User login
Do no harm: Benztropine revisited
Ms. P, a 63-year-old woman with a history of schizophrenia whose symptoms have been stable on haloperidol 10 mg/d and ziprasidone 40 mg twice daily, presents to the outpatient clinic for a medication review. She mentions that she has noticed problems with her “memory.” She says she has had difficulty remembering names of people and places as well as difficulty concentrating while reading and writing, which she did months ago with ease. A Montreal Cognitive Assessment (MoCA) is conducted, and Ms. P scores 13/30, indicating moderate cognitive impairment. Visuospatial tasks and clock drawing are intact, but she exhibits impairments in working memory, attention, and concentration. One year ago, Ms. P’s MoCA score was 27/30. She agrees to a neurologic assessment and is referred to neurology for work-up.
Ms. P’s physical examination and routine laboratory tests are all within normal limits. The neurologic exam reveals deficits in working memory, concentration, and attention, but is otherwise unremarkable. MRI reveals mild chronic microvascular changes. The neurology service does not rule out cognitive impairment but recommends adjusting the dosage of Ms. P’s psychiatric medications to elucidate if her impairment of memory and attention is due to medications. However, Ms. P had been managed on her current regimen for several years and had not been hospitalized in many years. Previous attempts to taper her antipsychotics had resulted in worsening symptoms. Ms. P is reluctant to attempt a taper of her antipsychotics because she fears decompensation of her chronic illness. The treating team reviews Ms. P’s medication regimen, and notes that she is receiving benztropine 1 mg twice daily for prophylaxis of extrapyramidal symptoms (EPS). Ms. P denies past or present symptoms of drug-induced parkinsonism, dystonia, or akathisia as well as constipation, sialorrhea, blurry vision, palpitations, or urinary retention.
Benztropine is a tropane alkaloid that was synthetized by combining the tropine portion of atropine with the benzhydryl portion of diphenhydramine hydrochloride. It has anticholinergic and antihistaminic properties1 and seems to inhibit the dopamine transporter. Benztropine is indicated for all forms of parkinsonism, including antipsychotic-induced parkinsonism, but is also prescribed for many off-label uses, including sialorrhea and akathisia (although many authors do not recommend anticholinergics for this purpose2,3), and for prophylaxis of EPS. Benztropine can be administered intravenously, intramuscularly, or orally. Given orally, the typical dosing is twice daily with a maximum dose of 6 mg/d. Benztropine is preferred over diphenhydramine and trihexyphenidyl due to adverse effects of sedation or potential for misuse of the medication.1
Second-generation antipsychotics (SGAs) have been associated with lower rates of neurologic adverse effects compared with first-generation antipsychotics (FGAs). Because SGAs are increasingly prescribed, the use of benztropine (along with other agents such as trihexyphenidyl) for EPS prophylaxis is not an evidence-based practice. However, despite a movement away from prophylactic management of movement disorders, benztropine continues to be prescribed for EPS and/or cholinergic symptoms, despite the peripheral and cognitive adverse effects of this agent and, in many instances, the lack of clear indication for its use.
According to the most recent edition of the American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia,4 anticholinergics should only be used for preventing acute dystonia in conjunction with a long-acting injectable antipsychotic. Furthermore, the APA Guideline states anticholinergics may be used for drug-induced parkinsonism when the dose of an antipsychotic cannot be reduced and an alternative agent is required. However, the first-line agent for drug-induced parkinsonism is amantadine, and benztropine should only be considered if amantadine is contraindicated.4 The rationale for this guideline and for judicious use of anticholinergics is that like any pharmacologic treatment, anticholinergics (including benztropine) carry the potential for adverse effects. For benztropine, these range from mild effects such as tachycardia and constipation to paralytic ileus, increased falls, worsening of tardive dyskinesia (TD), and potential cognitive impairment. Literature suggests that the first step in managing cognitive concerns in a patient with schizophrenia should be a close review of medications, and avoidance of agents with anticholinergic properties.5
Prescribing benztropine for EPS
EPS, which include dystonia, akathisia, drug-induced parkinsonism, and TD, are very frequent adverse effects noted with antipsychotics. Benztropine has demonstrated benefit in managing acute dystonia and the APA Guideline recommends IM administration of either benztropine 1 mg or diphenhydramine 25 mg for this purpose.4 However, in our experience, the most frequent indication for long-term prescribing of benztropine is prophylaxis of antipsychoticinduced dystonia. This use was suggested by some older studies. In a 1987 study by Boyer et al,6 patients who were administered benztropine with haloperidol did not develop acute dystonia, while patients who received haloperidol alone developed dystonia. However, this was a small retrospective study with methodological issues. Boyer et al6 suggested discontinuing prophylaxis with benztropine within 1 week, as acute dystonia occurred within 2.5 days. Other researchers7,8 have argued that short-term prophylaxis with benztropine for 1 week may work, especially during treatment with high-potency antipsychotics. However, in a review of the use of anticholinergics in conjunction with antipsychotics, Desmarais et al5 concluded that there is no need for prophylaxis and recommended alternative treatments. As we have noticed in Ms. P and other patients treated in our facilities, benztropine is frequently continued indefinitely without a clinical indication for its continuous use. Assessment and indication for continued use of benztropine should be considered regularly, and it should be discontinued when there is no clear indication for its use or when adverse effects emerge.
Prescribing benztropine for TD
TD is a subtype of tardive syndromes associated with the use of antipsychotics. It is characterized by repetitive involuntary movements such as lip smacking, puckering, chewing, or tongue protrusion. Proposed pathophysiological mechanisms include dopamine receptor hypersensitivity, N-methyl-D-aspartate (NMDA) receptor excitotoxicity, and gamma-aminobutyric acid (GABA)-containing neuron activity.
According to the APA Guideline, evidence of benztropine’s efficacy for the prevention of TD is lacking.4 A 2018 Cochrane systematic review9 was unable to provide a definitive conclusion regarding the effectiveness of benztropine and other anticholinergics for the treatment of antipsychotic-induced TD. While many clinicians believe that benztropine can be used to treat all types of EPS, there are no clear instances in reviewed literature where the efficacy of benztropine for treating TD could be reliably demonstrated. Furthermore, some literature suggests that anticholinergics such as benztropine increase the risk of developing TD.5,10 The mechanism underlying benztropine’s ability to precipitate or exacerbate abnormal movements is unclear, though it is theorized that anticholinergic medications may inhibit dopamine reuptake into neurons, thus leading to an excess of dopamine in the synaptic cleft that manifests as dyskinesias.10 Some authors also recommend that the first step in the management of TD should be to gradually discontinue anticholinergics, as this has been associated with improvement in TD.11
Continue to: Prescribing anticholinergics in specific patient populations...
Prescribing anticholinergics in specific patient populations
In addition to the adverse effects described above, benztropine can affect cognition, as we observed in Ms. P. The cholinergic system plays a role in human cognition, and blockade of muscarinic receptors has been associated with impairments in working memory and prefrontal tasks.12 These adverse cognitive effects are more pronounced in certain populations, including patients with schizophrenia and older adults.
Schizophrenia is associated with declining cognitive function, and the cognitive faculties of patients with schizophrenia may be worsened by anticholinergics. In patients with schizophrenia, social interactions and social integration are often impacted by profound negative symptoms such as social withdrawal and poverty of thought and speech.13 In a double-blind study by Baker et al,14 benztropine was found to have an impact on attention and concentration in patients with chronic schizophrenia. Baker et al14 found that patients with schizophrenia who were switched from benztropine to placebo increased their overall Wechsler Memory Scale scores compared to those maintained on benztropine. One crosssectional analysis found that a higher anticholinergic burden was associated with impairments across all cognitive domains, including memory, attention/control, executive and visuospatial functioning, and motor speed domains.15 Importantly, a higher anticholinergic medication burden was associated with worse cognitive performance.15 In addition to impairments in cognitive processing, anticholinergics have been associated with a decreased ability to benefit from psychosocial programs and impaired abilities to manage activities of daily living.4 In another study exploring the effects of discontinuing anticholinergics and the impact on movement disorders, Desmarais et al16 found patients experienced a significant improvement in scores on the Brief Assessment of Cognition in Schizophrenia after discontinuing anticholinergics. Vinogradov et al17 noted that “serum anticholinergic activity in schizophrenia patients shows a significant association with impaired performance in measures of verbal working memory and verbal learning memory and was significantly associated with a lowered response to an intensive course of computerized cognitive training.” They felt their findings underscored the cognitive cost of medications with high anticholinergic burden.
Geriatric patients. Careful consideration should be given before starting benztropine in patients age ≥65. The 2019 American Geriatric Society’s Beers Criteria18 recommend avoiding benztropine in geriatric patients; the level of recommendation is strong. Furthermore, the American Geriatric Society designates benztropine as a medication that should be avoided, and a nondrug approach or alternative medication be prescribed independent of the patient’s condition or diagnosis. In a recently published case report, Esang et al19 highlighted several salient findings from previous studies on the risks associated with anticholinergic use:
- any medications a patient takes with anticholinergic properties contribute to the overall anticholinergic load of a patient’s medication regimen
- the higher the anticholinergic burden, the greater the cognitive deficits
- switching from an FGA to an SGA may decrease the risk of EPS and may limit the need for anticholinergic medications such as benztropine for a particular patient.
One must also consider that the effects of multiple medications with anticholinergic properties is probably cumulative.
Alternatives for treating drug-induced parkinsonism
Antipsychotics exert their effects through antagonism of the D2 receptor, and this is the same mechanism that leads to parkinsonism. Specifically, the mechanism is believed to be D2 receptor antagonism in the striatum leading to disinhibition of striatal neurons containing GABA.11 This disinhibition of medium spiny neurons is propagated when acetylcholine is released from cholinergic interneurons. Anticholinergics such as benztropine can remedy symptoms by blocking the signal of acetylcholine on the M1 receptors on medium spiny neurons. However, benztropine also has the propensity to decrease cholinergic transmission, thereby impairing storage of new information into long-term memory as well as impair perception of time—similar to effects seen with (for instance) diphenhydramine.20
The first step in managing drug-induced parkinsonism is to monitor symptoms. The APA Guideline recommends monitoring for acute-onset EPS at weekly intervals when beginning treatment and until stable for 2 weeks, and then monitoring at every follow-up visit thereafter.4 The next recommendation for long-term management of drug-induced parkinsonism is reducing the antipsychotic dose, or replacing the patient’s antipsychotic with an antipsychotic that is less likely to precipitate parkinsonism,4 such as quetiapine, iloperidone, or clozapine.11 If dose reduction is not possible, and the patient’s symptoms are severe, pharmacologic management is indicated. The APA Guideline recommends amantadine as a first-line agent because it is associated with fewer peripheral adverse effects and less impairment in cognition compared with benztropine.4 In a small (N = 60) doubleblind crossover trial, Gelenberg et al20 found benztropine 4 mg/d—but not amantadine 200 mg/d—impaired free recall and perception of time, and participants’ perception of their own memory impairment was significantly greater with benztropine. Amantadine has also been compared to biperiden, a relatively selective M1 muscarinic receptor muscarinic agent. In a separate double-blind crossover study of 26 patients with chronic schizophrenia, Silver and Geraisy21 found that compared to amantadine, biperiden was associated with worse memory performance. The recommended starting dose of amantadine for parkinsonism is 100 mg in the morning, increased to 100 mg twice a day and titrated to a maximum daily dose of 300 mg/d in divided doses.4
Continue to: Alternatives for treating drug-induced akathisia...
Alternatives for treating drug-induced akathisia
Akathisia remains a relatively common adverse effect of SGAs, and the profound physical distress and impaired functioning caused by akathisia necessitates pharmacologic treatment. Despite frequent use in practice for presumed benefit in akathisia, benztropine is not effective for the treatment of akathisia and the APA Guideline recommends that long-term management should begin with an antipsychotic dose reduction, followed by a switch to an agent with less propensity to incite akathisia.4 Acute manifestations of akathisia must be treated, and mirtazapine, propranolol, or clonazepam may be considered as alternatives.4 Mirtazapine is dosed 7.5 mg to 10 mg nightly for akathisia, though it should be used in caution in patients at risk for mania.4 Mirtazapine’s potent 5-HT2A blockade at low doses may contribute to its utility in treating akathisia.2 Propranolol, a nonselective lipophilic beta-adrenergic antagonist, also has demonstrated efficacy in managing akathisia, with recommended dosing of 40 mg to 80 mg twice daily.2 Benzodiazepines such as clonazepam require judicious use for akathisia because they may also precipitate or exacerbate cognitive impairment.4
Alternatives for treating TD
As mentioned above, benztropine is not recommended for the treatment of TD.1 The Box4,22,23 outlines potential treatment options for TD.
Box
Monitoring is the first step in the prevention of tardive dyskinesia (TD). The American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia recommends patients receiving first-generation antipsychotics (FGAs) be monitored every 6 months, those prescribed second-generation antipsychotics (SGAs) be monitored every 12 months, and twice as frequent monitoring for geriatric patients and those who developed involuntary movements rapidly after starting an antipsychotic.4
The APA Guideline recommends decreasing or gradually tapering antipsychotics as another strategy for preventing TD.4 However, these recommendations should be weighed against the risk of short-term antipsychotic withdrawal. Withdrawal of D2 antagonists is associated with worsening of dyskinesias or withdrawal dyskinesia and psychotic decompensation.22
Current treatment recommendations give preference to the importance of preventing development of TD by tapering to the lowest dose of antipsychotic needed to control symptoms for the shortest duration possible.22 Thereafter, if treatment intervention is needed, consideration should be given to the following pharmacological interventions in order from highest level of recommendation (Grade A) to lowest (Grade C):
A: vesicular monoamine transporter-2 inhibitors deutetrabenazine and valbenazine
B: clonazepam, ginkgo biloba
C: amantadine, tetrabenazine, and globus pallidus interna deep brain stimulation.22
There is insufficient evidence to support or refute withdrawing causative agents or switching from FGAs to SGAs to treat TD.22 Furthermore, for many patients with schizophrenia, a gradual discontinuation of their antipsychotic must be weighed against the risk of relapse.23
Valbenazine and deutetrabenazine have been demonstrated to be efficacious and are FDA-approved for managing TD. The initial dose of valbenazine is 40 mg/d. Common adverse effects include somnolence and fatigue/ sedation. Valbenazine should be avoided in patients with QT prolongation or arrhythmias. Deutetrabenazine has less impact on the cytochrome P450 2D6 enzyme and therefore does not require genotyping as would be the case for patients who are receiving >50 mg/d of tetrabenazine. The starting dose of deutetrabenazine is 6 mg/d. Adverse effects include depression, suicidality, neuroleptic malignant syndrome, parkinsonism, and QT prolongation. Deutetrabenazine is contraindicated in patients who are suicidal or have untreated depression, hepatic impairment, or concomitant use of monoamine oxidase inhibitors.22 Deutetrabenazine is an isomer of tetrabenazine; however, evidence supporting the parent compound suggests limited use due to increased risk of adverse effects compared with valbenazine and deutetrabenazine.23 Tetrabenazine may be considered as an adjunctive treatment or used as a single agent if valbenazine or deutetrabenazine are not accessible.22
Discontinuing benztropine
Benztropine is recommended as a firstline agent for the management of acute dystonia, and it may be used temporarily for drug-induced parkinsonism, but it is not recommended to prevent EPS or TD. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic agent such as benztropine. Based on their review of earlier studies, Desmarais et al5 suggest a gradual 3-month discontinuation of benztropine. Multiple studies have demonstrated an ability to taper anticholinergics in days to months.4 However, gradual discontinuation is advisable to avoid cholinergic rebound and the reemergence of EPS, and to decrease the risk of neuroleptic malignant syndrome associated with sudden discontinuation.5 One suggested taper regimen is a decrease of 0.5 mg benztropine every week. Amantadine may be considered if parkinsonism is noted during the taper. Patients on benztropine may develop rebound symptoms, such as vivid dreams/nightmares; if this occurs, the taper rate can be slowed to a decrease of 0.5 mg every 2 weeks.4
Continue to: First do no harm...
First do no harm
Psychiatrists commonly prescribe benztropine to prevent EPS and TD, but available literature does not support the efficacy of benztropine for mitigating drug-induced parkinsonism, and studies report benztropine may significantly worsen cognitive processes and exacerbate TD.16 In addition, benztropine misuse has been correlated with euphoria and psychosis.16 More than 3 decades ago, the World Health Organization Heads of Centres Collaborating in WHO-Coordinated Studies on Biological Aspects of Mental Illness issued a consensus statement24 discouraging the prophylactic use of anticholinergics for patients receiving antipsychotics, yet we still see patients on an indefinite regimen of benztropine.
As clinicians, our goals should be to optimize a patient’s functioning and quality of life, and to use the lowest dose of medication along with the fewest medications necessary to avoid adverse effects such as EPS. Benztropine is recommended as a first-line agent for the management of acute dystonia, but its continued or indefinite use to prevent antipsychotic-induced adverse effects is not recommended. While all pharmacologic interventions carry a risk of adverse effects, weighing the risk of those effects against the clinical benefits is the prerogative of a skilled clinician. Benztropine and other anticholinergics prescribed for prophylactic purposes have numerous adverse effects, limited clinical utility, and a deleterious effect on quality of life. Furthermore, benztropine prophylaxis of drug-induced parkinsonism does not seem to be warranted, and the risks do not seem to outweigh the harm benztropine may cause, with the possible exception of “prophylactic” treatment of dystonia that is discontinued in a few days, as some researchers have suggested.6-8 The preventive value of benztropine has not been demonstrated. It is time we took inventory of medications that might cause more harm than good, rely on current treatment guidelines instead of habit, and use these agents judiciously while considering replacement with novel, safer medications whenever possible.
CASE CONTINUED
The clinical team considers benztropine’s ability to cause cognitive effects, and decides to taper and discontinue it over 1 month. Ms. P is seen in an outpatient clinic within 1 month of discontinuing benztropine. She reports that her difficulty remembering words and details has improved. She also says that she is now able to concentrate on writing and reading. The consulting neurologist also notes improvement. Ms. P continues to report improvement in symptoms over the next 2 months of follow-up, and says that her mood improved and she has less apathy.
Bottom Line
Benztropine is a first-line medication for acute dystonia, but its continued or indefinite use for preventing antipsychotic-induced adverse effects is not recommended. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic medication such as benztropine.
1. Cogentin [package insert]. McPherson, KS: Lundbeck Inc; 2013.
2. Poyurovsky M, Weizman A. Treatment of antipsychoticrelated akathisia revisited. J Clin Psychopharmacol. 2015; 35(6):711-714.
3. Salem H, Nagpal C, Pigott T, et al. Revisiting antipsychoticinduced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
4. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia. 3rd ed. American Psychiatric Association; 2021.
5. Desmarais JE, Beauclair L, Margolese HC. Anticholinergics in the era of atypical antipsychotics: short-term or long-term treatment? J Psychopharmacol. 2012;26(9):1167-1174.
6. Boyer WF, Bakalar NH, Lake CR. Anticholinergic prophylaxis of acute haloperidol-induced acute dystonic reactions. J Clin Psychopharmacol. 1987;7(3):164-166.
7. Winslow RS, Stillner V, Coons DJ, et al. Prevention of acute dystonic reactions in patients beginning high-potency neuroleptics. Am J Psychiatry. 1986;143(6):706-710.
8. Stern TA, Anderson WH. Benztropine prophylaxis of dystonic reactions. Psychopharmacology (Berl). 1979; 61(3):261-262.
9. Bergman H, Soares‐Weiser K. Anticholinergic medication for antipsychotic‐induced tardive dyskinesia. Cochrane Database Syst Rev. 2018;1(1):CD000204. doi:10.1002/ 14651858.CD000204.pub2
10. Howrie DL, Rowley AH, Krenzelok EP. Benztropineinduced acute dystonic reaction. Ann Emerg Med. 1986;15(5):594-596.
11. Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs. tardive dyskinesia--key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2): 233-248.
12. Wijegunaratne H, Qazi H, Koola M. Chronic and bedtime use of benztropine with antipsychotics: is it necessary? Schizophr Res. 2014;153(1-3):248-249.
13. Möller HJ. The relevance of negative symptoms in schizophrenia and how to treat them with psychopharmaceuticals? Psychiatr Danub. 2016;28(4):435-440.
14. Baker LA, Cheng LY, Amara IB. The withdrawal of benztropine mesylate in chronic schizophrenic patients. Br J Psychiatry. 1983;143:584-590.
15. Joshi YB, Thomas ML, Braff DL, et al. Anticholinergic medication burden-associated cognitive impairment in schizophrenia. Am J Psychiatry. 2021;178(9):838-847.
16. Desmarais JE, Beauclair E, Annable L, et al. Effects of discontinuing anticholinergic treatment on movement disorders, cognition and psychopathology in patients with schizophrenia. Ther Adv Psychopharmacol. 2014;4(6): 257-267.
17. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9): 1055-1062.
18. American Geriatrics Society 2019 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
19. Esang M, Person US, Izekor OO, et al. An unlikely case of benztropine misuse in an elderly schizophrenic. Cureus. 2021;13(2):e13434. doi:10.7759/cureus.13434
20. Gelenberg AJ, Van Putten T, Lavori PW, et al. Anticholinergic effects on memory: benztropine versus amantadine. J Clin Psychopharmacol. 1989;9(3):180-185.
21. Silver H, Geraisy N. Effects of biperiden and amantadine on memory in medicated chronic schizophrenic patients. A double-blind cross-over study. Br J Psychiatry. 1995; 166(2):241-243.
22. Bhidayasiri R, Jitkritsadakul O, Friedman J, et al. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67-75.
23. Ricciardi L, Pringsheim T, Barnes TRE, et al. Treatment recommendations for tardive dyskinesia. Canadian J Psychiatry. 2019;64(6):388-399.
24. Prophylactic use of anticholinergics in patients on long-term neuroleptic treatment. A consensus statement. World Health Organization heads of centres collaborating in WHO coordinated studies on biological aspects of mental illness. Br J Psychiatry. 1990;156:412.
Ms. P, a 63-year-old woman with a history of schizophrenia whose symptoms have been stable on haloperidol 10 mg/d and ziprasidone 40 mg twice daily, presents to the outpatient clinic for a medication review. She mentions that she has noticed problems with her “memory.” She says she has had difficulty remembering names of people and places as well as difficulty concentrating while reading and writing, which she did months ago with ease. A Montreal Cognitive Assessment (MoCA) is conducted, and Ms. P scores 13/30, indicating moderate cognitive impairment. Visuospatial tasks and clock drawing are intact, but she exhibits impairments in working memory, attention, and concentration. One year ago, Ms. P’s MoCA score was 27/30. She agrees to a neurologic assessment and is referred to neurology for work-up.
Ms. P’s physical examination and routine laboratory tests are all within normal limits. The neurologic exam reveals deficits in working memory, concentration, and attention, but is otherwise unremarkable. MRI reveals mild chronic microvascular changes. The neurology service does not rule out cognitive impairment but recommends adjusting the dosage of Ms. P’s psychiatric medications to elucidate if her impairment of memory and attention is due to medications. However, Ms. P had been managed on her current regimen for several years and had not been hospitalized in many years. Previous attempts to taper her antipsychotics had resulted in worsening symptoms. Ms. P is reluctant to attempt a taper of her antipsychotics because she fears decompensation of her chronic illness. The treating team reviews Ms. P’s medication regimen, and notes that she is receiving benztropine 1 mg twice daily for prophylaxis of extrapyramidal symptoms (EPS). Ms. P denies past or present symptoms of drug-induced parkinsonism, dystonia, or akathisia as well as constipation, sialorrhea, blurry vision, palpitations, or urinary retention.
Benztropine is a tropane alkaloid that was synthetized by combining the tropine portion of atropine with the benzhydryl portion of diphenhydramine hydrochloride. It has anticholinergic and antihistaminic properties1 and seems to inhibit the dopamine transporter. Benztropine is indicated for all forms of parkinsonism, including antipsychotic-induced parkinsonism, but is also prescribed for many off-label uses, including sialorrhea and akathisia (although many authors do not recommend anticholinergics for this purpose2,3), and for prophylaxis of EPS. Benztropine can be administered intravenously, intramuscularly, or orally. Given orally, the typical dosing is twice daily with a maximum dose of 6 mg/d. Benztropine is preferred over diphenhydramine and trihexyphenidyl due to adverse effects of sedation or potential for misuse of the medication.1
Second-generation antipsychotics (SGAs) have been associated with lower rates of neurologic adverse effects compared with first-generation antipsychotics (FGAs). Because SGAs are increasingly prescribed, the use of benztropine (along with other agents such as trihexyphenidyl) for EPS prophylaxis is not an evidence-based practice. However, despite a movement away from prophylactic management of movement disorders, benztropine continues to be prescribed for EPS and/or cholinergic symptoms, despite the peripheral and cognitive adverse effects of this agent and, in many instances, the lack of clear indication for its use.
According to the most recent edition of the American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia,4 anticholinergics should only be used for preventing acute dystonia in conjunction with a long-acting injectable antipsychotic. Furthermore, the APA Guideline states anticholinergics may be used for drug-induced parkinsonism when the dose of an antipsychotic cannot be reduced and an alternative agent is required. However, the first-line agent for drug-induced parkinsonism is amantadine, and benztropine should only be considered if amantadine is contraindicated.4 The rationale for this guideline and for judicious use of anticholinergics is that like any pharmacologic treatment, anticholinergics (including benztropine) carry the potential for adverse effects. For benztropine, these range from mild effects such as tachycardia and constipation to paralytic ileus, increased falls, worsening of tardive dyskinesia (TD), and potential cognitive impairment. Literature suggests that the first step in managing cognitive concerns in a patient with schizophrenia should be a close review of medications, and avoidance of agents with anticholinergic properties.5
Prescribing benztropine for EPS
EPS, which include dystonia, akathisia, drug-induced parkinsonism, and TD, are very frequent adverse effects noted with antipsychotics. Benztropine has demonstrated benefit in managing acute dystonia and the APA Guideline recommends IM administration of either benztropine 1 mg or diphenhydramine 25 mg for this purpose.4 However, in our experience, the most frequent indication for long-term prescribing of benztropine is prophylaxis of antipsychoticinduced dystonia. This use was suggested by some older studies. In a 1987 study by Boyer et al,6 patients who were administered benztropine with haloperidol did not develop acute dystonia, while patients who received haloperidol alone developed dystonia. However, this was a small retrospective study with methodological issues. Boyer et al6 suggested discontinuing prophylaxis with benztropine within 1 week, as acute dystonia occurred within 2.5 days. Other researchers7,8 have argued that short-term prophylaxis with benztropine for 1 week may work, especially during treatment with high-potency antipsychotics. However, in a review of the use of anticholinergics in conjunction with antipsychotics, Desmarais et al5 concluded that there is no need for prophylaxis and recommended alternative treatments. As we have noticed in Ms. P and other patients treated in our facilities, benztropine is frequently continued indefinitely without a clinical indication for its continuous use. Assessment and indication for continued use of benztropine should be considered regularly, and it should be discontinued when there is no clear indication for its use or when adverse effects emerge.
Prescribing benztropine for TD
TD is a subtype of tardive syndromes associated with the use of antipsychotics. It is characterized by repetitive involuntary movements such as lip smacking, puckering, chewing, or tongue protrusion. Proposed pathophysiological mechanisms include dopamine receptor hypersensitivity, N-methyl-D-aspartate (NMDA) receptor excitotoxicity, and gamma-aminobutyric acid (GABA)-containing neuron activity.
According to the APA Guideline, evidence of benztropine’s efficacy for the prevention of TD is lacking.4 A 2018 Cochrane systematic review9 was unable to provide a definitive conclusion regarding the effectiveness of benztropine and other anticholinergics for the treatment of antipsychotic-induced TD. While many clinicians believe that benztropine can be used to treat all types of EPS, there are no clear instances in reviewed literature where the efficacy of benztropine for treating TD could be reliably demonstrated. Furthermore, some literature suggests that anticholinergics such as benztropine increase the risk of developing TD.5,10 The mechanism underlying benztropine’s ability to precipitate or exacerbate abnormal movements is unclear, though it is theorized that anticholinergic medications may inhibit dopamine reuptake into neurons, thus leading to an excess of dopamine in the synaptic cleft that manifests as dyskinesias.10 Some authors also recommend that the first step in the management of TD should be to gradually discontinue anticholinergics, as this has been associated with improvement in TD.11
Continue to: Prescribing anticholinergics in specific patient populations...
Prescribing anticholinergics in specific patient populations
In addition to the adverse effects described above, benztropine can affect cognition, as we observed in Ms. P. The cholinergic system plays a role in human cognition, and blockade of muscarinic receptors has been associated with impairments in working memory and prefrontal tasks.12 These adverse cognitive effects are more pronounced in certain populations, including patients with schizophrenia and older adults.
Schizophrenia is associated with declining cognitive function, and the cognitive faculties of patients with schizophrenia may be worsened by anticholinergics. In patients with schizophrenia, social interactions and social integration are often impacted by profound negative symptoms such as social withdrawal and poverty of thought and speech.13 In a double-blind study by Baker et al,14 benztropine was found to have an impact on attention and concentration in patients with chronic schizophrenia. Baker et al14 found that patients with schizophrenia who were switched from benztropine to placebo increased their overall Wechsler Memory Scale scores compared to those maintained on benztropine. One crosssectional analysis found that a higher anticholinergic burden was associated with impairments across all cognitive domains, including memory, attention/control, executive and visuospatial functioning, and motor speed domains.15 Importantly, a higher anticholinergic medication burden was associated with worse cognitive performance.15 In addition to impairments in cognitive processing, anticholinergics have been associated with a decreased ability to benefit from psychosocial programs and impaired abilities to manage activities of daily living.4 In another study exploring the effects of discontinuing anticholinergics and the impact on movement disorders, Desmarais et al16 found patients experienced a significant improvement in scores on the Brief Assessment of Cognition in Schizophrenia after discontinuing anticholinergics. Vinogradov et al17 noted that “serum anticholinergic activity in schizophrenia patients shows a significant association with impaired performance in measures of verbal working memory and verbal learning memory and was significantly associated with a lowered response to an intensive course of computerized cognitive training.” They felt their findings underscored the cognitive cost of medications with high anticholinergic burden.
Geriatric patients. Careful consideration should be given before starting benztropine in patients age ≥65. The 2019 American Geriatric Society’s Beers Criteria18 recommend avoiding benztropine in geriatric patients; the level of recommendation is strong. Furthermore, the American Geriatric Society designates benztropine as a medication that should be avoided, and a nondrug approach or alternative medication be prescribed independent of the patient’s condition or diagnosis. In a recently published case report, Esang et al19 highlighted several salient findings from previous studies on the risks associated with anticholinergic use:
- any medications a patient takes with anticholinergic properties contribute to the overall anticholinergic load of a patient’s medication regimen
- the higher the anticholinergic burden, the greater the cognitive deficits
- switching from an FGA to an SGA may decrease the risk of EPS and may limit the need for anticholinergic medications such as benztropine for a particular patient.
One must also consider that the effects of multiple medications with anticholinergic properties is probably cumulative.
