User login
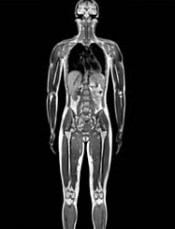
Third-hand smoke affects blood cell development in mice

and Antoine Snijders analyze
blood cells collected from mice
exposed to third-hand smoke.
Photo courtesy of
Marilyn Chung/Berkeley Lab
Exposure to third-hand smoke leads to biological effects on weight and blood cell development, according to preclinical research published in Scientific Reports.
Researchers found that newborn mice housed with smoke-treated cloths for 3 weeks weighed significantly less than mice in a control group.
Moreover, newborn and adult mice exposed to third-hand smoke experienced persistent changes in blood cell counts.
The blood cell count changes are associated with inflammatory and allergic reactions upon exposure to third-hand smoke, the researchers said.
For this study, the team set out to characterize the biological effects of exposure to third-hand smoke by placing 5-square-centimeter pieces of smoke-contaminated cotton cloth in cages with mice.
The researchers then compared smoke-exposed mice to control mice. The team assessed changes to body weight and the hematopoietic system after 3 weeks of exposure (or no exposure) for mice belonging to 2 age groups: birth to 3 weeks (neonatal) and 12 to 15 weeks (young adult).
The results showed that smoke exposure temporarily inhibited weight gain in the neonatal mice. There was no effect on weight gain in the young adult mice.
In addition, smoke exposure produced changes in blood cell populations that persisted over time and were evident in mice from both age groups.
In general, there were lower levels of platelets and specific types of white blood cells in the smoke-exposed mice.
For example, neonatal mice exposed to third-hand smoke had higher levels of eosinophils, female mice had higher levels of neutrophils, males had higher levels of basophils, and all mice had higher levels of B cells.
“Those are all types of white blood cells associated with inflammation and allergic reactions,” said study author Jian-Hua Mao, PhD, of Lawrence Berkeley National Laboratory in Berkeley, California.
“And the effects on blood cell count persisted even after exposure ended. Changes remained at least 14 weeks after exposure ended for the neonatal group and 2 weeks after it ended for the adults.”
The researchers pointed out that they did not study whether the observed biological changes led to specific diseases or other health outcomes, but other studies suggest links to adverse health effects.
“Third-hand smoke is an underappreciated risk factor in health,” said study author Antoine Snijders, PhD, of Lawrence Berkeley National Laboratory.
“It’s clear that more and bigger studies are needed, particularly in humans, so we can support policy decisions on third-hand smoke.” ![]()

and Antoine Snijders analyze
blood cells collected from mice
exposed to third-hand smoke.
Photo courtesy of
Marilyn Chung/Berkeley Lab
Exposure to third-hand smoke leads to biological effects on weight and blood cell development, according to preclinical research published in Scientific Reports.
Researchers found that newborn mice housed with smoke-treated cloths for 3 weeks weighed significantly less than mice in a control group.
Moreover, newborn and adult mice exposed to third-hand smoke experienced persistent changes in blood cell counts.
The blood cell count changes are associated with inflammatory and allergic reactions upon exposure to third-hand smoke, the researchers said.
For this study, the team set out to characterize the biological effects of exposure to third-hand smoke by placing 5-square-centimeter pieces of smoke-contaminated cotton cloth in cages with mice.
The researchers then compared smoke-exposed mice to control mice. The team assessed changes to body weight and the hematopoietic system after 3 weeks of exposure (or no exposure) for mice belonging to 2 age groups: birth to 3 weeks (neonatal) and 12 to 15 weeks (young adult).
The results showed that smoke exposure temporarily inhibited weight gain in the neonatal mice. There was no effect on weight gain in the young adult mice.
In addition, smoke exposure produced changes in blood cell populations that persisted over time and were evident in mice from both age groups.
In general, there were lower levels of platelets and specific types of white blood cells in the smoke-exposed mice.
For example, neonatal mice exposed to third-hand smoke had higher levels of eosinophils, female mice had higher levels of neutrophils, males had higher levels of basophils, and all mice had higher levels of B cells.
“Those are all types of white blood cells associated with inflammation and allergic reactions,” said study author Jian-Hua Mao, PhD, of Lawrence Berkeley National Laboratory in Berkeley, California.
“And the effects on blood cell count persisted even after exposure ended. Changes remained at least 14 weeks after exposure ended for the neonatal group and 2 weeks after it ended for the adults.”
The researchers pointed out that they did not study whether the observed biological changes led to specific diseases or other health outcomes, but other studies suggest links to adverse health effects.
“Third-hand smoke is an underappreciated risk factor in health,” said study author Antoine Snijders, PhD, of Lawrence Berkeley National Laboratory.
“It’s clear that more and bigger studies are needed, particularly in humans, so we can support policy decisions on third-hand smoke.” ![]()

and Antoine Snijders analyze
blood cells collected from mice
exposed to third-hand smoke.
Photo courtesy of
Marilyn Chung/Berkeley Lab
Exposure to third-hand smoke leads to biological effects on weight and blood cell development, according to preclinical research published in Scientific Reports.
Researchers found that newborn mice housed with smoke-treated cloths for 3 weeks weighed significantly less than mice in a control group.
Moreover, newborn and adult mice exposed to third-hand smoke experienced persistent changes in blood cell counts.
The blood cell count changes are associated with inflammatory and allergic reactions upon exposure to third-hand smoke, the researchers said.
For this study, the team set out to characterize the biological effects of exposure to third-hand smoke by placing 5-square-centimeter pieces of smoke-contaminated cotton cloth in cages with mice.
The researchers then compared smoke-exposed mice to control mice. The team assessed changes to body weight and the hematopoietic system after 3 weeks of exposure (or no exposure) for mice belonging to 2 age groups: birth to 3 weeks (neonatal) and 12 to 15 weeks (young adult).
The results showed that smoke exposure temporarily inhibited weight gain in the neonatal mice. There was no effect on weight gain in the young adult mice.
In addition, smoke exposure produced changes in blood cell populations that persisted over time and were evident in mice from both age groups.
In general, there were lower levels of platelets and specific types of white blood cells in the smoke-exposed mice.
For example, neonatal mice exposed to third-hand smoke had higher levels of eosinophils, female mice had higher levels of neutrophils, males had higher levels of basophils, and all mice had higher levels of B cells.
“Those are all types of white blood cells associated with inflammation and allergic reactions,” said study author Jian-Hua Mao, PhD, of Lawrence Berkeley National Laboratory in Berkeley, California.
“And the effects on blood cell count persisted even after exposure ended. Changes remained at least 14 weeks after exposure ended for the neonatal group and 2 weeks after it ended for the adults.”
The researchers pointed out that they did not study whether the observed biological changes led to specific diseases or other health outcomes, but other studies suggest links to adverse health effects.
“Third-hand smoke is an underappreciated risk factor in health,” said study author Antoine Snijders, PhD, of Lawrence Berkeley National Laboratory.
“It’s clear that more and bigger studies are needed, particularly in humans, so we can support policy decisions on third-hand smoke.” ![]()
Cheap manufacture of generic cancer drugs is feasible, study shows

