User login
Adjusting the dosing of antipsychotics and other psychotropics in renal disease
When to adjust the dosing of psychotropics in patients with renal impairment
Renal disease can play a large role in altering the pharmacokinetics of medications, especially in elimination or clearance and plasma protein binding. Specifically, renal impairment decreases the plasma protein binding secondary to decreased albumin and retention of urea, which competes with medications to bind to the protein.1
Electrolyte shifts—which could lead to a fatal arrhythmia—are common among patients with renal impairment. The risk can be further increased in this population if a patient is taking a medication that can induce arrhythmia. If a drug is primarily excreted by the kidneys, elimination could be significantly altered, especially if the medication has active metabolites.1
Normal renal function is defined as an estimated creatinine clearance (eCrCl) of >80 mL/min. Renal impairment is classified as:
- mild: eCrCl, 51 to 80 mL/min
- moderate: eCrCl, 31 to 50 mL/min
- severe: eCrCl, ≤30 mL/min
- end-stage renal disease (ESRD): eCrCl, <10 mL/min.2
Overall, there is minimal information about the effects of renal disease on psychotropic therapy; our goal here is to summarize available data. We have created quick reference tables highlighting psychotropics that have renal dosing recommendations based on manufacturers’ package inserts.
Antipsychotics
First-generation antipsychotics (FGAs). Dosage adjustments based on renal function are not required for any FGA, according to manufacturers’ package inserts. Some of these antipsychotics are excreted in urine, but typically as inactive metabolites.
Although there are no dosage recommendations based on renal function provided by the labeling, there has been concern about the use of some FGAs in patients with renal impairment. Specifically, concerns center around the piperidine phenothiazines (thioridazine and mesoridazine) because of the increased risk of electrocardiographic changes and medication-induced arrhythmias in renal disease due to electrolyte imbalances.3,4 Additionally, there is case evidence5 that phenothiazine antipsychotics could increase a patient’s risk for hypotension in chronic renal failure. Haloperidol is considered safe in renal disease because <1% of the medication is excreted unchanged through urine.6
Second-generation antipsychotics (SGAs). Overall, SGAs are considered safe in patients with renal disease. Most SGAs undergo extensive hepatic metabolism before excretion, allowing them to be used safely in patients with renal disease.
Sheehan et al7 analyzed the metabolism and excretion of SGAs, evaluating 8 antipsychotics divided into 4 groups: (1) excretion primarily as an unchanged drug in urine, (2) changed drug in urine, (3) changed drug in feces, (4) and unchanged drug in feces.
- Paliperidone was found to be primarily excreted as an unchanged drug in urine.
- Clozapine, iloperidone, olanzapine, quetiapine, and risperidone all were found to be primarily excreted as a changed drug in urine.
- Aripiprazole and ziprasidone were found to be primarily excreted as a changed drug in feces.
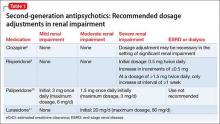
The manufacturers’ package inserts for clozapine, paliperidone, risperidone, and lurasidone have recommended dosage adjustments based on renal function (Table 1).8-11
Ziprasidone. Although ziprasidone does not have a recommended renal dosage adjustment, caution is recommended because of the risk of electrocardiographic changes and potential for medication-induced arrhythmias in patients with electrolyte disturbances secondary to renal disease. A single-dosage study of ziprasidone by Aweeka et al12 demonstrated that the pharmacokinetics of ziprasidone are unchanged in patients with renal impairment.
Asenapine. A small study by Peeters et al13 evaluated the pharmacokinetics of asenapine in hepatic and renal impairment and found no clinically relevant changes in asenapine’s pharmacokinetics among patients with any level of renal impairment compared with patients with normal renal function.
Aripiprazole. Mallikaarjun et al14 completed a small study evaluating the pharmacokinetics of aripiprazole in patients with renal impairment. They found that the pharmacokinetics of aripiprazole in these patients is no different than it is in patients with normal renal function who are taking aripiprazole.
Quetiapine. Thyrum et al15 conducted a similar study with quetiapine, which showed no significant difference detected in the pharmacokinetics of quetiapine in patients with renal impairment. Additionally, quetiapine had no negative effect on patients’ creatinine clearance.
Lurasidone. During clinical trials of lurasidone in patients with mild, moderate, and severe renal impairment, the mean Cmax and area under the curve was higher compared with healthy patients, which led to recommended dosage adjustments in patients with renal impairment.11
As mentioned above, renal impairment decreases the protein binding percentage of medications. Hypothetically, the greater the protein binding, the lower the recommended dosage in patients with renal impairment because the free or unbound form correlates with efficacy and toxicity. Most FGAs and SGAs have the protein-binding characteristic of ≥90%.16 Although it seems this characteristic should result in recommendations to adjust dosage based on renal function, the various pharmacokinetic studies of antipsychotics have not shown this factor to play a role in the manufacturers’ recommendations.
Antidepressants
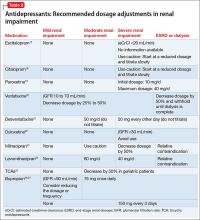
Comorbidity rates of depression in patients with renal disease range from 14% to 30%, making use of antidepressants in renal disease common.4 Antidepressants primarily are metabolized hepatically and excreted renally. Table 217-27 summarizes recommended dosing adjustments for antidepressants.
Selective serotonin reuptake inhibitors.Escitalopram is the (S)-enantiomer of the racemic antidepressant citalopram, both of which have been shown to decrease renal clearance in patients with mild or moderate renal impairment. However, according to the package insert, no dosage adjustments are needed.17 No extensive studies have been conducted on escitalopram or citalopram, but each should be initiated at a reduced dosage and the titration schedule should be prolonged in patients with severe renal impairment or ESRD.17,18
The plasma concentration of paroxetine has been noted to be elevated in patients with severe renal impairment, and the half-life can increase to nearly 50%.4 Paroxetine should be initiated at 10 mg/d, and then titrated slowly in patients with severe renal impairment.19,28
The pharmacokinetics of fluoxetine are unchanged in any stage of renal impairment. Patients in active renal dialysis report good tolerability and efficacy.4
Serotonin-norepinephrine reuptake inhibitors. Venlafaxine and its metabolite O-desmethylvenlafaxine (desvenlafaxine) are primarily excreted via renal elimination. Studies have shown that mild renal impairment can have an effect on plasma levels of the drug, and that moderate or severe impairment can increase the venlafaxine plasma concentration. According to the package insert, a dosage reduction of 50% is recommended for desvenlafaxine and venlafaxine.20,21
No significant pharmacokinetic changes with duloxetine have been noted in patients with mild or moderate renal impairment.22 However, duloxetine’s major metabolites, which are excreted renally, have been measured to be as much as 7 to 9 times higher in patients with ESRD compared with healthy subjects; therefore, it is recommended to avoid duloxetine in patients with severe renal disease.4,22 Our review of the literature produced limited recommendations on dosing milnacipran and its enantiomer levomilnacipran in renally impaired patients. The milnacipran package insert cautions its use in moderate renal impairment and recommends a 50% dosage reduction to 100 mg/d (50 mg twice daily) in patients with severe renal impairment.23 Dosage recommendations for levomilnacipran are 80 mg/d for moderate renal impairment and 40 mg/d for severe impairment. Both agents have relative contraindications for ESRD.23,24
Tricyclic antidepressants (TCAs) are predominantly metabolized hepatically, glucuronidated, and then eliminated renally. Desipramine, imipramine, and nortriptyline have nonspecific package insert recommendations for modified dosing in geriatric patients because of an age-related decrease in renal clearance.29-31 Review articles assert that elevated glucuronidated metabolites could increase patients’ sensitivity to side effects of TCAs. Because of concerns regarding elevated glucuronidated metabolites, it has been proposed to initiate TCAs at a low dosage, titrate slowly, and maintain the lowest effective dosage in patients with renal impairment.25
Monoamine oxidase inhibitors (MAOIs) and other antidepressants. The package inserts of the MAOIs isocarboxazid, phenelzine, selegiline, and tranylcypromine provide limited data and dosage recommendations for use in the context of renal impairment.32-36 Isocarboxazid should not be used in patients with severe renal impairment, according to the prescribing information.32 There are no dosing recommendations for transdermal selegiline in mild, moderate, or severe renal impairment.37 Extra vigilance is required when using MAOIs in patients with renal disease because of an increased risk of dialysis-induced hypotension (orthostatic hypotension is a common adverse effect of MAOIs).38
Bupropion is primarily metabolized hepatically to the active metabolite hydroxybupropion. Plasma levels of this metabolite at steady state are reported to be 10 times greater than bupropion’s concentration levels in healthy subjects; plasma levels are further increased in mild renal impairment.26 Hydroxybupropion is not dialyzable, which can increase the risk of toxicity with bupropion therapy in patients with renal impairment.3 If bupropion effectively treats depression in patients with declining renal function, specifically severe renal impairment and ESRD, then decreasing the dosage to 150 mg every 3 days is recommended to lessen the risk of toxicity. 27
Mood stabilizers
Lithium has the most published literature on dosing adjustments with renal impairment. Many providers are inclined to discontinue lithium use at the first sign of any change in renal function; however, monitoring, prevention, and treatment guidelines for lithium are well established after many years of research and clinical use.39 Lithium’s prescribing information recommends dosage adjustment in mild to moderate renal impairment and lists severe renal impairment and ESRD as relative contraindications.40
A recent study proposes more assertive use of lithium in patients with renal impairment of any severity. Rej et al41 compared continued lithium treatment to discontinuing treatment in geriatric patients with chronic renal failure, and reported (1) a statistically insignificant difference in renal function between groups at 2 years and (2) a “trending decrease” in renal function at 5 years in the lithium treatment group. With closely monitored plasma levels, lithium treatment is considered a workable treatment for patients with moderate renal impairment when mood stabilizer treatment has been effective.42
Lamotrigine and its main glucuronidated metabolite, lamotrigine-2N-glucuronide (L-2-N-G), are primarily excreted renally. In severe renal impairment and ESRD, the L-2-N-G levels are elevated but are not pharmacologically active and, therefore, do not affect plasma concentration or efficacy of lamotrigine.43 Although data are limited regarding the use of lamotrigine in severe renal impairment and ESRD, Kaufman44 reported a 17% to 20% decrease in concentration after dialysis—suggesting that post-dialysis titration might be needed in these patients.
Oxcarbazepine is metabolized by means of cytosolic enzymes in the liver to its primary pharmacologically active metabolite, 10-monohydroxy, which is further metabolized via glucuronidation and then renally excreted. There are no dosage adjustment recommendations for patients with an eCrCl >30 mL/min.45 Rouan et al46 suggest initiating oxcarbazepine at 50% of the recommended dosage and following a longer titration schedule in patients with an eCrCl 10 to 30 mL/min. No dosing suggestions for severe renal impairment and ESRD were provided because of study limitations; however, the general recommendation for psychotropic agents in patients in a severe stage of renal impairment is dosage reduction with close monitoring.46
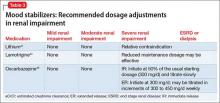
Table 341,44,46 summarizes dosage adjustments for mood stabilizers in patients with renal impairment.
1. Levy G. Pharmacokinetics in renal disease. Am J Med. 1977;62(4):461-465.
2. Preskorn SH. Clinically important differences in the pharmacokinetics of the ten newer “atypical” antipsychotics: part 3. Effects of renal and hepatic impairment. J Psychiatr Pract. 2012;18(6):430-437.
3. Cohen LM, Tessier EG, Germain MJ, et al. Update on psychotropic medication use in renal disease. Psychosomatics. 2004;45(1):34-48.
4. Baghdady NT, Banik S, Swartz SA, et al. Psychotropic drugs and renal failure: translating the evidence for clinical practice. Adv Ther. 2009;26(4):404-424.
5. Sheehan J, White A, Wilson R. Hazards of phenothiazines in chronic renal failure. Ir Med J. 1982;75(9):335.
6. Haloperidol [monograph]. In: Micromedex Drugdex [online database]. Greenwood Village, CO: Truven Health Analytics. Accessed December 17, 2014.
7. Sheehan JJ, Sliwa JK, Amatniek JC, et al. Atypical antipsychotic metabolism and excretion. Curr Drug Metab. 2010;11(6):516-525.
8. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2014.
9. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2014.
10. Invega [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2014.
11. Latuda [package insert]. Fort Lee, NJ: Sunovion Pharmaceuticals; 2013.
12. Aweeka F, Jayesekara D, Horton M, et al. The pharmacokinetics of ziprasidone in subjects with normal and impaired renal function. Br J Clin Pharmacol. 2004;49(suppl 1):27S-33S.
13. Peeters P, Bockbrader H, Spaans E, et al. Asenapine pharmacokinetics in hepatic and renal impairment. Clin Pharmacol. 2011;50(7):471-481.
14. Mallikaarjun S, Shoaf SE, Boulton DW, et al. Effects of hepatic or renal impairment on the pharmacokinetics of aripiprazole. Clin Pharmacokinet. 2008;47(8):533-542.
15. Thyrum PT, Wong YW, Yeh C. Single-dose pharmacokinetics of quetiapine in subjects with renal or hepatic impairment. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(4):521-533.
16. Lexi-Drugs. Lexicomp. Hudson, OH: Wolters Kluwer Health, Inc. http://online.lexi.com. Accessed May 28, 2015.
17. Lexapro [package insert]. Forest Pharmaceuticals, Inc.: St. Louis, MO; 2014.
18. Celexa [package insert]. Forest Pharmaceuticals, Inc.: St. Louis, MO; 2014.
19. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
20. Effexor [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2010.
21. Pristiq [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2014.
22. Cymbalta [package insert]. Indianapolis, IN: Lilly USA, LLC; 2014.
23. Savella [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2013.
24. Fetzima [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2014.
25. Kurella M, Bennett WM, Chertow GM. Analgesia in patients with ESRD: a review of available evidence. Am J Kidney Dis. 2003;42(2):217-228.
26. Wellbutrin [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
27. Worrall SP, Almond MK, Dhillon S. Pharmacokinetics of bupropion and its metabolites in haemodialysis patients who smoke. A single dose study. Nephron Clin Pract. 2004;97(3):c83-c89.
28. Nagler EV, Webster AC, Vanholder R, et al. Antidepressants for depression in stage 3-5 chronic kidney disease: a systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP). Nephrol Dial Transplant. 2012;27(10):3736-3745.
29. Norpramin. [package insert] Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2014.
30. Tofranil [package insert]. Hazelwood, MO: Mallinckrodt Inc.; 2014.
31. Pamelor [package insert]. Hazelwood, MO: Mallinckrodt Inc.; 2014.
32. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals, LLC; 2012.
33. Nardil [package insert]. New York, NY: Parke-Davis Division of Pfizer Inc.; 2009.
34. EMSAM [package insert]. Morgantown, WV: Mylan Specialty, L.P.; 2014.
35. Eldepryl [package insert]. Morgantown, WV: Somerset Pharmaceuticals, Inc.; 2009.
36. Parnate [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
37. Culpepper L. Reducing the burden of difficult-to-treat major depressive disorder: revisiting monoamine oxidase inhibitor therapy. Prim Care Companion CNS Disord. 2013;15(5). doi: 10.4088/PCC.13r01515.
38. Tossani E, Cassano P, Fava M. Depression and renal disease. Semin Dial. 2005;18(2):73-81.
39. Young AH, Hammond JM. Lithium in mood disorders: increasing evidence base, declining use? Br J Psychiatry. 2007;191:474-476.
40. Eskalith [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2003.
41. Rej S, Looper K, Segal M. The effect of serum lithium levels on renal function in geriatric outpatients: a retrospective longitudinal study. Drugs Aging. 2013;30(6):409-415.
42. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211.
43. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
44. Kaufman KR. Lamotrigine and hemodialysis in bipolar disorder: case analysis of dosing strategy with literature review. Bipolar Disord. 2010;12(4):446-449.
45. Trileptal [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
46. Rouan MC, Lecaillon JB, Godbillon J, et al. The effect of renal impairment on the pharmacokinetics of oxcarbazepine and its metabolites. Eur J Clin Pharmacol. 1994;47(2):161-167.
Renal disease can play a large role in altering the pharmacokinetics of medications, especially in elimination or clearance and plasma protein binding. Specifically, renal impairment decreases the plasma protein binding secondary to decreased albumin and retention of urea, which competes with medications to bind to the protein.1
Electrolyte shifts—which could lead to a fatal arrhythmia—are common among patients with renal impairment. The risk can be further increased in this population if a patient is taking a medication that can induce arrhythmia. If a drug is primarily excreted by the kidneys, elimination could be significantly altered, especially if the medication has active metabolites.1
Normal renal function is defined as an estimated creatinine clearance (eCrCl) of >80 mL/min. Renal impairment is classified as:
- mild: eCrCl, 51 to 80 mL/min
- moderate: eCrCl, 31 to 50 mL/min
- severe: eCrCl, ≤30 mL/min
- end-stage renal disease (ESRD): eCrCl, <10 mL/min.2
Overall, there is minimal information about the effects of renal disease on psychotropic therapy; our goal here is to summarize available data. We have created quick reference tables highlighting psychotropics that have renal dosing recommendations based on manufacturers’ package inserts.
Antipsychotics
First-generation antipsychotics (FGAs). Dosage adjustments based on renal function are not required for any FGA, according to manufacturers’ package inserts. Some of these antipsychotics are excreted in urine, but typically as inactive metabolites.
Although there are no dosage recommendations based on renal function provided by the labeling, there has been concern about the use of some FGAs in patients with renal impairment. Specifically, concerns center around the piperidine phenothiazines (thioridazine and mesoridazine) because of the increased risk of electrocardiographic changes and medication-induced arrhythmias in renal disease due to electrolyte imbalances.3,4 Additionally, there is case evidence5 that phenothiazine antipsychotics could increase a patient’s risk for hypotension in chronic renal failure. Haloperidol is considered safe in renal disease because <1% of the medication is excreted unchanged through urine.6
Second-generation antipsychotics (SGAs). Overall, SGAs are considered safe in patients with renal disease. Most SGAs undergo extensive hepatic metabolism before excretion, allowing them to be used safely in patients with renal disease.
Sheehan et al7 analyzed the metabolism and excretion of SGAs, evaluating 8 antipsychotics divided into 4 groups: (1) excretion primarily as an unchanged drug in urine, (2) changed drug in urine, (3) changed drug in feces, (4) and unchanged drug in feces.
- Paliperidone was found to be primarily excreted as an unchanged drug in urine.
- Clozapine, iloperidone, olanzapine, quetiapine, and risperidone all were found to be primarily excreted as a changed drug in urine.
- Aripiprazole and ziprasidone were found to be primarily excreted as a changed drug in feces.
The manufacturers’ package inserts for clozapine, paliperidone, risperidone, and lurasidone have recommended dosage adjustments based on renal function (Table 1).8-11
Ziprasidone. Although ziprasidone does not have a recommended renal dosage adjustment, caution is recommended because of the risk of electrocardiographic changes and potential for medication-induced arrhythmias in patients with electrolyte disturbances secondary to renal disease. A single-dosage study of ziprasidone by Aweeka et al12 demonstrated that the pharmacokinetics of ziprasidone are unchanged in patients with renal impairment.
Asenapine. A small study by Peeters et al13 evaluated the pharmacokinetics of asenapine in hepatic and renal impairment and found no clinically relevant changes in asenapine’s pharmacokinetics among patients with any level of renal impairment compared with patients with normal renal function.
Aripiprazole. Mallikaarjun et al14 completed a small study evaluating the pharmacokinetics of aripiprazole in patients with renal impairment. They found that the pharmacokinetics of aripiprazole in these patients is no different than it is in patients with normal renal function who are taking aripiprazole.
Quetiapine. Thyrum et al15 conducted a similar study with quetiapine, which showed no significant difference detected in the pharmacokinetics of quetiapine in patients with renal impairment. Additionally, quetiapine had no negative effect on patients’ creatinine clearance.
Lurasidone. During clinical trials of lurasidone in patients with mild, moderate, and severe renal impairment, the mean Cmax and area under the curve was higher compared with healthy patients, which led to recommended dosage adjustments in patients with renal impairment.11
As mentioned above, renal impairment decreases the protein binding percentage of medications. Hypothetically, the greater the protein binding, the lower the recommended dosage in patients with renal impairment because the free or unbound form correlates with efficacy and toxicity. Most FGAs and SGAs have the protein-binding characteristic of ≥90%.16 Although it seems this characteristic should result in recommendations to adjust dosage based on renal function, the various pharmacokinetic studies of antipsychotics have not shown this factor to play a role in the manufacturers’ recommendations.
Antidepressants
Comorbidity rates of depression in patients with renal disease range from 14% to 30%, making use of antidepressants in renal disease common.4 Antidepressants primarily are metabolized hepatically and excreted renally. Table 217-27 summarizes recommended dosing adjustments for antidepressants.
Selective serotonin reuptake inhibitors.Escitalopram is the (S)-enantiomer of the racemic antidepressant citalopram, both of which have been shown to decrease renal clearance in patients with mild or moderate renal impairment. However, according to the package insert, no dosage adjustments are needed.17 No extensive studies have been conducted on escitalopram or citalopram, but each should be initiated at a reduced dosage and the titration schedule should be prolonged in patients with severe renal impairment or ESRD.17,18
The plasma concentration of paroxetine has been noted to be elevated in patients with severe renal impairment, and the half-life can increase to nearly 50%.4 Paroxetine should be initiated at 10 mg/d, and then titrated slowly in patients with severe renal impairment.19,28
The pharmacokinetics of fluoxetine are unchanged in any stage of renal impairment. Patients in active renal dialysis report good tolerability and efficacy.4
Serotonin-norepinephrine reuptake inhibitors. Venlafaxine and its metabolite O-desmethylvenlafaxine (desvenlafaxine) are primarily excreted via renal elimination. Studies have shown that mild renal impairment can have an effect on plasma levels of the drug, and that moderate or severe impairment can increase the venlafaxine plasma concentration. According to the package insert, a dosage reduction of 50% is recommended for desvenlafaxine and venlafaxine.20,21
No significant pharmacokinetic changes with duloxetine have been noted in patients with mild or moderate renal impairment.22 However, duloxetine’s major metabolites, which are excreted renally, have been measured to be as much as 7 to 9 times higher in patients with ESRD compared with healthy subjects; therefore, it is recommended to avoid duloxetine in patients with severe renal disease.4,22 Our review of the literature produced limited recommendations on dosing milnacipran and its enantiomer levomilnacipran in renally impaired patients. The milnacipran package insert cautions its use in moderate renal impairment and recommends a 50% dosage reduction to 100 mg/d (50 mg twice daily) in patients with severe renal impairment.23 Dosage recommendations for levomilnacipran are 80 mg/d for moderate renal impairment and 40 mg/d for severe impairment. Both agents have relative contraindications for ESRD.23,24
Tricyclic antidepressants (TCAs) are predominantly metabolized hepatically, glucuronidated, and then eliminated renally. Desipramine, imipramine, and nortriptyline have nonspecific package insert recommendations for modified dosing in geriatric patients because of an age-related decrease in renal clearance.29-31 Review articles assert that elevated glucuronidated metabolites could increase patients’ sensitivity to side effects of TCAs. Because of concerns regarding elevated glucuronidated metabolites, it has been proposed to initiate TCAs at a low dosage, titrate slowly, and maintain the lowest effective dosage in patients with renal impairment.25
Monoamine oxidase inhibitors (MAOIs) and other antidepressants. The package inserts of the MAOIs isocarboxazid, phenelzine, selegiline, and tranylcypromine provide limited data and dosage recommendations for use in the context of renal impairment.32-36 Isocarboxazid should not be used in patients with severe renal impairment, according to the prescribing information.32 There are no dosing recommendations for transdermal selegiline in mild, moderate, or severe renal impairment.37 Extra vigilance is required when using MAOIs in patients with renal disease because of an increased risk of dialysis-induced hypotension (orthostatic hypotension is a common adverse effect of MAOIs).38
Bupropion is primarily metabolized hepatically to the active metabolite hydroxybupropion. Plasma levels of this metabolite at steady state are reported to be 10 times greater than bupropion’s concentration levels in healthy subjects; plasma levels are further increased in mild renal impairment.26 Hydroxybupropion is not dialyzable, which can increase the risk of toxicity with bupropion therapy in patients with renal impairment.3 If bupropion effectively treats depression in patients with declining renal function, specifically severe renal impairment and ESRD, then decreasing the dosage to 150 mg every 3 days is recommended to lessen the risk of toxicity. 27
Mood stabilizers
Lithium has the most published literature on dosing adjustments with renal impairment. Many providers are inclined to discontinue lithium use at the first sign of any change in renal function; however, monitoring, prevention, and treatment guidelines for lithium are well established after many years of research and clinical use.39 Lithium’s prescribing information recommends dosage adjustment in mild to moderate renal impairment and lists severe renal impairment and ESRD as relative contraindications.40
A recent study proposes more assertive use of lithium in patients with renal impairment of any severity. Rej et al41 compared continued lithium treatment to discontinuing treatment in geriatric patients with chronic renal failure, and reported (1) a statistically insignificant difference in renal function between groups at 2 years and (2) a “trending decrease” in renal function at 5 years in the lithium treatment group. With closely monitored plasma levels, lithium treatment is considered a workable treatment for patients with moderate renal impairment when mood stabilizer treatment has been effective.42
Lamotrigine and its main glucuronidated metabolite, lamotrigine-2N-glucuronide (L-2-N-G), are primarily excreted renally. In severe renal impairment and ESRD, the L-2-N-G levels are elevated but are not pharmacologically active and, therefore, do not affect plasma concentration or efficacy of lamotrigine.43 Although data are limited regarding the use of lamotrigine in severe renal impairment and ESRD, Kaufman44 reported a 17% to 20% decrease in concentration after dialysis—suggesting that post-dialysis titration might be needed in these patients.
Oxcarbazepine is metabolized by means of cytosolic enzymes in the liver to its primary pharmacologically active metabolite, 10-monohydroxy, which is further metabolized via glucuronidation and then renally excreted. There are no dosage adjustment recommendations for patients with an eCrCl >30 mL/min.45 Rouan et al46 suggest initiating oxcarbazepine at 50% of the recommended dosage and following a longer titration schedule in patients with an eCrCl 10 to 30 mL/min. No dosing suggestions for severe renal impairment and ESRD were provided because of study limitations; however, the general recommendation for psychotropic agents in patients in a severe stage of renal impairment is dosage reduction with close monitoring.46
Table 341,44,46 summarizes dosage adjustments for mood stabilizers in patients with renal impairment.
