User login
Preoperative Diabetes Management for Patients Undergoing Elective Surgeries at a Veterans Affairs Medical Center
Preoperative Diabetes Management for Patients Undergoing Elective Surgeries at a Veterans Affairs Medical Center
More than 38 million people in the United States (12%) have diabetes mellitus (DM), though 1 in 5 are unaware they have DM.1 The prevalence among veterans is even more substantial, impacting nearly 25% of those who received care from the US Department of Veterans Affairs (VA).2 DM can lead to increased health care costs in addition to various complications (eg, cardiovascular, renal), especially if left uncontrolled.1,3 similar impact is found in the perioperative period (defined as at or around the time of an operation), as multiple studies have found that uncontrolled preoperative DM can result in worsened surgical outcomes, including longer hospital stays, more infectious complications, and higher perioperative mortality.4-6
In contrast, adequate glycemic control assessed with blood glucose levels has been shown to decrease the incidence of postoperative infections.7 Optimizing glycemic control during hospital stays, especially postsurgery, has become the standard of care, with most health systems establishing specific protocols. In current literature, most studies examining DM management in the perioperative period are focused on postoperative care, with little attention to the preoperative period.4,6,7
One study found that patients with poor presurgery glycemic control assessed by hemoglobin A1c (HbA1c) levels were more likely to remain hyperglycemic during and after surgery. 8 Blood glucose levels < 200 mg/dL can lead to an increased risk of infection and impaired wound healing, meaning a well-controlled HbA1c before a procedure serves as a potential factor for success.9 The 2025 American Diabetes Association (ADA) Standards of Care (SOC) recommendation is to target HbA1c < 8% whenever possible, and some health systems require lower levels (eg, < 7% or 7.5%).10 With that goal in mind and knowing that preoperative hyperglycemia has been shown to be a contributing factor in the delay or cancellation of surgical cases, an argument can be made that attention to preoperative DM management also should be a focus for health care systems performing surgeries.8,9,11
Attention to glucose control during preoperative care offers an opportunity to screen for DM in patients who may not have been screened otherwise and to standardize perioperative DM management. Since DM disproportionately impacts veterans, this is a pertinent issue to the VA. Veterans can be more susceptible to complications if DM is left uncontrolled prior to surgery. To determine readiness for surgery and control of comorbid conditions such as DM before a planned surgery, facilities often perform a preoperative clinic assessment, often in a multidisciplinary clinic.
At Veteran Health Indiana (VHI), a presurgery clinic visit involving the primary surgery service (physician, nurse practitioner, and/or a physician assistant) is conducted 1 to 2 months prior to the planned procedure to determine whether a patient is ready for surgery. During this visit, patients receive a packet with instructions for various tasks and medications, such as applying topical antibiotic prophylaxis on the anticipated surgical site. This is documented in the form of a note in the VHI Computerized Patient Record System (CPRS). The medication instructions are provided according to the preferences of the surgical team. These may be templated notes that contain general directions on the timing and dosing of specific medications, in addition to instructions for holding or reducing doses when appropriate. The instructions can be tailored by the team conducting the preoperative visit (eg, “Take 20 units of insulin glargine the day before surgery” vs “Take half of your long-acting insulin the night before surgery”). Specific to DM, VHI has a nurse-driven day of surgery glucose assessment where point-of-care blood glucose is collected during preoperative holding for most patients.
There is limited research assessing the level of preoperative glycemic control and the incidence of complications in a veteran population. The objective of this study was to gain a baseline understanding of what, if any, standardization exists for preoperative instructions for DM medications and to assess the level of preoperative glycemic control and postoperative complications in patients with DM undergoing major elective surgical procedures.
Methods
This retrospective, single-center chart review was conducted at VHI. The Indiana University and VHI institutional review boards determined that this quality improvement project was exempt from review.
The primary outcome was the number of patients with surgical procedures delayed or canceled due to hyperglycemia or hypoglycemia. Hyperglycemia was defined as blood glucose > 180 mg/dL and hypoglycemia was defined as < 70 mg/dL, slight variations from the current ADA SOC preoperative specific recommendation of a blood glucose reading of 100 to 180 mg/dL within 4 hours of surgery.10 The standard outpatient hypoglycemia definition of blood glucose < 70 mg/dL was chosen because the current goal (< 100 mg/dL) was not the standard in previous ADA SOCs that were in place during the study period. Specifically, the 2018 ADA SOC did not provide preoperative recommendations and the 2019-2021 ADA SOC recommended 80 to 180 mg/dL.10,12-18 For patients who had multiple preoperative blood glucose measurements, the first recorded glucose on the day of the procedure was used.
The secondary outcomes of this study were focused on the preoperative process/care at VHI and postoperative glycemic control. The preoperative process included examining whether medication instructions were given and their quality. Additionally, the number of interventions for hyperglycemia and hypoglycemia were required immediately prior to surgery and the average preoperative HbA1c (measured within 3 months prior to surgery) were collected and analyzed. For postoperative glycemic control, average blood glucose measurements and number of hypoglycemic (< 70 mg/dL) and hyperglycemic (> 180 mg/dL) events were measured in addition to the frequency of changes made at discharge to patients’ DM medication regimens.
The safety outcome of this study assessed commonly observed postoperative complications and was examined up to 30 days postsurgery. These included acute kidney injury (defined using Kidney Disease: Improving Global Outcomes 2012, the standard during the study period), nonfatal myocardial infarction, nonfatal stroke, and surgical site infections, which were identified from the discharge summary written by the primary surgery service.19 All-cause mortality also was collected.
Patients were included if they were admitted for major elective surgeries and had a diagnosis of either type 1 or type 2 DM on their problem list, determined by International Classification of Diseases, Tenth Revision codes. Major elective surgery was defined as a procedure that would likely result in a hospital admission of > 24 hours. Of note, patients may have been included in this study more than once if they had > 1 procedure at least 30 days apart and met inclusion criteria within the time frame. Patients were excluded if they were taking no DM medications or chronic steroids (at any dose), residing in a long-term care facility, being managed by a non-VA clinician prior to surgery, or missing a preoperative blood glucose measurement.
All data were collected from the CPRS. A list of surgical cases involving patients with DM who were scheduled to undergo major elective surgeries from January 1, 2018, to December 31, 2021, at VHI was generated. The list was randomized to a smaller number (N = 394) for data collection due to the time and resource constraints for a pharmacy residency project. All data were deidentified and stored in a secured VA server to protect patient confidentiality. Descriptive statistics were used for all results.
Results
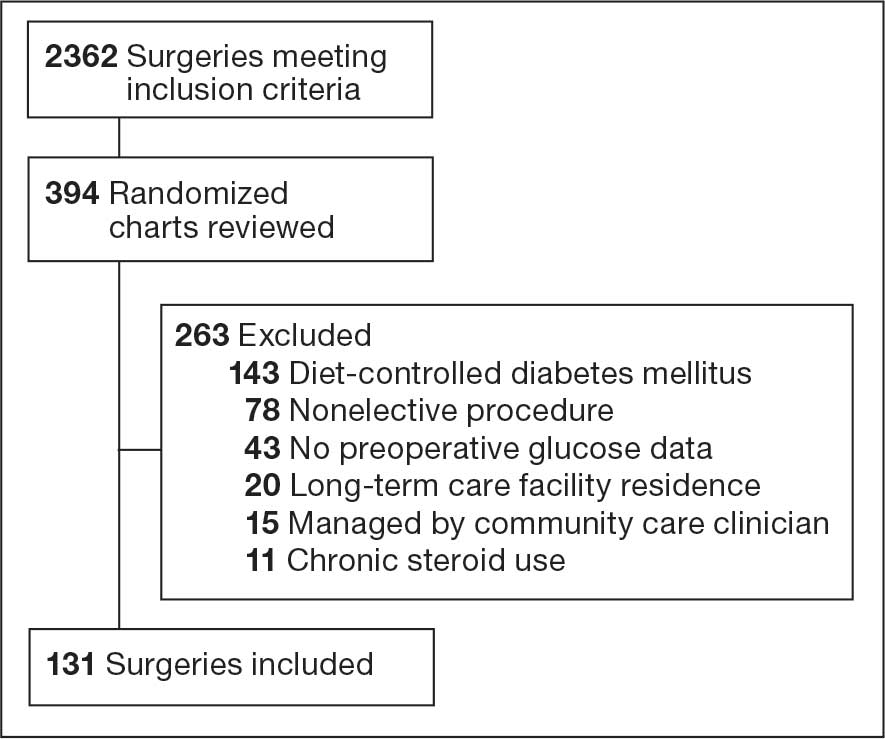
Initially, 2362 surgeries were identified. A randomized sample of 394 charts were reviewed and 131 cases met inclusion criteria. Each case involved a unique patient (Figure). The most common reasons for exclusion were 143 patients with diet-controlled DM and 78 nonelective surgeries. The mean (SD) age of patients was 68 (8) years, and the most were male (98.5%) and White (76.3%) (Table 1).
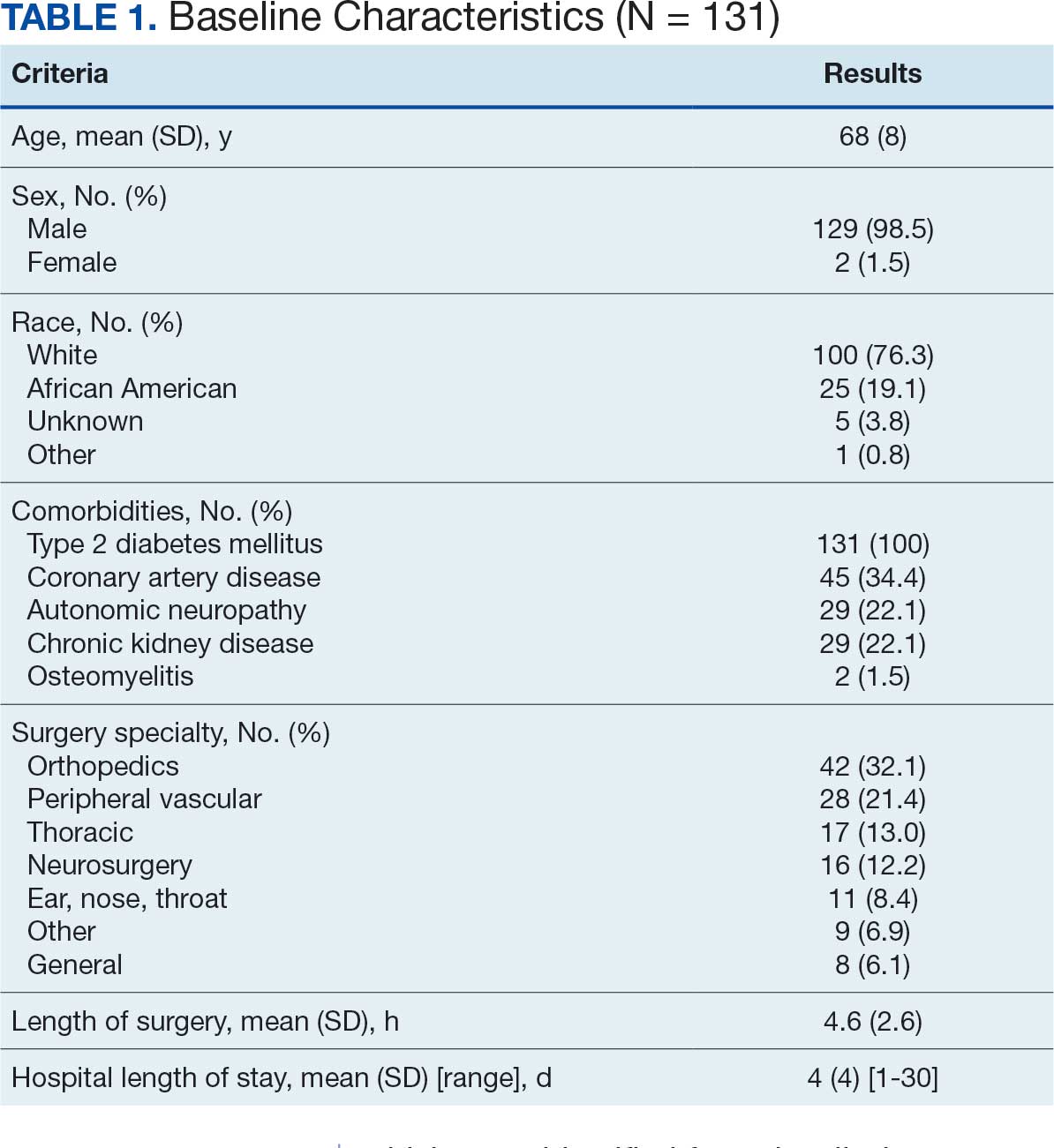
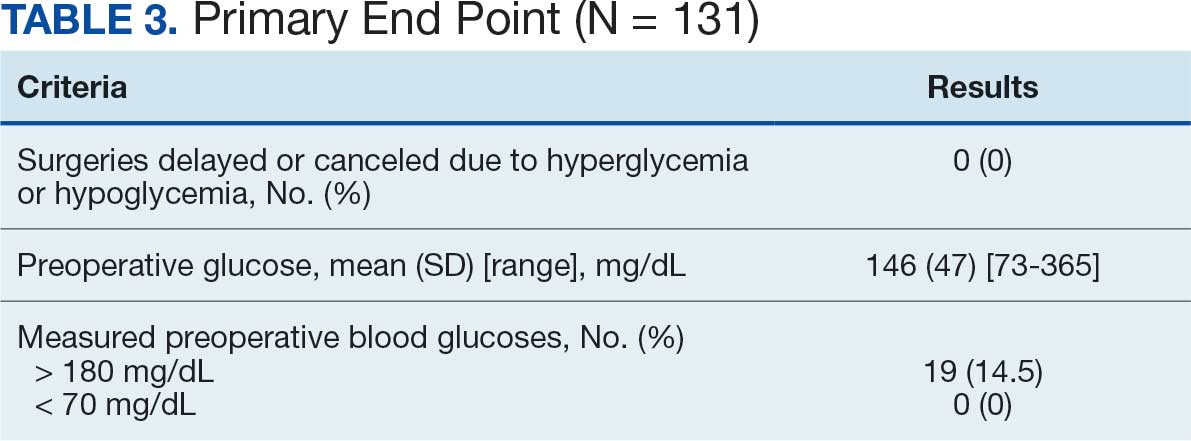
At baseline, 45 of 131 patients (34.4%) had coronary artery disease and 29 (22.1%) each had autonomic neuropathy and chronic kidney disease. Most surgeries were conducted by orthopedic (32.1%) and peripheral vascular (21.4%) specialties. The mean (SD) length of surgery was 4.6 (2.6) hours and of hospital length of stay was 4 (4) days. No patients stayed longer than the 30-day safety outcome follow-up period. All patients had type 2 DM and took a mean 2 DM medications. The 63 patients taking insulin had a mean (SD) total daily dose of 99 (77) U (Table 2). A preoperative HbA1c was collected in 116 patients within 3 months of surgery, with a mean HbA1c of 7.0% (range, 5.3-10.7).
