User login
Strategies to reduce and prevent polypharmacy in older patients
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
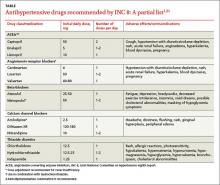
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.

Identifying polypharmacy
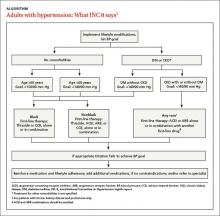
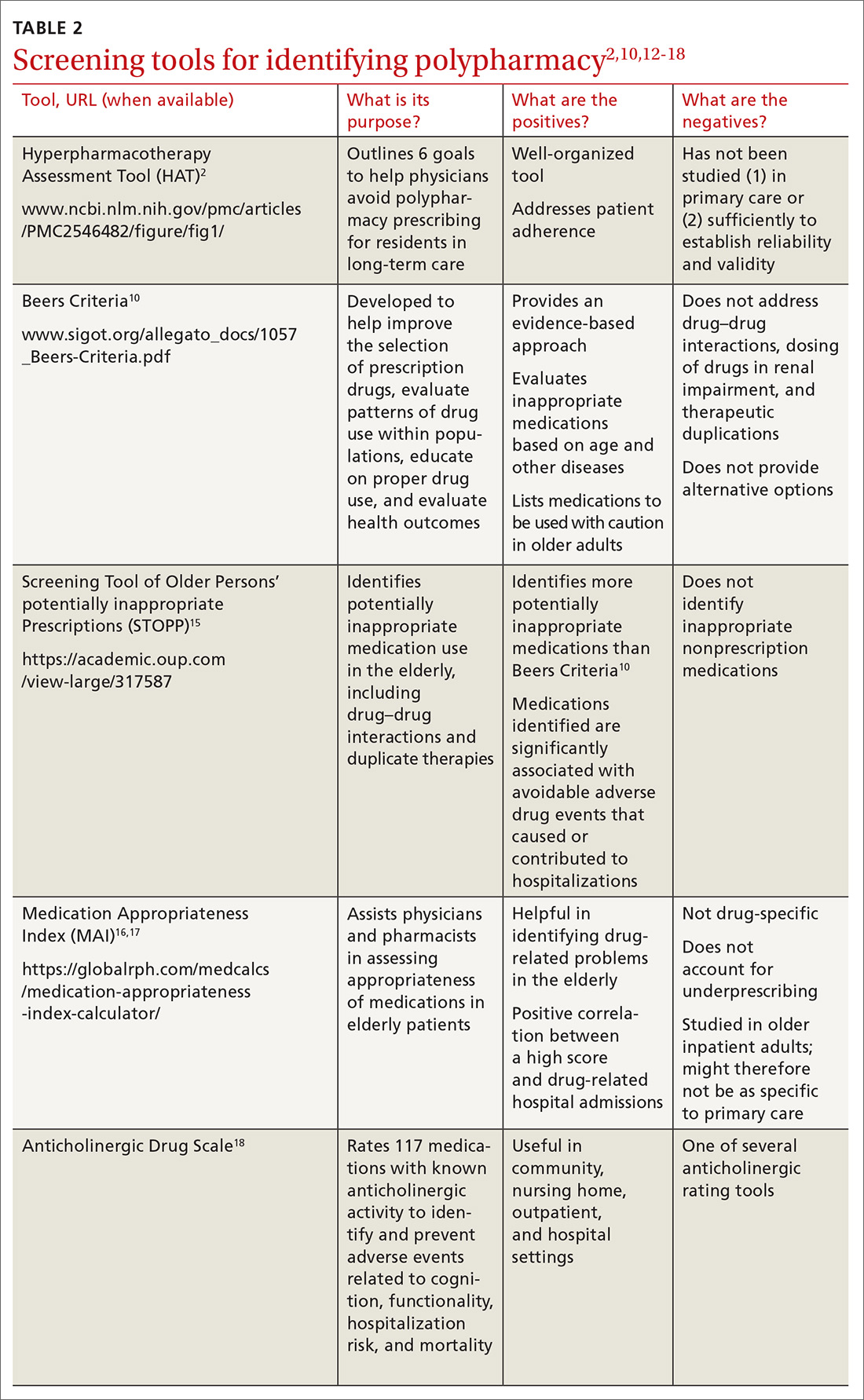
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.

Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.

For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.

Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.

For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
PRACTICE RECOMMENDATIONS
› Use one of the available tested and recommended screening tools to identify polypharmacy. C
› Engage in collaborative medication review to reduce the incidence of polypharmacy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Statin Adverse Effects: Sorting out the Evidence
CASE
Mr L., a 57-year-old obese patient (BMI > 40) who had not been to a clinician in a decade, comes to see you after a health fair screening revealed dyslipidemia (LDL cholesterol, 188 mg/dL; HDL cholesterol, 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg, and his fasting glucose is 101 mg/dL. Labs drawn that day reveal an A1C of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr L. also has a notable family history of heart disease.
Mr L. agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and to schedule a follow-up visit in eight weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose.
Based on the new recommendations, the use of statins is likely to rise.2 (A statin—rosuvastatin—is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for clinicians to know about the risks associated with statins and to be able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of more than 100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and > 240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients receiving placebo.4
Such discrepancies regarding particular risks, as well as the overall incidence of AEs and discontinuation rates, make the evidence difficult to sort out. We created this update with that in mind.
Continue for symptoms >>
MUSCULOSKELETAL SYMPTOMS ARE MOST COMMON
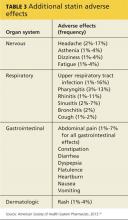
Musculoskeletal symptoms are the most common AEs reported by patients who are taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms are usually symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk for statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
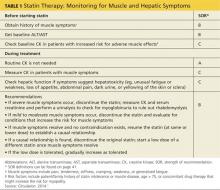
A baseline CK is recommended for patients with an increased risk for muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age older than 75, low levels of vitamin D, and concomitant use of medications that may increase the risk for myopathy (see Table 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from less than 1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of less than 0.06% over five years.1
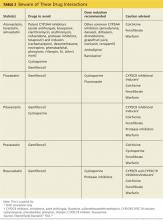
A 2006 systematic review estimated the absolute risk for rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See Table 212,13 for more on drug interactions.) But both the meta-analysis cited earlier4 and a previous systematic review14 (35 RCTs and > 74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk for statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the FDA cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N = 12,064) that found an increased risk for myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the
80-mg dose versus those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction greater than 50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80-mg simvastatin were excluded.17
The bottom line: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses lower than 300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE
On his next visit, Mr L. reports a new ache in his left shoulder and upper back, which he describes as mild but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr L. says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation but with no decline in strength. Mr L.’s BP has fallen to
134/84 mm Hg, and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, A1C of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only OTC, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Continue for hepatic effects >>
HEPATIC EFFECTS ARE RARE
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk for acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be less than 1.5% over the course of five years and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk for myopathy, and providing additional information about drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and the FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk for transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk for transaminase elevation (odds ratio [OR], 1.51) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
The bottom line: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
STATIN USE AND DIABETES: IS THERE A LINK?
Recent studies have found an increased risk for new-onset type 2 diabetes in statin users, with a greater risk associated with higher-potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1,000 patient-years for moderate-intensity therapy and three per 1,000 patient-years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk for diabetes was relatively small (OR, 1.09).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and more than 110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
The bottom line: Clinicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
STATINS MAY BE RENOPROTECTIVE
Statin use has been found to be associated with an increased risk for tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated but does recommend a lower dose for patients with CKD.28
The bottom line: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Continue for the risk of intracerebral hemorrhages >>
INTRACEREBRAL HEMORRHAGE: STATINS INCREASE RECURRENCE RISK
In recent years, there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n = 93) found that the absolute risk for recurrent ICH was 15.6% for patients randomized to atorvastatin versus 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, multivariate analysis indicated the increased risk was statistically significant (hazard ratio [HR], 1.69).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (> 40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
The bottom line: Clinicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
OTHER SERIOUS ADVERSE EFFECTS: WHICH REPORTS ARE ACCURATE?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk for malignancy has been considered for years. A large trial (N = 5,804) from 2002 found a correlation between pravastatin and an increased risk for new cancer diagnoses compared with placebo (HR, 1.25).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk for cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before modification of statin therapy is considered, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash (see Table 3).50
WHICH DRUG? POTENTIAL DIFFERENCES IN STATINS
A meta-analysis with more than 240,000 participants evaluated patients taking seven different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best-tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (> 40 mg/d) significantly increased the risk for CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk for rhabdomyolysis when taken at the highest dose.15,16
Continue for safety concerns >>
ARE STATINS SAFE FOR THESE PATIENTS?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk for AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk for AEs in Asian patients on statins.52
Patient factors that increase risk
Risk factors for statin-induced AEs include1
• Multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
• Unexplained ALT elevation more than 3x the upper limit of normal
• History of prior statin intolerance or concomitant use of drugs that affect statin metabolism
• Age older than 75
• Preexisting muscle disorders
• Low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE
The risk for elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr L.’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in six months, Mr L. reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
REFERENCES
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. www.medscape.com/viewarti cle/813571#3. Accessed October 19, 2014.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy.
J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173:1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:
393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. www.goldstand ard.com/product/gold-standard-drug-database/. Accessed October 19, 2014.
13. FDA. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed October 19,2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. FDA. FDA drug safety communication: ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed October 19, 2014.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:
526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. www.naturaldatabase.com.libproxy.uwyo.edu. Accessed October 19, 2014.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:
2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.