Renal disease can play a large role in altering the pharmacokinetics of medications, especially in elimination or clearance and plasma protein binding. Specifically, renal impairment decreases the plasma protein binding secondary to decreased albumin and retention of urea, which competes with medications to bind to the protein.1
Electrolyte shifts—which could lead to a fatal arrhythmia—are common among patients with renal impairment. The risk can be further increased in this population if a patient is taking a medication that can induce arrhythmia. If a drug is primarily excreted by the kidneys, elimination could be significantly altered, especially if the medication has active metabolites.1
Normal renal function is defined as an estimated creatinine clearance (eCrCl) of >80 mL/min. Renal impairment is classified as:
- mild: eCrCl, 51 to 80 mL/min
- moderate: eCrCl, 31 to 50 mL/min
- severe: eCrCl, ≤30 mL/min
- end-stage renal disease (ESRD): eCrCl, <10 mL/min.2
Overall, there is minimal information about the effects of renal disease on psychotropic therapy; our goal here is to summarize available data. We have created quick reference tables highlighting psychotropics that have renal dosing recommendations based on manufacturers’ package inserts.
Antipsychotics
First-generation antipsychotics (FGAs). Dosage adjustments based on renal function are not required for any FGA, according to manufacturers’ package inserts. Some of these antipsychotics are excreted in urine, but typically as inactive metabolites.
Although there are no dosage recommendations based on renal function provided by the labeling, there has been concern about the use of some FGAs in patients with renal impairment. Specifically, concerns center around the piperidine phenothiazines (thioridazine and mesoridazine) because of the increased risk of electrocardiographic changes and medication-induced arrhythmias in renal disease due to electrolyte imbalances.3,4 Additionally, there is case evidence5 that phenothiazine antipsychotics could increase a patient’s risk for hypotension in chronic renal failure. Haloperidol is considered safe in renal disease because <1% of the medication is excreted unchanged through urine.6
Second-generation antipsychotics (SGAs). Overall, SGAs are considered safe in patients with renal disease. Most SGAs undergo extensive hepatic metabolism before excretion, allowing them to be used safely in patients with renal disease.
Sheehan et al7 analyzed the metabolism and excretion of SGAs, evaluating 8 antipsychotics divided into 4 groups: (1) excretion primarily as an unchanged drug in urine, (2) changed drug in urine, (3) changed drug in feces, (4) and unchanged drug in feces.
- Paliperidone was found to be primarily excreted as an unchanged drug in urine.
- Clozapine, iloperidone, olanzapine, quetiapine, and risperidone all were found to be primarily excreted as a changed drug in urine.
- Aripiprazole and ziprasidone were found to be primarily excreted as a changed drug in feces.
The manufacturers’ package inserts for clozapine, paliperidone, risperidone, and lurasidone have recommended dosage adjustments based on renal function (Table 1).8-11
Ziprasidone. Although ziprasidone does not have a recommended renal dosage adjustment, caution is recommended because of the risk of electrocardiographic changes and potential for medication-induced arrhythmias in patients with electrolyte disturbances secondary to renal disease. A single-dosage study of ziprasidone by Aweeka et al12 demonstrated that the pharmacokinetics of ziprasidone are unchanged in patients with renal impairment.
Asenapine. A small study by Peeters et al13 evaluated the pharmacokinetics of asenapine in hepatic and renal impairment and found no clinically relevant changes in asenapine’s pharmacokinetics among patients with any level of renal impairment compared with patients with normal renal function.
Aripiprazole. Mallikaarjun et al14 completed a small study evaluating the pharmacokinetics of aripiprazole in patients with renal impairment. They found that the pharmacokinetics of aripiprazole in these patients is no different than it is in patients with normal renal function who are taking aripiprazole.
Quetiapine. Thyrum et al15 conducted a similar study with quetiapine, which showed no significant difference detected in the pharmacokinetics of quetiapine in patients with renal impairment. Additionally, quetiapine had no negative effect on patients’ creatinine clearance.
Lurasidone. During clinical trials of lurasidone in patients with mild, moderate, and severe renal impairment, the mean Cmax and area under the curve was higher compared with healthy patients, which led to recommended dosage adjustments in patients with renal impairment.11
As mentioned above, renal impairment decreases the protein binding percentage of medications. Hypothetically, the greater the protein binding, the lower the recommended dosage in patients with renal impairment because the free or unbound form correlates with efficacy and toxicity. Most FGAs and SGAs have the protein-binding characteristic of ≥90%.16 Although it seems this characteristic should result in recommendations to adjust dosage based on renal function, the various pharmacokinetic studies of antipsychotics have not shown this factor to play a role in the manufacturers’ recommendations.
Antidepressants
Comorbidity rates of depression in patients with renal disease range from 14% to 30%, making use of antidepressants in renal disease common.4 Antidepressants primarily are metabolized hepatically and excreted renally. Table 217-27 summarizes recommended dosing adjustments for antidepressants.
Selective serotonin reuptake inhibitors.Escitalopram is the (S)-enantiomer of the racemic antidepressant citalopram, both of which have been shown to decrease renal clearance in patients with mild or moderate renal impairment. However, according to the package insert, no dosage adjustments are needed.17 No extensive studies have been conducted on escitalopram or citalopram, but each should be initiated at a reduced dosage and the titration schedule should be prolonged in patients with severe renal impairment or ESRD.17,18
The plasma concentration of paroxetine has been noted to be elevated in patients with severe renal impairment, and the half-life can increase to nearly 50%.4 Paroxetine should be initiated at 10 mg/d, and then titrated slowly in patients with severe renal impairment.19,28
The pharmacokinetics of fluoxetine are unchanged in any stage of renal impairment. Patients in active renal dialysis report good tolerability and efficacy.4
Serotonin-norepinephrine reuptake inhibitors. Venlafaxine and its metabolite O-desmethylvenlafaxine (desvenlafaxine) are primarily excreted via renal elimination. Studies have shown that mild renal impairment can have an effect on plasma levels of the drug, and that moderate or severe impairment can increase the venlafaxine plasma concentration. According to the package insert, a dosage reduction of 50% is recommended for desvenlafaxine and venlafaxine.20,21
No significant pharmacokinetic changes with duloxetine have been noted in patients with mild or moderate renal impairment.22 However, duloxetine’s major metabolites, which are excreted renally, have been measured to be as much as 7 to 9 times higher in patients with ESRD compared with healthy subjects; therefore, it is recommended to avoid duloxetine in patients with severe renal disease.4,22 Our review of the literature produced limited recommendations on dosing milnacipran and its enantiomer levomilnacipran in renally impaired patients. The milnacipran package insert cautions its use in moderate renal impairment and recommends a 50% dosage reduction to 100 mg/d (50 mg twice daily) in patients with severe renal impairment.23 Dosage recommendations for levomilnacipran are 80 mg/d for moderate renal impairment and 40 mg/d for severe impairment. Both agents have relative contraindications for ESRD.23,24
Tricyclic antidepressants (TCAs) are predominantly metabolized hepatically, glucuronidated, and then eliminated renally. Desipramine, imipramine, and nortriptyline have nonspecific package insert recommendations for modified dosing in geriatric patients because of an age-related decrease in renal clearance.29-31 Review articles assert that elevated glucuronidated metabolites could increase patients’ sensitivity to side effects of TCAs. Because of concerns regarding elevated glucuronidated metabolites, it has been proposed to initiate TCAs at a low dosage, titrate slowly, and maintain the lowest effective dosage in patients with renal impairment.25
Monoamine oxidase inhibitors (MAOIs) and other antidepressants. The package inserts of the MAOIs isocarboxazid, phenelzine, selegiline, and tranylcypromine provide limited data and dosage recommendations for use in the context of renal impairment.32-36 Isocarboxazid should not be used in patients with severe renal impairment, according to the prescribing information.32 There are no dosing recommendations for transdermal selegiline in mild, moderate, or severe renal impairment.37 Extra vigilance is required when using MAOIs in patients with renal disease because of an increased risk of dialysis-induced hypotension (orthostatic hypotension is a common adverse effect of MAOIs).38
Bupropion is primarily metabolized hepatically to the active metabolite hydroxybupropion. Plasma levels of this metabolite at steady state are reported to be 10 times greater than bupropion’s concentration levels in healthy subjects; plasma levels are further increased in mild renal impairment.26 Hydroxybupropion is not dialyzable, which can increase the risk of toxicity with bupropion therapy in patients with renal impairment.3 If bupropion effectively treats depression in patients with declining renal function, specifically severe renal impairment and ESRD, then decreasing the dosage to 150 mg every 3 days is recommended to lessen the risk of toxicity. 27
Mood stabilizers
Lithium has the most published literature on dosing adjustments with renal impairment. Many providers are inclined to discontinue lithium use at the first sign of any change in renal function; however, monitoring, prevention, and treatment guidelines for lithium are well established after many years of research and clinical use.39 Lithium’s prescribing information recommends dosage adjustment in mild to moderate renal impairment and lists severe renal impairment and ESRD as relative contraindications.40
A recent study proposes more assertive use of lithium in patients with renal impairment of any severity. Rej et al41 compared continued lithium treatment to discontinuing treatment in geriatric patients with chronic renal failure, and reported (1) a statistically insignificant difference in renal function between groups at 2 years and (2) a “trending decrease” in renal function at 5 years in the lithium treatment group. With closely monitored plasma levels, lithium treatment is considered a workable treatment for patients with moderate renal impairment when mood stabilizer treatment has been effective.42
Lamotrigine and its main glucuronidated metabolite, lamotrigine-2N-glucuronide (L-2-N-G), are primarily excreted renally. In severe renal impairment and ESRD, the L-2-N-G levels are elevated but are not pharmacologically active and, therefore, do not affect plasma concentration or efficacy of lamotrigine.43 Although data are limited regarding the use of lamotrigine in severe renal impairment and ESRD, Kaufman44 reported a 17% to 20% decrease in concentration after dialysis—suggesting that post-dialysis titration might be needed in these patients.
Oxcarbazepine is metabolized by means of cytosolic enzymes in the liver to its primary pharmacologically active metabolite, 10-monohydroxy, which is further metabolized via glucuronidation and then renally excreted. There are no dosage adjustment recommendations for patients with an eCrCl >30 mL/min.45 Rouan et al46 suggest initiating oxcarbazepine at 50% of the recommended dosage and following a longer titration schedule in patients with an eCrCl 10 to 30 mL/min. No dosing suggestions for severe renal impairment and ESRD were provided because of study limitations; however, the general recommendation for psychotropic agents in patients in a severe stage of renal impairment is dosage reduction with close monitoring.46
Table 341,44,46 summarizes dosage adjustments for mood stabilizers in patients with renal impairment.
1. Levy G. Pharmacokinetics in renal disease. Am J Med. 1977;62(4):461-465.
2. Preskorn SH. Clinically important differences in the pharmacokinetics of the ten newer “atypical” antipsychotics: part 3. Effects of renal and hepatic impairment. J Psychiatr Pract. 2012;18(6):430-437.
3. Cohen LM, Tessier EG, Germain MJ, et al. Update on psychotropic medication use in renal disease. Psychosomatics. 2004;45(1):34-48.
4. Baghdady NT, Banik S, Swartz SA, et al. Psychotropic drugs and renal failure: translating the evidence for clinical practice. Adv Ther. 2009;26(4):404-424.
5. Sheehan J, White A, Wilson R. Hazards of phenothiazines in chronic renal failure. Ir Med J. 1982;75(9):335.
6. Haloperidol [monograph]. In: Micromedex Drugdex [online database]. Greenwood Village, CO: Truven Health Analytics. Accessed December 17, 2014.
7. Sheehan JJ, Sliwa JK, Amatniek JC, et al. Atypical antipsychotic metabolism and excretion. Curr Drug Metab. 2010;11(6):516-525.
8. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2014.
9. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2014.
10. Invega [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2014.
11. Latuda [package insert]. Fort Lee, NJ: Sunovion Pharmaceuticals; 2013.
12. Aweeka F, Jayesekara D, Horton M, et al. The pharmacokinetics of ziprasidone in subjects with normal and impaired renal function. Br J Clin Pharmacol. 2004;49(suppl 1):27S-33S.
13. Peeters P, Bockbrader H, Spaans E, et al. Asenapine pharmacokinetics in hepatic and renal impairment. Clin Pharmacol. 2011;50(7):471-481.
14. Mallikaarjun S, Shoaf SE, Boulton DW, et al. Effects of hepatic or renal impairment on the pharmacokinetics of aripiprazole. Clin Pharmacokinet. 2008;47(8):533-542.
15. Thyrum PT, Wong YW, Yeh C. Single-dose pharmacokinetics of quetiapine in subjects with renal or hepatic impairment. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(4):521-533.
16. Lexi-Drugs. Lexicomp. Hudson, OH: Wolters Kluwer Health, Inc. http://online.lexi.com. Accessed May 28, 2015.
17. Lexapro [package insert]. Forest Pharmaceuticals, Inc.: St. Louis, MO; 2014.
18. Celexa [package insert]. Forest Pharmaceuticals, Inc.: St. Louis, MO; 2014.
19. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
20. Effexor [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2010.
21. Pristiq [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2014.
22. Cymbalta [package insert]. Indianapolis, IN: Lilly USA, LLC; 2014.
23. Savella [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2013.
24. Fetzima [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2014.
25. Kurella M, Bennett WM, Chertow GM. Analgesia in patients with ESRD: a review of available evidence. Am J Kidney Dis. 2003;42(2):217-228.
26. Wellbutrin [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
27. Worrall SP, Almond MK, Dhillon S. Pharmacokinetics of bupropion and its metabolites in haemodialysis patients who smoke. A single dose study. Nephron Clin Pract. 2004;97(3):c83-c89.
28. Nagler EV, Webster AC, Vanholder R, et al. Antidepressants for depression in stage 3-5 chronic kidney disease: a systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP). Nephrol Dial Transplant. 2012;27(10):3736-3745.
29. Norpramin. [package insert] Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2014.
30. Tofranil [package insert]. Hazelwood, MO: Mallinckrodt Inc.; 2014.
31. Pamelor [package insert]. Hazelwood, MO: Mallinckrodt Inc.; 2014.
32. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals, LLC; 2012.
33. Nardil [package insert]. New York, NY: Parke-Davis Division of Pfizer Inc.; 2009.
34. EMSAM [package insert]. Morgantown, WV: Mylan Specialty, L.P.; 2014.
35. Eldepryl [package insert]. Morgantown, WV: Somerset Pharmaceuticals, Inc.; 2009.
36. Parnate [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
37. Culpepper L. Reducing the burden of difficult-to-treat major depressive disorder: revisiting monoamine oxidase inhibitor therapy. Prim Care Companion CNS Disord. 2013;15(5). doi: 10.4088/PCC.13r01515.
38. Tossani E, Cassano P, Fava M. Depression and renal disease. Semin Dial. 2005;18(2):73-81.
39. Young AH, Hammond JM. Lithium in mood disorders: increasing evidence base, declining use? Br J Psychiatry. 2007;191:474-476.
40. Eskalith [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2003.
41. Rej S, Looper K, Segal M. The effect of serum lithium levels on renal function in geriatric outpatients: a retrospective longitudinal study. Drugs Aging. 2013;30(6):409-415.
42. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211.
43. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
44. Kaufman KR. Lamotrigine and hemodialysis in bipolar disorder: case analysis of dosing strategy with literature review. Bipolar Disord. 2010;12(4):446-449.
45. Trileptal [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
46. Rouan MC, Lecaillon JB, Godbillon J, et al. The effect of renal impairment on the pharmacokinetics of oxcarbazepine and its metabolites. Eur J Clin Pharmacol. 1994;47(2):161-167.
1. Levy G. Pharmacokinetics in renal disease. Am J Med. 1977;62(4):461-465.
2. Preskorn SH. Clinically important differences in the pharmacokinetics of the ten newer “atypical” antipsychotics: part 3. Effects of renal and hepatic impairment. J Psychiatr Pract. 2012;18(6):430-437.
3. Cohen LM, Tessier EG, Germain MJ, et al. Update on psychotropic medication use in renal disease. Psychosomatics. 2004;45(1):34-48.
4. Baghdady NT, Banik S, Swartz SA, et al. Psychotropic drugs and renal failure: translating the evidence for clinical practice. Adv Ther. 2009;26(4):404-424.
5. Sheehan J, White A, Wilson R. Hazards of phenothiazines in chronic renal failure. Ir Med J. 1982;75(9):335.
6. Haloperidol [monograph]. In: Micromedex Drugdex [online database]. Greenwood Village, CO: Truven Health Analytics. Accessed December 17, 2014.
7. Sheehan JJ, Sliwa JK, Amatniek JC, et al. Atypical antipsychotic metabolism and excretion. Curr Drug Metab. 2010;11(6):516-525.
8. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2014.
9. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2014.
10. Invega [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2014.
11. Latuda [package insert]. Fort Lee, NJ: Sunovion Pharmaceuticals; 2013.
12. Aweeka F, Jayesekara D, Horton M, et al. The pharmacokinetics of ziprasidone in subjects with normal and impaired renal function. Br J Clin Pharmacol. 2004;49(suppl 1):27S-33S.
13. Peeters P, Bockbrader H, Spaans E, et al. Asenapine pharmacokinetics in hepatic and renal impairment. Clin Pharmacol. 2011;50(7):471-481.
14. Mallikaarjun S, Shoaf SE, Boulton DW, et al. Effects of hepatic or renal impairment on the pharmacokinetics of aripiprazole. Clin Pharmacokinet. 2008;47(8):533-542.
15. Thyrum PT, Wong YW, Yeh C. Single-dose pharmacokinetics of quetiapine in subjects with renal or hepatic impairment. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(4):521-533.
16. Lexi-Drugs. Lexicomp. Hudson, OH: Wolters Kluwer Health, Inc. http://online.lexi.com. Accessed May 28, 2015.
17. Lexapro [package insert]. Forest Pharmaceuticals, Inc.: St. Louis, MO; 2014.
18. Celexa [package insert]. Forest Pharmaceuticals, Inc.: St. Louis, MO; 2014.
19. Paxil [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
20. Effexor [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2010.
21. Pristiq [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2014.
22. Cymbalta [package insert]. Indianapolis, IN: Lilly USA, LLC; 2014.
23. Savella [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2013.
24. Fetzima [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2014.
25. Kurella M, Bennett WM, Chertow GM. Analgesia in patients with ESRD: a review of available evidence. Am J Kidney Dis. 2003;42(2):217-228.
26. Wellbutrin [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
27. Worrall SP, Almond MK, Dhillon S. Pharmacokinetics of bupropion and its metabolites in haemodialysis patients who smoke. A single dose study. Nephron Clin Pract. 2004;97(3):c83-c89.
28. Nagler EV, Webster AC, Vanholder R, et al. Antidepressants for depression in stage 3-5 chronic kidney disease: a systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP). Nephrol Dial Transplant. 2012;27(10):3736-3745.
29. Norpramin. [package insert] Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2014.
30. Tofranil [package insert]. Hazelwood, MO: Mallinckrodt Inc.; 2014.
31. Pamelor [package insert]. Hazelwood, MO: Mallinckrodt Inc.; 2014.
32. Marplan [package insert]. Parsippany, NJ: Validus Pharmaceuticals, LLC; 2012.
33. Nardil [package insert]. New York, NY: Parke-Davis Division of Pfizer Inc.; 2009.
34. EMSAM [package insert]. Morgantown, WV: Mylan Specialty, L.P.; 2014.
35. Eldepryl [package insert]. Morgantown, WV: Somerset Pharmaceuticals, Inc.; 2009.
36. Parnate [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
37. Culpepper L. Reducing the burden of difficult-to-treat major depressive disorder: revisiting monoamine oxidase inhibitor therapy. Prim Care Companion CNS Disord. 2013;15(5). doi: 10.4088/PCC.13r01515.
38. Tossani E, Cassano P, Fava M. Depression and renal disease. Semin Dial. 2005;18(2):73-81.
39. Young AH, Hammond JM. Lithium in mood disorders: increasing evidence base, declining use? Br J Psychiatry. 2007;191:474-476.
40. Eskalith [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2003.
41. Rej S, Looper K, Segal M. The effect of serum lithium levels on renal function in geriatric outpatients: a retrospective longitudinal study. Drugs Aging. 2013;30(6):409-415.
42. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211.
43. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
44. Kaufman KR. Lamotrigine and hemodialysis in bipolar disorder: case analysis of dosing strategy with literature review. Bipolar Disord. 2010;12(4):446-449.
45. Trileptal [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
46. Rouan MC, Lecaillon JB, Godbillon J, et al. The effect of renal impairment on the pharmacokinetics of oxcarbazepine and its metabolites. Eur J Clin Pharmacol. 1994;47(2):161-167.
Psychosis in treated neurosyphilis: Is now the time to stop his antipsychotic?
CASE Hallucinations, impaired memory
Mr. C is a 61-year-old African American man who visits the outpatient clinic for management of antipsychotic therapy for psychosis and depression. His most recent inpatient psychiatric hospitalization for auditory and visual hallucinations, paranoia, and agitation was more than 10 years ago. He has been taking chlorpromazine, 100 mg/d, for 11 years. Mr. C reports that he has had no psychotic symptoms in the past 3 years; he continues taking chlorpromazine, he says, because it helps him sleep.
How would you proceed with Mr. C’s care?
a) continue chlorpromazine because he has been symptom free
b) consider tapering and discontinuing chlorpromazine
c) obtain a more detailed history from Mr. C and perform additional tests
HISTORY Validation of diagnosis
Mr. C reports that, at age 48, he started hearing babies crying and started seeing dead infants crawling out of the incinerator at the hospital where he worked. He denies any psychiatric symptoms before that time. He stopped working 10 years ago because of his psychiatric symptoms and decline in cognition.
Subsequently, Mr. C had 3 inpatient psychiatric hospitalizations for auditory hallucinations; chlorpromazine, 100 mg/d, was prescribed for psychosis. Later efforts to discontinue chlorpromazine resulted in relapse of psychotic symptoms. Mr. C has no family history of psychiatric illness.
Mr. C’s medical history is significant for aortic regurgitation, congestive cardiac failure, hypertension, and left-sided sensorineural hearing loss. He has a history of cocaine abuse from age 21 to 45, but denies using any other substances, including alcohol and nicotine.
Urine toxicology and routine blood tests are within normal limits. The QTc is slightly prolonged over the past 2 years, recording 512, 520, and 505 milliseconds on serial electrocardiograms.
Mr. C is able to perform simple abstractions. He has a goal-directed thought process, devoid of any preoccupation, paranoia, and perceptual abnormalities. Cognitive screening reveals significant impairment of memory, registration, calculation, attention, and visuospatial skills.
Careful review of Mr. C’s history and medical records reveals a diagnosis of syphilis at age 48 after unprotected sexual intercourse. He recalls that he had a solitary genital lesion, which resolved over a few weeks. He then developed a slightly itchy, non-tender macular rash over his upper back, which he did not report to a physician. After a few months, he developed unsteady gait, blurry vision, and weakness of limbs, and had to crawl to the hospital. There, he was given a diagnosis of neurosyphilis. He also developed left-sided hearing loss during that time.
Mr. C was treated with aqueous penicillin G benzathine, 4 million units IV for 2 weeks. No follow-up cerebrospinal fluid (CSF) examination was documented after antibiotic treatment. He developed auditory and visual hallucinations and paranoia a few months after completing penicillin treatment. During the following year, he had 3 inpatient psychiatric hospitalizations for psychosis, agitation, and depressed mood.
How would you treat a patient with a history of neurosyphilis who presents with psychosis years after diagnosis?
a) repeat antibiotic treatment and stop the antipsychotic
b) repeat antibiotic treatment and continue the antipsychotic
c) attempt to discontinue the antipsychotic
d) continue the antipsychotic
The authors’ observations
Mr. C’s psychotic symptoms seem to be temporally related to his diagnosis of neurosyphilis at age 48. He and his family members deny that Mr. C had any history of psychosis or depression before the neurosyphilis diagnosis. All inpatient psychiatric hospitalizations were within 1 year of the neurosyphilis diagnosis.
Mr. C has been on a low dosage of chlorpromazine, which has significant antihistaminic action. Chlorpromazine also is known to cause QTc prolongation, especially in patients with heart disease.
TREATMENT Medication change
A serum rapid plasma reagin test is non-reactive, but Treponema pallidum particle agglutination is positive. MRI shows moderate atrophy suggestive of diffuse small-vessel disease.
Mr. C’s psychotic symptoms are considered to be sequelae of neurosyphilis, based on (1) the presence of positive antibody tests, (2) residual neurologic deficits, (3) other suggestive sequelae (aortic regurgitation, sensorineural deafness), and (4) age-inappropriate gradual cognitive decline in the absence of other psychiatric history.
Because we are concerned about the prolonged QTc, chlorpromazine is discontinued. Haloperidol, 5 mg at bedtime, is started. The neurology team does not recommend antibiotic treatment because symptoms have been stable for years. Mr. C refuses a lumbar puncture.
Mr. C returns to the outpatient clinic monthly. He is psychiatrically stable without any worsening of psychosis. Cognitive impairment remains stable over the next 6 months. Haloperidol is tapered to 2 mg at bedtime 6 months after initial evaluation. Mr. C remains psychiatrically stable on subsequent follow-up visits.
The authors’ observations
Mr. C’s psychotic symptoms persisted after standard antibiotic treatment of neurosyphilis and lapsed when he stopped taking antipsychotic medication 10 years after the initial treatment of neurosyphilis. He carried a diagnosis of schizophrenia for many years, even though his psychotic symptoms were atypical for the presentation of schizophrenia.
It is important to understand the natural course of syphilis, its implication on psychiatric symptom production, and long-term psychiatric prognosis.
Syphilis is a sexually transmitted infectious disease caused by T pallidum, a spirochete, that has varied clinical presentations. Osler called syphilis the “great imitator” for its array of system involvement, ranging from asymptomatic infection and afferent pupillary defect to depression, psychosis, and dementia. With wide use of penicillin, the rate of neurosyphilis declined steadily during the mid 1990s. By 1997, the overall rate reached its lowest point in the United States; in 1999 the Centers for Disease Control and Prevention released a national plan to eliminate syphilis.1 By 2004, however, prevalence had increased to 4.7/100,000. It is thought that this increase is mainly associated with substance use (especially crack cocaine) and HIV co-infection. Most cases were distributed in economically depressed geographical areas.
Psychiatric patients are at higher risk of acquiring the infection because of substance use, lack of education on safer sex practices, and impulsive behavior.
Stages of syphilis
Syphilis does not follow a step-wise progression. One-third of cases progress to the tertiary stage, even many years after initial infection, without adequate treatment.2
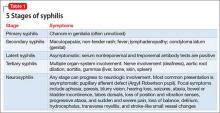
Almost 10% syphilis cases present with neurologic symptoms,3 and neurologic involvement can occur at any stage of disease progression. The most common symptoms of syphilis are presented in Table 1.
