User login
Preoperative Diabetes Management for Patients Undergoing Elective Surgeries at a Veterans Affairs Medical Center
Preoperative Diabetes Management for Patients Undergoing Elective Surgeries at a Veterans Affairs Medical Center
More than 38 million people in the United States (12%) have diabetes mellitus (DM), though 1 in 5 are unaware they have DM.1 The prevalence among veterans is even more substantial, impacting nearly 25% of those who received care from the US Department of Veterans Affairs (VA).2 DM can lead to increased health care costs in addition to various complications (eg, cardiovascular, renal), especially if left uncontrolled.1,3 similar impact is found in the perioperative period (defined as at or around the time of an operation), as multiple studies have found that uncontrolled preoperative DM can result in worsened surgical outcomes, including longer hospital stays, more infectious complications, and higher perioperative mortality.4-6
In contrast, adequate glycemic control assessed with blood glucose levels has been shown to decrease the incidence of postoperative infections.7 Optimizing glycemic control during hospital stays, especially postsurgery, has become the standard of care, with most health systems establishing specific protocols. In current literature, most studies examining DM management in the perioperative period are focused on postoperative care, with little attention to the preoperative period.4,6,7
One study found that patients with poor presurgery glycemic control assessed by hemoglobin A1c (HbA1c) levels were more likely to remain hyperglycemic during and after surgery. 8 Blood glucose levels < 200 mg/dL can lead to an increased risk of infection and impaired wound healing, meaning a well-controlled HbA1c before a procedure serves as a potential factor for success.9 The 2025 American Diabetes Association (ADA) Standards of Care (SOC) recommendation is to target HbA1c < 8% whenever possible, and some health systems require lower levels (eg, < 7% or 7.5%).10 With that goal in mind and knowing that preoperative hyperglycemia has been shown to be a contributing factor in the delay or cancellation of surgical cases, an argument can be made that attention to preoperative DM management also should be a focus for health care systems performing surgeries.8,9,11
Attention to glucose control during preoperative care offers an opportunity to screen for DM in patients who may not have been screened otherwise and to standardize perioperative DM management. Since DM disproportionately impacts veterans, this is a pertinent issue to the VA. Veterans can be more susceptible to complications if DM is left uncontrolled prior to surgery. To determine readiness for surgery and control of comorbid conditions such as DM before a planned surgery, facilities often perform a preoperative clinic assessment, often in a multidisciplinary clinic.
At Veteran Health Indiana (VHI), a presurgery clinic visit involving the primary surgery service (physician, nurse practitioner, and/or a physician assistant) is conducted 1 to 2 months prior to the planned procedure to determine whether a patient is ready for surgery. During this visit, patients receive a packet with instructions for various tasks and medications, such as applying topical antibiotic prophylaxis on the anticipated surgical site. This is documented in the form of a note in the VHI Computerized Patient Record System (CPRS). The medication instructions are provided according to the preferences of the surgical team. These may be templated notes that contain general directions on the timing and dosing of specific medications, in addition to instructions for holding or reducing doses when appropriate. The instructions can be tailored by the team conducting the preoperative visit (eg, “Take 20 units of insulin glargine the day before surgery” vs “Take half of your long-acting insulin the night before surgery”). Specific to DM, VHI has a nurse-driven day of surgery glucose assessment where point-of-care blood glucose is collected during preoperative holding for most patients.
There is limited research assessing the level of preoperative glycemic control and the incidence of complications in a veteran population. The objective of this study was to gain a baseline understanding of what, if any, standardization exists for preoperative instructions for DM medications and to assess the level of preoperative glycemic control and postoperative complications in patients with DM undergoing major elective surgical procedures.
Methods
This retrospective, single-center chart review was conducted at VHI. The Indiana University and VHI institutional review boards determined that this quality improvement project was exempt from review.
The primary outcome was the number of patients with surgical procedures delayed or canceled due to hyperglycemia or hypoglycemia. Hyperglycemia was defined as blood glucose > 180 mg/dL and hypoglycemia was defined as < 70 mg/dL, slight variations from the current ADA SOC preoperative specific recommendation of a blood glucose reading of 100 to 180 mg/dL within 4 hours of surgery.10 The standard outpatient hypoglycemia definition of blood glucose < 70 mg/dL was chosen because the current goal (< 100 mg/dL) was not the standard in previous ADA SOCs that were in place during the study period. Specifically, the 2018 ADA SOC did not provide preoperative recommendations and the 2019-2021 ADA SOC recommended 80 to 180 mg/dL.10,12-18 For patients who had multiple preoperative blood glucose measurements, the first recorded glucose on the day of the procedure was used.
The secondary outcomes of this study were focused on the preoperative process/care at VHI and postoperative glycemic control. The preoperative process included examining whether medication instructions were given and their quality. Additionally, the number of interventions for hyperglycemia and hypoglycemia were required immediately prior to surgery and the average preoperative HbA1c (measured within 3 months prior to surgery) were collected and analyzed. For postoperative glycemic control, average blood glucose measurements and number of hypoglycemic (< 70 mg/dL) and hyperglycemic (> 180 mg/dL) events were measured in addition to the frequency of changes made at discharge to patients’ DM medication regimens.
The safety outcome of this study assessed commonly observed postoperative complications and was examined up to 30 days postsurgery. These included acute kidney injury (defined using Kidney Disease: Improving Global Outcomes 2012, the standard during the study period), nonfatal myocardial infarction, nonfatal stroke, and surgical site infections, which were identified from the discharge summary written by the primary surgery service.19 All-cause mortality also was collected.
Patients were included if they were admitted for major elective surgeries and had a diagnosis of either type 1 or type 2 DM on their problem list, determined by International Classification of Diseases, Tenth Revision codes. Major elective surgery was defined as a procedure that would likely result in a hospital admission of > 24 hours. Of note, patients may have been included in this study more than once if they had > 1 procedure at least 30 days apart and met inclusion criteria within the time frame. Patients were excluded if they were taking no DM medications or chronic steroids (at any dose), residing in a long-term care facility, being managed by a non-VA clinician prior to surgery, or missing a preoperative blood glucose measurement.
All data were collected from the CPRS. A list of surgical cases involving patients with DM who were scheduled to undergo major elective surgeries from January 1, 2018, to December 31, 2021, at VHI was generated. The list was randomized to a smaller number (N = 394) for data collection due to the time and resource constraints for a pharmacy residency project. All data were deidentified and stored in a secured VA server to protect patient confidentiality. Descriptive statistics were used for all results.
Results
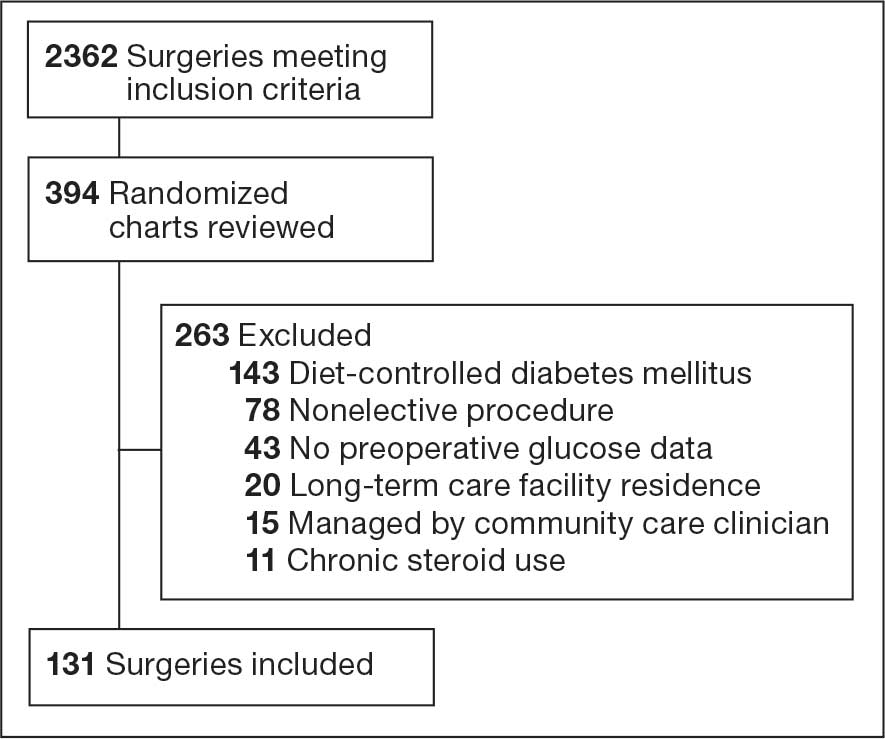
Initially, 2362 surgeries were identified. A randomized sample of 394 charts were reviewed and 131 cases met inclusion criteria. Each case involved a unique patient (Figure). The most common reasons for exclusion were 143 patients with diet-controlled DM and 78 nonelective surgeries. The mean (SD) age of patients was 68 (8) years, and the most were male (98.5%) and White (76.3%) (Table 1).