CASE
Mr L., a 57-year-old obese patient (BMI > 40) who had not been to a clinician in a decade, comes to see you after a health fair screening revealed dyslipidemia (LDL cholesterol, 188 mg/dL; HDL cholesterol, 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg, and his fasting glucose is 101 mg/dL. Labs drawn that day reveal an A1C of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr L. also has a notable family history of heart disease.
Mr L. agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and to schedule a follow-up visit in eight weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose.
Based on the new recommendations, the use of statins is likely to rise.2 (A statin—rosuvastatin—is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for clinicians to know about the risks associated with statins and to be able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of more than 100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and > 240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients receiving placebo.4
Such discrepancies regarding particular risks, as well as the overall incidence of AEs and discontinuation rates, make the evidence difficult to sort out. We created this update with that in mind.
Continue for symptoms >>
MUSCULOSKELETAL SYMPTOMS ARE MOST COMMON
Musculoskeletal symptoms are the most common AEs reported by patients who are taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms are usually symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk for statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
A baseline CK is recommended for patients with an increased risk for muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age older than 75, low levels of vitamin D, and concomitant use of medications that may increase the risk for myopathy (see Table 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from less than 1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of less than 0.06% over five years.1
A 2006 systematic review estimated the absolute risk for rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See Table 212,13 for more on drug interactions.) But both the meta-analysis cited earlier4 and a previous systematic review14 (35 RCTs and > 74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk for statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the FDA cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N = 12,064) that found an increased risk for myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the
80-mg dose versus those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction greater than 50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80-mg simvastatin were excluded.17
The bottom line: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses lower than 300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE
On his next visit, Mr L. reports a new ache in his left shoulder and upper back, which he describes as mild but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr L. says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation but with no decline in strength. Mr L.’s BP has fallen to
134/84 mm Hg, and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, A1C of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only OTC, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Continue for hepatic effects >>
HEPATIC EFFECTS ARE RARE
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk for acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be less than 1.5% over the course of five years and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk for myopathy, and providing additional information about drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and the FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk for transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk for transaminase elevation (odds ratio [OR], 1.51) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
The bottom line: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
STATIN USE AND DIABETES: IS THERE A LINK?
Recent studies have found an increased risk for new-onset type 2 diabetes in statin users, with a greater risk associated with higher-potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1,000 patient-years for moderate-intensity therapy and three per 1,000 patient-years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk for diabetes was relatively small (OR, 1.09).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and more than 110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
The bottom line: Clinicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
STATINS MAY BE RENOPROTECTIVE
Statin use has been found to be associated with an increased risk for tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated but does recommend a lower dose for patients with CKD.28
The bottom line: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Continue for the risk of intracerebral hemorrhages >>
INTRACEREBRAL HEMORRHAGE: STATINS INCREASE RECURRENCE RISK
In recent years, there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n = 93) found that the absolute risk for recurrent ICH was 15.6% for patients randomized to atorvastatin versus 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, multivariate analysis indicated the increased risk was statistically significant (hazard ratio [HR], 1.69).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (> 40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
The bottom line: Clinicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
OTHER SERIOUS ADVERSE EFFECTS: WHICH REPORTS ARE ACCURATE?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk for malignancy has been considered for years. A large trial (N = 5,804) from 2002 found a correlation between pravastatin and an increased risk for new cancer diagnoses compared with placebo (HR, 1.25).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk for cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before modification of statin therapy is considered, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash (see Table 3).50
WHICH DRUG? POTENTIAL DIFFERENCES IN STATINS
A meta-analysis with more than 240,000 participants evaluated patients taking seven different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best-tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (> 40 mg/d) significantly increased the risk for CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk for rhabdomyolysis when taken at the highest dose.15,16
Continue for safety concerns >>
ARE STATINS SAFE FOR THESE PATIENTS?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk for AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk for AEs in Asian patients on statins.52
Patient factors that increase risk
Risk factors for statin-induced AEs include1
• Multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
• Unexplained ALT elevation more than 3x the upper limit of normal
• History of prior statin intolerance or concomitant use of drugs that affect statin metabolism
• Age older than 75
• Preexisting muscle disorders
• Low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE
The risk for elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr L.’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in six months, Mr L. reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
REFERENCES
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. www.medscape.com/viewarti cle/813571#3. Accessed October 19, 2014.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy.
J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173:1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:
393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. www.goldstand ard.com/product/gold-standard-drug-database/. Accessed October 19, 2014.
13. FDA. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed October 19,2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. FDA. FDA drug safety communication: ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed October 19, 2014.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:
526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. www.naturaldatabase.com.libproxy.uwyo.edu. Accessed October 19, 2014.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:
2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.
CASE
Mr L., a 57-year-old obese patient (BMI > 40) who had not been to a clinician in a decade, comes to see you after a health fair screening revealed dyslipidemia (LDL cholesterol, 188 mg/dL; HDL cholesterol, 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg, and his fasting glucose is 101 mg/dL. Labs drawn that day reveal an A1C of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr L. also has a notable family history of heart disease.
Mr L. agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and to schedule a follow-up visit in eight weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose.
Based on the new recommendations, the use of statins is likely to rise.2 (A statin—rosuvastatin—is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for clinicians to know about the risks associated with statins and to be able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of more than 100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and > 240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients receiving placebo.4
Such discrepancies regarding particular risks, as well as the overall incidence of AEs and discontinuation rates, make the evidence difficult to sort out. We created this update with that in mind.
Continue for symptoms >>
MUSCULOSKELETAL SYMPTOMS ARE MOST COMMON
Musculoskeletal symptoms are the most common AEs reported by patients who are taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms are usually symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk for statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
A baseline CK is recommended for patients with an increased risk for muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age older than 75, low levels of vitamin D, and concomitant use of medications that may increase the risk for myopathy (see Table 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from less than 1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of less than 0.06% over five years.1
A 2006 systematic review estimated the absolute risk for rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See Table 212,13 for more on drug interactions.) But both the meta-analysis cited earlier4 and a previous systematic review14 (35 RCTs and > 74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk for statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the FDA cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N = 12,064) that found an increased risk for myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the
80-mg dose versus those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction greater than 50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80-mg simvastatin were excluded.17
The bottom line: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses lower than 300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE
On his next visit, Mr L. reports a new ache in his left shoulder and upper back, which he describes as mild but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr L. says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation but with no decline in strength. Mr L.’s BP has fallen to
134/84 mm Hg, and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, A1C of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only OTC, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Continue for hepatic effects >>
HEPATIC EFFECTS ARE RARE
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk for acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be less than 1.5% over the course of five years and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk for myopathy, and providing additional information about drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and the FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk for transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk for transaminase elevation (odds ratio [OR], 1.51) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
The bottom line: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
STATIN USE AND DIABETES: IS THERE A LINK?
Recent studies have found an increased risk for new-onset type 2 diabetes in statin users, with a greater risk associated with higher-potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1,000 patient-years for moderate-intensity therapy and three per 1,000 patient-years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk for diabetes was relatively small (OR, 1.09).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and more than 110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
The bottom line: Clinicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
STATINS MAY BE RENOPROTECTIVE
Statin use has been found to be associated with an increased risk for tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated but does recommend a lower dose for patients with CKD.28
The bottom line: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Continue for the risk of intracerebral hemorrhages >>
INTRACEREBRAL HEMORRHAGE: STATINS INCREASE RECURRENCE RISK
In recent years, there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n = 93) found that the absolute risk for recurrent ICH was 15.6% for patients randomized to atorvastatin versus 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, multivariate analysis indicated the increased risk was statistically significant (hazard ratio [HR], 1.69).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (> 40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
The bottom line: Clinicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
OTHER SERIOUS ADVERSE EFFECTS: WHICH REPORTS ARE ACCURATE?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk for malignancy has been considered for years. A large trial (N = 5,804) from 2002 found a correlation between pravastatin and an increased risk for new cancer diagnoses compared with placebo (HR, 1.25).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk for cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before modification of statin therapy is considered, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash (see Table 3).50
WHICH DRUG? POTENTIAL DIFFERENCES IN STATINS
A meta-analysis with more than 240,000 participants evaluated patients taking seven different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best-tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (> 40 mg/d) significantly increased the risk for CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk for rhabdomyolysis when taken at the highest dose.15,16
Continue for safety concerns >>
ARE STATINS SAFE FOR THESE PATIENTS?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk for AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk for AEs in Asian patients on statins.52
Patient factors that increase risk
Risk factors for statin-induced AEs include1
• Multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
• Unexplained ALT elevation more than 3x the upper limit of normal
• History of prior statin intolerance or concomitant use of drugs that affect statin metabolism
• Age older than 75
• Preexisting muscle disorders
• Low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE
The risk for elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr L.’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in six months, Mr L. reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
REFERENCES
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. www.medscape.com/viewarti cle/813571#3. Accessed October 19, 2014.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy.
J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173:1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:
393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. www.goldstand ard.com/product/gold-standard-drug-database/. Accessed October 19, 2014.
13. FDA. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed October 19,2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. FDA. FDA drug safety communication: ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed October 19, 2014.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:
526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. www.naturaldatabase.com.libproxy.uwyo.edu. Accessed October 19, 2014.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:
2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.
