User login
VALOR: Baby step forward or misstep in AML?
SAN FRANCISCO – Adding vosaroxin to cytarabine chemotherapy increased overall survival in first relapsed or refractory acute myeloid leukemia in the phase III VALOR study.
The difference in this primary endpoint, however, failed to achieve statistically significance (median 7.5 months vs. 6.1 months; P = .06), lead study author Dr. Farhad Ravandi reported at the annual meeting of the American Society of Hematology.
More patients receiving vosaroxin (Qinprezo) and cytarabine than cytarabine alone achieved complete remission (CR) (30.1% vs. 16.3%; P < .0001).
The benefit was significant across all subgroups (age at least 60 years, refractory disease, early and late relapse), except in those aged younger than 60 years, he said.
Vosaroxin is an investigational, first-in-class anticancer quinolone derivative that was granted fast track designation by the Food and Drug Administration in 2011 for the potential treatment of relapsed or refractory acute myeloid leukemia (AML) in combination with cytarabine.
Despite missing its primary endpoint, Dr. Ravandi argued during a press briefing that VALOR was a positive trial and that the survival benefit with combination vosaroxin was “highly significant.” He described relapsed/refractory AML as the equivalent of metastatic cancer, with patients presenting with disease “all over their body, right from day 1.”
“In solid tumors, we are excited about a 1- or 2-month survival improvement and I don’t see why we shouldn’t be excited in AML as well,” said Dr. Ravandi of the University of Texas M.D. Anderson Cancer Center in Houston.
He suggested that the bar has been set high in AML because about 40% of patients are cured and that great leaps forward remain rare. “We should not discount the small steps forward in treating our patients and providing better treatment options,” he said.
Press briefing moderator Dr. David Steensma of the Dana-Farber Cancer Institute, Boston, agreed that change is often incremental in AML but countered that the magnitude of benefit wasn’t great.
“AML is a really difficult population, no question about it, but there’s also no question that seven and a half months is pretty crummy and we need to do better than that,” Dr. Steensma said.
VALOR randomly assigned 711 adult patients from 124 sites in 15 countries to IV cytarabine 1 g/m² on days 1-5 plus placebo or IV vosaroxin 90 mg/m² on days 1 and 4 for induction and 70 mg/m² for subsequent cycles. Patients had AML refractory to initial induction therapy or were in first relapse, defined as relapse within 90 days to 24 months after first CR or CR with incomplete platelet recovery.
In all, 30.1% of patients in the vosaroxin group and 29% in the placebo group underwent allogeneic stem cell transplantation (ASCT). In those younger than 60 years, rates were 46.2% and 45.4%.
When stratified by age, there was a significant overall survival benefit with the vosaroxin combination for patients aged 60 years and older, who accounted for two-thirds of the study population (median 7.1 months vs. 5.0 months; hazard ratio, 0.75; P = .003), Dr. Ravandi said.
There was no survival advantage in patients younger than 60 years (median 9.1 months vs. 7.9 months; HR, 1.08, P = .60).
In a preplanned analysis censored for ASCT, median overall survival was significantly better in patients receiving the vosaroxin combination versus cytarabine alone (6.7 months vs. 5.3 months; HR, 0.83; P = .02).
“The benefit may be underestimated by the high rate of [ASCT], particularly in the younger patients,” Dr. Ravandi concluded during the formal presentation of the late-breaking abstract. “These data support the use of vosaroxin in combination with cytarabine as a new standard for salvage therapy in older patients with relapsed or refractory AML.”
During the discussion following the presentation, session comoderator Dr. Jonathan Friedberg of the University of Rochester Medical Center in Rochester, N.Y., asked, “How do you reconcile the observation that the patients you wanted to get to transplant got there, and yet you’re blaming the transplant for poor outcomes?”
Dr. Ravandi responded that transplantation is an issue in all AML studies and that a much larger study would have been needed to show a survival difference regardless of transplant status.
Treatment-related adverse events were more common in patients on vosaroxin and were mostly infection related. Stomatitis was identified as a dose-limiting toxicity in previous studies and occurred at any grade in 49% of vosaroxin patients and 19% of controls and at grade 3/4 in 15% and 3%.
Other notable grade 3/4 events were febrile neutropenia (47% vs. 33%) and thrombocytopenia (24% vs. 25%). This increase in toxicity did not translate into worse all-cause mortality at either 30 or 60 days, Dr. Ravandi said.
Sunesis Pharmaceuticals funded the study. Dr. Ravandi and several coauthors reported financial ties with Sunesis. Dr Steensma reported financial ties with several companies. Dr. Friedberg reported no disclosures.
SAN FRANCISCO – Adding vosaroxin to cytarabine chemotherapy increased overall survival in first relapsed or refractory acute myeloid leukemia in the phase III VALOR study.
The difference in this primary endpoint, however, failed to achieve statistically significance (median 7.5 months vs. 6.1 months; P = .06), lead study author Dr. Farhad Ravandi reported at the annual meeting of the American Society of Hematology.
More patients receiving vosaroxin (Qinprezo) and cytarabine than cytarabine alone achieved complete remission (CR) (30.1% vs. 16.3%; P < .0001).
The benefit was significant across all subgroups (age at least 60 years, refractory disease, early and late relapse), except in those aged younger than 60 years, he said.
Vosaroxin is an investigational, first-in-class anticancer quinolone derivative that was granted fast track designation by the Food and Drug Administration in 2011 for the potential treatment of relapsed or refractory acute myeloid leukemia (AML) in combination with cytarabine.
Despite missing its primary endpoint, Dr. Ravandi argued during a press briefing that VALOR was a positive trial and that the survival benefit with combination vosaroxin was “highly significant.” He described relapsed/refractory AML as the equivalent of metastatic cancer, with patients presenting with disease “all over their body, right from day 1.”
“In solid tumors, we are excited about a 1- or 2-month survival improvement and I don’t see why we shouldn’t be excited in AML as well,” said Dr. Ravandi of the University of Texas M.D. Anderson Cancer Center in Houston.
He suggested that the bar has been set high in AML because about 40% of patients are cured and that great leaps forward remain rare. “We should not discount the small steps forward in treating our patients and providing better treatment options,” he said.
Press briefing moderator Dr. David Steensma of the Dana-Farber Cancer Institute, Boston, agreed that change is often incremental in AML but countered that the magnitude of benefit wasn’t great.
“AML is a really difficult population, no question about it, but there’s also no question that seven and a half months is pretty crummy and we need to do better than that,” Dr. Steensma said.
VALOR randomly assigned 711 adult patients from 124 sites in 15 countries to IV cytarabine 1 g/m² on days 1-5 plus placebo or IV vosaroxin 90 mg/m² on days 1 and 4 for induction and 70 mg/m² for subsequent cycles. Patients had AML refractory to initial induction therapy or were in first relapse, defined as relapse within 90 days to 24 months after first CR or CR with incomplete platelet recovery.
In all, 30.1% of patients in the vosaroxin group and 29% in the placebo group underwent allogeneic stem cell transplantation (ASCT). In those younger than 60 years, rates were 46.2% and 45.4%.
When stratified by age, there was a significant overall survival benefit with the vosaroxin combination for patients aged 60 years and older, who accounted for two-thirds of the study population (median 7.1 months vs. 5.0 months; hazard ratio, 0.75; P = .003), Dr. Ravandi said.
There was no survival advantage in patients younger than 60 years (median 9.1 months vs. 7.9 months; HR, 1.08, P = .60).
In a preplanned analysis censored for ASCT, median overall survival was significantly better in patients receiving the vosaroxin combination versus cytarabine alone (6.7 months vs. 5.3 months; HR, 0.83; P = .02).
“The benefit may be underestimated by the high rate of [ASCT], particularly in the younger patients,” Dr. Ravandi concluded during the formal presentation of the late-breaking abstract. “These data support the use of vosaroxin in combination with cytarabine as a new standard for salvage therapy in older patients with relapsed or refractory AML.”
During the discussion following the presentation, session comoderator Dr. Jonathan Friedberg of the University of Rochester Medical Center in Rochester, N.Y., asked, “How do you reconcile the observation that the patients you wanted to get to transplant got there, and yet you’re blaming the transplant for poor outcomes?”
Dr. Ravandi responded that transplantation is an issue in all AML studies and that a much larger study would have been needed to show a survival difference regardless of transplant status.
Treatment-related adverse events were more common in patients on vosaroxin and were mostly infection related. Stomatitis was identified as a dose-limiting toxicity in previous studies and occurred at any grade in 49% of vosaroxin patients and 19% of controls and at grade 3/4 in 15% and 3%.
Other notable grade 3/4 events were febrile neutropenia (47% vs. 33%) and thrombocytopenia (24% vs. 25%). This increase in toxicity did not translate into worse all-cause mortality at either 30 or 60 days, Dr. Ravandi said.
Sunesis Pharmaceuticals funded the study. Dr. Ravandi and several coauthors reported financial ties with Sunesis. Dr Steensma reported financial ties with several companies. Dr. Friedberg reported no disclosures.
SAN FRANCISCO – Adding vosaroxin to cytarabine chemotherapy increased overall survival in first relapsed or refractory acute myeloid leukemia in the phase III VALOR study.
The difference in this primary endpoint, however, failed to achieve statistically significance (median 7.5 months vs. 6.1 months; P = .06), lead study author Dr. Farhad Ravandi reported at the annual meeting of the American Society of Hematology.
More patients receiving vosaroxin (Qinprezo) and cytarabine than cytarabine alone achieved complete remission (CR) (30.1% vs. 16.3%; P < .0001).
The benefit was significant across all subgroups (age at least 60 years, refractory disease, early and late relapse), except in those aged younger than 60 years, he said.
Vosaroxin is an investigational, first-in-class anticancer quinolone derivative that was granted fast track designation by the Food and Drug Administration in 2011 for the potential treatment of relapsed or refractory acute myeloid leukemia (AML) in combination with cytarabine.
Despite missing its primary endpoint, Dr. Ravandi argued during a press briefing that VALOR was a positive trial and that the survival benefit with combination vosaroxin was “highly significant.” He described relapsed/refractory AML as the equivalent of metastatic cancer, with patients presenting with disease “all over their body, right from day 1.”
“In solid tumors, we are excited about a 1- or 2-month survival improvement and I don’t see why we shouldn’t be excited in AML as well,” said Dr. Ravandi of the University of Texas M.D. Anderson Cancer Center in Houston.
He suggested that the bar has been set high in AML because about 40% of patients are cured and that great leaps forward remain rare. “We should not discount the small steps forward in treating our patients and providing better treatment options,” he said.
Press briefing moderator Dr. David Steensma of the Dana-Farber Cancer Institute, Boston, agreed that change is often incremental in AML but countered that the magnitude of benefit wasn’t great.
“AML is a really difficult population, no question about it, but there’s also no question that seven and a half months is pretty crummy and we need to do better than that,” Dr. Steensma said.
VALOR randomly assigned 711 adult patients from 124 sites in 15 countries to IV cytarabine 1 g/m² on days 1-5 plus placebo or IV vosaroxin 90 mg/m² on days 1 and 4 for induction and 70 mg/m² for subsequent cycles. Patients had AML refractory to initial induction therapy or were in first relapse, defined as relapse within 90 days to 24 months after first CR or CR with incomplete platelet recovery.
In all, 30.1% of patients in the vosaroxin group and 29% in the placebo group underwent allogeneic stem cell transplantation (ASCT). In those younger than 60 years, rates were 46.2% and 45.4%.
When stratified by age, there was a significant overall survival benefit with the vosaroxin combination for patients aged 60 years and older, who accounted for two-thirds of the study population (median 7.1 months vs. 5.0 months; hazard ratio, 0.75; P = .003), Dr. Ravandi said.
There was no survival advantage in patients younger than 60 years (median 9.1 months vs. 7.9 months; HR, 1.08, P = .60).
In a preplanned analysis censored for ASCT, median overall survival was significantly better in patients receiving the vosaroxin combination versus cytarabine alone (6.7 months vs. 5.3 months; HR, 0.83; P = .02).
“The benefit may be underestimated by the high rate of [ASCT], particularly in the younger patients,” Dr. Ravandi concluded during the formal presentation of the late-breaking abstract. “These data support the use of vosaroxin in combination with cytarabine as a new standard for salvage therapy in older patients with relapsed or refractory AML.”
During the discussion following the presentation, session comoderator Dr. Jonathan Friedberg of the University of Rochester Medical Center in Rochester, N.Y., asked, “How do you reconcile the observation that the patients you wanted to get to transplant got there, and yet you’re blaming the transplant for poor outcomes?”
Dr. Ravandi responded that transplantation is an issue in all AML studies and that a much larger study would have been needed to show a survival difference regardless of transplant status.
Treatment-related adverse events were more common in patients on vosaroxin and were mostly infection related. Stomatitis was identified as a dose-limiting toxicity in previous studies and occurred at any grade in 49% of vosaroxin patients and 19% of controls and at grade 3/4 in 15% and 3%.
Other notable grade 3/4 events were febrile neutropenia (47% vs. 33%) and thrombocytopenia (24% vs. 25%). This increase in toxicity did not translate into worse all-cause mortality at either 30 or 60 days, Dr. Ravandi said.
Sunesis Pharmaceuticals funded the study. Dr. Ravandi and several coauthors reported financial ties with Sunesis. Dr Steensma reported financial ties with several companies. Dr. Friedberg reported no disclosures.
AT ASH 2014
Key clinical point: Vosaroxin plus cytarabine failed to significantly improve overall survival in relapsed or refractory AML, but may offer older patients a new option.
Major finding: Median overall survival was 7.5 months for vosaroxin plus cytarabine vs. 6.1 months for cytarabine alone (HR, 0.865; P = .06).
