User login
Pseudoepitheliomatous Hyperplasia Arising From Purple Tattoo Pigment
To the Editor:
Pseudoepitheliomatous hyperplasia (PEH) is an uncommon type of reactive epidermal proliferation that can occur from a variety of causes, including an underlying infection, inflammation, neoplastic condition, or trauma induced from tattooing.1 Diagnosis can be challenging and requires clinicopathologic correlation, as PEH can mimic malignancy on histopathology.2-4 Histologically, PEH shows irregular hyperplasia of the epidermis and adnexal epithelium, elongation of the rete ridges, and extension of the reactive proliferation into the dermis. Absence of cytologic atypia is key to the diagnosis of PEH, helping to distinguish it from squamous cell carcinoma and keratoacanthoma. Clinically, patients typically present with well-demarcated, erythematous, scaly plaques or nodules in reactive areas, which can be symptomatically pruritic.
A 48-year-old woman presented with scaly and crusted verrucous plaques of 2 months’ duration that were isolated to the areas of purple pigment within a tattoo on the right lower leg. The patient reported pruritus in the affected areas that occurred immediately after obtaining the tattoo, which was her first and only tattoo. She denied any pertinent medical history, including an absence of immunosuppression and autoimmune or chronic inflammatory diseases.
Physical examination revealed scaly and crusted plaques isolated to areas of purple tattoo pigment (Figure 1). Areas of red, green, black, and blue pigmentation within the tattoo were uninvolved. With the initial suspicion of allergic contact dermatitis, two 6-mm punch biopsies were taken from adjacent linear plaques on the right leg for histology and tissue culture. Histopathologic evaluation revealed dermal tattoo pigment with overlying PEH and was negative for signs of infection (Figure 2). Infectious stains such as periodic acid–Schiff, Grocott-Gomori methenamine-silver, and Gram stains were performed and found to be negative. In addition, culture for mycobacteria came back negative. Prurigo was on the differential; however, histopathologic changes were more compatible with a PEH reaction to the tattoo.


Upon diagnosis, the patient was treated with clobetasol ointment 0.05% under occlusion for 1 month without reported improvement. The patient subsequently elected to undergo treatment with intralesional triamcinolone 5 mg/mL to all areas of PEH, except the areas immediately surrounding the healing biopsy sites. Twice-daily application of tacrolimus ointment 0.1% to all affected areas also was initiated. At follow-up 1 month later, she reported symptomatic relief of pruritus with a notable reduction in the thickness of the plaques in all treated areas (Figure 3). A second course of intralesional triamcinolone 5 mg/mL was performed. No additional plaques appeared during the treatment course, and the patient reported high satisfaction with the final result that was achieved.
An increase in the popularity of tattooing has led to more reports of various tattoo skin reactions.4-6 The differential diagnosis is broad for tattoo reactions and includes granulomatous inflammation, sarcoidosis, psoriasis (Köbner phenomenon), allergic contact dermatitis, lichen planus, morphealike reactions, squamous cell carcinoma, and keratoacanthoma,5 which makes clinicopathologic correlation essential for accurate diagnosis. Our case demonstrated the characteristic epithelial hyperplasia in the absence of cytologic atypia. In addition, the presence of mixed dermal inflammation histologically was noted in our patient.

Pseudoepitheliomatous hyperplasia development from a tattoo in areas of both mercury-based and non–mercury-based red pigment is a known association.7-9 Balfour et al10 also reported a case of PEH occurring secondary to manganese-based purple pigment. Because few cases have been reported, the epidemiology for PEH currently is unknown. Treatment of this condition primarily is anecdotal, with prior cases showing success with topical or intralesional steroids.5,7 As with any steroid-based treatment, we recommend less aggressive treatments initially with close follow-up and adaptation as needed to minimize adverse effects such as unwanted atrophy. Some success has been reported with the use of the Q-switched Nd:YAG laser in the setting of a PEH tattoo reaction.5 Similar to other tattoo reactions, surgical removal can be considered with failure of more conservative treatment methods and focal involvement.
