities, but no medications are FDA-approved for treating BPD. American Psychiatric Association guidelines recommend symptom-based pharmacologic strategies for BPD,18 although some researchers believe that these recommendations are out-of-date and not evidence-based.17,19 Some evidence suggests pharmacotherapy can have modest short-term benefits on specific BPD symptoms, but no data suggest that medication can reduce the severity of BPD or lead to remission.19-23 Just 1 randomized controlled trial (N = 17) has examined lithium for BPD and found no effect on mood.11,24
Misdiagnosis of BD in the context of BPD may create unrealistic expectations regarding the potential efficacy of medications for relieving symptoms. Patients may be diverted from potentially helpful psychotherapeutic treatments—such as DBT or mentalization therapy—which evidence suggests can effectively reduce symptoms, the need for additional treatments, and self-harm or suicidal behaviors.10,17,19 Evidence from long-term longitudinal studies suggests that psychosocial or psychotherapeutic treatment may protect against suicide in BPD patients.25
Table 2
DSM-IV-TR diagnostic criteria for a manic episode
|
The DSM-IV-TR diagnostic criteria for a hypomanic episode are similar to criteria for a manic episode, except:
|
| Source: Reference 7 |
Related Resources
- National Education Alliance Borderline Personality Disorder. www.borderlinepersonalitydisorder.com.
- Hoffman PD, Steiner-Grossman P. Borderline personality disorder: meeting the challenges to successful treatment. Philadelphia, PA: Haworth Press; 2008.
Drug Brand Names
- Cyclobenzaprine • Flexeril
- Hydrocodone/acetaminophen • Lorcet, Vicodin, others
- Lithium • Eskalith, Lithobid
- Temazepam • Restoril
- Tramadol • Ultram
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Unclear diagnosis
Police find Ms. S, age 31, extremely intoxicated and drinking alcohol in her car in a city park parking lot. In the emergency room, she becomes increasingly somnolent and clinicians intubate her trachea to protect her airway. Lab testing shows she has elevated acetaminophen and lithium serum levels, and she is transferred to our hospital for further management after being started on N-acetylcysteine to treat acetaminophen toxicity. Her “ex-fiancé,” the father of her 2 children, saw her earlier the day of the episode and says she was distraught, intoxicated, and had several empty pill bottles in her purse.
In our hospital, Ms. S’ lithium level increases from 2.3 mEq/L to a peak of 5.32 mEq/L, and she undergoes hemodialysis. On hospital day 2, her serum lithium level is trending downward. After Ms. S is able to breathe spontaneously, her trachea is extubated and her hemodialysis line is removed. A psychiatric consultation is obtained, but she is unable to provide a coherent history and the treating clinicians believe she has delirium caused by multiple factors.
On hospital day 3, Ms. S’ delirium clears enough for her to engage in an interview, and she is transferred to our inpatient psychiatry ward for further monitoring and stabilization.
She reports that she was diagnosed with bipolar disorder (BD) at age 12, when she faced multiple psychosocial stressors, including physical abuse by her mother’s boyfriend. She took several psychotropics—although she cannot remember which ones—until age 14, when she stopped all medications until the year before her current hospitalization. Although throughout adolescence and adulthood Ms. S experienced chronic irritability, anxiety, impulsive behavior, poor self-esteem, abusive relationships, self-cutting, and depressed mood, she maintains that she felt worse when she was taking psychotropics and doubts the BD diagnosis. She attributes her longstanding mood issues to low self-worth, a “codependent nature,” and a tendency to gravitate toward abusive relationships. Although she admits to experimenting with several illicit drugs during adolescence, she denies more recent substance use and states she drinks alcohol only once every few months.
The authors’ observations
BD is underdiagnosed in several patient populations, such as individuals previously diagnosed with MDD.1-3 Misdiagnosis can have severe implications, including delay in receiving treatment with effective medications (eg, mood stabilizers) or use of agents that can induce mania or rapid-cycling, such as antidepressants. Perhaps in response to this concern, in recent years clinicians increasingly have diagnosed BD in adolescents and adults. An analysis of a national database of physician practices found a 40-fold increase in office visits for BD among youth and a near doubling among adults from 1994 to 2003.4
Although underdiagnosis of BD remains important, some researchers have suggested that overdiagnosis may be more prevalent and equally harmful. In a study of 180 patients being treated for depression in a family care clinic, there was a 21.6% initial underdiagnosis rate among those eventually found to have BD.1 However, among 43 patients with a prior BD diagnosis, the diagnosis was not confirmed in 33%.1 In a study of 700 psychiatric outpatients in Rhode Island, only 43% of 145 patients who reported a prior BD diagnosis had that diagnosis confirmed.5 Three times as many patients were overdiagnosed with BD as underdiagnosed.
