User login
Systemic sclerosis overlap syndromes called distinct entity
ISTANBUL – Systemic sclerosis overlap syndromes follow a path of disease progression distinctly different from that of limited or diffuse cutaneous systemic sclerosis, according to a new analysis from the German Network for Systemic Scleroderma.
"This study shows for the first time that patients suffering from systemic sclerosis overlap syndromes should be viewed as a distinct systemic sclerosis subset," Dr. Pia Moinzadeh noted at the annual congress of the European Academy of Dermatology and Venereology.
Those with systemic sclerosis overlap syndromes (SSc-OS) are a heterogeneous subgroup of patients who have the clinical features of SSc according to American College of Rheumatology criteria simultaneously with the characteristic findings of at least one other connective tissue disease, such as dermatomyositis, Sjögren’s syndrome, rheumatoid arthritis, or lupus erythematosus.
The registry of the German Network for Systemic Scleroderma is a uniquely large and inclusive ongoing prospective data base directed by a joint committee of dermatologists and rheumatologists. More than 6 years ago, when the investigators realized that a sizable fraction of patients enrolled in the national registry didn’t fit within the classic bimodal SSc categorization scheme composed of two subtypes – limited cutaneous and diffuse cutaneous SSc – they proposed three additional SSc subtypes. These are SSc-OS, undifferentiated scleroderma, and sclerosis sine scleroderma.
The focus of Dr. Moinzadeh’s EADV presentation was on SSc-OS. Of 3,240 SSc patients in the German registry, patients with OS comprise 11%, while those with limited cutaneous SSc make up 43%, those with diffuse cutaneous disease account for 31%, and the remainder of patients have undifferentiated scleroderma or sclerosis sine scleroderma.
The validity of the SSc-OS diagnostic construct has been controversial. Some experts have contended that patients with SSc-OS ought to be lumped under the headings of limited cutaneous SSc or diffuse cutaneous SSc, depending on the extent of skin involvement. However, the unparalleled size, inclusiveness, and long-term prospective follow-up provided by the German registry have resulted in evidence that argues to the contrary, according to Dr. Moinzadeh, a rheumatologist at the University of Cologne (Germany).
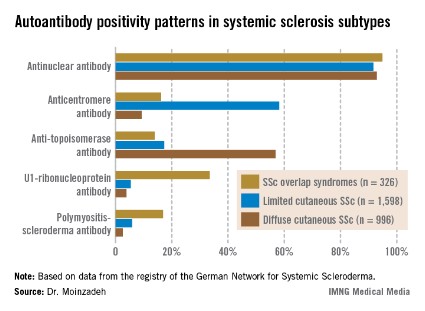
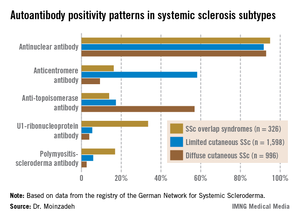
She reported on 326 patients with SSc-OS, 1,598 with limited cutaneous SSc, and 996 with diffuse cutaneous SSc prospectively followed through the German registry for a mean of 10.1 years. The follow-up data indicate SSc-OS evolves differently from either limited cutaneous SSc or diffuse cutaneous SSc. It also displays a distinctive pattern of organ involvement and autoantibody positivity, she noted.
The group with SSc-OS had a mean modified Rodnan skin score of 6.7, similar to the 7.2 score in the limited cutaneous SSc group and markedly less than the 15.8 in the diffuse cutaneous SSc patients. While this might suggest that patients with SSc-OS have a milder course of disease comparable to limited cutaneous SSc, in fact they progressed more rapidly, with earlier and more widespread significant organ involvement and a higher disease burden than the limited cutaneous SSc group, according to Dr. Moinzadeh.
Patients with SSc-OS developed musculoskeletal involvement far more frequently than others. Sixty-seven percent were affected, compared with 38% of patients with limited cutaneous SSc and 48% with diffuse cutaneous SSc. The patients in the SSc-OS group also developed musculoskeletal involvement earlier.
Lung fibrosis occurred in 38% of patients with SSc-OS, 27% of patients with limited cutaneous SSc, and 63% with diffuse cutaneous SSc. Cardiac involvement occurred in 15% with SSc-OS, 10% with limited cutaneous SSc, and 19% with diffuse cutaneous SSc. The onset of these manifestations of SSc in the SSc-OS subgroup was significantly earlier than in the limited cutaneous SSc patients but later than with diffuse cutaneous SSc.
Progression to esophageal and renal involvement, as well as pulmonary arterial hypertension, in the SSc-OS group occurred significantly earlier than in the limited cutaneous SSC patients, but again was later than with diffuse cutaneous SSC.
The autoantibody status of the three subgroups showed distinct differences (see graphic).
The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
ISTANBUL – Systemic sclerosis overlap syndromes follow a path of disease progression distinctly different from that of limited or diffuse cutaneous systemic sclerosis, according to a new analysis from the German Network for Systemic Scleroderma.
"This study shows for the first time that patients suffering from systemic sclerosis overlap syndromes should be viewed as a distinct systemic sclerosis subset," Dr. Pia Moinzadeh noted at the annual congress of the European Academy of Dermatology and Venereology.

Those with systemic sclerosis overlap syndromes (SSc-OS) are a heterogeneous subgroup of patients who have the clinical features of SSc according to American College of Rheumatology criteria simultaneously with the characteristic findings of at least one other connective tissue disease, such as dermatomyositis, Sjögren’s syndrome, rheumatoid arthritis, or lupus erythematosus.
The registry of the German Network for Systemic Scleroderma is a uniquely large and inclusive ongoing prospective data base directed by a joint committee of dermatologists and rheumatologists. More than 6 years ago, when the investigators realized that a sizable fraction of patients enrolled in the national registry didn’t fit within the classic bimodal SSc categorization scheme composed of two subtypes – limited cutaneous and diffuse cutaneous SSc – they proposed three additional SSc subtypes. These are SSc-OS, undifferentiated scleroderma, and sclerosis sine scleroderma.
The focus of Dr. Moinzadeh’s EADV presentation was on SSc-OS. Of 3,240 SSc patients in the German registry, patients with OS comprise 11%, while those with limited cutaneous SSc make up 43%, those with diffuse cutaneous disease account for 31%, and the remainder of patients have undifferentiated scleroderma or sclerosis sine scleroderma.
The validity of the SSc-OS diagnostic construct has been controversial. Some experts have contended that patients with SSc-OS ought to be lumped under the headings of limited cutaneous SSc or diffuse cutaneous SSc, depending on the extent of skin involvement. However, the unparalleled size, inclusiveness, and long-term prospective follow-up provided by the German registry have resulted in evidence that argues to the contrary, according to Dr. Moinzadeh, a rheumatologist at the University of Cologne (Germany).
She reported on 326 patients with SSc-OS, 1,598 with limited cutaneous SSc, and 996 with diffuse cutaneous SSc prospectively followed through the German registry for a mean of 10.1 years. The follow-up data indicate SSc-OS evolves differently from either limited cutaneous SSc or diffuse cutaneous SSc. It also displays a distinctive pattern of organ involvement and autoantibody positivity, she noted.
The group with SSc-OS had a mean modified Rodnan skin score of 6.7, similar to the 7.2 score in the limited cutaneous SSc group and markedly less than the 15.8 in the diffuse cutaneous SSc patients. While this might suggest that patients with SSc-OS have a milder course of disease comparable to limited cutaneous SSc, in fact they progressed more rapidly, with earlier and more widespread significant organ involvement and a higher disease burden than the limited cutaneous SSc group, according to Dr. Moinzadeh.
Patients with SSc-OS developed musculoskeletal involvement far more frequently than others. Sixty-seven percent were affected, compared with 38% of patients with limited cutaneous SSc and 48% with diffuse cutaneous SSc. The patients in the SSc-OS group also developed musculoskeletal involvement earlier.
Lung fibrosis occurred in 38% of patients with SSc-OS, 27% of patients with limited cutaneous SSc, and 63% with diffuse cutaneous SSc. Cardiac involvement occurred in 15% with SSc-OS, 10% with limited cutaneous SSc, and 19% with diffuse cutaneous SSc. The onset of these manifestations of SSc in the SSc-OS subgroup was significantly earlier than in the limited cutaneous SSc patients but later than with diffuse cutaneous SSc.
Progression to esophageal and renal involvement, as well as pulmonary arterial hypertension, in the SSc-OS group occurred significantly earlier than in the limited cutaneous SSC patients, but again was later than with diffuse cutaneous SSC.
The autoantibody status of the three subgroups showed distinct differences (see graphic).
The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
ISTANBUL – Systemic sclerosis overlap syndromes follow a path of disease progression distinctly different from that of limited or diffuse cutaneous systemic sclerosis, according to a new analysis from the German Network for Systemic Scleroderma.
"This study shows for the first time that patients suffering from systemic sclerosis overlap syndromes should be viewed as a distinct systemic sclerosis subset," Dr. Pia Moinzadeh noted at the annual congress of the European Academy of Dermatology and Venereology.

Those with systemic sclerosis overlap syndromes (SSc-OS) are a heterogeneous subgroup of patients who have the clinical features of SSc according to American College of Rheumatology criteria simultaneously with the characteristic findings of at least one other connective tissue disease, such as dermatomyositis, Sjögren’s syndrome, rheumatoid arthritis, or lupus erythematosus.
The registry of the German Network for Systemic Scleroderma is a uniquely large and inclusive ongoing prospective data base directed by a joint committee of dermatologists and rheumatologists. More than 6 years ago, when the investigators realized that a sizable fraction of patients enrolled in the national registry didn’t fit within the classic bimodal SSc categorization scheme composed of two subtypes – limited cutaneous and diffuse cutaneous SSc – they proposed three additional SSc subtypes. These are SSc-OS, undifferentiated scleroderma, and sclerosis sine scleroderma.
The focus of Dr. Moinzadeh’s EADV presentation was on SSc-OS. Of 3,240 SSc patients in the German registry, patients with OS comprise 11%, while those with limited cutaneous SSc make up 43%, those with diffuse cutaneous disease account for 31%, and the remainder of patients have undifferentiated scleroderma or sclerosis sine scleroderma.
The validity of the SSc-OS diagnostic construct has been controversial. Some experts have contended that patients with SSc-OS ought to be lumped under the headings of limited cutaneous SSc or diffuse cutaneous SSc, depending on the extent of skin involvement. However, the unparalleled size, inclusiveness, and long-term prospective follow-up provided by the German registry have resulted in evidence that argues to the contrary, according to Dr. Moinzadeh, a rheumatologist at the University of Cologne (Germany).
She reported on 326 patients with SSc-OS, 1,598 with limited cutaneous SSc, and 996 with diffuse cutaneous SSc prospectively followed through the German registry for a mean of 10.1 years. The follow-up data indicate SSc-OS evolves differently from either limited cutaneous SSc or diffuse cutaneous SSc. It also displays a distinctive pattern of organ involvement and autoantibody positivity, she noted.
The group with SSc-OS had a mean modified Rodnan skin score of 6.7, similar to the 7.2 score in the limited cutaneous SSc group and markedly less than the 15.8 in the diffuse cutaneous SSc patients. While this might suggest that patients with SSc-OS have a milder course of disease comparable to limited cutaneous SSc, in fact they progressed more rapidly, with earlier and more widespread significant organ involvement and a higher disease burden than the limited cutaneous SSc group, according to Dr. Moinzadeh.
Patients with SSc-OS developed musculoskeletal involvement far more frequently than others. Sixty-seven percent were affected, compared with 38% of patients with limited cutaneous SSc and 48% with diffuse cutaneous SSc. The patients in the SSc-OS group also developed musculoskeletal involvement earlier.
Lung fibrosis occurred in 38% of patients with SSc-OS, 27% of patients with limited cutaneous SSc, and 63% with diffuse cutaneous SSc. Cardiac involvement occurred in 15% with SSc-OS, 10% with limited cutaneous SSc, and 19% with diffuse cutaneous SSc. The onset of these manifestations of SSc in the SSc-OS subgroup was significantly earlier than in the limited cutaneous SSc patients but later than with diffuse cutaneous SSc.
Progression to esophageal and renal involvement, as well as pulmonary arterial hypertension, in the SSc-OS group occurred significantly earlier than in the limited cutaneous SSC patients, but again was later than with diffuse cutaneous SSC.
The autoantibody status of the three subgroups showed distinct differences (see graphic).
The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
AT THE EADV CONGRESS
Major finding: Musculoskeletal involvement occurred in 67% of patients with systemic sclerosis overlap syndromes, compared with 38% of those with limited cutaneous systemic sclerosis and 48% with diffuse cutaneous systemic sclerosis.
Data source: This analysis from the ongoing prospective national registry maintained by the German Network for Systemic Scleroderma included nearly 3,000 patients with systemic sclerosis followed for a mean of 10.1 years.
Disclosures: The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
Is PASI 90 becoming the new PASI 75?
ISTANBUL – With the majority of psoriasis patients now achieving PASI 90 responses in randomized trials of the latest-generation biologic agents, a push is on to replace PASI 75 with PASI 90 as the new goal defining treatment success. But some dermatologists have misgivings about raising the bar.
"More and more, editorialists are promoting the idea of silencing psoriasis in all patients. This is a tricky and challenging goal," Dr. Hervé Bachelez said at the annual congress of the European Academy of Dermatology and Venereology.
"You’ve probably noticed that PASI 90 and even PASI 100 are becoming important as secondary endpoints in virtually all clinical trials. It’s good, it’s legitimate, and the PASI 90 probably better reflects the wishes of the patient and the physician than the PASI 75. But we have to wait and see what the caveats of this are. You can say, ‘Let’s push the response rate up to PASI 100 in all patients,’ but the danger is that if you cross a line, you may be unable to precisely regulate the level of immunosuppression in some patients. Basically you can expect some safety issues in real life that you would not see in clinical trials," cautioned Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris.
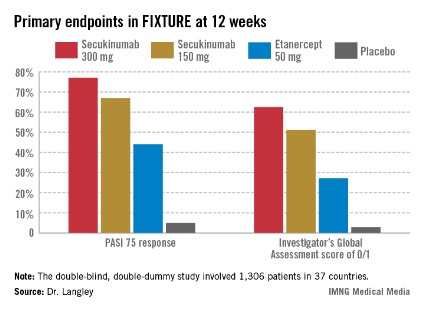
Not so many years ago the notion of PASI 90 responses in high double figures seemed a pipedream, he observed. For example, the week-12 PASI 90 rate in published randomized trials of methotrexate was only 9%, while for the first-generation tumor necrosis factor inhibitor etanercept, the rates were 19%-23%. In contrast, among the highlights of this year’s EADV congress were the presentation of results from clinical trials of two investigational interleukin-17 inhibitors: In the pivotal Phase III FIXTURE trial, secukinumab-treated patients had a PASI 90 response rate of 72% at week 16, while the week-16 PASI 90 rate in the Phase II OLE trial was 87% in patients on brodalimab, and even out to week 96, it was 78%.
Underscoring Dr. Bachelez’ concern that the randomized trial experience likely underestimates the true extent of safety hazards posed by potent therapies in daily clinical practice was a report by a consortium of 13 Spanish dermatology departments responsible for the BIOBADADERM registry. The Spanish registry is focused on safety and includes only psoriasis patients on systemic therapy, whether biologics or classic drugs. Among the first 1,042 enrollees receiving systemic therapy, fully 30% would not have been eligible for participation in randomized controlled trials for various reasons, including age greater than 70 years, having chronic kidney or liver disease, a history of hepatitis B or C, HIV infection, or cancer, or having psoriasis of a type other than chronic plaque disease.
The disturbing finding was that during 2,179 person-years of prospective follow-up, the large group of patients ineligible for randomized trials had a 2.7-fold increased risk of serious adverse events compared with patients on systemic therapy who were eligible for study participation.
The number needed to harm was calculated as follows: For every 40 patients treated with systemic therapy for 2.1 years despite not being eligible for randomized trials, one additional serious adverse event can be expected compared with similar treatment in randomized trial-eligible patients, according to the investigators (Arch. Dermatol. 2012;148:463-70). And that’s without pushing the envelope by trying to aim for a PASI 90 response, Dr. Bachelez noted.
A contrary view regarding PASI 90 as an emerging standard of treatment excellence was put forth elsewhere at the meeting by Dr. Peter van de Kerkhof, professor and head of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
He cited multiple studies demonstrating that substantial PASI reductions may not translate into tangible improvements in patients’ quality of life. For example, among psoriasis patients who achieved a PASI 75 response in one European study, 65% still had a Dermatology Life Quality Index (DLQI) score of 2 or more (Eur. J. Dermatol. 2010;20:62-7).
"This implies that there is something more to be wished for by patients, even when PASI 75 is reached," according to Dr. van de Kerkhof.
