User login
Painful Oral, Groin, and Scalp Lesions in a Young Man
Painful Oral, Groin, and Scalp Lesions in a Young Man
THE DIAGNOSIS: Pemphigus Vegetans
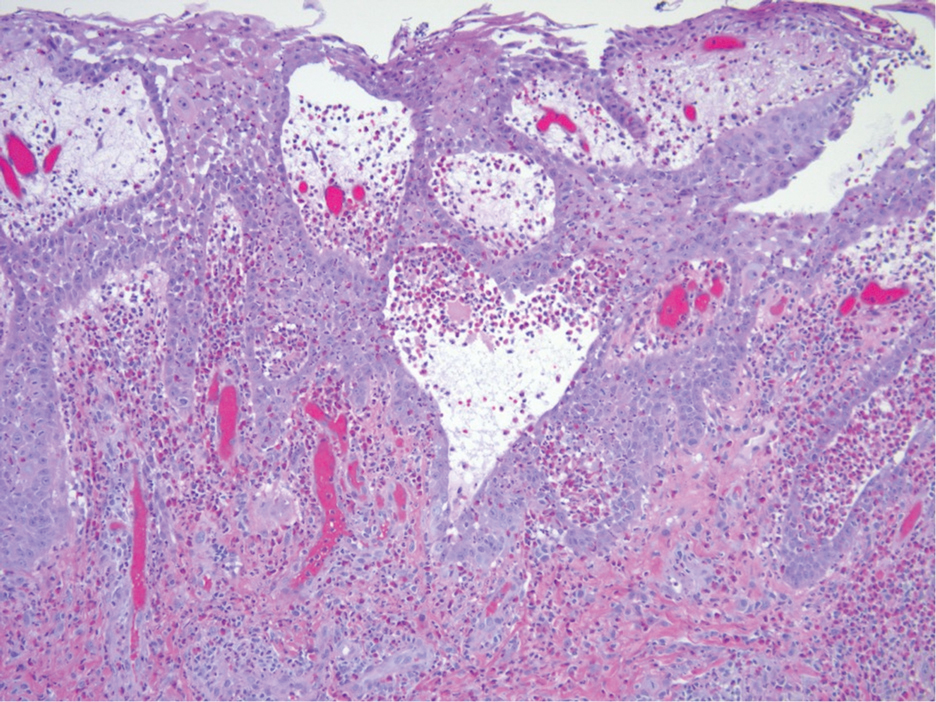
Histopathologic examination of the biopsies from the scalp and left anterior thigh revealed suprabasal clefting with acantholytic cells extending into the follicular infundibulum with eosinophilic pustules within the epidermis. The dermis contained perivascular lymphohistiocytic and eosinophilic inflammatory infiltrates without viral cytopathic effects (Figure 1). Direct immunofluorescence revealed strong IgG and moderate IgA pericellular deposition around keratinocyte cytoplasms (Figure 2). Serologic evaluation demonstrated anti–desmoglein 3 antibodies. Based on the clinical presentation and histopathologic correlation, a diagnosis of pemphigus vegetans was made.
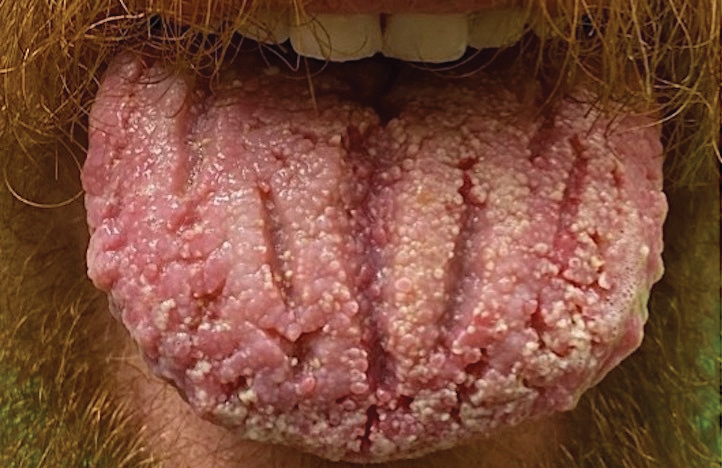
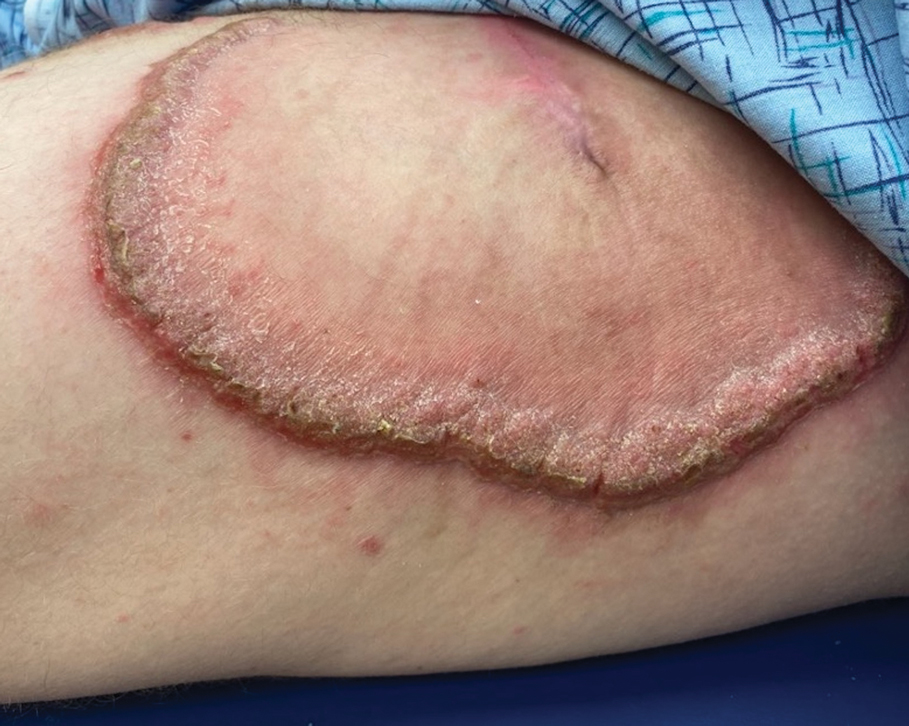
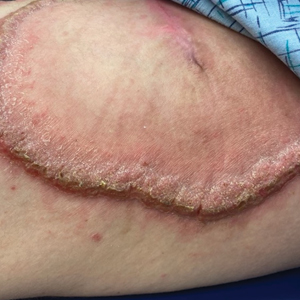
Pemphigus vegetans is a vesiculobullous autoimmune disease that is similar to pemphigus vulgaris but is characterized by the formation of vegetative plaques along the intertriginous areas and on the oral mucosa.1 It is the rarest variant of all pemphigus subtypes and was first described by Neumann in 1876.2 There are 2 subtypes of this variant: Hallopeau and Neumann, each with unique characteristics and physical manifestations. The Hallopeau type initially manifests with pustular lesions that rupture and evolve into erosions that commonly become infected. Gradually they merge and multiply to become more painful and vegetative.3 It has a more indolent course and typically responds well to treatment, and prolonged remission can be reached.4 The Neumann type is more severe and manifests with large vesiculobullous and erosive lesions that rupture and ulcerate, forming verrucous crusted vegetative plaques over the erosions.5 The erosions along the edge of the lesions induce new vegetation, becoming dry, hyperkeratotic, and fissured.3 The Neumann type often requires higher-dose steroids and typically is resistant to treatment.4 Patients can present with oral stomatitis and occasionally can develop a fissured or cerebriform appearance of the tongue, as seen in our patient (Figure 3).1,2 Nail changes include onychorrhexis, onychomadesis, subungual pustules, and ultimately nail atrophy.5
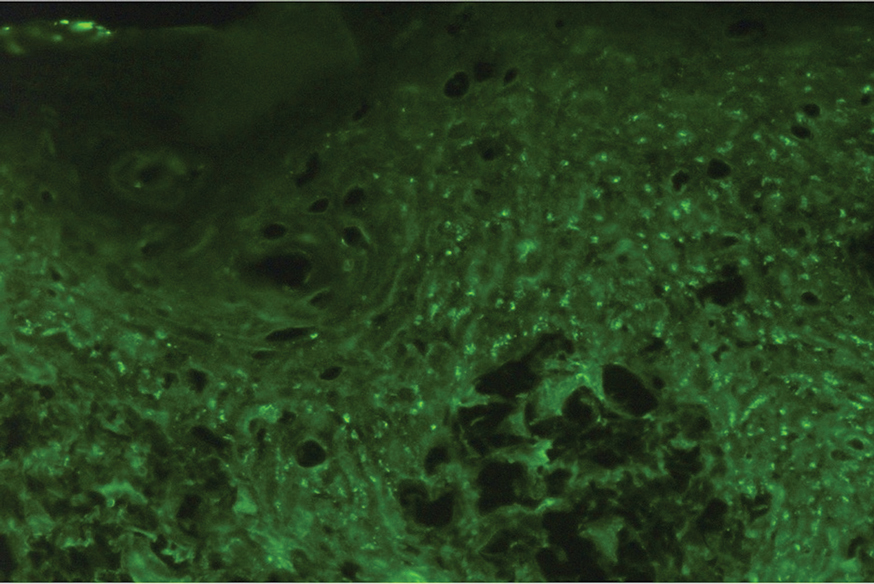
