User login
Invasive Compartment Pressure Testing for Chronic Exertional Compartment Syndrome: A Survey of Clinical Practice Among Military Orthopedic Surgeons
Chronic exertional compartment syndrome (CECS) is a common cause of leg pain during exertion in athletic and active-duty populations.1 It is caused by an increase in intramuscular pressure to a point that the tissues within the involved compartment become ischemic because of a decrease in arteriolar blood flow.2 This relative ischemia causes pain and may also be associated with neurologic symptoms. By definition, the pain associated with CECS resolves with rest. Patients typically describe a feeling of fullness or tightness, which eventually evolves into pain as they continue exercising. Pain onset is usually predictable and reproducible after a finite amount of time and/or intensity of exercise.
The differential diagnosis of leg pain during exercise includes CECS, medial tibial stress syndrome, popliteal entrapment syndrome, myopathy, peripheral nerve entrapment syndromes, stress fracture, and effort-induced rhabdomyolysis.3 CECS can be differentiated from other causes of leg pain with measurement of compartment pressures (the standard recommendation).4 Compartment pressure measurement, however, is invasive, time-consuming, and painful and may be associated with bleeding risk, infection, and nerve injury. Noninvasive means of testing for CECS (eg, magnetic resonance imaging [MRI], near-infrared spectroscopy [NIRS], thallium stress testing) remain experimental and expensive and are not easily accessible at all institutions.5-8 While invasive compartment pressure (ICP) testing remains an important tool in the diagnosis of CECS, its criteria and execution vary considerably. Aweid and colleagues4 performed a meta-analysis of use of ICP testing in the diagnosis of CECS and concluded that, though elevated ICP measurements are accepted as the gold standard for diagnosing CECS, the criteria outlined for a positive test lack high-level supporting evidence. In addition, how the test is performed has been inconsistent across studies—further clouding the literature.4
The review by Aweid and colleagues4 highlights the deficiencies in diagnosing CECS by ICP testing. In clinical practice, ICP testing is challenging for both the patient and physician. As other validated, less-invasive tests are lacking, emphasis should remain on the history and the physical examination. Although all athletic populations are at risk for CECS, the active-duty military population is at particularly high risk because of the physical requirements and demands of military service.1,9
We surveyed military orthopedic surgeons to investigate the clinical practice of performing ICP testing in patients with suspected CECS. We hypothesized that the rate of ICP testing among military orthopedic surgeons would not be 100% for patients with the typical signs and symptoms of CECS.
Materials and Methods
This study was approved by the institutional review board at Wright-Patterson Medical Center at Wright-Patterson Air Force Base in Ohio. A link to an online survey was distributed by email to members of the Society of Military Orthopaedic Surgeons. The anonymous survey polled the surgeons regarding basic demographic data and clinical practice as it pertains to the evaluation and treatment of CECS. No patient-protected health information was obtained. Survey results were compiled in a Microsoft Excel file for analysis.
Results
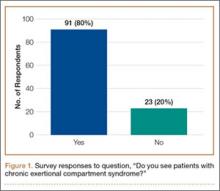
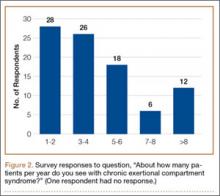
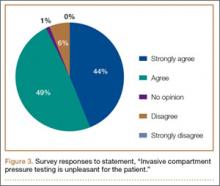
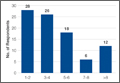
The survey was distributed to 606 email accounts; the response rate was 19% (114/606). Ninety-one surgeons (80%) indicated they have patients with CECS in their practice (Figure 1). Surgeons were asked how many CECS patients they see per year (responses are summarized in Figure 2) and how many years they have been in practice (Table).
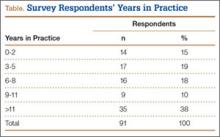
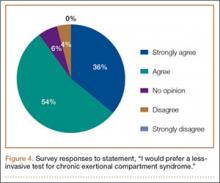
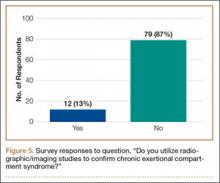
Ninety-three percent of the respondents agreed or strongly agreed that ICP testing is unpleasant for the patient (Figure 3), and 90% would prefer a less-invasive test for confirmatory testing for CECS (Figure 4). Only 13% of respondents indicated they actually use noninvasive modalities (eg, MRI, NIRS) to confirm the diagnosis of CECS (Figure 5).
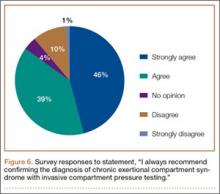
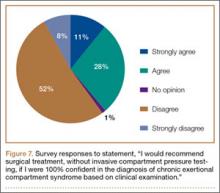
Respondents were asked about the practice of using ICP testing in the diagnosis of CECS (responses are summarized in Figures 6, 7). Although 85% of respondents agreed or strongly agreed with always confirming the diagnosis of CECS with ICP testing, 39% stated they would recommend surgical treatment without ICP testing if they were confident about the diagnosis based on clinical examination findings.
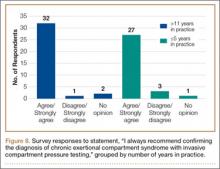
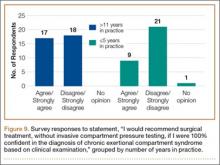
To better understand the apparent discrepancy between the percentage of surgeons who agreed or strongly agreed with always recommending ICP testing (85%) and the percentage who would recommend treatment without testing (39%), responses were stratified by clinical experience. Surgeons in practice more than 11 years (n = 35) were compared with those in practice 5 years or less (n = 31) (Table). Although the vast majority (85%) of respondents from both groups agreed or strongly agreed with always recommending ICP testing, 49% of those in practice more than 11 years and 29% in practice 5 years or less indicated they would recommend surgical treatment for CECS based solely on clinical examination findings (Figures 8, 9).
