User login
Catherine Cooper Nellist is editor of Pediatric News and Ob. Gyn. News. She has more than 30 years of experience reporting, writing, and editing stories about clinical medicine and the U.S. health care industry. Prior to taking the helm of these award-winning publications, Catherine covered major medical research meetings throughout the United States and Canada, and had been editor of Clinical Psychiatry News, and Dermatology News. She joined the company in 1984 after graduating magna cum laude from Dickinson College, Carlisle, Pa., with a BA in English.
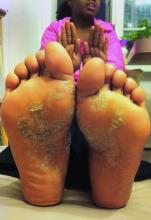
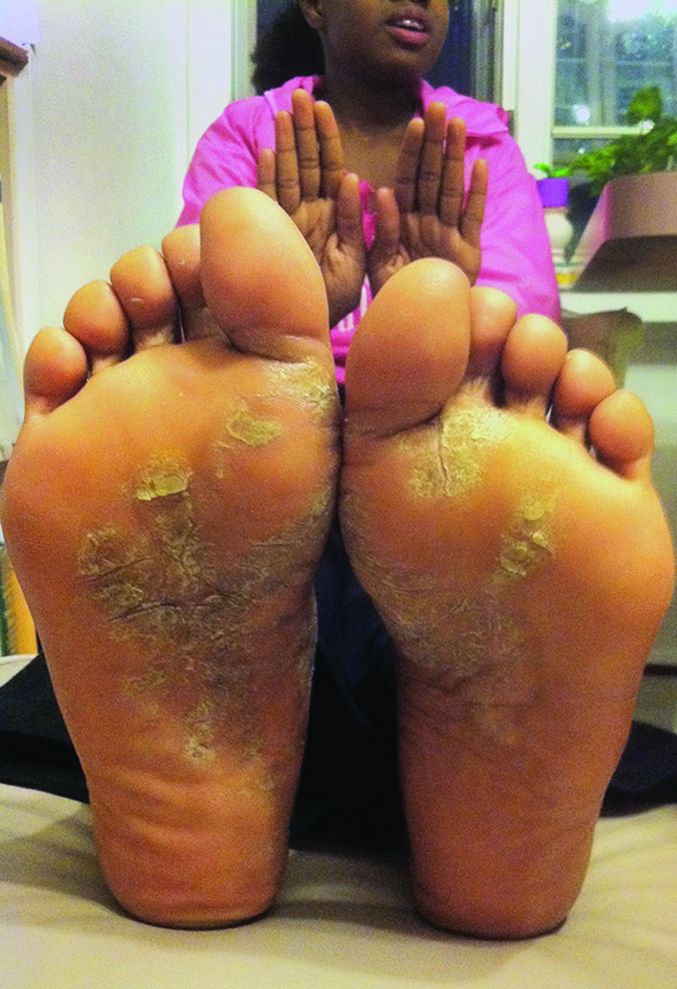
Adalimumab outperforms methotrexate in treating severe pediatric plaque psoriasis
Adalimumab appears to be a safe and effective treatment option for severe plaque psoriasis in children, outperforming methotrexate, based on the results of a phase III study, said Kim Papp, MD, PhD, of Probity Medical Research, Waterloo, Ont., and his associates.
“To our knowledge, this is the first randomized controlled study of either adalimumab or methotrexate in children and adolescents with psoriasis,” the researchers said, noting that the study did not include a placebo group because of ethical issues related to treating children with a severe chronic disorder.
At week 16 of the initial treatment period, an improvement of at least 75% in the Psoriasis Area and Severity Index (PASI75) score was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group – 22 (58%) – than in the methotrexate group – 12 (32%). In the 0.4-mg/kg adalimumab group, 17 (44%) of patients reached a PASI75. The PASI75 response was rapid in the 0.8 mg/kg adalimumab group, a significant difference, compared with the methotrexate group. It was reached by week 4 (P = .002).
“At week 16, the 26% difference between adalimumab 0.8 mg/kg and methotrexate in the proportion of patients who achieved PASI75 was significant and clinically relevant,” Dr. Papp and his associates concluded.
At week 16 of treatment, the proportion of patients who achieved a physician global assessment (PGA) score of 0 or 1 (clear or minimal) was higher in the adalimumab 0.8 mg/kg group (23 of 38 [61%]) than in the methotrexate group (15 of 37 [41%]) or in the adalimumab 0.4-mg/kg group (16 of 39 [41%]) (P = .083). At week 16, the difference between the adalimumab 0.8-mg/kg and methotrexate groups was not significant, the investigators said (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31189-3).
After the withdrawal period, PASI75 was achieved in 15 of 19 (79%) patients who were initial responders to adalimumab 0.8 mg/kg and 6 of 11 (55%) patients who were initial responders to adalimumab 0.4 mg/kg. PASI75 was achieved in six of eight (75%) patients who had responded to methotrexate treatment in the initial treatment period and who had loss of disease control in the withdrawal period.
During the initial treatment period, adverse events were reported by 26 of 38 (68%) in the adalimumab 0.8-mg/kg group, 30 of 39 (77%) in the adalimumab 0.4-mg/kg group, and 28 of 37 (76%) in the methotrexate group. Infections were the most frequently reported adverse events. Serious adverse events were infrequent, reported by three patients in the adalimumab 0.4-mg/kg group, and were not considered to be related to the study drug, the researchers said. Eleven severe adverse events were reported by 8 of the 114 (7%) children. Of these, headache was the most common. A case of urticaria during retreatment that led to discontinuation of adalimumab in the patient (who had received methotrexate in the first treatment period), was considered by the investigator as “probably related” to adalimumab.
