User login
Parents say cancer prevention is the best reason to give HPV vaccine
according to an analysis of a national survey.
Preventing a common infection also ranked highly as a reason for giving the vaccine, as did appeals to the vaccine’s lasting benefits and safety, the analysis showed.
The findings strongly support prioritizing cancer prevention as a reason for HPV vaccination, reported Melissa B. Gilkey, PhD, of the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and her associates.
“To achieve widespread coverage, healthcare providers need strategies for more effectively and efficiently communicating its value,” Dr. Gilkey and her colleagues reported in Cancer Epidemiology, Biomarkers & Prevention.
The findings were based on responses obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey completed by 1,259 parents of adolescents.
A total of 1,177 parent were included in this analysis after excluding surveys that were incomplete with regard to questions on provider communication about HPV vaccination.
In the online survey, parents were asked to rank, from best to worst, a list of 11 reasons providers commonly give to encourage parents to consider HPV vaccination for their child.
Overall, parents ranked cancer prevention as the best reason for guideline-consistent HPV vaccination (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
The worst reasons, as ranked by these parents, were “your child is due for it” (beta = –1.08), “I got it for my own child” (beta = –0.98), and “it is a scientific breakthrough” (beta = –0.67).
Researchers hypothesized that parents with low vaccination confidence would have different preferences. While those parents did less often endorse cancer prevention and a few other questions, the variation was minor and resulted in few differences versus the overall parent rankings, according to Dr. Gilkey and her colleagues.
“Although parents with low confidence may find top reasons for HPV vaccination less compelling, they would not necessarily benefit from targeted messaging,” they wrote.
The study was funded by the National Cancer Institute. Dr. Gilkey and coauthors had no potential conflicts of interest to disclose.
SOURCE: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
according to an analysis of a national survey.
Preventing a common infection also ranked highly as a reason for giving the vaccine, as did appeals to the vaccine’s lasting benefits and safety, the analysis showed.
The findings strongly support prioritizing cancer prevention as a reason for HPV vaccination, reported Melissa B. Gilkey, PhD, of the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and her associates.
“To achieve widespread coverage, healthcare providers need strategies for more effectively and efficiently communicating its value,” Dr. Gilkey and her colleagues reported in Cancer Epidemiology, Biomarkers & Prevention.
The findings were based on responses obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey completed by 1,259 parents of adolescents.
A total of 1,177 parent were included in this analysis after excluding surveys that were incomplete with regard to questions on provider communication about HPV vaccination.
In the online survey, parents were asked to rank, from best to worst, a list of 11 reasons providers commonly give to encourage parents to consider HPV vaccination for their child.
Overall, parents ranked cancer prevention as the best reason for guideline-consistent HPV vaccination (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
The worst reasons, as ranked by these parents, were “your child is due for it” (beta = –1.08), “I got it for my own child” (beta = –0.98), and “it is a scientific breakthrough” (beta = –0.67).
Researchers hypothesized that parents with low vaccination confidence would have different preferences. While those parents did less often endorse cancer prevention and a few other questions, the variation was minor and resulted in few differences versus the overall parent rankings, according to Dr. Gilkey and her colleagues.
“Although parents with low confidence may find top reasons for HPV vaccination less compelling, they would not necessarily benefit from targeted messaging,” they wrote.
The study was funded by the National Cancer Institute. Dr. Gilkey and coauthors had no potential conflicts of interest to disclose.
SOURCE: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
according to an analysis of a national survey.
Preventing a common infection also ranked highly as a reason for giving the vaccine, as did appeals to the vaccine’s lasting benefits and safety, the analysis showed.
The findings strongly support prioritizing cancer prevention as a reason for HPV vaccination, reported Melissa B. Gilkey, PhD, of the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and her associates.
“To achieve widespread coverage, healthcare providers need strategies for more effectively and efficiently communicating its value,” Dr. Gilkey and her colleagues reported in Cancer Epidemiology, Biomarkers & Prevention.
The findings were based on responses obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey completed by 1,259 parents of adolescents.
A total of 1,177 parent were included in this analysis after excluding surveys that were incomplete with regard to questions on provider communication about HPV vaccination.
In the online survey, parents were asked to rank, from best to worst, a list of 11 reasons providers commonly give to encourage parents to consider HPV vaccination for their child.
Overall, parents ranked cancer prevention as the best reason for guideline-consistent HPV vaccination (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
The worst reasons, as ranked by these parents, were “your child is due for it” (beta = –1.08), “I got it for my own child” (beta = –0.98), and “it is a scientific breakthrough” (beta = –0.67).
Researchers hypothesized that parents with low vaccination confidence would have different preferences. While those parents did less often endorse cancer prevention and a few other questions, the variation was minor and resulted in few differences versus the overall parent rankings, according to Dr. Gilkey and her colleagues.
“Although parents with low confidence may find top reasons for HPV vaccination less compelling, they would not necessarily benefit from targeted messaging,” they wrote.
The study was funded by the National Cancer Institute. Dr. Gilkey and coauthors had no potential conflicts of interest to disclose.
SOURCE: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION
Key clinical point: Among the reasons health care providers give parents for adolescent HPV vaccination, cancer prevention may be the best.
Major finding: Cancer prevention ranked highest (beta = 2.07), followed by preventing a common infection (beta = 0.68), having lasting benefits (beta = 0.67), and being a safe vaccine (beta = 0.41).
Study details: An analysis of 1,177 responses from parents of adolescents obtained in the Adolescent Cancer Prevention Communication Study, a 2016 online survey.
Disclosures: The study was funded by the National Cancer Institute. Study authors had no potential conflicts of interest to disclose.
Source: Gilkey MB et al. Cancer Epidemiol Biomarkers Prev. 2018 Jul;27(7):762-7.
ACIP votes to recommend new strains for the 2018-2019 flu vaccine
Thirteen members of the Advisory Committee on Immunization Practices (ACIP) voted to approve the influenza vaccine recommendations for 2018-2019, while one member abstained from voting at the summer ACIP meeting.
The 2018-2019 recommendation maintains the core recommendation that influenza vaccines should be administered to all persons 6 months or older who have no contraindications.
FluMist Quadrivalent (LAIV4) also is being updated for the 2018-2019 season. At the February meeting of ACIP, the committee approved language that providers may provide any licensed, age-appropriate influenza vaccine, and LAIV4 is considered in this set of vaccine options.
Prior to this approval, there was a discussion of the safety of the 2017-2018 vaccine. For many of the available vaccines, there were no new safety concerns raised from reports during the flu season. Monitoring during the 2018-2019 will yield more safety monitoring data concerning pregnancy and influenza vaccinations and anaphylaxis in persons with an egg allergy.
The committee’s recommendations must be approved by the Centers for Disease Control and Prevention’s director before they are considered official recommendations.
Thirteen members of the Advisory Committee on Immunization Practices (ACIP) voted to approve the influenza vaccine recommendations for 2018-2019, while one member abstained from voting at the summer ACIP meeting.
The 2018-2019 recommendation maintains the core recommendation that influenza vaccines should be administered to all persons 6 months or older who have no contraindications.
FluMist Quadrivalent (LAIV4) also is being updated for the 2018-2019 season. At the February meeting of ACIP, the committee approved language that providers may provide any licensed, age-appropriate influenza vaccine, and LAIV4 is considered in this set of vaccine options.
Prior to this approval, there was a discussion of the safety of the 2017-2018 vaccine. For many of the available vaccines, there were no new safety concerns raised from reports during the flu season. Monitoring during the 2018-2019 will yield more safety monitoring data concerning pregnancy and influenza vaccinations and anaphylaxis in persons with an egg allergy.
The committee’s recommendations must be approved by the Centers for Disease Control and Prevention’s director before they are considered official recommendations.
Thirteen members of the Advisory Committee on Immunization Practices (ACIP) voted to approve the influenza vaccine recommendations for 2018-2019, while one member abstained from voting at the summer ACIP meeting.
The 2018-2019 recommendation maintains the core recommendation that influenza vaccines should be administered to all persons 6 months or older who have no contraindications.
FluMist Quadrivalent (LAIV4) also is being updated for the 2018-2019 season. At the February meeting of ACIP, the committee approved language that providers may provide any licensed, age-appropriate influenza vaccine, and LAIV4 is considered in this set of vaccine options.
Prior to this approval, there was a discussion of the safety of the 2017-2018 vaccine. For many of the available vaccines, there were no new safety concerns raised from reports during the flu season. Monitoring during the 2018-2019 will yield more safety monitoring data concerning pregnancy and influenza vaccinations and anaphylaxis in persons with an egg allergy.
The committee’s recommendations must be approved by the Centers for Disease Control and Prevention’s director before they are considered official recommendations.
REPORTING FROM AN ACIP MEETING
Additional training may be warranted for clinicians administering DTaP
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
FROM PEDIATRICS
Key clinical point: No new or unexpected safety issues were found with DTaP.
Major finding: Nearly 90% of adverse events reported were not considered serious.
Study details: Large-scale data mining and records review from the Vaccine Adverse Event Reporting System.
Disclosures: The authors had no relevant financial disclosures. The study was funded by the Centers for Disease Control and Prevention and the Food and Drug Administration.
Source: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
Impact of varicella vaccination on herpes zoster is not what was expected
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.

The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.

The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
MALMO, SWEDEN – The unique 20-year U.S. experience with pediatric universal varicella vaccination hasn’t resulted in the anticipated increase in herpes zoster predicted by the exogenous boosting hypothesis, Lara J. Wolfson, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
In fact, the opposite has occurred. And this finding – based upon hard data – should be of considerable interest to European health officials who have been considering introducing universal varicella vaccination into their national health care systems but have refrained because of theoretical concerns raised by the venerable exogenous boosting hypothesis, noted Dr. Wolfson, director of outcomes research at the Merck Center for Observational and Real-World Evidence, Kenilworth, N.J.

The exogenous boosting hypothesis, which dates back to the mid-1960s, holds that reexposure to wild circulating varicella virus prevents development of herpes zoster later in life. Conversely, by vaccinating children against varicella, opportunities are diminished for reexposure to wild type virus among adults who weren’t vaccinated against varicella, so the hypothesis would predict an increase in the incidence of herpes zoster that should peak 15-35 years after introduction of universal varicella vaccination.
“The same virus that causes varicella in children later reactivates after going dormant in the dorsal root ganglia, and it reactivates as herpes zoster, which is 10 times more severe than chicken pox and leads to 10 times the health care costs. So if in fact implementing a universal varicella vaccine program would lead to an increased incidence of herpes zoster, this would be a bad thing,” the researcher explained.
However, the predictive models based upon the exogenous boosting hypothesis are built upon scanty data. And the models have great difficulty in adjusting for the changes in population dynamics that have occurred in the United States and Western Europe during the past quarter century: namely, declining birth rates coupled with survival to an older age.
Dr. Wolfson presented a retrospective study of deidentified administrative claims data from the MarketScan database covering roughly one-fifth of the U.S. population during 1991-2016. Her analysis broke down the annual incidence of varicella and herpes zoster in three eras: 1991-1995, which was the pre–varicella vaccination period; 1996-2006, when single-dose universal varicella vaccination of children was recommended; and 2007-2016, when two-dose vaccination became standard.
The first key study finding was that herpes zoster rates in the United States already were climbing across all age groups back in 1991-1995; that is, before introduction of universal varicella vaccination. Why? Probably because of those changes in population dynamics, although that’s speculative. The second key finding was that contrary to the exogenous boosting hypothesis prediction that the annual incidence of herpes zoster would accelerate after introduction of universal varicella vaccination, the rate of increase slowed, then plateaued during 2013-2016, most prominently in individuals aged 65 or older.
“In comparing the pre–universal varicella vaccination period to the one- or two-dose period or the total 20 years of vaccination, what we saw consistently across every age group is that herpes zoster is decelerating. There is actually less increase in the rate of herpes zoster than before varicella vaccination,” Dr. Wolfson said.
Uptake of the herpes zoster vaccine, introduced in the United States in 2008, was too low during the study years to account for this trend, she added.
Most dramatically, the incidence of herpes zoster among youths under age 18 years plummeted by 61.4%, from 88 per 100,000 person-years in 1991-1995 to 34 per 100,000 in 2016.
And of course, varicella disease has sharply declined in all age groups following the introduction of universal pediatric varicella vaccination, Dr. Wolfson observed.
Her study was supported by her employer, Merck.
REPORTING FROM ESPID 2018
Key clinical point: The exogenous boosting hypothesis that universal pediatric varicella vaccination would result in an increase in herpes zoster hasn’t been borne out by the U.S. experience.
Major finding: rather than accelerating as some had forecast.
Study details: This was a retrospective study of the annual incidence of varicella and herpes zoster during 1991-2016 in roughly one-fifth of the U.S. population.
Disclosures: The study was sponsored by Merck and presented by a company employee.
Make adult immunization a profit center
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
REPORTING FROM ACP INTERNAL MEDICINE
Vaccine nonmedical exemptions creating metro ‘hotspots’
Recent increases in nonmedical exemptions (NMEs) to vaccination have created metropolitan “hotspots” with large numbers of unvaccinated children, according to a report published June 12 in PLoS Medicine.
although rates seem to have plateaued in some states since 2014. As a result of those increases, there were, during the 2016-2017 school year, 15 metro areas with kindergarten NME populations over 400, reported Jacqueline K. Olive, and her associates at Baylor College of Medicine. Their report was based on data from state health departments and the Centers for Disease Control and Prevention.
Leading the way was Maricopa County, Ariz., home of Phoenix and 2,947 unvaccinated kindergartners, which was more than triple the number in county/city No. 2, Salt Lake County/Salt Lake City (NME total: 956). Close behind in third was King County, Wash. (Seattle) at 940, followed by Multnomah County, Ore. (Portland) at 711 and Oakland County, Mich. (Troy) at 686, the investigators said.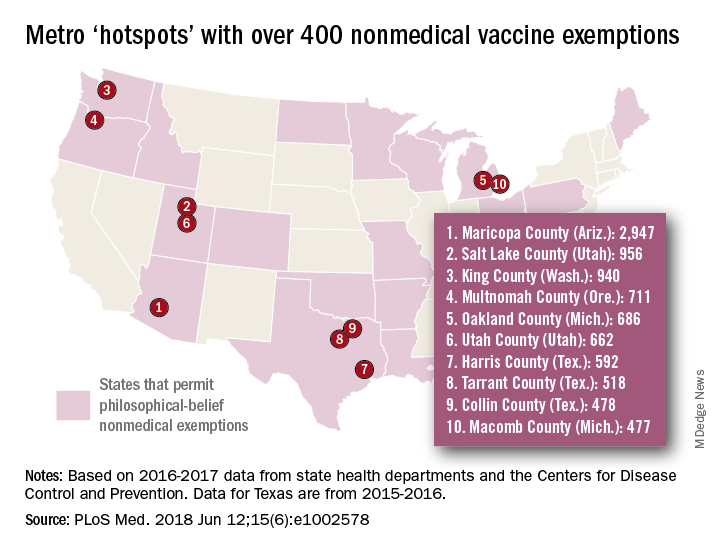
[There was only room for 10 in the map, so here are hotspots 11-15: Wayne County, Mich. (Detroit); Allegheny County, Pa. (Pittsburgh); Travis County, Tex. (Austin); Jackson County, Mo. (Kansas City); and Spokane County, Wash. (Spokane).]
In addition to the large-population hotspots, there are also a number of mainly rural counties with smaller populations but high NME rates. Eight of the 10 highest such rates can be found in Idaho, and at the top of that list is Camas County, which had an NME rate of 27% in 2016-2017, the researchers reported.
Analysis of the relationship between NMEs and MMR vaccination showed that “states with more NME students exhibited lower MMR vaccination rates. In contrast, states that have banned NMEs – Mississippi, California, and West Virginia – exhibit the highest MMR vaccine uptake and lowest incidence of vaccine preventable diseases,” the investigators wrote.
Ms. Olive and her associates said that there was no specific funding for the study and that no conflicts of interest existed.
SOURCE: Olive JK et al. PLoS Med. 2018 Jun 12;15(6): e1002578. doi: 10.1371/journal.pmed.1002578.
Recent increases in nonmedical exemptions (NMEs) to vaccination have created metropolitan “hotspots” with large numbers of unvaccinated children, according to a report published June 12 in PLoS Medicine.
although rates seem to have plateaued in some states since 2014. As a result of those increases, there were, during the 2016-2017 school year, 15 metro areas with kindergarten NME populations over 400, reported Jacqueline K. Olive, and her associates at Baylor College of Medicine. Their report was based on data from state health departments and the Centers for Disease Control and Prevention.
Leading the way was Maricopa County, Ariz., home of Phoenix and 2,947 unvaccinated kindergartners, which was more than triple the number in county/city No. 2, Salt Lake County/Salt Lake City (NME total: 956). Close behind in third was King County, Wash. (Seattle) at 940, followed by Multnomah County, Ore. (Portland) at 711 and Oakland County, Mich. (Troy) at 686, the investigators said.
