User login
Official Newspaper of the American College of Surgeons
FDA issues recommendations to avoid surgical fires
The Food and Drug Administration on May 29 issued a set of recommendations to medical professionals and health care facility staff to reduce the occurrence of surgical fires on or near a patient.
Surgical fires most often occur when there is an oxygen-enriched environment (a concentration of greater than 30%). In addition to an oxygen source, the other two necessary elements of the “fire triangle” are an ignition source and a fuel source.
The recommendations discuss the safe use of devices or items that may serve as a source of any one of those three elements.
Oxygen: Evaluate if supplemental oxygen is needed. If it is, titrate to the minimum concentration needed for adequate saturation. Closed oxygen delivery systems (such as a laryngeal mask or endotracheal tube) are safer than open oxygen delivery systems (such as a nasal cannula or mask). If you must use an open system, take additional precautions to exclude oxygen and flammable/combustible gases from the operative field, such as draping techniques that avoid accumulation of oxygen.
Ignition sources: Consider alternatives to using an ignition source for surgery of the head, neck, and upper chest if high concentrations of supplemental oxygen are being delivered. Check for insulation failure before use, and keep devices clean of char and tissue. When not in use, place the devices safely away from the patient and drapes. Devices are safer to use if you can allow time for the oxygen concentration in the room to decrease.
Fuel sources: Ensure dry conditions prior to draping, avoiding pooling of alcohol-based antiseptics during skin preparation. Use the appropriate-sized applicator for the surgical site. Be aware of products that may serve as a fuel source, such as oxygen-trapping gauze, plastic laryngeal masks, and aware of potential patient sources such as hair or gastrointestinal gases.
Training should include how to manage fires that do occur – stop the ignition source, then extinguish the fire – and evacuation procedures.
Read the full recommendations here.
The Food and Drug Administration on May 29 issued a set of recommendations to medical professionals and health care facility staff to reduce the occurrence of surgical fires on or near a patient.
Surgical fires most often occur when there is an oxygen-enriched environment (a concentration of greater than 30%). In addition to an oxygen source, the other two necessary elements of the “fire triangle” are an ignition source and a fuel source.
The recommendations discuss the safe use of devices or items that may serve as a source of any one of those three elements.
Oxygen: Evaluate if supplemental oxygen is needed. If it is, titrate to the minimum concentration needed for adequate saturation. Closed oxygen delivery systems (such as a laryngeal mask or endotracheal tube) are safer than open oxygen delivery systems (such as a nasal cannula or mask). If you must use an open system, take additional precautions to exclude oxygen and flammable/combustible gases from the operative field, such as draping techniques that avoid accumulation of oxygen.
Ignition sources: Consider alternatives to using an ignition source for surgery of the head, neck, and upper chest if high concentrations of supplemental oxygen are being delivered. Check for insulation failure before use, and keep devices clean of char and tissue. When not in use, place the devices safely away from the patient and drapes. Devices are safer to use if you can allow time for the oxygen concentration in the room to decrease.
Fuel sources: Ensure dry conditions prior to draping, avoiding pooling of alcohol-based antiseptics during skin preparation. Use the appropriate-sized applicator for the surgical site. Be aware of products that may serve as a fuel source, such as oxygen-trapping gauze, plastic laryngeal masks, and aware of potential patient sources such as hair or gastrointestinal gases.
Training should include how to manage fires that do occur – stop the ignition source, then extinguish the fire – and evacuation procedures.
Read the full recommendations here.
The Food and Drug Administration on May 29 issued a set of recommendations to medical professionals and health care facility staff to reduce the occurrence of surgical fires on or near a patient.
Surgical fires most often occur when there is an oxygen-enriched environment (a concentration of greater than 30%). In addition to an oxygen source, the other two necessary elements of the “fire triangle” are an ignition source and a fuel source.
The recommendations discuss the safe use of devices or items that may serve as a source of any one of those three elements.
Oxygen: Evaluate if supplemental oxygen is needed. If it is, titrate to the minimum concentration needed for adequate saturation. Closed oxygen delivery systems (such as a laryngeal mask or endotracheal tube) are safer than open oxygen delivery systems (such as a nasal cannula or mask). If you must use an open system, take additional precautions to exclude oxygen and flammable/combustible gases from the operative field, such as draping techniques that avoid accumulation of oxygen.
Ignition sources: Consider alternatives to using an ignition source for surgery of the head, neck, and upper chest if high concentrations of supplemental oxygen are being delivered. Check for insulation failure before use, and keep devices clean of char and tissue. When not in use, place the devices safely away from the patient and drapes. Devices are safer to use if you can allow time for the oxygen concentration in the room to decrease.
Fuel sources: Ensure dry conditions prior to draping, avoiding pooling of alcohol-based antiseptics during skin preparation. Use the appropriate-sized applicator for the surgical site. Be aware of products that may serve as a fuel source, such as oxygen-trapping gauze, plastic laryngeal masks, and aware of potential patient sources such as hair or gastrointestinal gases.
Training should include how to manage fires that do occur – stop the ignition source, then extinguish the fire – and evacuation procedures.
Read the full recommendations here.
The case for bariatric surgery to manage CV risk in diabetes
BOSTON – For patients with obesity and metabolic syndrome or type 2 diabetes ( health over the lifespan.
“Behavioral changes in diet and activity may be effective over the short term, but they are often ineffective over the long term,” said Daniel L. Hurley, MD. By contrast, “Bariatric surgery is very effective long-term,” he said.
At the annual clinical and scientific meeting of the American Association of Clinical Endocrinologists, Dr. Hurley made the case for bariatric surgery in effective and durable management of type 2 diabetes and cardiovascular risk, weighing risks and benefits for those with higher and lower levels of obesity.
Speaking during a morning session focused on bariatric surgery, Dr. Hurley, an endocrionologist at the Mayo Clinic, Rochester, Minn., noted that bariatric surgery reduces not just weight, but also visceral adiposity. This, he said, is important when thinking about type 2 diabetes (T2D), because diabetes prevalence has climbed in the United States as obesity has also increased, according to examination of data from the National Health and Nutrition Examination Survey (NHANES).
Additionally, increased abdominal adiposity is associated with increased risk for cardiovascular-related deaths, myocardial infarctions, and all-cause deaths. Some of this relationship is mediated by T2D, which itself “is a major cause of cardiovascular-related morbidity and mortality,” said Dr. Hurley.
From a population health perspective, the increased prevalence of T2D – expected to reach 10% in the United States by 2030 – will also boost cardiovascular morbidity and mortality, said Dr. Hurley. Those with T2D die 5 to 10 years earlier, and have double the risk for heart attack and stroke of their peers without diabetes. The risk of lower limb amputation can be as much as 40 times greater for an individual with T2D across the lifespan, he said.
The National Institutes of Health recognizes bariatric surgery as an appropriate weight loss therapy for individuals with a body mass index (BMI) of at least 35 kg/m2 and comorbidity. Whether bariatric surgery might be appropriate for individuals with T2D and BMIs of less than 35 kg/m2 is less settled, though at least some RCTs support the surgical approach, said Dr. Hurley.
The body of data that support long-term metabolic and cardiovascular benefits of bariatric surgery as obesity therapy is growing, said Dr. Hurley. A large prospective observational study by the American College of Surgeons’ Bariatric Surgery Center Network followed 28,616 patients, finding that Roux-en-Y gastric bypass (RYGB) was most effective in improving or resolving CVD comorbidities. At 1 year post surgery, 83% of RYGB patients saw improvement or resolution of T2D; the figure was 79% for hypertension and 66% for dyslipidemia (Ann Surg. 2011;254[3]:410-20).
Weight loss for patients receiving bariatric procedures has generally been durable: for laparoscopic RYGB patients tracked to 7 years after surgery, 75% had maintained at least a 20% weight loss (JAMA Surg. 2018;153[5]427-34).
Longer-term clinical follow-up points toward favorable metabolic and cardiovascular outcomes, said Dr. Hurley, citing data from the Swedish Obese Subjects (SOS) trial. This study followed over 4,000 patients with high BMIs (at least 34 kg/m2 for men and 38 kg/m2 for women) over 10 years. At that point, 36% of gastric bypass patients, compared with 13% of non-surgical high BMI patients, saw resolution of T2D, a significant difference. Triglyceride levels also fell significantly more for the bypass recipients. Hypertension was resolved in just 19% of patients at 10 years, a non-significant difference from the 11% of control patients. Data from the same patient set also showed a significant reduction in total cardiovascular events in the surgical versus non-surgical patients (n = 49 vs. 28, hazard ratio 0.83, log-rank P = .05). Fatal cardiovascular events were significantly lower for patients who had received bariatric surgery, with a 24% decline in mortality for bariatric surgery patients at about 11 years post surgery.
Canadian data showed even greater reductions in mortality, with an 89% decrease in mortality after RYGB, compared with non-surgical patients at the 5-year mark (Ann Surg 2004;240:416-24).
In trials that afforded a direct comparison of medical therapy and bariatric surgery obesity and diabetes, Dr. Hurley said that randomized trials generally show no change to modest change in HbA1c levels with medical management. By contrast, patients in the surgical arms showed a range of improvement ranging from a reduction of just under 1% to reductions of over 5%, with an average reduction of more than 2% across the trials.
Separating out data from the randomized controlled trials with patient BMIs averaging 35 kg/m2 or less, odds ratios still favored bariatric surgery over medication therapy for diabetes-related outcomes in this lower-BMI population, said Dr. Hurley (Diabetes Care 2016;39:924-33).
More data come from a recently reported randomized trial that assigned patients with T2D and a mean BMI of 37 kg/m2 (range, 27-43) to intensive medical therapy, or either sleeve gastrectomy (SG) or RYGB. The study, which had a 90% completion rate at the 5-year mark, found that both surgical procedures were significantly more effective at reducing HbA1c to 6% or less 12 months into the study (P less than .001).
