User login
Official Newspaper of the American College of Surgeons
Opioid use cut nearly 50% for urologic oncology surgery patients
PHOENIX – Opioid use in urologic oncology patients dropped by 46% after one high-volume surgical center introduced changes to order sets and adopted new patient communication strategies, a researcher has reported.
The changes, which promoted opioid-sparing pain regimens, led to a substantial drop in postoperative opioid use with no compromise in pain control, according to Kerri Stevenson, a nurse practitioner with Stanford Health Care.
“Patients can be successfully managed with minimal opioid medication,” Ms. Stevenson said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
However, “it takes a multidisciplinary team for effective change to occur – this cannot be done in silos,” she told attendees at the meeting.
Seeking to reduce their reliance on opioids to manage postoperative pain, Ms. Stevenson and her colleagues set out to reduce opioid use by 50%, from a baseline morphine equivalent daily dose (MEDD) of 95.1 in June to September 2017 to a target of 47.5 by March 2018.
The actual MEDD at the end of the quality improvement project was 51.5, a 46% reduction that was just shy of that goal, she reported.
Factors fueling opioid use included patient expectations that they would be used and the belief that adjunct medications were not as effective as opioids, Dr. Stevenson found in a team survey.
“We decided to target those,” she said. “Our key drivers were really focused on appropriate prescriptions, increasing patient and provider awareness, standardizing our pathways, and setting expectations.”
To tackle the problem, they revised EMR order sets to default to selection of adjunct medications, educated providers, and introduced new patient communication strategies.
Instead of asking “Would you like me to bring you some oxycodone?” providers would instead start by asking about the patient’s current pain control medications and whether they were working well. When prescribed, opioids should be started at lower doses and escalated only if needed.
“Once we started our interventions, we noticed an immediate effect,” Ms. Stevenson.
The decreases were consistent across a range of surgery types. For example, the MEDD dropped to 55.1 with robotic prostatectomy, a procedure with a 1-day admission and very small incisions, and to 50.6 for open radical cystectomy, which involves a large incision and a stay of approximately 4 days, she said.
To address concerns that they might just be undertreating patients, investigators looked retrospectively at pain scores. They saw no differences pre- and post intervention in pain or anxiety scores within the first 24-48 hours post procedure, Ms. Stevenson reported.
Ms. Stevenson had no disclosures related to the presentation. Coauthor Jay Bakul Shah, MD of Stanford Health Care reported a consulting or advisory role with Pacira Pharmaceuticals.
SOURCE: Stevenson K et al. Quality Care Symposium, Abstract 269.
PHOENIX – Opioid use in urologic oncology patients dropped by 46% after one high-volume surgical center introduced changes to order sets and adopted new patient communication strategies, a researcher has reported.
The changes, which promoted opioid-sparing pain regimens, led to a substantial drop in postoperative opioid use with no compromise in pain control, according to Kerri Stevenson, a nurse practitioner with Stanford Health Care.
“Patients can be successfully managed with minimal opioid medication,” Ms. Stevenson said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
However, “it takes a multidisciplinary team for effective change to occur – this cannot be done in silos,” she told attendees at the meeting.
Seeking to reduce their reliance on opioids to manage postoperative pain, Ms. Stevenson and her colleagues set out to reduce opioid use by 50%, from a baseline morphine equivalent daily dose (MEDD) of 95.1 in June to September 2017 to a target of 47.5 by March 2018.
The actual MEDD at the end of the quality improvement project was 51.5, a 46% reduction that was just shy of that goal, she reported.
Factors fueling opioid use included patient expectations that they would be used and the belief that adjunct medications were not as effective as opioids, Dr. Stevenson found in a team survey.
“We decided to target those,” she said. “Our key drivers were really focused on appropriate prescriptions, increasing patient and provider awareness, standardizing our pathways, and setting expectations.”
To tackle the problem, they revised EMR order sets to default to selection of adjunct medications, educated providers, and introduced new patient communication strategies.
Instead of asking “Would you like me to bring you some oxycodone?” providers would instead start by asking about the patient’s current pain control medications and whether they were working well. When prescribed, opioids should be started at lower doses and escalated only if needed.
“Once we started our interventions, we noticed an immediate effect,” Ms. Stevenson.
The decreases were consistent across a range of surgery types. For example, the MEDD dropped to 55.1 with robotic prostatectomy, a procedure with a 1-day admission and very small incisions, and to 50.6 for open radical cystectomy, which involves a large incision and a stay of approximately 4 days, she said.
To address concerns that they might just be undertreating patients, investigators looked retrospectively at pain scores. They saw no differences pre- and post intervention in pain or anxiety scores within the first 24-48 hours post procedure, Ms. Stevenson reported.
Ms. Stevenson had no disclosures related to the presentation. Coauthor Jay Bakul Shah, MD of Stanford Health Care reported a consulting or advisory role with Pacira Pharmaceuticals.
SOURCE: Stevenson K et al. Quality Care Symposium, Abstract 269.
PHOENIX – Opioid use in urologic oncology patients dropped by 46% after one high-volume surgical center introduced changes to order sets and adopted new patient communication strategies, a researcher has reported.
The changes, which promoted opioid-sparing pain regimens, led to a substantial drop in postoperative opioid use with no compromise in pain control, according to Kerri Stevenson, a nurse practitioner with Stanford Health Care.
“Patients can be successfully managed with minimal opioid medication,” Ms. Stevenson said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
However, “it takes a multidisciplinary team for effective change to occur – this cannot be done in silos,” she told attendees at the meeting.
Seeking to reduce their reliance on opioids to manage postoperative pain, Ms. Stevenson and her colleagues set out to reduce opioid use by 50%, from a baseline morphine equivalent daily dose (MEDD) of 95.1 in June to September 2017 to a target of 47.5 by March 2018.
The actual MEDD at the end of the quality improvement project was 51.5, a 46% reduction that was just shy of that goal, she reported.
Factors fueling opioid use included patient expectations that they would be used and the belief that adjunct medications were not as effective as opioids, Dr. Stevenson found in a team survey.
“We decided to target those,” she said. “Our key drivers were really focused on appropriate prescriptions, increasing patient and provider awareness, standardizing our pathways, and setting expectations.”
To tackle the problem, they revised EMR order sets to default to selection of adjunct medications, educated providers, and introduced new patient communication strategies.
Instead of asking “Would you like me to bring you some oxycodone?” providers would instead start by asking about the patient’s current pain control medications and whether they were working well. When prescribed, opioids should be started at lower doses and escalated only if needed.
“Once we started our interventions, we noticed an immediate effect,” Ms. Stevenson.
The decreases were consistent across a range of surgery types. For example, the MEDD dropped to 55.1 with robotic prostatectomy, a procedure with a 1-day admission and very small incisions, and to 50.6 for open radical cystectomy, which involves a large incision and a stay of approximately 4 days, she said.
To address concerns that they might just be undertreating patients, investigators looked retrospectively at pain scores. They saw no differences pre- and post intervention in pain or anxiety scores within the first 24-48 hours post procedure, Ms. Stevenson reported.
Ms. Stevenson had no disclosures related to the presentation. Coauthor Jay Bakul Shah, MD of Stanford Health Care reported a consulting or advisory role with Pacira Pharmaceuticals.
SOURCE: Stevenson K et al. Quality Care Symposium, Abstract 269.
REPORTING FROM THE QUALITY CARE SYMPOSIUM
Key clinical point: Substantial reductions in postoperative opioid use might be achievable through strategies that promote opioid-sparing pain regimens.
Major finding: Postoperative opioid use dropped 46% for urologic oncology patients after changing default order sets, introducing new patient communication strategies, and educating providers.
Study details: An analysis of opioid prescribing before and after introduction of a quality improvement project at one high-volume surgical center.
Disclosures: One study coauthor reported a consulting or advisory role with Pacira Pharmaceuticals.
Source: Stevenson K et al. Quality Care Symposium, Abstract 269.
Gastric banding, metformin “equal” for slowing early T2DM progression
BERLIN – Gastric banding surgery and metformin produce similar improvements in insulin sensitivity and parameters indicative of preserved beta-cell function in patients with impaired glucose tolerance (IGT) or newly diagnosed type 2 diabetes mellitus (T2DM), according to the results of a study conducted by the Restoring Insulin Secretion (RISE) Consortium.
“Both interventions resulted in about 50% improvements in insulin sensitivity at 1 year, which was attenuated at 2 years,” reported study investigator Thomas Buchanan, MD, of the University of Southern California, Los Angeles, at the annual meeting of the European Association for the Study of Diabetes.
“The beta-cell responses fell in a pattern that maintained relatively, but not perfectly, stable compensation for insulin resistance,” he added.
Although glucose levels improved “only slightly,” he said, “acute compensation to glucose improved significantly with gastric banding and beta-cell compensation at maximal stimulation fell significantly with metformin.”
Results of the BetaFat (Beta Cell Restoration through Fat Mitigation) study, which are now published online in Diabetes Care, also showed that greater weight loss could be achieved with surgery versus metformin, with a 8.9 kg difference between the groups at 2 years (10.6 vs. 1.7 kg, respectively, P less than .01).
HDL cholesterol levels also rose with both interventions, and gastric banding resulted in a greater effect on very low–density lipoprotein cholesterol and triglycerides, as well as serum ALT, Dr. Buchanan said.
The BetaFat study is one of three “proof-of principle” studies currently being conducted by the RISE Consortium in patients with IGT, sometimes called prediabetes, and T2DM, explained Steven E. Kahn, MB, ChB, the chair for the RISE studies.
The other two multicenter, randomized trials being conducted by the RISE Consortium are looking at the effects of medications on preserving beta-cell function in pediatric/adolescent (10-19 years) and adult (21-65 years) populations with IGT or mild, recently diagnosed T2DM. The design, and some results, of these trials can be viewed on a dedicated section of the Diabetes Care website.
Beta-cell function is being assessed using “state-of-the-art” methods; the coprimary endpoint of the surgery versus metformin study was the steady state C-peptide level and acute C-peptide response at maximal glycemia measured using a hyperglycemic “clamp.”
The goal of the RISE studies is to test different approaches to preserve beta-cell function. It is designed to answer the question of which is more effective in this setting: sustained weight loss through gastric banding such as in the BetaFat study or medication.
Patients were eligible for inclusion in the study if they were aged 21-65 years, had a body mass index of 30-40 kg/m2, and had IGT or a diagnosis of T2DM within the past year for which they had received no diabetes medication at recruitment.
A total of 88 individuals were randomized with exactly half undergoing gastric banding. This consisted of a gastric band placed laparoscopically and adjusted every 2 months for the first year, and then every 3 months for the following year depending on symptoms and weight change.
Normoglycemia was observed in none of the study subjects at baseline but in 22% and 15% of those who had gastric banding or metformin, respectively, at 2 years (P = .66).
As for tolerability, five patients who underwent gastric banding experienced serious adverse events, of which two were caused by band slippage and three were caused other reasons. In the metformin arm, there were two serious adverse events, both unrelated to the medication.
“Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild to moderate obesity and impaired glucose tolerance or early, mild type 2 diabetes,” Dr. Buchanan concluded. “The predominant beta-cell response was a reduction in secretion to maintain a relatively constant compensation for insulin resistance, with only a small improvement in glucose. Whether these interventions will have different effects on beta-cell function over the long-term remains to be determined.”
Commenting on the study, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University (England), noted that the changes in the lipid and liver parameters were important. Fasting plasma triglyceride levels fell from 1.3 mmol/L at baseline to 1.1 mmol/L at 2 years with surgery but stayed more or less the same with metformin (1.23 mmol/L and 1.28 mmol/L; P less than .009 comparing surgery and metformin groups at 2 years). Change in ALT levels were also significant comparing baseline values with results at 2 years, decreasing in the surgical group to a greater extent than in the metformin groups.
