User login
US cancer cases may near 1.7 million in 2017

patient and her father
Photo by Rhoda Baer
The US may see nearly 1.7 million new cancer cases in 2017 and more than 600,000 cancer-related deaths, according to a report from the American Cancer Society (ACS).
In addition to estimates for 2017, the report, “Cancer Statistics 2017,” includes the most recent data on cancer incidence, mortality, and survival in the US.
The report was published in CA: A Cancer Journal for Clinicians.
The report projects there will be 1,688,780 new cancer cases and 600,920 cancer deaths in the US this year.
This includes:
- 80,500 new cases of lymphoma and 21,210 lymphoma deaths
- 62,130 new cases of leukemia and 24,500 leukemia deaths
- 30,280 new cases of myeloma and 12,590 myeloma deaths.
The report also shows that, from 2004 to 2013, the overall cancer incidence rate was stable in women and declined by about 2% per year in men. From 2005 to 2014, the cancer death rate declined by about 1.5% annually in both men and women.
Overall, the cancer death rate dropped 25% from its peak of 215.1 (per 100,000 population) in 1991 to 161.2 (per 100,000 population) in 2014, the latest year for which data was available. This translates to about 2,143,200 fewer cancer deaths.
“The continuing drops in the cancer death rate are a powerful sign of the potential we have to reduce cancer’s deadly toll,” said Otis W. Brawley, MD, chief medical officer of the ACS.
He said the decrease in cancer death rates is the result of steady reductions in smoking and advances in early detection and treatment. The decrease is driven by decreasing death rates for the 4 major cancer sites—lung, breast, colorectal, and prostate.
The report also shows that racial disparities in cancer death rates continue to decline. The excess risk of cancer death in black men has dropped from 47% in 1990 to 21% in 2014. The black/white disparity declined similarly in women, from a peak of 20% in 1998 to 13% in 2014.
On the other hand, significant gender disparities persist for both cancer incidence and death in the US. For all cancer sites combined, the incidence rate is 20% higher in men than in women, and the cancer death rate is 40% higher in men.
Dr Brawley said the gender gap in cancer mortality largely reflects variation in the distribution of cancers that occur in men and women, much of which is due to differences in the prevalence of cancer risk factors.
The yearly “Cancer Statistics” reports have been published by ACS researchers since 1967 to inform and guide clinicians, investigators, and others in public health in prioritizing efforts to reduce the burden of cancer.
Cancer incidence data for the current report were collected by the Surveillance, Epidemiology, and End Results Program; the National Program of Cancer Registries; and the North American Association of Central Cancer Registries. Mortality data were collected by the National Center for Health Statistics. ![]()

patient and her father
Photo by Rhoda Baer
The US may see nearly 1.7 million new cancer cases in 2017 and more than 600,000 cancer-related deaths, according to a report from the American Cancer Society (ACS).
In addition to estimates for 2017, the report, “Cancer Statistics 2017,” includes the most recent data on cancer incidence, mortality, and survival in the US.
The report was published in CA: A Cancer Journal for Clinicians.
The report projects there will be 1,688,780 new cancer cases and 600,920 cancer deaths in the US this year.
This includes:
- 80,500 new cases of lymphoma and 21,210 lymphoma deaths
- 62,130 new cases of leukemia and 24,500 leukemia deaths
- 30,280 new cases of myeloma and 12,590 myeloma deaths.
The report also shows that, from 2004 to 2013, the overall cancer incidence rate was stable in women and declined by about 2% per year in men. From 2005 to 2014, the cancer death rate declined by about 1.5% annually in both men and women.
Overall, the cancer death rate dropped 25% from its peak of 215.1 (per 100,000 population) in 1991 to 161.2 (per 100,000 population) in 2014, the latest year for which data was available. This translates to about 2,143,200 fewer cancer deaths.
“The continuing drops in the cancer death rate are a powerful sign of the potential we have to reduce cancer’s deadly toll,” said Otis W. Brawley, MD, chief medical officer of the ACS.
He said the decrease in cancer death rates is the result of steady reductions in smoking and advances in early detection and treatment. The decrease is driven by decreasing death rates for the 4 major cancer sites—lung, breast, colorectal, and prostate.
The report also shows that racial disparities in cancer death rates continue to decline. The excess risk of cancer death in black men has dropped from 47% in 1990 to 21% in 2014. The black/white disparity declined similarly in women, from a peak of 20% in 1998 to 13% in 2014.
On the other hand, significant gender disparities persist for both cancer incidence and death in the US. For all cancer sites combined, the incidence rate is 20% higher in men than in women, and the cancer death rate is 40% higher in men.
Dr Brawley said the gender gap in cancer mortality largely reflects variation in the distribution of cancers that occur in men and women, much of which is due to differences in the prevalence of cancer risk factors.
The yearly “Cancer Statistics” reports have been published by ACS researchers since 1967 to inform and guide clinicians, investigators, and others in public health in prioritizing efforts to reduce the burden of cancer.
Cancer incidence data for the current report were collected by the Surveillance, Epidemiology, and End Results Program; the National Program of Cancer Registries; and the North American Association of Central Cancer Registries. Mortality data were collected by the National Center for Health Statistics. ![]()

patient and her father
Photo by Rhoda Baer
The US may see nearly 1.7 million new cancer cases in 2017 and more than 600,000 cancer-related deaths, according to a report from the American Cancer Society (ACS).
In addition to estimates for 2017, the report, “Cancer Statistics 2017,” includes the most recent data on cancer incidence, mortality, and survival in the US.
The report was published in CA: A Cancer Journal for Clinicians.
The report projects there will be 1,688,780 new cancer cases and 600,920 cancer deaths in the US this year.
This includes:
- 80,500 new cases of lymphoma and 21,210 lymphoma deaths
- 62,130 new cases of leukemia and 24,500 leukemia deaths
- 30,280 new cases of myeloma and 12,590 myeloma deaths.
The report also shows that, from 2004 to 2013, the overall cancer incidence rate was stable in women and declined by about 2% per year in men. From 2005 to 2014, the cancer death rate declined by about 1.5% annually in both men and women.
Overall, the cancer death rate dropped 25% from its peak of 215.1 (per 100,000 population) in 1991 to 161.2 (per 100,000 population) in 2014, the latest year for which data was available. This translates to about 2,143,200 fewer cancer deaths.
“The continuing drops in the cancer death rate are a powerful sign of the potential we have to reduce cancer’s deadly toll,” said Otis W. Brawley, MD, chief medical officer of the ACS.
He said the decrease in cancer death rates is the result of steady reductions in smoking and advances in early detection and treatment. The decrease is driven by decreasing death rates for the 4 major cancer sites—lung, breast, colorectal, and prostate.
The report also shows that racial disparities in cancer death rates continue to decline. The excess risk of cancer death in black men has dropped from 47% in 1990 to 21% in 2014. The black/white disparity declined similarly in women, from a peak of 20% in 1998 to 13% in 2014.
On the other hand, significant gender disparities persist for both cancer incidence and death in the US. For all cancer sites combined, the incidence rate is 20% higher in men than in women, and the cancer death rate is 40% higher in men.
Dr Brawley said the gender gap in cancer mortality largely reflects variation in the distribution of cancers that occur in men and women, much of which is due to differences in the prevalence of cancer risk factors.
The yearly “Cancer Statistics” reports have been published by ACS researchers since 1967 to inform and guide clinicians, investigators, and others in public health in prioritizing efforts to reduce the burden of cancer.
Cancer incidence data for the current report were collected by the Surveillance, Epidemiology, and End Results Program; the National Program of Cancer Registries; and the North American Association of Central Cancer Registries. Mortality data were collected by the National Center for Health Statistics. ![]()
Cancer genomic data released to public

Photo courtesy of the
National Institute of
General Medical Sciences
The American Association for Cancer Research (AACR) has announced the first public release of cancer genomic data aggregated through the AACR Project Genomics Evidence Neoplasia Information Exchange (GENIE).
The data set includes nearly 19,000 de-identified genomic records collected from patients who were treated at 8 international institutions, making it one of the largest public cancer genomic data sets released to date.
The release includes data for 59 major cancer types, including leukemias, lymphomas, and multiple myeloma.
The genomic data and a limited amount of linked clinical data for each patient can be accessed via the AACR Project GENIE cBioPortal or from Sage Bionetworks. (Users must create an account for either site to access the data.)
“We are excited to make publicly available this very large set of clinical-grade, next-generation sequencing data obtained during routine patient care,” said Charles L. Sawyers, MD, AACR Project GENIE Steering Committee chairperson.
“These data were generated as part of routine patient care and, without AACR Project GENIE, they would likely never have been shared with the global cancer research community.”
AACR Project GENIE is a multi-phase, international data-sharing project aimed at catalyzing precision oncology through the development of a registry that aggregates and links clinical-grade cancer genomic data with clinical outcomes from tens of thousands of cancer patients treated at multiple institutions.
The newly released data are fully de-identified in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
The data are derived from patients whose tumors were genetically sequenced as part of their care at any of the 8 institutions that participated in the first phase of AACR Project GENIE.
The goal of releasing these data to the cancer research community is to aid new research that will accelerate the pace of progress against cancer.
According to AACR, the data can be used to validate gene signatures of drug response or prognosis, identify new patient populations for drugs that are currently available, and uncover new drug targets and biomarkers.
“I am extremely proud that the American Association for Cancer Research, as the coordinating center for AACR Project GENIE, is delivering on its promise to make these important data publicly available just over a year after unveiling the initiative,” said Margaret Foti, PhD, MD, chief executive officer of the AACR.
To expand the AACR Project GENIE registry, the consortium is accepting applications for new participating centers. Any nonprofit institution that meets certain criteria can submit an application to become a project participant.
For more information on AACR Project GENIE, visit the project website or send an email to [email protected]. ![]()

Photo courtesy of the
National Institute of
General Medical Sciences
The American Association for Cancer Research (AACR) has announced the first public release of cancer genomic data aggregated through the AACR Project Genomics Evidence Neoplasia Information Exchange (GENIE).
The data set includes nearly 19,000 de-identified genomic records collected from patients who were treated at 8 international institutions, making it one of the largest public cancer genomic data sets released to date.
The release includes data for 59 major cancer types, including leukemias, lymphomas, and multiple myeloma.
The genomic data and a limited amount of linked clinical data for each patient can be accessed via the AACR Project GENIE cBioPortal or from Sage Bionetworks. (Users must create an account for either site to access the data.)
“We are excited to make publicly available this very large set of clinical-grade, next-generation sequencing data obtained during routine patient care,” said Charles L. Sawyers, MD, AACR Project GENIE Steering Committee chairperson.
“These data were generated as part of routine patient care and, without AACR Project GENIE, they would likely never have been shared with the global cancer research community.”
AACR Project GENIE is a multi-phase, international data-sharing project aimed at catalyzing precision oncology through the development of a registry that aggregates and links clinical-grade cancer genomic data with clinical outcomes from tens of thousands of cancer patients treated at multiple institutions.
The newly released data are fully de-identified in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
The data are derived from patients whose tumors were genetically sequenced as part of their care at any of the 8 institutions that participated in the first phase of AACR Project GENIE.
The goal of releasing these data to the cancer research community is to aid new research that will accelerate the pace of progress against cancer.
According to AACR, the data can be used to validate gene signatures of drug response or prognosis, identify new patient populations for drugs that are currently available, and uncover new drug targets and biomarkers.
“I am extremely proud that the American Association for Cancer Research, as the coordinating center for AACR Project GENIE, is delivering on its promise to make these important data publicly available just over a year after unveiling the initiative,” said Margaret Foti, PhD, MD, chief executive officer of the AACR.
To expand the AACR Project GENIE registry, the consortium is accepting applications for new participating centers. Any nonprofit institution that meets certain criteria can submit an application to become a project participant.
For more information on AACR Project GENIE, visit the project website or send an email to [email protected]. ![]()