Pemphigus diseases are characterized by IgG autoantibodies against desmoglein 3 and/or desmoglein 1. These are components of desmosomes that are responsible for keratinocyte adhesion, disruption of which results in the blister formation seen in pemphigus subtypes. The unique physical manifestation of pemphigus vegetans is thought to be due not only to autoantibodies against desmogleins 1 and 3 but also to autoantibodies against desmocollin 1 and 2.1
Histopathologic examination reveals hyperkeratosis and pseudoepitheliomatous hyperplasia with acantholysis that creates a suprabasal cleft. Basal cells remain intact to the basement membrane by hemidesmosomes, resulting in a tombstone appearance. The Hallopeau type typically manifests with a large eosinophilic inflammatory response, leading to eosinophilic spongiosis and intraepidermal microabscesses. The Neumann type manifests with more of a neutrophilic and lymphocytic infiltrate, accompanied by the eosinophilic response.1 For evaluation, obtain histopathology as well as direct immunofluorescence or enzyme-linked immunosorbent assay to look for intracellular deposition of desmoglein autoantibodies.
First-line treatment for pemphigus vulgaris and its variants is rituximab, an anti-CD20 monoclonal antibody. It has also been shown to have therapeutic benefit with combination of corticosteroids and rituximab. Corticosteroids should be given at a dose of 1 mg/kg daily for 2 to 4 weeks. Other immunosuppressive agents (steroid sparing) include azathioprine, dapsone, mycophenolate mofetil, methotrexate, cyclophosphamide, cyclosporine, and intravenous immunoglobulin. Pulse therapy with intermittent intravenous corticosteroids and immunosuppressants is another second-line therapeutic option. Topical therapeutic options include steroids, tacrolimus, and nicotinamide with oral tetracycline at onset and relapse. The goal of therapy is to maintain remission for 1 year then slowly taper treatment over another year.1
Our patient initially was treated with prednisone, and subsequent courses of azathioprine and mycophenolate mofetil failed. He then was treated with 2 infusions of rituximab that were given 2 weeks apart. He was able to taper off the prednisone 1 month after the last infusion with complete remission of disease. He has been disease free for more than 9 months postinfusion.
Differential diagnoses for pemphigus vegetans can include bullous pemphigoid, bullous systemic lupus erythematosus, dermatitis herpetiformis, and pemphigus vulgaris. Lesion characteristics are key to differentiating pemphigus vegetans from other autoimmune blistering disorders. Bullous pemphigoid will manifest with tense blisters where pemphigus vulgaris will be flaccid; this is due to the difference in autoantibody targets between the conditions. Diagnosis depends on clinical presentation and histopathologic findings.
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed December 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Rebello MS, Ramesh BM, Sukumar D, et al. Cerebriform cutaneous lesions in pemphigus vegetans. Indian J Dermatol. 2016;61:206-208.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Ajbani AA, Mehta KS, Marfatia YS. Verrucous lesions over external genitalia as a presenting feature of pemphigus vegetans. Indian J Sex Transm Dis AIDS. 2019;40:176-179.
- Vinay K, De D, Handa S, et al. Pemphigus vegetans presenting as a verrucous plaque on the finger. Clin Exp Dermatol. 2016;41:316-317.
THE DIAGNOSIS: Pemphigus Vegetans
Histopathologic examination of the biopsies from the scalp and left anterior thigh revealed suprabasal clefting with acantholytic cells extending into the follicular infundibulum with eosinophilic pustules within the epidermis. The dermis contained perivascular lymphohistiocytic and eosinophilic inflammatory infiltrates without viral cytopathic effects (Figure 1). Direct immunofluorescence revealed strong IgG and moderate IgA pericellular deposition around keratinocyte cytoplasms (Figure 2). Serologic evaluation demonstrated anti–desmoglein 3 antibodies. Based on the clinical presentation and histopathologic correlation, a diagnosis of pemphigus vegetans was made.


Pemphigus vegetans is a vesiculobullous autoimmune disease that is similar to pemphigus vulgaris but is characterized by the formation of vegetative plaques along the intertriginous areas and on the oral mucosa.1 It is the rarest variant of all pemphigus subtypes and was first described by Neumann in 1876.2 There are 2 subtypes of this variant: Hallopeau and Neumann, each with unique characteristics and physical manifestations. The Hallopeau type initially manifests with pustular lesions that rupture and evolve into erosions that commonly become infected. Gradually they merge and multiply to become more painful and vegetative.3 It has a more indolent course and typically responds well to treatment, and prolonged remission can be reached.4 The Neumann type is more severe and manifests with large vesiculobullous and erosive lesions that rupture and ulcerate, forming verrucous crusted vegetative plaques over the erosions.5 The erosions along the edge of the lesions induce new vegetation, becoming dry, hyperkeratotic, and fissured.3 The Neumann type often requires higher-dose steroids and typically is resistant to treatment.4 Patients can present with oral stomatitis and occasionally can develop a fissured or cerebriform appearance of the tongue, as seen in our patient (Figure 3).1,2 Nail changes include onychorrhexis, onychomadesis, subungual pustules, and ultimately nail atrophy.5

Pemphigus diseases are characterized by IgG autoantibodies against desmoglein 3 and/or desmoglein 1. These are components of desmosomes that are responsible for keratinocyte adhesion, disruption of which results in the blister formation seen in pemphigus subtypes. The unique physical manifestation of pemphigus vegetans is thought to be due not only to autoantibodies against desmogleins 1 and 3 but also to autoantibodies against desmocollin 1 and 2.1
Histopathologic examination reveals hyperkeratosis and pseudoepitheliomatous hyperplasia with acantholysis that creates a suprabasal cleft. Basal cells remain intact to the basement membrane by hemidesmosomes, resulting in a tombstone appearance. The Hallopeau type typically manifests with a large eosinophilic inflammatory response, leading to eosinophilic spongiosis and intraepidermal microabscesses. The Neumann type manifests with more of a neutrophilic and lymphocytic infiltrate, accompanied by the eosinophilic response.1 For evaluation, obtain histopathology as well as direct immunofluorescence or enzyme-linked immunosorbent assay to look for intracellular deposition of desmoglein autoantibodies.
First-line treatment for pemphigus vulgaris and its variants is rituximab, an anti-CD20 monoclonal antibody. It has also been shown to have therapeutic benefit with combination of corticosteroids and rituximab. Corticosteroids should be given at a dose of 1 mg/kg daily for 2 to 4 weeks. Other immunosuppressive agents (steroid sparing) include azathioprine, dapsone, mycophenolate mofetil, methotrexate, cyclophosphamide, cyclosporine, and intravenous immunoglobulin. Pulse therapy with intermittent intravenous corticosteroids and immunosuppressants is another second-line therapeutic option. Topical therapeutic options include steroids, tacrolimus, and nicotinamide with oral tetracycline at onset and relapse. The goal of therapy is to maintain remission for 1 year then slowly taper treatment over another year.1
Our patient initially was treated with prednisone, and subsequent courses of azathioprine and mycophenolate mofetil failed. He then was treated with 2 infusions of rituximab that were given 2 weeks apart. He was able to taper off the prednisone 1 month after the last infusion with complete remission of disease. He has been disease free for more than 9 months postinfusion.
Differential diagnoses for pemphigus vegetans can include bullous pemphigoid, bullous systemic lupus erythematosus, dermatitis herpetiformis, and pemphigus vulgaris. Lesion characteristics are key to differentiating pemphigus vegetans from other autoimmune blistering disorders. Bullous pemphigoid will manifest with tense blisters where pemphigus vulgaris will be flaccid; this is due to the difference in autoantibody targets between the conditions. Diagnosis depends on clinical presentation and histopathologic findings.
THE DIAGNOSIS: Pemphigus Vegetans
Histopathologic examination of the biopsies from the scalp and left anterior thigh revealed suprabasal clefting with acantholytic cells extending into the follicular infundibulum with eosinophilic pustules within the epidermis. The dermis contained perivascular lymphohistiocytic and eosinophilic inflammatory infiltrates without viral cytopathic effects (Figure 1). Direct immunofluorescence revealed strong IgG and moderate IgA pericellular deposition around keratinocyte cytoplasms (Figure 2). Serologic evaluation demonstrated anti–desmoglein 3 antibodies. Based on the clinical presentation and histopathologic correlation, a diagnosis of pemphigus vegetans was made.


Pemphigus vegetans is a vesiculobullous autoimmune disease that is similar to pemphigus vulgaris but is characterized by the formation of vegetative plaques along the intertriginous areas and on the oral mucosa.1 It is the rarest variant of all pemphigus subtypes and was first described by Neumann in 1876.2 There are 2 subtypes of this variant: Hallopeau and Neumann, each with unique characteristics and physical manifestations. The Hallopeau type initially manifests with pustular lesions that rupture and evolve into erosions that commonly become infected. Gradually they merge and multiply to become more painful and vegetative.3 It has a more indolent course and typically responds well to treatment, and prolonged remission can be reached.4 The Neumann type is more severe and manifests with large vesiculobullous and erosive lesions that rupture and ulcerate, forming verrucous crusted vegetative plaques over the erosions.5 The erosions along the edge of the lesions induce new vegetation, becoming dry, hyperkeratotic, and fissured.3 The Neumann type often requires higher-dose steroids and typically is resistant to treatment.4 Patients can present with oral stomatitis and occasionally can develop a fissured or cerebriform appearance of the tongue, as seen in our patient (Figure 3).1,2 Nail changes include onychorrhexis, onychomadesis, subungual pustules, and ultimately nail atrophy.5

Pemphigus diseases are characterized by IgG autoantibodies against desmoglein 3 and/or desmoglein 1. These are components of desmosomes that are responsible for keratinocyte adhesion, disruption of which results in the blister formation seen in pemphigus subtypes. The unique physical manifestation of pemphigus vegetans is thought to be due not only to autoantibodies against desmogleins 1 and 3 but also to autoantibodies against desmocollin 1 and 2.1
Histopathologic examination reveals hyperkeratosis and pseudoepitheliomatous hyperplasia with acantholysis that creates a suprabasal cleft. Basal cells remain intact to the basement membrane by hemidesmosomes, resulting in a tombstone appearance. The Hallopeau type typically manifests with a large eosinophilic inflammatory response, leading to eosinophilic spongiosis and intraepidermal microabscesses. The Neumann type manifests with more of a neutrophilic and lymphocytic infiltrate, accompanied by the eosinophilic response.1 For evaluation, obtain histopathology as well as direct immunofluorescence or enzyme-linked immunosorbent assay to look for intracellular deposition of desmoglein autoantibodies.
First-line treatment for pemphigus vulgaris and its variants is rituximab, an anti-CD20 monoclonal antibody. It has also been shown to have therapeutic benefit with combination of corticosteroids and rituximab. Corticosteroids should be given at a dose of 1 mg/kg daily for 2 to 4 weeks. Other immunosuppressive agents (steroid sparing) include azathioprine, dapsone, mycophenolate mofetil, methotrexate, cyclophosphamide, cyclosporine, and intravenous immunoglobulin. Pulse therapy with intermittent intravenous corticosteroids and immunosuppressants is another second-line therapeutic option. Topical therapeutic options include steroids, tacrolimus, and nicotinamide with oral tetracycline at onset and relapse. The goal of therapy is to maintain remission for 1 year then slowly taper treatment over another year.1
Our patient initially was treated with prednisone, and subsequent courses of azathioprine and mycophenolate mofetil failed. He then was treated with 2 infusions of rituximab that were given 2 weeks apart. He was able to taper off the prednisone 1 month after the last infusion with complete remission of disease. He has been disease free for more than 9 months postinfusion.
Differential diagnoses for pemphigus vegetans can include bullous pemphigoid, bullous systemic lupus erythematosus, dermatitis herpetiformis, and pemphigus vulgaris. Lesion characteristics are key to differentiating pemphigus vegetans from other autoimmune blistering disorders. Bullous pemphigoid will manifest with tense blisters where pemphigus vulgaris will be flaccid; this is due to the difference in autoantibody targets between the conditions. Diagnosis depends on clinical presentation and histopathologic findings.
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed December 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Rebello MS, Ramesh BM, Sukumar D, et al. Cerebriform cutaneous lesions in pemphigus vegetans. Indian J Dermatol. 2016;61:206-208.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Ajbani AA, Mehta KS, Marfatia YS. Verrucous lesions over external genitalia as a presenting feature of pemphigus vegetans. Indian J Sex Transm Dis AIDS. 2019;40:176-179.
- Vinay K, De D, Handa S, et al. Pemphigus vegetans presenting as a verrucous plaque on the finger. Clin Exp Dermatol. 2016;41:316-317.
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed December 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Rebello MS, Ramesh BM, Sukumar D, et al. Cerebriform cutaneous lesions in pemphigus vegetans. Indian J Dermatol. 2016;61:206-208.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Ajbani AA, Mehta KS, Marfatia YS. Verrucous lesions over external genitalia as a presenting feature of pemphigus vegetans. Indian J Sex Transm Dis AIDS. 2019;40:176-179.
- Vinay K, De D, Handa S, et al. Pemphigus vegetans presenting as a verrucous plaque on the finger. Clin Exp Dermatol. 2016;41:316-317.
Painful Oral, Groin, and Scalp Lesions in a Young Man
Painful Oral, Groin, and Scalp Lesions in a Young Man
A 27-year-old man presented to the dermatology department with painful oral and groin lesions of 2 years’ duration as well as lip ulceration that had been present for 1 month. The patient also reported moderately tender scalp and face lesions that had been present for several weeks. The lip ulceration was previously treated by his primary care provider with valacyclovir (1 g daily for 2 weeks) without improvement. Six months prior to the current presentation, we treated the groin lesions as condyloma involving the perineum and genital region at our clinic with no response to cryotherapy, topical imiquimod, or extensive surgical excision with skin grafting. Pathology at the time showed condyloma but was negative for human papillomavirus. Physical examination at the current presentation revealed superficial erosions along the vermilion border. The oral mucosa exhibited cobblestoning, and fissures were present on the tongue. Eroded pink plaques studded with vesicles were present on the vertex scalp and left chin. The bilateral inguinal regions extending to anterior-lateral upper thighs and posterior buttocks revealed erythematous, arcuate, and annular erosive plaques with verrucous hyperkeratotic borders and fissuring on the leading edge. Pink erosive and verrucous erythematous plaques were noted on the penile shaft, scrotum, and perineum. Punch biopsies of the scalp and left anterior thigh as well as direct immunofluorescence were performed.
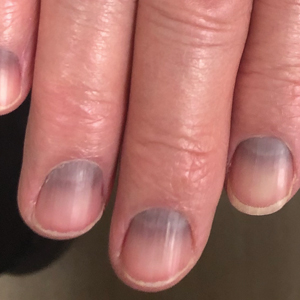
Blue to Slate Gray Discoloration of the Proximal Fingernails
The Diagnosis: Argyria-Induced Azure Lunulae
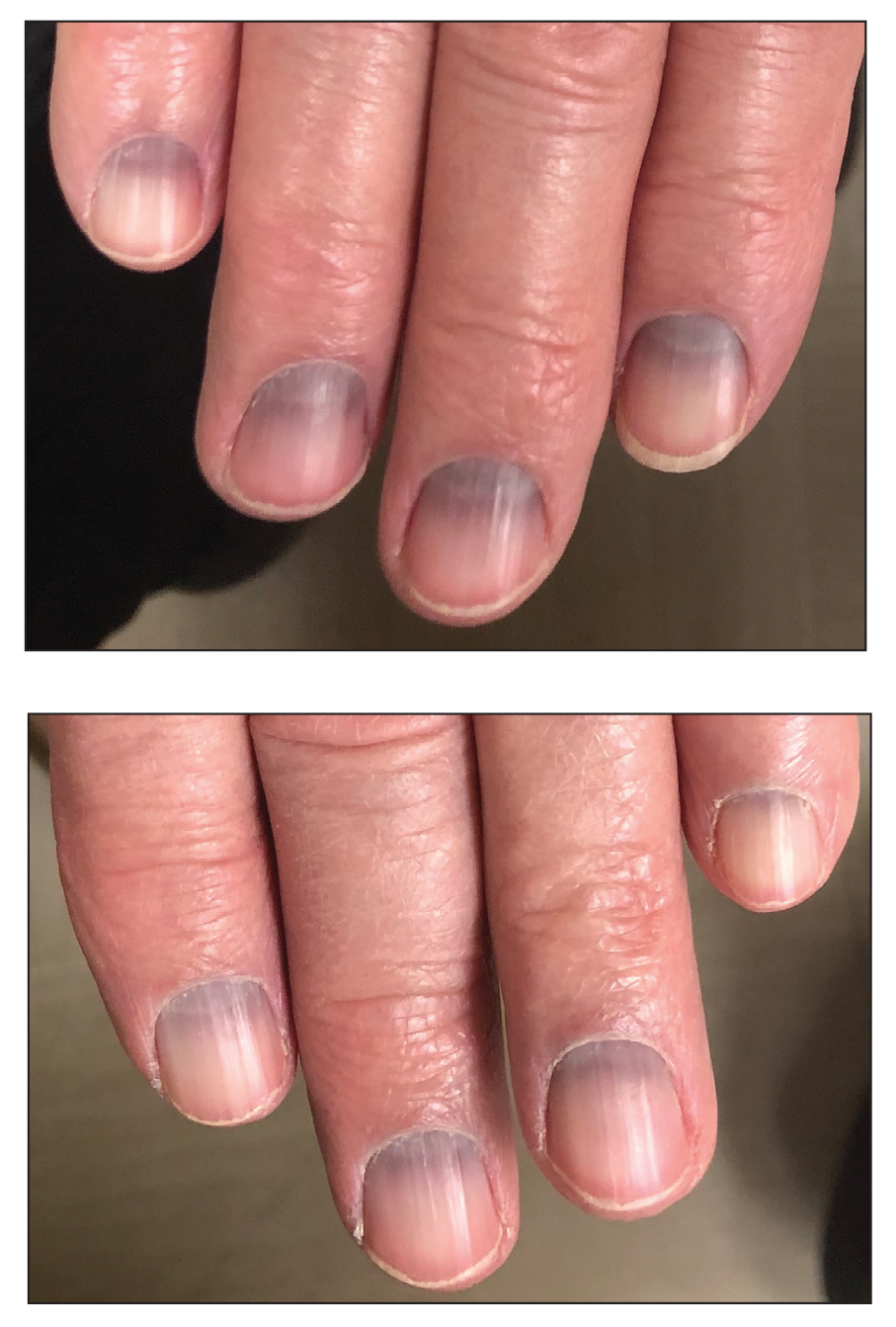
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
An 88-year-old man presented with asymptomatic and unchanging discoloration of the proximal fingernails of both hands of 50 years’ duration. Physical examination revealed blue to slate gray, subungual pigmentary changes of the fingernails of both hands sparing the nail bed distal to the lunulae. There was no overlying plate dystrophy, toenail involvement, or additional mucocutaneous abnormalities. His medical history was notable for heart failure, obstructive sleep apnea, and type 2 diabetes mellitus. He had no history of hepatic, ophthalmologic, or neurologic dysfunction.
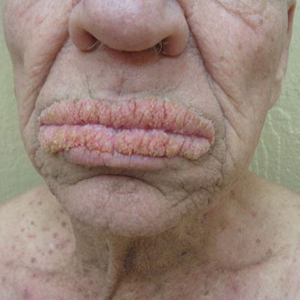
Oral Verrucous Plaques in a Patient With Urothelial Cancer
The Diagnosis: Paraneoplastic Acanthosis Nigricans
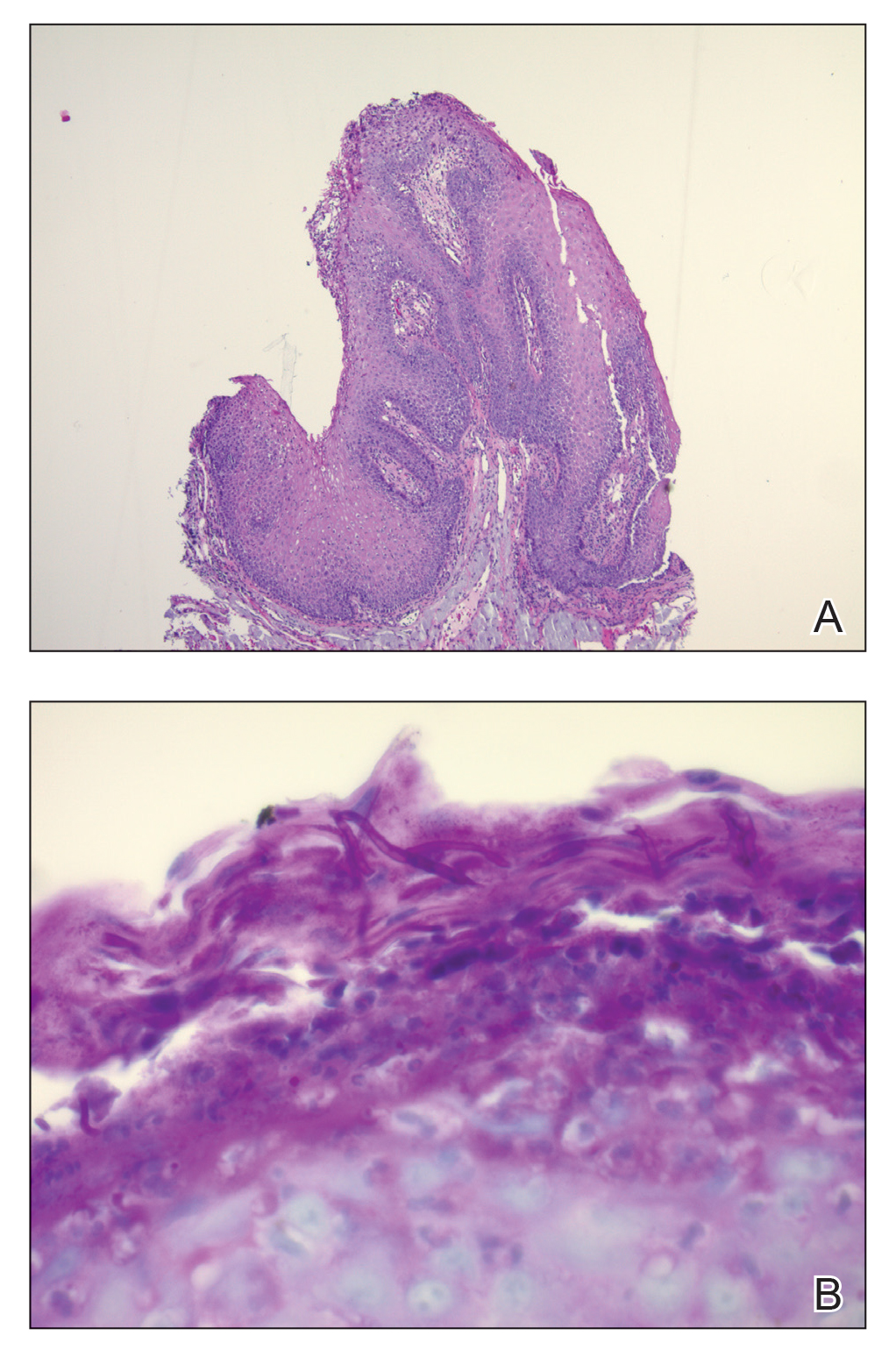
Histopathologic examination demonstrated verrucous epidermal hyperplasia (Figure, A). Fungal organisms were identified with an Alcian blue and periodic acid-Schiff stain (Figure, B). The organisms demonstrated a vertical orientation in relation to the mucosal surface, which was consistent with candidal organisms.
Given the rapid eruption of these plaques, the distribution on the oral and palmar surfaces (tripe palms), and the minimal improvement with both systemic steroids and antifungal treatment, a diagnosis of paraneoplastic acanthosis nigricans with secondary candidal infection was made. Drug-induced cheilitis was considered; however, improvement with discontinuation of the suspected offending drug would have been expected. Although chronic mucocutaneous candidiasis was possible, more prompt improvement upon initiation of systemic antifungal therapy would have been observed. Oral Crohn disease should be included in the differential, but it was unlikely given the lack of granulomas on pathology and absence of history of gastrointestinal tract symptoms. Melkersson-Rosenthal syndrome also was unlikely given the lack of facial nerve palsy as well as the lack of granulomas on pathology. Furthermore, none of these options would be associated with tripe palms, as seen in our patient.
Acanthosis nigricans is a localized skin disorder characterized by hyperpigmented velvety plaques arising in flexural and intertriginous regions. Although most cases (80%) are associated with idiopathic or benign conditions, the link between acanthosis nigricans and an underlying malignancy has been well documented.1-3 Most commonly associated with an underlying intra-abdominal malignancy (often gastric carcinoma), the lesions of paraneoplastic acanthosis nigricans are indistinguishable from their benign counterparts.1,4 When the condition presents abruptly and extensively in a nonobese patient, prompt workup for malignancy should be initiated. Rapid onset and atypical distribution (ie, palmar, perioral, or mucosal) more commonly is associated with a paraneoplastic etiology.5,6
Histopathology for acanthosis nigricans shows hyperkeratosis and epidermal papillomatosis. Horn pseudocyst formation is possible, but usually no hyperpigmentation is observed. The findings typically are indistinguishable from seborrheic keratoses, epidermal nevi, or lesions of confluent and reticulated papillomatosis of Gougerot and Carteaud.2
The underlying pathogenesis of acanthosis nigricans is poorly understood. In the benign subtype, insulin resistance commonly has been described. In the paraneoplastic subtype, it is proposed that the tumor produces a transforming growth factor that mimics epidermal growth factor and leads to keratinocyte proliferation.7,8 Paraneoplastic acanthosis nigricans has the potential to arise at any point of tumor development, further contributing to the diagnostic challenge. Treatment of the skin lesions involves management of the underlying malignancy. Unfortunately, many such malignancies often are at an advanced stage, and subsequent prognosis is poor.2
- Shah A, Jack A, Liu H, et al. Neoplastic/paraneoplastic dermatitis, fasciitis, and panniculitis. Rheum Dis Clin North Am. 2011;37:573-592.
- Chairatchaneeboon M, Kim EJ. Cutaneous paraneoplastic syndromes. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick's Dermatology. 9th ed. McGraw-Hill Education; 2019:2441-2464.
- Lee HC, Ker KJ, Chong WS. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Yu Q, Li XL, Ji G, et al. Malignant acanthosis nigricans: an early diagnostic clue for gastric adenocarcinoma. World J Surg Oncol. 2017;15:208.
- Mohrenschlager M, Vocks E, Wessner DB, et al. Tripe palms and malignant acanthosis nigricans: cutaneous signs of imminent metastasis in bladder cancer? J Urol. 2001;165:1629-1630.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101.
The Diagnosis: Paraneoplastic Acanthosis Nigricans
Histopathologic examination demonstrated verrucous epidermal hyperplasia (Figure, A). Fungal organisms were identified with an Alcian blue and periodic acid-Schiff stain (Figure, B). The organisms demonstrated a vertical orientation in relation to the mucosal surface, which was consistent with candidal organisms.

Given the rapid eruption of these plaques, the distribution on the oral and palmar surfaces (tripe palms), and the minimal improvement with both systemic steroids and antifungal treatment, a diagnosis of paraneoplastic acanthosis nigricans with secondary candidal infection was made. Drug-induced cheilitis was considered; however, improvement with discontinuation of the suspected offending drug would have been expected. Although chronic mucocutaneous candidiasis was possible, more prompt improvement upon initiation of systemic antifungal therapy would have been observed. Oral Crohn disease should be included in the differential, but it was unlikely given the lack of granulomas on pathology and absence of history of gastrointestinal tract symptoms. Melkersson-Rosenthal syndrome also was unlikely given the lack of facial nerve palsy as well as the lack of granulomas on pathology. Furthermore, none of these options would be associated with tripe palms, as seen in our patient.
Acanthosis nigricans is a localized skin disorder characterized by hyperpigmented velvety plaques arising in flexural and intertriginous regions. Although most cases (80%) are associated with idiopathic or benign conditions, the link between acanthosis nigricans and an underlying malignancy has been well documented.1-3 Most commonly associated with an underlying intra-abdominal malignancy (often gastric carcinoma), the lesions of paraneoplastic acanthosis nigricans are indistinguishable from their benign counterparts.1,4 When the condition presents abruptly and extensively in a nonobese patient, prompt workup for malignancy should be initiated. Rapid onset and atypical distribution (ie, palmar, perioral, or mucosal) more commonly is associated with a paraneoplastic etiology.5,6
Histopathology for acanthosis nigricans shows hyperkeratosis and epidermal papillomatosis. Horn pseudocyst formation is possible, but usually no hyperpigmentation is observed. The findings typically are indistinguishable from seborrheic keratoses, epidermal nevi, or lesions of confluent and reticulated papillomatosis of Gougerot and Carteaud.2
The underlying pathogenesis of acanthosis nigricans is poorly understood. In the benign subtype, insulin resistance commonly has been described. In the paraneoplastic subtype, it is proposed that the tumor produces a transforming growth factor that mimics epidermal growth factor and leads to keratinocyte proliferation.7,8 Paraneoplastic acanthosis nigricans has the potential to arise at any point of tumor development, further contributing to the diagnostic challenge. Treatment of the skin lesions involves management of the underlying malignancy. Unfortunately, many such malignancies often are at an advanced stage, and subsequent prognosis is poor.2
The Diagnosis: Paraneoplastic Acanthosis Nigricans
Histopathologic examination demonstrated verrucous epidermal hyperplasia (Figure, A). Fungal organisms were identified with an Alcian blue and periodic acid-Schiff stain (Figure, B). The organisms demonstrated a vertical orientation in relation to the mucosal surface, which was consistent with candidal organisms.

Given the rapid eruption of these plaques, the distribution on the oral and palmar surfaces (tripe palms), and the minimal improvement with both systemic steroids and antifungal treatment, a diagnosis of paraneoplastic acanthosis nigricans with secondary candidal infection was made. Drug-induced cheilitis was considered; however, improvement with discontinuation of the suspected offending drug would have been expected. Although chronic mucocutaneous candidiasis was possible, more prompt improvement upon initiation of systemic antifungal therapy would have been observed. Oral Crohn disease should be included in the differential, but it was unlikely given the lack of granulomas on pathology and absence of history of gastrointestinal tract symptoms. Melkersson-Rosenthal syndrome also was unlikely given the lack of facial nerve palsy as well as the lack of granulomas on pathology. Furthermore, none of these options would be associated with tripe palms, as seen in our patient.
Acanthosis nigricans is a localized skin disorder characterized by hyperpigmented velvety plaques arising in flexural and intertriginous regions. Although most cases (80%) are associated with idiopathic or benign conditions, the link between acanthosis nigricans and an underlying malignancy has been well documented.1-3 Most commonly associated with an underlying intra-abdominal malignancy (often gastric carcinoma), the lesions of paraneoplastic acanthosis nigricans are indistinguishable from their benign counterparts.1,4 When the condition presents abruptly and extensively in a nonobese patient, prompt workup for malignancy should be initiated. Rapid onset and atypical distribution (ie, palmar, perioral, or mucosal) more commonly is associated with a paraneoplastic etiology.5,6
Histopathology for acanthosis nigricans shows hyperkeratosis and epidermal papillomatosis. Horn pseudocyst formation is possible, but usually no hyperpigmentation is observed. The findings typically are indistinguishable from seborrheic keratoses, epidermal nevi, or lesions of confluent and reticulated papillomatosis of Gougerot and Carteaud.2
The underlying pathogenesis of acanthosis nigricans is poorly understood. In the benign subtype, insulin resistance commonly has been described. In the paraneoplastic subtype, it is proposed that the tumor produces a transforming growth factor that mimics epidermal growth factor and leads to keratinocyte proliferation.7,8 Paraneoplastic acanthosis nigricans has the potential to arise at any point of tumor development, further contributing to the diagnostic challenge. Treatment of the skin lesions involves management of the underlying malignancy. Unfortunately, many such malignancies often are at an advanced stage, and subsequent prognosis is poor.2
- Shah A, Jack A, Liu H, et al. Neoplastic/paraneoplastic dermatitis, fasciitis, and panniculitis. Rheum Dis Clin North Am. 2011;37:573-592.
- Chairatchaneeboon M, Kim EJ. Cutaneous paraneoplastic syndromes. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick's Dermatology. 9th ed. McGraw-Hill Education; 2019:2441-2464.
- Lee HC, Ker KJ, Chong WS. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Yu Q, Li XL, Ji G, et al. Malignant acanthosis nigricans: an early diagnostic clue for gastric adenocarcinoma. World J Surg Oncol. 2017;15:208.
- Mohrenschlager M, Vocks E, Wessner DB, et al. Tripe palms and malignant acanthosis nigricans: cutaneous signs of imminent metastasis in bladder cancer? J Urol. 2001;165:1629-1630.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101.
- Shah A, Jack A, Liu H, et al. Neoplastic/paraneoplastic dermatitis, fasciitis, and panniculitis. Rheum Dis Clin North Am. 2011;37:573-592.
- Chairatchaneeboon M, Kim EJ. Cutaneous paraneoplastic syndromes. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick's Dermatology. 9th ed. McGraw-Hill Education; 2019:2441-2464.
- Lee HC, Ker KJ, Chong WS. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Yu Q, Li XL, Ji G, et al. Malignant acanthosis nigricans: an early diagnostic clue for gastric adenocarcinoma. World J Surg Oncol. 2017;15:208.
- Mohrenschlager M, Vocks E, Wessner DB, et al. Tripe palms and malignant acanthosis nigricans: cutaneous signs of imminent metastasis in bladder cancer? J Urol. 2001;165:1629-1630.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101.
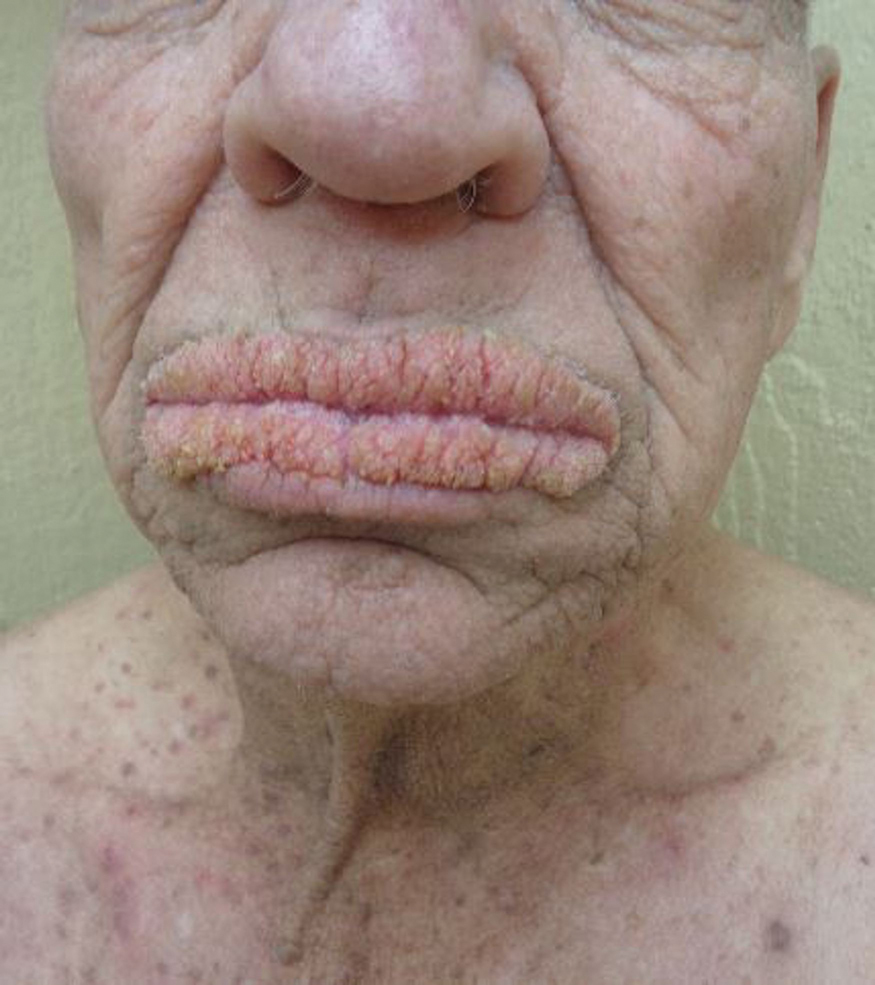
A 75-year-old nonobese man with metastatic urothelial carcinoma presented for evaluation and treatment of swollen lips. The patient stated that his lips began to swell and crack shortly after beginning pembrolizumab approximately 5 months prior. The swelling had progressively worsened, prompting discontinuation of the pembrolizumab by oncology about 2 months prior to presentation to our dermatology clinic. He reported slight improvement after the discontinuation of pembrolizumab, and he had since been started on carboplatin and gemcitabine. He previously was treated with oral corticosteroids without improvement. His oncologist started him on oral fluconazole for treatment of oral thrush on the day of presentation to our clinic. Physical examination revealed diffuse papillomatous and verrucous plaques of the upper and lower lips with involvement of the buccal mucosa. He also had deep fissures and white plaques on the tongue. Velvety hyperpigmented plaques were noted in the axillae, and he had confluent thickening of the palms. A 3-mm punch biopsy from the lower lip was performed. The patient subsequently was evaluated 2 weeks after the initial appointment, and minor improvement in the oral verrucous hyperplasia was noted following antifungal therapy, with resolution of the candidiasis.