“There’s a really important message here, the predictors of a better response to the weight loss [i.e. changes in triglycerides and liver enzymes] are all there,” Dr. Taylor observed. “RISE has looked at 2 years of this effect, but the conversion to type 2 diabetes is probably going to happen over a longer time course.”
He added that “although the primary outcome measure of change in insulin secretion was not achieved, the writing is on the wall. These people, provided they maintain their weight loss, are likely to succeed. We see all the hallmarks of a successful outcome for the weight loss group – remove the primary driver for type 2 diabetes, and that group is on track.”
The RISE Consortium conducted the BetaFat study. The RISE Consortium is supported by grants from the National Institutes for Health. Further support came from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies were provided by Allergan, Apollo Endosurgery, Abbott, and Novo Nordisk. Dr. Buchanan reported receiving research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
SOURCES: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
BERLIN – Gastric banding surgery and metformin produce similar improvements in insulin sensitivity and parameters indicative of preserved beta-cell function in patients with impaired glucose tolerance (IGT) or newly diagnosed type 2 diabetes mellitus (T2DM), according to the results of a study conducted by the Restoring Insulin Secretion (RISE) Consortium.
“Both interventions resulted in about 50% improvements in insulin sensitivity at 1 year, which was attenuated at 2 years,” reported study investigator Thomas Buchanan, MD, of the University of Southern California, Los Angeles, at the annual meeting of the European Association for the Study of Diabetes.
“The beta-cell responses fell in a pattern that maintained relatively, but not perfectly, stable compensation for insulin resistance,” he added.
Although glucose levels improved “only slightly,” he said, “acute compensation to glucose improved significantly with gastric banding and beta-cell compensation at maximal stimulation fell significantly with metformin.”
Results of the BetaFat (Beta Cell Restoration through Fat Mitigation) study, which are now published online in Diabetes Care, also showed that greater weight loss could be achieved with surgery versus metformin, with a 8.9 kg difference between the groups at 2 years (10.6 vs. 1.7 kg, respectively, P less than .01).
HDL cholesterol levels also rose with both interventions, and gastric banding resulted in a greater effect on very low–density lipoprotein cholesterol and triglycerides, as well as serum ALT, Dr. Buchanan said.
The BetaFat study is one of three “proof-of principle” studies currently being conducted by the RISE Consortium in patients with IGT, sometimes called prediabetes, and T2DM, explained Steven E. Kahn, MB, ChB, the chair for the RISE studies.
The other two multicenter, randomized trials being conducted by the RISE Consortium are looking at the effects of medications on preserving beta-cell function in pediatric/adolescent (10-19 years) and adult (21-65 years) populations with IGT or mild, recently diagnosed T2DM. The design, and some results, of these trials can be viewed on a dedicated section of the Diabetes Care website.
Beta-cell function is being assessed using “state-of-the-art” methods; the coprimary endpoint of the surgery versus metformin study was the steady state C-peptide level and acute C-peptide response at maximal glycemia measured using a hyperglycemic “clamp.”
The goal of the RISE studies is to test different approaches to preserve beta-cell function. It is designed to answer the question of which is more effective in this setting: sustained weight loss through gastric banding such as in the BetaFat study or medication.
Patients were eligible for inclusion in the study if they were aged 21-65 years, had a body mass index of 30-40 kg/m2, and had IGT or a diagnosis of T2DM within the past year for which they had received no diabetes medication at recruitment.
A total of 88 individuals were randomized with exactly half undergoing gastric banding. This consisted of a gastric band placed laparoscopically and adjusted every 2 months for the first year, and then every 3 months for the following year depending on symptoms and weight change.
Normoglycemia was observed in none of the study subjects at baseline but in 22% and 15% of those who had gastric banding or metformin, respectively, at 2 years (P = .66).
As for tolerability, five patients who underwent gastric banding experienced serious adverse events, of which two were caused by band slippage and three were caused other reasons. In the metformin arm, there were two serious adverse events, both unrelated to the medication.
“Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild to moderate obesity and impaired glucose tolerance or early, mild type 2 diabetes,” Dr. Buchanan concluded. “The predominant beta-cell response was a reduction in secretion to maintain a relatively constant compensation for insulin resistance, with only a small improvement in glucose. Whether these interventions will have different effects on beta-cell function over the long-term remains to be determined.”
Commenting on the study, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University (England), noted that the changes in the lipid and liver parameters were important. Fasting plasma triglyceride levels fell from 1.3 mmol/L at baseline to 1.1 mmol/L at 2 years with surgery but stayed more or less the same with metformin (1.23 mmol/L and 1.28 mmol/L; P less than .009 comparing surgery and metformin groups at 2 years). Change in ALT levels were also significant comparing baseline values with results at 2 years, decreasing in the surgical group to a greater extent than in the metformin groups.
“There’s a really important message here, the predictors of a better response to the weight loss [i.e. changes in triglycerides and liver enzymes] are all there,” Dr. Taylor observed. “RISE has looked at 2 years of this effect, but the conversion to type 2 diabetes is probably going to happen over a longer time course.”
He added that “although the primary outcome measure of change in insulin secretion was not achieved, the writing is on the wall. These people, provided they maintain their weight loss, are likely to succeed. We see all the hallmarks of a successful outcome for the weight loss group – remove the primary driver for type 2 diabetes, and that group is on track.”
The RISE Consortium conducted the BetaFat study. The RISE Consortium is supported by grants from the National Institutes for Health. Further support came from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies were provided by Allergan, Apollo Endosurgery, Abbott, and Novo Nordisk. Dr. Buchanan reported receiving research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
SOURCES: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
BERLIN – Gastric banding surgery and metformin produce similar improvements in insulin sensitivity and parameters indicative of preserved beta-cell function in patients with impaired glucose tolerance (IGT) or newly diagnosed type 2 diabetes mellitus (T2DM), according to the results of a study conducted by the Restoring Insulin Secretion (RISE) Consortium.
“Both interventions resulted in about 50% improvements in insulin sensitivity at 1 year, which was attenuated at 2 years,” reported study investigator Thomas Buchanan, MD, of the University of Southern California, Los Angeles, at the annual meeting of the European Association for the Study of Diabetes.
“The beta-cell responses fell in a pattern that maintained relatively, but not perfectly, stable compensation for insulin resistance,” he added.
Although glucose levels improved “only slightly,” he said, “acute compensation to glucose improved significantly with gastric banding and beta-cell compensation at maximal stimulation fell significantly with metformin.”
Results of the BetaFat (Beta Cell Restoration through Fat Mitigation) study, which are now published online in Diabetes Care, also showed that greater weight loss could be achieved with surgery versus metformin, with a 8.9 kg difference between the groups at 2 years (10.6 vs. 1.7 kg, respectively, P less than .01).
HDL cholesterol levels also rose with both interventions, and gastric banding resulted in a greater effect on very low–density lipoprotein cholesterol and triglycerides, as well as serum ALT, Dr. Buchanan said.
The BetaFat study is one of three “proof-of principle” studies currently being conducted by the RISE Consortium in patients with IGT, sometimes called prediabetes, and T2DM, explained Steven E. Kahn, MB, ChB, the chair for the RISE studies.
The other two multicenter, randomized trials being conducted by the RISE Consortium are looking at the effects of medications on preserving beta-cell function in pediatric/adolescent (10-19 years) and adult (21-65 years) populations with IGT or mild, recently diagnosed T2DM. The design, and some results, of these trials can be viewed on a dedicated section of the Diabetes Care website.
Beta-cell function is being assessed using “state-of-the-art” methods; the coprimary endpoint of the surgery versus metformin study was the steady state C-peptide level and acute C-peptide response at maximal glycemia measured using a hyperglycemic “clamp.”
The goal of the RISE studies is to test different approaches to preserve beta-cell function. It is designed to answer the question of which is more effective in this setting: sustained weight loss through gastric banding such as in the BetaFat study or medication.
Patients were eligible for inclusion in the study if they were aged 21-65 years, had a body mass index of 30-40 kg/m2, and had IGT or a diagnosis of T2DM within the past year for which they had received no diabetes medication at recruitment.
A total of 88 individuals were randomized with exactly half undergoing gastric banding. This consisted of a gastric band placed laparoscopically and adjusted every 2 months for the first year, and then every 3 months for the following year depending on symptoms and weight change.
Normoglycemia was observed in none of the study subjects at baseline but in 22% and 15% of those who had gastric banding or metformin, respectively, at 2 years (P = .66).
As for tolerability, five patients who underwent gastric banding experienced serious adverse events, of which two were caused by band slippage and three were caused other reasons. In the metformin arm, there were two serious adverse events, both unrelated to the medication.
“Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild to moderate obesity and impaired glucose tolerance or early, mild type 2 diabetes,” Dr. Buchanan concluded. “The predominant beta-cell response was a reduction in secretion to maintain a relatively constant compensation for insulin resistance, with only a small improvement in glucose. Whether these interventions will have different effects on beta-cell function over the long-term remains to be determined.”
Commenting on the study, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University (England), noted that the changes in the lipid and liver parameters were important. Fasting plasma triglyceride levels fell from 1.3 mmol/L at baseline to 1.1 mmol/L at 2 years with surgery but stayed more or less the same with metformin (1.23 mmol/L and 1.28 mmol/L; P less than .009 comparing surgery and metformin groups at 2 years). Change in ALT levels were also significant comparing baseline values with results at 2 years, decreasing in the surgical group to a greater extent than in the metformin groups.
“There’s a really important message here, the predictors of a better response to the weight loss [i.e. changes in triglycerides and liver enzymes] are all there,” Dr. Taylor observed. “RISE has looked at 2 years of this effect, but the conversion to type 2 diabetes is probably going to happen over a longer time course.”
He added that “although the primary outcome measure of change in insulin secretion was not achieved, the writing is on the wall. These people, provided they maintain their weight loss, are likely to succeed. We see all the hallmarks of a successful outcome for the weight loss group – remove the primary driver for type 2 diabetes, and that group is on track.”
The RISE Consortium conducted the BetaFat study. The RISE Consortium is supported by grants from the National Institutes for Health. Further support came from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies were provided by Allergan, Apollo Endosurgery, Abbott, and Novo Nordisk. Dr. Buchanan reported receiving research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
SOURCES: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
REPORTING FROM EASD 2018
Key clinical point: Over 2 years, gastric banding surgery and metformin produced similar improvements in insulin sensitivity and parameters suggestive of preserved beta-cell function in patients with prediabetes or early type 2 diabetes.
Major finding: Around a 50% improvement in insulin sensitivity was seen in both study groups at 1 year with attenuation of the effect at 2 years.
Study details: The BetaFat study included 88 obese adults with impaired glucose tolerance or newly diagnosed early type 2 diabetes.
Disclosures: The study was part of the RISE studies, which are supported by grants from the National Institutes for Health. Further support comes from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies are provided by Allergan, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk. Dr. Buchanan reported research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
Sources: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
Employer health insurance: Deductibles rising faster than wages
Health insurance deductibles have risen much faster than average wages over the last 10 years, according to the latest Employer Health Benefit Survey released by the Kaiser Family Foundation.
“The share of workers in plans with a general annual deductible has gone from 59% to 85%, and I think even more notably, the average deductible has more than doubled from $735 to $1,573 and deductibles have risen more markedly in smaller firms,” Drew Altman, president and CEO of the KFF said during an Oct. 3 press conference.
“These two trends combine for an effective 212% increase in worker deductibles over the past decade, and that is 8 times the increase in workers’ wages during the same period, which for me is the most important number,” he said.
Employer health care costs generally have remained stable, according to the annual survey, now in its 20th year. Annual family premiums for employer-sponsored health insurance rose 5% to an average $19,616 in 2018, extending a 7-year run of moderate increases. The average premium paid by the employee is $5,547.
