User login
Tick-borne disease has become a national issue
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.
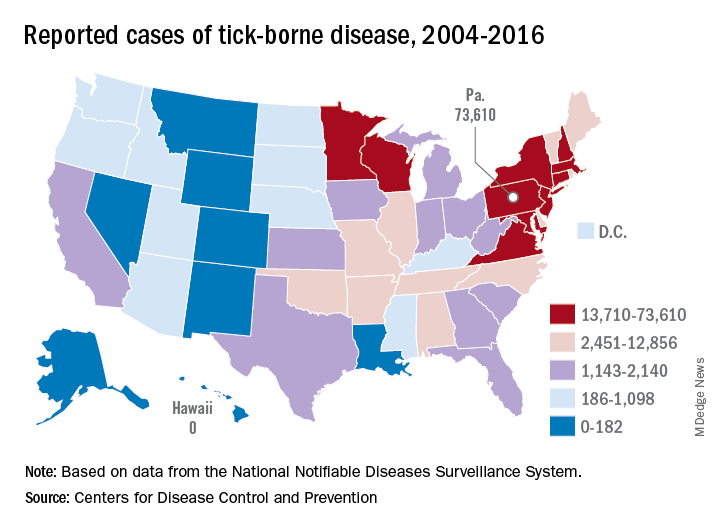
There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Methotrexate relieves pain of Chikungunya-associated arthritis
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
FROM JOURNAL OF CLINICAL RHEUMATOLOGY
Key clinical point:
Major finding: On a 10-point visual analog scale, the pain reduction from baseline on methotrexate at 8 weeks was 4.5 (P less than .0001).
Study details: Retrospective observational study.
Disclosures: The authors declared no potential conflicts of interest.
Source: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943
Hamstring tendinopathy implicated in persistent Lyme arthritis
CHICAGO – The big news regarding Lyme disease at the annual meeting of the American College of Rheumatology was a report that hamstring tendon calcification is extremely common among patients who have persistent Lyme arthritis despite having undergone appropriate antibiotic therapy.
“This is a fascinating study,” Robert A. Kalish, MD, a Lyme disease expert not involved in the research, said regarding the report by Sheila L. Arvikar, MD, and her coworkers at Massachusetts General Hospital, Boston.
One implication of this finding by a renowned group of Lyme disease researchers is that persistent posttreatment Lyme arthritis may in many cases be due to ongoing immunostimulation by spirochete remains located in hamstring tendons, a privileged, relatively avascular site where the foreign material may be able to evade immune clearance.
Also, as Dr. Arvikar pointed out in her presentation, calcific tendinopathy implies prior inflammation or degenerative changes. Thus, these calcific hamstring abnormalities implicate the hamstring tendons as a potential initial site of infection by hematogenously-spread Borrelia burgdorferi during the prearthritis phase of Lyme disease.
A further implication of the study is the possibility that hamstring tendon calcification could serve as a useful diagnostic aid in distinguishing Lyme arthritis from arthritis due to other causes. In the study, hamstring calcific tendinopathy was found in 28 of 31 adults and children with Lyme arthritis, 3 of 22 with knee osteoarthritis, and 1 of 14 patients with inflammatory arthritis, Dr. Arvikar noted.
She and her coinvestigators evaluated tendon pathology in their retrospective study of patients at the Massachusetts General Hospital Rheumatology Musculoskeletal Ultrasound Clinic. They used ultrasound because they have found it offers far better spatial resolution of calcification than does MRI or x-rays. The semimembranosus tendon was the hamstring tendon that most commonly exhibited calcification, although 11 patients with Lyme arthritis also had involvement of the semitendinosus tendon, compared with none of the controls with osteoarthritis or inflammatory arthritis.
In the eight patients with serial ultrasound evaluations over a period of up to 12 months, the calcification persisted but the symptoms of tendinitis and synovitis improved.
