User login
Official Newspaper of the American College of Surgeons
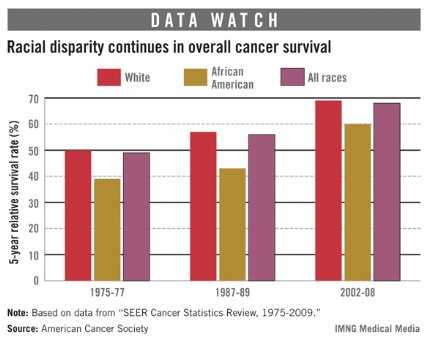
Age, race impact prostate cancer risk
Prostate cancers detected during screening are much more likely to be high risk when they affect black men and men aged 75 years or older.
Men over age 74 years were nine times more likely to have high-risk disease after a positive prostate-specific antigen test, and black men of all ages were twice as likely to have high-risk disease as were white men, based on a study of 4 years of data extracted from the Surveillance, Epidemiology and End Results (SEER) database.
The findings make the case for a more personalized approach to screening, Dr. Hong Zhang said at a press briefing during the 2013 Genitourinary Cancers Symposium.
Without prostate-specific antigen (PSA) screening, "we have no other way to detect prostate cancer sufficiently early to have the best chance of helping this group of high-risk patients," said Dr. Zhang of the University of Rochester (N.Y.).
The study brings a bit of context to current PSA screening guidelines, which are "all over the map," according to session moderator Dr. Bruce Roth of Washington University, St. Louis. In 2011, the United States Preventive Services Task Force determined that routine screening harms more men that it helps.
"The American Cancer Society recommends just screening older men and the USPTF recommends that nobody get screened," Dr. Roth said. Based on these results, the presumption that older men will die first of something other than their prostate cancer is not necessarily true. "In fact, a significant number of these men present with high-risk disease. Age is not the greatest determinant of who should and should not be screened."
During 2004-2008, 70,345 men were diagnosed with T1cN0M0 prostate cancer in SEER. Of these, 48% had low-risk disease (PSA less than 10 mg/L or Gleason score of 6 or less), 36% intermediate-risk disease (PSA between 10 mg/L and 20 mg/L or Gleason score 7), and 16% high-risk disease (PSA at least 20 mg/L, or Gleason score of 8 or higher).
The median age of patients with low-risk disease was 67 years; for those with intermediate-risk disease, median age was 70 years; and for high-risk disease, it was 72 years. Men 75 years or older accounted for 12% of the population, but for 24% of intermediate-risk and 26% of high-risk disease.
In a multivariate analysis, Dr. Zhang determined that, compared with younger men, those aged 75 years and older were almost five times more likely to have intermediate-risk disease and nine times more likely to have high-risk disease.
Blacks made up 13% of the low-risk category, 16% of the intermediate-risk category, and 18% of the high-risk category. Compared with whites, blacks were 1.5 times more likely to have intermediate-risk disease and twice as likely to have high-risk disease.
Dr. Zhang and Dr. Roth had no financial disclosures.
Prostate cancers detected during screening are much more likely to be high risk when they affect black men and men aged 75 years or older.
Men over age 74 years were nine times more likely to have high-risk disease after a positive prostate-specific antigen test, and black men of all ages were twice as likely to have high-risk disease as were white men, based on a study of 4 years of data extracted from the Surveillance, Epidemiology and End Results (SEER) database.
The findings make the case for a more personalized approach to screening, Dr. Hong Zhang said at a press briefing during the 2013 Genitourinary Cancers Symposium.
Without prostate-specific antigen (PSA) screening, "we have no other way to detect prostate cancer sufficiently early to have the best chance of helping this group of high-risk patients," said Dr. Zhang of the University of Rochester (N.Y.).
The study brings a bit of context to current PSA screening guidelines, which are "all over the map," according to session moderator Dr. Bruce Roth of Washington University, St. Louis. In 2011, the United States Preventive Services Task Force determined that routine screening harms more men that it helps.
"The American Cancer Society recommends just screening older men and the USPTF recommends that nobody get screened," Dr. Roth said. Based on these results, the presumption that older men will die first of something other than their prostate cancer is not necessarily true. "In fact, a significant number of these men present with high-risk disease. Age is not the greatest determinant of who should and should not be screened."
During 2004-2008, 70,345 men were diagnosed with T1cN0M0 prostate cancer in SEER. Of these, 48% had low-risk disease (PSA less than 10 mg/L or Gleason score of 6 or less), 36% intermediate-risk disease (PSA between 10 mg/L and 20 mg/L or Gleason score 7), and 16% high-risk disease (PSA at least 20 mg/L, or Gleason score of 8 or higher).
The median age of patients with low-risk disease was 67 years; for those with intermediate-risk disease, median age was 70 years; and for high-risk disease, it was 72 years. Men 75 years or older accounted for 12% of the population, but for 24% of intermediate-risk and 26% of high-risk disease.
In a multivariate analysis, Dr. Zhang determined that, compared with younger men, those aged 75 years and older were almost five times more likely to have intermediate-risk disease and nine times more likely to have high-risk disease.
Blacks made up 13% of the low-risk category, 16% of the intermediate-risk category, and 18% of the high-risk category. Compared with whites, blacks were 1.5 times more likely to have intermediate-risk disease and twice as likely to have high-risk disease.
Dr. Zhang and Dr. Roth had no financial disclosures.
Prostate cancers detected during screening are much more likely to be high risk when they affect black men and men aged 75 years or older.
Men over age 74 years were nine times more likely to have high-risk disease after a positive prostate-specific antigen test, and black men of all ages were twice as likely to have high-risk disease as were white men, based on a study of 4 years of data extracted from the Surveillance, Epidemiology and End Results (SEER) database.
The findings make the case for a more personalized approach to screening, Dr. Hong Zhang said at a press briefing during the 2013 Genitourinary Cancers Symposium.
Without prostate-specific antigen (PSA) screening, "we have no other way to detect prostate cancer sufficiently early to have the best chance of helping this group of high-risk patients," said Dr. Zhang of the University of Rochester (N.Y.).
The study brings a bit of context to current PSA screening guidelines, which are "all over the map," according to session moderator Dr. Bruce Roth of Washington University, St. Louis. In 2011, the United States Preventive Services Task Force determined that routine screening harms more men that it helps.
"The American Cancer Society recommends just screening older men and the USPTF recommends that nobody get screened," Dr. Roth said. Based on these results, the presumption that older men will die first of something other than their prostate cancer is not necessarily true. "In fact, a significant number of these men present with high-risk disease. Age is not the greatest determinant of who should and should not be screened."
During 2004-2008, 70,345 men were diagnosed with T1cN0M0 prostate cancer in SEER. Of these, 48% had low-risk disease (PSA less than 10 mg/L or Gleason score of 6 or less), 36% intermediate-risk disease (PSA between 10 mg/L and 20 mg/L or Gleason score 7), and 16% high-risk disease (PSA at least 20 mg/L, or Gleason score of 8 or higher).
The median age of patients with low-risk disease was 67 years; for those with intermediate-risk disease, median age was 70 years; and for high-risk disease, it was 72 years. Men 75 years or older accounted for 12% of the population, but for 24% of intermediate-risk and 26% of high-risk disease.
In a multivariate analysis, Dr. Zhang determined that, compared with younger men, those aged 75 years and older were almost five times more likely to have intermediate-risk disease and nine times more likely to have high-risk disease.
Blacks made up 13% of the low-risk category, 16% of the intermediate-risk category, and 18% of the high-risk category. Compared with whites, blacks were 1.5 times more likely to have intermediate-risk disease and twice as likely to have high-risk disease.
Dr. Zhang and Dr. Roth had no financial disclosures.
FROM THE 2013 GENITOURINARY CANCERS SYMPOSIUM
Major Finding: Men aged 75 years or older accounted for 12% of the population, but for 24% of intermediate-risk and 26% of high-risk disease.
Data Source: The study looked at more than 70,000 men diagnosed with prostate cancer from 2004-2008.
Disclosures: Dr. Zhang had no financial disclosures.
Survival shorter in young gastric cancer patients
Patients with gastric adenocarcinoma who were diagnosed before age 40 years died significantly sooner than did older patients, based on the results of a single-center, retrospective study of 520 cases.
Patients diagnosed before age 40 survived a median of 5 months after diagnosis; 24% were alive at 1 year. Older patients survived a median of 8 months, and 39% were alive at 1 year, reported Dr. Bryan Goldner and his associates.
Surgical exploration was often futile in the younger patients, said Dr. Goldner of Harbor-UCLA Medical Center in Torrance, Calif. Surgery removed all tumors with histologically free margins (R0 resection) in 33% of younger patients, compared with 60% of older patients, he reported in a poster presentation at a meeting on gastrointestinal cancers sponsored by the American Society of Clinical Oncology.
Recent reports in the medical literature suggest that younger patients with gastric adenocarcinoma are more likely to be diagnosed with node-positive and metastatic disease. One retrospective study of 350 patients found that younger patients had more aggressive gastric adenocarcinomas and died sooner than older patients (Arch. Surg. 2009;144:506-10).
A separate retrospective study of 33,236 U.S. patients found that younger patients presented with more advanced gastric adenocarcinoma but had better survival outcomes than older patients when stratified by disease stage (Ann. Surg. Oncol. 2011;18:2800-7).
Gastric cancer might not be suspected in younger patients, which could result in young patients going undiagnosed until their cancers are more advanced, Dr. Goldner suggested.
The meeting was cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Goldner reported having no financial disclosures.
On Twitter @sherryboschert
Patients with gastric adenocarcinoma who were diagnosed before age 40 years died significantly sooner than did older patients, based on the results of a single-center, retrospective study of 520 cases.
Patients diagnosed before age 40 survived a median of 5 months after diagnosis; 24% were alive at 1 year. Older patients survived a median of 8 months, and 39% were alive at 1 year, reported Dr. Bryan Goldner and his associates.
Surgical exploration was often futile in the younger patients, said Dr. Goldner of Harbor-UCLA Medical Center in Torrance, Calif. Surgery removed all tumors with histologically free margins (R0 resection) in 33% of younger patients, compared with 60% of older patients, he reported in a poster presentation at a meeting on gastrointestinal cancers sponsored by the American Society of Clinical Oncology.
Recent reports in the medical literature suggest that younger patients with gastric adenocarcinoma are more likely to be diagnosed with node-positive and metastatic disease. One retrospective study of 350 patients found that younger patients had more aggressive gastric adenocarcinomas and died sooner than older patients (Arch. Surg. 2009;144:506-10).
A separate retrospective study of 33,236 U.S. patients found that younger patients presented with more advanced gastric adenocarcinoma but had better survival outcomes than older patients when stratified by disease stage (Ann. Surg. Oncol. 2011;18:2800-7).
Gastric cancer might not be suspected in younger patients, which could result in young patients going undiagnosed until their cancers are more advanced, Dr. Goldner suggested.
The meeting was cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Goldner reported having no financial disclosures.
On Twitter @sherryboschert
Patients with gastric adenocarcinoma who were diagnosed before age 40 years died significantly sooner than did older patients, based on the results of a single-center, retrospective study of 520 cases.
Patients diagnosed before age 40 survived a median of 5 months after diagnosis; 24% were alive at 1 year. Older patients survived a median of 8 months, and 39% were alive at 1 year, reported Dr. Bryan Goldner and his associates.
Surgical exploration was often futile in the younger patients, said Dr. Goldner of Harbor-UCLA Medical Center in Torrance, Calif. Surgery removed all tumors with histologically free margins (R0 resection) in 33% of younger patients, compared with 60% of older patients, he reported in a poster presentation at a meeting on gastrointestinal cancers sponsored by the American Society of Clinical Oncology.
Recent reports in the medical literature suggest that younger patients with gastric adenocarcinoma are more likely to be diagnosed with node-positive and metastatic disease. One retrospective study of 350 patients found that younger patients had more aggressive gastric adenocarcinomas and died sooner than older patients (Arch. Surg. 2009;144:506-10).
