User login
Blacks balk at life-saving early lung cancer therapy
MADRID – Blacks may need additional guidance from clinicians to use radiotherapy for potentially curable lung cancer, a retrospective population-based study suggests.
Among 6,628 patients diagnosed with early-stage nonsquamous non–small cell lung cancer (NSCLC), primary radiation therapy doubled median survival from 11 months to 22.6 months for cases not receiving surgery (Log rank P value less than .0001).
Despite the survival advantage, blacks were significantly more likely than whites were to skip radiotherapy for stage IA NSCLC (46% vs. 37.5%; P = .02), Dr. Eric Flenaugh, chief of pulmonary and critical care medicine and vice chair of the department of medicine at Morehouse School of Medicine, Atlanta, reported at the world congress of the American College of Chest Physicians.
A subgroup analysis of nonsurgical stage IA cases in which surgery was not recommended or was contraindicated showed that 61% of whites went on to radiotherapy, compared with 47% of blacks (P = .007). When surgical resection was recommended but not performed, radiotherapy use was similar between races.
"What this basically says is that if they [blacks] chose not to have surgery, then they weren’t going to have anything," Dr. Flenaugh said in an interview. "We have to look at our approach to discussing with African Americans who have curable-stage cancer, particularly the IAs, that if you’re not a surgical candidate or choose not to have surgery, there are other options like radiotherapy that can improve your survival."
The data did not allow the investigators to determine patients’ chemotherapy status or which factors drove the lower uptake of radiotherapy, but prior research has shown that blacks undergo surgery for lung cancer less often than whites, even after access to care has been demonstrated (J. Clin. Oncol. 2006;24:413-8).
The current analysis, led by internal medicine resident Srinadh Annangi, MBBS, used data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) database for 6,628 patients diagnosed with NSCLC between 2004 and 2010, of which 4,210 did not receive surgery. NSCLC was staged as IA, IB, IIA, and IIB according to AJCC (American Joint Committee on Cancer) 6th edition classifications.
A little more than half of the 5,915 whites and 713 African-Americans were male, with a median age of 78 years and 67.5 years, respectively.
The proportion of tumors less than 2 cm in size for stages IA and IIA and less than 5 cm for stages IB and IIB was not significantly different between races, according to the poster presentation.
No significant racial disparities were seen for nonsurgical stage IB, IIA, and IIB cancers.
Among operable NSCLC cases, whites were significantly more likely to have surgery than were blacks (37% vs. 32%; P = .0004), whereas blacks were significantly more likely to have surgery recommended but refused or not performed (9% vs. 6%; P = .012).
Importantly, the proportion of blacks undergoing their recommended surgery was lower for both stage IA (78.3% vs. 86%; P less than .05) and IB cancers (74.6% vs. 81.3%; P less than .05).
The authors note that surgical resection remains the preferred treatment approach for operable stage I and II NSCLC, but conclude that eliminating the racial disparities in radiotherapy for early-stage NSCLC deemed inoperable or where surgery is refused can improve survival in the African American population.
Dr. Flenaugh and his coauthors reported no financial disclosures.
MADRID – Blacks may need additional guidance from clinicians to use radiotherapy for potentially curable lung cancer, a retrospective population-based study suggests.
Among 6,628 patients diagnosed with early-stage nonsquamous non–small cell lung cancer (NSCLC), primary radiation therapy doubled median survival from 11 months to 22.6 months for cases not receiving surgery (Log rank P value less than .0001).
Despite the survival advantage, blacks were significantly more likely than whites were to skip radiotherapy for stage IA NSCLC (46% vs. 37.5%; P = .02), Dr. Eric Flenaugh, chief of pulmonary and critical care medicine and vice chair of the department of medicine at Morehouse School of Medicine, Atlanta, reported at the world congress of the American College of Chest Physicians.
A subgroup analysis of nonsurgical stage IA cases in which surgery was not recommended or was contraindicated showed that 61% of whites went on to radiotherapy, compared with 47% of blacks (P = .007). When surgical resection was recommended but not performed, radiotherapy use was similar between races.
"What this basically says is that if they [blacks] chose not to have surgery, then they weren’t going to have anything," Dr. Flenaugh said in an interview. "We have to look at our approach to discussing with African Americans who have curable-stage cancer, particularly the IAs, that if you’re not a surgical candidate or choose not to have surgery, there are other options like radiotherapy that can improve your survival."
The data did not allow the investigators to determine patients’ chemotherapy status or which factors drove the lower uptake of radiotherapy, but prior research has shown that blacks undergo surgery for lung cancer less often than whites, even after access to care has been demonstrated (J. Clin. Oncol. 2006;24:413-8).
The current analysis, led by internal medicine resident Srinadh Annangi, MBBS, used data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) database for 6,628 patients diagnosed with NSCLC between 2004 and 2010, of which 4,210 did not receive surgery. NSCLC was staged as IA, IB, IIA, and IIB according to AJCC (American Joint Committee on Cancer) 6th edition classifications.
A little more than half of the 5,915 whites and 713 African-Americans were male, with a median age of 78 years and 67.5 years, respectively.
The proportion of tumors less than 2 cm in size for stages IA and IIA and less than 5 cm for stages IB and IIB was not significantly different between races, according to the poster presentation.
No significant racial disparities were seen for nonsurgical stage IB, IIA, and IIB cancers.
Among operable NSCLC cases, whites were significantly more likely to have surgery than were blacks (37% vs. 32%; P = .0004), whereas blacks were significantly more likely to have surgery recommended but refused or not performed (9% vs. 6%; P = .012).
Importantly, the proportion of blacks undergoing their recommended surgery was lower for both stage IA (78.3% vs. 86%; P less than .05) and IB cancers (74.6% vs. 81.3%; P less than .05).
The authors note that surgical resection remains the preferred treatment approach for operable stage I and II NSCLC, but conclude that eliminating the racial disparities in radiotherapy for early-stage NSCLC deemed inoperable or where surgery is refused can improve survival in the African American population.
Dr. Flenaugh and his coauthors reported no financial disclosures.
MADRID – Blacks may need additional guidance from clinicians to use radiotherapy for potentially curable lung cancer, a retrospective population-based study suggests.
Among 6,628 patients diagnosed with early-stage nonsquamous non–small cell lung cancer (NSCLC), primary radiation therapy doubled median survival from 11 months to 22.6 months for cases not receiving surgery (Log rank P value less than .0001).
Despite the survival advantage, blacks were significantly more likely than whites were to skip radiotherapy for stage IA NSCLC (46% vs. 37.5%; P = .02), Dr. Eric Flenaugh, chief of pulmonary and critical care medicine and vice chair of the department of medicine at Morehouse School of Medicine, Atlanta, reported at the world congress of the American College of Chest Physicians.
A subgroup analysis of nonsurgical stage IA cases in which surgery was not recommended or was contraindicated showed that 61% of whites went on to radiotherapy, compared with 47% of blacks (P = .007). When surgical resection was recommended but not performed, radiotherapy use was similar between races.
"What this basically says is that if they [blacks] chose not to have surgery, then they weren’t going to have anything," Dr. Flenaugh said in an interview. "We have to look at our approach to discussing with African Americans who have curable-stage cancer, particularly the IAs, that if you’re not a surgical candidate or choose not to have surgery, there are other options like radiotherapy that can improve your survival."
The data did not allow the investigators to determine patients’ chemotherapy status or which factors drove the lower uptake of radiotherapy, but prior research has shown that blacks undergo surgery for lung cancer less often than whites, even after access to care has been demonstrated (J. Clin. Oncol. 2006;24:413-8).
The current analysis, led by internal medicine resident Srinadh Annangi, MBBS, used data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) database for 6,628 patients diagnosed with NSCLC between 2004 and 2010, of which 4,210 did not receive surgery. NSCLC was staged as IA, IB, IIA, and IIB according to AJCC (American Joint Committee on Cancer) 6th edition classifications.
A little more than half of the 5,915 whites and 713 African-Americans were male, with a median age of 78 years and 67.5 years, respectively.
The proportion of tumors less than 2 cm in size for stages IA and IIA and less than 5 cm for stages IB and IIB was not significantly different between races, according to the poster presentation.
No significant racial disparities were seen for nonsurgical stage IB, IIA, and IIB cancers.
Among operable NSCLC cases, whites were significantly more likely to have surgery than were blacks (37% vs. 32%; P = .0004), whereas blacks were significantly more likely to have surgery recommended but refused or not performed (9% vs. 6%; P = .012).
Importantly, the proportion of blacks undergoing their recommended surgery was lower for both stage IA (78.3% vs. 86%; P less than .05) and IB cancers (74.6% vs. 81.3%; P less than .05).
The authors note that surgical resection remains the preferred treatment approach for operable stage I and II NSCLC, but conclude that eliminating the racial disparities in radiotherapy for early-stage NSCLC deemed inoperable or where surgery is refused can improve survival in the African American population.
Dr. Flenaugh and his coauthors reported no financial disclosures.
AT CHEST WORLD CONGRESS 2014
Major finding: Blacks were significantly less likely than were whites to receive radiotherapy for stage IA NSCLC (P = .02).
Data source: A population-based cohort study in 6,628 patients with lung cancer.
Disclosures: Dr. Flenaugh and his coauthors reported no financial disclosures.
Induction therapy does not complicate minimally invasive esophagectomy
PHOENIX, ARIZ. – Induction therapy does not appear to significantly increase the risk of postoperative complications in patients who undergo minimally invasive esophagectomy for esophageal adenocarcinoma, according to results of a study reported at the annual Society of Surgical Oncology Cancer Symposium.
"After balancing pretreatment variables that can potentially influence treatment decisions, we found that induction therapy does not significantly impact on perioperative outcomes compared with patients who are treated with minimally invasive esophagectomy as primary therapy," said Dr. Katie Sue Nason, of the department of cardiothoracic surgery at the University of Pittsburgh.
That conclusion comes from a propensity-matched analysis in which patients with similar pretreatment predictor variables were paired in an attempt to reduce comparison biases.
The investigators found no significant differences in mortality, major adverse events, readmissions, reoperations, or length of stay between 197 patients who received induction therapy and minimally invasive esophagectomy and 178 who had esophagectomy alone.
Although induction chemoradiation therapy may reduce the incidence of local and distant treatment failures in patients with esophageal cancer, it has the potential to increase the risk of postoperative adverse outcomes.
"What’s often not considered is that these factors that influence postoperative outcomes may also be influencing treatment allocation," Dr. Nason said.
In observational studies, for example, there may be larger differences in observed covariates between treatment groups that could lead to biased estimates of treatment effects.
"This could be adjusted for using propensity score matching, where you generate the conditional probability of one individual being treated with a particular treatment approach given multiple pretreatment covariates. By doing propensity score matching, you can then balance these covariates such as age and various comorbid illnesses between the two groups, and perhaps eliminate this treatment allocation bias that impacts on the relationship between the treatment and the postoperative outcomes," Dr. Nason explained.
She and her colleagues applied the technique to an analysis of outcomes from 375 patients with clinical stage II or greater esophageal adenocarcinoma treated with minimally invasive esophagectomy. They assessed tumor variables, comorbidities, treatments, and outcomes, and created propensity matching scores to match surgery-only patients one-on-one with no repeats to a patient who also underwent induction therapy and had a propensity score within 0.05 of that for the surgery-only patient.
Patients without suitable matches were excluded from the data set.
The extensive list of variables included age, smoking status, alcohol use, history of Barrett’s esophagus, myriad comorbidities, cancer location, pretreatment clinical stage, and many others.
