User login
Painless Purple Streaks on the Arms and Chest
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
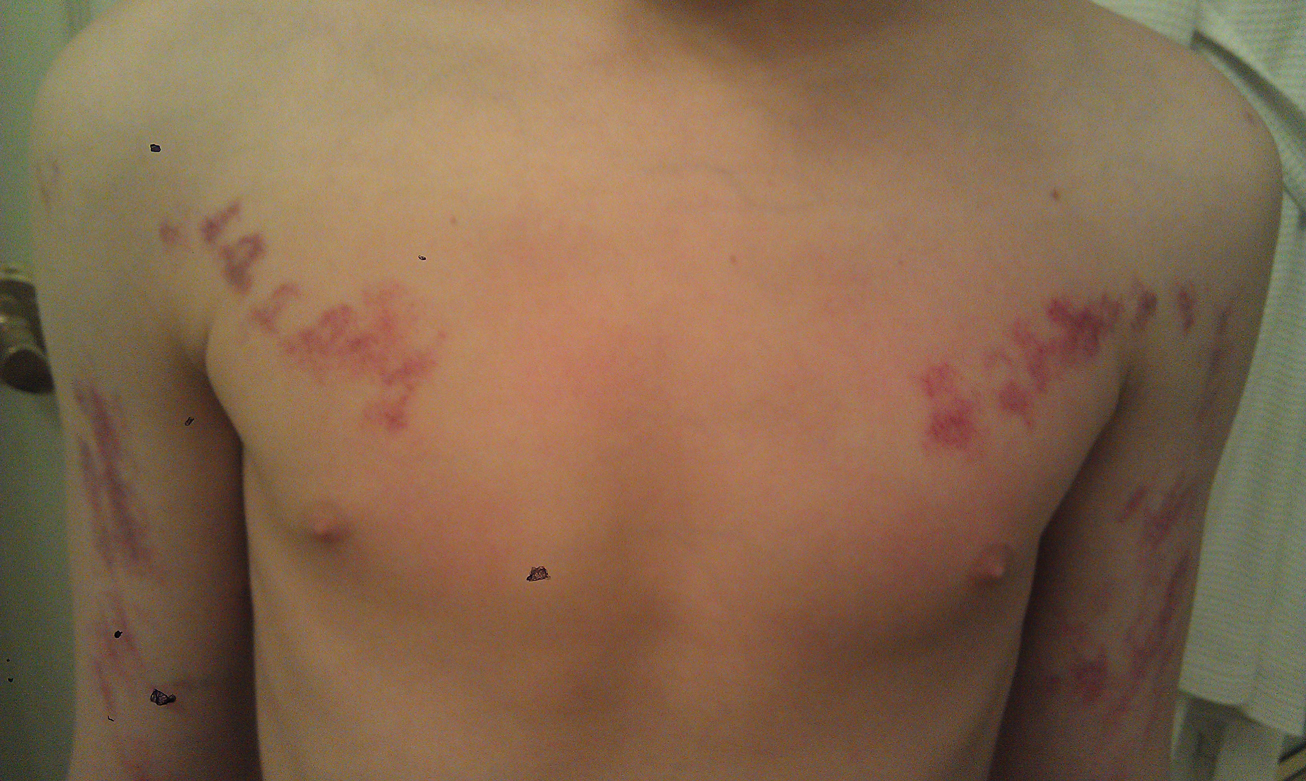
A 10-year-old boy presented with painless purple streaks on the arms and chest of 2 months' duration. The rash recurred several times per month and cleared without treatment in 3 to 5 days. There was no history of trauma or medication exposure, and he was growing and developing normally.
Depigmented plaques on vulva
A mother brought her 8-year-old daughter to our office for evaluation of vitiligo “down there” (FIGURE). The skin eruption first appeared on her vulva a year earlier and was intermittently pruritic. The lesions were initially smaller and red, but had since lightened in color, coalesced, and had begun to spread to the perianal area. The patient’s mother had received a call from her daughter’s teacher who observed that her daughter was scratching the area and might be masturbating in class.
The mother reported that 6 months earlier, her daughter had experienced bloody spots in her underwear accompanied by dysuria. The mother brought her to the emergency department, where she was treated with antibiotics for a urinary tract infection.