Alternatives for treating drug-induced parkinsonism
Antipsychotics exert their effects through antagonism of the D2 receptor, and this is the same mechanism that leads to parkinsonism. Specifically, the mechanism is believed to be D2 receptor antagonism in the striatum leading to disinhibition of striatal neurons containing GABA.11 This disinhibition of medium spiny neurons is propagated when acetylcholine is released from cholinergic interneurons. Anticholinergics such as benztropine can remedy symptoms by blocking the signal of acetylcholine on the M1 receptors on medium spiny neurons. However, benztropine also has the propensity to decrease cholinergic transmission, thereby impairing storage of new information into long-term memory as well as impair perception of time—similar to effects seen with (for instance) diphenhydramine.20
The first step in managing drug-induced parkinsonism is to monitor symptoms. The APA Guideline recommends monitoring for acute-onset EPS at weekly intervals when beginning treatment and until stable for 2 weeks, and then monitoring at every follow-up visit thereafter.4 The next recommendation for long-term management of drug-induced parkinsonism is reducing the antipsychotic dose, or replacing the patient’s antipsychotic with an antipsychotic that is less likely to precipitate parkinsonism,4 such as quetiapine, iloperidone, or clozapine.11 If dose reduction is not possible, and the patient’s symptoms are severe, pharmacologic management is indicated. The APA Guideline recommends amantadine as a first-line agent because it is associated with fewer peripheral adverse effects and less impairment in cognition compared with benztropine.4 In a small (N = 60) doubleblind crossover trial, Gelenberg et al20 found benztropine 4 mg/d—but not amantadine 200 mg/d—impaired free recall and perception of time, and participants’ perception of their own memory impairment was significantly greater with benztropine. Amantadine has also been compared to biperiden, a relatively selective M1 muscarinic receptor muscarinic agent. In a separate double-blind crossover study of 26 patients with chronic schizophrenia, Silver and Geraisy21 found that compared to amantadine, biperiden was associated with worse memory performance. The recommended starting dose of amantadine for parkinsonism is 100 mg in the morning, increased to 100 mg twice a day and titrated to a maximum daily dose of 300 mg/d in divided doses.4
Continue to: Alternatives for treating drug-induced akathisia...
Alternatives for treating drug-induced akathisia
Akathisia remains a relatively common adverse effect of SGAs, and the profound physical distress and impaired functioning caused by akathisia necessitates pharmacologic treatment. Despite frequent use in practice for presumed benefit in akathisia, benztropine is not effective for the treatment of akathisia and the APA Guideline recommends that long-term management should begin with an antipsychotic dose reduction, followed by a switch to an agent with less propensity to incite akathisia.4 Acute manifestations of akathisia must be treated, and mirtazapine, propranolol, or clonazepam may be considered as alternatives.4 Mirtazapine is dosed 7.5 mg to 10 mg nightly for akathisia, though it should be used in caution in patients at risk for mania.4 Mirtazapine’s potent 5-HT2A blockade at low doses may contribute to its utility in treating akathisia.2 Propranolol, a nonselective lipophilic beta-adrenergic antagonist, also has demonstrated efficacy in managing akathisia, with recommended dosing of 40 mg to 80 mg twice daily.2 Benzodiazepines such as clonazepam require judicious use for akathisia because they may also precipitate or exacerbate cognitive impairment.4
Alternatives for treating TD
As mentioned above, benztropine is not recommended for the treatment of TD.1 The Box4,22,23 outlines potential treatment options for TD.
Box
Monitoring is the first step in the prevention of tardive dyskinesia (TD). The American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia recommends patients receiving first-generation antipsychotics (FGAs) be monitored every 6 months, those prescribed second-generation antipsychotics (SGAs) be monitored every 12 months, and twice as frequent monitoring for geriatric patients and those who developed involuntary movements rapidly after starting an antipsychotic.4
The APA Guideline recommends decreasing or gradually tapering antipsychotics as another strategy for preventing TD.4 However, these recommendations should be weighed against the risk of short-term antipsychotic withdrawal. Withdrawal of D2 antagonists is associated with worsening of dyskinesias or withdrawal dyskinesia and psychotic decompensation.22
Current treatment recommendations give preference to the importance of preventing development of TD by tapering to the lowest dose of antipsychotic needed to control symptoms for the shortest duration possible.22 Thereafter, if treatment intervention is needed, consideration should be given to the following pharmacological interventions in order from highest level of recommendation (Grade A) to lowest (Grade C):
A: vesicular monoamine transporter-2 inhibitors deutetrabenazine and valbenazine
B: clonazepam, ginkgo biloba
C: amantadine, tetrabenazine, and globus pallidus interna deep brain stimulation.22
There is insufficient evidence to support or refute withdrawing causative agents or switching from FGAs to SGAs to treat TD.22 Furthermore, for many patients with schizophrenia, a gradual discontinuation of their antipsychotic must be weighed against the risk of relapse.23
Valbenazine and deutetrabenazine have been demonstrated to be efficacious and are FDA-approved for managing TD. The initial dose of valbenazine is 40 mg/d. Common adverse effects include somnolence and fatigue/ sedation. Valbenazine should be avoided in patients with QT prolongation or arrhythmias. Deutetrabenazine has less impact on the cytochrome P450 2D6 enzyme and therefore does not require genotyping as would be the case for patients who are receiving >50 mg/d of tetrabenazine. The starting dose of deutetrabenazine is 6 mg/d. Adverse effects include depression, suicidality, neuroleptic malignant syndrome, parkinsonism, and QT prolongation. Deutetrabenazine is contraindicated in patients who are suicidal or have untreated depression, hepatic impairment, or concomitant use of monoamine oxidase inhibitors.22 Deutetrabenazine is an isomer of tetrabenazine; however, evidence supporting the parent compound suggests limited use due to increased risk of adverse effects compared with valbenazine and deutetrabenazine.23 Tetrabenazine may be considered as an adjunctive treatment or used as a single agent if valbenazine or deutetrabenazine are not accessible.22
Discontinuing benztropine
Benztropine is recommended as a firstline agent for the management of acute dystonia, and it may be used temporarily for drug-induced parkinsonism, but it is not recommended to prevent EPS or TD. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic agent such as benztropine. Based on their review of earlier studies, Desmarais et al5 suggest a gradual 3-month discontinuation of benztropine. Multiple studies have demonstrated an ability to taper anticholinergics in days to months.4 However, gradual discontinuation is advisable to avoid cholinergic rebound and the reemergence of EPS, and to decrease the risk of neuroleptic malignant syndrome associated with sudden discontinuation.5 One suggested taper regimen is a decrease of 0.5 mg benztropine every week. Amantadine may be considered if parkinsonism is noted during the taper. Patients on benztropine may develop rebound symptoms, such as vivid dreams/nightmares; if this occurs, the taper rate can be slowed to a decrease of 0.5 mg every 2 weeks.4
Continue to: First do no harm...
First do no harm
Psychiatrists commonly prescribe benztropine to prevent EPS and TD, but available literature does not support the efficacy of benztropine for mitigating drug-induced parkinsonism, and studies report benztropine may significantly worsen cognitive processes and exacerbate TD.16 In addition, benztropine misuse has been correlated with euphoria and psychosis.16 More than 3 decades ago, the World Health Organization Heads of Centres Collaborating in WHO-Coordinated Studies on Biological Aspects of Mental Illness issued a consensus statement24 discouraging the prophylactic use of anticholinergics for patients receiving antipsychotics, yet we still see patients on an indefinite regimen of benztropine.
As clinicians, our goals should be to optimize a patient’s functioning and quality of life, and to use the lowest dose of medication along with the fewest medications necessary to avoid adverse effects such as EPS. Benztropine is recommended as a first-line agent for the management of acute dystonia, but its continued or indefinite use to prevent antipsychotic-induced adverse effects is not recommended. While all pharmacologic interventions carry a risk of adverse effects, weighing the risk of those effects against the clinical benefits is the prerogative of a skilled clinician. Benztropine and other anticholinergics prescribed for prophylactic purposes have numerous adverse effects, limited clinical utility, and a deleterious effect on quality of life. Furthermore, benztropine prophylaxis of drug-induced parkinsonism does not seem to be warranted, and the risks do not seem to outweigh the harm benztropine may cause, with the possible exception of “prophylactic” treatment of dystonia that is discontinued in a few days, as some researchers have suggested.6-8 The preventive value of benztropine has not been demonstrated. It is time we took inventory of medications that might cause more harm than good, rely on current treatment guidelines instead of habit, and use these agents judiciously while considering replacement with novel, safer medications whenever possible.
CASE CONTINUED
The clinical team considers benztropine’s ability to cause cognitive effects, and decides to taper and discontinue it over 1 month. Ms. P is seen in an outpatient clinic within 1 month of discontinuing benztropine. She reports that her difficulty remembering words and details has improved. She also says that she is now able to concentrate on writing and reading. The consulting neurologist also notes improvement. Ms. P continues to report improvement in symptoms over the next 2 months of follow-up, and says that her mood improved and she has less apathy.
Bottom Line
Benztropine is a first-line medication for acute dystonia, but its continued or indefinite use for preventing antipsychotic-induced adverse effects is not recommended. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic medication such as benztropine.
Ms. P, a 63-year-old woman with a history of schizophrenia whose symptoms have been stable on haloperidol 10 mg/d and ziprasidone 40 mg twice daily, presents to the outpatient clinic for a medication review. She mentions that she has noticed problems with her “memory.” She says she has had difficulty remembering names of people and places as well as difficulty concentrating while reading and writing, which she did months ago with ease. A Montreal Cognitive Assessment (MoCA) is conducted, and Ms. P scores 13/30, indicating moderate cognitive impairment. Visuospatial tasks and clock drawing are intact, but she exhibits impairments in working memory, attention, and concentration. One year ago, Ms. P’s MoCA score was 27/30. She agrees to a neurologic assessment and is referred to neurology for work-up.
Ms. P’s physical examination and routine laboratory tests are all within normal limits. The neurologic exam reveals deficits in working memory, concentration, and attention, but is otherwise unremarkable. MRI reveals mild chronic microvascular changes. The neurology service does not rule out cognitive impairment but recommends adjusting the dosage of Ms. P’s psychiatric medications to elucidate if her impairment of memory and attention is due to medications. However, Ms. P had been managed on her current regimen for several years and had not been hospitalized in many years. Previous attempts to taper her antipsychotics had resulted in worsening symptoms. Ms. P is reluctant to attempt a taper of her antipsychotics because she fears decompensation of her chronic illness. The treating team reviews Ms. P’s medication regimen, and notes that she is receiving benztropine 1 mg twice daily for prophylaxis of extrapyramidal symptoms (EPS). Ms. P denies past or present symptoms of drug-induced parkinsonism, dystonia, or akathisia as well as constipation, sialorrhea, blurry vision, palpitations, or urinary retention.
Benztropine is a tropane alkaloid that was synthetized by combining the tropine portion of atropine with the benzhydryl portion of diphenhydramine hydrochloride. It has anticholinergic and antihistaminic properties1 and seems to inhibit the dopamine transporter. Benztropine is indicated for all forms of parkinsonism, including antipsychotic-induced parkinsonism, but is also prescribed for many off-label uses, including sialorrhea and akathisia (although many authors do not recommend anticholinergics for this purpose2,3), and for prophylaxis of EPS. Benztropine can be administered intravenously, intramuscularly, or orally. Given orally, the typical dosing is twice daily with a maximum dose of 6 mg/d. Benztropine is preferred over diphenhydramine and trihexyphenidyl due to adverse effects of sedation or potential for misuse of the medication.1
Second-generation antipsychotics (SGAs) have been associated with lower rates of neurologic adverse effects compared with first-generation antipsychotics (FGAs). Because SGAs are increasingly prescribed, the use of benztropine (along with other agents such as trihexyphenidyl) for EPS prophylaxis is not an evidence-based practice. However, despite a movement away from prophylactic management of movement disorders, benztropine continues to be prescribed for EPS and/or cholinergic symptoms, despite the peripheral and cognitive adverse effects of this agent and, in many instances, the lack of clear indication for its use.
According to the most recent edition of the American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia,4 anticholinergics should only be used for preventing acute dystonia in conjunction with a long-acting injectable antipsychotic. Furthermore, the APA Guideline states anticholinergics may be used for drug-induced parkinsonism when the dose of an antipsychotic cannot be reduced and an alternative agent is required. However, the first-line agent for drug-induced parkinsonism is amantadine, and benztropine should only be considered if amantadine is contraindicated.4 The rationale for this guideline and for judicious use of anticholinergics is that like any pharmacologic treatment, anticholinergics (including benztropine) carry the potential for adverse effects. For benztropine, these range from mild effects such as tachycardia and constipation to paralytic ileus, increased falls, worsening of tardive dyskinesia (TD), and potential cognitive impairment. Literature suggests that the first step in managing cognitive concerns in a patient with schizophrenia should be a close review of medications, and avoidance of agents with anticholinergic properties.5
Prescribing benztropine for EPS
EPS, which include dystonia, akathisia, drug-induced parkinsonism, and TD, are very frequent adverse effects noted with antipsychotics. Benztropine has demonstrated benefit in managing acute dystonia and the APA Guideline recommends IM administration of either benztropine 1 mg or diphenhydramine 25 mg for this purpose.4 However, in our experience, the most frequent indication for long-term prescribing of benztropine is prophylaxis of antipsychoticinduced dystonia. This use was suggested by some older studies. In a 1987 study by Boyer et al,6 patients who were administered benztropine with haloperidol did not develop acute dystonia, while patients who received haloperidol alone developed dystonia. However, this was a small retrospective study with methodological issues. Boyer et al6 suggested discontinuing prophylaxis with benztropine within 1 week, as acute dystonia occurred within 2.5 days. Other researchers7,8 have argued that short-term prophylaxis with benztropine for 1 week may work, especially during treatment with high-potency antipsychotics. However, in a review of the use of anticholinergics in conjunction with antipsychotics, Desmarais et al5 concluded that there is no need for prophylaxis and recommended alternative treatments. As we have noticed in Ms. P and other patients treated in our facilities, benztropine is frequently continued indefinitely without a clinical indication for its continuous use. Assessment and indication for continued use of benztropine should be considered regularly, and it should be discontinued when there is no clear indication for its use or when adverse effects emerge.
Prescribing benztropine for TD
TD is a subtype of tardive syndromes associated with the use of antipsychotics. It is characterized by repetitive involuntary movements such as lip smacking, puckering, chewing, or tongue protrusion. Proposed pathophysiological mechanisms include dopamine receptor hypersensitivity, N-methyl-D-aspartate (NMDA) receptor excitotoxicity, and gamma-aminobutyric acid (GABA)-containing neuron activity.
According to the APA Guideline, evidence of benztropine’s efficacy for the prevention of TD is lacking.4 A 2018 Cochrane systematic review9 was unable to provide a definitive conclusion regarding the effectiveness of benztropine and other anticholinergics for the treatment of antipsychotic-induced TD. While many clinicians believe that benztropine can be used to treat all types of EPS, there are no clear instances in reviewed literature where the efficacy of benztropine for treating TD could be reliably demonstrated. Furthermore, some literature suggests that anticholinergics such as benztropine increase the risk of developing TD.5,10 The mechanism underlying benztropine’s ability to precipitate or exacerbate abnormal movements is unclear, though it is theorized that anticholinergic medications may inhibit dopamine reuptake into neurons, thus leading to an excess of dopamine in the synaptic cleft that manifests as dyskinesias.10 Some authors also recommend that the first step in the management of TD should be to gradually discontinue anticholinergics, as this has been associated with improvement in TD.11
Continue to: Prescribing anticholinergics in specific patient populations...
Prescribing anticholinergics in specific patient populations
In addition to the adverse effects described above, benztropine can affect cognition, as we observed in Ms. P. The cholinergic system plays a role in human cognition, and blockade of muscarinic receptors has been associated with impairments in working memory and prefrontal tasks.12 These adverse cognitive effects are more pronounced in certain populations, including patients with schizophrenia and older adults.
Schizophrenia is associated with declining cognitive function, and the cognitive faculties of patients with schizophrenia may be worsened by anticholinergics. In patients with schizophrenia, social interactions and social integration are often impacted by profound negative symptoms such as social withdrawal and poverty of thought and speech.13 In a double-blind study by Baker et al,14 benztropine was found to have an impact on attention and concentration in patients with chronic schizophrenia. Baker et al14 found that patients with schizophrenia who were switched from benztropine to placebo increased their overall Wechsler Memory Scale scores compared to those maintained on benztropine. One crosssectional analysis found that a higher anticholinergic burden was associated with impairments across all cognitive domains, including memory, attention/control, executive and visuospatial functioning, and motor speed domains.15 Importantly, a higher anticholinergic medication burden was associated with worse cognitive performance.15 In addition to impairments in cognitive processing, anticholinergics have been associated with a decreased ability to benefit from psychosocial programs and impaired abilities to manage activities of daily living.4 In another study exploring the effects of discontinuing anticholinergics and the impact on movement disorders, Desmarais et al16 found patients experienced a significant improvement in scores on the Brief Assessment of Cognition in Schizophrenia after discontinuing anticholinergics. Vinogradov et al17 noted that “serum anticholinergic activity in schizophrenia patients shows a significant association with impaired performance in measures of verbal working memory and verbal learning memory and was significantly associated with a lowered response to an intensive course of computerized cognitive training.” They felt their findings underscored the cognitive cost of medications with high anticholinergic burden.
Geriatric patients. Careful consideration should be given before starting benztropine in patients age ≥65. The 2019 American Geriatric Society’s Beers Criteria18 recommend avoiding benztropine in geriatric patients; the level of recommendation is strong. Furthermore, the American Geriatric Society designates benztropine as a medication that should be avoided, and a nondrug approach or alternative medication be prescribed independent of the patient’s condition or diagnosis. In a recently published case report, Esang et al19 highlighted several salient findings from previous studies on the risks associated with anticholinergic use:
- any medications a patient takes with anticholinergic properties contribute to the overall anticholinergic load of a patient’s medication regimen
- the higher the anticholinergic burden, the greater the cognitive deficits
- switching from an FGA to an SGA may decrease the risk of EPS and may limit the need for anticholinergic medications such as benztropine for a particular patient.
One must also consider that the effects of multiple medications with anticholinergic properties is probably cumulative.
Alternatives for treating drug-induced parkinsonism
Antipsychotics exert their effects through antagonism of the D2 receptor, and this is the same mechanism that leads to parkinsonism. Specifically, the mechanism is believed to be D2 receptor antagonism in the striatum leading to disinhibition of striatal neurons containing GABA.11 This disinhibition of medium spiny neurons is propagated when acetylcholine is released from cholinergic interneurons. Anticholinergics such as benztropine can remedy symptoms by blocking the signal of acetylcholine on the M1 receptors on medium spiny neurons. However, benztropine also has the propensity to decrease cholinergic transmission, thereby impairing storage of new information into long-term memory as well as impair perception of time—similar to effects seen with (for instance) diphenhydramine.20
The first step in managing drug-induced parkinsonism is to monitor symptoms. The APA Guideline recommends monitoring for acute-onset EPS at weekly intervals when beginning treatment and until stable for 2 weeks, and then monitoring at every follow-up visit thereafter.4 The next recommendation for long-term management of drug-induced parkinsonism is reducing the antipsychotic dose, or replacing the patient’s antipsychotic with an antipsychotic that is less likely to precipitate parkinsonism,4 such as quetiapine, iloperidone, or clozapine.11 If dose reduction is not possible, and the patient’s symptoms are severe, pharmacologic management is indicated. The APA Guideline recommends amantadine as a first-line agent because it is associated with fewer peripheral adverse effects and less impairment in cognition compared with benztropine.4 In a small (N = 60) doubleblind crossover trial, Gelenberg et al20 found benztropine 4 mg/d—but not amantadine 200 mg/d—impaired free recall and perception of time, and participants’ perception of their own memory impairment was significantly greater with benztropine. Amantadine has also been compared to biperiden, a relatively selective M1 muscarinic receptor muscarinic agent. In a separate double-blind crossover study of 26 patients with chronic schizophrenia, Silver and Geraisy21 found that compared to amantadine, biperiden was associated with worse memory performance. The recommended starting dose of amantadine for parkinsonism is 100 mg in the morning, increased to 100 mg twice a day and titrated to a maximum daily dose of 300 mg/d in divided doses.4
Continue to: Alternatives for treating drug-induced akathisia...
Alternatives for treating drug-induced akathisia
Akathisia remains a relatively common adverse effect of SGAs, and the profound physical distress and impaired functioning caused by akathisia necessitates pharmacologic treatment. Despite frequent use in practice for presumed benefit in akathisia, benztropine is not effective for the treatment of akathisia and the APA Guideline recommends that long-term management should begin with an antipsychotic dose reduction, followed by a switch to an agent with less propensity to incite akathisia.4 Acute manifestations of akathisia must be treated, and mirtazapine, propranolol, or clonazepam may be considered as alternatives.4 Mirtazapine is dosed 7.5 mg to 10 mg nightly for akathisia, though it should be used in caution in patients at risk for mania.4 Mirtazapine’s potent 5-HT2A blockade at low doses may contribute to its utility in treating akathisia.2 Propranolol, a nonselective lipophilic beta-adrenergic antagonist, also has demonstrated efficacy in managing akathisia, with recommended dosing of 40 mg to 80 mg twice daily.2 Benzodiazepines such as clonazepam require judicious use for akathisia because they may also precipitate or exacerbate cognitive impairment.4
Alternatives for treating TD
As mentioned above, benztropine is not recommended for the treatment of TD.1 The Box4,22,23 outlines potential treatment options for TD.
Box
Monitoring is the first step in the prevention of tardive dyskinesia (TD). The American Psychiatric Association’s (APA) Practice Guideline for the Treatment of Patients with Schizophrenia recommends patients receiving first-generation antipsychotics (FGAs) be monitored every 6 months, those prescribed second-generation antipsychotics (SGAs) be monitored every 12 months, and twice as frequent monitoring for geriatric patients and those who developed involuntary movements rapidly after starting an antipsychotic.4
The APA Guideline recommends decreasing or gradually tapering antipsychotics as another strategy for preventing TD.4 However, these recommendations should be weighed against the risk of short-term antipsychotic withdrawal. Withdrawal of D2 antagonists is associated with worsening of dyskinesias or withdrawal dyskinesia and psychotic decompensation.22
Current treatment recommendations give preference to the importance of preventing development of TD by tapering to the lowest dose of antipsychotic needed to control symptoms for the shortest duration possible.22 Thereafter, if treatment intervention is needed, consideration should be given to the following pharmacological interventions in order from highest level of recommendation (Grade A) to lowest (Grade C):
A: vesicular monoamine transporter-2 inhibitors deutetrabenazine and valbenazine
B: clonazepam, ginkgo biloba
C: amantadine, tetrabenazine, and globus pallidus interna deep brain stimulation.22
There is insufficient evidence to support or refute withdrawing causative agents or switching from FGAs to SGAs to treat TD.22 Furthermore, for many patients with schizophrenia, a gradual discontinuation of their antipsychotic must be weighed against the risk of relapse.23
Valbenazine and deutetrabenazine have been demonstrated to be efficacious and are FDA-approved for managing TD. The initial dose of valbenazine is 40 mg/d. Common adverse effects include somnolence and fatigue/ sedation. Valbenazine should be avoided in patients with QT prolongation or arrhythmias. Deutetrabenazine has less impact on the cytochrome P450 2D6 enzyme and therefore does not require genotyping as would be the case for patients who are receiving >50 mg/d of tetrabenazine. The starting dose of deutetrabenazine is 6 mg/d. Adverse effects include depression, suicidality, neuroleptic malignant syndrome, parkinsonism, and QT prolongation. Deutetrabenazine is contraindicated in patients who are suicidal or have untreated depression, hepatic impairment, or concomitant use of monoamine oxidase inhibitors.22 Deutetrabenazine is an isomer of tetrabenazine; however, evidence supporting the parent compound suggests limited use due to increased risk of adverse effects compared with valbenazine and deutetrabenazine.23 Tetrabenazine may be considered as an adjunctive treatment or used as a single agent if valbenazine or deutetrabenazine are not accessible.22
Discontinuing benztropine
Benztropine is recommended as a firstline agent for the management of acute dystonia, and it may be used temporarily for drug-induced parkinsonism, but it is not recommended to prevent EPS or TD. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic agent such as benztropine. Based on their review of earlier studies, Desmarais et al5 suggest a gradual 3-month discontinuation of benztropine. Multiple studies have demonstrated an ability to taper anticholinergics in days to months.4 However, gradual discontinuation is advisable to avoid cholinergic rebound and the reemergence of EPS, and to decrease the risk of neuroleptic malignant syndrome associated with sudden discontinuation.5 One suggested taper regimen is a decrease of 0.5 mg benztropine every week. Amantadine may be considered if parkinsonism is noted during the taper. Patients on benztropine may develop rebound symptoms, such as vivid dreams/nightmares; if this occurs, the taper rate can be slowed to a decrease of 0.5 mg every 2 weeks.4
Continue to: First do no harm...
First do no harm
Psychiatrists commonly prescribe benztropine to prevent EPS and TD, but available literature does not support the efficacy of benztropine for mitigating drug-induced parkinsonism, and studies report benztropine may significantly worsen cognitive processes and exacerbate TD.16 In addition, benztropine misuse has been correlated with euphoria and psychosis.16 More than 3 decades ago, the World Health Organization Heads of Centres Collaborating in WHO-Coordinated Studies on Biological Aspects of Mental Illness issued a consensus statement24 discouraging the prophylactic use of anticholinergics for patients receiving antipsychotics, yet we still see patients on an indefinite regimen of benztropine.
As clinicians, our goals should be to optimize a patient’s functioning and quality of life, and to use the lowest dose of medication along with the fewest medications necessary to avoid adverse effects such as EPS. Benztropine is recommended as a first-line agent for the management of acute dystonia, but its continued or indefinite use to prevent antipsychotic-induced adverse effects is not recommended. While all pharmacologic interventions carry a risk of adverse effects, weighing the risk of those effects against the clinical benefits is the prerogative of a skilled clinician. Benztropine and other anticholinergics prescribed for prophylactic purposes have numerous adverse effects, limited clinical utility, and a deleterious effect on quality of life. Furthermore, benztropine prophylaxis of drug-induced parkinsonism does not seem to be warranted, and the risks do not seem to outweigh the harm benztropine may cause, with the possible exception of “prophylactic” treatment of dystonia that is discontinued in a few days, as some researchers have suggested.6-8 The preventive value of benztropine has not been demonstrated. It is time we took inventory of medications that might cause more harm than good, rely on current treatment guidelines instead of habit, and use these agents judiciously while considering replacement with novel, safer medications whenever possible.
CASE CONTINUED
The clinical team considers benztropine’s ability to cause cognitive effects, and decides to taper and discontinue it over 1 month. Ms. P is seen in an outpatient clinic within 1 month of discontinuing benztropine. She reports that her difficulty remembering words and details has improved. She also says that she is now able to concentrate on writing and reading. The consulting neurologist also notes improvement. Ms. P continues to report improvement in symptoms over the next 2 months of follow-up, and says that her mood improved and she has less apathy.
Bottom Line
Benztropine is a first-line medication for acute dystonia, but its continued or indefinite use for preventing antipsychotic-induced adverse effects is not recommended. Given the multitude of adverse effects and cognitive impairment noted with anticholinergics, tapering should be considered for patients receiving an anticholinergic medication such as benztropine.
1. Cogentin [package insert]. McPherson, KS: Lundbeck Inc; 2013.
2. Poyurovsky M, Weizman A. Treatment of antipsychoticrelated akathisia revisited. J Clin Psychopharmacol. 2015; 35(6):711-714.
3. Salem H, Nagpal C, Pigott T, et al. Revisiting antipsychoticinduced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
4. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia. 3rd ed. American Psychiatric Association; 2021.
5. Desmarais JE, Beauclair L, Margolese HC. Anticholinergics in the era of atypical antipsychotics: short-term or long-term treatment? J Psychopharmacol. 2012;26(9):1167-1174.
6. Boyer WF, Bakalar NH, Lake CR. Anticholinergic prophylaxis of acute haloperidol-induced acute dystonic reactions. J Clin Psychopharmacol. 1987;7(3):164-166.
7. Winslow RS, Stillner V, Coons DJ, et al. Prevention of acute dystonic reactions in patients beginning high-potency neuroleptics. Am J Psychiatry. 1986;143(6):706-710.
8. Stern TA, Anderson WH. Benztropine prophylaxis of dystonic reactions. Psychopharmacology (Berl). 1979; 61(3):261-262.
9. Bergman H, Soares‐Weiser K. Anticholinergic medication for antipsychotic‐induced tardive dyskinesia. Cochrane Database Syst Rev. 2018;1(1):CD000204. doi:10.1002/ 14651858.CD000204.pub2
10. Howrie DL, Rowley AH, Krenzelok EP. Benztropineinduced acute dystonic reaction. Ann Emerg Med. 1986;15(5):594-596.
11. Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs. tardive dyskinesia--key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2): 233-248.
12. Wijegunaratne H, Qazi H, Koola M. Chronic and bedtime use of benztropine with antipsychotics: is it necessary? Schizophr Res. 2014;153(1-3):248-249.
13. Möller HJ. The relevance of negative symptoms in schizophrenia and how to treat them with psychopharmaceuticals? Psychiatr Danub. 2016;28(4):435-440.
14. Baker LA, Cheng LY, Amara IB. The withdrawal of benztropine mesylate in chronic schizophrenic patients. Br J Psychiatry. 1983;143:584-590.
15. Joshi YB, Thomas ML, Braff DL, et al. Anticholinergic medication burden-associated cognitive impairment in schizophrenia. Am J Psychiatry. 2021;178(9):838-847.
16. Desmarais JE, Beauclair E, Annable L, et al. Effects of discontinuing anticholinergic treatment on movement disorders, cognition and psychopathology in patients with schizophrenia. Ther Adv Psychopharmacol. 2014;4(6): 257-267.
17. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9): 1055-1062.
18. American Geriatrics Society 2019 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
19. Esang M, Person US, Izekor OO, et al. An unlikely case of benztropine misuse in an elderly schizophrenic. Cureus. 2021;13(2):e13434. doi:10.7759/cureus.13434
20. Gelenberg AJ, Van Putten T, Lavori PW, et al. Anticholinergic effects on memory: benztropine versus amantadine. J Clin Psychopharmacol. 1989;9(3):180-185.
21. Silver H, Geraisy N. Effects of biperiden and amantadine on memory in medicated chronic schizophrenic patients. A double-blind cross-over study. Br J Psychiatry. 1995; 166(2):241-243.
22. Bhidayasiri R, Jitkritsadakul O, Friedman J, et al. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67-75.
23. Ricciardi L, Pringsheim T, Barnes TRE, et al. Treatment recommendations for tardive dyskinesia. Canadian J Psychiatry. 2019;64(6):388-399.
24. Prophylactic use of anticholinergics in patients on long-term neuroleptic treatment. A consensus statement. World Health Organization heads of centres collaborating in WHO coordinated studies on biological aspects of mental illness. Br J Psychiatry. 1990;156:412.
1. Cogentin [package insert]. McPherson, KS: Lundbeck Inc; 2013.
2. Poyurovsky M, Weizman A. Treatment of antipsychoticrelated akathisia revisited. J Clin Psychopharmacol. 2015; 35(6):711-714.
