User login
Carotenoderma Associated With a Diet Rich in Red Palm Oil
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
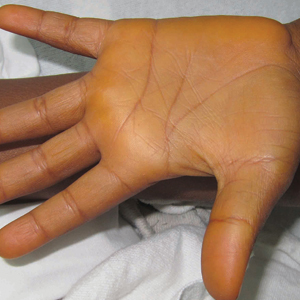
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
To the Editor:
Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands. We present a case of carotenoderma associated with a diet rich in red palm oil, a common food additive in parts of the world outside of the United States.
A previously healthy 8-year-old boy who recently immigrated to the United States from Liberia was hospitalized for treatment of a febrile illness that subsequently was attributed to a viral syndrome. On physical examination by the dermatology department, the patient was noted to have marked orange discoloration on the palms and soles (Figure). Laboratory workup revealed elevated serum β-carotene levels of 809 μg/dL (reference range, 10–85 μg/dL). Testing of hemoglobin/hematocrit levels and liver, thyroid, and kidney function was normal, and systemic examination revealed no further abnormalities. Upon further inquiry by the dermatology department, the patient’s family reported frequent addition of red palm oil to all of the child’s meals. The patient subsequently was diagnosed with carotenoderma and was instructed to limit inclusion of red palm oil in his diet.

Red palm oil is a rich source of β-carotene and is commonly used outside the United States as a dietary supplement or food flavoring. Excessive consumption of red palm oil or other sources rich in carotenes can result in elevated serum carotene levels or hypercarotenemia. An elevation in serum β-carotene levels may be recognized from 4 to 7 weeks after starting a β-carotene–rich diet.1
While dietary consumption of carotenes is the most common cause of carotenoderma, others include kidney or liver disease, hyperlipidemia, porphyria, diabetes mellitus, hypothyroidism, and anorexia nervosa.2-4 Moreover, since carotenoids are enzymatically converted to vitamin A in the small intestine, a mutation of the gene of the conversion enzyme β-carotene 15,15’-monooxygenase 1 (BCMO1) also can cause be a rare cause of hypercarotenemia.3
Carotenoderma, the clinical cutaneous manifestation of hypercarotenemia, occurs as a result of β-carotene deposits in the skin when serum concentration exceeds 250 μg/dL. More specifically, β-carotene accumulates mainly in the lipid-rich stratum corneum as well as in sweat and sebum, which explains the localized discoloration in fatty tissues and areas rich in sweat glands (eg, nasolabial folds, palms, soles).3,4 The sclerae of the eyes are not affected by the surplus of β-carotene in carotenoderma, which helps distinguish it from jaundice.5
The differential diagnosis of yellow discoloration of the skin includes jaundice, encompassing the prehepatic, hepatocellular, and posthepatic categories.4 Also noteworthy in the differential diagnosis is lycopenemia, which occurs as a result of eating lycopene-rich foods (eg, tomatoes), resulting in a deeper orange-yellow pigmentation when compared to the cutaneous manifestation of hypercarotenemia.2,4,6 Several drugs also have been reported to induce yellow discoloration of the skin, including sunitinib,7 sorafenib,8 quinacrine, saffron supplements, santonin, fluorescein, 2,4-dinitrophenol, canthaxanthin, tetryl and picric acids, and acriflavine.2,4
Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment. Contrary to popular belief, vitamin A toxicity does not occur in the presence of a surplus of β-carotenes because the enzymatic conversion of β-carotene to vitamin A is strictly regulated.9 Although acknowledging the various causes of carotenoderma is important, a simple history and laboratory testing for elevated serum β-carotene levels can eliminate further unnecessary testing and allow for prompt recognition of the condition. Appropriate dietary modifications also may be warranted.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
- Roe DA. Assessment of risk factors for carotenodermia and cutaneous signs of hypervitaminosis A in college-aged populations. Semin Dermatol. 1991;10:303-308.
- Manolios N, Samaras K. Hypercarotenaemia. Intern Med J. 2006;36:534.
- Wageesha ND, Ekanayake S, Jansz ER, et al. Studies on hypercarotenemia due to excessive ingestion of carrot, pumpkin and papaw [published online September 27, 2010]. Int J Food Sci Nutr. 2011;62:20-25.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Maruani A, Labarthe F, Dupré T, et al. Hypercarotenaemia in an infant [in French]. Ann Dermatol Venereol. 2010;137:32-35.
- Shaw JA, Koti M. Clinical images. CMAJ. 2009;180:895.
- Vignand-Courtin C, Martin C, Le Beller C, et al. Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int J Clin Pharm. 2012;34:286-289.
- Dasanu CA, Alexandrescu DT, Dutcher J. Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100:328-330.
- Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
Practice Points
- Carotenoderma is a cutaneous manifestation of elevated serum β-carotene levels and classically localizes to fatty tissues and areas rich in sweat glands.
- Carotenoderma caused by a diet rich in β-carotene is a benign condition in which a diet low in β-carotene is implicated for treatment.
Pediatric psoriasis may have a distinct presentation
Children may have a distinctive presentation of psoriasis, compared with adults, Dr. Wynnis Tom said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Infants may present with diaper involvement, also known as “napkin psoriasis,” which may be confused with irritant dermatitis or perineal infections. Moreover, guttate psoriasis, which can be preceded by infectious triggers such as Streptococcus and appears as small, salmon-pink bumps on the skin, is more frequent in children than adults, said Dr. Tom, associate professor of dermatology and pediatrics at the university.
Patients with psoriasis are at higher risk for psychiatric disorders, especially depression and anxiety. A study by Varni et al. discussed QOL ratings by 208 children aged 4-17 years with moderate to severe plaque disease. The study demonstrated a significant negative QOL impact in patients with plaque psoriasis, comparable to the impairment of QOL from arthritis or asthma (Eur J Pediatr. 2011 Sep 30;171[3]485-92).
Dr. Tom talked about other comorbidities associated with psoriasis, including psoriatic arthritis, and encouraged physicians to inquire about morning stiffness, joint pains, swelling, and gait abnormalities. “Psoriatic arthritis occurs in about 10% of children, and it is essential to detect early to prevent permanent joint damage,” she said. “Over the past decade, psoriasis has resurfaced as a systemic disorder as it may be associated with obesity, metabolic syndrome, and inflammatory bowel disease.” Psoriasis also entails an increased risk for cardiovascular disease, myocardial infarction, and stroke.
Dr. Tom emphasized, “because of these risks, we need to extend comorbidity screening to the pediatric population.”
Management of pediatric psoriasis has focused on topical and systemic therapies, in addition to phototherapies. Most systemic agents are used off-label on the basis of experience rather than evidence. Clinical trials are currently underway to extend indications for systemic therapy to the pediatric age group, she said.
Dr. Tom disclosed she is an investigator for Promius Pharma, Celgene, and Janssen.
Children may have a distinctive presentation of psoriasis, compared with adults, Dr. Wynnis Tom said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Infants may present with diaper involvement, also known as “napkin psoriasis,” which may be confused with irritant dermatitis or perineal infections. Moreover, guttate psoriasis, which can be preceded by infectious triggers such as Streptococcus and appears as small, salmon-pink bumps on the skin, is more frequent in children than adults, said Dr. Tom, associate professor of dermatology and pediatrics at the university.
Patients with psoriasis are at higher risk for psychiatric disorders, especially depression and anxiety. A study by Varni et al. discussed QOL ratings by 208 children aged 4-17 years with moderate to severe plaque disease. The study demonstrated a significant negative QOL impact in patients with plaque psoriasis, comparable to the impairment of QOL from arthritis or asthma (Eur J Pediatr. 2011 Sep 30;171[3]485-92).
Dr. Tom talked about other comorbidities associated with psoriasis, including psoriatic arthritis, and encouraged physicians to inquire about morning stiffness, joint pains, swelling, and gait abnormalities. “Psoriatic arthritis occurs in about 10% of children, and it is essential to detect early to prevent permanent joint damage,” she said. “Over the past decade, psoriasis has resurfaced as a systemic disorder as it may be associated with obesity, metabolic syndrome, and inflammatory bowel disease.” Psoriasis also entails an increased risk for cardiovascular disease, myocardial infarction, and stroke.
Dr. Tom emphasized, “because of these risks, we need to extend comorbidity screening to the pediatric population.”
Management of pediatric psoriasis has focused on topical and systemic therapies, in addition to phototherapies. Most systemic agents are used off-label on the basis of experience rather than evidence. Clinical trials are currently underway to extend indications for systemic therapy to the pediatric age group, she said.
Dr. Tom disclosed she is an investigator for Promius Pharma, Celgene, and Janssen.
Children may have a distinctive presentation of psoriasis, compared with adults, Dr. Wynnis Tom said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Infants may present with diaper involvement, also known as “napkin psoriasis,” which may be confused with irritant dermatitis or perineal infections. Moreover, guttate psoriasis, which can be preceded by infectious triggers such as Streptococcus and appears as small, salmon-pink bumps on the skin, is more frequent in children than adults, said Dr. Tom, associate professor of dermatology and pediatrics at the university.
Patients with psoriasis are at higher risk for psychiatric disorders, especially depression and anxiety. A study by Varni et al. discussed QOL ratings by 208 children aged 4-17 years with moderate to severe plaque disease. The study demonstrated a significant negative QOL impact in patients with plaque psoriasis, comparable to the impairment of QOL from arthritis or asthma (Eur J Pediatr. 2011 Sep 30;171[3]485-92).
Dr. Tom talked about other comorbidities associated with psoriasis, including psoriatic arthritis, and encouraged physicians to inquire about morning stiffness, joint pains, swelling, and gait abnormalities. “Psoriatic arthritis occurs in about 10% of children, and it is essential to detect early to prevent permanent joint damage,” she said. “Over the past decade, psoriasis has resurfaced as a systemic disorder as it may be associated with obesity, metabolic syndrome, and inflammatory bowel disease.” Psoriasis also entails an increased risk for cardiovascular disease, myocardial infarction, and stroke.
Dr. Tom emphasized, “because of these risks, we need to extend comorbidity screening to the pediatric population.”
Management of pediatric psoriasis has focused on topical and systemic therapies, in addition to phototherapies. Most systemic agents are used off-label on the basis of experience rather than evidence. Clinical trials are currently underway to extend indications for systemic therapy to the pediatric age group, she said.
Dr. Tom disclosed she is an investigator for Promius Pharma, Celgene, and Janssen.
Identifying the four key findings in patients with suspected severe drug reactions
There are four key findings in patients with suspected severe drug reactions: a high risk medication, mucosal involvement, presence of pustules, and laboratory abnormalities, especially a CBC with differential and liver function tests, James R. Treat, MD, said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Several cutaneous drug reactions that were discussed during the conference included acute generalized exanthematous pustulosis (AGEP), a drug reaction with eosinophilia and systemic symptoms (DRESS), and Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN).
AGEP is characterized by fever and generalized pustular eruption arising swiftly after administration of the causative drug. Such drugs include antibiotics, contrast agents, antifungals, and calcium channel blockers. Withdrawal of the offending drug and optimization of fluid and electrolyte balance are warranted in the management of AGEP. Topical steroids may decrease hospital length-of-stay and help with symptomatic treatment of AGEP, said Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia and an assistant professor of pediatrics and dermatology at the Perelman School of Medicine at the University of Pennsylvania.
A DRESS, also known as drug hypersensitivity syndrome, or drug-induced hypersensitivity syndrome, is a skin eruption that generally occurs 2-6 weeks after the patient starts the offending medication. Clinical signs of this condition include ill-appearance, fever (greater than 100.4° F), facial and hand edema, lymphadenopathy, and lab abnormalities, including hypereosinophilia, atypical lymphocytosis, transaminitis, and human herpesvirus 6 reactivation. DRESS may be misdiagnosed as viral infection, Kawasaki’s disease, or SJS.
Commonly implicated drugs include antiepileptic drugs, antibiotics, HIV medications, and sulfa-containing medications.
“While withdrawal of the offending drug is promptly warranted, this condition may require other therapeutics, particularly if there is significant systemic involvement,” Dr. Treat emphasized. There is evidence that systemic steroids (1-2 mg/kg/day) and cyclosporine can help improve the disease course, although their use is off-label.
SJS and TEN are other severe cutaneous adverse reactions caused by Mycoplasma infection or medications, such as anticonvulsants, antibiotics, HIV medications, and sulfa-containing drugs. “These entities are characterized by an ill-appearing, febrile patient with painful skin and mucosal membrane involvement,” Dr. Treat described.
Mucosal predominance may be seen in cases associated with Mycoplasma and have been termed “Mycoplasma-induced rash and mucositis,” although the terminology is controversial. In a case series by Darren G. Gregory, MD, treatment with amniotic membrane transplantation applied to the eyelid margins, palpebral conjunctiva, and ocular surface during the acute phases of SJS and TEN has been shown to be effective, decreasing the risk of significant oculovisual sequelae (Ophthalmol. 2011 May;118[5]:908-14).
Diagnostic criteria have been detailed to classify each of these adverse reactions. Dr. Treat concluded his lecture with a discussion of a retrospective study by Bouvresse et al. that projected AGEP, DRESS, and SJS-TEN as distinct entities (Orphanet J Rare Dis. 2012. doi: 10.1186/1750-1172-7-72).
Dr. Treat reported having no relevant financial disclosures.
There are four key findings in patients with suspected severe drug reactions: a high risk medication, mucosal involvement, presence of pustules, and laboratory abnormalities, especially a CBC with differential and liver function tests, James R. Treat, MD, said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Several cutaneous drug reactions that were discussed during the conference included acute generalized exanthematous pustulosis (AGEP), a drug reaction with eosinophilia and systemic symptoms (DRESS), and Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN).
AGEP is characterized by fever and generalized pustular eruption arising swiftly after administration of the causative drug. Such drugs include antibiotics, contrast agents, antifungals, and calcium channel blockers. Withdrawal of the offending drug and optimization of fluid and electrolyte balance are warranted in the management of AGEP. Topical steroids may decrease hospital length-of-stay and help with symptomatic treatment of AGEP, said Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia and an assistant professor of pediatrics and dermatology at the Perelman School of Medicine at the University of Pennsylvania.
A DRESS, also known as drug hypersensitivity syndrome, or drug-induced hypersensitivity syndrome, is a skin eruption that generally occurs 2-6 weeks after the patient starts the offending medication. Clinical signs of this condition include ill-appearance, fever (greater than 100.4° F), facial and hand edema, lymphadenopathy, and lab abnormalities, including hypereosinophilia, atypical lymphocytosis, transaminitis, and human herpesvirus 6 reactivation. DRESS may be misdiagnosed as viral infection, Kawasaki’s disease, or SJS.
Commonly implicated drugs include antiepileptic drugs, antibiotics, HIV medications, and sulfa-containing medications.
“While withdrawal of the offending drug is promptly warranted, this condition may require other therapeutics, particularly if there is significant systemic involvement,” Dr. Treat emphasized. There is evidence that systemic steroids (1-2 mg/kg/day) and cyclosporine can help improve the disease course, although their use is off-label.
SJS and TEN are other severe cutaneous adverse reactions caused by Mycoplasma infection or medications, such as anticonvulsants, antibiotics, HIV medications, and sulfa-containing drugs. “These entities are characterized by an ill-appearing, febrile patient with painful skin and mucosal membrane involvement,” Dr. Treat described.
Mucosal predominance may be seen in cases associated with Mycoplasma and have been termed “Mycoplasma-induced rash and mucositis,” although the terminology is controversial. In a case series by Darren G. Gregory, MD, treatment with amniotic membrane transplantation applied to the eyelid margins, palpebral conjunctiva, and ocular surface during the acute phases of SJS and TEN has been shown to be effective, decreasing the risk of significant oculovisual sequelae (Ophthalmol. 2011 May;118[5]:908-14).
Diagnostic criteria have been detailed to classify each of these adverse reactions. Dr. Treat concluded his lecture with a discussion of a retrospective study by Bouvresse et al. that projected AGEP, DRESS, and SJS-TEN as distinct entities (Orphanet J Rare Dis. 2012. doi: 10.1186/1750-1172-7-72).
Dr. Treat reported having no relevant financial disclosures.
There are four key findings in patients with suspected severe drug reactions: a high risk medication, mucosal involvement, presence of pustules, and laboratory abnormalities, especially a CBC with differential and liver function tests, James R. Treat, MD, said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Several cutaneous drug reactions that were discussed during the conference included acute generalized exanthematous pustulosis (AGEP), a drug reaction with eosinophilia and systemic symptoms (DRESS), and Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN).
AGEP is characterized by fever and generalized pustular eruption arising swiftly after administration of the causative drug. Such drugs include antibiotics, contrast agents, antifungals, and calcium channel blockers. Withdrawal of the offending drug and optimization of fluid and electrolyte balance are warranted in the management of AGEP. Topical steroids may decrease hospital length-of-stay and help with symptomatic treatment of AGEP, said Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia and an assistant professor of pediatrics and dermatology at the Perelman School of Medicine at the University of Pennsylvania.
A DRESS, also known as drug hypersensitivity syndrome, or drug-induced hypersensitivity syndrome, is a skin eruption that generally occurs 2-6 weeks after the patient starts the offending medication. Clinical signs of this condition include ill-appearance, fever (greater than 100.4° F), facial and hand edema, lymphadenopathy, and lab abnormalities, including hypereosinophilia, atypical lymphocytosis, transaminitis, and human herpesvirus 6 reactivation. DRESS may be misdiagnosed as viral infection, Kawasaki’s disease, or SJS.
Commonly implicated drugs include antiepileptic drugs, antibiotics, HIV medications, and sulfa-containing medications.
“While withdrawal of the offending drug is promptly warranted, this condition may require other therapeutics, particularly if there is significant systemic involvement,” Dr. Treat emphasized. There is evidence that systemic steroids (1-2 mg/kg/day) and cyclosporine can help improve the disease course, although their use is off-label.
SJS and TEN are other severe cutaneous adverse reactions caused by Mycoplasma infection or medications, such as anticonvulsants, antibiotics, HIV medications, and sulfa-containing drugs. “These entities are characterized by an ill-appearing, febrile patient with painful skin and mucosal membrane involvement,” Dr. Treat described.
Mucosal predominance may be seen in cases associated with Mycoplasma and have been termed “Mycoplasma-induced rash and mucositis,” although the terminology is controversial. In a case series by Darren G. Gregory, MD, treatment with amniotic membrane transplantation applied to the eyelid margins, palpebral conjunctiva, and ocular surface during the acute phases of SJS and TEN has been shown to be effective, decreasing the risk of significant oculovisual sequelae (Ophthalmol. 2011 May;118[5]:908-14).
Diagnostic criteria have been detailed to classify each of these adverse reactions. Dr. Treat concluded his lecture with a discussion of a retrospective study by Bouvresse et al. that projected AGEP, DRESS, and SJS-TEN as distinct entities (Orphanet J Rare Dis. 2012. doi: 10.1186/1750-1172-7-72).
Dr. Treat reported having no relevant financial disclosures.