Photo courtesy of the
National Institute of
General Medical Sciences
The American Association for Cancer Research (AACR) has announced the first public release of cancer genomic data aggregated through the AACR Project Genomics Evidence Neoplasia Information Exchange (GENIE).
The data set includes nearly 19,000 de-identified genomic records collected from patients who were treated at 8 international institutions, making it one of the largest public cancer genomic data sets released to date.
The release includes data for 59 major cancer types, including leukemias, lymphomas, and multiple myeloma.
The genomic data and a limited amount of linked clinical data for each patient can be accessed via the AACR Project GENIE cBioPortal or from Sage Bionetworks. (Users must create an account for either site to access the data.)
“We are excited to make publicly available this very large set of clinical-grade, next-generation sequencing data obtained during routine patient care,” said Charles L. Sawyers, MD, AACR Project GENIE Steering Committee chairperson.
“These data were generated as part of routine patient care and, without AACR Project GENIE, they would likely never have been shared with the global cancer research community.”
AACR Project GENIE is a multi-phase, international data-sharing project aimed at catalyzing precision oncology through the development of a registry that aggregates and links clinical-grade cancer genomic data with clinical outcomes from tens of thousands of cancer patients treated at multiple institutions.
The newly released data are fully de-identified in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
The data are derived from patients whose tumors were genetically sequenced as part of their care at any of the 8 institutions that participated in the first phase of AACR Project GENIE.
The goal of releasing these data to the cancer research community is to aid new research that will accelerate the pace of progress against cancer.
According to AACR, the data can be used to validate gene signatures of drug response or prognosis, identify new patient populations for drugs that are currently available, and uncover new drug targets and biomarkers.
“I am extremely proud that the American Association for Cancer Research, as the coordinating center for AACR Project GENIE, is delivering on its promise to make these important data publicly available just over a year after unveiling the initiative,” said Margaret Foti, PhD, MD, chief executive officer of the AACR.
To expand the AACR Project GENIE registry, the consortium is accepting applications for new participating centers. Any nonprofit institution that meets certain criteria can submit an application to become a project participant.
For more information on AACR Project GENIE, visit the project website or send an email to [email protected]. ![]()
Syringes recalled due to infection risk
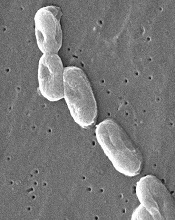
Image courtesy of
CDC/Janice Carr
The US Food and Drug Administration (FDA) has announced a Class I, nationwide recall of Nurse Assist Inc.’s normal saline flush syringes due to incidents of Burkholderia cepacia contamination.
Nurse Assist reported that patients developed B cepacia bloodstream infections while receiving intravenous care using the company’s prepackaged saline flush syringes, so the company voluntarily recalled all unexpired lots of the product.
This recall was first announced last October, but, yesterday, the FDA classified it as a Class I recall and said use of these syringes may cause serious injuries or death.
Nurse Assist said it is investigating the link between the syringes and the infections with the FDA, the US Centers for Disease Control, and various state health departments.
Until the investigation can be completed, all healthcare facilities with affected product should discontinue use and return the product to the supplier.
The normal saline flush is a plastic syringe filled with 0.9% sodium chloride (a 12 mL IV flush syringe with a 3 mL, 5 mL, or 10 mL fill volume). It is used to clear out medical devices that deliver medicine directly into the veins of a patient through a needle or catheter.
All unexpired lots of this product are covered by the recall. Product Numbers 1203, 1205, and 1210 are packaged 30 syringes to an inner carton and 6 inner cartons in a case (180 syringes).
For product number 1210-BP, 100 syringes are packaged in an inner carton with 4 inner cartons in a case (400 syringes). Lot code information can be found on the outer case panel, the back panel of the inner carton, and on each syringe label.
The lots being recalled were distributed to customers and distributors between February 16, 2016, and September 30, 2016. Product can be identified by the labeling on the packaging and device.
Customers with questions can contact Nurse Assist Inc. at 1-800-649-6800 ext. 10, Monday through Friday, between the hours of 8 am and 5 pm, Central Time, or via email at [email protected].
Healthcare professionals and consumers can report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program. ![]()

Image courtesy of
CDC/Janice Carr
The US Food and Drug Administration (FDA) has announced a Class I, nationwide recall of Nurse Assist Inc.’s normal saline flush syringes due to incidents of Burkholderia cepacia contamination.
Nurse Assist reported that patients developed B cepacia bloodstream infections while receiving intravenous care using the company’s prepackaged saline flush syringes, so the company voluntarily recalled all unexpired lots of the product.
This recall was first announced last October, but, yesterday, the FDA classified it as a Class I recall and said use of these syringes may cause serious injuries or death.
Nurse Assist said it is investigating the link between the syringes and the infections with the FDA, the US Centers for Disease Control, and various state health departments.
Until the investigation can be completed, all healthcare facilities with affected product should discontinue use and return the product to the supplier.
The normal saline flush is a plastic syringe filled with 0.9% sodium chloride (a 12 mL IV flush syringe with a 3 mL, 5 mL, or 10 mL fill volume). It is used to clear out medical devices that deliver medicine directly into the veins of a patient through a needle or catheter.
All unexpired lots of this product are covered by the recall. Product Numbers 1203, 1205, and 1210 are packaged 30 syringes to an inner carton and 6 inner cartons in a case (180 syringes).
For product number 1210-BP, 100 syringes are packaged in an inner carton with 4 inner cartons in a case (400 syringes). Lot code information can be found on the outer case panel, the back panel of the inner carton, and on each syringe label.
The lots being recalled were distributed to customers and distributors between February 16, 2016, and September 30, 2016. Product can be identified by the labeling on the packaging and device.
Customers with questions can contact Nurse Assist Inc. at 1-800-649-6800 ext. 10, Monday through Friday, between the hours of 8 am and 5 pm, Central Time, or via email at [email protected].
Healthcare professionals and consumers can report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program. ![]()

Image courtesy of
CDC/Janice Carr
The US Food and Drug Administration (FDA) has announced a Class I, nationwide recall of Nurse Assist Inc.’s normal saline flush syringes due to incidents of Burkholderia cepacia contamination.
Nurse Assist reported that patients developed B cepacia bloodstream infections while receiving intravenous care using the company’s prepackaged saline flush syringes, so the company voluntarily recalled all unexpired lots of the product.
This recall was first announced last October, but, yesterday, the FDA classified it as a Class I recall and said use of these syringes may cause serious injuries or death.
Nurse Assist said it is investigating the link between the syringes and the infections with the FDA, the US Centers for Disease Control, and various state health departments.
Until the investigation can be completed, all healthcare facilities with affected product should discontinue use and return the product to the supplier.
The normal saline flush is a plastic syringe filled with 0.9% sodium chloride (a 12 mL IV flush syringe with a 3 mL, 5 mL, or 10 mL fill volume). It is used to clear out medical devices that deliver medicine directly into the veins of a patient through a needle or catheter.
All unexpired lots of this product are covered by the recall. Product Numbers 1203, 1205, and 1210 are packaged 30 syringes to an inner carton and 6 inner cartons in a case (180 syringes).
For product number 1210-BP, 100 syringes are packaged in an inner carton with 4 inner cartons in a case (400 syringes). Lot code information can be found on the outer case panel, the back panel of the inner carton, and on each syringe label.
The lots being recalled were distributed to customers and distributors between February 16, 2016, and September 30, 2016. Product can be identified by the labeling on the packaging and device.
Customers with questions can contact Nurse Assist Inc. at 1-800-649-6800 ext. 10, Monday through Friday, between the hours of 8 am and 5 pm, Central Time, or via email at [email protected].
Healthcare professionals and consumers can report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program. ![]()
GAP malaria vaccine shows early promise
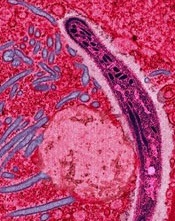
Image from Margaret Shear
and Ute Frevert
A next-generation malaria vaccine that uses genetically attenuated parasites (GAPs) has demonstrated promise in a phase 1 trial and in experiments with mice, according to researchers.
The team generated a genetically attenuated Plasmodium falciparum parasite by knocking out 3 genes that are required for the parasite to cause malaria in humans.
Healthy volunteers infected with this parasite, Pf GAP3KO, exhibited strong antibody responses, according to researchers, and experienced only mild or moderate adverse events (AEs).
When antibodies from these patients were transferred to humanized mice, they inhibited malaria infection in the liver.
The researchers described this work in Science Translational Medicine.
“This most recent publication builds on our previous work,” said study author Sebastian Mikolajczak, PhD, of the Center for Infectious Disease Research in Seattle, Washington.
“We had already good indicators in preclinical studies that this new ‘triple knock-out’ GAP (GAP3KO), which has 3 genes removed, is completely attenuated. The clinical study now shows that the GAP3KO vaccine is completely attenuated in humans and also shows that, even after only a single administration, it elicits a robust immune response against the malaria parasite. Together, these findings are critical milestones for malaria vaccine development.”
Dr Mikolajczak and his colleagues created GAP3KO by knocking out 3 genes in P falciparum that are required for the parasite to successfully infect and cause disease in humans—Pf p52−/p36−/sap1−.
The researchers then tested Pf GAP3KO sporozoites in 10 healthy human volunteers. The sporozoites were delivered via bites from infected mosquitoes.
All subjects experienced grade 1 AEs, and 70% had grade 2 AEs. There were no grade 3 or higher AEs.
Grade 2 AEs included 1 case of fatigue and several cases of administration-site reactions, including erythema (n=6), pruritus (n=2), and swelling (n=3).
All 10 volunteers completed the 28-day study period without exhibiting any symptoms of malaria. They also remained negative for blood-stage parasitemia throughout the study period.
All subjects experienced antibody responses as well. The researchers collected sera from the volunteers on day 0, 7, 13, and 28 after immunization and analyzed it for anti-circumsporozoite protein (CSP) antibody responses.
The team measured anti-CSP immunoglobulin G (IgG) by enzyme-linked immunosorbent assay (ELISA) using full-length recombinant PfCSP protein.
At day 0, volunteers’ sera showed an average of 1436 ± 257.3 arbitrary units (AU) of anti-CSP titers by ELISA. However, 3 volunteers were slightly above the 2000 AU cutoff for positivity.
At day 7, 8 of the 10 volunteers were positive for anti-CSP IgG, with an average titer of 3821 ± 808.1 AU. At day 13, all volunteers were positive, with an average of 11,547 ± 2084 AU. Positivity was maintained through day 28, with an average of 5774± 840 AU.
To determine whether these antibodies could inhibit Pf sporozoite invasion in vivo, the researchers transferred them to FRG huHep liver chimeric humanized mice, which allow for complete development of liver-stage parasites.
The researchers chose 5 volunteers whose immune serum showed high levels of invasion inhibition in vitro and varying CSP IgG titers. The mice received purified IgG from these volunteers from day 0 and day 13 immune samples (5 mice per volunteer per time point).
The mice were then challenged with bites from mosquitoes infected with sporozoites of a green fluorescent protein–luciferase–expressing P falciparum strain.
The researchers calculated inhibition of infection by comparing the liver-stage burden of mice receiving day 13 IgG samples to the mean value of liver-stage burden in mice receiving day 0 IgG samples.
The day 13 IgG samples inhibited liver infection by an average of 23.18 ± 57%, 32.23 ± 29.16%, 69.38 ± 18.64%, 76.63 ± 19.98%, and 87.99 ± 15.56% (per volunteer).
The 3 volunteers whose serum exhibited the highest inhibition had the lowest Pf CSP titers of the 5 samples.
The researchers said these promising results in mice and humans pave the way to a phase 1b trial of the GAP3KO vaccine candidate using controlled human malaria infection. ![]()