[There was only room for 10 in the map, so here are hotspots 11-15: Wayne County, Mich. (Detroit); Allegheny County, Pa. (Pittsburgh); Travis County, Tex. (Austin); Jackson County, Mo. (Kansas City); and Spokane County, Wash. (Spokane).]
In addition to the large-population hotspots, there are also a number of mainly rural counties with smaller populations but high NME rates. Eight of the 10 highest such rates can be found in Idaho, and at the top of that list is Camas County, which had an NME rate of 27% in 2016-2017, the researchers reported.
Analysis of the relationship between NMEs and MMR vaccination showed that “states with more NME students exhibited lower MMR vaccination rates. In contrast, states that have banned NMEs – Mississippi, California, and West Virginia – exhibit the highest MMR vaccine uptake and lowest incidence of vaccine preventable diseases,” the investigators wrote.
Ms. Olive and her associates said that there was no specific funding for the study and that no conflicts of interest existed.
SOURCE: Olive JK et al. PLoS Med. 2018 Jun 12;15(6): e1002578. doi: 10.1371/journal.pmed.1002578.
Recent increases in nonmedical exemptions (NMEs) to vaccination have created metropolitan “hotspots” with large numbers of unvaccinated children, according to a report published June 12 in PLoS Medicine.
although rates seem to have plateaued in some states since 2014. As a result of those increases, there were, during the 2016-2017 school year, 15 metro areas with kindergarten NME populations over 400, reported Jacqueline K. Olive, and her associates at Baylor College of Medicine. Their report was based on data from state health departments and the Centers for Disease Control and Prevention.
Leading the way was Maricopa County, Ariz., home of Phoenix and 2,947 unvaccinated kindergartners, which was more than triple the number in county/city No. 2, Salt Lake County/Salt Lake City (NME total: 956). Close behind in third was King County, Wash. (Seattle) at 940, followed by Multnomah County, Ore. (Portland) at 711 and Oakland County, Mich. (Troy) at 686, the investigators said.
[There was only room for 10 in the map, so here are hotspots 11-15: Wayne County, Mich. (Detroit); Allegheny County, Pa. (Pittsburgh); Travis County, Tex. (Austin); Jackson County, Mo. (Kansas City); and Spokane County, Wash. (Spokane).]
In addition to the large-population hotspots, there are also a number of mainly rural counties with smaller populations but high NME rates. Eight of the 10 highest such rates can be found in Idaho, and at the top of that list is Camas County, which had an NME rate of 27% in 2016-2017, the researchers reported.
Analysis of the relationship between NMEs and MMR vaccination showed that “states with more NME students exhibited lower MMR vaccination rates. In contrast, states that have banned NMEs – Mississippi, California, and West Virginia – exhibit the highest MMR vaccine uptake and lowest incidence of vaccine preventable diseases,” the investigators wrote.
Ms. Olive and her associates said that there was no specific funding for the study and that no conflicts of interest existed.
SOURCE: Olive JK et al. PLoS Med. 2018 Jun 12;15(6): e1002578. doi: 10.1371/journal.pmed.1002578.
FROM PLOS MEDICINE
Shingles hospitalization occurs more often among IBD patients
WASHINGTON –
This elevated risk for patients with inflammatory bowel disease (IBD) to develop a herpes zoster virus (HZV) reactivation severe enough to put them in the hospital makes it especially important for IBD patients to receive immunization against shingles, especially now that a more effective vaccine is available, Daniela G. Vinsard, MD, said at the annual Digestive Disease Week®. Ideally, IBD patients should receive the full course of the adjuvanted, recombinant zoster vaccine Shingrix before starting an immunosuppressive regimen, said Dr. Vinsard, a physician at the University of Connecticut, Farmington.
This finding, which underscored the susceptibility of IBD patients to shingles because of their immunosuppressive treatments and the importance of vaccination, recently became even more relevant when the Food and Drug Administration approved tofacitinib (Xeljanz) to treat ulcerative colitis in late May, commented Gil Y. Melmed, MD, director of clinical inflammatory bowel disease at Cedars-Sinai Medical Center, Los Angeles. Tofacitinib, which may be an attractive option to some patients as an oral immunomodulator, carries a black box warning about the added risk for certain serious infections while taking the drug, including HZV. Recent recommendations from the American College of Gastroenterology said that IBD patients aged 51 years or older should “strongly consider” HZV vaccination, including immunosuppressed patients (Am J Gastroenterol. 2017 Feb; 112[2]:241-58). The introduction of a potentially popular drug for ulcerative colitis that’s known to pose a risk for shingles might lead to a stronger recommendation for vaccination in the near future, Dr. Melmed said in an interview.
The study Dr. Vinsard reported used data collected by the National Inpatient Sample from 2012 to September 2015, which represented, with weighting, more than 142 million hospitalized American patients. From this data set she and her associates identified 7,180 IBD patients hospitalized with a primary diagnosis of a vaccine-preventable disease, and about 589,000 weighted patients hospitalized for a vaccine-preventable disease but without IBD. The selection also focused on patients aged 18-65 years. Dr. Vinsard said that she excluded older patients to eliminate advanced age as a cause of immunosuppression.
In a multivariate analysis that controlled for diabetes, HIV infection, cancer, and transplantation, the IBD patients had more than twice the rate of hospitalization for shingles, compared with the patients without IBD, Dr. Vinsard said. When broken down by specific disease type, the rate of HZV infection was 110% higher among ulcerative colitis patients, compared with the general population, and was 140% higher in Crohn’s disease patients, both statistically significant differences.
An additional finding from the analysis was that during the 4 years of study, the rate of hospitalizations of IBD patients for influenza steadily rose, from about 10% in 2012 to nearly 30% in 2015.
Dr. Vinsard reported no disclosures. Dr. Melmed reported consulting with Pfizer, the company that markets tofacitinib, and with several other companies that market biological agents.
WASHINGTON –
This elevated risk for patients with inflammatory bowel disease (IBD) to develop a herpes zoster virus (HZV) reactivation severe enough to put them in the hospital makes it especially important for IBD patients to receive immunization against shingles, especially now that a more effective vaccine is available, Daniela G. Vinsard, MD, said at the annual Digestive Disease Week®. Ideally, IBD patients should receive the full course of the adjuvanted, recombinant zoster vaccine Shingrix before starting an immunosuppressive regimen, said Dr. Vinsard, a physician at the University of Connecticut, Farmington.
This finding, which underscored the susceptibility of IBD patients to shingles because of their immunosuppressive treatments and the importance of vaccination, recently became even more relevant when the Food and Drug Administration approved tofacitinib (Xeljanz) to treat ulcerative colitis in late May, commented Gil Y. Melmed, MD, director of clinical inflammatory bowel disease at Cedars-Sinai Medical Center, Los Angeles. Tofacitinib, which may be an attractive option to some patients as an oral immunomodulator, carries a black box warning about the added risk for certain serious infections while taking the drug, including HZV. Recent recommendations from the American College of Gastroenterology said that IBD patients aged 51 years or older should “strongly consider” HZV vaccination, including immunosuppressed patients (Am J Gastroenterol. 2017 Feb; 112[2]:241-58). The introduction of a potentially popular drug for ulcerative colitis that’s known to pose a risk for shingles might lead to a stronger recommendation for vaccination in the near future, Dr. Melmed said in an interview.
The study Dr. Vinsard reported used data collected by the National Inpatient Sample from 2012 to September 2015, which represented, with weighting, more than 142 million hospitalized American patients. From this data set she and her associates identified 7,180 IBD patients hospitalized with a primary diagnosis of a vaccine-preventable disease, and about 589,000 weighted patients hospitalized for a vaccine-preventable disease but without IBD. The selection also focused on patients aged 18-65 years. Dr. Vinsard said that she excluded older patients to eliminate advanced age as a cause of immunosuppression.
In a multivariate analysis that controlled for diabetes, HIV infection, cancer, and transplantation, the IBD patients had more than twice the rate of hospitalization for shingles, compared with the patients without IBD, Dr. Vinsard said. When broken down by specific disease type, the rate of HZV infection was 110% higher among ulcerative colitis patients, compared with the general population, and was 140% higher in Crohn’s disease patients, both statistically significant differences.
An additional finding from the analysis was that during the 4 years of study, the rate of hospitalizations of IBD patients for influenza steadily rose, from about 10% in 2012 to nearly 30% in 2015.
Dr. Vinsard reported no disclosures. Dr. Melmed reported consulting with Pfizer, the company that markets tofacitinib, and with several other companies that market biological agents.
WASHINGTON –
This elevated risk for patients with inflammatory bowel disease (IBD) to develop a herpes zoster virus (HZV) reactivation severe enough to put them in the hospital makes it especially important for IBD patients to receive immunization against shingles, especially now that a more effective vaccine is available, Daniela G. Vinsard, MD, said at the annual Digestive Disease Week®. Ideally, IBD patients should receive the full course of the adjuvanted, recombinant zoster vaccine Shingrix before starting an immunosuppressive regimen, said Dr. Vinsard, a physician at the University of Connecticut, Farmington.
This finding, which underscored the susceptibility of IBD patients to shingles because of their immunosuppressive treatments and the importance of vaccination, recently became even more relevant when the Food and Drug Administration approved tofacitinib (Xeljanz) to treat ulcerative colitis in late May, commented Gil Y. Melmed, MD, director of clinical inflammatory bowel disease at Cedars-Sinai Medical Center, Los Angeles. Tofacitinib, which may be an attractive option to some patients as an oral immunomodulator, carries a black box warning about the added risk for certain serious infections while taking the drug, including HZV. Recent recommendations from the American College of Gastroenterology said that IBD patients aged 51 years or older should “strongly consider” HZV vaccination, including immunosuppressed patients (Am J Gastroenterol. 2017 Feb; 112[2]:241-58). The introduction of a potentially popular drug for ulcerative colitis that’s known to pose a risk for shingles might lead to a stronger recommendation for vaccination in the near future, Dr. Melmed said in an interview.
The study Dr. Vinsard reported used data collected by the National Inpatient Sample from 2012 to September 2015, which represented, with weighting, more than 142 million hospitalized American patients. From this data set she and her associates identified 7,180 IBD patients hospitalized with a primary diagnosis of a vaccine-preventable disease, and about 589,000 weighted patients hospitalized for a vaccine-preventable disease but without IBD. The selection also focused on patients aged 18-65 years. Dr. Vinsard said that she excluded older patients to eliminate advanced age as a cause of immunosuppression.
In a multivariate analysis that controlled for diabetes, HIV infection, cancer, and transplantation, the IBD patients had more than twice the rate of hospitalization for shingles, compared with the patients without IBD, Dr. Vinsard said. When broken down by specific disease type, the rate of HZV infection was 110% higher among ulcerative colitis patients, compared with the general population, and was 140% higher in Crohn’s disease patients, both statistically significant differences.
An additional finding from the analysis was that during the 4 years of study, the rate of hospitalizations of IBD patients for influenza steadily rose, from about 10% in 2012 to nearly 30% in 2015.
Dr. Vinsard reported no disclosures. Dr. Melmed reported consulting with Pfizer, the company that markets tofacitinib, and with several other companies that market biological agents.
REPORTING FROM DDW 2018
Key clinical point: Patients with inflammatory bowel disease have an increased risk for shingles that results in hospitalization.
Major finding: Patients with IBD hospitalized for a vaccine-preventable infection had twice the rate of shingles as the general population.
Study details: A review of data collected by the U.S. National Inpatient Sample during 2012-2015.
Disclosures: Dr. Vinsard reported no disclosures. Dr. Melmed reported consulting with Pfizer, the company that markets tofacitinib (Xeljanz), and with several other companies that market biological agents.
Vaccine-related febrile seizures have zero developmental impact
MALMO, SWEDEN – Children who experience a febrile seizure in conjunction with a vaccination have developmental outcomes comparable with those of children who have non–vaccine-related febrile seizures and healthy controls who’ve never had a febrile seizure, according to the first prospective case-control cohort study to examine the issue.
This finding has important implications for clinical practice, Lucy Deng, MD, observed at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Febrile seizures associated with a vaccine can decrease parent and provider confidence in vaccine safety,” the pediatrician noted. Based upon her study results, however, physicians now can offer a truly evidence-based message of reassurance.
“If you have a child with a vaccine-related febrile seizure, you can give the same advice to those parents as for anyone else who’s had a febrile seizure, in that there is no difference in the clinical outcomes of vaccine-proximate and non–vaccine-proximate febrile seizures. Vaccine-proximate febrile seizures are usually brief, they don’t require any antiepileptic drugs, their length of stay is usually less than a day, and developmentally at 12-24 months post initial febrile seizure, they’re exactly the same as children who’ve never had a seizure before or who’ve had a non-vaccine-related febrile seizure,” said Dr. Deng of the National Centre for Immunisation Research and Surveillance in Sydney.
The impetus for her study was straightforward: “We all know that most children with a history of febrile seizures have normal behavior, intelligence, and academic achievement and do not later develop epilepsy. What we didn’t know before is if all of these facts apply to vaccine-proximate febrile seizures,” she explained.
The clinical severity analysis portion of this prospective case-control cohort study included 1,085 children with febrile seizures seen at five Australian children’s hospitals. Sixty-eight of them had vaccine-proximate febrile seizures, for a 6.6% rate. The febrile seizures in the other 1,027 children didn’t occur within 2 weeks following a vaccination.
Measles vaccine was implicated in 56 of the 68 children with vaccine-proximate febrile seizures, or 82%. Because Australian children receive their first measles-containing vaccine at age 12 months, the average age of the cohort with vaccine-proximate febrile seizures was 13 months, significantly younger than the 20-month average for children with non–vaccine-related febrile seizures.
In a multivariate analysis adjusted for patient age, gender, and history of prior afebrile seizures, the groups with vaccine-proximate and vaccine-unrelated febrile seizures didn’t differ significantly in terms of the proportion with a hospital length of stay greater than 1 day (20% vs. 15%), ICU admission (1.5% vs. 2.3%), seizure duration of more than 15 minutes (16% vs. 12%), repeat seizures within 24 hours (9% vs. 10%), or discharge on antiepileptic medication (4.4% vs. 4.3%).
In the developmental outcomes analysis, 62 of the children with vaccine-proximate febrile seizures, 70 with vaccine-unrelated febrile seizures, and 85 healthy controls with no seizure history underwent formal assessment using the third edition of the Bayley Scales of Infant and Toddler Development 12-24 months after their initial febrile seizure. Scores adjusted for years of maternal education were closely similar in all three groups across all five test domains: cognitive, language, motor, social-emotional, and general-adaptive.
Dr. Deng reported having no financial conflicts of interest regarding the study, which was partially funded by the Australian National Centre for Immunisation Research and Surveillance.
MALMO, SWEDEN – Children who experience a febrile seizure in conjunction with a vaccination have developmental outcomes comparable with those of children who have non–vaccine-related febrile seizures and healthy controls who’ve never had a febrile seizure, according to the first prospective case-control cohort study to examine the issue.
This finding has important implications for clinical practice, Lucy Deng, MD, observed at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Febrile seizures associated with a vaccine can decrease parent and provider confidence in vaccine safety,” the pediatrician noted. Based upon her study results, however, physicians now can offer a truly evidence-based message of reassurance.
“If you have a child with a vaccine-related febrile seizure, you can give the same advice to those parents as for anyone else who’s had a febrile seizure, in that there is no difference in the clinical outcomes of vaccine-proximate and non–vaccine-proximate febrile seizures. Vaccine-proximate febrile seizures are usually brief, they don’t require any antiepileptic drugs, their length of stay is usually less than a day, and developmentally at 12-24 months post initial febrile seizure, they’re exactly the same as children who’ve never had a seizure before or who’ve had a non-vaccine-related febrile seizure,” said Dr. Deng of the National Centre for Immunisation Research and Surveillance in Sydney.
The impetus for her study was straightforward: “We all know that most children with a history of febrile seizures have normal behavior, intelligence, and academic achievement and do not later develop epilepsy. What we didn’t know before is if all of these facts apply to vaccine-proximate febrile seizures,” she explained.
The clinical severity analysis portion of this prospective case-control cohort study included 1,085 children with febrile seizures seen at five Australian children’s hospitals. Sixty-eight of them had vaccine-proximate febrile seizures, for a 6.6% rate. The febrile seizures in the other 1,027 children didn’t occur within 2 weeks following a vaccination.
Measles vaccine was implicated in 56 of the 68 children with vaccine-proximate febrile seizures, or 82%. Because Australian children receive their first measles-containing vaccine at age 12 months, the average age of the cohort with vaccine-proximate febrile seizures was 13 months, significantly younger than the 20-month average for children with non–vaccine-related febrile seizures.
In a multivariate analysis adjusted for patient age, gender, and history of prior afebrile seizures, the groups with vaccine-proximate and vaccine-unrelated febrile seizures didn’t differ significantly in terms of the proportion with a hospital length of stay greater than 1 day (20% vs. 15%), ICU admission (1.5% vs. 2.3%), seizure duration of more than 15 minutes (16% vs. 12%), repeat seizures within 24 hours (9% vs. 10%), or discharge on antiepileptic medication (4.4% vs. 4.3%).