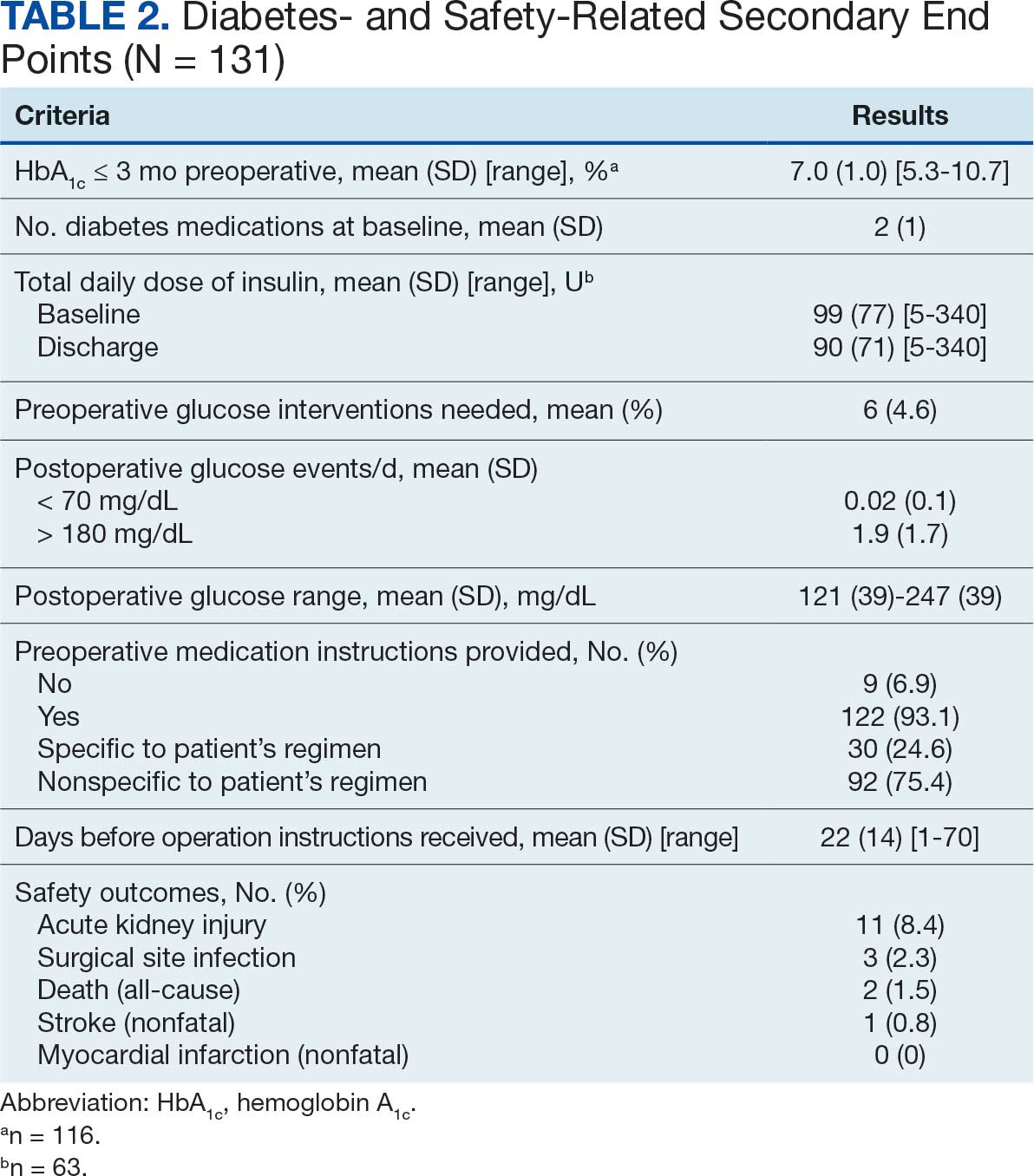
No patients had surgeries delayed or canceled because of uncontrolled DM on the day of surgery. The mean preoperative blood glucose level was 146 mg/dL (range, 73-365) (Table 3). No patients had a preoperative blood glucose level of < 70 mg/dL and 19 (14.5%) had a blood glucose level > 180 mg/dL. Among patients with hyperglycemia immediately prior to surgery, 6 (31.6%) had documentation of insulin being provided.
For this sample of patients, the preoperative clinic visit was conducted a mean 22 days prior to the planned surgery date. Among the 131 included patients, 122 (93.1%) had documentation of receiving instructions for DM medications. Among patients who had documented receipt of instructions, only 30 (24.6%) had instructions specifically tailored to their regimen rather than a generic templated form. The mean (SD) preoperative blood glucose was similar for those who received specific perioperative DM instructions at 146 (50) mg/dL when compared with those who did not at 147 (45) mg/dL. The mean (SD) preoperative blood glucose reading for those who had no documentation of receipt of perioperative instructions was 126 (54) mg/dL compared with 147 (46) mg/dL for those who did.
The mean number of postoperative blood glucose events per day was negligible for hypoglycemia and more frequent for hyperglycemia with a mean of 2 events per day. The mean postoperative blood glucose range was 121 to 247 mg/dL with most readings < 180 mg/dL. Upon discharge, most patients continued their home DM regimen with 5 patients (3.8%) having changes made to their regimen upon discharge.
Very few postoperative complications were identified from chart review. The most frequently observed postoperative complications were acute kidney injury, surgical site infections, and nonfatal stroke. There were no documented nonfatal myocardial infarctions. Two patients (1.5%) died within 30 days of the surgery; neither death was deemed to have been related to poor perioperative glycemic control.
Discussion
To our knowledge, this retrospective chart review was the first study to assess preoperative DM management and postoperative complications in a veteran population. VHI is a large, tertiary, level 1a, academic medical center that serves approximately 62,000 veterans annually and performs about 5000 to 6000 surgeries annually, a total that is increasing following the COVID-19 pandemic.20 This study found that the current process of a presurgery clinic visit and day of surgery glucose assessment has prevented surgical delays or cancellations.
Most patients included in this study were well controlled at baseline in accordance with the 2025 ADA SOC HbA1c recommendation of a preoperative HbA1c of < 8%, which may have contributed to no surgical delays or cancellations.10 However, not all patients had HbA1c collected within 3 months of surgery or even had one collected at all. Despite the ADA SOC providing no explicit recommendation for universal HbA1c screening prior to elective procedures, its importance cannot be understated given the body of evidence demonstrating poor outcomes with uncontrolled preoperative DM.8,10 The glycemic control at baseline may have contributed to the very few postsurgical complications observed in this study.
Although the current process at VHI prevented surgical delays and cancellations in this sample, there are still identified areas for improvement. One area is the instructions the patients received. Patients with DM are often prescribed ≥ 1 medication or a combination of insulins, noninsulin injectables, and oral DM medications, and this study population was no different. Because these medications may influence the anesthesia and perioperative periods, the ADA has specific guidance for altering administration schedules in the days leading up to surgery.10
Inappropriate administration of DM medications could lead to perioperative hypoglycemia or hyperglycemia, possibly causing surgical delays, case cancellations, and/or postoperative complications.21 Although these data reveal the specificity and documented receipt that the preoperative DM instructions did not impact the first recorded preoperative blood glucose, future studies should examine patient confidence in how to properly administer their DM medications prior to surgery. It is vital that patients receive clear instructions in accordance with the ADA SOC on whether to continue, hold, or adjust the dose of their medications to prevent fluctuations in blood glucose levels in the perioperative period, ensure safety with anesthesia, and prevent postoperative complications such as acute kidney injury. Of note, compliance with guideline recommendations for medication instructions was not examined because the data collection time frame expanded over multiple years and the recommendations have evolved each year as new data emerge.
Preoperative DM Management
The first key takeaway from this study is to ensure patients are ready for surgery with a formal assessment (typically in the form of a clinic visit) prior to the surgery. One private sector health system published their approach to this by administering an automatic preoperative HbA1c screening for those with a DM diagnosis and all patients with a random plasma glucose ≥ 200 mg/dL.22 Additionally, if the patient's HbA1c level was not at goal prior to surgery (≥ 8% for those with known DM and ≥ 6.5% with no known DM), patients were referred to endocrinology for further management. Increasing attention to the preoperative visit and extending HbA1c testing to all patients regardless of DM status also provides an opportunity to identify individuals living with undiagnosed DM.1
Even though there was no difference in the mean preoperative blood glucose level based on receipt or specificity of preoperative DM instructions, a second takeaway from this study is the importance of ensuring patients receive clear instructions on their DM medication schedule in the perioperative period. A practical first step may be updating the templates used by the primary surgery teams and providing education to the clinicians in the clinic on how to personalize the visits. Because the current preoperative DM process at VHI is managed by the primary surgical team in a clinic visit, there is an opportunity to shift this responsibility to other health care professionals, such as pharmacists—a change shown to reduce unintended omission of home medications following surgery during hospitalization and reduce costs.23,24
Limitations
This study relied on data included in the patient chart. These data include medication interventions made immediately prior to surgery, which can sometimes be inaccurately charted or difficult to find as they are not documented in the typical medication administration record. Also, the safety outcomes were collected from a discharge summary written by different clinicians, which may lead to information bias. Special attention was taken to ensure these data points were collected as accurately as possible, but it is possible some data may be inaccurate from unintentional human error. Additionally, the safety outcome was limited to a 30-day follow-up, but encompassed the entire length of postoperative stay for all included patients. Finally, given this study was retrospective with no comparison group and the intent was to improve processes at VHI, only hypotheses and potential interventions can be generated from this study. Future prospective studies with larger sample sizes and comparator groups are needed to draw further conclusions.
Conclusions
This study found that the current presurgery process at VHI appears to be successful in preventing surgical delays or cancellations due to hyperglycemia or hypoglycemia. Optimizing DM management can improve surgical outcomes by decreasing rates of postoperative complications, and this study added additional evidence in support of that in a unique population: veterans. Insight on the awareness of preoperative blood glucose management should be gleaned from this study, and based on this sample and site, the preadmission screening process and instructions provided to patients can serve as 2 starting points for optimizing elective surgery.
- Centers for Disease Control and Prevention. Diabetes basics. May 15, 2024. Accessed September 24, 2025. https://www.cdc.gov/diabetes/about/index.html
- Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135. doi:10.5888/pcd14.170230
- Farmaki P, Damaskos C, Garmpis N, et al . Complications of the Type 2 Diabetes Mellitus. Curr Cardiol Rev. 2020;16(4):249-251. doi:10.2174/1573403X1604201229115531
- Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783-1788. doi:10.2337/dc10-0304
- Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156:137 -142. doi:10.1530/eje.1.02321
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77-81. doi:10.1177/01486071980220027
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34:256-261. doi:10.2337/dc10-1407
- Pasquel FJ, Gomez-Huelgas R, Anzola I, et al. Predictive value of admission hemoglobin A1c on inpatient glycemic control and response to insulin therapy in medicine and surgery patients with type 2 diabetes. Diabetes Care. 2015;38:e202-e203. doi:10.2337/dc15-1835
- Alexiewicz JM, Kumar D, Smogorzewski M, et al. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med. 1995;123:919-924. doi:10.7326/0003-4819-123-12-199512150-00004
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2025. Diabetes Care. 2025;48(1 suppl 1):S321-S334. doi:10.2337/dc25-S016
- Kumar R, Gandhi R. Reasons for cancellation of operation on the day of intended surgery in a multidisciplinary 500 bedded hospital. J Anaesthesiol Clin Pharmacol. 2012;28:66-69. doi:10.4103/0970-9185.92442
- American Diabetes Association. 14. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2018. Diabetes Care. 2018;41(1 suppl 1):S144- S151. doi:10.2337/dc18-S014
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2019. Diabetes Care. 2019;42(suppl 1):S173- S181. doi:10.2337/dc19-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2020. Diabetes Care. 2020;43(suppl 1):S193- S202. doi:10.2337/dc20-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2021. Diabetes Care. 2021;44(suppl 1):S211- S220. doi:10.2337/dc21-S015
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
- ElSayed NA, Aleppo G, Aroda VR, et al. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(suppl 1):S267-S278. doi:10.2337/dc23-S016
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(suppl 1):S295-S306. doi:10.2337/dc24-S016
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138. Accessed September 24, 2025. https:// www.kisupplements.org/issue/S2157-1716(12)X7200-9
- US Department of Veterans Affairs. VA Indiana Healthcare: about us. Accessed September 24, 2025. https:// www.va.gov/indiana-health-care/about-us/
- Koh WX, Phelan R, Hopman WM, et al. Cancellation of elective surgery: rates, reasons and effect on patient satisfaction. Can J Surg. 2021;64:E155-E161. doi:10.1503/cjs.008119
- Pai S-L, Haehn DA, Pitruzzello NE, et al. Reducing infection rates with enhanced preoperative diabetes mellitus diagnosis and optimization processes. South Med J. 2023;116:215-219. doi:10.14423/SMJ.0000000000001507
- Forrester TG, Sullivan S, Snoswell CL, et al. Integrating a pharmacist into the perioperative setting. Aust Health Rev. 2020;44:563-568. doi:10.1071/AH19126
- Hale AR, Coombes ID, Stokes J, et al. Perioperative medication management: expanding the role of the preadmission clinic pharmacist in a single centre, randomised controlled trial of collaborative prescribing. BMJ Open. 2013;3:e003027. doi:10.1136/bmjopen-2013-003027
More than 38 million people in the United States (12%) have diabetes mellitus (DM), though 1 in 5 are unaware they have DM.1 The prevalence among veterans is even more substantial, impacting nearly 25% of those who received care from the US Department of Veterans Affairs (VA).2 DM can lead to increased health care costs in addition to various complications (eg, cardiovascular, renal), especially if left uncontrolled.1,3 similar impact is found in the perioperative period (defined as at or around the time of an operation), as multiple studies have found that uncontrolled preoperative DM can result in worsened surgical outcomes, including longer hospital stays, more infectious complications, and higher perioperative mortality.4-6
In contrast, adequate glycemic control assessed with blood glucose levels has been shown to decrease the incidence of postoperative infections.7 Optimizing glycemic control during hospital stays, especially postsurgery, has become the standard of care, with most health systems establishing specific protocols. In current literature, most studies examining DM management in the perioperative period are focused on postoperative care, with little attention to the preoperative period.4,6,7
One study found that patients with poor presurgery glycemic control assessed by hemoglobin A1c (HbA1c) levels were more likely to remain hyperglycemic during and after surgery. 8 Blood glucose levels < 200 mg/dL can lead to an increased risk of infection and impaired wound healing, meaning a well-controlled HbA1c before a procedure serves as a potential factor for success.9 The 2025 American Diabetes Association (ADA) Standards of Care (SOC) recommendation is to target HbA1c < 8% whenever possible, and some health systems require lower levels (eg, < 7% or 7.5%).10 With that goal in mind and knowing that preoperative hyperglycemia has been shown to be a contributing factor in the delay or cancellation of surgical cases, an argument can be made that attention to preoperative DM management also should be a focus for health care systems performing surgeries.8,9,11
Attention to glucose control during preoperative care offers an opportunity to screen for DM in patients who may not have been screened otherwise and to standardize perioperative DM management. Since DM disproportionately impacts veterans, this is a pertinent issue to the VA. Veterans can be more susceptible to complications if DM is left uncontrolled prior to surgery. To determine readiness for surgery and control of comorbid conditions such as DM before a planned surgery, facilities often perform a preoperative clinic assessment, often in a multidisciplinary clinic.
