User login
FDA warns of risks of excessive dosing of obeticholic acid
The Food and Drug Administration advised on Sept. 21 that incorrect excessive dosing of the liver disease medicine obeticholic acid (Ocaliva) creates an increased risk of serious liver injury and death. The agency recommends closer monitoring of dosages and following the current label’s recommendations.
Obeticholic acid is used to treat primary biliary cholangitis. Patients taking the drug should first be tested for their baseline liver function so that the effects of the drug can be monitored. The level of liver impairment determines the recommended dosage: Those with moderate to severe impairment should start by taking 5 mg weekly (with a possible increase to 10 mg weekly) instead of the standard 5 mg daily. Those who start at the standard dose and progress to moderate or severe liver impairment can have their dosage reduced or can discontinue the drug altogether.
The Food and Drug Administration advised on Sept. 21 that incorrect excessive dosing of the liver disease medicine obeticholic acid (Ocaliva) creates an increased risk of serious liver injury and death. The agency recommends closer monitoring of dosages and following the current label’s recommendations.
Obeticholic acid is used to treat primary biliary cholangitis. Patients taking the drug should first be tested for their baseline liver function so that the effects of the drug can be monitored. The level of liver impairment determines the recommended dosage: Those with moderate to severe impairment should start by taking 5 mg weekly (with a possible increase to 10 mg weekly) instead of the standard 5 mg daily. Those who start at the standard dose and progress to moderate or severe liver impairment can have their dosage reduced or can discontinue the drug altogether.
The Food and Drug Administration advised on Sept. 21 that incorrect excessive dosing of the liver disease medicine obeticholic acid (Ocaliva) creates an increased risk of serious liver injury and death. The agency recommends closer monitoring of dosages and following the current label’s recommendations.
Obeticholic acid is used to treat primary biliary cholangitis. Patients taking the drug should first be tested for their baseline liver function so that the effects of the drug can be monitored. The level of liver impairment determines the recommended dosage: Those with moderate to severe impairment should start by taking 5 mg weekly (with a possible increase to 10 mg weekly) instead of the standard 5 mg daily. Those who start at the standard dose and progress to moderate or severe liver impairment can have their dosage reduced or can discontinue the drug altogether.
Do not withhold opioid addiction drugs from patients taking benzodiazepines
The opioid addiction medications buprenorphine and methadone should not be withheld from patients who are taking benzodiazepines or other drugs that depress the central nervous system, the Food and Drug Administration advised in a safety alert posted Sept. 20.
The combined use of these medication-assisted treatment (MAT) drugs and central nervous system (CNS) depressants can lead to serious side effects. But the harm associated with opioid addiction that remains untreated usually outweighs those risks, the agency said. After reviewing this issue, the FDA said, it is requiring that this information be added to the drug labels of buprenorphine and methadone in addition to “recommendations for minimizing the use of [MAT] drugs and benzodiazepines together.”
Discontinuing MAT drugs is a goal, but patients might need medication-assisted treatment indefinitely. “Use should continue for as long as patients are benefiting and their use contributes to the intended treatment goals,” the alert said.
The opioid addiction medications buprenorphine and methadone should not be withheld from patients who are taking benzodiazepines or other drugs that depress the central nervous system, the Food and Drug Administration advised in a safety alert posted Sept. 20.
The combined use of these medication-assisted treatment (MAT) drugs and central nervous system (CNS) depressants can lead to serious side effects. But the harm associated with opioid addiction that remains untreated usually outweighs those risks, the agency said. After reviewing this issue, the FDA said, it is requiring that this information be added to the drug labels of buprenorphine and methadone in addition to “recommendations for minimizing the use of [MAT] drugs and benzodiazepines together.”
Discontinuing MAT drugs is a goal, but patients might need medication-assisted treatment indefinitely. “Use should continue for as long as patients are benefiting and their use contributes to the intended treatment goals,” the alert said.
The opioid addiction medications buprenorphine and methadone should not be withheld from patients who are taking benzodiazepines or other drugs that depress the central nervous system, the Food and Drug Administration advised in a safety alert posted Sept. 20.