In the developmental outcomes analysis, 62 of the children with vaccine-proximate febrile seizures, 70 with vaccine-unrelated febrile seizures, and 85 healthy controls with no seizure history underwent formal assessment using the third edition of the Bayley Scales of Infant and Toddler Development 12-24 months after their initial febrile seizure. Scores adjusted for years of maternal education were closely similar in all three groups across all five test domains: cognitive, language, motor, social-emotional, and general-adaptive.
Dr. Deng reported having no financial conflicts of interest regarding the study, which was partially funded by the Australian National Centre for Immunisation Research and Surveillance.
MALMO, SWEDEN – Children who experience a febrile seizure in conjunction with a vaccination have developmental outcomes comparable with those of children who have non–vaccine-related febrile seizures and healthy controls who’ve never had a febrile seizure, according to the first prospective case-control cohort study to examine the issue.
This finding has important implications for clinical practice, Lucy Deng, MD, observed at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Febrile seizures associated with a vaccine can decrease parent and provider confidence in vaccine safety,” the pediatrician noted. Based upon her study results, however, physicians now can offer a truly evidence-based message of reassurance.
“If you have a child with a vaccine-related febrile seizure, you can give the same advice to those parents as for anyone else who’s had a febrile seizure, in that there is no difference in the clinical outcomes of vaccine-proximate and non–vaccine-proximate febrile seizures. Vaccine-proximate febrile seizures are usually brief, they don’t require any antiepileptic drugs, their length of stay is usually less than a day, and developmentally at 12-24 months post initial febrile seizure, they’re exactly the same as children who’ve never had a seizure before or who’ve had a non-vaccine-related febrile seizure,” said Dr. Deng of the National Centre for Immunisation Research and Surveillance in Sydney.
The impetus for her study was straightforward: “We all know that most children with a history of febrile seizures have normal behavior, intelligence, and academic achievement and do not later develop epilepsy. What we didn’t know before is if all of these facts apply to vaccine-proximate febrile seizures,” she explained.
The clinical severity analysis portion of this prospective case-control cohort study included 1,085 children with febrile seizures seen at five Australian children’s hospitals. Sixty-eight of them had vaccine-proximate febrile seizures, for a 6.6% rate. The febrile seizures in the other 1,027 children didn’t occur within 2 weeks following a vaccination.
Measles vaccine was implicated in 56 of the 68 children with vaccine-proximate febrile seizures, or 82%. Because Australian children receive their first measles-containing vaccine at age 12 months, the average age of the cohort with vaccine-proximate febrile seizures was 13 months, significantly younger than the 20-month average for children with non–vaccine-related febrile seizures.
In a multivariate analysis adjusted for patient age, gender, and history of prior afebrile seizures, the groups with vaccine-proximate and vaccine-unrelated febrile seizures didn’t differ significantly in terms of the proportion with a hospital length of stay greater than 1 day (20% vs. 15%), ICU admission (1.5% vs. 2.3%), seizure duration of more than 15 minutes (16% vs. 12%), repeat seizures within 24 hours (9% vs. 10%), or discharge on antiepileptic medication (4.4% vs. 4.3%).
In the developmental outcomes analysis, 62 of the children with vaccine-proximate febrile seizures, 70 with vaccine-unrelated febrile seizures, and 85 healthy controls with no seizure history underwent formal assessment using the third edition of the Bayley Scales of Infant and Toddler Development 12-24 months after their initial febrile seizure. Scores adjusted for years of maternal education were closely similar in all three groups across all five test domains: cognitive, language, motor, social-emotional, and general-adaptive.
Dr. Deng reported having no financial conflicts of interest regarding the study, which was partially funded by the Australian National Centre for Immunisation Research and Surveillance.
REPORTING FROM ESPID 2018
Key clinical point: Parents now can confidently be reassured that vaccine-proximate febrile seizures have no long-term consequences.
Major finding: as in controls with no seizure history.
Study details: This prospective case-control study comprised 1,180 children at five Australian children’s hospitals.
Disclosures: The study was partially funded by the Australian National Centre for Immunisation Research and Surveillance. The presenter reported having no financial conflicts.
Many hospitals had no mandatory flu vaccine requirements in 2017
Many U.S. hospitals still did not have influenza vaccination requirements for health care personnel as of summer 2017, suggested the results of a national survey.
Nearly two-thirds of hospitals had mandatory influenza vaccination in place in 2017, up from just one-third in 2013, according to survey responses submitted by infection preventionists working at Veterans Affairs (VA) and non-VA hospitals.
Despite recommendations to vaccinate health care personnel against influenza, there are several challenges and barriers to implementing the practice, the authors wrote in JAMA Network Open.
“Mandating influenza vaccination remains a controversial topic, with uncertainty of the effectiveness of health care personnel influenza vaccination in reducing patient morbidity and mortality, different conclusions regarding the grading of the evidence, and numerous legal and ethical precedents to be carefully considered,” they wrote.
Their study was based on 1,062 responses to a panel survey of infection preventionists conducted every 4 years. The survey asked providers about practices used in their hospitals to prevent health care–associated infections.
Compared with 2013, when only 37.1% of non-VA hospitals had mandatory influenza vaccination requirements, the 2017 survey showed a significant increase to 61.4% (P less than .001), Dr. Greene and his colleagues wrote in their report.
By contrast, the proportion of VA hospitals with such requirements increased only slightly, from 1.3% in 2013 to just 4.1% in 2017 (P = .29), the report showed.
Penalties for not complying with the policy were not universal in hospitals with mandates, they added. Only 74% said they had such penalties, and 13% allowed health care personnel to decline influenza vaccination without a specified reason.
After the survey responses were received, the VA issued a directive stating that all health care personnel should receive annual influenza vaccination and should wear masks during influenza season, Dr. Greene noted.
That directive is in line with recommendations from the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, which have stated that all health care personnel should receive influenza vaccination each year.
In addition, the U.S. Department of Health & Human Services has set a goal of 90% of health care personnel to be vaccinated by 2020, Dr. Green and his coauthors noted.
Mandating influenza vaccination is just one proven successful strategy for increasing coverage at hospitals, according to the study authors. Other approaches include influenza education, incentives, free and easy access to vaccination, and annual campaigns directed at health care personnel, as well as written policies describing the vaccination goal.
“Regardless of whether an organization has an official mandate for vaccinations, establishing a written policy that states the organizational commitment to increasing vaccination rates is among the recommended strategies for improving vaccination coverage among health care personnel,” they wrote.
Dr. Greene and his coauthors reported receiving grants from the Blue Cross Blue Shield of Michigan Foundation and the U.S. Department of Veterans Affairs Patient Safety Center of Inquiry during the conduct of the study. One study coauthor reported personal fees from Jvion and from Doximity outside the submitted work.
SOURCE: Greene MT et al. JAMA Network Open. 2018;1(2):e180143.
This study suggests a significant increase in use of mandatory influenza vaccination policies during 2013-2017, driven mainly by increases at non–Veterans Affairs (VA) hospitals and little change at VA facilities. However, there are some caveats to the findings that should be considered, Hilary M. Babcock, MD, MPH, wrote in an editorial referencing the study.
The sample for the 2013 and 2017 surveys included different facilities and different size facilities, so direct comparisons cannot be made, according to Dr. Babcock.
Moreover, the survey questions were worded somewhat differently in the two surveys, and it does not appear that “mandate” was defined by the study authors, she said in her editorial.
The VA recently issued a directive that all health care personnel should receive influenza vaccination and wear masks during influenza season. This new directive provides an “excellent opportunity” to address knowledge gaps regarding the effects of influenza vaccination of health care personnel on patient outcomes, according to Dr. Babcock.
“While the assumption that decreasing the risk of influenza in health care personnel will result in decreased risk of influenza in patients cared for by those health care personnel is common sense, for acute care settings, it is still largely an assumption,” Dr. Babcock wrote. “Hopefully, the Veterans Health Administration will combine this initiative with thoughtful, planned, patient outcome assessments to help define the anticipated benefit of these efforts.”
Dr. Babcock is with Washington University and the BJC HealthCare Infection Prevention & Epidemiology Consortium, both in St. Louis. These comments are derived from her editorial in JAMA Network Open (2018;1[2]:e180144). Dr. Babcock reported no conflict of interest disclosures related to her editorial.
This study suggests a significant increase in use of mandatory influenza vaccination policies during 2013-2017, driven mainly by increases at non–Veterans Affairs (VA) hospitals and little change at VA facilities. However, there are some caveats to the findings that should be considered, Hilary M. Babcock, MD, MPH, wrote in an editorial referencing the study.
The sample for the 2013 and 2017 surveys included different facilities and different size facilities, so direct comparisons cannot be made, according to Dr. Babcock.
Moreover, the survey questions were worded somewhat differently in the two surveys, and it does not appear that “mandate” was defined by the study authors, she said in her editorial.
The VA recently issued a directive that all health care personnel should receive influenza vaccination and wear masks during influenza season. This new directive provides an “excellent opportunity” to address knowledge gaps regarding the effects of influenza vaccination of health care personnel on patient outcomes, according to Dr. Babcock.
“While the assumption that decreasing the risk of influenza in health care personnel will result in decreased risk of influenza in patients cared for by those health care personnel is common sense, for acute care settings, it is still largely an assumption,” Dr. Babcock wrote. “Hopefully, the Veterans Health Administration will combine this initiative with thoughtful, planned, patient outcome assessments to help define the anticipated benefit of these efforts.”
Dr. Babcock is with Washington University and the BJC HealthCare Infection Prevention & Epidemiology Consortium, both in St. Louis. These comments are derived from her editorial in JAMA Network Open (2018;1[2]:e180144). Dr. Babcock reported no conflict of interest disclosures related to her editorial.
This study suggests a significant increase in use of mandatory influenza vaccination policies during 2013-2017, driven mainly by increases at non–Veterans Affairs (VA) hospitals and little change at VA facilities. However, there are some caveats to the findings that should be considered, Hilary M. Babcock, MD, MPH, wrote in an editorial referencing the study.
The sample for the 2013 and 2017 surveys included different facilities and different size facilities, so direct comparisons cannot be made, according to Dr. Babcock.
Moreover, the survey questions were worded somewhat differently in the two surveys, and it does not appear that “mandate” was defined by the study authors, she said in her editorial.
The VA recently issued a directive that all health care personnel should receive influenza vaccination and wear masks during influenza season. This new directive provides an “excellent opportunity” to address knowledge gaps regarding the effects of influenza vaccination of health care personnel on patient outcomes, according to Dr. Babcock.
“While the assumption that decreasing the risk of influenza in health care personnel will result in decreased risk of influenza in patients cared for by those health care personnel is common sense, for acute care settings, it is still largely an assumption,” Dr. Babcock wrote. “Hopefully, the Veterans Health Administration will combine this initiative with thoughtful, planned, patient outcome assessments to help define the anticipated benefit of these efforts.”
Dr. Babcock is with Washington University and the BJC HealthCare Infection Prevention & Epidemiology Consortium, both in St. Louis. These comments are derived from her editorial in JAMA Network Open (2018;1[2]:e180144). Dr. Babcock reported no conflict of interest disclosures related to her editorial.
Many U.S. hospitals still did not have influenza vaccination requirements for health care personnel as of summer 2017, suggested the results of a national survey.
Nearly two-thirds of hospitals had mandatory influenza vaccination in place in 2017, up from just one-third in 2013, according to survey responses submitted by infection preventionists working at Veterans Affairs (VA) and non-VA hospitals.
Despite recommendations to vaccinate health care personnel against influenza, there are several challenges and barriers to implementing the practice, the authors wrote in JAMA Network Open.
“Mandating influenza vaccination remains a controversial topic, with uncertainty of the effectiveness of health care personnel influenza vaccination in reducing patient morbidity and mortality, different conclusions regarding the grading of the evidence, and numerous legal and ethical precedents to be carefully considered,” they wrote.
Their study was based on 1,062 responses to a panel survey of infection preventionists conducted every 4 years. The survey asked providers about practices used in their hospitals to prevent health care–associated infections.
Compared with 2013, when only 37.1% of non-VA hospitals had mandatory influenza vaccination requirements, the 2017 survey showed a significant increase to 61.4% (P less than .001), Dr. Greene and his colleagues wrote in their report.
By contrast, the proportion of VA hospitals with such requirements increased only slightly, from 1.3% in 2013 to just 4.1% in 2017 (P = .29), the report showed.
Penalties for not complying with the policy were not universal in hospitals with mandates, they added. Only 74% said they had such penalties, and 13% allowed health care personnel to decline influenza vaccination without a specified reason.
After the survey responses were received, the VA issued a directive stating that all health care personnel should receive annual influenza vaccination and should wear masks during influenza season, Dr. Greene noted.
That directive is in line with recommendations from the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, which have stated that all health care personnel should receive influenza vaccination each year.
In addition, the U.S. Department of Health & Human Services has set a goal of 90% of health care personnel to be vaccinated by 2020, Dr. Green and his coauthors noted.
Mandating influenza vaccination is just one proven successful strategy for increasing coverage at hospitals, according to the study authors. Other approaches include influenza education, incentives, free and easy access to vaccination, and annual campaigns directed at health care personnel, as well as written policies describing the vaccination goal.
“Regardless of whether an organization has an official mandate for vaccinations, establishing a written policy that states the organizational commitment to increasing vaccination rates is among the recommended strategies for improving vaccination coverage among health care personnel,” they wrote.
Dr. Greene and his coauthors reported receiving grants from the Blue Cross Blue Shield of Michigan Foundation and the U.S. Department of Veterans Affairs Patient Safety Center of Inquiry during the conduct of the study. One study coauthor reported personal fees from Jvion and from Doximity outside the submitted work.
SOURCE: Greene MT et al. JAMA Network Open. 2018;1(2):e180143.
Many U.S. hospitals still did not have influenza vaccination requirements for health care personnel as of summer 2017, suggested the results of a national survey.
Nearly two-thirds of hospitals had mandatory influenza vaccination in place in 2017, up from just one-third in 2013, according to survey responses submitted by infection preventionists working at Veterans Affairs (VA) and non-VA hospitals.
Despite recommendations to vaccinate health care personnel against influenza, there are several challenges and barriers to implementing the practice, the authors wrote in JAMA Network Open.
“Mandating influenza vaccination remains a controversial topic, with uncertainty of the effectiveness of health care personnel influenza vaccination in reducing patient morbidity and mortality, different conclusions regarding the grading of the evidence, and numerous legal and ethical precedents to be carefully considered,” they wrote.
Their study was based on 1,062 responses to a panel survey of infection preventionists conducted every 4 years. The survey asked providers about practices used in their hospitals to prevent health care–associated infections.
Compared with 2013, when only 37.1% of non-VA hospitals had mandatory influenza vaccination requirements, the 2017 survey showed a significant increase to 61.4% (P less than .001), Dr. Greene and his colleagues wrote in their report.
By contrast, the proportion of VA hospitals with such requirements increased only slightly, from 1.3% in 2013 to just 4.1% in 2017 (P = .29), the report showed.
Penalties for not complying with the policy were not universal in hospitals with mandates, they added. Only 74% said they had such penalties, and 13% allowed health care personnel to decline influenza vaccination without a specified reason.
After the survey responses were received, the VA issued a directive stating that all health care personnel should receive annual influenza vaccination and should wear masks during influenza season, Dr. Greene noted.
That directive is in line with recommendations from the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, which have stated that all health care personnel should receive influenza vaccination each year.
In addition, the U.S. Department of Health & Human Services has set a goal of 90% of health care personnel to be vaccinated by 2020, Dr. Green and his coauthors noted.
Mandating influenza vaccination is just one proven successful strategy for increasing coverage at hospitals, according to the study authors. Other approaches include influenza education, incentives, free and easy access to vaccination, and annual campaigns directed at health care personnel, as well as written policies describing the vaccination goal.
“Regardless of whether an organization has an official mandate for vaccinations, establishing a written policy that states the organizational commitment to increasing vaccination rates is among the recommended strategies for improving vaccination coverage among health care personnel,” they wrote.
Dr. Greene and his coauthors reported receiving grants from the Blue Cross Blue Shield of Michigan Foundation and the U.S. Department of Veterans Affairs Patient Safety Center of Inquiry during the conduct of the study. One study coauthor reported personal fees from Jvion and from Doximity outside the submitted work.
SOURCE: Greene MT et al. JAMA Network Open. 2018;1(2):e180143.
FROM JAMA Network Open
Key clinical point: Despite a significant increase in influenza vaccination at non-VA hospitals, many VA and non-VA hospitals still do not have mandatory influenza vaccination requirements for health care personnel.
Major finding: During 2013-2017, the proportion of non-VA hospitals with requirements increased from 37.1% to 61.4% (P less than .001), contrasting with a rise from 1.3% to just 4.1% at VA hospitals (P = .29).
Study details: A study of survey responses from 1,062 infection preventionists at VA and non-VA hospitals in the United States submitted between 2013 and 2017.
