User login
Ivonescimab: Possible New First-Line Standard in PD-L1–Positive Advanced NSCLC?
according to recent findings from the HARMONi-2 trial.
“This is the first randomized, phase 3 study to demonstrate a clinically significant improvement in efficacy with a novel drug compared to pembrolizumab in advanced NSCLC,” said study investigator Caicun Zhou, MD, PhD, with Shanghai Pulmonary Hospital in China,
The results highlight ivonescimab’s potential to become a “new standard of care” in advanced PD-L1–positive advanced NSCLC, said Dr. Zhou, who presented the analysis at the annual International Association for the Study of Lung Cancer (IASLC) World Conference on Lung Cancer in San Diego. Dr. Zhou is president-elect of the IASLC.
Ivonescimab (AK112) is a novel, potentially first-in-class investigational bispecific antibody that targets PD-1 and vascular endothelial growth factor (VEGF) developed by Akeso Biopharma, which funded the HARMONi-2 trial.
Conducted at 55 centers in China, HARMONi-2 enrolled 398 patients with untreated locally advanced or metastatic NSCLC, Eastern Cooperative Oncology Group Performance Status of 0 or 1, PD-L1 positive (with at least 1% of tumor cells expressing PD-L1), and no EGFR mutations or ALK rearrangements.
Patients were randomly allocated (1:1) to receive ivonescimab (20 mg/kg) or pembrolizumab (200 mg) every 3 weeks. The two groups were well balanced, and randomization was stratified by histology (squamous vs nonsquamous), clinical stage (IIIB/IIIC vs IV) and PD-L1 expression (PD-L1 of 1%-49% vs 50% or greater).
Dr. Zhou reported that patients who received ivonescimab were progression free for nearly twice as long as those on pembrolizumab — a median of 11.1 vs 5.8 months, indicating a 49% lower risk for progression or death (stratified hazard ratio [HR], 0.51; P < .0001).
The meaningful improvement in PFS with ivonescimab, compared with pembrolizumab, was “broadly consistent” in all prespecified subgroups, Dr. Zhou noted. That included patients with squamous NSCLC (HR, 0.48) and nonsquamous NSCLC (HR, 0.54), those with PD-L1 expression of 1%-49% (HR, 0.54) and 50% or higher (HR, 0.46), as well as those with liver metastases (HR, 0.47) and brain metastases (HR, 0.55).
The PFS benefit seen with ivonescimab in HARMONi-2 is “striking,” and the results “highlight the potential benefits of combined VEGF and PD-1 blockade together,” said John Heymach, MD, with the University of Texas MD Anderson Cancer Center in Houston, who served as discussant for the study.
Ivonescimab also led to a higher objective response rate (50% vs 38.5%) and disease control rate (89.9% vs 70.5%).
Grade 3 or higher treatment-related adverse events occurred in more patients receiving ivonescimab — 29.4% vs 15.6% on pembrolizumab. The difference largely stemmed from higher rates of proteinuria, hypertension, and lab abnormalities.
The rates of serious treatment-related adverse events were similar between the groups —20.8% in the ivonescimab group and 16.1% in the pembrolizumab group. Rates of grade 3 or higher immune-related adverse events were also similar, occurring in 7% of patients treated with ivonescimab and 8% of those receiving pembrolizumab.
In patients with squamous cell carcinoma, in particular, ivonescimab demonstrated a “very manageable” safety profile, Dr. Zhou noted. In this group, grade 3 or higher treatment-related adverse events occurred in 22.2% of patients (vs 18.7% receiving pembrolizumab).
Ivonescimab was associated with comparable but “numerically better” time to deterioration of global health status, based on the EORTC Core Quality of Life questionnaire, Dr. Zhou said.
Although the “really impressive and clinically meaningful” PFS benefits extended across different subgroups, “we await the overall survival results and additional studies done outside of China to confirm the benefit seen,” Dr. Heymach noted.
He also cautioned that, for patients with low to intermediate PD-L1 expression (1%-49%), pembrolizumab monotherapy “would not be the relevant comparator in the US and the rest of the world, and different study designs are going to be required for those populations.”
Based on the results of HARMONi-2, Akeso’s partner, Summit Therapeutics, plans to initiate HARMONi-7 in early 2025.
HARMONi-7 is currently planned as a multiregional, phase 3 clinical trial that will compare ivonescimab monotherapy to pembrolizumab monotherapy in patients with metastatic NSCLC whose tumors have high PD-L1 expression (50% or more).
Dr. Zhou has received consulting fees from Qilu Pharmaceutical, Hengrui, and TopAlliance Biosciences and honoraria from Eli Lilly China, Boehringer Ingelheim, Roche, Merck Sharp & Dohme, Qilu, Hengrui, Innovent Biologics, Alice, C-Stone, Luye Pharma, TopAlliance Biosciences, Amoy Diagnostics, and AnHeart Therapeutics. Dr. Heymach is a consultant for AbbVie, AnHeart Therapeutics, ArriVent Biopharma, AstraZeneca, BioCurity Pharmaceuticals, BioNTech, Blueprint Medicines, Boehringer Ingelheim, BMS, Eli Lilly, EMD Serono, Genentech, GlaxoSmithKline, Janssen Pharmaceuticals, Mirati Therapeutics, Novartis Pharmaceuticals, Regeneron Pharmaceuticals, Sanofi, Spectrum Pharmaceuticals, and Takeda.
A version of this article first appeared on Medscape.com.
according to recent findings from the HARMONi-2 trial.
“This is the first randomized, phase 3 study to demonstrate a clinically significant improvement in efficacy with a novel drug compared to pembrolizumab in advanced NSCLC,” said study investigator Caicun Zhou, MD, PhD, with Shanghai Pulmonary Hospital in China,
The results highlight ivonescimab’s potential to become a “new standard of care” in advanced PD-L1–positive advanced NSCLC, said Dr. Zhou, who presented the analysis at the annual International Association for the Study of Lung Cancer (IASLC) World Conference on Lung Cancer in San Diego. Dr. Zhou is president-elect of the IASLC.
Ivonescimab (AK112) is a novel, potentially first-in-class investigational bispecific antibody that targets PD-1 and vascular endothelial growth factor (VEGF) developed by Akeso Biopharma, which funded the HARMONi-2 trial.
Conducted at 55 centers in China, HARMONi-2 enrolled 398 patients with untreated locally advanced or metastatic NSCLC, Eastern Cooperative Oncology Group Performance Status of 0 or 1, PD-L1 positive (with at least 1% of tumor cells expressing PD-L1), and no EGFR mutations or ALK rearrangements.
Patients were randomly allocated (1:1) to receive ivonescimab (20 mg/kg) or pembrolizumab (200 mg) every 3 weeks. The two groups were well balanced, and randomization was stratified by histology (squamous vs nonsquamous), clinical stage (IIIB/IIIC vs IV) and PD-L1 expression (PD-L1 of 1%-49% vs 50% or greater).
Dr. Zhou reported that patients who received ivonescimab were progression free for nearly twice as long as those on pembrolizumab — a median of 11.1 vs 5.8 months, indicating a 49% lower risk for progression or death (stratified hazard ratio [HR], 0.51; P < .0001).
The meaningful improvement in PFS with ivonescimab, compared with pembrolizumab, was “broadly consistent” in all prespecified subgroups, Dr. Zhou noted. That included patients with squamous NSCLC (HR, 0.48) and nonsquamous NSCLC (HR, 0.54), those with PD-L1 expression of 1%-49% (HR, 0.54) and 50% or higher (HR, 0.46), as well as those with liver metastases (HR, 0.47) and brain metastases (HR, 0.55).
The PFS benefit seen with ivonescimab in HARMONi-2 is “striking,” and the results “highlight the potential benefits of combined VEGF and PD-1 blockade together,” said John Heymach, MD, with the University of Texas MD Anderson Cancer Center in Houston, who served as discussant for the study.
Ivonescimab also led to a higher objective response rate (50% vs 38.5%) and disease control rate (89.9% vs 70.5%).
Grade 3 or higher treatment-related adverse events occurred in more patients receiving ivonescimab — 29.4% vs 15.6% on pembrolizumab. The difference largely stemmed from higher rates of proteinuria, hypertension, and lab abnormalities.
The rates of serious treatment-related adverse events were similar between the groups —20.8% in the ivonescimab group and 16.1% in the pembrolizumab group. Rates of grade 3 or higher immune-related adverse events were also similar, occurring in 7% of patients treated with ivonescimab and 8% of those receiving pembrolizumab.
In patients with squamous cell carcinoma, in particular, ivonescimab demonstrated a “very manageable” safety profile, Dr. Zhou noted. In this group, grade 3 or higher treatment-related adverse events occurred in 22.2% of patients (vs 18.7% receiving pembrolizumab).
Ivonescimab was associated with comparable but “numerically better” time to deterioration of global health status, based on the EORTC Core Quality of Life questionnaire, Dr. Zhou said.
Although the “really impressive and clinically meaningful” PFS benefits extended across different subgroups, “we await the overall survival results and additional studies done outside of China to confirm the benefit seen,” Dr. Heymach noted.
He also cautioned that, for patients with low to intermediate PD-L1 expression (1%-49%), pembrolizumab monotherapy “would not be the relevant comparator in the US and the rest of the world, and different study designs are going to be required for those populations.”
Based on the results of HARMONi-2, Akeso’s partner, Summit Therapeutics, plans to initiate HARMONi-7 in early 2025.
HARMONi-7 is currently planned as a multiregional, phase 3 clinical trial that will compare ivonescimab monotherapy to pembrolizumab monotherapy in patients with metastatic NSCLC whose tumors have high PD-L1 expression (50% or more).
Dr. Zhou has received consulting fees from Qilu Pharmaceutical, Hengrui, and TopAlliance Biosciences and honoraria from Eli Lilly China, Boehringer Ingelheim, Roche, Merck Sharp & Dohme, Qilu, Hengrui, Innovent Biologics, Alice, C-Stone, Luye Pharma, TopAlliance Biosciences, Amoy Diagnostics, and AnHeart Therapeutics. Dr. Heymach is a consultant for AbbVie, AnHeart Therapeutics, ArriVent Biopharma, AstraZeneca, BioCurity Pharmaceuticals, BioNTech, Blueprint Medicines, Boehringer Ingelheim, BMS, Eli Lilly, EMD Serono, Genentech, GlaxoSmithKline, Janssen Pharmaceuticals, Mirati Therapeutics, Novartis Pharmaceuticals, Regeneron Pharmaceuticals, Sanofi, Spectrum Pharmaceuticals, and Takeda.
A version of this article first appeared on Medscape.com.
according to recent findings from the HARMONi-2 trial.
“This is the first randomized, phase 3 study to demonstrate a clinically significant improvement in efficacy with a novel drug compared to pembrolizumab in advanced NSCLC,” said study investigator Caicun Zhou, MD, PhD, with Shanghai Pulmonary Hospital in China,
The results highlight ivonescimab’s potential to become a “new standard of care” in advanced PD-L1–positive advanced NSCLC, said Dr. Zhou, who presented the analysis at the annual International Association for the Study of Lung Cancer (IASLC) World Conference on Lung Cancer in San Diego. Dr. Zhou is president-elect of the IASLC.
Ivonescimab (AK112) is a novel, potentially first-in-class investigational bispecific antibody that targets PD-1 and vascular endothelial growth factor (VEGF) developed by Akeso Biopharma, which funded the HARMONi-2 trial.
Conducted at 55 centers in China, HARMONi-2 enrolled 398 patients with untreated locally advanced or metastatic NSCLC, Eastern Cooperative Oncology Group Performance Status of 0 or 1, PD-L1 positive (with at least 1% of tumor cells expressing PD-L1), and no EGFR mutations or ALK rearrangements.
Patients were randomly allocated (1:1) to receive ivonescimab (20 mg/kg) or pembrolizumab (200 mg) every 3 weeks. The two groups were well balanced, and randomization was stratified by histology (squamous vs nonsquamous), clinical stage (IIIB/IIIC vs IV) and PD-L1 expression (PD-L1 of 1%-49% vs 50% or greater).
Dr. Zhou reported that patients who received ivonescimab were progression free for nearly twice as long as those on pembrolizumab — a median of 11.1 vs 5.8 months, indicating a 49% lower risk for progression or death (stratified hazard ratio [HR], 0.51; P < .0001).
The meaningful improvement in PFS with ivonescimab, compared with pembrolizumab, was “broadly consistent” in all prespecified subgroups, Dr. Zhou noted. That included patients with squamous NSCLC (HR, 0.48) and nonsquamous NSCLC (HR, 0.54), those with PD-L1 expression of 1%-49% (HR, 0.54) and 50% or higher (HR, 0.46), as well as those with liver metastases (HR, 0.47) and brain metastases (HR, 0.55).
The PFS benefit seen with ivonescimab in HARMONi-2 is “striking,” and the results “highlight the potential benefits of combined VEGF and PD-1 blockade together,” said John Heymach, MD, with the University of Texas MD Anderson Cancer Center in Houston, who served as discussant for the study.
Ivonescimab also led to a higher objective response rate (50% vs 38.5%) and disease control rate (89.9% vs 70.5%).
Grade 3 or higher treatment-related adverse events occurred in more patients receiving ivonescimab — 29.4% vs 15.6% on pembrolizumab. The difference largely stemmed from higher rates of proteinuria, hypertension, and lab abnormalities.
The rates of serious treatment-related adverse events were similar between the groups —20.8% in the ivonescimab group and 16.1% in the pembrolizumab group. Rates of grade 3 or higher immune-related adverse events were also similar, occurring in 7% of patients treated with ivonescimab and 8% of those receiving pembrolizumab.
In patients with squamous cell carcinoma, in particular, ivonescimab demonstrated a “very manageable” safety profile, Dr. Zhou noted. In this group, grade 3 or higher treatment-related adverse events occurred in 22.2% of patients (vs 18.7% receiving pembrolizumab).
Ivonescimab was associated with comparable but “numerically better” time to deterioration of global health status, based on the EORTC Core Quality of Life questionnaire, Dr. Zhou said.
Although the “really impressive and clinically meaningful” PFS benefits extended across different subgroups, “we await the overall survival results and additional studies done outside of China to confirm the benefit seen,” Dr. Heymach noted.
He also cautioned that, for patients with low to intermediate PD-L1 expression (1%-49%), pembrolizumab monotherapy “would not be the relevant comparator in the US and the rest of the world, and different study designs are going to be required for those populations.”
Based on the results of HARMONi-2, Akeso’s partner, Summit Therapeutics, plans to initiate HARMONi-7 in early 2025.
HARMONi-7 is currently planned as a multiregional, phase 3 clinical trial that will compare ivonescimab monotherapy to pembrolizumab monotherapy in patients with metastatic NSCLC whose tumors have high PD-L1 expression (50% or more).
Dr. Zhou has received consulting fees from Qilu Pharmaceutical, Hengrui, and TopAlliance Biosciences and honoraria from Eli Lilly China, Boehringer Ingelheim, Roche, Merck Sharp & Dohme, Qilu, Hengrui, Innovent Biologics, Alice, C-Stone, Luye Pharma, TopAlliance Biosciences, Amoy Diagnostics, and AnHeart Therapeutics. Dr. Heymach is a consultant for AbbVie, AnHeart Therapeutics, ArriVent Biopharma, AstraZeneca, BioCurity Pharmaceuticals, BioNTech, Blueprint Medicines, Boehringer Ingelheim, BMS, Eli Lilly, EMD Serono, Genentech, GlaxoSmithKline, Janssen Pharmaceuticals, Mirati Therapeutics, Novartis Pharmaceuticals, Regeneron Pharmaceuticals, Sanofi, Spectrum Pharmaceuticals, and Takeda.