At Veteran Health Indiana (VHI), a presurgery clinic visit involving the primary surgery service (physician, nurse practitioner, and/or a physician assistant) is conducted 1 to 2 months prior to the planned procedure to determine whether a patient is ready for surgery. During this visit, patients receive a packet with instructions for various tasks and medications, such as applying topical antibiotic prophylaxis on the anticipated surgical site. This is documented in the form of a note in the VHI Computerized Patient Record System (CPRS). The medication instructions are provided according to the preferences of the surgical team. These may be templated notes that contain general directions on the timing and dosing of specific medications, in addition to instructions for holding or reducing doses when appropriate. The instructions can be tailored by the team conducting the preoperative visit (eg, “Take 20 units of insulin glargine the day before surgery” vs “Take half of your long-acting insulin the night before surgery”). Specific to DM, VHI has a nurse-driven day of surgery glucose assessment where point-of-care blood glucose is collected during preoperative holding for most patients.
There is limited research assessing the level of preoperative glycemic control and the incidence of complications in a veteran population. The objective of this study was to gain a baseline understanding of what, if any, standardization exists for preoperative instructions for DM medications and to assess the level of preoperative glycemic control and postoperative complications in patients with DM undergoing major elective surgical procedures.
Methods
This retrospective, single-center chart review was conducted at VHI. The Indiana University and VHI institutional review boards determined that this quality improvement project was exempt from review.
The primary outcome was the number of patients with surgical procedures delayed or canceled due to hyperglycemia or hypoglycemia. Hyperglycemia was defined as blood glucose > 180 mg/dL and hypoglycemia was defined as < 70 mg/dL, slight variations from the current ADA SOC preoperative specific recommendation of a blood glucose reading of 100 to 180 mg/dL within 4 hours of surgery.10 The standard outpatient hypoglycemia definition of blood glucose < 70 mg/dL was chosen because the current goal (< 100 mg/dL) was not the standard in previous ADA SOCs that were in place during the study period. Specifically, the 2018 ADA SOC did not provide preoperative recommendations and the 2019-2021 ADA SOC recommended 80 to 180 mg/dL.10,12-18 For patients who had multiple preoperative blood glucose measurements, the first recorded glucose on the day of the procedure was used.
The secondary outcomes of this study were focused on the preoperative process/care at VHI and postoperative glycemic control. The preoperative process included examining whether medication instructions were given and their quality. Additionally, the number of interventions for hyperglycemia and hypoglycemia were required immediately prior to surgery and the average preoperative HbA1c (measured within 3 months prior to surgery) were collected and analyzed. For postoperative glycemic control, average blood glucose measurements and number of hypoglycemic (< 70 mg/dL) and hyperglycemic (> 180 mg/dL) events were measured in addition to the frequency of changes made at discharge to patients’ DM medication regimens.
The safety outcome of this study assessed commonly observed postoperative complications and was examined up to 30 days postsurgery. These included acute kidney injury (defined using Kidney Disease: Improving Global Outcomes 2012, the standard during the study period), nonfatal myocardial infarction, nonfatal stroke, and surgical site infections, which were identified from the discharge summary written by the primary surgery service.19 All-cause mortality also was collected.
Patients were included if they were admitted for major elective surgeries and had a diagnosis of either type 1 or type 2 DM on their problem list, determined by International Classification of Diseases, Tenth Revision codes. Major elective surgery was defined as a procedure that would likely result in a hospital admission of > 24 hours. Of note, patients may have been included in this study more than once if they had > 1 procedure at least 30 days apart and met inclusion criteria within the time frame. Patients were excluded if they were taking no DM medications or chronic steroids (at any dose), residing in a long-term care facility, being managed by a non-VA clinician prior to surgery, or missing a preoperative blood glucose measurement.
All data were collected from the CPRS. A list of surgical cases involving patients with DM who were scheduled to undergo major elective surgeries from January 1, 2018, to December 31, 2021, at VHI was generated. The list was randomized to a smaller number (N = 394) for data collection due to the time and resource constraints for a pharmacy residency project. All data were deidentified and stored in a secured VA server to protect patient confidentiality. Descriptive statistics were used for all results.
Results
Initially, 2362 surgeries were identified. A randomized sample of 394 charts were reviewed and 131 cases met inclusion criteria. Each case involved a unique patient (Figure). The most common reasons for exclusion were 143 patients with diet-controlled DM and 78 nonelective surgeries. The mean (SD) age of patients was 68 (8) years, and the most were male (98.5%) and White (76.3%) (Table 1).


At baseline, 45 of 131 patients (34.4%) had coronary artery disease and 29 (22.1%) each had autonomic neuropathy and chronic kidney disease. Most surgeries were conducted by orthopedic (32.1%) and peripheral vascular (21.4%) specialties. The mean (SD) length of surgery was 4.6 (2.6) hours and of hospital length of stay was 4 (4) days. No patients stayed longer than the 30-day safety outcome follow-up period. All patients had type 2 DM and took a mean 2 DM medications. The 63 patients taking insulin had a mean (SD) total daily dose of 99 (77) U (Table 2). A preoperative HbA1c was collected in 116 patients within 3 months of surgery, with a mean HbA1c of 7.0% (range, 5.3-10.7).

No patients had surgeries delayed or canceled because of uncontrolled DM on the day of surgery. The mean preoperative blood glucose level was 146 mg/dL (range, 73-365) (Table 3). No patients had a preoperative blood glucose level of < 70 mg/dL and 19 (14.5%) had a blood glucose level > 180 mg/dL. Among patients with hyperglycemia immediately prior to surgery, 6 (31.6%) had documentation of insulin being provided.

For this sample of patients, the preoperative clinic visit was conducted a mean 22 days prior to the planned surgery date. Among the 131 included patients, 122 (93.1%) had documentation of receiving instructions for DM medications. Among patients who had documented receipt of instructions, only 30 (24.6%) had instructions specifically tailored to their regimen rather than a generic templated form. The mean (SD) preoperative blood glucose was similar for those who received specific perioperative DM instructions at 146 (50) mg/dL when compared with those who did not at 147 (45) mg/dL. The mean (SD) preoperative blood glucose reading for those who had no documentation of receipt of perioperative instructions was 126 (54) mg/dL compared with 147 (46) mg/dL for those who did.
The mean number of postoperative blood glucose events per day was negligible for hypoglycemia and more frequent for hyperglycemia with a mean of 2 events per day. The mean postoperative blood glucose range was 121 to 247 mg/dL with most readings < 180 mg/dL. Upon discharge, most patients continued their home DM regimen with 5 patients (3.8%) having changes made to their regimen upon discharge.
Very few postoperative complications were identified from chart review. The most frequently observed postoperative complications were acute kidney injury, surgical site infections, and nonfatal stroke. There were no documented nonfatal myocardial infarctions. Two patients (1.5%) died within 30 days of the surgery; neither death was deemed to have been related to poor perioperative glycemic control.
Discussion
To our knowledge, this retrospective chart review was the first study to assess preoperative DM management and postoperative complications in a veteran population. VHI is a large, tertiary, level 1a, academic medical center that serves approximately 62,000 veterans annually and performs about 5000 to 6000 surgeries annually, a total that is increasing following the COVID-19 pandemic.20 This study found that the current process of a presurgery clinic visit and day of surgery glucose assessment has prevented surgical delays or cancellations.
Most patients included in this study were well controlled at baseline in accordance with the 2025 ADA SOC HbA1c recommendation of a preoperative HbA1c of < 8%, which may have contributed to no surgical delays or cancellations.10 However, not all patients had HbA1c collected within 3 months of surgery or even had one collected at all. Despite the ADA SOC providing no explicit recommendation for universal HbA1c screening prior to elective procedures, its importance cannot be understated given the body of evidence demonstrating poor outcomes with uncontrolled preoperative DM.8,10 The glycemic control at baseline may have contributed to the very few postsurgical complications observed in this study.
Although the current process at VHI prevented surgical delays and cancellations in this sample, there are still identified areas for improvement. One area is the instructions the patients received. Patients with DM are often prescribed ≥ 1 medication or a combination of insulins, noninsulin injectables, and oral DM medications, and this study population was no different. Because these medications may influence the anesthesia and perioperative periods, the ADA has specific guidance for altering administration schedules in the days leading up to surgery.10
Inappropriate administration of DM medications could lead to perioperative hypoglycemia or hyperglycemia, possibly causing surgical delays, case cancellations, and/or postoperative complications.21 Although these data reveal the specificity and documented receipt that the preoperative DM instructions did not impact the first recorded preoperative blood glucose, future studies should examine patient confidence in how to properly administer their DM medications prior to surgery. It is vital that patients receive clear instructions in accordance with the ADA SOC on whether to continue, hold, or adjust the dose of their medications to prevent fluctuations in blood glucose levels in the perioperative period, ensure safety with anesthesia, and prevent postoperative complications such as acute kidney injury. Of note, compliance with guideline recommendations for medication instructions was not examined because the data collection time frame expanded over multiple years and the recommendations have evolved each year as new data emerge.
Preoperative DM Management
The first key takeaway from this study is to ensure patients are ready for surgery with a formal assessment (typically in the form of a clinic visit) prior to the surgery. One private sector health system published their approach to this by administering an automatic preoperative HbA1c screening for those with a DM diagnosis and all patients with a random plasma glucose ≥ 200 mg/dL.22 Additionally, if the patient's HbA1c level was not at goal prior to surgery (≥ 8% for those with known DM and ≥ 6.5% with no known DM), patients were referred to endocrinology for further management. Increasing attention to the preoperative visit and extending HbA1c testing to all patients regardless of DM status also provides an opportunity to identify individuals living with undiagnosed DM.1
Even though there was no difference in the mean preoperative blood glucose level based on receipt or specificity of preoperative DM instructions, a second takeaway from this study is the importance of ensuring patients receive clear instructions on their DM medication schedule in the perioperative period. A practical first step may be updating the templates used by the primary surgery teams and providing education to the clinicians in the clinic on how to personalize the visits. Because the current preoperative DM process at VHI is managed by the primary surgical team in a clinic visit, there is an opportunity to shift this responsibility to other health care professionals, such as pharmacists—a change shown to reduce unintended omission of home medications following surgery during hospitalization and reduce costs.23,24
Limitations
This study relied on data included in the patient chart. These data include medication interventions made immediately prior to surgery, which can sometimes be inaccurately charted or difficult to find as they are not documented in the typical medication administration record. Also, the safety outcomes were collected from a discharge summary written by different clinicians, which may lead to information bias. Special attention was taken to ensure these data points were collected as accurately as possible, but it is possible some data may be inaccurate from unintentional human error. Additionally, the safety outcome was limited to a 30-day follow-up, but encompassed the entire length of postoperative stay for all included patients. Finally, given this study was retrospective with no comparison group and the intent was to improve processes at VHI, only hypotheses and potential interventions can be generated from this study. Future prospective studies with larger sample sizes and comparator groups are needed to draw further conclusions.
Conclusions
This study found that the current presurgery process at VHI appears to be successful in preventing surgical delays or cancellations due to hyperglycemia or hypoglycemia. Optimizing DM management can improve surgical outcomes by decreasing rates of postoperative complications, and this study added additional evidence in support of that in a unique population: veterans. Insight on the awareness of preoperative blood glucose management should be gleaned from this study, and based on this sample and site, the preadmission screening process and instructions provided to patients can serve as 2 starting points for optimizing elective surgery.
More than 38 million people in the United States (12%) have diabetes mellitus (DM), though 1 in 5 are unaware they have DM.1 The prevalence among veterans is even more substantial, impacting nearly 25% of those who received care from the US Department of Veterans Affairs (VA).2 DM can lead to increased health care costs in addition to various complications (eg, cardiovascular, renal), especially if left uncontrolled.1,3 similar impact is found in the perioperative period (defined as at or around the time of an operation), as multiple studies have found that uncontrolled preoperative DM can result in worsened surgical outcomes, including longer hospital stays, more infectious complications, and higher perioperative mortality.4-6
In contrast, adequate glycemic control assessed with blood glucose levels has been shown to decrease the incidence of postoperative infections.7 Optimizing glycemic control during hospital stays, especially postsurgery, has become the standard of care, with most health systems establishing specific protocols. In current literature, most studies examining DM management in the perioperative period are focused on postoperative care, with little attention to the preoperative period.4,6,7
One study found that patients with poor presurgery glycemic control assessed by hemoglobin A1c (HbA1c) levels were more likely to remain hyperglycemic during and after surgery. 8 Blood glucose levels < 200 mg/dL can lead to an increased risk of infection and impaired wound healing, meaning a well-controlled HbA1c before a procedure serves as a potential factor for success.9 The 2025 American Diabetes Association (ADA) Standards of Care (SOC) recommendation is to target HbA1c < 8% whenever possible, and some health systems require lower levels (eg, < 7% or 7.5%).10 With that goal in mind and knowing that preoperative hyperglycemia has been shown to be a contributing factor in the delay or cancellation of surgical cases, an argument can be made that attention to preoperative DM management also should be a focus for health care systems performing surgeries.8,9,11
Attention to glucose control during preoperative care offers an opportunity to screen for DM in patients who may not have been screened otherwise and to standardize perioperative DM management. Since DM disproportionately impacts veterans, this is a pertinent issue to the VA. Veterans can be more susceptible to complications if DM is left uncontrolled prior to surgery. To determine readiness for surgery and control of comorbid conditions such as DM before a planned surgery, facilities often perform a preoperative clinic assessment, often in a multidisciplinary clinic.
