User login
Tips and tools for safe opioid prescribing
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1

The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
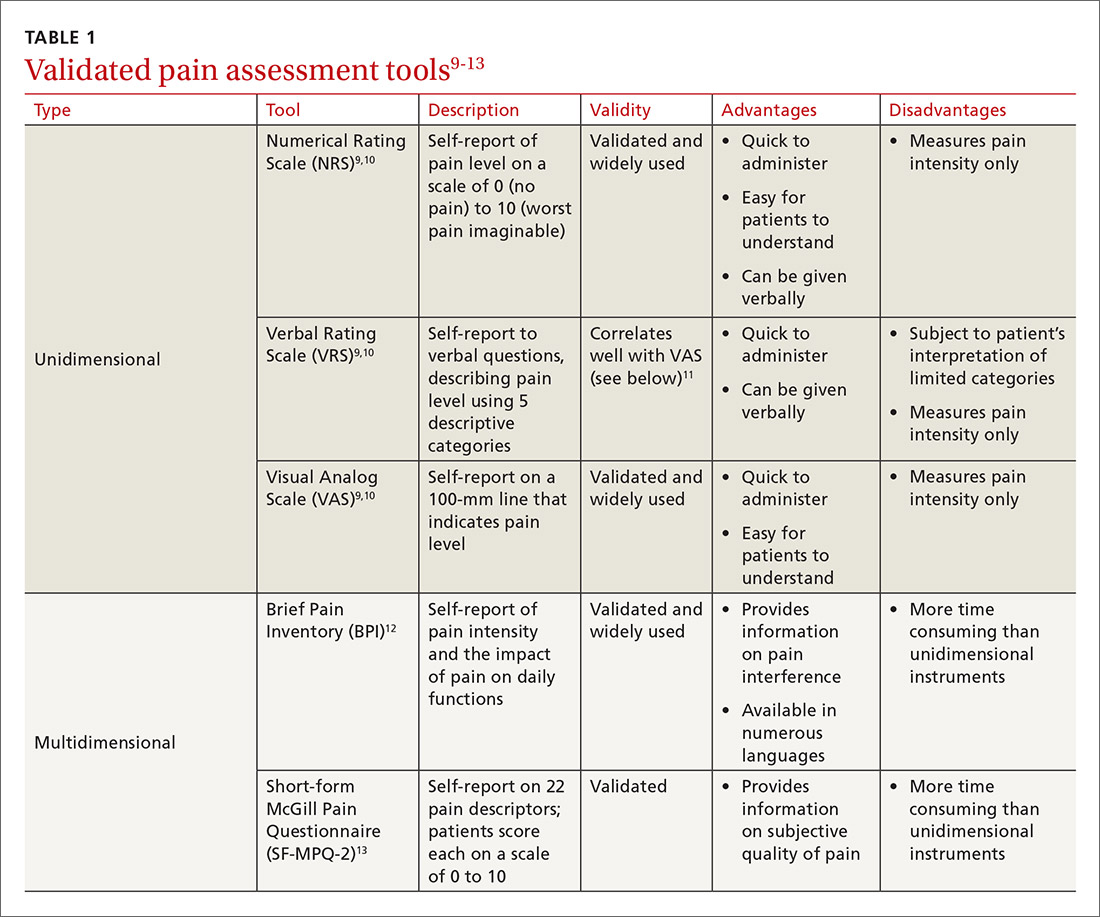
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30

Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,

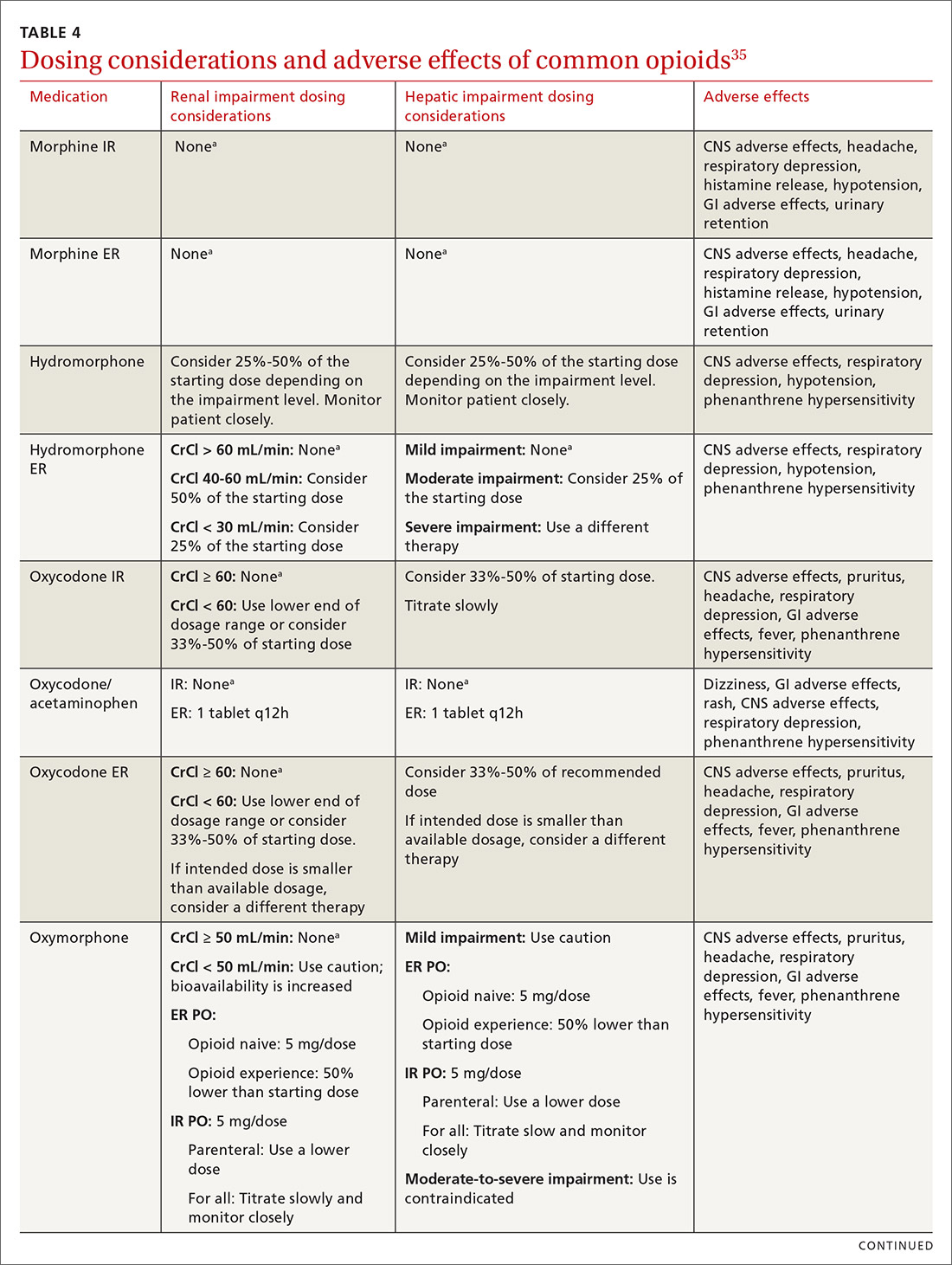
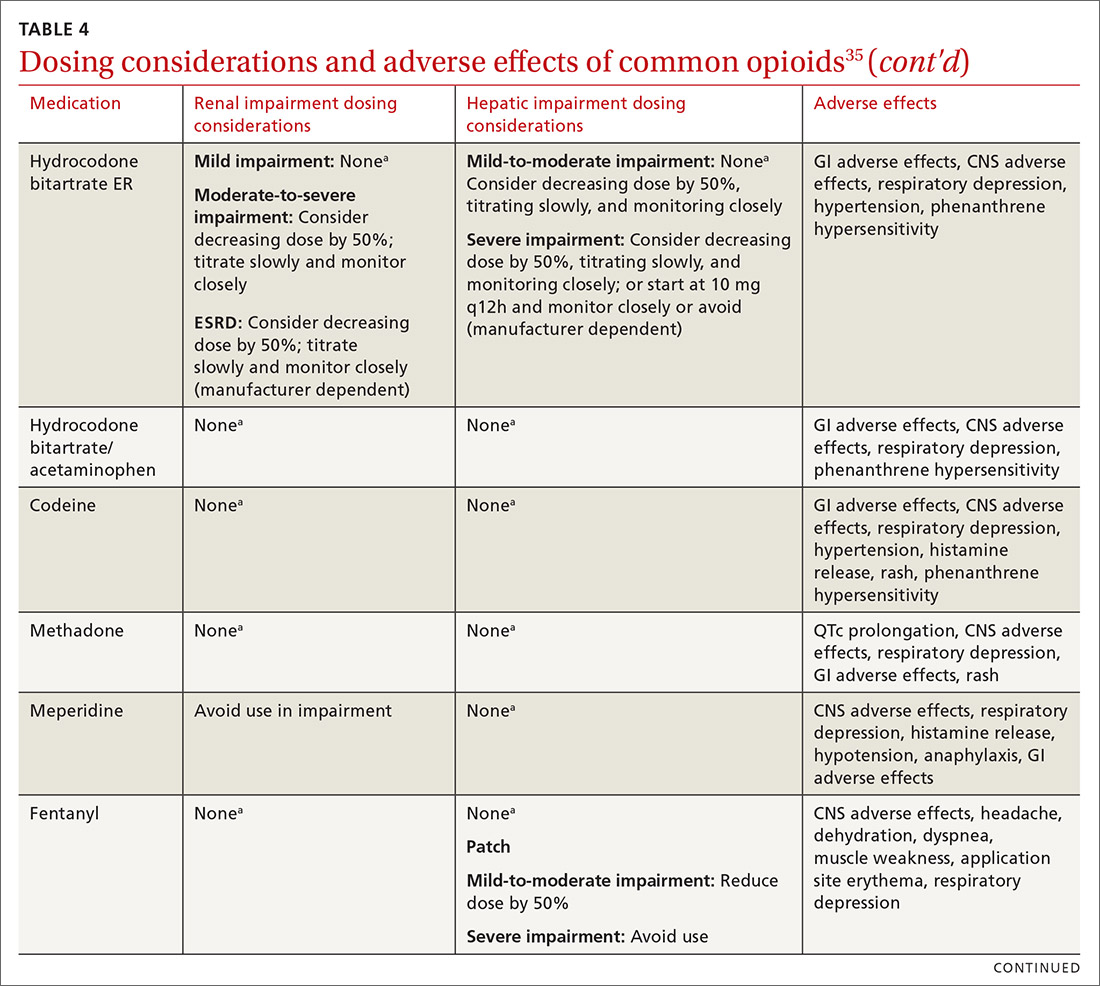
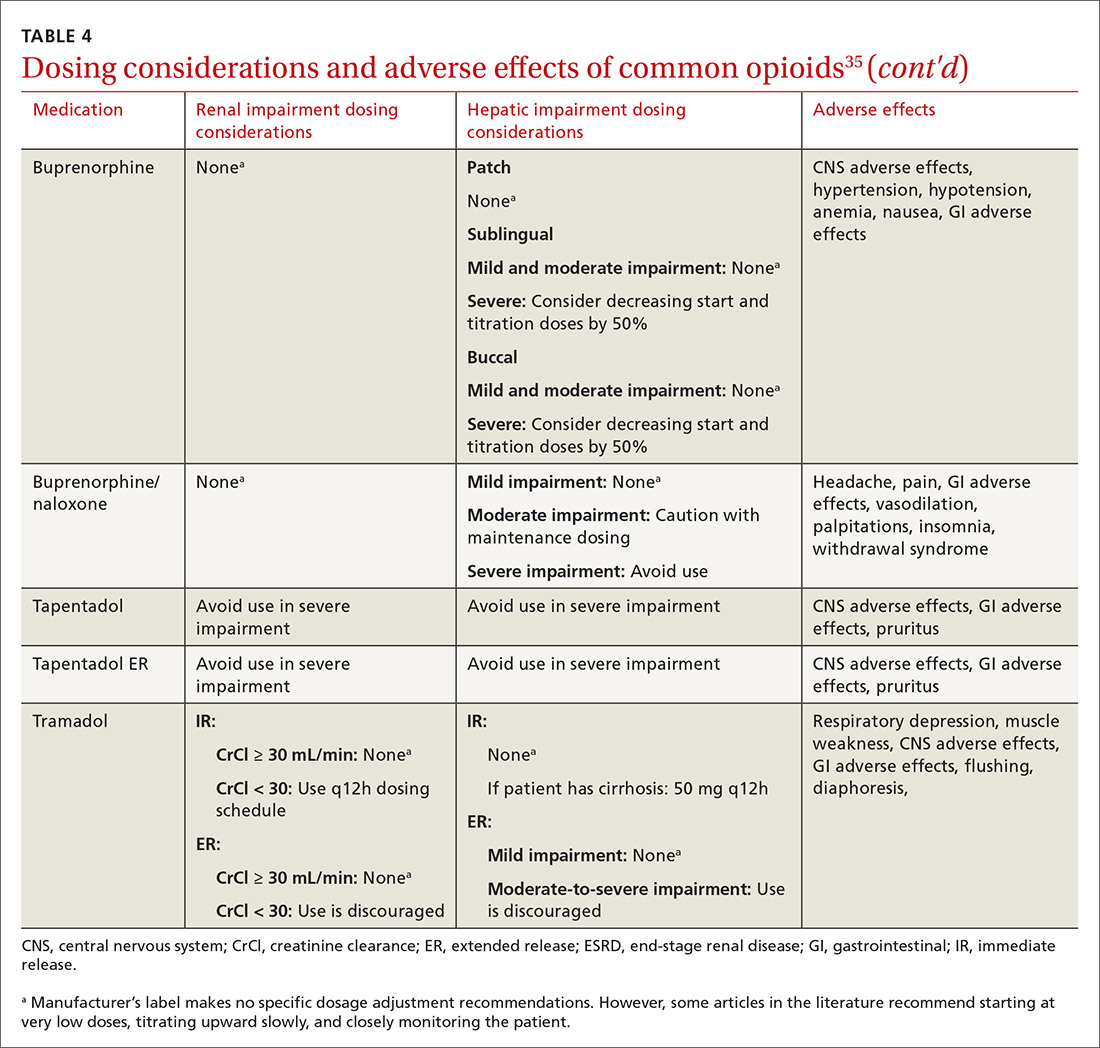
At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).
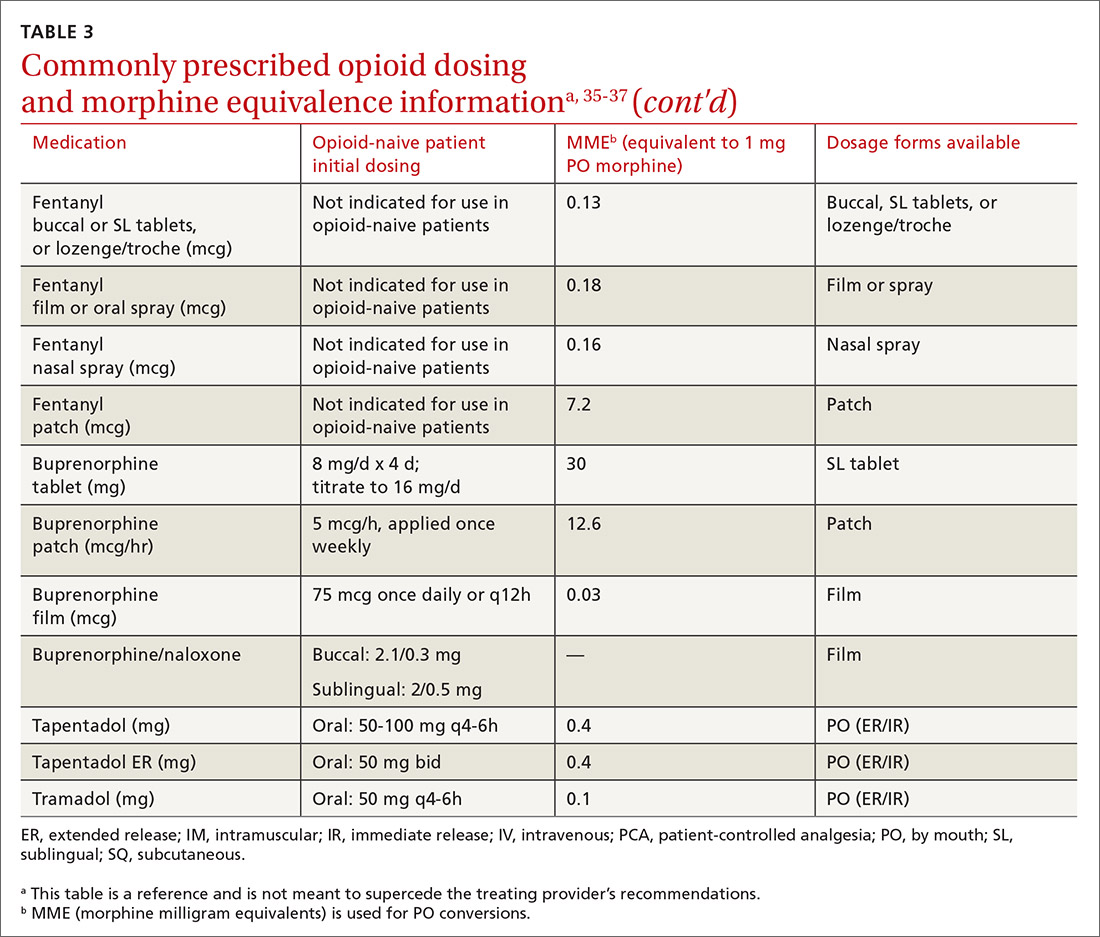
Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
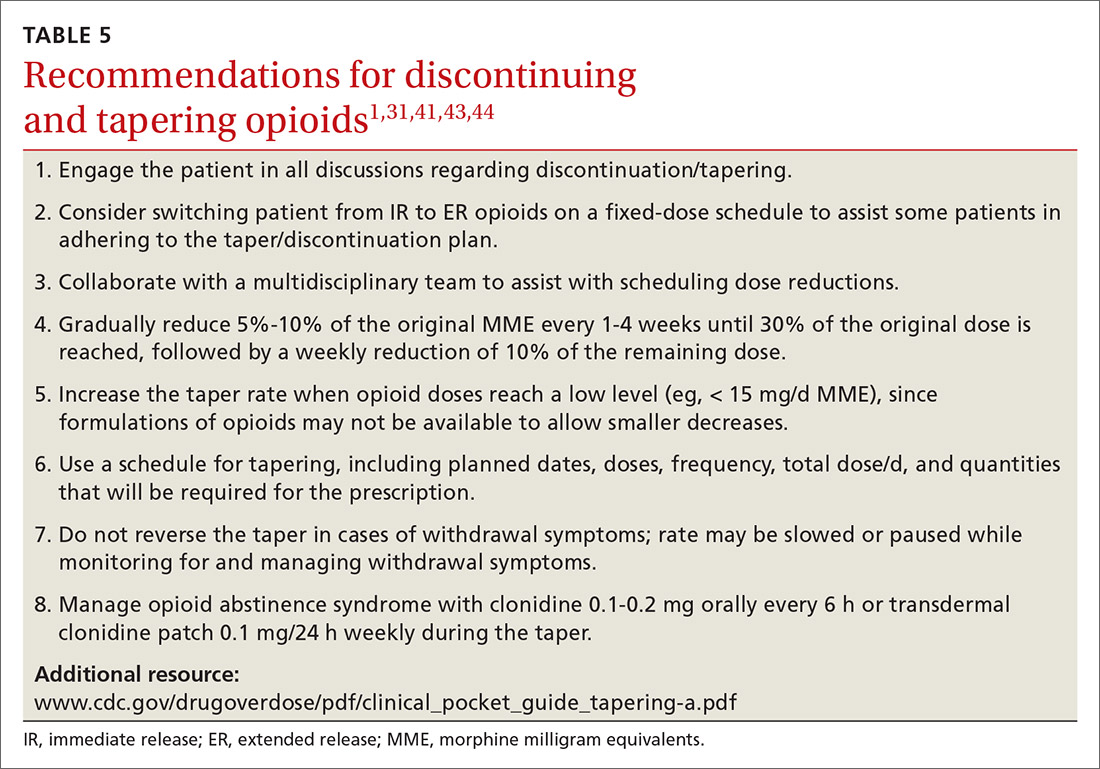
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.

CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1

The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16

Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30

Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,

At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).

Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28

CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.

Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31

Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44

General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.

CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1

The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16

Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30

Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,

At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).

Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28

CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.

Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31

Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44

General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.

CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
PRACTICE RECOMMENDATIONS
› Use a screening instrument such as the Opioid Risk Tool or the DIRE assessment to gauge a patient’s risk of opioid misuse and determine the frequency of monitoring. C
› Give as much priority to improving functional activity and minimizing adverse opioid effects as you do to relieving pain. C
› Prescribe an immediate-release, short-acting agent at first instead of a long-acting formulation; start with the lowest effective dosage and calculate total daily dose in terms of morphine milligram equivalents (MME). C
› Reduce the original MME dose by 5% to 10% every week when discontinuing an opioid. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Strategies to reduce and prevent polypharmacy in older patients
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.

Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.

Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.

For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.

Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.

For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
PRACTICE RECOMMENDATIONS
› Use one of the available tested and recommended screening tools to identify polypharmacy. C
› Engage in collaborative medication review to reduce the incidence of polypharmacy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series