Moreover, in another trial, even among patients with a PASI score of 0 at week 24, only 70% had an optimal DLQI of 0, not 100% as most dermatologists might expect (Br. J. Dermatol. 2006;154:1161-8).
A recent survey of 2,151 European psoriasis patients and their dermatologists highlighted a substantial degree of dissatisfaction with current therapies. Patients on biologics had higher rates of improvement from severe to moderate or mild disease than did those on any other forms of psoriasis therapy, yet 41% of patients on biologics were dissatisfied with their treatment (J. Dermatolog. Treat. 2013;24:193-8).
"I think it’s extremely important that we continue to innovate treatment possibilities for psoriasis in order to improve outcomes for our patients," Dr. van de Kerkhof said.
What patients really want, surveys suggest, are treatments that render them clear or nearly clear, and do so quickly. In one survey, patients rated as the most important characteristic about a therapy the rapidity with which it could achieve a moderate 50% improvement in symptoms. They rated that as higher in importance to them than the therapy’s long-term risks (Arch. Dermatol. 2007;143:1175-9), Dr. van de Kerkhof noted.
Patients also place a high priority on improvement of a broad array of symptoms that aren’t captured by either the PASI or the DLQI, Dr. Bruce E. Strober noted in a separate presentation. These include itching, plaque-related pain, altered skin appearance, flaking, and bleeding.
For this reason, he and a group of his coworkers have created and are now validating a new tool for the assessment of patient-related outcomes in psoriasis called the Psoriasis Symptom Diary (Value Health 2013;16:1014-22). The 16-item tool takes less than 5 minutes for a patient to fill out and is designed to replace the DLQI both in clinical trials and everyday practice. The goal is to be able to walk into the examination room, take a quick look at the Psoriasis Symptom Diary, and know from that how a patient is currently doing even before asking for the patient to disrobe.
"Let’s face it: Outside of skin cancer, when we’re doing dermatology, we’re in the quality of life business. That means we’re asking patients how they’re doing at every visit for psoriasis, atopic dermatitis, or severe acne. The DLQI does that, but it’s not psoriasis specific," said Dr. Strober, vice chair and director of the clinical trials unit in the department of dermatology at the University of Connecticut, Farmington.
As for the PASI, he doesn’t use it except in structured clinical trials. It’s too time consuming and has a high rate of inter- and intrarater variability.
"In the United States, dermatologists never do PASI scores in their clinics. The PASI score has numerous drawbacks that make it impractical in a regular practice setting," Dr. Strober said. "In my own practice, I routinely do a 5-point Physician’s Global Assessment along with an estimate of involved body surface area. I think that gives you a good picture of the objective level of psoriasis severity."
Dr. Bachelez, Dr. van de Kerkhof, and Dr. Strober each reported receiving research grants from and serving on advisory boards for 9-14 pharmaceutical companies engaged in developing new treatments for psoriasis.
ISTANBUL – With the majority of psoriasis patients now achieving PASI 90 responses in randomized trials of the latest-generation biologic agents, a push is on to replace PASI 75 with PASI 90 as the new goal defining treatment success. But some dermatologists have misgivings about raising the bar.
"More and more, editorialists are promoting the idea of silencing psoriasis in all patients. This is a tricky and challenging goal," Dr. Hervé Bachelez said at the annual congress of the European Academy of Dermatology and Venereology.
"You’ve probably noticed that PASI 90 and even PASI 100 are becoming important as secondary endpoints in virtually all clinical trials. It’s good, it’s legitimate, and the PASI 90 probably better reflects the wishes of the patient and the physician than the PASI 75. But we have to wait and see what the caveats of this are. You can say, ‘Let’s push the response rate up to PASI 100 in all patients,’ but the danger is that if you cross a line, you may be unable to precisely regulate the level of immunosuppression in some patients. Basically you can expect some safety issues in real life that you would not see in clinical trials," cautioned Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris.
Not so many years ago the notion of PASI 90 responses in high double figures seemed a pipedream, he observed. For example, the week-12 PASI 90 rate in published randomized trials of methotrexate was only 9%, while for the first-generation tumor necrosis factor inhibitor etanercept, the rates were 19%-23%. In contrast, among the highlights of this year’s EADV congress were the presentation of results from clinical trials of two investigational interleukin-17 inhibitors: In the pivotal Phase III FIXTURE trial, secukinumab-treated patients had a PASI 90 response rate of 72% at week 16, while the week-16 PASI 90 rate in the Phase II OLE trial was 87% in patients on brodalimab, and even out to week 96, it was 78%.
Underscoring Dr. Bachelez’ concern that the randomized trial experience likely underestimates the true extent of safety hazards posed by potent therapies in daily clinical practice was a report by a consortium of 13 Spanish dermatology departments responsible for the BIOBADADERM registry. The Spanish registry is focused on safety and includes only psoriasis patients on systemic therapy, whether biologics or classic drugs. Among the first 1,042 enrollees receiving systemic therapy, fully 30% would not have been eligible for participation in randomized controlled trials for various reasons, including age greater than 70 years, having chronic kidney or liver disease, a history of hepatitis B or C, HIV infection, or cancer, or having psoriasis of a type other than chronic plaque disease.
The disturbing finding was that during 2,179 person-years of prospective follow-up, the large group of patients ineligible for randomized trials had a 2.7-fold increased risk of serious adverse events compared with patients on systemic therapy who were eligible for study participation.
The number needed to harm was calculated as follows: For every 40 patients treated with systemic therapy for 2.1 years despite not being eligible for randomized trials, one additional serious adverse event can be expected compared with similar treatment in randomized trial-eligible patients, according to the investigators (Arch. Dermatol. 2012;148:463-70). And that’s without pushing the envelope by trying to aim for a PASI 90 response, Dr. Bachelez noted.
A contrary view regarding PASI 90 as an emerging standard of treatment excellence was put forth elsewhere at the meeting by Dr. Peter van de Kerkhof, professor and head of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
He cited multiple studies demonstrating that substantial PASI reductions may not translate into tangible improvements in patients’ quality of life. For example, among psoriasis patients who achieved a PASI 75 response in one European study, 65% still had a Dermatology Life Quality Index (DLQI) score of 2 or more (Eur. J. Dermatol. 2010;20:62-7).
"This implies that there is something more to be wished for by patients, even when PASI 75 is reached," according to Dr. van de Kerkhof.
Moreover, in another trial, even among patients with a PASI score of 0 at week 24, only 70% had an optimal DLQI of 0, not 100% as most dermatologists might expect (Br. J. Dermatol. 2006;154:1161-8).
A recent survey of 2,151 European psoriasis patients and their dermatologists highlighted a substantial degree of dissatisfaction with current therapies. Patients on biologics had higher rates of improvement from severe to moderate or mild disease than did those on any other forms of psoriasis therapy, yet 41% of patients on biologics were dissatisfied with their treatment (J. Dermatolog. Treat. 2013;24:193-8).
"I think it’s extremely important that we continue to innovate treatment possibilities for psoriasis in order to improve outcomes for our patients," Dr. van de Kerkhof said.
What patients really want, surveys suggest, are treatments that render them clear or nearly clear, and do so quickly. In one survey, patients rated as the most important characteristic about a therapy the rapidity with which it could achieve a moderate 50% improvement in symptoms. They rated that as higher in importance to them than the therapy’s long-term risks (Arch. Dermatol. 2007;143:1175-9), Dr. van de Kerkhof noted.
Patients also place a high priority on improvement of a broad array of symptoms that aren’t captured by either the PASI or the DLQI, Dr. Bruce E. Strober noted in a separate presentation. These include itching, plaque-related pain, altered skin appearance, flaking, and bleeding.
For this reason, he and a group of his coworkers have created and are now validating a new tool for the assessment of patient-related outcomes in psoriasis called the Psoriasis Symptom Diary (Value Health 2013;16:1014-22). The 16-item tool takes less than 5 minutes for a patient to fill out and is designed to replace the DLQI both in clinical trials and everyday practice. The goal is to be able to walk into the examination room, take a quick look at the Psoriasis Symptom Diary, and know from that how a patient is currently doing even before asking for the patient to disrobe.
"Let’s face it: Outside of skin cancer, when we’re doing dermatology, we’re in the quality of life business. That means we’re asking patients how they’re doing at every visit for psoriasis, atopic dermatitis, or severe acne. The DLQI does that, but it’s not psoriasis specific," said Dr. Strober, vice chair and director of the clinical trials unit in the department of dermatology at the University of Connecticut, Farmington.
As for the PASI, he doesn’t use it except in structured clinical trials. It’s too time consuming and has a high rate of inter- and intrarater variability.
"In the United States, dermatologists never do PASI scores in their clinics. The PASI score has numerous drawbacks that make it impractical in a regular practice setting," Dr. Strober said. "In my own practice, I routinely do a 5-point Physician’s Global Assessment along with an estimate of involved body surface area. I think that gives you a good picture of the objective level of psoriasis severity."
Dr. Bachelez, Dr. van de Kerkhof, and Dr. Strober each reported receiving research grants from and serving on advisory boards for 9-14 pharmaceutical companies engaged in developing new treatments for psoriasis.
ISTANBUL – With the majority of psoriasis patients now achieving PASI 90 responses in randomized trials of the latest-generation biologic agents, a push is on to replace PASI 75 with PASI 90 as the new goal defining treatment success. But some dermatologists have misgivings about raising the bar.
"More and more, editorialists are promoting the idea of silencing psoriasis in all patients. This is a tricky and challenging goal," Dr. Hervé Bachelez said at the annual congress of the European Academy of Dermatology and Venereology.
"You’ve probably noticed that PASI 90 and even PASI 100 are becoming important as secondary endpoints in virtually all clinical trials. It’s good, it’s legitimate, and the PASI 90 probably better reflects the wishes of the patient and the physician than the PASI 75. But we have to wait and see what the caveats of this are. You can say, ‘Let’s push the response rate up to PASI 100 in all patients,’ but the danger is that if you cross a line, you may be unable to precisely regulate the level of immunosuppression in some patients. Basically you can expect some safety issues in real life that you would not see in clinical trials," cautioned Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris.
Not so many years ago the notion of PASI 90 responses in high double figures seemed a pipedream, he observed. For example, the week-12 PASI 90 rate in published randomized trials of methotrexate was only 9%, while for the first-generation tumor necrosis factor inhibitor etanercept, the rates were 19%-23%. In contrast, among the highlights of this year’s EADV congress were the presentation of results from clinical trials of two investigational interleukin-17 inhibitors: In the pivotal Phase III FIXTURE trial, secukinumab-treated patients had a PASI 90 response rate of 72% at week 16, while the week-16 PASI 90 rate in the Phase II OLE trial was 87% in patients on brodalimab, and even out to week 96, it was 78%.
Underscoring Dr. Bachelez’ concern that the randomized trial experience likely underestimates the true extent of safety hazards posed by potent therapies in daily clinical practice was a report by a consortium of 13 Spanish dermatology departments responsible for the BIOBADADERM registry. The Spanish registry is focused on safety and includes only psoriasis patients on systemic therapy, whether biologics or classic drugs. Among the first 1,042 enrollees receiving systemic therapy, fully 30% would not have been eligible for participation in randomized controlled trials for various reasons, including age greater than 70 years, having chronic kidney or liver disease, a history of hepatitis B or C, HIV infection, or cancer, or having psoriasis of a type other than chronic plaque disease.
The disturbing finding was that during 2,179 person-years of prospective follow-up, the large group of patients ineligible for randomized trials had a 2.7-fold increased risk of serious adverse events compared with patients on systemic therapy who were eligible for study participation.
The number needed to harm was calculated as follows: For every 40 patients treated with systemic therapy for 2.1 years despite not being eligible for randomized trials, one additional serious adverse event can be expected compared with similar treatment in randomized trial-eligible patients, according to the investigators (Arch. Dermatol. 2012;148:463-70). And that’s without pushing the envelope by trying to aim for a PASI 90 response, Dr. Bachelez noted.
A contrary view regarding PASI 90 as an emerging standard of treatment excellence was put forth elsewhere at the meeting by Dr. Peter van de Kerkhof, professor and head of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
He cited multiple studies demonstrating that substantial PASI reductions may not translate into tangible improvements in patients’ quality of life. For example, among psoriasis patients who achieved a PASI 75 response in one European study, 65% still had a Dermatology Life Quality Index (DLQI) score of 2 or more (Eur. J. Dermatol. 2010;20:62-7).
"This implies that there is something more to be wished for by patients, even when PASI 75 is reached," according to Dr. van de Kerkhof.
Moreover, in another trial, even among patients with a PASI score of 0 at week 24, only 70% had an optimal DLQI of 0, not 100% as most dermatologists might expect (Br. J. Dermatol. 2006;154:1161-8).
A recent survey of 2,151 European psoriasis patients and their dermatologists highlighted a substantial degree of dissatisfaction with current therapies. Patients on biologics had higher rates of improvement from severe to moderate or mild disease than did those on any other forms of psoriasis therapy, yet 41% of patients on biologics were dissatisfied with their treatment (J. Dermatolog. Treat. 2013;24:193-8).
"I think it’s extremely important that we continue to innovate treatment possibilities for psoriasis in order to improve outcomes for our patients," Dr. van de Kerkhof said.
What patients really want, surveys suggest, are treatments that render them clear or nearly clear, and do so quickly. In one survey, patients rated as the most important characteristic about a therapy the rapidity with which it could achieve a moderate 50% improvement in symptoms. They rated that as higher in importance to them than the therapy’s long-term risks (Arch. Dermatol. 2007;143:1175-9), Dr. van de Kerkhof noted.
Patients also place a high priority on improvement of a broad array of symptoms that aren’t captured by either the PASI or the DLQI, Dr. Bruce E. Strober noted in a separate presentation. These include itching, plaque-related pain, altered skin appearance, flaking, and bleeding.
For this reason, he and a group of his coworkers have created and are now validating a new tool for the assessment of patient-related outcomes in psoriasis called the Psoriasis Symptom Diary (Value Health 2013;16:1014-22). The 16-item tool takes less than 5 minutes for a patient to fill out and is designed to replace the DLQI both in clinical trials and everyday practice. The goal is to be able to walk into the examination room, take a quick look at the Psoriasis Symptom Diary, and know from that how a patient is currently doing even before asking for the patient to disrobe.
"Let’s face it: Outside of skin cancer, when we’re doing dermatology, we’re in the quality of life business. That means we’re asking patients how they’re doing at every visit for psoriasis, atopic dermatitis, or severe acne. The DLQI does that, but it’s not psoriasis specific," said Dr. Strober, vice chair and director of the clinical trials unit in the department of dermatology at the University of Connecticut, Farmington.
As for the PASI, he doesn’t use it except in structured clinical trials. It’s too time consuming and has a high rate of inter- and intrarater variability.
"In the United States, dermatologists never do PASI scores in their clinics. The PASI score has numerous drawbacks that make it impractical in a regular practice setting," Dr. Strober said. "In my own practice, I routinely do a 5-point Physician’s Global Assessment along with an estimate of involved body surface area. I think that gives you a good picture of the objective level of psoriasis severity."
Dr. Bachelez, Dr. van de Kerkhof, and Dr. Strober each reported receiving research grants from and serving on advisory boards for 9-14 pharmaceutical companies engaged in developing new treatments for psoriasis.
EXPERT ANALYSIS FROM THE eadv CONGRESS
New drugs, strategies advance rheumatoid arthritis treatment
New biologics and more aggressive intervention strategies have advanced rheumatoid arthritis treatment over the last decade, Dr. Iain McInnes explains, but remission remains too infrequent. New drugs under development in the coming years offer promise for the future. For more on rheumatoid arthritis advances, visit http://www.rheumatologynews.com.
New biologics and more aggressive intervention strategies have advanced rheumatoid arthritis treatment over the last decade, Dr. Iain McInnes explains, but remission remains too infrequent. New drugs under development in the coming years offer promise for the future. For more on rheumatoid arthritis advances, visit http://www.rheumatologynews.com.
New biologics and more aggressive intervention strategies have advanced rheumatoid arthritis treatment over the last decade, Dr. Iain McInnes explains, but remission remains too infrequent. New drugs under development in the coming years offer promise for the future. For more on rheumatoid arthritis advances, visit http://www.rheumatologynews.com.
Options exist for psoriasis patients with multiple anti-TNF failures
LAS VEGAS – The use of an alternative anti–tumor necrosis factor drug is not necessarily precluded in a psoriasis patient with both skin and joint symptoms who has failed two previous anti-TNF drugs, according to Dr. Kenneth B. Gordon.
