User login
Fast-track protocol cuts lung resection complications, LOS
CHICAGO – An enhanced recovery pathway reduces short-term complications and hospital stays following cancer-related lung resection without raising readmissions or emergency visits after discharge, a study showed.
“A multimodal pathway for open, elective lobectomy seems to improve efficiency and quality of care,” Dr. Amin Madani, from McGill University in Montreal, said at the annual meeting of the Central Surgical Association (CSA).
Prior research suggests that an enhanced recovery pathway (ERP), also known as fast-track protocols, can improve surgical outcomes, but there is little evidence to support its use and effectiveness in lung resection.
Surgeons at McGill established an integrated, multimodal approach to perioperative care of these patients after creating a written, evidence-based, step-by-step pathway. Key elements are standardized preoperative patient education; removal of urine drains on postoperative day 1; removal of the last chest tube by postop (POD) day 3, if there is <300 cc of drainage in 24 hours and no air leak; ambulation goals of more than 75 m thrice-daily by POD 3; introduction of solid food on POD 1; and a target discharge of POD 4; Dr. Madani explained.
To examine the effectiveness of the pathway, the authors retrospectively analyzed outcomes in 127 patients undergoing elective lung resection for primary or secondary lung cancer receiving traditional care and 107 patients treated after the ERP was implemented in September 2012. At baseline, the two groups were similar with respect to age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) scores, pulmonary function, and smoking history.
Hospital length of stay was significantly reduced after the ERP from a median of 7 days with traditional care to 6 days (P < .01), driven largely by patients with an uncomplicated hospital course who were discharged after a median of 5 days after the pathway was implemented, Dr. Madani said.
It was not the case that patients went home too early, as readmissions (5% vs. 6%) and ED visits (3% vs. 5%) were similar between both groups, he added.
After the pathway was implemented, patients had earlier Foley catheter removal (POD 2 vs. 1), IV discontinuation (POD 3 vs. 2), ambulation (POD 2 vs. 1), last chest tube removal (POD 5 vs. 4), and epidural removal (POD 5 vs. 4).
The enhanced recovery pathway group had fewer overall complications than did the traditional care group (37% vs. 50%; P = .03), a threefold decrease in urinary tract infections (3% vs. 12%; P < .01), and a trend toward fewer pulmonary complications (25% vs. 31%; P = .38) and surgical site infections (1% vs. 6%; P = .07), he said.
Despite significantly earlier removal of chest tubes after the pathway, there was no difference in the incidence of pneumothorax or pleural effusion requiring tube re-insertion, affirming that “Chest tubes were not being removed too early, causing harm to patients,” Dr. Madani said.
In multivariate regression analysis adjusted for age, sex, BMI, and ASA score, there was a significant negative association between implementation of an enhanced recovery pathway and length of stay (beta, –0.18; P < .01) and complications (odds ratio, 0.46; P < .01), but not readmissions (OR, 1.59; P = .44).
Early removal of chest tubes and urinary catheter were independent predictors of decreased length of stay.
Dr. L. Michael Brunt, a discussant from Washington University in St. Louis, said the development of care pathways to enhance recovery after surgery is gaining a lot of interest in the surgical community, but went on to ask how much it cost to implement.
The overall cost of the surgeon-driven initiative, involving multiple pathways for various surgical procedures, is about $120,000 annually, or $100/patient for the 1,200 patients undergoing surgery using an ERP program at the McGill University Health Centre each year, Dr. Madani said. This cost also includes a full-time nurse practitioner now serving as the pathway coordinator and roughly $13,000 for patient education booklets, but no additional staff.
An audience member questioned whether the authors have identified factors predicting which patients would fail to meet pathway goals, observing that in the colorectal field, there are patients such as the 80-year-old, narcotic-naive woman with diabetes, who simply won’t progress.
“That’s a very good point, and I agree there are some patients whom you can’t fast track,” Dr. Madani replied. “Part of the deal here is that, yes, we have this protocolized pathway; however, the surgeon still has the right to change that if they feel it is important. We didn’t look at the specifics of which patient [factors] achieved adherence, but we could at some point in the future.”
CSA president and session moderator Christopher McHenry, from MetroHealth Medical Center in Cleveland, said he was impressed with the study and called the findings very believable.
“I think all of these recovery pathways can be very beneficial,” Dr. McHenry said in an interview. “It helps us re-look at how we’re managing our patients and see if there are ways that we can improve on their postoperative management that may lead to earlier discharge.”
The study was funded by an investigator-initiated research grant from Ethicon Canada. Dr. Madani, his coauthors, Dr. Brunt, and Dr. McHenry reported having no financial conflicts.
CHICAGO – An enhanced recovery pathway reduces short-term complications and hospital stays following cancer-related lung resection without raising readmissions or emergency visits after discharge, a study showed.
“A multimodal pathway for open, elective lobectomy seems to improve efficiency and quality of care,” Dr. Amin Madani, from McGill University in Montreal, said at the annual meeting of the Central Surgical Association (CSA).
Prior research suggests that an enhanced recovery pathway (ERP), also known as fast-track protocols, can improve surgical outcomes, but there is little evidence to support its use and effectiveness in lung resection.
Surgeons at McGill established an integrated, multimodal approach to perioperative care of these patients after creating a written, evidence-based, step-by-step pathway. Key elements are standardized preoperative patient education; removal of urine drains on postoperative day 1; removal of the last chest tube by postop (POD) day 3, if there is <300 cc of drainage in 24 hours and no air leak; ambulation goals of more than 75 m thrice-daily by POD 3; introduction of solid food on POD 1; and a target discharge of POD 4; Dr. Madani explained.
To examine the effectiveness of the pathway, the authors retrospectively analyzed outcomes in 127 patients undergoing elective lung resection for primary or secondary lung cancer receiving traditional care and 107 patients treated after the ERP was implemented in September 2012. At baseline, the two groups were similar with respect to age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) scores, pulmonary function, and smoking history.
Hospital length of stay was significantly reduced after the ERP from a median of 7 days with traditional care to 6 days (P < .01), driven largely by patients with an uncomplicated hospital course who were discharged after a median of 5 days after the pathway was implemented, Dr. Madani said.
It was not the case that patients went home too early, as readmissions (5% vs. 6%) and ED visits (3% vs. 5%) were similar between both groups, he added.
After the pathway was implemented, patients had earlier Foley catheter removal (POD 2 vs. 1), IV discontinuation (POD 3 vs. 2), ambulation (POD 2 vs. 1), last chest tube removal (POD 5 vs. 4), and epidural removal (POD 5 vs. 4).
The enhanced recovery pathway group had fewer overall complications than did the traditional care group (37% vs. 50%; P = .03), a threefold decrease in urinary tract infections (3% vs. 12%; P < .01), and a trend toward fewer pulmonary complications (25% vs. 31%; P = .38) and surgical site infections (1% vs. 6%; P = .07), he said.
Despite significantly earlier removal of chest tubes after the pathway, there was no difference in the incidence of pneumothorax or pleural effusion requiring tube re-insertion, affirming that “Chest tubes were not being removed too early, causing harm to patients,” Dr. Madani said.
In multivariate regression analysis adjusted for age, sex, BMI, and ASA score, there was a significant negative association between implementation of an enhanced recovery pathway and length of stay (beta, –0.18; P < .01) and complications (odds ratio, 0.46; P < .01), but not readmissions (OR, 1.59; P = .44).
Early removal of chest tubes and urinary catheter were independent predictors of decreased length of stay.
Dr. L. Michael Brunt, a discussant from Washington University in St. Louis, said the development of care pathways to enhance recovery after surgery is gaining a lot of interest in the surgical community, but went on to ask how much it cost to implement.
The overall cost of the surgeon-driven initiative, involving multiple pathways for various surgical procedures, is about $120,000 annually, or $100/patient for the 1,200 patients undergoing surgery using an ERP program at the McGill University Health Centre each year, Dr. Madani said. This cost also includes a full-time nurse practitioner now serving as the pathway coordinator and roughly $13,000 for patient education booklets, but no additional staff.
An audience member questioned whether the authors have identified factors predicting which patients would fail to meet pathway goals, observing that in the colorectal field, there are patients such as the 80-year-old, narcotic-naive woman with diabetes, who simply won’t progress.
“That’s a very good point, and I agree there are some patients whom you can’t fast track,” Dr. Madani replied. “Part of the deal here is that, yes, we have this protocolized pathway; however, the surgeon still has the right to change that if they feel it is important. We didn’t look at the specifics of which patient [factors] achieved adherence, but we could at some point in the future.”
CSA president and session moderator Christopher McHenry, from MetroHealth Medical Center in Cleveland, said he was impressed with the study and called the findings very believable.
“I think all of these recovery pathways can be very beneficial,” Dr. McHenry said in an interview. “It helps us re-look at how we’re managing our patients and see if there are ways that we can improve on their postoperative management that may lead to earlier discharge.”
The study was funded by an investigator-initiated research grant from Ethicon Canada. Dr. Madani, his coauthors, Dr. Brunt, and Dr. McHenry reported having no financial conflicts.
CHICAGO – An enhanced recovery pathway reduces short-term complications and hospital stays following cancer-related lung resection without raising readmissions or emergency visits after discharge, a study showed.
“A multimodal pathway for open, elective lobectomy seems to improve efficiency and quality of care,” Dr. Amin Madani, from McGill University in Montreal, said at the annual meeting of the Central Surgical Association (CSA).
Prior research suggests that an enhanced recovery pathway (ERP), also known as fast-track protocols, can improve surgical outcomes, but there is little evidence to support its use and effectiveness in lung resection.
Surgeons at McGill established an integrated, multimodal approach to perioperative care of these patients after creating a written, evidence-based, step-by-step pathway. Key elements are standardized preoperative patient education; removal of urine drains on postoperative day 1; removal of the last chest tube by postop (POD) day 3, if there is <300 cc of drainage in 24 hours and no air leak; ambulation goals of more than 75 m thrice-daily by POD 3; introduction of solid food on POD 1; and a target discharge of POD 4; Dr. Madani explained.
To examine the effectiveness of the pathway, the authors retrospectively analyzed outcomes in 127 patients undergoing elective lung resection for primary or secondary lung cancer receiving traditional care and 107 patients treated after the ERP was implemented in September 2012. At baseline, the two groups were similar with respect to age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) scores, pulmonary function, and smoking history.
Hospital length of stay was significantly reduced after the ERP from a median of 7 days with traditional care to 6 days (P < .01), driven largely by patients with an uncomplicated hospital course who were discharged after a median of 5 days after the pathway was implemented, Dr. Madani said.
It was not the case that patients went home too early, as readmissions (5% vs. 6%) and ED visits (3% vs. 5%) were similar between both groups, he added.
After the pathway was implemented, patients had earlier Foley catheter removal (POD 2 vs. 1), IV discontinuation (POD 3 vs. 2), ambulation (POD 2 vs. 1), last chest tube removal (POD 5 vs. 4), and epidural removal (POD 5 vs. 4).
The enhanced recovery pathway group had fewer overall complications than did the traditional care group (37% vs. 50%; P = .03), a threefold decrease in urinary tract infections (3% vs. 12%; P < .01), and a trend toward fewer pulmonary complications (25% vs. 31%; P = .38) and surgical site infections (1% vs. 6%; P = .07), he said.
Despite significantly earlier removal of chest tubes after the pathway, there was no difference in the incidence of pneumothorax or pleural effusion requiring tube re-insertion, affirming that “Chest tubes were not being removed too early, causing harm to patients,” Dr. Madani said.
In multivariate regression analysis adjusted for age, sex, BMI, and ASA score, there was a significant negative association between implementation of an enhanced recovery pathway and length of stay (beta, –0.18; P < .01) and complications (odds ratio, 0.46; P < .01), but not readmissions (OR, 1.59; P = .44).
Early removal of chest tubes and urinary catheter were independent predictors of decreased length of stay.
Dr. L. Michael Brunt, a discussant from Washington University in St. Louis, said the development of care pathways to enhance recovery after surgery is gaining a lot of interest in the surgical community, but went on to ask how much it cost to implement.