At the 60-month mark, 45% of the RYGB and 25% of the SG patients were on no diabetes medications, while just 2% of the medical therapy arm had stopped all medications, and 40% of this group remained on insulin 5 years into the study, said Dr. Hurley (N Engl J Med. 2017;376:641-651).
“For treatment of type 2 diabetes and cardiovascular co-morbidities, long-term goals often are met following bariatric surgery versus behavior change,” said Dr. Hurley.
Dr. Hurley reported that he had no financial disclosures.
SOURCE: Hurley, D. AACE 2018, Session SGS-4.
BOSTON – For patients with obesity and metabolic syndrome or type 2 diabetes ( health over the lifespan.
“Behavioral changes in diet and activity may be effective over the short term, but they are often ineffective over the long term,” said Daniel L. Hurley, MD. By contrast, “Bariatric surgery is very effective long-term,” he said.
At the annual clinical and scientific meeting of the American Association of Clinical Endocrinologists, Dr. Hurley made the case for bariatric surgery in effective and durable management of type 2 diabetes and cardiovascular risk, weighing risks and benefits for those with higher and lower levels of obesity.
Speaking during a morning session focused on bariatric surgery, Dr. Hurley, an endocrionologist at the Mayo Clinic, Rochester, Minn., noted that bariatric surgery reduces not just weight, but also visceral adiposity. This, he said, is important when thinking about type 2 diabetes (T2D), because diabetes prevalence has climbed in the United States as obesity has also increased, according to examination of data from the National Health and Nutrition Examination Survey (NHANES).
Additionally, increased abdominal adiposity is associated with increased risk for cardiovascular-related deaths, myocardial infarctions, and all-cause deaths. Some of this relationship is mediated by T2D, which itself “is a major cause of cardiovascular-related morbidity and mortality,” said Dr. Hurley.
From a population health perspective, the increased prevalence of T2D – expected to reach 10% in the United States by 2030 – will also boost cardiovascular morbidity and mortality, said Dr. Hurley. Those with T2D die 5 to 10 years earlier, and have double the risk for heart attack and stroke of their peers without diabetes. The risk of lower limb amputation can be as much as 40 times greater for an individual with T2D across the lifespan, he said.
The National Institutes of Health recognizes bariatric surgery as an appropriate weight loss therapy for individuals with a body mass index (BMI) of at least 35 kg/m2 and comorbidity. Whether bariatric surgery might be appropriate for individuals with T2D and BMIs of less than 35 kg/m2 is less settled, though at least some RCTs support the surgical approach, said Dr. Hurley.
The body of data that support long-term metabolic and cardiovascular benefits of bariatric surgery as obesity therapy is growing, said Dr. Hurley. A large prospective observational study by the American College of Surgeons’ Bariatric Surgery Center Network followed 28,616 patients, finding that Roux-en-Y gastric bypass (RYGB) was most effective in improving or resolving CVD comorbidities. At 1 year post surgery, 83% of RYGB patients saw improvement or resolution of T2D; the figure was 79% for hypertension and 66% for dyslipidemia (Ann Surg. 2011;254[3]:410-20).
Weight loss for patients receiving bariatric procedures has generally been durable: for laparoscopic RYGB patients tracked to 7 years after surgery, 75% had maintained at least a 20% weight loss (JAMA Surg. 2018;153[5]427-34).
Longer-term clinical follow-up points toward favorable metabolic and cardiovascular outcomes, said Dr. Hurley, citing data from the Swedish Obese Subjects (SOS) trial. This study followed over 4,000 patients with high BMIs (at least 34 kg/m2 for men and 38 kg/m2 for women) over 10 years. At that point, 36% of gastric bypass patients, compared with 13% of non-surgical high BMI patients, saw resolution of T2D, a significant difference. Triglyceride levels also fell significantly more for the bypass recipients. Hypertension was resolved in just 19% of patients at 10 years, a non-significant difference from the 11% of control patients. Data from the same patient set also showed a significant reduction in total cardiovascular events in the surgical versus non-surgical patients (n = 49 vs. 28, hazard ratio 0.83, log-rank P = .05). Fatal cardiovascular events were significantly lower for patients who had received bariatric surgery, with a 24% decline in mortality for bariatric surgery patients at about 11 years post surgery.
Canadian data showed even greater reductions in mortality, with an 89% decrease in mortality after RYGB, compared with non-surgical patients at the 5-year mark (Ann Surg 2004;240:416-24).
In trials that afforded a direct comparison of medical therapy and bariatric surgery obesity and diabetes, Dr. Hurley said that randomized trials generally show no change to modest change in HbA1c levels with medical management. By contrast, patients in the surgical arms showed a range of improvement ranging from a reduction of just under 1% to reductions of over 5%, with an average reduction of more than 2% across the trials.
Separating out data from the randomized controlled trials with patient BMIs averaging 35 kg/m2 or less, odds ratios still favored bariatric surgery over medication therapy for diabetes-related outcomes in this lower-BMI population, said Dr. Hurley (Diabetes Care 2016;39:924-33).
More data come from a recently reported randomized trial that assigned patients with T2D and a mean BMI of 37 kg/m2 (range, 27-43) to intensive medical therapy, or either sleeve gastrectomy (SG) or RYGB. The study, which had a 90% completion rate at the 5-year mark, found that both surgical procedures were significantly more effective at reducing HbA1c to 6% or less 12 months into the study (P less than .001).
At the 60-month mark, 45% of the RYGB and 25% of the SG patients were on no diabetes medications, while just 2% of the medical therapy arm had stopped all medications, and 40% of this group remained on insulin 5 years into the study, said Dr. Hurley (N Engl J Med. 2017;376:641-651).
“For treatment of type 2 diabetes and cardiovascular co-morbidities, long-term goals often are met following bariatric surgery versus behavior change,” said Dr. Hurley.
Dr. Hurley reported that he had no financial disclosures.
SOURCE: Hurley, D. AACE 2018, Session SGS-4.
BOSTON – For patients with obesity and metabolic syndrome or type 2 diabetes ( health over the lifespan.
“Behavioral changes in diet and activity may be effective over the short term, but they are often ineffective over the long term,” said Daniel L. Hurley, MD. By contrast, “Bariatric surgery is very effective long-term,” he said.
At the annual clinical and scientific meeting of the American Association of Clinical Endocrinologists, Dr. Hurley made the case for bariatric surgery in effective and durable management of type 2 diabetes and cardiovascular risk, weighing risks and benefits for those with higher and lower levels of obesity.
Speaking during a morning session focused on bariatric surgery, Dr. Hurley, an endocrionologist at the Mayo Clinic, Rochester, Minn., noted that bariatric surgery reduces not just weight, but also visceral adiposity. This, he said, is important when thinking about type 2 diabetes (T2D), because diabetes prevalence has climbed in the United States as obesity has also increased, according to examination of data from the National Health and Nutrition Examination Survey (NHANES).
Additionally, increased abdominal adiposity is associated with increased risk for cardiovascular-related deaths, myocardial infarctions, and all-cause deaths. Some of this relationship is mediated by T2D, which itself “is a major cause of cardiovascular-related morbidity and mortality,” said Dr. Hurley.
From a population health perspective, the increased prevalence of T2D – expected to reach 10% in the United States by 2030 – will also boost cardiovascular morbidity and mortality, said Dr. Hurley. Those with T2D die 5 to 10 years earlier, and have double the risk for heart attack and stroke of their peers without diabetes. The risk of lower limb amputation can be as much as 40 times greater for an individual with T2D across the lifespan, he said.
The National Institutes of Health recognizes bariatric surgery as an appropriate weight loss therapy for individuals with a body mass index (BMI) of at least 35 kg/m2 and comorbidity. Whether bariatric surgery might be appropriate for individuals with T2D and BMIs of less than 35 kg/m2 is less settled, though at least some RCTs support the surgical approach, said Dr. Hurley.
The body of data that support long-term metabolic and cardiovascular benefits of bariatric surgery as obesity therapy is growing, said Dr. Hurley. A large prospective observational study by the American College of Surgeons’ Bariatric Surgery Center Network followed 28,616 patients, finding that Roux-en-Y gastric bypass (RYGB) was most effective in improving or resolving CVD comorbidities. At 1 year post surgery, 83% of RYGB patients saw improvement or resolution of T2D; the figure was 79% for hypertension and 66% for dyslipidemia (Ann Surg. 2011;254[3]:410-20).
Weight loss for patients receiving bariatric procedures has generally been durable: for laparoscopic RYGB patients tracked to 7 years after surgery, 75% had maintained at least a 20% weight loss (JAMA Surg. 2018;153[5]427-34).
Longer-term clinical follow-up points toward favorable metabolic and cardiovascular outcomes, said Dr. Hurley, citing data from the Swedish Obese Subjects (SOS) trial. This study followed over 4,000 patients with high BMIs (at least 34 kg/m2 for men and 38 kg/m2 for women) over 10 years. At that point, 36% of gastric bypass patients, compared with 13% of non-surgical high BMI patients, saw resolution of T2D, a significant difference. Triglyceride levels also fell significantly more for the bypass recipients. Hypertension was resolved in just 19% of patients at 10 years, a non-significant difference from the 11% of control patients. Data from the same patient set also showed a significant reduction in total cardiovascular events in the surgical versus non-surgical patients (n = 49 vs. 28, hazard ratio 0.83, log-rank P = .05). Fatal cardiovascular events were significantly lower for patients who had received bariatric surgery, with a 24% decline in mortality for bariatric surgery patients at about 11 years post surgery.
Canadian data showed even greater reductions in mortality, with an 89% decrease in mortality after RYGB, compared with non-surgical patients at the 5-year mark (Ann Surg 2004;240:416-24).