A range of psychiatric symptoms have been reported among patients with syphilis, including anhedonia, suicidality, mania, grandiosity, persecutory delusions, auditory and visual hallucinations, paranoia, and cognitive impairment. The incidence of psychiatric symptoms is not clearly described in literature.
Diagnosis and treatment
Neurosyphilis, at any disease stage, should be suspected if a patient:
- exhibits suggestive symptoms
- does not respond to antibiotic treatment
- has late latent syphilis
- is immunocompromised.
Lumbar puncture and examination of CSF is the most useful diagnostic test. Dark field microscopy to reveal T pallidum is definitive, but only is applicable during the primary stage. The role of dark field microscopy of the CSF sample to diagnose neurologic involvement has not been established. Tests and treatment protocol are described in Table 2.2-5
Treatment of psychiatric symptoms of neurosyphilis
There are inconsistent and limited data about the prevalence of psychiatric symptoms in neurosyphilis. A retrospective study6 of 161 patients with neurosyphilis in South Africa reported that 50.9% exhibited a complex spectrum of symptoms that included delirium and dementia. Of treated patients, 17% continued to have residual symptoms during follow-up.
A review of the literature did not reveal any widely accepted guideline for screening for neurosyphilis in general psychiatry practice or a treatment protocol for psychiatric symptoms. This lack of guidance could be attributed to the rarity of the disease, cost-benefit analyses, and low specificity of antibody tests. In the literature, syphilis screening is recommended as a routine protocol when evaluating and treating dementia.7
In most studies, a diagnosis of neurosyphilis was confirmed by CSF examination; however, many of these studies did not report a specific follow-up CSF examination protocol. Most of these patients were treated with an antipsychotic with partial improvement in symptoms, even after standard antibiotic protocol.8
First- and second-generation antipsychotics and mood stabilizers have been shown to be useful in the acute treatment of psychosis and agitation.8 In few instances, the psychotropic medication was continued beyond several months and the patient was placed in a long-term care facility. Psychiatric symptoms persisted for many years with or without residual neurosyphilis symptoms, possibly because of permanent neuronal loss.
Clinical considerations
It often is difficult to distinguish a preexisting psychiatric disorder made worse by neurosyphilis from a secondary psychiatric disorder caused by neurosyphilis. The 2 might coexist, or psychiatric symptoms could be wrongly attributed to schizophrenia because of a lack of careful clinical evaluation.
Often, the follow-up diagnostic protocol for neurosyphilis is not followed; as a result, the need for re-treatment remains unclear. Rarity of the disease makes it difficult to perform a prospective, randomized study to determine the duration and effect of long-term psychiatric treatment.
Close follow-up and consideration of the risk vs benefit of psychotropic medication is key. Because there are no proven guidelines for the length of treatment with antipsychotics, it is prudent to minimize their use until psychiatrically indicated. Side effects, such as (in Mr. C’s case) changes in the QTc interval, should warrant consideration of discontinuing psychotropic medication. Interdisciplinary collaboration with neurology and infectious disease will improve the overall outcome of a complex clinical presentation.
1. Centers for Disease Control and Prevention. National plan to eliminate syphilis from the United States. http://www.cdc.gov/stopsyphilis/plan.htm. Updated December 7, 2007. Accessed July 7, 2016.
2. Friedrich F, Aigner M, Fearns N, et al. Psychosis in neurosyphilis—clinical aspects and implications. Psychopathology. 2014;47(1):3-9.
3. Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68(2):283-290.
4. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005-1009.
5. Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. Syphilis. http://www.cdc.gov/std/tg2015/syphilis.htm. Updated June 4, 2015. Accessed July 13, 2016.
6. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75(12):1727-1730.
7. Scott KR, Barrett AM. Dementia syndrome: evaluation and treatment. Expert Rev Neurother. 2007;7(4):407-422.
8. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48(5):440-445.
CASE Hallucinations, impaired memory
Mr. C is a 61-year-old African American man who visits the outpatient clinic for management of antipsychotic therapy for psychosis and depression. His most recent inpatient psychiatric hospitalization for auditory and visual hallucinations, paranoia, and agitation was more than 10 years ago. He has been taking chlorpromazine, 100 mg/d, for 11 years. Mr. C reports that he has had no psychotic symptoms in the past 3 years; he continues taking chlorpromazine, he says, because it helps him sleep.
How would you proceed with Mr. C’s care?
a) continue chlorpromazine because he has been symptom free
b) consider tapering and discontinuing chlorpromazine
c) obtain a more detailed history from Mr. C and perform additional tests
HISTORY Validation of diagnosis
Mr. C reports that, at age 48, he started hearing babies crying and started seeing dead infants crawling out of the incinerator at the hospital where he worked. He denies any psychiatric symptoms before that time. He stopped working 10 years ago because of his psychiatric symptoms and decline in cognition.
Subsequently, Mr. C had 3 inpatient psychiatric hospitalizations for auditory hallucinations; chlorpromazine, 100 mg/d, was prescribed for psychosis. Later efforts to discontinue chlorpromazine resulted in relapse of psychotic symptoms. Mr. C has no family history of psychiatric illness.
Mr. C’s medical history is significant for aortic regurgitation, congestive cardiac failure, hypertension, and left-sided sensorineural hearing loss. He has a history of cocaine abuse from age 21 to 45, but denies using any other substances, including alcohol and nicotine.
Urine toxicology and routine blood tests are within normal limits. The QTc is slightly prolonged over the past 2 years, recording 512, 520, and 505 milliseconds on serial electrocardiograms.
Mr. C is able to perform simple abstractions. He has a goal-directed thought process, devoid of any preoccupation, paranoia, and perceptual abnormalities. Cognitive screening reveals significant impairment of memory, registration, calculation, attention, and visuospatial skills.
Careful review of Mr. C’s history and medical records reveals a diagnosis of syphilis at age 48 after unprotected sexual intercourse. He recalls that he had a solitary genital lesion, which resolved over a few weeks. He then developed a slightly itchy, non-tender macular rash over his upper back, which he did not report to a physician. After a few months, he developed unsteady gait, blurry vision, and weakness of limbs, and had to crawl to the hospital. There, he was given a diagnosis of neurosyphilis. He also developed left-sided hearing loss during that time.
Mr. C was treated with aqueous penicillin G benzathine, 4 million units IV for 2 weeks. No follow-up cerebrospinal fluid (CSF) examination was documented after antibiotic treatment. He developed auditory and visual hallucinations and paranoia a few months after completing penicillin treatment. During the following year, he had 3 inpatient psychiatric hospitalizations for psychosis, agitation, and depressed mood.
How would you treat a patient with a history of neurosyphilis who presents with psychosis years after diagnosis?
a) repeat antibiotic treatment and stop the antipsychotic
b) repeat antibiotic treatment and continue the antipsychotic
c) attempt to discontinue the antipsychotic
d) continue the antipsychotic
The authors’ observations
Mr. C’s psychotic symptoms seem to be temporally related to his diagnosis of neurosyphilis at age 48. He and his family members deny that Mr. C had any history of psychosis or depression before the neurosyphilis diagnosis. All inpatient psychiatric hospitalizations were within 1 year of the neurosyphilis diagnosis.
Mr. C has been on a low dosage of chlorpromazine, which has significant antihistaminic action. Chlorpromazine also is known to cause QTc prolongation, especially in patients with heart disease.
TREATMENT Medication change
A serum rapid plasma reagin test is non-reactive, but Treponema pallidum particle agglutination is positive. MRI shows moderate atrophy suggestive of diffuse small-vessel disease.
Mr. C’s psychotic symptoms are considered to be sequelae of neurosyphilis, based on (1) the presence of positive antibody tests, (2) residual neurologic deficits, (3) other suggestive sequelae (aortic regurgitation, sensorineural deafness), and (4) age-inappropriate gradual cognitive decline in the absence of other psychiatric history.
Because we are concerned about the prolonged QTc, chlorpromazine is discontinued. Haloperidol, 5 mg at bedtime, is started. The neurology team does not recommend antibiotic treatment because symptoms have been stable for years. Mr. C refuses a lumbar puncture.
Mr. C returns to the outpatient clinic monthly. He is psychiatrically stable without any worsening of psychosis. Cognitive impairment remains stable over the next 6 months. Haloperidol is tapered to 2 mg at bedtime 6 months after initial evaluation. Mr. C remains psychiatrically stable on subsequent follow-up visits.
The authors’ observations
Mr. C’s psychotic symptoms persisted after standard antibiotic treatment of neurosyphilis and lapsed when he stopped taking antipsychotic medication 10 years after the initial treatment of neurosyphilis. He carried a diagnosis of schizophrenia for many years, even though his psychotic symptoms were atypical for the presentation of schizophrenia.
It is important to understand the natural course of syphilis, its implication on psychiatric symptom production, and long-term psychiatric prognosis.
Syphilis is a sexually transmitted infectious disease caused by T pallidum, a spirochete, that has varied clinical presentations. Osler called syphilis the “great imitator” for its array of system involvement, ranging from asymptomatic infection and afferent pupillary defect to depression, psychosis, and dementia. With wide use of penicillin, the rate of neurosyphilis declined steadily during the mid 1990s. By 1997, the overall rate reached its lowest point in the United States; in 1999 the Centers for Disease Control and Prevention released a national plan to eliminate syphilis.1 By 2004, however, prevalence had increased to 4.7/100,000. It is thought that this increase is mainly associated with substance use (especially crack cocaine) and HIV co-infection. Most cases were distributed in economically depressed geographical areas.
Psychiatric patients are at higher risk of acquiring the infection because of substance use, lack of education on safer sex practices, and impulsive behavior.
Stages of syphilis
Syphilis does not follow a step-wise progression. One-third of cases progress to the tertiary stage, even many years after initial infection, without adequate treatment.2
Almost 10% syphilis cases present with neurologic symptoms,3 and neurologic involvement can occur at any stage of disease progression. The most common symptoms of syphilis are presented in Table 1.
A range of psychiatric symptoms have been reported among patients with syphilis, including anhedonia, suicidality, mania, grandiosity, persecutory delusions, auditory and visual hallucinations, paranoia, and cognitive impairment. The incidence of psychiatric symptoms is not clearly described in literature.
Diagnosis and treatment
Neurosyphilis, at any disease stage, should be suspected if a patient:
- exhibits suggestive symptoms
- does not respond to antibiotic treatment
- has late latent syphilis
- is immunocompromised.
Lumbar puncture and examination of CSF is the most useful diagnostic test. Dark field microscopy to reveal T pallidum is definitive, but only is applicable during the primary stage. The role of dark field microscopy of the CSF sample to diagnose neurologic involvement has not been established. Tests and treatment protocol are described in Table 2.2-5
Treatment of psychiatric symptoms of neurosyphilis
There are inconsistent and limited data about the prevalence of psychiatric symptoms in neurosyphilis. A retrospective study6 of 161 patients with neurosyphilis in South Africa reported that 50.9% exhibited a complex spectrum of symptoms that included delirium and dementia. Of treated patients, 17% continued to have residual symptoms during follow-up.
A review of the literature did not reveal any widely accepted guideline for screening for neurosyphilis in general psychiatry practice or a treatment protocol for psychiatric symptoms. This lack of guidance could be attributed to the rarity of the disease, cost-benefit analyses, and low specificity of antibody tests. In the literature, syphilis screening is recommended as a routine protocol when evaluating and treating dementia.7
In most studies, a diagnosis of neurosyphilis was confirmed by CSF examination; however, many of these studies did not report a specific follow-up CSF examination protocol. Most of these patients were treated with an antipsychotic with partial improvement in symptoms, even after standard antibiotic protocol.8
First- and second-generation antipsychotics and mood stabilizers have been shown to be useful in the acute treatment of psychosis and agitation.8 In few instances, the psychotropic medication was continued beyond several months and the patient was placed in a long-term care facility. Psychiatric symptoms persisted for many years with or without residual neurosyphilis symptoms, possibly because of permanent neuronal loss.
Clinical considerations
It often is difficult to distinguish a preexisting psychiatric disorder made worse by neurosyphilis from a secondary psychiatric disorder caused by neurosyphilis. The 2 might coexist, or psychiatric symptoms could be wrongly attributed to schizophrenia because of a lack of careful clinical evaluation.
Often, the follow-up diagnostic protocol for neurosyphilis is not followed; as a result, the need for re-treatment remains unclear. Rarity of the disease makes it difficult to perform a prospective, randomized study to determine the duration and effect of long-term psychiatric treatment.
Close follow-up and consideration of the risk vs benefit of psychotropic medication is key. Because there are no proven guidelines for the length of treatment with antipsychotics, it is prudent to minimize their use until psychiatrically indicated. Side effects, such as (in Mr. C’s case) changes in the QTc interval, should warrant consideration of discontinuing psychotropic medication. Interdisciplinary collaboration with neurology and infectious disease will improve the overall outcome of a complex clinical presentation.
CASE Hallucinations, impaired memory
Mr. C is a 61-year-old African American man who visits the outpatient clinic for management of antipsychotic therapy for psychosis and depression. His most recent inpatient psychiatric hospitalization for auditory and visual hallucinations, paranoia, and agitation was more than 10 years ago. He has been taking chlorpromazine, 100 mg/d, for 11 years. Mr. C reports that he has had no psychotic symptoms in the past 3 years; he continues taking chlorpromazine, he says, because it helps him sleep.
How would you proceed with Mr. C’s care?
a) continue chlorpromazine because he has been symptom free
b) consider tapering and discontinuing chlorpromazine
c) obtain a more detailed history from Mr. C and perform additional tests
HISTORY Validation of diagnosis
Mr. C reports that, at age 48, he started hearing babies crying and started seeing dead infants crawling out of the incinerator at the hospital where he worked. He denies any psychiatric symptoms before that time. He stopped working 10 years ago because of his psychiatric symptoms and decline in cognition.
Subsequently, Mr. C had 3 inpatient psychiatric hospitalizations for auditory hallucinations; chlorpromazine, 100 mg/d, was prescribed for psychosis. Later efforts to discontinue chlorpromazine resulted in relapse of psychotic symptoms. Mr. C has no family history of psychiatric illness.
Mr. C’s medical history is significant for aortic regurgitation, congestive cardiac failure, hypertension, and left-sided sensorineural hearing loss. He has a history of cocaine abuse from age 21 to 45, but denies using any other substances, including alcohol and nicotine.
Urine toxicology and routine blood tests are within normal limits. The QTc is slightly prolonged over the past 2 years, recording 512, 520, and 505 milliseconds on serial electrocardiograms.
Mr. C is able to perform simple abstractions. He has a goal-directed thought process, devoid of any preoccupation, paranoia, and perceptual abnormalities. Cognitive screening reveals significant impairment of memory, registration, calculation, attention, and visuospatial skills.
Careful review of Mr. C’s history and medical records reveals a diagnosis of syphilis at age 48 after unprotected sexual intercourse. He recalls that he had a solitary genital lesion, which resolved over a few weeks. He then developed a slightly itchy, non-tender macular rash over his upper back, which he did not report to a physician. After a few months, he developed unsteady gait, blurry vision, and weakness of limbs, and had to crawl to the hospital. There, he was given a diagnosis of neurosyphilis. He also developed left-sided hearing loss during that time.
Mr. C was treated with aqueous penicillin G benzathine, 4 million units IV for 2 weeks. No follow-up cerebrospinal fluid (CSF) examination was documented after antibiotic treatment. He developed auditory and visual hallucinations and paranoia a few months after completing penicillin treatment. During the following year, he had 3 inpatient psychiatric hospitalizations for psychosis, agitation, and depressed mood.
How would you treat a patient with a history of neurosyphilis who presents with psychosis years after diagnosis?
a) repeat antibiotic treatment and stop the antipsychotic
b) repeat antibiotic treatment and continue the antipsychotic
c) attempt to discontinue the antipsychotic
d) continue the antipsychotic
The authors’ observations
Mr. C’s psychotic symptoms seem to be temporally related to his diagnosis of neurosyphilis at age 48. He and his family members deny that Mr. C had any history of psychosis or depression before the neurosyphilis diagnosis. All inpatient psychiatric hospitalizations were within 1 year of the neurosyphilis diagnosis.
Mr. C has been on a low dosage of chlorpromazine, which has significant antihistaminic action. Chlorpromazine also is known to cause QTc prolongation, especially in patients with heart disease.
TREATMENT Medication change
A serum rapid plasma reagin test is non-reactive, but Treponema pallidum particle agglutination is positive. MRI shows moderate atrophy suggestive of diffuse small-vessel disease.
Mr. C’s psychotic symptoms are considered to be sequelae of neurosyphilis, based on (1) the presence of positive antibody tests, (2) residual neurologic deficits, (3) other suggestive sequelae (aortic regurgitation, sensorineural deafness), and (4) age-inappropriate gradual cognitive decline in the absence of other psychiatric history.
Because we are concerned about the prolonged QTc, chlorpromazine is discontinued. Haloperidol, 5 mg at bedtime, is started. The neurology team does not recommend antibiotic treatment because symptoms have been stable for years. Mr. C refuses a lumbar puncture.
Mr. C returns to the outpatient clinic monthly. He is psychiatrically stable without any worsening of psychosis. Cognitive impairment remains stable over the next 6 months. Haloperidol is tapered to 2 mg at bedtime 6 months after initial evaluation. Mr. C remains psychiatrically stable on subsequent follow-up visits.
The authors’ observations
Mr. C’s psychotic symptoms persisted after standard antibiotic treatment of neurosyphilis and lapsed when he stopped taking antipsychotic medication 10 years after the initial treatment of neurosyphilis. He carried a diagnosis of schizophrenia for many years, even though his psychotic symptoms were atypical for the presentation of schizophrenia.
It is important to understand the natural course of syphilis, its implication on psychiatric symptom production, and long-term psychiatric prognosis.
Syphilis is a sexually transmitted infectious disease caused by T pallidum, a spirochete, that has varied clinical presentations. Osler called syphilis the “great imitator” for its array of system involvement, ranging from asymptomatic infection and afferent pupillary defect to depression, psychosis, and dementia. With wide use of penicillin, the rate of neurosyphilis declined steadily during the mid 1990s. By 1997, the overall rate reached its lowest point in the United States; in 1999 the Centers for Disease Control and Prevention released a national plan to eliminate syphilis.1 By 2004, however, prevalence had increased to 4.7/100,000. It is thought that this increase is mainly associated with substance use (especially crack cocaine) and HIV co-infection. Most cases were distributed in economically depressed geographical areas.
Psychiatric patients are at higher risk of acquiring the infection because of substance use, lack of education on safer sex practices, and impulsive behavior.
Stages of syphilis
Syphilis does not follow a step-wise progression. One-third of cases progress to the tertiary stage, even many years after initial infection, without adequate treatment.2
Almost 10% syphilis cases present with neurologic symptoms,3 and neurologic involvement can occur at any stage of disease progression. The most common symptoms of syphilis are presented in Table 1.
A range of psychiatric symptoms have been reported among patients with syphilis, including anhedonia, suicidality, mania, grandiosity, persecutory delusions, auditory and visual hallucinations, paranoia, and cognitive impairment. The incidence of psychiatric symptoms is not clearly described in literature.
Diagnosis and treatment
Neurosyphilis, at any disease stage, should be suspected if a patient:
- exhibits suggestive symptoms
- does not respond to antibiotic treatment
- has late latent syphilis
- is immunocompromised.
Lumbar puncture and examination of CSF is the most useful diagnostic test. Dark field microscopy to reveal T pallidum is definitive, but only is applicable during the primary stage. The role of dark field microscopy of the CSF sample to diagnose neurologic involvement has not been established. Tests and treatment protocol are described in Table 2.2-5
Treatment of psychiatric symptoms of neurosyphilis
There are inconsistent and limited data about the prevalence of psychiatric symptoms in neurosyphilis. A retrospective study6 of 161 patients with neurosyphilis in South Africa reported that 50.9% exhibited a complex spectrum of symptoms that included delirium and dementia. Of treated patients, 17% continued to have residual symptoms during follow-up.
A review of the literature did not reveal any widely accepted guideline for screening for neurosyphilis in general psychiatry practice or a treatment protocol for psychiatric symptoms. This lack of guidance could be attributed to the rarity of the disease, cost-benefit analyses, and low specificity of antibody tests. In the literature, syphilis screening is recommended as a routine protocol when evaluating and treating dementia.7
In most studies, a diagnosis of neurosyphilis was confirmed by CSF examination; however, many of these studies did not report a specific follow-up CSF examination protocol. Most of these patients were treated with an antipsychotic with partial improvement in symptoms, even after standard antibiotic protocol.8
First- and second-generation antipsychotics and mood stabilizers have been shown to be useful in the acute treatment of psychosis and agitation.8 In few instances, the psychotropic medication was continued beyond several months and the patient was placed in a long-term care facility. Psychiatric symptoms persisted for many years with or without residual neurosyphilis symptoms, possibly because of permanent neuronal loss.
Clinical considerations
It often is difficult to distinguish a preexisting psychiatric disorder made worse by neurosyphilis from a secondary psychiatric disorder caused by neurosyphilis. The 2 might coexist, or psychiatric symptoms could be wrongly attributed to schizophrenia because of a lack of careful clinical evaluation.
Often, the follow-up diagnostic protocol for neurosyphilis is not followed; as a result, the need for re-treatment remains unclear. Rarity of the disease makes it difficult to perform a prospective, randomized study to determine the duration and effect of long-term psychiatric treatment.
Close follow-up and consideration of the risk vs benefit of psychotropic medication is key. Because there are no proven guidelines for the length of treatment with antipsychotics, it is prudent to minimize their use until psychiatrically indicated. Side effects, such as (in Mr. C’s case) changes in the QTc interval, should warrant consideration of discontinuing psychotropic medication. Interdisciplinary collaboration with neurology and infectious disease will improve the overall outcome of a complex clinical presentation.
1. Centers for Disease Control and Prevention. National plan to eliminate syphilis from the United States. http://www.cdc.gov/stopsyphilis/plan.htm. Updated December 7, 2007. Accessed July 7, 2016.
2. Friedrich F, Aigner M, Fearns N, et al. Psychosis in neurosyphilis—clinical aspects and implications. Psychopathology. 2014;47(1):3-9.
3. Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68(2):283-290.
4. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005-1009.
5. Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. Syphilis. http://www.cdc.gov/std/tg2015/syphilis.htm. Updated June 4, 2015. Accessed July 13, 2016.
6. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75(12):1727-1730.
7. Scott KR, Barrett AM. Dementia syndrome: evaluation and treatment. Expert Rev Neurother. 2007;7(4):407-422.
8. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48(5):440-445.
1. Centers for Disease Control and Prevention. National plan to eliminate syphilis from the United States. http://www.cdc.gov/stopsyphilis/plan.htm. Updated December 7, 2007. Accessed July 7, 2016.
2. Friedrich F, Aigner M, Fearns N, et al. Psychosis in neurosyphilis—clinical aspects and implications. Psychopathology. 2014;47(1):3-9.
3. Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68(2):283-290.
4. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005-1009.
5. Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. Syphilis. http://www.cdc.gov/std/tg2015/syphilis.htm. Updated June 4, 2015. Accessed July 13, 2016.
6. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75(12):1727-1730.
7. Scott KR, Barrett AM. Dementia syndrome: evaluation and treatment. Expert Rev Neurother. 2007;7(4):407-422.
8. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48(5):440-445.
Using lipid guidelines to manage metabolic syndrome for patients taking an antipsychotic
Your patient who has schizophrenia, Mr. W, age 48, requests that you switch him from olanzapine, 10 mg/d, to another antipsychotic because he gained 25 lb over 1 month taking the drug. He now weighs 275 lb. Mr. W reports smoking at least 2 packs of cigarettes a day and takes lisinopril, 20 mg/d, for hypertension. You decide to start risperidone, 1 mg/d. First, however, your initial work-up includes:
- high-density lipoprotein (HDL), 24 mg/dL
- total cholesterol, 220 mg/dL
- blood pressure, 154/80 mm Hgwaist circumference, 39 in
- body mass index (BMI), 29
- hemoglobin A1c, of 5.6%.
A prolactin level is pending.
How do you interpret these values?
Metabolic syndrome is defined as the cluster of central obesity, insulin resistance, hypertension, and dyslipidemia. Metabolic syndrome increases a patient's risk of diabetes 5-fold and cardiovascular disease 3-fold.1 Physical inactivity and eating high-fat foods typically precede weight gain and obesity that, in turn, develop into insulin resistance, hypertension, and dyslipidemia.1
Patients with severe psychiatric illness have an increased rate of mortality from cardiovascular disease, compared with the general population.2-4 The cause of this phenomenon is multifactorial: In general, patients with severe mental illness receive insufficient preventive health care, do not eat a balanced diet, and are more likely to smoke cigarettes than other people.2-4
Also, compared with the general population, the diet of men with schizophrenia contains less vegetables and grains and women with schizophrenia consume less grains. An estimated 70% of patients with schizophrenia smoke.4 As measured by BMI, 86% of women with schizophrenia and 70% of men with schizophrenia are overweight or obese.4
Antipsychotics used to treat severe mental illness also have been implicated in metabolic syndrome, specifically second-generation antipsychotics (SGAs).5 Several theories aim to explain how antipsychotics lead to metabolic alterations.
Oxidative stress. One theory centers on the production of oxidative stress and the consequent reactive oxygen species that form after SGA treatment.6
Mitochondrial function. Another theory assesses the impact of antipsychotic treatment on mitochondrial function. Mitochondrial dysfunction causes decreased fatty acid oxidation, leading to lipid accumulation.7
The culminating affect of severe mental illness alone as well as treatment-emergent side effects of antipsychotics raises the question of how to best treat the dyslipidemia component of metabolic syndrome. This article will:
- review which antipsychotics impact lipids the most
- provide an overview of the most recent lipid guidelines
- describe how to best manage patients to prevent and treat dyslipidemia.
Impact of antipsychotics on lipids
Antipsychotic treatment can lead to metabolic syndrome; SGAs are implicated in most cases.8 A study by Liao et al9 investigated the risk of developing type 2 diabetes mellitus, hypertension, and hyperlipidemia in patients with schizophrenia who received treatment with a first-generation antipsychotic (FGA) compared with patients who received a SGA. The significance-adjusted hazard ratio for the development of hyperlipidemia in patients treated with a SGA was statistically significant compared with the general population (1.41; 95% CI, 1.09-1.83). The risk of hyperlipidemia in patients treated with a FGA was not significant.
