User login
Guselkumab prevails in phase II psoriasis trial
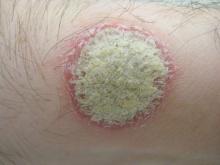
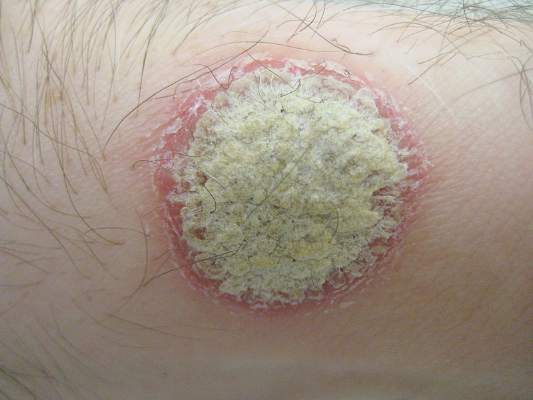
Guselkumab, a monoclonal antibody that specifically inhibits interleukin-23 signaling, was safe and more effective than placebo or adalimumab against plaque psoriasis in a phase II clinical trial, according to a report published online July 9 in the New England Journal of Medicine.
The 1-year randomized double-blind study, funded and administered by the maker of guselkumab, involved 293 adult patients treated at 31 sites in North America and 12 in Europe. The median duration of disease was 19 years. Study participants were randomly assigned to receive one of five subcutaneous doses of the agent (5 mg, 15 mg, 50 mg, 100 mg, or 200 mg) at two different dosing intervals (208 patients), matching placebo (42 patients), or adalimumab (43 patients) for 40 weeks and were followed for an additional 12 weeks. At 16 weeks, patients in the placebo group crossed over to receive 100 mg of guselkumab every 8 weeks, said Dr. Kenneth B. Gordon of Northwestern University, Chicago, and his associates.
The primary efficacy endpoint – the proportion of patients at week 16 with a Physician’s Global Assessment score of 0 or 1 – was significantly higher in every guselkumab dosing group (34%, 61%, 79%, 86%, and 83%, respectively) than in the placebo group (7%). In addition, the proportion of patients showing a 75%, 90%, or 100% improvement in Psoriasis Area and Severity Index score was significantly higher in each guselkumab group than in the placebo group. Scores on the Dermatology Life Quality Index also showed significantly greater improvement from baseline to week 16 in the guselkumab groups.
The findings were similar when patients taking the higher doses of guselkumab were compared with those taking adalimumab. At week 16, the proportion of patients with a PGA score of 0 or 1 was 58% with adalimumab, compared with 79%, 86%, and 83% at the highest doses of guselkumab (50 mg, 100 mg, or 200 mg). And at week 40, the proportion of patients with a PGA score of 0 or 1 was significantly higher in those taking 50 mg (71%), 100 mg (77%), or 200 mg (81%) guselkumab than in patients taking adalimumab (49%). The response to guselkumab was noted at the earliest patient assessment at week 4, peaked at week 20, and was maintained through week 40, Dr. Gordon and his associates said (N. Engl. J. Med. 2015 July 9 [doi:10.1056/NEJMoa1501646]).
The rates of adverse events, serious adverse events, and infections were similar across all the treatment groups. At week 16, infections were reported in 20% of patients taking guselkumab, 14% of those taking placebo, and 12% of those taking adalimumab. At final follow-up, infections were reported in 30% of patients taking guselkumab and 35% taking adalimumab. This study had a limited ability to assess uncommon adverse events; three patients taking guselkumab had major cardiovascular events during treatment, but “it is difficult to interpret the significance” of such events, the investigators said.
They added that larger and longer-term phase III trials are now underway to further assess the efficacy and safety of guselkumab for this indication. Moreover, their study results “validate the use of interleukin-23 as a therapeutic target by showing that therapy involving selective antagonism of this single cytokine in patients with moderate to severe plaque psoriasis is highly efficacious, as compared with adalimumab therapy,” Dr. Gordon and his associates said.
Guselkumab, a monoclonal antibody that specifically inhibits interleukin-23 signaling, was safe and more effective than placebo or adalimumab against plaque psoriasis in a phase II clinical trial, according to a report published online July 9 in the New England Journal of Medicine.
The 1-year randomized double-blind study, funded and administered by the maker of guselkumab, involved 293 adult patients treated at 31 sites in North America and 12 in Europe. The median duration of disease was 19 years. Study participants were randomly assigned to receive one of five subcutaneous doses of the agent (5 mg, 15 mg, 50 mg, 100 mg, or 200 mg) at two different dosing intervals (208 patients), matching placebo (42 patients), or adalimumab (43 patients) for 40 weeks and were followed for an additional 12 weeks. At 16 weeks, patients in the placebo group crossed over to receive 100 mg of guselkumab every 8 weeks, said Dr. Kenneth B. Gordon of Northwestern University, Chicago, and his associates.
The primary efficacy endpoint – the proportion of patients at week 16 with a Physician’s Global Assessment score of 0 or 1 – was significantly higher in every guselkumab dosing group (34%, 61%, 79%, 86%, and 83%, respectively) than in the placebo group (7%). In addition, the proportion of patients showing a 75%, 90%, or 100% improvement in Psoriasis Area and Severity Index score was significantly higher in each guselkumab group than in the placebo group. Scores on the Dermatology Life Quality Index also showed significantly greater improvement from baseline to week 16 in the guselkumab groups.
The findings were similar when patients taking the higher doses of guselkumab were compared with those taking adalimumab. At week 16, the proportion of patients with a PGA score of 0 or 1 was 58% with adalimumab, compared with 79%, 86%, and 83% at the highest doses of guselkumab (50 mg, 100 mg, or 200 mg). And at week 40, the proportion of patients with a PGA score of 0 or 1 was significantly higher in those taking 50 mg (71%), 100 mg (77%), or 200 mg (81%) guselkumab than in patients taking adalimumab (49%). The response to guselkumab was noted at the earliest patient assessment at week 4, peaked at week 20, and was maintained through week 40, Dr. Gordon and his associates said (N. Engl. J. Med. 2015 July 9 [doi:10.1056/NEJMoa1501646]).
The rates of adverse events, serious adverse events, and infections were similar across all the treatment groups. At week 16, infections were reported in 20% of patients taking guselkumab, 14% of those taking placebo, and 12% of those taking adalimumab. At final follow-up, infections were reported in 30% of patients taking guselkumab and 35% taking adalimumab. This study had a limited ability to assess uncommon adverse events; three patients taking guselkumab had major cardiovascular events during treatment, but “it is difficult to interpret the significance” of such events, the investigators said.
They added that larger and longer-term phase III trials are now underway to further assess the efficacy and safety of guselkumab for this indication. Moreover, their study results “validate the use of interleukin-23 as a therapeutic target by showing that therapy involving selective antagonism of this single cytokine in patients with moderate to severe plaque psoriasis is highly efficacious, as compared with adalimumab therapy,” Dr. Gordon and his associates said.
Guselkumab, a monoclonal antibody that specifically inhibits interleukin-23 signaling, was safe and more effective than placebo or adalimumab against plaque psoriasis in a phase II clinical trial, according to a report published online July 9 in the New England Journal of Medicine.
The 1-year randomized double-blind study, funded and administered by the maker of guselkumab, involved 293 adult patients treated at 31 sites in North America and 12 in Europe. The median duration of disease was 19 years. Study participants were randomly assigned to receive one of five subcutaneous doses of the agent (5 mg, 15 mg, 50 mg, 100 mg, or 200 mg) at two different dosing intervals (208 patients), matching placebo (42 patients), or adalimumab (43 patients) for 40 weeks and were followed for an additional 12 weeks. At 16 weeks, patients in the placebo group crossed over to receive 100 mg of guselkumab every 8 weeks, said Dr. Kenneth B. Gordon of Northwestern University, Chicago, and his associates.
The primary efficacy endpoint – the proportion of patients at week 16 with a Physician’s Global Assessment score of 0 or 1 – was significantly higher in every guselkumab dosing group (34%, 61%, 79%, 86%, and 83%, respectively) than in the placebo group (7%). In addition, the proportion of patients showing a 75%, 90%, or 100% improvement in Psoriasis Area and Severity Index score was significantly higher in each guselkumab group than in the placebo group. Scores on the Dermatology Life Quality Index also showed significantly greater improvement from baseline to week 16 in the guselkumab groups.
The findings were similar when patients taking the higher doses of guselkumab were compared with those taking adalimumab. At week 16, the proportion of patients with a PGA score of 0 or 1 was 58% with adalimumab, compared with 79%, 86%, and 83% at the highest doses of guselkumab (50 mg, 100 mg, or 200 mg). And at week 40, the proportion of patients with a PGA score of 0 or 1 was significantly higher in those taking 50 mg (71%), 100 mg (77%), or 200 mg (81%) guselkumab than in patients taking adalimumab (49%). The response to guselkumab was noted at the earliest patient assessment at week 4, peaked at week 20, and was maintained through week 40, Dr. Gordon and his associates said (N. Engl. J. Med. 2015 July 9 [doi:10.1056/NEJMoa1501646]).
The rates of adverse events, serious adverse events, and infections were similar across all the treatment groups. At week 16, infections were reported in 20% of patients taking guselkumab, 14% of those taking placebo, and 12% of those taking adalimumab. At final follow-up, infections were reported in 30% of patients taking guselkumab and 35% taking adalimumab. This study had a limited ability to assess uncommon adverse events; three patients taking guselkumab had major cardiovascular events during treatment, but “it is difficult to interpret the significance” of such events, the investigators said.
They added that larger and longer-term phase III trials are now underway to further assess the efficacy and safety of guselkumab for this indication. Moreover, their study results “validate the use of interleukin-23 as a therapeutic target by showing that therapy involving selective antagonism of this single cytokine in patients with moderate to severe plaque psoriasis is highly efficacious, as compared with adalimumab therapy,” Dr. Gordon and his associates said.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Guselkumab was safe and more effective than placebo and adalimumab against plaque psoriasis in a phase II industry-sponsored trial.
Major finding: The primary efficacy endpoint – the proportion of patients at week 16 with a Physician’s Global Assessment score of 0 or 1 – was significantly higher in every guselkumab group (34%, 61%, 79%, 86%, and 83%, respectively) than in the placebo group (7%).
Data source: A 1-year double-blind randomized phase II trial involving 293 adults at 43 sites in North America and Europe.
Disclosures: The trial was sponsored by Janssen, maker of guselkumab. Janssen supplied the study drugs, collected and analyzed the data, and helped write the report. Dr. Gordon reported receiving grants and personal fees from Janssen, AbbVie, Amgen, Celgene, Eli Lilly, Novartis, Pfizer, and Medac; his associates reported ties to numerous industry sources.
Clinical Trial Update: Topical Therapy for Plaque Psoriasis
Comparison of 122-0551 Foam Versus Vehicle for Plaque Psoriasis
Product: 122-0551 foam
Sponsor: Therapeutics, Inc
Stage of development: Phase 3
ClinicalTrials.gov Identifier: NCT02368210
Study start: January 2015
Study completion: October 2015 (August 2015 final collection date for primary outcome measure)
This phase 3 study will compare the efficacy and safety of 122-0551 foam (a glucocorticoid receptor agonist) and vehicle foam applied twice daily for 2 weeks in participants (aged ≥18 years) with plaque psoriasis.
The primary outcome measure will include the proportion of participants rated a “treatment success” based on the investigator global assessment.
Secondary outcome measures will include the proportion of participants rated a “treatment success” for each of the clinical signs of psoriasis: scaling, erythema, and plaque elevation.