JNC 8: What's covered, what's not, and what else to consider
› Initiate pharmacologic treatment for patients
60 years or older with systolic blood pressure (BP) ≥150 mm Hg and/or diastolic BP ≥90 mm Hg. A
› Start antihypertensive treatment for systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg in patients who are younger than 60 or have chronic kidney disease or diabetes. C
› Select either a thiazide diuretic or a calcium channel blocker as first-line therapy for African Americans, whether or not they have diabetes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carla S is a 64-year old African American whom you’re seeing for the first time. Her health has been excellent over the last 10 years, she reports, with one caveat: She has “borderline hypertension,” but has never been treated for it and denies any symptoms. Her blood pressure (BP) today is 154/82 mm Hg. A physical exam is unremarkable. Blood tests reveal a normal blood count and normal renal function, and a nonfasting glucose level of 145 mg/dL. You ask Ms. S to return in a week for a repeat BP and fasting lab work.
Hypertension is the most common condition seen by physicians in primary care,1 and a major risk factor for cardiovascular disease (CVD) and the morbidity and mortality associated with it. US treatment costs are an estimated $131 billion per year.2-4 With this in mind, the Joint National Committee on Hypertension (JNC) released its eighth report (JNC 8) in December 20131—the first update in a decade.
In many ways, JNC 8 guidelines are simpler than those of JNC 7,2 with more evidence-based recommendations and less reliance on expert opinion. The JNC has eliminated definitions such as stage 1, 2, and 3 hypertension, and focuses on outcomes instead. At the heart of the recommendations are 3 key questions:
1. At what BP should treatment be initiated to improve outcomes?
2. What should the target BP be for those undergoing treatment?
3. Which medications are best?
Answers to the first 2 questions, of course, go hand in hand. In other words, if the threshold for treatment is a systolic BP ≥140 mm Hg (more on that in a moment), then the target of treatment is a systolic BP of <140 mm Hg. In answer to the third question, JNC 8 offers guidance but gives physicians greater discretion in determining which type of drug to use when initiating treatment.1
In the text, algorithm, and table that follow, we present an overview of JNC 8. We also discuss the optimal treatment of hypertension in patients with heart failure (HF) and coronary artery disease (CAD)—populations JNC 8 does not address.
Age-based recommendations are a bit less stringent
60 years and older. Unlike JNC 7, which recommended initiating treatment for otherwise healthy patients of all ages with a BP ≥140/90 mm Hg,2 JNC 8 clearly delineates its recommendations by age. It calls for treating patients ages 60 or older with systolic BP ≥150 mm Hg and/or diastolic BP ≥90.1
The change is evidence-based: Moderate- to high-quality randomized controlled trials (RCTs) have found a reduced incidence of stroke, HF, and coronary heart disease when BP was treated to <150/90, but no additional benefit from a systolic BP target of <140 mm Hg for patients in this age group.5,6 Notably, JNC 8 does not recommend a change in medication for patients 60 years or older for whom the more stringent target is being maintained without adverse effects.1
18 to 59 years. For adults younger than 60, JNC 8 recommends treating systolic BP ≥140 and diastolic BP ≥90 mm Hg.1 The systolic BP guideline is based on expert opinion, however, as there is no high-quality evidence for a systolic threshold in this age group. This is largely because most patients younger than 60 who have systolic BP ≥140 also have diastolic BP ≥90, making it difficult to study the treatment of systolic BP alone. High-quality trials have shown improved health outcomes when patients ages 30 to 59 years were treated for diastolic BP ≥90, however.7-12 For patients younger than 30, the recommendation for treatment of diastolic pressure is based on expert consensus, as no sufficiently high-quality evidence exists.
Targets for patients with CKD and diabetes
Chronic kidney disease (CKD). JNC 8 recommends treating patients ages 18 to 69 years who have CKD and BP ≥140/90 mm Hg. JNC 7’s more stringent recommendation—treating such patients with BP ≥130/80 mm Hg2—was relaxed because there is little evidence of a lower mortality rate or cardiovascular or cerebrovascular benefits as a result of tighter control. In patients younger than 70, CKD is defined as an estimated (or measured) glomerular filtration rate (GFR) <60 mL/min/1.73 m2 or albuminuria (>30 mg of albumin per g of creatinine).1
It is important to note that this goal does not apply to individuals who have CKD and are 70 years or older. This is due to insufficient evidence, as well as uncertainty about the accuracy of an estimated GFR in this patient population. JNC 8 recommends that treatment of BP in patients 70 or older be based on comorbidities, including albuminuria, among other patient-specific considerations.1
Diabetes. JNC 8 recommends treating patients age 18 years or older who have diabetes and BP ≥140/90 mm Hg, as JNC 7 did.2 This is based largely on expert opinion.
Studies suggest that adults with both hypertension and diabetes have a reduction in mortality and improved cardiovascular and cerebrovascular outcomes when systolic BP is <150 mm Hg,13-15 but no strong data support a goal of <140/90 mm Hg. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial, for example, showed comparable outcomes in patients with systolic BP of 150 or 140 mm Hg.16 The use of expert opinion vs well-designed studies in this instance seems at odds with JNC 8’s general policy of placing greater emphasis on evidence.
CASE › On her second visit, Ms. S’s BP is 144/82 mm Hg and her cholesterol levels are within the normal range. Her fasting glucose level is 104 mg/dL and glycated hemoglobin (HbA1c) is 6%. At a repeat visit one month later, her BP is 146/76 mm Hg. Given these 2 acceptable readings (<150/90 mm Hg for individuals age 60 and older who do not have diabetes), you do not initiate antihypertensive treatment.
However, you explain to the patient that her fasting glucose and HbA1c are evidence of insulin resistance. Although a diagnosis of diabetes is not warranted, you arrange for Ms. S to meet with a diabetes nurse educator for help in improving her diet and following an exercise regimen.
Pharmacotherapy: JNC 8 offers wider latitude
Like its predecessor, JNC 8 stresses the importance of diet and exercise. (See “Controlling hypertension starts with lifestyle modification”17 in this article.) It diverges from JNC 7, however, in its recommendations for initiating treatment (ALGORITHM).1 The earlier version recommended thiazide diuretics as first-line therapy but included multiple indications for initiating therapy with other drug classes. JNC 8 guidelines are less specific.
Starting therapy with a thiazide diuretic, angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), or calcium channel blocker (CCB)—all of which have high-quality evidence of improved outcomes18-20—is recommended for most patients, including those with diabetes. (Blacks and patients with CKD are exceptions.) The recommended doses of these medications, summarized in the TABLE,1,21 are similar to those used in RCTs. Other types of drugs are not recommended, either because they were shown to be inferior to another class of antihypertensive or because there is insufficient evidence of their efficacy.
For most blacks... JNC 8 recommends thiazide diuretics and CCBs as first-line therapy—a recommendation that is evidence-based. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)22 revealed that black patients taking thiazide diuretics had fewer cerebrovascular and cardiovascular events and a lower rate of HF compared with those taking ACEIs, whether or not they had diabetes. Diuretics were more effective than CCBs in preventing HF, but no difference in rates of cerebrovascular and cardiovascular events, kidney disease, or overall mortality was found.22
For patients with CKD and proteinuria, regardless of race, JNC 8 calls for either an ACEI or an ARB as first-line agent to prevent progression to end-stage renal disease. This recommendation is based on expert consensus, and intended to prevent progression to end-stage renal disease.1,23
The optimal first-line agent for patients who have CKD without proteinuria is less clear. For such patients, JNC 8 notes, any of the 4 recommended drug classes can be used for initial therapy.1
Guidance on starting—and titrating—therapy
JNC 7 guidelines featured a complex means of diagnosing and monitoring hypertension.2 JNC 8 has simplified the recommendations, which call for patients to be reassessed within a month of initiating therapy.
The new guidelines include 3 distinct methods of dosing antihypertensive medications, none of which has demonstrated better outcomes than any other. All call for replacing one type of drug with another if the first trial is ineffective or results in adverse effects. And all stress the importance of avoiding ACEI and ARB combinations due to increases in serum creatinine and hyperkalemia and the need for monitoring. Note, however, that Method 3 is recommended for patients with more severe hypertension.1
Method 1. Initiate one medication from any of the 4 classes of antihypertensives recommended for initial treatment, and titrate to the maximum effective dose. If the BP goal is not achieved at maximum dose, add a medication from a second class and titrate that drug to the maximum effective dose, as well. If the goal is still not reached, add a medication from a third class and titrate up as needed.
Method 2. Initiate one medication, then add a second agent from a different drug class, if necessary, and titrate until both are at the maximum effective dose. If the goal still has not been reached, add a third agent and titrate that until BP is well controlled.
Method 3. Initiate 2 medications from 2 different classes of drugs simultaneously. If BP is not at goal after a reasonable trial, add a third agent and titrate to maximum effective dose. (Use this approach for patients who have systolic BP >160 mm Hg and/or diastolic BP >100 mm Hg or systolic BP >20 mm Hg above goal and/or diastolic BP >10 mm Hg above goal.)
As a general rule, a trial with monotherapy should be considered if BP is ≤160/100; a 2-agent combination is recommended as first-line therapy for pressure that exceeds that threshold. If a patient’s BP target is not reached even with the above strategies, a consultation with a hypertension specialist may be needed.