Dr. Gordon, professor of dermatology at Northwestern University, Chicago, described a case involving a 52-year-old man with a 20-year history of psoriasis who initially presented with mainly scalp and limited plaque psoriasis, and who was treated with topical corticosteroids and topical calcipotriene. After developing more extensive disease, he was treated successfully with ultraviolet B phototherapy.
Eleven years after first presenting with psoriasis, he presented with peripheral arthritis and enthesitis and was diagnosed with psoriatic arthritis.
The patient was treated initially with sulfasalazine, but failed to respond; methotrexate had only modest benefit, and at higher doses the patient developed liver function test abnormalities due to steatohepatitis, Dr. Gordon said at Perspectives in Rheumatic Diseases 2013.
Etanercept was initiated at 50 mg twice weekly, and the patient had an excellent initial response.
However, after stepping down to 50 mg weekly, his psoriasis flared.
"His joints were doing fine, but his skin was getting bad," Dr. Gordon said, noting that this type of response was also seen in early studies of etanercept, which showed that when doses were reduced to 50 mg weekly after 12 weeks, responses leveled off, with some patients achieving a 75% reduction in the Psoriasis Area and Severity Index (PASI) 75 response, and some losing PASI 75 response.
"It turns out that this occurs in 20%-30% of patients," he said, adding that some controversy exists regarding dosing, because "it’s not entirely clear that if you had just started at the lower dose and just let them go at the 50 mg once a week, that at 6 months they’d be any different than if you gave them the higher dose initially."
The REVEAL study of adalimumab for psoriasis also demonstrated loss of response after 33 weeks among those with an initial PASI 75 response (J. Am. Acad. Dermatol. 2012;66:241-51), he said.
"So clearly, patients lose response. Now, that lessens over time, but in the first year in psoriasis – and my guess is, in psoriatic arthritis as well – you have loss of effect at a relatively high level with all of these medicines," he said.
In fact, in the patient Dr. Gordon presented, a switch to adalimumab at a dose of 40 mg every other week resulted in an excellent initial response in both joints and skin, but skin symptoms returned after 8 months.
"This is something we’re all familiar with – patients doing well on an anti-TNF and then losing effect after a period of time on medication," he said.
So what are the alternatives?
An anti-TNF drug option after the patient cycles through etanercept, adalimumab, and infliximab is certolizumab, which was approved in September for the treatment of psoriatic arthritis. Study data suggest that the drug is "quite effective for psoriasis, was well tolerated, and similar to the other anti-TNFs," he said.
Using certolizumab is a reasonable approach that appears to work for both skin and joint symptoms, he said.
Golimumab is another possible option for treatment, but primary psoriasis data are lacking with this drug.
"It is my feeling that golimumab is probably not as potent for psoriasis as the other anti-TNF agents that we have, but that is an opinion," he said.
As for alternatives to anti-TNF agents, the PHEONIX 2 trial (Lancet 2008;371:1675-84) provided reasonable data in support of the interleukin-12 and -23 monoclonal antibody ustekinumab for skin disease, but patients who failed prior anti-TNF therapy actually had a poorer outcome, so that is something to keep in mind, he said, noting that adding methotrexate may help prevent a flare in patients who are switched from an anti-TNF agent to ustekinumab.
Ustekinumab, which also was approved in September for the treatment of psoriatic arthritis, is likely more useful for the skin, but is not exceptional for joint disease, he said at the meeting, held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Apremilast and tofacitinib, drugs now in development for psoriasis, may also prove useful in the future, as they may "theoretically be of benefit" based on currently available data, he said.
Dr. Gordon has received research support and/or honoraria from AbbVie (which markets adalimumab), Amgen (which markets etanercept), Celgene (which is developing apremilast), Eli Lilly, Janssen (which markets golimumab, infliximab, and ustekinumab), Novartis, and Pfizer (which markets tofacitinib).
LAS VEGAS – The use of an alternative anti–tumor necrosis factor drug is not necessarily precluded in a psoriasis patient with both skin and joint symptoms who has failed two previous anti-TNF drugs, according to Dr. Kenneth B. Gordon.
Dr. Gordon, professor of dermatology at Northwestern University, Chicago, described a case involving a 52-year-old man with a 20-year history of psoriasis who initially presented with mainly scalp and limited plaque psoriasis, and who was treated with topical corticosteroids and topical calcipotriene. After developing more extensive disease, he was treated successfully with ultraviolet B phototherapy.
Eleven years after first presenting with psoriasis, he presented with peripheral arthritis and enthesitis and was diagnosed with psoriatic arthritis.
The patient was treated initially with sulfasalazine, but failed to respond; methotrexate had only modest benefit, and at higher doses the patient developed liver function test abnormalities due to steatohepatitis, Dr. Gordon said at Perspectives in Rheumatic Diseases 2013.
Etanercept was initiated at 50 mg twice weekly, and the patient had an excellent initial response.
However, after stepping down to 50 mg weekly, his psoriasis flared.
"His joints were doing fine, but his skin was getting bad," Dr. Gordon said, noting that this type of response was also seen in early studies of etanercept, which showed that when doses were reduced to 50 mg weekly after 12 weeks, responses leveled off, with some patients achieving a 75% reduction in the Psoriasis Area and Severity Index (PASI) 75 response, and some losing PASI 75 response.
"It turns out that this occurs in 20%-30% of patients," he said, adding that some controversy exists regarding dosing, because "it’s not entirely clear that if you had just started at the lower dose and just let them go at the 50 mg once a week, that at 6 months they’d be any different than if you gave them the higher dose initially."
The REVEAL study of adalimumab for psoriasis also demonstrated loss of response after 33 weeks among those with an initial PASI 75 response (J. Am. Acad. Dermatol. 2012;66:241-51), he said.
"So clearly, patients lose response. Now, that lessens over time, but in the first year in psoriasis – and my guess is, in psoriatic arthritis as well – you have loss of effect at a relatively high level with all of these medicines," he said.
In fact, in the patient Dr. Gordon presented, a switch to adalimumab at a dose of 40 mg every other week resulted in an excellent initial response in both joints and skin, but skin symptoms returned after 8 months.
"This is something we’re all familiar with – patients doing well on an anti-TNF and then losing effect after a period of time on medication," he said.
So what are the alternatives?
An anti-TNF drug option after the patient cycles through etanercept, adalimumab, and infliximab is certolizumab, which was approved in September for the treatment of psoriatic arthritis. Study data suggest that the drug is "quite effective for psoriasis, was well tolerated, and similar to the other anti-TNFs," he said.
Using certolizumab is a reasonable approach that appears to work for both skin and joint symptoms, he said.
Golimumab is another possible option for treatment, but primary psoriasis data are lacking with this drug.
"It is my feeling that golimumab is probably not as potent for psoriasis as the other anti-TNF agents that we have, but that is an opinion," he said.
As for alternatives to anti-TNF agents, the PHEONIX 2 trial (Lancet 2008;371:1675-84) provided reasonable data in support of the interleukin-12 and -23 monoclonal antibody ustekinumab for skin disease, but patients who failed prior anti-TNF therapy actually had a poorer outcome, so that is something to keep in mind, he said, noting that adding methotrexate may help prevent a flare in patients who are switched from an anti-TNF agent to ustekinumab.
Ustekinumab, which also was approved in September for the treatment of psoriatic arthritis, is likely more useful for the skin, but is not exceptional for joint disease, he said at the meeting, held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Apremilast and tofacitinib, drugs now in development for psoriasis, may also prove useful in the future, as they may "theoretically be of benefit" based on currently available data, he said.
Dr. Gordon has received research support and/or honoraria from AbbVie (which markets adalimumab), Amgen (which markets etanercept), Celgene (which is developing apremilast), Eli Lilly, Janssen (which markets golimumab, infliximab, and ustekinumab), Novartis, and Pfizer (which markets tofacitinib).
LAS VEGAS – The use of an alternative anti–tumor necrosis factor drug is not necessarily precluded in a psoriasis patient with both skin and joint symptoms who has failed two previous anti-TNF drugs, according to Dr. Kenneth B. Gordon.
Dr. Gordon, professor of dermatology at Northwestern University, Chicago, described a case involving a 52-year-old man with a 20-year history of psoriasis who initially presented with mainly scalp and limited plaque psoriasis, and who was treated with topical corticosteroids and topical calcipotriene. After developing more extensive disease, he was treated successfully with ultraviolet B phototherapy.
Eleven years after first presenting with psoriasis, he presented with peripheral arthritis and enthesitis and was diagnosed with psoriatic arthritis.
The patient was treated initially with sulfasalazine, but failed to respond; methotrexate had only modest benefit, and at higher doses the patient developed liver function test abnormalities due to steatohepatitis, Dr. Gordon said at Perspectives in Rheumatic Diseases 2013.
Etanercept was initiated at 50 mg twice weekly, and the patient had an excellent initial response.
However, after stepping down to 50 mg weekly, his psoriasis flared.
"His joints were doing fine, but his skin was getting bad," Dr. Gordon said, noting that this type of response was also seen in early studies of etanercept, which showed that when doses were reduced to 50 mg weekly after 12 weeks, responses leveled off, with some patients achieving a 75% reduction in the Psoriasis Area and Severity Index (PASI) 75 response, and some losing PASI 75 response.
"It turns out that this occurs in 20%-30% of patients," he said, adding that some controversy exists regarding dosing, because "it’s not entirely clear that if you had just started at the lower dose and just let them go at the 50 mg once a week, that at 6 months they’d be any different than if you gave them the higher dose initially."
The REVEAL study of adalimumab for psoriasis also demonstrated loss of response after 33 weeks among those with an initial PASI 75 response (J. Am. Acad. Dermatol. 2012;66:241-51), he said.
"So clearly, patients lose response. Now, that lessens over time, but in the first year in psoriasis – and my guess is, in psoriatic arthritis as well – you have loss of effect at a relatively high level with all of these medicines," he said.
In fact, in the patient Dr. Gordon presented, a switch to adalimumab at a dose of 40 mg every other week resulted in an excellent initial response in both joints and skin, but skin symptoms returned after 8 months.
"This is something we’re all familiar with – patients doing well on an anti-TNF and then losing effect after a period of time on medication," he said.
So what are the alternatives?
An anti-TNF drug option after the patient cycles through etanercept, adalimumab, and infliximab is certolizumab, which was approved in September for the treatment of psoriatic arthritis. Study data suggest that the drug is "quite effective for psoriasis, was well tolerated, and similar to the other anti-TNFs," he said.
Using certolizumab is a reasonable approach that appears to work for both skin and joint symptoms, he said.
Golimumab is another possible option for treatment, but primary psoriasis data are lacking with this drug.
"It is my feeling that golimumab is probably not as potent for psoriasis as the other anti-TNF agents that we have, but that is an opinion," he said.
As for alternatives to anti-TNF agents, the PHEONIX 2 trial (Lancet 2008;371:1675-84) provided reasonable data in support of the interleukin-12 and -23 monoclonal antibody ustekinumab for skin disease, but patients who failed prior anti-TNF therapy actually had a poorer outcome, so that is something to keep in mind, he said, noting that adding methotrexate may help prevent a flare in patients who are switched from an anti-TNF agent to ustekinumab.
Ustekinumab, which also was approved in September for the treatment of psoriatic arthritis, is likely more useful for the skin, but is not exceptional for joint disease, he said at the meeting, held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Apremilast and tofacitinib, drugs now in development for psoriasis, may also prove useful in the future, as they may "theoretically be of benefit" based on currently available data, he said.
Dr. Gordon has received research support and/or honoraria from AbbVie (which markets adalimumab), Amgen (which markets etanercept), Celgene (which is developing apremilast), Eli Lilly, Janssen (which markets golimumab, infliximab, and ustekinumab), Novartis, and Pfizer (which markets tofacitinib).
EXPERT ANALYSIS FROM PERSPECTIVES IN RHEUMATIC DISEASES 2013
Treatment options expand for pulmonary arterial hypertension in scleroderma
LAS VEGAS – Scleroderma patients are at particularly high risk for developing pulmonary arterial hypertension, but treatment options are expanding, and with early referral to a PAH specialist, outcomes can be improved, according to Dr. Ronald J. Oudiz.
"In 1995 we had nothing. Now we have nine treatments specifically for PAH, and more that are being looked at," he said at Perspectives in Rheumatic Diseases 2013.
Three major signaling pathways, including the prostacyclin, endothelin, and nitric oxide pathways, form the basis for the available treatment options for this progressive and deadly disease, which has a 36-month survival rate of only about 60%. The treatments include prostacyclin analogues, endothelin receptor antagonists, and phosphodiesterase type 5 (PDE-5) inhibitors, said Dr. Oudiz, professor of medicine at the University of California, Los Angeles, and director of the Liu Center for Pulmonary Hypertension at Harbor-UCLA Medical Center.
The prostacyclin analogues include intravenous epoprostenol, inhaled iloprost, and treprostinil, which can be inhaled or delivered intravenously or subcutaneously. These have been shown to improve performance on the 6-minute walk test in a dose-dependent fashion, Dr. Oudiz noted.
Side effects with prostacyclin analogues can include flushing, headache, rash, thrombocytopenia, infection, and gastrointestinal effects such as diarrhea, nausea, and weight loss.
The endothelin receptor antagonists include bosentan and ambrisentan, which both are delivered orally. Their approval was "revolutionary, because for a while we only had IV drugs," he said.
Side effects with endothelin receptor antagonists include liver function test abnormalities, headache, nasal congestion, and edema. Although these drugs are generally tolerated well, liver function test abnormalities and edema can be troublesome for both patients and physicians as they require close monitoring and dose modification or interruption, he said.
PDE-5 inhibitors include sildenafil and tadalafil, which also are both oral drugs, and which have side effects that are similar to those seen with the endothelin-receptor antagonists, with the addition of myalgia, diarrhea, dyspepsia, and nose bleeds, he noted.
Overall, studies suggest that treatment with PAH drugs not only leads to improved exercise capacity, but probably improves long-term survival. In a meta-analysis of several major PAH drug trials, the majority of studies showed a potential mortality benefit (Eur. Heart. J. 2009;30:394-403), Dr. Oudiz noted.
"I say potential because we know none of the studies assessed in the meta-analysis were powered to examine mortality ... but nevertheless, we believe we’re doing more than just improving exercise capacity," he said.
In fact, in a study published in August in the New England Journal of Medicine, the new endothelin receptor antagonist macitentan was associated with a dose-dependent decrease in the number of outcome events measured, relative to placebo, including death, transplant, and heart failure in patients with PAH, he noted (N. Engl. J. Med. 2013;369:809-18).
"These are some really important endpoints we’re finally starting to meet," he said.
These advances underscore the importance of increased awareness of the risk of PAH in scleroderma and early recognition of the condition, and – since diagnosis and the subtleties of management are complex – they also underscore the importance of early referral to a PAH specialist, he said.
"Early treatment is always preferable to later treatment," he concluded at the meeting held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Oudiz has received grant or research support from, served as a consultant to, and/or served on a speakers’ bureau for Actelion, Bayer, Gilead, Ikaria, Lung, Pfizer, and United Therapeutics.
LAS VEGAS – Scleroderma patients are at particularly high risk for developing pulmonary arterial hypertension, but treatment options are expanding, and with early referral to a PAH specialist, outcomes can be improved, according to Dr. Ronald J. Oudiz.
"In 1995 we had nothing. Now we have nine treatments specifically for PAH, and more that are being looked at," he said at Perspectives in Rheumatic Diseases 2013.
Three major signaling pathways, including the prostacyclin, endothelin, and nitric oxide pathways, form the basis for the available treatment options for this progressive and deadly disease, which has a 36-month survival rate of only about 60%. The treatments include prostacyclin analogues, endothelin receptor antagonists, and phosphodiesterase type 5 (PDE-5) inhibitors, said Dr. Oudiz, professor of medicine at the University of California, Los Angeles, and director of the Liu Center for Pulmonary Hypertension at Harbor-UCLA Medical Center.
The prostacyclin analogues include intravenous epoprostenol, inhaled iloprost, and treprostinil, which can be inhaled or delivered intravenously or subcutaneously. These have been shown to improve performance on the 6-minute walk test in a dose-dependent fashion, Dr. Oudiz noted.
Side effects with prostacyclin analogues can include flushing, headache, rash, thrombocytopenia, infection, and gastrointestinal effects such as diarrhea, nausea, and weight loss.
The endothelin receptor antagonists include bosentan and ambrisentan, which both are delivered orally. Their approval was "revolutionary, because for a while we only had IV drugs," he said.
Side effects with endothelin receptor antagonists include liver function test abnormalities, headache, nasal congestion, and edema. Although these drugs are generally tolerated well, liver function test abnormalities and edema can be troublesome for both patients and physicians as they require close monitoring and dose modification or interruption, he said.
