User login
Lip Reconstruction After Mohs Micrographic Surgery: A Guide on Flaps
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).
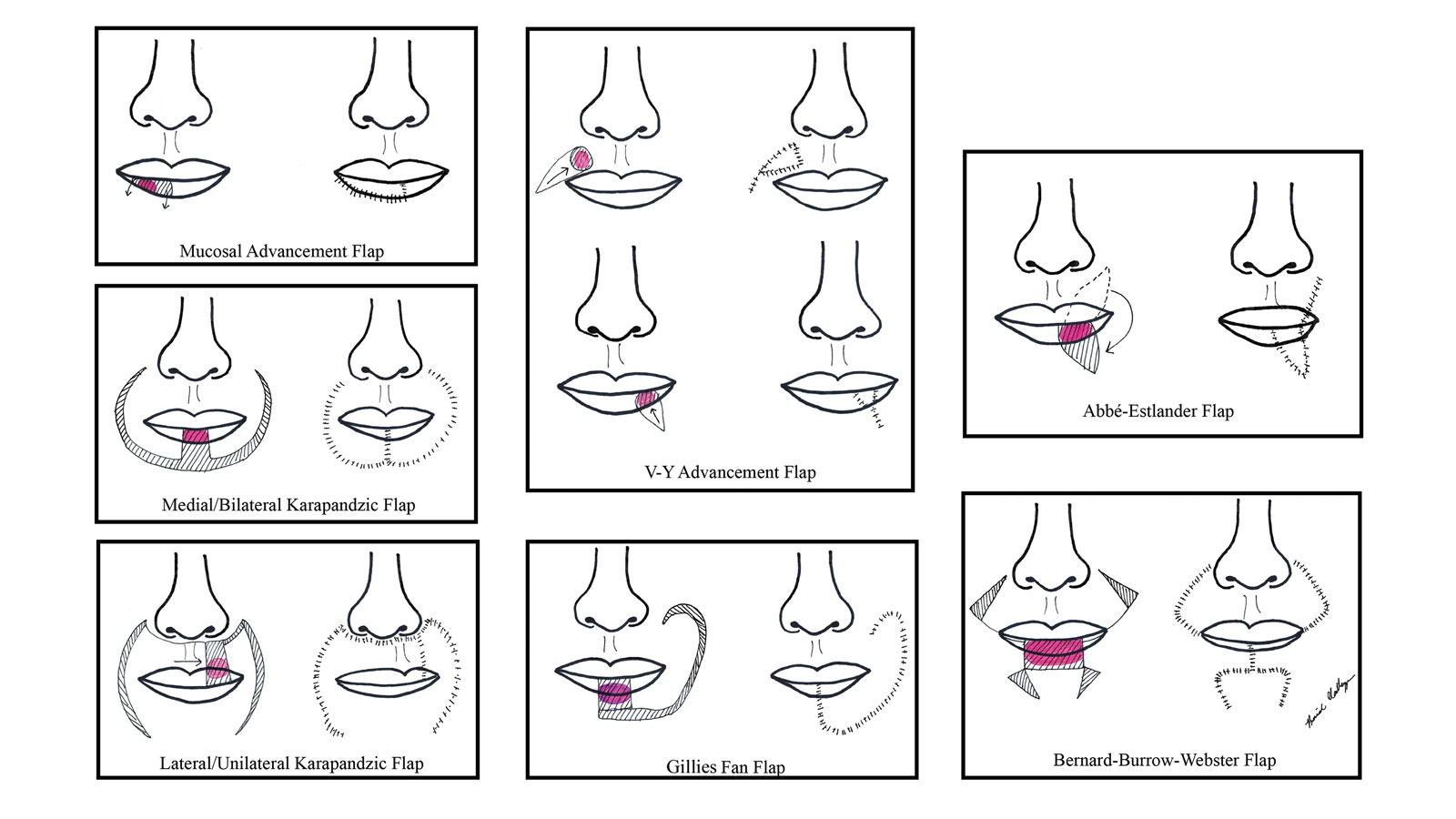
Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
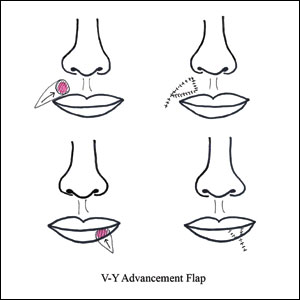
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1
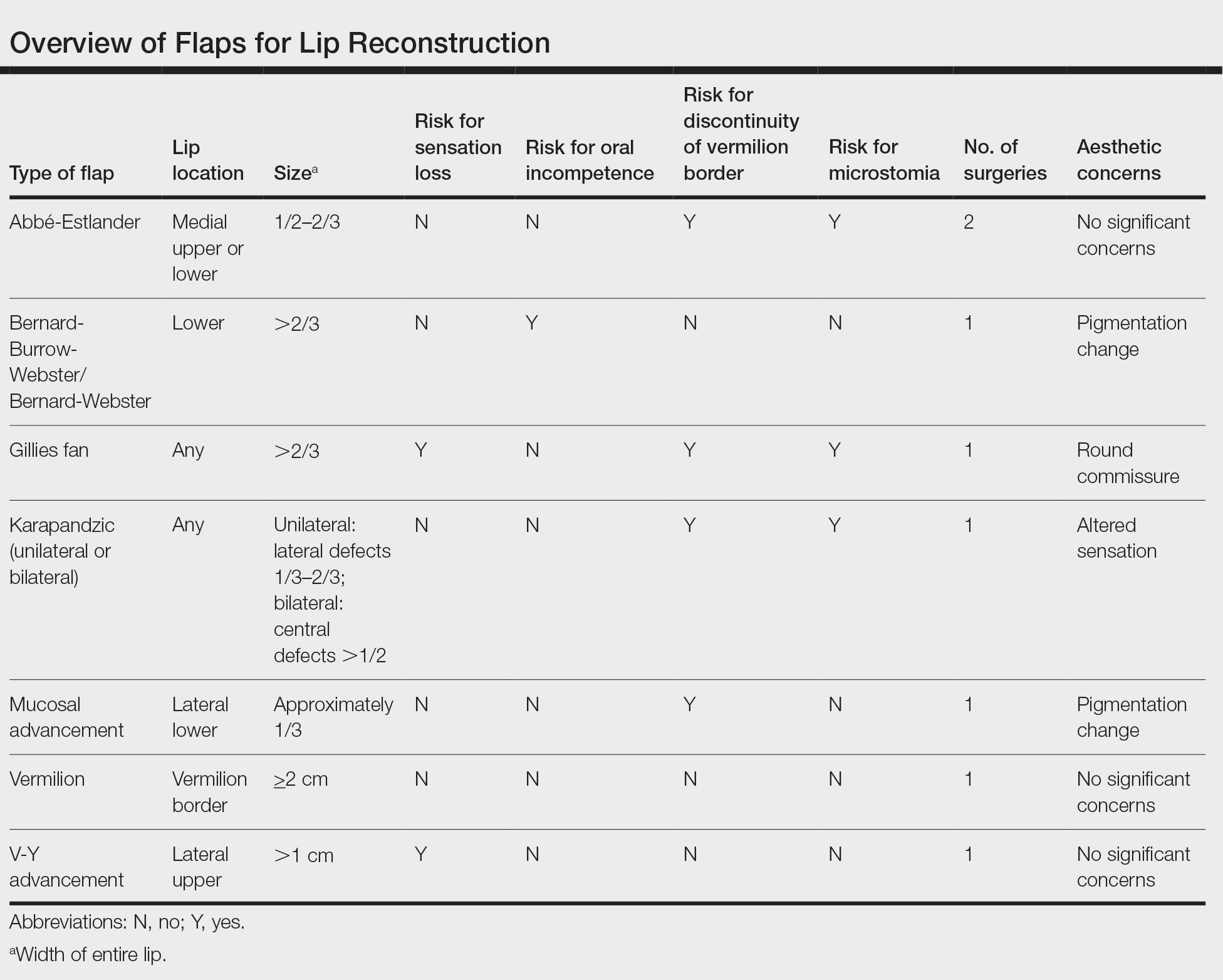
Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
Practice Points
- Even with early detection, many skin cancers on the lips require surgical removal with subsequent reconstruction.