3. Salem H, Nagpal C, Pigott T, et al. Revisiting antipsychoticinduced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
4. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia. 3rd ed. American Psychiatric Association; 2021.
5. Desmarais JE, Beauclair L, Margolese HC. Anticholinergics in the era of atypical antipsychotics: short-term or long-term treatment? J Psychopharmacol. 2012;26(9):1167-1174.
6. Boyer WF, Bakalar NH, Lake CR. Anticholinergic prophylaxis of acute haloperidol-induced acute dystonic reactions. J Clin Psychopharmacol. 1987;7(3):164-166.
7. Winslow RS, Stillner V, Coons DJ, et al. Prevention of acute dystonic reactions in patients beginning high-potency neuroleptics. Am J Psychiatry. 1986;143(6):706-710.
8. Stern TA, Anderson WH. Benztropine prophylaxis of dystonic reactions. Psychopharmacology (Berl). 1979; 61(3):261-262.
9. Bergman H, Soares‐Weiser K. Anticholinergic medication for antipsychotic‐induced tardive dyskinesia. Cochrane Database Syst Rev. 2018;1(1):CD000204. doi:10.1002/ 14651858.CD000204.pub2
10. Howrie DL, Rowley AH, Krenzelok EP. Benztropineinduced acute dystonic reaction. Ann Emerg Med. 1986;15(5):594-596.
11. Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs. tardive dyskinesia--key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2): 233-248.
12. Wijegunaratne H, Qazi H, Koola M. Chronic and bedtime use of benztropine with antipsychotics: is it necessary? Schizophr Res. 2014;153(1-3):248-249.
13. Möller HJ. The relevance of negative symptoms in schizophrenia and how to treat them with psychopharmaceuticals? Psychiatr Danub. 2016;28(4):435-440.
14. Baker LA, Cheng LY, Amara IB. The withdrawal of benztropine mesylate in chronic schizophrenic patients. Br J Psychiatry. 1983;143:584-590.
15. Joshi YB, Thomas ML, Braff DL, et al. Anticholinergic medication burden-associated cognitive impairment in schizophrenia. Am J Psychiatry. 2021;178(9):838-847.
16. Desmarais JE, Beauclair E, Annable L, et al. Effects of discontinuing anticholinergic treatment on movement disorders, cognition and psychopathology in patients with schizophrenia. Ther Adv Psychopharmacol. 2014;4(6): 257-267.
17. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9): 1055-1062.
18. American Geriatrics Society 2019 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
19. Esang M, Person US, Izekor OO, et al. An unlikely case of benztropine misuse in an elderly schizophrenic. Cureus. 2021;13(2):e13434. doi:10.7759/cureus.13434
20. Gelenberg AJ, Van Putten T, Lavori PW, et al. Anticholinergic effects on memory: benztropine versus amantadine. J Clin Psychopharmacol. 1989;9(3):180-185.
21. Silver H, Geraisy N. Effects of biperiden and amantadine on memory in medicated chronic schizophrenic patients. A double-blind cross-over study. Br J Psychiatry. 1995; 166(2):241-243.
22. Bhidayasiri R, Jitkritsadakul O, Friedman J, et al. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67-75.
23. Ricciardi L, Pringsheim T, Barnes TRE, et al. Treatment recommendations for tardive dyskinesia. Canadian J Psychiatry. 2019;64(6):388-399.
24. Prophylactic use of anticholinergics in patients on long-term neuroleptic treatment. A consensus statement. World Health Organization heads of centres collaborating in WHO coordinated studies on biological aspects of mental illness. Br J Psychiatry. 1990;156:412.
Should psychiatrists prescribe nonpsychotropic medications?
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.
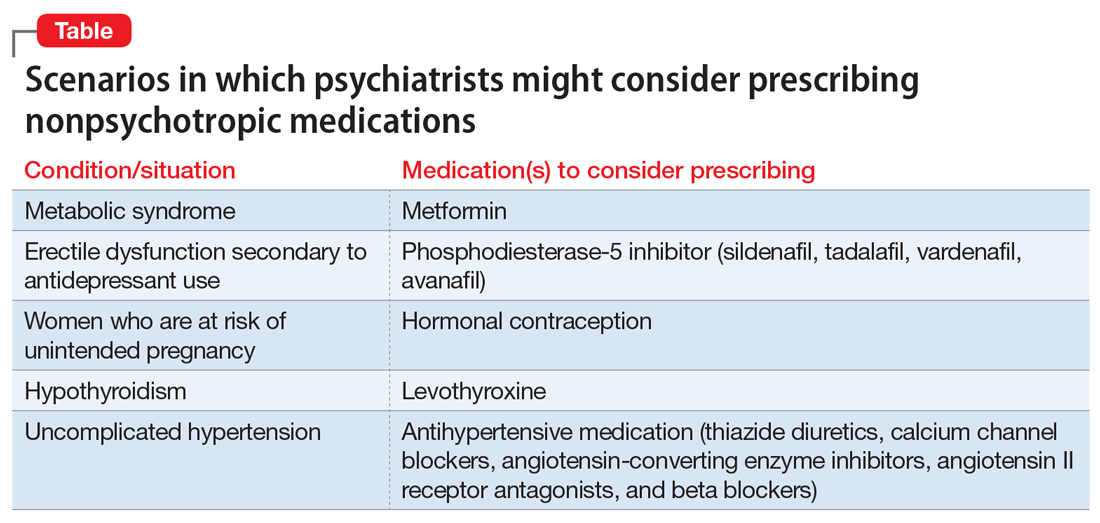
We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.

We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.

We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
Benzodiazepines for anxious depression
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
Psychosis in treated neurosyphilis: Is now the time to stop his antipsychotic?
CASE Hallucinations, impaired memory
Mr. C is a 61-year-old African American man who visits the outpatient clinic for management of antipsychotic therapy for psychosis and depression. His most recent inpatient psychiatric hospitalization for auditory and visual hallucinations, paranoia, and agitation was more than 10 years ago. He has been taking chlorpromazine, 100 mg/d, for 11 years. Mr. C reports that he has had no psychotic symptoms in the past 3 years; he continues taking chlorpromazine, he says, because it helps him sleep.
How would you proceed with Mr. C’s care?
a) continue chlorpromazine because he has been symptom free
b) consider tapering and discontinuing chlorpromazine
c) obtain a more detailed history from Mr. C and perform additional tests
HISTORY Validation of diagnosis
Mr. C reports that, at age 48, he started hearing babies crying and started seeing dead infants crawling out of the incinerator at the hospital where he worked. He denies any psychiatric symptoms before that time. He stopped working 10 years ago because of his psychiatric symptoms and decline in cognition.
Subsequently, Mr. C had 3 inpatient psychiatric hospitalizations for auditory hallucinations; chlorpromazine, 100 mg/d, was prescribed for psychosis. Later efforts to discontinue chlorpromazine resulted in relapse of psychotic symptoms. Mr. C has no family history of psychiatric illness.
Mr. C’s medical history is significant for aortic regurgitation, congestive cardiac failure, hypertension, and left-sided sensorineural hearing loss. He has a history of cocaine abuse from age 21 to 45, but denies using any other substances, including alcohol and nicotine.
Urine toxicology and routine blood tests are within normal limits. The QTc is slightly prolonged over the past 2 years, recording 512, 520, and 505 milliseconds on serial electrocardiograms.
Mr. C is able to perform simple abstractions. He has a goal-directed thought process, devoid of any preoccupation, paranoia, and perceptual abnormalities. Cognitive screening reveals significant impairment of memory, registration, calculation, attention, and visuospatial skills.
Careful review of Mr. C’s history and medical records reveals a diagnosis of syphilis at age 48 after unprotected sexual intercourse. He recalls that he had a solitary genital lesion, which resolved over a few weeks. He then developed a slightly itchy, non-tender macular rash over his upper back, which he did not report to a physician. After a few months, he developed unsteady gait, blurry vision, and weakness of limbs, and had to crawl to the hospital. There, he was given a diagnosis of neurosyphilis. He also developed left-sided hearing loss during that time.
Mr. C was treated with aqueous penicillin G benzathine, 4 million units IV for 2 weeks. No follow-up cerebrospinal fluid (CSF) examination was documented after antibiotic treatment. He developed auditory and visual hallucinations and paranoia a few months after completing penicillin treatment. During the following year, he had 3 inpatient psychiatric hospitalizations for psychosis, agitation, and depressed mood.
How would you treat a patient with a history of neurosyphilis who presents with psychosis years after diagnosis?
a) repeat antibiotic treatment and stop the antipsychotic
b) repeat antibiotic treatment and continue the antipsychotic
c) attempt to discontinue the antipsychotic
d) continue the antipsychotic
The authors’ observations
Mr. C’s psychotic symptoms seem to be temporally related to his diagnosis of neurosyphilis at age 48. He and his family members deny that Mr. C had any history of psychosis or depression before the neurosyphilis diagnosis. All inpatient psychiatric hospitalizations were within 1 year of the neurosyphilis diagnosis.
Mr. C has been on a low dosage of chlorpromazine, which has significant antihistaminic action. Chlorpromazine also is known to cause QTc prolongation, especially in patients with heart disease.
TREATMENT Medication change
A serum rapid plasma reagin test is non-reactive, but Treponema pallidum particle agglutination is positive. MRI shows moderate atrophy suggestive of diffuse small-vessel disease.
Mr. C’s psychotic symptoms are considered to be sequelae of neurosyphilis, based on (1) the presence of positive antibody tests, (2) residual neurologic deficits, (3) other suggestive sequelae (aortic regurgitation, sensorineural deafness), and (4) age-inappropriate gradual cognitive decline in the absence of other psychiatric history.
Because we are concerned about the prolonged QTc, chlorpromazine is discontinued. Haloperidol, 5 mg at bedtime, is started. The neurology team does not recommend antibiotic treatment because symptoms have been stable for years. Mr. C refuses a lumbar puncture.
Mr. C returns to the outpatient clinic monthly. He is psychiatrically stable without any worsening of psychosis. Cognitive impairment remains stable over the next 6 months. Haloperidol is tapered to 2 mg at bedtime 6 months after initial evaluation. Mr. C remains psychiatrically stable on subsequent follow-up visits.
The authors’ observations
Mr. C’s psychotic symptoms persisted after standard antibiotic treatment of neurosyphilis and lapsed when he stopped taking antipsychotic medication 10 years after the initial treatment of neurosyphilis. He carried a diagnosis of schizophrenia for many years, even though his psychotic symptoms were atypical for the presentation of schizophrenia.
It is important to understand the natural course of syphilis, its implication on psychiatric symptom production, and long-term psychiatric prognosis.
Syphilis is a sexually transmitted infectious disease caused by T pallidum, a spirochete, that has varied clinical presentations. Osler called syphilis the “great imitator” for its array of system involvement, ranging from asymptomatic infection and afferent pupillary defect to depression, psychosis, and dementia. With wide use of penicillin, the rate of neurosyphilis declined steadily during the mid 1990s. By 1997, the overall rate reached its lowest point in the United States; in 1999 the Centers for Disease Control and Prevention released a national plan to eliminate syphilis.1 By 2004, however, prevalence had increased to 4.7/100,000. It is thought that this increase is mainly associated with substance use (especially crack cocaine) and HIV co-infection. Most cases were distributed in economically depressed geographical areas.
Psychiatric patients are at higher risk of acquiring the infection because of substance use, lack of education on safer sex practices, and impulsive behavior.
Stages of syphilis
Syphilis does not follow a step-wise progression. One-third of cases progress to the tertiary stage, even many years after initial infection, without adequate treatment.2
Almost 10% syphilis cases present with neurologic symptoms,3 and neurologic involvement can occur at any stage of disease progression. The most common symptoms of syphilis are presented in Table 1.
A range of psychiatric symptoms have been reported among patients with syphilis, including anhedonia, suicidality, mania, grandiosity, persecutory delusions, auditory and visual hallucinations, paranoia, and cognitive impairment. The incidence of psychiatric symptoms is not clearly described in literature.
Diagnosis and treatment
Neurosyphilis, at any disease stage, should be suspected if a patient:
- exhibits suggestive symptoms
- does not respond to antibiotic treatment
- has late latent syphilis
- is immunocompromised.
Lumbar puncture and examination of CSF is the most useful diagnostic test. Dark field microscopy to reveal T pallidum is definitive, but only is applicable during the primary stage. The role of dark field microscopy of the CSF sample to diagnose neurologic involvement has not been established. Tests and treatment protocol are described in Table 2.2-5
Treatment of psychiatric symptoms of neurosyphilis
There are inconsistent and limited data about the prevalence of psychiatric symptoms in neurosyphilis. A retrospective study6 of 161 patients with neurosyphilis in South Africa reported that 50.9% exhibited a complex spectrum of symptoms that included delirium and dementia. Of treated patients, 17% continued to have residual symptoms during follow-up.
A review of the literature did not reveal any widely accepted guideline for screening for neurosyphilis in general psychiatry practice or a treatment protocol for psychiatric symptoms. This lack of guidance could be attributed to the rarity of the disease, cost-benefit analyses, and low specificity of antibody tests. In the literature, syphilis screening is recommended as a routine protocol when evaluating and treating dementia.7
In most studies, a diagnosis of neurosyphilis was confirmed by CSF examination; however, many of these studies did not report a specific follow-up CSF examination protocol. Most of these patients were treated with an antipsychotic with partial improvement in symptoms, even after standard antibiotic protocol.8
First- and second-generation antipsychotics and mood stabilizers have been shown to be useful in the acute treatment of psychosis and agitation.8 In few instances, the psychotropic medication was continued beyond several months and the patient was placed in a long-term care facility. Psychiatric symptoms persisted for many years with or without residual neurosyphilis symptoms, possibly because of permanent neuronal loss.
Clinical considerations
It often is difficult to distinguish a preexisting psychiatric disorder made worse by neurosyphilis from a secondary psychiatric disorder caused by neurosyphilis. The 2 might coexist, or psychiatric symptoms could be wrongly attributed to schizophrenia because of a lack of careful clinical evaluation.
Often, the follow-up diagnostic protocol for neurosyphilis is not followed; as a result, the need for re-treatment remains unclear. Rarity of the disease makes it difficult to perform a prospective, randomized study to determine the duration and effect of long-term psychiatric treatment.
Close follow-up and consideration of the risk vs benefit of psychotropic medication is key. Because there are no proven guidelines for the length of treatment with antipsychotics, it is prudent to minimize their use until psychiatrically indicated. Side effects, such as (in Mr. C’s case) changes in the QTc interval, should warrant consideration of discontinuing psychotropic medication. Interdisciplinary collaboration with neurology and infectious disease will improve the overall outcome of a complex clinical presentation.
1. Centers for Disease Control and Prevention. National plan to eliminate syphilis from the United States. http://www.cdc.gov/stopsyphilis/plan.htm. Updated December 7, 2007. Accessed July 7, 2016.
2. Friedrich F, Aigner M, Fearns N, et al. Psychosis in neurosyphilis—clinical aspects and implications. Psychopathology. 2014;47(1):3-9.
3. Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68(2):283-290.
4. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005-1009.
5. Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. Syphilis. http://www.cdc.gov/std/tg2015/syphilis.htm. Updated June 4, 2015. Accessed July 13, 2016.
6. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75(12):1727-1730.
7. Scott KR, Barrett AM. Dementia syndrome: evaluation and treatment. Expert Rev Neurother. 2007;7(4):407-422.
8. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48(5):440-445.
CASE Hallucinations, impaired memory
Mr. C is a 61-year-old African American man who visits the outpatient clinic for management of antipsychotic therapy for psychosis and depression. His most recent inpatient psychiatric hospitalization for auditory and visual hallucinations, paranoia, and agitation was more than 10 years ago. He has been taking chlorpromazine, 100 mg/d, for 11 years. Mr. C reports that he has had no psychotic symptoms in the past 3 years; he continues taking chlorpromazine, he says, because it helps him sleep.
How would you proceed with Mr. C’s care?
a) continue chlorpromazine because he has been symptom free
b) consider tapering and discontinuing chlorpromazine
c) obtain a more detailed history from Mr. C and perform additional tests
HISTORY Validation of diagnosis
Mr. C reports that, at age 48, he started hearing babies crying and started seeing dead infants crawling out of the incinerator at the hospital where he worked. He denies any psychiatric symptoms before that time. He stopped working 10 years ago because of his psychiatric symptoms and decline in cognition.
Subsequently, Mr. C had 3 inpatient psychiatric hospitalizations for auditory hallucinations; chlorpromazine, 100 mg/d, was prescribed for psychosis. Later efforts to discontinue chlorpromazine resulted in relapse of psychotic symptoms. Mr. C has no family history of psychiatric illness.
Mr. C’s medical history is significant for aortic regurgitation, congestive cardiac failure, hypertension, and left-sided sensorineural hearing loss. He has a history of cocaine abuse from age 21 to 45, but denies using any other substances, including alcohol and nicotine.
Urine toxicology and routine blood tests are within normal limits. The QTc is slightly prolonged over the past 2 years, recording 512, 520, and 505 milliseconds on serial electrocardiograms.
Mr. C is able to perform simple abstractions. He has a goal-directed thought process, devoid of any preoccupation, paranoia, and perceptual abnormalities. Cognitive screening reveals significant impairment of memory, registration, calculation, attention, and visuospatial skills.
Careful review of Mr. C’s history and medical records reveals a diagnosis of syphilis at age 48 after unprotected sexual intercourse. He recalls that he had a solitary genital lesion, which resolved over a few weeks. He then developed a slightly itchy, non-tender macular rash over his upper back, which he did not report to a physician. After a few months, he developed unsteady gait, blurry vision, and weakness of limbs, and had to crawl to the hospital. There, he was given a diagnosis of neurosyphilis. He also developed left-sided hearing loss during that time.
Mr. C was treated with aqueous penicillin G benzathine, 4 million units IV for 2 weeks. No follow-up cerebrospinal fluid (CSF) examination was documented after antibiotic treatment. He developed auditory and visual hallucinations and paranoia a few months after completing penicillin treatment. During the following year, he had 3 inpatient psychiatric hospitalizations for psychosis, agitation, and depressed mood.
How would you treat a patient with a history of neurosyphilis who presents with psychosis years after diagnosis?
a) repeat antibiotic treatment and stop the antipsychotic
b) repeat antibiotic treatment and continue the antipsychotic
c) attempt to discontinue the antipsychotic
d) continue the antipsychotic
The authors’ observations
Mr. C’s psychotic symptoms seem to be temporally related to his diagnosis of neurosyphilis at age 48. He and his family members deny that Mr. C had any history of psychosis or depression before the neurosyphilis diagnosis. All inpatient psychiatric hospitalizations were within 1 year of the neurosyphilis diagnosis.
Mr. C has been on a low dosage of chlorpromazine, which has significant antihistaminic action. Chlorpromazine also is known to cause QTc prolongation, especially in patients with heart disease.
TREATMENT Medication change
A serum rapid plasma reagin test is non-reactive, but Treponema pallidum particle agglutination is positive. MRI shows moderate atrophy suggestive of diffuse small-vessel disease.
Mr. C’s psychotic symptoms are considered to be sequelae of neurosyphilis, based on (1) the presence of positive antibody tests, (2) residual neurologic deficits, (3) other suggestive sequelae (aortic regurgitation, sensorineural deafness), and (4) age-inappropriate gradual cognitive decline in the absence of other psychiatric history.
Because we are concerned about the prolonged QTc, chlorpromazine is discontinued. Haloperidol, 5 mg at bedtime, is started. The neurology team does not recommend antibiotic treatment because symptoms have been stable for years. Mr. C refuses a lumbar puncture.
Mr. C returns to the outpatient clinic monthly. He is psychiatrically stable without any worsening of psychosis. Cognitive impairment remains stable over the next 6 months. Haloperidol is tapered to 2 mg at bedtime 6 months after initial evaluation. Mr. C remains psychiatrically stable on subsequent follow-up visits.
The authors’ observations
Mr. C’s psychotic symptoms persisted after standard antibiotic treatment of neurosyphilis and lapsed when he stopped taking antipsychotic medication 10 years after the initial treatment of neurosyphilis. He carried a diagnosis of schizophrenia for many years, even though his psychotic symptoms were atypical for the presentation of schizophrenia.
It is important to understand the natural course of syphilis, its implication on psychiatric symptom production, and long-term psychiatric prognosis.
Syphilis is a sexually transmitted infectious disease caused by T pallidum, a spirochete, that has varied clinical presentations. Osler called syphilis the “great imitator” for its array of system involvement, ranging from asymptomatic infection and afferent pupillary defect to depression, psychosis, and dementia. With wide use of penicillin, the rate of neurosyphilis declined steadily during the mid 1990s. By 1997, the overall rate reached its lowest point in the United States; in 1999 the Centers for Disease Control and Prevention released a national plan to eliminate syphilis.1 By 2004, however, prevalence had increased to 4.7/100,000. It is thought that this increase is mainly associated with substance use (especially crack cocaine) and HIV co-infection. Most cases were distributed in economically depressed geographical areas.
Psychiatric patients are at higher risk of acquiring the infection because of substance use, lack of education on safer sex practices, and impulsive behavior.
Stages of syphilis
Syphilis does not follow a step-wise progression. One-third of cases progress to the tertiary stage, even many years after initial infection, without adequate treatment.2
Almost 10% syphilis cases present with neurologic symptoms,3 and neurologic involvement can occur at any stage of disease progression. The most common symptoms of syphilis are presented in Table 1.
A range of psychiatric symptoms have been reported among patients with syphilis, including anhedonia, suicidality, mania, grandiosity, persecutory delusions, auditory and visual hallucinations, paranoia, and cognitive impairment. The incidence of psychiatric symptoms is not clearly described in literature.
Diagnosis and treatment
Neurosyphilis, at any disease stage, should be suspected if a patient:
- exhibits suggestive symptoms
- does not respond to antibiotic treatment
- has late latent syphilis
- is immunocompromised.
Lumbar puncture and examination of CSF is the most useful diagnostic test. Dark field microscopy to reveal T pallidum is definitive, but only is applicable during the primary stage. The role of dark field microscopy of the CSF sample to diagnose neurologic involvement has not been established. Tests and treatment protocol are described in Table 2.2-5
Treatment of psychiatric symptoms of neurosyphilis
There are inconsistent and limited data about the prevalence of psychiatric symptoms in neurosyphilis. A retrospective study6 of 161 patients with neurosyphilis in South Africa reported that 50.9% exhibited a complex spectrum of symptoms that included delirium and dementia. Of treated patients, 17% continued to have residual symptoms during follow-up.
A review of the literature did not reveal any widely accepted guideline for screening for neurosyphilis in general psychiatry practice or a treatment protocol for psychiatric symptoms. This lack of guidance could be attributed to the rarity of the disease, cost-benefit analyses, and low specificity of antibody tests. In the literature, syphilis screening is recommended as a routine protocol when evaluating and treating dementia.7
In most studies, a diagnosis of neurosyphilis was confirmed by CSF examination; however, many of these studies did not report a specific follow-up CSF examination protocol. Most of these patients were treated with an antipsychotic with partial improvement in symptoms, even after standard antibiotic protocol.8
First- and second-generation antipsychotics and mood stabilizers have been shown to be useful in the acute treatment of psychosis and agitation.8 In few instances, the psychotropic medication was continued beyond several months and the patient was placed in a long-term care facility. Psychiatric symptoms persisted for many years with or without residual neurosyphilis symptoms, possibly because of permanent neuronal loss.
Clinical considerations
It often is difficult to distinguish a preexisting psychiatric disorder made worse by neurosyphilis from a secondary psychiatric disorder caused by neurosyphilis. The 2 might coexist, or psychiatric symptoms could be wrongly attributed to schizophrenia because of a lack of careful clinical evaluation.
Often, the follow-up diagnostic protocol for neurosyphilis is not followed; as a result, the need for re-treatment remains unclear. Rarity of the disease makes it difficult to perform a prospective, randomized study to determine the duration and effect of long-term psychiatric treatment.
Close follow-up and consideration of the risk vs benefit of psychotropic medication is key. Because there are no proven guidelines for the length of treatment with antipsychotics, it is prudent to minimize their use until psychiatrically indicated. Side effects, such as (in Mr. C’s case) changes in the QTc interval, should warrant consideration of discontinuing psychotropic medication. Interdisciplinary collaboration with neurology and infectious disease will improve the overall outcome of a complex clinical presentation.
CASE Hallucinations, impaired memory
Mr. C is a 61-year-old African American man who visits the outpatient clinic for management of antipsychotic therapy for psychosis and depression. His most recent inpatient psychiatric hospitalization for auditory and visual hallucinations, paranoia, and agitation was more than 10 years ago. He has been taking chlorpromazine, 100 mg/d, for 11 years. Mr. C reports that he has had no psychotic symptoms in the past 3 years; he continues taking chlorpromazine, he says, because it helps him sleep.
How would you proceed with Mr. C’s care?
a) continue chlorpromazine because he has been symptom free
b) consider tapering and discontinuing chlorpromazine
c) obtain a more detailed history from Mr. C and perform additional tests
HISTORY Validation of diagnosis
Mr. C reports that, at age 48, he started hearing babies crying and started seeing dead infants crawling out of the incinerator at the hospital where he worked. He denies any psychiatric symptoms before that time. He stopped working 10 years ago because of his psychiatric symptoms and decline in cognition.
Subsequently, Mr. C had 3 inpatient psychiatric hospitalizations for auditory hallucinations; chlorpromazine, 100 mg/d, was prescribed for psychosis. Later efforts to discontinue chlorpromazine resulted in relapse of psychotic symptoms. Mr. C has no family history of psychiatric illness.
Mr. C’s medical history is significant for aortic regurgitation, congestive cardiac failure, hypertension, and left-sided sensorineural hearing loss. He has a history of cocaine abuse from age 21 to 45, but denies using any other substances, including alcohol and nicotine.
Urine toxicology and routine blood tests are within normal limits. The QTc is slightly prolonged over the past 2 years, recording 512, 520, and 505 milliseconds on serial electrocardiograms.
Mr. C is able to perform simple abstractions. He has a goal-directed thought process, devoid of any preoccupation, paranoia, and perceptual abnormalities. Cognitive screening reveals significant impairment of memory, registration, calculation, attention, and visuospatial skills.
Careful review of Mr. C’s history and medical records reveals a diagnosis of syphilis at age 48 after unprotected sexual intercourse. He recalls that he had a solitary genital lesion, which resolved over a few weeks. He then developed a slightly itchy, non-tender macular rash over his upper back, which he did not report to a physician. After a few months, he developed unsteady gait, blurry vision, and weakness of limbs, and had to crawl to the hospital. There, he was given a diagnosis of neurosyphilis. He also developed left-sided hearing loss during that time.
Mr. C was treated with aqueous penicillin G benzathine, 4 million units IV for 2 weeks. No follow-up cerebrospinal fluid (CSF) examination was documented after antibiotic treatment. He developed auditory and visual hallucinations and paranoia a few months after completing penicillin treatment. During the following year, he had 3 inpatient psychiatric hospitalizations for psychosis, agitation, and depressed mood.
How would you treat a patient with a history of neurosyphilis who presents with psychosis years after diagnosis?
a) repeat antibiotic treatment and stop the antipsychotic
b) repeat antibiotic treatment and continue the antipsychotic
c) attempt to discontinue the antipsychotic
d) continue the antipsychotic
The authors’ observations
Mr. C’s psychotic symptoms seem to be temporally related to his diagnosis of neurosyphilis at age 48. He and his family members deny that Mr. C had any history of psychosis or depression before the neurosyphilis diagnosis. All inpatient psychiatric hospitalizations were within 1 year of the neurosyphilis diagnosis.
Mr. C has been on a low dosage of chlorpromazine, which has significant antihistaminic action. Chlorpromazine also is known to cause QTc prolongation, especially in patients with heart disease.
TREATMENT Medication change
A serum rapid plasma reagin test is non-reactive, but Treponema pallidum particle agglutination is positive. MRI shows moderate atrophy suggestive of diffuse small-vessel disease.
Mr. C’s psychotic symptoms are considered to be sequelae of neurosyphilis, based on (1) the presence of positive antibody tests, (2) residual neurologic deficits, (3) other suggestive sequelae (aortic regurgitation, sensorineural deafness), and (4) age-inappropriate gradual cognitive decline in the absence of other psychiatric history.
Because we are concerned about the prolonged QTc, chlorpromazine is discontinued. Haloperidol, 5 mg at bedtime, is started. The neurology team does not recommend antibiotic treatment because symptoms have been stable for years. Mr. C refuses a lumbar puncture.
Mr. C returns to the outpatient clinic monthly. He is psychiatrically stable without any worsening of psychosis. Cognitive impairment remains stable over the next 6 months. Haloperidol is tapered to 2 mg at bedtime 6 months after initial evaluation. Mr. C remains psychiatrically stable on subsequent follow-up visits.
The authors’ observations
Mr. C’s psychotic symptoms persisted after standard antibiotic treatment of neurosyphilis and lapsed when he stopped taking antipsychotic medication 10 years after the initial treatment of neurosyphilis. He carried a diagnosis of schizophrenia for many years, even though his psychotic symptoms were atypical for the presentation of schizophrenia.
It is important to understand the natural course of syphilis, its implication on psychiatric symptom production, and long-term psychiatric prognosis.
Syphilis is a sexually transmitted infectious disease caused by T pallidum, a spirochete, that has varied clinical presentations. Osler called syphilis the “great imitator” for its array of system involvement, ranging from asymptomatic infection and afferent pupillary defect to depression, psychosis, and dementia. With wide use of penicillin, the rate of neurosyphilis declined steadily during the mid 1990s. By 1997, the overall rate reached its lowest point in the United States; in 1999 the Centers for Disease Control and Prevention released a national plan to eliminate syphilis.1 By 2004, however, prevalence had increased to 4.7/100,000. It is thought that this increase is mainly associated with substance use (especially crack cocaine) and HIV co-infection. Most cases were distributed in economically depressed geographical areas.
Psychiatric patients are at higher risk of acquiring the infection because of substance use, lack of education on safer sex practices, and impulsive behavior.
Stages of syphilis
Syphilis does not follow a step-wise progression. One-third of cases progress to the tertiary stage, even many years after initial infection, without adequate treatment.2
Almost 10% syphilis cases present with neurologic symptoms,3 and neurologic involvement can occur at any stage of disease progression. The most common symptoms of syphilis are presented in Table 1.
A range of psychiatric symptoms have been reported among patients with syphilis, including anhedonia, suicidality, mania, grandiosity, persecutory delusions, auditory and visual hallucinations, paranoia, and cognitive impairment. The incidence of psychiatric symptoms is not clearly described in literature.
Diagnosis and treatment
Neurosyphilis, at any disease stage, should be suspected if a patient:
- exhibits suggestive symptoms
- does not respond to antibiotic treatment
- has late latent syphilis
- is immunocompromised.