HPA Axis Suppression Following Treatment With Desoximetasone Topical Spray 0.25% in Pediatric Patients With Plaque Psoriasis
Product: Topicort® (desoximetasone) topical spray 0.25%
Sponsor: Taro Pharmaceuticals USA
Stage of development: Phase 4
ClinicalTrials.gov Identifier: NCT02340169
Study start: December 2014
Study completion: April 2016
This open-label, postmarketing safety study will evaluate the potential of Topicort (desoximetasone) topical spray 0.25% to suppress hypothalamic-pituitary-adrenal (HPA) axis function following twice daily dosing for 28 days in pediatric patients aged 2 to 17 years with moderate to severe plaque psoriasis. Participants will be serially enrolled into 3 cohorts.
The primary outcome measure will include HPA axis function suppression as measured by cortisol response test.
Secondary outcome measures will include evaluation of efficacy parameters, pharmacokinetics, and adverse events.
Long-term Safety and Efficacy of Calcitriol 3 µg/g Ointment in Pediatric Participants With Plaque Psoriasis
Product: Calcitriol 3 µg/g ointment
Sponsor: Galderma
Stage of development: Phase 4
ClinicalTrials.gov Identifier: NCT02125279
Study start: May 2014
Study completion: October 2016
This multicenter study will evaluate the safety and efficacy of calcitriol 3 µg/g ointment applied twice daily (without occlusion) to treat pediatric participants aged 2 to 17 years with mild to moderate plaque psoriasis for up to 26 weeks.
The primary outcome measure will include laboratory parameters related to calcium metabolism after 26 weeks of topical treatment with calcitriol 3 µg/g.
Evaluation of Calcipotriene Foam 0.005% Versus Vehicle Foam in Pediatric Patients With Plaque Psoriasis
Product: STF 115469 foam (calcipotriene foam 0.005%)
Sponsor: GlaxoSmithKline
Stage of development: Phase 3
ClinicalTrials.gov Identifier: NCT01582932
Study start: April 2013
Study completion: April 2019
This multicenter study will compare calcipotriene foam 0.005% with vehicle foam in pediatric participants aged 2 to 11 years (inclusive) with mild to moderate plaque psoriasis. Study participants or a primary caregiver will apply calcipotriene foam 0.005% as a thin layer twice daily (morning and evening) on the body and scalp (excluding the face) for up to 8 weeks.
The primary outcome measure will include treatment success.
Secondary outcome measures will include erythema, scaling, and plaque thickness.
Comparison of 122-0551 Foam Versus Vehicle for Plaque Psoriasis
Product: 122-0551 foam
Sponsor: Therapeutics, Inc
Stage of development: Phase 3
ClinicalTrials.gov Identifier: NCT02368210
Study start: January 2015
Study completion: October 2015 (August 2015 final collection date for primary outcome measure)
This phase 3 study will compare the efficacy and safety of 122-0551 foam (a glucocorticoid receptor agonist) and vehicle foam applied twice daily for 2 weeks in participants (aged ≥18 years) with plaque psoriasis.
The primary outcome measure will include the proportion of participants rated a “treatment success” based on the investigator global assessment.
Secondary outcome measures will include the proportion of participants rated a “treatment success” for each of the clinical signs of psoriasis: scaling, erythema, and plaque elevation.
HPA Axis Suppression Following Treatment With Desoximetasone Topical Spray 0.25% in Pediatric Patients With Plaque Psoriasis
Product: Topicort® (desoximetasone) topical spray 0.25%
Sponsor: Taro Pharmaceuticals USA
Stage of development: Phase 4
ClinicalTrials.gov Identifier: NCT02340169
Study start: December 2014
Study completion: April 2016
This open-label, postmarketing safety study will evaluate the potential of Topicort (desoximetasone) topical spray 0.25% to suppress hypothalamic-pituitary-adrenal (HPA) axis function following twice daily dosing for 28 days in pediatric patients aged 2 to 17 years with moderate to severe plaque psoriasis. Participants will be serially enrolled into 3 cohorts.
The primary outcome measure will include HPA axis function suppression as measured by cortisol response test.
Secondary outcome measures will include evaluation of efficacy parameters, pharmacokinetics, and adverse events.
Long-term Safety and Efficacy of Calcitriol 3 µg/g Ointment in Pediatric Participants With Plaque Psoriasis
Product: Calcitriol 3 µg/g ointment
Sponsor: Galderma
Stage of development: Phase 4
ClinicalTrials.gov Identifier: NCT02125279
Study start: May 2014
Study completion: October 2016
This multicenter study will evaluate the safety and efficacy of calcitriol 3 µg/g ointment applied twice daily (without occlusion) to treat pediatric participants aged 2 to 17 years with mild to moderate plaque psoriasis for up to 26 weeks.
The primary outcome measure will include laboratory parameters related to calcium metabolism after 26 weeks of topical treatment with calcitriol 3 µg/g.
Evaluation of Calcipotriene Foam 0.005% Versus Vehicle Foam in Pediatric Patients With Plaque Psoriasis
Product: STF 115469 foam (calcipotriene foam 0.005%)
Sponsor: GlaxoSmithKline
Stage of development: Phase 3
ClinicalTrials.gov Identifier: NCT01582932
Study start: April 2013
Study completion: April 2019
This multicenter study will compare calcipotriene foam 0.005% with vehicle foam in pediatric participants aged 2 to 11 years (inclusive) with mild to moderate plaque psoriasis. Study participants or a primary caregiver will apply calcipotriene foam 0.005% as a thin layer twice daily (morning and evening) on the body and scalp (excluding the face) for up to 8 weeks.
The primary outcome measure will include treatment success.
Secondary outcome measures will include erythema, scaling, and plaque thickness.
Comparison of 122-0551 Foam Versus Vehicle for Plaque Psoriasis
Product: 122-0551 foam
Sponsor: Therapeutics, Inc
Stage of development: Phase 3
ClinicalTrials.gov Identifier: NCT02368210
Study start: January 2015
Study completion: October 2015 (August 2015 final collection date for primary outcome measure)
This phase 3 study will compare the efficacy and safety of 122-0551 foam (a glucocorticoid receptor agonist) and vehicle foam applied twice daily for 2 weeks in participants (aged ≥18 years) with plaque psoriasis.
The primary outcome measure will include the proportion of participants rated a “treatment success” based on the investigator global assessment.
Secondary outcome measures will include the proportion of participants rated a “treatment success” for each of the clinical signs of psoriasis: scaling, erythema, and plaque elevation.
HPA Axis Suppression Following Treatment With Desoximetasone Topical Spray 0.25% in Pediatric Patients With Plaque Psoriasis
Product: Topicort® (desoximetasone) topical spray 0.25%
Sponsor: Taro Pharmaceuticals USA
Stage of development: Phase 4
ClinicalTrials.gov Identifier: NCT02340169
Study start: December 2014
Study completion: April 2016
This open-label, postmarketing safety study will evaluate the potential of Topicort (desoximetasone) topical spray 0.25% to suppress hypothalamic-pituitary-adrenal (HPA) axis function following twice daily dosing for 28 days in pediatric patients aged 2 to 17 years with moderate to severe plaque psoriasis. Participants will be serially enrolled into 3 cohorts.
The primary outcome measure will include HPA axis function suppression as measured by cortisol response test.
Secondary outcome measures will include evaluation of efficacy parameters, pharmacokinetics, and adverse events.
Long-term Safety and Efficacy of Calcitriol 3 µg/g Ointment in Pediatric Participants With Plaque Psoriasis
Product: Calcitriol 3 µg/g ointment
Sponsor: Galderma
Stage of development: Phase 4
ClinicalTrials.gov Identifier: NCT02125279
Study start: May 2014
Study completion: October 2016
This multicenter study will evaluate the safety and efficacy of calcitriol 3 µg/g ointment applied twice daily (without occlusion) to treat pediatric participants aged 2 to 17 years with mild to moderate plaque psoriasis for up to 26 weeks.
The primary outcome measure will include laboratory parameters related to calcium metabolism after 26 weeks of topical treatment with calcitriol 3 µg/g.
Evaluation of Calcipotriene Foam 0.005% Versus Vehicle Foam in Pediatric Patients With Plaque Psoriasis
Product: STF 115469 foam (calcipotriene foam 0.005%)
Sponsor: GlaxoSmithKline
Stage of development: Phase 3
ClinicalTrials.gov Identifier: NCT01582932
Study start: April 2013
Study completion: April 2019
This multicenter study will compare calcipotriene foam 0.005% with vehicle foam in pediatric participants aged 2 to 11 years (inclusive) with mild to moderate plaque psoriasis. Study participants or a primary caregiver will apply calcipotriene foam 0.005% as a thin layer twice daily (morning and evening) on the body and scalp (excluding the face) for up to 8 weeks.
The primary outcome measure will include treatment success.
Secondary outcome measures will include erythema, scaling, and plaque thickness.
EULAR’s psoriatic arthritis recommendations gain new drugs
ROME – Tumor necrosis factor inhibitors remain the mainstay of treatment for patients with psoriatic arthritis who don’t fully respond to treatment with a nonsteroidal anti-inflammatory drug and at least one conventional synthetic disease-modifying antirheumatic drug in newly updated treatment recommendations written by a task force of the European League Against Rheumatism.
The revised recommendations for psoriatic arthritis (PsA) replace what EULAR had last released in 2011 and published in 2012 (Ann. Rheum. Dis. 2012;71:4-12) and also add three new drug options not mentioned in the 2011 version that the panel now endorsed as alternatives to a tumor necrosis factor (TNF) inhibitor when a TNF inhibitor is not appropriate. The three additions are ustekinumab (Stelara), which acts by inhibiting interleukin- (IL-)12 and IL-23, secukinumab (Cosentyx), which acts by inhibiting IL-17, and apremilast (Otezla), which inhibits phosphodiesterase-4. Ustekinumab and secukinumab are considered biological disease-modifying antirheumatic drugs (bDMARDs), like the TNF inhibitors, while apremilast occupies a new classification niche as a targeted synthetic DMARD, said Dr. Laure Gossec, convener of the PsA treatment task force, at the European Congress of Rheumatology.
Although ustekinumab, secukinumab, and apremilast all have a role to play in managing selected PsA patients, they all remain second line to TNF inhibitors, stressed Dr. Gossec, professor of rheumatology at Pierre and Marie Curie University in Paris. A TNF inhibitor is the first agent to turn to when treatment with an NSAID and a conventional synthetic DMARD fails to bring about remission or minimal disease activity essentially because of its much longer track record of safety and efficacy, she said. Specifically, TNF inhibitors have been studied long term and have demonstrated their safety in registries, they have not been surpassed for efficacy by any other agent using indirect comparisons, and now that biosimilar TNF inhibitors have entered the market they also could potentially offer a price advantage, compared with newer agents.
But for some patients, TNF inhibitors are not appropriate, such as a patient with certain comorbidities or a history of infections that would make a TNF inhibitor contraindicated, she said. For these patients, one of the other types of biologic DMARDS now available would be the top alternative. In other patients, a comorbidity profile or a history of infections might make any biologic DMARD contraindicated, in which case the targeted synthetic DMARD apremilast is an appropriate alternative, she said.
The revised recommendations contain several other new items:
• For patients with active enthesitis, dactylitis, or both and an insufficient response to an NSAID or local glucocorticoid injections, then a TNF inhibitor or alternatively an IL-12 and IL-23 inhibitor (currently ustekinumab) or an IL-17 inhibitor (currently secukinumab) may be considered. Conventional synthetic DMARDS are ineffective for these patients, while in contrast all of the biologic DMARDs are effective, but the TNF inhibitors have an edge because of greater experience with the class in this setting.
• For patients with predominantly axial disease that is active and insufficiently responsive to NSAID treatment, a biologic DMARD should be considered, specifically a TNF inhibitor. Conventional synthetic DMARDs also have no efficacy for these patients, and few data exist regarding the efficacy of IL-12 and IL-23 inhibitors or IL-17 inhibitors.
• When patients fail to respond adequately to one biologic DMARD, switching to another biologic DMARD should be considered. This could involve switching from one TNF inhibitor to another, as some evidence exists that this could be effective. Alternatively, it could involve switching to a IL-12 and IL-23 inhibitor, an IL-17 inhibitor, or possibly a targeted synthetic DMARD (currently apremilast). The task force did not endorse any particular order of these drugs when switching occurs.