Disclosures: Authors reported receiving grants from the Blue Cross Blue Shield of Michigan Foundation and the U.S. Department of Veterans Affairs Patient Safety Center of Inquiry during the conduct of the study. One study coauthor reported personal fees from Jvion and from Doximity outside the submitted work.
Source: Greene MT et al. JAMA Network Open. 2018;1(2):e180143.
When the answer to vaccines is “No”
We all know how challenging and time-consuming it can be to convince vaccine-hesitant patients that vaccinations are what is best for them and their children. Patients are bombarded with misinformation through the news and social media that seeds or “confirms” their doubts about vaccines. And for our part, we have only a few minutes during an office visit to refute all of the false claims that are a mere click or scroll away.
To better prepare for this challenge, this article details a practical approach to discussing vaccines with your patients. Using the patient-friendly language and evidence described here, you will be well positioned to refute 13 common vaccine misconceptions and overcome the barriers that stand in the way of these lifesaving interventions.
A few important baseline concepts
In discussing vaccination with our patients, it is important to keep the following in mind:
Patients don’t refuse vaccinations just to make our lives difficult. They truly are trying to make the best decisions they can for themselves and their families. Recognizing this can significantly reduce frustration levels.
Time well spent. While educating patients about the value of vaccines takes time, the return is worth it. The more consistently we offer vaccines, along with the reasons they are important, the more likely patients are to give vaccines a second thought. In fact, studies show that provider recommendation is the most important factor in patients’ decisions to vaccinate.1
Approach matters. In all other aspects of medicine, we attempt to use a participatory approach, involving our patients in decisions regarding their health care. When discussing vaccines, however, a participatory approach (eg, “What do you want to do about vaccines today?”) can introduce doubt into patients’ minds. Studies show that a presumptive approach (eg, “Today we are going to provide the tetanus, human papillomavirus [HPV], and meningitis vaccines”) is a much more effective way to get patients to vaccinate.2
[polldaddy:10018427]
Continue to: Barriers to counseling
Barriers to counseling. Health care providers report a variety of barriers to effective vaccine counseling (limited time and resources, lack of confidence in addressing patients’ concerns, etc).3 In addition, providers sometimes worry that strong encouragement of vaccination will create an adversarial relationship with vaccine-hesitant patients. Developing a good rapport and trusting relationship, as well as using motivational interviewing approaches, can help communicate the importance of vaccines, while leaving patients with the sense that you have heard them and respect their intentions. (See “Facilitate vaccine discussions using these 2 approaches.” 4-7)
SIDEBAR
Facilitate vaccine discussions using these 2 approaches4-7
C.A.S.E.
Corroborate
Acknowledge concerns and find some point on which you can agree.
Example: "It sounds like we both want to keep your child healthy and safe."
About me
Describe what you have done to build your expertise on the subject.
Example: "I have been practicing medicine for 15 years and have spent a great deal of time researching the data on vaccinations."
Science
Review the data and science behind vaccines.
Example: "Vaccines are more rigorously studied and safer than almost any other intervention we have in medicine."
Explain/advise
Explain your recommendations, based on the science.
Example: "This is why I vaccinate my children, and this is why I recommend this vaccine for your child."
3As
Ask
Don't stop at a patient's first "No." Respectfully dig a bit deeper.
Example: "What questions do you have about the vaccines we are recommending today? Tell me what worries you about them."
Acknowledge
Acknowledge your patient's concerns.
Example: "You are obviously a very devoted parent, and I know that you are trying to make the best decision you can for your child. With everything we see on the news and social media, it's not always easy to know what to believe about vaccines."
Advise
Advise patients/parents of the facts about vaccines and provide a strong recommendation to vaccinate.
Example: "Depending on the year, influenza kills 12,000 to 56,000 people annually; the vast majority of those who die did not receive the flu vaccine.7 My family and I get the flu shot every year, and I strongly encourage you and your children to get this lifesaving vaccine."
Continue to: If at first you don't succeed...
If at first you don’t succeed, try again because patients often have an experience that changes their mind. Perhaps a friend died of throat cancer or a family member developed a complication of the flu that required hospitalization. You never know when something will influence patients’ choices.
Don’t wait for scheduled well visits. Use every patient encounter as a means to catch patients up on missing vaccinations.
Common misconceptions and concerns and how to counter them
1. I’ve heard that vaccines can actually make you sick.
When patients raise this concern, start with an explanation of how vaccines work. Explain that our bodies protect us from foreign invaders (such as viruses and bacteria) by mounting an immune response when we are exposed to these proteins. Vaccinations work by exploiting this immune response; they expose the body to killed or weakened viral or bacterial proteins in a safe and controlled manner. In this way, our immune system will have already developed antibodies to these invaders by the time we are exposed to an active infection.
To use an analogy to war, instead of being subjected to a surprise attack where we suffer large losses in the battle, vaccination prepares us with weapons (antibodies) to defend ourselves so that our bodies are now able to successfully fight off that attack.
Because the majority of vaccines are killed virus vaccines, they cannot cause the illness against which they are meant to protect. Triggering the immune system may make some recipients feel a little “under the weather” for a day or 2, but they do not make us “sick.”
Live attenuated vaccines are similarly safe for those with a healthy immune system. We don’t administer them, however, to people who have a weakened immune system (eg, pregnant women, newborns, people with acquired immunodeficiency virus, or patients receiving chemotherapy or other types of immunosuppression) because these patients could develop the illness that we are trying to protect against.
Continue to: 2. Don't vaccines cause autism? Aren't they toxic to the nervous system?
2. Don’t vaccines cause autism? Aren’t they toxic to the nervous system?
The largest setback to vaccination efforts in recent history was a 1998 study by Andrew Wakefield that suggested that vaccination (specifically the mercury [in the form of thimerosal] present in the measles, mumps, rubella [MMR] vaccine) was linked to the development of autism.8 This research was subsequently debunked,9 and the author of the 1998 study was stripped of his medical license for falsifying data. However, the damage to vaccination efforts had already been done.
Aluminum. Thimerosal is not the only agent that patients may find concerning. Some also worry about the aluminum content of vaccines. Aluminum works as an additive to boost the body’s immune response to a vaccine. It is used only in killed virus vaccines—not in live attenuated ones. The Agency for Toxic Substances and Disease Registry monitors minimum risk levels (MRLs) of aluminum and other compounds in potentially hazardous substances. The amount of aluminum in vaccines is far below the MRL for aluminum, which is 1 mg/kg/d.10 (See “The facts about thimerosal and aluminum in vaccines.”11-16)
SIDEBAR
The facts about thimerosal and aluminum in vaccines
Thimerosal
Ethyl-mercury was used (in the form of thimerosal) as a preservative to prevent bacterial and fungal contamination of vaccines. Since 2001, however, thimerosal has been removed from all US-licensed vaccines—except multidose vials of influenza vaccine—as a precautionary measure (and not for any reproducible evidence of harm). The multidose flu vial contains <0.01% thimerosal.11
Ethyl-mercury is cleared from the body much more rapidly than methyl-mercury (the kind found in certain types of fish) and is less toxic.12
Since the removal of thimerosal from vaccines, the Centers for Disease Control and Prevention notes that the rates of autism have actually increased.13
Even Autism Speaks, the leading organization dedicated to advocacy for patients with autism and their families, denies a link between vaccines and autism.14
Aluminum
We are exposed to aluminum in products we use extensively every day, such as pots and pans, aluminum foil, seasonings, cereal, baby formula, paints, fuels, and antiperspirants.15
Infants are exposed to about 4.4 mg of aluminum in the vaccines typically administered in the first 6 months of life.16 However, infants typically ingest more than that during the first 6 months of life. Breast milk contains about 7 mg over 6 months; milk-based formulas contain about 38 mg over 6 months; and soy-based formulas contain about 117 mg over 6 months.16
Contine to: 3. I'm healthy. I never get sick. Why do I need vaccinations?
3. I’m healthy. I never get sick. Why do I need vaccinations?
A good way to counter this comment is to respond: “Saying you don’t need vaccinations because you never get sick is like saying you don’t need to wear a seat belt because you’ve never been in a car accident.” Advise patients that we seek to vaccinate all members of a community—not just those who are sick or at high risk—to protect ourselves and to provide “herd immunity.” It’s important to explain that herd immunity is resistance to the spread of a contagious disease that results if a sufficiently high number of people (depending on the illness, typically 80%-95%) are immune to the disease, especially through vaccination.17,18 If vaccination levels fall, we see a rise in cases of vaccine-preventable illness (as was seen during the 2017 measles outbreak in a community in Minnesota).19
Even though many of us may not suffer severe consequences of an infection, we can still pass that infection to others. While the whooping cough that a healthy 35-year-old gets may cause only prolonged annoyance or time off from work, it can kill the baby that is sitting next to that adult on the plane or bus.
4. Isn’t it true that we see fewer serious illnesses because of improved hygiene and sanitation, rather than vaccines?
Our current US sanitation standards were established under the Safe Drinking Water Act of 1974.20 While improvements in hygiene, sanitation, nutrition, and other public health measures have undoubtedly decreased the spread of disease and improved survival rates, there is no denying the significant drop in disease that occurs after the introduction of a vaccine for a particular illness or the increase in cases of that disease when vaccination rates drop off.
By the early 1990s, our current sanitation standards were already well established. Yet we didn’t see a significant decrease in the incidence of infections with Haemophilus influenzae type b (Hib) until after the conjugate Hib vaccines were introduced (dropping from about 20,000 cases/year to 1419 cases/year by 1993).21
In Britain, a drop in the rate of pertussis (whooping cough) vaccination in 1974 resulted in an epidemic of more than 100,000 cases and 36 deaths by 1978. There was no decrease in hygiene or sanitation standards to explain this rise.21
Continue to: 5. Vaccines are just another way for "big pharma" to make "big money."
5. Vaccines are just another way for “big pharma” to make “big money.”
Patients may benefit from knowing that in the earlier days of vaccines, pharmaceutical companies actually moved away from production of vaccines because they were not very profitable. These days, with worldwide distribution, drug companies are back in the swing of making vaccines and, as we would expect from all companies, are in business to make a profit.
That said, health care providers receive no payments from drug companies for offering vaccines or for offering one vaccine over another. The reason we recommend vaccination is because we know it is best for our patients’ health and the health of the community.

6. We don’t see polio anymore. Why do I need the vaccine?
One of the factors contributing to the rise in antivaccine sentiment is that we rarely see vaccine-preventable illnesses (such as polio, measles, and mumps). But the absence of these illnesses is precisely due to prior years’ vaccination efforts.
Smallpox, a deadly and disfiguring disease that killed many millions of people and contributed to the downfall of the Roman, Aztec, and Incan empires, was eradicated from the planet in 1979, thanks to focused vaccination efforts by the World Health Organization. Vaccination works, but we have to keep at it.
While we no longer see as many of these vaccine-preventable illnesses in the United States, they are still present in other parts of the world. Our world is much smaller than it used to be. International travel is common, and illnesses can be reintroduced into a community with relative ease. We must remain vigilant.
Continue to: 7. I heard that vaccines are made from aborted fetal tissue.
7. I heard that vaccines are made from aborted fetal tissue.
There are 5 vaccines (varicella, rubella, hepatitis A, shingles, and rabies vaccines) that were originally made using aborted fetal tissue. In 1960, tissue from 2 fetuses aborted by maternal choice (and not for the purpose of vaccine production) was used to propagate cell lines that are still used in vaccine development today.
Human cells provide advantages for vaccine production that other cells do not. Some viruses do not grow well in animal cells. Animal cells can introduce contamination by bacteria and viruses that are not carried in human cell lines. Vaccine production can be hindered or halted, resulting in a vaccine shortage, if animal products used in development are threatened (eg, if an illness strikes egg-producing chickens; eggs are used to make the influenza vaccine).22
Some patients, particularly those who are Catholic, may have concerns about these vaccines. The National Catholic Bioethics Center has prepared a statement regarding the use of these vaccines that may help settle any moral dilemmas.23 It reads:
“The cell lines under consideration were begun using cells taken from one or more fetuses aborted almost 40 years ago. Since that time, the cell lines have grown independently. It is important to note that descendent cells are not the cells of the aborted child.”
“One is morally free to use the vaccine regardless of its historical association with abortion. The reason is that the risk to public health, if one chooses not to vaccinate, outweighs the legitimate concern about the origins of the vaccine. This is especially important for parents, who have a moral obligation to protect the life and health of their children and those around them.”
Continue to: 8. Vaccines aren't studied—or monitored—thoroughly enough.
8. Vaccines aren’t studied—or monitored— thoroughly enough.
Patients would benefit from knowing that vaccines are some of the most thoroughly studied products brought to market. They undergo rigorous testing and oversight, from both public and private organizations, for 10 to 15 years before being released for distribution. Post-licensure monitoring is ongoing, and the manufacturer may voluntarily participate in Phase IV trials to continue to test the safety and efficacy of a vaccine after release to market.
Monitoring adverse effects. In addition, in 1990, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration established the Vaccine Adverse Events Reporting System (VAERS) to “detect possible signals of adverse events associated with vaccines.”24 Most events reported are coincidental, but some common mild adverse events (like redness and swelling at the injection site) are often underreported.
Serious events are always thoroughly investigated and are often found unrelated. However, rare associations have been found. For example, an intestinal problem called intussusception, related to the original rotavirus vaccine, was discovered, and the vaccine causing it was removed from the market.25 A new, safer rotavirus vaccine option is now available. Patients need to know that we do have an effective system of checks and balances in which we can place our trust.
9. People can become paralyzed or stop breathing after receiving a vaccination. Why run those risks?
One of the most feared reactions to vaccination is Guillain-Barré syndrome (GBS), which can cause paralysis. The CDC estimates the risk for GBS associated with the flu vaccine, for example, to be 1 to 2 cases per 1 million people vaccinated.26 Another potential concern is the rate of anaphylaxis following vaccination. However, in a 2016 study in the Journal of Allergy and Clinical Immunology, the rate of anaphylaxis for all vaccines combined was only 1.31 per 1 million vaccines.27
The risk of developing severe complications from an illness is much greater than that of developing complications from the vaccine meant to protect a person against that illness. In the United States, the population-based risk for influenza-related hospitalization in children, for example, is as high as 150 in 100,000 with as many as 125 deaths annually.26
Continue to: 10. Isn't vaccination a personal choice? How does my health/illness impact the community?
10. Isn’t vaccination a personal choice? How does my health/illness impact the community?
Patients may not realize that most viruses are contagious from 1 to 2 days before symptoms appear, which means we can spread an illness before we even know we have it. Protecting oneself also protects those around us.
Economic concerns. There’s also the economic impact of these illnesses to consider. This includes the personal cost of being out of school or work for an extended period and the cost of a patient’s care, which can become astronomical if hospitalization is required and which can become the country’s problem if a person lacks sufficient health insurance coverage.
A study looking at the cost of 4 major adult vaccine-preventable illnesses (influenza, pneumococcal disease, shingles, and whooping cough) in the United States in 2013 estimated the annual cost for these illnesses in adults ≥50 years to be $26.5 billion.28 And that doesn’t include the cost of childhood vaccine-preventable diseases.
Countering 3 concerns about childhood vaccinations
1. I can’t afford vaccines for my child.
The Vaccines for Children program is a federally-funded program that covers the cost of all vaccines for children younger than 19 years of age who are Medicaid-eligible, American Indian, Alaskan Native, uninsured, or underinsured.29 Although there may be a small administration fee charged by the provider’s office, the vaccine is free.
2. Don’t all of the vaccines recommended for children overwhelm their immune systems?
Children are exposed to so many more proteins on a daily basis (by crawling around on the floor, putting their hands in their mouths, attending school or day care, etc) than they are ever exposed to in a series of vaccines.30 Exposure to these proteins in their environment and to those in vaccines only serves to boost their immunity and keep them healthier in the long run.
And thanks to advances in vaccine production, the immunologic load in vaccines is far less than it used to be. The 14 vaccines given today contain <200 bacterial and viral proteins or polysaccharides, compared with the >3000 of these immunologic components in the 7 vaccines administered in 1980.31
Continue to: Influenza vaccine: Patient-friendly talking points
SIDEBAR
Influenza vaccine: Patient-friendly talking points
- Some people think that getting the flu is no big deal. While it is true that the flu takes a greater toll on the very young and very old, the chronically ill, and the immune compromised, even healthy people can become seriously ill or die. The Centers for Disease Control and Prevention estimates that the flu is responsible for 140,000 to 720,000 hospitalizations and 12,000 to 56,000 deaths in the United States every year.7 Of those who die from the flu, approximately 80% did not receive a flu shot.36 Of children who died from the flu between 2004 and 2012, more than 40% had no risk factors for complications.37
- The flu shot is a killed virus vaccine, so it can't give you the flu. People sometimes feel under the weather (achy, low-grade fever) after a vaccine, but this is considered normal and evidence that your body's immune system is "revving up."
- It takes 2 weeks before the vaccine becomes effective so a person can still get the flu during that time. This is why it is so important to get the vaccine earlier in the fall, before the flu season takes hold.