PDE-5 inhibitors include sildenafil and tadalafil, which also are both oral drugs, and which have side effects that are similar to those seen with the endothelin-receptor antagonists, with the addition of myalgia, diarrhea, dyspepsia, and nose bleeds, he noted.
Overall, studies suggest that treatment with PAH drugs not only leads to improved exercise capacity, but probably improves long-term survival. In a meta-analysis of several major PAH drug trials, the majority of studies showed a potential mortality benefit (Eur. Heart. J. 2009;30:394-403), Dr. Oudiz noted.
"I say potential because we know none of the studies assessed in the meta-analysis were powered to examine mortality ... but nevertheless, we believe we’re doing more than just improving exercise capacity," he said.
In fact, in a study published in August in the New England Journal of Medicine, the new endothelin receptor antagonist macitentan was associated with a dose-dependent decrease in the number of outcome events measured, relative to placebo, including death, transplant, and heart failure in patients with PAH, he noted (N. Engl. J. Med. 2013;369:809-18).
"These are some really important endpoints we’re finally starting to meet," he said.
These advances underscore the importance of increased awareness of the risk of PAH in scleroderma and early recognition of the condition, and – since diagnosis and the subtleties of management are complex – they also underscore the importance of early referral to a PAH specialist, he said.
"Early treatment is always preferable to later treatment," he concluded at the meeting held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Oudiz has received grant or research support from, served as a consultant to, and/or served on a speakers’ bureau for Actelion, Bayer, Gilead, Ikaria, Lung, Pfizer, and United Therapeutics.
LAS VEGAS – Scleroderma patients are at particularly high risk for developing pulmonary arterial hypertension, but treatment options are expanding, and with early referral to a PAH specialist, outcomes can be improved, according to Dr. Ronald J. Oudiz.
"In 1995 we had nothing. Now we have nine treatments specifically for PAH, and more that are being looked at," he said at Perspectives in Rheumatic Diseases 2013.
Three major signaling pathways, including the prostacyclin, endothelin, and nitric oxide pathways, form the basis for the available treatment options for this progressive and deadly disease, which has a 36-month survival rate of only about 60%. The treatments include prostacyclin analogues, endothelin receptor antagonists, and phosphodiesterase type 5 (PDE-5) inhibitors, said Dr. Oudiz, professor of medicine at the University of California, Los Angeles, and director of the Liu Center for Pulmonary Hypertension at Harbor-UCLA Medical Center.
The prostacyclin analogues include intravenous epoprostenol, inhaled iloprost, and treprostinil, which can be inhaled or delivered intravenously or subcutaneously. These have been shown to improve performance on the 6-minute walk test in a dose-dependent fashion, Dr. Oudiz noted.
Side effects with prostacyclin analogues can include flushing, headache, rash, thrombocytopenia, infection, and gastrointestinal effects such as diarrhea, nausea, and weight loss.
The endothelin receptor antagonists include bosentan and ambrisentan, which both are delivered orally. Their approval was "revolutionary, because for a while we only had IV drugs," he said.
Side effects with endothelin receptor antagonists include liver function test abnormalities, headache, nasal congestion, and edema. Although these drugs are generally tolerated well, liver function test abnormalities and edema can be troublesome for both patients and physicians as they require close monitoring and dose modification or interruption, he said.
PDE-5 inhibitors include sildenafil and tadalafil, which also are both oral drugs, and which have side effects that are similar to those seen with the endothelin-receptor antagonists, with the addition of myalgia, diarrhea, dyspepsia, and nose bleeds, he noted.
Overall, studies suggest that treatment with PAH drugs not only leads to improved exercise capacity, but probably improves long-term survival. In a meta-analysis of several major PAH drug trials, the majority of studies showed a potential mortality benefit (Eur. Heart. J. 2009;30:394-403), Dr. Oudiz noted.
"I say potential because we know none of the studies assessed in the meta-analysis were powered to examine mortality ... but nevertheless, we believe we’re doing more than just improving exercise capacity," he said.
In fact, in a study published in August in the New England Journal of Medicine, the new endothelin receptor antagonist macitentan was associated with a dose-dependent decrease in the number of outcome events measured, relative to placebo, including death, transplant, and heart failure in patients with PAH, he noted (N. Engl. J. Med. 2013;369:809-18).
"These are some really important endpoints we’re finally starting to meet," he said.
These advances underscore the importance of increased awareness of the risk of PAH in scleroderma and early recognition of the condition, and – since diagnosis and the subtleties of management are complex – they also underscore the importance of early referral to a PAH specialist, he said.
"Early treatment is always preferable to later treatment," he concluded at the meeting held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Oudiz has received grant or research support from, served as a consultant to, and/or served on a speakers’ bureau for Actelion, Bayer, Gilead, Ikaria, Lung, Pfizer, and United Therapeutics.
EXPERT ANALYSIS FROM PERSEPCTIVES IN RHEUMATIC DISEASES 2013
Fixed maintenance secukinumab beats as-needed dosing
ISTANBUL – Psoriasis patients who initially achieve a high-level response to secukinumab are significantly more likely to retain it over the long haul if they follow a fixed maintenance therapy schedule of once-monthly subcutaneous dosing rather than dosing as needed in the event of the start of relapse, according to the phase III SCULPTURE trial.
Secukinumab is an investigational fully human IgG1 monoclonal antibody targeting interleukin-17A, a key player in psoriasis, because it generates downstream proinflammatory cytokines and stimulates keratinocyte growth. Earlier studies indicated the investigational biologic had unprecedented clinical efficacy and rapidity of response. These observations gave rise to the hypothesis in SCULPTURE that maintenance dosing as needed upon relapse would prove noninferior to conventional fixed once-monthly maintenance therapy.
If the study hypothesis proved valid, it would mean less exposure to potential medication side effects, lower drug costs, and greater patient convenience. However, the hypothesis wasn’t borne out in the 966-patient, double-blind, randomized SCULPTURE (Study Comparing Retreatment Upon Start of Relapse), Dr. Ulrich Mrowietz reported at the annual congress of the European Academy of Dermatology and Venereology.
SCULPTURE participants had moderate-to-severe chronic plaque psoriasis despite prior systemic therapies, including biologic agents in many cases. They were randomized double blind to induction therapy involving five once-weekly subcutaneous injections of secukinumab at either 150 or 300 mg. At week 8, 843 participants with a Psoriasis Area and Severity Index (PASI) 75 response were re-randomized to maintenance therapy at the same dose, to be delivered either once monthly or as needed for relapse. The definition of relapse in this study required two elements: loss of PASI 75 response, and at least a 20% fall from the maximum PASI improvement, compared with baseline.
At week 52, 78.2% of patients randomized to 300 mg of secukinumab on a fixed once-monthly schedule still maintained a PASI 75 response. This was a significantly better outcome than the 67.7% PASI 75 rate in patients assigned to secukinumab 300 mg as needed, the 62.1% rate in those on secukinumab 150 mg once monthly, and the 52% PASI 75 rate in patients on secukinumab 150 mg as needed, reported Dr. Mrowietz of the University Medical Center Schleswig-Holstein, Kiel (Germany).
He found a bright spot in the negative results: While patients assigned to as-needed maintenance therapy achieved roughly an absolute 10% lower PASI 75 rate at 1 year, those in the secukinumab 150-mg group did so with only 46% the number of doses received by patients in the fixed monthly therapy group, while those in the retreatment-as-needed with secukinumab 300-mg group got only 39% of the number of doses, compared with patients on fixed monthly therapy.
"Fixed monthly dosing is the best maintenance regimen. But, in selected patients, there may be an opportunity with secukinumab to deviate from the usual fixed dosing regimen in favor of an as-needed approach," he said.
This tradeoff of an absolute 10% reduction in efficacy in return for a dosing regimen that entails less than half as much medication over the course of a year could prove of interest to payers, he noted.
The key, according to Dr. Mrowietz, will be to try to identify criteria helpful in selecting patients with an increased likelihood of a high-level response to the retreat-as-needed management strategy. The phase III trial was completed so recently that those necessary subanalyses have yet to be done.
However, Dr. Kristian Reich, another investigator involved in the secukinumab clinical trials program, drew a different message from SCULPTURE. He observed that these newer biologic agents are so effective that the bar has been raised with regard to patient expectations. Many patients won’t be satisfied with a PASI 75 response once a PASI 90 is achievable. And with fixed monthly maintenance secukinumab, it often is.
Indeed, the week-52 PASI 90 rate in SCULPTURE was a highly robust 59.7% in patients on fixed monthly secukinumab 300 mg and 45.8% for fixed monthly low-dose therapy, compared with the unimpressive 13.8% and 11.2% PASI 90 rates with high- and low-dose as-needed therapy.
"My take from this is that the best way to use this drug for continuous disease control is to give the drug every 4 weeks. What this study tells us is for those patients where you have to stop, where you have to use on-and-off therapy because they go away for 2 months to Africa, or they have a major operation, or for other reasons, these data are reassuring that we can use the drug safely on an intermittent basis," said Dr. Reich of Georg-August University in Göttingen, Germany.
Prior to SCULPTURE, he and other investigators were concerned that intermittent secukinumab therapy might promote the development of harmful antidrug antibodies. But antidrug antibodies arose in only three patients on fixed monthly maintenance therapy and two patients on as-needed therapy, and had no impact upon clinical efficacy or safety, he noted.
Another phase III trial presented in Istanbul was ERASURE (Efficacy and Safety of Subcutaneous Secukinumab for Moderate to Severe Chronic Plaque-Type Psoriasis for Up to 1 Year). Dr. Boni E. Elewski reported on 738 patients with moderate-to-severe chronic plaque psoriasis who were randomized double blind to secukinumab at 150 mg or 300 mg, or to placebo. Participants averaged a PASI score of 22 at baseline, along with 33% body surface area involvement. Psoriatic arthritis was present in 23% of subjects.
The co-primary endpoints in ERASURE were the week 12 PASI 75 response rates and Investigator’s Global Assessment scores of 0/1, indicative of clear or almost clear on a modified 5-point scale. The week-12 PASI 75 rates were 81.6% in the secukinumab 300 mg group, 71.6% with secukinumab 150 mg, and 4.5% with placebo. The week-12 IGA 0/1 rates were 65.3%, 51.2%, and 2.4%, said Dr. Elewski of the University of Alabama, Birmingham.
Among the other notable findings in ERASURE were the 69.8% PASI 90 and 41.6% PASI 100 responses at week 16 in the group assigned to secukinumab 300 mg, the fact that only one patient, on secukinumab 150 mg, developed transient antidrug antibodies, and the complete absence of cardiovascular events during 52 weeks on secukinumab, observed Dr. Elewski, professor of dermatology at the University of Alabama, Birmingham.
Dr. Mrowietz, Dr. Reich, and Dr. Elewski reported having received research grants from and serving as consultants to Novartis, which sponsored the secukinumab clinical trials program. The dermatologists serve in similar capacities with other pharmaceutical companies developing new medications for psoriasis.
ISTANBUL – Psoriasis patients who initially achieve a high-level response to secukinumab are significantly more likely to retain it over the long haul if they follow a fixed maintenance therapy schedule of once-monthly subcutaneous dosing rather than dosing as needed in the event of the start of relapse, according to the phase III SCULPTURE trial.
Secukinumab is an investigational fully human IgG1 monoclonal antibody targeting interleukin-17A, a key player in psoriasis, because it generates downstream proinflammatory cytokines and stimulates keratinocyte growth. Earlier studies indicated the investigational biologic had unprecedented clinical efficacy and rapidity of response. These observations gave rise to the hypothesis in SCULPTURE that maintenance dosing as needed upon relapse would prove noninferior to conventional fixed once-monthly maintenance therapy.
If the study hypothesis proved valid, it would mean less exposure to potential medication side effects, lower drug costs, and greater patient convenience. However, the hypothesis wasn’t borne out in the 966-patient, double-blind, randomized SCULPTURE (Study Comparing Retreatment Upon Start of Relapse), Dr. Ulrich Mrowietz reported at the annual congress of the European Academy of Dermatology and Venereology.
SCULPTURE participants had moderate-to-severe chronic plaque psoriasis despite prior systemic therapies, including biologic agents in many cases. They were randomized double blind to induction therapy involving five once-weekly subcutaneous injections of secukinumab at either 150 or 300 mg. At week 8, 843 participants with a Psoriasis Area and Severity Index (PASI) 75 response were re-randomized to maintenance therapy at the same dose, to be delivered either once monthly or as needed for relapse. The definition of relapse in this study required two elements: loss of PASI 75 response, and at least a 20% fall from the maximum PASI improvement, compared with baseline.
At week 52, 78.2% of patients randomized to 300 mg of secukinumab on a fixed once-monthly schedule still maintained a PASI 75 response. This was a significantly better outcome than the 67.7% PASI 75 rate in patients assigned to secukinumab 300 mg as needed, the 62.1% rate in those on secukinumab 150 mg once monthly, and the 52% PASI 75 rate in patients on secukinumab 150 mg as needed, reported Dr. Mrowietz of the University Medical Center Schleswig-Holstein, Kiel (Germany).
He found a bright spot in the negative results: While patients assigned to as-needed maintenance therapy achieved roughly an absolute 10% lower PASI 75 rate at 1 year, those in the secukinumab 150-mg group did so with only 46% the number of doses received by patients in the fixed monthly therapy group, while those in the retreatment-as-needed with secukinumab 300-mg group got only 39% of the number of doses, compared with patients on fixed monthly therapy.
"Fixed monthly dosing is the best maintenance regimen. But, in selected patients, there may be an opportunity with secukinumab to deviate from the usual fixed dosing regimen in favor of an as-needed approach," he said.
This tradeoff of an absolute 10% reduction in efficacy in return for a dosing regimen that entails less than half as much medication over the course of a year could prove of interest to payers, he noted.
The key, according to Dr. Mrowietz, will be to try to identify criteria helpful in selecting patients with an increased likelihood of a high-level response to the retreat-as-needed management strategy. The phase III trial was completed so recently that those necessary subanalyses have yet to be done.
However, Dr. Kristian Reich, another investigator involved in the secukinumab clinical trials program, drew a different message from SCULPTURE. He observed that these newer biologic agents are so effective that the bar has been raised with regard to patient expectations. Many patients won’t be satisfied with a PASI 75 response once a PASI 90 is achievable. And with fixed monthly maintenance secukinumab, it often is.
Indeed, the week-52 PASI 90 rate in SCULPTURE was a highly robust 59.7% in patients on fixed monthly secukinumab 300 mg and 45.8% for fixed monthly low-dose therapy, compared with the unimpressive 13.8% and 11.2% PASI 90 rates with high- and low-dose as-needed therapy.
"My take from this is that the best way to use this drug for continuous disease control is to give the drug every 4 weeks. What this study tells us is for those patients where you have to stop, where you have to use on-and-off therapy because they go away for 2 months to Africa, or they have a major operation, or for other reasons, these data are reassuring that we can use the drug safely on an intermittent basis," said Dr. Reich of Georg-August University in Göttingen, Germany.
Prior to SCULPTURE, he and other investigators were concerned that intermittent secukinumab therapy might promote the development of harmful antidrug antibodies. But antidrug antibodies arose in only three patients on fixed monthly maintenance therapy and two patients on as-needed therapy, and had no impact upon clinical efficacy or safety, he noted.
Another phase III trial presented in Istanbul was ERASURE (Efficacy and Safety of Subcutaneous Secukinumab for Moderate to Severe Chronic Plaque-Type Psoriasis for Up to 1 Year). Dr. Boni E. Elewski reported on 738 patients with moderate-to-severe chronic plaque psoriasis who were randomized double blind to secukinumab at 150 mg or 300 mg, or to placebo. Participants averaged a PASI score of 22 at baseline, along with 33% body surface area involvement. Psoriatic arthritis was present in 23% of subjects.
The co-primary endpoints in ERASURE were the week 12 PASI 75 response rates and Investigator’s Global Assessment scores of 0/1, indicative of clear or almost clear on a modified 5-point scale. The week-12 PASI 75 rates were 81.6% in the secukinumab 300 mg group, 71.6% with secukinumab 150 mg, and 4.5% with placebo. The week-12 IGA 0/1 rates were 65.3%, 51.2%, and 2.4%, said Dr. Elewski of the University of Alabama, Birmingham.
Among the other notable findings in ERASURE were the 69.8% PASI 90 and 41.6% PASI 100 responses at week 16 in the group assigned to secukinumab 300 mg, the fact that only one patient, on secukinumab 150 mg, developed transient antidrug antibodies, and the complete absence of cardiovascular events during 52 weeks on secukinumab, observed Dr. Elewski, professor of dermatology at the University of Alabama, Birmingham.
Dr. Mrowietz, Dr. Reich, and Dr. Elewski reported having received research grants from and serving as consultants to Novartis, which sponsored the secukinumab clinical trials program. The dermatologists serve in similar capacities with other pharmaceutical companies developing new medications for psoriasis.