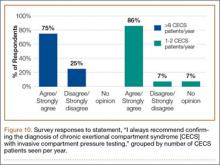
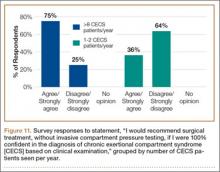
Responses were also stratified by number of CECS patients seen by each surgeon per year. Twenty-eight respondents saw 1 or 2 patients per year, and 12 saw more than 8 patients per year—31% and 13% of the total number of respondents, respectively. Of the respondents who saw 1 or 2 patients, 86% (24/28) agreed or strongly agreed with always recommending ICP testing—comparable to the 75% (9/12) who saw more than 8 patients (Figure 10). However, of the respondents who saw 1 or 2 patients, 36% (10/28) indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% (9/12) who saw more than 8 patients (Figure 11).
Discussion
CECS is a common cause of leg pain and a significant cause of disability among the active-duty military population. This was illustrated in 2 recent studies by Waterman and colleagues.1,9 The first1 investigated failure rates and disability after surgery for CECS among those on active duty. The authors showed that CECS is a substantial contributor to lower extremity disability in the military population and that there is a substantial risk for persistent symptoms despite surgical treatment. Nearly 1 in 5 patients experienced surgical failure after elective fasciotomy, and about 28% of patients were unable to return to the full activity required in the military. The second, more recent study9 was an epidemiologic study of risk factors associated with CECS in a physically active military population. The authors identified 4100 cases diagnosed between 2006 and 2011—representing an overall annual incidence of 0.49 per 1000 at-risk person-years, or about 683 cases per year; the authors also showed the incidence increased during the time period studied.
The diagnosis of CECS remains imperfect. A clinical history of exercise-induced lower leg pain that is relieved with rest suggests the diagnosis, but a confirmatory test is needed to distinguish CECS from other causes of exercise-induced leg pain. Although direct measurement of compartment pressures is the test used most often, it is invasive and time-consuming, can be uncomfortable for the patient, and may be associated with bleeding risk, infection, and nerve injury. Pedowitz and colleagues10 described the ICP testing criteria now generally used in the diagnosis of CECS. Unfortunately, there is little objective evidence supporting these criteria.4 Although less invasive tests (eg, MRI, NIRS) have been described,5-8 they may not be readily available across institutions, and further study is needed to validate their use in diagnosing CECS.
While an objective, validated test or measurement for confirming the diagnosis of CECS remains elusive, the outcomes after surgical treatment of CECS also remain imperfect. Surgery consists of both open and endoscopically assisted fasciotomy of the involved compartments.2,11-17 Reports of improvement after treatment range from 81% to 100%3; however, symptom relief does not come for all patients, particularly those in the military. Waterman and colleagues1 found a failure rate of about 20% among an active-duty military population. Packer and colleagues18 examined civilians with CECS, treated both operatively and nonoperatively. Patients in this series were diagnosed with CECS based on clinical symptoms as well as compartment pressure measurements according to the Pedowitz criteria. Although overall outcomes were better for operatively treated patients than for nonoperatively treated patients, only 47% of patients were completely pain-free, and 21% were unable to return to full activity.
More recent studies have explored use of other nonoperative treatment modalities. Diebal and colleagues19,20 used a running retraining program to treat patients with CECS. They based this treatment on the hypothesis that a heel-strike running pattern is associated with increased anterior compartment pressures.21 CECS patients who underwent a 6-week systematic treatment program focused on forefoot running, stride shortening, and hamstring activation during push-off experienced a decrease in clinical symptoms and posttreatment intracompartmental pressures.20 The improvements in clinical scores were maintained at 1-year follow-up. Another nonoperative intervention, recently described by Isner-Horobeti and colleagues,22 involves injecting botulinum toxin A (BoNT-A) into the anterior and lateral compartments of the leg. Sixteen patients with CECS received BoNT-A injections. On average, intracompartmental pressures were lower after injection than they were before injection. In addition, exertional pain was eliminated in 15 patients at an average follow-up of 4.4 months.
This survey-based study examined the practice patterns of military orthopedic surgeons who performed ICP testing for cases of suspected CECS. Our hypothesis was that, though ICP testing is the most commonly accepted method for confirming the diagnosis of CECS, the ICP testing rate would not be 100% among those surveyed.
The results of our study uncover an apparent inconsistency in survey responses among physicians who evaluate and treat patients with CECS. About 85% of respondents stated they would always recommend confirming the diagnosis of CECS with ICP testing. However, about 40% stated they would recommend surgical treatment without confirmatory testing if they were confident about the diagnosis based on clinical findings. In other words, only 60% of the respondents disagreed or strongly disagreed with pursuing surgical treatment without testing. One would expect a closer correlation between respondents who would always recommend ICP testing and those who disagreed with recommending surgical treatment without ICP testing. This raises the question of what actually occurs when CECS is suspected in clinical practice.
To better understand the apparent discrepancy between respondents who agreed or strongly agreed with always recommending ICP testing and respondents who would recommend treatment without testing, we grouped responses by clinical experience. Although 85% of respondents (no matter the number of years in practice) agreed or strongly agreed with the statement, “I always recommend confirming the diagnosis of CECS with ICP measurements,” 49% of those in practice more than 11 years (vs. 29% of those in practice 5 years or less) agreed or strongly agreed with recommending surgery without testing if they were 100% confident about the diagnosis of CECS based solely on clinical findings. This may suggest that, though most agreed that the gold standard for confirming the diagnosis of CECS remains ICP testing, those with more clinical experience were more comfortable forgoing this diagnostic measure and recommending treatment without testing.