Lumbar puncture and examination of CSF is the most useful diagnostic test. Dark field microscopy to reveal T pallidum is definitive, but only is applicable during the primary stage. The role of dark field microscopy of the CSF sample to diagnose neurologic involvement has not been established. Tests and treatment protocol are described in Table 2.2-5
Treatment of psychiatric symptoms of neurosyphilis
There are inconsistent and limited data about the prevalence of psychiatric symptoms in neurosyphilis. A retrospective study6 of 161 patients with neurosyphilis in South Africa reported that 50.9% exhibited a complex spectrum of symptoms that included delirium and dementia. Of treated patients, 17% continued to have residual symptoms during follow-up.
A review of the literature did not reveal any widely accepted guideline for screening for neurosyphilis in general psychiatry practice or a treatment protocol for psychiatric symptoms. This lack of guidance could be attributed to the rarity of the disease, cost-benefit analyses, and low specificity of antibody tests. In the literature, syphilis screening is recommended as a routine protocol when evaluating and treating dementia.7
In most studies, a diagnosis of neurosyphilis was confirmed by CSF examination; however, many of these studies did not report a specific follow-up CSF examination protocol. Most of these patients were treated with an antipsychotic with partial improvement in symptoms, even after standard antibiotic protocol.8
First- and second-generation antipsychotics and mood stabilizers have been shown to be useful in the acute treatment of psychosis and agitation.8 In few instances, the psychotropic medication was continued beyond several months and the patient was placed in a long-term care facility. Psychiatric symptoms persisted for many years with or without residual neurosyphilis symptoms, possibly because of permanent neuronal loss.
Clinical considerations
It often is difficult to distinguish a preexisting psychiatric disorder made worse by neurosyphilis from a secondary psychiatric disorder caused by neurosyphilis. The 2 might coexist, or psychiatric symptoms could be wrongly attributed to schizophrenia because of a lack of careful clinical evaluation.
Often, the follow-up diagnostic protocol for neurosyphilis is not followed; as a result, the need for re-treatment remains unclear. Rarity of the disease makes it difficult to perform a prospective, randomized study to determine the duration and effect of long-term psychiatric treatment.
Close follow-up and consideration of the risk vs benefit of psychotropic medication is key. Because there are no proven guidelines for the length of treatment with antipsychotics, it is prudent to minimize their use until psychiatrically indicated. Side effects, such as (in Mr. C’s case) changes in the QTc interval, should warrant consideration of discontinuing psychotropic medication. Interdisciplinary collaboration with neurology and infectious disease will improve the overall outcome of a complex clinical presentation.
1. Centers for Disease Control and Prevention. National plan to eliminate syphilis from the United States. http://www.cdc.gov/stopsyphilis/plan.htm. Updated December 7, 2007. Accessed July 7, 2016.
2. Friedrich F, Aigner M, Fearns N, et al. Psychosis in neurosyphilis—clinical aspects and implications. Psychopathology. 2014;47(1):3-9.
3. Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68(2):283-290.
4. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005-1009.
5. Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. Syphilis. http://www.cdc.gov/std/tg2015/syphilis.htm. Updated June 4, 2015. Accessed July 13, 2016.
6. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75(12):1727-1730.
7. Scott KR, Barrett AM. Dementia syndrome: evaluation and treatment. Expert Rev Neurother. 2007;7(4):407-422.
8. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48(5):440-445.
1. Centers for Disease Control and Prevention. National plan to eliminate syphilis from the United States. http://www.cdc.gov/stopsyphilis/plan.htm. Updated December 7, 2007. Accessed July 7, 2016.
2. Friedrich F, Aigner M, Fearns N, et al. Psychosis in neurosyphilis—clinical aspects and implications. Psychopathology. 2014;47(1):3-9.
3. Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68(2):283-290.
4. Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005-1009.
5. Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. Syphilis. http://www.cdc.gov/std/tg2015/syphilis.htm. Updated June 4, 2015. Accessed July 13, 2016.
6. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75(12):1727-1730.
7. Scott KR, Barrett AM. Dementia syndrome: evaluation and treatment. Expert Rev Neurother. 2007;7(4):407-422.
8. Sanchez FM, Zisselman MH. Treatment of psychiatric symptoms associated with neurosyphilis. Psychosomatics. 2007;48(5):440-445.
Flibanserin for hypoactive sexual desire disorder in premenopausal women
Flibanserin, FDA-approved in August 2015, is the first medication approved to treat acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women (Table 1). In clinical trials,1-4 the drug has shown modest efficacy in improving symptoms of low sexual desire (number of satisfying sexual events [SSEs], sexual desire, and overall sexual function). Flibanserin is not indicated to enhance sexual performance, for HSDD in postmenopausal women, or in men.
Clinical implications
Flibanserin could help premenopausal women who have distressing low sexual desire, which must be acquired and generalized:
- “Acquired low sexual desire” means that a patient had an adequate sexual desire that decreased or ceased for an unknown reason.
- “Generalized low sexual desire” means that lack of sexual desire occurs all the time and in all situations, not only with a certain partner or in some situations.
Women taking flibanserin could experience gradually increased sexual desire, increase in SSEs, and decrease of sexual distress. Flibanserin is indicated for long-term use; however, it should be discontinued after 8 weeks if the patient does not report any improvement in symptoms.
The number needed to treat with flibanserin likely would be rather large, but it is not available because of complex outcome measures in clinical trials. Flibanserin was not approved at 2 previous FDA committee hearings—mainly because of safety issues but also because of concerns about efficacy. For example, during the 2013 FDA hearing, the results presented showed statistically significant, but numerically small, treatment differences at 24 weeks compared with placebo. In an FDA responder analysis of the Phase-III trials, after accounting for the placebo effect, approximately 8% to 13% women were at least “much improved” on at least 1 of the primary outcomes.5
Flibanserin is not indicated for women whose sexual desire is due to (1) coexisting medical or psychiatric condition, (2) effects of medication or substance abuse, or (3) a relationship problem. It is unknown whether supplemental treatment would help these patients; however, it seems reasonable that combining flibanserin with psychosocial treatment, such as sex therapy or individual therapy, could be beneficial because it may be difficult to disentangle sexual dysfunction and relationship issues—2 problems that often are interwoven.
How it works
Flibanserin is a serotonin 1A receptor agonist and serotonin 2A receptor antagonist. In vitro, flibanserin demonstrated high affinity for the following 5-HT receptors:
- agonist activity at 5-HT1A
- antagonist activity at 5-HT2A, mostly in the prefrontal cortex.
Flibanserin also has moderate antagonist activities at the 5-HT2B, 5-HT2C, and dopamine D4 receptors. Flibanserin presumably acts centrally in the CNS; it has been suggested that flibanserin could rebalance neural circuitry involved in processing sexual desire by reducing serotonin activity and enhancing dopamine and epinephrine activity. The exact mechanism of how flibanserin improves sexual desire in women is unknown.
Pharmacokinetics
Flibanserin has a mean termination half-life of approximately 11 hours. It is administered once a day (50 to 100 mg) at bedtime. Steady state in healthy women was achieved after 3 days. Based on clinical observations, onset of action seems to be gradual and reaches maximum efficacy in approximately 8 weeks. Patients should discontinue the drug if no improvement is reported after 8 weeks. Flibanserin is readily absorbed from the gastrointestinal tract; however, food slows its absorption. The drug is 98% protein (mostly albumin)-bound.
Flibanserin is primarily metabolized in the liver by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP2C19. Co-administration of moderate (diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil) or strong (eg, ketoconazole, clarithromycin, nefazodone, ritonavir) CYP3A4 inhibitors increases the concentration of flibanserin. This could lead to severe hypotension and syncope; therefore, co-administering flibanserin with a strong CYP3A4 inhibitor is contraindicated. Grapefruit juice is a moderate inhibitor of CYP3A4, and in a study of 26 healthy females, 240 mL of grapefruit juice increased flibanserin concentration 1.4-fold. Flibanserin is excreted though urine and feces. Flibanserin should be taken once a day at bedtime because of sedation, somnolence, and possible syncope.
Efficacy
The efficacy of flibanserin for treating HSDD was established in three 24-week, randomized, double-blind, placebo-controlled studies (Table 2). The target population in these studies was premenopausal women (mean age 36, range 19 to 55) with acquired HSDD lasting at least 6 months (mean duration, approximately 5 years). The 3 studies included 1,187 women who received flibanserin, 100 mg at bedtime, and 1,188 women who received placebo. Participants were mostly white (88.6%), and included black (9.6%) and Asian (1.5%) women. The completion rates were 69% for flibanserin and 78% for placebo. Some of the trials included arms with a lower dosage of flibanserin (25 mg and 50 mg), which are not included in this analysis.
As noted in the package insert, these trials each had 2 co-primary efficacy endpoints, SSEs and sexual desire:
- change from baseline to Week 24 in the number of monthly SSEs (ie, sexual intercourse, oral sex, masturbation, or genital stimulation by the partner)
- change in sexual desire from baseline to 24-week endpoint.
In Study 1 and 2, change in sexual desire from baseline to Week 24 was measured daily by using an electronic diary. Every day, patients rated their sexual desire level by answering the question, “Indicate your most intense level of sexual desire” from 0 (no desire) to 3 (strong desire). These responses were totaled over a 28-day period to yield the monthly sexual desire score, which ranged from 0 to 84. These 2 studies also used the Female Sexual Function Index (FSFI) Desire domain as a secondary endpoint.
Study 3 used the FSFI Desire domain, comprising 2 questions, as the sexual desire co-primary endpoint:
- “Over the past 4 weeks, how often did you feel sexual desire or interest?” Responses ranged from 1 (almost never or never) to 5 (almost always or always).
- “Over the past 4 weeks, how would you rate your level (degree) of sexual desire or interest?” Responses ranged from 1 (very low or none at all) to 5 (very high).
In all 3 trials, flibanserin was associated with a small, yet statistically significant, improvement in change in monthly SSEs from baseline to Week 24 compared with placebo. In Study 1 and 2, there were no statistically significant differences between flibanserin and placebo for the electronic diary sexual desire endpoint. In the third study, there was statistically significant improvement in the change in sexual desire using the FSFI Desire domain with flibanserin compared with placebo. The FSFI Desire domain findings were consistent across all 3 trials. Flibanserin was associated with a decrease in sexual distress compared with placebo in all 3 studies.
Tolerability
Flibanserin was well tolerated in the 3 clinical trials. As the FDA noted, clinical trials are conducted under widely varying conditions and therefore adverse reaction rates observed in trials of flibanserin cannot be directly compared with those reported in clinical trials of another drug and might not reflect rates observed in clinical practice.
The discontinuation rate due to adverse reactions was 13% among patients treated with flibanserin, 100 mg at bedtime, and 6% among those taking placebo. The most common side effects were somnolence, dizziness, fatigue, nausea, insomnia, and dry mouth, which appear dose-dependent. Onset of most of these adverse events was within 14 days after the start of treatment.
Although hypotension and syncope rarely were seen with flibanserin alone in clinical trials, these adverse events occurred more frequently in the morning and when taken with alcohol and with some drugs (moderate or strong CYP3A4 inhibitors), and in patients with hepatic impairment. Therefore, women who drink alcohol or take a moderate or strong inhibitor of CYP3A4—both of which are contraindicated—and those with hepatic impairment should not take flibanserin.
Flibanserin should be taken at bedtime, because the risk of hypotension and syncope is higher when flibanserin is taken in the morning and because of associated sedation and somnolence.
Unique clinical issues
Flibanserin is the first FDA-approved medication for treating HSDD. It is important to note that the drug originally was developed as an antidepressant, but failed to show efficacy. Researchers noted that the drug was more effective than placebo when patients were asked, “How strong is your sexual desire?” The focus of development then shifted to a potential treatment of HSDD.
Flibanserin was not approved at 2 previous FDA hearings, mainly because of safety concerns. For the second hearing, the manufacturer, Boehringer Ingelheim, which sold the rights to the drug to Sprout Pharmaceuticals in 2011,6 did not present any new efficacy data, but provided additional safety data, such as research suggesting the absence of next-day driving impairment and data related to alcohol use (the study confirming hypotension associated with alcohol abuse used a small sample, and only 2 of 25 participants were women).
Contraindications
Flibanserin is contraindicated in patients using alcohol because of an increased risk of hypotension and syncope. A patient’s alcohol use should be evaluated before administering flibanserin, and patients should be counseled about the importance of abstaining from alcohol.
Similarly, concomitant use of flibanserin with a moderate or strong inhibitor of CYP3A4 increases the concentration of flibanserin and raises the risk of hypotension and syncope. Therefore, the use of a moderate or strong inhibitor of CYP3A4 in patients taking flibanserin is contraindicated. Similarly, patients with liver impairment should not take this drug.
Strong CYP2C19 inhibitors (proton-pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, antifungals) could increase flibanserin exposure, which may increase risk of hypotension, syncope, and CNS depression. Discuss these risks with your patients; doing so is particularly important when treating women of Chinese heritage, and some other Asian women, because 20% of these populations are genotypic CYP2C19 poor metabolizers.
Because of the increased risk of hypotension and syncope with alcohol use, flibanserin is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Addyi REMS Program. Flibanserin can be prescribed or dispensed only by physicians and pharmacists who watch this program’s online slide presentation and passed a comprehension test.a
Pregnant women should not take flibanserin because the effect on the fetus is unknown. Also, because the interaction with some oral contraceptives is unknown, patients should be cautioned about unwanted pregnancy. Women who are breastfeeding also should avoid using flibanserin because it is not known whether the drug is excreted in breast milk.
Women taking flibanserin also should avoid grapefruit juice, which increases flibanserin levels, and avoid using herbal products, resveratrol, and some over-the-counter drugs such as cimetidine. Women who have a depressive disorder also should avoid using flibanserin because their low sexual desire is more likely due to depression, which is not a therapeutic target for the drug.
Dosing
Flibanserin is provided in 100-mg film-coated tablets. It should be taken once a day at bedtime; titration is unnecessary. Length of treatment has not been determined, but it is recommended that patients stop flibanserin if they do not experience any benefit after 8 weeks. Although there is no guidance in the prescribing information, the medication probably could be stopped without tapering because withdrawal effects have not been observed.
Bottom Line
Flibanserin is FDA-approved for treating generalized, acquired hypoactive sexual desire disorder in premenopausal women. In clinical trials, the drug increased the number of satisfying sexual events and sexual desire, as measured by a diary and rating scales. Alcohol use and use of any moderate or strong inhibitor of cytochrome P450 3A4 are contraindicated in patients taking flibanserin because of an increased risk of hypotension and syncope.
1. Goldfisher ER, Breaux J, Katz M, et al. Continued efficacy and safety of flibanserin in premenopausal women with Hypoactive Sexual desire Disorder (HSDD): results from a randomized withdrawal trial. J Sex Med. 2011;8(11):3160- 3172.
2. Thorp J, Simon J, Dattani D, et al; DAISY trial investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med. 2012;9(3):793-804.
3. Derogatis LR, Komer L, Katz M, et al; VIOLET Trial Investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET study. J Sex Med. 2012;9(4):1074-1085.
4. Katz M, DeRogatis LR, Ackerman R, et al; BEGONIA trial investigators. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. 2013;10(7):1807-1815.
5. Gellad WF, Flynn KE, Alexander GC. Evaluation of flibanserin: science and advocacy at the FDA. JAMA. 2015;314(9):869-870
6. Joffe HV, Chang C, Sewell C, et al. FDA approval of flibanserin—treating hypoactive sexual desire disorder. N Engl J Med. 2016;374(2):101-104.
Flibanserin, FDA-approved in August 2015, is the first medication approved to treat acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women (Table 1). In clinical trials,1-4 the drug has shown modest efficacy in improving symptoms of low sexual desire (number of satisfying sexual events [SSEs], sexual desire, and overall sexual function). Flibanserin is not indicated to enhance sexual performance, for HSDD in postmenopausal women, or in men.
Clinical implications
Flibanserin could help premenopausal women who have distressing low sexual desire, which must be acquired and generalized:
- “Acquired low sexual desire” means that a patient had an adequate sexual desire that decreased or ceased for an unknown reason.
- “Generalized low sexual desire” means that lack of sexual desire occurs all the time and in all situations, not only with a certain partner or in some situations.
Women taking flibanserin could experience gradually increased sexual desire, increase in SSEs, and decrease of sexual distress. Flibanserin is indicated for long-term use; however, it should be discontinued after 8 weeks if the patient does not report any improvement in symptoms.
The number needed to treat with flibanserin likely would be rather large, but it is not available because of complex outcome measures in clinical trials. Flibanserin was not approved at 2 previous FDA committee hearings—mainly because of safety issues but also because of concerns about efficacy. For example, during the 2013 FDA hearing, the results presented showed statistically significant, but numerically small, treatment differences at 24 weeks compared with placebo. In an FDA responder analysis of the Phase-III trials, after accounting for the placebo effect, approximately 8% to 13% women were at least “much improved” on at least 1 of the primary outcomes.5
Flibanserin is not indicated for women whose sexual desire is due to (1) coexisting medical or psychiatric condition, (2) effects of medication or substance abuse, or (3) a relationship problem. It is unknown whether supplemental treatment would help these patients; however, it seems reasonable that combining flibanserin with psychosocial treatment, such as sex therapy or individual therapy, could be beneficial because it may be difficult to disentangle sexual dysfunction and relationship issues—2 problems that often are interwoven.
How it works
Flibanserin is a serotonin 1A receptor agonist and serotonin 2A receptor antagonist. In vitro, flibanserin demonstrated high affinity for the following 5-HT receptors:
- agonist activity at 5-HT1A
- antagonist activity at 5-HT2A, mostly in the prefrontal cortex.
Flibanserin also has moderate antagonist activities at the 5-HT2B, 5-HT2C, and dopamine D4 receptors. Flibanserin presumably acts centrally in the CNS; it has been suggested that flibanserin could rebalance neural circuitry involved in processing sexual desire by reducing serotonin activity and enhancing dopamine and epinephrine activity. The exact mechanism of how flibanserin improves sexual desire in women is unknown.
Pharmacokinetics
Flibanserin has a mean termination half-life of approximately 11 hours. It is administered once a day (50 to 100 mg) at bedtime. Steady state in healthy women was achieved after 3 days. Based on clinical observations, onset of action seems to be gradual and reaches maximum efficacy in approximately 8 weeks. Patients should discontinue the drug if no improvement is reported after 8 weeks. Flibanserin is readily absorbed from the gastrointestinal tract; however, food slows its absorption. The drug is 98% protein (mostly albumin)-bound.
Flibanserin is primarily metabolized in the liver by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP2C19. Co-administration of moderate (diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil) or strong (eg, ketoconazole, clarithromycin, nefazodone, ritonavir) CYP3A4 inhibitors increases the concentration of flibanserin. This could lead to severe hypotension and syncope; therefore, co-administering flibanserin with a strong CYP3A4 inhibitor is contraindicated. Grapefruit juice is a moderate inhibitor of CYP3A4, and in a study of 26 healthy females, 240 mL of grapefruit juice increased flibanserin concentration 1.4-fold. Flibanserin is excreted though urine and feces. Flibanserin should be taken once a day at bedtime because of sedation, somnolence, and possible syncope.
Efficacy
The efficacy of flibanserin for treating HSDD was established in three 24-week, randomized, double-blind, placebo-controlled studies (Table 2). The target population in these studies was premenopausal women (mean age 36, range 19 to 55) with acquired HSDD lasting at least 6 months (mean duration, approximately 5 years). The 3 studies included 1,187 women who received flibanserin, 100 mg at bedtime, and 1,188 women who received placebo. Participants were mostly white (88.6%), and included black (9.6%) and Asian (1.5%) women. The completion rates were 69% for flibanserin and 78% for placebo. Some of the trials included arms with a lower dosage of flibanserin (25 mg and 50 mg), which are not included in this analysis.
As noted in the package insert, these trials each had 2 co-primary efficacy endpoints, SSEs and sexual desire:
- change from baseline to Week 24 in the number of monthly SSEs (ie, sexual intercourse, oral sex, masturbation, or genital stimulation by the partner)
- change in sexual desire from baseline to 24-week endpoint.
In Study 1 and 2, change in sexual desire from baseline to Week 24 was measured daily by using an electronic diary. Every day, patients rated their sexual desire level by answering the question, “Indicate your most intense level of sexual desire” from 0 (no desire) to 3 (strong desire). These responses were totaled over a 28-day period to yield the monthly sexual desire score, which ranged from 0 to 84. These 2 studies also used the Female Sexual Function Index (FSFI) Desire domain as a secondary endpoint.
Study 3 used the FSFI Desire domain, comprising 2 questions, as the sexual desire co-primary endpoint:
- “Over the past 4 weeks, how often did you feel sexual desire or interest?” Responses ranged from 1 (almost never or never) to 5 (almost always or always).
- “Over the past 4 weeks, how would you rate your level (degree) of sexual desire or interest?” Responses ranged from 1 (very low or none at all) to 5 (very high).
In all 3 trials, flibanserin was associated with a small, yet statistically significant, improvement in change in monthly SSEs from baseline to Week 24 compared with placebo. In Study 1 and 2, there were no statistically significant differences between flibanserin and placebo for the electronic diary sexual desire endpoint. In the third study, there was statistically significant improvement in the change in sexual desire using the FSFI Desire domain with flibanserin compared with placebo. The FSFI Desire domain findings were consistent across all 3 trials. Flibanserin was associated with a decrease in sexual distress compared with placebo in all 3 studies.
Tolerability
Flibanserin was well tolerated in the 3 clinical trials. As the FDA noted, clinical trials are conducted under widely varying conditions and therefore adverse reaction rates observed in trials of flibanserin cannot be directly compared with those reported in clinical trials of another drug and might not reflect rates observed in clinical practice.
The discontinuation rate due to adverse reactions was 13% among patients treated with flibanserin, 100 mg at bedtime, and 6% among those taking placebo. The most common side effects were somnolence, dizziness, fatigue, nausea, insomnia, and dry mouth, which appear dose-dependent. Onset of most of these adverse events was within 14 days after the start of treatment.
Although hypotension and syncope rarely were seen with flibanserin alone in clinical trials, these adverse events occurred more frequently in the morning and when taken with alcohol and with some drugs (moderate or strong CYP3A4 inhibitors), and in patients with hepatic impairment. Therefore, women who drink alcohol or take a moderate or strong inhibitor of CYP3A4—both of which are contraindicated—and those with hepatic impairment should not take flibanserin.
Flibanserin should be taken at bedtime, because the risk of hypotension and syncope is higher when flibanserin is taken in the morning and because of associated sedation and somnolence.
Unique clinical issues
Flibanserin is the first FDA-approved medication for treating HSDD. It is important to note that the drug originally was developed as an antidepressant, but failed to show efficacy. Researchers noted that the drug was more effective than placebo when patients were asked, “How strong is your sexual desire?” The focus of development then shifted to a potential treatment of HSDD.
Flibanserin was not approved at 2 previous FDA hearings, mainly because of safety concerns. For the second hearing, the manufacturer, Boehringer Ingelheim, which sold the rights to the drug to Sprout Pharmaceuticals in 2011,6 did not present any new efficacy data, but provided additional safety data, such as research suggesting the absence of next-day driving impairment and data related to alcohol use (the study confirming hypotension associated with alcohol abuse used a small sample, and only 2 of 25 participants were women).
Contraindications
Flibanserin is contraindicated in patients using alcohol because of an increased risk of hypotension and syncope. A patient’s alcohol use should be evaluated before administering flibanserin, and patients should be counseled about the importance of abstaining from alcohol.
Similarly, concomitant use of flibanserin with a moderate or strong inhibitor of CYP3A4 increases the concentration of flibanserin and raises the risk of hypotension and syncope. Therefore, the use of a moderate or strong inhibitor of CYP3A4 in patients taking flibanserin is contraindicated. Similarly, patients with liver impairment should not take this drug.
Strong CYP2C19 inhibitors (proton-pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, antifungals) could increase flibanserin exposure, which may increase risk of hypotension, syncope, and CNS depression. Discuss these risks with your patients; doing so is particularly important when treating women of Chinese heritage, and some other Asian women, because 20% of these populations are genotypic CYP2C19 poor metabolizers.
Because of the increased risk of hypotension and syncope with alcohol use, flibanserin is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Addyi REMS Program. Flibanserin can be prescribed or dispensed only by physicians and pharmacists who watch this program’s online slide presentation and passed a comprehension test.a
Pregnant women should not take flibanserin because the effect on the fetus is unknown. Also, because the interaction with some oral contraceptives is unknown, patients should be cautioned about unwanted pregnancy. Women who are breastfeeding also should avoid using flibanserin because it is not known whether the drug is excreted in breast milk.
Women taking flibanserin also should avoid grapefruit juice, which increases flibanserin levels, and avoid using herbal products, resveratrol, and some over-the-counter drugs such as cimetidine. Women who have a depressive disorder also should avoid using flibanserin because their low sexual desire is more likely due to depression, which is not a therapeutic target for the drug.
Dosing
Flibanserin is provided in 100-mg film-coated tablets. It should be taken once a day at bedtime; titration is unnecessary. Length of treatment has not been determined, but it is recommended that patients stop flibanserin if they do not experience any benefit after 8 weeks. Although there is no guidance in the prescribing information, the medication probably could be stopped without tapering because withdrawal effects have not been observed.
Bottom Line
Flibanserin is FDA-approved for treating generalized, acquired hypoactive sexual desire disorder in premenopausal women. In clinical trials, the drug increased the number of satisfying sexual events and sexual desire, as measured by a diary and rating scales. Alcohol use and use of any moderate or strong inhibitor of cytochrome P450 3A4 are contraindicated in patients taking flibanserin because of an increased risk of hypotension and syncope.
Flibanserin, FDA-approved in August 2015, is the first medication approved to treat acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women (Table 1). In clinical trials,1-4 the drug has shown modest efficacy in improving symptoms of low sexual desire (number of satisfying sexual events [SSEs], sexual desire, and overall sexual function). Flibanserin is not indicated to enhance sexual performance, for HSDD in postmenopausal women, or in men.
Clinical implications
Flibanserin could help premenopausal women who have distressing low sexual desire, which must be acquired and generalized:
- “Acquired low sexual desire” means that a patient had an adequate sexual desire that decreased or ceased for an unknown reason.
- “Generalized low sexual desire” means that lack of sexual desire occurs all the time and in all situations, not only with a certain partner or in some situations.
Women taking flibanserin could experience gradually increased sexual desire, increase in SSEs, and decrease of sexual distress. Flibanserin is indicated for long-term use; however, it should be discontinued after 8 weeks if the patient does not report any improvement in symptoms.
The number needed to treat with flibanserin likely would be rather large, but it is not available because of complex outcome measures in clinical trials. Flibanserin was not approved at 2 previous FDA committee hearings—mainly because of safety issues but also because of concerns about efficacy. For example, during the 2013 FDA hearing, the results presented showed statistically significant, but numerically small, treatment differences at 24 weeks compared with placebo. In an FDA responder analysis of the Phase-III trials, after accounting for the placebo effect, approximately 8% to 13% women were at least “much improved” on at least 1 of the primary outcomes.5
Flibanserin is not indicated for women whose sexual desire is due to (1) coexisting medical or psychiatric condition, (2) effects of medication or substance abuse, or (3) a relationship problem. It is unknown whether supplemental treatment would help these patients; however, it seems reasonable that combining flibanserin with psychosocial treatment, such as sex therapy or individual therapy, could be beneficial because it may be difficult to disentangle sexual dysfunction and relationship issues—2 problems that often are interwoven.
How it works
Flibanserin is a serotonin 1A receptor agonist and serotonin 2A receptor antagonist. In vitro, flibanserin demonstrated high affinity for the following 5-HT receptors:
- agonist activity at 5-HT1A
- antagonist activity at 5-HT2A, mostly in the prefrontal cortex.
Flibanserin also has moderate antagonist activities at the 5-HT2B, 5-HT2C, and dopamine D4 receptors. Flibanserin presumably acts centrally in the CNS; it has been suggested that flibanserin could rebalance neural circuitry involved in processing sexual desire by reducing serotonin activity and enhancing dopamine and epinephrine activity. The exact mechanism of how flibanserin improves sexual desire in women is unknown.
Pharmacokinetics
Flibanserin has a mean termination half-life of approximately 11 hours. It is administered once a day (50 to 100 mg) at bedtime. Steady state in healthy women was achieved after 3 days. Based on clinical observations, onset of action seems to be gradual and reaches maximum efficacy in approximately 8 weeks. Patients should discontinue the drug if no improvement is reported after 8 weeks. Flibanserin is readily absorbed from the gastrointestinal tract; however, food slows its absorption. The drug is 98% protein (mostly albumin)-bound.
Flibanserin is primarily metabolized in the liver by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP2C19. Co-administration of moderate (diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil) or strong (eg, ketoconazole, clarithromycin, nefazodone, ritonavir) CYP3A4 inhibitors increases the concentration of flibanserin. This could lead to severe hypotension and syncope; therefore, co-administering flibanserin with a strong CYP3A4 inhibitor is contraindicated. Grapefruit juice is a moderate inhibitor of CYP3A4, and in a study of 26 healthy females, 240 mL of grapefruit juice increased flibanserin concentration 1.4-fold. Flibanserin is excreted though urine and feces. Flibanserin should be taken once a day at bedtime because of sedation, somnolence, and possible syncope.
Efficacy
The efficacy of flibanserin for treating HSDD was established in three 24-week, randomized, double-blind, placebo-controlled studies (Table 2). The target population in these studies was premenopausal women (mean age 36, range 19 to 55) with acquired HSDD lasting at least 6 months (mean duration, approximately 5 years). The 3 studies included 1,187 women who received flibanserin, 100 mg at bedtime, and 1,188 women who received placebo. Participants were mostly white (88.6%), and included black (9.6%) and Asian (1.5%) women. The completion rates were 69% for flibanserin and 78% for placebo. Some of the trials included arms with a lower dosage of flibanserin (25 mg and 50 mg), which are not included in this analysis.
As noted in the package insert, these trials each had 2 co-primary efficacy endpoints, SSEs and sexual desire:
- change from baseline to Week 24 in the number of monthly SSEs (ie, sexual intercourse, oral sex, masturbation, or genital stimulation by the partner)
- change in sexual desire from baseline to 24-week endpoint.
In Study 1 and 2, change in sexual desire from baseline to Week 24 was measured daily by using an electronic diary. Every day, patients rated their sexual desire level by answering the question, “Indicate your most intense level of sexual desire” from 0 (no desire) to 3 (strong desire). These responses were totaled over a 28-day period to yield the monthly sexual desire score, which ranged from 0 to 84. These 2 studies also used the Female Sexual Function Index (FSFI) Desire domain as a secondary endpoint.
Study 3 used the FSFI Desire domain, comprising 2 questions, as the sexual desire co-primary endpoint:
- “Over the past 4 weeks, how often did you feel sexual desire or interest?” Responses ranged from 1 (almost never or never) to 5 (almost always or always).
- “Over the past 4 weeks, how would you rate your level (degree) of sexual desire or interest?” Responses ranged from 1 (very low or none at all) to 5 (very high).
In all 3 trials, flibanserin was associated with a small, yet statistically significant, improvement in change in monthly SSEs from baseline to Week 24 compared with placebo. In Study 1 and 2, there were no statistically significant differences between flibanserin and placebo for the electronic diary sexual desire endpoint. In the third study, there was statistically significant improvement in the change in sexual desire using the FSFI Desire domain with flibanserin compared with placebo. The FSFI Desire domain findings were consistent across all 3 trials. Flibanserin was associated with a decrease in sexual distress compared with placebo in all 3 studies.