“No new safety risks were identified in our study; however, longer-term data are needed to fully assess the safety profile of adalimumab in the pediatric population,” Dr. Papp and his associates commented.
“Our results showed better quality of life outcomes in children and adolescents treated with adalimumab compared with methotrexate. The mean 10.8-point change in PedsQL [pediatric quality of life inventory] from baseline to week 16 in the adalimumab 0.8-mg/kg group exceeded the minimal clinically important difference of 4.36, whereas the 1.9-point change in the methotrexate group did not,” they noted.
The study was funded by AbbVie, the manufacturer of adalimumab (Humira). Dr. Papp has served as a consultant for AbbVie and a number of other pharmaceutical companies, for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
The initial treatment response to adalimumab was quick with “significant clinical difference compared with methotrexate seen as early as 4 weeks.” Improvement in the pediatric quality of life inventory (PedsQL) score from baseline “was significantly greater” in children in the adalimumab 0.8-mg/kg group than in children in the methotrexate group. Adalimumab provided “several clinically and statistically significant improvements” in children with severe plaque psoriasis, compared with methotrexate.
However, further studies are needed to determine both the short-term and long-term effectiveness and the safety of systemic treatments in children and adolescents with psoriasis.
Dario Kivelevitch, MD, is a third year dermatology resident, and Alan Menter, MD, is chief of dermatology at Baylor University Medical Center, Dallas. These comments are from an editorial that accompanied the study (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31190-X). Dr. Kivelevitch said he had no relevant financial disclosures. Dr. Menter disclosed grants and personal fees from AbbVie and other pharmaceutical companies, all outside the submitted work.
The initial treatment response to adalimumab was quick with “significant clinical difference compared with methotrexate seen as early as 4 weeks.” Improvement in the pediatric quality of life inventory (PedsQL) score from baseline “was significantly greater” in children in the adalimumab 0.8-mg/kg group than in children in the methotrexate group. Adalimumab provided “several clinically and statistically significant improvements” in children with severe plaque psoriasis, compared with methotrexate.
However, further studies are needed to determine both the short-term and long-term effectiveness and the safety of systemic treatments in children and adolescents with psoriasis.
Dario Kivelevitch, MD, is a third year dermatology resident, and Alan Menter, MD, is chief of dermatology at Baylor University Medical Center, Dallas. These comments are from an editorial that accompanied the study (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31190-X). Dr. Kivelevitch said he had no relevant financial disclosures. Dr. Menter disclosed grants and personal fees from AbbVie and other pharmaceutical companies, all outside the submitted work.
The initial treatment response to adalimumab was quick with “significant clinical difference compared with methotrexate seen as early as 4 weeks.” Improvement in the pediatric quality of life inventory (PedsQL) score from baseline “was significantly greater” in children in the adalimumab 0.8-mg/kg group than in children in the methotrexate group. Adalimumab provided “several clinically and statistically significant improvements” in children with severe plaque psoriasis, compared with methotrexate.
However, further studies are needed to determine both the short-term and long-term effectiveness and the safety of systemic treatments in children and adolescents with psoriasis.
Dario Kivelevitch, MD, is a third year dermatology resident, and Alan Menter, MD, is chief of dermatology at Baylor University Medical Center, Dallas. These comments are from an editorial that accompanied the study (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31190-X). Dr. Kivelevitch said he had no relevant financial disclosures. Dr. Menter disclosed grants and personal fees from AbbVie and other pharmaceutical companies, all outside the submitted work.
Adalimumab appears to be a safe and effective treatment option for severe plaque psoriasis in children, outperforming methotrexate, based on the results of a phase III study, said Kim Papp, MD, PhD, of Probity Medical Research, Waterloo, Ont., and his associates.
“To our knowledge, this is the first randomized controlled study of either adalimumab or methotrexate in children and adolescents with psoriasis,” the researchers said, noting that the study did not include a placebo group because of ethical issues related to treating children with a severe chronic disorder.
At week 16 of the initial treatment period, an improvement of at least 75% in the Psoriasis Area and Severity Index (PASI75) score was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group – 22 (58%) – than in the methotrexate group – 12 (32%). In the 0.4-mg/kg adalimumab group, 17 (44%) of patients reached a PASI75. The PASI75 response was rapid in the 0.8 mg/kg adalimumab group, a significant difference, compared with the methotrexate group. It was reached by week 4 (P = .002).
“At week 16, the 26% difference between adalimumab 0.8 mg/kg and methotrexate in the proportion of patients who achieved PASI75 was significant and clinically relevant,” Dr. Papp and his associates concluded.
At week 16 of treatment, the proportion of patients who achieved a physician global assessment (PGA) score of 0 or 1 (clear or minimal) was higher in the adalimumab 0.8 mg/kg group (23 of 38 [61%]) than in the methotrexate group (15 of 37 [41%]) or in the adalimumab 0.4-mg/kg group (16 of 39 [41%]) (P = .083). At week 16, the difference between the adalimumab 0.8-mg/kg and methotrexate groups was not significant, the investigators said (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31189-3).
After the withdrawal period, PASI75 was achieved in 15 of 19 (79%) patients who were initial responders to adalimumab 0.8 mg/kg and 6 of 11 (55%) patients who were initial responders to adalimumab 0.4 mg/kg. PASI75 was achieved in six of eight (75%) patients who had responded to methotrexate treatment in the initial treatment period and who had loss of disease control in the withdrawal period.