- There are several local flap reconstruction options available, and some may be used in combination for more complex defects.
- The most suitable technique should be chosen based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.
Aquatic Antagonists: Marine Rashes (Seabather’s Eruption and Diver’s Dermatitis)
Background and Clinical Presentation
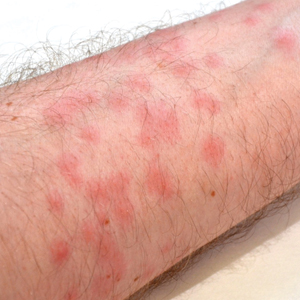
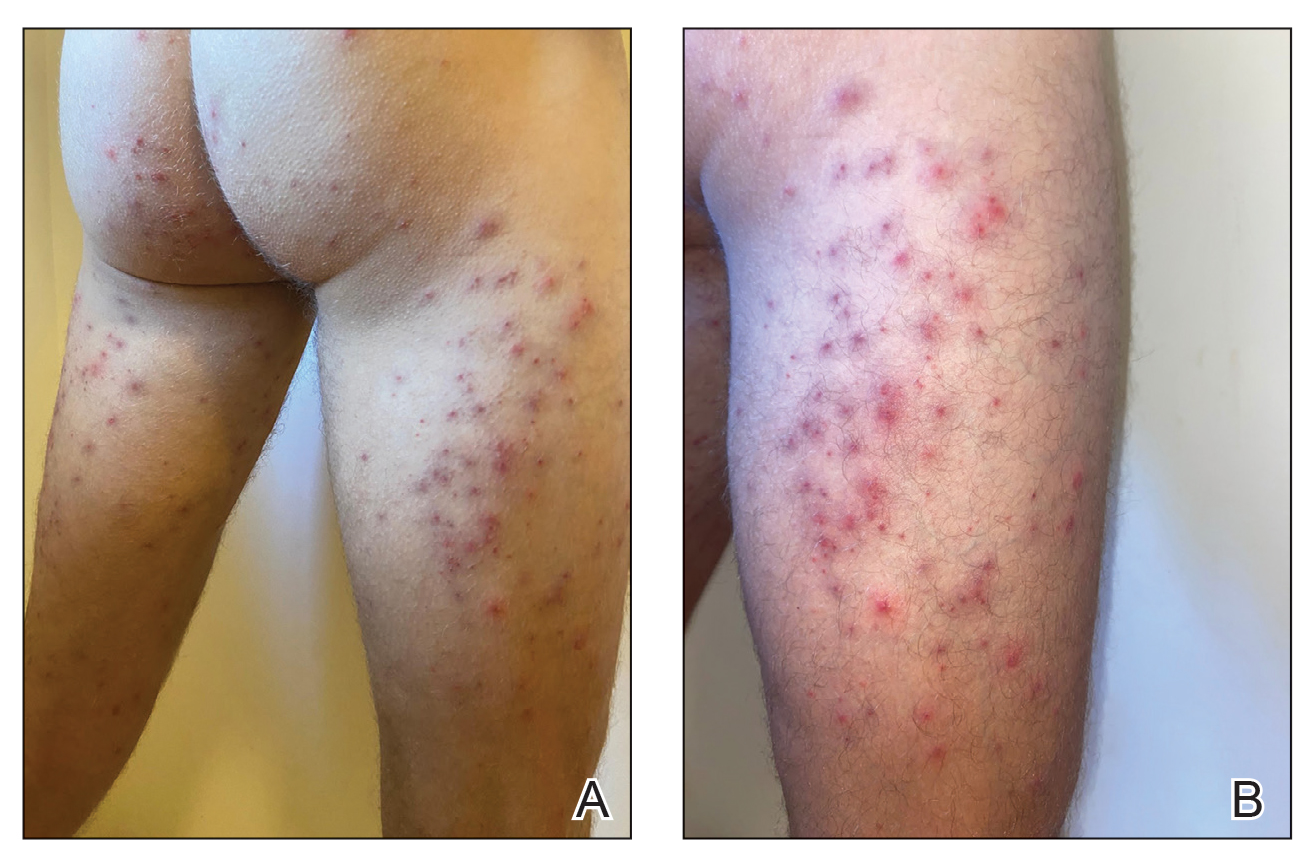
Seabather’s Eruption—Seabather’s eruption is a type I and IV hypersensitivity reaction caused by nematocysts of larval-stage thimble jellyfish (Linuche unguiculata), sea anemones (eg, Edwardsiella lineata), and larval cnidarians.1Linuche unguiculata commonly is found along the southeast coast of the United States and in the Caribbean, the Gulf of Mexico, and the coasts of Florida; less commonly, it has been reported along the coasts of Brazil and Papua New Guinea. Edwardsiella lineata more commonly is seen along the East Coast of the United States.2 Seabather’s eruption presents as numerous scattered, pruritic, red macules and papules (measuring 1 mm to 1.5 cm in size) distributed in areas covered by skin folds, wet clothing, or hair following exposure to marine water (Figure 1). This maculopapular rash generally appears shortly after exiting the water and can last up to several weeks in some cases.3 The cause for this delayed presentation is that the marine organisms become entrapped between the skin of the human contact and another object (eg, swimwear) but do not release their preformed antivenom until they are exposed to air after removal from the water, at which point the organisms die and cell lysis results in injection of the venom.