Dr. Arvikar and her colleagues are expanding the scope of their ongoing study by examining patients whose Lyme arthritis is milder than that of the initial population, including patients who haven’t yet received antibiotics. They are also evaluating more controls with inflammatory arthritis.
In a separate presentation, Dr. Kalish noted that Lyme arthritis, the manifestation of Lyme disease of greatest interest to rheumatologists, occurs in about 60% of untreated patients, with onset a mean of 6 months after the tick bite. It typically entails recurrent mono- or oligoarthritis of large joints. The knee is involved in roughly 95% of cases.
The natural history of untreated Lyme arthritis is a spontaneous resolution rate of 10%-20% per year. Since the 1980s, however, 4 weeks of oral doxycycline or amoxicillin has been the treatment of choice. About 10% of patients with Lyme arthritis continue to have active synovitis 3 months after their course of antibiotics.
“There are some patients you give the treatment to and their arthritis just melts away in a month, but some, no matter what you do with antibiotics, continue to have synovitis, often developing a highly proliferative palpable synovitis that is really gunked up and features obliterative microvascular lesions,” observed Dr. Kalish, a rheumatologist at Tufts University in Boston.
Dr. Kalish said that persistent posttreatment Lyme arthritis is most often due to a self-perpetuating immune response after the spirochete has been killed by antibiotics. He noted that patients with certain HLA-DRB1 haplotypes are more likely to experience persistent Lyme arthritis after standard recommended courses of antibiotics, and these DRB1 alleles correlate closely with the shared epitope associated with increased susceptibility to rheumatoid arthritis. Several candidate autoantigens have already been identified.
He noted that the Massachusetts General group, in an earlier study, demonstrated that the presence of B. burgdorferi DNA by PCR in synovial fluid from patients with persistent Lyme arthritis after antibiotic therapy was not a reliable indicator of active joint infection (Arthritis Rheum. 2011 Aug;63[8]:2238-47).
“This was a paradigm change for me in seeing this study, because prior to that I had used PCR somewhat to guide treatment and make management decisions,” Dr. Kalish said.
What’s a reasonable treatment strategy in patients with persistent Lyme arthritis despite 30 days of oral antibiotics? Dr. Kalish favors an algorithm similar to one published by Dr. Arvikar and Allen C. Steere, MD (Infect Dis Clin North Am. 2015 Jun;29[2]:269-80). In the case of mild persistent arthritis, he opts for another 30 days of oral doxycycline. If the arthritis is moderate or severe, he goes with either another 30 days of doxycycline or 30 days of intravenous ceftriaxone.
If the arthritis still hasn’t resolved despite two 30-day rounds of antibiotic therapy, he prescribes an NSAID or hydroxychloroquine if the persistent arthritis is mild, or methotrexate if it’s moderate to severe. And if the arthritis still persists after 3-6 months of disease-modifying antirheumatic drug therapy, he’ll consider synovectomy, which has a good success rate.
Neither Dr. Arvikar nor Dr. Kalish reported having any financial conflicts regarding their presentations.
SOURCE: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
CHICAGO – The big news regarding Lyme disease at the annual meeting of the American College of Rheumatology was a report that hamstring tendon calcification is extremely common among patients who have persistent Lyme arthritis despite having undergone appropriate antibiotic therapy.
“This is a fascinating study,” Robert A. Kalish, MD, a Lyme disease expert not involved in the research, said regarding the report by Sheila L. Arvikar, MD, and her coworkers at Massachusetts General Hospital, Boston.
One implication of this finding by a renowned group of Lyme disease researchers is that persistent posttreatment Lyme arthritis may in many cases be due to ongoing immunostimulation by spirochete remains located in hamstring tendons, a privileged, relatively avascular site where the foreign material may be able to evade immune clearance.
Also, as Dr. Arvikar pointed out in her presentation, calcific tendinopathy implies prior inflammation or degenerative changes. Thus, these calcific hamstring abnormalities implicate the hamstring tendons as a potential initial site of infection by hematogenously-spread Borrelia burgdorferi during the prearthritis phase of Lyme disease.