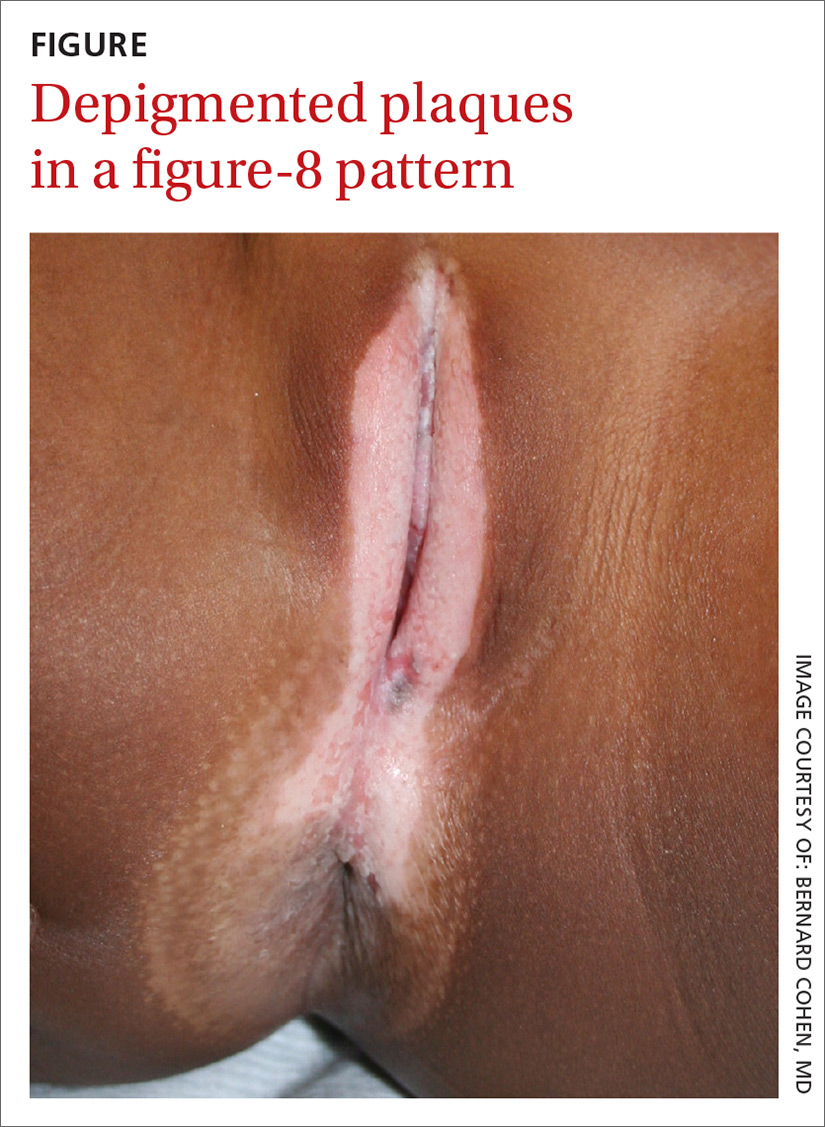
Our physical examination revealed well-circumscribed, symmetric, depigmented, confluent, crinkled, parchment-like plaques with small hemorrhagic erosions on the medial labia majora and minora. The lesions had spread to the perianal area with depigmentation superiorly and hypopigmentation inferiorly, creating a figure-8 pattern.
A review of systems was negative for pruritus, pain, dysuria, dyschezia, constipation, and vaginal discharge. The patient denied sexual activity, depression, or anxiety. Her mother denied behavioral changes in her daughter and said that her daughter hadn’t had any one-on-one time alone with any adults besides herself. Her mother was concerned that the white spots might spread to the rest of her daughter’s body, which could affect her socially.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen sclerosus
Based on the history and clinical findings, including the classic figure-8 pattern, we diagnosed childhood lichen sclerosus (LS) in this patient. LS is a chronic inflammatory skin disorder that primarily affects the genital mucosa. The disorder can present at any age, but is most common among postmenopausal women, with a prevalence estimated to be as high as one in 30.1-3 A second incidence peak is observed in prepubescent girls, with a prevalence of one in 900.3,4 LS is less common in men and boys, with a female-to-male ratio that can reach 10:1.5 The classic symptoms of LS are pruritus and pain, which may be intermittent or persistent.
In girls, initial manifestations may be constipation, dysuria, or even behavioral symptoms such as night fears, which can occur because children are less active at night and become more aware of urinary discomfort.1,2,6 Typical signs of LS are thin atrophic plaques that spare the vagina and cervix. The plaques can be ivory-white, erythematous, or violaceous. Some patients have perianal lesions as well, and can display the pathognomonic figure-8 pattern of porcelain plaques around the vulva and anus.5
With more advanced disease, erosions, lichenification, and even distortion of vulvar architecture may occur.2,4,7 In severe cases, labia resorption and clitoral phimosis may develop.5 Complications include secondary infection, dyspareunia, and psychosexual distress. The most worrisome sequela of LS is squamous cell carcinoma of the vulva (SCCV), which occurs in 5% of female patients with LS.4
In men and boys, LS typically involves the foreskin and the glans, while sparing the perianal region.5 Scarring of the foreskin can lead to phimosis, and patients may complain of painful erections and difficulty urinating. LS can also occur away from the genitalia in both males and females.
Autoimmune mechanisms, genetics, and hormones play a role
The exact pathogenesis of LS remains unknown, but multiple factors are likely at work.