The combined use of these medication-assisted treatment (MAT) drugs and central nervous system (CNS) depressants can lead to serious side effects. But the harm associated with opioid addiction that remains untreated usually outweighs those risks, the agency said. After reviewing this issue, the FDA said, it is requiring that this information be added to the drug labels of buprenorphine and methadone in addition to “recommendations for minimizing the use of [MAT] drugs and benzodiazepines together.”
Discontinuing MAT drugs is a goal, but patients might need medication-assisted treatment indefinitely. “Use should continue for as long as patients are benefiting and their use contributes to the intended treatment goals,” the alert said.
Parents of very preterm and VLBW infants have resilient quality of life decades later
A prospective cohort study of parents whose children were born very preterm (VP) or very low birth weight (VLBW) showed that their quality of life once their children reached adulthood was comparable to that of parents of term children. What did predict a poorer quality of life for these parents was whether their children had mental health problems or worse peer relationships in childhood.
The questionnaire used was the World Health Organization Quality of Life instrument, Short Edition, which assesses physical health, psychological health, social relationships, and environment in 26 items. Parents also answered the five-item Satisfaction with Life Scale.
“The overall Quality of Life factor score of parents of VP and VLBW and term offspring was not different (VP and VLBW: mean standardized factor score −0.02; 95% confidence interval, −0.13 to 0.08; term controls: mean standardized factor score 0.02; 95% CI, −0.07 to 0.12; P greater than .05),” wrote Dieter Wolke, PhD, of the University of Warwick (England) and his coauthors.
VP and VLBW, disabilities, poorer academic achievement, and poorer parent-child relationships did not predict a lower quality of life for the parents. However, “in offspring, better mental health and peer relationships in childhood (independently of whether they were born VP or VLBW or term) still predicted parents’ quality of life more than a decade later,” they said.
Thus, taking measures to improve children’s mental health and peer relationships likely would aid both the children and their parents, they observed.
One thing that the authors do not remark on is that a significant number of the VP and VLBW infants in the Bavarian study’s initial sample died in the hospital (172 of 682; 25%), with another 12 deaths between the infants’ discharge and adulthood; the cohort study’s focus, therefore, is limited to the quality of life of parents whose children are still alive. Some other eligible parents dropped out, although the authors noted that they tried to control for factors differing between the dropouts and the participants.
Read more at Pediatrics (2017 Sep;140[3]:e20171263)
A prospective cohort study of parents whose children were born very preterm (VP) or very low birth weight (VLBW) showed that their quality of life once their children reached adulthood was comparable to that of parents of term children. What did predict a poorer quality of life for these parents was whether their children had mental health problems or worse peer relationships in childhood.
The questionnaire used was the World Health Organization Quality of Life instrument, Short Edition, which assesses physical health, psychological health, social relationships, and environment in 26 items. Parents also answered the five-item Satisfaction with Life Scale.
“The overall Quality of Life factor score of parents of VP and VLBW and term offspring was not different (VP and VLBW: mean standardized factor score −0.02; 95% confidence interval, −0.13 to 0.08; term controls: mean standardized factor score 0.02; 95% CI, −0.07 to 0.12; P greater than .05),” wrote Dieter Wolke, PhD, of the University of Warwick (England) and his coauthors.
VP and VLBW, disabilities, poorer academic achievement, and poorer parent-child relationships did not predict a lower quality of life for the parents. However, “in offspring, better mental health and peer relationships in childhood (independently of whether they were born VP or VLBW or term) still predicted parents’ quality of life more than a decade later,” they said.
Thus, taking measures to improve children’s mental health and peer relationships likely would aid both the children and their parents, they observed.
One thing that the authors do not remark on is that a significant number of the VP and VLBW infants in the Bavarian study’s initial sample died in the hospital (172 of 682; 25%), with another 12 deaths between the infants’ discharge and adulthood; the cohort study’s focus, therefore, is limited to the quality of life of parents whose children are still alive. Some other eligible parents dropped out, although the authors noted that they tried to control for factors differing between the dropouts and the participants.