For a single individual, the average premium increased 3% to $6,896, with employees contributing an average of $1,186.
Although the year-over-year comparison has a premiums increase comparable to that of wages (2.6%) and inflation ($2.5%), over time, premiums are rising much faster.
KFF noted that 85% of employees have a deductible in their plan, up from 81% last year and 59% a decade ago. About 152 million Americans are covered by an employer-sponsored plan.
“Health care costs absolutely remain a burden for employers, but they are a bigger problem for workers as their cost sharing has been rising much faster than their wages have been rising in recent years,” Mr. Altman said.
The survey found that 70% of large employers offer some kind of complete health risk assessments and 38% offer incentives for workers to participate in these programs, with the value of incentives reaching $500 or more.
SOURCE: Kaiser Family Foundation, 2018 Employer Health Benefits.
Health insurance deductibles have risen much faster than average wages over the last 10 years, according to the latest Employer Health Benefit Survey released by the Kaiser Family Foundation.
“The share of workers in plans with a general annual deductible has gone from 59% to 85%, and I think even more notably, the average deductible has more than doubled from $735 to $1,573 and deductibles have risen more markedly in smaller firms,” Drew Altman, president and CEO of the KFF said during an Oct. 3 press conference.
“These two trends combine for an effective 212% increase in worker deductibles over the past decade, and that is 8 times the increase in workers’ wages during the same period, which for me is the most important number,” he said.
Employer health care costs generally have remained stable, according to the annual survey, now in its 20th year. Annual family premiums for employer-sponsored health insurance rose 5% to an average $19,616 in 2018, extending a 7-year run of moderate increases. The average premium paid by the employee is $5,547.
For a single individual, the average premium increased 3% to $6,896, with employees contributing an average of $1,186.
Although the year-over-year comparison has a premiums increase comparable to that of wages (2.6%) and inflation ($2.5%), over time, premiums are rising much faster.
KFF noted that 85% of employees have a deductible in their plan, up from 81% last year and 59% a decade ago. About 152 million Americans are covered by an employer-sponsored plan.
“Health care costs absolutely remain a burden for employers, but they are a bigger problem for workers as their cost sharing has been rising much faster than their wages have been rising in recent years,” Mr. Altman said.
The survey found that 70% of large employers offer some kind of complete health risk assessments and 38% offer incentives for workers to participate in these programs, with the value of incentives reaching $500 or more.
SOURCE: Kaiser Family Foundation, 2018 Employer Health Benefits.
Health insurance deductibles have risen much faster than average wages over the last 10 years, according to the latest Employer Health Benefit Survey released by the Kaiser Family Foundation.
“The share of workers in plans with a general annual deductible has gone from 59% to 85%, and I think even more notably, the average deductible has more than doubled from $735 to $1,573 and deductibles have risen more markedly in smaller firms,” Drew Altman, president and CEO of the KFF said during an Oct. 3 press conference.
“These two trends combine for an effective 212% increase in worker deductibles over the past decade, and that is 8 times the increase in workers’ wages during the same period, which for me is the most important number,” he said.
Employer health care costs generally have remained stable, according to the annual survey, now in its 20th year. Annual family premiums for employer-sponsored health insurance rose 5% to an average $19,616 in 2018, extending a 7-year run of moderate increases. The average premium paid by the employee is $5,547.
For a single individual, the average premium increased 3% to $6,896, with employees contributing an average of $1,186.
Although the year-over-year comparison has a premiums increase comparable to that of wages (2.6%) and inflation ($2.5%), over time, premiums are rising much faster.
KFF noted that 85% of employees have a deductible in their plan, up from 81% last year and 59% a decade ago. About 152 million Americans are covered by an employer-sponsored plan.
“Health care costs absolutely remain a burden for employers, but they are a bigger problem for workers as their cost sharing has been rising much faster than their wages have been rising in recent years,” Mr. Altman said.
The survey found that 70% of large employers offer some kind of complete health risk assessments and 38% offer incentives for workers to participate in these programs, with the value of incentives reaching $500 or more.
SOURCE: Kaiser Family Foundation, 2018 Employer Health Benefits.
Key clinical point: The average employee deductible rose from $735 to $1,573 in the last decade.
Major finding: Deductibles have risen 8 times faster than wages since 2008.
Study details: Kaiser Family Foundation surveyed 4,070 randomly selected nonfederal public and private firms with three or more employees; 2,160 responded to the full survey and 1,910 responded to a single question about offering coverage.
Disclosures: No financial conflicts of interest reported.
Source: Kaiser Family Foundation, 2018 Employer Health Benefits.
This is a drill
She had fallen on a garden implement, lacerating her superficial femoral artery. She used her cell phone to call 911. Thanks to an alert EMS crew and the Stop the Bleed training they recently received, a tourniquet was placed without delay. She got to our trauma bay in about 15 minutes after the tourniquet was applied. Although the patient made it abundantly clear that she was in pain, she was stable with moderate tachycardia and a good blood pressure.
Our trauma team leaped into action. The leader took report from the EMS while two nurses and a second surgeon assessed the patient, got her clothes cut off, and applied monitors. A third nurse got a second IV going. Primary survey was done in less than 90 seconds. The patient then underwent a focused exam including a log roll for back injuries.
The leg wound was still oozing a bit, so a second tourniquet was called for and pressure applied until it could be acquired. The patient was given 5 mg of morphine sulfate, which calmed her down a bit. Labs and x-rays were done quickly. The nursing staff suggested a tetanus booster, and the second surgeon who had gotten a basic past medical history suggested vancomycin since the patient said she was allergic to penicillin. Fifteen minutes after she hit the trauma bay, she was on her way to the OR for exploration, debridement, and vascular repair of her injury.
This was all done by four M2 medical students and five N4 nursing students, none of whom had had previous experience with this type of trauma patient
The students were managing this trauma situation in the simulation center of their medical school with four staff watching. This was their second run through for the afternoon. At debriefing, they compared their work on the first trauma of the day (a stab wound to the right chest) to their second attempt. They were satisfied with their efforts and so were we, the faculty who ran the simulation. Comparing their response to those I’ve seen in real life, I’d say these students understood their roles and responsibilities as well as the sort of thrown-together teams I’ve seen at places where trauma is not the main focus. While these young men and women are in the early stage of training and not ready for a real-world trauma emergency, they have gained knowledge about this kind of situation that I didn’t see until I was in residency and beyond. The times they are a-changing.
A couple of days later I was in Rochester, Minn., attending an American College of Surgeons Advanced Education Institute (ACS/AEI) course on simulations. At the end of that course, we participants were challenged by a manikin in extremis. Everyone there was an expert, had an advanced degree, had some experience in simulation, or were surgeons interested in simulation. I found that, even though this was a simulation and the patient was only a pretend human being, my adrenal cortex performed almost as if I were doing a real resuscitation. Previous training I’d had on teamwork, crew resource management, and ACLS all kicked in, and we got it done. But interestingly, we weren’t perfect. We debriefed and found that, even at our level of experience and training, a simple simulation could be very instructive. Seeing/doing is believing.
High-tech skills in high-risk occupations are well served by simulation training. Much of the airline piloting training is done by simulation. It works well for aviation, nuclear reactors, high voltage line work, and medicine. Most of these disciplines have embraced simulation as an essential part of training. Simulation is part of many surgical training programs, but it has other uses.
When was the last time you practiced a trauma resuscitation, an ultrasound fine-needle biopsy, laparoscopic maneuvers, or an unusual technique that you seldom perform, but when needed, must be pulled off very well? Most of us taking this simulation course agreed that time, money, and ego may get in the way of maintaining those skills for those rare instances when they are needed. Surgeons might want to consider simulation to keep some of our rarely used skills from getting rusty.
If you’re going to make a costly error, I would very much like you to do it on a piece of plastic, not on a patient. There are no consequences for messing up a procedure on a manikin and this kind of practice might teach you something critical. Practicing reduces stress and improves the performance of those placed on the spot by real-life events. Do you think Captain “Sully” Sullenberger could have landed that airliner in the Hudson River safely if he hadn’t practiced with countless mind-numbingly complex simulations? Sure, luck plays a part, and innate ability plays a part. But skill, knowledge, and practice are your best bet when all the eyes in the room swivel to you in a moment of crisis.
You may think that simulators have to cost $100,000 and be completely realistic to do the job. That’s not true. A banana, orange, or stick of butter can be fabulous sims for a med student. Felt and cardboard can make a realistic cricothyroidotomy model.
Surgeons all over the country are using simulation training to learn how to be better without getting real blood on their shoes. If you haven’t participated in a training simulation recently, I double-dog dare you to try it and tell me you found it without merit. The ACS Surgical Simulation Summit is being held in March 2019 in Chicago. You might want to check that out.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the University of Kansas School of Medicine, Salina, and Coeditor of ACS Surgery News.
She had fallen on a garden implement, lacerating her superficial femoral artery. She used her cell phone to call 911. Thanks to an alert EMS crew and the Stop the Bleed training they recently received, a tourniquet was placed without delay. She got to our trauma bay in about 15 minutes after the tourniquet was applied. Although the patient made it abundantly clear that she was in pain, she was stable with moderate tachycardia and a good blood pressure.
Our trauma team leaped into action. The leader took report from the EMS while two nurses and a second surgeon assessed the patient, got her clothes cut off, and applied monitors. A third nurse got a second IV going. Primary survey was done in less than 90 seconds. The patient then underwent a focused exam including a log roll for back injuries.
The leg wound was still oozing a bit, so a second tourniquet was called for and pressure applied until it could be acquired. The patient was given 5 mg of morphine sulfate, which calmed her down a bit. Labs and x-rays were done quickly. The nursing staff suggested a tetanus booster, and the second surgeon who had gotten a basic past medical history suggested vancomycin since the patient said she was allergic to penicillin. Fifteen minutes after she hit the trauma bay, she was on her way to the OR for exploration, debridement, and vascular repair of her injury.
This was all done by four M2 medical students and five N4 nursing students, none of whom had had previous experience with this type of trauma patient
The students were managing this trauma situation in the simulation center of their medical school with four staff watching. This was their second run through for the afternoon. At debriefing, they compared their work on the first trauma of the day (a stab wound to the right chest) to their second attempt. They were satisfied with their efforts and so were we, the faculty who ran the simulation. Comparing their response to those I’ve seen in real life, I’d say these students understood their roles and responsibilities as well as the sort of thrown-together teams I’ve seen at places where trauma is not the main focus. While these young men and women are in the early stage of training and not ready for a real-world trauma emergency, they have gained knowledge about this kind of situation that I didn’t see until I was in residency and beyond. The times they are a-changing.
A couple of days later I was in Rochester, Minn., attending an American College of Surgeons Advanced Education Institute (ACS/AEI) course on simulations. At the end of that course, we participants were challenged by a manikin in extremis. Everyone there was an expert, had an advanced degree, had some experience in simulation, or were surgeons interested in simulation. I found that, even though this was a simulation and the patient was only a pretend human being, my adrenal cortex performed almost as if I were doing a real resuscitation. Previous training I’d had on teamwork, crew resource management, and ACLS all kicked in, and we got it done. But interestingly, we weren’t perfect. We debriefed and found that, even at our level of experience and training, a simple simulation could be very instructive. Seeing/doing is believing.
High-tech skills in high-risk occupations are well served by simulation training. Much of the airline piloting training is done by simulation. It works well for aviation, nuclear reactors, high voltage line work, and medicine. Most of these disciplines have embraced simulation as an essential part of training. Simulation is part of many surgical training programs, but it has other uses.