Data source: Randomized, phase III trial in 711 patients with first relapsed or refractory acute myeloid leukemia.
Disclosures: Sunesis Pharmaceuticals funded the study. Dr. Ravandi and several coauthors reported financial ties with Sunesis. Dr Steensma reported financial ties with several companies. Dr. Friedberg reported no disclosures.
Ofatumumab maintenance halves risk of progression in relapsed CLL
SAN FRANCISCO – Ofatumumab maintenance therapy nearly doubled progression-free survival in patients with relapsed CLL, according to a preplanned interim analysis of the phase III PROLONG study.
At a median follow-up of 19.1 months, progression-free survival was 15.2 months with the standard approach of observation alone and 29.4 months with maintenance ofatumumab (Hazard ratio, 0.50; P < .0001).
Ofatumumab (Arzerra) also significantly increased the median time to next treatment from 31.1 months to 38 months (HR, 0.66; P = .0108), Dr. Marinus van Oers reported at the annual meeting of the American Society of Hematology.
The benefit in progression-free survival (PFS) with maintenance was “statistically significant and clinical relevant” and was present in all subgroups, he said. It was independent of age, gender, number and type of prior treatment, minimal residual disease status at study entry, and “response at study entry, although we have the impression that it’s more effective in patients on PR [partial response] than in patients on CR [complete response],” he added.
The rationale for the trial lies in the fact that despite recent advances, there is still no curative treatment for chronic lymphocytic leukemia (CLL). Ofatumumab, a type 1 CD20 monoclonal antibody, has a role as maintenance in follicular lymphoma (FL), which shares similarities in biological behavior with CLL. This role is debated, but a recent meta-analysis shows ofatumumab maintenance prolongs PFS and tends to prolong overall survival in relapsed patients with FL, Dr. van Oers of the Academic Medical Center in Amsterdam, The Netherlands, observed.
PROLONG randomized 474 patients with relapsed CLL to observation or ofatumumab 300 mg in week 1 and 1,000 mg in week 2, and every 8 weeks for 2 years. All patients were in second or third remission and within 3 months of response assessment after the last reinduction treatment. Patients with refractory disease or prior maintenance therapy or stem cell transplantation were excluded.
At baseline, the median age was about 65 years, 70% had at least two prior treatments, 80% were in partial remission from their last CLL treatment, and less than 10% had poor-risk cytogenetics 11p or 17p deletions. At the time of the analysis, 25% of patients had received all 13 cycles of ofatumumab.
Adverse events of any grade were increased with the addition of ofatumumab versus placebo (86% vs. 72%; P < .0001). Sixty percent were related to study treatment, but none resulted in study withdrawal, Dr. van Oers said. In all, 17 patients on the experimental arm dropped out due to physician decision or patient wish.
Among grade 3 events, neutropenia was significantly increased with maintenance therapy versus placebo (24% vs. 10%; P < .0001) and there was a non-significant increase in infections (13% vs. 8%). Five deaths occurred in the observation arm and two in the ofatumumab arm, one due to sepsis two months after the end of treatment and the other due to unrelated GI obstruction.
Median overall survival has not been reached for either arm (HR, 0.85; P = .487), he reported on behalf of HOVON and the NORDIC CLL group, co-developers of the study.
SAN FRANCISCO – Ofatumumab maintenance therapy nearly doubled progression-free survival in patients with relapsed CLL, according to a preplanned interim analysis of the phase III PROLONG study.
At a median follow-up of 19.1 months, progression-free survival was 15.2 months with the standard approach of observation alone and 29.4 months with maintenance ofatumumab (Hazard ratio, 0.50; P < .0001).
Ofatumumab (Arzerra) also significantly increased the median time to next treatment from 31.1 months to 38 months (HR, 0.66; P = .0108), Dr. Marinus van Oers reported at the annual meeting of the American Society of Hematology.
The benefit in progression-free survival (PFS) with maintenance was “statistically significant and clinical relevant” and was present in all subgroups, he said. It was independent of age, gender, number and type of prior treatment, minimal residual disease status at study entry, and “response at study entry, although we have the impression that it’s more effective in patients on PR [partial response] than in patients on CR [complete response],” he added.
The rationale for the trial lies in the fact that despite recent advances, there is still no curative treatment for chronic lymphocytic leukemia (CLL). Ofatumumab, a type 1 CD20 monoclonal antibody, has a role as maintenance in follicular lymphoma (FL), which shares similarities in biological behavior with CLL. This role is debated, but a recent meta-analysis shows ofatumumab maintenance prolongs PFS and tends to prolong overall survival in relapsed patients with FL, Dr. van Oers of the Academic Medical Center in Amsterdam, The Netherlands, observed.
PROLONG randomized 474 patients with relapsed CLL to observation or ofatumumab 300 mg in week 1 and 1,000 mg in week 2, and every 8 weeks for 2 years. All patients were in second or third remission and within 3 months of response assessment after the last reinduction treatment. Patients with refractory disease or prior maintenance therapy or stem cell transplantation were excluded.
At baseline, the median age was about 65 years, 70% had at least two prior treatments, 80% were in partial remission from their last CLL treatment, and less than 10% had poor-risk cytogenetics 11p or 17p deletions. At the time of the analysis, 25% of patients had received all 13 cycles of ofatumumab.
Adverse events of any grade were increased with the addition of ofatumumab versus placebo (86% vs. 72%; P < .0001). Sixty percent were related to study treatment, but none resulted in study withdrawal, Dr. van Oers said. In all, 17 patients on the experimental arm dropped out due to physician decision or patient wish.
Among grade 3 events, neutropenia was significantly increased with maintenance therapy versus placebo (24% vs. 10%; P < .0001) and there was a non-significant increase in infections (13% vs. 8%). Five deaths occurred in the observation arm and two in the ofatumumab arm, one due to sepsis two months after the end of treatment and the other due to unrelated GI obstruction.
Median overall survival has not been reached for either arm (HR, 0.85; P = .487), he reported on behalf of HOVON and the NORDIC CLL group, co-developers of the study.
SAN FRANCISCO – Ofatumumab maintenance therapy nearly doubled progression-free survival in patients with relapsed CLL, according to a preplanned interim analysis of the phase III PROLONG study.
At a median follow-up of 19.1 months, progression-free survival was 15.2 months with the standard approach of observation alone and 29.4 months with maintenance ofatumumab (Hazard ratio, 0.50; P < .0001).
Ofatumumab (Arzerra) also significantly increased the median time to next treatment from 31.1 months to 38 months (HR, 0.66; P = .0108), Dr. Marinus van Oers reported at the annual meeting of the American Society of Hematology.
The benefit in progression-free survival (PFS) with maintenance was “statistically significant and clinical relevant” and was present in all subgroups, he said. It was independent of age, gender, number and type of prior treatment, minimal residual disease status at study entry, and “response at study entry, although we have the impression that it’s more effective in patients on PR [partial response] than in patients on CR [complete response],” he added.
The rationale for the trial lies in the fact that despite recent advances, there is still no curative treatment for chronic lymphocytic leukemia (CLL). Ofatumumab, a type 1 CD20 monoclonal antibody, has a role as maintenance in follicular lymphoma (FL), which shares similarities in biological behavior with CLL. This role is debated, but a recent meta-analysis shows ofatumumab maintenance prolongs PFS and tends to prolong overall survival in relapsed patients with FL, Dr. van Oers of the Academic Medical Center in Amsterdam, The Netherlands, observed.
PROLONG randomized 474 patients with relapsed CLL to observation or ofatumumab 300 mg in week 1 and 1,000 mg in week 2, and every 8 weeks for 2 years. All patients were in second or third remission and within 3 months of response assessment after the last reinduction treatment. Patients with refractory disease or prior maintenance therapy or stem cell transplantation were excluded.
At baseline, the median age was about 65 years, 70% had at least two prior treatments, 80% were in partial remission from their last CLL treatment, and less than 10% had poor-risk cytogenetics 11p or 17p deletions. At the time of the analysis, 25% of patients had received all 13 cycles of ofatumumab.
Adverse events of any grade were increased with the addition of ofatumumab versus placebo (86% vs. 72%; P < .0001). Sixty percent were related to study treatment, but none resulted in study withdrawal, Dr. van Oers said. In all, 17 patients on the experimental arm dropped out due to physician decision or patient wish.
Among grade 3 events, neutropenia was significantly increased with maintenance therapy versus placebo (24% vs. 10%; P < .0001) and there was a non-significant increase in infections (13% vs. 8%). Five deaths occurred in the observation arm and two in the ofatumumab arm, one due to sepsis two months after the end of treatment and the other due to unrelated GI obstruction.
Median overall survival has not been reached for either arm (HR, 0.85; P = .487), he reported on behalf of HOVON and the NORDIC CLL group, co-developers of the study.
AT ASH 2014
Key clinical point: Maintenance ofatumumab cuts the risk of progression in half among patients with relapsed CLL.
Major finding: Progression-free survival was 15.2 months with observation alone and 29.4 months with maintenance ofatumumab (Hazard ratio, 0.50; P < .0001).
Data source: Randomized phase III trial in 474 patients with relapsed CLL.
Disclosures: GlaxoSmithKline sponsored the study. Dr. van Oers reported having no financial disclosures.
Brentuximab changes landscape for post-transplant Hodgkin’s lymphoma patients
SAN FRANCISCO – Early post-transplant brentuximab vedotin dramatically slows Hodgkin’s lymphoma progression in patients at high risk for relapse or progression, the phase III AETHERA study shows.
After a median follow-up of about 28 months, the primary end point of progression-free survival (PFS) per independent review increased from a median of 24 months with placebo and best supportive care (BSC) to 43 months with brentuximab and BSC (Hazard ratio, 0.57; P = .001).
Per investigator assessment, median PFS was 16 months with placebo and had not been reached with brentuximab (HR, 0.50),Dr. Craig Moskowitz reported at the annual meeting of the American Society of Hematology.
The benefit of brentuximab maintenance was consistent across every subgroup. Historically, roughly half of patients who undergo an autologous stem cell transplant will relapse.
“Once this study is published, in patients who met eligibility criteria to be on this study - and once again I’ll remind you that’s remission duration less than one year, disease outside the lymph node system, or primary refractory disease - in my opinion, this will be the standard of care,” Dr. Moskowitz said during a press briefing.
Brentuximab, an anti-CD30 antibody conjugate, is already approved in the U.S. for the management of Hodgkin’s lymphoma (HL) after failure of autologous stem cell transplantation (ASCT) or at least two prior lines of multi-agent chemotherapy in patients ineligible for ASCT.
Brentuximab is also indicated for systemic anaplastic large cell lymphoma after failure of at least one multi-agent chemotherapy regimen.
In September it was announced that AETHERA met its primary end point, but this was the first full look at the absolute survival rates and safety data.
The 2-year PFS rate for the brentuximab and placebo groups is now 63% vs. 51% per independent review and 65% vs. 45% per investigator.
“The bottom line is there’s a 20% difference in progression-free survival at 2 years upon investigator review. This has never been seen in patients with relapsed, refractory lymphoma, let alone Hodgkin lymphoma,” Dr. Moskowitz, clinical director of hematology oncology at Memorial Sloan-Kettering Cancer Center in New York City, said.
Overall survival data are immature, but was the same in both groups at 2 years (P = .62). The likelihood of showing a survival difference was not expected because 85% of patients who relapsed on the placebo arm crossed over to brentuximab, as allowed per protocol, and it’s known that brentuximab alone in auto-transplant failures improves outcomes by at least a year, he said. Also, twice as many patients who failed placebo received a second transplant.
Dr. Moskowitz stressed that nearly every patient in the trial had at least three risk factors that would place them at high risk of treatment failure and that studies have shown that for patients with this many risk factors, the chance of being cured by an auto-transplant is about 25%. The risk factors are: relapsed less than 12 months or refractory to frontline therapy, best response of partial remission or stable disease to most recent salvage therapy, extranodal disease at pre-ASCT relapse, B symptoms at pre-ASCT relapse, or two or more prior salvage therapies.
Press briefing moderator Dr. Brad Kahl of the University of Wisconsin-Madison, said Aethera is the first study to show a significant benefit for a post-transplant strategy.
“The biggest question in my mind is whether the application of maintenance brentuximab vedotin is just delaying the inevitable relapse, so the patients will still relapse, just later, or has the brentuximab taken patients who are destined to relapse and turned them into a cured patient,” Dr. Kahl said. “We don’t know the answer to that question. That will become apparent with more time.”
Dr. Moscowitz observed that relapses almost never happen after two year, adding, “So if you’re in remission at two years after stem cell transplantation for Hodgkin lymphoma, you are likely to be cured.”
AETHERA enrolled 329 patients with Hodgkin’s lymphoma and randomly assigned them after ASCT to brentuximab vedotin 1.8 mg/kg or placebo given every 3 weeks for up to 16 cycles, plus BSC. All patients were required to have achieved a complete response, partial remission, or stable disease to salvage therapy prior to ASCT. Their median age was 32 years and 53% were male.
Roughly 60% were refractory to upfront therapy, 43% in the brentuximab arm and 48% of controls had received 2 or more prior systemic therapies, and a third in each arm had extranodal involvement.
Consolidation therapy with brentuximab was generally well tolerated, Dr. Moskowitz said. Peripheral sensory neuropathy was the most common side effect, experienced at any grade in 67% on brentuximab vs. 19% of controls and at grade 3 in 13% vs. 1%. There were no grade 4 events and 85% of patients had resolution or improvement with dose reductions or stopping the drug.
Other adverse events in the brentuximab and control groups were neutropenia (35% vs. 12%), upper respiratory tract infections (26% vs. 23%), and fatigue (24% vs. 18%). Two patients died within 40 days of dosing with brentuximab, one from treatment-related acute respiratory distress syndrome associated with pneumonitis and one following an episode of treatment-related acute pancreatitis that had resolved at the time of death, he reported.