In trials that afforded a direct comparison of medical therapy and bariatric surgery obesity and diabetes, Dr. Hurley said that randomized trials generally show no change to modest change in HbA1c levels with medical management. By contrast, patients in the surgical arms showed a range of improvement ranging from a reduction of just under 1% to reductions of over 5%, with an average reduction of more than 2% across the trials.
Separating out data from the randomized controlled trials with patient BMIs averaging 35 kg/m2 or less, odds ratios still favored bariatric surgery over medication therapy for diabetes-related outcomes in this lower-BMI population, said Dr. Hurley (Diabetes Care 2016;39:924-33).
More data come from a recently reported randomized trial that assigned patients with T2D and a mean BMI of 37 kg/m2 (range, 27-43) to intensive medical therapy, or either sleeve gastrectomy (SG) or RYGB. The study, which had a 90% completion rate at the 5-year mark, found that both surgical procedures were significantly more effective at reducing HbA1c to 6% or less 12 months into the study (P less than .001).
At the 60-month mark, 45% of the RYGB and 25% of the SG patients were on no diabetes medications, while just 2% of the medical therapy arm had stopped all medications, and 40% of this group remained on insulin 5 years into the study, said Dr. Hurley (N Engl J Med. 2017;376:641-651).
“For treatment of type 2 diabetes and cardiovascular co-morbidities, long-term goals often are met following bariatric surgery versus behavior change,” said Dr. Hurley.
Dr. Hurley reported that he had no financial disclosures.
SOURCE: Hurley, D. AACE 2018, Session SGS-4.
EXPERT ANALYSIS FROM AACE 2018
DOJ won’t defend ACA from lawsuit challenging constitutionality
The Department of Justice is declining to interfere with a legal action that could have the significant impact on the Affordable Care Act, filing a brief stating that it will not defend the law in the case Texas v. The United States.
In February 2018, Texas and 19 other states filed a lawsuit in the U.S. District Court for the Northern District of Texas, Fort Worth Division, seeking to have the ACA’s individual mandate declared unconstitutional in light of the mandate’s penalty being reduced to zero effective Jan. 1, 2019. Congress eliminated the financial penalty for not carrying qualifying insurance coverage as part of the Tax Cuts and Jobs Act of 2017.
Taking it further, the plaintiffs argue in their court filing that if the individual mandate is found to be unconstitutional, the ACA “must be invalidated as a whole,” though they suggest that at minimum, “the guaranteed-issue and community rating provisions are non-severable from the mandate and must be invalidated along with the individual mandate.”
The Supreme Court in the 2012 case National Federation of Independent Business v. Sebelius ruled that the penalty associated with the individual mandate could be characterized as a tax and as such rejected the argument that the penalty and the individual mandate were unconstitutional. But since the repeal of the individual mandate and the government’s collection of revenue in conjunction with the mandate, the “ACA lacks a rational basis,” according to the plaintiffs.
DOJ signaled on June 7 that it is siding with the plaintiffs and will not be defending the Affordable Care Act in court.
In a letter sent the same day to House Minority Leader Nancy Pelosi, (D-Calif.), U.S. Attorney General Jeff Sessions said that after “careful consideration, and with the approval of the President of the United States, I have determined that ... the Department of Justice will not defend the constitutionality of the [individual mandate] and will argue that certain provisions of the Affordable Care Act (ACA) are inseverable from that provision.”
Mr. Sessions said in the letter that the plaintiffs “are correct” in determining that the individual mandate is unconstitutional in light of the legislative action to eliminate the penalty for not complying with the individual mandate.
However, the DOJ does not agree that the balance of the law outside of the individual mandate and the inseverable guaranteed issue and community rating provisions should remain in tact.
The court filing argues that the request for a temporary injunction to declare the individual mandate unconstitutional should not be allowed because the individual mandate’s penalty for non-coverage is in effect through 2018, therefore it remains constitutional.
“That said, because this is a pure question of law on which the Plaintiffs and Defendants do not disagree, the Court should consider construing Plaintiff’s motion as a request for summary judgment and then entering a declaratory judgment that the ACA’s provisions establishing the individual mandate as well as the guaranteed-issue and community-rating requirements will all be invalid as of January 1, 2019. That would be adequate relief against the government.”
Former CMS Administrator Andy Slavitt in a tweet called the government’s desire to push any decision until the new year, which would come after the midterm elections, an act of “savage cynicism.” He added in a later tweet that “people who care about public health don’t do this. People who care about the rule of law don’t do this.”
If the plaintiffs are successful in this lawsuit, it could have significant ramifications for Americans.
“If the judge buys the administration’s argument, and if his ruling is upheld on appeal, 52 million Americans with preexisting conditions could face denial of coverage or higher premiums,” Timothy Jost, emeritus professor, Washington and Lee University School of Law, said in a blog post published on The Commonwealth Fund website. “The administration’s argument would also allow insurers to charge women, older people, and people in certain occupations higher premiums. This policy change would jeopardize coverage not just for consumers in the individual market, but also people with preexisting conditions who have employer-sponsored coverage. If these people lost or left their jobs, they may not be able to get individual market coverage.”
The American Cancer Society Cancer Action Network, American Diabetes Association, American Heart Association, American Lung Association, and the National Multiple Sclerosis Society criticized the position taken by DOJ.
“Members of Congress on both sides of the aisle have been emphatic that critical protections should not be repealed without a replacement that would ensure patients can continue to have access to care,” the organizations said in a joint statement. “If the court strikes down these protections, that exact repeal without replace scenario will occur. Should this case be successful, people with cancer, heart disease, diabetes, and any serious or chronic condition are likely to be denied coverage due to their preexisting conditions or charged such high premiums because of their health status that they will be unable to afford any coverage that may be offered.”
The Department of Justice is declining to interfere with a legal action that could have the significant impact on the Affordable Care Act, filing a brief stating that it will not defend the law in the case Texas v. The United States.
In February 2018, Texas and 19 other states filed a lawsuit in the U.S. District Court for the Northern District of Texas, Fort Worth Division, seeking to have the ACA’s individual mandate declared unconstitutional in light of the mandate’s penalty being reduced to zero effective Jan. 1, 2019. Congress eliminated the financial penalty for not carrying qualifying insurance coverage as part of the Tax Cuts and Jobs Act of 2017.
Taking it further, the plaintiffs argue in their court filing that if the individual mandate is found to be unconstitutional, the ACA “must be invalidated as a whole,” though they suggest that at minimum, “the guaranteed-issue and community rating provisions are non-severable from the mandate and must be invalidated along with the individual mandate.”
The Supreme Court in the 2012 case National Federation of Independent Business v. Sebelius ruled that the penalty associated with the individual mandate could be characterized as a tax and as such rejected the argument that the penalty and the individual mandate were unconstitutional. But since the repeal of the individual mandate and the government’s collection of revenue in conjunction with the mandate, the “ACA lacks a rational basis,” according to the plaintiffs.
DOJ signaled on June 7 that it is siding with the plaintiffs and will not be defending the Affordable Care Act in court.
In a letter sent the same day to House Minority Leader Nancy Pelosi, (D-Calif.), U.S. Attorney General Jeff Sessions said that after “careful consideration, and with the approval of the President of the United States, I have determined that ... the Department of Justice will not defend the constitutionality of the [individual mandate] and will argue that certain provisions of the Affordable Care Act (ACA) are inseverable from that provision.”
Mr. Sessions said in the letter that the plaintiffs “are correct” in determining that the individual mandate is unconstitutional in light of the legislative action to eliminate the penalty for not complying with the individual mandate.
However, the DOJ does not agree that the balance of the law outside of the individual mandate and the inseverable guaranteed issue and community rating provisions should remain in tact.
The court filing argues that the request for a temporary injunction to declare the individual mandate unconstitutional should not be allowed because the individual mandate’s penalty for non-coverage is in effect through 2018, therefore it remains constitutional.
“That said, because this is a pure question of law on which the Plaintiffs and Defendants do not disagree, the Court should consider construing Plaintiff’s motion as a request for summary judgment and then entering a declaratory judgment that the ACA’s provisions establishing the individual mandate as well as the guaranteed-issue and community-rating requirements will all be invalid as of January 1, 2019. That would be adequate relief against the government.”
Former CMS Administrator Andy Slavitt in a tweet called the government’s desire to push any decision until the new year, which would come after the midterm elections, an act of “savage cynicism.” He added in a later tweet that “people who care about public health don’t do this. People who care about the rule of law don’t do this.”
If the plaintiffs are successful in this lawsuit, it could have significant ramifications for Americans.
“If the judge buys the administration’s argument, and if his ruling is upheld on appeal, 52 million Americans with preexisting conditions could face denial of coverage or higher premiums,” Timothy Jost, emeritus professor, Washington and Lee University School of Law, said in a blog post published on The Commonwealth Fund website. “The administration’s argument would also allow insurers to charge women, older people, and people in certain occupations higher premiums. This policy change would jeopardize coverage not just for consumers in the individual market, but also people with preexisting conditions who have employer-sponsored coverage. If these people lost or left their jobs, they may not be able to get individual market coverage.”
The American Cancer Society Cancer Action Network, American Diabetes Association, American Heart Association, American Lung Association, and the National Multiple Sclerosis Society criticized the position taken by DOJ.
“Members of Congress on both sides of the aisle have been emphatic that critical protections should not be repealed without a replacement that would ensure patients can continue to have access to care,” the organizations said in a joint statement. “If the court strikes down these protections, that exact repeal without replace scenario will occur. Should this case be successful, people with cancer, heart disease, diabetes, and any serious or chronic condition are likely to be denied coverage due to their preexisting conditions or charged such high premiums because of their health status that they will be unable to afford any coverage that may be offered.”
