User login
Leg-length discrepancy • asymmetric gluteal folds and popliteal fossae • positive Galeazzi test • Dx?
THE CASE
A healthy 6-month-old girl born via spontaneous vaginal delivery to a 33-year-old mother presented to her family physician (FP) for a routine well-child examination. The mother’s prenatal anatomy scan, delivery, and personal and family history were unremarkable. The patient was not firstborn or breech, and there was no family history of hip dysplasia. On prior infant well-child examinations, Ortolani and Barlow maneuvers were negative, and the patient demonstrated spontaneous movement of both legs. There was no evidence of hip dysplasia, lower extremity weakness, musculoskeletal abnormalities, or abnormal skin markings. The patient had normal growth and development (50th percentile for height and weight, average Ages & Stages Questionnaire scores) and no history of infection or trauma.
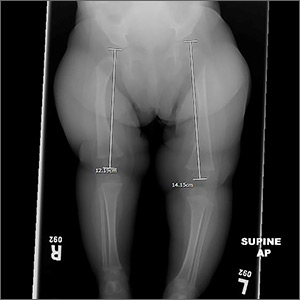
At the current presentation, the FP noted a leg-length discrepancy while palpating the bony (patellar and malleolar) landmarks of the lower extremities, but the right and left anterior superior iliac spine was symmetrical. The gluteal folds and popliteal fossae were asymmetric, a Galeazzi test was positive, and the right leg measured approximately 2 cm shorter than the left leg. There was no evidence of scoliosis or pelvic abnormalities. Physical examination revealed no ecchymosis or trauma. Orthopedic evaluation by the FP of the hips, knees, and ankles was normal, including negative repeat Ortolani and Barlow maneuvers and normal range of motion. We obtained x-rays of the lower extremities and ordered an orthopedic consultation.
THE DIAGNOSIS
The differential diagnosis included congenital, traumatic, infectious, inflammatory, idiopathic, and neurologic causes.1-3 The most common etiologies of leg-length discrepancies are summarized in TABLE 1.1-3 Radiographic imaging showed a femur length discrepancy, which was determined to be congenital without indication of trauma or disease; therefore, a diagnosis of congenital femoral bowing was made.
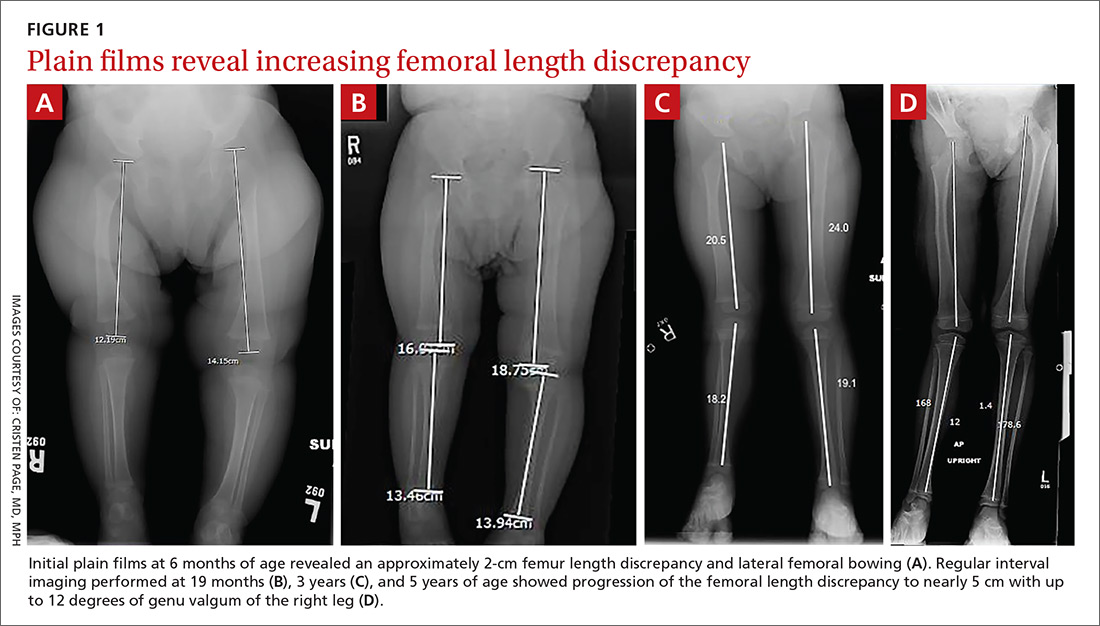
Initial orthopedic evaluation revealed a femur length discrepancy of approximately 2 cm. Plain films showed lateral femoral bowing (FIGURE 1A).
DISCUSSION
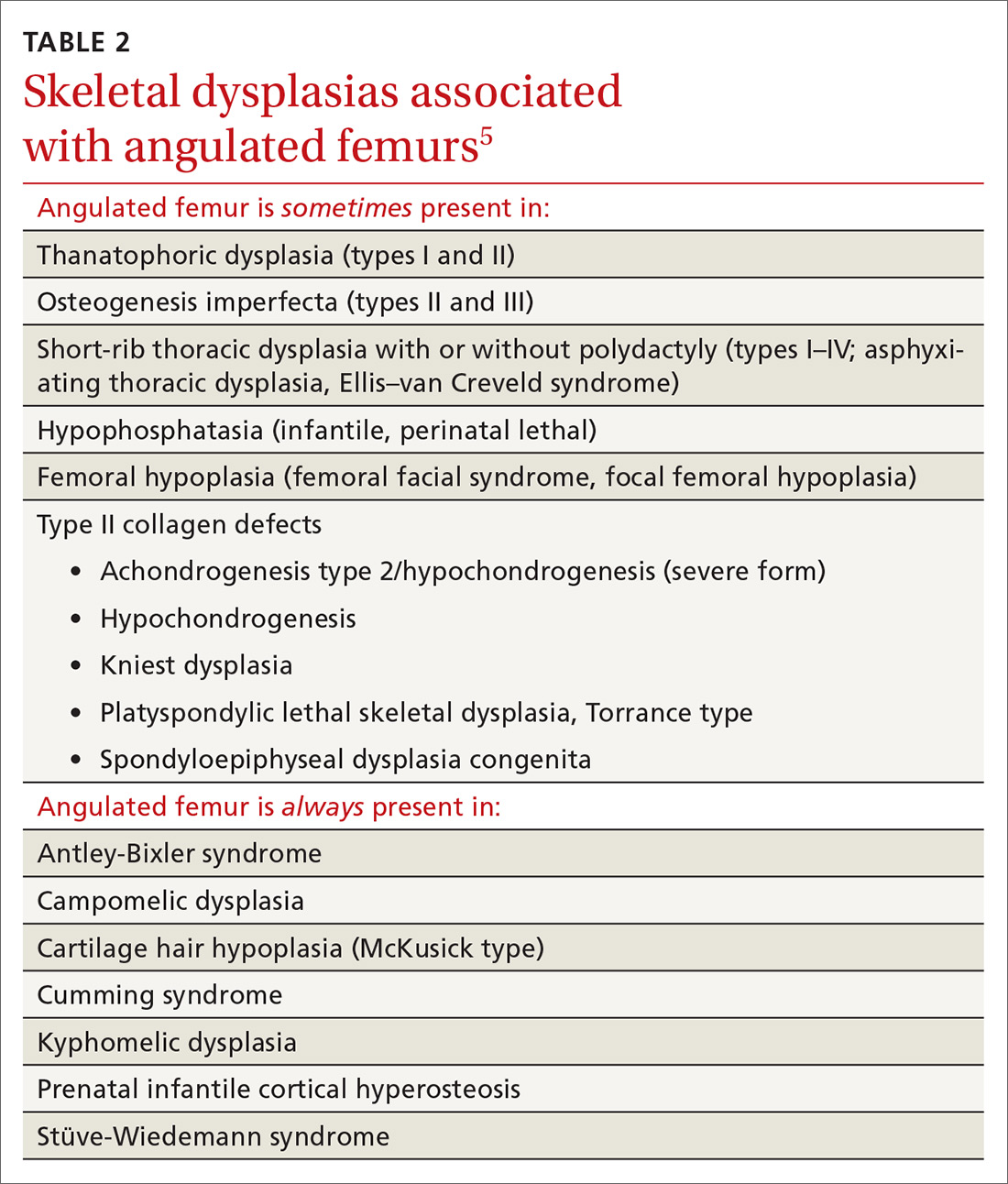
Congenital femoral bowing, which can present as a leg-length discrepancy in infants, is a relatively rare finding with an incidence of 1 per 52,000 births.4 Our patient presented with an isolated limb deformity, but congenital femoral bowing is recognized as a clinical feature of several skeletal dysplasias (TABLE 2).5
What’s recommended
The American Academy of Pediatrics recommends routine age-appropriate physical examination without specifying leg-length assessment.6 There is insufficient evidence, according to
Congenital femoral bowing requires plain film diagnosis
Following physical examination, diagnosis of congenital femoral bowing should be confirmed by plain films. Plain radiography remains the main imaging modality for proximal focal femoral deficiency and fibular hemimelia, and appropriate identification of the osseous abnormalities seen on radiographs allows for accurate classification of congenital femoral bowing, prognosis, and surgical planning.
