User login
Psychedelics for treating psychiatric disorders: Are they safe?
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
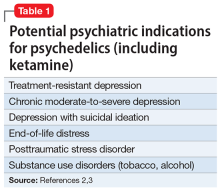
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
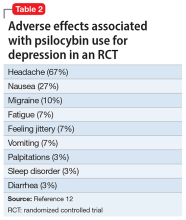
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
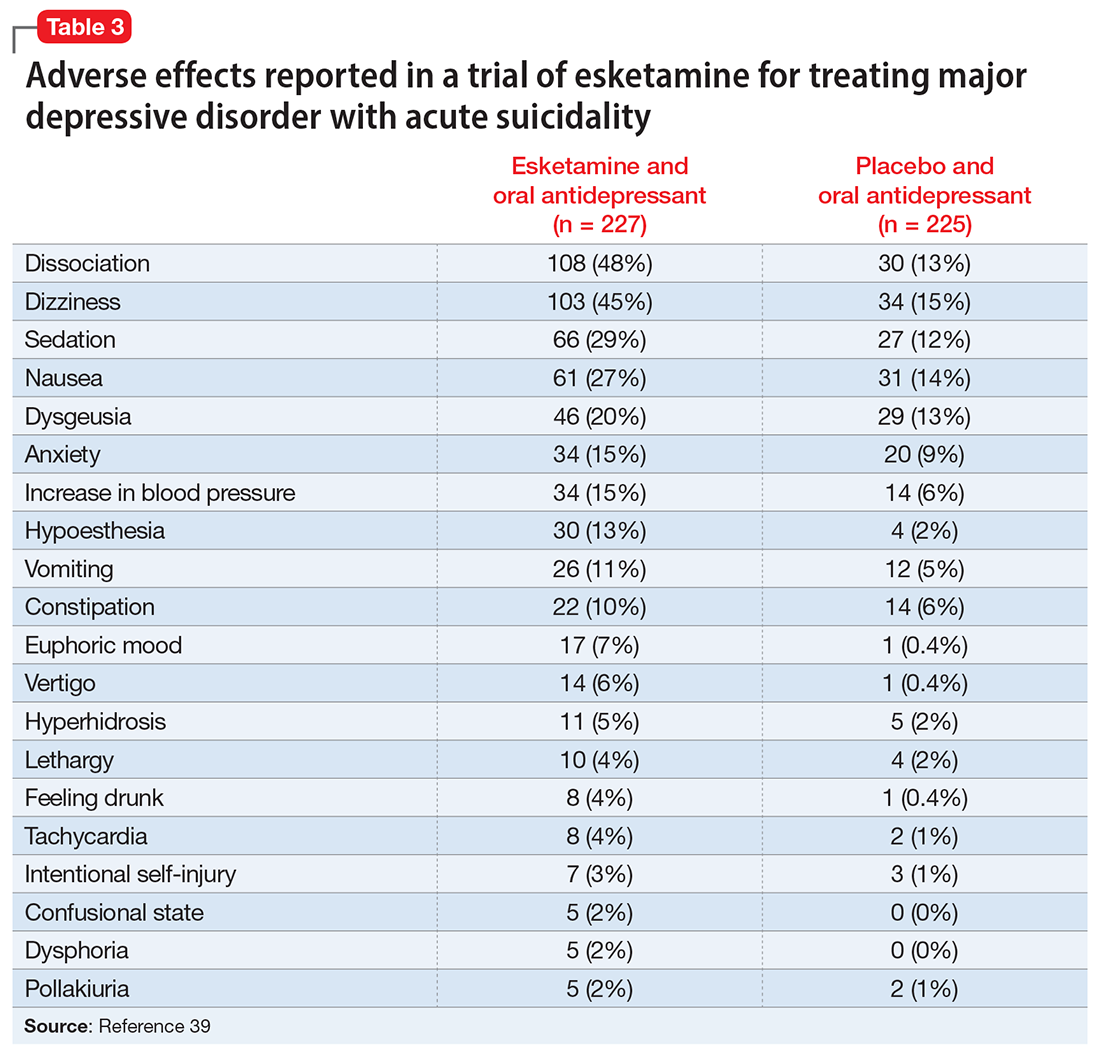
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40
Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40

Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40

Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
Alan F. Schatzberg, MD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Alan F. Schatzberg, MD. Dr. Schatzberg is the Kenneth T. Norris, Jr., Professor of Psychiatry and Behavioral Sciences at Stanford University. He served as the Chair of the Department at Stanford until 2010 and currently directs the Stanford Mood Disorders Center. He was the 136th president of the American Psychiatric Association (APA) (2009-2010). He has been an active investigator in the biology and psychopharmacology of depressive disorders, and has authored more than 700 publications and abstracts, including Schatzberg’s Manual of Clinical Psychopharmacology. Dr. Schatzberg is also the coeditor of the Textbook of Psychopharmacology with Charles B. Nemeroff, MD, PhD. He is a Past President of the American College of Neuropsychopharmacology (ACNP) and the Society of Biological Psychiatry, and was also the Secretary-General of the International Society of Psychoneuroendocrinology (ISPNE). In 2003, he was elected to the Institute of Medicine of the National Academy of Sciences (National Academy of Medicine). He has received numerous prestigious awards, including the 2005 Distinguished Service in Psychiatry Award from the American College of Psychiatrists, the 2005 Falcone Award from the National Alliance for Research in Schizophrenia and Affective Disorders, the 2014 Kraepelin Gold Medal from the Max Planck Institute of Psychiatry, the 2015 Gold Medal from the Society of Biological Psychiatry, the 2015 Lifetime Achievement Award of the ISPNE, the 2017 Julius Axelrod Mentorship Award from the ACNP, the 2018 Donald Klein, MD, Lifetime Achievement Award from the American Society of Clinical Psychopharmacology, and the 2018 Jules Marmor, MD, Award for Biopsychosocial Research from the APA.
Dr. Aftab: You have devoted much of your career to the development of psychopharmacology. What is your perspective on where the field of psychopharmacology stands at present, especially amid the widespread recognition of “treatment resistance” as a pervasive phenomenon and the scarcity of validated neurobiologic etiological models for psychiatric disorders?
Dr. Schatzberg: We have made considerable progress in the development of new classes of agents for major depression, but as we develop new agents, we still see a large percentage of patients who do not seem to demonstrate adequate responses, particularly in major depressive disorder. This has driven us to look for agents that work differently than previous ones. Although we have some new agents with seeming efficacy and newer mechanisms of action, eg, esketamine, these have largely been derived from clinical, often serendipitous, observations of antidepressant effects rather than from prospective development based on a known pharmacological effect or a biological construct of the disorder. Another intriguing and possibly effective anxiolytic and antidepressive agent is psilocybin, whose potential use is largely derived from clinicians who found it helpful in their practices in combination with psychotherapy. These 2 demonstrate how as we branch out into new territory, we find ourselves moving more and more toward drugs of known clinical risk; eg, mind-altering agents or drugs of abuse. These agents may offer risk-benefit ratios that can ultimately prove to be less attractive than what we might have wanted when we ventured on the journey. Unfortunately, there has been little dialogue about the limitations of several of these agents.
In the case of esketamine, the notion has been that the drug is a blocker of the N-methyl-
One approach that has been applied recently is target validation that purports to use functional MRI to assess behavioral and cognitive effects of drugs to allow inferences regarding efficacy in specific disorders. As we have discussed in a recent paper published in the American Journal of Psychiatry,4 this can be quite misleading and may provide both false positive and negative information. From my perspective, these tests do not appear sensitive enough to screen for patients having a disorder, nor for assessing possible drug effects in those patients. Thus, it is unclear if they can provide answers today that we can be confident in.
Continue to: Dr. Aftab...
Dr. Aftab: What do you see as some of the strengths of psychiatry as a profession?
Dr. Schatzberg: Psychiatry as a specialty combines 2 major perspectives—psychological processes and psychobiology—to develop methods for treating patients who suffer from disorders of the mind/brain. It is the most challenging of our specialties because we cannot study the brain directly. We cannot do procedures as we do in cardiology and pulmonology because they may prove dangerously invasive. That hands-off approach limits us, but for the curious it provides an opportunity to begin to unravel the processes that underlie brain functioning. Fortunately, we have therapies—both psychosocial and somatic—that can provide great relief to patients. These can be shown to be effective in sufficient numbers of patients to help many.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Schatzberg: We need to train our residents in a host of approaches, and not just medications and psychotherapy. They need to understand the basis of brain stimulation approaches (such as repetitive transcranial magnetic stimulation) as well as know how to apply them. We need to train residents more in substance abuse problems and the biology of addiction if they are to better understand the risks of certain new classes of medication. Lastly, we need to train residents in the application of genomics, proteomics, and brain imaging to somatic treatment development.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Schatzberg: The biggest threats come from ourselves. We need to do better with our classification approaches, such as the Diagnostic and Statistical Manual of Mental Disorders or the Research Domain Criteria. They need to become more rapidly adaptive to research in the field. We need to be more open to looking at what is a potentially dangerous trend in developing drugs of abuse and mind-altering drugs as therapeutics. We need to be able to demonstrate that telepsychiatry can be as effective as face-to-face treatment and should be reimbursed. Lastly, we need to develop better models for taking care of the psychiatric patient. We have too many patients and not enough psychiatrists.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Schatzberg: I see the future as bright. Over the past 10 years, led by efforts at the APA, some while I was President, reimbursement has increased dramatically. Over the past 10 years, we have done well developing some new drugs and somatic therapies, and these will continue. Less than a decade ago, large pharmaceutical had abandoned psychiatric drug development and investment into biotech start-ups had waned to near zero. However, the last year few years have seen a dramatic surge in investment, and these should yield novel agents and ones that may be combined with innovative biomarkers as companions.
1. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215. doi:10.1176/appi.ajp.2018.18020138
2. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786. doi:10.1038/s41380-019-0503-4
3. Bonaventura J, Lam S, Carlton M, et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 2021;10.1038/s41380-021-01093-2. doi:10.1038/s41380-021-01093-2
4. Schatzberg AF. Can target engagement studies miss their targets and mislead drug development? Am J Psychiatry. 2021;178(5):372-374. doi:10.1176/appi.ajp.2020.21030247
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Alan F. Schatzberg, MD. Dr. Schatzberg is the Kenneth T. Norris, Jr., Professor of Psychiatry and Behavioral Sciences at Stanford University. He served as the Chair of the Department at Stanford until 2010 and currently directs the Stanford Mood Disorders Center. He was the 136th president of the American Psychiatric Association (APA) (2009-2010). He has been an active investigator in the biology and psychopharmacology of depressive disorders, and has authored more than 700 publications and abstracts, including Schatzberg’s Manual of Clinical Psychopharmacology. Dr. Schatzberg is also the coeditor of the Textbook of Psychopharmacology with Charles B. Nemeroff, MD, PhD. He is a Past President of the American College of Neuropsychopharmacology (ACNP) and the Society of Biological Psychiatry, and was also the Secretary-General of the International Society of Psychoneuroendocrinology (ISPNE). In 2003, he was elected to the Institute of Medicine of the National Academy of Sciences (National Academy of Medicine). He has received numerous prestigious awards, including the 2005 Distinguished Service in Psychiatry Award from the American College of Psychiatrists, the 2005 Falcone Award from the National Alliance for Research in Schizophrenia and Affective Disorders, the 2014 Kraepelin Gold Medal from the Max Planck Institute of Psychiatry, the 2015 Gold Medal from the Society of Biological Psychiatry, the 2015 Lifetime Achievement Award of the ISPNE, the 2017 Julius Axelrod Mentorship Award from the ACNP, the 2018 Donald Klein, MD, Lifetime Achievement Award from the American Society of Clinical Psychopharmacology, and the 2018 Jules Marmor, MD, Award for Biopsychosocial Research from the APA.
Dr. Aftab: You have devoted much of your career to the development of psychopharmacology. What is your perspective on where the field of psychopharmacology stands at present, especially amid the widespread recognition of “treatment resistance” as a pervasive phenomenon and the scarcity of validated neurobiologic etiological models for psychiatric disorders?
Dr. Schatzberg: We have made considerable progress in the development of new classes of agents for major depression, but as we develop new agents, we still see a large percentage of patients who do not seem to demonstrate adequate responses, particularly in major depressive disorder. This has driven us to look for agents that work differently than previous ones. Although we have some new agents with seeming efficacy and newer mechanisms of action, eg, esketamine, these have largely been derived from clinical, often serendipitous, observations of antidepressant effects rather than from prospective development based on a known pharmacological effect or a biological construct of the disorder. Another intriguing and possibly effective anxiolytic and antidepressive agent is psilocybin, whose potential use is largely derived from clinicians who found it helpful in their practices in combination with psychotherapy. These 2 demonstrate how as we branch out into new territory, we find ourselves moving more and more toward drugs of known clinical risk; eg, mind-altering agents or drugs of abuse. These agents may offer risk-benefit ratios that can ultimately prove to be less attractive than what we might have wanted when we ventured on the journey. Unfortunately, there has been little dialogue about the limitations of several of these agents.
In the case of esketamine, the notion has been that the drug is a blocker of the N-methyl-
One approach that has been applied recently is target validation that purports to use functional MRI to assess behavioral and cognitive effects of drugs to allow inferences regarding efficacy in specific disorders. As we have discussed in a recent paper published in the American Journal of Psychiatry,4 this can be quite misleading and may provide both false positive and negative information. From my perspective, these tests do not appear sensitive enough to screen for patients having a disorder, nor for assessing possible drug effects in those patients. Thus, it is unclear if they can provide answers today that we can be confident in.
Continue to: Dr. Aftab...
Dr. Aftab: What do you see as some of the strengths of psychiatry as a profession?
Dr. Schatzberg: Psychiatry as a specialty combines 2 major perspectives—psychological processes and psychobiology—to develop methods for treating patients who suffer from disorders of the mind/brain. It is the most challenging of our specialties because we cannot study the brain directly. We cannot do procedures as we do in cardiology and pulmonology because they may prove dangerously invasive. That hands-off approach limits us, but for the curious it provides an opportunity to begin to unravel the processes that underlie brain functioning. Fortunately, we have therapies—both psychosocial and somatic—that can provide great relief to patients. These can be shown to be effective in sufficient numbers of patients to help many.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Schatzberg: We need to train our residents in a host of approaches, and not just medications and psychotherapy. They need to understand the basis of brain stimulation approaches (such as repetitive transcranial magnetic stimulation) as well as know how to apply them. We need to train residents more in substance abuse problems and the biology of addiction if they are to better understand the risks of certain new classes of medication. Lastly, we need to train residents in the application of genomics, proteomics, and brain imaging to somatic treatment development.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Schatzberg: The biggest threats come from ourselves. We need to do better with our classification approaches, such as the Diagnostic and Statistical Manual of Mental Disorders or the Research Domain Criteria. They need to become more rapidly adaptive to research in the field. We need to be more open to looking at what is a potentially dangerous trend in developing drugs of abuse and mind-altering drugs as therapeutics. We need to be able to demonstrate that telepsychiatry can be as effective as face-to-face treatment and should be reimbursed. Lastly, we need to develop better models for taking care of the psychiatric patient. We have too many patients and not enough psychiatrists.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Schatzberg: I see the future as bright. Over the past 10 years, led by efforts at the APA, some while I was President, reimbursement has increased dramatically. Over the past 10 years, we have done well developing some new drugs and somatic therapies, and these will continue. Less than a decade ago, large pharmaceutical had abandoned psychiatric drug development and investment into biotech start-ups had waned to near zero. However, the last year few years have seen a dramatic surge in investment, and these should yield novel agents and ones that may be combined with innovative biomarkers as companions.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Alan F. Schatzberg, MD. Dr. Schatzberg is the Kenneth T. Norris, Jr., Professor of Psychiatry and Behavioral Sciences at Stanford University. He served as the Chair of the Department at Stanford until 2010 and currently directs the Stanford Mood Disorders Center. He was the 136th president of the American Psychiatric Association (APA) (2009-2010). He has been an active investigator in the biology and psychopharmacology of depressive disorders, and has authored more than 700 publications and abstracts, including Schatzberg’s Manual of Clinical Psychopharmacology. Dr. Schatzberg is also the coeditor of the Textbook of Psychopharmacology with Charles B. Nemeroff, MD, PhD. He is a Past President of the American College of Neuropsychopharmacology (ACNP) and the Society of Biological Psychiatry, and was also the Secretary-General of the International Society of Psychoneuroendocrinology (ISPNE). In 2003, he was elected to the Institute of Medicine of the National Academy of Sciences (National Academy of Medicine). He has received numerous prestigious awards, including the 2005 Distinguished Service in Psychiatry Award from the American College of Psychiatrists, the 2005 Falcone Award from the National Alliance for Research in Schizophrenia and Affective Disorders, the 2014 Kraepelin Gold Medal from the Max Planck Institute of Psychiatry, the 2015 Gold Medal from the Society of Biological Psychiatry, the 2015 Lifetime Achievement Award of the ISPNE, the 2017 Julius Axelrod Mentorship Award from the ACNP, the 2018 Donald Klein, MD, Lifetime Achievement Award from the American Society of Clinical Psychopharmacology, and the 2018 Jules Marmor, MD, Award for Biopsychosocial Research from the APA.