Photo courtesy of FDA
AMSTERDAM—New research suggests some generic cancer drugs could be manufactured for less than 1% of the prices currently charged in the US and UK.
For example, researchers calculated that manufacturing a 400 mg tablet of imatinib costs $0.92.
Charging $1.04 per tablet would cover costs and allow for a 10% profit margin.
However, the current price of imatinib is $84.36 per tablet in the UK and $247.74 per tablet in the US.
Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, reported these findings at ECCO 2017: European Cancer Congress (abstract 1032).
Barber and her colleagues collected data on per-kilogram costs of exported active pharmaceutical ingredients (APIs) from an online database of Indian export logs.
The team then estimated generic prices for tablets through an established costing algorithm. They calculated per-dose API costs and added excipient costs of $2.63 per kg of finished pharmaceutical product and per-tablet costs of production of $0.01, plus a 10% profit margin accounting for a 26.6% average tax on profits (assuming manufacture in India.)
Finally, the researchers compared the calculated price to current unit prices in the US, UK, Spain, and India.
For imatinib, the team determined the cost of the API to be $2284 per kg and the API cost per tablet to be $0.91. They then added excipient cost ($0.002 per tablet), conversion cost ($0.01 per tablet), and a 10% profit margin accounting for a 26.6% tax on profits.
This resulted in the estimated generic price of $1.04 per tablet. The per-tablet price is below the estimated price in India ($0.22) but much higher than the estimated price in Spain ($57.53), the UK ($84.36), and the US ($247.74).
Barber noted that, according to her group’s calculations, imatinib could be produced for $54 a month.
Another drug that could be produced for a low cost is etoposide. Barber and her colleagues calculated a generic price for etoposide of $0.97 per 100 mg tablet.
However, the per-tablet price is $1.50 in India, $8.65 in Spain, $11.34 in the UK, and $87.14 in the US.
The researchers calculated a generic price for mercaptopurine of $0.03 per 50 mg tablet, which is the same as the per-tablet price in India. However, a 50 mg mercaptopurine tablet costs $3.14 in Spain, $2.56 in the UK, and $0.40 in the US.
“Showing that certain cancers could be treated for very low prices could transform the future of people with these cancers in very low-income countries where there are usually few or no treatment options,” Barber said. ![]()

Photo courtesy of FDA
AMSTERDAM—New research suggests some generic cancer drugs could be manufactured for less than 1% of the prices currently charged in the US and UK.
For example, researchers calculated that manufacturing a 400 mg tablet of imatinib costs $0.92.
Charging $1.04 per tablet would cover costs and allow for a 10% profit margin.
However, the current price of imatinib is $84.36 per tablet in the UK and $247.74 per tablet in the US.
Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, reported these findings at ECCO 2017: European Cancer Congress (abstract 1032).
Barber and her colleagues collected data on per-kilogram costs of exported active pharmaceutical ingredients (APIs) from an online database of Indian export logs.
The team then estimated generic prices for tablets through an established costing algorithm. They calculated per-dose API costs and added excipient costs of $2.63 per kg of finished pharmaceutical product and per-tablet costs of production of $0.01, plus a 10% profit margin accounting for a 26.6% average tax on profits (assuming manufacture in India.)
Finally, the researchers compared the calculated price to current unit prices in the US, UK, Spain, and India.
For imatinib, the team determined the cost of the API to be $2284 per kg and the API cost per tablet to be $0.91. They then added excipient cost ($0.002 per tablet), conversion cost ($0.01 per tablet), and a 10% profit margin accounting for a 26.6% tax on profits.
This resulted in the estimated generic price of $1.04 per tablet. The per-tablet price is below the estimated price in India ($0.22) but much higher than the estimated price in Spain ($57.53), the UK ($84.36), and the US ($247.74).
Barber noted that, according to her group’s calculations, imatinib could be produced for $54 a month.
Another drug that could be produced for a low cost is etoposide. Barber and her colleagues calculated a generic price for etoposide of $0.97 per 100 mg tablet.
However, the per-tablet price is $1.50 in India, $8.65 in Spain, $11.34 in the UK, and $87.14 in the US.
The researchers calculated a generic price for mercaptopurine of $0.03 per 50 mg tablet, which is the same as the per-tablet price in India. However, a 50 mg mercaptopurine tablet costs $3.14 in Spain, $2.56 in the UK, and $0.40 in the US.
“Showing that certain cancers could be treated for very low prices could transform the future of people with these cancers in very low-income countries where there are usually few or no treatment options,” Barber said. ![]()

Photo courtesy of FDA
AMSTERDAM—New research suggests some generic cancer drugs could be manufactured for less than 1% of the prices currently charged in the US and UK.
For example, researchers calculated that manufacturing a 400 mg tablet of imatinib costs $0.92.
Charging $1.04 per tablet would cover costs and allow for a 10% profit margin.
However, the current price of imatinib is $84.36 per tablet in the UK and $247.74 per tablet in the US.
Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, reported these findings at ECCO 2017: European Cancer Congress (abstract 1032).
Barber and her colleagues collected data on per-kilogram costs of exported active pharmaceutical ingredients (APIs) from an online database of Indian export logs.
The team then estimated generic prices for tablets through an established costing algorithm. They calculated per-dose API costs and added excipient costs of $2.63 per kg of finished pharmaceutical product and per-tablet costs of production of $0.01, plus a 10% profit margin accounting for a 26.6% average tax on profits (assuming manufacture in India.)
Finally, the researchers compared the calculated price to current unit prices in the US, UK, Spain, and India.
For imatinib, the team determined the cost of the API to be $2284 per kg and the API cost per tablet to be $0.91. They then added excipient cost ($0.002 per tablet), conversion cost ($0.01 per tablet), and a 10% profit margin accounting for a 26.6% tax on profits.
This resulted in the estimated generic price of $1.04 per tablet. The per-tablet price is below the estimated price in India ($0.22) but much higher than the estimated price in Spain ($57.53), the UK ($84.36), and the US ($247.74).
Barber noted that, according to her group’s calculations, imatinib could be produced for $54 a month.
Another drug that could be produced for a low cost is etoposide. Barber and her colleagues calculated a generic price for etoposide of $0.97 per 100 mg tablet.
However, the per-tablet price is $1.50 in India, $8.65 in Spain, $11.34 in the UK, and $87.14 in the US.
The researchers calculated a generic price for mercaptopurine of $0.03 per 50 mg tablet, which is the same as the per-tablet price in India. However, a 50 mg mercaptopurine tablet costs $3.14 in Spain, $2.56 in the UK, and $0.40 in the US.
“Showing that certain cancers could be treated for very low prices could transform the future of people with these cancers in very low-income countries where there are usually few or no treatment options,” Barber said. ![]()
Company withdraws MAA for pegfilgrastim biosimilar

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()
Recent price hikes for generic cancer meds exceed 100%