The overall cost of the surgeon-driven initiative, involving multiple pathways for various surgical procedures, is about $120,000 annually, or $100/patient for the 1,200 patients undergoing surgery using an ERP program at the McGill University Health Centre each year, Dr. Madani said. This cost also includes a full-time nurse practitioner now serving as the pathway coordinator and roughly $13,000 for patient education booklets, but no additional staff.
An audience member questioned whether the authors have identified factors predicting which patients would fail to meet pathway goals, observing that in the colorectal field, there are patients such as the 80-year-old, narcotic-naive woman with diabetes, who simply won’t progress.
“That’s a very good point, and I agree there are some patients whom you can’t fast track,” Dr. Madani replied. “Part of the deal here is that, yes, we have this protocolized pathway; however, the surgeon still has the right to change that if they feel it is important. We didn’t look at the specifics of which patient [factors] achieved adherence, but we could at some point in the future.”
CSA president and session moderator Christopher McHenry, from MetroHealth Medical Center in Cleveland, said he was impressed with the study and called the findings very believable.
“I think all of these recovery pathways can be very beneficial,” Dr. McHenry said in an interview. “It helps us re-look at how we’re managing our patients and see if there are ways that we can improve on their postoperative management that may lead to earlier discharge.”
The study was funded by an investigator-initiated research grant from Ethicon Canada. Dr. Madani, his coauthors, Dr. Brunt, and Dr. McHenry reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: An enhanced recovery pathway reduces complications and hospital stay following lung cancer resection without raising readmissions or ED visits.
Major finding: Patients in the enhanced recovery pathway vs. traditional care had fewer overall complications (37% vs. 50%; P = .03) and threefold fewer UTIs (3% vs. 12%; P < .01).
Data source: Observational study of 234 patients undergoing lung resection.
Disclosures: The study was funded by an investigator-initiated research grant from Ethicon Canada. Dr. Madani, his coauthors, Dr. Brunt, and Dr. McHenry reported having no financial conflicts.
Esophagogastric cancer patients on chemotherapy more likely to develop VTE
Incidence rates for developing venous thromboembolism (VTE) among esophagogastric cancer patients undergoing neoadjuvant chemotherapy in combination with curative intended surgery were significantly higher among patients with initial stage III and IV cancers and gastric cancer, according to a new study published in Thrombosis Research.
In the clinical prospective study, 129 patients with lower esophageal, gastroesophageal, and gastric cancer were examined between 2008 and 2011. Baseline assessments were recorded via bilateral compression ultrasound (biCUS) for deep vein thrombosis and computer tomography pulmonary angiography for pulmonary embolism. The patients received a chemotherapy regimen of oxaliplatin, capecitabine, and epirubicin, with curative intended surgery, and were examined before undergoing preoperative chemotherapy, surgery, and postoperative chemotherapy. The researchers encountered 21 VTE cases, or 16% of the total number of patients examined, with VTE incidences twice as likely to be asymptomatic than symptomatic.
The authors noted that state-of-the-art technology helped boost VTE detection rates among asymptomatic patients, and older studies may have underreported incidences of the disease.
“Although our study only included 129 patients, the systematic use of biCUS strongly suggests that the frequency of VTE is much greater than that previously reported for these types of cancer,” wrote Dr. Anders Christian Larsen and his associates at Aalborg University Hospital, Denmark.
Read more here (Thrombosis Research 2015 [doi:10.1016/j.thromres.2015.01.021]).
Incidence rates for developing venous thromboembolism (VTE) among esophagogastric cancer patients undergoing neoadjuvant chemotherapy in combination with curative intended surgery were significantly higher among patients with initial stage III and IV cancers and gastric cancer, according to a new study published in Thrombosis Research.
In the clinical prospective study, 129 patients with lower esophageal, gastroesophageal, and gastric cancer were examined between 2008 and 2011. Baseline assessments were recorded via bilateral compression ultrasound (biCUS) for deep vein thrombosis and computer tomography pulmonary angiography for pulmonary embolism. The patients received a chemotherapy regimen of oxaliplatin, capecitabine, and epirubicin, with curative intended surgery, and were examined before undergoing preoperative chemotherapy, surgery, and postoperative chemotherapy. The researchers encountered 21 VTE cases, or 16% of the total number of patients examined, with VTE incidences twice as likely to be asymptomatic than symptomatic.
The authors noted that state-of-the-art technology helped boost VTE detection rates among asymptomatic patients, and older studies may have underreported incidences of the disease.
“Although our study only included 129 patients, the systematic use of biCUS strongly suggests that the frequency of VTE is much greater than that previously reported for these types of cancer,” wrote Dr. Anders Christian Larsen and his associates at Aalborg University Hospital, Denmark.
Read more here (Thrombosis Research 2015 [doi:10.1016/j.thromres.2015.01.021]).
Incidence rates for developing venous thromboembolism (VTE) among esophagogastric cancer patients undergoing neoadjuvant chemotherapy in combination with curative intended surgery were significantly higher among patients with initial stage III and IV cancers and gastric cancer, according to a new study published in Thrombosis Research.
In the clinical prospective study, 129 patients with lower esophageal, gastroesophageal, and gastric cancer were examined between 2008 and 2011. Baseline assessments were recorded via bilateral compression ultrasound (biCUS) for deep vein thrombosis and computer tomography pulmonary angiography for pulmonary embolism. The patients received a chemotherapy regimen of oxaliplatin, capecitabine, and epirubicin, with curative intended surgery, and were examined before undergoing preoperative chemotherapy, surgery, and postoperative chemotherapy. The researchers encountered 21 VTE cases, or 16% of the total number of patients examined, with VTE incidences twice as likely to be asymptomatic than symptomatic.
The authors noted that state-of-the-art technology helped boost VTE detection rates among asymptomatic patients, and older studies may have underreported incidences of the disease.
“Although our study only included 129 patients, the systematic use of biCUS strongly suggests that the frequency of VTE is much greater than that previously reported for these types of cancer,” wrote Dr. Anders Christian Larsen and his associates at Aalborg University Hospital, Denmark.
Read more here (Thrombosis Research 2015 [doi:10.1016/j.thromres.2015.01.021]).
VIDEO: Eminent surgeon Dr. LaSalle D. Leffall reflects on his journey
LaSalle D. Leffall, Jr., M.D., FACS, is the Charles R. Drew Professor of Surgery, Howard University College of Medicine, Washington DC.
Dr. Leffall has had a long and distinguished career and has served as President of the American Cancer Society, the Society of Surgical Oncology, the Society of Surgical Chairmen, the Washington Academy of Surgery, the Society of Black Academic Surgeons, and the American College of Surgeons. He reflects on his career, the role of the American College of Surgeons in promoting diversity in the surgical field, and the Society for Black Academic Surgeons.
LaSalle D. Leffall, Jr., M.D., FACS, is the Charles R. Drew Professor of Surgery, Howard University College of Medicine, Washington DC.
Dr. Leffall has had a long and distinguished career and has served as President of the American Cancer Society, the Society of Surgical Oncology, the Society of Surgical Chairmen, the Washington Academy of Surgery, the Society of Black Academic Surgeons, and the American College of Surgeons. He reflects on his career, the role of the American College of Surgeons in promoting diversity in the surgical field, and the Society for Black Academic Surgeons.
LaSalle D. Leffall, Jr., M.D., FACS, is the Charles R. Drew Professor of Surgery, Howard University College of Medicine, Washington DC.
Dr. Leffall has had a long and distinguished career and has served as President of the American Cancer Society, the Society of Surgical Oncology, the Society of Surgical Chairmen, the Washington Academy of Surgery, the Society of Black Academic Surgeons, and the American College of Surgeons. He reflects on his career, the role of the American College of Surgeons in promoting diversity in the surgical field, and the Society for Black Academic Surgeons.
Baseline QOL measures not associated with outcomes in high-risk operable lung cancer patients
Poor baseline quality-of-life scores were not predictive of worse overall or recurrence-free survival, or of higher risk for adverse events following sublobar resection in high-risk surgical patients with lung cancer.
In addition, quality of life (QOL) and dyspnea scores did not deteriorate significantly overall, based upon the results of a prospective, multicenter study. Low dyspnea scores at baseline, however, did predict subsequent poor overall survival, according to Dr. Hiran C. Fernando of the Boston Medical Center and his colleagues.
The researchers assessed QOL using the 36-item Short-Form Health Survey (SF36) and the dyspnea score from the University of California, San Diego, Shortness of Breath Questionnaire (SOBQ). Both were measured at baseline, 3, 12, and 24 months. The SF36 scores were further broken down into the physical component summary (PCS) and the mental component summary (MCS), according to their report published online and in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2014 Nov. 13 [doi:10.1016/j.jtcvs.2014.11.003]) .
A total of 212 eligible patients in the American College of Surgeons Oncology Group Z4032 trial were randomized to sublobar resection (108 patients) or sublobar resection with brachytherapy (104). The mean age was about 70.5 years, and 56% were women. There were no significant differences in baseline QOL scores between arms. Baseline PCS and MCS scores were at least 1 standard deviation below those of the U.S. general population in 65% and 46.5% of the patients, respectively.
Overall, there were no significant differences in grade 3+ adverse events, overall survival, or recurrence-free survival seen in patients with baseline scores greater than or equal to median QOL scores or less than median scores. There was, however, significantly worse overall survival for patients with baseline SOBQ scores less than or equal to median. In addition, a 10-point drop in SOBQ score at 12 months also predicted poor overall survival, according to Dr. Fernando and his associates.
In terms of results for operative procedures and tumor types, there was a significantly higher percentage of patients with a decline of 10 points or more in SOBQ scores with segmentectomy, compared with wedge resection (40.5% vs. 21.9%) at 12 months, with thoracotomy vs. video-assisted thoracic surgery (VATS) (38.8% vs. 20.4%, P = .03) at 12 months, and for T1b vs. T1a tumors (46.9% vs. 23.5%) at 24 months. In addition, there was a significantly greater than or equal to 10-point improvement in PCS scores at 3 months with VATS vs. thoracotomy (16.5% vs. 3.6%).
The researchers pointed out that, although QOL measurements can be useful to help decide the optimal surgery procedure, it has even more relevance when considering surgical versus nonsurgical therapies, such as using stereotactic body radiation therapy, for high-risk patients with early-stage lung cancer.
“Some advantages relating to minimizing postoperative dyspnea, as measured by the SOBQ, were gained by using VATS (rather than thoracotomy) or wedge resection (rather than segmentectomy). In addition, VATS, as opposed to thoracotomy, patients had improved PCS scores at 3 months, lending support to the preferential use of VATS when SR is performed,” the researchers concluded.
The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
This research is a noteworthy contribution because there source of prospectively acquired QOL data for such a high-risk group facing lung surgery, Dr. Michael T. Jaklitsch said in his invited editorial commentary (J. Thorac. Cardiovasc. Surg. 2015 Dec. 2 [doi:10.1016/j.jtcvs.2014.11.068]).
 |
Dr. Michael T. Jaklitsch |
Although there was no evidence of predictive ability of QOL data for this population, “the predictive value of self-assessment may be more powerful in a broader population, however, than in a selected high-risk population such as the Alliance Z4032 trial,” he said. “The amount of pulmonary impairment required to enter this trial was likely the prime determinant of morbidity.” Thus QOL tools may be predictive with certain populations, but not in others. Overall, however, one benefit of self-assessment tools is that they allow patients to be seen more as people than as disease cases by the surgeons.
“Self-assessment tools allow our patients to tell us more completely about themselves and frequently become a springboard to discuss fears of the near future after surgery and what that might look like,” he added. “They allow us to ask our patients more completely, ‘How are you doing?’ ”
Dr. Jaklitsch is a thoracic surgeon at Brigham and Woman’s Hospital, Harvard Medical School, Boston.