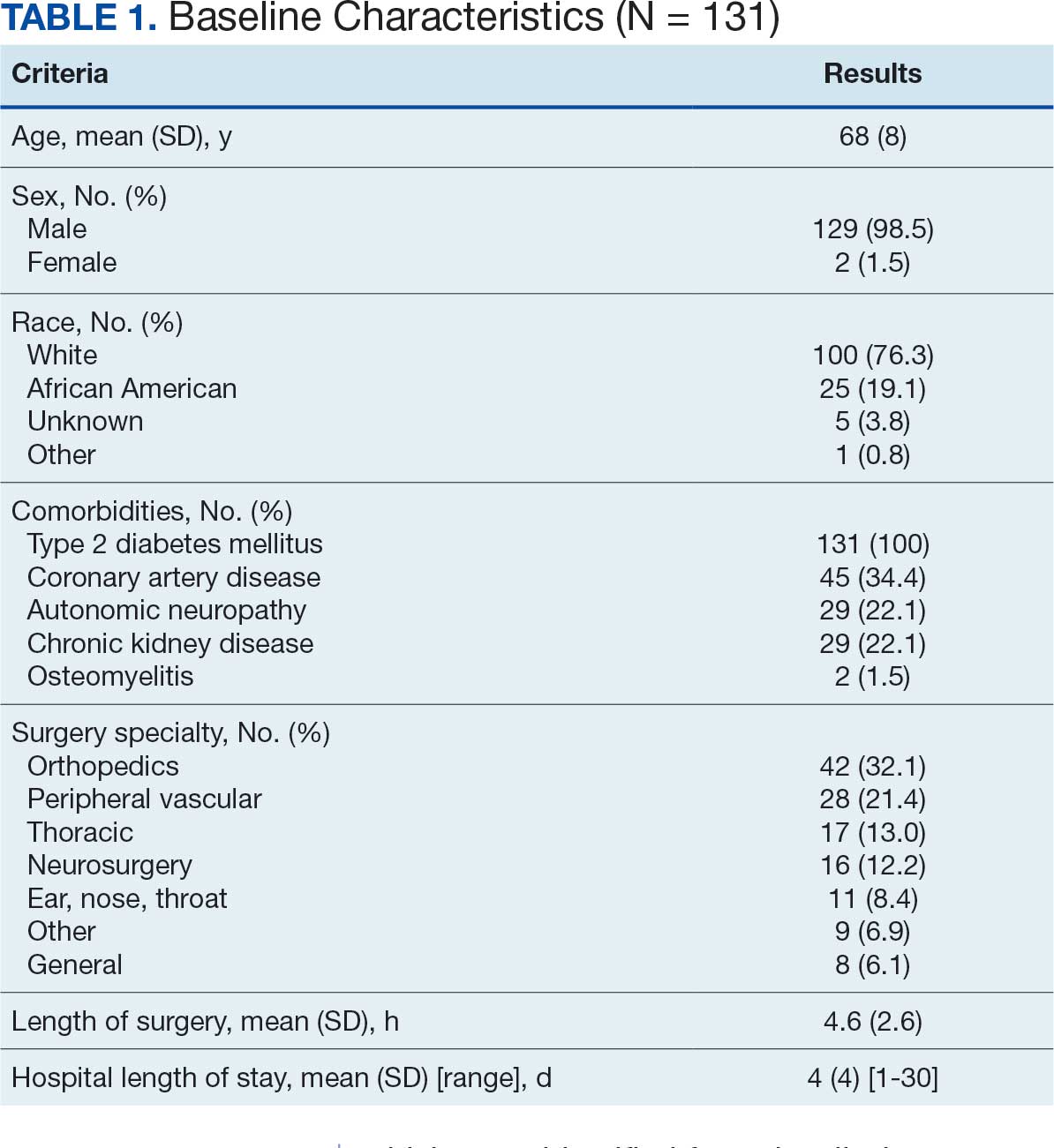
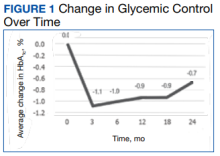
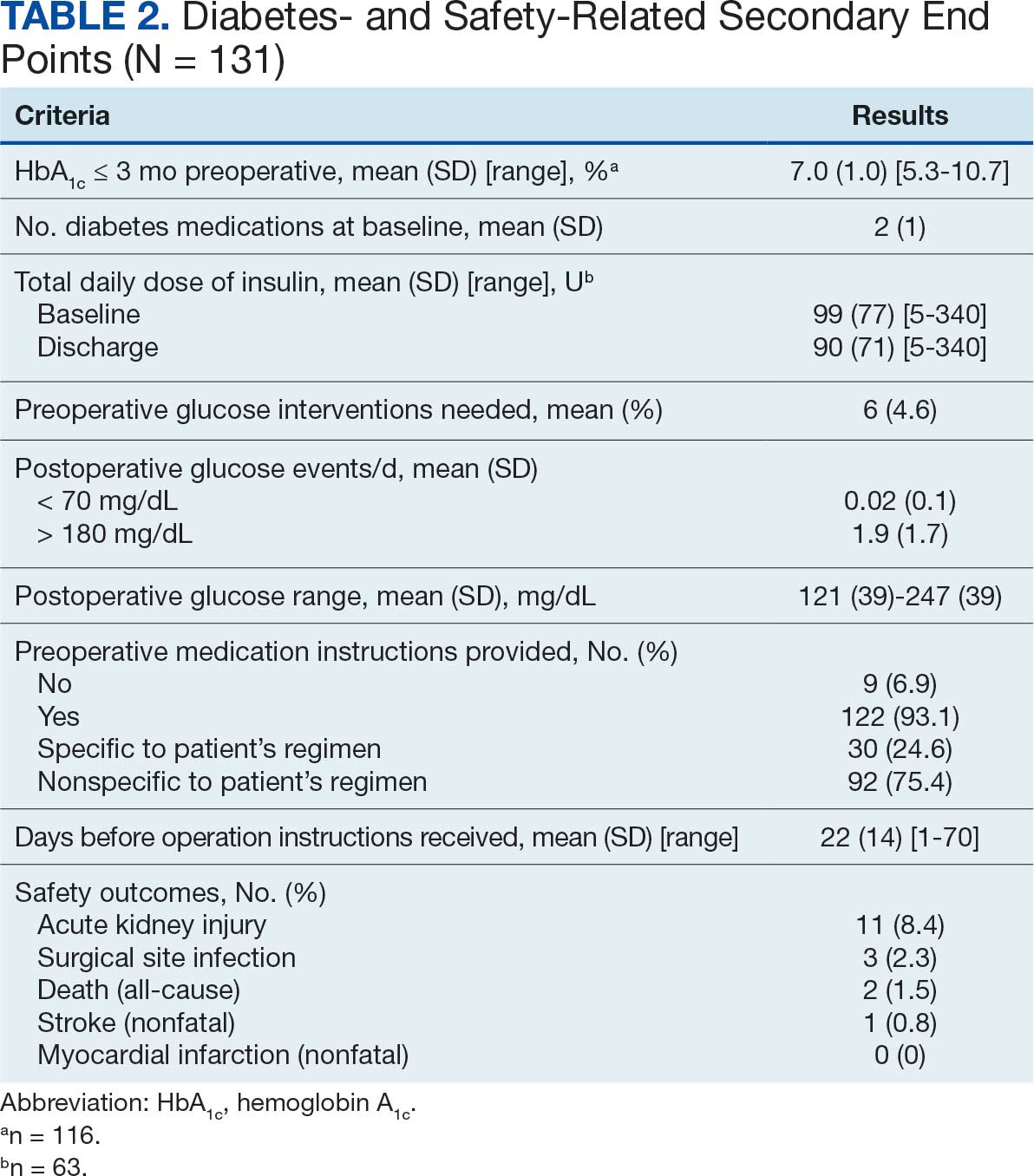
At baseline, 45 of 131 patients (34.4%) had coronary artery disease and 29 (22.1%) each had autonomic neuropathy and chronic kidney disease. Most surgeries were conducted by orthopedic (32.1%) and peripheral vascular (21.4%) specialties. The mean (SD) length of surgery was 4.6 (2.6) hours and of hospital length of stay was 4 (4) days. No patients stayed longer than the 30-day safety outcome follow-up period. All patients had type 2 DM and took a mean 2 DM medications. The 63 patients taking insulin had a mean (SD) total daily dose of 99 (77) U (Table 2). A preoperative HbA1c was collected in 116 patients within 3 months of surgery, with a mean HbA1c of 7.0% (range, 5.3-10.7).