Dr. Gossec has been a consultant to AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Novartis, Pfizer, Roche, and UCB.
On Twitter @mitchelzoler
ROME – Tumor necrosis factor inhibitors remain the mainstay of treatment for patients with psoriatic arthritis who don’t fully respond to treatment with a nonsteroidal anti-inflammatory drug and at least one conventional synthetic disease-modifying antirheumatic drug in newly updated treatment recommendations written by a task force of the European League Against Rheumatism.
The revised recommendations for psoriatic arthritis (PsA) replace what EULAR had last released in 2011 and published in 2012 (Ann. Rheum. Dis. 2012;71:4-12) and also add three new drug options not mentioned in the 2011 version that the panel now endorsed as alternatives to a tumor necrosis factor (TNF) inhibitor when a TNF inhibitor is not appropriate. The three additions are ustekinumab (Stelara), which acts by inhibiting interleukin- (IL-)12 and IL-23, secukinumab (Cosentyx), which acts by inhibiting IL-17, and apremilast (Otezla), which inhibits phosphodiesterase-4. Ustekinumab and secukinumab are considered biological disease-modifying antirheumatic drugs (bDMARDs), like the TNF inhibitors, while apremilast occupies a new classification niche as a targeted synthetic DMARD, said Dr. Laure Gossec, convener of the PsA treatment task force, at the European Congress of Rheumatology.
Although ustekinumab, secukinumab, and apremilast all have a role to play in managing selected PsA patients, they all remain second line to TNF inhibitors, stressed Dr. Gossec, professor of rheumatology at Pierre and Marie Curie University in Paris. A TNF inhibitor is the first agent to turn to when treatment with an NSAID and a conventional synthetic DMARD fails to bring about remission or minimal disease activity essentially because of its much longer track record of safety and efficacy, she said. Specifically, TNF inhibitors have been studied long term and have demonstrated their safety in registries, they have not been surpassed for efficacy by any other agent using indirect comparisons, and now that biosimilar TNF inhibitors have entered the market they also could potentially offer a price advantage, compared with newer agents.
But for some patients, TNF inhibitors are not appropriate, such as a patient with certain comorbidities or a history of infections that would make a TNF inhibitor contraindicated, she said. For these patients, one of the other types of biologic DMARDS now available would be the top alternative. In other patients, a comorbidity profile or a history of infections might make any biologic DMARD contraindicated, in which case the targeted synthetic DMARD apremilast is an appropriate alternative, she said.
The revised recommendations contain several other new items:
• For patients with active enthesitis, dactylitis, or both and an insufficient response to an NSAID or local glucocorticoid injections, then a TNF inhibitor or alternatively an IL-12 and IL-23 inhibitor (currently ustekinumab) or an IL-17 inhibitor (currently secukinumab) may be considered. Conventional synthetic DMARDS are ineffective for these patients, while in contrast all of the biologic DMARDs are effective, but the TNF inhibitors have an edge because of greater experience with the class in this setting.
• For patients with predominantly axial disease that is active and insufficiently responsive to NSAID treatment, a biologic DMARD should be considered, specifically a TNF inhibitor. Conventional synthetic DMARDs also have no efficacy for these patients, and few data exist regarding the efficacy of IL-12 and IL-23 inhibitors or IL-17 inhibitors.
• When patients fail to respond adequately to one biologic DMARD, switching to another biologic DMARD should be considered. This could involve switching from one TNF inhibitor to another, as some evidence exists that this could be effective. Alternatively, it could involve switching to a IL-12 and IL-23 inhibitor, an IL-17 inhibitor, or possibly a targeted synthetic DMARD (currently apremilast). The task force did not endorse any particular order of these drugs when switching occurs.
Dr. Gossec has been a consultant to AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Novartis, Pfizer, Roche, and UCB.
On Twitter @mitchelzoler
ROME – Tumor necrosis factor inhibitors remain the mainstay of treatment for patients with psoriatic arthritis who don’t fully respond to treatment with a nonsteroidal anti-inflammatory drug and at least one conventional synthetic disease-modifying antirheumatic drug in newly updated treatment recommendations written by a task force of the European League Against Rheumatism.
The revised recommendations for psoriatic arthritis (PsA) replace what EULAR had last released in 2011 and published in 2012 (Ann. Rheum. Dis. 2012;71:4-12) and also add three new drug options not mentioned in the 2011 version that the panel now endorsed as alternatives to a tumor necrosis factor (TNF) inhibitor when a TNF inhibitor is not appropriate. The three additions are ustekinumab (Stelara), which acts by inhibiting interleukin- (IL-)12 and IL-23, secukinumab (Cosentyx), which acts by inhibiting IL-17, and apremilast (Otezla), which inhibits phosphodiesterase-4. Ustekinumab and secukinumab are considered biological disease-modifying antirheumatic drugs (bDMARDs), like the TNF inhibitors, while apremilast occupies a new classification niche as a targeted synthetic DMARD, said Dr. Laure Gossec, convener of the PsA treatment task force, at the European Congress of Rheumatology.
Although ustekinumab, secukinumab, and apremilast all have a role to play in managing selected PsA patients, they all remain second line to TNF inhibitors, stressed Dr. Gossec, professor of rheumatology at Pierre and Marie Curie University in Paris. A TNF inhibitor is the first agent to turn to when treatment with an NSAID and a conventional synthetic DMARD fails to bring about remission or minimal disease activity essentially because of its much longer track record of safety and efficacy, she said. Specifically, TNF inhibitors have been studied long term and have demonstrated their safety in registries, they have not been surpassed for efficacy by any other agent using indirect comparisons, and now that biosimilar TNF inhibitors have entered the market they also could potentially offer a price advantage, compared with newer agents.
But for some patients, TNF inhibitors are not appropriate, such as a patient with certain comorbidities or a history of infections that would make a TNF inhibitor contraindicated, she said. For these patients, one of the other types of biologic DMARDS now available would be the top alternative. In other patients, a comorbidity profile or a history of infections might make any biologic DMARD contraindicated, in which case the targeted synthetic DMARD apremilast is an appropriate alternative, she said.
The revised recommendations contain several other new items:
• For patients with active enthesitis, dactylitis, or both and an insufficient response to an NSAID or local glucocorticoid injections, then a TNF inhibitor or alternatively an IL-12 and IL-23 inhibitor (currently ustekinumab) or an IL-17 inhibitor (currently secukinumab) may be considered. Conventional synthetic DMARDS are ineffective for these patients, while in contrast all of the biologic DMARDs are effective, but the TNF inhibitors have an edge because of greater experience with the class in this setting.
• For patients with predominantly axial disease that is active and insufficiently responsive to NSAID treatment, a biologic DMARD should be considered, specifically a TNF inhibitor. Conventional synthetic DMARDs also have no efficacy for these patients, and few data exist regarding the efficacy of IL-12 and IL-23 inhibitors or IL-17 inhibitors.
• When patients fail to respond adequately to one biologic DMARD, switching to another biologic DMARD should be considered. This could involve switching from one TNF inhibitor to another, as some evidence exists that this could be effective. Alternatively, it could involve switching to a IL-12 and IL-23 inhibitor, an IL-17 inhibitor, or possibly a targeted synthetic DMARD (currently apremilast). The task force did not endorse any particular order of these drugs when switching occurs.
Dr. Gossec has been a consultant to AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Novartis, Pfizer, Roche, and UCB.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE EULAR 2015 CONGRESS
WCD: Secukinumab shows effectiveness for nail, palmoplantar psoriasis
VANCOUVER, B.C. – The interleukin-17A inhibitor secukinumab demonstrated the greatest improvement in nail psoriasis ever reported from a randomized, placebo-controlled trial in the phase IIIb TRANSFIGURE study, Dr. Kristian Reich reported at the World Congress of Dermatology.
At 198 patients, TRANSFIGURE is the largest-ever prospective study in patients with moderate to severe chronic plaque psoriasis and significant nail involvement. And while only the 16-week results are available thus far, when TRANSFIGURE is completed after a planned 132 weeks of treatment, it will also be the longest-ever study in the treatment of nail psoriasis, noted Dr. Reich, a dermatologist in group practice in Hamburg, Germany.
Elsewhere at WCD 2015, Dr. Alice B. Gottlieb presented the week 16 results of the phase IIIb GESTURE study, in which 205 psoriasis patients with moderate to severe psoriasis of the palms and soles were randomized to subcutaneous secukinumab (Cosentyx) at 150 or 300 mg or placebo. Dosing was weekly for the first 5 weeks and monthly thereafter.
The primary endpoint, a palmoplantar Investigator’s Global Assessment scale score of 0 or 1 – clear or almost clear – at week 16 was 33.3% with secukinumab at 300 mg, 22.1% at 150 mg, and 1.5% with placebo. The average reduction in palmoplantar PASI (Psoriasis Area Severity Index) score from baseline was 54.6% with high-dose and 35.3% with low-dose secukinumab, compared with 4.1% in placebo-treated controls, reported Dr. Gottlieb, professor and chair of dermatology at Tufts University, Boston.
Like the TRANSFIGURE trial, GESTURE will continue for 132 weeks, with the initial placebo-treated controls being randomized to secukinumab at 150 or 300 mg after week 16.
Dr. Reich reported that by 16 weeks in TRANSFIGURE, mean scores on the Nail Psoriasis Severity Index had improved by 45.3%, compared with baseline, in patients on secukinumab 300 mg, 37.9% in those on secukinumab 150 mg, and 10.8% with placebo.
The results on the skin were dramatic: a PASI 75 rate of 87.1% with secukinumab 300 mg, 77% with secukinumab 150 mg, and 5.1% with placebo. The PASI 100 response rate – meaning totally clear skin – was 31.9% with high-dose and 25.2% with lower-dose secukinumab, while there was a zero PASI 100 rate in controls.
The only adverse events more common than with placebo were nasopharyngitis and upper respiratory infections.
Dr. Reich predicted that as the ongoing TRANSFIGURE study continues well beyond 16 weeks, the nail psoriasis response rates will climb, since nails are so slow growing.
TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
VANCOUVER, B.C. – The interleukin-17A inhibitor secukinumab demonstrated the greatest improvement in nail psoriasis ever reported from a randomized, placebo-controlled trial in the phase IIIb TRANSFIGURE study, Dr. Kristian Reich reported at the World Congress of Dermatology.
At 198 patients, TRANSFIGURE is the largest-ever prospective study in patients with moderate to severe chronic plaque psoriasis and significant nail involvement. And while only the 16-week results are available thus far, when TRANSFIGURE is completed after a planned 132 weeks of treatment, it will also be the longest-ever study in the treatment of nail psoriasis, noted Dr. Reich, a dermatologist in group practice in Hamburg, Germany.
Elsewhere at WCD 2015, Dr. Alice B. Gottlieb presented the week 16 results of the phase IIIb GESTURE study, in which 205 psoriasis patients with moderate to severe psoriasis of the palms and soles were randomized to subcutaneous secukinumab (Cosentyx) at 150 or 300 mg or placebo. Dosing was weekly for the first 5 weeks and monthly thereafter.
The primary endpoint, a palmoplantar Investigator’s Global Assessment scale score of 0 or 1 – clear or almost clear – at week 16 was 33.3% with secukinumab at 300 mg, 22.1% at 150 mg, and 1.5% with placebo. The average reduction in palmoplantar PASI (Psoriasis Area Severity Index) score from baseline was 54.6% with high-dose and 35.3% with low-dose secukinumab, compared with 4.1% in placebo-treated controls, reported Dr. Gottlieb, professor and chair of dermatology at Tufts University, Boston.
Like the TRANSFIGURE trial, GESTURE will continue for 132 weeks, with the initial placebo-treated controls being randomized to secukinumab at 150 or 300 mg after week 16.