Tolerability
Flibanserin was well tolerated in the 3 clinical trials. As the FDA noted, clinical trials are conducted under widely varying conditions and therefore adverse reaction rates observed in trials of flibanserin cannot be directly compared with those reported in clinical trials of another drug and might not reflect rates observed in clinical practice.
The discontinuation rate due to adverse reactions was 13% among patients treated with flibanserin, 100 mg at bedtime, and 6% among those taking placebo. The most common side effects were somnolence, dizziness, fatigue, nausea, insomnia, and dry mouth, which appear dose-dependent. Onset of most of these adverse events was within 14 days after the start of treatment.
Although hypotension and syncope rarely were seen with flibanserin alone in clinical trials, these adverse events occurred more frequently in the morning and when taken with alcohol and with some drugs (moderate or strong CYP3A4 inhibitors), and in patients with hepatic impairment. Therefore, women who drink alcohol or take a moderate or strong inhibitor of CYP3A4—both of which are contraindicated—and those with hepatic impairment should not take flibanserin.
Flibanserin should be taken at bedtime, because the risk of hypotension and syncope is higher when flibanserin is taken in the morning and because of associated sedation and somnolence.
Unique clinical issues
Flibanserin is the first FDA-approved medication for treating HSDD. It is important to note that the drug originally was developed as an antidepressant, but failed to show efficacy. Researchers noted that the drug was more effective than placebo when patients were asked, “How strong is your sexual desire?” The focus of development then shifted to a potential treatment of HSDD.
Flibanserin was not approved at 2 previous FDA hearings, mainly because of safety concerns. For the second hearing, the manufacturer, Boehringer Ingelheim, which sold the rights to the drug to Sprout Pharmaceuticals in 2011,6 did not present any new efficacy data, but provided additional safety data, such as research suggesting the absence of next-day driving impairment and data related to alcohol use (the study confirming hypotension associated with alcohol abuse used a small sample, and only 2 of 25 participants were women).
Contraindications
Flibanserin is contraindicated in patients using alcohol because of an increased risk of hypotension and syncope. A patient’s alcohol use should be evaluated before administering flibanserin, and patients should be counseled about the importance of abstaining from alcohol.
Similarly, concomitant use of flibanserin with a moderate or strong inhibitor of CYP3A4 increases the concentration of flibanserin and raises the risk of hypotension and syncope. Therefore, the use of a moderate or strong inhibitor of CYP3A4 in patients taking flibanserin is contraindicated. Similarly, patients with liver impairment should not take this drug.
Strong CYP2C19 inhibitors (proton-pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, antifungals) could increase flibanserin exposure, which may increase risk of hypotension, syncope, and CNS depression. Discuss these risks with your patients; doing so is particularly important when treating women of Chinese heritage, and some other Asian women, because 20% of these populations are genotypic CYP2C19 poor metabolizers.
Because of the increased risk of hypotension and syncope with alcohol use, flibanserin is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Addyi REMS Program. Flibanserin can be prescribed or dispensed only by physicians and pharmacists who watch this program’s online slide presentation and passed a comprehension test.a
Pregnant women should not take flibanserin because the effect on the fetus is unknown. Also, because the interaction with some oral contraceptives is unknown, patients should be cautioned about unwanted pregnancy. Women who are breastfeeding also should avoid using flibanserin because it is not known whether the drug is excreted in breast milk.
Women taking flibanserin also should avoid grapefruit juice, which increases flibanserin levels, and avoid using herbal products, resveratrol, and some over-the-counter drugs such as cimetidine. Women who have a depressive disorder also should avoid using flibanserin because their low sexual desire is more likely due to depression, which is not a therapeutic target for the drug.
Dosing
Flibanserin is provided in 100-mg film-coated tablets. It should be taken once a day at bedtime; titration is unnecessary. Length of treatment has not been determined, but it is recommended that patients stop flibanserin if they do not experience any benefit after 8 weeks. Although there is no guidance in the prescribing information, the medication probably could be stopped without tapering because withdrawal effects have not been observed.
Bottom Line
Flibanserin is FDA-approved for treating generalized, acquired hypoactive sexual desire disorder in premenopausal women. In clinical trials, the drug increased the number of satisfying sexual events and sexual desire, as measured by a diary and rating scales. Alcohol use and use of any moderate or strong inhibitor of cytochrome P450 3A4 are contraindicated in patients taking flibanserin because of an increased risk of hypotension and syncope.
1. Goldfisher ER, Breaux J, Katz M, et al. Continued efficacy and safety of flibanserin in premenopausal women with Hypoactive Sexual desire Disorder (HSDD): results from a randomized withdrawal trial. J Sex Med. 2011;8(11):3160- 3172.
2. Thorp J, Simon J, Dattani D, et al; DAISY trial investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med. 2012;9(3):793-804.
3. Derogatis LR, Komer L, Katz M, et al; VIOLET Trial Investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET study. J Sex Med. 2012;9(4):1074-1085.
4. Katz M, DeRogatis LR, Ackerman R, et al; BEGONIA trial investigators. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. 2013;10(7):1807-1815.
5. Gellad WF, Flynn KE, Alexander GC. Evaluation of flibanserin: science and advocacy at the FDA. JAMA. 2015;314(9):869-870
6. Joffe HV, Chang C, Sewell C, et al. FDA approval of flibanserin—treating hypoactive sexual desire disorder. N Engl J Med. 2016;374(2):101-104.
1. Goldfisher ER, Breaux J, Katz M, et al. Continued efficacy and safety of flibanserin in premenopausal women with Hypoactive Sexual desire Disorder (HSDD): results from a randomized withdrawal trial. J Sex Med. 2011;8(11):3160- 3172.
2. Thorp J, Simon J, Dattani D, et al; DAISY trial investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med. 2012;9(3):793-804.
3. Derogatis LR, Komer L, Katz M, et al; VIOLET Trial Investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET study. J Sex Med. 2012;9(4):1074-1085.
4. Katz M, DeRogatis LR, Ackerman R, et al; BEGONIA trial investigators. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. 2013;10(7):1807-1815.
5. Gellad WF, Flynn KE, Alexander GC. Evaluation of flibanserin: science and advocacy at the FDA. JAMA. 2015;314(9):869-870
6. Joffe HV, Chang C, Sewell C, et al. FDA approval of flibanserin—treating hypoactive sexual desire disorder. N Engl J Med. 2016;374(2):101-104.
Quo vadis, Psychiatry?
Psychiatrists frequently complain about their lack of recognition by other specialties, stigmatization of mental illness and the practice of psychiatry, and diminishing sense of identity as a specialty. Although I share these concerns, there is another trend that worries me perhaps more: the deliberate abandonment of more and more areas of what has traditionally been and should be psychiatry’s area of expertise and skills. Not all of this is our own doing; the fact is that other clinicians would like to get “a piece of our pie”—a trend seen in other specialties as well (eg, parts of radiology taken over by cardiologists). However, I view our role in this process as larger than other specialties’ or disciplines’ efforts.
Many of us choose not to treat substance abuse patients and instead refer them to “specialists”; yet, don’t we have enough of our own trained specialists and don’t we fill our addiction psychiatry fellowship training positions? Similarly, many do not like to treat patients with comorbid psychiatric illness and substance abuse, although this occurs frequently in our practice. Cognitive disorders often are left to neurologists and our role in managing these patients is diminishing. Pulmonologists gradually are taking over sleep disorders; one wonders why. We do not like to ask our patients about their sexual history, not even talking about treating their sexual problems! Most psychiatrists are afraid of prescribing phosphodiesterase-5 inhibitors. We are leaving the entire field of human sexuality to gynecologists, urologists, and other specialists. Paraphilic disorders are something we do not want to manage and we would rather get the whole area out of our classification systems, with the implication that these are not really mental health problems.
Many of us prefer not to treat personality disorder patients—especially those with borderline personality disorder—because they are “difficult.” Some do not even feel comfortable managing adverse effects of psychotropics such as the metabolic syndrome, or use “unusual” augmentations such as thyroid hormone. We prescribe fewer and fewer older, yet efficacious, psychotropic medications; only a small fraction of psychiatrists still prescribes monoamine oxidase inhibitors. Other disciplines, eg, primary care and pain medicine, prescribe some tricyclic antidepressants more than we do. We irrationally avoid benzodiazepines and do not like prescribing lithium, because it requires ordering blood levels and lab tests. We seem comfortable only with newer antidepressants and antipsychotics. How is this way of prescribing different from what is done in primary care? Some of our leaders sneer at the idea of psychiatrists practicing psychotherapy, perhaps feeling that such a “lowly art” should be provided by psychologists and social workers. We do not address relational issues. Last but not least, I hear colleagues saying that they do not like to treat “difficult” patients.
What are we aspiring to be and to do? To treat schizophrenia, bipolar disorder, and maybe depression, with a limited medication armamentarium we feel comfortable with and no psychotherapy? I am sure that many will say I am exaggerating, but I think not. We have, as Pogo said, met the enemy and he is us. We should get off the slippery slope of selling out psychiatry piece-by-piece, and fully embrace—clinically and research-wise (funding!)—all of what has been part of psychiatry.
Psychiatrists frequently complain about their lack of recognition by other specialties, stigmatization of mental illness and the practice of psychiatry, and diminishing sense of identity as a specialty. Although I share these concerns, there is another trend that worries me perhaps more: the deliberate abandonment of more and more areas of what has traditionally been and should be psychiatry’s area of expertise and skills. Not all of this is our own doing; the fact is that other clinicians would like to get “a piece of our pie”—a trend seen in other specialties as well (eg, parts of radiology taken over by cardiologists). However, I view our role in this process as larger than other specialties’ or disciplines’ efforts.
Many of us choose not to treat substance abuse patients and instead refer them to “specialists”; yet, don’t we have enough of our own trained specialists and don’t we fill our addiction psychiatry fellowship training positions? Similarly, many do not like to treat patients with comorbid psychiatric illness and substance abuse, although this occurs frequently in our practice. Cognitive disorders often are left to neurologists and our role in managing these patients is diminishing. Pulmonologists gradually are taking over sleep disorders; one wonders why. We do not like to ask our patients about their sexual history, not even talking about treating their sexual problems! Most psychiatrists are afraid of prescribing phosphodiesterase-5 inhibitors. We are leaving the entire field of human sexuality to gynecologists, urologists, and other specialists. Paraphilic disorders are something we do not want to manage and we would rather get the whole area out of our classification systems, with the implication that these are not really mental health problems.
Many of us prefer not to treat personality disorder patients—especially those with borderline personality disorder—because they are “difficult.” Some do not even feel comfortable managing adverse effects of psychotropics such as the metabolic syndrome, or use “unusual” augmentations such as thyroid hormone. We prescribe fewer and fewer older, yet efficacious, psychotropic medications; only a small fraction of psychiatrists still prescribes monoamine oxidase inhibitors. Other disciplines, eg, primary care and pain medicine, prescribe some tricyclic antidepressants more than we do. We irrationally avoid benzodiazepines and do not like prescribing lithium, because it requires ordering blood levels and lab tests. We seem comfortable only with newer antidepressants and antipsychotics. How is this way of prescribing different from what is done in primary care? Some of our leaders sneer at the idea of psychiatrists practicing psychotherapy, perhaps feeling that such a “lowly art” should be provided by psychologists and social workers. We do not address relational issues. Last but not least, I hear colleagues saying that they do not like to treat “difficult” patients.
What are we aspiring to be and to do? To treat schizophrenia, bipolar disorder, and maybe depression, with a limited medication armamentarium we feel comfortable with and no psychotherapy? I am sure that many will say I am exaggerating, but I think not. We have, as Pogo said, met the enemy and he is us. We should get off the slippery slope of selling out psychiatry piece-by-piece, and fully embrace—clinically and research-wise (funding!)—all of what has been part of psychiatry.
Psychiatrists frequently complain about their lack of recognition by other specialties, stigmatization of mental illness and the practice of psychiatry, and diminishing sense of identity as a specialty. Although I share these concerns, there is another trend that worries me perhaps more: the deliberate abandonment of more and more areas of what has traditionally been and should be psychiatry’s area of expertise and skills. Not all of this is our own doing; the fact is that other clinicians would like to get “a piece of our pie”—a trend seen in other specialties as well (eg, parts of radiology taken over by cardiologists). However, I view our role in this process as larger than other specialties’ or disciplines’ efforts.
Many of us choose not to treat substance abuse patients and instead refer them to “specialists”; yet, don’t we have enough of our own trained specialists and don’t we fill our addiction psychiatry fellowship training positions? Similarly, many do not like to treat patients with comorbid psychiatric illness and substance abuse, although this occurs frequently in our practice. Cognitive disorders often are left to neurologists and our role in managing these patients is diminishing. Pulmonologists gradually are taking over sleep disorders; one wonders why. We do not like to ask our patients about their sexual history, not even talking about treating their sexual problems! Most psychiatrists are afraid of prescribing phosphodiesterase-5 inhibitors. We are leaving the entire field of human sexuality to gynecologists, urologists, and other specialists. Paraphilic disorders are something we do not want to manage and we would rather get the whole area out of our classification systems, with the implication that these are not really mental health problems.
Many of us prefer not to treat personality disorder patients—especially those with borderline personality disorder—because they are “difficult.” Some do not even feel comfortable managing adverse effects of psychotropics such as the metabolic syndrome, or use “unusual” augmentations such as thyroid hormone. We prescribe fewer and fewer older, yet efficacious, psychotropic medications; only a small fraction of psychiatrists still prescribes monoamine oxidase inhibitors. Other disciplines, eg, primary care and pain medicine, prescribe some tricyclic antidepressants more than we do. We irrationally avoid benzodiazepines and do not like prescribing lithium, because it requires ordering blood levels and lab tests. We seem comfortable only with newer antidepressants and antipsychotics. How is this way of prescribing different from what is done in primary care? Some of our leaders sneer at the idea of psychiatrists practicing psychotherapy, perhaps feeling that such a “lowly art” should be provided by psychologists and social workers. We do not address relational issues. Last but not least, I hear colleagues saying that they do not like to treat “difficult” patients.
What are we aspiring to be and to do? To treat schizophrenia, bipolar disorder, and maybe depression, with a limited medication armamentarium we feel comfortable with and no psychotherapy? I am sure that many will say I am exaggerating, but I think not. We have, as Pogo said, met the enemy and he is us. We should get off the slippery slope of selling out psychiatry piece-by-piece, and fully embrace—clinically and research-wise (funding!)—all of what has been part of psychiatry.
Chronic non-cancer pain and substance use disorders: Challenges and strategies
Patients with chronic non-cancer pain (CNCP) and a comorbid substance use disorder (SUD) are difficult to treat. There is a lack of high-quality clinical trials to guide management. This article focuses on current research, guidelines, and recommendations to best manage these patients. We present an analysis of recent statistics, patient characteristics, screening methods, as well as a discussion of changes to DSM-5 regarding substance abuse and addiction (Box 1).1
Opioid use and opioid-related overdoses have increased dramatically over the last decade (Box 2).2-5 Opioids are the primary medication used to treat CNCP, but their use in patients with comorbid SUDs is controversial. It is crucial for psychiatrists and other clinicians to know how to best identify, manage, and treat patients with CNCP/SUD.
Risk factors for CNCP/SUD
Evidence regarding the efficacy of screening methods to identify patients with chronic pain who are at high risk for substance misuse is insufficient. Key risk factors for developing chronic pain may include:
• elevated psychological distress
• negative beliefs and expectations about pain
• pain fear and avoidance
• disability
• anger or hostility
• maladaptive coping strategies
• catastrophic behaviors.5
In addition, these individuals may have a spouse who enables the sick role behavior.
Risk factors for developing a SUD related to prescribed opioids include:
• a history of problematic substance use
• sedative-hypnotic use
• positive family history for substance abuse
• legal problems
• heavy tobacco use
• age <50
• major depressive disorder or anxiety.5
In a review of 38 articles, Morasco et al6 found low-grade evidence with mixed results in attempt to find a correlation among sex, depression, anxiety, and tobacco use with CNCP/SUD. Other data suggest that the risk of addiction once opioids have been started increases with long refill periods and opioid morphine equivalents >120 mg.7 A history of childhood sexual abuse also may be a risk factor for chronic pain and addiction.5
Prevalence
The prevalence of opioid abuse among CNCP patients ranges from 3% to 48%; the highest rates are found among patients visiting the emergency room for opioid refills.7 These patients are more likely to exhibit aberrant behavior with their medications and may be prescribed higher opioid doses than patients who have CNCP only. Adherent CNCP/SUD patients show no difference in response to pain treatment compared with those with CNCP alone.6 Approximately 11.5% of CNCP patients taking opioids demonstrate aberrant medication use.6
Screening: Which method is best?
Data are scarce regarding the best screening methods to identify patients with CNCP/SUD. A survey of 48 patients by Moore et al8 found the combination of a clinical interview and the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) is 90% sensitive in detecting CNCP/SUD. However, a systematic review by Chou et al9 found only 2 well-designed studies showing that the SOAPP-R weakly predicts future aberrant drug behavior and only 1 study showed that a high risk categorization on the Opioid Risk Tool (ORT) strongly increased the likelihood of predicting future abnormal drug-related behavior. Another well-designed study showed that the Current Opioid Misuse Measure (COMM) weakly raised the likelihood of detecting current aberrant drug behavior. No reliable data supported the efficacy of urine drug screens (UDS), pill counts, or prescription drug monitoring programs (PDMPs) for improving clinical outcomes.9 In a systematic review Starrels et al10 found only low-quality evidence supporting the effectiveness of opioid agreement contracts and UDS.
Treatment strategies
Once a patient with CNCP/SUD has been identified, it is important to categorize the severity of his (her) pain and substance use by using the decision tree (Figure) and screening tools such as SOAPP-R, ORT, and COMM. In a Veterans Administration (VA) study, only 35% of patients with an SUD received substance abuse treatment.11 The 2009 American Pain Society/American Academy of Pain Medicine guidelines recommended that opioids should considered for patients with substance abuse, serious aberrant drug-related behaviors, or psychiatric comorbidities only if frequent monitoring and treatment plan and mental health or addiction consultation were in place.12 These guidelines also recommended discontinuing opioids if repeated atypical behavior, substance abuse, diversion, lack of progress, or intolerable side effects occur. Repeated and more serious behaviors require a multidisciplinary team, expert consultation, therapy restructuring, and possibly discontinuation of opioids.12
The U.S. Office of National Drug Control Policy has created a council of federal agencies to spearhead the Prescription Drug Abuse Prevention Plan, which includes 4 major categories to reduce prescription drug abuse: education, monitoring, proper disposal, and enforcement.13 FDA commissioner Margaret Hamburg supports legislation to combine opioid education with Drug Enforcement Administration registration.14 The FDA began developing the risk evaluation and mitigation strategies in 2007 to educate physicians on proper prescribing of potentially dangerous medications.
Gourlay and Heit proposed a universal precautions method of opioid treatment for all pain patients.15 That includes:
• seeking differential diagnoses and comorbidities
• doing a baseline addiction assessment with UDS and PDMP evaluations
• obtaining informed consent for pain management
• creating pre- and post-treatment goals for pain and function
• evaluating the 4 “As” (analgesic response, increased activity, adverse events, and aberrant behavior)
• reviewing the evolution of the pain and comorbidities
• continuous documentation.5
Other helpful strategies include the Oregon’s SMART (Specific, Measurable, Action-oriented, Realistic, Time-Dependent) goal-setting, which helps physicians negotiate functional goals with patients and plan an exit strategy for those whose quality of life does not improve with opioids.5 Clinicians also can consider a sequential treatment model where patients with severe substance abuse and pain are detoxified of illicit drugs and alcohol before starting pain management. This approach is more effective if the pain is secondary to a more severe substance abuse problem that is not correlated to physical pain and acute rather than chronic.16
Psychotherapeutic interventions
In another VA study, a collaborative care intervention (CCI) combining education, self-efficacy, pain management, and feedback was not impeded by a history of SUD. The authors recommended CCI, stepped care, integrated interventions, and relapse prevention and stressed the importance of social support.17
A 10-week cognitive-behavioral therapy (CBT) program involving 44 patients enrolled in an integrated pain management program for recovering substance abusers found 50% of CNCP/SUD patients were opioid-free at 12 months.16 A combination of medication reduction and education resulted in less pain, increased functioning, decreased emotional distress, and less self-medicating. Additionally, patients reported 35% overall reduction in pain severity based on the McGill Pain Questionnaire but only 25% of patients showed a reliable improvement in their pain. Treatment changes lasted 1 year.16
A meta-analysis of psychological interventions such as CBT, behavioral treatment (BT), and self-regulated treatment (SRT) indicated that CBT and BT are moderately effective at lowering work-related disability and pain intensity for chronic low back pain alone or with multidisciplinary care and moderately lowered work-related disability. CBT had a moderate to large effect, while SRT with biofeedback and relaxation techniques had a large effect on lowering pain intensity. SRT also was shown to lower depression. Return-to-work rates were better with multidisciplinary care that included psychological interventions. These psychological interventions for chronic low back pain lowered self-reported pain, pain interference, depression, and disability while increasing quality of life; the largest effect was on pain intensity.18
A review by Williams et al19 analyzing the effects of BT and CBT on various outcome measures, including chronic pain, found small to moderate benefits for disability, mood, and catastrophic thinking with CBT, which lasted up to 6 months. Only weak improvements in pain were seen with CBT immediately after treatment. BT had a beneficial effect on catastrophic thinking but only right after treatment. CBT’s overall effect in these patients was positive, and changes lasted up to 6 months.
Pharmacologic treatments
Before and during opioid therapy, psychotherapy, physical therapy, and occupational therapy should be used with adjuvant medications appropriate to the pain condition, such as anticonvulsants (gabapentin, pregabalin, topiramate) and antidepressants including tricyclic antidepressants (amitriptyline, desipramine) and serotonin-norepinephrine reuptake inhibitors (duloxetine, venlafaxine, milnacipran).12 When considering opioids for patients with CNCP/SUD, adverse effects and safety is a primary consideration. Benzodiazepines generally should not be used with opioids because of their synergistic sedating effects.5
Opioids are misused more often by overingestion than by altering the delivery route, yet most efforts to create tamper-resistant medications has focused on
snorting or injection, which are considered more dangerous. Current tamper-resistance strategies include:
• creating a hard shell to prevent crushing and altering the medications
• chemical combinations, using agonists and antagonists such as buprenorphine combined with naloxone
• prodrugs, which become activated only in the GI system
• implants or patches.20,21
One prodrug in phase-I testing, compound PF329, becomes activated only in the GI tract by exposure to trypsin. Because it also contains trypsin inhibitors, overingestion will not lead to toxicity.20 These types of technologies may take years to develop and integrate into our therapeutic armamentarium.
If choosing opioid treatment for patients with CNCP/SUD, initially consider weak opioids such as codeine and tramadol.22 Tramadol, a partial μ agonist and weak inhibitor of serotonin and norepinephrine reuptake, is not a controlled substance and is indicated for moderate to severe pain; however, reports of its abuse potential are beginning to emerge. Tramadol has a frequency of abuse and withdrawal of approximately 2/100,000 patients taking the drug.23
Tapentadol has a dual mechanism of action—it combines a potent opioid agonist with a norepinephrine reuptake inhibitor—and is a schedule II medication. The norepinephrine and serotonin reuptake inhibition properties of tramadol and tapentadol can lead to undesired side effects and are less likely to be abused. Dart et al24 found tapentadol immediate release has the lowest abuse rate of all the opioids they studied, well below oxycodone and hydrocodone.
Methadone is a potent analgesic primarily used to treat opioid addiction, but it also is used for CNCP and cancer pain. With chronic use, methadone lacks the euphoric effect of other μ opioids; however, it can increase the QTc interval and has a long, variable half-life. As a result, methadone conversion tables are considered unreliable.
Methadone also has been associated with a disproportionate number of prescription opioid overdoses and deaths; it is present in 30% of all overdoses treated in emergency departments.4 Although methadone constitutes 5% of all opioid prescriptions in the United States, it is associated with one-third of opioid-related deaths, which is more than heroin and cocaine combined.14 Most methadone deaths occur within the first 7 days of initiating therapy, which suggests that patients were started on too high a dosage, were titrated too quickly, or had overestimated their tolerance.4 Reasons for methadone-related deaths are multifactorial and include:
• physician error and lack of knowledge
• patient nonadherence
• unanticipated comorbidities
• polypharmacy
• obstructive sleep apnea
• third-party payer policies listing it as first tier because of its low cost.4
In a Swedish study of 60 patients taking methadone, 75% had good pain relief on an average dose of 81.5 mg/d, whereas 25% had only moderate pain relief at a higher average dose of 157.5 mg/d. The authors described a methadone syndrome that included sedation, weakness, lethargy, weight gain, sweating, and sexual dysfunction, and that decreased the quality of life in 50% of patients.25 Another study found that among patients who died from sudden cardiac death and had methadone present in the toxicology screen, 45% were taking other psychotropics.26 Researchers also found a synergistic effect with benzodiazepines and an independent risk of sudden cardiac death and recommended obtaining pulmonary function tests and an electrocardiogram before starting methadone therapy, especially at higher doses.
Buprenorphine is a schedule III partial ì agonist opioid with a bell-shaped dose-response curve with a ceiling effect on respiratory depression, making it safe with an overdose. Although it is indicated for opioid dependence maintenance, it has been used off-label to treat chronic pain. It causes less euphoria than many other opioids including methadone. Buprenorphine is 25 to 50 times more potent than morphine and has a half-life of 20 to 44 hours but can be abused.27 It is available as a tablet, an injectable, and a 7-day patch. A combination of buprenorphine and naltrexone has a lower abuse potential,28 is administered sublingually and can be prescribed only by certified physicians.29 A subcutaneous implantable form of buprenorphine, which lasts 6 months, is under FDA review.30
Bottom Line
Multidisciplinary care paired with psychological interventions and a treatment plan has some evidence of efficacy in treating pain in patients with chronic non-cancer pain at high risk of substance abuse. Physician education in both pain and addiction is paramount. Frequent supervision, screening, monitoring and careful selection of medications will help physicians optimize outcomes and reduce risks.
Related Resources
- Agency Medical Directors Group. Intra-agency guideline on opioid dosing for chronic non-cancer pain. http://agencymeddirectors.wa.gov/files/opioidgdline.pdf.
- Stevenson E, Cole J, Walker R, et al. Association of chronic noncancer pain with substance abuse treatment outcomes among a community mental health center sample [published online January 3, 2013]. Addictive Disorders and their Treatment. doi: 10.1097/ADT.0b013e31827b0cd9.
Drug Brand Names
Amitriptyline • Elavil Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone Codeine • Tylenol with Codeine, others
Desipramine • Norpramin Duloxetine • Cymbalta
Gabapentin • Neurontin Methadone • Dolophine
Milnacipran • Savella Morphine • Roxanol
Oxycodone • Percolone, OxyContin Pregabalin • Lyrica
Tapentadol • Nucynta Topiramate • Topamax
Tramadol • Ultram Venlafaxine • Effexor
Hydrocodone/acetaminophen • Vicodin, Lorcet, others
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors thank Zita Juska for her editorial assistance with this article.
Featured Audio
Mark Juska, MD, discusses strategies for treating patients with comorbid pain and substance use disorders. Dr. Juska is a Fellow, Department of Anesthesiology, Wayne State University, Detroit, Michigan.
1. Giordano J. Pain and addiction: words, meanings, actions in the age of DSM-5. Practical Pain Management. http://www.practicalpainmanagement.com/resources/ethics/pain-addiction-words-meanings-actions-age-dsm-5. November 1, 2010. Accessed May 28, 2013.
2. The Joint Commission. Facts about pain management. http://www.jointcommission.org/pain_management. Updated February 27, 2013. Accessed May 28, 2013.
3. Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346-1347.
4. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-35.
5. Miotto K, Kaufman A, Kong A, et al. Managing co-occurring substance use and pain disorders. Psychiatr Clin North Am. 2012;35(2):393-410.
6. Morasco BJ, Gritzner S, Lewis L, et al. Systematic review of prevention, correlates and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorders. Pain. 2011;152:488-497.
7. Edlund MJ, Martin BC, Fan MY, et al. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP Study. Drug Alcohol Depend. 2010;112(1-2):90-98.
8. Moore TM, Jones T, Browder JH, et al. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10(8):1426-1433.
9. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic non cancer pain: prediction and identification of aberrant drug-related behaviors. A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guidelines. J Pain. 2009;10(2):131-146.
10. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712-720.
11. Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med. 2011; 26(9):965-971.
12. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):
113-130.
13. Office of National Drug Control Policy. Epidemic: responding to America’s prescription drug abuse crisis. http://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/rx_abuse_plan.pdf. Accessed May 28, 2013.
14. Kuehn BM. Methadone overdose deaths rise with increased prescribing for pain. JAMA. 2012;308(8):749-750.
15. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107-112.
16. Currie SR, Hodgins DC, Crabtree A, et al. Outcome from integrated pain management treatment for recovering substance abusers. J Pain. 2003;4(2):91-100.
17. Morasco BJ, Corson K, Turk DC, et al. Association between substance use disorder status and pain-related function following 12 months of treatment in primary care patients with musculoskeletal pain. J Pain. 2011;12(3):352-359.
18. Hoffman BM, Papas RK, Chatkoff DK, et al. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007;26(1):1-9.
19. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3.
20. Moorman-Li R, Motycka CA, Inge LD, et al. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T. 2012;37(7):412-418.
21. Stanos SP, Bruckenthal P, Barkin RL. Strategies to reduce the tampering and subsequent abuse of long-acting opioids: potential risks and benefits of formulations with physical or pharmacologic deterrents to tampering. Mayo Clinic Proc. 2012;87(7):683-694.
22. Substance Abuse and Mental Health Services Administration. Managing chronic pain in adults with or in recovery from substance use disorders. Treatment Improvement Protocol (TIP) Series 54. HHS Publication No. (SMA) 12-4671. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
23. Senay EC, Adams EH, Geller A, et al. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend. 2003;69:233-241.
24. Dart RC, Cicero TJ, Surratt HL, et al. Assessment of the abuse of tapentadol immediate release: the first 24 months. J Opioid Manag. 2012;8(6):395-402.
25. Rhodin A, Grönbladh L, Nilsson LH, et al. Methadone treatment of chronic non-malignant pain and opioid dependence—a long-term follow-up. Eur J Pain. 2006; 10(3):271-278.
26. Chuh SS, Socoteanu C, Reinier K, et al. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121(1):66-71.
27. Drug Enforcement Administration. Buprenorphine. http://www.deadiversion.usdoj.gov/drug_chem_info/buprenorphine.pdf. Accessed June 6, 2013.
28. Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15(3):169-178.
29. Substance Abuse and Mental Health Administration. Buprenorphine. http://buprenorphine.samhsa.gov/about.html. Accessed May 28, 2013.