A separate retrospective study of 33,236 U.S. patients found that younger patients presented with more advanced gastric adenocarcinoma but had better survival outcomes than older patients when stratified by disease stage (Ann. Surg. Oncol. 2011;18:2800-7).
Gastric cancer might not be suspected in younger patients, which could result in young patients going undiagnosed until their cancers are more advanced, Dr. Goldner suggested.
The meeting was cosponsored by ASCO, the American Gastroenterological Association Institute, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Goldner reported having no financial disclosures.
On Twitter @sherryboschert
AT A MEETING ON GASTROINTESTINAL CANCERS SPONSORED BY THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: Patients diagnosed with gastric adenocarcinoma before age 40 years lived a median of 5 months; older patients lived a median of 8 months.
Data Source: Retrospective study of 520 patients with gastric cancer at one public hospital.
Disclosures: Dr. Goldner reported having no financial disclosures.
No early cancer risk with donor lungs from heavy smokers
LOS ANGELES – Use of lungs from donors who smoked heavily does not worsen lung transplantation outcomes including risk for lung cancer death, at least in the medium term.
At a median follow-up of 2 years for 5,900 adults who had double-lung transplants, those who received lungs from heavy smokers had an actuarial median overall survival of roughly 5.5 years, and their lung function was essentially the same as that of patients who received lungs from other donors, Dr. Sharven Taghavi reported at the annual meeting of the Society of Thoracic Surgeons.
The study data came from the United Network for Organ Sharing (UNOS) database. A team led by Dr. Taghavi, of Temple University Hospital in Philadelphia, compared data for double-lung transplants from 2005-2011, comparing donors with a history of smoking exceeding 20 pack-years with other donors.
About 13% of the study patients received lungs from donors who had smoked heavily. Compared with other recipients, these recipients were more likely to have a primary diagnosis of chronic obstructive pulmonary disease and less likely to have a diagnosis of idiopathic pulmonary fibrosis. Otherwise, they were similar.
The rate of deaths due to cancer was based on case reports, as UNOS does not capture this outcome. Cancer deaths were 5.8% among recipients of lungs from heavy smokers and 3.6% among other recipients.
"There is a fairly low capture rate for this field, so it’s difficult to draw significant conclusions from it," cautioned Dr. Taghavi.
Patients who received lungs from heavy smokers had a 1-day longer length of stay in the hospital (18 days vs. 17 days), which "may not really be clinically relevant." Rates of acute rejection during hospitalization were comparable (10.7% vs. 8.8%), as was post-transplant airway dehiscence (1.8% vs. 1.8%).
Post-transplant peak forced expiratory volume in 1 second (FEV1) was the same (80% vs. 79%), as was decline in this measure over time. Median duration of freedom from bronchiolitis obliterans syndrome was 1,583 days vs. 1,827 days.
Risk-adjusted median all-cause survival – the study’s primary endpoint – did not differ significantly between the recipients given lungs from donors who smoked heavily and the other recipients (2,043 vs. 1,928 days).
The rate of cancer deaths did not differ significantly; however, the follow-up time is too short to address this concern in a meaningful way, Dr. Taghavi said.
"Currently, we recommend when evaluating a donor who has a heavy smoking history, that they undergo a thorough examination for lung tumors or evidence of cancer. This includes obtaining a chest x-ray, CT scans, and bronchoscopies. In addition, when the lungs are procured, they should undergo a very thorough visual inspection," he advised.
"Informed consent is very important. You have to discuss the donor’s smoking status with the recipient and explain the risks and the benefits," Dr. Taghavi said. Lung cancer risk, given the donor’s history, is about 1% to 2% annually, and that needs to be considered against the high likelihood of dying within 1 or 2 years without a transplant.
"One thing that is unquestionable is that survival will be better accepting these lungs than it will be sitting on a waiting list," he added. Only about half of the people listed for lung transplant in the United States each year actually undergo the surgery.
Recipients of lungs from heavy smokers do not need any extra follow-up or surveillance, as they are already diligently tested and monitored, according to Dr. Taghavi. The recipient’s immunosuppression does theoretically put one at additional risk for lung cancer.
Current guidelines of the International Society of Heart and Lung Transplantation advise against considering use of lungs from donors who have a smoking history of more than 20 pack-years, Dr. Taghavi noted. But he stopped short of saying that the study should prompt a formal revision of those guidelines.
"I think the findings start the conversation," he commented. "We should consider looking at these potential donors," especially when a recipient’s situation is dire.
Dr. Taghavi disclosed no conflicts of interest.
LOS ANGELES – Use of lungs from donors who smoked heavily does not worsen lung transplantation outcomes including risk for lung cancer death, at least in the medium term.
At a median follow-up of 2 years for 5,900 adults who had double-lung transplants, those who received lungs from heavy smokers had an actuarial median overall survival of roughly 5.5 years, and their lung function was essentially the same as that of patients who received lungs from other donors, Dr. Sharven Taghavi reported at the annual meeting of the Society of Thoracic Surgeons.
The study data came from the United Network for Organ Sharing (UNOS) database. A team led by Dr. Taghavi, of Temple University Hospital in Philadelphia, compared data for double-lung transplants from 2005-2011, comparing donors with a history of smoking exceeding 20 pack-years with other donors.
About 13% of the study patients received lungs from donors who had smoked heavily. Compared with other recipients, these recipients were more likely to have a primary diagnosis of chronic obstructive pulmonary disease and less likely to have a diagnosis of idiopathic pulmonary fibrosis. Otherwise, they were similar.
The rate of deaths due to cancer was based on case reports, as UNOS does not capture this outcome. Cancer deaths were 5.8% among recipients of lungs from heavy smokers and 3.6% among other recipients.
"There is a fairly low capture rate for this field, so it’s difficult to draw significant conclusions from it," cautioned Dr. Taghavi.
Patients who received lungs from heavy smokers had a 1-day longer length of stay in the hospital (18 days vs. 17 days), which "may not really be clinically relevant." Rates of acute rejection during hospitalization were comparable (10.7% vs. 8.8%), as was post-transplant airway dehiscence (1.8% vs. 1.8%).
Post-transplant peak forced expiratory volume in 1 second (FEV1) was the same (80% vs. 79%), as was decline in this measure over time. Median duration of freedom from bronchiolitis obliterans syndrome was 1,583 days vs. 1,827 days.
Risk-adjusted median all-cause survival – the study’s primary endpoint – did not differ significantly between the recipients given lungs from donors who smoked heavily and the other recipients (2,043 vs. 1,928 days).
The rate of cancer deaths did not differ significantly; however, the follow-up time is too short to address this concern in a meaningful way, Dr. Taghavi said.
"Currently, we recommend when evaluating a donor who has a heavy smoking history, that they undergo a thorough examination for lung tumors or evidence of cancer. This includes obtaining a chest x-ray, CT scans, and bronchoscopies. In addition, when the lungs are procured, they should undergo a very thorough visual inspection," he advised.
"Informed consent is very important. You have to discuss the donor’s smoking status with the recipient and explain the risks and the benefits," Dr. Taghavi said. Lung cancer risk, given the donor’s history, is about 1% to 2% annually, and that needs to be considered against the high likelihood of dying within 1 or 2 years without a transplant.
"One thing that is unquestionable is that survival will be better accepting these lungs than it will be sitting on a waiting list," he added. Only about half of the people listed for lung transplant in the United States each year actually undergo the surgery.
Recipients of lungs from heavy smokers do not need any extra follow-up or surveillance, as they are already diligently tested and monitored, according to Dr. Taghavi. The recipient’s immunosuppression does theoretically put one at additional risk for lung cancer.
Current guidelines of the International Society of Heart and Lung Transplantation advise against considering use of lungs from donors who have a smoking history of more than 20 pack-years, Dr. Taghavi noted. But he stopped short of saying that the study should prompt a formal revision of those guidelines.
"I think the findings start the conversation," he commented. "We should consider looking at these potential donors," especially when a recipient’s situation is dire.
Dr. Taghavi disclosed no conflicts of interest.
LOS ANGELES – Use of lungs from donors who smoked heavily does not worsen lung transplantation outcomes including risk for lung cancer death, at least in the medium term.
At a median follow-up of 2 years for 5,900 adults who had double-lung transplants, those who received lungs from heavy smokers had an actuarial median overall survival of roughly 5.5 years, and their lung function was essentially the same as that of patients who received lungs from other donors, Dr. Sharven Taghavi reported at the annual meeting of the Society of Thoracic Surgeons.
The study data came from the United Network for Organ Sharing (UNOS) database. A team led by Dr. Taghavi, of Temple University Hospital in Philadelphia, compared data for double-lung transplants from 2005-2011, comparing donors with a history of smoking exceeding 20 pack-years with other donors.
About 13% of the study patients received lungs from donors who had smoked heavily. Compared with other recipients, these recipients were more likely to have a primary diagnosis of chronic obstructive pulmonary disease and less likely to have a diagnosis of idiopathic pulmonary fibrosis. Otherwise, they were similar.
The rate of deaths due to cancer was based on case reports, as UNOS does not capture this outcome. Cancer deaths were 5.8% among recipients of lungs from heavy smokers and 3.6% among other recipients.
"There is a fairly low capture rate for this field, so it’s difficult to draw significant conclusions from it," cautioned Dr. Taghavi.
Patients who received lungs from heavy smokers had a 1-day longer length of stay in the hospital (18 days vs. 17 days), which "may not really be clinically relevant." Rates of acute rejection during hospitalization were comparable (10.7% vs. 8.8%), as was post-transplant airway dehiscence (1.8% vs. 1.8%).
Post-transplant peak forced expiratory volume in 1 second (FEV1) was the same (80% vs. 79%), as was decline in this measure over time. Median duration of freedom from bronchiolitis obliterans syndrome was 1,583 days vs. 1,827 days.
Risk-adjusted median all-cause survival – the study’s primary endpoint – did not differ significantly between the recipients given lungs from donors who smoked heavily and the other recipients (2,043 vs. 1,928 days).
The rate of cancer deaths did not differ significantly; however, the follow-up time is too short to address this concern in a meaningful way, Dr. Taghavi said.
"Currently, we recommend when evaluating a donor who has a heavy smoking history, that they undergo a thorough examination for lung tumors or evidence of cancer. This includes obtaining a chest x-ray, CT scans, and bronchoscopies. In addition, when the lungs are procured, they should undergo a very thorough visual inspection," he advised.
"Informed consent is very important. You have to discuss the donor’s smoking status with the recipient and explain the risks and the benefits," Dr. Taghavi said. Lung cancer risk, given the donor’s history, is about 1% to 2% annually, and that needs to be considered against the high likelihood of dying within 1 or 2 years without a transplant.
"One thing that is unquestionable is that survival will be better accepting these lungs than it will be sitting on a waiting list," he added. Only about half of the people listed for lung transplant in the United States each year actually undergo the surgery.
Recipients of lungs from heavy smokers do not need any extra follow-up or surveillance, as they are already diligently tested and monitored, according to Dr. Taghavi. The recipient’s immunosuppression does theoretically put one at additional risk for lung cancer.
Current guidelines of the International Society of Heart and Lung Transplantation advise against considering use of lungs from donors who have a smoking history of more than 20 pack-years, Dr. Taghavi noted. But he stopped short of saying that the study should prompt a formal revision of those guidelines.
"I think the findings start the conversation," he commented. "We should consider looking at these potential donors," especially when a recipient’s situation is dire.
Dr. Taghavi disclosed no conflicts of interest.
AT THE ANNUAL MEETING OF THE SOCIETY OF THROACIC SURGEONS
Major Finding: Risk-adjusted median all-cause survival did not differ significantly between patients given lungs from donors who smoked heavily and those receiving lungs from donors who did not smoke heavily (2,043 vs. 1,928 days).
Data Source: An observational cohort study of 5,900 adult primary double-lung transplant recipients in the UNOS database
Disclosures: Dr. Taghavi disclosed no relevant conflicts of interest.