We report an unusual case of PEH occurring secondary to purple tattoo pigment. Our report also demonstrates the clinical and symptomatic improvement of PEH that can be achieved through the use of intralesional corticosteroid therapy. Our patient represents a case of PEH reactive to tattooing with purple ink. Further research to elucidate the precise pathogenesis of PEH tattoo reactions would be helpful in identifying high-risk patients and determining the most efficacious treatments.
- Meani RE, Nixon RL, O’Keefe R, et al. Pseudoepitheliomatous hyperplasia secondary to allergic contact dermatitis to Grevillea Robyn Gordon. Australas J Dermatol. 2017;58:E8-E10.
- Chakrabarti S, Chakrabarti P, Agrawal D, et al. Pseudoepitheliomatous hyperplasia: a clinical entity mistaken for squamous cell carcinoma. J Cutan Aesthet Surg. 2014;7:232.
- Kluger N. Issues with keratoacanthoma, pseudoepitheliomatous hyperplasia and squamous cell carcinoma within tattoos: a clinical point of view. J Cutan Pathol. 2009;37:812-813.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-126.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Serup J. Diagnostic tools for doctors’ evaluation of tattoo complications. Curr Probl Dermatol. 2017;52:42-57.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
- Kluger N, Durand L, Minier-Thoumin C, et al. Pseudoepitheliomatous epidermal hyperplasia in tattoos: report of three cases. Am J Clin Dermatol. 2008;9:337-340.
- Cui W, McGregor DH, Stark SP, et al. Pseudoepitheliomatous hyperplasia—an unusual reaction following tattoo: report of a case and review of the literature. Int J Dermatol. 2007;46:743-745.
- Balfour E, Olhoffer I, Leffell D, et al. Massive pseudoepitheliomatous hyperplasia: an unusual reaction to a tattoo. Am J Dermatopathol. 2003;25:338-340.
To the Editor:
Pseudoepitheliomatous hyperplasia (PEH) is an uncommon type of reactive epidermal proliferation that can occur from a variety of causes, including an underlying infection, inflammation, neoplastic condition, or trauma induced from tattooing.1 Diagnosis can be challenging and requires clinicopathologic correlation, as PEH can mimic malignancy on histopathology.2-4 Histologically, PEH shows irregular hyperplasia of the epidermis and adnexal epithelium, elongation of the rete ridges, and extension of the reactive proliferation into the dermis. Absence of cytologic atypia is key to the diagnosis of PEH, helping to distinguish it from squamous cell carcinoma and keratoacanthoma. Clinically, patients typically present with well-demarcated, erythematous, scaly plaques or nodules in reactive areas, which can be symptomatically pruritic.
A 48-year-old woman presented with scaly and crusted verrucous plaques of 2 months’ duration that were isolated to the areas of purple pigment within a tattoo on the right lower leg. The patient reported pruritus in the affected areas that occurred immediately after obtaining the tattoo, which was her first and only tattoo. She denied any pertinent medical history, including an absence of immunosuppression and autoimmune or chronic inflammatory diseases.
Physical examination revealed scaly and crusted plaques isolated to areas of purple tattoo pigment (Figure 1). Areas of red, green, black, and blue pigmentation within the tattoo were uninvolved. With the initial suspicion of allergic contact dermatitis, two 6-mm punch biopsies were taken from adjacent linear plaques on the right leg for histology and tissue culture. Histopathologic evaluation revealed dermal tattoo pigment with overlying PEH and was negative for signs of infection (Figure 2). Infectious stains such as periodic acid–Schiff, Grocott-Gomori methenamine-silver, and Gram stains were performed and found to be negative. In addition, culture for mycobacteria came back negative. Prurigo was on the differential; however, histopathologic changes were more compatible with a PEH reaction to the tattoo.


Upon diagnosis, the patient was treated with clobetasol ointment 0.05% under occlusion for 1 month without reported improvement. The patient subsequently elected to undergo treatment with intralesional triamcinolone 5 mg/mL to all areas of PEH, except the areas immediately surrounding the healing biopsy sites. Twice-daily application of tacrolimus ointment 0.1% to all affected areas also was initiated. At follow-up 1 month later, she reported symptomatic relief of pruritus with a notable reduction in the thickness of the plaques in all treated areas (Figure 3). A second course of intralesional triamcinolone 5 mg/mL was performed. No additional plaques appeared during the treatment course, and the patient reported high satisfaction with the final result that was achieved.