Dr. Aftab: You have devoted much of your career to the development of psychopharmacology. What is your perspective on where the field of psychopharmacology stands at present, especially amid the widespread recognition of “treatment resistance” as a pervasive phenomenon and the scarcity of validated neurobiologic etiological models for psychiatric disorders?
Dr. Schatzberg: We have made considerable progress in the development of new classes of agents for major depression, but as we develop new agents, we still see a large percentage of patients who do not seem to demonstrate adequate responses, particularly in major depressive disorder. This has driven us to look for agents that work differently than previous ones. Although we have some new agents with seeming efficacy and newer mechanisms of action, eg, esketamine, these have largely been derived from clinical, often serendipitous, observations of antidepressant effects rather than from prospective development based on a known pharmacological effect or a biological construct of the disorder. Another intriguing and possibly effective anxiolytic and antidepressive agent is psilocybin, whose potential use is largely derived from clinicians who found it helpful in their practices in combination with psychotherapy. These 2 demonstrate how as we branch out into new territory, we find ourselves moving more and more toward drugs of known clinical risk; eg, mind-altering agents or drugs of abuse. These agents may offer risk-benefit ratios that can ultimately prove to be less attractive than what we might have wanted when we ventured on the journey. Unfortunately, there has been little dialogue about the limitations of several of these agents.
In the case of esketamine, the notion has been that the drug is a blocker of the N-methyl-
One approach that has been applied recently is target validation that purports to use functional MRI to assess behavioral and cognitive effects of drugs to allow inferences regarding efficacy in specific disorders. As we have discussed in a recent paper published in the American Journal of Psychiatry,4 this can be quite misleading and may provide both false positive and negative information. From my perspective, these tests do not appear sensitive enough to screen for patients having a disorder, nor for assessing possible drug effects in those patients. Thus, it is unclear if they can provide answers today that we can be confident in.
Continue to: Dr. Aftab...
Dr. Aftab: What do you see as some of the strengths of psychiatry as a profession?
Dr. Schatzberg: Psychiatry as a specialty combines 2 major perspectives—psychological processes and psychobiology—to develop methods for treating patients who suffer from disorders of the mind/brain. It is the most challenging of our specialties because we cannot study the brain directly. We cannot do procedures as we do in cardiology and pulmonology because they may prove dangerously invasive. That hands-off approach limits us, but for the curious it provides an opportunity to begin to unravel the processes that underlie brain functioning. Fortunately, we have therapies—both psychosocial and somatic—that can provide great relief to patients. These can be shown to be effective in sufficient numbers of patients to help many.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Schatzberg: We need to train our residents in a host of approaches, and not just medications and psychotherapy. They need to understand the basis of brain stimulation approaches (such as repetitive transcranial magnetic stimulation) as well as know how to apply them. We need to train residents more in substance abuse problems and the biology of addiction if they are to better understand the risks of certain new classes of medication. Lastly, we need to train residents in the application of genomics, proteomics, and brain imaging to somatic treatment development.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Schatzberg: The biggest threats come from ourselves. We need to do better with our classification approaches, such as the Diagnostic and Statistical Manual of Mental Disorders or the Research Domain Criteria. They need to become more rapidly adaptive to research in the field. We need to be more open to looking at what is a potentially dangerous trend in developing drugs of abuse and mind-altering drugs as therapeutics. We need to be able to demonstrate that telepsychiatry can be as effective as face-to-face treatment and should be reimbursed. Lastly, we need to develop better models for taking care of the psychiatric patient. We have too many patients and not enough psychiatrists.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Schatzberg: I see the future as bright. Over the past 10 years, led by efforts at the APA, some while I was President, reimbursement has increased dramatically. Over the past 10 years, we have done well developing some new drugs and somatic therapies, and these will continue. Less than a decade ago, large pharmaceutical had abandoned psychiatric drug development and investment into biotech start-ups had waned to near zero. However, the last year few years have seen a dramatic surge in investment, and these should yield novel agents and ones that may be combined with innovative biomarkers as companions.
1. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215. doi:10.1176/appi.ajp.2018.18020138
2. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786. doi:10.1038/s41380-019-0503-4
3. Bonaventura J, Lam S, Carlton M, et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 2021;10.1038/s41380-021-01093-2. doi:10.1038/s41380-021-01093-2
4. Schatzberg AF. Can target engagement studies miss their targets and mislead drug development? Am J Psychiatry. 2021;178(5):372-374. doi:10.1176/appi.ajp.2020.21030247
1. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215. doi:10.1176/appi.ajp.2018.18020138
2. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786. doi:10.1038/s41380-019-0503-4
3. Bonaventura J, Lam S, Carlton M, et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 2021;10.1038/s41380-021-01093-2. doi:10.1038/s41380-021-01093-2
4. Schatzberg AF. Can target engagement studies miss their targets and mislead drug development? Am J Psychiatry. 2021;178(5):372-374. doi:10.1176/appi.ajp.2020.21030247
Maria A. Oquendo, MD, PhD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
Altha J. Stewart, MD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Altha J. Stewart, MD. Dr. Stewart is Senior Associate Dean for Community Health Engagement at the University of Tennessee Health Science Center (UTHSC)–Memphis. She also serves as Chief of the Division of Social and Community Psychiatry and Director, Center for Health in Justice Involved Youth at UTHSC, where she manages community-based programs serving children impacted by trauma and mental illness and their families. In 2018, she was elected President of the American Psychiatric Association, the first African American individual elected in the 175-year history of the organization.
Dr. Aftab: Structural racism in academic and organized psychiatry is an issue that is close to your heart. What is your perspective on the current state of structural racism in American psychiatry, and what do you think we can do about it?
Dr. Stewart: That’s a good question to start with because I think the conversations that we need to have in academia in general and in academic psychiatry specifically really do frame the current issues that we are facing, whether we’re talking about eliminating health disparities or achieving mental health equity. Historically, from the very beginning these discussions have been structured in a racist manner. The early days of American psychiatry were very clearly directed towards maintaining a system that excluded large segments of the population of the time, since a particularly violent form of chattel slavery was being practiced in this country.
The mental health care system was primarily designed for the landowning white men of some standing in society, and so there was never any intent to do much in the way of providing quality humane service to people who were not part of that group. What we have today is a system that was designed for a racist societal structure, that was intended to perpetuate certain behaviors, policies, and practices that had at their core a racist framework. We have to acknowledge and start from this beginning point. This is not to blame anyone currently alive. These are larger structural problems. Before we can begin setting up strategic plans and other actions, we have to go back and acknowledge how we got here. We have to accept the responsibility for being here, and then we have to allow the conversations that need to happen to happen in a safe way, without further alienating people, or maligning and demeaning people who are for the most part well-intentioned but perhaps operating on automatic pilot in a system that is structurally racist.
Dr. Aftab: Do you think that the conversations that need to happen are taking place?
Dr. Stewart: Yes, I think they are beginning to happen. I do a fair number of talks and grand rounds, and what I discover when I meet with different academic departments and different groups is that most places now have a diversity committee, or the residents and students have assigned themselves as diversity leaders. They are really pushing to have these conversations, to insert these conversations into the training and education curricula. The structures in power are so deeply entrenched that many people, particularly younger people, are easily frustrated by the lack of forward motion. One of the things that seasoned leaders in psychiatry have to do is to help everyone understand that the movement forward might be glacial in the beginning, but any movement forward is good when it comes to this. The psychiatrists of my generation talked about cultural competence in psychiatry, but generations of today talk about structural competence. These are similar concepts, except that cultural competency worked within the traditional model, while structural competency recognizes that the system itself needs to change. I find this development very encouraging.
Dr. Aftab: What do you see as some of the strengths of our profession?
Continue to: Dr. Stewart
Dr. Stewart: I am a hopeful optimist when it comes to psychiatry. I have dedicated my professional life to psychiatry and specifically to community psychiatry. Throughout the time that I have practiced psychiatry, I have been encouraged that what we do as a medical specialty really does improve the quality of life for the people we serve. Situationally right now, we’re in a unique position because the COVID pandemic has laid open and then laid bare the whole issue of how we deal with psychological distress, whether it’s diagnosed mental illness or a natural, normal response to a catastrophic event. We are the experts in this. This is our sweet spot, our wheelhouse, whatever analogy you prefer. This is the moment where we assert our expertise as the leaders—not as service add-ons, not as followers, not as adjuncts, but as the leaders.
I am so impressed with the next generation of psychiatrists. They have a wonderful blend of pride and privilege at what they have been able to accomplish to get to the point where they are doctors and psychiatrists, but they have aligned that with a strong core sense of social justice, and they are moved by their responsibility to the people in the society around them.
Another strength of our profession is what we consider to be the “art” of psychiatry. That is, the way we marry the relational aspects of psychiatry with the biological, technical, and digital aspects to arrive at a happy collaboration that benefits people. It is our great skill to engage people, to interact with them therapeutically, to recognize and acknowledge the nonverbal cues. This skill will be even more important in the age of online mental health services. I’m an “old-school” therapist. I like that face-to-face interaction. I think it’s important to preserve that aspect of our practice, even as we move towards online services.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Stewart: I don’t think we can afford to remain in status quo, because we need to constantly think and rethink, evaluate and re-evaluate, assess things in the light of new information. Particularly if we’re talking about people who rely on public funding to get even the bare minimum services, status quo doesn’t cut it. It’s not good enough. I had a teacher during my residency, a child psychiatrist, who used to say, “Good, better, best. Never let it rest, until your good is better and your better is best.” Something about that has stuck with me. As my career progressed, I heard variations of it, including one from former Surgeon General of the United States David Satcher, who was not a psychiatrist, but pulled together the group that published the first Surgeon General’s report on mental health, followed by the Surgeon General’s report on mental health, culture, race, and ethnicity. He had the penetrating insight that risk factors are not to be accepted as predictive factors due to protective factors. If I am at risk for mental illness or a chronic medical condition based on my race or ethnicity or socioeconomic status or employment status, this does not mean that I am destined to experience that illness. In fact, we are not doing our job if we accept these outcomes as inevitable and make no attempt to change them. So, for me, if we accept the status quo, we give up on the message of “Good, better, best. Never let it rest, until your good is better and your better is best.”
Continue to: Dr. Aftab
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Stewart: Well, this is going to sound harsh, and I do hope that the readers do not feel that I intend it to be harsh. We get in our own way. I work in the public sector, for example, and the reality is that there aren’t enough psychiatrists to provide all the necessary psychiatric services for the people who need them. So many mental health clinics and practices employ other mental health professionals, whether they are psychologists or nurse practitioners or physician assistants with special training in mental health to provide those services. To have a blanket concern about anyone who is not an MD practicing in what is considered “our area” just begs the question that if we can’t do it and we don’t have enough psychiatrists to do it, should people just not get mental health treatment? Is that the solution? I don’t think so. I don’t think that’s what people want, either, but because of the energy that gets aroused around these issues, we lose sight of that end goal. I think the answer is that we must take leadership for ensuring that our colleagues are well-trained, maybe not as well-trained as physicians, but well-trained enough to provide good care working under our supervision.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Stewart: I think we are moving naturally into the space of integrated or collaborative care. I think we’re going to have to acknowledge that going forward, the path to being a good psychiatrist means that we will also be consultants. Not just the consultation-liaison kind of consultant that we typically think of, but a consultant to the rest of medicine around shaping programs, addressing how we treat comorbid illness, looking at ways to minimize the morbidity and mortality associated with some of the chronic medical and mental diseases. We’re moving naturally in that direction. For some people, that must be frightening. All throughout medicine people are witnessing change, and we need to adapt. I would hope that the specialty that is designed to help others deal with change will figure out how to use those skills to help themselves deal with the changes that are coming!
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Altha J. Stewart, MD. Dr. Stewart is Senior Associate Dean for Community Health Engagement at the University of Tennessee Health Science Center (UTHSC)–Memphis. She also serves as Chief of the Division of Social and Community Psychiatry and Director, Center for Health in Justice Involved Youth at UTHSC, where she manages community-based programs serving children impacted by trauma and mental illness and their families. In 2018, she was elected President of the American Psychiatric Association, the first African American individual elected in the 175-year history of the organization.
Dr. Aftab: Structural racism in academic and organized psychiatry is an issue that is close to your heart. What is your perspective on the current state of structural racism in American psychiatry, and what do you think we can do about it?
Dr. Stewart: That’s a good question to start with because I think the conversations that we need to have in academia in general and in academic psychiatry specifically really do frame the current issues that we are facing, whether we’re talking about eliminating health disparities or achieving mental health equity. Historically, from the very beginning these discussions have been structured in a racist manner. The early days of American psychiatry were very clearly directed towards maintaining a system that excluded large segments of the population of the time, since a particularly violent form of chattel slavery was being practiced in this country.
The mental health care system was primarily designed for the landowning white men of some standing in society, and so there was never any intent to do much in the way of providing quality humane service to people who were not part of that group. What we have today is a system that was designed for a racist societal structure, that was intended to perpetuate certain behaviors, policies, and practices that had at their core a racist framework. We have to acknowledge and start from this beginning point. This is not to blame anyone currently alive. These are larger structural problems. Before we can begin setting up strategic plans and other actions, we have to go back and acknowledge how we got here. We have to accept the responsibility for being here, and then we have to allow the conversations that need to happen to happen in a safe way, without further alienating people, or maligning and demeaning people who are for the most part well-intentioned but perhaps operating on automatic pilot in a system that is structurally racist.
Dr. Aftab: Do you think that the conversations that need to happen are taking place?
Dr. Stewart: Yes, I think they are beginning to happen. I do a fair number of talks and grand rounds, and what I discover when I meet with different academic departments and different groups is that most places now have a diversity committee, or the residents and students have assigned themselves as diversity leaders. They are really pushing to have these conversations, to insert these conversations into the training and education curricula. The structures in power are so deeply entrenched that many people, particularly younger people, are easily frustrated by the lack of forward motion. One of the things that seasoned leaders in psychiatry have to do is to help everyone understand that the movement forward might be glacial in the beginning, but any movement forward is good when it comes to this. The psychiatrists of my generation talked about cultural competence in psychiatry, but generations of today talk about structural competence. These are similar concepts, except that cultural competency worked within the traditional model, while structural competency recognizes that the system itself needs to change. I find this development very encouraging.
Dr. Aftab: What do you see as some of the strengths of our profession?
Continue to: Dr. Stewart
Dr. Stewart: I am a hopeful optimist when it comes to psychiatry. I have dedicated my professional life to psychiatry and specifically to community psychiatry. Throughout the time that I have practiced psychiatry, I have been encouraged that what we do as a medical specialty really does improve the quality of life for the people we serve. Situationally right now, we’re in a unique position because the COVID pandemic has laid open and then laid bare the whole issue of how we deal with psychological distress, whether it’s diagnosed mental illness or a natural, normal response to a catastrophic event. We are the experts in this. This is our sweet spot, our wheelhouse, whatever analogy you prefer. This is the moment where we assert our expertise as the leaders—not as service add-ons, not as followers, not as adjuncts, but as the leaders.
I am so impressed with the next generation of psychiatrists. They have a wonderful blend of pride and privilege at what they have been able to accomplish to get to the point where they are doctors and psychiatrists, but they have aligned that with a strong core sense of social justice, and they are moved by their responsibility to the people in the society around them.