ISTANBUL – Psoriasis patients who initially achieve a high-level response to secukinumab are significantly more likely to retain it over the long haul if they follow a fixed maintenance therapy schedule of once-monthly subcutaneous dosing rather than dosing as needed in the event of the start of relapse, according to the phase III SCULPTURE trial.
Secukinumab is an investigational fully human IgG1 monoclonal antibody targeting interleukin-17A, a key player in psoriasis, because it generates downstream proinflammatory cytokines and stimulates keratinocyte growth. Earlier studies indicated the investigational biologic had unprecedented clinical efficacy and rapidity of response. These observations gave rise to the hypothesis in SCULPTURE that maintenance dosing as needed upon relapse would prove noninferior to conventional fixed once-monthly maintenance therapy.
If the study hypothesis proved valid, it would mean less exposure to potential medication side effects, lower drug costs, and greater patient convenience. However, the hypothesis wasn’t borne out in the 966-patient, double-blind, randomized SCULPTURE (Study Comparing Retreatment Upon Start of Relapse), Dr. Ulrich Mrowietz reported at the annual congress of the European Academy of Dermatology and Venereology.
SCULPTURE participants had moderate-to-severe chronic plaque psoriasis despite prior systemic therapies, including biologic agents in many cases. They were randomized double blind to induction therapy involving five once-weekly subcutaneous injections of secukinumab at either 150 or 300 mg. At week 8, 843 participants with a Psoriasis Area and Severity Index (PASI) 75 response were re-randomized to maintenance therapy at the same dose, to be delivered either once monthly or as needed for relapse. The definition of relapse in this study required two elements: loss of PASI 75 response, and at least a 20% fall from the maximum PASI improvement, compared with baseline.
At week 52, 78.2% of patients randomized to 300 mg of secukinumab on a fixed once-monthly schedule still maintained a PASI 75 response. This was a significantly better outcome than the 67.7% PASI 75 rate in patients assigned to secukinumab 300 mg as needed, the 62.1% rate in those on secukinumab 150 mg once monthly, and the 52% PASI 75 rate in patients on secukinumab 150 mg as needed, reported Dr. Mrowietz of the University Medical Center Schleswig-Holstein, Kiel (Germany).
He found a bright spot in the negative results: While patients assigned to as-needed maintenance therapy achieved roughly an absolute 10% lower PASI 75 rate at 1 year, those in the secukinumab 150-mg group did so with only 46% the number of doses received by patients in the fixed monthly therapy group, while those in the retreatment-as-needed with secukinumab 300-mg group got only 39% of the number of doses, compared with patients on fixed monthly therapy.
"Fixed monthly dosing is the best maintenance regimen. But, in selected patients, there may be an opportunity with secukinumab to deviate from the usual fixed dosing regimen in favor of an as-needed approach," he said.
This tradeoff of an absolute 10% reduction in efficacy in return for a dosing regimen that entails less than half as much medication over the course of a year could prove of interest to payers, he noted.
The key, according to Dr. Mrowietz, will be to try to identify criteria helpful in selecting patients with an increased likelihood of a high-level response to the retreat-as-needed management strategy. The phase III trial was completed so recently that those necessary subanalyses have yet to be done.
However, Dr. Kristian Reich, another investigator involved in the secukinumab clinical trials program, drew a different message from SCULPTURE. He observed that these newer biologic agents are so effective that the bar has been raised with regard to patient expectations. Many patients won’t be satisfied with a PASI 75 response once a PASI 90 is achievable. And with fixed monthly maintenance secukinumab, it often is.
Indeed, the week-52 PASI 90 rate in SCULPTURE was a highly robust 59.7% in patients on fixed monthly secukinumab 300 mg and 45.8% for fixed monthly low-dose therapy, compared with the unimpressive 13.8% and 11.2% PASI 90 rates with high- and low-dose as-needed therapy.
"My take from this is that the best way to use this drug for continuous disease control is to give the drug every 4 weeks. What this study tells us is for those patients where you have to stop, where you have to use on-and-off therapy because they go away for 2 months to Africa, or they have a major operation, or for other reasons, these data are reassuring that we can use the drug safely on an intermittent basis," said Dr. Reich of Georg-August University in Göttingen, Germany.
Prior to SCULPTURE, he and other investigators were concerned that intermittent secukinumab therapy might promote the development of harmful antidrug antibodies. But antidrug antibodies arose in only three patients on fixed monthly maintenance therapy and two patients on as-needed therapy, and had no impact upon clinical efficacy or safety, he noted.
Another phase III trial presented in Istanbul was ERASURE (Efficacy and Safety of Subcutaneous Secukinumab for Moderate to Severe Chronic Plaque-Type Psoriasis for Up to 1 Year). Dr. Boni E. Elewski reported on 738 patients with moderate-to-severe chronic plaque psoriasis who were randomized double blind to secukinumab at 150 mg or 300 mg, or to placebo. Participants averaged a PASI score of 22 at baseline, along with 33% body surface area involvement. Psoriatic arthritis was present in 23% of subjects.
The co-primary endpoints in ERASURE were the week 12 PASI 75 response rates and Investigator’s Global Assessment scores of 0/1, indicative of clear or almost clear on a modified 5-point scale. The week-12 PASI 75 rates were 81.6% in the secukinumab 300 mg group, 71.6% with secukinumab 150 mg, and 4.5% with placebo. The week-12 IGA 0/1 rates were 65.3%, 51.2%, and 2.4%, said Dr. Elewski of the University of Alabama, Birmingham.
Among the other notable findings in ERASURE were the 69.8% PASI 90 and 41.6% PASI 100 responses at week 16 in the group assigned to secukinumab 300 mg, the fact that only one patient, on secukinumab 150 mg, developed transient antidrug antibodies, and the complete absence of cardiovascular events during 52 weeks on secukinumab, observed Dr. Elewski, professor of dermatology at the University of Alabama, Birmingham.
Dr. Mrowietz, Dr. Reich, and Dr. Elewski reported having received research grants from and serving as consultants to Novartis, which sponsored the secukinumab clinical trials program. The dermatologists serve in similar capacities with other pharmaceutical companies developing new medications for psoriasis.
AT THE EADV CONGRESS
Major finding: Among psoriasis patients who achieved a PASI 75 response to 5 weeks of induction therapy with subcutaneous secukinumab, 59.7% who were placed on a maintenance regimen of 300 mg every 4 weeks had a PASI 90 response at week 52, compared with 13.8% whose maintenance program involved taking 300 mg on an as-needed basis at the start of relapse.
Data source: The SCULPTURE study was a prospective, double-blind, 52-week, phase III clinical trial including 843 patients.
Disclosures: The study was sponsored by Novartis. Speakers reported having received research grants from and serving as consultants to the company.
Adapting HCQ dose did not reduce SLE flare rates
A strategy to maintain hydroxychloroquine blood levels above 1,000 ng/mL in adult patients with systemic lupus erythematosus did not reduce the number of SLE flares during a 7-month follow-up period, results from a French study demonstrated.
"This study confirms the pharmacokinetic/pharmacodynamic relation for hydroxychloroquine (HCQ) in patients with SLE," investigators led by Prof. Nathalie Costedoat-Chalumeau reported in the November 2013 issue of Annals of the Rheumatic Diseases.
"Our results do not justify recommending a therapeutic adaptation of HCQ dose. However, we suggest that [HCQ] be measured to detect non-adherence, especially in patients with active disease, and to help patients with poor adherence reach [HCQ levels greater than or equal to] 1,000 ng/mL."
The purpose of the trial, known at the PLUS Study, was to determine the potential benefits of individualizing HCQ dosing schedules to reach a target of 1,000 ng/mL or greater and thereby decrease rates of SLE flare. It was carried out in 573 patients with SLE at 37 centers in France from June 2007 through August 2010.
Of the 573 patients, researchers randomized 171 to one of two treatment groups: 84 to no daily dose change (group A), and 87 to increased HCQ dose to achieve the target of 1,000 g/mL or greater (group B). The primary endpoint was the number of patients with flares during 7 months of follow-up (Ann. Rheum. Dis. 2013;72:1786-92).
At the time of randomization, the mean age of patients was 40 years, 87% were female, and their average HCQ dose was 400 mg/day. At 7 months of follow-up, the proportion of SLE flare rates was similar between the two groups (25% in group A vs. 27.6% in group B; P = .7). In a subset analysis of 57 patients from group A whose HCQ values were below 1,000 ng/mL after randomization and 39 patients from group B who maintained the therapeutic target dose of 1,000 ng/mL or higher after randomization, patients in group B tended to fewer flares compared with their counterparts in group A (20.5% vs. 35.1%, respectively; P = .12).
One reason that HCQ dosing above 1,000 ng/mL did not reduce the rate of SLE flares during the study period "may be that higher doses do not have an added therapeutic effect," the researchers speculated. "However, several factors may provide an alternative explanation of why our study did not provide its primary hypothesis." For one, the maintenance of HCQ above 1,000 ng/mL during the 7 months of follow up "was difficult to achieve." This could be explained by two factors, they continued. "The first is the pharmacokinetic variations of HCQ, but this explanation is unlikely because HCQ has a long terminal half-life and these patients were thought to be in a steady state. The second potential explanation might be adherence problems, even though known or suspected non-adherence was a major criterion for exclusion in our study. We found 10 patients with [HCQ] sufficiently low at inclusion to constitute an objective marker of lack of compliance."
The researchers acknowledged certain limitations of the study, including the potential for being underpowered (171 patients studied though the calculated sample size called for 200), and that the trend toward lower SLE flares in patients with higher HCQ "must be interpreted cautiously, since this analysis was not performed according to randomization group. The result might also be explained by better adherence to other medications, especially steroids."
The study was funded by a grant from the French PHRC 2005 Ministère de la Santé. Sanofi provided the HCQ and placebo tablets.
A strategy to maintain hydroxychloroquine blood levels above 1,000 ng/mL in adult patients with systemic lupus erythematosus did not reduce the number of SLE flares during a 7-month follow-up period, results from a French study demonstrated.
"This study confirms the pharmacokinetic/pharmacodynamic relation for hydroxychloroquine (HCQ) in patients with SLE," investigators led by Prof. Nathalie Costedoat-Chalumeau reported in the November 2013 issue of Annals of the Rheumatic Diseases.
"Our results do not justify recommending a therapeutic adaptation of HCQ dose. However, we suggest that [HCQ] be measured to detect non-adherence, especially in patients with active disease, and to help patients with poor adherence reach [HCQ levels greater than or equal to] 1,000 ng/mL."
The purpose of the trial, known at the PLUS Study, was to determine the potential benefits of individualizing HCQ dosing schedules to reach a target of 1,000 ng/mL or greater and thereby decrease rates of SLE flare. It was carried out in 573 patients with SLE at 37 centers in France from June 2007 through August 2010.
Of the 573 patients, researchers randomized 171 to one of two treatment groups: 84 to no daily dose change (group A), and 87 to increased HCQ dose to achieve the target of 1,000 g/mL or greater (group B). The primary endpoint was the number of patients with flares during 7 months of follow-up (Ann. Rheum. Dis. 2013;72:1786-92).
At the time of randomization, the mean age of patients was 40 years, 87% were female, and their average HCQ dose was 400 mg/day. At 7 months of follow-up, the proportion of SLE flare rates was similar between the two groups (25% in group A vs. 27.6% in group B; P = .7). In a subset analysis of 57 patients from group A whose HCQ values were below 1,000 ng/mL after randomization and 39 patients from group B who maintained the therapeutic target dose of 1,000 ng/mL or higher after randomization, patients in group B tended to fewer flares compared with their counterparts in group A (20.5% vs. 35.1%, respectively; P = .12).
One reason that HCQ dosing above 1,000 ng/mL did not reduce the rate of SLE flares during the study period "may be that higher doses do not have an added therapeutic effect," the researchers speculated. "However, several factors may provide an alternative explanation of why our study did not provide its primary hypothesis." For one, the maintenance of HCQ above 1,000 ng/mL during the 7 months of follow up "was difficult to achieve." This could be explained by two factors, they continued. "The first is the pharmacokinetic variations of HCQ, but this explanation is unlikely because HCQ has a long terminal half-life and these patients were thought to be in a steady state. The second potential explanation might be adherence problems, even though known or suspected non-adherence was a major criterion for exclusion in our study. We found 10 patients with [HCQ] sufficiently low at inclusion to constitute an objective marker of lack of compliance."
The researchers acknowledged certain limitations of the study, including the potential for being underpowered (171 patients studied though the calculated sample size called for 200), and that the trend toward lower SLE flares in patients with higher HCQ "must be interpreted cautiously, since this analysis was not performed according to randomization group. The result might also be explained by better adherence to other medications, especially steroids."
The study was funded by a grant from the French PHRC 2005 Ministère de la Santé. Sanofi provided the HCQ and placebo tablets.
A strategy to maintain hydroxychloroquine blood levels above 1,000 ng/mL in adult patients with systemic lupus erythematosus did not reduce the number of SLE flares during a 7-month follow-up period, results from a French study demonstrated.
"This study confirms the pharmacokinetic/pharmacodynamic relation for hydroxychloroquine (HCQ) in patients with SLE," investigators led by Prof. Nathalie Costedoat-Chalumeau reported in the November 2013 issue of Annals of the Rheumatic Diseases.
"Our results do not justify recommending a therapeutic adaptation of HCQ dose. However, we suggest that [HCQ] be measured to detect non-adherence, especially in patients with active disease, and to help patients with poor adherence reach [HCQ levels greater than or equal to] 1,000 ng/mL."
The purpose of the trial, known at the PLUS Study, was to determine the potential benefits of individualizing HCQ dosing schedules to reach a target of 1,000 ng/mL or greater and thereby decrease rates of SLE flare. It was carried out in 573 patients with SLE at 37 centers in France from June 2007 through August 2010.
Of the 573 patients, researchers randomized 171 to one of two treatment groups: 84 to no daily dose change (group A), and 87 to increased HCQ dose to achieve the target of 1,000 g/mL or greater (group B). The primary endpoint was the number of patients with flares during 7 months of follow-up (Ann. Rheum. Dis. 2013;72:1786-92).
At the time of randomization, the mean age of patients was 40 years, 87% were female, and their average HCQ dose was 400 mg/day. At 7 months of follow-up, the proportion of SLE flare rates was similar between the two groups (25% in group A vs. 27.6% in group B; P = .7). In a subset analysis of 57 patients from group A whose HCQ values were below 1,000 ng/mL after randomization and 39 patients from group B who maintained the therapeutic target dose of 1,000 ng/mL or higher after randomization, patients in group B tended to fewer flares compared with their counterparts in group A (20.5% vs. 35.1%, respectively; P = .12).
One reason that HCQ dosing above 1,000 ng/mL did not reduce the rate of SLE flares during the study period "may be that higher doses do not have an added therapeutic effect," the researchers speculated. "However, several factors may provide an alternative explanation of why our study did not provide its primary hypothesis." For one, the maintenance of HCQ above 1,000 ng/mL during the 7 months of follow up "was difficult to achieve." This could be explained by two factors, they continued. "The first is the pharmacokinetic variations of HCQ, but this explanation is unlikely because HCQ has a long terminal half-life and these patients were thought to be in a steady state. The second potential explanation might be adherence problems, even though known or suspected non-adherence was a major criterion for exclusion in our study. We found 10 patients with [HCQ] sufficiently low at inclusion to constitute an objective marker of lack of compliance."
The researchers acknowledged certain limitations of the study, including the potential for being underpowered (171 patients studied though the calculated sample size called for 200), and that the trend toward lower SLE flares in patients with higher HCQ "must be interpreted cautiously, since this analysis was not performed according to randomization group. The result might also be explained by better adherence to other medications, especially steroids."
The study was funded by a grant from the French PHRC 2005 Ministère de la Santé. Sanofi provided the HCQ and placebo tablets.
FROM ANNALS OF THE RHEUMATIC DISEASES
Major finding: At 7 months of follow-up, the flare rates in patients with systemic lupus erythematosus were similar between those who received no change in hydroxychloroquine (HCQ) dosing and those whose HCQ dose was increased to achieve a target level of 1,000 ng/mL or greater (25% vs. 27.6%, respectively).
Data source: A study of 171 adults with SLE who were randomized to one of the two treatment groups at 37 centers in France from June 2007 through August 2010.
Disclosures: The study was funded by a grant from the French PHRC 2005 Ministère de la Santé. Sanofi provided the study drug and the placebo tablets.
Investigational biologic lessened symptoms in some patients with lupus
An investigational biologic that targets T cells in patients with systemic lupus erythematosus significantly lessened symptoms in a subset of patients who completed a 24-week randomized trial.