30. Ling W, Casadonte P, Bigelow G, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304(14):1576-1583.
Patients with chronic non-cancer pain (CNCP) and a comorbid substance use disorder (SUD) are difficult to treat. There is a lack of high-quality clinical trials to guide management. This article focuses on current research, guidelines, and recommendations to best manage these patients. We present an analysis of recent statistics, patient characteristics, screening methods, as well as a discussion of changes to DSM-5 regarding substance abuse and addiction (Box 1).1
Opioid use and opioid-related overdoses have increased dramatically over the last decade (Box 2).2-5 Opioids are the primary medication used to treat CNCP, but their use in patients with comorbid SUDs is controversial. It is crucial for psychiatrists and other clinicians to know how to best identify, manage, and treat patients with CNCP/SUD.
Risk factors for CNCP/SUD
Evidence regarding the efficacy of screening methods to identify patients with chronic pain who are at high risk for substance misuse is insufficient. Key risk factors for developing chronic pain may include:
• elevated psychological distress
• negative beliefs and expectations about pain
• pain fear and avoidance
• disability
• anger or hostility
• maladaptive coping strategies
• catastrophic behaviors.5
In addition, these individuals may have a spouse who enables the sick role behavior.
Risk factors for developing a SUD related to prescribed opioids include:
• a history of problematic substance use
• sedative-hypnotic use
• positive family history for substance abuse
• legal problems
• heavy tobacco use
• age <50
• major depressive disorder or anxiety.5
In a review of 38 articles, Morasco et al6 found low-grade evidence with mixed results in attempt to find a correlation among sex, depression, anxiety, and tobacco use with CNCP/SUD. Other data suggest that the risk of addiction once opioids have been started increases with long refill periods and opioid morphine equivalents >120 mg.7 A history of childhood sexual abuse also may be a risk factor for chronic pain and addiction.5
Prevalence
The prevalence of opioid abuse among CNCP patients ranges from 3% to 48%; the highest rates are found among patients visiting the emergency room for opioid refills.7 These patients are more likely to exhibit aberrant behavior with their medications and may be prescribed higher opioid doses than patients who have CNCP only. Adherent CNCP/SUD patients show no difference in response to pain treatment compared with those with CNCP alone.6 Approximately 11.5% of CNCP patients taking opioids demonstrate aberrant medication use.6
Screening: Which method is best?
Data are scarce regarding the best screening methods to identify patients with CNCP/SUD. A survey of 48 patients by Moore et al8 found the combination of a clinical interview and the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) is 90% sensitive in detecting CNCP/SUD. However, a systematic review by Chou et al9 found only 2 well-designed studies showing that the SOAPP-R weakly predicts future aberrant drug behavior and only 1 study showed that a high risk categorization on the Opioid Risk Tool (ORT) strongly increased the likelihood of predicting future abnormal drug-related behavior. Another well-designed study showed that the Current Opioid Misuse Measure (COMM) weakly raised the likelihood of detecting current aberrant drug behavior. No reliable data supported the efficacy of urine drug screens (UDS), pill counts, or prescription drug monitoring programs (PDMPs) for improving clinical outcomes.9 In a systematic review Starrels et al10 found only low-quality evidence supporting the effectiveness of opioid agreement contracts and UDS.
Treatment strategies
Once a patient with CNCP/SUD has been identified, it is important to categorize the severity of his (her) pain and substance use by using the decision tree (Figure) and screening tools such as SOAPP-R, ORT, and COMM. In a Veterans Administration (VA) study, only 35% of patients with an SUD received substance abuse treatment.11 The 2009 American Pain Society/American Academy of Pain Medicine guidelines recommended that opioids should considered for patients with substance abuse, serious aberrant drug-related behaviors, or psychiatric comorbidities only if frequent monitoring and treatment plan and mental health or addiction consultation were in place.12 These guidelines also recommended discontinuing opioids if repeated atypical behavior, substance abuse, diversion, lack of progress, or intolerable side effects occur. Repeated and more serious behaviors require a multidisciplinary team, expert consultation, therapy restructuring, and possibly discontinuation of opioids.12
The U.S. Office of National Drug Control Policy has created a council of federal agencies to spearhead the Prescription Drug Abuse Prevention Plan, which includes 4 major categories to reduce prescription drug abuse: education, monitoring, proper disposal, and enforcement.13 FDA commissioner Margaret Hamburg supports legislation to combine opioid education with Drug Enforcement Administration registration.14 The FDA began developing the risk evaluation and mitigation strategies in 2007 to educate physicians on proper prescribing of potentially dangerous medications.
Gourlay and Heit proposed a universal precautions method of opioid treatment for all pain patients.15 That includes:
• seeking differential diagnoses and comorbidities
• doing a baseline addiction assessment with UDS and PDMP evaluations
• obtaining informed consent for pain management
• creating pre- and post-treatment goals for pain and function
• evaluating the 4 “As” (analgesic response, increased activity, adverse events, and aberrant behavior)
• reviewing the evolution of the pain and comorbidities
• continuous documentation.5
Other helpful strategies include the Oregon’s SMART (Specific, Measurable, Action-oriented, Realistic, Time-Dependent) goal-setting, which helps physicians negotiate functional goals with patients and plan an exit strategy for those whose quality of life does not improve with opioids.5 Clinicians also can consider a sequential treatment model where patients with severe substance abuse and pain are detoxified of illicit drugs and alcohol before starting pain management. This approach is more effective if the pain is secondary to a more severe substance abuse problem that is not correlated to physical pain and acute rather than chronic.16
Psychotherapeutic interventions
In another VA study, a collaborative care intervention (CCI) combining education, self-efficacy, pain management, and feedback was not impeded by a history of SUD. The authors recommended CCI, stepped care, integrated interventions, and relapse prevention and stressed the importance of social support.17
A 10-week cognitive-behavioral therapy (CBT) program involving 44 patients enrolled in an integrated pain management program for recovering substance abusers found 50% of CNCP/SUD patients were opioid-free at 12 months.16 A combination of medication reduction and education resulted in less pain, increased functioning, decreased emotional distress, and less self-medicating. Additionally, patients reported 35% overall reduction in pain severity based on the McGill Pain Questionnaire but only 25% of patients showed a reliable improvement in their pain. Treatment changes lasted 1 year.16
A meta-analysis of psychological interventions such as CBT, behavioral treatment (BT), and self-regulated treatment (SRT) indicated that CBT and BT are moderately effective at lowering work-related disability and pain intensity for chronic low back pain alone or with multidisciplinary care and moderately lowered work-related disability. CBT had a moderate to large effect, while SRT with biofeedback and relaxation techniques had a large effect on lowering pain intensity. SRT also was shown to lower depression. Return-to-work rates were better with multidisciplinary care that included psychological interventions. These psychological interventions for chronic low back pain lowered self-reported pain, pain interference, depression, and disability while increasing quality of life; the largest effect was on pain intensity.18
A review by Williams et al19 analyzing the effects of BT and CBT on various outcome measures, including chronic pain, found small to moderate benefits for disability, mood, and catastrophic thinking with CBT, which lasted up to 6 months. Only weak improvements in pain were seen with CBT immediately after treatment. BT had a beneficial effect on catastrophic thinking but only right after treatment. CBT’s overall effect in these patients was positive, and changes lasted up to 6 months.
Pharmacologic treatments
Before and during opioid therapy, psychotherapy, physical therapy, and occupational therapy should be used with adjuvant medications appropriate to the pain condition, such as anticonvulsants (gabapentin, pregabalin, topiramate) and antidepressants including tricyclic antidepressants (amitriptyline, desipramine) and serotonin-norepinephrine reuptake inhibitors (duloxetine, venlafaxine, milnacipran).12 When considering opioids for patients with CNCP/SUD, adverse effects and safety is a primary consideration. Benzodiazepines generally should not be used with opioids because of their synergistic sedating effects.5
Opioids are misused more often by overingestion than by altering the delivery route, yet most efforts to create tamper-resistant medications has focused on
snorting or injection, which are considered more dangerous. Current tamper-resistance strategies include:
• creating a hard shell to prevent crushing and altering the medications
• chemical combinations, using agonists and antagonists such as buprenorphine combined with naloxone
• prodrugs, which become activated only in the GI system
• implants or patches.20,21
One prodrug in phase-I testing, compound PF329, becomes activated only in the GI tract by exposure to trypsin. Because it also contains trypsin inhibitors, overingestion will not lead to toxicity.20 These types of technologies may take years to develop and integrate into our therapeutic armamentarium.
If choosing opioid treatment for patients with CNCP/SUD, initially consider weak opioids such as codeine and tramadol.22 Tramadol, a partial μ agonist and weak inhibitor of serotonin and norepinephrine reuptake, is not a controlled substance and is indicated for moderate to severe pain; however, reports of its abuse potential are beginning to emerge. Tramadol has a frequency of abuse and withdrawal of approximately 2/100,000 patients taking the drug.23
Tapentadol has a dual mechanism of action—it combines a potent opioid agonist with a norepinephrine reuptake inhibitor—and is a schedule II medication. The norepinephrine and serotonin reuptake inhibition properties of tramadol and tapentadol can lead to undesired side effects and are less likely to be abused. Dart et al24 found tapentadol immediate release has the lowest abuse rate of all the opioids they studied, well below oxycodone and hydrocodone.
Methadone is a potent analgesic primarily used to treat opioid addiction, but it also is used for CNCP and cancer pain. With chronic use, methadone lacks the euphoric effect of other μ opioids; however, it can increase the QTc interval and has a long, variable half-life. As a result, methadone conversion tables are considered unreliable.
Methadone also has been associated with a disproportionate number of prescription opioid overdoses and deaths; it is present in 30% of all overdoses treated in emergency departments.4 Although methadone constitutes 5% of all opioid prescriptions in the United States, it is associated with one-third of opioid-related deaths, which is more than heroin and cocaine combined.14 Most methadone deaths occur within the first 7 days of initiating therapy, which suggests that patients were started on too high a dosage, were titrated too quickly, or had overestimated their tolerance.4 Reasons for methadone-related deaths are multifactorial and include:
• physician error and lack of knowledge
• patient nonadherence
• unanticipated comorbidities
• polypharmacy
• obstructive sleep apnea
• third-party payer policies listing it as first tier because of its low cost.4
In a Swedish study of 60 patients taking methadone, 75% had good pain relief on an average dose of 81.5 mg/d, whereas 25% had only moderate pain relief at a higher average dose of 157.5 mg/d. The authors described a methadone syndrome that included sedation, weakness, lethargy, weight gain, sweating, and sexual dysfunction, and that decreased the quality of life in 50% of patients.25 Another study found that among patients who died from sudden cardiac death and had methadone present in the toxicology screen, 45% were taking other psychotropics.26 Researchers also found a synergistic effect with benzodiazepines and an independent risk of sudden cardiac death and recommended obtaining pulmonary function tests and an electrocardiogram before starting methadone therapy, especially at higher doses.
Buprenorphine is a schedule III partial ì agonist opioid with a bell-shaped dose-response curve with a ceiling effect on respiratory depression, making it safe with an overdose. Although it is indicated for opioid dependence maintenance, it has been used off-label to treat chronic pain. It causes less euphoria than many other opioids including methadone. Buprenorphine is 25 to 50 times more potent than morphine and has a half-life of 20 to 44 hours but can be abused.27 It is available as a tablet, an injectable, and a 7-day patch. A combination of buprenorphine and naltrexone has a lower abuse potential,28 is administered sublingually and can be prescribed only by certified physicians.29 A subcutaneous implantable form of buprenorphine, which lasts 6 months, is under FDA review.30
Bottom Line
Multidisciplinary care paired with psychological interventions and a treatment plan has some evidence of efficacy in treating pain in patients with chronic non-cancer pain at high risk of substance abuse. Physician education in both pain and addiction is paramount. Frequent supervision, screening, monitoring and careful selection of medications will help physicians optimize outcomes and reduce risks.
Related Resources
- Agency Medical Directors Group. Intra-agency guideline on opioid dosing for chronic non-cancer pain. http://agencymeddirectors.wa.gov/files/opioidgdline.pdf.
- Stevenson E, Cole J, Walker R, et al. Association of chronic noncancer pain with substance abuse treatment outcomes among a community mental health center sample [published online January 3, 2013]. Addictive Disorders and their Treatment. doi: 10.1097/ADT.0b013e31827b0cd9.
Drug Brand Names
Amitriptyline • Elavil Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone Codeine • Tylenol with Codeine, others
Desipramine • Norpramin Duloxetine • Cymbalta
Gabapentin • Neurontin Methadone • Dolophine
Milnacipran • Savella Morphine • Roxanol
Oxycodone • Percolone, OxyContin Pregabalin • Lyrica
Tapentadol • Nucynta Topiramate • Topamax
Tramadol • Ultram Venlafaxine • Effexor
Hydrocodone/acetaminophen • Vicodin, Lorcet, others
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors thank Zita Juska for her editorial assistance with this article.
Featured Audio
Mark Juska, MD, discusses strategies for treating patients with comorbid pain and substance use disorders. Dr. Juska is a Fellow, Department of Anesthesiology, Wayne State University, Detroit, Michigan.
Patients with chronic non-cancer pain (CNCP) and a comorbid substance use disorder (SUD) are difficult to treat. There is a lack of high-quality clinical trials to guide management. This article focuses on current research, guidelines, and recommendations to best manage these patients. We present an analysis of recent statistics, patient characteristics, screening methods, as well as a discussion of changes to DSM-5 regarding substance abuse and addiction (Box 1).1
Opioid use and opioid-related overdoses have increased dramatically over the last decade (Box 2).2-5 Opioids are the primary medication used to treat CNCP, but their use in patients with comorbid SUDs is controversial. It is crucial for psychiatrists and other clinicians to know how to best identify, manage, and treat patients with CNCP/SUD.
Risk factors for CNCP/SUD
Evidence regarding the efficacy of screening methods to identify patients with chronic pain who are at high risk for substance misuse is insufficient. Key risk factors for developing chronic pain may include:
• elevated psychological distress
• negative beliefs and expectations about pain
• pain fear and avoidance
• disability
• anger or hostility
• maladaptive coping strategies
• catastrophic behaviors.5
In addition, these individuals may have a spouse who enables the sick role behavior.
Risk factors for developing a SUD related to prescribed opioids include:
• a history of problematic substance use
• sedative-hypnotic use
• positive family history for substance abuse
• legal problems
• heavy tobacco use
• age <50
• major depressive disorder or anxiety.5
In a review of 38 articles, Morasco et al6 found low-grade evidence with mixed results in attempt to find a correlation among sex, depression, anxiety, and tobacco use with CNCP/SUD. Other data suggest that the risk of addiction once opioids have been started increases with long refill periods and opioid morphine equivalents >120 mg.7 A history of childhood sexual abuse also may be a risk factor for chronic pain and addiction.5
Prevalence
The prevalence of opioid abuse among CNCP patients ranges from 3% to 48%; the highest rates are found among patients visiting the emergency room for opioid refills.7 These patients are more likely to exhibit aberrant behavior with their medications and may be prescribed higher opioid doses than patients who have CNCP only. Adherent CNCP/SUD patients show no difference in response to pain treatment compared with those with CNCP alone.6 Approximately 11.5% of CNCP patients taking opioids demonstrate aberrant medication use.6
Screening: Which method is best?
Data are scarce regarding the best screening methods to identify patients with CNCP/SUD. A survey of 48 patients by Moore et al8 found the combination of a clinical interview and the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) is 90% sensitive in detecting CNCP/SUD. However, a systematic review by Chou et al9 found only 2 well-designed studies showing that the SOAPP-R weakly predicts future aberrant drug behavior and only 1 study showed that a high risk categorization on the Opioid Risk Tool (ORT) strongly increased the likelihood of predicting future abnormal drug-related behavior. Another well-designed study showed that the Current Opioid Misuse Measure (COMM) weakly raised the likelihood of detecting current aberrant drug behavior. No reliable data supported the efficacy of urine drug screens (UDS), pill counts, or prescription drug monitoring programs (PDMPs) for improving clinical outcomes.9 In a systematic review Starrels et al10 found only low-quality evidence supporting the effectiveness of opioid agreement contracts and UDS.
Treatment strategies
Once a patient with CNCP/SUD has been identified, it is important to categorize the severity of his (her) pain and substance use by using the decision tree (Figure) and screening tools such as SOAPP-R, ORT, and COMM. In a Veterans Administration (VA) study, only 35% of patients with an SUD received substance abuse treatment.11 The 2009 American Pain Society/American Academy of Pain Medicine guidelines recommended that opioids should considered for patients with substance abuse, serious aberrant drug-related behaviors, or psychiatric comorbidities only if frequent monitoring and treatment plan and mental health or addiction consultation were in place.12 These guidelines also recommended discontinuing opioids if repeated atypical behavior, substance abuse, diversion, lack of progress, or intolerable side effects occur. Repeated and more serious behaviors require a multidisciplinary team, expert consultation, therapy restructuring, and possibly discontinuation of opioids.12
The U.S. Office of National Drug Control Policy has created a council of federal agencies to spearhead the Prescription Drug Abuse Prevention Plan, which includes 4 major categories to reduce prescription drug abuse: education, monitoring, proper disposal, and enforcement.13 FDA commissioner Margaret Hamburg supports legislation to combine opioid education with Drug Enforcement Administration registration.14 The FDA began developing the risk evaluation and mitigation strategies in 2007 to educate physicians on proper prescribing of potentially dangerous medications.
Gourlay and Heit proposed a universal precautions method of opioid treatment for all pain patients.15 That includes:
• seeking differential diagnoses and comorbidities
• doing a baseline addiction assessment with UDS and PDMP evaluations
• obtaining informed consent for pain management
• creating pre- and post-treatment goals for pain and function
• evaluating the 4 “As” (analgesic response, increased activity, adverse events, and aberrant behavior)
• reviewing the evolution of the pain and comorbidities
• continuous documentation.5
Other helpful strategies include the Oregon’s SMART (Specific, Measurable, Action-oriented, Realistic, Time-Dependent) goal-setting, which helps physicians negotiate functional goals with patients and plan an exit strategy for those whose quality of life does not improve with opioids.5 Clinicians also can consider a sequential treatment model where patients with severe substance abuse and pain are detoxified of illicit drugs and alcohol before starting pain management. This approach is more effective if the pain is secondary to a more severe substance abuse problem that is not correlated to physical pain and acute rather than chronic.16
Psychotherapeutic interventions
In another VA study, a collaborative care intervention (CCI) combining education, self-efficacy, pain management, and feedback was not impeded by a history of SUD. The authors recommended CCI, stepped care, integrated interventions, and relapse prevention and stressed the importance of social support.17
A 10-week cognitive-behavioral therapy (CBT) program involving 44 patients enrolled in an integrated pain management program for recovering substance abusers found 50% of CNCP/SUD patients were opioid-free at 12 months.16 A combination of medication reduction and education resulted in less pain, increased functioning, decreased emotional distress, and less self-medicating. Additionally, patients reported 35% overall reduction in pain severity based on the McGill Pain Questionnaire but only 25% of patients showed a reliable improvement in their pain. Treatment changes lasted 1 year.16
A meta-analysis of psychological interventions such as CBT, behavioral treatment (BT), and self-regulated treatment (SRT) indicated that CBT and BT are moderately effective at lowering work-related disability and pain intensity for chronic low back pain alone or with multidisciplinary care and moderately lowered work-related disability. CBT had a moderate to large effect, while SRT with biofeedback and relaxation techniques had a large effect on lowering pain intensity. SRT also was shown to lower depression. Return-to-work rates were better with multidisciplinary care that included psychological interventions. These psychological interventions for chronic low back pain lowered self-reported pain, pain interference, depression, and disability while increasing quality of life; the largest effect was on pain intensity.18
A review by Williams et al19 analyzing the effects of BT and CBT on various outcome measures, including chronic pain, found small to moderate benefits for disability, mood, and catastrophic thinking with CBT, which lasted up to 6 months. Only weak improvements in pain were seen with CBT immediately after treatment. BT had a beneficial effect on catastrophic thinking but only right after treatment. CBT’s overall effect in these patients was positive, and changes lasted up to 6 months.
Pharmacologic treatments
Before and during opioid therapy, psychotherapy, physical therapy, and occupational therapy should be used with adjuvant medications appropriate to the pain condition, such as anticonvulsants (gabapentin, pregabalin, topiramate) and antidepressants including tricyclic antidepressants (amitriptyline, desipramine) and serotonin-norepinephrine reuptake inhibitors (duloxetine, venlafaxine, milnacipran).12 When considering opioids for patients with CNCP/SUD, adverse effects and safety is a primary consideration. Benzodiazepines generally should not be used with opioids because of their synergistic sedating effects.5
Opioids are misused more often by overingestion than by altering the delivery route, yet most efforts to create tamper-resistant medications has focused on
snorting or injection, which are considered more dangerous. Current tamper-resistance strategies include:
• creating a hard shell to prevent crushing and altering the medications
• chemical combinations, using agonists and antagonists such as buprenorphine combined with naloxone
• prodrugs, which become activated only in the GI system
• implants or patches.20,21
One prodrug in phase-I testing, compound PF329, becomes activated only in the GI tract by exposure to trypsin. Because it also contains trypsin inhibitors, overingestion will not lead to toxicity.20 These types of technologies may take years to develop and integrate into our therapeutic armamentarium.
If choosing opioid treatment for patients with CNCP/SUD, initially consider weak opioids such as codeine and tramadol.22 Tramadol, a partial μ agonist and weak inhibitor of serotonin and norepinephrine reuptake, is not a controlled substance and is indicated for moderate to severe pain; however, reports of its abuse potential are beginning to emerge. Tramadol has a frequency of abuse and withdrawal of approximately 2/100,000 patients taking the drug.23
Tapentadol has a dual mechanism of action—it combines a potent opioid agonist with a norepinephrine reuptake inhibitor—and is a schedule II medication. The norepinephrine and serotonin reuptake inhibition properties of tramadol and tapentadol can lead to undesired side effects and are less likely to be abused. Dart et al24 found tapentadol immediate release has the lowest abuse rate of all the opioids they studied, well below oxycodone and hydrocodone.
Methadone is a potent analgesic primarily used to treat opioid addiction, but it also is used for CNCP and cancer pain. With chronic use, methadone lacks the euphoric effect of other μ opioids; however, it can increase the QTc interval and has a long, variable half-life. As a result, methadone conversion tables are considered unreliable.
Methadone also has been associated with a disproportionate number of prescription opioid overdoses and deaths; it is present in 30% of all overdoses treated in emergency departments.4 Although methadone constitutes 5% of all opioid prescriptions in the United States, it is associated with one-third of opioid-related deaths, which is more than heroin and cocaine combined.14 Most methadone deaths occur within the first 7 days of initiating therapy, which suggests that patients were started on too high a dosage, were titrated too quickly, or had overestimated their tolerance.4 Reasons for methadone-related deaths are multifactorial and include:
• physician error and lack of knowledge
• patient nonadherence
• unanticipated comorbidities
• polypharmacy
• obstructive sleep apnea
• third-party payer policies listing it as first tier because of its low cost.4
In a Swedish study of 60 patients taking methadone, 75% had good pain relief on an average dose of 81.5 mg/d, whereas 25% had only moderate pain relief at a higher average dose of 157.5 mg/d. The authors described a methadone syndrome that included sedation, weakness, lethargy, weight gain, sweating, and sexual dysfunction, and that decreased the quality of life in 50% of patients.25 Another study found that among patients who died from sudden cardiac death and had methadone present in the toxicology screen, 45% were taking other psychotropics.26 Researchers also found a synergistic effect with benzodiazepines and an independent risk of sudden cardiac death and recommended obtaining pulmonary function tests and an electrocardiogram before starting methadone therapy, especially at higher doses.
Buprenorphine is a schedule III partial ì agonist opioid with a bell-shaped dose-response curve with a ceiling effect on respiratory depression, making it safe with an overdose. Although it is indicated for opioid dependence maintenance, it has been used off-label to treat chronic pain. It causes less euphoria than many other opioids including methadone. Buprenorphine is 25 to 50 times more potent than morphine and has a half-life of 20 to 44 hours but can be abused.27 It is available as a tablet, an injectable, and a 7-day patch. A combination of buprenorphine and naltrexone has a lower abuse potential,28 is administered sublingually and can be prescribed only by certified physicians.29 A subcutaneous implantable form of buprenorphine, which lasts 6 months, is under FDA review.30
Bottom Line
Multidisciplinary care paired with psychological interventions and a treatment plan has some evidence of efficacy in treating pain in patients with chronic non-cancer pain at high risk of substance abuse. Physician education in both pain and addiction is paramount. Frequent supervision, screening, monitoring and careful selection of medications will help physicians optimize outcomes and reduce risks.
Related Resources
- Agency Medical Directors Group. Intra-agency guideline on opioid dosing for chronic non-cancer pain. http://agencymeddirectors.wa.gov/files/opioidgdline.pdf.
- Stevenson E, Cole J, Walker R, et al. Association of chronic noncancer pain with substance abuse treatment outcomes among a community mental health center sample [published online January 3, 2013]. Addictive Disorders and their Treatment. doi: 10.1097/ADT.0b013e31827b0cd9.
Drug Brand Names
Amitriptyline • Elavil Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone Codeine • Tylenol with Codeine, others
Desipramine • Norpramin Duloxetine • Cymbalta
Gabapentin • Neurontin Methadone • Dolophine
Milnacipran • Savella Morphine • Roxanol
Oxycodone • Percolone, OxyContin Pregabalin • Lyrica
Tapentadol • Nucynta Topiramate • Topamax
Tramadol • Ultram Venlafaxine • Effexor
Hydrocodone/acetaminophen • Vicodin, Lorcet, others
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors thank Zita Juska for her editorial assistance with this article.
Featured Audio
Mark Juska, MD, discusses strategies for treating patients with comorbid pain and substance use disorders. Dr. Juska is a Fellow, Department of Anesthesiology, Wayne State University, Detroit, Michigan.
1. Giordano J. Pain and addiction: words, meanings, actions in the age of DSM-5. Practical Pain Management. http://www.practicalpainmanagement.com/resources/ethics/pain-addiction-words-meanings-actions-age-dsm-5. November 1, 2010. Accessed May 28, 2013.
2. The Joint Commission. Facts about pain management. http://www.jointcommission.org/pain_management. Updated February 27, 2013. Accessed May 28, 2013.
3. Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346-1347.
4. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-35.
5. Miotto K, Kaufman A, Kong A, et al. Managing co-occurring substance use and pain disorders. Psychiatr Clin North Am. 2012;35(2):393-410.
6. Morasco BJ, Gritzner S, Lewis L, et al. Systematic review of prevention, correlates and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorders. Pain. 2011;152:488-497.
7. Edlund MJ, Martin BC, Fan MY, et al. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP Study. Drug Alcohol Depend. 2010;112(1-2):90-98.
8. Moore TM, Jones T, Browder JH, et al. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10(8):1426-1433.
9. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic non cancer pain: prediction and identification of aberrant drug-related behaviors. A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guidelines. J Pain. 2009;10(2):131-146.
10. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712-720.
11. Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med. 2011; 26(9):965-971.
12. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):
113-130.
13. Office of National Drug Control Policy. Epidemic: responding to America’s prescription drug abuse crisis. http://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/rx_abuse_plan.pdf. Accessed May 28, 2013.
14. Kuehn BM. Methadone overdose deaths rise with increased prescribing for pain. JAMA. 2012;308(8):749-750.
15. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107-112.
16. Currie SR, Hodgins DC, Crabtree A, et al. Outcome from integrated pain management treatment for recovering substance abusers. J Pain. 2003;4(2):91-100.
17. Morasco BJ, Corson K, Turk DC, et al. Association between substance use disorder status and pain-related function following 12 months of treatment in primary care patients with musculoskeletal pain. J Pain. 2011;12(3):352-359.
18. Hoffman BM, Papas RK, Chatkoff DK, et al. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007;26(1):1-9.
19. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3.
20. Moorman-Li R, Motycka CA, Inge LD, et al. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T. 2012;37(7):412-418.
21. Stanos SP, Bruckenthal P, Barkin RL. Strategies to reduce the tampering and subsequent abuse of long-acting opioids: potential risks and benefits of formulations with physical or pharmacologic deterrents to tampering. Mayo Clinic Proc. 2012;87(7):683-694.
22. Substance Abuse and Mental Health Services Administration. Managing chronic pain in adults with or in recovery from substance use disorders. Treatment Improvement Protocol (TIP) Series 54. HHS Publication No. (SMA) 12-4671. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
23. Senay EC, Adams EH, Geller A, et al. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend. 2003;69:233-241.
24. Dart RC, Cicero TJ, Surratt HL, et al. Assessment of the abuse of tapentadol immediate release: the first 24 months. J Opioid Manag. 2012;8(6):395-402.
25. Rhodin A, Grönbladh L, Nilsson LH, et al. Methadone treatment of chronic non-malignant pain and opioid dependence—a long-term follow-up. Eur J Pain. 2006; 10(3):271-278.
26. Chuh SS, Socoteanu C, Reinier K, et al. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121(1):66-71.
27. Drug Enforcement Administration. Buprenorphine. http://www.deadiversion.usdoj.gov/drug_chem_info/buprenorphine.pdf. Accessed June 6, 2013.
28. Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15(3):169-178.
29. Substance Abuse and Mental Health Administration. Buprenorphine. http://buprenorphine.samhsa.gov/about.html. Accessed May 28, 2013.
30. Ling W, Casadonte P, Bigelow G, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304(14):1576-1583.
1. Giordano J. Pain and addiction: words, meanings, actions in the age of DSM-5. Practical Pain Management. http://www.practicalpainmanagement.com/resources/ethics/pain-addiction-words-meanings-actions-age-dsm-5. November 1, 2010. Accessed May 28, 2013.
2. The Joint Commission. Facts about pain management. http://www.jointcommission.org/pain_management. Updated February 27, 2013. Accessed May 28, 2013.
3. Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346-1347.
4. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-35.
5. Miotto K, Kaufman A, Kong A, et al. Managing co-occurring substance use and pain disorders. Psychiatr Clin North Am. 2012;35(2):393-410.
6. Morasco BJ, Gritzner S, Lewis L, et al. Systematic review of prevention, correlates and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorders. Pain. 2011;152:488-497.
7. Edlund MJ, Martin BC, Fan MY, et al. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP Study. Drug Alcohol Depend. 2010;112(1-2):90-98.
8. Moore TM, Jones T, Browder JH, et al. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10(8):1426-1433.
9. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic non cancer pain: prediction and identification of aberrant drug-related behaviors. A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guidelines. J Pain. 2009;10(2):131-146.
10. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712-720.
11. Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med. 2011; 26(9):965-971.
12. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):
113-130.
13. Office of National Drug Control Policy. Epidemic: responding to America’s prescription drug abuse crisis. http://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/rx_abuse_plan.pdf. Accessed May 28, 2013.
14. Kuehn BM. Methadone overdose deaths rise with increased prescribing for pain. JAMA. 2012;308(8):749-750.
15. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107-112.
16. Currie SR, Hodgins DC, Crabtree A, et al. Outcome from integrated pain management treatment for recovering substance abusers. J Pain. 2003;4(2):91-100.
17. Morasco BJ, Corson K, Turk DC, et al. Association between substance use disorder status and pain-related function following 12 months of treatment in primary care patients with musculoskeletal pain. J Pain. 2011;12(3):352-359.