Assay may target early lung cancers for adjuvant therapy
LOS ANGELES – A novel genetic assay helps identify patients with early, aggressive lung cancer who might benefit from adjuvant therapy.
The assay, marketed as Pervino Lung RS by Life Technologies, is the only lung cancer signature to undergo blinded validation in two large cohorts from different countries, one in the United States and one in China (Lancet 2012;379:823-32).
It assesses expression of 14 genes involved in lung cancer tumorigenesis, including ones on the EGFR and KRAS signaling pathways. The assay provides considerably more prognostic information than do conventional criteria proposed by the National Comprehensive Cancer Network (NCCN) as defining high-risk tumors warranting treatment, according to Dr. Johannes R. Kratz, who reported the data at the annual meeting of the Society of Thoracic Surgeons.
The assay results were used to stratify the 269 study patients who had undergone resection of T1a node-negative and nonmetastatic, nonsquamous, non–small cell lung cancer (NSCLC) into groups with distinctly different 5-year survival rates.
Compared with their counterparts in the low-risk group, those in the intermediate- and high-risk groups had a respective doubling and more than tripling of the risk of death, said Dr. Kratz, who was the study’s lead investigator.
As a result of recommendations for CT screening in patients at high risk for lung cancer, resections of small node-negative tumors that are in fact deadly are likely to increase, he observed. Nearly 30% of all patients with stage IA tumors – the lowest level in the current classification system – will nonetheless die in the subsequent 5 years.
"These tumors with highly aggressive tumor biology can now be identified reliably with a prognostic gene signature. The identification of these small but deadly tumors may allow for personalized patient prognosis and could allow us to maximize the benefit of the early detection of these small but deadly tumors via low-dose CT screening," he added.
The current postoperative standard of care for stage IA disease is simply observation, according to Dr. Kratz, a former surgical resident at the Massachusetts General Hospital in Boston, and now a postdoctoral fellow at the University of California, San Francisco.
However, "we should strongly consider changing the way we think about patients with high-risk T1a tumors," he recommended. To that end, a randomized controlled trial of assay-guided adjuvant chemotherapy for early lung cancer is underway in China among roughly 1,000 patients.
Dr. Kratz said that studies to date have not examined a potential prognostic role of the assay in EGFR (epidermal growth factor receptor) mutations. "We haven’t performed an additional mutation analysis on these patients’ EGFR. The original assay was designed to work on patients with resected paraffin-embedded specimens and not fresh-frozen tissue specimens. As a result, it is difficult for us to do extensive EGFR mutation analysis. But that’s definitely something to consider, and it would be nice to explore that association."
It remains to be seen whether the assay, in fact, predicts chemotherapy benefit, he acknowledged in a related press conference. But research has suggested that such prognostic signatures in lung cancer are also predictive (J. Clin. Oncol. 2010;28:4417-24). "That is what we hope to show in the China trial as well," he said.
In the reported study, patients with T1a tumors were drawn from the initial validation cohorts. Fully 40% were under age 60. "This is important, because ... we’d like to be more aggressive in younger patients, both because they can tolerate it and we are more likely to treat them more aggressively," he noted.
The patients’ actual 5-year mortality rate was 32% overall, showing that "these tumors are as deadly as advertised."
The main study results, reported at the meeting and also published (JAMA 2012;308:1629-31), showed that the 5-year actuarial overall survival was 83%, 69%, and 52% among patients in assay-defined low-, medium-, and high-risk groups, respectively (P less than .0001).
In multivariate analyses, relative to their counterparts in the low-risk group, patients in the intermediate-risk group had a 2.0-fold higher risk of death (P = .04) and patients in the high-risk group had a 3.3-fold higher risk (P = .00).
The assay also showed good risk discrimination in analyses restricted to the smallest of tumors, those measuring 1.5 cm or less (P = .001 for difference across groups) and even those measuring 1.0 cm or less (P = .008).
And when compared with tumor size alone, the combination of the assay and tumor size significantly improved on the identification of patients who died (c-statistic, 0.68 vs. 0.57; P less than .0001).
Although these T1a tumors can be ablated nonoperatively, their genetic makeup offers a rich source of information about their subsequent behavior, Dr. Kratz said.
"Despite the popularity and endorsement of our radiology colleagues for techniques such as stereotactic radiation for small T1aN0M0 tumors, we should remember that these techniques don’t provide us with potentially important lung tissue that can provide prognostic and predictive information," he commented.
Dr. Kratz disclosed that he has been a consultant for Pinpoint Genomics, the company that developed the assay, and is a consultant for Life Technologies, which has acquired Pinpoint Genomics.
LOS ANGELES – A novel genetic assay helps identify patients with early, aggressive lung cancer who might benefit from adjuvant therapy.
The assay, marketed as Pervino Lung RS by Life Technologies, is the only lung cancer signature to undergo blinded validation in two large cohorts from different countries, one in the United States and one in China (Lancet 2012;379:823-32).
It assesses expression of 14 genes involved in lung cancer tumorigenesis, including ones on the EGFR and KRAS signaling pathways. The assay provides considerably more prognostic information than do conventional criteria proposed by the National Comprehensive Cancer Network (NCCN) as defining high-risk tumors warranting treatment, according to Dr. Johannes R. Kratz, who reported the data at the annual meeting of the Society of Thoracic Surgeons.
The assay results were used to stratify the 269 study patients who had undergone resection of T1a node-negative and nonmetastatic, nonsquamous, non–small cell lung cancer (NSCLC) into groups with distinctly different 5-year survival rates.
Compared with their counterparts in the low-risk group, those in the intermediate- and high-risk groups had a respective doubling and more than tripling of the risk of death, said Dr. Kratz, who was the study’s lead investigator.
As a result of recommendations for CT screening in patients at high risk for lung cancer, resections of small node-negative tumors that are in fact deadly are likely to increase, he observed. Nearly 30% of all patients with stage IA tumors – the lowest level in the current classification system – will nonetheless die in the subsequent 5 years.
"These tumors with highly aggressive tumor biology can now be identified reliably with a prognostic gene signature. The identification of these small but deadly tumors may allow for personalized patient prognosis and could allow us to maximize the benefit of the early detection of these small but deadly tumors via low-dose CT screening," he added.
The current postoperative standard of care for stage IA disease is simply observation, according to Dr. Kratz, a former surgical resident at the Massachusetts General Hospital in Boston, and now a postdoctoral fellow at the University of California, San Francisco.
However, "we should strongly consider changing the way we think about patients with high-risk T1a tumors," he recommended. To that end, a randomized controlled trial of assay-guided adjuvant chemotherapy for early lung cancer is underway in China among roughly 1,000 patients.
Dr. Kratz said that studies to date have not examined a potential prognostic role of the assay in EGFR (epidermal growth factor receptor) mutations. "We haven’t performed an additional mutation analysis on these patients’ EGFR. The original assay was designed to work on patients with resected paraffin-embedded specimens and not fresh-frozen tissue specimens. As a result, it is difficult for us to do extensive EGFR mutation analysis. But that’s definitely something to consider, and it would be nice to explore that association."
It remains to be seen whether the assay, in fact, predicts chemotherapy benefit, he acknowledged in a related press conference. But research has suggested that such prognostic signatures in lung cancer are also predictive (J. Clin. Oncol. 2010;28:4417-24). "That is what we hope to show in the China trial as well," he said.
In the reported study, patients with T1a tumors were drawn from the initial validation cohorts. Fully 40% were under age 60. "This is important, because ... we’d like to be more aggressive in younger patients, both because they can tolerate it and we are more likely to treat them more aggressively," he noted.
The patients’ actual 5-year mortality rate was 32% overall, showing that "these tumors are as deadly as advertised."
The main study results, reported at the meeting and also published (JAMA 2012;308:1629-31), showed that the 5-year actuarial overall survival was 83%, 69%, and 52% among patients in assay-defined low-, medium-, and high-risk groups, respectively (P less than .0001).
In multivariate analyses, relative to their counterparts in the low-risk group, patients in the intermediate-risk group had a 2.0-fold higher risk of death (P = .04) and patients in the high-risk group had a 3.3-fold higher risk (P = .00).
The assay also showed good risk discrimination in analyses restricted to the smallest of tumors, those measuring 1.5 cm or less (P = .001 for difference across groups) and even those measuring 1.0 cm or less (P = .008).
And when compared with tumor size alone, the combination of the assay and tumor size significantly improved on the identification of patients who died (c-statistic, 0.68 vs. 0.57; P less than .0001).
Although these T1a tumors can be ablated nonoperatively, their genetic makeup offers a rich source of information about their subsequent behavior, Dr. Kratz said.
"Despite the popularity and endorsement of our radiology colleagues for techniques such as stereotactic radiation for small T1aN0M0 tumors, we should remember that these techniques don’t provide us with potentially important lung tissue that can provide prognostic and predictive information," he commented.
Dr. Kratz disclosed that he has been a consultant for Pinpoint Genomics, the company that developed the assay, and is a consultant for Life Technologies, which has acquired Pinpoint Genomics.
LOS ANGELES – A novel genetic assay helps identify patients with early, aggressive lung cancer who might benefit from adjuvant therapy.
The assay, marketed as Pervino Lung RS by Life Technologies, is the only lung cancer signature to undergo blinded validation in two large cohorts from different countries, one in the United States and one in China (Lancet 2012;379:823-32).
It assesses expression of 14 genes involved in lung cancer tumorigenesis, including ones on the EGFR and KRAS signaling pathways. The assay provides considerably more prognostic information than do conventional criteria proposed by the National Comprehensive Cancer Network (NCCN) as defining high-risk tumors warranting treatment, according to Dr. Johannes R. Kratz, who reported the data at the annual meeting of the Society of Thoracic Surgeons.
The assay results were used to stratify the 269 study patients who had undergone resection of T1a node-negative and nonmetastatic, nonsquamous, non–small cell lung cancer (NSCLC) into groups with distinctly different 5-year survival rates.
Compared with their counterparts in the low-risk group, those in the intermediate- and high-risk groups had a respective doubling and more than tripling of the risk of death, said Dr. Kratz, who was the study’s lead investigator.
As a result of recommendations for CT screening in patients at high risk for lung cancer, resections of small node-negative tumors that are in fact deadly are likely to increase, he observed. Nearly 30% of all patients with stage IA tumors – the lowest level in the current classification system – will nonetheless die in the subsequent 5 years.
"These tumors with highly aggressive tumor biology can now be identified reliably with a prognostic gene signature. The identification of these small but deadly tumors may allow for personalized patient prognosis and could allow us to maximize the benefit of the early detection of these small but deadly tumors via low-dose CT screening," he added.
The current postoperative standard of care for stage IA disease is simply observation, according to Dr. Kratz, a former surgical resident at the Massachusetts General Hospital in Boston, and now a postdoctoral fellow at the University of California, San Francisco.
However, "we should strongly consider changing the way we think about patients with high-risk T1a tumors," he recommended. To that end, a randomized controlled trial of assay-guided adjuvant chemotherapy for early lung cancer is underway in China among roughly 1,000 patients.
Dr. Kratz said that studies to date have not examined a potential prognostic role of the assay in EGFR (epidermal growth factor receptor) mutations. "We haven’t performed an additional mutation analysis on these patients’ EGFR. The original assay was designed to work on patients with resected paraffin-embedded specimens and not fresh-frozen tissue specimens. As a result, it is difficult for us to do extensive EGFR mutation analysis. But that’s definitely something to consider, and it would be nice to explore that association."
It remains to be seen whether the assay, in fact, predicts chemotherapy benefit, he acknowledged in a related press conference. But research has suggested that such prognostic signatures in lung cancer are also predictive (J. Clin. Oncol. 2010;28:4417-24). "That is what we hope to show in the China trial as well," he said.
In the reported study, patients with T1a tumors were drawn from the initial validation cohorts. Fully 40% were under age 60. "This is important, because ... we’d like to be more aggressive in younger patients, both because they can tolerate it and we are more likely to treat them more aggressively," he noted.