Are there characteristics common to individuals incorrectly diagnosed with BD? In a study that compared patients who had been mistakenly diagnosed with BD with those who had not been diagnosed with BD, the overdiagnosis group was significantly more likely to be diagnosed with a personality disorder, in particular borderline or antisocial personality disorder.6 Only lifetime and current BPD, current posttraumatic stress disorder (PTSD), and lifetime impulse control disorders were independently associated with BD overdiagnosis. The odds ratio for overdiagnosis of BD in patients found to have BPD was 3.7.
EVALUATION: Rethink the diagnosis
In the last few months, Ms. S had complained to her primary care provider (PCP) of worsening anxiety and depressed mood. She was the victim of ongoing physical and emotional abuse by her ex-fiancé and was concerned that she may lose custody of her 2 sons. Approximately 8 months before admission, Ms. S’ PCP prescribed lithium, 450 mg, 3 times a day, for “mood stabilization” and depression because she’d already been diagnosed with BD. This was the first mood stabilizer she’d taken since she was 14. She also was taking unknown doses of hydrocodone/acetaminophen, cyclobenzaprine, and tramadol for pain and temazepam for insomnia. Ms. S continued to suffer from labile and depressed mood, and fought with her ex-fiancé and legal authorities to maintain custody of her 2 children until she was found in the park.
Throughout her hospitalization she denies that she attempted suicide that day, and maintains that this incident was caused by unintentional mismanagement of her medications. Although she continues to have a sense of low self-worth, she denies feeling depressed; in contrast, she says she feels like she has a “new lease on life.” During several interviews she cannot provide a history of any prolonged (ie, several days) episodes of elevated mood, increased goal-directed behavior, decreased need for sleep, tangential thought, pressured speech, or other symptoms that suggest hypomania or mania. She does not endorse prolonged periods of neurovegetative symptoms that would indicate a major depressive episode.
We feel that Ms. S’ symptoms of affective dysregulation, impulsivity, and interpersonal dysfunction are consistent with BPD, and we determine that she meets 6 of the 9 DSM-IV-TR diagnostic features of BPD (≥5 are required for a BPD diagnosis) (Table 1).7 Ms. S describes efforts to avoid abandonment, unstable and intense interpersonal relationships, marked and persistent unstable self-image, recurrent suicidal and self-mutilating behavior, affective instability, and chronic feelings of emptiness. She is discharged to follow up with a psychotherapist and family practitioner. She is not continued on any psychotropic medications.
The authors’ observations
Although it can be difficult to accurately diagnose psychiatric illness during a brief inpatient hospitalization, several clinicians who cared for Ms. S felt that her presentation was more consistent with BPD than BD. Her case is an example of the potential harm of incorrectly diagnosing personality-disordered patients with BD. Ms. S is impulsive and used lithium—a medication that is the standard of care for BD—in an overdose, which lead to a costly and dangerous hospitalization marked by a difficult tracheal intubation and hemodialysis.
Table 1
DSM-IV-TR diagnostic criteria for borderline personality disorder
| A pervasive pattern of instability of interpersonal relationships, self-image, and affects, and marked impulsivity, as indicated by ≥5 of the following: |
|
| Source: Reference 7 |
Distinguishing BD and BPD
There is considerable overlap in symptoms of BD and BPD. Although the episodic nature of BD is well differentiated from the more chronic course of BPD, many hypomania and mania symptoms are similar to those of BPD (Table 2).7 For example, patients with BD or BPD may exhibit impulsive behavior and labile moods. Substance use, risky and self-destructive behaviors, and inflammatory interpersonal relationships can occur in both disorders. Some researchers have suggested that pathophysiologically, BPD may fall on a spectrum of bipolar illness, and have proposed a clinical entity they call bipolar type IV or ultra-rapid cycling BD.2,8,9 There may be more co-occurrence of BD with BPD than would be expected by chance10; 1 review of BPD studies found the rate of comorbid BD ranged from 5.6% to 19%.11 However, because of differences in several factors—including phenomenology, family prevalence, longitudinal course, and medication response—some researchers have concluded that evidence does not support categorizing BPD as part of a bipolar spectrum.10-14 Nonetheless, BPD and other personality disorders often co-occur with axis I disorders, including MDD, BD, or PTSD.