Read more at Pediatrics (2017 Sep;140[3]:e20171263)
A prospective cohort study of parents whose children were born very preterm (VP) or very low birth weight (VLBW) showed that their quality of life once their children reached adulthood was comparable to that of parents of term children. What did predict a poorer quality of life for these parents was whether their children had mental health problems or worse peer relationships in childhood.
The questionnaire used was the World Health Organization Quality of Life instrument, Short Edition, which assesses physical health, psychological health, social relationships, and environment in 26 items. Parents also answered the five-item Satisfaction with Life Scale.
“The overall Quality of Life factor score of parents of VP and VLBW and term offspring was not different (VP and VLBW: mean standardized factor score −0.02; 95% confidence interval, −0.13 to 0.08; term controls: mean standardized factor score 0.02; 95% CI, −0.07 to 0.12; P greater than .05),” wrote Dieter Wolke, PhD, of the University of Warwick (England) and his coauthors.
VP and VLBW, disabilities, poorer academic achievement, and poorer parent-child relationships did not predict a lower quality of life for the parents. However, “in offspring, better mental health and peer relationships in childhood (independently of whether they were born VP or VLBW or term) still predicted parents’ quality of life more than a decade later,” they said.
Thus, taking measures to improve children’s mental health and peer relationships likely would aid both the children and their parents, they observed.
One thing that the authors do not remark on is that a significant number of the VP and VLBW infants in the Bavarian study’s initial sample died in the hospital (172 of 682; 25%), with another 12 deaths between the infants’ discharge and adulthood; the cohort study’s focus, therefore, is limited to the quality of life of parents whose children are still alive. Some other eligible parents dropped out, although the authors noted that they tried to control for factors differing between the dropouts and the participants.
Read more at Pediatrics (2017 Sep;140[3]:e20171263)
FROM PEDIATRICS
Ten-year outcomes support skipping axillary lymph node dissection with positive sentinel nodes
A follow-up to a study showing the noninferiority of sentinel lymph node dissection to axillary lymph node dissection for breast cancer in overall and disease-free survival at a median of 6.3 years found similar noninferiority in overall survival at 10 years.
Axillary lymph node dissection has a risk of complications including lymphedema, numbness, axillary web syndrome, and decreased upper-extremity range of motion. The American College of Surgeons Oncology Group Z0011 trial sought to determine if the procedure could be avoided without inferior survival outcomes.
Criticism of the study focused on the potential for later recurrence, particularly in patients with hormone receptor–positive breast cancer. All enrolled patients had one or two sentinel nodes with metastases. At randomization, 436 received sentinel lymph node dissection alone, and 420 received the additional axillary lymph node dissection. The patients were assessed every 6 months for the first 3 years, then annually.
After a median of 9.3 years, 110 of the patients had died of any cause – 51 in the sentinel lymph node dissection group and 59 in the axillary lymph node dissection group – a 10-year overall survival rate of 86.3% and 83.6%, respectively. This met the study’s primary endpoint of showing noninferior overall survival without the riskier procedure. In the study’s secondary endpoint, disease-free survival, there was not a significant difference either (80.2% vs. 78.2%).
“Axillary dissections are associated with considerable morbidity, and the results of this trial demonstrated that this morbidity can be avoided without decreasing cancer control. … These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes,” wrote Armando E. Guiliano, MD, of Cedars-Sinai Medical, Los Angeles and his coauthors (JAMA. 2017;318[10]:918-26. doi: 10.1001/jama.2017.11470).
A follow-up to a study showing the noninferiority of sentinel lymph node dissection to axillary lymph node dissection for breast cancer in overall and disease-free survival at a median of 6.3 years found similar noninferiority in overall survival at 10 years.
Axillary lymph node dissection has a risk of complications including lymphedema, numbness, axillary web syndrome, and decreased upper-extremity range of motion. The American College of Surgeons Oncology Group Z0011 trial sought to determine if the procedure could be avoided without inferior survival outcomes.
Criticism of the study focused on the potential for later recurrence, particularly in patients with hormone receptor–positive breast cancer. All enrolled patients had one or two sentinel nodes with metastases. At randomization, 436 received sentinel lymph node dissection alone, and 420 received the additional axillary lymph node dissection. The patients were assessed every 6 months for the first 3 years, then annually.