Continue to: Early diagnosis can improve treatment outcome
Early diagnosis can improve treatment outcome
Both early diagnosis of congenital femoral bowing and prediction of leg-length discrepancy at skeletal maturity can influence potential treatment options, which range from conservative management (eg, watchful waiting, physical therapy, shoe lifts, orthotics, bracing) to surgical intervention. Several models have been used to predict skeletal growth, including the Moseley straight line graph, Green and Anderson growth curve, Amstutz method, and Paley’s multiplier method.4,9-14
Intervention for leg-length discrepancy generally is dictated by the magnitude of the inequality and the presence of functional deficits and/or pain.2 If the degree of femur angulation begins to affect structural development, surgical intervention should be considered to align development and/or correct the discrepancy. Physical therapy, shoe lifts, orthotics, and bracing are treatment options for managing smaller discrepancies.2,15
Our patient. The physician (CP) reviewed treatment options with the family that included watchful waiting, use of a shoe lift and/or orthotics, and bracing. The family chose watchful waiting due to the structural integrity of the patient’s other major joints and her relatively preserved function.
Surgery. Ultimately the patient underwent medial distal femoral hemiepiphysiodesis of the right lower extremity at 6 years of age due to increasing leg-length discrepancy and lateralization of the patella from the valgus deformity. The patient’s mother reported that she did well postoperatively, with increased range of motion, improved physical capabilities, and reduced discomfort in the right leg.
A second surgery. At approximately 8 years and 9 months of age, the orthopedist noted that the patient’s leg-length discrepancy had increased, and she had right extensor mechanism malalignment and severe patellar subluxation. The patient subsequently underwent surgery to remove the existing hardware, including right extensor mechanism realignment via a Roux-Goldthwait procedure (with reconstruction of the medial patellofemoral ligament and anterior cruciate ligament), as well as left distal femoral epiphysiodesis. She did very well postoperatively and continues to participate in physical therapy approximately once weekly. She has had an improvement in her gait and stability using shoe lifts.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A routine well-child examination can be an opportunity to identify congenital musculoskeletal problems. Congenital femoral bowing is a relatively rare finding4 that may present as a leg-length discrepancy. With proper evaluation, including visual inspection, palpation, range-of-motion testing, and special tests as needed (eg, Galeazzi test, Ortolani and Barlow maneuvers), early intervention is possible if a leg-length discrepancy is noted. Close monitoring of gait abnormalities at routine well-child visits is essential.
Physical therapy, shoe lift therapy, and surgical approaches are treatment options for leg-length discrepancy,2 and early intervention can improve treatment outcomes.14 Understanding how to manage congenital femoral bowing over time is important in providing options and counselling patients and their families.15
Treatment of leg-length discrepancy in pediatric patients requires long-term management with a team approach that includes patients and their families. The goal of intervention is to reduce physical and emotional trauma, while addressing complications and maintaining function of the affected limb, as well as the whole body.15
CORRESPONDENCE
Beth P. Davis, DPT, MBA, FNAP, Emory University School of Medicine, Department of Rehabilitation Medicine, Division of Physical Therapy, 1462 Clifton Road NE, Suite 312, Atlanta, GA 30342; [email protected].
1. Shailam R, Jaramillo D, Kan JH. Growth arrest and leg-length discrepancy. Pediatr Radiol. 2013:43(suppl 1):S155-S165.
2. Brady RJ, Dean JB, Skinner TM, et al. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33:221-234.
3. Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143-153.
4. Bedoya MA, Chauvin NA, Jaramillo D, et al. Common patterns of congenital lower extremity shortening: diagnosis, classification, and follow-up. Radiographics. 2015;35:1191-1207.
5. Alanay Y, Krakow D, Rimoin DL, et al. Angulated femurs and the skeletal dysplasias: experience of the International Skeletal Dysplasia Registry (1988-2006). Am J Med Genet. 2007;143A:1159-1168.
6. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017.
7. Shipman SA, Hefland M, Moyer VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:557-576.
8. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117:898-902.
9. Paley D, Bhave A, Herzenberg JE, et al. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432-1446.
10. Castaneda P, Urquhart B, Sullivan E, et al. Hemiepiphysiodesis for the correcting of angular deformity about the knee. J Pediatr Orthop. 2008;28:188-191.
11. Mosley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977; 59:174-179.
12. Anderson M, Messner MB, Green WT. Distribution of lengths of the normal femur and tibia in children from one to eighteen years of age. J Bone Joint Surg Am. 1964;46:1197-1202.
13. Amstutz HC. The morphology, natural history, and treatment of proximal femoral focal deficiency. In: GT Aitken, ed. Proximal Femoral Focal Deficiency: A Congenital Anomaly. Washington, DC: National Academy of Sciences; 1969: 50-76.
14. Amstutz HC. Natural history and treatment of congenital absence of the fibula. J Bone and Joint Surg Am. 1972;54(A):1349.
15. Guidera KJ, Helal AA, Zuern KA. Management of pediatric limb length inequality. Adv Pediatr. 1995;42:501-543.
THE CASE
A healthy 6-month-old girl born via spontaneous vaginal delivery to a 33-year-old mother presented to her family physician (FP) for a routine well-child examination. The mother’s prenatal anatomy scan, delivery, and personal and family history were unremarkable. The patient was not firstborn or breech, and there was no family history of hip dysplasia. On prior infant well-child examinations, Ortolani and Barlow maneuvers were negative, and the patient demonstrated spontaneous movement of both legs. There was no evidence of hip dysplasia, lower extremity weakness, musculoskeletal abnormalities, or abnormal skin markings. The patient had normal growth and development (50th percentile for height and weight, average Ages & Stages Questionnaire scores) and no history of infection or trauma.
At the current presentation, the FP noted a leg-length discrepancy while palpating the bony (patellar and malleolar) landmarks of the lower extremities, but the right and left anterior superior iliac spine was symmetrical. The gluteal folds and popliteal fossae were asymmetric, a Galeazzi test was positive, and the right leg measured approximately 2 cm shorter than the left leg. There was no evidence of scoliosis or pelvic abnormalities. Physical examination revealed no ecchymosis or trauma. Orthopedic evaluation by the FP of the hips, knees, and ankles was normal, including negative repeat Ortolani and Barlow maneuvers and normal range of motion. We obtained x-rays of the lower extremities and ordered an orthopedic consultation.
THE DIAGNOSIS
The differential diagnosis included congenital, traumatic, infectious, inflammatory, idiopathic, and neurologic causes.1-3 The most common etiologies of leg-length discrepancies are summarized in TABLE 1.1-3 Radiographic imaging showed a femur length discrepancy, which was determined to be congenital without indication of trauma or disease; therefore, a diagnosis of congenital femoral bowing was made.