Some research has suggested that the increasing availability and marketing campaigns of medications to treat BD may promote diagnosis of the disorder.15 Zimmerman15 hypothesizes that physicians may be more likely to diagnose a condition that responds to medication (ie, BD) than one that is less responsive (ie, BPD). Financial compensation for treating axis I disorders is significantly better than for treating personality disorders.16 The inpatient setting confers barriers to accurately diagnosing personality disorders, including limits on the amount of time that clinicians can spend with patients or ability to communicate with sources of collateral information. A patient’s observed personality and behaviors while hospitalized may not accurately reflect his or her personality and behaviors in that patient’s “natural” environment.
Several diagnostic strategies can help distinguish BPD from BD. For BD to be the primary diagnosis, a patient must have had a hypomanic or manic episode. Sustained episodes of elation or extreme irritability without evident stressors suggest BD rather than BPD.10 According to Gunderson et al,10 “repeated angry outbursts, suicide attempts, or acts of deliberate self harm that are reactive to interpersonal stress and reflect extreme rejection sensitivity are axiomatic of borderline personality disorder.” In a review of clinical practice, Gunderson17 found that hypersensitivity to rejection and fearful preoccupation with expected abandonment are the most distinctive characteristics of BPD patients. He suggested that clinicians can establish the diagnosis by asking patients directly if they believe the criteria for BPD characterize them, which also can help a patient to accept the diagnosis.
Finally, during a short hospitalization, it can be helpful to obtain collateral information from the patient’s friends and family or further characterize the time course of symptoms and diagnostic features in the patient’s natural environment. Clinicians who are reluctant to diagnose BPD in an inpatient setting could suggest the presence of borderline traits or discuss the possibility of the BPD diagnosis in documentation (eg, in the assessment or formulation). Doing so would avoid a premature BPD diagnosis and allow outpatient providers to confirm or rule out personality disorder diagnoses over time. It is important to screen patients with BPD for co-occurring axis I disorders, including BD, MDD, PTSD, and substance abuse.
A false-positive BD diagnosis in patients with BPD has serious treatment implications. Antipsychotics, antidepressants, and anticonvulsants have been used to target BPD symptoms such as affective dysregulation, impulsivity, and cognitive/perceptual abnormalities, but no medications are FDA-approved for treating BPD. American Psychiatric Association guidelines recommend symptom-based pharmacologic strategies for BPD,18 although some researchers believe that these recommendations are out-of-date and not evidence-based.17,19 Some evidence suggests pharmacotherapy can have modest short-term benefits on specific BPD symptoms, but no data suggest that medication can reduce the severity of BPD or lead to remission.19-23 Just 1 randomized controlled trial (N = 17) has examined lithium for BPD and found no effect on mood.11,24
Misdiagnosis of BD in the context of BPD may create unrealistic expectations regarding the potential efficacy of medications for relieving symptoms. Patients may be diverted from potentially helpful psychotherapeutic treatments—such as DBT or mentalization therapy—which evidence suggests can effectively reduce symptoms, the need for additional treatments, and self-harm or suicidal behaviors.10,17,19 Evidence from long-term longitudinal studies suggests that psychosocial or psychotherapeutic treatment may protect against suicide in BPD patients.25
Table 2
DSM-IV-TR diagnostic criteria for a manic episode
|
The DSM-IV-TR diagnostic criteria for a hypomanic episode are similar to criteria for a manic episode, except:
|
| Source: Reference 7 |
Related Resources
- National Education Alliance Borderline Personality Disorder. www.borderlinepersonalitydisorder.com.
- Hoffman PD, Steiner-Grossman P. Borderline personality disorder: meeting the challenges to successful treatment. Philadelphia, PA: Haworth Press; 2008.
Drug Brand Names
- Cyclobenzaprine • Flexeril
- Hydrocodone/acetaminophen • Lorcet, Vicodin, others
- Lithium • Eskalith, Lithobid
- Temazepam • Restoril
- Tramadol • Ultram
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18(4):233-239.
2. Ghaemi SN, Ko JY, Goodwin FK. “Cade’s disease” and beyond: Misdiagnosis antidepressant use, and a proposed definition for bipolar spectrum disorder. Can J Psychiatry. 2002;47(2):125-134.
3. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv. 2001;52(1):51-55.
4. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
5. Zimmerman M, Ruggero CJ, Chelminski I, et al. Is bipolar disorder overdiagnosed? J Clin Psychiatry. 2008;69(6):935-940.