Dr. Reich reported that by 16 weeks in TRANSFIGURE, mean scores on the Nail Psoriasis Severity Index had improved by 45.3%, compared with baseline, in patients on secukinumab 300 mg, 37.9% in those on secukinumab 150 mg, and 10.8% with placebo.
The results on the skin were dramatic: a PASI 75 rate of 87.1% with secukinumab 300 mg, 77% with secukinumab 150 mg, and 5.1% with placebo. The PASI 100 response rate – meaning totally clear skin – was 31.9% with high-dose and 25.2% with lower-dose secukinumab, while there was a zero PASI 100 rate in controls.
The only adverse events more common than with placebo were nasopharyngitis and upper respiratory infections.
Dr. Reich predicted that as the ongoing TRANSFIGURE study continues well beyond 16 weeks, the nail psoriasis response rates will climb, since nails are so slow growing.
TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
VANCOUVER, B.C. – The interleukin-17A inhibitor secukinumab demonstrated the greatest improvement in nail psoriasis ever reported from a randomized, placebo-controlled trial in the phase IIIb TRANSFIGURE study, Dr. Kristian Reich reported at the World Congress of Dermatology.
At 198 patients, TRANSFIGURE is the largest-ever prospective study in patients with moderate to severe chronic plaque psoriasis and significant nail involvement. And while only the 16-week results are available thus far, when TRANSFIGURE is completed after a planned 132 weeks of treatment, it will also be the longest-ever study in the treatment of nail psoriasis, noted Dr. Reich, a dermatologist in group practice in Hamburg, Germany.
Elsewhere at WCD 2015, Dr. Alice B. Gottlieb presented the week 16 results of the phase IIIb GESTURE study, in which 205 psoriasis patients with moderate to severe psoriasis of the palms and soles were randomized to subcutaneous secukinumab (Cosentyx) at 150 or 300 mg or placebo. Dosing was weekly for the first 5 weeks and monthly thereafter.
The primary endpoint, a palmoplantar Investigator’s Global Assessment scale score of 0 or 1 – clear or almost clear – at week 16 was 33.3% with secukinumab at 300 mg, 22.1% at 150 mg, and 1.5% with placebo. The average reduction in palmoplantar PASI (Psoriasis Area Severity Index) score from baseline was 54.6% with high-dose and 35.3% with low-dose secukinumab, compared with 4.1% in placebo-treated controls, reported Dr. Gottlieb, professor and chair of dermatology at Tufts University, Boston.
Like the TRANSFIGURE trial, GESTURE will continue for 132 weeks, with the initial placebo-treated controls being randomized to secukinumab at 150 or 300 mg after week 16.
Dr. Reich reported that by 16 weeks in TRANSFIGURE, mean scores on the Nail Psoriasis Severity Index had improved by 45.3%, compared with baseline, in patients on secukinumab 300 mg, 37.9% in those on secukinumab 150 mg, and 10.8% with placebo.
The results on the skin were dramatic: a PASI 75 rate of 87.1% with secukinumab 300 mg, 77% with secukinumab 150 mg, and 5.1% with placebo. The PASI 100 response rate – meaning totally clear skin – was 31.9% with high-dose and 25.2% with lower-dose secukinumab, while there was a zero PASI 100 rate in controls.
The only adverse events more common than with placebo were nasopharyngitis and upper respiratory infections.
Dr. Reich predicted that as the ongoing TRANSFIGURE study continues well beyond 16 weeks, the nail psoriasis response rates will climb, since nails are so slow growing.
TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
AT WCD 2015
Key clinical point: Two phase IIIb trials show secukinumab at 300 mg is the most effective drug ever formally studied for nail or palmoplantar psoriasis.
Major finding: At 16 weeks, secukinumab at 300 mg improved nail psoriasis by 45.3% and palmoplantar psoriasis by 33.3%.
Data source: The phase IIIb TRANSFIGURE and GESTURE studies, ongoing randomized, prospective, initially double-blind studies in which 198 patients with significant nail psoriasis and 205 with palmoplantar psoriasis received secukinumab at 150 or 300 mg or placebo. Both studies will continue out to 132 weeks.
Disclosures: TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
WCD: IL-23p19 inhibitors yield ‘promising’ psoriasis results
VANCOUVER, B.C. – So far, monoclonal antibodies that specifically target IL-23p19 are showing promise for treating chronic plaque psoriasis and are usually well tolerated, Dr. Elisabeth Riedl said at the World Congress of Dermatology.
The agents target the interleukin (IL)-23 cytokine, which is important in the induction of T-helper-17 cells in psoriasis and several other inflammatory disorders, said Dr. Riedl of the department of dermatology at Mount Sinai Hospital in New York. IL-23 has two subunits – p40, which it shares with IL-12, and p19, which is specific for IL-23, she said. Based on earlier research, the effectiveness of psoriasis treatments that target the p40 subunit seems to depend mostly on their ability to neutralize IL-23, not IL-12, she noted.
That observation helped spur several psoriasis trials of monoclonal antibodies that target IL-23p19, according to Dr. Riedl. Among the most promising of the monoclonal antibodies is BI 655066, which significantly outperformed placebo in a randomized, double-blind phase I trial reported earlier in 2015. At week 12, 87% of patients who received intravenous or oral BI65506 achieved a PASI (Psoriasis Area and Severity Index) 75 score, while 58% achieved a PASI 90 score and 16% achieved PASI 100, compared with none of the placebo group.
Another IL-23p19 inhibitor, guselkumab (CNTO 1959), beat placebo in a small randomized, double-blind phase I trial, Dr. Riedl noted. All patients who received a single subcutaneous injection of 300 mg of guselkumab achieved a PASI 75 score, and about 80% achieved PASI 90, compared with none of the placebo group, she noted.
The third IL-23p19 inhibitor, tildrakizumab (MK-3222), also beat placebo at all doses tested in a three-part, randomized, placebo-controlled phase I trial, Dr. Riedl said. Furthermore, the histopathology of a subgroup of patients revealed significant decreases in lesions, compared with baseline, and a significant correlation between histopathologic and clinical scores, she noted. “In phase II, there was a significant response for all dosing cohorts, with a durable response to 52 weeks,” she added.
The primary safety concern for IL-23 inhibitors appears to be increased infection risk, although patients with genetic diseases connected to the IL-23/Th17 pathway already are at increased risk for infections such as candidiasis, mycobacteriosis, salmonellosis, and staphylococcal and Klebsiella infections, Dr. Riedl noted. “Ustekinumab targets IL-12/IL-23 and so far has shown a favorable safety profile,” she said, pointing to a 5-year follow-up study of the antibody that found no increase over time in adverse events and similar rates of adverse events among dosing groups.
Infections and headaches were the most commonly reported adverse events in phase I and II trials of targeted anti–IL-23p19 antibodies, Dr. Riedl said. In the phase I trial of BI 655066, rates of adverse events were similar for treatment and placebo except for nasopharyngitis, which affected 13% of the BI 655066 group and none of the placebo group, she noted. Guselkumab also was associated with similar rates of overall adverse events, compared with placebo, in its phase I trial, although 6 of 20 patients who received guselkumab developed infections, including two upper respiratory tract infections and one event each of bronchitis, folliculitis, viral gastroenteritis, herpes simplex infection, lower respiratory tract infection, vaginal infection, and nasopharyngitis, she said. Tildrakizumab was generally well tolerated in its phase IIb trial, she added. Serious adverse events that might have been related to treatment were rare but included bacterial arthritis, lymphedema, melanoma, stroke, epiglottitis, and knee infection.
Taken together, the data for IL-23p19 inhibitors are encouraging, according to Dr. Riedl. “The results suggest that selective inhibition of IL-23p19 is a promising target for novel therapies,” she said.
Dr. Riedl reported receiving grant support or serving as a consultant, advisory board member, or speakers bureau member for Abbvie, Celgen, Merck, Pfizer, and Janssen.
VANCOUVER, B.C. – So far, monoclonal antibodies that specifically target IL-23p19 are showing promise for treating chronic plaque psoriasis and are usually well tolerated, Dr. Elisabeth Riedl said at the World Congress of Dermatology.
The agents target the interleukin (IL)-23 cytokine, which is important in the induction of T-helper-17 cells in psoriasis and several other inflammatory disorders, said Dr. Riedl of the department of dermatology at Mount Sinai Hospital in New York. IL-23 has two subunits – p40, which it shares with IL-12, and p19, which is specific for IL-23, she said. Based on earlier research, the effectiveness of psoriasis treatments that target the p40 subunit seems to depend mostly on their ability to neutralize IL-23, not IL-12, she noted.
That observation helped spur several psoriasis trials of monoclonal antibodies that target IL-23p19, according to Dr. Riedl. Among the most promising of the monoclonal antibodies is BI 655066, which significantly outperformed placebo in a randomized, double-blind phase I trial reported earlier in 2015. At week 12, 87% of patients who received intravenous or oral BI65506 achieved a PASI (Psoriasis Area and Severity Index) 75 score, while 58% achieved a PASI 90 score and 16% achieved PASI 100, compared with none of the placebo group.
Another IL-23p19 inhibitor, guselkumab (CNTO 1959), beat placebo in a small randomized, double-blind phase I trial, Dr. Riedl noted. All patients who received a single subcutaneous injection of 300 mg of guselkumab achieved a PASI 75 score, and about 80% achieved PASI 90, compared with none of the placebo group, she noted.
The third IL-23p19 inhibitor, tildrakizumab (MK-3222), also beat placebo at all doses tested in a three-part, randomized, placebo-controlled phase I trial, Dr. Riedl said. Furthermore, the histopathology of a subgroup of patients revealed significant decreases in lesions, compared with baseline, and a significant correlation between histopathologic and clinical scores, she noted. “In phase II, there was a significant response for all dosing cohorts, with a durable response to 52 weeks,” she added.
The primary safety concern for IL-23 inhibitors appears to be increased infection risk, although patients with genetic diseases connected to the IL-23/Th17 pathway already are at increased risk for infections such as candidiasis, mycobacteriosis, salmonellosis, and staphylococcal and Klebsiella infections, Dr. Riedl noted. “Ustekinumab targets IL-12/IL-23 and so far has shown a favorable safety profile,” she said, pointing to a 5-year follow-up study of the antibody that found no increase over time in adverse events and similar rates of adverse events among dosing groups.
Infections and headaches were the most commonly reported adverse events in phase I and II trials of targeted anti–IL-23p19 antibodies, Dr. Riedl said. In the phase I trial of BI 655066, rates of adverse events were similar for treatment and placebo except for nasopharyngitis, which affected 13% of the BI 655066 group and none of the placebo group, she noted. Guselkumab also was associated with similar rates of overall adverse events, compared with placebo, in its phase I trial, although 6 of 20 patients who received guselkumab developed infections, including two upper respiratory tract infections and one event each of bronchitis, folliculitis, viral gastroenteritis, herpes simplex infection, lower respiratory tract infection, vaginal infection, and nasopharyngitis, she said. Tildrakizumab was generally well tolerated in its phase IIb trial, she added. Serious adverse events that might have been related to treatment were rare but included bacterial arthritis, lymphedema, melanoma, stroke, epiglottitis, and knee infection.
Taken together, the data for IL-23p19 inhibitors are encouraging, according to Dr. Riedl. “The results suggest that selective inhibition of IL-23p19 is a promising target for novel therapies,” she said.
Dr. Riedl reported receiving grant support or serving as a consultant, advisory board member, or speakers bureau member for Abbvie, Celgen, Merck, Pfizer, and Janssen.
VANCOUVER, B.C. – So far, monoclonal antibodies that specifically target IL-23p19 are showing promise for treating chronic plaque psoriasis and are usually well tolerated, Dr. Elisabeth Riedl said at the World Congress of Dermatology.