This research is a noteworthy contribution because there source of prospectively acquired QOL data for such a high-risk group facing lung surgery, Dr. Michael T. Jaklitsch said in his invited editorial commentary (J. Thorac. Cardiovasc. Surg. 2015 Dec. 2 [doi:10.1016/j.jtcvs.2014.11.068]).
 |
Dr. Michael T. Jaklitsch |
Although there was no evidence of predictive ability of QOL data for this population, “the predictive value of self-assessment may be more powerful in a broader population, however, than in a selected high-risk population such as the Alliance Z4032 trial,” he said. “The amount of pulmonary impairment required to enter this trial was likely the prime determinant of morbidity.” Thus QOL tools may be predictive with certain populations, but not in others. Overall, however, one benefit of self-assessment tools is that they allow patients to be seen more as people than as disease cases by the surgeons.
“Self-assessment tools allow our patients to tell us more completely about themselves and frequently become a springboard to discuss fears of the near future after surgery and what that might look like,” he added. “They allow us to ask our patients more completely, ‘How are you doing?’ ”
Dr. Jaklitsch is a thoracic surgeon at Brigham and Woman’s Hospital, Harvard Medical School, Boston.
This research is a noteworthy contribution because there source of prospectively acquired QOL data for such a high-risk group facing lung surgery, Dr. Michael T. Jaklitsch said in his invited editorial commentary (J. Thorac. Cardiovasc. Surg. 2015 Dec. 2 [doi:10.1016/j.jtcvs.2014.11.068]).
 |
Dr. Michael T. Jaklitsch |
Although there was no evidence of predictive ability of QOL data for this population, “the predictive value of self-assessment may be more powerful in a broader population, however, than in a selected high-risk population such as the Alliance Z4032 trial,” he said. “The amount of pulmonary impairment required to enter this trial was likely the prime determinant of morbidity.” Thus QOL tools may be predictive with certain populations, but not in others. Overall, however, one benefit of self-assessment tools is that they allow patients to be seen more as people than as disease cases by the surgeons.
“Self-assessment tools allow our patients to tell us more completely about themselves and frequently become a springboard to discuss fears of the near future after surgery and what that might look like,” he added. “They allow us to ask our patients more completely, ‘How are you doing?’ ”
Dr. Jaklitsch is a thoracic surgeon at Brigham and Woman’s Hospital, Harvard Medical School, Boston.
Poor baseline quality-of-life scores were not predictive of worse overall or recurrence-free survival, or of higher risk for adverse events following sublobar resection in high-risk surgical patients with lung cancer.
In addition, quality of life (QOL) and dyspnea scores did not deteriorate significantly overall, based upon the results of a prospective, multicenter study. Low dyspnea scores at baseline, however, did predict subsequent poor overall survival, according to Dr. Hiran C. Fernando of the Boston Medical Center and his colleagues.
The researchers assessed QOL using the 36-item Short-Form Health Survey (SF36) and the dyspnea score from the University of California, San Diego, Shortness of Breath Questionnaire (SOBQ). Both were measured at baseline, 3, 12, and 24 months. The SF36 scores were further broken down into the physical component summary (PCS) and the mental component summary (MCS), according to their report published online and in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2014 Nov. 13 [doi:10.1016/j.jtcvs.2014.11.003]) .
A total of 212 eligible patients in the American College of Surgeons Oncology Group Z4032 trial were randomized to sublobar resection (108 patients) or sublobar resection with brachytherapy (104). The mean age was about 70.5 years, and 56% were women. There were no significant differences in baseline QOL scores between arms. Baseline PCS and MCS scores were at least 1 standard deviation below those of the U.S. general population in 65% and 46.5% of the patients, respectively.
Overall, there were no significant differences in grade 3+ adverse events, overall survival, or recurrence-free survival seen in patients with baseline scores greater than or equal to median QOL scores or less than median scores. There was, however, significantly worse overall survival for patients with baseline SOBQ scores less than or equal to median. In addition, a 10-point drop in SOBQ score at 12 months also predicted poor overall survival, according to Dr. Fernando and his associates.
In terms of results for operative procedures and tumor types, there was a significantly higher percentage of patients with a decline of 10 points or more in SOBQ scores with segmentectomy, compared with wedge resection (40.5% vs. 21.9%) at 12 months, with thoracotomy vs. video-assisted thoracic surgery (VATS) (38.8% vs. 20.4%, P = .03) at 12 months, and for T1b vs. T1a tumors (46.9% vs. 23.5%) at 24 months. In addition, there was a significantly greater than or equal to 10-point improvement in PCS scores at 3 months with VATS vs. thoracotomy (16.5% vs. 3.6%).
The researchers pointed out that, although QOL measurements can be useful to help decide the optimal surgery procedure, it has even more relevance when considering surgical versus nonsurgical therapies, such as using stereotactic body radiation therapy, for high-risk patients with early-stage lung cancer.
“Some advantages relating to minimizing postoperative dyspnea, as measured by the SOBQ, were gained by using VATS (rather than thoracotomy) or wedge resection (rather than segmentectomy). In addition, VATS, as opposed to thoracotomy, patients had improved PCS scores at 3 months, lending support to the preferential use of VATS when SR is performed,” the researchers concluded.
The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
Poor baseline quality-of-life scores were not predictive of worse overall or recurrence-free survival, or of higher risk for adverse events following sublobar resection in high-risk surgical patients with lung cancer.
In addition, quality of life (QOL) and dyspnea scores did not deteriorate significantly overall, based upon the results of a prospective, multicenter study. Low dyspnea scores at baseline, however, did predict subsequent poor overall survival, according to Dr. Hiran C. Fernando of the Boston Medical Center and his colleagues.
The researchers assessed QOL using the 36-item Short-Form Health Survey (SF36) and the dyspnea score from the University of California, San Diego, Shortness of Breath Questionnaire (SOBQ). Both were measured at baseline, 3, 12, and 24 months. The SF36 scores were further broken down into the physical component summary (PCS) and the mental component summary (MCS), according to their report published online and in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2014 Nov. 13 [doi:10.1016/j.jtcvs.2014.11.003]) .
A total of 212 eligible patients in the American College of Surgeons Oncology Group Z4032 trial were randomized to sublobar resection (108 patients) or sublobar resection with brachytherapy (104). The mean age was about 70.5 years, and 56% were women. There were no significant differences in baseline QOL scores between arms. Baseline PCS and MCS scores were at least 1 standard deviation below those of the U.S. general population in 65% and 46.5% of the patients, respectively.
Overall, there were no significant differences in grade 3+ adverse events, overall survival, or recurrence-free survival seen in patients with baseline scores greater than or equal to median QOL scores or less than median scores. There was, however, significantly worse overall survival for patients with baseline SOBQ scores less than or equal to median. In addition, a 10-point drop in SOBQ score at 12 months also predicted poor overall survival, according to Dr. Fernando and his associates.
In terms of results for operative procedures and tumor types, there was a significantly higher percentage of patients with a decline of 10 points or more in SOBQ scores with segmentectomy, compared with wedge resection (40.5% vs. 21.9%) at 12 months, with thoracotomy vs. video-assisted thoracic surgery (VATS) (38.8% vs. 20.4%, P = .03) at 12 months, and for T1b vs. T1a tumors (46.9% vs. 23.5%) at 24 months. In addition, there was a significantly greater than or equal to 10-point improvement in PCS scores at 3 months with VATS vs. thoracotomy (16.5% vs. 3.6%).
The researchers pointed out that, although QOL measurements can be useful to help decide the optimal surgery procedure, it has even more relevance when considering surgical versus nonsurgical therapies, such as using stereotactic body radiation therapy, for high-risk patients with early-stage lung cancer.
“Some advantages relating to minimizing postoperative dyspnea, as measured by the SOBQ, were gained by using VATS (rather than thoracotomy) or wedge resection (rather than segmentectomy). In addition, VATS, as opposed to thoracotomy, patients had improved PCS scores at 3 months, lending support to the preferential use of VATS when SR is performed,” the researchers concluded.
The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Baseline quality-of-life measures were not predictive of outcomes after lung cancer surgery in high-risk operable patients.
Major finding: A significantly greater improvement in the physical component of quality of life at 3 months and in dyspnea at 1 year was seen from using VATS, compared with thoracotomy.
Data source: Researchers reviewed self-assessment QOL data from 212 eligible high-risk operable patients from the ACSOG Z4032 trial who had sublobar resections with or without brachytherapy using VATS or thoracotomy.
Disclosures: The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
More cancer patients surviving longer, but age-based disparities remain
Survival rates overall for cancer patients are higher now than 20 years ago, though younger patients are faring significantly better than older ones for many types of cancer, according to investigators.
The findings were published online Feb. 19 in JAMA Oncology.
Furthermore, racial disparities in survival persist for most cancer sites, wrote Chenjie Zeng and associates at Vanderbilt University, Nashville, Tenn.
Using data from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program, the researchers calculated survival rates for just over a million patients diagnosed with cancer of the colon or rectum, breast, prostate, lung, liver, pancreas or ovary over the time span from 1990 to 2009. Survival rates were obtained for each cancer site; results were grouped in 5-year cohorts by diagnosis date, and further broken down by race, sex, and age group (20-49, 50-64, 65-74, and 75-85 years at time of diagnosis).
Hazard ratios for cancer-specific death were obtained by comparing all later 5-year cohorts to the 1990-1994 group. Adjusted HRs for patients aged 50-64 years diagnosed with cancer in 2005-2009 compared with those diagnosed in 1990-1994 were 0.57 for colon or rectal cancer, 0.48 for breast cancer, 0.61 for liver cancer, and 0.32 for prostate cancer. By contrast, the oldest patients (aged 75-85 years) had HRs of 0.88, 0.88, 0.76, and 0.65, respectively, for these cancer sites. Age-related findings were less pronounced for lung and pancreatic cancers, Ms. Zeng and associates said (JAMA Oncology 2015 Feb. 19 [doi:10.1001/jamaoncol.2014.161]).
African Americans had a greater increase in prostate cancer survival than did whites or Asians; ovarian cancer survival, however, was reduced for African Americans but improved among whites over the study period. Overall survival rates were poorer for all cancer sites for African Americans when compared to whites, with racial disparities in screening and care a potential factor.
Some of the age-related survival differences may be attributed to younger patients’ being able to take greater advantage of newer therapies, since the largest age-related survival gap occurred for cancer sites with greater treatment advances (breast, colorectal, and prostate cancers), Ms. Zeng and associates said.
Study limitations included inability to exclude potential confounders such as socioeconomic status, lifestyle choices, and comorbidities, as well as oversampling of urban and foreign-born individuals in the study population, the researchers noted.
Survival rates overall for cancer patients are higher now than 20 years ago, though younger patients are faring significantly better than older ones for many types of cancer, according to investigators.
The findings were published online Feb. 19 in JAMA Oncology.
Furthermore, racial disparities in survival persist for most cancer sites, wrote Chenjie Zeng and associates at Vanderbilt University, Nashville, Tenn.
Using data from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program, the researchers calculated survival rates for just over a million patients diagnosed with cancer of the colon or rectum, breast, prostate, lung, liver, pancreas or ovary over the time span from 1990 to 2009. Survival rates were obtained for each cancer site; results were grouped in 5-year cohorts by diagnosis date, and further broken down by race, sex, and age group (20-49, 50-64, 65-74, and 75-85 years at time of diagnosis).
Hazard ratios for cancer-specific death were obtained by comparing all later 5-year cohorts to the 1990-1994 group. Adjusted HRs for patients aged 50-64 years diagnosed with cancer in 2005-2009 compared with those diagnosed in 1990-1994 were 0.57 for colon or rectal cancer, 0.48 for breast cancer, 0.61 for liver cancer, and 0.32 for prostate cancer. By contrast, the oldest patients (aged 75-85 years) had HRs of 0.88, 0.88, 0.76, and 0.65, respectively, for these cancer sites. Age-related findings were less pronounced for lung and pancreatic cancers, Ms. Zeng and associates said (JAMA Oncology 2015 Feb. 19 [doi:10.1001/jamaoncol.2014.161]).
African Americans had a greater increase in prostate cancer survival than did whites or Asians; ovarian cancer survival, however, was reduced for African Americans but improved among whites over the study period. Overall survival rates were poorer for all cancer sites for African Americans when compared to whites, with racial disparities in screening and care a potential factor.