In the overall analysis, the drug – rigerimod (Lupuzor) – did not perform significantly better than did placebo. But a post hoc analysis of patients with a higher clinical disease score found better results, with a response rate of 84% vs. 46% who took placebo, Dr. Robert Zimmer and his colleagues reported in the November issue of the Annals of the Rheumatic Diseases (2013 [doi:10.1136/annrheumdis-2012-202460]).
The drug was well tolerated, with 40% of the treatment group experiencing at least one side effect, compared with 49% of the placebo group, wrote Dr. Zimmer, president and chief science officer of ImmuPharma, the French company that is developing rigerimod.
Rigerimod is a made from a small nuclear ribonucleoprotein. Studies suggest that it inhibits T-cell reactivity, thereby reducing proteinuria, vasculitis, and dermatitis. It also prevented the production of antibodies to DNA from a lupus-model mouse.
The phase II study comprised an intent-to-treat group 149 patients who had baseline total scores of 6 or more on the SLEDAI-2K (Systemic Lupus Erythematosus Disease Activity Index-2K). They were randomized to three arms: subcutaneous injections of 200 mcg rigerimod every 4 weeks (group 1), every 2 weeks (group 2), or placebo injections.
All patients were treated for 12 weeks and then followed for an additional 12 weeks.
Most (96%) were women; their mean age at enrollment was about 38 years. The mean disease duration was about 8 years. The mean SLEDAI-2K total score was 11, and the mean physician’s global assessment (PGA), slightly more than 1. More than 80% were taking corticosteroids; about half were taking concomitant antimalarials and a quarter, concomitant azathioprine. Less than 5% were taking methotrexate.
Responders were considered those who had a reduction of at least 4 points in their baseline SLEDAI-2K score, with no increase in the PGA score. Treatment failures were those who had a severe disease flare, needed a steroid increase of at least 80 mg prednisone equivalent, or received biologics. Patients could continue their existing, stable medication regimens during the study.
The dropout rate was 9% (13 patients) – 8% of group 1, 2% of group 2 and 16% of the placebo group.
By the end of the 12-week treatment period, the response rate was significantly better in group 1 than in the placebo group (53% vs. 36%). Response in group 2 was not significantly different from group 1(45%).
By the end of week 24, the response rate had increased similarly in the entire study population (group 1, 59%: group 2, 59%; placebo, 53%). These rates were not significantly different from one another.
The target group analysis was conducted after the study’s end. The investigators restricted this group to patients whose SLEDAI-2K clinical –not overall – score was at least 6. "Despite the fact that this population had not been defined at the beginning of the study, the changes in the inclusion criteria led us to carefully analyze this population [clinical SLEDAI score of 6 and above] as this population will be the phase III population," they noted.
At week 12 in this population, there were significantly more responders in group 1 than in the placebo group (62% vs. 39%). The difference between group 2 and placebo was not statistically significant.
By week 24, groups 1 and 2 had similar response rates (69% and 62%), but these were not significantly higher than placebo (56%), with p values of 0.11 for group 1 vs. placebo and 0.28 for group 2 vs. placebo.
The investigators did not break down response by symptom, but did say "The apparent clinical benefit observed f or patients who received 200 mcg Lupuzor every 4 weeks, compared with those who received placebo every 2 weeks, were mainly due to an improvement in articular and cutaneous symptoms (arthritis and rash) at week 12."
The incidence of adverse events was low and similar between the groups; 40% of patients in each active group experienced at least one, compared with 49% of patients in the placebo group. The most common were injection site reactions, which occurred in 6% of group 1, 10% of group 2, and 2% of the placebo group.
One patient died during the trial. The cause of death was pneumonia, which was not deemed related to the study drug. The investigators noted that this patient had been on immunosuppressant therapy before entering the trial. Two other patients also developed pneumonia. "Thus," the investigators said, "while there did not appear to be an increased incidence of serious infections with Lupuzor in this short study, longer-term studies are needed to further characterise the overall tolerability profile of Lupuzor."
Finally, the investigators suggested that the clinical SLEDAI score, rather than the overall score, is the best way to assess this drug. "Comparing the number of responders in the target population and those of the overall population it appears that there is no change. Exactly the same number of responders was recorded in the three groups in the overall population and the target population. This indicates that the commonly used inclusion criterion [SLEDAI score of 6 and above] creates a bias in the evaluation of the study results in falsely reducing the response rate and therefore statistical analysis."
ImmuPharma is developing the drug and conducted the study.
An investigational biologic that targets T cells in patients with systemic lupus erythematosus significantly lessened symptoms in a subset of patients who completed a 24-week randomized trial.
In the overall analysis, the drug – rigerimod (Lupuzor) – did not perform significantly better than did placebo. But a post hoc analysis of patients with a higher clinical disease score found better results, with a response rate of 84% vs. 46% who took placebo, Dr. Robert Zimmer and his colleagues reported in the November issue of the Annals of the Rheumatic Diseases (2013 [doi:10.1136/annrheumdis-2012-202460]).
The drug was well tolerated, with 40% of the treatment group experiencing at least one side effect, compared with 49% of the placebo group, wrote Dr. Zimmer, president and chief science officer of ImmuPharma, the French company that is developing rigerimod.
Rigerimod is a made from a small nuclear ribonucleoprotein. Studies suggest that it inhibits T-cell reactivity, thereby reducing proteinuria, vasculitis, and dermatitis. It also prevented the production of antibodies to DNA from a lupus-model mouse.
The phase II study comprised an intent-to-treat group 149 patients who had baseline total scores of 6 or more on the SLEDAI-2K (Systemic Lupus Erythematosus Disease Activity Index-2K). They were randomized to three arms: subcutaneous injections of 200 mcg rigerimod every 4 weeks (group 1), every 2 weeks (group 2), or placebo injections.
All patients were treated for 12 weeks and then followed for an additional 12 weeks.
Most (96%) were women; their mean age at enrollment was about 38 years. The mean disease duration was about 8 years. The mean SLEDAI-2K total score was 11, and the mean physician’s global assessment (PGA), slightly more than 1. More than 80% were taking corticosteroids; about half were taking concomitant antimalarials and a quarter, concomitant azathioprine. Less than 5% were taking methotrexate.
Responders were considered those who had a reduction of at least 4 points in their baseline SLEDAI-2K score, with no increase in the PGA score. Treatment failures were those who had a severe disease flare, needed a steroid increase of at least 80 mg prednisone equivalent, or received biologics. Patients could continue their existing, stable medication regimens during the study.
The dropout rate was 9% (13 patients) – 8% of group 1, 2% of group 2 and 16% of the placebo group.
By the end of the 12-week treatment period, the response rate was significantly better in group 1 than in the placebo group (53% vs. 36%). Response in group 2 was not significantly different from group 1(45%).
By the end of week 24, the response rate had increased similarly in the entire study population (group 1, 59%: group 2, 59%; placebo, 53%). These rates were not significantly different from one another.
The target group analysis was conducted after the study’s end. The investigators restricted this group to patients whose SLEDAI-2K clinical –not overall – score was at least 6. "Despite the fact that this population had not been defined at the beginning of the study, the changes in the inclusion criteria led us to carefully analyze this population [clinical SLEDAI score of 6 and above] as this population will be the phase III population," they noted.
At week 12 in this population, there were significantly more responders in group 1 than in the placebo group (62% vs. 39%). The difference between group 2 and placebo was not statistically significant.
By week 24, groups 1 and 2 had similar response rates (69% and 62%), but these were not significantly higher than placebo (56%), with p values of 0.11 for group 1 vs. placebo and 0.28 for group 2 vs. placebo.
The investigators did not break down response by symptom, but did say "The apparent clinical benefit observed f or patients who received 200 mcg Lupuzor every 4 weeks, compared with those who received placebo every 2 weeks, were mainly due to an improvement in articular and cutaneous symptoms (arthritis and rash) at week 12."
The incidence of adverse events was low and similar between the groups; 40% of patients in each active group experienced at least one, compared with 49% of patients in the placebo group. The most common were injection site reactions, which occurred in 6% of group 1, 10% of group 2, and 2% of the placebo group.
One patient died during the trial. The cause of death was pneumonia, which was not deemed related to the study drug. The investigators noted that this patient had been on immunosuppressant therapy before entering the trial. Two other patients also developed pneumonia. "Thus," the investigators said, "while there did not appear to be an increased incidence of serious infections with Lupuzor in this short study, longer-term studies are needed to further characterise the overall tolerability profile of Lupuzor."
Finally, the investigators suggested that the clinical SLEDAI score, rather than the overall score, is the best way to assess this drug. "Comparing the number of responders in the target population and those of the overall population it appears that there is no change. Exactly the same number of responders was recorded in the three groups in the overall population and the target population. This indicates that the commonly used inclusion criterion [SLEDAI score of 6 and above] creates a bias in the evaluation of the study results in falsely reducing the response rate and therefore statistical analysis."
ImmuPharma is developing the drug and conducted the study.
An investigational biologic that targets T cells in patients with systemic lupus erythematosus significantly lessened symptoms in a subset of patients who completed a 24-week randomized trial.
In the overall analysis, the drug – rigerimod (Lupuzor) – did not perform significantly better than did placebo. But a post hoc analysis of patients with a higher clinical disease score found better results, with a response rate of 84% vs. 46% who took placebo, Dr. Robert Zimmer and his colleagues reported in the November issue of the Annals of the Rheumatic Diseases (2013 [doi:10.1136/annrheumdis-2012-202460]).
The drug was well tolerated, with 40% of the treatment group experiencing at least one side effect, compared with 49% of the placebo group, wrote Dr. Zimmer, president and chief science officer of ImmuPharma, the French company that is developing rigerimod.
Rigerimod is a made from a small nuclear ribonucleoprotein. Studies suggest that it inhibits T-cell reactivity, thereby reducing proteinuria, vasculitis, and dermatitis. It also prevented the production of antibodies to DNA from a lupus-model mouse.
The phase II study comprised an intent-to-treat group 149 patients who had baseline total scores of 6 or more on the SLEDAI-2K (Systemic Lupus Erythematosus Disease Activity Index-2K). They were randomized to three arms: subcutaneous injections of 200 mcg rigerimod every 4 weeks (group 1), every 2 weeks (group 2), or placebo injections.
All patients were treated for 12 weeks and then followed for an additional 12 weeks.
Most (96%) were women; their mean age at enrollment was about 38 years. The mean disease duration was about 8 years. The mean SLEDAI-2K total score was 11, and the mean physician’s global assessment (PGA), slightly more than 1. More than 80% were taking corticosteroids; about half were taking concomitant antimalarials and a quarter, concomitant azathioprine. Less than 5% were taking methotrexate.
Responders were considered those who had a reduction of at least 4 points in their baseline SLEDAI-2K score, with no increase in the PGA score. Treatment failures were those who had a severe disease flare, needed a steroid increase of at least 80 mg prednisone equivalent, or received biologics. Patients could continue their existing, stable medication regimens during the study.
The dropout rate was 9% (13 patients) – 8% of group 1, 2% of group 2 and 16% of the placebo group.
By the end of the 12-week treatment period, the response rate was significantly better in group 1 than in the placebo group (53% vs. 36%). Response in group 2 was not significantly different from group 1(45%).
By the end of week 24, the response rate had increased similarly in the entire study population (group 1, 59%: group 2, 59%; placebo, 53%). These rates were not significantly different from one another.
The target group analysis was conducted after the study’s end. The investigators restricted this group to patients whose SLEDAI-2K clinical –not overall – score was at least 6. "Despite the fact that this population had not been defined at the beginning of the study, the changes in the inclusion criteria led us to carefully analyze this population [clinical SLEDAI score of 6 and above] as this population will be the phase III population," they noted.
At week 12 in this population, there were significantly more responders in group 1 than in the placebo group (62% vs. 39%). The difference between group 2 and placebo was not statistically significant.
By week 24, groups 1 and 2 had similar response rates (69% and 62%), but these were not significantly higher than placebo (56%), with p values of 0.11 for group 1 vs. placebo and 0.28 for group 2 vs. placebo.
The investigators did not break down response by symptom, but did say "The apparent clinical benefit observed f or patients who received 200 mcg Lupuzor every 4 weeks, compared with those who received placebo every 2 weeks, were mainly due to an improvement in articular and cutaneous symptoms (arthritis and rash) at week 12."
The incidence of adverse events was low and similar between the groups; 40% of patients in each active group experienced at least one, compared with 49% of patients in the placebo group. The most common were injection site reactions, which occurred in 6% of group 1, 10% of group 2, and 2% of the placebo group.
One patient died during the trial. The cause of death was pneumonia, which was not deemed related to the study drug. The investigators noted that this patient had been on immunosuppressant therapy before entering the trial. Two other patients also developed pneumonia. "Thus," the investigators said, "while there did not appear to be an increased incidence of serious infections with Lupuzor in this short study, longer-term studies are needed to further characterise the overall tolerability profile of Lupuzor."
Finally, the investigators suggested that the clinical SLEDAI score, rather than the overall score, is the best way to assess this drug. "Comparing the number of responders in the target population and those of the overall population it appears that there is no change. Exactly the same number of responders was recorded in the three groups in the overall population and the target population. This indicates that the commonly used inclusion criterion [SLEDAI score of 6 and above] creates a bias in the evaluation of the study results in falsely reducing the response rate and therefore statistical analysis."
ImmuPharma is developing the drug and conducted the study.
Major finding: The investigational biologic rigerimod was superior to placebo in patients with a higher lupus clinical disease activity score, with a response rate of 84% vs. 46%.
Data source: The 24-week randomized, phase IIb trial involved 149 patients.
Disclosures: ImmuPharma is developing rigerimod and conducted the study. Dr. Zimmer is the company’s president and chief scientific officer.
Secukinumab soars in phase III psoriasis studies
ISTANBUL, TURKEY – The investigational interleukin-17A inhibitor secukinumab was the talk of Europe’s premier dermatology conference as a result of a series of world-premiere presentations of not one, but three, phase III clinical trials.
The entire secukinumab pivotal phase III program results were presented together at the annual congress of the European Academy of Dermatology and Venereology. Based on these promising results, Novartis plans to file for Food and Drug Administration and European regulatory approval before the end of 2013.
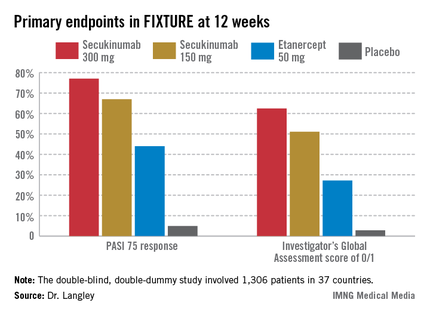
The three randomized, double-blind, multicenter pivotal trials of secukinumab in psoriasis collectively included 3,367 patients with moderate to severe chronic plaque psoriasis. All three studies were strongly positive. Efficacy rates were higher than with current biologics, clinical improvement was remarkably rapid, and the safety profile was reassuring.
"Those of us working in this area of research are really excited by this new data and what it says for the treatment of psoriasis patients," commented Dr. Richard Langley of Dalhousie University, Halifax, N.S.
He presented the centerpiece of this trio of phase III trials, known as the FIXTURE trial. FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using 2 Dosing Regimens to Determine Efficacy in Psoriasis) was a 1,306-patient, double-blind, double-dummy trial conducted by 153 investigators in 37 countries. The trial featured a 1-year head-to-head comparison of secukinumab and the widely prescribed tumor necrosis factor (TNF) inhibitor etanercept (Enbrel). The IL-17A inhibitor outperformed etanercept in all of the prespecified primary and secondary outcome measures. Moreover, the two biologics proved equally safe.
"I think it’s interesting in comparing these two molecules that when we look at the data to date, we see comparable safety results. Etanercept is one of the most widely used biologics and has an excellent safety record. That’s reassuring for those of us who are investigating IL-17 signaling," observed Dr. Langley, also the current president of the Canadian Dermatology Association.
FIXTURE participants had a mean baseline Psoriasis Area and Severity Index (PASI) score of 24, an average body mass index of 34 kg/m2, and a mean age in the mid-40s, and were previous inadequate responders to conventional systemic agents and/or biologics. The two co-primary endpoints were 12-week rates of at least a 75% reduction in PASI score, or PASI 75 response, and an Investigator’s Global Assessment (IGA) rating of 0/1, corresponding to clear or nearly clear, on a modified 5-point scale that provides more clinically meaningful information than the older 6-point IGA scale. Patients randomized to the secukinumab 300-mg or 150-mg groups did significantly better on both outcome measures than those assigned to etanercept.