A further implication of the study is the possibility that hamstring tendon calcification could serve as a useful diagnostic aid in distinguishing Lyme arthritis from arthritis due to other causes. In the study, hamstring calcific tendinopathy was found in 28 of 31 adults and children with Lyme arthritis, 3 of 22 with knee osteoarthritis, and 1 of 14 patients with inflammatory arthritis, Dr. Arvikar noted.
She and her coinvestigators evaluated tendon pathology in their retrospective study of patients at the Massachusetts General Hospital Rheumatology Musculoskeletal Ultrasound Clinic. They used ultrasound because they have found it offers far better spatial resolution of calcification than does MRI or x-rays. The semimembranosus tendon was the hamstring tendon that most commonly exhibited calcification, although 11 patients with Lyme arthritis also had involvement of the semitendinosus tendon, compared with none of the controls with osteoarthritis or inflammatory arthritis.
In the eight patients with serial ultrasound evaluations over a period of up to 12 months, the calcification persisted but the symptoms of tendinitis and synovitis improved.
Dr. Arvikar and her colleagues are expanding the scope of their ongoing study by examining patients whose Lyme arthritis is milder than that of the initial population, including patients who haven’t yet received antibiotics. They are also evaluating more controls with inflammatory arthritis.
In a separate presentation, Dr. Kalish noted that Lyme arthritis, the manifestation of Lyme disease of greatest interest to rheumatologists, occurs in about 60% of untreated patients, with onset a mean of 6 months after the tick bite. It typically entails recurrent mono- or oligoarthritis of large joints. The knee is involved in roughly 95% of cases.
The natural history of untreated Lyme arthritis is a spontaneous resolution rate of 10%-20% per year. Since the 1980s, however, 4 weeks of oral doxycycline or amoxicillin has been the treatment of choice. About 10% of patients with Lyme arthritis continue to have active synovitis 3 months after their course of antibiotics.
“There are some patients you give the treatment to and their arthritis just melts away in a month, but some, no matter what you do with antibiotics, continue to have synovitis, often developing a highly proliferative palpable synovitis that is really gunked up and features obliterative microvascular lesions,” observed Dr. Kalish, a rheumatologist at Tufts University in Boston.
Dr. Kalish said that persistent posttreatment Lyme arthritis is most often due to a self-perpetuating immune response after the spirochete has been killed by antibiotics. He noted that patients with certain HLA-DRB1 haplotypes are more likely to experience persistent Lyme arthritis after standard recommended courses of antibiotics, and these DRB1 alleles correlate closely with the shared epitope associated with increased susceptibility to rheumatoid arthritis. Several candidate autoantigens have already been identified.
He noted that the Massachusetts General group, in an earlier study, demonstrated that the presence of B. burgdorferi DNA by PCR in synovial fluid from patients with persistent Lyme arthritis after antibiotic therapy was not a reliable indicator of active joint infection (Arthritis Rheum. 2011 Aug;63[8]:2238-47).
“This was a paradigm change for me in seeing this study, because prior to that I had used PCR somewhat to guide treatment and make management decisions,” Dr. Kalish said.
What’s a reasonable treatment strategy in patients with persistent Lyme arthritis despite 30 days of oral antibiotics? Dr. Kalish favors an algorithm similar to one published by Dr. Arvikar and Allen C. Steere, MD (Infect Dis Clin North Am. 2015 Jun;29[2]:269-80). In the case of mild persistent arthritis, he opts for another 30 days of oral doxycycline. If the arthritis is moderate or severe, he goes with either another 30 days of doxycycline or 30 days of intravenous ceftriaxone.
If the arthritis still hasn’t resolved despite two 30-day rounds of antibiotic therapy, he prescribes an NSAID or hydroxychloroquine if the persistent arthritis is mild, or methotrexate if it’s moderate to severe. And if the arthritis still persists after 3-6 months of disease-modifying antirheumatic drug therapy, he’ll consider synovectomy, which has a good success rate.