Among the 375 patients, the investigators were able to generate propensity-matching scores for 82 pairs for the comparison of treatments and outcome.
They found that there were no significant differences between induction and surgery-only patients in the primary outcome of adverse events within 30 days of surgery, including in-hospital and 30-day mortality, major adverse events or at least 1 postoperative adverse event, readmission within 30 days, reoperation in-hospital or within 30 days, or length of stay greater than 10 days.
Dr. Nason noted that although unmatched patients differed in age, presentation with alarm symptoms, daily alcohol use, clinical stage and comorbid illnesses, the pretreatment variables were all well balanced in the propensity-matched analysis.
Mortality after minimally invasive esophagectomy was 1.8% among all 375 patients, 1.5% among patients who underwent induction, and 2.3% among patients who underwent surgery alone; these differences were not significant.
Major adverse events occurred in 28% of patients overall, 27% of those who received induction, and 30% of those who had surgery alone, also not significant.
The study was internally funded. Dr. Nason reported having no financial disclosures.
PHOENIX, ARIZ. – Induction therapy does not appear to significantly increase the risk of postoperative complications in patients who undergo minimally invasive esophagectomy for esophageal adenocarcinoma, according to results of a study reported at the annual Society of Surgical Oncology Cancer Symposium.
"After balancing pretreatment variables that can potentially influence treatment decisions, we found that induction therapy does not significantly impact on perioperative outcomes compared with patients who are treated with minimally invasive esophagectomy as primary therapy," said Dr. Katie Sue Nason, of the department of cardiothoracic surgery at the University of Pittsburgh.
That conclusion comes from a propensity-matched analysis in which patients with similar pretreatment predictor variables were paired in an attempt to reduce comparison biases.
The investigators found no significant differences in mortality, major adverse events, readmissions, reoperations, or length of stay between 197 patients who received induction therapy and minimally invasive esophagectomy and 178 who had esophagectomy alone.
Although induction chemoradiation therapy may reduce the incidence of local and distant treatment failures in patients with esophageal cancer, it has the potential to increase the risk of postoperative adverse outcomes.
"What’s often not considered is that these factors that influence postoperative outcomes may also be influencing treatment allocation," Dr. Nason said.
In observational studies, for example, there may be larger differences in observed covariates between treatment groups that could lead to biased estimates of treatment effects.
"This could be adjusted for using propensity score matching, where you generate the conditional probability of one individual being treated with a particular treatment approach given multiple pretreatment covariates. By doing propensity score matching, you can then balance these covariates such as age and various comorbid illnesses between the two groups, and perhaps eliminate this treatment allocation bias that impacts on the relationship between the treatment and the postoperative outcomes," Dr. Nason explained.
She and her colleagues applied the technique to an analysis of outcomes from 375 patients with clinical stage II or greater esophageal adenocarcinoma treated with minimally invasive esophagectomy. They assessed tumor variables, comorbidities, treatments, and outcomes, and created propensity matching scores to match surgery-only patients one-on-one with no repeats to a patient who also underwent induction therapy and had a propensity score within 0.05 of that for the surgery-only patient.
Patients without suitable matches were excluded from the data set.
The extensive list of variables included age, smoking status, alcohol use, history of Barrett’s esophagus, myriad comorbidities, cancer location, pretreatment clinical stage, and many others.
Among the 375 patients, the investigators were able to generate propensity-matching scores for 82 pairs for the comparison of treatments and outcome.
They found that there were no significant differences between induction and surgery-only patients in the primary outcome of adverse events within 30 days of surgery, including in-hospital and 30-day mortality, major adverse events or at least 1 postoperative adverse event, readmission within 30 days, reoperation in-hospital or within 30 days, or length of stay greater than 10 days.
Dr. Nason noted that although unmatched patients differed in age, presentation with alarm symptoms, daily alcohol use, clinical stage and comorbid illnesses, the pretreatment variables were all well balanced in the propensity-matched analysis.
Mortality after minimally invasive esophagectomy was 1.8% among all 375 patients, 1.5% among patients who underwent induction, and 2.3% among patients who underwent surgery alone; these differences were not significant.
Major adverse events occurred in 28% of patients overall, 27% of those who received induction, and 30% of those who had surgery alone, also not significant.
The study was internally funded. Dr. Nason reported having no financial disclosures.
PHOENIX, ARIZ. – Induction therapy does not appear to significantly increase the risk of postoperative complications in patients who undergo minimally invasive esophagectomy for esophageal adenocarcinoma, according to results of a study reported at the annual Society of Surgical Oncology Cancer Symposium.
"After balancing pretreatment variables that can potentially influence treatment decisions, we found that induction therapy does not significantly impact on perioperative outcomes compared with patients who are treated with minimally invasive esophagectomy as primary therapy," said Dr. Katie Sue Nason, of the department of cardiothoracic surgery at the University of Pittsburgh.
That conclusion comes from a propensity-matched analysis in which patients with similar pretreatment predictor variables were paired in an attempt to reduce comparison biases.
The investigators found no significant differences in mortality, major adverse events, readmissions, reoperations, or length of stay between 197 patients who received induction therapy and minimally invasive esophagectomy and 178 who had esophagectomy alone.
Although induction chemoradiation therapy may reduce the incidence of local and distant treatment failures in patients with esophageal cancer, it has the potential to increase the risk of postoperative adverse outcomes.
"What’s often not considered is that these factors that influence postoperative outcomes may also be influencing treatment allocation," Dr. Nason said.
In observational studies, for example, there may be larger differences in observed covariates between treatment groups that could lead to biased estimates of treatment effects.
"This could be adjusted for using propensity score matching, where you generate the conditional probability of one individual being treated with a particular treatment approach given multiple pretreatment covariates. By doing propensity score matching, you can then balance these covariates such as age and various comorbid illnesses between the two groups, and perhaps eliminate this treatment allocation bias that impacts on the relationship between the treatment and the postoperative outcomes," Dr. Nason explained.
She and her colleagues applied the technique to an analysis of outcomes from 375 patients with clinical stage II or greater esophageal adenocarcinoma treated with minimally invasive esophagectomy. They assessed tumor variables, comorbidities, treatments, and outcomes, and created propensity matching scores to match surgery-only patients one-on-one with no repeats to a patient who also underwent induction therapy and had a propensity score within 0.05 of that for the surgery-only patient.
Patients without suitable matches were excluded from the data set.
The extensive list of variables included age, smoking status, alcohol use, history of Barrett’s esophagus, myriad comorbidities, cancer location, pretreatment clinical stage, and many others.
Among the 375 patients, the investigators were able to generate propensity-matching scores for 82 pairs for the comparison of treatments and outcome.
They found that there were no significant differences between induction and surgery-only patients in the primary outcome of adverse events within 30 days of surgery, including in-hospital and 30-day mortality, major adverse events or at least 1 postoperative adverse event, readmission within 30 days, reoperation in-hospital or within 30 days, or length of stay greater than 10 days.
Dr. Nason noted that although unmatched patients differed in age, presentation with alarm symptoms, daily alcohol use, clinical stage and comorbid illnesses, the pretreatment variables were all well balanced in the propensity-matched analysis.
Mortality after minimally invasive esophagectomy was 1.8% among all 375 patients, 1.5% among patients who underwent induction, and 2.3% among patients who underwent surgery alone; these differences were not significant.
Major adverse events occurred in 28% of patients overall, 27% of those who received induction, and 30% of those who had surgery alone, also not significant.
The study was internally funded. Dr. Nason reported having no financial disclosures.
AT SSO 2014
Major finding: There were no significant differences in postoperative deaths or adverse outcomes among patients who underwent induction therapy and surgery for esophageal adenocarcinoma or surgery alone.
Data source: Comparison study of 375 patients with propensity-matched scoring.
Disclosures: The study was internally funded. Dr. Nason reported having no financial disclosures.
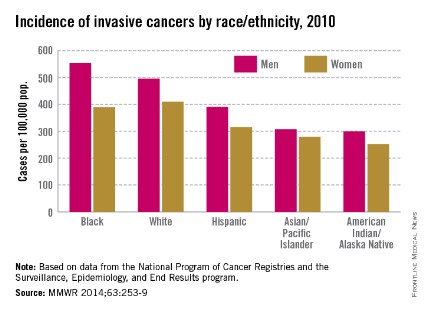
Invasive cancer incidence highest in black men
Men had a 24% higher incidence of invasive cancer than women in 2010, and black men had the highest rate among men of all races/ethnicities, the Centers for Disease Control and Prevention reported March 27.
Among women, however, the incidence of invasive cancers was highest among whites – 410 cases per 100,000 population – in 2010, the last year for which data are available. Black women had the second-highest rate: 389 per 100,000. In comparison, black men had an incidence of 553 per 100,000 and white men had a rate of 495 per 100,000, the CDC said (MMWR 2014:63;253-9).
The overall incidence rates were 503 per 100,000 for all men and 405 for all women. The total U.S. rate was 446 per 100,000 in 2010, compared with 459 in 2009, according to the CDC, which defined invasive cancers as "all cancers except in situ cancers (other than in the urinary bladder) and basal and squamous cell skin cancers."

Men had a 24% higher incidence of invasive cancer than women in 2010, and black men had the highest rate among men of all races/ethnicities, the Centers for Disease Control and Prevention reported March 27.
Among women, however, the incidence of invasive cancers was highest among whites – 410 cases per 100,000 population – in 2010, the last year for which data are available. Black women had the second-highest rate: 389 per 100,000. In comparison, black men had an incidence of 553 per 100,000 and white men had a rate of 495 per 100,000, the CDC said (MMWR 2014:63;253-9).
The overall incidence rates were 503 per 100,000 for all men and 405 for all women. The total U.S. rate was 446 per 100,000 in 2010, compared with 459 in 2009, according to the CDC, which defined invasive cancers as "all cancers except in situ cancers (other than in the urinary bladder) and basal and squamous cell skin cancers."

Men had a 24% higher incidence of invasive cancer than women in 2010, and black men had the highest rate among men of all races/ethnicities, the Centers for Disease Control and Prevention reported March 27.
Among women, however, the incidence of invasive cancers was highest among whites – 410 cases per 100,000 population – in 2010, the last year for which data are available. Black women had the second-highest rate: 389 per 100,000. In comparison, black men had an incidence of 553 per 100,000 and white men had a rate of 495 per 100,000, the CDC said (MMWR 2014:63;253-9).
The overall incidence rates were 503 per 100,000 for all men and 405 for all women. The total U.S. rate was 446 per 100,000 in 2010, compared with 459 in 2009, according to the CDC, which defined invasive cancers as "all cancers except in situ cancers (other than in the urinary bladder) and basal and squamous cell skin cancers."

FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Patient selection can keep the lid on esophagectomy costs
PHOENIX, ARIZ. – Cost and quality are not always synonymous, particularly when it comes to complex surgical procedures such as esophagectomy.
A review of records on more than 6,700 patients who underwent esophagectomy during a 4-year period showed that factors such as patient age, severity of illness, and hospital/surgeon volume can have a major effect on resource utilization and costs, said Dr. Daniel E. Abbott, assistant professor of surgery at the University of Cincinnati.
"There are certainly actionable risk factors for poor outcomes, such as mortality, and increased resource utilization, including dollar costs, the opportunity costs of increased length of stay, readmission, and rehabilitation and skilled nursing facilities," he reported at the annual Society of Surgical Oncology Cancer Symposium.
"I would argue that careful patient selection can have profound influences on cost-effectiveness. I think that as our health care is evolving and our outcomes are increasingly scrutinized, there will be increasing pressure to have better outcomes at lower costs," he added.
Dr. Abbott and his colleagues examined clinical variables in the cases of 6,737 esophagectomy patients treated from 2009 through 2012 in the University Healthsystems Consortium (UHC), an organization comprising 120 university hospitals and 299 affiliates.
They evaluated patient characteristics such as age and race, severity of illness index (1-4), esophagectomy type, and center and surgeon volume, and evaluated the effects of these variables on clinical outcomes that contribute to resource utilization, including deaths, readmissions, length of stay (LOS), and discharge disposition.
They found that the median LOS for all patients was 10 days (interquartile range, 8-17 days), but for patients over age 70, the median LOS was 11 days (P less than .01 vs. patients 70 and under).
Older patients did not have significantly higher readmission rates, but of the 4.2% of all patients who died in hospital, those over age 70 had more than twice the death rate of younger patients (7.0% vs. 3.2%, P less than .01).
Older patients were also significantly more likely to be discharged to a skilled nursing or rehabilitation facility than were younger patients (31.9% vs. 10.6%; P less than .01).
Total median cost per patient was $25,952, but again, older patients accounted for more of the expenses, at a median of $27,628 vs. $25,841 for those 70 and under (P less than .01).
In a multivariate analysis, patients over 70 had a more than twofold increase in risk of death (odds ratio, 2.12; P less than .01). Other factors significantly associated with greater risk for death were greater severity of illness (OR, 14.0; P less than .01) and black race vs. other races (OR, 1.88; P less than .01).
Factors associated with more frequent readmissions included greater severity of illness (OR, 1.33; P less than .01), and black race (OR, 1.34; P = .01), while patients of high-volume surgeons were less likely to need readmission (OR, 0.87; P = .04).
Lengths of stay were greater among patients over age 70, with every year over 70 translating into a 6% greater LOS (OR, 1.06; P =.03); older patients had a 16% increase in LOS for every year over 70 (OR, 1.16; P less than .01).
Similarly, each increase in severity of illness index score above 2 was associated with a 31% increase in LOS (OR, 1.31) and a 475% increase in intensive care unit (ICU) days (OR, 4.75; P less than .01 for both LOS and ICU days).
Black patients had a 22% increase in LOS vs. other races (OR, 1.22; P less than .01) and a 31% relative increase in ICU days (OR, 1.31; P = .01).
Because age and severity of illness were both strong predictors for mortality, readmission, and other perioperative outcomes, the authors conducted a further analysis combining the two variables, and found that for every 5 years of age, there were significant increases in the risk for death among patients with a greater severity of illness, compared with low severity.
Dr. Abbott noted that some of the odds ratios for older, sicker patients were "ridiculously high," probably because of the smaller sample sizes.
In a multivariate analysis of cost, factors associated with higher costs were age (OR, 1.14; P less than .01), greater severity of illness (OR, 2.14; P less than .01), and black race (OR, 1.15; P less than .01).
And in an analysis of cumulative resource use, the authors found what Dr. Abbott called a "snowball effect," in that hospitals with the lowest total costs discharged the majority of patients home, and that as costs increased, hospitals were less likely to discharge patients home and more likely to discharge them either to home health services or to extended care. In addition, as costs rose, the percentage of patients who died in hospital also rose.
He acknowledged that because the study used administrative data from university hospitals, it was skewed toward high-volume centers, and that the database did not include survival data, information about longitudinal resource use, or procedure-specific complications.
The authors did not disclose the funding source of the study. Dr. Abbott reported having no financial disclosures.
PHOENIX, ARIZ. – Cost and quality are not always synonymous, particularly when it comes to complex surgical procedures such as esophagectomy.
A review of records on more than 6,700 patients who underwent esophagectomy during a 4-year period showed that factors such as patient age, severity of illness, and hospital/surgeon volume can have a major effect on resource utilization and costs, said Dr. Daniel E. Abbott, assistant professor of surgery at the University of Cincinnati.
"There are certainly actionable risk factors for poor outcomes, such as mortality, and increased resource utilization, including dollar costs, the opportunity costs of increased length of stay, readmission, and rehabilitation and skilled nursing facilities," he reported at the annual Society of Surgical Oncology Cancer Symposium.
"I would argue that careful patient selection can have profound influences on cost-effectiveness. I think that as our health care is evolving and our outcomes are increasingly scrutinized, there will be increasing pressure to have better outcomes at lower costs," he added.
Dr. Abbott and his colleagues examined clinical variables in the cases of 6,737 esophagectomy patients treated from 2009 through 2012 in the University Healthsystems Consortium (UHC), an organization comprising 120 university hospitals and 299 affiliates.
They evaluated patient characteristics such as age and race, severity of illness index (1-4), esophagectomy type, and center and surgeon volume, and evaluated the effects of these variables on clinical outcomes that contribute to resource utilization, including deaths, readmissions, length of stay (LOS), and discharge disposition.
They found that the median LOS for all patients was 10 days (interquartile range, 8-17 days), but for patients over age 70, the median LOS was 11 days (P less than .01 vs. patients 70 and under).
Older patients did not have significantly higher readmission rates, but of the 4.2% of all patients who died in hospital, those over age 70 had more than twice the death rate of younger patients (7.0% vs. 3.2%, P less than .01).
Older patients were also significantly more likely to be discharged to a skilled nursing or rehabilitation facility than were younger patients (31.9% vs. 10.6%; P less than .01).
Total median cost per patient was $25,952, but again, older patients accounted for more of the expenses, at a median of $27,628 vs. $25,841 for those 70 and under (P less than .01).
In a multivariate analysis, patients over 70 had a more than twofold increase in risk of death (odds ratio, 2.12; P less than .01). Other factors significantly associated with greater risk for death were greater severity of illness (OR, 14.0; P less than .01) and black race vs. other races (OR, 1.88; P less than .01).
Factors associated with more frequent readmissions included greater severity of illness (OR, 1.33; P less than .01), and black race (OR, 1.34; P = .01), while patients of high-volume surgeons were less likely to need readmission (OR, 0.87; P = .04).
Lengths of stay were greater among patients over age 70, with every year over 70 translating into a 6% greater LOS (OR, 1.06; P =.03); older patients had a 16% increase in LOS for every year over 70 (OR, 1.16; P less than .01).
Similarly, each increase in severity of illness index score above 2 was associated with a 31% increase in LOS (OR, 1.31) and a 475% increase in intensive care unit (ICU) days (OR, 4.75; P less than .01 for both LOS and ICU days).
Black patients had a 22% increase in LOS vs. other races (OR, 1.22; P less than .01) and a 31% relative increase in ICU days (OR, 1.31; P = .01).
Because age and severity of illness were both strong predictors for mortality, readmission, and other perioperative outcomes, the authors conducted a further analysis combining the two variables, and found that for every 5 years of age, there were significant increases in the risk for death among patients with a greater severity of illness, compared with low severity.
Dr. Abbott noted that some of the odds ratios for older, sicker patients were "ridiculously high," probably because of the smaller sample sizes.
In a multivariate analysis of cost, factors associated with higher costs were age (OR, 1.14; P less than .01), greater severity of illness (OR, 2.14; P less than .01), and black race (OR, 1.15; P less than .01).
And in an analysis of cumulative resource use, the authors found what Dr. Abbott called a "snowball effect," in that hospitals with the lowest total costs discharged the majority of patients home, and that as costs increased, hospitals were less likely to discharge patients home and more likely to discharge them either to home health services or to extended care. In addition, as costs rose, the percentage of patients who died in hospital also rose.
He acknowledged that because the study used administrative data from university hospitals, it was skewed toward high-volume centers, and that the database did not include survival data, information about longitudinal resource use, or procedure-specific complications.
The authors did not disclose the funding source of the study. Dr. Abbott reported having no financial disclosures.
PHOENIX, ARIZ. – Cost and quality are not always synonymous, particularly when it comes to complex surgical procedures such as esophagectomy.
A review of records on more than 6,700 patients who underwent esophagectomy during a 4-year period showed that factors such as patient age, severity of illness, and hospital/surgeon volume can have a major effect on resource utilization and costs, said Dr. Daniel E. Abbott, assistant professor of surgery at the University of Cincinnati.
"There are certainly actionable risk factors for poor outcomes, such as mortality, and increased resource utilization, including dollar costs, the opportunity costs of increased length of stay, readmission, and rehabilitation and skilled nursing facilities," he reported at the annual Society of Surgical Oncology Cancer Symposium.
"I would argue that careful patient selection can have profound influences on cost-effectiveness. I think that as our health care is evolving and our outcomes are increasingly scrutinized, there will be increasing pressure to have better outcomes at lower costs," he added.
Dr. Abbott and his colleagues examined clinical variables in the cases of 6,737 esophagectomy patients treated from 2009 through 2012 in the University Healthsystems Consortium (UHC), an organization comprising 120 university hospitals and 299 affiliates.
They evaluated patient characteristics such as age and race, severity of illness index (1-4), esophagectomy type, and center and surgeon volume, and evaluated the effects of these variables on clinical outcomes that contribute to resource utilization, including deaths, readmissions, length of stay (LOS), and discharge disposition.
They found that the median LOS for all patients was 10 days (interquartile range, 8-17 days), but for patients over age 70, the median LOS was 11 days (P less than .01 vs. patients 70 and under).
Older patients did not have significantly higher readmission rates, but of the 4.2% of all patients who died in hospital, those over age 70 had more than twice the death rate of younger patients (7.0% vs. 3.2%, P less than .01).
Older patients were also significantly more likely to be discharged to a skilled nursing or rehabilitation facility than were younger patients (31.9% vs. 10.6%; P less than .01).
Total median cost per patient was $25,952, but again, older patients accounted for more of the expenses, at a median of $27,628 vs. $25,841 for those 70 and under (P less than .01).
In a multivariate analysis, patients over 70 had a more than twofold increase in risk of death (odds ratio, 2.12; P less than .01). Other factors significantly associated with greater risk for death were greater severity of illness (OR, 14.0; P less than .01) and black race vs. other races (OR, 1.88; P less than .01).
Factors associated with more frequent readmissions included greater severity of illness (OR, 1.33; P less than .01), and black race (OR, 1.34; P = .01), while patients of high-volume surgeons were less likely to need readmission (OR, 0.87; P = .04).
Lengths of stay were greater among patients over age 70, with every year over 70 translating into a 6% greater LOS (OR, 1.06; P =.03); older patients had a 16% increase in LOS for every year over 70 (OR, 1.16; P less than .01).
Similarly, each increase in severity of illness index score above 2 was associated with a 31% increase in LOS (OR, 1.31) and a 475% increase in intensive care unit (ICU) days (OR, 4.75; P less than .01 for both LOS and ICU days).
Black patients had a 22% increase in LOS vs. other races (OR, 1.22; P less than .01) and a 31% relative increase in ICU days (OR, 1.31; P = .01).
Because age and severity of illness were both strong predictors for mortality, readmission, and other perioperative outcomes, the authors conducted a further analysis combining the two variables, and found that for every 5 years of age, there were significant increases in the risk for death among patients with a greater severity of illness, compared with low severity.
Dr. Abbott noted that some of the odds ratios for older, sicker patients were "ridiculously high," probably because of the smaller sample sizes.
In a multivariate analysis of cost, factors associated with higher costs were age (OR, 1.14; P less than .01), greater severity of illness (OR, 2.14; P less than .01), and black race (OR, 1.15; P less than .01).
And in an analysis of cumulative resource use, the authors found what Dr. Abbott called a "snowball effect," in that hospitals with the lowest total costs discharged the majority of patients home, and that as costs increased, hospitals were less likely to discharge patients home and more likely to discharge them either to home health services or to extended care. In addition, as costs rose, the percentage of patients who died in hospital also rose.
He acknowledged that because the study used administrative data from university hospitals, it was skewed toward high-volume centers, and that the database did not include survival data, information about longitudinal resource use, or procedure-specific complications.
The authors did not disclose the funding source of the study. Dr. Abbott reported having no financial disclosures.
AT SSO 2014
Major finding: Factors associated with higher costs for esophagectomy were age, greater severity of illness, and black race.
Data source: Retrospective analysis of demographic and clinical factors associated with costs of esophagectomy in 6,737 patients treated in university-based hospitals and affiliates.
Disclosures: The authors did not disclose the funding source of the study. Dr. Abbott reported having no financial disclosures.
Small PNETs may still harbor malignant potential
PHOENIX, ARIZ. – Even small pancreatic neuroendocrine tumors have the potential for malignancy and need excision or close monitoring, investigators caution.
A retrospective cohort study following outcomes for patients with nonfunctional pancreatic neuroendocrine tumors (PNETs) 2 cm in diameter or less found that in an unadjusted analysis stratified by surgery type, those who did not have surgery had significantly worse overall survival 5 years after diagnosis, although there were no significant survival differences among all surgery types, reported Dr. Lauren Gratian of Duke University, Durham, N.C.
PNETs 2 cm in size or smaller are increasing in incidence in the United States, Dr. Gratian said at the annual Society of Surgical Oncology Cancer Symposium. "This is important, because their malignant potential is uncertain, and optimal surgical management remains unclear."
She pointed to National Comprehensive Cancer Network (NCCN) guidelines, which say that nonfunctional PNETs 2 cm or smaller are candidates for either enucleation or partial pancreatic resection, and that in certain cases, observation may be considered. Specifically, tumors smaller than 1 cm that were discovered incidentally may be candidates for observation, with the decision based on estimated surgical risk, site of the tumor, and patient comorbidities.
To get a better handle on the incidence of nonfunctional PNETs over time, discern their malignant potential, and see what factors are associated with survival, the investigators drew from the American College of Surgeons’ National Cancer Data Base (NCDB) for records on patients diagnosed from 1998 through 2011 with nonfunctional PNETs that met the criteria. Patients with functional PNETs, carcinoids, and/or exocrine tumors were excluded.
Of the 1,854 patients identified, 487 with more than 1 malignant primary, unknown surgical status, or surgery/pancreatectomy not otherwise specified were excluded, leaving a survival cohort of 1,367. Of this group, 368 (27%) had no surgery, 586 (43%) had a partial pancreatic resection, 324 (24%) had a pancreatoduodenectomy (Whipple procedure) with or without a partial gastrectomy, and 89 (6%) underwent total pancreatectomy.
The researchers found that the incidence of small nonfunctional PNETs as a percentage of all PNETs increased significantly over time, from 7% in 1998 to 20% in 2010.
When they grouped tumors by size (5 mm or smaller, more than 5 mm up to 1 cm, and more than 1 cm up to 2 cm), they found that there were no differences in tumor propensity for either distant metastases (11%, 9%, and 13%, respectively) or regional lymph node involvement (34%, 21%, and 29%).
Patients with distant metastases were significantly more likely to have had no surgery (P less than .001), and patients with partial pancreatic sections were significantly more likely than those who had undergone Whipple or total procedures to have positive surgical margins (P = .009).
An unadjusted analysis of 5-year overall survival by surgery type showed no significant differences among patients treated with the various procedures, but significantly worse outcomes for patients who had no surgery vs. any surgery (P less than .01).
There were no significant differences in survival between patients who had some regional lymph nodes examined compared with those who had no nodal dissection.
In a proportional hazards analysis adjusted for demographic and clinical features, factors significantly associated with overall survival were older age at diagnosis, which was associated with a higher risk of death (hazard ratio, 1.04; 95% confidence interval, 1.02-1.06); later year of diagnosis, associated with a lower risk (HR, 0.85; 95% CI, 0.77-0.94); and Whipple procedure, which was associated with an increased risk for death vs. partial pancreatectomy (HR, 1.88; 95% CI, 1.13-3.11).
Dr. Gratian noted that the study was limited by the inclusion of only malignant tumors in the database; potential coding errors; missing data on grade and stage of diseases; and the lack of information on variables such as disease recurrence, mitotic rates, or the Ki67 proliferative index.
The funding source for the study was not disclosed. Dr. Gratian and her coauthors reported having no financial disclosures.
PHOENIX, ARIZ. – Even small pancreatic neuroendocrine tumors have the potential for malignancy and need excision or close monitoring, investigators caution.
A retrospective cohort study following outcomes for patients with nonfunctional pancreatic neuroendocrine tumors (PNETs) 2 cm in diameter or less found that in an unadjusted analysis stratified by surgery type, those who did not have surgery had significantly worse overall survival 5 years after diagnosis, although there were no significant survival differences among all surgery types, reported Dr. Lauren Gratian of Duke University, Durham, N.C.
PNETs 2 cm in size or smaller are increasing in incidence in the United States, Dr. Gratian said at the annual Society of Surgical Oncology Cancer Symposium. "This is important, because their malignant potential is uncertain, and optimal surgical management remains unclear."
She pointed to National Comprehensive Cancer Network (NCCN) guidelines, which say that nonfunctional PNETs 2 cm or smaller are candidates for either enucleation or partial pancreatic resection, and that in certain cases, observation may be considered. Specifically, tumors smaller than 1 cm that were discovered incidentally may be candidates for observation, with the decision based on estimated surgical risk, site of the tumor, and patient comorbidities.
To get a better handle on the incidence of nonfunctional PNETs over time, discern their malignant potential, and see what factors are associated with survival, the investigators drew from the American College of Surgeons’ National Cancer Data Base (NCDB) for records on patients diagnosed from 1998 through 2011 with nonfunctional PNETs that met the criteria. Patients with functional PNETs, carcinoids, and/or exocrine tumors were excluded.
Of the 1,854 patients identified, 487 with more than 1 malignant primary, unknown surgical status, or surgery/pancreatectomy not otherwise specified were excluded, leaving a survival cohort of 1,367. Of this group, 368 (27%) had no surgery, 586 (43%) had a partial pancreatic resection, 324 (24%) had a pancreatoduodenectomy (Whipple procedure) with or without a partial gastrectomy, and 89 (6%) underwent total pancreatectomy.
The researchers found that the incidence of small nonfunctional PNETs as a percentage of all PNETs increased significantly over time, from 7% in 1998 to 20% in 2010.
When they grouped tumors by size (5 mm or smaller, more than 5 mm up to 1 cm, and more than 1 cm up to 2 cm), they found that there were no differences in tumor propensity for either distant metastases (11%, 9%, and 13%, respectively) or regional lymph node involvement (34%, 21%, and 29%).
Patients with distant metastases were significantly more likely to have had no surgery (P less than .001), and patients with partial pancreatic sections were significantly more likely than those who had undergone Whipple or total procedures to have positive surgical margins (P = .009).
An unadjusted analysis of 5-year overall survival by surgery type showed no significant differences among patients treated with the various procedures, but significantly worse outcomes for patients who had no surgery vs. any surgery (P less than .01).
There were no significant differences in survival between patients who had some regional lymph nodes examined compared with those who had no nodal dissection.
In a proportional hazards analysis adjusted for demographic and clinical features, factors significantly associated with overall survival were older age at diagnosis, which was associated with a higher risk of death (hazard ratio, 1.04; 95% confidence interval, 1.02-1.06); later year of diagnosis, associated with a lower risk (HR, 0.85; 95% CI, 0.77-0.94); and Whipple procedure, which was associated with an increased risk for death vs. partial pancreatectomy (HR, 1.88; 95% CI, 1.13-3.11).
Dr. Gratian noted that the study was limited by the inclusion of only malignant tumors in the database; potential coding errors; missing data on grade and stage of diseases; and the lack of information on variables such as disease recurrence, mitotic rates, or the Ki67 proliferative index.
The funding source for the study was not disclosed. Dr. Gratian and her coauthors reported having no financial disclosures.
PHOENIX, ARIZ. – Even small pancreatic neuroendocrine tumors have the potential for malignancy and need excision or close monitoring, investigators caution.
A retrospective cohort study following outcomes for patients with nonfunctional pancreatic neuroendocrine tumors (PNETs) 2 cm in diameter or less found that in an unadjusted analysis stratified by surgery type, those who did not have surgery had significantly worse overall survival 5 years after diagnosis, although there were no significant survival differences among all surgery types, reported Dr. Lauren Gratian of Duke University, Durham, N.C.
PNETs 2 cm in size or smaller are increasing in incidence in the United States, Dr. Gratian said at the annual Society of Surgical Oncology Cancer Symposium. "This is important, because their malignant potential is uncertain, and optimal surgical management remains unclear."
She pointed to National Comprehensive Cancer Network (NCCN) guidelines, which say that nonfunctional PNETs 2 cm or smaller are candidates for either enucleation or partial pancreatic resection, and that in certain cases, observation may be considered. Specifically, tumors smaller than 1 cm that were discovered incidentally may be candidates for observation, with the decision based on estimated surgical risk, site of the tumor, and patient comorbidities.
To get a better handle on the incidence of nonfunctional PNETs over time, discern their malignant potential, and see what factors are associated with survival, the investigators drew from the American College of Surgeons’ National Cancer Data Base (NCDB) for records on patients diagnosed from 1998 through 2011 with nonfunctional PNETs that met the criteria. Patients with functional PNETs, carcinoids, and/or exocrine tumors were excluded.
Of the 1,854 patients identified, 487 with more than 1 malignant primary, unknown surgical status, or surgery/pancreatectomy not otherwise specified were excluded, leaving a survival cohort of 1,367. Of this group, 368 (27%) had no surgery, 586 (43%) had a partial pancreatic resection, 324 (24%) had a pancreatoduodenectomy (Whipple procedure) with or without a partial gastrectomy, and 89 (6%) underwent total pancreatectomy.
The researchers found that the incidence of small nonfunctional PNETs as a percentage of all PNETs increased significantly over time, from 7% in 1998 to 20% in 2010.
When they grouped tumors by size (5 mm or smaller, more than 5 mm up to 1 cm, and more than 1 cm up to 2 cm), they found that there were no differences in tumor propensity for either distant metastases (11%, 9%, and 13%, respectively) or regional lymph node involvement (34%, 21%, and 29%).
Patients with distant metastases were significantly more likely to have had no surgery (P less than .001), and patients with partial pancreatic sections were significantly more likely than those who had undergone Whipple or total procedures to have positive surgical margins (P = .009).
An unadjusted analysis of 5-year overall survival by surgery type showed no significant differences among patients treated with the various procedures, but significantly worse outcomes for patients who had no surgery vs. any surgery (P less than .01).
There were no significant differences in survival between patients who had some regional lymph nodes examined compared with those who had no nodal dissection.
In a proportional hazards analysis adjusted for demographic and clinical features, factors significantly associated with overall survival were older age at diagnosis, which was associated with a higher risk of death (hazard ratio, 1.04; 95% confidence interval, 1.02-1.06); later year of diagnosis, associated with a lower risk (HR, 0.85; 95% CI, 0.77-0.94); and Whipple procedure, which was associated with an increased risk for death vs. partial pancreatectomy (HR, 1.88; 95% CI, 1.13-3.11).
Dr. Gratian noted that the study was limited by the inclusion of only malignant tumors in the database; potential coding errors; missing data on grade and stage of diseases; and the lack of information on variables such as disease recurrence, mitotic rates, or the Ki67 proliferative index.
The funding source for the study was not disclosed. Dr. Gratian and her coauthors reported having no financial disclosures.
AT SSO 2014
Major finding: Among patients with pancreatic neuroendocrine tumors 2 cm or smaller, 5-year overall survival was significantly worse for patients treated with observation alone than for those who had any type of surgery (P less than .01).
Data source: A retrospective database cohort study of 1,367 patients from 1998 through 2011 with nonfunctional PNETs.
Disclosures: The funding source for the study was not disclosed. Dr. Gratian and her coauthors reported having no financial disclosures.
Bariatric surgery reduces uterine cancer risk
TAMPA – Women who undergo bariatric surgery to lose weight are about 70% less likely to develop uterine cancer than are obese women who do not undergo such surgery, according to findings from a large retrospective cohort study.
The risk reduction was even greater (81%) among those who maintained their weight loss after surgery. The findings suggest that obesity may be a modifiable risk factor for uterine cancer, reported Dr. Kristy Kay Ward of the Moores Cancer Center at the University of California, San Diego.
Of more than 7.4 million inpatient admissions among women aged 18 years or older who were registered in the University Health System Consortium dataset from Jan. 1, 2009, to June 1, 2013, 103,797 had a history of bariatric surgery, and 44,345 had a diagnosis of uterine malignancy. The overall rate of uterine malignancy was 599/100,000 patients among those without a history of bariatric surgery, and which was 2.8 times higher among obese vs. nonobese patients within this group (1,409 vs. 496 per 100,000).
The overall rate of uterine cancer among those with a history of bariatric surgery was 408/100,000, but the rate was 2.5 times higher among those with persistent obesity after surgery, compared with those who maintained weight loss after surgery (682/100,000 vs. 270/100,000), Dr. Ward said at the annual meeting of the Society of Gynecologic Oncology.
Compared with obese women without a history of bariatric surgery, the relative risk of uterine cancer was 0.29 for women with prior bariatric surgery, 0.19 for women with normal weight after surgery, and 0.48 for women who remained obese after surgery, so the overall risk reduction with surgery was 70%, the maximum risk reduction (for those with normal weight after surgery) was 81%, and the lowest reduction in risk (for those who had surgery but remained obese) was 52%, Dr. Ward said.
Though limited by the retrospective nature of the study and the fact that the data didn’t differentiate between types of bariatric surgery, the findings are notable, because about 50,000 women were diagnosed with uterine cancer in 2013, making it the most common cancer affecting female reproductive organs. Furthermore, endometrial cancer, which accounts for 95% of uterine cancers, is associated with obesity in about 50% of cases, she explained.
In fact, obese women are two- to fourfold more likely to develop endometrial cancer than are women of normal weight, she said.
The current findings suggest that "a history of bariatric surgery is associated with substantial and clinically significantly reduced risk of uterine malignancy," she said, adding: "Our previous work, in agreement with the findings of others, had indicated that the risk of uterine malignancy increases linearly with BMI [body mass index]. Along with the findings of the current study, this supports that obesity may be a modifiable risk factor related to the development of endometrial cancer."
The mechanism for the link between bariatric surgery and reduced uterine cancer risk remains unclear, but fat loss likely plays a role, as adiposity is known to increase endogenous estrogen circulation. The bariatric surgery itself may also "somehow be influencing the immune system and decreasing inflammation," thereby contributing to decreased cancer risk, Dr. Ward noted.
The findings suggest that weight reduction measures, including bariatric surgery in appropriate candidates, are vitally important in obese women, she said.
"Screening of patients, counseling patients about the dangers of obesity, and appropriate referral for bariatric surgery may have great impact on the overall health of this population," she concluded, adding that future research should examine the benefits of bariatric surgery for the reduction of cancer, including endometrial cancer.
Dr. Ward reported having no disclosures.
TAMPA – Women who undergo bariatric surgery to lose weight are about 70% less likely to develop uterine cancer than are obese women who do not undergo such surgery, according to findings from a large retrospective cohort study.
The risk reduction was even greater (81%) among those who maintained their weight loss after surgery. The findings suggest that obesity may be a modifiable risk factor for uterine cancer, reported Dr. Kristy Kay Ward of the Moores Cancer Center at the University of California, San Diego.
Of more than 7.4 million inpatient admissions among women aged 18 years or older who were registered in the University Health System Consortium dataset from Jan. 1, 2009, to June 1, 2013, 103,797 had a history of bariatric surgery, and 44,345 had a diagnosis of uterine malignancy. The overall rate of uterine malignancy was 599/100,000 patients among those without a history of bariatric surgery, and which was 2.8 times higher among obese vs. nonobese patients within this group (1,409 vs. 496 per 100,000).
The overall rate of uterine cancer among those with a history of bariatric surgery was 408/100,000, but the rate was 2.5 times higher among those with persistent obesity after surgery, compared with those who maintained weight loss after surgery (682/100,000 vs. 270/100,000), Dr. Ward said at the annual meeting of the Society of Gynecologic Oncology.
Compared with obese women without a history of bariatric surgery, the relative risk of uterine cancer was 0.29 for women with prior bariatric surgery, 0.19 for women with normal weight after surgery, and 0.48 for women who remained obese after surgery, so the overall risk reduction with surgery was 70%, the maximum risk reduction (for those with normal weight after surgery) was 81%, and the lowest reduction in risk (for those who had surgery but remained obese) was 52%, Dr. Ward said.
Though limited by the retrospective nature of the study and the fact that the data didn’t differentiate between types of bariatric surgery, the findings are notable, because about 50,000 women were diagnosed with uterine cancer in 2013, making it the most common cancer affecting female reproductive organs. Furthermore, endometrial cancer, which accounts for 95% of uterine cancers, is associated with obesity in about 50% of cases, she explained.
In fact, obese women are two- to fourfold more likely to develop endometrial cancer than are women of normal weight, she said.
The current findings suggest that "a history of bariatric surgery is associated with substantial and clinically significantly reduced risk of uterine malignancy," she said, adding: "Our previous work, in agreement with the findings of others, had indicated that the risk of uterine malignancy increases linearly with BMI [body mass index]. Along with the findings of the current study, this supports that obesity may be a modifiable risk factor related to the development of endometrial cancer."
The mechanism for the link between bariatric surgery and reduced uterine cancer risk remains unclear, but fat loss likely plays a role, as adiposity is known to increase endogenous estrogen circulation. The bariatric surgery itself may also "somehow be influencing the immune system and decreasing inflammation," thereby contributing to decreased cancer risk, Dr. Ward noted.
The findings suggest that weight reduction measures, including bariatric surgery in appropriate candidates, are vitally important in obese women, she said.
"Screening of patients, counseling patients about the dangers of obesity, and appropriate referral for bariatric surgery may have great impact on the overall health of this population," she concluded, adding that future research should examine the benefits of bariatric surgery for the reduction of cancer, including endometrial cancer.
Dr. Ward reported having no disclosures.
TAMPA – Women who undergo bariatric surgery to lose weight are about 70% less likely to develop uterine cancer than are obese women who do not undergo such surgery, according to findings from a large retrospective cohort study.
The risk reduction was even greater (81%) among those who maintained their weight loss after surgery. The findings suggest that obesity may be a modifiable risk factor for uterine cancer, reported Dr. Kristy Kay Ward of the Moores Cancer Center at the University of California, San Diego.
Of more than 7.4 million inpatient admissions among women aged 18 years or older who were registered in the University Health System Consortium dataset from Jan. 1, 2009, to June 1, 2013, 103,797 had a history of bariatric surgery, and 44,345 had a diagnosis of uterine malignancy. The overall rate of uterine malignancy was 599/100,000 patients among those without a history of bariatric surgery, and which was 2.8 times higher among obese vs. nonobese patients within this group (1,409 vs. 496 per 100,000).
The overall rate of uterine cancer among those with a history of bariatric surgery was 408/100,000, but the rate was 2.5 times higher among those with persistent obesity after surgery, compared with those who maintained weight loss after surgery (682/100,000 vs. 270/100,000), Dr. Ward said at the annual meeting of the Society of Gynecologic Oncology.
Compared with obese women without a history of bariatric surgery, the relative risk of uterine cancer was 0.29 for women with prior bariatric surgery, 0.19 for women with normal weight after surgery, and 0.48 for women who remained obese after surgery, so the overall risk reduction with surgery was 70%, the maximum risk reduction (for those with normal weight after surgery) was 81%, and the lowest reduction in risk (for those who had surgery but remained obese) was 52%, Dr. Ward said.
Though limited by the retrospective nature of the study and the fact that the data didn’t differentiate between types of bariatric surgery, the findings are notable, because about 50,000 women were diagnosed with uterine cancer in 2013, making it the most common cancer affecting female reproductive organs. Furthermore, endometrial cancer, which accounts for 95% of uterine cancers, is associated with obesity in about 50% of cases, she explained.
In fact, obese women are two- to fourfold more likely to develop endometrial cancer than are women of normal weight, she said.
The current findings suggest that "a history of bariatric surgery is associated with substantial and clinically significantly reduced risk of uterine malignancy," she said, adding: "Our previous work, in agreement with the findings of others, had indicated that the risk of uterine malignancy increases linearly with BMI [body mass index]. Along with the findings of the current study, this supports that obesity may be a modifiable risk factor related to the development of endometrial cancer."
The mechanism for the link between bariatric surgery and reduced uterine cancer risk remains unclear, but fat loss likely plays a role, as adiposity is known to increase endogenous estrogen circulation. The bariatric surgery itself may also "somehow be influencing the immune system and decreasing inflammation," thereby contributing to decreased cancer risk, Dr. Ward noted.
The findings suggest that weight reduction measures, including bariatric surgery in appropriate candidates, are vitally important in obese women, she said.
"Screening of patients, counseling patients about the dangers of obesity, and appropriate referral for bariatric surgery may have great impact on the overall health of this population," she concluded, adding that future research should examine the benefits of bariatric surgery for the reduction of cancer, including endometrial cancer.
Dr. Ward reported having no disclosures.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Major finding: Bariatric surgery was associated with a 70% reduction in risk of uterine cancer.
Data source: A retrospective cohort study involving more than 7.4 million inpatient admissions.
Disclosures: Dr. Ward reported having no disclosures.
VIDEO: The DecisionDx-Melanoma test can predict metastasis of sentinel node-negative melanomas
DENVER – A 134-patient study of patients with stage I, II, or III cutaneous melanoma found that the DecisionDx-Melanoma test was useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results.
In a video interview, Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University Skin Cancer Institute, Chicago, explains the best uses for the test and its patient management advantages.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
DENVER – A 134-patient study of patients with stage I, II, or III cutaneous melanoma found that the DecisionDx-Melanoma test was useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results.
In a video interview, Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University Skin Cancer Institute, Chicago, explains the best uses for the test and its patient management advantages.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
DENVER – A 134-patient study of patients with stage I, II, or III cutaneous melanoma found that the DecisionDx-Melanoma test was useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results.
In a video interview, Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University Skin Cancer Institute, Chicago, explains the best uses for the test and its patient management advantages.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @naseemmiller
AT THE AAD ANNUAL MEETING
Gene test predicts metastasis of sentinel node-negative melanomas
DENVER – A gene expression profile test was an independent predictor of metastasis of primary cutaneous melanomas in patients with negative sentinel lymph node biopsies.
The DecisionDx-Melanoma test is useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results, said Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University, Chicago, Skin Cancer Institute. The test "is an independent predictor of metastasis and death, and significantly improves upon sentinel lymph node biopsy for staging melanoma patients."
The results of the DecisionDx-Melanoma test, a noninvasive 31-gene expression profile (GEP) test, were compared with the results of sentinel lymph node biopsy (SLNB) in 134 patients who had stage I, II, or III cutaneous melanoma and underwent a documented sentinel lymph node procedure. Of the 134 patients, 28 had positive sentinel lymph nodes and 91 had positive (class 2, high risk) GEP results.
Metastases developed over a subsequent 5-year period in 18 of the 28 patients with positive SLNB and in 62 of the 91 patients with positive GEP results. Metastases developed in 51 of the 106 patients with negative SLNB and in 7 of 43 patients with negative (class 1, low risk) GEP results.
While the positive predictive value of the two tests were comparable, the ability of GEP to predict negative outcomes was significantly better than that of SLNB (P less than .0001), Dr. Gerami reported at the annual meeting of the American Academy of Dermatology.
The rate of 5-year metastasis-free survival (MFS) was 55% for 106 patients with negative SLNB, compared to 37% for 28 patients with positive SLNB (P = .003). The GEP test results showed improved prognostic accuracy in these same patients with an MFS of 87% for the 43 patients with negative GEP (class 1, low risk) results and of 31% for the 91 patients with positive GEP (class 2, high risk) results (P less than .0001).
Differences in overall survival (OS) paralleled the MFS rates, with SLNB-negative patients having a 5-year OS of 67% and SLNB-positive patients having a 5-year OS of 55% (P = .024). OS for negative GEP (class 1, low risk) patients was 92% and for positive GEP (high risk, class 2) was 49% (P less than .0001).
Use of the GEP test also was analyzed in combination with SLNB status. As expected, the 20% of patients (n = 27) who had high-risk results for both tests (GEP class 2 and SLNB-positive findings) had lower survival rates (MFS, 34%; OS, 53%). Similarly, the 31% of patients (n = 42) who had low-risk results for both tests (GEP class 1 and SLNB-negative findings) had higher survival rates (MFS, 82%; OS, 92%).
Importantly, the MFS was 31% and the OS was 49% at 5 years in the 64 patients who had SLNB-negative results but class 2 GEP test results, Dr. Gerami said. Cox multivariate analysis comparing the GEP test to SLNB showed the GEP test to be the only independent and highly significant prognostic factor in this analysis (P less than .000003).
Dr. Gerami has been a consultant to Castle Biosciences. The DecisionDx-Melanoma test is a product of Castle Biosciences, the sponsor of the study. More information about the test can be found at www.skinmelanoma.com.
On Twitter @maryjodales
DENVER – A gene expression profile test was an independent predictor of metastasis of primary cutaneous melanomas in patients with negative sentinel lymph node biopsies.
The DecisionDx-Melanoma test is useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results, said Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University, Chicago, Skin Cancer Institute. The test "is an independent predictor of metastasis and death, and significantly improves upon sentinel lymph node biopsy for staging melanoma patients."
The results of the DecisionDx-Melanoma test, a noninvasive 31-gene expression profile (GEP) test, were compared with the results of sentinel lymph node biopsy (SLNB) in 134 patients who had stage I, II, or III cutaneous melanoma and underwent a documented sentinel lymph node procedure. Of the 134 patients, 28 had positive sentinel lymph nodes and 91 had positive (class 2, high risk) GEP results.
Metastases developed over a subsequent 5-year period in 18 of the 28 patients with positive SLNB and in 62 of the 91 patients with positive GEP results. Metastases developed in 51 of the 106 patients with negative SLNB and in 7 of 43 patients with negative (class 1, low risk) GEP results.
While the positive predictive value of the two tests were comparable, the ability of GEP to predict negative outcomes was significantly better than that of SLNB (P less than .0001), Dr. Gerami reported at the annual meeting of the American Academy of Dermatology.
The rate of 5-year metastasis-free survival (MFS) was 55% for 106 patients with negative SLNB, compared to 37% for 28 patients with positive SLNB (P = .003). The GEP test results showed improved prognostic accuracy in these same patients with an MFS of 87% for the 43 patients with negative GEP (class 1, low risk) results and of 31% for the 91 patients with positive GEP (class 2, high risk) results (P less than .0001).
Differences in overall survival (OS) paralleled the MFS rates, with SLNB-negative patients having a 5-year OS of 67% and SLNB-positive patients having a 5-year OS of 55% (P = .024). OS for negative GEP (class 1, low risk) patients was 92% and for positive GEP (high risk, class 2) was 49% (P less than .0001).
Use of the GEP test also was analyzed in combination with SLNB status. As expected, the 20% of patients (n = 27) who had high-risk results for both tests (GEP class 2 and SLNB-positive findings) had lower survival rates (MFS, 34%; OS, 53%). Similarly, the 31% of patients (n = 42) who had low-risk results for both tests (GEP class 1 and SLNB-negative findings) had higher survival rates (MFS, 82%; OS, 92%).
Importantly, the MFS was 31% and the OS was 49% at 5 years in the 64 patients who had SLNB-negative results but class 2 GEP test results, Dr. Gerami said. Cox multivariate analysis comparing the GEP test to SLNB showed the GEP test to be the only independent and highly significant prognostic factor in this analysis (P less than .000003).
Dr. Gerami has been a consultant to Castle Biosciences. The DecisionDx-Melanoma test is a product of Castle Biosciences, the sponsor of the study. More information about the test can be found at www.skinmelanoma.com.
On Twitter @maryjodales
DENVER – A gene expression profile test was an independent predictor of metastasis of primary cutaneous melanomas in patients with negative sentinel lymph node biopsies.
The DecisionDx-Melanoma test is useful for identifying a high-risk group of patients with negative sentinel lymph node biopsy results, said Dr. Pedram Gerami of the department of dermatology and director of melanoma research at the Northwestern University, Chicago, Skin Cancer Institute. The test "is an independent predictor of metastasis and death, and significantly improves upon sentinel lymph node biopsy for staging melanoma patients."
The results of the DecisionDx-Melanoma test, a noninvasive 31-gene expression profile (GEP) test, were compared with the results of sentinel lymph node biopsy (SLNB) in 134 patients who had stage I, II, or III cutaneous melanoma and underwent a documented sentinel lymph node procedure. Of the 134 patients, 28 had positive sentinel lymph nodes and 91 had positive (class 2, high risk) GEP results.
Metastases developed over a subsequent 5-year period in 18 of the 28 patients with positive SLNB and in 62 of the 91 patients with positive GEP results. Metastases developed in 51 of the 106 patients with negative SLNB and in 7 of 43 patients with negative (class 1, low risk) GEP results.
While the positive predictive value of the two tests were comparable, the ability of GEP to predict negative outcomes was significantly better than that of SLNB (P less than .0001), Dr. Gerami reported at the annual meeting of the American Academy of Dermatology.
The rate of 5-year metastasis-free survival (MFS) was 55% for 106 patients with negative SLNB, compared to 37% for 28 patients with positive SLNB (P = .003). The GEP test results showed improved prognostic accuracy in these same patients with an MFS of 87% for the 43 patients with negative GEP (class 1, low risk) results and of 31% for the 91 patients with positive GEP (class 2, high risk) results (P less than .0001).
Differences in overall survival (OS) paralleled the MFS rates, with SLNB-negative patients having a 5-year OS of 67% and SLNB-positive patients having a 5-year OS of 55% (P = .024). OS for negative GEP (class 1, low risk) patients was 92% and for positive GEP (high risk, class 2) was 49% (P less than .0001).
Use of the GEP test also was analyzed in combination with SLNB status. As expected, the 20% of patients (n = 27) who had high-risk results for both tests (GEP class 2 and SLNB-positive findings) had lower survival rates (MFS, 34%; OS, 53%). Similarly, the 31% of patients (n = 42) who had low-risk results for both tests (GEP class 1 and SLNB-negative findings) had higher survival rates (MFS, 82%; OS, 92%).
Importantly, the MFS was 31% and the OS was 49% at 5 years in the 64 patients who had SLNB-negative results but class 2 GEP test results, Dr. Gerami said. Cox multivariate analysis comparing the GEP test to SLNB showed the GEP test to be the only independent and highly significant prognostic factor in this analysis (P less than .000003).
Dr. Gerami has been a consultant to Castle Biosciences. The DecisionDx-Melanoma test is a product of Castle Biosciences, the sponsor of the study. More information about the test can be found at www.skinmelanoma.com.
On Twitter @maryjodales
AT THE AAD ANNUAL MEETING
Major finding: Metastasis-free survival was 31% and overall survival was 49% at 5 years in the 64 patients who had negative sentinel node lymph biopsy results and high-risk, class 2 gene expression profile test results.
Data source: A study of 134 patients who had stage I, II, or III cutaneous melanoma and underwent a documented sentinel lymph node procedure.
Disclosures: Dr. Gerami has been a consultant to Castle Biosciences. The DecisionDx-Melanoma test is a product of Castle Biosciences, the study sponsor.
Blood test predicts Merkel cell carcinoma metastases
DENVER – A newly-available test based on the results of a simple blood draw has proved useful for detecting early recurrences of Merkel cell carcinomas in those patients who produce antibodies to the Merkel polyomavirus oncoprotein at initial diagnosis.
Known as AMERK, the test can be used at diagnosis to determine which patients have the oncoprotein antibodies and performed at routine followups as an indicator of recurrence in asymptomatic, antibody-positive patients.
The test already has been shown to alert clinicians to the presence of surgically manageable metastases that would have otherwise gone undetected until symptoms prompted a tomographic scan, Dr. Astrid Blom reported at the annual meeting of the American Academy of Dermatology. She presented two cases of surgically operable metastatic Merkel cell tumors, one metastatic to the kidney and the other to the pancreas; both were detected via increasing titers of oncoprotein antibodies in otherwise asymptomatic patients.
Nearly 40% of Merkel cell carcinomas recur. Performing a predictive blood test at routine followups can be a reassuring measure in those patients with negative findings. It also can be an early indicator of metastasis in otherwise asymptomatic patients who made oncoprotein antibodies at diagnosis and were successfully treated for Merkel call carcinoma, she said.
Merkel cell polyomavirus drives about 80% of the approximately 2,000 Merkel cell carcinomas that are diagnosed each year. About half of affected patients produce oncoprotein antibodies to the polyomavirus, which are detectable at diagnosis. The AMERK test is useful only for followup of those patients with oncoprotein antibodies. Patients who lack initially detectable levels of oncoprotein antibodies at diagnosis do not later produce antibodies should their disease recur, reported Dr. Blom of the University of Washington, Seattle, where the test was developed and is performed.
The clinical utility of AMERK, a 75-step assay that takes nearly 2 days to perform, was verified using 1,342 samples from 104 controls and 519 patients from around the world, with data correlating to 3,018 status updates. The data analysis was limited to the 217 patients with adequate follow-up data.
A simple blood draw was collected and sent for analysis at the University of Washington, Seattle. Oncoprotein antibodies were detected in 52% of 217 patients with incident cases of Merkel cell carcinoma and in 2% of 530 control subjects. The antibody titers in controls were barely detectable, however, unlike the levels seen in the patients. The sensitivity of the test was 82%, and the specificity was 98%; the negative predictive value was 99%, and the positive predictive value was 78%.
Levels decrease by 90% or more over the course of the year after successful surgical treatment of Merkel cell carcinomas. When the cancers recur, at least a 10-fold increase in oncoprotein antibody titers are noted.
Support for the development of the AMERK test came from the National Institutes of Health, the National Cancer Institute, and the American Cancer Society and from private donors and patients.
RESOURCES:
Information about the test is available online at http://www.merkelcell.org/sero/
A video that discusses the antibody test can be viewed here.
Dr. Kelly Paulsen, et.al. published the scientific paper that first described the test (Cancer Res. 70(21): 8388-97).
How to order the Merkel virus serology test
Physicians and staff based outside of the University of Washington system should contact their local laboratory where the patient will have their blood sample collected to initiate the process.
The local laboratory "send out test coordinator" should contact the UW Reference Laboratory Services Call Center at 206-685-6066. UW Reference Laboratory Services will set up an account for the ordering physician or clinic; collect the required billing and reporting information; and provide requirements for sample collection, processing and shipping from the local laboratory. Once this one-time administrative process is complete, patient samples can be collected routinely from that facility for the Merkel antibody test (name of test is "AMERK").
On Twitter @maryjodales
DENVER – A newly-available test based on the results of a simple blood draw has proved useful for detecting early recurrences of Merkel cell carcinomas in those patients who produce antibodies to the Merkel polyomavirus oncoprotein at initial diagnosis.
Known as AMERK, the test can be used at diagnosis to determine which patients have the oncoprotein antibodies and performed at routine followups as an indicator of recurrence in asymptomatic, antibody-positive patients.
The test already has been shown to alert clinicians to the presence of surgically manageable metastases that would have otherwise gone undetected until symptoms prompted a tomographic scan, Dr. Astrid Blom reported at the annual meeting of the American Academy of Dermatology. She presented two cases of surgically operable metastatic Merkel cell tumors, one metastatic to the kidney and the other to the pancreas; both were detected via increasing titers of oncoprotein antibodies in otherwise asymptomatic patients.
Nearly 40% of Merkel cell carcinomas recur. Performing a predictive blood test at routine followups can be a reassuring measure in those patients with negative findings. It also can be an early indicator of metastasis in otherwise asymptomatic patients who made oncoprotein antibodies at diagnosis and were successfully treated for Merkel call carcinoma, she said.
Merkel cell polyomavirus drives about 80% of the approximately 2,000 Merkel cell carcinomas that are diagnosed each year. About half of affected patients produce oncoprotein antibodies to the polyomavirus, which are detectable at diagnosis. The AMERK test is useful only for followup of those patients with oncoprotein antibodies. Patients who lack initially detectable levels of oncoprotein antibodies at diagnosis do not later produce antibodies should their disease recur, reported Dr. Blom of the University of Washington, Seattle, where the test was developed and is performed.
The clinical utility of AMERK, a 75-step assay that takes nearly 2 days to perform, was verified using 1,342 samples from 104 controls and 519 patients from around the world, with data correlating to 3,018 status updates. The data analysis was limited to the 217 patients with adequate follow-up data.
A simple blood draw was collected and sent for analysis at the University of Washington, Seattle. Oncoprotein antibodies were detected in 52% of 217 patients with incident cases of Merkel cell carcinoma and in 2% of 530 control subjects. The antibody titers in controls were barely detectable, however, unlike the levels seen in the patients. The sensitivity of the test was 82%, and the specificity was 98%; the negative predictive value was 99%, and the positive predictive value was 78%.
Levels decrease by 90% or more over the course of the year after successful surgical treatment of Merkel cell carcinomas. When the cancers recur, at least a 10-fold increase in oncoprotein antibody titers are noted.
Support for the development of the AMERK test came from the National Institutes of Health, the National Cancer Institute, and the American Cancer Society and from private donors and patients.
RESOURCES:
Information about the test is available online at http://www.merkelcell.org/sero/
A video that discusses the antibody test can be viewed here.
Dr. Kelly Paulsen, et.al. published the scientific paper that first described the test (Cancer Res. 70(21): 8388-97).
How to order the Merkel virus serology test
Physicians and staff based outside of the University of Washington system should contact their local laboratory where the patient will have their blood sample collected to initiate the process.
The local laboratory "send out test coordinator" should contact the UW Reference Laboratory Services Call Center at 206-685-6066. UW Reference Laboratory Services will set up an account for the ordering physician or clinic; collect the required billing and reporting information; and provide requirements for sample collection, processing and shipping from the local laboratory. Once this one-time administrative process is complete, patient samples can be collected routinely from that facility for the Merkel antibody test (name of test is "AMERK").
On Twitter @maryjodales
DENVER – A newly-available test based on the results of a simple blood draw has proved useful for detecting early recurrences of Merkel cell carcinomas in those patients who produce antibodies to the Merkel polyomavirus oncoprotein at initial diagnosis.
Known as AMERK, the test can be used at diagnosis to determine which patients have the oncoprotein antibodies and performed at routine followups as an indicator of recurrence in asymptomatic, antibody-positive patients.
The test already has been shown to alert clinicians to the presence of surgically manageable metastases that would have otherwise gone undetected until symptoms prompted a tomographic scan, Dr. Astrid Blom reported at the annual meeting of the American Academy of Dermatology. She presented two cases of surgically operable metastatic Merkel cell tumors, one metastatic to the kidney and the other to the pancreas; both were detected via increasing titers of oncoprotein antibodies in otherwise asymptomatic patients.
Nearly 40% of Merkel cell carcinomas recur. Performing a predictive blood test at routine followups can be a reassuring measure in those patients with negative findings. It also can be an early indicator of metastasis in otherwise asymptomatic patients who made oncoprotein antibodies at diagnosis and were successfully treated for Merkel call carcinoma, she said.
Merkel cell polyomavirus drives about 80% of the approximately 2,000 Merkel cell carcinomas that are diagnosed each year. About half of affected patients produce oncoprotein antibodies to the polyomavirus, which are detectable at diagnosis. The AMERK test is useful only for followup of those patients with oncoprotein antibodies. Patients who lack initially detectable levels of oncoprotein antibodies at diagnosis do not later produce antibodies should their disease recur, reported Dr. Blom of the University of Washington, Seattle, where the test was developed and is performed.
The clinical utility of AMERK, a 75-step assay that takes nearly 2 days to perform, was verified using 1,342 samples from 104 controls and 519 patients from around the world, with data correlating to 3,018 status updates. The data analysis was limited to the 217 patients with adequate follow-up data.
A simple blood draw was collected and sent for analysis at the University of Washington, Seattle. Oncoprotein antibodies were detected in 52% of 217 patients with incident cases of Merkel cell carcinoma and in 2% of 530 control subjects. The antibody titers in controls were barely detectable, however, unlike the levels seen in the patients. The sensitivity of the test was 82%, and the specificity was 98%; the negative predictive value was 99%, and the positive predictive value was 78%.
Levels decrease by 90% or more over the course of the year after successful surgical treatment of Merkel cell carcinomas. When the cancers recur, at least a 10-fold increase in oncoprotein antibody titers are noted.
Support for the development of the AMERK test came from the National Institutes of Health, the National Cancer Institute, and the American Cancer Society and from private donors and patients.
RESOURCES:
Information about the test is available online at http://www.merkelcell.org/sero/
A video that discusses the antibody test can be viewed here.
Dr. Kelly Paulsen, et.al. published the scientific paper that first described the test (Cancer Res. 70(21): 8388-97).
How to order the Merkel virus serology test
Physicians and staff based outside of the University of Washington system should contact their local laboratory where the patient will have their blood sample collected to initiate the process.
The local laboratory "send out test coordinator" should contact the UW Reference Laboratory Services Call Center at 206-685-6066. UW Reference Laboratory Services will set up an account for the ordering physician or clinic; collect the required billing and reporting information; and provide requirements for sample collection, processing and shipping from the local laboratory. Once this one-time administrative process is complete, patient samples can be collected routinely from that facility for the Merkel antibody test (name of test is "AMERK").
On Twitter @maryjodales
AT THE AAD ANNUAL MEETING
Major finding: Two cases of surgically operable, metastatic Merkel cell tumors, one metastatic to the kidney and the other to the pancreas, were detected via increasing serum titers of oncoprotein antibodies as part of the followup of otherwise asymptomatic patients.
Data source: Followup study of the 52% of 217 patients who had incident cases of Merkel cell carcinoma and had positive titers for oncoprotein antibodies.
Disclosures: Support for the development of the AMERK test came from the National Institutes of Health, the National Cancer Institute, the American Cancer Society, and from private donors and patients.
Imaging studies don’t predict pancreatic resectability post-FOLFIRINOX
PHOENIX – Seeing is not always believing when it comes to determining whether pancreatic adenocarcinomas are resectable.
In patients with locally advanced or borderline resectable pancreatic adenocarcinoma who underwent neoadjuvant therapy with the FOLFIRINOX regimen (leucovorin, fluorouracil, irinotecan, and oxaliplatin), presurgical imaging was not a reliable determinant of resectability, said Dr. Cristina R. Ferrone from the department of general and gastrointestinal surgery at Massachusetts General Hospital in Boston.
"It really is a paradigm shift that we can’t rely on CT scans and MRIs the way we did before," she said at the annual Society of Surgical Oncology Cancer Symposium.
An important case
For her team, the paradigm shift was sparked by the case of a 41-year-old woman who presented with a 3.6-cm pancreatic adenocarcinoma involving the superior mesenteric artery (SMA), and a cancer antigen 19-9 (CA 19-9) level of 985 U/mL (normal values for the nonspecific biomarker range from 0 to 37 U/mL, according to National Comprehensive Cancer Network guidelines).
Following 4 months of FOLFIRINOX and 50.4 Gy of chemoradiation, her CA 19-9 level normalized to 37 U/mL. But her postchemotherapy imaging studies appeared identical to her prechemotherapy studies, with no apparent evidence of tumor regression and apparent continued involvement of the SMA following FOLFIRINOX.
"We had a big debate about this woman, because she had a normalized CA 19-9. Do we attempt to resect a patient like this?" Dr. Ferrone said.
After considerable discussion, the decision was made to take the patient into the operating room, approach the pancreas from the left side, and perform intraoperative biopsies on the SMA. If the biopsies were positive, the surgical team would either perform intraoperative radiation therapy (IORT) or use a soft-tissue ablation system (Nanoknife). If the biopsies were negative, the team would proceed to resection.
The patient went on to resection, and was found to have only minimal residual tumor on postoperative pathology samples, Dr. Ferrone reported.
Case comparisons
To see whether other patients could benefit from a similar approach, the investigators compared outcomes for 87 patients who underwent upfront resection for presumed organ-confined disease and 38 patients who received neoadjuvant FOLFIRINOX before surgery. Of the latter group, 5 received FOLFIRINOX chemotherapy only; 16 received FOLFIRINOX and 50.4 Gy of radiation; 10 received FOLFIRINOX, radiation, and IORT; 4 received FOLFIRINOX and proton-beam therapy; and 3 had FOLFIRINOX with other drug therapies.
The median CA 19-9 level fell from 169 U/mL before FOLFIRINOX to 16 U/mL after (P = .003), and more than twice as many patients had levels below 40 U/mL after therapy (31% vs. 72%; P = .03)
Median tumor diameter on CT also decreased after chemotherapy, from 3.6 cm to 2.2 cm (P = .001).
Pathology review of images from 20 cases with the observer blinded to treatment status showed 14 cases judged to be locally advanced disease and 6 borderline pre-FOLFIRINOX, and 12 locally advanced cases, 3 borderline, and 5 cases deemed resectable post-FOLFIRINOX.
When the investigators compared clinical and operative results in the surgery-only patients vs. results in those who had neoadjuvant FOLFIRINOX, they found that the latter group had higher mean operative times (300 minutes for surgery-only patients vs. 393 minutes for FOLFIRINOX-treated patients; P less than .001) and higher mean blood loss (400 mL vs. 600 mL, respectively; P = .01).
However, patients who received neoadjuvant therapy had significantly fewer postoperative complications (63% vs. 35% of patients; P = .004) and fewer pancreatic fistulas (29% vs. 0%; P less than .001).
Patients who received FOLFIRINOX also had a lower proportion of readmissions within 90 days (30% vs. 21%); shorter lengths of stay (7 days vs. 6 days); and no deaths, compared with 1 in the up-front surgery group, but these differences were not significant.
Pathology results showed that 79% of the surgery-only patients had positive lymph nodes, compared with only 37% of those in the neoadjuvant group (P less than .001). The neoadjuvant group also had lower proportions of both lymphatic invasion (70% vs. 37%, P less than .001) and perineural invasion (P less than .001).
There were proportionally more R0 (clean margin) resections in the FOLFIRINOX group, but the difference was not significant.
After a median follow-up of 10 months for the up-front surgery patients and 12 months for the FOLFIRINOX-treated patients, there have been no differences in local or distant progression or deaths from pancreatic cancer. Dr. Ferrone acknowledged that a larger patient sample and longer follow-up may be needed to detect significant differences.
However, Kaplan-Meier overall survival estimates from the time of diagnosis out to 34 months significantly favor the FOLFIRINOX-treated patients (P = .02).
"With this therapeutic modality and intensive surgical interventions, we hope that we are making an impact in these patients’ lives and improving their survival," she said.
The study was internally supported. Dr. Ferrone reported having no financial disclosures.
PHOENIX – Seeing is not always believing when it comes to determining whether pancreatic adenocarcinomas are resectable.
In patients with locally advanced or borderline resectable pancreatic adenocarcinoma who underwent neoadjuvant therapy with the FOLFIRINOX regimen (leucovorin, fluorouracil, irinotecan, and oxaliplatin), presurgical imaging was not a reliable determinant of resectability, said Dr. Cristina R. Ferrone from the department of general and gastrointestinal surgery at Massachusetts General Hospital in Boston.
"It really is a paradigm shift that we can’t rely on CT scans and MRIs the way we did before," she said at the annual Society of Surgical Oncology Cancer Symposium.
An important case
For her team, the paradigm shift was sparked by the case of a 41-year-old woman who presented with a 3.6-cm pancreatic adenocarcinoma involving the superior mesenteric artery (SMA), and a cancer antigen 19-9 (CA 19-9) level of 985 U/mL (normal values for the nonspecific biomarker range from 0 to 37 U/mL, according to National Comprehensive Cancer Network guidelines).
Following 4 months of FOLFIRINOX and 50.4 Gy of chemoradiation, her CA 19-9 level normalized to 37 U/mL. But her postchemotherapy imaging studies appeared identical to her prechemotherapy studies, with no apparent evidence of tumor regression and apparent continued involvement of the SMA following FOLFIRINOX.
"We had a big debate about this woman, because she had a normalized CA 19-9. Do we attempt to resect a patient like this?" Dr. Ferrone said.
After considerable discussion, the decision was made to take the patient into the operating room, approach the pancreas from the left side, and perform intraoperative biopsies on the SMA. If the biopsies were positive, the surgical team would either perform intraoperative radiation therapy (IORT) or use a soft-tissue ablation system (Nanoknife). If the biopsies were negative, the team would proceed to resection.
The patient went on to resection, and was found to have only minimal residual tumor on postoperative pathology samples, Dr. Ferrone reported.
Case comparisons
To see whether other patients could benefit from a similar approach, the investigators compared outcomes for 87 patients who underwent upfront resection for presumed organ-confined disease and 38 patients who received neoadjuvant FOLFIRINOX before surgery. Of the latter group, 5 received FOLFIRINOX chemotherapy only; 16 received FOLFIRINOX and 50.4 Gy of radiation; 10 received FOLFIRINOX, radiation, and IORT; 4 received FOLFIRINOX and proton-beam therapy; and 3 had FOLFIRINOX with other drug therapies.
The median CA 19-9 level fell from 169 U/mL before FOLFIRINOX to 16 U/mL after (P = .003), and more than twice as many patients had levels below 40 U/mL after therapy (31% vs. 72%; P = .03)
Median tumor diameter on CT also decreased after chemotherapy, from 3.6 cm to 2.2 cm (P = .001).
Pathology review of images from 20 cases with the observer blinded to treatment status showed 14 cases judged to be locally advanced disease and 6 borderline pre-FOLFIRINOX, and 12 locally advanced cases, 3 borderline, and 5 cases deemed resectable post-FOLFIRINOX.
When the investigators compared clinical and operative results in the surgery-only patients vs. results in those who had neoadjuvant FOLFIRINOX, they found that the latter group had higher mean operative times (300 minutes for surgery-only patients vs. 393 minutes for FOLFIRINOX-treated patients; P less than .001) and higher mean blood loss (400 mL vs. 600 mL, respectively; P = .01).
However, patients who received neoadjuvant therapy had significantly fewer postoperative complications (63% vs. 35% of patients; P = .004) and fewer pancreatic fistulas (29% vs. 0%; P less than .001).
Patients who received FOLFIRINOX also had a lower proportion of readmissions within 90 days (30% vs. 21%); shorter lengths of stay (7 days vs. 6 days); and no deaths, compared with 1 in the up-front surgery group, but these differences were not significant.
Pathology results showed that 79% of the surgery-only patients had positive lymph nodes, compared with only 37% of those in the neoadjuvant group (P less than .001). The neoadjuvant group also had lower proportions of both lymphatic invasion (70% vs. 37%, P less than .001) and perineural invasion (P less than .001).
There were proportionally more R0 (clean margin) resections in the FOLFIRINOX group, but the difference was not significant.
After a median follow-up of 10 months for the up-front surgery patients and 12 months for the FOLFIRINOX-treated patients, there have been no differences in local or distant progression or deaths from pancreatic cancer. Dr. Ferrone acknowledged that a larger patient sample and longer follow-up may be needed to detect significant differences.
However, Kaplan-Meier overall survival estimates from the time of diagnosis out to 34 months significantly favor the FOLFIRINOX-treated patients (P = .02).
"With this therapeutic modality and intensive surgical interventions, we hope that we are making an impact in these patients’ lives and improving their survival," she said.
The study was internally supported. Dr. Ferrone reported having no financial disclosures.
PHOENIX – Seeing is not always believing when it comes to determining whether pancreatic adenocarcinomas are resectable.
In patients with locally advanced or borderline resectable pancreatic adenocarcinoma who underwent neoadjuvant therapy with the FOLFIRINOX regimen (leucovorin, fluorouracil, irinotecan, and oxaliplatin), presurgical imaging was not a reliable determinant of resectability, said Dr. Cristina R. Ferrone from the department of general and gastrointestinal surgery at Massachusetts General Hospital in Boston.
"It really is a paradigm shift that we can’t rely on CT scans and MRIs the way we did before," she said at the annual Society of Surgical Oncology Cancer Symposium.
An important case
For her team, the paradigm shift was sparked by the case of a 41-year-old woman who presented with a 3.6-cm pancreatic adenocarcinoma involving the superior mesenteric artery (SMA), and a cancer antigen 19-9 (CA 19-9) level of 985 U/mL (normal values for the nonspecific biomarker range from 0 to 37 U/mL, according to National Comprehensive Cancer Network guidelines).
Following 4 months of FOLFIRINOX and 50.4 Gy of chemoradiation, her CA 19-9 level normalized to 37 U/mL. But her postchemotherapy imaging studies appeared identical to her prechemotherapy studies, with no apparent evidence of tumor regression and apparent continued involvement of the SMA following FOLFIRINOX.
"We had a big debate about this woman, because she had a normalized CA 19-9. Do we attempt to resect a patient like this?" Dr. Ferrone said.
After considerable discussion, the decision was made to take the patient into the operating room, approach the pancreas from the left side, and perform intraoperative biopsies on the SMA. If the biopsies were positive, the surgical team would either perform intraoperative radiation therapy (IORT) or use a soft-tissue ablation system (Nanoknife). If the biopsies were negative, the team would proceed to resection.
The patient went on to resection, and was found to have only minimal residual tumor on postoperative pathology samples, Dr. Ferrone reported.
Case comparisons
To see whether other patients could benefit from a similar approach, the investigators compared outcomes for 87 patients who underwent upfront resection for presumed organ-confined disease and 38 patients who received neoadjuvant FOLFIRINOX before surgery. Of the latter group, 5 received FOLFIRINOX chemotherapy only; 16 received FOLFIRINOX and 50.4 Gy of radiation; 10 received FOLFIRINOX, radiation, and IORT; 4 received FOLFIRINOX and proton-beam therapy; and 3 had FOLFIRINOX with other drug therapies.
The median CA 19-9 level fell from 169 U/mL before FOLFIRINOX to 16 U/mL after (P = .003), and more than twice as many patients had levels below 40 U/mL after therapy (31% vs. 72%; P = .03)
Median tumor diameter on CT also decreased after chemotherapy, from 3.6 cm to 2.2 cm (P = .001).
Pathology review of images from 20 cases with the observer blinded to treatment status showed 14 cases judged to be locally advanced disease and 6 borderline pre-FOLFIRINOX, and 12 locally advanced cases, 3 borderline, and 5 cases deemed resectable post-FOLFIRINOX.
When the investigators compared clinical and operative results in the surgery-only patients vs. results in those who had neoadjuvant FOLFIRINOX, they found that the latter group had higher mean operative times (300 minutes for surgery-only patients vs. 393 minutes for FOLFIRINOX-treated patients; P less than .001) and higher mean blood loss (400 mL vs. 600 mL, respectively; P = .01).
However, patients who received neoadjuvant therapy had significantly fewer postoperative complications (63% vs. 35% of patients; P = .004) and fewer pancreatic fistulas (29% vs. 0%; P less than .001).
Patients who received FOLFIRINOX also had a lower proportion of readmissions within 90 days (30% vs. 21%); shorter lengths of stay (7 days vs. 6 days); and no deaths, compared with 1 in the up-front surgery group, but these differences were not significant.
Pathology results showed that 79% of the surgery-only patients had positive lymph nodes, compared with only 37% of those in the neoadjuvant group (P less than .001). The neoadjuvant group also had lower proportions of both lymphatic invasion (70% vs. 37%, P less than .001) and perineural invasion (P less than .001).
There were proportionally more R0 (clean margin) resections in the FOLFIRINOX group, but the difference was not significant.
After a median follow-up of 10 months for the up-front surgery patients and 12 months for the FOLFIRINOX-treated patients, there have been no differences in local or distant progression or deaths from pancreatic cancer. Dr. Ferrone acknowledged that a larger patient sample and longer follow-up may be needed to detect significant differences.
However, Kaplan-Meier overall survival estimates from the time of diagnosis out to 34 months significantly favor the FOLFIRINOX-treated patients (P = .02).
"With this therapeutic modality and intensive surgical interventions, we hope that we are making an impact in these patients’ lives and improving their survival," she said.
The study was internally supported. Dr. Ferrone reported having no financial disclosures.
AT SSO 2014
Major finding: Patients who underwent neoadjuvant FOLFIRINOX therapy before resection of pancreatic adenocarcinomas had significantly better estimated overall survival than patients treated with upfront surgery.
Data source: Case series comparing 87 patients who had upfront resections with 38 who received FOLFIRINOX with or without radiation before/during surgery.
Disclosures: The study was internally supported. Dr. Ferrone reported having no financial disclosures.