At Veteran Health Indiana (VHI), a presurgery clinic visit involving the primary surgery service (physician, nurse practitioner, and/or a physician assistant) is conducted 1 to 2 months prior to the planned procedure to determine whether a patient is ready for surgery. During this visit, patients receive a packet with instructions for various tasks and medications, such as applying topical antibiotic prophylaxis on the anticipated surgical site. This is documented in the form of a note in the VHI Computerized Patient Record System (CPRS). The medication instructions are provided according to the preferences of the surgical team. These may be templated notes that contain general directions on the timing and dosing of specific medications, in addition to instructions for holding or reducing doses when appropriate. The instructions can be tailored by the team conducting the preoperative visit (eg, “Take 20 units of insulin glargine the day before surgery” vs “Take half of your long-acting insulin the night before surgery”). Specific to DM, VHI has a nurse-driven day of surgery glucose assessment where point-of-care blood glucose is collected during preoperative holding for most patients.
There is limited research assessing the level of preoperative glycemic control and the incidence of complications in a veteran population. The objective of this study was to gain a baseline understanding of what, if any, standardization exists for preoperative instructions for DM medications and to assess the level of preoperative glycemic control and postoperative complications in patients with DM undergoing major elective surgical procedures.
Methods
This retrospective, single-center chart review was conducted at VHI. The Indiana University and VHI institutional review boards determined that this quality improvement project was exempt from review.
The primary outcome was the number of patients with surgical procedures delayed or canceled due to hyperglycemia or hypoglycemia. Hyperglycemia was defined as blood glucose > 180 mg/dL and hypoglycemia was defined as < 70 mg/dL, slight variations from the current ADA SOC preoperative specific recommendation of a blood glucose reading of 100 to 180 mg/dL within 4 hours of surgery.10 The standard outpatient hypoglycemia definition of blood glucose < 70 mg/dL was chosen because the current goal (< 100 mg/dL) was not the standard in previous ADA SOCs that were in place during the study period. Specifically, the 2018 ADA SOC did not provide preoperative recommendations and the 2019-2021 ADA SOC recommended 80 to 180 mg/dL.10,12-18 For patients who had multiple preoperative blood glucose measurements, the first recorded glucose on the day of the procedure was used.
The secondary outcomes of this study were focused on the preoperative process/care at VHI and postoperative glycemic control. The preoperative process included examining whether medication instructions were given and their quality. Additionally, the number of interventions for hyperglycemia and hypoglycemia were required immediately prior to surgery and the average preoperative HbA1c (measured within 3 months prior to surgery) were collected and analyzed. For postoperative glycemic control, average blood glucose measurements and number of hypoglycemic (< 70 mg/dL) and hyperglycemic (> 180 mg/dL) events were measured in addition to the frequency of changes made at discharge to patients’ DM medication regimens.
The safety outcome of this study assessed commonly observed postoperative complications and was examined up to 30 days postsurgery. These included acute kidney injury (defined using Kidney Disease: Improving Global Outcomes 2012, the standard during the study period), nonfatal myocardial infarction, nonfatal stroke, and surgical site infections, which were identified from the discharge summary written by the primary surgery service.19 All-cause mortality also was collected.
Patients were included if they were admitted for major elective surgeries and had a diagnosis of either type 1 or type 2 DM on their problem list, determined by International Classification of Diseases, Tenth Revision codes. Major elective surgery was defined as a procedure that would likely result in a hospital admission of > 24 hours. Of note, patients may have been included in this study more than once if they had > 1 procedure at least 30 days apart and met inclusion criteria within the time frame. Patients were excluded if they were taking no DM medications or chronic steroids (at any dose), residing in a long-term care facility, being managed by a non-VA clinician prior to surgery, or missing a preoperative blood glucose measurement.
All data were collected from the CPRS. A list of surgical cases involving patients with DM who were scheduled to undergo major elective surgeries from January 1, 2018, to December 31, 2021, at VHI was generated. The list was randomized to a smaller number (N = 394) for data collection due to the time and resource constraints for a pharmacy residency project. All data were deidentified and stored in a secured VA server to protect patient confidentiality. Descriptive statistics were used for all results.
Results
Initially, 2362 surgeries were identified. A randomized sample of 394 charts were reviewed and 131 cases met inclusion criteria. Each case involved a unique patient (Figure). The most common reasons for exclusion were 143 patients with diet-controlled DM and 78 nonelective surgeries. The mean (SD) age of patients was 68 (8) years, and the most were male (98.5%) and White (76.3%) (Table 1).


At baseline, 45 of 131 patients (34.4%) had coronary artery disease and 29 (22.1%) each had autonomic neuropathy and chronic kidney disease. Most surgeries were conducted by orthopedic (32.1%) and peripheral vascular (21.4%) specialties. The mean (SD) length of surgery was 4.6 (2.6) hours and of hospital length of stay was 4 (4) days. No patients stayed longer than the 30-day safety outcome follow-up period. All patients had type 2 DM and took a mean 2 DM medications. The 63 patients taking insulin had a mean (SD) total daily dose of 99 (77) U (Table 2). A preoperative HbA1c was collected in 116 patients within 3 months of surgery, with a mean HbA1c of 7.0% (range, 5.3-10.7).

No patients had surgeries delayed or canceled because of uncontrolled DM on the day of surgery. The mean preoperative blood glucose level was 146 mg/dL (range, 73-365) (Table 3). No patients had a preoperative blood glucose level of < 70 mg/dL and 19 (14.5%) had a blood glucose level > 180 mg/dL. Among patients with hyperglycemia immediately prior to surgery, 6 (31.6%) had documentation of insulin being provided.

For this sample of patients, the preoperative clinic visit was conducted a mean 22 days prior to the planned surgery date. Among the 131 included patients, 122 (93.1%) had documentation of receiving instructions for DM medications. Among patients who had documented receipt of instructions, only 30 (24.6%) had instructions specifically tailored to their regimen rather than a generic templated form. The mean (SD) preoperative blood glucose was similar for those who received specific perioperative DM instructions at 146 (50) mg/dL when compared with those who did not at 147 (45) mg/dL. The mean (SD) preoperative blood glucose reading for those who had no documentation of receipt of perioperative instructions was 126 (54) mg/dL compared with 147 (46) mg/dL for those who did.
The mean number of postoperative blood glucose events per day was negligible for hypoglycemia and more frequent for hyperglycemia with a mean of 2 events per day. The mean postoperative blood glucose range was 121 to 247 mg/dL with most readings < 180 mg/dL. Upon discharge, most patients continued their home DM regimen with 5 patients (3.8%) having changes made to their regimen upon discharge.
Very few postoperative complications were identified from chart review. The most frequently observed postoperative complications were acute kidney injury, surgical site infections, and nonfatal stroke. There were no documented nonfatal myocardial infarctions. Two patients (1.5%) died within 30 days of the surgery; neither death was deemed to have been related to poor perioperative glycemic control.
Discussion
To our knowledge, this retrospective chart review was the first study to assess preoperative DM management and postoperative complications in a veteran population. VHI is a large, tertiary, level 1a, academic medical center that serves approximately 62,000 veterans annually and performs about 5000 to 6000 surgeries annually, a total that is increasing following the COVID-19 pandemic.20 This study found that the current process of a presurgery clinic visit and day of surgery glucose assessment has prevented surgical delays or cancellations.
Most patients included in this study were well controlled at baseline in accordance with the 2025 ADA SOC HbA1c recommendation of a preoperative HbA1c of < 8%, which may have contributed to no surgical delays or cancellations.10 However, not all patients had HbA1c collected within 3 months of surgery or even had one collected at all. Despite the ADA SOC providing no explicit recommendation for universal HbA1c screening prior to elective procedures, its importance cannot be understated given the body of evidence demonstrating poor outcomes with uncontrolled preoperative DM.8,10 The glycemic control at baseline may have contributed to the very few postsurgical complications observed in this study.
Although the current process at VHI prevented surgical delays and cancellations in this sample, there are still identified areas for improvement. One area is the instructions the patients received. Patients with DM are often prescribed ≥ 1 medication or a combination of insulins, noninsulin injectables, and oral DM medications, and this study population was no different. Because these medications may influence the anesthesia and perioperative periods, the ADA has specific guidance for altering administration schedules in the days leading up to surgery.10
Inappropriate administration of DM medications could lead to perioperative hypoglycemia or hyperglycemia, possibly causing surgical delays, case cancellations, and/or postoperative complications.21 Although these data reveal the specificity and documented receipt that the preoperative DM instructions did not impact the first recorded preoperative blood glucose, future studies should examine patient confidence in how to properly administer their DM medications prior to surgery. It is vital that patients receive clear instructions in accordance with the ADA SOC on whether to continue, hold, or adjust the dose of their medications to prevent fluctuations in blood glucose levels in the perioperative period, ensure safety with anesthesia, and prevent postoperative complications such as acute kidney injury. Of note, compliance with guideline recommendations for medication instructions was not examined because the data collection time frame expanded over multiple years and the recommendations have evolved each year as new data emerge.
Preoperative DM Management
The first key takeaway from this study is to ensure patients are ready for surgery with a formal assessment (typically in the form of a clinic visit) prior to the surgery. One private sector health system published their approach to this by administering an automatic preoperative HbA1c screening for those with a DM diagnosis and all patients with a random plasma glucose ≥ 200 mg/dL.22 Additionally, if the patient's HbA1c level was not at goal prior to surgery (≥ 8% for those with known DM and ≥ 6.5% with no known DM), patients were referred to endocrinology for further management. Increasing attention to the preoperative visit and extending HbA1c testing to all patients regardless of DM status also provides an opportunity to identify individuals living with undiagnosed DM.1
Even though there was no difference in the mean preoperative blood glucose level based on receipt or specificity of preoperative DM instructions, a second takeaway from this study is the importance of ensuring patients receive clear instructions on their DM medication schedule in the perioperative period. A practical first step may be updating the templates used by the primary surgery teams and providing education to the clinicians in the clinic on how to personalize the visits. Because the current preoperative DM process at VHI is managed by the primary surgical team in a clinic visit, there is an opportunity to shift this responsibility to other health care professionals, such as pharmacists—a change shown to reduce unintended omission of home medications following surgery during hospitalization and reduce costs.23,24
Limitations
This study relied on data included in the patient chart. These data include medication interventions made immediately prior to surgery, which can sometimes be inaccurately charted or difficult to find as they are not documented in the typical medication administration record. Also, the safety outcomes were collected from a discharge summary written by different clinicians, which may lead to information bias. Special attention was taken to ensure these data points were collected as accurately as possible, but it is possible some data may be inaccurate from unintentional human error. Additionally, the safety outcome was limited to a 30-day follow-up, but encompassed the entire length of postoperative stay for all included patients. Finally, given this study was retrospective with no comparison group and the intent was to improve processes at VHI, only hypotheses and potential interventions can be generated from this study. Future prospective studies with larger sample sizes and comparator groups are needed to draw further conclusions.
Conclusions
This study found that the current presurgery process at VHI appears to be successful in preventing surgical delays or cancellations due to hyperglycemia or hypoglycemia. Optimizing DM management can improve surgical outcomes by decreasing rates of postoperative complications, and this study added additional evidence in support of that in a unique population: veterans. Insight on the awareness of preoperative blood glucose management should be gleaned from this study, and based on this sample and site, the preadmission screening process and instructions provided to patients can serve as 2 starting points for optimizing elective surgery.