Neither Dr. Arvikar nor Dr. Kalish reported having any financial conflicts regarding their presentations.
SOURCE: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
CHICAGO – The big news regarding Lyme disease at the annual meeting of the American College of Rheumatology was a report that hamstring tendon calcification is extremely common among patients who have persistent Lyme arthritis despite having undergone appropriate antibiotic therapy.
“This is a fascinating study,” Robert A. Kalish, MD, a Lyme disease expert not involved in the research, said regarding the report by Sheila L. Arvikar, MD, and her coworkers at Massachusetts General Hospital, Boston.
One implication of this finding by a renowned group of Lyme disease researchers is that persistent posttreatment Lyme arthritis may in many cases be due to ongoing immunostimulation by spirochete remains located in hamstring tendons, a privileged, relatively avascular site where the foreign material may be able to evade immune clearance.
Also, as Dr. Arvikar pointed out in her presentation, calcific tendinopathy implies prior inflammation or degenerative changes. Thus, these calcific hamstring abnormalities implicate the hamstring tendons as a potential initial site of infection by hematogenously-spread Borrelia burgdorferi during the prearthritis phase of Lyme disease.
A further implication of the study is the possibility that hamstring tendon calcification could serve as a useful diagnostic aid in distinguishing Lyme arthritis from arthritis due to other causes. In the study, hamstring calcific tendinopathy was found in 28 of 31 adults and children with Lyme arthritis, 3 of 22 with knee osteoarthritis, and 1 of 14 patients with inflammatory arthritis, Dr. Arvikar noted.
She and her coinvestigators evaluated tendon pathology in their retrospective study of patients at the Massachusetts General Hospital Rheumatology Musculoskeletal Ultrasound Clinic. They used ultrasound because they have found it offers far better spatial resolution of calcification than does MRI or x-rays. The semimembranosus tendon was the hamstring tendon that most commonly exhibited calcification, although 11 patients with Lyme arthritis also had involvement of the semitendinosus tendon, compared with none of the controls with osteoarthritis or inflammatory arthritis.
In the eight patients with serial ultrasound evaluations over a period of up to 12 months, the calcification persisted but the symptoms of tendinitis and synovitis improved.
Dr. Arvikar and her colleagues are expanding the scope of their ongoing study by examining patients whose Lyme arthritis is milder than that of the initial population, including patients who haven’t yet received antibiotics. They are also evaluating more controls with inflammatory arthritis.
In a separate presentation, Dr. Kalish noted that Lyme arthritis, the manifestation of Lyme disease of greatest interest to rheumatologists, occurs in about 60% of untreated patients, with onset a mean of 6 months after the tick bite. It typically entails recurrent mono- or oligoarthritis of large joints. The knee is involved in roughly 95% of cases.
The natural history of untreated Lyme arthritis is a spontaneous resolution rate of 10%-20% per year. Since the 1980s, however, 4 weeks of oral doxycycline or amoxicillin has been the treatment of choice. About 10% of patients with Lyme arthritis continue to have active synovitis 3 months after their course of antibiotics.
“There are some patients you give the treatment to and their arthritis just melts away in a month, but some, no matter what you do with antibiotics, continue to have synovitis, often developing a highly proliferative palpable synovitis that is really gunked up and features obliterative microvascular lesions,” observed Dr. Kalish, a rheumatologist at Tufts University in Boston.
Dr. Kalish said that persistent posttreatment Lyme arthritis is most often due to a self-perpetuating immune response after the spirochete has been killed by antibiotics. He noted that patients with certain HLA-DRB1 haplotypes are more likely to experience persistent Lyme arthritis after standard recommended courses of antibiotics, and these DRB1 alleles correlate closely with the shared epitope associated with increased susceptibility to rheumatoid arthritis. Several candidate autoantigens have already been identified.
He noted that the Massachusetts General group, in an earlier study, demonstrated that the presence of B. burgdorferi DNA by PCR in synovial fluid from patients with persistent Lyme arthritis after antibiotic therapy was not a reliable indicator of active joint infection (Arthritis Rheum. 2011 Aug;63[8]:2238-47).