Autoimmune mechanisms. Up to 60% of women with LS have an autoimmune disorder, which is most commonly vitiligo, alopecia areata, or thyroid disease.5 In addition, 67% of patients have autoantibodies against extracellular matrix protein 1, and 30% have them against bullous pemphigoid antigen 180.1,8
Genetics. LS is associated with certain human leukocyte antigen class II haplotypes (especially DQ7) and with polymorphisms at the interleukin-1 receptor antagonist gene locus.5,6,9
Hormones. The clear peaks of incidence during times of low estrogen, and a higher incidence in patients with Turner syndrome or kidney disease, suggest that low estrogen may play a role in the development of LS, as well.1,5,6
While it is generally accepted that trauma may trigger LS via the Koebner phenomenon (the appearance of lesions at the site of injury), there is debate as to whether microbes—especially Borrelia burgdorferi and human papillomavirus (HPV)—might play a role.1,5
Diagnosis is often delayed, misdiagnosis is common
The average delay from symptom onset to diagnosis of LS is 1.3 years, and up to 84% of childhood LS is misdiagnosed before referral.2,9 The differential diagnosis includes:
Sexual abuse. In prepubertal girls presenting with genital redness, the can’t-miss diagnosis is sexual abuse, which occurs in more than 25% of children in the United States.10 Initial manifestations may be regression in developmental milestones, such as new-onset bedwetting, or behavioral changes such as social withdrawal or declining academic performance.11
However, physicians must be conscientious about ruling out medical etiologies before prematurely diagnosing abuse. Fourteen percent of girls with LS are incorrectly diagnosed as having been sexually abused.2 A clinical pearl is that while LS may resemble abuse on exam, it rarely affects the hymenal structure.12 It is also important to keep in mind that the 2 entities are not incompatible, as sexual abuse leading to LS via Koebnerization is a well-described phenomenon.12
Lichen planus. LP, which is also an immune-mediated inflammatory disorder affecting the vulva, classically presents with the 6 Ps: pruritic, polygonal, planar, purple papules and plaques.4 LP is distinguished from LS by being rare in childhood, having a predilection for the flexor wrists, and involving the oral and vaginal mucosa.4
Lichen simplex chronicus (LSC) is a chronic, circumscribed, pruritic, eczematous condition that becomes lichenified with thickened skin secondary to repeated scratching.13 Children with atopic dermatitis can develop LSC, but other children can also develop the scratch-itch cycle that results in the thickened plaques of LSC. Like LS, LSC can occur in areas other than the genitalia, including the neck and feet.14
Allergic contact dermatitis can occur in the genital area from diaper creams, soaps, and perfumes. Irritant contact dermatitis can occur from exposure to diarrhea, bedwetting, and other irritants. Contact dermatitis is less likely to have the classic figure-8 pattern seen in LS.
Psoriasis in the genital area can be confused with LS. However, psoriasis favors the groin creases in what is called inverse psoriasis. In addition, psoriasis tends to involve multiple areas, including the extensor surfaces of the elbows and knees, the nails, and the scalp.
Vitiligo can present on the genitals as circumscribed hypopigmented and depigmented patches that are flat. Vitiligo is asymptomatic, and the only pathology is the change in skin color. With LS, there is lichenification, atrophy, and sclerosis.4 Vitiligo often occurs with bilateral symmetric involvement in areas of trauma including the face, neck, scalp, elbows, wrists, hands, knees, ankles, and feet.
Treatment aims to improve symptoms
LS is usually diagnosed clinically (especially in children, as a biopsy is a great challenge to perform). However, when the clinical presentation is unclear, a skin biopsy will demonstrate the diagnostic findings of thinning of the epidermis, loss of rete pegs, hyperkeratosis, and dermal fibrosis with a T-lymphocyte-dominant inflammatory infiltrate.1,2,4,5
LS is a remitting and relapsing condition with no cure. The goals of treatment are to provide symptom relief and minimize scarring and atrophy,2 but it is unknown whether treatment reduces the risk of malignancy.9
First-line treatment for both genders and all ages is ultrapotent topical corticosteroids; clobetasol propionate 0.05% is most commonly used.1,6 Regimens vary, but the vast majority of patients improve within 3 months of once-daily treatment.4
For refractory LS, calcineurin inhibitors such as tacrolimus may be used. Although it has a black box warning regarding a potential cancer risk, long-term studies of children using tacrolimus for atopic dermatitis have not demonstrated an increased risk of malignancy.6,9 Because of a considerable adverse effect profile, oral retinoids are limited to refractory cases in adults.6 Surgery is reserved for scarring and adhesions.4
Follow-up plays an important role in management
Historically, it was believed that pediatric LS had an excellent prognosis, with patients achieving complete resolution after puberty.1,4 Recent findings have shown mixed results, with LS persisting in many patients beyond puberty.2,4 Therefore, regular follow-up is recommended every 6 to 12 months.