During the initial treatment period, adverse events were reported by 26 of 38 (68%) in the adalimumab 0.8-mg/kg group, 30 of 39 (77%) in the adalimumab 0.4-mg/kg group, and 28 of 37 (76%) in the methotrexate group. Infections were the most frequently reported adverse events. Serious adverse events were infrequent, reported by three patients in the adalimumab 0.4-mg/kg group, and were not considered to be related to the study drug, the researchers said. Eleven severe adverse events were reported by 8 of the 114 (7%) children. Of these, headache was the most common. A case of urticaria during retreatment that led to discontinuation of adalimumab in the patient (who had received methotrexate in the first treatment period), was considered by the investigator as “probably related” to adalimumab.
“No new safety risks were identified in our study; however, longer-term data are needed to fully assess the safety profile of adalimumab in the pediatric population,” Dr. Papp and his associates commented.
“Our results showed better quality of life outcomes in children and adolescents treated with adalimumab compared with methotrexate. The mean 10.8-point change in PedsQL [pediatric quality of life inventory] from baseline to week 16 in the adalimumab 0.8-mg/kg group exceeded the minimal clinically important difference of 4.36, whereas the 1.9-point change in the methotrexate group did not,” they noted.
The study was funded by AbbVie, the manufacturer of adalimumab (Humira). Dr. Papp has served as a consultant for AbbVie and a number of other pharmaceutical companies, for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
Adalimumab appears to be a safe and effective treatment option for severe plaque psoriasis in children, outperforming methotrexate, based on the results of a phase III study, said Kim Papp, MD, PhD, of Probity Medical Research, Waterloo, Ont., and his associates.
“To our knowledge, this is the first randomized controlled study of either adalimumab or methotrexate in children and adolescents with psoriasis,” the researchers said, noting that the study did not include a placebo group because of ethical issues related to treating children with a severe chronic disorder.
At week 16 of the initial treatment period, an improvement of at least 75% in the Psoriasis Area and Severity Index (PASI75) score was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group – 22 (58%) – than in the methotrexate group – 12 (32%). In the 0.4-mg/kg adalimumab group, 17 (44%) of patients reached a PASI75. The PASI75 response was rapid in the 0.8 mg/kg adalimumab group, a significant difference, compared with the methotrexate group. It was reached by week 4 (P = .002).
“At week 16, the 26% difference between adalimumab 0.8 mg/kg and methotrexate in the proportion of patients who achieved PASI75 was significant and clinically relevant,” Dr. Papp and his associates concluded.
At week 16 of treatment, the proportion of patients who achieved a physician global assessment (PGA) score of 0 or 1 (clear or minimal) was higher in the adalimumab 0.8 mg/kg group (23 of 38 [61%]) than in the methotrexate group (15 of 37 [41%]) or in the adalimumab 0.4-mg/kg group (16 of 39 [41%]) (P = .083). At week 16, the difference between the adalimumab 0.8-mg/kg and methotrexate groups was not significant, the investigators said (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31189-3).
After the withdrawal period, PASI75 was achieved in 15 of 19 (79%) patients who were initial responders to adalimumab 0.8 mg/kg and 6 of 11 (55%) patients who were initial responders to adalimumab 0.4 mg/kg. PASI75 was achieved in six of eight (75%) patients who had responded to methotrexate treatment in the initial treatment period and who had loss of disease control in the withdrawal period.
During the initial treatment period, adverse events were reported by 26 of 38 (68%) in the adalimumab 0.8-mg/kg group, 30 of 39 (77%) in the adalimumab 0.4-mg/kg group, and 28 of 37 (76%) in the methotrexate group. Infections were the most frequently reported adverse events. Serious adverse events were infrequent, reported by three patients in the adalimumab 0.4-mg/kg group, and were not considered to be related to the study drug, the researchers said. Eleven severe adverse events were reported by 8 of the 114 (7%) children. Of these, headache was the most common. A case of urticaria during retreatment that led to discontinuation of adalimumab in the patient (who had received methotrexate in the first treatment period), was considered by the investigator as “probably related” to adalimumab.
“No new safety risks were identified in our study; however, longer-term data are needed to fully assess the safety profile of adalimumab in the pediatric population,” Dr. Papp and his associates commented.
“Our results showed better quality of life outcomes in children and adolescents treated with adalimumab compared with methotrexate. The mean 10.8-point change in PedsQL [pediatric quality of life inventory] from baseline to week 16 in the adalimumab 0.8-mg/kg group exceeded the minimal clinically important difference of 4.36, whereas the 1.9-point change in the methotrexate group did not,” they noted.
The study was funded by AbbVie, the manufacturer of adalimumab (Humira). Dr. Papp has served as a consultant for AbbVie and a number of other pharmaceutical companies, for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
FROM THE LANCET
Key clinical point:
Major finding: At week 16 of the initial treatment period, Psoriasis Area and Severity Index (PASI)175 was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group (22 of 38 [58%]) than in the methotrexate group (12 of 37 [32%]).
Data source: A double-blind, phase III trial was done at 38 clinics in 13 countries with 114 children aged 4-17 years, with severe plaque psoriasis that had not responded to topical therapy.
Disclosures: The study was funded by AbbVie. Dr. Papp has served as a consultant for adalimumab manufacturer AbbVie and a number of other pharmaceutical companies for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
Nausea with pediatric functional abdominal pain may mean depression, anxiety
(FAP), reported Alexandra C. Russell, MD, and her associates at Vanderbilt University, Nashville.
In a study of 398 children with FAP and nausea and 479 with FAP alone, those with comorbid nausea had significantly more GI symptoms and depression symptoms. Anxiety symptoms were not studied. Mean age at baseline was 12 years.
The FAP plus nausea patients were 1.79-times more likely to meet criteria for a current DSM-IV anxiety disorder and 2.61-times more likely to meet DSM-IV diagnostic criteria for generalized anxiety disorder than were FAP-only patients. The FAP and nausea patients were 1.81 times more likely to meet criteria for a DSM-IV for a major depressive disorder in their lifetime than were FAP-only patients.