The Department of Justice is declining to interfere with a legal action that could have the significant impact on the Affordable Care Act, filing a brief stating that it will not defend the law in the case Texas v. The United States.
In February 2018, Texas and 19 other states filed a lawsuit in the U.S. District Court for the Northern District of Texas, Fort Worth Division, seeking to have the ACA’s individual mandate declared unconstitutional in light of the mandate’s penalty being reduced to zero effective Jan. 1, 2019. Congress eliminated the financial penalty for not carrying qualifying insurance coverage as part of the Tax Cuts and Jobs Act of 2017.
Taking it further, the plaintiffs argue in their court filing that if the individual mandate is found to be unconstitutional, the ACA “must be invalidated as a whole,” though they suggest that at minimum, “the guaranteed-issue and community rating provisions are non-severable from the mandate and must be invalidated along with the individual mandate.”
The Supreme Court in the 2012 case National Federation of Independent Business v. Sebelius ruled that the penalty associated with the individual mandate could be characterized as a tax and as such rejected the argument that the penalty and the individual mandate were unconstitutional. But since the repeal of the individual mandate and the government’s collection of revenue in conjunction with the mandate, the “ACA lacks a rational basis,” according to the plaintiffs.
DOJ signaled on June 7 that it is siding with the plaintiffs and will not be defending the Affordable Care Act in court.
In a letter sent the same day to House Minority Leader Nancy Pelosi, (D-Calif.), U.S. Attorney General Jeff Sessions said that after “careful consideration, and with the approval of the President of the United States, I have determined that ... the Department of Justice will not defend the constitutionality of the [individual mandate] and will argue that certain provisions of the Affordable Care Act (ACA) are inseverable from that provision.”
Mr. Sessions said in the letter that the plaintiffs “are correct” in determining that the individual mandate is unconstitutional in light of the legislative action to eliminate the penalty for not complying with the individual mandate.
However, the DOJ does not agree that the balance of the law outside of the individual mandate and the inseverable guaranteed issue and community rating provisions should remain in tact.
The court filing argues that the request for a temporary injunction to declare the individual mandate unconstitutional should not be allowed because the individual mandate’s penalty for non-coverage is in effect through 2018, therefore it remains constitutional.
“That said, because this is a pure question of law on which the Plaintiffs and Defendants do not disagree, the Court should consider construing Plaintiff’s motion as a request for summary judgment and then entering a declaratory judgment that the ACA’s provisions establishing the individual mandate as well as the guaranteed-issue and community-rating requirements will all be invalid as of January 1, 2019. That would be adequate relief against the government.”
Former CMS Administrator Andy Slavitt in a tweet called the government’s desire to push any decision until the new year, which would come after the midterm elections, an act of “savage cynicism.” He added in a later tweet that “people who care about public health don’t do this. People who care about the rule of law don’t do this.”
If the plaintiffs are successful in this lawsuit, it could have significant ramifications for Americans.
“If the judge buys the administration’s argument, and if his ruling is upheld on appeal, 52 million Americans with preexisting conditions could face denial of coverage or higher premiums,” Timothy Jost, emeritus professor, Washington and Lee University School of Law, said in a blog post published on The Commonwealth Fund website. “The administration’s argument would also allow insurers to charge women, older people, and people in certain occupations higher premiums. This policy change would jeopardize coverage not just for consumers in the individual market, but also people with preexisting conditions who have employer-sponsored coverage. If these people lost or left their jobs, they may not be able to get individual market coverage.”
The American Cancer Society Cancer Action Network, American Diabetes Association, American Heart Association, American Lung Association, and the National Multiple Sclerosis Society criticized the position taken by DOJ.
“Members of Congress on both sides of the aisle have been emphatic that critical protections should not be repealed without a replacement that would ensure patients can continue to have access to care,” the organizations said in a joint statement. “If the court strikes down these protections, that exact repeal without replace scenario will occur. Should this case be successful, people with cancer, heart disease, diabetes, and any serious or chronic condition are likely to be denied coverage due to their preexisting conditions or charged such high premiums because of their health status that they will be unable to afford any coverage that may be offered.”
Customized airway stents show promise in feasibility trial
SAN DIEGO – for whom conventional stents were not suitable or failed, results from a small study demonstrated.
“Anatomically complex airway stenosis remains a challenging situation,” lead study author Nicolas Guibert, MD, said at an international conference of the American Thoracic Society. “Conventional devices are either not suited or may result in a significant complication rate, including poor clinical tolerance, migration, or granulation tissue reaction due to lack of congruence.”
Dr. Guibert reported results from eight patients. Of these, three had posttransplant complex airway stenoses involving the bronchus intermedius. Each improved after placement of the customized stents. For example, one patient with vanishing bronchus intermedius syndrome experienced improvements in NYHA dyspnea score from 3 to 1, the VQ11 score from 22 to 11/55, and forced expiratory volume in 1 second (FEV1) from 70% to 107%. The stent was removed after 3 months. Meanwhile, a patient with localized malacia and stenosis of the right main bronchus experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 score from 27 to 15/55, and FEV1 from 70% to 102%. That person’s stent is still in place with no complications. Another patient with localized malacia and stenosis of the bronchus intermedius experienced improvements in FEV1 from 84% to 100%. That person’s device was removed after 3 months, with no residual stenosis.
A fourth patient underwent stent placement for localized malacia (cartilage ring rupture). That person experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 from 23 to 15/55, and FEV1 from 66% to 92%, and peak flow from 49% to 82%. The device is still in place with no complications. A fifth patient received stent placement for extensive tracheobronchomalacia, but it had imperfect congruence and was removed after 3 months because it caused intense cough.
One patient with post-tracheotomy stenosis experienced improvements in NYHA dyspnea score from 3 to 0, VQ11 from 29 to 12/55, and peak flow from 45% to 81%. That person’s device is still in place, Dr. Guibert said. Two other patients treated for post-tracheotomy experienced stent migration (conventional stents also migrated in these two cases), despite good bronchoscopic congruence after placement.
“Tracheal diseases result in suboptimal congruence, probably due to higher respiratory variation,” Dr. Guibert said. “These devices need to be studied in less selected populations and the technology has to be improved.” He reported having no financial disclosures.
SOURCE: Guibert N et al. ATS 2018, Abstract 4433.
SAN DIEGO – for whom conventional stents were not suitable or failed, results from a small study demonstrated.
“Anatomically complex airway stenosis remains a challenging situation,” lead study author Nicolas Guibert, MD, said at an international conference of the American Thoracic Society. “Conventional devices are either not suited or may result in a significant complication rate, including poor clinical tolerance, migration, or granulation tissue reaction due to lack of congruence.”
Dr. Guibert reported results from eight patients. Of these, three had posttransplant complex airway stenoses involving the bronchus intermedius. Each improved after placement of the customized stents. For example, one patient with vanishing bronchus intermedius syndrome experienced improvements in NYHA dyspnea score from 3 to 1, the VQ11 score from 22 to 11/55, and forced expiratory volume in 1 second (FEV1) from 70% to 107%. The stent was removed after 3 months. Meanwhile, a patient with localized malacia and stenosis of the right main bronchus experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 score from 27 to 15/55, and FEV1 from 70% to 102%. That person’s stent is still in place with no complications. Another patient with localized malacia and stenosis of the bronchus intermedius experienced improvements in FEV1 from 84% to 100%. That person’s device was removed after 3 months, with no residual stenosis.
A fourth patient underwent stent placement for localized malacia (cartilage ring rupture). That person experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 from 23 to 15/55, and FEV1 from 66% to 92%, and peak flow from 49% to 82%. The device is still in place with no complications. A fifth patient received stent placement for extensive tracheobronchomalacia, but it had imperfect congruence and was removed after 3 months because it caused intense cough.
One patient with post-tracheotomy stenosis experienced improvements in NYHA dyspnea score from 3 to 0, VQ11 from 29 to 12/55, and peak flow from 45% to 81%. That person’s device is still in place, Dr. Guibert said. Two other patients treated for post-tracheotomy experienced stent migration (conventional stents also migrated in these two cases), despite good bronchoscopic congruence after placement.
“Tracheal diseases result in suboptimal congruence, probably due to higher respiratory variation,” Dr. Guibert said. “These devices need to be studied in less selected populations and the technology has to be improved.” He reported having no financial disclosures.
SOURCE: Guibert N et al. ATS 2018, Abstract 4433.
SAN DIEGO – for whom conventional stents were not suitable or failed, results from a small study demonstrated.
“Anatomically complex airway stenosis remains a challenging situation,” lead study author Nicolas Guibert, MD, said at an international conference of the American Thoracic Society. “Conventional devices are either not suited or may result in a significant complication rate, including poor clinical tolerance, migration, or granulation tissue reaction due to lack of congruence.”
Dr. Guibert reported results from eight patients. Of these, three had posttransplant complex airway stenoses involving the bronchus intermedius. Each improved after placement of the customized stents. For example, one patient with vanishing bronchus intermedius syndrome experienced improvements in NYHA dyspnea score from 3 to 1, the VQ11 score from 22 to 11/55, and forced expiratory volume in 1 second (FEV1) from 70% to 107%. The stent was removed after 3 months. Meanwhile, a patient with localized malacia and stenosis of the right main bronchus experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 score from 27 to 15/55, and FEV1 from 70% to 102%. That person’s stent is still in place with no complications. Another patient with localized malacia and stenosis of the bronchus intermedius experienced improvements in FEV1 from 84% to 100%. That person’s device was removed after 3 months, with no residual stenosis.
A fourth patient underwent stent placement for localized malacia (cartilage ring rupture). That person experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 from 23 to 15/55, and FEV1 from 66% to 92%, and peak flow from 49% to 82%. The device is still in place with no complications. A fifth patient received stent placement for extensive tracheobronchomalacia, but it had imperfect congruence and was removed after 3 months because it caused intense cough.