Initial orthopedic evaluation revealed a femur length discrepancy of approximately 2 cm. Plain films showed lateral femoral bowing (FIGURE 1A).

DISCUSSION
Congenital femoral bowing, which can present as a leg-length discrepancy in infants, is a relatively rare finding with an incidence of 1 per 52,000 births.4 Our patient presented with an isolated limb deformity, but congenital femoral bowing is recognized as a clinical feature of several skeletal dysplasias (TABLE 2).5

What’s recommended
The American Academy of Pediatrics recommends routine age-appropriate physical examination without specifying leg-length assessment.6 There is insufficient evidence, according to
Congenital femoral bowing requires plain film diagnosis
Following physical examination, diagnosis of congenital femoral bowing should be confirmed by plain films. Plain radiography remains the main imaging modality for proximal focal femoral deficiency and fibular hemimelia, and appropriate identification of the osseous abnormalities seen on radiographs allows for accurate classification of congenital femoral bowing, prognosis, and surgical planning.
Continue to: Early diagnosis can improve treatment outcome
Early diagnosis can improve treatment outcome
Both early diagnosis of congenital femoral bowing and prediction of leg-length discrepancy at skeletal maturity can influence potential treatment options, which range from conservative management (eg, watchful waiting, physical therapy, shoe lifts, orthotics, bracing) to surgical intervention. Several models have been used to predict skeletal growth, including the Moseley straight line graph, Green and Anderson growth curve, Amstutz method, and Paley’s multiplier method.4,9-14
Intervention for leg-length discrepancy generally is dictated by the magnitude of the inequality and the presence of functional deficits and/or pain.2 If the degree of femur angulation begins to affect structural development, surgical intervention should be considered to align development and/or correct the discrepancy. Physical therapy, shoe lifts, orthotics, and bracing are treatment options for managing smaller discrepancies.2,15
Our patient. The physician (CP) reviewed treatment options with the family that included watchful waiting, use of a shoe lift and/or orthotics, and bracing. The family chose watchful waiting due to the structural integrity of the patient’s other major joints and her relatively preserved function.
Surgery. Ultimately the patient underwent medial distal femoral hemiepiphysiodesis of the right lower extremity at 6 years of age due to increasing leg-length discrepancy and lateralization of the patella from the valgus deformity. The patient’s mother reported that she did well postoperatively, with increased range of motion, improved physical capabilities, and reduced discomfort in the right leg.
A second surgery. At approximately 8 years and 9 months of age, the orthopedist noted that the patient’s leg-length discrepancy had increased, and she had right extensor mechanism malalignment and severe patellar subluxation. The patient subsequently underwent surgery to remove the existing hardware, including right extensor mechanism realignment via a Roux-Goldthwait procedure (with reconstruction of the medial patellofemoral ligament and anterior cruciate ligament), as well as left distal femoral epiphysiodesis. She did very well postoperatively and continues to participate in physical therapy approximately once weekly. She has had an improvement in her gait and stability using shoe lifts.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A routine well-child examination can be an opportunity to identify congenital musculoskeletal problems. Congenital femoral bowing is a relatively rare finding4 that may present as a leg-length discrepancy. With proper evaluation, including visual inspection, palpation, range-of-motion testing, and special tests as needed (eg, Galeazzi test, Ortolani and Barlow maneuvers), early intervention is possible if a leg-length discrepancy is noted. Close monitoring of gait abnormalities at routine well-child visits is essential.
Physical therapy, shoe lift therapy, and surgical approaches are treatment options for leg-length discrepancy,2 and early intervention can improve treatment outcomes.14 Understanding how to manage congenital femoral bowing over time is important in providing options and counselling patients and their families.15
Treatment of leg-length discrepancy in pediatric patients requires long-term management with a team approach that includes patients and their families. The goal of intervention is to reduce physical and emotional trauma, while addressing complications and maintaining function of the affected limb, as well as the whole body.15
CORRESPONDENCE
Beth P. Davis, DPT, MBA, FNAP, Emory University School of Medicine, Department of Rehabilitation Medicine, Division of Physical Therapy, 1462 Clifton Road NE, Suite 312, Atlanta, GA 30342; [email protected].
THE CASE
A healthy 6-month-old girl born via spontaneous vaginal delivery to a 33-year-old mother presented to her family physician (FP) for a routine well-child examination. The mother’s prenatal anatomy scan, delivery, and personal and family history were unremarkable. The patient was not firstborn or breech, and there was no family history of hip dysplasia. On prior infant well-child examinations, Ortolani and Barlow maneuvers were negative, and the patient demonstrated spontaneous movement of both legs. There was no evidence of hip dysplasia, lower extremity weakness, musculoskeletal abnormalities, or abnormal skin markings. The patient had normal growth and development (50th percentile for height and weight, average Ages & Stages Questionnaire scores) and no history of infection or trauma.
At the current presentation, the FP noted a leg-length discrepancy while palpating the bony (patellar and malleolar) landmarks of the lower extremities, but the right and left anterior superior iliac spine was symmetrical. The gluteal folds and popliteal fossae were asymmetric, a Galeazzi test was positive, and the right leg measured approximately 2 cm shorter than the left leg. There was no evidence of scoliosis or pelvic abnormalities. Physical examination revealed no ecchymosis or trauma. Orthopedic evaluation by the FP of the hips, knees, and ankles was normal, including negative repeat Ortolani and Barlow maneuvers and normal range of motion. We obtained x-rays of the lower extremities and ordered an orthopedic consultation.
THE DIAGNOSIS
The differential diagnosis included congenital, traumatic, infectious, inflammatory, idiopathic, and neurologic causes.1-3 The most common etiologies of leg-length discrepancies are summarized in TABLE 1.1-3 Radiographic imaging showed a femur length discrepancy, which was determined to be congenital without indication of trauma or disease; therefore, a diagnosis of congenital femoral bowing was made.

Initial orthopedic evaluation revealed a femur length discrepancy of approximately 2 cm. Plain films showed lateral femoral bowing (FIGURE 1A).

DISCUSSION
Congenital femoral bowing, which can present as a leg-length discrepancy in infants, is a relatively rare finding with an incidence of 1 per 52,000 births.4 Our patient presented with an isolated limb deformity, but congenital femoral bowing is recognized as a clinical feature of several skeletal dysplasias (TABLE 2).5

What’s recommended
The American Academy of Pediatrics recommends routine age-appropriate physical examination without specifying leg-length assessment.6 There is insufficient evidence, according to
Congenital femoral bowing requires plain film diagnosis
Following physical examination, diagnosis of congenital femoral bowing should be confirmed by plain films. Plain radiography remains the main imaging modality for proximal focal femoral deficiency and fibular hemimelia, and appropriate identification of the osseous abnormalities seen on radiographs allows for accurate classification of congenital femoral bowing, prognosis, and surgical planning.
Continue to: Early diagnosis can improve treatment outcome
Early diagnosis can improve treatment outcome
Both early diagnosis of congenital femoral bowing and prediction of leg-length discrepancy at skeletal maturity can influence potential treatment options, which range from conservative management (eg, watchful waiting, physical therapy, shoe lifts, orthotics, bracing) to surgical intervention. Several models have been used to predict skeletal growth, including the Moseley straight line graph, Green and Anderson growth curve, Amstutz method, and Paley’s multiplier method.4,9-14
Intervention for leg-length discrepancy generally is dictated by the magnitude of the inequality and the presence of functional deficits and/or pain.2 If the degree of femur angulation begins to affect structural development, surgical intervention should be considered to align development and/or correct the discrepancy. Physical therapy, shoe lifts, orthotics, and bracing are treatment options for managing smaller discrepancies.2,15
Our patient. The physician (CP) reviewed treatment options with the family that included watchful waiting, use of a shoe lift and/or orthotics, and bracing. The family chose watchful waiting due to the structural integrity of the patient’s other major joints and her relatively preserved function.