Studies have aimed to describe which SGAs carry the greatest risk of hyperlipidemia.10,11 To summarize findings, in 2004 the American Diabetes Association (ADA) and American Psychiatric Association released a consensus statement on the impact of antipsychotic medications on obesity and diabetes.12 The statement listed the following antipsychotics in order of greatest to least impact on hyperlipidemia:
- clozapine
- olanzapine
- quetiapine
- risperidone
- ziprasidone
- aripiprazole.
To evaluate newer SGAs, a systematic review and meta-analysis by De Hert et al13 aimed to assess the metabolic risks associated with asenapine, iloperidone, lurasidone, and paliperidone. In general, the studies included in the meta-analysis showed little or no clinically meaningful differences among these newer agents in terms of total cholesterol in short-term trials, except for asenapine and iloperidone.
Asenapine was found to increase the total cholesterol level in long-term trials (>12 weeks) by an average of 6.53 mg/dL. These trials also demonstrated a decrease in HDL cholesterol (−0.13 mg/dL) and a decrease in low-density lipoprotein cholesterol (LDL-C) (−1.72 mg/dL to −0.86 mg/dL). The impact of asenapine on these lab results does not appear to be clinically significant.13,14
Iloperidone. A study evaluating the impact iloperidone on lipid values showed a statistically significant increase in total cholesterol, HDL, and LDL-C levels after 12 weeks.13,15
Overview: Latest lipid guidelines
Current literature lacks information regarding statin use for overall prevention of metabolic syndrome. However, the most recent update to the American Heart Association's guideline on treating blood cholesterol to reduce atherosclerotic cardiovascular risk in adults describes the role of statin therapy to address dyslipidemia, which is one component of metabolic syndrome.16,17
Some of the greatest changes seen with the latest blood cholesterol guidelines include:
- focus on atherosclerotic cardiovascular disease (ASCVD) risk reduction to identify 4 statin benefit groups
- transition away from treating to a target LDL value
- use of the Pooled Cohort Equation to estimate 10-year ASCVD risk, rather than the Framingham Risk Score.
Placing patients in 1 of 4 statin benefit groups
Unlike the 2002 National Cholesterol Education Program Adult Treatment Panel III (ATP III) guidelines, the latest guidelines have identified 4 statin treatment benefit groups:
- patients with clinical ASCVD (including those who have had acute coronary syndrome, stroke, or myocardial infarction, or who have stable or unstable angina, transient ischemic attacks, or peripheral artery disease, or a combination of these findings)patients with LDL-C >190 mg/dL
- patients age 40 to 75 with type 1 or type 2 diabetes mellitus
- patients with an estimated 10-year ASCVD risk of ≥7.5% that was estimated using the Pooled Cohort Equation.16,17
Table 1 represents each statin benefit group and recommended treatment options.
Selected statin therapy for each statin benefit group is further delineated into low-, moderate-, and high-intensity therapy. Intensity of statin therapy represents the expected LDL lowering capacity of selected statins. Low-intensity statin therapy, on average, is expected to lower LDL-C by <30%. Moderate-intensity statin therapy is expected to lower LDL-C by 30% to <50%. High-intensity statin therapy is expected to lower LDL-C by >50%.
When selecting treatment for patients, it is important to first determine the statin benefit group that the patient falls under, and then select the appropriate statin intensity. The categorization of the different statins based on LDL-C lowering capacity is described in Table 2.
Whenever a patient is started on statin therapy, order a liver function test and lipid profile at baseline. Repeat these tests 4 to 12 weeks after statin initiation, then every 3 to 12 months.
Transition away from treating to a target LDL-C goal
ATP III guidelines suggested that elevated LDL was the leading cause of coronary heart disease and recommended therapy with LDL-lowering medications.18 The panel that developed the 2013 lipid guideline concluded that there was no evidence that showed benefit in treating to a designated LDL-C goal.16,17 Arguably, treating to a target may lead to overtreatment in some patients and under-treatment in others. Treatment is now recommended based on statin intensity.
Using the Pooled Cohort Equation
In moving away from the Framingham Risk Score, the latest lipid guidelines established a new calculation to assess cardiovascular disease. The Pooled Cohort Equation estimates the 10-year ASCVD risk for patients based on selected risk factors: age, sex, race, lipids, diabetes, smoking status, and blood pressure. Although other potential cardiovascular disease risk factors have been identified, the Pooled Cohort Equation focused on those risk factors that have been correlated with cardiovascular disease since the 1960s.16,17,19 The Pooled Cohort Equation is intended to (1) more accurately identify higher-risk patients and (2) assess who would best benefit from statin therapy.
Recommended lab tests and subsequent treatment
With the new lipid guidelines in place to direct dyslipidemia treatment and a better understanding of how certain antipsychotics impact lipid values, the next step is monitoring parameters for patients. Before initiating antipsychotic treatment and in accordance with the 2014 National Institute for Health and Care Excellence (NICE) guidelines, baseline measurements should include weight, waist circumference, pulse, blood pressure, fasting blood glucose, hemoglobin A1c, blood lipid profile, and, if risperidone or paliperidone is initiated, prolactin level.20 Additionally, patients should be assessed at baseline for any movement disorders as well as current nutritional status, diet, and level of physical activity.
Once treatment is selected on a patient-specific basis, weight should be measured weekly for the first 6 weeks, again at 12 weeks and 1 year, and then annually. Pulse and blood pressure should be obtained 12 weeks after treatment initiation and at 1 year. Fasting blood glucose, hemoglobin A1c, and blood lipid levels should be collected 12 weeks after treatment onset, then at the 1-year mark.20 These laboratory parameters should be measured annually while the patient is receiving antipsychotic treatment.
Alternately, you can follow the monitoring parameters in the more dated 2004 ADA consensus statement:
- baseline assessment to include BMI, waist circumference, blood pressure, fasting plasma glucose, fasting lipid profile, and personal and family history
- BMI measured again at 4 weeks, 8 weeks, 12 weeks, and then quarterly
- 12-week follow-up measurement of fasting plasma glucose, fasting lipids, and blood pressure
- annual measurement of fasting blood glucose, blood pressure, and waist circumference.12
In addition to the NICE guidelines and the ADA consensus statement, use of the current lipid guidelines and the Pooled Cohort Equation to assess 10-year ASCVD risk should be obtained at baseline and throughout antipsychotic treatment. If you identify an abnormality in the lipid profile, you have several options:
- Decrease the antipsychotic dosage
- Switch to an antipsychotic considered to be less risky
- Discontinue therapy
- Implement diet and exercise
- Refer the patient to a dietitian or other clinician skilled in managing overweight or obesity and hyperlipidemia.21
Furthermore, patients identified as being in 1 of the 4 statin benefit groups should be started on appropriate pharmacotherapy. Non-statin therapy as adjunct or in lieu of statin therapy is not considered to be first-line.16
CASE CONTINUED
After reviewing Mr. W's lab results, you calculate that he has a 24% ten-year ASCVD risk, using the Pooled Cohort Equation. Following the treatment algorithm for statin benefit groups, you see that Mr. W meets criteria for high-intensity statin therapy. You stop olanzapine, switch to risperidone, 1 mg/d, and initiate atorvastatin, 40 mg/d. You plan to assess Mr. W's weight weekly over the next 6 weeks and order a liver profile and lipid profile in 6 weeks.
Related Resource
- AHA/ACC 2013 Prevention Guidelines Tools CV Risk Calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_Prevention-Guidelines.jsp.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Atorvastatin • Lipitor
Clozapine • Clozaril
Fluvastatin • Lescol
Iloperidone • Fanapt
Lovastatin • Mevacor
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Pitavastatin • Livalo
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Rosuvastatin • Crestor
Simvastatin • Zocor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio.
1. O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1-12.
2. McCreadie RG; Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry. 2003;183:534-539.
3. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP Study. JAMA Psychiatry. 2014;7(12):1350-1363.
4. Nordentoft M, Wahlbeck K, Hällgren J, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE. 2013;8(1):e55176. doi: 10.1371/journal.pone.0055176.
5. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
6. Baig MR, Navaira E, Escamilla MA, et al. Clozapine treatment causes oxidation of proteins involved in energy metabolism in lymphoblastoid cells: a possible mechanism for antipsychotic-induced metabolic alterations. J Psychiatr Pract. 2010;16(5):325-333.
7. Schrauwen P, Schrauwen-Hinderling V, Hoeks J, et al. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta. 2010;1801(3):266-271.
8. Watanabe J, Suzuki Y, Someya T. Lipid effects of psychiatric medications. Curr Atheroscler Rep. 2013;15(1):292.
9. Liao HH, Chang CS, Wei WC, et al. Schizophrenia patients at higher risk of diabetes, hypertension and hyperlipidemia: a population-based study. Schizophr Res. 2011;126(1-3):110-116.
10. Lidenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160(2):290-296.
11. Olfson M, Marcus SC, Corey-Lisle P, et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163(10):1821-1825.
12. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone, and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
14. Kemp DE, Zhao J, Cazorla P, et al. Weight change and metabolic effects of asenapine in patients with schizophrenia and bipolar disorder. J Clin Psychiary. 2014;75(3):238-245.
15. Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo-and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S20-S28.
16. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
17. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S72.
18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
19. Ioannidis JP. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA. 2014;311(5):463-464.
20. National Collaborating Centre for Mental Health. Psychosis and schizophrenia in adults: treatment and management: the NICE Guideline on Treatment and Management. https://www.nice.org.uk/guidance/cg178/evidence/full-guideline-490503565. Published 2014. Accessed June 8, 2016.
21. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
Your patient who has schizophrenia, Mr. W, age 48, requests that you switch him from olanzapine, 10 mg/d, to another antipsychotic because he gained 25 lb over 1 month taking the drug. He now weighs 275 lb. Mr. W reports smoking at least 2 packs of cigarettes a day and takes lisinopril, 20 mg/d, for hypertension. You decide to start risperidone, 1 mg/d. First, however, your initial work-up includes:
- high-density lipoprotein (HDL), 24 mg/dL
- total cholesterol, 220 mg/dL
- blood pressure, 154/80 mm Hgwaist circumference, 39 in
- body mass index (BMI), 29
- hemoglobin A1c, of 5.6%.
A prolactin level is pending.
How do you interpret these values?
Metabolic syndrome is defined as the cluster of central obesity, insulin resistance, hypertension, and dyslipidemia. Metabolic syndrome increases a patient's risk of diabetes 5-fold and cardiovascular disease 3-fold.1 Physical inactivity and eating high-fat foods typically precede weight gain and obesity that, in turn, develop into insulin resistance, hypertension, and dyslipidemia.1
Patients with severe psychiatric illness have an increased rate of mortality from cardiovascular disease, compared with the general population.2-4 The cause of this phenomenon is multifactorial: In general, patients with severe mental illness receive insufficient preventive health care, do not eat a balanced diet, and are more likely to smoke cigarettes than other people.2-4
Also, compared with the general population, the diet of men with schizophrenia contains less vegetables and grains and women with schizophrenia consume less grains. An estimated 70% of patients with schizophrenia smoke.4 As measured by BMI, 86% of women with schizophrenia and 70% of men with schizophrenia are overweight or obese.4
Antipsychotics used to treat severe mental illness also have been implicated in metabolic syndrome, specifically second-generation antipsychotics (SGAs).5 Several theories aim to explain how antipsychotics lead to metabolic alterations.
Oxidative stress. One theory centers on the production of oxidative stress and the consequent reactive oxygen species that form after SGA treatment.6
Mitochondrial function. Another theory assesses the impact of antipsychotic treatment on mitochondrial function. Mitochondrial dysfunction causes decreased fatty acid oxidation, leading to lipid accumulation.7
The culminating affect of severe mental illness alone as well as treatment-emergent side effects of antipsychotics raises the question of how to best treat the dyslipidemia component of metabolic syndrome. This article will:
- review which antipsychotics impact lipids the most
- provide an overview of the most recent lipid guidelines
- describe how to best manage patients to prevent and treat dyslipidemia.
Impact of antipsychotics on lipids
Antipsychotic treatment can lead to metabolic syndrome; SGAs are implicated in most cases.8 A study by Liao et al9 investigated the risk of developing type 2 diabetes mellitus, hypertension, and hyperlipidemia in patients with schizophrenia who received treatment with a first-generation antipsychotic (FGA) compared with patients who received a SGA. The significance-adjusted hazard ratio for the development of hyperlipidemia in patients treated with a SGA was statistically significant compared with the general population (1.41; 95% CI, 1.09-1.83). The risk of hyperlipidemia in patients treated with a FGA was not significant.
Studies have aimed to describe which SGAs carry the greatest risk of hyperlipidemia.10,11 To summarize findings, in 2004 the American Diabetes Association (ADA) and American Psychiatric Association released a consensus statement on the impact of antipsychotic medications on obesity and diabetes.12 The statement listed the following antipsychotics in order of greatest to least impact on hyperlipidemia:
- clozapine
- olanzapine
- quetiapine
- risperidone
- ziprasidone
- aripiprazole.
To evaluate newer SGAs, a systematic review and meta-analysis by De Hert et al13 aimed to assess the metabolic risks associated with asenapine, iloperidone, lurasidone, and paliperidone. In general, the studies included in the meta-analysis showed little or no clinically meaningful differences among these newer agents in terms of total cholesterol in short-term trials, except for asenapine and iloperidone.
Asenapine was found to increase the total cholesterol level in long-term trials (>12 weeks) by an average of 6.53 mg/dL. These trials also demonstrated a decrease in HDL cholesterol (−0.13 mg/dL) and a decrease in low-density lipoprotein cholesterol (LDL-C) (−1.72 mg/dL to −0.86 mg/dL). The impact of asenapine on these lab results does not appear to be clinically significant.13,14
Iloperidone. A study evaluating the impact iloperidone on lipid values showed a statistically significant increase in total cholesterol, HDL, and LDL-C levels after 12 weeks.13,15
Overview: Latest lipid guidelines
Current literature lacks information regarding statin use for overall prevention of metabolic syndrome. However, the most recent update to the American Heart Association's guideline on treating blood cholesterol to reduce atherosclerotic cardiovascular risk in adults describes the role of statin therapy to address dyslipidemia, which is one component of metabolic syndrome.16,17
Some of the greatest changes seen with the latest blood cholesterol guidelines include:
- focus on atherosclerotic cardiovascular disease (ASCVD) risk reduction to identify 4 statin benefit groups
- transition away from treating to a target LDL value
- use of the Pooled Cohort Equation to estimate 10-year ASCVD risk, rather than the Framingham Risk Score.
Placing patients in 1 of 4 statin benefit groups
Unlike the 2002 National Cholesterol Education Program Adult Treatment Panel III (ATP III) guidelines, the latest guidelines have identified 4 statin treatment benefit groups:
- patients with clinical ASCVD (including those who have had acute coronary syndrome, stroke, or myocardial infarction, or who have stable or unstable angina, transient ischemic attacks, or peripheral artery disease, or a combination of these findings)patients with LDL-C >190 mg/dL
- patients age 40 to 75 with type 1 or type 2 diabetes mellitus
- patients with an estimated 10-year ASCVD risk of ≥7.5% that was estimated using the Pooled Cohort Equation.16,17
Table 1 represents each statin benefit group and recommended treatment options.
Selected statin therapy for each statin benefit group is further delineated into low-, moderate-, and high-intensity therapy. Intensity of statin therapy represents the expected LDL lowering capacity of selected statins. Low-intensity statin therapy, on average, is expected to lower LDL-C by <30%. Moderate-intensity statin therapy is expected to lower LDL-C by 30% to <50%. High-intensity statin therapy is expected to lower LDL-C by >50%.
When selecting treatment for patients, it is important to first determine the statin benefit group that the patient falls under, and then select the appropriate statin intensity. The categorization of the different statins based on LDL-C lowering capacity is described in Table 2.
Whenever a patient is started on statin therapy, order a liver function test and lipid profile at baseline. Repeat these tests 4 to 12 weeks after statin initiation, then every 3 to 12 months.
Transition away from treating to a target LDL-C goal
ATP III guidelines suggested that elevated LDL was the leading cause of coronary heart disease and recommended therapy with LDL-lowering medications.18 The panel that developed the 2013 lipid guideline concluded that there was no evidence that showed benefit in treating to a designated LDL-C goal.16,17 Arguably, treating to a target may lead to overtreatment in some patients and under-treatment in others. Treatment is now recommended based on statin intensity.
Using the Pooled Cohort Equation
In moving away from the Framingham Risk Score, the latest lipid guidelines established a new calculation to assess cardiovascular disease. The Pooled Cohort Equation estimates the 10-year ASCVD risk for patients based on selected risk factors: age, sex, race, lipids, diabetes, smoking status, and blood pressure. Although other potential cardiovascular disease risk factors have been identified, the Pooled Cohort Equation focused on those risk factors that have been correlated with cardiovascular disease since the 1960s.16,17,19 The Pooled Cohort Equation is intended to (1) more accurately identify higher-risk patients and (2) assess who would best benefit from statin therapy.
Recommended lab tests and subsequent treatment
With the new lipid guidelines in place to direct dyslipidemia treatment and a better understanding of how certain antipsychotics impact lipid values, the next step is monitoring parameters for patients. Before initiating antipsychotic treatment and in accordance with the 2014 National Institute for Health and Care Excellence (NICE) guidelines, baseline measurements should include weight, waist circumference, pulse, blood pressure, fasting blood glucose, hemoglobin A1c, blood lipid profile, and, if risperidone or paliperidone is initiated, prolactin level.20 Additionally, patients should be assessed at baseline for any movement disorders as well as current nutritional status, diet, and level of physical activity.
Once treatment is selected on a patient-specific basis, weight should be measured weekly for the first 6 weeks, again at 12 weeks and 1 year, and then annually. Pulse and blood pressure should be obtained 12 weeks after treatment initiation and at 1 year. Fasting blood glucose, hemoglobin A1c, and blood lipid levels should be collected 12 weeks after treatment onset, then at the 1-year mark.20 These laboratory parameters should be measured annually while the patient is receiving antipsychotic treatment.
Alternately, you can follow the monitoring parameters in the more dated 2004 ADA consensus statement:
- baseline assessment to include BMI, waist circumference, blood pressure, fasting plasma glucose, fasting lipid profile, and personal and family history
- BMI measured again at 4 weeks, 8 weeks, 12 weeks, and then quarterly
- 12-week follow-up measurement of fasting plasma glucose, fasting lipids, and blood pressure
- annual measurement of fasting blood glucose, blood pressure, and waist circumference.12
In addition to the NICE guidelines and the ADA consensus statement, use of the current lipid guidelines and the Pooled Cohort Equation to assess 10-year ASCVD risk should be obtained at baseline and throughout antipsychotic treatment. If you identify an abnormality in the lipid profile, you have several options:
- Decrease the antipsychotic dosage
- Switch to an antipsychotic considered to be less risky
- Discontinue therapy
- Implement diet and exercise
- Refer the patient to a dietitian or other clinician skilled in managing overweight or obesity and hyperlipidemia.21
Furthermore, patients identified as being in 1 of the 4 statin benefit groups should be started on appropriate pharmacotherapy. Non-statin therapy as adjunct or in lieu of statin therapy is not considered to be first-line.16
CASE CONTINUED
After reviewing Mr. W's lab results, you calculate that he has a 24% ten-year ASCVD risk, using the Pooled Cohort Equation. Following the treatment algorithm for statin benefit groups, you see that Mr. W meets criteria for high-intensity statin therapy. You stop olanzapine, switch to risperidone, 1 mg/d, and initiate atorvastatin, 40 mg/d. You plan to assess Mr. W's weight weekly over the next 6 weeks and order a liver profile and lipid profile in 6 weeks.
Related Resource
- AHA/ACC 2013 Prevention Guidelines Tools CV Risk Calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_Prevention-Guidelines.jsp.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Atorvastatin • Lipitor
Clozapine • Clozaril
Fluvastatin • Lescol
Iloperidone • Fanapt
Lovastatin • Mevacor
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Pitavastatin • Livalo
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Rosuvastatin • Crestor
Simvastatin • Zocor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio.
Your patient who has schizophrenia, Mr. W, age 48, requests that you switch him from olanzapine, 10 mg/d, to another antipsychotic because he gained 25 lb over 1 month taking the drug. He now weighs 275 lb. Mr. W reports smoking at least 2 packs of cigarettes a day and takes lisinopril, 20 mg/d, for hypertension. You decide to start risperidone, 1 mg/d. First, however, your initial work-up includes:
- high-density lipoprotein (HDL), 24 mg/dL
- total cholesterol, 220 mg/dL
- blood pressure, 154/80 mm Hgwaist circumference, 39 in
- body mass index (BMI), 29
- hemoglobin A1c, of 5.6%.
A prolactin level is pending.
How do you interpret these values?
Metabolic syndrome is defined as the cluster of central obesity, insulin resistance, hypertension, and dyslipidemia. Metabolic syndrome increases a patient's risk of diabetes 5-fold and cardiovascular disease 3-fold.1 Physical inactivity and eating high-fat foods typically precede weight gain and obesity that, in turn, develop into insulin resistance, hypertension, and dyslipidemia.1
Patients with severe psychiatric illness have an increased rate of mortality from cardiovascular disease, compared with the general population.2-4 The cause of this phenomenon is multifactorial: In general, patients with severe mental illness receive insufficient preventive health care, do not eat a balanced diet, and are more likely to smoke cigarettes than other people.2-4
Also, compared with the general population, the diet of men with schizophrenia contains less vegetables and grains and women with schizophrenia consume less grains. An estimated 70% of patients with schizophrenia smoke.4 As measured by BMI, 86% of women with schizophrenia and 70% of men with schizophrenia are overweight or obese.4
Antipsychotics used to treat severe mental illness also have been implicated in metabolic syndrome, specifically second-generation antipsychotics (SGAs).5 Several theories aim to explain how antipsychotics lead to metabolic alterations.
Oxidative stress. One theory centers on the production of oxidative stress and the consequent reactive oxygen species that form after SGA treatment.6
Mitochondrial function. Another theory assesses the impact of antipsychotic treatment on mitochondrial function. Mitochondrial dysfunction causes decreased fatty acid oxidation, leading to lipid accumulation.7
The culminating affect of severe mental illness alone as well as treatment-emergent side effects of antipsychotics raises the question of how to best treat the dyslipidemia component of metabolic syndrome. This article will:
- review which antipsychotics impact lipids the most
- provide an overview of the most recent lipid guidelines
- describe how to best manage patients to prevent and treat dyslipidemia.
Impact of antipsychotics on lipids
Antipsychotic treatment can lead to metabolic syndrome; SGAs are implicated in most cases.8 A study by Liao et al9 investigated the risk of developing type 2 diabetes mellitus, hypertension, and hyperlipidemia in patients with schizophrenia who received treatment with a first-generation antipsychotic (FGA) compared with patients who received a SGA. The significance-adjusted hazard ratio for the development of hyperlipidemia in patients treated with a SGA was statistically significant compared with the general population (1.41; 95% CI, 1.09-1.83). The risk of hyperlipidemia in patients treated with a FGA was not significant.
Studies have aimed to describe which SGAs carry the greatest risk of hyperlipidemia.10,11 To summarize findings, in 2004 the American Diabetes Association (ADA) and American Psychiatric Association released a consensus statement on the impact of antipsychotic medications on obesity and diabetes.12 The statement listed the following antipsychotics in order of greatest to least impact on hyperlipidemia:
- clozapine
- olanzapine
- quetiapine
- risperidone
- ziprasidone
- aripiprazole.
To evaluate newer SGAs, a systematic review and meta-analysis by De Hert et al13 aimed to assess the metabolic risks associated with asenapine, iloperidone, lurasidone, and paliperidone. In general, the studies included in the meta-analysis showed little or no clinically meaningful differences among these newer agents in terms of total cholesterol in short-term trials, except for asenapine and iloperidone.
Asenapine was found to increase the total cholesterol level in long-term trials (>12 weeks) by an average of 6.53 mg/dL. These trials also demonstrated a decrease in HDL cholesterol (−0.13 mg/dL) and a decrease in low-density lipoprotein cholesterol (LDL-C) (−1.72 mg/dL to −0.86 mg/dL). The impact of asenapine on these lab results does not appear to be clinically significant.13,14
Iloperidone. A study evaluating the impact iloperidone on lipid values showed a statistically significant increase in total cholesterol, HDL, and LDL-C levels after 12 weeks.13,15
Overview: Latest lipid guidelines
Current literature lacks information regarding statin use for overall prevention of metabolic syndrome. However, the most recent update to the American Heart Association's guideline on treating blood cholesterol to reduce atherosclerotic cardiovascular risk in adults describes the role of statin therapy to address dyslipidemia, which is one component of metabolic syndrome.16,17
Some of the greatest changes seen with the latest blood cholesterol guidelines include:
- focus on atherosclerotic cardiovascular disease (ASCVD) risk reduction to identify 4 statin benefit groups
- transition away from treating to a target LDL value
- use of the Pooled Cohort Equation to estimate 10-year ASCVD risk, rather than the Framingham Risk Score.
Placing patients in 1 of 4 statin benefit groups
Unlike the 2002 National Cholesterol Education Program Adult Treatment Panel III (ATP III) guidelines, the latest guidelines have identified 4 statin treatment benefit groups:
- patients with clinical ASCVD (including those who have had acute coronary syndrome, stroke, or myocardial infarction, or who have stable or unstable angina, transient ischemic attacks, or peripheral artery disease, or a combination of these findings)patients with LDL-C >190 mg/dL
- patients age 40 to 75 with type 1 or type 2 diabetes mellitus
- patients with an estimated 10-year ASCVD risk of ≥7.5% that was estimated using the Pooled Cohort Equation.16,17
Table 1 represents each statin benefit group and recommended treatment options.
Selected statin therapy for each statin benefit group is further delineated into low-, moderate-, and high-intensity therapy. Intensity of statin therapy represents the expected LDL lowering capacity of selected statins. Low-intensity statin therapy, on average, is expected to lower LDL-C by <30%. Moderate-intensity statin therapy is expected to lower LDL-C by 30% to <50%. High-intensity statin therapy is expected to lower LDL-C by >50%.
When selecting treatment for patients, it is important to first determine the statin benefit group that the patient falls under, and then select the appropriate statin intensity. The categorization of the different statins based on LDL-C lowering capacity is described in Table 2.
Whenever a patient is started on statin therapy, order a liver function test and lipid profile at baseline. Repeat these tests 4 to 12 weeks after statin initiation, then every 3 to 12 months.
Transition away from treating to a target LDL-C goal
ATP III guidelines suggested that elevated LDL was the leading cause of coronary heart disease and recommended therapy with LDL-lowering medications.18 The panel that developed the 2013 lipid guideline concluded that there was no evidence that showed benefit in treating to a designated LDL-C goal.16,17 Arguably, treating to a target may lead to overtreatment in some patients and under-treatment in others. Treatment is now recommended based on statin intensity.