- The "stomach flu" is not the flu. The flu vaccine does not protect against the "stomach flu" or other flu-like illnesses.
- The flu vaccine is not perfect. It is an educated guess as to which strains will be circulating that year. (At its best, the flu vaccine is about 60% effective.38) However, it makes the chance of getting the flu less likely and significantly decreases the odds of severe complications/death.
- Egg allergies are no longer a reason to avoid the flu vaccine. There is an egg-free vaccine called Flublok (for those ≥18 years of age). In 2016-17, the Advisory Committee on Immunization Practices changed the recommendations for flu vaccine in egg-allergic people. The recommendations say that if reactions are mild, or you can eat cooked eggs without a problem, you can receive a flu vaccine. If you have severe reactions, such as trouble breathing or recurrent vomiting, you can still receive the flu vaccine, but must be monitored by a health care provider who can recognize and respond to a severe allergic reaction.39
Continue to: 3. Why don't we adhere to Dr. Sears' vaccine schedule?
3. Why don’t we adhere to Dr. Sears’ vaccine schedule?
There are multiple ways in which Dr. Robert Sears’ book, The Vaccine Book: Making the Right Decision for your Child, published in 2007, misrepresents vaccine science and leads patients astray in making decisions regarding vaccinations.32 Most important to note is that Dr. Sears’ Alternative Vaccine Schedule, which seeks to make it so that children do not receive more than 2 vaccinations per office visit, would require visits to a health care provider at 2, 3, 4, 5, 6, 7, 9, 12, 15, 18, and 21 months, and at 2, 2.5, 3, 3.5, 4, 5, and 6 years of age. This significantly increases the number of office visits and needle sticks, and raises the age at which vaccines are given, increasing the risk of illness outbreaks and decreasing the likelihood that parents would return to the office to complete the full series.
Acceptance of influenza and HPV vaccines remains a challenge
We are significantly less successful at getting parents and patients to agree to influenza and HPV vaccines than to the other vaccines we offer. The influenza vaccine success rate in 2016 was 59% in children and 43.3% in adults.33 Compared to the Tdap vaccine (88%) and the meningococcal vaccine (82%), which are offered at the same age as the HPV vaccine, success rates for HPV vaccine are significantly lower. In 2016, only 60.4% of boys and girls were current on their first HPV injection and only 43.3% were up to date with the full series.34
Newness of vaccines a factor?
Perhaps it is because the recommendations for these 2 vaccines are relatively new, and people don’t yet grasp the seriousness and scope of the diseases. Until 2010, the flu shot was recommended only for the very young, the elderly, and the medically high risk.
Similarly, the HPV vaccine was originally introduced for girls in 2006 and wasn’t recommended for boys until 2011.
Continue to: Human papillomavirus vaccine: Patient-friendly talking points
SIDEBAR
Human papillomavirus vaccine: Patient-friendly talking points
- Human papillomavirus (HPV) causes genital warts and cancer of the cervix, vagina, vulva, anus, rectum, penis, and oropharynx.
- The HPV vaccine is a cancer prevention vaccine. The 9-valent vaccine is active against 2 genital wart-causing strains and 7 cancer-causing strains of HPV.
- HPV is highly prevalent; 79 million Americans are currently infected, nearly 14 million people become newly infected each year, and nearly all of us will be exposed at some point in our sexual lives.40
- There are often no outward signs of infection, so it is a difficult infection to avoid.
- It takes no high-risk sexual activity to be exposed to the HPV virus.
- The HPV vaccine is recommended for both boys and girls usually around age 11 to 12 years (but as early as 9 years and as late as 26 years is acceptable). If the first vaccine is administered before 15 years of age, only 2 injections are needed 6 to 12 months apart. If the first vaccine is administered after 15 years of age, 3 injections are needed at 0, 2 months, and 6 months.41
- Completing the series before sexual activity begins is the best way to protect our children because the vaccine is a preventive measure, not a treatment.
- The HPV vaccine is highly effective with >90% efficacy against high-risk cancer-causing strains.42
- The HPV vaccine offers long-term protection. The vaccine has been on the market since 2006, and immunity has not yet diminished. Further monitoring is ongoing.43
- The HPV vaccine is covered under the Vaccines For Children program until age 19 years. Then it is up to individual insurance plans to cover it.
- The HPV vaccine does not cause infertility.44 HPV infection, on the other hand, can lead to fertility problems if, for example, treatment for cervical precancer or cancer requires partial removal of the cervix or a hysterectomy.
- The HPV vaccine does not cause autoimmune diseases.45,46 Studies show no difference between vaccinated and unvaccinated groups in rates of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes mellitus, multiple sclerosis, Hashimoto's thyroiditis, Graves' disease, and others.
- The HPV vaccine does not encourage earlier sexual activity. There was no earlier incidence of outcomes related to sexual activity (pregnancy, sexually transmitted infection testing or diagnosis, or contraceptive counseling) in vaccinated vs unvaccinated patients studied.47
Continue to: A sensitive subject
A sensitive subject. Discussion of a vaccine related to a child’s sexual health makes some parents uncomfortable. Studies show that focusing on the cancer prevention aspects of the vaccine, rather than on sexual transmission of HPV, results in greater vaccine acceptance.35
However, if discussion of sexual transmission is unavoidable, remind parents to consider their own adolescence and whether they chose to share everything with their parents. Point out that there were probably things they did that they later looked back on and thought, “What was I thinking?” Their children, no matter how wonderful and levelheaded they are, will be no different. And, as much as parents don’t want to think about it, some kids will suffer unwanted sexual contact. Shouldn’t parents protect their children as best as they can?
A teen’s right to choose? Some states have passed a Mature Minor Doctrine, which provides for mature, unemancipated teens to make their own medical decisions regarding such issues as sexuality, mental health, and drug and alcohol use without their parents’ consent. In these states, teens may elect to receive the HPV vaccine without parental permission. (Check your state’s laws for specifics, and see the 2 boxes with patient-friendly talking points for influenza vaccine7,36-39 and human papillomavirus vaccine.40-47)
CORRESPONDENCE
Gretchen LaSalle, MD, MultiCare Rockwood Clinic, 2214 East 29th Avenue, Spokane, WA 99203; [email protected].
1. Paterson P, Meurice F, Stanberry LR, et al. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6706.
2. Opel DJ, Heritage J, Taylor J, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132:1037-1046.
3. Palmer J, Carrico C, Costanzo C. Identifying and overcoming perceived barriers of providers towards vaccination: a literature review. J Vaccines. 2015;1-7.
4. Autism Science Foundation. Making the CASE for vaccines: a new model for talking to patients about vaccines. Available at: http://autismsciencefoundation.org/wp-content/uploads/2015/12/Making-the-CASE-for-Vaccines-Guide_final.pdf. Accessed April 8, 2018.
5. Jacobson RM, Van Etta L, Bahta L. The C.A.S.E approach: guidance for talking to vaccine-hesitant patients. Minn Med. 2013;96:49-50.
6. Henrickson NB, Opel DJ, Grothaus L, et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics. 2015;136:70-79.
7. Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. Available at: https://www.cdc.gov/flu/protect/keyfacts.htm. Accessed April 8, 2018.
8. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
9. Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32:3623-3629.
10. Agency for Toxic Substances & Disease Registry. Minimal risk levels for hazardous substances. Available at: https://www.atsdr.cdc.gov/mrls/mrllist.asp#34tag. Accessed April 8, 2018.
11. US Food and Drug Administration. Thimerosal and vaccines. Available at: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed April 8, 2018.
12. Hviid A, Stellfeld M, Wohlfahrt J, et al. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763-1766.
13. Centers for Disease Control and Prevention. Thimerosal in vaccines. Available at: https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed May 8, 2018.
14. Autism Speaks. Frequently asked questions. Available at: https://www.autismspeaks.org/what-autism/faq. Accessed April 8, 2018.
15. Agency for Toxic Substances & Disease Registry. Toxic substances portal-aluminum. Public Health Statement for Aluminum, CAS #7429-90-5. Available at: https://www.atsdr.cdc.gov/PHS/PHS.asp?id=1076&tid=34. Accessed April 8, 2018.
16. Children’s Hospital of Philadelphia. Vaccine ingredients-aluminum. Available at: www.chop.edu/centers-programs/vaccine-education-center/vaccine-ingredients/aluminum. Accessed April 8, 2018.
17. Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med. 2014;371:1661-1663.
18. Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55:72-77.
19. Hall V, Banerjee E, Kenyon C, et al. Measles outbreak – Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:713-717.
20. The National Academies of Sciences Engineering Medicine. History of U.S. water and wastewater systems. Privatization of Water Services in the United States: an Assessment of Issues and Experience. Washington, DC: The National Academies Press; 2002:29-40. Available at: https://www.nap.edu/read/10135/chapter/4#35. Accessed May 7, 2018.
21. World Health Organization. Global vaccine safety. Six common misconceptions about immunization. Available at: http://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index1.html. Accessed May 7, 2018.
22. The history of vaccines. Human cell strains in vaccine development. Available at: https://www.historyofvaccines.org/content/articles/human-cell-strains-vaccine-development. Accessed April 8, 2018.
23. The National Catholic Bioethics Center. Frequently asked questions. Available at: https://www.ncbcenter.org/resources/frequently-asked-questions/use-vaccines/. Accessed April 8, 2018.
24. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33:4398-4405.
25. Foster S. Rotavirus vaccine and intussusception. J Pediatr Pharmacol Ther. 2007;12:4-7.
26. Mistry RD, Fischer JB, Prasad PA, et al. Severe complications of influenza-like illnesses. Pediatrics. 2014;134:e684-e690.
27. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
28. McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36:259-273.
29. Centers for Disease Control and Prevention. Vaccines for Children (VFC) Program. Available at: https://www.cdc.gov/features/vfcprogram/index.html. Accessed April 8, 2018.
30. Plotkin S, Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456-461.
31. Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109:124-129.
32. Offit PA, Moser CA. The problem with Dr. Bob’s alternative vaccine schedule. Pediatrics. 2009;123:e164-e169.
33. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. April 8. 2018.
34. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
35. Thomas TL. Cancer prevention: HPV vaccination. Semin Oncol Nurs. 2016:32:273-280.
36. Centers for Disease Control and Prevention. Estimating seasonal influenza-associated deaths in the United States. Available at: https://www.cdc.gov/flu/about/disease/US_flu-related_deaths.htm. Accessed May 8, 2018.
37. Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States: 2004-2012. Pediatrics. 2013;132:796-804.
38. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed April 8, 2018.
39. Centers for Disease Control and Prevention. Influenza (flu). Flu vaccine and people with egg allergies. Available at: https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm. Accessed April 8, 2018.
40. Centers for Disease Control and Prevention. For parents: vaccines for your children. HPV vaccine for preteens and teens. Available at: https://www.cdc.gov/vaccines/parents/diseases/teen/hpv.html. Accessed April 8, 2018.
41. Centers for Disease Control and Prevention. Vaccines and preventable diseases. HPV vaccine recommendations. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed May 7, 2018.
42. Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719-726.
43. De Vincenzo R, Conte C, Ricci C, et al. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999-1010.
44. McInerney KA, Hatch EE, Wesselink AK. The effect of vaccination against human papillomavirus on fecundability. Paedeatr Perinat Epidemiol. 2017;31:531-536.
45. Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193-203.
46. Vichnin M, Bonanni P, Klein NP, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006-2015. Ped Infect Dis J. 2015;34:983-991.
47. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11-to-12-year-olds. Pediatrics. 2012;130:798-805.
We all know how challenging and time-consuming it can be to convince vaccine-hesitant patients that vaccinations are what is best for them and their children. Patients are bombarded with misinformation through the news and social media that seeds or “confirms” their doubts about vaccines. And for our part, we have only a few minutes during an office visit to refute all of the false claims that are a mere click or scroll away.
To better prepare for this challenge, this article details a practical approach to discussing vaccines with your patients. Using the patient-friendly language and evidence described here, you will be well positioned to refute 13 common vaccine misconceptions and overcome the barriers that stand in the way of these lifesaving interventions.
A few important baseline concepts
In discussing vaccination with our patients, it is important to keep the following in mind:
Patients don’t refuse vaccinations just to make our lives difficult. They truly are trying to make the best decisions they can for themselves and their families. Recognizing this can significantly reduce frustration levels.
Time well spent. While educating patients about the value of vaccines takes time, the return is worth it. The more consistently we offer vaccines, along with the reasons they are important, the more likely patients are to give vaccines a second thought. In fact, studies show that provider recommendation is the most important factor in patients’ decisions to vaccinate.1
Approach matters. In all other aspects of medicine, we attempt to use a participatory approach, involving our patients in decisions regarding their health care. When discussing vaccines, however, a participatory approach (eg, “What do you want to do about vaccines today?”) can introduce doubt into patients’ minds. Studies show that a presumptive approach (eg, “Today we are going to provide the tetanus, human papillomavirus [HPV], and meningitis vaccines”) is a much more effective way to get patients to vaccinate.2
[polldaddy:10018427]
Continue to: Barriers to counseling
Barriers to counseling. Health care providers report a variety of barriers to effective vaccine counseling (limited time and resources, lack of confidence in addressing patients’ concerns, etc).3 In addition, providers sometimes worry that strong encouragement of vaccination will create an adversarial relationship with vaccine-hesitant patients. Developing a good rapport and trusting relationship, as well as using motivational interviewing approaches, can help communicate the importance of vaccines, while leaving patients with the sense that you have heard them and respect their intentions. (See “Facilitate vaccine discussions using these 2 approaches.” 4-7)
SIDEBAR
Facilitate vaccine discussions using these 2 approaches4-7
C.A.S.E.
Corroborate
Acknowledge concerns and find some point on which you can agree.
Example: "It sounds like we both want to keep your child healthy and safe."
About me
Describe what you have done to build your expertise on the subject.
Example: "I have been practicing medicine for 15 years and have spent a great deal of time researching the data on vaccinations."
Science
Review the data and science behind vaccines.
Example: "Vaccines are more rigorously studied and safer than almost any other intervention we have in medicine."
Explain/advise
Explain your recommendations, based on the science.
Example: "This is why I vaccinate my children, and this is why I recommend this vaccine for your child."
3As
Ask
Don't stop at a patient's first "No." Respectfully dig a bit deeper.
Example: "What questions do you have about the vaccines we are recommending today? Tell me what worries you about them."
Acknowledge
Acknowledge your patient's concerns.
Example: "You are obviously a very devoted parent, and I know that you are trying to make the best decision you can for your child. With everything we see on the news and social media, it's not always easy to know what to believe about vaccines."
Advise
Advise patients/parents of the facts about vaccines and provide a strong recommendation to vaccinate.
Example: "Depending on the year, influenza kills 12,000 to 56,000 people annually; the vast majority of those who die did not receive the flu vaccine.7 My family and I get the flu shot every year, and I strongly encourage you and your children to get this lifesaving vaccine."
Continue to: If at first you don't succeed...
If at first you don’t succeed, try again because patients often have an experience that changes their mind. Perhaps a friend died of throat cancer or a family member developed a complication of the flu that required hospitalization. You never know when something will influence patients’ choices.
Don’t wait for scheduled well visits. Use every patient encounter as a means to catch patients up on missing vaccinations.
Common misconceptions and concerns and how to counter them
1. I’ve heard that vaccines can actually make you sick.
When patients raise this concern, start with an explanation of how vaccines work. Explain that our bodies protect us from foreign invaders (such as viruses and bacteria) by mounting an immune response when we are exposed to these proteins. Vaccinations work by exploiting this immune response; they expose the body to killed or weakened viral or bacterial proteins in a safe and controlled manner. In this way, our immune system will have already developed antibodies to these invaders by the time we are exposed to an active infection.
To use an analogy to war, instead of being subjected to a surprise attack where we suffer large losses in the battle, vaccination prepares us with weapons (antibodies) to defend ourselves so that our bodies are now able to successfully fight off that attack.
Because the majority of vaccines are killed virus vaccines, they cannot cause the illness against which they are meant to protect. Triggering the immune system may make some recipients feel a little “under the weather” for a day or 2, but they do not make us “sick.”
Live attenuated vaccines are similarly safe for those with a healthy immune system. We don’t administer them, however, to people who have a weakened immune system (eg, pregnant women, newborns, people with acquired immunodeficiency virus, or patients receiving chemotherapy or other types of immunosuppression) because these patients could develop the illness that we are trying to protect against.
Continue to: 2. Don't vaccines cause autism? Aren't they toxic to the nervous system?
2. Don’t vaccines cause autism? Aren’t they toxic to the nervous system?
The largest setback to vaccination efforts in recent history was a 1998 study by Andrew Wakefield that suggested that vaccination (specifically the mercury [in the form of thimerosal] present in the measles, mumps, rubella [MMR] vaccine) was linked to the development of autism.8 This research was subsequently debunked,9 and the author of the 1998 study was stripped of his medical license for falsifying data. However, the damage to vaccination efforts had already been done.