An increase in the popularity of tattooing has led to more reports of various tattoo skin reactions.4-6 The differential diagnosis is broad for tattoo reactions and includes granulomatous inflammation, sarcoidosis, psoriasis (Köbner phenomenon), allergic contact dermatitis, lichen planus, morphealike reactions, squamous cell carcinoma, and keratoacanthoma,5 which makes clinicopathologic correlation essential for accurate diagnosis. Our case demonstrated the characteristic epithelial hyperplasia in the absence of cytologic atypia. In addition, the presence of mixed dermal inflammation histologically was noted in our patient.

Pseudoepitheliomatous hyperplasia development from a tattoo in areas of both mercury-based and non–mercury-based red pigment is a known association.7-9 Balfour et al10 also reported a case of PEH occurring secondary to manganese-based purple pigment. Because few cases have been reported, the epidemiology for PEH currently is unknown. Treatment of this condition primarily is anecdotal, with prior cases showing success with topical or intralesional steroids.5,7 As with any steroid-based treatment, we recommend less aggressive treatments initially with close follow-up and adaptation as needed to minimize adverse effects such as unwanted atrophy. Some success has been reported with the use of the Q-switched Nd:YAG laser in the setting of a PEH tattoo reaction.5 Similar to other tattoo reactions, surgical removal can be considered with failure of more conservative treatment methods and focal involvement.
We report an unusual case of PEH occurring secondary to purple tattoo pigment. Our report also demonstrates the clinical and symptomatic improvement of PEH that can be achieved through the use of intralesional corticosteroid therapy. Our patient represents a case of PEH reactive to tattooing with purple ink. Further research to elucidate the precise pathogenesis of PEH tattoo reactions would be helpful in identifying high-risk patients and determining the most efficacious treatments.
To the Editor:
Pseudoepitheliomatous hyperplasia (PEH) is an uncommon type of reactive epidermal proliferation that can occur from a variety of causes, including an underlying infection, inflammation, neoplastic condition, or trauma induced from tattooing.1 Diagnosis can be challenging and requires clinicopathologic correlation, as PEH can mimic malignancy on histopathology.2-4 Histologically, PEH shows irregular hyperplasia of the epidermis and adnexal epithelium, elongation of the rete ridges, and extension of the reactive proliferation into the dermis. Absence of cytologic atypia is key to the diagnosis of PEH, helping to distinguish it from squamous cell carcinoma and keratoacanthoma. Clinically, patients typically present with well-demarcated, erythematous, scaly plaques or nodules in reactive areas, which can be symptomatically pruritic.
A 48-year-old woman presented with scaly and crusted verrucous plaques of 2 months’ duration that were isolated to the areas of purple pigment within a tattoo on the right lower leg. The patient reported pruritus in the affected areas that occurred immediately after obtaining the tattoo, which was her first and only tattoo. She denied any pertinent medical history, including an absence of immunosuppression and autoimmune or chronic inflammatory diseases.
Physical examination revealed scaly and crusted plaques isolated to areas of purple tattoo pigment (Figure 1). Areas of red, green, black, and blue pigmentation within the tattoo were uninvolved. With the initial suspicion of allergic contact dermatitis, two 6-mm punch biopsies were taken from adjacent linear plaques on the right leg for histology and tissue culture. Histopathologic evaluation revealed dermal tattoo pigment with overlying PEH and was negative for signs of infection (Figure 2). Infectious stains such as periodic acid–Schiff, Grocott-Gomori methenamine-silver, and Gram stains were performed and found to be negative. In addition, culture for mycobacteria came back negative. Prurigo was on the differential; however, histopathologic changes were more compatible with a PEH reaction to the tattoo.


Upon diagnosis, the patient was treated with clobetasol ointment 0.05% under occlusion for 1 month without reported improvement. The patient subsequently elected to undergo treatment with intralesional triamcinolone 5 mg/mL to all areas of PEH, except the areas immediately surrounding the healing biopsy sites. Twice-daily application of tacrolimus ointment 0.1% to all affected areas also was initiated. At follow-up 1 month later, she reported symptomatic relief of pruritus with a notable reduction in the thickness of the plaques in all treated areas (Figure 3). A second course of intralesional triamcinolone 5 mg/mL was performed. No additional plaques appeared during the treatment course, and the patient reported high satisfaction with the final result that was achieved.