Using the Pooled Cohort Equation
In moving away from the Framingham Risk Score, the latest lipid guidelines established a new calculation to assess cardiovascular disease. The Pooled Cohort Equation estimates the 10-year ASCVD risk for patients based on selected risk factors: age, sex, race, lipids, diabetes, smoking status, and blood pressure. Although other potential cardiovascular disease risk factors have been identified, the Pooled Cohort Equation focused on those risk factors that have been correlated with cardiovascular disease since the 1960s.16,17,19 The Pooled Cohort Equation is intended to (1) more accurately identify higher-risk patients and (2) assess who would best benefit from statin therapy.
Recommended lab tests and subsequent treatment
With the new lipid guidelines in place to direct dyslipidemia treatment and a better understanding of how certain antipsychotics impact lipid values, the next step is monitoring parameters for patients. Before initiating antipsychotic treatment and in accordance with the 2014 National Institute for Health and Care Excellence (NICE) guidelines, baseline measurements should include weight, waist circumference, pulse, blood pressure, fasting blood glucose, hemoglobin A1c, blood lipid profile, and, if risperidone or paliperidone is initiated, prolactin level.20 Additionally, patients should be assessed at baseline for any movement disorders as well as current nutritional status, diet, and level of physical activity.
Once treatment is selected on a patient-specific basis, weight should be measured weekly for the first 6 weeks, again at 12 weeks and 1 year, and then annually. Pulse and blood pressure should be obtained 12 weeks after treatment initiation and at 1 year. Fasting blood glucose, hemoglobin A1c, and blood lipid levels should be collected 12 weeks after treatment onset, then at the 1-year mark.20 These laboratory parameters should be measured annually while the patient is receiving antipsychotic treatment.
Alternately, you can follow the monitoring parameters in the more dated 2004 ADA consensus statement:
- baseline assessment to include BMI, waist circumference, blood pressure, fasting plasma glucose, fasting lipid profile, and personal and family history
- BMI measured again at 4 weeks, 8 weeks, 12 weeks, and then quarterly
- 12-week follow-up measurement of fasting plasma glucose, fasting lipids, and blood pressure
- annual measurement of fasting blood glucose, blood pressure, and waist circumference.12
In addition to the NICE guidelines and the ADA consensus statement, use of the current lipid guidelines and the Pooled Cohort Equation to assess 10-year ASCVD risk should be obtained at baseline and throughout antipsychotic treatment. If you identify an abnormality in the lipid profile, you have several options:
- Decrease the antipsychotic dosage
- Switch to an antipsychotic considered to be less risky
- Discontinue therapy
- Implement diet and exercise
- Refer the patient to a dietitian or other clinician skilled in managing overweight or obesity and hyperlipidemia.21
Furthermore, patients identified as being in 1 of the 4 statin benefit groups should be started on appropriate pharmacotherapy. Non-statin therapy as adjunct or in lieu of statin therapy is not considered to be first-line.16
CASE CONTINUED
After reviewing Mr. W's lab results, you calculate that he has a 24% ten-year ASCVD risk, using the Pooled Cohort Equation. Following the treatment algorithm for statin benefit groups, you see that Mr. W meets criteria for high-intensity statin therapy. You stop olanzapine, switch to risperidone, 1 mg/d, and initiate atorvastatin, 40 mg/d. You plan to assess Mr. W's weight weekly over the next 6 weeks and order a liver profile and lipid profile in 6 weeks.
Related Resource
- AHA/ACC 2013 Prevention Guidelines Tools CV Risk Calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_Prevention-Guidelines.jsp.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Atorvastatin • Lipitor
Clozapine • Clozaril
Fluvastatin • Lescol
Iloperidone • Fanapt
Lovastatin • Mevacor
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Pitavastatin • Livalo
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Rosuvastatin • Crestor
Simvastatin • Zocor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio.
1. O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1-12.
2. McCreadie RG; Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry. 2003;183:534-539.
3. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP Study. JAMA Psychiatry. 2014;7(12):1350-1363.
4. Nordentoft M, Wahlbeck K, Hällgren J, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE. 2013;8(1):e55176. doi: 10.1371/journal.pone.0055176.
5. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
6. Baig MR, Navaira E, Escamilla MA, et al. Clozapine treatment causes oxidation of proteins involved in energy metabolism in lymphoblastoid cells: a possible mechanism for antipsychotic-induced metabolic alterations. J Psychiatr Pract. 2010;16(5):325-333.
7. Schrauwen P, Schrauwen-Hinderling V, Hoeks J, et al. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta. 2010;1801(3):266-271.
8. Watanabe J, Suzuki Y, Someya T. Lipid effects of psychiatric medications. Curr Atheroscler Rep. 2013;15(1):292.
9. Liao HH, Chang CS, Wei WC, et al. Schizophrenia patients at higher risk of diabetes, hypertension and hyperlipidemia: a population-based study. Schizophr Res. 2011;126(1-3):110-116.
10. Lidenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160(2):290-296.
11. Olfson M, Marcus SC, Corey-Lisle P, et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163(10):1821-1825.
12. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone, and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
14. Kemp DE, Zhao J, Cazorla P, et al. Weight change and metabolic effects of asenapine in patients with schizophrenia and bipolar disorder. J Clin Psychiary. 2014;75(3):238-245.
15. Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo-and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S20-S28.
16. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
17. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S72.
18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
19. Ioannidis JP. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA. 2014;311(5):463-464.
20. National Collaborating Centre for Mental Health. Psychosis and schizophrenia in adults: treatment and management: the NICE Guideline on Treatment and Management. https://www.nice.org.uk/guidance/cg178/evidence/full-guideline-490503565. Published 2014. Accessed June 8, 2016.
21. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
1. O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1-12.
2. McCreadie RG; Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry. 2003;183:534-539.
3. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP Study. JAMA Psychiatry. 2014;7(12):1350-1363.
4. Nordentoft M, Wahlbeck K, Hällgren J, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE. 2013;8(1):e55176. doi: 10.1371/journal.pone.0055176.
5. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
6. Baig MR, Navaira E, Escamilla MA, et al. Clozapine treatment causes oxidation of proteins involved in energy metabolism in lymphoblastoid cells: a possible mechanism for antipsychotic-induced metabolic alterations. J Psychiatr Pract. 2010;16(5):325-333.
7. Schrauwen P, Schrauwen-Hinderling V, Hoeks J, et al. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta. 2010;1801(3):266-271.
8. Watanabe J, Suzuki Y, Someya T. Lipid effects of psychiatric medications. Curr Atheroscler Rep. 2013;15(1):292.
9. Liao HH, Chang CS, Wei WC, et al. Schizophrenia patients at higher risk of diabetes, hypertension and hyperlipidemia: a population-based study. Schizophr Res. 2011;126(1-3):110-116.
10. Lidenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160(2):290-296.
11. Olfson M, Marcus SC, Corey-Lisle P, et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163(10):1821-1825.
12. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone, and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
14. Kemp DE, Zhao J, Cazorla P, et al. Weight change and metabolic effects of asenapine in patients with schizophrenia and bipolar disorder. J Clin Psychiary. 2014;75(3):238-245.
15. Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo-and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S20-S28.
16. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
17. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S72.
18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
19. Ioannidis JP. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA. 2014;311(5):463-464.
20. National Collaborating Centre for Mental Health. Psychosis and schizophrenia in adults: treatment and management: the NICE Guideline on Treatment and Management. https://www.nice.org.uk/guidance/cg178/evidence/full-guideline-490503565. Published 2014. Accessed June 8, 2016.
21. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
No evidence of pregnancy, but she is suicidal and depressed after ‘my baby died’
CASE Depressed after she says her baby died
Ms. R, age 50, is an African-American woman who presents to a psychiatric hospital under an involuntary commitment executed by local law enforcement. Her sister called the authorities because Ms. R reportedly told her that she is “very depressed” and wants to “end [her] life” by taking an overdose of medications after the death of her newborn 1 week earlier.
Ms. R states that she delivered a child at “full term” in the emergency department of an outside community hospital, and that her current psychiatric symptoms began after the child died from “SIDS” [sudden infant death syndrome] shortly after birth.
Ms. R describes depressive symptoms including depressed mood, anhedonia, decreased energy, feelings of guilt, decreased concentration, poor sleep, and suicidal ideation. She denies substance use or a medical condition that could have induced these symptoms, and denies symptoms of mania, anxiety, or psychosis at admission or during the previous year.
Ms. R reports a history of manic episodes that includes periods of elevated mood or irritability, impulsivity, increased energy, excessive spending despite negative consequences, lack of need for sleep, rapid thoughts, and rapid speech that impaired her social and occupational functioning. Her most recent manic episode was approximately 3 years before this admission. She reports a previous suicide attempt and a history of physical abuse from a former intimate partner.
Neither the findings of a physical examination nor the results of a screening test for serum β-human chorionic gonadotropin (βHCG) are consistent with pregnancy. Ms. R’s medical record reveals that she was hospitalized for a “cardiac workup” a week earlier and requested investigation of possible pregnancy, which was negative. Records also reveal that she had a hysterectomy 10 years earlier.
Although Ms. R’s sister and boyfriend support her claim of pregnancy, the patient’s young adult son refutes it and states that she “does stuff like this for attention.” Her son also reports receiving a forged sonogram picture that his mother found online 1 month earlier. Ms. R presents an obituary from a local newspaper for the child but, on further investigation, the photograph of the infant was discovered to be of another child, also obtained online. Ms. R’s family denies knowledge of potential external reward Ms. R could gain by claiming to be pregnant.
Which of the following diagnoses can be considered after Ms. R’s initial presentation?
a) somatic symptom disorder
b) major depressive disorder
c) bipolar I disorder
d) delusional disorder
The authors’ observations
Ms. R reported the recent death of a newborn that was incompatible with her medical history. Her family members revealed that Ms. R made an active effort to deceive them about the reported pregnancy. She also exhibited symptoms of a major depressive episode in the context of previous manic episodes and expressed suicidal ideation.
The first step in the diagnostic pathway was to rule out possible medical explanations, including pregnancy, which could account for the patient’s symptoms. Although the serum βHCG level usually returns to non-pregnant levels 2 to 4 weeks after delivery, it can take even longer in some women.1 The absence of βHCG along with the recorded history of hysterectomy indicated that Ms. R was not pregnant at the time of testing or within the preceding few weeks. Once medical anomalies and substance use were ruled out, further classification of the psychiatric condition was undertaken.
One aspect of establishing a diagnosis for Ms. R is determining the presence of psychosis (eg, delusional thinking) (Table 1). Ms. R deliberately fabricated evidence of her pregnancy and manipulated family members, which indicated a low likelihood of delusions and supported a diagnostic alternative to psychosis.
Ms. R has a well-described history of manic episodes with current symptoms of a major depressive episode. The treatment team makes a diagnosis of bipolar I disorder, most recent episode depressed. The depressive symptoms Ms. R described were consistent with bipolar depression but did not explain her report of a pregnancy that is inconsistent with reality.
As is the case with Ms. R, diagnostic clarity often requires observation and evaluation over time. Building a strong therapeutic relationship with Ms. R in the context of an appropriate treatment plan allows the treatment team to explore the origin, motivations, and evolution of her thought content while managing her illness.
Confronting a patient about her false claims is likely to result in which of the following?
a) spontaneous resolution of symptoms
b) improved therapeutic alliance
c) degradation of the patient’s coping mechanism
d) violent outbursts by the patient
EVALUATION Confrontation
At admission, Ms. R remains resolute that she was pregnant and is suffering immense psychological distress secondary to the death of her child. Early in the treatment course, she is confronted with evidence indicating that her pregnancy was impossible. Shortly after this interaction, nursing staff alerts the treating physician that Ms. R experienced a “seizure-like spell” characterized by gross non-stereotyped jerking of the upper extremities, intact orientation, retention of bowel and bladder function, and coherent speech consistent with a diagnosis of pseudoseizure.2
Ms. R is transferred to a tertiary care facility for neurologic evaluation and observation. Ms. R repeatedly presents a photograph that she claims to be of her deceased child and implores the allied treatment team to advocate for discharge. Evaluation of Ms. R’s neurologic symptoms revealed no medical explanation for the “seizure-like spell” and she is transferred to the inpatient psychiatric hospital.
Upon return to the inpatient psychiatric unit, Ms. R receives intensive psychological exploration of her symptoms, thought content, and the foundation of her pregnancy claim. Within days, she acknowledges that the pregnancy was “not real” and that she was conscious of this fact in the months prior to hospitalization. She cites turmoil in her romantic relationship as the primary stimulus for her actions.
The authors’ observations
Ms. R’s reported pregnancy was not a delusion, but rather a deceitful exposition constructed with appropriate reality testing and a conscious awareness of the manipulation. This eliminated delusions as the explanation of her pregnancy claim. Although Ms. R initially rejected evidence refuting her belief of pregnancy, she recognized and accepted reality with appropriate intervention.
Factitious disorder vs malingering
Factitious disorder and malingering can present with intentional induction or report of symptoms or signs of a physical abnormality:
Factitious disorder imposed on the self is a willful misrepresentation or fabrication of signs or symptoms of an illness by a person in the absence of obvious personal gain that cannot be explained by a separate physical or mental illness (Table 2).3,4
Malingering is the intentional production or exaggeration of physical or psychological signs or symptoms with obvious secondary gain.
Malingering can be excluded in Ms. R’s case: She did not gain external reward by falsely reporting pregnancy. Although DSM-IV-TR (Table 2) assumes that the motivation for the patient with factitious disorder is to assume the sick role, DSM-5 merely states that the she (he) should present themselves as ill, impaired, or injured.3,4
Ms. R’s treatment team diagnosed factitious disorder imposed on self after careful exclusion of other causes for her symptoms. Bipolar I disorder, most recent episode depressed, also was diagnosed after considering Ms. R’s previous history of manic episodes and depressive symptoms at presentation.
Factitious disorder and other psychiatric conditions often are comorbid. Bipolar disorder, as in Ms. R’s case, as well as major depressive disorder commonly are comorbid with factitious disorder. It is also important to note that factitious disorder often occurs in the context of a personality disorder.5
Which of the following medications are FDA-approved for treating factitious disorder?
a) olanzapine-fluoxetine combination
b) lurasidone
c) valproic acid
d) all of the above
e) no medications are approved for treating factitious disorder
TREATMENT Support, drug therapy
Treatment of Ms. R’s factitious disorder consists of psychological interventions via psychotherapy and strengthening of social support. She participates in daily individual therapy sessions as well as several group therapy activities. Ms. R engages with her social worker to facilitate a successful transition to an appropriate support network and access community resources to aid her wellness.
The treatment team feels that her diagnosis of bipolar I disorder, most recent episode depressed, warrants pharmacologic intervention. Ms. R agrees to begin a mood stabilizer, valproic acid, instead of medications FDA-approved to treat bipolar depression, such as lurasidone or quetiapine, because she reports good efficacy and tolerability when she took it during a major depressive episode approximately 4 years earlier.
Valproic acid is started at 250 mg/d and increased to 1,000 mg/d. Ms. R tolerates the medication without observed or reported adverse effects.
The authors’ observations
Managing factitious disorder can be challenging; patients can evoke strong feelings of countertransference during treatment.3,6,7 Providers might feel that the patient does not need to be treated, or that the patient is “not really sick.” This may induce anger and animosity toward the patient (therapeutic nihilism).8 These negative emotions are likely to disrupt the patient–provider relationship and exacerbate the patient’s symptoms.
It is generally accepted that the patient should be made aware of the treatment plan, in an indirect and tactful way, so that the patient does not feel “outed.” Unmasking the patient—the process of instilling insight—is a delicate step and can be a stressful time for the patient.9 A confrontational approach often places the patient’s sick role in doubt and does not address the pathological aspect of the disorder.
It is rare for a patient to admit to fabricating symptoms; confronted, the patient is likely to double their efforts to maintain the rouse of a fictional disease.10,11 It is important for the treatment team to be aware that patients frequently leave the treatment facility against medical advice, seek a different provider, or even pursue legal action for defamation against the treating physician.
Treating comorbid medical and psychiatric conditions is important for successful management of a patient with factitious disorder. Initiating valproic acid to address Ms. R’s bipolar depression contributed to her overall psychiatric stability. Initial treatment with a medication that is FDA-approved for treating bipolar depression, such as lurasidone, quetiapine, or olanzapine-fluoxetine combination, should be considered as an alternative. We chose valproic acid for Ms. R because of its previous efficacy, good tolerability, and the patient’s high level of comfort with the medication.
Which of the following are risk factors for factitious disorder?
a) lengthy medical treatments or hospitalizations as a child
b) female sex
c) experience as a health care worker
d) all of the above
OUTCOME Stabilization
Successful treatment during Ms. R’s inpatient psychiatric admission results in improved insight, remission of suicidal ideation, and stabilization of mood lability. She is discharged to the care of her family with a plan to follow up with a psychotherapist and psychiatrist. Continued administration of valproic acid continues to be effective after discharge.
Ms. R engages in frequent follow-up with outpatient psychiatric services. She remains engaged in psychotherapy and psychiatric care 1 year after discharge. Ms. R has made no report of pregnancy or required hospitalization during this time. She expresses trust in the mental health care system and acknowledges the role treatment played in her improvement.
1. Reyes FI, Winter JS, Faiman C. Postpartum disappearance of chorionic gonadotropin from the maternal and neonatal circulations. Am J Obstet Gynecol. 1985;153(5):486-489.
2. Avbersek A, Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry. 2010;81(7):719-725.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Kapfhammer HP, Rothenhausler HM, Dietrich E, et al. Artifactual disorders—between deception and self-mutilation. Experiences in consultation psychiatry at a university clinic [in German]. Nervenarzt. 1998;69(5):401-409.
6. Feldman MD, Feldman JM. Tangled in the web: countertransference in the therapy of factitious disorders. Int J Psychiatry Med. 1995;25(4):389-399.
7. Wedel KR. A therapeutic confrontation approach to treating patients with factitious illness. Soc Work. 1971;16(2):69-73.
8. Feldman MD, Hamilton JC, Deemer HN. Factitious disorder. In: Phillips KA, ed. Somatoform and factitious disorder. Washington, DC: American Psychiatric Press; 2001:129-159.
9. Scher LM, Knudsen P, Leamon M. Somatic symptom and related disorders. In: Hales RE, Yudofsky SC, Weiss Roberts L, eds. The American Publishing Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing; 2014:531-556.
10. Lipsitt DR. Introduction. In: Feldman MD, Eisendrath SJ, eds. The spectrum of factitious disorders. Washington, DC: American Psychiatric Press; 1996:xix-xxviii.
11. van der Feltz-Cornelis CM. Confronting patients about a factitious disorder [in Dutch]. Ned Tidjschr Geneeskd. 2000;144(12):545-548.
CASE Depressed after she says her baby died
Ms. R, age 50, is an African-American woman who presents to a psychiatric hospital under an involuntary commitment executed by local law enforcement. Her sister called the authorities because Ms. R reportedly told her that she is “very depressed” and wants to “end [her] life” by taking an overdose of medications after the death of her newborn 1 week earlier.
Ms. R states that she delivered a child at “full term” in the emergency department of an outside community hospital, and that her current psychiatric symptoms began after the child died from “SIDS” [sudden infant death syndrome] shortly after birth.
Ms. R describes depressive symptoms including depressed mood, anhedonia, decreased energy, feelings of guilt, decreased concentration, poor sleep, and suicidal ideation. She denies substance use or a medical condition that could have induced these symptoms, and denies symptoms of mania, anxiety, or psychosis at admission or during the previous year.
Ms. R reports a history of manic episodes that includes periods of elevated mood or irritability, impulsivity, increased energy, excessive spending despite negative consequences, lack of need for sleep, rapid thoughts, and rapid speech that impaired her social and occupational functioning. Her most recent manic episode was approximately 3 years before this admission. She reports a previous suicide attempt and a history of physical abuse from a former intimate partner.
Neither the findings of a physical examination nor the results of a screening test for serum β-human chorionic gonadotropin (βHCG) are consistent with pregnancy. Ms. R’s medical record reveals that she was hospitalized for a “cardiac workup” a week earlier and requested investigation of possible pregnancy, which was negative. Records also reveal that she had a hysterectomy 10 years earlier.
Although Ms. R’s sister and boyfriend support her claim of pregnancy, the patient’s young adult son refutes it and states that she “does stuff like this for attention.” Her son also reports receiving a forged sonogram picture that his mother found online 1 month earlier. Ms. R presents an obituary from a local newspaper for the child but, on further investigation, the photograph of the infant was discovered to be of another child, also obtained online. Ms. R’s family denies knowledge of potential external reward Ms. R could gain by claiming to be pregnant.
Which of the following diagnoses can be considered after Ms. R’s initial presentation?
a) somatic symptom disorder
b) major depressive disorder
c) bipolar I disorder
d) delusional disorder
The authors’ observations
Ms. R reported the recent death of a newborn that was incompatible with her medical history. Her family members revealed that Ms. R made an active effort to deceive them about the reported pregnancy. She also exhibited symptoms of a major depressive episode in the context of previous manic episodes and expressed suicidal ideation.
The first step in the diagnostic pathway was to rule out possible medical explanations, including pregnancy, which could account for the patient’s symptoms. Although the serum βHCG level usually returns to non-pregnant levels 2 to 4 weeks after delivery, it can take even longer in some women.1 The absence of βHCG along with the recorded history of hysterectomy indicated that Ms. R was not pregnant at the time of testing or within the preceding few weeks. Once medical anomalies and substance use were ruled out, further classification of the psychiatric condition was undertaken.
One aspect of establishing a diagnosis for Ms. R is determining the presence of psychosis (eg, delusional thinking) (Table 1). Ms. R deliberately fabricated evidence of her pregnancy and manipulated family members, which indicated a low likelihood of delusions and supported a diagnostic alternative to psychosis.
Ms. R has a well-described history of manic episodes with current symptoms of a major depressive episode. The treatment team makes a diagnosis of bipolar I disorder, most recent episode depressed. The depressive symptoms Ms. R described were consistent with bipolar depression but did not explain her report of a pregnancy that is inconsistent with reality.
As is the case with Ms. R, diagnostic clarity often requires observation and evaluation over time. Building a strong therapeutic relationship with Ms. R in the context of an appropriate treatment plan allows the treatment team to explore the origin, motivations, and evolution of her thought content while managing her illness.
Confronting a patient about her false claims is likely to result in which of the following?
a) spontaneous resolution of symptoms
b) improved therapeutic alliance
c) degradation of the patient’s coping mechanism
d) violent outbursts by the patient
EVALUATION Confrontation
At admission, Ms. R remains resolute that she was pregnant and is suffering immense psychological distress secondary to the death of her child. Early in the treatment course, she is confronted with evidence indicating that her pregnancy was impossible. Shortly after this interaction, nursing staff alerts the treating physician that Ms. R experienced a “seizure-like spell” characterized by gross non-stereotyped jerking of the upper extremities, intact orientation, retention of bowel and bladder function, and coherent speech consistent with a diagnosis of pseudoseizure.2
Ms. R is transferred to a tertiary care facility for neurologic evaluation and observation. Ms. R repeatedly presents a photograph that she claims to be of her deceased child and implores the allied treatment team to advocate for discharge. Evaluation of Ms. R’s neurologic symptoms revealed no medical explanation for the “seizure-like spell” and she is transferred to the inpatient psychiatric hospital.
Upon return to the inpatient psychiatric unit, Ms. R receives intensive psychological exploration of her symptoms, thought content, and the foundation of her pregnancy claim. Within days, she acknowledges that the pregnancy was “not real” and that she was conscious of this fact in the months prior to hospitalization. She cites turmoil in her romantic relationship as the primary stimulus for her actions.
The authors’ observations
Ms. R’s reported pregnancy was not a delusion, but rather a deceitful exposition constructed with appropriate reality testing and a conscious awareness of the manipulation. This eliminated delusions as the explanation of her pregnancy claim. Although Ms. R initially rejected evidence refuting her belief of pregnancy, she recognized and accepted reality with appropriate intervention.
Factitious disorder vs malingering
Factitious disorder and malingering can present with intentional induction or report of symptoms or signs of a physical abnormality:
Factitious disorder imposed on the self is a willful misrepresentation or fabrication of signs or symptoms of an illness by a person in the absence of obvious personal gain that cannot be explained by a separate physical or mental illness (Table 2).3,4
Malingering is the intentional production or exaggeration of physical or psychological signs or symptoms with obvious secondary gain.
Malingering can be excluded in Ms. R’s case: She did not gain external reward by falsely reporting pregnancy. Although DSM-IV-TR (Table 2) assumes that the motivation for the patient with factitious disorder is to assume the sick role, DSM-5 merely states that the she (he) should present themselves as ill, impaired, or injured.3,4
Ms. R’s treatment team diagnosed factitious disorder imposed on self after careful exclusion of other causes for her symptoms. Bipolar I disorder, most recent episode depressed, also was diagnosed after considering Ms. R’s previous history of manic episodes and depressive symptoms at presentation.
Factitious disorder and other psychiatric conditions often are comorbid. Bipolar disorder, as in Ms. R’s case, as well as major depressive disorder commonly are comorbid with factitious disorder. It is also important to note that factitious disorder often occurs in the context of a personality disorder.5
Which of the following medications are FDA-approved for treating factitious disorder?
a) olanzapine-fluoxetine combination
b) lurasidone
c) valproic acid
d) all of the above
e) no medications are approved for treating factitious disorder
TREATMENT Support, drug therapy
Treatment of Ms. R’s factitious disorder consists of psychological interventions via psychotherapy and strengthening of social support. She participates in daily individual therapy sessions as well as several group therapy activities. Ms. R engages with her social worker to facilitate a successful transition to an appropriate support network and access community resources to aid her wellness.
The treatment team feels that her diagnosis of bipolar I disorder, most recent episode depressed, warrants pharmacologic intervention. Ms. R agrees to begin a mood stabilizer, valproic acid, instead of medications FDA-approved to treat bipolar depression, such as lurasidone or quetiapine, because she reports good efficacy and tolerability when she took it during a major depressive episode approximately 4 years earlier.
Valproic acid is started at 250 mg/d and increased to 1,000 mg/d. Ms. R tolerates the medication without observed or reported adverse effects.
The authors’ observations
Managing factitious disorder can be challenging; patients can evoke strong feelings of countertransference during treatment.3,6,7 Providers might feel that the patient does not need to be treated, or that the patient is “not really sick.” This may induce anger and animosity toward the patient (therapeutic nihilism).8 These negative emotions are likely to disrupt the patient–provider relationship and exacerbate the patient’s symptoms.