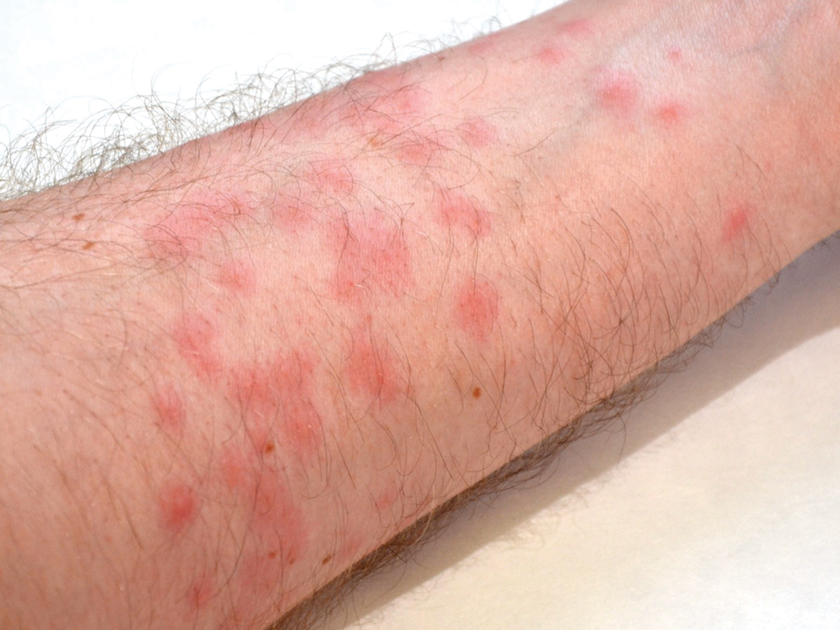
Diver’s Dermatitis—Diver’s dermatitis (also referred to as “swimmer’s itch”) is a type I and IV hypersensitivity reaction caused by schistosome cercariae released by aquatic snails.4 There are several different cercarial species known to be capable of causing diver dermatitis, but the most commonly implicated genera are Trichobilharzia and Gigantobilharzia. These parasites most commonly are found in freshwater lakes but also occur in oceans, particularly in brackish areas adjacent to freshwater access. Factors associated with increased concentrations of these parasites include shallow, slow-moving water and prolonged onshore wind causing accumulation near the shoreline. It also is thought that the snail host will shed greater concentrations of the parasitic worm in the morning hours and after prolonged exposure to sunlight.4 These flatworm trematodes have a 2-host life cycle. The snails function as intermediate hosts for the parasites before they enter their final host, which are birds. Humans only function as incidental and nonviable hosts for these worms. The parasites gain access to the human body by burrowing into exposed skin. Because the parasite is unable to survive on human hosts, it dies shortly after penetrating the skin, which leads to an intense inflammatory response causing symptoms of pruritus within hours of exposure (Figure 2). The initial eruption progresses over a few days into a diffuse, maculopapular, pruritic rash, similar to that seen in seabather’s eruption. This rash then regresses completely in 1 to 3 weeks. Subsequent exposure to the same parasite is associated with increased severity of future rashes, likely due to antibody-mediated sensitization.4
Diagnosis—Marine-derived dermatoses from various sources can present very similarly; thus, it is difficult to discern the specific etiology behind the clinical presentation. No commonly utilized imaging modalities can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough patient history often can aid in differentiation of the cause of the eruption. For example, lesions located only on nonexposed areas of the skin increases the likelihood of seabather’s eruption due to nematocysts being trapped between clothing and the skin. In contrast, diver’s dermatitis generally appears on areas of the skin that were directly exposed to water and uncovered by clothing.5 Patient reports of a lack of symptoms until shortly after exiting the water further support a diagnosis of seabather’s eruption, as this delayed presentation of symptoms is caused by lysis of the culprit organisms following removal from the marine environment. The cell lysis is responsible for the widespread injection of preformed venom via the numerous nematocysts trapped between clothing and the patient’s body.1
Treatment
For both conditions, the symptoms are treated with hydrocortisone or other topical steroid solutions in conjunction with oral hydroxyzine. Alternative treatments include calamine lotion with 1% menthol and nonsteroidal anti-inflammatory drugs. Taking baths with oatmeal, Epsom salts, or baking soda also may alleviate some of the pruritic symptoms.2
Prevention
The ability to diagnose the precise cause of these similar marine rashes can bring peace of mind to both patients and physicians regardless of their similar management strategies. Severe contact dermatitis of unknown etiology can be disconcerting for patients. Additionally, documenting the causes of marine rashes in particular geographic locations can be beneficial for establishing which organisms are most likely to affect visitors to those areas. This type of data collection can be utilized to develop preventative recommendations, such as deciding when to avoid the water. Education of the public can be done with the use of informational posters located near popular swimming areas and online public service announcements. Informing the general public about the dangers of entering the ocean, especially during certain times of the year when nematocyst-equipped sea creatures are in abundance, could serve to prevent numerous cases of seabather’s eruption. Likewise, advising against immersion in shallow, slow-moving water during the morning hours or after prolonged sun exposure in trematode-endemic areas could prevent numerous cases of diver’s dermatitis. Basic information on what to expect if afflicted by a marine rash also may reduce the number of emergency department visits for these conditions, thus providing economic benefit for patients and for hospitals since patients would better know how to acutely treat these rashes and lessen the patient load at hospital emergency departments. If individuals can assure themselves of the self-limited nature of these types of dermatoses, they may be less inclined to seek medical consultation.
Final Thoughts
As the climate continues to change, the incidence of marine rashes such as seabather’s eruption and diver’s dermatitis is expected to increase due to warmer surface temperatures causing more frequent and earlier blooms of L unguiculata and E lineata. Cases of diver’s dermatitis also could increase due to a longer season of more frequent human exposure from an increase in warmer temperatures. The projected uptick in incidences of these marine rashes makes understanding these pathologies even more pertinent for physicians.6 Increasing our understanding of the different types of marine rashes and their causes will help guide future recommendations for the general public when visiting the ocean.
Future research may wish to investigate unique ways in which to prevent contact between these organisms and humans. Past research on mice indicated that topical application of DEET (N,N-diethyl-meta-toluamide) prior to trematode exposure prevented penetration of the skin by parasitic worms.7 Future studies are needed to examine the effectiveness of this preventative technique on humans. For now, dermatologists may counsel our ocean-going patients on preventative behaviors as well as provide reassurance and symptomatic relief when they present to our clinics with marine rashes.
- Parrish DO. Seabather’s eruption or diver’s dermatitis? JAMA. 1993;270:2300-2301. doi:10.1001/jama.1993.03510190054021
- Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice’. JAMA. 1993;269:1669-1672. doi:10.1001/jama.1993.03500130083037
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by algae and Bryozoans. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology: Biotic, Chemical, and Physical Agents. Springer; 2016:127-137.
- Tracz ES, Al-Jubury A, Buchmann K, et al. Outbreak of swimmer’s itch in Denmark. Acta Derm Venereol. 2019;99:1116-1120. doi:10.2340/00015555-3309
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. JAAD. 2016;76:140-147. doi:10.1016/j.jaad.2016.08.014
- Salafsky B, Ramaswamy K, He YX, et al. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg. 1999;61:743-750. doi:10.4269/ajtmh.1999.61.743
Background and Clinical Presentation
Seabather’s Eruption—Seabather’s eruption is a type I and IV hypersensitivity reaction caused by nematocysts of larval-stage thimble jellyfish (Linuche unguiculata), sea anemones (eg, Edwardsiella lineata), and larval cnidarians.1Linuche unguiculata commonly is found along the southeast coast of the United States and in the Caribbean, the Gulf of Mexico, and the coasts of Florida; less commonly, it has been reported along the coasts of Brazil and Papua New Guinea. Edwardsiella lineata more commonly is seen along the East Coast of the United States.2 Seabather’s eruption presents as numerous scattered, pruritic, red macules and papules (measuring 1 mm to 1.5 cm in size) distributed in areas covered by skin folds, wet clothing, or hair following exposure to marine water (Figure 1). This maculopapular rash generally appears shortly after exiting the water and can last up to several weeks in some cases.3 The cause for this delayed presentation is that the marine organisms become entrapped between the skin of the human contact and another object (eg, swimwear) but do not release their preformed antivenom until they are exposed to air after removal from the water, at which point the organisms die and cell lysis results in injection of the venom.

