User login
ACR: Years of TNF blockers did not increase risk of lymphoma in RA
SAN FRANCISCO – Rheumatoid arthritis conferred a doubling of the risk of lymphoma when compared against the general population in a large Swedish registry study, regardless of the patients’ experience with biological disease-modifying antirheumatic drugs.
But patients who took biological disease-modifying antirheumatic drugs (bDMARDs) had a sixfold greater risk of natural killer or T-cell lymphoma than did the general population, and that association was about four times stronger than for patients who had never taken biologics, reported Dr. Karin Hellgren, who led the study at the Karolinska Institute in Stockholm. That finding in particular shows the need to keep studying the links between bDMARDs and specific lymphoma subtypes, he said.
Severe rheumatoid arthritis seems to strongly increase the risk of lymphoma (Arthritis Rheum. 2006;54[3]:692-701), but researchers have debated whether the reason relates to bDMARDs or RA itself. Clinical trials have produced conflicting results; observational studies have reported no overall link between bDMARDs and lymphoma, but have raised questions about long-term exposure, the effects of individual agents, and lymphoma subtypes, Dr. Hellgren said at the annual meeting of the American College of Rheumatology.
To delve deeper into these issues, he and his associates compared 15 years of data for 13,240 RA patients on bDMARDs from the Swedish Biologics (ARTIS), Patient, and Cancer Registers and a national cohort of 46,568 bDMARD-naive patients. The researchers also compared both groups with 458,846 age- and gender-matched adults from the Swedish Population Register, following individuals until the end of 2012 or until lymphoma diagnosis, death, emigration, or bDMARD initiation, in the case of the naive patients.
Overall, patients with RA averaged one diagnosis of lymphoma per 1,000 population, compared with 0.5 cases per 1,000 for the overall population of Sweden, Dr. Hellgren said. In terms of absolute numbers, there were 241 cases of lymphoma among bDMARD-naive patients, 1,413 cases in the general population, and 69 cases among patients on bDMARDs, including 68 who were taking TNF inhibitors. The average age of the latter group of patients was 57 years. They had been diagnosed with RA at about age 50, had a mean 28-joint Disease Activity Score score of 5.3, and averaged 5.9 years of exposure to TNF inhibitors. Their risk of any type of malignant lymphoma was about 20% higher than for bDMARD-naive patients, but the difference was insignificant overall and in subgroups stratified by gender, age, and year starting treatment. Likewise, although patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), there was no significant difference in risk compared with bDMARD-naive patients.
“There also were no statistically significant differences between drugs,” including infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira), Dr. Hellgren said. “In terms of the newer TNF inhibitors and other biologics, data are still too scarce to evaluate,” he added. Both exposed and bDMARD-naive patients were at especially high risk of Hodgkin lymphoma and diffuse large B-cell lymphoma, compared with the general population, but the strongest association of all was between bDMARD exposure and natural killer or T-cell lymphoma (HR, 6.0; 95% CI, 2.7-13.3). “The distribution of lymphoma subtypes warrants further assessment,” Dr. Hellgren concluded.
The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
SAN FRANCISCO – Rheumatoid arthritis conferred a doubling of the risk of lymphoma when compared against the general population in a large Swedish registry study, regardless of the patients’ experience with biological disease-modifying antirheumatic drugs.
But patients who took biological disease-modifying antirheumatic drugs (bDMARDs) had a sixfold greater risk of natural killer or T-cell lymphoma than did the general population, and that association was about four times stronger than for patients who had never taken biologics, reported Dr. Karin Hellgren, who led the study at the Karolinska Institute in Stockholm. That finding in particular shows the need to keep studying the links between bDMARDs and specific lymphoma subtypes, he said.
Severe rheumatoid arthritis seems to strongly increase the risk of lymphoma (Arthritis Rheum. 2006;54[3]:692-701), but researchers have debated whether the reason relates to bDMARDs or RA itself. Clinical trials have produced conflicting results; observational studies have reported no overall link between bDMARDs and lymphoma, but have raised questions about long-term exposure, the effects of individual agents, and lymphoma subtypes, Dr. Hellgren said at the annual meeting of the American College of Rheumatology.
To delve deeper into these issues, he and his associates compared 15 years of data for 13,240 RA patients on bDMARDs from the Swedish Biologics (ARTIS), Patient, and Cancer Registers and a national cohort of 46,568 bDMARD-naive patients. The researchers also compared both groups with 458,846 age- and gender-matched adults from the Swedish Population Register, following individuals until the end of 2012 or until lymphoma diagnosis, death, emigration, or bDMARD initiation, in the case of the naive patients.
Overall, patients with RA averaged one diagnosis of lymphoma per 1,000 population, compared with 0.5 cases per 1,000 for the overall population of Sweden, Dr. Hellgren said. In terms of absolute numbers, there were 241 cases of lymphoma among bDMARD-naive patients, 1,413 cases in the general population, and 69 cases among patients on bDMARDs, including 68 who were taking TNF inhibitors. The average age of the latter group of patients was 57 years. They had been diagnosed with RA at about age 50, had a mean 28-joint Disease Activity Score score of 5.3, and averaged 5.9 years of exposure to TNF inhibitors. Their risk of any type of malignant lymphoma was about 20% higher than for bDMARD-naive patients, but the difference was insignificant overall and in subgroups stratified by gender, age, and year starting treatment. Likewise, although patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), there was no significant difference in risk compared with bDMARD-naive patients.
“There also were no statistically significant differences between drugs,” including infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira), Dr. Hellgren said. “In terms of the newer TNF inhibitors and other biologics, data are still too scarce to evaluate,” he added. Both exposed and bDMARD-naive patients were at especially high risk of Hodgkin lymphoma and diffuse large B-cell lymphoma, compared with the general population, but the strongest association of all was between bDMARD exposure and natural killer or T-cell lymphoma (HR, 6.0; 95% CI, 2.7-13.3). “The distribution of lymphoma subtypes warrants further assessment,” Dr. Hellgren concluded.
The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
SAN FRANCISCO – Rheumatoid arthritis conferred a doubling of the risk of lymphoma when compared against the general population in a large Swedish registry study, regardless of the patients’ experience with biological disease-modifying antirheumatic drugs.
But patients who took biological disease-modifying antirheumatic drugs (bDMARDs) had a sixfold greater risk of natural killer or T-cell lymphoma than did the general population, and that association was about four times stronger than for patients who had never taken biologics, reported Dr. Karin Hellgren, who led the study at the Karolinska Institute in Stockholm. That finding in particular shows the need to keep studying the links between bDMARDs and specific lymphoma subtypes, he said.
Severe rheumatoid arthritis seems to strongly increase the risk of lymphoma (Arthritis Rheum. 2006;54[3]:692-701), but researchers have debated whether the reason relates to bDMARDs or RA itself. Clinical trials have produced conflicting results; observational studies have reported no overall link between bDMARDs and lymphoma, but have raised questions about long-term exposure, the effects of individual agents, and lymphoma subtypes, Dr. Hellgren said at the annual meeting of the American College of Rheumatology.
To delve deeper into these issues, he and his associates compared 15 years of data for 13,240 RA patients on bDMARDs from the Swedish Biologics (ARTIS), Patient, and Cancer Registers and a national cohort of 46,568 bDMARD-naive patients. The researchers also compared both groups with 458,846 age- and gender-matched adults from the Swedish Population Register, following individuals until the end of 2012 or until lymphoma diagnosis, death, emigration, or bDMARD initiation, in the case of the naive patients.
Overall, patients with RA averaged one diagnosis of lymphoma per 1,000 population, compared with 0.5 cases per 1,000 for the overall population of Sweden, Dr. Hellgren said. In terms of absolute numbers, there were 241 cases of lymphoma among bDMARD-naive patients, 1,413 cases in the general population, and 69 cases among patients on bDMARDs, including 68 who were taking TNF inhibitors. The average age of the latter group of patients was 57 years. They had been diagnosed with RA at about age 50, had a mean 28-joint Disease Activity Score score of 5.3, and averaged 5.9 years of exposure to TNF inhibitors. Their risk of any type of malignant lymphoma was about 20% higher than for bDMARD-naive patients, but the difference was insignificant overall and in subgroups stratified by gender, age, and year starting treatment. Likewise, although patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), there was no significant difference in risk compared with bDMARD-naive patients.
“There also were no statistically significant differences between drugs,” including infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira), Dr. Hellgren said. “In terms of the newer TNF inhibitors and other biologics, data are still too scarce to evaluate,” he added. Both exposed and bDMARD-naive patients were at especially high risk of Hodgkin lymphoma and diffuse large B-cell lymphoma, compared with the general population, but the strongest association of all was between bDMARD exposure and natural killer or T-cell lymphoma (HR, 6.0; 95% CI, 2.7-13.3). “The distribution of lymphoma subtypes warrants further assessment,” Dr. Hellgren concluded.
The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
AT THE ACR ANNUAL MEETING
Key clinical point: Exposure to biological disease-modifying antirheumatic drugs did not increase the overall risk of lymphoma among patients with rheumatoid arthritis.
Major finding: Patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), but there was no significant difference in risk compared with bDMARD-naive patients. However, patients on bDMARDs had about a fourfold greater risk of NK/T-cell lymphoma compared with bDMARD-naive patients.
Data source: A matched registry analysis of 13,240 patients from the Swedish Biologics (ARTIS), Patient, and Cancer Registers; 46,568 bio-naive patients; and 458,846 members of the general population.
Disclosures: The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
What’s on tap at ASH 2015
FROM A TELECONFERENCE – The American Society of Hematology’s (ASH) 57th annual meeting in Orlando is chock-full of much-anticipated results in cancer immunotherapies such as CAR T cell therapies and checkpoint inhibitors, advances in sickle cell disease, and practical advice on managing the latest drugs in the clinic, ASH officials said in a teleconference. Here are some of the day-by-day picks selected by ASH president Dr. David Williams and ASH secretary Dr. Stephanie J. Lee, who gave their recommendations during a conference call for the press. Meeting abstracts are now available online.
Saturday, Dec. 5
Clinical applications of newly approved drugs
The popular special education session on clinical applications of newly approved drugs returns on Saturday, Dec. 5 at 9:30 a.m., with didactic presentations that address issues clinicians may face such as drug-drug interactions, side effects, and adverse events. The three drugs to be discussed this year are: idarucizumab (Praxbind), the first specific reversal agent approved for dabigatran reversal; blinatumomab (Blincyto), approved for second-line treatment of Philadelphia chromosomenegative acute lymphoblastic leukemia; and the histone deacetylase (HDAC) inhibitor panobinostat (Farydak), approved for the treatment of multiple myeloma.
Adoptive immunotherapy
One presentation to look out for next month is abstract 99at 12:30 p.m. on Saturday, Dec. 5 in the adoptive immunotherapy session, Dr. Williams told reporters. The chimeric antigen receptor (CAR)-T-cell approach has relied on genetically engineering the patient’s own T cells to rev up the immune system. This group’s approach is to treat B-cell malignancies after allogeneic hematopoietic stem cell transplantation using a single infusion of anti-CD19 CAR-T cells from the patient’s transplant donor.
Eight of 20 patients treated with this strategy achieved remission, including six complete remissions and two partial remissions. Importantly, none of these patients developed acute graft-versus-host disease, a potential consequence of using allogeneic rather than autologous T cells, he said. The authors also noted that patients who responded and went into remission were marked by higher numbers of these infused CAR-T cells in their circulation, suggesting a biomarker of response.