Another measure of clinical experience used in this survey was based on number of CECS patients seen per year. Responses of surgeons who saw 1 or 2 patients with CECS per year were compared with responses of surgeons who saw more than 8 patients with CECS per year. Of the respondents who saw 1 or 2 patients, 86% agreed or strongly agreed with always recommending ICP testing to confirm CECS—comparable to the 75% who saw more than 8 patients. However, of the respondents who saw 1 or 2 patients, 36% indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% who saw more than 8 patients.
Responses regarding the absolute of always recommending ICP testing and the absolute of being 100% confident about the clinical diagnosis of CECS highlight differences between the surgeons with more experience (>11 years in practice, >8 CECS patients per year) and those with less experience (≤5 years in practice, 1 or 2 CECS patients per year). Surgeons in practice longer, and surgeons who saw more patients with suspected CECS per year, were more likely to recommend surgical treatment based solely on clinical findings.
Conclusion
CECS can cause debilitating activity-related leg pain in both civilian and military populations. Treatment with fasciotomy is often curative, but a significant number of patients may continue to have pain and disability. As the incidence of treatment failures may be higher in the military than in civilians, proper evaluation of patients with suspected CECS is particularly important for military orthopedic surgeons. The diagnosis of CECS can be challenging to both the clinician and patient, and diagnostic modalities remain imperfect. The results of this study highlight this, revealing less than 100% agreement regarding use of ICP testing (the gold standard) for diagnosis of CECS.
This study also highlights the need for an improved method of diagnosing CECS since 93% of respondents agreed or strongly agreed that ICP testing is unpleasant for the patient, and 90% would prefer a less-invasive test. In addition, the ICP testing criteria for establishing the diagnosis of CECS remain inconsistent. If a reliable, consistent, and less-invasive test were available, perhaps there would be less variability in practitioners’ evaluations of patients with CECS.
This study shows an inconsistency among military orthopedic surgeons evaluating and treating patients with CECS. As testing modalities for CECS remain imperfect, clinical acumen and experience assume an important role in the assessment of patients with suspected CECS.
1. Waterman BR, Laughlin M, Kilcoyne K, Cameron KL, Owens BD. Surgical treatment of chronic exertional compartment syndrome of the leg: failure rates and postoperative disability in an active patient population. J Bone Joint Surg Am. 2013;95(7):592-596.
2. Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36-40.
3. Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11(4):268-276.
4. Aweid O, Del Buono A, Malliaras P, et al. Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med. 2012;22(4):356-370.
5. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42(3):385-392.
6. van den Brand JG, Verleisdonk EJ, van der Werken C. Near infrared spectroscopy in the diagnosis of chronic exertional compartment syndrome. Am J Sports Med. 2004;32(2):452-456.
7. van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33(5):699-704.
8. Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30(6):321-325.
9. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
10. Pedowitz RA, Hargens AR, Mubarek SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18(1):35-40.1. Rorabeck CH, Bourne RB, Fowler PJ. The surgical treatment of exertional compartment syndrome in athletes. J Bone Joint Surg Am. 1983;65(9):1245-1251.
12. Rorabeck CH, Fowler PJ, Nott L. The results of fasciotomy in the management of chronic exertional compartment syndrome. Am J Sports Med. 1988;16(3):224-227.
13. Detmer DE, Sharpe K, Sufit RL, Girdley FM. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13(3):162-170.
14. Stein DA, Sennett BJ. One-portal endoscopically assisted fasciotomy for exertional compartment syndrome. Arthroscopy. 2005;21(1):108-112.
15. Fronek J, Mubarak SJ, Hargens AR, et al. Management of chronic exertional anterior compartment syndrome of the lower extremity. Clin Orthop Relat Res. 1989;(220):217-227.
16. Leversedge FJ, Casey PJ, Seiler JG 3rd, Xerogeanes JW. Endoscopically assisted fasciotomy: description of technique and in vitro assessment of lower-leg compartment decompression. Am J Sports Med. 2002;30(2):272-278.
17. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21(6):811-817.
18. Packer JD, Day MS, Nguyen JT, Hobart SJ, Hannafin JA, Metzl JD. Functional outcomes and patient satisfaction after fasciotomy for chronic exertional compartment syndrome. Am J Sports Med. 2013;41(2):430-436.
19. Diebal AR, Gregory R, Alitz C, Gerber JP. Effects of forefoot running on chronic exertional compartment syndrome: a case series. Int J Sports Phys Ther. 2011;6(4):312-321.
20. Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated with chronic exertional compartment syndrome. Am J Sports Med. 2012;40(5):1060-1067.
21. Kirby RL, McDermott AG. Anterior tibial compartment pressures during running with rearfoot and forefoot landing styles. Arch Phys Med Rehabil. 1983;64(7):296-299.
22. Isner-Horobeti ME, Dufour SP, Blaes C, Lecocq J. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41(11):2558-2566.
Chronic exertional compartment syndrome (CECS) is a common cause of leg pain during exertion in athletic and active-duty populations.1 It is caused by an increase in intramuscular pressure to a point that the tissues within the involved compartment become ischemic because of a decrease in arteriolar blood flow.2 This relative ischemia causes pain and may also be associated with neurologic symptoms. By definition, the pain associated with CECS resolves with rest. Patients typically describe a feeling of fullness or tightness, which eventually evolves into pain as they continue exercising. Pain onset is usually predictable and reproducible after a finite amount of time and/or intensity of exercise.