Another strength of our profession is what we consider to be the “art” of psychiatry. That is, the way we marry the relational aspects of psychiatry with the biological, technical, and digital aspects to arrive at a happy collaboration that benefits people. It is our great skill to engage people, to interact with them therapeutically, to recognize and acknowledge the nonverbal cues. This skill will be even more important in the age of online mental health services. I’m an “old-school” therapist. I like that face-to-face interaction. I think it’s important to preserve that aspect of our practice, even as we move towards online services.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Stewart: I don’t think we can afford to remain in status quo, because we need to constantly think and rethink, evaluate and re-evaluate, assess things in the light of new information. Particularly if we’re talking about people who rely on public funding to get even the bare minimum services, status quo doesn’t cut it. It’s not good enough. I had a teacher during my residency, a child psychiatrist, who used to say, “Good, better, best. Never let it rest, until your good is better and your better is best.” Something about that has stuck with me. As my career progressed, I heard variations of it, including one from former Surgeon General of the United States David Satcher, who was not a psychiatrist, but pulled together the group that published the first Surgeon General’s report on mental health, followed by the Surgeon General’s report on mental health, culture, race, and ethnicity. He had the penetrating insight that risk factors are not to be accepted as predictive factors due to protective factors. If I am at risk for mental illness or a chronic medical condition based on my race or ethnicity or socioeconomic status or employment status, this does not mean that I am destined to experience that illness. In fact, we are not doing our job if we accept these outcomes as inevitable and make no attempt to change them. So, for me, if we accept the status quo, we give up on the message of “Good, better, best. Never let it rest, until your good is better and your better is best.”
Continue to: Dr. Aftab
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Stewart: Well, this is going to sound harsh, and I do hope that the readers do not feel that I intend it to be harsh. We get in our own way. I work in the public sector, for example, and the reality is that there aren’t enough psychiatrists to provide all the necessary psychiatric services for the people who need them. So many mental health clinics and practices employ other mental health professionals, whether they are psychologists or nurse practitioners or physician assistants with special training in mental health to provide those services. To have a blanket concern about anyone who is not an MD practicing in what is considered “our area” just begs the question that if we can’t do it and we don’t have enough psychiatrists to do it, should people just not get mental health treatment? Is that the solution? I don’t think so. I don’t think that’s what people want, either, but because of the energy that gets aroused around these issues, we lose sight of that end goal. I think the answer is that we must take leadership for ensuring that our colleagues are well-trained, maybe not as well-trained as physicians, but well-trained enough to provide good care working under our supervision.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Stewart: I think we are moving naturally into the space of integrated or collaborative care. I think we’re going to have to acknowledge that going forward, the path to being a good psychiatrist means that we will also be consultants. Not just the consultation-liaison kind of consultant that we typically think of, but a consultant to the rest of medicine around shaping programs, addressing how we treat comorbid illness, looking at ways to minimize the morbidity and mortality associated with some of the chronic medical and mental diseases. We’re moving naturally in that direction. For some people, that must be frightening. All throughout medicine people are witnessing change, and we need to adapt. I would hope that the specialty that is designed to help others deal with change will figure out how to use those skills to help themselves deal with the changes that are coming!
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Altha J. Stewart, MD. Dr. Stewart is Senior Associate Dean for Community Health Engagement at the University of Tennessee Health Science Center (UTHSC)–Memphis. She also serves as Chief of the Division of Social and Community Psychiatry and Director, Center for Health in Justice Involved Youth at UTHSC, where she manages community-based programs serving children impacted by trauma and mental illness and their families. In 2018, she was elected President of the American Psychiatric Association, the first African American individual elected in the 175-year history of the organization.
Dr. Aftab: Structural racism in academic and organized psychiatry is an issue that is close to your heart. What is your perspective on the current state of structural racism in American psychiatry, and what do you think we can do about it?
Dr. Stewart: That’s a good question to start with because I think the conversations that we need to have in academia in general and in academic psychiatry specifically really do frame the current issues that we are facing, whether we’re talking about eliminating health disparities or achieving mental health equity. Historically, from the very beginning these discussions have been structured in a racist manner. The early days of American psychiatry were very clearly directed towards maintaining a system that excluded large segments of the population of the time, since a particularly violent form of chattel slavery was being practiced in this country.
The mental health care system was primarily designed for the landowning white men of some standing in society, and so there was never any intent to do much in the way of providing quality humane service to people who were not part of that group. What we have today is a system that was designed for a racist societal structure, that was intended to perpetuate certain behaviors, policies, and practices that had at their core a racist framework. We have to acknowledge and start from this beginning point. This is not to blame anyone currently alive. These are larger structural problems. Before we can begin setting up strategic plans and other actions, we have to go back and acknowledge how we got here. We have to accept the responsibility for being here, and then we have to allow the conversations that need to happen to happen in a safe way, without further alienating people, or maligning and demeaning people who are for the most part well-intentioned but perhaps operating on automatic pilot in a system that is structurally racist.
Dr. Aftab: Do you think that the conversations that need to happen are taking place?
Dr. Stewart: Yes, I think they are beginning to happen. I do a fair number of talks and grand rounds, and what I discover when I meet with different academic departments and different groups is that most places now have a diversity committee, or the residents and students have assigned themselves as diversity leaders. They are really pushing to have these conversations, to insert these conversations into the training and education curricula. The structures in power are so deeply entrenched that many people, particularly younger people, are easily frustrated by the lack of forward motion. One of the things that seasoned leaders in psychiatry have to do is to help everyone understand that the movement forward might be glacial in the beginning, but any movement forward is good when it comes to this. The psychiatrists of my generation talked about cultural competence in psychiatry, but generations of today talk about structural competence. These are similar concepts, except that cultural competency worked within the traditional model, while structural competency recognizes that the system itself needs to change. I find this development very encouraging.
Dr. Aftab: What do you see as some of the strengths of our profession?
Continue to: Dr. Stewart
Dr. Stewart: I am a hopeful optimist when it comes to psychiatry. I have dedicated my professional life to psychiatry and specifically to community psychiatry. Throughout the time that I have practiced psychiatry, I have been encouraged that what we do as a medical specialty really does improve the quality of life for the people we serve. Situationally right now, we’re in a unique position because the COVID pandemic has laid open and then laid bare the whole issue of how we deal with psychological distress, whether it’s diagnosed mental illness or a natural, normal response to a catastrophic event. We are the experts in this. This is our sweet spot, our wheelhouse, whatever analogy you prefer. This is the moment where we assert our expertise as the leaders—not as service add-ons, not as followers, not as adjuncts, but as the leaders.
I am so impressed with the next generation of psychiatrists. They have a wonderful blend of pride and privilege at what they have been able to accomplish to get to the point where they are doctors and psychiatrists, but they have aligned that with a strong core sense of social justice, and they are moved by their responsibility to the people in the society around them.
Another strength of our profession is what we consider to be the “art” of psychiatry. That is, the way we marry the relational aspects of psychiatry with the biological, technical, and digital aspects to arrive at a happy collaboration that benefits people. It is our great skill to engage people, to interact with them therapeutically, to recognize and acknowledge the nonverbal cues. This skill will be even more important in the age of online mental health services. I’m an “old-school” therapist. I like that face-to-face interaction. I think it’s important to preserve that aspect of our practice, even as we move towards online services.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Stewart: I don’t think we can afford to remain in status quo, because we need to constantly think and rethink, evaluate and re-evaluate, assess things in the light of new information. Particularly if we’re talking about people who rely on public funding to get even the bare minimum services, status quo doesn’t cut it. It’s not good enough. I had a teacher during my residency, a child psychiatrist, who used to say, “Good, better, best. Never let it rest, until your good is better and your better is best.” Something about that has stuck with me. As my career progressed, I heard variations of it, including one from former Surgeon General of the United States David Satcher, who was not a psychiatrist, but pulled together the group that published the first Surgeon General’s report on mental health, followed by the Surgeon General’s report on mental health, culture, race, and ethnicity. He had the penetrating insight that risk factors are not to be accepted as predictive factors due to protective factors. If I am at risk for mental illness or a chronic medical condition based on my race or ethnicity or socioeconomic status or employment status, this does not mean that I am destined to experience that illness. In fact, we are not doing our job if we accept these outcomes as inevitable and make no attempt to change them. So, for me, if we accept the status quo, we give up on the message of “Good, better, best. Never let it rest, until your good is better and your better is best.”
Continue to: Dr. Aftab
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Stewart: Well, this is going to sound harsh, and I do hope that the readers do not feel that I intend it to be harsh. We get in our own way. I work in the public sector, for example, and the reality is that there aren’t enough psychiatrists to provide all the necessary psychiatric services for the people who need them. So many mental health clinics and practices employ other mental health professionals, whether they are psychologists or nurse practitioners or physician assistants with special training in mental health to provide those services. To have a blanket concern about anyone who is not an MD practicing in what is considered “our area” just begs the question that if we can’t do it and we don’t have enough psychiatrists to do it, should people just not get mental health treatment? Is that the solution? I don’t think so. I don’t think that’s what people want, either, but because of the energy that gets aroused around these issues, we lose sight of that end goal. I think the answer is that we must take leadership for ensuring that our colleagues are well-trained, maybe not as well-trained as physicians, but well-trained enough to provide good care working under our supervision.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Stewart: I think we are moving naturally into the space of integrated or collaborative care. I think we’re going to have to acknowledge that going forward, the path to being a good psychiatrist means that we will also be consultants. Not just the consultation-liaison kind of consultant that we typically think of, but a consultant to the rest of medicine around shaping programs, addressing how we treat comorbid illness, looking at ways to minimize the morbidity and mortality associated with some of the chronic medical and mental diseases. We’re moving naturally in that direction. For some people, that must be frightening. All throughout medicine people are witnessing change, and we need to adapt. I would hope that the specialty that is designed to help others deal with change will figure out how to use those skills to help themselves deal with the changes that are coming!
Paul Summergrad, MD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Paul Summergrad, MD. Dr. Summergrad is the Dr. Frances S. Arkin Professor and Chair of the Department of Psychiatry and Professor of Psychiatry and Medicine at Tufts University School of Medicine and Psychiatrist-in-Chief at Tufts Medical Center, Boston, Massachusetts. From 2014 to 2015, Dr. Summergrad served as the 141st president of the American Psychiatric Association, and is a past president of the American Association of Chairs of Departments of Psychiatry. Dr. Summergrad’s research focuses on mood disorders, medical/psychiatric comorbidity, and health system design. He received the Distinguished Faculty Award from Tufts University School of Medicine in 2015 and the Leadership Award of the American Association of Chairs of Departments of Psychiatry in 2018. In 2020, he was elected to the Honorary Fellowship of the Royal College of Psychiatrists, their highest honor. He is the lead editor of Textbook of Medical Psychiatry, which was published by American Psychiatric Association Publishing in 2020.
Dr. Aftab: Much of your career has been devoted to the practice of “medical psychiatry” in which you have fruitfully integrated your medical training as well as psychoanalytic training. How has this influenced your understanding of the medical model in psychiatry and psychiatry’s relationship with medicine?
Dr. Summergrad: It is a really complex and ongoing influence. I think there is a misunderstanding of what is meant by the medical model in psychiatry. It has nothing to do with the etiology of mental disorders or their treatment. At its core, the medical model is based on a syndromic view of disorders: that we attend to those symptoms of illness that occur together more frequently than they might by chance and then, based on that provisional diagnostic cluster, look for causes, risk factors, and interventions that affect the putative disorder’s course. As a consequence of that process, disorders are refined, often separated into a group of disorders, or in some cases discarded. An excellent example that we have all been living through has been our evolving understanding of COVID-19, which is now understood to be as much a clotting and inflammatory disorder as a respiratory condition.
Medical psychiatry is a different and discrete area of clinical psychiatric interest. It covers a variety of areas: the complexity of the management of patients with comorbid medical and psychiatric illness, the impact of medical illness on the course of psychiatric illness and life expectancy, and conversely the effects of psychiatric illness on the course of medical disorders—for example, the increased mortality in patients with myocardial infarction (MI) and post-MI major depression. At its core is the study of medical disorders, including neurologic conditions, that directly cause syndromes in the realm that we define as mental disorders. This was the focus of our recent Textbook of Medical Psychiatry. This has been a long-standing interest of mine since I did my medical residency at Boston City Hospital before I trained in psychiatry, and it has informed my career in many other ways.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Summergrad: Psychiatry has so many riches: a long clinical tradition based on close and long-term observation and interaction with patients, effective psychotherapies such as cognitive-behavioral therapy and psychodynamic therapies, and evidence-based pharmacologic and other somatic therapies.
Second, there has been substantial growth in our fundamental understanding of the neurobiology of psychiatric illness with regards to circuitry, imaging, and genetics. While many of these advances have arisen from more basic research, it is also the case that the evolution of a consistent diagnostic nomenclature in the 1970s, even with all its limitations, allowed for advances in research, diagnosis, and treatment.
Finally, our other great strengths are our roots in medicine and the importance of those skills in assessing patients and caring for active comorbidities. We are one of the last fields in clinical medicine where doctors can take the time to establish a detailed and close working relationship with our patients. We are fortunate to have this great mix of science, medicine, and interpersonal skills, which is highly rewarding.
Continue to: Dr. Aftab: Are there ways...
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Summergrad: There are many, as there are in many other fields of medicine. For too many, there is a reification of a diagnostic nomenclature as being identical to detailed and thoughtful clinical evaluation. For many, the pressures of health care economics mean that they may be taking care of more inpatients than is optimal, or are under pressure to see a larger number of patients as a so-called “prescriber,” a term I think should be banished.
We have struggled significantly to have a coherent link between our clinical work, including our interventions, our emerging understanding of neuroscience and genetics, and the experiences of our patients, including the onset and timing of many of the disorders we treat. Part of this is that we lack a unified model of mental functioning that unites the onset of illness, its clinical phenomena, and any underlying pathophysiology. We operate at multiple levels of abstraction (brain-mind) compared with other medical fields. While other medical fields incorporate experience, they are more fully operating, from a pathophysiologic perspective, at a physical level alone. Even in common parlance, we can easily talk about the heart as a pump, or the kidney as a filter, but there is no corresponding way to describe what the brain-mind is and does. This could be construed as a weakness; I see it more as an intrinsic complexity of our field.
What we refer to as psychiatric disorders deal with the most intimate aspects of people’s beings: their sense of self and capacity. Many people experience our diagnostic work and nomenclature as wounding, demeaning, distancing, or defining their very essence or being as ill. There is a wonderful story that I heard from the great Elyn Saks, the constitutional law professor, regarding her own illness, about which she has been admirably open. She described a long course of significant psychotic illness, eventually diagnosed as schizophrenia, for which she received years of psychotherapy, psychopharmacology, and hospital care, both when she studied at Oxford and while she was a law student at Yale. She described that after 10 years of care, she was eventually prescribed clozapine, which made a major difference in her illness. It was about the same time that she finally accepted that she had a psychiatric illness, and it was at that very moment of acceptance that she realized that it wasn’t about her, that it didn’t define who she was in her essence. Very moving and important. In defining pathophysiology or what we call psychopathology, we need to make sure it is clear that we are not labeling or diagnosing anyone’s essential being.
I think we need to tread very carefully in these areas, including being very sensitive with our language. Much of this is in the nature of the illnesses we deal with and their profound intimacy, but again we need to be mindful of this. These areas are ones which I think contribute to a resentment of psychiatry, and are possibly related to some of the anti-psychiatry sentiments and criticisms of the so-called medical model in psychiatry, which as I noted above is, I think, not well understood.
Continue to: Dr. Aftab: What is your perception...
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Summergrad: I actually am very bullish on psychiatry’s future. While we are far from perfect, the illnesses we care for are so ubiquitous that many if not most people will experience them personally or in close family members over their lifetime. As such, there is a real and broad understanding about the need for psychiatric care; we do, however, have to always strive to do it better and with greater sensitivity to the human experiences of those who seek our care.
Dr. Aftab: What do you envision for the future of psychiatry? What opportunities lie ahead for us?
Dr. Summergrad: I think we will see an expansion of awareness of mental disorders globally. While it may seem counterintuitive to say this in the midst of a global pandemic, over the course of the last 80 years, the global burden of disease related to communicable diseases has fallen across much of the developed world and the burden of disease attributable to noncommunicable disease has increased. Psychiatric disorders are among the most frequent noncommunicable disorders and are increasing as a proportion of total illness burden. As such, the need for mental health–related care will increase dramatically over the next half century, if not longer.
Second, I think our understanding of neurobiology, the impact of medical disorders, and pathophysiology related to mechanisms such as inflammation in psychiatric disorders will increase. Likewise, our appreciation will grow for non-allelic influences on the genetics or heritability of psychiatric disorders. I don’t think we have come near to tapping the effects of epigenetics on psychiatric illnesses, and that will become increasingly important.