Aluminum. Thimerosal is not the only agent that patients may find concerning. Some also worry about the aluminum content of vaccines. Aluminum works as an additive to boost the body’s immune response to a vaccine. It is used only in killed virus vaccines—not in live attenuated ones. The Agency for Toxic Substances and Disease Registry monitors minimum risk levels (MRLs) of aluminum and other compounds in potentially hazardous substances. The amount of aluminum in vaccines is far below the MRL for aluminum, which is 1 mg/kg/d.10 (See “The facts about thimerosal and aluminum in vaccines.”11-16)
SIDEBAR
The facts about thimerosal and aluminum in vaccines
Thimerosal
Ethyl-mercury was used (in the form of thimerosal) as a preservative to prevent bacterial and fungal contamination of vaccines. Since 2001, however, thimerosal has been removed from all US-licensed vaccines—except multidose vials of influenza vaccine—as a precautionary measure (and not for any reproducible evidence of harm). The multidose flu vial contains <0.01% thimerosal.11
Ethyl-mercury is cleared from the body much more rapidly than methyl-mercury (the kind found in certain types of fish) and is less toxic.12
Since the removal of thimerosal from vaccines, the Centers for Disease Control and Prevention notes that the rates of autism have actually increased.13
Even Autism Speaks, the leading organization dedicated to advocacy for patients with autism and their families, denies a link between vaccines and autism.14
Aluminum
We are exposed to aluminum in products we use extensively every day, such as pots and pans, aluminum foil, seasonings, cereal, baby formula, paints, fuels, and antiperspirants.15
Infants are exposed to about 4.4 mg of aluminum in the vaccines typically administered in the first 6 months of life.16 However, infants typically ingest more than that during the first 6 months of life. Breast milk contains about 7 mg over 6 months; milk-based formulas contain about 38 mg over 6 months; and soy-based formulas contain about 117 mg over 6 months.16
Contine to: 3. I'm healthy. I never get sick. Why do I need vaccinations?
3. I’m healthy. I never get sick. Why do I need vaccinations?
A good way to counter this comment is to respond: “Saying you don’t need vaccinations because you never get sick is like saying you don’t need to wear a seat belt because you’ve never been in a car accident.” Advise patients that we seek to vaccinate all members of a community—not just those who are sick or at high risk—to protect ourselves and to provide “herd immunity.” It’s important to explain that herd immunity is resistance to the spread of a contagious disease that results if a sufficiently high number of people (depending on the illness, typically 80%-95%) are immune to the disease, especially through vaccination.17,18 If vaccination levels fall, we see a rise in cases of vaccine-preventable illness (as was seen during the 2017 measles outbreak in a community in Minnesota).19
Even though many of us may not suffer severe consequences of an infection, we can still pass that infection to others. While the whooping cough that a healthy 35-year-old gets may cause only prolonged annoyance or time off from work, it can kill the baby that is sitting next to that adult on the plane or bus.
4. Isn’t it true that we see fewer serious illnesses because of improved hygiene and sanitation, rather than vaccines?
Our current US sanitation standards were established under the Safe Drinking Water Act of 1974.20 While improvements in hygiene, sanitation, nutrition, and other public health measures have undoubtedly decreased the spread of disease and improved survival rates, there is no denying the significant drop in disease that occurs after the introduction of a vaccine for a particular illness or the increase in cases of that disease when vaccination rates drop off.
By the early 1990s, our current sanitation standards were already well established. Yet we didn’t see a significant decrease in the incidence of infections with Haemophilus influenzae type b (Hib) until after the conjugate Hib vaccines were introduced (dropping from about 20,000 cases/year to 1419 cases/year by 1993).21
In Britain, a drop in the rate of pertussis (whooping cough) vaccination in 1974 resulted in an epidemic of more than 100,000 cases and 36 deaths by 1978. There was no decrease in hygiene or sanitation standards to explain this rise.21
Continue to: 5. Vaccines are just another way for "big pharma" to make "big money."
5. Vaccines are just another way for “big pharma” to make “big money.”
Patients may benefit from knowing that in the earlier days of vaccines, pharmaceutical companies actually moved away from production of vaccines because they were not very profitable. These days, with worldwide distribution, drug companies are back in the swing of making vaccines and, as we would expect from all companies, are in business to make a profit.
That said, health care providers receive no payments from drug companies for offering vaccines or for offering one vaccine over another. The reason we recommend vaccination is because we know it is best for our patients’ health and the health of the community.

6. We don’t see polio anymore. Why do I need the vaccine?
One of the factors contributing to the rise in antivaccine sentiment is that we rarely see vaccine-preventable illnesses (such as polio, measles, and mumps). But the absence of these illnesses is precisely due to prior years’ vaccination efforts.
Smallpox, a deadly and disfiguring disease that killed many millions of people and contributed to the downfall of the Roman, Aztec, and Incan empires, was eradicated from the planet in 1979, thanks to focused vaccination efforts by the World Health Organization. Vaccination works, but we have to keep at it.
While we no longer see as many of these vaccine-preventable illnesses in the United States, they are still present in other parts of the world. Our world is much smaller than it used to be. International travel is common, and illnesses can be reintroduced into a community with relative ease. We must remain vigilant.
Continue to: 7. I heard that vaccines are made from aborted fetal tissue.
7. I heard that vaccines are made from aborted fetal tissue.
There are 5 vaccines (varicella, rubella, hepatitis A, shingles, and rabies vaccines) that were originally made using aborted fetal tissue. In 1960, tissue from 2 fetuses aborted by maternal choice (and not for the purpose of vaccine production) was used to propagate cell lines that are still used in vaccine development today.
Human cells provide advantages for vaccine production that other cells do not. Some viruses do not grow well in animal cells. Animal cells can introduce contamination by bacteria and viruses that are not carried in human cell lines. Vaccine production can be hindered or halted, resulting in a vaccine shortage, if animal products used in development are threatened (eg, if an illness strikes egg-producing chickens; eggs are used to make the influenza vaccine).22
Some patients, particularly those who are Catholic, may have concerns about these vaccines. The National Catholic Bioethics Center has prepared a statement regarding the use of these vaccines that may help settle any moral dilemmas.23 It reads:
“The cell lines under consideration were begun using cells taken from one or more fetuses aborted almost 40 years ago. Since that time, the cell lines have grown independently. It is important to note that descendent cells are not the cells of the aborted child.”
“One is morally free to use the vaccine regardless of its historical association with abortion. The reason is that the risk to public health, if one chooses not to vaccinate, outweighs the legitimate concern about the origins of the vaccine. This is especially important for parents, who have a moral obligation to protect the life and health of their children and those around them.”
Continue to: 8. Vaccines aren't studied—or monitored—thoroughly enough.
8. Vaccines aren’t studied—or monitored— thoroughly enough.
Patients would benefit from knowing that vaccines are some of the most thoroughly studied products brought to market. They undergo rigorous testing and oversight, from both public and private organizations, for 10 to 15 years before being released for distribution. Post-licensure monitoring is ongoing, and the manufacturer may voluntarily participate in Phase IV trials to continue to test the safety and efficacy of a vaccine after release to market.
Monitoring adverse effects. In addition, in 1990, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration established the Vaccine Adverse Events Reporting System (VAERS) to “detect possible signals of adverse events associated with vaccines.”24 Most events reported are coincidental, but some common mild adverse events (like redness and swelling at the injection site) are often underreported.
Serious events are always thoroughly investigated and are often found unrelated. However, rare associations have been found. For example, an intestinal problem called intussusception, related to the original rotavirus vaccine, was discovered, and the vaccine causing it was removed from the market.25 A new, safer rotavirus vaccine option is now available. Patients need to know that we do have an effective system of checks and balances in which we can place our trust.
9. People can become paralyzed or stop breathing after receiving a vaccination. Why run those risks?
One of the most feared reactions to vaccination is Guillain-Barré syndrome (GBS), which can cause paralysis. The CDC estimates the risk for GBS associated with the flu vaccine, for example, to be 1 to 2 cases per 1 million people vaccinated.26 Another potential concern is the rate of anaphylaxis following vaccination. However, in a 2016 study in the Journal of Allergy and Clinical Immunology, the rate of anaphylaxis for all vaccines combined was only 1.31 per 1 million vaccines.27
The risk of developing severe complications from an illness is much greater than that of developing complications from the vaccine meant to protect a person against that illness. In the United States, the population-based risk for influenza-related hospitalization in children, for example, is as high as 150 in 100,000 with as many as 125 deaths annually.26
Continue to: 10. Isn't vaccination a personal choice? How does my health/illness impact the community?
10. Isn’t vaccination a personal choice? How does my health/illness impact the community?
Patients may not realize that most viruses are contagious from 1 to 2 days before symptoms appear, which means we can spread an illness before we even know we have it. Protecting oneself also protects those around us.
Economic concerns. There’s also the economic impact of these illnesses to consider. This includes the personal cost of being out of school or work for an extended period and the cost of a patient’s care, which can become astronomical if hospitalization is required and which can become the country’s problem if a person lacks sufficient health insurance coverage.
A study looking at the cost of 4 major adult vaccine-preventable illnesses (influenza, pneumococcal disease, shingles, and whooping cough) in the United States in 2013 estimated the annual cost for these illnesses in adults ≥50 years to be $26.5 billion.28 And that doesn’t include the cost of childhood vaccine-preventable diseases.
Countering 3 concerns about childhood vaccinations
1. I can’t afford vaccines for my child.
The Vaccines for Children program is a federally-funded program that covers the cost of all vaccines for children younger than 19 years of age who are Medicaid-eligible, American Indian, Alaskan Native, uninsured, or underinsured.29 Although there may be a small administration fee charged by the provider’s office, the vaccine is free.
2. Don’t all of the vaccines recommended for children overwhelm their immune systems?
Children are exposed to so many more proteins on a daily basis (by crawling around on the floor, putting their hands in their mouths, attending school or day care, etc) than they are ever exposed to in a series of vaccines.30 Exposure to these proteins in their environment and to those in vaccines only serves to boost their immunity and keep them healthier in the long run.
And thanks to advances in vaccine production, the immunologic load in vaccines is far less than it used to be. The 14 vaccines given today contain <200 bacterial and viral proteins or polysaccharides, compared with the >3000 of these immunologic components in the 7 vaccines administered in 1980.31
Continue to: Influenza vaccine: Patient-friendly talking points
SIDEBAR
Influenza vaccine: Patient-friendly talking points
- Some people think that getting the flu is no big deal. While it is true that the flu takes a greater toll on the very young and very old, the chronically ill, and the immune compromised, even healthy people can become seriously ill or die. The Centers for Disease Control and Prevention estimates that the flu is responsible for 140,000 to 720,000 hospitalizations and 12,000 to 56,000 deaths in the United States every year.7 Of those who die from the flu, approximately 80% did not receive a flu shot.36 Of children who died from the flu between 2004 and 2012, more than 40% had no risk factors for complications.37
- The flu shot is a killed virus vaccine, so it can't give you the flu. People sometimes feel under the weather (achy, low-grade fever) after a vaccine, but this is considered normal and evidence that your body's immune system is "revving up."
- It takes 2 weeks before the vaccine becomes effective so a person can still get the flu during that time. This is why it is so important to get the vaccine earlier in the fall, before the flu season takes hold.
- The "stomach flu" is not the flu. The flu vaccine does not protect against the "stomach flu" or other flu-like illnesses.
- The flu vaccine is not perfect. It is an educated guess as to which strains will be circulating that year. (At its best, the flu vaccine is about 60% effective.38) However, it makes the chance of getting the flu less likely and significantly decreases the odds of severe complications/death.
- Egg allergies are no longer a reason to avoid the flu vaccine. There is an egg-free vaccine called Flublok (for those ≥18 years of age). In 2016-17, the Advisory Committee on Immunization Practices changed the recommendations for flu vaccine in egg-allergic people. The recommendations say that if reactions are mild, or you can eat cooked eggs without a problem, you can receive a flu vaccine. If you have severe reactions, such as trouble breathing or recurrent vomiting, you can still receive the flu vaccine, but must be monitored by a health care provider who can recognize and respond to a severe allergic reaction.39
Continue to: 3. Why don't we adhere to Dr. Sears' vaccine schedule?
3. Why don’t we adhere to Dr. Sears’ vaccine schedule?
There are multiple ways in which Dr. Robert Sears’ book, The Vaccine Book: Making the Right Decision for your Child, published in 2007, misrepresents vaccine science and leads patients astray in making decisions regarding vaccinations.32 Most important to note is that Dr. Sears’ Alternative Vaccine Schedule, which seeks to make it so that children do not receive more than 2 vaccinations per office visit, would require visits to a health care provider at 2, 3, 4, 5, 6, 7, 9, 12, 15, 18, and 21 months, and at 2, 2.5, 3, 3.5, 4, 5, and 6 years of age. This significantly increases the number of office visits and needle sticks, and raises the age at which vaccines are given, increasing the risk of illness outbreaks and decreasing the likelihood that parents would return to the office to complete the full series.
Acceptance of influenza and HPV vaccines remains a challenge
We are significantly less successful at getting parents and patients to agree to influenza and HPV vaccines than to the other vaccines we offer. The influenza vaccine success rate in 2016 was 59% in children and 43.3% in adults.33 Compared to the Tdap vaccine (88%) and the meningococcal vaccine (82%), which are offered at the same age as the HPV vaccine, success rates for HPV vaccine are significantly lower. In 2016, only 60.4% of boys and girls were current on their first HPV injection and only 43.3% were up to date with the full series.34
Newness of vaccines a factor?
Perhaps it is because the recommendations for these 2 vaccines are relatively new, and people don’t yet grasp the seriousness and scope of the diseases. Until 2010, the flu shot was recommended only for the very young, the elderly, and the medically high risk.
Similarly, the HPV vaccine was originally introduced for girls in 2006 and wasn’t recommended for boys until 2011.
Continue to: Human papillomavirus vaccine: Patient-friendly talking points
SIDEBAR
Human papillomavirus vaccine: Patient-friendly talking points
- Human papillomavirus (HPV) causes genital warts and cancer of the cervix, vagina, vulva, anus, rectum, penis, and oropharynx.
- The HPV vaccine is a cancer prevention vaccine. The 9-valent vaccine is active against 2 genital wart-causing strains and 7 cancer-causing strains of HPV.
- HPV is highly prevalent; 79 million Americans are currently infected, nearly 14 million people become newly infected each year, and nearly all of us will be exposed at some point in our sexual lives.40
- There are often no outward signs of infection, so it is a difficult infection to avoid.
- It takes no high-risk sexual activity to be exposed to the HPV virus.
- The HPV vaccine is recommended for both boys and girls usually around age 11 to 12 years (but as early as 9 years and as late as 26 years is acceptable). If the first vaccine is administered before 15 years of age, only 2 injections are needed 6 to 12 months apart. If the first vaccine is administered after 15 years of age, 3 injections are needed at 0, 2 months, and 6 months.41
- Completing the series before sexual activity begins is the best way to protect our children because the vaccine is a preventive measure, not a treatment.
- The HPV vaccine is highly effective with >90% efficacy against high-risk cancer-causing strains.42
- The HPV vaccine offers long-term protection. The vaccine has been on the market since 2006, and immunity has not yet diminished. Further monitoring is ongoing.43
- The HPV vaccine is covered under the Vaccines For Children program until age 19 years. Then it is up to individual insurance plans to cover it.
- The HPV vaccine does not cause infertility.44 HPV infection, on the other hand, can lead to fertility problems if, for example, treatment for cervical precancer or cancer requires partial removal of the cervix or a hysterectomy.
- The HPV vaccine does not cause autoimmune diseases.45,46 Studies show no difference between vaccinated and unvaccinated groups in rates of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes mellitus, multiple sclerosis, Hashimoto's thyroiditis, Graves' disease, and others.
- The HPV vaccine does not encourage earlier sexual activity. There was no earlier incidence of outcomes related to sexual activity (pregnancy, sexually transmitted infection testing or diagnosis, or contraceptive counseling) in vaccinated vs unvaccinated patients studied.47
Continue to: A sensitive subject
A sensitive subject. Discussion of a vaccine related to a child’s sexual health makes some parents uncomfortable. Studies show that focusing on the cancer prevention aspects of the vaccine, rather than on sexual transmission of HPV, results in greater vaccine acceptance.35
However, if discussion of sexual transmission is unavoidable, remind parents to consider their own adolescence and whether they chose to share everything with their parents. Point out that there were probably things they did that they later looked back on and thought, “What was I thinking?” Their children, no matter how wonderful and levelheaded they are, will be no different. And, as much as parents don’t want to think about it, some kids will suffer unwanted sexual contact. Shouldn’t parents protect their children as best as they can?
A teen’s right to choose? Some states have passed a Mature Minor Doctrine, which provides for mature, unemancipated teens to make their own medical decisions regarding such issues as sexuality, mental health, and drug and alcohol use without their parents’ consent. In these states, teens may elect to receive the HPV vaccine without parental permission. (Check your state’s laws for specifics, and see the 2 boxes with patient-friendly talking points for influenza vaccine7,36-39 and human papillomavirus vaccine.40-47)
CORRESPONDENCE
Gretchen LaSalle, MD, MultiCare Rockwood Clinic, 2214 East 29th Avenue, Spokane, WA 99203; [email protected].