A version of this article first appeared on Medscape.com.
FROM WCLC 2024
Do Clonal Hematopoiesis and Mosaic Chromosomal Alterations Increase Solid Tumor Risk?
Clonal hematopoiesis of indeterminate potential (CHIP) and mosaic chromosomal alterations (mCAs) are associated with an increased risk for breast cancer, and CHIP is associated with increased mortality in patients with colon cancer, according to the authors of new research.
These findings, drawn from almost 11,000 patients in the Women’s Health Initiative (WHI) study, add further evidence that CHIP and mCA drive solid tumor risk, alongside known associations with hematologic malignancies, reported lead author Pinkal Desai, MD, associate professor of medicine and clinical director of molecular aging at Englander Institute for Precision Medicine, Weill Cornell Medical College, New York City, and colleagues.
How This Study Differs From Others of Breast Cancer Risk Factors
“The independent effect of CHIP and mCA on risk and mortality from solid tumors has not been elucidated due to lack of detailed data on mortality outcomes and risk factors,” the investigators wrote in Cancer, although some previous studies have suggested a link.
In particular, the investigators highlighted a 2022 UK Biobank study, which reported an association between CHIP and lung cancer and a borderline association with breast cancer that did not quite reach statistical significance.
But the UK Biobank study was confined to a UK population, Dr. Desai noted in an interview, and the data were less detailed than those in the present investigation.
“In terms of risk, the part that was lacking in previous studies was a comprehensive assessment of risk factors that increase risk for all these cancers,” Dr. Desai said. “For example, for breast cancer, we had very detailed data on [participants’] Gail risk score, which is known to impact breast cancer risk. We also had mammogram data and colonoscopy data.”
In an accompanying editorial, Koichi Takahashi, MD, PhD , and Nehali Shah, BS, of The University of Texas MD Anderson Cancer Center, Houston, Texas, pointed out the same UK Biobank findings, then noted that CHIP has also been linked with worse overall survival in unselected cancer patients. Still, they wrote, “the impact of CH on cancer risk and mortality remains controversial due to conflicting data and context‐dependent effects,” necessitating studies like this one by Dr. Desai and colleagues.
How Was the Relationship Between CHIP, MCA, and Solid Tumor Risk Assessed?
To explore possible associations between CHIP, mCA, and solid tumors, the investigators analyzed whole genome sequencing data from 10,866 women in the WHI, a multi-study program that began in 1992 and involved 161,808 women in both observational and clinical trial cohorts.
In 2002, the first big data release from the WHI suggested that hormone replacement therapy (HRT) increased breast cancer risk, leading to widespread reduction in HRT use.
More recent reports continue to shape our understanding of these risks, suggesting differences across cancer types. For breast cancer, the WHI data suggested that HRT-associated risk was largely driven by formulations involving progesterone and estrogen, whereas estrogen-only formulations, now more common, are generally considered to present an acceptable risk profile for suitable patients.
The new study accounted for this potential HRT-associated risk, including by adjusting for patients who received HRT, type of HRT received, and duration of HRT received. According to Desai, this approach is commonly used when analyzing data from the WHI, nullifying concerns about the potentially deleterious effects of the hormones used in the study.
“Our question was not ‘does HRT cause cancer?’ ” Dr. Desai said in an interview. “But HRT can be linked to breast cancer risk and has a potential to be a confounder, and hence the above methodology.
“So I can say that the confounding/effect modification that HRT would have contributed to in the relationship between exposure (CH and mCA) and outcome (cancer) is well adjusted for as described above. This is standard in WHI analyses,” she continued.
“Every Women’s Health Initiative analysis that comes out — not just for our study — uses a standard method ... where you account for hormonal therapy,” Dr. Desai added, again noting that many other potential risk factors were considered, enabling a “detailed, robust” analysis.
Dr. Takahashi and Ms. Shah agreed. “A notable strength of this study is its adjustment for many confounding factors,” they wrote. “The cohort’s well‐annotated data on other known cancer risk factors allowed for a robust assessment of CH’s independent risk.”
How Do Findings Compare With Those of the UK Biobank Study?
CHIP was associated with a 30% increased risk for breast cancer (hazard ratio [HR], 1.30; 95% CI, 1.03-1.64; P = .02), strengthening the borderline association reported by the UK Biobank study.
In contrast with the UK Biobank study, CHIP was not associated with lung cancer risk, although this may have been caused by fewer cases of lung cancer and a lack of male patients, Dr. Desai suggested.
“The discrepancy between the studies lies in the risk of lung cancer, although the point estimate in the current study suggested a positive association,” wrote Dr. Takahashi and Ms. Shah.
As in the UK Biobank study, CHIP was not associated with increased risk of developing colorectal cancer.
Mortality analysis, however, which was not conducted in the UK Biobank study, offered a new insight: Patients with existing colorectal cancer and CHIP had a significantly higher mortality risk than those without CHIP. Before stage adjustment, risk for mortality among those with colorectal cancer and CHIP was fourfold higher than those without CHIP (HR, 3.99; 95% CI, 2.41-6.62; P < .001). After stage adjustment, CHIP was still associated with a twofold higher mortality risk (HR, 2.50; 95% CI, 1.32-4.72; P = .004).
The investigators’ first mCA analyses, which employed a cell fraction cutoff greater than 3%, were unfruitful. But raising the cell fraction threshold to 5% in an exploratory analysis showed that autosomal mCA was associated with a 39% increased risk for breast cancer (HR, 1.39; 95% CI, 1.06-1.83; P = .01). No such associations were found between mCA and colorectal or lung cancer, regardless of cell fraction threshold.
The original 3% cell fraction threshold was selected on the basis of previous studies reporting a link between mCA and hematologic malignancies at this cutoff, Dr. Desai said.
She and her colleagues said a higher 5% cutoff might be needed, as they suspected that the link between mCA and solid tumors may not be causal, requiring a higher mutation rate.
Why Do Results Differ Between These Types of Studies?
Dr. Takahashi and Ms. Shah suggested that one possible limitation of the new study, and an obstacle to comparing results with the UK Biobank study and others like it, goes beyond population heterogeneity; incongruent findings could also be explained by differences in whole genome sequencing (WGS) technique.
“Although WGS allows sensitive detection of mCA through broad genomic coverage, it is less effective at detecting CHIP with low variant allele frequency (VAF) due to its relatively shallow depth (30x),” they wrote. “Consequently, the prevalence of mCA (18.8%) was much higher than that of CHIP (8.3%) in this cohort, contrasting with other studies using deeper sequencing.” As a result, the present study may have underestimated CHIP prevalence because of shallow sequencing depth.
“This inconsistency is a common challenge in CH population studies due to the lack of standardized methodologies and the frequent reliance on preexisting data not originally intended for CH detection,” Dr. Takahashi and Ms. Shah said.
Even so, despite the “heavily context-dependent” nature of these reported risks, the body of evidence to date now offers a convincing biological rationale linking CH with cancer development and outcomes, they added.
How Do the CHIP- and mCA-associated Risks Differ Between Solid Tumors and Blood Cancers?
“[These solid tumor risks are] not causal in the way CHIP mutations are causal for blood cancers,” Dr. Desai said. “Here we are talking about solid tumor risk, and it’s kind of scattered. It’s not just breast cancer ... there’s also increased colon cancer mortality. So I feel these mutations are doing something different ... they are sort of an added factor.”
Specific mechanisms remain unclear, Dr. Desai said, although she speculated about possible impacts on the inflammatory state or alterations to the tumor microenvironment.
“These are blood cells, right?” Dr. Desai asked. “They’re everywhere, and they’re changing something inherently in these tumors.”
Future research and therapeutic development
Siddhartha Jaiswal, MD, PhD, assistant professor in the Department of Pathology at Stanford University in California, whose lab focuses on clonal hematopoiesis, said the causality question is central to future research.
“The key question is, are these mutations acting because they alter the function of blood cells in some way to promote cancer risk, or is it reflective of some sort of shared etiology that’s not causal?” Dr. Jaiswal said in an interview.
Available data support both possibilities.
On one side, “reasonable evidence” supports the noncausal view, Dr. Jaiswal noted, because telomere length is one of the most common genetic risk factors for clonal hematopoiesis and also for solid tumors, suggesting a shared genetic factor. On the other hand, CHIP and mCA could be directly protumorigenic via conferred disturbances of immune cell function.
When asked if both causal and noncausal factors could be at play, Dr. Jaiswal said, “yeah, absolutely.”
The presence of a causal association could be promising from a therapeutic standpoint.
“If it turns out that this association is driven by a direct causal effect of the mutations, perhaps related to immune cell function or dysfunction, then targeting that dysfunction could be a therapeutic path to improve outcomes in people, and there’s a lot of interest in this,” Dr. Jaiswal said. He went on to explain how a trial exploring this approach via interleukin-8 inhibition in lung cancer fell short.
Yet earlier intervention may still hold promise, according to experts.
“[This study] provokes the hypothesis that CH‐targeted interventions could potentially reduce cancer risk in the future,” Dr. Takahashi and Ms. Shah said in their editorial.
The WHI program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The investigators disclosed relationships with Eli Lilly, AbbVie, Celgene, and others. Dr. Jaiswal reported stock equity in a company that has an interest in clonal hematopoiesis.
A version of this article first appeared on Medscape.com.
Clonal hematopoiesis of indeterminate potential (CHIP) and mosaic chromosomal alterations (mCAs) are associated with an increased risk for breast cancer, and CHIP is associated with increased mortality in patients with colon cancer, according to the authors of new research.
These findings, drawn from almost 11,000 patients in the Women’s Health Initiative (WHI) study, add further evidence that CHIP and mCA drive solid tumor risk, alongside known associations with hematologic malignancies, reported lead author Pinkal Desai, MD, associate professor of medicine and clinical director of molecular aging at Englander Institute for Precision Medicine, Weill Cornell Medical College, New York City, and colleagues.
How This Study Differs From Others of Breast Cancer Risk Factors
“The independent effect of CHIP and mCA on risk and mortality from solid tumors has not been elucidated due to lack of detailed data on mortality outcomes and risk factors,” the investigators wrote in Cancer, although some previous studies have suggested a link.
In particular, the investigators highlighted a 2022 UK Biobank study, which reported an association between CHIP and lung cancer and a borderline association with breast cancer that did not quite reach statistical significance.
But the UK Biobank study was confined to a UK population, Dr. Desai noted in an interview, and the data were less detailed than those in the present investigation.
“In terms of risk, the part that was lacking in previous studies was a comprehensive assessment of risk factors that increase risk for all these cancers,” Dr. Desai said. “For example, for breast cancer, we had very detailed data on [participants’] Gail risk score, which is known to impact breast cancer risk. We also had mammogram data and colonoscopy data.”
In an accompanying editorial, Koichi Takahashi, MD, PhD , and Nehali Shah, BS, of The University of Texas MD Anderson Cancer Center, Houston, Texas, pointed out the same UK Biobank findings, then noted that CHIP has also been linked with worse overall survival in unselected cancer patients. Still, they wrote, “the impact of CH on cancer risk and mortality remains controversial due to conflicting data and context‐dependent effects,” necessitating studies like this one by Dr. Desai and colleagues.
How Was the Relationship Between CHIP, MCA, and Solid Tumor Risk Assessed?
To explore possible associations between CHIP, mCA, and solid tumors, the investigators analyzed whole genome sequencing data from 10,866 women in the WHI, a multi-study program that began in 1992 and involved 161,808 women in both observational and clinical trial cohorts.
In 2002, the first big data release from the WHI suggested that hormone replacement therapy (HRT) increased breast cancer risk, leading to widespread reduction in HRT use.
More recent reports continue to shape our understanding of these risks, suggesting differences across cancer types. For breast cancer, the WHI data suggested that HRT-associated risk was largely driven by formulations involving progesterone and estrogen, whereas estrogen-only formulations, now more common, are generally considered to present an acceptable risk profile for suitable patients.
The new study accounted for this potential HRT-associated risk, including by adjusting for patients who received HRT, type of HRT received, and duration of HRT received. According to Desai, this approach is commonly used when analyzing data from the WHI, nullifying concerns about the potentially deleterious effects of the hormones used in the study.
“Our question was not ‘does HRT cause cancer?’ ” Dr. Desai said in an interview. “But HRT can be linked to breast cancer risk and has a potential to be a confounder, and hence the above methodology.
“So I can say that the confounding/effect modification that HRT would have contributed to in the relationship between exposure (CH and mCA) and outcome (cancer) is well adjusted for as described above. This is standard in WHI analyses,” she continued.
“Every Women’s Health Initiative analysis that comes out — not just for our study — uses a standard method ... where you account for hormonal therapy,” Dr. Desai added, again noting that many other potential risk factors were considered, enabling a “detailed, robust” analysis.
Dr. Takahashi and Ms. Shah agreed. “A notable strength of this study is its adjustment for many confounding factors,” they wrote. “The cohort’s well‐annotated data on other known cancer risk factors allowed for a robust assessment of CH’s independent risk.”
How Do Findings Compare With Those of the UK Biobank Study?
CHIP was associated with a 30% increased risk for breast cancer (hazard ratio [HR], 1.30; 95% CI, 1.03-1.64; P = .02), strengthening the borderline association reported by the UK Biobank study.
In contrast with the UK Biobank study, CHIP was not associated with lung cancer risk, although this may have been caused by fewer cases of lung cancer and a lack of male patients, Dr. Desai suggested.
“The discrepancy between the studies lies in the risk of lung cancer, although the point estimate in the current study suggested a positive association,” wrote Dr. Takahashi and Ms. Shah.
As in the UK Biobank study, CHIP was not associated with increased risk of developing colorectal cancer.
Mortality analysis, however, which was not conducted in the UK Biobank study, offered a new insight: Patients with existing colorectal cancer and CHIP had a significantly higher mortality risk than those without CHIP. Before stage adjustment, risk for mortality among those with colorectal cancer and CHIP was fourfold higher than those without CHIP (HR, 3.99; 95% CI, 2.41-6.62; P < .001). After stage adjustment, CHIP was still associated with a twofold higher mortality risk (HR, 2.50; 95% CI, 1.32-4.72; P = .004).
The investigators’ first mCA analyses, which employed a cell fraction cutoff greater than 3%, were unfruitful. But raising the cell fraction threshold to 5% in an exploratory analysis showed that autosomal mCA was associated with a 39% increased risk for breast cancer (HR, 1.39; 95% CI, 1.06-1.83; P = .01). No such associations were found between mCA and colorectal or lung cancer, regardless of cell fraction threshold.
The original 3% cell fraction threshold was selected on the basis of previous studies reporting a link between mCA and hematologic malignancies at this cutoff, Dr. Desai said.
She and her colleagues said a higher 5% cutoff might be needed, as they suspected that the link between mCA and solid tumors may not be causal, requiring a higher mutation rate.
Why Do Results Differ Between These Types of Studies?
Dr. Takahashi and Ms. Shah suggested that one possible limitation of the new study, and an obstacle to comparing results with the UK Biobank study and others like it, goes beyond population heterogeneity; incongruent findings could also be explained by differences in whole genome sequencing (WGS) technique.