Checkpoint, please?
Immunotherapy is a “very hot area,” so ASH has put together a special session at 4 p.m. Saturday called “Checkpoint, Please?” Dr. Williams said. Topics include the role of programmed death (PD)-1 and PD-ligand 1 in acute and chronic graft-versus-host disease, checkpoint blockade with neoantigen cancer vaccines, and insights into the mechanisms of action of anti-CTLA-4 (cytotoxic T-lymphocyte–associated protein 4) antibody therapy.
Sunday, Dec. 6
Precision medicine
Sunday’s plenary scientific session will include several noteworthy personalized medicine abstracts featuring emerging therapies targeted to specific genetic subtypes, Dr. Lee, from the University of Washington, Seattle, said.
Plenary abstract 6 is a large, multinational study looking at whether adding the multikinase inhibitor midostaurin to standard induction therapy and carried through 1 year of maintenance would improve outcomes in newly diagnosed acute myeloid leukemia with FLT3 mutations. Patients with these deleterious mutations do enter remission with chemotherapy, but often relapse.
Overall and event-free survival were better at 5 years by about 7% to 8% in the experimental arm using midostaurin, she said. Caveats are that complete response rates were similar in both arms and lower than reported in other trials.
“Because we know that patients with this FLT3 mutation have a very poor prognosis with standard chemotherapy, more than half of the patients in this trial received an allogeneic transplant,” Dr. Lee noted. “But the abstract does say that the results are similar if you censor at the time of the transplant.”
In this same vein of precision medicine is plenary abstract 1, testing whether adding rituximab to standard chemotherapy improves outcomes in adults with CD-20–positive, Philadelphia chromosome–negative, B-cell precursor acute lymphoblastic leukemia (ALL). Rituximab (Rituxan) binds to CD-20, which is found in about 30% to 50% of adult B-cell ALL, she said.
At 2 years, patients treated with rituximab had longer event-free survival than controls (65% vs. 52%; P = .038), but similar overall survival (71% vs. 64%; P = .09), according to the abstract. The rituximab arm also received more allogeneic transplants, but again, after censoring the data, the abstract states that both event-free and overall survival were longer with rituximab, Dr. Lee said.
Sickle cell anemia
Sunday’s plenary session will also feature the very important TWiTCH (TCD with Transfusions Changing to Hydroxyurea) study evaluating hydroxyurea therapy as an alternative to chronic blood transfusions to prevent stroke. Stroke is one of the most dreaded complications of sickle cell disease, occurring in up to 10% of children, Dr. Williams said. Though transfusions are effective, they have to be continued indefinitely and lead to iron overload. Hydroxyurea increases the amount of fetal hemoglobin and fetal red blood cells and has become a standard therapy to attenuate the complications of sickle cell.
The phase III noninferiority study, which used Transcranial Doppler (TCD) screening to identify children at elevated risk for stroke, showed that hydroxyurea “was as good as current therapy with red cell transfusions and there was some indication, although not significant, that it might even be superior in lowering the TCD levels,” Dr. Williams said. An added benefit of the hydroxyurea was that it improved the patients’ iron overload status. There were no strokes in either group.
Sunday’s abstract 202 is another presentation “that I’m sure will get a lot of attention,” Dr. Williams said. It offers updated details on outcomes from patients with sickle cell disease (SCD) treated with a novel gene therapy transduced with the LentiGlobin BB305 (Bluebird Bio) lentiviral vector. Patients with beta thalassemia major have remained transfusion-independent for more than a year after this treatment, with results now available from four patients with SCD. One patient with a severe phenotype has had no sickle cell complications and has been able to stop his transfusion therapy, while two of the other four patients are also transfusion-independent.
“This is an early study showing what appears to be efficacy of the gene therapy approach not in thalassemia, but in sickle cell disease,” Dr. Williams said, noting that abstract 3233 will also feature results using LentiGlobin gene therapy in severe SCD.
ASH/EHA joint symposium
Also noteworthy is a special joint ASH/European Hematology Association symposium looking at how well genomic data are being incorporated into practice in the U.S. and Europe.
Monday, Dec. 7
ASH/FDA joint symposium
A joint ASH/FDA symposium on late-breaking drug approvals is new this year and features drugs that gained approval in November 2015. FDA product-reviewers will discuss safety and efficacy issues in the clinical approval trials and toxicity studies, while clinicians will share their experiences in the real-world use of these drugs.
“This is information that is really going to be very hot off the press and presented in conjunction with the FDA,” Dr. Lee said.
Dr. Williams reported research funding from Bluebird Bio. Dr. Lee reported having no conflicts of interest.
FROM A TELECONFERENCE – The American Society of Hematology’s (ASH) 57th annual meeting in Orlando is chock-full of much-anticipated results in cancer immunotherapies such as CAR T cell therapies and checkpoint inhibitors, advances in sickle cell disease, and practical advice on managing the latest drugs in the clinic, ASH officials said in a teleconference. Here are some of the day-by-day picks selected by ASH president Dr. David Williams and ASH secretary Dr. Stephanie J. Lee, who gave their recommendations during a conference call for the press. Meeting abstracts are now available online.
Saturday, Dec. 5
Clinical applications of newly approved drugs
The popular special education session on clinical applications of newly approved drugs returns on Saturday, Dec. 5 at 9:30 a.m., with didactic presentations that address issues clinicians may face such as drug-drug interactions, side effects, and adverse events. The three drugs to be discussed this year are: idarucizumab (Praxbind), the first specific reversal agent approved for dabigatran reversal; blinatumomab (Blincyto), approved for second-line treatment of Philadelphia chromosomenegative acute lymphoblastic leukemia; and the histone deacetylase (HDAC) inhibitor panobinostat (Farydak), approved for the treatment of multiple myeloma.
Adoptive immunotherapy
One presentation to look out for next month is abstract 99at 12:30 p.m. on Saturday, Dec. 5 in the adoptive immunotherapy session, Dr. Williams told reporters. The chimeric antigen receptor (CAR)-T-cell approach has relied on genetically engineering the patient’s own T cells to rev up the immune system. This group’s approach is to treat B-cell malignancies after allogeneic hematopoietic stem cell transplantation using a single infusion of anti-CD19 CAR-T cells from the patient’s transplant donor.
Eight of 20 patients treated with this strategy achieved remission, including six complete remissions and two partial remissions. Importantly, none of these patients developed acute graft-versus-host disease, a potential consequence of using allogeneic rather than autologous T cells, he said. The authors also noted that patients who responded and went into remission were marked by higher numbers of these infused CAR-T cells in their circulation, suggesting a biomarker of response.
Checkpoint, please?
Immunotherapy is a “very hot area,” so ASH has put together a special session at 4 p.m. Saturday called “Checkpoint, Please?” Dr. Williams said. Topics include the role of programmed death (PD)-1 and PD-ligand 1 in acute and chronic graft-versus-host disease, checkpoint blockade with neoantigen cancer vaccines, and insights into the mechanisms of action of anti-CTLA-4 (cytotoxic T-lymphocyte–associated protein 4) antibody therapy.
Sunday, Dec. 6
Precision medicine
Sunday’s plenary scientific session will include several noteworthy personalized medicine abstracts featuring emerging therapies targeted to specific genetic subtypes, Dr. Lee, from the University of Washington, Seattle, said.
Plenary abstract 6 is a large, multinational study looking at whether adding the multikinase inhibitor midostaurin to standard induction therapy and carried through 1 year of maintenance would improve outcomes in newly diagnosed acute myeloid leukemia with FLT3 mutations. Patients with these deleterious mutations do enter remission with chemotherapy, but often relapse.
Overall and event-free survival were better at 5 years by about 7% to 8% in the experimental arm using midostaurin, she said. Caveats are that complete response rates were similar in both arms and lower than reported in other trials.
“Because we know that patients with this FLT3 mutation have a very poor prognosis with standard chemotherapy, more than half of the patients in this trial received an allogeneic transplant,” Dr. Lee noted. “But the abstract does say that the results are similar if you censor at the time of the transplant.”
In this same vein of precision medicine is plenary abstract 1, testing whether adding rituximab to standard chemotherapy improves outcomes in adults with CD-20–positive, Philadelphia chromosome–negative, B-cell precursor acute lymphoblastic leukemia (ALL). Rituximab (Rituxan) binds to CD-20, which is found in about 30% to 50% of adult B-cell ALL, she said.
At 2 years, patients treated with rituximab had longer event-free survival than controls (65% vs. 52%; P = .038), but similar overall survival (71% vs. 64%; P = .09), according to the abstract. The rituximab arm also received more allogeneic transplants, but again, after censoring the data, the abstract states that both event-free and overall survival were longer with rituximab, Dr. Lee said.
Sickle cell anemia
Sunday’s plenary session will also feature the very important TWiTCH (TCD with Transfusions Changing to Hydroxyurea) study evaluating hydroxyurea therapy as an alternative to chronic blood transfusions to prevent stroke. Stroke is one of the most dreaded complications of sickle cell disease, occurring in up to 10% of children, Dr. Williams said. Though transfusions are effective, they have to be continued indefinitely and lead to iron overload. Hydroxyurea increases the amount of fetal hemoglobin and fetal red blood cells and has become a standard therapy to attenuate the complications of sickle cell.
The phase III noninferiority study, which used Transcranial Doppler (TCD) screening to identify children at elevated risk for stroke, showed that hydroxyurea “was as good as current therapy with red cell transfusions and there was some indication, although not significant, that it might even be superior in lowering the TCD levels,” Dr. Williams said. An added benefit of the hydroxyurea was that it improved the patients’ iron overload status. There were no strokes in either group.
Sunday’s abstract 202 is another presentation “that I’m sure will get a lot of attention,” Dr. Williams said. It offers updated details on outcomes from patients with sickle cell disease (SCD) treated with a novel gene therapy transduced with the LentiGlobin BB305 (Bluebird Bio) lentiviral vector. Patients with beta thalassemia major have remained transfusion-independent for more than a year after this treatment, with results now available from four patients with SCD. One patient with a severe phenotype has had no sickle cell complications and has been able to stop his transfusion therapy, while two of the other four patients are also transfusion-independent.
“This is an early study showing what appears to be efficacy of the gene therapy approach not in thalassemia, but in sickle cell disease,” Dr. Williams said, noting that abstract 3233 will also feature results using LentiGlobin gene therapy in severe SCD.
ASH/EHA joint symposium
Also noteworthy is a special joint ASH/European Hematology Association symposium looking at how well genomic data are being incorporated into practice in the U.S. and Europe.
Monday, Dec. 7
ASH/FDA joint symposium
A joint ASH/FDA symposium on late-breaking drug approvals is new this year and features drugs that gained approval in November 2015. FDA product-reviewers will discuss safety and efficacy issues in the clinical approval trials and toxicity studies, while clinicians will share their experiences in the real-world use of these drugs.
“This is information that is really going to be very hot off the press and presented in conjunction with the FDA,” Dr. Lee said.
Dr. Williams reported research funding from Bluebird Bio. Dr. Lee reported having no conflicts of interest.
