User login
Insulin Injection-Site Acanthosis Nigricans: Skin Reactions and Clinical Implications
Insulin injection therapy is one of the most widely used health care interventions to manage both type 1 and type 2 diabetes mellitus (T1DM/T2DM). Globally, more than 150 to 200 million people inject insulin into their upper posterior arms, buttocks, anterior and lateral thighs, or abdomen.1,2 In an ideal world, every patient would be using the correct site and rotating their insulin injection sites in accordance with health care professional (HCP) recommendations—systematic injections in one general body location, at least 1 cm away from the previous injection.2 Unfortunately, same-site insulin injection (repeatedly in the same region within 1 cm of previous injections) is a common mistake made by patients with DM—in one study, 63% of participants either did not rotate sites correctly or failed to do so at all.
Insulin-resistant cutaneous complications may occur as a result of same-site insulin injections. The most common is lipohypertrophy, reported in some studies in nearly 50% of patients with DM on insulin therapy.4 Other common cutaneous complications include lipoatrophy and amyloidosis. Injection-site acanthosis nigricans, although uncommon, has been reported in 18 cases in the literature.
Most articles suggest that same-site insulin injections decrease local insulin sensitivity and result in tissue hypertrophy because of the anabolic properties of insulin and increase in insulin binding to insulin-like growth factor-1 (IGF-1) receptor.5-20 The hyperkeratotic growth and varying insulin absorption rates associated with these cutaneous complications increase chances of either hyper- or hypoglycemic episodes in patients.10,11,13 It is the responsibility of the DM care professional to provide proper insulin-injection technique education and perform routine inspection of injection sites to reduce cutaneous complications of insulin therapy. The purpose of this article is to (1) describe a case of acanthosis nigricans resulting from insulin injection at the same site; (2) review case reports
Case Presentation
A 75-year-old patient with an 8-year history of T2DM, as well as stable coronary artery disease, atrial fibrillation, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and stage 3 chronic kidney disease, presented with 2 discrete abdominal hyperpigmented plaques. At the time of the initial clinic visit, the patient was taking metformin 1000 mg twice daily and insulin glargine 40 units once daily. When insulin was initiated 7 years prior, the patient received
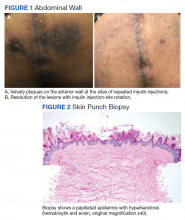
The patient reported 5 years of progressive, asymptomatic hyperpigmentation of the skin surrounding his insulin glargine injection sites and injecting in these same sites daily without rotation. He reported no additional skin changes or symptoms. He had noticed no skin changes while using NPH insulin during his first year of insulin therapy. On examination, the abdominal wall skin demonstrated 2 well-demarcated, nearly black, soft, velvety plaques, measuring 9 × 8 cm on the left side and 4 × 3.5 cm on the right, suggesting acanthosis nigricans (Figure 1A). The remainder of the skin examination, including the flexures, was normal. Of note, the patient received biweekly intramuscular testosterone injections in the gluteal region for secondary hypogonadism with no adverse dermatologic effects. A skin punch biopsy was performed and revealed epidermal papillomatosis and hyperkeratosis, confirming the clinical diagnosis of acanthosis nigricans (Figure 2).
After a review of insulin-injection technique at his clinic visit, the patient started rotating insulin injection sites over his entire abdomen, and the acanthosis nigricans partially improved. A few months later, the patient stopped rotating the insulin injection site, and the acanthosis nigricans worsened again. Because of worsening glycemic control, the patient was then started on insulin aspart. He did not develop any skin changes at the insulin aspart injection site, although he was not rotating its site of injection.
Subsequently, with reeducation and proper injection-site rotation, the patient had resolution of his acanthosis nigricans (Figure 1b).
Discussion
A review of the literature revealed 18 reported cases of acanthosis nigricans at sites of repeated insulin injection (Table).5-20 Acanthosis nigricans at the site of insulin injection afflicts patients of any age, with cases observed in patients aged 14 to 75 years. Sixteen (84%) of 19 cases were male. Fourteen cases (73%) had T2DM; the rest of the patients had T1DM. The duration of insulin injection therapy prior to onset ranged from immediate to 13 years (median 4 years). Fourteen cases (73%) were reported on the abdomen; however, other sites, such as thighs and upper arm, also were reported. Lesions size varied from 12 to 360 cm2. Two cases had associated amyloidosis. The average HbA1c reported at presentation was 10%. Following insulin injection-site rotation, most of the cases reported improvement of both glycemic control and acanthosis nigricans appearance.
In the case described by Kudo and colleagues, a 59-year-old male patient with T2DM had been injecting insulin into the same spot on his abdomen for 10 years. He developed acanthosis nigricans and an amyloidoma so large and firm that it bent the needle when he injected insulin.11
Most of the cases we found in the literature were after 2005 and associated with the use of human or analog insulin. These cases may be related to a bias, as cases may be easier to find in digital archives in the later years, when human or analog insulins have been in common use. Also noteworthy, in cases that reported dosage, most were not very high, and the highest daily dose was 240 IU/d. Ten reports of injection-site acanthosis nigricans were in dermatology journals; only 5 reports were in endocrinology journals and 3 in general medical journals, indicating possible less awareness of this phenomenon in other HCPs who care for patients with DM.
Complications of Same-Site Injections
Acanthosis nigricans. Commonly found in the armpits, neck folds, and groin, acanthosis nigricans is known as one of the calling cards for insulin resistance, obesity, and hyperinsulinemia.21 Acanthosis nigricans can be seen in people with or without DM and is not limited to those on insulin therapy. However, same-site insulin injections for 4 to 6 years also may result in injection-site acanthosis nigricans–like lesions because of factors such as insulin exposure at the local tissue level.16
Acanthosis nigricans development is characterized by hyperpigmented, hyperkeratotic, velvety, and sometimes verrucous plaques.6 Acanthosis nigricans surrounding repeated injection sites is hypothesized to develop as a result of localized hyperinsulinemia secondary to insulin resistance, which increases the stimulation of IGF, thereby causing epidermal hypertrophy.5-20 If insulin injection therapy continues to be administered through the acanthosis nigricans lesion, it results in decreased insulin absorption, leading to poor glycemic control.13
Acanthosis nigricans associated with insulin injection is reversible. After rotation of injection sites, lesions either decrease in size or severity of appearance.5-8,11 Also, by avoiding injection into the hyperkeratotic plaques and using normal subcutaneous tissue for injection, patients’ response to insulin improves, as measured by HbA1c and by decreased daily insulin requirement.6-8,10,12,18-20
Lipohypertrophy. This is characterized by an increase in localized adipose tissue and is the most common cutaneous complication of insulin therapy.2 Lipohypertrophy presents as a firm, rubbery mass in the location of same-site insulin injections.22 Development of lipohypertrophy is suspected to be the result of either (1) anabolic effect of insulin on local adipocytes, promoting fat and protein synthesis; (2) an autoimmune response by immunoglobulin (Ig) G or IgE antibodies to insulin, immune response to insulin of different species, or to insulin injection techniques; or (3) repeated trauma to the injection site from repeated needle usage.4,23
In a study assessing the prevalence of lipohypertrophy and its relation to insulin technique, 49.1% of participants with
Primary prevention measures include injection site inspection and patient education about rotation and abstaining from needle reuse.22 If a patient already has signs of lipohypertrophy, data supports education and insulin injection technique practice as simple and effective means to reduce insulin action variability and increase glycemic control.24
Lipoatrophy. Lipoatrophy is described as a local loss of subcutaneous adipose tissue often in the face, buttocks, legs and arm regions and can be rooted in genetic, immune, or drug-associated etiologies.25 Insulin-induced lipoatrophy is suspected to be the result of tumor necrosis factor-α hyperproduction in reaction to insulin crystal presence at the injection site.26,27 Overall, lipoatrophy development has decreased since the use of recombinant human insulin and analog insulin therapy.28 The decrease is hypothesized to be due to increased subcutaneous tissue absorption rate of human insulin and its analog, decreasing overall adipocyte exposure to localized high insulin concentration.27 Treatments for same-site insulin-derived lipoatrophy include changing injection sites and preparation of insulin.26 When injection into the lipoatrophic site was avoided, glycemic control and lipoatrophy appearance improved.26
Amyloidosis. Amyloidosis indicates the presence of an extracellular bundle of insoluble polymeric protein fibrils in tissues and organs.29 Insulin-induced amyloidosis presents as a hard mass or ball near the injection site.29 Insulin is one of many hormones that can form amyloid fibrils, and there have been several dozen cases reported of amyloid formation at the site of insulin injection.29-31 Although insulin-derived amyloidosis is rare, it may be misdiagnosed as lipohypertrophy due to a lack of histopathologic testing or general awareness of the complication.29
In a case series of 7 patients with amyloidosis, all patients had a mean HbA1c of 9.3% (range, 8.5-10.2%) and averaged 1 IU/kg bodyweight before intervention.30 After the discovery of the mass, participants were instructed to avoid injection into the amyloidoma, and average insulin requirements decreased to 0.48 IU/kg body weight (P = .40).30 Patients with amyloidosis who rotated their injection sites experienced better glycemic control and decreased insulin requirements.30
Pathophysiology of Localized Insulin Resistance
Insulin regulates glucose homeostasis in skeletal muscle and adipose tissue, increases hepatic and adipocyte lipid synthesis, and decreases adipocyte fatty acid release.32 Generalized insulin resistance occurs when target tissues have decreased glucose uptake in response to circulating insulin.32 Insulin resistance increases the amount of free insulin in surrounding tissues. At high concentrations, insulin fosters tissue growth by binding to IGF-1 receptors, stimulating hypertrophy and reproduction of keratinocytes and fibroblasts.33 This pathophysiology helps explain the origin of localized acanthosis nigricans at same-site insulin injections.
Conclusions
Cutaneous complications are a local adverse effect of long-term failure to rotate insulin injection sites. Our case serves as a call to action for HCPs to improve education regarding insulin injection-site rotation, conduct routine injection-site inspection, and actively document cases as they occur to increase public awareness of these important complications.
If a patient with DM presents with unexplained poor glycemic control, consider questioning the patient about injection-site location and how often they are rotating the insulin injection site. Inspect the site for cutaneous complications. Of note, if a patient has a cutaneous complication due to insulin injection, adjust or decrease the insulin dosage when rotating sites to mitigate the risk of hypoglycemic episodes.
Improvement of glycemic control, cosmetic appearance of injection site, and insulin use all begin with skin inspection, injection technique education, and periodic review by a HCP.
1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384
2. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. doi:10.1016/j.mayocp.2016.06.010
3. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. doi:10.1016/j.diabet.2013.05.006
4. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025-2027. doi:10.2337/diacare.28.8.2025
5. Erickson L, Lipschutz DE, Wrigley W, Kearse WO. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209(6):934-935. doi:10.1001/jama.1969.03160190056019
6. Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122(9):1054-1056. doi:10.1001/archderm.1986.01660210104028 7. Gannon D, Ross MW, Mahajan T. Acanthosis nigricans-like plaque and lipohypertrophy in type 1 diabetes. Pract Diabetes International. 2005;22(6).
8. Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144(1):126-127. doi:10.1001/archdermatol.2007.27
9. Pachón Burgos A, Chan Aguilar MP. Visual vignette. Hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14(4):514. doi:10.4158/EP.14.4.514
10. Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94(2):e34-e36. doi:10.1016/j.diabres.2011.07.023
11. Kudo-Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2012;38(1):25-29. doi:10.1111/j.1365-2230.2012.04373.x
12. Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11(12):e85-e87.
13. Kanwar A, Sawatkar G, Dogra S, Bhadada S. Acanthosis nigricans—an uncommon cutaneous adverse effect of a common medication: report of two cases. Indian J Dermatol Venereol Leprol. 2013;79(4):553. doi:10.4103/0378-6323.113112
14. Dhingra M, Garg G, Gupta M, Khurana U, Thami GP. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19(1):9. Published 2013 Jan 15.
15. Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57(1):127-129. doi:10.4103/0377-4929.130920
16. Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39(1):5-9.
17. Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39(12):e163. doi:10.1097/DAD.0000000000000659
18. Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190(47):E1390. doi:10.1503/cmaj.180705
19. Pal R, Bhattacharjee R, Chatterjee D, Bhadada SK, Bhansali A, Dutta P. Exogenous insulin-induced localized acanthosis nigricans: a rare injection site complication. Can J Diabetes. 2020;44(3):219-221. doi:10.1016/j.jcjd.2019.08.010
20. Bomar L, Lewallen R, Jorizzo J. Localized acanthosis nigricans at the site of repetitive insulin injections. Cutis. 2020;105(2);E20-E22.
21. Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48-53. doi:10.1016/j.clindermatol.2017.09.008
22. Kalra S, Kumar A, Gupta Y. Prevention of lipohypertrophy. J Pak Med Assoc. 2016;66(7):910-911.
23. Singha A, Bhattarcharjee R, Ghosh S, Chakrabarti SK, Baidya A, Chowdhury S. Concurrence of lipoatrophy and lipohypertrophy in children with type 1 diabetes using recombinant human insulin: two case reports. Clin Diabetes. 2016;34(1):51-53. doi:10.2337/diaclin.34.1.51
24. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486-1492. doi:10.2337/dc16-0610.
25. Reitman ML, Arioglu E, Gavrilova O, Taylor SI. Lipoatrophy revisited. Trends Endocrinol Metab. 2000;11(10):410-416. doi:10.1016/s1043-2760(00)00309-x
26. Kondo A, Nakamura A, Takeuchi J, Miyoshi H, Atsumi T. Insulin-Induced Distant Site Lipoatrophy. Diabetes Care. 2017;40(6):e67-e68. doi:10.2337/dc16-2385
27. Jermendy G, Nádas J, Sápi Z. “Lipoblastoma-like” lipoatrophy induced by human insulin: morphological evidence for local dedifferentiation of adipocytes?. Diabetologia. 2000;43(7):955-956. doi:10.1007/s001250051476
28. Mokta JK, Mokta KK, Panda P. Insulin lipodystrophy and lipohypertrophy. Indian J Endocrinol Metab. 2013;17(4):773-774. doi:10.4103/2230-8210.113788
29. Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19(1):174-177. doi:10.4103/2230-8210.146879
30. Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127(5):450-454. doi:10.1016/j.amjmed.2013.10.029
31. Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19(10):881-882. doi:10.1046/j.1464-5491.2002.07581.x
32. Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20(4):665-679. doi:10.1016/j.beem.2006.09.007
33. Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239-249. doi:10.4103/2229-5178.137765
Insulin injection therapy is one of the most widely used health care interventions to manage both type 1 and type 2 diabetes mellitus (T1DM/T2DM). Globally, more than 150 to 200 million people inject insulin into their upper posterior arms, buttocks, anterior and lateral thighs, or abdomen.1,2 In an ideal world, every patient would be using the correct site and rotating their insulin injection sites in accordance with health care professional (HCP) recommendations—systematic injections in one general body location, at least 1 cm away from the previous injection.2 Unfortunately, same-site insulin injection (repeatedly in the same region within 1 cm of previous injections) is a common mistake made by patients with DM—in one study, 63% of participants either did not rotate sites correctly or failed to do so at all.
Insulin-resistant cutaneous complications may occur as a result of same-site insulin injections. The most common is lipohypertrophy, reported in some studies in nearly 50% of patients with DM on insulin therapy.4 Other common cutaneous complications include lipoatrophy and amyloidosis. Injection-site acanthosis nigricans, although uncommon, has been reported in 18 cases in the literature.
Most articles suggest that same-site insulin injections decrease local insulin sensitivity and result in tissue hypertrophy because of the anabolic properties of insulin and increase in insulin binding to insulin-like growth factor-1 (IGF-1) receptor.5-20 The hyperkeratotic growth and varying insulin absorption rates associated with these cutaneous complications increase chances of either hyper- or hypoglycemic episodes in patients.10,11,13 It is the responsibility of the DM care professional to provide proper insulin-injection technique education and perform routine inspection of injection sites to reduce cutaneous complications of insulin therapy. The purpose of this article is to (1) describe a case of acanthosis nigricans resulting from insulin injection at the same site; (2) review case reports
Case Presentation
A 75-year-old patient with an 8-year history of T2DM, as well as stable coronary artery disease, atrial fibrillation, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and stage 3 chronic kidney disease, presented with 2 discrete abdominal hyperpigmented plaques. At the time of the initial clinic visit, the patient was taking metformin 1000 mg twice daily and insulin glargine 40 units once daily. When insulin was initiated 7 years prior, the patient received
The patient reported 5 years of progressive, asymptomatic hyperpigmentation of the skin surrounding his insulin glargine injection sites and injecting in these same sites daily without rotation. He reported no additional skin changes or symptoms. He had noticed no skin changes while using NPH insulin during his first year of insulin therapy. On examination, the abdominal wall skin demonstrated 2 well-demarcated, nearly black, soft, velvety plaques, measuring 9 × 8 cm on the left side and 4 × 3.5 cm on the right, suggesting acanthosis nigricans (Figure 1A). The remainder of the skin examination, including the flexures, was normal. Of note, the patient received biweekly intramuscular testosterone injections in the gluteal region for secondary hypogonadism with no adverse dermatologic effects. A skin punch biopsy was performed and revealed epidermal papillomatosis and hyperkeratosis, confirming the clinical diagnosis of acanthosis nigricans (Figure 2).
After a review of insulin-injection technique at his clinic visit, the patient started rotating insulin injection sites over his entire abdomen, and the acanthosis nigricans partially improved. A few months later, the patient stopped rotating the insulin injection site, and the acanthosis nigricans worsened again. Because of worsening glycemic control, the patient was then started on insulin aspart. He did not develop any skin changes at the insulin aspart injection site, although he was not rotating its site of injection.
Subsequently, with reeducation and proper injection-site rotation, the patient had resolution of his acanthosis nigricans (Figure 1b).
Discussion
A review of the literature revealed 18 reported cases of acanthosis nigricans at sites of repeated insulin injection (Table).5-20 Acanthosis nigricans at the site of insulin injection afflicts patients of any age, with cases observed in patients aged 14 to 75 years. Sixteen (84%) of 19 cases were male. Fourteen cases (73%) had T2DM; the rest of the patients had T1DM. The duration of insulin injection therapy prior to onset ranged from immediate to 13 years (median 4 years). Fourteen cases (73%) were reported on the abdomen; however, other sites, such as thighs and upper arm, also were reported. Lesions size varied from 12 to 360 cm2. Two cases had associated amyloidosis. The average HbA1c reported at presentation was 10%. Following insulin injection-site rotation, most of the cases reported improvement of both glycemic control and acanthosis nigricans appearance.
In the case described by Kudo and colleagues, a 59-year-old male patient with T2DM had been injecting insulin into the same spot on his abdomen for 10 years. He developed acanthosis nigricans and an amyloidoma so large and firm that it bent the needle when he injected insulin.11
Most of the cases we found in the literature were after 2005 and associated with the use of human or analog insulin. These cases may be related to a bias, as cases may be easier to find in digital archives in the later years, when human or analog insulins have been in common use. Also noteworthy, in cases that reported dosage, most were not very high, and the highest daily dose was 240 IU/d. Ten reports of injection-site acanthosis nigricans were in dermatology journals; only 5 reports were in endocrinology journals and 3 in general medical journals, indicating possible less awareness of this phenomenon in other HCPs who care for patients with DM.
Complications of Same-Site Injections
Acanthosis nigricans. Commonly found in the armpits, neck folds, and groin, acanthosis nigricans is known as one of the calling cards for insulin resistance, obesity, and hyperinsulinemia.21 Acanthosis nigricans can be seen in people with or without DM and is not limited to those on insulin therapy. However, same-site insulin injections for 4 to 6 years also may result in injection-site acanthosis nigricans–like lesions because of factors such as insulin exposure at the local tissue level.16
Acanthosis nigricans development is characterized by hyperpigmented, hyperkeratotic, velvety, and sometimes verrucous plaques.6 Acanthosis nigricans surrounding repeated injection sites is hypothesized to develop as a result of localized hyperinsulinemia secondary to insulin resistance, which increases the stimulation of IGF, thereby causing epidermal hypertrophy.5-20 If insulin injection therapy continues to be administered through the acanthosis nigricans lesion, it results in decreased insulin absorption, leading to poor glycemic control.13
Acanthosis nigricans associated with insulin injection is reversible. After rotation of injection sites, lesions either decrease in size or severity of appearance.5-8,11 Also, by avoiding injection into the hyperkeratotic plaques and using normal subcutaneous tissue for injection, patients’ response to insulin improves, as measured by HbA1c and by decreased daily insulin requirement.6-8,10,12,18-20
Lipohypertrophy. This is characterized by an increase in localized adipose tissue and is the most common cutaneous complication of insulin therapy.2 Lipohypertrophy presents as a firm, rubbery mass in the location of same-site insulin injections.22 Development of lipohypertrophy is suspected to be the result of either (1) anabolic effect of insulin on local adipocytes, promoting fat and protein synthesis; (2) an autoimmune response by immunoglobulin (Ig) G or IgE antibodies to insulin, immune response to insulin of different species, or to insulin injection techniques; or (3) repeated trauma to the injection site from repeated needle usage.4,23
In a study assessing the prevalence of lipohypertrophy and its relation to insulin technique, 49.1% of participants with
Primary prevention measures include injection site inspection and patient education about rotation and abstaining from needle reuse.22 If a patient already has signs of lipohypertrophy, data supports education and insulin injection technique practice as simple and effective means to reduce insulin action variability and increase glycemic control.24
Lipoatrophy. Lipoatrophy is described as a local loss of subcutaneous adipose tissue often in the face, buttocks, legs and arm regions and can be rooted in genetic, immune, or drug-associated etiologies.25 Insulin-induced lipoatrophy is suspected to be the result of tumor necrosis factor-α hyperproduction in reaction to insulin crystal presence at the injection site.26,27 Overall, lipoatrophy development has decreased since the use of recombinant human insulin and analog insulin therapy.28 The decrease is hypothesized to be due to increased subcutaneous tissue absorption rate of human insulin and its analog, decreasing overall adipocyte exposure to localized high insulin concentration.27 Treatments for same-site insulin-derived lipoatrophy include changing injection sites and preparation of insulin.26 When injection into the lipoatrophic site was avoided, glycemic control and lipoatrophy appearance improved.26
Amyloidosis. Amyloidosis indicates the presence of an extracellular bundle of insoluble polymeric protein fibrils in tissues and organs.29 Insulin-induced amyloidosis presents as a hard mass or ball near the injection site.29 Insulin is one of many hormones that can form amyloid fibrils, and there have been several dozen cases reported of amyloid formation at the site of insulin injection.29-31 Although insulin-derived amyloidosis is rare, it may be misdiagnosed as lipohypertrophy due to a lack of histopathologic testing or general awareness of the complication.29
In a case series of 7 patients with amyloidosis, all patients had a mean HbA1c of 9.3% (range, 8.5-10.2%) and averaged 1 IU/kg bodyweight before intervention.30 After the discovery of the mass, participants were instructed to avoid injection into the amyloidoma, and average insulin requirements decreased to 0.48 IU/kg body weight (P = .40).30 Patients with amyloidosis who rotated their injection sites experienced better glycemic control and decreased insulin requirements.30
Pathophysiology of Localized Insulin Resistance
Insulin regulates glucose homeostasis in skeletal muscle and adipose tissue, increases hepatic and adipocyte lipid synthesis, and decreases adipocyte fatty acid release.32 Generalized insulin resistance occurs when target tissues have decreased glucose uptake in response to circulating insulin.32 Insulin resistance increases the amount of free insulin in surrounding tissues. At high concentrations, insulin fosters tissue growth by binding to IGF-1 receptors, stimulating hypertrophy and reproduction of keratinocytes and fibroblasts.33 This pathophysiology helps explain the origin of localized acanthosis nigricans at same-site insulin injections.
Conclusions
Cutaneous complications are a local adverse effect of long-term failure to rotate insulin injection sites. Our case serves as a call to action for HCPs to improve education regarding insulin injection-site rotation, conduct routine injection-site inspection, and actively document cases as they occur to increase public awareness of these important complications.
If a patient with DM presents with unexplained poor glycemic control, consider questioning the patient about injection-site location and how often they are rotating the insulin injection site. Inspect the site for cutaneous complications. Of note, if a patient has a cutaneous complication due to insulin injection, adjust or decrease the insulin dosage when rotating sites to mitigate the risk of hypoglycemic episodes.
Improvement of glycemic control, cosmetic appearance of injection site, and insulin use all begin with skin inspection, injection technique education, and periodic review by a HCP.
Insulin injection therapy is one of the most widely used health care interventions to manage both type 1 and type 2 diabetes mellitus (T1DM/T2DM). Globally, more than 150 to 200 million people inject insulin into their upper posterior arms, buttocks, anterior and lateral thighs, or abdomen.1,2 In an ideal world, every patient would be using the correct site and rotating their insulin injection sites in accordance with health care professional (HCP) recommendations—systematic injections in one general body location, at least 1 cm away from the previous injection.2 Unfortunately, same-site insulin injection (repeatedly in the same region within 1 cm of previous injections) is a common mistake made by patients with DM—in one study, 63% of participants either did not rotate sites correctly or failed to do so at all.
Insulin-resistant cutaneous complications may occur as a result of same-site insulin injections. The most common is lipohypertrophy, reported in some studies in nearly 50% of patients with DM on insulin therapy.4 Other common cutaneous complications include lipoatrophy and amyloidosis. Injection-site acanthosis nigricans, although uncommon, has been reported in 18 cases in the literature.
Most articles suggest that same-site insulin injections decrease local insulin sensitivity and result in tissue hypertrophy because of the anabolic properties of insulin and increase in insulin binding to insulin-like growth factor-1 (IGF-1) receptor.5-20 The hyperkeratotic growth and varying insulin absorption rates associated with these cutaneous complications increase chances of either hyper- or hypoglycemic episodes in patients.10,11,13 It is the responsibility of the DM care professional to provide proper insulin-injection technique education and perform routine inspection of injection sites to reduce cutaneous complications of insulin therapy. The purpose of this article is to (1) describe a case of acanthosis nigricans resulting from insulin injection at the same site; (2) review case reports
Case Presentation
A 75-year-old patient with an 8-year history of T2DM, as well as stable coronary artery disease, atrial fibrillation, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and stage 3 chronic kidney disease, presented with 2 discrete abdominal hyperpigmented plaques. At the time of the initial clinic visit, the patient was taking metformin 1000 mg twice daily and insulin glargine 40 units once daily. When insulin was initiated 7 years prior, the patient received
The patient reported 5 years of progressive, asymptomatic hyperpigmentation of the skin surrounding his insulin glargine injection sites and injecting in these same sites daily without rotation. He reported no additional skin changes or symptoms. He had noticed no skin changes while using NPH insulin during his first year of insulin therapy. On examination, the abdominal wall skin demonstrated 2 well-demarcated, nearly black, soft, velvety plaques, measuring 9 × 8 cm on the left side and 4 × 3.5 cm on the right, suggesting acanthosis nigricans (Figure 1A). The remainder of the skin examination, including the flexures, was normal. Of note, the patient received biweekly intramuscular testosterone injections in the gluteal region for secondary hypogonadism with no adverse dermatologic effects. A skin punch biopsy was performed and revealed epidermal papillomatosis and hyperkeratosis, confirming the clinical diagnosis of acanthosis nigricans (Figure 2).
After a review of insulin-injection technique at his clinic visit, the patient started rotating insulin injection sites over his entire abdomen, and the acanthosis nigricans partially improved. A few months later, the patient stopped rotating the insulin injection site, and the acanthosis nigricans worsened again. Because of worsening glycemic control, the patient was then started on insulin aspart. He did not develop any skin changes at the insulin aspart injection site, although he was not rotating its site of injection.
Subsequently, with reeducation and proper injection-site rotation, the patient had resolution of his acanthosis nigricans (Figure 1b).
Discussion
A review of the literature revealed 18 reported cases of acanthosis nigricans at sites of repeated insulin injection (Table).5-20 Acanthosis nigricans at the site of insulin injection afflicts patients of any age, with cases observed in patients aged 14 to 75 years. Sixteen (84%) of 19 cases were male. Fourteen cases (73%) had T2DM; the rest of the patients had T1DM. The duration of insulin injection therapy prior to onset ranged from immediate to 13 years (median 4 years). Fourteen cases (73%) were reported on the abdomen; however, other sites, such as thighs and upper arm, also were reported. Lesions size varied from 12 to 360 cm2. Two cases had associated amyloidosis. The average HbA1c reported at presentation was 10%. Following insulin injection-site rotation, most of the cases reported improvement of both glycemic control and acanthosis nigricans appearance.
In the case described by Kudo and colleagues, a 59-year-old male patient with T2DM had been injecting insulin into the same spot on his abdomen for 10 years. He developed acanthosis nigricans and an amyloidoma so large and firm that it bent the needle when he injected insulin.11
Most of the cases we found in the literature were after 2005 and associated with the use of human or analog insulin. These cases may be related to a bias, as cases may be easier to find in digital archives in the later years, when human or analog insulins have been in common use. Also noteworthy, in cases that reported dosage, most were not very high, and the highest daily dose was 240 IU/d. Ten reports of injection-site acanthosis nigricans were in dermatology journals; only 5 reports were in endocrinology journals and 3 in general medical journals, indicating possible less awareness of this phenomenon in other HCPs who care for patients with DM.
Complications of Same-Site Injections
Acanthosis nigricans. Commonly found in the armpits, neck folds, and groin, acanthosis nigricans is known as one of the calling cards for insulin resistance, obesity, and hyperinsulinemia.21 Acanthosis nigricans can be seen in people with or without DM and is not limited to those on insulin therapy. However, same-site insulin injections for 4 to 6 years also may result in injection-site acanthosis nigricans–like lesions because of factors such as insulin exposure at the local tissue level.16
Acanthosis nigricans development is characterized by hyperpigmented, hyperkeratotic, velvety, and sometimes verrucous plaques.6 Acanthosis nigricans surrounding repeated injection sites is hypothesized to develop as a result of localized hyperinsulinemia secondary to insulin resistance, which increases the stimulation of IGF, thereby causing epidermal hypertrophy.5-20 If insulin injection therapy continues to be administered through the acanthosis nigricans lesion, it results in decreased insulin absorption, leading to poor glycemic control.13
Acanthosis nigricans associated with insulin injection is reversible. After rotation of injection sites, lesions either decrease in size or severity of appearance.5-8,11 Also, by avoiding injection into the hyperkeratotic plaques and using normal subcutaneous tissue for injection, patients’ response to insulin improves, as measured by HbA1c and by decreased daily insulin requirement.6-8,10,12,18-20
Lipohypertrophy. This is characterized by an increase in localized adipose tissue and is the most common cutaneous complication of insulin therapy.2 Lipohypertrophy presents as a firm, rubbery mass in the location of same-site insulin injections.22 Development of lipohypertrophy is suspected to be the result of either (1) anabolic effect of insulin on local adipocytes, promoting fat and protein synthesis; (2) an autoimmune response by immunoglobulin (Ig) G or IgE antibodies to insulin, immune response to insulin of different species, or to insulin injection techniques; or (3) repeated trauma to the injection site from repeated needle usage.4,23
In a study assessing the prevalence of lipohypertrophy and its relation to insulin technique, 49.1% of participants with
Primary prevention measures include injection site inspection and patient education about rotation and abstaining from needle reuse.22 If a patient already has signs of lipohypertrophy, data supports education and insulin injection technique practice as simple and effective means to reduce insulin action variability and increase glycemic control.24
Lipoatrophy. Lipoatrophy is described as a local loss of subcutaneous adipose tissue often in the face, buttocks, legs and arm regions and can be rooted in genetic, immune, or drug-associated etiologies.25 Insulin-induced lipoatrophy is suspected to be the result of tumor necrosis factor-α hyperproduction in reaction to insulin crystal presence at the injection site.26,27 Overall, lipoatrophy development has decreased since the use of recombinant human insulin and analog insulin therapy.28 The decrease is hypothesized to be due to increased subcutaneous tissue absorption rate of human insulin and its analog, decreasing overall adipocyte exposure to localized high insulin concentration.27 Treatments for same-site insulin-derived lipoatrophy include changing injection sites and preparation of insulin.26 When injection into the lipoatrophic site was avoided, glycemic control and lipoatrophy appearance improved.26
Amyloidosis. Amyloidosis indicates the presence of an extracellular bundle of insoluble polymeric protein fibrils in tissues and organs.29 Insulin-induced amyloidosis presents as a hard mass or ball near the injection site.29 Insulin is one of many hormones that can form amyloid fibrils, and there have been several dozen cases reported of amyloid formation at the site of insulin injection.29-31 Although insulin-derived amyloidosis is rare, it may be misdiagnosed as lipohypertrophy due to a lack of histopathologic testing or general awareness of the complication.29
In a case series of 7 patients with amyloidosis, all patients had a mean HbA1c of 9.3% (range, 8.5-10.2%) and averaged 1 IU/kg bodyweight before intervention.30 After the discovery of the mass, participants were instructed to avoid injection into the amyloidoma, and average insulin requirements decreased to 0.48 IU/kg body weight (P = .40).30 Patients with amyloidosis who rotated their injection sites experienced better glycemic control and decreased insulin requirements.30
Pathophysiology of Localized Insulin Resistance
Insulin regulates glucose homeostasis in skeletal muscle and adipose tissue, increases hepatic and adipocyte lipid synthesis, and decreases adipocyte fatty acid release.32 Generalized insulin resistance occurs when target tissues have decreased glucose uptake in response to circulating insulin.32 Insulin resistance increases the amount of free insulin in surrounding tissues. At high concentrations, insulin fosters tissue growth by binding to IGF-1 receptors, stimulating hypertrophy and reproduction of keratinocytes and fibroblasts.33 This pathophysiology helps explain the origin of localized acanthosis nigricans at same-site insulin injections.
Conclusions
Cutaneous complications are a local adverse effect of long-term failure to rotate insulin injection sites. Our case serves as a call to action for HCPs to improve education regarding insulin injection-site rotation, conduct routine injection-site inspection, and actively document cases as they occur to increase public awareness of these important complications.
If a patient with DM presents with unexplained poor glycemic control, consider questioning the patient about injection-site location and how often they are rotating the insulin injection site. Inspect the site for cutaneous complications. Of note, if a patient has a cutaneous complication due to insulin injection, adjust or decrease the insulin dosage when rotating sites to mitigate the risk of hypoglycemic episodes.
Improvement of glycemic control, cosmetic appearance of injection site, and insulin use all begin with skin inspection, injection technique education, and periodic review by a HCP.
1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384
2. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. doi:10.1016/j.mayocp.2016.06.010
3. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. doi:10.1016/j.diabet.2013.05.006
4. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025-2027. doi:10.2337/diacare.28.8.2025
5. Erickson L, Lipschutz DE, Wrigley W, Kearse WO. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209(6):934-935. doi:10.1001/jama.1969.03160190056019
6. Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122(9):1054-1056. doi:10.1001/archderm.1986.01660210104028 7. Gannon D, Ross MW, Mahajan T. Acanthosis nigricans-like plaque and lipohypertrophy in type 1 diabetes. Pract Diabetes International. 2005;22(6).
8. Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144(1):126-127. doi:10.1001/archdermatol.2007.27
9. Pachón Burgos A, Chan Aguilar MP. Visual vignette. Hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14(4):514. doi:10.4158/EP.14.4.514
10. Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94(2):e34-e36. doi:10.1016/j.diabres.2011.07.023
11. Kudo-Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2012;38(1):25-29. doi:10.1111/j.1365-2230.2012.04373.x
12. Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11(12):e85-e87.
13. Kanwar A, Sawatkar G, Dogra S, Bhadada S. Acanthosis nigricans—an uncommon cutaneous adverse effect of a common medication: report of two cases. Indian J Dermatol Venereol Leprol. 2013;79(4):553. doi:10.4103/0378-6323.113112
14. Dhingra M, Garg G, Gupta M, Khurana U, Thami GP. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19(1):9. Published 2013 Jan 15.
15. Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57(1):127-129. doi:10.4103/0377-4929.130920
16. Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39(1):5-9.
17. Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39(12):e163. doi:10.1097/DAD.0000000000000659
18. Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190(47):E1390. doi:10.1503/cmaj.180705
19. Pal R, Bhattacharjee R, Chatterjee D, Bhadada SK, Bhansali A, Dutta P. Exogenous insulin-induced localized acanthosis nigricans: a rare injection site complication. Can J Diabetes. 2020;44(3):219-221. doi:10.1016/j.jcjd.2019.08.010
20. Bomar L, Lewallen R, Jorizzo J. Localized acanthosis nigricans at the site of repetitive insulin injections. Cutis. 2020;105(2);E20-E22.
21. Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48-53. doi:10.1016/j.clindermatol.2017.09.008
22. Kalra S, Kumar A, Gupta Y. Prevention of lipohypertrophy. J Pak Med Assoc. 2016;66(7):910-911.
23. Singha A, Bhattarcharjee R, Ghosh S, Chakrabarti SK, Baidya A, Chowdhury S. Concurrence of lipoatrophy and lipohypertrophy in children with type 1 diabetes using recombinant human insulin: two case reports. Clin Diabetes. 2016;34(1):51-53. doi:10.2337/diaclin.34.1.51
24. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486-1492. doi:10.2337/dc16-0610.
25. Reitman ML, Arioglu E, Gavrilova O, Taylor SI. Lipoatrophy revisited. Trends Endocrinol Metab. 2000;11(10):410-416. doi:10.1016/s1043-2760(00)00309-x
26. Kondo A, Nakamura A, Takeuchi J, Miyoshi H, Atsumi T. Insulin-Induced Distant Site Lipoatrophy. Diabetes Care. 2017;40(6):e67-e68. doi:10.2337/dc16-2385
27. Jermendy G, Nádas J, Sápi Z. “Lipoblastoma-like” lipoatrophy induced by human insulin: morphological evidence for local dedifferentiation of adipocytes?. Diabetologia. 2000;43(7):955-956. doi:10.1007/s001250051476
28. Mokta JK, Mokta KK, Panda P. Insulin lipodystrophy and lipohypertrophy. Indian J Endocrinol Metab. 2013;17(4):773-774. doi:10.4103/2230-8210.113788
29. Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19(1):174-177. doi:10.4103/2230-8210.146879
30. Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127(5):450-454. doi:10.1016/j.amjmed.2013.10.029
31. Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19(10):881-882. doi:10.1046/j.1464-5491.2002.07581.x
32. Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20(4):665-679. doi:10.1016/j.beem.2006.09.007
33. Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239-249. doi:10.4103/2229-5178.137765
1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384
2. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. doi:10.1016/j.mayocp.2016.06.010
3. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. doi:10.1016/j.diabet.2013.05.006
4. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025-2027. doi:10.2337/diacare.28.8.2025
5. Erickson L, Lipschutz DE, Wrigley W, Kearse WO. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209(6):934-935. doi:10.1001/jama.1969.03160190056019
6. Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122(9):1054-1056. doi:10.1001/archderm.1986.01660210104028 7. Gannon D, Ross MW, Mahajan T. Acanthosis nigricans-like plaque and lipohypertrophy in type 1 diabetes. Pract Diabetes International. 2005;22(6).
8. Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144(1):126-127. doi:10.1001/archdermatol.2007.27
9. Pachón Burgos A, Chan Aguilar MP. Visual vignette. Hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14(4):514. doi:10.4158/EP.14.4.514
10. Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94(2):e34-e36. doi:10.1016/j.diabres.2011.07.023
11. Kudo-Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2012;38(1):25-29. doi:10.1111/j.1365-2230.2012.04373.x
12. Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11(12):e85-e87.
13. Kanwar A, Sawatkar G, Dogra S, Bhadada S. Acanthosis nigricans—an uncommon cutaneous adverse effect of a common medication: report of two cases. Indian J Dermatol Venereol Leprol. 2013;79(4):553. doi:10.4103/0378-6323.113112
14. Dhingra M, Garg G, Gupta M, Khurana U, Thami GP. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19(1):9. Published 2013 Jan 15.
15. Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57(1):127-129. doi:10.4103/0377-4929.130920
16. Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39(1):5-9.
17. Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39(12):e163. doi:10.1097/DAD.0000000000000659
18. Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190(47):E1390. doi:10.1503/cmaj.180705
19. Pal R, Bhattacharjee R, Chatterjee D, Bhadada SK, Bhansali A, Dutta P. Exogenous insulin-induced localized acanthosis nigricans: a rare injection site complication. Can J Diabetes. 2020;44(3):219-221. doi:10.1016/j.jcjd.2019.08.010
20. Bomar L, Lewallen R, Jorizzo J. Localized acanthosis nigricans at the site of repetitive insulin injections. Cutis. 2020;105(2);E20-E22.
21. Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48-53. doi:10.1016/j.clindermatol.2017.09.008
22. Kalra S, Kumar A, Gupta Y. Prevention of lipohypertrophy. J Pak Med Assoc. 2016;66(7):910-911.
23. Singha A, Bhattarcharjee R, Ghosh S, Chakrabarti SK, Baidya A, Chowdhury S. Concurrence of lipoatrophy and lipohypertrophy in children with type 1 diabetes using recombinant human insulin: two case reports. Clin Diabetes. 2016;34(1):51-53. doi:10.2337/diaclin.34.1.51
24. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486-1492. doi:10.2337/dc16-0610.
25. Reitman ML, Arioglu E, Gavrilova O, Taylor SI. Lipoatrophy revisited. Trends Endocrinol Metab. 2000;11(10):410-416. doi:10.1016/s1043-2760(00)00309-x
26. Kondo A, Nakamura A, Takeuchi J, Miyoshi H, Atsumi T. Insulin-Induced Distant Site Lipoatrophy. Diabetes Care. 2017;40(6):e67-e68. doi:10.2337/dc16-2385
27. Jermendy G, Nádas J, Sápi Z. “Lipoblastoma-like” lipoatrophy induced by human insulin: morphological evidence for local dedifferentiation of adipocytes?. Diabetologia. 2000;43(7):955-956. doi:10.1007/s001250051476
28. Mokta JK, Mokta KK, Panda P. Insulin lipodystrophy and lipohypertrophy. Indian J Endocrinol Metab. 2013;17(4):773-774. doi:10.4103/2230-8210.113788
29. Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19(1):174-177. doi:10.4103/2230-8210.146879
30. Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127(5):450-454. doi:10.1016/j.amjmed.2013.10.029
31. Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19(10):881-882. doi:10.1046/j.1464-5491.2002.07581.x
32. Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20(4):665-679. doi:10.1016/j.beem.2006.09.007
33. Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239-249. doi:10.4103/2229-5178.137765
The Design and Implementation of a Heart Disease Reversal Program in the Veterans Health Administration: Before and During the COVID-19 Pandemic
While cardiovascular mortality rates have declined, heart disease continues to be the leading cause of death in the US, and the number of people with cardiovascular disease (CVD) is rising.1 CVD is more prevalent among military veterans than it is among nonveterans aged ≥ 25 years, and veteran status is associated with higher risk of incident heart disease after controlling for socioeconomic status, other medical diseases, depression, and lifestyle.2-4 Combat exposure, posttraumatic stress disorder (PTSD), and Purple Heart commendation are associated with higher rates of CVD, including adverse cardiovascular events.5-7 Many patients seeking care in the Veterans Health Administration (VHA), including those who undergo cardiac catheterization, meet the criteria for multimorbidity (defined as having ≥ 2 chronic diseases8), which is common among veterans.9,10 Multimorbidity presents a challenge for lifestyle intervention, as different diets may be prescribed to treat different conditions, such as Dietary Approaches to Stop Hypertension, and low-glycemic diet for diabetes mellitus (DM). Veterans with CVD are often clinically complex and may require more multifaceted secondary prevention programs.
During the coronavirus 2019 (COVID-19) pandemic, effective secondary prevention intervention is needed more than ever. Older age, CVD, and common comorbidities, including hypertension, DM, and obesity, place patients at the highest risk for severe COVID-19 infection.11 COVID-19 social distancing encourages vulnerable populations to stay home, which can make engaging in any levels of physical activity more challenging. The International Food Council found that 85% of adults have made a change to their food consumption pattern, including eating more, during the COVID-19 pandemic.12 Thus, secondary CVD prevention programs for veterans need to provide treatment that addresses these specific challenges and can be delivered via telehealth for continuity of care after disruption of traditional services.
Clinical practice guidelines for the treatment of patients with recent cardiovascular adverse events (AEs) include a referral to cardiac rehabilitation (CR).13 CR emphasizes exercise as the main intervention, along with coaching to promote multiple risk reduction. The most comprehensive CR program is intensive CR (ICR), including the Ornish ICR program.14 ICR includes 4 components: vegetarian diet, exercise, stress management (yoga, meditation), and group support. Ornish ICR has been shown to be efficacious in randomized controlled trials (RCTs).15-17 Three effectiveness studies, with 5,372 participants, demonstrated the real-world effectiveness of Ornish ICR in US hospitals.14,18,19 The program also was adapted successfully for the active-duty military and veteran population.20,21 Yet Ornish ICR is time intensive, and there are no certified VHA ICR Ornish sites. Furthermore, there is no formal strategy for targeting people with atherosclerotic CVD who no longer meet the criteria for CR or ICR. While Ornish ICR is highly effective for patients who are eligible and have access, a more effective and streamlined approach is needed for targeting many patients.
Nutrition may be the most powerful Ornish ICR component. The initial RCT conducted by Ornish and colleagues included only stress management training and a whole-food, plant-based (WFPB) diet, including grains, legumes, vegetables, fruits, nuts, and seeds. The trial found 91% of participants experienced reduced angina after only 24 days.15 The only single-component intervention study resulting in partial reversal of atherosclerosis was a WFPB diet-only study, which documented regression of atherosclerotic plaques after 5 years, using coronary angiography in 73% of participants, with arrested progression in the other 27%.22 Participants reported no cardiovascular AEs after 12 years.23 Furthermore, a number of other recent studies have demonstrated the benefits of WFPB diet-only interventions for type 2 DM (T2DM), hypertension, and obesity.24-27 The Heart Disease Reversal Program (HDRP) was developed to create an interdisciplinary lifestyle intervention that emphasized nutrition for a broad population of veterans with atherosclerotic CVD, of varying levels of functional ability, to promote comprehensive CVD risk reduction and bring heart disease reversal intervention into routine clinical practice.
Program Description
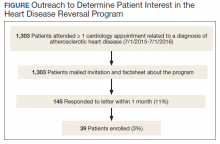
The Mental Health, Cardiology, and Nutrition and Food services all approved the launch of HDRP. We contacted veterans by mail, and 11% expressed interest (Figure). Among patients who received the initial mailed letter (prior to our accepting staff referrals), only 5% of patients who enrolled in HDRP reported previously being told about or prescribed a WFPB diet by any health care provider (HCP). Currently, patients are primarily referred to HDRP by Cardiology, Primary Care, and Mental Health services.
Design
HDRP is an adaptation of interdisciplinary lifestyle interventions that have resulted in regression of atherosclerotic blockages confirmed with invasive coronary angiography.15-17,22,28 HDRP currently is offered in a Behavioral Medicine Clinic at the Sacramento US Department of Veterans Affairs (VA) Medical Center (VAMC) in California. Program staff include a clinical health psychologist who organizes, coordinates, and act as the lead facilitator of the program; registered dietitians; clinical pharmacists; and a consulting physician. Patients engage in the 4-month core HDRP program in small cohorts (ie, 6-10 patients), and spouses/partners are highly encouraged to attend all sessions.
Components
Telephone screening. Patients are screened for the inclusion and exclusion criteria (Table 1). Patients engaging in a traditional CR program are included in the screening. Patients are informed that the program consists of lifestyle intervention, including emphasis on following a WFPB diet.
Health assessment. Once approved, all patients are instructed to complete baseline laboratory tests and questionnaires. Along with an electronic health record (EHR) review, a psychosocial assessment is completed by a licensed clinical health psychologist who assesses CVD history, eating behavior, exercise/physical activity, sleep, mental health, substance use, and social history, with the aim of enhancing our ability to help the patient to benefit from HDRP.29 The patient data are used to develop a case conceptualization (ie, integrated understanding of the particular patient’s psychiatric and medical diagnoses, behavioral patterns, social supports, lifestyle habits, strengths and weaknesses, and their interrelationships with each other and the patient’s environment), resulting in an individualized plan. Patients are encouraged to ask questions about the program, and those who are still interested are invited to attend a seminar. A request for medical clearance to participate in the program is initiated through the EHR or by patients scheduling an appointment with their HCP. All patients are medically cleared by their HCP for participation. Safe exercise recommendations also are provided and guide patient goals.
CVD risk profile. Patients complete psychosocial questionnaires and fasting laboratory tests to produce a tailored CVD risk profile. Laboratory tests include fasting lipid, fasting glucose, hemoglobin A1c (HbA1c) C-reactive protein, vitamin B12, and vitamin D. The same tests (excluding HbA1c) are completed 1 month later (after completing 4 group sessions) and again posttreatment (including HbA1c). Self-reported questionnaires are completed at the same time points, which include the Rate Your Plate dietary composition questionnaire, CHAMPS physical activity questionnaire for older adults, Beck Depression Inventory-II, and the Perceived Stress Scale.
Seminar. A 2-hour seminar provides patients and families with an opportunity to meet HDRP program staff, learn the background and rationale for chronic disease reversal, obtain a summary of the program, and hear a patient testimonial. Patients are asked to make a commitment, and the informed consent process includes all patients signing a behavioral contract.
Assessment and feedback. A licensed clinical health psychologist provides feedback to patients on their comprehensive CVD risk profile, using motivational interviewing.30,31 Smokers are encouraged to quit, and those interested are referred to their HCP and/or facility smoking cessation program.
Group sessions. Twelve weekly group sessions cover nutrition education and cooking, physical activity and exercise, stress management training, and medication reconciliation and adjustment. The nutrition component is the centerpiece of HDRP and is delivered by registered dietitians (Table 2). Patients are instructed to use the 3-week period between the HDRP seminar and the first core group session to try new recipes and prepare their kitchens, pantries, and mind-set to adopt the HDRP diet with 100% adherence. The WFPB diet used is consistent with the current guidelines of Caldwell Esselstyn, MD, and Dean Ornish, MD.32-34
A psychologist delivers the physical activity component. Patients are encouraged to meet the American Heart Association/American College of Cardiology recommendations for aerobic exercise (at least 150 minutes of moderate intensity physical activity per week) through a walking program.35 Patients with medical contraindications (eg, severe pain, mobility restrictions) are encouraged to follow the exercise/activity recommendations they had been given by their primary care provider (PCP), physical therapist, or other HCP.
A psychologist provides evidence-based cognitive behavioral stress management (CBSM) training, adapted from models developed for patients with stable ischemic heart disease, HIV/AIDS, and cancer.36-38 CBSM is a psychotherapy grounded in stress/coping theory and cognitive behavioral theory of psychopathology that integrates cognitive restructuring, coping skills training, communication/assertiveness training, anger management, and mindfulness/acceptance-based approaches. Additional emphasis is placed on assisting patients’ adjustment to the lifestyle challenges for following a plant-based diet, dealing with food cravings and emotional eating, and connecting lifestyle change to patients’ deepest values and goals.
A clinical pharmacist conducts a medication reconciliation for each patient at baseline. The pharmacist consults with each patient’s PCP, cardiologist, and HDRP consulting physician, as needed, to ensure safe adjustments to medications. Pharmacists also provide education on medications at group sessions.
After completion of the 12-week core program, graduates are encouraged to attend the monthly graduates’ group indefinitely, and as often as they desire to promote maintenance of the disease reversal lifestyle. Patients are encouraged to complete our recommended fasting laboratory work every 3 to 6 months to facilitate maintenance of treatment gains.
Program Evaluation
Patients frequently reported that the group format was vital to their success. Patients requested a cooking class, yet we lacked a full teaching kitchen. Integrating plant-based meal samples at every session and cooking videos helped. Patients reported that 100% adherence to the WFPB diet led to significant changes in their food preferences, including a loss of interest in meat.39 Patients encouraged us to keep the “disease reversal” language and focus. One veteran stated: “Disease reversal, that is the reason I called you when I got your letter.” Showing before and after images of coronary angiograms and cardiac positron emission tomography scans depicting regression of atherosclerotic plaque and restored myocardial perfusion were described as highly motivating and generated willingness to commit to a more aggressive lifestyle change.31
Patients routinely stated that they lacked understanding of their laboratory test results, which HDRP remedied. Some patients reported their adult children followed a plant-based diet, and our program resulted in a new commonality and source of bonding that was highly valued. Some patients reported that HDRP was helpful for controlling their COVID-19 anxiety and feeling in control of their health. Satisfaction surveys were completed by participants at the end of the core program, which demonstrated very high satisfaction with and acceptability of HDRP (Table 3).
The program also has received positive feedback from HCPs when we alert them to improvements in outcome measures for their patients. These HCPs expressed satisfaction with having a program to refer patients to that can help with chronic illness in more depth.
COVID-19 Response
Face-to-face group appointments were converted to videoconferencing as a result of the COVID-19 pandemic. While HDRP always promoted the use of technology and mHealth tools, the pandemic led us to develop novel technology-based interventions.40 One cohort transitioned from in-person to videoconferencing sessions, and 2 cohorts recently started this format and are ongoing. We have successfully used videoconferencing with Cisco Webex, the VA-approved backup platform, as we encountered technical barriers when using VA Video Connect. Program materials were shared electronically, and participants sent blood pressure/sugar logs by secure messaging. Guidance for online grocery shopping with home delivery was provided, and research on the benefits of the HDRP lifestyle on immune function was incorporated.
The stress management component incorporated coping with COVID-19, including normalizing common emotional difficulties with sheltering-in-place and quarantine, acknowledging and processing fear and anxiety related to being at very high risk for severe COVID-19. We presented heart disease reversal as an urgent and feasible goal during the pandemic both reducing risk of premature death and major adverse cardiovascular events in the long-term and also reducing personal risk of severe COVID complications. The new VA COVID Coach app was also presented as a resource. Reputable sources of COVID-19 and public health information were shared. Walking continued to be the primary recommended form of exercise, while indoor home exercise options were promoted during the periods of very poor air quality due to the widespread California fires and smoke.
Considering the research suggesting benefits of our intervention for treating T2DM,promoting sustained weight loss, and promoting comprehensive cardiometabolic risk reduction, we have begun accepting referrals for patients with any type of atherosclerotic CVD (eg, peripheral artery disease, carotid artery disease), patients with T2DM (without CVD), and patients with only a history of ischemic stroke or transient ischemic attack.24-27 Vascular surgery has become a new referral source, primarily for patients with peripheral and carotid artery diseases. Finally, we are leveraging videoconferencing and accepting referrals across the VA Northern California Health Care System (VANCHCS)catchment (from the California-Oregon state border to the San Francisco Bay Area). This also helps address a long-standing problem with reaching the many rural veterans who live far from a VA clinic. We successfully implemented a consult/referral process within the EHR that is available to providers across VANCHCS.
Discussion
The efficacy and effectiveness of reversal programs are well established in intensive programs (eg, ICR), yet such programs have yet to be streamlined and disseminated broadly into routine clinical care. HDRP has endeavored to address this by emphasizing nutrition relative to other program components. We have learned that the words “disease reversal” are very often the reason patients initially reach out or accept referral to our program.
Consistent with past research on plant-based nutrition interventions, the group format was indispensable.41 Individual sessions with a clinical health psychologist enabled tailored feedback and education on how behavior changes could impact laboratory results and how certain psychosocial factors could support success. Participants reported that seeing significantly favorable laboratory results was highly motivating and confirmed the power of their lifestyle changes. Furthermore, a psychosocial health assessment with individual sessions promoted a tailored treatment plan with targeted clinical interventions, such as behavioral health education, motivational interviewing, and advanced methods, including cognitive behavioral therapy and techniques drawn from dialectical behavior therapy and acceptance and commitment therapy.
Veterans with multimorbidity face the difficult task of learning and maintaining a complex disease self-management program and implementing a lifestyle approach that is feasible, effective, promotes weight loss, and treats multiple conditions. HDRP is a model approach for this population, as demonstrated by a recent case report of a 65-year-old male veteran with atherosclerotic CVD, T2DM, hypertension, and myasthenia gravis who had 2 heart attacks within 2 months.42 His neurologic disease precluded significant physical activity. Although he achieved some initial weight loss through lifestyle changes, he continued to have daily angina despite optimal and aggressive cardiology management. After enrolling in HDRP and adopting the WFPB diet, the patient reported almost complete resolution of angina within 1 month, similar to that found in other studies.15
The literature suggests that concern over the acceptability of plant-based diets and patients’ ability to adhere to them long-term may be misplaced. A review paper on dietary interventions lasting > 1 year found that 51 to 61% of vegetarian and vegan study participants had maintained dietary adherence, while 20 to 55% of omnivorous diet intervention participants adhered to their study diets.43 Remarkably, there were no statistically significant differences in the acceptability of the vegan, vegetarian, or omnivorous diets in the studies reviewed.43 Recent dietary research also suggests that providing patients with higher goals (eg, adopting a vegan diet instead of only moderate dietary changes) results in greater weight loss and maintenance.26 HDRP provides training on consumption of whole plant foods, which may offer patients a unique advantage for maximizing results and higher adherence over time.
Limitations
Hands-on cooking instruction was not provided at our VAMC. The total time of the intervention was significantly less in HDRP (25 hours) than it was for the Ornish ICR program (72 hours), which may hinder long-term adherence. Without an exercise facility, we were not able to provide more detailed exercise instruction and supervised exercise.
Program Improvements Planned
There are a number of improvements that are planned for HDRP. First, the program anticipates requesting medical clearance at the telephone screening stage for self-referred patients. Second,
Conclusions
Although our patient population was self-selected for participation, early program evaluation demonstrates high acceptability. Very few patients had ever been told about a heart disease reversing lifestyle, and we found direct-to-patient clinical outreach an effective method for launching a disease reversal program (optimally timed with HCP presentations). Furthermore, the program is adaptable to current restrictions on in-person appointments due to the COVID-19 pandemic, and much more convenient for rural veterans who live far from any VA clinic. Being able to offer sustainable health care for individuals during unexpected public health crises is critically important. Additionally, treating veterans who are most vulnerable to pandemic illness due to existing medical conditions, such as CVD, should be a high priority. Last, HDRP also may represent a novel integrated treatment for COVID-19 anxiety and secondary CVD prevention, as lifestyle habits are optimized to improve chronic diseases that elevate risk for severe COVID-19 infection and mortality, as well as including coping strategies consistent with evidence-based psychotherapies for anxiety disorders.44
We believe that beyond the clinical benefits to patients, there is significant value and benefit added to the health care system by offering an intervention within the “disease reversal” paradigm. Efforts of the health care team to reverse a disease can be considered the highest aim of medicine and health care.45
Acknowledgments
This work was supported by the US Department of Veterans Affairs. We give special thanks to David M. Gellerman, MD, PhD, and David W. Schafer, PsyD, for providing Mental Health Service support for initiating the Heart Disease Reversal Program, and to Joseph Giorgio, PsyD (Program Manager, Integrated Care Program) for sustaining it. We thank Amogh Bhat, MD, Chief of Cardiology, for his continued support and partnership with the Cardiology Department. We express thanks to Stephanie Mohney, RDN (Chief, Nutrition and Food Service), Amy Klotz, RDN (Supervisory Dietician), Sian M. Carr-Lopez, PharmD (Associate Chief of Pharmacy, Primary Care), and Michelle Rand, PharmD, CACP (Anticoagulation Clinical Pharmacist-Supervisor) for their staff support of this interdisciplinary program. We thank the patients and their families for their participation in the program and commitment to the lifestyle changes. We also thank the following individuals for their contributions to this program: Lisa Wagaman, RDN, Karen Soong, PharmD, Sara S. Ali, PharmD, Suzan Hua, PharmD, and Stephen Cooperman.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12 ): e493]. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
2. Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chronic Illn. 2020;16(1):55-68. doi:10.1177/1742395318785237.
3. Hinojosa R. Sex, age, race/ethnicity, veteran status, and the likelihood of reporting cardiovascular conditions in the National Health Interview Survey. J Cardiovasc Nurs. 2019;34(3):215-221. doi:10.1097/JCN.0000000000000561 4. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
5. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans Study. Prim Care Companion CNS Disord. 2017;19(3):10.4088/PCC.17m02118. Published 2017 Jun 22. doi:10.4088/PCC.17m02118
6. Bukhbinder AS, Wang AC, Qureshi SU, et al. Increased vascular pathology in older veterans with a purple heart commendation or chronic post-traumatic stress disorder. J Geriatr Psychiatry Neurol. 2020;33(4):195-206. doi:10.1177/0891988719868308
7. Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320-329. doi:10.1016/S2215-0366(16)30377-7
8. Forman DE, Maurer MS, Boyd C, et a;. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149-2161. doi:10.1016/j.jacc.2018.03.022
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114(11):1750-1757. doi:10.1016/j.amjcard.2014.08.045
11. Centers for Disease Control and Prevention. Coronavirus 2019 (COVID-19):people at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. Updated September 11, 2020. Accessed November 12, 2020.
12. International Food Information Council. 2020 food and health survey. https://foodinsight.org/2020-food-and-health-survey. Updated June 9, 2020. Accessed November 12, 2020.
13. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
14. Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot. 2010;24(4):260-266. doi:10.4278/ajhp.24.4.arb
15. Ornish D, Scherwitz LW, Doody RS, et al. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54-59.
16. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129-133. doi:10.1016/0140-6736(90)91656-u.
17. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease [published correction appears in JAMA 1999 Apr 21;281(15):1380]. JAMA. 1998;280(23):2001-2007. doi:10.1001/jama.280.23.2001
18. Frattaroli J, Weidner G, Merritt-Worden TA, Frenda S, Ornish D. Angina pectoris and atherosclerotic risk factors in the multisite cardiac lifestyle intervention program. Am J Cardiol. 2008;101(7):911-918. doi:10.1016/j.amjcard.2007.11.039
19. Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the Multicenter Lifestyle Demonstration Project. Am J Cardiol. 2003;91(11):1316-1322. doi:10.1016/s0002-9149(03)00320-5
20. Marshall DA, Walizer EM, Vernalis MN. Achievement of heart health characteristics through participation in an intensive lifestyle change program (Coronary Artery Disease Reversal Study). J Cardiopulm Rehabil Prev. 2009;29(2):84-96. doi:10.1097/HCR.0b013e31819a00b2
21. Marshall D, Elaine W, Vernalis M. The effect of a one-year lifestyle intervention program on carotid intima media thickness. Mil Med. 2011;176(7):798-804. doi:10.7205/milmed-d-10-00447
22. Esselstyn CB Jr, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6):560-568.
23. Esselstyn CB Jr. Updating a 12-year experience with arrest and reversal therapy for coronary heart disease (an overdue requiem for palliative cardiology). Am J Cardiol. 1999;84(3):339-A8. doi:10.1016/s0002-9149(99)00290-8
24. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777-1783. doi:10.2337/dc06-0606
25. McDougall J, Thomas LE, McDougall C, et al. Effects of 7 days on an ad libitum low-fat vegan diet: the McDougall Program cohort [published correction appears in Nutr J. 2017 Feb 10;16(1):12]. Nutr J. 2014;13:99. Published 2014 Oct 14. doi:10.1186/1475-2891-13-99
26. Turner-McGrievy GM, Davidson CR, Wingard EE, Wilcox S, Frongillo EA. Comparative effectiveness of plant-based diets for weight loss: a randomized controlled trial of five different diets. Nutrition. 2015;31(2):350-358. doi:10.1016/j.nut.2014.09.002
27. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. Published 2017 Mar 20. doi:10.1038/nutd.2017.3

28. Schaefer S, Hussein H, Gershony GR, Rutledge JC, Kappagoda CT. Regression of severe atherosclerotic plaque in patients with mild elevation of LDL cholesterol. J Investig Med. 1997;45(9):536-541.
29. Kitazono R. Know thy patient: Enhancing lifestyle interventions with psychological assessment. Int J Dis Rev Prev. 2020;2(1):76-81.
30. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013.
31. Mascola AJ, Yiaslas TA, Meir RL, et al. Framing physical activity as a distinct and uniquely valuable behavior independent of weight management: A pilot randomized controlled trial for overweight and obese sedentary persons. Eat Weight Disord. 2009;14(2-3):e148-e152. doi:10.1007/BF03327814
32. Esselstyn AC, Esselstyn J. The Prevent and Reverse Heart Disease Cookbook: Over 125 Delicious, Life-Changing, Plant-Based Recipes. New York, NY: Avery; 2014.
33. Esselstyn CB Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356-364.
34. Ornish D, Ornish A. Undo It! How Simple Lifestyle Changes Can Reverse Most Chronic Diseases. New York, NY: Ballantine Books; 2019.
35. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association [published correction appears in J Am Coll Cardiol. 2015 Apr 14;65(14):1495. Dosage error in article text.]. J Am Coll Cardiol. 2011;58(23):2432-2446. doi:10.1016/j.jacc.2011.10.824
36. Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164-168. doi:10.1016/s0002-9149(01)02194-4
37. Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173-188. doi:10.1080/1025389031000156727
38. Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261-270. doi:10.1207/s15324796abm3103_8
39. Yiaslas TA. “Look doctor, I’m a carnivore.” Int J Dis Rev Prev. 2020;2(2):35-39.
40. Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010;16(9):931-938. doi:10.1089/tmj.2010.0065
41. Barnard ND, Sherwitz L, Ornish D. Adherence and acceptability of a low-fat, vegetarian diet among patients with cardiac disease. J Cardiopulm Rehabil. 1992;12(6):423-431.
42. Yiaslas TA, Taylor J, Embree J, Schaefer S. Elimination of angina, comprehensive cardio-metabolic risk reduction, and 50-pound weight loss in a US Navy veteran with myasthenia gravis. Int J Dis Rev Prev. 2019;1(1):77-83.
43. Berkow SE, Barnard N, Eckart J, Katcher H. Four therapeutic diets: adherence and acceptability. Can J Diet Pract Res. 2010;71(4):199-204. doi:10.3148/71.4.2010.199
44. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
45. Yiaslas TA. The pursuit of arete in medicine and health care. Int J Dis Rev Prev. 2019;1(2):53-56.
While cardiovascular mortality rates have declined, heart disease continues to be the leading cause of death in the US, and the number of people with cardiovascular disease (CVD) is rising.1 CVD is more prevalent among military veterans than it is among nonveterans aged ≥ 25 years, and veteran status is associated with higher risk of incident heart disease after controlling for socioeconomic status, other medical diseases, depression, and lifestyle.2-4 Combat exposure, posttraumatic stress disorder (PTSD), and Purple Heart commendation are associated with higher rates of CVD, including adverse cardiovascular events.5-7 Many patients seeking care in the Veterans Health Administration (VHA), including those who undergo cardiac catheterization, meet the criteria for multimorbidity (defined as having ≥ 2 chronic diseases8), which is common among veterans.9,10 Multimorbidity presents a challenge for lifestyle intervention, as different diets may be prescribed to treat different conditions, such as Dietary Approaches to Stop Hypertension, and low-glycemic diet for diabetes mellitus (DM). Veterans with CVD are often clinically complex and may require more multifaceted secondary prevention programs.
During the coronavirus 2019 (COVID-19) pandemic, effective secondary prevention intervention is needed more than ever. Older age, CVD, and common comorbidities, including hypertension, DM, and obesity, place patients at the highest risk for severe COVID-19 infection.11 COVID-19 social distancing encourages vulnerable populations to stay home, which can make engaging in any levels of physical activity more challenging. The International Food Council found that 85% of adults have made a change to their food consumption pattern, including eating more, during the COVID-19 pandemic.12 Thus, secondary CVD prevention programs for veterans need to provide treatment that addresses these specific challenges and can be delivered via telehealth for continuity of care after disruption of traditional services.
Clinical practice guidelines for the treatment of patients with recent cardiovascular adverse events (AEs) include a referral to cardiac rehabilitation (CR).13 CR emphasizes exercise as the main intervention, along with coaching to promote multiple risk reduction. The most comprehensive CR program is intensive CR (ICR), including the Ornish ICR program.14 ICR includes 4 components: vegetarian diet, exercise, stress management (yoga, meditation), and group support. Ornish ICR has been shown to be efficacious in randomized controlled trials (RCTs).15-17 Three effectiveness studies, with 5,372 participants, demonstrated the real-world effectiveness of Ornish ICR in US hospitals.14,18,19 The program also was adapted successfully for the active-duty military and veteran population.20,21 Yet Ornish ICR is time intensive, and there are no certified VHA ICR Ornish sites. Furthermore, there is no formal strategy for targeting people with atherosclerotic CVD who no longer meet the criteria for CR or ICR. While Ornish ICR is highly effective for patients who are eligible and have access, a more effective and streamlined approach is needed for targeting many patients.
Nutrition may be the most powerful Ornish ICR component. The initial RCT conducted by Ornish and colleagues included only stress management training and a whole-food, plant-based (WFPB) diet, including grains, legumes, vegetables, fruits, nuts, and seeds. The trial found 91% of participants experienced reduced angina after only 24 days.15 The only single-component intervention study resulting in partial reversal of atherosclerosis was a WFPB diet-only study, which documented regression of atherosclerotic plaques after 5 years, using coronary angiography in 73% of participants, with arrested progression in the other 27%.22 Participants reported no cardiovascular AEs after 12 years.23 Furthermore, a number of other recent studies have demonstrated the benefits of WFPB diet-only interventions for type 2 DM (T2DM), hypertension, and obesity.24-27 The Heart Disease Reversal Program (HDRP) was developed to create an interdisciplinary lifestyle intervention that emphasized nutrition for a broad population of veterans with atherosclerotic CVD, of varying levels of functional ability, to promote comprehensive CVD risk reduction and bring heart disease reversal intervention into routine clinical practice.
Program Description
The Mental Health, Cardiology, and Nutrition and Food services all approved the launch of HDRP. We contacted veterans by mail, and 11% expressed interest (Figure). Among patients who received the initial mailed letter (prior to our accepting staff referrals), only 5% of patients who enrolled in HDRP reported previously being told about or prescribed a WFPB diet by any health care provider (HCP). Currently, patients are primarily referred to HDRP by Cardiology, Primary Care, and Mental Health services.
Design
HDRP is an adaptation of interdisciplinary lifestyle interventions that have resulted in regression of atherosclerotic blockages confirmed with invasive coronary angiography.15-17,22,28 HDRP currently is offered in a Behavioral Medicine Clinic at the Sacramento US Department of Veterans Affairs (VA) Medical Center (VAMC) in California. Program staff include a clinical health psychologist who organizes, coordinates, and act as the lead facilitator of the program; registered dietitians; clinical pharmacists; and a consulting physician. Patients engage in the 4-month core HDRP program in small cohorts (ie, 6-10 patients), and spouses/partners are highly encouraged to attend all sessions.
Components
Telephone screening. Patients are screened for the inclusion and exclusion criteria (Table 1). Patients engaging in a traditional CR program are included in the screening. Patients are informed that the program consists of lifestyle intervention, including emphasis on following a WFPB diet.
Health assessment. Once approved, all patients are instructed to complete baseline laboratory tests and questionnaires. Along with an electronic health record (EHR) review, a psychosocial assessment is completed by a licensed clinical health psychologist who assesses CVD history, eating behavior, exercise/physical activity, sleep, mental health, substance use, and social history, with the aim of enhancing our ability to help the patient to benefit from HDRP.29 The patient data are used to develop a case conceptualization (ie, integrated understanding of the particular patient’s psychiatric and medical diagnoses, behavioral patterns, social supports, lifestyle habits, strengths and weaknesses, and their interrelationships with each other and the patient’s environment), resulting in an individualized plan. Patients are encouraged to ask questions about the program, and those who are still interested are invited to attend a seminar. A request for medical clearance to participate in the program is initiated through the EHR or by patients scheduling an appointment with their HCP. All patients are medically cleared by their HCP for participation. Safe exercise recommendations also are provided and guide patient goals.
CVD risk profile. Patients complete psychosocial questionnaires and fasting laboratory tests to produce a tailored CVD risk profile. Laboratory tests include fasting lipid, fasting glucose, hemoglobin A1c (HbA1c) C-reactive protein, vitamin B12, and vitamin D. The same tests (excluding HbA1c) are completed 1 month later (after completing 4 group sessions) and again posttreatment (including HbA1c). Self-reported questionnaires are completed at the same time points, which include the Rate Your Plate dietary composition questionnaire, CHAMPS physical activity questionnaire for older adults, Beck Depression Inventory-II, and the Perceived Stress Scale.
Seminar. A 2-hour seminar provides patients and families with an opportunity to meet HDRP program staff, learn the background and rationale for chronic disease reversal, obtain a summary of the program, and hear a patient testimonial. Patients are asked to make a commitment, and the informed consent process includes all patients signing a behavioral contract.
Assessment and feedback. A licensed clinical health psychologist provides feedback to patients on their comprehensive CVD risk profile, using motivational interviewing.30,31 Smokers are encouraged to quit, and those interested are referred to their HCP and/or facility smoking cessation program.
Group sessions. Twelve weekly group sessions cover nutrition education and cooking, physical activity and exercise, stress management training, and medication reconciliation and adjustment. The nutrition component is the centerpiece of HDRP and is delivered by registered dietitians (Table 2). Patients are instructed to use the 3-week period between the HDRP seminar and the first core group session to try new recipes and prepare their kitchens, pantries, and mind-set to adopt the HDRP diet with 100% adherence. The WFPB diet used is consistent with the current guidelines of Caldwell Esselstyn, MD, and Dean Ornish, MD.32-34
A psychologist delivers the physical activity component. Patients are encouraged to meet the American Heart Association/American College of Cardiology recommendations for aerobic exercise (at least 150 minutes of moderate intensity physical activity per week) through a walking program.35 Patients with medical contraindications (eg, severe pain, mobility restrictions) are encouraged to follow the exercise/activity recommendations they had been given by their primary care provider (PCP), physical therapist, or other HCP.
A psychologist provides evidence-based cognitive behavioral stress management (CBSM) training, adapted from models developed for patients with stable ischemic heart disease, HIV/AIDS, and cancer.36-38 CBSM is a psychotherapy grounded in stress/coping theory and cognitive behavioral theory of psychopathology that integrates cognitive restructuring, coping skills training, communication/assertiveness training, anger management, and mindfulness/acceptance-based approaches. Additional emphasis is placed on assisting patients’ adjustment to the lifestyle challenges for following a plant-based diet, dealing with food cravings and emotional eating, and connecting lifestyle change to patients’ deepest values and goals.
A clinical pharmacist conducts a medication reconciliation for each patient at baseline. The pharmacist consults with each patient’s PCP, cardiologist, and HDRP consulting physician, as needed, to ensure safe adjustments to medications. Pharmacists also provide education on medications at group sessions.
After completion of the 12-week core program, graduates are encouraged to attend the monthly graduates’ group indefinitely, and as often as they desire to promote maintenance of the disease reversal lifestyle. Patients are encouraged to complete our recommended fasting laboratory work every 3 to 6 months to facilitate maintenance of treatment gains.
Program Evaluation
Patients frequently reported that the group format was vital to their success. Patients requested a cooking class, yet we lacked a full teaching kitchen. Integrating plant-based meal samples at every session and cooking videos helped. Patients reported that 100% adherence to the WFPB diet led to significant changes in their food preferences, including a loss of interest in meat.39 Patients encouraged us to keep the “disease reversal” language and focus. One veteran stated: “Disease reversal, that is the reason I called you when I got your letter.” Showing before and after images of coronary angiograms and cardiac positron emission tomography scans depicting regression of atherosclerotic plaque and restored myocardial perfusion were described as highly motivating and generated willingness to commit to a more aggressive lifestyle change.31
Patients routinely stated that they lacked understanding of their laboratory test results, which HDRP remedied. Some patients reported their adult children followed a plant-based diet, and our program resulted in a new commonality and source of bonding that was highly valued. Some patients reported that HDRP was helpful for controlling their COVID-19 anxiety and feeling in control of their health. Satisfaction surveys were completed by participants at the end of the core program, which demonstrated very high satisfaction with and acceptability of HDRP (Table 3).
The program also has received positive feedback from HCPs when we alert them to improvements in outcome measures for their patients. These HCPs expressed satisfaction with having a program to refer patients to that can help with chronic illness in more depth.
COVID-19 Response
Face-to-face group appointments were converted to videoconferencing as a result of the COVID-19 pandemic. While HDRP always promoted the use of technology and mHealth tools, the pandemic led us to develop novel technology-based interventions.40 One cohort transitioned from in-person to videoconferencing sessions, and 2 cohorts recently started this format and are ongoing. We have successfully used videoconferencing with Cisco Webex, the VA-approved backup platform, as we encountered technical barriers when using VA Video Connect. Program materials were shared electronically, and participants sent blood pressure/sugar logs by secure messaging. Guidance for online grocery shopping with home delivery was provided, and research on the benefits of the HDRP lifestyle on immune function was incorporated.
The stress management component incorporated coping with COVID-19, including normalizing common emotional difficulties with sheltering-in-place and quarantine, acknowledging and processing fear and anxiety related to being at very high risk for severe COVID-19. We presented heart disease reversal as an urgent and feasible goal during the pandemic both reducing risk of premature death and major adverse cardiovascular events in the long-term and also reducing personal risk of severe COVID complications. The new VA COVID Coach app was also presented as a resource. Reputable sources of COVID-19 and public health information were shared. Walking continued to be the primary recommended form of exercise, while indoor home exercise options were promoted during the periods of very poor air quality due to the widespread California fires and smoke.
Considering the research suggesting benefits of our intervention for treating T2DM,promoting sustained weight loss, and promoting comprehensive cardiometabolic risk reduction, we have begun accepting referrals for patients with any type of atherosclerotic CVD (eg, peripheral artery disease, carotid artery disease), patients with T2DM (without CVD), and patients with only a history of ischemic stroke or transient ischemic attack.24-27 Vascular surgery has become a new referral source, primarily for patients with peripheral and carotid artery diseases. Finally, we are leveraging videoconferencing and accepting referrals across the VA Northern California Health Care System (VANCHCS)catchment (from the California-Oregon state border to the San Francisco Bay Area). This also helps address a long-standing problem with reaching the many rural veterans who live far from a VA clinic. We successfully implemented a consult/referral process within the EHR that is available to providers across VANCHCS.
Discussion
The efficacy and effectiveness of reversal programs are well established in intensive programs (eg, ICR), yet such programs have yet to be streamlined and disseminated broadly into routine clinical care. HDRP has endeavored to address this by emphasizing nutrition relative to other program components. We have learned that the words “disease reversal” are very often the reason patients initially reach out or accept referral to our program.
Consistent with past research on plant-based nutrition interventions, the group format was indispensable.41 Individual sessions with a clinical health psychologist enabled tailored feedback and education on how behavior changes could impact laboratory results and how certain psychosocial factors could support success. Participants reported that seeing significantly favorable laboratory results was highly motivating and confirmed the power of their lifestyle changes. Furthermore, a psychosocial health assessment with individual sessions promoted a tailored treatment plan with targeted clinical interventions, such as behavioral health education, motivational interviewing, and advanced methods, including cognitive behavioral therapy and techniques drawn from dialectical behavior therapy and acceptance and commitment therapy.
Veterans with multimorbidity face the difficult task of learning and maintaining a complex disease self-management program and implementing a lifestyle approach that is feasible, effective, promotes weight loss, and treats multiple conditions. HDRP is a model approach for this population, as demonstrated by a recent case report of a 65-year-old male veteran with atherosclerotic CVD, T2DM, hypertension, and myasthenia gravis who had 2 heart attacks within 2 months.42 His neurologic disease precluded significant physical activity. Although he achieved some initial weight loss through lifestyle changes, he continued to have daily angina despite optimal and aggressive cardiology management. After enrolling in HDRP and adopting the WFPB diet, the patient reported almost complete resolution of angina within 1 month, similar to that found in other studies.15
The literature suggests that concern over the acceptability of plant-based diets and patients’ ability to adhere to them long-term may be misplaced. A review paper on dietary interventions lasting > 1 year found that 51 to 61% of vegetarian and vegan study participants had maintained dietary adherence, while 20 to 55% of omnivorous diet intervention participants adhered to their study diets.43 Remarkably, there were no statistically significant differences in the acceptability of the vegan, vegetarian, or omnivorous diets in the studies reviewed.43 Recent dietary research also suggests that providing patients with higher goals (eg, adopting a vegan diet instead of only moderate dietary changes) results in greater weight loss and maintenance.26 HDRP provides training on consumption of whole plant foods, which may offer patients a unique advantage for maximizing results and higher adherence over time.
Limitations
Hands-on cooking instruction was not provided at our VAMC. The total time of the intervention was significantly less in HDRP (25 hours) than it was for the Ornish ICR program (72 hours), which may hinder long-term adherence. Without an exercise facility, we were not able to provide more detailed exercise instruction and supervised exercise.
Program Improvements Planned
There are a number of improvements that are planned for HDRP. First, the program anticipates requesting medical clearance at the telephone screening stage for self-referred patients. Second,
Conclusions
Although our patient population was self-selected for participation, early program evaluation demonstrates high acceptability. Very few patients had ever been told about a heart disease reversing lifestyle, and we found direct-to-patient clinical outreach an effective method for launching a disease reversal program (optimally timed with HCP presentations). Furthermore, the program is adaptable to current restrictions on in-person appointments due to the COVID-19 pandemic, and much more convenient for rural veterans who live far from any VA clinic. Being able to offer sustainable health care for individuals during unexpected public health crises is critically important. Additionally, treating veterans who are most vulnerable to pandemic illness due to existing medical conditions, such as CVD, should be a high priority. Last, HDRP also may represent a novel integrated treatment for COVID-19 anxiety and secondary CVD prevention, as lifestyle habits are optimized to improve chronic diseases that elevate risk for severe COVID-19 infection and mortality, as well as including coping strategies consistent with evidence-based psychotherapies for anxiety disorders.44
We believe that beyond the clinical benefits to patients, there is significant value and benefit added to the health care system by offering an intervention within the “disease reversal” paradigm. Efforts of the health care team to reverse a disease can be considered the highest aim of medicine and health care.45
Acknowledgments
This work was supported by the US Department of Veterans Affairs. We give special thanks to David M. Gellerman, MD, PhD, and David W. Schafer, PsyD, for providing Mental Health Service support for initiating the Heart Disease Reversal Program, and to Joseph Giorgio, PsyD (Program Manager, Integrated Care Program) for sustaining it. We thank Amogh Bhat, MD, Chief of Cardiology, for his continued support and partnership with the Cardiology Department. We express thanks to Stephanie Mohney, RDN (Chief, Nutrition and Food Service), Amy Klotz, RDN (Supervisory Dietician), Sian M. Carr-Lopez, PharmD (Associate Chief of Pharmacy, Primary Care), and Michelle Rand, PharmD, CACP (Anticoagulation Clinical Pharmacist-Supervisor) for their staff support of this interdisciplinary program. We thank the patients and their families for their participation in the program and commitment to the lifestyle changes. We also thank the following individuals for their contributions to this program: Lisa Wagaman, RDN, Karen Soong, PharmD, Sara S. Ali, PharmD, Suzan Hua, PharmD, and Stephen Cooperman.
While cardiovascular mortality rates have declined, heart disease continues to be the leading cause of death in the US, and the number of people with cardiovascular disease (CVD) is rising.1 CVD is more prevalent among military veterans than it is among nonveterans aged ≥ 25 years, and veteran status is associated with higher risk of incident heart disease after controlling for socioeconomic status, other medical diseases, depression, and lifestyle.2-4 Combat exposure, posttraumatic stress disorder (PTSD), and Purple Heart commendation are associated with higher rates of CVD, including adverse cardiovascular events.5-7 Many patients seeking care in the Veterans Health Administration (VHA), including those who undergo cardiac catheterization, meet the criteria for multimorbidity (defined as having ≥ 2 chronic diseases8), which is common among veterans.9,10 Multimorbidity presents a challenge for lifestyle intervention, as different diets may be prescribed to treat different conditions, such as Dietary Approaches to Stop Hypertension, and low-glycemic diet for diabetes mellitus (DM). Veterans with CVD are often clinically complex and may require more multifaceted secondary prevention programs.
During the coronavirus 2019 (COVID-19) pandemic, effective secondary prevention intervention is needed more than ever. Older age, CVD, and common comorbidities, including hypertension, DM, and obesity, place patients at the highest risk for severe COVID-19 infection.11 COVID-19 social distancing encourages vulnerable populations to stay home, which can make engaging in any levels of physical activity more challenging. The International Food Council found that 85% of adults have made a change to their food consumption pattern, including eating more, during the COVID-19 pandemic.12 Thus, secondary CVD prevention programs for veterans need to provide treatment that addresses these specific challenges and can be delivered via telehealth for continuity of care after disruption of traditional services.
Clinical practice guidelines for the treatment of patients with recent cardiovascular adverse events (AEs) include a referral to cardiac rehabilitation (CR).13 CR emphasizes exercise as the main intervention, along with coaching to promote multiple risk reduction. The most comprehensive CR program is intensive CR (ICR), including the Ornish ICR program.14 ICR includes 4 components: vegetarian diet, exercise, stress management (yoga, meditation), and group support. Ornish ICR has been shown to be efficacious in randomized controlled trials (RCTs).15-17 Three effectiveness studies, with 5,372 participants, demonstrated the real-world effectiveness of Ornish ICR in US hospitals.14,18,19 The program also was adapted successfully for the active-duty military and veteran population.20,21 Yet Ornish ICR is time intensive, and there are no certified VHA ICR Ornish sites. Furthermore, there is no formal strategy for targeting people with atherosclerotic CVD who no longer meet the criteria for CR or ICR. While Ornish ICR is highly effective for patients who are eligible and have access, a more effective and streamlined approach is needed for targeting many patients.
Nutrition may be the most powerful Ornish ICR component. The initial RCT conducted by Ornish and colleagues included only stress management training and a whole-food, plant-based (WFPB) diet, including grains, legumes, vegetables, fruits, nuts, and seeds. The trial found 91% of participants experienced reduced angina after only 24 days.15 The only single-component intervention study resulting in partial reversal of atherosclerosis was a WFPB diet-only study, which documented regression of atherosclerotic plaques after 5 years, using coronary angiography in 73% of participants, with arrested progression in the other 27%.22 Participants reported no cardiovascular AEs after 12 years.23 Furthermore, a number of other recent studies have demonstrated the benefits of WFPB diet-only interventions for type 2 DM (T2DM), hypertension, and obesity.24-27 The Heart Disease Reversal Program (HDRP) was developed to create an interdisciplinary lifestyle intervention that emphasized nutrition for a broad population of veterans with atherosclerotic CVD, of varying levels of functional ability, to promote comprehensive CVD risk reduction and bring heart disease reversal intervention into routine clinical practice.
Program Description
The Mental Health, Cardiology, and Nutrition and Food services all approved the launch of HDRP. We contacted veterans by mail, and 11% expressed interest (Figure). Among patients who received the initial mailed letter (prior to our accepting staff referrals), only 5% of patients who enrolled in HDRP reported previously being told about or prescribed a WFPB diet by any health care provider (HCP). Currently, patients are primarily referred to HDRP by Cardiology, Primary Care, and Mental Health services.
Design
HDRP is an adaptation of interdisciplinary lifestyle interventions that have resulted in regression of atherosclerotic blockages confirmed with invasive coronary angiography.15-17,22,28 HDRP currently is offered in a Behavioral Medicine Clinic at the Sacramento US Department of Veterans Affairs (VA) Medical Center (VAMC) in California. Program staff include a clinical health psychologist who organizes, coordinates, and act as the lead facilitator of the program; registered dietitians; clinical pharmacists; and a consulting physician. Patients engage in the 4-month core HDRP program in small cohorts (ie, 6-10 patients), and spouses/partners are highly encouraged to attend all sessions.
Components
Telephone screening. Patients are screened for the inclusion and exclusion criteria (Table 1). Patients engaging in a traditional CR program are included in the screening. Patients are informed that the program consists of lifestyle intervention, including emphasis on following a WFPB diet.
Health assessment. Once approved, all patients are instructed to complete baseline laboratory tests and questionnaires. Along with an electronic health record (EHR) review, a psychosocial assessment is completed by a licensed clinical health psychologist who assesses CVD history, eating behavior, exercise/physical activity, sleep, mental health, substance use, and social history, with the aim of enhancing our ability to help the patient to benefit from HDRP.29 The patient data are used to develop a case conceptualization (ie, integrated understanding of the particular patient’s psychiatric and medical diagnoses, behavioral patterns, social supports, lifestyle habits, strengths and weaknesses, and their interrelationships with each other and the patient’s environment), resulting in an individualized plan. Patients are encouraged to ask questions about the program, and those who are still interested are invited to attend a seminar. A request for medical clearance to participate in the program is initiated through the EHR or by patients scheduling an appointment with their HCP. All patients are medically cleared by their HCP for participation. Safe exercise recommendations also are provided and guide patient goals.
CVD risk profile. Patients complete psychosocial questionnaires and fasting laboratory tests to produce a tailored CVD risk profile. Laboratory tests include fasting lipid, fasting glucose, hemoglobin A1c (HbA1c) C-reactive protein, vitamin B12, and vitamin D. The same tests (excluding HbA1c) are completed 1 month later (after completing 4 group sessions) and again posttreatment (including HbA1c). Self-reported questionnaires are completed at the same time points, which include the Rate Your Plate dietary composition questionnaire, CHAMPS physical activity questionnaire for older adults, Beck Depression Inventory-II, and the Perceived Stress Scale.
Seminar. A 2-hour seminar provides patients and families with an opportunity to meet HDRP program staff, learn the background and rationale for chronic disease reversal, obtain a summary of the program, and hear a patient testimonial. Patients are asked to make a commitment, and the informed consent process includes all patients signing a behavioral contract.
Assessment and feedback. A licensed clinical health psychologist provides feedback to patients on their comprehensive CVD risk profile, using motivational interviewing.30,31 Smokers are encouraged to quit, and those interested are referred to their HCP and/or facility smoking cessation program.
Group sessions. Twelve weekly group sessions cover nutrition education and cooking, physical activity and exercise, stress management training, and medication reconciliation and adjustment. The nutrition component is the centerpiece of HDRP and is delivered by registered dietitians (Table 2). Patients are instructed to use the 3-week period between the HDRP seminar and the first core group session to try new recipes and prepare their kitchens, pantries, and mind-set to adopt the HDRP diet with 100% adherence. The WFPB diet used is consistent with the current guidelines of Caldwell Esselstyn, MD, and Dean Ornish, MD.32-34
A psychologist delivers the physical activity component. Patients are encouraged to meet the American Heart Association/American College of Cardiology recommendations for aerobic exercise (at least 150 minutes of moderate intensity physical activity per week) through a walking program.35 Patients with medical contraindications (eg, severe pain, mobility restrictions) are encouraged to follow the exercise/activity recommendations they had been given by their primary care provider (PCP), physical therapist, or other HCP.
A psychologist provides evidence-based cognitive behavioral stress management (CBSM) training, adapted from models developed for patients with stable ischemic heart disease, HIV/AIDS, and cancer.36-38 CBSM is a psychotherapy grounded in stress/coping theory and cognitive behavioral theory of psychopathology that integrates cognitive restructuring, coping skills training, communication/assertiveness training, anger management, and mindfulness/acceptance-based approaches. Additional emphasis is placed on assisting patients’ adjustment to the lifestyle challenges for following a plant-based diet, dealing with food cravings and emotional eating, and connecting lifestyle change to patients’ deepest values and goals.
A clinical pharmacist conducts a medication reconciliation for each patient at baseline. The pharmacist consults with each patient’s PCP, cardiologist, and HDRP consulting physician, as needed, to ensure safe adjustments to medications. Pharmacists also provide education on medications at group sessions.
After completion of the 12-week core program, graduates are encouraged to attend the monthly graduates’ group indefinitely, and as often as they desire to promote maintenance of the disease reversal lifestyle. Patients are encouraged to complete our recommended fasting laboratory work every 3 to 6 months to facilitate maintenance of treatment gains.
Program Evaluation
Patients frequently reported that the group format was vital to their success. Patients requested a cooking class, yet we lacked a full teaching kitchen. Integrating plant-based meal samples at every session and cooking videos helped. Patients reported that 100% adherence to the WFPB diet led to significant changes in their food preferences, including a loss of interest in meat.39 Patients encouraged us to keep the “disease reversal” language and focus. One veteran stated: “Disease reversal, that is the reason I called you when I got your letter.” Showing before and after images of coronary angiograms and cardiac positron emission tomography scans depicting regression of atherosclerotic plaque and restored myocardial perfusion were described as highly motivating and generated willingness to commit to a more aggressive lifestyle change.31
Patients routinely stated that they lacked understanding of their laboratory test results, which HDRP remedied. Some patients reported their adult children followed a plant-based diet, and our program resulted in a new commonality and source of bonding that was highly valued. Some patients reported that HDRP was helpful for controlling their COVID-19 anxiety and feeling in control of their health. Satisfaction surveys were completed by participants at the end of the core program, which demonstrated very high satisfaction with and acceptability of HDRP (Table 3).
The program also has received positive feedback from HCPs when we alert them to improvements in outcome measures for their patients. These HCPs expressed satisfaction with having a program to refer patients to that can help with chronic illness in more depth.
COVID-19 Response
Face-to-face group appointments were converted to videoconferencing as a result of the COVID-19 pandemic. While HDRP always promoted the use of technology and mHealth tools, the pandemic led us to develop novel technology-based interventions.40 One cohort transitioned from in-person to videoconferencing sessions, and 2 cohorts recently started this format and are ongoing. We have successfully used videoconferencing with Cisco Webex, the VA-approved backup platform, as we encountered technical barriers when using VA Video Connect. Program materials were shared electronically, and participants sent blood pressure/sugar logs by secure messaging. Guidance for online grocery shopping with home delivery was provided, and research on the benefits of the HDRP lifestyle on immune function was incorporated.
The stress management component incorporated coping with COVID-19, including normalizing common emotional difficulties with sheltering-in-place and quarantine, acknowledging and processing fear and anxiety related to being at very high risk for severe COVID-19. We presented heart disease reversal as an urgent and feasible goal during the pandemic both reducing risk of premature death and major adverse cardiovascular events in the long-term and also reducing personal risk of severe COVID complications. The new VA COVID Coach app was also presented as a resource. Reputable sources of COVID-19 and public health information were shared. Walking continued to be the primary recommended form of exercise, while indoor home exercise options were promoted during the periods of very poor air quality due to the widespread California fires and smoke.
Considering the research suggesting benefits of our intervention for treating T2DM,promoting sustained weight loss, and promoting comprehensive cardiometabolic risk reduction, we have begun accepting referrals for patients with any type of atherosclerotic CVD (eg, peripheral artery disease, carotid artery disease), patients with T2DM (without CVD), and patients with only a history of ischemic stroke or transient ischemic attack.24-27 Vascular surgery has become a new referral source, primarily for patients with peripheral and carotid artery diseases. Finally, we are leveraging videoconferencing and accepting referrals across the VA Northern California Health Care System (VANCHCS)catchment (from the California-Oregon state border to the San Francisco Bay Area). This also helps address a long-standing problem with reaching the many rural veterans who live far from a VA clinic. We successfully implemented a consult/referral process within the EHR that is available to providers across VANCHCS.
Discussion
The efficacy and effectiveness of reversal programs are well established in intensive programs (eg, ICR), yet such programs have yet to be streamlined and disseminated broadly into routine clinical care. HDRP has endeavored to address this by emphasizing nutrition relative to other program components. We have learned that the words “disease reversal” are very often the reason patients initially reach out or accept referral to our program.
Consistent with past research on plant-based nutrition interventions, the group format was indispensable.41 Individual sessions with a clinical health psychologist enabled tailored feedback and education on how behavior changes could impact laboratory results and how certain psychosocial factors could support success. Participants reported that seeing significantly favorable laboratory results was highly motivating and confirmed the power of their lifestyle changes. Furthermore, a psychosocial health assessment with individual sessions promoted a tailored treatment plan with targeted clinical interventions, such as behavioral health education, motivational interviewing, and advanced methods, including cognitive behavioral therapy and techniques drawn from dialectical behavior therapy and acceptance and commitment therapy.
Veterans with multimorbidity face the difficult task of learning and maintaining a complex disease self-management program and implementing a lifestyle approach that is feasible, effective, promotes weight loss, and treats multiple conditions. HDRP is a model approach for this population, as demonstrated by a recent case report of a 65-year-old male veteran with atherosclerotic CVD, T2DM, hypertension, and myasthenia gravis who had 2 heart attacks within 2 months.42 His neurologic disease precluded significant physical activity. Although he achieved some initial weight loss through lifestyle changes, he continued to have daily angina despite optimal and aggressive cardiology management. After enrolling in HDRP and adopting the WFPB diet, the patient reported almost complete resolution of angina within 1 month, similar to that found in other studies.15
The literature suggests that concern over the acceptability of plant-based diets and patients’ ability to adhere to them long-term may be misplaced. A review paper on dietary interventions lasting > 1 year found that 51 to 61% of vegetarian and vegan study participants had maintained dietary adherence, while 20 to 55% of omnivorous diet intervention participants adhered to their study diets.43 Remarkably, there were no statistically significant differences in the acceptability of the vegan, vegetarian, or omnivorous diets in the studies reviewed.43 Recent dietary research also suggests that providing patients with higher goals (eg, adopting a vegan diet instead of only moderate dietary changes) results in greater weight loss and maintenance.26 HDRP provides training on consumption of whole plant foods, which may offer patients a unique advantage for maximizing results and higher adherence over time.
Limitations
Hands-on cooking instruction was not provided at our VAMC. The total time of the intervention was significantly less in HDRP (25 hours) than it was for the Ornish ICR program (72 hours), which may hinder long-term adherence. Without an exercise facility, we were not able to provide more detailed exercise instruction and supervised exercise.
Program Improvements Planned
There are a number of improvements that are planned for HDRP. First, the program anticipates requesting medical clearance at the telephone screening stage for self-referred patients. Second,
Conclusions
Although our patient population was self-selected for participation, early program evaluation demonstrates high acceptability. Very few patients had ever been told about a heart disease reversing lifestyle, and we found direct-to-patient clinical outreach an effective method for launching a disease reversal program (optimally timed with HCP presentations). Furthermore, the program is adaptable to current restrictions on in-person appointments due to the COVID-19 pandemic, and much more convenient for rural veterans who live far from any VA clinic. Being able to offer sustainable health care for individuals during unexpected public health crises is critically important. Additionally, treating veterans who are most vulnerable to pandemic illness due to existing medical conditions, such as CVD, should be a high priority. Last, HDRP also may represent a novel integrated treatment for COVID-19 anxiety and secondary CVD prevention, as lifestyle habits are optimized to improve chronic diseases that elevate risk for severe COVID-19 infection and mortality, as well as including coping strategies consistent with evidence-based psychotherapies for anxiety disorders.44
We believe that beyond the clinical benefits to patients, there is significant value and benefit added to the health care system by offering an intervention within the “disease reversal” paradigm. Efforts of the health care team to reverse a disease can be considered the highest aim of medicine and health care.45
Acknowledgments
This work was supported by the US Department of Veterans Affairs. We give special thanks to David M. Gellerman, MD, PhD, and David W. Schafer, PsyD, for providing Mental Health Service support for initiating the Heart Disease Reversal Program, and to Joseph Giorgio, PsyD (Program Manager, Integrated Care Program) for sustaining it. We thank Amogh Bhat, MD, Chief of Cardiology, for his continued support and partnership with the Cardiology Department. We express thanks to Stephanie Mohney, RDN (Chief, Nutrition and Food Service), Amy Klotz, RDN (Supervisory Dietician), Sian M. Carr-Lopez, PharmD (Associate Chief of Pharmacy, Primary Care), and Michelle Rand, PharmD, CACP (Anticoagulation Clinical Pharmacist-Supervisor) for their staff support of this interdisciplinary program. We thank the patients and their families for their participation in the program and commitment to the lifestyle changes. We also thank the following individuals for their contributions to this program: Lisa Wagaman, RDN, Karen Soong, PharmD, Sara S. Ali, PharmD, Suzan Hua, PharmD, and Stephen Cooperman.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12 ): e493]. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
2. Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chronic Illn. 2020;16(1):55-68. doi:10.1177/1742395318785237.
3. Hinojosa R. Sex, age, race/ethnicity, veteran status, and the likelihood of reporting cardiovascular conditions in the National Health Interview Survey. J Cardiovasc Nurs. 2019;34(3):215-221. doi:10.1097/JCN.0000000000000561 4. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
5. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans Study. Prim Care Companion CNS Disord. 2017;19(3):10.4088/PCC.17m02118. Published 2017 Jun 22. doi:10.4088/PCC.17m02118
6. Bukhbinder AS, Wang AC, Qureshi SU, et al. Increased vascular pathology in older veterans with a purple heart commendation or chronic post-traumatic stress disorder. J Geriatr Psychiatry Neurol. 2020;33(4):195-206. doi:10.1177/0891988719868308
7. Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320-329. doi:10.1016/S2215-0366(16)30377-7
8. Forman DE, Maurer MS, Boyd C, et a;. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149-2161. doi:10.1016/j.jacc.2018.03.022
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114(11):1750-1757. doi:10.1016/j.amjcard.2014.08.045
11. Centers for Disease Control and Prevention. Coronavirus 2019 (COVID-19):people at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. Updated September 11, 2020. Accessed November 12, 2020.
12. International Food Information Council. 2020 food and health survey. https://foodinsight.org/2020-food-and-health-survey. Updated June 9, 2020. Accessed November 12, 2020.
13. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
14. Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot. 2010;24(4):260-266. doi:10.4278/ajhp.24.4.arb
15. Ornish D, Scherwitz LW, Doody RS, et al. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54-59.
16. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129-133. doi:10.1016/0140-6736(90)91656-u.
17. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease [published correction appears in JAMA 1999 Apr 21;281(15):1380]. JAMA. 1998;280(23):2001-2007. doi:10.1001/jama.280.23.2001
18. Frattaroli J, Weidner G, Merritt-Worden TA, Frenda S, Ornish D. Angina pectoris and atherosclerotic risk factors in the multisite cardiac lifestyle intervention program. Am J Cardiol. 2008;101(7):911-918. doi:10.1016/j.amjcard.2007.11.039
19. Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the Multicenter Lifestyle Demonstration Project. Am J Cardiol. 2003;91(11):1316-1322. doi:10.1016/s0002-9149(03)00320-5
20. Marshall DA, Walizer EM, Vernalis MN. Achievement of heart health characteristics through participation in an intensive lifestyle change program (Coronary Artery Disease Reversal Study). J Cardiopulm Rehabil Prev. 2009;29(2):84-96. doi:10.1097/HCR.0b013e31819a00b2
21. Marshall D, Elaine W, Vernalis M. The effect of a one-year lifestyle intervention program on carotid intima media thickness. Mil Med. 2011;176(7):798-804. doi:10.7205/milmed-d-10-00447
22. Esselstyn CB Jr, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6):560-568.
23. Esselstyn CB Jr. Updating a 12-year experience with arrest and reversal therapy for coronary heart disease (an overdue requiem for palliative cardiology). Am J Cardiol. 1999;84(3):339-A8. doi:10.1016/s0002-9149(99)00290-8
24. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777-1783. doi:10.2337/dc06-0606
25. McDougall J, Thomas LE, McDougall C, et al. Effects of 7 days on an ad libitum low-fat vegan diet: the McDougall Program cohort [published correction appears in Nutr J. 2017 Feb 10;16(1):12]. Nutr J. 2014;13:99. Published 2014 Oct 14. doi:10.1186/1475-2891-13-99
26. Turner-McGrievy GM, Davidson CR, Wingard EE, Wilcox S, Frongillo EA. Comparative effectiveness of plant-based diets for weight loss: a randomized controlled trial of five different diets. Nutrition. 2015;31(2):350-358. doi:10.1016/j.nut.2014.09.002
27. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. Published 2017 Mar 20. doi:10.1038/nutd.2017.3

28. Schaefer S, Hussein H, Gershony GR, Rutledge JC, Kappagoda CT. Regression of severe atherosclerotic plaque in patients with mild elevation of LDL cholesterol. J Investig Med. 1997;45(9):536-541.
29. Kitazono R. Know thy patient: Enhancing lifestyle interventions with psychological assessment. Int J Dis Rev Prev. 2020;2(1):76-81.
30. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013.
31. Mascola AJ, Yiaslas TA, Meir RL, et al. Framing physical activity as a distinct and uniquely valuable behavior independent of weight management: A pilot randomized controlled trial for overweight and obese sedentary persons. Eat Weight Disord. 2009;14(2-3):e148-e152. doi:10.1007/BF03327814
32. Esselstyn AC, Esselstyn J. The Prevent and Reverse Heart Disease Cookbook: Over 125 Delicious, Life-Changing, Plant-Based Recipes. New York, NY: Avery; 2014.
33. Esselstyn CB Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356-364.
34. Ornish D, Ornish A. Undo It! How Simple Lifestyle Changes Can Reverse Most Chronic Diseases. New York, NY: Ballantine Books; 2019.
35. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association [published correction appears in J Am Coll Cardiol. 2015 Apr 14;65(14):1495. Dosage error in article text.]. J Am Coll Cardiol. 2011;58(23):2432-2446. doi:10.1016/j.jacc.2011.10.824
36. Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164-168. doi:10.1016/s0002-9149(01)02194-4
37. Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173-188. doi:10.1080/1025389031000156727
38. Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261-270. doi:10.1207/s15324796abm3103_8
39. Yiaslas TA. “Look doctor, I’m a carnivore.” Int J Dis Rev Prev. 2020;2(2):35-39.
40. Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010;16(9):931-938. doi:10.1089/tmj.2010.0065
41. Barnard ND, Sherwitz L, Ornish D. Adherence and acceptability of a low-fat, vegetarian diet among patients with cardiac disease. J Cardiopulm Rehabil. 1992;12(6):423-431.
42. Yiaslas TA, Taylor J, Embree J, Schaefer S. Elimination of angina, comprehensive cardio-metabolic risk reduction, and 50-pound weight loss in a US Navy veteran with myasthenia gravis. Int J Dis Rev Prev. 2019;1(1):77-83.
43. Berkow SE, Barnard N, Eckart J, Katcher H. Four therapeutic diets: adherence and acceptability. Can J Diet Pract Res. 2010;71(4):199-204. doi:10.3148/71.4.2010.199
44. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
45. Yiaslas TA. The pursuit of arete in medicine and health care. Int J Dis Rev Prev. 2019;1(2):53-56.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12 ): e493]. Circulation. 2018;137(12):e67-e492. doi:10.1161/CIR.0000000000000558
2. Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chronic Illn. 2020;16(1):55-68. doi:10.1177/1742395318785237.
3. Hinojosa R. Sex, age, race/ethnicity, veteran status, and the likelihood of reporting cardiovascular conditions in the National Health Interview Survey. J Cardiovasc Nurs. 2019;34(3):215-221. doi:10.1097/JCN.0000000000000561 4. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
5. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans Study. Prim Care Companion CNS Disord. 2017;19(3):10.4088/PCC.17m02118. Published 2017 Jun 22. doi:10.4088/PCC.17m02118
6. Bukhbinder AS, Wang AC, Qureshi SU, et al. Increased vascular pathology in older veterans with a purple heart commendation or chronic post-traumatic stress disorder. J Geriatr Psychiatry Neurol. 2020;33(4):195-206. doi:10.1177/0891988719868308
7. Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320-329. doi:10.1016/S2215-0366(16)30377-7
8. Forman DE, Maurer MS, Boyd C, et a;. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149-2161. doi:10.1016/j.jacc.2018.03.022
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114(11):1750-1757. doi:10.1016/j.amjcard.2014.08.045
11. Centers for Disease Control and Prevention. Coronavirus 2019 (COVID-19):people at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. Updated September 11, 2020. Accessed November 12, 2020.
12. International Food Information Council. 2020 food and health survey. https://foodinsight.org/2020-food-and-health-survey. Updated June 9, 2020. Accessed November 12, 2020.
13. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
14. Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot. 2010;24(4):260-266. doi:10.4278/ajhp.24.4.arb
15. Ornish D, Scherwitz LW, Doody RS, et al. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54-59.
16. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129-133. doi:10.1016/0140-6736(90)91656-u.
17. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease [published correction appears in JAMA 1999 Apr 21;281(15):1380]. JAMA. 1998;280(23):2001-2007. doi:10.1001/jama.280.23.2001
18. Frattaroli J, Weidner G, Merritt-Worden TA, Frenda S, Ornish D. Angina pectoris and atherosclerotic risk factors in the multisite cardiac lifestyle intervention program. Am J Cardiol. 2008;101(7):911-918. doi:10.1016/j.amjcard.2007.11.039
19. Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the Multicenter Lifestyle Demonstration Project. Am J Cardiol. 2003;91(11):1316-1322. doi:10.1016/s0002-9149(03)00320-5
20. Marshall DA, Walizer EM, Vernalis MN. Achievement of heart health characteristics through participation in an intensive lifestyle change program (Coronary Artery Disease Reversal Study). J Cardiopulm Rehabil Prev. 2009;29(2):84-96. doi:10.1097/HCR.0b013e31819a00b2
21. Marshall D, Elaine W, Vernalis M. The effect of a one-year lifestyle intervention program on carotid intima media thickness. Mil Med. 2011;176(7):798-804. doi:10.7205/milmed-d-10-00447
22. Esselstyn CB Jr, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6):560-568.
23. Esselstyn CB Jr. Updating a 12-year experience with arrest and reversal therapy for coronary heart disease (an overdue requiem for palliative cardiology). Am J Cardiol. 1999;84(3):339-A8. doi:10.1016/s0002-9149(99)00290-8
24. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777-1783. doi:10.2337/dc06-0606
25. McDougall J, Thomas LE, McDougall C, et al. Effects of 7 days on an ad libitum low-fat vegan diet: the McDougall Program cohort [published correction appears in Nutr J. 2017 Feb 10;16(1):12]. Nutr J. 2014;13:99. Published 2014 Oct 14. doi:10.1186/1475-2891-13-99
26. Turner-McGrievy GM, Davidson CR, Wingard EE, Wilcox S, Frongillo EA. Comparative effectiveness of plant-based diets for weight loss: a randomized controlled trial of five different diets. Nutrition. 2015;31(2):350-358. doi:10.1016/j.nut.2014.09.002
27. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. Published 2017 Mar 20. doi:10.1038/nutd.2017.3

28. Schaefer S, Hussein H, Gershony GR, Rutledge JC, Kappagoda CT. Regression of severe atherosclerotic plaque in patients with mild elevation of LDL cholesterol. J Investig Med. 1997;45(9):536-541.
29. Kitazono R. Know thy patient: Enhancing lifestyle interventions with psychological assessment. Int J Dis Rev Prev. 2020;2(1):76-81.
30. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013.
31. Mascola AJ, Yiaslas TA, Meir RL, et al. Framing physical activity as a distinct and uniquely valuable behavior independent of weight management: A pilot randomized controlled trial for overweight and obese sedentary persons. Eat Weight Disord. 2009;14(2-3):e148-e152. doi:10.1007/BF03327814
32. Esselstyn AC, Esselstyn J. The Prevent and Reverse Heart Disease Cookbook: Over 125 Delicious, Life-Changing, Plant-Based Recipes. New York, NY: Avery; 2014.
33. Esselstyn CB Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356-364.
34. Ornish D, Ornish A. Undo It! How Simple Lifestyle Changes Can Reverse Most Chronic Diseases. New York, NY: Ballantine Books; 2019.
35. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association [published correction appears in J Am Coll Cardiol. 2015 Apr 14;65(14):1495. Dosage error in article text.]. J Am Coll Cardiol. 2011;58(23):2432-2446. doi:10.1016/j.jacc.2011.10.824
36. Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164-168. doi:10.1016/s0002-9149(01)02194-4
37. Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173-188. doi:10.1080/1025389031000156727
38. Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261-270. doi:10.1207/s15324796abm3103_8
39. Yiaslas TA. “Look doctor, I’m a carnivore.” Int J Dis Rev Prev. 2020;2(2):35-39.
40. Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010;16(9):931-938. doi:10.1089/tmj.2010.0065
41. Barnard ND, Sherwitz L, Ornish D. Adherence and acceptability of a low-fat, vegetarian diet among patients with cardiac disease. J Cardiopulm Rehabil. 1992;12(6):423-431.
42. Yiaslas TA, Taylor J, Embree J, Schaefer S. Elimination of angina, comprehensive cardio-metabolic risk reduction, and 50-pound weight loss in a US Navy veteran with myasthenia gravis. Int J Dis Rev Prev. 2019;1(1):77-83.
43. Berkow SE, Barnard N, Eckart J, Katcher H. Four therapeutic diets: adherence and acceptability. Can J Diet Pract Res. 2010;71(4):199-204. doi:10.3148/71.4.2010.199
44. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514. doi:10.1002/da.22728
45. Yiaslas TA. The pursuit of arete in medicine and health care. Int J Dis Rev Prev. 2019;1(2):53-56.