The differential diagnosis of leg pain during exercise includes CECS, medial tibial stress syndrome, popliteal entrapment syndrome, myopathy, peripheral nerve entrapment syndromes, stress fracture, and effort-induced rhabdomyolysis.3 CECS can be differentiated from other causes of leg pain with measurement of compartment pressures (the standard recommendation).4 Compartment pressure measurement, however, is invasive, time-consuming, and painful and may be associated with bleeding risk, infection, and nerve injury. Noninvasive means of testing for CECS (eg, magnetic resonance imaging [MRI], near-infrared spectroscopy [NIRS], thallium stress testing) remain experimental and expensive and are not easily accessible at all institutions.5-8 While invasive compartment pressure (ICP) testing remains an important tool in the diagnosis of CECS, its criteria and execution vary considerably. Aweid and colleagues4 performed a meta-analysis of use of ICP testing in the diagnosis of CECS and concluded that, though elevated ICP measurements are accepted as the gold standard for diagnosing CECS, the criteria outlined for a positive test lack high-level supporting evidence. In addition, how the test is performed has been inconsistent across studies—further clouding the literature.4
The review by Aweid and colleagues4 highlights the deficiencies in diagnosing CECS by ICP testing. In clinical practice, ICP testing is challenging for both the patient and physician. As other validated, less-invasive tests are lacking, emphasis should remain on the history and the physical examination. Although all athletic populations are at risk for CECS, the active-duty military population is at particularly high risk because of the physical requirements and demands of military service.1,9
We surveyed military orthopedic surgeons to investigate the clinical practice of performing ICP testing in patients with suspected CECS. We hypothesized that the rate of ICP testing among military orthopedic surgeons would not be 100% for patients with the typical signs and symptoms of CECS.
Materials and Methods
This study was approved by the institutional review board at Wright-Patterson Medical Center at Wright-Patterson Air Force Base in Ohio. A link to an online survey was distributed by email to members of the Society of Military Orthopaedic Surgeons. The anonymous survey polled the surgeons regarding basic demographic data and clinical practice as it pertains to the evaluation and treatment of CECS. No patient-protected health information was obtained. Survey results were compiled in a Microsoft Excel file for analysis.
Results
The survey was distributed to 606 email accounts; the response rate was 19% (114/606). Ninety-one surgeons (80%) indicated they have patients with CECS in their practice (Figure 1). Surgeons were asked how many CECS patients they see per year (responses are summarized in Figure 2) and how many years they have been in practice (Table).
Ninety-three percent of the respondents agreed or strongly agreed that ICP testing is unpleasant for the patient (Figure 3), and 90% would prefer a less-invasive test for confirmatory testing for CECS (Figure 4). Only 13% of respondents indicated they actually use noninvasive modalities (eg, MRI, NIRS) to confirm the diagnosis of CECS (Figure 5).
Respondents were asked about the practice of using ICP testing in the diagnosis of CECS (responses are summarized in Figures 6, 7). Although 85% of respondents agreed or strongly agreed with always confirming the diagnosis of CECS with ICP testing, 39% stated they would recommend surgical treatment without ICP testing if they were confident about the diagnosis based on clinical examination findings.
To better understand the apparent discrepancy between the percentage of surgeons who agreed or strongly agreed with always recommending ICP testing (85%) and the percentage who would recommend treatment without testing (39%), responses were stratified by clinical experience. Surgeons in practice more than 11 years (n = 35) were compared with those in practice 5 years or less (n = 31) (Table). Although the vast majority (85%) of respondents from both groups agreed or strongly agreed with always recommending ICP testing, 49% of those in practice more than 11 years and 29% in practice 5 years or less indicated they would recommend surgical treatment for CECS based solely on clinical examination findings (Figures 8, 9).
Responses were also stratified by number of CECS patients seen by each surgeon per year. Twenty-eight respondents saw 1 or 2 patients per year, and 12 saw more than 8 patients per year—31% and 13% of the total number of respondents, respectively. Of the respondents who saw 1 or 2 patients, 86% (24/28) agreed or strongly agreed with always recommending ICP testing—comparable to the 75% (9/12) who saw more than 8 patients (Figure 10). However, of the respondents who saw 1 or 2 patients, 36% (10/28) indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% (9/12) who saw more than 8 patients (Figure 11).
Discussion
CECS is a common cause of leg pain and a significant cause of disability among the active-duty military population. This was illustrated in 2 recent studies by Waterman and colleagues.1,9 The first1 investigated failure rates and disability after surgery for CECS among those on active duty. The authors showed that CECS is a substantial contributor to lower extremity disability in the military population and that there is a substantial risk for persistent symptoms despite surgical treatment. Nearly 1 in 5 patients experienced surgical failure after elective fasciotomy, and about 28% of patients were unable to return to the full activity required in the military. The second, more recent study9 was an epidemiologic study of risk factors associated with CECS in a physically active military population. The authors identified 4100 cases diagnosed between 2006 and 2011—representing an overall annual incidence of 0.49 per 1000 at-risk person-years, or about 683 cases per year; the authors also showed the incidence increased during the time period studied.
The diagnosis of CECS remains imperfect. A clinical history of exercise-induced lower leg pain that is relieved with rest suggests the diagnosis, but a confirmatory test is needed to distinguish CECS from other causes of exercise-induced leg pain. Although direct measurement of compartment pressures is the test used most often, it is invasive and time-consuming, can be uncomfortable for the patient, and may be associated with bleeding risk, infection, and nerve injury. Pedowitz and colleagues10 described the ICP testing criteria now generally used in the diagnosis of CECS. Unfortunately, there is little objective evidence supporting these criteria.4 Although less invasive tests (eg, MRI, NIRS) have been described,5-8 they may not be readily available across institutions, and further study is needed to validate their use in diagnosing CECS.