Image from Margaret Shear
and Ute Frevert
A next-generation malaria vaccine that uses genetically attenuated parasites (GAPs) has demonstrated promise in a phase 1 trial and in experiments with mice, according to researchers.
The team generated a genetically attenuated Plasmodium falciparum parasite by knocking out 3 genes that are required for the parasite to cause malaria in humans.
Healthy volunteers infected with this parasite, Pf GAP3KO, exhibited strong antibody responses, according to researchers, and experienced only mild or moderate adverse events (AEs).
When antibodies from these patients were transferred to humanized mice, they inhibited malaria infection in the liver.
The researchers described this work in Science Translational Medicine.
“This most recent publication builds on our previous work,” said study author Sebastian Mikolajczak, PhD, of the Center for Infectious Disease Research in Seattle, Washington.
“We had already good indicators in preclinical studies that this new ‘triple knock-out’ GAP (GAP3KO), which has 3 genes removed, is completely attenuated. The clinical study now shows that the GAP3KO vaccine is completely attenuated in humans and also shows that, even after only a single administration, it elicits a robust immune response against the malaria parasite. Together, these findings are critical milestones for malaria vaccine development.”
Dr Mikolajczak and his colleagues created GAP3KO by knocking out 3 genes in P falciparum that are required for the parasite to successfully infect and cause disease in humans—Pf p52−/p36−/sap1−.
The researchers then tested Pf GAP3KO sporozoites in 10 healthy human volunteers. The sporozoites were delivered via bites from infected mosquitoes.
All subjects experienced grade 1 AEs, and 70% had grade 2 AEs. There were no grade 3 or higher AEs.
Grade 2 AEs included 1 case of fatigue and several cases of administration-site reactions, including erythema (n=6), pruritus (n=2), and swelling (n=3).
All 10 volunteers completed the 28-day study period without exhibiting any symptoms of malaria. They also remained negative for blood-stage parasitemia throughout the study period.
All subjects experienced antibody responses as well. The researchers collected sera from the volunteers on day 0, 7, 13, and 28 after immunization and analyzed it for anti-circumsporozoite protein (CSP) antibody responses.
The team measured anti-CSP immunoglobulin G (IgG) by enzyme-linked immunosorbent assay (ELISA) using full-length recombinant PfCSP protein.
At day 0, volunteers’ sera showed an average of 1436 ± 257.3 arbitrary units (AU) of anti-CSP titers by ELISA. However, 3 volunteers were slightly above the 2000 AU cutoff for positivity.
At day 7, 8 of the 10 volunteers were positive for anti-CSP IgG, with an average titer of 3821 ± 808.1 AU. At day 13, all volunteers were positive, with an average of 11,547 ± 2084 AU. Positivity was maintained through day 28, with an average of 5774± 840 AU.
To determine whether these antibodies could inhibit Pf sporozoite invasion in vivo, the researchers transferred them to FRG huHep liver chimeric humanized mice, which allow for complete development of liver-stage parasites.
The researchers chose 5 volunteers whose immune serum showed high levels of invasion inhibition in vitro and varying CSP IgG titers. The mice received purified IgG from these volunteers from day 0 and day 13 immune samples (5 mice per volunteer per time point).
The mice were then challenged with bites from mosquitoes infected with sporozoites of a green fluorescent protein–luciferase–expressing P falciparum strain.
The researchers calculated inhibition of infection by comparing the liver-stage burden of mice receiving day 13 IgG samples to the mean value of liver-stage burden in mice receiving day 0 IgG samples.
The day 13 IgG samples inhibited liver infection by an average of 23.18 ± 57%, 32.23 ± 29.16%, 69.38 ± 18.64%, 76.63 ± 19.98%, and 87.99 ± 15.56% (per volunteer).
The 3 volunteers whose serum exhibited the highest inhibition had the lowest Pf CSP titers of the 5 samples.
The researchers said these promising results in mice and humans pave the way to a phase 1b trial of the GAP3KO vaccine candidate using controlled human malaria infection. ![]()

Image from Margaret Shear
and Ute Frevert
A next-generation malaria vaccine that uses genetically attenuated parasites (GAPs) has demonstrated promise in a phase 1 trial and in experiments with mice, according to researchers.
The team generated a genetically attenuated Plasmodium falciparum parasite by knocking out 3 genes that are required for the parasite to cause malaria in humans.
Healthy volunteers infected with this parasite, Pf GAP3KO, exhibited strong antibody responses, according to researchers, and experienced only mild or moderate adverse events (AEs).
When antibodies from these patients were transferred to humanized mice, they inhibited malaria infection in the liver.
The researchers described this work in Science Translational Medicine.
“This most recent publication builds on our previous work,” said study author Sebastian Mikolajczak, PhD, of the Center for Infectious Disease Research in Seattle, Washington.
“We had already good indicators in preclinical studies that this new ‘triple knock-out’ GAP (GAP3KO), which has 3 genes removed, is completely attenuated. The clinical study now shows that the GAP3KO vaccine is completely attenuated in humans and also shows that, even after only a single administration, it elicits a robust immune response against the malaria parasite. Together, these findings are critical milestones for malaria vaccine development.”
Dr Mikolajczak and his colleagues created GAP3KO by knocking out 3 genes in P falciparum that are required for the parasite to successfully infect and cause disease in humans—Pf p52−/p36−/sap1−.
The researchers then tested Pf GAP3KO sporozoites in 10 healthy human volunteers. The sporozoites were delivered via bites from infected mosquitoes.
All subjects experienced grade 1 AEs, and 70% had grade 2 AEs. There were no grade 3 or higher AEs.
Grade 2 AEs included 1 case of fatigue and several cases of administration-site reactions, including erythema (n=6), pruritus (n=2), and swelling (n=3).
All 10 volunteers completed the 28-day study period without exhibiting any symptoms of malaria. They also remained negative for blood-stage parasitemia throughout the study period.
All subjects experienced antibody responses as well. The researchers collected sera from the volunteers on day 0, 7, 13, and 28 after immunization and analyzed it for anti-circumsporozoite protein (CSP) antibody responses.
The team measured anti-CSP immunoglobulin G (IgG) by enzyme-linked immunosorbent assay (ELISA) using full-length recombinant PfCSP protein.
At day 0, volunteers’ sera showed an average of 1436 ± 257.3 arbitrary units (AU) of anti-CSP titers by ELISA. However, 3 volunteers were slightly above the 2000 AU cutoff for positivity.
At day 7, 8 of the 10 volunteers were positive for anti-CSP IgG, with an average titer of 3821 ± 808.1 AU. At day 13, all volunteers were positive, with an average of 11,547 ± 2084 AU. Positivity was maintained through day 28, with an average of 5774± 840 AU.
To determine whether these antibodies could inhibit Pf sporozoite invasion in vivo, the researchers transferred them to FRG huHep liver chimeric humanized mice, which allow for complete development of liver-stage parasites.
The researchers chose 5 volunteers whose immune serum showed high levels of invasion inhibition in vitro and varying CSP IgG titers. The mice received purified IgG from these volunteers from day 0 and day 13 immune samples (5 mice per volunteer per time point).
The mice were then challenged with bites from mosquitoes infected with sporozoites of a green fluorescent protein–luciferase–expressing P falciparum strain.
The researchers calculated inhibition of infection by comparing the liver-stage burden of mice receiving day 13 IgG samples to the mean value of liver-stage burden in mice receiving day 0 IgG samples.
The day 13 IgG samples inhibited liver infection by an average of 23.18 ± 57%, 32.23 ± 29.16%, 69.38 ± 18.64%, 76.63 ± 19.98%, and 87.99 ± 15.56% (per volunteer).
The 3 volunteers whose serum exhibited the highest inhibition had the lowest Pf CSP titers of the 5 samples.
The researchers said these promising results in mice and humans pave the way to a phase 1b trial of the GAP3KO vaccine candidate using controlled human malaria infection. ![]()
Intervention relieves distress in cancer patients