When was the last time you practiced a trauma resuscitation, an ultrasound fine-needle biopsy, laparoscopic maneuvers, or an unusual technique that you seldom perform, but when needed, must be pulled off very well? Most of us taking this simulation course agreed that time, money, and ego may get in the way of maintaining those skills for those rare instances when they are needed. Surgeons might want to consider simulation to keep some of our rarely used skills from getting rusty.
If you’re going to make a costly error, I would very much like you to do it on a piece of plastic, not on a patient. There are no consequences for messing up a procedure on a manikin and this kind of practice might teach you something critical. Practicing reduces stress and improves the performance of those placed on the spot by real-life events. Do you think Captain “Sully” Sullenberger could have landed that airliner in the Hudson River safely if he hadn’t practiced with countless mind-numbingly complex simulations? Sure, luck plays a part, and innate ability plays a part. But skill, knowledge, and practice are your best bet when all the eyes in the room swivel to you in a moment of crisis.
You may think that simulators have to cost $100,000 and be completely realistic to do the job. That’s not true. A banana, orange, or stick of butter can be fabulous sims for a med student. Felt and cardboard can make a realistic cricothyroidotomy model.
Surgeons all over the country are using simulation training to learn how to be better without getting real blood on their shoes. If you haven’t participated in a training simulation recently, I double-dog dare you to try it and tell me you found it without merit. The ACS Surgical Simulation Summit is being held in March 2019 in Chicago. You might want to check that out.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the University of Kansas School of Medicine, Salina, and Coeditor of ACS Surgery News.
She had fallen on a garden implement, lacerating her superficial femoral artery. She used her cell phone to call 911. Thanks to an alert EMS crew and the Stop the Bleed training they recently received, a tourniquet was placed without delay. She got to our trauma bay in about 15 minutes after the tourniquet was applied. Although the patient made it abundantly clear that she was in pain, she was stable with moderate tachycardia and a good blood pressure.
Our trauma team leaped into action. The leader took report from the EMS while two nurses and a second surgeon assessed the patient, got her clothes cut off, and applied monitors. A third nurse got a second IV going. Primary survey was done in less than 90 seconds. The patient then underwent a focused exam including a log roll for back injuries.
The leg wound was still oozing a bit, so a second tourniquet was called for and pressure applied until it could be acquired. The patient was given 5 mg of morphine sulfate, which calmed her down a bit. Labs and x-rays were done quickly. The nursing staff suggested a tetanus booster, and the second surgeon who had gotten a basic past medical history suggested vancomycin since the patient said she was allergic to penicillin. Fifteen minutes after she hit the trauma bay, she was on her way to the OR for exploration, debridement, and vascular repair of her injury.
This was all done by four M2 medical students and five N4 nursing students, none of whom had had previous experience with this type of trauma patient
The students were managing this trauma situation in the simulation center of their medical school with four staff watching. This was their second run through for the afternoon. At debriefing, they compared their work on the first trauma of the day (a stab wound to the right chest) to their second attempt. They were satisfied with their efforts and so were we, the faculty who ran the simulation. Comparing their response to those I’ve seen in real life, I’d say these students understood their roles and responsibilities as well as the sort of thrown-together teams I’ve seen at places where trauma is not the main focus. While these young men and women are in the early stage of training and not ready for a real-world trauma emergency, they have gained knowledge about this kind of situation that I didn’t see until I was in residency and beyond. The times they are a-changing.
A couple of days later I was in Rochester, Minn., attending an American College of Surgeons Advanced Education Institute (ACS/AEI) course on simulations. At the end of that course, we participants were challenged by a manikin in extremis. Everyone there was an expert, had an advanced degree, had some experience in simulation, or were surgeons interested in simulation. I found that, even though this was a simulation and the patient was only a pretend human being, my adrenal cortex performed almost as if I were doing a real resuscitation. Previous training I’d had on teamwork, crew resource management, and ACLS all kicked in, and we got it done. But interestingly, we weren’t perfect. We debriefed and found that, even at our level of experience and training, a simple simulation could be very instructive. Seeing/doing is believing.
High-tech skills in high-risk occupations are well served by simulation training. Much of the airline piloting training is done by simulation. It works well for aviation, nuclear reactors, high voltage line work, and medicine. Most of these disciplines have embraced simulation as an essential part of training. Simulation is part of many surgical training programs, but it has other uses.
When was the last time you practiced a trauma resuscitation, an ultrasound fine-needle biopsy, laparoscopic maneuvers, or an unusual technique that you seldom perform, but when needed, must be pulled off very well? Most of us taking this simulation course agreed that time, money, and ego may get in the way of maintaining those skills for those rare instances when they are needed. Surgeons might want to consider simulation to keep some of our rarely used skills from getting rusty.
If you’re going to make a costly error, I would very much like you to do it on a piece of plastic, not on a patient. There are no consequences for messing up a procedure on a manikin and this kind of practice might teach you something critical. Practicing reduces stress and improves the performance of those placed on the spot by real-life events. Do you think Captain “Sully” Sullenberger could have landed that airliner in the Hudson River safely if he hadn’t practiced with countless mind-numbingly complex simulations? Sure, luck plays a part, and innate ability plays a part. But skill, knowledge, and practice are your best bet when all the eyes in the room swivel to you in a moment of crisis.
You may think that simulators have to cost $100,000 and be completely realistic to do the job. That’s not true. A banana, orange, or stick of butter can be fabulous sims for a med student. Felt and cardboard can make a realistic cricothyroidotomy model.
Surgeons all over the country are using simulation training to learn how to be better without getting real blood on their shoes. If you haven’t participated in a training simulation recently, I double-dog dare you to try it and tell me you found it without merit. The ACS Surgical Simulation Summit is being held in March 2019 in Chicago. You might want to check that out.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the University of Kansas School of Medicine, Salina, and Coeditor of ACS Surgery News.
Communication and consent
We knew that the case would be a difficult one. The patient was a man in his mid-40s who had several serious chronic conditions and was on high-dose steroids. He had been operated on 10 days earlier by one of my partners for a bowel obstruction and had required a resection of a small portion of the terminal ileum. Unfortunately, on the day after surgery, it became obvious that the patient needed a reexploration for bleeding. He had developed clear evidence of a significant anastomotic leak and had to be taken emergently back to the operating room.
His condition had been worsening during the day. We had booked the case in the OR but had been put off by a trauma emergency and a neurosurgical emergency. During the 3 hours of waiting to take him to the OR, the patient’s sister and mother came to the hospital and were now waiting with him in the preop area. I was on my way up to see him when my resident called. Despite the patient having signed an operative consent form a few hours earlier when we booked the case, he was now “declining” an operation. I was surprised. This man had undergone several operations in the last few years and two in the last 2 weeks. I arrived to find the patient stating that he did not want surgery. Lying in bed, he was adamant that he should not have surgery. The surgical resident who had spoken with the patient several times over the last few hours was also surprised. The patient’s family members were yelling that, of course, he wanted surgery and why would he change his mind.
This is a difficult situation since one of the central tenets of the ethical practice of surgery is to allow patients to make decisions about their own care. The right to make autonomous choices even extends to circumstances in which patients make what we might consider “bad” decisions. As long as the patient has the capacity to make an autonomous choice, he or she should have that choice respected.
This patient, who just a few hours ago had agreed to surgery, now seemed to have changed his mind. Although it can be frustrating, we do allow patients to change their minds. On the one hand, this was a straightforward case. The patient was refusing a potentially life-saving operation. Such a situation is never pleasant for a surgeon, but as long as the patient understands the risks, we respect such choices.
However, my resident made an astute observation. She pointed out that, when asked why he now did not want surgery, he replied that “this is all a movie – it’s not really happening.” The patient appeared to be oriented to person and place, but nevertheless, his reasoning seemed to have been altered. It appeared that this patient was no longer making sense because his underlying medical condition had deteriorated. We considered whether he was becoming septic and that this change in medical condition had rendered him unable to make an informed decision. My resident, who had discussed the operation with the patient several times, stated that the patient’s decision making seemed very different than even an hour ago. His family members agreed, stating that, up until a few minutes before, he was in favor of surgery. They pleaded with us to just take him into the operating room.
We considered our options. We could delay surgery and consult psychiatry to ask them to assess his competency. However, on a weekend night, this would likely take several hours. We considered the option of waiting in the preop area for the patient’s medical condition to further worsen. If he became overtly septic and lost consciousness, then we could readily turn to the family members – his surrogate decision makers – and ask them to consent to the procedure. Although this “by the book” approach might take away any worry that we were overriding an autonomous patient’s choice, we knew that it would unnecessarily expose him to greater operative risks. This option was not in his best interest and therefore not much of an option.
Ultimately, the surgical resident, the attending anesthesiologist, the family, and I decided that his decision to not have surgery at this moment was not consistent with his prior decisions, and he could provide no reason for changing his mind. We brought the patient into the operating room and explored him. He did have a large anastomotic leak with a large volume of enteric contents in the peritoneal cavity. He survived the operation and, not unexpectedly, required a long postoperative stay in the hospital. Once he was a few days out, I inquired about whether he was glad that he had surgery. He was quick to state his confidence that it had been the right choice for him. He did not even remember having ever refused the surgery.
Although this case raised many concerns for all of us involved in the patient’s care, one overriding lesson that came through to me. Informed consent should not be viewed as a solitary event, but a conversation. This patient had expressed his desire to have surgery multiple times to my surgical resident and to his family. Even though we should never take the position that patients cannot change their minds, we should carefully question those choices that are inconsistent with the prior discussions that have been undertaken. Good communication skills – including listening to the patient, understanding the patient’s reasoning, and reflecting on the entire conversation – are essential in obtaining informed consent.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
We knew that the case would be a difficult one. The patient was a man in his mid-40s who had several serious chronic conditions and was on high-dose steroids. He had been operated on 10 days earlier by one of my partners for a bowel obstruction and had required a resection of a small portion of the terminal ileum. Unfortunately, on the day after surgery, it became obvious that the patient needed a reexploration for bleeding. He had developed clear evidence of a significant anastomotic leak and had to be taken emergently back to the operating room.
His condition had been worsening during the day. We had booked the case in the OR but had been put off by a trauma emergency and a neurosurgical emergency. During the 3 hours of waiting to take him to the OR, the patient’s sister and mother came to the hospital and were now waiting with him in the preop area. I was on my way up to see him when my resident called. Despite the patient having signed an operative consent form a few hours earlier when we booked the case, he was now “declining” an operation. I was surprised. This man had undergone several operations in the last few years and two in the last 2 weeks. I arrived to find the patient stating that he did not want surgery. Lying in bed, he was adamant that he should not have surgery. The surgical resident who had spoken with the patient several times over the last few hours was also surprised. The patient’s family members were yelling that, of course, he wanted surgery and why would he change his mind.
This is a difficult situation since one of the central tenets of the ethical practice of surgery is to allow patients to make decisions about their own care. The right to make autonomous choices even extends to circumstances in which patients make what we might consider “bad” decisions. As long as the patient has the capacity to make an autonomous choice, he or she should have that choice respected.
This patient, who just a few hours ago had agreed to surgery, now seemed to have changed his mind. Although it can be frustrating, we do allow patients to change their minds. On the one hand, this was a straightforward case. The patient was refusing a potentially life-saving operation. Such a situation is never pleasant for a surgeon, but as long as the patient understands the risks, we respect such choices.
However, my resident made an astute observation. She pointed out that, when asked why he now did not want surgery, he replied that “this is all a movie – it’s not really happening.” The patient appeared to be oriented to person and place, but nevertheless, his reasoning seemed to have been altered. It appeared that this patient was no longer making sense because his underlying medical condition had deteriorated. We considered whether he was becoming septic and that this change in medical condition had rendered him unable to make an informed decision. My resident, who had discussed the operation with the patient several times, stated that the patient’s decision making seemed very different than even an hour ago. His family members agreed, stating that, up until a few minutes before, he was in favor of surgery. They pleaded with us to just take him into the operating room.