6. Zimmerman M, Ruggero CJ, Chelminski I, et al. Psychiatric diagnoses in patients previously overdiagnosed with bipolar disorder. J Clin Psychiatry. 2010;71(1):26-31.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Akiskal HS. The bipolar spectrum-the shaping of a new paradigm in psychiatry. Curr Psychiatry Rep. 2002;4(1):1-3.
9. Akiskal HS, Pinto O. The evolving bipolar spectrum. Prototypes I II, III, and IV. Psychiatr Clin North Am. 1999;22(3):517-534, vii.
10. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
11. Paris J, Gunderson J, Weinberg I. The interface between borderline personality disorder and bipolar spectrum disorders. Compr Psychiatry. 2007;48(2):145-154.
12. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-39.
13. Paris J. Borderline or bipolar? Distinguishing borderline personality disorder from bipolar spectrum disorders. Harv Rev Psychiatry. 2004;12(3):140-145.
14. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
15. Zimmerman M. Problems diagnosing bipolar disorder in clinical practice. Expert Rev Neurother. 2010;10(7):1019-1021.
16. Stone MH. Relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1126-1128.
17. Gunderson JG. Clinical practice. Borderline personality disorder. N Engl J Med. 2011;364(21):2037-2042.
18. American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Washington D.C.: American Psychiatric Association; 2001.
19. Paris J. The treatment of borderline personality disorder: implications of research on diagnosis etiology, and outcome. Annu Rev Clin Psychol. 2009;5:277-290.
20. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653.-
21. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
22. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174.
23. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
24. Links PS, Steiner M, Boiago I, et al. Lithium therapy for borderline patients: preliminary findings. J Pers Disord. 1990;4(2):173-181.
25. Goodman M, Roiff T, Oakes AH, et al. Suicidal risk and management in borderline personality disorder. Curr Psychiatry Rep. 2012;14(1):79-85.
1. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18(4):233-239.
2. Ghaemi SN, Ko JY, Goodwin FK. “Cade’s disease” and beyond: Misdiagnosis antidepressant use, and a proposed definition for bipolar spectrum disorder. Can J Psychiatry. 2002;47(2):125-134.
3. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv. 2001;52(1):51-55.
4. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
5. Zimmerman M, Ruggero CJ, Chelminski I, et al. Is bipolar disorder overdiagnosed? J Clin Psychiatry. 2008;69(6):935-940.
6. Zimmerman M, Ruggero CJ, Chelminski I, et al. Psychiatric diagnoses in patients previously overdiagnosed with bipolar disorder. J Clin Psychiatry. 2010;71(1):26-31.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
8. Akiskal HS. The bipolar spectrum-the shaping of a new paradigm in psychiatry. Curr Psychiatry Rep. 2002;4(1):1-3.
9. Akiskal HS, Pinto O. The evolving bipolar spectrum. Prototypes I II, III, and IV. Psychiatr Clin North Am. 1999;22(3):517-534, vii.
10. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
11. Paris J, Gunderson J, Weinberg I. The interface between borderline personality disorder and bipolar spectrum disorders. Compr Psychiatry. 2007;48(2):145-154.
12. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-39.
13. Paris J. Borderline or bipolar? Distinguishing borderline personality disorder from bipolar spectrum disorders. Harv Rev Psychiatry. 2004;12(3):140-145.
14. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
15. Zimmerman M. Problems diagnosing bipolar disorder in clinical practice. Expert Rev Neurother. 2010;10(7):1019-1021.
16. Stone MH. Relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1126-1128.
17. Gunderson JG. Clinical practice. Borderline personality disorder. N Engl J Med. 2011;364(21):2037-2042.
18. American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Washington D.C.: American Psychiatric Association; 2001.
19. Paris J. The treatment of borderline personality disorder: implications of research on diagnosis etiology, and outcome. Annu Rev Clin Psychol. 2009;5:277-290.
20. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653.-
21. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
22. Mercer D, Douglass AB, Links PS. Meta-analyses of mood stabilizers antidepressants and antipsychotics in the treatment of borderline personality disorder: effectiveness for depression and anger symptoms. J Pers Disord. 2009;23(2):156-174.
23. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
24. Links PS, Steiner M, Boiago I, et al. Lithium therapy for borderline patients: preliminary findings. J Pers Disord. 1990;4(2):173-181.
25. Goodman M, Roiff T, Oakes AH, et al. Suicidal risk and management in borderline personality disorder. Curr Psychiatry Rep. 2012;14(1):79-85.