Diver’s Dermatitis—Diver’s dermatitis (also referred to as “swimmer’s itch”) is a type I and IV hypersensitivity reaction caused by schistosome cercariae released by aquatic snails.4 There are several different cercarial species known to be capable of causing diver dermatitis, but the most commonly implicated genera are Trichobilharzia and Gigantobilharzia. These parasites most commonly are found in freshwater lakes but also occur in oceans, particularly in brackish areas adjacent to freshwater access. Factors associated with increased concentrations of these parasites include shallow, slow-moving water and prolonged onshore wind causing accumulation near the shoreline. It also is thought that the snail host will shed greater concentrations of the parasitic worm in the morning hours and after prolonged exposure to sunlight.4 These flatworm trematodes have a 2-host life cycle. The snails function as intermediate hosts for the parasites before they enter their final host, which are birds. Humans only function as incidental and nonviable hosts for these worms. The parasites gain access to the human body by burrowing into exposed skin. Because the parasite is unable to survive on human hosts, it dies shortly after penetrating the skin, which leads to an intense inflammatory response causing symptoms of pruritus within hours of exposure (Figure 2). The initial eruption progresses over a few days into a diffuse, maculopapular, pruritic rash, similar to that seen in seabather’s eruption. This rash then regresses completely in 1 to 3 weeks. Subsequent exposure to the same parasite is associated with increased severity of future rashes, likely due to antibody-mediated sensitization.4

Diagnosis—Marine-derived dermatoses from various sources can present very similarly; thus, it is difficult to discern the specific etiology behind the clinical presentation. No commonly utilized imaging modalities can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough patient history often can aid in differentiation of the cause of the eruption. For example, lesions located only on nonexposed areas of the skin increases the likelihood of seabather’s eruption due to nematocysts being trapped between clothing and the skin. In contrast, diver’s dermatitis generally appears on areas of the skin that were directly exposed to water and uncovered by clothing.5 Patient reports of a lack of symptoms until shortly after exiting the water further support a diagnosis of seabather’s eruption, as this delayed presentation of symptoms is caused by lysis of the culprit organisms following removal from the marine environment. The cell lysis is responsible for the widespread injection of preformed venom via the numerous nematocysts trapped between clothing and the patient’s body.1
Treatment
For both conditions, the symptoms are treated with hydrocortisone or other topical steroid solutions in conjunction with oral hydroxyzine. Alternative treatments include calamine lotion with 1% menthol and nonsteroidal anti-inflammatory drugs. Taking baths with oatmeal, Epsom salts, or baking soda also may alleviate some of the pruritic symptoms.2
Prevention
The ability to diagnose the precise cause of these similar marine rashes can bring peace of mind to both patients and physicians regardless of their similar management strategies. Severe contact dermatitis of unknown etiology can be disconcerting for patients. Additionally, documenting the causes of marine rashes in particular geographic locations can be beneficial for establishing which organisms are most likely to affect visitors to those areas. This type of data collection can be utilized to develop preventative recommendations, such as deciding when to avoid the water. Education of the public can be done with the use of informational posters located near popular swimming areas and online public service announcements. Informing the general public about the dangers of entering the ocean, especially during certain times of the year when nematocyst-equipped sea creatures are in abundance, could serve to prevent numerous cases of seabather’s eruption. Likewise, advising against immersion in shallow, slow-moving water during the morning hours or after prolonged sun exposure in trematode-endemic areas could prevent numerous cases of diver’s dermatitis. Basic information on what to expect if afflicted by a marine rash also may reduce the number of emergency department visits for these conditions, thus providing economic benefit for patients and for hospitals since patients would better know how to acutely treat these rashes and lessen the patient load at hospital emergency departments. If individuals can assure themselves of the self-limited nature of these types of dermatoses, they may be less inclined to seek medical consultation.
Final Thoughts
As the climate continues to change, the incidence of marine rashes such as seabather’s eruption and diver’s dermatitis is expected to increase due to warmer surface temperatures causing more frequent and earlier blooms of L unguiculata and E lineata. Cases of diver’s dermatitis also could increase due to a longer season of more frequent human exposure from an increase in warmer temperatures. The projected uptick in incidences of these marine rashes makes understanding these pathologies even more pertinent for physicians.6 Increasing our understanding of the different types of marine rashes and their causes will help guide future recommendations for the general public when visiting the ocean.
Future research may wish to investigate unique ways in which to prevent contact between these organisms and humans. Past research on mice indicated that topical application of DEET (N,N-diethyl-meta-toluamide) prior to trematode exposure prevented penetration of the skin by parasitic worms.7 Future studies are needed to examine the effectiveness of this preventative technique on humans. For now, dermatologists may counsel our ocean-going patients on preventative behaviors as well as provide reassurance and symptomatic relief when they present to our clinics with marine rashes.
Background and Clinical Presentation
Seabather’s Eruption—Seabather’s eruption is a type I and IV hypersensitivity reaction caused by nematocysts of larval-stage thimble jellyfish (Linuche unguiculata), sea anemones (eg, Edwardsiella lineata), and larval cnidarians.1Linuche unguiculata commonly is found along the southeast coast of the United States and in the Caribbean, the Gulf of Mexico, and the coasts of Florida; less commonly, it has been reported along the coasts of Brazil and Papua New Guinea. Edwardsiella lineata more commonly is seen along the East Coast of the United States.2 Seabather’s eruption presents as numerous scattered, pruritic, red macules and papules (measuring 1 mm to 1.5 cm in size) distributed in areas covered by skin folds, wet clothing, or hair following exposure to marine water (Figure 1). This maculopapular rash generally appears shortly after exiting the water and can last up to several weeks in some cases.3 The cause for this delayed presentation is that the marine organisms become entrapped between the skin of the human contact and another object (eg, swimwear) but do not release their preformed antivenom until they are exposed to air after removal from the water, at which point the organisms die and cell lysis results in injection of the venom.

Diver’s Dermatitis—Diver’s dermatitis (also referred to as “swimmer’s itch”) is a type I and IV hypersensitivity reaction caused by schistosome cercariae released by aquatic snails.4 There are several different cercarial species known to be capable of causing diver dermatitis, but the most commonly implicated genera are Trichobilharzia and Gigantobilharzia. These parasites most commonly are found in freshwater lakes but also occur in oceans, particularly in brackish areas adjacent to freshwater access. Factors associated with increased concentrations of these parasites include shallow, slow-moving water and prolonged onshore wind causing accumulation near the shoreline. It also is thought that the snail host will shed greater concentrations of the parasitic worm in the morning hours and after prolonged exposure to sunlight.4 These flatworm trematodes have a 2-host life cycle. The snails function as intermediate hosts for the parasites before they enter their final host, which are birds. Humans only function as incidental and nonviable hosts for these worms. The parasites gain access to the human body by burrowing into exposed skin. Because the parasite is unable to survive on human hosts, it dies shortly after penetrating the skin, which leads to an intense inflammatory response causing symptoms of pruritus within hours of exposure (Figure 2). The initial eruption progresses over a few days into a diffuse, maculopapular, pruritic rash, similar to that seen in seabather’s eruption. This rash then regresses completely in 1 to 3 weeks. Subsequent exposure to the same parasite is associated with increased severity of future rashes, likely due to antibody-mediated sensitization.4

Diagnosis—Marine-derived dermatoses from various sources can present very similarly; thus, it is difficult to discern the specific etiology behind the clinical presentation. No commonly utilized imaging modalities can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough patient history often can aid in differentiation of the cause of the eruption. For example, lesions located only on nonexposed areas of the skin increases the likelihood of seabather’s eruption due to nematocysts being trapped between clothing and the skin. In contrast, diver’s dermatitis generally appears on areas of the skin that were directly exposed to water and uncovered by clothing.5 Patient reports of a lack of symptoms until shortly after exiting the water further support a diagnosis of seabather’s eruption, as this delayed presentation of symptoms is caused by lysis of the culprit organisms following removal from the marine environment. The cell lysis is responsible for the widespread injection of preformed venom via the numerous nematocysts trapped between clothing and the patient’s body.1
Treatment
For both conditions, the symptoms are treated with hydrocortisone or other topical steroid solutions in conjunction with oral hydroxyzine. Alternative treatments include calamine lotion with 1% menthol and nonsteroidal anti-inflammatory drugs. Taking baths with oatmeal, Epsom salts, or baking soda also may alleviate some of the pruritic symptoms.2
Prevention
The ability to diagnose the precise cause of these similar marine rashes can bring peace of mind to both patients and physicians regardless of their similar management strategies. Severe contact dermatitis of unknown etiology can be disconcerting for patients. Additionally, documenting the causes of marine rashes in particular geographic locations can be beneficial for establishing which organisms are most likely to affect visitors to those areas. This type of data collection can be utilized to develop preventative recommendations, such as deciding when to avoid the water. Education of the public can be done with the use of informational posters located near popular swimming areas and online public service announcements. Informing the general public about the dangers of entering the ocean, especially during certain times of the year when nematocyst-equipped sea creatures are in abundance, could serve to prevent numerous cases of seabather’s eruption. Likewise, advising against immersion in shallow, slow-moving water during the morning hours or after prolonged sun exposure in trematode-endemic areas could prevent numerous cases of diver’s dermatitis. Basic information on what to expect if afflicted by a marine rash also may reduce the number of emergency department visits for these conditions, thus providing economic benefit for patients and for hospitals since patients would better know how to acutely treat these rashes and lessen the patient load at hospital emergency departments. If individuals can assure themselves of the self-limited nature of these types of dermatoses, they may be less inclined to seek medical consultation.