Surgery. Ultimately the patient underwent medial distal femoral hemiepiphysiodesis of the right lower extremity at 6 years of age due to increasing leg-length discrepancy and lateralization of the patella from the valgus deformity. The patient’s mother reported that she did well postoperatively, with increased range of motion, improved physical capabilities, and reduced discomfort in the right leg.
A second surgery. At approximately 8 years and 9 months of age, the orthopedist noted that the patient’s leg-length discrepancy had increased, and she had right extensor mechanism malalignment and severe patellar subluxation. The patient subsequently underwent surgery to remove the existing hardware, including right extensor mechanism realignment via a Roux-Goldthwait procedure (with reconstruction of the medial patellofemoral ligament and anterior cruciate ligament), as well as left distal femoral epiphysiodesis. She did very well postoperatively and continues to participate in physical therapy approximately once weekly. She has had an improvement in her gait and stability using shoe lifts.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A routine well-child examination can be an opportunity to identify congenital musculoskeletal problems. Congenital femoral bowing is a relatively rare finding4 that may present as a leg-length discrepancy. With proper evaluation, including visual inspection, palpation, range-of-motion testing, and special tests as needed (eg, Galeazzi test, Ortolani and Barlow maneuvers), early intervention is possible if a leg-length discrepancy is noted. Close monitoring of gait abnormalities at routine well-child visits is essential.
Physical therapy, shoe lift therapy, and surgical approaches are treatment options for leg-length discrepancy,2 and early intervention can improve treatment outcomes.14 Understanding how to manage congenital femoral bowing over time is important in providing options and counselling patients and their families.15
Treatment of leg-length discrepancy in pediatric patients requires long-term management with a team approach that includes patients and their families. The goal of intervention is to reduce physical and emotional trauma, while addressing complications and maintaining function of the affected limb, as well as the whole body.15
CORRESPONDENCE
Beth P. Davis, DPT, MBA, FNAP, Emory University School of Medicine, Department of Rehabilitation Medicine, Division of Physical Therapy, 1462 Clifton Road NE, Suite 312, Atlanta, GA 30342; [email protected].
1. Shailam R, Jaramillo D, Kan JH. Growth arrest and leg-length discrepancy. Pediatr Radiol. 2013:43(suppl 1):S155-S165.
2. Brady RJ, Dean JB, Skinner TM, et al. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33:221-234.
3. Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143-153.
4. Bedoya MA, Chauvin NA, Jaramillo D, et al. Common patterns of congenital lower extremity shortening: diagnosis, classification, and follow-up. Radiographics. 2015;35:1191-1207.
5. Alanay Y, Krakow D, Rimoin DL, et al. Angulated femurs and the skeletal dysplasias: experience of the International Skeletal Dysplasia Registry (1988-2006). Am J Med Genet. 2007;143A:1159-1168.
6. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017.
7. Shipman SA, Hefland M, Moyer VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:557-576.
8. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117:898-902.
9. Paley D, Bhave A, Herzenberg JE, et al. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432-1446.
10. Castaneda P, Urquhart B, Sullivan E, et al. Hemiepiphysiodesis for the correcting of angular deformity about the knee. J Pediatr Orthop. 2008;28:188-191.
11. Mosley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977; 59:174-179.
12. Anderson M, Messner MB, Green WT. Distribution of lengths of the normal femur and tibia in children from one to eighteen years of age. J Bone Joint Surg Am. 1964;46:1197-1202.
13. Amstutz HC. The morphology, natural history, and treatment of proximal femoral focal deficiency. In: GT Aitken, ed. Proximal Femoral Focal Deficiency: A Congenital Anomaly. Washington, DC: National Academy of Sciences; 1969: 50-76.
14. Amstutz HC. Natural history and treatment of congenital absence of the fibula. J Bone and Joint Surg Am. 1972;54(A):1349.
15. Guidera KJ, Helal AA, Zuern KA. Management of pediatric limb length inequality. Adv Pediatr. 1995;42:501-543.
1. Shailam R, Jaramillo D, Kan JH. Growth arrest and leg-length discrepancy. Pediatr Radiol. 2013:43(suppl 1):S155-S165.
2. Brady RJ, Dean JB, Skinner TM, et al. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33:221-234.
3. Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143-153.
4. Bedoya MA, Chauvin NA, Jaramillo D, et al. Common patterns of congenital lower extremity shortening: diagnosis, classification, and follow-up. Radiographics. 2015;35:1191-1207.
5. Alanay Y, Krakow D, Rimoin DL, et al. Angulated femurs and the skeletal dysplasias: experience of the International Skeletal Dysplasia Registry (1988-2006). Am J Med Genet. 2007;143A:1159-1168.
6. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017.
7. Shipman SA, Hefland M, Moyer VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:557-576.
8. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117:898-902.
9. Paley D, Bhave A, Herzenberg JE, et al. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432-1446.
10. Castaneda P, Urquhart B, Sullivan E, et al. Hemiepiphysiodesis for the correcting of angular deformity about the knee. J Pediatr Orthop. 2008;28:188-191.
11. Mosley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977; 59:174-179.
12. Anderson M, Messner MB, Green WT. Distribution of lengths of the normal femur and tibia in children from one to eighteen years of age. J Bone Joint Surg Am. 1964;46:1197-1202.
13. Amstutz HC. The morphology, natural history, and treatment of proximal femoral focal deficiency. In: GT Aitken, ed. Proximal Femoral Focal Deficiency: A Congenital Anomaly. Washington, DC: National Academy of Sciences; 1969: 50-76.
14. Amstutz HC. Natural history and treatment of congenital absence of the fibula. J Bone and Joint Surg Am. 1972;54(A):1349.
15. Guidera KJ, Helal AA, Zuern KA. Management of pediatric limb length inequality. Adv Pediatr. 1995;42:501-543.
Yeast Infection in Pregnancy? Think Twice About Fluconazole

A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although the latter are recommended as firstline therapy, the ease of oral therapy makes it an attractive option.3,4
However, the safety of oral fluconazole during pregnancy has recently come under scrutiny. Case reports have linked high-dose use with congenital malformation.5,6 These case reports led to epidemiologic studies in which no such association was found.7,8
A large cohort study involving 1,079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk for congenital malformation or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriage.10 However, the validity of both studies’ findings was limited by small numbers of participants.
The current study is the largest to date to evaluate whether use of fluconazole in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth, compared to topical azoles.
STUDY SUMMARY
Increased risk for miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole–exposed pregnancy was matched with up to four unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented by filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. Of the total cohort, 3,315 pregnancies were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortion occurred in 147 of these pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR], 1.48).
Rates of stillbirth. Of 5,382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR, 1.32). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were four times more likely than lower doses (150 mg) to be associated with stillbirth (HRs, 4.10 and 0.99, respectively).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk for spontaneous abortion, compared to topical azole use (130 of 2,823 pregnancies vs 118 of 2,823 pregnancies; HR, 1.62)—but not an increased risk for stillbirth (20 of 4,301 pregnancies vs 22 of 4,301 pregnancies; HR, 1.18).
WHAT'S NEW
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk for spontaneous abortion. By comparing treatments in a sensitivity analysis, the researchers were able to eliminate Candida infections causing spontaneous abortion as a confounding factor. In addition, this study challenges the balance between ease of use and safety.
CAVEATS
A skewed population?
This cohort study using a Danish hospital registry may not be generalizable to a larger, non-Scandinavian population. Those not seeking care through a hospital were likely missed; if those seeking care through the hospital had a higher risk for abortion, the results could be biased. However, this would not have affected the results of the comparison between the two active treatments.
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically.
In all, given the large sample size and the care taken to match each exposed pregnancy with up to four unexposed pregnancies, these limitations likely had little influence on the overall findings.
CHALLENGES TO IMPLEMENTATION
Balancing ease of use with safety
Given the ease of using oral fluconazole, compared with daily topical azole therapy, many clinicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(9):624-626.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al; Vaginal Infections and Prematurity Study Group. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidiasis. Practitioner. 1985;229:655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.