Based on the results, study sponsor Seattle Genetics is expected to seek approval for brentuximab in this consolidation setting in the first half of 2015, according to a statement from the company. The ongoing phase III ECHELON-1 and ECHELON 2 trials in HL and mature T-cell lymphomas are looking at the use of brentuximab in frontline disease.
SAN FRANCISCO – Early post-transplant brentuximab vedotin dramatically slows Hodgkin’s lymphoma progression in patients at high risk for relapse or progression, the phase III AETHERA study shows.
After a median follow-up of about 28 months, the primary end point of progression-free survival (PFS) per independent review increased from a median of 24 months with placebo and best supportive care (BSC) to 43 months with brentuximab and BSC (Hazard ratio, 0.57; P = .001).
Per investigator assessment, median PFS was 16 months with placebo and had not been reached with brentuximab (HR, 0.50),Dr. Craig Moskowitz reported at the annual meeting of the American Society of Hematology.
The benefit of brentuximab maintenance was consistent across every subgroup. Historically, roughly half of patients who undergo an autologous stem cell transplant will relapse.
“Once this study is published, in patients who met eligibility criteria to be on this study - and once again I’ll remind you that’s remission duration less than one year, disease outside the lymph node system, or primary refractory disease - in my opinion, this will be the standard of care,” Dr. Moskowitz said during a press briefing.
Brentuximab, an anti-CD30 antibody conjugate, is already approved in the U.S. for the management of Hodgkin’s lymphoma (HL) after failure of autologous stem cell transplantation (ASCT) or at least two prior lines of multi-agent chemotherapy in patients ineligible for ASCT.
Brentuximab is also indicated for systemic anaplastic large cell lymphoma after failure of at least one multi-agent chemotherapy regimen.
In September it was announced that AETHERA met its primary end point, but this was the first full look at the absolute survival rates and safety data.
The 2-year PFS rate for the brentuximab and placebo groups is now 63% vs. 51% per independent review and 65% vs. 45% per investigator.
“The bottom line is there’s a 20% difference in progression-free survival at 2 years upon investigator review. This has never been seen in patients with relapsed, refractory lymphoma, let alone Hodgkin lymphoma,” Dr. Moskowitz, clinical director of hematology oncology at Memorial Sloan-Kettering Cancer Center in New York City, said.
Overall survival data are immature, but was the same in both groups at 2 years (P = .62). The likelihood of showing a survival difference was not expected because 85% of patients who relapsed on the placebo arm crossed over to brentuximab, as allowed per protocol, and it’s known that brentuximab alone in auto-transplant failures improves outcomes by at least a year, he said. Also, twice as many patients who failed placebo received a second transplant.
Dr. Moskowitz stressed that nearly every patient in the trial had at least three risk factors that would place them at high risk of treatment failure and that studies have shown that for patients with this many risk factors, the chance of being cured by an auto-transplant is about 25%. The risk factors are: relapsed less than 12 months or refractory to frontline therapy, best response of partial remission or stable disease to most recent salvage therapy, extranodal disease at pre-ASCT relapse, B symptoms at pre-ASCT relapse, or two or more prior salvage therapies.
Press briefing moderator Dr. Brad Kahl of the University of Wisconsin-Madison, said Aethera is the first study to show a significant benefit for a post-transplant strategy.
“The biggest question in my mind is whether the application of maintenance brentuximab vedotin is just delaying the inevitable relapse, so the patients will still relapse, just later, or has the brentuximab taken patients who are destined to relapse and turned them into a cured patient,” Dr. Kahl said. “We don’t know the answer to that question. That will become apparent with more time.”
Dr. Moscowitz observed that relapses almost never happen after two year, adding, “So if you’re in remission at two years after stem cell transplantation for Hodgkin lymphoma, you are likely to be cured.”
AETHERA enrolled 329 patients with Hodgkin’s lymphoma and randomly assigned them after ASCT to brentuximab vedotin 1.8 mg/kg or placebo given every 3 weeks for up to 16 cycles, plus BSC. All patients were required to have achieved a complete response, partial remission, or stable disease to salvage therapy prior to ASCT. Their median age was 32 years and 53% were male.
Roughly 60% were refractory to upfront therapy, 43% in the brentuximab arm and 48% of controls had received 2 or more prior systemic therapies, and a third in each arm had extranodal involvement.
Consolidation therapy with brentuximab was generally well tolerated, Dr. Moskowitz said. Peripheral sensory neuropathy was the most common side effect, experienced at any grade in 67% on brentuximab vs. 19% of controls and at grade 3 in 13% vs. 1%. There were no grade 4 events and 85% of patients had resolution or improvement with dose reductions or stopping the drug.
Other adverse events in the brentuximab and control groups were neutropenia (35% vs. 12%), upper respiratory tract infections (26% vs. 23%), and fatigue (24% vs. 18%). Two patients died within 40 days of dosing with brentuximab, one from treatment-related acute respiratory distress syndrome associated with pneumonitis and one following an episode of treatment-related acute pancreatitis that had resolved at the time of death, he reported.
Based on the results, study sponsor Seattle Genetics is expected to seek approval for brentuximab in this consolidation setting in the first half of 2015, according to a statement from the company. The ongoing phase III ECHELON-1 and ECHELON 2 trials in HL and mature T-cell lymphomas are looking at the use of brentuximab in frontline disease.
SAN FRANCISCO – Early post-transplant brentuximab vedotin dramatically slows Hodgkin’s lymphoma progression in patients at high risk for relapse or progression, the phase III AETHERA study shows.
After a median follow-up of about 28 months, the primary end point of progression-free survival (PFS) per independent review increased from a median of 24 months with placebo and best supportive care (BSC) to 43 months with brentuximab and BSC (Hazard ratio, 0.57; P = .001).
Per investigator assessment, median PFS was 16 months with placebo and had not been reached with brentuximab (HR, 0.50),Dr. Craig Moskowitz reported at the annual meeting of the American Society of Hematology.
The benefit of brentuximab maintenance was consistent across every subgroup. Historically, roughly half of patients who undergo an autologous stem cell transplant will relapse.
“Once this study is published, in patients who met eligibility criteria to be on this study - and once again I’ll remind you that’s remission duration less than one year, disease outside the lymph node system, or primary refractory disease - in my opinion, this will be the standard of care,” Dr. Moskowitz said during a press briefing.
Brentuximab, an anti-CD30 antibody conjugate, is already approved in the U.S. for the management of Hodgkin’s lymphoma (HL) after failure of autologous stem cell transplantation (ASCT) or at least two prior lines of multi-agent chemotherapy in patients ineligible for ASCT.
Brentuximab is also indicated for systemic anaplastic large cell lymphoma after failure of at least one multi-agent chemotherapy regimen.
In September it was announced that AETHERA met its primary end point, but this was the first full look at the absolute survival rates and safety data.
The 2-year PFS rate for the brentuximab and placebo groups is now 63% vs. 51% per independent review and 65% vs. 45% per investigator.
“The bottom line is there’s a 20% difference in progression-free survival at 2 years upon investigator review. This has never been seen in patients with relapsed, refractory lymphoma, let alone Hodgkin lymphoma,” Dr. Moskowitz, clinical director of hematology oncology at Memorial Sloan-Kettering Cancer Center in New York City, said.
Overall survival data are immature, but was the same in both groups at 2 years (P = .62). The likelihood of showing a survival difference was not expected because 85% of patients who relapsed on the placebo arm crossed over to brentuximab, as allowed per protocol, and it’s known that brentuximab alone in auto-transplant failures improves outcomes by at least a year, he said. Also, twice as many patients who failed placebo received a second transplant.
Dr. Moskowitz stressed that nearly every patient in the trial had at least three risk factors that would place them at high risk of treatment failure and that studies have shown that for patients with this many risk factors, the chance of being cured by an auto-transplant is about 25%. The risk factors are: relapsed less than 12 months or refractory to frontline therapy, best response of partial remission or stable disease to most recent salvage therapy, extranodal disease at pre-ASCT relapse, B symptoms at pre-ASCT relapse, or two or more prior salvage therapies.
Press briefing moderator Dr. Brad Kahl of the University of Wisconsin-Madison, said Aethera is the first study to show a significant benefit for a post-transplant strategy.
“The biggest question in my mind is whether the application of maintenance brentuximab vedotin is just delaying the inevitable relapse, so the patients will still relapse, just later, or has the brentuximab taken patients who are destined to relapse and turned them into a cured patient,” Dr. Kahl said. “We don’t know the answer to that question. That will become apparent with more time.”
Dr. Moscowitz observed that relapses almost never happen after two year, adding, “So if you’re in remission at two years after stem cell transplantation for Hodgkin lymphoma, you are likely to be cured.”
AETHERA enrolled 329 patients with Hodgkin’s lymphoma and randomly assigned them after ASCT to brentuximab vedotin 1.8 mg/kg or placebo given every 3 weeks for up to 16 cycles, plus BSC. All patients were required to have achieved a complete response, partial remission, or stable disease to salvage therapy prior to ASCT. Their median age was 32 years and 53% were male.
Roughly 60% were refractory to upfront therapy, 43% in the brentuximab arm and 48% of controls had received 2 or more prior systemic therapies, and a third in each arm had extranodal involvement.
Consolidation therapy with brentuximab was generally well tolerated, Dr. Moskowitz said. Peripheral sensory neuropathy was the most common side effect, experienced at any grade in 67% on brentuximab vs. 19% of controls and at grade 3 in 13% vs. 1%. There were no grade 4 events and 85% of patients had resolution or improvement with dose reductions or stopping the drug.
Other adverse events in the brentuximab and control groups were neutropenia (35% vs. 12%), upper respiratory tract infections (26% vs. 23%), and fatigue (24% vs. 18%). Two patients died within 40 days of dosing with brentuximab, one from treatment-related acute respiratory distress syndrome associated with pneumonitis and one following an episode of treatment-related acute pancreatitis that had resolved at the time of death, he reported.
Based on the results, study sponsor Seattle Genetics is expected to seek approval for brentuximab in this consolidation setting in the first half of 2015, according to a statement from the company. The ongoing phase III ECHELON-1 and ECHELON 2 trials in HL and mature T-cell lymphomas are looking at the use of brentuximab in frontline disease.
AT ASH 2014
Key clinical point: Brentuximab vedotin given immediately post-transplant significantly improves progression-free survival in patients with Hodgkin’s lymphoma at high risk for progression.
Major finding: Median progression-free survival per independent review was 24 months with placebo vs. 43 months with brentuximab (HR, 0.57; P = .001).
Data source: Randomized, double-blind, phase III study in 329 patients with Hodgkin’s lymphoma.
Disclosures: Seattle Genetics sponsored the study. Dr. Moskowitz reported research funding from Genentech and Merck, and research funding from and consultancy for Seattle Genetics. Several co-authors reported financial ties with industry, including employment with or equity ownership in Seattle Genetics.
PD-1 checkpoint inhibitors show mettle against relapsed Hodgkin’s lymphoma
SAN FRANCISCO– PD-1 checkpoint inhibitors, which have shown remarkable efficacy against advanced malignant melanoma, appear to hold similar promise in the treatment of relapsed or refractory Hodgkin’s lymphoma, results from two early studies suggest.
In a phase I study, the PD-1 blocking antibody nivolumab produced an 87% response rate in 23 heavily pre-treated patients with relapsed Hodgkin’s lymphoma (HL). In a separate phase Ib study, pembrolizumab, which blocks the PD-1 and PD-2 ligands, produced a 66% overall response rate, 21% complete remission rate, and 86% clinical benefit rate among 29 patients with HL for whom therapy with brentuximab vedotin (Adcetris) had failed.
The studies were presented at a media briefing prior to the presentation of data in oral sessions at the annual meeting of the American Society of Hematology.
“Classical Hodgkin lymphoma appears to be a tumor with genetically determined vulnerability to PD-1 blockade. We hope that PD-1 blockade in the future can become an important part of the treatment of patients with Hodgkin lymphoma,” said Dr. Phillipe Armand from the Dana-Farber Cancer Institute in Boston, an investigator for the nivolumab study.
Evidence from preclinical studies suggests that the Reed-Sternberg malignant cells characteristic of HL may use the PD-1 (programmed death 1) pathway to evade detection by immune cells, as suggested by pathologic studies showing the cells surrounded by an extensive but ineffective infiltrate of inflammatory cells.
“We’ve wondered for a long time how Hodgkin lymphoma could attract such a brisk immune response and yet have this immune response fail to kill the tumor,” he said.
Genetic Achilles heel
Genetic analyses had shown that HL frequently has a mutation that results in amplification of a region on chromosome 9 (9p24.1) which leads to increased expression of PD-1 ligands 1 and 2, and leads to a downregulation or weakening of the immune response. The mutation appears to work through the Janus kinase (JAK)-signal transducer and activator transcription (STAT) signalling. These findings suggested to researchers that classical HL has a genetically driven and, ideally, targetable dependence on the PD-1 pathway for survival, Dr. Armand explained.
To test this idea, he and colleagues studied 23 patients with relapsed or refractory HL that had been heavily pre-treated who were part of an independent expansion cohort of a study of nivolumab in hematologic malignancies. Of these patients, 78% were enrolled after a relapse following autologous stem cell transplantation, and 22% after treatment with brentuximab vedotin had failed.
The patients received nivolumab 3 mg/kg every 2 weeks until they had either a complete response, tumor progression, or excessive side effects. In all, 20 of the 23 patients (87%) had an objective response to the single-agent therapy, including 4 (17%) complete responses and 16 (70%) partial responses. The remaining three patients (13%) had stable disease.