18. Hoffman BM, Papas RK, Chatkoff DK, et al. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007;26(1):1-9.
19. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3.
20. Moorman-Li R, Motycka CA, Inge LD, et al. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T. 2012;37(7):412-418.
21. Stanos SP, Bruckenthal P, Barkin RL. Strategies to reduce the tampering and subsequent abuse of long-acting opioids: potential risks and benefits of formulations with physical or pharmacologic deterrents to tampering. Mayo Clinic Proc. 2012;87(7):683-694.
22. Substance Abuse and Mental Health Services Administration. Managing chronic pain in adults with or in recovery from substance use disorders. Treatment Improvement Protocol (TIP) Series 54. HHS Publication No. (SMA) 12-4671. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
23. Senay EC, Adams EH, Geller A, et al. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend. 2003;69:233-241.
24. Dart RC, Cicero TJ, Surratt HL, et al. Assessment of the abuse of tapentadol immediate release: the first 24 months. J Opioid Manag. 2012;8(6):395-402.
25. Rhodin A, Grönbladh L, Nilsson LH, et al. Methadone treatment of chronic non-malignant pain and opioid dependence—a long-term follow-up. Eur J Pain. 2006; 10(3):271-278.
26. Chuh SS, Socoteanu C, Reinier K, et al. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121(1):66-71.
27. Drug Enforcement Administration. Buprenorphine. http://www.deadiversion.usdoj.gov/drug_chem_info/buprenorphine.pdf. Accessed June 6, 2013.
28. Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15(3):169-178.
29. Substance Abuse and Mental Health Administration. Buprenorphine. http://buprenorphine.samhsa.gov/about.html. Accessed May 28, 2013.
30. Ling W, Casadonte P, Bigelow G, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304(14):1576-1583.
Exercise prescription: A practical, effective therapy for depression
Mrs. S, age 44, is on leave from her job as a bank cashier because depressive symptoms interfered with her performance. At a university-based psychiatric clinic she reports feeling depressed, reduced interest in daily activities, problems with sleep onset and maintenance, inconsistent appetite, low energy, hopelessness, and decreased memory and concentration.
The resident psychiatrist diagnoses major depressive disorder (MDD) and starts Mrs. S on sertraline, 50 mg/d. The dosage is gradually titrated to 200 mg/d, and after 8 weeks she reports substantial improvement.
Mrs. S returns to her job but experiences residual low energy, lethargy, and inconsistent sleep. Her work schedule and caring for her 2 children at home prevent her from continuing weekly cognitive-behavioral therapy (CBT), but she soon notices that she feels more energetic. She reports that because of high gasoline prices she has been walking several miles daily to commute by train to work. The resident psychiatrist sees this as an opportunity to reinforce the benefits of exercise for depression.
Antidepressants alone do not adequately treat many patients with depression. In the STAR*D Project—which compared long-term outcomes of various depression treatments—only 28% to 33% of outpatients achieved remission with selective serotonin reuptake inhibitor (SSRI) monotherapy. Rates were somewhat higher with bupropion or serotonin norepinephrine reuptake inhibitor (SNRI) monotherapy, but greater benefit was obtained from augmenting SSRIs.1
Combining antidepressants with psychotherapy2 and lifestyle changes—particularly exercise—makes sense intuitively and is supported by well-designed studies:
- The 60% of adults in the National Comorbidity Survey who said they exercised regularly reported lower rates of depression and anxiety, compared with less active adults.3
- A meta-analysis of 11 randomized, controlled trials supports the use of exercise as an effective intervention for clinical depression.4
Elevation of endorphins in the CNS
Changes in neurotransmitters such as serotonin and norepinephrine
Increased levels of brain-derived neurotrophic factor
Reduction of serum cortisol
Elevation of body temperature
Improved self-esteem
Distraction from daily stress
Induction of a relaxed state via biofeedback
This article examines the evidence supporting exercise for treating and preventing clinical depression. We begin by addressing clinicians’ concerns about motivating depressed patients to exercise.
Overcoming barriers
Physician issues. Busy physicians often omit discussions about exercise during brief office visits. Only 34% of 9,299 patients in a population-based survey5 reported that their doctors counseled them about exercise during their most recent visits. Counseling patients does not have to be time-intensive, however. A study of the Physician-based Assessment and Counseling for Exercise (PACE) project showed that 70% of physicians could provide exercise counseling in 3 to 5 minutes, and most patients reported following their physicians’ advice.6
Highly depressed individuals are at risk to quit when they encounter barriers to exercise and to respond to difficulties with frustration and self-disappointment. Thus, depressed patients may need support and encouragement to initiate and maintain regular exercise routines.7 Set small, realistic goals for them, and discuss how to solve problems and remove barriers to increase their likelihood to exercise.
Interventions are most likely to be effective when you counsel patients about exercise as prescription and discuss exercise at each visit.8 Previously sedentary patients have shown short-term moderate increases in physical activity in response to physician counseling. In a study of 212 adults (mean age 39, 84% female), the PACE project significantly increased minutes of weekly walking.9 More than one-half (52%) of patients increased their physical activity, compared with 12% of controls whose physicians did not provide the PACE intervention.
Patient issues. Lack of time and no appropriate space to exercise are common complaints, particularly among residents of regions with long, cold winters. Some patients perceive regular exercise as monotonous or boring, and others may lack the necessary initiative because of poor physical health, fear, negative experiences, or lack of knowledge about exercising. These barriers can be pronounced in older depressed persons. In a cross-sectional study of 645 residents of Jyväskylä, Finland, those age >75 with depressive symptoms were more than twice as likely to be physically inactive as nondepressed residents.10
An intensive exercise program is not the optimal starting point for many patients. Even walking or light jogging can be an effective exercise for depressed individuals with physical limitations. For these patients, a consultation with their primary physician may be necessary if a more intensive program has to be recommended.
Exercise as monotherapy
A dose-response relationship? Various mechanisms have been suggested for the benefits of exercise in depression (Box 1). Exercise alone—without medication—may be an effective treatment for mild and in some cases moderate MDD, and aerobic exercise may reduce depressive symptoms in a dose-response relationship.11
A study of exercise in a supervised laboratory setting demonstrated this relationship in 80 adults age 20 to 45 with mild-to-moderate depressive symptoms. Subjects were randomly assigned to an exercise control group (3 days/week of flexibility exercise) or 1 of 4 aerobic exercise groups that varied in total energy expenditure (a “low dose” of 7.0 kcal/kg/week or a “public health dose” of 17.5 kcal/kg/week). The 17-item Hamilton Rating Scale for Depression (HRSD) was the primary outcome measure.
After 12 weeks, HRSD scores declined from baseline by 47% in subjects engaged in the public health dose of aerobic exercise—a significant reduction. Depressive symptoms declined by 30% in the low-dose exercisers, but this was comparable to the 29% reduction in the control group.
Comment. The effective exercise dose in this study is similar to the public health recommendation of 30 minutes of moderate-to-vigorous activity on all or most days per week (see Related Resources). Antidepressant effects have been associated with more modest physical activity, however, which may be easier to initiate and maintain for individuals with depression. The study did not find significant differences in outcomes based on the subjects’ age, gender, or exercise frequency. Nevertheless, the exercise dose may be important to produce an antidepressant effect.
An inverse relationship? Compared with occasional exercise, habitual physical activity usually is associated with greater cardiorespiratory fitness. Whether habitual activity also results in fewer depressive symptoms and greater emotional well-being remains to be seen.
A large, cross-sectional, National Institutes of Health-funded study of 5,451 men and 1,277 women12 suggests an inverse relationship between physical activity and depressive symptoms. Subjects underwent a treadmill exercise test to evaluate physical fitness. A 20-point self-report scale assessed depressive symptoms, and the General Well-Being Schedule13 was used to assess emotional well-being. Depressive symptoms were more severe in “inactive” and “insufficiently active” subjects compared with “sufficiently active” and “highly active” subjects.
On the other hand, although regular exercise may be associated with reduced depressive symptoms in the population at large, no cause-effect relationship was found in a population-based, longitudinal study of 5,952 twins.14
A prospective, randomized, controlled trial15 suggests that exercise could be an important treatment tool in patients diagnosed with MDD. The 202 adult subjects (153 women, 49 men) were randomly assigned to 1 of 4 treatments:
- supervised exercise in a group setting
- home-based exercise
- antidepressant medication (sertraline, 50 to 200 mg/d)
- placebo pills.
Patients underwent the structured clinical interview for depression and completed the HRSD. After 16 weeks, 41% of participants achieved remission, defined as no longer meeting MDD criteria and a HRSD score <8. Compared with placebo controls, patients receiving active treatments tended to have higher remission rates:
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with medication
- 31% with placebo.
Comment. The placebo response rate was relatively high in this study, and antidepressant dosages might not have been optimal. These factors could explain why remission rates with supervised exercise and antidepressant medication were comparable. The study might have been more reliable if it had included a medication plus exercise arm. Patients treated in an office setting might not fare as well as these study subjects whose exercise was supervised.
Postpartum depression occurs in an estimated 13% of new mothers.16 In a controlled trial, 80 women with depression at 4 weeks postpartum were assigned to either:
- an exercise support program (1 hour supervised exercise and 2 sessions at home each week for 3 months)
- standard care.
Most depressed patients can benefit from aerobic exercise or high-intensity progressive resistance training (PRT). Consult with your patient’s primary care physician before designing an exercise regimen. Incorporate warm-up and cool-down periods during each exercise session.
Aerobics. A 30- to 45-minute daily regimen of running, walking, swimming, biking, dancing, or elliptical training is recommended for most people. An optimum regimen achieves a target heart rate of 70% to 85% of the individual’s maximum heart rate. A goal of 40% to 50% of maximum heart rate is an appropriate goal for patients starting an exercise program. At least 10 minutes of aerobic activity is necessary to produce the desired benefit.
PRT. High-intensity progressive resistance training may be recommended in consultation with a physical therapist or certified trainer. This usually consists of 30 to 45 minutes of systematic training of various muscle groups 3 days a week. An optimal resistance of 80% of maximal load is desirable, but this may be adjusted for individual patients. Lifting weights, push-ups, sit-ups, using resistance bands, and heavy gardening may be part of this regimen.
No subjects received medication. Women in the exercise support program were less likely to have high scores on the Edinburgh Postnatal Depression Scale, compared with controls. Women who exercised also reported a greater sense of well-being. Differences between the 2 groups were not statistically significant at 4 weeks post partum but achieved significance at 5 months.17
Depressive symptoms may exacerbate fatigue in postpartum women.18 A study of 88 women with postpartum depression showed the benefits of a home-based exercise program on physical and mental fatigue.19 This finding may be important because fatigue often is associated with treatment-resistant depression and may increase the likelihood of relapse in women with postpartum depression.20
Late-life depression. Exercise can benefit the depressed elderly as well. In a 10-week randomized, controlled trial21 of volunteers age ≥60 with major or minor depression or dysthymia, progressive resistance training (PRT) significantly reduced depression, as measured by the Beck Depression Inventory (BDI) and HRSD. PRT also improved quality of life, vitality, social functioning, and emotional well-being when compared with a control group (Box 2).
A dose-response relationship of exercise for treating late-life depression was shown in a blinded, controlled trial22 of 60 community-dwelling, depressed subjects age >60. These patients were randomly assigned to high-intensity PRT, low-intensity PRT, or standard care by a general practitioner (GP). A ≥50% reduction in HRSD score was achieved by:
- 61% of the high-intensity PRT group
- 29% of the low-intensity PRT group
- 21% of the GP care group.
Sleep quality improved in all participants, with the greatest relative change in the high-intensity PRT group.
Exercise vs psychotherapy. The benefits of exercise may be comparable or superior to those of cognitive or group psychotherapy.23,24 This may be good news for patients such as Mrs. S who lack time or financial resources for regular psychotherapy.
Adjunctive exercise
In depressed patients, exercise may increase the perceived quality of life when combined with medication. This was demonstrated in a randomized, 32-week naturalistic study of 30 women, age 40 to 60, with treatment-resistant MDD.25 The 10 women who received various antidepressants plus physical exercise showed significantly greater long-term improvement in depression symptoms, as measured by the HRSD and Global Assessment of Functioning (GAF) scores, compared with 20 women who received pharmacotherapy alone.26 Study limitations included the absence of a placebo arm, small sample size, and inclusion of subjects with comorbid anxiety disorders.
Group aerobic exercise programs can be an effective and feasible treatment for depression, particularly for older adults. In a controlled trial,27 156 men and women age >50 with MDD were randomly assigned to 3 groups: a program of aerobic exercise; sertraline, ≤200 mg/d; or exercise plus sertraline. HRSD and BDI scores before and after treatment were the primary outcome measures. Secondary measures included aerobic capacity, life satisfaction, self-esteem, anxiety, and dysfunctional cognitions. After 16 weeks of treatment, similar percentages of patients in each group no longer met DSM-IV-TR criteria for MDD:
- 60.4% of patients in the exercise-only group
- 68.8% of patients in the medication-only group
- 65.5% of patients receiving exercise plus medication.
Depression severity appeared to predict the rate of response to the different treatments. Patients who received medication alone seemed to have the most rapid response to treatment. Patients with less severe depression appeared to respond more quickly to exercise plus medication than those with more severe depression.
Long-term benefits
Because depression is a chronic, relapsing illness, any treatment will be widely accepted only if its benefits are long-term. A study of aerobic exercise in 156 adults age ≥50 with MDD28 found that benefits were sustained for >6 months.
Participants were randomly assigned to 4 months of aerobic exercise; sertraline, ≤200 mg/d; or a combination of exercise and sertraline. Aerobic exercise consisted of 30 minutes of brisk walking and jogging on a treadmill, with training ranges equivalent to 70% to 85% of individuals’ maximum heart rate. Appropriate warm-up and cool-down sessions of 5 to 10 minutes were included.
Depressive symptoms improved significantly from baseline in all 3 groups—as assessed by clinical interview, HRSD, and BDI—and after 4 months a comparable number in each group no longer met diagnostic criteria for MDD. When subjects were reassessed 6 months later, the exercisers had significantly lower relapse rates than those receiving medication (P=.01). Those who continued to exercise also were less likely to meet MDD criteria at the end of the 10-month study.
Ask about physical activity at every visit to gauge motivation to exercise
Discuss benefits of exercise for depression and other ailments, and use motivational interviewing techniques when appropriate
Screen for barriers to an exercise routine, and discuss strategies to overcome barriers
Recommend exercise as a prescription, rather than simply advice, because adherence may be greater
Encourage patients to increase physical activity each day, participate in exercise support groups, and seek support from coworkers, family, and friends
Even when unsupervised, exercise can have long-term benefits—as was shown in a randomized, blinded, controlled study of 32 elderly subjects.29 An active treatment group underwent 10 weeks of supervised weight lifting, followed by 10 weeks of unsupervised exercise. Controls received no active treatment. Depression scores as measured by BDI were significantly lower at 20 weeks and 26 months in exercisers compared with controls. An antidepressant effect was seen in 73% of exercisers vs 36% of controls at 20 weeks of treatment.
Comment. These studies show that exercise can maintain an anti depressant effect for 10 to 26 months, but additional randomized controlled studies are needed.
Preventing depression? Inactive nondepressed individuals may be at greater risk to develop depression compared with active individuals, according to a 29-year longitudinal study of Californians age 17 to 94. This association was somewhat diminished when findings were adjusted for the Alameda County residents’ physical health, socioeconomic status, social supports, life events, and other health habits.30 The authors recommended that exercise programs be offered in community mental health programs.
Box 4
Simple steps to build physical activity into daily life
| The American Heart Association offers helpful tips for increasing daily exercise at home, at work, and at play. For additional suggestions, go to www.americanheart.org. | ||
|---|---|---|
| At home | At the office | At play |
| Do housework yourself instead of hiring someone else to do it | Brainstorm project ideas with a coworker while taking a walk | Plan family outings and vacations that include physical |
| Work in the garden or mow the grass (using a riding mower doesn’t count); rake leaves, prune, dig, and pick up trash | Stand while talking on the telephone | activity (hiking, backpacking, swimming, etc.) |
| Go out for a short walk before breakfast, after dinner or both; start with 5 to 10 minutes and work up to 30 minutes | Walk down the hall to speak with someone rather than using the telephone | See the sights in new cities by walking, jogging, or bicycling |
| Walk or bike to the corner store instead of driving | Take the stairs instead of the elevator, or get off a few floors early and take the stairs the rest of the way | Make a date with a friend to enjoy your favorite physical activities, and do them regularly |
| When walking, increase the pace from leisurely to brisk; choose a hilly route | Schedule exercise time on your business calendar, and treat it as any other important appointment | Play your favorite music while exercising, something that motivates you |
| Dance with someone or by yourself; take dancing lessons | ||
| Join a recreational club that emphasizes physical activity | ||
| When golfing, walk the course instead of using a cart | ||
CASE CONTINUED: Removing barriers to exercise
The resident psychiatrist treating Mrs. S encourages her to join an aerobic exercise class at the nearby fitness facility. Because cost is a potential barrier, he helps her negotiate a discount for the first 6 months of membership. Her husband agrees in a joint counseling session to help more with the care of their children so that she can attend the classes.
With continued sertraline, 200 mg/d, and aerobic exercise, Mrs. S’s residual depressive symptoms gradually improve. She still has days when she is unable to attend the exercise classes, but she benefits from the program and is functioning better at work and home.
Getting started
We recommend that psychiatrists inquire about physical activity at every visit to gauge patients’ perception and motivation to exercise. Find ways to overcome patients’ fears and negative experiences with exercise. Provide information to help increase physical activity among patients with depressive symptoms10 (see Related Resources).
Encourage patients to take steps each day to increase their physical activity (Box 3). Depending on the severity of the individual’s depression and inactivity, a realistic starting point may be to take the stairs instead of an elevator, play with children and pets, or take short brisk walks in the yard or neighborhood (Box 4). Consider stationary bikes or swimming as alternatives for physically handicapped individuals and patients who have undergone knee replacements.
- 2008 physical activity guidelines for Americans. U.S. Department of Health and Human Services; 2008:vi-viii. www.health.gov/paguidelines.
- American College of Sports Medicine/American Health Association physical activity guidelines and keys to exercise success. www.acsm.org.
- American Heart Association. www.americanheart.org.
- U.S. Department of Health and Human Services. Quick Guide for Healthy Living. Get Active. www.healthfinder.gov/prevention/ViewTopic.aspx?topicID=22.
Drug brand names
- Bupropion • Wellbutrin
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459.
2. Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164(5):739-752.
3. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med. 2003;36(6):698-703.
4. Stathopoulou G, Powers MB, Berry AC, et al. Exercise interventions for mental health: a quantitative and qualitative review. Clinical Psychology Science and Practice. 2006;13(2):179-193.
5. Wee CC, McCarthy EP, Davis RB, et al. Physician counseling about exercise. JAMA. 1999;282(16):1583-1588.
6. Long BJ, Calfas KJ, Wooten W, et al. A multisite field test of the acceptability of physical activity counseling in primary care: project PACE. Am J Prev Med. 1996;12(2):73-81.
7. Vickers KS, Nies MA, Patten CA, et al. Patients with diabetes and depression may need additional support for exercise. Am J Health Behav. 2006;30(4):353-362.
8. Weidinger KA, Lovegreen SL, Elliott MB, et al. How to make exercise counseling more effective: lessons from rural America. J Fam Pract. 2008;57(6):394-402.
9. Calfas KJ, Long BJ, Sallis JF, et al. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med. 1996;25(3):225-233.
10. Rosqvist E, Heikkinen E, Lyyra TM, et al. Factors affecting the increased risk of physical inactivity among older people with depressive symptoms. Scand J Med Sci Sports. 2008 May 22 [Epub ahead of print].
11. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
12. Galper DI, Trivedi MH, Barlow CE, et al. Inverse association between physical inactivity and mental health in men and women. Med Sci Sports Exerc. 2006;38(1):173-178.
Fazio AF. A concurrent validation study of the NCHS General Well-Being Schedule. Vital and Health Statistics. Hyattsville, MD: National Center for Health Statistics, US Public Health Service; September 1977. Series 2, No. 73, DHEW Publication No. (HRA) 78-1347:1-13.
14. De Moor MH, Boomsma DI, Stubbe JH, et al. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry. 2008;65(8):897-905.
15. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
16. O’Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry. 1996;8(1):37-54.
17. Heh SS, Huang LH, Ho SM, et al. Effectiveness of an exercise support program in reducing the severity of postnatal depression in Taiwanese women. Birth. 2008;35(1):60-65.
18. Saurel-Cubizolles MJ, Romito P, Lelong N, et al. Women’s health after childbirth: a longitudinal study in France and Italy. BJOG. 2000;107(10):1202-1209.
19. Dritsa M, Da Costa D, Dupuis G, et al. Effects of a home-based exercise intervention on fatigue in postpartum depressed women: results of a randomized controlled trial. Ann Behav Med. 2008;35(2):179-187.
20. Corwin EJ, Brownstead J, Barton N, et al. The impact of fatigue on the development of postpartum depression. J Obstet Gynecol Neonatal Nurs. 2005;34(5):577-586.
21. Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci. 1997;52(1):M27-35.
22. Singh NA, Stavrinos TM, Scarbek Y, et al. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(6):768-776.
23. Fremont J, Wilcoxon Craighead L. Aerobic exercise and cognitive therapy in the treatment of dysphoric moods. Cognit Ther Res. 1987;11(2):241-251.
24. Klein MH, Greist JH, Gurman RA, et al. A comparative outcome study of group psychotherapy vs. exercise treatments for depression. Int J Ment Health. 1985;13:148-177.
25. Carta MG, Hardoy MC, Pilu A, et al. Improving physical quality of life with group physical activity in the adjunctive treatment of major depressive disorder. Clin Pract Epidemiol Ment Health. 2008;4:1.
26. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
27. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349-2356.
28. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633-638.
29. Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2001;56(8):M497-504.
30. Camacho TC, Roberts RE, Lazarus NB, et al. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol. 1991;134(2):220-231.
Mrs. S, age 44, is on leave from her job as a bank cashier because depressive symptoms interfered with her performance. At a university-based psychiatric clinic she reports feeling depressed, reduced interest in daily activities, problems with sleep onset and maintenance, inconsistent appetite, low energy, hopelessness, and decreased memory and concentration.
The resident psychiatrist diagnoses major depressive disorder (MDD) and starts Mrs. S on sertraline, 50 mg/d. The dosage is gradually titrated to 200 mg/d, and after 8 weeks she reports substantial improvement.
Mrs. S returns to her job but experiences residual low energy, lethargy, and inconsistent sleep. Her work schedule and caring for her 2 children at home prevent her from continuing weekly cognitive-behavioral therapy (CBT), but she soon notices that she feels more energetic. She reports that because of high gasoline prices she has been walking several miles daily to commute by train to work. The resident psychiatrist sees this as an opportunity to reinforce the benefits of exercise for depression.
Antidepressants alone do not adequately treat many patients with depression. In the STAR*D Project—which compared long-term outcomes of various depression treatments—only 28% to 33% of outpatients achieved remission with selective serotonin reuptake inhibitor (SSRI) monotherapy. Rates were somewhat higher with bupropion or serotonin norepinephrine reuptake inhibitor (SNRI) monotherapy, but greater benefit was obtained from augmenting SSRIs.1
Combining antidepressants with psychotherapy2 and lifestyle changes—particularly exercise—makes sense intuitively and is supported by well-designed studies:
- The 60% of adults in the National Comorbidity Survey who said they exercised regularly reported lower rates of depression and anxiety, compared with less active adults.3
- A meta-analysis of 11 randomized, controlled trials supports the use of exercise as an effective intervention for clinical depression.4
Elevation of endorphins in the CNS
Changes in neurotransmitters such as serotonin and norepinephrine
Increased levels of brain-derived neurotrophic factor
Reduction of serum cortisol
Elevation of body temperature
Improved self-esteem
Distraction from daily stress
Induction of a relaxed state via biofeedback
This article examines the evidence supporting exercise for treating and preventing clinical depression. We begin by addressing clinicians’ concerns about motivating depressed patients to exercise.
Overcoming barriers
Physician issues. Busy physicians often omit discussions about exercise during brief office visits. Only 34% of 9,299 patients in a population-based survey5 reported that their doctors counseled them about exercise during their most recent visits. Counseling patients does not have to be time-intensive, however. A study of the Physician-based Assessment and Counseling for Exercise (PACE) project showed that 70% of physicians could provide exercise counseling in 3 to 5 minutes, and most patients reported following their physicians’ advice.6
Highly depressed individuals are at risk to quit when they encounter barriers to exercise and to respond to difficulties with frustration and self-disappointment. Thus, depressed patients may need support and encouragement to initiate and maintain regular exercise routines.7 Set small, realistic goals for them, and discuss how to solve problems and remove barriers to increase their likelihood to exercise.
Interventions are most likely to be effective when you counsel patients about exercise as prescription and discuss exercise at each visit.8 Previously sedentary patients have shown short-term moderate increases in physical activity in response to physician counseling. In a study of 212 adults (mean age 39, 84% female), the PACE project significantly increased minutes of weekly walking.9 More than one-half (52%) of patients increased their physical activity, compared with 12% of controls whose physicians did not provide the PACE intervention.
Patient issues. Lack of time and no appropriate space to exercise are common complaints, particularly among residents of regions with long, cold winters. Some patients perceive regular exercise as monotonous or boring, and others may lack the necessary initiative because of poor physical health, fear, negative experiences, or lack of knowledge about exercising. These barriers can be pronounced in older depressed persons. In a cross-sectional study of 645 residents of Jyväskylä, Finland, those age >75 with depressive symptoms were more than twice as likely to be physically inactive as nondepressed residents.10
An intensive exercise program is not the optimal starting point for many patients. Even walking or light jogging can be an effective exercise for depressed individuals with physical limitations. For these patients, a consultation with their primary physician may be necessary if a more intensive program has to be recommended.
Exercise as monotherapy
A dose-response relationship? Various mechanisms have been suggested for the benefits of exercise in depression (Box 1). Exercise alone—without medication—may be an effective treatment for mild and in some cases moderate MDD, and aerobic exercise may reduce depressive symptoms in a dose-response relationship.11
A study of exercise in a supervised laboratory setting demonstrated this relationship in 80 adults age 20 to 45 with mild-to-moderate depressive symptoms. Subjects were randomly assigned to an exercise control group (3 days/week of flexibility exercise) or 1 of 4 aerobic exercise groups that varied in total energy expenditure (a “low dose” of 7.0 kcal/kg/week or a “public health dose” of 17.5 kcal/kg/week). The 17-item Hamilton Rating Scale for Depression (HRSD) was the primary outcome measure.
After 12 weeks, HRSD scores declined from baseline by 47% in subjects engaged in the public health dose of aerobic exercise—a significant reduction. Depressive symptoms declined by 30% in the low-dose exercisers, but this was comparable to the 29% reduction in the control group.
Comment. The effective exercise dose in this study is similar to the public health recommendation of 30 minutes of moderate-to-vigorous activity on all or most days per week (see Related Resources). Antidepressant effects have been associated with more modest physical activity, however, which may be easier to initiate and maintain for individuals with depression. The study did not find significant differences in outcomes based on the subjects’ age, gender, or exercise frequency. Nevertheless, the exercise dose may be important to produce an antidepressant effect.
An inverse relationship? Compared with occasional exercise, habitual physical activity usually is associated with greater cardiorespiratory fitness. Whether habitual activity also results in fewer depressive symptoms and greater emotional well-being remains to be seen.
A large, cross-sectional, National Institutes of Health-funded study of 5,451 men and 1,277 women12 suggests an inverse relationship between physical activity and depressive symptoms. Subjects underwent a treadmill exercise test to evaluate physical fitness. A 20-point self-report scale assessed depressive symptoms, and the General Well-Being Schedule13 was used to assess emotional well-being. Depressive symptoms were more severe in “inactive” and “insufficiently active” subjects compared with “sufficiently active” and “highly active” subjects.
On the other hand, although regular exercise may be associated with reduced depressive symptoms in the population at large, no cause-effect relationship was found in a population-based, longitudinal study of 5,952 twins.14
A prospective, randomized, controlled trial15 suggests that exercise could be an important treatment tool in patients diagnosed with MDD. The 202 adult subjects (153 women, 49 men) were randomly assigned to 1 of 4 treatments:
- supervised exercise in a group setting
- home-based exercise
- antidepressant medication (sertraline, 50 to 200 mg/d)
- placebo pills.
Patients underwent the structured clinical interview for depression and completed the HRSD. After 16 weeks, 41% of participants achieved remission, defined as no longer meeting MDD criteria and a HRSD score <8. Compared with placebo controls, patients receiving active treatments tended to have higher remission rates:
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with medication
- 31% with placebo.
Comment. The placebo response rate was relatively high in this study, and antidepressant dosages might not have been optimal. These factors could explain why remission rates with supervised exercise and antidepressant medication were comparable. The study might have been more reliable if it had included a medication plus exercise arm. Patients treated in an office setting might not fare as well as these study subjects whose exercise was supervised.
Postpartum depression occurs in an estimated 13% of new mothers.16 In a controlled trial, 80 women with depression at 4 weeks postpartum were assigned to either:
- an exercise support program (1 hour supervised exercise and 2 sessions at home each week for 3 months)
- standard care.
Most depressed patients can benefit from aerobic exercise or high-intensity progressive resistance training (PRT). Consult with your patient’s primary care physician before designing an exercise regimen. Incorporate warm-up and cool-down periods during each exercise session.
Aerobics. A 30- to 45-minute daily regimen of running, walking, swimming, biking, dancing, or elliptical training is recommended for most people. An optimum regimen achieves a target heart rate of 70% to 85% of the individual’s maximum heart rate. A goal of 40% to 50% of maximum heart rate is an appropriate goal for patients starting an exercise program. At least 10 minutes of aerobic activity is necessary to produce the desired benefit.
PRT. High-intensity progressive resistance training may be recommended in consultation with a physical therapist or certified trainer. This usually consists of 30 to 45 minutes of systematic training of various muscle groups 3 days a week. An optimal resistance of 80% of maximal load is desirable, but this may be adjusted for individual patients. Lifting weights, push-ups, sit-ups, using resistance bands, and heavy gardening may be part of this regimen.
No subjects received medication. Women in the exercise support program were less likely to have high scores on the Edinburgh Postnatal Depression Scale, compared with controls. Women who exercised also reported a greater sense of well-being. Differences between the 2 groups were not statistically significant at 4 weeks post partum but achieved significance at 5 months.17
Depressive symptoms may exacerbate fatigue in postpartum women.18 A study of 88 women with postpartum depression showed the benefits of a home-based exercise program on physical and mental fatigue.19 This finding may be important because fatigue often is associated with treatment-resistant depression and may increase the likelihood of relapse in women with postpartum depression.20
Late-life depression. Exercise can benefit the depressed elderly as well. In a 10-week randomized, controlled trial21 of volunteers age ≥60 with major or minor depression or dysthymia, progressive resistance training (PRT) significantly reduced depression, as measured by the Beck Depression Inventory (BDI) and HRSD. PRT also improved quality of life, vitality, social functioning, and emotional well-being when compared with a control group (Box 2).