The patients’ actual 5-year mortality rate was 32% overall, showing that "these tumors are as deadly as advertised."
The main study results, reported at the meeting and also published (JAMA 2012;308:1629-31), showed that the 5-year actuarial overall survival was 83%, 69%, and 52% among patients in assay-defined low-, medium-, and high-risk groups, respectively (P less than .0001).
In multivariate analyses, relative to their counterparts in the low-risk group, patients in the intermediate-risk group had a 2.0-fold higher risk of death (P = .04) and patients in the high-risk group had a 3.3-fold higher risk (P = .00).
The assay also showed good risk discrimination in analyses restricted to the smallest of tumors, those measuring 1.5 cm or less (P = .001 for difference across groups) and even those measuring 1.0 cm or less (P = .008).
And when compared with tumor size alone, the combination of the assay and tumor size significantly improved on the identification of patients who died (c-statistic, 0.68 vs. 0.57; P less than .0001).
Although these T1a tumors can be ablated nonoperatively, their genetic makeup offers a rich source of information about their subsequent behavior, Dr. Kratz said.
"Despite the popularity and endorsement of our radiology colleagues for techniques such as stereotactic radiation for small T1aN0M0 tumors, we should remember that these techniques don’t provide us with potentially important lung tissue that can provide prognostic and predictive information," he commented.
Dr. Kratz disclosed that he has been a consultant for Pinpoint Genomics, the company that developed the assay, and is a consultant for Life Technologies, which has acquired Pinpoint Genomics.
AT THE ANNUAL MEETING OF THE SOCIETY OF THORACIC SURGEONS
Major Finding: The 5-year actuarial overall survival was 83%, 69%, and 52% among patients in assay-defined low-, medium-, and high-risk groups, respectively (P less than .0001).
Data Source: A cohort study of 269 patients who underwent resection of T1aN0M0 nonsquamous NSCLC.
Disclosures: Dr. Kratz disclosed that he has been a consultant for Pinpoint Genomics, the company that developed the assay, and is a consultant for Life Technologies, which has acquired Pinpoint Genomics.
The American College of Surgeons: The next hundred years
The American College of Surgeons (ACS) is in its centennial year. As part of our commemoration of this historic occasion, we are looking back not only at some of the remarkable achievements of the College’s founders, but also ahead to see what the next hundred years may hold for surgery. Four issues of pressing urgency are access to care, rural surgery (workforce shortage/maldistribution), surgical education, and internationalism – all of which are closely connected.
Access
The College is committed to ensuring that surgical patients have access to high-quality, safe, appropriate, and affordable surgical care. Barriers to receiving this level of care–in addition to gender, race, age and income–include lack of insurance, long wait times, geography, and maldistribution or shortages of surgeons.
As the United States proceeds in implementing the Affordable Care Act, the ACS must speak wisely and forcefully about what does and does not work for surgical patients with respect to public policy. In matters political we must always advocate for what is best for our patients. When we do that we will never be wrong.
In our discussions with legislators and policymakers, we should focus on the “Value Proposition,” which is Value = Quality/Cost. The surprise in this proposition is that increasing quality decreases cost as best practices come into play. Inappropriate variation–that is, waste–is eliminated. One good example of the College’s contributions to fulfillment of this proposition is the development and proliferation of the ACS National Surgical Quality Improvement Program.
Rural health care
Rural communities often bear the brunt of surgeon shortages and maldistribution. For surgical trainees who are suited to rural life, we must provide support and broad training.
The College has a model that may be useful in addressing surgeon shortages and maldistribution. A hallmark of the ACS Committee on Trauma’s (COT) Inclusive Trauma System Model is regionalization, which ensures that the right patient gets to the right place in the right time. We should ensure that similar systems are in place to support rural surgeons in caring for patients when they can, but also to move patients out through established channels to a higher level of care when appropriate. The newly formed ACS Advisory Council for Rural Surgery is exploring these and other opportunities and will help develop policies and programs that are supportive of rural surgeons and their patients.
Surgical education
As wonderfully as the Halsted model worked for training 20th century surgeons, it does not fit the 21st century reality. It was outdated even before the introduction of the 80-hour workweek. And then there is debt. The average U.S. medical graduate has debt exceeding $100,000, and nearly a quarter owe $200,000 or more.
Meanwhile, young surgeons are appropriately seeking a balanced lifestyle while being expected to lead multidisciplinary teams caring for ever-more complex surgical disease. I believe the ACS, the specialty societies, the American Board of Surgery, the American Surgical Association, the Royal College of Physicians and Surgeons of Canada, and all international surgical leaders should collaborate in recreating surgical education for this century.
Internationalism
The world is flat, and all these concerns about access, rural surgery, and surgical education are profoundly international as well. The Advanced Trauma Life Support® course (ATLS®) is the College’s most widespread international program, with 1.3 million physicians trained in 63 countries since the program began in 1980. Haile T. Debas, MD, FACS, has said that trauma is a global endemic. The ACS, through the Committee on Trauma, must address this global endemic in peace, war, and disaster. As Dr. Debas has said, “We should have a diplomacy of health,” and there is no better example of fulfillment of this policy in the ACS than the COT’s ATLS program. ATLS is truly an ACS international phalanx.
International collaboration is essential to the future of our profession. For that reason, I have made a high priority during my presidential year of supporting the excellent work of the ACS International Relations Committee and of collaborating with the leaders of international surgical colleges and societies. To this end, the ACS will host a meeting of Presidents of international surgical societies at our Washington Office in July 2013 where we will seek solutions to common problems. We have much to do together.
Because there are serious challenges ahead in this new century, the ACS needs diverse leadership at every level. It can be a wonderful two-way street, with younger Fellows contributing their energy and fresh vision, and the more senior among us offering support and crucial leads as to how things get done. My greatest wish for all Fellows of the American College of Surgeons is the joy of a life in surgery that has been mine.
Dr. Eastman is President of the American College of Surgeons.
The American College of Surgeons (ACS) is in its centennial year. As part of our commemoration of this historic occasion, we are looking back not only at some of the remarkable achievements of the College’s founders, but also ahead to see what the next hundred years may hold for surgery. Four issues of pressing urgency are access to care, rural surgery (workforce shortage/maldistribution), surgical education, and internationalism – all of which are closely connected.
Access
The College is committed to ensuring that surgical patients have access to high-quality, safe, appropriate, and affordable surgical care. Barriers to receiving this level of care–in addition to gender, race, age and income–include lack of insurance, long wait times, geography, and maldistribution or shortages of surgeons.
As the United States proceeds in implementing the Affordable Care Act, the ACS must speak wisely and forcefully about what does and does not work for surgical patients with respect to public policy. In matters political we must always advocate for what is best for our patients. When we do that we will never be wrong.
In our discussions with legislators and policymakers, we should focus on the “Value Proposition,” which is Value = Quality/Cost. The surprise in this proposition is that increasing quality decreases cost as best practices come into play. Inappropriate variation–that is, waste–is eliminated. One good example of the College’s contributions to fulfillment of this proposition is the development and proliferation of the ACS National Surgical Quality Improvement Program.
Rural health care
Rural communities often bear the brunt of surgeon shortages and maldistribution. For surgical trainees who are suited to rural life, we must provide support and broad training.
The College has a model that may be useful in addressing surgeon shortages and maldistribution. A hallmark of the ACS Committee on Trauma’s (COT) Inclusive Trauma System Model is regionalization, which ensures that the right patient gets to the right place in the right time. We should ensure that similar systems are in place to support rural surgeons in caring for patients when they can, but also to move patients out through established channels to a higher level of care when appropriate. The newly formed ACS Advisory Council for Rural Surgery is exploring these and other opportunities and will help develop policies and programs that are supportive of rural surgeons and their patients.
Surgical education
As wonderfully as the Halsted model worked for training 20th century surgeons, it does not fit the 21st century reality. It was outdated even before the introduction of the 80-hour workweek. And then there is debt. The average U.S. medical graduate has debt exceeding $100,000, and nearly a quarter owe $200,000 or more.
Meanwhile, young surgeons are appropriately seeking a balanced lifestyle while being expected to lead multidisciplinary teams caring for ever-more complex surgical disease. I believe the ACS, the specialty societies, the American Board of Surgery, the American Surgical Association, the Royal College of Physicians and Surgeons of Canada, and all international surgical leaders should collaborate in recreating surgical education for this century.
Internationalism
The world is flat, and all these concerns about access, rural surgery, and surgical education are profoundly international as well. The Advanced Trauma Life Support® course (ATLS®) is the College’s most widespread international program, with 1.3 million physicians trained in 63 countries since the program began in 1980. Haile T. Debas, MD, FACS, has said that trauma is a global endemic. The ACS, through the Committee on Trauma, must address this global endemic in peace, war, and disaster. As Dr. Debas has said, “We should have a diplomacy of health,” and there is no better example of fulfillment of this policy in the ACS than the COT’s ATLS program. ATLS is truly an ACS international phalanx.
International collaboration is essential to the future of our profession. For that reason, I have made a high priority during my presidential year of supporting the excellent work of the ACS International Relations Committee and of collaborating with the leaders of international surgical colleges and societies. To this end, the ACS will host a meeting of Presidents of international surgical societies at our Washington Office in July 2013 where we will seek solutions to common problems. We have much to do together.
Because there are serious challenges ahead in this new century, the ACS needs diverse leadership at every level. It can be a wonderful two-way street, with younger Fellows contributing their energy and fresh vision, and the more senior among us offering support and crucial leads as to how things get done. My greatest wish for all Fellows of the American College of Surgeons is the joy of a life in surgery that has been mine.
Dr. Eastman is President of the American College of Surgeons.
The American College of Surgeons (ACS) is in its centennial year. As part of our commemoration of this historic occasion, we are looking back not only at some of the remarkable achievements of the College’s founders, but also ahead to see what the next hundred years may hold for surgery. Four issues of pressing urgency are access to care, rural surgery (workforce shortage/maldistribution), surgical education, and internationalism – all of which are closely connected.
Access
The College is committed to ensuring that surgical patients have access to high-quality, safe, appropriate, and affordable surgical care. Barriers to receiving this level of care–in addition to gender, race, age and income–include lack of insurance, long wait times, geography, and maldistribution or shortages of surgeons.
As the United States proceeds in implementing the Affordable Care Act, the ACS must speak wisely and forcefully about what does and does not work for surgical patients with respect to public policy. In matters political we must always advocate for what is best for our patients. When we do that we will never be wrong.
In our discussions with legislators and policymakers, we should focus on the “Value Proposition,” which is Value = Quality/Cost. The surprise in this proposition is that increasing quality decreases cost as best practices come into play. Inappropriate variation–that is, waste–is eliminated. One good example of the College’s contributions to fulfillment of this proposition is the development and proliferation of the ACS National Surgical Quality Improvement Program.
Rural health care
Rural communities often bear the brunt of surgeon shortages and maldistribution. For surgical trainees who are suited to rural life, we must provide support and broad training.
The College has a model that may be useful in addressing surgeon shortages and maldistribution. A hallmark of the ACS Committee on Trauma’s (COT) Inclusive Trauma System Model is regionalization, which ensures that the right patient gets to the right place in the right time. We should ensure that similar systems are in place to support rural surgeons in caring for patients when they can, but also to move patients out through established channels to a higher level of care when appropriate. The newly formed ACS Advisory Council for Rural Surgery is exploring these and other opportunities and will help develop policies and programs that are supportive of rural surgeons and their patients.
Surgical education
As wonderfully as the Halsted model worked for training 20th century surgeons, it does not fit the 21st century reality. It was outdated even before the introduction of the 80-hour workweek. And then there is debt. The average U.S. medical graduate has debt exceeding $100,000, and nearly a quarter owe $200,000 or more.
Meanwhile, young surgeons are appropriately seeking a balanced lifestyle while being expected to lead multidisciplinary teams caring for ever-more complex surgical disease. I believe the ACS, the specialty societies, the American Board of Surgery, the American Surgical Association, the Royal College of Physicians and Surgeons of Canada, and all international surgical leaders should collaborate in recreating surgical education for this century.