Treating patients with cardiovascular comorbidities
As noted earlier, JNC 8 offers no guidance in treating patients with HF or CAD and multiple comorbidities. In such cases, we turn to the American College of Cardiology (ACC) and American Heart Association (AHA).24
Recent ACC/AHA guidelines recommend a beta-blocker and ACEI for patients with a history of symptomatic stable HF and a left ventricular ejection fraction (EF) ≤40%, unless contraindications exist.24 Beta-blockers and an ACEI or an ARB should be used to prevent HF in patients with a history of myocardial infarction (MI) or acute coronary syndrome and a reduced EF. Beta-blockers with evidence to support their use in such cases include carvedilol, bisoprolol, and sustained-release metoprolol succinate.24
For symptomatic patients with dyspnea or other mild fluid retention, a loop diuretic or a thiazide diuretic can be used. Nondihydropyridine CCBs should be avoided in post-MI patients with low left ventricular EF due to the medication’s negative inotropic effects.24 The optimal drug regimen for secondary stroke prevention is not clear due to a lack of studies comparing drug regimens, but data suggest that a diuretic or a diuretic-ACEI combination is beneficial.25
Evaluating treatment-resistant hypertension
When a patient presents with treatment-resistant hypertension—elevated BP that is not controlled with a 3-drug regimen, all at maximum doses—start by asking several questions.26 Is the patient:
- having difficulty following a drug regimen that calls for multiple daily doses?
- drinking excessive amounts of alcohol?
- failing to adhere to a low-salt dietary regimen?
- taking any other medications or supplements that might elevate BP (eg, nonsteroidal anti-inflammatory agents, pseudoephedrine, ephedra, or licorice)?
- unable to afford all the drugs prescribed?
If no such issues are identified, consider a referral to a specialist for further evaluation and to rule out disorders associated with treatment-resistant hypertension, including CKD, renal artery stenosis, hyperaldosteronemia, sleep apnea, and coarctation of the aorta.26
For most people, cardiovascular health is dependent on exercise and weight control. That’s particularly true for those with hypertension, for whom limiting alcohol and salt consumption is crucial, as well.
JNC 8 calls for lifestyle management,1 but specific recommendations come from the American College of Cardiology (ACC)/American Heart Association (AHA)’s 2013 Lifestyle Work Group.17 The guidelines call for patients with elevated blood pressure (BP) to follow a diet rich in vegetables, fruits, and whole grains, including low-fat dairy, poultry, fish, legumes, nuts, and nontropical vegetable oils, such as the DASH (Dietary Approaches to Stop Hypertension) or AHA diet. Salt consumption should not exceed 2400 mg/d—and, ideally, be limited to 1500 mg/d or reflect a reduction of at least 1000 mg/d.17
Stress the importance of regular physical activity in controlling BP, as well. The ACC/AHA call for adults to engage in moderate to vigorous aerobic activity 3 to 4 times a week, averaging about 40 minutes per session.17
CASE › When Ms. S returns 3 months later, her BP is 140/70 mm Hg, her fasting glucose is 94 mg/dL, and her HbA1c is 5.7%. You encourage her to continue her new dietary and exercise regimen and schedule a follow-up visit in 6 months.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy, Health Sciences Center, Room 292, 1000 East University Avenue, Department 3375, Laramie, WY 82071; [email protected]
1. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
2. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
4. Heidenreich PA, Trogdon JG, Khavjou OA, et al; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944.
5. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
6. Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
7. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
8. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. III. Reduction in stroke incidence among persons with high blood pressure. JAMA. 1982;247:633-638.
9. Hypertension-Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. JAMA. 1974;229:409-418.
10. Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Br Med J (Clin Res Ed). 1985;291:97-104.
11. The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet. 1980;1:1261-1267.
12. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
13. Curb JD, Pressel SL, Cutler JA, et al; Systolic Hypertension in the Elderly Program Cooperative Research Group. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276:1886-1892.
14. Tuomilehto J, Rastenyte D, Birkenhäger WH, et al; Systolic Hypertension in Europe Trial Investigators. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med. 1999;340:677-684.
15. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
16. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99.
18. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255-3264.
19. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
20. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
21. Mann JFE. Choice of drug therapy in primary (essential) hypertension: recommendations. UpToDate Web site. Available at: http://www.uptodate.com/contents/choice-of-drug-therapy-in-primary-essential-hypertension-recommendations. Accessed March 3, 2014.
22. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997.
23. Wright JT Jr, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
24. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e319.
25. Furie KL, Kasner SE, Adams RJ, et al; American Heart Assocaition Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Stroke. 2011;42:227-276.
26. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14-26.
› Initiate pharmacologic treatment for patients
60 years or older with systolic blood pressure (BP) ≥150 mm Hg and/or diastolic BP ≥90 mm Hg. A
› Start antihypertensive treatment for systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg in patients who are younger than 60 or have chronic kidney disease or diabetes. C
› Select either a thiazide diuretic or a calcium channel blocker as first-line therapy for African Americans, whether or not they have diabetes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carla S is a 64-year old African American whom you’re seeing for the first time. Her health has been excellent over the last 10 years, she reports, with one caveat: She has “borderline hypertension,” but has never been treated for it and denies any symptoms. Her blood pressure (BP) today is 154/82 mm Hg. A physical exam is unremarkable. Blood tests reveal a normal blood count and normal renal function, and a nonfasting glucose level of 145 mg/dL. You ask Ms. S to return in a week for a repeat BP and fasting lab work.
Hypertension is the most common condition seen by physicians in primary care,1 and a major risk factor for cardiovascular disease (CVD) and the morbidity and mortality associated with it. US treatment costs are an estimated $131 billion per year.2-4 With this in mind, the Joint National Committee on Hypertension (JNC) released its eighth report (JNC 8) in December 20131—the first update in a decade.
In many ways, JNC 8 guidelines are simpler than those of JNC 7,2 with more evidence-based recommendations and less reliance on expert opinion. The JNC has eliminated definitions such as stage 1, 2, and 3 hypertension, and focuses on outcomes instead. At the heart of the recommendations are 3 key questions:
1. At what BP should treatment be initiated to improve outcomes?
2. What should the target BP be for those undergoing treatment?
3. Which medications are best?
Answers to the first 2 questions, of course, go hand in hand. In other words, if the threshold for treatment is a systolic BP ≥140 mm Hg (more on that in a moment), then the target of treatment is a systolic BP of <140 mm Hg. In answer to the third question, JNC 8 offers guidance but gives physicians greater discretion in determining which type of drug to use when initiating treatment.1
In the text, algorithm, and table that follow, we present an overview of JNC 8. We also discuss the optimal treatment of hypertension in patients with heart failure (HF) and coronary artery disease (CAD)—populations JNC 8 does not address.
Age-based recommendations are a bit less stringent
60 years and older. Unlike JNC 7, which recommended initiating treatment for otherwise healthy patients of all ages with a BP ≥140/90 mm Hg,2 JNC 8 clearly delineates its recommendations by age. It calls for treating patients ages 60 or older with systolic BP ≥150 mm Hg and/or diastolic BP ≥90.1
The change is evidence-based: Moderate- to high-quality randomized controlled trials (RCTs) have found a reduced incidence of stroke, HF, and coronary heart disease when BP was treated to <150/90, but no additional benefit from a systolic BP target of <140 mm Hg for patients in this age group.5,6 Notably, JNC 8 does not recommend a change in medication for patients 60 years or older for whom the more stringent target is being maintained without adverse effects.1
18 to 59 years. For adults younger than 60, JNC 8 recommends treating systolic BP ≥140 and diastolic BP ≥90 mm Hg.1 The systolic BP guideline is based on expert opinion, however, as there is no high-quality evidence for a systolic threshold in this age group. This is largely because most patients younger than 60 who have systolic BP ≥140 also have diastolic BP ≥90, making it difficult to study the treatment of systolic BP alone. High-quality trials have shown improved health outcomes when patients ages 30 to 59 years were treated for diastolic BP ≥90, however.7-12 For patients younger than 30, the recommendation for treatment of diastolic pressure is based on expert consensus, as no sufficiently high-quality evidence exists.
Targets for patients with CKD and diabetes
Chronic kidney disease (CKD). JNC 8 recommends treating patients ages 18 to 69 years who have CKD and BP ≥140/90 mm Hg. JNC 7’s more stringent recommendation—treating such patients with BP ≥130/80 mm Hg2—was relaxed because there is little evidence of a lower mortality rate or cardiovascular or cerebrovascular benefits as a result of tighter control. In patients younger than 70, CKD is defined as an estimated (or measured) glomerular filtration rate (GFR) <60 mL/min/1.73 m2 or albuminuria (>30 mg of albumin per g of creatinine).1
It is important to note that this goal does not apply to individuals who have CKD and are 70 years or older. This is due to insufficient evidence, as well as uncertainty about the accuracy of an estimated GFR in this patient population. JNC 8 recommends that treatment of BP in patients 70 or older be based on comorbidities, including albuminuria, among other patient-specific considerations.1
Diabetes. JNC 8 recommends treating patients age 18 years or older who have diabetes and BP ≥140/90 mm Hg, as JNC 7 did.2 This is based largely on expert opinion.
Studies suggest that adults with both hypertension and diabetes have a reduction in mortality and improved cardiovascular and cerebrovascular outcomes when systolic BP is <150 mm Hg,13-15 but no strong data support a goal of <140/90 mm Hg. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial, for example, showed comparable outcomes in patients with systolic BP of 150 or 140 mm Hg.16 The use of expert opinion vs well-designed studies in this instance seems at odds with JNC 8’s general policy of placing greater emphasis on evidence.
CASE › On her second visit, Ms. S’s BP is 144/82 mm Hg and her cholesterol levels are within the normal range. Her fasting glucose level is 104 mg/dL and glycated hemoglobin (HbA1c) is 6%. At a repeat visit one month later, her BP is 146/76 mm Hg. Given these 2 acceptable readings (<150/90 mm Hg for individuals age 60 and older who do not have diabetes), you do not initiate antihypertensive treatment.
However, you explain to the patient that her fasting glucose and HbA1c are evidence of insulin resistance. Although a diagnosis of diabetes is not warranted, you arrange for Ms. S to meet with a diabetes nurse educator for help in improving her diet and following an exercise regimen.