- Centers for Disease Control and Prevention. Diabetes basics. May 15, 2024. Accessed September 24, 2025. https://www.cdc.gov/diabetes/about/index.html
- Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135. doi:10.5888/pcd14.170230
- Farmaki P, Damaskos C, Garmpis N, et al . Complications of the Type 2 Diabetes Mellitus. Curr Cardiol Rev. 2020;16(4):249-251. doi:10.2174/1573403X1604201229115531
- Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783-1788. doi:10.2337/dc10-0304
- Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156:137 -142. doi:10.1530/eje.1.02321
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77-81. doi:10.1177/01486071980220027
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34:256-261. doi:10.2337/dc10-1407
- Pasquel FJ, Gomez-Huelgas R, Anzola I, et al. Predictive value of admission hemoglobin A1c on inpatient glycemic control and response to insulin therapy in medicine and surgery patients with type 2 diabetes. Diabetes Care. 2015;38:e202-e203. doi:10.2337/dc15-1835
- Alexiewicz JM, Kumar D, Smogorzewski M, et al. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med. 1995;123:919-924. doi:10.7326/0003-4819-123-12-199512150-00004
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2025. Diabetes Care. 2025;48(1 suppl 1):S321-S334. doi:10.2337/dc25-S016
- Kumar R, Gandhi R. Reasons for cancellation of operation on the day of intended surgery in a multidisciplinary 500 bedded hospital. J Anaesthesiol Clin Pharmacol. 2012;28:66-69. doi:10.4103/0970-9185.92442
- American Diabetes Association. 14. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2018. Diabetes Care. 2018;41(1 suppl 1):S144- S151. doi:10.2337/dc18-S014
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2019. Diabetes Care. 2019;42(suppl 1):S173- S181. doi:10.2337/dc19-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2020. Diabetes Care. 2020;43(suppl 1):S193- S202. doi:10.2337/dc20-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2021. Diabetes Care. 2021;44(suppl 1):S211- S220. doi:10.2337/dc21-S015
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
- ElSayed NA, Aleppo G, Aroda VR, et al. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(suppl 1):S267-S278. doi:10.2337/dc23-S016
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(suppl 1):S295-S306. doi:10.2337/dc24-S016
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138. Accessed September 24, 2025. https:// www.kisupplements.org/issue/S2157-1716(12)X7200-9
- US Department of Veterans Affairs. VA Indiana Healthcare: about us. Accessed September 24, 2025. https:// www.va.gov/indiana-health-care/about-us/
- Koh WX, Phelan R, Hopman WM, et al. Cancellation of elective surgery: rates, reasons and effect on patient satisfaction. Can J Surg. 2021;64:E155-E161. doi:10.1503/cjs.008119
- Pai S-L, Haehn DA, Pitruzzello NE, et al. Reducing infection rates with enhanced preoperative diabetes mellitus diagnosis and optimization processes. South Med J. 2023;116:215-219. doi:10.14423/SMJ.0000000000001507
- Forrester TG, Sullivan S, Snoswell CL, et al. Integrating a pharmacist into the perioperative setting. Aust Health Rev. 2020;44:563-568. doi:10.1071/AH19126
- Hale AR, Coombes ID, Stokes J, et al. Perioperative medication management: expanding the role of the preadmission clinic pharmacist in a single centre, randomised controlled trial of collaborative prescribing. BMJ Open. 2013;3:e003027. doi:10.1136/bmjopen-2013-003027
- Centers for Disease Control and Prevention. Diabetes basics. May 15, 2024. Accessed September 24, 2025. https://www.cdc.gov/diabetes/about/index.html
- Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135. doi:10.5888/pcd14.170230
- Farmaki P, Damaskos C, Garmpis N, et al . Complications of the Type 2 Diabetes Mellitus. Curr Cardiol Rev. 2020;16(4):249-251. doi:10.2174/1573403X1604201229115531
- Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783-1788. doi:10.2337/dc10-0304
- Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156:137 -142. doi:10.1530/eje.1.02321
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77-81. doi:10.1177/01486071980220027
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34:256-261. doi:10.2337/dc10-1407
- Pasquel FJ, Gomez-Huelgas R, Anzola I, et al. Predictive value of admission hemoglobin A1c on inpatient glycemic control and response to insulin therapy in medicine and surgery patients with type 2 diabetes. Diabetes Care. 2015;38:e202-e203. doi:10.2337/dc15-1835
- Alexiewicz JM, Kumar D, Smogorzewski M, et al. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med. 1995;123:919-924. doi:10.7326/0003-4819-123-12-199512150-00004
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2025. Diabetes Care. 2025;48(1 suppl 1):S321-S334. doi:10.2337/dc25-S016
- Kumar R, Gandhi R. Reasons for cancellation of operation on the day of intended surgery in a multidisciplinary 500 bedded hospital. J Anaesthesiol Clin Pharmacol. 2012;28:66-69. doi:10.4103/0970-9185.92442
- American Diabetes Association. 14. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2018. Diabetes Care. 2018;41(1 suppl 1):S144- S151. doi:10.2337/dc18-S014
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2019. Diabetes Care. 2019;42(suppl 1):S173- S181. doi:10.2337/dc19-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2020. Diabetes Care. 2020;43(suppl 1):S193- S202. doi:10.2337/dc20-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2021. Diabetes Care. 2021;44(suppl 1):S211- S220. doi:10.2337/dc21-S015
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
- ElSayed NA, Aleppo G, Aroda VR, et al. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(suppl 1):S267-S278. doi:10.2337/dc23-S016
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(suppl 1):S295-S306. doi:10.2337/dc24-S016
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138. Accessed September 24, 2025. https:// www.kisupplements.org/issue/S2157-1716(12)X7200-9
- US Department of Veterans Affairs. VA Indiana Healthcare: about us. Accessed September 24, 2025. https:// www.va.gov/indiana-health-care/about-us/
- Koh WX, Phelan R, Hopman WM, et al. Cancellation of elective surgery: rates, reasons and effect on patient satisfaction. Can J Surg. 2021;64:E155-E161. doi:10.1503/cjs.008119
- Pai S-L, Haehn DA, Pitruzzello NE, et al. Reducing infection rates with enhanced preoperative diabetes mellitus diagnosis and optimization processes. South Med J. 2023;116:215-219. doi:10.14423/SMJ.0000000000001507
- Forrester TG, Sullivan S, Snoswell CL, et al. Integrating a pharmacist into the perioperative setting. Aust Health Rev. 2020;44:563-568. doi:10.1071/AH19126
- Hale AR, Coombes ID, Stokes J, et al. Perioperative medication management: expanding the role of the preadmission clinic pharmacist in a single centre, randomised controlled trial of collaborative prescribing. BMJ Open. 2013;3:e003027. doi:10.1136/bmjopen-2013-003027
Preoperative Diabetes Management for Patients Undergoing Elective Surgeries at a Veterans Affairs Medical Center
Preoperative Diabetes Management for Patients Undergoing Elective Surgeries at a Veterans Affairs Medical Center
Development of a Pharmacist-Led Emergency Department Antimicrobial Surveillance Program
On September 18, 2014, President Barack Obama signed an executive order that made addressing antibiotic-resistant bacteria a national security policy.1 This legislation resulted in the creation of a large multidepartment task force to combat the global and domestic problem of antimicrobial resistance. The order required hospitals and other inpatient health care delivery facilities, including the Department of Veterans Affairs (VA), to implement robust antimicrobial stewardship programs that adhere to best practices, such as those identified by the Centers for Disease Control and Prevention (CDC). More specifically, the VA was mandated to take steps to encourage other areas of health care, such as ambulatory surgery centers and outpatient clinics, to adopt antimicrobial stewardship programs.1 This order also reinforced the importance for VA facilities to continue to develop, improve, and sustain efforts in antimicrobial stewardship.
Prior to the order, in 2012 the Richard L. Roudebush VA Medical Center (RRVAMC) in Indianapolis, Indiana, implemented an inpatient antimicrobial stewardship program that included thrice-weekly meetings to review inpatient records and make stewardship recommendations with an infectious diseases physician champion and clinical pharmacists. These efforts led to the improved use of antimicrobial agents on the inpatient side of the medical center. During the first 4 years of implementation, the program helped to decrease the defined daily doses of broad-spectrum antibiotics per 1,000 patient days nearly 36%, from 532 in 2012 to 343 in 2015, as well as decrease the days of therapy of fluoroquinolones per 1,000 patient days 28.75%, from 80 in 2012 to 57 in 2015. Additionally, the program showed a significant decrease in the standardized antimicrobial administration ratio, a benchmark measure developed by the CDC to reflect a facility’s actual antimicrobial use to the expected use of a similar facility based on bed size, number of intensive care unit beds, location type, and medical school affiliation.2
While the RRVAMC antimicrobial stewardship team has been able to intervene on most of the inpatients admitted to the medical center, the outpatient arena has had few antimicrobial stewardship interventions. Recognizing a need to establish and expand pharmacy services and for improvement of outpatient antimicrobial stewardship, RRVAMC leadership decided to establish a pharmacist-led outpatient antimicrobial surveillance program, starting specifically within the emergency department (ED).
Clinical pharmacists in the ED setting are uniquely positioned to improve patient care and encourage the judicious use of antimicrobials for empiric treatment of urinary tract infections (UTIs). The CDC’s Core Elements of Outpatient Antibiotic Stewardship recommends pharmacist availability in the ED setting, and previous literature has demonstrated pharmacist utility in ED postdischarge culture monitoring and surveillance.3-5
This article will highlight one such program review at the RRVAMC and demonstrate the need for pharmacist-led antimicrobial stewardship and monitoring in the ED. The purpose of this study was to test the hypothesis that pharmacist intervention would be necessary to prospectively check for “bug-drug mismatch” and assure proper follow-up of urine cultures at this institution. The project was deemed to be quality improvement and thereby granted exemption by the RRVAMC Institutional Review Board.
Methods
This project took place at the RRVAMC, a 229-bed tertiary academic medical center that serves > 60,000 patients annually. The RRVAMC ED has 20 beds and received about 29,000 visits in 2014. Patients were eligible for initial evaluation if they had a urine culture collected in the ED within the 91-day period from September 1, 2015 to November 30, 2015. Patients were included for data analysis if it was documented that they were treated for actual or clinically suspected, based on signs and symptoms, uncomplicated UTI, complicated UTI, or UTI with pyelonephritis. Patients did not need to have a positive urine culture for inclusion, as infections could still be present despite negative culture results.6 Patients with positive cultures who were not clinically symptomatic of a UTI and were not treated as such by the ED provider (ie, asymptomatic bacteriuria) were excluded from the study.
Data collection took place via daily chart review of patient records in both the Computerized Patient Record System and Decentralized Hospital Computer Program medical applications as urine cultures were performed. Data were gathered and assessed by a postgraduate year-2 internal medicine pharmacy resident on rotation in the ED who reviewed cultures daily and made interventions based on the results as needed. The pharmacy resident was physically present within the ED during the first 30 days of the project. The pharmacy resident was not within the direct practice area during the final 61 days of the project but was in a different area of the hospital and available for consultation.
Primary data collected included urine culture results and susceptibilities, empiric antimicrobial choices, and admission status. Other data collected included duration of treatment and secondary antibiotics chosen, each of which specifically evaluated those patients who were not admitted to the hospital and were thus treated as outpatients. Additional data generated from this study were used to identify empiric antibiotics utilized for the treatment of UTIs and assess for appropriate selection and duration of therapy within this institution.
Results
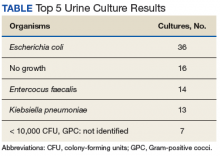
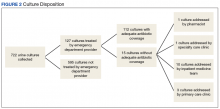
During the study period, 722 urine cultures were collected in the ED and were included for initial evaluation. Of these, 127 were treated by the ED provider pursuant to one of the indications specified and were included in the data analysis. Treatment with an antimicrobial agent provided adequate coverage for the identified pathogen in 112 patients, yielding a match rate of 88%. As all included cultures were collected in suspicion of an infection, those cultures yielding no growth were considered to have been adequately covered.
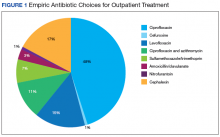
Nearly half (45%) of treatment plans included a fluoroquinolone. Of those treated on an outpatient basis, fluoroquinolones were even more frequently used, comprising 50 of 82 (61%) courses. Ciprofloxacin was the most frequently used treatment, used in 39 of the 82 outpatient regimens (48%). Cephalexin was the second most common and was used in 14 outpatient regimens (17%), followed by levofloxacin (15%) (Figure 1).
Mismatched cultures, or those where the prescribed antibiotic did not provide adequate coverage of the identified pathogens based on susceptibilities, occurred at a rate of 12%. Follow-up on these cultures was determined largely by the patient’s admission status. The majority of mismatched cultures were addressed by the inpatient team (10/15) upon admission.
Discussion
Empiric antibiotic selection for the treatment of UTIs continues to be the cornerstone of antibiotic management for the treatment of such a disease state.7 The noted drug-bug match rate of 88% in this study demonstrates effective initial empiric coverage and ensures a vast majority of veterans receive adequate coverage for identified pathogens. Additionally, this rate shows that the current system seems to be functioning appropriately and refutes the author’s preconceived ideas that the mismatch rate was higher at RRVAMC. However, these findings also demonstrate a predominant use of fluoroquinolones for empiric treatment in a majority of patients who could be better served with narrower spectrum agents. Only 2 of the outpatient regimens were for the treatment of pyelonephritis, the only indication in which a fluoroquinolone would be the standard of care per guideline recommendations.7
These findings were consistent with a similar study in which 83% of ED collected urine cultures ultimately grew bacteria susceptibleto empiric treatment.8 This number was similar to the current study despite the latter study consisting of predominantly female patients (93%) and excluding patients with a history of benign prostatic hypertrophy, catheter use, or history of genitourinary cancer, which are frequently found within the VA population. Thus, despite having a differing patient population at the current study’s facility with characteristics that would classify most to be treated as a complicated UTI, empiric coverage rates remained similar. The lower than anticipated intervention rate by the pharmacist on rotation in the ED can be directly attributed to this high empiric match rate, which could in turn be attributed to the extensive use of broad-spectrum antibiotics for treatment.
Empiric antimicrobial selection is based largely on local resistance patterns.7 Of particular importance is the resistance patterns of E coli, as it is the primary isolate responsible for UTIs worldwide. Thus, it is not unexpected that the most frequently isolated pathogen in the current study also was E coli. While clinical practice guidelines state that hospital-wide antibiograms often are skewed by cultures collected from inpatients or those with complicated infection, the current study found hospital-wide E coli resistance patterns, specifically those related to fluoroquinolone use, to be similar to those collected in the ED alone (78.5% hospital-wide susceptibility vs. 75% ED susceptibility). This was expected, as similar studies comparing E coli resistance patterns from ED-collected urine cultures to those institution-wide also have found similar rates of resistance.8,9 These findings are of particular importance as E coli resistance is noted to be increasing, varies with geographic area, and local resistance patterns are rarely known.7 Thus, these findings may aid ED providers in their empiric antimicrobial selections.
Ciprofloxacin was the most frequently used medication for the treatment of UTIs. While overall empiric selections were found to have favorable resistance patterns, it is difficult to interpret the appropriateness of ciprofloxacin’s use in the present study. First, there is a distinct lack of US-based clinical practice guidelines for the treatment of complicated UTIs. As the majority of this study population was male, it is difficult to directly extrapolate from the current Infectious Diseases Society of America treatment guidelines for uncomplicated cystitis and apply to the study population. Although recommended for the treatment of pyelonephritis, it is unclear whether ciprofloxacin should be utilized as a first-line empiric option for the treatment of UTIs in males.
Despite the lack of disease-specific recommendations for ciprofloxacin, recommendations exist regarding its use when local resistance patterns are known.7 It is currently recommended that these agents not be used when resistance rates of E coli exceed 20% for trimethoprim-sulfamethoxazole or 10% for fluoroquinolones. As this study demonstrated a nearly 25% resistance rate for E coli to fluoroquinolones in both the ED and institution-wide sample populations, it could potentially be ascertained that ciprofloxacin is an inappropriate choice for the empiric treatment of UTIs in this patient population. However, as noted, it is unknown whether this recommendation would still be applicable when applied to the treatment of complicated cystitis and greater male population, as overall rates of susceptible cultures to all organisms was similar to other published studies.8,9
While there is scant specific guidance related to the treatment of complicated UTIs, there is emerging guidance on the use of fluoroquinolones, both in general and specifically related to the treatment of UTIs. In July 2016, the FDA issued a drug safety communication regarding the use of and warnings for fluoroquinolones, which explicitly stated that “health care professionals should not prescribe systemic fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated UTIs because the risks outweigh the benefits in these patients.”10
This guidance has the potential to impact fluoroquinolone prescribing significantly at RRVAMC. Given the large number of fluoroquinolones prescribed for UTIs, the downstream effects that this shift in prescribing would have is unknown. As most nonfluoroquinolones used for UTI typically are narrower in antimicrobial spectrum (eg, trimethoprim/sulfamethoxazole, nitrofurantoin, cephalexin, etc) the possibility exists that the match rate for empiric therapy may decrease. Thus, a larger need for closer follow-up to assure adequate coverage may arise, posing a more expanded role for an ED-based pharmacist than was demonstrated in the current study.