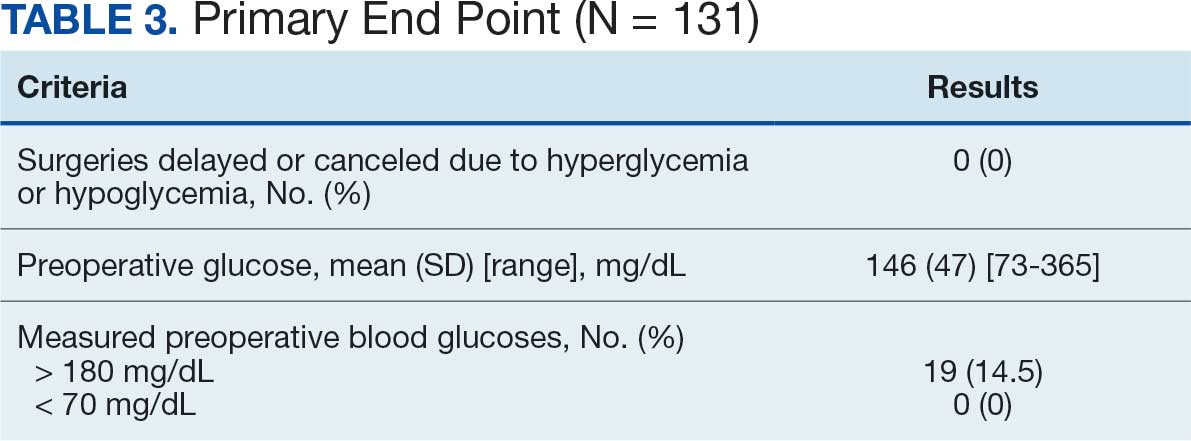
No patients had surgeries delayed or canceled because of uncontrolled DM on the day of surgery. The mean preoperative blood glucose level was 146 mg/dL (range, 73-365) (Table 3). No patients had a preoperative blood glucose level of < 70 mg/dL and 19 (14.5%) had a blood glucose level > 180 mg/dL. Among patients with hyperglycemia immediately prior to surgery, 6 (31.6%) had documentation of insulin being provided.
For this sample of patients, the preoperative clinic visit was conducted a mean 22 days prior to the planned surgery date. Among the 131 included patients, 122 (93.1%) had documentation of receiving instructions for DM medications. Among patients who had documented receipt of instructions, only 30 (24.6%) had instructions specifically tailored to their regimen rather than a generic templated form. The mean (SD) preoperative blood glucose was similar for those who received specific perioperative DM instructions at 146 (50) mg/dL when compared with those who did not at 147 (45) mg/dL. The mean (SD) preoperative blood glucose reading for those who had no documentation of receipt of perioperative instructions was 126 (54) mg/dL compared with 147 (46) mg/dL for those who did.
The mean number of postoperative blood glucose events per day was negligible for hypoglycemia and more frequent for hyperglycemia with a mean of 2 events per day. The mean postoperative blood glucose range was 121 to 247 mg/dL with most readings < 180 mg/dL. Upon discharge, most patients continued their home DM regimen with 5 patients (3.8%) having changes made to their regimen upon discharge.
Very few postoperative complications were identified from chart review. The most frequently observed postoperative complications were acute kidney injury, surgical site infections, and nonfatal stroke. There were no documented nonfatal myocardial infarctions. Two patients (1.5%) died within 30 days of the surgery; neither death was deemed to have been related to poor perioperative glycemic control.
Discussion
To our knowledge, this retrospective chart review was the first study to assess preoperative DM management and postoperative complications in a veteran population. VHI is a large, tertiary, level 1a, academic medical center that serves approximately 62,000 veterans annually and performs about 5000 to 6000 surgeries annually, a total that is increasing following the COVID-19 pandemic.20 This study found that the current process of a presurgery clinic visit and day of surgery glucose assessment has prevented surgical delays or cancellations.
Most patients included in this study were well controlled at baseline in accordance with the 2025 ADA SOC HbA1c recommendation of a preoperative HbA1c of < 8%, which may have contributed to no surgical delays or cancellations.10 However, not all patients had HbA1c collected within 3 months of surgery or even had one collected at all. Despite the ADA SOC providing no explicit recommendation for universal HbA1c screening prior to elective procedures, its importance cannot be understated given the body of evidence demonstrating poor outcomes with uncontrolled preoperative DM.8,10 The glycemic control at baseline may have contributed to the very few postsurgical complications observed in this study.
Although the current process at VHI prevented surgical delays and cancellations in this sample, there are still identified areas for improvement. One area is the instructions the patients received. Patients with DM are often prescribed ≥ 1 medication or a combination of insulins, noninsulin injectables, and oral DM medications, and this study population was no different. Because these medications may influence the anesthesia and perioperative periods, the ADA has specific guidance for altering administration schedules in the days leading up to surgery.10
Inappropriate administration of DM medications could lead to perioperative hypoglycemia or hyperglycemia, possibly causing surgical delays, case cancellations, and/or postoperative complications.21 Although these data reveal the specificity and documented receipt that the preoperative DM instructions did not impact the first recorded preoperative blood glucose, future studies should examine patient confidence in how to properly administer their DM medications prior to surgery. It is vital that patients receive clear instructions in accordance with the ADA SOC on whether to continue, hold, or adjust the dose of their medications to prevent fluctuations in blood glucose levels in the perioperative period, ensure safety with anesthesia, and prevent postoperative complications such as acute kidney injury. Of note, compliance with guideline recommendations for medication instructions was not examined because the data collection time frame expanded over multiple years and the recommendations have evolved each year as new data emerge.
Preoperative DM Management
The first key takeaway from this study is to ensure patients are ready for surgery with a formal assessment (typically in the form of a clinic visit) prior to the surgery. One private sector health system published their approach to this by administering an automatic preoperative HbA1c screening for those with a DM diagnosis and all patients with a random plasma glucose ≥ 200 mg/dL.22 Additionally, if the patient's HbA1c level was not at goal prior to surgery (≥ 8% for those with known DM and ≥ 6.5% with no known DM), patients were referred to endocrinology for further management. Increasing attention to the preoperative visit and extending HbA1c testing to all patients regardless of DM status also provides an opportunity to identify individuals living with undiagnosed DM.1
Even though there was no difference in the mean preoperative blood glucose level based on receipt or specificity of preoperative DM instructions, a second takeaway from this study is the importance of ensuring patients receive clear instructions on their DM medication schedule in the perioperative period. A practical first step may be updating the templates used by the primary surgery teams and providing education to the clinicians in the clinic on how to personalize the visits. Because the current preoperative DM process at VHI is managed by the primary surgical team in a clinic visit, there is an opportunity to shift this responsibility to other health care professionals, such as pharmacists—a change shown to reduce unintended omission of home medications following surgery during hospitalization and reduce costs.23,24
Limitations
This study relied on data included in the patient chart. These data include medication interventions made immediately prior to surgery, which can sometimes be inaccurately charted or difficult to find as they are not documented in the typical medication administration record. Also, the safety outcomes were collected from a discharge summary written by different clinicians, which may lead to information bias. Special attention was taken to ensure these data points were collected as accurately as possible, but it is possible some data may be inaccurate from unintentional human error. Additionally, the safety outcome was limited to a 30-day follow-up, but encompassed the entire length of postoperative stay for all included patients. Finally, given this study was retrospective with no comparison group and the intent was to improve processes at VHI, only hypotheses and potential interventions can be generated from this study. Future prospective studies with larger sample sizes and comparator groups are needed to draw further conclusions.
Conclusions
This study found that the current presurgery process at VHI appears to be successful in preventing surgical delays or cancellations due to hyperglycemia or hypoglycemia. Optimizing DM management can improve surgical outcomes by decreasing rates of postoperative complications, and this study added additional evidence in support of that in a unique population: veterans. Insight on the awareness of preoperative blood glucose management should be gleaned from this study, and based on this sample and site, the preadmission screening process and instructions provided to patients can serve as 2 starting points for optimizing elective surgery.
