User login
Acute Onset of Vitiligolike Depigmentation After Nivolumab Therapy for Systemic Melanoma
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
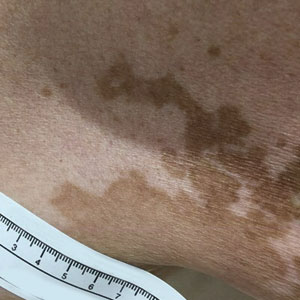
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.
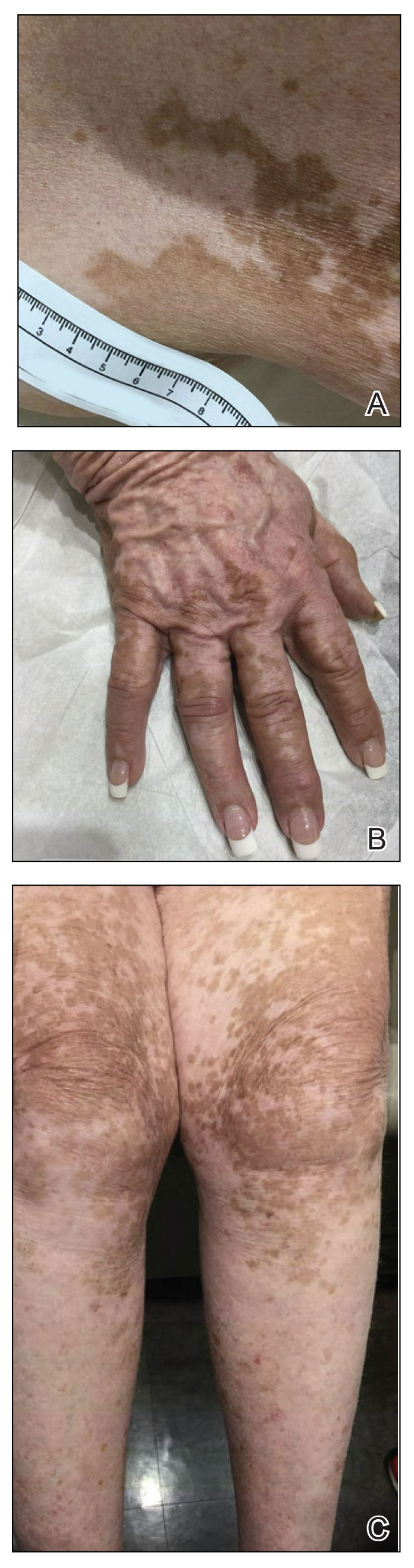
At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.

At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.

At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
Practice Points
- New-onset vitiligo coinciding with malignant melanoma should be considered a good prognostic indicator.
- Daily use of hydroquinone cream 4% in conjunction with diligent photoprotection was shown to even overall skin tone in a patient experiencing leukoderma from nivolumab therapy.