Final Thoughts
As the climate continues to change, the incidence of marine rashes such as seabather’s eruption and diver’s dermatitis is expected to increase due to warmer surface temperatures causing more frequent and earlier blooms of L unguiculata and E lineata. Cases of diver’s dermatitis also could increase due to a longer season of more frequent human exposure from an increase in warmer temperatures. The projected uptick in incidences of these marine rashes makes understanding these pathologies even more pertinent for physicians.6 Increasing our understanding of the different types of marine rashes and their causes will help guide future recommendations for the general public when visiting the ocean.
Future research may wish to investigate unique ways in which to prevent contact between these organisms and humans. Past research on mice indicated that topical application of DEET (N,N-diethyl-meta-toluamide) prior to trematode exposure prevented penetration of the skin by parasitic worms.7 Future studies are needed to examine the effectiveness of this preventative technique on humans. For now, dermatologists may counsel our ocean-going patients on preventative behaviors as well as provide reassurance and symptomatic relief when they present to our clinics with marine rashes.
- Parrish DO. Seabather’s eruption or diver’s dermatitis? JAMA. 1993;270:2300-2301. doi:10.1001/jama.1993.03510190054021
- Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice’. JAMA. 1993;269:1669-1672. doi:10.1001/jama.1993.03500130083037
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by algae and Bryozoans. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology: Biotic, Chemical, and Physical Agents. Springer; 2016:127-137.
- Tracz ES, Al-Jubury A, Buchmann K, et al. Outbreak of swimmer’s itch in Denmark. Acta Derm Venereol. 2019;99:1116-1120. doi:10.2340/00015555-3309
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. JAAD. 2016;76:140-147. doi:10.1016/j.jaad.2016.08.014
- Salafsky B, Ramaswamy K, He YX, et al. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg. 1999;61:743-750. doi:10.4269/ajtmh.1999.61.743
- Parrish DO. Seabather’s eruption or diver’s dermatitis? JAMA. 1993;270:2300-2301. doi:10.1001/jama.1993.03510190054021
- Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice’. JAMA. 1993;269:1669-1672. doi:10.1001/jama.1993.03500130083037
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by algae and Bryozoans. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology: Biotic, Chemical, and Physical Agents. Springer; 2016:127-137.
- Tracz ES, Al-Jubury A, Buchmann K, et al. Outbreak of swimmer’s itch in Denmark. Acta Derm Venereol. 2019;99:1116-1120. doi:10.2340/00015555-3309
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. JAAD. 2016;76:140-147. doi:10.1016/j.jaad.2016.08.014
- Salafsky B, Ramaswamy K, He YX, et al. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg. 1999;61:743-750. doi:10.4269/ajtmh.1999.61.743
Practice Points
- Seabather’s eruption and diver’s dermatitis have similar clinical presentations but differ in the ways that organisms come in contact with the skin.
- No commonly utilized imaging modality can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough history often can aid in differentiating these marine rashes.
- Physicians should understand the pathologies of common marine rashes due to a projected uptick in the number of cases related to climate change.
Impact of the COVID-19 Pandemic on Characteristics of Cutaneous Tumors Treated by Mohs Micrographic Surgery
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
Practice Points
- Mohs surgeons should follow best practice guidelines dictated by our governing professional societies in selecting skin cancers for treatment by Mohs micrographic surgery (MMS) during the COVID-19 pandemic and beyond.
- The COVID-19 pandemic has impacted the characteristics of skin cancers treated by MMS, largely driven by new guidelines.
- Changing characteristics of skin cancers treated by MMS are of clinical significance, potentially affecting the extent of reconstructive surgery, cost, operating time, and future tumor characteristics.