- Centers for Disease Control and Prevention. Diabetes basics. May 15, 2024. Accessed September 24, 2025. https://www.cdc.gov/diabetes/about/index.html
- Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135. doi:10.5888/pcd14.170230
- Farmaki P, Damaskos C, Garmpis N, et al . Complications of the Type 2 Diabetes Mellitus. Curr Cardiol Rev. 2020;16(4):249-251. doi:10.2174/1573403X1604201229115531
- Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783-1788. doi:10.2337/dc10-0304
- Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156:137 -142. doi:10.1530/eje.1.02321
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77-81. doi:10.1177/01486071980220027
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34:256-261. doi:10.2337/dc10-1407
- Pasquel FJ, Gomez-Huelgas R, Anzola I, et al. Predictive value of admission hemoglobin A1c on inpatient glycemic control and response to insulin therapy in medicine and surgery patients with type 2 diabetes. Diabetes Care. 2015;38:e202-e203. doi:10.2337/dc15-1835
- Alexiewicz JM, Kumar D, Smogorzewski M, et al. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med. 1995;123:919-924. doi:10.7326/0003-4819-123-12-199512150-00004
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2025. Diabetes Care. 2025;48(1 suppl 1):S321-S334. doi:10.2337/dc25-S016
- Kumar R, Gandhi R. Reasons for cancellation of operation on the day of intended surgery in a multidisciplinary 500 bedded hospital. J Anaesthesiol Clin Pharmacol. 2012;28:66-69. doi:10.4103/0970-9185.92442
- American Diabetes Association. 14. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2018. Diabetes Care. 2018;41(1 suppl 1):S144- S151. doi:10.2337/dc18-S014
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2019. Diabetes Care. 2019;42(suppl 1):S173- S181. doi:10.2337/dc19-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2020. Diabetes Care. 2020;43(suppl 1):S193- S202. doi:10.2337/dc20-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2021. Diabetes Care. 2021;44(suppl 1):S211- S220. doi:10.2337/dc21-S015
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
- ElSayed NA, Aleppo G, Aroda VR, et al. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(suppl 1):S267-S278. doi:10.2337/dc23-S016
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(suppl 1):S295-S306. doi:10.2337/dc24-S016
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138. Accessed September 24, 2025. https:// www.kisupplements.org/issue/S2157-1716(12)X7200-9
- US Department of Veterans Affairs. VA Indiana Healthcare: about us. Accessed September 24, 2025. https:// www.va.gov/indiana-health-care/about-us/
- Koh WX, Phelan R, Hopman WM, et al. Cancellation of elective surgery: rates, reasons and effect on patient satisfaction. Can J Surg. 2021;64:E155-E161. doi:10.1503/cjs.008119
- Pai S-L, Haehn DA, Pitruzzello NE, et al. Reducing infection rates with enhanced preoperative diabetes mellitus diagnosis and optimization processes. South Med J. 2023;116:215-219. doi:10.14423/SMJ.0000000000001507
- Forrester TG, Sullivan S, Snoswell CL, et al. Integrating a pharmacist into the perioperative setting. Aust Health Rev. 2020;44:563-568. doi:10.1071/AH19126
- Hale AR, Coombes ID, Stokes J, et al. Perioperative medication management: expanding the role of the preadmission clinic pharmacist in a single centre, randomised controlled trial of collaborative prescribing. BMJ Open. 2013;3:e003027. doi:10.1136/bmjopen-2013-003027
More than 38 million people in the United States (12%) have diabetes mellitus (DM), though 1 in 5 are unaware they have DM.1 The prevalence among veterans is even more substantial, impacting nearly 25% of those who received care from the US Department of Veterans Affairs (VA).2 DM can lead to increased health care costs in addition to various complications (eg, cardiovascular, renal), especially if left uncontrolled.1,3 similar impact is found in the perioperative period (defined as at or around the time of an operation), as multiple studies have found that uncontrolled preoperative DM can result in worsened surgical outcomes, including longer hospital stays, more infectious complications, and higher perioperative mortality.4-6
In contrast, adequate glycemic control assessed with blood glucose levels has been shown to decrease the incidence of postoperative infections.7 Optimizing glycemic control during hospital stays, especially postsurgery, has become the standard of care, with most health systems establishing specific protocols. In current literature, most studies examining DM management in the perioperative period are focused on postoperative care, with little attention to the preoperative period.4,6,7
One study found that patients with poor presurgery glycemic control assessed by hemoglobin A1c (HbA1c) levels were more likely to remain hyperglycemic during and after surgery. 8 Blood glucose levels < 200 mg/dL can lead to an increased risk of infection and impaired wound healing, meaning a well-controlled HbA1c before a procedure serves as a potential factor for success.9 The 2025 American Diabetes Association (ADA) Standards of Care (SOC) recommendation is to target HbA1c < 8% whenever possible, and some health systems require lower levels (eg, < 7% or 7.5%).10 With that goal in mind and knowing that preoperative hyperglycemia has been shown to be a contributing factor in the delay or cancellation of surgical cases, an argument can be made that attention to preoperative DM management also should be a focus for health care systems performing surgeries.8,9,11
Attention to glucose control during preoperative care offers an opportunity to screen for DM in patients who may not have been screened otherwise and to standardize perioperative DM management. Since DM disproportionately impacts veterans, this is a pertinent issue to the VA. Veterans can be more susceptible to complications if DM is left uncontrolled prior to surgery. To determine readiness for surgery and control of comorbid conditions such as DM before a planned surgery, facilities often perform a preoperative clinic assessment, often in a multidisciplinary clinic.
At Veteran Health Indiana (VHI), a presurgery clinic visit involving the primary surgery service (physician, nurse practitioner, and/or a physician assistant) is conducted 1 to 2 months prior to the planned procedure to determine whether a patient is ready for surgery. During this visit, patients receive a packet with instructions for various tasks and medications, such as applying topical antibiotic prophylaxis on the anticipated surgical site. This is documented in the form of a note in the VHI Computerized Patient Record System (CPRS). The medication instructions are provided according to the preferences of the surgical team. These may be templated notes that contain general directions on the timing and dosing of specific medications, in addition to instructions for holding or reducing doses when appropriate. The instructions can be tailored by the team conducting the preoperative visit (eg, “Take 20 units of insulin glargine the day before surgery” vs “Take half of your long-acting insulin the night before surgery”). Specific to DM, VHI has a nurse-driven day of surgery glucose assessment where point-of-care blood glucose is collected during preoperative holding for most patients.
There is limited research assessing the level of preoperative glycemic control and the incidence of complications in a veteran population. The objective of this study was to gain a baseline understanding of what, if any, standardization exists for preoperative instructions for DM medications and to assess the level of preoperative glycemic control and postoperative complications in patients with DM undergoing major elective surgical procedures.
Methods
This retrospective, single-center chart review was conducted at VHI. The Indiana University and VHI institutional review boards determined that this quality improvement project was exempt from review.
The primary outcome was the number of patients with surgical procedures delayed or canceled due to hyperglycemia or hypoglycemia. Hyperglycemia was defined as blood glucose > 180 mg/dL and hypoglycemia was defined as < 70 mg/dL, slight variations from the current ADA SOC preoperative specific recommendation of a blood glucose reading of 100 to 180 mg/dL within 4 hours of surgery.10 The standard outpatient hypoglycemia definition of blood glucose < 70 mg/dL was chosen because the current goal (< 100 mg/dL) was not the standard in previous ADA SOCs that were in place during the study period. Specifically, the 2018 ADA SOC did not provide preoperative recommendations and the 2019-2021 ADA SOC recommended 80 to 180 mg/dL.10,12-18 For patients who had multiple preoperative blood glucose measurements, the first recorded glucose on the day of the procedure was used.
The secondary outcomes of this study were focused on the preoperative process/care at VHI and postoperative glycemic control. The preoperative process included examining whether medication instructions were given and their quality. Additionally, the number of interventions for hyperglycemia and hypoglycemia were required immediately prior to surgery and the average preoperative HbA1c (measured within 3 months prior to surgery) were collected and analyzed. For postoperative glycemic control, average blood glucose measurements and number of hypoglycemic (< 70 mg/dL) and hyperglycemic (> 180 mg/dL) events were measured in addition to the frequency of changes made at discharge to patients’ DM medication regimens.
The safety outcome of this study assessed commonly observed postoperative complications and was examined up to 30 days postsurgery. These included acute kidney injury (defined using Kidney Disease: Improving Global Outcomes 2012, the standard during the study period), nonfatal myocardial infarction, nonfatal stroke, and surgical site infections, which were identified from the discharge summary written by the primary surgery service.19 All-cause mortality also was collected.
Patients were included if they were admitted for major elective surgeries and had a diagnosis of either type 1 or type 2 DM on their problem list, determined by International Classification of Diseases, Tenth Revision codes. Major elective surgery was defined as a procedure that would likely result in a hospital admission of > 24 hours. Of note, patients may have been included in this study more than once if they had > 1 procedure at least 30 days apart and met inclusion criteria within the time frame. Patients were excluded if they were taking no DM medications or chronic steroids (at any dose), residing in a long-term care facility, being managed by a non-VA clinician prior to surgery, or missing a preoperative blood glucose measurement.