FROM A TELECONFERENCE – The American Society of Hematology’s (ASH) 57th annual meeting in Orlando is chock-full of much-anticipated results in cancer immunotherapies such as CAR T cell therapies and checkpoint inhibitors, advances in sickle cell disease, and practical advice on managing the latest drugs in the clinic, ASH officials said in a teleconference. Here are some of the day-by-day picks selected by ASH president Dr. David Williams and ASH secretary Dr. Stephanie J. Lee, who gave their recommendations during a conference call for the press. Meeting abstracts are now available online.
Saturday, Dec. 5
Clinical applications of newly approved drugs
The popular special education session on clinical applications of newly approved drugs returns on Saturday, Dec. 5 at 9:30 a.m., with didactic presentations that address issues clinicians may face such as drug-drug interactions, side effects, and adverse events. The three drugs to be discussed this year are: idarucizumab (Praxbind), the first specific reversal agent approved for dabigatran reversal; blinatumomab (Blincyto), approved for second-line treatment of Philadelphia chromosomenegative acute lymphoblastic leukemia; and the histone deacetylase (HDAC) inhibitor panobinostat (Farydak), approved for the treatment of multiple myeloma.
Adoptive immunotherapy
One presentation to look out for next month is abstract 99at 12:30 p.m. on Saturday, Dec. 5 in the adoptive immunotherapy session, Dr. Williams told reporters. The chimeric antigen receptor (CAR)-T-cell approach has relied on genetically engineering the patient’s own T cells to rev up the immune system. This group’s approach is to treat B-cell malignancies after allogeneic hematopoietic stem cell transplantation using a single infusion of anti-CD19 CAR-T cells from the patient’s transplant donor.
Eight of 20 patients treated with this strategy achieved remission, including six complete remissions and two partial remissions. Importantly, none of these patients developed acute graft-versus-host disease, a potential consequence of using allogeneic rather than autologous T cells, he said. The authors also noted that patients who responded and went into remission were marked by higher numbers of these infused CAR-T cells in their circulation, suggesting a biomarker of response.
Checkpoint, please?
Immunotherapy is a “very hot area,” so ASH has put together a special session at 4 p.m. Saturday called “Checkpoint, Please?” Dr. Williams said. Topics include the role of programmed death (PD)-1 and PD-ligand 1 in acute and chronic graft-versus-host disease, checkpoint blockade with neoantigen cancer vaccines, and insights into the mechanisms of action of anti-CTLA-4 (cytotoxic T-lymphocyte–associated protein 4) antibody therapy.
Sunday, Dec. 6
Precision medicine
Sunday’s plenary scientific session will include several noteworthy personalized medicine abstracts featuring emerging therapies targeted to specific genetic subtypes, Dr. Lee, from the University of Washington, Seattle, said.
Plenary abstract 6 is a large, multinational study looking at whether adding the multikinase inhibitor midostaurin to standard induction therapy and carried through 1 year of maintenance would improve outcomes in newly diagnosed acute myeloid leukemia with FLT3 mutations. Patients with these deleterious mutations do enter remission with chemotherapy, but often relapse.
Overall and event-free survival were better at 5 years by about 7% to 8% in the experimental arm using midostaurin, she said. Caveats are that complete response rates were similar in both arms and lower than reported in other trials.
“Because we know that patients with this FLT3 mutation have a very poor prognosis with standard chemotherapy, more than half of the patients in this trial received an allogeneic transplant,” Dr. Lee noted. “But the abstract does say that the results are similar if you censor at the time of the transplant.”
In this same vein of precision medicine is plenary abstract 1, testing whether adding rituximab to standard chemotherapy improves outcomes in adults with CD-20–positive, Philadelphia chromosome–negative, B-cell precursor acute lymphoblastic leukemia (ALL). Rituximab (Rituxan) binds to CD-20, which is found in about 30% to 50% of adult B-cell ALL, she said.
At 2 years, patients treated with rituximab had longer event-free survival than controls (65% vs. 52%; P = .038), but similar overall survival (71% vs. 64%; P = .09), according to the abstract. The rituximab arm also received more allogeneic transplants, but again, after censoring the data, the abstract states that both event-free and overall survival were longer with rituximab, Dr. Lee said.
Sickle cell anemia
Sunday’s plenary session will also feature the very important TWiTCH (TCD with Transfusions Changing to Hydroxyurea) study evaluating hydroxyurea therapy as an alternative to chronic blood transfusions to prevent stroke. Stroke is one of the most dreaded complications of sickle cell disease, occurring in up to 10% of children, Dr. Williams said. Though transfusions are effective, they have to be continued indefinitely and lead to iron overload. Hydroxyurea increases the amount of fetal hemoglobin and fetal red blood cells and has become a standard therapy to attenuate the complications of sickle cell.
The phase III noninferiority study, which used Transcranial Doppler (TCD) screening to identify children at elevated risk for stroke, showed that hydroxyurea “was as good as current therapy with red cell transfusions and there was some indication, although not significant, that it might even be superior in lowering the TCD levels,” Dr. Williams said. An added benefit of the hydroxyurea was that it improved the patients’ iron overload status. There were no strokes in either group.
Sunday’s abstract 202 is another presentation “that I’m sure will get a lot of attention,” Dr. Williams said. It offers updated details on outcomes from patients with sickle cell disease (SCD) treated with a novel gene therapy transduced with the LentiGlobin BB305 (Bluebird Bio) lentiviral vector. Patients with beta thalassemia major have remained transfusion-independent for more than a year after this treatment, with results now available from four patients with SCD. One patient with a severe phenotype has had no sickle cell complications and has been able to stop his transfusion therapy, while two of the other four patients are also transfusion-independent.
“This is an early study showing what appears to be efficacy of the gene therapy approach not in thalassemia, but in sickle cell disease,” Dr. Williams said, noting that abstract 3233 will also feature results using LentiGlobin gene therapy in severe SCD.
ASH/EHA joint symposium
Also noteworthy is a special joint ASH/European Hematology Association symposium looking at how well genomic data are being incorporated into practice in the U.S. and Europe.
Monday, Dec. 7
ASH/FDA joint symposium
A joint ASH/FDA symposium on late-breaking drug approvals is new this year and features drugs that gained approval in November 2015. FDA product-reviewers will discuss safety and efficacy issues in the clinical approval trials and toxicity studies, while clinicians will share their experiences in the real-world use of these drugs.
“This is information that is really going to be very hot off the press and presented in conjunction with the FDA,” Dr. Lee said.
Dr. Williams reported research funding from Bluebird Bio. Dr. Lee reported having no conflicts of interest.
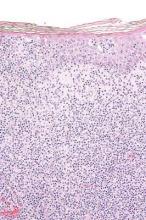
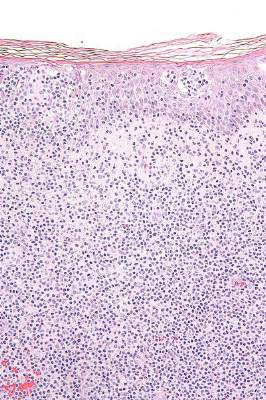
Topical resiquimod effective for early-stage cutaneous T-cell lymphoma
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
FROM BLOOD
Key clinical point: Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL).
Major finding: In total, 11 of 12 patients had significant improvement: 2 had resolution of all evidence of disease, and 9 experienced improvement greater than or equal to 50%.
Data source: The open-label, phase I trial evaluated 12 patients with early stage CTCL.
Disclosures: Dr. Rook and one coauthor have patents pending on HTS in cutaneous lymphoma. His coauthor is employed by Adaptive Biotechnologies.
Immunotherapy: Inject locally, treat globally?
NEW YORK – “Inject locally, treat globally” may become a new mantra for cancer immunotherapy. Dr. Ronald Levy, professor and chief of the department of oncology at Stanford (Calif.) University, discussed impressive and durable systemic results from local treatment of tumors, reviewing his group’s recent work and discussing ongoing early stage clinical trials.
In the hope of triggering an immune response that induces a systemic CD8 T-cell response, Dr. Levy and other investigators began experimenting with intralesional injections of immunotherapies, with and without adjunctive radiation or chemotherapy. The principle has been evaluated in a mouse model, in which up to 80% of mice receiving intratumoral immunotherapy were cured. Now, human trials are underway for solid tumors as well as lymphoma.
An early clinical trial involving 15 patients with recurrent low-grade B-cell lymphoma combined targeted low-dose radiation of a single tumor site with injection of CpG, immune-boosting snippets of DNA, into the same site. Looking for partial or complete regression, investigators saw promising results, though some patients who were responders didn’t see full effect until 24 weeks after injection (J Clin Oncol. 2010 Oct 1;28[28]:4324-32). The overall objective response rate was 27%, although 80% of patients (12 of 15) had stable disease or partial or complete response through a median follow-up of 33.7 months. Some individual patients had marked regression or disappearance of bulky tumors at distant sites.
CpG – motifs of cytosines and guanines – were strung together, said Dr. Levy, with a sulfur rather than a phosphate backbone to make them more stable for injection. These bits of DNA, which are present in both bacteria and vertebrates, are an agonist for toll-like receptor 9, activating B cells and dendritic cells, and then tumor-specific T-cells.
Dr. Levy and his collaborators used a two-tumor mouse model to track intralesional injection effectiveness for a variety of immunotherapies. Mice seeded with tumor cells bilaterally over the abdomen were injected with CpG and two other immune therapies at a single abdominal tumor site, and bilateral regression, if any, was tracked in comparison to systemic immunotherapy (J Clin Invest. 2013;123[6]:2447-63).
Though both treatment arms had good initial response, 70% of the systemically treated mice relapsed by 150 days after injection, compared with just 10% of those receiving intratumoral therapy (P = .002).
Of the 23 mice whose therapy consisted of intratumoral administration of CpG together with anti-CTLA4 and anti-OX40 antibodies (aCTLA4 and aOX40), 21 (91%) were alive 50 days after treatment. These mice fared better than did those receiving any other intratumoral treatment combination (P = .004, compared with CpG+aOX40; P = .03, compared with aCTLA4), suggesting a synergistic benefit to the triple combination.
Somewhat surprisingly, even murine models that also had tumor seeding into brain tissue saw marked reduction or even resolution of brain tumors, showing that the blood-brain barrier does not impede the effect within the CNS.
What didn’t work? PD-1 inhibitors were not particularly effective at provoking a systemic effect when injected into tumors. Peritumoral injection, though theoretically taking advantage of some aspects of the tumor microenvironment, also did not show global effect.
Based on the human CpG + radiation trials and the mouse experiments, the research group is proceeding with phase I and II clinical trials of CpG in combinations with aCTLA4 and aOX40. These, Dr. Levy said, were more likely to be available clinically, and human intratumoral T regulatory cells are known to express both CTLA4 and OX40.
All agents and combinations had an enhancing effect, said Dr. Levy. “We like this treatment and trial design,” he said, noting that everyone sees benefit at the local injection site, and some see global results.
Dr. Levy discussed multiple studies, and he disclosed receiving grant support from Pfizer, Dynavax, and Bristol-Myers Squibb. He also has served as a consultant to Five Prime, Kite, BeiGene, Innate Pharma, Bullet Biotech, and Immune Design.
NEW YORK – “Inject locally, treat globally” may become a new mantra for cancer immunotherapy. Dr. Ronald Levy, professor and chief of the department of oncology at Stanford (Calif.) University, discussed impressive and durable systemic results from local treatment of tumors, reviewing his group’s recent work and discussing ongoing early stage clinical trials.