The onset of benefit with secukinumab was swift; a statistically significant improvement compared with etanercept was seen within 2 weeks in terms of IGA scores and within 3 weeks for PASI 75. Secukinumab at 300 mg per subcutaneous injection – the most effective dose across all three pivotal trials – achieved roughly a 50% reduction in PASI scores after 3 weeks, compared with 8 weeks for etanercept, the dermatologist explained.
The PASI 75 response rate at 12 weeks was 77.1% in patients receiving secukinumab at 300 mg, 67% for those receiving secukinumab at 150 mg, 44% for etanercept 50 mg, and 4.9% for placebo.
The highest response rates with secukinumab were seen at week 16 rather than week 12. In the secukinumab 300-mg group, the PASI 75 rate at week 16 was 86.7%, with that high level of response being retained throughout the remainder of the 52-week study. In contrast, the PASI 75 rate at week 16 for etanercept was less than 60%. The IGA 0/1 rate at week 16 was 75.5% in the secukinumab 300-mg group and 60% with secukinumab 150 mg. The peak IGA 0/1 rate in the etanercept arm was only about 45%, and it came much later, at week 26.
Looking at more stringent secondary endpoints, the investigators found that a PASI 90 response was achieved by week 16 in 72.4% of the high-dose secukinumab group, compared with about 30% with etanercept. Twenty-four percent of patients on high-dose secukinumab had a PASI 100 response at week 12, compared with 4% on etanercept. By week 16, 36.8% of the high-dose secukinumab group had a PASI 100 response. At week 52, 65% of the secukinumab 300-mg group maintained a PASI 90 response, compared with 33% of the etanercept group.
The most common adverse event documented in FIXTURE was nasopharyngitis, which occurred in roughly one-third of patients in all four study arms. The serious infection rate was 1%-1.2% in all four study arms. No signals of an increase in malignancies or major adverse cardiovascular events were noted in any of the active treatment groups. Overall, adverse events were similar in both secukinumab arms and comparable to etanercept. Injection-site reactions occurred in 6.1% of the etanercept group and in none of the patients assigned to secukinumab or placebo.
The incidence of treatment-emergent antidrug antibodies in the secukinumab arms was 0.4%, and when it occurred, it had no impact on treatment efficacy or safety. Patients on etanercept weren’t tested for the emergence of antidrug antibodies.
Candida infections occurred in 4.7% of patients on secukinumab 300 mg, 2.3% of those on 150 mg, and 1.2% of the etanercept group. All cases in patients on secukinumab were mild or moderate and easily treated. Candida infections are a side effect of special interest because patients with a genetic defect in the IL-17 pathway can develop chronic mucocutaneous candidiasis. A theoretical concern that pharmacologic inhibition of IL-17 might create a big problem in this regard has not materialized.
The other two phase III secukinumab clinical trials presented in Istanbul – ERASURE and SCULPTURE – showed efficacy and safety results similar to those of FIXTURE. Unlike FIXTURE, neither of those trials featured a head-to-head comparison with another biologic.
These were the first completed phase III studies for any of the three biologics in this novel, highly promising class targeting IL-17 in the treatment of psoriasis and other immune-mediated inflammatory diseases. The other two agents in the pipeline targeting IL-17, brodalumab and ixekizumab, remain in ongoing phase III trials.
Because of the pivotal role IL-17 plays in generating inflammatory cytokines downstream, all three drugs are being studied for additional indications beyond psoriasis. For example, secukinumab, an IgG1 human monoclonal antibody, is in phase III clinical trials for psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis, and in phase II for muscular sclerosis and asthma.
One audience member asked Dr. Langley for his thoughts regarding the absence of injection-site reactions in the secukinumab-treated patients. He replied that one obvious factor is that secukinumab injections are given much less frequently: once monthly during maintenance therapy as compared to once weekly with etanercept. In any event, he doesn’t consider injection-site reactions to be clinically relevant.
"Injection-site reactions don’t seem to be a major issue with any of the subcutaneous therapies. It has never in 15 years of using different biologics been an issue that’s made me stop treatment for a patient," he added.
Secukinumab was dosed at baseline, again weekly through week 4, then once monthly from week 8 through the study’s end. Etanercept was dosed at 50 mg twice weekly for 12 weeks, then once weekly in accord with the labeling instructions in most countries. Nonresponders at 12 weeks in the placebo arm were at that point randomized to secukinumab at 300 mg or 150 mg. The statistical analysis in FIXTURE was performed by nonresponder imputation.
"Those who are interested in statistics will know that that’s the most rigorous way that you can look at the data, because anybody who drops out of the study, whether they withdraw consent, have a protocol violation, or have an adverse event, even if they were responding at that time, are considered a failure or nonresponder," Dr. Langley explained.
Asked how he sees secukinumab fitting into patient management, Dr. Langley replied, "Most of us are forced to use conventional therapies and phototherapy to get approval for biologics, but after that I think the safety and efficacy of this drug put it right at the top. I could definitely use this drug as a first-line biologic for patients with plain psoriasis without psoriatic arthritis."
Coinvestigator Dr. Kristian Reich, professor of dermatology at Georg-August University in Göttingen, Germany, said he will probably continue to use TNF inhibitors in psoriasis patients with prominent arthritis, where he believes the TNF inhibitors are particularly beneficial, but he anticipates using secukinumab widely in others.
Dr. Bruce E. Strober, another active clinical trial researcher in psoriasis, said he likes what he sees in the IL-17 inhibitor data.
"I predict as a specialty we’ll become very confident in these drugs 5 years from now. A lot of us will be using them as first line in many patients. That’s my prediction. For now, though, I’m a big fan of TNF inhibition," added Dr. Strober, vice chair of dermatology at the University of Connecticut Medical Center, Farmington.
The secukinumab development program is sponsored by Novartis. Dr. Langley, Dr. Reich, and Dr. Strober disclosed having received research grants from and serving as consultants to Novartis and numerous other companies developing biologic agents for psoriasis.
ISTANBUL, TURKEY – The investigational interleukin-17A inhibitor secukinumab was the talk of Europe’s premier dermatology conference as a result of a series of world-premiere presentations of not one, but three, phase III clinical trials.
The entire secukinumab pivotal phase III program results were presented together at the annual congress of the European Academy of Dermatology and Venereology. Based on these promising results, Novartis plans to file for Food and Drug Administration and European regulatory approval before the end of 2013.

The three randomized, double-blind, multicenter pivotal trials of secukinumab in psoriasis collectively included 3,367 patients with moderate to severe chronic plaque psoriasis. All three studies were strongly positive. Efficacy rates were higher than with current biologics, clinical improvement was remarkably rapid, and the safety profile was reassuring.
"Those of us working in this area of research are really excited by this new data and what it says for the treatment of psoriasis patients," commented Dr. Richard Langley of Dalhousie University, Halifax, N.S.
He presented the centerpiece of this trio of phase III trials, known as the FIXTURE trial. FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using 2 Dosing Regimens to Determine Efficacy in Psoriasis) was a 1,306-patient, double-blind, double-dummy trial conducted by 153 investigators in 37 countries. The trial featured a 1-year head-to-head comparison of secukinumab and the widely prescribed tumor necrosis factor (TNF) inhibitor etanercept (Enbrel). The IL-17A inhibitor outperformed etanercept in all of the prespecified primary and secondary outcome measures. Moreover, the two biologics proved equally safe.
"I think it’s interesting in comparing these two molecules that when we look at the data to date, we see comparable safety results. Etanercept is one of the most widely used biologics and has an excellent safety record. That’s reassuring for those of us who are investigating IL-17 signaling," observed Dr. Langley, also the current president of the Canadian Dermatology Association.
FIXTURE participants had a mean baseline Psoriasis Area and Severity Index (PASI) score of 24, an average body mass index of 34 kg/m2, and a mean age in the mid-40s, and were previous inadequate responders to conventional systemic agents and/or biologics. The two co-primary endpoints were 12-week rates of at least a 75% reduction in PASI score, or PASI 75 response, and an Investigator’s Global Assessment (IGA) rating of 0/1, corresponding to clear or nearly clear, on a modified 5-point scale that provides more clinically meaningful information than the older 6-point IGA scale. Patients randomized to the secukinumab 300-mg or 150-mg groups did significantly better on both outcome measures than those assigned to etanercept.
The onset of benefit with secukinumab was swift; a statistically significant improvement compared with etanercept was seen within 2 weeks in terms of IGA scores and within 3 weeks for PASI 75. Secukinumab at 300 mg per subcutaneous injection – the most effective dose across all three pivotal trials – achieved roughly a 50% reduction in PASI scores after 3 weeks, compared with 8 weeks for etanercept, the dermatologist explained.
The PASI 75 response rate at 12 weeks was 77.1% in patients receiving secukinumab at 300 mg, 67% for those receiving secukinumab at 150 mg, 44% for etanercept 50 mg, and 4.9% for placebo.
The highest response rates with secukinumab were seen at week 16 rather than week 12. In the secukinumab 300-mg group, the PASI 75 rate at week 16 was 86.7%, with that high level of response being retained throughout the remainder of the 52-week study. In contrast, the PASI 75 rate at week 16 for etanercept was less than 60%. The IGA 0/1 rate at week 16 was 75.5% in the secukinumab 300-mg group and 60% with secukinumab 150 mg. The peak IGA 0/1 rate in the etanercept arm was only about 45%, and it came much later, at week 26.
Looking at more stringent secondary endpoints, the investigators found that a PASI 90 response was achieved by week 16 in 72.4% of the high-dose secukinumab group, compared with about 30% with etanercept. Twenty-four percent of patients on high-dose secukinumab had a PASI 100 response at week 12, compared with 4% on etanercept. By week 16, 36.8% of the high-dose secukinumab group had a PASI 100 response. At week 52, 65% of the secukinumab 300-mg group maintained a PASI 90 response, compared with 33% of the etanercept group.
The most common adverse event documented in FIXTURE was nasopharyngitis, which occurred in roughly one-third of patients in all four study arms. The serious infection rate was 1%-1.2% in all four study arms. No signals of an increase in malignancies or major adverse cardiovascular events were noted in any of the active treatment groups. Overall, adverse events were similar in both secukinumab arms and comparable to etanercept. Injection-site reactions occurred in 6.1% of the etanercept group and in none of the patients assigned to secukinumab or placebo.
The incidence of treatment-emergent antidrug antibodies in the secukinumab arms was 0.4%, and when it occurred, it had no impact on treatment efficacy or safety. Patients on etanercept weren’t tested for the emergence of antidrug antibodies.
Candida infections occurred in 4.7% of patients on secukinumab 300 mg, 2.3% of those on 150 mg, and 1.2% of the etanercept group. All cases in patients on secukinumab were mild or moderate and easily treated. Candida infections are a side effect of special interest because patients with a genetic defect in the IL-17 pathway can develop chronic mucocutaneous candidiasis. A theoretical concern that pharmacologic inhibition of IL-17 might create a big problem in this regard has not materialized.
The other two phase III secukinumab clinical trials presented in Istanbul – ERASURE and SCULPTURE – showed efficacy and safety results similar to those of FIXTURE. Unlike FIXTURE, neither of those trials featured a head-to-head comparison with another biologic.
These were the first completed phase III studies for any of the three biologics in this novel, highly promising class targeting IL-17 in the treatment of psoriasis and other immune-mediated inflammatory diseases. The other two agents in the pipeline targeting IL-17, brodalumab and ixekizumab, remain in ongoing phase III trials.
Because of the pivotal role IL-17 plays in generating inflammatory cytokines downstream, all three drugs are being studied for additional indications beyond psoriasis. For example, secukinumab, an IgG1 human monoclonal antibody, is in phase III clinical trials for psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis, and in phase II for muscular sclerosis and asthma.
One audience member asked Dr. Langley for his thoughts regarding the absence of injection-site reactions in the secukinumab-treated patients. He replied that one obvious factor is that secukinumab injections are given much less frequently: once monthly during maintenance therapy as compared to once weekly with etanercept. In any event, he doesn’t consider injection-site reactions to be clinically relevant.
"Injection-site reactions don’t seem to be a major issue with any of the subcutaneous therapies. It has never in 15 years of using different biologics been an issue that’s made me stop treatment for a patient," he added.
Secukinumab was dosed at baseline, again weekly through week 4, then once monthly from week 8 through the study’s end. Etanercept was dosed at 50 mg twice weekly for 12 weeks, then once weekly in accord with the labeling instructions in most countries. Nonresponders at 12 weeks in the placebo arm were at that point randomized to secukinumab at 300 mg or 150 mg. The statistical analysis in FIXTURE was performed by nonresponder imputation.
"Those who are interested in statistics will know that that’s the most rigorous way that you can look at the data, because anybody who drops out of the study, whether they withdraw consent, have a protocol violation, or have an adverse event, even if they were responding at that time, are considered a failure or nonresponder," Dr. Langley explained.
Asked how he sees secukinumab fitting into patient management, Dr. Langley replied, "Most of us are forced to use conventional therapies and phototherapy to get approval for biologics, but after that I think the safety and efficacy of this drug put it right at the top. I could definitely use this drug as a first-line biologic for patients with plain psoriasis without psoriatic arthritis."
Coinvestigator Dr. Kristian Reich, professor of dermatology at Georg-August University in Göttingen, Germany, said he will probably continue to use TNF inhibitors in psoriasis patients with prominent arthritis, where he believes the TNF inhibitors are particularly beneficial, but he anticipates using secukinumab widely in others.
Dr. Bruce E. Strober, another active clinical trial researcher in psoriasis, said he likes what he sees in the IL-17 inhibitor data.
"I predict as a specialty we’ll become very confident in these drugs 5 years from now. A lot of us will be using them as first line in many patients. That’s my prediction. For now, though, I’m a big fan of TNF inhibition," added Dr. Strober, vice chair of dermatology at the University of Connecticut Medical Center, Farmington.
The secukinumab development program is sponsored by Novartis. Dr. Langley, Dr. Reich, and Dr. Strober disclosed having received research grants from and serving as consultants to Novartis and numerous other companies developing biologic agents for psoriasis.
ISTANBUL, TURKEY – The investigational interleukin-17A inhibitor secukinumab was the talk of Europe’s premier dermatology conference as a result of a series of world-premiere presentations of not one, but three, phase III clinical trials.
The entire secukinumab pivotal phase III program results were presented together at the annual congress of the European Academy of Dermatology and Venereology. Based on these promising results, Novartis plans to file for Food and Drug Administration and European regulatory approval before the end of 2013.

The three randomized, double-blind, multicenter pivotal trials of secukinumab in psoriasis collectively included 3,367 patients with moderate to severe chronic plaque psoriasis. All three studies were strongly positive. Efficacy rates were higher than with current biologics, clinical improvement was remarkably rapid, and the safety profile was reassuring.
"Those of us working in this area of research are really excited by this new data and what it says for the treatment of psoriasis patients," commented Dr. Richard Langley of Dalhousie University, Halifax, N.S.
He presented the centerpiece of this trio of phase III trials, known as the FIXTURE trial. FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using 2 Dosing Regimens to Determine Efficacy in Psoriasis) was a 1,306-patient, double-blind, double-dummy trial conducted by 153 investigators in 37 countries. The trial featured a 1-year head-to-head comparison of secukinumab and the widely prescribed tumor necrosis factor (TNF) inhibitor etanercept (Enbrel). The IL-17A inhibitor outperformed etanercept in all of the prespecified primary and secondary outcome measures. Moreover, the two biologics proved equally safe.
"I think it’s interesting in comparing these two molecules that when we look at the data to date, we see comparable safety results. Etanercept is one of the most widely used biologics and has an excellent safety record. That’s reassuring for those of us who are investigating IL-17 signaling," observed Dr. Langley, also the current president of the Canadian Dermatology Association.
FIXTURE participants had a mean baseline Psoriasis Area and Severity Index (PASI) score of 24, an average body mass index of 34 kg/m2, and a mean age in the mid-40s, and were previous inadequate responders to conventional systemic agents and/or biologics. The two co-primary endpoints were 12-week rates of at least a 75% reduction in PASI score, or PASI 75 response, and an Investigator’s Global Assessment (IGA) rating of 0/1, corresponding to clear or nearly clear, on a modified 5-point scale that provides more clinically meaningful information than the older 6-point IGA scale. Patients randomized to the secukinumab 300-mg or 150-mg groups did significantly better on both outcome measures than those assigned to etanercept.
The onset of benefit with secukinumab was swift; a statistically significant improvement compared with etanercept was seen within 2 weeks in terms of IGA scores and within 3 weeks for PASI 75. Secukinumab at 300 mg per subcutaneous injection – the most effective dose across all three pivotal trials – achieved roughly a 50% reduction in PASI scores after 3 weeks, compared with 8 weeks for etanercept, the dermatologist explained.
The PASI 75 response rate at 12 weeks was 77.1% in patients receiving secukinumab at 300 mg, 67% for those receiving secukinumab at 150 mg, 44% for etanercept 50 mg, and 4.9% for placebo.