A dose-response relationship of exercise for treating late-life depression was shown in a blinded, controlled trial22 of 60 community-dwelling, depressed subjects age >60. These patients were randomly assigned to high-intensity PRT, low-intensity PRT, or standard care by a general practitioner (GP). A ≥50% reduction in HRSD score was achieved by:
- 61% of the high-intensity PRT group
- 29% of the low-intensity PRT group
- 21% of the GP care group.
Sleep quality improved in all participants, with the greatest relative change in the high-intensity PRT group.
Exercise vs psychotherapy. The benefits of exercise may be comparable or superior to those of cognitive or group psychotherapy.23,24 This may be good news for patients such as Mrs. S who lack time or financial resources for regular psychotherapy.
Adjunctive exercise
In depressed patients, exercise may increase the perceived quality of life when combined with medication. This was demonstrated in a randomized, 32-week naturalistic study of 30 women, age 40 to 60, with treatment-resistant MDD.25 The 10 women who received various antidepressants plus physical exercise showed significantly greater long-term improvement in depression symptoms, as measured by the HRSD and Global Assessment of Functioning (GAF) scores, compared with 20 women who received pharmacotherapy alone.26 Study limitations included the absence of a placebo arm, small sample size, and inclusion of subjects with comorbid anxiety disorders.
Group aerobic exercise programs can be an effective and feasible treatment for depression, particularly for older adults. In a controlled trial,27 156 men and women age >50 with MDD were randomly assigned to 3 groups: a program of aerobic exercise; sertraline, ≤200 mg/d; or exercise plus sertraline. HRSD and BDI scores before and after treatment were the primary outcome measures. Secondary measures included aerobic capacity, life satisfaction, self-esteem, anxiety, and dysfunctional cognitions. After 16 weeks of treatment, similar percentages of patients in each group no longer met DSM-IV-TR criteria for MDD:
- 60.4% of patients in the exercise-only group
- 68.8% of patients in the medication-only group
- 65.5% of patients receiving exercise plus medication.
Depression severity appeared to predict the rate of response to the different treatments. Patients who received medication alone seemed to have the most rapid response to treatment. Patients with less severe depression appeared to respond more quickly to exercise plus medication than those with more severe depression.
Long-term benefits
Because depression is a chronic, relapsing illness, any treatment will be widely accepted only if its benefits are long-term. A study of aerobic exercise in 156 adults age ≥50 with MDD28 found that benefits were sustained for >6 months.
Participants were randomly assigned to 4 months of aerobic exercise; sertraline, ≤200 mg/d; or a combination of exercise and sertraline. Aerobic exercise consisted of 30 minutes of brisk walking and jogging on a treadmill, with training ranges equivalent to 70% to 85% of individuals’ maximum heart rate. Appropriate warm-up and cool-down sessions of 5 to 10 minutes were included.
Depressive symptoms improved significantly from baseline in all 3 groups—as assessed by clinical interview, HRSD, and BDI—and after 4 months a comparable number in each group no longer met diagnostic criteria for MDD. When subjects were reassessed 6 months later, the exercisers had significantly lower relapse rates than those receiving medication (P=.01). Those who continued to exercise also were less likely to meet MDD criteria at the end of the 10-month study.
Ask about physical activity at every visit to gauge motivation to exercise
Discuss benefits of exercise for depression and other ailments, and use motivational interviewing techniques when appropriate
Screen for barriers to an exercise routine, and discuss strategies to overcome barriers
Recommend exercise as a prescription, rather than simply advice, because adherence may be greater
Encourage patients to increase physical activity each day, participate in exercise support groups, and seek support from coworkers, family, and friends
Even when unsupervised, exercise can have long-term benefits—as was shown in a randomized, blinded, controlled study of 32 elderly subjects.29 An active treatment group underwent 10 weeks of supervised weight lifting, followed by 10 weeks of unsupervised exercise. Controls received no active treatment. Depression scores as measured by BDI were significantly lower at 20 weeks and 26 months in exercisers compared with controls. An antidepressant effect was seen in 73% of exercisers vs 36% of controls at 20 weeks of treatment.
Comment. These studies show that exercise can maintain an anti depressant effect for 10 to 26 months, but additional randomized controlled studies are needed.
Preventing depression? Inactive nondepressed individuals may be at greater risk to develop depression compared with active individuals, according to a 29-year longitudinal study of Californians age 17 to 94. This association was somewhat diminished when findings were adjusted for the Alameda County residents’ physical health, socioeconomic status, social supports, life events, and other health habits.30 The authors recommended that exercise programs be offered in community mental health programs.
Box 4
Simple steps to build physical activity into daily life
| The American Heart Association offers helpful tips for increasing daily exercise at home, at work, and at play. For additional suggestions, go to www.americanheart.org. | ||
|---|---|---|
| At home | At the office | At play |
| Do housework yourself instead of hiring someone else to do it | Brainstorm project ideas with a coworker while taking a walk | Plan family outings and vacations that include physical |
| Work in the garden or mow the grass (using a riding mower doesn’t count); rake leaves, prune, dig, and pick up trash | Stand while talking on the telephone | activity (hiking, backpacking, swimming, etc.) |
| Go out for a short walk before breakfast, after dinner or both; start with 5 to 10 minutes and work up to 30 minutes | Walk down the hall to speak with someone rather than using the telephone | See the sights in new cities by walking, jogging, or bicycling |
| Walk or bike to the corner store instead of driving | Take the stairs instead of the elevator, or get off a few floors early and take the stairs the rest of the way | Make a date with a friend to enjoy your favorite physical activities, and do them regularly |
| When walking, increase the pace from leisurely to brisk; choose a hilly route | Schedule exercise time on your business calendar, and treat it as any other important appointment | Play your favorite music while exercising, something that motivates you |
| Dance with someone or by yourself; take dancing lessons | ||
| Join a recreational club that emphasizes physical activity | ||
| When golfing, walk the course instead of using a cart | ||
CASE CONTINUED: Removing barriers to exercise
The resident psychiatrist treating Mrs. S encourages her to join an aerobic exercise class at the nearby fitness facility. Because cost is a potential barrier, he helps her negotiate a discount for the first 6 months of membership. Her husband agrees in a joint counseling session to help more with the care of their children so that she can attend the classes.
With continued sertraline, 200 mg/d, and aerobic exercise, Mrs. S’s residual depressive symptoms gradually improve. She still has days when she is unable to attend the exercise classes, but she benefits from the program and is functioning better at work and home.
Getting started
We recommend that psychiatrists inquire about physical activity at every visit to gauge patients’ perception and motivation to exercise. Find ways to overcome patients’ fears and negative experiences with exercise. Provide information to help increase physical activity among patients with depressive symptoms10 (see Related Resources).
Encourage patients to take steps each day to increase their physical activity (Box 3). Depending on the severity of the individual’s depression and inactivity, a realistic starting point may be to take the stairs instead of an elevator, play with children and pets, or take short brisk walks in the yard or neighborhood (Box 4). Consider stationary bikes or swimming as alternatives for physically handicapped individuals and patients who have undergone knee replacements.
- 2008 physical activity guidelines for Americans. U.S. Department of Health and Human Services; 2008:vi-viii. www.health.gov/paguidelines.
- American College of Sports Medicine/American Health Association physical activity guidelines and keys to exercise success. www.acsm.org.
- American Heart Association. www.americanheart.org.
- U.S. Department of Health and Human Services. Quick Guide for Healthy Living. Get Active. www.healthfinder.gov/prevention/ViewTopic.aspx?topicID=22.
Drug brand names
- Bupropion • Wellbutrin
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mrs. S, age 44, is on leave from her job as a bank cashier because depressive symptoms interfered with her performance. At a university-based psychiatric clinic she reports feeling depressed, reduced interest in daily activities, problems with sleep onset and maintenance, inconsistent appetite, low energy, hopelessness, and decreased memory and concentration.
The resident psychiatrist diagnoses major depressive disorder (MDD) and starts Mrs. S on sertraline, 50 mg/d. The dosage is gradually titrated to 200 mg/d, and after 8 weeks she reports substantial improvement.
Mrs. S returns to her job but experiences residual low energy, lethargy, and inconsistent sleep. Her work schedule and caring for her 2 children at home prevent her from continuing weekly cognitive-behavioral therapy (CBT), but she soon notices that she feels more energetic. She reports that because of high gasoline prices she has been walking several miles daily to commute by train to work. The resident psychiatrist sees this as an opportunity to reinforce the benefits of exercise for depression.
Antidepressants alone do not adequately treat many patients with depression. In the STAR*D Project—which compared long-term outcomes of various depression treatments—only 28% to 33% of outpatients achieved remission with selective serotonin reuptake inhibitor (SSRI) monotherapy. Rates were somewhat higher with bupropion or serotonin norepinephrine reuptake inhibitor (SNRI) monotherapy, but greater benefit was obtained from augmenting SSRIs.1
Combining antidepressants with psychotherapy2 and lifestyle changes—particularly exercise—makes sense intuitively and is supported by well-designed studies:
- The 60% of adults in the National Comorbidity Survey who said they exercised regularly reported lower rates of depression and anxiety, compared with less active adults.3
- A meta-analysis of 11 randomized, controlled trials supports the use of exercise as an effective intervention for clinical depression.4
Elevation of endorphins in the CNS
Changes in neurotransmitters such as serotonin and norepinephrine
Increased levels of brain-derived neurotrophic factor
Reduction of serum cortisol
Elevation of body temperature
Improved self-esteem
Distraction from daily stress
Induction of a relaxed state via biofeedback
This article examines the evidence supporting exercise for treating and preventing clinical depression. We begin by addressing clinicians’ concerns about motivating depressed patients to exercise.
Overcoming barriers
Physician issues. Busy physicians often omit discussions about exercise during brief office visits. Only 34% of 9,299 patients in a population-based survey5 reported that their doctors counseled them about exercise during their most recent visits. Counseling patients does not have to be time-intensive, however. A study of the Physician-based Assessment and Counseling for Exercise (PACE) project showed that 70% of physicians could provide exercise counseling in 3 to 5 minutes, and most patients reported following their physicians’ advice.6
Highly depressed individuals are at risk to quit when they encounter barriers to exercise and to respond to difficulties with frustration and self-disappointment. Thus, depressed patients may need support and encouragement to initiate and maintain regular exercise routines.7 Set small, realistic goals for them, and discuss how to solve problems and remove barriers to increase their likelihood to exercise.
Interventions are most likely to be effective when you counsel patients about exercise as prescription and discuss exercise at each visit.8 Previously sedentary patients have shown short-term moderate increases in physical activity in response to physician counseling. In a study of 212 adults (mean age 39, 84% female), the PACE project significantly increased minutes of weekly walking.9 More than one-half (52%) of patients increased their physical activity, compared with 12% of controls whose physicians did not provide the PACE intervention.
Patient issues. Lack of time and no appropriate space to exercise are common complaints, particularly among residents of regions with long, cold winters. Some patients perceive regular exercise as monotonous or boring, and others may lack the necessary initiative because of poor physical health, fear, negative experiences, or lack of knowledge about exercising. These barriers can be pronounced in older depressed persons. In a cross-sectional study of 645 residents of Jyväskylä, Finland, those age >75 with depressive symptoms were more than twice as likely to be physically inactive as nondepressed residents.10
An intensive exercise program is not the optimal starting point for many patients. Even walking or light jogging can be an effective exercise for depressed individuals with physical limitations. For these patients, a consultation with their primary physician may be necessary if a more intensive program has to be recommended.
Exercise as monotherapy
A dose-response relationship? Various mechanisms have been suggested for the benefits of exercise in depression (Box 1). Exercise alone—without medication—may be an effective treatment for mild and in some cases moderate MDD, and aerobic exercise may reduce depressive symptoms in a dose-response relationship.11
A study of exercise in a supervised laboratory setting demonstrated this relationship in 80 adults age 20 to 45 with mild-to-moderate depressive symptoms. Subjects were randomly assigned to an exercise control group (3 days/week of flexibility exercise) or 1 of 4 aerobic exercise groups that varied in total energy expenditure (a “low dose” of 7.0 kcal/kg/week or a “public health dose” of 17.5 kcal/kg/week). The 17-item Hamilton Rating Scale for Depression (HRSD) was the primary outcome measure.
After 12 weeks, HRSD scores declined from baseline by 47% in subjects engaged in the public health dose of aerobic exercise—a significant reduction. Depressive symptoms declined by 30% in the low-dose exercisers, but this was comparable to the 29% reduction in the control group.
Comment. The effective exercise dose in this study is similar to the public health recommendation of 30 minutes of moderate-to-vigorous activity on all or most days per week (see Related Resources). Antidepressant effects have been associated with more modest physical activity, however, which may be easier to initiate and maintain for individuals with depression. The study did not find significant differences in outcomes based on the subjects’ age, gender, or exercise frequency. Nevertheless, the exercise dose may be important to produce an antidepressant effect.
An inverse relationship? Compared with occasional exercise, habitual physical activity usually is associated with greater cardiorespiratory fitness. Whether habitual activity also results in fewer depressive symptoms and greater emotional well-being remains to be seen.
A large, cross-sectional, National Institutes of Health-funded study of 5,451 men and 1,277 women12 suggests an inverse relationship between physical activity and depressive symptoms. Subjects underwent a treadmill exercise test to evaluate physical fitness. A 20-point self-report scale assessed depressive symptoms, and the General Well-Being Schedule13 was used to assess emotional well-being. Depressive symptoms were more severe in “inactive” and “insufficiently active” subjects compared with “sufficiently active” and “highly active” subjects.
On the other hand, although regular exercise may be associated with reduced depressive symptoms in the population at large, no cause-effect relationship was found in a population-based, longitudinal study of 5,952 twins.14
A prospective, randomized, controlled trial15 suggests that exercise could be an important treatment tool in patients diagnosed with MDD. The 202 adult subjects (153 women, 49 men) were randomly assigned to 1 of 4 treatments:
- supervised exercise in a group setting
- home-based exercise
- antidepressant medication (sertraline, 50 to 200 mg/d)
- placebo pills.
Patients underwent the structured clinical interview for depression and completed the HRSD. After 16 weeks, 41% of participants achieved remission, defined as no longer meeting MDD criteria and a HRSD score <8. Compared with placebo controls, patients receiving active treatments tended to have higher remission rates:
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with medication
- 31% with placebo.
Comment. The placebo response rate was relatively high in this study, and antidepressant dosages might not have been optimal. These factors could explain why remission rates with supervised exercise and antidepressant medication were comparable. The study might have been more reliable if it had included a medication plus exercise arm. Patients treated in an office setting might not fare as well as these study subjects whose exercise was supervised.
Postpartum depression occurs in an estimated 13% of new mothers.16 In a controlled trial, 80 women with depression at 4 weeks postpartum were assigned to either:
- an exercise support program (1 hour supervised exercise and 2 sessions at home each week for 3 months)
- standard care.
Most depressed patients can benefit from aerobic exercise or high-intensity progressive resistance training (PRT). Consult with your patient’s primary care physician before designing an exercise regimen. Incorporate warm-up and cool-down periods during each exercise session.
Aerobics. A 30- to 45-minute daily regimen of running, walking, swimming, biking, dancing, or elliptical training is recommended for most people. An optimum regimen achieves a target heart rate of 70% to 85% of the individual’s maximum heart rate. A goal of 40% to 50% of maximum heart rate is an appropriate goal for patients starting an exercise program. At least 10 minutes of aerobic activity is necessary to produce the desired benefit.
PRT. High-intensity progressive resistance training may be recommended in consultation with a physical therapist or certified trainer. This usually consists of 30 to 45 minutes of systematic training of various muscle groups 3 days a week. An optimal resistance of 80% of maximal load is desirable, but this may be adjusted for individual patients. Lifting weights, push-ups, sit-ups, using resistance bands, and heavy gardening may be part of this regimen.
No subjects received medication. Women in the exercise support program were less likely to have high scores on the Edinburgh Postnatal Depression Scale, compared with controls. Women who exercised also reported a greater sense of well-being. Differences between the 2 groups were not statistically significant at 4 weeks post partum but achieved significance at 5 months.17
Depressive symptoms may exacerbate fatigue in postpartum women.18 A study of 88 women with postpartum depression showed the benefits of a home-based exercise program on physical and mental fatigue.19 This finding may be important because fatigue often is associated with treatment-resistant depression and may increase the likelihood of relapse in women with postpartum depression.20
Late-life depression. Exercise can benefit the depressed elderly as well. In a 10-week randomized, controlled trial21 of volunteers age ≥60 with major or minor depression or dysthymia, progressive resistance training (PRT) significantly reduced depression, as measured by the Beck Depression Inventory (BDI) and HRSD. PRT also improved quality of life, vitality, social functioning, and emotional well-being when compared with a control group (Box 2).
A dose-response relationship of exercise for treating late-life depression was shown in a blinded, controlled trial22 of 60 community-dwelling, depressed subjects age >60. These patients were randomly assigned to high-intensity PRT, low-intensity PRT, or standard care by a general practitioner (GP). A ≥50% reduction in HRSD score was achieved by:
- 61% of the high-intensity PRT group
- 29% of the low-intensity PRT group
- 21% of the GP care group.
Sleep quality improved in all participants, with the greatest relative change in the high-intensity PRT group.
Exercise vs psychotherapy. The benefits of exercise may be comparable or superior to those of cognitive or group psychotherapy.23,24 This may be good news for patients such as Mrs. S who lack time or financial resources for regular psychotherapy.
Adjunctive exercise
In depressed patients, exercise may increase the perceived quality of life when combined with medication. This was demonstrated in a randomized, 32-week naturalistic study of 30 women, age 40 to 60, with treatment-resistant MDD.25 The 10 women who received various antidepressants plus physical exercise showed significantly greater long-term improvement in depression symptoms, as measured by the HRSD and Global Assessment of Functioning (GAF) scores, compared with 20 women who received pharmacotherapy alone.26 Study limitations included the absence of a placebo arm, small sample size, and inclusion of subjects with comorbid anxiety disorders.
Group aerobic exercise programs can be an effective and feasible treatment for depression, particularly for older adults. In a controlled trial,27 156 men and women age >50 with MDD were randomly assigned to 3 groups: a program of aerobic exercise; sertraline, ≤200 mg/d; or exercise plus sertraline. HRSD and BDI scores before and after treatment were the primary outcome measures. Secondary measures included aerobic capacity, life satisfaction, self-esteem, anxiety, and dysfunctional cognitions. After 16 weeks of treatment, similar percentages of patients in each group no longer met DSM-IV-TR criteria for MDD:
- 60.4% of patients in the exercise-only group
- 68.8% of patients in the medication-only group
- 65.5% of patients receiving exercise plus medication.
Depression severity appeared to predict the rate of response to the different treatments. Patients who received medication alone seemed to have the most rapid response to treatment. Patients with less severe depression appeared to respond more quickly to exercise plus medication than those with more severe depression.
Long-term benefits
Because depression is a chronic, relapsing illness, any treatment will be widely accepted only if its benefits are long-term. A study of aerobic exercise in 156 adults age ≥50 with MDD28 found that benefits were sustained for >6 months.
Participants were randomly assigned to 4 months of aerobic exercise; sertraline, ≤200 mg/d; or a combination of exercise and sertraline. Aerobic exercise consisted of 30 minutes of brisk walking and jogging on a treadmill, with training ranges equivalent to 70% to 85% of individuals’ maximum heart rate. Appropriate warm-up and cool-down sessions of 5 to 10 minutes were included.
Depressive symptoms improved significantly from baseline in all 3 groups—as assessed by clinical interview, HRSD, and BDI—and after 4 months a comparable number in each group no longer met diagnostic criteria for MDD. When subjects were reassessed 6 months later, the exercisers had significantly lower relapse rates than those receiving medication (P=.01). Those who continued to exercise also were less likely to meet MDD criteria at the end of the 10-month study.
Ask about physical activity at every visit to gauge motivation to exercise
Discuss benefits of exercise for depression and other ailments, and use motivational interviewing techniques when appropriate
Screen for barriers to an exercise routine, and discuss strategies to overcome barriers
Recommend exercise as a prescription, rather than simply advice, because adherence may be greater
Encourage patients to increase physical activity each day, participate in exercise support groups, and seek support from coworkers, family, and friends
Even when unsupervised, exercise can have long-term benefits—as was shown in a randomized, blinded, controlled study of 32 elderly subjects.29 An active treatment group underwent 10 weeks of supervised weight lifting, followed by 10 weeks of unsupervised exercise. Controls received no active treatment. Depression scores as measured by BDI were significantly lower at 20 weeks and 26 months in exercisers compared with controls. An antidepressant effect was seen in 73% of exercisers vs 36% of controls at 20 weeks of treatment.
Comment. These studies show that exercise can maintain an anti depressant effect for 10 to 26 months, but additional randomized controlled studies are needed.
Preventing depression? Inactive nondepressed individuals may be at greater risk to develop depression compared with active individuals, according to a 29-year longitudinal study of Californians age 17 to 94. This association was somewhat diminished when findings were adjusted for the Alameda County residents’ physical health, socioeconomic status, social supports, life events, and other health habits.30 The authors recommended that exercise programs be offered in community mental health programs.
Box 4
Simple steps to build physical activity into daily life
| The American Heart Association offers helpful tips for increasing daily exercise at home, at work, and at play. For additional suggestions, go to www.americanheart.org. | ||
|---|---|---|
| At home | At the office | At play |
| Do housework yourself instead of hiring someone else to do it | Brainstorm project ideas with a coworker while taking a walk | Plan family outings and vacations that include physical |
| Work in the garden or mow the grass (using a riding mower doesn’t count); rake leaves, prune, dig, and pick up trash | Stand while talking on the telephone | activity (hiking, backpacking, swimming, etc.) |
| Go out for a short walk before breakfast, after dinner or both; start with 5 to 10 minutes and work up to 30 minutes | Walk down the hall to speak with someone rather than using the telephone | See the sights in new cities by walking, jogging, or bicycling |
| Walk or bike to the corner store instead of driving | Take the stairs instead of the elevator, or get off a few floors early and take the stairs the rest of the way | Make a date with a friend to enjoy your favorite physical activities, and do them regularly |
| When walking, increase the pace from leisurely to brisk; choose a hilly route | Schedule exercise time on your business calendar, and treat it as any other important appointment | Play your favorite music while exercising, something that motivates you |
| Dance with someone or by yourself; take dancing lessons | ||
| Join a recreational club that emphasizes physical activity | ||
| When golfing, walk the course instead of using a cart | ||
CASE CONTINUED: Removing barriers to exercise
The resident psychiatrist treating Mrs. S encourages her to join an aerobic exercise class at the nearby fitness facility. Because cost is a potential barrier, he helps her negotiate a discount for the first 6 months of membership. Her husband agrees in a joint counseling session to help more with the care of their children so that she can attend the classes.
With continued sertraline, 200 mg/d, and aerobic exercise, Mrs. S’s residual depressive symptoms gradually improve. She still has days when she is unable to attend the exercise classes, but she benefits from the program and is functioning better at work and home.
Getting started
We recommend that psychiatrists inquire about physical activity at every visit to gauge patients’ perception and motivation to exercise. Find ways to overcome patients’ fears and negative experiences with exercise. Provide information to help increase physical activity among patients with depressive symptoms10 (see Related Resources).
Encourage patients to take steps each day to increase their physical activity (Box 3). Depending on the severity of the individual’s depression and inactivity, a realistic starting point may be to take the stairs instead of an elevator, play with children and pets, or take short brisk walks in the yard or neighborhood (Box 4). Consider stationary bikes or swimming as alternatives for physically handicapped individuals and patients who have undergone knee replacements.
- 2008 physical activity guidelines for Americans. U.S. Department of Health and Human Services; 2008:vi-viii. www.health.gov/paguidelines.
- American College of Sports Medicine/American Health Association physical activity guidelines and keys to exercise success. www.acsm.org.
- American Heart Association. www.americanheart.org.
- U.S. Department of Health and Human Services. Quick Guide for Healthy Living. Get Active. www.healthfinder.gov/prevention/ViewTopic.aspx?topicID=22.
Drug brand names
- Bupropion • Wellbutrin
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459.
2. Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164(5):739-752.
3. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med. 2003;36(6):698-703.
4. Stathopoulou G, Powers MB, Berry AC, et al. Exercise interventions for mental health: a quantitative and qualitative review. Clinical Psychology Science and Practice. 2006;13(2):179-193.
5. Wee CC, McCarthy EP, Davis RB, et al. Physician counseling about exercise. JAMA. 1999;282(16):1583-1588.
6. Long BJ, Calfas KJ, Wooten W, et al. A multisite field test of the acceptability of physical activity counseling in primary care: project PACE. Am J Prev Med. 1996;12(2):73-81.
7. Vickers KS, Nies MA, Patten CA, et al. Patients with diabetes and depression may need additional support for exercise. Am J Health Behav. 2006;30(4):353-362.
8. Weidinger KA, Lovegreen SL, Elliott MB, et al. How to make exercise counseling more effective: lessons from rural America. J Fam Pract. 2008;57(6):394-402.
9. Calfas KJ, Long BJ, Sallis JF, et al. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med. 1996;25(3):225-233.
10. Rosqvist E, Heikkinen E, Lyyra TM, et al. Factors affecting the increased risk of physical inactivity among older people with depressive symptoms. Scand J Med Sci Sports. 2008 May 22 [Epub ahead of print].
11. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
12. Galper DI, Trivedi MH, Barlow CE, et al. Inverse association between physical inactivity and mental health in men and women. Med Sci Sports Exerc. 2006;38(1):173-178.
Fazio AF. A concurrent validation study of the NCHS General Well-Being Schedule. Vital and Health Statistics. Hyattsville, MD: National Center for Health Statistics, US Public Health Service; September 1977. Series 2, No. 73, DHEW Publication No. (HRA) 78-1347:1-13.
14. De Moor MH, Boomsma DI, Stubbe JH, et al. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry. 2008;65(8):897-905.
15. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
16. O’Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry. 1996;8(1):37-54.
17. Heh SS, Huang LH, Ho SM, et al. Effectiveness of an exercise support program in reducing the severity of postnatal depression in Taiwanese women. Birth. 2008;35(1):60-65.
18. Saurel-Cubizolles MJ, Romito P, Lelong N, et al. Women’s health after childbirth: a longitudinal study in France and Italy. BJOG. 2000;107(10):1202-1209.
19. Dritsa M, Da Costa D, Dupuis G, et al. Effects of a home-based exercise intervention on fatigue in postpartum depressed women: results of a randomized controlled trial. Ann Behav Med. 2008;35(2):179-187.
20. Corwin EJ, Brownstead J, Barton N, et al. The impact of fatigue on the development of postpartum depression. J Obstet Gynecol Neonatal Nurs. 2005;34(5):577-586.
21. Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci. 1997;52(1):M27-35.
22. Singh NA, Stavrinos TM, Scarbek Y, et al. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(6):768-776.
23. Fremont J, Wilcoxon Craighead L. Aerobic exercise and cognitive therapy in the treatment of dysphoric moods. Cognit Ther Res. 1987;11(2):241-251.
24. Klein MH, Greist JH, Gurman RA, et al. A comparative outcome study of group psychotherapy vs. exercise treatments for depression. Int J Ment Health. 1985;13:148-177.
25. Carta MG, Hardoy MC, Pilu A, et al. Improving physical quality of life with group physical activity in the adjunctive treatment of major depressive disorder. Clin Pract Epidemiol Ment Health. 2008;4:1.
26. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
27. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349-2356.
28. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633-638.
29. Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2001;56(8):M497-504.
30. Camacho TC, Roberts RE, Lazarus NB, et al. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol. 1991;134(2):220-231.
1. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459.
2. Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164(5):739-752.
3. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med. 2003;36(6):698-703.
4. Stathopoulou G, Powers MB, Berry AC, et al. Exercise interventions for mental health: a quantitative and qualitative review. Clinical Psychology Science and Practice. 2006;13(2):179-193.
5. Wee CC, McCarthy EP, Davis RB, et al. Physician counseling about exercise. JAMA. 1999;282(16):1583-1588.
6. Long BJ, Calfas KJ, Wooten W, et al. A multisite field test of the acceptability of physical activity counseling in primary care: project PACE. Am J Prev Med. 1996;12(2):73-81.
7. Vickers KS, Nies MA, Patten CA, et al. Patients with diabetes and depression may need additional support for exercise. Am J Health Behav. 2006;30(4):353-362.
8. Weidinger KA, Lovegreen SL, Elliott MB, et al. How to make exercise counseling more effective: lessons from rural America. J Fam Pract. 2008;57(6):394-402.
9. Calfas KJ, Long BJ, Sallis JF, et al. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med. 1996;25(3):225-233.
10. Rosqvist E, Heikkinen E, Lyyra TM, et al. Factors affecting the increased risk of physical inactivity among older people with depressive symptoms. Scand J Med Sci Sports. 2008 May 22 [Epub ahead of print].
11. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
12. Galper DI, Trivedi MH, Barlow CE, et al. Inverse association between physical inactivity and mental health in men and women. Med Sci Sports Exerc. 2006;38(1):173-178.
Fazio AF. A concurrent validation study of the NCHS General Well-Being Schedule. Vital and Health Statistics. Hyattsville, MD: National Center for Health Statistics, US Public Health Service; September 1977. Series 2, No. 73, DHEW Publication No. (HRA) 78-1347:1-13.
14. De Moor MH, Boomsma DI, Stubbe JH, et al. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry. 2008;65(8):897-905.
15. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
16. O’Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry. 1996;8(1):37-54.
17. Heh SS, Huang LH, Ho SM, et al. Effectiveness of an exercise support program in reducing the severity of postnatal depression in Taiwanese women. Birth. 2008;35(1):60-65.
18. Saurel-Cubizolles MJ, Romito P, Lelong N, et al. Women’s health after childbirth: a longitudinal study in France and Italy. BJOG. 2000;107(10):1202-1209.
19. Dritsa M, Da Costa D, Dupuis G, et al. Effects of a home-based exercise intervention on fatigue in postpartum depressed women: results of a randomized controlled trial. Ann Behav Med. 2008;35(2):179-187.
20. Corwin EJ, Brownstead J, Barton N, et al. The impact of fatigue on the development of postpartum depression. J Obstet Gynecol Neonatal Nurs. 2005;34(5):577-586.
21. Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci. 1997;52(1):M27-35.
22. Singh NA, Stavrinos TM, Scarbek Y, et al. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(6):768-776.
23. Fremont J, Wilcoxon Craighead L. Aerobic exercise and cognitive therapy in the treatment of dysphoric moods. Cognit Ther Res. 1987;11(2):241-251.
24. Klein MH, Greist JH, Gurman RA, et al. A comparative outcome study of group psychotherapy vs. exercise treatments for depression. Int J Ment Health. 1985;13:148-177.
25. Carta MG, Hardoy MC, Pilu A, et al. Improving physical quality of life with group physical activity in the adjunctive treatment of major depressive disorder. Clin Pract Epidemiol Ment Health. 2008;4:1.
26. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
27. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349-2356.
28. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633-638.
29. Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2001;56(8):M497-504.
30. Camacho TC, Roberts RE, Lazarus NB, et al. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol. 1991;134(2):220-231.