It is generally accepted that the patient should be made aware of the treatment plan, in an indirect and tactful way, so that the patient does not feel “outed.” Unmasking the patient—the process of instilling insight—is a delicate step and can be a stressful time for the patient.9 A confrontational approach often places the patient’s sick role in doubt and does not address the pathological aspect of the disorder.
It is rare for a patient to admit to fabricating symptoms; confronted, the patient is likely to double their efforts to maintain the rouse of a fictional disease.10,11 It is important for the treatment team to be aware that patients frequently leave the treatment facility against medical advice, seek a different provider, or even pursue legal action for defamation against the treating physician.
Treating comorbid medical and psychiatric conditions is important for successful management of a patient with factitious disorder. Initiating valproic acid to address Ms. R’s bipolar depression contributed to her overall psychiatric stability. Initial treatment with a medication that is FDA-approved for treating bipolar depression, such as lurasidone, quetiapine, or olanzapine-fluoxetine combination, should be considered as an alternative. We chose valproic acid for Ms. R because of its previous efficacy, good tolerability, and the patient’s high level of comfort with the medication.
Which of the following are risk factors for factitious disorder?
a) lengthy medical treatments or hospitalizations as a child
b) female sex
c) experience as a health care worker
d) all of the above
OUTCOME Stabilization
Successful treatment during Ms. R’s inpatient psychiatric admission results in improved insight, remission of suicidal ideation, and stabilization of mood lability. She is discharged to the care of her family with a plan to follow up with a psychotherapist and psychiatrist. Continued administration of valproic acid continues to be effective after discharge.
Ms. R engages in frequent follow-up with outpatient psychiatric services. She remains engaged in psychotherapy and psychiatric care 1 year after discharge. Ms. R has made no report of pregnancy or required hospitalization during this time. She expresses trust in the mental health care system and acknowledges the role treatment played in her improvement.
CASE Depressed after she says her baby died
Ms. R, age 50, is an African-American woman who presents to a psychiatric hospital under an involuntary commitment executed by local law enforcement. Her sister called the authorities because Ms. R reportedly told her that she is “very depressed” and wants to “end [her] life” by taking an overdose of medications after the death of her newborn 1 week earlier.
Ms. R states that she delivered a child at “full term” in the emergency department of an outside community hospital, and that her current psychiatric symptoms began after the child died from “SIDS” [sudden infant death syndrome] shortly after birth.
Ms. R describes depressive symptoms including depressed mood, anhedonia, decreased energy, feelings of guilt, decreased concentration, poor sleep, and suicidal ideation. She denies substance use or a medical condition that could have induced these symptoms, and denies symptoms of mania, anxiety, or psychosis at admission or during the previous year.
Ms. R reports a history of manic episodes that includes periods of elevated mood or irritability, impulsivity, increased energy, excessive spending despite negative consequences, lack of need for sleep, rapid thoughts, and rapid speech that impaired her social and occupational functioning. Her most recent manic episode was approximately 3 years before this admission. She reports a previous suicide attempt and a history of physical abuse from a former intimate partner.
Neither the findings of a physical examination nor the results of a screening test for serum β-human chorionic gonadotropin (βHCG) are consistent with pregnancy. Ms. R’s medical record reveals that she was hospitalized for a “cardiac workup” a week earlier and requested investigation of possible pregnancy, which was negative. Records also reveal that she had a hysterectomy 10 years earlier.
Although Ms. R’s sister and boyfriend support her claim of pregnancy, the patient’s young adult son refutes it and states that she “does stuff like this for attention.” Her son also reports receiving a forged sonogram picture that his mother found online 1 month earlier. Ms. R presents an obituary from a local newspaper for the child but, on further investigation, the photograph of the infant was discovered to be of another child, also obtained online. Ms. R’s family denies knowledge of potential external reward Ms. R could gain by claiming to be pregnant.
Which of the following diagnoses can be considered after Ms. R’s initial presentation?
a) somatic symptom disorder
b) major depressive disorder
c) bipolar I disorder
d) delusional disorder
The authors’ observations
Ms. R reported the recent death of a newborn that was incompatible with her medical history. Her family members revealed that Ms. R made an active effort to deceive them about the reported pregnancy. She also exhibited symptoms of a major depressive episode in the context of previous manic episodes and expressed suicidal ideation.
The first step in the diagnostic pathway was to rule out possible medical explanations, including pregnancy, which could account for the patient’s symptoms. Although the serum βHCG level usually returns to non-pregnant levels 2 to 4 weeks after delivery, it can take even longer in some women.1 The absence of βHCG along with the recorded history of hysterectomy indicated that Ms. R was not pregnant at the time of testing or within the preceding few weeks. Once medical anomalies and substance use were ruled out, further classification of the psychiatric condition was undertaken.
One aspect of establishing a diagnosis for Ms. R is determining the presence of psychosis (eg, delusional thinking) (Table 1). Ms. R deliberately fabricated evidence of her pregnancy and manipulated family members, which indicated a low likelihood of delusions and supported a diagnostic alternative to psychosis.
Ms. R has a well-described history of manic episodes with current symptoms of a major depressive episode. The treatment team makes a diagnosis of bipolar I disorder, most recent episode depressed. The depressive symptoms Ms. R described were consistent with bipolar depression but did not explain her report of a pregnancy that is inconsistent with reality.
As is the case with Ms. R, diagnostic clarity often requires observation and evaluation over time. Building a strong therapeutic relationship with Ms. R in the context of an appropriate treatment plan allows the treatment team to explore the origin, motivations, and evolution of her thought content while managing her illness.
Confronting a patient about her false claims is likely to result in which of the following?
a) spontaneous resolution of symptoms
b) improved therapeutic alliance
c) degradation of the patient’s coping mechanism
d) violent outbursts by the patient
EVALUATION Confrontation
At admission, Ms. R remains resolute that she was pregnant and is suffering immense psychological distress secondary to the death of her child. Early in the treatment course, she is confronted with evidence indicating that her pregnancy was impossible. Shortly after this interaction, nursing staff alerts the treating physician that Ms. R experienced a “seizure-like spell” characterized by gross non-stereotyped jerking of the upper extremities, intact orientation, retention of bowel and bladder function, and coherent speech consistent with a diagnosis of pseudoseizure.2
Ms. R is transferred to a tertiary care facility for neurologic evaluation and observation. Ms. R repeatedly presents a photograph that she claims to be of her deceased child and implores the allied treatment team to advocate for discharge. Evaluation of Ms. R’s neurologic symptoms revealed no medical explanation for the “seizure-like spell” and she is transferred to the inpatient psychiatric hospital.
Upon return to the inpatient psychiatric unit, Ms. R receives intensive psychological exploration of her symptoms, thought content, and the foundation of her pregnancy claim. Within days, she acknowledges that the pregnancy was “not real” and that she was conscious of this fact in the months prior to hospitalization. She cites turmoil in her romantic relationship as the primary stimulus for her actions.
The authors’ observations
Ms. R’s reported pregnancy was not a delusion, but rather a deceitful exposition constructed with appropriate reality testing and a conscious awareness of the manipulation. This eliminated delusions as the explanation of her pregnancy claim. Although Ms. R initially rejected evidence refuting her belief of pregnancy, she recognized and accepted reality with appropriate intervention.
Factitious disorder vs malingering
Factitious disorder and malingering can present with intentional induction or report of symptoms or signs of a physical abnormality:
Factitious disorder imposed on the self is a willful misrepresentation or fabrication of signs or symptoms of an illness by a person in the absence of obvious personal gain that cannot be explained by a separate physical or mental illness (Table 2).3,4
Malingering is the intentional production or exaggeration of physical or psychological signs or symptoms with obvious secondary gain.
Malingering can be excluded in Ms. R’s case: She did not gain external reward by falsely reporting pregnancy. Although DSM-IV-TR (Table 2) assumes that the motivation for the patient with factitious disorder is to assume the sick role, DSM-5 merely states that the she (he) should present themselves as ill, impaired, or injured.3,4
Ms. R’s treatment team diagnosed factitious disorder imposed on self after careful exclusion of other causes for her symptoms. Bipolar I disorder, most recent episode depressed, also was diagnosed after considering Ms. R’s previous history of manic episodes and depressive symptoms at presentation.
Factitious disorder and other psychiatric conditions often are comorbid. Bipolar disorder, as in Ms. R’s case, as well as major depressive disorder commonly are comorbid with factitious disorder. It is also important to note that factitious disorder often occurs in the context of a personality disorder.5
Which of the following medications are FDA-approved for treating factitious disorder?
a) olanzapine-fluoxetine combination
b) lurasidone
c) valproic acid
d) all of the above
e) no medications are approved for treating factitious disorder
TREATMENT Support, drug therapy
Treatment of Ms. R’s factitious disorder consists of psychological interventions via psychotherapy and strengthening of social support. She participates in daily individual therapy sessions as well as several group therapy activities. Ms. R engages with her social worker to facilitate a successful transition to an appropriate support network and access community resources to aid her wellness.
The treatment team feels that her diagnosis of bipolar I disorder, most recent episode depressed, warrants pharmacologic intervention. Ms. R agrees to begin a mood stabilizer, valproic acid, instead of medications FDA-approved to treat bipolar depression, such as lurasidone or quetiapine, because she reports good efficacy and tolerability when she took it during a major depressive episode approximately 4 years earlier.
Valproic acid is started at 250 mg/d and increased to 1,000 mg/d. Ms. R tolerates the medication without observed or reported adverse effects.
The authors’ observations
Managing factitious disorder can be challenging; patients can evoke strong feelings of countertransference during treatment.3,6,7 Providers might feel that the patient does not need to be treated, or that the patient is “not really sick.” This may induce anger and animosity toward the patient (therapeutic nihilism).8 These negative emotions are likely to disrupt the patient–provider relationship and exacerbate the patient’s symptoms.
It is generally accepted that the patient should be made aware of the treatment plan, in an indirect and tactful way, so that the patient does not feel “outed.” Unmasking the patient—the process of instilling insight—is a delicate step and can be a stressful time for the patient.9 A confrontational approach often places the patient’s sick role in doubt and does not address the pathological aspect of the disorder.
It is rare for a patient to admit to fabricating symptoms; confronted, the patient is likely to double their efforts to maintain the rouse of a fictional disease.10,11 It is important for the treatment team to be aware that patients frequently leave the treatment facility against medical advice, seek a different provider, or even pursue legal action for defamation against the treating physician.
Treating comorbid medical and psychiatric conditions is important for successful management of a patient with factitious disorder. Initiating valproic acid to address Ms. R’s bipolar depression contributed to her overall psychiatric stability. Initial treatment with a medication that is FDA-approved for treating bipolar depression, such as lurasidone, quetiapine, or olanzapine-fluoxetine combination, should be considered as an alternative. We chose valproic acid for Ms. R because of its previous efficacy, good tolerability, and the patient’s high level of comfort with the medication.
Which of the following are risk factors for factitious disorder?
a) lengthy medical treatments or hospitalizations as a child
b) female sex
c) experience as a health care worker
d) all of the above
OUTCOME Stabilization
Successful treatment during Ms. R’s inpatient psychiatric admission results in improved insight, remission of suicidal ideation, and stabilization of mood lability. She is discharged to the care of her family with a plan to follow up with a psychotherapist and psychiatrist. Continued administration of valproic acid continues to be effective after discharge.
Ms. R engages in frequent follow-up with outpatient psychiatric services. She remains engaged in psychotherapy and psychiatric care 1 year after discharge. Ms. R has made no report of pregnancy or required hospitalization during this time. She expresses trust in the mental health care system and acknowledges the role treatment played in her improvement.
1. Reyes FI, Winter JS, Faiman C. Postpartum disappearance of chorionic gonadotropin from the maternal and neonatal circulations. Am J Obstet Gynecol. 1985;153(5):486-489.
2. Avbersek A, Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry. 2010;81(7):719-725.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Kapfhammer HP, Rothenhausler HM, Dietrich E, et al. Artifactual disorders—between deception and self-mutilation. Experiences in consultation psychiatry at a university clinic [in German]. Nervenarzt. 1998;69(5):401-409.
6. Feldman MD, Feldman JM. Tangled in the web: countertransference in the therapy of factitious disorders. Int J Psychiatry Med. 1995;25(4):389-399.
7. Wedel KR. A therapeutic confrontation approach to treating patients with factitious illness. Soc Work. 1971;16(2):69-73.
8. Feldman MD, Hamilton JC, Deemer HN. Factitious disorder. In: Phillips KA, ed. Somatoform and factitious disorder. Washington, DC: American Psychiatric Press; 2001:129-159.
9. Scher LM, Knudsen P, Leamon M. Somatic symptom and related disorders. In: Hales RE, Yudofsky SC, Weiss Roberts L, eds. The American Publishing Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing; 2014:531-556.
10. Lipsitt DR. Introduction. In: Feldman MD, Eisendrath SJ, eds. The spectrum of factitious disorders. Washington, DC: American Psychiatric Press; 1996:xix-xxviii.
11. van der Feltz-Cornelis CM. Confronting patients about a factitious disorder [in Dutch]. Ned Tidjschr Geneeskd. 2000;144(12):545-548.
1. Reyes FI, Winter JS, Faiman C. Postpartum disappearance of chorionic gonadotropin from the maternal and neonatal circulations. Am J Obstet Gynecol. 1985;153(5):486-489.
2. Avbersek A, Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry. 2010;81(7):719-725.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Kapfhammer HP, Rothenhausler HM, Dietrich E, et al. Artifactual disorders—between deception and self-mutilation. Experiences in consultation psychiatry at a university clinic [in German]. Nervenarzt. 1998;69(5):401-409.
6. Feldman MD, Feldman JM. Tangled in the web: countertransference in the therapy of factitious disorders. Int J Psychiatry Med. 1995;25(4):389-399.
7. Wedel KR. A therapeutic confrontation approach to treating patients with factitious illness. Soc Work. 1971;16(2):69-73.
8. Feldman MD, Hamilton JC, Deemer HN. Factitious disorder. In: Phillips KA, ed. Somatoform and factitious disorder. Washington, DC: American Psychiatric Press; 2001:129-159.
9. Scher LM, Knudsen P, Leamon M. Somatic symptom and related disorders. In: Hales RE, Yudofsky SC, Weiss Roberts L, eds. The American Publishing Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing; 2014:531-556.
10. Lipsitt DR. Introduction. In: Feldman MD, Eisendrath SJ, eds. The spectrum of factitious disorders. Washington, DC: American Psychiatric Press; 1996:xix-xxviii.
11. van der Feltz-Cornelis CM. Confronting patients about a factitious disorder [in Dutch]. Ned Tidjschr Geneeskd. 2000;144(12):545-548.
Binge eating most effectively treated by CBT, lisdexamfetamine, SGAs
Cognitive-behavioral therapy, lisdexamfetamine, and second-generation antidepressants are the most effective treatments for adult binge-eating disorder, a systematic review by Kimberly A. Brownley, PhD, and her associates found.
A total of 34 trials were included in the review. Patients who received therapist-led cognitive-behavioral therapy (CBT) achieved binge eating abstinence at a rate of 58.8%, compared with 11.2% of those on a wait list. Just over 40% of patients achieved abstinence on lisdexamfetamine, compared with 14.9% on a placebo, and 39.9% of patients achieved abstinence on second-generation antipsychotics (SGAs), compared with 23.6% on a placebo.
Total eating-related obsessions and compulsions were significantly reduced in patients receiving lisdexamfetamine and SGAs, and CBT significantly improved eating-related psychopathology. Body mass index was not reduced in patients receiving SGAs or CBT, but was reduced in those receiving lisdexamfetamine and topiramate, compared with placebo. Symptoms of depression were reduced by SGAs, but not by CBT.
In a related editorial, Dr. Michael J. Devlin of the New York State Psychiatric Institute and Columbia University, New York, praised the review by Dr. Brownley and her associates as an expert summary of the “current evidence on binge-eating disorder.” He went on to make the connection between eating disorders and obesity, and discuss the prospects for interventions.
“The seeds of unhealthy eating that eventually lead to obesity, disordered eating, or both often are sown during childhood or adolescence, and interventions at the community and family levels in the context of enlightened public policy likely would yield significant benefit,” Dr. Devlin wrote. “Only by understanding binge-eating disorder at various levels of analysis and through different professional lenses will we ensure that its life span is shortened, to the benefit of our own.”
Find the full study (doi: 10.7326/M15-2455) and editorial (doi: 10.7326/M16-1398) in the Annals of Internal Medicine.
Cognitive-behavioral therapy, lisdexamfetamine, and second-generation antidepressants are the most effective treatments for adult binge-eating disorder, a systematic review by Kimberly A. Brownley, PhD, and her associates found.
A total of 34 trials were included in the review. Patients who received therapist-led cognitive-behavioral therapy (CBT) achieved binge eating abstinence at a rate of 58.8%, compared with 11.2% of those on a wait list. Just over 40% of patients achieved abstinence on lisdexamfetamine, compared with 14.9% on a placebo, and 39.9% of patients achieved abstinence on second-generation antipsychotics (SGAs), compared with 23.6% on a placebo.
Total eating-related obsessions and compulsions were significantly reduced in patients receiving lisdexamfetamine and SGAs, and CBT significantly improved eating-related psychopathology. Body mass index was not reduced in patients receiving SGAs or CBT, but was reduced in those receiving lisdexamfetamine and topiramate, compared with placebo. Symptoms of depression were reduced by SGAs, but not by CBT.
In a related editorial, Dr. Michael J. Devlin of the New York State Psychiatric Institute and Columbia University, New York, praised the review by Dr. Brownley and her associates as an expert summary of the “current evidence on binge-eating disorder.” He went on to make the connection between eating disorders and obesity, and discuss the prospects for interventions.
“The seeds of unhealthy eating that eventually lead to obesity, disordered eating, or both often are sown during childhood or adolescence, and interventions at the community and family levels in the context of enlightened public policy likely would yield significant benefit,” Dr. Devlin wrote. “Only by understanding binge-eating disorder at various levels of analysis and through different professional lenses will we ensure that its life span is shortened, to the benefit of our own.”
Find the full study (doi: 10.7326/M15-2455) and editorial (doi: 10.7326/M16-1398) in the Annals of Internal Medicine.
Cognitive-behavioral therapy, lisdexamfetamine, and second-generation antidepressants are the most effective treatments for adult binge-eating disorder, a systematic review by Kimberly A. Brownley, PhD, and her associates found.
A total of 34 trials were included in the review. Patients who received therapist-led cognitive-behavioral therapy (CBT) achieved binge eating abstinence at a rate of 58.8%, compared with 11.2% of those on a wait list. Just over 40% of patients achieved abstinence on lisdexamfetamine, compared with 14.9% on a placebo, and 39.9% of patients achieved abstinence on second-generation antipsychotics (SGAs), compared with 23.6% on a placebo.
Total eating-related obsessions and compulsions were significantly reduced in patients receiving lisdexamfetamine and SGAs, and CBT significantly improved eating-related psychopathology. Body mass index was not reduced in patients receiving SGAs or CBT, but was reduced in those receiving lisdexamfetamine and topiramate, compared with placebo. Symptoms of depression were reduced by SGAs, but not by CBT.
In a related editorial, Dr. Michael J. Devlin of the New York State Psychiatric Institute and Columbia University, New York, praised the review by Dr. Brownley and her associates as an expert summary of the “current evidence on binge-eating disorder.” He went on to make the connection between eating disorders and obesity, and discuss the prospects for interventions.
“The seeds of unhealthy eating that eventually lead to obesity, disordered eating, or both often are sown during childhood or adolescence, and interventions at the community and family levels in the context of enlightened public policy likely would yield significant benefit,” Dr. Devlin wrote. “Only by understanding binge-eating disorder at various levels of analysis and through different professional lenses will we ensure that its life span is shortened, to the benefit of our own.”
Find the full study (doi: 10.7326/M15-2455) and editorial (doi: 10.7326/M16-1398) in the Annals of Internal Medicine.
FROM THE ANNALS OF INTERNAL MEDICINE
Mirtazapine improved functional dyspepsia, psychological distress
SAN DIEGO – The tetracyclic antidepressant mirtazapine significantly improved indicators of functional dyspepsia and psychological distress in a single-center, randomized, placebo-controlled, double-blinded trial of 116 adults.
After 3 months of treatment, the mirtazapine group had significantly less nausea, early satiety, depression, somatization, hostility, and phobic anxiety, compared with the control group (all P values < .05), Dr. Yaoyao Gong reported at the annual Digestive Disease Week. Most patients began improving after 1-2 weeks of mirtazapine therapy, she added.
“We assume that mirtazapine might improve functional dyspepsia by improving depression and anxiety, reducing visceral sensitivity, and through its prokinetic effects on gastrointestinal transit,” said Dr. Gong, who is at the gastroenterology department of Nanjing Medical University, China.
Mirtazapine is a presynaptic alpha-2 antagonist that also blocks the 5-HT2a, 5-HT2c, 5-HT3, and H-1 receptors. This atypical antidepressant reduced visceral hypersensitivity and increased gastric accommodation, gastric emptying, and colonic transit time in animal studies, and improved weight loss, early satiety, and nausea in a recent U.S. placebo-controlled pilot trial (Clin Gastroenterol Hepatol. 2016 Mar;14[3]:385-92).
As a biopsychosocial disorder, functional dyspepsia involves both physical and psychological symptoms, Dr. Gong noted. To explore how mirtazapine affects both realms, she and her associates randomized outpatients meeting Rome III functional dyspepsia criteria who also been diagnosed by a psychiatrist with anxiety, depression, or somatization disorder to receive either mirtazapine, 15 mg per day (61 patients) or placebo (55 patients). Both groups also received omeprazole, 30 mg per day, and mosapride, 5 mg three times a day. Patients were mostly in their early 40s, none was taking SSRIs or MAOIs, they had no history of abdominal surgery or upper endoscopy lesions, and they had negative 13C urea breath tests for Helicobacter pylori infection.
After 3 months of treatment, mirtazapine was associated with significantly lower 7-point Likert scales for nausea and early satiety, compared with standard care alone, said Dr. Gong. Mirtazapine did not significantly improve the other Rome III criteria for functional dyspepsia, but general overall score was significantly lower than for the control group.
In addition, the mirtazapine group had a “markedly better” average Symptom Checklist (SCL)-90 score, compared with the control group, and individual measures of depression, somatization, hostility, and phobic anxiety also were significantly lower than for controls (P < .05).
Mirtazapine did not significantly affect overall anxiety, a frequent psychological feature of functional dyspepsia. Mirtazapine was most often associated with dry mouth, although the researchers did not measure weight gain, another common adverse effect of the medication.
None of the patients stopped treatment because of adverse effects. The study group was too small to look at the separate effects of mirtazapine on epigastric pain and postprandial distress syndrome, Dr. Gong noted.
“We plan to assess these patients again after 12 months of treatment,” she said.
Dr. Gong did not report funding sources and had no disclosures.
SAN DIEGO – The tetracyclic antidepressant mirtazapine significantly improved indicators of functional dyspepsia and psychological distress in a single-center, randomized, placebo-controlled, double-blinded trial of 116 adults.
After 3 months of treatment, the mirtazapine group had significantly less nausea, early satiety, depression, somatization, hostility, and phobic anxiety, compared with the control group (all P values < .05), Dr. Yaoyao Gong reported at the annual Digestive Disease Week. Most patients began improving after 1-2 weeks of mirtazapine therapy, she added.
“We assume that mirtazapine might improve functional dyspepsia by improving depression and anxiety, reducing visceral sensitivity, and through its prokinetic effects on gastrointestinal transit,” said Dr. Gong, who is at the gastroenterology department of Nanjing Medical University, China.
Mirtazapine is a presynaptic alpha-2 antagonist that also blocks the 5-HT2a, 5-HT2c, 5-HT3, and H-1 receptors. This atypical antidepressant reduced visceral hypersensitivity and increased gastric accommodation, gastric emptying, and colonic transit time in animal studies, and improved weight loss, early satiety, and nausea in a recent U.S. placebo-controlled pilot trial (Clin Gastroenterol Hepatol. 2016 Mar;14[3]:385-92).
As a biopsychosocial disorder, functional dyspepsia involves both physical and psychological symptoms, Dr. Gong noted. To explore how mirtazapine affects both realms, she and her associates randomized outpatients meeting Rome III functional dyspepsia criteria who also been diagnosed by a psychiatrist with anxiety, depression, or somatization disorder to receive either mirtazapine, 15 mg per day (61 patients) or placebo (55 patients). Both groups also received omeprazole, 30 mg per day, and mosapride, 5 mg three times a day. Patients were mostly in their early 40s, none was taking SSRIs or MAOIs, they had no history of abdominal surgery or upper endoscopy lesions, and they had negative 13C urea breath tests for Helicobacter pylori infection.
After 3 months of treatment, mirtazapine was associated with significantly lower 7-point Likert scales for nausea and early satiety, compared with standard care alone, said Dr. Gong. Mirtazapine did not significantly improve the other Rome III criteria for functional dyspepsia, but general overall score was significantly lower than for the control group.
In addition, the mirtazapine group had a “markedly better” average Symptom Checklist (SCL)-90 score, compared with the control group, and individual measures of depression, somatization, hostility, and phobic anxiety also were significantly lower than for controls (P < .05).
Mirtazapine did not significantly affect overall anxiety, a frequent psychological feature of functional dyspepsia. Mirtazapine was most often associated with dry mouth, although the researchers did not measure weight gain, another common adverse effect of the medication.
None of the patients stopped treatment because of adverse effects. The study group was too small to look at the separate effects of mirtazapine on epigastric pain and postprandial distress syndrome, Dr. Gong noted.
“We plan to assess these patients again after 12 months of treatment,” she said.
Dr. Gong did not report funding sources and had no disclosures.
SAN DIEGO – The tetracyclic antidepressant mirtazapine significantly improved indicators of functional dyspepsia and psychological distress in a single-center, randomized, placebo-controlled, double-blinded trial of 116 adults.
After 3 months of treatment, the mirtazapine group had significantly less nausea, early satiety, depression, somatization, hostility, and phobic anxiety, compared with the control group (all P values < .05), Dr. Yaoyao Gong reported at the annual Digestive Disease Week. Most patients began improving after 1-2 weeks of mirtazapine therapy, she added.
“We assume that mirtazapine might improve functional dyspepsia by improving depression and anxiety, reducing visceral sensitivity, and through its prokinetic effects on gastrointestinal transit,” said Dr. Gong, who is at the gastroenterology department of Nanjing Medical University, China.