The agents target the interleukin (IL)-23 cytokine, which is important in the induction of T-helper-17 cells in psoriasis and several other inflammatory disorders, said Dr. Riedl of the department of dermatology at Mount Sinai Hospital in New York. IL-23 has two subunits – p40, which it shares with IL-12, and p19, which is specific for IL-23, she said. Based on earlier research, the effectiveness of psoriasis treatments that target the p40 subunit seems to depend mostly on their ability to neutralize IL-23, not IL-12, she noted.
That observation helped spur several psoriasis trials of monoclonal antibodies that target IL-23p19, according to Dr. Riedl. Among the most promising of the monoclonal antibodies is BI 655066, which significantly outperformed placebo in a randomized, double-blind phase I trial reported earlier in 2015. At week 12, 87% of patients who received intravenous or oral BI65506 achieved a PASI (Psoriasis Area and Severity Index) 75 score, while 58% achieved a PASI 90 score and 16% achieved PASI 100, compared with none of the placebo group.
Another IL-23p19 inhibitor, guselkumab (CNTO 1959), beat placebo in a small randomized, double-blind phase I trial, Dr. Riedl noted. All patients who received a single subcutaneous injection of 300 mg of guselkumab achieved a PASI 75 score, and about 80% achieved PASI 90, compared with none of the placebo group, she noted.
The third IL-23p19 inhibitor, tildrakizumab (MK-3222), also beat placebo at all doses tested in a three-part, randomized, placebo-controlled phase I trial, Dr. Riedl said. Furthermore, the histopathology of a subgroup of patients revealed significant decreases in lesions, compared with baseline, and a significant correlation between histopathologic and clinical scores, she noted. “In phase II, there was a significant response for all dosing cohorts, with a durable response to 52 weeks,” she added.
The primary safety concern for IL-23 inhibitors appears to be increased infection risk, although patients with genetic diseases connected to the IL-23/Th17 pathway already are at increased risk for infections such as candidiasis, mycobacteriosis, salmonellosis, and staphylococcal and Klebsiella infections, Dr. Riedl noted. “Ustekinumab targets IL-12/IL-23 and so far has shown a favorable safety profile,” she said, pointing to a 5-year follow-up study of the antibody that found no increase over time in adverse events and similar rates of adverse events among dosing groups.
Infections and headaches were the most commonly reported adverse events in phase I and II trials of targeted anti–IL-23p19 antibodies, Dr. Riedl said. In the phase I trial of BI 655066, rates of adverse events were similar for treatment and placebo except for nasopharyngitis, which affected 13% of the BI 655066 group and none of the placebo group, she noted. Guselkumab also was associated with similar rates of overall adverse events, compared with placebo, in its phase I trial, although 6 of 20 patients who received guselkumab developed infections, including two upper respiratory tract infections and one event each of bronchitis, folliculitis, viral gastroenteritis, herpes simplex infection, lower respiratory tract infection, vaginal infection, and nasopharyngitis, she said. Tildrakizumab was generally well tolerated in its phase IIb trial, she added. Serious adverse events that might have been related to treatment were rare but included bacterial arthritis, lymphedema, melanoma, stroke, epiglottitis, and knee infection.
Taken together, the data for IL-23p19 inhibitors are encouraging, according to Dr. Riedl. “The results suggest that selective inhibition of IL-23p19 is a promising target for novel therapies,” she said.
Dr. Riedl reported receiving grant support or serving as a consultant, advisory board member, or speakers bureau member for Abbvie, Celgen, Merck, Pfizer, and Janssen.
EXPERT ANALYSIS FROM WCD 2015
WCD: Ixekizumab beats etanercept for psoriatic itch
VANCOUVER, B.C. – Ixekizumab-treated patients with plaque psoriasis reported markedly faster and larger improvements in itch severity than those on high-dose etanercept or placebo in a large head-to-head phase III randomized trial.
Ixekizumab is an investigational humanized IgG4 monoclonal antibody directed against the proinflammatory cytokines interleukin-17A. In the 1,224-patient phase III UNCOVER-2 trial, itch severity was rapidly reduced in ixekizumab-treated patients as early as week 1, when the first assessment occurred, Dr. Gil Yosipovitch reported at the World Congress of Dermatology.
Moreover, a clinically meaningful itch response – defined by Dr. Yosipovitch and coinvestigators as at least a 4-point drop from baseline on a 0-10 Itch Numeric Rating Scale based upon 24-hour recall – occurred in the ixekizumab-treated patients by week 4. Patients randomized to etanercept (Enbrel) at 50 mg twice per week never reached that bar during the 12-week study. Neither did the placebo-treated controls, noted Dr. Yosipovitch, professor and chairman of the department of dermatology at Temple University in Philadelphia.
The average baseline itch severity score in UNCOVER-2 was 6.6 points. The mean 12-week reduction in itch score was 0.4 points, or 14%, with placebo; 3.6 points, or 51%, with etanercept; 4.9 points, or 75%, with ixekizumab at 80 mg every 4 weeks; and 5.2 points, or 76%, with ixekizumab 80 mg every 2 weeks.
“The most important part of my presentation today is the rapid response to ixekizumab. There’s a significant reduction in itch in the first week and a 75% reduction at 12 weeks. I have to tell you, as someone who’s been in the itch field for a very long time, those are very impressive results,” the dermatologist declared. “Fast improvement is the issue, really. It we want a drug that claims to have an antipruritic effect, it has to work fast, because that’s what really bothers the patient.”
Another impressive statistic: 41% of ixekizumab-treated patients reported “no itching” at 12 weeks, compared with 17% on high-dose etanercept and 2% with placebo, he continued.
A recurring theme at WCD 2015, bolstered by an impressively large survey of North American and European dermatologists, rheumatologists, and patients, is that psoriasis patients feel itch hasn’t gotten the attention it deserves from physicians. Itch is the most common symptom of psoriasis, and more psoriasis patients rate itch as the most bothersome aspect of their chronic inflammatory skin disease than they do any other symptom, Dr. Yosipovitch noted.
Aside from this UNCOVER-2 analysis, very few data exist regarding the antipruritic effects of various biologic agents, he observed.
“I am very excited about this type of approach. Hopefully other companies wil now put more focus on itch. I think this is what the patients want, and if we can achieve fast responses, we are doing our job well,” the dermatologist said.
One audience member asked why the itch score is based upon recall over the previous 24 hours. Why not a week?
Dr. Yosipovitch replied that from other studies he’s done, he has found itch recall beyond 24 hours to be highly problematic.
“I actually recommend doing it twice a day, because itch is not constant throughout the day. It fluctuates. It is worst at nighttime, and that affects quality of life. In trials, I try to have patients keep a diary capturing itch evening and morning,” Dr. Yosipovitch said.
Another audience member asked whether a rapid response to itch might be a biomarker predictive of a later favorable response in terms of lesion size. If so, he continued, perhaps failure to experience a reduction in itch within the first week or so on an agent such a ixekizumab might be a signal to switch to another drug, rather than waiting for 4-8 weeks.
Dr. Yosipovitch replied that it’s an excellent thought and worthy of study, but the answer simply isn’t known yet.
The primary outcomes of UNCOVER-2 have previously been presented (Lancet 2015 June 10 [doi: 10.1016/S0140-6736(15)60125-8]). Briefly, the Psoriasis Area Severity Index (PASI) 75 response rate was 2% with placebo, 42% with etanercept, 78% with ixekizumab every 4 weeks, and 90% with ixekizumab every 2 weeks. When the bar was set higher – a PASI 100 – the response rate was 1% with placebo, 5% with etanercept, and 31% and 41% with ixekizumab every 4 and 2 weeks.
The UNCOVER-2 trial was sponsored by Eli Lilly. Dr. Yosipovitch is a recipient of research grants from and/or a consultant to Eli Lilly and roughly a dozen other pharmaceutical companies.
VANCOUVER, B.C. – Ixekizumab-treated patients with plaque psoriasis reported markedly faster and larger improvements in itch severity than those on high-dose etanercept or placebo in a large head-to-head phase III randomized trial.
Ixekizumab is an investigational humanized IgG4 monoclonal antibody directed against the proinflammatory cytokines interleukin-17A. In the 1,224-patient phase III UNCOVER-2 trial, itch severity was rapidly reduced in ixekizumab-treated patients as early as week 1, when the first assessment occurred, Dr. Gil Yosipovitch reported at the World Congress of Dermatology.
Moreover, a clinically meaningful itch response – defined by Dr. Yosipovitch and coinvestigators as at least a 4-point drop from baseline on a 0-10 Itch Numeric Rating Scale based upon 24-hour recall – occurred in the ixekizumab-treated patients by week 4. Patients randomized to etanercept (Enbrel) at 50 mg twice per week never reached that bar during the 12-week study. Neither did the placebo-treated controls, noted Dr. Yosipovitch, professor and chairman of the department of dermatology at Temple University in Philadelphia.
The average baseline itch severity score in UNCOVER-2 was 6.6 points. The mean 12-week reduction in itch score was 0.4 points, or 14%, with placebo; 3.6 points, or 51%, with etanercept; 4.9 points, or 75%, with ixekizumab at 80 mg every 4 weeks; and 5.2 points, or 76%, with ixekizumab 80 mg every 2 weeks.
“The most important part of my presentation today is the rapid response to ixekizumab. There’s a significant reduction in itch in the first week and a 75% reduction at 12 weeks. I have to tell you, as someone who’s been in the itch field for a very long time, those are very impressive results,” the dermatologist declared. “Fast improvement is the issue, really. It we want a drug that claims to have an antipruritic effect, it has to work fast, because that’s what really bothers the patient.”
Another impressive statistic: 41% of ixekizumab-treated patients reported “no itching” at 12 weeks, compared with 17% on high-dose etanercept and 2% with placebo, he continued.
A recurring theme at WCD 2015, bolstered by an impressively large survey of North American and European dermatologists, rheumatologists, and patients, is that psoriasis patients feel itch hasn’t gotten the attention it deserves from physicians. Itch is the most common symptom of psoriasis, and more psoriasis patients rate itch as the most bothersome aspect of their chronic inflammatory skin disease than they do any other symptom, Dr. Yosipovitch noted.
Aside from this UNCOVER-2 analysis, very few data exist regarding the antipruritic effects of various biologic agents, he observed.
“I am very excited about this type of approach. Hopefully other companies wil now put more focus on itch. I think this is what the patients want, and if we can achieve fast responses, we are doing our job well,” the dermatologist said.
One audience member asked why the itch score is based upon recall over the previous 24 hours. Why not a week?
Dr. Yosipovitch replied that from other studies he’s done, he has found itch recall beyond 24 hours to be highly problematic.
“I actually recommend doing it twice a day, because itch is not constant throughout the day. It fluctuates. It is worst at nighttime, and that affects quality of life. In trials, I try to have patients keep a diary capturing itch evening and morning,” Dr. Yosipovitch said.
Another audience member asked whether a rapid response to itch might be a biomarker predictive of a later favorable response in terms of lesion size. If so, he continued, perhaps failure to experience a reduction in itch within the first week or so on an agent such a ixekizumab might be a signal to switch to another drug, rather than waiting for 4-8 weeks.
Dr. Yosipovitch replied that it’s an excellent thought and worthy of study, but the answer simply isn’t known yet.
The primary outcomes of UNCOVER-2 have previously been presented (Lancet 2015 June 10 [doi: 10.1016/S0140-6736(15)60125-8]). Briefly, the Psoriasis Area Severity Index (PASI) 75 response rate was 2% with placebo, 42% with etanercept, 78% with ixekizumab every 4 weeks, and 90% with ixekizumab every 2 weeks. When the bar was set higher – a PASI 100 – the response rate was 1% with placebo, 5% with etanercept, and 31% and 41% with ixekizumab every 4 and 2 weeks.
The UNCOVER-2 trial was sponsored by Eli Lilly. Dr. Yosipovitch is a recipient of research grants from and/or a consultant to Eli Lilly and roughly a dozen other pharmaceutical companies.