Pharmacotherapy: JNC 8 offers wider latitude
Like its predecessor, JNC 8 stresses the importance of diet and exercise. (See “Controlling hypertension starts with lifestyle modification”17 in this article.) It diverges from JNC 7, however, in its recommendations for initiating treatment (ALGORITHM).1 The earlier version recommended thiazide diuretics as first-line therapy but included multiple indications for initiating therapy with other drug classes. JNC 8 guidelines are less specific.
Starting therapy with a thiazide diuretic, angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), or calcium channel blocker (CCB)—all of which have high-quality evidence of improved outcomes18-20—is recommended for most patients, including those with diabetes. (Blacks and patients with CKD are exceptions.) The recommended doses of these medications, summarized in the TABLE,1,21 are similar to those used in RCTs. Other types of drugs are not recommended, either because they were shown to be inferior to another class of antihypertensive or because there is insufficient evidence of their efficacy.
For most blacks... JNC 8 recommends thiazide diuretics and CCBs as first-line therapy—a recommendation that is evidence-based. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)22 revealed that black patients taking thiazide diuretics had fewer cerebrovascular and cardiovascular events and a lower rate of HF compared with those taking ACEIs, whether or not they had diabetes. Diuretics were more effective than CCBs in preventing HF, but no difference in rates of cerebrovascular and cardiovascular events, kidney disease, or overall mortality was found.22
For patients with CKD and proteinuria, regardless of race, JNC 8 calls for either an ACEI or an ARB as first-line agent to prevent progression to end-stage renal disease. This recommendation is based on expert consensus, and intended to prevent progression to end-stage renal disease.1,23
The optimal first-line agent for patients who have CKD without proteinuria is less clear. For such patients, JNC 8 notes, any of the 4 recommended drug classes can be used for initial therapy.1
Guidance on starting—and titrating—therapy
JNC 7 guidelines featured a complex means of diagnosing and monitoring hypertension.2 JNC 8 has simplified the recommendations, which call for patients to be reassessed within a month of initiating therapy.
The new guidelines include 3 distinct methods of dosing antihypertensive medications, none of which has demonstrated better outcomes than any other. All call for replacing one type of drug with another if the first trial is ineffective or results in adverse effects. And all stress the importance of avoiding ACEI and ARB combinations due to increases in serum creatinine and hyperkalemia and the need for monitoring. Note, however, that Method 3 is recommended for patients with more severe hypertension.1
Method 1. Initiate one medication from any of the 4 classes of antihypertensives recommended for initial treatment, and titrate to the maximum effective dose. If the BP goal is not achieved at maximum dose, add a medication from a second class and titrate that drug to the maximum effective dose, as well. If the goal is still not reached, add a medication from a third class and titrate up as needed.
Method 2. Initiate one medication, then add a second agent from a different drug class, if necessary, and titrate until both are at the maximum effective dose. If the goal still has not been reached, add a third agent and titrate that until BP is well controlled.
Method 3. Initiate 2 medications from 2 different classes of drugs simultaneously. If BP is not at goal after a reasonable trial, add a third agent and titrate to maximum effective dose. (Use this approach for patients who have systolic BP >160 mm Hg and/or diastolic BP >100 mm Hg or systolic BP >20 mm Hg above goal and/or diastolic BP >10 mm Hg above goal.)
As a general rule, a trial with monotherapy should be considered if BP is ≤160/100; a 2-agent combination is recommended as first-line therapy for pressure that exceeds that threshold. If a patient’s BP target is not reached even with the above strategies, a consultation with a hypertension specialist may be needed.
Treating patients with cardiovascular comorbidities
As noted earlier, JNC 8 offers no guidance in treating patients with HF or CAD and multiple comorbidities. In such cases, we turn to the American College of Cardiology (ACC) and American Heart Association (AHA).24
Recent ACC/AHA guidelines recommend a beta-blocker and ACEI for patients with a history of symptomatic stable HF and a left ventricular ejection fraction (EF) ≤40%, unless contraindications exist.24 Beta-blockers and an ACEI or an ARB should be used to prevent HF in patients with a history of myocardial infarction (MI) or acute coronary syndrome and a reduced EF. Beta-blockers with evidence to support their use in such cases include carvedilol, bisoprolol, and sustained-release metoprolol succinate.24
For symptomatic patients with dyspnea or other mild fluid retention, a loop diuretic or a thiazide diuretic can be used. Nondihydropyridine CCBs should be avoided in post-MI patients with low left ventricular EF due to the medication’s negative inotropic effects.24 The optimal drug regimen for secondary stroke prevention is not clear due to a lack of studies comparing drug regimens, but data suggest that a diuretic or a diuretic-ACEI combination is beneficial.25
Evaluating treatment-resistant hypertension
When a patient presents with treatment-resistant hypertension—elevated BP that is not controlled with a 3-drug regimen, all at maximum doses—start by asking several questions.26 Is the patient:
- having difficulty following a drug regimen that calls for multiple daily doses?
- drinking excessive amounts of alcohol?
- failing to adhere to a low-salt dietary regimen?
- taking any other medications or supplements that might elevate BP (eg, nonsteroidal anti-inflammatory agents, pseudoephedrine, ephedra, or licorice)?
- unable to afford all the drugs prescribed?
If no such issues are identified, consider a referral to a specialist for further evaluation and to rule out disorders associated with treatment-resistant hypertension, including CKD, renal artery stenosis, hyperaldosteronemia, sleep apnea, and coarctation of the aorta.26
For most people, cardiovascular health is dependent on exercise and weight control. That’s particularly true for those with hypertension, for whom limiting alcohol and salt consumption is crucial, as well.
JNC 8 calls for lifestyle management,1 but specific recommendations come from the American College of Cardiology (ACC)/American Heart Association (AHA)’s 2013 Lifestyle Work Group.17 The guidelines call for patients with elevated blood pressure (BP) to follow a diet rich in vegetables, fruits, and whole grains, including low-fat dairy, poultry, fish, legumes, nuts, and nontropical vegetable oils, such as the DASH (Dietary Approaches to Stop Hypertension) or AHA diet. Salt consumption should not exceed 2400 mg/d—and, ideally, be limited to 1500 mg/d or reflect a reduction of at least 1000 mg/d.17
Stress the importance of regular physical activity in controlling BP, as well. The ACC/AHA call for adults to engage in moderate to vigorous aerobic activity 3 to 4 times a week, averaging about 40 minutes per session.17
CASE › When Ms. S returns 3 months later, her BP is 140/70 mm Hg, her fasting glucose is 94 mg/dL, and her HbA1c is 5.7%. You encourage her to continue her new dietary and exercise regimen and schedule a follow-up visit in 6 months.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy, Health Sciences Center, Room 292, 1000 East University Avenue, Department 3375, Laramie, WY 82071; [email protected]
› Initiate pharmacologic treatment for patients
60 years or older with systolic blood pressure (BP) ≥150 mm Hg and/or diastolic BP ≥90 mm Hg. A
› Start antihypertensive treatment for systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg in patients who are younger than 60 or have chronic kidney disease or diabetes. C
› Select either a thiazide diuretic or a calcium channel blocker as first-line therapy for African Americans, whether or not they have diabetes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carla S is a 64-year old African American whom you’re seeing for the first time. Her health has been excellent over the last 10 years, she reports, with one caveat: She has “borderline hypertension,” but has never been treated for it and denies any symptoms. Her blood pressure (BP) today is 154/82 mm Hg. A physical exam is unremarkable. Blood tests reveal a normal blood count and normal renal function, and a nonfasting glucose level of 145 mg/dL. You ask Ms. S to return in a week for a repeat BP and fasting lab work.
Hypertension is the most common condition seen by physicians in primary care,1 and a major risk factor for cardiovascular disease (CVD) and the morbidity and mortality associated with it. US treatment costs are an estimated $131 billion per year.2-4 With this in mind, the Joint National Committee on Hypertension (JNC) released its eighth report (JNC 8) in December 20131—the first update in a decade.
In many ways, JNC 8 guidelines are simpler than those of JNC 7,2 with more evidence-based recommendations and less reliance on expert opinion. The JNC has eliminated definitions such as stage 1, 2, and 3 hypertension, and focuses on outcomes instead. At the heart of the recommendations are 3 key questions:
1. At what BP should treatment be initiated to improve outcomes?
2. What should the target BP be for those undergoing treatment?
3. Which medications are best?
Answers to the first 2 questions, of course, go hand in hand. In other words, if the threshold for treatment is a systolic BP ≥140 mm Hg (more on that in a moment), then the target of treatment is a systolic BP of <140 mm Hg. In answer to the third question, JNC 8 offers guidance but gives physicians greater discretion in determining which type of drug to use when initiating treatment.1
In the text, algorithm, and table that follow, we present an overview of JNC 8. We also discuss the optimal treatment of hypertension in patients with heart failure (HF) and coronary artery disease (CAD)—populations JNC 8 does not address.