“This was a paradigm change for me in seeing this study, because prior to that I had used PCR somewhat to guide treatment and make management decisions,” Dr. Kalish said.
What’s a reasonable treatment strategy in patients with persistent Lyme arthritis despite 30 days of oral antibiotics? Dr. Kalish favors an algorithm similar to one published by Dr. Arvikar and Allen C. Steere, MD (Infect Dis Clin North Am. 2015 Jun;29[2]:269-80). In the case of mild persistent arthritis, he opts for another 30 days of oral doxycycline. If the arthritis is moderate or severe, he goes with either another 30 days of doxycycline or 30 days of intravenous ceftriaxone.
If the arthritis still hasn’t resolved despite two 30-day rounds of antibiotic therapy, he prescribes an NSAID or hydroxychloroquine if the persistent arthritis is mild, or methotrexate if it’s moderate to severe. And if the arthritis still persists after 3-6 months of disease-modifying antirheumatic drug therapy, he’ll consider synovectomy, which has a good success rate.
Neither Dr. Arvikar nor Dr. Kalish reported having any financial conflicts regarding their presentations.
SOURCE: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Ultrasound evidence of hamstring calcific tendinopathy was found in 28 of 31 patients with persistent posttreatment Lyme arthritis, compared with 3 of 22 patients with knee osteoarthritis.
Study details: This was a retrospective imaging study of hamstring tendon status in 31 patients with persistent posttreatment Lyme arthritis, 22 patients with osteoarthritis, and 14 with inflammatory arthritis.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
Source: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
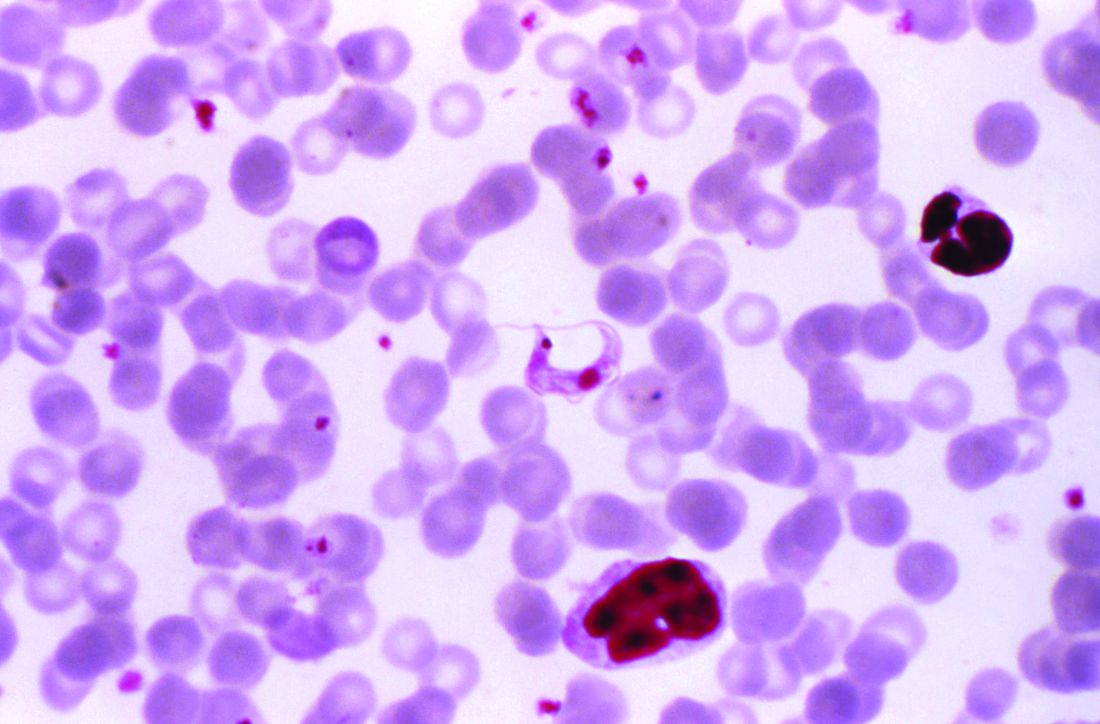
AHA: Chagas disease and its heart effects have come to the U.S.