VANCOUVER, B.C. – Ixekizumab-treated patients with plaque psoriasis reported markedly faster and larger improvements in itch severity than those on high-dose etanercept or placebo in a large head-to-head phase III randomized trial.
Ixekizumab is an investigational humanized IgG4 monoclonal antibody directed against the proinflammatory cytokines interleukin-17A. In the 1,224-patient phase III UNCOVER-2 trial, itch severity was rapidly reduced in ixekizumab-treated patients as early as week 1, when the first assessment occurred, Dr. Gil Yosipovitch reported at the World Congress of Dermatology.
Moreover, a clinically meaningful itch response – defined by Dr. Yosipovitch and coinvestigators as at least a 4-point drop from baseline on a 0-10 Itch Numeric Rating Scale based upon 24-hour recall – occurred in the ixekizumab-treated patients by week 4. Patients randomized to etanercept (Enbrel) at 50 mg twice per week never reached that bar during the 12-week study. Neither did the placebo-treated controls, noted Dr. Yosipovitch, professor and chairman of the department of dermatology at Temple University in Philadelphia.
The average baseline itch severity score in UNCOVER-2 was 6.6 points. The mean 12-week reduction in itch score was 0.4 points, or 14%, with placebo; 3.6 points, or 51%, with etanercept; 4.9 points, or 75%, with ixekizumab at 80 mg every 4 weeks; and 5.2 points, or 76%, with ixekizumab 80 mg every 2 weeks.
“The most important part of my presentation today is the rapid response to ixekizumab. There’s a significant reduction in itch in the first week and a 75% reduction at 12 weeks. I have to tell you, as someone who’s been in the itch field for a very long time, those are very impressive results,” the dermatologist declared. “Fast improvement is the issue, really. It we want a drug that claims to have an antipruritic effect, it has to work fast, because that’s what really bothers the patient.”
Another impressive statistic: 41% of ixekizumab-treated patients reported “no itching” at 12 weeks, compared with 17% on high-dose etanercept and 2% with placebo, he continued.
A recurring theme at WCD 2015, bolstered by an impressively large survey of North American and European dermatologists, rheumatologists, and patients, is that psoriasis patients feel itch hasn’t gotten the attention it deserves from physicians. Itch is the most common symptom of psoriasis, and more psoriasis patients rate itch as the most bothersome aspect of their chronic inflammatory skin disease than they do any other symptom, Dr. Yosipovitch noted.
Aside from this UNCOVER-2 analysis, very few data exist regarding the antipruritic effects of various biologic agents, he observed.
“I am very excited about this type of approach. Hopefully other companies wil now put more focus on itch. I think this is what the patients want, and if we can achieve fast responses, we are doing our job well,” the dermatologist said.
One audience member asked why the itch score is based upon recall over the previous 24 hours. Why not a week?
Dr. Yosipovitch replied that from other studies he’s done, he has found itch recall beyond 24 hours to be highly problematic.
“I actually recommend doing it twice a day, because itch is not constant throughout the day. It fluctuates. It is worst at nighttime, and that affects quality of life. In trials, I try to have patients keep a diary capturing itch evening and morning,” Dr. Yosipovitch said.
Another audience member asked whether a rapid response to itch might be a biomarker predictive of a later favorable response in terms of lesion size. If so, he continued, perhaps failure to experience a reduction in itch within the first week or so on an agent such a ixekizumab might be a signal to switch to another drug, rather than waiting for 4-8 weeks.
Dr. Yosipovitch replied that it’s an excellent thought and worthy of study, but the answer simply isn’t known yet.
The primary outcomes of UNCOVER-2 have previously been presented (Lancet 2015 June 10 [doi: 10.1016/S0140-6736(15)60125-8]). Briefly, the Psoriasis Area Severity Index (PASI) 75 response rate was 2% with placebo, 42% with etanercept, 78% with ixekizumab every 4 weeks, and 90% with ixekizumab every 2 weeks. When the bar was set higher – a PASI 100 – the response rate was 1% with placebo, 5% with etanercept, and 31% and 41% with ixekizumab every 4 and 2 weeks.
The UNCOVER-2 trial was sponsored by Eli Lilly. Dr. Yosipovitch is a recipient of research grants from and/or a consultant to Eli Lilly and roughly a dozen other pharmaceutical companies.
AT WCD 2015
Key clinical point: Ixekizumab reduces psoriatic itch markedly faster and more profoundly than etanercept.
Major finding: Psoriasis patients randomized to ixekizumab showed a significant reduction in itch scores within the first week.
Data source: The UNCOVER-2 trial was a 12-week, phase III randomized trial involving 1,224 patients with plaque psoriasis.
Disclosures: UNCOVER-2 was sponsored by Eli Lilly. The presenter reported receiving research grants from and/or serving as a consultant to Eli Lilly and roughly a dozen other pharmaceutical companies.
VIDEO: Could a deep Koebner phenomenon trigger psoriatic arthritis?
ROME – Psoriasis patients with a past history of bone or joint trauma had about 50% higher risk of later developing psoriatic arthritis than did those without a history of trauma in a longitudinal, population-based study of more than 70,000 psoriasis patients in the United Kingdom.
The relationship between trauma and later development of psoriatic arthritis could involve a deep Koebner phenomenon similar to what is observed with the Koebner phenomenon in the skin, suggested lead investigator Dr. Thorvardur Löve in an interview at the European Congress of Rheumatology.
Based on these findings, “one of the things that we are very excited about is the potential to think of strategies and test strategies that might be used in psoriasis patients once they are injured. So should we do anything different in an injured psoriasis patient, for instance, some sort of preventive treatment?” said Dr. Löve of Landspitali University Hospital in Reykjavik, Iceland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Psoriasis patients with a past history of bone or joint trauma had about 50% higher risk of later developing psoriatic arthritis than did those without a history of trauma in a longitudinal, population-based study of more than 70,000 psoriasis patients in the United Kingdom.
The relationship between trauma and later development of psoriatic arthritis could involve a deep Koebner phenomenon similar to what is observed with the Koebner phenomenon in the skin, suggested lead investigator Dr. Thorvardur Löve in an interview at the European Congress of Rheumatology.
Based on these findings, “one of the things that we are very excited about is the potential to think of strategies and test strategies that might be used in psoriasis patients once they are injured. So should we do anything different in an injured psoriasis patient, for instance, some sort of preventive treatment?” said Dr. Löve of Landspitali University Hospital in Reykjavik, Iceland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Psoriasis patients with a past history of bone or joint trauma had about 50% higher risk of later developing psoriatic arthritis than did those without a history of trauma in a longitudinal, population-based study of more than 70,000 psoriasis patients in the United Kingdom.
The relationship between trauma and later development of psoriatic arthritis could involve a deep Koebner phenomenon similar to what is observed with the Koebner phenomenon in the skin, suggested lead investigator Dr. Thorvardur Löve in an interview at the European Congress of Rheumatology.
Based on these findings, “one of the things that we are very excited about is the potential to think of strategies and test strategies that might be used in psoriasis patients once they are injured. So should we do anything different in an injured psoriasis patient, for instance, some sort of preventive treatment?” said Dr. Löve of Landspitali University Hospital in Reykjavik, Iceland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2015 CONGRESS
Amgen’s termination of brodalumab stuns psoriasis world
VANCOUVER, B.C. – Amgen’s recent announcement that it is pulling the plug on further development of the investigational interleukin-17 receptor A inhibitor brodalumab was the red hot topic among psoriasis experts at the World Congress of Dermatology.
The biologic agent had seemingly been breezing through phase III clinical trials, racking up head-turning efficacy and reasonable safety numbers in a series of very large randomized studies conducted in patients with moderate-to-severe chronic plaque psoriasis. Then came the surprise announcement a scant couple of weeks before WCD 2015.
Brodalumab was also in codevelopment by Amgen and AstraZeneca for psoriatic arthritis and axial spondyloarthritis. Amgen is halting all participation in those research projects as well.
The company’s decision was based upon preliminary indications from the Food and Drug Administration that, as a condition for marketing approval, there would likely need to be restrictive labeling calling for physician monitoring for suicidality, something few dermatologists or rheumatologists are comfortable with.
“During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” Dr. Sean E. Harper, executive vice president of research and development at Amgen, said in the company’s announcement.
AstraZeneca officials said in a separate statement that they will review all the brodalumab data in detail and make a decision as soon as possible regarding whether to pursue unilateral development of the biologic agent.
Dr. Richard G. Langley, who at WCD 2015 presented the results of the AMAGINE-3 trial – 1,881 psoriasis patients randomized to ustekinumab (Stelara) at the approved dosing, placebo, or brodalumab at either 140 mg or 210 mg subcutaneously – noted that rates of depression and suicidal ideation were similarly low in the ustekinumab and brodalumab groups during the 52-week study, and there were no suicide attempts.
The problem for Amgen was a single-digit numeric imbalance in these adverse events in some of the earlier, considerably smaller studies of brodalumab, according to Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
“We have to remember that we can sometimes make spurious assumptions about rare events and perhaps cast a shadow on a pathway or molecule before we have full information,” he said diplomatically. “The Amgen decision is particularly unfortunate for patients because with brodalumab the only patients I’ve had who were depressed were the ones I’ve told are now no longer going to get the medication.”
Session chair Dr. Yves Poulin of Laval University in Quebec City, Quebec, said his experience has been similar: Patients love the unprecedented effectiveness of brodalumab and are crestfallen at learning they have to go off it.
Indeed, in AMAGINE-3, the 12-week PASI 75 rate among patients on brodalumab at 210 mg every 2 weeks – the more effective dose of the IL-17A inhibitor – was 85.1%, compared with 69.2% for ustekinumab, which is one of the most widely prescribed biologics. The PASI 100 rate was 36.7% for brodalumab, twice the 18.5% rate with ustekinumab. And the Investigator’s Global Assessment rating of 0 or 1 – clear or almost clear – was 79.6% with brodalumab, compared with 59% for ustekinumab, Dr. Langley reported.
“A consistent finding with brodalumab and the other IL-17 inhibitors is the remarkably fast improvement: within the first 2-4 weeks there’s a robust response,” he added.
Elsewhere at WCD 2015, Dr. Alan Menter presented the findings of the AMAGINE-2 trial, an 1,831-patient study identical in design to AMAGINE-3. The efficacy and safety results were virtually the same as in AMAGINE-3 as well, both in the 12-week induction phase and out to 52 weeks.
“We have a major issue that I think people haven’t understood well over the years relating to depression and psoriasis. Suicidal ideation has always been an issue. If you’re a 25-year-old and you’re trying to make your way in life and you’ve got significant psoriasis, you’re going to get depressed. But the suicidal ideation is twice as high in young people with psoriasis at baseline than in all the other autoimmune diseases put together,” said Dr. Menter, chair of dermatology at Baylor University Medical Center, Dallas, and the founding president of the International Psoriasis Council.
There has been no suicidality signal with secukinumab (Cosentyx), which in January became the first and, to date, only IL-17 inhibitor approved for marketing, nor with ixekizumab, which is in phase III clinical trials. There are distinct differences between the IL-17 inhibitor molecules – for example, brodalumab is IgG2, secukinumab is IgG1, and ixekizumab is IgG4. And brodalumab is a receptor antibody that not only inhibits the activity of IL-17A, but of IL-17F and -E as well, whereas secukinumab and ixekizumab work by other mechanisms. Investigators are now taking a close look at these differences to see if they have effects in terms of clinical outcomes and side effect profiles, he said.
Dr. Langley and Dr. Menter reported having financial relationships with Amgen and numerous other pharmaceutical companies.
VANCOUVER, B.C. – Amgen’s recent announcement that it is pulling the plug on further development of the investigational interleukin-17 receptor A inhibitor brodalumab was the red hot topic among psoriasis experts at the World Congress of Dermatology.
The biologic agent had seemingly been breezing through phase III clinical trials, racking up head-turning efficacy and reasonable safety numbers in a series of very large randomized studies conducted in patients with moderate-to-severe chronic plaque psoriasis. Then came the surprise announcement a scant couple of weeks before WCD 2015.