After a median of 9.3 years, 110 of the patients had died of any cause – 51 in the sentinel lymph node dissection group and 59 in the axillary lymph node dissection group – a 10-year overall survival rate of 86.3% and 83.6%, respectively. This met the study’s primary endpoint of showing noninferior overall survival without the riskier procedure. In the study’s secondary endpoint, disease-free survival, there was not a significant difference either (80.2% vs. 78.2%).
“Axillary dissections are associated with considerable morbidity, and the results of this trial demonstrated that this morbidity can be avoided without decreasing cancer control. … These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes,” wrote Armando E. Guiliano, MD, of Cedars-Sinai Medical, Los Angeles and his coauthors (JAMA. 2017;318[10]:918-26. doi: 10.1001/jama.2017.11470).
A follow-up to a study showing the noninferiority of sentinel lymph node dissection to axillary lymph node dissection for breast cancer in overall and disease-free survival at a median of 6.3 years found similar noninferiority in overall survival at 10 years.
Axillary lymph node dissection has a risk of complications including lymphedema, numbness, axillary web syndrome, and decreased upper-extremity range of motion. The American College of Surgeons Oncology Group Z0011 trial sought to determine if the procedure could be avoided without inferior survival outcomes.
Criticism of the study focused on the potential for later recurrence, particularly in patients with hormone receptor–positive breast cancer. All enrolled patients had one or two sentinel nodes with metastases. At randomization, 436 received sentinel lymph node dissection alone, and 420 received the additional axillary lymph node dissection. The patients were assessed every 6 months for the first 3 years, then annually.
After a median of 9.3 years, 110 of the patients had died of any cause – 51 in the sentinel lymph node dissection group and 59 in the axillary lymph node dissection group – a 10-year overall survival rate of 86.3% and 83.6%, respectively. This met the study’s primary endpoint of showing noninferior overall survival without the riskier procedure. In the study’s secondary endpoint, disease-free survival, there was not a significant difference either (80.2% vs. 78.2%).
“Axillary dissections are associated with considerable morbidity, and the results of this trial demonstrated that this morbidity can be avoided without decreasing cancer control. … These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes,” wrote Armando E. Guiliano, MD, of Cedars-Sinai Medical, Los Angeles and his coauthors (JAMA. 2017;318[10]:918-26. doi: 10.1001/jama.2017.11470).
FROM JAMA
Three percent of high school seniors report using synthetic cannabinoids
, according to data from the national Monitoring the Future survey.
SCs are more potent than marijuana (as much as 100 times stronger); do not contain the anti-anxiety and antipsychotic constituent of marijuana, cannabidiol; and have a greater risk of a range of adverse effects, such as tachycardia, agitation, nausea, generalized tonic-clonic seizures, psychiatric problems, and death.
Joseph J. Palamar, PhD, MPH, of New York University, and his colleagues set out to examine data about current use of SCs, as previous studies had focused on lifetime or past-year use. Monitoring the Future surveys approximately 15,000 high school seniors annually in the United States. Of the six survey forms (which are distributed randomly), only two asked seniors about their current use of SCs, limiting the current study to a third of the survey sample.
The researchers found that, while 81% of current SC users also reported current use of marijuana, only 9% of current marijuana users reported current use of SCs. SC use was correlated much more highly than marijuana use with the current use of other drugs such as LSD (15% vs. 3%), opioids (14% vs. 6%), cocaine (11% vs. 3%), and heroin (6% vs. 0.1%).
“Current SC use appears to be part of a more extensive polydrug use repertoire involving other illegal drugs that are less prevalent among marijuana-only users. ... These associations suggest the need to target marijuana users who also use other drugs to help prevent initiation of SCs,” concluded Dr. Palamar and his associates.
Read more at (Pediatrics. 2017 Oct;140[4]:e20171330).
, according to data from the national Monitoring the Future survey.
SCs are more potent than marijuana (as much as 100 times stronger); do not contain the anti-anxiety and antipsychotic constituent of marijuana, cannabidiol; and have a greater risk of a range of adverse effects, such as tachycardia, agitation, nausea, generalized tonic-clonic seizures, psychiatric problems, and death.