Internationalism
The world is flat, and all these concerns about access, rural surgery, and surgical education are profoundly international as well. The Advanced Trauma Life Support® course (ATLS®) is the College’s most widespread international program, with 1.3 million physicians trained in 63 countries since the program began in 1980. Haile T. Debas, MD, FACS, has said that trauma is a global endemic. The ACS, through the Committee on Trauma, must address this global endemic in peace, war, and disaster. As Dr. Debas has said, “We should have a diplomacy of health,” and there is no better example of fulfillment of this policy in the ACS than the COT’s ATLS program. ATLS is truly an ACS international phalanx.
International collaboration is essential to the future of our profession. For that reason, I have made a high priority during my presidential year of supporting the excellent work of the ACS International Relations Committee and of collaborating with the leaders of international surgical colleges and societies. To this end, the ACS will host a meeting of Presidents of international surgical societies at our Washington Office in July 2013 where we will seek solutions to common problems. We have much to do together.
Because there are serious challenges ahead in this new century, the ACS needs diverse leadership at every level. It can be a wonderful two-way street, with younger Fellows contributing their energy and fresh vision, and the more senior among us offering support and crucial leads as to how things get done. My greatest wish for all Fellows of the American College of Surgeons is the joy of a life in surgery that has been mine.
Dr. Eastman is President of the American College of Surgeons.
Breast-conserving therapy improved survival over mastectomy
Women who underwent lumpectomy for stage I or II breast cancer were 28% less likely to die from any cause and up to 16% less likely to die from breast cancer, compared with women who underwent mastectomy, Dr. E. Shelley Hwang and her colleagues reported Jan. 28 in the online issue of Cancer.
The 3-year disease-specific survival benefit for breast-conserving therapy (BCT) was most pronounced for chronic respiratory disease, for which lumpectomy was associated with a 54% decreased risk of death; heart disease, with a 49% decreased risk; and cerebrovascular disease, with a decreased risk of 36%.
The survival benefit varied with age, tumor size, and hormone receptor status but was significant in every subgroup, Dr. Hwang and her colleagues wrote (Cancer Jan. 28, 2013 [doi:10.1002/cncr.27795]).
"Our findings have important implications for understanding the overall benefit of BCT at the population level," wrote Dr. Hwang of the Duke University Comprehensive Cancer Center, Durham, N.C., and her coauthors. "These results provide confidence in the efficacy of BCT even among younger patients with HR-negative disease thought to be at relatively higher risk for local failure."
The team reviewed the records of 112,154 women who were treated for a new, unilateral T1/T2 stage I or II breast cancer diagnosed from 1990 to 2004. Most of these women (61,771) underwent a lumpectomy and radiation; the remainder underwent mastectomy without radiation. They were followed for a median of 10 years.
About a quarter of each group was younger than 50 years when diagnosed; another quarter was aged 70-80 years. A small portion (6%) was younger than 40 years.
Surgical approach evolved over the study period. Breast-conserving therapy increased from 37% in 1990-92 to 62% by 2002-04, while the rate of mastectomies declined.
The median tumor size was 1.5 cm; patients with larger tumors were more likely to have mastectomies. "Interestingly, the use of BCT varied by age even among tumors [smaller than and equal to] 2 cm where the youngest and oldest age groups had the lowest BCT rate. In [tumors larger than] 2 cm, BCT rate declined by age," Dr. Hwang and her associates said.
Over the follow-up period, there were 31,416 deaths; 39% of these were caused by breast cancer; 5-year overall survival was 89%.
To further explore the treatment-mortality interaction, the investigators divided the cohort into four groups according to age and tumor characteristics:
• 50 years or older, hormone receptor negative.
• 50 years or older, hormone receptor negative.
• Younger than 50 years, hormone receptor positive.
• Younger than 50 years, hormone receptor positive.
Women 50 years and older with HR-positive tumors who had BCT experienced the greatest survival benefit, compared with mastectomy patients (hazard ratio, 0.81). Women younger than 50 years with HR- positive tumors experienced the smallest benefit (HR, 0.93), but one which was still statistically significant.
The investigators also looked at 3-year overall and disease-specific survival. "Notably, BCT was associated with significantly lower 3-year mortality rates from all causes," including heart disease (HR, 0.51), chronic respiratory disease (HR, 0.46), and cerebrovascular disease (HR, 0.64).
The findings align with those of randomized trials showing the benefits of BCT, the authors noted.
"Despite this, recent studies have shown an increased rate of mastectomy for patient subgroups including younger women with early-stage tumors, many of which would have presumably been amenable to BCT," they wrote. This could be the result of a perception that women with unfavorable characteristics, like younger age and high-risk tumors, don’t do as well with BCT.
The investigators noted that some differences in disease burden at baseline could have contributed to the findings.
"Interestingly, for every cause of mortality that we evaluated, women who had mastectomy were more likely to die within 3 years of their breast cancer diagnosis than women who chose BCT. Based on these findings, it is reasonable to infer that the mastectomy group was likely to have a greater burden of nonfatal comorbidities at presentation, and that this factor may well have influenced surgical decision-making. Nevertheless, this factor alone cannot account for why women with mastectomy had lower [disease specific survival] after adjusting for age and tumor characteristics," Dr. Hwang and her associates said.
Based on the strong associations with survival, "these findings support the notion that BCT, when combined with radiation, confers at least equivalent and perhaps even superior survival to mastectomy as definitive breast cancer treatment," they said.
The National Cancer Institute funded the study. Dr. Hwang had no financial disclosures.
This article is interesting and important, but I don’t see any mention of systemic therapy, which has a huge impact on breast cancer–specific survival. Confounding factors could have created bias in this registry analysis that makes interpretation of improved survival impossible.

|
|
Having said that, I believe this is an important publication. The benefits of mastectomy are clearly overemphasized, and mastectomy is used in many situations where breast-conserving surgery will result in at least an identical outcome. Patients often understand that their outcome will be improved if more surgery is done, or if they remove the offending breast.
Education for patients in this regard is critical. Surgeons and medical oncologists are critical components of a change in practice – a change that has the potential to significantly improve quality of life and cosmetic outcome for women with breast cancer, representing over 200,000 patients per year in the United States. Hopefully, data such as these will disabuse practitioners of the all-too-common approach that mastectomy is an easier, simpler solution than breast-conserving surgery when managing early-stage breast cancer.
Hope S. Rugo, M.D., associate editor of The Oncology Report, is professor of medicine and director of breast oncology and clinical trials education at the comprehensive cancer center of the University of California, San Francisco.
This article is interesting and important, but I don’t see any mention of systemic therapy, which has a huge impact on breast cancer–specific survival. Confounding factors could have created bias in this registry analysis that makes interpretation of improved survival impossible.

|
|
Having said that, I believe this is an important publication. The benefits of mastectomy are clearly overemphasized, and mastectomy is used in many situations where breast-conserving surgery will result in at least an identical outcome. Patients often understand that their outcome will be improved if more surgery is done, or if they remove the offending breast.
Education for patients in this regard is critical. Surgeons and medical oncologists are critical components of a change in practice – a change that has the potential to significantly improve quality of life and cosmetic outcome for women with breast cancer, representing over 200,000 patients per year in the United States. Hopefully, data such as these will disabuse practitioners of the all-too-common approach that mastectomy is an easier, simpler solution than breast-conserving surgery when managing early-stage breast cancer.
Hope S. Rugo, M.D., associate editor of The Oncology Report, is professor of medicine and director of breast oncology and clinical trials education at the comprehensive cancer center of the University of California, San Francisco.
This article is interesting and important, but I don’t see any mention of systemic therapy, which has a huge impact on breast cancer–specific survival. Confounding factors could have created bias in this registry analysis that makes interpretation of improved survival impossible.

|
|
Having said that, I believe this is an important publication. The benefits of mastectomy are clearly overemphasized, and mastectomy is used in many situations where breast-conserving surgery will result in at least an identical outcome. Patients often understand that their outcome will be improved if more surgery is done, or if they remove the offending breast.
Education for patients in this regard is critical. Surgeons and medical oncologists are critical components of a change in practice – a change that has the potential to significantly improve quality of life and cosmetic outcome for women with breast cancer, representing over 200,000 patients per year in the United States. Hopefully, data such as these will disabuse practitioners of the all-too-common approach that mastectomy is an easier, simpler solution than breast-conserving surgery when managing early-stage breast cancer.
Hope S. Rugo, M.D., associate editor of The Oncology Report, is professor of medicine and director of breast oncology and clinical trials education at the comprehensive cancer center of the University of California, San Francisco.
Women who underwent lumpectomy for stage I or II breast cancer were 28% less likely to die from any cause and up to 16% less likely to die from breast cancer, compared with women who underwent mastectomy, Dr. E. Shelley Hwang and her colleagues reported Jan. 28 in the online issue of Cancer.
The 3-year disease-specific survival benefit for breast-conserving therapy (BCT) was most pronounced for chronic respiratory disease, for which lumpectomy was associated with a 54% decreased risk of death; heart disease, with a 49% decreased risk; and cerebrovascular disease, with a decreased risk of 36%.
The survival benefit varied with age, tumor size, and hormone receptor status but was significant in every subgroup, Dr. Hwang and her colleagues wrote (Cancer Jan. 28, 2013 [doi:10.1002/cncr.27795]).
"Our findings have important implications for understanding the overall benefit of BCT at the population level," wrote Dr. Hwang of the Duke University Comprehensive Cancer Center, Durham, N.C., and her coauthors. "These results provide confidence in the efficacy of BCT even among younger patients with HR-negative disease thought to be at relatively higher risk for local failure."
The team reviewed the records of 112,154 women who were treated for a new, unilateral T1/T2 stage I or II breast cancer diagnosed from 1990 to 2004. Most of these women (61,771) underwent a lumpectomy and radiation; the remainder underwent mastectomy without radiation. They were followed for a median of 10 years.
About a quarter of each group was younger than 50 years when diagnosed; another quarter was aged 70-80 years. A small portion (6%) was younger than 40 years.
Surgical approach evolved over the study period. Breast-conserving therapy increased from 37% in 1990-92 to 62% by 2002-04, while the rate of mastectomies declined.
The median tumor size was 1.5 cm; patients with larger tumors were more likely to have mastectomies. "Interestingly, the use of BCT varied by age even among tumors [smaller than and equal to] 2 cm where the youngest and oldest age groups had the lowest BCT rate. In [tumors larger than] 2 cm, BCT rate declined by age," Dr. Hwang and her associates said.
Over the follow-up period, there were 31,416 deaths; 39% of these were caused by breast cancer; 5-year overall survival was 89%.
To further explore the treatment-mortality interaction, the investigators divided the cohort into four groups according to age and tumor characteristics:
• 50 years or older, hormone receptor negative.
• 50 years or older, hormone receptor negative.
• Younger than 50 years, hormone receptor positive.
• Younger than 50 years, hormone receptor positive.
Women 50 years and older with HR-positive tumors who had BCT experienced the greatest survival benefit, compared with mastectomy patients (hazard ratio, 0.81). Women younger than 50 years with HR- positive tumors experienced the smallest benefit (HR, 0.93), but one which was still statistically significant.
The investigators also looked at 3-year overall and disease-specific survival. "Notably, BCT was associated with significantly lower 3-year mortality rates from all causes," including heart disease (HR, 0.51), chronic respiratory disease (HR, 0.46), and cerebrovascular disease (HR, 0.64).
The findings align with those of randomized trials showing the benefits of BCT, the authors noted.
"Despite this, recent studies have shown an increased rate of mastectomy for patient subgroups including younger women with early-stage tumors, many of which would have presumably been amenable to BCT," they wrote. This could be the result of a perception that women with unfavorable characteristics, like younger age and high-risk tumors, don’t do as well with BCT.
The investigators noted that some differences in disease burden at baseline could have contributed to the findings.