An increase in the popularity of tattooing has led to more reports of various tattoo skin reactions.4-6 The differential diagnosis is broad for tattoo reactions and includes granulomatous inflammation, sarcoidosis, psoriasis (Köbner phenomenon), allergic contact dermatitis, lichen planus, morphealike reactions, squamous cell carcinoma, and keratoacanthoma,5 which makes clinicopathologic correlation essential for accurate diagnosis. Our case demonstrated the characteristic epithelial hyperplasia in the absence of cytologic atypia. In addition, the presence of mixed dermal inflammation histologically was noted in our patient.

Pseudoepitheliomatous hyperplasia development from a tattoo in areas of both mercury-based and non–mercury-based red pigment is a known association.7-9 Balfour et al10 also reported a case of PEH occurring secondary to manganese-based purple pigment. Because few cases have been reported, the epidemiology for PEH currently is unknown. Treatment of this condition primarily is anecdotal, with prior cases showing success with topical or intralesional steroids.5,7 As with any steroid-based treatment, we recommend less aggressive treatments initially with close follow-up and adaptation as needed to minimize adverse effects such as unwanted atrophy. Some success has been reported with the use of the Q-switched Nd:YAG laser in the setting of a PEH tattoo reaction.5 Similar to other tattoo reactions, surgical removal can be considered with failure of more conservative treatment methods and focal involvement.
We report an unusual case of PEH occurring secondary to purple tattoo pigment. Our report also demonstrates the clinical and symptomatic improvement of PEH that can be achieved through the use of intralesional corticosteroid therapy. Our patient represents a case of PEH reactive to tattooing with purple ink. Further research to elucidate the precise pathogenesis of PEH tattoo reactions would be helpful in identifying high-risk patients and determining the most efficacious treatments.
- Meani RE, Nixon RL, O’Keefe R, et al. Pseudoepitheliomatous hyperplasia secondary to allergic contact dermatitis to Grevillea Robyn Gordon. Australas J Dermatol. 2017;58:E8-E10.
- Chakrabarti S, Chakrabarti P, Agrawal D, et al. Pseudoepitheliomatous hyperplasia: a clinical entity mistaken for squamous cell carcinoma. J Cutan Aesthet Surg. 2014;7:232.
- Kluger N. Issues with keratoacanthoma, pseudoepitheliomatous hyperplasia and squamous cell carcinoma within tattoos: a clinical point of view. J Cutan Pathol. 2009;37:812-813.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-126.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Serup J. Diagnostic tools for doctors’ evaluation of tattoo complications. Curr Probl Dermatol. 2017;52:42-57.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
- Kluger N, Durand L, Minier-Thoumin C, et al. Pseudoepitheliomatous epidermal hyperplasia in tattoos: report of three cases. Am J Clin Dermatol. 2008;9:337-340.
- Cui W, McGregor DH, Stark SP, et al. Pseudoepitheliomatous hyperplasia—an unusual reaction following tattoo: report of a case and review of the literature. Int J Dermatol. 2007;46:743-745.
- Balfour E, Olhoffer I, Leffell D, et al. Massive pseudoepitheliomatous hyperplasia: an unusual reaction to a tattoo. Am J Dermatopathol. 2003;25:338-340.
- Meani RE, Nixon RL, O’Keefe R, et al. Pseudoepitheliomatous hyperplasia secondary to allergic contact dermatitis to Grevillea Robyn Gordon. Australas J Dermatol. 2017;58:E8-E10.
- Chakrabarti S, Chakrabarti P, Agrawal D, et al. Pseudoepitheliomatous hyperplasia: a clinical entity mistaken for squamous cell carcinoma. J Cutan Aesthet Surg. 2014;7:232.
- Kluger N. Issues with keratoacanthoma, pseudoepitheliomatous hyperplasia and squamous cell carcinoma within tattoos: a clinical point of view. J Cutan Pathol. 2009;37:812-813.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-126.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Serup J. Diagnostic tools for doctors’ evaluation of tattoo complications. Curr Probl Dermatol. 2017;52:42-57.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
- Kluger N, Durand L, Minier-Thoumin C, et al. Pseudoepitheliomatous epidermal hyperplasia in tattoos: report of three cases. Am J Clin Dermatol. 2008;9:337-340.
- Cui W, McGregor DH, Stark SP, et al. Pseudoepitheliomatous hyperplasia—an unusual reaction following tattoo: report of a case and review of the literature. Int J Dermatol. 2007;46:743-745.
- Balfour E, Olhoffer I, Leffell D, et al. Massive pseudoepitheliomatous hyperplasia: an unusual reaction to a tattoo. Am J Dermatopathol. 2003;25:338-340.
Practice Points
- Pseudoepitheliomatous hyperplasia (PEH) is a rare benign condition that can arise in response to multiple underlying triggers such as tattoo pigment.
- Histopathologic evaluation is essential for diagnosis and shows characteristic hyperplasia of the epidermis.
- Clinicians should consider intralesional steroids in the treatment of PEH once atypical mycobacterial and deep fungal infections have been ruled out.
Trichodysplasia Spinulosa in the Setting of Colon Cancer
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
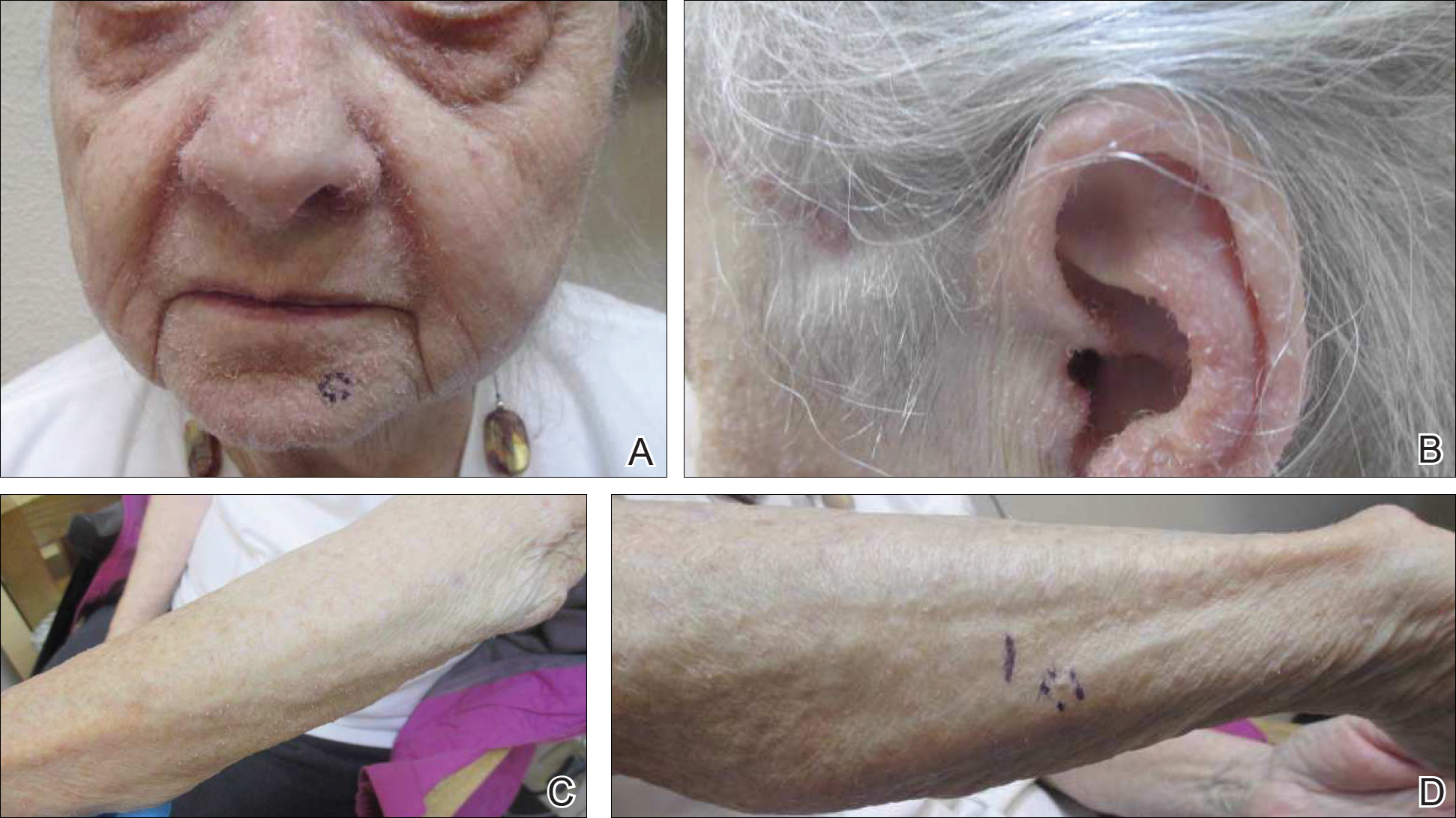
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).
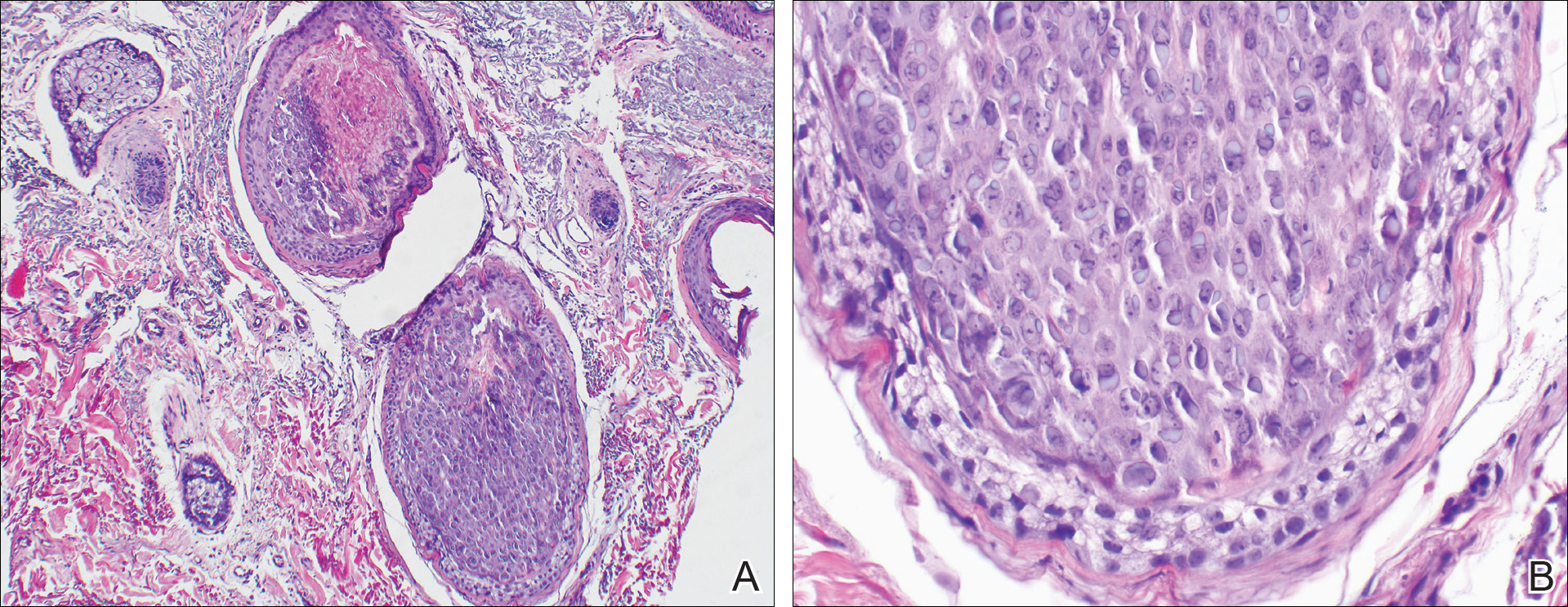
A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.
The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).

A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.

The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).

A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.

The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
Practice Points
- Rashes have a life span and can evolve with time.
- If apparent straightforward conditions do not appear to respond to standard therapy, start to think outside the box for underlying potential causes.