In the hope of triggering an immune response that induces a systemic CD8 T-cell response, Dr. Levy and other investigators began experimenting with intralesional injections of immunotherapies, with and without adjunctive radiation or chemotherapy. The principle has been evaluated in a mouse model, in which up to 80% of mice receiving intratumoral immunotherapy were cured. Now, human trials are underway for solid tumors as well as lymphoma.
An early clinical trial involving 15 patients with recurrent low-grade B-cell lymphoma combined targeted low-dose radiation of a single tumor site with injection of CpG, immune-boosting snippets of DNA, into the same site. Looking for partial or complete regression, investigators saw promising results, though some patients who were responders didn’t see full effect until 24 weeks after injection (J Clin Oncol. 2010 Oct 1;28[28]:4324-32). The overall objective response rate was 27%, although 80% of patients (12 of 15) had stable disease or partial or complete response through a median follow-up of 33.7 months. Some individual patients had marked regression or disappearance of bulky tumors at distant sites.
CpG – motifs of cytosines and guanines – were strung together, said Dr. Levy, with a sulfur rather than a phosphate backbone to make them more stable for injection. These bits of DNA, which are present in both bacteria and vertebrates, are an agonist for toll-like receptor 9, activating B cells and dendritic cells, and then tumor-specific T-cells.
Dr. Levy and his collaborators used a two-tumor mouse model to track intralesional injection effectiveness for a variety of immunotherapies. Mice seeded with tumor cells bilaterally over the abdomen were injected with CpG and two other immune therapies at a single abdominal tumor site, and bilateral regression, if any, was tracked in comparison to systemic immunotherapy (J Clin Invest. 2013;123[6]:2447-63).
Though both treatment arms had good initial response, 70% of the systemically treated mice relapsed by 150 days after injection, compared with just 10% of those receiving intratumoral therapy (P = .002).
Of the 23 mice whose therapy consisted of intratumoral administration of CpG together with anti-CTLA4 and anti-OX40 antibodies (aCTLA4 and aOX40), 21 (91%) were alive 50 days after treatment. These mice fared better than did those receiving any other intratumoral treatment combination (P = .004, compared with CpG+aOX40; P = .03, compared with aCTLA4), suggesting a synergistic benefit to the triple combination.
Somewhat surprisingly, even murine models that also had tumor seeding into brain tissue saw marked reduction or even resolution of brain tumors, showing that the blood-brain barrier does not impede the effect within the CNS.
What didn’t work? PD-1 inhibitors were not particularly effective at provoking a systemic effect when injected into tumors. Peritumoral injection, though theoretically taking advantage of some aspects of the tumor microenvironment, also did not show global effect.
Based on the human CpG + radiation trials and the mouse experiments, the research group is proceeding with phase I and II clinical trials of CpG in combinations with aCTLA4 and aOX40. These, Dr. Levy said, were more likely to be available clinically, and human intratumoral T regulatory cells are known to express both CTLA4 and OX40.
All agents and combinations had an enhancing effect, said Dr. Levy. “We like this treatment and trial design,” he said, noting that everyone sees benefit at the local injection site, and some see global results.
Dr. Levy discussed multiple studies, and he disclosed receiving grant support from Pfizer, Dynavax, and Bristol-Myers Squibb. He also has served as a consultant to Five Prime, Kite, BeiGene, Innate Pharma, Bullet Biotech, and Immune Design.
NEW YORK – “Inject locally, treat globally” may become a new mantra for cancer immunotherapy. Dr. Ronald Levy, professor and chief of the department of oncology at Stanford (Calif.) University, discussed impressive and durable systemic results from local treatment of tumors, reviewing his group’s recent work and discussing ongoing early stage clinical trials.
In the hope of triggering an immune response that induces a systemic CD8 T-cell response, Dr. Levy and other investigators began experimenting with intralesional injections of immunotherapies, with and without adjunctive radiation or chemotherapy. The principle has been evaluated in a mouse model, in which up to 80% of mice receiving intratumoral immunotherapy were cured. Now, human trials are underway for solid tumors as well as lymphoma.
An early clinical trial involving 15 patients with recurrent low-grade B-cell lymphoma combined targeted low-dose radiation of a single tumor site with injection of CpG, immune-boosting snippets of DNA, into the same site. Looking for partial or complete regression, investigators saw promising results, though some patients who were responders didn’t see full effect until 24 weeks after injection (J Clin Oncol. 2010 Oct 1;28[28]:4324-32). The overall objective response rate was 27%, although 80% of patients (12 of 15) had stable disease or partial or complete response through a median follow-up of 33.7 months. Some individual patients had marked regression or disappearance of bulky tumors at distant sites.
CpG – motifs of cytosines and guanines – were strung together, said Dr. Levy, with a sulfur rather than a phosphate backbone to make them more stable for injection. These bits of DNA, which are present in both bacteria and vertebrates, are an agonist for toll-like receptor 9, activating B cells and dendritic cells, and then tumor-specific T-cells.
Dr. Levy and his collaborators used a two-tumor mouse model to track intralesional injection effectiveness for a variety of immunotherapies. Mice seeded with tumor cells bilaterally over the abdomen were injected with CpG and two other immune therapies at a single abdominal tumor site, and bilateral regression, if any, was tracked in comparison to systemic immunotherapy (J Clin Invest. 2013;123[6]:2447-63).
Though both treatment arms had good initial response, 70% of the systemically treated mice relapsed by 150 days after injection, compared with just 10% of those receiving intratumoral therapy (P = .002).
Of the 23 mice whose therapy consisted of intratumoral administration of CpG together with anti-CTLA4 and anti-OX40 antibodies (aCTLA4 and aOX40), 21 (91%) were alive 50 days after treatment. These mice fared better than did those receiving any other intratumoral treatment combination (P = .004, compared with CpG+aOX40; P = .03, compared with aCTLA4), suggesting a synergistic benefit to the triple combination.
Somewhat surprisingly, even murine models that also had tumor seeding into brain tissue saw marked reduction or even resolution of brain tumors, showing that the blood-brain barrier does not impede the effect within the CNS.
What didn’t work? PD-1 inhibitors were not particularly effective at provoking a systemic effect when injected into tumors. Peritumoral injection, though theoretically taking advantage of some aspects of the tumor microenvironment, also did not show global effect.
Based on the human CpG + radiation trials and the mouse experiments, the research group is proceeding with phase I and II clinical trials of CpG in combinations with aCTLA4 and aOX40. These, Dr. Levy said, were more likely to be available clinically, and human intratumoral T regulatory cells are known to express both CTLA4 and OX40.
All agents and combinations had an enhancing effect, said Dr. Levy. “We like this treatment and trial design,” he said, noting that everyone sees benefit at the local injection site, and some see global results.
Dr. Levy discussed multiple studies, and he disclosed receiving grant support from Pfizer, Dynavax, and Bristol-Myers Squibb. He also has served as a consultant to Five Prime, Kite, BeiGene, Innate Pharma, Bullet Biotech, and Immune Design.
EXPERT ANALYSIS FROM THE FIRST INTERNATIONAL CANCER IMMUNOTHERAPY CONFERENCE
No signal for the superiority of autologous versus allogenic stem-cell transplants in T-cell lymphoma
A randomized trial designed to compare autologous to allogeneic stem cell transplantation as first-line therapy in younger patients with peripheral T-cell lymphoma was discontinued early because nearly 40% of the patients had early disease progression and did not undergo transplantation.
Peripheral T-cell lymphoma generally yields a poor prognosis when treated with conventional chemotherapy, but autologous or allogeneic stem cell transplants were thought to be an option for patients with relapsing or refractory disease. Based on this hypothesis, the AATT (Autologous or Allogeneic Transplantation in T-Cell Lymphoma) study explored stem cell transplant as a first-line therapy, enrolling 104 patients aged 18-60 between 2011 and 2014.
All patients received four courses of chemotherapy with CHOEP-14 (cyclophosphamide, adriamycin, vincristine, etoposide, and prednisone).
Those in the autologous stem cell group and those without a suitable donor proceeded to one course of DHAP (high-dose ara-C, cis-platinum, and dexamethasone) and stem cell collection. Patients randomized to autologous transplantation received high dose therapy (BCNU, etoposide, cytarabine, melphalan: BEAM) followed 4-6 weeks later by transplantation of autologous stem cells.
Patients randomized to allogeneic transplantation received high dose therapy (fludarabine, busulfan, cyclophosphamide: FBC) followed by transplantation of allogeneic stem cells. GvHD prophylaxis included antithymocyte globuline (ATG), cyclosporine A, and mycophenolate mofetil.
Among the 58 patients eligible for the interim analysis, the mean age was 50 and 64% were male. Thirteen of the 28 patients randomized for allogeneic transplants underwent transplants; the others were not allografted because of progressive disease or lack of a donor. Of the 30 patients randomized to autologous SCT, 19 had the procedure; 11 did not receive transplants because of progressive disease or infection, Dr. Norbert Schmitz of Asklepios Klinik St. Georg, Hamburg, Germany, reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
The primary outcome, 1-year event-free survival (EFS), was 41% in the intent-to-treat population (95% CI, 27%–54%).
Causes of death included lymphoma (seven autologous, five allogeneic), salvage therapy (two), early or late infections (four), and graft vs. host disease (two).
Survival rates did not significantly differ in the two stem cell transplant groups, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedure. Based on the low probability of meeting the primary outcome, the data safety monitoring board decided to stop patient accrual and discontinue the trial.
*This article was updated 7/8/2015.
As outcomes for patients with PTCL are suboptimal with standard therapy, usually CHOP/CHOEP, young and fit patients are commonly offered high dose chemotherapy with stem cell support (SCT) to consolidate 1st remission, though no firm data support this approach. As a trial of SCT vs observation would be difficult to accomplish, the AATT trail was undertaken to compare autologous vs allogeneic transplantation. The trial was not able to answer this question as it was halted early due to the high proportion of patients unable to proceed to SCT. One lesson here is that data reported for PTCL patients who receive SCT in 1st remission suffers from selection bias, unless accompanied by an intent-to-treat analysis. There is a clear need for improved induction therapy for PTCL.
As outcomes for patients with PTCL are suboptimal with standard therapy, usually CHOP/CHOEP, young and fit patients are commonly offered high dose chemotherapy with stem cell support (SCT) to consolidate 1st remission, though no firm data support this approach. As a trial of SCT vs observation would be difficult to accomplish, the AATT trail was undertaken to compare autologous vs allogeneic transplantation. The trial was not able to answer this question as it was halted early due to the high proportion of patients unable to proceed to SCT. One lesson here is that data reported for PTCL patients who receive SCT in 1st remission suffers from selection bias, unless accompanied by an intent-to-treat analysis. There is a clear need for improved induction therapy for PTCL.
As outcomes for patients with PTCL are suboptimal with standard therapy, usually CHOP/CHOEP, young and fit patients are commonly offered high dose chemotherapy with stem cell support (SCT) to consolidate 1st remission, though no firm data support this approach. As a trial of SCT vs observation would be difficult to accomplish, the AATT trail was undertaken to compare autologous vs allogeneic transplantation. The trial was not able to answer this question as it was halted early due to the high proportion of patients unable to proceed to SCT. One lesson here is that data reported for PTCL patients who receive SCT in 1st remission suffers from selection bias, unless accompanied by an intent-to-treat analysis. There is a clear need for improved induction therapy for PTCL.