While an objective, validated test or measurement for confirming the diagnosis of CECS remains elusive, the outcomes after surgical treatment of CECS also remain imperfect. Surgery consists of both open and endoscopically assisted fasciotomy of the involved compartments.2,11-17 Reports of improvement after treatment range from 81% to 100%3; however, symptom relief does not come for all patients, particularly those in the military. Waterman and colleagues1 found a failure rate of about 20% among an active-duty military population. Packer and colleagues18 examined civilians with CECS, treated both operatively and nonoperatively. Patients in this series were diagnosed with CECS based on clinical symptoms as well as compartment pressure measurements according to the Pedowitz criteria. Although overall outcomes were better for operatively treated patients than for nonoperatively treated patients, only 47% of patients were completely pain-free, and 21% were unable to return to full activity.
More recent studies have explored use of other nonoperative treatment modalities. Diebal and colleagues19,20 used a running retraining program to treat patients with CECS. They based this treatment on the hypothesis that a heel-strike running pattern is associated with increased anterior compartment pressures.21 CECS patients who underwent a 6-week systematic treatment program focused on forefoot running, stride shortening, and hamstring activation during push-off experienced a decrease in clinical symptoms and posttreatment intracompartmental pressures.20 The improvements in clinical scores were maintained at 1-year follow-up. Another nonoperative intervention, recently described by Isner-Horobeti and colleagues,22 involves injecting botulinum toxin A (BoNT-A) into the anterior and lateral compartments of the leg. Sixteen patients with CECS received BoNT-A injections. On average, intracompartmental pressures were lower after injection than they were before injection. In addition, exertional pain was eliminated in 15 patients at an average follow-up of 4.4 months.
This survey-based study examined the practice patterns of military orthopedic surgeons who performed ICP testing for cases of suspected CECS. Our hypothesis was that, though ICP testing is the most commonly accepted method for confirming the diagnosis of CECS, the ICP testing rate would not be 100% among those surveyed.
The results of our study uncover an apparent inconsistency in survey responses among physicians who evaluate and treat patients with CECS. About 85% of respondents stated they would always recommend confirming the diagnosis of CECS with ICP testing. However, about 40% stated they would recommend surgical treatment without confirmatory testing if they were confident about the diagnosis based on clinical findings. In other words, only 60% of the respondents disagreed or strongly disagreed with pursuing surgical treatment without testing. One would expect a closer correlation between respondents who would always recommend ICP testing and those who disagreed with recommending surgical treatment without ICP testing. This raises the question of what actually occurs when CECS is suspected in clinical practice.
To better understand the apparent discrepancy between respondents who agreed or strongly agreed with always recommending ICP testing and respondents who would recommend treatment without testing, we grouped responses by clinical experience. Although 85% of respondents (no matter the number of years in practice) agreed or strongly agreed with the statement, “I always recommend confirming the diagnosis of CECS with ICP measurements,” 49% of those in practice more than 11 years (vs. 29% of those in practice 5 years or less) agreed or strongly agreed with recommending surgery without testing if they were 100% confident about the diagnosis of CECS based solely on clinical findings. This may suggest that, though most agreed that the gold standard for confirming the diagnosis of CECS remains ICP testing, those with more clinical experience were more comfortable forgoing this diagnostic measure and recommending treatment without testing.
Another measure of clinical experience used in this survey was based on number of CECS patients seen per year. Responses of surgeons who saw 1 or 2 patients with CECS per year were compared with responses of surgeons who saw more than 8 patients with CECS per year. Of the respondents who saw 1 or 2 patients, 86% agreed or strongly agreed with always recommending ICP testing to confirm CECS—comparable to the 75% who saw more than 8 patients. However, of the respondents who saw 1 or 2 patients, 36% indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% who saw more than 8 patients.
Responses regarding the absolute of always recommending ICP testing and the absolute of being 100% confident about the clinical diagnosis of CECS highlight differences between the surgeons with more experience (>11 years in practice, >8 CECS patients per year) and those with less experience (≤5 years in practice, 1 or 2 CECS patients per year). Surgeons in practice longer, and surgeons who saw more patients with suspected CECS per year, were more likely to recommend surgical treatment based solely on clinical findings.
Conclusion
CECS can cause debilitating activity-related leg pain in both civilian and military populations. Treatment with fasciotomy is often curative, but a significant number of patients may continue to have pain and disability. As the incidence of treatment failures may be higher in the military than in civilians, proper evaluation of patients with suspected CECS is particularly important for military orthopedic surgeons. The diagnosis of CECS can be challenging to both the clinician and patient, and diagnostic modalities remain imperfect. The results of this study highlight this, revealing less than 100% agreement regarding use of ICP testing (the gold standard) for diagnosis of CECS.
This study also highlights the need for an improved method of diagnosing CECS since 93% of respondents agreed or strongly agreed that ICP testing is unpleasant for the patient, and 90% would prefer a less-invasive test. In addition, the ICP testing criteria for establishing the diagnosis of CECS remain inconsistent. If a reliable, consistent, and less-invasive test were available, perhaps there would be less variability in practitioners’ evaluations of patients with CECS.
This study shows an inconsistency among military orthopedic surgeons evaluating and treating patients with CECS. As testing modalities for CECS remain imperfect, clinical acumen and experience assume an important role in the assessment of patients with suspected CECS.
Chronic exertional compartment syndrome (CECS) is a common cause of leg pain during exertion in athletic and active-duty populations.1 It is caused by an increase in intramuscular pressure to a point that the tissues within the involved compartment become ischemic because of a decrease in arteriolar blood flow.2 This relative ischemia causes pain and may also be associated with neurologic symptoms. By definition, the pain associated with CECS resolves with rest. Patients typically describe a feeling of fullness or tightness, which eventually evolves into pain as they continue exercising. Pain onset is usually predictable and reproducible after a finite amount of time and/or intensity of exercise.