"Interestingly, for every cause of mortality that we evaluated, women who had mastectomy were more likely to die within 3 years of their breast cancer diagnosis than women who chose BCT. Based on these findings, it is reasonable to infer that the mastectomy group was likely to have a greater burden of nonfatal comorbidities at presentation, and that this factor may well have influenced surgical decision-making. Nevertheless, this factor alone cannot account for why women with mastectomy had lower [disease specific survival] after adjusting for age and tumor characteristics," Dr. Hwang and her associates said.
Based on the strong associations with survival, "these findings support the notion that BCT, when combined with radiation, confers at least equivalent and perhaps even superior survival to mastectomy as definitive breast cancer treatment," they said.
The National Cancer Institute funded the study. Dr. Hwang had no financial disclosures.
Women who underwent lumpectomy for stage I or II breast cancer were 28% less likely to die from any cause and up to 16% less likely to die from breast cancer, compared with women who underwent mastectomy, Dr. E. Shelley Hwang and her colleagues reported Jan. 28 in the online issue of Cancer.
The 3-year disease-specific survival benefit for breast-conserving therapy (BCT) was most pronounced for chronic respiratory disease, for which lumpectomy was associated with a 54% decreased risk of death; heart disease, with a 49% decreased risk; and cerebrovascular disease, with a decreased risk of 36%.
The survival benefit varied with age, tumor size, and hormone receptor status but was significant in every subgroup, Dr. Hwang and her colleagues wrote (Cancer Jan. 28, 2013 [doi:10.1002/cncr.27795]).
"Our findings have important implications for understanding the overall benefit of BCT at the population level," wrote Dr. Hwang of the Duke University Comprehensive Cancer Center, Durham, N.C., and her coauthors. "These results provide confidence in the efficacy of BCT even among younger patients with HR-negative disease thought to be at relatively higher risk for local failure."
The team reviewed the records of 112,154 women who were treated for a new, unilateral T1/T2 stage I or II breast cancer diagnosed from 1990 to 2004. Most of these women (61,771) underwent a lumpectomy and radiation; the remainder underwent mastectomy without radiation. They were followed for a median of 10 years.
About a quarter of each group was younger than 50 years when diagnosed; another quarter was aged 70-80 years. A small portion (6%) was younger than 40 years.
Surgical approach evolved over the study period. Breast-conserving therapy increased from 37% in 1990-92 to 62% by 2002-04, while the rate of mastectomies declined.
The median tumor size was 1.5 cm; patients with larger tumors were more likely to have mastectomies. "Interestingly, the use of BCT varied by age even among tumors [smaller than and equal to] 2 cm where the youngest and oldest age groups had the lowest BCT rate. In [tumors larger than] 2 cm, BCT rate declined by age," Dr. Hwang and her associates said.
Over the follow-up period, there were 31,416 deaths; 39% of these were caused by breast cancer; 5-year overall survival was 89%.
To further explore the treatment-mortality interaction, the investigators divided the cohort into four groups according to age and tumor characteristics:
• 50 years or older, hormone receptor negative.
• 50 years or older, hormone receptor negative.
• Younger than 50 years, hormone receptor positive.
• Younger than 50 years, hormone receptor positive.
Women 50 years and older with HR-positive tumors who had BCT experienced the greatest survival benefit, compared with mastectomy patients (hazard ratio, 0.81). Women younger than 50 years with HR- positive tumors experienced the smallest benefit (HR, 0.93), but one which was still statistically significant.
The investigators also looked at 3-year overall and disease-specific survival. "Notably, BCT was associated with significantly lower 3-year mortality rates from all causes," including heart disease (HR, 0.51), chronic respiratory disease (HR, 0.46), and cerebrovascular disease (HR, 0.64).
The findings align with those of randomized trials showing the benefits of BCT, the authors noted.
"Despite this, recent studies have shown an increased rate of mastectomy for patient subgroups including younger women with early-stage tumors, many of which would have presumably been amenable to BCT," they wrote. This could be the result of a perception that women with unfavorable characteristics, like younger age and high-risk tumors, don’t do as well with BCT.
The investigators noted that some differences in disease burden at baseline could have contributed to the findings.
"Interestingly, for every cause of mortality that we evaluated, women who had mastectomy were more likely to die within 3 years of their breast cancer diagnosis than women who chose BCT. Based on these findings, it is reasonable to infer that the mastectomy group was likely to have a greater burden of nonfatal comorbidities at presentation, and that this factor may well have influenced surgical decision-making. Nevertheless, this factor alone cannot account for why women with mastectomy had lower [disease specific survival] after adjusting for age and tumor characteristics," Dr. Hwang and her associates said.
Based on the strong associations with survival, "these findings support the notion that BCT, when combined with radiation, confers at least equivalent and perhaps even superior survival to mastectomy as definitive breast cancer treatment," they said.
The National Cancer Institute funded the study. Dr. Hwang had no financial disclosures.
FROM CANCER
Major Finding: Women who underwent lumpectomy for stage I or II breast cancer were 28% less likely to die from any cause and up to 16% less likely to die from breast cancer than women who underwent mastectomy.
Data Source: Multivariate survival analysis including more than 112,000 women.
Disclosures: The National Cancer Institute funded the work. None of the authors had any financial disclosures.
Tedizolid found noninferior to linezolid for complicated skin infections
Tedizolid, a second-generation oxazolidinone available in both oral and IV formulations, was found to be noninferior to linezolid for the treatment of acute, complicated infections of the skin and skin structure, including methicillin-resistant Staphylococcus aureus infections, according to a report in the Feb. 13 issue of JAMA.
In a phase III, randomized clinical trial, oral tedizolid phosphate (also known as TR-701) was at least as effective as oral linezolid early in the course of infection (at 48-72 hours after initiating therapy), and response to the drug persisted at a later follow-up at 7-14 days after treatment ended. Early effectiveness is important because clinicians decide whether to continue with their chosen agent or switch to different drugs during that period.
Tedizolid offers an advantage over linezolid in that it requires only once-daily dosing of a 200-mg tablet for a total of 6 days; the standard course of linezolid requires twice-daily dosing of a 600-mg tablet for 10 days. In this trial, tedizolid produced fewer gastrointestinal adverse effects and halved the rate of low platelet counts, compared with linezolid, said Dr. Philippe Prokocimer of Trius Therapeutics, San Diego, and his associates.
Trius, the maker of tedizolid, funded and conducted this 1-year, double-blind study at 54 medical centers in North America, Latin America, and Europe.
The researchers compared the two antibiotics in 667 adults (age range, 18-100 years) who had cellulitis/erysipelas, major cutaneous abscess, or wound infection surrounded by erythema, with a minimum total lesion surface of 75 cm2. The study subjects also had at least one local and one regional or one systemic sign of infection, and the infecting organism was either thought to be or proven to be a gram-positive pathogen.
The subjects were stratified by the presence or absence of fever at baseline, the geographic region where they resided, and the type of infection they had, and then randomly assigned to receive tedizolid (332 patients) or linezolid (335 patients). Both groups presented a similar clinical picture, with a median infected area of 188 cm; 87% of the study population had lymphadenopathy adjacent to the lesion, 41% had abnormal white blood cell counts, and 18% were febrile.
In the primary efficacy intention-to-treat analysis, 79.5% of the subjects receiving tedizolid and 79.4% receiving linezolid were classified as responders – meaning they were afebrile and their primary skin lesions had stopped spreading – when evaluated at 48-72 hours after the first dose of the antibiotics.
Conversely, 8.1% of the subjects receiving tedizolid and 10.4% of those receiving linezolid were true nonresponders, with fever or continued spread of the primary lesion, at 48-72 hours.
Thus, tedizolid met the criteria for noninferiority to linezolid at this time point, Dr. Prokocimer and his colleagues reported (JAMA 2013;309:559-69).
The remaining subjects were classified as "indeterminate" responders, usually because temperature data were missing or were only available outside the time window specified in the study protocol.
Secondary efficacy measures, sustained treatment response rates at two later follow-up times, followed a similar pattern. At the end of treatment, 69.3% of the tedizolid group and 71.9% of the linezolid group were deemed responders, and at final follow-up 1-2 weeks later, 80.2% and 81.1% were responders, respectively.
Results of several sensitivity and subgroup analyses confirmed those of these primary and secondary analyses. "Of particular interest are the similar treatment response rates in the tedizolid group (78%) and in the linezolid group (76.1%) in the sensitivity analysis that was based on the Foundation for the National Institutes of Health recommended outcome," which is a 20% or greater reduction in the lesion area.
Also of note was the finding that response rates were similarly high (approximately 85%) with both antibiotics in the subgroup of 178 patients who had methicillin-resistant Staphylococcus aureus (MRSA) infections.
Rates of adverse events and of serious adverse events were similar between the two study groups, with fewer patients in the tedizolid group reporting GI symptoms.
Postmarketing studies have reported possible links between linezolid and myelosuppression. in this study, low platelet counts were less than half as frequent in the subjects who received tedizolid as in those who received linezolid, but the analysis "was not adequately powered to make conclusions about the risk of myelosuppression with tedizolid," the authors said.
The investigators reported ties to Trius, Cerexa, Achaogen, and other companies.
This study shows that a short course of the new oral antibiotic tedizolid appears to be effective and may have a better safety profile than linezolid, said Dr. Shira Doron and Dr. Helen W. Boucher.
Noninferior efficacy with a shorter course of therapy is also important because it may prevent the "collateral damage" attributable to longer courses of antibiotics, such as the development of multidrug-resistant organisms and of Clostridium difficile infections, they noted.
These remarks are distilled from an invited editorial on the research (JAMA 2013;30:609-11 Dr. Doron and Dr. Boucher are in the division of geographic medicine and infectious diseases at Tufts Medical Center, Boston. Dr. Doron reported ties to Forest, Merck, and Optimer, and Dr. Boucher reported ties to Basilea, Cerexa, Durata, and other companies.
oral tedizolid phosphate, TR-701, linezolid
This study shows that a short course of the new oral antibiotic tedizolid appears to be effective and may have a better safety profile than linezolid, said Dr. Shira Doron and Dr. Helen W. Boucher.
Noninferior efficacy with a shorter course of therapy is also important because it may prevent the "collateral damage" attributable to longer courses of antibiotics, such as the development of multidrug-resistant organisms and of Clostridium difficile infections, they noted.
These remarks are distilled from an invited editorial on the research (JAMA 2013;30:609-11 Dr. Doron and Dr. Boucher are in the division of geographic medicine and infectious diseases at Tufts Medical Center, Boston. Dr. Doron reported ties to Forest, Merck, and Optimer, and Dr. Boucher reported ties to Basilea, Cerexa, Durata, and other companies.
This study shows that a short course of the new oral antibiotic tedizolid appears to be effective and may have a better safety profile than linezolid, said Dr. Shira Doron and Dr. Helen W. Boucher.
Noninferior efficacy with a shorter course of therapy is also important because it may prevent the "collateral damage" attributable to longer courses of antibiotics, such as the development of multidrug-resistant organisms and of Clostridium difficile infections, they noted.
These remarks are distilled from an invited editorial on the research (JAMA 2013;30:609-11 Dr. Doron and Dr. Boucher are in the division of geographic medicine and infectious diseases at Tufts Medical Center, Boston. Dr. Doron reported ties to Forest, Merck, and Optimer, and Dr. Boucher reported ties to Basilea, Cerexa, Durata, and other companies.
Tedizolid, a second-generation oxazolidinone available in both oral and IV formulations, was found to be noninferior to linezolid for the treatment of acute, complicated infections of the skin and skin structure, including methicillin-resistant Staphylococcus aureus infections, according to a report in the Feb. 13 issue of JAMA.
In a phase III, randomized clinical trial, oral tedizolid phosphate (also known as TR-701) was at least as effective as oral linezolid early in the course of infection (at 48-72 hours after initiating therapy), and response to the drug persisted at a later follow-up at 7-14 days after treatment ended. Early effectiveness is important because clinicians decide whether to continue with their chosen agent or switch to different drugs during that period.