Mirtazapine is a presynaptic alpha-2 antagonist that also blocks the 5-HT2a, 5-HT2c, 5-HT3, and H-1 receptors. This atypical antidepressant reduced visceral hypersensitivity and increased gastric accommodation, gastric emptying, and colonic transit time in animal studies, and improved weight loss, early satiety, and nausea in a recent U.S. placebo-controlled pilot trial (Clin Gastroenterol Hepatol. 2016 Mar;14[3]:385-92).
As a biopsychosocial disorder, functional dyspepsia involves both physical and psychological symptoms, Dr. Gong noted. To explore how mirtazapine affects both realms, she and her associates randomized outpatients meeting Rome III functional dyspepsia criteria who also been diagnosed by a psychiatrist with anxiety, depression, or somatization disorder to receive either mirtazapine, 15 mg per day (61 patients) or placebo (55 patients). Both groups also received omeprazole, 30 mg per day, and mosapride, 5 mg three times a day. Patients were mostly in their early 40s, none was taking SSRIs or MAOIs, they had no history of abdominal surgery or upper endoscopy lesions, and they had negative 13C urea breath tests for Helicobacter pylori infection.
After 3 months of treatment, mirtazapine was associated with significantly lower 7-point Likert scales for nausea and early satiety, compared with standard care alone, said Dr. Gong. Mirtazapine did not significantly improve the other Rome III criteria for functional dyspepsia, but general overall score was significantly lower than for the control group.
In addition, the mirtazapine group had a “markedly better” average Symptom Checklist (SCL)-90 score, compared with the control group, and individual measures of depression, somatization, hostility, and phobic anxiety also were significantly lower than for controls (P < .05).
Mirtazapine did not significantly affect overall anxiety, a frequent psychological feature of functional dyspepsia. Mirtazapine was most often associated with dry mouth, although the researchers did not measure weight gain, another common adverse effect of the medication.
None of the patients stopped treatment because of adverse effects. The study group was too small to look at the separate effects of mirtazapine on epigastric pain and postprandial distress syndrome, Dr. Gong noted.
“We plan to assess these patients again after 12 months of treatment,” she said.
Dr. Gong did not report funding sources and had no disclosures.
AT DDW® 2016
Key clinical point: A 15-mg daily dose of mirtazapine controlled comorbid functional dyspepsia and psychological distress more effectively than placebo.
Major finding: At 3 months, the mirtazapine group had significantly less nausea, early satiety, depression, somatization, hostility, and phobic anxiety than patients who received only standard of care (P < .05 for all comparisons).
Data source: A single-center, randomized, double-blind, placebo-controlled trial of 116 adults with functional dyspepsia and depression, anxiety, or somatization disorder.
Disclosures: Dr. Gong reported no funding sources and had no disclosures.
Precipitously and certainly psychotic—but what’s the cause?
CASE Sudden personality change
Ms. L, age 38, is brought to the university hospital’s emergency department (ED) under police escort after she awoke in the middle of the night screaming, “I found it out! I’m a lie! Life is a lie!” and began threatening suicide. This prompted her spouse to call emergency services because of concerns about her safety.
Over the preceding 9 days—and, most precipitously, over the last 24 hours—Ms. L has experienced a dramatic “change in her personality,” according to her spouse. In the ED, she is oriented to person, place, and time. Her vital signs are within normal limits, other than a mild tachycardia. Complete blood count and complete metabolic profile are unremarkable and a urine drug screen is positive only for benzodiazepines (she recently was prescribed alprazolam). Ms. L smiles inappropriately at the ED physicians and confides that she is hearing music by The Lumineers, despite silence in her room.
The psychiatry service is consulted after she is seen making threats of harm to her family members.
EVALUATION Confusion
Over past several weeks, Ms. L has experienced rapid onset of neurovegetative symptoms, with poor oral intake, increased somnolence, neglect of hygiene, excessive time spent in bed, and weight loss of 15 to 20 lb, according to her spouse. She also has been complaining of foggy mentation, weakening handgrip, and tinnitus. She has no previous psychiatric history.
She recently established care with an outpatient neurologist and infectious disease specialist to address these symptoms. Outpatient EEG and sexually transmitted infection (STI) tests were scheduled but not yet obtained. Ms. L’s spouse observes that her drastic “personality change” over the preceding 24 hours coincided with her feeling upset and offended by a physician’s recommendation to obtain STI tests (it is unclear why the physician recommended these tests).
Ms. L had presented to another local ED 4 times over several weeks for various complaints, and had been prescribed alprazolam, 0.5 mg, 3 times a day as needed, and buspirone, 15 mg/d, for anxiety. She also had received a short course of doxycycline, 200 mg/d, which she did not finish, for treatment of presumed Lyme disease. According to her spouse, Ms. L had completed a course of doxycycline for Lyme disease 1 year earlier, but the medical records are not available for review.
During the interview, Ms. L is fairly well groomed but appears confused; she asks her spouse if she is “real” and states that she feels “crazy.” She seems uncomfortable and is guarded, with a minimally reactive, anxious affect. She has general psychomotor slowing and her speech is soft and monotonous, with prominent latency. She reports passive suicidal ideations as well as active auditory hallucinations of a musical quality.
The Mini-Mental State Examination (MMSE) score is 19/30, indicating moderate cognitive impairment, and she is unable to complete attention, executive function, 3-stage command, and delayed word recall tasks. She reports fatigue, diarrhea, and decreased appetite. Her physical examination is notable for an overweight white woman without focal neurologic deficits. Her family psychiatric history reveals bipolar disorder in 2 distant relatives.
In the ED, Ms. L is given 3 provisional diagnoses:
- adjustment disorder, because of her reaction to the proposed STI testing
- psychotic disorder not otherwise specified, because of her obvious psychosis of unknown cause
- rule out delirium due to a general medical condition, because of her sudden onset of attention, perception, and memory difficulties.
As Ms. L sits in her room, her abnormal behaviors become more apparent. She starts to endorse active suicidal ideations and becomes aggressive, trying to choke her spouse, shouting, jumping on her bed, and attempting to strike herself. For her safety, she is physically restrained and given IM haloperidol, 10 mg, and IM lorazepam, 2 mg.
What would you do next to treat Ms. L?
a) Admit her to the psychiatric unit for monitoring and treatment of psychosis and consider additional antipsychotics for agitation
b) Perform a bedside lumbar puncture to assess for findings suggestive of a CNS infection or anomaly
c) Sedate her with IM ketamine, intubate her, and admit her to the intensive care unit (ICU) for further medical workup
d) Begin IV antibiotic therapy with ceftriaxone for early-disseminated Lyme disease with CNS involvement
The authors’ observations
Clearly, Ms. L was psychotic. However, psychosis is a nonspecific term used to describe a heterogeneous group of phenomena in which one experiences an impaired sense of reality. Although commonly caused by psychiatric disorders, psychosis can arise from a variety of causes.1 Ms. L’s initial physical examination and laboratory studies were within the normal range, but her mental status exam and MMSE were abnormal. At this point, selecting the appropriate setting for further observation, workup, and treatment became important.
TREATMENT The right setting
Given the abrupt onset of Ms. L’s symptoms, the treatment team is concerned about active neurologic or infectious disease. However, no acute laboratory or physical examination findings support this hypothesis, and the ED physicians conclude that no further emergent workup is indicated. Because Ms. L is threatening harm to herself and others, she cannot be safely discharged. The treatment team decides the safest option is to admit Ms. L to the inpatient psychiatric unit for observation, further non-emergent workup, and consultation with the neurology service.
At admission. Ms. L is cooperative and calm, lying in bed comfortably. She obeys simple commands; a brief neurologic examination is remarkable for a sedated female without focal motor or sensory deficits. Although her answers to questions are brief, they are appropriate. She sleeps without incident for approximately 10 hours.
The next morning. Ms. L does not awaken to verbal or gentle physical stimuli. Upon sternal rub, she awakens and forcefully squeezes the examiner’s arm, after which she closes her eyes and does not answer further questions (but does resist passive eye opening). After several minutes, she begins exhibiting verbigeration, shouting repeated phrases such as “The birds are in my ears” and “No, I am not okay.”
An emergent EEG is ordered because the team is concerned about nonconvulsive status epilepticus and the neurology service is consulted about the need for an urgent lumbar puncture. Without any obvious abnormal physical examination findings, however, the neurology team’s initial assessment attributes Ms. L’s presentation to a primary psychiatric illness and does not recommend a lumbar puncture or EEG.
That day and night, Ms. L has several episodes of agitation with a disorganized thought process and perseverative speech. She appears distraught and exhibits menacing behaviors. She is poorly redirectable and physically hostile toward staff, requiring several emergent doses of IM haloperidol and IM lorazepam, to which she responds minimally. Ms. L is placed on constant observation, requiring frequent redirection from the rooms of other patients and intermittent seclusion because of her violent, destructive behavior.
The next day. Ms. L remains grossly agitated and psychotic. Although an EEG is ordered, it is not performed because the technicians are concerned about their safety. With her unclear history of Lyme disease and concern for an infectious encephalopathy, Ms. L’s history and symptoms are discussed with the infectious disease service. Given her abrupt onset of symptoms, including auditory hallucinations, they express concern for herpes simplex encephalitis and recommend emergent treatment with IV acyclovir and ceftriaxone.
This recommendation, however, causes a practical conundrum. Because of state laws and differences in staff training, the treatment team believes that the inpatient psychiatric unit is not the appropriate setting to administer these IV treatments. At the same time, hospital security, nursing staff, and the receiving medical team are concerned about transporting Ms. L to the general medical floor.
In the ICU. After discussion, the teams decide that the safest and least traumatic option is to transport Ms. L to the ICU after she is sedated and intubated. In the ICU, she undergoes empirical treatment for herpes simplex encephalitis and further medical workup.
An EEG reveals findings suggestive of severe encephalopathy. A lumbar puncture shows lymphocytic pleocytosis with an opening pressure of 28 cm H2O and normal protein and glucose levels. Her serum C-reactive protein is slightly elevated at 1.4 mg/dL. She also is found to have an elevated herpes simplex virus (HSV)-2 IgG antibody.
Subsequent hospital stay. Ms. L has 2 episodes of seizure-like activity, for which she is treated with levetiracetam, 2,000 mg/d, increased to 3,000 mg/d. She is sedated for several days to allow broad treatment with antiviral and antibiotic medications. Although she experiences intermittent fevers and tachycardia, cultures of blood, urine, and cerebrospinal fluid (CSF) show no growth. Similarly, a test of serum HSV IgM antibodies is negative.
CT of the chest, abdomen, and pelvis reveals no findings suggestive of malignancy but does show a solid-appearing 6-mm nodule in her right lung. Magnetic resonance angiography of the head and neck shows no evidence of abnormalities other than atrophy of the superior cerebellar vermis and a subtle focus of T2/FLAIR signal abnormality in the medial portion of the left occipital lobe.
The following weeks. Ms. L’s cognitive status improves markedly. Extensive studies—including serum ammonia, thyroid-stimulating hormone, Lyme disease antibody, vitamin B12, folate, beta-hCG, HIV, hepatitis B and C, Varicella zoster, syphilis, Lyme disease serology, CSF Eastern equine encephalitis, St. Louis encephalitis virus, West Nile virus, Ehrlichia chaffeensis, Babesia microti, Rocky Mountain spotted fever, John Cunningham virus, typhus fever, cryptococcal antigen, rabies, 2 serum tests for anti-N-methyl-D-aspartate (NMDA) receptor antibodies, and serum ceruloplasmin—are normal.
At discharge, Ms. L’s clinical presentation is thought to be most consistent with viral encephalitis, because of her CSF lymphocytic pleocytosis, fever, and improvement with supportive care. Because she improves, the team does not find it necessary to wait for results of pending studies, including a paraneoplastic autoantibody panel and a CSF anti-NMDA receptor antibody, before discharging her.
Readmission. Although the results of the paraneoplastic autoantibody panel are unremarkable, several weeks after discharge Ms. L’s CSF anti-NMDA receptor antibodies return positive, despite 2 earlier negative serum studies. She is readmitted to the neurology service for treatment with immunomodulators.
A positron-emission tomography scan is negative for malignancy. She is treated on an ongoing basis with immunomodulators; cognition improves such that she is able to start working again with good overall functioning. Despite this improvement, she experiences residual sequelae, including noise sensitivity, amnesia of the events surrounding her hospitalization, mild short-term memory deficits, and persistent affective blunting.
The authors’ observations
Psychosis is not exclusive to psychiatric syndromes and frequently is a symptom of an underlying neurologic, immunologic, metabolic, infectious, or oncologic abnormality.1 Anti-NMDA receptor encephalitis is an autoimmune disease in which antibodies attack NMDA-type glutamate receptors at central neuronal synapses and can produce psychosis, as seen with Ms. L2 (Table 12,3). The etiology of the disease is not fully understood. Determining the appropriate setting to perform a complete medical workup in a severely agitated patient after an initial negative medical workup can be challenging.
What’s the most appropriate treatment setting?
This case illustrates the importance, with any new-onset psychosis, of weighing heavily a carefully obtained psychiatric history, even in the absence of focal physical examination and initial laboratory abnormalities. It also highlights the challenge of determining the most appropriate initial setting for performing the important task of a complete medical workup for first-episode psychosis.
Ms. L initially was treated in the inpatient psychiatric unit because of safety concerns and practical limitations, but was later found to have a disease that could not be managed in that setting. She proved to be too agitated to obtain a full medical workup on the inpatient psychiatric or general medical floors and required transfer to the ICU. Despite her normal basic laboratory tests, her EEG and CSF studies did demonstrate abnormalities, suggesting these can be useful to the basic workup for psychosis of unknown cause (Table 21,2).
This case also demonstrates that negative serum anti-NMDA receptor antibody tests do not rule out the disease; one study found that only 85% of patients with CSF anti-NMDA receptor antibodies also had detectable antibodies in their serum and that detectability changed during the course of the disease.4 This supports the utility of a lumbar puncture as part of a basic initial workup for some cases of new-onset psychosis. Because clinical outcomes often correlate with early treatment, as with anti-NMDA receptor encephalitis, a timely diagnostic workup of psychosis often can be important.3,5 The ICU can be considered an appropriate setting for working up some patients who develop new, rapid-onset psychosis and severe agitation, even in the absence of initial laboratory or physical examination findings.
Ms. L’s case also illustrates the importance of completing a thorough medical workup for patients with new-onset psychosis before transferring them to an independent psychiatric hospital. Initially, the university’s psychiatric unit was at capacity and a bed was sought at outside psychiatric hospitals while Ms. L waited in the ED. Had Ms. L not been admitted to a large academic medical center, she may not have had access to the multidisciplinary collaboration that proved necessary for the appropriate diagnosis and treatment of her anti-NMDA receptor encephalitis (Table 35,6).
What prodromal symptoms occur as long as 2 weeks as an initial presentation in many patients with anti-NMDA receptor encephalitis?
a) Flu-like symptoms of lethargy, headache, gastrointestinal symptoms, myalgias, fevers, and upper respiratory symptoms
b) Delusions, hallucinations, disorganized behaviors and thoughts, behavioral outbursts, hypersexuality, mood lability, personality change, paranoia, echolalia, mutism, anxiety, agitation, aggression, hyperactivity, sleep dysfunction, and blunted affect
c) Dyskinesias, autonomic instability, central hypoventilation, and seizures
The authors’ observations
Lab results, vital signs, and physical examination should not supplant a careful history when determining an appropriate clinical course of action. As experts in the cognitive sciences, psychiatrists may be the most qualified in determining whether a patient with new-onset psychosis should undergo further medical testing before a condition is deemed to be solely of a psychiatric cause. As a neurologic disease of immunologic origin with psychiatric manifestations, anti-NMDA receptor encephalitis is a complex condition requiring collaboration among several specialists for appropriate management.
1. Freudenreich O. Differential diagnosis of psychotic symptoms: medical “mimics.” Psychiatric Times. http://www.psychiatrictimes.com/forensic-psychiatry/differential-diagnosis-psychotic-symptoms-medical-%E2%80%9Cmimics%E2%80%9D. Published December 3, 2012. Accessed March 31, 2016.
2. Kayser MS, Dalmau J. Anti-NMDA receptor encephalitis in psychiatry. Curr Psychiatry Rev. 2011;7(3):189-193.
3. Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74.
4. Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167-177.
5. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-1098.
6. Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
CASE Sudden personality change
Ms. L, age 38, is brought to the university hospital’s emergency department (ED) under police escort after she awoke in the middle of the night screaming, “I found it out! I’m a lie! Life is a lie!” and began threatening suicide. This prompted her spouse to call emergency services because of concerns about her safety.
Over the preceding 9 days—and, most precipitously, over the last 24 hours—Ms. L has experienced a dramatic “change in her personality,” according to her spouse. In the ED, she is oriented to person, place, and time. Her vital signs are within normal limits, other than a mild tachycardia. Complete blood count and complete metabolic profile are unremarkable and a urine drug screen is positive only for benzodiazepines (she recently was prescribed alprazolam). Ms. L smiles inappropriately at the ED physicians and confides that she is hearing music by The Lumineers, despite silence in her room.
The psychiatry service is consulted after she is seen making threats of harm to her family members.
EVALUATION Confusion
Over past several weeks, Ms. L has experienced rapid onset of neurovegetative symptoms, with poor oral intake, increased somnolence, neglect of hygiene, excessive time spent in bed, and weight loss of 15 to 20 lb, according to her spouse. She also has been complaining of foggy mentation, weakening handgrip, and tinnitus. She has no previous psychiatric history.
She recently established care with an outpatient neurologist and infectious disease specialist to address these symptoms. Outpatient EEG and sexually transmitted infection (STI) tests were scheduled but not yet obtained. Ms. L’s spouse observes that her drastic “personality change” over the preceding 24 hours coincided with her feeling upset and offended by a physician’s recommendation to obtain STI tests (it is unclear why the physician recommended these tests).
Ms. L had presented to another local ED 4 times over several weeks for various complaints, and had been prescribed alprazolam, 0.5 mg, 3 times a day as needed, and buspirone, 15 mg/d, for anxiety. She also had received a short course of doxycycline, 200 mg/d, which she did not finish, for treatment of presumed Lyme disease. According to her spouse, Ms. L had completed a course of doxycycline for Lyme disease 1 year earlier, but the medical records are not available for review.
During the interview, Ms. L is fairly well groomed but appears confused; she asks her spouse if she is “real” and states that she feels “crazy.” She seems uncomfortable and is guarded, with a minimally reactive, anxious affect. She has general psychomotor slowing and her speech is soft and monotonous, with prominent latency. She reports passive suicidal ideations as well as active auditory hallucinations of a musical quality.
The Mini-Mental State Examination (MMSE) score is 19/30, indicating moderate cognitive impairment, and she is unable to complete attention, executive function, 3-stage command, and delayed word recall tasks. She reports fatigue, diarrhea, and decreased appetite. Her physical examination is notable for an overweight white woman without focal neurologic deficits. Her family psychiatric history reveals bipolar disorder in 2 distant relatives.
In the ED, Ms. L is given 3 provisional diagnoses:
- adjustment disorder, because of her reaction to the proposed STI testing
- psychotic disorder not otherwise specified, because of her obvious psychosis of unknown cause
- rule out delirium due to a general medical condition, because of her sudden onset of attention, perception, and memory difficulties.
As Ms. L sits in her room, her abnormal behaviors become more apparent. She starts to endorse active suicidal ideations and becomes aggressive, trying to choke her spouse, shouting, jumping on her bed, and attempting to strike herself. For her safety, she is physically restrained and given IM haloperidol, 10 mg, and IM lorazepam, 2 mg.
What would you do next to treat Ms. L?
a) Admit her to the psychiatric unit for monitoring and treatment of psychosis and consider additional antipsychotics for agitation
b) Perform a bedside lumbar puncture to assess for findings suggestive of a CNS infection or anomaly
c) Sedate her with IM ketamine, intubate her, and admit her to the intensive care unit (ICU) for further medical workup
d) Begin IV antibiotic therapy with ceftriaxone for early-disseminated Lyme disease with CNS involvement
The authors’ observations
Clearly, Ms. L was psychotic. However, psychosis is a nonspecific term used to describe a heterogeneous group of phenomena in which one experiences an impaired sense of reality. Although commonly caused by psychiatric disorders, psychosis can arise from a variety of causes.1 Ms. L’s initial physical examination and laboratory studies were within the normal range, but her mental status exam and MMSE were abnormal. At this point, selecting the appropriate setting for further observation, workup, and treatment became important.
TREATMENT The right setting
Given the abrupt onset of Ms. L’s symptoms, the treatment team is concerned about active neurologic or infectious disease. However, no acute laboratory or physical examination findings support this hypothesis, and the ED physicians conclude that no further emergent workup is indicated. Because Ms. L is threatening harm to herself and others, she cannot be safely discharged. The treatment team decides the safest option is to admit Ms. L to the inpatient psychiatric unit for observation, further non-emergent workup, and consultation with the neurology service.
At admission. Ms. L is cooperative and calm, lying in bed comfortably. She obeys simple commands; a brief neurologic examination is remarkable for a sedated female without focal motor or sensory deficits. Although her answers to questions are brief, they are appropriate. She sleeps without incident for approximately 10 hours.
The next morning. Ms. L does not awaken to verbal or gentle physical stimuli. Upon sternal rub, she awakens and forcefully squeezes the examiner’s arm, after which she closes her eyes and does not answer further questions (but does resist passive eye opening). After several minutes, she begins exhibiting verbigeration, shouting repeated phrases such as “The birds are in my ears” and “No, I am not okay.”
An emergent EEG is ordered because the team is concerned about nonconvulsive status epilepticus and the neurology service is consulted about the need for an urgent lumbar puncture. Without any obvious abnormal physical examination findings, however, the neurology team’s initial assessment attributes Ms. L’s presentation to a primary psychiatric illness and does not recommend a lumbar puncture or EEG.
That day and night, Ms. L has several episodes of agitation with a disorganized thought process and perseverative speech. She appears distraught and exhibits menacing behaviors. She is poorly redirectable and physically hostile toward staff, requiring several emergent doses of IM haloperidol and IM lorazepam, to which she responds minimally. Ms. L is placed on constant observation, requiring frequent redirection from the rooms of other patients and intermittent seclusion because of her violent, destructive behavior.
The next day. Ms. L remains grossly agitated and psychotic. Although an EEG is ordered, it is not performed because the technicians are concerned about their safety. With her unclear history of Lyme disease and concern for an infectious encephalopathy, Ms. L’s history and symptoms are discussed with the infectious disease service. Given her abrupt onset of symptoms, including auditory hallucinations, they express concern for herpes simplex encephalitis and recommend emergent treatment with IV acyclovir and ceftriaxone.
This recommendation, however, causes a practical conundrum. Because of state laws and differences in staff training, the treatment team believes that the inpatient psychiatric unit is not the appropriate setting to administer these IV treatments. At the same time, hospital security, nursing staff, and the receiving medical team are concerned about transporting Ms. L to the general medical floor.
In the ICU. After discussion, the teams decide that the safest and least traumatic option is to transport Ms. L to the ICU after she is sedated and intubated. In the ICU, she undergoes empirical treatment for herpes simplex encephalitis and further medical workup.
An EEG reveals findings suggestive of severe encephalopathy. A lumbar puncture shows lymphocytic pleocytosis with an opening pressure of 28 cm H2O and normal protein and glucose levels. Her serum C-reactive protein is slightly elevated at 1.4 mg/dL. She also is found to have an elevated herpes simplex virus (HSV)-2 IgG antibody.
Subsequent hospital stay. Ms. L has 2 episodes of seizure-like activity, for which she is treated with levetiracetam, 2,000 mg/d, increased to 3,000 mg/d. She is sedated for several days to allow broad treatment with antiviral and antibiotic medications. Although she experiences intermittent fevers and tachycardia, cultures of blood, urine, and cerebrospinal fluid (CSF) show no growth. Similarly, a test of serum HSV IgM antibodies is negative.
CT of the chest, abdomen, and pelvis reveals no findings suggestive of malignancy but does show a solid-appearing 6-mm nodule in her right lung. Magnetic resonance angiography of the head and neck shows no evidence of abnormalities other than atrophy of the superior cerebellar vermis and a subtle focus of T2/FLAIR signal abnormality in the medial portion of the left occipital lobe.
The following weeks. Ms. L’s cognitive status improves markedly. Extensive studies—including serum ammonia, thyroid-stimulating hormone, Lyme disease antibody, vitamin B12, folate, beta-hCG, HIV, hepatitis B and C, Varicella zoster, syphilis, Lyme disease serology, CSF Eastern equine encephalitis, St. Louis encephalitis virus, West Nile virus, Ehrlichia chaffeensis, Babesia microti, Rocky Mountain spotted fever, John Cunningham virus, typhus fever, cryptococcal antigen, rabies, 2 serum tests for anti-N-methyl-D-aspartate (NMDA) receptor antibodies, and serum ceruloplasmin—are normal.
At discharge, Ms. L’s clinical presentation is thought to be most consistent with viral encephalitis, because of her CSF lymphocytic pleocytosis, fever, and improvement with supportive care. Because she improves, the team does not find it necessary to wait for results of pending studies, including a paraneoplastic autoantibody panel and a CSF anti-NMDA receptor antibody, before discharging her.
Readmission. Although the results of the paraneoplastic autoantibody panel are unremarkable, several weeks after discharge Ms. L’s CSF anti-NMDA receptor antibodies return positive, despite 2 earlier negative serum studies. She is readmitted to the neurology service for treatment with immunomodulators.
A positron-emission tomography scan is negative for malignancy. She is treated on an ongoing basis with immunomodulators; cognition improves such that she is able to start working again with good overall functioning. Despite this improvement, she experiences residual sequelae, including noise sensitivity, amnesia of the events surrounding her hospitalization, mild short-term memory deficits, and persistent affective blunting.
The authors’ observations
Psychosis is not exclusive to psychiatric syndromes and frequently is a symptom of an underlying neurologic, immunologic, metabolic, infectious, or oncologic abnormality.1 Anti-NMDA receptor encephalitis is an autoimmune disease in which antibodies attack NMDA-type glutamate receptors at central neuronal synapses and can produce psychosis, as seen with Ms. L2 (Table 12,3). The etiology of the disease is not fully understood. Determining the appropriate setting to perform a complete medical workup in a severely agitated patient after an initial negative medical workup can be challenging.
What’s the most appropriate treatment setting?
This case illustrates the importance, with any new-onset psychosis, of weighing heavily a carefully obtained psychiatric history, even in the absence of focal physical examination and initial laboratory abnormalities. It also highlights the challenge of determining the most appropriate initial setting for performing the important task of a complete medical workup for first-episode psychosis.