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()
CHMP recommends lenalidomide maintenance

Photo courtesy of Celgene
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended a new indication for lenalidomide (Revlimid®).
The CHMP advised the European Commission (EC) to approve the use of lenalidomide as maintenance therapy in adults who had newly diagnosed multiple myeloma (MM) prior to receiving an autologous stem cell transplant (ASCT).
If approved by the EC, lenalidomide will be the first licensed maintenance treatment available to this patient population in the European Union.
The EC, which generally follows the CHMP’s recommendations, is expected to make its final decision on this use of lenalidomide in approximately 2 months.
If approval is granted, detailed conditions for the use of lenalidomide will be described in the Summary of Product Characteristics, which will be published in the revised European Public Assessment Report.
Lenalidomide is a product of Celgene.
The CHMP’s recommendation to approve lenalidomide as maintenance in MM was based on the results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211. Results from both studies were published in NEJM in May 2012.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing ASCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen post-ASCT of lenalidomide monotherapy, followed by continuous daily treatment with lenalidomide or placebo until relapse.
“Studies show that maintenance treatment after ASCT with Revlimid may help control residual malignant cells and delay tumor growth by enhancing immune function,” said Michel Attal, MD, of the Institut Universitaire du Cancer Toulouse Oncopole and Institut Claudius Regaud in France.
“Our primary goal is to delay disease progression for as long as possible, and we have seen in several independent studies that Revlimid maintenance after ASCT can halve the risk of disease progression by sustaining the response.” ![]()

Photo courtesy of Celgene
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended a new indication for lenalidomide (Revlimid®).
The CHMP advised the European Commission (EC) to approve the use of lenalidomide as maintenance therapy in adults who had newly diagnosed multiple myeloma (MM) prior to receiving an autologous stem cell transplant (ASCT).
If approved by the EC, lenalidomide will be the first licensed maintenance treatment available to this patient population in the European Union.
The EC, which generally follows the CHMP’s recommendations, is expected to make its final decision on this use of lenalidomide in approximately 2 months.
If approval is granted, detailed conditions for the use of lenalidomide will be described in the Summary of Product Characteristics, which will be published in the revised European Public Assessment Report.
Lenalidomide is a product of Celgene.
The CHMP’s recommendation to approve lenalidomide as maintenance in MM was based on the results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211. Results from both studies were published in NEJM in May 2012.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing ASCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen post-ASCT of lenalidomide monotherapy, followed by continuous daily treatment with lenalidomide or placebo until relapse.
“Studies show that maintenance treatment after ASCT with Revlimid may help control residual malignant cells and delay tumor growth by enhancing immune function,” said Michel Attal, MD, of the Institut Universitaire du Cancer Toulouse Oncopole and Institut Claudius Regaud in France.
“Our primary goal is to delay disease progression for as long as possible, and we have seen in several independent studies that Revlimid maintenance after ASCT can halve the risk of disease progression by sustaining the response.” ![]()

Photo courtesy of Celgene
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended a new indication for lenalidomide (Revlimid®).
The CHMP advised the European Commission (EC) to approve the use of lenalidomide as maintenance therapy in adults who had newly diagnosed multiple myeloma (MM) prior to receiving an autologous stem cell transplant (ASCT).
If approved by the EC, lenalidomide will be the first licensed maintenance treatment available to this patient population in the European Union.
The EC, which generally follows the CHMP’s recommendations, is expected to make its final decision on this use of lenalidomide in approximately 2 months.
If approval is granted, detailed conditions for the use of lenalidomide will be described in the Summary of Product Characteristics, which will be published in the revised European Public Assessment Report.
Lenalidomide is a product of Celgene.
The CHMP’s recommendation to approve lenalidomide as maintenance in MM was based on the results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211. Results from both studies were published in NEJM in May 2012.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing ASCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen post-ASCT of lenalidomide monotherapy, followed by continuous daily treatment with lenalidomide or placebo until relapse.
“Studies show that maintenance treatment after ASCT with Revlimid may help control residual malignant cells and delay tumor growth by enhancing immune function,” said Michel Attal, MD, of the Institut Universitaire du Cancer Toulouse Oncopole and Institut Claudius Regaud in France.
“Our primary goal is to delay disease progression for as long as possible, and we have seen in several independent studies that Revlimid maintenance after ASCT can halve the risk of disease progression by sustaining the response.” ![]()
Software could improve image analysis, team says
Researchers say they have developed new software that will analyze medical and scientific images faster and more accurately than ever before.
The team says this software, Tracking Equilibrium and Nonequilibrium shifts in Data (TREND), can analyze any series of images, including nuclear magnetic resonance images, computerized tomography scans, ultrasound images, video images, and imaging from scientific equipment of all kinds.
The researchers described the TREND software in Biophysical Journal.
The team said TREND can study sets of images to resolve and track the changes among the images.
And the software can analyze videos to plot and resolve changes as well as reconstruct videos to focus only on the individual processes and changes of interest.
“TREND allows accurate, rapid analysis of incredibly complex and nuanced images, which can potentially save doctors, patients, and scientists countless hours and money,” said Steve Van Doren, PhD, of the University of Missouri in Columbia, Missouri.
“TREND has allowed us to advance our own research into enzyme interactions considerably. Previously, it would take us weeks to analyze a single group of images. With TREND, that analysis now takes only a few minutes and is more accurate and consistent than if a human performed the work.” ![]()

Researchers say they have developed new software that will analyze medical and scientific images faster and more accurately than ever before.
The team says this software, Tracking Equilibrium and Nonequilibrium shifts in Data (TREND), can analyze any series of images, including nuclear magnetic resonance images, computerized tomography scans, ultrasound images, video images, and imaging from scientific equipment of all kinds.
The researchers described the TREND software in Biophysical Journal.
The team said TREND can study sets of images to resolve and track the changes among the images.
And the software can analyze videos to plot and resolve changes as well as reconstruct videos to focus only on the individual processes and changes of interest.
“TREND allows accurate, rapid analysis of incredibly complex and nuanced images, which can potentially save doctors, patients, and scientists countless hours and money,” said Steve Van Doren, PhD, of the University of Missouri in Columbia, Missouri.
“TREND has allowed us to advance our own research into enzyme interactions considerably. Previously, it would take us weeks to analyze a single group of images. With TREND, that analysis now takes only a few minutes and is more accurate and consistent than if a human performed the work.” ![]()

Researchers say they have developed new software that will analyze medical and scientific images faster and more accurately than ever before.
The team says this software, Tracking Equilibrium and Nonequilibrium shifts in Data (TREND), can analyze any series of images, including nuclear magnetic resonance images, computerized tomography scans, ultrasound images, video images, and imaging from scientific equipment of all kinds.
The researchers described the TREND software in Biophysical Journal.
The team said TREND can study sets of images to resolve and track the changes among the images.
And the software can analyze videos to plot and resolve changes as well as reconstruct videos to focus only on the individual processes and changes of interest.
“TREND allows accurate, rapid analysis of incredibly complex and nuanced images, which can potentially save doctors, patients, and scientists countless hours and money,” said Steve Van Doren, PhD, of the University of Missouri in Columbia, Missouri.
“TREND has allowed us to advance our own research into enzyme interactions considerably. Previously, it would take us weeks to analyze a single group of images. With TREND, that analysis now takes only a few minutes and is more accurate and consistent than if a human performed the work.” ![]()
Roche launches new blood analyzer

