User login
Lip Reconstruction After Mohs Micrographic Surgery: A Guide on Flaps
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
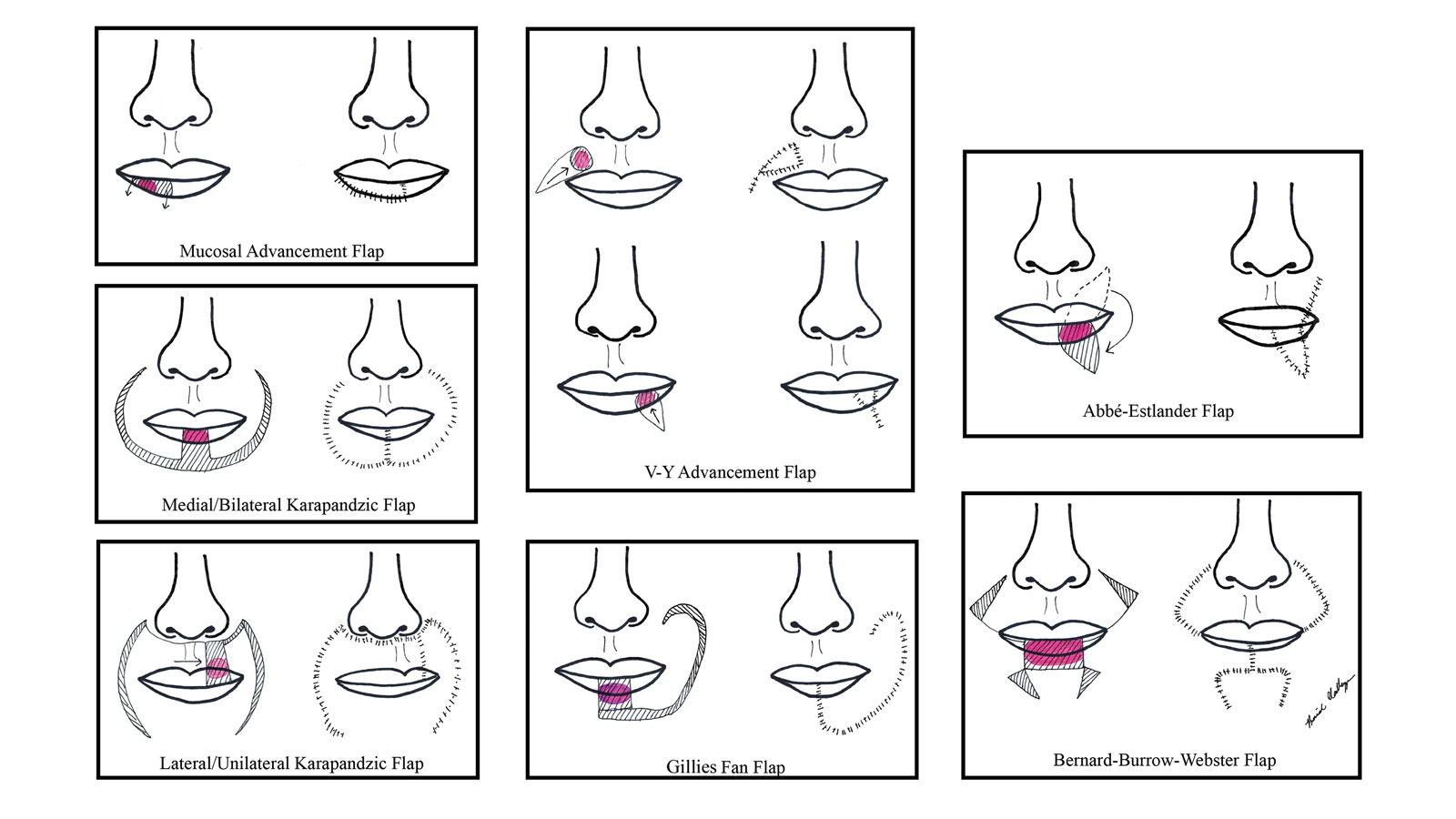
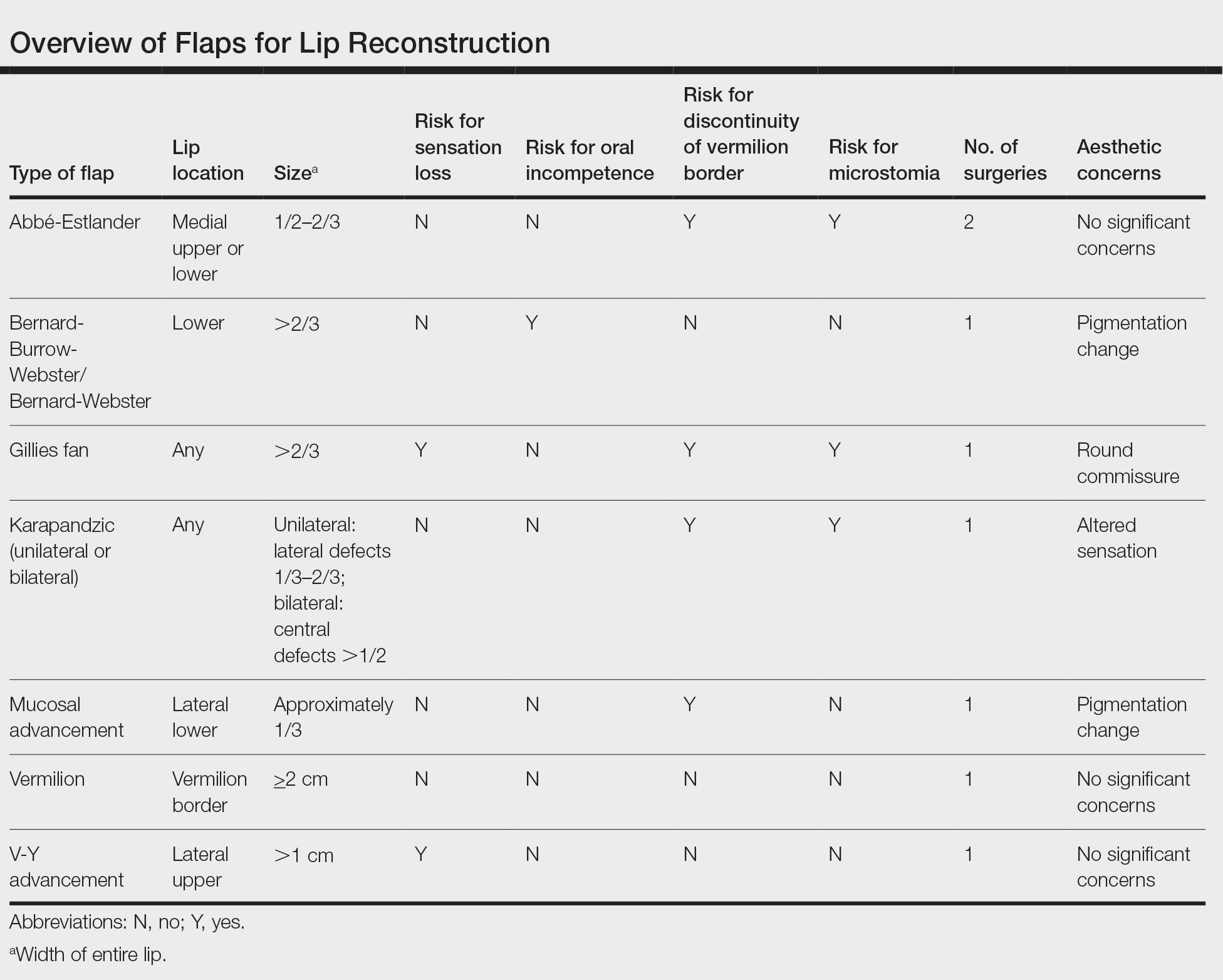
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).
Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
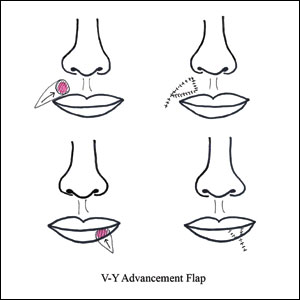
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1
Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
Practice Points
- Even with early detection, many skin cancers on the lips require surgical removal with subsequent reconstruction.
- There are several local flap reconstruction options available, and some may be used in combination for more complex defects.