chemotherapy
Photo by Rhoda Baer
Results of a small study suggest a single dose of the hallucinogenic drug psilocybin, when combined with counseling, can significantly lessen psychological distress in cancer patients for months at a time.
The study showed that psychological counseling and a single dose of psilocybin brought relief from distress that lasted for more than 6 months in a majority of the subjects monitored.
This was based on clinical evaluation scores for anxiety and depression.
“Our results represent the strongest evidence to date of a clinical benefit from psilocybin therapy, with the potential to transform care for patients with cancer-related psychological distress,” said study author Stephen Ross, MD, of New York University School of Medicine in New York, New York.
“If larger clinical trials prove successful, then we could ultimately have available a safe, effective, and inexpensive medication—dispensed under strict control—to alleviate the distress that increases suicide rates among cancer patients.”
Dr Ross and his colleagues reported the results of their study in the Journal of Psychopharmacology alongside a related study and 11 accompanying editorials.
Dr Ross’s study included 29 patients with cancer-related anxiety and depression. Their mean age was 56, and 62% were female. Ninety percent were Caucasian, and 10% were classified as “other” race.
Patients had breast cancer (31%), reproductive cancers (28%), digestive cancers (17%), leukemia/lymphoma (14%), and other cancers (10%).
All patients had been diagnosed as suffering from serious psychological distress related to their disease.
Treatment
Half of the patients were randomly assigned to receive a 0.3 mg/kg dose of psilocybin, and half received a vitamin placebo (250 mg of niacin) known to produce a “rush” that mimics a hallucinogenic drug experience.
Approximately half way through the study’s monitoring period (after 7 weeks), all patients switched treatments. Those who initially received psilocybin took a single dose of niacin, and vice-versa. Neither patients nor researchers knew who had first received psilocybin or placebo.
All patients were provided with tailored counseling from a psychiatrist, psychologist, nurse, or social worker. And the patients were monitored for side effects and improvements in their mental state.
Safety
The researchers said there were no serious adverse events (AEs), either medical or psychiatric, that were attributed to psilocybin.
The most common medical AEs that were attributable to psilocybin were non-clinically significant elevations in blood pressure and heart rate (76%), headaches/migraines (28%), and nausea (14%).
The most common psychiatric AEs attributable to psilocybin were transient anxiety (17%) and transient psychotic-like symptoms (7%; 1 case of transient paranoid ideation and 1 case of transient thought disorder).
Efficacy
The researchers said that, prior to the crossover, psilocybin produced immediate, substantial, and sustained improvements in anxiety and depression.
Specifically, patients who received psilocybin first had significant improvements in responses on the Hospital Anxiety and Depression Scale and the Beck Depression Inventory, when compared to patients who received niacin first.
The differences were significant 1 day after the patients’ first session and 7 weeks after the first session (P≤0.01 for all).
At the 6.5-month follow-up, 60% to 80% of participants continued with clinically significant reductions in depression or anxiety.
The researchers said a key finding of this study was that improvements in clinical evaluation scores for anxiety and depression lasted for the study’s extended monitoring period, which was 8 months for those who took psilocybin first.
Patients also reported post-psilocybin improvements in their quality of life, such as going out more, greater energy, getting along better with family members, and doing well at work. Some reported variations of spirituality, unusual peacefulness, and increased feelings of altruism.
“Our study showed that psilocybin facilitated experiences that drove reductions in psychological distress,” said study author Anthony Bossis, PhD, of New York University School of Medicine. “And if it’s true for cancer care, then it could apply to other stressful medical conditions.”
He cautioned that patients should not consume psilocybin on their own or without supervision from a physician and a trained counselor.
“Psilocybin therapy may not work for everyone,” he noted. “And some groups, such as people with schizophrenia, as well as adolescents, should not be treated with it.” ![]()

chemotherapy
Photo by Rhoda Baer
Results of a small study suggest a single dose of the hallucinogenic drug psilocybin, when combined with counseling, can significantly lessen psychological distress in cancer patients for months at a time.
The study showed that psychological counseling and a single dose of psilocybin brought relief from distress that lasted for more than 6 months in a majority of the subjects monitored.
This was based on clinical evaluation scores for anxiety and depression.
“Our results represent the strongest evidence to date of a clinical benefit from psilocybin therapy, with the potential to transform care for patients with cancer-related psychological distress,” said study author Stephen Ross, MD, of New York University School of Medicine in New York, New York.
“If larger clinical trials prove successful, then we could ultimately have available a safe, effective, and inexpensive medication—dispensed under strict control—to alleviate the distress that increases suicide rates among cancer patients.”
Dr Ross and his colleagues reported the results of their study in the Journal of Psychopharmacology alongside a related study and 11 accompanying editorials.
Dr Ross’s study included 29 patients with cancer-related anxiety and depression. Their mean age was 56, and 62% were female. Ninety percent were Caucasian, and 10% were classified as “other” race.
Patients had breast cancer (31%), reproductive cancers (28%), digestive cancers (17%), leukemia/lymphoma (14%), and other cancers (10%).
All patients had been diagnosed as suffering from serious psychological distress related to their disease.
Treatment
Half of the patients were randomly assigned to receive a 0.3 mg/kg dose of psilocybin, and half received a vitamin placebo (250 mg of niacin) known to produce a “rush” that mimics a hallucinogenic drug experience.
Approximately half way through the study’s monitoring period (after 7 weeks), all patients switched treatments. Those who initially received psilocybin took a single dose of niacin, and vice-versa. Neither patients nor researchers knew who had first received psilocybin or placebo.
All patients were provided with tailored counseling from a psychiatrist, psychologist, nurse, or social worker. And the patients were monitored for side effects and improvements in their mental state.
Safety
The researchers said there were no serious adverse events (AEs), either medical or psychiatric, that were attributed to psilocybin.
The most common medical AEs that were attributable to psilocybin were non-clinically significant elevations in blood pressure and heart rate (76%), headaches/migraines (28%), and nausea (14%).
The most common psychiatric AEs attributable to psilocybin were transient anxiety (17%) and transient psychotic-like symptoms (7%; 1 case of transient paranoid ideation and 1 case of transient thought disorder).
Efficacy
The researchers said that, prior to the crossover, psilocybin produced immediate, substantial, and sustained improvements in anxiety and depression.
Specifically, patients who received psilocybin first had significant improvements in responses on the Hospital Anxiety and Depression Scale and the Beck Depression Inventory, when compared to patients who received niacin first.
The differences were significant 1 day after the patients’ first session and 7 weeks after the first session (P≤0.01 for all).
At the 6.5-month follow-up, 60% to 80% of participants continued with clinically significant reductions in depression or anxiety.
The researchers said a key finding of this study was that improvements in clinical evaluation scores for anxiety and depression lasted for the study’s extended monitoring period, which was 8 months for those who took psilocybin first.
Patients also reported post-psilocybin improvements in their quality of life, such as going out more, greater energy, getting along better with family members, and doing well at work. Some reported variations of spirituality, unusual peacefulness, and increased feelings of altruism.
“Our study showed that psilocybin facilitated experiences that drove reductions in psychological distress,” said study author Anthony Bossis, PhD, of New York University School of Medicine. “And if it’s true for cancer care, then it could apply to other stressful medical conditions.”
He cautioned that patients should not consume psilocybin on their own or without supervision from a physician and a trained counselor.
“Psilocybin therapy may not work for everyone,” he noted. “And some groups, such as people with schizophrenia, as well as adolescents, should not be treated with it.” ![]()

chemotherapy
Photo by Rhoda Baer
Results of a small study suggest a single dose of the hallucinogenic drug psilocybin, when combined with counseling, can significantly lessen psychological distress in cancer patients for months at a time.
The study showed that psychological counseling and a single dose of psilocybin brought relief from distress that lasted for more than 6 months in a majority of the subjects monitored.
This was based on clinical evaluation scores for anxiety and depression.
“Our results represent the strongest evidence to date of a clinical benefit from psilocybin therapy, with the potential to transform care for patients with cancer-related psychological distress,” said study author Stephen Ross, MD, of New York University School of Medicine in New York, New York.
“If larger clinical trials prove successful, then we could ultimately have available a safe, effective, and inexpensive medication—dispensed under strict control—to alleviate the distress that increases suicide rates among cancer patients.”
Dr Ross and his colleagues reported the results of their study in the Journal of Psychopharmacology alongside a related study and 11 accompanying editorials.
Dr Ross’s study included 29 patients with cancer-related anxiety and depression. Their mean age was 56, and 62% were female. Ninety percent were Caucasian, and 10% were classified as “other” race.
Patients had breast cancer (31%), reproductive cancers (28%), digestive cancers (17%), leukemia/lymphoma (14%), and other cancers (10%).
All patients had been diagnosed as suffering from serious psychological distress related to their disease.
Treatment
Half of the patients were randomly assigned to receive a 0.3 mg/kg dose of psilocybin, and half received a vitamin placebo (250 mg of niacin) known to produce a “rush” that mimics a hallucinogenic drug experience.
Approximately half way through the study’s monitoring period (after 7 weeks), all patients switched treatments. Those who initially received psilocybin took a single dose of niacin, and vice-versa. Neither patients nor researchers knew who had first received psilocybin or placebo.
All patients were provided with tailored counseling from a psychiatrist, psychologist, nurse, or social worker. And the patients were monitored for side effects and improvements in their mental state.
Safety
The researchers said there were no serious adverse events (AEs), either medical or psychiatric, that were attributed to psilocybin.
The most common medical AEs that were attributable to psilocybin were non-clinically significant elevations in blood pressure and heart rate (76%), headaches/migraines (28%), and nausea (14%).
The most common psychiatric AEs attributable to psilocybin were transient anxiety (17%) and transient psychotic-like symptoms (7%; 1 case of transient paranoid ideation and 1 case of transient thought disorder).
Efficacy
The researchers said that, prior to the crossover, psilocybin produced immediate, substantial, and sustained improvements in anxiety and depression.
Specifically, patients who received psilocybin first had significant improvements in responses on the Hospital Anxiety and Depression Scale and the Beck Depression Inventory, when compared to patients who received niacin first.
The differences were significant 1 day after the patients’ first session and 7 weeks after the first session (P≤0.01 for all).
At the 6.5-month follow-up, 60% to 80% of participants continued with clinically significant reductions in depression or anxiety.
The researchers said a key finding of this study was that improvements in clinical evaluation scores for anxiety and depression lasted for the study’s extended monitoring period, which was 8 months for those who took psilocybin first.
Patients also reported post-psilocybin improvements in their quality of life, such as going out more, greater energy, getting along better with family members, and doing well at work. Some reported variations of spirituality, unusual peacefulness, and increased feelings of altruism.
“Our study showed that psilocybin facilitated experiences that drove reductions in psychological distress,” said study author Anthony Bossis, PhD, of New York University School of Medicine. “And if it’s true for cancer care, then it could apply to other stressful medical conditions.”
He cautioned that patients should not consume psilocybin on their own or without supervision from a physician and a trained counselor.
“Psilocybin therapy may not work for everyone,” he noted. “And some groups, such as people with schizophrenia, as well as adolescents, should not be treated with it.” ![]()
Infection in AML patient prompts discovery