Joseph J. Palamar, PhD, MPH, of New York University, and his colleagues set out to examine data about current use of SCs, as previous studies had focused on lifetime or past-year use. Monitoring the Future surveys approximately 15,000 high school seniors annually in the United States. Of the six survey forms (which are distributed randomly), only two asked seniors about their current use of SCs, limiting the current study to a third of the survey sample.
The researchers found that, while 81% of current SC users also reported current use of marijuana, only 9% of current marijuana users reported current use of SCs. SC use was correlated much more highly than marijuana use with the current use of other drugs such as LSD (15% vs. 3%), opioids (14% vs. 6%), cocaine (11% vs. 3%), and heroin (6% vs. 0.1%).
“Current SC use appears to be part of a more extensive polydrug use repertoire involving other illegal drugs that are less prevalent among marijuana-only users. ... These associations suggest the need to target marijuana users who also use other drugs to help prevent initiation of SCs,” concluded Dr. Palamar and his associates.
Read more at (Pediatrics. 2017 Oct;140[4]:e20171330).
, according to data from the national Monitoring the Future survey.
SCs are more potent than marijuana (as much as 100 times stronger); do not contain the anti-anxiety and antipsychotic constituent of marijuana, cannabidiol; and have a greater risk of a range of adverse effects, such as tachycardia, agitation, nausea, generalized tonic-clonic seizures, psychiatric problems, and death.
Joseph J. Palamar, PhD, MPH, of New York University, and his colleagues set out to examine data about current use of SCs, as previous studies had focused on lifetime or past-year use. Monitoring the Future surveys approximately 15,000 high school seniors annually in the United States. Of the six survey forms (which are distributed randomly), only two asked seniors about their current use of SCs, limiting the current study to a third of the survey sample.
The researchers found that, while 81% of current SC users also reported current use of marijuana, only 9% of current marijuana users reported current use of SCs. SC use was correlated much more highly than marijuana use with the current use of other drugs such as LSD (15% vs. 3%), opioids (14% vs. 6%), cocaine (11% vs. 3%), and heroin (6% vs. 0.1%).
“Current SC use appears to be part of a more extensive polydrug use repertoire involving other illegal drugs that are less prevalent among marijuana-only users. ... These associations suggest the need to target marijuana users who also use other drugs to help prevent initiation of SCs,” concluded Dr. Palamar and his associates.
Read more at (Pediatrics. 2017 Oct;140[4]:e20171330).
FROM PEDIATRICS
Tivozanib gets EU approval for advanced RCC
The European Commission has approved tivozanib for the treatment of advanced renal cell carcinoma (RCC) in adult patients in the European Union, Norway, and Iceland.
Tivozanib (Fotivda) is a vascular endothelial growth factor receptor tyrosine kinase inhibitor, taken orally once daily. It is indicated for first-line treatment of patients, naive to both vascular endothelial growth factor receptors and mTOR pathway inhibitors, experiencing disease progression following one cytokine therapy treatment, according to the press release.
Its approval is based on superior progression-free survival (PFS) in TIVO-1, a phase 3 trial comparing the efficacy and tolerability of tivozanib (1.5 mg once daily) with that of sorafenib (400 mg twice daily). In the overall trial population of 517 patients with advanced RCC, PFS was 11.9 months for patients treated with tivozanib, compared with 9.1 months for those treated with sorafenib (hazard ratio, 0.797; 95% confidence interval, 0.639-0.993; P = .042).
Patients in the tivozanib arm also experienced fewer cases of diarrhea and hand-foot syndrome, and required fewer dose reductions because of adverse effects than did those taking sorafenib.
The approval follows a recommendation from the Committee for Medical Products for Human Use.
The Food and Drug Administration rejected the New Drug Application for tivozanib in 2013, based on TIVO-1 data. Aveo Oncology plans to reapply in the United States with data from TIVO-3, expected in early 2018, they said in the press release.
The European Commission has approved tivozanib for the treatment of advanced renal cell carcinoma (RCC) in adult patients in the European Union, Norway, and Iceland.