A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although the latter are recommended as firstline therapy, the ease of oral therapy makes it an attractive option.3,4
However, the safety of oral fluconazole during pregnancy has recently come under scrutiny. Case reports have linked high-dose use with congenital malformation.5,6 These case reports led to epidemiologic studies in which no such association was found.7,8
A large cohort study involving 1,079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk for congenital malformation or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriage.10 However, the validity of both studies’ findings was limited by small numbers of participants.
The current study is the largest to date to evaluate whether use of fluconazole in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth, compared to topical azoles.
STUDY SUMMARY
Increased risk for miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole–exposed pregnancy was matched with up to four unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented by filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. Of the total cohort, 3,315 pregnancies were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortion occurred in 147 of these pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR], 1.48).
Rates of stillbirth. Of 5,382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR, 1.32). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were four times more likely than lower doses (150 mg) to be associated with stillbirth (HRs, 4.10 and 0.99, respectively).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk for spontaneous abortion, compared to topical azole use (130 of 2,823 pregnancies vs 118 of 2,823 pregnancies; HR, 1.62)—but not an increased risk for stillbirth (20 of 4,301 pregnancies vs 22 of 4,301 pregnancies; HR, 1.18).
WHAT'S NEW
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk for spontaneous abortion. By comparing treatments in a sensitivity analysis, the researchers were able to eliminate Candida infections causing spontaneous abortion as a confounding factor. In addition, this study challenges the balance between ease of use and safety.
CAVEATS
A skewed population?
This cohort study using a Danish hospital registry may not be generalizable to a larger, non-Scandinavian population. Those not seeking care through a hospital were likely missed; if those seeking care through the hospital had a higher risk for abortion, the results could be biased. However, this would not have affected the results of the comparison between the two active treatments.
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically.
In all, given the large sample size and the care taken to match each exposed pregnancy with up to four unexposed pregnancies, these limitations likely had little influence on the overall findings.
CHALLENGES TO IMPLEMENTATION
Balancing ease of use with safety
Given the ease of using oral fluconazole, compared with daily topical azole therapy, many clinicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(9):624-626.

A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although the latter are recommended as firstline therapy, the ease of oral therapy makes it an attractive option.3,4
However, the safety of oral fluconazole during pregnancy has recently come under scrutiny. Case reports have linked high-dose use with congenital malformation.5,6 These case reports led to epidemiologic studies in which no such association was found.7,8
A large cohort study involving 1,079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk for congenital malformation or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriage.10 However, the validity of both studies’ findings was limited by small numbers of participants.
The current study is the largest to date to evaluate whether use of fluconazole in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth, compared to topical azoles.
STUDY SUMMARY
Increased risk for miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole–exposed pregnancy was matched with up to four unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented by filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. Of the total cohort, 3,315 pregnancies were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortion occurred in 147 of these pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR], 1.48).
Rates of stillbirth. Of 5,382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR, 1.32). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were four times more likely than lower doses (150 mg) to be associated with stillbirth (HRs, 4.10 and 0.99, respectively).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk for spontaneous abortion, compared to topical azole use (130 of 2,823 pregnancies vs 118 of 2,823 pregnancies; HR, 1.62)—but not an increased risk for stillbirth (20 of 4,301 pregnancies vs 22 of 4,301 pregnancies; HR, 1.18).
WHAT'S NEW
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk for spontaneous abortion. By comparing treatments in a sensitivity analysis, the researchers were able to eliminate Candida infections causing spontaneous abortion as a confounding factor. In addition, this study challenges the balance between ease of use and safety.
CAVEATS
A skewed population?
This cohort study using a Danish hospital registry may not be generalizable to a larger, non-Scandinavian population. Those not seeking care through a hospital were likely missed; if those seeking care through the hospital had a higher risk for abortion, the results could be biased. However, this would not have affected the results of the comparison between the two active treatments.
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically.
In all, given the large sample size and the care taken to match each exposed pregnancy with up to four unexposed pregnancies, these limitations likely had little influence on the overall findings.
CHALLENGES TO IMPLEMENTATION
Balancing ease of use with safety
Given the ease of using oral fluconazole, compared with daily topical azole therapy, many clinicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(9):624-626.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al; Vaginal Infections and Prematurity Study Group. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidiasis. Practitioner. 1985;229:655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al; Vaginal Infections and Prematurity Study Group. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidiasis. Practitioner. 1985;229:655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
Yeast infection in pregnancy? Think twice about fluconazole
PRACTICE CHANGER
Avoid prescribing oral fluconazole in early pregnancy because it is associated with a higher rate of spontaneous abortion than is topical azole therapy.1
Strength of recommendation
B: Based on large cohort study performed in Denmark.
Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
Illustrative Case
A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although topical azoles are recommended as first-line therapy,3 the ease of oral therapy makes it an attractive treatment option.4 The safety of oral fluconazole during pregnancy, however, has recently come under scrutiny.
Case reports have linked high-dose fluconazole use during pregnancy with congenital malformations.5,6 These case reports led to epidemiological studies evaluating fluconazole’s safety, but, in these studies, no association with congenital malformations was found.7,8
A large cohort study involving 1079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk of congenital malformations or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriages.10 However, the validity of both studies’ findings was limited by small numbers of participants. The current study is the largest to date to evaluate whether use of fluconazole compared to that of topical azoles in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth.
Study Summary
Fluconazole significantly increases risk of miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole-exposed pregnancy was matched with up to 4 unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented based on filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. From the total cohort of more than 1.4 million pregnancies, 3315 were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortions occurred in 147 of the 3315 fluconazole-exposed pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR]=1.48; 95% confidence interval [CI], 1.23-1.77).
Rates of stillbirth. Of 5382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR=1.32; 95% CI, 0.82-2.14). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were 4 times more likely to be associated with stillbirth (HR=4.10; 95% CI, 1.89-8.90) than lower doses (150 mg) (HR= 0.99; 95% CI, 0.56-1.74).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk of spontaneous abortion when compared to topical azole use: 130 of 2823 pregnancies vs 118 of 2823 pregnancies, respectively (HR=1.62; 95% CI, 1.26-2.07), but not an increased risk of stillbirths: 20 of 4301 pregnancies vs 22 of 4301 pregnancies, respectively (HR=1.18; 95% CI, 0.64-2.16).
What’s New
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk of spontaneous abortion. By comparing treatments in a sensitivity analysis, the confounder of Candida infections causing spontaneous abortion was removed. In addition, when considering the ease of dosing of fluconazole as compared with topical imidazoles, this study challenges the balance of ease of use with safety.
Caveats
A skewed population and limited generalizability?
This large cohort study using the National Patient Register in Denmark may not be generalizable to a larger, non-Scandinavian population. Since a hospital registry was used, those not seeking care through the hospital were likely missed. If patients
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically. In all, given the large sample size and the care taken to match each exposed pregnancy with up to 4 unexposed pregnancies, these limitations are likely to have had little influence on the overall findings of the study.
Challenges to Implementation
Balancing ease of use with safety
Given the ease of using oral fluconazole vs daily topical azole therapy, many physicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidosis. Practitioner. 1985;229: 655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
PRACTICE CHANGER
Avoid prescribing oral fluconazole in early pregnancy because it is associated with a higher rate of spontaneous abortion than is topical azole therapy.1
Strength of recommendation
B: Based on large cohort study performed in Denmark.
Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
Illustrative Case
A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although topical azoles are recommended as first-line therapy,3 the ease of oral therapy makes it an attractive treatment option.4 The safety of oral fluconazole during pregnancy, however, has recently come under scrutiny.
Case reports have linked high-dose fluconazole use during pregnancy with congenital malformations.5,6 These case reports led to epidemiological studies evaluating fluconazole’s safety, but, in these studies, no association with congenital malformations was found.7,8
A large cohort study involving 1079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk of congenital malformations or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriages.10 However, the validity of both studies’ findings was limited by small numbers of participants. The current study is the largest to date to evaluate whether use of fluconazole compared to that of topical azoles in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth.