One patient with post-tracheotomy stenosis experienced improvements in NYHA dyspnea score from 3 to 0, VQ11 from 29 to 12/55, and peak flow from 45% to 81%. That person’s device is still in place, Dr. Guibert said. Two other patients treated for post-tracheotomy experienced stent migration (conventional stents also migrated in these two cases), despite good bronchoscopic congruence after placement.
“Tracheal diseases result in suboptimal congruence, probably due to higher respiratory variation,” Dr. Guibert said. “These devices need to be studied in less selected populations and the technology has to be improved.” He reported having no financial disclosures.
SOURCE: Guibert N et al. ATS 2018, Abstract 4433.
REPORTING FROM ATS 2018
Key clinical point: Customized, 3-D airway stents have the potential for improving tolerance and decreasing the complication rate.
Major finding: Congruence and outcomes tended to be better in stenoses involving the bronchial level (three of three, no complications).
Study details: A feasibility study of eight patients with nonmalignant, anatomically complex, and symptomatic stenosis for which conventional stents were not suitable.
Disclosures: Dr. Guibert reported having no financial disclosures.
Source: Guibert N et al. ATS 2018, Abstract 4433.
Hospital-acquired conditions drop 8% since 2014, saving 8,000 lives and $3 billion
From 2014 to 2016, the rate of potentially deadly hospital-acquired conditions in the United States dropped by 8% – a change that translated into 350,000 fewer such conditions, 8,000 fewer inpatient deaths, and a national savings of almost $3 billion.
The preliminary new baseline rate for hospital-acquired conditions (HACs) is 90 per 1,000 discharges – down from 98 per 1,000 discharges at the end of 2014, according to the Agency for Healthcare Research and Quality’s new report, “AHRQ National Scorecard on Hospital-Acquired Conditions – Updated Baseline Rates and Preliminary Results 2014-2016.”
The largest improvements occurred in central line–associated bloodstream infections (down 31% from 2014), postoperative venous thromboembolism (21% decline), adverse drug events (15% decline), and pressure ulcers (10% decline). A new category, C. difficile infections, also showed a large decline over 2014 (11%).
These numbers build on earlier successes associated with a national goal set by the Centers for Medicare & Medicaid Services to reduce HACs by 20% by 2019. They should be hailed as proof that attention to prevention strategies can save lives and money, said Seema Verma, CMS administrator.
“Today’s results show that this is a tremendous accomplishment by America’s hospitals in delivering high-quality, affordable healthcare,” Ms. Verma said in a press statement. “CMS is committed to moving the healthcare system to one that improves quality and fosters innovation while reducing administrative burden and lowering costs. This work could not be accomplished without the concerted effort of our many hospital, patient, provider, private, and federal partners – all working together to ensure the best possible care by protecting patients from harm and making care safer.”
The numbers continue to go in the right direction, the report noted. Data reported in late 2016 found a 17% decline in HACs from 2010 to 2014. This equated to 2.1 million HACs, 87,000 fewer deaths, and a savings of $19.9 billion.
Much work remains to be done to achieve the stated 2019 goal, the report noted, but the rewards are great. Reaching the 20% reduction goal would secure a total decrease in the HAC rate from 98 to 78 per 1,000 discharges. This would result in 1.78 million fewer HAC in the years from 2015-2019. That decrease would ultimately save 53,000 lives and $19.1 billion over 5 years.
From 2014 to 2016, the rate of potentially deadly hospital-acquired conditions in the United States dropped by 8% – a change that translated into 350,000 fewer such conditions, 8,000 fewer inpatient deaths, and a national savings of almost $3 billion.
The preliminary new baseline rate for hospital-acquired conditions (HACs) is 90 per 1,000 discharges – down from 98 per 1,000 discharges at the end of 2014, according to the Agency for Healthcare Research and Quality’s new report, “AHRQ National Scorecard on Hospital-Acquired Conditions – Updated Baseline Rates and Preliminary Results 2014-2016.”
The largest improvements occurred in central line–associated bloodstream infections (down 31% from 2014), postoperative venous thromboembolism (21% decline), adverse drug events (15% decline), and pressure ulcers (10% decline). A new category, C. difficile infections, also showed a large decline over 2014 (11%).
These numbers build on earlier successes associated with a national goal set by the Centers for Medicare & Medicaid Services to reduce HACs by 20% by 2019. They should be hailed as proof that attention to prevention strategies can save lives and money, said Seema Verma, CMS administrator.
“Today’s results show that this is a tremendous accomplishment by America’s hospitals in delivering high-quality, affordable healthcare,” Ms. Verma said in a press statement. “CMS is committed to moving the healthcare system to one that improves quality and fosters innovation while reducing administrative burden and lowering costs. This work could not be accomplished without the concerted effort of our many hospital, patient, provider, private, and federal partners – all working together to ensure the best possible care by protecting patients from harm and making care safer.”
The numbers continue to go in the right direction, the report noted. Data reported in late 2016 found a 17% decline in HACs from 2010 to 2014. This equated to 2.1 million HACs, 87,000 fewer deaths, and a savings of $19.9 billion.
Much work remains to be done to achieve the stated 2019 goal, the report noted, but the rewards are great. Reaching the 20% reduction goal would secure a total decrease in the HAC rate from 98 to 78 per 1,000 discharges. This would result in 1.78 million fewer HAC in the years from 2015-2019. That decrease would ultimately save 53,000 lives and $19.1 billion over 5 years.
From 2014 to 2016, the rate of potentially deadly hospital-acquired conditions in the United States dropped by 8% – a change that translated into 350,000 fewer such conditions, 8,000 fewer inpatient deaths, and a national savings of almost $3 billion.
The preliminary new baseline rate for hospital-acquired conditions (HACs) is 90 per 1,000 discharges – down from 98 per 1,000 discharges at the end of 2014, according to the Agency for Healthcare Research and Quality’s new report, “AHRQ National Scorecard on Hospital-Acquired Conditions – Updated Baseline Rates and Preliminary Results 2014-2016.”
The largest improvements occurred in central line–associated bloodstream infections (down 31% from 2014), postoperative venous thromboembolism (21% decline), adverse drug events (15% decline), and pressure ulcers (10% decline). A new category, C. difficile infections, also showed a large decline over 2014 (11%).
These numbers build on earlier successes associated with a national goal set by the Centers for Medicare & Medicaid Services to reduce HACs by 20% by 2019. They should be hailed as proof that attention to prevention strategies can save lives and money, said Seema Verma, CMS administrator.
“Today’s results show that this is a tremendous accomplishment by America’s hospitals in delivering high-quality, affordable healthcare,” Ms. Verma said in a press statement. “CMS is committed to moving the healthcare system to one that improves quality and fosters innovation while reducing administrative burden and lowering costs. This work could not be accomplished without the concerted effort of our many hospital, patient, provider, private, and federal partners – all working together to ensure the best possible care by protecting patients from harm and making care safer.”
The numbers continue to go in the right direction, the report noted. Data reported in late 2016 found a 17% decline in HACs from 2010 to 2014. This equated to 2.1 million HACs, 87,000 fewer deaths, and a savings of $19.9 billion.
Much work remains to be done to achieve the stated 2019 goal, the report noted, but the rewards are great. Reaching the 20% reduction goal would secure a total decrease in the HAC rate from 98 to 78 per 1,000 discharges. This would result in 1.78 million fewer HAC in the years from 2015-2019. That decrease would ultimately save 53,000 lives and $19.1 billion over 5 years.
HHS to allow insurers’ workaround on 2019 prices
Federal officials will not block insurance companies from again using a workaround to cushion a steep rise in health premiums caused by President Donald Trump’s cancellation of a program established under the Affordable Care Act, Health and Human Services Secretary Alex Azar announced June 6.
The technique – called “silver loading” because it pushed price increases onto the silver-level plans in the ACA marketplaces – was used by many states for 2018 policies. But federal officials had hinted they might bar the practice next year.
At a hearing June 6 before the House Education and Workforce Committee, Mr. Azar said stopping this practice “would require regulations, which simply couldn’t be done in time for the 2019 plan period.”
States moved to silver loading after the president in October cut off federal reimbursement for so-called cost-sharing reduction subsidies that the ACA guaranteed to insurance companies. Those payments offset the cost of discounts that insurers are required by the law to provide to some low-income people to help cover their deductibles and other out-of-pocket costs.
States scrambled to let insurers raise rates so they would stay in the market. And many let them use this technique to recoup the lost funding by adding to the premium costs of midlevel silver plans in the health exchanges.
Because the formula for federal premium subsidies offered to people who purchase through the marketplaces is based on the prices of those silver plans, as those premiums rose so did the subsidies to help people afford them. That meant the federal government ended up paying much of the increase in prices.
At the committee hearing June 6, under questioning from Rep. Joe Courtney (D-Conn.), Mr. Azar declined to say if the department was considering a future ban.
“It’s not an easy question,” Mr. Azar said.
The fact that the federal government ended up effectively making the payments aggravated many Republicans, and there have been rumors over the past several months that HHS might require the premium increases to be applied across all plans, boosting costs for all buyers in the individual market.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services, told reporters in April that the department was examining the possibility.
Apparently that will not happen, at least not for plan year 2019.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Federal officials will not block insurance companies from again using a workaround to cushion a steep rise in health premiums caused by President Donald Trump’s cancellation of a program established under the Affordable Care Act, Health and Human Services Secretary Alex Azar announced June 6.
The technique – called “silver loading” because it pushed price increases onto the silver-level plans in the ACA marketplaces – was used by many states for 2018 policies. But federal officials had hinted they might bar the practice next year.