Tivozanib (Fotivda) is a vascular endothelial growth factor receptor tyrosine kinase inhibitor, taken orally once daily. It is indicated for first-line treatment of patients, naive to both vascular endothelial growth factor receptors and mTOR pathway inhibitors, experiencing disease progression following one cytokine therapy treatment, according to the press release.
Its approval is based on superior progression-free survival (PFS) in TIVO-1, a phase 3 trial comparing the efficacy and tolerability of tivozanib (1.5 mg once daily) with that of sorafenib (400 mg twice daily). In the overall trial population of 517 patients with advanced RCC, PFS was 11.9 months for patients treated with tivozanib, compared with 9.1 months for those treated with sorafenib (hazard ratio, 0.797; 95% confidence interval, 0.639-0.993; P = .042).
Patients in the tivozanib arm also experienced fewer cases of diarrhea and hand-foot syndrome, and required fewer dose reductions because of adverse effects than did those taking sorafenib.
The approval follows a recommendation from the Committee for Medical Products for Human Use.
The Food and Drug Administration rejected the New Drug Application for tivozanib in 2013, based on TIVO-1 data. Aveo Oncology plans to reapply in the United States with data from TIVO-3, expected in early 2018, they said in the press release.
The European Commission has approved tivozanib for the treatment of advanced renal cell carcinoma (RCC) in adult patients in the European Union, Norway, and Iceland.
Tivozanib (Fotivda) is a vascular endothelial growth factor receptor tyrosine kinase inhibitor, taken orally once daily. It is indicated for first-line treatment of patients, naive to both vascular endothelial growth factor receptors and mTOR pathway inhibitors, experiencing disease progression following one cytokine therapy treatment, according to the press release.
Its approval is based on superior progression-free survival (PFS) in TIVO-1, a phase 3 trial comparing the efficacy and tolerability of tivozanib (1.5 mg once daily) with that of sorafenib (400 mg twice daily). In the overall trial population of 517 patients with advanced RCC, PFS was 11.9 months for patients treated with tivozanib, compared with 9.1 months for those treated with sorafenib (hazard ratio, 0.797; 95% confidence interval, 0.639-0.993; P = .042).
Patients in the tivozanib arm also experienced fewer cases of diarrhea and hand-foot syndrome, and required fewer dose reductions because of adverse effects than did those taking sorafenib.
The approval follows a recommendation from the Committee for Medical Products for Human Use.
The Food and Drug Administration rejected the New Drug Application for tivozanib in 2013, based on TIVO-1 data. Aveo Oncology plans to reapply in the United States with data from TIVO-3, expected in early 2018, they said in the press release.
Exelixis seeks expanded indication for cabozantinib in RCC
Exelixis has submitted a supplemental New Drug Application to the Food and Drug Administration for cabozantinib (Cabometyx) for the treatment of previously untreated advanced renal cell carcinoma (RCC).
The application, announced on Aug. 16, seeks to allow the manufacturer to modify the label. Cabozantinib was approved in April 2016 for treatment of patients with advanced RCC who had previously received antiangiogenic therapy.
The results of the trial were published in the Journal of Clinical Oncology (2017 Feb 20;35[6]:591-7). An independent review committee confirmed the primary efficacy endpoint results in June 2017.
Exelixis has submitted a supplemental New Drug Application to the Food and Drug Administration for cabozantinib (Cabometyx) for the treatment of previously untreated advanced renal cell carcinoma (RCC).
The application, announced on Aug. 16, seeks to allow the manufacturer to modify the label. Cabozantinib was approved in April 2016 for treatment of patients with advanced RCC who had previously received antiangiogenic therapy.
The results of the trial were published in the Journal of Clinical Oncology (2017 Feb 20;35[6]:591-7). An independent review committee confirmed the primary efficacy endpoint results in June 2017.
Exelixis has submitted a supplemental New Drug Application to the Food and Drug Administration for cabozantinib (Cabometyx) for the treatment of previously untreated advanced renal cell carcinoma (RCC).
The application, announced on Aug. 16, seeks to allow the manufacturer to modify the label. Cabozantinib was approved in April 2016 for treatment of patients with advanced RCC who had previously received antiangiogenic therapy.