Age-based recommendations are a bit less stringent
60 years and older. Unlike JNC 7, which recommended initiating treatment for otherwise healthy patients of all ages with a BP ≥140/90 mm Hg,2 JNC 8 clearly delineates its recommendations by age. It calls for treating patients ages 60 or older with systolic BP ≥150 mm Hg and/or diastolic BP ≥90.1
The change is evidence-based: Moderate- to high-quality randomized controlled trials (RCTs) have found a reduced incidence of stroke, HF, and coronary heart disease when BP was treated to <150/90, but no additional benefit from a systolic BP target of <140 mm Hg for patients in this age group.5,6 Notably, JNC 8 does not recommend a change in medication for patients 60 years or older for whom the more stringent target is being maintained without adverse effects.1
18 to 59 years. For adults younger than 60, JNC 8 recommends treating systolic BP ≥140 and diastolic BP ≥90 mm Hg.1 The systolic BP guideline is based on expert opinion, however, as there is no high-quality evidence for a systolic threshold in this age group. This is largely because most patients younger than 60 who have systolic BP ≥140 also have diastolic BP ≥90, making it difficult to study the treatment of systolic BP alone. High-quality trials have shown improved health outcomes when patients ages 30 to 59 years were treated for diastolic BP ≥90, however.7-12 For patients younger than 30, the recommendation for treatment of diastolic pressure is based on expert consensus, as no sufficiently high-quality evidence exists.
Targets for patients with CKD and diabetes
Chronic kidney disease (CKD). JNC 8 recommends treating patients ages 18 to 69 years who have CKD and BP ≥140/90 mm Hg. JNC 7’s more stringent recommendation—treating such patients with BP ≥130/80 mm Hg2—was relaxed because there is little evidence of a lower mortality rate or cardiovascular or cerebrovascular benefits as a result of tighter control. In patients younger than 70, CKD is defined as an estimated (or measured) glomerular filtration rate (GFR) <60 mL/min/1.73 m2 or albuminuria (>30 mg of albumin per g of creatinine).1
It is important to note that this goal does not apply to individuals who have CKD and are 70 years or older. This is due to insufficient evidence, as well as uncertainty about the accuracy of an estimated GFR in this patient population. JNC 8 recommends that treatment of BP in patients 70 or older be based on comorbidities, including albuminuria, among other patient-specific considerations.1
Diabetes. JNC 8 recommends treating patients age 18 years or older who have diabetes and BP ≥140/90 mm Hg, as JNC 7 did.2 This is based largely on expert opinion.
Studies suggest that adults with both hypertension and diabetes have a reduction in mortality and improved cardiovascular and cerebrovascular outcomes when systolic BP is <150 mm Hg,13-15 but no strong data support a goal of <140/90 mm Hg. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial, for example, showed comparable outcomes in patients with systolic BP of 150 or 140 mm Hg.16 The use of expert opinion vs well-designed studies in this instance seems at odds with JNC 8’s general policy of placing greater emphasis on evidence.
CASE › On her second visit, Ms. S’s BP is 144/82 mm Hg and her cholesterol levels are within the normal range. Her fasting glucose level is 104 mg/dL and glycated hemoglobin (HbA1c) is 6%. At a repeat visit one month later, her BP is 146/76 mm Hg. Given these 2 acceptable readings (<150/90 mm Hg for individuals age 60 and older who do not have diabetes), you do not initiate antihypertensive treatment.
However, you explain to the patient that her fasting glucose and HbA1c are evidence of insulin resistance. Although a diagnosis of diabetes is not warranted, you arrange for Ms. S to meet with a diabetes nurse educator for help in improving her diet and following an exercise regimen.
Pharmacotherapy: JNC 8 offers wider latitude
Like its predecessor, JNC 8 stresses the importance of diet and exercise. (See “Controlling hypertension starts with lifestyle modification”17 in this article.) It diverges from JNC 7, however, in its recommendations for initiating treatment (ALGORITHM).1 The earlier version recommended thiazide diuretics as first-line therapy but included multiple indications for initiating therapy with other drug classes. JNC 8 guidelines are less specific.
Starting therapy with a thiazide diuretic, angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), or calcium channel blocker (CCB)—all of which have high-quality evidence of improved outcomes18-20—is recommended for most patients, including those with diabetes. (Blacks and patients with CKD are exceptions.) The recommended doses of these medications, summarized in the TABLE,1,21 are similar to those used in RCTs. Other types of drugs are not recommended, either because they were shown to be inferior to another class of antihypertensive or because there is insufficient evidence of their efficacy.
For most blacks... JNC 8 recommends thiazide diuretics and CCBs as first-line therapy—a recommendation that is evidence-based. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)22 revealed that black patients taking thiazide diuretics had fewer cerebrovascular and cardiovascular events and a lower rate of HF compared with those taking ACEIs, whether or not they had diabetes. Diuretics were more effective than CCBs in preventing HF, but no difference in rates of cerebrovascular and cardiovascular events, kidney disease, or overall mortality was found.22
For patients with CKD and proteinuria, regardless of race, JNC 8 calls for either an ACEI or an ARB as first-line agent to prevent progression to end-stage renal disease. This recommendation is based on expert consensus, and intended to prevent progression to end-stage renal disease.1,23
The optimal first-line agent for patients who have CKD without proteinuria is less clear. For such patients, JNC 8 notes, any of the 4 recommended drug classes can be used for initial therapy.1
Guidance on starting—and titrating—therapy
JNC 7 guidelines featured a complex means of diagnosing and monitoring hypertension.2 JNC 8 has simplified the recommendations, which call for patients to be reassessed within a month of initiating therapy.
The new guidelines include 3 distinct methods of dosing antihypertensive medications, none of which has demonstrated better outcomes than any other. All call for replacing one type of drug with another if the first trial is ineffective or results in adverse effects. And all stress the importance of avoiding ACEI and ARB combinations due to increases in serum creatinine and hyperkalemia and the need for monitoring. Note, however, that Method 3 is recommended for patients with more severe hypertension.1
Method 1. Initiate one medication from any of the 4 classes of antihypertensives recommended for initial treatment, and titrate to the maximum effective dose. If the BP goal is not achieved at maximum dose, add a medication from a second class and titrate that drug to the maximum effective dose, as well. If the goal is still not reached, add a medication from a third class and titrate up as needed.
Method 2. Initiate one medication, then add a second agent from a different drug class, if necessary, and titrate until both are at the maximum effective dose. If the goal still has not been reached, add a third agent and titrate that until BP is well controlled.
Method 3. Initiate 2 medications from 2 different classes of drugs simultaneously. If BP is not at goal after a reasonable trial, add a third agent and titrate to maximum effective dose. (Use this approach for patients who have systolic BP >160 mm Hg and/or diastolic BP >100 mm Hg or systolic BP >20 mm Hg above goal and/or diastolic BP >10 mm Hg above goal.)
As a general rule, a trial with monotherapy should be considered if BP is ≤160/100; a 2-agent combination is recommended as first-line therapy for pressure that exceeds that threshold. If a patient’s BP target is not reached even with the above strategies, a consultation with a hypertension specialist may be needed.
Treating patients with cardiovascular comorbidities
As noted earlier, JNC 8 offers no guidance in treating patients with HF or CAD and multiple comorbidities. In such cases, we turn to the American College of Cardiology (ACC) and American Heart Association (AHA).24
Recent ACC/AHA guidelines recommend a beta-blocker and ACEI for patients with a history of symptomatic stable HF and a left ventricular ejection fraction (EF) ≤40%, unless contraindications exist.24 Beta-blockers and an ACEI or an ARB should be used to prevent HF in patients with a history of myocardial infarction (MI) or acute coronary syndrome and a reduced EF. Beta-blockers with evidence to support their use in such cases include carvedilol, bisoprolol, and sustained-release metoprolol succinate.24
For symptomatic patients with dyspnea or other mild fluid retention, a loop diuretic or a thiazide diuretic can be used. Nondihydropyridine CCBs should be avoided in post-MI patients with low left ventricular EF due to the medication’s negative inotropic effects.24 The optimal drug regimen for secondary stroke prevention is not clear due to a lack of studies comparing drug regimens, but data suggest that a diuretic or a diuretic-ACEI combination is beneficial.25
Evaluating treatment-resistant hypertension
When a patient presents with treatment-resistant hypertension—elevated BP that is not controlled with a 3-drug regimen, all at maximum doses—start by asking several questions.26 Is the patient:
- having difficulty following a drug regimen that calls for multiple daily doses?
- drinking excessive amounts of alcohol?
- failing to adhere to a low-salt dietary regimen?
- taking any other medications or supplements that might elevate BP (eg, nonsteroidal anti-inflammatory agents, pseudoephedrine, ephedra, or licorice)?
- unable to afford all the drugs prescribed?
If no such issues are identified, consider a referral to a specialist for further evaluation and to rule out disorders associated with treatment-resistant hypertension, including CKD, renal artery stenosis, hyperaldosteronemia, sleep apnea, and coarctation of the aorta.26
For most people, cardiovascular health is dependent on exercise and weight control. That’s particularly true for those with hypertension, for whom limiting alcohol and salt consumption is crucial, as well.
JNC 8 calls for lifestyle management,1 but specific recommendations come from the American College of Cardiology (ACC)/American Heart Association (AHA)’s 2013 Lifestyle Work Group.17 The guidelines call for patients with elevated blood pressure (BP) to follow a diet rich in vegetables, fruits, and whole grains, including low-fat dairy, poultry, fish, legumes, nuts, and nontropical vegetable oils, such as the DASH (Dietary Approaches to Stop Hypertension) or AHA diet. Salt consumption should not exceed 2400 mg/d—and, ideally, be limited to 1500 mg/d or reflect a reduction of at least 1000 mg/d.17
Stress the importance of regular physical activity in controlling BP, as well. The ACC/AHA call for adults to engage in moderate to vigorous aerobic activity 3 to 4 times a week, averaging about 40 minutes per session.17
CASE › When Ms. S returns 3 months later, her BP is 140/70 mm Hg, her fasting glucose is 94 mg/dL, and her HbA1c is 5.7%. You encourage her to continue her new dietary and exercise regimen and schedule a follow-up visit in 6 months.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy, Health Sciences Center, Room 292, 1000 East University Avenue, Department 3375, Laramie, WY 82071; [email protected]
1. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
2. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
4. Heidenreich PA, Trogdon JG, Khavjou OA, et al; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944.
5. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
6. Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
7. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
8. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. III. Reduction in stroke incidence among persons with high blood pressure. JAMA. 1982;247:633-638.
9. Hypertension-Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. JAMA. 1974;229:409-418.
10. Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Br Med J (Clin Res Ed). 1985;291:97-104.
11. The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet. 1980;1:1261-1267.
12. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
13. Curb JD, Pressel SL, Cutler JA, et al; Systolic Hypertension in the Elderly Program Cooperative Research Group. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276:1886-1892.
14. Tuomilehto J, Rastenyte D, Birkenhäger WH, et al; Systolic Hypertension in Europe Trial Investigators. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med. 1999;340:677-684.
15. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
16. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99.
18. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255-3264.
19. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
20. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
21. Mann JFE. Choice of drug therapy in primary (essential) hypertension: recommendations. UpToDate Web site. Available at: http://www.uptodate.com/contents/choice-of-drug-therapy-in-primary-essential-hypertension-recommendations. Accessed March 3, 2014.
22. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997.
23. Wright JT Jr, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
24. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e319.
25. Furie KL, Kasner SE, Adams RJ, et al; American Heart Assocaition Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Stroke. 2011;42:227-276.
26. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14-26.
1. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
2. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
4. Heidenreich PA, Trogdon JG, Khavjou OA, et al; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944.
5. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
6. Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
7. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
8. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. III. Reduction in stroke incidence among persons with high blood pressure. JAMA. 1982;247:633-638.
9. Hypertension-Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. JAMA. 1974;229:409-418.
10. Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Br Med J (Clin Res Ed). 1985;291:97-104.
11. The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet. 1980;1:1261-1267.
12. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
13. Curb JD, Pressel SL, Cutler JA, et al; Systolic Hypertension in the Elderly Program Cooperative Research Group. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276:1886-1892.
14. Tuomilehto J, Rastenyte D, Birkenhäger WH, et al; Systolic Hypertension in Europe Trial Investigators. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med. 1999;340:677-684.
15. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
16. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99.
18. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255-3264.
19. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
20. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
21. Mann JFE. Choice of drug therapy in primary (essential) hypertension: recommendations. UpToDate Web site. Available at: http://www.uptodate.com/contents/choice-of-drug-therapy-in-primary-essential-hypertension-recommendations. Accessed March 3, 2014.
22. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997.
23. Wright JT Jr, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
24. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e319.
25. Furie KL, Kasner SE, Adams RJ, et al; American Heart Assocaition Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Stroke. 2011;42:227-276.
26. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14-26.
Statin adverse effects: Sorting out the evidence
› Advise patients starting statin therapy to stop taking the medication and call your office immediately if they develop severe muscle pain or weakness, as statins are associated with a small increased risk of rhabdomyolysis. B
› Obtain a baseline creatine kinase level for patients with an increased risk of musculoskeletal disorders; routine monitoring is needed only for those who experience muscle pain or weakness while on statin therapy. C
› Prescribe statins for patients with chronic kidney or liver disease when indicated; statin therapy is not associated with an increased risk of renal or hepatic failure. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carl L, a 57-year-old obese patient (body mass index [BMI] >40) who had not been to a doctor in a decade, comes to see you after a health fair screening revealed dyslipidemia (low-density lipoprotein [LDL] cholesterol, 188 mg/dL; high-density lipoprotein cholesterol [HDL], 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg and his fasting glucose is 101 mg/dL. Labs drawn that day reveal a glycated hemoglobin (HbA1c) of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr. L also has a prominent family history of heart disease.
Mr. L agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and schedule a follow-up visit in 8 weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose. (To read about the controversy the recommendations generated, see “The new cholesterol guideline: Beyond the headlines,” J Fam Pract. 2013;62:730.)
Based on the new recommendations, the use of statins is likely to rise.2 (A statin [rosuvastatin] is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for physicians to be knowledgeable about the risks associated with statins and able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of >100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and >240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients on placebo.4
Such discrepancies regarding particular risks as well as the overall incidence of AEs and discontinuation rates make the evidence difficult to sort out. We created this update with that in mind.
Musculoskeletal symptoms are most common
Skeletal muscle symptoms are the most common AEs reported by patients taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms usually are symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk of statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
A baseline CK is recommended for patients with an increased risk of muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age >75 years, low levels of vitamin D, and concomitant use of medications that may increase the risk of myopathy (TABLE 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from <1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of <.06% over 5 years.1
A 2006 systematic review estimated the absolute risk of rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See TABLE 212,13 for more on drug-drug interactions.) But both the meta-analysis cited earlier4 and an earlier systematic review14 (35 RCTs and >74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk of statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug-drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the US Food and Drug Administration (FDA) cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N=12,064) that found an increased risk of myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the 80-mg dose vs those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction >50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80 mg simvastatin were excluded.17
THE BOTTOM LINE: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses <300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE › On his next visit, Mr. L reports a new ache in his left shoulder and upper back, which he describes as mild, but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr. L says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation, but with no decline in strength. Mr. L’s BP has fallen to 134/84 mm Hg and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, HbA1c of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only over the counter, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Hepatic effects are rare
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk of acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be <1.5% over the course of 5 years, and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk of myopathy, and providing additional information about drug-drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk of transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk of transaminase elevation (odds ratio [OR], 1.51; 95% confidence interval [CI], 1.24-1.84) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
THE BOTTOM LINE: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
Statin use and diabetes: Is there a link?
Recent studies have found an increased risk of new-onset type 2 diabetes in statin users, with a greater risk associated with higher potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1000 patient-years for moderate-intensity therapy and 3 per 1000 patient years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk of diabetes was relatively small (OR=1.09; 95% CI, 1.02-1.16).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and >110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
THE BOTTOM LINE: Physicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
Statins may be renoprotective
Statin use has been found to be associated with an increased risk of tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated, but does recommend a lower dose for patients with CKD.28
THE BOTTOM LINE: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Intracerebral hemorrhage: Statins increase recurrence risk
In recent years there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n=93) found that the absolute risk of recurrent ICH was 15.6% for patients randomized to atorvastatin vs 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, the increased risk was statistically significant in multivariate analysis (hazard ratio [HR]=1.69; 95% CI, 1.1-2.6).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (>40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
THE BOTTOM LINE: Physicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
Other serious adverse effects: Which reports are accurate?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk of malignancy has been considered for years. A large trial (N=5804) from 2002 found a correlation between pravastatin and an increased risk of new cancer diagnoses compared with placebo (HR=1.25; 95% CI, 1.04-1.51; P=.02).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk of developing cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before considering a modification of statin therapy, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash, among other AEs (TABLE 3).50
Which drug? Potential differences in statins
The meta-analysis with >240,000 participants evaluated patients taking 7 different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (>40 mg/d) significantly increased the risk of CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk of rhabdomyolysis at the highest dose.15,16
Are statins safe for these patients?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding,1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk of AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk of AEs in Asian patients on statins.52 (To read more about statin use in particular patient populations, see “Statin therapy: When to think twice,” J Fam Pract. 2013;62:726-732.)
Patient factors that increase risk
Risk factors for statin-induced AEs include:1
- multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
- unexplained ALT elevation >3 times the upper limit of normal
- history of prior statin intolerance or concomitant use of drugs that affect statin metabolism
- age >75 years
- preexisting muscle disorders
- low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE › The risk of elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr. L’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in 6 months, Mr. L reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected]
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. Medscape Web site. Available at: http://www.medscape.com/viewarticle/813571#3. Accessed December 11, 2013.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy. J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173: 1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19: 403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. Elsevier/Gold Standard Web site. Available at: http://www.goldstandard.com/product/gold-standard-drug-database/. Accessed December 4, 2013.
13. US Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed July 23, 2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. US Food and Drug Administration. FDA drug safety communication: Ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed December 9, 2013.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. University of Wyoming Libraries Web site. Available at: http://www.naturaldatabase.com.libproxy.uwyo.edu. Accessed December 4, 2013.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5): S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.
› Advise patients starting statin therapy to stop taking the medication and call your office immediately if they develop severe muscle pain or weakness, as statins are associated with a small increased risk of rhabdomyolysis. B
› Obtain a baseline creatine kinase level for patients with an increased risk of musculoskeletal disorders; routine monitoring is needed only for those who experience muscle pain or weakness while on statin therapy. C
› Prescribe statins for patients with chronic kidney or liver disease when indicated; statin therapy is not associated with an increased risk of renal or hepatic failure. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carl L, a 57-year-old obese patient (body mass index [BMI] >40) who had not been to a doctor in a decade, comes to see you after a health fair screening revealed dyslipidemia (low-density lipoprotein [LDL] cholesterol, 188 mg/dL; high-density lipoprotein cholesterol [HDL], 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg and his fasting glucose is 101 mg/dL. Labs drawn that day reveal a glycated hemoglobin (HbA1c) of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr. L also has a prominent family history of heart disease.
Mr. L agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and schedule a follow-up visit in 8 weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose. (To read about the controversy the recommendations generated, see “The new cholesterol guideline: Beyond the headlines,” J Fam Pract. 2013;62:730.)