The highest response rates with secukinumab were seen at week 16 rather than week 12. In the secukinumab 300-mg group, the PASI 75 rate at week 16 was 86.7%, with that high level of response being retained throughout the remainder of the 52-week study. In contrast, the PASI 75 rate at week 16 for etanercept was less than 60%. The IGA 0/1 rate at week 16 was 75.5% in the secukinumab 300-mg group and 60% with secukinumab 150 mg. The peak IGA 0/1 rate in the etanercept arm was only about 45%, and it came much later, at week 26.
Looking at more stringent secondary endpoints, the investigators found that a PASI 90 response was achieved by week 16 in 72.4% of the high-dose secukinumab group, compared with about 30% with etanercept. Twenty-four percent of patients on high-dose secukinumab had a PASI 100 response at week 12, compared with 4% on etanercept. By week 16, 36.8% of the high-dose secukinumab group had a PASI 100 response. At week 52, 65% of the secukinumab 300-mg group maintained a PASI 90 response, compared with 33% of the etanercept group.
The most common adverse event documented in FIXTURE was nasopharyngitis, which occurred in roughly one-third of patients in all four study arms. The serious infection rate was 1%-1.2% in all four study arms. No signals of an increase in malignancies or major adverse cardiovascular events were noted in any of the active treatment groups. Overall, adverse events were similar in both secukinumab arms and comparable to etanercept. Injection-site reactions occurred in 6.1% of the etanercept group and in none of the patients assigned to secukinumab or placebo.
The incidence of treatment-emergent antidrug antibodies in the secukinumab arms was 0.4%, and when it occurred, it had no impact on treatment efficacy or safety. Patients on etanercept weren’t tested for the emergence of antidrug antibodies.
Candida infections occurred in 4.7% of patients on secukinumab 300 mg, 2.3% of those on 150 mg, and 1.2% of the etanercept group. All cases in patients on secukinumab were mild or moderate and easily treated. Candida infections are a side effect of special interest because patients with a genetic defect in the IL-17 pathway can develop chronic mucocutaneous candidiasis. A theoretical concern that pharmacologic inhibition of IL-17 might create a big problem in this regard has not materialized.
The other two phase III secukinumab clinical trials presented in Istanbul – ERASURE and SCULPTURE – showed efficacy and safety results similar to those of FIXTURE. Unlike FIXTURE, neither of those trials featured a head-to-head comparison with another biologic.
These were the first completed phase III studies for any of the three biologics in this novel, highly promising class targeting IL-17 in the treatment of psoriasis and other immune-mediated inflammatory diseases. The other two agents in the pipeline targeting IL-17, brodalumab and ixekizumab, remain in ongoing phase III trials.
Because of the pivotal role IL-17 plays in generating inflammatory cytokines downstream, all three drugs are being studied for additional indications beyond psoriasis. For example, secukinumab, an IgG1 human monoclonal antibody, is in phase III clinical trials for psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis, and in phase II for muscular sclerosis and asthma.
One audience member asked Dr. Langley for his thoughts regarding the absence of injection-site reactions in the secukinumab-treated patients. He replied that one obvious factor is that secukinumab injections are given much less frequently: once monthly during maintenance therapy as compared to once weekly with etanercept. In any event, he doesn’t consider injection-site reactions to be clinically relevant.
"Injection-site reactions don’t seem to be a major issue with any of the subcutaneous therapies. It has never in 15 years of using different biologics been an issue that’s made me stop treatment for a patient," he added.
Secukinumab was dosed at baseline, again weekly through week 4, then once monthly from week 8 through the study’s end. Etanercept was dosed at 50 mg twice weekly for 12 weeks, then once weekly in accord with the labeling instructions in most countries. Nonresponders at 12 weeks in the placebo arm were at that point randomized to secukinumab at 300 mg or 150 mg. The statistical analysis in FIXTURE was performed by nonresponder imputation.
"Those who are interested in statistics will know that that’s the most rigorous way that you can look at the data, because anybody who drops out of the study, whether they withdraw consent, have a protocol violation, or have an adverse event, even if they were responding at that time, are considered a failure or nonresponder," Dr. Langley explained.
Asked how he sees secukinumab fitting into patient management, Dr. Langley replied, "Most of us are forced to use conventional therapies and phototherapy to get approval for biologics, but after that I think the safety and efficacy of this drug put it right at the top. I could definitely use this drug as a first-line biologic for patients with plain psoriasis without psoriatic arthritis."
Coinvestigator Dr. Kristian Reich, professor of dermatology at Georg-August University in Göttingen, Germany, said he will probably continue to use TNF inhibitors in psoriasis patients with prominent arthritis, where he believes the TNF inhibitors are particularly beneficial, but he anticipates using secukinumab widely in others.
Dr. Bruce E. Strober, another active clinical trial researcher in psoriasis, said he likes what he sees in the IL-17 inhibitor data.
"I predict as a specialty we’ll become very confident in these drugs 5 years from now. A lot of us will be using them as first line in many patients. That’s my prediction. For now, though, I’m a big fan of TNF inhibition," added Dr. Strober, vice chair of dermatology at the University of Connecticut Medical Center, Farmington.
The secukinumab development program is sponsored by Novartis. Dr. Langley, Dr. Reich, and Dr. Strober disclosed having received research grants from and serving as consultants to Novartis and numerous other companies developing biologic agents for psoriasis.
AT THE EADV CONGRESS
Major finding: The PASI 75 response rate at 12 weeks in patients with moderate to severe chronic plaque psoriasis was 77.1% in those randomized to the investigational interleukin-17A inhibitor secukinumab at 300 mg, 67% for secukinumab at 150 mg, 44% for etanercept 50 mg, and 4.9% for placebo.
Data source: The FIXTURE trial was a randomized, double-blind, double-dummy, year-long, phase III trial involving 1,306 patients with chronic plaque psoriasis not adequately responsive to prior systemic therapies.
Disclosures: The secukinumab development program is sponsored by Novartis. Dr. Langley, Dr. Reich, and Dr. Strober disclosed having received research grants from and serving as consultants to Novartis and numerous other companies developing biologic agents for psoriasis.
Psoriasis linked to nonalcoholic fatty liver disease
ISTANBUL, TURKEY – The prevalence of psoriasis was significantly greater in subjects with nonalcoholic fatty liver disease by a margin of 7% to 4.2%, according to findings from a long-term, population-based cohort study including nearly 4,000 individuals.
This newly identified link between two common diseases – one of which manifests as visible, angry skin lesions; the other silent, insidious, and often unrecognized until its late stages – contains an important message for clinicians, said Dr. Ella van der Voort of Erasmus University in Rotterdam, the Netherlands.
"Physicians should be aware of this increased risk before prescribing for psoriasis patients medications with potential for liver toxicity – and I’m not talking about just psoriasis medications, but also medications in general, like statins," she said at the annual congress of the European Academy of Dermatology and Venereology.
Psoriasis was present in 5.1% of the study group. Most cases were mild, with affected patients having a mean psoriasis area severity index of 2.9 out of a possible 72 and no use of systemic psoriasis therapies. Nevertheless, the prevalence of nonalcoholic fatty liver disease (NAFLD) was 46.2% among the 118 psoriasis patients, compared with 33% in the 2,174 subjects without psoriasis.
NAFLD is now the most common form of chronic liver disease in Western countries. By 2020, when new well-tolerated and highly effective medications for hepatitis C are expected to have made a mark, NAFLD may be the leading reason for liver transplantation in developed countries, Dr. van der Voort observed.
The Rotterdam Study is designed to examine contributors to chronic diseases in the elderly, including cardiovascular, endocrinologic, psychiatric, neurologic, and eye diseases. The first wave of participants enrolled in 1989-93. Dr. van der Voort’s study focused on the third cohort, consisting of 3,932 elderly Rotterdam-area residents enrolled in 2006-2008. The mean age at enrollment was 76 years, and the ongoing routine evaluation of the cohort includes liver ultrasound, lab tests for liver enzyme levels and serologic evidence of hepatitis, and a skin examination.
Dr. van der Voort’s analysis was restricted to the 2,292 third-wave subjects who were not heavy drinkers and didn’t have chronic hepatitis, either of which rules out the diagnosis of NAFLD. The participants were not taking hepatotoxic medications, and there was no uncertainty as to the skin examination findings.
The hallmark of NAFLD is the accumulation of triglycerides in the hepatocytes. The previously well-described risk factors for NAFLD include the metabolic syndrome and its individual components, as well as sedentary lifestyle, smoking, and a poor-quality diet. There is also a genetic component: individuals with a mutation in the phospholipase domain-containing protein 3 (PNPLA3) gene, which codes for an enzyme functioning in hydrolyzation of triglycerides, are predisposed to NAFLD.
The prevalence of metabolic syndrome was 62% in subjects with psoriasis and 52% in those without psoriasis. Individuals with psoriasis also were more likely to be smokers by a margin of 15% to 8%. After adjusting for those variables, as well as alcohol intake, age, gender, liver enzyme levels, and other potential confounders, two strong independent predictors of NAFLD were metabolic syndrome, with an adjusted odds ratio of 3.5, and psoriasis, with an odds ratio of 1.7.
In a multivariate analysis, psoriasis was not only independently associated with a 70% increased likelihood of NAFLD, but psoriasis patients with NAFLD were also 60% more likely to have the more severe forms of NAFLD.
The Rotterdam study is funded chiefly by Dutch scientific research foundations, with secondary pharmaceutical industry support. Dr. van der Voort reported having no financial conflicts of interest.
ISTANBUL, TURKEY – The prevalence of psoriasis was significantly greater in subjects with nonalcoholic fatty liver disease by a margin of 7% to 4.2%, according to findings from a long-term, population-based cohort study including nearly 4,000 individuals.
This newly identified link between two common diseases – one of which manifests as visible, angry skin lesions; the other silent, insidious, and often unrecognized until its late stages – contains an important message for clinicians, said Dr. Ella van der Voort of Erasmus University in Rotterdam, the Netherlands.
"Physicians should be aware of this increased risk before prescribing for psoriasis patients medications with potential for liver toxicity – and I’m not talking about just psoriasis medications, but also medications in general, like statins," she said at the annual congress of the European Academy of Dermatology and Venereology.
Psoriasis was present in 5.1% of the study group. Most cases were mild, with affected patients having a mean psoriasis area severity index of 2.9 out of a possible 72 and no use of systemic psoriasis therapies. Nevertheless, the prevalence of nonalcoholic fatty liver disease (NAFLD) was 46.2% among the 118 psoriasis patients, compared with 33% in the 2,174 subjects without psoriasis.
NAFLD is now the most common form of chronic liver disease in Western countries. By 2020, when new well-tolerated and highly effective medications for hepatitis C are expected to have made a mark, NAFLD may be the leading reason for liver transplantation in developed countries, Dr. van der Voort observed.
The Rotterdam Study is designed to examine contributors to chronic diseases in the elderly, including cardiovascular, endocrinologic, psychiatric, neurologic, and eye diseases. The first wave of participants enrolled in 1989-93. Dr. van der Voort’s study focused on the third cohort, consisting of 3,932 elderly Rotterdam-area residents enrolled in 2006-2008. The mean age at enrollment was 76 years, and the ongoing routine evaluation of the cohort includes liver ultrasound, lab tests for liver enzyme levels and serologic evidence of hepatitis, and a skin examination.
Dr. van der Voort’s analysis was restricted to the 2,292 third-wave subjects who were not heavy drinkers and didn’t have chronic hepatitis, either of which rules out the diagnosis of NAFLD. The participants were not taking hepatotoxic medications, and there was no uncertainty as to the skin examination findings.
The hallmark of NAFLD is the accumulation of triglycerides in the hepatocytes. The previously well-described risk factors for NAFLD include the metabolic syndrome and its individual components, as well as sedentary lifestyle, smoking, and a poor-quality diet. There is also a genetic component: individuals with a mutation in the phospholipase domain-containing protein 3 (PNPLA3) gene, which codes for an enzyme functioning in hydrolyzation of triglycerides, are predisposed to NAFLD.
The prevalence of metabolic syndrome was 62% in subjects with psoriasis and 52% in those without psoriasis. Individuals with psoriasis also were more likely to be smokers by a margin of 15% to 8%. After adjusting for those variables, as well as alcohol intake, age, gender, liver enzyme levels, and other potential confounders, two strong independent predictors of NAFLD were metabolic syndrome, with an adjusted odds ratio of 3.5, and psoriasis, with an odds ratio of 1.7.
In a multivariate analysis, psoriasis was not only independently associated with a 70% increased likelihood of NAFLD, but psoriasis patients with NAFLD were also 60% more likely to have the more severe forms of NAFLD.
The Rotterdam study is funded chiefly by Dutch scientific research foundations, with secondary pharmaceutical industry support. Dr. van der Voort reported having no financial conflicts of interest.
ISTANBUL, TURKEY – The prevalence of psoriasis was significantly greater in subjects with nonalcoholic fatty liver disease by a margin of 7% to 4.2%, according to findings from a long-term, population-based cohort study including nearly 4,000 individuals.
This newly identified link between two common diseases – one of which manifests as visible, angry skin lesions; the other silent, insidious, and often unrecognized until its late stages – contains an important message for clinicians, said Dr. Ella van der Voort of Erasmus University in Rotterdam, the Netherlands.
"Physicians should be aware of this increased risk before prescribing for psoriasis patients medications with potential for liver toxicity – and I’m not talking about just psoriasis medications, but also medications in general, like statins," she said at the annual congress of the European Academy of Dermatology and Venereology.
Psoriasis was present in 5.1% of the study group. Most cases were mild, with affected patients having a mean psoriasis area severity index of 2.9 out of a possible 72 and no use of systemic psoriasis therapies. Nevertheless, the prevalence of nonalcoholic fatty liver disease (NAFLD) was 46.2% among the 118 psoriasis patients, compared with 33% in the 2,174 subjects without psoriasis.
NAFLD is now the most common form of chronic liver disease in Western countries. By 2020, when new well-tolerated and highly effective medications for hepatitis C are expected to have made a mark, NAFLD may be the leading reason for liver transplantation in developed countries, Dr. van der Voort observed.
The Rotterdam Study is designed to examine contributors to chronic diseases in the elderly, including cardiovascular, endocrinologic, psychiatric, neurologic, and eye diseases. The first wave of participants enrolled in 1989-93. Dr. van der Voort’s study focused on the third cohort, consisting of 3,932 elderly Rotterdam-area residents enrolled in 2006-2008. The mean age at enrollment was 76 years, and the ongoing routine evaluation of the cohort includes liver ultrasound, lab tests for liver enzyme levels and serologic evidence of hepatitis, and a skin examination.
Dr. van der Voort’s analysis was restricted to the 2,292 third-wave subjects who were not heavy drinkers and didn’t have chronic hepatitis, either of which rules out the diagnosis of NAFLD. The participants were not taking hepatotoxic medications, and there was no uncertainty as to the skin examination findings.
The hallmark of NAFLD is the accumulation of triglycerides in the hepatocytes. The previously well-described risk factors for NAFLD include the metabolic syndrome and its individual components, as well as sedentary lifestyle, smoking, and a poor-quality diet. There is also a genetic component: individuals with a mutation in the phospholipase domain-containing protein 3 (PNPLA3) gene, which codes for an enzyme functioning in hydrolyzation of triglycerides, are predisposed to NAFLD.
The prevalence of metabolic syndrome was 62% in subjects with psoriasis and 52% in those without psoriasis. Individuals with psoriasis also were more likely to be smokers by a margin of 15% to 8%. After adjusting for those variables, as well as alcohol intake, age, gender, liver enzyme levels, and other potential confounders, two strong independent predictors of NAFLD were metabolic syndrome, with an adjusted odds ratio of 3.5, and psoriasis, with an odds ratio of 1.7.
In a multivariate analysis, psoriasis was not only independently associated with a 70% increased likelihood of NAFLD, but psoriasis patients with NAFLD were also 60% more likely to have the more severe forms of NAFLD.
The Rotterdam study is funded chiefly by Dutch scientific research foundations, with secondary pharmaceutical industry support. Dr. van der Voort reported having no financial conflicts of interest.
AT THE EADV CONGRESS
Major finding: The prevalence of nonalcoholic fatty liver disease in a large cohort of elderly subjects was 46.2% among those with psoriasis and 33% in those without. In a multivariate regression analysis, psoriasis was independently associated with a 70% increased likelihood of the hepatic disease.
Data source: The Rotterdam Study is a long-standing prospective, population-based cohort study. The aim is to determine key factors in the development of a range of chronic diseases common in the elderly.
Disclosures: The Rotterdam Study is funded primarily by Dutch scientific research foundations. The presenter reported having no financial conflicts.