- The most suitable technique should be chosen based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.
Dermatologic Management of Hidradenitis Suppurativa and Impact on Pregnancy and Breastfeeding
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
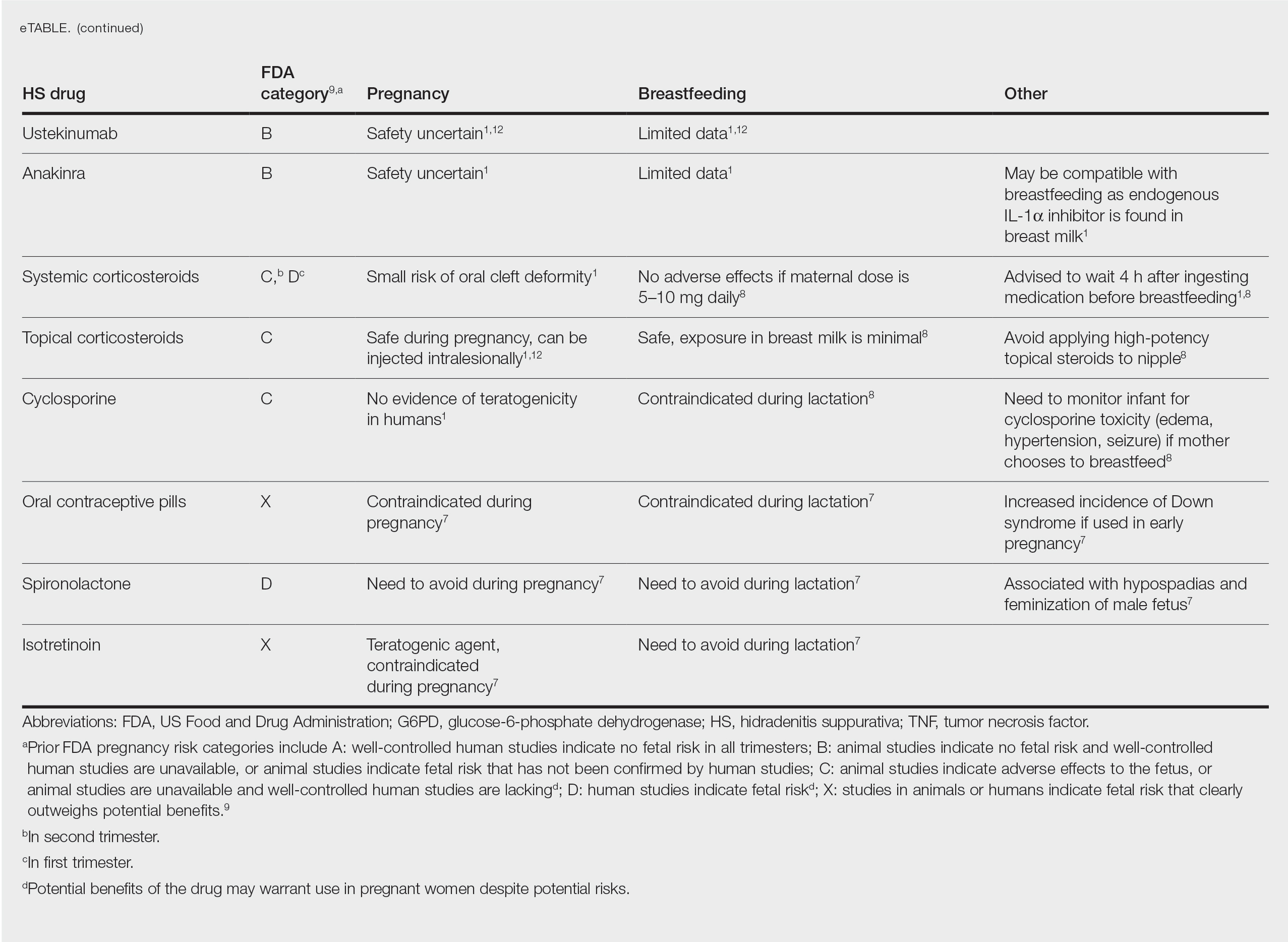
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
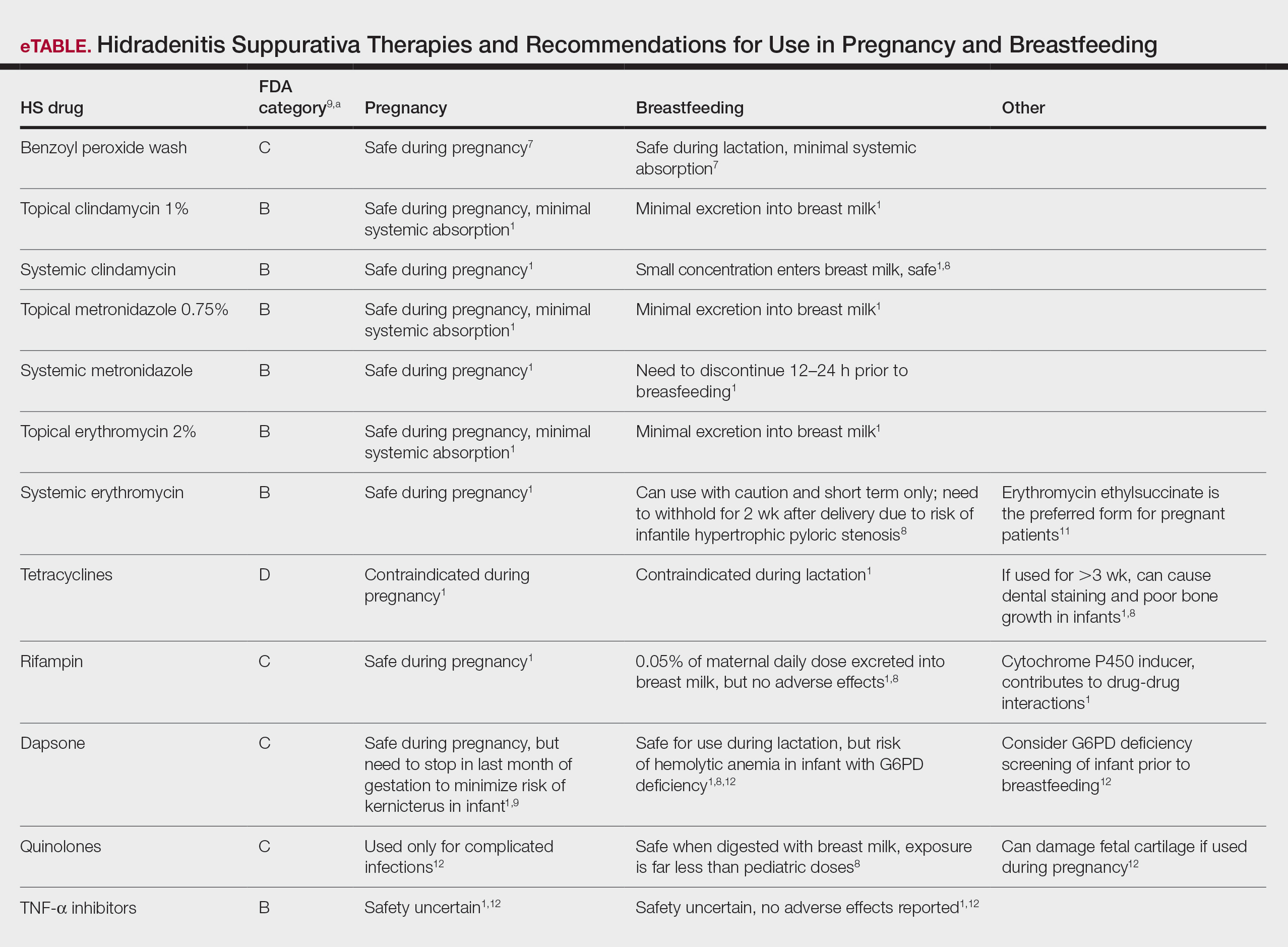
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9
Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5
CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9

Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5

CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9

Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5

CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
Practice Points
- Some medications used to treat hidradenitis suppurativa (HS) may have teratogenic effects and be contraindicated during breastfeeding.
- We summarize what treatments are proven to be safe in pregnancy and breastfeeding and highlight the need for more guidelines and safety data for dermatologists to manage their pregnant patients with HS.