Some of the age-related survival differences may be attributed to younger patients’ being able to take greater advantage of newer therapies, since the largest age-related survival gap occurred for cancer sites with greater treatment advances (breast, colorectal, and prostate cancers), Ms. Zeng and associates said.
Study limitations included inability to exclude potential confounders such as socioeconomic status, lifestyle choices, and comorbidities, as well as oversampling of urban and foreign-born individuals in the study population, the researchers noted.
Survival rates overall for cancer patients are higher now than 20 years ago, though younger patients are faring significantly better than older ones for many types of cancer, according to investigators.
The findings were published online Feb. 19 in JAMA Oncology.
Furthermore, racial disparities in survival persist for most cancer sites, wrote Chenjie Zeng and associates at Vanderbilt University, Nashville, Tenn.
Using data from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program, the researchers calculated survival rates for just over a million patients diagnosed with cancer of the colon or rectum, breast, prostate, lung, liver, pancreas or ovary over the time span from 1990 to 2009. Survival rates were obtained for each cancer site; results were grouped in 5-year cohorts by diagnosis date, and further broken down by race, sex, and age group (20-49, 50-64, 65-74, and 75-85 years at time of diagnosis).
Hazard ratios for cancer-specific death were obtained by comparing all later 5-year cohorts to the 1990-1994 group. Adjusted HRs for patients aged 50-64 years diagnosed with cancer in 2005-2009 compared with those diagnosed in 1990-1994 were 0.57 for colon or rectal cancer, 0.48 for breast cancer, 0.61 for liver cancer, and 0.32 for prostate cancer. By contrast, the oldest patients (aged 75-85 years) had HRs of 0.88, 0.88, 0.76, and 0.65, respectively, for these cancer sites. Age-related findings were less pronounced for lung and pancreatic cancers, Ms. Zeng and associates said (JAMA Oncology 2015 Feb. 19 [doi:10.1001/jamaoncol.2014.161]).
African Americans had a greater increase in prostate cancer survival than did whites or Asians; ovarian cancer survival, however, was reduced for African Americans but improved among whites over the study period. Overall survival rates were poorer for all cancer sites for African Americans when compared to whites, with racial disparities in screening and care a potential factor.
Some of the age-related survival differences may be attributed to younger patients’ being able to take greater advantage of newer therapies, since the largest age-related survival gap occurred for cancer sites with greater treatment advances (breast, colorectal, and prostate cancers), Ms. Zeng and associates said.
Study limitations included inability to exclude potential confounders such as socioeconomic status, lifestyle choices, and comorbidities, as well as oversampling of urban and foreign-born individuals in the study population, the researchers noted.
FROM JAMA ONCOLOGY
Key clinical point: Cancer survival rates improved less for older than younger patients in a large longitudinal study.
Major finding: All age groups showed improved survival for all cancers with the exception of ovarian cancer, but differences were greater for younger ages.
Data source: Longitudinal analysis of 20 years of data from 1.02 million cancer patients in nine population-based National Cancer Institute registries.
Disclosures: This study was supported by the National Institutes of Health and by funds from Vanderbilt University’s Ingram Professorship and Anne Potter Wilson Chair. Ms. Zeng received support from the Vanderbilt International Scholarship Program. The authors reported no conflicts of interest.
Teamwork key to head and neck cancer management
PARIS – Successful head and neck cancer management can be achieved only if a multidisciplinary approach is taken, experts emphasized at a recent international conference on anticancer treatment.
Because of its very location and complex anatomy, squamous cell cancer of the head and neck (SCCHN) is a difficult tumor to treat, Dr. Jean-Pierre Lefebvre of Centre Oscar Lambret in Lille, France, explained. Two-thirds of tumors are diagnosed at a late stage and often require a combination of therapeutic approaches and thus “combined toxicities.” Patients also frequently have comorbid illnesses that can affect their compliance and tolerance to treatments.
“There is only one solution: a multidisciplinary approach at any time of the management,” Dr. Lefebvre said.
The multidisciplinary approach requires a tight-knit team of imaging specialists; biologists and pathologists; anesthesiologists and surgeons; medical and radiation oncologists; nurses, general practitioners, and other support professions, such as dentists, dietitians, psychologists, speech and physical therapy specialists; and of course, the patients themselves.
Dr. Lefebvre noted that it was vital to provide patients with good information about their disease and its treatment, from the time of diagnosis to explain the various management decisions made by the multidisciplinary team and likely outcomes of the recommended interventions.
The primary goals of treatment are to control disease above the clavicles and to ensure survival, Dr. Lefebvre observed. Other treatment goals include preserving organ function, controlling symptoms, and creating a minimal impact on a patient’s quality of life by providing treatments that offer minimal long-term toxicity, good tolerability, and perhaps most important, good patient satisfaction.
Selecting treatment can be challenging and cannot be done without a multidisciplinary decision. The two main pathways are a surgery-based or radiotherapy-based treatment, but within each there are multiple options and combinations that need careful consideration on a case-by-case basis.
“It’s not a cookbook decision,” agreed Dr. Jan B. Vermorken, emeritus professor of oncology at Antwerp University Hospital, Belgium, who discussed the systemic treatment of head and neck cancer in a separate lecture. He agreed that head and neck cancer treatment is a multidisciplinary challenge that needs to balance the efficacy and tolerability of treatment on an individual basis, and always while considering the patient’s preferences.
“Patients can be very well informed,” Dr. Vermorken noted and suggested that clinicians need to be prepared to help patients understand the information that they find themselves in order to be able to counter any misinformation they might have found.
“There is no treatment without side effects,” Dr. Vermorken stressed. “When there are no side effects, [the treatment] doesn’t work. So you have to warn patients there are always side effects of the treatment they will be given.”
In addition to the importance of the multidisciplinary team in the management of head and neck cancer, understanding the biology of the disease and using systemic treatment are important for treatment, he said. Recent advances in this area include the recognition of the human papillomavirus as a risk factor for and strong predictor of survival in oropharyngeal cancer, and the role of epidermal growth factor receptor to enable targeting with anti-EGFR drugs, such as cetuximab (Erbitux). Systemic treatment for locally advanced disease includes concurrent chemoradiotherapy (CCRT), bioradiotherapy (BRT) with cetuximab and sequential chemotherapy (induction chemotherapy followed by CCRT or BRT).
In most cases of locally advanced SCCHN, the recommended chemotherapy of choice is high-dose cisplatin, given every 3 weeks. Although alternatives to this have been proposed – such as lowering the dose of cisplatin or using carboplatin or cetuximab instead – they have been insufficiently studied and many questions remain unanswered at the moment.
As for the treatment of recurrent or metastatic SCCHN, if it is resectable, then this would be followed by radiotherapy or CCRT. In patients deemed fit enough to handle the regimen, a combination of a platinum agent, 5-fluorouracil (5-FU) and cetuximab) is a new standard first-line regimen, although the role of maintenance cetuximab is unclear.
Better chemotherapy partners for cetuximab or alternatives for anti-EGFR–targeting agents are under investigation. This includes using docetaxel (Taxotere) instead of 5-FU with cetuximab or using lapatinib (Tykerb), afatinib (Gilotrif) or dacomitinib to block multiple human epidermal growth factor receptors or a variety of monoclonal antibodies to try to overcome resistance to anti-EGFR drugs.
Reactivation of immune surveillance by blocking the PD-1 pathway with drugs such as nivolumab (Opdivo) and pembrolizumab (Keytruda) seems to be a promising approach for treating head and neck cancer and is under investigation in other tumors, including non–small cell lung cancer, triple-negative breast cancer, and melanoma, Dr. Vermorken said.Dr. Lefebvre has acted as a consultant to Merck Serono and Sanofi. Dr. Vermorken has participated in advisory boards of AstraZeneca; Boehringer Ingelheim; Debiopharm; Genentech; Merck Serono; Merck, Sharp & Dohme; Oncolytics Biotech; Pierre Fabre; and Vaccinogen; and received lecturer fees from Merck Serono.
PARIS – Successful head and neck cancer management can be achieved only if a multidisciplinary approach is taken, experts emphasized at a recent international conference on anticancer treatment.
Because of its very location and complex anatomy, squamous cell cancer of the head and neck (SCCHN) is a difficult tumor to treat, Dr. Jean-Pierre Lefebvre of Centre Oscar Lambret in Lille, France, explained. Two-thirds of tumors are diagnosed at a late stage and often require a combination of therapeutic approaches and thus “combined toxicities.” Patients also frequently have comorbid illnesses that can affect their compliance and tolerance to treatments.
“There is only one solution: a multidisciplinary approach at any time of the management,” Dr. Lefebvre said.
The multidisciplinary approach requires a tight-knit team of imaging specialists; biologists and pathologists; anesthesiologists and surgeons; medical and radiation oncologists; nurses, general practitioners, and other support professions, such as dentists, dietitians, psychologists, speech and physical therapy specialists; and of course, the patients themselves.
Dr. Lefebvre noted that it was vital to provide patients with good information about their disease and its treatment, from the time of diagnosis to explain the various management decisions made by the multidisciplinary team and likely outcomes of the recommended interventions.
The primary goals of treatment are to control disease above the clavicles and to ensure survival, Dr. Lefebvre observed. Other treatment goals include preserving organ function, controlling symptoms, and creating a minimal impact on a patient’s quality of life by providing treatments that offer minimal long-term toxicity, good tolerability, and perhaps most important, good patient satisfaction.
Selecting treatment can be challenging and cannot be done without a multidisciplinary decision. The two main pathways are a surgery-based or radiotherapy-based treatment, but within each there are multiple options and combinations that need careful consideration on a case-by-case basis.
“It’s not a cookbook decision,” agreed Dr. Jan B. Vermorken, emeritus professor of oncology at Antwerp University Hospital, Belgium, who discussed the systemic treatment of head and neck cancer in a separate lecture. He agreed that head and neck cancer treatment is a multidisciplinary challenge that needs to balance the efficacy and tolerability of treatment on an individual basis, and always while considering the patient’s preferences.
“Patients can be very well informed,” Dr. Vermorken noted and suggested that clinicians need to be prepared to help patients understand the information that they find themselves in order to be able to counter any misinformation they might have found.
“There is no treatment without side effects,” Dr. Vermorken stressed. “When there are no side effects, [the treatment] doesn’t work. So you have to warn patients there are always side effects of the treatment they will be given.”
In addition to the importance of the multidisciplinary team in the management of head and neck cancer, understanding the biology of the disease and using systemic treatment are important for treatment, he said. Recent advances in this area include the recognition of the human papillomavirus as a risk factor for and strong predictor of survival in oropharyngeal cancer, and the role of epidermal growth factor receptor to enable targeting with anti-EGFR drugs, such as cetuximab (Erbitux). Systemic treatment for locally advanced disease includes concurrent chemoradiotherapy (CCRT), bioradiotherapy (BRT) with cetuximab and sequential chemotherapy (induction chemotherapy followed by CCRT or BRT).
In most cases of locally advanced SCCHN, the recommended chemotherapy of choice is high-dose cisplatin, given every 3 weeks. Although alternatives to this have been proposed – such as lowering the dose of cisplatin or using carboplatin or cetuximab instead – they have been insufficiently studied and many questions remain unanswered at the moment.
As for the treatment of recurrent or metastatic SCCHN, if it is resectable, then this would be followed by radiotherapy or CCRT. In patients deemed fit enough to handle the regimen, a combination of a platinum agent, 5-fluorouracil (5-FU) and cetuximab) is a new standard first-line regimen, although the role of maintenance cetuximab is unclear.
Better chemotherapy partners for cetuximab or alternatives for anti-EGFR–targeting agents are under investigation. This includes using docetaxel (Taxotere) instead of 5-FU with cetuximab or using lapatinib (Tykerb), afatinib (Gilotrif) or dacomitinib to block multiple human epidermal growth factor receptors or a variety of monoclonal antibodies to try to overcome resistance to anti-EGFR drugs.