For uncomplicated LS, specialist follow-up is not indicated. Female patients should regularly conduct self-examinations and, at a minimum, undergo annual examinations by their primary care physician. Those who require specialist follow-up include patients with difficult-to-control symptoms, hypertrophic lesions, a history of SCCV or differentiated vulvar intraepithelial neoplasia (dVIN), or pathology showing possible dVIN.15
Our patient. We prescribed clobetasol propionate 0.05% ointment to be used once daily for 8 weeks. We stressed the importance of genital self-examinations using a mirror to monitor for any concerning changes such as skin thickening. We showed the patient and her mother photos of normal female genitalia to help normalize the genital exam, and taught the patient how to find her plaques in the mirror. We set expectations by emphasizing the chronic nature of LS and the likelihood of recurrence. We also encouraged HPV vaccination in the upcoming years to prevent both cervical cancer and HPV-related SCCV.
CORRESPONDENCE
Somya Abubucker, MD, University of Hawaii, 1356 Lusitana Street, 7th floor, Honolulu, HI 96813; [email protected].
1. Murphy R. Lichen sclerosus. Dermatol Clin. 2010;28:707-715.
2. Dendrinos ML, Quint EH. Lichen sclerosus in children and adolescents. Curr Opin Obstet Gynecol. 2013;25:370-374.
3. Eva LJ. Screening and follow up of vulval skin disorders. Best Pract Res Clin Obstet Gynaecol. 2012;26:175-188.
4. Focseneanu MA, Gupta M, Squires KC, et al. The course of lichen sclerosus diagnosed prior to puberty. J Pediatr Adolesc Gynecol. 2013;26:153-155.
5. Funaro D. Lichen sclerosus: a review and practical approach. Dermatol Ther. 2004;17:28-37.
6. Heymann WR. Lichen sclerosus. J Am Acad Dermatol. 2007;56:683-684.
7. Tong LX, Sun GS, Teng JM. Pediatric lichen sclerosus: a review of the epidemiology and treatment options. Pediatr Dermatol. 2015;32:593-599.
8. Lagerstedt M, Karvinen K, Joki-Erkkilä M, et al. Childhood lichen sclerosus—a challenge for clinicians. Pediatr Dermatol. 2013;30:444-450.
9. Keith PJ, Wolz MM, Peters MS. Eosinophils in lichen sclerosus et atrophicus. J Cutan Pathol. 2015;42:693-698.
10. National Sexual Violence Resource Center. Child sexual abuse prevention. 2011. Available at: https://www.nsvrc.org/sites/default/files/Publications_NSVRC_Overview_Child-sexual-abuse-prevention_0.pdf. Accessed February 8, 2018.
11. Dubowitz H, Lane WG. Abused and neglected children. In: Kliegman RM, Stanton BF, St. Geme JW, et al, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016:236-249.
12. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44:803-806.
13. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
14. Warshaw E, Hook K. Dermatitis. In: Soutor C, Hordinsky MK, eds. Clinical Dermatology. 1st ed. New York, NY: McGraw-Hill; 2013.
15. Jones RW, Scurry J, Neill S, et al. Guidelines for the follow-up of women with vulvar lichen sclerosus in specialist clinics. Am J Obstet Gynecol. 2008;198:496.e1-e3.
A mother brought her 8-year-old daughter to our office for evaluation of vitiligo “down there” (FIGURE). The skin eruption first appeared on her vulva a year earlier and was intermittently pruritic. The lesions were initially smaller and red, but had since lightened in color, coalesced, and had begun to spread to the perianal area. The patient’s mother had received a call from her daughter’s teacher who observed that her daughter was scratching the area and might be masturbating in class.

The mother reported that 6 months earlier, her daughter had experienced bloody spots in her underwear accompanied by dysuria. The mother brought her to the emergency department, where she was treated with antibiotics for a urinary tract infection.
Our physical examination revealed well-circumscribed, symmetric, depigmented, confluent, crinkled, parchment-like plaques with small hemorrhagic erosions on the medial labia majora and minora. The lesions had spread to the perianal area with depigmentation superiorly and hypopigmentation inferiorly, creating a figure-8 pattern.
A review of systems was negative for pruritus, pain, dysuria, dyschezia, constipation, and vaginal discharge. The patient denied sexual activity, depression, or anxiety. Her mother denied behavioral changes in her daughter and said that her daughter hadn’t had any one-on-one time alone with any adults besides herself. Her mother was concerned that the white spots might spread to the rest of her daughter’s body, which could affect her socially.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen sclerosus
Based on the history and clinical findings, including the classic figure-8 pattern, we diagnosed childhood lichen sclerosus (LS) in this patient. LS is a chronic inflammatory skin disorder that primarily affects the genital mucosa. The disorder can present at any age, but is most common among postmenopausal women, with a prevalence estimated to be as high as one in 30.1-3 A second incidence peak is observed in prepubescent girls, with a prevalence of one in 900.3,4 LS is less common in men and boys, with a female-to-male ratio that can reach 10:1.5 The classic symptoms of LS are pruritus and pain, which may be intermittent or persistent.