The differential diagnosis of leg pain during exercise includes CECS, medial tibial stress syndrome, popliteal entrapment syndrome, myopathy, peripheral nerve entrapment syndromes, stress fracture, and effort-induced rhabdomyolysis.3 CECS can be differentiated from other causes of leg pain with measurement of compartment pressures (the standard recommendation).4 Compartment pressure measurement, however, is invasive, time-consuming, and painful and may be associated with bleeding risk, infection, and nerve injury. Noninvasive means of testing for CECS (eg, magnetic resonance imaging [MRI], near-infrared spectroscopy [NIRS], thallium stress testing) remain experimental and expensive and are not easily accessible at all institutions.5-8 While invasive compartment pressure (ICP) testing remains an important tool in the diagnosis of CECS, its criteria and execution vary considerably. Aweid and colleagues4 performed a meta-analysis of use of ICP testing in the diagnosis of CECS and concluded that, though elevated ICP measurements are accepted as the gold standard for diagnosing CECS, the criteria outlined for a positive test lack high-level supporting evidence. In addition, how the test is performed has been inconsistent across studies—further clouding the literature.4
The review by Aweid and colleagues4 highlights the deficiencies in diagnosing CECS by ICP testing. In clinical practice, ICP testing is challenging for both the patient and physician. As other validated, less-invasive tests are lacking, emphasis should remain on the history and the physical examination. Although all athletic populations are at risk for CECS, the active-duty military population is at particularly high risk because of the physical requirements and demands of military service.1,9
We surveyed military orthopedic surgeons to investigate the clinical practice of performing ICP testing in patients with suspected CECS. We hypothesized that the rate of ICP testing among military orthopedic surgeons would not be 100% for patients with the typical signs and symptoms of CECS.
Materials and Methods
This study was approved by the institutional review board at Wright-Patterson Medical Center at Wright-Patterson Air Force Base in Ohio. A link to an online survey was distributed by email to members of the Society of Military Orthopaedic Surgeons. The anonymous survey polled the surgeons regarding basic demographic data and clinical practice as it pertains to the evaluation and treatment of CECS. No patient-protected health information was obtained. Survey results were compiled in a Microsoft Excel file for analysis.
Results
The survey was distributed to 606 email accounts; the response rate was 19% (114/606). Ninety-one surgeons (80%) indicated they have patients with CECS in their practice (Figure 1). Surgeons were asked how many CECS patients they see per year (responses are summarized in Figure 2) and how many years they have been in practice (Table).
Ninety-three percent of the respondents agreed or strongly agreed that ICP testing is unpleasant for the patient (Figure 3), and 90% would prefer a less-invasive test for confirmatory testing for CECS (Figure 4). Only 13% of respondents indicated they actually use noninvasive modalities (eg, MRI, NIRS) to confirm the diagnosis of CECS (Figure 5).
Respondents were asked about the practice of using ICP testing in the diagnosis of CECS (responses are summarized in Figures 6, 7). Although 85% of respondents agreed or strongly agreed with always confirming the diagnosis of CECS with ICP testing, 39% stated they would recommend surgical treatment without ICP testing if they were confident about the diagnosis based on clinical examination findings.
To better understand the apparent discrepancy between the percentage of surgeons who agreed or strongly agreed with always recommending ICP testing (85%) and the percentage who would recommend treatment without testing (39%), responses were stratified by clinical experience. Surgeons in practice more than 11 years (n = 35) were compared with those in practice 5 years or less (n = 31) (Table). Although the vast majority (85%) of respondents from both groups agreed or strongly agreed with always recommending ICP testing, 49% of those in practice more than 11 years and 29% in practice 5 years or less indicated they would recommend surgical treatment for CECS based solely on clinical examination findings (Figures 8, 9).
Responses were also stratified by number of CECS patients seen by each surgeon per year. Twenty-eight respondents saw 1 or 2 patients per year, and 12 saw more than 8 patients per year—31% and 13% of the total number of respondents, respectively. Of the respondents who saw 1 or 2 patients, 86% (24/28) agreed or strongly agreed with always recommending ICP testing—comparable to the 75% (9/12) who saw more than 8 patients (Figure 10). However, of the respondents who saw 1 or 2 patients, 36% (10/28) indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% (9/12) who saw more than 8 patients (Figure 11).
Discussion
CECS is a common cause of leg pain and a significant cause of disability among the active-duty military population. This was illustrated in 2 recent studies by Waterman and colleagues.1,9 The first1 investigated failure rates and disability after surgery for CECS among those on active duty. The authors showed that CECS is a substantial contributor to lower extremity disability in the military population and that there is a substantial risk for persistent symptoms despite surgical treatment. Nearly 1 in 5 patients experienced surgical failure after elective fasciotomy, and about 28% of patients were unable to return to the full activity required in the military. The second, more recent study9 was an epidemiologic study of risk factors associated with CECS in a physically active military population. The authors identified 4100 cases diagnosed between 2006 and 2011—representing an overall annual incidence of 0.49 per 1000 at-risk person-years, or about 683 cases per year; the authors also showed the incidence increased during the time period studied.