I also think that over time, our understanding of particular neurobiological pathways and our ability to modulate these pathways will increase. How that will eventually yield the ability to treat patients with greater precision I don’t know, but I expect that will occur. Over time, we may even learn enough to have a workable theory of mind and brain, but I am not sure that all of these mysteries will yield anytime soon, and for some of them, answers may have to come from other domains of human experience.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Paul Summergrad, MD. Dr. Summergrad is the Dr. Frances S. Arkin Professor and Chair of the Department of Psychiatry and Professor of Psychiatry and Medicine at Tufts University School of Medicine and Psychiatrist-in-Chief at Tufts Medical Center, Boston, Massachusetts. From 2014 to 2015, Dr. Summergrad served as the 141st president of the American Psychiatric Association, and is a past president of the American Association of Chairs of Departments of Psychiatry. Dr. Summergrad’s research focuses on mood disorders, medical/psychiatric comorbidity, and health system design. He received the Distinguished Faculty Award from Tufts University School of Medicine in 2015 and the Leadership Award of the American Association of Chairs of Departments of Psychiatry in 2018. In 2020, he was elected to the Honorary Fellowship of the Royal College of Psychiatrists, their highest honor. He is the lead editor of Textbook of Medical Psychiatry, which was published by American Psychiatric Association Publishing in 2020.
Dr. Aftab: Much of your career has been devoted to the practice of “medical psychiatry” in which you have fruitfully integrated your medical training as well as psychoanalytic training. How has this influenced your understanding of the medical model in psychiatry and psychiatry’s relationship with medicine?
Dr. Summergrad: It is a really complex and ongoing influence. I think there is a misunderstanding of what is meant by the medical model in psychiatry. It has nothing to do with the etiology of mental disorders or their treatment. At its core, the medical model is based on a syndromic view of disorders: that we attend to those symptoms of illness that occur together more frequently than they might by chance and then, based on that provisional diagnostic cluster, look for causes, risk factors, and interventions that affect the putative disorder’s course. As a consequence of that process, disorders are refined, often separated into a group of disorders, or in some cases discarded. An excellent example that we have all been living through has been our evolving understanding of COVID-19, which is now understood to be as much a clotting and inflammatory disorder as a respiratory condition.
Medical psychiatry is a different and discrete area of clinical psychiatric interest. It covers a variety of areas: the complexity of the management of patients with comorbid medical and psychiatric illness, the impact of medical illness on the course of psychiatric illness and life expectancy, and conversely the effects of psychiatric illness on the course of medical disorders—for example, the increased mortality in patients with myocardial infarction (MI) and post-MI major depression. At its core is the study of medical disorders, including neurologic conditions, that directly cause syndromes in the realm that we define as mental disorders. This was the focus of our recent Textbook of Medical Psychiatry. This has been a long-standing interest of mine since I did my medical residency at Boston City Hospital before I trained in psychiatry, and it has informed my career in many other ways.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Summergrad: Psychiatry has so many riches: a long clinical tradition based on close and long-term observation and interaction with patients, effective psychotherapies such as cognitive-behavioral therapy and psychodynamic therapies, and evidence-based pharmacologic and other somatic therapies.
Second, there has been substantial growth in our fundamental understanding of the neurobiology of psychiatric illness with regards to circuitry, imaging, and genetics. While many of these advances have arisen from more basic research, it is also the case that the evolution of a consistent diagnostic nomenclature in the 1970s, even with all its limitations, allowed for advances in research, diagnosis, and treatment.
Finally, our other great strengths are our roots in medicine and the importance of those skills in assessing patients and caring for active comorbidities. We are one of the last fields in clinical medicine where doctors can take the time to establish a detailed and close working relationship with our patients. We are fortunate to have this great mix of science, medicine, and interpersonal skills, which is highly rewarding.
Continue to: Dr. Aftab: Are there ways...
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Summergrad: There are many, as there are in many other fields of medicine. For too many, there is a reification of a diagnostic nomenclature as being identical to detailed and thoughtful clinical evaluation. For many, the pressures of health care economics mean that they may be taking care of more inpatients than is optimal, or are under pressure to see a larger number of patients as a so-called “prescriber,” a term I think should be banished.
We have struggled significantly to have a coherent link between our clinical work, including our interventions, our emerging understanding of neuroscience and genetics, and the experiences of our patients, including the onset and timing of many of the disorders we treat. Part of this is that we lack a unified model of mental functioning that unites the onset of illness, its clinical phenomena, and any underlying pathophysiology. We operate at multiple levels of abstraction (brain-mind) compared with other medical fields. While other medical fields incorporate experience, they are more fully operating, from a pathophysiologic perspective, at a physical level alone. Even in common parlance, we can easily talk about the heart as a pump, or the kidney as a filter, but there is no corresponding way to describe what the brain-mind is and does. This could be construed as a weakness; I see it more as an intrinsic complexity of our field.
What we refer to as psychiatric disorders deal with the most intimate aspects of people’s beings: their sense of self and capacity. Many people experience our diagnostic work and nomenclature as wounding, demeaning, distancing, or defining their very essence or being as ill. There is a wonderful story that I heard from the great Elyn Saks, the constitutional law professor, regarding her own illness, about which she has been admirably open. She described a long course of significant psychotic illness, eventually diagnosed as schizophrenia, for which she received years of psychotherapy, psychopharmacology, and hospital care, both when she studied at Oxford and while she was a law student at Yale. She described that after 10 years of care, she was eventually prescribed clozapine, which made a major difference in her illness. It was about the same time that she finally accepted that she had a psychiatric illness, and it was at that very moment of acceptance that she realized that it wasn’t about her, that it didn’t define who she was in her essence. Very moving and important. In defining pathophysiology or what we call psychopathology, we need to make sure it is clear that we are not labeling or diagnosing anyone’s essential being.
I think we need to tread very carefully in these areas, including being very sensitive with our language. Much of this is in the nature of the illnesses we deal with and their profound intimacy, but again we need to be mindful of this. These areas are ones which I think contribute to a resentment of psychiatry, and are possibly related to some of the anti-psychiatry sentiments and criticisms of the so-called medical model in psychiatry, which as I noted above is, I think, not well understood.
Continue to: Dr. Aftab: What is your perception...
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Summergrad: I actually am very bullish on psychiatry’s future. While we are far from perfect, the illnesses we care for are so ubiquitous that many if not most people will experience them personally or in close family members over their lifetime. As such, there is a real and broad understanding about the need for psychiatric care; we do, however, have to always strive to do it better and with greater sensitivity to the human experiences of those who seek our care.
Dr. Aftab: What do you envision for the future of psychiatry? What opportunities lie ahead for us?
Dr. Summergrad: I think we will see an expansion of awareness of mental disorders globally. While it may seem counterintuitive to say this in the midst of a global pandemic, over the course of the last 80 years, the global burden of disease related to communicable diseases has fallen across much of the developed world and the burden of disease attributable to noncommunicable disease has increased. Psychiatric disorders are among the most frequent noncommunicable disorders and are increasing as a proportion of total illness burden. As such, the need for mental health–related care will increase dramatically over the next half century, if not longer.
Second, I think our understanding of neurobiology, the impact of medical disorders, and pathophysiology related to mechanisms such as inflammation in psychiatric disorders will increase. Likewise, our appreciation will grow for non-allelic influences on the genetics or heritability of psychiatric disorders. I don’t think we have come near to tapping the effects of epigenetics on psychiatric illnesses, and that will become increasingly important.
I also think that over time, our understanding of particular neurobiological pathways and our ability to modulate these pathways will increase. How that will eventually yield the ability to treat patients with greater precision I don’t know, but I expect that will occur. Over time, we may even learn enough to have a workable theory of mind and brain, but I am not sure that all of these mysteries will yield anytime soon, and for some of them, answers may have to come from other domains of human experience.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Paul Summergrad, MD. Dr. Summergrad is the Dr. Frances S. Arkin Professor and Chair of the Department of Psychiatry and Professor of Psychiatry and Medicine at Tufts University School of Medicine and Psychiatrist-in-Chief at Tufts Medical Center, Boston, Massachusetts. From 2014 to 2015, Dr. Summergrad served as the 141st president of the American Psychiatric Association, and is a past president of the American Association of Chairs of Departments of Psychiatry. Dr. Summergrad’s research focuses on mood disorders, medical/psychiatric comorbidity, and health system design. He received the Distinguished Faculty Award from Tufts University School of Medicine in 2015 and the Leadership Award of the American Association of Chairs of Departments of Psychiatry in 2018. In 2020, he was elected to the Honorary Fellowship of the Royal College of Psychiatrists, their highest honor. He is the lead editor of Textbook of Medical Psychiatry, which was published by American Psychiatric Association Publishing in 2020.
Dr. Aftab: Much of your career has been devoted to the practice of “medical psychiatry” in which you have fruitfully integrated your medical training as well as psychoanalytic training. How has this influenced your understanding of the medical model in psychiatry and psychiatry’s relationship with medicine?
Dr. Summergrad: It is a really complex and ongoing influence. I think there is a misunderstanding of what is meant by the medical model in psychiatry. It has nothing to do with the etiology of mental disorders or their treatment. At its core, the medical model is based on a syndromic view of disorders: that we attend to those symptoms of illness that occur together more frequently than they might by chance and then, based on that provisional diagnostic cluster, look for causes, risk factors, and interventions that affect the putative disorder’s course. As a consequence of that process, disorders are refined, often separated into a group of disorders, or in some cases discarded. An excellent example that we have all been living through has been our evolving understanding of COVID-19, which is now understood to be as much a clotting and inflammatory disorder as a respiratory condition.
Medical psychiatry is a different and discrete area of clinical psychiatric interest. It covers a variety of areas: the complexity of the management of patients with comorbid medical and psychiatric illness, the impact of medical illness on the course of psychiatric illness and life expectancy, and conversely the effects of psychiatric illness on the course of medical disorders—for example, the increased mortality in patients with myocardial infarction (MI) and post-MI major depression. At its core is the study of medical disorders, including neurologic conditions, that directly cause syndromes in the realm that we define as mental disorders. This was the focus of our recent Textbook of Medical Psychiatry. This has been a long-standing interest of mine since I did my medical residency at Boston City Hospital before I trained in psychiatry, and it has informed my career in many other ways.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Summergrad: Psychiatry has so many riches: a long clinical tradition based on close and long-term observation and interaction with patients, effective psychotherapies such as cognitive-behavioral therapy and psychodynamic therapies, and evidence-based pharmacologic and other somatic therapies.
Second, there has been substantial growth in our fundamental understanding of the neurobiology of psychiatric illness with regards to circuitry, imaging, and genetics. While many of these advances have arisen from more basic research, it is also the case that the evolution of a consistent diagnostic nomenclature in the 1970s, even with all its limitations, allowed for advances in research, diagnosis, and treatment.
Finally, our other great strengths are our roots in medicine and the importance of those skills in assessing patients and caring for active comorbidities. We are one of the last fields in clinical medicine where doctors can take the time to establish a detailed and close working relationship with our patients. We are fortunate to have this great mix of science, medicine, and interpersonal skills, which is highly rewarding.
Continue to: Dr. Aftab: Are there ways...
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Summergrad: There are many, as there are in many other fields of medicine. For too many, there is a reification of a diagnostic nomenclature as being identical to detailed and thoughtful clinical evaluation. For many, the pressures of health care economics mean that they may be taking care of more inpatients than is optimal, or are under pressure to see a larger number of patients as a so-called “prescriber,” a term I think should be banished.
We have struggled significantly to have a coherent link between our clinical work, including our interventions, our emerging understanding of neuroscience and genetics, and the experiences of our patients, including the onset and timing of many of the disorders we treat. Part of this is that we lack a unified model of mental functioning that unites the onset of illness, its clinical phenomena, and any underlying pathophysiology. We operate at multiple levels of abstraction (brain-mind) compared with other medical fields. While other medical fields incorporate experience, they are more fully operating, from a pathophysiologic perspective, at a physical level alone. Even in common parlance, we can easily talk about the heart as a pump, or the kidney as a filter, but there is no corresponding way to describe what the brain-mind is and does. This could be construed as a weakness; I see it more as an intrinsic complexity of our field.
What we refer to as psychiatric disorders deal with the most intimate aspects of people’s beings: their sense of self and capacity. Many people experience our diagnostic work and nomenclature as wounding, demeaning, distancing, or defining their very essence or being as ill. There is a wonderful story that I heard from the great Elyn Saks, the constitutional law professor, regarding her own illness, about which she has been admirably open. She described a long course of significant psychotic illness, eventually diagnosed as schizophrenia, for which she received years of psychotherapy, psychopharmacology, and hospital care, both when she studied at Oxford and while she was a law student at Yale. She described that after 10 years of care, she was eventually prescribed clozapine, which made a major difference in her illness. It was about the same time that she finally accepted that she had a psychiatric illness, and it was at that very moment of acceptance that she realized that it wasn’t about her, that it didn’t define who she was in her essence. Very moving and important. In defining pathophysiology or what we call psychopathology, we need to make sure it is clear that we are not labeling or diagnosing anyone’s essential being.
I think we need to tread very carefully in these areas, including being very sensitive with our language. Much of this is in the nature of the illnesses we deal with and their profound intimacy, but again we need to be mindful of this. These areas are ones which I think contribute to a resentment of psychiatry, and are possibly related to some of the anti-psychiatry sentiments and criticisms of the so-called medical model in psychiatry, which as I noted above is, I think, not well understood.
Continue to: Dr. Aftab: What is your perception...
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Summergrad: I actually am very bullish on psychiatry’s future. While we are far from perfect, the illnesses we care for are so ubiquitous that many if not most people will experience them personally or in close family members over their lifetime. As such, there is a real and broad understanding about the need for psychiatric care; we do, however, have to always strive to do it better and with greater sensitivity to the human experiences of those who seek our care.
Dr. Aftab: What do you envision for the future of psychiatry? What opportunities lie ahead for us?
Dr. Summergrad: I think we will see an expansion of awareness of mental disorders globally. While it may seem counterintuitive to say this in the midst of a global pandemic, over the course of the last 80 years, the global burden of disease related to communicable diseases has fallen across much of the developed world and the burden of disease attributable to noncommunicable disease has increased. Psychiatric disorders are among the most frequent noncommunicable disorders and are increasing as a proportion of total illness burden. As such, the need for mental health–related care will increase dramatically over the next half century, if not longer.
Second, I think our understanding of neurobiology, the impact of medical disorders, and pathophysiology related to mechanisms such as inflammation in psychiatric disorders will increase. Likewise, our appreciation will grow for non-allelic influences on the genetics or heritability of psychiatric disorders. I don’t think we have come near to tapping the effects of epigenetics on psychiatric illnesses, and that will become increasingly important.
I also think that over time, our understanding of particular neurobiological pathways and our ability to modulate these pathways will increase. How that will eventually yield the ability to treat patients with greater precision I don’t know, but I expect that will occur. Over time, we may even learn enough to have a workable theory of mind and brain, but I am not sure that all of these mysteries will yield anytime soon, and for some of them, answers may have to come from other domains of human experience.
Dilip V. Jeste, MD, on the state of psychiatry
Editor’s note: Psychiatry Leaders’ Perspectives is a new department in
In this first Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Dilip V. Jeste, MD. Dr. Jeste is Senior Associate Dean for Healthy Aging and Senior Care, Estelle and Edgar Levi Memorial Chair in Aging, Director of the Sam and Rose Stein Institute for Research on Aging, Distinguished Professor of Psychiatry and Neurosciences, University of California San Diego; and Co-Director of the UC San Diego-IBM Center on Artificial Intelligence for Healthy Living. His main areas of research include schizophrenia, neuropsychiatric interventions, and successful aging. He served as the 139th President of the American Psychiatric Association (APA) and also is a past president of the American Association for Geriatric Psychiatry, the West Coast College of Biological Psychiatry, and founding president of International College of Geriatric Psychoneuropharmacology.
Dr. Aftab: The focus of your term as president of the APA was on “positive psychiatry.” You are also one of the world’s foremost experts in this area. How successful have you been in your mission to promote positive psychiatry, and how has your message been received?
Dr. Jeste: Let me start with a little bit of background about why I got into positive psychiatry. As a geriatric psychiatrist, my research work has brought me face to face with the paradox of aging: although physical health declines with age, mental health and well-being improve on average. This is the case not just for individuals in the community but also for individuals with serious mental illnesses. That got me into thinking more and more about the ways in which we can bring positive change in the lives of patients. When I became the president of the APA, one of my main tasks was to finalize and publish the DSM-5, which rightly focuses on the disorders we treat, but it also provided me with an opportunity to highlight the side of psychiatry that focuses on the positive aspects of our own and our patients’ lives, such as wisdom, resilience, meaning, and social connectedness.
As is the case with any new idea, there is a lot of resistance in the beginning and this will always be the case. However, I would say that positive psychiatry has been received very well. We now have an APA Caucus and a World Psychiatric Association Section on positive psychiatry. Our book, Positive Psychiatry, turned out to be one of the best sellers for American Psychiatric Publishing! Every year, there are symposia on positive psychiatry and papers and books from other countries. Overall, the reception has been very promising.
Dr. Aftab: Thank you for this interesting background, Dr. Jeste. Now let me ask you about the current state of psychiatry. What do you see as some of the strengths of our profession?
Dr. Jeste: Psychiatry’s unique strength is our skill in promoting adaptive behavior change, with a focus on positive factors such as resilience, wisdom, optimism, social engagement, improved health, and longevity. If you look at the research literature, the effect sizes of factors such as optimism, resilience, and social engagement are equal to or greater than interventions such as statins, smoking cessation, and exercise. Cardiothoracic surgeons and radiologists can’t help people increase their resilience, optimism, and social engagement, but psychiatrists can. Behavior change is our expertise. When people are suicidal, we give them hope; we help depressed individuals become active, productive, and happy. We treat people with schizophrenia and bipolar disorder, reduce their psychopathological behaviors, and improve their everyday functioning.
Continue to: Dr. Aftab
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Jeste: Unfortunately, there are a lot of restrictions posed by the current reimbursement system. As a result, psychiatrists spend most of their time prescribing medications in clinical practice. I have nothing against psychopharmacology, but we also need to focus on important aspects of our lives, such as lifestyle, cognitive attitudes, self-care, and social engagement. We need to go beyond symptom reduction. A prominent example is loneliness, which is a major risk factor for morbidity and mortality; the treatment for loneliness is not increasing social network, it’s actually changing one’s perception of and ability to enhance appropriate socialization. Who can do that? Psychiatrists! But we don’t do that right now because the health insurance system doesn’t reimburse psychiatrists to do that.
Dr. Aftab: What is your perception of the threats that psychiatry faces? You had to fend off a variety of challenges during your year as APA president, such as issues surrounding revision of DSM-5. How has that experience shaped your assessment?
Dr. Jeste: I was honored to oversee the finalization and publication of DSM-5 as the president of the APA, even though I lost a lot of sleep working on it! What I found was that there was a lot of antagonism in the media, as well as among several advocacy groups, about the DSM. The misperception was that psychiatry and the APA were trying to expand diagnoses so that the drug companies could sell medications to more people, and psychiatry would benefit from this because of its relationship with the industry. That was actually not the case at all. What I tried to do was to understand where these groups were coming from, and to treat them as collaborators and partners, not as enemies. One thing I am particularly proud of is that we established the Summit Group for DSM-5, which brought together perspectives of the various stakeholders, and our communication both within and outside of the APA improved significantly. It’s gratifying to note that much of the controversy in the media died down after DSM-5 was published. The often-critical New York Times wrote that while DSM-5 is far from perfect, it is the best we have today clinically, and I’m very proud of the work we did on it.
Dr. Aftab: What sort of opportunities lie ahead for psychiatry? What do you envision for the future of the field?
Continue to: Dr. Jeste
Dr. Jeste: As a neuroscientist, I’m excited about the new developments in brain science. Our understanding of the neurobiologic basis of mental illnesses is slowly but surely increasing. I’m also very heartened by all the research going on with regard to the prevention of mental illnesses. I think we will be able to reduce the risk of many psychiatric disorders in the future. This is an exciting time for the field, and psychiatry is going to look very different 20 years from now!
Dr. Aftab: Some people think there’s a conflict between a neuroscientific and psychosocial understanding of psychiatry. How do you think the 2 relate to each other?
Dr. Jeste: The reality, I think, is that there is no conflict. Ultimately, the mind is a function of the brain, and the mind operates within a society. Neuroscientists are also realizing the importance of psychosocial aspects, and there is a growing social neuroscience, looking at the neurobiology of things such as loneliness, social isolation, and wisdom. The effects of psychosocial interventions such as meditation and long-term cognitive-behavioral therapy on the brain are now indisputable. I like to say that psychosocial interventions are often more biological in their effects than the drugs!
Dr. Aftab: Any words of wisdom for psychiatry trainees and early career psychiatrists?
Dr. Jeste: First of all, I congratulate them for going into psychiatry, which is rapidly advancing and is the field of the future. Looking at new developments, such as in artificial intelligence, I wish I could be a young person again just getting into psychiatry! The role of psychiatrists is also evolving, and psychiatrists will become leaders of multidisciplinary teams. I would advise trainees and early career psychiatrists not to get frustrated by issues such as insurance reimbursements; these obstacles will pass. Society is becoming far more conscious of the importance of mental health to our well-being. So I see a reason to be optimistic. I would also mention that the younger generation has a lot to teach the older generation while at the same time benefitting from the wisdom they have to offer. One of the best things we can promote is intergenerational activity, both within and outside of our profession.
Editor’s note: Psychiatry Leaders’ Perspectives is a new department in
In this first Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Dilip V. Jeste, MD. Dr. Jeste is Senior Associate Dean for Healthy Aging and Senior Care, Estelle and Edgar Levi Memorial Chair in Aging, Director of the Sam and Rose Stein Institute for Research on Aging, Distinguished Professor of Psychiatry and Neurosciences, University of California San Diego; and Co-Director of the UC San Diego-IBM Center on Artificial Intelligence for Healthy Living. His main areas of research include schizophrenia, neuropsychiatric interventions, and successful aging. He served as the 139th President of the American Psychiatric Association (APA) and also is a past president of the American Association for Geriatric Psychiatry, the West Coast College of Biological Psychiatry, and founding president of International College of Geriatric Psychoneuropharmacology.
Dr. Aftab: The focus of your term as president of the APA was on “positive psychiatry.” You are also one of the world’s foremost experts in this area. How successful have you been in your mission to promote positive psychiatry, and how has your message been received?
Dr. Jeste: Let me start with a little bit of background about why I got into positive psychiatry. As a geriatric psychiatrist, my research work has brought me face to face with the paradox of aging: although physical health declines with age, mental health and well-being improve on average. This is the case not just for individuals in the community but also for individuals with serious mental illnesses. That got me into thinking more and more about the ways in which we can bring positive change in the lives of patients. When I became the president of the APA, one of my main tasks was to finalize and publish the DSM-5, which rightly focuses on the disorders we treat, but it also provided me with an opportunity to highlight the side of psychiatry that focuses on the positive aspects of our own and our patients’ lives, such as wisdom, resilience, meaning, and social connectedness.
As is the case with any new idea, there is a lot of resistance in the beginning and this will always be the case. However, I would say that positive psychiatry has been received very well. We now have an APA Caucus and a World Psychiatric Association Section on positive psychiatry. Our book, Positive Psychiatry, turned out to be one of the best sellers for American Psychiatric Publishing! Every year, there are symposia on positive psychiatry and papers and books from other countries. Overall, the reception has been very promising.
Dr. Aftab: Thank you for this interesting background, Dr. Jeste. Now let me ask you about the current state of psychiatry. What do you see as some of the strengths of our profession?
Dr. Jeste: Psychiatry’s unique strength is our skill in promoting adaptive behavior change, with a focus on positive factors such as resilience, wisdom, optimism, social engagement, improved health, and longevity. If you look at the research literature, the effect sizes of factors such as optimism, resilience, and social engagement are equal to or greater than interventions such as statins, smoking cessation, and exercise. Cardiothoracic surgeons and radiologists can’t help people increase their resilience, optimism, and social engagement, but psychiatrists can. Behavior change is our expertise. When people are suicidal, we give them hope; we help depressed individuals become active, productive, and happy. We treat people with schizophrenia and bipolar disorder, reduce their psychopathological behaviors, and improve their everyday functioning.
Continue to: Dr. Aftab
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Jeste: Unfortunately, there are a lot of restrictions posed by the current reimbursement system. As a result, psychiatrists spend most of their time prescribing medications in clinical practice. I have nothing against psychopharmacology, but we also need to focus on important aspects of our lives, such as lifestyle, cognitive attitudes, self-care, and social engagement. We need to go beyond symptom reduction. A prominent example is loneliness, which is a major risk factor for morbidity and mortality; the treatment for loneliness is not increasing social network, it’s actually changing one’s perception of and ability to enhance appropriate socialization. Who can do that? Psychiatrists! But we don’t do that right now because the health insurance system doesn’t reimburse psychiatrists to do that.
Dr. Aftab: What is your perception of the threats that psychiatry faces? You had to fend off a variety of challenges during your year as APA president, such as issues surrounding revision of DSM-5. How has that experience shaped your assessment?
Dr. Jeste: I was honored to oversee the finalization and publication of DSM-5 as the president of the APA, even though I lost a lot of sleep working on it! What I found was that there was a lot of antagonism in the media, as well as among several advocacy groups, about the DSM. The misperception was that psychiatry and the APA were trying to expand diagnoses so that the drug companies could sell medications to more people, and psychiatry would benefit from this because of its relationship with the industry. That was actually not the case at all. What I tried to do was to understand where these groups were coming from, and to treat them as collaborators and partners, not as enemies. One thing I am particularly proud of is that we established the Summit Group for DSM-5, which brought together perspectives of the various stakeholders, and our communication both within and outside of the APA improved significantly. It’s gratifying to note that much of the controversy in the media died down after DSM-5 was published. The often-critical New York Times wrote that while DSM-5 is far from perfect, it is the best we have today clinically, and I’m very proud of the work we did on it.
Dr. Aftab: What sort of opportunities lie ahead for psychiatry? What do you envision for the future of the field?
Continue to: Dr. Jeste
Dr. Jeste: As a neuroscientist, I’m excited about the new developments in brain science. Our understanding of the neurobiologic basis of mental illnesses is slowly but surely increasing. I’m also very heartened by all the research going on with regard to the prevention of mental illnesses. I think we will be able to reduce the risk of many psychiatric disorders in the future. This is an exciting time for the field, and psychiatry is going to look very different 20 years from now!
Dr. Aftab: Some people think there’s a conflict between a neuroscientific and psychosocial understanding of psychiatry. How do you think the 2 relate to each other?
Dr. Jeste: The reality, I think, is that there is no conflict. Ultimately, the mind is a function of the brain, and the mind operates within a society. Neuroscientists are also realizing the importance of psychosocial aspects, and there is a growing social neuroscience, looking at the neurobiology of things such as loneliness, social isolation, and wisdom. The effects of psychosocial interventions such as meditation and long-term cognitive-behavioral therapy on the brain are now indisputable. I like to say that psychosocial interventions are often more biological in their effects than the drugs!
Dr. Aftab: Any words of wisdom for psychiatry trainees and early career psychiatrists?
Dr. Jeste: First of all, I congratulate them for going into psychiatry, which is rapidly advancing and is the field of the future. Looking at new developments, such as in artificial intelligence, I wish I could be a young person again just getting into psychiatry! The role of psychiatrists is also evolving, and psychiatrists will become leaders of multidisciplinary teams. I would advise trainees and early career psychiatrists not to get frustrated by issues such as insurance reimbursements; these obstacles will pass. Society is becoming far more conscious of the importance of mental health to our well-being. So I see a reason to be optimistic. I would also mention that the younger generation has a lot to teach the older generation while at the same time benefitting from the wisdom they have to offer. One of the best things we can promote is intergenerational activity, both within and outside of our profession.
Editor’s note: Psychiatry Leaders’ Perspectives is a new department in
In this first Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Dilip V. Jeste, MD. Dr. Jeste is Senior Associate Dean for Healthy Aging and Senior Care, Estelle and Edgar Levi Memorial Chair in Aging, Director of the Sam and Rose Stein Institute for Research on Aging, Distinguished Professor of Psychiatry and Neurosciences, University of California San Diego; and Co-Director of the UC San Diego-IBM Center on Artificial Intelligence for Healthy Living. His main areas of research include schizophrenia, neuropsychiatric interventions, and successful aging. He served as the 139th President of the American Psychiatric Association (APA) and also is a past president of the American Association for Geriatric Psychiatry, the West Coast College of Biological Psychiatry, and founding president of International College of Geriatric Psychoneuropharmacology.
Dr. Aftab: The focus of your term as president of the APA was on “positive psychiatry.” You are also one of the world’s foremost experts in this area. How successful have you been in your mission to promote positive psychiatry, and how has your message been received?
Dr. Jeste: Let me start with a little bit of background about why I got into positive psychiatry. As a geriatric psychiatrist, my research work has brought me face to face with the paradox of aging: although physical health declines with age, mental health and well-being improve on average. This is the case not just for individuals in the community but also for individuals with serious mental illnesses. That got me into thinking more and more about the ways in which we can bring positive change in the lives of patients. When I became the president of the APA, one of my main tasks was to finalize and publish the DSM-5, which rightly focuses on the disorders we treat, but it also provided me with an opportunity to highlight the side of psychiatry that focuses on the positive aspects of our own and our patients’ lives, such as wisdom, resilience, meaning, and social connectedness.
As is the case with any new idea, there is a lot of resistance in the beginning and this will always be the case. However, I would say that positive psychiatry has been received very well. We now have an APA Caucus and a World Psychiatric Association Section on positive psychiatry. Our book, Positive Psychiatry, turned out to be one of the best sellers for American Psychiatric Publishing! Every year, there are symposia on positive psychiatry and papers and books from other countries. Overall, the reception has been very promising.
Dr. Aftab: Thank you for this interesting background, Dr. Jeste. Now let me ask you about the current state of psychiatry. What do you see as some of the strengths of our profession?
Dr. Jeste: Psychiatry’s unique strength is our skill in promoting adaptive behavior change, with a focus on positive factors such as resilience, wisdom, optimism, social engagement, improved health, and longevity. If you look at the research literature, the effect sizes of factors such as optimism, resilience, and social engagement are equal to or greater than interventions such as statins, smoking cessation, and exercise. Cardiothoracic surgeons and radiologists can’t help people increase their resilience, optimism, and social engagement, but psychiatrists can. Behavior change is our expertise. When people are suicidal, we give them hope; we help depressed individuals become active, productive, and happy. We treat people with schizophrenia and bipolar disorder, reduce their psychopathological behaviors, and improve their everyday functioning.
Continue to: Dr. Aftab
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Jeste: Unfortunately, there are a lot of restrictions posed by the current reimbursement system. As a result, psychiatrists spend most of their time prescribing medications in clinical practice. I have nothing against psychopharmacology, but we also need to focus on important aspects of our lives, such as lifestyle, cognitive attitudes, self-care, and social engagement. We need to go beyond symptom reduction. A prominent example is loneliness, which is a major risk factor for morbidity and mortality; the treatment for loneliness is not increasing social network, it’s actually changing one’s perception of and ability to enhance appropriate socialization. Who can do that? Psychiatrists! But we don’t do that right now because the health insurance system doesn’t reimburse psychiatrists to do that.
Dr. Aftab: What is your perception of the threats that psychiatry faces? You had to fend off a variety of challenges during your year as APA president, such as issues surrounding revision of DSM-5. How has that experience shaped your assessment?
Dr. Jeste: I was honored to oversee the finalization and publication of DSM-5 as the president of the APA, even though I lost a lot of sleep working on it! What I found was that there was a lot of antagonism in the media, as well as among several advocacy groups, about the DSM. The misperception was that psychiatry and the APA were trying to expand diagnoses so that the drug companies could sell medications to more people, and psychiatry would benefit from this because of its relationship with the industry. That was actually not the case at all. What I tried to do was to understand where these groups were coming from, and to treat them as collaborators and partners, not as enemies. One thing I am particularly proud of is that we established the Summit Group for DSM-5, which brought together perspectives of the various stakeholders, and our communication both within and outside of the APA improved significantly. It’s gratifying to note that much of the controversy in the media died down after DSM-5 was published. The often-critical New York Times wrote that while DSM-5 is far from perfect, it is the best we have today clinically, and I’m very proud of the work we did on it.
Dr. Aftab: What sort of opportunities lie ahead for psychiatry? What do you envision for the future of the field?
Continue to: Dr. Jeste
Dr. Jeste: As a neuroscientist, I’m excited about the new developments in brain science. Our understanding of the neurobiologic basis of mental illnesses is slowly but surely increasing. I’m also very heartened by all the research going on with regard to the prevention of mental illnesses. I think we will be able to reduce the risk of many psychiatric disorders in the future. This is an exciting time for the field, and psychiatry is going to look very different 20 years from now!
Dr. Aftab: Some people think there’s a conflict between a neuroscientific and psychosocial understanding of psychiatry. How do you think the 2 relate to each other?
Dr. Jeste: The reality, I think, is that there is no conflict. Ultimately, the mind is a function of the brain, and the mind operates within a society. Neuroscientists are also realizing the importance of psychosocial aspects, and there is a growing social neuroscience, looking at the neurobiology of things such as loneliness, social isolation, and wisdom. The effects of psychosocial interventions such as meditation and long-term cognitive-behavioral therapy on the brain are now indisputable. I like to say that psychosocial interventions are often more biological in their effects than the drugs!
Dr. Aftab: Any words of wisdom for psychiatry trainees and early career psychiatrists?
Dr. Jeste: First of all, I congratulate them for going into psychiatry, which is rapidly advancing and is the field of the future. Looking at new developments, such as in artificial intelligence, I wish I could be a young person again just getting into psychiatry! The role of psychiatrists is also evolving, and psychiatrists will become leaders of multidisciplinary teams. I would advise trainees and early career psychiatrists not to get frustrated by issues such as insurance reimbursements; these obstacles will pass. Society is becoming far more conscious of the importance of mental health to our well-being. So I see a reason to be optimistic. I would also mention that the younger generation has a lot to teach the older generation while at the same time benefitting from the wisdom they have to offer. One of the best things we can promote is intergenerational activity, both within and outside of our profession.
Delusions, hypersexuality, and a steep cognitive decline
CASE Inconsistent stories
Ms. P, age 56, is an Asian American woman who was brought in by police after being found standing by her car in the middle of a busy road displaying bizarre behavior. She provides an inconsistent story about why she was brought to the hospital, saying that the police did so because she wasn’t driving fast enough and because her English is weak. At another point, she says that she had stopped her car to pick up a penny from the road and the police brought her to the hospital “to experience life, to rest, to meet people.”
Upon further questioning, Ms. P reveals that she is experiencing racing thoughts, feels full of energy, has pressured speech, and does not need much sleep. She also is sexually preoccupied, talks about having extra-marital affairs, and expresses her infatuation with TV news anchors. She says she is sexually active but is unable to offer any further details, and—while giggling—asks the treatment team not to reveal this information to her husband. Ms. P also reports hearing angels singing from the sky.
Chart review reveals that Ms. P had been admitted to same hospital 5 years earlier, at which time she was given diagnoses of late-onset schizophrenia (LOS) and mild cognitive impairment. Ms. P also had 3 psychiatric inpatient admissions in the past 2 years at a different hospital, but her records are inaccessible because she refuses to allow her chart to be released.
Ms. P has not taken the psychiatric medications prescribed for her for several months; she says, “I don’t need medication. I am self-healing.” She denies using illicit substances, including marijuana, smoking, and current alcohol use, but reports occasional social drinking in the past. Her urine drug screen is negative.
The most striking revelation in Ms. P’s social history is her high premorbid functional status. She has 2 master’s degrees and had been working as a senior accountant at a major hospital system until 7 years ago. In contrast, when interviewed at the hospital, Ms. P reports that she is working at a child care center.
On mental status exam, Ms. P is half-draped in a hospital gown, casual, overly friendly, smiling, and twirling her hair. Her mood is elevated with inappropriate affect. Her thought process is bizarre and illogical. She is alert, fully oriented, and her sensorium is clear. She has persistent ambivalence and contradictory thoughts regarding suicidal ideation. Recent and remote memory are largely intact. She does not express homicidal ideation.
What could be causing Ms. P’s psychosis and functional decline?
a) major neurocognitive disorder
b) schizophrenia
c) schizoaffective disorder
d) bipolar disorder, current manic episode
HISTORY Fired from her job
According to Ms. P’s chart from her admission 5 years earlier, police brought her to the hospital because she was causing a disturbance at a restaurant. When interviewed, Ms. P reported a false story that she fought with her husband, kicked him, and spat on his face. She said that her husband then punched her in the face, she ran out of the house, and a bystander called the police. At the time, her husband was contacted and denied the incident. He said that Ms. P had gone to the store and not returned, and he did not know what happened to her.
Her husband reported a steady and progressive decline in function and behavior dating back to 8 years ago with no known prior behavioral disturbances. In the chart from 5 years ago, her husband reported that Ms. P had been a high-functioning senior executive accountant at a major hospital system 7 years before the current admission, at which time she was fired from her job. He said that, just before being fired, Ms. P had been reading the mystery novel The Da Vinci Code and believed that events in the book specifically applied to her. Ms. P would stay up all night making clothes; when she would go to work, she was caught sleeping on the job and performing poorly, including submitting reports with incorrect information. She yelled at co-workers and was unable to take direction from her supervisors.
Ms. P’s husband also reported that she believed people were trying to “look like her,” by having plastic surgery. He reported unusual behavior at home, including eating food off the countertop that had been out for hours and was not fit for consumption.
Ms. P’s husband could not be contacted during this admission because he was out of country and they were separated. Collateral information is obtained from Ms. P’s mother, who lives apart from her but in the same city and speaks no English. She confirms Ms. P’s high premorbid functioning, and reports that her daughter’s change in behavior went back as far as 10 years. She reports that Ms. P had problems controlling anger and had frequent altercations with her husband and mother, including threatening her with a knife. Self-care and hygiene then declined strikingly. She began to have odd religious beliefs (eg, she was the daughter of Jesus Christ) and insisted on dressing in peculiar ways.
No family history of psychiatric disorders, such as schizophrenia, bipolar disorder, or dementia, was reported (Table 1).
The authors’ observations
The existence of LOS as a distinct subtype of schizophrenia has been the subject of discussion and controversy as far back as Manfred Bleuler in 1943 who coined the term “late-onset schizophrenia.”1 In 2000, a consensus statement by the International Late-Onset Schizophrenia Group standardized the nomenclature, defining LOS as onset between age 40 and 60, and very-late-onset schizophrenia-like psychosis (VLOS) as onset after age 60.2 Although there is no diagnostic subcategory for LOS in DSM, DSM-5 notes that (1) women are overrepresented in late-onset cases and (2) the course generally is characterized by a predominance of psychotic symptoms with preservation of affect and social functioning.3 DSM authors comment that it is not yet clear whether LOS is the same condition as schizophrenia diagnosed earlier in life. Approximately 23% of schizophrenia cases have onset after age 40.4
Cognitive symptoms in LOS
The presence of cognitive deficits in schizophrenia is common and well-recognized. The intellectual impairment is generalized and global, and there also is specific impairment in a range of cognitive functions, such as executive functions, memory, psychomotor speed, attention, and social cognition.5 Typically these cognitive impairments are present before onset of psychotic symptoms. Although cognitive symptoms are not part of the formal diagnostic criteria, DSM-5 acknowledges their presence.3 In a systematic review on nature and course of cognitive function in LOS, Rajji and Mulsant6 report that global deficits and specific deficits in executive functions, visuospatial constructional abilities, verbal fluency, and psychomotor speech have been found consistently in studies of LOS, although the presence of deficits in memory, attention, and working memory has been less consistent.
The presence of cognitive symptoms in LOS is less well-studied and understood (Table 2). The International Consensus Statement reported that no difference in type of cognitive deficit has been found in early–onset cases (onset before age 40) compared with late-onset cases, although LOS is associated with relatively milder cognitive deficits. Additionally, premorbid educational, occupational, and psychosocial functioning are less impaired in LOS than they are in early-onset schizophrenia.2
Rajji et al7 performed a meta-analysis comparison of patients with youth-onset schizophrenia, adults with first-episode schizophrenia, and those with LOS on their cognitive profiles. They reported that patients with youth-onset schizophrenia have globally severe cognitive deficits, whereas those with LOS demonstrate minimal deficits on arithmetic, digit symbol coding, and vocabulary but larger deficits on attention, fluency, global cognition, IQ, and visuospatial construction.7
There are conflicting views in the literature with regards to the course of cognitive deficits in schizophrenia. One group of researchers believes that there is progressive deterioration in cognitive functioning over time, while another maintains that cognitive impairment in schizophrenia is largely “a static encephalopathy” with no significant progression of symptoms.8 A number of studies referenced by Rajji and Mulsant6 in their systematic review report that cognitive deficits seen in patients with LOS largely are stable on follow-up with an average duration of up to 3 years. However, 2 studies with longer follow-up report evidence of cognitive decline.9,10
Relevant findings from the literature. Brodaty et al9 followed 27 patients with LOS without dementia and 34 otherwise healthy participants at baseline, 1 year, and 5 years. They reported that 9 patients with LOS and none of the control group were found to have dementia (5 Alzheimer type, 1 vascular, and 3 dementia of unknown type) at 5-year follow-up. Some patients had no clinical signs of dementia at baseline or at 1-year follow-up, but were found to have dementia at 5-year follow-up. The authors speculated that LOS might be a prodrome of Alzheimer-type dementia.
Kørner et al10 studied 12,600 patients with LOS and 7,700 with VLOS, selected from the Danish nationwide registry; follow-up was 3 to 4.58 years. They concluded that patients with LOS and VLOS were at 2 to 3 times greater risk of developing dementia than patients with osteoarthritis or the general population. The most common diagnosis among patients with schizophrenia was unspecified dementia, with Alzheimer’s dementia (AD) being the most common diagnosis in control groups. The findings suggest that dementia in LOS and VLOS has a different basis than AD.
Zakzanis et al11 investigated which neuropsychological tests best differentiate patients with LOS and those with AD or frontotemporal dementia. They reported that Wechsler Adult Intelligence Scale-Revised (WAIS-R) Similarities subtest and the California Verbal Learning Test (both short- and long-delay free recall) can differentiate LOS from AD, and a test battery comprising the WAIS-R Vocabulary, Information, Digit Span, and Comprehension subtests, and the Hooper Visual Organization test can differentiate LOS and frontotemporal dementia.12
EVALUATION Significant impairment
CT head and MRI brain scans without contrast suggest mild generalized atrophy that is more prominent in frontal and parietal areas, but the scans are otherwise unremarkable overall. A PET scan is significant for hypoactivity in the temporal and parietal lobes but, again, the images are interpreted as unremarkable overall.
Ms. P scores 21 on the Montreal Cognitive Assessment (MoCA), indicative of significant cognitive impairment (normal score, ≥26). This is a 3-point decline on a MoCA performed during her admission 5 years earlier.
Ms. P scores 8 on the Middlesex Elderly Assessment of Mental State, the lowest score in the borderline range of cognitive function for geriatric patients. She scores 13 on the Kohlman Evaluation of Living Skills, indicating that she needs maximal supervision, structure, and support to live in the community. Particularly notable is that Ms. P failed 5 out of 6 subtests in money management—a marked decline for someone who had worked as a senior accountant.
Given Ms. P’s significant cognitive decline from premorbid functioning, verified by collateral information, and current cognitive deficits established on standardized tests, we determine that, in addition to a diagnosis of schizoaffective disorder, she might meet DSM-5 criteria for unspecified major neurocognitive disorder if her functioning does not improve with treatment.
The authors’ observations
There is scant literature on late-onset schizoaffective disorder. Webster and Grossberg13 conducted a retrospective chart review of 1,730 patients age >65 who were admitted to a geriatric psychiatry unit from 1988 to 1995. Of these patients, 166 (approximately 10%) were found to have late life-onset psychosis. The psychosis was attributed to various causes, such as dementia, depression, bipolar disorder, medical causes, delirium, medication toxicity. Two patients were diagnosed with schizophrenia and 2 were diagnosed with schizoaffective disorder (the authors did not provide additional information about the patients with schizoaffective disorder). Brenner et al14 reports a case of late-onset schizoaffective disorder in a 70-year-old female patient. Evans et al15 compared outpatients age 45 to 77 with a diagnosis of schizoaffective disorder (n = 29), schizophrenia (n = 154), or nonpsychotic mood disorder (n = 27) and concluded that late-onset schizoaffective disorder might represent a variant of LOS in clinical symptom profiles and cognitive impairment but with additional mood symptoms.16
How would you begin treating Ms. P?
a) start a mood stabilizer
b) start an atypical antipsychotic
c) obtain more history and collateral information
d) recommend outpatient treatment
The authors’ observations
Given Ms. P’s manic symptoms, thought disorder, and history of psychotic symptoms with diagnosis of LOS, we assigned her a presumptive diagnosis of schizoaffective disorder, bipolar type. From the patient report, collateral information from her mother, earlier documented collateral from her husband, and chart review, it was apparent to us that Ms. P’s psychiatric history went back only 10 years—therefore meeting temporal criteria for LOS.
Clinical assessment (Figure) and standardized tests revealed the presence of neurocognitive deficits sufficient to meet criteria for major neurocognitive disorder (Table 33). The pattern of neurocognitive deficits is consistent with an AD-like amnestic picture, although no clear-cut diagnosis was present, and the neurocognitive disorder was better classified as unspecified rather than of a particular type. It remains uncertain whether cognitive deficits of severity that meet criteria for major neurocognitive disorder are sufficiently accounted for by the diagnosis of LOS alone. Unless diagnostic criteria for schizophrenia are expanded to include cognitive deficits, a separate diagnosis of major neurocognitive disorder is warranted at present.
TREATMENT Pharmacotherapy
On the unit, Ms. P is observed by nursing staff wandering, with some pressured speech but no behavioral agitation. Her clothing had been bizarre, with multiple layers, and, at one point, she walks with her gown open and without undergarments. She also reports to the nurses that she has a lot of sexual thoughts. When the interview team enters her room, they find her masturbating.
Ms. P is started on aripiprazole, 10 mg/d, titrated to 20 mg/d, and divalproex sodium, 500 mg/d. The decision to initiate a cognitive enhancer, such as an acetylcholinesterase inhibitor or memantine, is deferred to outpatient care to allow for the possibility that her cognitive features will improve after the psychosis is treated.
By the end of first week, Ms. P’s manic features are no longer prominent but her thought process continues to be bizarre, with poor insight and judgment. She demonstrates severe ambivalence in all matters, consistently gives inconsistent accounts of the past, and makes dramatic false statements.
For example, when asked about her children, Ms. P tells us that she has 6 children—the youngest 3 months old, at home by himself and “probably dead by now.” In reality, she has only a 20-year-old son who is studying abroad. Talking about her marriage, Ms. P says she and her husband are not divorced on paper but that, because they haven’t had sex for 8 years, the law has provided them with an automatic divorce.
OUTCOME Significant improvement
Ms. P shows significant response to aripiprazole and divalproex, which are well tolerated without significant adverse effects. Her limitations in executive functioning and rational thought process lead the treatment team to consider nursing home placement under guardianship. Days before discharge, however, reexamination of her neuropsychiatric state suggests significant improvement in thought process, with improvement in cognitive features. Ms. P also becomes cooperative with treatment planning.
The treatment team has meetings with Ms. P’s mother to discuss monitoring and plans for discharge. Ms. P is discharged with follow-up arranged at community mental health services.
Bottom Line
Global as well as specific cognitive deficits are associated with late-onset schizophrenia. Studies have reported increased risk of dementia in these patients over the course of 3 to 5 years, usually unspecified or Alzheimer’s type. It is imperative to assess patients with schizophrenia, especially those age ≥40, for presence of neurocognitive disorder by means of neurocognitive testing.
Related Resources
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
- Radhakrishnan R, Butler R, Head L. Dementia in schizophrenia. Adv Psychiatr Treat. 2012;18(2):144-153.
Drug Brand Names
Aripiprazole • Abilify
Divalproex sodium • Depakote
Mematine • Namenda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturer of competing products.
1. Bleuler M. Die spätschizophrenen Krankheitsbilder. Fortschr Neurol Psychiatr. 1943;15:259-290.
2. Howard R, Rabins PV, Seeman MV, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000; 157(2):172-178.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14(1):39-55.
5. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1):4-19.
6. Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102(1-3):122-140.
7. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286-293.
8. Goldberg TE, Hyde TM, Kleinman JE, et al. Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull. 1993;19(4):797-804.
9. Brodaty H, Sachdev P, Koschera A, et al. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry. 2003;183(3):213-219.
10. Kørner A, Lopez AG, Lauritzen L, et al. Late and very-late first‐contact schizophrenia and the risk of dementia—a nationwide register based study. Int J Geriatr Psychiatry. 2009;24(1):61-67.
11. Zakzanis KK, Andrikopoulos J, Young DA, et al. Neuropsychological differentiation of late-onset schizophrenia and dementia of the Alzheimer’s type. Appl Neuropsychol. 2003;10(2):105-114.
12. Zakzanis KK, Kielar A, Young DA, et al. Neuropsychological differentiation of late onset schizophrenia and frontotemporal dementia. Cognitive Neuropsychiatry. 2001;6(1):63-77.
13. Webster J, Grossberg GT. Late-life onset of psychotic symptoms. Am J Geriatr Psychiatry. 1998;6(3):196-202.
14. Brenner R, Campbell K, Konakondla K, et al. Late onset schizoaffective disorder. Consultant. 2014;53(6):487-488.
15. Evans JD, Heaton RK, Paulsen JS, et al. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874-882.
16. Jeste DV, Blazer DG, First M. Aging-related diagnostic variations: need for diagnostic criteria appropriate for elderly psychiatric patients. Biol Psychiatry. 2005;58(4):265-271.
CASE Inconsistent stories
Ms. P, age 56, is an Asian American woman who was brought in by police after being found standing by her car in the middle of a busy road displaying bizarre behavior. She provides an inconsistent story about why she was brought to the hospital, saying that the police did so because she wasn’t driving fast enough and because her English is weak. At another point, she says that she had stopped her car to pick up a penny from the road and the police brought her to the hospital “to experience life, to rest, to meet people.”
Upon further questioning, Ms. P reveals that she is experiencing racing thoughts, feels full of energy, has pressured speech, and does not need much sleep. She also is sexually preoccupied, talks about having extra-marital affairs, and expresses her infatuation with TV news anchors. She says she is sexually active but is unable to offer any further details, and—while giggling—asks the treatment team not to reveal this information to her husband. Ms. P also reports hearing angels singing from the sky.
Chart review reveals that Ms. P had been admitted to same hospital 5 years earlier, at which time she was given diagnoses of late-onset schizophrenia (LOS) and mild cognitive impairment. Ms. P also had 3 psychiatric inpatient admissions in the past 2 years at a different hospital, but her records are inaccessible because she refuses to allow her chart to be released.
Ms. P has not taken the psychiatric medications prescribed for her for several months; she says, “I don’t need medication. I am self-healing.” She denies using illicit substances, including marijuana, smoking, and current alcohol use, but reports occasional social drinking in the past. Her urine drug screen is negative.
The most striking revelation in Ms. P’s social history is her high premorbid functional status. She has 2 master’s degrees and had been working as a senior accountant at a major hospital system until 7 years ago. In contrast, when interviewed at the hospital, Ms. P reports that she is working at a child care center.
On mental status exam, Ms. P is half-draped in a hospital gown, casual, overly friendly, smiling, and twirling her hair. Her mood is elevated with inappropriate affect. Her thought process is bizarre and illogical. She is alert, fully oriented, and her sensorium is clear. She has persistent ambivalence and contradictory thoughts regarding suicidal ideation. Recent and remote memory are largely intact. She does not express homicidal ideation.
What could be causing Ms. P’s psychosis and functional decline?
a) major neurocognitive disorder
b) schizophrenia
c) schizoaffective disorder
d) bipolar disorder, current manic episode
HISTORY Fired from her job
According to Ms. P’s chart from her admission 5 years earlier, police brought her to the hospital because she was causing a disturbance at a restaurant. When interviewed, Ms. P reported a false story that she fought with her husband, kicked him, and spat on his face. She said that her husband then punched her in the face, she ran out of the house, and a bystander called the police. At the time, her husband was contacted and denied the incident. He said that Ms. P had gone to the store and not returned, and he did not know what happened to her.
Her husband reported a steady and progressive decline in function and behavior dating back to 8 years ago with no known prior behavioral disturbances. In the chart from 5 years ago, her husband reported that Ms. P had been a high-functioning senior executive accountant at a major hospital system 7 years before the current admission, at which time she was fired from her job. He said that, just before being fired, Ms. P had been reading the mystery novel The Da Vinci Code and believed that events in the book specifically applied to her. Ms. P would stay up all night making clothes; when she would go to work, she was caught sleeping on the job and performing poorly, including submitting reports with incorrect information. She yelled at co-workers and was unable to take direction from her supervisors.
Ms. P’s husband also reported that she believed people were trying to “look like her,” by having plastic surgery. He reported unusual behavior at home, including eating food off the countertop that had been out for hours and was not fit for consumption.
Ms. P’s husband could not be contacted during this admission because he was out of country and they were separated. Collateral information is obtained from Ms. P’s mother, who lives apart from her but in the same city and speaks no English. She confirms Ms. P’s high premorbid functioning, and reports that her daughter’s change in behavior went back as far as 10 years. She reports that Ms. P had problems controlling anger and had frequent altercations with her husband and mother, including threatening her with a knife. Self-care and hygiene then declined strikingly. She began to have odd religious beliefs (eg, she was the daughter of Jesus Christ) and insisted on dressing in peculiar ways.
No family history of psychiatric disorders, such as schizophrenia, bipolar disorder, or dementia, was reported (Table 1).
The authors’ observations
The existence of LOS as a distinct subtype of schizophrenia has been the subject of discussion and controversy as far back as Manfred Bleuler in 1943 who coined the term “late-onset schizophrenia.”1 In 2000, a consensus statement by the International Late-Onset Schizophrenia Group standardized the nomenclature, defining LOS as onset between age 40 and 60, and very-late-onset schizophrenia-like psychosis (VLOS) as onset after age 60.2 Although there is no diagnostic subcategory for LOS in DSM, DSM-5 notes that (1) women are overrepresented in late-onset cases and (2) the course generally is characterized by a predominance of psychotic symptoms with preservation of affect and social functioning.3 DSM authors comment that it is not yet clear whether LOS is the same condition as schizophrenia diagnosed earlier in life. Approximately 23% of schizophrenia cases have onset after age 40.4
Cognitive symptoms in LOS
The presence of cognitive deficits in schizophrenia is common and well-recognized. The intellectual impairment is generalized and global, and there also is specific impairment in a range of cognitive functions, such as executive functions, memory, psychomotor speed, attention, and social cognition.5 Typically these cognitive impairments are present before onset of psychotic symptoms. Although cognitive symptoms are not part of the formal diagnostic criteria, DSM-5 acknowledges their presence.3 In a systematic review on nature and course of cognitive function in LOS, Rajji and Mulsant6 report that global deficits and specific deficits in executive functions, visuospatial constructional abilities, verbal fluency, and psychomotor speech have been found consistently in studies of LOS, although the presence of deficits in memory, attention, and working memory has been less consistent.
The presence of cognitive symptoms in LOS is less well-studied and understood (Table 2). The International Consensus Statement reported that no difference in type of cognitive deficit has been found in early–onset cases (onset before age 40) compared with late-onset cases, although LOS is associated with relatively milder cognitive deficits. Additionally, premorbid educational, occupational, and psychosocial functioning are less impaired in LOS than they are in early-onset schizophrenia.2
Rajji et al7 performed a meta-analysis comparison of patients with youth-onset schizophrenia, adults with first-episode schizophrenia, and those with LOS on their cognitive profiles. They reported that patients with youth-onset schizophrenia have globally severe cognitive deficits, whereas those with LOS demonstrate minimal deficits on arithmetic, digit symbol coding, and vocabulary but larger deficits on attention, fluency, global cognition, IQ, and visuospatial construction.7
There are conflicting views in the literature with regards to the course of cognitive deficits in schizophrenia. One group of researchers believes that there is progressive deterioration in cognitive functioning over time, while another maintains that cognitive impairment in schizophrenia is largely “a static encephalopathy” with no significant progression of symptoms.8 A number of studies referenced by Rajji and Mulsant6 in their systematic review report that cognitive deficits seen in patients with LOS largely are stable on follow-up with an average duration of up to 3 years. However, 2 studies with longer follow-up report evidence of cognitive decline.9,10
Relevant findings from the literature. Brodaty et al9 followed 27 patients with LOS without dementia and 34 otherwise healthy participants at baseline, 1 year, and 5 years. They reported that 9 patients with LOS and none of the control group were found to have dementia (5 Alzheimer type, 1 vascular, and 3 dementia of unknown type) at 5-year follow-up. Some patients had no clinical signs of dementia at baseline or at 1-year follow-up, but were found to have dementia at 5-year follow-up. The authors speculated that LOS might be a prodrome of Alzheimer-type dementia.
Kørner et al10 studied 12,600 patients with LOS and 7,700 with VLOS, selected from the Danish nationwide registry; follow-up was 3 to 4.58 years. They concluded that patients with LOS and VLOS were at 2 to 3 times greater risk of developing dementia than patients with osteoarthritis or the general population. The most common diagnosis among patients with schizophrenia was unspecified dementia, with Alzheimer’s dementia (AD) being the most common diagnosis in control groups. The findings suggest that dementia in LOS and VLOS has a different basis than AD.
Zakzanis et al11 investigated which neuropsychological tests best differentiate patients with LOS and those with AD or frontotemporal dementia. They reported that Wechsler Adult Intelligence Scale-Revised (WAIS-R) Similarities subtest and the California Verbal Learning Test (both short- and long-delay free recall) can differentiate LOS from AD, and a test battery comprising the WAIS-R Vocabulary, Information, Digit Span, and Comprehension subtests, and the Hooper Visual Organization test can differentiate LOS and frontotemporal dementia.12
EVALUATION Significant impairment
CT head and MRI brain scans without contrast suggest mild generalized atrophy that is more prominent in frontal and parietal areas, but the scans are otherwise unremarkable overall. A PET scan is significant for hypoactivity in the temporal and parietal lobes but, again, the images are interpreted as unremarkable overall.
Ms. P scores 21 on the Montreal Cognitive Assessment (MoCA), indicative of significant cognitive impairment (normal score, ≥26). This is a 3-point decline on a MoCA performed during her admission 5 years earlier.
Ms. P scores 8 on the Middlesex Elderly Assessment of Mental State, the lowest score in the borderline range of cognitive function for geriatric patients. She scores 13 on the Kohlman Evaluation of Living Skills, indicating that she needs maximal supervision, structure, and support to live in the community. Particularly notable is that Ms. P failed 5 out of 6 subtests in money management—a marked decline for someone who had worked as a senior accountant.
Given Ms. P’s significant cognitive decline from premorbid functioning, verified by collateral information, and current cognitive deficits established on standardized tests, we determine that, in addition to a diagnosis of schizoaffective disorder, she might meet DSM-5 criteria for unspecified major neurocognitive disorder if her functioning does not improve with treatment.
The authors’ observations
There is scant literature on late-onset schizoaffective disorder. Webster and Grossberg13 conducted a retrospective chart review of 1,730 patients age >65 who were admitted to a geriatric psychiatry unit from 1988 to 1995. Of these patients, 166 (approximately 10%) were found to have late life-onset psychosis. The psychosis was attributed to various causes, such as dementia, depression, bipolar disorder, medical causes, delirium, medication toxicity. Two patients were diagnosed with schizophrenia and 2 were diagnosed with schizoaffective disorder (the authors did not provide additional information about the patients with schizoaffective disorder). Brenner et al14 reports a case of late-onset schizoaffective disorder in a 70-year-old female patient. Evans et al15 compared outpatients age 45 to 77 with a diagnosis of schizoaffective disorder (n = 29), schizophrenia (n = 154), or nonpsychotic mood disorder (n = 27) and concluded that late-onset schizoaffective disorder might represent a variant of LOS in clinical symptom profiles and cognitive impairment but with additional mood symptoms.16
How would you begin treating Ms. P?
a) start a mood stabilizer
b) start an atypical antipsychotic
c) obtain more history and collateral information
d) recommend outpatient treatment
The authors’ observations
Given Ms. P’s manic symptoms, thought disorder, and history of psychotic symptoms with diagnosis of LOS, we assigned her a presumptive diagnosis of schizoaffective disorder, bipolar type. From the patient report, collateral information from her mother, earlier documented collateral from her husband, and chart review, it was apparent to us that Ms. P’s psychiatric history went back only 10 years—therefore meeting temporal criteria for LOS.
Clinical assessment (Figure) and standardized tests revealed the presence of neurocognitive deficits sufficient to meet criteria for major neurocognitive disorder (Table 33). The pattern of neurocognitive deficits is consistent with an AD-like amnestic picture, although no clear-cut diagnosis was present, and the neurocognitive disorder was better classified as unspecified rather than of a particular type. It remains uncertain whether cognitive deficits of severity that meet criteria for major neurocognitive disorder are sufficiently accounted for by the diagnosis of LOS alone. Unless diagnostic criteria for schizophrenia are expanded to include cognitive deficits, a separate diagnosis of major neurocognitive disorder is warranted at present.
TREATMENT Pharmacotherapy
On the unit, Ms. P is observed by nursing staff wandering, with some pressured speech but no behavioral agitation. Her clothing had been bizarre, with multiple layers, and, at one point, she walks with her gown open and without undergarments. She also reports to the nurses that she has a lot of sexual thoughts. When the interview team enters her room, they find her masturbating.
Ms. P is started on aripiprazole, 10 mg/d, titrated to 20 mg/d, and divalproex sodium, 500 mg/d. The decision to initiate a cognitive enhancer, such as an acetylcholinesterase inhibitor or memantine, is deferred to outpatient care to allow for the possibility that her cognitive features will improve after the psychosis is treated.
By the end of first week, Ms. P’s manic features are no longer prominent but her thought process continues to be bizarre, with poor insight and judgment. She demonstrates severe ambivalence in all matters, consistently gives inconsistent accounts of the past, and makes dramatic false statements.
For example, when asked about her children, Ms. P tells us that she has 6 children—the youngest 3 months old, at home by himself and “probably dead by now.” In reality, she has only a 20-year-old son who is studying abroad. Talking about her marriage, Ms. P says she and her husband are not divorced on paper but that, because they haven’t had sex for 8 years, the law has provided them with an automatic divorce.
OUTCOME Significant improvement
Ms. P shows significant response to aripiprazole and divalproex, which are well tolerated without significant adverse effects. Her limitations in executive functioning and rational thought process lead the treatment team to consider nursing home placement under guardianship. Days before discharge, however, reexamination of her neuropsychiatric state suggests significant improvement in thought process, with improvement in cognitive features. Ms. P also becomes cooperative with treatment planning.
The treatment team has meetings with Ms. P’s mother to discuss monitoring and plans for discharge. Ms. P is discharged with follow-up arranged at community mental health services.
Bottom Line
Global as well as specific cognitive deficits are associated with late-onset schizophrenia. Studies have reported increased risk of dementia in these patients over the course of 3 to 5 years, usually unspecified or Alzheimer’s type. It is imperative to assess patients with schizophrenia, especially those age ≥40, for presence of neurocognitive disorder by means of neurocognitive testing.
Related Resources
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
- Radhakrishnan R, Butler R, Head L. Dementia in schizophrenia. Adv Psychiatr Treat. 2012;18(2):144-153.
Drug Brand Names
Aripiprazole • Abilify
Divalproex sodium • Depakote
Mematine • Namenda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturer of competing products.
CASE Inconsistent stories
Ms. P, age 56, is an Asian American woman who was brought in by police after being found standing by her car in the middle of a busy road displaying bizarre behavior. She provides an inconsistent story about why she was brought to the hospital, saying that the police did so because she wasn’t driving fast enough and because her English is weak. At another point, she says that she had stopped her car to pick up a penny from the road and the police brought her to the hospital “to experience life, to rest, to meet people.”
Upon further questioning, Ms. P reveals that she is experiencing racing thoughts, feels full of energy, has pressured speech, and does not need much sleep. She also is sexually preoccupied, talks about having extra-marital affairs, and expresses her infatuation with TV news anchors. She says she is sexually active but is unable to offer any further details, and—while giggling—asks the treatment team not to reveal this information to her husband. Ms. P also reports hearing angels singing from the sky.
Chart review reveals that Ms. P had been admitted to same hospital 5 years earlier, at which time she was given diagnoses of late-onset schizophrenia (LOS) and mild cognitive impairment. Ms. P also had 3 psychiatric inpatient admissions in the past 2 years at a different hospital, but her records are inaccessible because she refuses to allow her chart to be released.
Ms. P has not taken the psychiatric medications prescribed for her for several months; she says, “I don’t need medication. I am self-healing.” She denies using illicit substances, including marijuana, smoking, and current alcohol use, but reports occasional social drinking in the past. Her urine drug screen is negative.
The most striking revelation in Ms. P’s social history is her high premorbid functional status. She has 2 master’s degrees and had been working as a senior accountant at a major hospital system until 7 years ago. In contrast, when interviewed at the hospital, Ms. P reports that she is working at a child care center.
On mental status exam, Ms. P is half-draped in a hospital gown, casual, overly friendly, smiling, and twirling her hair. Her mood is elevated with inappropriate affect. Her thought process is bizarre and illogical. She is alert, fully oriented, and her sensorium is clear. She has persistent ambivalence and contradictory thoughts regarding suicidal ideation. Recent and remote memory are largely intact. She does not express homicidal ideation.
What could be causing Ms. P’s psychosis and functional decline?
a) major neurocognitive disorder
b) schizophrenia
c) schizoaffective disorder
d) bipolar disorder, current manic episode
HISTORY Fired from her job
According to Ms. P’s chart from her admission 5 years earlier, police brought her to the hospital because she was causing a disturbance at a restaurant. When interviewed, Ms. P reported a false story that she fought with her husband, kicked him, and spat on his face. She said that her husband then punched her in the face, she ran out of the house, and a bystander called the police. At the time, her husband was contacted and denied the incident. He said that Ms. P had gone to the store and not returned, and he did not know what happened to her.
Her husband reported a steady and progressive decline in function and behavior dating back to 8 years ago with no known prior behavioral disturbances. In the chart from 5 years ago, her husband reported that Ms. P had been a high-functioning senior executive accountant at a major hospital system 7 years before the current admission, at which time she was fired from her job. He said that, just before being fired, Ms. P had been reading the mystery novel The Da Vinci Code and believed that events in the book specifically applied to her. Ms. P would stay up all night making clothes; when she would go to work, she was caught sleeping on the job and performing poorly, including submitting reports with incorrect information. She yelled at co-workers and was unable to take direction from her supervisors.
Ms. P’s husband also reported that she believed people were trying to “look like her,” by having plastic surgery. He reported unusual behavior at home, including eating food off the countertop that had been out for hours and was not fit for consumption.
Ms. P’s husband could not be contacted during this admission because he was out of country and they were separated. Collateral information is obtained from Ms. P’s mother, who lives apart from her but in the same city and speaks no English. She confirms Ms. P’s high premorbid functioning, and reports that her daughter’s change in behavior went back as far as 10 years. She reports that Ms. P had problems controlling anger and had frequent altercations with her husband and mother, including threatening her with a knife. Self-care and hygiene then declined strikingly. She began to have odd religious beliefs (eg, she was the daughter of Jesus Christ) and insisted on dressing in peculiar ways.
No family history of psychiatric disorders, such as schizophrenia, bipolar disorder, or dementia, was reported (Table 1).
The authors’ observations
The existence of LOS as a distinct subtype of schizophrenia has been the subject of discussion and controversy as far back as Manfred Bleuler in 1943 who coined the term “late-onset schizophrenia.”1 In 2000, a consensus statement by the International Late-Onset Schizophrenia Group standardized the nomenclature, defining LOS as onset between age 40 and 60, and very-late-onset schizophrenia-like psychosis (VLOS) as onset after age 60.2 Although there is no diagnostic subcategory for LOS in DSM, DSM-5 notes that (1) women are overrepresented in late-onset cases and (2) the course generally is characterized by a predominance of psychotic symptoms with preservation of affect and social functioning.3 DSM authors comment that it is not yet clear whether LOS is the same condition as schizophrenia diagnosed earlier in life. Approximately 23% of schizophrenia cases have onset after age 40.4
Cognitive symptoms in LOS
The presence of cognitive deficits in schizophrenia is common and well-recognized. The intellectual impairment is generalized and global, and there also is specific impairment in a range of cognitive functions, such as executive functions, memory, psychomotor speed, attention, and social cognition.5 Typically these cognitive impairments are present before onset of psychotic symptoms. Although cognitive symptoms are not part of the formal diagnostic criteria, DSM-5 acknowledges their presence.3 In a systematic review on nature and course of cognitive function in LOS, Rajji and Mulsant6 report that global deficits and specific deficits in executive functions, visuospatial constructional abilities, verbal fluency, and psychomotor speech have been found consistently in studies of LOS, although the presence of deficits in memory, attention, and working memory has been less consistent.
The presence of cognitive symptoms in LOS is less well-studied and understood (Table 2). The International Consensus Statement reported that no difference in type of cognitive deficit has been found in early–onset cases (onset before age 40) compared with late-onset cases, although LOS is associated with relatively milder cognitive deficits. Additionally, premorbid educational, occupational, and psychosocial functioning are less impaired in LOS than they are in early-onset schizophrenia.2
Rajji et al7 performed a meta-analysis comparison of patients with youth-onset schizophrenia, adults with first-episode schizophrenia, and those with LOS on their cognitive profiles. They reported that patients with youth-onset schizophrenia have globally severe cognitive deficits, whereas those with LOS demonstrate minimal deficits on arithmetic, digit symbol coding, and vocabulary but larger deficits on attention, fluency, global cognition, IQ, and visuospatial construction.7
There are conflicting views in the literature with regards to the course of cognitive deficits in schizophrenia. One group of researchers believes that there is progressive deterioration in cognitive functioning over time, while another maintains that cognitive impairment in schizophrenia is largely “a static encephalopathy” with no significant progression of symptoms.8 A number of studies referenced by Rajji and Mulsant6 in their systematic review report that cognitive deficits seen in patients with LOS largely are stable on follow-up with an average duration of up to 3 years. However, 2 studies with longer follow-up report evidence of cognitive decline.9,10
Relevant findings from the literature. Brodaty et al9 followed 27 patients with LOS without dementia and 34 otherwise healthy participants at baseline, 1 year, and 5 years. They reported that 9 patients with LOS and none of the control group were found to have dementia (5 Alzheimer type, 1 vascular, and 3 dementia of unknown type) at 5-year follow-up. Some patients had no clinical signs of dementia at baseline or at 1-year follow-up, but were found to have dementia at 5-year follow-up. The authors speculated that LOS might be a prodrome of Alzheimer-type dementia.
Kørner et al10 studied 12,600 patients with LOS and 7,700 with VLOS, selected from the Danish nationwide registry; follow-up was 3 to 4.58 years. They concluded that patients with LOS and VLOS were at 2 to 3 times greater risk of developing dementia than patients with osteoarthritis or the general population. The most common diagnosis among patients with schizophrenia was unspecified dementia, with Alzheimer’s dementia (AD) being the most common diagnosis in control groups. The findings suggest that dementia in LOS and VLOS has a different basis than AD.
Zakzanis et al11 investigated which neuropsychological tests best differentiate patients with LOS and those with AD or frontotemporal dementia. They reported that Wechsler Adult Intelligence Scale-Revised (WAIS-R) Similarities subtest and the California Verbal Learning Test (both short- and long-delay free recall) can differentiate LOS from AD, and a test battery comprising the WAIS-R Vocabulary, Information, Digit Span, and Comprehension subtests, and the Hooper Visual Organization test can differentiate LOS and frontotemporal dementia.12
EVALUATION Significant impairment
CT head and MRI brain scans without contrast suggest mild generalized atrophy that is more prominent in frontal and parietal areas, but the scans are otherwise unremarkable overall. A PET scan is significant for hypoactivity in the temporal and parietal lobes but, again, the images are interpreted as unremarkable overall.
Ms. P scores 21 on the Montreal Cognitive Assessment (MoCA), indicative of significant cognitive impairment (normal score, ≥26). This is a 3-point decline on a MoCA performed during her admission 5 years earlier.
Ms. P scores 8 on the Middlesex Elderly Assessment of Mental State, the lowest score in the borderline range of cognitive function for geriatric patients. She scores 13 on the Kohlman Evaluation of Living Skills, indicating that she needs maximal supervision, structure, and support to live in the community. Particularly notable is that Ms. P failed 5 out of 6 subtests in money management—a marked decline for someone who had worked as a senior accountant.
Given Ms. P’s significant cognitive decline from premorbid functioning, verified by collateral information, and current cognitive deficits established on standardized tests, we determine that, in addition to a diagnosis of schizoaffective disorder, she might meet DSM-5 criteria for unspecified major neurocognitive disorder if her functioning does not improve with treatment.
The authors’ observations
There is scant literature on late-onset schizoaffective disorder. Webster and Grossberg13 conducted a retrospective chart review of 1,730 patients age >65 who were admitted to a geriatric psychiatry unit from 1988 to 1995. Of these patients, 166 (approximately 10%) were found to have late life-onset psychosis. The psychosis was attributed to various causes, such as dementia, depression, bipolar disorder, medical causes, delirium, medication toxicity. Two patients were diagnosed with schizophrenia and 2 were diagnosed with schizoaffective disorder (the authors did not provide additional information about the patients with schizoaffective disorder). Brenner et al14 reports a case of late-onset schizoaffective disorder in a 70-year-old female patient. Evans et al15 compared outpatients age 45 to 77 with a diagnosis of schizoaffective disorder (n = 29), schizophrenia (n = 154), or nonpsychotic mood disorder (n = 27) and concluded that late-onset schizoaffective disorder might represent a variant of LOS in clinical symptom profiles and cognitive impairment but with additional mood symptoms.16
How would you begin treating Ms. P?
a) start a mood stabilizer
b) start an atypical antipsychotic
c) obtain more history and collateral information
d) recommend outpatient treatment
The authors’ observations
Given Ms. P’s manic symptoms, thought disorder, and history of psychotic symptoms with diagnosis of LOS, we assigned her a presumptive diagnosis of schizoaffective disorder, bipolar type. From the patient report, collateral information from her mother, earlier documented collateral from her husband, and chart review, it was apparent to us that Ms. P’s psychiatric history went back only 10 years—therefore meeting temporal criteria for LOS.
Clinical assessment (Figure) and standardized tests revealed the presence of neurocognitive deficits sufficient to meet criteria for major neurocognitive disorder (Table 33). The pattern of neurocognitive deficits is consistent with an AD-like amnestic picture, although no clear-cut diagnosis was present, and the neurocognitive disorder was better classified as unspecified rather than of a particular type. It remains uncertain whether cognitive deficits of severity that meet criteria for major neurocognitive disorder are sufficiently accounted for by the diagnosis of LOS alone. Unless diagnostic criteria for schizophrenia are expanded to include cognitive deficits, a separate diagnosis of major neurocognitive disorder is warranted at present.
TREATMENT Pharmacotherapy
On the unit, Ms. P is observed by nursing staff wandering, with some pressured speech but no behavioral agitation. Her clothing had been bizarre, with multiple layers, and, at one point, she walks with her gown open and without undergarments. She also reports to the nurses that she has a lot of sexual thoughts. When the interview team enters her room, they find her masturbating.
Ms. P is started on aripiprazole, 10 mg/d, titrated to 20 mg/d, and divalproex sodium, 500 mg/d. The decision to initiate a cognitive enhancer, such as an acetylcholinesterase inhibitor or memantine, is deferred to outpatient care to allow for the possibility that her cognitive features will improve after the psychosis is treated.
By the end of first week, Ms. P’s manic features are no longer prominent but her thought process continues to be bizarre, with poor insight and judgment. She demonstrates severe ambivalence in all matters, consistently gives inconsistent accounts of the past, and makes dramatic false statements.
For example, when asked about her children, Ms. P tells us that she has 6 children—the youngest 3 months old, at home by himself and “probably dead by now.” In reality, she has only a 20-year-old son who is studying abroad. Talking about her marriage, Ms. P says she and her husband are not divorced on paper but that, because they haven’t had sex for 8 years, the law has provided them with an automatic divorce.
OUTCOME Significant improvement
Ms. P shows significant response to aripiprazole and divalproex, which are well tolerated without significant adverse effects. Her limitations in executive functioning and rational thought process lead the treatment team to consider nursing home placement under guardianship. Days before discharge, however, reexamination of her neuropsychiatric state suggests significant improvement in thought process, with improvement in cognitive features. Ms. P also becomes cooperative with treatment planning.
The treatment team has meetings with Ms. P’s mother to discuss monitoring and plans for discharge. Ms. P is discharged with follow-up arranged at community mental health services.
Bottom Line
Global as well as specific cognitive deficits are associated with late-onset schizophrenia. Studies have reported increased risk of dementia in these patients over the course of 3 to 5 years, usually unspecified or Alzheimer’s type. It is imperative to assess patients with schizophrenia, especially those age ≥40, for presence of neurocognitive disorder by means of neurocognitive testing.
Related Resources
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
- Radhakrishnan R, Butler R, Head L. Dementia in schizophrenia. Adv Psychiatr Treat. 2012;18(2):144-153.
Drug Brand Names
Aripiprazole • Abilify
Divalproex sodium • Depakote
Mematine • Namenda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturer of competing products.
1. Bleuler M. Die spätschizophrenen Krankheitsbilder. Fortschr Neurol Psychiatr. 1943;15:259-290.
2. Howard R, Rabins PV, Seeman MV, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000; 157(2):172-178.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14(1):39-55.
5. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1):4-19.
6. Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102(1-3):122-140.
7. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286-293.
8. Goldberg TE, Hyde TM, Kleinman JE, et al. Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull. 1993;19(4):797-804.
9. Brodaty H, Sachdev P, Koschera A, et al. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry. 2003;183(3):213-219.
10. Kørner A, Lopez AG, Lauritzen L, et al. Late and very-late first‐contact schizophrenia and the risk of dementia—a nationwide register based study. Int J Geriatr Psychiatry. 2009;24(1):61-67.
11. Zakzanis KK, Andrikopoulos J, Young DA, et al. Neuropsychological differentiation of late-onset schizophrenia and dementia of the Alzheimer’s type. Appl Neuropsychol. 2003;10(2):105-114.
12. Zakzanis KK, Kielar A, Young DA, et al. Neuropsychological differentiation of late onset schizophrenia and frontotemporal dementia. Cognitive Neuropsychiatry. 2001;6(1):63-77.
13. Webster J, Grossberg GT. Late-life onset of psychotic symptoms. Am J Geriatr Psychiatry. 1998;6(3):196-202.
14. Brenner R, Campbell K, Konakondla K, et al. Late onset schizoaffective disorder. Consultant. 2014;53(6):487-488.
15. Evans JD, Heaton RK, Paulsen JS, et al. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874-882.
16. Jeste DV, Blazer DG, First M. Aging-related diagnostic variations: need for diagnostic criteria appropriate for elderly psychiatric patients. Biol Psychiatry. 2005;58(4):265-271.
1. Bleuler M. Die spätschizophrenen Krankheitsbilder. Fortschr Neurol Psychiatr. 1943;15:259-290.
2. Howard R, Rabins PV, Seeman MV, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000; 157(2):172-178.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14(1):39-55.
5. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1):4-19.
6. Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102(1-3):122-140.
7. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286-293.
8. Goldberg TE, Hyde TM, Kleinman JE, et al. Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull. 1993;19(4):797-804.
9. Brodaty H, Sachdev P, Koschera A, et al. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry. 2003;183(3):213-219.
10. Kørner A, Lopez AG, Lauritzen L, et al. Late and very-late first‐contact schizophrenia and the risk of dementia—a nationwide register based study. Int J Geriatr Psychiatry. 2009;24(1):61-67.
11. Zakzanis KK, Andrikopoulos J, Young DA, et al. Neuropsychological differentiation of late-onset schizophrenia and dementia of the Alzheimer’s type. Appl Neuropsychol. 2003;10(2):105-114.
12. Zakzanis KK, Kielar A, Young DA, et al. Neuropsychological differentiation of late onset schizophrenia and frontotemporal dementia. Cognitive Neuropsychiatry. 2001;6(1):63-77.
13. Webster J, Grossberg GT. Late-life onset of psychotic symptoms. Am J Geriatr Psychiatry. 1998;6(3):196-202.
14. Brenner R, Campbell K, Konakondla K, et al. Late onset schizoaffective disorder. Consultant. 2014;53(6):487-488.
15. Evans JD, Heaton RK, Paulsen JS, et al. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874-882.
16. Jeste DV, Blazer DG, First M. Aging-related diagnostic variations: need for diagnostic criteria appropriate for elderly psychiatric patients. Biol Psychiatry. 2005;58(4):265-271.
Venlafaxine discontinuation syndrome: Prevention and management
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.