Tedizolid offers an advantage over linezolid in that it requires only once-daily dosing of a 200-mg tablet for a total of 6 days; the standard course of linezolid requires twice-daily dosing of a 600-mg tablet for 10 days. In this trial, tedizolid produced fewer gastrointestinal adverse effects and halved the rate of low platelet counts, compared with linezolid, said Dr. Philippe Prokocimer of Trius Therapeutics, San Diego, and his associates.
Trius, the maker of tedizolid, funded and conducted this 1-year, double-blind study at 54 medical centers in North America, Latin America, and Europe.
The researchers compared the two antibiotics in 667 adults (age range, 18-100 years) who had cellulitis/erysipelas, major cutaneous abscess, or wound infection surrounded by erythema, with a minimum total lesion surface of 75 cm2. The study subjects also had at least one local and one regional or one systemic sign of infection, and the infecting organism was either thought to be or proven to be a gram-positive pathogen.
The subjects were stratified by the presence or absence of fever at baseline, the geographic region where they resided, and the type of infection they had, and then randomly assigned to receive tedizolid (332 patients) or linezolid (335 patients). Both groups presented a similar clinical picture, with a median infected area of 188 cm; 87% of the study population had lymphadenopathy adjacent to the lesion, 41% had abnormal white blood cell counts, and 18% were febrile.
In the primary efficacy intention-to-treat analysis, 79.5% of the subjects receiving tedizolid and 79.4% receiving linezolid were classified as responders – meaning they were afebrile and their primary skin lesions had stopped spreading – when evaluated at 48-72 hours after the first dose of the antibiotics.
Conversely, 8.1% of the subjects receiving tedizolid and 10.4% of those receiving linezolid were true nonresponders, with fever or continued spread of the primary lesion, at 48-72 hours.
Thus, tedizolid met the criteria for noninferiority to linezolid at this time point, Dr. Prokocimer and his colleagues reported (JAMA 2013;309:559-69).
The remaining subjects were classified as "indeterminate" responders, usually because temperature data were missing or were only available outside the time window specified in the study protocol.
Secondary efficacy measures, sustained treatment response rates at two later follow-up times, followed a similar pattern. At the end of treatment, 69.3% of the tedizolid group and 71.9% of the linezolid group were deemed responders, and at final follow-up 1-2 weeks later, 80.2% and 81.1% were responders, respectively.
Results of several sensitivity and subgroup analyses confirmed those of these primary and secondary analyses. "Of particular interest are the similar treatment response rates in the tedizolid group (78%) and in the linezolid group (76.1%) in the sensitivity analysis that was based on the Foundation for the National Institutes of Health recommended outcome," which is a 20% or greater reduction in the lesion area.
Also of note was the finding that response rates were similarly high (approximately 85%) with both antibiotics in the subgroup of 178 patients who had methicillin-resistant Staphylococcus aureus (MRSA) infections.
Rates of adverse events and of serious adverse events were similar between the two study groups, with fewer patients in the tedizolid group reporting GI symptoms.
Postmarketing studies have reported possible links between linezolid and myelosuppression. in this study, low platelet counts were less than half as frequent in the subjects who received tedizolid as in those who received linezolid, but the analysis "was not adequately powered to make conclusions about the risk of myelosuppression with tedizolid," the authors said.
The investigators reported ties to Trius, Cerexa, Achaogen, and other companies.
Tedizolid, a second-generation oxazolidinone available in both oral and IV formulations, was found to be noninferior to linezolid for the treatment of acute, complicated infections of the skin and skin structure, including methicillin-resistant Staphylococcus aureus infections, according to a report in the Feb. 13 issue of JAMA.
In a phase III, randomized clinical trial, oral tedizolid phosphate (also known as TR-701) was at least as effective as oral linezolid early in the course of infection (at 48-72 hours after initiating therapy), and response to the drug persisted at a later follow-up at 7-14 days after treatment ended. Early effectiveness is important because clinicians decide whether to continue with their chosen agent or switch to different drugs during that period.
Tedizolid offers an advantage over linezolid in that it requires only once-daily dosing of a 200-mg tablet for a total of 6 days; the standard course of linezolid requires twice-daily dosing of a 600-mg tablet for 10 days. In this trial, tedizolid produced fewer gastrointestinal adverse effects and halved the rate of low platelet counts, compared with linezolid, said Dr. Philippe Prokocimer of Trius Therapeutics, San Diego, and his associates.
Trius, the maker of tedizolid, funded and conducted this 1-year, double-blind study at 54 medical centers in North America, Latin America, and Europe.
The researchers compared the two antibiotics in 667 adults (age range, 18-100 years) who had cellulitis/erysipelas, major cutaneous abscess, or wound infection surrounded by erythema, with a minimum total lesion surface of 75 cm2. The study subjects also had at least one local and one regional or one systemic sign of infection, and the infecting organism was either thought to be or proven to be a gram-positive pathogen.
The subjects were stratified by the presence or absence of fever at baseline, the geographic region where they resided, and the type of infection they had, and then randomly assigned to receive tedizolid (332 patients) or linezolid (335 patients). Both groups presented a similar clinical picture, with a median infected area of 188 cm; 87% of the study population had lymphadenopathy adjacent to the lesion, 41% had abnormal white blood cell counts, and 18% were febrile.
In the primary efficacy intention-to-treat analysis, 79.5% of the subjects receiving tedizolid and 79.4% receiving linezolid were classified as responders – meaning they were afebrile and their primary skin lesions had stopped spreading – when evaluated at 48-72 hours after the first dose of the antibiotics.
Conversely, 8.1% of the subjects receiving tedizolid and 10.4% of those receiving linezolid were true nonresponders, with fever or continued spread of the primary lesion, at 48-72 hours.
Thus, tedizolid met the criteria for noninferiority to linezolid at this time point, Dr. Prokocimer and his colleagues reported (JAMA 2013;309:559-69).
The remaining subjects were classified as "indeterminate" responders, usually because temperature data were missing or were only available outside the time window specified in the study protocol.
Secondary efficacy measures, sustained treatment response rates at two later follow-up times, followed a similar pattern. At the end of treatment, 69.3% of the tedizolid group and 71.9% of the linezolid group were deemed responders, and at final follow-up 1-2 weeks later, 80.2% and 81.1% were responders, respectively.
Results of several sensitivity and subgroup analyses confirmed those of these primary and secondary analyses. "Of particular interest are the similar treatment response rates in the tedizolid group (78%) and in the linezolid group (76.1%) in the sensitivity analysis that was based on the Foundation for the National Institutes of Health recommended outcome," which is a 20% or greater reduction in the lesion area.
Also of note was the finding that response rates were similarly high (approximately 85%) with both antibiotics in the subgroup of 178 patients who had methicillin-resistant Staphylococcus aureus (MRSA) infections.
Rates of adverse events and of serious adverse events were similar between the two study groups, with fewer patients in the tedizolid group reporting GI symptoms.
Postmarketing studies have reported possible links between linezolid and myelosuppression. in this study, low platelet counts were less than half as frequent in the subjects who received tedizolid as in those who received linezolid, but the analysis "was not adequately powered to make conclusions about the risk of myelosuppression with tedizolid," the authors said.
The investigators reported ties to Trius, Cerexa, Achaogen, and other companies.
oral tedizolid phosphate, TR-701, linezolid
oral tedizolid phosphate, TR-701, linezolid
FROM JAMA
Major Finding: A total of 79.5% of patients receiving tedizolid and 79.4% receiving linezolid showed a treatment response within 48-72 hours; 80.2% and 81.1%, respectively, showed a persistent treatment response 7-14 days after treatment ended.
Data Source: A 1-year randomized, double-blind, multinational phase III noninferiority trial comparing tedizolid and linezolid in 667 adults with acute, complicated infections of the skin and skin structure.
Disclosures: The investigators reported ties to Trius, Cerexa, Achaogen, and other companies.
Medicaid expansion, Medicare reform: The Policy & Practice Podcast
While the Obama administration was out pitching the Affordable Care Act last week, a leading Republican lawmaker unveiled what is likely to be the GOP game plan for the new Congress.
HHS Secretary Kathleen Sebelius said the administration is trying to get the word out so that the millions of Americans who will be eligible for new health insurance this fall understand how to get that coverage. She also urged governors to take the administration up on its offer to pay the cost of those who will be newly eligible for Medicaid.
She came close to calling it an offer they could not refuse.
Meanwhile, House Majority Leader Eric Cantor talked up the Republican vision for health policy in the coming year. Reforming Medicare will be high on the list, but the words "premium support" did not cross his lips.
And Republicans and Democrats announced that they are willing to work together to repeal the Sustainable Growth Rate formula.
For more on those stories, tune in to this week’s edition of the Policy & Practice podcast.
On Twitter @aliciaault
While the Obama administration was out pitching the Affordable Care Act last week, a leading Republican lawmaker unveiled what is likely to be the GOP game plan for the new Congress.
HHS Secretary Kathleen Sebelius said the administration is trying to get the word out so that the millions of Americans who will be eligible for new health insurance this fall understand how to get that coverage. She also urged governors to take the administration up on its offer to pay the cost of those who will be newly eligible for Medicaid.
She came close to calling it an offer they could not refuse.
Meanwhile, House Majority Leader Eric Cantor talked up the Republican vision for health policy in the coming year. Reforming Medicare will be high on the list, but the words "premium support" did not cross his lips.
And Republicans and Democrats announced that they are willing to work together to repeal the Sustainable Growth Rate formula.
For more on those stories, tune in to this week’s edition of the Policy & Practice podcast.
On Twitter @aliciaault
While the Obama administration was out pitching the Affordable Care Act last week, a leading Republican lawmaker unveiled what is likely to be the GOP game plan for the new Congress.
HHS Secretary Kathleen Sebelius said the administration is trying to get the word out so that the millions of Americans who will be eligible for new health insurance this fall understand how to get that coverage. She also urged governors to take the administration up on its offer to pay the cost of those who will be newly eligible for Medicaid.
She came close to calling it an offer they could not refuse.
Meanwhile, House Majority Leader Eric Cantor talked up the Republican vision for health policy in the coming year. Reforming Medicare will be high on the list, but the words "premium support" did not cross his lips.
And Republicans and Democrats announced that they are willing to work together to repeal the Sustainable Growth Rate formula.
For more on those stories, tune in to this week’s edition of the Policy & Practice podcast.
On Twitter @aliciaault
Cancer survival still lower for African Americans
The 5-year cancer survival rate for African Americans has risen 54% since the 1970s, but continues to be lower than that of whites, according to a report from the American Cancer Society.
Data from the Surveillance, Epidemiology, and End Results (SEER) database show that the 5-year relative survival rate in 2002-2008 was 60% for blacks, 69% for whites, and 68% for all races. Those numbers are all up significantly since 1975-77, when the rates for all cancers were 39% for African Americans, 50% for whites, and 49% for all races, the ACS reported.
The differences between blacks and whites varied among specific cancer sites. In 2002-2008, the 5-year survival for melanoma was 70% for blacks and 93% for whites. For colon cancer, the rate was 55% for blacks and 66% for whites. For kidney and renal pelvis cancer, however, the rates were much closer: 70% for blacks and 72% for whites. The only sites for which African Americans had higher survival rates were the brain (41%, compared with 34%) and the stomach (28%, compared with 27%), the ACS said.
The 5-year cancer survival rate for African Americans has risen 54% since the 1970s, but continues to be lower than that of whites, according to a report from the American Cancer Society.
Data from the Surveillance, Epidemiology, and End Results (SEER) database show that the 5-year relative survival rate in 2002-2008 was 60% for blacks, 69% for whites, and 68% for all races. Those numbers are all up significantly since 1975-77, when the rates for all cancers were 39% for African Americans, 50% for whites, and 49% for all races, the ACS reported.