A randomized trial designed to compare autologous to allogeneic stem cell transplantation as first-line therapy in younger patients with peripheral T-cell lymphoma was discontinued early because nearly 40% of the patients had early disease progression and did not undergo transplantation.
Peripheral T-cell lymphoma generally yields a poor prognosis when treated with conventional chemotherapy, but autologous or allogeneic stem cell transplants were thought to be an option for patients with relapsing or refractory disease. Based on this hypothesis, the AATT (Autologous or Allogeneic Transplantation in T-Cell Lymphoma) study explored stem cell transplant as a first-line therapy, enrolling 104 patients aged 18-60 between 2011 and 2014.
All patients received four courses of chemotherapy with CHOEP-14 (cyclophosphamide, adriamycin, vincristine, etoposide, and prednisone).
Those in the autologous stem cell group and those without a suitable donor proceeded to one course of DHAP (high-dose ara-C, cis-platinum, and dexamethasone) and stem cell collection. Patients randomized to autologous transplantation received high dose therapy (BCNU, etoposide, cytarabine, melphalan: BEAM) followed 4-6 weeks later by transplantation of autologous stem cells.
Patients randomized to allogeneic transplantation received high dose therapy (fludarabine, busulfan, cyclophosphamide: FBC) followed by transplantation of allogeneic stem cells. GvHD prophylaxis included antithymocyte globuline (ATG), cyclosporine A, and mycophenolate mofetil.
Among the 58 patients eligible for the interim analysis, the mean age was 50 and 64% were male. Thirteen of the 28 patients randomized for allogeneic transplants underwent transplants; the others were not allografted because of progressive disease or lack of a donor. Of the 30 patients randomized to autologous SCT, 19 had the procedure; 11 did not receive transplants because of progressive disease or infection, Dr. Norbert Schmitz of Asklepios Klinik St. Georg, Hamburg, Germany, reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
The primary outcome, 1-year event-free survival (EFS), was 41% in the intent-to-treat population (95% CI, 27%–54%).
Causes of death included lymphoma (seven autologous, five allogeneic), salvage therapy (two), early or late infections (four), and graft vs. host disease (two).
Survival rates did not significantly differ in the two stem cell transplant groups, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedure. Based on the low probability of meeting the primary outcome, the data safety monitoring board decided to stop patient accrual and discontinue the trial.
*This article was updated 7/8/2015.
A randomized trial designed to compare autologous to allogeneic stem cell transplantation as first-line therapy in younger patients with peripheral T-cell lymphoma was discontinued early because nearly 40% of the patients had early disease progression and did not undergo transplantation.
Peripheral T-cell lymphoma generally yields a poor prognosis when treated with conventional chemotherapy, but autologous or allogeneic stem cell transplants were thought to be an option for patients with relapsing or refractory disease. Based on this hypothesis, the AATT (Autologous or Allogeneic Transplantation in T-Cell Lymphoma) study explored stem cell transplant as a first-line therapy, enrolling 104 patients aged 18-60 between 2011 and 2014.
All patients received four courses of chemotherapy with CHOEP-14 (cyclophosphamide, adriamycin, vincristine, etoposide, and prednisone).
Those in the autologous stem cell group and those without a suitable donor proceeded to one course of DHAP (high-dose ara-C, cis-platinum, and dexamethasone) and stem cell collection. Patients randomized to autologous transplantation received high dose therapy (BCNU, etoposide, cytarabine, melphalan: BEAM) followed 4-6 weeks later by transplantation of autologous stem cells.
Patients randomized to allogeneic transplantation received high dose therapy (fludarabine, busulfan, cyclophosphamide: FBC) followed by transplantation of allogeneic stem cells. GvHD prophylaxis included antithymocyte globuline (ATG), cyclosporine A, and mycophenolate mofetil.
Among the 58 patients eligible for the interim analysis, the mean age was 50 and 64% were male. Thirteen of the 28 patients randomized for allogeneic transplants underwent transplants; the others were not allografted because of progressive disease or lack of a donor. Of the 30 patients randomized to autologous SCT, 19 had the procedure; 11 did not receive transplants because of progressive disease or infection, Dr. Norbert Schmitz of Asklepios Klinik St. Georg, Hamburg, Germany, reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
The primary outcome, 1-year event-free survival (EFS), was 41% in the intent-to-treat population (95% CI, 27%–54%).
Causes of death included lymphoma (seven autologous, five allogeneic), salvage therapy (two), early or late infections (four), and graft vs. host disease (two).
Survival rates did not significantly differ in the two stem cell transplant groups, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedure. Based on the low probability of meeting the primary outcome, the data safety monitoring board decided to stop patient accrual and discontinue the trial.
*This article was updated 7/8/2015.
FROM 13-ICML
Key clinical point: Survival rates did not significantly differ for autologous versus allogenic stem cell transplant in patients with peripheral T-cell lymphoma, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedures.
Major finding: Early disease progression led to the discontinuation of a randomized trial comparing autologous to allogeneic stem cell transplantation in younger patients with peripheral T-cell lymphoma.
Data source: Results from 58 patients eligible for the interim analysis.
Disclosures: There were no relevant financial disclosures.
Naloxone lotion improves disabling itch in CTCL
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
AT WCD 2015
Key clinical point: Naloxone lotion shows promise for the severe pruritis that accompanies cutaneous T-cell lymphoma.
Major finding: Patients with cutaneous T-cell lymphoma reported an absolute 21% greater reduction in pruritis with naloxone lotion than with its vehicle.
Data source: This was a 15-patient, multicenter, double-blind, crossover study.
Disclosures: The study was funded by Elorac. The presenter reported having no financial conflicts.
FDA approves belinostat for peripheral T-cell lymphoma
Belinostat, a histone deacetylase inhibitor, has been approved for treating peripheral T-cell lymphoma, based on the results of the BELIEF study that found an overall response rate of nearly 26% among treated patients.
This is the third drug approved for this rare, aggressive form of non-Hodgkin’s lymphoma (NHL) since 2009, according to the Food and Drug Administration statement announcing the approval on July 3.
The other two drugs are pralatrexate injection (Folotyn), a folate analogue metabolic inhibitor approved in 2009 for treating relapsed or refractory peripheral T-cell lymphoma (PTCL); and romidepsin (Istodax), a histone deacetylase (HDAC) inhibitor approved in 2011 for treating PTCL in patients who have received at least one previous treatment.
This is an accelerated approval, which is based on surrogate or intermediate endpoints considered by the FDA as "reasonably likely to predict clinical benefit for patients with serious conditions with unmet medical needs." Confirmatory trials that verify the clinical benefit are required for full approval; otherwise, the approval can be withdrawn by the FDA. Belinostat will be marketed as Beleodaq by Spectrum Pharmaceuticals, which also markets Folotyn.
HDAC inhibitors "catalyze the removal of acetyl groups from the lysine residues of histones and some nonhistone proteins," and in vitro, belinostat "caused the accumulation of acetylated histones and other proteins, inducing cell cycle arrest and/or apoptosis of some transformed cells," according to a statement on the approval, issued by Spectrum on July 7. "Belinostat shows preferential cytotoxicity towards tumor cells compared to normal cells," and it "inhibited the enzymatic activity of histone deacetylases at nanomolar concentrations," the statement said.
In the BELIEF study, an open-label, single-arm, nonrandomized study, 129 patients with relapsed or refractory PTCL were treated with belinostat, administered via an IV infusion, once a day on days 1-5 of a 21-day cycle, repeated every 3 weeks until the disease progressed or adverse effects became unacceptable. The overall response rate (complete and partial responses), the primary efficacy endpoint, was 25.8%. Nausea, vomiting, fatigue, pyrexia, and anemia were the most common adverse events associated with treatment, according to the FDA.
The company said that the drug is expected to be available in less than 3 weeks of approval (before July 24). The confirmatory trial is a phase III study that will evaluate belinostat plus CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone), compared with CHOP alone.
PTCL accounts for about 10%-15% of NHL cases in North America, according to the FDA, which cites National Cancer Institute estimates that 70,800 Americans will be diagnosed with NHL and 18,990 will die of NHL in 2014.
The prescribing information for belinostat is available here.
Belinostat, a histone deacetylase inhibitor, has been approved for treating peripheral T-cell lymphoma, based on the results of the BELIEF study that found an overall response rate of nearly 26% among treated patients.
This is the third drug approved for this rare, aggressive form of non-Hodgkin’s lymphoma (NHL) since 2009, according to the Food and Drug Administration statement announcing the approval on July 3.
The other two drugs are pralatrexate injection (Folotyn), a folate analogue metabolic inhibitor approved in 2009 for treating relapsed or refractory peripheral T-cell lymphoma (PTCL); and romidepsin (Istodax), a histone deacetylase (HDAC) inhibitor approved in 2011 for treating PTCL in patients who have received at least one previous treatment.
This is an accelerated approval, which is based on surrogate or intermediate endpoints considered by the FDA as "reasonably likely to predict clinical benefit for patients with serious conditions with unmet medical needs." Confirmatory trials that verify the clinical benefit are required for full approval; otherwise, the approval can be withdrawn by the FDA. Belinostat will be marketed as Beleodaq by Spectrum Pharmaceuticals, which also markets Folotyn.
HDAC inhibitors "catalyze the removal of acetyl groups from the lysine residues of histones and some nonhistone proteins," and in vitro, belinostat "caused the accumulation of acetylated histones and other proteins, inducing cell cycle arrest and/or apoptosis of some transformed cells," according to a statement on the approval, issued by Spectrum on July 7. "Belinostat shows preferential cytotoxicity towards tumor cells compared to normal cells," and it "inhibited the enzymatic activity of histone deacetylases at nanomolar concentrations," the statement said.
In the BELIEF study, an open-label, single-arm, nonrandomized study, 129 patients with relapsed or refractory PTCL were treated with belinostat, administered via an IV infusion, once a day on days 1-5 of a 21-day cycle, repeated every 3 weeks until the disease progressed or adverse effects became unacceptable. The overall response rate (complete and partial responses), the primary efficacy endpoint, was 25.8%. Nausea, vomiting, fatigue, pyrexia, and anemia were the most common adverse events associated with treatment, according to the FDA.
The company said that the drug is expected to be available in less than 3 weeks of approval (before July 24). The confirmatory trial is a phase III study that will evaluate belinostat plus CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone), compared with CHOP alone.
PTCL accounts for about 10%-15% of NHL cases in North America, according to the FDA, which cites National Cancer Institute estimates that 70,800 Americans will be diagnosed with NHL and 18,990 will die of NHL in 2014.
The prescribing information for belinostat is available here.
Belinostat, a histone deacetylase inhibitor, has been approved for treating peripheral T-cell lymphoma, based on the results of the BELIEF study that found an overall response rate of nearly 26% among treated patients.
This is the third drug approved for this rare, aggressive form of non-Hodgkin’s lymphoma (NHL) since 2009, according to the Food and Drug Administration statement announcing the approval on July 3.
The other two drugs are pralatrexate injection (Folotyn), a folate analogue metabolic inhibitor approved in 2009 for treating relapsed or refractory peripheral T-cell lymphoma (PTCL); and romidepsin (Istodax), a histone deacetylase (HDAC) inhibitor approved in 2011 for treating PTCL in patients who have received at least one previous treatment.