The suggestion that nausea may be an independent risk factor for later anxiety and depression in children with FAP “may be owing to chronic nausea being a distressing symptom that permeates multiple areas of physical and psychosocial functioning,” Dr. Russell and her colleagues surmised.
“It may be difficult for these patients to maintain good nutrition, sleep habits, exercise, and resiliency when they frequently are nauseated,” the study authors noted. “Alternatively, FAP patients with comorbid nausea may represent a phenotype with autonomic nervous system dysfunction that contributes to both nausea and emotional distress.”
Read more in Clinical Gastroenterology and Hepatology (2017 May;15[5]:706-11).
(FAP), reported Alexandra C. Russell, MD, and her associates at Vanderbilt University, Nashville.
In a study of 398 children with FAP and nausea and 479 with FAP alone, those with comorbid nausea had significantly more GI symptoms and depression symptoms. Anxiety symptoms were not studied. Mean age at baseline was 12 years.
The FAP plus nausea patients were 1.79-times more likely to meet criteria for a current DSM-IV anxiety disorder and 2.61-times more likely to meet DSM-IV diagnostic criteria for generalized anxiety disorder than were FAP-only patients. The FAP and nausea patients were 1.81 times more likely to meet criteria for a DSM-IV for a major depressive disorder in their lifetime than were FAP-only patients.
The suggestion that nausea may be an independent risk factor for later anxiety and depression in children with FAP “may be owing to chronic nausea being a distressing symptom that permeates multiple areas of physical and psychosocial functioning,” Dr. Russell and her colleagues surmised.
“It may be difficult for these patients to maintain good nutrition, sleep habits, exercise, and resiliency when they frequently are nauseated,” the study authors noted. “Alternatively, FAP patients with comorbid nausea may represent a phenotype with autonomic nervous system dysfunction that contributes to both nausea and emotional distress.”
Read more in Clinical Gastroenterology and Hepatology (2017 May;15[5]:706-11).
(FAP), reported Alexandra C. Russell, MD, and her associates at Vanderbilt University, Nashville.
In a study of 398 children with FAP and nausea and 479 with FAP alone, those with comorbid nausea had significantly more GI symptoms and depression symptoms. Anxiety symptoms were not studied. Mean age at baseline was 12 years.
The FAP plus nausea patients were 1.79-times more likely to meet criteria for a current DSM-IV anxiety disorder and 2.61-times more likely to meet DSM-IV diagnostic criteria for generalized anxiety disorder than were FAP-only patients. The FAP and nausea patients were 1.81 times more likely to meet criteria for a DSM-IV for a major depressive disorder in their lifetime than were FAP-only patients.
The suggestion that nausea may be an independent risk factor for later anxiety and depression in children with FAP “may be owing to chronic nausea being a distressing symptom that permeates multiple areas of physical and psychosocial functioning,” Dr. Russell and her colleagues surmised.
“It may be difficult for these patients to maintain good nutrition, sleep habits, exercise, and resiliency when they frequently are nauseated,” the study authors noted. “Alternatively, FAP patients with comorbid nausea may represent a phenotype with autonomic nervous system dysfunction that contributes to both nausea and emotional distress.”
Read more in Clinical Gastroenterology and Hepatology (2017 May;15[5]:706-11).
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Racial, ethnic differences exist ADHD treatment of Medicaid-enrolled youth
, reported Janet R. Cummings, PhD, and her associates at the Rollins School of Public Health at Emory University, Atlanta.
Overall, quality of care for Medicaid-enrolled children receiving ADHD treatment is poor. Of those who initiated medications, 59% visited a provider within 30 days, 64% received at least two other doctor visits, and 38% received combined treatment with any psychotherapy visit. Sixty percent did not fill the ADHD prescription for enough days, 70% had no psychotherapy visit, and 42% stopped treatment.
The percentage that had any follow-up visit in the initiation phase was lower among African American children than among white children (56% vs. 61%, P less than .001), while Hispanic children were more likely than were white children to receive adequate follow-up in the initiation phase (63% vs. 61%; P less than .001) as well as in the C&M phase (71% vs. 63%; P less than .001). In children who continued medication, African American and Hispanic children were more likely than were white children to receive any psychotherapy (42% and 49% vs. 35%; P less than .001).
“The adjusted rate of discontinuing medication was 22.4% points higher (P less than .001) among African American versus white youth and 16.7% points higher (P less than .001) among Hispanic versus white youth,” Dr. Cummings and her associates said. “These findings are in line with research indicating that racial/ethnic minority parents may prefer psychosocial treatments over medication for ADHD.”
In terms of stopping treatment, the percentages were significantly higher among African American (51%) and Hispanic (45%) children than among white children at 36% (P less than .001).
“Higher rates of medication discontinuation among minority youth could be due to differences in cultural health beliefs and/or concerns about ADHD medication treatment. African American parents are less likely than white parents to conceptualize ADHD as a medical condition requiring treatment and may be less willing to administer psychotropic medication to a child due to beliefs about medication efficacy and side effects. ADHD medication is associated with an increased risk of adverse effects ... and a substantial proportion of treatment discontinuation is due to these adverse effects,” the researchers said.
Read more in Pediatrics (2017 May 16. doi: 10.1542/ peds. 2016-2444).
, reported Janet R. Cummings, PhD, and her associates at the Rollins School of Public Health at Emory University, Atlanta.
Overall, quality of care for Medicaid-enrolled children receiving ADHD treatment is poor. Of those who initiated medications, 59% visited a provider within 30 days, 64% received at least two other doctor visits, and 38% received combined treatment with any psychotherapy visit. Sixty percent did not fill the ADHD prescription for enough days, 70% had no psychotherapy visit, and 42% stopped treatment.