The diagnosis of CECS remains imperfect. A clinical history of exercise-induced lower leg pain that is relieved with rest suggests the diagnosis, but a confirmatory test is needed to distinguish CECS from other causes of exercise-induced leg pain. Although direct measurement of compartment pressures is the test used most often, it is invasive and time-consuming, can be uncomfortable for the patient, and may be associated with bleeding risk, infection, and nerve injury. Pedowitz and colleagues10 described the ICP testing criteria now generally used in the diagnosis of CECS. Unfortunately, there is little objective evidence supporting these criteria.4 Although less invasive tests (eg, MRI, NIRS) have been described,5-8 they may not be readily available across institutions, and further study is needed to validate their use in diagnosing CECS.
While an objective, validated test or measurement for confirming the diagnosis of CECS remains elusive, the outcomes after surgical treatment of CECS also remain imperfect. Surgery consists of both open and endoscopically assisted fasciotomy of the involved compartments.2,11-17 Reports of improvement after treatment range from 81% to 100%3; however, symptom relief does not come for all patients, particularly those in the military. Waterman and colleagues1 found a failure rate of about 20% among an active-duty military population. Packer and colleagues18 examined civilians with CECS, treated both operatively and nonoperatively. Patients in this series were diagnosed with CECS based on clinical symptoms as well as compartment pressure measurements according to the Pedowitz criteria. Although overall outcomes were better for operatively treated patients than for nonoperatively treated patients, only 47% of patients were completely pain-free, and 21% were unable to return to full activity.
More recent studies have explored use of other nonoperative treatment modalities. Diebal and colleagues19,20 used a running retraining program to treat patients with CECS. They based this treatment on the hypothesis that a heel-strike running pattern is associated with increased anterior compartment pressures.21 CECS patients who underwent a 6-week systematic treatment program focused on forefoot running, stride shortening, and hamstring activation during push-off experienced a decrease in clinical symptoms and posttreatment intracompartmental pressures.20 The improvements in clinical scores were maintained at 1-year follow-up. Another nonoperative intervention, recently described by Isner-Horobeti and colleagues,22 involves injecting botulinum toxin A (BoNT-A) into the anterior and lateral compartments of the leg. Sixteen patients with CECS received BoNT-A injections. On average, intracompartmental pressures were lower after injection than they were before injection. In addition, exertional pain was eliminated in 15 patients at an average follow-up of 4.4 months.
This survey-based study examined the practice patterns of military orthopedic surgeons who performed ICP testing for cases of suspected CECS. Our hypothesis was that, though ICP testing is the most commonly accepted method for confirming the diagnosis of CECS, the ICP testing rate would not be 100% among those surveyed.
The results of our study uncover an apparent inconsistency in survey responses among physicians who evaluate and treat patients with CECS. About 85% of respondents stated they would always recommend confirming the diagnosis of CECS with ICP testing. However, about 40% stated they would recommend surgical treatment without confirmatory testing if they were confident about the diagnosis based on clinical findings. In other words, only 60% of the respondents disagreed or strongly disagreed with pursuing surgical treatment without testing. One would expect a closer correlation between respondents who would always recommend ICP testing and those who disagreed with recommending surgical treatment without ICP testing. This raises the question of what actually occurs when CECS is suspected in clinical practice.
To better understand the apparent discrepancy between respondents who agreed or strongly agreed with always recommending ICP testing and respondents who would recommend treatment without testing, we grouped responses by clinical experience. Although 85% of respondents (no matter the number of years in practice) agreed or strongly agreed with the statement, “I always recommend confirming the diagnosis of CECS with ICP measurements,” 49% of those in practice more than 11 years (vs. 29% of those in practice 5 years or less) agreed or strongly agreed with recommending surgery without testing if they were 100% confident about the diagnosis of CECS based solely on clinical findings. This may suggest that, though most agreed that the gold standard for confirming the diagnosis of CECS remains ICP testing, those with more clinical experience were more comfortable forgoing this diagnostic measure and recommending treatment without testing.
Another measure of clinical experience used in this survey was based on number of CECS patients seen per year. Responses of surgeons who saw 1 or 2 patients with CECS per year were compared with responses of surgeons who saw more than 8 patients with CECS per year. Of the respondents who saw 1 or 2 patients, 86% agreed or strongly agreed with always recommending ICP testing to confirm CECS—comparable to the 75% who saw more than 8 patients. However, of the respondents who saw 1 or 2 patients, 36% indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% who saw more than 8 patients.
Responses regarding the absolute of always recommending ICP testing and the absolute of being 100% confident about the clinical diagnosis of CECS highlight differences between the surgeons with more experience (>11 years in practice, >8 CECS patients per year) and those with less experience (≤5 years in practice, 1 or 2 CECS patients per year). Surgeons in practice longer, and surgeons who saw more patients with suspected CECS per year, were more likely to recommend surgical treatment based solely on clinical findings.
Conclusion
CECS can cause debilitating activity-related leg pain in both civilian and military populations. Treatment with fasciotomy is often curative, but a significant number of patients may continue to have pain and disability. As the incidence of treatment failures may be higher in the military than in civilians, proper evaluation of patients with suspected CECS is particularly important for military orthopedic surgeons. The diagnosis of CECS can be challenging to both the clinician and patient, and diagnostic modalities remain imperfect. The results of this study highlight this, revealing less than 100% agreement regarding use of ICP testing (the gold standard) for diagnosis of CECS.
This study also highlights the need for an improved method of diagnosing CECS since 93% of respondents agreed or strongly agreed that ICP testing is unpleasant for the patient, and 90% would prefer a less-invasive test. In addition, the ICP testing criteria for establishing the diagnosis of CECS remain inconsistent. If a reliable, consistent, and less-invasive test were available, perhaps there would be less variability in practitioners’ evaluations of patients with CECS.
This study shows an inconsistency among military orthopedic surgeons evaluating and treating patients with CECS. As testing modalities for CECS remain imperfect, clinical acumen and experience assume an important role in the assessment of patients with suspected CECS.