We considered our options. We could delay surgery and consult psychiatry to ask them to assess his competency. However, on a weekend night, this would likely take several hours. We considered the option of waiting in the preop area for the patient’s medical condition to further worsen. If he became overtly septic and lost consciousness, then we could readily turn to the family members – his surrogate decision makers – and ask them to consent to the procedure. Although this “by the book” approach might take away any worry that we were overriding an autonomous patient’s choice, we knew that it would unnecessarily expose him to greater operative risks. This option was not in his best interest and therefore not much of an option.
Ultimately, the surgical resident, the attending anesthesiologist, the family, and I decided that his decision to not have surgery at this moment was not consistent with his prior decisions, and he could provide no reason for changing his mind. We brought the patient into the operating room and explored him. He did have a large anastomotic leak with a large volume of enteric contents in the peritoneal cavity. He survived the operation and, not unexpectedly, required a long postoperative stay in the hospital. Once he was a few days out, I inquired about whether he was glad that he had surgery. He was quick to state his confidence that it had been the right choice for him. He did not even remember having ever refused the surgery.
Although this case raised many concerns for all of us involved in the patient’s care, one overriding lesson that came through to me. Informed consent should not be viewed as a solitary event, but a conversation. This patient had expressed his desire to have surgery multiple times to my surgical resident and to his family. Even though we should never take the position that patients cannot change their minds, we should carefully question those choices that are inconsistent with the prior discussions that have been undertaken. Good communication skills – including listening to the patient, understanding the patient’s reasoning, and reflecting on the entire conversation – are essential in obtaining informed consent.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
We knew that the case would be a difficult one. The patient was a man in his mid-40s who had several serious chronic conditions and was on high-dose steroids. He had been operated on 10 days earlier by one of my partners for a bowel obstruction and had required a resection of a small portion of the terminal ileum. Unfortunately, on the day after surgery, it became obvious that the patient needed a reexploration for bleeding. He had developed clear evidence of a significant anastomotic leak and had to be taken emergently back to the operating room.
His condition had been worsening during the day. We had booked the case in the OR but had been put off by a trauma emergency and a neurosurgical emergency. During the 3 hours of waiting to take him to the OR, the patient’s sister and mother came to the hospital and were now waiting with him in the preop area. I was on my way up to see him when my resident called. Despite the patient having signed an operative consent form a few hours earlier when we booked the case, he was now “declining” an operation. I was surprised. This man had undergone several operations in the last few years and two in the last 2 weeks. I arrived to find the patient stating that he did not want surgery. Lying in bed, he was adamant that he should not have surgery. The surgical resident who had spoken with the patient several times over the last few hours was also surprised. The patient’s family members were yelling that, of course, he wanted surgery and why would he change his mind.
This is a difficult situation since one of the central tenets of the ethical practice of surgery is to allow patients to make decisions about their own care. The right to make autonomous choices even extends to circumstances in which patients make what we might consider “bad” decisions. As long as the patient has the capacity to make an autonomous choice, he or she should have that choice respected.
This patient, who just a few hours ago had agreed to surgery, now seemed to have changed his mind. Although it can be frustrating, we do allow patients to change their minds. On the one hand, this was a straightforward case. The patient was refusing a potentially life-saving operation. Such a situation is never pleasant for a surgeon, but as long as the patient understands the risks, we respect such choices.
However, my resident made an astute observation. She pointed out that, when asked why he now did not want surgery, he replied that “this is all a movie – it’s not really happening.” The patient appeared to be oriented to person and place, but nevertheless, his reasoning seemed to have been altered. It appeared that this patient was no longer making sense because his underlying medical condition had deteriorated. We considered whether he was becoming septic and that this change in medical condition had rendered him unable to make an informed decision. My resident, who had discussed the operation with the patient several times, stated that the patient’s decision making seemed very different than even an hour ago. His family members agreed, stating that, up until a few minutes before, he was in favor of surgery. They pleaded with us to just take him into the operating room.
We considered our options. We could delay surgery and consult psychiatry to ask them to assess his competency. However, on a weekend night, this would likely take several hours. We considered the option of waiting in the preop area for the patient’s medical condition to further worsen. If he became overtly septic and lost consciousness, then we could readily turn to the family members – his surrogate decision makers – and ask them to consent to the procedure. Although this “by the book” approach might take away any worry that we were overriding an autonomous patient’s choice, we knew that it would unnecessarily expose him to greater operative risks. This option was not in his best interest and therefore not much of an option.
Ultimately, the surgical resident, the attending anesthesiologist, the family, and I decided that his decision to not have surgery at this moment was not consistent with his prior decisions, and he could provide no reason for changing his mind. We brought the patient into the operating room and explored him. He did have a large anastomotic leak with a large volume of enteric contents in the peritoneal cavity. He survived the operation and, not unexpectedly, required a long postoperative stay in the hospital. Once he was a few days out, I inquired about whether he was glad that he had surgery. He was quick to state his confidence that it had been the right choice for him. He did not even remember having ever refused the surgery.
Although this case raised many concerns for all of us involved in the patient’s care, one overriding lesson that came through to me. Informed consent should not be viewed as a solitary event, but a conversation. This patient had expressed his desire to have surgery multiple times to my surgical resident and to his family. Even though we should never take the position that patients cannot change their minds, we should carefully question those choices that are inconsistent with the prior discussions that have been undertaken. Good communication skills – including listening to the patient, understanding the patient’s reasoning, and reflecting on the entire conversation – are essential in obtaining informed consent.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
U.S. vs. Europe: Costs of cardiac implant devices compared
Prices that hospitals pay for cardiac implant devices are two to six times higher in the United States than in Europe, according to analysis of a large hospital panel survey.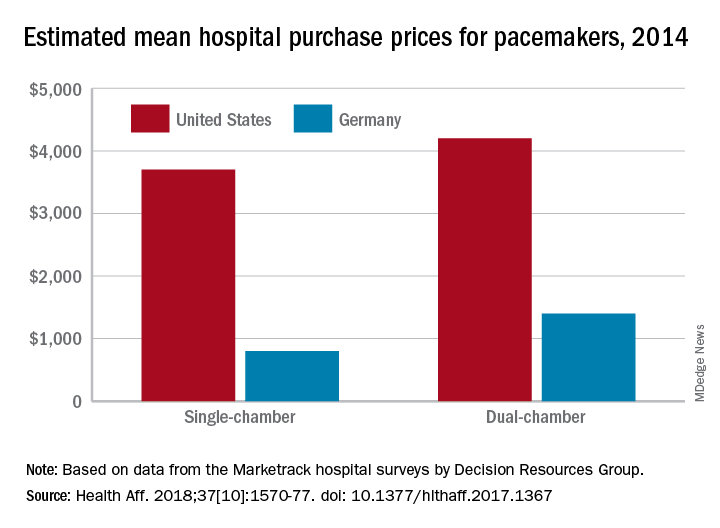
U.S. hospitals had an estimated mean cost of $670 for a bare-metal stent in 2014, compared with $120 in Germany, and the mean costs for dual-chamber pacemakers that year were $4,200 in the United States and $1,400 in Germany, which had lower costs for cardiac devices than the other three European countries – United Kingdom, France, and Italy – included in the study, Martin Wenzl, MSc, and Elias Mossialos, MD, PhD, reported in Health Affairs.
France generally had the highest costs among the European countries, with Italy next and then the United Kingdom. The estimated cost of bare-metal stents was actually higher for French hospitals ($750) than for those in the United States, and Italy had mean prices similar to the United Sates for dual-chamber implantable cardioverter-defibrillators. The prices of implantable cardioverter-defibrillators and cardiac resynchronization devices with defibrillating function were the other exceptions, with the United Kingdom similar to or higher than the United States, said Mr. Wenzl and Dr. Mossialos, both of the London School of Economics and Political Science.
The analysis of data from Decision Resources Group’s Marketrack hospital surveys also showed significant variation between the hospitals in each country, with the exception of France, where payments are based on the specific device rather than the procedure and the system “creates weak incentives for hospitals to negotiate lower prices,” they said. In most of the device categories, “variation between hospitals in each country was similar to variation between countries,” they wrote, adding that prices in general “were only weakly correlated with volumes purchased by hospitals.”
The study was supported by a grant from the Commonwealth Fund. The investigators did not disclose any possible conflicts of interest.
SOURCE: Wenzi M, Mossialos E. Health Aff. 2018;37[10]:1570-77. doi: 10.1377/hlthaff.2017.1367.
Prices that hospitals pay for cardiac implant devices are two to six times higher in the United States than in Europe, according to analysis of a large hospital panel survey.
U.S. hospitals had an estimated mean cost of $670 for a bare-metal stent in 2014, compared with $120 in Germany, and the mean costs for dual-chamber pacemakers that year were $4,200 in the United States and $1,400 in Germany, which had lower costs for cardiac devices than the other three European countries – United Kingdom, France, and Italy – included in the study, Martin Wenzl, MSc, and Elias Mossialos, MD, PhD, reported in Health Affairs.
France generally had the highest costs among the European countries, with Italy next and then the United Kingdom. The estimated cost of bare-metal stents was actually higher for French hospitals ($750) than for those in the United States, and Italy had mean prices similar to the United Sates for dual-chamber implantable cardioverter-defibrillators. The prices of implantable cardioverter-defibrillators and cardiac resynchronization devices with defibrillating function were the other exceptions, with the United Kingdom similar to or higher than the United States, said Mr. Wenzl and Dr. Mossialos, both of the London School of Economics and Political Science.
The analysis of data from Decision Resources Group’s Marketrack hospital surveys also showed significant variation between the hospitals in each country, with the exception of France, where payments are based on the specific device rather than the procedure and the system “creates weak incentives for hospitals to negotiate lower prices,” they said. In most of the device categories, “variation between hospitals in each country was similar to variation between countries,” they wrote, adding that prices in general “were only weakly correlated with volumes purchased by hospitals.”
The study was supported by a grant from the Commonwealth Fund. The investigators did not disclose any possible conflicts of interest.
SOURCE: Wenzi M, Mossialos E. Health Aff. 2018;37[10]:1570-77. doi: 10.1377/hlthaff.2017.1367.
Prices that hospitals pay for cardiac implant devices are two to six times higher in the United States than in Europe, according to analysis of a large hospital panel survey.
U.S. hospitals had an estimated mean cost of $670 for a bare-metal stent in 2014, compared with $120 in Germany, and the mean costs for dual-chamber pacemakers that year were $4,200 in the United States and $1,400 in Germany, which had lower costs for cardiac devices than the other three European countries – United Kingdom, France, and Italy – included in the study, Martin Wenzl, MSc, and Elias Mossialos, MD, PhD, reported in Health Affairs.
France generally had the highest costs among the European countries, with Italy next and then the United Kingdom. The estimated cost of bare-metal stents was actually higher for French hospitals ($750) than for those in the United States, and Italy had mean prices similar to the United Sates for dual-chamber implantable cardioverter-defibrillators. The prices of implantable cardioverter-defibrillators and cardiac resynchronization devices with defibrillating function were the other exceptions, with the United Kingdom similar to or higher than the United States, said Mr. Wenzl and Dr. Mossialos, both of the London School of Economics and Political Science.
The analysis of data from Decision Resources Group’s Marketrack hospital surveys also showed significant variation between the hospitals in each country, with the exception of France, where payments are based on the specific device rather than the procedure and the system “creates weak incentives for hospitals to negotiate lower prices,” they said. In most of the device categories, “variation between hospitals in each country was similar to variation between countries,” they wrote, adding that prices in general “were only weakly correlated with volumes purchased by hospitals.”
The study was supported by a grant from the Commonwealth Fund. The investigators did not disclose any possible conflicts of interest.
