User login
Going Digital With Dermoscopy
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.

Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
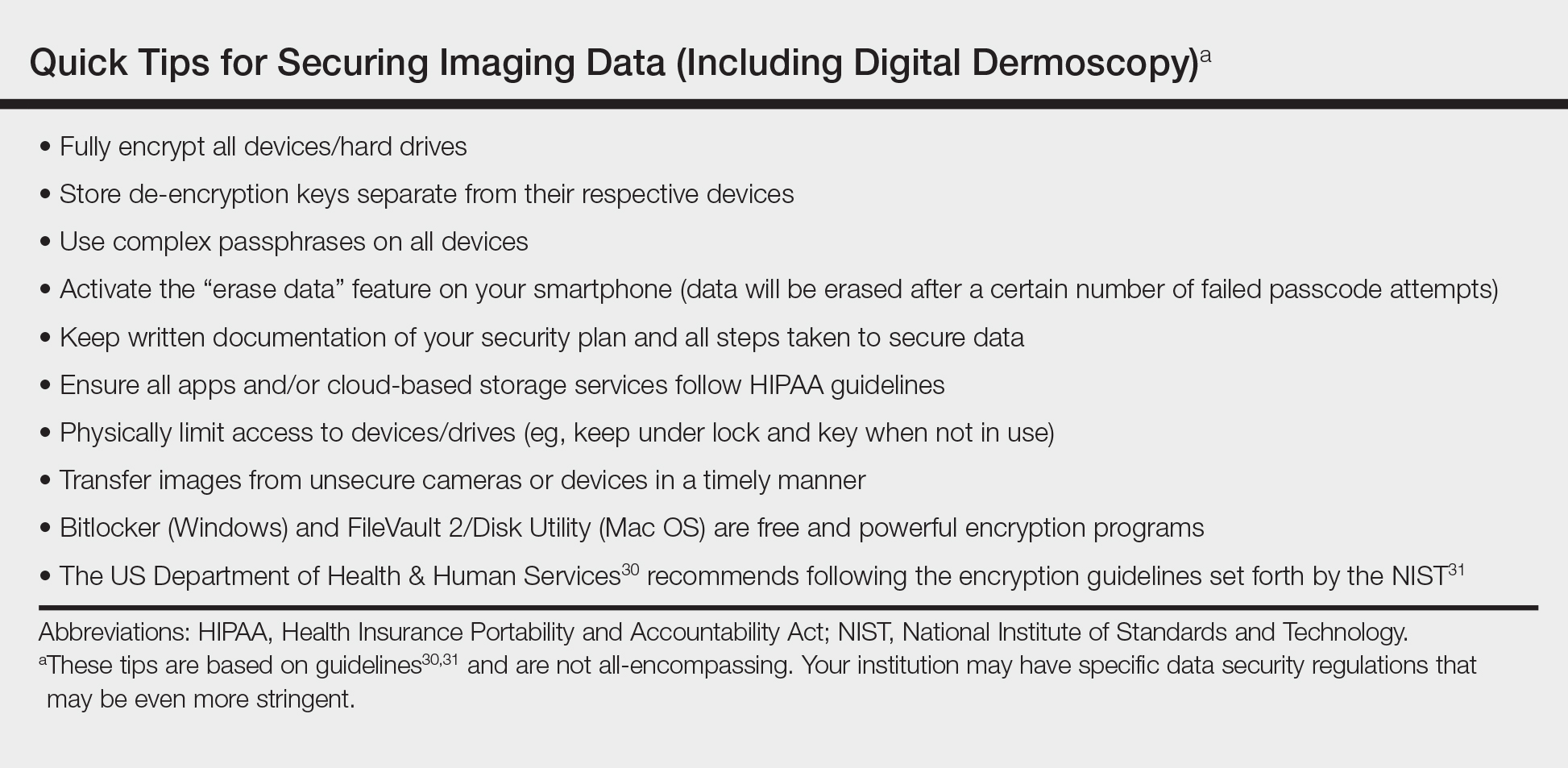
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.

Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31

Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.

Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31

Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.