The longest time on study at the data cutoff point was 72 weeks. Among all responders, 60% had a response by 8 weeks of therapy, 48% are ongoing, and 43% of patients are still on treatment.
Drug-related adverse events were reported in 18 patients, most commonly rash and decreased platelet count. Five patients had grade 3 events. There were no drug-related grade 4 events or deaths.
In an editorial accompanying the study, which was also published online in The New England Journal of Medicine, Dr. Mario Sznoll and Dr. Dan L. Longo from the Yale University School of Medicine in New Haven, Connecticut write that “with recent data showing impressive clinical activity of PD-1 or PD-L1 antagonists in subgroups of patients with a variety of different cancers, the critical and foundational role of immune interventions in cancer treatment is no longer deniable,” (NEJM, Dec. 6, 2014 [DOI: 10.1056/NEJMoa1411087]).
Pembrolizumab trial
Dr. Craig H. Moskowitz from Memorial Sloan-Kettering Cancer Center in New York City discussed results of the second study, dubbed KEYNOTE-013 (A Phase Ib Multi-Cohort Trial of MK-3475 in Subjects With Hematologic Malignancies).
In this study, patients with HL who were not transplant eligible or for whom transplant had failed and who either had a relapse or were refractory to therapy with brentuximab vedotin received 19 mg/kg IV infusion of pembrolizumab every 2 weeks until complete response, partial response/stable disease, or disease progression.
Of the 31 patients enrolled, 29 were available for the analysis. As of the data cutoff in November 2014, 6 patients (21%) had achieved a complete remission, and 13 (45%) had a partial response, for an overall response rate of 66%. The median time to response was 12 weeks, and as of the data cutoff 17 of 19 patients had ongoing responses. The median response duration has not yet been reached. An additional 6 patients (21%) had stable disease, leading to an overall clinical benefit rate (responses plus stable disease) of 86%.
The patients generally tolerated the drug well. There were 4 treatment-related adverse events in 3 patients, including axillary pain, hypoxia, joint swelling, and pneumonitis. There were no grade 4 treatment-related events or deaths.
Of the tumor samples evaluable, all expressed PD-L1, supporting the rationale for PD-1 blockade in this population, Dr. Moskowitz said.
The results of both his and Dr. Armand’s study support the continued development of PD-1 inhibitors in various subsets of patients with classical Hodgkin’s lymphoma, he said.
SAN FRANCISCO– PD-1 checkpoint inhibitors, which have shown remarkable efficacy against advanced malignant melanoma, appear to hold similar promise in the treatment of relapsed or refractory Hodgkin’s lymphoma, results from two early studies suggest.
In a phase I study, the PD-1 blocking antibody nivolumab produced an 87% response rate in 23 heavily pre-treated patients with relapsed Hodgkin’s lymphoma (HL). In a separate phase Ib study, pembrolizumab, which blocks the PD-1 and PD-2 ligands, produced a 66% overall response rate, 21% complete remission rate, and 86% clinical benefit rate among 29 patients with HL for whom therapy with brentuximab vedotin (Adcetris) had failed.
The studies were presented at a media briefing prior to the presentation of data in oral sessions at the annual meeting of the American Society of Hematology.
“Classical Hodgkin lymphoma appears to be a tumor with genetically determined vulnerability to PD-1 blockade. We hope that PD-1 blockade in the future can become an important part of the treatment of patients with Hodgkin lymphoma,” said Dr. Phillipe Armand from the Dana-Farber Cancer Institute in Boston, an investigator for the nivolumab study.
Evidence from preclinical studies suggests that the Reed-Sternberg malignant cells characteristic of HL may use the PD-1 (programmed death 1) pathway to evade detection by immune cells, as suggested by pathologic studies showing the cells surrounded by an extensive but ineffective infiltrate of inflammatory cells.
“We’ve wondered for a long time how Hodgkin lymphoma could attract such a brisk immune response and yet have this immune response fail to kill the tumor,” he said.
Genetic Achilles heel
Genetic analyses had shown that HL frequently has a mutation that results in amplification of a region on chromosome 9 (9p24.1) which leads to increased expression of PD-1 ligands 1 and 2, and leads to a downregulation or weakening of the immune response. The mutation appears to work through the Janus kinase (JAK)-signal transducer and activator transcription (STAT) signalling. These findings suggested to researchers that classical HL has a genetically driven and, ideally, targetable dependence on the PD-1 pathway for survival, Dr. Armand explained.
To test this idea, he and colleagues studied 23 patients with relapsed or refractory HL that had been heavily pre-treated who were part of an independent expansion cohort of a study of nivolumab in hematologic malignancies. Of these patients, 78% were enrolled after a relapse following autologous stem cell transplantation, and 22% after treatment with brentuximab vedotin had failed.
The patients received nivolumab 3 mg/kg every 2 weeks until they had either a complete response, tumor progression, or excessive side effects. In all, 20 of the 23 patients (87%) had an objective response to the single-agent therapy, including 4 (17%) complete responses and 16 (70%) partial responses. The remaining three patients (13%) had stable disease.
The longest time on study at the data cutoff point was 72 weeks. Among all responders, 60% had a response by 8 weeks of therapy, 48% are ongoing, and 43% of patients are still on treatment.
Drug-related adverse events were reported in 18 patients, most commonly rash and decreased platelet count. Five patients had grade 3 events. There were no drug-related grade 4 events or deaths.
In an editorial accompanying the study, which was also published online in The New England Journal of Medicine, Dr. Mario Sznoll and Dr. Dan L. Longo from the Yale University School of Medicine in New Haven, Connecticut write that “with recent data showing impressive clinical activity of PD-1 or PD-L1 antagonists in subgroups of patients with a variety of different cancers, the critical and foundational role of immune interventions in cancer treatment is no longer deniable,” (NEJM, Dec. 6, 2014 [DOI: 10.1056/NEJMoa1411087]).
Pembrolizumab trial
Dr. Craig H. Moskowitz from Memorial Sloan-Kettering Cancer Center in New York City discussed results of the second study, dubbed KEYNOTE-013 (A Phase Ib Multi-Cohort Trial of MK-3475 in Subjects With Hematologic Malignancies).
In this study, patients with HL who were not transplant eligible or for whom transplant had failed and who either had a relapse or were refractory to therapy with brentuximab vedotin received 19 mg/kg IV infusion of pembrolizumab every 2 weeks until complete response, partial response/stable disease, or disease progression.
Of the 31 patients enrolled, 29 were available for the analysis. As of the data cutoff in November 2014, 6 patients (21%) had achieved a complete remission, and 13 (45%) had a partial response, for an overall response rate of 66%. The median time to response was 12 weeks, and as of the data cutoff 17 of 19 patients had ongoing responses. The median response duration has not yet been reached. An additional 6 patients (21%) had stable disease, leading to an overall clinical benefit rate (responses plus stable disease) of 86%.
The patients generally tolerated the drug well. There were 4 treatment-related adverse events in 3 patients, including axillary pain, hypoxia, joint swelling, and pneumonitis. There were no grade 4 treatment-related events or deaths.
Of the tumor samples evaluable, all expressed PD-L1, supporting the rationale for PD-1 blockade in this population, Dr. Moskowitz said.
The results of both his and Dr. Armand’s study support the continued development of PD-1 inhibitors in various subsets of patients with classical Hodgkin’s lymphoma, he said.
SAN FRANCISCO– PD-1 checkpoint inhibitors, which have shown remarkable efficacy against advanced malignant melanoma, appear to hold similar promise in the treatment of relapsed or refractory Hodgkin’s lymphoma, results from two early studies suggest.
In a phase I study, the PD-1 blocking antibody nivolumab produced an 87% response rate in 23 heavily pre-treated patients with relapsed Hodgkin’s lymphoma (HL). In a separate phase Ib study, pembrolizumab, which blocks the PD-1 and PD-2 ligands, produced a 66% overall response rate, 21% complete remission rate, and 86% clinical benefit rate among 29 patients with HL for whom therapy with brentuximab vedotin (Adcetris) had failed.
The studies were presented at a media briefing prior to the presentation of data in oral sessions at the annual meeting of the American Society of Hematology.
“Classical Hodgkin lymphoma appears to be a tumor with genetically determined vulnerability to PD-1 blockade. We hope that PD-1 blockade in the future can become an important part of the treatment of patients with Hodgkin lymphoma,” said Dr. Phillipe Armand from the Dana-Farber Cancer Institute in Boston, an investigator for the nivolumab study.
Evidence from preclinical studies suggests that the Reed-Sternberg malignant cells characteristic of HL may use the PD-1 (programmed death 1) pathway to evade detection by immune cells, as suggested by pathologic studies showing the cells surrounded by an extensive but ineffective infiltrate of inflammatory cells.
“We’ve wondered for a long time how Hodgkin lymphoma could attract such a brisk immune response and yet have this immune response fail to kill the tumor,” he said.
Genetic Achilles heel
Genetic analyses had shown that HL frequently has a mutation that results in amplification of a region on chromosome 9 (9p24.1) which leads to increased expression of PD-1 ligands 1 and 2, and leads to a downregulation or weakening of the immune response. The mutation appears to work through the Janus kinase (JAK)-signal transducer and activator transcription (STAT) signalling. These findings suggested to researchers that classical HL has a genetically driven and, ideally, targetable dependence on the PD-1 pathway for survival, Dr. Armand explained.
To test this idea, he and colleagues studied 23 patients with relapsed or refractory HL that had been heavily pre-treated who were part of an independent expansion cohort of a study of nivolumab in hematologic malignancies. Of these patients, 78% were enrolled after a relapse following autologous stem cell transplantation, and 22% after treatment with brentuximab vedotin had failed.
The patients received nivolumab 3 mg/kg every 2 weeks until they had either a complete response, tumor progression, or excessive side effects. In all, 20 of the 23 patients (87%) had an objective response to the single-agent therapy, including 4 (17%) complete responses and 16 (70%) partial responses. The remaining three patients (13%) had stable disease.
The longest time on study at the data cutoff point was 72 weeks. Among all responders, 60% had a response by 8 weeks of therapy, 48% are ongoing, and 43% of patients are still on treatment.
Drug-related adverse events were reported in 18 patients, most commonly rash and decreased platelet count. Five patients had grade 3 events. There were no drug-related grade 4 events or deaths.
In an editorial accompanying the study, which was also published online in The New England Journal of Medicine, Dr. Mario Sznoll and Dr. Dan L. Longo from the Yale University School of Medicine in New Haven, Connecticut write that “with recent data showing impressive clinical activity of PD-1 or PD-L1 antagonists in subgroups of patients with a variety of different cancers, the critical and foundational role of immune interventions in cancer treatment is no longer deniable,” (NEJM, Dec. 6, 2014 [DOI: 10.1056/NEJMoa1411087]).
Pembrolizumab trial
Dr. Craig H. Moskowitz from Memorial Sloan-Kettering Cancer Center in New York City discussed results of the second study, dubbed KEYNOTE-013 (A Phase Ib Multi-Cohort Trial of MK-3475 in Subjects With Hematologic Malignancies).
In this study, patients with HL who were not transplant eligible or for whom transplant had failed and who either had a relapse or were refractory to therapy with brentuximab vedotin received 19 mg/kg IV infusion of pembrolizumab every 2 weeks until complete response, partial response/stable disease, or disease progression.
Of the 31 patients enrolled, 29 were available for the analysis. As of the data cutoff in November 2014, 6 patients (21%) had achieved a complete remission, and 13 (45%) had a partial response, for an overall response rate of 66%. The median time to response was 12 weeks, and as of the data cutoff 17 of 19 patients had ongoing responses. The median response duration has not yet been reached. An additional 6 patients (21%) had stable disease, leading to an overall clinical benefit rate (responses plus stable disease) of 86%.
The patients generally tolerated the drug well. There were 4 treatment-related adverse events in 3 patients, including axillary pain, hypoxia, joint swelling, and pneumonitis. There were no grade 4 treatment-related events or deaths.
Of the tumor samples evaluable, all expressed PD-L1, supporting the rationale for PD-1 blockade in this population, Dr. Moskowitz said.
The results of both his and Dr. Armand’s study support the continued development of PD-1 inhibitors in various subsets of patients with classical Hodgkin’s lymphoma, he said.
Key clinical point: PD-1 checkpoint inhibition appears to be an effective strategy against treatment-refractory Hodgkin’s lymphoma.
Major finding: Nivolumab produced an 87% objective response rate and pembrolizumab a 66% response rate in patients with heavily pre-treated Hodgkin’s lymphoma.
Data source: A phase I study with 23 patients and a phase Ib study with 29 patients with relapsed or refractory Hodgkin’s lymphoma.
Disclosures: Dr. Armand’s study is supported by Bristol-Myers Squibb. He reported grant support from Bristol-Myers Squibb during the conduct of the study and personal fees from Merck outside the study. Dr. Moskowitz’ study is supported by Merck. He reported receiving research funding from the company. Dr. Sznol reported personal fees from Bristol-Myers Squibb, Dr. Longo reported no relevant disclosures.
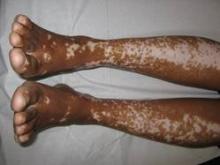
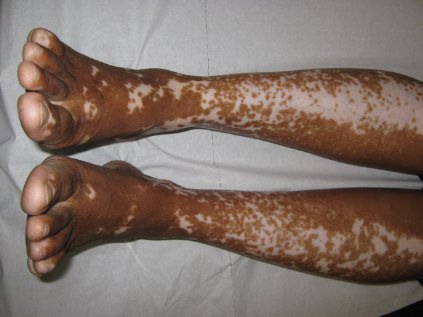
Vitiligo, alopecia more likely in GVHD when donor is female and recipient is male
The development of vitiligo or alopecia areata is not common in patients with chronic graft-versus-host disease, but it is significantly more likely to occur when the donor was female, especially when the recipient was male, according to a report published online Sept. 10 in JAMA Dermatology.