SOURCE: Wenzi M, Mossialos E. Health Aff. 2018;37[10]:1570-77. doi: 10.1377/hlthaff.2017.1367.
FROM HEALTH AFFAIRS
Drug-coated balloons shown noninferior to DES in thin coronaries
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Drug-coated balloon treatment worked as well as drug-eluting stents in thin coronaries.
Major finding: Twelve-month MACE occurred in 7.33% of balloon-treated patients and in 7.45% of stent-treated patients.
Study details: BASKET-SMALL 2, an international, multicenter randomized trial with 758 patients.
Disclosures: The investigator-initiated study received partial funding from B. Braun, the company that markets the drug-coated balloon (SeQuent Please) tested in the study. Dr. Jeger has received research funding from B. Braun. Dr. Mehran has been a consultant to Abbott, Bayer, BSC, and CSL Behring and has received research funding from Abbott, Astra Zeneca, Bayer, BCC, DSI, and Janssen.
The hidden cost of excellence
SAN DIEGO – Readiness and excellence in trauma services come at a high cost.
Level I trauma centers in Georgia lay out an average of $10 million each year to maintain the essential services necessary to operate at full throttle 24 hours a day, 7 days a week. At the Medical Center of Central Georgia, Macon, that amounts to about $4,480 per patient, Dennis W. Ashley, MD, said at the annual meeting of American Association for the Surgery of Trauma. The yearly tab comes to about $5 million for level II centers.
Medical staff pay was the biggest single driver of that cost, accounting for an average $5.5 million annually for level I centers and $3 million for level II centers.
“These readiness costs are incurred well before the first patient is even treated,” said Dr. Ashley, director of trauma and adult critical care at the Medical Center of Central Georgia. “These are costs required by trauma center regulations to maintain essential infrastructure – nonpatient care costs that the hospital would not have to pay if it were not a trauma center.”
Hospitals incur these costs to comply with standards outlined in “Resources for Optimal Care of the Injured Patient,” otherwise known as the “Orange Book.”
To assess these by center, the Georgia Trauma Commission created a cost-reporting survey that was distributed to the state’s 6 level I centers and 10 level II centers in 2017. The surveys examined 2016 costs, and were carried out in conjunction with independent financial reviews to guarantee accurate reporting. Data were gathered in four general areas: administrative/program support staff, clinical medical staff, in-house operating room, and outreach and education.
During 2016, the 16 centers treated a total of 24,488 trauma patients: 15,660 in level I centers and 8,828 in level II centers.
Overall, the six level I centers spent a total of about $60.5 million in 2017 on resource readiness expense. The 10 level II centers spent a total of about $51 million. In all, trauma center status costs these institutions more than $112 million that year.
Salary and benefits for clinical and medical staff were the biggest cost driver for both types of trauma centers. Level I centers laid out a total of $33.2 million – about $5.5 million for each center. Level II centers paid a total of $31.7 million – about $3.1 million each.
Administrative and program support staff comprised the next largest expense. Level I centers paid abut $21.6 million altogether – $3.6 million each. Level II centers paid $13.9 million – about $1.4 million each.
The cost of maintaining constant operating room coverage accounted for $4.9 million in the level I centers ($830,000 each), and $2.5 million in level II centers (about $252,000 each).
Outreach and education comprised the smallest portion of spending. This accounted for a total of about $700,000 for level I centers and $1 million for level II centers ($115,000 and $109,000, respectively).
Dr. Ashley further broke down each general category. In the administrative and program support bucket, trauma program managers and trauma coordinators were the main cost drivers for both types of center. Trauma managers cost a grand total of $1.56 million: $113,000 for each level I center and $88,169 for level II centers. Trauma coordinators cost a grand total of $921,800 and senior administrative support accounted for a total of about $590,000.
Program support staff consisted of physical, occupational, and speech therapists, as well as research coordinators and others. The big-ticket items here were case managers, and staff providing physical, occupational, and speech therapy. Each of those services cost a total of about $6 million for all centers (around $600,000 for each level I center and $240,000 for each level II center).
Staffing trauma surgery made up the bulk of costs for clinical medical staff (a total of almost $19 million), followed by orthopedics ($13.8 million) and neurosurgery ($6.5 million).
Education and outreach were comparatively poorly funded, Dr. Ashley said. “We should do a better job on this,” he noted. All the centers combined spent about $340,000 on injury prevention, for example. Educational programs for emergency department staff made up about $590,000, and for intensive care unit staff, the total tab was about $174,000.
The survey plainly shows the financial commitment necessary to maintain high-quality trauma care, he said. “The significant cost of trauma center readiness highlights the need for additional trauma funding.”
He had no financial disclosures.
[email protected]
SAN DIEGO – Readiness and excellence in trauma services come at a high cost.
Level I trauma centers in Georgia lay out an average of $10 million each year to maintain the essential services necessary to operate at full throttle 24 hours a day, 7 days a week. At the Medical Center of Central Georgia, Macon, that amounts to about $4,480 per patient, Dennis W. Ashley, MD, said at the annual meeting of American Association for the Surgery of Trauma. The yearly tab comes to about $5 million for level II centers.
Medical staff pay was the biggest single driver of that cost, accounting for an average $5.5 million annually for level I centers and $3 million for level II centers.
“These readiness costs are incurred well before the first patient is even treated,” said Dr. Ashley, director of trauma and adult critical care at the Medical Center of Central Georgia. “These are costs required by trauma center regulations to maintain essential infrastructure – nonpatient care costs that the hospital would not have to pay if it were not a trauma center.”
Hospitals incur these costs to comply with standards outlined in “Resources for Optimal Care of the Injured Patient,” otherwise known as the “Orange Book.”
To assess these by center, the Georgia Trauma Commission created a cost-reporting survey that was distributed to the state’s 6 level I centers and 10 level II centers in 2017. The surveys examined 2016 costs, and were carried out in conjunction with independent financial reviews to guarantee accurate reporting. Data were gathered in four general areas: administrative/program support staff, clinical medical staff, in-house operating room, and outreach and education.
During 2016, the 16 centers treated a total of 24,488 trauma patients: 15,660 in level I centers and 8,828 in level II centers.
Overall, the six level I centers spent a total of about $60.5 million in 2017 on resource readiness expense. The 10 level II centers spent a total of about $51 million. In all, trauma center status costs these institutions more than $112 million that year.
Salary and benefits for clinical and medical staff were the biggest cost driver for both types of trauma centers. Level I centers laid out a total of $33.2 million – about $5.5 million for each center. Level II centers paid a total of $31.7 million – about $3.1 million each.
Administrative and program support staff comprised the next largest expense. Level I centers paid abut $21.6 million altogether – $3.6 million each. Level II centers paid $13.9 million – about $1.4 million each.
The cost of maintaining constant operating room coverage accounted for $4.9 million in the level I centers ($830,000 each), and $2.5 million in level II centers (about $252,000 each).
Outreach and education comprised the smallest portion of spending. This accounted for a total of about $700,000 for level I centers and $1 million for level II centers ($115,000 and $109,000, respectively).
Dr. Ashley further broke down each general category. In the administrative and program support bucket, trauma program managers and trauma coordinators were the main cost drivers for both types of center. Trauma managers cost a grand total of $1.56 million: $113,000 for each level I center and $88,169 for level II centers. Trauma coordinators cost a grand total of $921,800 and senior administrative support accounted for a total of about $590,000.
Program support staff consisted of physical, occupational, and speech therapists, as well as research coordinators and others. The big-ticket items here were case managers, and staff providing physical, occupational, and speech therapy. Each of those services cost a total of about $6 million for all centers (around $600,000 for each level I center and $240,000 for each level II center).
Staffing trauma surgery made up the bulk of costs for clinical medical staff (a total of almost $19 million), followed by orthopedics ($13.8 million) and neurosurgery ($6.5 million).
Education and outreach were comparatively poorly funded, Dr. Ashley said. “We should do a better job on this,” he noted. All the centers combined spent about $340,000 on injury prevention, for example. Educational programs for emergency department staff made up about $590,000, and for intensive care unit staff, the total tab was about $174,000.
The survey plainly shows the financial commitment necessary to maintain high-quality trauma care, he said. “The significant cost of trauma center readiness highlights the need for additional trauma funding.”
He had no financial disclosures.
[email protected]
SAN DIEGO – Readiness and excellence in trauma services come at a high cost.
Level I trauma centers in Georgia lay out an average of $10 million each year to maintain the essential services necessary to operate at full throttle 24 hours a day, 7 days a week. At the Medical Center of Central Georgia, Macon, that amounts to about $4,480 per patient, Dennis W. Ashley, MD, said at the annual meeting of American Association for the Surgery of Trauma. The yearly tab comes to about $5 million for level II centers.
Medical staff pay was the biggest single driver of that cost, accounting for an average $5.5 million annually for level I centers and $3 million for level II centers.
“These readiness costs are incurred well before the first patient is even treated,” said Dr. Ashley, director of trauma and adult critical care at the Medical Center of Central Georgia. “These are costs required by trauma center regulations to maintain essential infrastructure – nonpatient care costs that the hospital would not have to pay if it were not a trauma center.”
Hospitals incur these costs to comply with standards outlined in “Resources for Optimal Care of the Injured Patient,” otherwise known as the “Orange Book.”
To assess these by center, the Georgia Trauma Commission created a cost-reporting survey that was distributed to the state’s 6 level I centers and 10 level II centers in 2017. The surveys examined 2016 costs, and were carried out in conjunction with independent financial reviews to guarantee accurate reporting. Data were gathered in four general areas: administrative/program support staff, clinical medical staff, in-house operating room, and outreach and education.
During 2016, the 16 centers treated a total of 24,488 trauma patients: 15,660 in level I centers and 8,828 in level II centers.
Overall, the six level I centers spent a total of about $60.5 million in 2017 on resource readiness expense. The 10 level II centers spent a total of about $51 million. In all, trauma center status costs these institutions more than $112 million that year.
Salary and benefits for clinical and medical staff were the biggest cost driver for both types of trauma centers. Level I centers laid out a total of $33.2 million – about $5.5 million for each center. Level II centers paid a total of $31.7 million – about $3.1 million each.
Administrative and program support staff comprised the next largest expense. Level I centers paid abut $21.6 million altogether – $3.6 million each. Level II centers paid $13.9 million – about $1.4 million each.
The cost of maintaining constant operating room coverage accounted for $4.9 million in the level I centers ($830,000 each), and $2.5 million in level II centers (about $252,000 each).
Outreach and education comprised the smallest portion of spending. This accounted for a total of about $700,000 for level I centers and $1 million for level II centers ($115,000 and $109,000, respectively).
Dr. Ashley further broke down each general category. In the administrative and program support bucket, trauma program managers and trauma coordinators were the main cost drivers for both types of center. Trauma managers cost a grand total of $1.56 million: $113,000 for each level I center and $88,169 for level II centers. Trauma coordinators cost a grand total of $921,800 and senior administrative support accounted for a total of about $590,000.
Program support staff consisted of physical, occupational, and speech therapists, as well as research coordinators and others. The big-ticket items here were case managers, and staff providing physical, occupational, and speech therapy. Each of those services cost a total of about $6 million for all centers (around $600,000 for each level I center and $240,000 for each level II center).
Staffing trauma surgery made up the bulk of costs for clinical medical staff (a total of almost $19 million), followed by orthopedics ($13.8 million) and neurosurgery ($6.5 million).
Education and outreach were comparatively poorly funded, Dr. Ashley said. “We should do a better job on this,” he noted. All the centers combined spent about $340,000 on injury prevention, for example. Educational programs for emergency department staff made up about $590,000, and for intensive care unit staff, the total tab was about $174,000.