The percentage that had any follow-up visit in the initiation phase was lower among African American children than among white children (56% vs. 61%, P less than .001), while Hispanic children were more likely than were white children to receive adequate follow-up in the initiation phase (63% vs. 61%; P less than .001) as well as in the C&M phase (71% vs. 63%; P less than .001). In children who continued medication, African American and Hispanic children were more likely than were white children to receive any psychotherapy (42% and 49% vs. 35%; P less than .001).
“The adjusted rate of discontinuing medication was 22.4% points higher (P less than .001) among African American versus white youth and 16.7% points higher (P less than .001) among Hispanic versus white youth,” Dr. Cummings and her associates said. “These findings are in line with research indicating that racial/ethnic minority parents may prefer psychosocial treatments over medication for ADHD.”
In terms of stopping treatment, the percentages were significantly higher among African American (51%) and Hispanic (45%) children than among white children at 36% (P less than .001).
“Higher rates of medication discontinuation among minority youth could be due to differences in cultural health beliefs and/or concerns about ADHD medication treatment. African American parents are less likely than white parents to conceptualize ADHD as a medical condition requiring treatment and may be less willing to administer psychotropic medication to a child due to beliefs about medication efficacy and side effects. ADHD medication is associated with an increased risk of adverse effects ... and a substantial proportion of treatment discontinuation is due to these adverse effects,” the researchers said.
Read more in Pediatrics (2017 May 16. doi: 10.1542/ peds. 2016-2444).
, reported Janet R. Cummings, PhD, and her associates at the Rollins School of Public Health at Emory University, Atlanta.
Overall, quality of care for Medicaid-enrolled children receiving ADHD treatment is poor. Of those who initiated medications, 59% visited a provider within 30 days, 64% received at least two other doctor visits, and 38% received combined treatment with any psychotherapy visit. Sixty percent did not fill the ADHD prescription for enough days, 70% had no psychotherapy visit, and 42% stopped treatment.
The percentage that had any follow-up visit in the initiation phase was lower among African American children than among white children (56% vs. 61%, P less than .001), while Hispanic children were more likely than were white children to receive adequate follow-up in the initiation phase (63% vs. 61%; P less than .001) as well as in the C&M phase (71% vs. 63%; P less than .001). In children who continued medication, African American and Hispanic children were more likely than were white children to receive any psychotherapy (42% and 49% vs. 35%; P less than .001).
“The adjusted rate of discontinuing medication was 22.4% points higher (P less than .001) among African American versus white youth and 16.7% points higher (P less than .001) among Hispanic versus white youth,” Dr. Cummings and her associates said. “These findings are in line with research indicating that racial/ethnic minority parents may prefer psychosocial treatments over medication for ADHD.”
In terms of stopping treatment, the percentages were significantly higher among African American (51%) and Hispanic (45%) children than among white children at 36% (P less than .001).
“Higher rates of medication discontinuation among minority youth could be due to differences in cultural health beliefs and/or concerns about ADHD medication treatment. African American parents are less likely than white parents to conceptualize ADHD as a medical condition requiring treatment and may be less willing to administer psychotropic medication to a child due to beliefs about medication efficacy and side effects. ADHD medication is associated with an increased risk of adverse effects ... and a substantial proportion of treatment discontinuation is due to these adverse effects,” the researchers said.
Read more in Pediatrics (2017 May 16. doi: 10.1542/ peds. 2016-2444).
FROM PEDIATRICS
Aerosolized MMR vaccine showed good seropositivity
Aerosolized MMR vaccine can be used in booster campaigns in school-age children for measles and rubella, said José Luis Díaz Ortega, MD, of the Instituto Nacional de Salud Pública, Mexico, and his associates.
However, more studies of school-age children with longer follow-up of mumps antibody persistence are needed, they said.
Aerosolized vaccines are not available in the United States.
Read more in the journal Vaccine (2017 Apr 28. doi: 10.1016/j.vaccine.2017.04.027).
Aerosolized MMR vaccine can be used in booster campaigns in school-age children for measles and rubella, said José Luis Díaz Ortega, MD, of the Instituto Nacional de Salud Pública, Mexico, and his associates.
However, more studies of school-age children with longer follow-up of mumps antibody persistence are needed, they said.
Aerosolized vaccines are not available in the United States.
Read more in the journal Vaccine (2017 Apr 28. doi: 10.1016/j.vaccine.2017.04.027).
Aerosolized MMR vaccine can be used in booster campaigns in school-age children for measles and rubella, said José Luis Díaz Ortega, MD, of the Instituto Nacional de Salud Pública, Mexico, and his associates.
However, more studies of school-age children with longer follow-up of mumps antibody persistence are needed, they said.
Aerosolized vaccines are not available in the United States.
Read more in the journal Vaccine (2017 Apr 28. doi: 10.1016/j.vaccine.2017.04.027).
FROM VACCINE
Two-dose HPV vaccine trials in teens show effective immunological responses
, said Maddalena D’Addario of the University of Bern, Switzerland, and her associates.
In seven controlled trials in 11 countries that directly compared two-dose and three-dose HPV vaccine schedules, teen girls receiving two doses of HPV vaccine with a 6-month interval between them had noninferior antibody responses to HPV16 and HPV18 for at least 2 years, compared with girls receiving three doses.
Read more in the journal Vaccine (2017 May 19;35[22]:2892-901).
, said Maddalena D’Addario of the University of Bern, Switzerland, and her associates.
In seven controlled trials in 11 countries that directly compared two-dose and three-dose HPV vaccine schedules, teen girls receiving two doses of HPV vaccine with a 6-month interval between them had noninferior antibody responses to HPV16 and HPV18 for at least 2 years, compared with girls receiving three doses.