Ms. L initially was treated in the inpatient psychiatric unit because of safety concerns and practical limitations, but was later found to have a disease that could not be managed in that setting. She proved to be too agitated to obtain a full medical workup on the inpatient psychiatric or general medical floors and required transfer to the ICU. Despite her normal basic laboratory tests, her EEG and CSF studies did demonstrate abnormalities, suggesting these can be useful to the basic workup for psychosis of unknown cause (Table 21,2).
This case also demonstrates that negative serum anti-NMDA receptor antibody tests do not rule out the disease; one study found that only 85% of patients with CSF anti-NMDA receptor antibodies also had detectable antibodies in their serum and that detectability changed during the course of the disease.4 This supports the utility of a lumbar puncture as part of a basic initial workup for some cases of new-onset psychosis. Because clinical outcomes often correlate with early treatment, as with anti-NMDA receptor encephalitis, a timely diagnostic workup of psychosis often can be important.3,5 The ICU can be considered an appropriate setting for working up some patients who develop new, rapid-onset psychosis and severe agitation, even in the absence of initial laboratory or physical examination findings.
Ms. L’s case also illustrates the importance of completing a thorough medical workup for patients with new-onset psychosis before transferring them to an independent psychiatric hospital. Initially, the university’s psychiatric unit was at capacity and a bed was sought at outside psychiatric hospitals while Ms. L waited in the ED. Had Ms. L not been admitted to a large academic medical center, she may not have had access to the multidisciplinary collaboration that proved necessary for the appropriate diagnosis and treatment of her anti-NMDA receptor encephalitis (Table 35,6).
What prodromal symptoms occur as long as 2 weeks as an initial presentation in many patients with anti-NMDA receptor encephalitis?
a) Flu-like symptoms of lethargy, headache, gastrointestinal symptoms, myalgias, fevers, and upper respiratory symptoms
b) Delusions, hallucinations, disorganized behaviors and thoughts, behavioral outbursts, hypersexuality, mood lability, personality change, paranoia, echolalia, mutism, anxiety, agitation, aggression, hyperactivity, sleep dysfunction, and blunted affect
c) Dyskinesias, autonomic instability, central hypoventilation, and seizures
The authors’ observations
Lab results, vital signs, and physical examination should not supplant a careful history when determining an appropriate clinical course of action. As experts in the cognitive sciences, psychiatrists may be the most qualified in determining whether a patient with new-onset psychosis should undergo further medical testing before a condition is deemed to be solely of a psychiatric cause. As a neurologic disease of immunologic origin with psychiatric manifestations, anti-NMDA receptor encephalitis is a complex condition requiring collaboration among several specialists for appropriate management.
CASE Sudden personality change
Ms. L, age 38, is brought to the university hospital’s emergency department (ED) under police escort after she awoke in the middle of the night screaming, “I found it out! I’m a lie! Life is a lie!” and began threatening suicide. This prompted her spouse to call emergency services because of concerns about her safety.
Over the preceding 9 days—and, most precipitously, over the last 24 hours—Ms. L has experienced a dramatic “change in her personality,” according to her spouse. In the ED, she is oriented to person, place, and time. Her vital signs are within normal limits, other than a mild tachycardia. Complete blood count and complete metabolic profile are unremarkable and a urine drug screen is positive only for benzodiazepines (she recently was prescribed alprazolam). Ms. L smiles inappropriately at the ED physicians and confides that she is hearing music by The Lumineers, despite silence in her room.
The psychiatry service is consulted after she is seen making threats of harm to her family members.
EVALUATION Confusion
Over past several weeks, Ms. L has experienced rapid onset of neurovegetative symptoms, with poor oral intake, increased somnolence, neglect of hygiene, excessive time spent in bed, and weight loss of 15 to 20 lb, according to her spouse. She also has been complaining of foggy mentation, weakening handgrip, and tinnitus. She has no previous psychiatric history.
She recently established care with an outpatient neurologist and infectious disease specialist to address these symptoms. Outpatient EEG and sexually transmitted infection (STI) tests were scheduled but not yet obtained. Ms. L’s spouse observes that her drastic “personality change” over the preceding 24 hours coincided with her feeling upset and offended by a physician’s recommendation to obtain STI tests (it is unclear why the physician recommended these tests).
Ms. L had presented to another local ED 4 times over several weeks for various complaints, and had been prescribed alprazolam, 0.5 mg, 3 times a day as needed, and buspirone, 15 mg/d, for anxiety. She also had received a short course of doxycycline, 200 mg/d, which she did not finish, for treatment of presumed Lyme disease. According to her spouse, Ms. L had completed a course of doxycycline for Lyme disease 1 year earlier, but the medical records are not available for review.
During the interview, Ms. L is fairly well groomed but appears confused; she asks her spouse if she is “real” and states that she feels “crazy.” She seems uncomfortable and is guarded, with a minimally reactive, anxious affect. She has general psychomotor slowing and her speech is soft and monotonous, with prominent latency. She reports passive suicidal ideations as well as active auditory hallucinations of a musical quality.
The Mini-Mental State Examination (MMSE) score is 19/30, indicating moderate cognitive impairment, and she is unable to complete attention, executive function, 3-stage command, and delayed word recall tasks. She reports fatigue, diarrhea, and decreased appetite. Her physical examination is notable for an overweight white woman without focal neurologic deficits. Her family psychiatric history reveals bipolar disorder in 2 distant relatives.
In the ED, Ms. L is given 3 provisional diagnoses:
- adjustment disorder, because of her reaction to the proposed STI testing
- psychotic disorder not otherwise specified, because of her obvious psychosis of unknown cause
- rule out delirium due to a general medical condition, because of her sudden onset of attention, perception, and memory difficulties.
As Ms. L sits in her room, her abnormal behaviors become more apparent. She starts to endorse active suicidal ideations and becomes aggressive, trying to choke her spouse, shouting, jumping on her bed, and attempting to strike herself. For her safety, she is physically restrained and given IM haloperidol, 10 mg, and IM lorazepam, 2 mg.
What would you do next to treat Ms. L?
a) Admit her to the psychiatric unit for monitoring and treatment of psychosis and consider additional antipsychotics for agitation
b) Perform a bedside lumbar puncture to assess for findings suggestive of a CNS infection or anomaly
c) Sedate her with IM ketamine, intubate her, and admit her to the intensive care unit (ICU) for further medical workup
d) Begin IV antibiotic therapy with ceftriaxone for early-disseminated Lyme disease with CNS involvement
The authors’ observations
Clearly, Ms. L was psychotic. However, psychosis is a nonspecific term used to describe a heterogeneous group of phenomena in which one experiences an impaired sense of reality. Although commonly caused by psychiatric disorders, psychosis can arise from a variety of causes.1 Ms. L’s initial physical examination and laboratory studies were within the normal range, but her mental status exam and MMSE were abnormal. At this point, selecting the appropriate setting for further observation, workup, and treatment became important.
TREATMENT The right setting
Given the abrupt onset of Ms. L’s symptoms, the treatment team is concerned about active neurologic or infectious disease. However, no acute laboratory or physical examination findings support this hypothesis, and the ED physicians conclude that no further emergent workup is indicated. Because Ms. L is threatening harm to herself and others, she cannot be safely discharged. The treatment team decides the safest option is to admit Ms. L to the inpatient psychiatric unit for observation, further non-emergent workup, and consultation with the neurology service.
At admission. Ms. L is cooperative and calm, lying in bed comfortably. She obeys simple commands; a brief neurologic examination is remarkable for a sedated female without focal motor or sensory deficits. Although her answers to questions are brief, they are appropriate. She sleeps without incident for approximately 10 hours.
The next morning. Ms. L does not awaken to verbal or gentle physical stimuli. Upon sternal rub, she awakens and forcefully squeezes the examiner’s arm, after which she closes her eyes and does not answer further questions (but does resist passive eye opening). After several minutes, she begins exhibiting verbigeration, shouting repeated phrases such as “The birds are in my ears” and “No, I am not okay.”
An emergent EEG is ordered because the team is concerned about nonconvulsive status epilepticus and the neurology service is consulted about the need for an urgent lumbar puncture. Without any obvious abnormal physical examination findings, however, the neurology team’s initial assessment attributes Ms. L’s presentation to a primary psychiatric illness and does not recommend a lumbar puncture or EEG.
That day and night, Ms. L has several episodes of agitation with a disorganized thought process and perseverative speech. She appears distraught and exhibits menacing behaviors. She is poorly redirectable and physically hostile toward staff, requiring several emergent doses of IM haloperidol and IM lorazepam, to which she responds minimally. Ms. L is placed on constant observation, requiring frequent redirection from the rooms of other patients and intermittent seclusion because of her violent, destructive behavior.
The next day. Ms. L remains grossly agitated and psychotic. Although an EEG is ordered, it is not performed because the technicians are concerned about their safety. With her unclear history of Lyme disease and concern for an infectious encephalopathy, Ms. L’s history and symptoms are discussed with the infectious disease service. Given her abrupt onset of symptoms, including auditory hallucinations, they express concern for herpes simplex encephalitis and recommend emergent treatment with IV acyclovir and ceftriaxone.
This recommendation, however, causes a practical conundrum. Because of state laws and differences in staff training, the treatment team believes that the inpatient psychiatric unit is not the appropriate setting to administer these IV treatments. At the same time, hospital security, nursing staff, and the receiving medical team are concerned about transporting Ms. L to the general medical floor.
In the ICU. After discussion, the teams decide that the safest and least traumatic option is to transport Ms. L to the ICU after she is sedated and intubated. In the ICU, she undergoes empirical treatment for herpes simplex encephalitis and further medical workup.
An EEG reveals findings suggestive of severe encephalopathy. A lumbar puncture shows lymphocytic pleocytosis with an opening pressure of 28 cm H2O and normal protein and glucose levels. Her serum C-reactive protein is slightly elevated at 1.4 mg/dL. She also is found to have an elevated herpes simplex virus (HSV)-2 IgG antibody.
Subsequent hospital stay. Ms. L has 2 episodes of seizure-like activity, for which she is treated with levetiracetam, 2,000 mg/d, increased to 3,000 mg/d. She is sedated for several days to allow broad treatment with antiviral and antibiotic medications. Although she experiences intermittent fevers and tachycardia, cultures of blood, urine, and cerebrospinal fluid (CSF) show no growth. Similarly, a test of serum HSV IgM antibodies is negative.
CT of the chest, abdomen, and pelvis reveals no findings suggestive of malignancy but does show a solid-appearing 6-mm nodule in her right lung. Magnetic resonance angiography of the head and neck shows no evidence of abnormalities other than atrophy of the superior cerebellar vermis and a subtle focus of T2/FLAIR signal abnormality in the medial portion of the left occipital lobe.
The following weeks. Ms. L’s cognitive status improves markedly. Extensive studies—including serum ammonia, thyroid-stimulating hormone, Lyme disease antibody, vitamin B12, folate, beta-hCG, HIV, hepatitis B and C, Varicella zoster, syphilis, Lyme disease serology, CSF Eastern equine encephalitis, St. Louis encephalitis virus, West Nile virus, Ehrlichia chaffeensis, Babesia microti, Rocky Mountain spotted fever, John Cunningham virus, typhus fever, cryptococcal antigen, rabies, 2 serum tests for anti-N-methyl-D-aspartate (NMDA) receptor antibodies, and serum ceruloplasmin—are normal.
At discharge, Ms. L’s clinical presentation is thought to be most consistent with viral encephalitis, because of her CSF lymphocytic pleocytosis, fever, and improvement with supportive care. Because she improves, the team does not find it necessary to wait for results of pending studies, including a paraneoplastic autoantibody panel and a CSF anti-NMDA receptor antibody, before discharging her.
Readmission. Although the results of the paraneoplastic autoantibody panel are unremarkable, several weeks after discharge Ms. L’s CSF anti-NMDA receptor antibodies return positive, despite 2 earlier negative serum studies. She is readmitted to the neurology service for treatment with immunomodulators.
A positron-emission tomography scan is negative for malignancy. She is treated on an ongoing basis with immunomodulators; cognition improves such that she is able to start working again with good overall functioning. Despite this improvement, she experiences residual sequelae, including noise sensitivity, amnesia of the events surrounding her hospitalization, mild short-term memory deficits, and persistent affective blunting.
The authors’ observations
Psychosis is not exclusive to psychiatric syndromes and frequently is a symptom of an underlying neurologic, immunologic, metabolic, infectious, or oncologic abnormality.1 Anti-NMDA receptor encephalitis is an autoimmune disease in which antibodies attack NMDA-type glutamate receptors at central neuronal synapses and can produce psychosis, as seen with Ms. L2 (Table 12,3). The etiology of the disease is not fully understood. Determining the appropriate setting to perform a complete medical workup in a severely agitated patient after an initial negative medical workup can be challenging.
What’s the most appropriate treatment setting?
This case illustrates the importance, with any new-onset psychosis, of weighing heavily a carefully obtained psychiatric history, even in the absence of focal physical examination and initial laboratory abnormalities. It also highlights the challenge of determining the most appropriate initial setting for performing the important task of a complete medical workup for first-episode psychosis.
Ms. L initially was treated in the inpatient psychiatric unit because of safety concerns and practical limitations, but was later found to have a disease that could not be managed in that setting. She proved to be too agitated to obtain a full medical workup on the inpatient psychiatric or general medical floors and required transfer to the ICU. Despite her normal basic laboratory tests, her EEG and CSF studies did demonstrate abnormalities, suggesting these can be useful to the basic workup for psychosis of unknown cause (Table 21,2).
This case also demonstrates that negative serum anti-NMDA receptor antibody tests do not rule out the disease; one study found that only 85% of patients with CSF anti-NMDA receptor antibodies also had detectable antibodies in their serum and that detectability changed during the course of the disease.4 This supports the utility of a lumbar puncture as part of a basic initial workup for some cases of new-onset psychosis. Because clinical outcomes often correlate with early treatment, as with anti-NMDA receptor encephalitis, a timely diagnostic workup of psychosis often can be important.3,5 The ICU can be considered an appropriate setting for working up some patients who develop new, rapid-onset psychosis and severe agitation, even in the absence of initial laboratory or physical examination findings.
Ms. L’s case also illustrates the importance of completing a thorough medical workup for patients with new-onset psychosis before transferring them to an independent psychiatric hospital. Initially, the university’s psychiatric unit was at capacity and a bed was sought at outside psychiatric hospitals while Ms. L waited in the ED. Had Ms. L not been admitted to a large academic medical center, she may not have had access to the multidisciplinary collaboration that proved necessary for the appropriate diagnosis and treatment of her anti-NMDA receptor encephalitis (Table 35,6).
What prodromal symptoms occur as long as 2 weeks as an initial presentation in many patients with anti-NMDA receptor encephalitis?
a) Flu-like symptoms of lethargy, headache, gastrointestinal symptoms, myalgias, fevers, and upper respiratory symptoms
b) Delusions, hallucinations, disorganized behaviors and thoughts, behavioral outbursts, hypersexuality, mood lability, personality change, paranoia, echolalia, mutism, anxiety, agitation, aggression, hyperactivity, sleep dysfunction, and blunted affect
c) Dyskinesias, autonomic instability, central hypoventilation, and seizures
The authors’ observations
Lab results, vital signs, and physical examination should not supplant a careful history when determining an appropriate clinical course of action. As experts in the cognitive sciences, psychiatrists may be the most qualified in determining whether a patient with new-onset psychosis should undergo further medical testing before a condition is deemed to be solely of a psychiatric cause. As a neurologic disease of immunologic origin with psychiatric manifestations, anti-NMDA receptor encephalitis is a complex condition requiring collaboration among several specialists for appropriate management.
1. Freudenreich O. Differential diagnosis of psychotic symptoms: medical “mimics.” Psychiatric Times. http://www.psychiatrictimes.com/forensic-psychiatry/differential-diagnosis-psychotic-symptoms-medical-%E2%80%9Cmimics%E2%80%9D. Published December 3, 2012. Accessed March 31, 2016.
2. Kayser MS, Dalmau J. Anti-NMDA receptor encephalitis in psychiatry. Curr Psychiatry Rev. 2011;7(3):189-193.
3. Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74.
4. Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167-177.
5. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-1098.
6. Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
1. Freudenreich O. Differential diagnosis of psychotic symptoms: medical “mimics.” Psychiatric Times. http://www.psychiatrictimes.com/forensic-psychiatry/differential-diagnosis-psychotic-symptoms-medical-%E2%80%9Cmimics%E2%80%9D. Published December 3, 2012. Accessed March 31, 2016.
2. Kayser MS, Dalmau J. Anti-NMDA receptor encephalitis in psychiatry. Curr Psychiatry Rev. 2011;7(3):189-193.
3. Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74.
4. Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167-177.
5. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-1098.
6. Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
COPD comorbid with mental illness: What psychiatrists can do
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
Be an activist to prevent edentulism among the mentally ill
Poor dental hygiene is a serious and prevalent problem among people with mental illness or cognitive impairment: Dental caries and periodontal disease are 3.4 times more common among the mentally ill than among the general population.1 Little has been published on the causes and prevention of these diseases among the mentally ill, however. Interprofessional education provides the opportunity to reinforce the connection between oral health and systemic health.
Untreated dental disease can result in edentulism (partial or complete tooth loss). Often, this condition leads to embarrassment, poor self-image, and social isolation—all of which can exacerbate the psychotic state and its symptoms. Working with your patient to improve oral health can, in turn, lead to better mental and physical health.
CASE REPORT
Edentulism in a man with schizophrenia
A 34-year-old man, given a diagnosis of schizophrenia at age 17, is admitted to the inpatient psychiatry unit for bizarre behavior. The next day, 4 maxillary and incisor teeth fall out suddenly while he is brushing his teeth. The patient is brought to emergency dental services.
Factors contributing to his tooth loss include:
- schizophrenia
- neglected oral hygiene
- adverse effects of antipsychotic medication
- lack of advice on the importance of oral hygiene
- failure to recognize signs of a dental problem.
What else can lead to edentulism?
Breakdown of the periodontal attachment2 also can be caused by disinterest in oral hygiene practices; craving of, and preference for, carbohydrates because of reduced central serotonin activity3,4; and xerostomia.
Xerostomia, or dry mouth, caused by psychotropic agents and an altered immune response, facilitates growth of pathogenic bacteria and can lead to several dental diseases (Table). These conditions are exacerbated by consumption of chewing gum, sweets, and sugary drinks in response to constantly feeling thirsty from xerostomia. Advise patients to take frequent sips of fluid or let ice cubes melt in their mouth.
Bruxism. Patients taking a selective serotonin reuptake inhibitor or an atypical antipsychotic can develop a movement disorder (eg, extrapyramidal symptoms or tardive dyskinesia) that includes clenching, grinding of the teeth (bruxism), or both, which can worsen their periodontal condition.
Lack of skills, physical dexterity, and motivation to maintain good oral hygiene are common among people with mental illness. Most patients visit a dentist only when they experience a serious oral problem or an emergency (ie, trauma). Many dentists treat psychiatric patients by extracting the tooth that is causing the pain, instead of pursuing complex tooth preservation or restoration techniques because of (1) the extent of the disease, (2) lack of knowledge related to psychiatric illnesses, and (3) frequent and timely follow-ups.5
Providing education about oral health to patients, implementing preventive steps, and educating other medical specialities about the link between oral health and systemic health can help to reduce the burden of dental problems among mentally ill patients.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products
1. Persson K, Axtelius B, Söderfeldt B, et al. Oral health-related quality of life and dental status in an outpatient psychiatric population: a multivariate approach. Int J Ment Health Nurs. 2010;19(1):62-70.
2. Lalloo R, Kisely S, Amarasinghe H, et al. Oral health of patients on psychotropic medications: a study of outpatients in Queensland. Australas Psychiatry. 2013;21(4):338-342.
3. O’Neil A, Berk M, Venugopal K, et al. The association between poor dental health and depression: findings from a large-scale, population-based study (the NHANES study). Gen Hosp Psychiatry. 2014;36(3):266-270.
4. Kisely S, Quek LH, Paris J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
5. Arnaiz A, Zumárraga M, Díez-Altuna I, et al. Oral health and the symptoms of schizophrenia. Psychiatry Res. 2011;188(1):24-28.
Poor dental hygiene is a serious and prevalent problem among people with mental illness or cognitive impairment: Dental caries and periodontal disease are 3.4 times more common among the mentally ill than among the general population.1 Little has been published on the causes and prevention of these diseases among the mentally ill, however. Interprofessional education provides the opportunity to reinforce the connection between oral health and systemic health.
Untreated dental disease can result in edentulism (partial or complete tooth loss). Often, this condition leads to embarrassment, poor self-image, and social isolation—all of which can exacerbate the psychotic state and its symptoms. Working with your patient to improve oral health can, in turn, lead to better mental and physical health.
CASE REPORT
Edentulism in a man with schizophrenia
A 34-year-old man, given a diagnosis of schizophrenia at age 17, is admitted to the inpatient psychiatry unit for bizarre behavior. The next day, 4 maxillary and incisor teeth fall out suddenly while he is brushing his teeth. The patient is brought to emergency dental services.
Factors contributing to his tooth loss include:
- schizophrenia
- neglected oral hygiene
- adverse effects of antipsychotic medication
- lack of advice on the importance of oral hygiene
- failure to recognize signs of a dental problem.
What else can lead to edentulism?
Breakdown of the periodontal attachment2 also can be caused by disinterest in oral hygiene practices; craving of, and preference for, carbohydrates because of reduced central serotonin activity3,4; and xerostomia.
Xerostomia, or dry mouth, caused by psychotropic agents and an altered immune response, facilitates growth of pathogenic bacteria and can lead to several dental diseases (Table). These conditions are exacerbated by consumption of chewing gum, sweets, and sugary drinks in response to constantly feeling thirsty from xerostomia. Advise patients to take frequent sips of fluid or let ice cubes melt in their mouth.
Bruxism. Patients taking a selective serotonin reuptake inhibitor or an atypical antipsychotic can develop a movement disorder (eg, extrapyramidal symptoms or tardive dyskinesia) that includes clenching, grinding of the teeth (bruxism), or both, which can worsen their periodontal condition.
Lack of skills, physical dexterity, and motivation to maintain good oral hygiene are common among people with mental illness. Most patients visit a dentist only when they experience a serious oral problem or an emergency (ie, trauma). Many dentists treat psychiatric patients by extracting the tooth that is causing the pain, instead of pursuing complex tooth preservation or restoration techniques because of (1) the extent of the disease, (2) lack of knowledge related to psychiatric illnesses, and (3) frequent and timely follow-ups.5
Providing education about oral health to patients, implementing preventive steps, and educating other medical specialities about the link between oral health and systemic health can help to reduce the burden of dental problems among mentally ill patients.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products
Poor dental hygiene is a serious and prevalent problem among people with mental illness or cognitive impairment: Dental caries and periodontal disease are 3.4 times more common among the mentally ill than among the general population.1 Little has been published on the causes and prevention of these diseases among the mentally ill, however. Interprofessional education provides the opportunity to reinforce the connection between oral health and systemic health.
Untreated dental disease can result in edentulism (partial or complete tooth loss). Often, this condition leads to embarrassment, poor self-image, and social isolation—all of which can exacerbate the psychotic state and its symptoms. Working with your patient to improve oral health can, in turn, lead to better mental and physical health.
CASE REPORT
Edentulism in a man with schizophrenia
A 34-year-old man, given a diagnosis of schizophrenia at age 17, is admitted to the inpatient psychiatry unit for bizarre behavior. The next day, 4 maxillary and incisor teeth fall out suddenly while he is brushing his teeth. The patient is brought to emergency dental services.
Factors contributing to his tooth loss include:
- schizophrenia
- neglected oral hygiene
- adverse effects of antipsychotic medication
- lack of advice on the importance of oral hygiene
- failure to recognize signs of a dental problem.
What else can lead to edentulism?
Breakdown of the periodontal attachment2 also can be caused by disinterest in oral hygiene practices; craving of, and preference for, carbohydrates because of reduced central serotonin activity3,4; and xerostomia.
Xerostomia, or dry mouth, caused by psychotropic agents and an altered immune response, facilitates growth of pathogenic bacteria and can lead to several dental diseases (Table). These conditions are exacerbated by consumption of chewing gum, sweets, and sugary drinks in response to constantly feeling thirsty from xerostomia. Advise patients to take frequent sips of fluid or let ice cubes melt in their mouth.
Bruxism. Patients taking a selective serotonin reuptake inhibitor or an atypical antipsychotic can develop a movement disorder (eg, extrapyramidal symptoms or tardive dyskinesia) that includes clenching, grinding of the teeth (bruxism), or both, which can worsen their periodontal condition.
Lack of skills, physical dexterity, and motivation to maintain good oral hygiene are common among people with mental illness. Most patients visit a dentist only when they experience a serious oral problem or an emergency (ie, trauma). Many dentists treat psychiatric patients by extracting the tooth that is causing the pain, instead of pursuing complex tooth preservation or restoration techniques because of (1) the extent of the disease, (2) lack of knowledge related to psychiatric illnesses, and (3) frequent and timely follow-ups.5
Providing education about oral health to patients, implementing preventive steps, and educating other medical specialities about the link between oral health and systemic health can help to reduce the burden of dental problems among mentally ill patients.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products
1. Persson K, Axtelius B, Söderfeldt B, et al. Oral health-related quality of life and dental status in an outpatient psychiatric population: a multivariate approach. Int J Ment Health Nurs. 2010;19(1):62-70.
2. Lalloo R, Kisely S, Amarasinghe H, et al. Oral health of patients on psychotropic medications: a study of outpatients in Queensland. Australas Psychiatry. 2013;21(4):338-342.
3. O’Neil A, Berk M, Venugopal K, et al. The association between poor dental health and depression: findings from a large-scale, population-based study (the NHANES study). Gen Hosp Psychiatry. 2014;36(3):266-270.
4. Kisely S, Quek LH, Paris J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
5. Arnaiz A, Zumárraga M, Díez-Altuna I, et al. Oral health and the symptoms of schizophrenia. Psychiatry Res. 2011;188(1):24-28.
1. Persson K, Axtelius B, Söderfeldt B, et al. Oral health-related quality of life and dental status in an outpatient psychiatric population: a multivariate approach. Int J Ment Health Nurs. 2010;19(1):62-70.
2. Lalloo R, Kisely S, Amarasinghe H, et al. Oral health of patients on psychotropic medications: a study of outpatients in Queensland. Australas Psychiatry. 2013;21(4):338-342.
3. O’Neil A, Berk M, Venugopal K, et al. The association between poor dental health and depression: findings from a large-scale, population-based study (the NHANES study). Gen Hosp Psychiatry. 2014;36(3):266-270.
4. Kisely S, Quek LH, Paris J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
5. Arnaiz A, Zumárraga M, Díez-Altuna I, et al. Oral health and the symptoms of schizophrenia. Psychiatry Res. 2011;188(1):24-28.