In girls, initial manifestations may be constipation, dysuria, or even behavioral symptoms such as night fears, which can occur because children are less active at night and become more aware of urinary discomfort.1,2,6 Typical signs of LS are thin atrophic plaques that spare the vagina and cervix. The plaques can be ivory-white, erythematous, or violaceous. Some patients have perianal lesions as well, and can display the pathognomonic figure-8 pattern of porcelain plaques around the vulva and anus.5
With more advanced disease, erosions, lichenification, and even distortion of vulvar architecture may occur.2,4,7 In severe cases, labia resorption and clitoral phimosis may develop.5 Complications include secondary infection, dyspareunia, and psychosexual distress. The most worrisome sequela of LS is squamous cell carcinoma of the vulva (SCCV), which occurs in 5% of female patients with LS.4
In men and boys, LS typically involves the foreskin and the glans, while sparing the perianal region.5 Scarring of the foreskin can lead to phimosis, and patients may complain of painful erections and difficulty urinating. LS can also occur away from the genitalia in both males and females.
Autoimmune mechanisms, genetics, and hormones play a role
The exact pathogenesis of LS remains unknown, but multiple factors are likely at work.
Autoimmune mechanisms. Up to 60% of women with LS have an autoimmune disorder, which is most commonly vitiligo, alopecia areata, or thyroid disease.5 In addition, 67% of patients have autoantibodies against extracellular matrix protein 1, and 30% have them against bullous pemphigoid antigen 180.1,8
Genetics. LS is associated with certain human leukocyte antigen class II haplotypes (especially DQ7) and with polymorphisms at the interleukin-1 receptor antagonist gene locus.5,6,9
Hormones. The clear peaks of incidence during times of low estrogen, and a higher incidence in patients with Turner syndrome or kidney disease, suggest that low estrogen may play a role in the development of LS, as well.1,5,6
While it is generally accepted that trauma may trigger LS via the Koebner phenomenon (the appearance of lesions at the site of injury), there is debate as to whether microbes—especially Borrelia burgdorferi and human papillomavirus (HPV)—might play a role.1,5
Diagnosis is often delayed, misdiagnosis is common
The average delay from symptom onset to diagnosis of LS is 1.3 years, and up to 84% of childhood LS is misdiagnosed before referral.2,9 The differential diagnosis includes:
Sexual abuse. In prepubertal girls presenting with genital redness, the can’t-miss diagnosis is sexual abuse, which occurs in more than 25% of children in the United States.10 Initial manifestations may be regression in developmental milestones, such as new-onset bedwetting, or behavioral changes such as social withdrawal or declining academic performance.11
However, physicians must be conscientious about ruling out medical etiologies before prematurely diagnosing abuse. Fourteen percent of girls with LS are incorrectly diagnosed as having been sexually abused.2 A clinical pearl is that while LS may resemble abuse on exam, it rarely affects the hymenal structure.12 It is also important to keep in mind that the 2 entities are not incompatible, as sexual abuse leading to LS via Koebnerization is a well-described phenomenon.12
Lichen planus. LP, which is also an immune-mediated inflammatory disorder affecting the vulva, classically presents with the 6 Ps: pruritic, polygonal, planar, purple papules and plaques.4 LP is distinguished from LS by being rare in childhood, having a predilection for the flexor wrists, and involving the oral and vaginal mucosa.4
Lichen simplex chronicus (LSC) is a chronic, circumscribed, pruritic, eczematous condition that becomes lichenified with thickened skin secondary to repeated scratching.13 Children with atopic dermatitis can develop LSC, but other children can also develop the scratch-itch cycle that results in the thickened plaques of LSC. Like LS, LSC can occur in areas other than the genitalia, including the neck and feet.14
Allergic contact dermatitis can occur in the genital area from diaper creams, soaps, and perfumes. Irritant contact dermatitis can occur from exposure to diarrhea, bedwetting, and other irritants. Contact dermatitis is less likely to have the classic figure-8 pattern seen in LS.
Psoriasis in the genital area can be confused with LS. However, psoriasis favors the groin creases in what is called inverse psoriasis. In addition, psoriasis tends to involve multiple areas, including the extensor surfaces of the elbows and knees, the nails, and the scalp.
Vitiligo can present on the genitals as circumscribed hypopigmented and depigmented patches that are flat. Vitiligo is asymptomatic, and the only pathology is the change in skin color. With LS, there is lichenification, atrophy, and sclerosis.4 Vitiligo often occurs with bilateral symmetric involvement in areas of trauma including the face, neck, scalp, elbows, wrists, hands, knees, ankles, and feet.
Treatment aims to improve symptoms
LS is usually diagnosed clinically (especially in children, as a biopsy is a great challenge to perform). However, when the clinical presentation is unclear, a skin biopsy will demonstrate the diagnostic findings of thinning of the epidermis, loss of rete pegs, hyperkeratosis, and dermal fibrosis with a T-lymphocyte-dominant inflammatory infiltrate.1,2,4,5
LS is a remitting and relapsing condition with no cure. The goals of treatment are to provide symptom relief and minimize scarring and atrophy,2 but it is unknown whether treatment reduces the risk of malignancy.9
First-line treatment for both genders and all ages is ultrapotent topical corticosteroids; clobetasol propionate 0.05% is most commonly used.1,6 Regimens vary, but the vast majority of patients improve within 3 months of once-daily treatment.4
For refractory LS, calcineurin inhibitors such as tacrolimus may be used. Although it has a black box warning regarding a potential cancer risk, long-term studies of children using tacrolimus for atopic dermatitis have not demonstrated an increased risk of malignancy.6,9 Because of a considerable adverse effect profile, oral retinoids are limited to refractory cases in adults.6 Surgery is reserved for scarring and adhesions.4
Follow-up plays an important role in management
Historically, it was believed that pediatric LS had an excellent prognosis, with patients achieving complete resolution after puberty.1,4 Recent findings have shown mixed results, with LS persisting in many patients beyond puberty.2,4 Therefore, regular follow-up is recommended every 6 to 12 months.