This new guidance also may place providers in an area of larger uncertainty with regards to treating both complicated and uncomplicated cystitis. Given the enhanced warnings on fluoroquinolone use, it is unknown whether prescribers would gravitate to utilizing similar options as their peers as alternatives to fluoroquinolones. Similarly, duration of therapy with nonfluoroquinolone agents is unclear as well; as the present study demonstrated a large range in treatment duration of outpatients (3-14 days). While the average observed duration of 8.3 days is intuitively fitting, as the majority of cases were in males, no published guideline exists that affirms the appropriateness of this finding. Such uncertainty and potential inconsistency between providers affords a large opportunity for developing a standardized treatment pathway for the treatment of UTIs to ensure both effective and guideline concordant treatment for patients, specifically with regards to antimicrobial selection and duration of treatment.
It is noteworthy to mention that all follow-ups on positive cultures inadequately covered by empiric therapy took place on the day organism identification and susceptibility data were released. This finding was somewhat surprising, as it was originally theorized that most ED-collected urine cultures were not monitored to completion by a pharmacist and that would be necessary in order to ensure proper follow-up of culture results. What is not clear is whether there is a robust process for the follow-up of urine cultures in the ED. Most of the bug-drug mismatches coincidentally were admitted to the inpatient teams where there were appropriate personnel to follow up and adjust the antibiotic selection. If there was a bug-drug mismatch, and the patient was not admitted, it is unclear whether there is a consistent process for follow-up.
Given the limited number of mismatched cultures that required change in therapy, it is unknown if this role would expand if more narrow-spectrum agents were utilized, theoretically leading to a higher mismatch rate and necessitating closer follow-up. Furthermore, given the common practice of mailed prescriptions at the VA, it is all the more imperative that the cultures be acted upon on the day they were identified, as the mailing and processing time of prescriptions may limit the clinical utility in switching from a more broad-spectrum agent, to one more targeted for an identified organism. While a patient traveling back to the medical center for expedited prescription pickup at the pharmacy would alleviate this problem, many patients at the facility travel great distances or may not have readily available travel means to return to the medical center.
Future Directions
While minimal follow-up was required after a patient had left the ED, this study demonstrated a fundamental need for further refinements in antimicrobial stewardship activities within the ED. Duration of therapy, empiric selection, and proper dosing are key areas where the ED-based pharmacy resident was able to intervene during the time physically stationed in the ED. The data collected from this study demonstrated this and was ultimately combined with other ED-based interventions and utilized as supporting evidence in the pharmacy service business plan, outlining the necessity of a full-time pharmacy presence in the ED. The business plan submission, along with other ongoing RRVAMC initiatives, ultimately led to the approval for clinical pharmacy specialists to expand practice into the ED. These positions will continue to advance pharmacy practice within the ED, while affording opportunities for pharmacists to practice at the top of their licensure, provide individualized provider education, and deliver real-time antimicrobial stewardship interventions. Furthermore, as the majority of the study period was monitored outside of the ED, the project may provide a model for other VA institutions without full-time ED pharmacists to implement as a means to improve antimicrobial stewardship and further build an evidence base for expanding their pharmacy services to the ED.
Given the large number of fluoroquinolones utilized in the ED, this study has raised the question of what prescribing patterns look like with regards to outpatient UTI treatment within the realm of primary care at RRVAMC. Despite the great strides made with regards to antimicrobial stewardship at this facility on the inpatient side, no formal antimicrobial stewardship program exists for review in the outpatient setting, where literature suggests the majority of antibiotics are prescribed.3,11 While more robust protocols are in place for follow-up of culture data in the primary care realm at this facility, the prescribing patterns are relatively unknown.
A recent study completed at a similar VA facility found that 60% of antibiotics prescribed for cystitis, pharyngitis, or sinusitis on an outpatient basis were guideline-discordant, and CDC guidance has further recommended specific focus should be undertaken with regards to outpatient stewardship practices in the treatment of genitourinary infections.3,12 These findings highlight the need for outpatient antimicrobial stewardship and presents a compelling reason to further investigate outpatient prescribing within primary care at RRVAMC.
Strengths and Limitations
Strengths of the current study include the ability to monitor urine cultures in real time and to provide timely interventions in the event of a rare bug-drug mismatch. The evaluation of cultures in this study shows that the majority of cases had a drug selected with adequate coverage. The study did assure ED providers that, even though guidelines may suggest otherwise, urine cultures drawn in the ED at RRVAMC followed similar resistance patterns seen for the facility as a whole. Moreover, it is valuable as it captures data that are directly applicable to the VA patient population, in which there is little published data with regards to UTI treatment and no formal VA guidance.
A primary limitation of this study is the lack of differentiation between cultures collected from patients with or without indwelling catheters. However, only including patients who presented with signs and/or symptoms of a UTI limits the number of cultures that could potentially be deemed as colonization, thus minimizing the potential for nonpathogenic organisms to confound the results. This study also did not differentiate the setting from which the patient presented (eg, community, extended care facility, etc) that could have potentially provided guidance on resistance patterns for community-acquired UTIs and whether this may have differed from hospital-acquired or facility-acquired UTIs. Another limitation was the relatively short time frame for data collection. A data collection period greater than 91 days would allow for a larger sample size, thus making the data more robust and potentially allowing for the identification of other trends not seen in the current study. A longer data collection period also would have afforded the opportunity to track more robust clinical outcomes throughout the study, identifying whether treatment failure may have been linked to the use of certain classes or spectrums of activity of antibiotics.
Conclusion
Despite the E coli resistance rate to ciprofloxacin (> 20%), the empiric treatments chosen were > 85% effective, needing minimal follow-up once a patient left the ED. Nonetheless, a change in prescribing patterns based on recent national recommendations may provide expanded opportunities in antimicrobial stewardship for ED-based pharmacists. Further research is needed in antimicrobial stewardship within this facility’s outpatient primary care realm, potentially uncovering other opportunities for pharmacist intervention to assure guideline concordant care for the treatment of UTIs as well as other infections treated in primary care patients.
1. Obama B. Executive order–combating antibiotic-resistant bacteria. https://www.whitehouse.gov/the -press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. Published September 18, 2014. Accessed June 7, 2018.
2. Livorsi DJ, O’Leary E, Pierce T, et al. A novel metric to monitor the influence of antimicrobial stewardship activities. Infect Control Hosp Epidemiol. 2017;38(6):721-723.
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hick LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12.
4. Wymore ES, Casanova TJ, Broekenmeier RL, Martin JK Jr. Clinical pharmacist’s daily role in the emergency department of a community hospital. Am J Health-Syst Pharm. 2008;65(5):395-396, 398-399.
5. Frandzel S. ED pharmacists’ value on display at ASHP Midyear. http://www.pharmacypracticenews.com/ViewArticle.aspx?ses=ogst&d_id=53&a_id=22524. Published February 14, 2013. Accessed June 15, 2018.
6. Heytens S, DeSutter A, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017;23(9)647-652.
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120.
8. Lingenfelter E, Drapkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient urinary tract infection. Am J Emerg Med. 2016;34(8):1600-1603.
9. Zatorski C, Jordan JA, Cosgrove SE, Zocchi M, May L. Comparison of antibiotic susceptibility of Escherichia coli in urinary isolates from an emergency department with other institutional susceptibility data. Am J Health-Syst Pharm. 2015;72(24):2176-2180.
10. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Updated March 8, 2018. Accessed June 13, 2018.
11. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241.
12. Meyer HE, Lund BC, Heintz BH, Alexander B, Egge JA, Livorsi DJ. Identifying opportunities to improve guideline-concordant antibiotic prescribing in veterans with acute respiratory infections or cystitis. Infect Control Hosp Epidemiol. 2017;38(6):724-728.
On September 18, 2014, President Barack Obama signed an executive order that made addressing antibiotic-resistant bacteria a national security policy.1 This legislation resulted in the creation of a large multidepartment task force to combat the global and domestic problem of antimicrobial resistance. The order required hospitals and other inpatient health care delivery facilities, including the Department of Veterans Affairs (VA), to implement robust antimicrobial stewardship programs that adhere to best practices, such as those identified by the Centers for Disease Control and Prevention (CDC). More specifically, the VA was mandated to take steps to encourage other areas of health care, such as ambulatory surgery centers and outpatient clinics, to adopt antimicrobial stewardship programs.1 This order also reinforced the importance for VA facilities to continue to develop, improve, and sustain efforts in antimicrobial stewardship.
Prior to the order, in 2012 the Richard L. Roudebush VA Medical Center (RRVAMC) in Indianapolis, Indiana, implemented an inpatient antimicrobial stewardship program that included thrice-weekly meetings to review inpatient records and make stewardship recommendations with an infectious diseases physician champion and clinical pharmacists. These efforts led to the improved use of antimicrobial agents on the inpatient side of the medical center. During the first 4 years of implementation, the program helped to decrease the defined daily doses of broad-spectrum antibiotics per 1,000 patient days nearly 36%, from 532 in 2012 to 343 in 2015, as well as decrease the days of therapy of fluoroquinolones per 1,000 patient days 28.75%, from 80 in 2012 to 57 in 2015. Additionally, the program showed a significant decrease in the standardized antimicrobial administration ratio, a benchmark measure developed by the CDC to reflect a facility’s actual antimicrobial use to the expected use of a similar facility based on bed size, number of intensive care unit beds, location type, and medical school affiliation.2
While the RRVAMC antimicrobial stewardship team has been able to intervene on most of the inpatients admitted to the medical center, the outpatient arena has had few antimicrobial stewardship interventions. Recognizing a need to establish and expand pharmacy services and for improvement of outpatient antimicrobial stewardship, RRVAMC leadership decided to establish a pharmacist-led outpatient antimicrobial surveillance program, starting specifically within the emergency department (ED).
Clinical pharmacists in the ED setting are uniquely positioned to improve patient care and encourage the judicious use of antimicrobials for empiric treatment of urinary tract infections (UTIs). The CDC’s Core Elements of Outpatient Antibiotic Stewardship recommends pharmacist availability in the ED setting, and previous literature has demonstrated pharmacist utility in ED postdischarge culture monitoring and surveillance.3-5
This article will highlight one such program review at the RRVAMC and demonstrate the need for pharmacist-led antimicrobial stewardship and monitoring in the ED. The purpose of this study was to test the hypothesis that pharmacist intervention would be necessary to prospectively check for “bug-drug mismatch” and assure proper follow-up of urine cultures at this institution. The project was deemed to be quality improvement and thereby granted exemption by the RRVAMC Institutional Review Board.
Methods
This project took place at the RRVAMC, a 229-bed tertiary academic medical center that serves > 60,000 patients annually. The RRVAMC ED has 20 beds and received about 29,000 visits in 2014. Patients were eligible for initial evaluation if they had a urine culture collected in the ED within the 91-day period from September 1, 2015 to November 30, 2015. Patients were included for data analysis if it was documented that they were treated for actual or clinically suspected, based on signs and symptoms, uncomplicated UTI, complicated UTI, or UTI with pyelonephritis. Patients did not need to have a positive urine culture for inclusion, as infections could still be present despite negative culture results.6 Patients with positive cultures who were not clinically symptomatic of a UTI and were not treated as such by the ED provider (ie, asymptomatic bacteriuria) were excluded from the study.
Data collection took place via daily chart review of patient records in both the Computerized Patient Record System and Decentralized Hospital Computer Program medical applications as urine cultures were performed. Data were gathered and assessed by a postgraduate year-2 internal medicine pharmacy resident on rotation in the ED who reviewed cultures daily and made interventions based on the results as needed. The pharmacy resident was physically present within the ED during the first 30 days of the project. The pharmacy resident was not within the direct practice area during the final 61 days of the project but was in a different area of the hospital and available for consultation.
Primary data collected included urine culture results and susceptibilities, empiric antimicrobial choices, and admission status. Other data collected included duration of treatment and secondary antibiotics chosen, each of which specifically evaluated those patients who were not admitted to the hospital and were thus treated as outpatients. Additional data generated from this study were used to identify empiric antibiotics utilized for the treatment of UTIs and assess for appropriate selection and duration of therapy within this institution.
Results
During the study period, 722 urine cultures were collected in the ED and were included for initial evaluation. Of these, 127 were treated by the ED provider pursuant to one of the indications specified and were included in the data analysis. Treatment with an antimicrobial agent provided adequate coverage for the identified pathogen in 112 patients, yielding a match rate of 88%. As all included cultures were collected in suspicion of an infection, those cultures yielding no growth were considered to have been adequately covered.
Nearly half (45%) of treatment plans included a fluoroquinolone. Of those treated on an outpatient basis, fluoroquinolones were even more frequently used, comprising 50 of 82 (61%) courses. Ciprofloxacin was the most frequently used treatment, used in 39 of the 82 outpatient regimens (48%). Cephalexin was the second most common and was used in 14 outpatient regimens (17%), followed by levofloxacin (15%) (Figure 1).
Mismatched cultures, or those where the prescribed antibiotic did not provide adequate coverage of the identified pathogens based on susceptibilities, occurred at a rate of 12%. Follow-up on these cultures was determined largely by the patient’s admission status. The majority of mismatched cultures were addressed by the inpatient team (10/15) upon admission.
Discussion
Empiric antibiotic selection for the treatment of UTIs continues to be the cornerstone of antibiotic management for the treatment of such a disease state.7 The noted drug-bug match rate of 88% in this study demonstrates effective initial empiric coverage and ensures a vast majority of veterans receive adequate coverage for identified pathogens. Additionally, this rate shows that the current system seems to be functioning appropriately and refutes the author’s preconceived ideas that the mismatch rate was higher at RRVAMC. However, these findings also demonstrate a predominant use of fluoroquinolones for empiric treatment in a majority of patients who could be better served with narrower spectrum agents. Only 2 of the outpatient regimens were for the treatment of pyelonephritis, the only indication in which a fluoroquinolone would be the standard of care per guideline recommendations.7
These findings were consistent with a similar study in which 83% of ED collected urine cultures ultimately grew bacteria susceptibleto empiric treatment.8 This number was similar to the current study despite the latter study consisting of predominantly female patients (93%) and excluding patients with a history of benign prostatic hypertrophy, catheter use, or history of genitourinary cancer, which are frequently found within the VA population. Thus, despite having a differing patient population at the current study’s facility with characteristics that would classify most to be treated as a complicated UTI, empiric coverage rates remained similar. The lower than anticipated intervention rate by the pharmacist on rotation in the ED can be directly attributed to this high empiric match rate, which could in turn be attributed to the extensive use of broad-spectrum antibiotics for treatment.