Fifteen case reports and small series in the literature have reported the development of vitiligo or alopecia areata after allogeneic hematopoietic stem cell transplantation, most often among patients who first developed chronic graft-versus-host disease (GVHD) after the procedure. To further explore the frequency of these two skin autoimmune manifestations in GVHD and to identify associated risk factors, researchers performed a retrospective cross-sectional analysis involving 282 adult and pediatric patients referred to the National Institutes of Health Clinical Center for GVHD in 2004-2013.
They identified 15 GVHD patients with vitiligo (4.9%) and 2 with alopecia areata (0.7%); one of these patients had both skin disorders. Most of the 15 patients had undergone stem-cell transplantation to treat chronic myelogenous leukemia (CML) (5 patients) or acute leukemia or myelodysplastic syndrome (5 patients), and most had had a human leukocyte antigen–identical donor. Twelve of the 15 developed the skin disorder after having GVHD for more than 1 year, said Rena C. Zuo of the National Cancer Institute’s dermatology branch and her associates.
"Notably, CML accounts for only about 300 of 7,892 (3.8%) allogeneic hematopoietic stem-cell transplantations per year in the United States, a relative minority among indicated diseases," they said.
In what they described as the first study to identify an association between donor/recipient sex mismatch and the development of concomitant autoimmunity in patients with chronic GVHD, the investigators found that 14 of the 15 patients who developed vitiligo or alopecia areata had female donors, 2 of whom had previously given birth; the gender of the donor in the 15th case was unknown. Nine of these 14 recipients were male, "resulting in a female-to-male sex mismatch in [at least] 64% of cases," Ms. Zuo and her colleagues said (JAMA Dermatol. 2014 Sept. 10 [doi:10.1001/jamadermatol.2014.1550]).
Both parous female donors and donor-recipient sex mismatch are known risk factors for GVHD. The risk of autoimmunity in female-to-male transplants "may reflect the activity of skin-homing donor T cells specific for recipient minor histocompatibility antigens encoded by Y-chromosome genes, a mechanism previously implicated in both GVHD and graft-versus-tumor responses," the investigators added.
This study was supported by the National Institutes of Health and the National Cancer Institute. Ms. Zuo and her associates reported no financial conflicts of interest.
The development of vitiligo or alopecia areata is not common in patients with chronic graft-versus-host disease, but it is significantly more likely to occur when the donor was female, especially when the recipient was male, according to a report published online Sept. 10 in JAMA Dermatology.
Fifteen case reports and small series in the literature have reported the development of vitiligo or alopecia areata after allogeneic hematopoietic stem cell transplantation, most often among patients who first developed chronic graft-versus-host disease (GVHD) after the procedure. To further explore the frequency of these two skin autoimmune manifestations in GVHD and to identify associated risk factors, researchers performed a retrospective cross-sectional analysis involving 282 adult and pediatric patients referred to the National Institutes of Health Clinical Center for GVHD in 2004-2013.
They identified 15 GVHD patients with vitiligo (4.9%) and 2 with alopecia areata (0.7%); one of these patients had both skin disorders. Most of the 15 patients had undergone stem-cell transplantation to treat chronic myelogenous leukemia (CML) (5 patients) or acute leukemia or myelodysplastic syndrome (5 patients), and most had had a human leukocyte antigen–identical donor. Twelve of the 15 developed the skin disorder after having GVHD for more than 1 year, said Rena C. Zuo of the National Cancer Institute’s dermatology branch and her associates.
"Notably, CML accounts for only about 300 of 7,892 (3.8%) allogeneic hematopoietic stem-cell transplantations per year in the United States, a relative minority among indicated diseases," they said.
In what they described as the first study to identify an association between donor/recipient sex mismatch and the development of concomitant autoimmunity in patients with chronic GVHD, the investigators found that 14 of the 15 patients who developed vitiligo or alopecia areata had female donors, 2 of whom had previously given birth; the gender of the donor in the 15th case was unknown. Nine of these 14 recipients were male, "resulting in a female-to-male sex mismatch in [at least] 64% of cases," Ms. Zuo and her colleagues said (JAMA Dermatol. 2014 Sept. 10 [doi:10.1001/jamadermatol.2014.1550]).
Both parous female donors and donor-recipient sex mismatch are known risk factors for GVHD. The risk of autoimmunity in female-to-male transplants "may reflect the activity of skin-homing donor T cells specific for recipient minor histocompatibility antigens encoded by Y-chromosome genes, a mechanism previously implicated in both GVHD and graft-versus-tumor responses," the investigators added.
This study was supported by the National Institutes of Health and the National Cancer Institute. Ms. Zuo and her associates reported no financial conflicts of interest.
The development of vitiligo or alopecia areata is not common in patients with chronic graft-versus-host disease, but it is significantly more likely to occur when the donor was female, especially when the recipient was male, according to a report published online Sept. 10 in JAMA Dermatology.
Fifteen case reports and small series in the literature have reported the development of vitiligo or alopecia areata after allogeneic hematopoietic stem cell transplantation, most often among patients who first developed chronic graft-versus-host disease (GVHD) after the procedure. To further explore the frequency of these two skin autoimmune manifestations in GVHD and to identify associated risk factors, researchers performed a retrospective cross-sectional analysis involving 282 adult and pediatric patients referred to the National Institutes of Health Clinical Center for GVHD in 2004-2013.
They identified 15 GVHD patients with vitiligo (4.9%) and 2 with alopecia areata (0.7%); one of these patients had both skin disorders. Most of the 15 patients had undergone stem-cell transplantation to treat chronic myelogenous leukemia (CML) (5 patients) or acute leukemia or myelodysplastic syndrome (5 patients), and most had had a human leukocyte antigen–identical donor. Twelve of the 15 developed the skin disorder after having GVHD for more than 1 year, said Rena C. Zuo of the National Cancer Institute’s dermatology branch and her associates.
"Notably, CML accounts for only about 300 of 7,892 (3.8%) allogeneic hematopoietic stem-cell transplantations per year in the United States, a relative minority among indicated diseases," they said.
In what they described as the first study to identify an association between donor/recipient sex mismatch and the development of concomitant autoimmunity in patients with chronic GVHD, the investigators found that 14 of the 15 patients who developed vitiligo or alopecia areata had female donors, 2 of whom had previously given birth; the gender of the donor in the 15th case was unknown. Nine of these 14 recipients were male, "resulting in a female-to-male sex mismatch in [at least] 64% of cases," Ms. Zuo and her colleagues said (JAMA Dermatol. 2014 Sept. 10 [doi:10.1001/jamadermatol.2014.1550]).
Both parous female donors and donor-recipient sex mismatch are known risk factors for GVHD. The risk of autoimmunity in female-to-male transplants "may reflect the activity of skin-homing donor T cells specific for recipient minor histocompatibility antigens encoded by Y-chromosome genes, a mechanism previously implicated in both GVHD and graft-versus-tumor responses," the investigators added.
This study was supported by the National Institutes of Health and the National Cancer Institute. Ms. Zuo and her associates reported no financial conflicts of interest.
FROM JAMA DERMATOLOGY
Key clinical point: Female-to-male stem cell donation ups the risk for vitiligo in GVHD.
Major finding: 14 of the 15 patients who developed vitiligo or alopecia areata had female donors (the gender of the 15th donor was unknown), and 9 of these 14 recipients were male.
Data source: A retrospective cross-sectional analysis involving 282 adults and children with chronic GVHD, 15 of whom developed vitiligo or alopecia areata.
Disclosures: This study was supported by the National Institutes of Health and the National Cancer Institute. Dr. Zuo and her associates reported no financial conflicts of interest.
Imatinib appears safe, effective for the long haul
CHICAGO – After a decade on therapy with imatinib, a majority of patients with chronic myeloid leukemia will experience an adverse drug reaction, but most reactions are mild and manageable, according to results from a study presented at the annual meeting of the American Society of Clinical Oncology.
Of 1,375 patients with CML who received imatinib (Gleevec) monotherapy at some point, 1,018 (74%) had nonhematologic toxicities sometime during therapy, but only 199 (14%) had grade 3 or 4 toxicities, and there were no deaths attributed to imatinib, reported Dr. Rüdiger Hehlmann of the University of Heidelberg, Germany, and his colleagues.
Adverse drug reactions were manageable even when imatinib was combined with interferon-alfa (IFN-alfa), the investigators from the German CML Study Group reported in a poster at the meeting.
"After 10 years, imatinib continues to be an excellent choice for most patients with CML," they wrote.
In the 13 years that have elapsed since imatinib was approved in the United States as the first-in-class tyrosine kinase inhibitor, second-generation TKIs and other targeted agents have emerged, drawing attention to the safety of the older regimen.
The investigators evaluated long-term follow-up data and analyzed adverse drug reaction data for 1,501 patients treated with imatinib monotherapy in doses of 400 or 800 mg/day, as well as imatinib 400 mg in combination with IFN-alfa.
At the most recent evaluation, in November 2013, 164 patients had died, 1,003 were still on imatinib, 275 had been switched to a second-generation TKI, and 106 underwent bone marrow transplant (some patients received more than one therapy, accounting for the difference in total numbers).
The median follow-up time was 6.5 years, with some patients on study for as long as 11.5 years.
The probability of 10-year survival was 84%, and of 10-year progression-free survival was 81%.
An analysis of survival by molecular response rates showed an overall survival rate of 89% for those who achieved a major molecular response (MR, defined as a BCR-ABL RNA level of 0.1% or less), and 74% for those who achieved MR 4.5 (a 4.5 log10reduction or greater in BCR-ABL transcripts).
The 8-year probabilities for all grades of adverse events among the patients who received imatinib monotherapy were 41% for edema or fluid overload, 38% for gastrointestinal toxicities, 25% for myalgia/arthralgia, 20% for rash, 17% for musculoskeletal events, 17% for fatigue, 11% for neurological toxicities, and 10% for flulike symptoms.
Five patients had grade 2 or 3 peripheral arterial occlusive disease, but it was not clear whether these events were associated with imatinib.
For most patients the first adverse drug reaction occurred within 3 years of starting on imatinib, with the frequency of reactions decreasing thereafter.
Dr. Hehlmann disclosed receiving research support from Novartis, marketer of imatinib.
CHICAGO – After a decade on therapy with imatinib, a majority of patients with chronic myeloid leukemia will experience an adverse drug reaction, but most reactions are mild and manageable, according to results from a study presented at the annual meeting of the American Society of Clinical Oncology.
Of 1,375 patients with CML who received imatinib (Gleevec) monotherapy at some point, 1,018 (74%) had nonhematologic toxicities sometime during therapy, but only 199 (14%) had grade 3 or 4 toxicities, and there were no deaths attributed to imatinib, reported Dr. Rüdiger Hehlmann of the University of Heidelberg, Germany, and his colleagues.
Adverse drug reactions were manageable even when imatinib was combined with interferon-alfa (IFN-alfa), the investigators from the German CML Study Group reported in a poster at the meeting.
"After 10 years, imatinib continues to be an excellent choice for most patients with CML," they wrote.
In the 13 years that have elapsed since imatinib was approved in the United States as the first-in-class tyrosine kinase inhibitor, second-generation TKIs and other targeted agents have emerged, drawing attention to the safety of the older regimen.
The investigators evaluated long-term follow-up data and analyzed adverse drug reaction data for 1,501 patients treated with imatinib monotherapy in doses of 400 or 800 mg/day, as well as imatinib 400 mg in combination with IFN-alfa.
At the most recent evaluation, in November 2013, 164 patients had died, 1,003 were still on imatinib, 275 had been switched to a second-generation TKI, and 106 underwent bone marrow transplant (some patients received more than one therapy, accounting for the difference in total numbers).
The median follow-up time was 6.5 years, with some patients on study for as long as 11.5 years.
The probability of 10-year survival was 84%, and of 10-year progression-free survival was 81%.
An analysis of survival by molecular response rates showed an overall survival rate of 89% for those who achieved a major molecular response (MR, defined as a BCR-ABL RNA level of 0.1% or less), and 74% for those who achieved MR 4.5 (a 4.5 log10reduction or greater in BCR-ABL transcripts).
The 8-year probabilities for all grades of adverse events among the patients who received imatinib monotherapy were 41% for edema or fluid overload, 38% for gastrointestinal toxicities, 25% for myalgia/arthralgia, 20% for rash, 17% for musculoskeletal events, 17% for fatigue, 11% for neurological toxicities, and 10% for flulike symptoms.
Five patients had grade 2 or 3 peripheral arterial occlusive disease, but it was not clear whether these events were associated with imatinib.
For most patients the first adverse drug reaction occurred within 3 years of starting on imatinib, with the frequency of reactions decreasing thereafter.
Dr. Hehlmann disclosed receiving research support from Novartis, marketer of imatinib.
CHICAGO – After a decade on therapy with imatinib, a majority of patients with chronic myeloid leukemia will experience an adverse drug reaction, but most reactions are mild and manageable, according to results from a study presented at the annual meeting of the American Society of Clinical Oncology.
Of 1,375 patients with CML who received imatinib (Gleevec) monotherapy at some point, 1,018 (74%) had nonhematologic toxicities sometime during therapy, but only 199 (14%) had grade 3 or 4 toxicities, and there were no deaths attributed to imatinib, reported Dr. Rüdiger Hehlmann of the University of Heidelberg, Germany, and his colleagues.
Adverse drug reactions were manageable even when imatinib was combined with interferon-alfa (IFN-alfa), the investigators from the German CML Study Group reported in a poster at the meeting.
"After 10 years, imatinib continues to be an excellent choice for most patients with CML," they wrote.
In the 13 years that have elapsed since imatinib was approved in the United States as the first-in-class tyrosine kinase inhibitor, second-generation TKIs and other targeted agents have emerged, drawing attention to the safety of the older regimen.
The investigators evaluated long-term follow-up data and analyzed adverse drug reaction data for 1,501 patients treated with imatinib monotherapy in doses of 400 or 800 mg/day, as well as imatinib 400 mg in combination with IFN-alfa.
At the most recent evaluation, in November 2013, 164 patients had died, 1,003 were still on imatinib, 275 had been switched to a second-generation TKI, and 106 underwent bone marrow transplant (some patients received more than one therapy, accounting for the difference in total numbers).
The median follow-up time was 6.5 years, with some patients on study for as long as 11.5 years.
The probability of 10-year survival was 84%, and of 10-year progression-free survival was 81%.
An analysis of survival by molecular response rates showed an overall survival rate of 89% for those who achieved a major molecular response (MR, defined as a BCR-ABL RNA level of 0.1% or less), and 74% for those who achieved MR 4.5 (a 4.5 log10reduction or greater in BCR-ABL transcripts).
The 8-year probabilities for all grades of adverse events among the patients who received imatinib monotherapy were 41% for edema or fluid overload, 38% for gastrointestinal toxicities, 25% for myalgia/arthralgia, 20% for rash, 17% for musculoskeletal events, 17% for fatigue, 11% for neurological toxicities, and 10% for flulike symptoms.
Five patients had grade 2 or 3 peripheral arterial occlusive disease, but it was not clear whether these events were associated with imatinib.
For most patients the first adverse drug reaction occurred within 3 years of starting on imatinib, with the frequency of reactions decreasing thereafter.
Dr. Hehlmann disclosed receiving research support from Novartis, marketer of imatinib.
AT THE ASCO ANNUAL MEETING 2014
Key clinical finding: Imatinib is safe and effective for treating patients with chronic myeloid leukemia over the course of a decade.
Major finding: Of 1,375 patients with CML who received imatinib (Gleevec) monotherapy, 74% had nonhematologic toxicities sometime during therapy, but only 199 (14%) had grade 3 or 4 toxicities.
Data source: Review of prospectively collected 10-year follow-up data from a phase II trial of imatinib in 1,501 patients with CML.
Disclosures: Dr. Hehlmann disclosed receiving research support from Novartis, marketer of imatinib.
Swapping bortezomib for vincristine improves PFS in mantle cell lymphoma
CHICAGO – Tweaking the R-CHOP recipe to substitute bortezomib for vincristine may bring clinical benefit to patients with newly diagnosed mantle cell lymphoma who are ineligible for bone marrow transplant.
In a randomized phase III trial in patients with MCL who could not undergo bone marrow transplant due to age or comorbidities, those patients who received a combination of rituximab, doxorubicin, bortezomib (Velcade), cyclophosphamide, and prednisone (the VR-CAP regimen) had significantly better progression-free survival than did patients treated with R-CHOP, the same regimen but with vincristine instead of bortezomib.
The VR-CAP regimen "could be considered a new standard of care for newly diagnosed mantle cell lymphoma patients not considered for intensive treatment and bone marrow transplant," Dr. Franco Cavalli reported at the annual meeting of the American Society of Clinical Oncology.
The bortezomib-containing regimen was associated with more grade 3 or 4 toxicities than standard R-CHOP, but adverse events were manageable, and most patients in each study arm were able to stay on chemotherapy for all prescribed cycles, said Dr. Cavalli of the Oncology Institute of Southern Switzerland, Bellinzona.
R-CHOP is a standard frontline therapy for patients with MCL who are deemed to be ineligible for intensive therapy and/or bone marrow transplant. But the regimen offers only limited progression-free survival (PFS) in this population, Dr. Cavalli said.
Because bortezomib is approved for the treatment of relapsed MCL in the United States and 53 other nations, the authors investigated whether it could improve outcomes when given to patients with newly diagnosed disease.
The LYM-3002 trial was a phase III study conducted at 128 centers in 28 countries in Europe, Asia, the Americas, and Africa. Patients with newly diagnosed MCL stage II-IV, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and who were ineligible or not considered for bone marrow transplant, were randomized to receive either R-CHOP or VR-CAP. In R-CHOP, vincristine 1.4 mg/m2 was delivered to a maximum of 2 mg intravenously on day 1 of each cycle. In VR-CAP, bortezomib 1.3 mg/m2 was delivered via intravenous infusion on days 1, 4, 8, and 11 of each cycle. Patients were assigned to receive at least six cycles of therapy, with an additional two cycles possible if investigator-assessed responses were first documented at the end of cycle 6.
A total of 487 patients (244 assigned to R-CHOP and 243 to VR-CAP) were included in the intention-to-treat analysis.
At a median follow-up of 40 months, median PFS, the primary endpoint, was 24.7 months in the VR-CAP arm, compared with 14.4 months for R-CHOP (hazard ratio, 0.63; P less than .001), as judged by an independent review committee. Investigator-rated PFS was 30.7 months vs. 16.1 months, respectively (HR, 0.51; P less than .001).
Clinical responses according to International Working Group revised response criteria for malignant lymphoma included overall response rates (complete response, complete unconfirmed response, and partial response) of 90% in the R-CHOP–treated patients and 92% in those who received VR-CAP.
However, there was a higher proportion of combined complete response and complete unconfirmed response in the VR-CAP group: 42% for R-CHOP vs. 53% for VR-CAP (odds ratio, 1.69; P = .007).
Median time to response was also shorter with VR-CAP (1.6 vs. 1.4 months; HR, 1.54; P less than .001).
Independent reviewer-rated median time-to-progression was 16.1 months for R-CHOP vs. 30.5 months for VR-CAP (HR, 0.58; P less than .001). Median time to next therapy was 24.8 vs. 44.5 months, respectively (HR, 0.50; P less than .001), and median treatment-free interval was 20.5 vs. 40.6 months (HR, 0.50; P less than .001).
Median overall survival was 56.3 months among R-CHOP–treated patients, vs. not reached among VR-CAP–treated patients.
Grade 3 or higher drug-related adverse events occurred in 85% and 93% of patients, respectively. The events were considered serious in 21% of R-CHOP–treated patients and in 33% of VR-CAP–treated patients. In all, 7% of patients on R-CHOP and 9% of those on VR-CAP discontinued therapy because of adverse events.
Grade 3 adverse events were more frequent with VR-CAP and included neutropenia, leukopenia, lymphopenia, and thrombocytopenia, the last of which occurred in 6% of patients on R-CHOP, compared with 57% for VR-CAP. Despite this difference, however, rates of grade 3 or higher bleeding were similar between the groups, occurring in 1.2% and 1.7%, respectively.
The invited discussant, Dr. Michael E. Williams, chief of hematology/oncology at the University of Virginia Cancer Center, Charlottesville, commented that the study provides proof of principle "that if you add an active single agent and substitute bortezomib for vincristine, which would appear to be a less active agent, that you can certainly improve PFS significantly."
Dr. Williams said that it remains to be seen, however, whether, as Dr. Cavalli suggested, certain treatment strategies could be used to lower the incidence of drug-related adverse events and improve PFS rates further, such as the use of subcutaneous rather than intravenous bortezomib, different dosing schedules, or rituximab in the maintenance phase.
The study was supported by Janssen Global Services and Millennium. Dr. Cavalli disclosed receiving travel support for attending the ASCO annual meeting, but reported having no other conflicts of interest. Dr. Williams disclosed consulting/advising for Millennium and receiving research funding from Janssen and Millennium.
CHICAGO – Tweaking the R-CHOP recipe to substitute bortezomib for vincristine may bring clinical benefit to patients with newly diagnosed mantle cell lymphoma who are ineligible for bone marrow transplant.
In a randomized phase III trial in patients with MCL who could not undergo bone marrow transplant due to age or comorbidities, those patients who received a combination of rituximab, doxorubicin, bortezomib (Velcade), cyclophosphamide, and prednisone (the VR-CAP regimen) had significantly better progression-free survival than did patients treated with R-CHOP, the same regimen but with vincristine instead of bortezomib.
The VR-CAP regimen "could be considered a new standard of care for newly diagnosed mantle cell lymphoma patients not considered for intensive treatment and bone marrow transplant," Dr. Franco Cavalli reported at the annual meeting of the American Society of Clinical Oncology.
The bortezomib-containing regimen was associated with more grade 3 or 4 toxicities than standard R-CHOP, but adverse events were manageable, and most patients in each study arm were able to stay on chemotherapy for all prescribed cycles, said Dr. Cavalli of the Oncology Institute of Southern Switzerland, Bellinzona.
R-CHOP is a standard frontline therapy for patients with MCL who are deemed to be ineligible for intensive therapy and/or bone marrow transplant. But the regimen offers only limited progression-free survival (PFS) in this population, Dr. Cavalli said.
Because bortezomib is approved for the treatment of relapsed MCL in the United States and 53 other nations, the authors investigated whether it could improve outcomes when given to patients with newly diagnosed disease.
The LYM-3002 trial was a phase III study conducted at 128 centers in 28 countries in Europe, Asia, the Americas, and Africa. Patients with newly diagnosed MCL stage II-IV, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and who were ineligible or not considered for bone marrow transplant, were randomized to receive either R-CHOP or VR-CAP. In R-CHOP, vincristine 1.4 mg/m2 was delivered to a maximum of 2 mg intravenously on day 1 of each cycle. In VR-CAP, bortezomib 1.3 mg/m2 was delivered via intravenous infusion on days 1, 4, 8, and 11 of each cycle. Patients were assigned to receive at least six cycles of therapy, with an additional two cycles possible if investigator-assessed responses were first documented at the end of cycle 6.
A total of 487 patients (244 assigned to R-CHOP and 243 to VR-CAP) were included in the intention-to-treat analysis.
At a median follow-up of 40 months, median PFS, the primary endpoint, was 24.7 months in the VR-CAP arm, compared with 14.4 months for R-CHOP (hazard ratio, 0.63; P less than .001), as judged by an independent review committee. Investigator-rated PFS was 30.7 months vs. 16.1 months, respectively (HR, 0.51; P less than .001).
Clinical responses according to International Working Group revised response criteria for malignant lymphoma included overall response rates (complete response, complete unconfirmed response, and partial response) of 90% in the R-CHOP–treated patients and 92% in those who received VR-CAP.
However, there was a higher proportion of combined complete response and complete unconfirmed response in the VR-CAP group: 42% for R-CHOP vs. 53% for VR-CAP (odds ratio, 1.69; P = .007).
Median time to response was also shorter with VR-CAP (1.6 vs. 1.4 months; HR, 1.54; P less than .001).
Independent reviewer-rated median time-to-progression was 16.1 months for R-CHOP vs. 30.5 months for VR-CAP (HR, 0.58; P less than .001). Median time to next therapy was 24.8 vs. 44.5 months, respectively (HR, 0.50; P less than .001), and median treatment-free interval was 20.5 vs. 40.6 months (HR, 0.50; P less than .001).
Median overall survival was 56.3 months among R-CHOP–treated patients, vs. not reached among VR-CAP–treated patients.
Grade 3 or higher drug-related adverse events occurred in 85% and 93% of patients, respectively. The events were considered serious in 21% of R-CHOP–treated patients and in 33% of VR-CAP–treated patients. In all, 7% of patients on R-CHOP and 9% of those on VR-CAP discontinued therapy because of adverse events.
Grade 3 adverse events were more frequent with VR-CAP and included neutropenia, leukopenia, lymphopenia, and thrombocytopenia, the last of which occurred in 6% of patients on R-CHOP, compared with 57% for VR-CAP. Despite this difference, however, rates of grade 3 or higher bleeding were similar between the groups, occurring in 1.2% and 1.7%, respectively.
The invited discussant, Dr. Michael E. Williams, chief of hematology/oncology at the University of Virginia Cancer Center, Charlottesville, commented that the study provides proof of principle "that if you add an active single agent and substitute bortezomib for vincristine, which would appear to be a less active agent, that you can certainly improve PFS significantly."
Dr. Williams said that it remains to be seen, however, whether, as Dr. Cavalli suggested, certain treatment strategies could be used to lower the incidence of drug-related adverse events and improve PFS rates further, such as the use of subcutaneous rather than intravenous bortezomib, different dosing schedules, or rituximab in the maintenance phase.
The study was supported by Janssen Global Services and Millennium. Dr. Cavalli disclosed receiving travel support for attending the ASCO annual meeting, but reported having no other conflicts of interest. Dr. Williams disclosed consulting/advising for Millennium and receiving research funding from Janssen and Millennium.
CHICAGO – Tweaking the R-CHOP recipe to substitute bortezomib for vincristine may bring clinical benefit to patients with newly diagnosed mantle cell lymphoma who are ineligible for bone marrow transplant.
In a randomized phase III trial in patients with MCL who could not undergo bone marrow transplant due to age or comorbidities, those patients who received a combination of rituximab, doxorubicin, bortezomib (Velcade), cyclophosphamide, and prednisone (the VR-CAP regimen) had significantly better progression-free survival than did patients treated with R-CHOP, the same regimen but with vincristine instead of bortezomib.
The VR-CAP regimen "could be considered a new standard of care for newly diagnosed mantle cell lymphoma patients not considered for intensive treatment and bone marrow transplant," Dr. Franco Cavalli reported at the annual meeting of the American Society of Clinical Oncology.
The bortezomib-containing regimen was associated with more grade 3 or 4 toxicities than standard R-CHOP, but adverse events were manageable, and most patients in each study arm were able to stay on chemotherapy for all prescribed cycles, said Dr. Cavalli of the Oncology Institute of Southern Switzerland, Bellinzona.