All data were collected from the CPRS. A list of surgical cases involving patients with DM who were scheduled to undergo major elective surgeries from January 1, 2018, to December 31, 2021, at VHI was generated. The list was randomized to a smaller number (N = 394) for data collection due to the time and resource constraints for a pharmacy residency project. All data were deidentified and stored in a secured VA server to protect patient confidentiality. Descriptive statistics were used for all results.
Results
Initially, 2362 surgeries were identified. A randomized sample of 394 charts were reviewed and 131 cases met inclusion criteria. Each case involved a unique patient (Figure). The most common reasons for exclusion were 143 patients with diet-controlled DM and 78 nonelective surgeries. The mean (SD) age of patients was 68 (8) years, and the most were male (98.5%) and White (76.3%) (Table 1).


At baseline, 45 of 131 patients (34.4%) had coronary artery disease and 29 (22.1%) each had autonomic neuropathy and chronic kidney disease. Most surgeries were conducted by orthopedic (32.1%) and peripheral vascular (21.4%) specialties. The mean (SD) length of surgery was 4.6 (2.6) hours and of hospital length of stay was 4 (4) days. No patients stayed longer than the 30-day safety outcome follow-up period. All patients had type 2 DM and took a mean 2 DM medications. The 63 patients taking insulin had a mean (SD) total daily dose of 99 (77) U (Table 2). A preoperative HbA1c was collected in 116 patients within 3 months of surgery, with a mean HbA1c of 7.0% (range, 5.3-10.7).

No patients had surgeries delayed or canceled because of uncontrolled DM on the day of surgery. The mean preoperative blood glucose level was 146 mg/dL (range, 73-365) (Table 3). No patients had a preoperative blood glucose level of < 70 mg/dL and 19 (14.5%) had a blood glucose level > 180 mg/dL. Among patients with hyperglycemia immediately prior to surgery, 6 (31.6%) had documentation of insulin being provided.

For this sample of patients, the preoperative clinic visit was conducted a mean 22 days prior to the planned surgery date. Among the 131 included patients, 122 (93.1%) had documentation of receiving instructions for DM medications. Among patients who had documented receipt of instructions, only 30 (24.6%) had instructions specifically tailored to their regimen rather than a generic templated form. The mean (SD) preoperative blood glucose was similar for those who received specific perioperative DM instructions at 146 (50) mg/dL when compared with those who did not at 147 (45) mg/dL. The mean (SD) preoperative blood glucose reading for those who had no documentation of receipt of perioperative instructions was 126 (54) mg/dL compared with 147 (46) mg/dL for those who did.
The mean number of postoperative blood glucose events per day was negligible for hypoglycemia and more frequent for hyperglycemia with a mean of 2 events per day. The mean postoperative blood glucose range was 121 to 247 mg/dL with most readings < 180 mg/dL. Upon discharge, most patients continued their home DM regimen with 5 patients (3.8%) having changes made to their regimen upon discharge.
Very few postoperative complications were identified from chart review. The most frequently observed postoperative complications were acute kidney injury, surgical site infections, and nonfatal stroke. There were no documented nonfatal myocardial infarctions. Two patients (1.5%) died within 30 days of the surgery; neither death was deemed to have been related to poor perioperative glycemic control.
Discussion
To our knowledge, this retrospective chart review was the first study to assess preoperative DM management and postoperative complications in a veteran population. VHI is a large, tertiary, level 1a, academic medical center that serves approximately 62,000 veterans annually and performs about 5000 to 6000 surgeries annually, a total that is increasing following the COVID-19 pandemic.20 This study found that the current process of a presurgery clinic visit and day of surgery glucose assessment has prevented surgical delays or cancellations.
Most patients included in this study were well controlled at baseline in accordance with the 2025 ADA SOC HbA1c recommendation of a preoperative HbA1c of < 8%, which may have contributed to no surgical delays or cancellations.10 However, not all patients had HbA1c collected within 3 months of surgery or even had one collected at all. Despite the ADA SOC providing no explicit recommendation for universal HbA1c screening prior to elective procedures, its importance cannot be understated given the body of evidence demonstrating poor outcomes with uncontrolled preoperative DM.8,10 The glycemic control at baseline may have contributed to the very few postsurgical complications observed in this study.
Although the current process at VHI prevented surgical delays and cancellations in this sample, there are still identified areas for improvement. One area is the instructions the patients received. Patients with DM are often prescribed ≥ 1 medication or a combination of insulins, noninsulin injectables, and oral DM medications, and this study population was no different. Because these medications may influence the anesthesia and perioperative periods, the ADA has specific guidance for altering administration schedules in the days leading up to surgery.10
Inappropriate administration of DM medications could lead to perioperative hypoglycemia or hyperglycemia, possibly causing surgical delays, case cancellations, and/or postoperative complications.21 Although these data reveal the specificity and documented receipt that the preoperative DM instructions did not impact the first recorded preoperative blood glucose, future studies should examine patient confidence in how to properly administer their DM medications prior to surgery. It is vital that patients receive clear instructions in accordance with the ADA SOC on whether to continue, hold, or adjust the dose of their medications to prevent fluctuations in blood glucose levels in the perioperative period, ensure safety with anesthesia, and prevent postoperative complications such as acute kidney injury. Of note, compliance with guideline recommendations for medication instructions was not examined because the data collection time frame expanded over multiple years and the recommendations have evolved each year as new data emerge.
Preoperative DM Management
The first key takeaway from this study is to ensure patients are ready for surgery with a formal assessment (typically in the form of a clinic visit) prior to the surgery. One private sector health system published their approach to this by administering an automatic preoperative HbA1c screening for those with a DM diagnosis and all patients with a random plasma glucose ≥ 200 mg/dL.22 Additionally, if the patient's HbA1c level was not at goal prior to surgery (≥ 8% for those with known DM and ≥ 6.5% with no known DM), patients were referred to endocrinology for further management. Increasing attention to the preoperative visit and extending HbA1c testing to all patients regardless of DM status also provides an opportunity to identify individuals living with undiagnosed DM.1
Even though there was no difference in the mean preoperative blood glucose level based on receipt or specificity of preoperative DM instructions, a second takeaway from this study is the importance of ensuring patients receive clear instructions on their DM medication schedule in the perioperative period. A practical first step may be updating the templates used by the primary surgery teams and providing education to the clinicians in the clinic on how to personalize the visits. Because the current preoperative DM process at VHI is managed by the primary surgical team in a clinic visit, there is an opportunity to shift this responsibility to other health care professionals, such as pharmacists—a change shown to reduce unintended omission of home medications following surgery during hospitalization and reduce costs.23,24
Limitations
This study relied on data included in the patient chart. These data include medication interventions made immediately prior to surgery, which can sometimes be inaccurately charted or difficult to find as they are not documented in the typical medication administration record. Also, the safety outcomes were collected from a discharge summary written by different clinicians, which may lead to information bias. Special attention was taken to ensure these data points were collected as accurately as possible, but it is possible some data may be inaccurate from unintentional human error. Additionally, the safety outcome was limited to a 30-day follow-up, but encompassed the entire length of postoperative stay for all included patients. Finally, given this study was retrospective with no comparison group and the intent was to improve processes at VHI, only hypotheses and potential interventions can be generated from this study. Future prospective studies with larger sample sizes and comparator groups are needed to draw further conclusions.
Conclusions
This study found that the current presurgery process at VHI appears to be successful in preventing surgical delays or cancellations due to hyperglycemia or hypoglycemia. Optimizing DM management can improve surgical outcomes by decreasing rates of postoperative complications, and this study added additional evidence in support of that in a unique population: veterans. Insight on the awareness of preoperative blood glucose management should be gleaned from this study, and based on this sample and site, the preadmission screening process and instructions provided to patients can serve as 2 starting points for optimizing elective surgery.
More than 38 million people in the United States (12%) have diabetes mellitus (DM), though 1 in 5 are unaware they have DM.1 The prevalence among veterans is even more substantial, impacting nearly 25% of those who received care from the US Department of Veterans Affairs (VA).2 DM can lead to increased health care costs in addition to various complications (eg, cardiovascular, renal), especially if left uncontrolled.1,3 similar impact is found in the perioperative period (defined as at or around the time of an operation), as multiple studies have found that uncontrolled preoperative DM can result in worsened surgical outcomes, including longer hospital stays, more infectious complications, and higher perioperative mortality.4-6
In contrast, adequate glycemic control assessed with blood glucose levels has been shown to decrease the incidence of postoperative infections.7 Optimizing glycemic control during hospital stays, especially postsurgery, has become the standard of care, with most health systems establishing specific protocols. In current literature, most studies examining DM management in the perioperative period are focused on postoperative care, with little attention to the preoperative period.4,6,7
One study found that patients with poor presurgery glycemic control assessed by hemoglobin A1c (HbA1c) levels were more likely to remain hyperglycemic during and after surgery. 8 Blood glucose levels < 200 mg/dL can lead to an increased risk of infection and impaired wound healing, meaning a well-controlled HbA1c before a procedure serves as a potential factor for success.9 The 2025 American Diabetes Association (ADA) Standards of Care (SOC) recommendation is to target HbA1c < 8% whenever possible, and some health systems require lower levels (eg, < 7% or 7.5%).10 With that goal in mind and knowing that preoperative hyperglycemia has been shown to be a contributing factor in the delay or cancellation of surgical cases, an argument can be made that attention to preoperative DM management also should be a focus for health care systems performing surgeries.8,9,11
Attention to glucose control during preoperative care offers an opportunity to screen for DM in patients who may not have been screened otherwise and to standardize perioperative DM management. Since DM disproportionately impacts veterans, this is a pertinent issue to the VA. Veterans can be more susceptible to complications if DM is left uncontrolled prior to surgery. To determine readiness for surgery and control of comorbid conditions such as DM before a planned surgery, facilities often perform a preoperative clinic assessment, often in a multidisciplinary clinic.