Chagas disease, a cause of serious cardiovascular problems and sudden death, was previously localized mainly in the tropics, but now affects at least 300,000 people in the United States and is growing in prevalence in other traditionally nonendemic areas, including Europe, Australia, and Japan. The American Heart Association and the Inter-American Society of Cardiology have released a statement to “increase global awareness among providers who may encounter patients with Chagas disease outside of traditionally endemic environments.”
The document summarizes the most up-to-date information on diagnosis, screening, and treatment of Trypanosoma cruzi (the protozoan cause of Chagas) infection, focusing primarily on its cardiovascular aspects, and was developed by Maria Carmo Pereira Nunes, MD, chair, and her colleagues on the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee.
Chagas disease is transmitted by a blood-sucking insect vector Triatoma infestans and, less frequently, from mother to fetus or by contaminated food or drink, and about one third of infected individuals develop chronic heart disease.
Although 60%-70% of people infected with T. cruzi never develop any symptoms, those who do can develop heart disease, including heart failure, stroke, life threatening ventricular arrhythmias, and cardiac arrest, according to the statement published in Circulation.
Chronic Chagas-related heart disease develops after several decades of the indeterminate, or subclinical, form of the disease following the initial acute infection. Potential risk factors for progression to the chronic stage include African ancestry, age, severity of acute infection, nutritional status, alcoholism, and their concomitant diseases.
In most studies, sudden death is the most common overall cause of death in patients with Chagas-related cardiomyopathy (55%-60%), followed by heart failure (25%-30%) and embolic events (10%-15%), according to the authors.
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease, with benznidazole as the first-line treatment because it has better tolerance, is more widely available, and has more published data published on its efficacy. Furthermore, it is available in the United States, after the Food and Drug Administration granted fast-track approved 2017 for treatment of Chagas disease. Use of nifurtimox in the United States entails consultation with the Centers for Disease Control and prevention, according to the statement.
“More data are needed on the best practices for the treatment of Chagas cardiomyopathy. Because no specific clinical trials have been conducted, care for
patients with Chagas-induced ventricular dysfunction is extrapolated from general heart failure recommendations with unclear efficacy (and potential harm),” Dr. Pereira Nunes and her colleagues concluded.
One author disclosed receiving a research grant from Merck and speakers’ bureau and/or honoraria from Bayer; Biotronik, and Medtronic. The others had no relevant disclosures.
SOURCE: Nunes, MCP, et al., Circulation. 2018 Aug 20; doi: 10.1161/CIR.0000000000000599.
Chagas disease, a cause of serious cardiovascular problems and sudden death, was previously localized mainly in the tropics, but now affects at least 300,000 people in the United States and is growing in prevalence in other traditionally nonendemic areas, including Europe, Australia, and Japan. The American Heart Association and the Inter-American Society of Cardiology have released a statement to “increase global awareness among providers who may encounter patients with Chagas disease outside of traditionally endemic environments.”
The document summarizes the most up-to-date information on diagnosis, screening, and treatment of Trypanosoma cruzi (the protozoan cause of Chagas) infection, focusing primarily on its cardiovascular aspects, and was developed by Maria Carmo Pereira Nunes, MD, chair, and her colleagues on the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee.
Chagas disease is transmitted by a blood-sucking insect vector Triatoma infestans and, less frequently, from mother to fetus or by contaminated food or drink, and about one third of infected individuals develop chronic heart disease.
Although 60%-70% of people infected with T. cruzi never develop any symptoms, those who do can develop heart disease, including heart failure, stroke, life threatening ventricular arrhythmias, and cardiac arrest, according to the statement published in Circulation.