“Although WGS allows sensitive detection of mCA through broad genomic coverage, it is less effective at detecting CHIP with low variant allele frequency (VAF) due to its relatively shallow depth (30x),” they wrote. “Consequently, the prevalence of mCA (18.8%) was much higher than that of CHIP (8.3%) in this cohort, contrasting with other studies using deeper sequencing.” As a result, the present study may have underestimated CHIP prevalence because of shallow sequencing depth.
“This inconsistency is a common challenge in CH population studies due to the lack of standardized methodologies and the frequent reliance on preexisting data not originally intended for CH detection,” Dr. Takahashi and Ms. Shah said.
Even so, despite the “heavily context-dependent” nature of these reported risks, the body of evidence to date now offers a convincing biological rationale linking CH with cancer development and outcomes, they added.
How Do the CHIP- and mCA-associated Risks Differ Between Solid Tumors and Blood Cancers?
“[These solid tumor risks are] not causal in the way CHIP mutations are causal for blood cancers,” Dr. Desai said. “Here we are talking about solid tumor risk, and it’s kind of scattered. It’s not just breast cancer ... there’s also increased colon cancer mortality. So I feel these mutations are doing something different ... they are sort of an added factor.”
Specific mechanisms remain unclear, Dr. Desai said, although she speculated about possible impacts on the inflammatory state or alterations to the tumor microenvironment.
“These are blood cells, right?” Dr. Desai asked. “They’re everywhere, and they’re changing something inherently in these tumors.”
Future research and therapeutic development
Siddhartha Jaiswal, MD, PhD, assistant professor in the Department of Pathology at Stanford University in California, whose lab focuses on clonal hematopoiesis, said the causality question is central to future research.
“The key question is, are these mutations acting because they alter the function of blood cells in some way to promote cancer risk, or is it reflective of some sort of shared etiology that’s not causal?” Dr. Jaiswal said in an interview.
Available data support both possibilities.
On one side, “reasonable evidence” supports the noncausal view, Dr. Jaiswal noted, because telomere length is one of the most common genetic risk factors for clonal hematopoiesis and also for solid tumors, suggesting a shared genetic factor. On the other hand, CHIP and mCA could be directly protumorigenic via conferred disturbances of immune cell function.
When asked if both causal and noncausal factors could be at play, Dr. Jaiswal said, “yeah, absolutely.”
The presence of a causal association could be promising from a therapeutic standpoint.
“If it turns out that this association is driven by a direct causal effect of the mutations, perhaps related to immune cell function or dysfunction, then targeting that dysfunction could be a therapeutic path to improve outcomes in people, and there’s a lot of interest in this,” Dr. Jaiswal said. He went on to explain how a trial exploring this approach via interleukin-8 inhibition in lung cancer fell short.
Yet earlier intervention may still hold promise, according to experts.
“[This study] provokes the hypothesis that CH‐targeted interventions could potentially reduce cancer risk in the future,” Dr. Takahashi and Ms. Shah said in their editorial.
The WHI program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The investigators disclosed relationships with Eli Lilly, AbbVie, Celgene, and others. Dr. Jaiswal reported stock equity in a company that has an interest in clonal hematopoiesis.
A version of this article first appeared on Medscape.com.
Clonal hematopoiesis of indeterminate potential (CHIP) and mosaic chromosomal alterations (mCAs) are associated with an increased risk for breast cancer, and CHIP is associated with increased mortality in patients with colon cancer, according to the authors of new research.
These findings, drawn from almost 11,000 patients in the Women’s Health Initiative (WHI) study, add further evidence that CHIP and mCA drive solid tumor risk, alongside known associations with hematologic malignancies, reported lead author Pinkal Desai, MD, associate professor of medicine and clinical director of molecular aging at Englander Institute for Precision Medicine, Weill Cornell Medical College, New York City, and colleagues.
How This Study Differs From Others of Breast Cancer Risk Factors
“The independent effect of CHIP and mCA on risk and mortality from solid tumors has not been elucidated due to lack of detailed data on mortality outcomes and risk factors,” the investigators wrote in Cancer, although some previous studies have suggested a link.
In particular, the investigators highlighted a 2022 UK Biobank study, which reported an association between CHIP and lung cancer and a borderline association with breast cancer that did not quite reach statistical significance.
But the UK Biobank study was confined to a UK population, Dr. Desai noted in an interview, and the data were less detailed than those in the present investigation.
“In terms of risk, the part that was lacking in previous studies was a comprehensive assessment of risk factors that increase risk for all these cancers,” Dr. Desai said. “For example, for breast cancer, we had very detailed data on [participants’] Gail risk score, which is known to impact breast cancer risk. We also had mammogram data and colonoscopy data.”
In an accompanying editorial, Koichi Takahashi, MD, PhD , and Nehali Shah, BS, of The University of Texas MD Anderson Cancer Center, Houston, Texas, pointed out the same UK Biobank findings, then noted that CHIP has also been linked with worse overall survival in unselected cancer patients. Still, they wrote, “the impact of CH on cancer risk and mortality remains controversial due to conflicting data and context‐dependent effects,” necessitating studies like this one by Dr. Desai and colleagues.
How Was the Relationship Between CHIP, MCA, and Solid Tumor Risk Assessed?
To explore possible associations between CHIP, mCA, and solid tumors, the investigators analyzed whole genome sequencing data from 10,866 women in the WHI, a multi-study program that began in 1992 and involved 161,808 women in both observational and clinical trial cohorts.
In 2002, the first big data release from the WHI suggested that hormone replacement therapy (HRT) increased breast cancer risk, leading to widespread reduction in HRT use.
More recent reports continue to shape our understanding of these risks, suggesting differences across cancer types. For breast cancer, the WHI data suggested that HRT-associated risk was largely driven by formulations involving progesterone and estrogen, whereas estrogen-only formulations, now more common, are generally considered to present an acceptable risk profile for suitable patients.
The new study accounted for this potential HRT-associated risk, including by adjusting for patients who received HRT, type of HRT received, and duration of HRT received. According to Desai, this approach is commonly used when analyzing data from the WHI, nullifying concerns about the potentially deleterious effects of the hormones used in the study.
“Our question was not ‘does HRT cause cancer?’ ” Dr. Desai said in an interview. “But HRT can be linked to breast cancer risk and has a potential to be a confounder, and hence the above methodology.
“So I can say that the confounding/effect modification that HRT would have contributed to in the relationship between exposure (CH and mCA) and outcome (cancer) is well adjusted for as described above. This is standard in WHI analyses,” she continued.
“Every Women’s Health Initiative analysis that comes out — not just for our study — uses a standard method ... where you account for hormonal therapy,” Dr. Desai added, again noting that many other potential risk factors were considered, enabling a “detailed, robust” analysis.
Dr. Takahashi and Ms. Shah agreed. “A notable strength of this study is its adjustment for many confounding factors,” they wrote. “The cohort’s well‐annotated data on other known cancer risk factors allowed for a robust assessment of CH’s independent risk.”
How Do Findings Compare With Those of the UK Biobank Study?
CHIP was associated with a 30% increased risk for breast cancer (hazard ratio [HR], 1.30; 95% CI, 1.03-1.64; P = .02), strengthening the borderline association reported by the UK Biobank study.
In contrast with the UK Biobank study, CHIP was not associated with lung cancer risk, although this may have been caused by fewer cases of lung cancer and a lack of male patients, Dr. Desai suggested.
“The discrepancy between the studies lies in the risk of lung cancer, although the point estimate in the current study suggested a positive association,” wrote Dr. Takahashi and Ms. Shah.
As in the UK Biobank study, CHIP was not associated with increased risk of developing colorectal cancer.
Mortality analysis, however, which was not conducted in the UK Biobank study, offered a new insight: Patients with existing colorectal cancer and CHIP had a significantly higher mortality risk than those without CHIP. Before stage adjustment, risk for mortality among those with colorectal cancer and CHIP was fourfold higher than those without CHIP (HR, 3.99; 95% CI, 2.41-6.62; P < .001). After stage adjustment, CHIP was still associated with a twofold higher mortality risk (HR, 2.50; 95% CI, 1.32-4.72; P = .004).
The investigators’ first mCA analyses, which employed a cell fraction cutoff greater than 3%, were unfruitful. But raising the cell fraction threshold to 5% in an exploratory analysis showed that autosomal mCA was associated with a 39% increased risk for breast cancer (HR, 1.39; 95% CI, 1.06-1.83; P = .01). No such associations were found between mCA and colorectal or lung cancer, regardless of cell fraction threshold.
The original 3% cell fraction threshold was selected on the basis of previous studies reporting a link between mCA and hematologic malignancies at this cutoff, Dr. Desai said.
She and her colleagues said a higher 5% cutoff might be needed, as they suspected that the link between mCA and solid tumors may not be causal, requiring a higher mutation rate.
Why Do Results Differ Between These Types of Studies?
Dr. Takahashi and Ms. Shah suggested that one possible limitation of the new study, and an obstacle to comparing results with the UK Biobank study and others like it, goes beyond population heterogeneity; incongruent findings could also be explained by differences in whole genome sequencing (WGS) technique.
“Although WGS allows sensitive detection of mCA through broad genomic coverage, it is less effective at detecting CHIP with low variant allele frequency (VAF) due to its relatively shallow depth (30x),” they wrote. “Consequently, the prevalence of mCA (18.8%) was much higher than that of CHIP (8.3%) in this cohort, contrasting with other studies using deeper sequencing.” As a result, the present study may have underestimated CHIP prevalence because of shallow sequencing depth.
“This inconsistency is a common challenge in CH population studies due to the lack of standardized methodologies and the frequent reliance on preexisting data not originally intended for CH detection,” Dr. Takahashi and Ms. Shah said.
Even so, despite the “heavily context-dependent” nature of these reported risks, the body of evidence to date now offers a convincing biological rationale linking CH with cancer development and outcomes, they added.
How Do the CHIP- and mCA-associated Risks Differ Between Solid Tumors and Blood Cancers?
“[These solid tumor risks are] not causal in the way CHIP mutations are causal for blood cancers,” Dr. Desai said. “Here we are talking about solid tumor risk, and it’s kind of scattered. It’s not just breast cancer ... there’s also increased colon cancer mortality. So I feel these mutations are doing something different ... they are sort of an added factor.”
Specific mechanisms remain unclear, Dr. Desai said, although she speculated about possible impacts on the inflammatory state or alterations to the tumor microenvironment.
“These are blood cells, right?” Dr. Desai asked. “They’re everywhere, and they’re changing something inherently in these tumors.”
Future research and therapeutic development
Siddhartha Jaiswal, MD, PhD, assistant professor in the Department of Pathology at Stanford University in California, whose lab focuses on clonal hematopoiesis, said the causality question is central to future research.
“The key question is, are these mutations acting because they alter the function of blood cells in some way to promote cancer risk, or is it reflective of some sort of shared etiology that’s not causal?” Dr. Jaiswal said in an interview.
Available data support both possibilities.
On one side, “reasonable evidence” supports the noncausal view, Dr. Jaiswal noted, because telomere length is one of the most common genetic risk factors for clonal hematopoiesis and also for solid tumors, suggesting a shared genetic factor. On the other hand, CHIP and mCA could be directly protumorigenic via conferred disturbances of immune cell function.
When asked if both causal and noncausal factors could be at play, Dr. Jaiswal said, “yeah, absolutely.”
The presence of a causal association could be promising from a therapeutic standpoint.
“If it turns out that this association is driven by a direct causal effect of the mutations, perhaps related to immune cell function or dysfunction, then targeting that dysfunction could be a therapeutic path to improve outcomes in people, and there’s a lot of interest in this,” Dr. Jaiswal said. He went on to explain how a trial exploring this approach via interleukin-8 inhibition in lung cancer fell short.
Yet earlier intervention may still hold promise, according to experts.
“[This study] provokes the hypothesis that CH‐targeted interventions could potentially reduce cancer risk in the future,” Dr. Takahashi and Ms. Shah said in their editorial.
The WHI program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The investigators disclosed relationships with Eli Lilly, AbbVie, Celgene, and others. Dr. Jaiswal reported stock equity in a company that has an interest in clonal hematopoiesis.
A version of this article first appeared on Medscape.com.
