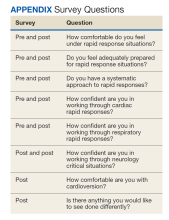
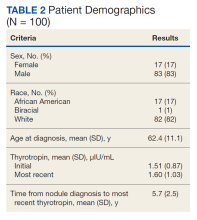
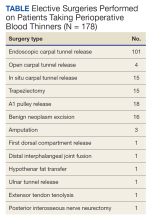
User login
The challenges of managing CMV infection during pregnancy
CASE Anomalous findings on fetal anatomic survey
A 27-year-old previously healthy primigravid woman is at 18 weeks’ gestation. She is a first-grade schoolteacher. On her fetal anatomic survey, the estimated fetal weight was in the eighth percentile. Echogenic bowel and a small amount of ascitic fluid were noted in the fetal abdomen. The lateral and third ventricles were mildly dilated, the head circumference was 2 standard deviations below normal, and the placenta was slightly thickened and edematous.
What is the most likely diagnosis?
What diagnostic tests are indicated?
What management options are available for this patient?
Cytomegalovirus (CMV) is the most common of the perinatally transmitted infections, affecting 1% to 4% of all pregnancies. Although the virus typically causes either asymptomatic infection or only mild illness in immunocompetent individuals, it can cause life-threatening disease in immunocompromised persons and in the developing fetus. In this article, we review the virology and epidemiology of CMV infection and then focus on the key methods to diagnose infection in the mother and fetus. We conclude by considering measures that may be of at least modest value in treating CMV in pregnancy.
Virology of CMV infection
Cytomegalovirus is a double-stranded DNA virus in the Herpesviridae family. This ubiquitous virus is present in virtually all secretions and excretions of an infected host, including blood, urine, saliva, breast milk, genital secretions, and tissues and organs used for donation. Infection is transmitted through direct contact with any of the substances listed; contact with infected urine or saliva is the most common mode of transmission. Disease occurrence does not show seasonal variation.
After exposure, an incubation period of 28 to 60 days ensues, followed by development of viremia and clinical symptoms. In the majority of exposed individuals, CMV establishes a lifelong latent infection, and recurrent episodes of illness can occur as a result of reactivation of latent virus (also known as secondary infection) or, more rarely, infection with a new viral strain. In fact, most CMV illness episodes in pregnancy represent a reactivation of a previous infection rather than a new infection.
Following initial infection, both IgM (immunoglobulin M) and IgG (immunoglobulin G) antibodies develop rapidly and can be detected in blood within 1 to 2 weeks. IgM levels typically wane within 30 to 60 days, although persistence for several months is not unusual, and levels also can increase with viral reactivation (secondary infection). IgG antibodies typically persist for many years after a primary infection.
Intrauterine CMV infection occurs through hematogenous transplacental passage during maternal viremia. The risk of transmission and severity of fetal effects depend on whether or not the infection is primary or secondary in nature as well as the gestational age at fetal exposure.1,2
Additionally, postnatal vertical transmission can occur through exposure to viral particles in genital secretions as well as breast milk. CMV acquired in the postnatal period rarely produces severe sequelae in a healthy term neonate, but it has been associated with an increased rate of complications in very low birth weight and premature newborns.3
Continue to: Who is at risk...
Who is at risk
Congenital CMV, which occurs in 2.1 to 7.7 per 10,000 live births in the United States, is both the most common congenital infection and the leading cause of nonhereditary congenital hearing loss in children.4,5 The main reservoir of CMV in the United States is young children in day care settings, with approximately 50% of this population showing evidence of viral shedding in saliva.1 Adult populations in North America have a high prevalence of CMV IgG antibodies indicative of prior infection, with rates reaching 50% to 80%. Among seronegative individuals aged 12 to 49, the rate of seroconversion is approximately 1 in 60 annually.6 Significant racial disparities have been noted in rates of seroprevalence and seroconversion, with higher rates of infection in non-Hispanic Black and Mexican American individuals.6 Overall, the rate of new CMV infection among pregnant women in the United States is 0.7% to 4%.7
Clinical manifestations
Manifestations of infection differ depending on whether or not infection is primary or recurrent (secondary) and whether or not the host is immunocompetent or has a compromised immune system. Unique manifestations develop in the fetus.
CMV infection in children and adults. Among individuals with a normal immune response, the typical course of CMV is either no symptoms or a mononucleosis-like illness. In symptomatic patients, the most common symptoms include malaise, fever, and night sweats, and the most common associated laboratory abnormalities are elevation in liver function tests and a decreased white blood cell count, with a predominance of lymphocytes.8
Immunocompromised individuals are at risk for significant morbidity and mortality resulting from CMV. Illness may be the result of reactivation of latent infection due to decreased immune function or may be acquired as a result of treatment such as transplantation of CMV-positive organs or tissues, including bone marrow. Virtually any organ system can be affected, with potential for permanent organ damage and death. Severe systemic infection also can occur.
CMV infection in the fetus and neonate. As noted previously, fetal infection develops as a result of transplacental passage coincident with maternal infection. The risk of CMV transmission to the fetus and the severity of fetal injury vary based on gestational age at fetal infection and whether or not maternal infection is primary or secondary.
In most studies, primary maternal infections are associated with higher rates of fetal infection and more severe fetal and neonatal disease manifestations.2,7,9,10 Primary infections carry an overall 30% to 40% risk of transmission to the fetus.7,11 The risk of fetal transmission is much lower with a recurrent infection and is usually less than 2%.11 Due to their greater overall incidence, secondary infections account for the majority of cases of fetal and neonatal CMV disease.7 Importantly, although secondary infections generally have been regarded as having a lower risk and lower severity of fetal and neonatal disease, several recent studies have demonstrated rates of complications similar to, and even exceeding, those of primary infections.12-15 The TABLE provides a summary of the risks of fetal transmission and symptomatic fetal infection based on trimester of pregnancy.2,11,16-18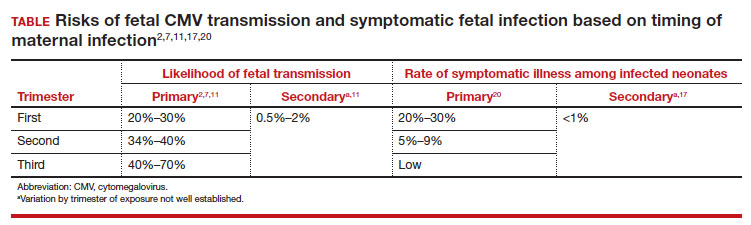
In the fetus, CMV may affect multiple organ systems. Among sonographic and magnetic resonance imaging (MRI) findings, central nervous system (CNS) anomalies are the most common.19,20 These can include microcephaly, ventriculomegaly, and periventricular calcifications. The gastrointestinal system also is frequently affected, and findings include echogenic bowel, hepatosplenomegaly, and liver calcifications. Lastly, isolated effusions, placentomegaly, fetal growth restriction, and even frank hydrops can develop. More favorable neurologic outcomes have been demonstrated in infants with no prenatal brain imaging abnormalities.20,21 However, the role of MRI in prenatal prognosis currently is not well defined.
FIGURE 1 illustrates selected sonographic findings associated with fetal CMV infection.
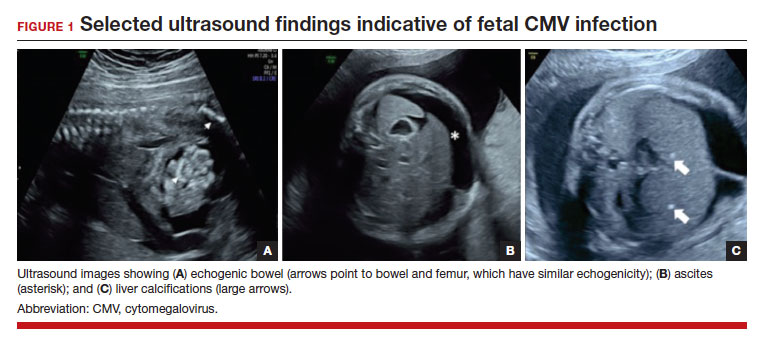
About 85% to 90% of infants with congenital CMV that results from primary maternal infection have no symptoms at birth. Among the 10% to 15% of infants that do have symptoms, petechial rash, jaundice, and hepatosplenomegaly are the most common manifestations (“blueberry muffin baby”). Approximately 10% to 20% of infants in this group have evidence of chorioretinitis on ophthalmologic examination, and 50% show either microcephaly or low birth weight.22Among survivors of symptomatic congenital CMV, more than 50% have long-term neurologic morbidities that may include sensorineural hearing loss, seizures, vision impairment, and developmental disabilities. Note that even when neonates appear asymptomatic at birth (regardless of whether infection is primary or secondary), 5% may develop microcephaly and motor deficits, 10% go on to develop sensorineural hearing loss, and the overall rate of neurologic morbidity reaches 13% to 15%.12,23 Some of the observed deficits manifest at several years of age, and, currently, no models exist for prediction of outcome.
Continue to: Diagnosing CMV infection...
Diagnosing CMV infection
Maternal infection
If maternal CMV infection is suspected based on a symptomatic illness or an abnormal fetal ultrasound exam, the first diagnostic test should be an assessment of IgM and IgG serology. If the former test results are positive and the latter negative, the diagnosis of acute CMV infection is confirmed. A positive serum CMV DNA polymerase chain reaction (PCR) test adds additional assurance that the diagnosis is correct. Primary infection, as noted above, poses the greatest risk of serious injury to the fetus.1
A frequent diagnostic dilemma arises when both the IgM and IgG antibody are positive. Remember that CMV IgM antibody can remain positive for 9 to 12 months after a primary infection and can reappear in the maternal serum in the face of a recurrent or reactivated infection. When confronted by both a positive IgM and positive IgG result, the clinician should then order IgG avidity testing. If the avidity is low to moderate, which reflects poor binding of antibody to the virus, the patient likely has an acute infection. If the avidity is high, which reflects enhanced binding of antibody to virus, the patient probably has a recurrent or reactivated infection; this scenario poses less danger to the developing fetus. The presence of CMV DNA in serum is also more consistent with acute infection, although viremia still can occur with recurrent infection. FIGURE 2 presents a suggested algorithm for the diagnosis of CMV in the pregnant patient.1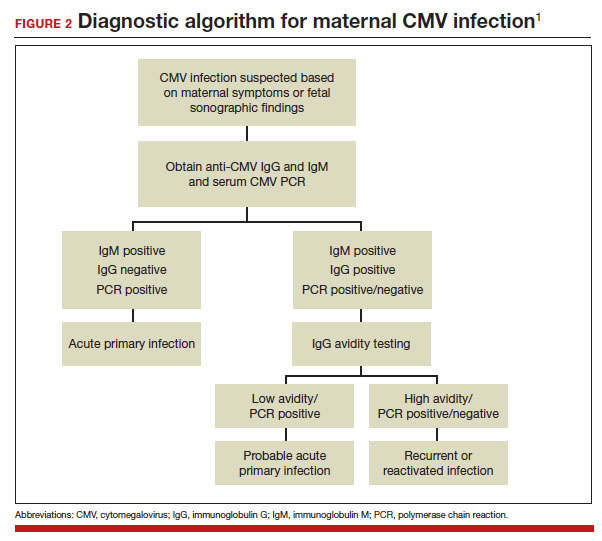
If a diagnosis of maternal CMV infection is confirmed, liver function tests should be obtained to determine if CMV hepatitis is present. If the liver function tests are abnormal, a coagulation profile also should be performed to identify the mother who might be at risk for peripartum hemorrhage.
Fetal infection
The single best test for confirmation of congenital CMV infection is detection of viral DNA and quantitation of viral load in the amniotic fluid by PCR. If the amniocentesis is performed prior to 20 weeks’ gestation and is negative, the test should be repeated in approximately 4 weeks.1,19,24
Detection of viral DNA indicates congenital infection. The ultimate task, however, is to determine if the infection has injured the fetus. Detailed ultrasound examination is the key to identifying fetal injury. As noted previously, the principal ultrasonographic findings that suggest congenital CMV infection include2,19,20,21,25:
- hydropic placenta
- fetal growth restriction
- microcephaly (head circumference more than 3 standard deviations below the mean)
- periventricular calcifications
- enlarged liver
- echogenic bowel
- ascites
- fetal hydrops.
Management: Evidence on CMV hyperimmune globulin, valacyclovir
If the immunocompetent mother has clinical manifestations of infection, she should receive symptomatic treatment. She should be encouraged to rest as much as possible, stay well hydrated, and use acetaminophen (1,000 mg every 6 to 8 hours) as needed for malaise and fever.
However, if the mother is immunocompromised and has signs of serious complications, such as chorioretinitis, hepatitis, or pneumonia, more aggressive therapy is indicated. Drugs used in this setting include foscarnet and ganciclovir and are best prescribed in consultation with a medical infectious disease specialist.
At this time, no consistently effective therapy for congenital infection is available. Therefore, if a patient has primary CMV infection in the first half of pregnancy, particularly in the first trimester, she should be counseled that the risk of fetal infection is approximately 40% and that approximately 5% to 15% of infants will be severely affected at birth. Given this information, some patients may opt for pregnancy termination.
In 2005, a report from Nigro and colleagues stimulated great hope that CMV-specific hyperimmune globulin (CytoGam) might be of value for both treatment and prophylaxis for congenital infection.26 These authors studied 157 women with confirmed primary CMV infection. One-hundred forty-eight women were asymptomatic and were identified by routine serologic screening, 8 had symptomatic infection, and 1 was identified because of abnormal fetal ultrasound findings. Forty-five women had CMV detected in amniotic fluid by PCR or culture more than 6 weeks before study enrollment. Thirty-one of these women were treated with intravenous hyperimmune globulin (200 U or 200 mg/kg maternal body weight); 14 declined treatment. Seven of the latter women had infants who were acutely symptomatic at the time of delivery; only 1 of the 31 treated women had an affected neonate (adjusted odds ratio [OR], 0.02; P<.001). In this same study, 84 women did not have a diagnostic amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Thirty-seven of these women received hyperimmune globulin (100 U or 100 mg/kg) every month until delivery, and 47 declined treatment. Six of the treated women delivered infected infants compared with 19 of the untreated women (adjusted OR, 0.32; P<.04).
Although these results were quite encouraging, several problems existed with the study’s design, as noted in an editorial that accompanied the study’s publication.27 First, the study was not randomized or placebo controlled. Second, patients were not stratified based on the severity of fetal ultrasound abnormalities. Third, the dosing of hyperimmune globulin varied; 9 of the 31 patients in the treatment group received additional infusions of drug into either the amniotic fluid or fetal umbilical vein. Moreover, patients in the prophylaxis group actually received a higher cumulative dose of hyperimmune globulin than patients in the treatment group.
Two subsequent investigations that were better designed were unable to verify the effectiveness of hyperimmune globulin. In 2014, Revello and colleagues reported the results of a prospective, randomized, placebo-controlled, double-blinded study of 124 women at 5 to 26 weeks’ gestation with confirmed primary CMV infection.28 The rate of congenital infection was 30% in the group treated with hyperimmune globulin and 44% in the placebo group (P=.13). There also was no significant difference in the concentration of serum CMV DNA in treated versus untreated mothers. Moreover, the number of adverse obstetric events (preterm delivery, fetal growth restriction, intrahepatic cholestasis of pregnancy, and postpartum preeclampsia) in the treatment group was higher than in the placebo group, 13% versus 2%.
In 2021, Hughes and colleagues published the results of a multicenter, double-blind trial in 399 women who had a diagnosis of primary CMV infection before 23 weeks’ gestation.29 The primary outcome was defined as a composite of congenital CMV infection or fetal/neonatal death. An adverse primary outcome occurred in 22.7% of the patients who received hyperimmune globulin and 19.4% of those who received placebo (relative risk, 1.17; 95% confidence interval [CI], 0.80–1.72; P=.42).
Continue to: Jacquemard and colleagues...
Jacquemard and colleagues then proposed a different approach.30 In a small pilot study of 20 patients, these authors used high doses of oral valacylovir (2 g 4 times daily) and documented therapeutic drug concentrations and a decline in CMV viral load in fetal serum. Patients were not stratified by severity of fetal injury at onset of treatment, so the authors were unable to define which fetuses were most likely to benefit from treatment.
In a follow-up investigation, Leruez-Ville and colleagues reported another small series in which high-dose oral valacyclovir (8 g daily) was used for treatment.31 They excluded fetuses with severe brain anomalies and fetuses with no sonographic evidence of injury. The median gestational age at diagnosis was 26 weeks. Thirty-four of 43 treated fetuses were free of injury at birth. In addition, the viral load in the neonate’s serum decreased significantly after treatment, and the platelet count increased. The authors then compared these outcomes to a historical cohort and confirmed that treatment increased the proportion of asymptomatic neonates from 43% without treatment to 82% with treatment (P<.05 with no overlapping confidence intervals).
We conclude from these investigations that hyperimmune globulin is unlikely to be of value in treating congenital CMV infection, especially if the fetus already has sonographic findings of severe injury. High-dose oral valacyclovir also is unlikely to be of value in severely affected fetuses, particularly those with evidence of CNS injury. However, antiviral therapy may be of modest value in situations when the fetus is less severely injured.
Preventive measures
Since no definitive treatment is available for congenital CMV infection, our efforts as clinicians should focus on measures that may prevent transmission of infection to the pregnant patient. These measures include:
- Encouraging patients to use careful handwashing techniques when handling infant diapers and toys.
- Encouraging patients to adopt safe sexual practices if not already engaged in a mutually faithful, monogamous relationship.
- Using CMV-negative blood when transfusing a pregnant woman or a fetus.
At the present time, unfortunately, a readily available and highly effective therapy for prevention of CMV infection is not available.
CASE Congenital infection diagnosed
The ultrasound findings are most consistent with congenital CMV infection, especially given the patient’s work as an elementary schoolteacher. The diagnosis of maternal infection is best established by conventional serology (positive IgM, negative IgM) and detection of viral DNA in maternal blood by PCR testing. The diagnosis of congenital infection is best confirmed by documentation of viral DNA in the amniotic fluid by PCR testing. Given that this fetus already has evidence of moderate to severe injury, no treatment is likely to be effective in reversing the abnormal ultrasound findings. Pregnancy termination may be an option, depending upon the patient’s desires and the legal restrictions prevalent in the patient’s geographic area. ●
- Cytomegalovirus infection is the most common of the perinatally transmitted infections.
- Maternal infection is often asymptomatic. When symptoms are present, they resemble those of an influenza-like illness. In immunocompromised persons, however, CMV may cause serious complications, including pneumonia, hepatitis, and chorioretinitis.
- The virus is transmitted by contact with contaminated body fluids, such as saliva, urine, blood, and genital secretions.
- The greatest risk of severe fetal injury results from primary maternal infection in the first trimester of pregnancy.
- Manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, hepatosplenomegaly, ascites, chorioretinitis, thrombocytopenia, purpura, and hydrops (“blueberry muffin baby”).
- Late manifestations of infection, which usually follow recurrent maternal infection, may appear as a child enters elementary school and include visual and auditory deficits, developmental delays, and learning disabilities.
- The diagnosis of maternal infection is confirmed by serology and detection of viral DNA in the serum by PCR testing.
- The diagnosis of fetal infection is best made by a combination of abnormal ultrasound findings and detection of CMV DNA in amniotic fluid. The characteristic ultrasound findings include placentomegaly, microcephaly, ventriculomegaly, growth restriction, echogenic bowel, and serous effusions/hydrops.
- Treatment of the mother with antiviral medications such as valacyclovir may be of modest value in reducing placental edema, decreasing viral load in the fetus, and hastening the resolution of some ultrasound findings, such as echogenic bowel.
- While initial studies seemed promising, the use of hyperimmune globulin has not proven to be consistently effective in treating congenital infection.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. 2019:888-890.
- Chatzakis C, Ville Y, Makrydimas G, et al. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am J Obstet Gynecol. 2020;223:870-883.e11. doi:10.1016/j.ajog.2020.05.038
- Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169:e153785. doi:10.1001 /jamapediatrics.2015.3785
- Messinger CJ, Lipsitch M, Bateman BT, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174:1159-1167. doi:10.1001/jamapediatrics.2020.3009
- De Cuyper E, Acke F, Keymeulen A, et al. Risk factors for hearing loss at birth in newborns with congenital cytomegalovirus infection. JAMA Otolaryngol Head Neck Surg. 2023;149:122-130. doi:10.1001/jamaoto.2022.4109
- Colugnati FA, Staras SA, Dollard SC, et al. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi:10.1186/1471-2334-7-71
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904-1908.
- Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603-1606. doi:10.1086/379711
- Fowler KB, Stagno S, Pass RF, et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663-667. doi:10.1056 /NEJM199203053261003
- Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526-1532. doi:10.1093/ cid/ciy1128
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253-276. doi:10.1002/ rmv.535
- Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93-99. doi:10.1097/00006454-199202000-00007
- Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332-336. doi:10.1016/j.jpeds.2005.09.003
- Zalel Y, Gilboa Y, Berkenshtat M, et al. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet Gynecol. 31:417-420. doi:10.1002/uog.5255
- Scaramuzzino F, Di Pastena M, Chiurchiu S, et al. Secondary cytomegalovirus infections: how much do we still not know? Comparison of children with symptomatic congenital cytomegalovirus born to mothers with primary and secondary infection. Front Pediatr. 2022;10:885926. doi:10.3389/fped.2022.885926
- Gindes L, Teperberg-Oikawa M, Sherman D, et al. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830-835. doi:10.1111/j.1471-0528.2007.01651.x
- Hadar E, Dorfman E, Bardin R, et al. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: a retrospective cohort study. BMC Infect Dis. 2017;17:31. doi:10.1186/s12879-016-2161-3
- Elkan Miller T, Weisz B, Yinon Y, et al. Congenital cytomegalovirus infection following second and third trimester maternal infection is associated with mild childhood adverse outcome not predicted by prenatal imaging. J Pediatric Infect Dis Soc. 2021;10:562-568. doi:10.1093/jpids/ piaa154
- Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirusassociated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol. 2013;41:508-514. doi:10.1002/uog.12377
- Lipitz S, Elkan Miller T, Yinon Y, et al. Revisiting short- and long-term outcome after fetal first-trimester primary cytomegalovirus infection in relation to prenatal imaging findings. Ultrasound Obstet Gynecol. 2020;56:572-578. doi:10.1002/uog.21946
- Buca D, Di Mascio D, Rizzo G, et al. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:551-559. doi:10.1002/uog.23143
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57 (suppl 4):S178-S181. doi:10.1093/cid/cit629
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355-363. doi:10.1002/rmv.544
- Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. 2016;214:B5-11. doi:10.1016 /j.ajog.2016.02.042
- Rouse DJ, Fette LM, Hughes BL, et al. Noninvasive prediction of congenital cytomegalovirus infection after maternal primary infection. Obstet Gynecol. 2022;139:400-406. doi:10.1097/AOG.0000000000004691
- Nigro G, Adler SP, La Torre R, et al; Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362. doi:10.1056/NEJMoa043337
- Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;355:1402-1404. doi:10.1056 /NEJMe058172
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316-1326. doi:10.1056/NEJMoa1310214
- Hughes BL, Clifton RG, Rouse DJ, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med. 2021;385:436-444. doi:10.1056/NEJMoa1913569
- Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113-1121. doi:10.1111/j.1471-0528.2007.01308.x
- Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215:462.e1-462.e10. doi:10.1016/j.ajog.2016.04.003
CASE Anomalous findings on fetal anatomic survey
A 27-year-old previously healthy primigravid woman is at 18 weeks’ gestation. She is a first-grade schoolteacher. On her fetal anatomic survey, the estimated fetal weight was in the eighth percentile. Echogenic bowel and a small amount of ascitic fluid were noted in the fetal abdomen. The lateral and third ventricles were mildly dilated, the head circumference was 2 standard deviations below normal, and the placenta was slightly thickened and edematous.