This is an accelerated approval, which is based on surrogate or intermediate endpoints considered by the FDA as "reasonably likely to predict clinical benefit for patients with serious conditions with unmet medical needs." Confirmatory trials that verify the clinical benefit are required for full approval; otherwise, the approval can be withdrawn by the FDA. Belinostat will be marketed as Beleodaq by Spectrum Pharmaceuticals, which also markets Folotyn.
HDAC inhibitors "catalyze the removal of acetyl groups from the lysine residues of histones and some nonhistone proteins," and in vitro, belinostat "caused the accumulation of acetylated histones and other proteins, inducing cell cycle arrest and/or apoptosis of some transformed cells," according to a statement on the approval, issued by Spectrum on July 7. "Belinostat shows preferential cytotoxicity towards tumor cells compared to normal cells," and it "inhibited the enzymatic activity of histone deacetylases at nanomolar concentrations," the statement said.
In the BELIEF study, an open-label, single-arm, nonrandomized study, 129 patients with relapsed or refractory PTCL were treated with belinostat, administered via an IV infusion, once a day on days 1-5 of a 21-day cycle, repeated every 3 weeks until the disease progressed or adverse effects became unacceptable. The overall response rate (complete and partial responses), the primary efficacy endpoint, was 25.8%. Nausea, vomiting, fatigue, pyrexia, and anemia were the most common adverse events associated with treatment, according to the FDA.
The company said that the drug is expected to be available in less than 3 weeks of approval (before July 24). The confirmatory trial is a phase III study that will evaluate belinostat plus CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone), compared with CHOP alone.
PTCL accounts for about 10%-15% of NHL cases in North America, according to the FDA, which cites National Cancer Institute estimates that 70,800 Americans will be diagnosed with NHL and 18,990 will die of NHL in 2014.
The prescribing information for belinostat is available here.
Cutaneous erythema predicted low-dose alemtuzumab response in leukemic cutaneous T-cell lymphoma
In patients with leukemic cutaneous T-cell lymphoma, diffuse cutaneous erythema without plaques or tumors predicted complete remission with low-dose alemtuzumab therapy, investigators reported online April 23 in a JAMA Dermatology research letter.
"Initial clinical presentation was more predictive of response than was complex cellular phenotyping of T cells from blood and skin," wrote Dr. Rei Watanabe at Brigham and Women’s Hospital in Boston and associates. "In other words, the eyes of a well-trained dermatologist were more powerful than a comprehensive translational research program in identifying complete responders to LDA [low-dose alemtuzumab] therapy."
The researchers treated 23 patients with leukemic cutaneous T-cell lymphoma with 10 mg subcutaneous LDA three times weekly. Seventeen patients presented with diffuse erythema without superimposed plaques or tumors, of whom 13 experienced complete remission after LDA treatment and 4 had residual or emergent disease that could be controlled with skin-directed therapy (JAMA Dermatol. 2014 [doi:10.1001/jamadermatol.2013.10099]).
However, none of the six patients who presented with discrete patches, plaques, or tumors experienced full remission with LDA, the researchers reported. One patient later remitted with electron beam therapy, while five had their disease recur or progress, including two who developed large cell transformation.
Patients also were more likely to respond to LDA if more than 80% of their malignant T cells had TCM markers (CCR7+/L-selectin+), but clinical presentation better predicted response, the investigators reported.
"On a scientific level, diffuse erythema is likely caused by migrating T cells, and only T cells that migrate into blood are cleared by LDA," noted Dr. Watanabe and her associates. "Fixed skin lesions are more likely to be caused by TRM [nonmigratory skin resident memory T cells], cells that escape LDA clearance by remaining long-term in skin."
Based on the findings, the investigators recommended using LDA with or without adjuvant skin-directed treatments in patients who present with diffuse cutaneous erythema. But "we caution against its use in patients with preexisting plaques and/or tumors," the researchers said.
The Leukemia & Lymphoma Society, the National Institutes of Health, the Damon Runyon Cancer Research Foundation, and a private contribution from Edward P. Lawrence, Esq., funded the study. Coauthor Dr. Clark reported receiving honoraria from Novartis and Stiefel Laboratories. The authors disclosed no other conflicts of interest.
In patients with leukemic cutaneous T-cell lymphoma, diffuse cutaneous erythema without plaques or tumors predicted complete remission with low-dose alemtuzumab therapy, investigators reported online April 23 in a JAMA Dermatology research letter.
"Initial clinical presentation was more predictive of response than was complex cellular phenotyping of T cells from blood and skin," wrote Dr. Rei Watanabe at Brigham and Women’s Hospital in Boston and associates. "In other words, the eyes of a well-trained dermatologist were more powerful than a comprehensive translational research program in identifying complete responders to LDA [low-dose alemtuzumab] therapy."
The researchers treated 23 patients with leukemic cutaneous T-cell lymphoma with 10 mg subcutaneous LDA three times weekly. Seventeen patients presented with diffuse erythema without superimposed plaques or tumors, of whom 13 experienced complete remission after LDA treatment and 4 had residual or emergent disease that could be controlled with skin-directed therapy (JAMA Dermatol. 2014 [doi:10.1001/jamadermatol.2013.10099]).
However, none of the six patients who presented with discrete patches, plaques, or tumors experienced full remission with LDA, the researchers reported. One patient later remitted with electron beam therapy, while five had their disease recur or progress, including two who developed large cell transformation.
Patients also were more likely to respond to LDA if more than 80% of their malignant T cells had TCM markers (CCR7+/L-selectin+), but clinical presentation better predicted response, the investigators reported.
"On a scientific level, diffuse erythema is likely caused by migrating T cells, and only T cells that migrate into blood are cleared by LDA," noted Dr. Watanabe and her associates. "Fixed skin lesions are more likely to be caused by TRM [nonmigratory skin resident memory T cells], cells that escape LDA clearance by remaining long-term in skin."
Based on the findings, the investigators recommended using LDA with or without adjuvant skin-directed treatments in patients who present with diffuse cutaneous erythema. But "we caution against its use in patients with preexisting plaques and/or tumors," the researchers said.
The Leukemia & Lymphoma Society, the National Institutes of Health, the Damon Runyon Cancer Research Foundation, and a private contribution from Edward P. Lawrence, Esq., funded the study. Coauthor Dr. Clark reported receiving honoraria from Novartis and Stiefel Laboratories. The authors disclosed no other conflicts of interest.
In patients with leukemic cutaneous T-cell lymphoma, diffuse cutaneous erythema without plaques or tumors predicted complete remission with low-dose alemtuzumab therapy, investigators reported online April 23 in a JAMA Dermatology research letter.
"Initial clinical presentation was more predictive of response than was complex cellular phenotyping of T cells from blood and skin," wrote Dr. Rei Watanabe at Brigham and Women’s Hospital in Boston and associates. "In other words, the eyes of a well-trained dermatologist were more powerful than a comprehensive translational research program in identifying complete responders to LDA [low-dose alemtuzumab] therapy."
The researchers treated 23 patients with leukemic cutaneous T-cell lymphoma with 10 mg subcutaneous LDA three times weekly. Seventeen patients presented with diffuse erythema without superimposed plaques or tumors, of whom 13 experienced complete remission after LDA treatment and 4 had residual or emergent disease that could be controlled with skin-directed therapy (JAMA Dermatol. 2014 [doi:10.1001/jamadermatol.2013.10099]).
However, none of the six patients who presented with discrete patches, plaques, or tumors experienced full remission with LDA, the researchers reported. One patient later remitted with electron beam therapy, while five had their disease recur or progress, including two who developed large cell transformation.
Patients also were more likely to respond to LDA if more than 80% of their malignant T cells had TCM markers (CCR7+/L-selectin+), but clinical presentation better predicted response, the investigators reported.
"On a scientific level, diffuse erythema is likely caused by migrating T cells, and only T cells that migrate into blood are cleared by LDA," noted Dr. Watanabe and her associates. "Fixed skin lesions are more likely to be caused by TRM [nonmigratory skin resident memory T cells], cells that escape LDA clearance by remaining long-term in skin."
Based on the findings, the investigators recommended using LDA with or without adjuvant skin-directed treatments in patients who present with diffuse cutaneous erythema. But "we caution against its use in patients with preexisting plaques and/or tumors," the researchers said.
The Leukemia & Lymphoma Society, the National Institutes of Health, the Damon Runyon Cancer Research Foundation, and a private contribution from Edward P. Lawrence, Esq., funded the study. Coauthor Dr. Clark reported receiving honoraria from Novartis and Stiefel Laboratories. The authors disclosed no other conflicts of interest.
FROM JAMA DERMATOLOGY
Major finding: Of 17 patients who presented with diffuse cutaneous erythema without superimposed plaques or tumors, 13 fully remitted on low-dose alemtuzumab LDA and 4 had disease that was controllable with skin-directed therapy. None of the six patients who presented with discrete patches, plaques, or tumors experienced full remission with LDA.
Data source: A study of 23 patients with leukemic cutaneous T-cell lymphoma.
Disclosures: The Leukemia & Lymphoma Society, the National Institutes of Health, the Damon Runyon Cancer Research Foundation, and a private contribution from Edward P. Lawrence, Esq., funded the study. Coauthor Dr. Clark reported receiving honoraria from Novartis and Stiefel Laboratories. The authors disclosed no other conflicts of interest.
New guidelines address primary cutaneous T-cell lymphoproliferative disorders
HOLLYWOOD, FLA. – The treatment of patients with lymphomatoid papulosis depends on the presentation, according to new National Comprehensive Cancer Network guidelines for managing primary cutaneous CD30+ T-cell lymphoproliferative disorders.
No treatment is needed in patients with lymphomatoid papulosis (LyP) who present without symptoms because spontaneous remission is extremely common in this disease, and these patients typically won’t have problems with progressive disease, Dr. Andrew D. Zelenetz said at the annual conference of the National Comprehensive Cancer Network.
For those who are symptomatic, topical or systemic treatments are useful in some cases.
Topical steroids "are effective, but not great," commented Dr. Zelenetz, vice chairman of medical informatics at Memorial Sloan Kettering Cancer Center, New York; professor of medicine at Cornell University, New York; and chair of the NCCN Non-Hodgkin's Lymphomas Guidelines panel.
Reported response rates are in the 50%-60% range, he said.
Bexarotene is another treatment option, although experience with this drug is quite limited. The largest series included only 11 patients. Unpublished data from that series at Memorial Sloan Kettering Cancer Center show a response rate of 45% at a maximum oral dose of 600 mg daily, Dr. Zelenetz said.
However, where this is a response, it is "dramatic and quite obvious," he noted, adding that treatment duration needs to be adequate before a patient is considered a nonresponder; the median duration of treatment in the 11-patient series was 35.5 weeks.
In a series of 57 patients from Memorial Sloan Kettering Cancer Center (including the 11 treated systemically with bexarotene), 16 received no therapy; 19 received topical treatment with steroids (13 patients), bexarotene (2 patients), UVB (2 patients), cryotherapy (1 patient), or nitrogen mustard (1 patient); and 5 received systemic treatment with methotrexate.
At follow-up, 14% of patients had no evidence of disease, and, with the exception of one who died of another cause, the remaining patients were alive with disease, Dr. Zelenetz said.
LyP is a rare CD30+ cutaneous lymphoproliferative disorder characterized by self-healing cropped or generalized eruptions of papules that come and go on the trunk or proximal extremities. In rare cases they present as solitary lesions.
"Even though many patients actually have intermittent recurrent disease ... the death rate from LyP is zero. So this is a very manageable disease; don’t overtreat these tumors," he said.