Brodalumab was also in codevelopment by Amgen and AstraZeneca for psoriatic arthritis and axial spondyloarthritis. Amgen is halting all participation in those research projects as well.
The company’s decision was based upon preliminary indications from the Food and Drug Administration that, as a condition for marketing approval, there would likely need to be restrictive labeling calling for physician monitoring for suicidality, something few dermatologists or rheumatologists are comfortable with.
“During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” Dr. Sean E. Harper, executive vice president of research and development at Amgen, said in the company’s announcement.
AstraZeneca officials said in a separate statement that they will review all the brodalumab data in detail and make a decision as soon as possible regarding whether to pursue unilateral development of the biologic agent.
Dr. Richard G. Langley, who at WCD 2015 presented the results of the AMAGINE-3 trial – 1,881 psoriasis patients randomized to ustekinumab (Stelara) at the approved dosing, placebo, or brodalumab at either 140 mg or 210 mg subcutaneously – noted that rates of depression and suicidal ideation were similarly low in the ustekinumab and brodalumab groups during the 52-week study, and there were no suicide attempts.
The problem for Amgen was a single-digit numeric imbalance in these adverse events in some of the earlier, considerably smaller studies of brodalumab, according to Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
“We have to remember that we can sometimes make spurious assumptions about rare events and perhaps cast a shadow on a pathway or molecule before we have full information,” he said diplomatically. “The Amgen decision is particularly unfortunate for patients because with brodalumab the only patients I’ve had who were depressed were the ones I’ve told are now no longer going to get the medication.”
Session chair Dr. Yves Poulin of Laval University in Quebec City, Quebec, said his experience has been similar: Patients love the unprecedented effectiveness of brodalumab and are crestfallen at learning they have to go off it.
Indeed, in AMAGINE-3, the 12-week PASI 75 rate among patients on brodalumab at 210 mg every 2 weeks – the more effective dose of the IL-17A inhibitor – was 85.1%, compared with 69.2% for ustekinumab, which is one of the most widely prescribed biologics. The PASI 100 rate was 36.7% for brodalumab, twice the 18.5% rate with ustekinumab. And the Investigator’s Global Assessment rating of 0 or 1 – clear or almost clear – was 79.6% with brodalumab, compared with 59% for ustekinumab, Dr. Langley reported.
“A consistent finding with brodalumab and the other IL-17 inhibitors is the remarkably fast improvement: within the first 2-4 weeks there’s a robust response,” he added.
Elsewhere at WCD 2015, Dr. Alan Menter presented the findings of the AMAGINE-2 trial, an 1,831-patient study identical in design to AMAGINE-3. The efficacy and safety results were virtually the same as in AMAGINE-3 as well, both in the 12-week induction phase and out to 52 weeks.
“We have a major issue that I think people haven’t understood well over the years relating to depression and psoriasis. Suicidal ideation has always been an issue. If you’re a 25-year-old and you’re trying to make your way in life and you’ve got significant psoriasis, you’re going to get depressed. But the suicidal ideation is twice as high in young people with psoriasis at baseline than in all the other autoimmune diseases put together,” said Dr. Menter, chair of dermatology at Baylor University Medical Center, Dallas, and the founding president of the International Psoriasis Council.
There has been no suicidality signal with secukinumab (Cosentyx), which in January became the first and, to date, only IL-17 inhibitor approved for marketing, nor with ixekizumab, which is in phase III clinical trials. There are distinct differences between the IL-17 inhibitor molecules – for example, brodalumab is IgG2, secukinumab is IgG1, and ixekizumab is IgG4. And brodalumab is a receptor antibody that not only inhibits the activity of IL-17A, but of IL-17F and -E as well, whereas secukinumab and ixekizumab work by other mechanisms. Investigators are now taking a close look at these differences to see if they have effects in terms of clinical outcomes and side effect profiles, he said.
Dr. Langley and Dr. Menter reported having financial relationships with Amgen and numerous other pharmaceutical companies.
VANCOUVER, B.C. – Amgen’s recent announcement that it is pulling the plug on further development of the investigational interleukin-17 receptor A inhibitor brodalumab was the red hot topic among psoriasis experts at the World Congress of Dermatology.
The biologic agent had seemingly been breezing through phase III clinical trials, racking up head-turning efficacy and reasonable safety numbers in a series of very large randomized studies conducted in patients with moderate-to-severe chronic plaque psoriasis. Then came the surprise announcement a scant couple of weeks before WCD 2015.
Brodalumab was also in codevelopment by Amgen and AstraZeneca for psoriatic arthritis and axial spondyloarthritis. Amgen is halting all participation in those research projects as well.
The company’s decision was based upon preliminary indications from the Food and Drug Administration that, as a condition for marketing approval, there would likely need to be restrictive labeling calling for physician monitoring for suicidality, something few dermatologists or rheumatologists are comfortable with.
“During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” Dr. Sean E. Harper, executive vice president of research and development at Amgen, said in the company’s announcement.
AstraZeneca officials said in a separate statement that they will review all the brodalumab data in detail and make a decision as soon as possible regarding whether to pursue unilateral development of the biologic agent.
Dr. Richard G. Langley, who at WCD 2015 presented the results of the AMAGINE-3 trial – 1,881 psoriasis patients randomized to ustekinumab (Stelara) at the approved dosing, placebo, or brodalumab at either 140 mg or 210 mg subcutaneously – noted that rates of depression and suicidal ideation were similarly low in the ustekinumab and brodalumab groups during the 52-week study, and there were no suicide attempts.
The problem for Amgen was a single-digit numeric imbalance in these adverse events in some of the earlier, considerably smaller studies of brodalumab, according to Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
“We have to remember that we can sometimes make spurious assumptions about rare events and perhaps cast a shadow on a pathway or molecule before we have full information,” he said diplomatically. “The Amgen decision is particularly unfortunate for patients because with brodalumab the only patients I’ve had who were depressed were the ones I’ve told are now no longer going to get the medication.”
Session chair Dr. Yves Poulin of Laval University in Quebec City, Quebec, said his experience has been similar: Patients love the unprecedented effectiveness of brodalumab and are crestfallen at learning they have to go off it.
Indeed, in AMAGINE-3, the 12-week PASI 75 rate among patients on brodalumab at 210 mg every 2 weeks – the more effective dose of the IL-17A inhibitor – was 85.1%, compared with 69.2% for ustekinumab, which is one of the most widely prescribed biologics. The PASI 100 rate was 36.7% for brodalumab, twice the 18.5% rate with ustekinumab. And the Investigator’s Global Assessment rating of 0 or 1 – clear or almost clear – was 79.6% with brodalumab, compared with 59% for ustekinumab, Dr. Langley reported.
“A consistent finding with brodalumab and the other IL-17 inhibitors is the remarkably fast improvement: within the first 2-4 weeks there’s a robust response,” he added.
Elsewhere at WCD 2015, Dr. Alan Menter presented the findings of the AMAGINE-2 trial, an 1,831-patient study identical in design to AMAGINE-3. The efficacy and safety results were virtually the same as in AMAGINE-3 as well, both in the 12-week induction phase and out to 52 weeks.
“We have a major issue that I think people haven’t understood well over the years relating to depression and psoriasis. Suicidal ideation has always been an issue. If you’re a 25-year-old and you’re trying to make your way in life and you’ve got significant psoriasis, you’re going to get depressed. But the suicidal ideation is twice as high in young people with psoriasis at baseline than in all the other autoimmune diseases put together,” said Dr. Menter, chair of dermatology at Baylor University Medical Center, Dallas, and the founding president of the International Psoriasis Council.
There has been no suicidality signal with secukinumab (Cosentyx), which in January became the first and, to date, only IL-17 inhibitor approved for marketing, nor with ixekizumab, which is in phase III clinical trials. There are distinct differences between the IL-17 inhibitor molecules – for example, brodalumab is IgG2, secukinumab is IgG1, and ixekizumab is IgG4. And brodalumab is a receptor antibody that not only inhibits the activity of IL-17A, but of IL-17F and -E as well, whereas secukinumab and ixekizumab work by other mechanisms. Investigators are now taking a close look at these differences to see if they have effects in terms of clinical outcomes and side effect profiles, he said.
Dr. Langley and Dr. Menter reported having financial relationships with Amgen and numerous other pharmaceutical companies.
EXPERT ANALYSIS FROM WCD 2015
Lasting ustekinumab benefits seen in psoriatic arthritis
The final results of a 2-year, phase III study assessing the clinical efficacy and safety of ustekinumab in 615 patients with active psoriatic arthritis confirm that joint- and skin-related improvements are maintained.
Data from the randomized, double blind, placebo-controlled PSUMMIT 1 study showed that 56.7%-63.6% of psoriatic arthritis (PsA) patients treated with a 45-mg or 90-mg dose every 12 weeks achieved American College of Rheumatology criteria for a 20% improvement in joint symptoms (ACR20). ACR50 and ACR70 responses ranged from 37.3% to 46% and 18.6% to 24.7%, respectively.
“The proportions of patients with either DAS28-CRP [28-joint Disease Activity Score using C-reactive protein] response or remission were maintained from week 52 to week 100,” noted lead study author Dr. Arthur Kavanaugh of the University of California-San Diego in La Jolla and his associates (Arthritis Care Res. 2015 June 19 [doi:10.1002/acr.22645]).
A moderate or good response according to DAS28-CRP was achieved by 71.9%-76.7% of patients. A 75% improvement in the Psoriasis Area and Severity Index (PASI) was achieved by 63.9%-72.5% of patients, and 41%-51.9% achieved a PASI 90. Mean changes in radiographic damage also were maintained.
“No unexpected safety events were observed through 2 years, and results were consistent with the known safety profile of ustekinumab,” Dr. Kavanaugh and his colleagues wrote, adding that the study “demonstrated a favorable benefit-risk profile of ustekinumab treatment in patients with active PsA.”
The trial was funded by Janssen Research & Development. Dr. Kavanaugh has received research support from AbbVie, Janssen, and UCB. Many of the other authors also had financial ties to Janssen and other manufacturers of biologics for psoriatic arthritis.
The final results of a 2-year, phase III study assessing the clinical efficacy and safety of ustekinumab in 615 patients with active psoriatic arthritis confirm that joint- and skin-related improvements are maintained.
Data from the randomized, double blind, placebo-controlled PSUMMIT 1 study showed that 56.7%-63.6% of psoriatic arthritis (PsA) patients treated with a 45-mg or 90-mg dose every 12 weeks achieved American College of Rheumatology criteria for a 20% improvement in joint symptoms (ACR20). ACR50 and ACR70 responses ranged from 37.3% to 46% and 18.6% to 24.7%, respectively.
“The proportions of patients with either DAS28-CRP [28-joint Disease Activity Score using C-reactive protein] response or remission were maintained from week 52 to week 100,” noted lead study author Dr. Arthur Kavanaugh of the University of California-San Diego in La Jolla and his associates (Arthritis Care Res. 2015 June 19 [doi:10.1002/acr.22645]).
A moderate or good response according to DAS28-CRP was achieved by 71.9%-76.7% of patients. A 75% improvement in the Psoriasis Area and Severity Index (PASI) was achieved by 63.9%-72.5% of patients, and 41%-51.9% achieved a PASI 90. Mean changes in radiographic damage also were maintained.
“No unexpected safety events were observed through 2 years, and results were consistent with the known safety profile of ustekinumab,” Dr. Kavanaugh and his colleagues wrote, adding that the study “demonstrated a favorable benefit-risk profile of ustekinumab treatment in patients with active PsA.”
The trial was funded by Janssen Research & Development. Dr. Kavanaugh has received research support from AbbVie, Janssen, and UCB. Many of the other authors also had financial ties to Janssen and other manufacturers of biologics for psoriatic arthritis.
The final results of a 2-year, phase III study assessing the clinical efficacy and safety of ustekinumab in 615 patients with active psoriatic arthritis confirm that joint- and skin-related improvements are maintained.