What is the most likely diagnosis?
What diagnostic tests are indicated?
What management options are available for this patient?
Cytomegalovirus (CMV) is the most common of the perinatally transmitted infections, affecting 1% to 4% of all pregnancies. Although the virus typically causes either asymptomatic infection or only mild illness in immunocompetent individuals, it can cause life-threatening disease in immunocompromised persons and in the developing fetus. In this article, we review the virology and epidemiology of CMV infection and then focus on the key methods to diagnose infection in the mother and fetus. We conclude by considering measures that may be of at least modest value in treating CMV in pregnancy.
Virology of CMV infection
Cytomegalovirus is a double-stranded DNA virus in the Herpesviridae family. This ubiquitous virus is present in virtually all secretions and excretions of an infected host, including blood, urine, saliva, breast milk, genital secretions, and tissues and organs used for donation. Infection is transmitted through direct contact with any of the substances listed; contact with infected urine or saliva is the most common mode of transmission. Disease occurrence does not show seasonal variation.
After exposure, an incubation period of 28 to 60 days ensues, followed by development of viremia and clinical symptoms. In the majority of exposed individuals, CMV establishes a lifelong latent infection, and recurrent episodes of illness can occur as a result of reactivation of latent virus (also known as secondary infection) or, more rarely, infection with a new viral strain. In fact, most CMV illness episodes in pregnancy represent a reactivation of a previous infection rather than a new infection.
Following initial infection, both IgM (immunoglobulin M) and IgG (immunoglobulin G) antibodies develop rapidly and can be detected in blood within 1 to 2 weeks. IgM levels typically wane within 30 to 60 days, although persistence for several months is not unusual, and levels also can increase with viral reactivation (secondary infection). IgG antibodies typically persist for many years after a primary infection.
Intrauterine CMV infection occurs through hematogenous transplacental passage during maternal viremia. The risk of transmission and severity of fetal effects depend on whether or not the infection is primary or secondary in nature as well as the gestational age at fetal exposure.1,2
Additionally, postnatal vertical transmission can occur through exposure to viral particles in genital secretions as well as breast milk. CMV acquired in the postnatal period rarely produces severe sequelae in a healthy term neonate, but it has been associated with an increased rate of complications in very low birth weight and premature newborns.3
Continue to: Who is at risk...
Who is at risk
Congenital CMV, which occurs in 2.1 to 7.7 per 10,000 live births in the United States, is both the most common congenital infection and the leading cause of nonhereditary congenital hearing loss in children.4,5 The main reservoir of CMV in the United States is young children in day care settings, with approximately 50% of this population showing evidence of viral shedding in saliva.1 Adult populations in North America have a high prevalence of CMV IgG antibodies indicative of prior infection, with rates reaching 50% to 80%. Among seronegative individuals aged 12 to 49, the rate of seroconversion is approximately 1 in 60 annually.6 Significant racial disparities have been noted in rates of seroprevalence and seroconversion, with higher rates of infection in non-Hispanic Black and Mexican American individuals.6 Overall, the rate of new CMV infection among pregnant women in the United States is 0.7% to 4%.7
Clinical manifestations
Manifestations of infection differ depending on whether or not infection is primary or recurrent (secondary) and whether or not the host is immunocompetent or has a compromised immune system. Unique manifestations develop in the fetus.
CMV infection in children and adults. Among individuals with a normal immune response, the typical course of CMV is either no symptoms or a mononucleosis-like illness. In symptomatic patients, the most common symptoms include malaise, fever, and night sweats, and the most common associated laboratory abnormalities are elevation in liver function tests and a decreased white blood cell count, with a predominance of lymphocytes.8
Immunocompromised individuals are at risk for significant morbidity and mortality resulting from CMV. Illness may be the result of reactivation of latent infection due to decreased immune function or may be acquired as a result of treatment such as transplantation of CMV-positive organs or tissues, including bone marrow. Virtually any organ system can be affected, with potential for permanent organ damage and death. Severe systemic infection also can occur.
CMV infection in the fetus and neonate. As noted previously, fetal infection develops as a result of transplacental passage coincident with maternal infection. The risk of CMV transmission to the fetus and the severity of fetal injury vary based on gestational age at fetal infection and whether or not maternal infection is primary or secondary.
In most studies, primary maternal infections are associated with higher rates of fetal infection and more severe fetal and neonatal disease manifestations.2,7,9,10 Primary infections carry an overall 30% to 40% risk of transmission to the fetus.7,11 The risk of fetal transmission is much lower with a recurrent infection and is usually less than 2%.11 Due to their greater overall incidence, secondary infections account for the majority of cases of fetal and neonatal CMV disease.7 Importantly, although secondary infections generally have been regarded as having a lower risk and lower severity of fetal and neonatal disease, several recent studies have demonstrated rates of complications similar to, and even exceeding, those of primary infections.12-15 The TABLE provides a summary of the risks of fetal transmission and symptomatic fetal infection based on trimester of pregnancy.2,11,16-18
In the fetus, CMV may affect multiple organ systems. Among sonographic and magnetic resonance imaging (MRI) findings, central nervous system (CNS) anomalies are the most common.19,20 These can include microcephaly, ventriculomegaly, and periventricular calcifications. The gastrointestinal system also is frequently affected, and findings include echogenic bowel, hepatosplenomegaly, and liver calcifications. Lastly, isolated effusions, placentomegaly, fetal growth restriction, and even frank hydrops can develop. More favorable neurologic outcomes have been demonstrated in infants with no prenatal brain imaging abnormalities.20,21 However, the role of MRI in prenatal prognosis currently is not well defined.
FIGURE 1 illustrates selected sonographic findings associated with fetal CMV infection.

About 85% to 90% of infants with congenital CMV that results from primary maternal infection have no symptoms at birth. Among the 10% to 15% of infants that do have symptoms, petechial rash, jaundice, and hepatosplenomegaly are the most common manifestations (“blueberry muffin baby”). Approximately 10% to 20% of infants in this group have evidence of chorioretinitis on ophthalmologic examination, and 50% show either microcephaly or low birth weight.22Among survivors of symptomatic congenital CMV, more than 50% have long-term neurologic morbidities that may include sensorineural hearing loss, seizures, vision impairment, and developmental disabilities. Note that even when neonates appear asymptomatic at birth (regardless of whether infection is primary or secondary), 5% may develop microcephaly and motor deficits, 10% go on to develop sensorineural hearing loss, and the overall rate of neurologic morbidity reaches 13% to 15%.12,23 Some of the observed deficits manifest at several years of age, and, currently, no models exist for prediction of outcome.
Continue to: Diagnosing CMV infection...
Diagnosing CMV infection
Maternal infection
If maternal CMV infection is suspected based on a symptomatic illness or an abnormal fetal ultrasound exam, the first diagnostic test should be an assessment of IgM and IgG serology. If the former test results are positive and the latter negative, the diagnosis of acute CMV infection is confirmed. A positive serum CMV DNA polymerase chain reaction (PCR) test adds additional assurance that the diagnosis is correct. Primary infection, as noted above, poses the greatest risk of serious injury to the fetus.1
A frequent diagnostic dilemma arises when both the IgM and IgG antibody are positive. Remember that CMV IgM antibody can remain positive for 9 to 12 months after a primary infection and can reappear in the maternal serum in the face of a recurrent or reactivated infection. When confronted by both a positive IgM and positive IgG result, the clinician should then order IgG avidity testing. If the avidity is low to moderate, which reflects poor binding of antibody to the virus, the patient likely has an acute infection. If the avidity is high, which reflects enhanced binding of antibody to virus, the patient probably has a recurrent or reactivated infection; this scenario poses less danger to the developing fetus. The presence of CMV DNA in serum is also more consistent with acute infection, although viremia still can occur with recurrent infection. FIGURE 2 presents a suggested algorithm for the diagnosis of CMV in the pregnant patient.1
If a diagnosis of maternal CMV infection is confirmed, liver function tests should be obtained to determine if CMV hepatitis is present. If the liver function tests are abnormal, a coagulation profile also should be performed to identify the mother who might be at risk for peripartum hemorrhage.
Fetal infection
The single best test for confirmation of congenital CMV infection is detection of viral DNA and quantitation of viral load in the amniotic fluid by PCR. If the amniocentesis is performed prior to 20 weeks’ gestation and is negative, the test should be repeated in approximately 4 weeks.1,19,24
Detection of viral DNA indicates congenital infection. The ultimate task, however, is to determine if the infection has injured the fetus. Detailed ultrasound examination is the key to identifying fetal injury. As noted previously, the principal ultrasonographic findings that suggest congenital CMV infection include2,19,20,21,25:
- hydropic placenta
- fetal growth restriction
- microcephaly (head circumference more than 3 standard deviations below the mean)
- periventricular calcifications
- enlarged liver
- echogenic bowel
- ascites
- fetal hydrops.
Management: Evidence on CMV hyperimmune globulin, valacyclovir
If the immunocompetent mother has clinical manifestations of infection, she should receive symptomatic treatment. She should be encouraged to rest as much as possible, stay well hydrated, and use acetaminophen (1,000 mg every 6 to 8 hours) as needed for malaise and fever.
However, if the mother is immunocompromised and has signs of serious complications, such as chorioretinitis, hepatitis, or pneumonia, more aggressive therapy is indicated. Drugs used in this setting include foscarnet and ganciclovir and are best prescribed in consultation with a medical infectious disease specialist.
At this time, no consistently effective therapy for congenital infection is available. Therefore, if a patient has primary CMV infection in the first half of pregnancy, particularly in the first trimester, she should be counseled that the risk of fetal infection is approximately 40% and that approximately 5% to 15% of infants will be severely affected at birth. Given this information, some patients may opt for pregnancy termination.
In 2005, a report from Nigro and colleagues stimulated great hope that CMV-specific hyperimmune globulin (CytoGam) might be of value for both treatment and prophylaxis for congenital infection.26 These authors studied 157 women with confirmed primary CMV infection. One-hundred forty-eight women were asymptomatic and were identified by routine serologic screening, 8 had symptomatic infection, and 1 was identified because of abnormal fetal ultrasound findings. Forty-five women had CMV detected in amniotic fluid by PCR or culture more than 6 weeks before study enrollment. Thirty-one of these women were treated with intravenous hyperimmune globulin (200 U or 200 mg/kg maternal body weight); 14 declined treatment. Seven of the latter women had infants who were acutely symptomatic at the time of delivery; only 1 of the 31 treated women had an affected neonate (adjusted odds ratio [OR], 0.02; P<.001). In this same study, 84 women did not have a diagnostic amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Thirty-seven of these women received hyperimmune globulin (100 U or 100 mg/kg) every month until delivery, and 47 declined treatment. Six of the treated women delivered infected infants compared with 19 of the untreated women (adjusted OR, 0.32; P<.04).
Although these results were quite encouraging, several problems existed with the study’s design, as noted in an editorial that accompanied the study’s publication.27 First, the study was not randomized or placebo controlled. Second, patients were not stratified based on the severity of fetal ultrasound abnormalities. Third, the dosing of hyperimmune globulin varied; 9 of the 31 patients in the treatment group received additional infusions of drug into either the amniotic fluid or fetal umbilical vein. Moreover, patients in the prophylaxis group actually received a higher cumulative dose of hyperimmune globulin than patients in the treatment group.
Two subsequent investigations that were better designed were unable to verify the effectiveness of hyperimmune globulin. In 2014, Revello and colleagues reported the results of a prospective, randomized, placebo-controlled, double-blinded study of 124 women at 5 to 26 weeks’ gestation with confirmed primary CMV infection.28 The rate of congenital infection was 30% in the group treated with hyperimmune globulin and 44% in the placebo group (P=.13). There also was no significant difference in the concentration of serum CMV DNA in treated versus untreated mothers. Moreover, the number of adverse obstetric events (preterm delivery, fetal growth restriction, intrahepatic cholestasis of pregnancy, and postpartum preeclampsia) in the treatment group was higher than in the placebo group, 13% versus 2%.
In 2021, Hughes and colleagues published the results of a multicenter, double-blind trial in 399 women who had a diagnosis of primary CMV infection before 23 weeks’ gestation.29 The primary outcome was defined as a composite of congenital CMV infection or fetal/neonatal death. An adverse primary outcome occurred in 22.7% of the patients who received hyperimmune globulin and 19.4% of those who received placebo (relative risk, 1.17; 95% confidence interval [CI], 0.80–1.72; P=.42).
Continue to: Jacquemard and colleagues...
Jacquemard and colleagues then proposed a different approach.30 In a small pilot study of 20 patients, these authors used high doses of oral valacylovir (2 g 4 times daily) and documented therapeutic drug concentrations and a decline in CMV viral load in fetal serum. Patients were not stratified by severity of fetal injury at onset of treatment, so the authors were unable to define which fetuses were most likely to benefit from treatment.
In a follow-up investigation, Leruez-Ville and colleagues reported another small series in which high-dose oral valacyclovir (8 g daily) was used for treatment.31 They excluded fetuses with severe brain anomalies and fetuses with no sonographic evidence of injury. The median gestational age at diagnosis was 26 weeks. Thirty-four of 43 treated fetuses were free of injury at birth. In addition, the viral load in the neonate’s serum decreased significantly after treatment, and the platelet count increased. The authors then compared these outcomes to a historical cohort and confirmed that treatment increased the proportion of asymptomatic neonates from 43% without treatment to 82% with treatment (P<.05 with no overlapping confidence intervals).
We conclude from these investigations that hyperimmune globulin is unlikely to be of value in treating congenital CMV infection, especially if the fetus already has sonographic findings of severe injury. High-dose oral valacyclovir also is unlikely to be of value in severely affected fetuses, particularly those with evidence of CNS injury. However, antiviral therapy may be of modest value in situations when the fetus is less severely injured.
Preventive measures
Since no definitive treatment is available for congenital CMV infection, our efforts as clinicians should focus on measures that may prevent transmission of infection to the pregnant patient. These measures include:
- Encouraging patients to use careful handwashing techniques when handling infant diapers and toys.
- Encouraging patients to adopt safe sexual practices if not already engaged in a mutually faithful, monogamous relationship.
- Using CMV-negative blood when transfusing a pregnant woman or a fetus.
At the present time, unfortunately, a readily available and highly effective therapy for prevention of CMV infection is not available.
CASE Congenital infection diagnosed
The ultrasound findings are most consistent with congenital CMV infection, especially given the patient’s work as an elementary schoolteacher. The diagnosis of maternal infection is best established by conventional serology (positive IgM, negative IgM) and detection of viral DNA in maternal blood by PCR testing. The diagnosis of congenital infection is best confirmed by documentation of viral DNA in the amniotic fluid by PCR testing. Given that this fetus already has evidence of moderate to severe injury, no treatment is likely to be effective in reversing the abnormal ultrasound findings. Pregnancy termination may be an option, depending upon the patient’s desires and the legal restrictions prevalent in the patient’s geographic area. ●
- Cytomegalovirus infection is the most common of the perinatally transmitted infections.
- Maternal infection is often asymptomatic. When symptoms are present, they resemble those of an influenza-like illness. In immunocompromised persons, however, CMV may cause serious complications, including pneumonia, hepatitis, and chorioretinitis.
- The virus is transmitted by contact with contaminated body fluids, such as saliva, urine, blood, and genital secretions.
- The greatest risk of severe fetal injury results from primary maternal infection in the first trimester of pregnancy.
- Manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, hepatosplenomegaly, ascites, chorioretinitis, thrombocytopenia, purpura, and hydrops (“blueberry muffin baby”).
- Late manifestations of infection, which usually follow recurrent maternal infection, may appear as a child enters elementary school and include visual and auditory deficits, developmental delays, and learning disabilities.
- The diagnosis of maternal infection is confirmed by serology and detection of viral DNA in the serum by PCR testing.
- The diagnosis of fetal infection is best made by a combination of abnormal ultrasound findings and detection of CMV DNA in amniotic fluid. The characteristic ultrasound findings include placentomegaly, microcephaly, ventriculomegaly, growth restriction, echogenic bowel, and serous effusions/hydrops.
- Treatment of the mother with antiviral medications such as valacyclovir may be of modest value in reducing placental edema, decreasing viral load in the fetus, and hastening the resolution of some ultrasound findings, such as echogenic bowel.
- While initial studies seemed promising, the use of hyperimmune globulin has not proven to be consistently effective in treating congenital infection.
CASE Anomalous findings on fetal anatomic survey
A 27-year-old previously healthy primigravid woman is at 18 weeks’ gestation. She is a first-grade schoolteacher. On her fetal anatomic survey, the estimated fetal weight was in the eighth percentile. Echogenic bowel and a small amount of ascitic fluid were noted in the fetal abdomen. The lateral and third ventricles were mildly dilated, the head circumference was 2 standard deviations below normal, and the placenta was slightly thickened and edematous.
What is the most likely diagnosis?
What diagnostic tests are indicated?
What management options are available for this patient?
Cytomegalovirus (CMV) is the most common of the perinatally transmitted infections, affecting 1% to 4% of all pregnancies. Although the virus typically causes either asymptomatic infection or only mild illness in immunocompetent individuals, it can cause life-threatening disease in immunocompromised persons and in the developing fetus. In this article, we review the virology and epidemiology of CMV infection and then focus on the key methods to diagnose infection in the mother and fetus. We conclude by considering measures that may be of at least modest value in treating CMV in pregnancy.
Virology of CMV infection
Cytomegalovirus is a double-stranded DNA virus in the Herpesviridae family. This ubiquitous virus is present in virtually all secretions and excretions of an infected host, including blood, urine, saliva, breast milk, genital secretions, and tissues and organs used for donation. Infection is transmitted through direct contact with any of the substances listed; contact with infected urine or saliva is the most common mode of transmission. Disease occurrence does not show seasonal variation.
After exposure, an incubation period of 28 to 60 days ensues, followed by development of viremia and clinical symptoms. In the majority of exposed individuals, CMV establishes a lifelong latent infection, and recurrent episodes of illness can occur as a result of reactivation of latent virus (also known as secondary infection) or, more rarely, infection with a new viral strain. In fact, most CMV illness episodes in pregnancy represent a reactivation of a previous infection rather than a new infection.
Following initial infection, both IgM (immunoglobulin M) and IgG (immunoglobulin G) antibodies develop rapidly and can be detected in blood within 1 to 2 weeks. IgM levels typically wane within 30 to 60 days, although persistence for several months is not unusual, and levels also can increase with viral reactivation (secondary infection). IgG antibodies typically persist for many years after a primary infection.
Intrauterine CMV infection occurs through hematogenous transplacental passage during maternal viremia. The risk of transmission and severity of fetal effects depend on whether or not the infection is primary or secondary in nature as well as the gestational age at fetal exposure.1,2
Additionally, postnatal vertical transmission can occur through exposure to viral particles in genital secretions as well as breast milk. CMV acquired in the postnatal period rarely produces severe sequelae in a healthy term neonate, but it has been associated with an increased rate of complications in very low birth weight and premature newborns.3
Continue to: Who is at risk...
Who is at risk
Congenital CMV, which occurs in 2.1 to 7.7 per 10,000 live births in the United States, is both the most common congenital infection and the leading cause of nonhereditary congenital hearing loss in children.4,5 The main reservoir of CMV in the United States is young children in day care settings, with approximately 50% of this population showing evidence of viral shedding in saliva.1 Adult populations in North America have a high prevalence of CMV IgG antibodies indicative of prior infection, with rates reaching 50% to 80%. Among seronegative individuals aged 12 to 49, the rate of seroconversion is approximately 1 in 60 annually.6 Significant racial disparities have been noted in rates of seroprevalence and seroconversion, with higher rates of infection in non-Hispanic Black and Mexican American individuals.6 Overall, the rate of new CMV infection among pregnant women in the United States is 0.7% to 4%.7
Clinical manifestations
Manifestations of infection differ depending on whether or not infection is primary or recurrent (secondary) and whether or not the host is immunocompetent or has a compromised immune system. Unique manifestations develop in the fetus.
CMV infection in children and adults. Among individuals with a normal immune response, the typical course of CMV is either no symptoms or a mononucleosis-like illness. In symptomatic patients, the most common symptoms include malaise, fever, and night sweats, and the most common associated laboratory abnormalities are elevation in liver function tests and a decreased white blood cell count, with a predominance of lymphocytes.8
Immunocompromised individuals are at risk for significant morbidity and mortality resulting from CMV. Illness may be the result of reactivation of latent infection due to decreased immune function or may be acquired as a result of treatment such as transplantation of CMV-positive organs or tissues, including bone marrow. Virtually any organ system can be affected, with potential for permanent organ damage and death. Severe systemic infection also can occur.
CMV infection in the fetus and neonate. As noted previously, fetal infection develops as a result of transplacental passage coincident with maternal infection. The risk of CMV transmission to the fetus and the severity of fetal injury vary based on gestational age at fetal infection and whether or not maternal infection is primary or secondary.
In most studies, primary maternal infections are associated with higher rates of fetal infection and more severe fetal and neonatal disease manifestations.2,7,9,10 Primary infections carry an overall 30% to 40% risk of transmission to the fetus.7,11 The risk of fetal transmission is much lower with a recurrent infection and is usually less than 2%.11 Due to their greater overall incidence, secondary infections account for the majority of cases of fetal and neonatal CMV disease.7 Importantly, although secondary infections generally have been regarded as having a lower risk and lower severity of fetal and neonatal disease, several recent studies have demonstrated rates of complications similar to, and even exceeding, those of primary infections.12-15 The TABLE provides a summary of the risks of fetal transmission and symptomatic fetal infection based on trimester of pregnancy.2,11,16-18
In the fetus, CMV may affect multiple organ systems. Among sonographic and magnetic resonance imaging (MRI) findings, central nervous system (CNS) anomalies are the most common.19,20 These can include microcephaly, ventriculomegaly, and periventricular calcifications. The gastrointestinal system also is frequently affected, and findings include echogenic bowel, hepatosplenomegaly, and liver calcifications. Lastly, isolated effusions, placentomegaly, fetal growth restriction, and even frank hydrops can develop. More favorable neurologic outcomes have been demonstrated in infants with no prenatal brain imaging abnormalities.20,21 However, the role of MRI in prenatal prognosis currently is not well defined.
FIGURE 1 illustrates selected sonographic findings associated with fetal CMV infection.

About 85% to 90% of infants with congenital CMV that results from primary maternal infection have no symptoms at birth. Among the 10% to 15% of infants that do have symptoms, petechial rash, jaundice, and hepatosplenomegaly are the most common manifestations (“blueberry muffin baby”). Approximately 10% to 20% of infants in this group have evidence of chorioretinitis on ophthalmologic examination, and 50% show either microcephaly or low birth weight.22Among survivors of symptomatic congenital CMV, more than 50% have long-term neurologic morbidities that may include sensorineural hearing loss, seizures, vision impairment, and developmental disabilities. Note that even when neonates appear asymptomatic at birth (regardless of whether infection is primary or secondary), 5% may develop microcephaly and motor deficits, 10% go on to develop sensorineural hearing loss, and the overall rate of neurologic morbidity reaches 13% to 15%.12,23 Some of the observed deficits manifest at several years of age, and, currently, no models exist for prediction of outcome.
Continue to: Diagnosing CMV infection...
Diagnosing CMV infection
Maternal infection
If maternal CMV infection is suspected based on a symptomatic illness or an abnormal fetal ultrasound exam, the first diagnostic test should be an assessment of IgM and IgG serology. If the former test results are positive and the latter negative, the diagnosis of acute CMV infection is confirmed. A positive serum CMV DNA polymerase chain reaction (PCR) test adds additional assurance that the diagnosis is correct. Primary infection, as noted above, poses the greatest risk of serious injury to the fetus.1
A frequent diagnostic dilemma arises when both the IgM and IgG antibody are positive. Remember that CMV IgM antibody can remain positive for 9 to 12 months after a primary infection and can reappear in the maternal serum in the face of a recurrent or reactivated infection. When confronted by both a positive IgM and positive IgG result, the clinician should then order IgG avidity testing. If the avidity is low to moderate, which reflects poor binding of antibody to the virus, the patient likely has an acute infection. If the avidity is high, which reflects enhanced binding of antibody to virus, the patient probably has a recurrent or reactivated infection; this scenario poses less danger to the developing fetus. The presence of CMV DNA in serum is also more consistent with acute infection, although viremia still can occur with recurrent infection. FIGURE 2 presents a suggested algorithm for the diagnosis of CMV in the pregnant patient.1
If a diagnosis of maternal CMV infection is confirmed, liver function tests should be obtained to determine if CMV hepatitis is present. If the liver function tests are abnormal, a coagulation profile also should be performed to identify the mother who might be at risk for peripartum hemorrhage.
Fetal infection
The single best test for confirmation of congenital CMV infection is detection of viral DNA and quantitation of viral load in the amniotic fluid by PCR. If the amniocentesis is performed prior to 20 weeks’ gestation and is negative, the test should be repeated in approximately 4 weeks.1,19,24
Detection of viral DNA indicates congenital infection. The ultimate task, however, is to determine if the infection has injured the fetus. Detailed ultrasound examination is the key to identifying fetal injury. As noted previously, the principal ultrasonographic findings that suggest congenital CMV infection include2,19,20,21,25:
- hydropic placenta
- fetal growth restriction
- microcephaly (head circumference more than 3 standard deviations below the mean)
- periventricular calcifications
- enlarged liver
- echogenic bowel
- ascites
- fetal hydrops.
Management: Evidence on CMV hyperimmune globulin, valacyclovir
If the immunocompetent mother has clinical manifestations of infection, she should receive symptomatic treatment. She should be encouraged to rest as much as possible, stay well hydrated, and use acetaminophen (1,000 mg every 6 to 8 hours) as needed for malaise and fever.
However, if the mother is immunocompromised and has signs of serious complications, such as chorioretinitis, hepatitis, or pneumonia, more aggressive therapy is indicated. Drugs used in this setting include foscarnet and ganciclovir and are best prescribed in consultation with a medical infectious disease specialist.
At this time, no consistently effective therapy for congenital infection is available. Therefore, if a patient has primary CMV infection in the first half of pregnancy, particularly in the first trimester, she should be counseled that the risk of fetal infection is approximately 40% and that approximately 5% to 15% of infants will be severely affected at birth. Given this information, some patients may opt for pregnancy termination.
In 2005, a report from Nigro and colleagues stimulated great hope that CMV-specific hyperimmune globulin (CytoGam) might be of value for both treatment and prophylaxis for congenital infection.26 These authors studied 157 women with confirmed primary CMV infection. One-hundred forty-eight women were asymptomatic and were identified by routine serologic screening, 8 had symptomatic infection, and 1 was identified because of abnormal fetal ultrasound findings. Forty-five women had CMV detected in amniotic fluid by PCR or culture more than 6 weeks before study enrollment. Thirty-one of these women were treated with intravenous hyperimmune globulin (200 U or 200 mg/kg maternal body weight); 14 declined treatment. Seven of the latter women had infants who were acutely symptomatic at the time of delivery; only 1 of the 31 treated women had an affected neonate (adjusted odds ratio [OR], 0.02; P<.001). In this same study, 84 women did not have a diagnostic amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Thirty-seven of these women received hyperimmune globulin (100 U or 100 mg/kg) every month until delivery, and 47 declined treatment. Six of the treated women delivered infected infants compared with 19 of the untreated women (adjusted OR, 0.32; P<.04).
Although these results were quite encouraging, several problems existed with the study’s design, as noted in an editorial that accompanied the study’s publication.27 First, the study was not randomized or placebo controlled. Second, patients were not stratified based on the severity of fetal ultrasound abnormalities. Third, the dosing of hyperimmune globulin varied; 9 of the 31 patients in the treatment group received additional infusions of drug into either the amniotic fluid or fetal umbilical vein. Moreover, patients in the prophylaxis group actually received a higher cumulative dose of hyperimmune globulin than patients in the treatment group.
Two subsequent investigations that were better designed were unable to verify the effectiveness of hyperimmune globulin. In 2014, Revello and colleagues reported the results of a prospective, randomized, placebo-controlled, double-blinded study of 124 women at 5 to 26 weeks’ gestation with confirmed primary CMV infection.28 The rate of congenital infection was 30% in the group treated with hyperimmune globulin and 44% in the placebo group (P=.13). There also was no significant difference in the concentration of serum CMV DNA in treated versus untreated mothers. Moreover, the number of adverse obstetric events (preterm delivery, fetal growth restriction, intrahepatic cholestasis of pregnancy, and postpartum preeclampsia) in the treatment group was higher than in the placebo group, 13% versus 2%.
In 2021, Hughes and colleagues published the results of a multicenter, double-blind trial in 399 women who had a diagnosis of primary CMV infection before 23 weeks’ gestation.29 The primary outcome was defined as a composite of congenital CMV infection or fetal/neonatal death. An adverse primary outcome occurred in 22.7% of the patients who received hyperimmune globulin and 19.4% of those who received placebo (relative risk, 1.17; 95% confidence interval [CI], 0.80–1.72; P=.42).
Continue to: Jacquemard and colleagues...
Jacquemard and colleagues then proposed a different approach.30 In a small pilot study of 20 patients, these authors used high doses of oral valacylovir (2 g 4 times daily) and documented therapeutic drug concentrations and a decline in CMV viral load in fetal serum. Patients were not stratified by severity of fetal injury at onset of treatment, so the authors were unable to define which fetuses were most likely to benefit from treatment.
In a follow-up investigation, Leruez-Ville and colleagues reported another small series in which high-dose oral valacyclovir (8 g daily) was used for treatment.31 They excluded fetuses with severe brain anomalies and fetuses with no sonographic evidence of injury. The median gestational age at diagnosis was 26 weeks. Thirty-four of 43 treated fetuses were free of injury at birth. In addition, the viral load in the neonate’s serum decreased significantly after treatment, and the platelet count increased. The authors then compared these outcomes to a historical cohort and confirmed that treatment increased the proportion of asymptomatic neonates from 43% without treatment to 82% with treatment (P<.05 with no overlapping confidence intervals).
We conclude from these investigations that hyperimmune globulin is unlikely to be of value in treating congenital CMV infection, especially if the fetus already has sonographic findings of severe injury. High-dose oral valacyclovir also is unlikely to be of value in severely affected fetuses, particularly those with evidence of CNS injury. However, antiviral therapy may be of modest value in situations when the fetus is less severely injured.
Preventive measures
Since no definitive treatment is available for congenital CMV infection, our efforts as clinicians should focus on measures that may prevent transmission of infection to the pregnant patient. These measures include:
- Encouraging patients to use careful handwashing techniques when handling infant diapers and toys.
- Encouraging patients to adopt safe sexual practices if not already engaged in a mutually faithful, monogamous relationship.
- Using CMV-negative blood when transfusing a pregnant woman or a fetus.
At the present time, unfortunately, a readily available and highly effective therapy for prevention of CMV infection is not available.
CASE Congenital infection diagnosed
The ultrasound findings are most consistent with congenital CMV infection, especially given the patient’s work as an elementary schoolteacher. The diagnosis of maternal infection is best established by conventional serology (positive IgM, negative IgM) and detection of viral DNA in maternal blood by PCR testing. The diagnosis of congenital infection is best confirmed by documentation of viral DNA in the amniotic fluid by PCR testing. Given that this fetus already has evidence of moderate to severe injury, no treatment is likely to be effective in reversing the abnormal ultrasound findings. Pregnancy termination may be an option, depending upon the patient’s desires and the legal restrictions prevalent in the patient’s geographic area. ●
- Cytomegalovirus infection is the most common of the perinatally transmitted infections.
- Maternal infection is often asymptomatic. When symptoms are present, they resemble those of an influenza-like illness. In immunocompromised persons, however, CMV may cause serious complications, including pneumonia, hepatitis, and chorioretinitis.
- The virus is transmitted by contact with contaminated body fluids, such as saliva, urine, blood, and genital secretions.
- The greatest risk of severe fetal injury results from primary maternal infection in the first trimester of pregnancy.
- Manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, hepatosplenomegaly, ascites, chorioretinitis, thrombocytopenia, purpura, and hydrops (“blueberry muffin baby”).
- Late manifestations of infection, which usually follow recurrent maternal infection, may appear as a child enters elementary school and include visual and auditory deficits, developmental delays, and learning disabilities.
- The diagnosis of maternal infection is confirmed by serology and detection of viral DNA in the serum by PCR testing.
- The diagnosis of fetal infection is best made by a combination of abnormal ultrasound findings and detection of CMV DNA in amniotic fluid. The characteristic ultrasound findings include placentomegaly, microcephaly, ventriculomegaly, growth restriction, echogenic bowel, and serous effusions/hydrops.
- Treatment of the mother with antiviral medications such as valacyclovir may be of modest value in reducing placental edema, decreasing viral load in the fetus, and hastening the resolution of some ultrasound findings, such as echogenic bowel.
- While initial studies seemed promising, the use of hyperimmune globulin has not proven to be consistently effective in treating congenital infection.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. 2019:888-890.
- Chatzakis C, Ville Y, Makrydimas G, et al. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am J Obstet Gynecol. 2020;223:870-883.e11. doi:10.1016/j.ajog.2020.05.038
- Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169:e153785. doi:10.1001 /jamapediatrics.2015.3785
- Messinger CJ, Lipsitch M, Bateman BT, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174:1159-1167. doi:10.1001/jamapediatrics.2020.3009
- De Cuyper E, Acke F, Keymeulen A, et al. Risk factors for hearing loss at birth in newborns with congenital cytomegalovirus infection. JAMA Otolaryngol Head Neck Surg. 2023;149:122-130. doi:10.1001/jamaoto.2022.4109
- Colugnati FA, Staras SA, Dollard SC, et al. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi:10.1186/1471-2334-7-71
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904-1908.
- Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603-1606. doi:10.1086/379711
- Fowler KB, Stagno S, Pass RF, et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663-667. doi:10.1056 /NEJM199203053261003
- Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526-1532. doi:10.1093/ cid/ciy1128
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253-276. doi:10.1002/ rmv.535
- Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93-99. doi:10.1097/00006454-199202000-00007
- Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332-336. doi:10.1016/j.jpeds.2005.09.003
- Zalel Y, Gilboa Y, Berkenshtat M, et al. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet Gynecol. 31:417-420. doi:10.1002/uog.5255
- Scaramuzzino F, Di Pastena M, Chiurchiu S, et al. Secondary cytomegalovirus infections: how much do we still not know? Comparison of children with symptomatic congenital cytomegalovirus born to mothers with primary and secondary infection. Front Pediatr. 2022;10:885926. doi:10.3389/fped.2022.885926
- Gindes L, Teperberg-Oikawa M, Sherman D, et al. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830-835. doi:10.1111/j.1471-0528.2007.01651.x
- Hadar E, Dorfman E, Bardin R, et al. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: a retrospective cohort study. BMC Infect Dis. 2017;17:31. doi:10.1186/s12879-016-2161-3
- Elkan Miller T, Weisz B, Yinon Y, et al. Congenital cytomegalovirus infection following second and third trimester maternal infection is associated with mild childhood adverse outcome not predicted by prenatal imaging. J Pediatric Infect Dis Soc. 2021;10:562-568. doi:10.1093/jpids/ piaa154
- Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirusassociated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol. 2013;41:508-514. doi:10.1002/uog.12377
- Lipitz S, Elkan Miller T, Yinon Y, et al. Revisiting short- and long-term outcome after fetal first-trimester primary cytomegalovirus infection in relation to prenatal imaging findings. Ultrasound Obstet Gynecol. 2020;56:572-578. doi:10.1002/uog.21946
- Buca D, Di Mascio D, Rizzo G, et al. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:551-559. doi:10.1002/uog.23143
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57 (suppl 4):S178-S181. doi:10.1093/cid/cit629
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355-363. doi:10.1002/rmv.544
- Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. 2016;214:B5-11. doi:10.1016 /j.ajog.2016.02.042
- Rouse DJ, Fette LM, Hughes BL, et al. Noninvasive prediction of congenital cytomegalovirus infection after maternal primary infection. Obstet Gynecol. 2022;139:400-406. doi:10.1097/AOG.0000000000004691
- Nigro G, Adler SP, La Torre R, et al; Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362. doi:10.1056/NEJMoa043337
- Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;355:1402-1404. doi:10.1056 /NEJMe058172
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316-1326. doi:10.1056/NEJMoa1310214
- Hughes BL, Clifton RG, Rouse DJ, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med. 2021;385:436-444. doi:10.1056/NEJMoa1913569
- Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113-1121. doi:10.1111/j.1471-0528.2007.01308.x
- Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215:462.e1-462.e10. doi:10.1016/j.ajog.2016.04.003
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. 2019:888-890.
- Chatzakis C, Ville Y, Makrydimas G, et al. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am J Obstet Gynecol. 2020;223:870-883.e11. doi:10.1016/j.ajog.2020.05.038
- Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169:e153785. doi:10.1001 /jamapediatrics.2015.3785
- Messinger CJ, Lipsitch M, Bateman BT, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174:1159-1167. doi:10.1001/jamapediatrics.2020.3009
- De Cuyper E, Acke F, Keymeulen A, et al. Risk factors for hearing loss at birth in newborns with congenital cytomegalovirus infection. JAMA Otolaryngol Head Neck Surg. 2023;149:122-130. doi:10.1001/jamaoto.2022.4109
- Colugnati FA, Staras SA, Dollard SC, et al. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi:10.1186/1471-2334-7-71
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904-1908.
- Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603-1606. doi:10.1086/379711
- Fowler KB, Stagno S, Pass RF, et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663-667. doi:10.1056 /NEJM199203053261003
- Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526-1532. doi:10.1093/ cid/ciy1128
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253-276. doi:10.1002/ rmv.535
- Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93-99. doi:10.1097/00006454-199202000-00007
- Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332-336. doi:10.1016/j.jpeds.2005.09.003
- Zalel Y, Gilboa Y, Berkenshtat M, et al. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet Gynecol. 31:417-420. doi:10.1002/uog.5255
- Scaramuzzino F, Di Pastena M, Chiurchiu S, et al. Secondary cytomegalovirus infections: how much do we still not know? Comparison of children with symptomatic congenital cytomegalovirus born to mothers with primary and secondary infection. Front Pediatr. 2022;10:885926. doi:10.3389/fped.2022.885926
- Gindes L, Teperberg-Oikawa M, Sherman D, et al. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830-835. doi:10.1111/j.1471-0528.2007.01651.x
- Hadar E, Dorfman E, Bardin R, et al. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: a retrospective cohort study. BMC Infect Dis. 2017;17:31. doi:10.1186/s12879-016-2161-3
- Elkan Miller T, Weisz B, Yinon Y, et al. Congenital cytomegalovirus infection following second and third trimester maternal infection is associated with mild childhood adverse outcome not predicted by prenatal imaging. J Pediatric Infect Dis Soc. 2021;10:562-568. doi:10.1093/jpids/ piaa154
- Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirusassociated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol. 2013;41:508-514. doi:10.1002/uog.12377
- Lipitz S, Elkan Miller T, Yinon Y, et al. Revisiting short- and long-term outcome after fetal first-trimester primary cytomegalovirus infection in relation to prenatal imaging findings. Ultrasound Obstet Gynecol. 2020;56:572-578. doi:10.1002/uog.21946
- Buca D, Di Mascio D, Rizzo G, et al. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:551-559. doi:10.1002/uog.23143
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57 (suppl 4):S178-S181. doi:10.1093/cid/cit629
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355-363. doi:10.1002/rmv.544
- Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. 2016;214:B5-11. doi:10.1016 /j.ajog.2016.02.042
- Rouse DJ, Fette LM, Hughes BL, et al. Noninvasive prediction of congenital cytomegalovirus infection after maternal primary infection. Obstet Gynecol. 2022;139:400-406. doi:10.1097/AOG.0000000000004691
- Nigro G, Adler SP, La Torre R, et al; Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362. doi:10.1056/NEJMoa043337
- Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;355:1402-1404. doi:10.1056 /NEJMe058172
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316-1326. doi:10.1056/NEJMoa1310214
- Hughes BL, Clifton RG, Rouse DJ, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med. 2021;385:436-444. doi:10.1056/NEJMoa1913569
- Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113-1121. doi:10.1111/j.1471-0528.2007.01308.x
- Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215:462.e1-462.e10. doi:10.1016/j.ajog.2016.04.003
Discomfort in right breast
Breast cancer is the most common tumor type and second only to lung cancer as a cause of cancer death in women. Nearly 300,000 women (and 2800 men) will receive a new diagnosis of breast cancer in the United States in 2023. Despite improvements in treatment options, breast cancer still will lead to 43,700 deaths among women this year.
Breast tumors generally are either ductal or lobular in origin. Ductal carcinomas arise in the lining of the milk ducts and are the most commonly found tumor type in breast cancer. Almost 3 in 4 diagnosed breast cancers histologically are invasive ductal carcinomas, which have spread from the source into surrounding structures. It has no specific histologic indicators and is differentiated from ductal carcinoma in situ by its having spread outside the duct. The presence of lymph node involvement in this patient also confirms invasive ductal carcinoma without metastatic spread. Invasive lobular carcinomas occur much less frequently and have a different histologic appearance of smaller cells that appear to be arranged in linear groups.
Mammography involves low-dose radiation and is used in diagnosis and is the most widely used screening technique for breast cancer. As a screening tool, mammography may detect lesions 1-2 years before they become palpable on breast self-examination. While the incidence of breast cancer in women has slowly increased over the past 20 years, mortality has decreased largely as a result of improved awareness and uptake of screening mammography. Current American Cancer Society guidelines recommend continued mammography screenings at least every other year after age 55 and continued for as long as a woman has a life expectancy > 10 years. Screening or diagnosis using MRI is usually reserved for individuals at high risk for breast cancer or a history of breast cancer before age 50.
For all newly diagnosed primary invasive breast cancers, biomarker testing for estrogen and progesterone receptor expression and HER2 expression are part of the standard workup recommended by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) to help guide treatment decisions. Biomarker testing showed that her tumor was ER+ and HER2-negative, the most common finding in breast cancer. The patient's history did not suggest a risk for BRCA or other familial cancer mutations, but molecular testing was done and was negative for BRCA1 and BRCA2. In postmenopausal women, ASCO and NCCN also recommend use of risk assessment tools, such as Oncotype DX, to determine whether chemotherapy will add benefit to systemic endocrine therapy. Postmenopausal patients with ER+/HER2-negative breast cancer, one to three positive nodes, and a score < 26 on the 21-gene Oncotype DX can safely forego cytotoxic chemotherapy and derive maximum survival benefit from hormonal therapy alone.
This patient was diagnosed with a primary tumor of approximately 25 mm, metastasis to two ipsilateral nodes, and no distant metastasis, or stage IIIA. Her risk recurrence score was 20, indicating that she could safely forego the rigors of cytotoxic chemotherapy. She underwent localized breast-conserving surgery and began adjuvant tamoxifen therapy (x 2 years) to be followed with an aromatase inhibitor (x 3 years).
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Breast cancer is the most common tumor type and second only to lung cancer as a cause of cancer death in women. Nearly 300,000 women (and 2800 men) will receive a new diagnosis of breast cancer in the United States in 2023. Despite improvements in treatment options, breast cancer still will lead to 43,700 deaths among women this year.
Breast tumors generally are either ductal or lobular in origin. Ductal carcinomas arise in the lining of the milk ducts and are the most commonly found tumor type in breast cancer. Almost 3 in 4 diagnosed breast cancers histologically are invasive ductal carcinomas, which have spread from the source into surrounding structures. It has no specific histologic indicators and is differentiated from ductal carcinoma in situ by its having spread outside the duct. The presence of lymph node involvement in this patient also confirms invasive ductal carcinoma without metastatic spread. Invasive lobular carcinomas occur much less frequently and have a different histologic appearance of smaller cells that appear to be arranged in linear groups.
Mammography involves low-dose radiation and is used in diagnosis and is the most widely used screening technique for breast cancer. As a screening tool, mammography may detect lesions 1-2 years before they become palpable on breast self-examination. While the incidence of breast cancer in women has slowly increased over the past 20 years, mortality has decreased largely as a result of improved awareness and uptake of screening mammography. Current American Cancer Society guidelines recommend continued mammography screenings at least every other year after age 55 and continued for as long as a woman has a life expectancy > 10 years. Screening or diagnosis using MRI is usually reserved for individuals at high risk for breast cancer or a history of breast cancer before age 50.
For all newly diagnosed primary invasive breast cancers, biomarker testing for estrogen and progesterone receptor expression and HER2 expression are part of the standard workup recommended by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) to help guide treatment decisions. Biomarker testing showed that her tumor was ER+ and HER2-negative, the most common finding in breast cancer. The patient's history did not suggest a risk for BRCA or other familial cancer mutations, but molecular testing was done and was negative for BRCA1 and BRCA2. In postmenopausal women, ASCO and NCCN also recommend use of risk assessment tools, such as Oncotype DX, to determine whether chemotherapy will add benefit to systemic endocrine therapy. Postmenopausal patients with ER+/HER2-negative breast cancer, one to three positive nodes, and a score < 26 on the 21-gene Oncotype DX can safely forego cytotoxic chemotherapy and derive maximum survival benefit from hormonal therapy alone.
This patient was diagnosed with a primary tumor of approximately 25 mm, metastasis to two ipsilateral nodes, and no distant metastasis, or stage IIIA. Her risk recurrence score was 20, indicating that she could safely forego the rigors of cytotoxic chemotherapy. She underwent localized breast-conserving surgery and began adjuvant tamoxifen therapy (x 2 years) to be followed with an aromatase inhibitor (x 3 years).
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Breast cancer is the most common tumor type and second only to lung cancer as a cause of cancer death in women. Nearly 300,000 women (and 2800 men) will receive a new diagnosis of breast cancer in the United States in 2023. Despite improvements in treatment options, breast cancer still will lead to 43,700 deaths among women this year.
Breast tumors generally are either ductal or lobular in origin. Ductal carcinomas arise in the lining of the milk ducts and are the most commonly found tumor type in breast cancer. Almost 3 in 4 diagnosed breast cancers histologically are invasive ductal carcinomas, which have spread from the source into surrounding structures. It has no specific histologic indicators and is differentiated from ductal carcinoma in situ by its having spread outside the duct. The presence of lymph node involvement in this patient also confirms invasive ductal carcinoma without metastatic spread. Invasive lobular carcinomas occur much less frequently and have a different histologic appearance of smaller cells that appear to be arranged in linear groups.
Mammography involves low-dose radiation and is used in diagnosis and is the most widely used screening technique for breast cancer. As a screening tool, mammography may detect lesions 1-2 years before they become palpable on breast self-examination. While the incidence of breast cancer in women has slowly increased over the past 20 years, mortality has decreased largely as a result of improved awareness and uptake of screening mammography. Current American Cancer Society guidelines recommend continued mammography screenings at least every other year after age 55 and continued for as long as a woman has a life expectancy > 10 years. Screening or diagnosis using MRI is usually reserved for individuals at high risk for breast cancer or a history of breast cancer before age 50.
For all newly diagnosed primary invasive breast cancers, biomarker testing for estrogen and progesterone receptor expression and HER2 expression are part of the standard workup recommended by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) to help guide treatment decisions. Biomarker testing showed that her tumor was ER+ and HER2-negative, the most common finding in breast cancer. The patient's history did not suggest a risk for BRCA or other familial cancer mutations, but molecular testing was done and was negative for BRCA1 and BRCA2. In postmenopausal women, ASCO and NCCN also recommend use of risk assessment tools, such as Oncotype DX, to determine whether chemotherapy will add benefit to systemic endocrine therapy. Postmenopausal patients with ER+/HER2-negative breast cancer, one to three positive nodes, and a score < 26 on the 21-gene Oncotype DX can safely forego cytotoxic chemotherapy and derive maximum survival benefit from hormonal therapy alone.
This patient was diagnosed with a primary tumor of approximately 25 mm, metastasis to two ipsilateral nodes, and no distant metastasis, or stage IIIA. Her risk recurrence score was 20, indicating that she could safely forego the rigors of cytotoxic chemotherapy. She underwent localized breast-conserving surgery and began adjuvant tamoxifen therapy (x 2 years) to be followed with an aromatase inhibitor (x 3 years).
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 70-year-old woman presents for an annual exam and reports development of a firm lump in her right breast. She remarks that she has had discomfort in the same area for "at least a year," but the lump has become noticeable with even a cursory self-exam over the past 2 months. She has no previous history of breast cancer, hypertension, type 2 diabetes, or other cardiometabolic disease. She had no previous abnormal findings on mammograms through age 65 but has not had one since. Her family history includes a grandmother who died of breast cancer at age 64 and her father who lived with prostate cancer for 20 years after diagnosis at age 60. The physical exam reveals a firm, palpable lump in the upper right quadrant of her right breast. The exam is otherwise normal for the patient's age, with minor osteoarthritis that she remarks has worsened over the past year. Mammography, image-guided biopsy, and biomarker and molecular testing are ordered. Lymph node testing reveals two involved nodes, and histology reveals the following (see image).
Keep COVID-19 vaccination on your patients’ radar
The Advisory Committee on Immunization Practices (ACIP) recently issued updated recommendations on the use of vaccines to protect against COVID-19.1 In addition, 3 new COVID-19 vaccine products have been approved for use in the United States since September. Before we discuss both of these items, it’s important to understand why we’re still talking about COVID-19 vaccines.
The impact of vaccination can’t be understated. Vaccines to protect against COVID-19 have been hugely successful in preventing mortality and morbidity from illness caused by SARS-CoV-2. It is estimated that in the first year alone, after vaccines became widely available, they saved more than 14 million lives globally.2 By the end of 2022, they had prevented 18.5 million hospitalizations and 3.2 million deaths in the United States.3 However, waning levels of vaccine-induced immunity and the continuous mutation of the virus have prompted the need for booster doses of vaccine and development of new vaccines.
Enter this year’s vaccines. The new products include updated (2023-2024 formula) COVID-19 mRNA vaccines from Moderna and Pfizer-BioNTech, for use in those ages 6 months and older, and Novavax COVID-19 vaccine for use in those ages 12 years and older. All 3 provide protection against the currently circulating XBB variants, which by September 2023 accounted for > 99% of circulating SARS-CoV-2 strains in the United States.1
Novavax is an option for those who are hesitant to use an mRNA-based vaccine, although the exact recommendations for its use are still pending. Of note, the previously approved bivalent vaccines and the previous Novavax monovalent vaccine are no longer approved for use in the United States.
Current recommendations. For those ages 5 years and older, the recommendation is for a single dose of the 2023-2024 COVID-19 vaccine regardless of previous vaccination history—except for those who were previously unvaccinated and choose Novavax. (Those individuals should receive 2 doses, 3 to 8 weeks apart.) For those ages 6 months through 4 years, the recommended number of doses varies by vaccine and previous vaccination history1; a table can be found at www.cdc.gov/mmwr/volumes/72/wr/mm7242e1.htm.
Those who are moderately to severely immunocompromised should receive a 3-dose series with one of the 2023-2024 COVID-19 vaccines and may receive 1 or more additional updated doses.1 These recommendations are more nuanced, and a full description of them can be found at www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html.
Major changes in this year’s recommendations,4 compared to those previously made on the use of the bivalent vaccines, include:
- Eliminating complex recommendations for 5-year-olds, who are now included in the standard recommendation
- Reducing the number of COVID-19 vaccine products in use by standardizing the dose (25 mcg) for those ages 6 months to 11 years
- Choosing to monitor epidemiology and vaccine effectiveness data to determine whether an additional dose of this year’s vaccine will be needed for those ages 65 years and older, rather than making a recommendation now.
Who’s paying? Another change is how COVID-19 vaccines are paid for. The United States is moving from a system of federal procurement and distribution to the commercial marketplace. This may lead to some disruption and confusion.
All commercial health plans, as well as Medicare and Medicaid, must cover vaccines recommend by the ACIP with no out-of-pocket cost. The Vaccines for Children program provides free vaccine for uninsured and underinsured children up to age 19 years.
However, that leaves no payer for uninsured adults. In response, the CDC has announced the establishment of the Bridge Access Program, which is a private/government partnership to provide the vaccine to this age group. Details about where an adult can obtain a free COVID-19 vaccine through this program can be found by visiting www.cdc.gov/vaccines/programs/bridge/index.html or by calling 800-CDC-INFO.
A dynamic situation. COVID-19 vaccines and associated recommendations are likely to change with time, as we learn how best to formulate them to adjust to virus mutations and determine the optimal intervals to adjust and administer these vaccines. The result may (or may not) eventually resemble the approach recommended for influenza vaccines, which is annual assessment and adjustment of the targeted antigens, when needed, and annual universal vaccination.
1. Regan JJ, Moulia DL, Link-Guelles R, et al. Use of updated COVID-19 vaccines 2023-2024 formula for persons aged > 6 months: recommendations of the Advisory Committee on Immunization Practices—United States, September 2023. MMWR Morb Mortal Wkly Rep. 2023;72:1140-1146. doi: 10.15585/mmwr.mm7242e1
2. Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293-302. doi: 10.1016/S1473-3099(22)00320-6
3. Fitzpatrick M, Moghadas S, Pandey A, et al. Two years of US COVID-19 vaccines have prevented millions of hospitalizations and deaths. The Commonwealth Fund; 2022. Published December 13, 2022. Accessed November 2, 2023. www.commonwealthfund.org/blog/2022/two-years-covid-vaccines-prevented-millions-deaths-hospitalizations https://doi.org/10.26099/whsf-fp90
4. Wallace M. Evidence to recommendations framework: 2023-2024 (monovalent, XBB containing) COVID-19 vaccine. Presented to the Advisory Committee on Immunization Practices, September 12, 2023. Accessed November 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/11-COVID-Wallace-508.pdf
The Advisory Committee on Immunization Practices (ACIP) recently issued updated recommendations on the use of vaccines to protect against COVID-19.1 In addition, 3 new COVID-19 vaccine products have been approved for use in the United States since September. Before we discuss both of these items, it’s important to understand why we’re still talking about COVID-19 vaccines.
The impact of vaccination can’t be understated. Vaccines to protect against COVID-19 have been hugely successful in preventing mortality and morbidity from illness caused by SARS-CoV-2. It is estimated that in the first year alone, after vaccines became widely available, they saved more than 14 million lives globally.2 By the end of 2022, they had prevented 18.5 million hospitalizations and 3.2 million deaths in the United States.3 However, waning levels of vaccine-induced immunity and the continuous mutation of the virus have prompted the need for booster doses of vaccine and development of new vaccines.
Enter this year’s vaccines. The new products include updated (2023-2024 formula) COVID-19 mRNA vaccines from Moderna and Pfizer-BioNTech, for use in those ages 6 months and older, and Novavax COVID-19 vaccine for use in those ages 12 years and older. All 3 provide protection against the currently circulating XBB variants, which by September 2023 accounted for > 99% of circulating SARS-CoV-2 strains in the United States.1
Novavax is an option for those who are hesitant to use an mRNA-based vaccine, although the exact recommendations for its use are still pending. Of note, the previously approved bivalent vaccines and the previous Novavax monovalent vaccine are no longer approved for use in the United States.
Current recommendations. For those ages 5 years and older, the recommendation is for a single dose of the 2023-2024 COVID-19 vaccine regardless of previous vaccination history—except for those who were previously unvaccinated and choose Novavax. (Those individuals should receive 2 doses, 3 to 8 weeks apart.) For those ages 6 months through 4 years, the recommended number of doses varies by vaccine and previous vaccination history1; a table can be found at www.cdc.gov/mmwr/volumes/72/wr/mm7242e1.htm.
Those who are moderately to severely immunocompromised should receive a 3-dose series with one of the 2023-2024 COVID-19 vaccines and may receive 1 or more additional updated doses.1 These recommendations are more nuanced, and a full description of them can be found at www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html.
Major changes in this year’s recommendations,4 compared to those previously made on the use of the bivalent vaccines, include:
- Eliminating complex recommendations for 5-year-olds, who are now included in the standard recommendation
- Reducing the number of COVID-19 vaccine products in use by standardizing the dose (25 mcg) for those ages 6 months to 11 years
- Choosing to monitor epidemiology and vaccine effectiveness data to determine whether an additional dose of this year’s vaccine will be needed for those ages 65 years and older, rather than making a recommendation now.
Who’s paying? Another change is how COVID-19 vaccines are paid for. The United States is moving from a system of federal procurement and distribution to the commercial marketplace. This may lead to some disruption and confusion.
All commercial health plans, as well as Medicare and Medicaid, must cover vaccines recommend by the ACIP with no out-of-pocket cost. The Vaccines for Children program provides free vaccine for uninsured and underinsured children up to age 19 years.
However, that leaves no payer for uninsured adults. In response, the CDC has announced the establishment of the Bridge Access Program, which is a private/government partnership to provide the vaccine to this age group. Details about where an adult can obtain a free COVID-19 vaccine through this program can be found by visiting www.cdc.gov/vaccines/programs/bridge/index.html or by calling 800-CDC-INFO.
A dynamic situation. COVID-19 vaccines and associated recommendations are likely to change with time, as we learn how best to formulate them to adjust to virus mutations and determine the optimal intervals to adjust and administer these vaccines. The result may (or may not) eventually resemble the approach recommended for influenza vaccines, which is annual assessment and adjustment of the targeted antigens, when needed, and annual universal vaccination.
The Advisory Committee on Immunization Practices (ACIP) recently issued updated recommendations on the use of vaccines to protect against COVID-19.1 In addition, 3 new COVID-19 vaccine products have been approved for use in the United States since September. Before we discuss both of these items, it’s important to understand why we’re still talking about COVID-19 vaccines.
The impact of vaccination can’t be understated. Vaccines to protect against COVID-19 have been hugely successful in preventing mortality and morbidity from illness caused by SARS-CoV-2. It is estimated that in the first year alone, after vaccines became widely available, they saved more than 14 million lives globally.2 By the end of 2022, they had prevented 18.5 million hospitalizations and 3.2 million deaths in the United States.3 However, waning levels of vaccine-induced immunity and the continuous mutation of the virus have prompted the need for booster doses of vaccine and development of new vaccines.
Enter this year’s vaccines. The new products include updated (2023-2024 formula) COVID-19 mRNA vaccines from Moderna and Pfizer-BioNTech, for use in those ages 6 months and older, and Novavax COVID-19 vaccine for use in those ages 12 years and older. All 3 provide protection against the currently circulating XBB variants, which by September 2023 accounted for > 99% of circulating SARS-CoV-2 strains in the United States.1
Novavax is an option for those who are hesitant to use an mRNA-based vaccine, although the exact recommendations for its use are still pending. Of note, the previously approved bivalent vaccines and the previous Novavax monovalent vaccine are no longer approved for use in the United States.
Current recommendations. For those ages 5 years and older, the recommendation is for a single dose of the 2023-2024 COVID-19 vaccine regardless of previous vaccination history—except for those who were previously unvaccinated and choose Novavax. (Those individuals should receive 2 doses, 3 to 8 weeks apart.) For those ages 6 months through 4 years, the recommended number of doses varies by vaccine and previous vaccination history1; a table can be found at www.cdc.gov/mmwr/volumes/72/wr/mm7242e1.htm.
Those who are moderately to severely immunocompromised should receive a 3-dose series with one of the 2023-2024 COVID-19 vaccines and may receive 1 or more additional updated doses.1 These recommendations are more nuanced, and a full description of them can be found at www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html.
Major changes in this year’s recommendations,4 compared to those previously made on the use of the bivalent vaccines, include:
- Eliminating complex recommendations for 5-year-olds, who are now included in the standard recommendation
- Reducing the number of COVID-19 vaccine products in use by standardizing the dose (25 mcg) for those ages 6 months to 11 years
- Choosing to monitor epidemiology and vaccine effectiveness data to determine whether an additional dose of this year’s vaccine will be needed for those ages 65 years and older, rather than making a recommendation now.
Who’s paying? Another change is how COVID-19 vaccines are paid for. The United States is moving from a system of federal procurement and distribution to the commercial marketplace. This may lead to some disruption and confusion.
All commercial health plans, as well as Medicare and Medicaid, must cover vaccines recommend by the ACIP with no out-of-pocket cost. The Vaccines for Children program provides free vaccine for uninsured and underinsured children up to age 19 years.
However, that leaves no payer for uninsured adults. In response, the CDC has announced the establishment of the Bridge Access Program, which is a private/government partnership to provide the vaccine to this age group. Details about where an adult can obtain a free COVID-19 vaccine through this program can be found by visiting www.cdc.gov/vaccines/programs/bridge/index.html or by calling 800-CDC-INFO.
A dynamic situation. COVID-19 vaccines and associated recommendations are likely to change with time, as we learn how best to formulate them to adjust to virus mutations and determine the optimal intervals to adjust and administer these vaccines. The result may (or may not) eventually resemble the approach recommended for influenza vaccines, which is annual assessment and adjustment of the targeted antigens, when needed, and annual universal vaccination.
1. Regan JJ, Moulia DL, Link-Guelles R, et al. Use of updated COVID-19 vaccines 2023-2024 formula for persons aged > 6 months: recommendations of the Advisory Committee on Immunization Practices—United States, September 2023. MMWR Morb Mortal Wkly Rep. 2023;72:1140-1146. doi: 10.15585/mmwr.mm7242e1
2. Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293-302. doi: 10.1016/S1473-3099(22)00320-6
3. Fitzpatrick M, Moghadas S, Pandey A, et al. Two years of US COVID-19 vaccines have prevented millions of hospitalizations and deaths. The Commonwealth Fund; 2022. Published December 13, 2022. Accessed November 2, 2023. www.commonwealthfund.org/blog/2022/two-years-covid-vaccines-prevented-millions-deaths-hospitalizations https://doi.org/10.26099/whsf-fp90
4. Wallace M. Evidence to recommendations framework: 2023-2024 (monovalent, XBB containing) COVID-19 vaccine. Presented to the Advisory Committee on Immunization Practices, September 12, 2023. Accessed November 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/11-COVID-Wallace-508.pdf
1. Regan JJ, Moulia DL, Link-Guelles R, et al. Use of updated COVID-19 vaccines 2023-2024 formula for persons aged > 6 months: recommendations of the Advisory Committee on Immunization Practices—United States, September 2023. MMWR Morb Mortal Wkly Rep. 2023;72:1140-1146. doi: 10.15585/mmwr.mm7242e1
2. Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293-302. doi: 10.1016/S1473-3099(22)00320-6
3. Fitzpatrick M, Moghadas S, Pandey A, et al. Two years of US COVID-19 vaccines have prevented millions of hospitalizations and deaths. The Commonwealth Fund; 2022. Published December 13, 2022. Accessed November 2, 2023. www.commonwealthfund.org/blog/2022/two-years-covid-vaccines-prevented-millions-deaths-hospitalizations https://doi.org/10.26099/whsf-fp90
4. Wallace M. Evidence to recommendations framework: 2023-2024 (monovalent, XBB containing) COVID-19 vaccine. Presented to the Advisory Committee on Immunization Practices, September 12, 2023. Accessed November 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/11-COVID-Wallace-508.pdf
Abdominal Pain and Fever 48 Hours After Hysterosalpingography
A 37-year-old woman presented to the emergency department (ED) with 12 hours of fever and lower abdominal cramp pain. She had a history significant for hypothyroidism, infertility, and dysmenorrhea and had a hysterosalpingography (HSG) 48 hours prior for a comprehensive infertility workup.
On examination, the patient’s vital signs were a 94 bpm heart rate; 109/64 mm Hg blood pressure; 14 breaths per minute respiratory rate; 99% oxygen saturation on room air; and 101.2 °F temperature. The patient reported pain in the bilateral lower abdominal quadrants and no history of sexually transmitted infection, pelvic inflammatory disease, vaginal discharge or bleeding, dysuria, hematuria, melena, or bright red blood per rectum. A human chorionic gonadotropin urine test was negative on intake. The HSG 48 hours prior showed no concerning findings with normal uterine cavity and normal caliber fallopian tubes bilaterally.
On physical examination, the patient’s abdomen was nondistended, nonperitonitic, and without evidence of acute trauma or surgical scars. On palpation, the patient was tender in her suprapubic region and lower abdominal quadrants without evidence of guarding or rebound
The patient’s initial laboratory tests were as follows: white blood cells, 20.1 × 103/μL (reference range, 4-11 × 103/μL); hemoglobin, 12.1 g/dL (reference range, 12.1-15.1 g/dL); hematocrit, 37.1%, (reference range, 36%-48%); alanine aminotransferase, 84 U/L (reference range, 7-56 U/L); aspartate aminotransferase, 66 U/L (reference range, 8-33 U/L); and lipase, 25 U/L (5-60 U/L). Urinalysis was notable for only 5 red blood cells and negative for white blood cells, leukocyte esterase, and nitrites. The patient’s pain and fever were controlled with 1 g IV acetaminophen.
The patient’s fever, leukocytosis, and physical examination were concerning for possible intra-abdominal processes, so a
Discussion
The patient was diagnosed with bilateral tubo-ovarian abscess (TOA) likely secondary to her HSG procedure 48 hours before. TOA is a severe infectious, inflammatory condition involving a mass of the ovaries, fallopian tubes, or adjacent tissues of the upper female genital tract.1 Traditionally, TOAs are sequelae of undiagnosed or subclinical acute or chronic pelvic inflammatory disease (PID). This is known to occur via pathogen ascension from the lower to the upper female genital tract resulting in cervicitis, endometritis, salpingitis, oophoritis, and if left untreated, peritonitis.1 About 70,000 women are diagnosed with TOAs in the US every year. These patients require hospitalization as well as IV antibiotics for gold-standard treatment; however, some cases may require percutaneous drainage based on size, severity, and location.2
Diagnostic Considerations
Clinically, patients with TOAs present with fever, chills, lower abdominal pain, vaginal discharge with cervical motion tenderness, and an adnexal mass on examination.3 When a TOA is suspected, a urine human chorionic gonadotropin test and testing for C trachomatis and N gonorrhoeae are warranted. An ED workup often reveals leukocytosis, elevated C-reactive protein, and elevated erythrocyte sedimentation rate. Imaging is recommended once a TOA is suspected. Ultrasound is the gold-standard imaging modality and boasts a sensitivity of 93% and specificity of 98% for the detection of TOAs; however, CT has also been shown to be an effective diagnostic modality.4
Despite being common, TOAs are difficult to predict, detect, and diagnose; thus the clinician must often rely on thorough history taking and physical examination to raise suspicion.5 Although most frequently associated with sexual transmission, TOAs occur in not sexually active women in adolescence and adulthood. Specifically, TOAs also can present secondary to other intra-abdominal pathologies, such as appendicitis, diverticulitis, and pyelonephritis, as well as a complication of intrauterine procedures, such as an HSG, or less commonly, following intrauterine device (IUD) insertion.5-7
Given that sexually transmitted infections are the most common etiology of TOAs, C trachomatis and N gonorrhoeae are the most likely microorganisms to be isolated (Table).8-12 In not sexually active populations, Escherichia coli and Gardnerella vaginalis should be considered instead. Although rare, women with IUDs have been shown to have an increased incidence of PID/TOA secondary to Actinomyces israleii relative to women without IUDs.12 In patients with TOAs secondary to intraabdominal surgery, anaerobic bacteria, such as Bacterioides and Peptostreptococcus species in addition to Escherichia coli, are likely culprits.11
In patients after HSG, infectious complications are uncommon enough that the American College of Obstetricians and Gynecologists recommends against antibiotic prophylaxis unless there are risk factors of dilated fallopian tubes or a history of PID.13 Identifying a precise percentage of TOA as a complication of HSG is rather elusive in the literature, though, it is frequently noted that infection in general is uncommon, and the risk of developing PID is about 1.4% to 3.4%.14 However, in the retrospective study most often cited, all women who developed PID following HSG had evidence of dilated fallopian tubes. Given our patient had no history of PID or dilated fallopian tubes, her risk of developing infection (PID or postprocedural abscess) would be considered very low; therefore, the index of suspicion also was low.15
Following diagnosis, the patient was promptly admitted and treated with a course of IV ceftriaxone 1 g every 24 hours, IV doxycycline 100 mg every 12 hours, and a single dose of IV metronidazole 500 mg. Her leukocytosis, fever, and pain improved within 48 hours without the need for percutaneous drainage and the patient made a complete recovery.
Complications
TOAs can carry significant morbidity and mortality, and there are both acute and chronic complications associated. Even if properly treated, TOAs can rupture leading to severe illness, such as peritonitis and septic shock. This often requires surgical intervention and hemodynamic pressure support in the intensive care unit setting.16 One of the most feared long-term complications of TOAs is infertility secondary to structural abnormalities of the female reproductive tract.10 Adhesions, strictures, and scarring are associated with TOAs irrespective of medical or surgical management, and thus any women with a history of PID or TOA require advanced fertility workup if they are having difficulties with conception or implantation.17
Minimizing infectious transmission also is essential in the treatment of TOA/PID. All women who receive a diagnosis of PID or TOA should be evaluated for gonorrhea, chlamydia, HIV, and syphilis. Women should be instructed to abstain from sexual intercourse until therapy is complete, symptoms have resolved, and sex partners have been treated for potential chlamydial or gonococcal infections. All contraceptive methods can be continued during treatment.
Conclusions
This case presented several challenges as the bilateral TOAs developed postprocedure in a patient without risk factors. Furthermore, this case did not follow the classic presentation of ascending bacterial translocation to the ovaries over days to weeks. The diagnosis was complicated by a largely benign physical examination and a pelvic examination without evidence of abnormal vaginal discharge or cervicitis. The only indicators were fever, leukocytosis, and abdominal pain in the setting of a recent, uncomplicated HSG procedure. Vigilance is required to obtain the necessary history, and the differential of TOA must be broadened to include women without a history or symptoms of a sexually transmitted infection (contrary to the classic association). We aim to encourage heightened clinical suspicion for TOAs in patients who present with fever, leukocytosis, and abdominal pain after recent HSG or other intrauterine instrumentation procedures and therefore improve patient outcomes.
1. Gkrozou F, Tsonis O, Daniilidis A, Navrozoglou I, Paschopoulos M. Tubo-ovarian abscess: exploring optimal treatment options based on current evidence. J Endometr Pelvic Pain Disord. 2020;13(1):10-19. doi:10.1177/2284026520960649
2. Taylor KJ, Wasson JF, De Graaff C, Rosenfield AT, Andriole VT. Accuracy of grey-scale ultrasound diagnosis of abdominal and pelvic abscesses in 220 patients. Lancet. 1978;1(8055):83-84. doi:10.1016/s0140-6736(78)90016-8
3. Bridwell RE, Koyfman A, Long B. High risk and low prevalence diseases: tubo-ovarian abscess. Am J Emerg Med. 2022;57:70-75. doi:10.1016/j.ajem.2022.04.026
4. Lambert MJ, Villa M. Gynecologic ultrasound in emergency medicine. Emerg Med Clin North Am. 2004;22(3):683-696. doi:10.1016/j.emc.2004.04.016
5. Munro K, Gharaibeh A, Nagabushanam S, Martin C. Diagnosis and management of tubo-ovarian abscesses. Obstet Gynaecol. 2018;20(1):11-19. doi:10.1111/tog.12447
6. Fink D, Lim PPC, Desai A, Stephans AB, Wien MA. Recurrent tubo-ovarian abscess in a nonsexually active adolescent. Consultant. 2022;62(1):e26-e28. doi:10.25270/con.2021.04.00010
7. Hiller N, Fux T, Finkelstein A, Mezeh H, Simanovsky N. CT differentiation between tubo-ovarian and appendiceal origin of right lower quadrant abscess: CT, clinical, and laboratory correlation. Emerg Radiol. 2016;23:133-139. doi:10.1007/s10140-015-1372-z
8. Kairys N, Roepke C. Tubo-ovarian abscess. In: StatPearls. Treasure Island (FL): StatPearls Publishing; June 12, 2023.
9. Gao Y, Qu P, Zhou Y, Ding W. Risk factors for the development of tubo-ovarian abscesses in women with ovarian endometriosis: a retrospective matched case-control study. BMC Womens Health. 2021:21:43. doi:10.1186/s12905-021-01188-6
10. Curry A, Williams T, Penny ML. Pelvic inflammatory disease: diagnosis, management, and prevention. Am Fam Physician. 2019;100(6):357-364.
11. Landers DV, Sweet RL. Tubo-ovarian abscess: contemporary approach to management. Rev Infect Dis. 1983;5(5):876-884. doi:10.1093/clinids/5.5.876
12. Burkman R, Schlesselman S, McCaffrey L, Gupta PK, Spence M. The relationship of genital tract actinomycetes and the development of pelvic inflammatory disease. Am J Obstet Gynecol. 1982;143(5):585-589. doi:10.1016/0002-9378(82)90552-x
13. Armstrong C. ACOG releases guidelines on antibiotic prophylaxis for gynecologic procedures. Am Fam Physician. 2007;75(7):1094-1096.
14. Pittaway DE, Winfield AC, Maxson W, Daniell J, Herbert C, Wentz AC. Prevention of acute pelvic inflammatory disease after hysterosalpingography: efficacy of doxycycline prophylaxis. Am J Obstet Gynecol. 1983;147(6):623-626. doi:10.1016/0002-9378(83)90438-6
15. Committee on Practice Bulletins—Gynecology. Prevention of Infection After Gynecologic Procedures: ACOG Practice Bulletin, Number 195. Obstet Gynecol. 2018;131(6):e172-e189. doi:10.1097/AOG.0000000000002670
16. Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population-based nested controlled study. Clinics (Sao Paulo). 2018;73:e364. Published 2018 Aug 9. doi:10.6061/clinics/2018/e364
17. Fouks Y, Azem F, Many A, Cohen Y, Levin I, Cohen A. Fertility outcomes in patients with tubo-ovarian abscesses after an oocyte retrieval: a longitudinal cohort analysis. Arch Gynecol Obstet. 2019;300(3):763-769. doi:10.1007/s00404-019-05230-9
A 37-year-old woman presented to the emergency department (ED) with 12 hours of fever and lower abdominal cramp pain. She had a history significant for hypothyroidism, infertility, and dysmenorrhea and had a hysterosalpingography (HSG) 48 hours prior for a comprehensive infertility workup.
On examination, the patient’s vital signs were a 94 bpm heart rate; 109/64 mm Hg blood pressure; 14 breaths per minute respiratory rate; 99% oxygen saturation on room air; and 101.2 °F temperature. The patient reported pain in the bilateral lower abdominal quadrants and no history of sexually transmitted infection, pelvic inflammatory disease, vaginal discharge or bleeding, dysuria, hematuria, melena, or bright red blood per rectum. A human chorionic gonadotropin urine test was negative on intake. The HSG 48 hours prior showed no concerning findings with normal uterine cavity and normal caliber fallopian tubes bilaterally.
On physical examination, the patient’s abdomen was nondistended, nonperitonitic, and without evidence of acute trauma or surgical scars. On palpation, the patient was tender in her suprapubic region and lower abdominal quadrants without evidence of guarding or rebound
The patient’s initial laboratory tests were as follows: white blood cells, 20.1 × 103/μL (reference range, 4-11 × 103/μL); hemoglobin, 12.1 g/dL (reference range, 12.1-15.1 g/dL); hematocrit, 37.1%, (reference range, 36%-48%); alanine aminotransferase, 84 U/L (reference range, 7-56 U/L); aspartate aminotransferase, 66 U/L (reference range, 8-33 U/L); and lipase, 25 U/L (5-60 U/L). Urinalysis was notable for only 5 red blood cells and negative for white blood cells, leukocyte esterase, and nitrites. The patient’s pain and fever were controlled with 1 g IV acetaminophen.
The patient’s fever, leukocytosis, and physical examination were concerning for possible intra-abdominal processes, so a
Discussion
The patient was diagnosed with bilateral tubo-ovarian abscess (TOA) likely secondary to her HSG procedure 48 hours before. TOA is a severe infectious, inflammatory condition involving a mass of the ovaries, fallopian tubes, or adjacent tissues of the upper female genital tract.1 Traditionally, TOAs are sequelae of undiagnosed or subclinical acute or chronic pelvic inflammatory disease (PID). This is known to occur via pathogen ascension from the lower to the upper female genital tract resulting in cervicitis, endometritis, salpingitis, oophoritis, and if left untreated, peritonitis.1 About 70,000 women are diagnosed with TOAs in the US every year. These patients require hospitalization as well as IV antibiotics for gold-standard treatment; however, some cases may require percutaneous drainage based on size, severity, and location.2
Diagnostic Considerations
Clinically, patients with TOAs present with fever, chills, lower abdominal pain, vaginal discharge with cervical motion tenderness, and an adnexal mass on examination.3 When a TOA is suspected, a urine human chorionic gonadotropin test and testing for C trachomatis and N gonorrhoeae are warranted. An ED workup often reveals leukocytosis, elevated C-reactive protein, and elevated erythrocyte sedimentation rate. Imaging is recommended once a TOA is suspected. Ultrasound is the gold-standard imaging modality and boasts a sensitivity of 93% and specificity of 98% for the detection of TOAs; however, CT has also been shown to be an effective diagnostic modality.4
Despite being common, TOAs are difficult to predict, detect, and diagnose; thus the clinician must often rely on thorough history taking and physical examination to raise suspicion.5 Although most frequently associated with sexual transmission, TOAs occur in not sexually active women in adolescence and adulthood. Specifically, TOAs also can present secondary to other intra-abdominal pathologies, such as appendicitis, diverticulitis, and pyelonephritis, as well as a complication of intrauterine procedures, such as an HSG, or less commonly, following intrauterine device (IUD) insertion.5-7
Given that sexually transmitted infections are the most common etiology of TOAs, C trachomatis and N gonorrhoeae are the most likely microorganisms to be isolated (Table).8-12 In not sexually active populations, Escherichia coli and Gardnerella vaginalis should be considered instead. Although rare, women with IUDs have been shown to have an increased incidence of PID/TOA secondary to Actinomyces israleii relative to women without IUDs.12 In patients with TOAs secondary to intraabdominal surgery, anaerobic bacteria, such as Bacterioides and Peptostreptococcus species in addition to Escherichia coli, are likely culprits.11
In patients after HSG, infectious complications are uncommon enough that the American College of Obstetricians and Gynecologists recommends against antibiotic prophylaxis unless there are risk factors of dilated fallopian tubes or a history of PID.13 Identifying a precise percentage of TOA as a complication of HSG is rather elusive in the literature, though, it is frequently noted that infection in general is uncommon, and the risk of developing PID is about 1.4% to 3.4%.14 However, in the retrospective study most often cited, all women who developed PID following HSG had evidence of dilated fallopian tubes. Given our patient had no history of PID or dilated fallopian tubes, her risk of developing infection (PID or postprocedural abscess) would be considered very low; therefore, the index of suspicion also was low.15
Following diagnosis, the patient was promptly admitted and treated with a course of IV ceftriaxone 1 g every 24 hours, IV doxycycline 100 mg every 12 hours, and a single dose of IV metronidazole 500 mg. Her leukocytosis, fever, and pain improved within 48 hours without the need for percutaneous drainage and the patient made a complete recovery.
Complications
TOAs can carry significant morbidity and mortality, and there are both acute and chronic complications associated. Even if properly treated, TOAs can rupture leading to severe illness, such as peritonitis and septic shock. This often requires surgical intervention and hemodynamic pressure support in the intensive care unit setting.16 One of the most feared long-term complications of TOAs is infertility secondary to structural abnormalities of the female reproductive tract.10 Adhesions, strictures, and scarring are associated with TOAs irrespective of medical or surgical management, and thus any women with a history of PID or TOA require advanced fertility workup if they are having difficulties with conception or implantation.17
Minimizing infectious transmission also is essential in the treatment of TOA/PID. All women who receive a diagnosis of PID or TOA should be evaluated for gonorrhea, chlamydia, HIV, and syphilis. Women should be instructed to abstain from sexual intercourse until therapy is complete, symptoms have resolved, and sex partners have been treated for potential chlamydial or gonococcal infections. All contraceptive methods can be continued during treatment.
Conclusions
This case presented several challenges as the bilateral TOAs developed postprocedure in a patient without risk factors. Furthermore, this case did not follow the classic presentation of ascending bacterial translocation to the ovaries over days to weeks. The diagnosis was complicated by a largely benign physical examination and a pelvic examination without evidence of abnormal vaginal discharge or cervicitis. The only indicators were fever, leukocytosis, and abdominal pain in the setting of a recent, uncomplicated HSG procedure. Vigilance is required to obtain the necessary history, and the differential of TOA must be broadened to include women without a history or symptoms of a sexually transmitted infection (contrary to the classic association). We aim to encourage heightened clinical suspicion for TOAs in patients who present with fever, leukocytosis, and abdominal pain after recent HSG or other intrauterine instrumentation procedures and therefore improve patient outcomes.
A 37-year-old woman presented to the emergency department (ED) with 12 hours of fever and lower abdominal cramp pain. She had a history significant for hypothyroidism, infertility, and dysmenorrhea and had a hysterosalpingography (HSG) 48 hours prior for a comprehensive infertility workup.
On examination, the patient’s vital signs were a 94 bpm heart rate; 109/64 mm Hg blood pressure; 14 breaths per minute respiratory rate; 99% oxygen saturation on room air; and 101.2 °F temperature. The patient reported pain in the bilateral lower abdominal quadrants and no history of sexually transmitted infection, pelvic inflammatory disease, vaginal discharge or bleeding, dysuria, hematuria, melena, or bright red blood per rectum. A human chorionic gonadotropin urine test was negative on intake. The HSG 48 hours prior showed no concerning findings with normal uterine cavity and normal caliber fallopian tubes bilaterally.
On physical examination, the patient’s abdomen was nondistended, nonperitonitic, and without evidence of acute trauma or surgical scars. On palpation, the patient was tender in her suprapubic region and lower abdominal quadrants without evidence of guarding or rebound
The patient’s initial laboratory tests were as follows: white blood cells, 20.1 × 103/μL (reference range, 4-11 × 103/μL); hemoglobin, 12.1 g/dL (reference range, 12.1-15.1 g/dL); hematocrit, 37.1%, (reference range, 36%-48%); alanine aminotransferase, 84 U/L (reference range, 7-56 U/L); aspartate aminotransferase, 66 U/L (reference range, 8-33 U/L); and lipase, 25 U/L (5-60 U/L). Urinalysis was notable for only 5 red blood cells and negative for white blood cells, leukocyte esterase, and nitrites. The patient’s pain and fever were controlled with 1 g IV acetaminophen.
The patient’s fever, leukocytosis, and physical examination were concerning for possible intra-abdominal processes, so a
Discussion
The patient was diagnosed with bilateral tubo-ovarian abscess (TOA) likely secondary to her HSG procedure 48 hours before. TOA is a severe infectious, inflammatory condition involving a mass of the ovaries, fallopian tubes, or adjacent tissues of the upper female genital tract.1 Traditionally, TOAs are sequelae of undiagnosed or subclinical acute or chronic pelvic inflammatory disease (PID). This is known to occur via pathogen ascension from the lower to the upper female genital tract resulting in cervicitis, endometritis, salpingitis, oophoritis, and if left untreated, peritonitis.1 About 70,000 women are diagnosed with TOAs in the US every year. These patients require hospitalization as well as IV antibiotics for gold-standard treatment; however, some cases may require percutaneous drainage based on size, severity, and location.2
Diagnostic Considerations
Clinically, patients with TOAs present with fever, chills, lower abdominal pain, vaginal discharge with cervical motion tenderness, and an adnexal mass on examination.3 When a TOA is suspected, a urine human chorionic gonadotropin test and testing for C trachomatis and N gonorrhoeae are warranted. An ED workup often reveals leukocytosis, elevated C-reactive protein, and elevated erythrocyte sedimentation rate. Imaging is recommended once a TOA is suspected. Ultrasound is the gold-standard imaging modality and boasts a sensitivity of 93% and specificity of 98% for the detection of TOAs; however, CT has also been shown to be an effective diagnostic modality.4
Despite being common, TOAs are difficult to predict, detect, and diagnose; thus the clinician must often rely on thorough history taking and physical examination to raise suspicion.5 Although most frequently associated with sexual transmission, TOAs occur in not sexually active women in adolescence and adulthood. Specifically, TOAs also can present secondary to other intra-abdominal pathologies, such as appendicitis, diverticulitis, and pyelonephritis, as well as a complication of intrauterine procedures, such as an HSG, or less commonly, following intrauterine device (IUD) insertion.5-7
Given that sexually transmitted infections are the most common etiology of TOAs, C trachomatis and N gonorrhoeae are the most likely microorganisms to be isolated (Table).8-12 In not sexually active populations, Escherichia coli and Gardnerella vaginalis should be considered instead. Although rare, women with IUDs have been shown to have an increased incidence of PID/TOA secondary to Actinomyces israleii relative to women without IUDs.12 In patients with TOAs secondary to intraabdominal surgery, anaerobic bacteria, such as Bacterioides and Peptostreptococcus species in addition to Escherichia coli, are likely culprits.11
In patients after HSG, infectious complications are uncommon enough that the American College of Obstetricians and Gynecologists recommends against antibiotic prophylaxis unless there are risk factors of dilated fallopian tubes or a history of PID.13 Identifying a precise percentage of TOA as a complication of HSG is rather elusive in the literature, though, it is frequently noted that infection in general is uncommon, and the risk of developing PID is about 1.4% to 3.4%.14 However, in the retrospective study most often cited, all women who developed PID following HSG had evidence of dilated fallopian tubes. Given our patient had no history of PID or dilated fallopian tubes, her risk of developing infection (PID or postprocedural abscess) would be considered very low; therefore, the index of suspicion also was low.15
Following diagnosis, the patient was promptly admitted and treated with a course of IV ceftriaxone 1 g every 24 hours, IV doxycycline 100 mg every 12 hours, and a single dose of IV metronidazole 500 mg. Her leukocytosis, fever, and pain improved within 48 hours without the need for percutaneous drainage and the patient made a complete recovery.
Complications
TOAs can carry significant morbidity and mortality, and there are both acute and chronic complications associated. Even if properly treated, TOAs can rupture leading to severe illness, such as peritonitis and septic shock. This often requires surgical intervention and hemodynamic pressure support in the intensive care unit setting.16 One of the most feared long-term complications of TOAs is infertility secondary to structural abnormalities of the female reproductive tract.10 Adhesions, strictures, and scarring are associated with TOAs irrespective of medical or surgical management, and thus any women with a history of PID or TOA require advanced fertility workup if they are having difficulties with conception or implantation.17
Minimizing infectious transmission also is essential in the treatment of TOA/PID. All women who receive a diagnosis of PID or TOA should be evaluated for gonorrhea, chlamydia, HIV, and syphilis. Women should be instructed to abstain from sexual intercourse until therapy is complete, symptoms have resolved, and sex partners have been treated for potential chlamydial or gonococcal infections. All contraceptive methods can be continued during treatment.
Conclusions
This case presented several challenges as the bilateral TOAs developed postprocedure in a patient without risk factors. Furthermore, this case did not follow the classic presentation of ascending bacterial translocation to the ovaries over days to weeks. The diagnosis was complicated by a largely benign physical examination and a pelvic examination without evidence of abnormal vaginal discharge or cervicitis. The only indicators were fever, leukocytosis, and abdominal pain in the setting of a recent, uncomplicated HSG procedure. Vigilance is required to obtain the necessary history, and the differential of TOA must be broadened to include women without a history or symptoms of a sexually transmitted infection (contrary to the classic association). We aim to encourage heightened clinical suspicion for TOAs in patients who present with fever, leukocytosis, and abdominal pain after recent HSG or other intrauterine instrumentation procedures and therefore improve patient outcomes.
1. Gkrozou F, Tsonis O, Daniilidis A, Navrozoglou I, Paschopoulos M. Tubo-ovarian abscess: exploring optimal treatment options based on current evidence. J Endometr Pelvic Pain Disord. 2020;13(1):10-19. doi:10.1177/2284026520960649
2. Taylor KJ, Wasson JF, De Graaff C, Rosenfield AT, Andriole VT. Accuracy of grey-scale ultrasound diagnosis of abdominal and pelvic abscesses in 220 patients. Lancet. 1978;1(8055):83-84. doi:10.1016/s0140-6736(78)90016-8
3. Bridwell RE, Koyfman A, Long B. High risk and low prevalence diseases: tubo-ovarian abscess. Am J Emerg Med. 2022;57:70-75. doi:10.1016/j.ajem.2022.04.026
4. Lambert MJ, Villa M. Gynecologic ultrasound in emergency medicine. Emerg Med Clin North Am. 2004;22(3):683-696. doi:10.1016/j.emc.2004.04.016
5. Munro K, Gharaibeh A, Nagabushanam S, Martin C. Diagnosis and management of tubo-ovarian abscesses. Obstet Gynaecol. 2018;20(1):11-19. doi:10.1111/tog.12447
6. Fink D, Lim PPC, Desai A, Stephans AB, Wien MA. Recurrent tubo-ovarian abscess in a nonsexually active adolescent. Consultant. 2022;62(1):e26-e28. doi:10.25270/con.2021.04.00010
7. Hiller N, Fux T, Finkelstein A, Mezeh H, Simanovsky N. CT differentiation between tubo-ovarian and appendiceal origin of right lower quadrant abscess: CT, clinical, and laboratory correlation. Emerg Radiol. 2016;23:133-139. doi:10.1007/s10140-015-1372-z
8. Kairys N, Roepke C. Tubo-ovarian abscess. In: StatPearls. Treasure Island (FL): StatPearls Publishing; June 12, 2023.
9. Gao Y, Qu P, Zhou Y, Ding W. Risk factors for the development of tubo-ovarian abscesses in women with ovarian endometriosis: a retrospective matched case-control study. BMC Womens Health. 2021:21:43. doi:10.1186/s12905-021-01188-6
10. Curry A, Williams T, Penny ML. Pelvic inflammatory disease: diagnosis, management, and prevention. Am Fam Physician. 2019;100(6):357-364.
11. Landers DV, Sweet RL. Tubo-ovarian abscess: contemporary approach to management. Rev Infect Dis. 1983;5(5):876-884. doi:10.1093/clinids/5.5.876
12. Burkman R, Schlesselman S, McCaffrey L, Gupta PK, Spence M. The relationship of genital tract actinomycetes and the development of pelvic inflammatory disease. Am J Obstet Gynecol. 1982;143(5):585-589. doi:10.1016/0002-9378(82)90552-x
13. Armstrong C. ACOG releases guidelines on antibiotic prophylaxis for gynecologic procedures. Am Fam Physician. 2007;75(7):1094-1096.
14. Pittaway DE, Winfield AC, Maxson W, Daniell J, Herbert C, Wentz AC. Prevention of acute pelvic inflammatory disease after hysterosalpingography: efficacy of doxycycline prophylaxis. Am J Obstet Gynecol. 1983;147(6):623-626. doi:10.1016/0002-9378(83)90438-6
15. Committee on Practice Bulletins—Gynecology. Prevention of Infection After Gynecologic Procedures: ACOG Practice Bulletin, Number 195. Obstet Gynecol. 2018;131(6):e172-e189. doi:10.1097/AOG.0000000000002670
16. Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population-based nested controlled study. Clinics (Sao Paulo). 2018;73:e364. Published 2018 Aug 9. doi:10.6061/clinics/2018/e364
17. Fouks Y, Azem F, Many A, Cohen Y, Levin I, Cohen A. Fertility outcomes in patients with tubo-ovarian abscesses after an oocyte retrieval: a longitudinal cohort analysis. Arch Gynecol Obstet. 2019;300(3):763-769. doi:10.1007/s00404-019-05230-9
1. Gkrozou F, Tsonis O, Daniilidis A, Navrozoglou I, Paschopoulos M. Tubo-ovarian abscess: exploring optimal treatment options based on current evidence. J Endometr Pelvic Pain Disord. 2020;13(1):10-19. doi:10.1177/2284026520960649
2. Taylor KJ, Wasson JF, De Graaff C, Rosenfield AT, Andriole VT. Accuracy of grey-scale ultrasound diagnosis of abdominal and pelvic abscesses in 220 patients. Lancet. 1978;1(8055):83-84. doi:10.1016/s0140-6736(78)90016-8
3. Bridwell RE, Koyfman A, Long B. High risk and low prevalence diseases: tubo-ovarian abscess. Am J Emerg Med. 2022;57:70-75. doi:10.1016/j.ajem.2022.04.026
4. Lambert MJ, Villa M. Gynecologic ultrasound in emergency medicine. Emerg Med Clin North Am. 2004;22(3):683-696. doi:10.1016/j.emc.2004.04.016
5. Munro K, Gharaibeh A, Nagabushanam S, Martin C. Diagnosis and management of tubo-ovarian abscesses. Obstet Gynaecol. 2018;20(1):11-19. doi:10.1111/tog.12447
6. Fink D, Lim PPC, Desai A, Stephans AB, Wien MA. Recurrent tubo-ovarian abscess in a nonsexually active adolescent. Consultant. 2022;62(1):e26-e28. doi:10.25270/con.2021.04.00010
7. Hiller N, Fux T, Finkelstein A, Mezeh H, Simanovsky N. CT differentiation between tubo-ovarian and appendiceal origin of right lower quadrant abscess: CT, clinical, and laboratory correlation. Emerg Radiol. 2016;23:133-139. doi:10.1007/s10140-015-1372-z
8. Kairys N, Roepke C. Tubo-ovarian abscess. In: StatPearls. Treasure Island (FL): StatPearls Publishing; June 12, 2023.
9. Gao Y, Qu P, Zhou Y, Ding W. Risk factors for the development of tubo-ovarian abscesses in women with ovarian endometriosis: a retrospective matched case-control study. BMC Womens Health. 2021:21:43. doi:10.1186/s12905-021-01188-6
10. Curry A, Williams T, Penny ML. Pelvic inflammatory disease: diagnosis, management, and prevention. Am Fam Physician. 2019;100(6):357-364.
11. Landers DV, Sweet RL. Tubo-ovarian abscess: contemporary approach to management. Rev Infect Dis. 1983;5(5):876-884. doi:10.1093/clinids/5.5.876
12. Burkman R, Schlesselman S, McCaffrey L, Gupta PK, Spence M. The relationship of genital tract actinomycetes and the development of pelvic inflammatory disease. Am J Obstet Gynecol. 1982;143(5):585-589. doi:10.1016/0002-9378(82)90552-x
13. Armstrong C. ACOG releases guidelines on antibiotic prophylaxis for gynecologic procedures. Am Fam Physician. 2007;75(7):1094-1096.
14. Pittaway DE, Winfield AC, Maxson W, Daniell J, Herbert C, Wentz AC. Prevention of acute pelvic inflammatory disease after hysterosalpingography: efficacy of doxycycline prophylaxis. Am J Obstet Gynecol. 1983;147(6):623-626. doi:10.1016/0002-9378(83)90438-6
15. Committee on Practice Bulletins—Gynecology. Prevention of Infection After Gynecologic Procedures: ACOG Practice Bulletin, Number 195. Obstet Gynecol. 2018;131(6):e172-e189. doi:10.1097/AOG.0000000000002670
16. Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population-based nested controlled study. Clinics (Sao Paulo). 2018;73:e364. Published 2018 Aug 9. doi:10.6061/clinics/2018/e364
17. Fouks Y, Azem F, Many A, Cohen Y, Levin I, Cohen A. Fertility outcomes in patients with tubo-ovarian abscesses after an oocyte retrieval: a longitudinal cohort analysis. Arch Gynecol Obstet. 2019;300(3):763-769. doi:10.1007/s00404-019-05230-9
Nightmare on CIL Street: A Simulation Series to Increase Confidence and Skill in Responding to Clinical Emergencies
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
Missing Table and a Clarification
Correction
In: Barbeito A, Raghunathan K, Connolly S, et al. Barriers to implementation of telehealth pre-anesthesia evaluation visits in the Department of Veterans Affairs. Fed Pract. 2023;40(7):210-217a. doi:10.12788/fp.0387. Federal Practitioner inadvertently excluded Table 2. It has been updated online and in PubMed Central.
Clarification
In: Weaver M, Geppert CMA. Salute to service dogs. Fed Pract . 2023;40(9):278-280. doi:10.12788/fp.0414, The PAWS Act was noted and the authors want to provide the following
Correction
In: Barbeito A, Raghunathan K, Connolly S, et al. Barriers to implementation of telehealth pre-anesthesia evaluation visits in the Department of Veterans Affairs. Fed Pract. 2023;40(7):210-217a. doi:10.12788/fp.0387. Federal Practitioner inadvertently excluded Table 2. It has been updated online and in PubMed Central.
Clarification
In: Weaver M, Geppert CMA. Salute to service dogs. Fed Pract . 2023;40(9):278-280. doi:10.12788/fp.0414, The PAWS Act was noted and the authors want to provide the following
Correction
In: Barbeito A, Raghunathan K, Connolly S, et al. Barriers to implementation of telehealth pre-anesthesia evaluation visits in the Department of Veterans Affairs. Fed Pract. 2023;40(7):210-217a. doi:10.12788/fp.0387. Federal Practitioner inadvertently excluded Table 2. It has been updated online and in PubMed Central.
Clarification
In: Weaver M, Geppert CMA. Salute to service dogs. Fed Pract . 2023;40(9):278-280. doi:10.12788/fp.0414, The PAWS Act was noted and the authors want to provide the following
Monitoring Thyrotropin in Veterans With Thyroid Nodules
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
Elective Hand Surgery and Antithrombotic Use in Veterans
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
Who Gets to Determine Whether Home Is “Unsafe” at the End of Life?
Sometimes a patient at the end of life (EOL) just wants to go home. We recently treated such a patient, “Joe,” a 66-year-old veteran with end-stage chronic obstructive pulmonary disorder (COPD), severe hearing loss, and heavy alcohol use. A neighbor brought Joe to the hospital when he developed a urinary tract infection. Before hospitalization, Joe spent his days in bed. His neighbor was his designated health care agent (HCA) and caregiver, dropping off meals and bringing Joe to medical appointments. Joe had no other social support. In the hospital, Joe could not participate in physical therapy (PT) evaluations due to severe dyspnea on exertion. He was recommended for home PT, a home health aide, and home nursing, but Joe declined these services out of concern for encroachment on his independence. Given his heavy alcohol use, limited support, and functional limitations, the hospitalist team felt that Joe would be best served in a skilled nursing facility. As the palliative care team, we were consulted and felt that he was eligible for hospice. Joe simply wanted to go home.
Many patients like Joe experience functional decline at EOL, leading to increased care needs and transitions between sites of care.1 Some hospitalized patients at EOL want to transition directly to home, but due to their limited functioning and social support, discharge home may be deemed unsafe by health care professionals (HCPs). Clinicians then face the difficult balancing act of honoring patient wishes and avoiding a bad outcome. For patients at EOL, issues of capacity and risk become particularly salient. Furthermore, the unique structure of the US Department of Veterans Affairs (VA) health system and the psychosocial needs of some veterans add additional considerations for complex EOL discharges.2
End-of-life Decision Making
While patients may express strong preferences regarding their health care, their decision-making ability may worsen as they approach EOL. Contributing factors include older age, effects of hospitalization, treatment adverse effects, and comorbidities, including cognitive impairment. Studies of terminally ill patients show high rates of impaired decisional capacity.3,4 It is critical to assess capacity as part of discharge planning. Even when patients have the capacity, families and caregivers have an important voice, since they are often instrumental in maintaining patients at home.
Defining Risk
Determining whether a discharge is risky or unsafe is highly subjective, with differing opinions among clinicians and between patients and clinicians.5-7 In a qualitative study by Coombs and colleagues, HCPs tended toward a risk-averse approach to discharge decisions, sometimes favoring discharge to care facilities despite patient preferences.6 This approach also reflects pressures from the health care system to decrease the length of stay and reduce readmissions, important metrics for patient care and cost containment. However, keeping patients hospitalized or in nursing facilities does not completely mitigate risks (eg, falls) and carries other hazards (eg, nosocomial infections), as highlighted during the COVID-19 pandemic.7,8 The prospect of malpractice lawsuits and HCP moral distress about perceived risky home situations can also understandably affect decision making.
At the same time, risk calculation changes depending on the patient’s clinical status and priorities. Coombs and colleagues found that in contrast to clinicians, patients nearing EOL are willing to accept increasing risks and suboptimal living conditions to remain at home.6 What may be intolerable for a younger, healthier patient with a long life expectancy may be acceptable for someone who is approaching EOL. In our framework, a risky home discharge at EOL is considered one in which other adverse events, such as falls or inadequate symptom management, are likely.
Ethical Considerations
Unsafe discharges are challenging in part because some of the pillars of medical ethics can conflict. Prior articles have analyzed the ethical concerns of unsafe discharges in detail.9-11 Briefly, when patients wish to return home against initial medical recommendations, treatment teams may focus on the principles of beneficence and nonmaleficence, as exemplified by the desire to minimize harm, and justice, in which clinicians consider resource allocation and risks that a home discharge poses to family members, caregivers, and home health professionals. However, autonomy is important to consider as well. The concept of dignity of risk highlights the imperative to respect others’ decisions even when they increase the chance of harm, particularly given the overall shift in medicine from paternalism to shared decision making.12 Accommodating patient choice in how and where health care is received allows patients to regain some control over their lives, thereby enhancing their quality of life and promoting patient dignity, especially in their remaining days.13
Discharge Risk Framework
Our risk assessment framework helps clinicians more objectively identify factors that increase or decrease risk, inform discharge planning, partner with patients and families, give patients a prominent role in EOL decisions, and mitigate the risk of a bad outcome. This concept has been used in psychiatry, in which formal suicide assessment includes identifying risk factors and protective factors to estimate suicide risk and determine interventions.14 Similar to suicide risk estimation, this framework is based on clinical judgment rather than a specific calculation.
While this framework serves as a guide for determining and mitigating risk, we encourage teams to consider legal or ethical consultations in challenging cases, such as those in which patients lack both capacity and an involved HCA.
Step 1: Determine the patient’s capacity regarding disposition planning. Patients at EOL are at a higher risk of impaired decision-making capabilities; therefore, capacity evaluation is a critical step.
Step 2: Identify risk factors and protective factors for discharge home. Risk factors are intrinsic and extrinsic factors that increase risk such as functional or sensory impairments. Protective factors are intrinsic and extrinsic factors that decrease risk, including a good understanding of illness and consistent connection with the health care system (Table 1).
Step 3: Determine discharge to home risk level based on identified risk factors and protective factors. Patients may be at low, moderate, or high risk of having an adverse event, such as a fall or inadequate symptom control (Table 2).
Step 4: Identify risk mitigation strategies. These should be tailored to the patient based on the factors identified in Step 2. Examples include home nursing and therapy, mental health treatment, a medical alert system, and frequent contact between the patient and health care team.
Step 5: Meet with inpatient and outpatient HCP teams. Meetings should include the primary care professional (PCP) or relevant subspecialist, such as an oncologist for patients with cancer. For veterans receiving care solely at a local VA medical center, this can be easier to facilitate, but for veterans who receive care through both VA and non-VA systems, this step may require additional coordination. We also recommend including interdisciplinary team members, such as social workers, case managers, and the relevant home care or hospice agency. Certain agencies may decline admission if they perceive increased risk, such as no 24-hour care, perceived self-neglect, and limited instrumental support. During this meeting, HCPs discuss risk mitigation strategies identified in Step 4 and create a plan to propose to patients and families.
Step 6: Meet with patient, HCA, and family members. In addition to sharing information about prognosis, assessing caregiver capabilities and burden can guide conversations about discharge. The discharge plan should be determined through shared decision making.11 If the patient lacks capacity regarding disposition planning, this should be shared with the HCA. However, even when patients lack capacity, it is important to continue to engage them to understand their goals and preferences.
Step 7: Maximize risk mitigation strategies. If a moderate- or high-risk discharge is requested, the health care team should maximize risk mitigation strategies. For low-risk discharges, risk mitigation strategies can still promote safety, especially since risk increases as patients progress toward EOL. In some instances, patients, their HCAs, or caregivers may decline all risk mitigation strategies despite best efforts to communicate and negotiate options. In such circumstances, we recommend discussing the case with the outpatient team for a warm handoff. HCPs should also document all efforts (helpful from a legal standpoint as well as for the patient’s future treatment teams) and respect the decision to discharge home.
Applying the Framework
Our patient Joe provides a good illustration of how to implement this EOL framework. He was deemed to have the capacity to make decisions regarding discharge (Step 1). We determined his risk factors and protective factors for discharge (Step 2). His poor functional status, limited instrumental support, heavy alcohol use, rejection of home services, and communication barriers due to severe hearing impairment all increased his risk. Protective factors included an appreciation of functional limitations, intact cognition, and an involved HCA. Based on his limited instrumental support and poor function but good insight into limitations, discharge home was deemed to be of moderate risk (Step 3). Although risk factors such as alcohol use and severe hearing impairment could have raised his level to high risk, we felt that his involved HCA maintained him in the moderate-risk category.
We worked with the hospitalist team, PT, and audiology to identify multiple risk mitigation strategies: frequent phone calls between the HCA and outpatient palliative care team, home PT to improve transfers from bed to bedside commode, home nursing services either through a routine agency or hospice, and hearing aids for better communication (Steps 4 and 5). We then proposed these strategies to Joe and his HCA (Step 6). Due to concerns about infringement on his independence, Joe declined all home services but agreed to twice-daily check-ins by his HCA, frequent communication between his HCA and our team, and new hearing aids.
Joe returned home with the agreed-upon risk mitigation strategies in place (Step 7). Despite clinicians’ original reservations about sending Joe home without formal services, his HCA maintained close contact with our team, noting that Joe remained stable and happy to be at home in the months following discharge.
Conclusions
Fortunately, VA HCPs operate in an integrated health care system with access to psychological, social, and at-home medical support that can help mitigate risks. Still, we have benefitted from having a tool to help us evaluate risk systematically. Even if patients, families, and HCPs disagree on ideal discharge plans, this tool helps clinicians approach discharges methodically while maintaining open communication and partnership with patients. In doing so, our framework reflects the shift in medical culture from a patriarchal approach to shared decision-making practices regarding all aspects of medical care. Furthermore, we hope that this can help reduce clinician moral distress stemming from these challenging cases.
Future research on best practices for discharge risk assessment and optimizing home safety are needed. We also hope to evaluate the impact and effectiveness of our framework through interviews with key stakeholders. For Joe and other veterans like him, where to spend their final days may be the last important decision they make in life, and our framework allows for their voices to be better heard throughout the decision-making process.
Acknowledgments
We thank Brooke Lifland, MD, for her theoretical contributions to the concept behind this paper.
1. Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press (US); March 19, 2015.
2. Casarett D, Pickard A, Amos Bailey F, et al. Important aspects of end-of-life care among veterans: implications for measurement and quality improvement. J Pain Symptom Manage. 2008;35(2):115-125. doi:10.1016/j.jpainsymman.2007.03.008
3. Kolva E, Rosenfeld B, Brescia R, Comfort C. Assessing decision-making capacity at end of life. Gen Hosp Psychiatry. 2014;36(4):392-397. doi:10.1016/j.genhosppsych.2014.02.013
4. Kolva E, Rosenfeld B, Saracino R. Assessing the decision-making capacity of terminally ill patients with cancer. Am J Geriatr Psychiatry. 2018;26(5):523-531. doi:10.1016/j.jagp.2017.11.012
5. Macmillan MS. Hospital staff’s perceptions of risk associated with the discharge of elderly people from acute hospital care. J Adv Nurs. 1994;19(2):249-256. doi:10.1111/j.1365-2648.1994.tb01078.x
6. Coombs MA, Parker R, de Vries K. Managing risk during care transitions when approaching end of life: A qualitative study of patients’ and health care professionals’ decision making. Palliat Med. 2017;31(7):617-624. doi:10.1177/0269216316673476
7. Hyslop B. ‘Not safe for discharge’? Words, values, and person-centred care. Age Ageing. 2020;49(3):334-336. doi:10.1093/ageing/afz170
8. Goodacre S. Safe discharge: an irrational, unhelpful and unachievable concept. Emerg Med J. 2006;23(10):753-755. doi:10.1136/emj.2006.037903
9. Swidler RN, Seastrum T, Shelton W. Difficult hospital inpatient discharge decisions: ethical, legal and clinical practice issues. Am J Bioeth. 2007;7(3):23-28. doi:10.1080/15265160601171739
10. Hill J, Filer W. Safety and ethical considerations in discharging patients to suboptimal living situations. AMA J Ethics. 2015;17(6):506-510. Published 2015 Jun 1. doi:10.1001/journalofethics.2015.17.6.ecas2-1506
11. West JC. What is an ethically informed approach to managing patient safety risk during discharge planning?. AMA J Ethics. 2020;22(11):E919-E923. Published 2020 Nov 1. doi:10.1001/amajethics.2020.919
12. Mukherjee D. Discharge decisions and the dignity of risk. Hastings Cent Rep. 2015;45(3):7-8. doi:10.1002/hast.441
13. Wheatley VJ, Baker JI. “Please, I want to go home”: ethical issues raised when considering choice of place of care in palliative care. Postgrad Med J. 2007;83(984):643-648. doi:10.1136/pgmj.2007.058487
14. Work Group on Suicidal Behaviors. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(suppl 11):1-60.
Sometimes a patient at the end of life (EOL) just wants to go home. We recently treated such a patient, “Joe,” a 66-year-old veteran with end-stage chronic obstructive pulmonary disorder (COPD), severe hearing loss, and heavy alcohol use. A neighbor brought Joe to the hospital when he developed a urinary tract infection. Before hospitalization, Joe spent his days in bed. His neighbor was his designated health care agent (HCA) and caregiver, dropping off meals and bringing Joe to medical appointments. Joe had no other social support. In the hospital, Joe could not participate in physical therapy (PT) evaluations due to severe dyspnea on exertion. He was recommended for home PT, a home health aide, and home nursing, but Joe declined these services out of concern for encroachment on his independence. Given his heavy alcohol use, limited support, and functional limitations, the hospitalist team felt that Joe would be best served in a skilled nursing facility. As the palliative care team, we were consulted and felt that he was eligible for hospice. Joe simply wanted to go home.
Many patients like Joe experience functional decline at EOL, leading to increased care needs and transitions between sites of care.1 Some hospitalized patients at EOL want to transition directly to home, but due to their limited functioning and social support, discharge home may be deemed unsafe by health care professionals (HCPs). Clinicians then face the difficult balancing act of honoring patient wishes and avoiding a bad outcome. For patients at EOL, issues of capacity and risk become particularly salient. Furthermore, the unique structure of the US Department of Veterans Affairs (VA) health system and the psychosocial needs of some veterans add additional considerations for complex EOL discharges.2
End-of-life Decision Making
While patients may express strong preferences regarding their health care, their decision-making ability may worsen as they approach EOL. Contributing factors include older age, effects of hospitalization, treatment adverse effects, and comorbidities, including cognitive impairment. Studies of terminally ill patients show high rates of impaired decisional capacity.3,4 It is critical to assess capacity as part of discharge planning. Even when patients have the capacity, families and caregivers have an important voice, since they are often instrumental in maintaining patients at home.
Defining Risk
Determining whether a discharge is risky or unsafe is highly subjective, with differing opinions among clinicians and between patients and clinicians.5-7 In a qualitative study by Coombs and colleagues, HCPs tended toward a risk-averse approach to discharge decisions, sometimes favoring discharge to care facilities despite patient preferences.6 This approach also reflects pressures from the health care system to decrease the length of stay and reduce readmissions, important metrics for patient care and cost containment. However, keeping patients hospitalized or in nursing facilities does not completely mitigate risks (eg, falls) and carries other hazards (eg, nosocomial infections), as highlighted during the COVID-19 pandemic.7,8 The prospect of malpractice lawsuits and HCP moral distress about perceived risky home situations can also understandably affect decision making.
At the same time, risk calculation changes depending on the patient’s clinical status and priorities. Coombs and colleagues found that in contrast to clinicians, patients nearing EOL are willing to accept increasing risks and suboptimal living conditions to remain at home.6 What may be intolerable for a younger, healthier patient with a long life expectancy may be acceptable for someone who is approaching EOL. In our framework, a risky home discharge at EOL is considered one in which other adverse events, such as falls or inadequate symptom management, are likely.
Ethical Considerations
Unsafe discharges are challenging in part because some of the pillars of medical ethics can conflict. Prior articles have analyzed the ethical concerns of unsafe discharges in detail.9-11 Briefly, when patients wish to return home against initial medical recommendations, treatment teams may focus on the principles of beneficence and nonmaleficence, as exemplified by the desire to minimize harm, and justice, in which clinicians consider resource allocation and risks that a home discharge poses to family members, caregivers, and home health professionals. However, autonomy is important to consider as well. The concept of dignity of risk highlights the imperative to respect others’ decisions even when they increase the chance of harm, particularly given the overall shift in medicine from paternalism to shared decision making.12 Accommodating patient choice in how and where health care is received allows patients to regain some control over their lives, thereby enhancing their quality of life and promoting patient dignity, especially in their remaining days.13
Discharge Risk Framework
Our risk assessment framework helps clinicians more objectively identify factors that increase or decrease risk, inform discharge planning, partner with patients and families, give patients a prominent role in EOL decisions, and mitigate the risk of a bad outcome. This concept has been used in psychiatry, in which formal suicide assessment includes identifying risk factors and protective factors to estimate suicide risk and determine interventions.14 Similar to suicide risk estimation, this framework is based on clinical judgment rather than a specific calculation.
While this framework serves as a guide for determining and mitigating risk, we encourage teams to consider legal or ethical consultations in challenging cases, such as those in which patients lack both capacity and an involved HCA.
Step 1: Determine the patient’s capacity regarding disposition planning. Patients at EOL are at a higher risk of impaired decision-making capabilities; therefore, capacity evaluation is a critical step.
Step 2: Identify risk factors and protective factors for discharge home. Risk factors are intrinsic and extrinsic factors that increase risk such as functional or sensory impairments. Protective factors are intrinsic and extrinsic factors that decrease risk, including a good understanding of illness and consistent connection with the health care system (Table 1).
Step 3: Determine discharge to home risk level based on identified risk factors and protective factors. Patients may be at low, moderate, or high risk of having an adverse event, such as a fall or inadequate symptom control (Table 2).
Step 4: Identify risk mitigation strategies. These should be tailored to the patient based on the factors identified in Step 2. Examples include home nursing and therapy, mental health treatment, a medical alert system, and frequent contact between the patient and health care team.
Step 5: Meet with inpatient and outpatient HCP teams. Meetings should include the primary care professional (PCP) or relevant subspecialist, such as an oncologist for patients with cancer. For veterans receiving care solely at a local VA medical center, this can be easier to facilitate, but for veterans who receive care through both VA and non-VA systems, this step may require additional coordination. We also recommend including interdisciplinary team members, such as social workers, case managers, and the relevant home care or hospice agency. Certain agencies may decline admission if they perceive increased risk, such as no 24-hour care, perceived self-neglect, and limited instrumental support. During this meeting, HCPs discuss risk mitigation strategies identified in Step 4 and create a plan to propose to patients and families.
Step 6: Meet with patient, HCA, and family members. In addition to sharing information about prognosis, assessing caregiver capabilities and burden can guide conversations about discharge. The discharge plan should be determined through shared decision making.11 If the patient lacks capacity regarding disposition planning, this should be shared with the HCA. However, even when patients lack capacity, it is important to continue to engage them to understand their goals and preferences.
Step 7: Maximize risk mitigation strategies. If a moderate- or high-risk discharge is requested, the health care team should maximize risk mitigation strategies. For low-risk discharges, risk mitigation strategies can still promote safety, especially since risk increases as patients progress toward EOL. In some instances, patients, their HCAs, or caregivers may decline all risk mitigation strategies despite best efforts to communicate and negotiate options. In such circumstances, we recommend discussing the case with the outpatient team for a warm handoff. HCPs should also document all efforts (helpful from a legal standpoint as well as for the patient’s future treatment teams) and respect the decision to discharge home.
Applying the Framework
Our patient Joe provides a good illustration of how to implement this EOL framework. He was deemed to have the capacity to make decisions regarding discharge (Step 1). We determined his risk factors and protective factors for discharge (Step 2). His poor functional status, limited instrumental support, heavy alcohol use, rejection of home services, and communication barriers due to severe hearing impairment all increased his risk. Protective factors included an appreciation of functional limitations, intact cognition, and an involved HCA. Based on his limited instrumental support and poor function but good insight into limitations, discharge home was deemed to be of moderate risk (Step 3). Although risk factors such as alcohol use and severe hearing impairment could have raised his level to high risk, we felt that his involved HCA maintained him in the moderate-risk category.
We worked with the hospitalist team, PT, and audiology to identify multiple risk mitigation strategies: frequent phone calls between the HCA and outpatient palliative care team, home PT to improve transfers from bed to bedside commode, home nursing services either through a routine agency or hospice, and hearing aids for better communication (Steps 4 and 5). We then proposed these strategies to Joe and his HCA (Step 6). Due to concerns about infringement on his independence, Joe declined all home services but agreed to twice-daily check-ins by his HCA, frequent communication between his HCA and our team, and new hearing aids.
Joe returned home with the agreed-upon risk mitigation strategies in place (Step 7). Despite clinicians’ original reservations about sending Joe home without formal services, his HCA maintained close contact with our team, noting that Joe remained stable and happy to be at home in the months following discharge.
Conclusions
Fortunately, VA HCPs operate in an integrated health care system with access to psychological, social, and at-home medical support that can help mitigate risks. Still, we have benefitted from having a tool to help us evaluate risk systematically. Even if patients, families, and HCPs disagree on ideal discharge plans, this tool helps clinicians approach discharges methodically while maintaining open communication and partnership with patients. In doing so, our framework reflects the shift in medical culture from a patriarchal approach to shared decision-making practices regarding all aspects of medical care. Furthermore, we hope that this can help reduce clinician moral distress stemming from these challenging cases.
Future research on best practices for discharge risk assessment and optimizing home safety are needed. We also hope to evaluate the impact and effectiveness of our framework through interviews with key stakeholders. For Joe and other veterans like him, where to spend their final days may be the last important decision they make in life, and our framework allows for their voices to be better heard throughout the decision-making process.
Acknowledgments
We thank Brooke Lifland, MD, for her theoretical contributions to the concept behind this paper.
Sometimes a patient at the end of life (EOL) just wants to go home. We recently treated such a patient, “Joe,” a 66-year-old veteran with end-stage chronic obstructive pulmonary disorder (COPD), severe hearing loss, and heavy alcohol use. A neighbor brought Joe to the hospital when he developed a urinary tract infection. Before hospitalization, Joe spent his days in bed. His neighbor was his designated health care agent (HCA) and caregiver, dropping off meals and bringing Joe to medical appointments. Joe had no other social support. In the hospital, Joe could not participate in physical therapy (PT) evaluations due to severe dyspnea on exertion. He was recommended for home PT, a home health aide, and home nursing, but Joe declined these services out of concern for encroachment on his independence. Given his heavy alcohol use, limited support, and functional limitations, the hospitalist team felt that Joe would be best served in a skilled nursing facility. As the palliative care team, we were consulted and felt that he was eligible for hospice. Joe simply wanted to go home.
Many patients like Joe experience functional decline at EOL, leading to increased care needs and transitions between sites of care.1 Some hospitalized patients at EOL want to transition directly to home, but due to their limited functioning and social support, discharge home may be deemed unsafe by health care professionals (HCPs). Clinicians then face the difficult balancing act of honoring patient wishes and avoiding a bad outcome. For patients at EOL, issues of capacity and risk become particularly salient. Furthermore, the unique structure of the US Department of Veterans Affairs (VA) health system and the psychosocial needs of some veterans add additional considerations for complex EOL discharges.2
End-of-life Decision Making
While patients may express strong preferences regarding their health care, their decision-making ability may worsen as they approach EOL. Contributing factors include older age, effects of hospitalization, treatment adverse effects, and comorbidities, including cognitive impairment. Studies of terminally ill patients show high rates of impaired decisional capacity.3,4 It is critical to assess capacity as part of discharge planning. Even when patients have the capacity, families and caregivers have an important voice, since they are often instrumental in maintaining patients at home.
Defining Risk
Determining whether a discharge is risky or unsafe is highly subjective, with differing opinions among clinicians and between patients and clinicians.5-7 In a qualitative study by Coombs and colleagues, HCPs tended toward a risk-averse approach to discharge decisions, sometimes favoring discharge to care facilities despite patient preferences.6 This approach also reflects pressures from the health care system to decrease the length of stay and reduce readmissions, important metrics for patient care and cost containment. However, keeping patients hospitalized or in nursing facilities does not completely mitigate risks (eg, falls) and carries other hazards (eg, nosocomial infections), as highlighted during the COVID-19 pandemic.7,8 The prospect of malpractice lawsuits and HCP moral distress about perceived risky home situations can also understandably affect decision making.
At the same time, risk calculation changes depending on the patient’s clinical status and priorities. Coombs and colleagues found that in contrast to clinicians, patients nearing EOL are willing to accept increasing risks and suboptimal living conditions to remain at home.6 What may be intolerable for a younger, healthier patient with a long life expectancy may be acceptable for someone who is approaching EOL. In our framework, a risky home discharge at EOL is considered one in which other adverse events, such as falls or inadequate symptom management, are likely.
Ethical Considerations
Unsafe discharges are challenging in part because some of the pillars of medical ethics can conflict. Prior articles have analyzed the ethical concerns of unsafe discharges in detail.9-11 Briefly, when patients wish to return home against initial medical recommendations, treatment teams may focus on the principles of beneficence and nonmaleficence, as exemplified by the desire to minimize harm, and justice, in which clinicians consider resource allocation and risks that a home discharge poses to family members, caregivers, and home health professionals. However, autonomy is important to consider as well. The concept of dignity of risk highlights the imperative to respect others’ decisions even when they increase the chance of harm, particularly given the overall shift in medicine from paternalism to shared decision making.12 Accommodating patient choice in how and where health care is received allows patients to regain some control over their lives, thereby enhancing their quality of life and promoting patient dignity, especially in their remaining days.13
Discharge Risk Framework
Our risk assessment framework helps clinicians more objectively identify factors that increase or decrease risk, inform discharge planning, partner with patients and families, give patients a prominent role in EOL decisions, and mitigate the risk of a bad outcome. This concept has been used in psychiatry, in which formal suicide assessment includes identifying risk factors and protective factors to estimate suicide risk and determine interventions.14 Similar to suicide risk estimation, this framework is based on clinical judgment rather than a specific calculation.
While this framework serves as a guide for determining and mitigating risk, we encourage teams to consider legal or ethical consultations in challenging cases, such as those in which patients lack both capacity and an involved HCA.
Step 1: Determine the patient’s capacity regarding disposition planning. Patients at EOL are at a higher risk of impaired decision-making capabilities; therefore, capacity evaluation is a critical step.
Step 2: Identify risk factors and protective factors for discharge home. Risk factors are intrinsic and extrinsic factors that increase risk such as functional or sensory impairments. Protective factors are intrinsic and extrinsic factors that decrease risk, including a good understanding of illness and consistent connection with the health care system (Table 1).
Step 3: Determine discharge to home risk level based on identified risk factors and protective factors. Patients may be at low, moderate, or high risk of having an adverse event, such as a fall or inadequate symptom control (Table 2).
Step 4: Identify risk mitigation strategies. These should be tailored to the patient based on the factors identified in Step 2. Examples include home nursing and therapy, mental health treatment, a medical alert system, and frequent contact between the patient and health care team.
Step 5: Meet with inpatient and outpatient HCP teams. Meetings should include the primary care professional (PCP) or relevant subspecialist, such as an oncologist for patients with cancer. For veterans receiving care solely at a local VA medical center, this can be easier to facilitate, but for veterans who receive care through both VA and non-VA systems, this step may require additional coordination. We also recommend including interdisciplinary team members, such as social workers, case managers, and the relevant home care or hospice agency. Certain agencies may decline admission if they perceive increased risk, such as no 24-hour care, perceived self-neglect, and limited instrumental support. During this meeting, HCPs discuss risk mitigation strategies identified in Step 4 and create a plan to propose to patients and families.
Step 6: Meet with patient, HCA, and family members. In addition to sharing information about prognosis, assessing caregiver capabilities and burden can guide conversations about discharge. The discharge plan should be determined through shared decision making.11 If the patient lacks capacity regarding disposition planning, this should be shared with the HCA. However, even when patients lack capacity, it is important to continue to engage them to understand their goals and preferences.
Step 7: Maximize risk mitigation strategies. If a moderate- or high-risk discharge is requested, the health care team should maximize risk mitigation strategies. For low-risk discharges, risk mitigation strategies can still promote safety, especially since risk increases as patients progress toward EOL. In some instances, patients, their HCAs, or caregivers may decline all risk mitigation strategies despite best efforts to communicate and negotiate options. In such circumstances, we recommend discussing the case with the outpatient team for a warm handoff. HCPs should also document all efforts (helpful from a legal standpoint as well as for the patient’s future treatment teams) and respect the decision to discharge home.
Applying the Framework
Our patient Joe provides a good illustration of how to implement this EOL framework. He was deemed to have the capacity to make decisions regarding discharge (Step 1). We determined his risk factors and protective factors for discharge (Step 2). His poor functional status, limited instrumental support, heavy alcohol use, rejection of home services, and communication barriers due to severe hearing impairment all increased his risk. Protective factors included an appreciation of functional limitations, intact cognition, and an involved HCA. Based on his limited instrumental support and poor function but good insight into limitations, discharge home was deemed to be of moderate risk (Step 3). Although risk factors such as alcohol use and severe hearing impairment could have raised his level to high risk, we felt that his involved HCA maintained him in the moderate-risk category.
We worked with the hospitalist team, PT, and audiology to identify multiple risk mitigation strategies: frequent phone calls between the HCA and outpatient palliative care team, home PT to improve transfers from bed to bedside commode, home nursing services either through a routine agency or hospice, and hearing aids for better communication (Steps 4 and 5). We then proposed these strategies to Joe and his HCA (Step 6). Due to concerns about infringement on his independence, Joe declined all home services but agreed to twice-daily check-ins by his HCA, frequent communication between his HCA and our team, and new hearing aids.
Joe returned home with the agreed-upon risk mitigation strategies in place (Step 7). Despite clinicians’ original reservations about sending Joe home without formal services, his HCA maintained close contact with our team, noting that Joe remained stable and happy to be at home in the months following discharge.
Conclusions
Fortunately, VA HCPs operate in an integrated health care system with access to psychological, social, and at-home medical support that can help mitigate risks. Still, we have benefitted from having a tool to help us evaluate risk systematically. Even if patients, families, and HCPs disagree on ideal discharge plans, this tool helps clinicians approach discharges methodically while maintaining open communication and partnership with patients. In doing so, our framework reflects the shift in medical culture from a patriarchal approach to shared decision-making practices regarding all aspects of medical care. Furthermore, we hope that this can help reduce clinician moral distress stemming from these challenging cases.
Future research on best practices for discharge risk assessment and optimizing home safety are needed. We also hope to evaluate the impact and effectiveness of our framework through interviews with key stakeholders. For Joe and other veterans like him, where to spend their final days may be the last important decision they make in life, and our framework allows for their voices to be better heard throughout the decision-making process.
Acknowledgments
We thank Brooke Lifland, MD, for her theoretical contributions to the concept behind this paper.
1. Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press (US); March 19, 2015.
2. Casarett D, Pickard A, Amos Bailey F, et al. Important aspects of end-of-life care among veterans: implications for measurement and quality improvement. J Pain Symptom Manage. 2008;35(2):115-125. doi:10.1016/j.jpainsymman.2007.03.008
3. Kolva E, Rosenfeld B, Brescia R, Comfort C. Assessing decision-making capacity at end of life. Gen Hosp Psychiatry. 2014;36(4):392-397. doi:10.1016/j.genhosppsych.2014.02.013
4. Kolva E, Rosenfeld B, Saracino R. Assessing the decision-making capacity of terminally ill patients with cancer. Am J Geriatr Psychiatry. 2018;26(5):523-531. doi:10.1016/j.jagp.2017.11.012
5. Macmillan MS. Hospital staff’s perceptions of risk associated with the discharge of elderly people from acute hospital care. J Adv Nurs. 1994;19(2):249-256. doi:10.1111/j.1365-2648.1994.tb01078.x
6. Coombs MA, Parker R, de Vries K. Managing risk during care transitions when approaching end of life: A qualitative study of patients’ and health care professionals’ decision making. Palliat Med. 2017;31(7):617-624. doi:10.1177/0269216316673476
7. Hyslop B. ‘Not safe for discharge’? Words, values, and person-centred care. Age Ageing. 2020;49(3):334-336. doi:10.1093/ageing/afz170
8. Goodacre S. Safe discharge: an irrational, unhelpful and unachievable concept. Emerg Med J. 2006;23(10):753-755. doi:10.1136/emj.2006.037903
9. Swidler RN, Seastrum T, Shelton W. Difficult hospital inpatient discharge decisions: ethical, legal and clinical practice issues. Am J Bioeth. 2007;7(3):23-28. doi:10.1080/15265160601171739
10. Hill J, Filer W. Safety and ethical considerations in discharging patients to suboptimal living situations. AMA J Ethics. 2015;17(6):506-510. Published 2015 Jun 1. doi:10.1001/journalofethics.2015.17.6.ecas2-1506
11. West JC. What is an ethically informed approach to managing patient safety risk during discharge planning?. AMA J Ethics. 2020;22(11):E919-E923. Published 2020 Nov 1. doi:10.1001/amajethics.2020.919
12. Mukherjee D. Discharge decisions and the dignity of risk. Hastings Cent Rep. 2015;45(3):7-8. doi:10.1002/hast.441
13. Wheatley VJ, Baker JI. “Please, I want to go home”: ethical issues raised when considering choice of place of care in palliative care. Postgrad Med J. 2007;83(984):643-648. doi:10.1136/pgmj.2007.058487
14. Work Group on Suicidal Behaviors. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(suppl 11):1-60.
1. Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press (US); March 19, 2015.
2. Casarett D, Pickard A, Amos Bailey F, et al. Important aspects of end-of-life care among veterans: implications for measurement and quality improvement. J Pain Symptom Manage. 2008;35(2):115-125. doi:10.1016/j.jpainsymman.2007.03.008
3. Kolva E, Rosenfeld B, Brescia R, Comfort C. Assessing decision-making capacity at end of life. Gen Hosp Psychiatry. 2014;36(4):392-397. doi:10.1016/j.genhosppsych.2014.02.013
4. Kolva E, Rosenfeld B, Saracino R. Assessing the decision-making capacity of terminally ill patients with cancer. Am J Geriatr Psychiatry. 2018;26(5):523-531. doi:10.1016/j.jagp.2017.11.012
5. Macmillan MS. Hospital staff’s perceptions of risk associated with the discharge of elderly people from acute hospital care. J Adv Nurs. 1994;19(2):249-256. doi:10.1111/j.1365-2648.1994.tb01078.x
6. Coombs MA, Parker R, de Vries K. Managing risk during care transitions when approaching end of life: A qualitative study of patients’ and health care professionals’ decision making. Palliat Med. 2017;31(7):617-624. doi:10.1177/0269216316673476
7. Hyslop B. ‘Not safe for discharge’? Words, values, and person-centred care. Age Ageing. 2020;49(3):334-336. doi:10.1093/ageing/afz170
8. Goodacre S. Safe discharge: an irrational, unhelpful and unachievable concept. Emerg Med J. 2006;23(10):753-755. doi:10.1136/emj.2006.037903
9. Swidler RN, Seastrum T, Shelton W. Difficult hospital inpatient discharge decisions: ethical, legal and clinical practice issues. Am J Bioeth. 2007;7(3):23-28. doi:10.1080/15265160601171739
10. Hill J, Filer W. Safety and ethical considerations in discharging patients to suboptimal living situations. AMA J Ethics. 2015;17(6):506-510. Published 2015 Jun 1. doi:10.1001/journalofethics.2015.17.6.ecas2-1506
11. West JC. What is an ethically informed approach to managing patient safety risk during discharge planning?. AMA J Ethics. 2020;22(11):E919-E923. Published 2020 Nov 1. doi:10.1001/amajethics.2020.919
12. Mukherjee D. Discharge decisions and the dignity of risk. Hastings Cent Rep. 2015;45(3):7-8. doi:10.1002/hast.441
13. Wheatley VJ, Baker JI. “Please, I want to go home”: ethical issues raised when considering choice of place of care in palliative care. Postgrad Med J. 2007;83(984):643-648. doi:10.1136/pgmj.2007.058487
14. Work Group on Suicidal Behaviors. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(suppl 11):1-60.
Where Have All the Future Veterans Gone?
Word to the Nation: Guard zealously your right to serve in the Armed Forces, for without them, there will be no other rights to guard.
John F. Kennedy 1
The title of this Veterans Day editorial is a paraphrase of the legendary folk artist Pete Seeger’s protest song popularized during the Vietnam War. On January 27, 1973, in the wake of the widespread antiwar movement, Secretary of Defense Melvin Laird announced an end to the dreaded draft.2
For nearly 50 years, the all-volunteer military was celebrated as an outstanding achievement that professionalized the armed services and arguably made the US military among the most highly trained and effective fighting forces in the world. That was until an ongoing recruitment crisis threatened to write a different and far more disturbing conclusion to what the government had heralded as a “success story.”3
The recruiting crisis is a complicated problem with many facets that have received increasing attention from journalists, the media, experts, think tanks, and the government. Given this complexity, this will be a 2-part editorial: This column examines the scope of the crisis and the putative causes of the problem with recruiting Americans to serve in uniform. The next column will examine the potential impact of the shortage of service members on federal health care practice.
The Recruiting Crisis
Over the past several years, nearly every branch of the armed forces has struggled with recruitment, especially the Army. In April of this year, the US Department of Defense (DoD) reported that the Army, Navy, and Air Force would all fail to meet recruitment goals; only the Marines and Space Forces were expected to reach their targets.4 At the end of its fiscal year (October 1), the Army acknowledged that its 55,000 recruits were 10,000 fewer soldiers than it had aimed to enlist.5 But this was still more people joining the ranks than in 2022 when the Army was 15,000 recruits below the mark.6
Challenging Trends
There are many putative causes and proposed solutions for the recruitment crisis. Among the most serious is a marked drop in the American public’s confidence in the military. A June 2023 Gallup poll found that only 60% of citizens expressed “a great deal” or “quite a lot” of confidence in the military. This was the nadir of a 5-year decline that this year reached the lowest point since 1997/1998.7 For many Americans in and out of uniform, the ignoble end to the long war in Afghanistan leaving behind friends and allies contrary to the military ethos is cited as a significant contributor to both the loss of confidence in the military and the recruiting crisis.8
These cultural developments reinforce each other. Now, many veterans do not want their relatives and friends to follow them into the armed services. A 2021 survey by the Military Family Advisory Network found that slightly more than 60% of veterans and active-duty service members would recommend a military career to a potential recruit. This was down from 75% in 2019.9 Veterans cite a variety of reasons for discouraging their fellow citizens from serving, including low pay compared with civilian employment, especially in a labor-hungry job market; and the military failure to fulfill health care promises, housing, and other social services, especially for the growing number experiencing mental health disorders related to their service.10
Two facts about recruitment heighten the negative impact of some veterans’ change of attitude toward joining the services. First, since the end of the draft, military life in the US has become a family tradition. Published in 2011, a Pew Research Center study found that even then, a decreasing number of Americans had a family connection to the military. More respondents aged ≥ 50 years had a parent, child, spouse, or sibling who had served compared with those aged 30 to 49 years and those aged 18 to 29 (77%, 57%, and 33%, respectively).11 Second, since the end of the draft, far fewer Americans have had military experience. Only 1% of the nation is currently in military service, and the veteran population is steadily declining. In 1980, 18% of adult Americans were veterans; 20 years later, that number is only 7%.12 This makes it less likely that a high school or college student will have a personal or even a passing relationship with a teacher, coach, or other mentoring adult who is or has been a military member. This demographic discrepancy has generated what sociologists call the military-civilian gap.10 That division has been manipulated in the increasingly vehement culture wars and generational struggles that are splitting the country.12
This relatively recent sociological trend is reflected in a growing lack of interest among many young Americans in armed forces service. A DoD survey of participants aged 16 to 24 years regarding their intention to serve in the military found that 89% were probably not going to pursue a career in uniform. More than 65% of respondents indicated that the possibility of physical injury, death, or psychological trauma was the primary deterrent for considering enlisting.13 The latter barrier is directly related to our work as practitioners caring for service members and veterans, and through our compassion and competence, we may help bridge the widening divide between the military and civilian spheres. These numbers speak to the unwilling; there is also a significant group of Americans who want to serve yet are unable to due to their history, diagnoses, or condition.14 Their motivation to be military members in the face of the recruitment challenges highlighted here present federal practitioners with ethical questions that will be the subject of the next column.
Armed Forces and Veterans Day
This column’s epigraph is from President John F. Kennedy, a decorated World War II Navy combat veteran who decreed Armed Forces Day an official holiday a decade before conscription ended.1 The commemoration was to thank and honor all individuals currently serving in the military for their patriotism and sacrifice. President Kennedy’s Word to the Nation could not be timelier on Veterans Day 2023. The data reviewed here raise profound questions as to where tomorrow’s service members and the veterans of the future will come from, and how we will persuade them that though there are real risks to military service, the rewards are both tangible and transcendent.
1. US Department of Defense. Armed Forces Day. Accessed October 17, 2023. https://afd.defense.gov/History
2. Zipkin A. The military draft ended 50 years ago, dividing a generation. The Washington Post. January 27, 2023. Accessed October 17, 2023. https://www.washingtonpost.com/history/2023/01/27/draft-end-conscription-1973
3. Lopez TC. All-volunteer force proves successful for U.S. military. March 2, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3316678/all-volunteer-force-proves-successful-for-us-military
4. Garamone J. Vice-chiefs talk recruiting shortfalls, readiness issues. April 20, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3369472/vice-chiefs-talk-recruiting-shortfalls-readiness-issues
5. Winkie D. Army recruiters at two-thirds of contract goals as the fiscal year closes. Military Times. September 7, 2023. Accessed October 17, 2023. https://www.armytimes.com/news/recruiting/2023/09/07/army-recruiters-at-two-thirds-of-contract-goals-as-fiscal-year-closes
6. Baldor LC. Army misses recruiting goal by 15,000 soldiers. Accessed October 17, 2023. https://www.armytimes.com/news/your-army/2022/10/02/army-misses-recruiting-goal-by-15000-soldiers
7. Younis M. Confidence in U.S. military lowest in over two decades. Accessed October 17, 2023. https://news.gallup.com/poll/509189/confidence-military-lowest-two-decades.aspx
8. Rogin A, Corkery A. Why recruiting and confidence in America’s armed forces is so low right now? Accessed October 17, 2023. https://www.pbs.org/newshour/show/why-recruiting-and-confidence-in-americas-armed-forces-is-so-low-right-now
9. Military Family Advisory Network. 2021 military family support programming survey. Accessed October 17, 2023. https://www.mfan.org/wp-content/uploads/2022/07/Executive-Summary-MFAN-Programming-Survey-Results-2021.pdf
10. Kesling B. The military recruiting crisis: even veterans don’t want their family to join. Wall Street Journal. 30 June 2023. Accessed October 17, 2023. https://www.wsj.com/articles/military-recruiting-crisis-veterans-dont-want-their-children-to-join-510e1a25
11. Pew Research Center. The military-civilian gap: fewer family connections. Accessed October 17, 2023. https://www.pewresearch.org/social-trends/2011/11/23/the-military-civilian-gap-fewer-family-connections
12. Myers M. Is the military too ‘woke’ to recruit? Accessed October 17, 2023. https://www.militarytimes.com/news/your-military/2022/10/13/is-the-military-too-woke-to-recruit
13. Schaeffer K. The changing face of America’s veteran population. Accessed October 17, 2023. https://www.pewresearch.org/short-reads/2021/04/05/the-changing-face-of-americas-veteran-population
14. Phillips D. With few able and fewer willing, U.S. military can’t find recruits. New York Times. July 14, 2023. Accessed October 17, 2023. https://www.nytimes.com/2022/07/14/us/us-military-recruiting-enlistment.html
Word to the Nation: Guard zealously your right to serve in the Armed Forces, for without them, there will be no other rights to guard.
John F. Kennedy 1
The title of this Veterans Day editorial is a paraphrase of the legendary folk artist Pete Seeger’s protest song popularized during the Vietnam War. On January 27, 1973, in the wake of the widespread antiwar movement, Secretary of Defense Melvin Laird announced an end to the dreaded draft.2
For nearly 50 years, the all-volunteer military was celebrated as an outstanding achievement that professionalized the armed services and arguably made the US military among the most highly trained and effective fighting forces in the world. That was until an ongoing recruitment crisis threatened to write a different and far more disturbing conclusion to what the government had heralded as a “success story.”3
The recruiting crisis is a complicated problem with many facets that have received increasing attention from journalists, the media, experts, think tanks, and the government. Given this complexity, this will be a 2-part editorial: This column examines the scope of the crisis and the putative causes of the problem with recruiting Americans to serve in uniform. The next column will examine the potential impact of the shortage of service members on federal health care practice.
The Recruiting Crisis
Over the past several years, nearly every branch of the armed forces has struggled with recruitment, especially the Army. In April of this year, the US Department of Defense (DoD) reported that the Army, Navy, and Air Force would all fail to meet recruitment goals; only the Marines and Space Forces were expected to reach their targets.4 At the end of its fiscal year (October 1), the Army acknowledged that its 55,000 recruits were 10,000 fewer soldiers than it had aimed to enlist.5 But this was still more people joining the ranks than in 2022 when the Army was 15,000 recruits below the mark.6
Challenging Trends
There are many putative causes and proposed solutions for the recruitment crisis. Among the most serious is a marked drop in the American public’s confidence in the military. A June 2023 Gallup poll found that only 60% of citizens expressed “a great deal” or “quite a lot” of confidence in the military. This was the nadir of a 5-year decline that this year reached the lowest point since 1997/1998.7 For many Americans in and out of uniform, the ignoble end to the long war in Afghanistan leaving behind friends and allies contrary to the military ethos is cited as a significant contributor to both the loss of confidence in the military and the recruiting crisis.8
These cultural developments reinforce each other. Now, many veterans do not want their relatives and friends to follow them into the armed services. A 2021 survey by the Military Family Advisory Network found that slightly more than 60% of veterans and active-duty service members would recommend a military career to a potential recruit. This was down from 75% in 2019.9 Veterans cite a variety of reasons for discouraging their fellow citizens from serving, including low pay compared with civilian employment, especially in a labor-hungry job market; and the military failure to fulfill health care promises, housing, and other social services, especially for the growing number experiencing mental health disorders related to their service.10
Two facts about recruitment heighten the negative impact of some veterans’ change of attitude toward joining the services. First, since the end of the draft, military life in the US has become a family tradition. Published in 2011, a Pew Research Center study found that even then, a decreasing number of Americans had a family connection to the military. More respondents aged ≥ 50 years had a parent, child, spouse, or sibling who had served compared with those aged 30 to 49 years and those aged 18 to 29 (77%, 57%, and 33%, respectively).11 Second, since the end of the draft, far fewer Americans have had military experience. Only 1% of the nation is currently in military service, and the veteran population is steadily declining. In 1980, 18% of adult Americans were veterans; 20 years later, that number is only 7%.12 This makes it less likely that a high school or college student will have a personal or even a passing relationship with a teacher, coach, or other mentoring adult who is or has been a military member. This demographic discrepancy has generated what sociologists call the military-civilian gap.10 That division has been manipulated in the increasingly vehement culture wars and generational struggles that are splitting the country.12
This relatively recent sociological trend is reflected in a growing lack of interest among many young Americans in armed forces service. A DoD survey of participants aged 16 to 24 years regarding their intention to serve in the military found that 89% were probably not going to pursue a career in uniform. More than 65% of respondents indicated that the possibility of physical injury, death, or psychological trauma was the primary deterrent for considering enlisting.13 The latter barrier is directly related to our work as practitioners caring for service members and veterans, and through our compassion and competence, we may help bridge the widening divide between the military and civilian spheres. These numbers speak to the unwilling; there is also a significant group of Americans who want to serve yet are unable to due to their history, diagnoses, or condition.14 Their motivation to be military members in the face of the recruitment challenges highlighted here present federal practitioners with ethical questions that will be the subject of the next column.
Armed Forces and Veterans Day
This column’s epigraph is from President John F. Kennedy, a decorated World War II Navy combat veteran who decreed Armed Forces Day an official holiday a decade before conscription ended.1 The commemoration was to thank and honor all individuals currently serving in the military for their patriotism and sacrifice. President Kennedy’s Word to the Nation could not be timelier on Veterans Day 2023. The data reviewed here raise profound questions as to where tomorrow’s service members and the veterans of the future will come from, and how we will persuade them that though there are real risks to military service, the rewards are both tangible and transcendent.
Word to the Nation: Guard zealously your right to serve in the Armed Forces, for without them, there will be no other rights to guard.
John F. Kennedy 1
The title of this Veterans Day editorial is a paraphrase of the legendary folk artist Pete Seeger’s protest song popularized during the Vietnam War. On January 27, 1973, in the wake of the widespread antiwar movement, Secretary of Defense Melvin Laird announced an end to the dreaded draft.2
For nearly 50 years, the all-volunteer military was celebrated as an outstanding achievement that professionalized the armed services and arguably made the US military among the most highly trained and effective fighting forces in the world. That was until an ongoing recruitment crisis threatened to write a different and far more disturbing conclusion to what the government had heralded as a “success story.”3
The recruiting crisis is a complicated problem with many facets that have received increasing attention from journalists, the media, experts, think tanks, and the government. Given this complexity, this will be a 2-part editorial: This column examines the scope of the crisis and the putative causes of the problem with recruiting Americans to serve in uniform. The next column will examine the potential impact of the shortage of service members on federal health care practice.
The Recruiting Crisis
Over the past several years, nearly every branch of the armed forces has struggled with recruitment, especially the Army. In April of this year, the US Department of Defense (DoD) reported that the Army, Navy, and Air Force would all fail to meet recruitment goals; only the Marines and Space Forces were expected to reach their targets.4 At the end of its fiscal year (October 1), the Army acknowledged that its 55,000 recruits were 10,000 fewer soldiers than it had aimed to enlist.5 But this was still more people joining the ranks than in 2022 when the Army was 15,000 recruits below the mark.6
Challenging Trends
There are many putative causes and proposed solutions for the recruitment crisis. Among the most serious is a marked drop in the American public’s confidence in the military. A June 2023 Gallup poll found that only 60% of citizens expressed “a great deal” or “quite a lot” of confidence in the military. This was the nadir of a 5-year decline that this year reached the lowest point since 1997/1998.7 For many Americans in and out of uniform, the ignoble end to the long war in Afghanistan leaving behind friends and allies contrary to the military ethos is cited as a significant contributor to both the loss of confidence in the military and the recruiting crisis.8
These cultural developments reinforce each other. Now, many veterans do not want their relatives and friends to follow them into the armed services. A 2021 survey by the Military Family Advisory Network found that slightly more than 60% of veterans and active-duty service members would recommend a military career to a potential recruit. This was down from 75% in 2019.9 Veterans cite a variety of reasons for discouraging their fellow citizens from serving, including low pay compared with civilian employment, especially in a labor-hungry job market; and the military failure to fulfill health care promises, housing, and other social services, especially for the growing number experiencing mental health disorders related to their service.10
Two facts about recruitment heighten the negative impact of some veterans’ change of attitude toward joining the services. First, since the end of the draft, military life in the US has become a family tradition. Published in 2011, a Pew Research Center study found that even then, a decreasing number of Americans had a family connection to the military. More respondents aged ≥ 50 years had a parent, child, spouse, or sibling who had served compared with those aged 30 to 49 years and those aged 18 to 29 (77%, 57%, and 33%, respectively).11 Second, since the end of the draft, far fewer Americans have had military experience. Only 1% of the nation is currently in military service, and the veteran population is steadily declining. In 1980, 18% of adult Americans were veterans; 20 years later, that number is only 7%.12 This makes it less likely that a high school or college student will have a personal or even a passing relationship with a teacher, coach, or other mentoring adult who is or has been a military member. This demographic discrepancy has generated what sociologists call the military-civilian gap.10 That division has been manipulated in the increasingly vehement culture wars and generational struggles that are splitting the country.12
This relatively recent sociological trend is reflected in a growing lack of interest among many young Americans in armed forces service. A DoD survey of participants aged 16 to 24 years regarding their intention to serve in the military found that 89% were probably not going to pursue a career in uniform. More than 65% of respondents indicated that the possibility of physical injury, death, or psychological trauma was the primary deterrent for considering enlisting.13 The latter barrier is directly related to our work as practitioners caring for service members and veterans, and through our compassion and competence, we may help bridge the widening divide between the military and civilian spheres. These numbers speak to the unwilling; there is also a significant group of Americans who want to serve yet are unable to due to their history, diagnoses, or condition.14 Their motivation to be military members in the face of the recruitment challenges highlighted here present federal practitioners with ethical questions that will be the subject of the next column.
Armed Forces and Veterans Day
This column’s epigraph is from President John F. Kennedy, a decorated World War II Navy combat veteran who decreed Armed Forces Day an official holiday a decade before conscription ended.1 The commemoration was to thank and honor all individuals currently serving in the military for their patriotism and sacrifice. President Kennedy’s Word to the Nation could not be timelier on Veterans Day 2023. The data reviewed here raise profound questions as to where tomorrow’s service members and the veterans of the future will come from, and how we will persuade them that though there are real risks to military service, the rewards are both tangible and transcendent.
1. US Department of Defense. Armed Forces Day. Accessed October 17, 2023. https://afd.defense.gov/History
2. Zipkin A. The military draft ended 50 years ago, dividing a generation. The Washington Post. January 27, 2023. Accessed October 17, 2023. https://www.washingtonpost.com/history/2023/01/27/draft-end-conscription-1973
3. Lopez TC. All-volunteer force proves successful for U.S. military. March 2, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3316678/all-volunteer-force-proves-successful-for-us-military
4. Garamone J. Vice-chiefs talk recruiting shortfalls, readiness issues. April 20, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3369472/vice-chiefs-talk-recruiting-shortfalls-readiness-issues
5. Winkie D. Army recruiters at two-thirds of contract goals as the fiscal year closes. Military Times. September 7, 2023. Accessed October 17, 2023. https://www.armytimes.com/news/recruiting/2023/09/07/army-recruiters-at-two-thirds-of-contract-goals-as-fiscal-year-closes
6. Baldor LC. Army misses recruiting goal by 15,000 soldiers. Accessed October 17, 2023. https://www.armytimes.com/news/your-army/2022/10/02/army-misses-recruiting-goal-by-15000-soldiers
7. Younis M. Confidence in U.S. military lowest in over two decades. Accessed October 17, 2023. https://news.gallup.com/poll/509189/confidence-military-lowest-two-decades.aspx
8. Rogin A, Corkery A. Why recruiting and confidence in America’s armed forces is so low right now? Accessed October 17, 2023. https://www.pbs.org/newshour/show/why-recruiting-and-confidence-in-americas-armed-forces-is-so-low-right-now
9. Military Family Advisory Network. 2021 military family support programming survey. Accessed October 17, 2023. https://www.mfan.org/wp-content/uploads/2022/07/Executive-Summary-MFAN-Programming-Survey-Results-2021.pdf
10. Kesling B. The military recruiting crisis: even veterans don’t want their family to join. Wall Street Journal. 30 June 2023. Accessed October 17, 2023. https://www.wsj.com/articles/military-recruiting-crisis-veterans-dont-want-their-children-to-join-510e1a25
11. Pew Research Center. The military-civilian gap: fewer family connections. Accessed October 17, 2023. https://www.pewresearch.org/social-trends/2011/11/23/the-military-civilian-gap-fewer-family-connections
12. Myers M. Is the military too ‘woke’ to recruit? Accessed October 17, 2023. https://www.militarytimes.com/news/your-military/2022/10/13/is-the-military-too-woke-to-recruit
13. Schaeffer K. The changing face of America’s veteran population. Accessed October 17, 2023. https://www.pewresearch.org/short-reads/2021/04/05/the-changing-face-of-americas-veteran-population
14. Phillips D. With few able and fewer willing, U.S. military can’t find recruits. New York Times. July 14, 2023. Accessed October 17, 2023. https://www.nytimes.com/2022/07/14/us/us-military-recruiting-enlistment.html
1. US Department of Defense. Armed Forces Day. Accessed October 17, 2023. https://afd.defense.gov/History
2. Zipkin A. The military draft ended 50 years ago, dividing a generation. The Washington Post. January 27, 2023. Accessed October 17, 2023. https://www.washingtonpost.com/history/2023/01/27/draft-end-conscription-1973
3. Lopez TC. All-volunteer force proves successful for U.S. military. March 2, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3316678/all-volunteer-force-proves-successful-for-us-military
4. Garamone J. Vice-chiefs talk recruiting shortfalls, readiness issues. April 20, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3369472/vice-chiefs-talk-recruiting-shortfalls-readiness-issues
5. Winkie D. Army recruiters at two-thirds of contract goals as the fiscal year closes. Military Times. September 7, 2023. Accessed October 17, 2023. https://www.armytimes.com/news/recruiting/2023/09/07/army-recruiters-at-two-thirds-of-contract-goals-as-fiscal-year-closes
6. Baldor LC. Army misses recruiting goal by 15,000 soldiers. Accessed October 17, 2023. https://www.armytimes.com/news/your-army/2022/10/02/army-misses-recruiting-goal-by-15000-soldiers
7. Younis M. Confidence in U.S. military lowest in over two decades. Accessed October 17, 2023. https://news.gallup.com/poll/509189/confidence-military-lowest-two-decades.aspx
8. Rogin A, Corkery A. Why recruiting and confidence in America’s armed forces is so low right now? Accessed October 17, 2023. https://www.pbs.org/newshour/show/why-recruiting-and-confidence-in-americas-armed-forces-is-so-low-right-now
9. Military Family Advisory Network. 2021 military family support programming survey. Accessed October 17, 2023. https://www.mfan.org/wp-content/uploads/2022/07/Executive-Summary-MFAN-Programming-Survey-Results-2021.pdf
10. Kesling B. The military recruiting crisis: even veterans don’t want their family to join. Wall Street Journal. 30 June 2023. Accessed October 17, 2023. https://www.wsj.com/articles/military-recruiting-crisis-veterans-dont-want-their-children-to-join-510e1a25
11. Pew Research Center. The military-civilian gap: fewer family connections. Accessed October 17, 2023. https://www.pewresearch.org/social-trends/2011/11/23/the-military-civilian-gap-fewer-family-connections
12. Myers M. Is the military too ‘woke’ to recruit? Accessed October 17, 2023. https://www.militarytimes.com/news/your-military/2022/10/13/is-the-military-too-woke-to-recruit
13. Schaeffer K. The changing face of America’s veteran population. Accessed October 17, 2023. https://www.pewresearch.org/short-reads/2021/04/05/the-changing-face-of-americas-veteran-population
14. Phillips D. With few able and fewer willing, U.S. military can’t find recruits. New York Times. July 14, 2023. Accessed October 17, 2023. https://www.nytimes.com/2022/07/14/us/us-military-recruiting-enlistment.html