Photo by Graham Colm
Roche has announced the launch of its cobas m 511 integrated hematology analyzer in countries that recognize the CE mark.*
The cobas m 511 combines 3 components of the hematology testing process—a digital morphology analyzer, cell counter, and classifier—into a single system that prepares, stains, and analyzes microscopy blood slides.
Roche said cobas m 511 provides greater accuracy and consistency than current technologies by identifying, counting, isolating, and categorizing blood cells, then presenting the digital images of all these cell types.
The company said this automation and digitalization reduces the need for resource-intensive manual microscope reviews, supports clinicians to share challenging cases around the world, and enables the delivery of quicker results, which ultimately aid patient diagnoses.
The cobas m 511 uses Bloodhound® technology for printing, staining, and imaging. This technology uses 30 µL of blood to print a monolayer onto the slide, stains for further analysis of the morphology, and enables classification of cells displayed on a viewing station.
Unlike the indirect methods commonly used in blood analysis today, the cobas m 511 images individual cells directly.
Based on these direct images, the Bloodhound® technology counts, analyzes morphology, and then classifies every cell in the viewing area to provide a standard complete blood count and 5-part differential and reticulocyte count.
While hematologists will continue to have the option of looking at slides under their microscopes, the cobas m 511 provides cell-by-cell images that, in many cases, may eliminate the need for microscopic review.
“With this launch, patients will benefit from a faster and more accurate diagnosis of blood diseases as diverse as anemia and leukemia,” said Roland Diggelmann, CEO of Roche Diagnostics.
“We are entering a new area of innovation with Roche in hematology testing, supporting customers with integrated and efficient laboratory solutions, which deliver increased medical value.” ![]()
*Local product availability may vary independently from CE mark approval. The cobas m 511 integrated hematology analyzer is not available in countries with previously agreed third-party vendor agreements.

Photo by Graham Colm
Roche has announced the launch of its cobas m 511 integrated hematology analyzer in countries that recognize the CE mark.*
The cobas m 511 combines 3 components of the hematology testing process—a digital morphology analyzer, cell counter, and classifier—into a single system that prepares, stains, and analyzes microscopy blood slides.
Roche said cobas m 511 provides greater accuracy and consistency than current technologies by identifying, counting, isolating, and categorizing blood cells, then presenting the digital images of all these cell types.
The company said this automation and digitalization reduces the need for resource-intensive manual microscope reviews, supports clinicians to share challenging cases around the world, and enables the delivery of quicker results, which ultimately aid patient diagnoses.
The cobas m 511 uses Bloodhound® technology for printing, staining, and imaging. This technology uses 30 µL of blood to print a monolayer onto the slide, stains for further analysis of the morphology, and enables classification of cells displayed on a viewing station.
Unlike the indirect methods commonly used in blood analysis today, the cobas m 511 images individual cells directly.
Based on these direct images, the Bloodhound® technology counts, analyzes morphology, and then classifies every cell in the viewing area to provide a standard complete blood count and 5-part differential and reticulocyte count.
While hematologists will continue to have the option of looking at slides under their microscopes, the cobas m 511 provides cell-by-cell images that, in many cases, may eliminate the need for microscopic review.
“With this launch, patients will benefit from a faster and more accurate diagnosis of blood diseases as diverse as anemia and leukemia,” said Roland Diggelmann, CEO of Roche Diagnostics.
“We are entering a new area of innovation with Roche in hematology testing, supporting customers with integrated and efficient laboratory solutions, which deliver increased medical value.” ![]()
*Local product availability may vary independently from CE mark approval. The cobas m 511 integrated hematology analyzer is not available in countries with previously agreed third-party vendor agreements.

Photo by Graham Colm
Roche has announced the launch of its cobas m 511 integrated hematology analyzer in countries that recognize the CE mark.*
The cobas m 511 combines 3 components of the hematology testing process—a digital morphology analyzer, cell counter, and classifier—into a single system that prepares, stains, and analyzes microscopy blood slides.
Roche said cobas m 511 provides greater accuracy and consistency than current technologies by identifying, counting, isolating, and categorizing blood cells, then presenting the digital images of all these cell types.
The company said this automation and digitalization reduces the need for resource-intensive manual microscope reviews, supports clinicians to share challenging cases around the world, and enables the delivery of quicker results, which ultimately aid patient diagnoses.
The cobas m 511 uses Bloodhound® technology for printing, staining, and imaging. This technology uses 30 µL of blood to print a monolayer onto the slide, stains for further analysis of the morphology, and enables classification of cells displayed on a viewing station.
Unlike the indirect methods commonly used in blood analysis today, the cobas m 511 images individual cells directly.
Based on these direct images, the Bloodhound® technology counts, analyzes morphology, and then classifies every cell in the viewing area to provide a standard complete blood count and 5-part differential and reticulocyte count.
While hematologists will continue to have the option of looking at slides under their microscopes, the cobas m 511 provides cell-by-cell images that, in many cases, may eliminate the need for microscopic review.
“With this launch, patients will benefit from a faster and more accurate diagnosis of blood diseases as diverse as anemia and leukemia,” said Roland Diggelmann, CEO of Roche Diagnostics.
“We are entering a new area of innovation with Roche in hematology testing, supporting customers with integrated and efficient laboratory solutions, which deliver increased medical value.”
*Local product availability may vary independently from CE mark approval. The cobas m 511 integrated hematology analyzer is not available in countries with previously agreed third-party vendor agreements.
CMA report reveals successes and shortcomings

The European Medicines Agency (EMA) has released a report showing both successes and room for improvement regarding conditional marketing authorizations (CMAs).
CMA is one of the tools available to regulators to support the development of and early access to drugs that address unmet medical needs of patients in the European Union.
Drugs are granted CMA if the public health benefit of their immediate availability is thought to outweigh the risk of an authorization on the basis of less comprehensive data than normally required.
A CMA is valid for 1 year. As part of the authorization, the drug’s developer is obliged to carry out further studies to obtain complete data.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) assesses the data generated by these specific post-authorization obligations at least annually to ensure the balance of benefits and risks of the drug continues to remain positive.
At the end of its assessment, the CHMP issues a recommendation regarding the renewal of the CMA or its conversion into a standard marketing authorization.
Overview
The EMA’s report summarizes the experience with CMAs from the first use of this authorization type in 2006 until June 30, 2016.
During this time, a total of 30 drugs have received a CMA, including several
hematology drugs—Adcetris (brentuximab vedotin),
Arzerra (ofatumumab), Blincyto (blinatumomab), Bosulif (bosutinib), Darzalex (daratumumab), and Pixuvri (pixantrone).
Eleven CMAs have been converted into standard marketing authorizations (including Arzerra’s CMA), 2 have been withdrawn for commercial reasons, and 17 are still conditional authorizations.
None of the drugs that still have CMAs have been authorized for more than 5 years. And none of the CMAs issued since 2006 have had to be revoked or suspended.
Successes
According to the EMA’s analysis, marketing authorization holders comply with the specific obligations imposed by the agency.
More than 90% of completed specific obligations did not result in major changes of scope, and about 70% of specific obligations did not require an extension to the originally specified timelines.
The report shows that it took an average of 4 years to generate the additional data needed and to convert a CMA into a full marketing authorization.
This suggests patients with life-threatening or seriously debilitating conditions had access to promising drugs much earlier than they would have under standard authorization.
Areas for improvement
The EMA’s analysis also revealed room for improvement.
The report showed that, relatively frequently, CMA was first
considered only during the assessment of the drug application, which meant granting a CMA took longer than intended.
Therefore, the EMA recommends that drug developers engage in early dialogue with the EMA
and prospectively plan to apply for a CMA.
The agency said this should support
prompt assessment of such applications and could also facilitate prompt
completion of additional studies and timely availability of
comprehensive data.
The EMA said another area for improvement is engaging other stakeholders involved in bringing drugs to patients—in particular, Health Technology Assessment bodies—to facilitate the generation of all data needed for decision-making through one development program.

The European Medicines Agency (EMA) has released a report showing both successes and room for improvement regarding conditional marketing authorizations (CMAs).
CMA is one of the tools available to regulators to support the development of and early access to drugs that address unmet medical needs of patients in the European Union.
Drugs are granted CMA if the public health benefit of their immediate availability is thought to outweigh the risk of an authorization on the basis of less comprehensive data than normally required.
A CMA is valid for 1 year. As part of the authorization, the drug’s developer is obliged to carry out further studies to obtain complete data.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) assesses the data generated by these specific post-authorization obligations at least annually to ensure the balance of benefits and risks of the drug continues to remain positive.
At the end of its assessment, the CHMP issues a recommendation regarding the renewal of the CMA or its conversion into a standard marketing authorization.
Overview
The EMA’s report summarizes the experience with CMAs from the first use of this authorization type in 2006 until June 30, 2016.
During this time, a total of 30 drugs have received a CMA, including several
hematology drugs—Adcetris (brentuximab vedotin),
Arzerra (ofatumumab), Blincyto (blinatumomab), Bosulif (bosutinib), Darzalex (daratumumab), and Pixuvri (pixantrone).
Eleven CMAs have been converted into standard marketing authorizations (including Arzerra’s CMA), 2 have been withdrawn for commercial reasons, and 17 are still conditional authorizations.
None of the drugs that still have CMAs have been authorized for more than 5 years. And none of the CMAs issued since 2006 have had to be revoked or suspended.
Successes
According to the EMA’s analysis, marketing authorization holders comply with the specific obligations imposed by the agency.
More than 90% of completed specific obligations did not result in major changes of scope, and about 70% of specific obligations did not require an extension to the originally specified timelines.
The report shows that it took an average of 4 years to generate the additional data needed and to convert a CMA into a full marketing authorization.
This suggests patients with life-threatening or seriously debilitating conditions had access to promising drugs much earlier than they would have under standard authorization.
Areas for improvement
The EMA’s analysis also revealed room for improvement.
The report showed that, relatively frequently, CMA was first
considered only during the assessment of the drug application, which meant granting a CMA took longer than intended.
Therefore, the EMA recommends that drug developers engage in early dialogue with the EMA
and prospectively plan to apply for a CMA.
The agency said this should support
prompt assessment of such applications and could also facilitate prompt
completion of additional studies and timely availability of
comprehensive data.
The EMA said another area for improvement is engaging other stakeholders involved in bringing drugs to patients—in particular, Health Technology Assessment bodies—to facilitate the generation of all data needed for decision-making through one development program.

The European Medicines Agency (EMA) has released a report showing both successes and room for improvement regarding conditional marketing authorizations (CMAs).
CMA is one of the tools available to regulators to support the development of and early access to drugs that address unmet medical needs of patients in the European Union.
Drugs are granted CMA if the public health benefit of their immediate availability is thought to outweigh the risk of an authorization on the basis of less comprehensive data than normally required.
A CMA is valid for 1 year. As part of the authorization, the drug’s developer is obliged to carry out further studies to obtain complete data.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) assesses the data generated by these specific post-authorization obligations at least annually to ensure the balance of benefits and risks of the drug continues to remain positive.
At the end of its assessment, the CHMP issues a recommendation regarding the renewal of the CMA or its conversion into a standard marketing authorization.
Overview
The EMA’s report summarizes the experience with CMAs from the first use of this authorization type in 2006 until June 30, 2016.
During this time, a total of 30 drugs have received a CMA, including several
hematology drugs—Adcetris (brentuximab vedotin),
Arzerra (ofatumumab), Blincyto (blinatumomab), Bosulif (bosutinib), Darzalex (daratumumab), and Pixuvri (pixantrone).
Eleven CMAs have been converted into standard marketing authorizations (including Arzerra’s CMA), 2 have been withdrawn for commercial reasons, and 17 are still conditional authorizations.
None of the drugs that still have CMAs have been authorized for more than 5 years. And none of the CMAs issued since 2006 have had to be revoked or suspended.
Successes
According to the EMA’s analysis, marketing authorization holders comply with the specific obligations imposed by the agency.
More than 90% of completed specific obligations did not result in major changes of scope, and about 70% of specific obligations did not require an extension to the originally specified timelines.
The report shows that it took an average of 4 years to generate the additional data needed and to convert a CMA into a full marketing authorization.
This suggests patients with life-threatening or seriously debilitating conditions had access to promising drugs much earlier than they would have under standard authorization.
Areas for improvement
The EMA’s analysis also revealed room for improvement.
The report showed that, relatively frequently, CMA was first
considered only during the assessment of the drug application, which meant granting a CMA took longer than intended.
Therefore, the EMA recommends that drug developers engage in early dialogue with the EMA
and prospectively plan to apply for a CMA.
The agency said this should support
prompt assessment of such applications and could also facilitate prompt
completion of additional studies and timely availability of
comprehensive data.
The EMA said another area for improvement is engaging other stakeholders involved in bringing drugs to patients—in particular, Health Technology Assessment bodies—to facilitate the generation of all data needed for decision-making through one development program.
Vaccine candidate can protect humans from malaria

Plasmodium falciparum
Photo by Mae Melvin, CDC
In a phase 2 trial, a malaria vaccine candidate was able to prevent volunteers from contracting Plasmodium falciparum malaria.
The vaccine, known as PfSPZ vaccine, is composed of live but weakened P falciparum sporozoites.
Three to 5 doses of PfSPZ vaccine protected subjects against malaria parasites similar to those in the vaccine as well as parasites different from those in the vaccine.
A majority of subjects were protected at 3 weeks after vaccination. For some subjects, this protection was sustained at 24 weeks.
PfSPZ vaccine was considered well-tolerated, as all adverse events (AEs) in this trial were grade 1 or 2.
These results were published in JCI Insight.
The study was funded by the US Department of Defense through the Joint Warfighter Program, the Military Infectious Disease Research Program, and US Navy Advanced Medical Development, with additional support from Sanaria, Inc., the company developing PfSPZ vaccine.
“Our military continues to be at risk from malaria as it deploys worldwide,” said Kenneth A. Bertram, MD, of the US Army Medical Research and Materiel Command in Ft Detrick, Maryland.
“We are excited about the results of this clinical trial and are now investing in the ongoing clinical trial to finalize the vaccination regimen for PfSPZ vaccine.”
The current trial included 67 volunteers with a median age of 29.6 (range, 19 to 45).
They were randomized to 3 treatment groups. Forty-five subjects were set to receive the PfSPZ vaccine, and 22 subjects served as controls.
The volunteers underwent controlled human malaria infection (CHMI) 3 weeks after vaccinated subjects received their final vaccine dose and then again at 24 weeks.
Efficacy at 3 weeks
Group 1
In this group, 13 subjects received 5 doses of the vaccine at 2.7 × 105. They and 6 control subjects underwent CHMI with a homologous strain of P falciparum, Pf3D7.
Twelve of the 13 fully immunized subjects, or 92.3%, did not develop parasitemia. However, all 6 control subjects did, with a median prepatent period of 11.6 days. The prepatent period in the immunized subject who developed parasitemia was 13.9 days.
Group 2
In this group, 5 subjects received 5 doses of the vaccine at 2.7 × 105. They and 4 control subjects underwent CHMI with a heterologous strain of P falciparum, Pf7G8.
Four of the 5 fully immunized subjects, or 80%, did not develop parasitemia. However, all 4 control subjects did, with a median prepatent period of 11.9 days. The prepatent period in the fully immunized subject who developed parasitemia was 11.9 days.
Group 3
In this group, 15 subjects received 3 doses of the vaccine at 4.5 × 105 and underwent homologous Pf3D7 CHMI. The control subjects for this group were the same as those in group 1.
Thirteen of the 15 immunized subjects, or 86.7%, did not develop parasitemia. The prepatent periods in the 2 immunized subjects who did develop parasitemia were 13.9 days and 16.9 days.
Efficacy at 24 weeks
Study subjects underwent a second CHMI at 24 weeks after immunized participants received their final dose of vaccine.
Group 1
Seven of the 10 fully immunized subjects who underwent a second CHMI did not develop parasitemia (70%). However, all 6 control subjects did, with a median prepatent period of 11.6 days. The median prepatent period in the 3 fully immunized subjects who developed parasitemia was 15.4 days.
Group 2
One of the 10 fully immunized subjects who underwent a second CHMI did not develop parasitemia (10%). However, all control subjects did, with a median prepatent period of 10.9 days. The median prepatent period in the 9 fully immunized subjects who developed parasitemia was 11.9 days.
Group 3
Eight of the 14 fully immunized subjects who underwent a second CHMI did not develop parasitemia (57.1%). The median prepatent period in the 6 fully immunized subjects who did develop parasitemia was 14.0 days.
Safety
There were 66 solicited AEs reported within 7 days of immunization that were considered possibly, probably, or definitely related to vaccination. Ninety-two percent of these AEs were grade 1, and 8% were grade 2. All unsolicited AEs reported within 7 days of immunization were grade 1.
The incidence of AEs was not higher in group 3 than in groups 1 or 2, and the incidence of AEs did not increase as subjects received additional doses of the vaccine.
The most common AEs (with an incidence of 10% or higher in at least 1 group) were injection site pain, headache, fatigue, malaise, myalgia, injection site hemorrhage, and cough.
“The results of this clinical trial, along with recent results from other trials of this vaccine in the US and Africa, were critical to our decision to move forward with a trial involving 400 infants in Kenya,” said Tina Oneko, MD, of the Kenya Medical Research Institute, who is the principal investigator of the Kenya trial but was not involved in the current trial.
“This represents significant progress toward the development of a regimen for PfSPZ vaccine that we anticipate will provide a high level [of] efficacy for malaria prevention in all age groups in Africa.”

Plasmodium falciparum
Photo by Mae Melvin, CDC
In a phase 2 trial, a malaria vaccine candidate was able to prevent volunteers from contracting Plasmodium falciparum malaria.
The vaccine, known as PfSPZ vaccine, is composed of live but weakened P falciparum sporozoites.
Three to 5 doses of PfSPZ vaccine protected subjects against malaria parasites similar to those in the vaccine as well as parasites different from those in the vaccine.
A majority of subjects were protected at 3 weeks after vaccination. For some subjects, this protection was sustained at 24 weeks.
PfSPZ vaccine was considered well-tolerated, as all adverse events (AEs) in this trial were grade 1 or 2.
These results were published in JCI Insight.
The study was funded by the US Department of Defense through the Joint Warfighter Program, the Military Infectious Disease Research Program, and US Navy Advanced Medical Development, with additional support from Sanaria, Inc., the company developing PfSPZ vaccine.
“Our military continues to be at risk from malaria as it deploys worldwide,” said Kenneth A. Bertram, MD, of the US Army Medical Research and Materiel Command in Ft Detrick, Maryland.
“We are excited about the results of this clinical trial and are now investing in the ongoing clinical trial to finalize the vaccination regimen for PfSPZ vaccine.”
The current trial included 67 volunteers with a median age of 29.6 (range, 19 to 45).
They were randomized to 3 treatment groups. Forty-five subjects were set to receive the PfSPZ vaccine, and 22 subjects served as controls.
The volunteers underwent controlled human malaria infection (CHMI) 3 weeks after vaccinated subjects received their final vaccine dose and then again at 24 weeks.
Efficacy at 3 weeks
Group 1
In this group, 13 subjects received 5 doses of the vaccine at 2.7 × 105. They and 6 control subjects underwent CHMI with a homologous strain of P falciparum, Pf3D7.
Twelve of the 13 fully immunized subjects, or 92.3%, did not develop parasitemia. However, all 6 control subjects did, with a median prepatent period of 11.6 days. The prepatent period in the immunized subject who developed parasitemia was 13.9 days.
Group 2
In this group, 5 subjects received 5 doses of the vaccine at 2.7 × 105. They and 4 control subjects underwent CHMI with a heterologous strain of P falciparum, Pf7G8.
Four of the 5 fully immunized subjects, or 80%, did not develop parasitemia. However, all 4 control subjects did, with a median prepatent period of 11.9 days. The prepatent period in the fully immunized subject who developed parasitemia was 11.9 days.
Group 3
In this group, 15 subjects received 3 doses of the vaccine at 4.5 × 105 and underwent homologous Pf3D7 CHMI. The control subjects for this group were the same as those in group 1.
Thirteen of the 15 immunized subjects, or 86.7%, did not develop parasitemia. The prepatent periods in the 2 immunized subjects who did develop parasitemia were 13.9 days and 16.9 days.
Efficacy at 24 weeks
Study subjects underwent a second CHMI at 24 weeks after immunized participants received their final dose of vaccine.
Group 1
Seven of the 10 fully immunized subjects who underwent a second CHMI did not develop parasitemia (70%). However, all 6 control subjects did, with a median prepatent period of 11.6 days. The median prepatent period in the 3 fully immunized subjects who developed parasitemia was 15.4 days.
Group 2
One of the 10 fully immunized subjects who underwent a second CHMI did not develop parasitemia (10%). However, all control subjects did, with a median prepatent period of 10.9 days. The median prepatent period in the 9 fully immunized subjects who developed parasitemia was 11.9 days.
Group 3
Eight of the 14 fully immunized subjects who underwent a second CHMI did not develop parasitemia (57.1%). The median prepatent period in the 6 fully immunized subjects who did develop parasitemia was 14.0 days.
Safety
There were 66 solicited AEs reported within 7 days of immunization that were considered possibly, probably, or definitely related to vaccination. Ninety-two percent of these AEs were grade 1, and 8% were grade 2. All unsolicited AEs reported within 7 days of immunization were grade 1.
The incidence of AEs was not higher in group 3 than in groups 1 or 2, and the incidence of AEs did not increase as subjects received additional doses of the vaccine.
The most common AEs (with an incidence of 10% or higher in at least 1 group) were injection site pain, headache, fatigue, malaise, myalgia, injection site hemorrhage, and cough.
“The results of this clinical trial, along with recent results from other trials of this vaccine in the US and Africa, were critical to our decision to move forward with a trial involving 400 infants in Kenya,” said Tina Oneko, MD, of the Kenya Medical Research Institute, who is the principal investigator of the Kenya trial but was not involved in the current trial.
“This represents significant progress toward the development of a regimen for PfSPZ vaccine that we anticipate will provide a high level [of] efficacy for malaria prevention in all age groups in Africa.”

Plasmodium falciparum
Photo by Mae Melvin, CDC
In a phase 2 trial, a malaria vaccine candidate was able to prevent volunteers from contracting Plasmodium falciparum malaria.
The vaccine, known as PfSPZ vaccine, is composed of live but weakened P falciparum sporozoites.
Three to 5 doses of PfSPZ vaccine protected subjects against malaria parasites similar to those in the vaccine as well as parasites different from those in the vaccine.
A majority of subjects were protected at 3 weeks after vaccination. For some subjects, this protection was sustained at 24 weeks.
PfSPZ vaccine was considered well-tolerated, as all adverse events (AEs) in this trial were grade 1 or 2.
These results were published in JCI Insight.
The study was funded by the US Department of Defense through the Joint Warfighter Program, the Military Infectious Disease Research Program, and US Navy Advanced Medical Development, with additional support from Sanaria, Inc., the company developing PfSPZ vaccine.
“Our military continues to be at risk from malaria as it deploys worldwide,” said Kenneth A. Bertram, MD, of the US Army Medical Research and Materiel Command in Ft Detrick, Maryland.
“We are excited about the results of this clinical trial and are now investing in the ongoing clinical trial to finalize the vaccination regimen for PfSPZ vaccine.”
The current trial included 67 volunteers with a median age of 29.6 (range, 19 to 45).
They were randomized to 3 treatment groups. Forty-five subjects were set to receive the PfSPZ vaccine, and 22 subjects served as controls.
The volunteers underwent controlled human malaria infection (CHMI) 3 weeks after vaccinated subjects received their final vaccine dose and then again at 24 weeks.
Efficacy at 3 weeks
Group 1
In this group, 13 subjects received 5 doses of the vaccine at 2.7 × 105. They and 6 control subjects underwent CHMI with a homologous strain of P falciparum, Pf3D7.
Twelve of the 13 fully immunized subjects, or 92.3%, did not develop parasitemia. However, all 6 control subjects did, with a median prepatent period of 11.6 days. The prepatent period in the immunized subject who developed parasitemia was 13.9 days.
Group 2
In this group, 5 subjects received 5 doses of the vaccine at 2.7 × 105. They and 4 control subjects underwent CHMI with a heterologous strain of P falciparum, Pf7G8.
Four of the 5 fully immunized subjects, or 80%, did not develop parasitemia. However, all 4 control subjects did, with a median prepatent period of 11.9 days. The prepatent period in the fully immunized subject who developed parasitemia was 11.9 days.
Group 3
In this group, 15 subjects received 3 doses of the vaccine at 4.5 × 105 and underwent homologous Pf3D7 CHMI. The control subjects for this group were the same as those in group 1.
Thirteen of the 15 immunized subjects, or 86.7%, did not develop parasitemia. The prepatent periods in the 2 immunized subjects who did develop parasitemia were 13.9 days and 16.9 days.
Efficacy at 24 weeks
Study subjects underwent a second CHMI at 24 weeks after immunized participants received their final dose of vaccine.
Group 1
Seven of the 10 fully immunized subjects who underwent a second CHMI did not develop parasitemia (70%). However, all 6 control subjects did, with a median prepatent period of 11.6 days. The median prepatent period in the 3 fully immunized subjects who developed parasitemia was 15.4 days.
Group 2
One of the 10 fully immunized subjects who underwent a second CHMI did not develop parasitemia (10%). However, all control subjects did, with a median prepatent period of 10.9 days. The median prepatent period in the 9 fully immunized subjects who developed parasitemia was 11.9 days.
Group 3
Eight of the 14 fully immunized subjects who underwent a second CHMI did not develop parasitemia (57.1%). The median prepatent period in the 6 fully immunized subjects who did develop parasitemia was 14.0 days.
Safety
There were 66 solicited AEs reported within 7 days of immunization that were considered possibly, probably, or definitely related to vaccination. Ninety-two percent of these AEs were grade 1, and 8% were grade 2. All unsolicited AEs reported within 7 days of immunization were grade 1.
The incidence of AEs was not higher in group 3 than in groups 1 or 2, and the incidence of AEs did not increase as subjects received additional doses of the vaccine.
The most common AEs (with an incidence of 10% or higher in at least 1 group) were injection site pain, headache, fatigue, malaise, myalgia, injection site hemorrhage, and cough.
“The results of this clinical trial, along with recent results from other trials of this vaccine in the US and Africa, were critical to our decision to move forward with a trial involving 400 infants in Kenya,” said Tina Oneko, MD, of the Kenya Medical Research Institute, who is the principal investigator of the Kenya trial but was not involved in the current trial.
“This represents significant progress toward the development of a regimen for PfSPZ vaccine that we anticipate will provide a high level [of] efficacy for malaria prevention in all age groups in Africa.”
PI ties to industry linked to positive trial results

