User login
Botulinum toxin for chronic pain: What's on the horizon?
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.
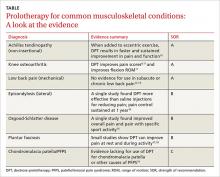
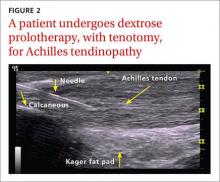
The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.
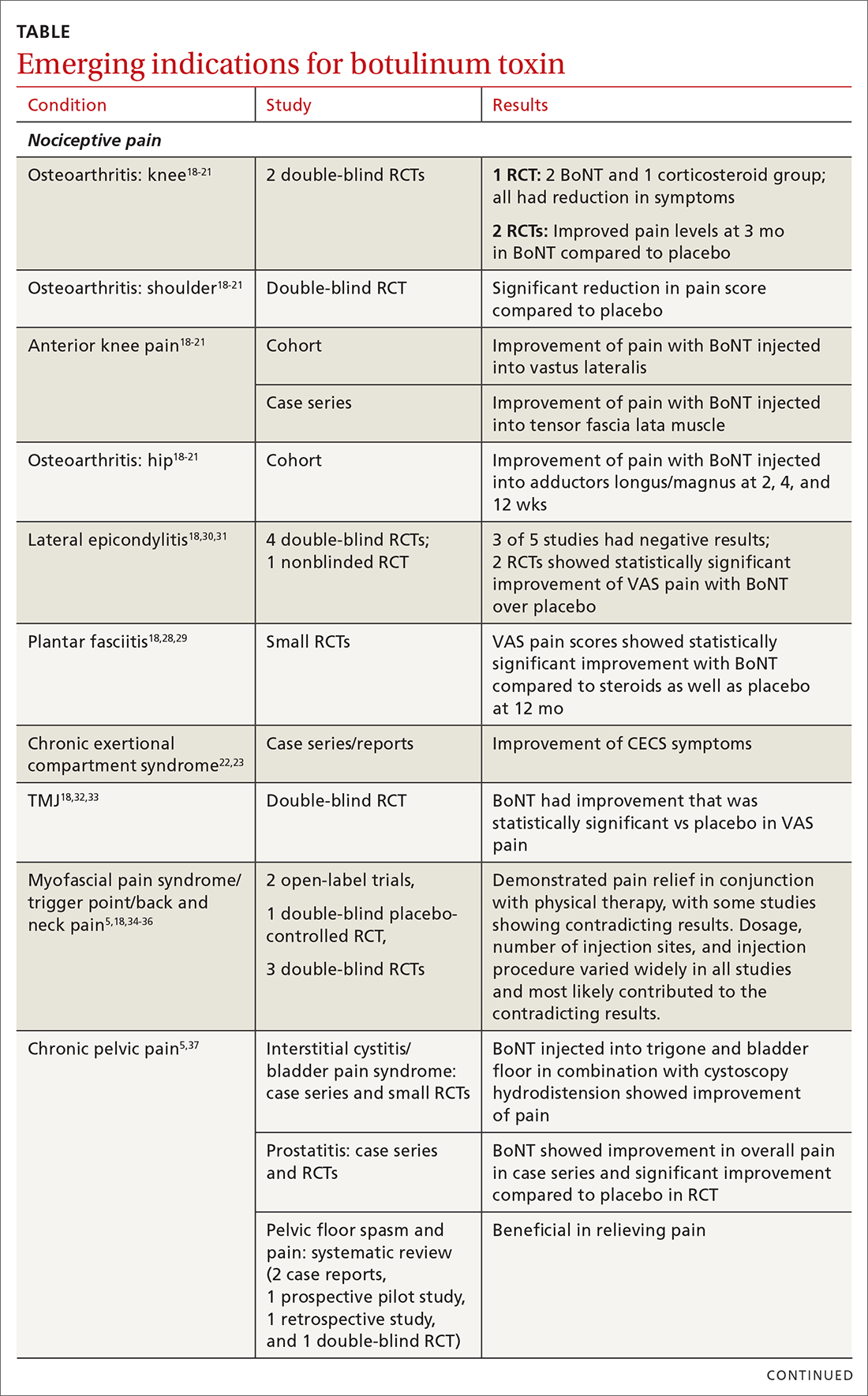
Chronic joint pain