At Veteran Health Indiana (VHI), a presurgery clinic visit involving the primary surgery service (physician, nurse practitioner, and/or a physician assistant) is conducted 1 to 2 months prior to the planned procedure to determine whether a patient is ready for surgery. During this visit, patients receive a packet with instructions for various tasks and medications, such as applying topical antibiotic prophylaxis on the anticipated surgical site. This is documented in the form of a note in the VHI Computerized Patient Record System (CPRS). The medication instructions are provided according to the preferences of the surgical team. These may be templated notes that contain general directions on the timing and dosing of specific medications, in addition to instructions for holding or reducing doses when appropriate. The instructions can be tailored by the team conducting the preoperative visit (eg, “Take 20 units of insulin glargine the day before surgery” vs “Take half of your long-acting insulin the night before surgery”). Specific to DM, VHI has a nurse-driven day of surgery glucose assessment where point-of-care blood glucose is collected during preoperative holding for most patients.
There is limited research assessing the level of preoperative glycemic control and the incidence of complications in a veteran population. The objective of this study was to gain a baseline understanding of what, if any, standardization exists for preoperative instructions for DM medications and to assess the level of preoperative glycemic control and postoperative complications in patients with DM undergoing major elective surgical procedures.
Methods
This retrospective, single-center chart review was conducted at VHI. The Indiana University and VHI institutional review boards determined that this quality improvement project was exempt from review.
The primary outcome was the number of patients with surgical procedures delayed or canceled due to hyperglycemia or hypoglycemia. Hyperglycemia was defined as blood glucose > 180 mg/dL and hypoglycemia was defined as < 70 mg/dL, slight variations from the current ADA SOC preoperative specific recommendation of a blood glucose reading of 100 to 180 mg/dL within 4 hours of surgery.10 The standard outpatient hypoglycemia definition of blood glucose < 70 mg/dL was chosen because the current goal (< 100 mg/dL) was not the standard in previous ADA SOCs that were in place during the study period. Specifically, the 2018 ADA SOC did not provide preoperative recommendations and the 2019-2021 ADA SOC recommended 80 to 180 mg/dL.10,12-18 For patients who had multiple preoperative blood glucose measurements, the first recorded glucose on the day of the procedure was used.
The secondary outcomes of this study were focused on the preoperative process/care at VHI and postoperative glycemic control. The preoperative process included examining whether medication instructions were given and their quality. Additionally, the number of interventions for hyperglycemia and hypoglycemia were required immediately prior to surgery and the average preoperative HbA1c (measured within 3 months prior to surgery) were collected and analyzed. For postoperative glycemic control, average blood glucose measurements and number of hypoglycemic (< 70 mg/dL) and hyperglycemic (> 180 mg/dL) events were measured in addition to the frequency of changes made at discharge to patients’ DM medication regimens.
The safety outcome of this study assessed commonly observed postoperative complications and was examined up to 30 days postsurgery. These included acute kidney injury (defined using Kidney Disease: Improving Global Outcomes 2012, the standard during the study period), nonfatal myocardial infarction, nonfatal stroke, and surgical site infections, which were identified from the discharge summary written by the primary surgery service.19 All-cause mortality also was collected.
Patients were included if they were admitted for major elective surgeries and had a diagnosis of either type 1 or type 2 DM on their problem list, determined by International Classification of Diseases, Tenth Revision codes. Major elective surgery was defined as a procedure that would likely result in a hospital admission of > 24 hours. Of note, patients may have been included in this study more than once if they had > 1 procedure at least 30 days apart and met inclusion criteria within the time frame. Patients were excluded if they were taking no DM medications or chronic steroids (at any dose), residing in a long-term care facility, being managed by a non-VA clinician prior to surgery, or missing a preoperative blood glucose measurement.
All data were collected from the CPRS. A list of surgical cases involving patients with DM who were scheduled to undergo major elective surgeries from January 1, 2018, to December 31, 2021, at VHI was generated. The list was randomized to a smaller number (N = 394) for data collection due to the time and resource constraints for a pharmacy residency project. All data were deidentified and stored in a secured VA server to protect patient confidentiality. Descriptive statistics were used for all results.
Results
Initially, 2362 surgeries were identified. A randomized sample of 394 charts were reviewed and 131 cases met inclusion criteria. Each case involved a unique patient (Figure). The most common reasons for exclusion were 143 patients with diet-controlled DM and 78 nonelective surgeries. The mean (SD) age of patients was 68 (8) years, and the most were male (98.5%) and White (76.3%) (Table 1).


At baseline, 45 of 131 patients (34.4%) had coronary artery disease and 29 (22.1%) each had autonomic neuropathy and chronic kidney disease. Most surgeries were conducted by orthopedic (32.1%) and peripheral vascular (21.4%) specialties. The mean (SD) length of surgery was 4.6 (2.6) hours and of hospital length of stay was 4 (4) days. No patients stayed longer than the 30-day safety outcome follow-up period. All patients had type 2 DM and took a mean 2 DM medications. The 63 patients taking insulin had a mean (SD) total daily dose of 99 (77) U (Table 2). A preoperative HbA1c was collected in 116 patients within 3 months of surgery, with a mean HbA1c of 7.0% (range, 5.3-10.7).

No patients had surgeries delayed or canceled because of uncontrolled DM on the day of surgery. The mean preoperative blood glucose level was 146 mg/dL (range, 73-365) (Table 3). No patients had a preoperative blood glucose level of < 70 mg/dL and 19 (14.5%) had a blood glucose level > 180 mg/dL. Among patients with hyperglycemia immediately prior to surgery, 6 (31.6%) had documentation of insulin being provided.

For this sample of patients, the preoperative clinic visit was conducted a mean 22 days prior to the planned surgery date. Among the 131 included patients, 122 (93.1%) had documentation of receiving instructions for DM medications. Among patients who had documented receipt of instructions, only 30 (24.6%) had instructions specifically tailored to their regimen rather than a generic templated form. The mean (SD) preoperative blood glucose was similar for those who received specific perioperative DM instructions at 146 (50) mg/dL when compared with those who did not at 147 (45) mg/dL. The mean (SD) preoperative blood glucose reading for those who had no documentation of receipt of perioperative instructions was 126 (54) mg/dL compared with 147 (46) mg/dL for those who did.
The mean number of postoperative blood glucose events per day was negligible for hypoglycemia and more frequent for hyperglycemia with a mean of 2 events per day. The mean postoperative blood glucose range was 121 to 247 mg/dL with most readings < 180 mg/dL. Upon discharge, most patients continued their home DM regimen with 5 patients (3.8%) having changes made to their regimen upon discharge.
Very few postoperative complications were identified from chart review. The most frequently observed postoperative complications were acute kidney injury, surgical site infections, and nonfatal stroke. There were no documented nonfatal myocardial infarctions. Two patients (1.5%) died within 30 days of the surgery; neither death was deemed to have been related to poor perioperative glycemic control.
Discussion
To our knowledge, this retrospective chart review was the first study to assess preoperative DM management and postoperative complications in a veteran population. VHI is a large, tertiary, level 1a, academic medical center that serves approximately 62,000 veterans annually and performs about 5000 to 6000 surgeries annually, a total that is increasing following the COVID-19 pandemic.20 This study found that the current process of a presurgery clinic visit and day of surgery glucose assessment has prevented surgical delays or cancellations.
Most patients included in this study were well controlled at baseline in accordance with the 2025 ADA SOC HbA1c recommendation of a preoperative HbA1c of < 8%, which may have contributed to no surgical delays or cancellations.10 However, not all patients had HbA1c collected within 3 months of surgery or even had one collected at all. Despite the ADA SOC providing no explicit recommendation for universal HbA1c screening prior to elective procedures, its importance cannot be understated given the body of evidence demonstrating poor outcomes with uncontrolled preoperative DM.8,10 The glycemic control at baseline may have contributed to the very few postsurgical complications observed in this study.