Data from the randomized, double blind, placebo-controlled PSUMMIT 1 study showed that 56.7%-63.6% of psoriatic arthritis (PsA) patients treated with a 45-mg or 90-mg dose every 12 weeks achieved American College of Rheumatology criteria for a 20% improvement in joint symptoms (ACR20). ACR50 and ACR70 responses ranged from 37.3% to 46% and 18.6% to 24.7%, respectively.
“The proportions of patients with either DAS28-CRP [28-joint Disease Activity Score using C-reactive protein] response or remission were maintained from week 52 to week 100,” noted lead study author Dr. Arthur Kavanaugh of the University of California-San Diego in La Jolla and his associates (Arthritis Care Res. 2015 June 19 [doi:10.1002/acr.22645]).
A moderate or good response according to DAS28-CRP was achieved by 71.9%-76.7% of patients. A 75% improvement in the Psoriasis Area and Severity Index (PASI) was achieved by 63.9%-72.5% of patients, and 41%-51.9% achieved a PASI 90. Mean changes in radiographic damage also were maintained.
“No unexpected safety events were observed through 2 years, and results were consistent with the known safety profile of ustekinumab,” Dr. Kavanaugh and his colleagues wrote, adding that the study “demonstrated a favorable benefit-risk profile of ustekinumab treatment in patients with active PsA.”
The trial was funded by Janssen Research & Development. Dr. Kavanaugh has received research support from AbbVie, Janssen, and UCB. Many of the other authors also had financial ties to Janssen and other manufacturers of biologics for psoriatic arthritis.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: The effects of ustekinumab on joint and skin symptoms are maintained for up to 2 years in adult patients with PsA.
Major finding: ACR20, 50, and 70 responses ranged from 56.7% to 63.6%, 37.3% to 46%, and 18.6% to 24.7%, respectively.
Data source: Randomized, double blind, placebo-controlled phase III PSUMMIT1 trial assessing the clinical efficacy and safety of ustekinumab in 615 patients with active PsA.
Disclosures: The trial was funded by Janssen Research & Development. Dr. Kavanaugh has received research support from AbbVie, Janssen, and UCB. Many of the other authors also had financial ties to Janssen and other manufacturers of biologics for psoriatic arthritis.
EULAR: Panel previews updated CVD recommendations
ROME – The interval for assessing cardiovascular disease risk in patients with at least one inflamed joint can be as long as 5 years, depending on the patient, according to revised recommendations issued by a European League Against Rheumatism expert panel. The first edition of the recommendations called for annual assessment.
“We leave the assessment interval up to each clinician. Annually is very hard to incorporate into clinical practice,” said Dr. Michael T. Nurmohamed, convenor of both the initial recommendation panel as well as the group that produced the new revision. He previewed the updated recommendations during a talk at the European Congress of Rheumatology. The final form of the revised recommendations, which apply to patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, will soon be posted online and published, he said.
The context for cardiovascular disease risk assessment of patients with these rheumatoid diseases who are in primary-prevention mode has changed since the initial version was released in 2009 (Ann. Rheum. Dis. 2010;69:325-31). That changed context led to a rethinking of the appropriate interval for risk-factor assessment, said Dr. Nurmohamed, professor and head of rheumatology research at VU University Medical Center in Amsterdam. “In 2009, tight control of rheumatoid diseases generally did not exist,” he said in an interview.
In patients with well-controlled rheumatoid disease, “you can assess their cardiovascular disease [CVD] risk, and if the risk is very low” you can defer the next follow-up assessment for 5 years or longer. But if a patient’s CVD risk is high, assess the patient more often, Dr. Nurmohamed suggested. The recommendations also call for an updated CVD risk assessment following a “major change” in antirheumatoid therapy.
The updated recommendations include two other notable changes. First, there is now a much deeper evidence base behind the need for CVD risk assessment and management in patients with psoriatic arthritis or ankylosing spondylitis. “We mentioned them before, but the data weren’t all that hard. We now have more evidence,” he said in an interview.
Second, the revised recommendations say that ultrasound examination of atherosclerotic plaque number and volume in a patient’s carotid artery “may be considered.” Dr. Nurmohamed acknowledged that carotid examination by ultrasound is very discretionary; it can provide useful risk information, but “not every rheumatologist will do it.”
Most other aspects of the revised recommendations remain similar to the 2009 edition. They emphasize controlling inflammation to lower CVD risk, multiplying a patient’s CVD risk score by a factor of 1.5 to better estimate their increased risk level due to their rheumatic disease, cautious use of nonsteroidal anti-inflammatory drugs, and minimized use of glucocorticoids. Multiplying a patient’s CVD risk score results in a “rough” risk estimate, he cautioned. “The problem is we use 1.5 for all patients, regardless of their rheumatoid-disease duration,” he said.
Recommended interventions to reduce a patient’s CVD risk include lifestyle steps of healthy diet, regular exercise, and smoking cessation, and the standard drug interventions for reducing CVD risk: antihypertensive medications and lipid-lowering drugs, especially statins. Target levels for treating hypertension and hyperlipidemia remain the same as for primary prevention in the general population, he said. CVD risk assessment and management steps for patients with one of the covered rheumatoid diseases and established CVD are the same as for standard secondary-prevention measures in the general population.
A notable gap in the revision is the continued absence of recommendations for patients with gout. “Gout is on our research agenda, but represents an enormous task,” Dr. Nurmohamed said. He anticipates that his panel will eventually develop recommendations for managing CVD risk in gout patients.
On Twitter @mitchelzoler
ROME – The interval for assessing cardiovascular disease risk in patients with at least one inflamed joint can be as long as 5 years, depending on the patient, according to revised recommendations issued by a European League Against Rheumatism expert panel. The first edition of the recommendations called for annual assessment.
“We leave the assessment interval up to each clinician. Annually is very hard to incorporate into clinical practice,” said Dr. Michael T. Nurmohamed, convenor of both the initial recommendation panel as well as the group that produced the new revision. He previewed the updated recommendations during a talk at the European Congress of Rheumatology. The final form of the revised recommendations, which apply to patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, will soon be posted online and published, he said.
The context for cardiovascular disease risk assessment of patients with these rheumatoid diseases who are in primary-prevention mode has changed since the initial version was released in 2009 (Ann. Rheum. Dis. 2010;69:325-31). That changed context led to a rethinking of the appropriate interval for risk-factor assessment, said Dr. Nurmohamed, professor and head of rheumatology research at VU University Medical Center in Amsterdam. “In 2009, tight control of rheumatoid diseases generally did not exist,” he said in an interview.
In patients with well-controlled rheumatoid disease, “you can assess their cardiovascular disease [CVD] risk, and if the risk is very low” you can defer the next follow-up assessment for 5 years or longer. But if a patient’s CVD risk is high, assess the patient more often, Dr. Nurmohamed suggested. The recommendations also call for an updated CVD risk assessment following a “major change” in antirheumatoid therapy.
The updated recommendations include two other notable changes. First, there is now a much deeper evidence base behind the need for CVD risk assessment and management in patients with psoriatic arthritis or ankylosing spondylitis. “We mentioned them before, but the data weren’t all that hard. We now have more evidence,” he said in an interview.
Second, the revised recommendations say that ultrasound examination of atherosclerotic plaque number and volume in a patient’s carotid artery “may be considered.” Dr. Nurmohamed acknowledged that carotid examination by ultrasound is very discretionary; it can provide useful risk information, but “not every rheumatologist will do it.”
Most other aspects of the revised recommendations remain similar to the 2009 edition. They emphasize controlling inflammation to lower CVD risk, multiplying a patient’s CVD risk score by a factor of 1.5 to better estimate their increased risk level due to their rheumatic disease, cautious use of nonsteroidal anti-inflammatory drugs, and minimized use of glucocorticoids. Multiplying a patient’s CVD risk score results in a “rough” risk estimate, he cautioned. “The problem is we use 1.5 for all patients, regardless of their rheumatoid-disease duration,” he said.
Recommended interventions to reduce a patient’s CVD risk include lifestyle steps of healthy diet, regular exercise, and smoking cessation, and the standard drug interventions for reducing CVD risk: antihypertensive medications and lipid-lowering drugs, especially statins. Target levels for treating hypertension and hyperlipidemia remain the same as for primary prevention in the general population, he said. CVD risk assessment and management steps for patients with one of the covered rheumatoid diseases and established CVD are the same as for standard secondary-prevention measures in the general population.
A notable gap in the revision is the continued absence of recommendations for patients with gout. “Gout is on our research agenda, but represents an enormous task,” Dr. Nurmohamed said. He anticipates that his panel will eventually develop recommendations for managing CVD risk in gout patients.
On Twitter @mitchelzoler
ROME – The interval for assessing cardiovascular disease risk in patients with at least one inflamed joint can be as long as 5 years, depending on the patient, according to revised recommendations issued by a European League Against Rheumatism expert panel. The first edition of the recommendations called for annual assessment.
“We leave the assessment interval up to each clinician. Annually is very hard to incorporate into clinical practice,” said Dr. Michael T. Nurmohamed, convenor of both the initial recommendation panel as well as the group that produced the new revision. He previewed the updated recommendations during a talk at the European Congress of Rheumatology. The final form of the revised recommendations, which apply to patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, will soon be posted online and published, he said.
The context for cardiovascular disease risk assessment of patients with these rheumatoid diseases who are in primary-prevention mode has changed since the initial version was released in 2009 (Ann. Rheum. Dis. 2010;69:325-31). That changed context led to a rethinking of the appropriate interval for risk-factor assessment, said Dr. Nurmohamed, professor and head of rheumatology research at VU University Medical Center in Amsterdam. “In 2009, tight control of rheumatoid diseases generally did not exist,” he said in an interview.
In patients with well-controlled rheumatoid disease, “you can assess their cardiovascular disease [CVD] risk, and if the risk is very low” you can defer the next follow-up assessment for 5 years or longer. But if a patient’s CVD risk is high, assess the patient more often, Dr. Nurmohamed suggested. The recommendations also call for an updated CVD risk assessment following a “major change” in antirheumatoid therapy.
The updated recommendations include two other notable changes. First, there is now a much deeper evidence base behind the need for CVD risk assessment and management in patients with psoriatic arthritis or ankylosing spondylitis. “We mentioned them before, but the data weren’t all that hard. We now have more evidence,” he said in an interview.
Second, the revised recommendations say that ultrasound examination of atherosclerotic plaque number and volume in a patient’s carotid artery “may be considered.” Dr. Nurmohamed acknowledged that carotid examination by ultrasound is very discretionary; it can provide useful risk information, but “not every rheumatologist will do it.”
Most other aspects of the revised recommendations remain similar to the 2009 edition. They emphasize controlling inflammation to lower CVD risk, multiplying a patient’s CVD risk score by a factor of 1.5 to better estimate their increased risk level due to their rheumatic disease, cautious use of nonsteroidal anti-inflammatory drugs, and minimized use of glucocorticoids. Multiplying a patient’s CVD risk score results in a “rough” risk estimate, he cautioned. “The problem is we use 1.5 for all patients, regardless of their rheumatoid-disease duration,” he said.
Recommended interventions to reduce a patient’s CVD risk include lifestyle steps of healthy diet, regular exercise, and smoking cessation, and the standard drug interventions for reducing CVD risk: antihypertensive medications and lipid-lowering drugs, especially statins. Target levels for treating hypertension and hyperlipidemia remain the same as for primary prevention in the general population, he said. CVD risk assessment and management steps for patients with one of the covered rheumatoid diseases and established CVD are the same as for standard secondary-prevention measures in the general population.
A notable gap in the revision is the continued absence of recommendations for patients with gout. “Gout is on our research agenda, but represents an enormous task,” Dr. Nurmohamed said. He anticipates that his panel will eventually develop recommendations for managing CVD risk in gout patients.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE EULAR 2015 Congress