Study Summary
Fluconazole significantly increases risk of miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole-exposed pregnancy was matched with up to 4 unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented based on filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. From the total cohort of more than 1.4 million pregnancies, 3315 were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortions occurred in 147 of the 3315 fluconazole-exposed pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR]=1.48; 95% confidence interval [CI], 1.23-1.77).
Rates of stillbirth. Of 5382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR=1.32; 95% CI, 0.82-2.14). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were 4 times more likely to be associated with stillbirth (HR=4.10; 95% CI, 1.89-8.90) than lower doses (150 mg) (HR= 0.99; 95% CI, 0.56-1.74).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk of spontaneous abortion when compared to topical azole use: 130 of 2823 pregnancies vs 118 of 2823 pregnancies, respectively (HR=1.62; 95% CI, 1.26-2.07), but not an increased risk of stillbirths: 20 of 4301 pregnancies vs 22 of 4301 pregnancies, respectively (HR=1.18; 95% CI, 0.64-2.16).
What’s New
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk of spontaneous abortion. By comparing treatments in a sensitivity analysis, the confounder of Candida infections causing spontaneous abortion was removed. In addition, when considering the ease of dosing of fluconazole as compared with topical imidazoles, this study challenges the balance of ease of use with safety.
Caveats
A skewed population and limited generalizability?
This large cohort study using the National Patient Register in Denmark may not be generalizable to a larger, non-Scandinavian population. Since a hospital registry was used, those not seeking care through the hospital were likely missed. If patients
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically. In all, given the large sample size and the care taken to match each exposed pregnancy with up to 4 unexposed pregnancies, these limitations are likely to have had little influence on the overall findings of the study.
Challenges to Implementation
Balancing ease of use with safety
Given the ease of using oral fluconazole vs daily topical azole therapy, many physicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
Avoid prescribing oral fluconazole in early pregnancy because it is associated with a higher rate of spontaneous abortion than is topical azole therapy.1
Strength of recommendation
B: Based on large cohort study performed in Denmark.
Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
Illustrative Case
A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although topical azoles are recommended as first-line therapy,3 the ease of oral therapy makes it an attractive treatment option.4 The safety of oral fluconazole during pregnancy, however, has recently come under scrutiny.
Case reports have linked high-dose fluconazole use during pregnancy with congenital malformations.5,6 These case reports led to epidemiological studies evaluating fluconazole’s safety, but, in these studies, no association with congenital malformations was found.7,8
A large cohort study involving 1079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk of congenital malformations or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriages.10 However, the validity of both studies’ findings was limited by small numbers of participants. The current study is the largest to date to evaluate whether use of fluconazole compared to that of topical azoles in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth.
Study Summary
Fluconazole significantly increases risk of miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole-exposed pregnancy was matched with up to 4 unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented based on filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. From the total cohort of more than 1.4 million pregnancies, 3315 were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortions occurred in 147 of the 3315 fluconazole-exposed pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR]=1.48; 95% confidence interval [CI], 1.23-1.77).
Rates of stillbirth. Of 5382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR=1.32; 95% CI, 0.82-2.14). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were 4 times more likely to be associated with stillbirth (HR=4.10; 95% CI, 1.89-8.90) than lower doses (150 mg) (HR= 0.99; 95% CI, 0.56-1.74).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk of spontaneous abortion when compared to topical azole use: 130 of 2823 pregnancies vs 118 of 2823 pregnancies, respectively (HR=1.62; 95% CI, 1.26-2.07), but not an increased risk of stillbirths: 20 of 4301 pregnancies vs 22 of 4301 pregnancies, respectively (HR=1.18; 95% CI, 0.64-2.16).
What’s New
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk of spontaneous abortion. By comparing treatments in a sensitivity analysis, the confounder of Candida infections causing spontaneous abortion was removed. In addition, when considering the ease of dosing of fluconazole as compared with topical imidazoles, this study challenges the balance of ease of use with safety.
Caveats
A skewed population and limited generalizability?
This large cohort study using the National Patient Register in Denmark may not be generalizable to a larger, non-Scandinavian population. Since a hospital registry was used, those not seeking care through the hospital were likely missed. If patients
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically. In all, given the large sample size and the care taken to match each exposed pregnancy with up to 4 unexposed pregnancies, these limitations are likely to have had little influence on the overall findings of the study.
Challenges to Implementation
Balancing ease of use with safety
Given the ease of using oral fluconazole vs daily topical azole therapy, many physicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidosis. Practitioner. 1985;229: 655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidosis. Practitioner. 1985;229: 655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Sterile or Nonsterile Gloves for Minor Skin Excisions?
PRACTICE CHANGER
Consider using nonsterile gloves during minor skin excisions (even those requiring sutures), because the infection rate is not increased compared to using sterile gloves.1
STRENGTH OF RECOMMENDATION
B: Based on a randomized controlled trial (RCT) conducted in a primary care practice.1
ILLUSTRATIVE CASE
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean nonsterile box in the exam room?
Nonsterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that nonsterile gloves do not increase the risk for infection during several types of minor skin procedures.
A partially blinded RCT in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) and nonsterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using nonsterile (0.49%) versus sterile (0.50%) gloves.3,4
Guidelines on the use of sterile versus nonsterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the CDC and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of nonmelanoma skin cancer, due out this winter, which may provide additional guidance.8 A 2003 review instructed primary care providers to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile versus nonsterile glove use for minor skin excisions in a primary care outpatient practice.
Continue for study summary >>
STUDY SUMMARY
Nonsterile is not inferior
Heal et al1 conducted a prospective, noninferiority RCT to compare the incidence of infection after minor skin surgery performed by six physicians from a single general practice in Australia using sterile versus nonsterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge; pain or tenderness; localized swelling, redness, or heat at the site; or a diagnosis of skin or soft-tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or nonsterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65, and 59% were men. At baseline, there were no large differences between patients in the sterile and nonsterile glove groups in terms of smoking status, anticoagulant or corticosteroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis; skin cancer and precursor; or other.
The incidence of infection in the nonsterile gloves group was 21/241 (8.7%) versus 22/237 in the control group (9.3%). The confidence interval (CI; 95%) for the difference in infection rate (–0.6%) was –4.0% to 2.9%—significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
Continue for what's new >>
WHAT’S NEW
New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, nonsterile gloves are not inferior to sterile gloves for excisional procedures that require sutures. While standard practice has many family practice providers using sterile gloves for these procedures, this study promotes changing this behavior.
Continue for caveats >>
CAVEATS
High infection rate, other factors may limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why nonsterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and clinicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the nonsterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves; outcomes were similar in terms of the difference in infection rate between sterile and nonsterile gloves.4
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found nonsterile gloves to be noninferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway clinicians from ingrained habits.
REFERENCES
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40: 234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infection: prevention and treatment of surgical site infection. www.nice.org.uk/guidance/cg74/chapter/1-recommendations. Accessed November 17, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed November 17, 2015.
8. American Academy of Dermatology. Clinical guidelines. www.aad.org/education/clinical-guidelines. Accessed November 17, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(11):723-724, 727.
PRACTICE CHANGER
Consider using nonsterile gloves during minor skin excisions (even those requiring sutures), because the infection rate is not increased compared to using sterile gloves.1
STRENGTH OF RECOMMENDATION
B: Based on a randomized controlled trial (RCT) conducted in a primary care practice.1
ILLUSTRATIVE CASE
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean nonsterile box in the exam room?
Nonsterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that nonsterile gloves do not increase the risk for infection during several types of minor skin procedures.
A partially blinded RCT in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) and nonsterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using nonsterile (0.49%) versus sterile (0.50%) gloves.3,4
Guidelines on the use of sterile versus nonsterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the CDC and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of nonmelanoma skin cancer, due out this winter, which may provide additional guidance.8 A 2003 review instructed primary care providers to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile versus nonsterile glove use for minor skin excisions in a primary care outpatient practice.