Read more in the journal Vaccine (2017 May 19;35[22]:2892-901).
, said Maddalena D’Addario of the University of Bern, Switzerland, and her associates.
In seven controlled trials in 11 countries that directly compared two-dose and three-dose HPV vaccine schedules, teen girls receiving two doses of HPV vaccine with a 6-month interval between them had noninferior antibody responses to HPV16 and HPV18 for at least 2 years, compared with girls receiving three doses.
Read more in the journal Vaccine (2017 May 19;35[22]:2892-901).
FROM VACCINE
Meningococcal B, C vaccines together lead to adequate immune response
and it demonstrated adequate immune response to MenB, according to Marco Aurelio P. Safadi, MD, of Santa Casa de São Paulo (Brazil) School of Medical Sciences, and his associates.
Of 117 healthy infants who received 4CMenB concomitantly with MenC CRM and 111 who received MenC CRM alone, 99%-100% of infants had serum bactericidal antibody assay using human complement (hSBA) titres 1:8 or greater against MenC after the second vaccination of the primary series (3 months, 5 months, and 12 months of age), and 100% of infants reached these titres after the booster vaccination.
There were more local reactions, such as tenderness, with the combination MenB and MenC vaccinations than with the MenC vaccine alone, as well as systemic adverse reactions such as unusual crying and fever.
Read more in the journal Vaccine (2017 Apr 11;35[16]:2052-9).
and it demonstrated adequate immune response to MenB, according to Marco Aurelio P. Safadi, MD, of Santa Casa de São Paulo (Brazil) School of Medical Sciences, and his associates.
Of 117 healthy infants who received 4CMenB concomitantly with MenC CRM and 111 who received MenC CRM alone, 99%-100% of infants had serum bactericidal antibody assay using human complement (hSBA) titres 1:8 or greater against MenC after the second vaccination of the primary series (3 months, 5 months, and 12 months of age), and 100% of infants reached these titres after the booster vaccination.
There were more local reactions, such as tenderness, with the combination MenB and MenC vaccinations than with the MenC vaccine alone, as well as systemic adverse reactions such as unusual crying and fever.
Read more in the journal Vaccine (2017 Apr 11;35[16]:2052-9).
and it demonstrated adequate immune response to MenB, according to Marco Aurelio P. Safadi, MD, of Santa Casa de São Paulo (Brazil) School of Medical Sciences, and his associates.
Of 117 healthy infants who received 4CMenB concomitantly with MenC CRM and 111 who received MenC CRM alone, 99%-100% of infants had serum bactericidal antibody assay using human complement (hSBA) titres 1:8 or greater against MenC after the second vaccination of the primary series (3 months, 5 months, and 12 months of age), and 100% of infants reached these titres after the booster vaccination.
There were more local reactions, such as tenderness, with the combination MenB and MenC vaccinations than with the MenC vaccine alone, as well as systemic adverse reactions such as unusual crying and fever.
Read more in the journal Vaccine (2017 Apr 11;35[16]:2052-9).
FROM VACCINE
High allele level linked to lamotrigine-induced SCAR
, reported Byung-Keun Kim, MD, of Seoul National University and associates.
In a study of 18 Korean patients with lamotrigine-induced SCAR, a control group of Korean lamotrigine-tolerant patients, and a control group of the general Korean population, the frequency of the HLA-A*31:01 allele was significantly higher in the lamotrigine-induced SCAR patients than in the lamotrigine-tolerant patients (odds ratio, 11.43; P = .0037) or the other control group (OR, 7.27; P = .00034).
High levels of the HLA-A*31:01 allele also have been reported in Korean patients with carbamazepine-induced SCAR, suggesting an association with the HLA allele and drug-induced SCAR that is specific to ethnicity.
That idea is supported by reports that the HLA-B*15:02 allele is a well-known risk allele of carbamazepine-induced SCAR in Han Chinese and Southeast Asians and that other HLA alleles have been significantly associated with SCAR only with patients of European ancestry or only with patients of Mestizo Mexican ancestry, Dr. Kim and associates said.
The SCAR in this study were Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug rash with eosinophilia and systemic syndrome, also known as DRESS.
Read more in the Annals of Allergy, Asthma & Immunology (2017 May;118[5]:629-30).
, reported Byung-Keun Kim, MD, of Seoul National University and associates.
In a study of 18 Korean patients with lamotrigine-induced SCAR, a control group of Korean lamotrigine-tolerant patients, and a control group of the general Korean population, the frequency of the HLA-A*31:01 allele was significantly higher in the lamotrigine-induced SCAR patients than in the lamotrigine-tolerant patients (odds ratio, 11.43; P = .0037) or the other control group (OR, 7.27; P = .00034).
High levels of the HLA-A*31:01 allele also have been reported in Korean patients with carbamazepine-induced SCAR, suggesting an association with the HLA allele and drug-induced SCAR that is specific to ethnicity.
That idea is supported by reports that the HLA-B*15:02 allele is a well-known risk allele of carbamazepine-induced SCAR in Han Chinese and Southeast Asians and that other HLA alleles have been significantly associated with SCAR only with patients of European ancestry or only with patients of Mestizo Mexican ancestry, Dr. Kim and associates said.
The SCAR in this study were Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug rash with eosinophilia and systemic syndrome, also known as DRESS.
Read more in the Annals of Allergy, Asthma & Immunology (2017 May;118[5]:629-30).
, reported Byung-Keun Kim, MD, of Seoul National University and associates.
In a study of 18 Korean patients with lamotrigine-induced SCAR, a control group of Korean lamotrigine-tolerant patients, and a control group of the general Korean population, the frequency of the HLA-A*31:01 allele was significantly higher in the lamotrigine-induced SCAR patients than in the lamotrigine-tolerant patients (odds ratio, 11.43; P = .0037) or the other control group (OR, 7.27; P = .00034).
High levels of the HLA-A*31:01 allele also have been reported in Korean patients with carbamazepine-induced SCAR, suggesting an association with the HLA allele and drug-induced SCAR that is specific to ethnicity.
That idea is supported by reports that the HLA-B*15:02 allele is a well-known risk allele of carbamazepine-induced SCAR in Han Chinese and Southeast Asians and that other HLA alleles have been significantly associated with SCAR only with patients of European ancestry or only with patients of Mestizo Mexican ancestry, Dr. Kim and associates said.
The SCAR in this study were Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug rash with eosinophilia and systemic syndrome, also known as DRESS.
Read more in the Annals of Allergy, Asthma & Immunology (2017 May;118[5]:629-30).
FROM THE ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
Safe, effective backup for U.S. MMR vaccine exists
Antibodies against measles, mumps, and rubella persisted for up to 2 years after vaccination of children 12-15 months with the MMR vaccine approved in the United States and a similar one approved in Europe, both of which are manufactured without human serum albumin, Andrea A. Berry, MD, at the University of Maryland, Baltimore, and her associates said.
In 752 children aged a mean 12 months who received either the United States MMR vaccine and the European one, seropositivity for measles, mumps, and rubella persisted for up to 2 years with both vaccines. Both vaccines also were well tolerated.
Because there is only one MMR vaccine licensed in the United States, interruption to this single supply line could present a public health risk, Dr. Berry and her associates said. As both the vaccines used in this study do not contain human serum albumin, the theoretical risk of microbial contamination is reduced, compared with previous formulations of MMR.
Read more at Human Vaccines Immunotherapeutics. (2017. doi: 10.1080/21645515.2017.1309486).
Antibodies against measles, mumps, and rubella persisted for up to 2 years after vaccination of children 12-15 months with the MMR vaccine approved in the United States and a similar one approved in Europe, both of which are manufactured without human serum albumin, Andrea A. Berry, MD, at the University of Maryland, Baltimore, and her associates said.
In 752 children aged a mean 12 months who received either the United States MMR vaccine and the European one, seropositivity for measles, mumps, and rubella persisted for up to 2 years with both vaccines. Both vaccines also were well tolerated.
Because there is only one MMR vaccine licensed in the United States, interruption to this single supply line could present a public health risk, Dr. Berry and her associates said. As both the vaccines used in this study do not contain human serum albumin, the theoretical risk of microbial contamination is reduced, compared with previous formulations of MMR.
Read more at Human Vaccines Immunotherapeutics. (2017. doi: 10.1080/21645515.2017.1309486).
Antibodies against measles, mumps, and rubella persisted for up to 2 years after vaccination of children 12-15 months with the MMR vaccine approved in the United States and a similar one approved in Europe, both of which are manufactured without human serum albumin, Andrea A. Berry, MD, at the University of Maryland, Baltimore, and her associates said.
In 752 children aged a mean 12 months who received either the United States MMR vaccine and the European one, seropositivity for measles, mumps, and rubella persisted for up to 2 years with both vaccines. Both vaccines also were well tolerated.
Because there is only one MMR vaccine licensed in the United States, interruption to this single supply line could present a public health risk, Dr. Berry and her associates said. As both the vaccines used in this study do not contain human serum albumin, the theoretical risk of microbial contamination is reduced, compared with previous formulations of MMR.
Read more at Human Vaccines Immunotherapeutics. (2017. doi: 10.1080/21645515.2017.1309486).
FROM HUMAN VACCINES & IMMUNOTHERAPEUTICS
Lower-dose aluminum hydroxide–adjuvanted polio vaccine noninferior to standard IPV
Aluminum hydroxide–adjuvanted vaccines with reduced doses of inactivated polio vaccine (IPV-Al) were noninferior to standard inactivated poliovirus vaccine (IPV) in a large trial in the Dominican Republic, said Luis Rivera, MD, of the Hospital Maternidad Nuestra Señora de la Altagracia, Santo Domingo, Dominican Republic, and his associates.
If these findings are replicated in phase III trials and regulatory approval follows, such vaccines could be low-cost replacements for standard IPV and oral poliovirus vaccines in low-resource countries, the study authors suggested.
In this phase II, blinded, randomized trial with three investigational IPV-Al groups and one IPV group, the vaccines were given at 6 weeks, 10 weeks, 14 weeks, and 18 weeks to 823 infants who had not previously received any polio vaccination. The three new IPV-Al vaccines all proved to be noninferior to IPV for poliovirus types 1, 2, and 3.
For 1/10 IPV-Al, the seroconversion rates for the different poliovirus types were 98.5% (type 1), 94.6% (type 2), and 99.5% (type 3), compared with 100% (type 1), 98.5% (type 2), and 100% (type 3) for IPV.
This and the results from other studies “have paved the way for further clinical investigations of IPV-Al in phase III trials,” Dr. Rivera and his associates wrote.
“The low frequency of adverse events in this phase II trial suggests that a safety evaluation is not necessarily justified,” they concluded.
The Bill & Melinda Gates Foundation funded the study. The authors had no disclosures.
Read more in the Lancet Infectious Diseases (2017 Apr 25. doi: 10.1016/S1473-3099(17)30177-9).
Aluminum hydroxide–adjuvanted vaccines with reduced doses of inactivated polio vaccine (IPV-Al) were noninferior to standard inactivated poliovirus vaccine (IPV) in a large trial in the Dominican Republic, said Luis Rivera, MD, of the Hospital Maternidad Nuestra Señora de la Altagracia, Santo Domingo, Dominican Republic, and his associates.