We all know how challenging and time-consuming it can be to convince vaccine-hesitant patients that vaccinations are what is best for them and their children. Patients are bombarded with misinformation through the news and social media that seeds or “confirms” their doubts about vaccines. And for our part, we have only a few minutes during an office visit to refute all of the false claims that are a mere click or scroll away.
To better prepare for this challenge, this article details a practical approach to discussing vaccines with your patients. Using the patient-friendly language and evidence described here, you will be well positioned to refute 13 common vaccine misconceptions and overcome the barriers that stand in the way of these lifesaving interventions.
A few important baseline concepts
In discussing vaccination with our patients, it is important to keep the following in mind:
Patients don’t refuse vaccinations just to make our lives difficult. They truly are trying to make the best decisions they can for themselves and their families. Recognizing this can significantly reduce frustration levels.
Time well spent. While educating patients about the value of vaccines takes time, the return is worth it. The more consistently we offer vaccines, along with the reasons they are important, the more likely patients are to give vaccines a second thought. In fact, studies show that provider recommendation is the most important factor in patients’ decisions to vaccinate.1
Approach matters. In all other aspects of medicine, we attempt to use a participatory approach, involving our patients in decisions regarding their health care. When discussing vaccines, however, a participatory approach (eg, “What do you want to do about vaccines today?”) can introduce doubt into patients’ minds. Studies show that a presumptive approach (eg, “Today we are going to provide the tetanus, human papillomavirus [HPV], and meningitis vaccines”) is a much more effective way to get patients to vaccinate.2
[polldaddy:10018427]
Continue to: Barriers to counseling
Barriers to counseling. Health care providers report a variety of barriers to effective vaccine counseling (limited time and resources, lack of confidence in addressing patients’ concerns, etc).3 In addition, providers sometimes worry that strong encouragement of vaccination will create an adversarial relationship with vaccine-hesitant patients. Developing a good rapport and trusting relationship, as well as using motivational interviewing approaches, can help communicate the importance of vaccines, while leaving patients with the sense that you have heard them and respect their intentions. (See “Facilitate vaccine discussions using these 2 approaches.” 4-7)
SIDEBAR
Facilitate vaccine discussions using these 2 approaches4-7
C.A.S.E.
Corroborate
Acknowledge concerns and find some point on which you can agree.
Example: "It sounds like we both want to keep your child healthy and safe."
About me
Describe what you have done to build your expertise on the subject.
Example: "I have been practicing medicine for 15 years and have spent a great deal of time researching the data on vaccinations."
Science
Review the data and science behind vaccines.
Example: "Vaccines are more rigorously studied and safer than almost any other intervention we have in medicine."
Explain/advise
Explain your recommendations, based on the science.
Example: "This is why I vaccinate my children, and this is why I recommend this vaccine for your child."
3As
Ask
Don't stop at a patient's first "No." Respectfully dig a bit deeper.
Example: "What questions do you have about the vaccines we are recommending today? Tell me what worries you about them."
Acknowledge
Acknowledge your patient's concerns.
Example: "You are obviously a very devoted parent, and I know that you are trying to make the best decision you can for your child. With everything we see on the news and social media, it's not always easy to know what to believe about vaccines."
Advise
Advise patients/parents of the facts about vaccines and provide a strong recommendation to vaccinate.
Example: "Depending on the year, influenza kills 12,000 to 56,000 people annually; the vast majority of those who die did not receive the flu vaccine.7 My family and I get the flu shot every year, and I strongly encourage you and your children to get this lifesaving vaccine."
Continue to: If at first you don't succeed...
If at first you don’t succeed, try again because patients often have an experience that changes their mind. Perhaps a friend died of throat cancer or a family member developed a complication of the flu that required hospitalization. You never know when something will influence patients’ choices.
Don’t wait for scheduled well visits. Use every patient encounter as a means to catch patients up on missing vaccinations.
Common misconceptions and concerns and how to counter them
1. I’ve heard that vaccines can actually make you sick.
When patients raise this concern, start with an explanation of how vaccines work. Explain that our bodies protect us from foreign invaders (such as viruses and bacteria) by mounting an immune response when we are exposed to these proteins. Vaccinations work by exploiting this immune response; they expose the body to killed or weakened viral or bacterial proteins in a safe and controlled manner. In this way, our immune system will have already developed antibodies to these invaders by the time we are exposed to an active infection.
To use an analogy to war, instead of being subjected to a surprise attack where we suffer large losses in the battle, vaccination prepares us with weapons (antibodies) to defend ourselves so that our bodies are now able to successfully fight off that attack.
Because the majority of vaccines are killed virus vaccines, they cannot cause the illness against which they are meant to protect. Triggering the immune system may make some recipients feel a little “under the weather” for a day or 2, but they do not make us “sick.”
Live attenuated vaccines are similarly safe for those with a healthy immune system. We don’t administer them, however, to people who have a weakened immune system (eg, pregnant women, newborns, people with acquired immunodeficiency virus, or patients receiving chemotherapy or other types of immunosuppression) because these patients could develop the illness that we are trying to protect against.
Continue to: 2. Don't vaccines cause autism? Aren't they toxic to the nervous system?
2. Don’t vaccines cause autism? Aren’t they toxic to the nervous system?
The largest setback to vaccination efforts in recent history was a 1998 study by Andrew Wakefield that suggested that vaccination (specifically the mercury [in the form of thimerosal] present in the measles, mumps, rubella [MMR] vaccine) was linked to the development of autism.8 This research was subsequently debunked,9 and the author of the 1998 study was stripped of his medical license for falsifying data. However, the damage to vaccination efforts had already been done.
Aluminum. Thimerosal is not the only agent that patients may find concerning. Some also worry about the aluminum content of vaccines. Aluminum works as an additive to boost the body’s immune response to a vaccine. It is used only in killed virus vaccines—not in live attenuated ones. The Agency for Toxic Substances and Disease Registry monitors minimum risk levels (MRLs) of aluminum and other compounds in potentially hazardous substances. The amount of aluminum in vaccines is far below the MRL for aluminum, which is 1 mg/kg/d.10 (See “The facts about thimerosal and aluminum in vaccines.”11-16)
SIDEBAR
The facts about thimerosal and aluminum in vaccines
Thimerosal
Ethyl-mercury was used (in the form of thimerosal) as a preservative to prevent bacterial and fungal contamination of vaccines. Since 2001, however, thimerosal has been removed from all US-licensed vaccines—except multidose vials of influenza vaccine—as a precautionary measure (and not for any reproducible evidence of harm). The multidose flu vial contains <0.01% thimerosal.11
Ethyl-mercury is cleared from the body much more rapidly than methyl-mercury (the kind found in certain types of fish) and is less toxic.12
Since the removal of thimerosal from vaccines, the Centers for Disease Control and Prevention notes that the rates of autism have actually increased.13
Even Autism Speaks, the leading organization dedicated to advocacy for patients with autism and their families, denies a link between vaccines and autism.14
Aluminum
We are exposed to aluminum in products we use extensively every day, such as pots and pans, aluminum foil, seasonings, cereal, baby formula, paints, fuels, and antiperspirants.15
Infants are exposed to about 4.4 mg of aluminum in the vaccines typically administered in the first 6 months of life.16 However, infants typically ingest more than that during the first 6 months of life. Breast milk contains about 7 mg over 6 months; milk-based formulas contain about 38 mg over 6 months; and soy-based formulas contain about 117 mg over 6 months.16
Contine to: 3. I'm healthy. I never get sick. Why do I need vaccinations?
3. I’m healthy. I never get sick. Why do I need vaccinations?
A good way to counter this comment is to respond: “Saying you don’t need vaccinations because you never get sick is like saying you don’t need to wear a seat belt because you’ve never been in a car accident.” Advise patients that we seek to vaccinate all members of a community—not just those who are sick or at high risk—to protect ourselves and to provide “herd immunity.” It’s important to explain that herd immunity is resistance to the spread of a contagious disease that results if a sufficiently high number of people (depending on the illness, typically 80%-95%) are immune to the disease, especially through vaccination.17,18 If vaccination levels fall, we see a rise in cases of vaccine-preventable illness (as was seen during the 2017 measles outbreak in a community in Minnesota).19
Even though many of us may not suffer severe consequences of an infection, we can still pass that infection to others. While the whooping cough that a healthy 35-year-old gets may cause only prolonged annoyance or time off from work, it can kill the baby that is sitting next to that adult on the plane or bus.
4. Isn’t it true that we see fewer serious illnesses because of improved hygiene and sanitation, rather than vaccines?
Our current US sanitation standards were established under the Safe Drinking Water Act of 1974.20 While improvements in hygiene, sanitation, nutrition, and other public health measures have undoubtedly decreased the spread of disease and improved survival rates, there is no denying the significant drop in disease that occurs after the introduction of a vaccine for a particular illness or the increase in cases of that disease when vaccination rates drop off.
By the early 1990s, our current sanitation standards were already well established. Yet we didn’t see a significant decrease in the incidence of infections with Haemophilus influenzae type b (Hib) until after the conjugate Hib vaccines were introduced (dropping from about 20,000 cases/year to 1419 cases/year by 1993).21
In Britain, a drop in the rate of pertussis (whooping cough) vaccination in 1974 resulted in an epidemic of more than 100,000 cases and 36 deaths by 1978. There was no decrease in hygiene or sanitation standards to explain this rise.21
Continue to: 5. Vaccines are just another way for "big pharma" to make "big money."
5. Vaccines are just another way for “big pharma” to make “big money.”
Patients may benefit from knowing that in the earlier days of vaccines, pharmaceutical companies actually moved away from production of vaccines because they were not very profitable. These days, with worldwide distribution, drug companies are back in the swing of making vaccines and, as we would expect from all companies, are in business to make a profit.
That said, health care providers receive no payments from drug companies for offering vaccines or for offering one vaccine over another. The reason we recommend vaccination is because we know it is best for our patients’ health and the health of the community.

6. We don’t see polio anymore. Why do I need the vaccine?
One of the factors contributing to the rise in antivaccine sentiment is that we rarely see vaccine-preventable illnesses (such as polio, measles, and mumps). But the absence of these illnesses is precisely due to prior years’ vaccination efforts.
Smallpox, a deadly and disfiguring disease that killed many millions of people and contributed to the downfall of the Roman, Aztec, and Incan empires, was eradicated from the planet in 1979, thanks to focused vaccination efforts by the World Health Organization. Vaccination works, but we have to keep at it.
While we no longer see as many of these vaccine-preventable illnesses in the United States, they are still present in other parts of the world. Our world is much smaller than it used to be. International travel is common, and illnesses can be reintroduced into a community with relative ease. We must remain vigilant.
Continue to: 7. I heard that vaccines are made from aborted fetal tissue.
7. I heard that vaccines are made from aborted fetal tissue.
There are 5 vaccines (varicella, rubella, hepatitis A, shingles, and rabies vaccines) that were originally made using aborted fetal tissue. In 1960, tissue from 2 fetuses aborted by maternal choice (and not for the purpose of vaccine production) was used to propagate cell lines that are still used in vaccine development today.
Human cells provide advantages for vaccine production that other cells do not. Some viruses do not grow well in animal cells. Animal cells can introduce contamination by bacteria and viruses that are not carried in human cell lines. Vaccine production can be hindered or halted, resulting in a vaccine shortage, if animal products used in development are threatened (eg, if an illness strikes egg-producing chickens; eggs are used to make the influenza vaccine).22
Some patients, particularly those who are Catholic, may have concerns about these vaccines. The National Catholic Bioethics Center has prepared a statement regarding the use of these vaccines that may help settle any moral dilemmas.23 It reads:
“The cell lines under consideration were begun using cells taken from one or more fetuses aborted almost 40 years ago. Since that time, the cell lines have grown independently. It is important to note that descendent cells are not the cells of the aborted child.”
“One is morally free to use the vaccine regardless of its historical association with abortion. The reason is that the risk to public health, if one chooses not to vaccinate, outweighs the legitimate concern about the origins of the vaccine. This is especially important for parents, who have a moral obligation to protect the life and health of their children and those around them.”
Continue to: 8. Vaccines aren't studied—or monitored—thoroughly enough.
8. Vaccines aren’t studied—or monitored— thoroughly enough.
Patients would benefit from knowing that vaccines are some of the most thoroughly studied products brought to market. They undergo rigorous testing and oversight, from both public and private organizations, for 10 to 15 years before being released for distribution. Post-licensure monitoring is ongoing, and the manufacturer may voluntarily participate in Phase IV trials to continue to test the safety and efficacy of a vaccine after release to market.
Monitoring adverse effects. In addition, in 1990, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration established the Vaccine Adverse Events Reporting System (VAERS) to “detect possible signals of adverse events associated with vaccines.”24 Most events reported are coincidental, but some common mild adverse events (like redness and swelling at the injection site) are often underreported.
Serious events are always thoroughly investigated and are often found unrelated. However, rare associations have been found. For example, an intestinal problem called intussusception, related to the original rotavirus vaccine, was discovered, and the vaccine causing it was removed from the market.25 A new, safer rotavirus vaccine option is now available. Patients need to know that we do have an effective system of checks and balances in which we can place our trust.
9. People can become paralyzed or stop breathing after receiving a vaccination. Why run those risks?
One of the most feared reactions to vaccination is Guillain-Barré syndrome (GBS), which can cause paralysis. The CDC estimates the risk for GBS associated with the flu vaccine, for example, to be 1 to 2 cases per 1 million people vaccinated.26 Another potential concern is the rate of anaphylaxis following vaccination. However, in a 2016 study in the Journal of Allergy and Clinical Immunology, the rate of anaphylaxis for all vaccines combined was only 1.31 per 1 million vaccines.27
The risk of developing severe complications from an illness is much greater than that of developing complications from the vaccine meant to protect a person against that illness. In the United States, the population-based risk for influenza-related hospitalization in children, for example, is as high as 150 in 100,000 with as many as 125 deaths annually.26
Continue to: 10. Isn't vaccination a personal choice? How does my health/illness impact the community?
10. Isn’t vaccination a personal choice? How does my health/illness impact the community?
Patients may not realize that most viruses are contagious from 1 to 2 days before symptoms appear, which means we can spread an illness before we even know we have it. Protecting oneself also protects those around us.
Economic concerns. There’s also the economic impact of these illnesses to consider. This includes the personal cost of being out of school or work for an extended period and the cost of a patient’s care, which can become astronomical if hospitalization is required and which can become the country’s problem if a person lacks sufficient health insurance coverage.
A study looking at the cost of 4 major adult vaccine-preventable illnesses (influenza, pneumococcal disease, shingles, and whooping cough) in the United States in 2013 estimated the annual cost for these illnesses in adults ≥50 years to be $26.5 billion.28 And that doesn’t include the cost of childhood vaccine-preventable diseases.
Countering 3 concerns about childhood vaccinations
1. I can’t afford vaccines for my child.
The Vaccines for Children program is a federally-funded program that covers the cost of all vaccines for children younger than 19 years of age who are Medicaid-eligible, American Indian, Alaskan Native, uninsured, or underinsured.29 Although there may be a small administration fee charged by the provider’s office, the vaccine is free.
2. Don’t all of the vaccines recommended for children overwhelm their immune systems?
Children are exposed to so many more proteins on a daily basis (by crawling around on the floor, putting their hands in their mouths, attending school or day care, etc) than they are ever exposed to in a series of vaccines.30 Exposure to these proteins in their environment and to those in vaccines only serves to boost their immunity and keep them healthier in the long run.
And thanks to advances in vaccine production, the immunologic load in vaccines is far less than it used to be. The 14 vaccines given today contain <200 bacterial and viral proteins or polysaccharides, compared with the >3000 of these immunologic components in the 7 vaccines administered in 1980.31
Continue to: Influenza vaccine: Patient-friendly talking points
SIDEBAR
Influenza vaccine: Patient-friendly talking points
- Some people think that getting the flu is no big deal. While it is true that the flu takes a greater toll on the very young and very old, the chronically ill, and the immune compromised, even healthy people can become seriously ill or die. The Centers for Disease Control and Prevention estimates that the flu is responsible for 140,000 to 720,000 hospitalizations and 12,000 to 56,000 deaths in the United States every year.7 Of those who die from the flu, approximately 80% did not receive a flu shot.36 Of children who died from the flu between 2004 and 2012, more than 40% had no risk factors for complications.37
- The flu shot is a killed virus vaccine, so it can't give you the flu. People sometimes feel under the weather (achy, low-grade fever) after a vaccine, but this is considered normal and evidence that your body's immune system is "revving up."
- It takes 2 weeks before the vaccine becomes effective so a person can still get the flu during that time. This is why it is so important to get the vaccine earlier in the fall, before the flu season takes hold.
- The "stomach flu" is not the flu. The flu vaccine does not protect against the "stomach flu" or other flu-like illnesses.