Although the current process at VHI prevented surgical delays and cancellations in this sample, there are still identified areas for improvement. One area is the instructions the patients received. Patients with DM are often prescribed ≥ 1 medication or a combination of insulins, noninsulin injectables, and oral DM medications, and this study population was no different. Because these medications may influence the anesthesia and perioperative periods, the ADA has specific guidance for altering administration schedules in the days leading up to surgery.10
Inappropriate administration of DM medications could lead to perioperative hypoglycemia or hyperglycemia, possibly causing surgical delays, case cancellations, and/or postoperative complications.21 Although these data reveal the specificity and documented receipt that the preoperative DM instructions did not impact the first recorded preoperative blood glucose, future studies should examine patient confidence in how to properly administer their DM medications prior to surgery. It is vital that patients receive clear instructions in accordance with the ADA SOC on whether to continue, hold, or adjust the dose of their medications to prevent fluctuations in blood glucose levels in the perioperative period, ensure safety with anesthesia, and prevent postoperative complications such as acute kidney injury. Of note, compliance with guideline recommendations for medication instructions was not examined because the data collection time frame expanded over multiple years and the recommendations have evolved each year as new data emerge.
Preoperative DM Management
The first key takeaway from this study is to ensure patients are ready for surgery with a formal assessment (typically in the form of a clinic visit) prior to the surgery. One private sector health system published their approach to this by administering an automatic preoperative HbA1c screening for those with a DM diagnosis and all patients with a random plasma glucose ≥ 200 mg/dL.22 Additionally, if the patient's HbA1c level was not at goal prior to surgery (≥ 8% for those with known DM and ≥ 6.5% with no known DM), patients were referred to endocrinology for further management. Increasing attention to the preoperative visit and extending HbA1c testing to all patients regardless of DM status also provides an opportunity to identify individuals living with undiagnosed DM.1
Even though there was no difference in the mean preoperative blood glucose level based on receipt or specificity of preoperative DM instructions, a second takeaway from this study is the importance of ensuring patients receive clear instructions on their DM medication schedule in the perioperative period. A practical first step may be updating the templates used by the primary surgery teams and providing education to the clinicians in the clinic on how to personalize the visits. Because the current preoperative DM process at VHI is managed by the primary surgical team in a clinic visit, there is an opportunity to shift this responsibility to other health care professionals, such as pharmacists—a change shown to reduce unintended omission of home medications following surgery during hospitalization and reduce costs.23,24
Limitations
This study relied on data included in the patient chart. These data include medication interventions made immediately prior to surgery, which can sometimes be inaccurately charted or difficult to find as they are not documented in the typical medication administration record. Also, the safety outcomes were collected from a discharge summary written by different clinicians, which may lead to information bias. Special attention was taken to ensure these data points were collected as accurately as possible, but it is possible some data may be inaccurate from unintentional human error. Additionally, the safety outcome was limited to a 30-day follow-up, but encompassed the entire length of postoperative stay for all included patients. Finally, given this study was retrospective with no comparison group and the intent was to improve processes at VHI, only hypotheses and potential interventions can be generated from this study. Future prospective studies with larger sample sizes and comparator groups are needed to draw further conclusions.
Conclusions
This study found that the current presurgery process at VHI appears to be successful in preventing surgical delays or cancellations due to hyperglycemia or hypoglycemia. Optimizing DM management can improve surgical outcomes by decreasing rates of postoperative complications, and this study added additional evidence in support of that in a unique population: veterans. Insight on the awareness of preoperative blood glucose management should be gleaned from this study, and based on this sample and site, the preadmission screening process and instructions provided to patients can serve as 2 starting points for optimizing elective surgery.
- Centers for Disease Control and Prevention. Diabetes basics. May 15, 2024. Accessed September 24, 2025. https://www.cdc.gov/diabetes/about/index.html
- Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135. doi:10.5888/pcd14.170230
- Farmaki P, Damaskos C, Garmpis N, et al . Complications of the Type 2 Diabetes Mellitus. Curr Cardiol Rev. 2020;16(4):249-251. doi:10.2174/1573403X1604201229115531
- Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783-1788. doi:10.2337/dc10-0304
- Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156:137 -142. doi:10.1530/eje.1.02321
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77-81. doi:10.1177/01486071980220027
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34:256-261. doi:10.2337/dc10-1407
- Pasquel FJ, Gomez-Huelgas R, Anzola I, et al. Predictive value of admission hemoglobin A1c on inpatient glycemic control and response to insulin therapy in medicine and surgery patients with type 2 diabetes. Diabetes Care. 2015;38:e202-e203. doi:10.2337/dc15-1835
- Alexiewicz JM, Kumar D, Smogorzewski M, et al. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med. 1995;123:919-924. doi:10.7326/0003-4819-123-12-199512150-00004
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2025. Diabetes Care. 2025;48(1 suppl 1):S321-S334. doi:10.2337/dc25-S016
- Kumar R, Gandhi R. Reasons for cancellation of operation on the day of intended surgery in a multidisciplinary 500 bedded hospital. J Anaesthesiol Clin Pharmacol. 2012;28:66-69. doi:10.4103/0970-9185.92442
- American Diabetes Association. 14. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2018. Diabetes Care. 2018;41(1 suppl 1):S144- S151. doi:10.2337/dc18-S014
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2019. Diabetes Care. 2019;42(suppl 1):S173- S181. doi:10.2337/dc19-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2020. Diabetes Care. 2020;43(suppl 1):S193- S202. doi:10.2337/dc20-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2021. Diabetes Care. 2021;44(suppl 1):S211- S220. doi:10.2337/dc21-S015
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
- ElSayed NA, Aleppo G, Aroda VR, et al. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(suppl 1):S267-S278. doi:10.2337/dc23-S016
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(suppl 1):S295-S306. doi:10.2337/dc24-S016
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138. Accessed September 24, 2025. https:// www.kisupplements.org/issue/S2157-1716(12)X7200-9
- US Department of Veterans Affairs. VA Indiana Healthcare: about us. Accessed September 24, 2025. https:// www.va.gov/indiana-health-care/about-us/
- Koh WX, Phelan R, Hopman WM, et al. Cancellation of elective surgery: rates, reasons and effect on patient satisfaction. Can J Surg. 2021;64:E155-E161. doi:10.1503/cjs.008119
- Pai S-L, Haehn DA, Pitruzzello NE, et al. Reducing infection rates with enhanced preoperative diabetes mellitus diagnosis and optimization processes. South Med J. 2023;116:215-219. doi:10.14423/SMJ.0000000000001507
- Forrester TG, Sullivan S, Snoswell CL, et al. Integrating a pharmacist into the perioperative setting. Aust Health Rev. 2020;44:563-568. doi:10.1071/AH19126
- Hale AR, Coombes ID, Stokes J, et al. Perioperative medication management: expanding the role of the preadmission clinic pharmacist in a single centre, randomised controlled trial of collaborative prescribing. BMJ Open. 2013;3:e003027. doi:10.1136/bmjopen-2013-003027
- Centers for Disease Control and Prevention. Diabetes basics. May 15, 2024. Accessed September 24, 2025. https://www.cdc.gov/diabetes/about/index.html
- Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135. doi:10.5888/pcd14.170230
- Farmaki P, Damaskos C, Garmpis N, et al . Complications of the Type 2 Diabetes Mellitus. Curr Cardiol Rev. 2020;16(4):249-251. doi:10.2174/1573403X1604201229115531
- Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783-1788. doi:10.2337/dc10-0304
- Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156:137 -142. doi:10.1530/eje.1.02321
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77-81. doi:10.1177/01486071980220027
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34:256-261. doi:10.2337/dc10-1407
- Pasquel FJ, Gomez-Huelgas R, Anzola I, et al. Predictive value of admission hemoglobin A1c on inpatient glycemic control and response to insulin therapy in medicine and surgery patients with type 2 diabetes. Diabetes Care. 2015;38:e202-e203. doi:10.2337/dc15-1835
- Alexiewicz JM, Kumar D, Smogorzewski M, et al. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med. 1995;123:919-924. doi:10.7326/0003-4819-123-12-199512150-00004
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2025. Diabetes Care. 2025;48(1 suppl 1):S321-S334. doi:10.2337/dc25-S016
- Kumar R, Gandhi R. Reasons for cancellation of operation on the day of intended surgery in a multidisciplinary 500 bedded hospital. J Anaesthesiol Clin Pharmacol. 2012;28:66-69. doi:10.4103/0970-9185.92442
- American Diabetes Association. 14. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2018. Diabetes Care. 2018;41(1 suppl 1):S144- S151. doi:10.2337/dc18-S014
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2019. Diabetes Care. 2019;42(suppl 1):S173- S181. doi:10.2337/dc19-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2020. Diabetes Care. 2020;43(suppl 1):S193- S202. doi:10.2337/dc20-S015
- American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes— 2021. Diabetes Care. 2021;44(suppl 1):S211- S220. doi:10.2337/dc21-S015
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
- ElSayed NA, Aleppo G, Aroda VR, et al. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(suppl 1):S267-S278. doi:10.2337/dc23-S016
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(suppl 1):S295-S306. doi:10.2337/dc24-S016
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138. Accessed September 24, 2025. https:// www.kisupplements.org/issue/S2157-1716(12)X7200-9
- US Department of Veterans Affairs. VA Indiana Healthcare: about us. Accessed September 24, 2025. https:// www.va.gov/indiana-health-care/about-us/
- Koh WX, Phelan R, Hopman WM, et al. Cancellation of elective surgery: rates, reasons and effect on patient satisfaction. Can J Surg. 2021;64:E155-E161. doi:10.1503/cjs.008119
- Pai S-L, Haehn DA, Pitruzzello NE, et al. Reducing infection rates with enhanced preoperative diabetes mellitus diagnosis and optimization processes. South Med J. 2023;116:215-219. doi:10.14423/SMJ.0000000000001507
- Forrester TG, Sullivan S, Snoswell CL, et al. Integrating a pharmacist into the perioperative setting. Aust Health Rev. 2020;44:563-568. doi:10.1071/AH19126
- Hale AR, Coombes ID, Stokes J, et al. Perioperative medication management: expanding the role of the preadmission clinic pharmacist in a single centre, randomised controlled trial of collaborative prescribing. BMJ Open. 2013;3:e003027. doi:10.1136/bmjopen-2013-003027
Preoperative Diabetes Management for Patients Undergoing Elective Surgeries at a Veterans Affairs Medical Center
Preoperative Diabetes Management for Patients Undergoing Elective Surgeries at a Veterans Affairs Medical Center
Assessment of Glucagon-like Peptide-1 Receptor Agonists in Veterans Taking Basal/Bolus Insulin Regimens
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent HbA1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of type 1 DM (T1DM), were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline HbA1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units.