Reactivation of immune surveillance by blocking the PD-1 pathway with drugs such as nivolumab (Opdivo) and pembrolizumab (Keytruda) seems to be a promising approach for treating head and neck cancer and is under investigation in other tumors, including non–small cell lung cancer, triple-negative breast cancer, and melanoma, Dr. Vermorken said.Dr. Lefebvre has acted as a consultant to Merck Serono and Sanofi. Dr. Vermorken has participated in advisory boards of AstraZeneca; Boehringer Ingelheim; Debiopharm; Genentech; Merck Serono; Merck, Sharp & Dohme; Oncolytics Biotech; Pierre Fabre; and Vaccinogen; and received lecturer fees from Merck Serono.
PARIS – Successful head and neck cancer management can be achieved only if a multidisciplinary approach is taken, experts emphasized at a recent international conference on anticancer treatment.
Because of its very location and complex anatomy, squamous cell cancer of the head and neck (SCCHN) is a difficult tumor to treat, Dr. Jean-Pierre Lefebvre of Centre Oscar Lambret in Lille, France, explained. Two-thirds of tumors are diagnosed at a late stage and often require a combination of therapeutic approaches and thus “combined toxicities.” Patients also frequently have comorbid illnesses that can affect their compliance and tolerance to treatments.
“There is only one solution: a multidisciplinary approach at any time of the management,” Dr. Lefebvre said.
The multidisciplinary approach requires a tight-knit team of imaging specialists; biologists and pathologists; anesthesiologists and surgeons; medical and radiation oncologists; nurses, general practitioners, and other support professions, such as dentists, dietitians, psychologists, speech and physical therapy specialists; and of course, the patients themselves.
Dr. Lefebvre noted that it was vital to provide patients with good information about their disease and its treatment, from the time of diagnosis to explain the various management decisions made by the multidisciplinary team and likely outcomes of the recommended interventions.
The primary goals of treatment are to control disease above the clavicles and to ensure survival, Dr. Lefebvre observed. Other treatment goals include preserving organ function, controlling symptoms, and creating a minimal impact on a patient’s quality of life by providing treatments that offer minimal long-term toxicity, good tolerability, and perhaps most important, good patient satisfaction.
Selecting treatment can be challenging and cannot be done without a multidisciplinary decision. The two main pathways are a surgery-based or radiotherapy-based treatment, but within each there are multiple options and combinations that need careful consideration on a case-by-case basis.
“It’s not a cookbook decision,” agreed Dr. Jan B. Vermorken, emeritus professor of oncology at Antwerp University Hospital, Belgium, who discussed the systemic treatment of head and neck cancer in a separate lecture. He agreed that head and neck cancer treatment is a multidisciplinary challenge that needs to balance the efficacy and tolerability of treatment on an individual basis, and always while considering the patient’s preferences.
“Patients can be very well informed,” Dr. Vermorken noted and suggested that clinicians need to be prepared to help patients understand the information that they find themselves in order to be able to counter any misinformation they might have found.
“There is no treatment without side effects,” Dr. Vermorken stressed. “When there are no side effects, [the treatment] doesn’t work. So you have to warn patients there are always side effects of the treatment they will be given.”
In addition to the importance of the multidisciplinary team in the management of head and neck cancer, understanding the biology of the disease and using systemic treatment are important for treatment, he said. Recent advances in this area include the recognition of the human papillomavirus as a risk factor for and strong predictor of survival in oropharyngeal cancer, and the role of epidermal growth factor receptor to enable targeting with anti-EGFR drugs, such as cetuximab (Erbitux). Systemic treatment for locally advanced disease includes concurrent chemoradiotherapy (CCRT), bioradiotherapy (BRT) with cetuximab and sequential chemotherapy (induction chemotherapy followed by CCRT or BRT).
In most cases of locally advanced SCCHN, the recommended chemotherapy of choice is high-dose cisplatin, given every 3 weeks. Although alternatives to this have been proposed – such as lowering the dose of cisplatin or using carboplatin or cetuximab instead – they have been insufficiently studied and many questions remain unanswered at the moment.
As for the treatment of recurrent or metastatic SCCHN, if it is resectable, then this would be followed by radiotherapy or CCRT. In patients deemed fit enough to handle the regimen, a combination of a platinum agent, 5-fluorouracil (5-FU) and cetuximab) is a new standard first-line regimen, although the role of maintenance cetuximab is unclear.
Better chemotherapy partners for cetuximab or alternatives for anti-EGFR–targeting agents are under investigation. This includes using docetaxel (Taxotere) instead of 5-FU with cetuximab or using lapatinib (Tykerb), afatinib (Gilotrif) or dacomitinib to block multiple human epidermal growth factor receptors or a variety of monoclonal antibodies to try to overcome resistance to anti-EGFR drugs.
Reactivation of immune surveillance by blocking the PD-1 pathway with drugs such as nivolumab (Opdivo) and pembrolizumab (Keytruda) seems to be a promising approach for treating head and neck cancer and is under investigation in other tumors, including non–small cell lung cancer, triple-negative breast cancer, and melanoma, Dr. Vermorken said.Dr. Lefebvre has acted as a consultant to Merck Serono and Sanofi. Dr. Vermorken has participated in advisory boards of AstraZeneca; Boehringer Ingelheim; Debiopharm; Genentech; Merck Serono; Merck, Sharp & Dohme; Oncolytics Biotech; Pierre Fabre; and Vaccinogen; and received lecturer fees from Merck Serono.
EXPERT ANALYSIS FROM ICACT 2015
Cancer mortality lowest in western United States
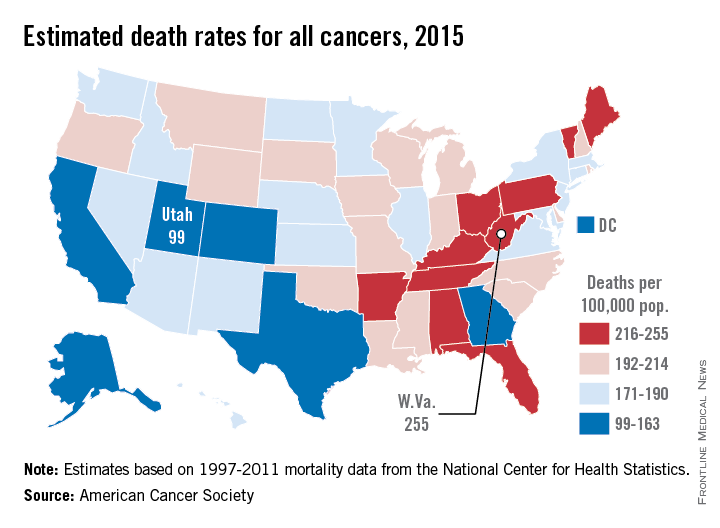
Utah will have the lowest cancer mortality rate in the United States in 2015, according to a report from the American Cancer Society.
While 11 states are predicted to have fewer cancer-related deaths in 2015 than 2014, the mortality rate will be lowest in Utah, with just 99 deaths per 100,000 people. Alaska and Colorado will have the next lowest mortality rates at 141 and 142 per 100,000 people, respectively. West Virginia is estimated to have the highest cancer mortality rate at 255 per 100,000 people, followed by Kentucky and Arkansas at 231 and 228, respectively. The national cancer mortality rate will be 185 per 100,000.
There will be about 1.66 million new cases of cancer in 2015 and about 590,000 deaths. Female breast cancer will probably be the most common with 231,000 new cases, but with an estimated 221,000 new cases each, lung/bronchus and prostate cancer also will rank high. Lung and bronchus cancer will be the most common cause of death, with 158,000 deaths predicted in 2015, more than a quarter of overall cancer deaths, according to the ACS.
Socioeconomic status makes a big difference in both cancer incidence and mortality. Cancer can stem from a higher likelihood to engage in risky behavior such as smoking, an unhealthy diet, or a sedentary lifestyle. Demographics matter more than ethnicity for cancer mortality, as “cancer mortality rates among both black and non-Hispanic white men with 12 or fewer years of education are almost 3 times higher than those of college graduates for all cancers combined and 4-5 times higher for lung cancer,” the ACS said.
Estimated data were based on 1995-2011 cancer incidence rates collected by the National Center for Health Statistics and the ACS.
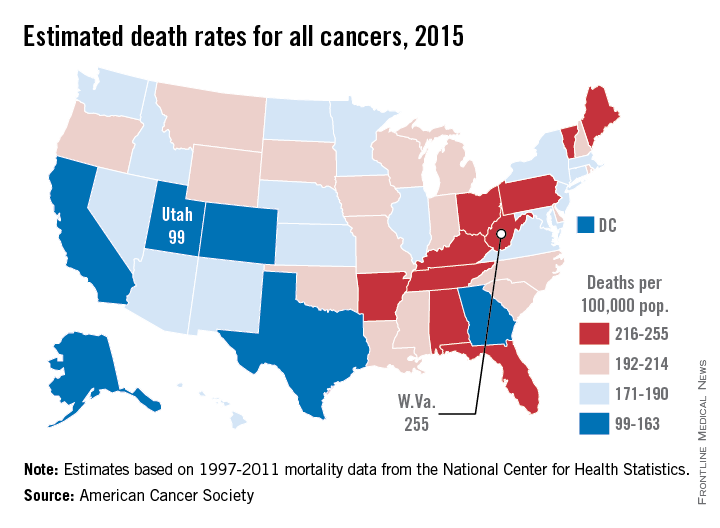
Utah will have the lowest cancer mortality rate in the United States in 2015, according to a report from the American Cancer Society.
While 11 states are predicted to have fewer cancer-related deaths in 2015 than 2014, the mortality rate will be lowest in Utah, with just 99 deaths per 100,000 people. Alaska and Colorado will have the next lowest mortality rates at 141 and 142 per 100,000 people, respectively. West Virginia is estimated to have the highest cancer mortality rate at 255 per 100,000 people, followed by Kentucky and Arkansas at 231 and 228, respectively. The national cancer mortality rate will be 185 per 100,000.

There will be about 1.66 million new cases of cancer in 2015 and about 590,000 deaths. Female breast cancer will probably be the most common with 231,000 new cases, but with an estimated 221,000 new cases each, lung/bronchus and prostate cancer also will rank high. Lung and bronchus cancer will be the most common cause of death, with 158,000 deaths predicted in 2015, more than a quarter of overall cancer deaths, according to the ACS.
Socioeconomic status makes a big difference in both cancer incidence and mortality. Cancer can stem from a higher likelihood to engage in risky behavior such as smoking, an unhealthy diet, or a sedentary lifestyle. Demographics matter more than ethnicity for cancer mortality, as “cancer mortality rates among both black and non-Hispanic white men with 12 or fewer years of education are almost 3 times higher than those of college graduates for all cancers combined and 4-5 times higher for lung cancer,” the ACS said.
Estimated data were based on 1995-2011 cancer incidence rates collected by the National Center for Health Statistics and the ACS.
Utah will have the lowest cancer mortality rate in the United States in 2015, according to a report from the American Cancer Society.
While 11 states are predicted to have fewer cancer-related deaths in 2015 than 2014, the mortality rate will be lowest in Utah, with just 99 deaths per 100,000 people. Alaska and Colorado will have the next lowest mortality rates at 141 and 142 per 100,000 people, respectively. West Virginia is estimated to have the highest cancer mortality rate at 255 per 100,000 people, followed by Kentucky and Arkansas at 231 and 228, respectively. The national cancer mortality rate will be 185 per 100,000.

There will be about 1.66 million new cases of cancer in 2015 and about 590,000 deaths. Female breast cancer will probably be the most common with 231,000 new cases, but with an estimated 221,000 new cases each, lung/bronchus and prostate cancer also will rank high. Lung and bronchus cancer will be the most common cause of death, with 158,000 deaths predicted in 2015, more than a quarter of overall cancer deaths, according to the ACS.
Socioeconomic status makes a big difference in both cancer incidence and mortality. Cancer can stem from a higher likelihood to engage in risky behavior such as smoking, an unhealthy diet, or a sedentary lifestyle. Demographics matter more than ethnicity for cancer mortality, as “cancer mortality rates among both black and non-Hispanic white men with 12 or fewer years of education are almost 3 times higher than those of college graduates for all cancers combined and 4-5 times higher for lung cancer,” the ACS said.