For uncomplicated LS, specialist follow-up is not indicated. Female patients should regularly conduct self-examinations and, at a minimum, undergo annual examinations by their primary care physician. Those who require specialist follow-up include patients with difficult-to-control symptoms, hypertrophic lesions, a history of SCCV or differentiated vulvar intraepithelial neoplasia (dVIN), or pathology showing possible dVIN.15
Our patient. We prescribed clobetasol propionate 0.05% ointment to be used once daily for 8 weeks. We stressed the importance of genital self-examinations using a mirror to monitor for any concerning changes such as skin thickening. We showed the patient and her mother photos of normal female genitalia to help normalize the genital exam, and taught the patient how to find her plaques in the mirror. We set expectations by emphasizing the chronic nature of LS and the likelihood of recurrence. We also encouraged HPV vaccination in the upcoming years to prevent both cervical cancer and HPV-related SCCV.
CORRESPONDENCE
Somya Abubucker, MD, University of Hawaii, 1356 Lusitana Street, 7th floor, Honolulu, HI 96813; [email protected].
A mother brought her 8-year-old daughter to our office for evaluation of vitiligo “down there” (FIGURE). The skin eruption first appeared on her vulva a year earlier and was intermittently pruritic. The lesions were initially smaller and red, but had since lightened in color, coalesced, and had begun to spread to the perianal area. The patient’s mother had received a call from her daughter’s teacher who observed that her daughter was scratching the area and might be masturbating in class.

The mother reported that 6 months earlier, her daughter had experienced bloody spots in her underwear accompanied by dysuria. The mother brought her to the emergency department, where she was treated with antibiotics for a urinary tract infection.
Our physical examination revealed well-circumscribed, symmetric, depigmented, confluent, crinkled, parchment-like plaques with small hemorrhagic erosions on the medial labia majora and minora. The lesions had spread to the perianal area with depigmentation superiorly and hypopigmentation inferiorly, creating a figure-8 pattern.
A review of systems was negative for pruritus, pain, dysuria, dyschezia, constipation, and vaginal discharge. The patient denied sexual activity, depression, or anxiety. Her mother denied behavioral changes in her daughter and said that her daughter hadn’t had any one-on-one time alone with any adults besides herself. Her mother was concerned that the white spots might spread to the rest of her daughter’s body, which could affect her socially.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen sclerosus
Based on the history and clinical findings, including the classic figure-8 pattern, we diagnosed childhood lichen sclerosus (LS) in this patient. LS is a chronic inflammatory skin disorder that primarily affects the genital mucosa. The disorder can present at any age, but is most common among postmenopausal women, with a prevalence estimated to be as high as one in 30.1-3 A second incidence peak is observed in prepubescent girls, with a prevalence of one in 900.3,4 LS is less common in men and boys, with a female-to-male ratio that can reach 10:1.5 The classic symptoms of LS are pruritus and pain, which may be intermittent or persistent.
In girls, initial manifestations may be constipation, dysuria, or even behavioral symptoms such as night fears, which can occur because children are less active at night and become more aware of urinary discomfort.1,2,6 Typical signs of LS are thin atrophic plaques that spare the vagina and cervix. The plaques can be ivory-white, erythematous, or violaceous. Some patients have perianal lesions as well, and can display the pathognomonic figure-8 pattern of porcelain plaques around the vulva and anus.5
With more advanced disease, erosions, lichenification, and even distortion of vulvar architecture may occur.2,4,7 In severe cases, labia resorption and clitoral phimosis may develop.5 Complications include secondary infection, dyspareunia, and psychosexual distress. The most worrisome sequela of LS is squamous cell carcinoma of the vulva (SCCV), which occurs in 5% of female patients with LS.4
In men and boys, LS typically involves the foreskin and the glans, while sparing the perianal region.5 Scarring of the foreskin can lead to phimosis, and patients may complain of painful erections and difficulty urinating. LS can also occur away from the genitalia in both males and females.
Autoimmune mechanisms, genetics, and hormones play a role
The exact pathogenesis of LS remains unknown, but multiple factors are likely at work.