At a hearing June 6 before the House Education and Workforce Committee, Mr. Azar said stopping this practice “would require regulations, which simply couldn’t be done in time for the 2019 plan period.”
States moved to silver loading after the president in October cut off federal reimbursement for so-called cost-sharing reduction subsidies that the ACA guaranteed to insurance companies. Those payments offset the cost of discounts that insurers are required by the law to provide to some low-income people to help cover their deductibles and other out-of-pocket costs.
States scrambled to let insurers raise rates so they would stay in the market. And many let them use this technique to recoup the lost funding by adding to the premium costs of midlevel silver plans in the health exchanges.
Because the formula for federal premium subsidies offered to people who purchase through the marketplaces is based on the prices of those silver plans, as those premiums rose so did the subsidies to help people afford them. That meant the federal government ended up paying much of the increase in prices.
At the committee hearing June 6, under questioning from Rep. Joe Courtney (D-Conn.), Mr. Azar declined to say if the department was considering a future ban.
“It’s not an easy question,” Mr. Azar said.
The fact that the federal government ended up effectively making the payments aggravated many Republicans, and there have been rumors over the past several months that HHS might require the premium increases to be applied across all plans, boosting costs for all buyers in the individual market.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services, told reporters in April that the department was examining the possibility.
Apparently that will not happen, at least not for plan year 2019.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Federal officials will not block insurance companies from again using a workaround to cushion a steep rise in health premiums caused by President Donald Trump’s cancellation of a program established under the Affordable Care Act, Health and Human Services Secretary Alex Azar announced June 6.
The technique – called “silver loading” because it pushed price increases onto the silver-level plans in the ACA marketplaces – was used by many states for 2018 policies. But federal officials had hinted they might bar the practice next year.
At a hearing June 6 before the House Education and Workforce Committee, Mr. Azar said stopping this practice “would require regulations, which simply couldn’t be done in time for the 2019 plan period.”
States moved to silver loading after the president in October cut off federal reimbursement for so-called cost-sharing reduction subsidies that the ACA guaranteed to insurance companies. Those payments offset the cost of discounts that insurers are required by the law to provide to some low-income people to help cover their deductibles and other out-of-pocket costs.
States scrambled to let insurers raise rates so they would stay in the market. And many let them use this technique to recoup the lost funding by adding to the premium costs of midlevel silver plans in the health exchanges.
Because the formula for federal premium subsidies offered to people who purchase through the marketplaces is based on the prices of those silver plans, as those premiums rose so did the subsidies to help people afford them. That meant the federal government ended up paying much of the increase in prices.
At the committee hearing June 6, under questioning from Rep. Joe Courtney (D-Conn.), Mr. Azar declined to say if the department was considering a future ban.
“It’s not an easy question,” Mr. Azar said.
The fact that the federal government ended up effectively making the payments aggravated many Republicans, and there have been rumors over the past several months that HHS might require the premium increases to be applied across all plans, boosting costs for all buyers in the individual market.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services, told reporters in April that the department was examining the possibility.
Apparently that will not happen, at least not for plan year 2019.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Bezafibrate shows promise as second-line option for PBC
Nearly one-third of patients with primary biliary cholangitis treated with bezafibrate showed clinical improvement after 24 months, according to data from a randomized trial of 100 adults.
Ursodeoxycholic acid remains the standard first-line therapy for primary biliary cholangitis (PBC), but many patients have an incomplete response to the treatment, and consequently their long-term survival is limited, wrote Christophe Corpechot, MD, of Sorbonne University, Paris, and his colleagues. PBC is also known as primary biliary cirrhosis.
In the BEZURSO trial (Bezafibrate in Combination with Ursodeoxycholic Acid in Primary Biliary Cirrhosis), published in the New England Journal of Medicine, the researchers randomized 100 primary PBC patients with an inadequate response to ursodeoxycholic acid to receive 400 mg per day of bezafibrate or a placebo for 24 months. Inadequate response was defined as “a serum level of alkaline phosphatase or aspartate aminotransferase more than 1.5 times the upper limit of the normal range or an abnormal total bilirubin level, assessed after at least 6 months of treatment with ursodeoxycholic acid,” the researchers said.
Baseline demographics were not significantly different between the groups. The average age of the patients was 53 years, and 95% were white women.
After 24 months, 31% of the patients in the treatment group met the primary outcome, which was the achievement of normal levels of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, and albumin, plus a normal prothrombin index. By contrast, none of the patients in the placebo group achieved the primary outcome.
In particular, bezafibrate patients showed a 60% reduction in alkaline phosphatase levels from baseline to 3 months, and a 14% decrease in total bilirubin from baseline during the course of the study.
Clinical outcomes were similar between the groups; 20% of the bezafibrate group and 18% of the placebo group developed portal hypertension, and two patients in each group developed liver complications. No deaths occurred in either group during the study. Approximately half of the patients in each group reported adverse events. Serious adverse events occurred in 14 bezafibrate patients and 12 placebo patients.
The findings were limited by the small study population, which prevented assessment of bezafibrate on liver transplantation and death, and by the limited histologic data to look at the impact on liver fibrosis and hepatic inflammation, the researchers said.
However, the results support the use of bezafibrate as an add-on to ursodeoxycholic acid in PBC patients, and merit larger, longer studies, they noted.
The study was supported by the Programme Hospitalier de Recherche Clinique 2010, Ministry of Health, and Arrow Génériques. Dr. Corpechot disclosed relationships with companies including Intercept France, Inventiva Pharma, and GlaxoSmithKline.
SOURCE: Corpechot C et al. N Engl J Med. 2018 June 6. doi: 10.1056/NEJMoa1714519.
The BEZURSO study findings “merit cautious excitement,” Elizabeth J. Carey, MD, wrote in an editorial.
“This pivotal trial effectively doubles the limited options for second-line therapy of primary biliary cholangitis,” she said.
Approximately 40% of primary biliary cholangitis patients fail to respond adequately to ursodeoxycholic acid, the first-line therapy, and they remain at risk for progression of liver disease and liver failure, wrote Dr. Carey. Bezafibrate is the first drug to generate improvement in these patients not only in measures of biochemical markers, but also measures of fibrosis and disease symptoms, she said. Patient reports of reduced itching and lower levels of fatigue are worth noting, although they were not the primary outcomes, said Dr. Carey.
“Improvement in patient-reported outcomes prompts the question of whether there is a role for the use of bezafibrate for the management of fatigue or pruritus, even in patients who have a biochemical response to ursodeoxycholic acid,” she noted (N Engl J Med. 2018 June 6. doi: 10.1056/NEJMe1804945).
Despite the promising results, challenges remain for primary biliary cholangitis patients, as approximately 70% did not meet the primary outcome, and those with more severe disease were less likely to respond, Dr. Carey said. However, she added, any agent “that both delays disease progression and alleviates symptoms is a potential boon for patients with the debilitating symptoms of primary biliary cholangitis.”
Dr. Carey is affiliated with the Mayo Clinic in Phoenix, Ariz. Disclosure forms provided by the author are available at NEJM.org.
The BEZURSO study findings “merit cautious excitement,” Elizabeth J. Carey, MD, wrote in an editorial.
“This pivotal trial effectively doubles the limited options for second-line therapy of primary biliary cholangitis,” she said.
Approximately 40% of primary biliary cholangitis patients fail to respond adequately to ursodeoxycholic acid, the first-line therapy, and they remain at risk for progression of liver disease and liver failure, wrote Dr. Carey. Bezafibrate is the first drug to generate improvement in these patients not only in measures of biochemical markers, but also measures of fibrosis and disease symptoms, she said. Patient reports of reduced itching and lower levels of fatigue are worth noting, although they were not the primary outcomes, said Dr. Carey.
“Improvement in patient-reported outcomes prompts the question of whether there is a role for the use of bezafibrate for the management of fatigue or pruritus, even in patients who have a biochemical response to ursodeoxycholic acid,” she noted (N Engl J Med. 2018 June 6. doi: 10.1056/NEJMe1804945).
Despite the promising results, challenges remain for primary biliary cholangitis patients, as approximately 70% did not meet the primary outcome, and those with more severe disease were less likely to respond, Dr. Carey said. However, she added, any agent “that both delays disease progression and alleviates symptoms is a potential boon for patients with the debilitating symptoms of primary biliary cholangitis.”
Dr. Carey is affiliated with the Mayo Clinic in Phoenix, Ariz. Disclosure forms provided by the author are available at NEJM.org.
The BEZURSO study findings “merit cautious excitement,” Elizabeth J. Carey, MD, wrote in an editorial.
“This pivotal trial effectively doubles the limited options for second-line therapy of primary biliary cholangitis,” she said.
Approximately 40% of primary biliary cholangitis patients fail to respond adequately to ursodeoxycholic acid, the first-line therapy, and they remain at risk for progression of liver disease and liver failure, wrote Dr. Carey. Bezafibrate is the first drug to generate improvement in these patients not only in measures of biochemical markers, but also measures of fibrosis and disease symptoms, she said. Patient reports of reduced itching and lower levels of fatigue are worth noting, although they were not the primary outcomes, said Dr. Carey.
“Improvement in patient-reported outcomes prompts the question of whether there is a role for the use of bezafibrate for the management of fatigue or pruritus, even in patients who have a biochemical response to ursodeoxycholic acid,” she noted (N Engl J Med. 2018 June 6. doi: 10.1056/NEJMe1804945).
Despite the promising results, challenges remain for primary biliary cholangitis patients, as approximately 70% did not meet the primary outcome, and those with more severe disease were less likely to respond, Dr. Carey said. However, she added, any agent “that both delays disease progression and alleviates symptoms is a potential boon for patients with the debilitating symptoms of primary biliary cholangitis.”