Estimated data were based on 1995-2011 cancer incidence rates collected by the National Center for Health Statistics and the ACS.
Aggressive surgery doesn’t necessarily improve survival from advanced ovarian cancer
For women with advanced epithelial ovarian cancer, aggressive cytoreductive surgery improves survival only if complete resection of disease is achieved. Aggressive debulking that achieves anything less than complete resection (R0) – even if the residual tumor is minimal (< 1 cm) – will not improve survival, according to a report published online Feb. 9 in Journal of Clinical Oncology.
“Over the last decade, there has been a growing trend toward more aggressive primary debulking surgery for women with epithelial ovarian cancer,” even though the impact of this approach on survival “has been unclear.” To examine the issue, researchers analyzed data from the multicenter Gynecologic Oncology Group-182 cohort, which they described as the largest clinical trial of ovarian cancer to date. They assessed survival outcomes in 2,655 patients who underwent aggressive cytoreductive surgery before receiving chemotherapy. The resection was complete (R0), in 32.4% of the women; it left minimal residual tumor (< 1 cm) in the remaining 67.6%, said Dr. Neil S. Horowitz of Brigham and Women’s Hospital, Boston, and his associates.
Both overall survival and progression-free survival were significantly improved when R0 was achieved, but not when there was minimal residual tumor. In the literature, as many as 25% of women who undergo aggressive surgical cytoreduction experience significant postoperative morbidity, and up to 2% fail to survive the procedure. These findings therefore “suggest that complex surgical procedures should be selectively used in patients with significant disease distribution and limited to those where only microscopic residual can be achieved,” the investigators said (J. Clin. Oncol. 2015 Feb. 9 [doi: 10.1200/JCO.2014.56.3106]).
“We suggest a potential paradigm shift, in which, if R0 is difficult to attain at primary cytoreduction, use of neoadjuvant chemotherapy with interval debulking to allow for R0 may be superior to primary surgery after which the patient is left with gross residual disease,” Dr. Horowitz and his associates added.
For women with advanced epithelial ovarian cancer, aggressive cytoreductive surgery improves survival only if complete resection of disease is achieved. Aggressive debulking that achieves anything less than complete resection (R0) – even if the residual tumor is minimal (< 1 cm) – will not improve survival, according to a report published online Feb. 9 in Journal of Clinical Oncology.
“Over the last decade, there has been a growing trend toward more aggressive primary debulking surgery for women with epithelial ovarian cancer,” even though the impact of this approach on survival “has been unclear.” To examine the issue, researchers analyzed data from the multicenter Gynecologic Oncology Group-182 cohort, which they described as the largest clinical trial of ovarian cancer to date. They assessed survival outcomes in 2,655 patients who underwent aggressive cytoreductive surgery before receiving chemotherapy. The resection was complete (R0), in 32.4% of the women; it left minimal residual tumor (< 1 cm) in the remaining 67.6%, said Dr. Neil S. Horowitz of Brigham and Women’s Hospital, Boston, and his associates.
Both overall survival and progression-free survival were significantly improved when R0 was achieved, but not when there was minimal residual tumor. In the literature, as many as 25% of women who undergo aggressive surgical cytoreduction experience significant postoperative morbidity, and up to 2% fail to survive the procedure. These findings therefore “suggest that complex surgical procedures should be selectively used in patients with significant disease distribution and limited to those where only microscopic residual can be achieved,” the investigators said (J. Clin. Oncol. 2015 Feb. 9 [doi: 10.1200/JCO.2014.56.3106]).
“We suggest a potential paradigm shift, in which, if R0 is difficult to attain at primary cytoreduction, use of neoadjuvant chemotherapy with interval debulking to allow for R0 may be superior to primary surgery after which the patient is left with gross residual disease,” Dr. Horowitz and his associates added.
For women with advanced epithelial ovarian cancer, aggressive cytoreductive surgery improves survival only if complete resection of disease is achieved. Aggressive debulking that achieves anything less than complete resection (R0) – even if the residual tumor is minimal (< 1 cm) – will not improve survival, according to a report published online Feb. 9 in Journal of Clinical Oncology.
“Over the last decade, there has been a growing trend toward more aggressive primary debulking surgery for women with epithelial ovarian cancer,” even though the impact of this approach on survival “has been unclear.” To examine the issue, researchers analyzed data from the multicenter Gynecologic Oncology Group-182 cohort, which they described as the largest clinical trial of ovarian cancer to date. They assessed survival outcomes in 2,655 patients who underwent aggressive cytoreductive surgery before receiving chemotherapy. The resection was complete (R0), in 32.4% of the women; it left minimal residual tumor (< 1 cm) in the remaining 67.6%, said Dr. Neil S. Horowitz of Brigham and Women’s Hospital, Boston, and his associates.
Both overall survival and progression-free survival were significantly improved when R0 was achieved, but not when there was minimal residual tumor. In the literature, as many as 25% of women who undergo aggressive surgical cytoreduction experience significant postoperative morbidity, and up to 2% fail to survive the procedure. These findings therefore “suggest that complex surgical procedures should be selectively used in patients with significant disease distribution and limited to those where only microscopic residual can be achieved,” the investigators said (J. Clin. Oncol. 2015 Feb. 9 [doi: 10.1200/JCO.2014.56.3106]).
“We suggest a potential paradigm shift, in which, if R0 is difficult to attain at primary cytoreduction, use of neoadjuvant chemotherapy with interval debulking to allow for R0 may be superior to primary surgery after which the patient is left with gross residual disease,” Dr. Horowitz and his associates added.
Key clinical point: Both overall survival and progression-free survival were significantly improved when complete resection was achieved, but not when there was minimal residual tumor.
Data source: A retrospective secondary analysis of survival data in a subgroup of 2,655 women enrolled in a cohort study of advanced epithelial ovarian cancer.
Disclosures: This study was supported by the National Cancer Institute. Dr. Horowitz and his associates reported having no financial disclosures.
GC score predicts best approach to post-RP RT
A validated genomic classifier score based on 22 prespecified biomarkers is prognostic for the development of clinical metastasis after radical prostatectomy, and could help inform decision making about the timing of subsequent radiotherapy, according to a review of 188 patients who were treated with post–radical prostatectomy radiotherapy.
The findings suggest that patients with a low genomic classifier (GC) score are best treated with salvage radiotherapy (SRT), and those with a high score are best treated with adjuvant radiotherapy (ART), reported Dr. Robert B. Den of Thomas Jefferson University, Philadelphia, and his colleagues. The study was published online Feb. 9 in the Journal of Clinical Oncology.
The 5-year cumulative incidence of metastasis in the study subjects, who were identified from the GenomeDx prostate cancer genomic database, was 0%, 9%, and 29% in those with low (less than 0.4), average (0.4-0.6), and high (greater than 0.6) GC scores, respectively. On multivariable analysis, pre–radical prostatectomy prostate-specific antigen levels and GC were independent predictors of metastasis (hazard ratio, 2.12; hazard ratio, 1.90 for every 10% increase in GC score, respectively). No differences were seen in the cumulative incidence of metastasis when patients with GC scores less than 0.4 were compared based on whether they received ART or SRT, but among those with GC scores of 0.4 or higher, the cumulative incidence of metastasis at 5 years was 6% in those who received ART, and 23% in those who received SRT (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.59.0026]).
Use of the GC scoring model either alone or in combination with the Cancer of the Prostate Risk Assessment Postsurgical (CAPRA-S) scoring model was superior to other clinicopathological models for predicting metastasis, and had “a higher net benefit than clinical models across a wide range of decision threshold probabilities,” they noted.
The patients were men with pT3 or margin-positive prostate cancer who received radiotherapy after radical prostatectomy (post-RP RT) at either Thomas Jefferson University, Philadelphia, or the Mayo Clinic, Rochester, Minn., between 1990 and 2009. They were treated at a median dose of 66.6 Gy with conventional fractionation by either three-dimensional conformal RT or by intensity-modulated RT techniques, and followed for a median of 10 years after radical prostatectomy and 8 years after radiotherapy.
The findings have important implications for the treatment of contemporary prostate cancer patients who harbor adverse pathologic characteristics at the time of radical prostatectomy; these patients are often treated with postoperative radiotherapy alone or with hormonal therapy, but the optimal timing of post-RP RT has been unclear, the investigators explained.
“Advocates for adjuvant RT argue that this treatment modality might maximize cancer control outcomes. However, salvage RT can minimize overtreatment while offering acceptable oncologic outcomes,” they wrote, adding that trials comparing the two are underway, but because of the rarity of data in the field and the unresolved controversy about the best approach to treatment, they “sought to integrate a novel biomarker test to improve clinical decision making regarding post-RP RT.
“We demonstrate that the GC is highly prognostic in the setting of postprostatectomy RT and that the GC may be a predictive marker that can help determine which patient will benefit from ART as opposed to SRT. This supports the importance of local therapy in the setting of presumed occult metastatic disease,” they said, noting that the findings are “particularly intriguing and provide a unique, more individualized approach in the management of postprostatectomy patients with adverse pathologic findings.”
While a biomarker shouldn’t replace shared patient-physician decision making, the use of the GC could provide insight into the aggressiveness of disease and aid in decision making regarding postprostatectomy therapy, they said.
Intensification of therapy in men with a high GC score who are receiving salvage radiotherapy is currently being examined in the Radiation Therapy Oncology Group 9601 randomized, phase III trial comparing SRT with SRT plus high-dose bicalutamide, the noted.
“Given that this cohort consists of high-risk patients by clinicopathologic nomograms and the utilization of a GC allowed for significant downstaging, this study has major ramifications in terms of both potential for overtreatment and substantial cost savings to the U.S. health care system. Thus, the GC is a valuable tool to aid in management of men with prostate cancer undergoing prostatectomy,” they concluded.
Dr. Den and several coauthors disclosed ties with GenomeDx Biosciences, Janssen, Medivation, CE Outcomes, Photocure, Dendreon, Astellas, Celgene, Varian, Merck KGaA, Vertex, Glenview Consulting, Bayer, NRG Oncology, and Myriad Genetics.
A validated genomic classifier score based on 22 prespecified biomarkers is prognostic for the development of clinical metastasis after radical prostatectomy, and could help inform decision making about the timing of subsequent radiotherapy, according to a review of 188 patients who were treated with post–radical prostatectomy radiotherapy.
The findings suggest that patients with a low genomic classifier (GC) score are best treated with salvage radiotherapy (SRT), and those with a high score are best treated with adjuvant radiotherapy (ART), reported Dr. Robert B. Den of Thomas Jefferson University, Philadelphia, and his colleagues. The study was published online Feb. 9 in the Journal of Clinical Oncology.
The 5-year cumulative incidence of metastasis in the study subjects, who were identified from the GenomeDx prostate cancer genomic database, was 0%, 9%, and 29% in those with low (less than 0.4), average (0.4-0.6), and high (greater than 0.6) GC scores, respectively. On multivariable analysis, pre–radical prostatectomy prostate-specific antigen levels and GC were independent predictors of metastasis (hazard ratio, 2.12; hazard ratio, 1.90 for every 10% increase in GC score, respectively). No differences were seen in the cumulative incidence of metastasis when patients with GC scores less than 0.4 were compared based on whether they received ART or SRT, but among those with GC scores of 0.4 or higher, the cumulative incidence of metastasis at 5 years was 6% in those who received ART, and 23% in those who received SRT (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.59.0026]).
Use of the GC scoring model either alone or in combination with the Cancer of the Prostate Risk Assessment Postsurgical (CAPRA-S) scoring model was superior to other clinicopathological models for predicting metastasis, and had “a higher net benefit than clinical models across a wide range of decision threshold probabilities,” they noted.
The patients were men with pT3 or margin-positive prostate cancer who received radiotherapy after radical prostatectomy (post-RP RT) at either Thomas Jefferson University, Philadelphia, or the Mayo Clinic, Rochester, Minn., between 1990 and 2009. They were treated at a median dose of 66.6 Gy with conventional fractionation by either three-dimensional conformal RT or by intensity-modulated RT techniques, and followed for a median of 10 years after radical prostatectomy and 8 years after radiotherapy.