Dermatologic Management of Hidradenitis Suppurativa and Impact on Pregnancy and Breastfeeding
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9
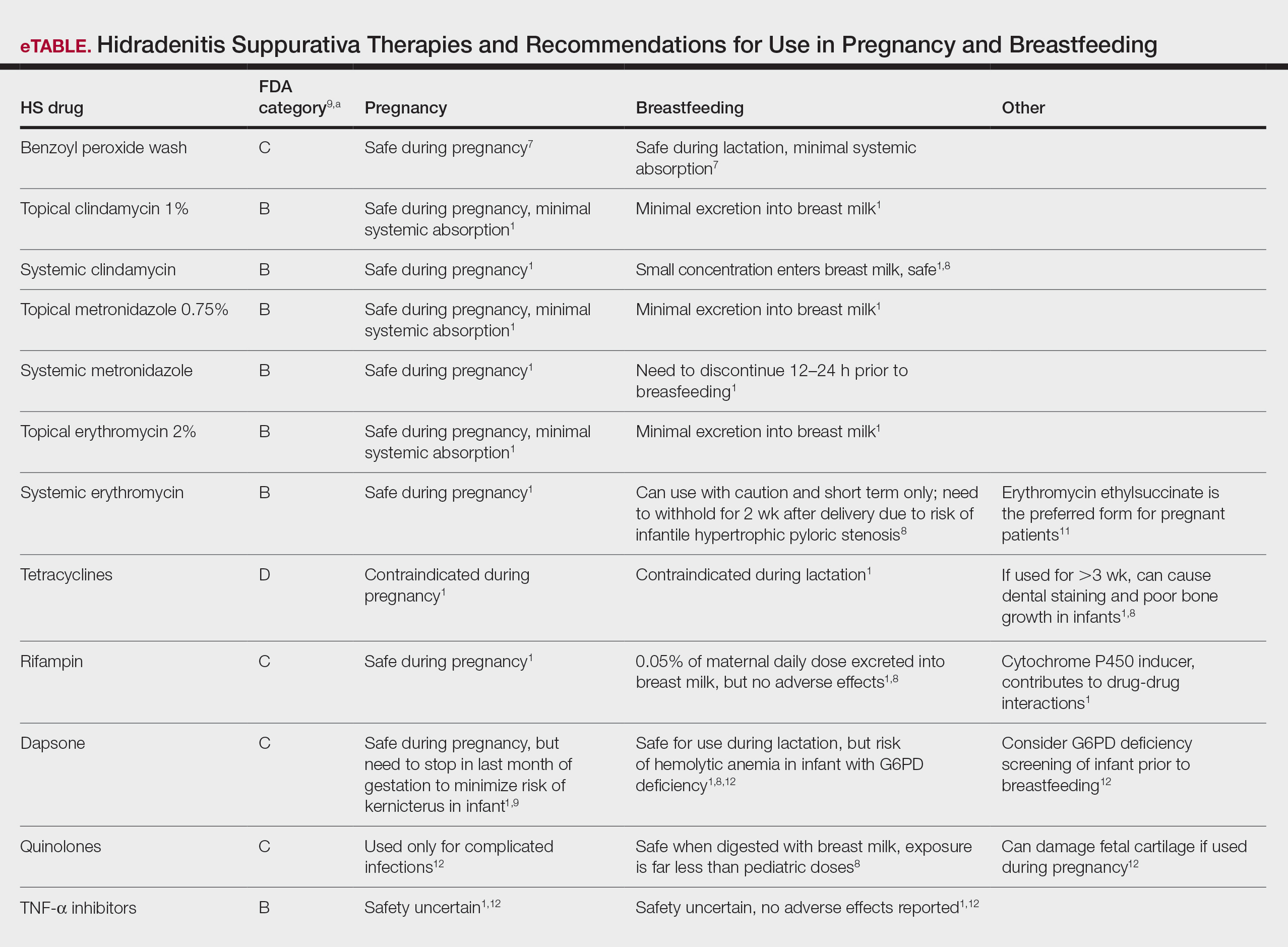
Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5
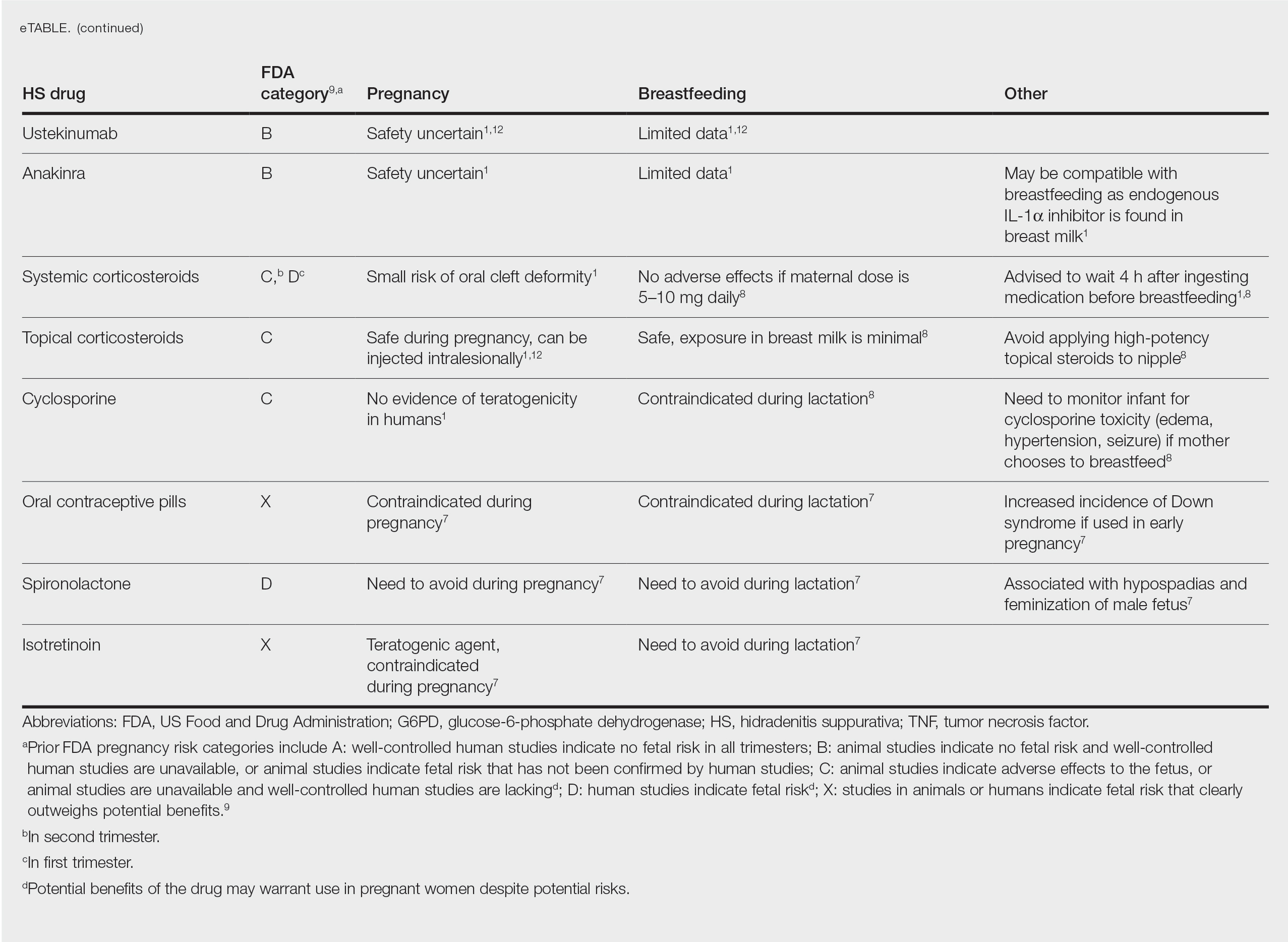
CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9

Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5

CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9

Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5

CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
Practice Points
- Some medications used to treat hidradenitis suppurativa (HS) may have teratogenic effects and be contraindicated during breastfeeding.
- We summarize what treatments are proven to be safe in pregnancy and breastfeeding and highlight the need for more guidelines and safety data for dermatologists to manage their pregnant patients with HS.
Utilization of Telehealth Services During the COVID-19 Pandemic
In 2017, lawmakers and insurers in the state of Texas approved the use of telehealth services in times of crisis.1 During the coronavirus disease 2019 (COVID-19) pandemic, our clinic has used telemedicine to provide remote care to dermatology patients. We posit that the quick introduction and implementation of telemedicine during this time of need will change the way we practice dermatology in the future.
At the University of Texas Medical Branch in Galveston, Texas, we primarily have used 2 forms of telemedicine during the COVID-19 pandemic: live face-to-face video communication (our institution primarily uses FaceTime), and a combination of telephone calls with store-and-forward images. All dermatology services at our institution were converted to telemedicine visits, and in-person office visits were only done if deemed necessary after triage by telemedicine in April and May 2020. This strategy removed the necessity for patients to leave their homes for their appointments, which not only saved them travel costs and time but also reduced the potential spread of COVID-19. Since this time, the clinic has reopened for in-person visits; however, patients still have the option to schedule a telehealth appointment if they prefer. Many patients still select the telehealth option for the above reasons.