1. Waterman BR, Laughlin M, Kilcoyne K, Cameron KL, Owens BD. Surgical treatment of chronic exertional compartment syndrome of the leg: failure rates and postoperative disability in an active patient population. J Bone Joint Surg Am. 2013;95(7):592-596.
2. Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36-40.
3. Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11(4):268-276.
4. Aweid O, Del Buono A, Malliaras P, et al. Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med. 2012;22(4):356-370.
5. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42(3):385-392.
6. van den Brand JG, Verleisdonk EJ, van der Werken C. Near infrared spectroscopy in the diagnosis of chronic exertional compartment syndrome. Am J Sports Med. 2004;32(2):452-456.
7. van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33(5):699-704.
8. Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30(6):321-325.
9. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
10. Pedowitz RA, Hargens AR, Mubarek SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18(1):35-40.1. Rorabeck CH, Bourne RB, Fowler PJ. The surgical treatment of exertional compartment syndrome in athletes. J Bone Joint Surg Am. 1983;65(9):1245-1251.
12. Rorabeck CH, Fowler PJ, Nott L. The results of fasciotomy in the management of chronic exertional compartment syndrome. Am J Sports Med. 1988;16(3):224-227.
13. Detmer DE, Sharpe K, Sufit RL, Girdley FM. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13(3):162-170.
14. Stein DA, Sennett BJ. One-portal endoscopically assisted fasciotomy for exertional compartment syndrome. Arthroscopy. 2005;21(1):108-112.
15. Fronek J, Mubarak SJ, Hargens AR, et al. Management of chronic exertional anterior compartment syndrome of the lower extremity. Clin Orthop Relat Res. 1989;(220):217-227.
16. Leversedge FJ, Casey PJ, Seiler JG 3rd, Xerogeanes JW. Endoscopically assisted fasciotomy: description of technique and in vitro assessment of lower-leg compartment decompression. Am J Sports Med. 2002;30(2):272-278.
17. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21(6):811-817.
18. Packer JD, Day MS, Nguyen JT, Hobart SJ, Hannafin JA, Metzl JD. Functional outcomes and patient satisfaction after fasciotomy for chronic exertional compartment syndrome. Am J Sports Med. 2013;41(2):430-436.
19. Diebal AR, Gregory R, Alitz C, Gerber JP. Effects of forefoot running on chronic exertional compartment syndrome: a case series. Int J Sports Phys Ther. 2011;6(4):312-321.
20. Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated with chronic exertional compartment syndrome. Am J Sports Med. 2012;40(5):1060-1067.
21. Kirby RL, McDermott AG. Anterior tibial compartment pressures during running with rearfoot and forefoot landing styles. Arch Phys Med Rehabil. 1983;64(7):296-299.
22. Isner-Horobeti ME, Dufour SP, Blaes C, Lecocq J. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41(11):2558-2566.
1. Waterman BR, Laughlin M, Kilcoyne K, Cameron KL, Owens BD. Surgical treatment of chronic exertional compartment syndrome of the leg: failure rates and postoperative disability in an active patient population. J Bone Joint Surg Am. 2013;95(7):592-596.
2. Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36-40.
3. Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11(4):268-276.
4. Aweid O, Del Buono A, Malliaras P, et al. Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med. 2012;22(4):356-370.
5. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42(3):385-392.
6. van den Brand JG, Verleisdonk EJ, van der Werken C. Near infrared spectroscopy in the diagnosis of chronic exertional compartment syndrome. Am J Sports Med. 2004;32(2):452-456.
7. van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33(5):699-704.
8. Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30(6):321-325.
9. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
10. Pedowitz RA, Hargens AR, Mubarek SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18(1):35-40.1. Rorabeck CH, Bourne RB, Fowler PJ. The surgical treatment of exertional compartment syndrome in athletes. J Bone Joint Surg Am. 1983;65(9):1245-1251.
12. Rorabeck CH, Fowler PJ, Nott L. The results of fasciotomy in the management of chronic exertional compartment syndrome. Am J Sports Med. 1988;16(3):224-227.
13. Detmer DE, Sharpe K, Sufit RL, Girdley FM. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13(3):162-170.
14. Stein DA, Sennett BJ. One-portal endoscopically assisted fasciotomy for exertional compartment syndrome. Arthroscopy. 2005;21(1):108-112.
15. Fronek J, Mubarak SJ, Hargens AR, et al. Management of chronic exertional anterior compartment syndrome of the lower extremity. Clin Orthop Relat Res. 1989;(220):217-227.
16. Leversedge FJ, Casey PJ, Seiler JG 3rd, Xerogeanes JW. Endoscopically assisted fasciotomy: description of technique and in vitro assessment of lower-leg compartment decompression. Am J Sports Med. 2002;30(2):272-278.
17. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21(6):811-817.
18. Packer JD, Day MS, Nguyen JT, Hobart SJ, Hannafin JA, Metzl JD. Functional outcomes and patient satisfaction after fasciotomy for chronic exertional compartment syndrome. Am J Sports Med. 2013;41(2):430-436.
19. Diebal AR, Gregory R, Alitz C, Gerber JP. Effects of forefoot running on chronic exertional compartment syndrome: a case series. Int J Sports Phys Ther. 2011;6(4):312-321.
20. Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated with chronic exertional compartment syndrome. Am J Sports Med. 2012;40(5):1060-1067.
21. Kirby RL, McDermott AG. Anterior tibial compartment pressures during running with rearfoot and forefoot landing styles. Arch Phys Med Rehabil. 1983;64(7):296-299.
22. Isner-Horobeti ME, Dufour SP, Blaes C, Lecocq J. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41(11):2558-2566.