R-CHOP is a standard frontline therapy for patients with MCL who are deemed to be ineligible for intensive therapy and/or bone marrow transplant. But the regimen offers only limited progression-free survival (PFS) in this population, Dr. Cavalli said.
Because bortezomib is approved for the treatment of relapsed MCL in the United States and 53 other nations, the authors investigated whether it could improve outcomes when given to patients with newly diagnosed disease.
The LYM-3002 trial was a phase III study conducted at 128 centers in 28 countries in Europe, Asia, the Americas, and Africa. Patients with newly diagnosed MCL stage II-IV, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and who were ineligible or not considered for bone marrow transplant, were randomized to receive either R-CHOP or VR-CAP. In R-CHOP, vincristine 1.4 mg/m2 was delivered to a maximum of 2 mg intravenously on day 1 of each cycle. In VR-CAP, bortezomib 1.3 mg/m2 was delivered via intravenous infusion on days 1, 4, 8, and 11 of each cycle. Patients were assigned to receive at least six cycles of therapy, with an additional two cycles possible if investigator-assessed responses were first documented at the end of cycle 6.
A total of 487 patients (244 assigned to R-CHOP and 243 to VR-CAP) were included in the intention-to-treat analysis.
At a median follow-up of 40 months, median PFS, the primary endpoint, was 24.7 months in the VR-CAP arm, compared with 14.4 months for R-CHOP (hazard ratio, 0.63; P less than .001), as judged by an independent review committee. Investigator-rated PFS was 30.7 months vs. 16.1 months, respectively (HR, 0.51; P less than .001).
Clinical responses according to International Working Group revised response criteria for malignant lymphoma included overall response rates (complete response, complete unconfirmed response, and partial response) of 90% in the R-CHOP–treated patients and 92% in those who received VR-CAP.
However, there was a higher proportion of combined complete response and complete unconfirmed response in the VR-CAP group: 42% for R-CHOP vs. 53% for VR-CAP (odds ratio, 1.69; P = .007).
Median time to response was also shorter with VR-CAP (1.6 vs. 1.4 months; HR, 1.54; P less than .001).
Independent reviewer-rated median time-to-progression was 16.1 months for R-CHOP vs. 30.5 months for VR-CAP (HR, 0.58; P less than .001). Median time to next therapy was 24.8 vs. 44.5 months, respectively (HR, 0.50; P less than .001), and median treatment-free interval was 20.5 vs. 40.6 months (HR, 0.50; P less than .001).
Median overall survival was 56.3 months among R-CHOP–treated patients, vs. not reached among VR-CAP–treated patients.
Grade 3 or higher drug-related adverse events occurred in 85% and 93% of patients, respectively. The events were considered serious in 21% of R-CHOP–treated patients and in 33% of VR-CAP–treated patients. In all, 7% of patients on R-CHOP and 9% of those on VR-CAP discontinued therapy because of adverse events.
Grade 3 adverse events were more frequent with VR-CAP and included neutropenia, leukopenia, lymphopenia, and thrombocytopenia, the last of which occurred in 6% of patients on R-CHOP, compared with 57% for VR-CAP. Despite this difference, however, rates of grade 3 or higher bleeding were similar between the groups, occurring in 1.2% and 1.7%, respectively.
The invited discussant, Dr. Michael E. Williams, chief of hematology/oncology at the University of Virginia Cancer Center, Charlottesville, commented that the study provides proof of principle "that if you add an active single agent and substitute bortezomib for vincristine, which would appear to be a less active agent, that you can certainly improve PFS significantly."
Dr. Williams said that it remains to be seen, however, whether, as Dr. Cavalli suggested, certain treatment strategies could be used to lower the incidence of drug-related adverse events and improve PFS rates further, such as the use of subcutaneous rather than intravenous bortezomib, different dosing schedules, or rituximab in the maintenance phase.
The study was supported by Janssen Global Services and Millennium. Dr. Cavalli disclosed receiving travel support for attending the ASCO annual meeting, but reported having no other conflicts of interest. Dr. Williams disclosed consulting/advising for Millennium and receiving research funding from Janssen and Millennium.
AT THE ASCO ANNUAL MEETING 2014
Key clinical point: Substituting bortezomib for vincristine may bring clinical benefit to patients with newly diagnosed mantle cell lymphoma who are ineligible for bone marrow transplant.
Major finding: At a median follow-up of 40 months, median progression-free survival was 24.7 months in the bortezomib-containing VR-CAP arm, compared with 14.4 months for R-CHOP, a significant difference.
Data source: Randomized, open-label phase III study in 487 patients with newly diagnosed mantle cell lymphoma.
Disclosures: The study was supported by Janssen Global Services and Millennium. Dr. Cavalli disclosed receiving travel support for attending the ASCO annual meeting, but reported having no other conflicts of interest. Dr. Williams disclosed consulting/advising for Millennium and receiving research funding from Janssen and Millennium.
Monosomal karyotype, high prognostic risk score predicted transplantation failure
Monosomal karyotype and high prognostic risk according to the revised International Prognostic Scoring System are independent predictors of relapse and mortality in patients with myelodysplastic syndrome or oligoblastic acute myeloid leukemia who undergo allogeneic hematopoietic stem cell transplantation, according to findings from the GITMO (Gruppo Italiano Trapianto di Midollo Osseo) registry.
Treatment failure after allogeneic hematopoietic stem cell transplantation may be from transplant complications or relapse. To understand the predictors of failure, investigators studied outcomes in 519 patients with myelodysplastic syndrome or oligoblastic acute myeloid leukemia who underwent hematopoietic stem cell transplantation between 2000 and 2011.
Those with monosomal karyotype had a 49% relapse rate and a 10% 5-year overall survival rate; both rates were significantly worse, compared with patients without monosomal karyotype (P less than .001 for each). Those considered high or very-high risk based on the International Prognostic Scoring System (IPSS-R), had 39% and 23% 5-year overall survival, respectively, and 23% and 39% relapse rates, respectively (P less than .001 in all cases vs. patients not at high or very-high risk), Dr. Matteo G. Della Porta of Fondazione IRCCS Policlinico San Matteo, Pavia, Italy and colleagues reported on behalf of the GITMO.
Age of 50 years or older and high hematopoietic cell transplantation-comorbidity index scores were independent predictors of nonrelapse mortality (P = .02; P = .017, respectively), they found (Blood 2014 [doi:10.1182/blood-2013-12-542720]).
Accounting for various combinations of patients’ ages, IPSS-R category, monosomal karyotype, and high hematopoietic cell transplantation–comorbidity index, the 5-year probability of survival after allogeneic hematopoietic stem cell transplantation ranged from 0 to 94%. The analyses performed reinforce the concept that allogenic hematopoietic stem cell transplantation – the only potentially curative treatment for MDS – "offers optimal eradication of myelodysplastic hematopoiesis when the procedure is performed before MDS patients progress to advanced disease stages," the investigators concluded.
The investigators reported having no disclosures.
Monosomal karyotype and high prognostic risk according to the revised International Prognostic Scoring System are independent predictors of relapse and mortality in patients with myelodysplastic syndrome or oligoblastic acute myeloid leukemia who undergo allogeneic hematopoietic stem cell transplantation, according to findings from the GITMO (Gruppo Italiano Trapianto di Midollo Osseo) registry.
Treatment failure after allogeneic hematopoietic stem cell transplantation may be from transplant complications or relapse. To understand the predictors of failure, investigators studied outcomes in 519 patients with myelodysplastic syndrome or oligoblastic acute myeloid leukemia who underwent hematopoietic stem cell transplantation between 2000 and 2011.
Those with monosomal karyotype had a 49% relapse rate and a 10% 5-year overall survival rate; both rates were significantly worse, compared with patients without monosomal karyotype (P less than .001 for each). Those considered high or very-high risk based on the International Prognostic Scoring System (IPSS-R), had 39% and 23% 5-year overall survival, respectively, and 23% and 39% relapse rates, respectively (P less than .001 in all cases vs. patients not at high or very-high risk), Dr. Matteo G. Della Porta of Fondazione IRCCS Policlinico San Matteo, Pavia, Italy and colleagues reported on behalf of the GITMO.
Age of 50 years or older and high hematopoietic cell transplantation-comorbidity index scores were independent predictors of nonrelapse mortality (P = .02; P = .017, respectively), they found (Blood 2014 [doi:10.1182/blood-2013-12-542720]).
Accounting for various combinations of patients’ ages, IPSS-R category, monosomal karyotype, and high hematopoietic cell transplantation–comorbidity index, the 5-year probability of survival after allogeneic hematopoietic stem cell transplantation ranged from 0 to 94%. The analyses performed reinforce the concept that allogenic hematopoietic stem cell transplantation – the only potentially curative treatment for MDS – "offers optimal eradication of myelodysplastic hematopoiesis when the procedure is performed before MDS patients progress to advanced disease stages," the investigators concluded.
The investigators reported having no disclosures.
Monosomal karyotype and high prognostic risk according to the revised International Prognostic Scoring System are independent predictors of relapse and mortality in patients with myelodysplastic syndrome or oligoblastic acute myeloid leukemia who undergo allogeneic hematopoietic stem cell transplantation, according to findings from the GITMO (Gruppo Italiano Trapianto di Midollo Osseo) registry.
Treatment failure after allogeneic hematopoietic stem cell transplantation may be from transplant complications or relapse. To understand the predictors of failure, investigators studied outcomes in 519 patients with myelodysplastic syndrome or oligoblastic acute myeloid leukemia who underwent hematopoietic stem cell transplantation between 2000 and 2011.
Those with monosomal karyotype had a 49% relapse rate and a 10% 5-year overall survival rate; both rates were significantly worse, compared with patients without monosomal karyotype (P less than .001 for each). Those considered high or very-high risk based on the International Prognostic Scoring System (IPSS-R), had 39% and 23% 5-year overall survival, respectively, and 23% and 39% relapse rates, respectively (P less than .001 in all cases vs. patients not at high or very-high risk), Dr. Matteo G. Della Porta of Fondazione IRCCS Policlinico San Matteo, Pavia, Italy and colleagues reported on behalf of the GITMO.
Age of 50 years or older and high hematopoietic cell transplantation-comorbidity index scores were independent predictors of nonrelapse mortality (P = .02; P = .017, respectively), they found (Blood 2014 [doi:10.1182/blood-2013-12-542720]).
Accounting for various combinations of patients’ ages, IPSS-R category, monosomal karyotype, and high hematopoietic cell transplantation–comorbidity index, the 5-year probability of survival after allogeneic hematopoietic stem cell transplantation ranged from 0 to 94%. The analyses performed reinforce the concept that allogenic hematopoietic stem cell transplantation – the only potentially curative treatment for MDS – "offers optimal eradication of myelodysplastic hematopoiesis when the procedure is performed before MDS patients progress to advanced disease stages," the investigators concluded.
The investigators reported having no disclosures.
FROM BLOOD
Major finding: Patients with a monosomal karyotype had a 49% relapse rate and a 10% 5-year overall survival rate, and those considered high or very-high risk based on the IPSS-R, had 39% and 23% 5-year overall survival, respectively, and 23% and 39% relapse rates, respectively.
Data source: An analysis of GITMO registry data.
Disclosures: The investigators reported having no disclosures.
No survival benefit for routine surveillance scans in classical Hodgkin disease
CHICAGO – Routine surveillance imaging does not improve clinical outcomes in patients with classic Hodgkin disease who are in first complete remission and it sharply increases costs, researchers reported at the annual meeting of the American Society of Clinical Oncology.
The team, led by Dr. Sai Ravi Pingali, retrospectively reviewed the charts of 241 adult patients who achieved a complete remission after first-line therapy.
In 68%, the treating physicians’ planned approach was routine surveillance imaging, which consisted of radiologic imaging with scans every few months, plus clinical exams and laboratory testing. In the other 32%, the planned approach was clinical surveillance, meaning that radiologic imaging was performed only if concerning signs or symptoms occurred.
The two groups had statistically indistinguishable overall survival, and in both groups, all patients experiencing relapse successfully achieved a second complete remission with salvage therapy.
In the routinely imaged group, scanning increased costs by nearly $20,000 per patient and by almost $600,000 per each relapse detected. In addition, patients were exposed to the associated radiation.
"We were unable to detect an overall survival benefit associated with routine surveillance imaging, although I have to acknowledge that our study was limited in power given the small number of deaths and relapses," commented Dr. Pingali, an oncologist with the Medical College of Wisconsin Affiliated Hospitals in Milwaukee.
"Relapses in both ... groups were effectively salvaged with autologous stem cell transplantation, arguing against a critical advantage of detection of asymptomatic relapse. Also, we need to keep in mind that the costs associated with routine surveillance imaging are significant, and it is also associated with potential risks, both in terms of radiation exposure and unnecessary work-up," he added.
"We do not feel that potential risks and costs without overall survival benefit or any other clinical benefit justify the practice of routine surveillance imaging in classical Hodgkin lymphoma patients who have achieved a complete remission after first-line therapy. We recommend that such patients be followed clinically," Dr. Pingali concluded.
Invited discussant Dr. Leo Gordon of Northwestern University in Chicago, agreed that accumulating data argue against routine imaging for surveillance in this context and noted that insurers will likely not continue to cover scans having no proven benefit. The data should prompt a revision of guidelines and reeducation of clinicians and patients, he said.
"For translational researchers and investigators and academics, I think we need to convince journal reviewers that a manuscript is acceptable if scans are not so frequent. And for industry trials, I think we need to discuss with the Food and Drug Administration the endpoint of progression-free survival and that those endpoints may not only be driven by scans but by more mundane parameters," he said—namely, the history and physical examination.
But session comoderator Dr. Gilles A. Salles of Hospices Civils de Lyon, Université Claude Bernard, France, expressed reservations, noting that the study did not provide information on how patients were allocated to groups and the time frame of relapse.
"It may be different whether relapses occur early, in the first year, or they occur later, and that may have some implications for the surveillance," he said. "I understand that you and many others jumped over the idea that we should immediately stop. A few people may think that we need more solid data, despite the provocative and quality data that were presented, to really make this jump in clinical practice. That’s my personal opinion."
Dr. Pingali and his team retrospectively reviewed the charts of adult patients who received a new diagnosis of classical Hodgkin lymphoma between 2000 and 2010 at three institutions, achieved complete remission after first-line therapy and had at least 2 years of follow-up.
The routine surveillance imaging and clinical surveillance groups had similar demographic and disease characteristics, Dr. Pingali reported. The former were significantly more likely to have received ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, and dacarbazine) and less likely to have received the Stanford V regimen as first-line therapy, and they were significantly less likely to have received radiation therapy.
With a median duration of follow-up of about 4 years, the groups did not differ significantly with respect to overall survival. "When we look at the 5-year time point, when typically the surveillance CT scans are discontinued, the curves are pretty much superimposable," he pointed out.
There were five deaths in the routine surveillance imaging group, one of which was from relapsed disease; the other deaths were from cancer, heart failure, hip fracture, and myelodysplastic syndrome. There were four deaths in the clinical surveillance group: two were from non-Hodgkin lymphoma and two from unknown causes while the patient was in confirmed remission.
All of the six patients in the routinely imaged group and all of the five patients in the clinically followed group experiencing a relapse achieved another complete remission with second-line therapy.
The mean number of scans received was 1.14 in the clinical surveillance group – usually the scan performed after first-line treatment to confirm remission, according to Dr. Pingali – and 4.25 in the routine surveillance imaging group. The ratio of scans to detected relapses was 18 vs. 124.
The extra charges incurred from scans using the routine surveillance imaging approach were $18,896/patient and $593,698/relapse.
"It is important to note that this does not include additional costs from the work-up of the false-positive scans and also the wages lost," he noted.
Dr. Pingali disclosed no relevant conflicts of interest. Dr. Gordon disclosed that he receives honoraria from Genentech and research funding from Millennium and Pharmacyclics. Dr. Salles disclosed serving as an advisor or consultant for Calistoga Pharmaceuticals, Celgene; Genentech, Janssen Pharmaceutica, and Roche. He has served as a speaker or a member of a speakers bureau and has received grants for clinical research from Celgene and Roche.
CHICAGO – Routine surveillance imaging does not improve clinical outcomes in patients with classic Hodgkin disease who are in first complete remission and it sharply increases costs, researchers reported at the annual meeting of the American Society of Clinical Oncology.
The team, led by Dr. Sai Ravi Pingali, retrospectively reviewed the charts of 241 adult patients who achieved a complete remission after first-line therapy.
In 68%, the treating physicians’ planned approach was routine surveillance imaging, which consisted of radiologic imaging with scans every few months, plus clinical exams and laboratory testing. In the other 32%, the planned approach was clinical surveillance, meaning that radiologic imaging was performed only if concerning signs or symptoms occurred.
The two groups had statistically indistinguishable overall survival, and in both groups, all patients experiencing relapse successfully achieved a second complete remission with salvage therapy.
In the routinely imaged group, scanning increased costs by nearly $20,000 per patient and by almost $600,000 per each relapse detected. In addition, patients were exposed to the associated radiation.
"We were unable to detect an overall survival benefit associated with routine surveillance imaging, although I have to acknowledge that our study was limited in power given the small number of deaths and relapses," commented Dr. Pingali, an oncologist with the Medical College of Wisconsin Affiliated Hospitals in Milwaukee.
"Relapses in both ... groups were effectively salvaged with autologous stem cell transplantation, arguing against a critical advantage of detection of asymptomatic relapse. Also, we need to keep in mind that the costs associated with routine surveillance imaging are significant, and it is also associated with potential risks, both in terms of radiation exposure and unnecessary work-up," he added.
"We do not feel that potential risks and costs without overall survival benefit or any other clinical benefit justify the practice of routine surveillance imaging in classical Hodgkin lymphoma patients who have achieved a complete remission after first-line therapy. We recommend that such patients be followed clinically," Dr. Pingali concluded.
Invited discussant Dr. Leo Gordon of Northwestern University in Chicago, agreed that accumulating data argue against routine imaging for surveillance in this context and noted that insurers will likely not continue to cover scans having no proven benefit. The data should prompt a revision of guidelines and reeducation of clinicians and patients, he said.
"For translational researchers and investigators and academics, I think we need to convince journal reviewers that a manuscript is acceptable if scans are not so frequent. And for industry trials, I think we need to discuss with the Food and Drug Administration the endpoint of progression-free survival and that those endpoints may not only be driven by scans but by more mundane parameters," he said—namely, the history and physical examination.
But session comoderator Dr. Gilles A. Salles of Hospices Civils de Lyon, Université Claude Bernard, France, expressed reservations, noting that the study did not provide information on how patients were allocated to groups and the time frame of relapse.
"It may be different whether relapses occur early, in the first year, or they occur later, and that may have some implications for the surveillance," he said. "I understand that you and many others jumped over the idea that we should immediately stop. A few people may think that we need more solid data, despite the provocative and quality data that were presented, to really make this jump in clinical practice. That’s my personal opinion."
Dr. Pingali and his team retrospectively reviewed the charts of adult patients who received a new diagnosis of classical Hodgkin lymphoma between 2000 and 2010 at three institutions, achieved complete remission after first-line therapy and had at least 2 years of follow-up.
The routine surveillance imaging and clinical surveillance groups had similar demographic and disease characteristics, Dr. Pingali reported. The former were significantly more likely to have received ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, and dacarbazine) and less likely to have received the Stanford V regimen as first-line therapy, and they were significantly less likely to have received radiation therapy.
With a median duration of follow-up of about 4 years, the groups did not differ significantly with respect to overall survival. "When we look at the 5-year time point, when typically the surveillance CT scans are discontinued, the curves are pretty much superimposable," he pointed out.
There were five deaths in the routine surveillance imaging group, one of which was from relapsed disease; the other deaths were from cancer, heart failure, hip fracture, and myelodysplastic syndrome. There were four deaths in the clinical surveillance group: two were from non-Hodgkin lymphoma and two from unknown causes while the patient was in confirmed remission.
All of the six patients in the routinely imaged group and all of the five patients in the clinically followed group experiencing a relapse achieved another complete remission with second-line therapy.
The mean number of scans received was 1.14 in the clinical surveillance group – usually the scan performed after first-line treatment to confirm remission, according to Dr. Pingali – and 4.25 in the routine surveillance imaging group. The ratio of scans to detected relapses was 18 vs. 124.
The extra charges incurred from scans using the routine surveillance imaging approach were $18,896/patient and $593,698/relapse.
"It is important to note that this does not include additional costs from the work-up of the false-positive scans and also the wages lost," he noted.
Dr. Pingali disclosed no relevant conflicts of interest. Dr. Gordon disclosed that he receives honoraria from Genentech and research funding from Millennium and Pharmacyclics. Dr. Salles disclosed serving as an advisor or consultant for Calistoga Pharmaceuticals, Celgene; Genentech, Janssen Pharmaceutica, and Roche. He has served as a speaker or a member of a speakers bureau and has received grants for clinical research from Celgene and Roche.
CHICAGO – Routine surveillance imaging does not improve clinical outcomes in patients with classic Hodgkin disease who are in first complete remission and it sharply increases costs, researchers reported at the annual meeting of the American Society of Clinical Oncology.
The team, led by Dr. Sai Ravi Pingali, retrospectively reviewed the charts of 241 adult patients who achieved a complete remission after first-line therapy.
In 68%, the treating physicians’ planned approach was routine surveillance imaging, which consisted of radiologic imaging with scans every few months, plus clinical exams and laboratory testing. In the other 32%, the planned approach was clinical surveillance, meaning that radiologic imaging was performed only if concerning signs or symptoms occurred.
The two groups had statistically indistinguishable overall survival, and in both groups, all patients experiencing relapse successfully achieved a second complete remission with salvage therapy.
In the routinely imaged group, scanning increased costs by nearly $20,000 per patient and by almost $600,000 per each relapse detected. In addition, patients were exposed to the associated radiation.
"We were unable to detect an overall survival benefit associated with routine surveillance imaging, although I have to acknowledge that our study was limited in power given the small number of deaths and relapses," commented Dr. Pingali, an oncologist with the Medical College of Wisconsin Affiliated Hospitals in Milwaukee.
"Relapses in both ... groups were effectively salvaged with autologous stem cell transplantation, arguing against a critical advantage of detection of asymptomatic relapse. Also, we need to keep in mind that the costs associated with routine surveillance imaging are significant, and it is also associated with potential risks, both in terms of radiation exposure and unnecessary work-up," he added.
"We do not feel that potential risks and costs without overall survival benefit or any other clinical benefit justify the practice of routine surveillance imaging in classical Hodgkin lymphoma patients who have achieved a complete remission after first-line therapy. We recommend that such patients be followed clinically," Dr. Pingali concluded.
Invited discussant Dr. Leo Gordon of Northwestern University in Chicago, agreed that accumulating data argue against routine imaging for surveillance in this context and noted that insurers will likely not continue to cover scans having no proven benefit. The data should prompt a revision of guidelines and reeducation of clinicians and patients, he said.
"For translational researchers and investigators and academics, I think we need to convince journal reviewers that a manuscript is acceptable if scans are not so frequent. And for industry trials, I think we need to discuss with the Food and Drug Administration the endpoint of progression-free survival and that those endpoints may not only be driven by scans but by more mundane parameters," he said—namely, the history and physical examination.
But session comoderator Dr. Gilles A. Salles of Hospices Civils de Lyon, Université Claude Bernard, France, expressed reservations, noting that the study did not provide information on how patients were allocated to groups and the time frame of relapse.
"It may be different whether relapses occur early, in the first year, or they occur later, and that may have some implications for the surveillance," he said. "I understand that you and many others jumped over the idea that we should immediately stop. A few people may think that we need more solid data, despite the provocative and quality data that were presented, to really make this jump in clinical practice. That’s my personal opinion."
Dr. Pingali and his team retrospectively reviewed the charts of adult patients who received a new diagnosis of classical Hodgkin lymphoma between 2000 and 2010 at three institutions, achieved complete remission after first-line therapy and had at least 2 years of follow-up.
The routine surveillance imaging and clinical surveillance groups had similar demographic and disease characteristics, Dr. Pingali reported. The former were significantly more likely to have received ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, and dacarbazine) and less likely to have received the Stanford V regimen as first-line therapy, and they were significantly less likely to have received radiation therapy.
With a median duration of follow-up of about 4 years, the groups did not differ significantly with respect to overall survival. "When we look at the 5-year time point, when typically the surveillance CT scans are discontinued, the curves are pretty much superimposable," he pointed out.
There were five deaths in the routine surveillance imaging group, one of which was from relapsed disease; the other deaths were from cancer, heart failure, hip fracture, and myelodysplastic syndrome. There were four deaths in the clinical surveillance group: two were from non-Hodgkin lymphoma and two from unknown causes while the patient was in confirmed remission.
All of the six patients in the routinely imaged group and all of the five patients in the clinically followed group experiencing a relapse achieved another complete remission with second-line therapy.
The mean number of scans received was 1.14 in the clinical surveillance group – usually the scan performed after first-line treatment to confirm remission, according to Dr. Pingali – and 4.25 in the routine surveillance imaging group. The ratio of scans to detected relapses was 18 vs. 124.
The extra charges incurred from scans using the routine surveillance imaging approach were $18,896/patient and $593,698/relapse.
"It is important to note that this does not include additional costs from the work-up of the false-positive scans and also the wages lost," he noted.
Dr. Pingali disclosed no relevant conflicts of interest. Dr. Gordon disclosed that he receives honoraria from Genentech and research funding from Millennium and Pharmacyclics. Dr. Salles disclosed serving as an advisor or consultant for Calistoga Pharmaceuticals, Celgene; Genentech, Janssen Pharmaceutica, and Roche. He has served as a speaker or a member of a speakers bureau and has received grants for clinical research from Celgene and Roche.
AT THE ASCO ANNUAL MEETING 2013
Major finding: The ratio of scans to detected relapses was 18 for the clinical surveillance group and 124 for the routine scan group. The extra charges incurred from scans using the routine surveillance imaging approach were $18,896/patient and $593,698/relapse.
Data source: A retrospective analysis of 241 patients with classical Hodgkin lymphoma in first complete remission
Disclosures: Dr. Pingali disclosed no relevant conflicts of interest. Dr. Gordon disclosed that he receives honoraria from Genentech and research funding from Millennium and Pharmacyclics. Dr. Salles disclosed serving as an advisor or consultant for Calistoga Pharmaceuticals, Celgene; Genentech, Janssen Pharmaceutica, and Roche. He has served as a speaker or a member of a speakers bureau and has received grants for clinical research from Celgene and Roche.
Transfusion Medicine
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transfusion-transmitted infections and transfusion-related reactions, and controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products and indications for transfusion along with the associated risks and also discusses specific clinical situations, such as massive transfusion.
To read the full article in PDF:
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transfusion-transmitted infections and transfusion-related reactions, and controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products and indications for transfusion along with the associated risks and also discusses specific clinical situations, such as massive transfusion.
To read the full article in PDF:
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transfusion-transmitted infections and transfusion-related reactions, and controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products and indications for transfusion along with the associated risks and also discusses specific clinical situations, such as massive transfusion.
To read the full article in PDF: