User login
Cost is high for Japanese encephalitis vaccinations
Vaccination costs surrounding Japanese encephalitis is high, according to an economic analysis presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The analytic horizon for this research was 6 years, but productivity losses were evaluated over average life expectancy.
The researchers analyzed their data from two analytic perspectives: societal and traveler perspective. Factors analyzed included vaccine per dose, vaccine administration cost, and vaccine adverse events costs per vaccine.
The researchers assessed the risk to travelers based upon disease incidence among different groups. The highest-risk travelers (Group 1) – those who planned to spend a month or more in JE-endemic areas – had an incidence rate of 0.53/1 million travelers. Group 2 travelers – those who planned to stay less than a month and more than a fifth of their time outdoors – had an incidence rate of 0.25/1 million. Group 3 travelers, at lowest risk, had the lowest incidence rate at 0.04/1 million.
The calculated societal perspective cost per outcome averted was quite high for each risk group. For Group 1, the cost was $596 million per case averted. This cost rose to $1.3 billion for each case of long-term sequelae averted, and rose even higher to avert death, to $1.8 billion per death averted. These costs nearly doubled for Group 2, and in Group 3, the cost ballooned to $7.9 billion to avert one case of JE, $17 billion per prevention of long-term sequelae, and $23 billion per death averted.
The individual costs for JE vaccination are $292 per dose, with an administration fee of $46. Short-term treatment of JE costs nearly $30,000, and long-term treatment of JE also comes with a large bill of $8,437.
These costs are not simply monetary but are also felt in lost economic productivity. The cost of complete short-term recovery is nearly $60,000. Over an individual’s lifetime, this number rose to more than $1.5 million based on total loss of productivity.
Of the 67% of patients who survive JE, 32% recover completely while 68% deal with long-term sequelae. Of those, 28% experience mild symptoms while the remaining 72% have severe sequelae.
JE vaccine effectiveness does not appear to be an issue, with a 0.91 proportion of neutralizing antibodies seen after 1 year. With booster doses, vaccine effectiveness is 0.96.
There are limitations to this study, such as results being affected by the uncertainty of JE incidence. “The single most important variable is incidence,” stated Dr. Meltzer.
Vaccination costs surrounding Japanese encephalitis is high, according to an economic analysis presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The analytic horizon for this research was 6 years, but productivity losses were evaluated over average life expectancy.
The researchers analyzed their data from two analytic perspectives: societal and traveler perspective. Factors analyzed included vaccine per dose, vaccine administration cost, and vaccine adverse events costs per vaccine.
The researchers assessed the risk to travelers based upon disease incidence among different groups. The highest-risk travelers (Group 1) – those who planned to spend a month or more in JE-endemic areas – had an incidence rate of 0.53/1 million travelers. Group 2 travelers – those who planned to stay less than a month and more than a fifth of their time outdoors – had an incidence rate of 0.25/1 million. Group 3 travelers, at lowest risk, had the lowest incidence rate at 0.04/1 million.
The calculated societal perspective cost per outcome averted was quite high for each risk group. For Group 1, the cost was $596 million per case averted. This cost rose to $1.3 billion for each case of long-term sequelae averted, and rose even higher to avert death, to $1.8 billion per death averted. These costs nearly doubled for Group 2, and in Group 3, the cost ballooned to $7.9 billion to avert one case of JE, $17 billion per prevention of long-term sequelae, and $23 billion per death averted.
The individual costs for JE vaccination are $292 per dose, with an administration fee of $46. Short-term treatment of JE costs nearly $30,000, and long-term treatment of JE also comes with a large bill of $8,437.
These costs are not simply monetary but are also felt in lost economic productivity. The cost of complete short-term recovery is nearly $60,000. Over an individual’s lifetime, this number rose to more than $1.5 million based on total loss of productivity.
Of the 67% of patients who survive JE, 32% recover completely while 68% deal with long-term sequelae. Of those, 28% experience mild symptoms while the remaining 72% have severe sequelae.
JE vaccine effectiveness does not appear to be an issue, with a 0.91 proportion of neutralizing antibodies seen after 1 year. With booster doses, vaccine effectiveness is 0.96.
There are limitations to this study, such as results being affected by the uncertainty of JE incidence. “The single most important variable is incidence,” stated Dr. Meltzer.
Vaccination costs surrounding Japanese encephalitis is high, according to an economic analysis presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The analytic horizon for this research was 6 years, but productivity losses were evaluated over average life expectancy.
The researchers analyzed their data from two analytic perspectives: societal and traveler perspective. Factors analyzed included vaccine per dose, vaccine administration cost, and vaccine adverse events costs per vaccine.
The researchers assessed the risk to travelers based upon disease incidence among different groups. The highest-risk travelers (Group 1) – those who planned to spend a month or more in JE-endemic areas – had an incidence rate of 0.53/1 million travelers. Group 2 travelers – those who planned to stay less than a month and more than a fifth of their time outdoors – had an incidence rate of 0.25/1 million. Group 3 travelers, at lowest risk, had the lowest incidence rate at 0.04/1 million.
The calculated societal perspective cost per outcome averted was quite high for each risk group. For Group 1, the cost was $596 million per case averted. This cost rose to $1.3 billion for each case of long-term sequelae averted, and rose even higher to avert death, to $1.8 billion per death averted. These costs nearly doubled for Group 2, and in Group 3, the cost ballooned to $7.9 billion to avert one case of JE, $17 billion per prevention of long-term sequelae, and $23 billion per death averted.
The individual costs for JE vaccination are $292 per dose, with an administration fee of $46. Short-term treatment of JE costs nearly $30,000, and long-term treatment of JE also comes with a large bill of $8,437.
These costs are not simply monetary but are also felt in lost economic productivity. The cost of complete short-term recovery is nearly $60,000. Over an individual’s lifetime, this number rose to more than $1.5 million based on total loss of productivity.
Of the 67% of patients who survive JE, 32% recover completely while 68% deal with long-term sequelae. Of those, 28% experience mild symptoms while the remaining 72% have severe sequelae.
JE vaccine effectiveness does not appear to be an issue, with a 0.91 proportion of neutralizing antibodies seen after 1 year. With booster doses, vaccine effectiveness is 0.96.
There are limitations to this study, such as results being affected by the uncertainty of JE incidence. “The single most important variable is incidence,” stated Dr. Meltzer.
REPORTING FROM AN ACIP MEETING
Anthrax vaccine recommendations updated in the event of a wide-area release
at their meeting.
The recommendations to the committee sought to optimize the use of Anthrax Vaccine Adsorbed (AVA) in post-exposure prophylaxis (PEP) in the event of a wide-area release of Bacillus anthracis spores. In this event, a mass vaccination effort would be undertaken, requiring expedited administration of AVA. ACIP now recommends that the intramuscular administration may be used over the traditional subcutaneous approach if there are any operational or logistical challenges that delay effective vaccination. Another recommendation from ACIP would allow two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. The committee also recommended that AbxPEP, an antimicrobial, be stopped 42 days after the first dose of AVA or 2 weeks after the last dose.
William A. Bower, MD, of the division of high-consequence pathogens and pathology at the Centers for Disease Control and Prevention, and the anthrax work group looked at three nonhuman primate studies and eight human immunogenicity and adverse event studies during the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE). All of the animal studies were used to predict human survival by vaccinating the nonhuman primates with AVA, then challenging them with B. anthracis. Using animal studies to predict human survival is common practice under the “animal rule.”
When Dr. Bower and the work group assessed the studies comparing intramuscular administration with subcutaneous administration of AVA, they rated the overall evidence as GRADE 2. However, they rated the adverse events data as GRADE 1.
Dr. Bower and his colleagues also reviewed dose-sparing studies to identify the feasibility of allowing two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. For these studies, the anthrax work group gave a GRADE score of 2.
The overall evidence score for microbial duration for PEP was judged to be GRADE 2.
“These forthcoming recommendations will be used by the CDC to inform state and local health departments to better prepare for an emergency response to a wide-area release of Bacillus anthracis spores,” said Dr. Bower.
The committee’s recommendations must be approved by the CDC’s director before they are considered official recommendations.
Dr. Bower did not report any relevant financial conflicts of interest.
at their meeting.
The recommendations to the committee sought to optimize the use of Anthrax Vaccine Adsorbed (AVA) in post-exposure prophylaxis (PEP) in the event of a wide-area release of Bacillus anthracis spores. In this event, a mass vaccination effort would be undertaken, requiring expedited administration of AVA. ACIP now recommends that the intramuscular administration may be used over the traditional subcutaneous approach if there are any operational or logistical challenges that delay effective vaccination. Another recommendation from ACIP would allow two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. The committee also recommended that AbxPEP, an antimicrobial, be stopped 42 days after the first dose of AVA or 2 weeks after the last dose.
William A. Bower, MD, of the division of high-consequence pathogens and pathology at the Centers for Disease Control and Prevention, and the anthrax work group looked at three nonhuman primate studies and eight human immunogenicity and adverse event studies during the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE). All of the animal studies were used to predict human survival by vaccinating the nonhuman primates with AVA, then challenging them with B. anthracis. Using animal studies to predict human survival is common practice under the “animal rule.”
When Dr. Bower and the work group assessed the studies comparing intramuscular administration with subcutaneous administration of AVA, they rated the overall evidence as GRADE 2. However, they rated the adverse events data as GRADE 1.
Dr. Bower and his colleagues also reviewed dose-sparing studies to identify the feasibility of allowing two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. For these studies, the anthrax work group gave a GRADE score of 2.
The overall evidence score for microbial duration for PEP was judged to be GRADE 2.
“These forthcoming recommendations will be used by the CDC to inform state and local health departments to better prepare for an emergency response to a wide-area release of Bacillus anthracis spores,” said Dr. Bower.
The committee’s recommendations must be approved by the CDC’s director before they are considered official recommendations.
Dr. Bower did not report any relevant financial conflicts of interest.
at their meeting.
The recommendations to the committee sought to optimize the use of Anthrax Vaccine Adsorbed (AVA) in post-exposure prophylaxis (PEP) in the event of a wide-area release of Bacillus anthracis spores. In this event, a mass vaccination effort would be undertaken, requiring expedited administration of AVA. ACIP now recommends that the intramuscular administration may be used over the traditional subcutaneous approach if there are any operational or logistical challenges that delay effective vaccination. Another recommendation from ACIP would allow two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. The committee also recommended that AbxPEP, an antimicrobial, be stopped 42 days after the first dose of AVA or 2 weeks after the last dose.
William A. Bower, MD, of the division of high-consequence pathogens and pathology at the Centers for Disease Control and Prevention, and the anthrax work group looked at three nonhuman primate studies and eight human immunogenicity and adverse event studies during the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE). All of the animal studies were used to predict human survival by vaccinating the nonhuman primates with AVA, then challenging them with B. anthracis. Using animal studies to predict human survival is common practice under the “animal rule.”
When Dr. Bower and the work group assessed the studies comparing intramuscular administration with subcutaneous administration of AVA, they rated the overall evidence as GRADE 2. However, they rated the adverse events data as GRADE 1.
Dr. Bower and his colleagues also reviewed dose-sparing studies to identify the feasibility of allowing two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. For these studies, the anthrax work group gave a GRADE score of 2.
The overall evidence score for microbial duration for PEP was judged to be GRADE 2.
“These forthcoming recommendations will be used by the CDC to inform state and local health departments to better prepare for an emergency response to a wide-area release of Bacillus anthracis spores,” said Dr. Bower.
The committee’s recommendations must be approved by the CDC’s director before they are considered official recommendations.
Dr. Bower did not report any relevant financial conflicts of interest.
REPORTING FROM AN ACIP MEETING
Norovirus vaccine appears promising in children
MALMO, SWEDEN – in an interim analysis of an ongoing phase 2 study, Taisei Masuda, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The randomized, double-blind, multinational trial remains blinded because follow-up is continuing, so – to the disappointment of the ESPID audience – there are as yet no data on duration of antibody persistence or clinical efficacy.
However, an earlier phase 2 study in 420 healthy participants aged 18-64 years showed that the Takeda vaccine elicited persistent immune responses 1 year post vaccination and that higher antibody levels correlated with a reduced frequency of moderate to severe vomiting and diarrheal illness following oral challenge with norovirus (Clin Vaccine Immunol. 2015 Aug;22[8]:923-9). Follow-up will continue in order to learn how long the protective immune response lasts in adults, according to Dr. Masuda, of Takeda Pharmaceuticals in Zurich.
The bivalent Takeda vaccine is the first candidate vaccine to reach the randomized trial stage. An oral vaccine in tablet form under development by Vaxart, a San Francisco Bay Area biotech company, recently completed preliminary phase 1 studies.
Dr. Masuda explained that the Takeda vaccine contains virus-like particle antigens from norovirus strains GI.1 and GII.4c, which together account for the majority of human norovirus illness. These virus-like particles are formed on the outer surface of the virus. Of note, virus-like particle–based vaccines against hepatitis B and human papillomavirus have won regulatory approval in the United States, Europe, and elsewhere.
He presented data on 120 healthy subjects aged 1 year to less than 4 years old and another 120 aged 4 years to less than 9 years. They are part of a larger phase 2 study of 840 children as young as age 6 weeks. This was a dose-finding study, so participants received various doses of the vaccine on day 1 and either a second dose or a saline injection 28 days later. The vaccine, which contains aluminum hydroxide to enhance immunogenicity, comes in prefilled syringes.
At 57 days of follow-up in this interim analysis, protective seroresponse rates as defined by at least a fourfold increase in histo-blood group antigen–blocking titers approached 100%. In the older group, this was typically achieved with a single dose of vaccine. However, the younger group of children generally derived further benefit from a second dose, according to Dr. Masuda.
In terms of safety concerns, he said no serious adverse events occurred in the study and no one withdrew from the trial because of vaccine-related side effects. The overall safety picture was the same in the two age groups. The incidence of fever of 38° C or higher was similar after administration of vaccine and placebo. Injection site pain occurred in one-quarter of younger vaccine recipients, in 38%-63% of those aged 4 years or older, and in 17%-22% who got placebo injections. Those and other local and systemic adverse events were mostly mild and transient. Their incidence and severity weren’t related to vaccine dosage.
In sum, Dr. Masuda deemed the safety profile “clinically acceptable.”
Session chair Karina Butler, MD, of Temple Street Children’s University Hospital, Dublin, raised the question of how might this vaccine, which may require two doses in younger children, fit into an already crowded pediatric immunization schedule – will parents and physicians embrace it?
Dr. Masuda replied that noroviruses are the No. 1 cause of acute gastroenteritis worldwide and there is a clamor for development of effective vaccines to protect the groups that bear the greatest burden of disease, including children, the elderly, military personnel, cruise ship vacationers, and others who experience crowded conditions. He expressed confidence that a safe and effective vaccine will be in high demand.
“In the future, we’ll look at the possibility of a combination vaccine,” he added.
In response to audience questions, Dr. Masuda said that in adult studies higher levels of immunogenicity have been achieved after vaccination, compared with natural infection; however, there are as yet no pediatric data on that score. Also, investigators have seen evidence of cross-reactivity to the vaccine in some but not all naturally circulating nonvaccine strains.
The vaccine formulation being carried forward into advanced clinical trials in adults is 15 mcg of GI.1/50 mcg of GII.4c (J Infect Dis. 2018 Jan 30;217[4]:597-607).
The phase 2 study presented by Dr. Masuda was supported by the U.S. Army.
MALMO, SWEDEN – in an interim analysis of an ongoing phase 2 study, Taisei Masuda, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The randomized, double-blind, multinational trial remains blinded because follow-up is continuing, so – to the disappointment of the ESPID audience – there are as yet no data on duration of antibody persistence or clinical efficacy.
However, an earlier phase 2 study in 420 healthy participants aged 18-64 years showed that the Takeda vaccine elicited persistent immune responses 1 year post vaccination and that higher antibody levels correlated with a reduced frequency of moderate to severe vomiting and diarrheal illness following oral challenge with norovirus (Clin Vaccine Immunol. 2015 Aug;22[8]:923-9). Follow-up will continue in order to learn how long the protective immune response lasts in adults, according to Dr. Masuda, of Takeda Pharmaceuticals in Zurich.
The bivalent Takeda vaccine is the first candidate vaccine to reach the randomized trial stage. An oral vaccine in tablet form under development by Vaxart, a San Francisco Bay Area biotech company, recently completed preliminary phase 1 studies.
Dr. Masuda explained that the Takeda vaccine contains virus-like particle antigens from norovirus strains GI.1 and GII.4c, which together account for the majority of human norovirus illness. These virus-like particles are formed on the outer surface of the virus. Of note, virus-like particle–based vaccines against hepatitis B and human papillomavirus have won regulatory approval in the United States, Europe, and elsewhere.
He presented data on 120 healthy subjects aged 1 year to less than 4 years old and another 120 aged 4 years to less than 9 years. They are part of a larger phase 2 study of 840 children as young as age 6 weeks. This was a dose-finding study, so participants received various doses of the vaccine on day 1 and either a second dose or a saline injection 28 days later. The vaccine, which contains aluminum hydroxide to enhance immunogenicity, comes in prefilled syringes.
At 57 days of follow-up in this interim analysis, protective seroresponse rates as defined by at least a fourfold increase in histo-blood group antigen–blocking titers approached 100%. In the older group, this was typically achieved with a single dose of vaccine. However, the younger group of children generally derived further benefit from a second dose, according to Dr. Masuda.
In terms of safety concerns, he said no serious adverse events occurred in the study and no one withdrew from the trial because of vaccine-related side effects. The overall safety picture was the same in the two age groups. The incidence of fever of 38° C or higher was similar after administration of vaccine and placebo. Injection site pain occurred in one-quarter of younger vaccine recipients, in 38%-63% of those aged 4 years or older, and in 17%-22% who got placebo injections. Those and other local and systemic adverse events were mostly mild and transient. Their incidence and severity weren’t related to vaccine dosage.
In sum, Dr. Masuda deemed the safety profile “clinically acceptable.”
Session chair Karina Butler, MD, of Temple Street Children’s University Hospital, Dublin, raised the question of how might this vaccine, which may require two doses in younger children, fit into an already crowded pediatric immunization schedule – will parents and physicians embrace it?
Dr. Masuda replied that noroviruses are the No. 1 cause of acute gastroenteritis worldwide and there is a clamor for development of effective vaccines to protect the groups that bear the greatest burden of disease, including children, the elderly, military personnel, cruise ship vacationers, and others who experience crowded conditions. He expressed confidence that a safe and effective vaccine will be in high demand.
“In the future, we’ll look at the possibility of a combination vaccine,” he added.
In response to audience questions, Dr. Masuda said that in adult studies higher levels of immunogenicity have been achieved after vaccination, compared with natural infection; however, there are as yet no pediatric data on that score. Also, investigators have seen evidence of cross-reactivity to the vaccine in some but not all naturally circulating nonvaccine strains.
The vaccine formulation being carried forward into advanced clinical trials in adults is 15 mcg of GI.1/50 mcg of GII.4c (J Infect Dis. 2018 Jan 30;217[4]:597-607).
The phase 2 study presented by Dr. Masuda was supported by the U.S. Army.
MALMO, SWEDEN – in an interim analysis of an ongoing phase 2 study, Taisei Masuda, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The randomized, double-blind, multinational trial remains blinded because follow-up is continuing, so – to the disappointment of the ESPID audience – there are as yet no data on duration of antibody persistence or clinical efficacy.
However, an earlier phase 2 study in 420 healthy participants aged 18-64 years showed that the Takeda vaccine elicited persistent immune responses 1 year post vaccination and that higher antibody levels correlated with a reduced frequency of moderate to severe vomiting and diarrheal illness following oral challenge with norovirus (Clin Vaccine Immunol. 2015 Aug;22[8]:923-9). Follow-up will continue in order to learn how long the protective immune response lasts in adults, according to Dr. Masuda, of Takeda Pharmaceuticals in Zurich.
The bivalent Takeda vaccine is the first candidate vaccine to reach the randomized trial stage. An oral vaccine in tablet form under development by Vaxart, a San Francisco Bay Area biotech company, recently completed preliminary phase 1 studies.
Dr. Masuda explained that the Takeda vaccine contains virus-like particle antigens from norovirus strains GI.1 and GII.4c, which together account for the majority of human norovirus illness. These virus-like particles are formed on the outer surface of the virus. Of note, virus-like particle–based vaccines against hepatitis B and human papillomavirus have won regulatory approval in the United States, Europe, and elsewhere.
He presented data on 120 healthy subjects aged 1 year to less than 4 years old and another 120 aged 4 years to less than 9 years. They are part of a larger phase 2 study of 840 children as young as age 6 weeks. This was a dose-finding study, so participants received various doses of the vaccine on day 1 and either a second dose or a saline injection 28 days later. The vaccine, which contains aluminum hydroxide to enhance immunogenicity, comes in prefilled syringes.
At 57 days of follow-up in this interim analysis, protective seroresponse rates as defined by at least a fourfold increase in histo-blood group antigen–blocking titers approached 100%. In the older group, this was typically achieved with a single dose of vaccine. However, the younger group of children generally derived further benefit from a second dose, according to Dr. Masuda.
In terms of safety concerns, he said no serious adverse events occurred in the study and no one withdrew from the trial because of vaccine-related side effects. The overall safety picture was the same in the two age groups. The incidence of fever of 38° C or higher was similar after administration of vaccine and placebo. Injection site pain occurred in one-quarter of younger vaccine recipients, in 38%-63% of those aged 4 years or older, and in 17%-22% who got placebo injections. Those and other local and systemic adverse events were mostly mild and transient. Their incidence and severity weren’t related to vaccine dosage.
In sum, Dr. Masuda deemed the safety profile “clinically acceptable.”
Session chair Karina Butler, MD, of Temple Street Children’s University Hospital, Dublin, raised the question of how might this vaccine, which may require two doses in younger children, fit into an already crowded pediatric immunization schedule – will parents and physicians embrace it?
Dr. Masuda replied that noroviruses are the No. 1 cause of acute gastroenteritis worldwide and there is a clamor for development of effective vaccines to protect the groups that bear the greatest burden of disease, including children, the elderly, military personnel, cruise ship vacationers, and others who experience crowded conditions. He expressed confidence that a safe and effective vaccine will be in high demand.
“In the future, we’ll look at the possibility of a combination vaccine,” he added.
In response to audience questions, Dr. Masuda said that in adult studies higher levels of immunogenicity have been achieved after vaccination, compared with natural infection; however, there are as yet no pediatric data on that score. Also, investigators have seen evidence of cross-reactivity to the vaccine in some but not all naturally circulating nonvaccine strains.
The vaccine formulation being carried forward into advanced clinical trials in adults is 15 mcg of GI.1/50 mcg of GII.4c (J Infect Dis. 2018 Jan 30;217[4]:597-607).
The phase 2 study presented by Dr. Masuda was supported by the U.S. Army.
REPORTING FROM ESPID 2018
Key clinical point: Hope runs high that an effective norovirus vaccine is in the works.
Major finding: Protective seroresponse rates against the two chief disease-causing strains of norovirus were seen in nearly 100% of vaccinated children aged 1-8 years.
Study details: This is an ongoing prospective, multicenter, double-blind, phase 2 randomized trial including 840 children.
Disclosures: The study was supported by the U.S. Army and presented by an employee of Takeda Pharmaceuticals.
Pediatric asthma patients should be considered priority for flu vaccine
Children with asthma who present to emergency departments for treatment are significantly more likely to test positive for one or more respiratory pathogens, reported Dr. Joanna Merckx of the Montreal Children’s Hospital at the McGill University Health Centre, and her associates.
Nearly two-thirds of patients tested positive for one or more respiratory viruses in a study conducted by Dr. Merckx and her associates. “Given the documented safety of influenza immunization in children with asthma and its expected protective effect,” such cases should be among those prioritized to receive influenza immunization.
In reviewing the findings of the study, Dr. Merckx and her colleagues sought to determine whether closely evaluating the effects of specific respiratory pathogens could be useful in further developing appropriate preventive treatments for children with asthma; improving efforts to diagnose pathogens at the time of ED treatment; and identifying patients at higher risk of treatment failure who could be candidates for more intensive treatment protocols.
Children aged 1-17 years presenting to one of five EDs in the Pediatric Emergency Research Canada network during 2011-2013 with moderate or severe asthma flares were considered for the study. All eligible DOORWAY study participants with a valid respiratory specimen were included in the study and received a standardized dose of oral and bronchodilator treatment with salbutamol; those with severe exacerbations also received ipratropium bromide (Atrovent).
Within 1 hour of study inclusion, patients were tested by way of nasopharyngeal aspirate or swab. Patients identified with coinfection presented with two or more pathogens. Failure of ED management was defined as patients admitted to the hospital for asthma; ED treatment lasting 8 or more hours after corticosteroid treatment; or returns to the ED within 72 hours after discharge that led to hospital admission or prolonged ED stay.
Of 1,012 children enrolled in the study, 958 were assessed for worsening of asthma symptoms. Of the 958 respiratory specimens tested, 62% tested positive for one or more pathogens, 8.5% were found to have coinfection, of which respiratory syncytial virus (RSV) and coronavirus were the most frequent copathogens. Rhinovirus was the most prevalent pathogen, occurring in 29%, and of these, rhinovirus C was the most frequent species (18.2%), followed by RSV (17.9%); only two patients tested positive for Mycoplasma pneumoniae.
Children with a laboratory-confirmed pathogen were younger, had higher tobacco exposure, and were slightly more likely to present with fever (29% vs. 24%), compared with children without a laboratory-confirmed pathogen. Children with rhinovirus were less often febrile (16% vs 41%) and less frequently diagnosed with pneumonia (5% vs. 16%.) than those without a rhinovirus infection The proportion of children presenting with a severe exacerbation of asthma was 33%.
Overall, 17% of patients experienced treatment failure. Those with current respiratory infection were at increased risk of treatment failure, for a risk difference of 8% (95% confidence interval, 3.3%-13.1%). RSV, influenza, and parainfluenza virus (PIV) were associated with 21%, 38%, and 47% higher risks of treatment failure, respectively, noted Dr. Merckx and her associates. These resulted in absolute risks of 9%, 25%, and 34%, respectively, the authors reported in Pediatrics.
Coronavirus, adenovirus, enterovirus D68, and the presence of a coinfection, however, were not found to increase the risk of treatment failure, they noted.
Although rhinovirus may play a role in triggering reactions that require medical attention, such cases still appear to respond favorably to treatment, they said.
A separate study cited by Dr. Merckx and her associates observed the same outcome for rhinovirus patients but more patients diagnosed with nonrhinovirus pathogens, especially human metapneumovirus (hMPV) and PIV, had moderate, rather than severe, symptoms and were much more likely to experience higher treatment failure, particularly those infected with RSV, influenza, and PIV.
“It appears reasonable to pursue strategies to improve immunization coverage for influenza and invest in efforts for the development of vaccines for RSV and rhinovirus,” they said.
In cases in which respiratory pathogens were present (especially nonrhinovirus pathogens), greater treatment failure occurred, despite use of inhaler and corticosteroids. The researchers noted that severity of condition at time of treatment and patient response to treatment should be considered as two separate, distinct dimensions of viral infection impact in children with acute asthma. “The high prevalence of rhinovirus C in children presenting with asthma exacerbation, its presumed association with asthma-related hospitalization, and its peak in the fall,” also should be considered as a leading cause for more potential severe disease.
Dr. Merckx and her associates did point to several possibly significant implications with their findings. Intensifying treatment using inhaled anticholinergics or magnesium sulfate could block the vagally mediated reflex bronchoconstriction typically seen in cases of asthma exacerbation worsened by viral infection. Although these therapies currently only are used only in severe reactions, it may be useful to examine their efficacy in any cases triggered by RSV, influenza, and PIV because these have been associated with a poor treatment response.
While it still is necessary to clarify its mechanism of action, azithromycin’s demonstrated benefit in preschoolers with severe reactions suggests it could be a possible alternative pathogen–nonspecific therapy to address antineutrophilic inflammation, they said.
Any treatment intensification would first require clear identification of responsible pathogens using on-site diagnostic measures in the ED. Until such testing is possible, preventive measures need to be prioritized, they advised.
Dr. Merckx had no relevant disclosures; two of her associates reported receiving grants, salary rewards, and/or unrestricted donations from various pharmaceutical companies or foundations.
SOURCE: Merckx J et al. Pediatrics. 2018 Jun;142(1):e20174105.
Children with asthma who present to emergency departments for treatment are significantly more likely to test positive for one or more respiratory pathogens, reported Dr. Joanna Merckx of the Montreal Children’s Hospital at the McGill University Health Centre, and her associates.
Nearly two-thirds of patients tested positive for one or more respiratory viruses in a study conducted by Dr. Merckx and her associates. “Given the documented safety of influenza immunization in children with asthma and its expected protective effect,” such cases should be among those prioritized to receive influenza immunization.
In reviewing the findings of the study, Dr. Merckx and her colleagues sought to determine whether closely evaluating the effects of specific respiratory pathogens could be useful in further developing appropriate preventive treatments for children with asthma; improving efforts to diagnose pathogens at the time of ED treatment; and identifying patients at higher risk of treatment failure who could be candidates for more intensive treatment protocols.
Children aged 1-17 years presenting to one of five EDs in the Pediatric Emergency Research Canada network during 2011-2013 with moderate or severe asthma flares were considered for the study. All eligible DOORWAY study participants with a valid respiratory specimen were included in the study and received a standardized dose of oral and bronchodilator treatment with salbutamol; those with severe exacerbations also received ipratropium bromide (Atrovent).
Within 1 hour of study inclusion, patients were tested by way of nasopharyngeal aspirate or swab. Patients identified with coinfection presented with two or more pathogens. Failure of ED management was defined as patients admitted to the hospital for asthma; ED treatment lasting 8 or more hours after corticosteroid treatment; or returns to the ED within 72 hours after discharge that led to hospital admission or prolonged ED stay.
Of 1,012 children enrolled in the study, 958 were assessed for worsening of asthma symptoms. Of the 958 respiratory specimens tested, 62% tested positive for one or more pathogens, 8.5% were found to have coinfection, of which respiratory syncytial virus (RSV) and coronavirus were the most frequent copathogens. Rhinovirus was the most prevalent pathogen, occurring in 29%, and of these, rhinovirus C was the most frequent species (18.2%), followed by RSV (17.9%); only two patients tested positive for Mycoplasma pneumoniae.
Children with a laboratory-confirmed pathogen were younger, had higher tobacco exposure, and were slightly more likely to present with fever (29% vs. 24%), compared with children without a laboratory-confirmed pathogen. Children with rhinovirus were less often febrile (16% vs 41%) and less frequently diagnosed with pneumonia (5% vs. 16%.) than those without a rhinovirus infection The proportion of children presenting with a severe exacerbation of asthma was 33%.
Overall, 17% of patients experienced treatment failure. Those with current respiratory infection were at increased risk of treatment failure, for a risk difference of 8% (95% confidence interval, 3.3%-13.1%). RSV, influenza, and parainfluenza virus (PIV) were associated with 21%, 38%, and 47% higher risks of treatment failure, respectively, noted Dr. Merckx and her associates. These resulted in absolute risks of 9%, 25%, and 34%, respectively, the authors reported in Pediatrics.
Coronavirus, adenovirus, enterovirus D68, and the presence of a coinfection, however, were not found to increase the risk of treatment failure, they noted.
Although rhinovirus may play a role in triggering reactions that require medical attention, such cases still appear to respond favorably to treatment, they said.
A separate study cited by Dr. Merckx and her associates observed the same outcome for rhinovirus patients but more patients diagnosed with nonrhinovirus pathogens, especially human metapneumovirus (hMPV) and PIV, had moderate, rather than severe, symptoms and were much more likely to experience higher treatment failure, particularly those infected with RSV, influenza, and PIV.
“It appears reasonable to pursue strategies to improve immunization coverage for influenza and invest in efforts for the development of vaccines for RSV and rhinovirus,” they said.
In cases in which respiratory pathogens were present (especially nonrhinovirus pathogens), greater treatment failure occurred, despite use of inhaler and corticosteroids. The researchers noted that severity of condition at time of treatment and patient response to treatment should be considered as two separate, distinct dimensions of viral infection impact in children with acute asthma. “The high prevalence of rhinovirus C in children presenting with asthma exacerbation, its presumed association with asthma-related hospitalization, and its peak in the fall,” also should be considered as a leading cause for more potential severe disease.
Dr. Merckx and her associates did point to several possibly significant implications with their findings. Intensifying treatment using inhaled anticholinergics or magnesium sulfate could block the vagally mediated reflex bronchoconstriction typically seen in cases of asthma exacerbation worsened by viral infection. Although these therapies currently only are used only in severe reactions, it may be useful to examine their efficacy in any cases triggered by RSV, influenza, and PIV because these have been associated with a poor treatment response.
While it still is necessary to clarify its mechanism of action, azithromycin’s demonstrated benefit in preschoolers with severe reactions suggests it could be a possible alternative pathogen–nonspecific therapy to address antineutrophilic inflammation, they said.
Any treatment intensification would first require clear identification of responsible pathogens using on-site diagnostic measures in the ED. Until such testing is possible, preventive measures need to be prioritized, they advised.
Dr. Merckx had no relevant disclosures; two of her associates reported receiving grants, salary rewards, and/or unrestricted donations from various pharmaceutical companies or foundations.
SOURCE: Merckx J et al. Pediatrics. 2018 Jun;142(1):e20174105.
Children with asthma who present to emergency departments for treatment are significantly more likely to test positive for one or more respiratory pathogens, reported Dr. Joanna Merckx of the Montreal Children’s Hospital at the McGill University Health Centre, and her associates.
Nearly two-thirds of patients tested positive for one or more respiratory viruses in a study conducted by Dr. Merckx and her associates. “Given the documented safety of influenza immunization in children with asthma and its expected protective effect,” such cases should be among those prioritized to receive influenza immunization.
In reviewing the findings of the study, Dr. Merckx and her colleagues sought to determine whether closely evaluating the effects of specific respiratory pathogens could be useful in further developing appropriate preventive treatments for children with asthma; improving efforts to diagnose pathogens at the time of ED treatment; and identifying patients at higher risk of treatment failure who could be candidates for more intensive treatment protocols.
Children aged 1-17 years presenting to one of five EDs in the Pediatric Emergency Research Canada network during 2011-2013 with moderate or severe asthma flares were considered for the study. All eligible DOORWAY study participants with a valid respiratory specimen were included in the study and received a standardized dose of oral and bronchodilator treatment with salbutamol; those with severe exacerbations also received ipratropium bromide (Atrovent).
Within 1 hour of study inclusion, patients were tested by way of nasopharyngeal aspirate or swab. Patients identified with coinfection presented with two or more pathogens. Failure of ED management was defined as patients admitted to the hospital for asthma; ED treatment lasting 8 or more hours after corticosteroid treatment; or returns to the ED within 72 hours after discharge that led to hospital admission or prolonged ED stay.
Of 1,012 children enrolled in the study, 958 were assessed for worsening of asthma symptoms. Of the 958 respiratory specimens tested, 62% tested positive for one or more pathogens, 8.5% were found to have coinfection, of which respiratory syncytial virus (RSV) and coronavirus were the most frequent copathogens. Rhinovirus was the most prevalent pathogen, occurring in 29%, and of these, rhinovirus C was the most frequent species (18.2%), followed by RSV (17.9%); only two patients tested positive for Mycoplasma pneumoniae.
Children with a laboratory-confirmed pathogen were younger, had higher tobacco exposure, and were slightly more likely to present with fever (29% vs. 24%), compared with children without a laboratory-confirmed pathogen. Children with rhinovirus were less often febrile (16% vs 41%) and less frequently diagnosed with pneumonia (5% vs. 16%.) than those without a rhinovirus infection The proportion of children presenting with a severe exacerbation of asthma was 33%.
Overall, 17% of patients experienced treatment failure. Those with current respiratory infection were at increased risk of treatment failure, for a risk difference of 8% (95% confidence interval, 3.3%-13.1%). RSV, influenza, and parainfluenza virus (PIV) were associated with 21%, 38%, and 47% higher risks of treatment failure, respectively, noted Dr. Merckx and her associates. These resulted in absolute risks of 9%, 25%, and 34%, respectively, the authors reported in Pediatrics.
Coronavirus, adenovirus, enterovirus D68, and the presence of a coinfection, however, were not found to increase the risk of treatment failure, they noted.
Although rhinovirus may play a role in triggering reactions that require medical attention, such cases still appear to respond favorably to treatment, they said.
A separate study cited by Dr. Merckx and her associates observed the same outcome for rhinovirus patients but more patients diagnosed with nonrhinovirus pathogens, especially human metapneumovirus (hMPV) and PIV, had moderate, rather than severe, symptoms and were much more likely to experience higher treatment failure, particularly those infected with RSV, influenza, and PIV.
“It appears reasonable to pursue strategies to improve immunization coverage for influenza and invest in efforts for the development of vaccines for RSV and rhinovirus,” they said.
In cases in which respiratory pathogens were present (especially nonrhinovirus pathogens), greater treatment failure occurred, despite use of inhaler and corticosteroids. The researchers noted that severity of condition at time of treatment and patient response to treatment should be considered as two separate, distinct dimensions of viral infection impact in children with acute asthma. “The high prevalence of rhinovirus C in children presenting with asthma exacerbation, its presumed association with asthma-related hospitalization, and its peak in the fall,” also should be considered as a leading cause for more potential severe disease.
Dr. Merckx and her associates did point to several possibly significant implications with their findings. Intensifying treatment using inhaled anticholinergics or magnesium sulfate could block the vagally mediated reflex bronchoconstriction typically seen in cases of asthma exacerbation worsened by viral infection. Although these therapies currently only are used only in severe reactions, it may be useful to examine their efficacy in any cases triggered by RSV, influenza, and PIV because these have been associated with a poor treatment response.
While it still is necessary to clarify its mechanism of action, azithromycin’s demonstrated benefit in preschoolers with severe reactions suggests it could be a possible alternative pathogen–nonspecific therapy to address antineutrophilic inflammation, they said.
Any treatment intensification would first require clear identification of responsible pathogens using on-site diagnostic measures in the ED. Until such testing is possible, preventive measures need to be prioritized, they advised.
Dr. Merckx had no relevant disclosures; two of her associates reported receiving grants, salary rewards, and/or unrestricted donations from various pharmaceutical companies or foundations.
SOURCE: Merckx J et al. Pediatrics. 2018 Jun;142(1):e20174105.
FROM PEDIATRICS
Key clinical point: Nearly two-thirds of emergency asthma cases test positive for multiple pathogens.
Major finding: Risk of treatment failure is higher with respiratory syncytial virus, influenza, and parainfluenza virus.
Study details: Ancillary multicenter, prospective, ethics-approved cohort study of 958 children with asthma.
Disclosures: Dr. Merckx had no relevant disclosures; two of her associates reported receiving grants, salary rewards, and/or unrestricted donations from various pharmaceutical companies or foundations.
Source: Merckx J et al. Pediatrics. 2018;142(1):e20174105.
Parents say cancer prevention is the best reason to give HPV vaccine
according to an analysis of a national survey.
Preventing a common infection also ranked highly as a reason for giving the vaccine, as did appeals to the vaccine’s lasting benefits and safety, the analysis showed.
The findings strongly support prioritizing cancer prevention as a reason for HPV vaccination, reported Melissa B. Gilkey, PhD, of the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and her associates.
“To achieve widespread coverage, healthcare providers need strategies for more effectively and efficiently communicating its value,” Dr. Gilkey and her colleagues reported in Cancer Epidemiology, Biomarkers & Prevention.
The findings were based on responses obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey completed by 1,259 parents of adolescents.
A total of 1,177 parent were included in this analysis after excluding surveys that were incomplete with regard to questions on provider communication about HPV vaccination.
In the online survey, parents were asked to rank, from best to worst, a list of 11 reasons providers commonly give to encourage parents to consider HPV vaccination for their child.
Overall, parents ranked cancer prevention as the best reason for guideline-consistent HPV vaccination (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
The worst reasons, as ranked by these parents, were “your child is due for it” (beta = –1.08), “I got it for my own child” (beta = –0.98), and “it is a scientific breakthrough” (beta = –0.67).
Researchers hypothesized that parents with low vaccination confidence would have different preferences. While those parents did less often endorse cancer prevention and a few other questions, the variation was minor and resulted in few differences versus the overall parent rankings, according to Dr. Gilkey and her colleagues.
“Although parents with low confidence may find top reasons for HPV vaccination less compelling, they would not necessarily benefit from targeted messaging,” they wrote.
The study was funded by the National Cancer Institute. Dr. Gilkey and coauthors had no potential conflicts of interest to disclose.
SOURCE: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
according to an analysis of a national survey.
Preventing a common infection also ranked highly as a reason for giving the vaccine, as did appeals to the vaccine’s lasting benefits and safety, the analysis showed.
The findings strongly support prioritizing cancer prevention as a reason for HPV vaccination, reported Melissa B. Gilkey, PhD, of the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and her associates.
“To achieve widespread coverage, healthcare providers need strategies for more effectively and efficiently communicating its value,” Dr. Gilkey and her colleagues reported in Cancer Epidemiology, Biomarkers & Prevention.
The findings were based on responses obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey completed by 1,259 parents of adolescents.
A total of 1,177 parent were included in this analysis after excluding surveys that were incomplete with regard to questions on provider communication about HPV vaccination.
In the online survey, parents were asked to rank, from best to worst, a list of 11 reasons providers commonly give to encourage parents to consider HPV vaccination for their child.
Overall, parents ranked cancer prevention as the best reason for guideline-consistent HPV vaccination (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
The worst reasons, as ranked by these parents, were “your child is due for it” (beta = –1.08), “I got it for my own child” (beta = –0.98), and “it is a scientific breakthrough” (beta = –0.67).
Researchers hypothesized that parents with low vaccination confidence would have different preferences. While those parents did less often endorse cancer prevention and a few other questions, the variation was minor and resulted in few differences versus the overall parent rankings, according to Dr. Gilkey and her colleagues.
“Although parents with low confidence may find top reasons for HPV vaccination less compelling, they would not necessarily benefit from targeted messaging,” they wrote.
The study was funded by the National Cancer Institute. Dr. Gilkey and coauthors had no potential conflicts of interest to disclose.
SOURCE: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
according to an analysis of a national survey.
Preventing a common infection also ranked highly as a reason for giving the vaccine, as did appeals to the vaccine’s lasting benefits and safety, the analysis showed.
The findings strongly support prioritizing cancer prevention as a reason for HPV vaccination, reported Melissa B. Gilkey, PhD, of the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and her associates.
“To achieve widespread coverage, healthcare providers need strategies for more effectively and efficiently communicating its value,” Dr. Gilkey and her colleagues reported in Cancer Epidemiology, Biomarkers & Prevention.
The findings were based on responses obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey completed by 1,259 parents of adolescents.
A total of 1,177 parent were included in this analysis after excluding surveys that were incomplete with regard to questions on provider communication about HPV vaccination.
In the online survey, parents were asked to rank, from best to worst, a list of 11 reasons providers commonly give to encourage parents to consider HPV vaccination for their child.
Overall, parents ranked cancer prevention as the best reason for guideline-consistent HPV vaccination (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
The worst reasons, as ranked by these parents, were “your child is due for it” (beta = –1.08), “I got it for my own child” (beta = –0.98), and “it is a scientific breakthrough” (beta = –0.67).
Researchers hypothesized that parents with low vaccination confidence would have different preferences. While those parents did less often endorse cancer prevention and a few other questions, the variation was minor and resulted in few differences versus the overall parent rankings, according to Dr. Gilkey and her colleagues.
“Although parents with low confidence may find top reasons for HPV vaccination less compelling, they would not necessarily benefit from targeted messaging,” they wrote.
The study was funded by the National Cancer Institute. Dr. Gilkey and coauthors had no potential conflicts of interest to disclose.
SOURCE: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION
Key clinical point: Among the reasons health care providers give parents for adolescent HPV vaccination, cancer prevention may be the best.
Major finding: Cancer prevention ranked highest (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
Study details: An analysis of 1,177 responses from parents of adolescents obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey.
Disclosures: The study was funded by the National Cancer Institute. Study authors had no potential conflicts of interest to disclose.
Source: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
ACIP votes to recommend new strains for the 2018-2019 flu vaccine
Thirteen members of the Advisory Committee on Immunization Practices (ACIP) voted to approve the influenza vaccine recommendations for 2018-2019, while one member abstained from voting at the summer ACIP meeting.
The 2018-2019 recommendation maintains the core recommendation that influenza vaccines should be administered to all persons 6 months or older who have no contraindications.
FluMist Quadrivalent (LAIV4) also is being updated for the 2018-2019 season. At the February meeting of ACIP, the committee approved language that providers may provide any licensed, age-appropriate influenza vaccine, and LAIV4 is considered in this set of vaccine options.
Prior to this approval, there was a discussion of the safety of the 2017-2018 vaccine. For many of the available vaccines, there were no new safety concerns raised from reports during the flu season. Monitoring during the 2018-2019 will yield more safety monitoring data concerning pregnancy and influenza vaccinations and anaphylaxis in persons with an egg allergy.
The committee’s recommendations must be approved by the Centers for Disease Control and Prevention’s director before they are considered official recommendations.
Thirteen members of the Advisory Committee on Immunization Practices (ACIP) voted to approve the influenza vaccine recommendations for 2018-2019, while one member abstained from voting at the summer ACIP meeting.
The 2018-2019 recommendation maintains the core recommendation that influenza vaccines should be administered to all persons 6 months or older who have no contraindications.
FluMist Quadrivalent (LAIV4) also is being updated for the 2018-2019 season. At the February meeting of ACIP, the committee approved language that providers may provide any licensed, age-appropriate influenza vaccine, and LAIV4 is considered in this set of vaccine options.
Prior to this approval, there was a discussion of the safety of the 2017-2018 vaccine. For many of the available vaccines, there were no new safety concerns raised from reports during the flu season. Monitoring during the 2018-2019 will yield more safety monitoring data concerning pregnancy and influenza vaccinations and anaphylaxis in persons with an egg allergy.
The committee’s recommendations must be approved by the Centers for Disease Control and Prevention’s director before they are considered official recommendations.
Thirteen members of the Advisory Committee on Immunization Practices (ACIP) voted to approve the influenza vaccine recommendations for 2018-2019, while one member abstained from voting at the summer ACIP meeting.
The 2018-2019 recommendation maintains the core recommendation that influenza vaccines should be administered to all persons 6 months or older who have no contraindications.
FluMist Quadrivalent (LAIV4) also is being updated for the 2018-2019 season. At the February meeting of ACIP, the committee approved language that providers may provide any licensed, age-appropriate influenza vaccine, and LAIV4 is considered in this set of vaccine options.
Prior to this approval, there was a discussion of the safety of the 2017-2018 vaccine. For many of the available vaccines, there were no new safety concerns raised from reports during the flu season. Monitoring during the 2018-2019 will yield more safety monitoring data concerning pregnancy and influenza vaccinations and anaphylaxis in persons with an egg allergy.
The committee’s recommendations must be approved by the Centers for Disease Control and Prevention’s director before they are considered official recommendations.
REPORTING FROM AN ACIP MEETING
Additional training may be warranted for clinicians administering DTaP
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
FROM PEDIATRICS
Key clinical point: No new or unexpected safety issues were found with DTaP.
Major finding: Nearly 90% of adverse events reported were not considered serious.
Study details: Large-scale data mining and records review from the Vaccine Adverse Event Reporting System.
Disclosures: The authors had no relevant financial disclosures. The study was funded by the Centers for Disease Control and Prevention and the Food and Drug Administration.
Source: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
Impact of varicella vaccination on herpes zoster is not what was expected
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.
The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.
The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.
The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
REPORTING FROM ESPID 2018
Key clinical point: The exogenous boosting hypothesis that universal pediatric varicella vaccination would result in an increase in herpes zoster hasn’t been borne out by the U.S. experience.
Major finding: rather than accelerating as some had forecast.
Study details: This was a retrospective study of the annual incidence of varicella and herpes zoster during 1991-2016 in roughly one-fifth of the U.S. population.
Disclosures: The study was sponsored by Merck and presented by a company employee.
Make adult immunization a profit center
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
REPORTING FROM ACP INTERNAL MEDICINE
Vaccine nonmedical exemptions creating metro ‘hotspots’
Recent increases in nonmedical exemptions (NMEs) to vaccination have created metropolitan “hotspots” with large numbers of unvaccinated children, according to a report published June 12 in PLoS Medicine.
although rates seem to have plateaued in some states since 2014. As a result of those increases, there were, during the 2016-2017 school year, 15 metro areas with kindergarten NME populations over 400, reported Jacqueline K. Olive, and her associates at Baylor College of Medicine. Their report was based on data from state health departments and the Centers for Disease Control and Prevention.
Leading the way was Maricopa County, Ariz., home of Phoenix and 2,947 unvaccinated kindergartners, which was more than triple the number in county/city No. 2, Salt Lake County/Salt Lake City (NME total: 956). Close behind in third was King County, Wash. (Seattle) at 940, followed by Multnomah County, Ore. (Portland) at 711 and Oakland County, Mich. (Troy) at 686, the investigators said.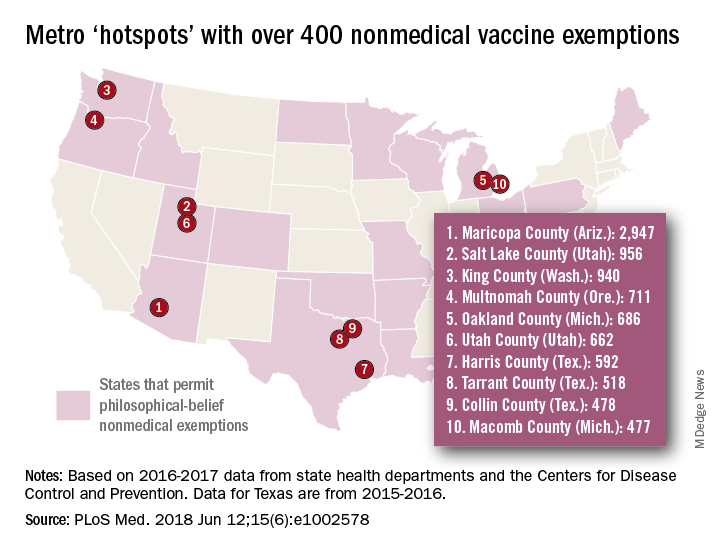
[There was only room for 10 in the map, so here are hotspots 11-15: Wayne County, Mich. (Detroit); Allegheny County, Pa. (Pittsburgh); Travis County, Tex. (Austin); Jackson County, Mo. (Kansas City); and Spokane County, Wash. (Spokane).]
In addition to the large-population hotspots, there are also a number of mainly rural counties with smaller populations but high NME rates. Eight of the 10 highest such rates can be found in Idaho, and at the top of that list is Camas County, which had an NME rate of 27% in 2016-2017, the researchers reported.
Analysis of the relationship between NMEs and MMR vaccination showed that “states with more NME students exhibited lower MMR vaccination rates. In contrast, states that have banned NMEs – Mississippi, California, and West Virginia – exhibit the highest MMR vaccine uptake and lowest incidence of vaccine preventable diseases,” the investigators wrote.
Ms. Olive and her associates said that there was no specific funding for the study and that no conflicts of interest existed.
SOURCE: Olive JK et al. PLoS Med. 2018 Jun 12;15(6): e1002578. doi: 10.1371/journal.pmed.1002578.
Recent increases in nonmedical exemptions (NMEs) to vaccination have created metropolitan “hotspots” with large numbers of unvaccinated children, according to a report published June 12 in PLoS Medicine.
although rates seem to have plateaued in some states since 2014. As a result of those increases, there were, during the 2016-2017 school year, 15 metro areas with kindergarten NME populations over 400, reported Jacqueline K. Olive, and her associates at Baylor College of Medicine. Their report was based on data from state health departments and the Centers for Disease Control and Prevention.
Leading the way was Maricopa County, Ariz., home of Phoenix and 2,947 unvaccinated kindergartners, which was more than triple the number in county/city No. 2, Salt Lake County/Salt Lake City (NME total: 956). Close behind in third was King County, Wash. (Seattle) at 940, followed by Multnomah County, Ore. (Portland) at 711 and Oakland County, Mich. (Troy) at 686, the investigators said.
[There was only room for 10 in the map, so here are hotspots 11-15: Wayne County, Mich. (Detroit); Allegheny County, Pa. (Pittsburgh); Travis County, Tex. (Austin); Jackson County, Mo. (Kansas City); and Spokane County, Wash. (Spokane).]
In addition to the large-population hotspots, there are also a number of mainly rural counties with smaller populations but high NME rates. Eight of the 10 highest such rates can be found in Idaho, and at the top of that list is Camas County, which had an NME rate of 27% in 2016-2017, the researchers reported.
Analysis of the relationship between NMEs and MMR vaccination showed that “states with more NME students exhibited lower MMR vaccination rates. In contrast, states that have banned NMEs – Mississippi, California, and West Virginia – exhibit the highest MMR vaccine uptake and lowest incidence of vaccine preventable diseases,” the investigators wrote.
Ms. Olive and her associates said that there was no specific funding for the study and that no conflicts of interest existed.
SOURCE: Olive JK et al. PLoS Med. 2018 Jun 12;15(6): e1002578. doi: 10.1371/journal.pmed.1002578.
Recent increases in nonmedical exemptions (NMEs) to vaccination have created metropolitan “hotspots” with large numbers of unvaccinated children, according to a report published June 12 in PLoS Medicine.
although rates seem to have plateaued in some states since 2014. As a result of those increases, there were, during the 2016-2017 school year, 15 metro areas with kindergarten NME populations over 400, reported Jacqueline K. Olive, and her associates at Baylor College of Medicine. Their report was based on data from state health departments and the Centers for Disease Control and Prevention.
Leading the way was Maricopa County, Ariz., home of Phoenix and 2,947 unvaccinated kindergartners, which was more than triple the number in county/city No. 2, Salt Lake County/Salt Lake City (NME total: 956). Close behind in third was King County, Wash. (Seattle) at 940, followed by Multnomah County, Ore. (Portland) at 711 and Oakland County, Mich. (Troy) at 686, the investigators said.
[There was only room for 10 in the map, so here are hotspots 11-15: Wayne County, Mich. (Detroit); Allegheny County, Pa. (Pittsburgh); Travis County, Tex. (Austin); Jackson County, Mo. (Kansas City); and Spokane County, Wash. (Spokane).]
In addition to the large-population hotspots, there are also a number of mainly rural counties with smaller populations but high NME rates. Eight of the 10 highest such rates can be found in Idaho, and at the top of that list is Camas County, which had an NME rate of 27% in 2016-2017, the researchers reported.
Analysis of the relationship between NMEs and MMR vaccination showed that “states with more NME students exhibited lower MMR vaccination rates. In contrast, states that have banned NMEs – Mississippi, California, and West Virginia – exhibit the highest MMR vaccine uptake and lowest incidence of vaccine preventable diseases,” the investigators wrote.
Ms. Olive and her associates said that there was no specific funding for the study and that no conflicts of interest existed.
SOURCE: Olive JK et al. PLoS Med. 2018 Jun 12;15(6): e1002578. doi: 10.1371/journal.pmed.1002578.
FROM PLOS MEDICINE