Continue for study summary >>
STUDY SUMMARY
Nonsterile is not inferior
Heal et al1 conducted a prospective, noninferiority RCT to compare the incidence of infection after minor skin surgery performed by six physicians from a single general practice in Australia using sterile versus nonsterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge; pain or tenderness; localized swelling, redness, or heat at the site; or a diagnosis of skin or soft-tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or nonsterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65, and 59% were men. At baseline, there were no large differences between patients in the sterile and nonsterile glove groups in terms of smoking status, anticoagulant or corticosteroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis; skin cancer and precursor; or other.
The incidence of infection in the nonsterile gloves group was 21/241 (8.7%) versus 22/237 in the control group (9.3%). The confidence interval (CI; 95%) for the difference in infection rate (–0.6%) was –4.0% to 2.9%—significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
Continue for what's new >>
WHAT’S NEW
New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, nonsterile gloves are not inferior to sterile gloves for excisional procedures that require sutures. While standard practice has many family practice providers using sterile gloves for these procedures, this study promotes changing this behavior.
Continue for caveats >>
CAVEATS
High infection rate, other factors may limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why nonsterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and clinicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the nonsterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves; outcomes were similar in terms of the difference in infection rate between sterile and nonsterile gloves.4
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found nonsterile gloves to be noninferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway clinicians from ingrained habits.
REFERENCES
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40: 234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infection: prevention and treatment of surgical site infection. www.nice.org.uk/guidance/cg74/chapter/1-recommendations. Accessed November 17, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed November 17, 2015.
8. American Academy of Dermatology. Clinical guidelines. www.aad.org/education/clinical-guidelines. Accessed November 17, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(11):723-724, 727.
PRACTICE CHANGER
Consider using nonsterile gloves during minor skin excisions (even those requiring sutures), because the infection rate is not increased compared to using sterile gloves.1
STRENGTH OF RECOMMENDATION
B: Based on a randomized controlled trial (RCT) conducted in a primary care practice.1
ILLUSTRATIVE CASE
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean nonsterile box in the exam room?
Nonsterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that nonsterile gloves do not increase the risk for infection during several types of minor skin procedures.
A partially blinded RCT in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) and nonsterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using nonsterile (0.49%) versus sterile (0.50%) gloves.3,4
Guidelines on the use of sterile versus nonsterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the CDC and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of nonmelanoma skin cancer, due out this winter, which may provide additional guidance.8 A 2003 review instructed primary care providers to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile versus nonsterile glove use for minor skin excisions in a primary care outpatient practice.
Continue for study summary >>
STUDY SUMMARY
Nonsterile is not inferior
Heal et al1 conducted a prospective, noninferiority RCT to compare the incidence of infection after minor skin surgery performed by six physicians from a single general practice in Australia using sterile versus nonsterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge; pain or tenderness; localized swelling, redness, or heat at the site; or a diagnosis of skin or soft-tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or nonsterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65, and 59% were men. At baseline, there were no large differences between patients in the sterile and nonsterile glove groups in terms of smoking status, anticoagulant or corticosteroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis; skin cancer and precursor; or other.
The incidence of infection in the nonsterile gloves group was 21/241 (8.7%) versus 22/237 in the control group (9.3%). The confidence interval (CI; 95%) for the difference in infection rate (–0.6%) was –4.0% to 2.9%—significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
Continue for what's new >>
WHAT’S NEW
New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, nonsterile gloves are not inferior to sterile gloves for excisional procedures that require sutures. While standard practice has many family practice providers using sterile gloves for these procedures, this study promotes changing this behavior.
Continue for caveats >>
CAVEATS
High infection rate, other factors may limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why nonsterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and clinicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the nonsterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves; outcomes were similar in terms of the difference in infection rate between sterile and nonsterile gloves.4
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found nonsterile gloves to be noninferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway clinicians from ingrained habits.
REFERENCES
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40: 234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infection: prevention and treatment of surgical site infection. www.nice.org.uk/guidance/cg74/chapter/1-recommendations. Accessed November 17, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed November 17, 2015.
8. American Academy of Dermatology. Clinical guidelines. www.aad.org/education/clinical-guidelines. Accessed November 17, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(11):723-724, 727.
Sterile or non-sterile gloves for minor skin excisions?
Consider using non-sterile gloves during minor skin excisions (even those that require sutures) because the infection rate is not increased compared to using sterile gloves.1
Strength of recommendation
B: Based on a randomized controlled trial done in a primary care practice.
Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled noninferiority trial. Med J Aust. 2015;202:27-31.
Illustrative case
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean non-sterile box in the exam room?
Non-sterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that non-sterile gloves do not increase the risk of infection during several types of minor skin procedures.
A partially blinded, randomized controlled trial (RCT) in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) vs non-sterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using non-sterile (0.49%) vs sterile (0.50%) gloves.3,4
Guidelines on the use of sterile vs non-sterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the Centers for Disease Control and Prevention (CDC) and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of non-melanoma skin cancer that’s due out this winter, and this may provide additional guidance.8 A 2003 review instructed primary care physicians to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile vs non-sterile glove use for minor skin excisions in a primary care outpatient practice.
STUDY SUMMARY: Non-sterile gloves are not inferior to sterile gloves
Heal et al1 conducted a prospective, randomized, controlled, noninferiority trial to compare the incidence of infection after minor skin surgery performed by 6 physicians from a single general practice in Australia using sterile vs non-sterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge, pain or tenderness, localized swelling or redness or heat at the site, or a diagnosis of skin or soft tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or non-sterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65 years and 59% were men. At baseline, there were no large differences between patients in the sterile and non-sterile glove groups in terms of smoking status, anticoagulant or steroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis, skin cancer and precursor, or other.
The incidence of infection in the non-sterile gloves group was 21/241 (8.7%; 95% confidence interval [CI], 4.9%-12.6%) vs 22/237 in the control group (9.3%; 95% CI, 7.4%-11.1%). The CI (95%) for the difference in infection rate (-0.6%) was -4.0% to 2.9%. This was significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
WHAT'S NEW: New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, non-sterile gloves are not inferior to sterile gloves for performing excisional procedures that require sutures. While standard practice has many family physicians using sterile gloves for these procedures, this study promotes changing this behavior.
CAVEATS: A high infection rate, other factors might limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why non-sterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and physicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the non-sterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves, and had similar outcomes in terms of the difference in infection rate between sterile and non-sterile gloves.4
CHALLENGES TO IMPLEMENTATION: Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found non-sterile gloves are non-inferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway family physicians from ingrained habits.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40:234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. October 2008. Available at: https://www.nice.org.uk/guidance/cg74. Accessed July 28, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). Updated August 28, 2013. Available at: http://www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed July 31, 2015.
8. American Academy of Dermatology. Clinical Guidelines. American Academy of Dermatology Web site. Available at: https://www.aad.org/education/clinical-guidelines. Accessed July 28, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
Consider using non-sterile gloves during minor skin excisions (even those that require sutures) because the infection rate is not increased compared to using sterile gloves.1
Strength of recommendation
B: Based on a randomized controlled trial done in a primary care practice.
Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled noninferiority trial. Med J Aust. 2015;202:27-31.
Illustrative case
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean non-sterile box in the exam room?
Non-sterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that non-sterile gloves do not increase the risk of infection during several types of minor skin procedures.
A partially blinded, randomized controlled trial (RCT) in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) vs non-sterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using non-sterile (0.49%) vs sterile (0.50%) gloves.3,4
Guidelines on the use of sterile vs non-sterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the Centers for Disease Control and Prevention (CDC) and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of non-melanoma skin cancer that’s due out this winter, and this may provide additional guidance.8 A 2003 review instructed primary care physicians to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile vs non-sterile glove use for minor skin excisions in a primary care outpatient practice.