Although routine skin checks were not always possible by video and/or store-and-forward images, telemedicine worked very well for follow-up visits, especially isotretinoin follow-ups. During the COVID-19 outbreak, iPLEDGE (https://www.ipledgeprogram.com/iPledgeUI/home.u) rapidly adapted to the use of telemedicine and even began to allow home pregnancy tests to be entered into the iPLEDGE system by health care providers. Isotretinoin follow-ups are especially useful for patients who do not require laboratory monitoring at the visit. Patients are easily evaluated, screened for side effects, and continued on their treatment if no concerns are found during the telemedicine visit. Patients who require laboratory monitoring are still able to schedule tests at our clinics or at free-standing laboratories near their homes without having an in-office dermatology appointment. At-home pregnancy tests are still being utilized as an option for patients electing for telehealth follow-ups. This strategy is both health conscious by protecting the patient from exposure to COVID-19 at a testing center and cost-effective, especially for our uninsured patients, while still meeting the safety check for iPLEDGE.
Additionally, we utilized store-and-forward telemedicine for hospital consultations. If the patient’s condition can easily be diagnosed by viewing unedited clinical images remotely, the clinician can further decrease the risk of COVID-19 spread and exposure by providing the consultation and treatment recommendations by telephone. In cases in which a diagnosis could not be made by reviewing clinical photographs remotely, an in-person visit would be done. We continue to use this strategy for our confirmed COVID-positive hospital consultations to help protect our faculty and residents and decrease the use of personal protective equipment. We propose this model could be instituted for patients admitted to hospitals without access to dermatology consultations. Store-and-forward photographs of worrisome lesions and rashes also can be used to triage visits. For example, a patient with a new-onset keratoacanthoma and a history of nonmelanoma skin cancer contacted our clinic during the pandemic and sent store-and-forward images for review. The patient was triaged by a telemedicine visit and was then brought into the clinic for biopsy based on his clinical photographs and history. Patients also have requested prescriptions for bimatoprost and tretinoin via telehealth, a service that many medical spas and online telehealth companies provide already but was not offered at our practice until now.
Telemedicine also has potentially helped decrease the number of patients going to urgent care clinics for dermatology-related issues. Additionally, we have utilized one provider per day to be the “on-call” dermatologist who would be doing telemedicine appointments for patients with new-onset conditions. This strategy not only minimized possible patient exposure to COVID-19 but also helped preserve resources at urgent care clinics and emergency departments, which currently are inundated with patients. Since we have reopened for in-person visits, we have been unable to sustain an on-call dermatologist for telemedicine but may re-employ this strategy in the future.
The unique experience of practicing medicine during a pandemic has and will affect the way we practice moving forward. The way telemedicine has been quickly and easily implemented by the health care community during the COVID-19 pandemic has taught our dermatologists the value of this method of health care delivery. We will likely continue to use telemedicine after the pandemic has been contained. Telemedicine has the potential to expand access to care to rural and underserved areas, hospitals without on-call dermatologists, and homebound patients. We also may be better able to provide isotretinoin to our patients who have deferred treatment due to difficulty with transportation to the monthly visits. Store-and-forward images could help patients referred to dermatology avoid long wait times for obvious skin cancers that would benefit from early treatment. Telemedicine visits also could potentially improve attendance for patients who forget about their appointment by calling them after they miss their scheduled appointment time and complete a telehealth encounter on the same day instead, which could help recover costs of no-show appointments for clinics.
It is still unclear how private insurance companies will adapt to the new use of telemedicine, but we hope they follow the lead of Medicare, which released a statement on March 6, 2020, supporting the implementation of telehealth services.2 Although Medicare has made adjustments to allow for equal reimbursement for telehealth appointments, private insurance companies still vary greatly. Many practices are struggling and some remained open despite shelter-in-place orders, but we propose telemedicine may be a safer alternative for patients and providers during the current health crisis that would keep billable services in place. It is still uncertain whether the laws enacted to make telemedicine accessible during this time will hold after COVID-19 is contained, but we are hopeful that living through the pandemic will bring some positive benefit to our practice and the patients we serve.
- Texas laws and regulations relating to telemedicine. Texas Medical Association website. https://www.texmed.org/Template.aspx?id=47554. Updated March 19, 2020. Accessed July 14, 2020.
- Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID 19 outbreak. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Published March 17, 2020. Accessed July 14, 2020.
In 2017, lawmakers and insurers in the state of Texas approved the use of telehealth services in times of crisis.1 During the coronavirus disease 2019 (COVID-19) pandemic, our clinic has used telemedicine to provide remote care to dermatology patients. We posit that the quick introduction and implementation of telemedicine during this time of need will change the way we practice dermatology in the future.
At the University of Texas Medical Branch in Galveston, Texas, we primarily have used 2 forms of telemedicine during the COVID-19 pandemic: live face-to-face video communication (our institution primarily uses FaceTime), and a combination of telephone calls with store-and-forward images. All dermatology services at our institution were converted to telemedicine visits, and in-person office visits were only done if deemed necessary after triage by telemedicine in April and May 2020. This strategy removed the necessity for patients to leave their homes for their appointments, which not only saved them travel costs and time but also reduced the potential spread of COVID-19. Since this time, the clinic has reopened for in-person visits; however, patients still have the option to schedule a telehealth appointment if they prefer. Many patients still select the telehealth option for the above reasons.
Although routine skin checks were not always possible by video and/or store-and-forward images, telemedicine worked very well for follow-up visits, especially isotretinoin follow-ups. During the COVID-19 outbreak, iPLEDGE (https://www.ipledgeprogram.com/iPledgeUI/home.u) rapidly adapted to the use of telemedicine and even began to allow home pregnancy tests to be entered into the iPLEDGE system by health care providers. Isotretinoin follow-ups are especially useful for patients who do not require laboratory monitoring at the visit. Patients are easily evaluated, screened for side effects, and continued on their treatment if no concerns are found during the telemedicine visit. Patients who require laboratory monitoring are still able to schedule tests at our clinics or at free-standing laboratories near their homes without having an in-office dermatology appointment. At-home pregnancy tests are still being utilized as an option for patients electing for telehealth follow-ups. This strategy is both health conscious by protecting the patient from exposure to COVID-19 at a testing center and cost-effective, especially for our uninsured patients, while still meeting the safety check for iPLEDGE.
Additionally, we utilized store-and-forward telemedicine for hospital consultations. If the patient’s condition can easily be diagnosed by viewing unedited clinical images remotely, the clinician can further decrease the risk of COVID-19 spread and exposure by providing the consultation and treatment recommendations by telephone. In cases in which a diagnosis could not be made by reviewing clinical photographs remotely, an in-person visit would be done. We continue to use this strategy for our confirmed COVID-positive hospital consultations to help protect our faculty and residents and decrease the use of personal protective equipment. We propose this model could be instituted for patients admitted to hospitals without access to dermatology consultations. Store-and-forward photographs of worrisome lesions and rashes also can be used to triage visits. For example, a patient with a new-onset keratoacanthoma and a history of nonmelanoma skin cancer contacted our clinic during the pandemic and sent store-and-forward images for review. The patient was triaged by a telemedicine visit and was then brought into the clinic for biopsy based on his clinical photographs and history. Patients also have requested prescriptions for bimatoprost and tretinoin via telehealth, a service that many medical spas and online telehealth companies provide already but was not offered at our practice until now.
Telemedicine also has potentially helped decrease the number of patients going to urgent care clinics for dermatology-related issues. Additionally, we have utilized one provider per day to be the “on-call” dermatologist who would be doing telemedicine appointments for patients with new-onset conditions. This strategy not only minimized possible patient exposure to COVID-19 but also helped preserve resources at urgent care clinics and emergency departments, which currently are inundated with patients. Since we have reopened for in-person visits, we have been unable to sustain an on-call dermatologist for telemedicine but may re-employ this strategy in the future.
