User login
Carotenoderma Associated With a Diet Rich in Red Palm Oil
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
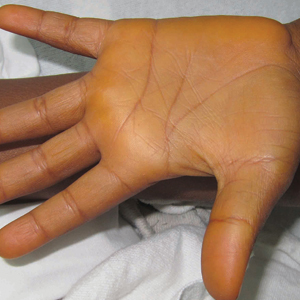
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
Practice Points
- Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands.
- Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment.
Wearable Health Device Dermatitis: A Case of Acrylate-Related Contact Allergy
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
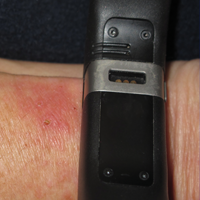
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).
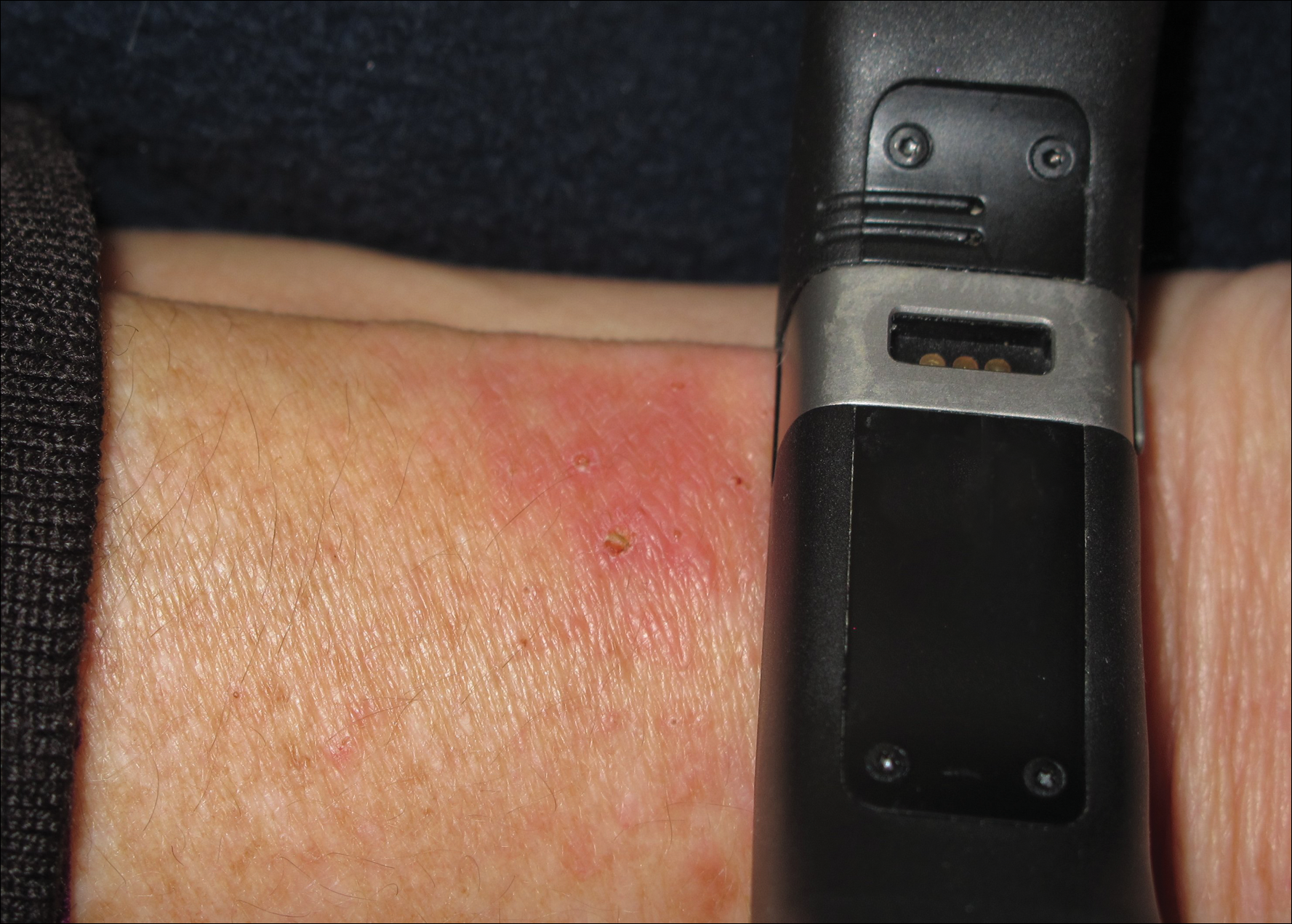
Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).



Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).



Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).



Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Practice Points
- Mobile wearable health devices are likely to become an important potential source of contact sensitization as their use increases given their often prolonged contact time with the skin.
- Mobile wearable health devices may pose a risk for allergic contact dermatitis as a result of a variety of components that come into contact with the skin, including but not limited to metals, rubber components, adhesives, and dyes.