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up,
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lower than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older, males and White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result of adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent HbA1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of type 1 DM (T1DM), were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline HbA1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units.
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up,
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lower than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older, males and White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result of adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent HbA1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of type 1 DM (T1DM), were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline HbA1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units.
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up,
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lower than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older, males and White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result of adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
Assessment of Glucagon-like Peptide-1 Receptor Agonists in Veterans TakingBasal/Bolus Insulin Regimens
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent Hb A1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of T1DM, were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline Hb A1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units. Mean duration of DM was 10 years, and mean use of basal/bolus insulin regimen was 6.1 years. Most participants (91%) used an insulin regimen containing insulin glargine and insulin aspart; the remaining participants used insulin detemir and insulin aspart. Semaglutide and liraglutide were the most commonly used GLP-1 RAs (44% and 39%, respectively) (Table 1).
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up, a different number of patient charts were available for review at each period (Table 2). Glycemic control was significantly improved at all time points when compared with baseline, but over time the benefit declined. The mean change in HbA1c was −1.1% (95% CI, −1.3 to −0.8; P < .001) at 3 months; −1.0% (95% CI, −1.3 to −0.7; P < .001) at 6 months; −0.9% (95% CI, −1.3 to −0.6; P < .001) at 12 months; −0.9% (95% CI −1.4 to −0.3; P = .002) at 18 months; and −0.7% (95% CI, −1.4 to 0.1; P = .07) at 24 months (Figure 1). Mean weight decreased from baseline −2.7 kg (95% CI, −3.7 to −1.6; P < .001); −4.4 kg (95% CI −5.7 to −3.2; P < .001) at 6 months; −3.9 kg (95% CI −6.0 to −1.9; P < .001) at 12 months; −4.7 kg (95% CI −6.7 to −2.6; P < .001) at 18 months; and −2.8 kg (95% CI, −5.9 to 0.3; P = .07) at 24 months (Figure 2). Mean TDD decreased at 3 months −12 units (95% CI, −19 to −5; P < .001); −18 units (95% CI, −27 to −9; P < .001) at 6 months; −14 units (95% CI, −24 to −5; P = .004) at 12 months; −9 units (95% CI, −21 to 3; P = .15) at 18 months; and −18 units (95% CI, −43 to 5 units; P = .12) at 24 months (Figure 3). The most common AEs were hypoglycemia (30%), diarrhea (11%), nausea (4%), and abdominal pain (3%).
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lover than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents being available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older males of White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the length of study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent Hb A1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of T1DM, were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline Hb A1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units. Mean duration of DM was 10 years, and mean use of basal/bolus insulin regimen was 6.1 years. Most participants (91%) used an insulin regimen containing insulin glargine and insulin aspart; the remaining participants used insulin detemir and insulin aspart. Semaglutide and liraglutide were the most commonly used GLP-1 RAs (44% and 39%, respectively) (Table 1).
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up, a different number of patient charts were available for review at each period (Table 2). Glycemic control was significantly improved at all time points when compared with baseline, but over time the benefit declined. The mean change in HbA1c was −1.1% (95% CI, −1.3 to −0.8; P < .001) at 3 months; −1.0% (95% CI, −1.3 to −0.7; P < .001) at 6 months; −0.9% (95% CI, −1.3 to −0.6; P < .001) at 12 months; −0.9% (95% CI −1.4 to −0.3; P = .002) at 18 months; and −0.7% (95% CI, −1.4 to 0.1; P = .07) at 24 months (Figure 1). Mean weight decreased from baseline −2.7 kg (95% CI, −3.7 to −1.6; P < .001); −4.4 kg (95% CI −5.7 to −3.2; P < .001) at 6 months; −3.9 kg (95% CI −6.0 to −1.9; P < .001) at 12 months; −4.7 kg (95% CI −6.7 to −2.6; P < .001) at 18 months; and −2.8 kg (95% CI, −5.9 to 0.3; P = .07) at 24 months (Figure 2). Mean TDD decreased at 3 months −12 units (95% CI, −19 to −5; P < .001); −18 units (95% CI, −27 to −9; P < .001) at 6 months; −14 units (95% CI, −24 to −5; P = .004) at 12 months; −9 units (95% CI, −21 to 3; P = .15) at 18 months; and −18 units (95% CI, −43 to 5 units; P = .12) at 24 months (Figure 3). The most common AEs were hypoglycemia (30%), diarrhea (11%), nausea (4%), and abdominal pain (3%).
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lover than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents being available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older males of White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the length of study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent Hb A1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of T1DM, were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline Hb A1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units. Mean duration of DM was 10 years, and mean use of basal/bolus insulin regimen was 6.1 years. Most participants (91%) used an insulin regimen containing insulin glargine and insulin aspart; the remaining participants used insulin detemir and insulin aspart. Semaglutide and liraglutide were the most commonly used GLP-1 RAs (44% and 39%, respectively) (Table 1).
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up, a different number of patient charts were available for review at each period (Table 2). Glycemic control was significantly improved at all time points when compared with baseline, but over time the benefit declined. The mean change in HbA1c was −1.1% (95% CI, −1.3 to −0.8; P < .001) at 3 months; −1.0% (95% CI, −1.3 to −0.7; P < .001) at 6 months; −0.9% (95% CI, −1.3 to −0.6; P < .001) at 12 months; −0.9% (95% CI −1.4 to −0.3; P = .002) at 18 months; and −0.7% (95% CI, −1.4 to 0.1; P = .07) at 24 months (Figure 1). Mean weight decreased from baseline −2.7 kg (95% CI, −3.7 to −1.6; P < .001); −4.4 kg (95% CI −5.7 to −3.2; P < .001) at 6 months; −3.9 kg (95% CI −6.0 to −1.9; P < .001) at 12 months; −4.7 kg (95% CI −6.7 to −2.6; P < .001) at 18 months; and −2.8 kg (95% CI, −5.9 to 0.3; P = .07) at 24 months (Figure 2). Mean TDD decreased at 3 months −12 units (95% CI, −19 to −5; P < .001); −18 units (95% CI, −27 to −9; P < .001) at 6 months; −14 units (95% CI, −24 to −5; P = .004) at 12 months; −9 units (95% CI, −21 to 3; P = .15) at 18 months; and −18 units (95% CI, −43 to 5 units; P = .12) at 24 months (Figure 3). The most common AEs were hypoglycemia (30%), diarrhea (11%), nausea (4%), and abdominal pain (3%).
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lover than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents being available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older males of White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the length of study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf