User login
Physician leadership: Racial disparities and racism. Where do we go from here?
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
COVID-19 and pregnancy: Is miscarriage a risk?

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020
Steps to leadership during the COVID-19 era and beyond
SARS CoV-2 (severe acute respiratory syndrome corona-
virus 2) has challenged us all and will continue to do so for at least the next several months. This novel virus has uncovered our medical hubris and our collective failure to acknowledge our vulnerability in the face of biological threats. As government, public health, health systems, medical professionals, and individuals struggle to grasp its enormous impact, we must recognize and seize the opportunities for leadership that the coronavirus disease (COVID-19) pandemic presents to us as physicians.
For too long we have abdicated responsibility for driving change in the US health system to politicians, administrators, and those not on the front line of care delivery. We can, however, reclaim our voice and position of influence in 2 primary spheres: first, as ObGyns we have the specific clinical knowledge and experience required to help guide our institutions in the care of our patients under new and ever-changing circumstances; second, beyond our clinical role as ObGyns, we are servant leaders to whom the public, the government, our trainees, and our clinical teams turn for guidance.
Foundations for policy development
Disaster planning in hospitals and public health systems rarely includes consideration for pregnant and delivering patients. As ObGyns, we must create policies and procedures using the best available evidence—which is slim—and, in the absence of evidence, use our clinical and scientific expertise both to optimize patient care and to minimize risk to the health care team.
At this point in time there is much we do not know, such as whether viral particles in blood are contagious, amniotic fluid contains infectious droplets, or newborns are in danger if they room-in with an infected mother. What we do know is that the evidence will evolve and that our policies and procedures must be fluid and allow for rapid change. Here are some guiding principles for such policies.
Maximize telemedicine and remote monitoring
Labor and delivery (L&D) is an emergency department in which people are triaged from the outside. Systems should incorporate the best guidance from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists while reducing infection exposure to staff, laboring patients, and newborns. One way to limit traffic in the triage area is to have a seasoned clinician perform phone triage for women who think they need evaluation for labor.
Maintain universal caution and precautions
All people entering L&D should be presumed to be COVID-19 positive, according to early evidence reported from Columbia University in New York City.1 After remote or off-site phone triage determines that evaluation is needed in L&D, a transporter could ensure that all people escorted to L&D undergo a rapid COVID-19 test, wear a mask, and wash their hands. Until point-of-care testing is available, we must adopt safety precautions, since current data suggest that asymptomatic people may shed the infectious virus.
Both vaginal and cesarean deliveries expose everyone in the room to respiratory droplets. Common sense tells us that the laboring patient and her support person should wear a mask and that caregivers should be protected with N95 masks as well as face shields. If this were standard for every laboring patient, exposure during emergency situations might be minimized.
Continue to: Maximize support during labor...
Maximize support during labor
We should not need to ban partners and support people. Solid evidence demonstrates that support in labor improves outcomes, reduces the need for cesarean delivery, and increases patient satisfaction. We can and should protect staff and patients by requiring everyone to wear a mask.
Symptomatic patients, of course, require additional measures and personal protective equipment (PPE) to reduce the risk of infection among the health care team. These should be identical to the measures the hospital infectious disease experts have implemented in the intensive care unit.
Champion continuous quality improvement
It is our responsibility to implement continuous quality improvement processes so that we can respond to data that become available, and this begins with collecting our own local data.
We have sparse data on the risks of miscarriage, congenital anomalies, and preterm birth, but there have been anecdotal reports of both early miscarriage and premature labor. Given the known increased risk for severe disease with influenza during pregnancy, we understandably are concerned about how our pregnant patients will fare. There are also unknowns with respect to fetal exposure risk. During this pandemic we must capture such data within our own systems and share aggregated, de-identified data broadly and swiftly if real signals indicate a need for change in procedures or policy.
In the meantime, we can apply our expertise and best judgment to work within teams that include all stakeholders—administrators, nurses, engineers, pediatricians, infectious disease experts, and public members—to establish policies that respond to the best current evidence.
Protect vulnerable team members
SARS CoV-2 is highly contagious. Thus far, data do not suggest that pregnant women are at higher risk for severe disease, but we must assume that working in the hospital environment among many COVID-19 patients increases the risk for exposure. With so many current unknowns, it may be prudent to keep pregnant health care workers out of clinical areas in the hospital and reassign them to other duties when feasible. Medical students nationwide similarly have been removed from clinical rotations to minimize their exposure risk as well as to preserve scarce PPE.
These decisions are difficult for all involved, and shared decision making between administrators, clinical leaders, and pregnant staff that promotes transparency, honesty, and openness is key. Since the risk is unknown and financial consequences may result for both the hospital and the staff member, open discussion and thoughtful policies that can be revised as new information is obtained will help achieve the best possible resolution to a difficult situation.
Continue to: ObGyns as servant leaders...
ObGyns as servant leaders
COVID-19 challenges us to balance individual and public health considerations while also considering the economic and social consequences of actions. The emergence of this novel pathogen and its rapid global spread are frightening both to an uninformed public and to our skeptical government officials. Beyond our immediate clinical responsibilities, how should we as knowledgeable professionals respond?
Servant leaders commit to service and support and mentor those around them with empathy and collaboration. Servant leaders have the strategic vision to continuously grow, change, and improve at all times, but especially during a crisis. COVID-19 challenges us to be those servant leaders. To do so we must:
Promote and exhibit transparency by speaking truth to power and communicating with empathy for patients, staff, and those on the front lines who daily place themselves and their families at risk to ensure that we have essential services. Amplifying the needs and concerns of the frontline workers can drive those in power to develop practical and useful solutions.
Nurses and physicians have been threatened, and some actually terminated from their positions, because they publicly disclosed their institutions’ working conditions, lack of PPE, and unpreparedness. For example, a decorated US Navy captain was stripped of his command for writing a letter to drive action in managing a COVID-19 outbreak on the confined quarters of his ship. Such public health heroes have exhibited professionalism and leadership, placing the health and well-being of their colleagues, peers, and patients above their own careers. If we all spoke up with honesty and openness, we could have profound impact.
Hold ourselves and others accountable for scientific rigor and honesty. We must acknowledge what we do not know and be straightforward in discussing risks and benefits. The uncertainty surrounding the COVID-19 public health crisis has created anxiety among health care workers, public-facing workers, government officials, and the public. We should not speculate but rather speak clearly and openly about our knowledge deficits.
The US culture in health care drives us to prefer action over inaction. “Doing something” feels proactive, and we are conditioned to think of doing something as a less risky strategy than watchful waiting. In this time of uncertainty, we must be wary of unproven and potentially harmful interventions, and we must use our best judgment and expertise to study procedures and medications that have potential benefit.
Be collaborative and creative in crafting practical workarounds that can be implemented at scale. New processes implemented in the past month to accommodate our new socially and physically distant reality include telemedicine for prenatal care, home monitoring of blood pressure, remote physiologic monitoring of blood sugars for diabetic patients, reviewing digital images to provide remote wound care, and home pulse oximetry to assess COVID-19–positive patients at home.
More workarounds are needed to support women’s ongoing health needs. Our expertise should guide those strategies while we strive to optimize outcomes, minimize resource utilization, and reduce exposure risk for ourselves, our staff, and our patients.
Advocate for systems to collect and analyze robust data so we can adjust interventions rapidly as new information arises. As we navigate the pandemic, the lack of evidence to inform decisions and treatment challenges us daily. We should use the current crisis to promote strategies that will support rapid, comprehensive data collection during disasters. Knowledge truly is power, and without it we are forced to improvise and speculate.
ObGyns must insist that data collection includes all pregnancies—not only those positive for COVID-19 since the testing has been sporadic and imperfect—and that the data are stratified by age, gender, race and ethnicity, and sociodemographics. This would enable us to learn as much as possible as quickly as possible and would therefore inform our responses for the current SARS CoV-2 pandemic as well as for the next disaster.
Continue to: Acknowledge the limitations of the system...
Acknowledge the limitations of the system and be wise stewards of resources. Our health care system does not have sufficient resources to manage patients with severe COVID-19 and the “usual” emergencies like stroke, myocardial infarction, ectopic pregnancy, and broken bones.
Disaster planning should include a regional triage system that can take incoming calls and direct emergency medical technicians, ambulances, and private citizens to appropriate facilities and direct those who do not require urgent medical care away from those facilities.
We must incorporate principles from battlefield medicine, because this is a battle, and we are at war. That means there will be difficult decisions. It is better to engage a regional team of experts to create a system for triage and care delivery than for each provider and institution to be forced by a void in leadership to go it individually. We should engage with government and public health officials to optimize both cure and care. Although we are unable to save everyone, we can work to ensure comfort and care for all.
Demonstrate compassion and caring for patients and each other. During the COVID-19 pandemic crisis, we can each channel our best selves to support and protect each other physically and emotionally. Many of us chose ObGyn because it is generally a “happy” specialty. None of us entered medicine to watch people die or to be unable to comfort them, to be unable to allow their families to be with them, to be unable to “do something.”
A crucial part of disaster planning and response is to prepare for the second victims: those of us forced to keep going through our emotional distress because there is no time to debrief and process our pain. Frontline caregivers need support and help now as well as after the surge passes. We need to speak up to ensure there is adequate PPE, creative staffing, and supportive resources to help caregivers process their anxiety, fatigue, and distress.
Take the lead
Every crisis brings both risk and opportunity. The COVID-19 pandemic provides ObGyns the chance to have a louder voice and a meaningful seat at the table as new and creative policies must be implemented at every level. We can use this opportunity to recapture our roles as champions for women and leaders within our health care system.
Critical steps in servant leadership include speaking up with honesty, transparency, and openness; taking risks to disclose inequities, dangerous conditions, and inadequate resources; and committing ourselves to each other, our teams, and the public. When we take these steps, we will be the driving force for a cohesive, reasoned, structured, and compassionate response to the COVID-19 crisis. As we seize this opportunity to lead, we will rekindle our passion for medicine, caring for the sick, and protecting the well. ●
- Sutton D, Fuchs K, D’Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery [letter]. N Engl J Med. April 13, 2020. doi:10.1056/NEJMc2009316.
SARS CoV-2 (severe acute respiratory syndrome corona-
virus 2) has challenged us all and will continue to do so for at least the next several months. This novel virus has uncovered our medical hubris and our collective failure to acknowledge our vulnerability in the face of biological threats. As government, public health, health systems, medical professionals, and individuals struggle to grasp its enormous impact, we must recognize and seize the opportunities for leadership that the coronavirus disease (COVID-19) pandemic presents to us as physicians.
For too long we have abdicated responsibility for driving change in the US health system to politicians, administrators, and those not on the front line of care delivery. We can, however, reclaim our voice and position of influence in 2 primary spheres: first, as ObGyns we have the specific clinical knowledge and experience required to help guide our institutions in the care of our patients under new and ever-changing circumstances; second, beyond our clinical role as ObGyns, we are servant leaders to whom the public, the government, our trainees, and our clinical teams turn for guidance.
Foundations for policy development
Disaster planning in hospitals and public health systems rarely includes consideration for pregnant and delivering patients. As ObGyns, we must create policies and procedures using the best available evidence—which is slim—and, in the absence of evidence, use our clinical and scientific expertise both to optimize patient care and to minimize risk to the health care team.
At this point in time there is much we do not know, such as whether viral particles in blood are contagious, amniotic fluid contains infectious droplets, or newborns are in danger if they room-in with an infected mother. What we do know is that the evidence will evolve and that our policies and procedures must be fluid and allow for rapid change. Here are some guiding principles for such policies.
Maximize telemedicine and remote monitoring
Labor and delivery (L&D) is an emergency department in which people are triaged from the outside. Systems should incorporate the best guidance from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists while reducing infection exposure to staff, laboring patients, and newborns. One way to limit traffic in the triage area is to have a seasoned clinician perform phone triage for women who think they need evaluation for labor.
Maintain universal caution and precautions
All people entering L&D should be presumed to be COVID-19 positive, according to early evidence reported from Columbia University in New York City.1 After remote or off-site phone triage determines that evaluation is needed in L&D, a transporter could ensure that all people escorted to L&D undergo a rapid COVID-19 test, wear a mask, and wash their hands. Until point-of-care testing is available, we must adopt safety precautions, since current data suggest that asymptomatic people may shed the infectious virus.
Both vaginal and cesarean deliveries expose everyone in the room to respiratory droplets. Common sense tells us that the laboring patient and her support person should wear a mask and that caregivers should be protected with N95 masks as well as face shields. If this were standard for every laboring patient, exposure during emergency situations might be minimized.
Continue to: Maximize support during labor...
Maximize support during labor
We should not need to ban partners and support people. Solid evidence demonstrates that support in labor improves outcomes, reduces the need for cesarean delivery, and increases patient satisfaction. We can and should protect staff and patients by requiring everyone to wear a mask.
Symptomatic patients, of course, require additional measures and personal protective equipment (PPE) to reduce the risk of infection among the health care team. These should be identical to the measures the hospital infectious disease experts have implemented in the intensive care unit.
Champion continuous quality improvement
It is our responsibility to implement continuous quality improvement processes so that we can respond to data that become available, and this begins with collecting our own local data.
We have sparse data on the risks of miscarriage, congenital anomalies, and preterm birth, but there have been anecdotal reports of both early miscarriage and premature labor. Given the known increased risk for severe disease with influenza during pregnancy, we understandably are concerned about how our pregnant patients will fare. There are also unknowns with respect to fetal exposure risk. During this pandemic we must capture such data within our own systems and share aggregated, de-identified data broadly and swiftly if real signals indicate a need for change in procedures or policy.
In the meantime, we can apply our expertise and best judgment to work within teams that include all stakeholders—administrators, nurses, engineers, pediatricians, infectious disease experts, and public members—to establish policies that respond to the best current evidence.
Protect vulnerable team members
SARS CoV-2 is highly contagious. Thus far, data do not suggest that pregnant women are at higher risk for severe disease, but we must assume that working in the hospital environment among many COVID-19 patients increases the risk for exposure. With so many current unknowns, it may be prudent to keep pregnant health care workers out of clinical areas in the hospital and reassign them to other duties when feasible. Medical students nationwide similarly have been removed from clinical rotations to minimize their exposure risk as well as to preserve scarce PPE.
These decisions are difficult for all involved, and shared decision making between administrators, clinical leaders, and pregnant staff that promotes transparency, honesty, and openness is key. Since the risk is unknown and financial consequences may result for both the hospital and the staff member, open discussion and thoughtful policies that can be revised as new information is obtained will help achieve the best possible resolution to a difficult situation.
Continue to: ObGyns as servant leaders...
ObGyns as servant leaders
COVID-19 challenges us to balance individual and public health considerations while also considering the economic and social consequences of actions. The emergence of this novel pathogen and its rapid global spread are frightening both to an uninformed public and to our skeptical government officials. Beyond our immediate clinical responsibilities, how should we as knowledgeable professionals respond?
Servant leaders commit to service and support and mentor those around them with empathy and collaboration. Servant leaders have the strategic vision to continuously grow, change, and improve at all times, but especially during a crisis. COVID-19 challenges us to be those servant leaders. To do so we must:
Promote and exhibit transparency by speaking truth to power and communicating with empathy for patients, staff, and those on the front lines who daily place themselves and their families at risk to ensure that we have essential services. Amplifying the needs and concerns of the frontline workers can drive those in power to develop practical and useful solutions.
Nurses and physicians have been threatened, and some actually terminated from their positions, because they publicly disclosed their institutions’ working conditions, lack of PPE, and unpreparedness. For example, a decorated US Navy captain was stripped of his command for writing a letter to drive action in managing a COVID-19 outbreak on the confined quarters of his ship. Such public health heroes have exhibited professionalism and leadership, placing the health and well-being of their colleagues, peers, and patients above their own careers. If we all spoke up with honesty and openness, we could have profound impact.
Hold ourselves and others accountable for scientific rigor and honesty. We must acknowledge what we do not know and be straightforward in discussing risks and benefits. The uncertainty surrounding the COVID-19 public health crisis has created anxiety among health care workers, public-facing workers, government officials, and the public. We should not speculate but rather speak clearly and openly about our knowledge deficits.
The US culture in health care drives us to prefer action over inaction. “Doing something” feels proactive, and we are conditioned to think of doing something as a less risky strategy than watchful waiting. In this time of uncertainty, we must be wary of unproven and potentially harmful interventions, and we must use our best judgment and expertise to study procedures and medications that have potential benefit.
Be collaborative and creative in crafting practical workarounds that can be implemented at scale. New processes implemented in the past month to accommodate our new socially and physically distant reality include telemedicine for prenatal care, home monitoring of blood pressure, remote physiologic monitoring of blood sugars for diabetic patients, reviewing digital images to provide remote wound care, and home pulse oximetry to assess COVID-19–positive patients at home.
More workarounds are needed to support women’s ongoing health needs. Our expertise should guide those strategies while we strive to optimize outcomes, minimize resource utilization, and reduce exposure risk for ourselves, our staff, and our patients.
Advocate for systems to collect and analyze robust data so we can adjust interventions rapidly as new information arises. As we navigate the pandemic, the lack of evidence to inform decisions and treatment challenges us daily. We should use the current crisis to promote strategies that will support rapid, comprehensive data collection during disasters. Knowledge truly is power, and without it we are forced to improvise and speculate.
ObGyns must insist that data collection includes all pregnancies—not only those positive for COVID-19 since the testing has been sporadic and imperfect—and that the data are stratified by age, gender, race and ethnicity, and sociodemographics. This would enable us to learn as much as possible as quickly as possible and would therefore inform our responses for the current SARS CoV-2 pandemic as well as for the next disaster.
Continue to: Acknowledge the limitations of the system...
Acknowledge the limitations of the system and be wise stewards of resources. Our health care system does not have sufficient resources to manage patients with severe COVID-19 and the “usual” emergencies like stroke, myocardial infarction, ectopic pregnancy, and broken bones.
Disaster planning should include a regional triage system that can take incoming calls and direct emergency medical technicians, ambulances, and private citizens to appropriate facilities and direct those who do not require urgent medical care away from those facilities.
We must incorporate principles from battlefield medicine, because this is a battle, and we are at war. That means there will be difficult decisions. It is better to engage a regional team of experts to create a system for triage and care delivery than for each provider and institution to be forced by a void in leadership to go it individually. We should engage with government and public health officials to optimize both cure and care. Although we are unable to save everyone, we can work to ensure comfort and care for all.
Demonstrate compassion and caring for patients and each other. During the COVID-19 pandemic crisis, we can each channel our best selves to support and protect each other physically and emotionally. Many of us chose ObGyn because it is generally a “happy” specialty. None of us entered medicine to watch people die or to be unable to comfort them, to be unable to allow their families to be with them, to be unable to “do something.”
A crucial part of disaster planning and response is to prepare for the second victims: those of us forced to keep going through our emotional distress because there is no time to debrief and process our pain. Frontline caregivers need support and help now as well as after the surge passes. We need to speak up to ensure there is adequate PPE, creative staffing, and supportive resources to help caregivers process their anxiety, fatigue, and distress.
Take the lead
Every crisis brings both risk and opportunity. The COVID-19 pandemic provides ObGyns the chance to have a louder voice and a meaningful seat at the table as new and creative policies must be implemented at every level. We can use this opportunity to recapture our roles as champions for women and leaders within our health care system.
Critical steps in servant leadership include speaking up with honesty, transparency, and openness; taking risks to disclose inequities, dangerous conditions, and inadequate resources; and committing ourselves to each other, our teams, and the public. When we take these steps, we will be the driving force for a cohesive, reasoned, structured, and compassionate response to the COVID-19 crisis. As we seize this opportunity to lead, we will rekindle our passion for medicine, caring for the sick, and protecting the well. ●
SARS CoV-2 (severe acute respiratory syndrome corona-
virus 2) has challenged us all and will continue to do so for at least the next several months. This novel virus has uncovered our medical hubris and our collective failure to acknowledge our vulnerability in the face of biological threats. As government, public health, health systems, medical professionals, and individuals struggle to grasp its enormous impact, we must recognize and seize the opportunities for leadership that the coronavirus disease (COVID-19) pandemic presents to us as physicians.
For too long we have abdicated responsibility for driving change in the US health system to politicians, administrators, and those not on the front line of care delivery. We can, however, reclaim our voice and position of influence in 2 primary spheres: first, as ObGyns we have the specific clinical knowledge and experience required to help guide our institutions in the care of our patients under new and ever-changing circumstances; second, beyond our clinical role as ObGyns, we are servant leaders to whom the public, the government, our trainees, and our clinical teams turn for guidance.
Foundations for policy development
Disaster planning in hospitals and public health systems rarely includes consideration for pregnant and delivering patients. As ObGyns, we must create policies and procedures using the best available evidence—which is slim—and, in the absence of evidence, use our clinical and scientific expertise both to optimize patient care and to minimize risk to the health care team.
At this point in time there is much we do not know, such as whether viral particles in blood are contagious, amniotic fluid contains infectious droplets, or newborns are in danger if they room-in with an infected mother. What we do know is that the evidence will evolve and that our policies and procedures must be fluid and allow for rapid change. Here are some guiding principles for such policies.
Maximize telemedicine and remote monitoring
Labor and delivery (L&D) is an emergency department in which people are triaged from the outside. Systems should incorporate the best guidance from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists while reducing infection exposure to staff, laboring patients, and newborns. One way to limit traffic in the triage area is to have a seasoned clinician perform phone triage for women who think they need evaluation for labor.
Maintain universal caution and precautions
All people entering L&D should be presumed to be COVID-19 positive, according to early evidence reported from Columbia University in New York City.1 After remote or off-site phone triage determines that evaluation is needed in L&D, a transporter could ensure that all people escorted to L&D undergo a rapid COVID-19 test, wear a mask, and wash their hands. Until point-of-care testing is available, we must adopt safety precautions, since current data suggest that asymptomatic people may shed the infectious virus.
Both vaginal and cesarean deliveries expose everyone in the room to respiratory droplets. Common sense tells us that the laboring patient and her support person should wear a mask and that caregivers should be protected with N95 masks as well as face shields. If this were standard for every laboring patient, exposure during emergency situations might be minimized.
Continue to: Maximize support during labor...
Maximize support during labor
We should not need to ban partners and support people. Solid evidence demonstrates that support in labor improves outcomes, reduces the need for cesarean delivery, and increases patient satisfaction. We can and should protect staff and patients by requiring everyone to wear a mask.
Symptomatic patients, of course, require additional measures and personal protective equipment (PPE) to reduce the risk of infection among the health care team. These should be identical to the measures the hospital infectious disease experts have implemented in the intensive care unit.
Champion continuous quality improvement
It is our responsibility to implement continuous quality improvement processes so that we can respond to data that become available, and this begins with collecting our own local data.
We have sparse data on the risks of miscarriage, congenital anomalies, and preterm birth, but there have been anecdotal reports of both early miscarriage and premature labor. Given the known increased risk for severe disease with influenza during pregnancy, we understandably are concerned about how our pregnant patients will fare. There are also unknowns with respect to fetal exposure risk. During this pandemic we must capture such data within our own systems and share aggregated, de-identified data broadly and swiftly if real signals indicate a need for change in procedures or policy.
In the meantime, we can apply our expertise and best judgment to work within teams that include all stakeholders—administrators, nurses, engineers, pediatricians, infectious disease experts, and public members—to establish policies that respond to the best current evidence.
Protect vulnerable team members
SARS CoV-2 is highly contagious. Thus far, data do not suggest that pregnant women are at higher risk for severe disease, but we must assume that working in the hospital environment among many COVID-19 patients increases the risk for exposure. With so many current unknowns, it may be prudent to keep pregnant health care workers out of clinical areas in the hospital and reassign them to other duties when feasible. Medical students nationwide similarly have been removed from clinical rotations to minimize their exposure risk as well as to preserve scarce PPE.
These decisions are difficult for all involved, and shared decision making between administrators, clinical leaders, and pregnant staff that promotes transparency, honesty, and openness is key. Since the risk is unknown and financial consequences may result for both the hospital and the staff member, open discussion and thoughtful policies that can be revised as new information is obtained will help achieve the best possible resolution to a difficult situation.
Continue to: ObGyns as servant leaders...
ObGyns as servant leaders
COVID-19 challenges us to balance individual and public health considerations while also considering the economic and social consequences of actions. The emergence of this novel pathogen and its rapid global spread are frightening both to an uninformed public and to our skeptical government officials. Beyond our immediate clinical responsibilities, how should we as knowledgeable professionals respond?
Servant leaders commit to service and support and mentor those around them with empathy and collaboration. Servant leaders have the strategic vision to continuously grow, change, and improve at all times, but especially during a crisis. COVID-19 challenges us to be those servant leaders. To do so we must:
Promote and exhibit transparency by speaking truth to power and communicating with empathy for patients, staff, and those on the front lines who daily place themselves and their families at risk to ensure that we have essential services. Amplifying the needs and concerns of the frontline workers can drive those in power to develop practical and useful solutions.
Nurses and physicians have been threatened, and some actually terminated from their positions, because they publicly disclosed their institutions’ working conditions, lack of PPE, and unpreparedness. For example, a decorated US Navy captain was stripped of his command for writing a letter to drive action in managing a COVID-19 outbreak on the confined quarters of his ship. Such public health heroes have exhibited professionalism and leadership, placing the health and well-being of their colleagues, peers, and patients above their own careers. If we all spoke up with honesty and openness, we could have profound impact.
Hold ourselves and others accountable for scientific rigor and honesty. We must acknowledge what we do not know and be straightforward in discussing risks and benefits. The uncertainty surrounding the COVID-19 public health crisis has created anxiety among health care workers, public-facing workers, government officials, and the public. We should not speculate but rather speak clearly and openly about our knowledge deficits.
The US culture in health care drives us to prefer action over inaction. “Doing something” feels proactive, and we are conditioned to think of doing something as a less risky strategy than watchful waiting. In this time of uncertainty, we must be wary of unproven and potentially harmful interventions, and we must use our best judgment and expertise to study procedures and medications that have potential benefit.
Be collaborative and creative in crafting practical workarounds that can be implemented at scale. New processes implemented in the past month to accommodate our new socially and physically distant reality include telemedicine for prenatal care, home monitoring of blood pressure, remote physiologic monitoring of blood sugars for diabetic patients, reviewing digital images to provide remote wound care, and home pulse oximetry to assess COVID-19–positive patients at home.
More workarounds are needed to support women’s ongoing health needs. Our expertise should guide those strategies while we strive to optimize outcomes, minimize resource utilization, and reduce exposure risk for ourselves, our staff, and our patients.
Advocate for systems to collect and analyze robust data so we can adjust interventions rapidly as new information arises. As we navigate the pandemic, the lack of evidence to inform decisions and treatment challenges us daily. We should use the current crisis to promote strategies that will support rapid, comprehensive data collection during disasters. Knowledge truly is power, and without it we are forced to improvise and speculate.
ObGyns must insist that data collection includes all pregnancies—not only those positive for COVID-19 since the testing has been sporadic and imperfect—and that the data are stratified by age, gender, race and ethnicity, and sociodemographics. This would enable us to learn as much as possible as quickly as possible and would therefore inform our responses for the current SARS CoV-2 pandemic as well as for the next disaster.
Continue to: Acknowledge the limitations of the system...
Acknowledge the limitations of the system and be wise stewards of resources. Our health care system does not have sufficient resources to manage patients with severe COVID-19 and the “usual” emergencies like stroke, myocardial infarction, ectopic pregnancy, and broken bones.
Disaster planning should include a regional triage system that can take incoming calls and direct emergency medical technicians, ambulances, and private citizens to appropriate facilities and direct those who do not require urgent medical care away from those facilities.
We must incorporate principles from battlefield medicine, because this is a battle, and we are at war. That means there will be difficult decisions. It is better to engage a regional team of experts to create a system for triage and care delivery than for each provider and institution to be forced by a void in leadership to go it individually. We should engage with government and public health officials to optimize both cure and care. Although we are unable to save everyone, we can work to ensure comfort and care for all.
Demonstrate compassion and caring for patients and each other. During the COVID-19 pandemic crisis, we can each channel our best selves to support and protect each other physically and emotionally. Many of us chose ObGyn because it is generally a “happy” specialty. None of us entered medicine to watch people die or to be unable to comfort them, to be unable to allow their families to be with them, to be unable to “do something.”
A crucial part of disaster planning and response is to prepare for the second victims: those of us forced to keep going through our emotional distress because there is no time to debrief and process our pain. Frontline caregivers need support and help now as well as after the surge passes. We need to speak up to ensure there is adequate PPE, creative staffing, and supportive resources to help caregivers process their anxiety, fatigue, and distress.
Take the lead
Every crisis brings both risk and opportunity. The COVID-19 pandemic provides ObGyns the chance to have a louder voice and a meaningful seat at the table as new and creative policies must be implemented at every level. We can use this opportunity to recapture our roles as champions for women and leaders within our health care system.
Critical steps in servant leadership include speaking up with honesty, transparency, and openness; taking risks to disclose inequities, dangerous conditions, and inadequate resources; and committing ourselves to each other, our teams, and the public. When we take these steps, we will be the driving force for a cohesive, reasoned, structured, and compassionate response to the COVID-19 crisis. As we seize this opportunity to lead, we will rekindle our passion for medicine, caring for the sick, and protecting the well. ●
- Sutton D, Fuchs K, D’Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery [letter]. N Engl J Med. April 13, 2020. doi:10.1056/NEJMc2009316.
- Sutton D, Fuchs K, D’Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery [letter]. N Engl J Med. April 13, 2020. doi:10.1056/NEJMc2009316.
What’s in store for ObGyn reimbursement in the EHR age and beyond
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
The HPV vaccine is now recommended for adults aged 27–45: Counseling implications
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.
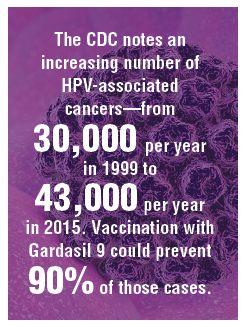
HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.
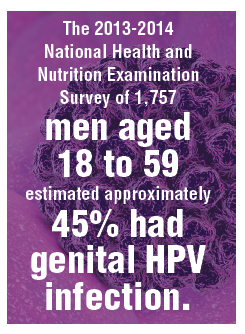
The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.

HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.

The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.

HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.

The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
What makes a quality “quality measure”?
The future of health care is value-based care. If Value equals Quality divided by Cost, then a defined, validated way to measure Quality is paramount to that equation. (Fortunately, Cost comes with convenient measurement units called dollars.) Payers now are asking health care providers to shift from a fee-for-service to a value-based reimbursement structure to encourage providers to deliver the best care at the lowest cost. Providers who can embrace this data-driven paradigm will succeed in this new environment.
So how do we define high-quality care? What makes a good quality measure? How do you actually measure what happens in a clinical encounter that impacts health outcomes?
To answer these questions, organizations have constructed standardized clinical quality measures. Clinical quality measures facilitate value-based care by providing a metric on which to measure a patient’s quality of care. They can be used 1) to decrease the overuse, underuse, and misuse of health care services and 2) to measure patient engagement and satisfaction with care.
What are quality measures?
The Academy of Medicine (formerly named the Institute of Medicine) defines health care quality as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.”1
Clearly defined components and terminology. From a quantitative standpoint, quality measures must have a clearly defined numerator and denominator and appropriate inclusions, exclusions, and exceptions. These components need to be expressed clearly in terms of publicly available terminologies, such as ICD (International Classification of Diseases) codes or SNOMED CT (Systematized Nomenclature of Medicine—Clinical Terms) terms. A measure that asks if “antihypertensive meds” have been given will not nearly be as specific as one that asks if “labetalol IV, or hydralazine IV, or nifedipine SL” has been administered. The decision to tie the data elements in a measure to administrative data, such as ICD codes, or to clinical data, such as SNOMED CT, also affects how these measures can be calculated.
Moving targets. The target of the measure also must carefully be considered. Quality measures can be used to evaluate care across the full range of health care settings—from individual providers, to care teams, to hospitals and hospital systems, to health plans. While some measures easily can be assigned to a specific provider, others are not as straightforward. For example, who gets assigned the cesarean delivery when a midwife turns the case over to an obstetrician?
Timeframe in outcomes measurement. The data infrastructure is currently set up to support measurement of immediate events, 30-day or 90-day episodes, and health insurance plan member years. Longer-term outcomes, such as over 5- and 10- year periods, are out of reach for most measures. To obtain an accurate view of the impact of medical interventions or disease conditions, however, it will be important to follow patients over time. For example, to know the failure rate of intrauterine systems, sterilization, or hormonal contraceptives, it is important to be able to track pregnancy occurrence during use of these methods for longer than 90 days. Failures can occur years after a method is initiated.
Another example is to create a performance measure focused on the overall improvement in quality of life and costs related to different treatments for abnormal uterine bleeding. How does the patient experience vary over time between treatment with hormonal contraception, endometrial ablation, or hysterectomy? Which option is most “valuable” over time when the patient experience and the cost are assessed for more than a 90-day episode? These important questions need to be answered as we maneuver into a value-based health system.
Risk adjustment. Quality measures also may need to be risk adjusted. The “My patients are sicker” refrain must be accounted for with full transparency and based on the best available data. Quality measures can be adjusted using an Observed/Expected factor, which helps to account for complicated cases.2
Clearly, social and behavioral determinants of health also play a role in these adjustments, but it can be more challenging to acquire the data elements needed for those types of adjustments. Including these data enables us to evaluate health disparities between populations, both demographically and socioeconomically.3 This is important for future development of minority inclusive quality measures. Some racial and ethnic minority populations have poorer health outcomes from preventable and treatable diseases. Evidence shows that these groups have differences in access to health care, quality of care, and health measures, including life expectancy and maternal mortality. Access to clinical data through quality measures allows for these health disparities to be brought into quantifiable perspective and assists in the development of future incentive programs to combat health inequalities and provide improved delivery of care.
Read about how to develop quality measures
Developing quality measures
Quality measures generally fall into 4 broad categories: structure, process, outcome, and patient experience (TABLE).4,5 Quality measure development begins with an assessment of the evidence, which is usually derived from clinical guidelines that link a particular process, structure, or outcome with improved patient health or experience of care. For example, the American College of Obstetricians and Gynecologists (ACOG) has developed a clinical practice guideline for screening, diagnosing, and managing gestational diabetes. The guideline addresses drug therapies, such as insulin, and alternative treatments, such as nutrition therapy. Much like the process for creating the guideline itself, translating the guideline into a quality measure requires a thoughtful, transparent, and well-defined process.
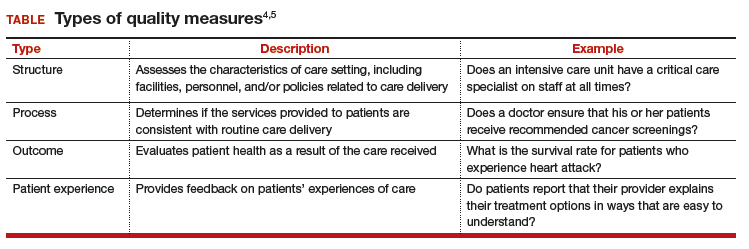
Role of the quality measure steward. Coordinating the process of translating evidence-based guidelines into quality measures requires a measure steward. Measure stewards usually are government agencies, nonprofit organizations, and/or for-profit companies. During the development process, the steward usually reaches out to additional stakeholders for feedback and consensus. Development process steps include:
- evaluation of the evidence, including the clinical practice guideline(s)
- consensus on the best measurement approach (consider the feasibility of the measurement and how it will be collected)
- development of detailed measure specifications (that is, what will be measured and how)
- feedback on the specifications from stakeholders, including professional societies and patient advocates
- testing of the measure logic and clinical validity against clinical data
- final approval by the measure steward.
Endorsement of quality measures. After a quality measure is developed, it is often endorsed by government agencies, professional societies, and/or consumer groups. Endorsement is a consensus-based process in which stakeholders evaluate a proposed measure based on established standards. Generally, stakeholders include health care professionals, consumers, payers, hospitals, health plans, and government agencies.
Evaluation of quality measures includes these important considerations:
- Are the necessary data fields available in a typical electronic health record (EHR) system?
- What is the data quality for those data fields?
- Can the measure be calculated reliably across different data sets or EHRs?
- Does the measure address one of the National Academy of Medicine quality properties? According to the academy, quality in the context of clinical care can be defined in terms of properties of effectiveness, equity, safety, efficiency, patient centeredness, and timeliness.1
Read about ACOG’s role in developing quality measures
ACOG’s role in developing quality measures
In October 2016, the Centers for Medicare and Medicaid Services released the final Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). Under this rule, the Merit-based Incentive Payment System (MIPS) was created, which was intended to drive “value” rather than “volume” in payment incentives. Measures are critical to defining value-based care. However, the law has limited or no impact on providers who do not care for Medicare patients.
Clinicians eligible to participate in MACRA must bill more than $90,000 a year in Medicare Part B allowed charges and provide care for more than 200 Medicare patients per year.6 This means that the MIPS largely overlooks ObGyns, as the bulk of our patients are insured either by private insurance or by Medicaid. However, maternity care spending is a significant part of both Medicaid and private insurers’ outlay, and both payers are actively considering using value-based financial models that will need to be fed by quality metrics. ACOG wants to be at the forefront of measure development for quality metrics that affect members and has committed resources to formation of a measure development team.
ACOG wants providers to be in control of how their practices are evaluated. For this reason, ACOG is focusing on measures that are based on clinical data entered by providers into an EHR at the point of care. At the same time, ACOG is cognizant of not increasing the documentation burden for providers. Understanding the quality of the data, as opposed to the quality of care, will be a fundamental task for the maternity care registry that ACOG is launching in 2018.
What can ObGyns do?
Quality measures are about more than just money. Public reporting of these measures on government and payer websites may influence public perception of a practice.7 The focus on patient-centered care means that patients have a voice in their care, financially as well as literally, so expect to see increased scrutiny of provider performance by patients as well as payers. One way to measure patient experience of treatments, symptoms, and quality of life is through patient-reported outcome measures (PROMs). Assessing PROMs in routine care ensures that information only the patient can provide is collected and analyzed, thus further enhancing the delivery of care and evaluating how that care is impacting the lives of your patients.
The transition from fee-for-service to a value-based system will not happen overnight, but it will happen. This transition—from being paid for the quantity of documentation to the quality of documentation—will require some change management, rethinking of workflows, and better documentation tools (such as apps instead of EHR customization).
Many in the medical profession are actively exploring these changes and new developments. These changes are too important to leave to administrators, coders, scribes, app developers, and policy makers. Someone in your practice, hospital, or health system is working on these issues today. Tomorrow, you need to be at the table. The voices of practicing ObGyns are critical as we work to address the current challenging environment in which we spend more per capita than any other nation with far inferior results. Measures that matter to us and to our patients will help us provide better and more cost-effective care that payers and patients value.8
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Academy of Sciences. Crossing the quality chasm: the IOM Health Care Quality Initiative. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Updated January 2, 2018. Accessed January 11, 2018.
- Agency for Healthcare Research and Quality. Selecting quality and resource use measures: a decision guide for community quality collaboratives. Part 2. Introduction to measures of quality (continued). https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/perfmeasguide/perfmeaspt2a.html. Reviewed 2014. Accessed December 12, 2017.
- Thomas SB, Fine MJ, Ibrahim SA. Health disparities: the importance of culture and health communication. Am J Public Health. 2004;94(12):2050.
- Agency for Healthcare Research and Quality. Types of quality measures. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/types.html. Reviewed 2011. Accessed December 12, 2017.
- Agency for Healthcare Research and Quality. Understanding quality measurement. https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/chtoolbx/understand/index.html. Reviewed November 2017. Accessed December 12, 2017.
- Centers for Medicare and Medicaid Services. Quality payment program. https://www.cms.gov/Medicare/Quality-Payment-Program/resource-library/QPP-Year-2-Final-Rule-Fact-Sheet.pdf. Published December 2017. Accessed December 12, 2017.
- Howell EA, Zeitlin J, Hebert PL, Balbierz, A, Egorova N. Association between hospital-level obstetric quality indicators and maternal and neonatal morbidity. JAMA. 2014;312(15):1531–1541.
- Tooker J. The importance of measuring quality and performance in healthcare. MedGenMed. 2005;7(2):49.
The future of health care is value-based care. If Value equals Quality divided by Cost, then a defined, validated way to measure Quality is paramount to that equation. (Fortunately, Cost comes with convenient measurement units called dollars.) Payers now are asking health care providers to shift from a fee-for-service to a value-based reimbursement structure to encourage providers to deliver the best care at the lowest cost. Providers who can embrace this data-driven paradigm will succeed in this new environment.
So how do we define high-quality care? What makes a good quality measure? How do you actually measure what happens in a clinical encounter that impacts health outcomes?
To answer these questions, organizations have constructed standardized clinical quality measures. Clinical quality measures facilitate value-based care by providing a metric on which to measure a patient’s quality of care. They can be used 1) to decrease the overuse, underuse, and misuse of health care services and 2) to measure patient engagement and satisfaction with care.
What are quality measures?
The Academy of Medicine (formerly named the Institute of Medicine) defines health care quality as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.”1
Clearly defined components and terminology. From a quantitative standpoint, quality measures must have a clearly defined numerator and denominator and appropriate inclusions, exclusions, and exceptions. These components need to be expressed clearly in terms of publicly available terminologies, such as ICD (International Classification of Diseases) codes or SNOMED CT (Systematized Nomenclature of Medicine—Clinical Terms) terms. A measure that asks if “antihypertensive meds” have been given will not nearly be as specific as one that asks if “labetalol IV, or hydralazine IV, or nifedipine SL” has been administered. The decision to tie the data elements in a measure to administrative data, such as ICD codes, or to clinical data, such as SNOMED CT, also affects how these measures can be calculated.
Moving targets. The target of the measure also must carefully be considered. Quality measures can be used to evaluate care across the full range of health care settings—from individual providers, to care teams, to hospitals and hospital systems, to health plans. While some measures easily can be assigned to a specific provider, others are not as straightforward. For example, who gets assigned the cesarean delivery when a midwife turns the case over to an obstetrician?
Timeframe in outcomes measurement. The data infrastructure is currently set up to support measurement of immediate events, 30-day or 90-day episodes, and health insurance plan member years. Longer-term outcomes, such as over 5- and 10- year periods, are out of reach for most measures. To obtain an accurate view of the impact of medical interventions or disease conditions, however, it will be important to follow patients over time. For example, to know the failure rate of intrauterine systems, sterilization, or hormonal contraceptives, it is important to be able to track pregnancy occurrence during use of these methods for longer than 90 days. Failures can occur years after a method is initiated.
Another example is to create a performance measure focused on the overall improvement in quality of life and costs related to different treatments for abnormal uterine bleeding. How does the patient experience vary over time between treatment with hormonal contraception, endometrial ablation, or hysterectomy? Which option is most “valuable” over time when the patient experience and the cost are assessed for more than a 90-day episode? These important questions need to be answered as we maneuver into a value-based health system.
Risk adjustment. Quality measures also may need to be risk adjusted. The “My patients are sicker” refrain must be accounted for with full transparency and based on the best available data. Quality measures can be adjusted using an Observed/Expected factor, which helps to account for complicated cases.2
Clearly, social and behavioral determinants of health also play a role in these adjustments, but it can be more challenging to acquire the data elements needed for those types of adjustments. Including these data enables us to evaluate health disparities between populations, both demographically and socioeconomically.3 This is important for future development of minority inclusive quality measures. Some racial and ethnic minority populations have poorer health outcomes from preventable and treatable diseases. Evidence shows that these groups have differences in access to health care, quality of care, and health measures, including life expectancy and maternal mortality. Access to clinical data through quality measures allows for these health disparities to be brought into quantifiable perspective and assists in the development of future incentive programs to combat health inequalities and provide improved delivery of care.
Read about how to develop quality measures
Developing quality measures
Quality measures generally fall into 4 broad categories: structure, process, outcome, and patient experience (TABLE).4,5 Quality measure development begins with an assessment of the evidence, which is usually derived from clinical guidelines that link a particular process, structure, or outcome with improved patient health or experience of care. For example, the American College of Obstetricians and Gynecologists (ACOG) has developed a clinical practice guideline for screening, diagnosing, and managing gestational diabetes. The guideline addresses drug therapies, such as insulin, and alternative treatments, such as nutrition therapy. Much like the process for creating the guideline itself, translating the guideline into a quality measure requires a thoughtful, transparent, and well-defined process.

Role of the quality measure steward. Coordinating the process of translating evidence-based guidelines into quality measures requires a measure steward. Measure stewards usually are government agencies, nonprofit organizations, and/or for-profit companies. During the development process, the steward usually reaches out to additional stakeholders for feedback and consensus. Development process steps include:
- evaluation of the evidence, including the clinical practice guideline(s)
- consensus on the best measurement approach (consider the feasibility of the measurement and how it will be collected)
- development of detailed measure specifications (that is, what will be measured and how)
- feedback on the specifications from stakeholders, including professional societies and patient advocates
- testing of the measure logic and clinical validity against clinical data
- final approval by the measure steward.
Endorsement of quality measures. After a quality measure is developed, it is often endorsed by government agencies, professional societies, and/or consumer groups. Endorsement is a consensus-based process in which stakeholders evaluate a proposed measure based on established standards. Generally, stakeholders include health care professionals, consumers, payers, hospitals, health plans, and government agencies.
Evaluation of quality measures includes these important considerations:
- Are the necessary data fields available in a typical electronic health record (EHR) system?
- What is the data quality for those data fields?
- Can the measure be calculated reliably across different data sets or EHRs?
- Does the measure address one of the National Academy of Medicine quality properties? According to the academy, quality in the context of clinical care can be defined in terms of properties of effectiveness, equity, safety, efficiency, patient centeredness, and timeliness.1
Read about ACOG’s role in developing quality measures
ACOG’s role in developing quality measures
In October 2016, the Centers for Medicare and Medicaid Services released the final Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). Under this rule, the Merit-based Incentive Payment System (MIPS) was created, which was intended to drive “value” rather than “volume” in payment incentives. Measures are critical to defining value-based care. However, the law has limited or no impact on providers who do not care for Medicare patients.
Clinicians eligible to participate in MACRA must bill more than $90,000 a year in Medicare Part B allowed charges and provide care for more than 200 Medicare patients per year.6 This means that the MIPS largely overlooks ObGyns, as the bulk of our patients are insured either by private insurance or by Medicaid. However, maternity care spending is a significant part of both Medicaid and private insurers’ outlay, and both payers are actively considering using value-based financial models that will need to be fed by quality metrics. ACOG wants to be at the forefront of measure development for quality metrics that affect members and has committed resources to formation of a measure development team.
ACOG wants providers to be in control of how their practices are evaluated. For this reason, ACOG is focusing on measures that are based on clinical data entered by providers into an EHR at the point of care. At the same time, ACOG is cognizant of not increasing the documentation burden for providers. Understanding the quality of the data, as opposed to the quality of care, will be a fundamental task for the maternity care registry that ACOG is launching in 2018.
What can ObGyns do?
Quality measures are about more than just money. Public reporting of these measures on government and payer websites may influence public perception of a practice.7 The focus on patient-centered care means that patients have a voice in their care, financially as well as literally, so expect to see increased scrutiny of provider performance by patients as well as payers. One way to measure patient experience of treatments, symptoms, and quality of life is through patient-reported outcome measures (PROMs). Assessing PROMs in routine care ensures that information only the patient can provide is collected and analyzed, thus further enhancing the delivery of care and evaluating how that care is impacting the lives of your patients.
The transition from fee-for-service to a value-based system will not happen overnight, but it will happen. This transition—from being paid for the quantity of documentation to the quality of documentation—will require some change management, rethinking of workflows, and better documentation tools (such as apps instead of EHR customization).
Many in the medical profession are actively exploring these changes and new developments. These changes are too important to leave to administrators, coders, scribes, app developers, and policy makers. Someone in your practice, hospital, or health system is working on these issues today. Tomorrow, you need to be at the table. The voices of practicing ObGyns are critical as we work to address the current challenging environment in which we spend more per capita than any other nation with far inferior results. Measures that matter to us and to our patients will help us provide better and more cost-effective care that payers and patients value.8
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The future of health care is value-based care. If Value equals Quality divided by Cost, then a defined, validated way to measure Quality is paramount to that equation. (Fortunately, Cost comes with convenient measurement units called dollars.) Payers now are asking health care providers to shift from a fee-for-service to a value-based reimbursement structure to encourage providers to deliver the best care at the lowest cost. Providers who can embrace this data-driven paradigm will succeed in this new environment.
So how do we define high-quality care? What makes a good quality measure? How do you actually measure what happens in a clinical encounter that impacts health outcomes?
To answer these questions, organizations have constructed standardized clinical quality measures. Clinical quality measures facilitate value-based care by providing a metric on which to measure a patient’s quality of care. They can be used 1) to decrease the overuse, underuse, and misuse of health care services and 2) to measure patient engagement and satisfaction with care.
What are quality measures?
The Academy of Medicine (formerly named the Institute of Medicine) defines health care quality as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.”1
Clearly defined components and terminology. From a quantitative standpoint, quality measures must have a clearly defined numerator and denominator and appropriate inclusions, exclusions, and exceptions. These components need to be expressed clearly in terms of publicly available terminologies, such as ICD (International Classification of Diseases) codes or SNOMED CT (Systematized Nomenclature of Medicine—Clinical Terms) terms. A measure that asks if “antihypertensive meds” have been given will not nearly be as specific as one that asks if “labetalol IV, or hydralazine IV, or nifedipine SL” has been administered. The decision to tie the data elements in a measure to administrative data, such as ICD codes, or to clinical data, such as SNOMED CT, also affects how these measures can be calculated.
Moving targets. The target of the measure also must carefully be considered. Quality measures can be used to evaluate care across the full range of health care settings—from individual providers, to care teams, to hospitals and hospital systems, to health plans. While some measures easily can be assigned to a specific provider, others are not as straightforward. For example, who gets assigned the cesarean delivery when a midwife turns the case over to an obstetrician?
Timeframe in outcomes measurement. The data infrastructure is currently set up to support measurement of immediate events, 30-day or 90-day episodes, and health insurance plan member years. Longer-term outcomes, such as over 5- and 10- year periods, are out of reach for most measures. To obtain an accurate view of the impact of medical interventions or disease conditions, however, it will be important to follow patients over time. For example, to know the failure rate of intrauterine systems, sterilization, or hormonal contraceptives, it is important to be able to track pregnancy occurrence during use of these methods for longer than 90 days. Failures can occur years after a method is initiated.
Another example is to create a performance measure focused on the overall improvement in quality of life and costs related to different treatments for abnormal uterine bleeding. How does the patient experience vary over time between treatment with hormonal contraception, endometrial ablation, or hysterectomy? Which option is most “valuable” over time when the patient experience and the cost are assessed for more than a 90-day episode? These important questions need to be answered as we maneuver into a value-based health system.
Risk adjustment. Quality measures also may need to be risk adjusted. The “My patients are sicker” refrain must be accounted for with full transparency and based on the best available data. Quality measures can be adjusted using an Observed/Expected factor, which helps to account for complicated cases.2
Clearly, social and behavioral determinants of health also play a role in these adjustments, but it can be more challenging to acquire the data elements needed for those types of adjustments. Including these data enables us to evaluate health disparities between populations, both demographically and socioeconomically.3 This is important for future development of minority inclusive quality measures. Some racial and ethnic minority populations have poorer health outcomes from preventable and treatable diseases. Evidence shows that these groups have differences in access to health care, quality of care, and health measures, including life expectancy and maternal mortality. Access to clinical data through quality measures allows for these health disparities to be brought into quantifiable perspective and assists in the development of future incentive programs to combat health inequalities and provide improved delivery of care.
Read about how to develop quality measures
Developing quality measures
Quality measures generally fall into 4 broad categories: structure, process, outcome, and patient experience (TABLE).4,5 Quality measure development begins with an assessment of the evidence, which is usually derived from clinical guidelines that link a particular process, structure, or outcome with improved patient health or experience of care. For example, the American College of Obstetricians and Gynecologists (ACOG) has developed a clinical practice guideline for screening, diagnosing, and managing gestational diabetes. The guideline addresses drug therapies, such as insulin, and alternative treatments, such as nutrition therapy. Much like the process for creating the guideline itself, translating the guideline into a quality measure requires a thoughtful, transparent, and well-defined process.

Role of the quality measure steward. Coordinating the process of translating evidence-based guidelines into quality measures requires a measure steward. Measure stewards usually are government agencies, nonprofit organizations, and/or for-profit companies. During the development process, the steward usually reaches out to additional stakeholders for feedback and consensus. Development process steps include:
- evaluation of the evidence, including the clinical practice guideline(s)
- consensus on the best measurement approach (consider the feasibility of the measurement and how it will be collected)
- development of detailed measure specifications (that is, what will be measured and how)
- feedback on the specifications from stakeholders, including professional societies and patient advocates
- testing of the measure logic and clinical validity against clinical data
- final approval by the measure steward.
Endorsement of quality measures. After a quality measure is developed, it is often endorsed by government agencies, professional societies, and/or consumer groups. Endorsement is a consensus-based process in which stakeholders evaluate a proposed measure based on established standards. Generally, stakeholders include health care professionals, consumers, payers, hospitals, health plans, and government agencies.
Evaluation of quality measures includes these important considerations:
- Are the necessary data fields available in a typical electronic health record (EHR) system?
- What is the data quality for those data fields?
- Can the measure be calculated reliably across different data sets or EHRs?
- Does the measure address one of the National Academy of Medicine quality properties? According to the academy, quality in the context of clinical care can be defined in terms of properties of effectiveness, equity, safety, efficiency, patient centeredness, and timeliness.1
Read about ACOG’s role in developing quality measures
ACOG’s role in developing quality measures
In October 2016, the Centers for Medicare and Medicaid Services released the final Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). Under this rule, the Merit-based Incentive Payment System (MIPS) was created, which was intended to drive “value” rather than “volume” in payment incentives. Measures are critical to defining value-based care. However, the law has limited or no impact on providers who do not care for Medicare patients.
Clinicians eligible to participate in MACRA must bill more than $90,000 a year in Medicare Part B allowed charges and provide care for more than 200 Medicare patients per year.6 This means that the MIPS largely overlooks ObGyns, as the bulk of our patients are insured either by private insurance or by Medicaid. However, maternity care spending is a significant part of both Medicaid and private insurers’ outlay, and both payers are actively considering using value-based financial models that will need to be fed by quality metrics. ACOG wants to be at the forefront of measure development for quality metrics that affect members and has committed resources to formation of a measure development team.
ACOG wants providers to be in control of how their practices are evaluated. For this reason, ACOG is focusing on measures that are based on clinical data entered by providers into an EHR at the point of care. At the same time, ACOG is cognizant of not increasing the documentation burden for providers. Understanding the quality of the data, as opposed to the quality of care, will be a fundamental task for the maternity care registry that ACOG is launching in 2018.
What can ObGyns do?
Quality measures are about more than just money. Public reporting of these measures on government and payer websites may influence public perception of a practice.7 The focus on patient-centered care means that patients have a voice in their care, financially as well as literally, so expect to see increased scrutiny of provider performance by patients as well as payers. One way to measure patient experience of treatments, symptoms, and quality of life is through patient-reported outcome measures (PROMs). Assessing PROMs in routine care ensures that information only the patient can provide is collected and analyzed, thus further enhancing the delivery of care and evaluating how that care is impacting the lives of your patients.
The transition from fee-for-service to a value-based system will not happen overnight, but it will happen. This transition—from being paid for the quantity of documentation to the quality of documentation—will require some change management, rethinking of workflows, and better documentation tools (such as apps instead of EHR customization).
Many in the medical profession are actively exploring these changes and new developments. These changes are too important to leave to administrators, coders, scribes, app developers, and policy makers. Someone in your practice, hospital, or health system is working on these issues today. Tomorrow, you need to be at the table. The voices of practicing ObGyns are critical as we work to address the current challenging environment in which we spend more per capita than any other nation with far inferior results. Measures that matter to us and to our patients will help us provide better and more cost-effective care that payers and patients value.8
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Academy of Sciences. Crossing the quality chasm: the IOM Health Care Quality Initiative. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Updated January 2, 2018. Accessed January 11, 2018.
- Agency for Healthcare Research and Quality. Selecting quality and resource use measures: a decision guide for community quality collaboratives. Part 2. Introduction to measures of quality (continued). https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/perfmeasguide/perfmeaspt2a.html. Reviewed 2014. Accessed December 12, 2017.
- Thomas SB, Fine MJ, Ibrahim SA. Health disparities: the importance of culture and health communication. Am J Public Health. 2004;94(12):2050.
- Agency for Healthcare Research and Quality. Types of quality measures. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/types.html. Reviewed 2011. Accessed December 12, 2017.
- Agency for Healthcare Research and Quality. Understanding quality measurement. https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/chtoolbx/understand/index.html. Reviewed November 2017. Accessed December 12, 2017.
- Centers for Medicare and Medicaid Services. Quality payment program. https://www.cms.gov/Medicare/Quality-Payment-Program/resource-library/QPP-Year-2-Final-Rule-Fact-Sheet.pdf. Published December 2017. Accessed December 12, 2017.
- Howell EA, Zeitlin J, Hebert PL, Balbierz, A, Egorova N. Association between hospital-level obstetric quality indicators and maternal and neonatal morbidity. JAMA. 2014;312(15):1531–1541.
- Tooker J. The importance of measuring quality and performance in healthcare. MedGenMed. 2005;7(2):49.
- National Academy of Sciences. Crossing the quality chasm: the IOM Health Care Quality Initiative. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Updated January 2, 2018. Accessed January 11, 2018.
- Agency for Healthcare Research and Quality. Selecting quality and resource use measures: a decision guide for community quality collaboratives. Part 2. Introduction to measures of quality (continued). https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/perfmeasguide/perfmeaspt2a.html. Reviewed 2014. Accessed December 12, 2017.
- Thomas SB, Fine MJ, Ibrahim SA. Health disparities: the importance of culture and health communication. Am J Public Health. 2004;94(12):2050.
- Agency for Healthcare Research and Quality. Types of quality measures. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/types.html. Reviewed 2011. Accessed December 12, 2017.
- Agency for Healthcare Research and Quality. Understanding quality measurement. https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/chtoolbx/understand/index.html. Reviewed November 2017. Accessed December 12, 2017.
- Centers for Medicare and Medicaid Services. Quality payment program. https://www.cms.gov/Medicare/Quality-Payment-Program/resource-library/QPP-Year-2-Final-Rule-Fact-Sheet.pdf. Published December 2017. Accessed December 12, 2017.
- Howell EA, Zeitlin J, Hebert PL, Balbierz, A, Egorova N. Association between hospital-level obstetric quality indicators and maternal and neonatal morbidity. JAMA. 2014;312(15):1531–1541.
- Tooker J. The importance of measuring quality and performance in healthcare. MedGenMed. 2005;7(2):49.
Read all parts of this series
PART 1 Value-based payment: What does it mean and how can ObGyns get out ahead
PART 2 What makes a “quality” quality measure?
PART 3 The role of patient-reported outcomes in women’s health
PART 4 It costs what?! How we can educate residents and students on how much things cost
Goodbye measures of data quantity, hello data quality measures of MACRA
Practicing clinical medicine is increasingly challenging. Besides the onslaught of new clinical information, we have credentialing, accreditation, certification, team-based care, and patient satisfaction that contribute to the complexity of current medical practice. At the heart of many of these challenges is the issue of accountability. Never has our work product as physicians been under such intense scrutiny as it is today.
To demonstrate proof of the care we have provided, we have enlisted a host of administrators, assistants, abstractors, and other helpers to decipher our work and demonstrate its value to professional organizations, boards, hospitals, insurers, and the government. They comb through our charts, decipher our handwriting and dictations, guesstimate our intentions, and sometimes devalue our care because we have not adequately documented what we have done. To solve this accountability problem, our government and the payer community have promoted the electronic health record (EHR) as the “single source of truth” for the care we provide.
This effort received a huge boost in 2009 with the Health Information Technology for Economic and Clinical Health (HITECH) Act. HITECH authorized incentive payments through Medicare and Medicaid to health care providers that could demonstrate Meaningful Use (MU) of a certified EHR. This resulted in a boom in EHR purchases and installations.
By 2012, 71.8% of office-based physicians reported using some type of EHR system, up from 34.8% in 2007.1 In many respects this action was designed as a stimulus for the slow economy, but Congress also wanted some type of accountability that the money spent to subsidize EHR purchases was going to be well spent, and would hopefully have an impact on some of the serious health issues we face.
The initial stage of this MU program seemed to work out reasonably well. So, if a little is good, more must be better, right? Unfortunately, no. But, where did MU go wrong, and how is it being fixed? Contrary to popular belief, MU is not going away, it is being transformed. To help you navigate the tethered landscape of MU past and, more importantly, bring you up to speed on MU future (the Medicare Access and CHIP Reauthorization Act of 2015 [MACRA]) and your payment incentives in this data-centric world, we address MU transformation in this article.
Where Meaningful Use stage 2 went wrong
MU stage 2 turned out to significantly increase the documentation burden on health care professionals. In addition, one of the tragic unintended consequences was that all available EHR development resources by vendors went toward meeting MU data capture requirements rather than to improving the usability and efficiency of the EHRs. Neither result has been well received by health care professionals.
Stage 3 of MU is now in place. It is an attempt to simplify the requirements and focus on quality, safety, interoperability, and patient engagement. See “Meaningful Use stage 3 specifications”. The current progression of MU stages is depicted in TABLE 1.2
Meaningful Use stage 3 specifications
Objective 1: Protect patient health information. Protect electronic health information created or maintained by the Certified Electronic Health Record Technology (CEHRT) through the implementation of appropriate technical, administrative, and physical safeguards.
Objective 2: Electronic prescribing. Eligible providers (EPs) must generate and transmit permissible prescriptions electronically, and eligible hospitals must generate and transmit permissible discharge prescriptions electronically.
Objective 3: Clinical decision support. Implement clinical decision support interventions focused on improving performance on high-priority health conditions.
Objective 4: Computerized provider order entry. Use computerized provider order entry for medication, laboratory, and diagnostic imaging orders directly entered by any licensed health care professional, credentialed medical assistant, or a medical staff member credentialed and performing the equivalent duties of a credentialed medical assistant, who can enter orders into the medical record per state, local, and professional guidelines.
Objective 5: Patient electronic access to health information. The EP provides patients (or patient-authorized representatives) with timely electronic access to their health information and patient-specific education.
Objective 6: Coordination of care through patient engagement. Use the CEHRT to engage with patients or their authorized representatives about the patient's care.
Objective 7: Health information exchange. The EP provides a summary of care record when transitioning or referring their patient to another setting of care, receives or retrieves a summary of care record upon the receipt of a transition or referral or upon the first patient encounter with a new patient, and incorporates summary of care information from other providers into their EHR using the functions of CEHRT.
Objective 8: Public health and clinical data registry reporting. The EP is in active engagement with a public health agency or clinical data registry to submit electronic public health data in a meaningful way using certified EHR technology, except where prohibited, and in accordance with applicable law and practice.
Reference
1. Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 3. Federal Register website. https://www.federalregister.gov/articles/2015/03/30/2015-06685/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-3#t-4. Accessed March 19, 2016.
Our new paradigm
Now that EHR implementation is fairly widespread, attention is focused on streamlining the reporting and documentation required for accountability, both from the data entry standpoint and the data analysis standpoint. Discrete data elements, entered by clinicians at the point of care, and downloaded directly from the EHR increasingly will be the way our patient care is assessed. Understanding this new paradigm is critical for both practice and professional viability.
Challenges in this new era
To understand the challenges ahead, we must first take a critical look at how physicians think about documentation, and what changes these models of documentation will have to undergo. Physicians are taught to think in complex models that we document as narratives or stories. While these models are composed of individual “elements” (patient age, due date, hemoglobin value, systolic blood pressure), the real information is in how these elements are related. Understanding a patient, a disease process, or a clinical workflow involves elements that must have context and relationships to be meaningful. Isolated hemoglobin or systolic blood pressure values tell us little, and may in fact obscure the forest for the trees. Physicians want to tell, and understand, the story.
However, an EHR is much more than a collection of narrative text documents. Entering data as discrete elements will allow each data element to be standardized, delegated, automated, analyzed, and monetized. In fact, these processes cannot be accomplished without the data being in this discrete form. While a common complaint about EHRs is that the “story” is hard to decipher, discrete elements are here to stay. Algorithms that can “read” a story and automatically populate these elements (known as natural language processing, or NLP) may someday allow us to go back to our dictations, but that day is frustratingly still far off.
Hello eCQMs
Up to now, physicians have relied on an army of abstractors, coders, billers, quality and safety helpers, and the like to read our notes and supply discrete data to the many clients who want to see accountability for our work. This process of course adds considerable cost to the health care system, and the data collected may not always supply accurate information. The gap between administrative data (gathered from the International Classificationof Diseases Ninth and Tenth revisions and Current Procedural Terminology [copyright American Medical Association] codes) and clinical reality is well documented.3–5
In an attempt to simplify this process, and to create a stronger connection to actual clinical data, the Centers for Medicare and Medicaid Services (CMS)6 is moving toward direct extraction of discrete data that have been entered by health care providers themselves.7 Using clinical data to report on quality metrics allows for improvement in risk adjustment as well as accuracy. Specific measures of this type have been designated eCQMs.
An eCQM is a format for a quality measure, utilizing data entered directly by health care professionals, and extracted directly from the EHR, without the need for additional personnel to review and abstract the chart. eCQMs rapidly are being phased into use for Medicare reimbursement; it is assumed that Medicaid and private payers soon will follow. Instead of payment solely for the quantity of documentation and intervention, we will soon also be paid for the quality of the care we provide (and document). TABLE 2 includes the proposed eCQM reporting timelines for Medicare and Medicaid.2
MACRA
eCQMs are a part of a larger federal effort to reform physician payments—MACRA. Over the past few years, there have been numerous federal programs to measure the quality and appropriateness of care. The Evaluation and Management (E&M) coding guidelines have been supplemented with factors for quality (Physician Quality Reporting System [PQRS]), resource use (the Value-based Payment Modifier), and EHR engagement (MU stages 1, 2, and 3). All of these programs are now being rolled up into a single program under MACRA.
MACRA has 2 distinct parts, known as the Merit-based Incentive Payment System (MIPS) and the Alternative Payment Model. MIPS keeps the underlying fee-for-service model but adds in a factor based on the following metrics:
- clinical quality (which will be based on eCQMs)
- resource use (a gauge of how many economic resources you use in comparison to your peers)
- clinical practice improvement (a measure of how well you are engaged in quality improvement, which includes capturing patient satisfaction data, and being part of a qualified clinical data registry is one way to demonstrate that engagement)
- meaningful use of EHR.
It is important to understand this last bulleted metric: MU is not going away (although that is a popular belief), it is just being transformed into MACRA, with the MU criteria simplified to emphasize a patient-centered medical record. Getting your patients involved through a portal and being able yourself to download, transmit, and accept patients’ data in electronic form are significant parts of MU. Vendors will continue to bear some of this burden, as their requirement to produce systems capable of these functions also increases their accountability.
Measurement and payment incentive
In the MIPS part of MACRA, the 4 factors of clinical quality, resource use, clinical practice improvement, and meaningful use of EHR will be combined in a formula to determine where each practitioner lies in comparison to his or her peers.
Now the bad news: Instead of receiving a bonus by meeting a benchmark, the bonus funds will be subtracted from those providers on the low end of the curve, and given to those at the top end. No matter how well the group does as a whole, no additional money will be available, and the bottom tier will be paying the bonuses of the top tier. The total pool of money to be distributed by CMS in the MIPS program will only grow by 0.5% per year for the foreseeable future. But MACRA does provide an alternative model for reimbursement, the Alternative Payment Model.
Alternative Payment Model
The Alternative Payment Model is basically an Accountable Care Organization—a group of providers agree to meet a certain standard of care (eCQMs again) and, in turn, receive a lump sum of money to deliver that care to a population. If there is some money left over at the end of a year, the group runs a profit. If not, they run a loss. One advantage of this model is that, under MACRA, the pool of money paid to “qualified” groups will increase at 5% per year for the next 5 years. This is certainly a better deal than the 0.5% increase of MIPS.
For specialists in general obstetrics and gynecology it may very well be that the volume of Medicare patients we see will be insufficient to participate meaningfully in either MIPS or the Alternative Payment Model. Regulations are still being crafted to exempt low-volume providers from the burdens associated with MACRA, and the American Congress of Obstetricians and Gynecologists (ACOG) is working diligently to advocate for systems that will allow members to see Medicare patients without requiring the substantial investments these programs likely will require.
The EHR: The single source of truth
The push to make the EHR the single source of truth will streamline many peripheral activities on the health care delivery side as well as the payer side. These requirements will present a new challenge to health care professionals, however. No one went to medical school to become a data entry clerk. Still, EHRs show the promise to transform many aspects of health care delivery. They speed communication,8 reduce errors,9 and may well improve the safety and quality of care. There also is some evidence developing that they may slow the rising cost of health care.10
But they are also quickly becoming a major source of physician dissatisfaction,11 with an apparent dose-response relationship.12 Authors of a recent RAND study note, “the current state of EHR technology significantly worsened professional satisfaction in multiple ways, due to poor usability, time-consuming data entry, interference with face-to-face patient care, inefficient and less fulfilling work content, insufficient health information exchange, and degradation of clinical documentation.”13
This pushback against EHRs has beenheard all the way to Congress. The Senate recently has introduced the ‘‘Improving Health Information Technology Act.’’14 This bill includes proposals for rating EHR systems, decreasing “unnecessary” documentation, prohibiting “information blocking,” and increasing interoperability. It remains to be seen what specific actions will be included, and how this bill will fare in an election year.
So the practice of medicine continues to evolve, and our accountability obligations show no sign of slowing down. The vision of the EHR as a single source of truth—the tool to streamline both the data entry and the data analysis—is being pushed hard by the folks who control the purse strings. This certainly will change the way we conduct our work as physicians and health care professionals. There are innovative efforts being developed to ease this burden. Cloud-based object-oriented data models, independent “apps,” open Application Programming Interfaces, or other technologies may supplant the transactional billing platforms15 we now rely upon.
ACOG is engaged at many levels with these issues, and we will continue to keep the interests of our members and the health of our patients at the center of our efforts. But it seems that, at least for now, a move to capturing discrete data elements and relying on eCQMs for quality measurements will shape the foreseeable payment incentive future.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hsiao CJ, Hing E, Ashman J. Trends in electronic health record system use among office-based physicians: United States, 2007–2012. Natl Health Stat Report. 2014;(75):1–18.
- Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 3. Federal Register website. https://www.federalregister.gov/articles/2015/03/30/2015-06685/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-3#t-4. Published March 10, 2015. Accessed March 19, 2016.
- Assareh H, Achat HM, Stubbs JM, Guevarra VM, Hill K.Incidence and variation of discrepancies in recording chronic conditions in Australian hospital administrative data. PLoS One. 2016;11(1):e0147087.
- Williams DJ, Shah SS, Myers A, et al. Identifying pediatric community-acquired pneumonia hospitalizations: Accuracy of administrative billing codes. JAMA Pediatr. 2013;167(9):851–858.
- Liede A, Hernandez RK, Roth M, Calkins G, Larrabee K, Nicacio L. Validation of International Classification of Diseases coding for bone metastases in electronic health records using technology-enabled abstraction. Clin Epidemiol. 2015;7:441–448.
- Revisions of Quality Reporting Requirements for Specific Providers, Including Changes Related to the Electronic Health Record Incentive Program. Federal Register website. https://federalregister.gov/a/2015-19049. Published August 17, 2015. Accessed March 19, 2016.
- Panjamapirom A. Hospitals: Electronic CQM Reporting Has Arrived. Are You Ready? http://www.ihealthbeat.org/perspectives/2015/hospitals-electronic-cqm-reporting-has -arrived-are-you-ready. Published August 24, 2015. Accessed March 17, 2016.
- Bernstein PS, Farinelli C, Merkatz IR. Using an electronic medical record to improve communication within a prenatal care network. Obstet Gynecol. 2005;105(3):607–612.
- George J, Bernstein PS. Using electronic medical records to reduce errors and risks in a prenatal network. Curr Opin Obstet Gynecol. 2009;21(6):527–531.
- Adler-Milstein J, Salzberg C, Franz C, Orav EJ, Newhouse JP, Bates DW. Effect of electronic health records on health care costs: longitudinal comparative evidence from community practices. Ann Intern Med. 2013;159(2):97–104.
- Pedulli L. Survey reveals widespread dissatisfaction with EHR systems. http://www.clinical-innovation.com/topics/ehr-emr/survey-reveals-widespread-dissatisfaction-ehr-systems. Published February 11, 2014. Accessed March 17, 2016.
- Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Inform Assoc. 2014;21(e1):e100–e106.
- Friedberg MW, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. RAND Corporation website. http://www.rand.org/pubs/research_reports/RR439.html. Published 2013. Accessed March 17, 2016.
- Majority and Minority Staff of the Senate Committee on Health, Education, Labor, and Pensions. Summary of Improving Health Information Technology Act. http://www.help.senate.gov/imo/media/doc/Improving%20Health%20Information%20Technology%20Act%20--%20Summary.pdf. Accessed March 18, 2016.
- LetDoctorsbeDoctors.com. http://www.letdoctorsbedoctors.com/?sf21392355=1. Published 2016. Accessed March 18, 2016.
Practicing clinical medicine is increasingly challenging. Besides the onslaught of new clinical information, we have credentialing, accreditation, certification, team-based care, and patient satisfaction that contribute to the complexity of current medical practice. At the heart of many of these challenges is the issue of accountability. Never has our work product as physicians been under such intense scrutiny as it is today.
To demonstrate proof of the care we have provided, we have enlisted a host of administrators, assistants, abstractors, and other helpers to decipher our work and demonstrate its value to professional organizations, boards, hospitals, insurers, and the government. They comb through our charts, decipher our handwriting and dictations, guesstimate our intentions, and sometimes devalue our care because we have not adequately documented what we have done. To solve this accountability problem, our government and the payer community have promoted the electronic health record (EHR) as the “single source of truth” for the care we provide.
This effort received a huge boost in 2009 with the Health Information Technology for Economic and Clinical Health (HITECH) Act. HITECH authorized incentive payments through Medicare and Medicaid to health care providers that could demonstrate Meaningful Use (MU) of a certified EHR. This resulted in a boom in EHR purchases and installations.
By 2012, 71.8% of office-based physicians reported using some type of EHR system, up from 34.8% in 2007.1 In many respects this action was designed as a stimulus for the slow economy, but Congress also wanted some type of accountability that the money spent to subsidize EHR purchases was going to be well spent, and would hopefully have an impact on some of the serious health issues we face.
The initial stage of this MU program seemed to work out reasonably well. So, if a little is good, more must be better, right? Unfortunately, no. But, where did MU go wrong, and how is it being fixed? Contrary to popular belief, MU is not going away, it is being transformed. To help you navigate the tethered landscape of MU past and, more importantly, bring you up to speed on MU future (the Medicare Access and CHIP Reauthorization Act of 2015 [MACRA]) and your payment incentives in this data-centric world, we address MU transformation in this article.
Where Meaningful Use stage 2 went wrong
MU stage 2 turned out to significantly increase the documentation burden on health care professionals. In addition, one of the tragic unintended consequences was that all available EHR development resources by vendors went toward meeting MU data capture requirements rather than to improving the usability and efficiency of the EHRs. Neither result has been well received by health care professionals.
Stage 3 of MU is now in place. It is an attempt to simplify the requirements and focus on quality, safety, interoperability, and patient engagement. See “Meaningful Use stage 3 specifications”. The current progression of MU stages is depicted in TABLE 1.2
Meaningful Use stage 3 specifications
Objective 1: Protect patient health information. Protect electronic health information created or maintained by the Certified Electronic Health Record Technology (CEHRT) through the implementation of appropriate technical, administrative, and physical safeguards.
Objective 2: Electronic prescribing. Eligible providers (EPs) must generate and transmit permissible prescriptions electronically, and eligible hospitals must generate and transmit permissible discharge prescriptions electronically.
Objective 3: Clinical decision support. Implement clinical decision support interventions focused on improving performance on high-priority health conditions.
Objective 4: Computerized provider order entry. Use computerized provider order entry for medication, laboratory, and diagnostic imaging orders directly entered by any licensed health care professional, credentialed medical assistant, or a medical staff member credentialed and performing the equivalent duties of a credentialed medical assistant, who can enter orders into the medical record per state, local, and professional guidelines.
Objective 5: Patient electronic access to health information. The EP provides patients (or patient-authorized representatives) with timely electronic access to their health information and patient-specific education.
Objective 6: Coordination of care through patient engagement. Use the CEHRT to engage with patients or their authorized representatives about the patient's care.
Objective 7: Health information exchange. The EP provides a summary of care record when transitioning or referring their patient to another setting of care, receives or retrieves a summary of care record upon the receipt of a transition or referral or upon the first patient encounter with a new patient, and incorporates summary of care information from other providers into their EHR using the functions of CEHRT.
Objective 8: Public health and clinical data registry reporting. The EP is in active engagement with a public health agency or clinical data registry to submit electronic public health data in a meaningful way using certified EHR technology, except where prohibited, and in accordance with applicable law and practice.
Reference
1. Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 3. Federal Register website. https://www.federalregister.gov/articles/2015/03/30/2015-06685/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-3#t-4. Accessed March 19, 2016.
Our new paradigm
Now that EHR implementation is fairly widespread, attention is focused on streamlining the reporting and documentation required for accountability, both from the data entry standpoint and the data analysis standpoint. Discrete data elements, entered by clinicians at the point of care, and downloaded directly from the EHR increasingly will be the way our patient care is assessed. Understanding this new paradigm is critical for both practice and professional viability.
Challenges in this new era
To understand the challenges ahead, we must first take a critical look at how physicians think about documentation, and what changes these models of documentation will have to undergo. Physicians are taught to think in complex models that we document as narratives or stories. While these models are composed of individual “elements” (patient age, due date, hemoglobin value, systolic blood pressure), the real information is in how these elements are related. Understanding a patient, a disease process, or a clinical workflow involves elements that must have context and relationships to be meaningful. Isolated hemoglobin or systolic blood pressure values tell us little, and may in fact obscure the forest for the trees. Physicians want to tell, and understand, the story.
However, an EHR is much more than a collection of narrative text documents. Entering data as discrete elements will allow each data element to be standardized, delegated, automated, analyzed, and monetized. In fact, these processes cannot be accomplished without the data being in this discrete form. While a common complaint about EHRs is that the “story” is hard to decipher, discrete elements are here to stay. Algorithms that can “read” a story and automatically populate these elements (known as natural language processing, or NLP) may someday allow us to go back to our dictations, but that day is frustratingly still far off.
Hello eCQMs
Up to now, physicians have relied on an army of abstractors, coders, billers, quality and safety helpers, and the like to read our notes and supply discrete data to the many clients who want to see accountability for our work. This process of course adds considerable cost to the health care system, and the data collected may not always supply accurate information. The gap between administrative data (gathered from the International Classificationof Diseases Ninth and Tenth revisions and Current Procedural Terminology [copyright American Medical Association] codes) and clinical reality is well documented.3–5
In an attempt to simplify this process, and to create a stronger connection to actual clinical data, the Centers for Medicare and Medicaid Services (CMS)6 is moving toward direct extraction of discrete data that have been entered by health care providers themselves.7 Using clinical data to report on quality metrics allows for improvement in risk adjustment as well as accuracy. Specific measures of this type have been designated eCQMs.
An eCQM is a format for a quality measure, utilizing data entered directly by health care professionals, and extracted directly from the EHR, without the need for additional personnel to review and abstract the chart. eCQMs rapidly are being phased into use for Medicare reimbursement; it is assumed that Medicaid and private payers soon will follow. Instead of payment solely for the quantity of documentation and intervention, we will soon also be paid for the quality of the care we provide (and document). TABLE 2 includes the proposed eCQM reporting timelines for Medicare and Medicaid.2
MACRA
eCQMs are a part of a larger federal effort to reform physician payments—MACRA. Over the past few years, there have been numerous federal programs to measure the quality and appropriateness of care. The Evaluation and Management (E&M) coding guidelines have been supplemented with factors for quality (Physician Quality Reporting System [PQRS]), resource use (the Value-based Payment Modifier), and EHR engagement (MU stages 1, 2, and 3). All of these programs are now being rolled up into a single program under MACRA.
MACRA has 2 distinct parts, known as the Merit-based Incentive Payment System (MIPS) and the Alternative Payment Model. MIPS keeps the underlying fee-for-service model but adds in a factor based on the following metrics:
- clinical quality (which will be based on eCQMs)
- resource use (a gauge of how many economic resources you use in comparison to your peers)
- clinical practice improvement (a measure of how well you are engaged in quality improvement, which includes capturing patient satisfaction data, and being part of a qualified clinical data registry is one way to demonstrate that engagement)
- meaningful use of EHR.
It is important to understand this last bulleted metric: MU is not going away (although that is a popular belief), it is just being transformed into MACRA, with the MU criteria simplified to emphasize a patient-centered medical record. Getting your patients involved through a portal and being able yourself to download, transmit, and accept patients’ data in electronic form are significant parts of MU. Vendors will continue to bear some of this burden, as their requirement to produce systems capable of these functions also increases their accountability.
Measurement and payment incentive
In the MIPS part of MACRA, the 4 factors of clinical quality, resource use, clinical practice improvement, and meaningful use of EHR will be combined in a formula to determine where each practitioner lies in comparison to his or her peers.
Now the bad news: Instead of receiving a bonus by meeting a benchmark, the bonus funds will be subtracted from those providers on the low end of the curve, and given to those at the top end. No matter how well the group does as a whole, no additional money will be available, and the bottom tier will be paying the bonuses of the top tier. The total pool of money to be distributed by CMS in the MIPS program will only grow by 0.5% per year for the foreseeable future. But MACRA does provide an alternative model for reimbursement, the Alternative Payment Model.
Alternative Payment Model
The Alternative Payment Model is basically an Accountable Care Organization—a group of providers agree to meet a certain standard of care (eCQMs again) and, in turn, receive a lump sum of money to deliver that care to a population. If there is some money left over at the end of a year, the group runs a profit. If not, they run a loss. One advantage of this model is that, under MACRA, the pool of money paid to “qualified” groups will increase at 5% per year for the next 5 years. This is certainly a better deal than the 0.5% increase of MIPS.
For specialists in general obstetrics and gynecology it may very well be that the volume of Medicare patients we see will be insufficient to participate meaningfully in either MIPS or the Alternative Payment Model. Regulations are still being crafted to exempt low-volume providers from the burdens associated with MACRA, and the American Congress of Obstetricians and Gynecologists (ACOG) is working diligently to advocate for systems that will allow members to see Medicare patients without requiring the substantial investments these programs likely will require.
The EHR: The single source of truth
The push to make the EHR the single source of truth will streamline many peripheral activities on the health care delivery side as well as the payer side. These requirements will present a new challenge to health care professionals, however. No one went to medical school to become a data entry clerk. Still, EHRs show the promise to transform many aspects of health care delivery. They speed communication,8 reduce errors,9 and may well improve the safety and quality of care. There also is some evidence developing that they may slow the rising cost of health care.10
But they are also quickly becoming a major source of physician dissatisfaction,11 with an apparent dose-response relationship.12 Authors of a recent RAND study note, “the current state of EHR technology significantly worsened professional satisfaction in multiple ways, due to poor usability, time-consuming data entry, interference with face-to-face patient care, inefficient and less fulfilling work content, insufficient health information exchange, and degradation of clinical documentation.”13
This pushback against EHRs has beenheard all the way to Congress. The Senate recently has introduced the ‘‘Improving Health Information Technology Act.’’14 This bill includes proposals for rating EHR systems, decreasing “unnecessary” documentation, prohibiting “information blocking,” and increasing interoperability. It remains to be seen what specific actions will be included, and how this bill will fare in an election year.
So the practice of medicine continues to evolve, and our accountability obligations show no sign of slowing down. The vision of the EHR as a single source of truth—the tool to streamline both the data entry and the data analysis—is being pushed hard by the folks who control the purse strings. This certainly will change the way we conduct our work as physicians and health care professionals. There are innovative efforts being developed to ease this burden. Cloud-based object-oriented data models, independent “apps,” open Application Programming Interfaces, or other technologies may supplant the transactional billing platforms15 we now rely upon.
ACOG is engaged at many levels with these issues, and we will continue to keep the interests of our members and the health of our patients at the center of our efforts. But it seems that, at least for now, a move to capturing discrete data elements and relying on eCQMs for quality measurements will shape the foreseeable payment incentive future.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Practicing clinical medicine is increasingly challenging. Besides the onslaught of new clinical information, we have credentialing, accreditation, certification, team-based care, and patient satisfaction that contribute to the complexity of current medical practice. At the heart of many of these challenges is the issue of accountability. Never has our work product as physicians been under such intense scrutiny as it is today.
To demonstrate proof of the care we have provided, we have enlisted a host of administrators, assistants, abstractors, and other helpers to decipher our work and demonstrate its value to professional organizations, boards, hospitals, insurers, and the government. They comb through our charts, decipher our handwriting and dictations, guesstimate our intentions, and sometimes devalue our care because we have not adequately documented what we have done. To solve this accountability problem, our government and the payer community have promoted the electronic health record (EHR) as the “single source of truth” for the care we provide.
This effort received a huge boost in 2009 with the Health Information Technology for Economic and Clinical Health (HITECH) Act. HITECH authorized incentive payments through Medicare and Medicaid to health care providers that could demonstrate Meaningful Use (MU) of a certified EHR. This resulted in a boom in EHR purchases and installations.
By 2012, 71.8% of office-based physicians reported using some type of EHR system, up from 34.8% in 2007.1 In many respects this action was designed as a stimulus for the slow economy, but Congress also wanted some type of accountability that the money spent to subsidize EHR purchases was going to be well spent, and would hopefully have an impact on some of the serious health issues we face.
The initial stage of this MU program seemed to work out reasonably well. So, if a little is good, more must be better, right? Unfortunately, no. But, where did MU go wrong, and how is it being fixed? Contrary to popular belief, MU is not going away, it is being transformed. To help you navigate the tethered landscape of MU past and, more importantly, bring you up to speed on MU future (the Medicare Access and CHIP Reauthorization Act of 2015 [MACRA]) and your payment incentives in this data-centric world, we address MU transformation in this article.
Where Meaningful Use stage 2 went wrong
MU stage 2 turned out to significantly increase the documentation burden on health care professionals. In addition, one of the tragic unintended consequences was that all available EHR development resources by vendors went toward meeting MU data capture requirements rather than to improving the usability and efficiency of the EHRs. Neither result has been well received by health care professionals.
Stage 3 of MU is now in place. It is an attempt to simplify the requirements and focus on quality, safety, interoperability, and patient engagement. See “Meaningful Use stage 3 specifications”. The current progression of MU stages is depicted in TABLE 1.2
Meaningful Use stage 3 specifications
Objective 1: Protect patient health information. Protect electronic health information created or maintained by the Certified Electronic Health Record Technology (CEHRT) through the implementation of appropriate technical, administrative, and physical safeguards.
Objective 2: Electronic prescribing. Eligible providers (EPs) must generate and transmit permissible prescriptions electronically, and eligible hospitals must generate and transmit permissible discharge prescriptions electronically.
Objective 3: Clinical decision support. Implement clinical decision support interventions focused on improving performance on high-priority health conditions.
Objective 4: Computerized provider order entry. Use computerized provider order entry for medication, laboratory, and diagnostic imaging orders directly entered by any licensed health care professional, credentialed medical assistant, or a medical staff member credentialed and performing the equivalent duties of a credentialed medical assistant, who can enter orders into the medical record per state, local, and professional guidelines.
Objective 5: Patient electronic access to health information. The EP provides patients (or patient-authorized representatives) with timely electronic access to their health information and patient-specific education.
Objective 6: Coordination of care through patient engagement. Use the CEHRT to engage with patients or their authorized representatives about the patient's care.
Objective 7: Health information exchange. The EP provides a summary of care record when transitioning or referring their patient to another setting of care, receives or retrieves a summary of care record upon the receipt of a transition or referral or upon the first patient encounter with a new patient, and incorporates summary of care information from other providers into their EHR using the functions of CEHRT.
Objective 8: Public health and clinical data registry reporting. The EP is in active engagement with a public health agency or clinical data registry to submit electronic public health data in a meaningful way using certified EHR technology, except where prohibited, and in accordance with applicable law and practice.
Reference
1. Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 3. Federal Register website. https://www.federalregister.gov/articles/2015/03/30/2015-06685/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-3#t-4. Accessed March 19, 2016.
Our new paradigm
Now that EHR implementation is fairly widespread, attention is focused on streamlining the reporting and documentation required for accountability, both from the data entry standpoint and the data analysis standpoint. Discrete data elements, entered by clinicians at the point of care, and downloaded directly from the EHR increasingly will be the way our patient care is assessed. Understanding this new paradigm is critical for both practice and professional viability.
Challenges in this new era
To understand the challenges ahead, we must first take a critical look at how physicians think about documentation, and what changes these models of documentation will have to undergo. Physicians are taught to think in complex models that we document as narratives or stories. While these models are composed of individual “elements” (patient age, due date, hemoglobin value, systolic blood pressure), the real information is in how these elements are related. Understanding a patient, a disease process, or a clinical workflow involves elements that must have context and relationships to be meaningful. Isolated hemoglobin or systolic blood pressure values tell us little, and may in fact obscure the forest for the trees. Physicians want to tell, and understand, the story.
However, an EHR is much more than a collection of narrative text documents. Entering data as discrete elements will allow each data element to be standardized, delegated, automated, analyzed, and monetized. In fact, these processes cannot be accomplished without the data being in this discrete form. While a common complaint about EHRs is that the “story” is hard to decipher, discrete elements are here to stay. Algorithms that can “read” a story and automatically populate these elements (known as natural language processing, or NLP) may someday allow us to go back to our dictations, but that day is frustratingly still far off.
Hello eCQMs
Up to now, physicians have relied on an army of abstractors, coders, billers, quality and safety helpers, and the like to read our notes and supply discrete data to the many clients who want to see accountability for our work. This process of course adds considerable cost to the health care system, and the data collected may not always supply accurate information. The gap between administrative data (gathered from the International Classificationof Diseases Ninth and Tenth revisions and Current Procedural Terminology [copyright American Medical Association] codes) and clinical reality is well documented.3–5
In an attempt to simplify this process, and to create a stronger connection to actual clinical data, the Centers for Medicare and Medicaid Services (CMS)6 is moving toward direct extraction of discrete data that have been entered by health care providers themselves.7 Using clinical data to report on quality metrics allows for improvement in risk adjustment as well as accuracy. Specific measures of this type have been designated eCQMs.
An eCQM is a format for a quality measure, utilizing data entered directly by health care professionals, and extracted directly from the EHR, without the need for additional personnel to review and abstract the chart. eCQMs rapidly are being phased into use for Medicare reimbursement; it is assumed that Medicaid and private payers soon will follow. Instead of payment solely for the quantity of documentation and intervention, we will soon also be paid for the quality of the care we provide (and document). TABLE 2 includes the proposed eCQM reporting timelines for Medicare and Medicaid.2
MACRA
eCQMs are a part of a larger federal effort to reform physician payments—MACRA. Over the past few years, there have been numerous federal programs to measure the quality and appropriateness of care. The Evaluation and Management (E&M) coding guidelines have been supplemented with factors for quality (Physician Quality Reporting System [PQRS]), resource use (the Value-based Payment Modifier), and EHR engagement (MU stages 1, 2, and 3). All of these programs are now being rolled up into a single program under MACRA.
MACRA has 2 distinct parts, known as the Merit-based Incentive Payment System (MIPS) and the Alternative Payment Model. MIPS keeps the underlying fee-for-service model but adds in a factor based on the following metrics:
- clinical quality (which will be based on eCQMs)
- resource use (a gauge of how many economic resources you use in comparison to your peers)
- clinical practice improvement (a measure of how well you are engaged in quality improvement, which includes capturing patient satisfaction data, and being part of a qualified clinical data registry is one way to demonstrate that engagement)
- meaningful use of EHR.
It is important to understand this last bulleted metric: MU is not going away (although that is a popular belief), it is just being transformed into MACRA, with the MU criteria simplified to emphasize a patient-centered medical record. Getting your patients involved through a portal and being able yourself to download, transmit, and accept patients’ data in electronic form are significant parts of MU. Vendors will continue to bear some of this burden, as their requirement to produce systems capable of these functions also increases their accountability.
Measurement and payment incentive
In the MIPS part of MACRA, the 4 factors of clinical quality, resource use, clinical practice improvement, and meaningful use of EHR will be combined in a formula to determine where each practitioner lies in comparison to his or her peers.
Now the bad news: Instead of receiving a bonus by meeting a benchmark, the bonus funds will be subtracted from those providers on the low end of the curve, and given to those at the top end. No matter how well the group does as a whole, no additional money will be available, and the bottom tier will be paying the bonuses of the top tier. The total pool of money to be distributed by CMS in the MIPS program will only grow by 0.5% per year for the foreseeable future. But MACRA does provide an alternative model for reimbursement, the Alternative Payment Model.
Alternative Payment Model
The Alternative Payment Model is basically an Accountable Care Organization—a group of providers agree to meet a certain standard of care (eCQMs again) and, in turn, receive a lump sum of money to deliver that care to a population. If there is some money left over at the end of a year, the group runs a profit. If not, they run a loss. One advantage of this model is that, under MACRA, the pool of money paid to “qualified” groups will increase at 5% per year for the next 5 years. This is certainly a better deal than the 0.5% increase of MIPS.
For specialists in general obstetrics and gynecology it may very well be that the volume of Medicare patients we see will be insufficient to participate meaningfully in either MIPS or the Alternative Payment Model. Regulations are still being crafted to exempt low-volume providers from the burdens associated with MACRA, and the American Congress of Obstetricians and Gynecologists (ACOG) is working diligently to advocate for systems that will allow members to see Medicare patients without requiring the substantial investments these programs likely will require.
The EHR: The single source of truth
The push to make the EHR the single source of truth will streamline many peripheral activities on the health care delivery side as well as the payer side. These requirements will present a new challenge to health care professionals, however. No one went to medical school to become a data entry clerk. Still, EHRs show the promise to transform many aspects of health care delivery. They speed communication,8 reduce errors,9 and may well improve the safety and quality of care. There also is some evidence developing that they may slow the rising cost of health care.10
But they are also quickly becoming a major source of physician dissatisfaction,11 with an apparent dose-response relationship.12 Authors of a recent RAND study note, “the current state of EHR technology significantly worsened professional satisfaction in multiple ways, due to poor usability, time-consuming data entry, interference with face-to-face patient care, inefficient and less fulfilling work content, insufficient health information exchange, and degradation of clinical documentation.”13
This pushback against EHRs has beenheard all the way to Congress. The Senate recently has introduced the ‘‘Improving Health Information Technology Act.’’14 This bill includes proposals for rating EHR systems, decreasing “unnecessary” documentation, prohibiting “information blocking,” and increasing interoperability. It remains to be seen what specific actions will be included, and how this bill will fare in an election year.
So the practice of medicine continues to evolve, and our accountability obligations show no sign of slowing down. The vision of the EHR as a single source of truth—the tool to streamline both the data entry and the data analysis—is being pushed hard by the folks who control the purse strings. This certainly will change the way we conduct our work as physicians and health care professionals. There are innovative efforts being developed to ease this burden. Cloud-based object-oriented data models, independent “apps,” open Application Programming Interfaces, or other technologies may supplant the transactional billing platforms15 we now rely upon.
ACOG is engaged at many levels with these issues, and we will continue to keep the interests of our members and the health of our patients at the center of our efforts. But it seems that, at least for now, a move to capturing discrete data elements and relying on eCQMs for quality measurements will shape the foreseeable payment incentive future.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hsiao CJ, Hing E, Ashman J. Trends in electronic health record system use among office-based physicians: United States, 2007–2012. Natl Health Stat Report. 2014;(75):1–18.
- Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 3. Federal Register website. https://www.federalregister.gov/articles/2015/03/30/2015-06685/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-3#t-4. Published March 10, 2015. Accessed March 19, 2016.
- Assareh H, Achat HM, Stubbs JM, Guevarra VM, Hill K.Incidence and variation of discrepancies in recording chronic conditions in Australian hospital administrative data. PLoS One. 2016;11(1):e0147087.
- Williams DJ, Shah SS, Myers A, et al. Identifying pediatric community-acquired pneumonia hospitalizations: Accuracy of administrative billing codes. JAMA Pediatr. 2013;167(9):851–858.
- Liede A, Hernandez RK, Roth M, Calkins G, Larrabee K, Nicacio L. Validation of International Classification of Diseases coding for bone metastases in electronic health records using technology-enabled abstraction. Clin Epidemiol. 2015;7:441–448.
- Revisions of Quality Reporting Requirements for Specific Providers, Including Changes Related to the Electronic Health Record Incentive Program. Federal Register website. https://federalregister.gov/a/2015-19049. Published August 17, 2015. Accessed March 19, 2016.
- Panjamapirom A. Hospitals: Electronic CQM Reporting Has Arrived. Are You Ready? http://www.ihealthbeat.org/perspectives/2015/hospitals-electronic-cqm-reporting-has -arrived-are-you-ready. Published August 24, 2015. Accessed March 17, 2016.
- Bernstein PS, Farinelli C, Merkatz IR. Using an electronic medical record to improve communication within a prenatal care network. Obstet Gynecol. 2005;105(3):607–612.
- George J, Bernstein PS. Using electronic medical records to reduce errors and risks in a prenatal network. Curr Opin Obstet Gynecol. 2009;21(6):527–531.
- Adler-Milstein J, Salzberg C, Franz C, Orav EJ, Newhouse JP, Bates DW. Effect of electronic health records on health care costs: longitudinal comparative evidence from community practices. Ann Intern Med. 2013;159(2):97–104.
- Pedulli L. Survey reveals widespread dissatisfaction with EHR systems. http://www.clinical-innovation.com/topics/ehr-emr/survey-reveals-widespread-dissatisfaction-ehr-systems. Published February 11, 2014. Accessed March 17, 2016.
- Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Inform Assoc. 2014;21(e1):e100–e106.
- Friedberg MW, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. RAND Corporation website. http://www.rand.org/pubs/research_reports/RR439.html. Published 2013. Accessed March 17, 2016.
- Majority and Minority Staff of the Senate Committee on Health, Education, Labor, and Pensions. Summary of Improving Health Information Technology Act. http://www.help.senate.gov/imo/media/doc/Improving%20Health%20Information%20Technology%20Act%20--%20Summary.pdf. Accessed March 18, 2016.
- LetDoctorsbeDoctors.com. http://www.letdoctorsbedoctors.com/?sf21392355=1. Published 2016. Accessed March 18, 2016.
- Hsiao CJ, Hing E, Ashman J. Trends in electronic health record system use among office-based physicians: United States, 2007–2012. Natl Health Stat Report. 2014;(75):1–18.
- Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 3. Federal Register website. https://www.federalregister.gov/articles/2015/03/30/2015-06685/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-3#t-4. Published March 10, 2015. Accessed March 19, 2016.
- Assareh H, Achat HM, Stubbs JM, Guevarra VM, Hill K.Incidence and variation of discrepancies in recording chronic conditions in Australian hospital administrative data. PLoS One. 2016;11(1):e0147087.
- Williams DJ, Shah SS, Myers A, et al. Identifying pediatric community-acquired pneumonia hospitalizations: Accuracy of administrative billing codes. JAMA Pediatr. 2013;167(9):851–858.
- Liede A, Hernandez RK, Roth M, Calkins G, Larrabee K, Nicacio L. Validation of International Classification of Diseases coding for bone metastases in electronic health records using technology-enabled abstraction. Clin Epidemiol. 2015;7:441–448.
- Revisions of Quality Reporting Requirements for Specific Providers, Including Changes Related to the Electronic Health Record Incentive Program. Federal Register website. https://federalregister.gov/a/2015-19049. Published August 17, 2015. Accessed March 19, 2016.
- Panjamapirom A. Hospitals: Electronic CQM Reporting Has Arrived. Are You Ready? http://www.ihealthbeat.org/perspectives/2015/hospitals-electronic-cqm-reporting-has -arrived-are-you-ready. Published August 24, 2015. Accessed March 17, 2016.
- Bernstein PS, Farinelli C, Merkatz IR. Using an electronic medical record to improve communication within a prenatal care network. Obstet Gynecol. 2005;105(3):607–612.
- George J, Bernstein PS. Using electronic medical records to reduce errors and risks in a prenatal network. Curr Opin Obstet Gynecol. 2009;21(6):527–531.
- Adler-Milstein J, Salzberg C, Franz C, Orav EJ, Newhouse JP, Bates DW. Effect of electronic health records on health care costs: longitudinal comparative evidence from community practices. Ann Intern Med. 2013;159(2):97–104.
- Pedulli L. Survey reveals widespread dissatisfaction with EHR systems. http://www.clinical-innovation.com/topics/ehr-emr/survey-reveals-widespread-dissatisfaction-ehr-systems. Published February 11, 2014. Accessed March 17, 2016.
- Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Inform Assoc. 2014;21(e1):e100–e106.
- Friedberg MW, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. RAND Corporation website. http://www.rand.org/pubs/research_reports/RR439.html. Published 2013. Accessed March 17, 2016.
- Majority and Minority Staff of the Senate Committee on Health, Education, Labor, and Pensions. Summary of Improving Health Information Technology Act. http://www.help.senate.gov/imo/media/doc/Improving%20Health%20Information%20Technology%20Act%20--%20Summary.pdf. Accessed March 18, 2016.
- LetDoctorsbeDoctors.com. http://www.letdoctorsbedoctors.com/?sf21392355=1. Published 2016. Accessed March 18, 2016.
Sildenafil for anorgasmia
THE QUESTION:
Is sildenafil an effective treatment for arousal disorder in premenopausal women?
Past studies:
These have shown that sildenafil is not as promising in women as in men. However, previous trials examined its usefulness in postmenopausal women. Little research has been conducted in premenopausal women.
This study:
The benefits of sildenafil treatment were examined in 53 women, ages 22 to 38, who had developed arousal disorders and were unable to achieve orgasm. Included in the trial, were heterosexual women who had been incapable of experiencing vaginal lubrication or genital sensation for a period of 6 months or more. Normal ovarian function was documented by ultrasonography. Women on oral contraceptives or hormone therapy and those who did not have a sexual partner were excluded from the trial. Also, women with medical conditions, including hypertension, diabetes, cancer, alcohol abuse, and liver disease, could not enroll in the study.
In a double-blind crossover study design, patients were followed monthly for 3 months and treated with 25 mg of sildenafil, 50 mg of sildenafil, and placebo in a randomized fashion. Each participant took either dose of sildenafil or placebo 1 hour before planned intercourse. The researchers used a 5-point scale to detect changes in sexual behavior. The results: Women reported increased sexual arousal, enjoyment, and frequency of sexual fantasies with both doses of sildenafil compared with placebo (4.2 versus 2.6, respectively). The frequency of orgasm also improved significantly with both doses of sildenafil compared with placebo (3.8 versus 2.4, respectively).
Find this study:
June 2001 issue of British Journal of Obstetrics and Gynaecology; abstract online at www.elsevier.com.
Who may be affected by these findings?
Healthy young women with normal hormones and libido experiencing sexual arousal disorders. Additionally, postmenopausal women with normal sexual desire, steady partners, and hormones replaced to normal levels may respond well to sildenafil. Clearly, future research is needed in this postmenopausal population.
Expert commentary:
As female sexual dysfunction is discussed more widely on the talk show circuit, women are being encouraged to seek medical attention and treatment for this complex problem. Unfortunately, little research exists to better understand and treat sexual dissatisfaction among women, specifically arousal and orgasmic disorders. This trial, however, is one step toward the development of effective therapeutic strategies to combat these issues. The researchers successfully isolated arousal deficiencies from desire and orgasmic disorders in a group of young premenopausal women. The participants responded well to a treatment series of sildenafil. The medication regulates smooth muscle contractions by selectively blocking phosphodiesterase type 5. It also permits vasodilation, resulting in subsequent clitoral, vulvar, and vaginal erectile tissue engorgement.
The results of this trial are most interesting in that previous trials of sildenafil did not demonstrate any improvement in postmenopausal women with sexual dysfunction. However, those trials did not define arousal disorder as strictly as the authors of this study. Based on sildenafil’s mechanism of action, postmenopausal women with vascular disease, hypertension, and diabetes—a population similar to males with erectile dysfunction—who have normal libido and hormones, may be ideal candidates for sildenafil therapy.
Caveats:
In this study, 4 women stopped taking sildenafil 50 mg, 2 women stopped taking sildenafil 25 mg, and 2 women stopped taking placebo due to vision problems, headaches, and fear of adverse reactions.
The bottom line:
Physicians may consider prescribing 25 to 50 mg of sildenafil to premenopausal women with normal hormones and libido who experience difficulty with arousal, lubrication, and genital sensation.
THE QUESTION:
Is sildenafil an effective treatment for arousal disorder in premenopausal women?
Past studies:
These have shown that sildenafil is not as promising in women as in men. However, previous trials examined its usefulness in postmenopausal women. Little research has been conducted in premenopausal women.
This study:
The benefits of sildenafil treatment were examined in 53 women, ages 22 to 38, who had developed arousal disorders and were unable to achieve orgasm. Included in the trial, were heterosexual women who had been incapable of experiencing vaginal lubrication or genital sensation for a period of 6 months or more. Normal ovarian function was documented by ultrasonography. Women on oral contraceptives or hormone therapy and those who did not have a sexual partner were excluded from the trial. Also, women with medical conditions, including hypertension, diabetes, cancer, alcohol abuse, and liver disease, could not enroll in the study.
In a double-blind crossover study design, patients were followed monthly for 3 months and treated with 25 mg of sildenafil, 50 mg of sildenafil, and placebo in a randomized fashion. Each participant took either dose of sildenafil or placebo 1 hour before planned intercourse. The researchers used a 5-point scale to detect changes in sexual behavior. The results: Women reported increased sexual arousal, enjoyment, and frequency of sexual fantasies with both doses of sildenafil compared with placebo (4.2 versus 2.6, respectively). The frequency of orgasm also improved significantly with both doses of sildenafil compared with placebo (3.8 versus 2.4, respectively).
Find this study:
June 2001 issue of British Journal of Obstetrics and Gynaecology; abstract online at www.elsevier.com.
Who may be affected by these findings?
Healthy young women with normal hormones and libido experiencing sexual arousal disorders. Additionally, postmenopausal women with normal sexual desire, steady partners, and hormones replaced to normal levels may respond well to sildenafil. Clearly, future research is needed in this postmenopausal population.
Expert commentary:
As female sexual dysfunction is discussed more widely on the talk show circuit, women are being encouraged to seek medical attention and treatment for this complex problem. Unfortunately, little research exists to better understand and treat sexual dissatisfaction among women, specifically arousal and orgasmic disorders. This trial, however, is one step toward the development of effective therapeutic strategies to combat these issues. The researchers successfully isolated arousal deficiencies from desire and orgasmic disorders in a group of young premenopausal women. The participants responded well to a treatment series of sildenafil. The medication regulates smooth muscle contractions by selectively blocking phosphodiesterase type 5. It also permits vasodilation, resulting in subsequent clitoral, vulvar, and vaginal erectile tissue engorgement.
The results of this trial are most interesting in that previous trials of sildenafil did not demonstrate any improvement in postmenopausal women with sexual dysfunction. However, those trials did not define arousal disorder as strictly as the authors of this study. Based on sildenafil’s mechanism of action, postmenopausal women with vascular disease, hypertension, and diabetes—a population similar to males with erectile dysfunction—who have normal libido and hormones, may be ideal candidates for sildenafil therapy.
Caveats:
In this study, 4 women stopped taking sildenafil 50 mg, 2 women stopped taking sildenafil 25 mg, and 2 women stopped taking placebo due to vision problems, headaches, and fear of adverse reactions.
The bottom line:
Physicians may consider prescribing 25 to 50 mg of sildenafil to premenopausal women with normal hormones and libido who experience difficulty with arousal, lubrication, and genital sensation.
THE QUESTION:
Is sildenafil an effective treatment for arousal disorder in premenopausal women?
Past studies:
These have shown that sildenafil is not as promising in women as in men. However, previous trials examined its usefulness in postmenopausal women. Little research has been conducted in premenopausal women.
This study:
The benefits of sildenafil treatment were examined in 53 women, ages 22 to 38, who had developed arousal disorders and were unable to achieve orgasm. Included in the trial, were heterosexual women who had been incapable of experiencing vaginal lubrication or genital sensation for a period of 6 months or more. Normal ovarian function was documented by ultrasonography. Women on oral contraceptives or hormone therapy and those who did not have a sexual partner were excluded from the trial. Also, women with medical conditions, including hypertension, diabetes, cancer, alcohol abuse, and liver disease, could not enroll in the study.
In a double-blind crossover study design, patients were followed monthly for 3 months and treated with 25 mg of sildenafil, 50 mg of sildenafil, and placebo in a randomized fashion. Each participant took either dose of sildenafil or placebo 1 hour before planned intercourse. The researchers used a 5-point scale to detect changes in sexual behavior. The results: Women reported increased sexual arousal, enjoyment, and frequency of sexual fantasies with both doses of sildenafil compared with placebo (4.2 versus 2.6, respectively). The frequency of orgasm also improved significantly with both doses of sildenafil compared with placebo (3.8 versus 2.4, respectively).
Find this study:
June 2001 issue of British Journal of Obstetrics and Gynaecology; abstract online at www.elsevier.com.
Who may be affected by these findings?
Healthy young women with normal hormones and libido experiencing sexual arousal disorders. Additionally, postmenopausal women with normal sexual desire, steady partners, and hormones replaced to normal levels may respond well to sildenafil. Clearly, future research is needed in this postmenopausal population.
Expert commentary:
As female sexual dysfunction is discussed more widely on the talk show circuit, women are being encouraged to seek medical attention and treatment for this complex problem. Unfortunately, little research exists to better understand and treat sexual dissatisfaction among women, specifically arousal and orgasmic disorders. This trial, however, is one step toward the development of effective therapeutic strategies to combat these issues. The researchers successfully isolated arousal deficiencies from desire and orgasmic disorders in a group of young premenopausal women. The participants responded well to a treatment series of sildenafil. The medication regulates smooth muscle contractions by selectively blocking phosphodiesterase type 5. It also permits vasodilation, resulting in subsequent clitoral, vulvar, and vaginal erectile tissue engorgement.
The results of this trial are most interesting in that previous trials of sildenafil did not demonstrate any improvement in postmenopausal women with sexual dysfunction. However, those trials did not define arousal disorder as strictly as the authors of this study. Based on sildenafil’s mechanism of action, postmenopausal women with vascular disease, hypertension, and diabetes—a population similar to males with erectile dysfunction—who have normal libido and hormones, may be ideal candidates for sildenafil therapy.
Caveats:
In this study, 4 women stopped taking sildenafil 50 mg, 2 women stopped taking sildenafil 25 mg, and 2 women stopped taking placebo due to vision problems, headaches, and fear of adverse reactions.
The bottom line:
Physicians may consider prescribing 25 to 50 mg of sildenafil to premenopausal women with normal hormones and libido who experience difficulty with arousal, lubrication, and genital sensation.