At the other end of the spectrum of CD30+ lymphoproliferative disorders addressed in the new NCCN guidelines is anaplastic large cell lymphoma (ALCL).
Primary cutaneous ALCL is characterized by skin-only presentation that is often localized but which can be disseminated in some cases. Lesions also tend to be larger and "more piled up" than those seen with LyP.
"You can get clustering in a specific area, but we don’t tend to have these big crops of lesions that we see with LyP," Dr. Zelenetz said.
The pathology is also different, with diffuse infiltration of the subcutaneous tissue. The cells are large and anaplastic, and there is intense expression of CD30.
The course of disease is usually indolent, with progression to extracutaneous sites in about 10%-15% of cases.
Nodules or tumors in cases of ALCL are less likely than LyP lesions to regress spontaneously, Dr. Zelenetz said.
This disease must be distinguished from a skin presentation of systemic ALCL, he noted.
"So what’s the big difference? In anaplastic large cell lymphoma that’s systemic, you will have multiple nodules all over and happen to have skin disease. With primary cutaneous ALCL, you have skin only or skin and some regional lymph nodes but nothing beyond that," he said, adding that these primary cutaneous tumors do extremely well nevertheless, with cumulative survival rates above 90%.
Those with systemic ALCL, however, have much lower cumulative survival, in the 25% range.
As with LyP, treatment for primary cutaneous ALCL is based on presentation.
For solitary or grouped lesions, the preferred treatment is surgical excision if needed for diagnosis or radiation if the diagnosis is already established.
Methotrexate is the preferred treatment for multifocal lesions.
The subtype that includes regional lymph nodes is typically treated with very mild chemotherapy including methotrexate or pralatrexate. Radiation can be used for locoregional disease, Dr. Zelenetz said.
An exception to the rule that patients with primary cutaneous ALCL do well is in cases of extensive limb disease. Patients with involvement of a single limb – usually lower extremity, but not always – have poor survival, and their disease is refractory to chemotherapy and radiation. It is unclear why there is a distinction in this presentation, but it is important to be aware of it, he said.
Dr. Zelenetz is a scientific adviser for Cancer Genetics Inc. and Gilead and has received consulting fees, honoraria, and/or grant or other research support from Celgene Corp., Cephalon Inc., Genentech Inc., GlaxoSmithKline, Roche Laboratories Inc., sanofi-aventis U.S., and Seattle Genetics Inc.
HOLLYWOOD, FLA. – The treatment of patients with lymphomatoid papulosis depends on the presentation, according to new National Comprehensive Cancer Network guidelines for managing primary cutaneous CD30+ T-cell lymphoproliferative disorders.
No treatment is needed in patients with lymphomatoid papulosis (LyP) who present without symptoms because spontaneous remission is extremely common in this disease, and these patients typically won’t have problems with progressive disease, Dr. Andrew D. Zelenetz said at the annual conference of the National Comprehensive Cancer Network.
For those who are symptomatic, topical or systemic treatments are useful in some cases.
Topical steroids "are effective, but not great," commented Dr. Zelenetz, vice chairman of medical informatics at Memorial Sloan Kettering Cancer Center, New York; professor of medicine at Cornell University, New York; and chair of the NCCN Non-Hodgkin's Lymphomas Guidelines panel.
Reported response rates are in the 50%-60% range, he said.
Bexarotene is another treatment option, although experience with this drug is quite limited. The largest series included only 11 patients. Unpublished data from that series at Memorial Sloan Kettering Cancer Center show a response rate of 45% at a maximum oral dose of 600 mg daily, Dr. Zelenetz said.
However, where this is a response, it is "dramatic and quite obvious," he noted, adding that treatment duration needs to be adequate before a patient is considered a nonresponder; the median duration of treatment in the 11-patient series was 35.5 weeks.
In a series of 57 patients from Memorial Sloan Kettering Cancer Center (including the 11 treated systemically with bexarotene), 16 received no therapy; 19 received topical treatment with steroids (13 patients), bexarotene (2 patients), UVB (2 patients), cryotherapy (1 patient), or nitrogen mustard (1 patient); and 5 received systemic treatment with methotrexate.
At follow-up, 14% of patients had no evidence of disease, and, with the exception of one who died of another cause, the remaining patients were alive with disease, Dr. Zelenetz said.
LyP is a rare CD30+ cutaneous lymphoproliferative disorder characterized by self-healing cropped or generalized eruptions of papules that come and go on the trunk or proximal extremities. In rare cases they present as solitary lesions.
"Even though many patients actually have intermittent recurrent disease ... the death rate from LyP is zero. So this is a very manageable disease; don’t overtreat these tumors," he said.
At the other end of the spectrum of CD30+ lymphoproliferative disorders addressed in the new NCCN guidelines is anaplastic large cell lymphoma (ALCL).
Primary cutaneous ALCL is characterized by skin-only presentation that is often localized but which can be disseminated in some cases. Lesions also tend to be larger and "more piled up" than those seen with LyP.
"You can get clustering in a specific area, but we don’t tend to have these big crops of lesions that we see with LyP," Dr. Zelenetz said.
The pathology is also different, with diffuse infiltration of the subcutaneous tissue. The cells are large and anaplastic, and there is intense expression of CD30.
The course of disease is usually indolent, with progression to extracutaneous sites in about 10%-15% of cases.
Nodules or tumors in cases of ALCL are less likely than LyP lesions to regress spontaneously, Dr. Zelenetz said.
This disease must be distinguished from a skin presentation of systemic ALCL, he noted.
"So what’s the big difference? In anaplastic large cell lymphoma that’s systemic, you will have multiple nodules all over and happen to have skin disease. With primary cutaneous ALCL, you have skin only or skin and some regional lymph nodes but nothing beyond that," he said, adding that these primary cutaneous tumors do extremely well nevertheless, with cumulative survival rates above 90%.
Those with systemic ALCL, however, have much lower cumulative survival, in the 25% range.
As with LyP, treatment for primary cutaneous ALCL is based on presentation.
For solitary or grouped lesions, the preferred treatment is surgical excision if needed for diagnosis or radiation if the diagnosis is already established.
Methotrexate is the preferred treatment for multifocal lesions.
The subtype that includes regional lymph nodes is typically treated with very mild chemotherapy including methotrexate or pralatrexate. Radiation can be used for locoregional disease, Dr. Zelenetz said.
An exception to the rule that patients with primary cutaneous ALCL do well is in cases of extensive limb disease. Patients with involvement of a single limb – usually lower extremity, but not always – have poor survival, and their disease is refractory to chemotherapy and radiation. It is unclear why there is a distinction in this presentation, but it is important to be aware of it, he said.
Dr. Zelenetz is a scientific adviser for Cancer Genetics Inc. and Gilead and has received consulting fees, honoraria, and/or grant or other research support from Celgene Corp., Cephalon Inc., Genentech Inc., GlaxoSmithKline, Roche Laboratories Inc., sanofi-aventis U.S., and Seattle Genetics Inc.
HOLLYWOOD, FLA. – The treatment of patients with lymphomatoid papulosis depends on the presentation, according to new National Comprehensive Cancer Network guidelines for managing primary cutaneous CD30+ T-cell lymphoproliferative disorders.
No treatment is needed in patients with lymphomatoid papulosis (LyP) who present without symptoms because spontaneous remission is extremely common in this disease, and these patients typically won’t have problems with progressive disease, Dr. Andrew D. Zelenetz said at the annual conference of the National Comprehensive Cancer Network.
For those who are symptomatic, topical or systemic treatments are useful in some cases.
Topical steroids "are effective, but not great," commented Dr. Zelenetz, vice chairman of medical informatics at Memorial Sloan Kettering Cancer Center, New York; professor of medicine at Cornell University, New York; and chair of the NCCN Non-Hodgkin's Lymphomas Guidelines panel.
Reported response rates are in the 50%-60% range, he said.
Bexarotene is another treatment option, although experience with this drug is quite limited. The largest series included only 11 patients. Unpublished data from that series at Memorial Sloan Kettering Cancer Center show a response rate of 45% at a maximum oral dose of 600 mg daily, Dr. Zelenetz said.
However, where this is a response, it is "dramatic and quite obvious," he noted, adding that treatment duration needs to be adequate before a patient is considered a nonresponder; the median duration of treatment in the 11-patient series was 35.5 weeks.
In a series of 57 patients from Memorial Sloan Kettering Cancer Center (including the 11 treated systemically with bexarotene), 16 received no therapy; 19 received topical treatment with steroids (13 patients), bexarotene (2 patients), UVB (2 patients), cryotherapy (1 patient), or nitrogen mustard (1 patient); and 5 received systemic treatment with methotrexate.
At follow-up, 14% of patients had no evidence of disease, and, with the exception of one who died of another cause, the remaining patients were alive with disease, Dr. Zelenetz said.
LyP is a rare CD30+ cutaneous lymphoproliferative disorder characterized by self-healing cropped or generalized eruptions of papules that come and go on the trunk or proximal extremities. In rare cases they present as solitary lesions.
"Even though many patients actually have intermittent recurrent disease ... the death rate from LyP is zero. So this is a very manageable disease; don’t overtreat these tumors," he said.
At the other end of the spectrum of CD30+ lymphoproliferative disorders addressed in the new NCCN guidelines is anaplastic large cell lymphoma (ALCL).
Primary cutaneous ALCL is characterized by skin-only presentation that is often localized but which can be disseminated in some cases. Lesions also tend to be larger and "more piled up" than those seen with LyP.
"You can get clustering in a specific area, but we don’t tend to have these big crops of lesions that we see with LyP," Dr. Zelenetz said.
The pathology is also different, with diffuse infiltration of the subcutaneous tissue. The cells are large and anaplastic, and there is intense expression of CD30.
The course of disease is usually indolent, with progression to extracutaneous sites in about 10%-15% of cases.
Nodules or tumors in cases of ALCL are less likely than LyP lesions to regress spontaneously, Dr. Zelenetz said.
This disease must be distinguished from a skin presentation of systemic ALCL, he noted.
"So what’s the big difference? In anaplastic large cell lymphoma that’s systemic, you will have multiple nodules all over and happen to have skin disease. With primary cutaneous ALCL, you have skin only or skin and some regional lymph nodes but nothing beyond that," he said, adding that these primary cutaneous tumors do extremely well nevertheless, with cumulative survival rates above 90%.
Those with systemic ALCL, however, have much lower cumulative survival, in the 25% range.
As with LyP, treatment for primary cutaneous ALCL is based on presentation.
For solitary or grouped lesions, the preferred treatment is surgical excision if needed for diagnosis or radiation if the diagnosis is already established.
Methotrexate is the preferred treatment for multifocal lesions.
The subtype that includes regional lymph nodes is typically treated with very mild chemotherapy including methotrexate or pralatrexate. Radiation can be used for locoregional disease, Dr. Zelenetz said.
An exception to the rule that patients with primary cutaneous ALCL do well is in cases of extensive limb disease. Patients with involvement of a single limb – usually lower extremity, but not always – have poor survival, and their disease is refractory to chemotherapy and radiation. It is unclear why there is a distinction in this presentation, but it is important to be aware of it, he said.
Dr. Zelenetz is a scientific adviser for Cancer Genetics Inc. and Gilead and has received consulting fees, honoraria, and/or grant or other research support from Celgene Corp., Cephalon Inc., Genentech Inc., GlaxoSmithKline, Roche Laboratories Inc., sanofi-aventis U.S., and Seattle Genetics Inc.