STUDY SUMMARY: Non-sterile gloves are not inferior to sterile gloves
Heal et al1 conducted a prospective, randomized, controlled, noninferiority trial to compare the incidence of infection after minor skin surgery performed by 6 physicians from a single general practice in Australia using sterile vs non-sterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge, pain or tenderness, localized swelling or redness or heat at the site, or a diagnosis of skin or soft tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or non-sterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65 years and 59% were men. At baseline, there were no large differences between patients in the sterile and non-sterile glove groups in terms of smoking status, anticoagulant or steroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis, skin cancer and precursor, or other.
The incidence of infection in the non-sterile gloves group was 21/241 (8.7%; 95% confidence interval [CI], 4.9%-12.6%) vs 22/237 in the control group (9.3%; 95% CI, 7.4%-11.1%). The CI (95%) for the difference in infection rate (-0.6%) was -4.0% to 2.9%. This was significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
WHAT'S NEW: New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, non-sterile gloves are not inferior to sterile gloves for performing excisional procedures that require sutures. While standard practice has many family physicians using sterile gloves for these procedures, this study promotes changing this behavior.
CAVEATS: A high infection rate, other factors might limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why non-sterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and physicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the non-sterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves, and had similar outcomes in terms of the difference in infection rate between sterile and non-sterile gloves.4
CHALLENGES TO IMPLEMENTATION: Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found non-sterile gloves are non-inferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway family physicians from ingrained habits.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Consider using non-sterile gloves during minor skin excisions (even those that require sutures) because the infection rate is not increased compared to using sterile gloves.1
Strength of recommendation
B: Based on a randomized controlled trial done in a primary care practice.
Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled noninferiority trial. Med J Aust. 2015;202:27-31.
Illustrative case
A 50-year-old man comes to your office to have a mole removed from his arm. You decide to excise the lesion in your office today. Do you need to use sterile gloves for this procedure, or can you use gloves from the clean non-sterile box in the exam room?
Non-sterile gloves are readily available during a typical office visit and cost up to a dollar less per pair than sterile gloves.1-3 Studies conducted in settings other than primary care offices have shown that non-sterile gloves do not increase the risk of infection during several types of minor skin procedures.
A partially blinded, randomized controlled trial (RCT) in an emergency department found no significant difference in infection rates between the use of sterile (6.1%) vs non-sterile (4.4%) gloves during laceration repairs.2 Similarly, a small RCT in an outpatient dermatology clinic and a larger prospective trial by a Mohs dermatologist showed that infection rates were not increased after Mohs surgery using non-sterile (0.49%) vs sterile (0.50%) gloves.3,4
Guidelines on the use of sterile vs non-sterile gloves for minor skin excisions in outpatient primary care are difficult to come by. Current guidelines from the Centers for Disease Control and Prevention (CDC) and other agencies regarding surgical site infections are broad and focus on the operating room environment.5-7
The American Academy of Dermatology is working on a guideline for treatment of non-melanoma skin cancer that’s due out this winter, and this may provide additional guidance.8 A 2003 review instructed primary care physicians to use sterile gloves for excisional skin biopsies that require sutures.9
The 2015 study by Heal et al1 appears to be the first RCT to address the question of sterile vs non-sterile glove use for minor skin excisions in a primary care outpatient practice.
STUDY SUMMARY: Non-sterile gloves are not inferior to sterile gloves
Heal et al1 conducted a prospective, randomized, controlled, noninferiority trial to compare the incidence of infection after minor skin surgery performed by 6 physicians from a single general practice in Australia using sterile vs non-sterile clean gloves. They evaluated 576 consecutive patients who presented for skin excision between June 2012 and March 2013. Eighty-three patients were excluded because they had a latex allergy, were using oral antibiotics or immunosuppressive drugs, or required a skin flap procedure or excision of a sebaceous cyst. The physicians followed a standard process for performing the procedures and did not use topical antibiotics or antiseptic cleansing after the procedure.
The primary outcome was surgical site infection within 30 days of the excision, defined as purulent discharge, pain or tenderness, localized swelling or redness or heat at the site, or a diagnosis of skin or soft tissue infection by a general practitioner. The clinicians who assessed for infection were blinded to the patient’s assignment to the sterile or non-sterile glove group, and a stitch abscess was not counted as an infection.
The patients’ mean age was 65 years and 59% were men. At baseline, there were no large differences between patients in the sterile and non-sterile glove groups in terms of smoking status, anticoagulant or steroid use, diabetes, excision site, size of excision, and median days until removal of sutures. The lesions were identified histologically as nevus or seborrheic keratosis, skin cancer and precursor, or other.
The incidence of infection in the non-sterile gloves group was 21/241 (8.7%; 95% confidence interval [CI], 4.9%-12.6%) vs 22/237 in the control group (9.3%; 95% CI, 7.4%-11.1%). The CI (95%) for the difference in infection rate (-0.6%) was -4.0% to 2.9%. This was significantly below the predetermined noninferiority margin of 7%. In a sensitivity analysis of patients lost to follow-up (15 patients, 3%) that assumed all of these patients were without infection, or with infection, the CI was still below the noninferiority margin of 7%. The per-protocol analysis showed similar results.
WHAT'S NEW: New evidence questions the need for sterile gloves for in-office excisions
Heal et al1 demonstrated that in a primary care setting, non-sterile gloves are not inferior to sterile gloves for performing excisional procedures that require sutures. While standard practice has many family physicians using sterile gloves for these procedures, this study promotes changing this behavior.
CAVEATS: A high infection rate, other factors might limit generalizability
The overall rate of infection in this study (9%) was higher than that found in the studies from emergency medicine and dermatology literature cited earlier.2-4 A similarly high infection rate has been found in other studies of minor surgery by Heal et al, including a 2006 study that showed a wound infection rate of 8.6%.10 The significance of the higher infection rate is unknown, but there is no clear reason why non-sterile gloves might be less effective in preventing infection in environments with lower infection rates.
This was not a double-blinded study, and physicians might change their behavior during a procedure depending on the type of gloves they are wearing. The sterile gloves used in this study contained powder, while the non-sterile gloves were powderless, but this variable is not known to affect infection rates. A study of Mohs surgery avoided this variable by only using powderless gloves, and had similar outcomes in terms of the difference in infection rate between sterile and non-sterile gloves.4
CHALLENGES TO IMPLEMENTATION: Ingrained habits can be hard to change
Tradition and training die hard. While multiple studies in several settings have found non-sterile gloves are non-inferior to sterile gloves in preventing surgical site infection after minor skin surgeries, this single study in the primary care office setting may not be enough to sway family physicians from ingrained habits.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40:234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. October 2008. Available at: https://www.nice.org.uk/guidance/cg74. Accessed July 28, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). Updated August 28, 2013. Available at: http://www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed July 31, 2015.
8. American Academy of Dermatology. Clinical Guidelines. American Academy of Dermatology Web site. Available at: https://www.aad.org/education/clinical-guidelines. Accessed July 28, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
1. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomized controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
2. Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: a randomized controlled trial. Ann Emerg Med. 2004;43:362-370.
3. Mehta D, Chambers N, Adams B, et al. Comparison of the prevalence of surgical site infection with use of sterile versus nonsterile gloves for resection and reconstruction during Mohs surgery. Dermatol Surg. 2014;40:234-239.
4. Xia Y, Cho S, Greenway HT, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg. 2011;37:651-656.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-132.
6. National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. October 2008. Available at: https://www.nice.org.uk/guidance/cg74. Accessed July 28, 2015.
7. National Health and Medical Research Council. Australian Guidelines for the Prevention and Control of Infection in Healthcare (2010). Updated August 28, 2013. Available at: http://www.nhmrc.gov.au/book/html-australian-guideline-sprevention-and-control-infection-healthcare-2010. Accessed July 31, 2015.
8. American Academy of Dermatology. Clinical Guidelines. American Academy of Dermatology Web site. Available at: https://www.aad.org/education/clinical-guidelines. Accessed July 28, 2015.
9. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67:1539-1544.
10. Heal C, Buettner P, Browning S. Risk factors for wound infection after minor surgery in general practice. Med J Aust. 2006;18:255-258.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.