The survey plainly shows the financial commitment necessary to maintain high-quality trauma care, he said. “The significant cost of trauma center readiness highlights the need for additional trauma funding.”
He had no financial disclosures.
[email protected]
REPORTING FROM THE AAST ANNUAL MEETING
Key clinical point: Meeting excellence requirements costs trauma centers millions of dollars.
Major finding: A level I center invests about $10 million annually to meet readiness requirements.
Study details: The Georgia survey queried six level I centers and 10 level II centers.
Disclosures: Dr. Ashley had no financial disclosures.
Source: Ashely DW et al. AAST 2018. Abstract 18.
Low-dose ketamine controls pain from severe chest injury, while sparing opioid consumption
SAN DIEGO – while reducing opioid consumption.
The anesthetic didn’t make much difference in pain control or opioid use overall in a randomized study of 93 patients with thoracic injury Nathan Kugler, MD, said at the annual meeting of the American Association for the Surgery of Trauma. But among severely injured patients, it cut the opioid mean equivalency dose by about 164 mg over the 48-hour infusion and by 328 mg over a mean hospital stay while maintaining pain control, said Dr. Kugler, a surgical resident at the Medical College of Wisconsin, Milwaukee.
“With increasing focus on multimodal pain strategies, opioid-based regimens continue to be the backbone of pain control,” he said. “We have used ketamine effectively for failure of maximum therapy and demonstrated an opioid-sparing effect.” This new research shows that the drug can be an effective adjunct for acute pain control for severely injured patients in the emergency setting.
The study recruited 93 patients with thoracic injury; they had a mean of six broken ribs, mostly caused by motor vehicle accidents. Most of the patients were male (75%), and their mean age was 46 years. The mean Injury Severity Score was about 15; about 30% had flail chest.
All patients received a standardized acute pain medication regime comprising acetaminophen, nonsteroidal anti-inflammatories, methocarbamol (Robaxin), and intravenous opioids. Regional therapies included rib block with an epidural catheter. In addition, they were randomized to placebo infusions or to 48 hours of IV ketamine at 2.5 mcg/kg per minute. “To put this in perspective, for a 70-kg patient, that is a mean of 10.5 mg/hour,” Dr. Kugler said.
The primary endpoint was a reduction of at least 2 points on an 11-point pain scale. Secondary endpoints included opioid use in oral morphine equivalents (OME); respiratory complications; and psychoactive events. The primary outcome was assessed with an area under the curve model.
In the overall group, there was no significant between-group difference in pain score. Nor were there differences in the total OME at 12-24 hours (184 mg ketamine vs. 230 mg placebo), or at 48 hours (86 vs. 113 mg).
Dr. Kugler also looked at these outcomes in patients who had only rib fractures independent of other chest injury. He saw no significant differences in pain scores or OME at 24 or 48 hours.
However, significant differences did emerge in the group of severely injured patients with an Injury Severity Score of more than 15. There were no differences in pain scores at either time point. However, ketamine allowed patients to achieve the same level of pain control with significantly less opioid medication. The OME at 12-24 hours was 50.5 mg vs 94 mg. At 24-48 hours, it was 87 mg vs. 64 mg.
This worked out to a mean OME savings of 148 mg over a patient’s entire hospitalization.
“We saw a very nice separation of opioid consumption that began early and continued to separate over the 48-hour infusion and even after it was discontinued,” Dr. Kugler said.
This benefit was achieved without any additional adverse events, he added. There were no significant differences in confusion; epidural placement; length of stay; respiratory event, sedation, hallucinations, delusions or disturbing dreams; or unplanned transfers to the ICU.
Dr. Kugler disclosed that he and primary investigator Thomas Carver, MD, also of the Medical College of Wisconsin, Milwaukee, are both paid consultants for InnoVital Systems.
SAN DIEGO – while reducing opioid consumption.
The anesthetic didn’t make much difference in pain control or opioid use overall in a randomized study of 93 patients with thoracic injury Nathan Kugler, MD, said at the annual meeting of the American Association for the Surgery of Trauma. But among severely injured patients, it cut the opioid mean equivalency dose by about 164 mg over the 48-hour infusion and by 328 mg over a mean hospital stay while maintaining pain control, said Dr. Kugler, a surgical resident at the Medical College of Wisconsin, Milwaukee.
“With increasing focus on multimodal pain strategies, opioid-based regimens continue to be the backbone of pain control,” he said. “We have used ketamine effectively for failure of maximum therapy and demonstrated an opioid-sparing effect.” This new research shows that the drug can be an effective adjunct for acute pain control for severely injured patients in the emergency setting.
The study recruited 93 patients with thoracic injury; they had a mean of six broken ribs, mostly caused by motor vehicle accidents. Most of the patients were male (75%), and their mean age was 46 years. The mean Injury Severity Score was about 15; about 30% had flail chest.
All patients received a standardized acute pain medication regime comprising acetaminophen, nonsteroidal anti-inflammatories, methocarbamol (Robaxin), and intravenous opioids. Regional therapies included rib block with an epidural catheter. In addition, they were randomized to placebo infusions or to 48 hours of IV ketamine at 2.5 mcg/kg per minute. “To put this in perspective, for a 70-kg patient, that is a mean of 10.5 mg/hour,” Dr. Kugler said.
The primary endpoint was a reduction of at least 2 points on an 11-point pain scale. Secondary endpoints included opioid use in oral morphine equivalents (OME); respiratory complications; and psychoactive events. The primary outcome was assessed with an area under the curve model.
In the overall group, there was no significant between-group difference in pain score. Nor were there differences in the total OME at 12-24 hours (184 mg ketamine vs. 230 mg placebo), or at 48 hours (86 vs. 113 mg).
Dr. Kugler also looked at these outcomes in patients who had only rib fractures independent of other chest injury. He saw no significant differences in pain scores or OME at 24 or 48 hours.
However, significant differences did emerge in the group of severely injured patients with an Injury Severity Score of more than 15. There were no differences in pain scores at either time point. However, ketamine allowed patients to achieve the same level of pain control with significantly less opioid medication. The OME at 12-24 hours was 50.5 mg vs 94 mg. At 24-48 hours, it was 87 mg vs. 64 mg.
This worked out to a mean OME savings of 148 mg over a patient’s entire hospitalization.
“We saw a very nice separation of opioid consumption that began early and continued to separate over the 48-hour infusion and even after it was discontinued,” Dr. Kugler said.
This benefit was achieved without any additional adverse events, he added. There were no significant differences in confusion; epidural placement; length of stay; respiratory event, sedation, hallucinations, delusions or disturbing dreams; or unplanned transfers to the ICU.
Dr. Kugler disclosed that he and primary investigator Thomas Carver, MD, also of the Medical College of Wisconsin, Milwaukee, are both paid consultants for InnoVital Systems.
SAN DIEGO – while reducing opioid consumption.
The anesthetic didn’t make much difference in pain control or opioid use overall in a randomized study of 93 patients with thoracic injury Nathan Kugler, MD, said at the annual meeting of the American Association for the Surgery of Trauma. But among severely injured patients, it cut the opioid mean equivalency dose by about 164 mg over the 48-hour infusion and by 328 mg over a mean hospital stay while maintaining pain control, said Dr. Kugler, a surgical resident at the Medical College of Wisconsin, Milwaukee.
“With increasing focus on multimodal pain strategies, opioid-based regimens continue to be the backbone of pain control,” he said. “We have used ketamine effectively for failure of maximum therapy and demonstrated an opioid-sparing effect.” This new research shows that the drug can be an effective adjunct for acute pain control for severely injured patients in the emergency setting.
The study recruited 93 patients with thoracic injury; they had a mean of six broken ribs, mostly caused by motor vehicle accidents. Most of the patients were male (75%), and their mean age was 46 years. The mean Injury Severity Score was about 15; about 30% had flail chest.
All patients received a standardized acute pain medication regime comprising acetaminophen, nonsteroidal anti-inflammatories, methocarbamol (Robaxin), and intravenous opioids. Regional therapies included rib block with an epidural catheter. In addition, they were randomized to placebo infusions or to 48 hours of IV ketamine at 2.5 mcg/kg per minute. “To put this in perspective, for a 70-kg patient, that is a mean of 10.5 mg/hour,” Dr. Kugler said.
The primary endpoint was a reduction of at least 2 points on an 11-point pain scale. Secondary endpoints included opioid use in oral morphine equivalents (OME); respiratory complications; and psychoactive events. The primary outcome was assessed with an area under the curve model.
In the overall group, there was no significant between-group difference in pain score. Nor were there differences in the total OME at 12-24 hours (184 mg ketamine vs. 230 mg placebo), or at 48 hours (86 vs. 113 mg).
Dr. Kugler also looked at these outcomes in patients who had only rib fractures independent of other chest injury. He saw no significant differences in pain scores or OME at 24 or 48 hours.
However, significant differences did emerge in the group of severely injured patients with an Injury Severity Score of more than 15. There were no differences in pain scores at either time point. However, ketamine allowed patients to achieve the same level of pain control with significantly less opioid medication. The OME at 12-24 hours was 50.5 mg vs 94 mg. At 24-48 hours, it was 87 mg vs. 64 mg.
This worked out to a mean OME savings of 148 mg over a patient’s entire hospitalization.
“We saw a very nice separation of opioid consumption that began early and continued to separate over the 48-hour infusion and even after it was discontinued,” Dr. Kugler said.
This benefit was achieved without any additional adverse events, he added. There were no significant differences in confusion; epidural placement; length of stay; respiratory event, sedation, hallucinations, delusions or disturbing dreams; or unplanned transfers to the ICU.
Dr. Kugler disclosed that he and primary investigator Thomas Carver, MD, also of the Medical College of Wisconsin, Milwaukee, are both paid consultants for InnoVital Systems.
REPORTING FROM THE AAST ANNUAL MEETING
Key clinical point: Low-dose ketamine controlled pain while reducing opioid use among patients with severe thoracic injury.
Major finding: Compared with placebo, ketamine reduced opioids conferred OME savings of 148 mg over a patient’s entire hospitalization.
Study details: The randomized study comprised 93 patients with thoracic injury.
Disclosures: Dr. Kugler disclosed that he and primary investigator Thomas Carver, MD, are both paid consultants for InnoVital Systems.
Source: Carver T et al. AAST 2018, Oral abstract 2
It may be time to rethink damage-control laparotomy
SAN ANTONIO – A , but it can cost fewer resources: fewer days in intensive care, fewer days on a ventilator, and a shorter overall length of stay.
Among damage-control abdominal trauma cases that could have been closed, definitive laparotomy (DEF) was associated with a 56% probability of major abdominal complications – very close to the probability associated with damage-control closure, John Harvin, MD, said at the annual meeting of the American Association for the Surgery of Trauma.
Definitive closure was also associated with about a 72% chance of more ventilator- and hospital-free days and a 77% chance of more ICU-free days than damage-control closure, said Dr. Harvin of the University of Texas, Austin.
“Our analysis tells us that there’s a minimal chance of reducing complications with a definitive laparotomy, compared to leaving them open, but this also comes with more than a 70% chance of having a shorter hospital staying and getting off the ventilator faster.”
The data from a 2-year quality review process reaffirms what trauma surgeons have been seeing, and reporting, since damage-control closure became more popular in the past decade, said Peter Rhee, MD, commenting on the study.
“There are three congregations in this faith of damage-control laparotomy,” said Dr. Rhee, a surgeon in Atlanta, Ga. “The first believes it should be the default for all these types of operations and that being preemptive is better than anything. The second believes that it doesn’t hurt the patient too much, and that it can be done when absolutely needed to save a life. The third belief is that there’s minimal data to support it, that it should rarely be used, and that it’s always costly. We all know that the pendulum of damage-control laparotomy is finally swinging back to the center.”