Empiric antimicrobial selection is based largely on local resistance patterns.7 Of particular importance is the resistance patterns of E coli, as it is the primary isolate responsible for UTIs worldwide. Thus, it is not unexpected that the most frequently isolated pathogen in the current study also was E coli. While clinical practice guidelines state that hospital-wide antibiograms often are skewed by cultures collected from inpatients or those with complicated infection, the current study found hospital-wide E coli resistance patterns, specifically those related to fluoroquinolone use, to be similar to those collected in the ED alone (78.5% hospital-wide susceptibility vs. 75% ED susceptibility). This was expected, as similar studies comparing E coli resistance patterns from ED-collected urine cultures to those institution-wide also have found similar rates of resistance.8,9 These findings are of particular importance as E coli resistance is noted to be increasing, varies with geographic area, and local resistance patterns are rarely known.7 Thus, these findings may aid ED providers in their empiric antimicrobial selections.
Ciprofloxacin was the most frequently used medication for the treatment of UTIs. While overall empiric selections were found to have favorable resistance patterns, it is difficult to interpret the appropriateness of ciprofloxacin’s use in the present study. First, there is a distinct lack of US-based clinical practice guidelines for the treatment of complicated UTIs. As the majority of this study population was male, it is difficult to directly extrapolate from the current Infectious Diseases Society of America treatment guidelines for uncomplicated cystitis and apply to the study population. Although recommended for the treatment of pyelonephritis, it is unclear whether ciprofloxacin should be utilized as a first-line empiric option for the treatment of UTIs in males.
Despite the lack of disease-specific recommendations for ciprofloxacin, recommendations exist regarding its use when local resistance patterns are known.7 It is currently recommended that these agents not be used when resistance rates of E coli exceed 20% for trimethoprim-sulfamethoxazole or 10% for fluoroquinolones. As this study demonstrated a nearly 25% resistance rate for E coli to fluoroquinolones in both the ED and institution-wide sample populations, it could potentially be ascertained that ciprofloxacin is an inappropriate choice for the empiric treatment of UTIs in this patient population. However, as noted, it is unknown whether this recommendation would still be applicable when applied to the treatment of complicated cystitis and greater male population, as overall rates of susceptible cultures to all organisms was similar to other published studies.8,9
While there is scant specific guidance related to the treatment of complicated UTIs, there is emerging guidance on the use of fluoroquinolones, both in general and specifically related to the treatment of UTIs. In July 2016, the FDA issued a drug safety communication regarding the use of and warnings for fluoroquinolones, which explicitly stated that “health care professionals should not prescribe systemic fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated UTIs because the risks outweigh the benefits in these patients.”10
This guidance has the potential to impact fluoroquinolone prescribing significantly at RRVAMC. Given the large number of fluoroquinolones prescribed for UTIs, the downstream effects that this shift in prescribing would have is unknown. As most nonfluoroquinolones used for UTI typically are narrower in antimicrobial spectrum (eg, trimethoprim/sulfamethoxazole, nitrofurantoin, cephalexin, etc) the possibility exists that the match rate for empiric therapy may decrease. Thus, a larger need for closer follow-up to assure adequate coverage may arise, posing a more expanded role for an ED-based pharmacist than was demonstrated in the current study.
This new guidance also may place providers in an area of larger uncertainty with regards to treating both complicated and uncomplicated cystitis. Given the enhanced warnings on fluoroquinolone use, it is unknown whether prescribers would gravitate to utilizing similar options as their peers as alternatives to fluoroquinolones. Similarly, duration of therapy with nonfluoroquinolone agents is unclear as well; as the present study demonstrated a large range in treatment duration of outpatients (3-14 days). While the average observed duration of 8.3 days is intuitively fitting, as the majority of cases were in males, no published guideline exists that affirms the appropriateness of this finding. Such uncertainty and potential inconsistency between providers affords a large opportunity for developing a standardized treatment pathway for the treatment of UTIs to ensure both effective and guideline concordant treatment for patients, specifically with regards to antimicrobial selection and duration of treatment.
It is noteworthy to mention that all follow-ups on positive cultures inadequately covered by empiric therapy took place on the day organism identification and susceptibility data were released. This finding was somewhat surprising, as it was originally theorized that most ED-collected urine cultures were not monitored to completion by a pharmacist and that would be necessary in order to ensure proper follow-up of culture results. What is not clear is whether there is a robust process for the follow-up of urine cultures in the ED. Most of the bug-drug mismatches coincidentally were admitted to the inpatient teams where there were appropriate personnel to follow up and adjust the antibiotic selection. If there was a bug-drug mismatch, and the patient was not admitted, it is unclear whether there is a consistent process for follow-up.
Given the limited number of mismatched cultures that required change in therapy, it is unknown if this role would expand if more narrow-spectrum agents were utilized, theoretically leading to a higher mismatch rate and necessitating closer follow-up. Furthermore, given the common practice of mailed prescriptions at the VA, it is all the more imperative that the cultures be acted upon on the day they were identified, as the mailing and processing time of prescriptions may limit the clinical utility in switching from a more broad-spectrum agent, to one more targeted for an identified organism. While a patient traveling back to the medical center for expedited prescription pickup at the pharmacy would alleviate this problem, many patients at the facility travel great distances or may not have readily available travel means to return to the medical center.
Future Directions
While minimal follow-up was required after a patient had left the ED, this study demonstrated a fundamental need for further refinements in antimicrobial stewardship activities within the ED. Duration of therapy, empiric selection, and proper dosing are key areas where the ED-based pharmacy resident was able to intervene during the time physically stationed in the ED. The data collected from this study demonstrated this and was ultimately combined with other ED-based interventions and utilized as supporting evidence in the pharmacy service business plan, outlining the necessity of a full-time pharmacy presence in the ED. The business plan submission, along with other ongoing RRVAMC initiatives, ultimately led to the approval for clinical pharmacy specialists to expand practice into the ED. These positions will continue to advance pharmacy practice within the ED, while affording opportunities for pharmacists to practice at the top of their licensure, provide individualized provider education, and deliver real-time antimicrobial stewardship interventions. Furthermore, as the majority of the study period was monitored outside of the ED, the project may provide a model for other VA institutions without full-time ED pharmacists to implement as a means to improve antimicrobial stewardship and further build an evidence base for expanding their pharmacy services to the ED.
Given the large number of fluoroquinolones utilized in the ED, this study has raised the question of what prescribing patterns look like with regards to outpatient UTI treatment within the realm of primary care at RRVAMC. Despite the great strides made with regards to antimicrobial stewardship at this facility on the inpatient side, no formal antimicrobial stewardship program exists for review in the outpatient setting, where literature suggests the majority of antibiotics are prescribed.3,11 While more robust protocols are in place for follow-up of culture data in the primary care realm at this facility, the prescribing patterns are relatively unknown.
A recent study completed at a similar VA facility found that 60% of antibiotics prescribed for cystitis, pharyngitis, or sinusitis on an outpatient basis were guideline-discordant, and CDC guidance has further recommended specific focus should be undertaken with regards to outpatient stewardship practices in the treatment of genitourinary infections.3,12 These findings highlight the need for outpatient antimicrobial stewardship and presents a compelling reason to further investigate outpatient prescribing within primary care at RRVAMC.
Strengths and Limitations
Strengths of the current study include the ability to monitor urine cultures in real time and to provide timely interventions in the event of a rare bug-drug mismatch. The evaluation of cultures in this study shows that the majority of cases had a drug selected with adequate coverage. The study did assure ED providers that, even though guidelines may suggest otherwise, urine cultures drawn in the ED at RRVAMC followed similar resistance patterns seen for the facility as a whole. Moreover, it is valuable as it captures data that are directly applicable to the VA patient population, in which there is little published data with regards to UTI treatment and no formal VA guidance.
A primary limitation of this study is the lack of differentiation between cultures collected from patients with or without indwelling catheters. However, only including patients who presented with signs and/or symptoms of a UTI limits the number of cultures that could potentially be deemed as colonization, thus minimizing the potential for nonpathogenic organisms to confound the results. This study also did not differentiate the setting from which the patient presented (eg, community, extended care facility, etc) that could have potentially provided guidance on resistance patterns for community-acquired UTIs and whether this may have differed from hospital-acquired or facility-acquired UTIs. Another limitation was the relatively short time frame for data collection. A data collection period greater than 91 days would allow for a larger sample size, thus making the data more robust and potentially allowing for the identification of other trends not seen in the current study. A longer data collection period also would have afforded the opportunity to track more robust clinical outcomes throughout the study, identifying whether treatment failure may have been linked to the use of certain classes or spectrums of activity of antibiotics.
Conclusion
Despite the E coli resistance rate to ciprofloxacin (> 20%), the empiric treatments chosen were > 85% effective, needing minimal follow-up once a patient left the ED. Nonetheless, a change in prescribing patterns based on recent national recommendations may provide expanded opportunities in antimicrobial stewardship for ED-based pharmacists. Further research is needed in antimicrobial stewardship within this facility’s outpatient primary care realm, potentially uncovering other opportunities for pharmacist intervention to assure guideline concordant care for the treatment of UTIs as well as other infections treated in primary care patients.
On September 18, 2014, President Barack Obama signed an executive order that made addressing antibiotic-resistant bacteria a national security policy.1 This legislation resulted in the creation of a large multidepartment task force to combat the global and domestic problem of antimicrobial resistance. The order required hospitals and other inpatient health care delivery facilities, including the Department of Veterans Affairs (VA), to implement robust antimicrobial stewardship programs that adhere to best practices, such as those identified by the Centers for Disease Control and Prevention (CDC). More specifically, the VA was mandated to take steps to encourage other areas of health care, such as ambulatory surgery centers and outpatient clinics, to adopt antimicrobial stewardship programs.1 This order also reinforced the importance for VA facilities to continue to develop, improve, and sustain efforts in antimicrobial stewardship.
Prior to the order, in 2012 the Richard L. Roudebush VA Medical Center (RRVAMC) in Indianapolis, Indiana, implemented an inpatient antimicrobial stewardship program that included thrice-weekly meetings to review inpatient records and make stewardship recommendations with an infectious diseases physician champion and clinical pharmacists. These efforts led to the improved use of antimicrobial agents on the inpatient side of the medical center. During the first 4 years of implementation, the program helped to decrease the defined daily doses of broad-spectrum antibiotics per 1,000 patient days nearly 36%, from 532 in 2012 to 343 in 2015, as well as decrease the days of therapy of fluoroquinolones per 1,000 patient days 28.75%, from 80 in 2012 to 57 in 2015. Additionally, the program showed a significant decrease in the standardized antimicrobial administration ratio, a benchmark measure developed by the CDC to reflect a facility’s actual antimicrobial use to the expected use of a similar facility based on bed size, number of intensive care unit beds, location type, and medical school affiliation.2
While the RRVAMC antimicrobial stewardship team has been able to intervene on most of the inpatients admitted to the medical center, the outpatient arena has had few antimicrobial stewardship interventions. Recognizing a need to establish and expand pharmacy services and for improvement of outpatient antimicrobial stewardship, RRVAMC leadership decided to establish a pharmacist-led outpatient antimicrobial surveillance program, starting specifically within the emergency department (ED).
Clinical pharmacists in the ED setting are uniquely positioned to improve patient care and encourage the judicious use of antimicrobials for empiric treatment of urinary tract infections (UTIs). The CDC’s Core Elements of Outpatient Antibiotic Stewardship recommends pharmacist availability in the ED setting, and previous literature has demonstrated pharmacist utility in ED postdischarge culture monitoring and surveillance.3-5
This article will highlight one such program review at the RRVAMC and demonstrate the need for pharmacist-led antimicrobial stewardship and monitoring in the ED. The purpose of this study was to test the hypothesis that pharmacist intervention would be necessary to prospectively check for “bug-drug mismatch” and assure proper follow-up of urine cultures at this institution. The project was deemed to be quality improvement and thereby granted exemption by the RRVAMC Institutional Review Board.
Methods
This project took place at the RRVAMC, a 229-bed tertiary academic medical center that serves > 60,000 patients annually. The RRVAMC ED has 20 beds and received about 29,000 visits in 2014. Patients were eligible for initial evaluation if they had a urine culture collected in the ED within the 91-day period from September 1, 2015 to November 30, 2015. Patients were included for data analysis if it was documented that they were treated for actual or clinically suspected, based on signs and symptoms, uncomplicated UTI, complicated UTI, or UTI with pyelonephritis. Patients did not need to have a positive urine culture for inclusion, as infections could still be present despite negative culture results.6 Patients with positive cultures who were not clinically symptomatic of a UTI and were not treated as such by the ED provider (ie, asymptomatic bacteriuria) were excluded from the study.
Data collection took place via daily chart review of patient records in both the Computerized Patient Record System and Decentralized Hospital Computer Program medical applications as urine cultures were performed. Data were gathered and assessed by a postgraduate year-2 internal medicine pharmacy resident on rotation in the ED who reviewed cultures daily and made interventions based on the results as needed. The pharmacy resident was physically present within the ED during the first 30 days of the project. The pharmacy resident was not within the direct practice area during the final 61 days of the project but was in a different area of the hospital and available for consultation.
Primary data collected included urine culture results and susceptibilities, empiric antimicrobial choices, and admission status. Other data collected included duration of treatment and secondary antibiotics chosen, each of which specifically evaluated those patients who were not admitted to the hospital and were thus treated as outpatients. Additional data generated from this study were used to identify empiric antibiotics utilized for the treatment of UTIs and assess for appropriate selection and duration of therapy within this institution.
Results
During the study period, 722 urine cultures were collected in the ED and were included for initial evaluation. Of these, 127 were treated by the ED provider pursuant to one of the indications specified and were included in the data analysis. Treatment with an antimicrobial agent provided adequate coverage for the identified pathogen in 112 patients, yielding a match rate of 88%. As all included cultures were collected in suspicion of an infection, those cultures yielding no growth were considered to have been adequately covered.