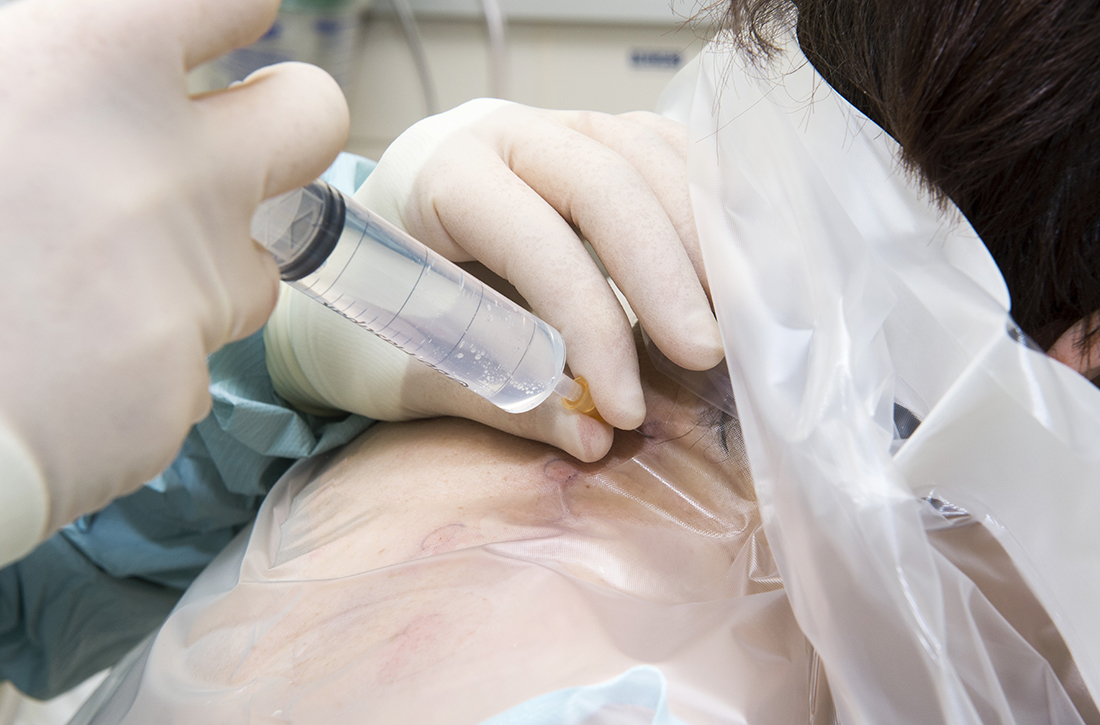
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
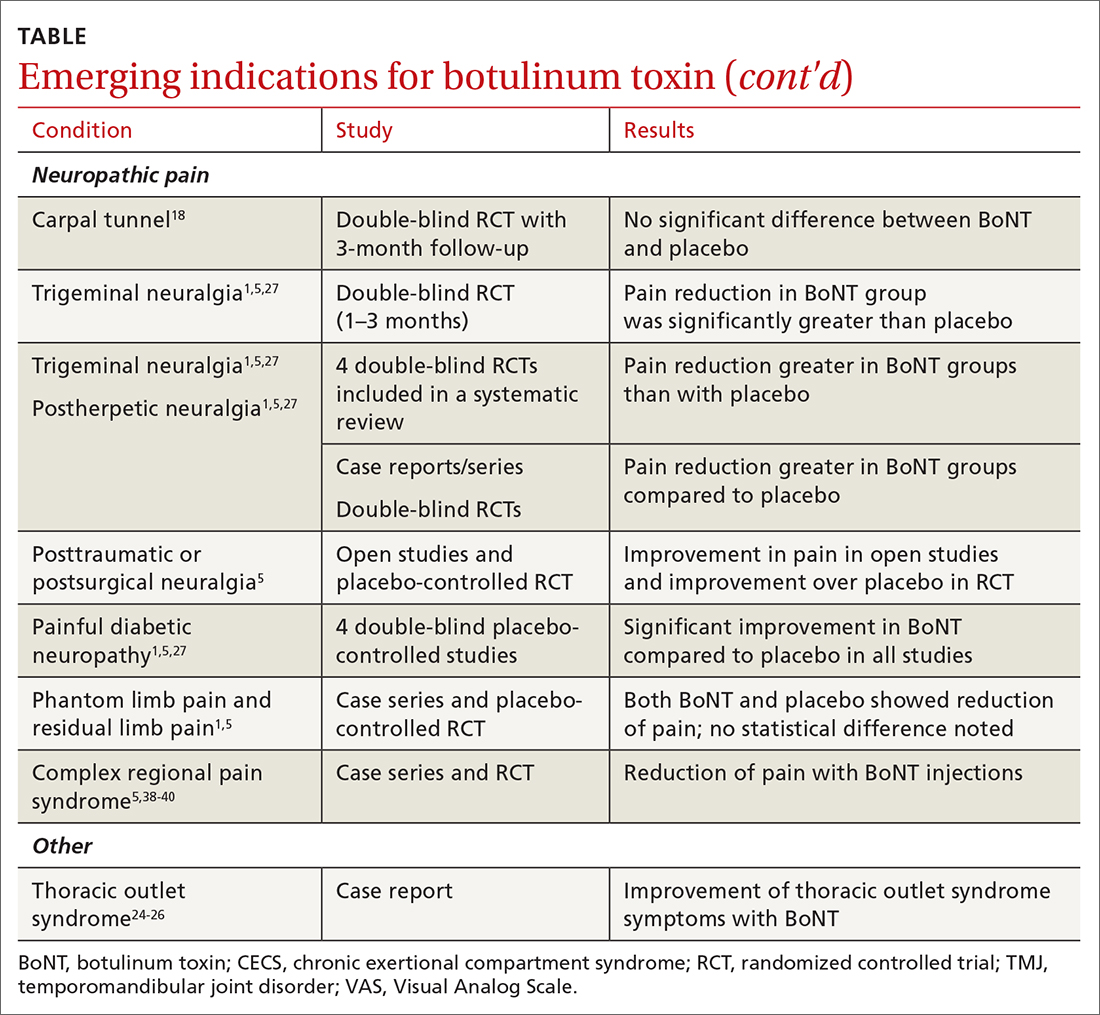
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.