The study arose from an effort at the Red Duke Trauma Institute, Austin, Tex., to reduce its rate of damage-control laparotomy (DCL). Surgeons examined all emergent trauma laparotomies conducted from 2013 to 2015. They discussed each case and compared morbidity and mortality rates to a published control group. This work was published last year in the Journal of the American College of Surgeons.
By adopting this review procedure, the hospital was able to decrease its 39% DCL rate to 23% over 2 years (an absolute reduction of 68 cases) without any change in infection rates, fascial dehiscence, unplanned reoperation, or mortality. The improvements continued, Dr. Harvin noted, with a farther 17% reduction in DCL after the project concluded.
Dr. Harvin’s analysis looked at 44 of these procedures which, according to the adjudication panel, could have been managed by DEF. Each was matched to a DEF case, and the outcomes were calculated with a Bayesian statistical model.
The primary outcome was major abdominal complication, a composite measure of fascial dehiscence, organ or space surgical-site infection, reopening of fascia, and enteric suture line failure. Secondary outcomes were days off the ventilator and out of the ICU and hospital.
Of the 872 patients in the study, most (639; 73%) were managed by DEF; the rest (209; 24%) were managed by DCL. Of these, the panel agreed that 44 (22%) could have been safely closed at the time of surgery and survived at least 5 days. The propensity-matching scheme comprised 39 of these cases matched with 39 DEF cases.
Most were male (74%); the mean age was 38 years. Penetrating injuries were most common (54%). The abdominal Abbreviated Injury Score was 3. The mean Injury Severity Score was 22 in the DEF cases and 25 in the DCL cases, but this was not a significant difference. There were no differences in blood pressure at presentation or at the end of the surgery, no differences in blood transfusion needs, and no differences in body temperature.
A major abdominal complication occurred in 31% of the DEF cases and 21% of the DCL cases, a relative risk of 0.99. This amounted to a 56% posterior probability of a complication associated with DEF.
Comparing DCL cases with DEF cases, the mean number of hospital-free days was 15 vs.13; ICU-free days, 26 vs. 21; and ventilator-free days, 29 vs. 26. These differences amount to a 72% chance that DEL would result in more hospital-free days and more ventilator-free days, and a 77% chance that DEL would result in more ICU-free days.
The numbers underscore the need to rethink DCL for abdominal trauma, Dr. Rhee said.
“I too once believed in this procedure and used to do it all the time. After 2 decades, we now know that it contributed to the frozen bellies, abdominal wound hernias, fascial dehiscence, missed complications, and to the never-ending enteroatmospheric fistulas. If we want to reduce fistulas, we must first reduce damage-control laparotomy. Nurses will love you for not creating the narcotic-addicted, total parenteral nutrition–dependent patient with a fragrant open belly and a fistula.”
Neither Dr. Harvin nor Dr Rhee had any relevant financial disclosures.
SAN ANTONIO – A , but it can cost fewer resources: fewer days in intensive care, fewer days on a ventilator, and a shorter overall length of stay.
Among damage-control abdominal trauma cases that could have been closed, definitive laparotomy (DEF) was associated with a 56% probability of major abdominal complications – very close to the probability associated with damage-control closure, John Harvin, MD, said at the annual meeting of the American Association for the Surgery of Trauma.
Definitive closure was also associated with about a 72% chance of more ventilator- and hospital-free days and a 77% chance of more ICU-free days than damage-control closure, said Dr. Harvin of the University of Texas, Austin.
“Our analysis tells us that there’s a minimal chance of reducing complications with a definitive laparotomy, compared to leaving them open, but this also comes with more than a 70% chance of having a shorter hospital staying and getting off the ventilator faster.”
The data from a 2-year quality review process reaffirms what trauma surgeons have been seeing, and reporting, since damage-control closure became more popular in the past decade, said Peter Rhee, MD, commenting on the study.
“There are three congregations in this faith of damage-control laparotomy,” said Dr. Rhee, a surgeon in Atlanta, Ga. “The first believes it should be the default for all these types of operations and that being preemptive is better than anything. The second believes that it doesn’t hurt the patient too much, and that it can be done when absolutely needed to save a life. The third belief is that there’s minimal data to support it, that it should rarely be used, and that it’s always costly. We all know that the pendulum of damage-control laparotomy is finally swinging back to the center.”
The study arose from an effort at the Red Duke Trauma Institute, Austin, Tex., to reduce its rate of damage-control laparotomy (DCL). Surgeons examined all emergent trauma laparotomies conducted from 2013 to 2015. They discussed each case and compared morbidity and mortality rates to a published control group. This work was published last year in the Journal of the American College of Surgeons.
By adopting this review procedure, the hospital was able to decrease its 39% DCL rate to 23% over 2 years (an absolute reduction of 68 cases) without any change in infection rates, fascial dehiscence, unplanned reoperation, or mortality. The improvements continued, Dr. Harvin noted, with a farther 17% reduction in DCL after the project concluded.
Dr. Harvin’s analysis looked at 44 of these procedures which, according to the adjudication panel, could have been managed by DEF. Each was matched to a DEF case, and the outcomes were calculated with a Bayesian statistical model.
The primary outcome was major abdominal complication, a composite measure of fascial dehiscence, organ or space surgical-site infection, reopening of fascia, and enteric suture line failure. Secondary outcomes were days off the ventilator and out of the ICU and hospital.
Of the 872 patients in the study, most (639; 73%) were managed by DEF; the rest (209; 24%) were managed by DCL. Of these, the panel agreed that 44 (22%) could have been safely closed at the time of surgery and survived at least 5 days. The propensity-matching scheme comprised 39 of these cases matched with 39 DEF cases.
Most were male (74%); the mean age was 38 years. Penetrating injuries were most common (54%). The abdominal Abbreviated Injury Score was 3. The mean Injury Severity Score was 22 in the DEF cases and 25 in the DCL cases, but this was not a significant difference. There were no differences in blood pressure at presentation or at the end of the surgery, no differences in blood transfusion needs, and no differences in body temperature.
A major abdominal complication occurred in 31% of the DEF cases and 21% of the DCL cases, a relative risk of 0.99. This amounted to a 56% posterior probability of a complication associated with DEF.
Comparing DCL cases with DEF cases, the mean number of hospital-free days was 15 vs.13; ICU-free days, 26 vs. 21; and ventilator-free days, 29 vs. 26. These differences amount to a 72% chance that DEL would result in more hospital-free days and more ventilator-free days, and a 77% chance that DEL would result in more ICU-free days.
The numbers underscore the need to rethink DCL for abdominal trauma, Dr. Rhee said.
“I too once believed in this procedure and used to do it all the time. After 2 decades, we now know that it contributed to the frozen bellies, abdominal wound hernias, fascial dehiscence, missed complications, and to the never-ending enteroatmospheric fistulas. If we want to reduce fistulas, we must first reduce damage-control laparotomy. Nurses will love you for not creating the narcotic-addicted, total parenteral nutrition–dependent patient with a fragrant open belly and a fistula.”
Neither Dr. Harvin nor Dr Rhee had any relevant financial disclosures.
SAN ANTONIO – A , but it can cost fewer resources: fewer days in intensive care, fewer days on a ventilator, and a shorter overall length of stay.
Among damage-control abdominal trauma cases that could have been closed, definitive laparotomy (DEF) was associated with a 56% probability of major abdominal complications – very close to the probability associated with damage-control closure, John Harvin, MD, said at the annual meeting of the American Association for the Surgery of Trauma.
Definitive closure was also associated with about a 72% chance of more ventilator- and hospital-free days and a 77% chance of more ICU-free days than damage-control closure, said Dr. Harvin of the University of Texas, Austin.
“Our analysis tells us that there’s a minimal chance of reducing complications with a definitive laparotomy, compared to leaving them open, but this also comes with more than a 70% chance of having a shorter hospital staying and getting off the ventilator faster.”
The data from a 2-year quality review process reaffirms what trauma surgeons have been seeing, and reporting, since damage-control closure became more popular in the past decade, said Peter Rhee, MD, commenting on the study.
“There are three congregations in this faith of damage-control laparotomy,” said Dr. Rhee, a surgeon in Atlanta, Ga. “The first believes it should be the default for all these types of operations and that being preemptive is better than anything. The second believes that it doesn’t hurt the patient too much, and that it can be done when absolutely needed to save a life. The third belief is that there’s minimal data to support it, that it should rarely be used, and that it’s always costly. We all know that the pendulum of damage-control laparotomy is finally swinging back to the center.”
The study arose from an effort at the Red Duke Trauma Institute, Austin, Tex., to reduce its rate of damage-control laparotomy (DCL). Surgeons examined all emergent trauma laparotomies conducted from 2013 to 2015. They discussed each case and compared morbidity and mortality rates to a published control group. This work was published last year in the Journal of the American College of Surgeons.
By adopting this review procedure, the hospital was able to decrease its 39% DCL rate to 23% over 2 years (an absolute reduction of 68 cases) without any change in infection rates, fascial dehiscence, unplanned reoperation, or mortality. The improvements continued, Dr. Harvin noted, with a farther 17% reduction in DCL after the project concluded.
Dr. Harvin’s analysis looked at 44 of these procedures which, according to the adjudication panel, could have been managed by DEF. Each was matched to a DEF case, and the outcomes were calculated with a Bayesian statistical model.
The primary outcome was major abdominal complication, a composite measure of fascial dehiscence, organ or space surgical-site infection, reopening of fascia, and enteric suture line failure. Secondary outcomes were days off the ventilator and out of the ICU and hospital.
Of the 872 patients in the study, most (639; 73%) were managed by DEF; the rest (209; 24%) were managed by DCL. Of these, the panel agreed that 44 (22%) could have been safely closed at the time of surgery and survived at least 5 days. The propensity-matching scheme comprised 39 of these cases matched with 39 DEF cases.
Most were male (74%); the mean age was 38 years. Penetrating injuries were most common (54%). The abdominal Abbreviated Injury Score was 3. The mean Injury Severity Score was 22 in the DEF cases and 25 in the DCL cases, but this was not a significant difference. There were no differences in blood pressure at presentation or at the end of the surgery, no differences in blood transfusion needs, and no differences in body temperature.
A major abdominal complication occurred in 31% of the DEF cases and 21% of the DCL cases, a relative risk of 0.99. This amounted to a 56% posterior probability of a complication associated with DEF.
Comparing DCL cases with DEF cases, the mean number of hospital-free days was 15 vs.13; ICU-free days, 26 vs. 21; and ventilator-free days, 29 vs. 26. These differences amount to a 72% chance that DEL would result in more hospital-free days and more ventilator-free days, and a 77% chance that DEL would result in more ICU-free days.
The numbers underscore the need to rethink DCL for abdominal trauma, Dr. Rhee said.
“I too once believed in this procedure and used to do it all the time. After 2 decades, we now know that it contributed to the frozen bellies, abdominal wound hernias, fascial dehiscence, missed complications, and to the never-ending enteroatmospheric fistulas. If we want to reduce fistulas, we must first reduce damage-control laparotomy. Nurses will love you for not creating the narcotic-addicted, total parenteral nutrition–dependent patient with a fragrant open belly and a fistula.”
Neither Dr. Harvin nor Dr Rhee had any relevant financial disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point: Definitive laparotomy for abdominal trauma added little risk of complications but shortened ventilator and ICU days.
Major finding: Definitive closure was associated with a 72% chance of more ventilator- and hospital-free days and a 77% chance of more ICU-free days than damage-control closure.
Study details: The Bayseian analysis comprised 39 definitive and 39 damage-control laparotomy patients.
Disclosures: Neither Dr. Harvin nor Dr. Rhee had any financial disclosures.
Source: Harvin J et al. AAST 2018, Oral paper 12.



