Autoimmune mechanisms. Up to 60% of women with LS have an autoimmune disorder, which is most commonly vitiligo, alopecia areata, or thyroid disease.5 In addition, 67% of patients have autoantibodies against extracellular matrix protein 1, and 30% have them against bullous pemphigoid antigen 180.1,8
Genetics. LS is associated with certain human leukocyte antigen class II haplotypes (especially DQ7) and with polymorphisms at the interleukin-1 receptor antagonist gene locus.5,6,9
Hormones. The clear peaks of incidence during times of low estrogen, and a higher incidence in patients with Turner syndrome or kidney disease, suggest that low estrogen may play a role in the development of LS, as well.1,5,6
While it is generally accepted that trauma may trigger LS via the Koebner phenomenon (the appearance of lesions at the site of injury), there is debate as to whether microbes—especially Borrelia burgdorferi and human papillomavirus (HPV)—might play a role.1,5
Diagnosis is often delayed, misdiagnosis is common
The average delay from symptom onset to diagnosis of LS is 1.3 years, and up to 84% of childhood LS is misdiagnosed before referral.2,9 The differential diagnosis includes:
Sexual abuse. In prepubertal girls presenting with genital redness, the can’t-miss diagnosis is sexual abuse, which occurs in more than 25% of children in the United States.10 Initial manifestations may be regression in developmental milestones, such as new-onset bedwetting, or behavioral changes such as social withdrawal or declining academic performance.11
However, physicians must be conscientious about ruling out medical etiologies before prematurely diagnosing abuse. Fourteen percent of girls with LS are incorrectly diagnosed as having been sexually abused.2 A clinical pearl is that while LS may resemble abuse on exam, it rarely affects the hymenal structure.12 It is also important to keep in mind that the 2 entities are not incompatible, as sexual abuse leading to LS via Koebnerization is a well-described phenomenon.12
Lichen planus. LP, which is also an immune-mediated inflammatory disorder affecting the vulva, classically presents with the 6 Ps: pruritic, polygonal, planar, purple papules and plaques.4 LP is distinguished from LS by being rare in childhood, having a predilection for the flexor wrists, and involving the oral and vaginal mucosa.4
Lichen simplex chronicus (LSC) is a chronic, circumscribed, pruritic, eczematous condition that becomes lichenified with thickened skin secondary to repeated scratching.13 Children with atopic dermatitis can develop LSC, but other children can also develop the scratch-itch cycle that results in the thickened plaques of LSC. Like LS, LSC can occur in areas other than the genitalia, including the neck and feet.14
Allergic contact dermatitis can occur in the genital area from diaper creams, soaps, and perfumes. Irritant contact dermatitis can occur from exposure to diarrhea, bedwetting, and other irritants. Contact dermatitis is less likely to have the classic figure-8 pattern seen in LS.
Psoriasis in the genital area can be confused with LS. However, psoriasis favors the groin creases in what is called inverse psoriasis. In addition, psoriasis tends to involve multiple areas, including the extensor surfaces of the elbows and knees, the nails, and the scalp.
Vitiligo can present on the genitals as circumscribed hypopigmented and depigmented patches that are flat. Vitiligo is asymptomatic, and the only pathology is the change in skin color. With LS, there is lichenification, atrophy, and sclerosis.4 Vitiligo often occurs with bilateral symmetric involvement in areas of trauma including the face, neck, scalp, elbows, wrists, hands, knees, ankles, and feet.
Treatment aims to improve symptoms
LS is usually diagnosed clinically (especially in children, as a biopsy is a great challenge to perform). However, when the clinical presentation is unclear, a skin biopsy will demonstrate the diagnostic findings of thinning of the epidermis, loss of rete pegs, hyperkeratosis, and dermal fibrosis with a T-lymphocyte-dominant inflammatory infiltrate.1,2,4,5
LS is a remitting and relapsing condition with no cure. The goals of treatment are to provide symptom relief and minimize scarring and atrophy,2 but it is unknown whether treatment reduces the risk of malignancy.9
First-line treatment for both genders and all ages is ultrapotent topical corticosteroids; clobetasol propionate 0.05% is most commonly used.1,6 Regimens vary, but the vast majority of patients improve within 3 months of once-daily treatment.4
For refractory LS, calcineurin inhibitors such as tacrolimus may be used. Although it has a black box warning regarding a potential cancer risk, long-term studies of children using tacrolimus for atopic dermatitis have not demonstrated an increased risk of malignancy.6,9 Because of a considerable adverse effect profile, oral retinoids are limited to refractory cases in adults.6 Surgery is reserved for scarring and adhesions.4
Follow-up plays an important role in management
Historically, it was believed that pediatric LS had an excellent prognosis, with patients achieving complete resolution after puberty.1,4 Recent findings have shown mixed results, with LS persisting in many patients beyond puberty.2,4 Therefore, regular follow-up is recommended every 6 to 12 months.