The unique experience of practicing medicine during a pandemic has and will affect the way we practice moving forward. The way telemedicine has been quickly and easily implemented by the health care community during the COVID-19 pandemic has taught our dermatologists the value of this method of health care delivery. We will likely continue to use telemedicine after the pandemic has been contained. Telemedicine has the potential to expand access to care to rural and underserved areas, hospitals without on-call dermatologists, and homebound patients. We also may be better able to provide isotretinoin to our patients who have deferred treatment due to difficulty with transportation to the monthly visits. Store-and-forward images could help patients referred to dermatology avoid long wait times for obvious skin cancers that would benefit from early treatment. Telemedicine visits also could potentially improve attendance for patients who forget about their appointment by calling them after they miss their scheduled appointment time and complete a telehealth encounter on the same day instead, which could help recover costs of no-show appointments for clinics.
It is still unclear how private insurance companies will adapt to the new use of telemedicine, but we hope they follow the lead of Medicare, which released a statement on March 6, 2020, supporting the implementation of telehealth services.2 Although Medicare has made adjustments to allow for equal reimbursement for telehealth appointments, private insurance companies still vary greatly. Many practices are struggling and some remained open despite shelter-in-place orders, but we propose telemedicine may be a safer alternative for patients and providers during the current health crisis that would keep billable services in place. It is still uncertain whether the laws enacted to make telemedicine accessible during this time will hold after COVID-19 is contained, but we are hopeful that living through the pandemic will bring some positive benefit to our practice and the patients we serve.
In 2017, lawmakers and insurers in the state of Texas approved the use of telehealth services in times of crisis.1 During the coronavirus disease 2019 (COVID-19) pandemic, our clinic has used telemedicine to provide remote care to dermatology patients. We posit that the quick introduction and implementation of telemedicine during this time of need will change the way we practice dermatology in the future.
At the University of Texas Medical Branch in Galveston, Texas, we primarily have used 2 forms of telemedicine during the COVID-19 pandemic: live face-to-face video communication (our institution primarily uses FaceTime), and a combination of telephone calls with store-and-forward images. All dermatology services at our institution were converted to telemedicine visits, and in-person office visits were only done if deemed necessary after triage by telemedicine in April and May 2020. This strategy removed the necessity for patients to leave their homes for their appointments, which not only saved them travel costs and time but also reduced the potential spread of COVID-19. Since this time, the clinic has reopened for in-person visits; however, patients still have the option to schedule a telehealth appointment if they prefer. Many patients still select the telehealth option for the above reasons.
Although routine skin checks were not always possible by video and/or store-and-forward images, telemedicine worked very well for follow-up visits, especially isotretinoin follow-ups. During the COVID-19 outbreak, iPLEDGE (https://www.ipledgeprogram.com/iPledgeUI/home.u) rapidly adapted to the use of telemedicine and even began to allow home pregnancy tests to be entered into the iPLEDGE system by health care providers. Isotretinoin follow-ups are especially useful for patients who do not require laboratory monitoring at the visit. Patients are easily evaluated, screened for side effects, and continued on their treatment if no concerns are found during the telemedicine visit. Patients who require laboratory monitoring are still able to schedule tests at our clinics or at free-standing laboratories near their homes without having an in-office dermatology appointment. At-home pregnancy tests are still being utilized as an option for patients electing for telehealth follow-ups. This strategy is both health conscious by protecting the patient from exposure to COVID-19 at a testing center and cost-effective, especially for our uninsured patients, while still meeting the safety check for iPLEDGE.
Additionally, we utilized store-and-forward telemedicine for hospital consultations. If the patient’s condition can easily be diagnosed by viewing unedited clinical images remotely, the clinician can further decrease the risk of COVID-19 spread and exposure by providing the consultation and treatment recommendations by telephone. In cases in which a diagnosis could not be made by reviewing clinical photographs remotely, an in-person visit would be done. We continue to use this strategy for our confirmed COVID-positive hospital consultations to help protect our faculty and residents and decrease the use of personal protective equipment. We propose this model could be instituted for patients admitted to hospitals without access to dermatology consultations. Store-and-forward photographs of worrisome lesions and rashes also can be used to triage visits. For example, a patient with a new-onset keratoacanthoma and a history of nonmelanoma skin cancer contacted our clinic during the pandemic and sent store-and-forward images for review. The patient was triaged by a telemedicine visit and was then brought into the clinic for biopsy based on his clinical photographs and history. Patients also have requested prescriptions for bimatoprost and tretinoin via telehealth, a service that many medical spas and online telehealth companies provide already but was not offered at our practice until now.
Telemedicine also has potentially helped decrease the number of patients going to urgent care clinics for dermatology-related issues. Additionally, we have utilized one provider per day to be the “on-call” dermatologist who would be doing telemedicine appointments for patients with new-onset conditions. This strategy not only minimized possible patient exposure to COVID-19 but also helped preserve resources at urgent care clinics and emergency departments, which currently are inundated with patients. Since we have reopened for in-person visits, we have been unable to sustain an on-call dermatologist for telemedicine but may re-employ this strategy in the future.
The unique experience of practicing medicine during a pandemic has and will affect the way we practice moving forward. The way telemedicine has been quickly and easily implemented by the health care community during the COVID-19 pandemic has taught our dermatologists the value of this method of health care delivery. We will likely continue to use telemedicine after the pandemic has been contained. Telemedicine has the potential to expand access to care to rural and underserved areas, hospitals without on-call dermatologists, and homebound patients. We also may be better able to provide isotretinoin to our patients who have deferred treatment due to difficulty with transportation to the monthly visits. Store-and-forward images could help patients referred to dermatology avoid long wait times for obvious skin cancers that would benefit from early treatment. Telemedicine visits also could potentially improve attendance for patients who forget about their appointment by calling them after they miss their scheduled appointment time and complete a telehealth encounter on the same day instead, which could help recover costs of no-show appointments for clinics.
It is still unclear how private insurance companies will adapt to the new use of telemedicine, but we hope they follow the lead of Medicare, which released a statement on March 6, 2020, supporting the implementation of telehealth services.2 Although Medicare has made adjustments to allow for equal reimbursement for telehealth appointments, private insurance companies still vary greatly. Many practices are struggling and some remained open despite shelter-in-place orders, but we propose telemedicine may be a safer alternative for patients and providers during the current health crisis that would keep billable services in place. It is still uncertain whether the laws enacted to make telemedicine accessible during this time will hold after COVID-19 is contained, but we are hopeful that living through the pandemic will bring some positive benefit to our practice and the patients we serve.
- Texas laws and regulations relating to telemedicine. Texas Medical Association website. https://www.texmed.org/Template.aspx?id=47554. Updated March 19, 2020. Accessed July 14, 2020.
- Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID 19 outbreak. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Published March 17, 2020. Accessed July 14, 2020.
- Texas laws and regulations relating to telemedicine. Texas Medical Association website. https://www.texmed.org/Template.aspx?id=47554. Updated March 19, 2020. Accessed July 14, 2020.
- Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID 19 outbreak. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Published March 17, 2020. Accessed July 14, 2020.
Practice Points
- Telehealth can increase access to dermatologic care for both inpatient hospital consultations and outpatient clinic visits, especially in areas lacking dermatologists.
- With the current iPLEDGE accommodations for coronavirus disease 19, we have been able to treat patients who live 3 hours away and cannot travel for monthly isotretinoin visits.
- Telehealth allows our providers to better triage benign vs potentially malignant conditions to schedule patients in a more appropriate time frame.