- The flu vaccine is not perfect. It is an educated guess as to which strains will be circulating that year. (At its best, the flu vaccine is about 60% effective.38) However, it makes the chance of getting the flu less likely and significantly decreases the odds of severe complications/death.
- Egg allergies are no longer a reason to avoid the flu vaccine. There is an egg-free vaccine called Flublok (for those ≥18 years of age). In 2016-17, the Advisory Committee on Immunization Practices changed the recommendations for flu vaccine in egg-allergic people. The recommendations say that if reactions are mild, or you can eat cooked eggs without a problem, you can receive a flu vaccine. If you have severe reactions, such as trouble breathing or recurrent vomiting, you can still receive the flu vaccine, but must be monitored by a health care provider who can recognize and respond to a severe allergic reaction.39
Continue to: 3. Why don't we adhere to Dr. Sears' vaccine schedule?
3. Why don’t we adhere to Dr. Sears’ vaccine schedule?
There are multiple ways in which Dr. Robert Sears’ book, The Vaccine Book: Making the Right Decision for your Child, published in 2007, misrepresents vaccine science and leads patients astray in making decisions regarding vaccinations.32 Most important to note is that Dr. Sears’ Alternative Vaccine Schedule, which seeks to make it so that children do not receive more than 2 vaccinations per office visit, would require visits to a health care provider at 2, 3, 4, 5, 6, 7, 9, 12, 15, 18, and 21 months, and at 2, 2.5, 3, 3.5, 4, 5, and 6 years of age. This significantly increases the number of office visits and needle sticks, and raises the age at which vaccines are given, increasing the risk of illness outbreaks and decreasing the likelihood that parents would return to the office to complete the full series.
Acceptance of influenza and HPV vaccines remains a challenge
We are significantly less successful at getting parents and patients to agree to influenza and HPV vaccines than to the other vaccines we offer. The influenza vaccine success rate in 2016 was 59% in children and 43.3% in adults.33 Compared to the Tdap vaccine (88%) and the meningococcal vaccine (82%), which are offered at the same age as the HPV vaccine, success rates for HPV vaccine are significantly lower. In 2016, only 60.4% of boys and girls were current on their first HPV injection and only 43.3% were up to date with the full series.34
Newness of vaccines a factor?
Perhaps it is because the recommendations for these 2 vaccines are relatively new, and people don’t yet grasp the seriousness and scope of the diseases. Until 2010, the flu shot was recommended only for the very young, the elderly, and the medically high risk.
Similarly, the HPV vaccine was originally introduced for girls in 2006 and wasn’t recommended for boys until 2011.
Continue to: Human papillomavirus vaccine: Patient-friendly talking points
SIDEBAR
Human papillomavirus vaccine: Patient-friendly talking points
- Human papillomavirus (HPV) causes genital warts and cancer of the cervix, vagina, vulva, anus, rectum, penis, and oropharynx.
- The HPV vaccine is a cancer prevention vaccine. The 9-valent vaccine is active against 2 genital wart-causing strains and 7 cancer-causing strains of HPV.
- HPV is highly prevalent; 79 million Americans are currently infected, nearly 14 million people become newly infected each year, and nearly all of us will be exposed at some point in our sexual lives.40
- There are often no outward signs of infection, so it is a difficult infection to avoid.
- It takes no high-risk sexual activity to be exposed to the HPV virus.
- The HPV vaccine is recommended for both boys and girls usually around age 11 to 12 years (but as early as 9 years and as late as 26 years is acceptable). If the first vaccine is administered before 15 years of age, only 2 injections are needed 6 to 12 months apart. If the first vaccine is administered after 15 years of age, 3 injections are needed at 0, 2 months, and 6 months.41
- Completing the series before sexual activity begins is the best way to protect our children because the vaccine is a preventive measure, not a treatment.
- The HPV vaccine is highly effective with >90% efficacy against high-risk cancer-causing strains.42
- The HPV vaccine offers long-term protection. The vaccine has been on the market since 2006, and immunity has not yet diminished. Further monitoring is ongoing.43
- The HPV vaccine is covered under the Vaccines For Children program until age 19 years. Then it is up to individual insurance plans to cover it.
- The HPV vaccine does not cause infertility.44 HPV infection, on the other hand, can lead to fertility problems if, for example, treatment for cervical precancer or cancer requires partial removal of the cervix or a hysterectomy.
- The HPV vaccine does not cause autoimmune diseases.45,46 Studies show no difference between vaccinated and unvaccinated groups in rates of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes mellitus, multiple sclerosis, Hashimoto's thyroiditis, Graves' disease, and others.
- The HPV vaccine does not encourage earlier sexual activity. There was no earlier incidence of outcomes related to sexual activity (pregnancy, sexually transmitted infection testing or diagnosis, or contraceptive counseling) in vaccinated vs unvaccinated patients studied.47
Continue to: A sensitive subject
A sensitive subject. Discussion of a vaccine related to a child’s sexual health makes some parents uncomfortable. Studies show that focusing on the cancer prevention aspects of the vaccine, rather than on sexual transmission of HPV, results in greater vaccine acceptance.35
However, if discussion of sexual transmission is unavoidable, remind parents to consider their own adolescence and whether they chose to share everything with their parents. Point out that there were probably things they did that they later looked back on and thought, “What was I thinking?” Their children, no matter how wonderful and levelheaded they are, will be no different. And, as much as parents don’t want to think about it, some kids will suffer unwanted sexual contact. Shouldn’t parents protect their children as best as they can?
A teen’s right to choose? Some states have passed a Mature Minor Doctrine, which provides for mature, unemancipated teens to make their own medical decisions regarding such issues as sexuality, mental health, and drug and alcohol use without their parents’ consent. In these states, teens may elect to receive the HPV vaccine without parental permission. (Check your state’s laws for specifics, and see the 2 boxes with patient-friendly talking points for influenza vaccine7,36-39 and human papillomavirus vaccine.40-47)
CORRESPONDENCE
Gretchen LaSalle, MD, MultiCare Rockwood Clinic, 2214 East 29th Avenue, Spokane, WA 99203; [email protected].
1. Paterson P, Meurice F, Stanberry LR, et al. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6706.
2. Opel DJ, Heritage J, Taylor J, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132:1037-1046.
3. Palmer J, Carrico C, Costanzo C. Identifying and overcoming perceived barriers of providers towards vaccination: a literature review. J Vaccines. 2015;1-7.
4. Autism Science Foundation. Making the CASE for vaccines: a new model for talking to patients about vaccines. Available at: http://autismsciencefoundation.org/wp-content/uploads/2015/12/Making-the-CASE-for-Vaccines-Guide_final.pdf. Accessed April 8, 2018.
5. Jacobson RM, Van Etta L, Bahta L. The C.A.S.E approach: guidance for talking to vaccine-hesitant patients. Minn Med. 2013;96:49-50.
6. Henrickson NB, Opel DJ, Grothaus L, et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics. 2015;136:70-79.
7. Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. Available at: https://www.cdc.gov/flu/protect/keyfacts.htm. Accessed April 8, 2018.
8. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
9. Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32:3623-3629.
10. Agency for Toxic Substances & Disease Registry. Minimal risk levels for hazardous substances. Available at: https://www.atsdr.cdc.gov/mrls/mrllist.asp#34tag. Accessed April 8, 2018.
11. US Food and Drug Administration. Thimerosal and vaccines. Available at: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed April 8, 2018.
12. Hviid A, Stellfeld M, Wohlfahrt J, et al. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763-1766.
13. Centers for Disease Control and Prevention. Thimerosal in vaccines. Available at: https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed May 8, 2018.
14. Autism Speaks. Frequently asked questions. Available at: https://www.autismspeaks.org/what-autism/faq. Accessed April 8, 2018.
15. Agency for Toxic Substances & Disease Registry. Toxic substances portal-aluminum. Public Health Statement for Aluminum, CAS #7429-90-5. Available at: https://www.atsdr.cdc.gov/PHS/PHS.asp?id=1076&tid=34. Accessed April 8, 2018.
16. Children’s Hospital of Philadelphia. Vaccine ingredients-aluminum. Available at: www.chop.edu/centers-programs/vaccine-education-center/vaccine-ingredients/aluminum. Accessed April 8, 2018.
17. Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med. 2014;371:1661-1663.
18. Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55:72-77.
19. Hall V, Banerjee E, Kenyon C, et al. Measles outbreak – Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:713-717.
20. The National Academies of Sciences Engineering Medicine. History of U.S. water and wastewater systems. Privatization of Water Services in the United States: an Assessment of Issues and Experience. Washington, DC: The National Academies Press; 2002:29-40. Available at: https://www.nap.edu/read/10135/chapter/4#35. Accessed May 7, 2018.
21. World Health Organization. Global vaccine safety. Six common misconceptions about immunization. Available at: http://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index1.html. Accessed May 7, 2018.
22. The history of vaccines. Human cell strains in vaccine development. Available at: https://www.historyofvaccines.org/content/articles/human-cell-strains-vaccine-development. Accessed April 8, 2018.
23. The National Catholic Bioethics Center. Frequently asked questions. Available at: https://www.ncbcenter.org/resources/frequently-asked-questions/use-vaccines/. Accessed April 8, 2018.
24. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33:4398-4405.
25. Foster S. Rotavirus vaccine and intussusception. J Pediatr Pharmacol Ther. 2007;12:4-7.
26. Mistry RD, Fischer JB, Prasad PA, et al. Severe complications of influenza-like illnesses. Pediatrics. 2014;134:e684-e690.
27. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
28. McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36:259-273.
29. Centers for Disease Control and Prevention. Vaccines for Children (VFC) Program. Available at: https://www.cdc.gov/features/vfcprogram/index.html. Accessed April 8, 2018.
30. Plotkin S, Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456-461.
31. Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109:124-129.
32. Offit PA, Moser CA. The problem with Dr. Bob’s alternative vaccine schedule. Pediatrics. 2009;123:e164-e169.
33. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. April 8. 2018.
34. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
35. Thomas TL. Cancer prevention: HPV vaccination. Semin Oncol Nurs. 2016:32:273-280.
36. Centers for Disease Control and Prevention. Estimating seasonal influenza-associated deaths in the United States. Available at: https://www.cdc.gov/flu/about/disease/US_flu-related_deaths.htm. Accessed May 8, 2018.
37. Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States: 2004-2012. Pediatrics. 2013;132:796-804.
38. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed April 8, 2018.
39. Centers for Disease Control and Prevention. Influenza (flu). Flu vaccine and people with egg allergies. Available at: https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm. Accessed April 8, 2018.
40. Centers for Disease Control and Prevention. For parents: vaccines for your children. HPV vaccine for preteens and teens. Available at: https://www.cdc.gov/vaccines/parents/diseases/teen/hpv.html. Accessed April 8, 2018.
41. Centers for Disease Control and Prevention. Vaccines and preventable diseases. HPV vaccine recommendations. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed May 7, 2018.
42. Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719-726.
43. De Vincenzo R, Conte C, Ricci C, et al. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999-1010.
44. McInerney KA, Hatch EE, Wesselink AK. The effect of vaccination against human papillomavirus on fecundability. Paedeatr Perinat Epidemiol. 2017;31:531-536.
45. Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193-203.
46. Vichnin M, Bonanni P, Klein NP, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006-2015. Ped Infect Dis J. 2015;34:983-991.
47. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11-to-12-year-olds. Pediatrics. 2012;130:798-805.
1. Paterson P, Meurice F, Stanberry LR, et al. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6706.
2. Opel DJ, Heritage J, Taylor J, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132:1037-1046.
3. Palmer J, Carrico C, Costanzo C. Identifying and overcoming perceived barriers of providers towards vaccination: a literature review. J Vaccines. 2015;1-7.
4. Autism Science Foundation. Making the CASE for vaccines: a new model for talking to patients about vaccines. Available at: http://autismsciencefoundation.org/wp-content/uploads/2015/12/Making-the-CASE-for-Vaccines-Guide_final.pdf. Accessed April 8, 2018.
5. Jacobson RM, Van Etta L, Bahta L. The C.A.S.E approach: guidance for talking to vaccine-hesitant patients. Minn Med. 2013;96:49-50.
6. Henrickson NB, Opel DJ, Grothaus L, et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics. 2015;136:70-79.
7. Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. Available at: https://www.cdc.gov/flu/protect/keyfacts.htm. Accessed April 8, 2018.
8. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
9. Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32:3623-3629.
10. Agency for Toxic Substances & Disease Registry. Minimal risk levels for hazardous substances. Available at: https://www.atsdr.cdc.gov/mrls/mrllist.asp#34tag. Accessed April 8, 2018.
11. US Food and Drug Administration. Thimerosal and vaccines. Available at: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed April 8, 2018.
12. Hviid A, Stellfeld M, Wohlfahrt J, et al. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763-1766.
13. Centers for Disease Control and Prevention. Thimerosal in vaccines. Available at: https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed May 8, 2018.
14. Autism Speaks. Frequently asked questions. Available at: https://www.autismspeaks.org/what-autism/faq. Accessed April 8, 2018.
15. Agency for Toxic Substances & Disease Registry. Toxic substances portal-aluminum. Public Health Statement for Aluminum, CAS #7429-90-5. Available at: https://www.atsdr.cdc.gov/PHS/PHS.asp?id=1076&tid=34. Accessed April 8, 2018.
16. Children’s Hospital of Philadelphia. Vaccine ingredients-aluminum. Available at: www.chop.edu/centers-programs/vaccine-education-center/vaccine-ingredients/aluminum. Accessed April 8, 2018.
17. Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med. 2014;371:1661-1663.
18. Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55:72-77.
19. Hall V, Banerjee E, Kenyon C, et al. Measles outbreak – Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:713-717.
20. The National Academies of Sciences Engineering Medicine. History of U.S. water and wastewater systems. Privatization of Water Services in the United States: an Assessment of Issues and Experience. Washington, DC: The National Academies Press; 2002:29-40. Available at: https://www.nap.edu/read/10135/chapter/4#35. Accessed May 7, 2018.
21. World Health Organization. Global vaccine safety. Six common misconceptions about immunization. Available at: http://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index1.html. Accessed May 7, 2018.
22. The history of vaccines. Human cell strains in vaccine development. Available at: https://www.historyofvaccines.org/content/articles/human-cell-strains-vaccine-development. Accessed April 8, 2018.
23. The National Catholic Bioethics Center. Frequently asked questions. Available at: https://www.ncbcenter.org/resources/frequently-asked-questions/use-vaccines/. Accessed April 8, 2018.
24. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33:4398-4405.
25. Foster S. Rotavirus vaccine and intussusception. J Pediatr Pharmacol Ther. 2007;12:4-7.
26. Mistry RD, Fischer JB, Prasad PA, et al. Severe complications of influenza-like illnesses. Pediatrics. 2014;134:e684-e690.
27. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
28. McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36:259-273.
29. Centers for Disease Control and Prevention. Vaccines for Children (VFC) Program. Available at: https://www.cdc.gov/features/vfcprogram/index.html. Accessed April 8, 2018.
30. Plotkin S, Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456-461.
31. Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109:124-129.
32. Offit PA, Moser CA. The problem with Dr. Bob’s alternative vaccine schedule. Pediatrics. 2009;123:e164-e169.
33. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. April 8. 2018.
34. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
35. Thomas TL. Cancer prevention: HPV vaccination. Semin Oncol Nurs. 2016:32:273-280.
36. Centers for Disease Control and Prevention. Estimating seasonal influenza-associated deaths in the United States. Available at: https://www.cdc.gov/flu/about/disease/US_flu-related_deaths.htm. Accessed May 8, 2018.
37. Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States: 2004-2012. Pediatrics. 2013;132:796-804.
38. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed April 8, 2018.
39. Centers for Disease Control and Prevention. Influenza (flu). Flu vaccine and people with egg allergies. Available at: https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm. Accessed April 8, 2018.
40. Centers for Disease Control and Prevention. For parents: vaccines for your children. HPV vaccine for preteens and teens. Available at: https://www.cdc.gov/vaccines/parents/diseases/teen/hpv.html. Accessed April 8, 2018.
41. Centers for Disease Control and Prevention. Vaccines and preventable diseases. HPV vaccine recommendations. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed May 7, 2018.
42. Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719-726.
43. De Vincenzo R, Conte C, Ricci C, et al. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999-1010.
44. McInerney KA, Hatch EE, Wesselink AK. The effect of vaccination against human papillomavirus on fecundability. Paedeatr Perinat Epidemiol. 2017;31:531-536.
45. Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193-203.
46. Vichnin M, Bonanni P, Klein NP, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006-2015. Ped Infect Dis J. 2015;34:983-991.
47. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11-to-12-year-olds. Pediatrics. 2012;130:798-805.
From The Journal of Family Practice | 2018;67(6):348-351,359-364.
PRACTICE RECOMMENDATIONS
› Use a presumptive approach when discussing vaccines with patients/parents. A
› Offer vaccines at every opportunity; provider recommendation is the most important factor in getting patients to vaccinate. A
› Focus on the cancer prevention aspect of the human papillomavirus vaccine to improve rates of vaccine acceptance. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series