AT THE NCCN ANNUAL CONFERENCE
Gene therapy for SCID-X1 may successfully reset immune system
NEW ORLEANS – Tweaking experimental gene therapy for X-linked severe combined immunodeficiency may help to restore patient immune function while reducing the risk for subsequent leukemias.
In a small, multinational phase I/II trial, seven of nine children with SCID-X1 showed evidence of T-cell recovery and function, as well as a lower risk for promoting growth of leukemic cells, when a self-inactivating gamma-retroviral vector (SCID-2) was used to promote reconstitution of the child’s immune system without insertional oncogenesis, reported Dr. Sung-Yun Pai at the annual meeting of the American Society of Hematology.
"Outcomes for boys who do not have well-matched donors are suboptimal, and it’s particularly for these boys that we are targeting gene therapy," said Dr. Pai, of Boston Children’s Hospital and the Dana-Farber Cancer Institute, Boston.
In previous gene therapy trials, investigators used the Moloney leukemia virus (MLV)-based gamma-retroviral vector (SCID-1) with strong promoters and enhancers to express an IL-2 receptor that reconstituted the immune system successfully in 18 of 20 boys.
However, 5 of the 20 boys developed T-cell acute lymphoblastic leukemia; 1 of the children died, and the remaining 4 were successfully treated.
The investigators found that in the patients with leukemia, the SCID-1 vector had inserted into a chromosomal region close to proto-oncogenes such as LMO2, and the enhancers were driving expression of the neighboring oncogene, promoting expression of aberrant T cells. The vector was subsequently modified with the goal of improved safety but similar efficacy to the original, said Dr. Pai. The strong viral enhancers were removed to prevent accident enhancement should the inserted genes manage to find their way into oncogenes.
The phase I/II study is being conducted in London, Paris, Boston, Cincinnati, and Los Angeles, and to date has enrolled 9 male children of a planned 20.
Of the 9, one child died from a preexisting adenoviral infection before immune recovery was complete, and one child did not have engraftment of the gene-marked cells and went on to transplant.
"The other patients have 9 months to 36 months of follow-up, they have evidence of T-cell recovery, of T-cell function, have cleared SCID-related infections, and are all out of hospital, healthy at home, [and] leading essentially normal lives."
When the investigators looked at the comparative efficacy of the SCID-1 and SCID-2 vectors, they saw that 6 months after gene therapy, there was no significant difference in the median number of T cells generated.
"It’s far too early to comment on whether this vector will truly be safer in terms of leukemia," Dr. Pai said, noting that in the SCID-1 trial the leukemias developed 3-5 years after gene therapy, and the longest follow-up in the SCID-2 study is only 3 years.
The investigators are, however, conducting molecular surrogate safety analyses looking at gene insertion sites from the blood of patients treated with SCID-1 and are comparing those sites with the vector-insertion sites in cells from patients in SCID-2.
Looking at a global genomewide map of integrations, they found no significant differences between SCID-1 and SCID-2. However, when they focused on 38 genes that are to be proto-oncogenes in lymphoid cancer, they found that significantly more integration of the modified genes occurred in proximity to the oncogenes in SCID-1 than in SCID-2 (P = .003).
"We hope that these data suggest that the modified SCID vector will show less capacity to drive aberrant cell growth and lead to less leukemogenesis," said Dr. Pai.
SCID-X1 is caused by inherited mutations in the gamma subunit of the interleukin (IL)-2 receptor. As a result, males are born without T lymphocytes or natural killer cells. Without a bone marrow or stem cell transplantation, children with the disease die early from opportunistic or community-acquired infections.
"These are really paradigm-changing results for mortally wounded children," said Dr. Laurence James Neil Cooper of the University of Texas M.D. Anderson Cancer Center in Houston, who moderated the briefing but was not involved in the study.
The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.
NEW ORLEANS – Tweaking experimental gene therapy for X-linked severe combined immunodeficiency may help to restore patient immune function while reducing the risk for subsequent leukemias.
In a small, multinational phase I/II trial, seven of nine children with SCID-X1 showed evidence of T-cell recovery and function, as well as a lower risk for promoting growth of leukemic cells, when a self-inactivating gamma-retroviral vector (SCID-2) was used to promote reconstitution of the child’s immune system without insertional oncogenesis, reported Dr. Sung-Yun Pai at the annual meeting of the American Society of Hematology.
"Outcomes for boys who do not have well-matched donors are suboptimal, and it’s particularly for these boys that we are targeting gene therapy," said Dr. Pai, of Boston Children’s Hospital and the Dana-Farber Cancer Institute, Boston.
In previous gene therapy trials, investigators used the Moloney leukemia virus (MLV)-based gamma-retroviral vector (SCID-1) with strong promoters and enhancers to express an IL-2 receptor that reconstituted the immune system successfully in 18 of 20 boys.
However, 5 of the 20 boys developed T-cell acute lymphoblastic leukemia; 1 of the children died, and the remaining 4 were successfully treated.
The investigators found that in the patients with leukemia, the SCID-1 vector had inserted into a chromosomal region close to proto-oncogenes such as LMO2, and the enhancers were driving expression of the neighboring oncogene, promoting expression of aberrant T cells. The vector was subsequently modified with the goal of improved safety but similar efficacy to the original, said Dr. Pai. The strong viral enhancers were removed to prevent accident enhancement should the inserted genes manage to find their way into oncogenes.
The phase I/II study is being conducted in London, Paris, Boston, Cincinnati, and Los Angeles, and to date has enrolled 9 male children of a planned 20.
Of the 9, one child died from a preexisting adenoviral infection before immune recovery was complete, and one child did not have engraftment of the gene-marked cells and went on to transplant.
"The other patients have 9 months to 36 months of follow-up, they have evidence of T-cell recovery, of T-cell function, have cleared SCID-related infections, and are all out of hospital, healthy at home, [and] leading essentially normal lives."
When the investigators looked at the comparative efficacy of the SCID-1 and SCID-2 vectors, they saw that 6 months after gene therapy, there was no significant difference in the median number of T cells generated.
"It’s far too early to comment on whether this vector will truly be safer in terms of leukemia," Dr. Pai said, noting that in the SCID-1 trial the leukemias developed 3-5 years after gene therapy, and the longest follow-up in the SCID-2 study is only 3 years.
The investigators are, however, conducting molecular surrogate safety analyses looking at gene insertion sites from the blood of patients treated with SCID-1 and are comparing those sites with the vector-insertion sites in cells from patients in SCID-2.
Looking at a global genomewide map of integrations, they found no significant differences between SCID-1 and SCID-2. However, when they focused on 38 genes that are to be proto-oncogenes in lymphoid cancer, they found that significantly more integration of the modified genes occurred in proximity to the oncogenes in SCID-1 than in SCID-2 (P = .003).
"We hope that these data suggest that the modified SCID vector will show less capacity to drive aberrant cell growth and lead to less leukemogenesis," said Dr. Pai.
SCID-X1 is caused by inherited mutations in the gamma subunit of the interleukin (IL)-2 receptor. As a result, males are born without T lymphocytes or natural killer cells. Without a bone marrow or stem cell transplantation, children with the disease die early from opportunistic or community-acquired infections.
"These are really paradigm-changing results for mortally wounded children," said Dr. Laurence James Neil Cooper of the University of Texas M.D. Anderson Cancer Center in Houston, who moderated the briefing but was not involved in the study.
The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.
NEW ORLEANS – Tweaking experimental gene therapy for X-linked severe combined immunodeficiency may help to restore patient immune function while reducing the risk for subsequent leukemias.
In a small, multinational phase I/II trial, seven of nine children with SCID-X1 showed evidence of T-cell recovery and function, as well as a lower risk for promoting growth of leukemic cells, when a self-inactivating gamma-retroviral vector (SCID-2) was used to promote reconstitution of the child’s immune system without insertional oncogenesis, reported Dr. Sung-Yun Pai at the annual meeting of the American Society of Hematology.
"Outcomes for boys who do not have well-matched donors are suboptimal, and it’s particularly for these boys that we are targeting gene therapy," said Dr. Pai, of Boston Children’s Hospital and the Dana-Farber Cancer Institute, Boston.
In previous gene therapy trials, investigators used the Moloney leukemia virus (MLV)-based gamma-retroviral vector (SCID-1) with strong promoters and enhancers to express an IL-2 receptor that reconstituted the immune system successfully in 18 of 20 boys.
However, 5 of the 20 boys developed T-cell acute lymphoblastic leukemia; 1 of the children died, and the remaining 4 were successfully treated.
The investigators found that in the patients with leukemia, the SCID-1 vector had inserted into a chromosomal region close to proto-oncogenes such as LMO2, and the enhancers were driving expression of the neighboring oncogene, promoting expression of aberrant T cells. The vector was subsequently modified with the goal of improved safety but similar efficacy to the original, said Dr. Pai. The strong viral enhancers were removed to prevent accident enhancement should the inserted genes manage to find their way into oncogenes.
The phase I/II study is being conducted in London, Paris, Boston, Cincinnati, and Los Angeles, and to date has enrolled 9 male children of a planned 20.
Of the 9, one child died from a preexisting adenoviral infection before immune recovery was complete, and one child did not have engraftment of the gene-marked cells and went on to transplant.
"The other patients have 9 months to 36 months of follow-up, they have evidence of T-cell recovery, of T-cell function, have cleared SCID-related infections, and are all out of hospital, healthy at home, [and] leading essentially normal lives."
When the investigators looked at the comparative efficacy of the SCID-1 and SCID-2 vectors, they saw that 6 months after gene therapy, there was no significant difference in the median number of T cells generated.
"It’s far too early to comment on whether this vector will truly be safer in terms of leukemia," Dr. Pai said, noting that in the SCID-1 trial the leukemias developed 3-5 years after gene therapy, and the longest follow-up in the SCID-2 study is only 3 years.
The investigators are, however, conducting molecular surrogate safety analyses looking at gene insertion sites from the blood of patients treated with SCID-1 and are comparing those sites with the vector-insertion sites in cells from patients in SCID-2.
Looking at a global genomewide map of integrations, they found no significant differences between SCID-1 and SCID-2. However, when they focused on 38 genes that are to be proto-oncogenes in lymphoid cancer, they found that significantly more integration of the modified genes occurred in proximity to the oncogenes in SCID-1 than in SCID-2 (P = .003).
"We hope that these data suggest that the modified SCID vector will show less capacity to drive aberrant cell growth and lead to less leukemogenesis," said Dr. Pai.
SCID-X1 is caused by inherited mutations in the gamma subunit of the interleukin (IL)-2 receptor. As a result, males are born without T lymphocytes or natural killer cells. Without a bone marrow or stem cell transplantation, children with the disease die early from opportunistic or community-acquired infections.
"These are really paradigm-changing results for mortally wounded children," said Dr. Laurence James Neil Cooper of the University of Texas M.D. Anderson Cancer Center in Houston, who moderated the briefing but was not involved in the study.
The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.
AT ASH 2013
Major finding: Of nine boys with X-linked severe combined immunodeficiency who were treated with gene therapy, seven patients have evidence of T-cell function, have cleared SCID-related infections, and are out of hospital.
Data source: Preliminary results of a prospective phase I/II clinical trial of nine children.
Disclosures: The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.