The differences between blacks and whites varied among specific cancer sites. In 2002-2008, the 5-year survival for melanoma was 70% for blacks and 93% for whites. For colon cancer, the rate was 55% for blacks and 66% for whites. For kidney and renal pelvis cancer, however, the rates were much closer: 70% for blacks and 72% for whites. The only sites for which African Americans had higher survival rates were the brain (41%, compared with 34%) and the stomach (28%, compared with 27%), the ACS said.
The 5-year cancer survival rate for African Americans has risen 54% since the 1970s, but continues to be lower than that of whites, according to a report from the American Cancer Society.
Data from the Surveillance, Epidemiology, and End Results (SEER) database show that the 5-year relative survival rate in 2002-2008 was 60% for blacks, 69% for whites, and 68% for all races. Those numbers are all up significantly since 1975-77, when the rates for all cancers were 39% for African Americans, 50% for whites, and 49% for all races, the ACS reported.
The differences between blacks and whites varied among specific cancer sites. In 2002-2008, the 5-year survival for melanoma was 70% for blacks and 93% for whites. For colon cancer, the rate was 55% for blacks and 66% for whites. For kidney and renal pelvis cancer, however, the rates were much closer: 70% for blacks and 72% for whites. The only sites for which African Americans had higher survival rates were the brain (41%, compared with 34%) and the stomach (28%, compared with 27%), the ACS said.
Daily chlorhexidine bath cuts bloodstream, MRSA infections in ICUs
For patients in intensive care units and bone marrow transplantation units, daily bathing with chlorhexidine-impregnated washcloths significantly reduced the incidence of bloodstream infections and decreased the acquisition of multidrug-resistant organisms in a randomized controlled trial reported online Feb. 7 in the New England Journal of Medicine.
The multicenter study, which included nine medical, coronary care, surgical, cardiac surgery, and bone marrow transplantation units in several geographic regions of the United States, confirms the results of previous single-center studies and demonstrates that the benefit of daily chlorhexidine bathing is widely generalizable, said Dr. Michael W. Climo of the Hunter Holmes McGuire Veterans Affairs Medical Center, Richmond, Va., and his associates.
Each hospital unit participating in the trial was randomized to perform daily bathing of patients using either washcloths impregnated with 2% chlorhexidine gluconate (intervention) or washcloths with no impregnated antimicrobial (control) for 6 months, followed by a crossover period in which patients were bathed daily with the alternate product for another 6 months. A total of 7,727 patients were included in the study.
Nurses were trained in the manufacturer’s instructions for bathing, which involved using the cloths in a sequential order to rinse all body surfaces except the face (to avoid getting chlorhexidine in the patient’s eyes and mouth). Implementing this cost-effective strategy was "relatively straightforward" because it did not require much of a change from current routine patient-bathing practices, the investigators said.
All the hospital units performed active surveillance for methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE) throughout the study.
There were 119 hospital-acquired bloodstream infections during the intervention and 165 during the control period, representing a 28% reduction with chlorhexidine bathing. The rate of primary bloodstream infections was 31% lower and the rate of central catheter–associated bloodstream infections was 53% lower with the intervention, Dr. Climo and his associates reported (N. Engl. J. Med. 2013 Feb. 6 [doi: 10.1056/NEJMoa1113849]).
This benefit was greatest among patients who had longer lengths of stay in the hospital units.
One "unanticipated" finding was that rates of fungal infection in particular were dramatically reduced with chlorhexidine bathing. The incidence of primary bloodstream infection caused by fungi was 53% lower and the incidence of central catheter–associated fungal bloodstream infection was 90% lower during the intervention period than during the control period.
This antifungal effect has not been reported previously. "If our results are confirmed, topical use of chlorhexidine could be added to strategies to prevent fungal infection," the researchers noted.
The rate of MRSA or VRE acquisition was 23% lower during the intervention period (5.10 cases per 1,000 patient-days) than during the control period (6.60 cases per 1,000 patient-days). However, reductions in the incidence of MRSA and VRE bacteremia did not reach statistical significance, most likely because the overall number of cases was so low, Dr. Climo and his colleagues said.
No serious adverse effects were observed. The incidence of skin reactions was higher with the control washcloths (3.4%) than with the chlorhexidine washcloths (2.0%), and none of the skin reactions were considered to be related to the bathing intervention.
It was reassuring that none of the MRSA or VRE isolates that were detected in this study showed resistance to chlorhexidine. However, the development of resistance to biocides and disinfectants among nosocomial organisms remains "a substantial concern" and should continue to be monitored, they added.
This study was supported by the Centers for Disease Control and Prevention and Sage Products. Sage Products supplied the washcloths, provided technical and educational support, and participated in weekly teleconferences with the research group but was not involved in the study design, data analysis, or reporting of the study results. Dr. Climo and his associates reported ties to Sage Products, Centene, and other companies.
For patients in intensive care units and bone marrow transplantation units, daily bathing with chlorhexidine-impregnated washcloths significantly reduced the incidence of bloodstream infections and decreased the acquisition of multidrug-resistant organisms in a randomized controlled trial reported online Feb. 7 in the New England Journal of Medicine.
The multicenter study, which included nine medical, coronary care, surgical, cardiac surgery, and bone marrow transplantation units in several geographic regions of the United States, confirms the results of previous single-center studies and demonstrates that the benefit of daily chlorhexidine bathing is widely generalizable, said Dr. Michael W. Climo of the Hunter Holmes McGuire Veterans Affairs Medical Center, Richmond, Va., and his associates.
Each hospital unit participating in the trial was randomized to perform daily bathing of patients using either washcloths impregnated with 2% chlorhexidine gluconate (intervention) or washcloths with no impregnated antimicrobial (control) for 6 months, followed by a crossover period in which patients were bathed daily with the alternate product for another 6 months. A total of 7,727 patients were included in the study.
Nurses were trained in the manufacturer’s instructions for bathing, which involved using the cloths in a sequential order to rinse all body surfaces except the face (to avoid getting chlorhexidine in the patient’s eyes and mouth). Implementing this cost-effective strategy was "relatively straightforward" because it did not require much of a change from current routine patient-bathing practices, the investigators said.
All the hospital units performed active surveillance for methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE) throughout the study.
There were 119 hospital-acquired bloodstream infections during the intervention and 165 during the control period, representing a 28% reduction with chlorhexidine bathing. The rate of primary bloodstream infections was 31% lower and the rate of central catheter–associated bloodstream infections was 53% lower with the intervention, Dr. Climo and his associates reported (N. Engl. J. Med. 2013 Feb. 6 [doi: 10.1056/NEJMoa1113849]).
This benefit was greatest among patients who had longer lengths of stay in the hospital units.
One "unanticipated" finding was that rates of fungal infection in particular were dramatically reduced with chlorhexidine bathing. The incidence of primary bloodstream infection caused by fungi was 53% lower and the incidence of central catheter–associated fungal bloodstream infection was 90% lower during the intervention period than during the control period.
This antifungal effect has not been reported previously. "If our results are confirmed, topical use of chlorhexidine could be added to strategies to prevent fungal infection," the researchers noted.
The rate of MRSA or VRE acquisition was 23% lower during the intervention period (5.10 cases per 1,000 patient-days) than during the control period (6.60 cases per 1,000 patient-days). However, reductions in the incidence of MRSA and VRE bacteremia did not reach statistical significance, most likely because the overall number of cases was so low, Dr. Climo and his colleagues said.
No serious adverse effects were observed. The incidence of skin reactions was higher with the control washcloths (3.4%) than with the chlorhexidine washcloths (2.0%), and none of the skin reactions were considered to be related to the bathing intervention.
It was reassuring that none of the MRSA or VRE isolates that were detected in this study showed resistance to chlorhexidine. However, the development of resistance to biocides and disinfectants among nosocomial organisms remains "a substantial concern" and should continue to be monitored, they added.
This study was supported by the Centers for Disease Control and Prevention and Sage Products. Sage Products supplied the washcloths, provided technical and educational support, and participated in weekly teleconferences with the research group but was not involved in the study design, data analysis, or reporting of the study results. Dr. Climo and his associates reported ties to Sage Products, Centene, and other companies.
For patients in intensive care units and bone marrow transplantation units, daily bathing with chlorhexidine-impregnated washcloths significantly reduced the incidence of bloodstream infections and decreased the acquisition of multidrug-resistant organisms in a randomized controlled trial reported online Feb. 7 in the New England Journal of Medicine.
The multicenter study, which included nine medical, coronary care, surgical, cardiac surgery, and bone marrow transplantation units in several geographic regions of the United States, confirms the results of previous single-center studies and demonstrates that the benefit of daily chlorhexidine bathing is widely generalizable, said Dr. Michael W. Climo of the Hunter Holmes McGuire Veterans Affairs Medical Center, Richmond, Va., and his associates.
Each hospital unit participating in the trial was randomized to perform daily bathing of patients using either washcloths impregnated with 2% chlorhexidine gluconate (intervention) or washcloths with no impregnated antimicrobial (control) for 6 months, followed by a crossover period in which patients were bathed daily with the alternate product for another 6 months. A total of 7,727 patients were included in the study.
Nurses were trained in the manufacturer’s instructions for bathing, which involved using the cloths in a sequential order to rinse all body surfaces except the face (to avoid getting chlorhexidine in the patient’s eyes and mouth). Implementing this cost-effective strategy was "relatively straightforward" because it did not require much of a change from current routine patient-bathing practices, the investigators said.
All the hospital units performed active surveillance for methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE) throughout the study.
There were 119 hospital-acquired bloodstream infections during the intervention and 165 during the control period, representing a 28% reduction with chlorhexidine bathing. The rate of primary bloodstream infections was 31% lower and the rate of central catheter–associated bloodstream infections was 53% lower with the intervention, Dr. Climo and his associates reported (N. Engl. J. Med. 2013 Feb. 6 [doi: 10.1056/NEJMoa1113849]).
This benefit was greatest among patients who had longer lengths of stay in the hospital units.
One "unanticipated" finding was that rates of fungal infection in particular were dramatically reduced with chlorhexidine bathing. The incidence of primary bloodstream infection caused by fungi was 53% lower and the incidence of central catheter–associated fungal bloodstream infection was 90% lower during the intervention period than during the control period.
This antifungal effect has not been reported previously. "If our results are confirmed, topical use of chlorhexidine could be added to strategies to prevent fungal infection," the researchers noted.
The rate of MRSA or VRE acquisition was 23% lower during the intervention period (5.10 cases per 1,000 patient-days) than during the control period (6.60 cases per 1,000 patient-days). However, reductions in the incidence of MRSA and VRE bacteremia did not reach statistical significance, most likely because the overall number of cases was so low, Dr. Climo and his colleagues said.
No serious adverse effects were observed. The incidence of skin reactions was higher with the control washcloths (3.4%) than with the chlorhexidine washcloths (2.0%), and none of the skin reactions were considered to be related to the bathing intervention.
It was reassuring that none of the MRSA or VRE isolates that were detected in this study showed resistance to chlorhexidine. However, the development of resistance to biocides and disinfectants among nosocomial organisms remains "a substantial concern" and should continue to be monitored, they added.
This study was supported by the Centers for Disease Control and Prevention and Sage Products. Sage Products supplied the washcloths, provided technical and educational support, and participated in weekly teleconferences with the research group but was not involved in the study design, data analysis, or reporting of the study results. Dr. Climo and his associates reported ties to Sage Products, Centene, and other companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: Bathing patients daily with chlorhexidine-impregnated washcloths reduced the rate of bloodstream infections by 28%, the rate of central catheter–associated infections by 53%, the rate of fungal bloodstream infections by 53%, and the rate of MRSA or VRE acquisition by 23%.
Data Source: A multicenter, randomized controlled trial assessing the ability of chlorhexidine bathing of 7,727 intensive care patients to reduce bloodstream infections and the acquisition of resistant organisms.
Disclosures: This study was supported by the Centers for Disease Control and Prevention and Sage Products. Sage Products supplied the washcloths, provided technical and educational support, and participated in weekly teleconferences with the research group but was not involved in the study design, data analysis, or reporting of the study results. Dr. Climo and his associates reported ties to Sage Products, Centene, and other companies.