Dr. Carey is affiliated with the Mayo Clinic in Phoenix, Ariz. Disclosure forms provided by the author are available at NEJM.org.
Nearly one-third of patients with primary biliary cholangitis treated with bezafibrate showed clinical improvement after 24 months, according to data from a randomized trial of 100 adults.
Ursodeoxycholic acid remains the standard first-line therapy for primary biliary cholangitis (PBC), but many patients have an incomplete response to the treatment, and consequently their long-term survival is limited, wrote Christophe Corpechot, MD, of Sorbonne University, Paris, and his colleagues. PBC is also known as primary biliary cirrhosis.
In the BEZURSO trial (Bezafibrate in Combination with Ursodeoxycholic Acid in Primary Biliary Cirrhosis), published in the New England Journal of Medicine, the researchers randomized 100 primary PBC patients with an inadequate response to ursodeoxycholic acid to receive 400 mg per day of bezafibrate or a placebo for 24 months. Inadequate response was defined as “a serum level of alkaline phosphatase or aspartate aminotransferase more than 1.5 times the upper limit of the normal range or an abnormal total bilirubin level, assessed after at least 6 months of treatment with ursodeoxycholic acid,” the researchers said.
Baseline demographics were not significantly different between the groups. The average age of the patients was 53 years, and 95% were white women.
After 24 months, 31% of the patients in the treatment group met the primary outcome, which was the achievement of normal levels of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, and albumin, plus a normal prothrombin index. By contrast, none of the patients in the placebo group achieved the primary outcome.
In particular, bezafibrate patients showed a 60% reduction in alkaline phosphatase levels from baseline to 3 months, and a 14% decrease in total bilirubin from baseline during the course of the study.
Clinical outcomes were similar between the groups; 20% of the bezafibrate group and 18% of the placebo group developed portal hypertension, and two patients in each group developed liver complications. No deaths occurred in either group during the study. Approximately half of the patients in each group reported adverse events. Serious adverse events occurred in 14 bezafibrate patients and 12 placebo patients.
The findings were limited by the small study population, which prevented assessment of bezafibrate on liver transplantation and death, and by the limited histologic data to look at the impact on liver fibrosis and hepatic inflammation, the researchers said.
However, the results support the use of bezafibrate as an add-on to ursodeoxycholic acid in PBC patients, and merit larger, longer studies, they noted.
The study was supported by the Programme Hospitalier de Recherche Clinique 2010, Ministry of Health, and Arrow Génériques. Dr. Corpechot disclosed relationships with companies including Intercept France, Inventiva Pharma, and GlaxoSmithKline.
SOURCE: Corpechot C et al. N Engl J Med. 2018 June 6. doi: 10.1056/NEJMoa1714519.
Nearly one-third of patients with primary biliary cholangitis treated with bezafibrate showed clinical improvement after 24 months, according to data from a randomized trial of 100 adults.
Ursodeoxycholic acid remains the standard first-line therapy for primary biliary cholangitis (PBC), but many patients have an incomplete response to the treatment, and consequently their long-term survival is limited, wrote Christophe Corpechot, MD, of Sorbonne University, Paris, and his colleagues. PBC is also known as primary biliary cirrhosis.
In the BEZURSO trial (Bezafibrate in Combination with Ursodeoxycholic Acid in Primary Biliary Cirrhosis), published in the New England Journal of Medicine, the researchers randomized 100 primary PBC patients with an inadequate response to ursodeoxycholic acid to receive 400 mg per day of bezafibrate or a placebo for 24 months. Inadequate response was defined as “a serum level of alkaline phosphatase or aspartate aminotransferase more than 1.5 times the upper limit of the normal range or an abnormal total bilirubin level, assessed after at least 6 months of treatment with ursodeoxycholic acid,” the researchers said.
Baseline demographics were not significantly different between the groups. The average age of the patients was 53 years, and 95% were white women.
After 24 months, 31% of the patients in the treatment group met the primary outcome, which was the achievement of normal levels of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, and albumin, plus a normal prothrombin index. By contrast, none of the patients in the placebo group achieved the primary outcome.
In particular, bezafibrate patients showed a 60% reduction in alkaline phosphatase levels from baseline to 3 months, and a 14% decrease in total bilirubin from baseline during the course of the study.
Clinical outcomes were similar between the groups; 20% of the bezafibrate group and 18% of the placebo group developed portal hypertension, and two patients in each group developed liver complications. No deaths occurred in either group during the study. Approximately half of the patients in each group reported adverse events. Serious adverse events occurred in 14 bezafibrate patients and 12 placebo patients.
The findings were limited by the small study population, which prevented assessment of bezafibrate on liver transplantation and death, and by the limited histologic data to look at the impact on liver fibrosis and hepatic inflammation, the researchers said.
However, the results support the use of bezafibrate as an add-on to ursodeoxycholic acid in PBC patients, and merit larger, longer studies, they noted.
The study was supported by the Programme Hospitalier de Recherche Clinique 2010, Ministry of Health, and Arrow Génériques. Dr. Corpechot disclosed relationships with companies including Intercept France, Inventiva Pharma, and GlaxoSmithKline.
SOURCE: Corpechot C et al. N Engl J Med. 2018 June 6. doi: 10.1056/NEJMoa1714519.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Primary biliary cholangitis patients who took bezafibrate showed decreases in alkaline phosphatase levels and total bilirubin.
Major finding: A total of 31% of patients who took bezafibrate achieved normal levels of disease biomarkers after 24 months compared with 0% of placebo patients.
Study details: The data come from a double-blind, placebo-controlled trial of 100 adults with primary biliary cholangitis at 21 medical centers in France.
Disclosures: Programme Hospitalier de Recherche Clinique 2010 (Ministry of Health) and Arrow Génériques supported the study. Dr. Corpechot disclosed relationships with companies including Intercept France, Inventiva Pharma, and GlaxoSmithKline.
Source: Corpechot C et al. N Engl J Med. 2018 June 6. doi: 10.1056/NEJMoa1714519.
Verma unveils Medicaid scorecard but refuses to judge efforts
“This is about bringing a level of transparency and accountability to the Medicaid program that we have never had before,” said Seema Verma, administrator of the Centers for Medicare & Medicaid Services.
Yet in a meeting with reporters, Ms. Verma refused to discuss the findings in any detail or comment on any individual states that performed poorly or exceptionally.
“I will let you look at the data and make your own conclusions,” she told journalists a few minutes before the report was posted online.
When reporters pressed Ms. Verma to comment on the document, she refused to give an assessment of the Medicaid program, the federal-state health program for low-income residents. She has run Medicaid for the past 15 months.
“The idea here is to give you a sense of where states are on different areas,” she said. “The idea is to be used for best practices,” and it’s “an opportunity for us to identify” and have discussions with states that aren’t performing well.
Medicaid covers about 75 million people, about half of them children.
The report looked at how well states provide a wide variety of health services to children and adults. It also reviewed how quickly the federal government was approving state waiver requests to change their programs.
But not all states provided data for each service because sharing information was voluntary.
For example, half the states did not show how well they control Medicaid enrollees’ blood pressure.
The National Association of Medicaid Directors panned the scorecard. It acknowledged the need for a system to measure performance but said its members have concerns about its accuracy and usefulness.
“There are significant methodological issues with the underlying data, including completeness, timeliness, and quality,” the association said in a statement. It noted that most of the data come from 2015.
As expected, the data showed great variation in how states provide care, including immunizing teenagers and getting dental care to children. A big reason is that state Medicaid benefits and payments to doctors vary dramatically, the Medicaid directors said, so that “it will not be possible to make apples-to-apples comparisons between states.”
In her first public speech, Ms. Verma promised last November to release a Medicaid scorecard. She said states won’t immediately face any consequences for poor performance – but that could change.
“The data … begins to offer taxpayers insights into how their dollars are being spent and the impact those dollars have on health outcomes,” Ms. Verma said on June 4.
Sara Rosenbaum, a professor of health law and policy at George Washington University in Washington, who previously led a congressional advisory board on Medicaid, suggested that the information is still too incomplete to be of great value.
“It is amazing to me that in 2018 this is all we have when trying to understand how the nation’s largest insurer performs for its poorest and most vulnerable residents,” she said.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
“This is about bringing a level of transparency and accountability to the Medicaid program that we have never had before,” said Seema Verma, administrator of the Centers for Medicare & Medicaid Services.
Yet in a meeting with reporters, Ms. Verma refused to discuss the findings in any detail or comment on any individual states that performed poorly or exceptionally.
“I will let you look at the data and make your own conclusions,” she told journalists a few minutes before the report was posted online.
When reporters pressed Ms. Verma to comment on the document, she refused to give an assessment of the Medicaid program, the federal-state health program for low-income residents. She has run Medicaid for the past 15 months.
“The idea here is to give you a sense of where states are on different areas,” she said. “The idea is to be used for best practices,” and it’s “an opportunity for us to identify” and have discussions with states that aren’t performing well.
Medicaid covers about 75 million people, about half of them children.
The report looked at how well states provide a wide variety of health services to children and adults. It also reviewed how quickly the federal government was approving state waiver requests to change their programs.
But not all states provided data for each service because sharing information was voluntary.
For example, half the states did not show how well they control Medicaid enrollees’ blood pressure.
The National Association of Medicaid Directors panned the scorecard. It acknowledged the need for a system to measure performance but said its members have concerns about its accuracy and usefulness.
“There are significant methodological issues with the underlying data, including completeness, timeliness, and quality,” the association said in a statement. It noted that most of the data come from 2015.
As expected, the data showed great variation in how states provide care, including immunizing teenagers and getting dental care to children. A big reason is that state Medicaid benefits and payments to doctors vary dramatically, the Medicaid directors said, so that “it will not be possible to make apples-to-apples comparisons between states.”