For uncomplicated LS, specialist follow-up is not indicated. Female patients should regularly conduct self-examinations and, at a minimum, undergo annual examinations by their primary care physician. Those who require specialist follow-up include patients with difficult-to-control symptoms, hypertrophic lesions, a history of SCCV or differentiated vulvar intraepithelial neoplasia (dVIN), or pathology showing possible dVIN.15
Our patient. We prescribed clobetasol propionate 0.05% ointment to be used once daily for 8 weeks. We stressed the importance of genital self-examinations using a mirror to monitor for any concerning changes such as skin thickening. We showed the patient and her mother photos of normal female genitalia to help normalize the genital exam, and taught the patient how to find her plaques in the mirror. We set expectations by emphasizing the chronic nature of LS and the likelihood of recurrence. We also encouraged HPV vaccination in the upcoming years to prevent both cervical cancer and HPV-related SCCV.
CORRESPONDENCE
Somya Abubucker, MD, University of Hawaii, 1356 Lusitana Street, 7th floor, Honolulu, HI 96813; [email protected].
1. Murphy R. Lichen sclerosus. Dermatol Clin. 2010;28:707-715.
2. Dendrinos ML, Quint EH. Lichen sclerosus in children and adolescents. Curr Opin Obstet Gynecol. 2013;25:370-374.
3. Eva LJ. Screening and follow up of vulval skin disorders. Best Pract Res Clin Obstet Gynaecol. 2012;26:175-188.
4. Focseneanu MA, Gupta M, Squires KC, et al. The course of lichen sclerosus diagnosed prior to puberty. J Pediatr Adolesc Gynecol. 2013;26:153-155.
5. Funaro D. Lichen sclerosus: a review and practical approach. Dermatol Ther. 2004;17:28-37.
6. Heymann WR. Lichen sclerosus. J Am Acad Dermatol. 2007;56:683-684.
7. Tong LX, Sun GS, Teng JM. Pediatric lichen sclerosus: a review of the epidemiology and treatment options. Pediatr Dermatol. 2015;32:593-599.
8. Lagerstedt M, Karvinen K, Joki-Erkkilä M, et al. Childhood lichen sclerosus—a challenge for clinicians. Pediatr Dermatol. 2013;30:444-450.
9. Keith PJ, Wolz MM, Peters MS. Eosinophils in lichen sclerosus et atrophicus. J Cutan Pathol. 2015;42:693-698.
10. National Sexual Violence Resource Center. Child sexual abuse prevention. 2011. Available at: https://www.nsvrc.org/sites/default/files/Publications_NSVRC_Overview_Child-sexual-abuse-prevention_0.pdf. Accessed February 8, 2018.
11. Dubowitz H, Lane WG. Abused and neglected children. In: Kliegman RM, Stanton BF, St. Geme JW, et al, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016:236-249.
12. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44:803-806.
13. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
14. Warshaw E, Hook K. Dermatitis. In: Soutor C, Hordinsky MK, eds. Clinical Dermatology. 1st ed. New York, NY: McGraw-Hill; 2013.
15. Jones RW, Scurry J, Neill S, et al. Guidelines for the follow-up of women with vulvar lichen sclerosus in specialist clinics. Am J Obstet Gynecol. 2008;198:496.e1-e3.
1. Murphy R. Lichen sclerosus. Dermatol Clin. 2010;28:707-715.
2. Dendrinos ML, Quint EH. Lichen sclerosus in children and adolescents. Curr Opin Obstet Gynecol. 2013;25:370-374.
3. Eva LJ. Screening and follow up of vulval skin disorders. Best Pract Res Clin Obstet Gynaecol. 2012;26:175-188.
4. Focseneanu MA, Gupta M, Squires KC, et al. The course of lichen sclerosus diagnosed prior to puberty. J Pediatr Adolesc Gynecol. 2013;26:153-155.
5. Funaro D. Lichen sclerosus: a review and practical approach. Dermatol Ther. 2004;17:28-37.
6. Heymann WR. Lichen sclerosus. J Am Acad Dermatol. 2007;56:683-684.
7. Tong LX, Sun GS, Teng JM. Pediatric lichen sclerosus: a review of the epidemiology and treatment options. Pediatr Dermatol. 2015;32:593-599.
8. Lagerstedt M, Karvinen K, Joki-Erkkilä M, et al. Childhood lichen sclerosus—a challenge for clinicians. Pediatr Dermatol. 2013;30:444-450.
9. Keith PJ, Wolz MM, Peters MS. Eosinophils in lichen sclerosus et atrophicus. J Cutan Pathol. 2015;42:693-698.
10. National Sexual Violence Resource Center. Child sexual abuse prevention. 2011. Available at: https://www.nsvrc.org/sites/default/files/Publications_NSVRC_Overview_Child-sexual-abuse-prevention_0.pdf. Accessed February 8, 2018.
11. Dubowitz H, Lane WG. Abused and neglected children. In: Kliegman RM, Stanton BF, St. Geme JW, et al, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016:236-249.
12. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44:803-806.
13. Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
14. Warshaw E, Hook K. Dermatitis. In: Soutor C, Hordinsky MK, eds. Clinical Dermatology. 1st ed. New York, NY: McGraw-Hill; 2013.
15. Jones RW, Scurry J, Neill S, et al. Guidelines for the follow-up of women with vulvar lichen sclerosus in specialist clinics. Am J Obstet Gynecol. 2008;198:496.e1-e3.