If these findings are replicated in phase III trials and regulatory approval follows, such vaccines could be low-cost replacements for standard IPV and oral poliovirus vaccines in low-resource countries, the study authors suggested.
In this phase II, blinded, randomized trial with three investigational IPV-Al groups and one IPV group, the vaccines were given at 6 weeks, 10 weeks, 14 weeks, and 18 weeks to 823 infants who had not previously received any polio vaccination. The three new IPV-Al vaccines all proved to be noninferior to IPV for poliovirus types 1, 2, and 3.
For 1/10 IPV-Al, the seroconversion rates for the different poliovirus types were 98.5% (type 1), 94.6% (type 2), and 99.5% (type 3), compared with 100% (type 1), 98.5% (type 2), and 100% (type 3) for IPV.
This and the results from other studies “have paved the way for further clinical investigations of IPV-Al in phase III trials,” Dr. Rivera and his associates wrote.
“The low frequency of adverse events in this phase II trial suggests that a safety evaluation is not necessarily justified,” they concluded.
The Bill & Melinda Gates Foundation funded the study. The authors had no disclosures.
Read more in the Lancet Infectious Diseases (2017 Apr 25. doi: 10.1016/S1473-3099(17)30177-9).
Aluminum hydroxide–adjuvanted vaccines with reduced doses of inactivated polio vaccine (IPV-Al) were noninferior to standard inactivated poliovirus vaccine (IPV) in a large trial in the Dominican Republic, said Luis Rivera, MD, of the Hospital Maternidad Nuestra Señora de la Altagracia, Santo Domingo, Dominican Republic, and his associates.
If these findings are replicated in phase III trials and regulatory approval follows, such vaccines could be low-cost replacements for standard IPV and oral poliovirus vaccines in low-resource countries, the study authors suggested.
In this phase II, blinded, randomized trial with three investigational IPV-Al groups and one IPV group, the vaccines were given at 6 weeks, 10 weeks, 14 weeks, and 18 weeks to 823 infants who had not previously received any polio vaccination. The three new IPV-Al vaccines all proved to be noninferior to IPV for poliovirus types 1, 2, and 3.
For 1/10 IPV-Al, the seroconversion rates for the different poliovirus types were 98.5% (type 1), 94.6% (type 2), and 99.5% (type 3), compared with 100% (type 1), 98.5% (type 2), and 100% (type 3) for IPV.
This and the results from other studies “have paved the way for further clinical investigations of IPV-Al in phase III trials,” Dr. Rivera and his associates wrote.
“The low frequency of adverse events in this phase II trial suggests that a safety evaluation is not necessarily justified,” they concluded.
The Bill & Melinda Gates Foundation funded the study. The authors had no disclosures.
Read more in the Lancet Infectious Diseases (2017 Apr 25. doi: 10.1016/S1473-3099(17)30177-9).
FROM THE LANCET INFECTIOUS DISEASES
Adalimumab is good first-line anti-TNF therapy for pediatric Crohn’s disease
Adalimumab (ADA) as a first-line anti–tumor necrosis factor therapy induced and maintained clinical remission in children with Crohn’s disease, said Víctor Manuel Navas-López, MD, PhD, of the Hospital Materno Infantil, Málaga, Spain, and his associates.
Infliximab is the usual first-line anti–tumor necrosis factor treatment given to children with Crohn’s disease, with ADA used in patients who don’t respond or who develop tolerance to infliximab.
Dose escalation was necessary for 26% of the 62 patients. Thirty-nine percent of patients had growth retardation.
“ADA treatment significantly improved z-score growth rate in children with Crohn’s disease, especially in those with severe growth failure at baseline,” the researchers said. Only 13% of patients reported adverse events, none of them severe.
Read more in the Anales de Pediatría (2017 Apr 14. doi: 10.1016/j.anpedi.2017.01.013).
Adalimumab (ADA) as a first-line anti–tumor necrosis factor therapy induced and maintained clinical remission in children with Crohn’s disease, said Víctor Manuel Navas-López, MD, PhD, of the Hospital Materno Infantil, Málaga, Spain, and his associates.
Infliximab is the usual first-line anti–tumor necrosis factor treatment given to children with Crohn’s disease, with ADA used in patients who don’t respond or who develop tolerance to infliximab.
Dose escalation was necessary for 26% of the 62 patients. Thirty-nine percent of patients had growth retardation.
“ADA treatment significantly improved z-score growth rate in children with Crohn’s disease, especially in those with severe growth failure at baseline,” the researchers said. Only 13% of patients reported adverse events, none of them severe.
Read more in the Anales de Pediatría (2017 Apr 14. doi: 10.1016/j.anpedi.2017.01.013).
Adalimumab (ADA) as a first-line anti–tumor necrosis factor therapy induced and maintained clinical remission in children with Crohn’s disease, said Víctor Manuel Navas-López, MD, PhD, of the Hospital Materno Infantil, Málaga, Spain, and his associates.
Infliximab is the usual first-line anti–tumor necrosis factor treatment given to children with Crohn’s disease, with ADA used in patients who don’t respond or who develop tolerance to infliximab.
Dose escalation was necessary for 26% of the 62 patients. Thirty-nine percent of patients had growth retardation.
“ADA treatment significantly improved z-score growth rate in children with Crohn’s disease, especially in those with severe growth failure at baseline,” the researchers said. Only 13% of patients reported adverse events, none of them severe.
Read more in the Anales de Pediatría (2017 Apr 14. doi: 10.1016/j.anpedi.2017.01.013).
FROM ANALES DE PEDIATRIA