Photo courtesy of
Janice Carr/CDC
The quest to understand a prolonged infection in an infant with acute myeloid leukemia (AML) has led to the discovery of a mutation that allows bacteria to tolerate antibiotic therapy.
Researchers described this discovery in the journal mBio.
“These findings detail a ‘perfect storm’ for development of antibiotic tolerance by bacteria that already pose a clinical challenge,” said study author Jason Rosch, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The same conditions may be present in other patients with immune systems that have been compromised by chemotherapy or disease,” added co-author Joshua Wolf, MBBS, also of St. Jude.
The “perfect storm” involved a patient who was 6 weeks old when she was diagnosed with AML. The treatment wiped out her white blood cells, and, despite infection-control measures, she developed a bloodstream infection with vancomycin-resistant Enterococcus faecium (VRE).
The infection persisted for 26 days and only resolved after her immune system recovered. She then successfully completed AML treatment.
In-depth DNA sequencing of 22 VRE samples collected during the patient’s infection helped researchers link the prolonged infection to a point mutation in the relA gene of VRE.
The mutation inappropriately activated the stringent response pathway, which bacteria use to survive under stress and to tolerate antibiotics.
The mutation resulted in elevated levels of the signaling molecule alarmone, and this likely primed the bacteria to survive exposure to multiple antibiotics, the researchers said.
The team also noted that relA-mutant VRE was susceptible to the antibiotics linezolid and daptomycin in minimum inhibitory concentration testing and during planktonic growth.
However, when growing in biofilm, relA-mutant VRE could tolerate high doses of both antibiotics.
“This mutation has particular clinical significance because the antibiotics involved, linezolid and daptomycin, are the last line of defense against VRE infection,” Dr Wolf said.
Among the compounds in development for the treatment of bacterial biofilms is the experimental antibiotic ADEP-4. In this study, ADEP-4 killed relA-mutant and non-mutant VRE growing in biofilm in the lab.
“In the future, compounds like ADEP-4 may provide a new approach to resolving persistent infections,” Dr Wolf said.
Dr Rosch noted that evidence gleaned from tracking the evolution of VRE throughout the infection suggested the patient’s immune-compromised state was essential to survival of the mutant VRE.
Gene transcription was altered significantly in relA-mutant VRE and produced biofilms that were less robust and possibly unlikely to otherwise survive.
“The case expands our understanding of the role of the stringent response in susceptibility and tolerance to a wide range of antibiotics, especially in biofilms,” Dr Rosch said. “It also demonstrates that these mutations can develop and gain a foothold during a human infection.” ![]()

Photo courtesy of
Janice Carr/CDC
The quest to understand a prolonged infection in an infant with acute myeloid leukemia (AML) has led to the discovery of a mutation that allows bacteria to tolerate antibiotic therapy.
Researchers described this discovery in the journal mBio.
“These findings detail a ‘perfect storm’ for development of antibiotic tolerance by bacteria that already pose a clinical challenge,” said study author Jason Rosch, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The same conditions may be present in other patients with immune systems that have been compromised by chemotherapy or disease,” added co-author Joshua Wolf, MBBS, also of St. Jude.
The “perfect storm” involved a patient who was 6 weeks old when she was diagnosed with AML. The treatment wiped out her white blood cells, and, despite infection-control measures, she developed a bloodstream infection with vancomycin-resistant Enterococcus faecium (VRE).
The infection persisted for 26 days and only resolved after her immune system recovered. She then successfully completed AML treatment.
In-depth DNA sequencing of 22 VRE samples collected during the patient’s infection helped researchers link the prolonged infection to a point mutation in the relA gene of VRE.
The mutation inappropriately activated the stringent response pathway, which bacteria use to survive under stress and to tolerate antibiotics.
The mutation resulted in elevated levels of the signaling molecule alarmone, and this likely primed the bacteria to survive exposure to multiple antibiotics, the researchers said.
The team also noted that relA-mutant VRE was susceptible to the antibiotics linezolid and daptomycin in minimum inhibitory concentration testing and during planktonic growth.
However, when growing in biofilm, relA-mutant VRE could tolerate high doses of both antibiotics.
“This mutation has particular clinical significance because the antibiotics involved, linezolid and daptomycin, are the last line of defense against VRE infection,” Dr Wolf said.
Among the compounds in development for the treatment of bacterial biofilms is the experimental antibiotic ADEP-4. In this study, ADEP-4 killed relA-mutant and non-mutant VRE growing in biofilm in the lab.
“In the future, compounds like ADEP-4 may provide a new approach to resolving persistent infections,” Dr Wolf said.
Dr Rosch noted that evidence gleaned from tracking the evolution of VRE throughout the infection suggested the patient’s immune-compromised state was essential to survival of the mutant VRE.
Gene transcription was altered significantly in relA-mutant VRE and produced biofilms that were less robust and possibly unlikely to otherwise survive.
“The case expands our understanding of the role of the stringent response in susceptibility and tolerance to a wide range of antibiotics, especially in biofilms,” Dr Rosch said. “It also demonstrates that these mutations can develop and gain a foothold during a human infection.” ![]()

Photo courtesy of
Janice Carr/CDC
The quest to understand a prolonged infection in an infant with acute myeloid leukemia (AML) has led to the discovery of a mutation that allows bacteria to tolerate antibiotic therapy.
Researchers described this discovery in the journal mBio.
“These findings detail a ‘perfect storm’ for development of antibiotic tolerance by bacteria that already pose a clinical challenge,” said study author Jason Rosch, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The same conditions may be present in other patients with immune systems that have been compromised by chemotherapy or disease,” added co-author Joshua Wolf, MBBS, also of St. Jude.
The “perfect storm” involved a patient who was 6 weeks old when she was diagnosed with AML. The treatment wiped out her white blood cells, and, despite infection-control measures, she developed a bloodstream infection with vancomycin-resistant Enterococcus faecium (VRE).
The infection persisted for 26 days and only resolved after her immune system recovered. She then successfully completed AML treatment.
In-depth DNA sequencing of 22 VRE samples collected during the patient’s infection helped researchers link the prolonged infection to a point mutation in the relA gene of VRE.
The mutation inappropriately activated the stringent response pathway, which bacteria use to survive under stress and to tolerate antibiotics.
The mutation resulted in elevated levels of the signaling molecule alarmone, and this likely primed the bacteria to survive exposure to multiple antibiotics, the researchers said.
The team also noted that relA-mutant VRE was susceptible to the antibiotics linezolid and daptomycin in minimum inhibitory concentration testing and during planktonic growth.
However, when growing in biofilm, relA-mutant VRE could tolerate high doses of both antibiotics.
“This mutation has particular clinical significance because the antibiotics involved, linezolid and daptomycin, are the last line of defense against VRE infection,” Dr Wolf said.
Among the compounds in development for the treatment of bacterial biofilms is the experimental antibiotic ADEP-4. In this study, ADEP-4 killed relA-mutant and non-mutant VRE growing in biofilm in the lab.
“In the future, compounds like ADEP-4 may provide a new approach to resolving persistent infections,” Dr Wolf said.
Dr Rosch noted that evidence gleaned from tracking the evolution of VRE throughout the infection suggested the patient’s immune-compromised state was essential to survival of the mutant VRE.
Gene transcription was altered significantly in relA-mutant VRE and produced biofilms that were less robust and possibly unlikely to otherwise survive.
“The case expands our understanding of the role of the stringent response in susceptibility and tolerance to a wide range of antibiotics, especially in biofilms,” Dr Rosch said. “It also demonstrates that these mutations can develop and gain a foothold during a human infection.” ![]()
Drug eases existential anxiety in cancer patients

chemotherapy
Photo by Rhoda Baer
One-time treatment with the hallucinogenic drug psilocybin may provide long-term relief of existential anxiety in patients with life-threatening cancers, according to a small study.
After receiving a single high dose of the drug, most of the patients studied reported decreases in depression and anxiety as well as increases in quality of life and optimism.
These improvements were sustained at 6 months of follow-up.
“The most interesting and remarkable finding is that a single dose of psilocybin, which lasts 4 to 6 hours, produced enduring decreases in depression and anxiety symptoms, and this may represent a fascinating new model for treating some psychiatric conditions,” said study author Roland Griffiths, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Griffiths said this study grew out of a decade of research at Johns Hopkins on the effects of psilocybin in healthy volunteers, which showed that psilocybin can consistently produce positive changes in mood, behavior, and spirituality when administered to carefully screened and prepared participants.
The current study was designed to see if psilocybin could produce similar results in psychologically distressed cancer patients.
The results were published in the Journal of Psychopharmacology alongside a similar study and 11 accompanying editorials.
For their study, Dr Griffiths and his colleagues recruited 51 participants diagnosed with life-threatening cancers, most of which were recurrent or metastatic.
Types of cancer included breast (n=13), upper aerodigestive (n=7), gastrointestinal (n=4), genitourinary (n=18), and “other” cancers (n=1), as well as hematologic malignancies (n=8).
All participants had been given a formal psychiatric diagnosis, including an anxiety or depressive disorder.
Half of the participants were female, and they had an average age of 56. Ninety-two percent were white, 4% were black, and 2% were Asian.
Treatment
Each participant had 2 treatment sessions scheduled 5 weeks apart. In 1 session, they received a capsule containing a very low dose (1 or 3 mg per 70 kg) of psilocybin that was meant to act as a “control” because the dose was too low to produce effects.
In the other session, participants received a capsule with what is considered a moderate or high dose (22 or 30 mg per 70 kg).
To minimize expectancy effects, the participants and the staff members supervising the sessions were told that participants would receive psilocybin on both sessions, but they did not know that all participants would receive a high dose and a low dose.
Blood pressure and mood were monitored throughout the sessions.
Two monitors aided participants during each session, encouraging them to lie down, wear an eye mask, listen to music through headphones, and direct their attention on their inner experience. If anxiety or confusion arose, the monitors provided reassurance to the participants.
Participants, staff, and community observers rated participants’ moods, attitudes, and behaviors throughout the study.
The researchers assessed each participant via questionnaires and structured interviews before the first session, 7 hours after taking the psilocybin, 5 weeks after each session, and 6 months after the second session.
Adverse events
Thirty-four percent of participants had an episode of elevated systolic blood pressure (>160 mm Hg at 1 or more time-point) in the high-dose psilocybin session, and 17% of participants had such an episode in the low-dose session.
Thirteen percent and 2%, respectively, had an episode of elevated diastolic blood pressure (>100 mm Hg at 1 or more time-point). None of these episodes met criteria for medical intervention.
During the high-dose psilocybin session, 15% of patients experienced nausea or vomiting. There were no such events during the low-dose session.
Three participants reported mild to moderate headaches after the high-dose session.
Twenty-one percent of patients reported physical discomfort during the high-dose session, as did 8% of patients during the low-dose session.
Psychological discomfort occurred in 32% and 12% of participants, respectively. The researchers said there were no cases of hallucinogen persisting perception disorder or prolonged psychosis.
Efficacy outcomes
Most participants reported experiencing changes in visual perception, emotions, and thinking after taking high-dose psilocybin. They also reported experiences of psychological insight and profound, deeply meaningful experiences.
Six months after the final session of treatment, about 80% of participants continued to show clinically significant decreases in depressed mood and anxiety, according to clinician assessment.
According to the participants themselves, 83% had increases in well-being or life satisfaction at 6 months after treatment.
Sixty-seven percent of participants rated the experience as one of the top 5 meaningful experiences in their lives, and 70% rated the experience as one of their top 5 spiritually significant lifetime events.
“Before beginning the study, it wasn’t clear to me that this treatment would be helpful, since cancer patients may experience profound hopelessness in response to their diagnosis, which is often followed by multiple surgeries and prolonged chemotherapy,” Dr Griffiths said.
“I could imagine that cancer patients would receive psilocybin, look into the existential void, and come out even more fearful. However, the positive changes in attitudes, moods, and behavior that we documented in healthy volunteers were replicated in cancer patients.” ![]()