FROM CANCER
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
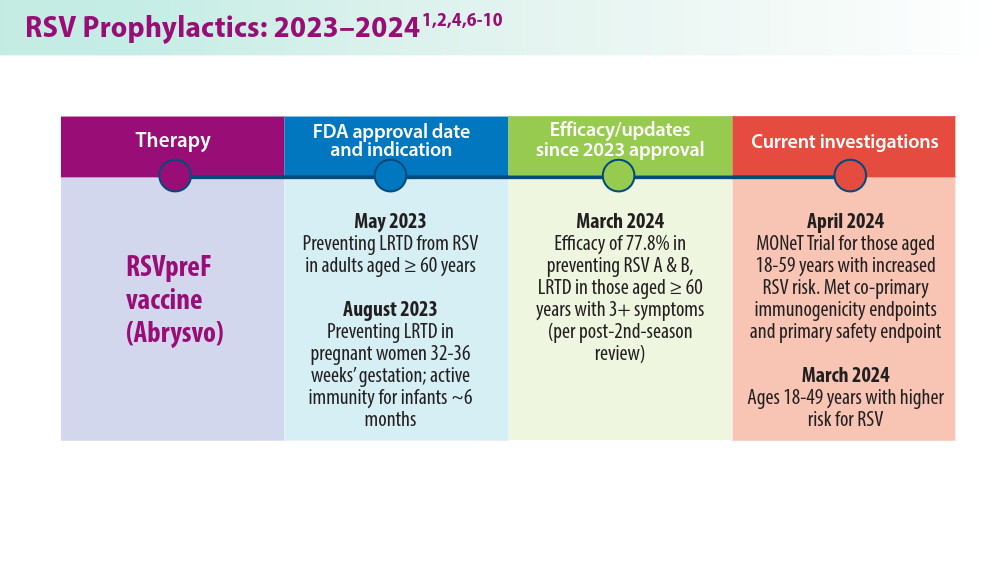
1. Pfizer announces positive top-line results from phase 3 study of ABRYSVO® in adults aged 18 to 59 at increased risk for RSV disease. Press release. Pfizer; April 9, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-results-phase-3-study-1
2. Pfizer announces positive top-line data for full season two efficacy of ABRYSVO® for RSV in older adults. Press release. Pfizer; February 29, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two
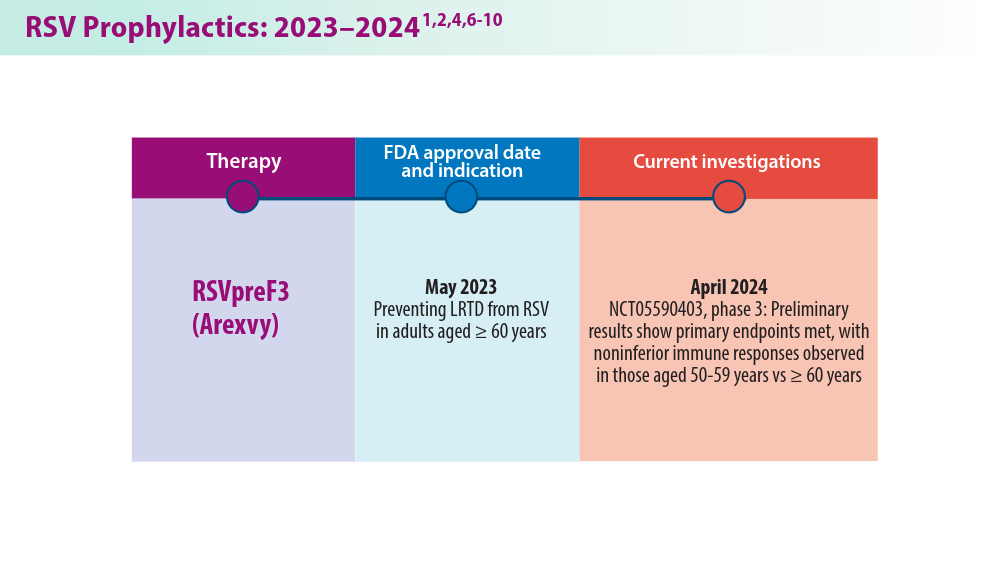
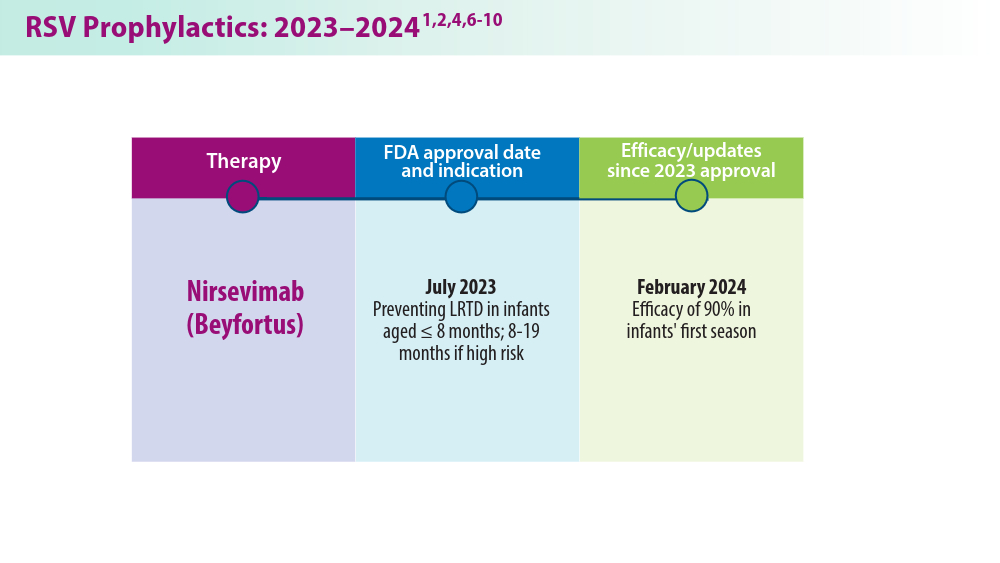
3. CDC study shows effectiveness of RSV immunization for infants. Press release. US Centers for Disease Control and Prevention; March 7, 2024. Accessed May 22, 2024. https://www.cdc.gov/media/releases/2024/s0307-rsv-immunization.html
4. Moline HL, Tannis A, Toepfer AP, et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus–associated hospitalization among infants entering their first respiratory syncytial virus season — new vaccine surveillance network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73(9):209-214. doi:10.15585/mmwr.mm7309a4
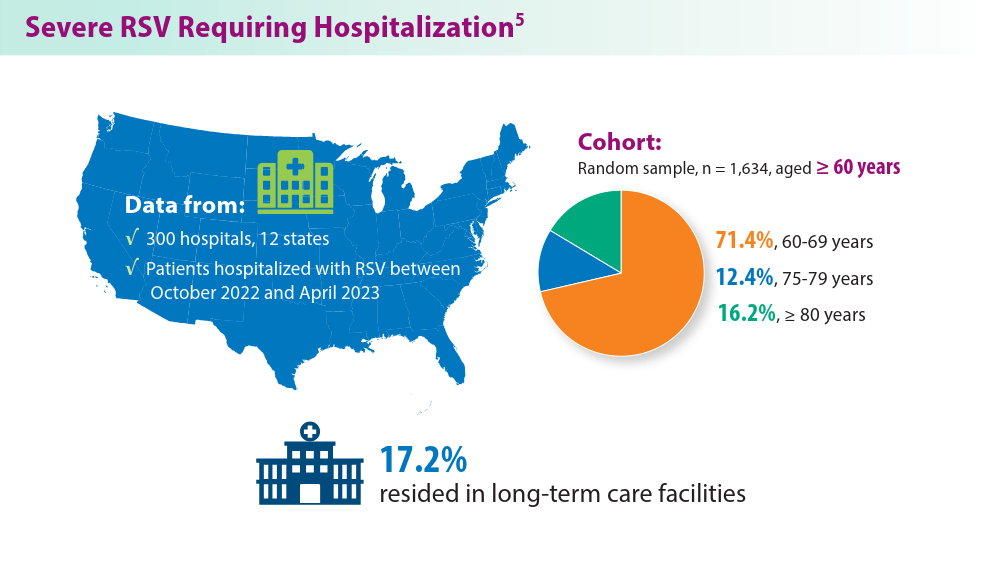
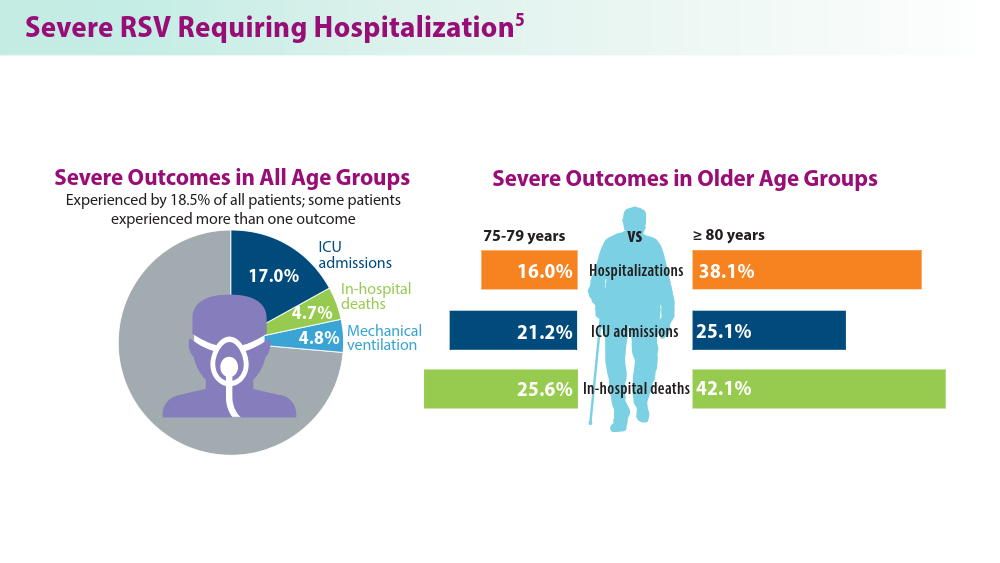
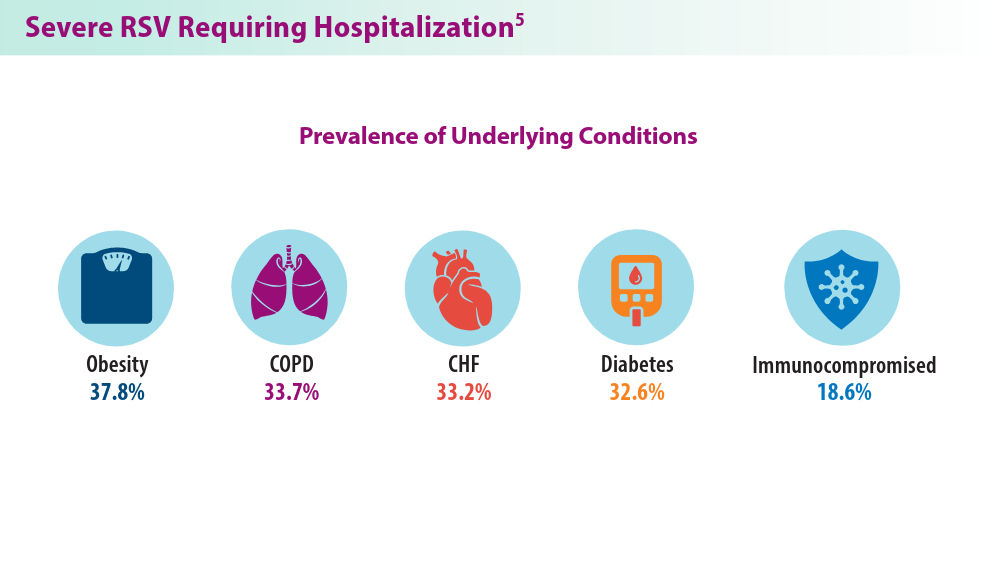
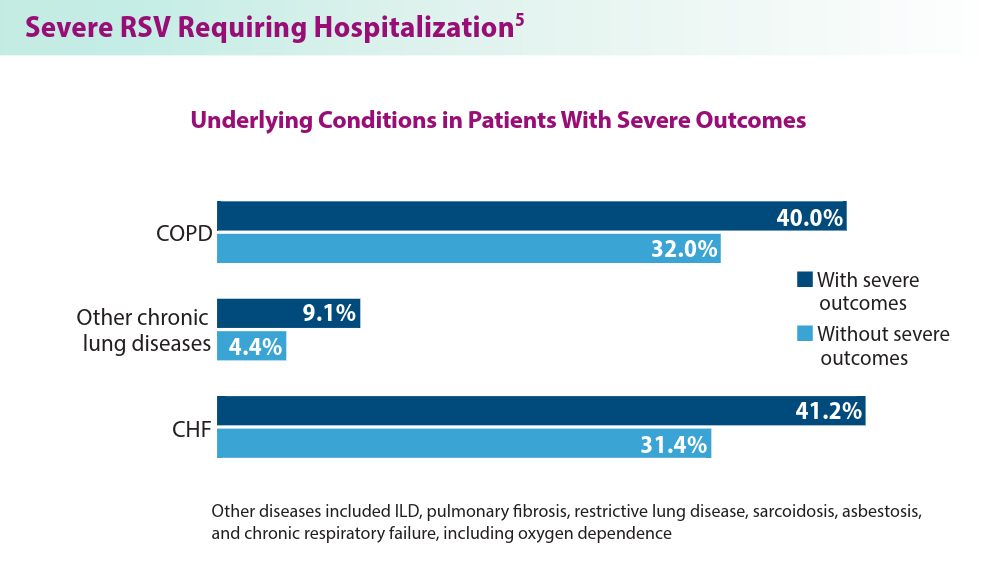
5. Havers FP, Whitaker M, Melgar M, et al; for the RSV-NET Surveillance Team. Characteristics and outcomes among adults aged ≥60 years hospitalized with laboratory-confirmed respiratory syncytial virus ─ RSV-NET, 12 states, July 2022–June 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1075-1082. doi:10.15585/mmwr.mm7240a1
6. Walsh EE, Pérez Marc G, Zareba AM, et al; for the RENOIR Clinical Trial Group. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
7. Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(41):1115-1122. doi:10.15585/mmwr.mm7241e1
8. Baker J, Aliabadi N, Munjal I, et al. Equivalent immunogenicity across three RSVpreF vaccine lots in healthy adults 18-49 years of age: results of a randomized phase 3 study. Vaccine. 2024;42(13):3172-3179. doi:10.1016/j.vaccine.2024.03.070
9. New data for AREXVY, GSK’s RSV vaccine, show potential to help protect adults aged 50 to 59 at increased risk for RSV disease. Press release. GSK; October 25, 2023. Accessed May 22, 2024. https://us.gsk.com/en-us/media/press-releases/new-data-for-arexvy/
1. Pfizer announces positive top-line results from phase 3 study of ABRYSVO® in adults aged 18 to 59 at increased risk for RSV disease. Press release. Pfizer; April 9, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-results-phase-3-study-1
2. Pfizer announces positive top-line data for full season two efficacy of ABRYSVO® for RSV in older adults. Press release. Pfizer; February 29, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two
3. CDC study shows effectiveness of RSV immunization for infants. Press release. US Centers for Disease Control and Prevention; March 7, 2024. Accessed May 22, 2024. https://www.cdc.gov/media/releases/2024/s0307-rsv-immunization.html
4. Moline HL, Tannis A, Toepfer AP, et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus–associated hospitalization among infants entering their first respiratory syncytial virus season — new vaccine surveillance network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73(9):209-214. doi:10.15585/mmwr.mm7309a4
5. Havers FP, Whitaker M, Melgar M, et al; for the RSV-NET Surveillance Team. Characteristics and outcomes among adults aged ≥60 years hospitalized with laboratory-confirmed respiratory syncytial virus ─ RSV-NET, 12 states, July 2022–June 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1075-1082. doi:10.15585/mmwr.mm7240a1
6. Walsh EE, Pérez Marc G, Zareba AM, et al; for the RENOIR Clinical Trial Group. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
7. Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(41):1115-1122. doi:10.15585/mmwr.mm7241e1
8. Baker J, Aliabadi N, Munjal I, et al. Equivalent immunogenicity across three RSVpreF vaccine lots in healthy adults 18-49 years of age: results of a randomized phase 3 study. Vaccine. 2024;42(13):3172-3179. doi:10.1016/j.vaccine.2024.03.070
9. New data for AREXVY, GSK’s RSV vaccine, show potential to help protect adults aged 50 to 59 at increased risk for RSV disease. Press release. GSK; October 25, 2023. Accessed May 22, 2024. https://us.gsk.com/en-us/media/press-releases/new-data-for-arexvy/
1. Pfizer announces positive top-line results from phase 3 study of ABRYSVO® in adults aged 18 to 59 at increased risk for RSV disease. Press release. Pfizer; April 9, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-results-phase-3-study-1
2. Pfizer announces positive top-line data for full season two efficacy of ABRYSVO® for RSV in older adults. Press release. Pfizer; February 29, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two
3. CDC study shows effectiveness of RSV immunization for infants. Press release. US Centers for Disease Control and Prevention; March 7, 2024. Accessed May 22, 2024. https://www.cdc.gov/media/releases/2024/s0307-rsv-immunization.html
4. Moline HL, Tannis A, Toepfer AP, et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus–associated hospitalization among infants entering their first respiratory syncytial virus season — new vaccine surveillance network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73(9):209-214. doi:10.15585/mmwr.mm7309a4
5. Havers FP, Whitaker M, Melgar M, et al; for the RSV-NET Surveillance Team. Characteristics and outcomes among adults aged ≥60 years hospitalized with laboratory-confirmed respiratory syncytial virus ─ RSV-NET, 12 states, July 2022–June 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1075-1082. doi:10.15585/mmwr.mm7240a1
6. Walsh EE, Pérez Marc G, Zareba AM, et al; for the RENOIR Clinical Trial Group. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
7. Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(41):1115-1122. doi:10.15585/mmwr.mm7241e1
8. Baker J, Aliabadi N, Munjal I, et al. Equivalent immunogenicity across three RSVpreF vaccine lots in healthy adults 18-49 years of age: results of a randomized phase 3 study. Vaccine. 2024;42(13):3172-3179. doi:10.1016/j.vaccine.2024.03.070
9. New data for AREXVY, GSK’s RSV vaccine, show potential to help protect adults aged 50 to 59 at increased risk for RSV disease. Press release. GSK; October 25, 2023. Accessed May 22, 2024. https://us.gsk.com/en-us/media/press-releases/new-data-for-arexvy/
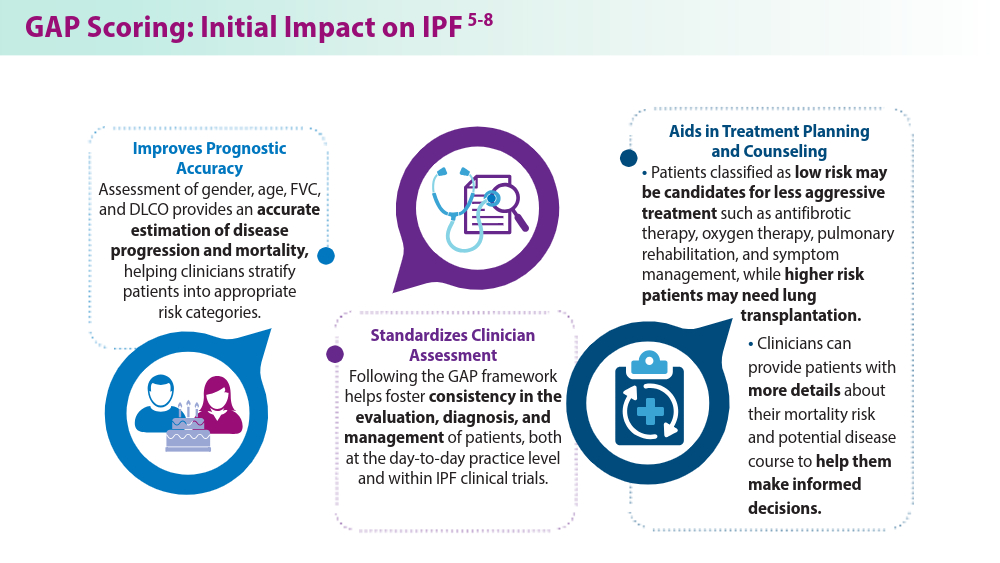
Closing the GAP in Idiopathic Pulmonary Fibrosis
5 things you should know about IPF. American Lung Association. April 12, 2023. Accessed June 21, 2024. https://www.lung.org/blog/idiopathic-pulmonary-fibrosis-things-to-know
Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566-572. doi:10.1016/S2213-2600(14)70101-8
Morrow T. Improving outcomes and managing costs in idiopathic pulmonary fibrosis. Am J Manag Care. 2019;25(11 suppl):S204-S209. PMID: 31419090
Man RK, Gogikar A, Nanda A, et al. A comparison of the effectiveness of nintedanib and pirfenidone in treating idiopathic pulmonary fibrosis: a systematic review. Cureus. 2024;16(2):e54268. doi:10.7759/cureus.54268
Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684-691. doi:10.7326/0003-4819-156-10-201205150-00004
Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44-e68. doi:10.1164/rccm.201807-1255ST
Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med. 2016;194(3):265-275. doi:10.1164/rccm.201604-0801CI
Abuserewa ST, Duff R, Becker G. Treatment of idiopathic pulmonary fibrosis. Cureus. 2021;13(5):e15360. doi:10.7759/cureus.15360
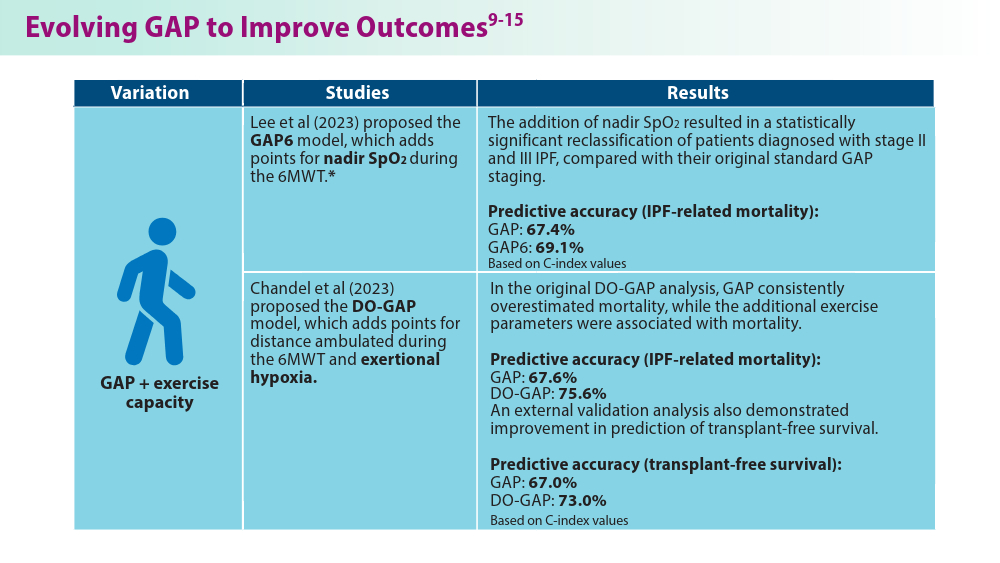
Lee JH, Jang JH, Jang HJ, et al. New prognostic scoring system for mortality in idiopathic pulmonary fibrosis by modifying the gender, age, and physiology model with desaturation during the six-minute walk test. Front Med (Lausanne). 2023;10:1052129. doi:10.3389/fmed.2023.1052129
Chandel A, Pastre J, Valery S, King CS, Nathan SD. Derivation and validation of a simple multidimensional index incorporating exercise capacity parameters for survival prediction in idiopathic pulmonary fibrosis. Thorax. 2023;78(4):368-375. doi:10.1136/thoraxjnl-2021-218440
Chandel A, King CS, Ignacio RV, et al. External validation and longitudinal application of the DO-GAP index to individualise survival prediction in idiopathic pulmonary fibrosis. ERJ Open Res. 2023;9(3):00124-2023. doi:10.1183/23120541.00124-2023
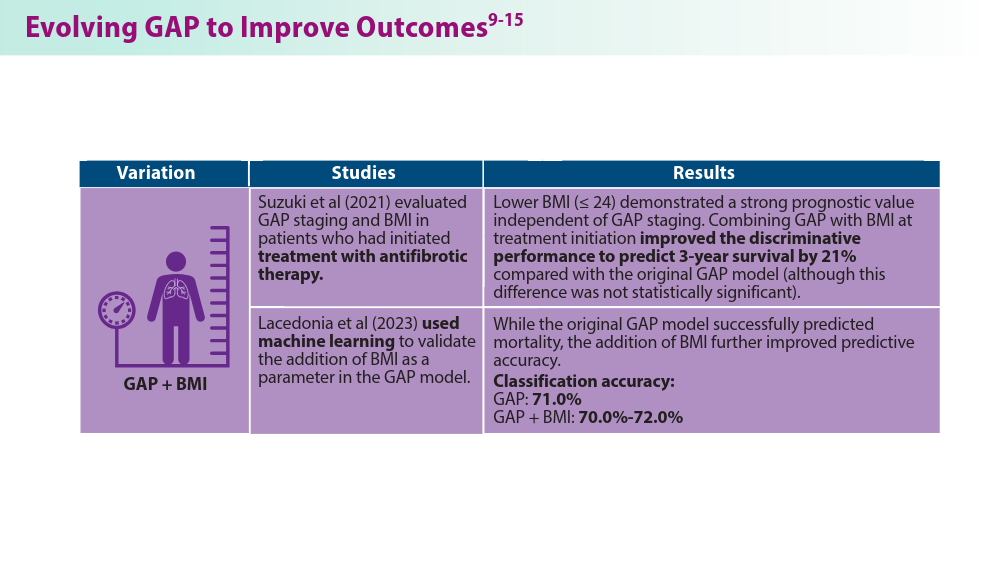
Suzuki Y, Mori K, Aono Y, et al. Combined assessment of the GAP index and body mass index at antifibrotic therapy initiation for prognosis of idiopathic pulmonary fibrosis. Sci Rep. 2021;11(1):18579. doi:10.1038/s41598-021-98161-y
Lacedonia D, De Pace CC, Rea G, et al. Machine learning and BMI improve the prognostic value of GAP index in treated IPF patients. Bioengineering (Basel). 2023;10(2):251. doi:10.3390/bioengineering10020251
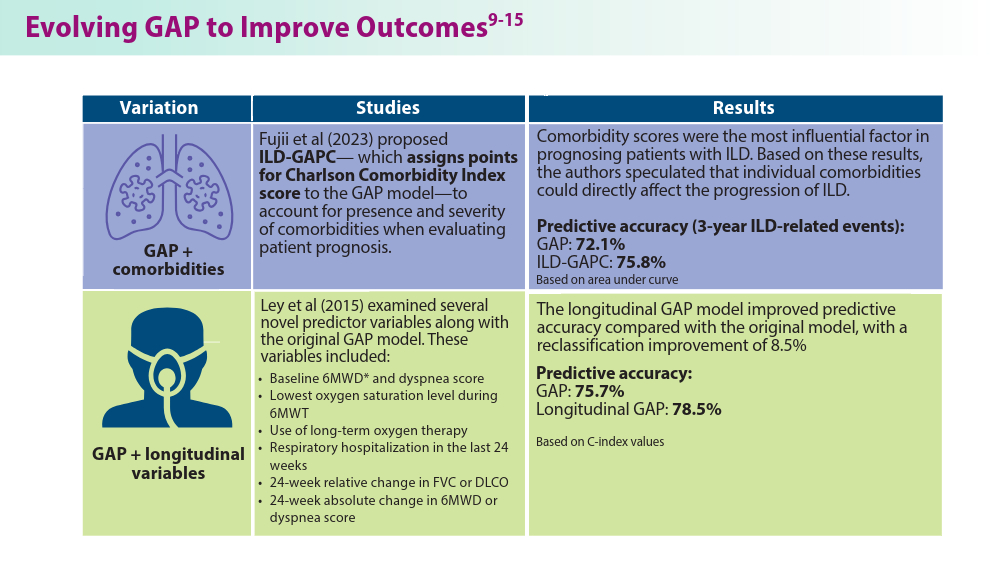
Fujii H, Hara Y, Saigusa Y, et al. ILD-GAP combined with the Charlson Comorbidity Index score (ILD-GAPC) as a prognostic prediction model in patients with interstitial lung disease. Can Respir J. 2023;2023:5088207. doi:10.1155/2023/5088207
Ley B, Bradford WZ, Weycker D, Vittinghoff E, du Bois RM, Collard HR. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1374-1381. doi:10.1183/09031936.00146314
Kreuter M, Lee JS, Tzouvelekis A, et al. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2021;204(1):74-81. doi:10.1164/rccm.202003-0669OC
Kreuter M, Lee JS, Tzouvelekis A, et al. A modified blood cell GAP (cGAP) to prognosticate outcomes in IPF. Poster presented at: European Respiratory Society International Congress; September 4-6, 2022. https://medically.gene.com/global/en/unrestricted/respiratory/ERS-2022/ers-2022-poster-kreuter-a-modified-blood-cell-gap.html
Nishikiori H, Chiba H, Lee SH, et al. A modified GAP model for East-Asian populations with idiopathic pulmonary fibrosis. Respir Investig. 2020;58(5):395-402. doi:10.1016/j.resinv.2020.04.001
5 things you should know about IPF. American Lung Association. April 12, 2023. Accessed June 21, 2024. https://www.lung.org/blog/idiopathic-pulmonary-fibrosis-things-to-know
Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566-572. doi:10.1016/S2213-2600(14)70101-8
Morrow T. Improving outcomes and managing costs in idiopathic pulmonary fibrosis. Am J Manag Care. 2019;25(11 suppl):S204-S209. PMID: 31419090
Man RK, Gogikar A, Nanda A, et al. A comparison of the effectiveness of nintedanib and pirfenidone in treating idiopathic pulmonary fibrosis: a systematic review. Cureus. 2024;16(2):e54268. doi:10.7759/cureus.54268
Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684-691. doi:10.7326/0003-4819-156-10-201205150-00004
Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44-e68. doi:10.1164/rccm.201807-1255ST
Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med. 2016;194(3):265-275. doi:10.1164/rccm.201604-0801CI
Abuserewa ST, Duff R, Becker G. Treatment of idiopathic pulmonary fibrosis. Cureus. 2021;13(5):e15360. doi:10.7759/cureus.15360
Lee JH, Jang JH, Jang HJ, et al. New prognostic scoring system for mortality in idiopathic pulmonary fibrosis by modifying the gender, age, and physiology model with desaturation during the six-minute walk test. Front Med (Lausanne). 2023;10:1052129. doi:10.3389/fmed.2023.1052129
Chandel A, Pastre J, Valery S, King CS, Nathan SD. Derivation and validation of a simple multidimensional index incorporating exercise capacity parameters for survival prediction in idiopathic pulmonary fibrosis. Thorax. 2023;78(4):368-375. doi:10.1136/thoraxjnl-2021-218440
Chandel A, King CS, Ignacio RV, et al. External validation and longitudinal application of the DO-GAP index to individualise survival prediction in idiopathic pulmonary fibrosis. ERJ Open Res. 2023;9(3):00124-2023. doi:10.1183/23120541.00124-2023
Suzuki Y, Mori K, Aono Y, et al. Combined assessment of the GAP index and body mass index at antifibrotic therapy initiation for prognosis of idiopathic pulmonary fibrosis. Sci Rep. 2021;11(1):18579. doi:10.1038/s41598-021-98161-y
Lacedonia D, De Pace CC, Rea G, et al. Machine learning and BMI improve the prognostic value of GAP index in treated IPF patients. Bioengineering (Basel). 2023;10(2):251. doi:10.3390/bioengineering10020251
Fujii H, Hara Y, Saigusa Y, et al. ILD-GAP combined with the Charlson Comorbidity Index score (ILD-GAPC) as a prognostic prediction model in patients with interstitial lung disease. Can Respir J. 2023;2023:5088207. doi:10.1155/2023/5088207
Ley B, Bradford WZ, Weycker D, Vittinghoff E, du Bois RM, Collard HR. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1374-1381. doi:10.1183/09031936.00146314
Kreuter M, Lee JS, Tzouvelekis A, et al. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2021;204(1):74-81. doi:10.1164/rccm.202003-0669OC
Kreuter M, Lee JS, Tzouvelekis A, et al. A modified blood cell GAP (cGAP) to prognosticate outcomes in IPF. Poster presented at: European Respiratory Society International Congress; September 4-6, 2022. https://medically.gene.com/global/en/unrestricted/respiratory/ERS-2022/ers-2022-poster-kreuter-a-modified-blood-cell-gap.html
Nishikiori H, Chiba H, Lee SH, et al. A modified GAP model for East-Asian populations with idiopathic pulmonary fibrosis. Respir Investig. 2020;58(5):395-402. doi:10.1016/j.resinv.2020.04.001
5 things you should know about IPF. American Lung Association. April 12, 2023. Accessed June 21, 2024. https://www.lung.org/blog/idiopathic-pulmonary-fibrosis-things-to-know
Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566-572. doi:10.1016/S2213-2600(14)70101-8
Morrow T. Improving outcomes and managing costs in idiopathic pulmonary fibrosis. Am J Manag Care. 2019;25(11 suppl):S204-S209. PMID: 31419090
Man RK, Gogikar A, Nanda A, et al. A comparison of the effectiveness of nintedanib and pirfenidone in treating idiopathic pulmonary fibrosis: a systematic review. Cureus. 2024;16(2):e54268. doi:10.7759/cureus.54268
Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684-691. doi:10.7326/0003-4819-156-10-201205150-00004
Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44-e68. doi:10.1164/rccm.201807-1255ST
Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med. 2016;194(3):265-275. doi:10.1164/rccm.201604-0801CI
Abuserewa ST, Duff R, Becker G. Treatment of idiopathic pulmonary fibrosis. Cureus. 2021;13(5):e15360. doi:10.7759/cureus.15360
Lee JH, Jang JH, Jang HJ, et al. New prognostic scoring system for mortality in idiopathic pulmonary fibrosis by modifying the gender, age, and physiology model with desaturation during the six-minute walk test. Front Med (Lausanne). 2023;10:1052129. doi:10.3389/fmed.2023.1052129
Chandel A, Pastre J, Valery S, King CS, Nathan SD. Derivation and validation of a simple multidimensional index incorporating exercise capacity parameters for survival prediction in idiopathic pulmonary fibrosis. Thorax. 2023;78(4):368-375. doi:10.1136/thoraxjnl-2021-218440
Chandel A, King CS, Ignacio RV, et al. External validation and longitudinal application of the DO-GAP index to individualise survival prediction in idiopathic pulmonary fibrosis. ERJ Open Res. 2023;9(3):00124-2023. doi:10.1183/23120541.00124-2023
Suzuki Y, Mori K, Aono Y, et al. Combined assessment of the GAP index and body mass index at antifibrotic therapy initiation for prognosis of idiopathic pulmonary fibrosis. Sci Rep. 2021;11(1):18579. doi:10.1038/s41598-021-98161-y
Lacedonia D, De Pace CC, Rea G, et al. Machine learning and BMI improve the prognostic value of GAP index in treated IPF patients. Bioengineering (Basel). 2023;10(2):251. doi:10.3390/bioengineering10020251
Fujii H, Hara Y, Saigusa Y, et al. ILD-GAP combined with the Charlson Comorbidity Index score (ILD-GAPC) as a prognostic prediction model in patients with interstitial lung disease. Can Respir J. 2023;2023:5088207. doi:10.1155/2023/5088207
Ley B, Bradford WZ, Weycker D, Vittinghoff E, du Bois RM, Collard HR. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1374-1381. doi:10.1183/09031936.00146314
Kreuter M, Lee JS, Tzouvelekis A, et al. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2021;204(1):74-81. doi:10.1164/rccm.202003-0669OC
Kreuter M, Lee JS, Tzouvelekis A, et al. A modified blood cell GAP (cGAP) to prognosticate outcomes in IPF. Poster presented at: European Respiratory Society International Congress; September 4-6, 2022. https://medically.gene.com/global/en/unrestricted/respiratory/ERS-2022/ers-2022-poster-kreuter-a-modified-blood-cell-gap.html
Nishikiori H, Chiba H, Lee SH, et al. A modified GAP model for East-Asian populations with idiopathic pulmonary fibrosis. Respir Investig. 2020;58(5):395-402. doi:10.1016/j.resinv.2020.04.001
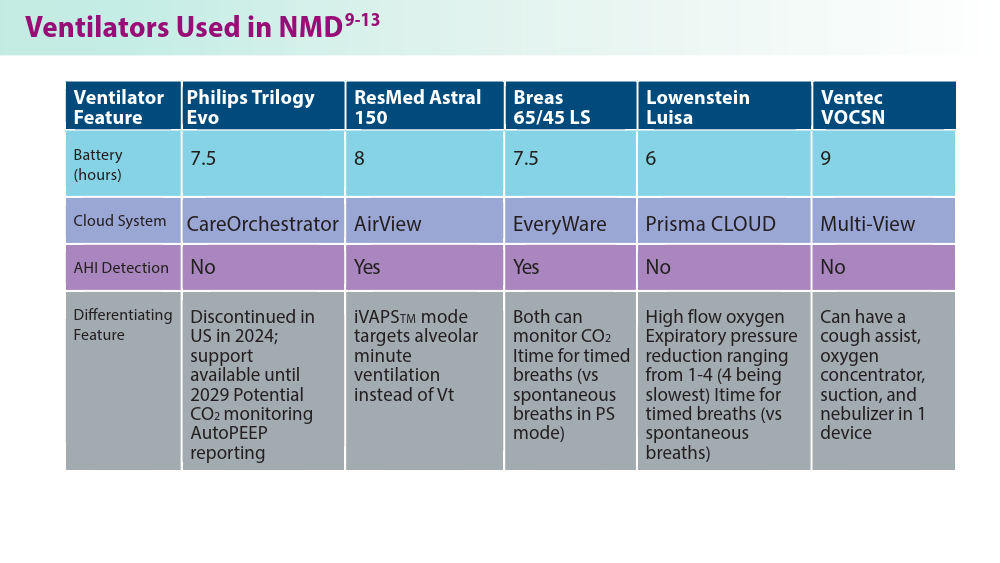
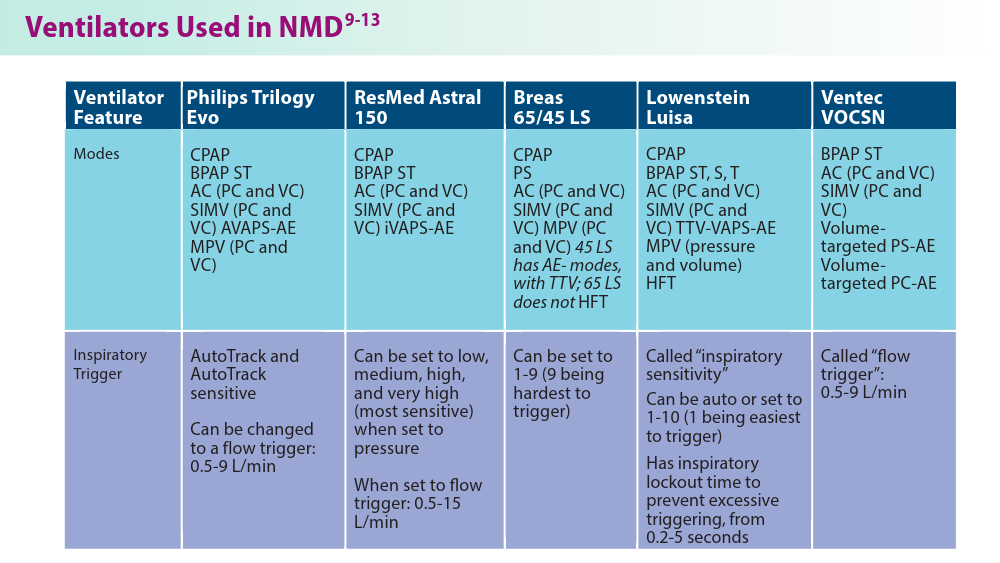
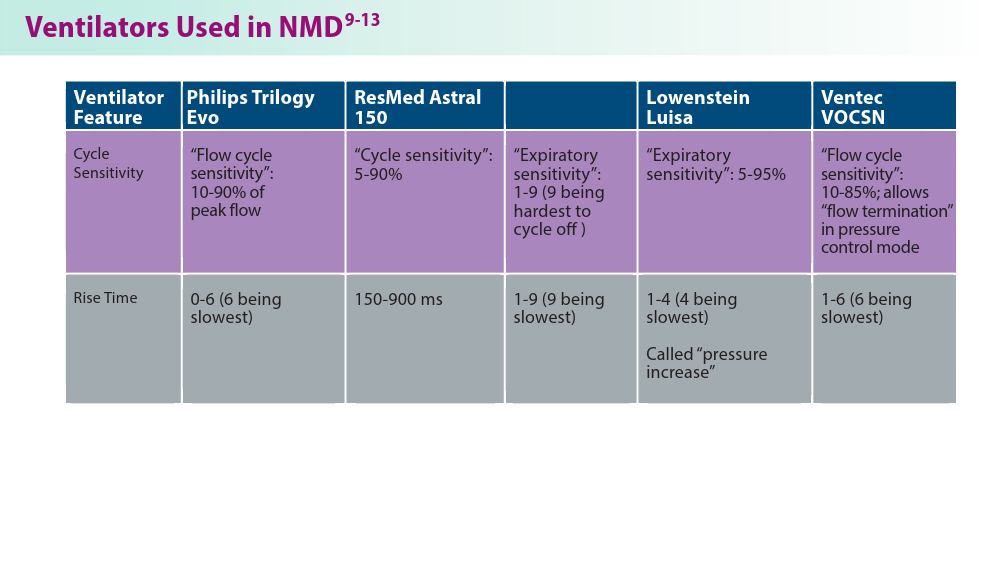
Noninvasive Ventilation in Neuromuscular Disease
- Gong Y, Sankari A. Noninvasive ventilation. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.Updated December 11, 2022. Accessed June 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK578188/
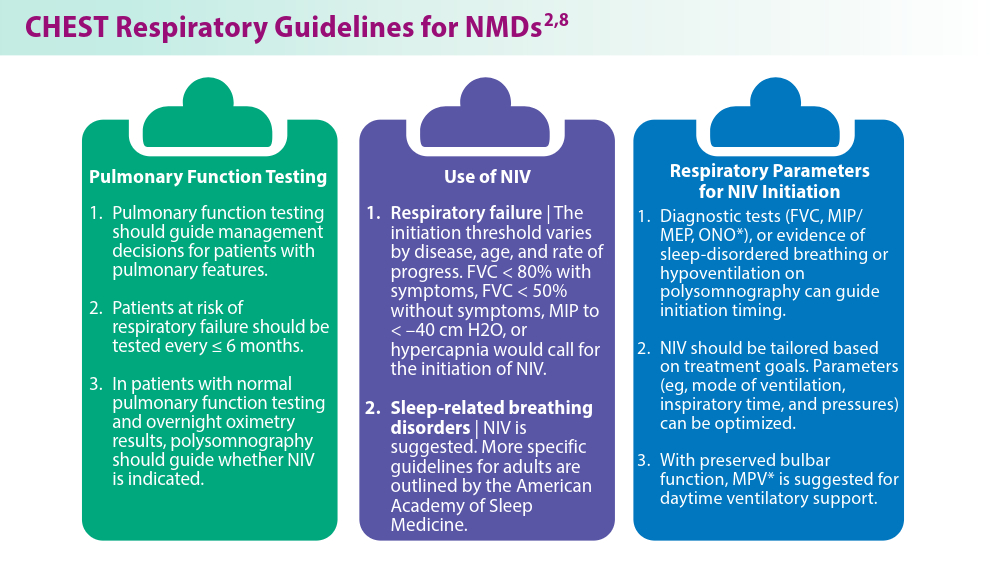
- Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American College of Chest Physicians clinical practice guideline and expert panel report. Chest. 2023;164(2):394-413. doi:10.1016/j.chest.2023.03.011
- Taran S, McCredie VA, Goligher EC. Noninvasive and invasive mechanical ventilation for neurologic disorders. Handb Clin Neurol. 2022;189:361-386. doi:10.1016/B978-0-323-91532-8.00015-X
- Rao F, Garuti G, Vitacca M, et al; for the UILDM Respiratory Group. Management of respiratory complications and rehabilitation in individuals with muscular dystrophies: 1st Consensus Conference report from UILDM - Italian Muscular Dystrophy Association (Milan, January 25-26, 2019). Acta Myol. 2021;40(1):8-42. doi:10.36185/2532-1900-045
- Respiratory assist devices. Centers for Medicare & Medicaid Services. Revised January 1, 2024. Accessed June 19, 2024. https://www.cms.gov/ medicare-coverage-database/view/lcd.aspx?lcdid=33800
- What you need to know about the Philips PAP device recalls. American College of Chest Physicians. February 1, 2024. Accessed June 19, 2024. https://www.chestnet.org/Newsroom/CHEST-News/2021/07/What-YouNeed-to-Know-About-the-Philips-PAP-Device-Recall
- Orr JE, Chen K, Vaida F, et al. Effectiveness of long-term noninvasive ventilation measured by remote monitoring in neuromuscular disease. ERJ Open Res. 2023;9(5):00163-2023. doi:10.1183/23120541.00163-2023
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504. doi:10.5664/jcsm.6506
- Phillips Respironics. Trilogy Evo Clinical Manual. 2019
- ResMed. Astral Series Clinical Guide. 2018
- Breas. Vivo 45 LS User Manual. 2023
- Lowenstein Medical. Luisa Life Support Ventilation.
- Ventec Life Systems. VOCSN Clinical and Technical Manual. 2019
- Gong Y, Sankari A. Noninvasive ventilation. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.Updated December 11, 2022. Accessed June 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK578188/
- Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American College of Chest Physicians clinical practice guideline and expert panel report. Chest. 2023;164(2):394-413. doi:10.1016/j.chest.2023.03.011
- Taran S, McCredie VA, Goligher EC. Noninvasive and invasive mechanical ventilation for neurologic disorders. Handb Clin Neurol. 2022;189:361-386. doi:10.1016/B978-0-323-91532-8.00015-X
- Rao F, Garuti G, Vitacca M, et al; for the UILDM Respiratory Group. Management of respiratory complications and rehabilitation in individuals with muscular dystrophies: 1st Consensus Conference report from UILDM - Italian Muscular Dystrophy Association (Milan, January 25-26, 2019). Acta Myol. 2021;40(1):8-42. doi:10.36185/2532-1900-045
- Respiratory assist devices. Centers for Medicare & Medicaid Services. Revised January 1, 2024. Accessed June 19, 2024. https://www.cms.gov/ medicare-coverage-database/view/lcd.aspx?lcdid=33800
- What you need to know about the Philips PAP device recalls. American College of Chest Physicians. February 1, 2024. Accessed June 19, 2024. https://www.chestnet.org/Newsroom/CHEST-News/2021/07/What-YouNeed-to-Know-About-the-Philips-PAP-Device-Recall
- Orr JE, Chen K, Vaida F, et al. Effectiveness of long-term noninvasive ventilation measured by remote monitoring in neuromuscular disease. ERJ Open Res. 2023;9(5):00163-2023. doi:10.1183/23120541.00163-2023
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504. doi:10.5664/jcsm.6506
- Phillips Respironics. Trilogy Evo Clinical Manual. 2019
- ResMed. Astral Series Clinical Guide. 2018
- Breas. Vivo 45 LS User Manual. 2023
- Lowenstein Medical. Luisa Life Support Ventilation.
- Ventec Life Systems. VOCSN Clinical and Technical Manual. 2019
- Gong Y, Sankari A. Noninvasive ventilation. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.Updated December 11, 2022. Accessed June 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK578188/
- Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American College of Chest Physicians clinical practice guideline and expert panel report. Chest. 2023;164(2):394-413. doi:10.1016/j.chest.2023.03.011
- Taran S, McCredie VA, Goligher EC. Noninvasive and invasive mechanical ventilation for neurologic disorders. Handb Clin Neurol. 2022;189:361-386. doi:10.1016/B978-0-323-91532-8.00015-X
- Rao F, Garuti G, Vitacca M, et al; for the UILDM Respiratory Group. Management of respiratory complications and rehabilitation in individuals with muscular dystrophies: 1st Consensus Conference report from UILDM - Italian Muscular Dystrophy Association (Milan, January 25-26, 2019). Acta Myol. 2021;40(1):8-42. doi:10.36185/2532-1900-045
- Respiratory assist devices. Centers for Medicare & Medicaid Services. Revised January 1, 2024. Accessed June 19, 2024. https://www.cms.gov/ medicare-coverage-database/view/lcd.aspx?lcdid=33800
- What you need to know about the Philips PAP device recalls. American College of Chest Physicians. February 1, 2024. Accessed June 19, 2024. https://www.chestnet.org/Newsroom/CHEST-News/2021/07/What-YouNeed-to-Know-About-the-Philips-PAP-Device-Recall
- Orr JE, Chen K, Vaida F, et al. Effectiveness of long-term noninvasive ventilation measured by remote monitoring in neuromuscular disease. ERJ Open Res. 2023;9(5):00163-2023. doi:10.1183/23120541.00163-2023
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504. doi:10.5664/jcsm.6506
- Phillips Respironics. Trilogy Evo Clinical Manual. 2019
- ResMed. Astral Series Clinical Guide. 2018
- Breas. Vivo 45 LS User Manual. 2023
- Lowenstein Medical. Luisa Life Support Ventilation.
- Ventec Life Systems. VOCSN Clinical and Technical Manual. 2019
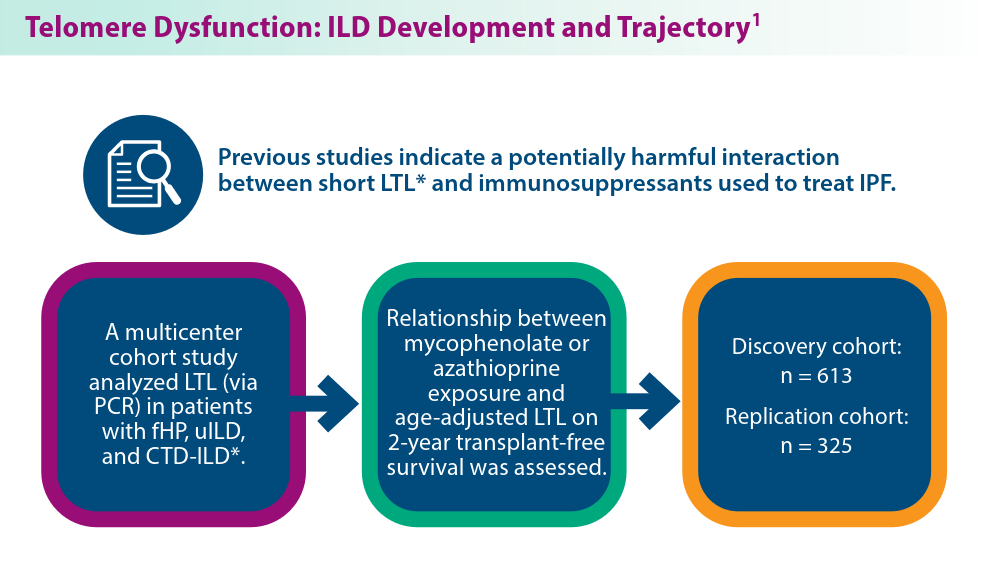
The Genetic Side of Interstitial Lung Disease
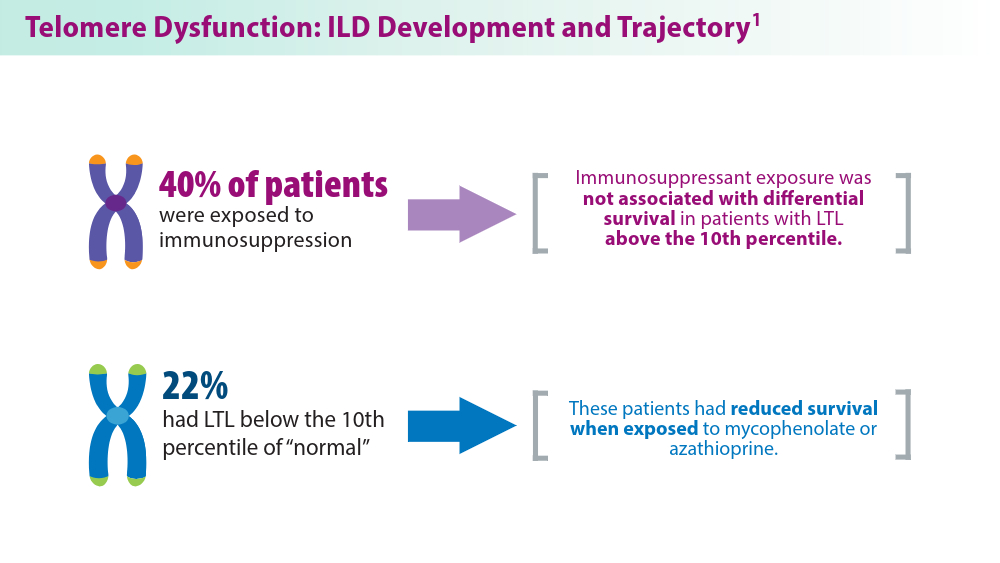
- Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441. doi:10.1183/13993003.00441-2023
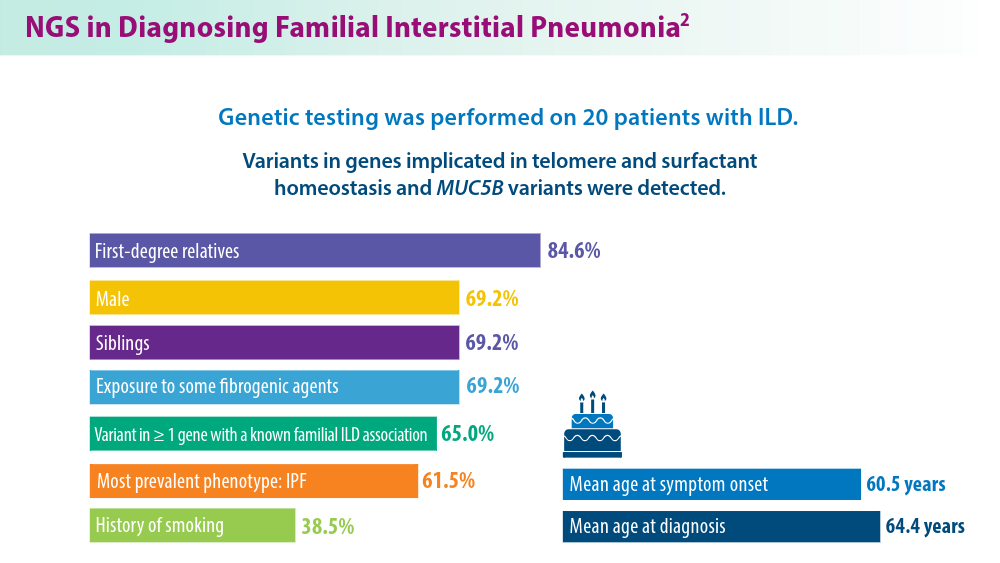
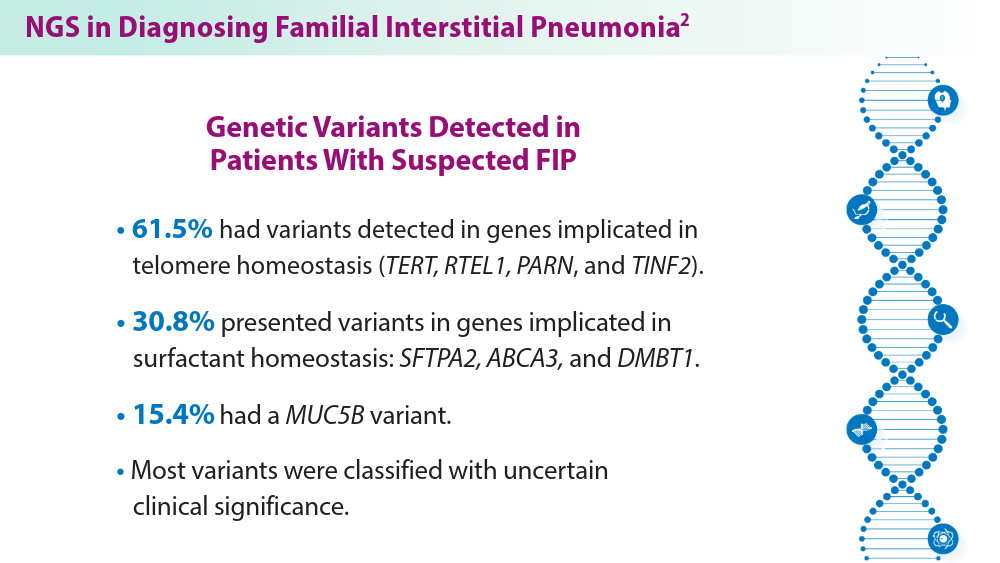
- Gigante AR, Tinoco EM, Fonseca A, et al. Use of next-generation sequencing to support the diagnosis of familial interstitial pneumonia. Genes (Basel). 2023;14(2):326. doi:10.3390/genes14020326
- Adegunsoye A, Kropski JA, Behr J, et al. Genetics and genomics of pulmonary fibrosis: charting the molecular landscape and shaping precision medicine. Am J Respir Crit Care Med. Published online April 4, 2024. doi:10.1164/rccm.202401-0238SO
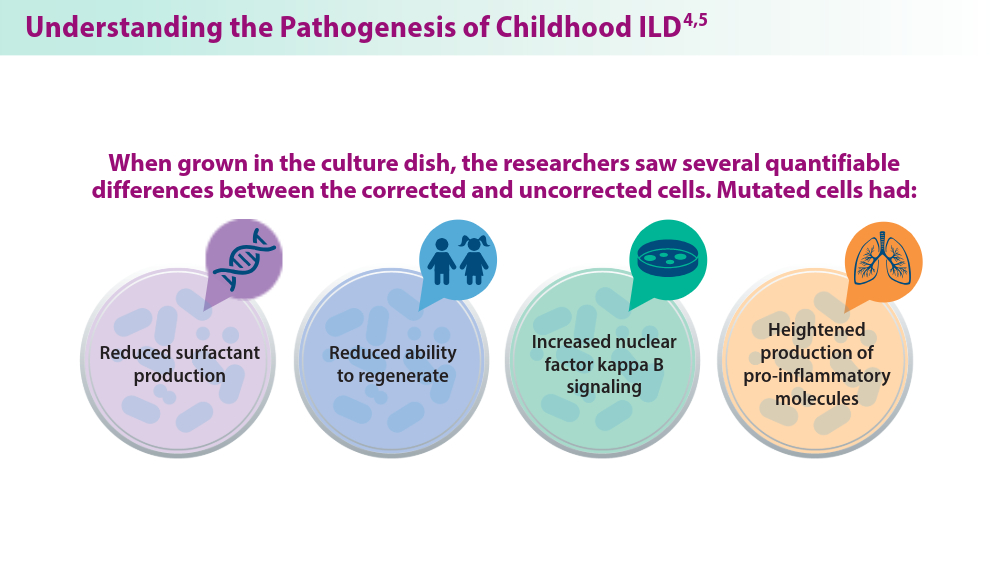
- Sun YL, Hennessey EE, Heins H, et al. Human pluripotent stem cell modeling of alveolar type 2 cell dysfunction caused by ABCA3 mutations. J Clin Invest. 2024;134(2):e164274. doi:10.1172/JCI164274
- Raghu G, Torres JM, Bennett RL. Genetic factors for ILD—the path of precision medicine. Lancet Respir Med. Published online March 20, 2024. doi:10.1016/S2213-2600(24)00071-7
- Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441. doi:10.1183/13993003.00441-2023
- Gigante AR, Tinoco EM, Fonseca A, et al. Use of next-generation sequencing to support the diagnosis of familial interstitial pneumonia. Genes (Basel). 2023;14(2):326. doi:10.3390/genes14020326
- Adegunsoye A, Kropski JA, Behr J, et al. Genetics and genomics of pulmonary fibrosis: charting the molecular landscape and shaping precision medicine. Am J Respir Crit Care Med. Published online April 4, 2024. doi:10.1164/rccm.202401-0238SO
- Sun YL, Hennessey EE, Heins H, et al. Human pluripotent stem cell modeling of alveolar type 2 cell dysfunction caused by ABCA3 mutations. J Clin Invest. 2024;134(2):e164274. doi:10.1172/JCI164274
- Raghu G, Torres JM, Bennett RL. Genetic factors for ILD—the path of precision medicine. Lancet Respir Med. Published online March 20, 2024. doi:10.1016/S2213-2600(24)00071-7
- Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441. doi:10.1183/13993003.00441-2023
- Gigante AR, Tinoco EM, Fonseca A, et al. Use of next-generation sequencing to support the diagnosis of familial interstitial pneumonia. Genes (Basel). 2023;14(2):326. doi:10.3390/genes14020326
- Adegunsoye A, Kropski JA, Behr J, et al. Genetics and genomics of pulmonary fibrosis: charting the molecular landscape and shaping precision medicine. Am J Respir Crit Care Med. Published online April 4, 2024. doi:10.1164/rccm.202401-0238SO
- Sun YL, Hennessey EE, Heins H, et al. Human pluripotent stem cell modeling of alveolar type 2 cell dysfunction caused by ABCA3 mutations. J Clin Invest. 2024;134(2):e164274. doi:10.1172/JCI164274
- Raghu G, Torres JM, Bennett RL. Genetic factors for ILD—the path of precision medicine. Lancet Respir Med. Published online March 20, 2024. doi:10.1016/S2213-2600(24)00071-7
SBRT vs Surgery in CRC Lung Metastases: Which Is Better?
TOPLINE:
However, those who received surgery had significantly better progression-free and disease-free survival rates, as well as a longer time to intrathoracic progression.
METHODOLOGY:
- SBRT has been shown to provide effective local control and improve short-term survival for patients with pulmonary oligometastases from CRC and has become an alternative for these patients who are ineligible or reluctant to undergo surgery. It’s unclear, however, whether SBRT should be prioritized over surgery in patients with CRC pulmonary metastases, largely because of a lack of prospective data.
- In the current analysis, researchers compared outcomes among 335 patients (median age, 61 years) with lung metastases from CRC who underwent surgery or SBRT, using data from the Peking University Cancer Hospital and Institute between March 2011 and September 2022.
- A total of 251 patients were included in the final analysis after propensity score matching, 173 (68.9%) underwent surgery and 78 (31.1%) received SBRT. The median follow-up was 61.6 months in the surgery group and 54.4 months in the SBRT group.
- The study outcomes were freedom from intrathoracic progression, progression-free survival, and overall survival.
TAKEAWAY:
- At 5 years, rates of freedom from intrathoracic progression were more than twofold higher in the surgery group than in the SBRT group (53% vs 23.4%; hazard ratio [HR], 0.46; P < .001). Progression-free survival rates were also more than twofold higher in the surgery group vs the SBRT group (43.8% vs 18.5%; HR, 0.47; P < .001), respectively. In the SBRT group, a higher percentage of patients had a disease-free interval of less than 12 months compared with the surgery group, with rates of 48.7% and 32.9%, respectively (P = 0.025).
- Overall survival, however, was not significantly different between the two groups at 5 years (72.5% in the surgery group vs 63.7% in the SBRT group; P = .260). The number of pulmonary metastases (HR, 1.87; 95% CI, 1.11-3.14, P = .019 and tumor size (HR, 1.03; 95% CI, 1.00-1.05, P = .023) were significant prognostic factors for overall survival.
- Local recurrence was more prevalent after SBRT (33.3%) than surgery (16.9%), while new intrathoracic tumors occurred more frequently after surgery than SBRT (71.8% vs 43.1%). Repeated local treatments were common among patients with intrathoracic progression, which might have contributed to favorable survival outcomes in both groups.
- Both treatments were well-tolerated with no treatment-related mortality or grade ≥ 3 toxicities. In the surgery group, 14 patients experienced complications, including atrial fibrillation (n = 4) and prolonged air leaks (n = 7). In the SBRT group, radiation pneumonitis was the most common adverse event (n = 21).
IN PRACTICE:
SBRT yielded overall survival benefits similar to surgery despite a “higher likelihood of prior extrapulmonary metastases, a shorter disease-free interval, and a greater number of metastatic lesions,” the authors wrote. Still, SBRT should be regarded as an “effective alternative in cases in which surgical intervention is either unviable or declined by the patient,” the authors concluded.
SOURCE:
The study was co-led by Yaqi Wang and Xin Dong, Peking University Cancer Hospital & Institute, Beijing, China, and was published online in the International Journal of Radiation Oncology, Biology, Physics.
LIMITATIONS:
This single-center retrospective study had an inherent selection bias. The lack of balanced sample sizes of the surgery and SBRT groups might have affected the robustness of the statistical analyses. Detailed data on adverse events were not available.
DISCLOSURES:
The study was supported by grants from the National Natural Science Foundation of China, Beijing Natural Science Foundation, and Beijing Municipal Administration of Hospital’s Ascent Plan. The authors did not declare any conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
However, those who received surgery had significantly better progression-free and disease-free survival rates, as well as a longer time to intrathoracic progression.
METHODOLOGY:
- SBRT has been shown to provide effective local control and improve short-term survival for patients with pulmonary oligometastases from CRC and has become an alternative for these patients who are ineligible or reluctant to undergo surgery. It’s unclear, however, whether SBRT should be prioritized over surgery in patients with CRC pulmonary metastases, largely because of a lack of prospective data.
- In the current analysis, researchers compared outcomes among 335 patients (median age, 61 years) with lung metastases from CRC who underwent surgery or SBRT, using data from the Peking University Cancer Hospital and Institute between March 2011 and September 2022.
- A total of 251 patients were included in the final analysis after propensity score matching, 173 (68.9%) underwent surgery and 78 (31.1%) received SBRT. The median follow-up was 61.6 months in the surgery group and 54.4 months in the SBRT group.
- The study outcomes were freedom from intrathoracic progression, progression-free survival, and overall survival.
TAKEAWAY:
- At 5 years, rates of freedom from intrathoracic progression were more than twofold higher in the surgery group than in the SBRT group (53% vs 23.4%; hazard ratio [HR], 0.46; P < .001). Progression-free survival rates were also more than twofold higher in the surgery group vs the SBRT group (43.8% vs 18.5%; HR, 0.47; P < .001), respectively. In the SBRT group, a higher percentage of patients had a disease-free interval of less than 12 months compared with the surgery group, with rates of 48.7% and 32.9%, respectively (P = 0.025).
- Overall survival, however, was not significantly different between the two groups at 5 years (72.5% in the surgery group vs 63.7% in the SBRT group; P = .260). The number of pulmonary metastases (HR, 1.87; 95% CI, 1.11-3.14, P = .019 and tumor size (HR, 1.03; 95% CI, 1.00-1.05, P = .023) were significant prognostic factors for overall survival.
- Local recurrence was more prevalent after SBRT (33.3%) than surgery (16.9%), while new intrathoracic tumors occurred more frequently after surgery than SBRT (71.8% vs 43.1%). Repeated local treatments were common among patients with intrathoracic progression, which might have contributed to favorable survival outcomes in both groups.
- Both treatments were well-tolerated with no treatment-related mortality or grade ≥ 3 toxicities. In the surgery group, 14 patients experienced complications, including atrial fibrillation (n = 4) and prolonged air leaks (n = 7). In the SBRT group, radiation pneumonitis was the most common adverse event (n = 21).
IN PRACTICE:
SBRT yielded overall survival benefits similar to surgery despite a “higher likelihood of prior extrapulmonary metastases, a shorter disease-free interval, and a greater number of metastatic lesions,” the authors wrote. Still, SBRT should be regarded as an “effective alternative in cases in which surgical intervention is either unviable or declined by the patient,” the authors concluded.
SOURCE:
The study was co-led by Yaqi Wang and Xin Dong, Peking University Cancer Hospital & Institute, Beijing, China, and was published online in the International Journal of Radiation Oncology, Biology, Physics.
LIMITATIONS:
This single-center retrospective study had an inherent selection bias. The lack of balanced sample sizes of the surgery and SBRT groups might have affected the robustness of the statistical analyses. Detailed data on adverse events were not available.
DISCLOSURES:
The study was supported by grants from the National Natural Science Foundation of China, Beijing Natural Science Foundation, and Beijing Municipal Administration of Hospital’s Ascent Plan. The authors did not declare any conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
However, those who received surgery had significantly better progression-free and disease-free survival rates, as well as a longer time to intrathoracic progression.
METHODOLOGY:
- SBRT has been shown to provide effective local control and improve short-term survival for patients with pulmonary oligometastases from CRC and has become an alternative for these patients who are ineligible or reluctant to undergo surgery. It’s unclear, however, whether SBRT should be prioritized over surgery in patients with CRC pulmonary metastases, largely because of a lack of prospective data.
- In the current analysis, researchers compared outcomes among 335 patients (median age, 61 years) with lung metastases from CRC who underwent surgery or SBRT, using data from the Peking University Cancer Hospital and Institute between March 2011 and September 2022.
- A total of 251 patients were included in the final analysis after propensity score matching, 173 (68.9%) underwent surgery and 78 (31.1%) received SBRT. The median follow-up was 61.6 months in the surgery group and 54.4 months in the SBRT group.
- The study outcomes were freedom from intrathoracic progression, progression-free survival, and overall survival.
TAKEAWAY:
- At 5 years, rates of freedom from intrathoracic progression were more than twofold higher in the surgery group than in the SBRT group (53% vs 23.4%; hazard ratio [HR], 0.46; P < .001). Progression-free survival rates were also more than twofold higher in the surgery group vs the SBRT group (43.8% vs 18.5%; HR, 0.47; P < .001), respectively. In the SBRT group, a higher percentage of patients had a disease-free interval of less than 12 months compared with the surgery group, with rates of 48.7% and 32.9%, respectively (P = 0.025).
- Overall survival, however, was not significantly different between the two groups at 5 years (72.5% in the surgery group vs 63.7% in the SBRT group; P = .260). The number of pulmonary metastases (HR, 1.87; 95% CI, 1.11-3.14, P = .019 and tumor size (HR, 1.03; 95% CI, 1.00-1.05, P = .023) were significant prognostic factors for overall survival.
- Local recurrence was more prevalent after SBRT (33.3%) than surgery (16.9%), while new intrathoracic tumors occurred more frequently after surgery than SBRT (71.8% vs 43.1%). Repeated local treatments were common among patients with intrathoracic progression, which might have contributed to favorable survival outcomes in both groups.
- Both treatments were well-tolerated with no treatment-related mortality or grade ≥ 3 toxicities. In the surgery group, 14 patients experienced complications, including atrial fibrillation (n = 4) and prolonged air leaks (n = 7). In the SBRT group, radiation pneumonitis was the most common adverse event (n = 21).
IN PRACTICE:
SBRT yielded overall survival benefits similar to surgery despite a “higher likelihood of prior extrapulmonary metastases, a shorter disease-free interval, and a greater number of metastatic lesions,” the authors wrote. Still, SBRT should be regarded as an “effective alternative in cases in which surgical intervention is either unviable or declined by the patient,” the authors concluded.
SOURCE:
The study was co-led by Yaqi Wang and Xin Dong, Peking University Cancer Hospital & Institute, Beijing, China, and was published online in the International Journal of Radiation Oncology, Biology, Physics.
LIMITATIONS:
This single-center retrospective study had an inherent selection bias. The lack of balanced sample sizes of the surgery and SBRT groups might have affected the robustness of the statistical analyses. Detailed data on adverse events were not available.
DISCLOSURES:
The study was supported by grants from the National Natural Science Foundation of China, Beijing Natural Science Foundation, and Beijing Municipal Administration of Hospital’s Ascent Plan. The authors did not declare any conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FDA OKs First-Line Lazertinib With Amivantamab for NSCLC
This marks the first approval for lazertinib. Amivantamab was initially approved by the FDA in 2021 and carries a few indications for locally advanced or metastatic NSCLC. Both drugs are manufactured by Janssen Biotech Inc.
“Patients will now have the option of a potential new first-line standard of care with significant clinical benefits over osimertinib,” study investigator Alexander Spira, MD, PhD, director, Virginia Cancer Specialists Research Institute, said in a news release from Johnson & Johnson .
Lazertinib is an oral, highly selective, third-generation EGFR tyrosine kinase inhibitor that can penetrate the brain and amivantamab is a bispecific antibody targeting EGFR and MET.
The approval was based on results from the phase 3 MARIPOSA trial, which showed that the combination reduced the risk of disease progression or death by 30% compared with osimertinib.
The MARIPOSA trial randomly allocated 1074 patients with exon 19 deletion or exon 21 L858R substitution mutation-positive locally advanced or metastatic NSCLC and no prior systemic therapy for advanced disease to amivantamab plus lazertinib, osimertinib alone, or lazertinib alone.
Lazertinib plus amivantamab demonstrated a statistically significant improvement in progression-free survival compared with osimertinib (hazard ratio, 0.70; P < .001). Median progression-free survival was 23.7 months with the combination vs 16.6 months osimertinib alone and 18.5 months with lazertinib alone.
The median duration of response was 9 months longer with the combination compared with osimertinib (25.8 months vs 16.7 months).
The most common adverse reactions (≥ 20%) were rash, nail toxicity, infusion-related reactions (amivantamab), musculoskeletal pain, edema, stomatitis, venous thromboembolism, paresthesia, fatigue, diarrhea, constipation, COVID-19, hemorrhage, dry skin, decreased appetite, pruritus, nausea, and ocular toxicity.
“A serious safety signal of venous thromboembolic events was observed with lazertinib in combination with amivantamab and prophylactic anticoagulation should be administered for the first four months of therapy,” the FDA noted in a statement announcing the approval.
Results from MARIPOSA were first presented at the European Society for Medical Oncology 2023 Congress and published in The New England Journal of Medicine in June. Longer-term follow-up data from MARIPOSA will be presented at the International Association for the Study of Lung Cancer 2024 World Congress on Lung Cancer in September.
A version of this article appeared on Medscape.com.
This marks the first approval for lazertinib. Amivantamab was initially approved by the FDA in 2021 and carries a few indications for locally advanced or metastatic NSCLC. Both drugs are manufactured by Janssen Biotech Inc.
“Patients will now have the option of a potential new first-line standard of care with significant clinical benefits over osimertinib,” study investigator Alexander Spira, MD, PhD, director, Virginia Cancer Specialists Research Institute, said in a news release from Johnson & Johnson .
Lazertinib is an oral, highly selective, third-generation EGFR tyrosine kinase inhibitor that can penetrate the brain and amivantamab is a bispecific antibody targeting EGFR and MET.
The approval was based on results from the phase 3 MARIPOSA trial, which showed that the combination reduced the risk of disease progression or death by 30% compared with osimertinib.
The MARIPOSA trial randomly allocated 1074 patients with exon 19 deletion or exon 21 L858R substitution mutation-positive locally advanced or metastatic NSCLC and no prior systemic therapy for advanced disease to amivantamab plus lazertinib, osimertinib alone, or lazertinib alone.
Lazertinib plus amivantamab demonstrated a statistically significant improvement in progression-free survival compared with osimertinib (hazard ratio, 0.70; P < .001). Median progression-free survival was 23.7 months with the combination vs 16.6 months osimertinib alone and 18.5 months with lazertinib alone.
The median duration of response was 9 months longer with the combination compared with osimertinib (25.8 months vs 16.7 months).
The most common adverse reactions (≥ 20%) were rash, nail toxicity, infusion-related reactions (amivantamab), musculoskeletal pain, edema, stomatitis, venous thromboembolism, paresthesia, fatigue, diarrhea, constipation, COVID-19, hemorrhage, dry skin, decreased appetite, pruritus, nausea, and ocular toxicity.
“A serious safety signal of venous thromboembolic events was observed with lazertinib in combination with amivantamab and prophylactic anticoagulation should be administered for the first four months of therapy,” the FDA noted in a statement announcing the approval.
Results from MARIPOSA were first presented at the European Society for Medical Oncology 2023 Congress and published in The New England Journal of Medicine in June. Longer-term follow-up data from MARIPOSA will be presented at the International Association for the Study of Lung Cancer 2024 World Congress on Lung Cancer in September.
A version of this article appeared on Medscape.com.
This marks the first approval for lazertinib. Amivantamab was initially approved by the FDA in 2021 and carries a few indications for locally advanced or metastatic NSCLC. Both drugs are manufactured by Janssen Biotech Inc.
“Patients will now have the option of a potential new first-line standard of care with significant clinical benefits over osimertinib,” study investigator Alexander Spira, MD, PhD, director, Virginia Cancer Specialists Research Institute, said in a news release from Johnson & Johnson .
Lazertinib is an oral, highly selective, third-generation EGFR tyrosine kinase inhibitor that can penetrate the brain and amivantamab is a bispecific antibody targeting EGFR and MET.
The approval was based on results from the phase 3 MARIPOSA trial, which showed that the combination reduced the risk of disease progression or death by 30% compared with osimertinib.
The MARIPOSA trial randomly allocated 1074 patients with exon 19 deletion or exon 21 L858R substitution mutation-positive locally advanced or metastatic NSCLC and no prior systemic therapy for advanced disease to amivantamab plus lazertinib, osimertinib alone, or lazertinib alone.
Lazertinib plus amivantamab demonstrated a statistically significant improvement in progression-free survival compared with osimertinib (hazard ratio, 0.70; P < .001). Median progression-free survival was 23.7 months with the combination vs 16.6 months osimertinib alone and 18.5 months with lazertinib alone.
The median duration of response was 9 months longer with the combination compared with osimertinib (25.8 months vs 16.7 months).
The most common adverse reactions (≥ 20%) were rash, nail toxicity, infusion-related reactions (amivantamab), musculoskeletal pain, edema, stomatitis, venous thromboembolism, paresthesia, fatigue, diarrhea, constipation, COVID-19, hemorrhage, dry skin, decreased appetite, pruritus, nausea, and ocular toxicity.
“A serious safety signal of venous thromboembolic events was observed with lazertinib in combination with amivantamab and prophylactic anticoagulation should be administered for the first four months of therapy,” the FDA noted in a statement announcing the approval.
Results from MARIPOSA were first presented at the European Society for Medical Oncology 2023 Congress and published in The New England Journal of Medicine in June. Longer-term follow-up data from MARIPOSA will be presented at the International Association for the Study of Lung Cancer 2024 World Congress on Lung Cancer in September.
A version of this article appeared on Medscape.com.
Alert System Could Warn of Impact of Severe Weather on Health
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
It’s Never Too Late to Convince Patients to Quit Smoking
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.