Based on the new recommendations, the use of statins is likely to rise.2 (A statin [rosuvastatin] is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for physicians to be knowledgeable about the risks associated with statins and able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of >100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and >240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients on placebo.4
Such discrepancies regarding particular risks as well as the overall incidence of AEs and discontinuation rates make the evidence difficult to sort out. We created this update with that in mind.
Musculoskeletal symptoms are most common
Skeletal muscle symptoms are the most common AEs reported by patients taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms usually are symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk of statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
A baseline CK is recommended for patients with an increased risk of muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age >75 years, low levels of vitamin D, and concomitant use of medications that may increase the risk of myopathy (TABLE 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from <1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of <.06% over 5 years.1
A 2006 systematic review estimated the absolute risk of rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See TABLE 212,13 for more on drug-drug interactions.) But both the meta-analysis cited earlier4 and an earlier systematic review14 (35 RCTs and >74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk of statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug-drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the US Food and Drug Administration (FDA) cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N=12,064) that found an increased risk of myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the 80-mg dose vs those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction >50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80 mg simvastatin were excluded.17
THE BOTTOM LINE: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses <300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE › On his next visit, Mr. L reports a new ache in his left shoulder and upper back, which he describes as mild, but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr. L says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation, but with no decline in strength. Mr. L’s BP has fallen to 134/84 mm Hg and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, HbA1c of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only over the counter, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Hepatic effects are rare
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk of acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be <1.5% over the course of 5 years, and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk of myopathy, and providing additional information about drug-drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk of transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk of transaminase elevation (odds ratio [OR], 1.51; 95% confidence interval [CI], 1.24-1.84) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
THE BOTTOM LINE: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
Statin use and diabetes: Is there a link?
Recent studies have found an increased risk of new-onset type 2 diabetes in statin users, with a greater risk associated with higher potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1000 patient-years for moderate-intensity therapy and 3 per 1000 patient years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk of diabetes was relatively small (OR=1.09; 95% CI, 1.02-1.16).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and >110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
THE BOTTOM LINE: Physicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
Statins may be renoprotective
Statin use has been found to be associated with an increased risk of tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated, but does recommend a lower dose for patients with CKD.28
THE BOTTOM LINE: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Intracerebral hemorrhage: Statins increase recurrence risk
In recent years there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n=93) found that the absolute risk of recurrent ICH was 15.6% for patients randomized to atorvastatin vs 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, the increased risk was statistically significant in multivariate analysis (hazard ratio [HR]=1.69; 95% CI, 1.1-2.6).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (>40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
THE BOTTOM LINE: Physicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
Other serious adverse effects: Which reports are accurate?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk of malignancy has been considered for years. A large trial (N=5804) from 2002 found a correlation between pravastatin and an increased risk of new cancer diagnoses compared with placebo (HR=1.25; 95% CI, 1.04-1.51; P=.02).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk of developing cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before considering a modification of statin therapy, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash, among other AEs (TABLE 3).50
Which drug? Potential differences in statins
The meta-analysis with >240,000 participants evaluated patients taking 7 different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (>40 mg/d) significantly increased the risk of CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk of rhabdomyolysis at the highest dose.15,16
Are statins safe for these patients?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding,1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk of AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk of AEs in Asian patients on statins.52 (To read more about statin use in particular patient populations, see “Statin therapy: When to think twice,” J Fam Pract. 2013;62:726-732.)
Patient factors that increase risk
Risk factors for statin-induced AEs include:1
- multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
- unexplained ALT elevation >3 times the upper limit of normal
- history of prior statin intolerance or concomitant use of drugs that affect statin metabolism
- age >75 years
- preexisting muscle disorders
- low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE › The risk of elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr. L’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in 6 months, Mr. L reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected]
› Advise patients starting statin therapy to stop taking the medication and call your office immediately if they develop severe muscle pain or weakness, as statins are associated with a small increased risk of rhabdomyolysis. B
› Obtain a baseline creatine kinase level for patients with an increased risk of musculoskeletal disorders; routine monitoring is needed only for those who experience muscle pain or weakness while on statin therapy. C
› Prescribe statins for patients with chronic kidney or liver disease when indicated; statin therapy is not associated with an increased risk of renal or hepatic failure. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carl L, a 57-year-old obese patient (body mass index [BMI] >40) who had not been to a doctor in a decade, comes to see you after a health fair screening revealed dyslipidemia (low-density lipoprotein [LDL] cholesterol, 188 mg/dL; high-density lipoprotein cholesterol [HDL], 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg and his fasting glucose is 101 mg/dL. Labs drawn that day reveal a glycated hemoglobin (HbA1c) of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr. L also has a prominent family history of heart disease.
Mr. L agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and schedule a follow-up visit in 8 weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose. (To read about the controversy the recommendations generated, see “The new cholesterol guideline: Beyond the headlines,” J Fam Pract. 2013;62:730.)
Based on the new recommendations, the use of statins is likely to rise.2 (A statin [rosuvastatin] is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for physicians to be knowledgeable about the risks associated with statins and able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of >100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and >240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients on placebo.4
Such discrepancies regarding particular risks as well as the overall incidence of AEs and discontinuation rates make the evidence difficult to sort out. We created this update with that in mind.
Musculoskeletal symptoms are most common
Skeletal muscle symptoms are the most common AEs reported by patients taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms usually are symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk of statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
A baseline CK is recommended for patients with an increased risk of muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age >75 years, low levels of vitamin D, and concomitant use of medications that may increase the risk of myopathy (TABLE 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from <1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of <.06% over 5 years.1
A 2006 systematic review estimated the absolute risk of rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See TABLE 212,13 for more on drug-drug interactions.) But both the meta-analysis cited earlier4 and an earlier systematic review14 (35 RCTs and >74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk of statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug-drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the US Food and Drug Administration (FDA) cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N=12,064) that found an increased risk of myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the 80-mg dose vs those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction >50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80 mg simvastatin were excluded.17
THE BOTTOM LINE: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses <300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE › On his next visit, Mr. L reports a new ache in his left shoulder and upper back, which he describes as mild, but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr. L says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation, but with no decline in strength. Mr. L’s BP has fallen to 134/84 mm Hg and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, HbA1c of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only over the counter, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Hepatic effects are rare
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk of acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be <1.5% over the course of 5 years, and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk of myopathy, and providing additional information about drug-drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk of transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk of transaminase elevation (odds ratio [OR], 1.51; 95% confidence interval [CI], 1.24-1.84) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
THE BOTTOM LINE: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
Statin use and diabetes: Is there a link?
Recent studies have found an increased risk of new-onset type 2 diabetes in statin users, with a greater risk associated with higher potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1000 patient-years for moderate-intensity therapy and 3 per 1000 patient years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk of diabetes was relatively small (OR=1.09; 95% CI, 1.02-1.16).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and >110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
THE BOTTOM LINE: Physicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
Statins may be renoprotective
Statin use has been found to be associated with an increased risk of tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated, but does recommend a lower dose for patients with CKD.28
THE BOTTOM LINE: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Intracerebral hemorrhage: Statins increase recurrence risk
In recent years there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n=93) found that the absolute risk of recurrent ICH was 15.6% for patients randomized to atorvastatin vs 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, the increased risk was statistically significant in multivariate analysis (hazard ratio [HR]=1.69; 95% CI, 1.1-2.6).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (>40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
THE BOTTOM LINE: Physicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
Other serious adverse effects: Which reports are accurate?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk of malignancy has been considered for years. A large trial (N=5804) from 2002 found a correlation between pravastatin and an increased risk of new cancer diagnoses compared with placebo (HR=1.25; 95% CI, 1.04-1.51; P=.02).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk of developing cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before considering a modification of statin therapy, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash, among other AEs (TABLE 3).50
Which drug? Potential differences in statins
The meta-analysis with >240,000 participants evaluated patients taking 7 different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (>40 mg/d) significantly increased the risk of CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk of rhabdomyolysis at the highest dose.15,16
Are statins safe for these patients?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding,1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk of AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk of AEs in Asian patients on statins.52 (To read more about statin use in particular patient populations, see “Statin therapy: When to think twice,” J Fam Pract. 2013;62:726-732.)
Patient factors that increase risk
Risk factors for statin-induced AEs include:1
- multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
- unexplained ALT elevation >3 times the upper limit of normal
- history of prior statin intolerance or concomitant use of drugs that affect statin metabolism
- age >75 years
- preexisting muscle disorders
- low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE › The risk of elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr. L’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in 6 months, Mr. L reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected]
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. Medscape Web site. Available at: http://www.medscape.com/viewarticle/813571#3. Accessed December 11, 2013.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy. J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173: 1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19: 403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. Elsevier/Gold Standard Web site. Available at: http://www.goldstandard.com/product/gold-standard-drug-database/. Accessed December 4, 2013.
13. US Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed July 23, 2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. US Food and Drug Administration. FDA drug safety communication: Ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed December 9, 2013.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. University of Wyoming Libraries Web site. Available at: http://www.naturaldatabase.com.libproxy.uwyo.edu. Accessed December 4, 2013.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5): S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. Medscape Web site. Available at: http://www.medscape.com/viewarticle/813571#3. Accessed December 11, 2013.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy. J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173: 1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19: 403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. Elsevier/Gold Standard Web site. Available at: http://www.goldstandard.com/product/gold-standard-drug-database/. Accessed December 4, 2013.
13. US Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed July 23, 2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. US Food and Drug Administration. FDA drug safety communication: Ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed December 9, 2013.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. University of Wyoming Libraries Web site. Available at: http://www.naturaldatabase.com.libproxy.uwyo.edu. Accessed December 4, 2013.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5): S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.