The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.

Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.

The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.

Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
PRACTICE RECOMMENDATIONS
› Consider botulinum toxin (BoNT) for patients with headache, spasticity, or cervical dystonia, as the FDA has approved BoNT for pain relief in these conditions. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
MSK injury? Make splinting choices based on the evidence
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).
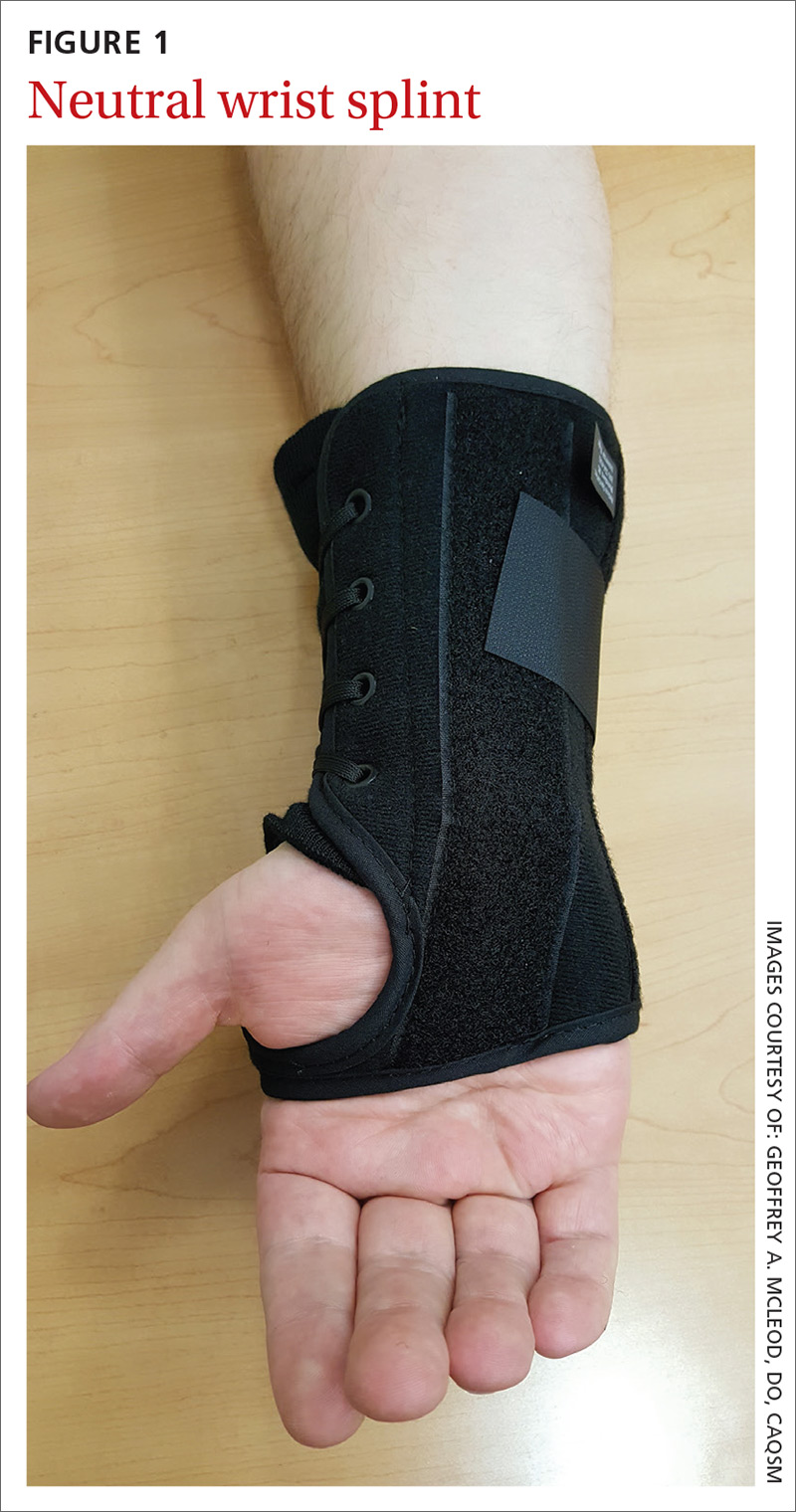
Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.
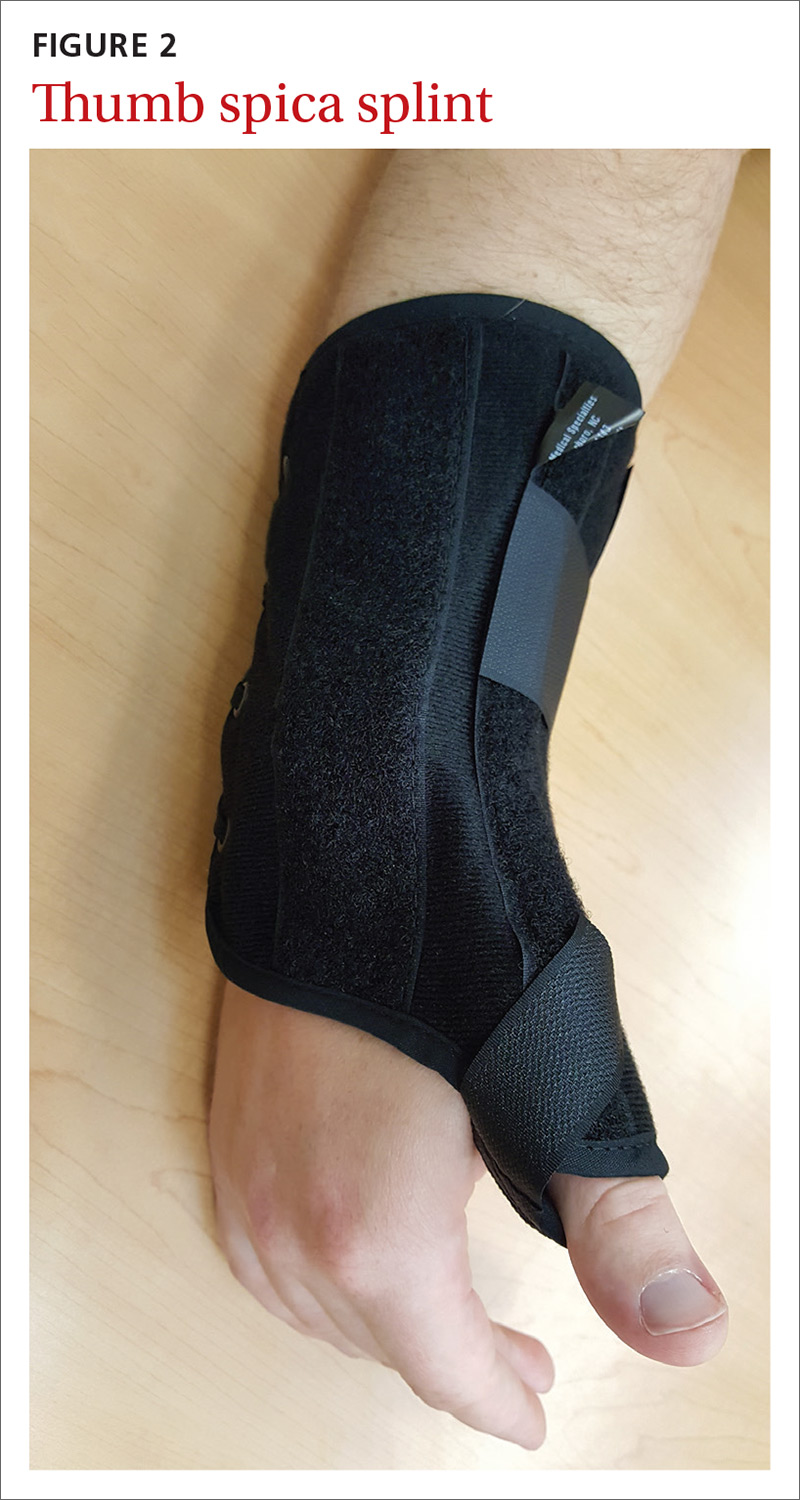
Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.
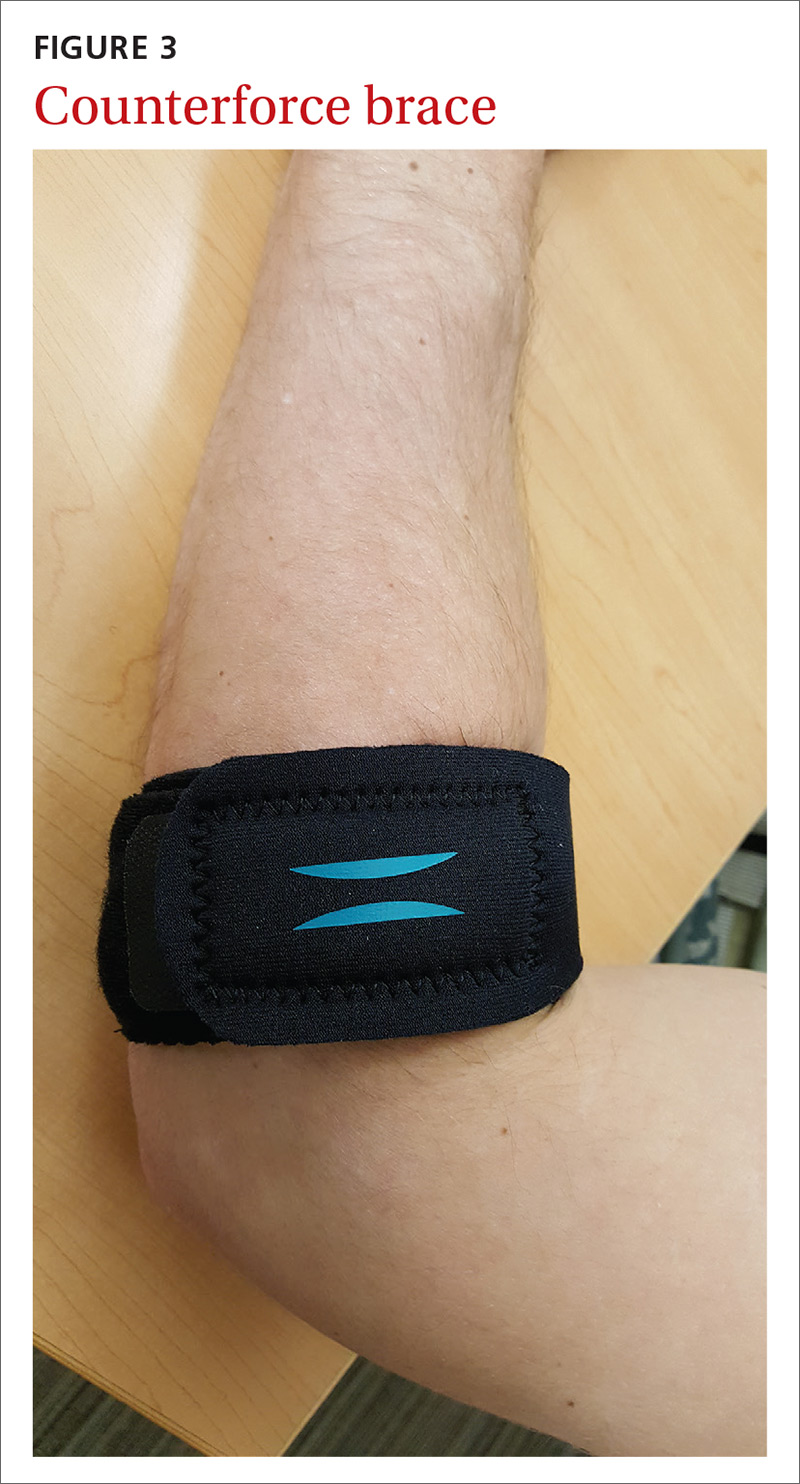
Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.
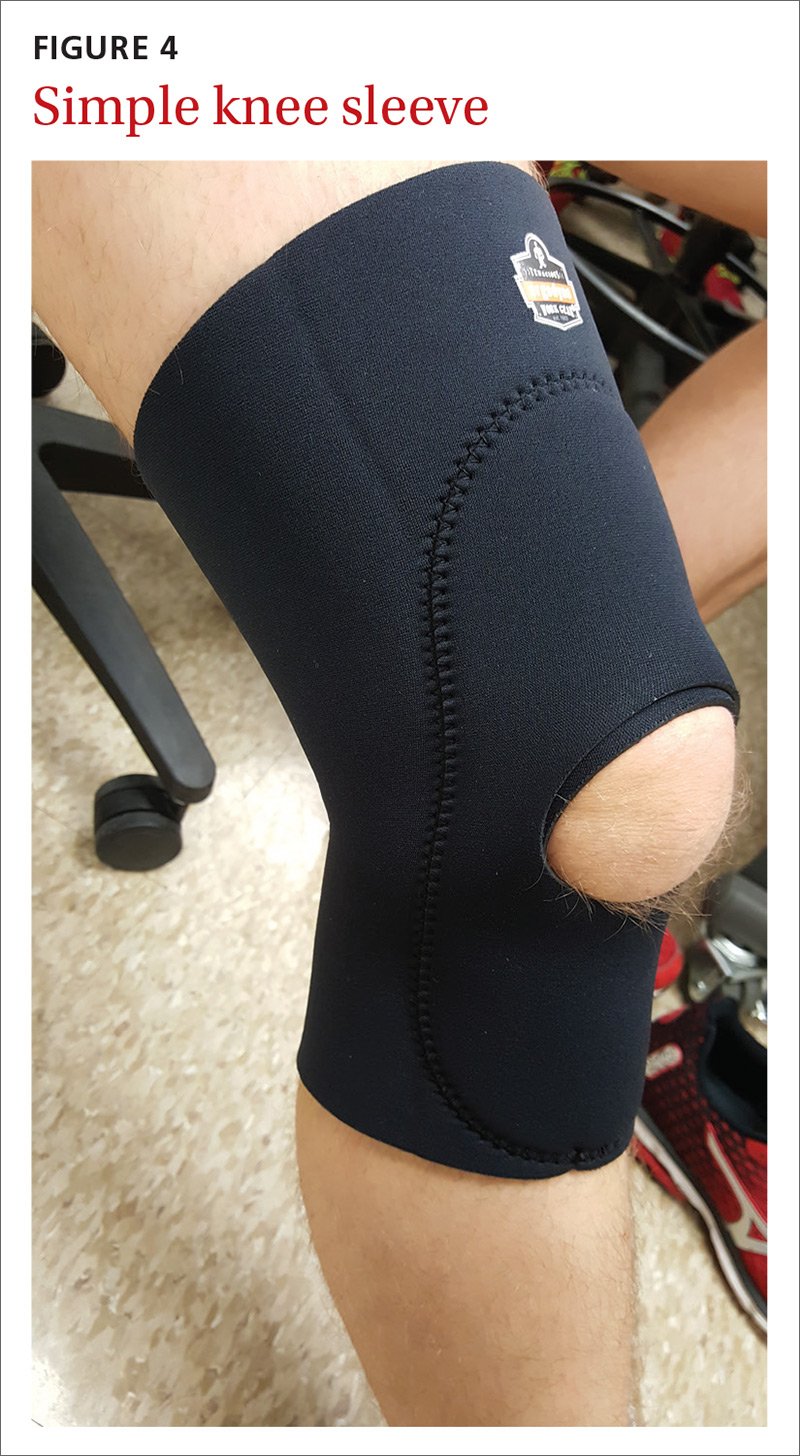
Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).
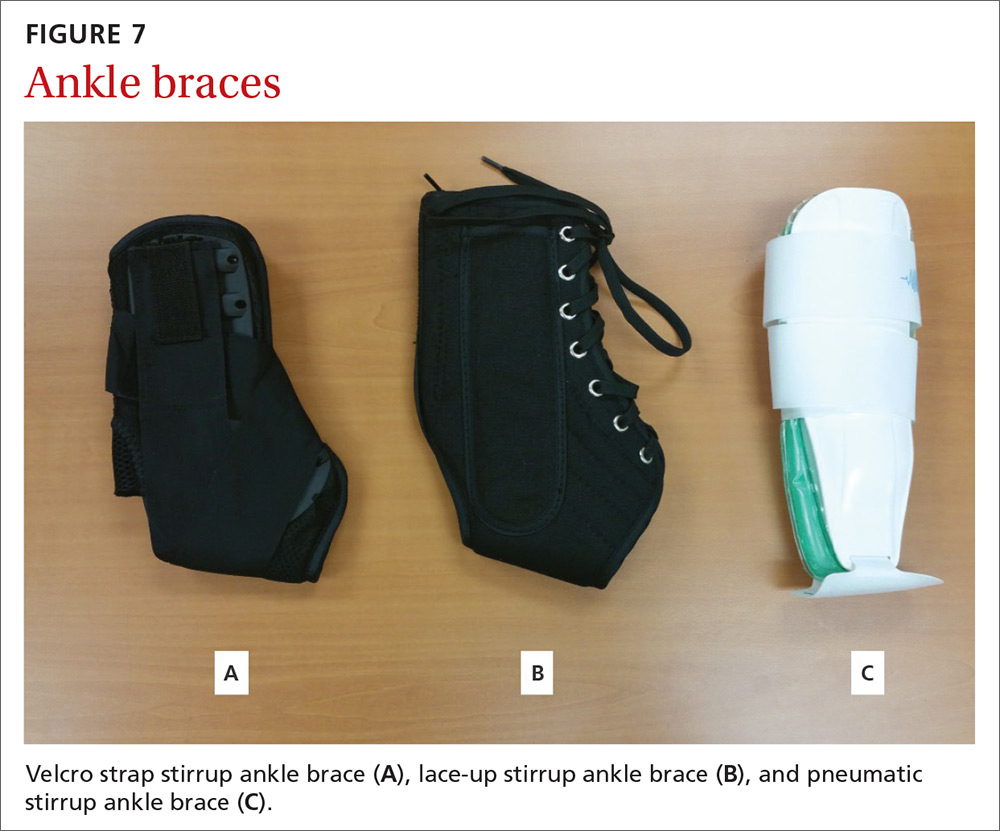
Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).

Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.

Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.

Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.

Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).

Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).

Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.

Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.

Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.

Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).

Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
From The Journal of Family Practice | 2018;67(11):678-683.
PRACTICE RECOMMENDATIONS
› Consider a wrist splint for carpal tunnel syndrome secondary to repetitive motion. B
› Recommend a simple knee sleeve to help patients with osteoarthritis reduce their pain and improve daily function. B
› Use ankle bracing for secondary prevention of a recurrent ankle sprain. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Prolotherapy: Can it help your patient?
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.