Nearly half (45%) of treatment plans included a fluoroquinolone. Of those treated on an outpatient basis, fluoroquinolones were even more frequently used, comprising 50 of 82 (61%) courses. Ciprofloxacin was the most frequently used treatment, used in 39 of the 82 outpatient regimens (48%). Cephalexin was the second most common and was used in 14 outpatient regimens (17%), followed by levofloxacin (15%) (Figure 1).
Mismatched cultures, or those where the prescribed antibiotic did not provide adequate coverage of the identified pathogens based on susceptibilities, occurred at a rate of 12%. Follow-up on these cultures was determined largely by the patient’s admission status. The majority of mismatched cultures were addressed by the inpatient team (10/15) upon admission.
Discussion
Empiric antibiotic selection for the treatment of UTIs continues to be the cornerstone of antibiotic management for the treatment of such a disease state.7 The noted drug-bug match rate of 88% in this study demonstrates effective initial empiric coverage and ensures a vast majority of veterans receive adequate coverage for identified pathogens. Additionally, this rate shows that the current system seems to be functioning appropriately and refutes the author’s preconceived ideas that the mismatch rate was higher at RRVAMC. However, these findings also demonstrate a predominant use of fluoroquinolones for empiric treatment in a majority of patients who could be better served with narrower spectrum agents. Only 2 of the outpatient regimens were for the treatment of pyelonephritis, the only indication in which a fluoroquinolone would be the standard of care per guideline recommendations.7
These findings were consistent with a similar study in which 83% of ED collected urine cultures ultimately grew bacteria susceptibleto empiric treatment.8 This number was similar to the current study despite the latter study consisting of predominantly female patients (93%) and excluding patients with a history of benign prostatic hypertrophy, catheter use, or history of genitourinary cancer, which are frequently found within the VA population. Thus, despite having a differing patient population at the current study’s facility with characteristics that would classify most to be treated as a complicated UTI, empiric coverage rates remained similar. The lower than anticipated intervention rate by the pharmacist on rotation in the ED can be directly attributed to this high empiric match rate, which could in turn be attributed to the extensive use of broad-spectrum antibiotics for treatment.
Empiric antimicrobial selection is based largely on local resistance patterns.7 Of particular importance is the resistance patterns of E coli, as it is the primary isolate responsible for UTIs worldwide. Thus, it is not unexpected that the most frequently isolated pathogen in the current study also was E coli. While clinical practice guidelines state that hospital-wide antibiograms often are skewed by cultures collected from inpatients or those with complicated infection, the current study found hospital-wide E coli resistance patterns, specifically those related to fluoroquinolone use, to be similar to those collected in the ED alone (78.5% hospital-wide susceptibility vs. 75% ED susceptibility). This was expected, as similar studies comparing E coli resistance patterns from ED-collected urine cultures to those institution-wide also have found similar rates of resistance.8,9 These findings are of particular importance as E coli resistance is noted to be increasing, varies with geographic area, and local resistance patterns are rarely known.7 Thus, these findings may aid ED providers in their empiric antimicrobial selections.
Ciprofloxacin was the most frequently used medication for the treatment of UTIs. While overall empiric selections were found to have favorable resistance patterns, it is difficult to interpret the appropriateness of ciprofloxacin’s use in the present study. First, there is a distinct lack of US-based clinical practice guidelines for the treatment of complicated UTIs. As the majority of this study population was male, it is difficult to directly extrapolate from the current Infectious Diseases Society of America treatment guidelines for uncomplicated cystitis and apply to the study population. Although recommended for the treatment of pyelonephritis, it is unclear whether ciprofloxacin should be utilized as a first-line empiric option for the treatment of UTIs in males.
Despite the lack of disease-specific recommendations for ciprofloxacin, recommendations exist regarding its use when local resistance patterns are known.7 It is currently recommended that these agents not be used when resistance rates of E coli exceed 20% for trimethoprim-sulfamethoxazole or 10% for fluoroquinolones. As this study demonstrated a nearly 25% resistance rate for E coli to fluoroquinolones in both the ED and institution-wide sample populations, it could potentially be ascertained that ciprofloxacin is an inappropriate choice for the empiric treatment of UTIs in this patient population. However, as noted, it is unknown whether this recommendation would still be applicable when applied to the treatment of complicated cystitis and greater male population, as overall rates of susceptible cultures to all organisms was similar to other published studies.8,9
While there is scant specific guidance related to the treatment of complicated UTIs, there is emerging guidance on the use of fluoroquinolones, both in general and specifically related to the treatment of UTIs. In July 2016, the FDA issued a drug safety communication regarding the use of and warnings for fluoroquinolones, which explicitly stated that “health care professionals should not prescribe systemic fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated UTIs because the risks outweigh the benefits in these patients.”10
This guidance has the potential to impact fluoroquinolone prescribing significantly at RRVAMC. Given the large number of fluoroquinolones prescribed for UTIs, the downstream effects that this shift in prescribing would have is unknown. As most nonfluoroquinolones used for UTI typically are narrower in antimicrobial spectrum (eg, trimethoprim/sulfamethoxazole, nitrofurantoin, cephalexin, etc) the possibility exists that the match rate for empiric therapy may decrease. Thus, a larger need for closer follow-up to assure adequate coverage may arise, posing a more expanded role for an ED-based pharmacist than was demonstrated in the current study.
This new guidance also may place providers in an area of larger uncertainty with regards to treating both complicated and uncomplicated cystitis. Given the enhanced warnings on fluoroquinolone use, it is unknown whether prescribers would gravitate to utilizing similar options as their peers as alternatives to fluoroquinolones. Similarly, duration of therapy with nonfluoroquinolone agents is unclear as well; as the present study demonstrated a large range in treatment duration of outpatients (3-14 days). While the average observed duration of 8.3 days is intuitively fitting, as the majority of cases were in males, no published guideline exists that affirms the appropriateness of this finding. Such uncertainty and potential inconsistency between providers affords a large opportunity for developing a standardized treatment pathway for the treatment of UTIs to ensure both effective and guideline concordant treatment for patients, specifically with regards to antimicrobial selection and duration of treatment.
It is noteworthy to mention that all follow-ups on positive cultures inadequately covered by empiric therapy took place on the day organism identification and susceptibility data were released. This finding was somewhat surprising, as it was originally theorized that most ED-collected urine cultures were not monitored to completion by a pharmacist and that would be necessary in order to ensure proper follow-up of culture results. What is not clear is whether there is a robust process for the follow-up of urine cultures in the ED. Most of the bug-drug mismatches coincidentally were admitted to the inpatient teams where there were appropriate personnel to follow up and adjust the antibiotic selection. If there was a bug-drug mismatch, and the patient was not admitted, it is unclear whether there is a consistent process for follow-up.
Given the limited number of mismatched cultures that required change in therapy, it is unknown if this role would expand if more narrow-spectrum agents were utilized, theoretically leading to a higher mismatch rate and necessitating closer follow-up. Furthermore, given the common practice of mailed prescriptions at the VA, it is all the more imperative that the cultures be acted upon on the day they were identified, as the mailing and processing time of prescriptions may limit the clinical utility in switching from a more broad-spectrum agent, to one more targeted for an identified organism. While a patient traveling back to the medical center for expedited prescription pickup at the pharmacy would alleviate this problem, many patients at the facility travel great distances or may not have readily available travel means to return to the medical center.
Future Directions
While minimal follow-up was required after a patient had left the ED, this study demonstrated a fundamental need for further refinements in antimicrobial stewardship activities within the ED. Duration of therapy, empiric selection, and proper dosing are key areas where the ED-based pharmacy resident was able to intervene during the time physically stationed in the ED. The data collected from this study demonstrated this and was ultimately combined with other ED-based interventions and utilized as supporting evidence in the pharmacy service business plan, outlining the necessity of a full-time pharmacy presence in the ED. The business plan submission, along with other ongoing RRVAMC initiatives, ultimately led to the approval for clinical pharmacy specialists to expand practice into the ED. These positions will continue to advance pharmacy practice within the ED, while affording opportunities for pharmacists to practice at the top of their licensure, provide individualized provider education, and deliver real-time antimicrobial stewardship interventions. Furthermore, as the majority of the study period was monitored outside of the ED, the project may provide a model for other VA institutions without full-time ED pharmacists to implement as a means to improve antimicrobial stewardship and further build an evidence base for expanding their pharmacy services to the ED.
Given the large number of fluoroquinolones utilized in the ED, this study has raised the question of what prescribing patterns look like with regards to outpatient UTI treatment within the realm of primary care at RRVAMC. Despite the great strides made with regards to antimicrobial stewardship at this facility on the inpatient side, no formal antimicrobial stewardship program exists for review in the outpatient setting, where literature suggests the majority of antibiotics are prescribed.3,11 While more robust protocols are in place for follow-up of culture data in the primary care realm at this facility, the prescribing patterns are relatively unknown.
A recent study completed at a similar VA facility found that 60% of antibiotics prescribed for cystitis, pharyngitis, or sinusitis on an outpatient basis were guideline-discordant, and CDC guidance has further recommended specific focus should be undertaken with regards to outpatient stewardship practices in the treatment of genitourinary infections.3,12 These findings highlight the need for outpatient antimicrobial stewardship and presents a compelling reason to further investigate outpatient prescribing within primary care at RRVAMC.
Strengths and Limitations
Strengths of the current study include the ability to monitor urine cultures in real time and to provide timely interventions in the event of a rare bug-drug mismatch. The evaluation of cultures in this study shows that the majority of cases had a drug selected with adequate coverage. The study did assure ED providers that, even though guidelines may suggest otherwise, urine cultures drawn in the ED at RRVAMC followed similar resistance patterns seen for the facility as a whole. Moreover, it is valuable as it captures data that are directly applicable to the VA patient population, in which there is little published data with regards to UTI treatment and no formal VA guidance.
A primary limitation of this study is the lack of differentiation between cultures collected from patients with or without indwelling catheters. However, only including patients who presented with signs and/or symptoms of a UTI limits the number of cultures that could potentially be deemed as colonization, thus minimizing the potential for nonpathogenic organisms to confound the results. This study also did not differentiate the setting from which the patient presented (eg, community, extended care facility, etc) that could have potentially provided guidance on resistance patterns for community-acquired UTIs and whether this may have differed from hospital-acquired or facility-acquired UTIs. Another limitation was the relatively short time frame for data collection. A data collection period greater than 91 days would allow for a larger sample size, thus making the data more robust and potentially allowing for the identification of other trends not seen in the current study. A longer data collection period also would have afforded the opportunity to track more robust clinical outcomes throughout the study, identifying whether treatment failure may have been linked to the use of certain classes or spectrums of activity of antibiotics.
Conclusion
Despite the E coli resistance rate to ciprofloxacin (> 20%), the empiric treatments chosen were > 85% effective, needing minimal follow-up once a patient left the ED. Nonetheless, a change in prescribing patterns based on recent national recommendations may provide expanded opportunities in antimicrobial stewardship for ED-based pharmacists. Further research is needed in antimicrobial stewardship within this facility’s outpatient primary care realm, potentially uncovering other opportunities for pharmacist intervention to assure guideline concordant care for the treatment of UTIs as well as other infections treated in primary care patients.
1. Obama B. Executive order–combating antibiotic-resistant bacteria. https://www.whitehouse.gov/the -press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. Published September 18, 2014. Accessed June 7, 2018.
2. Livorsi DJ, O’Leary E, Pierce T, et al. A novel metric to monitor the influence of antimicrobial stewardship activities. Infect Control Hosp Epidemiol. 2017;38(6):721-723.
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hick LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12.
4. Wymore ES, Casanova TJ, Broekenmeier RL, Martin JK Jr. Clinical pharmacist’s daily role in the emergency department of a community hospital. Am J Health-Syst Pharm. 2008;65(5):395-396, 398-399.
5. Frandzel S. ED pharmacists’ value on display at ASHP Midyear. http://www.pharmacypracticenews.com/ViewArticle.aspx?ses=ogst&d_id=53&a_id=22524. Published February 14, 2013. Accessed June 15, 2018.
6. Heytens S, DeSutter A, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017;23(9)647-652.
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120.
8. Lingenfelter E, Drapkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient urinary tract infection. Am J Emerg Med. 2016;34(8):1600-1603.
9. Zatorski C, Jordan JA, Cosgrove SE, Zocchi M, May L. Comparison of antibiotic susceptibility of Escherichia coli in urinary isolates from an emergency department with other institutional susceptibility data. Am J Health-Syst Pharm. 2015;72(24):2176-2180.
10. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Updated March 8, 2018. Accessed June 13, 2018.
11. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241.
12. Meyer HE, Lund BC, Heintz BH, Alexander B, Egge JA, Livorsi DJ. Identifying opportunities to improve guideline-concordant antibiotic prescribing in veterans with acute respiratory infections or cystitis. Infect Control Hosp Epidemiol. 2017;38(6):724-728.
1. Obama B. Executive order–combating antibiotic-resistant bacteria. https://www.whitehouse.gov/the -press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. Published September 18, 2014. Accessed June 7, 2018.
2. Livorsi DJ, O’Leary E, Pierce T, et al. A novel metric to monitor the influence of antimicrobial stewardship activities. Infect Control Hosp Epidemiol. 2017;38(6):721-723.
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hick LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12.
4. Wymore ES, Casanova TJ, Broekenmeier RL, Martin JK Jr. Clinical pharmacist’s daily role in the emergency department of a community hospital. Am J Health-Syst Pharm. 2008;65(5):395-396, 398-399.
5. Frandzel S. ED pharmacists’ value on display at ASHP Midyear. http://www.pharmacypracticenews.com/ViewArticle.aspx?ses=ogst&d_id=53&a_id=22524. Published February 14, 2013. Accessed June 15, 2018.
6. Heytens S, DeSutter A, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017;23(9)647-652.
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120.
8. Lingenfelter E, Drapkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient urinary tract infection. Am J Emerg Med. 2016;34(8):1600-1603.
9. Zatorski C, Jordan JA, Cosgrove SE, Zocchi M, May L. Comparison of antibiotic susceptibility of Escherichia coli in urinary isolates from an emergency department with other institutional susceptibility data. Am J Health-Syst Pharm. 2015;72(24):2176-2180.
10. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Updated March 8, 2018. Accessed June 13, 2018.
11. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241.
12. Meyer HE, Lund BC, Heintz BH, Alexander B, Egge JA, Livorsi DJ. Identifying opportunities to improve guideline-concordant antibiotic prescribing in veterans with acute respiratory infections or cystitis. Infect Control Hosp Epidemiol. 2017;38(6):724-728.