chemotherapy
Photo by Rhoda Baer
One-time treatment with the hallucinogenic drug psilocybin may provide long-term relief of existential anxiety in patients with life-threatening cancers, according to a small study.
After receiving a single high dose of the drug, most of the patients studied reported decreases in depression and anxiety as well as increases in quality of life and optimism.
These improvements were sustained at 6 months of follow-up.
“The most interesting and remarkable finding is that a single dose of psilocybin, which lasts 4 to 6 hours, produced enduring decreases in depression and anxiety symptoms, and this may represent a fascinating new model for treating some psychiatric conditions,” said study author Roland Griffiths, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Griffiths said this study grew out of a decade of research at Johns Hopkins on the effects of psilocybin in healthy volunteers, which showed that psilocybin can consistently produce positive changes in mood, behavior, and spirituality when administered to carefully screened and prepared participants.
The current study was designed to see if psilocybin could produce similar results in psychologically distressed cancer patients.
The results were published in the Journal of Psychopharmacology alongside a similar study and 11 accompanying editorials.
For their study, Dr Griffiths and his colleagues recruited 51 participants diagnosed with life-threatening cancers, most of which were recurrent or metastatic.
Types of cancer included breast (n=13), upper aerodigestive (n=7), gastrointestinal (n=4), genitourinary (n=18), and “other” cancers (n=1), as well as hematologic malignancies (n=8).
All participants had been given a formal psychiatric diagnosis, including an anxiety or depressive disorder.
Half of the participants were female, and they had an average age of 56. Ninety-two percent were white, 4% were black, and 2% were Asian.
Treatment
Each participant had 2 treatment sessions scheduled 5 weeks apart. In 1 session, they received a capsule containing a very low dose (1 or 3 mg per 70 kg) of psilocybin that was meant to act as a “control” because the dose was too low to produce effects.
In the other session, participants received a capsule with what is considered a moderate or high dose (22 or 30 mg per 70 kg).
To minimize expectancy effects, the participants and the staff members supervising the sessions were told that participants would receive psilocybin on both sessions, but they did not know that all participants would receive a high dose and a low dose.
Blood pressure and mood were monitored throughout the sessions.
Two monitors aided participants during each session, encouraging them to lie down, wear an eye mask, listen to music through headphones, and direct their attention on their inner experience. If anxiety or confusion arose, the monitors provided reassurance to the participants.
Participants, staff, and community observers rated participants’ moods, attitudes, and behaviors throughout the study.
The researchers assessed each participant via questionnaires and structured interviews before the first session, 7 hours after taking the psilocybin, 5 weeks after each session, and 6 months after the second session.
Adverse events
Thirty-four percent of participants had an episode of elevated systolic blood pressure (>160 mm Hg at 1 or more time-point) in the high-dose psilocybin session, and 17% of participants had such an episode in the low-dose session.
Thirteen percent and 2%, respectively, had an episode of elevated diastolic blood pressure (>100 mm Hg at 1 or more time-point). None of these episodes met criteria for medical intervention.
During the high-dose psilocybin session, 15% of patients experienced nausea or vomiting. There were no such events during the low-dose session.
Three participants reported mild to moderate headaches after the high-dose session.
Twenty-one percent of patients reported physical discomfort during the high-dose session, as did 8% of patients during the low-dose session.
Psychological discomfort occurred in 32% and 12% of participants, respectively. The researchers said there were no cases of hallucinogen persisting perception disorder or prolonged psychosis.
Efficacy outcomes
Most participants reported experiencing changes in visual perception, emotions, and thinking after taking high-dose psilocybin. They also reported experiences of psychological insight and profound, deeply meaningful experiences.
Six months after the final session of treatment, about 80% of participants continued to show clinically significant decreases in depressed mood and anxiety, according to clinician assessment.
According to the participants themselves, 83% had increases in well-being or life satisfaction at 6 months after treatment.
Sixty-seven percent of participants rated the experience as one of the top 5 meaningful experiences in their lives, and 70% rated the experience as one of their top 5 spiritually significant lifetime events.
“Before beginning the study, it wasn’t clear to me that this treatment would be helpful, since cancer patients may experience profound hopelessness in response to their diagnosis, which is often followed by multiple surgeries and prolonged chemotherapy,” Dr Griffiths said.
“I could imagine that cancer patients would receive psilocybin, look into the existential void, and come out even more fearful. However, the positive changes in attitudes, moods, and behavior that we documented in healthy volunteers were replicated in cancer patients.” ![]()

chemotherapy
Photo by Rhoda Baer
One-time treatment with the hallucinogenic drug psilocybin may provide long-term relief of existential anxiety in patients with life-threatening cancers, according to a small study.
After receiving a single high dose of the drug, most of the patients studied reported decreases in depression and anxiety as well as increases in quality of life and optimism.
These improvements were sustained at 6 months of follow-up.
“The most interesting and remarkable finding is that a single dose of psilocybin, which lasts 4 to 6 hours, produced enduring decreases in depression and anxiety symptoms, and this may represent a fascinating new model for treating some psychiatric conditions,” said study author Roland Griffiths, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Griffiths said this study grew out of a decade of research at Johns Hopkins on the effects of psilocybin in healthy volunteers, which showed that psilocybin can consistently produce positive changes in mood, behavior, and spirituality when administered to carefully screened and prepared participants.
The current study was designed to see if psilocybin could produce similar results in psychologically distressed cancer patients.
The results were published in the Journal of Psychopharmacology alongside a similar study and 11 accompanying editorials.
For their study, Dr Griffiths and his colleagues recruited 51 participants diagnosed with life-threatening cancers, most of which were recurrent or metastatic.
Types of cancer included breast (n=13), upper aerodigestive (n=7), gastrointestinal (n=4), genitourinary (n=18), and “other” cancers (n=1), as well as hematologic malignancies (n=8).
All participants had been given a formal psychiatric diagnosis, including an anxiety or depressive disorder.
Half of the participants were female, and they had an average age of 56. Ninety-two percent were white, 4% were black, and 2% were Asian.
Treatment
Each participant had 2 treatment sessions scheduled 5 weeks apart. In 1 session, they received a capsule containing a very low dose (1 or 3 mg per 70 kg) of psilocybin that was meant to act as a “control” because the dose was too low to produce effects.
In the other session, participants received a capsule with what is considered a moderate or high dose (22 or 30 mg per 70 kg).
To minimize expectancy effects, the participants and the staff members supervising the sessions were told that participants would receive psilocybin on both sessions, but they did not know that all participants would receive a high dose and a low dose.
Blood pressure and mood were monitored throughout the sessions.
Two monitors aided participants during each session, encouraging them to lie down, wear an eye mask, listen to music through headphones, and direct their attention on their inner experience. If anxiety or confusion arose, the monitors provided reassurance to the participants.
Participants, staff, and community observers rated participants’ moods, attitudes, and behaviors throughout the study.
The researchers assessed each participant via questionnaires and structured interviews before the first session, 7 hours after taking the psilocybin, 5 weeks after each session, and 6 months after the second session.
Adverse events
Thirty-four percent of participants had an episode of elevated systolic blood pressure (>160 mm Hg at 1 or more time-point) in the high-dose psilocybin session, and 17% of participants had such an episode in the low-dose session.
Thirteen percent and 2%, respectively, had an episode of elevated diastolic blood pressure (>100 mm Hg at 1 or more time-point). None of these episodes met criteria for medical intervention.
During the high-dose psilocybin session, 15% of patients experienced nausea or vomiting. There were no such events during the low-dose session.
Three participants reported mild to moderate headaches after the high-dose session.
Twenty-one percent of patients reported physical discomfort during the high-dose session, as did 8% of patients during the low-dose session.
Psychological discomfort occurred in 32% and 12% of participants, respectively. The researchers said there were no cases of hallucinogen persisting perception disorder or prolonged psychosis.
Efficacy outcomes
Most participants reported experiencing changes in visual perception, emotions, and thinking after taking high-dose psilocybin. They also reported experiences of psychological insight and profound, deeply meaningful experiences.
Six months after the final session of treatment, about 80% of participants continued to show clinically significant decreases in depressed mood and anxiety, according to clinician assessment.
According to the participants themselves, 83% had increases in well-being or life satisfaction at 6 months after treatment.
Sixty-seven percent of participants rated the experience as one of the top 5 meaningful experiences in their lives, and 70% rated the experience as one of their top 5 spiritually significant lifetime events.
“Before beginning the study, it wasn’t clear to me that this treatment would be helpful, since cancer patients may experience profound hopelessness in response to their diagnosis, which is often followed by multiple surgeries and prolonged chemotherapy,” Dr Griffiths said.
“I could imagine that cancer patients would receive psilocybin, look into the existential void, and come out even more fearful. However, the positive changes in attitudes, moods, and behavior that we documented in healthy volunteers were replicated in cancer patients.”
Explaining lack of response to malaria vaccines
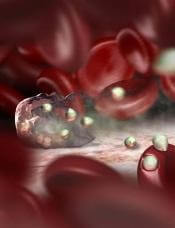
Image by Peter H. Seeberger
Researchers say they have uncovered one potential reason why it has been difficult to generate protective immunity against the early liver stage of malaria infection in regions where the incidence of malaria is high.
Their research, conducted in mice and published in Cell Reports, suggests that exposure to the blood stage of malaria infection inhibits the formation of the protective immune cells (and their antibodies) that can prevent liver-stage infection.
“The blood stage of malaria infection has a very profound impact on the liver stage immune response, and that impact had never been dissected and visualized at this level,” said study author Marion Pepper, PhD, of the University of Washington School of Medicine in Seattle.
“These studies really suggest that you need a vaccine that is protective against both stages of infection to effectively prevent malaria.”
To track how the blood stage of malaria infection overpowers the liver-stage immune response, Dr Pepper and her colleagues infected 2 groups of mice with different forms of malaria parasites.
One group of mice was infected with Plasmodium yoelii wild-type sporozoites, which complete the pre-erythrocytic stage of infection and establish a blood-stage infection.
The other group was infected with a genetically attenuated Plasmodium yoelii parasite that arrests late in liver stage development and does not cause blood-stage infection.
Six days after infection, the researchers found the levels of antibodies were significantly lower in the mice with the blood stage infection than in mice that only had the parasite targeted to the liver.
To understand this discrepancy, the team tracked the differentiation of Plasmodium liver stage-specific B cells. B cells can differentiate into antibody-secreting early effector cells or long-lived memory cells, both of which contribute to protection against malaria.
The researchers discovered that, 14 days after infection, the B cells in the blood-stage-infected mice never went through the necessary changes to make rapidly responsive memory cells.
However, in the mice that received the liver-stage attenuated version of the parasite, the B cells were still able to differentiate and create the necessary antibodies and memory cells for an effective immune response.
“This work really highlights the importance of looking at antigen-specific B cells,” Dr Pepper said. “These data also suggest that if you’re getting a vaccine while you have an ongoing blood-stage infection, there is a chance that the vaccine will not generate good memory cells because the blood stage disrupts all the processes that are involved in making that immunological memory.”
Dr Pepper and her colleagues are now looking into the possibility of treatment to solve this problem.
The team found that when they treated the second stage of the infection with the anti-malarial drug atovaquone, the B cells were able to create the optimally responsive memory cells.
For now, the researchers hope their work can be used to answer immediate questions about the efficacy of malaria vaccines in regions that are most significantly affected by the disease.
“Malaria has evolved with us throughout human existence and therefore has some potent immune evasion strategies,” Dr Pepper said. “We really tried to tease apart some of the factors that could be driving the loss of protective immunity during natural infection and with current vaccine strategies in areas of high malaria transmission.”
“Our next step is to compare malaria-specific B cells after vaccination or natural infection in humans so we can translate these findings and start to determine how to solve this problem.”

Image by Peter H. Seeberger
Researchers say they have uncovered one potential reason why it has been difficult to generate protective immunity against the early liver stage of malaria infection in regions where the incidence of malaria is high.
Their research, conducted in mice and published in Cell Reports, suggests that exposure to the blood stage of malaria infection inhibits the formation of the protective immune cells (and their antibodies) that can prevent liver-stage infection.
“The blood stage of malaria infection has a very profound impact on the liver stage immune response, and that impact had never been dissected and visualized at this level,” said study author Marion Pepper, PhD, of the University of Washington School of Medicine in Seattle.
“These studies really suggest that you need a vaccine that is protective against both stages of infection to effectively prevent malaria.”
To track how the blood stage of malaria infection overpowers the liver-stage immune response, Dr Pepper and her colleagues infected 2 groups of mice with different forms of malaria parasites.
One group of mice was infected with Plasmodium yoelii wild-type sporozoites, which complete the pre-erythrocytic stage of infection and establish a blood-stage infection.
The other group was infected with a genetically attenuated Plasmodium yoelii parasite that arrests late in liver stage development and does not cause blood-stage infection.
Six days after infection, the researchers found the levels of antibodies were significantly lower in the mice with the blood stage infection than in mice that only had the parasite targeted to the liver.
To understand this discrepancy, the team tracked the differentiation of Plasmodium liver stage-specific B cells. B cells can differentiate into antibody-secreting early effector cells or long-lived memory cells, both of which contribute to protection against malaria.
The researchers discovered that, 14 days after infection, the B cells in the blood-stage-infected mice never went through the necessary changes to make rapidly responsive memory cells.
However, in the mice that received the liver-stage attenuated version of the parasite, the B cells were still able to differentiate and create the necessary antibodies and memory cells for an effective immune response.
“This work really highlights the importance of looking at antigen-specific B cells,” Dr Pepper said. “These data also suggest that if you’re getting a vaccine while you have an ongoing blood-stage infection, there is a chance that the vaccine will not generate good memory cells because the blood stage disrupts all the processes that are involved in making that immunological memory.”
Dr Pepper and her colleagues are now looking into the possibility of treatment to solve this problem.
The team found that when they treated the second stage of the infection with the anti-malarial drug atovaquone, the B cells were able to create the optimally responsive memory cells.
For now, the researchers hope their work can be used to answer immediate questions about the efficacy of malaria vaccines in regions that are most significantly affected by the disease.
“Malaria has evolved with us throughout human existence and therefore has some potent immune evasion strategies,” Dr Pepper said. “We really tried to tease apart some of the factors that could be driving the loss of protective immunity during natural infection and with current vaccine strategies in areas of high malaria transmission.”
“Our next step is to compare malaria-specific B cells after vaccination or natural infection in humans so we can translate these findings and start to determine how to solve this problem.”

Image by Peter H. Seeberger
Researchers say they have uncovered one potential reason why it has been difficult to generate protective immunity against the early liver stage of malaria infection in regions where the incidence of malaria is high.
Their research, conducted in mice and published in Cell Reports, suggests that exposure to the blood stage of malaria infection inhibits the formation of the protective immune cells (and their antibodies) that can prevent liver-stage infection.
“The blood stage of malaria infection has a very profound impact on the liver stage immune response, and that impact had never been dissected and visualized at this level,” said study author Marion Pepper, PhD, of the University of Washington School of Medicine in Seattle.
“These studies really suggest that you need a vaccine that is protective against both stages of infection to effectively prevent malaria.”
To track how the blood stage of malaria infection overpowers the liver-stage immune response, Dr Pepper and her colleagues infected 2 groups of mice with different forms of malaria parasites.
One group of mice was infected with Plasmodium yoelii wild-type sporozoites, which complete the pre-erythrocytic stage of infection and establish a blood-stage infection.
The other group was infected with a genetically attenuated Plasmodium yoelii parasite that arrests late in liver stage development and does not cause blood-stage infection.
Six days after infection, the researchers found the levels of antibodies were significantly lower in the mice with the blood stage infection than in mice that only had the parasite targeted to the liver.
To understand this discrepancy, the team tracked the differentiation of Plasmodium liver stage-specific B cells. B cells can differentiate into antibody-secreting early effector cells or long-lived memory cells, both of which contribute to protection against malaria.
The researchers discovered that, 14 days after infection, the B cells in the blood-stage-infected mice never went through the necessary changes to make rapidly responsive memory cells.
However, in the mice that received the liver-stage attenuated version of the parasite, the B cells were still able to differentiate and create the necessary antibodies and memory cells for an effective immune response.
“This work really highlights the importance of looking at antigen-specific B cells,” Dr Pepper said. “These data also suggest that if you’re getting a vaccine while you have an ongoing blood-stage infection, there is a chance that the vaccine will not generate good memory cells because the blood stage disrupts all the processes that are involved in making that immunological memory.”
Dr Pepper and her colleagues are now looking into the possibility of treatment to solve this problem.
The team found that when they treated the second stage of the infection with the anti-malarial drug atovaquone, the B cells were able to create the optimally responsive memory cells.
For now, the researchers hope their work can be used to answer immediate questions about the efficacy of malaria vaccines in regions that are most significantly affected by the disease.
“Malaria has evolved with us throughout human existence and therefore has some potent immune evasion strategies,” Dr Pepper said. “We really tried to tease apart some of the factors that could be driving the loss of protective immunity during natural infection and with current vaccine strategies in areas of high malaria transmission.”
“Our next step is to compare malaria-specific B cells after vaccination or natural infection in humans so we can translate these findings and start to determine how to solve this problem.”
Hospitalized patients may fare better with female doctors

Photo courtesy of CDC
New research suggests that hospitalized patients on Medicare may fare better when treated by female internists.
Researchers analyzed data on more than 1.5 million hospitalizations of Medicare beneficiaries and found that patients treated by female physicians had lower rates of 30-day mortality and hospital readmission than those treated by male physicians.
The results were published in JAMA Internal Medicine alongside a related editorial.
“There’s a lot of evidence out there that male and female physicians practice medicine differently,” noted study author Ashish K. Jha, MD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Female physicians are more likely to adhere to clinical practice guidelines. They’re more likely to practice evidence-based medicine. And while that data has been out there, we don’t really know to what extent that actually matters for patient outcomes.”
So with this study, Dr Jha and his colleagues set out to determine if differences in practice patterns translate into differences in patient outcomes.
The researchers analyzed data on 1,583,028 hospitalizations to assess 30-day mortality rates and 1,540,797 hospitalizations to assess readmissions. The hospitalizations occurred from January 1, 2011, to December 31, 2014.
In the 30-day mortality analysis, the patients’ mean age was 80.2 years, 621,412 patients were male, and 961,616 were female.
In the hospital readmission analysis, the mean patient age was 80.1 years, 602,115 patients were male, and 938,682 were female.
Physician characteristics
During the study period, 58,344 internists treated at least 1 hospitalized Medicare beneficiary. Among those physicians, 18,751 were women (32.1%).
Female physicians tended to be younger than males, with mean ages of 42.8 and 47.8, respectively. Females were also more likely than males to have had osteopathic training—8.4% and 7.0%, respectively.
Females were more likely than males to work in large hospitals (41.9% vs 35.7%), nonprofit hospitals (78.2% vs 75.6%), major teaching hospitals (29.0% vs 21.1%), and hospitals located in the Northeast (26.8% vs 22.7%).
Female physicians tended to treat fewer patients than males—131.9 and 180.5 hospitalizations per year, respectively.
Patient characteristics were largely similar between male and female physicians. However, female physicians treated a higher proportion of female patients than male physicians did—62.1% and 60.2%, respectively.
Results
An adjusted analysis showed that patients treated by female physicians had lower 30-day mortality rates than those treated by males—11.07% and 11.49%, respectively (risk difference, –0.43%; 95% confidence interval, –0.57% to –0.28%; P<0.001; number needed to treat to prevent 1 death, 233).
An adjusted analysis for 30-day hospital readmission rates showed a lower rate for patients treated by females than those treated by males—15.02% and 15.57%, respectively (risk difference, –0.55%; 95% confidence interval, –0.71% to –0.39%; P<0.001; number needed to treat to prevent 1 readmission, 182).
These analyses were adjusted for patient characteristics, hospital-fixed effects, and physician characteristics.
The researchers noted that patients treated by female physicians had lower 30-day mortality and readmission rates regardless of their medical condition or the severity of their illness.
“Across a wide range of conditions, we see a very consistent pattern—that patients who are treated by female physicians had modest but consistently better outcomes than patients treated by male physicians,” Dr Jha said.
“That was true across conditions. It was also true across severity of illness. In fact, among the patients who were the sickest, that’s where we saw some of the largest gaps between female and male physicians.”
The researchers also adjusted their analyses for patients’ length of stay, use of care, discharge location, patient volume, and physicians’ years of practice. But this did not affect the results.
Dr Jha and his colleagues said the results of this study suggest differences in practice patterns between male and female physicians may have important clinical implications for patients. And understanding why these differences exist may provide valuable insights into improving the quality of patient care.

Photo courtesy of CDC
New research suggests that hospitalized patients on Medicare may fare better when treated by female internists.
Researchers analyzed data on more than 1.5 million hospitalizations of Medicare beneficiaries and found that patients treated by female physicians had lower rates of 30-day mortality and hospital readmission than those treated by male physicians.
The results were published in JAMA Internal Medicine alongside a related editorial.
“There’s a lot of evidence out there that male and female physicians practice medicine differently,” noted study author Ashish K. Jha, MD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Female physicians are more likely to adhere to clinical practice guidelines. They’re more likely to practice evidence-based medicine. And while that data has been out there, we don’t really know to what extent that actually matters for patient outcomes.”
So with this study, Dr Jha and his colleagues set out to determine if differences in practice patterns translate into differences in patient outcomes.
The researchers analyzed data on 1,583,028 hospitalizations to assess 30-day mortality rates and 1,540,797 hospitalizations to assess readmissions. The hospitalizations occurred from January 1, 2011, to December 31, 2014.
In the 30-day mortality analysis, the patients’ mean age was 80.2 years, 621,412 patients were male, and 961,616 were female.
In the hospital readmission analysis, the mean patient age was 80.1 years, 602,115 patients were male, and 938,682 were female.
Physician characteristics
During the study period, 58,344 internists treated at least 1 hospitalized Medicare beneficiary. Among those physicians, 18,751 were women (32.1%).
Female physicians tended to be younger than males, with mean ages of 42.8 and 47.8, respectively. Females were also more likely than males to have had osteopathic training—8.4% and 7.0%, respectively.
Females were more likely than males to work in large hospitals (41.9% vs 35.7%), nonprofit hospitals (78.2% vs 75.6%), major teaching hospitals (29.0% vs 21.1%), and hospitals located in the Northeast (26.8% vs 22.7%).
Female physicians tended to treat fewer patients than males—131.9 and 180.5 hospitalizations per year, respectively.
Patient characteristics were largely similar between male and female physicians. However, female physicians treated a higher proportion of female patients than male physicians did—62.1% and 60.2%, respectively.
Results
An adjusted analysis showed that patients treated by female physicians had lower 30-day mortality rates than those treated by males—11.07% and 11.49%, respectively (risk difference, –0.43%; 95% confidence interval, –0.57% to –0.28%; P<0.001; number needed to treat to prevent 1 death, 233).
An adjusted analysis for 30-day hospital readmission rates showed a lower rate for patients treated by females than those treated by males—15.02% and 15.57%, respectively (risk difference, –0.55%; 95% confidence interval, –0.71% to –0.39%; P<0.001; number needed to treat to prevent 1 readmission, 182).
These analyses were adjusted for patient characteristics, hospital-fixed effects, and physician characteristics.
The researchers noted that patients treated by female physicians had lower 30-day mortality and readmission rates regardless of their medical condition or the severity of their illness.
“Across a wide range of conditions, we see a very consistent pattern—that patients who are treated by female physicians had modest but consistently better outcomes than patients treated by male physicians,” Dr Jha said.
“That was true across conditions. It was also true across severity of illness. In fact, among the patients who were the sickest, that’s where we saw some of the largest gaps between female and male physicians.”
The researchers also adjusted their analyses for patients’ length of stay, use of care, discharge location, patient volume, and physicians’ years of practice. But this did not affect the results.
Dr Jha and his colleagues said the results of this study suggest differences in practice patterns between male and female physicians may have important clinical implications for patients. And understanding why these differences exist may provide valuable insights into improving the quality of patient care.

Photo courtesy of CDC
New research suggests that hospitalized patients on Medicare may fare better when treated by female internists.
Researchers analyzed data on more than 1.5 million hospitalizations of Medicare beneficiaries and found that patients treated by female physicians had lower rates of 30-day mortality and hospital readmission than those treated by male physicians.
The results were published in JAMA Internal Medicine alongside a related editorial.
“There’s a lot of evidence out there that male and female physicians practice medicine differently,” noted study author Ashish K. Jha, MD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Female physicians are more likely to adhere to clinical practice guidelines. They’re more likely to practice evidence-based medicine. And while that data has been out there, we don’t really know to what extent that actually matters for patient outcomes.”
So with this study, Dr Jha and his colleagues set out to determine if differences in practice patterns translate into differences in patient outcomes.
The researchers analyzed data on 1,583,028 hospitalizations to assess 30-day mortality rates and 1,540,797 hospitalizations to assess readmissions. The hospitalizations occurred from January 1, 2011, to December 31, 2014.
In the 30-day mortality analysis, the patients’ mean age was 80.2 years, 621,412 patients were male, and 961,616 were female.
In the hospital readmission analysis, the mean patient age was 80.1 years, 602,115 patients were male, and 938,682 were female.
Physician characteristics
During the study period, 58,344 internists treated at least 1 hospitalized Medicare beneficiary. Among those physicians, 18,751 were women (32.1%).
Female physicians tended to be younger than males, with mean ages of 42.8 and 47.8, respectively. Females were also more likely than males to have had osteopathic training—8.4% and 7.0%, respectively.
Females were more likely than males to work in large hospitals (41.9% vs 35.7%), nonprofit hospitals (78.2% vs 75.6%), major teaching hospitals (29.0% vs 21.1%), and hospitals located in the Northeast (26.8% vs 22.7%).
Female physicians tended to treat fewer patients than males—131.9 and 180.5 hospitalizations per year, respectively.
Patient characteristics were largely similar between male and female physicians. However, female physicians treated a higher proportion of female patients than male physicians did—62.1% and 60.2%, respectively.
Results
An adjusted analysis showed that patients treated by female physicians had lower 30-day mortality rates than those treated by males—11.07% and 11.49%, respectively (risk difference, –0.43%; 95% confidence interval, –0.57% to –0.28%; P<0.001; number needed to treat to prevent 1 death, 233).
An adjusted analysis for 30-day hospital readmission rates showed a lower rate for patients treated by females than those treated by males—15.02% and 15.57%, respectively (risk difference, –0.55%; 95% confidence interval, –0.71% to –0.39%; P<0.001; number needed to treat to prevent 1 readmission, 182).
These analyses were adjusted for patient characteristics, hospital-fixed effects, and physician characteristics.
The researchers noted that patients treated by female physicians had lower 30-day mortality and readmission rates regardless of their medical condition or the severity of their illness.
“Across a wide range of conditions, we see a very consistent pattern—that patients who are treated by female physicians had modest but consistently better outcomes than patients treated by male physicians,” Dr Jha said.
“That was true across conditions. It was also true across severity of illness. In fact, among the patients who were the sickest, that’s where we saw some of the largest gaps between female and male physicians.”
The researchers also adjusted their analyses for patients’ length of stay, use of care, discharge location, patient volume, and physicians’ years of practice. But this did not affect the results.
Dr Jha and his colleagues said the results of this study suggest differences in practice patterns between male and female physicians may have important clinical implications for patients. And understanding why these differences exist may provide valuable insights into improving the quality of patient care.
Chemical could aid malaria control

Photo courtesy of the CDC
A chemical that disrupts hormone signaling in mosquitoes may reduce their ability to transmit malaria, according to a study published in PLOS Pathogens.
The findings suggest a potential new approach to combat spread of the disease.
“As insecticide resistance is spreading, new intervention methods to control mosquitoes are urgently needed,” said study author Flaminia Catteruccia, PhD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Our study provides a new strategy based on the use of a non-toxic compound that prevents transmission of malaria parasites without killing the mosquito.”
Dr Catteruccia and her colleagues treated adult female Anopheles gambiae mosquitoes with a chemical known as dibenzoylhydrazine (DBH) to see how it would impact their biological processes.
DBH mimics the action of the steroid hormone 20-hydroxyecdysone, which plays a key role in the reproductive cycle of the female mosquito.
The researchers found various aspects of the mosquitoes’ life cycle to be disrupted after treatment with DBH.
DBH-treated mosquitoes produced and laid fewer eggs, didn’t mate successfully, and died more rapidly than non-treated mosquitoes. The effects were greater the higher the DBH dose.
And DBH-treated mosquitoes were less likely to be infected by malaria parasites.
To further explore the potential of hormone targeting as a malaria control tactic, the researchers fed their experimental results into a mathematical model of the mosquito life cycle.
The results suggest that applying DBH to bed nets or spraying it indoors could potentially reduce malaria transmission as effectively as insecticides.
The researchers noted that DBH compounds are not toxic to mammals, which would make them ideally suited for use in bed nets, where low toxicity is essential.

Photo courtesy of the CDC
A chemical that disrupts hormone signaling in mosquitoes may reduce their ability to transmit malaria, according to a study published in PLOS Pathogens.
The findings suggest a potential new approach to combat spread of the disease.
“As insecticide resistance is spreading, new intervention methods to control mosquitoes are urgently needed,” said study author Flaminia Catteruccia, PhD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Our study provides a new strategy based on the use of a non-toxic compound that prevents transmission of malaria parasites without killing the mosquito.”
Dr Catteruccia and her colleagues treated adult female Anopheles gambiae mosquitoes with a chemical known as dibenzoylhydrazine (DBH) to see how it would impact their biological processes.
DBH mimics the action of the steroid hormone 20-hydroxyecdysone, which plays a key role in the reproductive cycle of the female mosquito.
The researchers found various aspects of the mosquitoes’ life cycle to be disrupted after treatment with DBH.
DBH-treated mosquitoes produced and laid fewer eggs, didn’t mate successfully, and died more rapidly than non-treated mosquitoes. The effects were greater the higher the DBH dose.
And DBH-treated mosquitoes were less likely to be infected by malaria parasites.
To further explore the potential of hormone targeting as a malaria control tactic, the researchers fed their experimental results into a mathematical model of the mosquito life cycle.
The results suggest that applying DBH to bed nets or spraying it indoors could potentially reduce malaria transmission as effectively as insecticides.
The researchers noted that DBH compounds are not toxic to mammals, which would make them ideally suited for use in bed nets, where low toxicity is essential.

Photo courtesy of the CDC
A chemical that disrupts hormone signaling in mosquitoes may reduce their ability to transmit malaria, according to a study published in PLOS Pathogens.
The findings suggest a potential new approach to combat spread of the disease.
“As insecticide resistance is spreading, new intervention methods to control mosquitoes are urgently needed,” said study author Flaminia Catteruccia, PhD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Our study provides a new strategy based on the use of a non-toxic compound that prevents transmission of malaria parasites without killing the mosquito.”
Dr Catteruccia and her colleagues treated adult female Anopheles gambiae mosquitoes with a chemical known as dibenzoylhydrazine (DBH) to see how it would impact their biological processes.
DBH mimics the action of the steroid hormone 20-hydroxyecdysone, which plays a key role in the reproductive cycle of the female mosquito.
The researchers found various aspects of the mosquitoes’ life cycle to be disrupted after treatment with DBH.
DBH-treated mosquitoes produced and laid fewer eggs, didn’t mate successfully, and died more rapidly than non-treated mosquitoes. The effects were greater the higher the DBH dose.
And DBH-treated mosquitoes were less likely to be infected by malaria parasites.
To further explore the potential of hormone targeting as a malaria control tactic, the researchers fed their experimental results into a mathematical model of the mosquito life cycle.
The results suggest that applying DBH to bed nets or spraying it indoors could potentially reduce malaria transmission as effectively as insecticides.
The researchers noted that DBH compounds are not toxic to mammals, which would make them ideally suited for use in bed nets, where low toxicity is essential.