The findings have important implications for the treatment of contemporary prostate cancer patients who harbor adverse pathologic characteristics at the time of radical prostatectomy; these patients are often treated with postoperative radiotherapy alone or with hormonal therapy, but the optimal timing of post-RP RT has been unclear, the investigators explained.
“Advocates for adjuvant RT argue that this treatment modality might maximize cancer control outcomes. However, salvage RT can minimize overtreatment while offering acceptable oncologic outcomes,” they wrote, adding that trials comparing the two are underway, but because of the rarity of data in the field and the unresolved controversy about the best approach to treatment, they “sought to integrate a novel biomarker test to improve clinical decision making regarding post-RP RT.
“We demonstrate that the GC is highly prognostic in the setting of postprostatectomy RT and that the GC may be a predictive marker that can help determine which patient will benefit from ART as opposed to SRT. This supports the importance of local therapy in the setting of presumed occult metastatic disease,” they said, noting that the findings are “particularly intriguing and provide a unique, more individualized approach in the management of postprostatectomy patients with adverse pathologic findings.”
While a biomarker shouldn’t replace shared patient-physician decision making, the use of the GC could provide insight into the aggressiveness of disease and aid in decision making regarding postprostatectomy therapy, they said.
Intensification of therapy in men with a high GC score who are receiving salvage radiotherapy is currently being examined in the Radiation Therapy Oncology Group 9601 randomized, phase III trial comparing SRT with SRT plus high-dose bicalutamide, the noted.
“Given that this cohort consists of high-risk patients by clinicopathologic nomograms and the utilization of a GC allowed for significant downstaging, this study has major ramifications in terms of both potential for overtreatment and substantial cost savings to the U.S. health care system. Thus, the GC is a valuable tool to aid in management of men with prostate cancer undergoing prostatectomy,” they concluded.
Dr. Den and several coauthors disclosed ties with GenomeDx Biosciences, Janssen, Medivation, CE Outcomes, Photocure, Dendreon, Astellas, Celgene, Varian, Merck KGaA, Vertex, Glenview Consulting, Bayer, NRG Oncology, and Myriad Genetics.
A validated genomic classifier score based on 22 prespecified biomarkers is prognostic for the development of clinical metastasis after radical prostatectomy, and could help inform decision making about the timing of subsequent radiotherapy, according to a review of 188 patients who were treated with post–radical prostatectomy radiotherapy.
The findings suggest that patients with a low genomic classifier (GC) score are best treated with salvage radiotherapy (SRT), and those with a high score are best treated with adjuvant radiotherapy (ART), reported Dr. Robert B. Den of Thomas Jefferson University, Philadelphia, and his colleagues. The study was published online Feb. 9 in the Journal of Clinical Oncology.
The 5-year cumulative incidence of metastasis in the study subjects, who were identified from the GenomeDx prostate cancer genomic database, was 0%, 9%, and 29% in those with low (less than 0.4), average (0.4-0.6), and high (greater than 0.6) GC scores, respectively. On multivariable analysis, pre–radical prostatectomy prostate-specific antigen levels and GC were independent predictors of metastasis (hazard ratio, 2.12; hazard ratio, 1.90 for every 10% increase in GC score, respectively). No differences were seen in the cumulative incidence of metastasis when patients with GC scores less than 0.4 were compared based on whether they received ART or SRT, but among those with GC scores of 0.4 or higher, the cumulative incidence of metastasis at 5 years was 6% in those who received ART, and 23% in those who received SRT (J. Clin. Oncol. 2015 Feb. 9 [doi:10.1200/JCO.2014.59.0026]).
Use of the GC scoring model either alone or in combination with the Cancer of the Prostate Risk Assessment Postsurgical (CAPRA-S) scoring model was superior to other clinicopathological models for predicting metastasis, and had “a higher net benefit than clinical models across a wide range of decision threshold probabilities,” they noted.
The patients were men with pT3 or margin-positive prostate cancer who received radiotherapy after radical prostatectomy (post-RP RT) at either Thomas Jefferson University, Philadelphia, or the Mayo Clinic, Rochester, Minn., between 1990 and 2009. They were treated at a median dose of 66.6 Gy with conventional fractionation by either three-dimensional conformal RT or by intensity-modulated RT techniques, and followed for a median of 10 years after radical prostatectomy and 8 years after radiotherapy.
The findings have important implications for the treatment of contemporary prostate cancer patients who harbor adverse pathologic characteristics at the time of radical prostatectomy; these patients are often treated with postoperative radiotherapy alone or with hormonal therapy, but the optimal timing of post-RP RT has been unclear, the investigators explained.
“Advocates for adjuvant RT argue that this treatment modality might maximize cancer control outcomes. However, salvage RT can minimize overtreatment while offering acceptable oncologic outcomes,” they wrote, adding that trials comparing the two are underway, but because of the rarity of data in the field and the unresolved controversy about the best approach to treatment, they “sought to integrate a novel biomarker test to improve clinical decision making regarding post-RP RT.
“We demonstrate that the GC is highly prognostic in the setting of postprostatectomy RT and that the GC may be a predictive marker that can help determine which patient will benefit from ART as opposed to SRT. This supports the importance of local therapy in the setting of presumed occult metastatic disease,” they said, noting that the findings are “particularly intriguing and provide a unique, more individualized approach in the management of postprostatectomy patients with adverse pathologic findings.”
While a biomarker shouldn’t replace shared patient-physician decision making, the use of the GC could provide insight into the aggressiveness of disease and aid in decision making regarding postprostatectomy therapy, they said.
Intensification of therapy in men with a high GC score who are receiving salvage radiotherapy is currently being examined in the Radiation Therapy Oncology Group 9601 randomized, phase III trial comparing SRT with SRT plus high-dose bicalutamide, the noted.
“Given that this cohort consists of high-risk patients by clinicopathologic nomograms and the utilization of a GC allowed for significant downstaging, this study has major ramifications in terms of both potential for overtreatment and substantial cost savings to the U.S. health care system. Thus, the GC is a valuable tool to aid in management of men with prostate cancer undergoing prostatectomy,” they concluded.
Dr. Den and several coauthors disclosed ties with GenomeDx Biosciences, Janssen, Medivation, CE Outcomes, Photocure, Dendreon, Astellas, Celgene, Varian, Merck KGaA, Vertex, Glenview Consulting, Bayer, NRG Oncology, and Myriad Genetics.
Key clinical point: Use of a validated GC score can help determine if ART or SRT is best following radical prostatectomy.
Major finding: Among patients with GC scores of 0.4 or higher, the cumulative incidence of metastasis at 5 years was 6% in those who received ART, and 23% in those who received SRT.
Data source: A review of 188 cases in a genomic database.
Disclosures: Dr. Den and several coauthors disclosed ties with GenomeDx Biosciences, Janssen, Medivation, CE Outcomes, Photocure, Dendreon, Astellas, Celgene, Varian, Merck KGaA, Vertex, Glenview Consulting, Bayer, NRG Oncology, and Myriad Genetics.
Jury still out on survival benefit of resecting primary in mCRC
SAN FRANCISCO – Resecting the primary tumor in patients with metastatic colon or colorectal cancer may prolong survival. But then again, it may not.
This was the overarching take-home message from a trio of cohort studies presented at the Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology. Results were reported in a poster session.
“Whereas surgery is the primary treatment of localized colorectal cancer, resection of the primary tumor in patients with incurable metastatic disease is usually recommended for palliative purposes to manage obstruction, perforation, or bleeding,” Dr. Shahid Ahmed, lead investigator of one of the studies, noted in comments provided by e-mail. “The role of surgical resection of the primary tumor in patients with newly diagnosed incurable stage IV colorectal cancer remains controversial.”
In earlier research, he and colleagues found a survival benefit of primary resection among Canadian patients whose cancer was diagnosed between 1992 and 2005 (Cancer 2014;120:683-91). But the majority did not receive systemic therapy, and those who did were often given older regimens.
In a new study aimed at testing the association in the contemporary treatment era, the researchers analyzed data from 569 patients with stage IV colorectal cancer diagnosed between 2006 and 2010 who had a median follow-up of 11 months. Overall, 55% had resection of the primary tumor.
Among the 57% of patients who received systemic therapy, 91% received FOLFIRI or FOLFOX, 65% received bevacizumab (Avastin), and 10% received cetuximab (Erbitux) or panitumumab (Vectibix), according to Dr. Ahmed, professor of medicine, University of Saskatchewan, Canada.
Results for the entire cohort showed that median overall survival was 18 months in patients who had resection of their primary versus 4 months in those who did not (multivariate hazard ratio, 0.44; P less than .001).
Among the subgroup of patients who received chemotherapy, median survival was 27 months with primary resection versus 14 months without it (P less than .0001). And among the subgroup that specifically received FOLFIRI or FOLFOX and a biologic agent, it was 35 months with primary resection and 23 months without it (P less than .001).
“Surgical resection of primary tumor improves survival of patients with stage IV colorectal cancer, independent of other prognostic variables including age, performance status, comorbid illness, and chemotherapy,” maintained Dr. Ahmed. “The current study validates our findings and supports surgical resection of primary tumor in patients with stage IV colorectal cancer who are treated with modern chemotherapy and biologics.
“A well-designed prospective randomized trial is warranted to confirm the survival benefit conferred by the primary tumor resection,” he added, noting that two such trials in Europe – SYNCHRONOUS and CAIRO4 – are underway.
“If the magnitude of survival benefit is confirmed in these future randomized studies, surgical resection of the primary tumor could potentially be a more cost-effective intervention compared with novel systemic therapy in the management of metastatic colorectal cancer,” he concluded.
In a second study, Dr. Aaron Lewis, a surgical oncology fellow at the City of Hope, Duarte, Calif., and colleagues analyzed data from patients with stage IV colon cancer in the Surveillance, Epidemiology, and End Results (SEER) database for the years 1998 through 2011. They excluded those who died within 30 days of diagnosis or had resection of metastases. Overall, 70% of the 28,068 included patients had resection of their primary.
In multivariate analyses, patients who underwent resection had half the risk of death when compared with peers who did not have this surgery (hazard ratio, 0.49), reported Dr. Lewis.
Findings were essentially the same when the analysis was repeated in a subset of matched patients: Median survival was 17 months with resection versus 9 months without it (hazard ratio, 0.48; P less than .0001). Estimated 3-year survival was 23% and 6%, respectively.
“There are limitations, factors that we couldn’t completely control for. For example, there is no chemotherapy data in the SEER database. We didn’t know the timing of surgery in relation to chemotherapy. And we didn’t know whether these patients were asymptomatic or symptomatic,” Dr. Lewis noted in an interview. “But analysis of this huge group of patients in the United States that are getting treated shows that there is a survival benefit.”
Possible reasons why surgery might prolong life in this setting are unknown but may include the effects of tumor debulking or some enhancement of the immune response, he proposed.
To definitively confirm a survival benefit, a randomized controlled trial is needed, he agreed. “This seems to be a popular question in the literature in the last couple of years, so maybe somebody will be willing to take it on.”
In a third study, a team led by Dr. Zeinab Alawadi, a surgeon and postdoctoral fellow at the University of Texas MD Anderson Cancer Center, Houston, analyzed data from 14,399 patients in the National Cancer Data Base. They had been diagnosed with stage IV colon cancer between 2003 and 2005. The researchers excluded patients who had nonelective resection or surgery at other sites, such as metastasectomy.
The primary tumor was resected in 55% of all patients studied and in 74% of patients included in a 1-year landmark analysis done to account for early deaths related to comorbidity or disease burden, reported Dr. Alawadi.
In the entire cohort, primary resection conferred a significant survival benefit after standard multivariate adjustment (hazard ratio, 0.39) that persisted after propensity score weighting to account for treatment selection bias (hazard ratio, 0.41). The benefit was also significant, but much attenuated, in an instrumental variable analysis, another method for accounting for treatment selection bias (relative mortality rate, 0.88).