In her first public speech, Ms. Verma promised last November to release a Medicaid scorecard. She said states won’t immediately face any consequences for poor performance – but that could change.
“The data … begins to offer taxpayers insights into how their dollars are being spent and the impact those dollars have on health outcomes,” Ms. Verma said on June 4.
Sara Rosenbaum, a professor of health law and policy at George Washington University in Washington, who previously led a congressional advisory board on Medicaid, suggested that the information is still too incomplete to be of great value.
“It is amazing to me that in 2018 this is all we have when trying to understand how the nation’s largest insurer performs for its poorest and most vulnerable residents,” she said.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
“This is about bringing a level of transparency and accountability to the Medicaid program that we have never had before,” said Seema Verma, administrator of the Centers for Medicare & Medicaid Services.
Yet in a meeting with reporters, Ms. Verma refused to discuss the findings in any detail or comment on any individual states that performed poorly or exceptionally.
“I will let you look at the data and make your own conclusions,” she told journalists a few minutes before the report was posted online.
When reporters pressed Ms. Verma to comment on the document, she refused to give an assessment of the Medicaid program, the federal-state health program for low-income residents. She has run Medicaid for the past 15 months.
“The idea here is to give you a sense of where states are on different areas,” she said. “The idea is to be used for best practices,” and it’s “an opportunity for us to identify” and have discussions with states that aren’t performing well.
Medicaid covers about 75 million people, about half of them children.
The report looked at how well states provide a wide variety of health services to children and adults. It also reviewed how quickly the federal government was approving state waiver requests to change their programs.
But not all states provided data for each service because sharing information was voluntary.
For example, half the states did not show how well they control Medicaid enrollees’ blood pressure.
The National Association of Medicaid Directors panned the scorecard. It acknowledged the need for a system to measure performance but said its members have concerns about its accuracy and usefulness.
“There are significant methodological issues with the underlying data, including completeness, timeliness, and quality,” the association said in a statement. It noted that most of the data come from 2015.
As expected, the data showed great variation in how states provide care, including immunizing teenagers and getting dental care to children. A big reason is that state Medicaid benefits and payments to doctors vary dramatically, the Medicaid directors said, so that “it will not be possible to make apples-to-apples comparisons between states.”
In her first public speech, Ms. Verma promised last November to release a Medicaid scorecard. She said states won’t immediately face any consequences for poor performance – but that could change.
“The data … begins to offer taxpayers insights into how their dollars are being spent and the impact those dollars have on health outcomes,” Ms. Verma said on June 4.
Sara Rosenbaum, a professor of health law and policy at George Washington University in Washington, who previously led a congressional advisory board on Medicaid, suggested that the information is still too incomplete to be of great value.
“It is amazing to me that in 2018 this is all we have when trying to understand how the nation’s largest insurer performs for its poorest and most vulnerable residents,” she said.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
AMA: Opioid prescriptions down since 2013
Opioid prescriptions dropped by 22% over the last 5 years, with decreases seen in all 50 states, according to a new report from the American Medical Association’s opioid task force.
“The largest decrease in opioid prescriptions in 25 years reflects the fact that physicians and other health care professionals are increasingly judicious when prescribing opioids,” Patrice A. Harris, MD, chair of the task force, said in the report.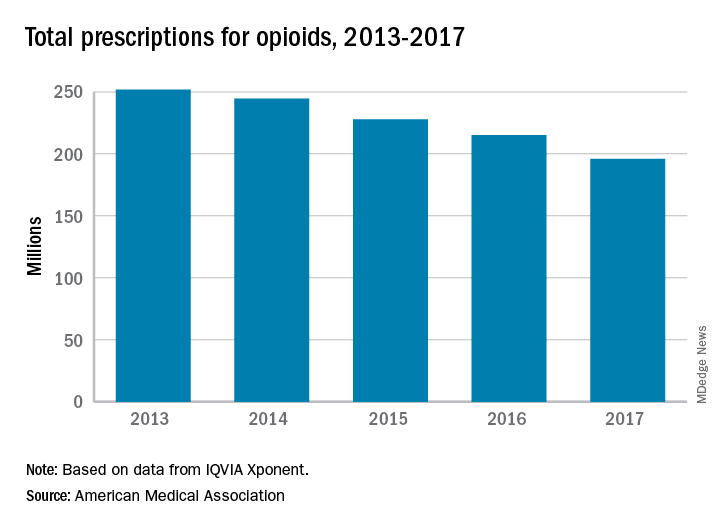
Other signs of progress against the opioid epidemic include the increasing number of health care professionals registered for prescription drug monitoring programs: It has more than tripled, from 472,000 in 2014 to 1.55 million in 2017. Furthermore, the number of naloxone prescriptions in 2017 had more than doubled, from about 3,500 per week to 8,000, the task force reported.
“Unfortunately, deaths related to heroin and illicit fentanyl, and to prescription opioids, continue to rise. We need well-designed initiatives that bring together public and private insurers, policymakers, public health infrastructure, and communities with the shared goal to improve access and coverage for comprehensive pain management and treatment for substance use disorders,” Dr. Harris said.
Opioid prescriptions dropped by 22% over the last 5 years, with decreases seen in all 50 states, according to a new report from the American Medical Association’s opioid task force.
“The largest decrease in opioid prescriptions in 25 years reflects the fact that physicians and other health care professionals are increasingly judicious when prescribing opioids,” Patrice A. Harris, MD, chair of the task force, said in the report.
Other signs of progress against the opioid epidemic include the increasing number of health care professionals registered for prescription drug monitoring programs: It has more than tripled, from 472,000 in 2014 to 1.55 million in 2017. Furthermore, the number of naloxone prescriptions in 2017 had more than doubled, from about 3,500 per week to 8,000, the task force reported.
“Unfortunately, deaths related to heroin and illicit fentanyl, and to prescription opioids, continue to rise. We need well-designed initiatives that bring together public and private insurers, policymakers, public health infrastructure, and communities with the shared goal to improve access and coverage for comprehensive pain management and treatment for substance use disorders,” Dr. Harris said.
Opioid prescriptions dropped by 22% over the last 5 years, with decreases seen in all 50 states, according to a new report from the American Medical Association’s opioid task force.
“The largest decrease in opioid prescriptions in 25 years reflects the fact that physicians and other health care professionals are increasingly judicious when prescribing opioids,” Patrice A. Harris, MD, chair of the task force, said in the report.
Other signs of progress against the opioid epidemic include the increasing number of health care professionals registered for prescription drug monitoring programs: It has more than tripled, from 472,000 in 2014 to 1.55 million in 2017. Furthermore, the number of naloxone prescriptions in 2017 had more than doubled, from about 3,500 per week to 8,000, the task force reported.
“Unfortunately, deaths related to heroin and illicit fentanyl, and to prescription opioids, continue to rise. We need well-designed initiatives that bring together public and private insurers, policymakers, public health infrastructure, and communities with the shared goal to improve access and coverage for comprehensive pain management and treatment for substance use disorders,” Dr. Harris said.
FDA alerts clinicians to gastric balloon deaths
according to an alert from the Food and Drug Administration issued on June 4.
Seven of these deaths occurred in patients in the United States; four involved the ORBERA Intragastric Balloon System, and three involved the ReShape Integrated Dual Balloon System.
The FDA has approved updated labeling for the ORBERA and ReShape balloon systems in the United States. The labels contain more information about possible death associated with the use of these devices in the United States. The manufacturers’ sites, Apollo Endosurgery and ReShape Lifesciences, provide more details about the new labeling.
In a letter to health care providers, the FDA advised clinicians to educate bariatric surgery patients about the symptoms of complications from balloon procedures, including not only gastric perforation but also esophageal perforation, balloon deflation, gastrointestinal obstruction, and ulceration. In addition, the FDA reminded clinicians to monitor patients during the entire course of treatment for additional complications, including acute pancreatitis and spontaneous hyperinflation.
Any adverse events involving intragastric balloon systems should be reported to the FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
according to an alert from the Food and Drug Administration issued on June 4.
Seven of these deaths occurred in patients in the United States; four involved the ORBERA Intragastric Balloon System, and three involved the ReShape Integrated Dual Balloon System.
The FDA has approved updated labeling for the ORBERA and ReShape balloon systems in the United States. The labels contain more information about possible death associated with the use of these devices in the United States. The manufacturers’ sites, Apollo Endosurgery and ReShape Lifesciences, provide more details about the new labeling.
In a letter to health care providers, the FDA advised clinicians to educate bariatric surgery patients about the symptoms of complications from balloon procedures, including not only gastric perforation but also esophageal perforation, balloon deflation, gastrointestinal obstruction, and ulceration. In addition, the FDA reminded clinicians to monitor patients during the entire course of treatment for additional complications, including acute pancreatitis and spontaneous hyperinflation.
Any adverse events involving intragastric balloon systems should be reported to the FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
according to an alert from the Food and Drug Administration issued on June 4.
Seven of these deaths occurred in patients in the United States; four involved the ORBERA Intragastric Balloon System, and three involved the ReShape Integrated Dual Balloon System.
The FDA has approved updated labeling for the ORBERA and ReShape balloon systems in the United States. The labels contain more information about possible death associated with the use of these devices in the United States. The manufacturers’ sites, Apollo Endosurgery and ReShape Lifesciences, provide more details about the new labeling.
In a letter to health care providers, the FDA advised clinicians to educate bariatric surgery patients about the symptoms of complications from balloon procedures, including not only gastric perforation but also esophageal perforation, balloon deflation, gastrointestinal obstruction, and ulceration. In addition, the FDA reminded clinicians to monitor patients during the entire course of treatment for additional complications, including acute pancreatitis and spontaneous hyperinflation.
Any adverse events involving intragastric balloon systems should be reported to the FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting program.