Chronic Chagas-related heart disease develops after several decades of the indeterminate, or subclinical, form of the disease following the initial acute infection. Potential risk factors for progression to the chronic stage include African ancestry, age, severity of acute infection, nutritional status, alcoholism, and their concomitant diseases.
In most studies, sudden death is the most common overall cause of death in patients with Chagas-related cardiomyopathy (55%-60%), followed by heart failure (25%-30%) and embolic events (10%-15%), according to the authors.
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease, with benznidazole as the first-line treatment because it has better tolerance, is more widely available, and has more published data published on its efficacy. Furthermore, it is available in the United States, after the Food and Drug Administration granted fast-track approved 2017 for treatment of Chagas disease. Use of nifurtimox in the United States entails consultation with the Centers for Disease Control and prevention, according to the statement.
“More data are needed on the best practices for the treatment of Chagas cardiomyopathy. Because no specific clinical trials have been conducted, care for
patients with Chagas-induced ventricular dysfunction is extrapolated from general heart failure recommendations with unclear efficacy (and potential harm),” Dr. Pereira Nunes and her colleagues concluded.
One author disclosed receiving a research grant from Merck and speakers’ bureau and/or honoraria from Bayer; Biotronik, and Medtronic. The others had no relevant disclosures.
SOURCE: Nunes, MCP, et al., Circulation. 2018 Aug 20; doi: 10.1161/CIR.0000000000000599.
Chagas disease, a cause of serious cardiovascular problems and sudden death, was previously localized mainly in the tropics, but now affects at least 300,000 people in the United States and is growing in prevalence in other traditionally nonendemic areas, including Europe, Australia, and Japan. The American Heart Association and the Inter-American Society of Cardiology have released a statement to “increase global awareness among providers who may encounter patients with Chagas disease outside of traditionally endemic environments.”
The document summarizes the most up-to-date information on diagnosis, screening, and treatment of Trypanosoma cruzi (the protozoan cause of Chagas) infection, focusing primarily on its cardiovascular aspects, and was developed by Maria Carmo Pereira Nunes, MD, chair, and her colleagues on the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee.
Chagas disease is transmitted by a blood-sucking insect vector Triatoma infestans and, less frequently, from mother to fetus or by contaminated food or drink, and about one third of infected individuals develop chronic heart disease.
Although 60%-70% of people infected with T. cruzi never develop any symptoms, those who do can develop heart disease, including heart failure, stroke, life threatening ventricular arrhythmias, and cardiac arrest, according to the statement published in Circulation.
Chronic Chagas-related heart disease develops after several decades of the indeterminate, or subclinical, form of the disease following the initial acute infection. Potential risk factors for progression to the chronic stage include African ancestry, age, severity of acute infection, nutritional status, alcoholism, and their concomitant diseases.
In most studies, sudden death is the most common overall cause of death in patients with Chagas-related cardiomyopathy (55%-60%), followed by heart failure (25%-30%) and embolic events (10%-15%), according to the authors.
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease, with benznidazole as the first-line treatment because it has better tolerance, is more widely available, and has more published data published on its efficacy. Furthermore, it is available in the United States, after the Food and Drug Administration granted fast-track approved 2017 for treatment of Chagas disease. Use of nifurtimox in the United States entails consultation with the Centers for Disease Control and prevention, according to the statement.
“More data are needed on the best practices for the treatment of Chagas cardiomyopathy. Because no specific clinical trials have been conducted, care for
patients with Chagas-induced ventricular dysfunction is extrapolated from general heart failure recommendations with unclear efficacy (and potential harm),” Dr. Pereira Nunes and her colleagues concluded.
One author disclosed receiving a research grant from Merck and speakers’ bureau and/or honoraria from Bayer; Biotronik, and Medtronic. The others had no relevant disclosures.
SOURCE: Nunes, MCP, et al., Circulation. 2018 Aug 20; doi: 10.1161/CIR.0000000000000599.
FROM CIRCULATION