In the 1-year landmark population, primary resection conferred a smaller significant survival benefit after standard multivariate adjustment (hazard ratio, 0.60) that persisted after propensity score weighting (hazard ratio, 0.59). But there was no longer a significant benefit in the instrumental variable analysis here.
“Among the entire cohort of patients with stage 4 colon cancer, primary tumor resection offered no survival benefit over systemic chemotherapy alone when the [instrumental variable] method was applied at the 1 year landmark,” the investigators write.
“Subject to selection and survivor treatment bias, standard regression analysis may overestimate the benefit of [primary tumor resection],” they concluded.
SAN FRANCISCO – Resecting the primary tumor in patients with metastatic colon or colorectal cancer may prolong survival. But then again, it may not.
This was the overarching take-home message from a trio of cohort studies presented at the Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology. Results were reported in a poster session.
“Whereas surgery is the primary treatment of localized colorectal cancer, resection of the primary tumor in patients with incurable metastatic disease is usually recommended for palliative purposes to manage obstruction, perforation, or bleeding,” Dr. Shahid Ahmed, lead investigator of one of the studies, noted in comments provided by e-mail. “The role of surgical resection of the primary tumor in patients with newly diagnosed incurable stage IV colorectal cancer remains controversial.”
In earlier research, he and colleagues found a survival benefit of primary resection among Canadian patients whose cancer was diagnosed between 1992 and 2005 (Cancer 2014;120:683-91). But the majority did not receive systemic therapy, and those who did were often given older regimens.
In a new study aimed at testing the association in the contemporary treatment era, the researchers analyzed data from 569 patients with stage IV colorectal cancer diagnosed between 2006 and 2010 who had a median follow-up of 11 months. Overall, 55% had resection of the primary tumor.
Among the 57% of patients who received systemic therapy, 91% received FOLFIRI or FOLFOX, 65% received bevacizumab (Avastin), and 10% received cetuximab (Erbitux) or panitumumab (Vectibix), according to Dr. Ahmed, professor of medicine, University of Saskatchewan, Canada.
Results for the entire cohort showed that median overall survival was 18 months in patients who had resection of their primary versus 4 months in those who did not (multivariate hazard ratio, 0.44; P less than .001).
Among the subgroup of patients who received chemotherapy, median survival was 27 months with primary resection versus 14 months without it (P less than .0001). And among the subgroup that specifically received FOLFIRI or FOLFOX and a biologic agent, it was 35 months with primary resection and 23 months without it (P less than .001).
“Surgical resection of primary tumor improves survival of patients with stage IV colorectal cancer, independent of other prognostic variables including age, performance status, comorbid illness, and chemotherapy,” maintained Dr. Ahmed. “The current study validates our findings and supports surgical resection of primary tumor in patients with stage IV colorectal cancer who are treated with modern chemotherapy and biologics.
“A well-designed prospective randomized trial is warranted to confirm the survival benefit conferred by the primary tumor resection,” he added, noting that two such trials in Europe – SYNCHRONOUS and CAIRO4 – are underway.
“If the magnitude of survival benefit is confirmed in these future randomized studies, surgical resection of the primary tumor could potentially be a more cost-effective intervention compared with novel systemic therapy in the management of metastatic colorectal cancer,” he concluded.
In a second study, Dr. Aaron Lewis, a surgical oncology fellow at the City of Hope, Duarte, Calif., and colleagues analyzed data from patients with stage IV colon cancer in the Surveillance, Epidemiology, and End Results (SEER) database for the years 1998 through 2011. They excluded those who died within 30 days of diagnosis or had resection of metastases. Overall, 70% of the 28,068 included patients had resection of their primary.
In multivariate analyses, patients who underwent resection had half the risk of death when compared with peers who did not have this surgery (hazard ratio, 0.49), reported Dr. Lewis.
Findings were essentially the same when the analysis was repeated in a subset of matched patients: Median survival was 17 months with resection versus 9 months without it (hazard ratio, 0.48; P less than .0001). Estimated 3-year survival was 23% and 6%, respectively.
“There are limitations, factors that we couldn’t completely control for. For example, there is no chemotherapy data in the SEER database. We didn’t know the timing of surgery in relation to chemotherapy. And we didn’t know whether these patients were asymptomatic or symptomatic,” Dr. Lewis noted in an interview. “But analysis of this huge group of patients in the United States that are getting treated shows that there is a survival benefit.”
Possible reasons why surgery might prolong life in this setting are unknown but may include the effects of tumor debulking or some enhancement of the immune response, he proposed.
To definitively confirm a survival benefit, a randomized controlled trial is needed, he agreed. “This seems to be a popular question in the literature in the last couple of years, so maybe somebody will be willing to take it on.”
In a third study, a team led by Dr. Zeinab Alawadi, a surgeon and postdoctoral fellow at the University of Texas MD Anderson Cancer Center, Houston, analyzed data from 14,399 patients in the National Cancer Data Base. They had been diagnosed with stage IV colon cancer between 2003 and 2005. The researchers excluded patients who had nonelective resection or surgery at other sites, such as metastasectomy.
The primary tumor was resected in 55% of all patients studied and in 74% of patients included in a 1-year landmark analysis done to account for early deaths related to comorbidity or disease burden, reported Dr. Alawadi.
In the entire cohort, primary resection conferred a significant survival benefit after standard multivariate adjustment (hazard ratio, 0.39) that persisted after propensity score weighting to account for treatment selection bias (hazard ratio, 0.41). The benefit was also significant, but much attenuated, in an instrumental variable analysis, another method for accounting for treatment selection bias (relative mortality rate, 0.88).
In the 1-year landmark population, primary resection conferred a smaller significant survival benefit after standard multivariate adjustment (hazard ratio, 0.60) that persisted after propensity score weighting (hazard ratio, 0.59). But there was no longer a significant benefit in the instrumental variable analysis here.
“Among the entire cohort of patients with stage 4 colon cancer, primary tumor resection offered no survival benefit over systemic chemotherapy alone when the [instrumental variable] method was applied at the 1 year landmark,” the investigators write.
“Subject to selection and survivor treatment bias, standard regression analysis may overestimate the benefit of [primary tumor resection],” they concluded.
SAN FRANCISCO – Resecting the primary tumor in patients with metastatic colon or colorectal cancer may prolong survival. But then again, it may not.
This was the overarching take-home message from a trio of cohort studies presented at the Gastrointestinal Cancers Symposium cosponsored by the AGA Institute, the American Society of Clinical Oncology, ASTRO, and the Society of Surgical Oncology. Results were reported in a poster session.
“Whereas surgery is the primary treatment of localized colorectal cancer, resection of the primary tumor in patients with incurable metastatic disease is usually recommended for palliative purposes to manage obstruction, perforation, or bleeding,” Dr. Shahid Ahmed, lead investigator of one of the studies, noted in comments provided by e-mail. “The role of surgical resection of the primary tumor in patients with newly diagnosed incurable stage IV colorectal cancer remains controversial.”
In earlier research, he and colleagues found a survival benefit of primary resection among Canadian patients whose cancer was diagnosed between 1992 and 2005 (Cancer 2014;120:683-91). But the majority did not receive systemic therapy, and those who did were often given older regimens.
In a new study aimed at testing the association in the contemporary treatment era, the researchers analyzed data from 569 patients with stage IV colorectal cancer diagnosed between 2006 and 2010 who had a median follow-up of 11 months. Overall, 55% had resection of the primary tumor.
Among the 57% of patients who received systemic therapy, 91% received FOLFIRI or FOLFOX, 65% received bevacizumab (Avastin), and 10% received cetuximab (Erbitux) or panitumumab (Vectibix), according to Dr. Ahmed, professor of medicine, University of Saskatchewan, Canada.
Results for the entire cohort showed that median overall survival was 18 months in patients who had resection of their primary versus 4 months in those who did not (multivariate hazard ratio, 0.44; P less than .001).
Among the subgroup of patients who received chemotherapy, median survival was 27 months with primary resection versus 14 months without it (P less than .0001). And among the subgroup that specifically received FOLFIRI or FOLFOX and a biologic agent, it was 35 months with primary resection and 23 months without it (P less than .001).
“Surgical resection of primary tumor improves survival of patients with stage IV colorectal cancer, independent of other prognostic variables including age, performance status, comorbid illness, and chemotherapy,” maintained Dr. Ahmed. “The current study validates our findings and supports surgical resection of primary tumor in patients with stage IV colorectal cancer who are treated with modern chemotherapy and biologics.
“A well-designed prospective randomized trial is warranted to confirm the survival benefit conferred by the primary tumor resection,” he added, noting that two such trials in Europe – SYNCHRONOUS and CAIRO4 – are underway.
“If the magnitude of survival benefit is confirmed in these future randomized studies, surgical resection of the primary tumor could potentially be a more cost-effective intervention compared with novel systemic therapy in the management of metastatic colorectal cancer,” he concluded.
In a second study, Dr. Aaron Lewis, a surgical oncology fellow at the City of Hope, Duarte, Calif., and colleagues analyzed data from patients with stage IV colon cancer in the Surveillance, Epidemiology, and End Results (SEER) database for the years 1998 through 2011. They excluded those who died within 30 days of diagnosis or had resection of metastases. Overall, 70% of the 28,068 included patients had resection of their primary.
In multivariate analyses, patients who underwent resection had half the risk of death when compared with peers who did not have this surgery (hazard ratio, 0.49), reported Dr. Lewis.
Findings were essentially the same when the analysis was repeated in a subset of matched patients: Median survival was 17 months with resection versus 9 months without it (hazard ratio, 0.48; P less than .0001). Estimated 3-year survival was 23% and 6%, respectively.
“There are limitations, factors that we couldn’t completely control for. For example, there is no chemotherapy data in the SEER database. We didn’t know the timing of surgery in relation to chemotherapy. And we didn’t know whether these patients were asymptomatic or symptomatic,” Dr. Lewis noted in an interview. “But analysis of this huge group of patients in the United States that are getting treated shows that there is a survival benefit.”
Possible reasons why surgery might prolong life in this setting are unknown but may include the effects of tumor debulking or some enhancement of the immune response, he proposed.
To definitively confirm a survival benefit, a randomized controlled trial is needed, he agreed. “This seems to be a popular question in the literature in the last couple of years, so maybe somebody will be willing to take it on.”
In a third study, a team led by Dr. Zeinab Alawadi, a surgeon and postdoctoral fellow at the University of Texas MD Anderson Cancer Center, Houston, analyzed data from 14,399 patients in the National Cancer Data Base. They had been diagnosed with stage IV colon cancer between 2003 and 2005. The researchers excluded patients who had nonelective resection or surgery at other sites, such as metastasectomy.
The primary tumor was resected in 55% of all patients studied and in 74% of patients included in a 1-year landmark analysis done to account for early deaths related to comorbidity or disease burden, reported Dr. Alawadi.
In the entire cohort, primary resection conferred a significant survival benefit after standard multivariate adjustment (hazard ratio, 0.39) that persisted after propensity score weighting to account for treatment selection bias (hazard ratio, 0.41). The benefit was also significant, but much attenuated, in an instrumental variable analysis, another method for accounting for treatment selection bias (relative mortality rate, 0.88).
In the 1-year landmark population, primary resection conferred a smaller significant survival benefit after standard multivariate adjustment (hazard ratio, 0.60) that persisted after propensity score weighting (hazard ratio, 0.59). But there was no longer a significant benefit in the instrumental variable analysis here.
“Among the entire cohort of patients with stage 4 colon cancer, primary tumor resection offered no survival benefit over systemic chemotherapy alone when the [instrumental variable] method was applied at the 1 year landmark,” the investigators write.
“Subject to selection and survivor treatment bias, standard regression analysis may overestimate the benefit of [primary tumor resection],” they concluded.
AT THE GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: Data are mixed regarding an overall survival benefit of resecting the primary tumor.
Major finding: Two studies found a halving of the risk of death, whereas one study found lesser or even no benefit.
Data source: A trio of cohort studies in 569 patients, 28,068 patients, and 14,399 patients with metastatic colon or colorectal cancer.
Disclosures: Dr. Ahmed, Dr. Lewis, and Dr. Alawadi disclosed that they had no conflicts of interest.