capsules for a clinical trial
Photo by Esther Dyson
Financial ties between principal investigators (PIs) and drug companies are independently associated with positive clinical trial results, according to new research.
The study showed a significant association between positive trial outcomes and PIs having financial ties to the manufacturer of the study drug, even after accounting for the source of research funding.
Researchers reported these findings in The BMJ.
Salomeh Keyhani, MD, of the University of California, San Francisco, and her colleagues conducted this research, analyzing a random sample of 195 drug trials published in 2013.
The team found financial ties between PIs and manufacturers of the study drug for 67.7% of the studies (n=132). In all, 58% of the PIs had financial ties to the manufacturers (231/397).
Types of financial ties included:
- Advisor/consultancy payments (39%)
- Speakers’ fees (20%)
- Unspecified financial ties (20%)
- Honoraria (13%)
- Employee relationships (13%)
- Travel fees (13%)
- Stock ownership (10%)
- Having a patent related to the study drug (5%).
PIs reported financial ties to the drug manufacturer in 76% (103/136) of studies with positive results and 49% (29/59) of studies with negative results (P<0.001).
In a multivariate analysis adjusted for the study’s funding source, a financial tie was significantly associated with a positive trial outcome. The odds ratio was 3.57 (P=0.001).
In a multivariate analysis adjusted for a range of other study-related factors as well, a financial tie remained significantly associated with a positive trial outcome. The odds ratio was 3.37 (P=0.006).
Dr Keyhani and her colleagues stressed that this analysis was observational and cannot be used to draw conclusions about causation.
However, they said, given the importance of industry and academic collaboration in advancing the development of new treatments, “more thought needs to be given to the roles that investigators, policy makers, and journal editors can play in ensuring the credibility of the evidence base.”
Authors of a related editorial said more research is needed to determine how industry funding and financial ties could influence trial results.
The authors—Andreas Lundh, PhD, of the University of Southern Denmark, and Lisa Bero, PhD, of the University of Sydney in Australia—urged trial investigators to share their data and participate in industry-funded trials only if data are made publicly available.
The authors also suggested journals could help by rejecting research by investigators who are unwilling to share their data and by penalizing investigators who fail to disclose financial ties. The role of sponsors, or companies with which investigators have ties, in the research must also be transparent.
In the meantime, trials with industry funding or investigators with financial ties “should be interpreted with caution until all relevant information is fully disclosed and easily accessible,” the authors concluded.

capsules for a clinical trial
Photo by Esther Dyson
Financial ties between principal investigators (PIs) and drug companies are independently associated with positive clinical trial results, according to new research.
The study showed a significant association between positive trial outcomes and PIs having financial ties to the manufacturer of the study drug, even after accounting for the source of research funding.
Researchers reported these findings in The BMJ.
Salomeh Keyhani, MD, of the University of California, San Francisco, and her colleagues conducted this research, analyzing a random sample of 195 drug trials published in 2013.
The team found financial ties between PIs and manufacturers of the study drug for 67.7% of the studies (n=132). In all, 58% of the PIs had financial ties to the manufacturers (231/397).
Types of financial ties included:
- Advisor/consultancy payments (39%)
- Speakers’ fees (20%)
- Unspecified financial ties (20%)
- Honoraria (13%)
- Employee relationships (13%)
- Travel fees (13%)
- Stock ownership (10%)
- Having a patent related to the study drug (5%).
PIs reported financial ties to the drug manufacturer in 76% (103/136) of studies with positive results and 49% (29/59) of studies with negative results (P<0.001).
In a multivariate analysis adjusted for the study’s funding source, a financial tie was significantly associated with a positive trial outcome. The odds ratio was 3.57 (P=0.001).
In a multivariate analysis adjusted for a range of other study-related factors as well, a financial tie remained significantly associated with a positive trial outcome. The odds ratio was 3.37 (P=0.006).
Dr Keyhani and her colleagues stressed that this analysis was observational and cannot be used to draw conclusions about causation.
However, they said, given the importance of industry and academic collaboration in advancing the development of new treatments, “more thought needs to be given to the roles that investigators, policy makers, and journal editors can play in ensuring the credibility of the evidence base.”
Authors of a related editorial said more research is needed to determine how industry funding and financial ties could influence trial results.
The authors—Andreas Lundh, PhD, of the University of Southern Denmark, and Lisa Bero, PhD, of the University of Sydney in Australia—urged trial investigators to share their data and participate in industry-funded trials only if data are made publicly available.
The authors also suggested journals could help by rejecting research by investigators who are unwilling to share their data and by penalizing investigators who fail to disclose financial ties. The role of sponsors, or companies with which investigators have ties, in the research must also be transparent.
In the meantime, trials with industry funding or investigators with financial ties “should be interpreted with caution until all relevant information is fully disclosed and easily accessible,” the authors concluded.

capsules for a clinical trial
Photo by Esther Dyson
Financial ties between principal investigators (PIs) and drug companies are independently associated with positive clinical trial results, according to new research.
The study showed a significant association between positive trial outcomes and PIs having financial ties to the manufacturer of the study drug, even after accounting for the source of research funding.
Researchers reported these findings in The BMJ.
Salomeh Keyhani, MD, of the University of California, San Francisco, and her colleagues conducted this research, analyzing a random sample of 195 drug trials published in 2013.
The team found financial ties between PIs and manufacturers of the study drug for 67.7% of the studies (n=132). In all, 58% of the PIs had financial ties to the manufacturers (231/397).
Types of financial ties included:
- Advisor/consultancy payments (39%)
- Speakers’ fees (20%)
- Unspecified financial ties (20%)
- Honoraria (13%)
- Employee relationships (13%)
- Travel fees (13%)
- Stock ownership (10%)
- Having a patent related to the study drug (5%).
PIs reported financial ties to the drug manufacturer in 76% (103/136) of studies with positive results and 49% (29/59) of studies with negative results (P<0.001).
In a multivariate analysis adjusted for the study’s funding source, a financial tie was significantly associated with a positive trial outcome. The odds ratio was 3.57 (P=0.001).
In a multivariate analysis adjusted for a range of other study-related factors as well, a financial tie remained significantly associated with a positive trial outcome. The odds ratio was 3.37 (P=0.006).
Dr Keyhani and her colleagues stressed that this analysis was observational and cannot be used to draw conclusions about causation.
However, they said, given the importance of industry and academic collaboration in advancing the development of new treatments, “more thought needs to be given to the roles that investigators, policy makers, and journal editors can play in ensuring the credibility of the evidence base.”
Authors of a related editorial said more research is needed to determine how industry funding and financial ties could influence trial results.
The authors—Andreas Lundh, PhD, of the University of Southern Denmark, and Lisa Bero, PhD, of the University of Sydney in Australia—urged trial investigators to share their data and participate in industry-funded trials only if data are made publicly available.
The authors also suggested journals could help by rejecting research by investigators who are unwilling to share their data and by penalizing investigators who fail to disclose financial ties. The role of sponsors, or companies with which investigators have ties, in the research must also be transparent.
In the meantime, trials with industry funding or investigators with financial ties “should be interpreted with caution until all relevant information is fully disclosed and easily accessible,” the authors concluded.