The results of the trial were published in the Journal of Clinical Oncology (2017 Feb 20;35[6]:591-7). An independent review committee confirmed the primary efficacy endpoint results in June 2017.
Cognitive impairment in MS may affect fitness to drive motor vehicles
Assessing fitness to drive a motor vehicle is an important part of clinical practice for multiple sclerosis, but MS and cognitive impairment alone don’t indicate that a patient will fail a formal road test. However, the results from a recent study indicate that one cognitive test may be able to predict the patients who would fail road tests though only with a high false-positive rate.
During 2015-2016, Sarah A. Morrow, MD, of Western University, London, Ont., and her coauthors recruited licensed drivers aged 18-59 who were diagnosed with MS. They had low physical disability (Expanded Disability Status Scale score of less than 4) but cognitive impairment in both processing speed and either memory or executive function. This set of requirements, along with the need for signed informed consent, yielded a smaller sample size (36) than the researchers’ goal, a possible weakness of the study, the investigators said (Mult Scler. 2017 Aug 7. doi: 10.1177/1352458517723991).
The researchers noted that, if a patient does not show impairment on the BVMTR-IR, then they will definitely pass the road test, but they also noted that showing impairment is much less likely to predict a failed test. Instead, impairment both on this test and on the Symbol Digit Modalities Test, which measures processing speed, should indicate the need for a formal driving assessment.
“This study further contributes to the clinician’s ability to identify [persons with MS] in whom fitness-to-drive should be addressed,” the investigators wrote.
Assessing fitness to drive a motor vehicle is an important part of clinical practice for multiple sclerosis, but MS and cognitive impairment alone don’t indicate that a patient will fail a formal road test. However, the results from a recent study indicate that one cognitive test may be able to predict the patients who would fail road tests though only with a high false-positive rate.
During 2015-2016, Sarah A. Morrow, MD, of Western University, London, Ont., and her coauthors recruited licensed drivers aged 18-59 who were diagnosed with MS. They had low physical disability (Expanded Disability Status Scale score of less than 4) but cognitive impairment in both processing speed and either memory or executive function. This set of requirements, along with the need for signed informed consent, yielded a smaller sample size (36) than the researchers’ goal, a possible weakness of the study, the investigators said (Mult Scler. 2017 Aug 7. doi: 10.1177/1352458517723991).
The researchers noted that, if a patient does not show impairment on the BVMTR-IR, then they will definitely pass the road test, but they also noted that showing impairment is much less likely to predict a failed test. Instead, impairment both on this test and on the Symbol Digit Modalities Test, which measures processing speed, should indicate the need for a formal driving assessment.
“This study further contributes to the clinician’s ability to identify [persons with MS] in whom fitness-to-drive should be addressed,” the investigators wrote.
Assessing fitness to drive a motor vehicle is an important part of clinical practice for multiple sclerosis, but MS and cognitive impairment alone don’t indicate that a patient will fail a formal road test. However, the results from a recent study indicate that one cognitive test may be able to predict the patients who would fail road tests though only with a high false-positive rate.
During 2015-2016, Sarah A. Morrow, MD, of Western University, London, Ont., and her coauthors recruited licensed drivers aged 18-59 who were diagnosed with MS. They had low physical disability (Expanded Disability Status Scale score of less than 4) but cognitive impairment in both processing speed and either memory or executive function. This set of requirements, along with the need for signed informed consent, yielded a smaller sample size (36) than the researchers’ goal, a possible weakness of the study, the investigators said (Mult Scler. 2017 Aug 7. doi: 10.1177/1352458517723991).
The researchers noted that, if a patient does not show impairment on the BVMTR-IR, then they will definitely pass the road test, but they also noted that showing impairment is much less likely to predict a failed test. Instead, impairment both on this test and on the Symbol Digit Modalities Test, which measures processing speed, should indicate the need for a formal driving assessment.
“This study further contributes to the clinician’s ability to identify [persons with MS] in whom fitness-to-drive should be addressed,” the investigators wrote.
FROM MULTIPLE SCLEROSIS JOURNAL
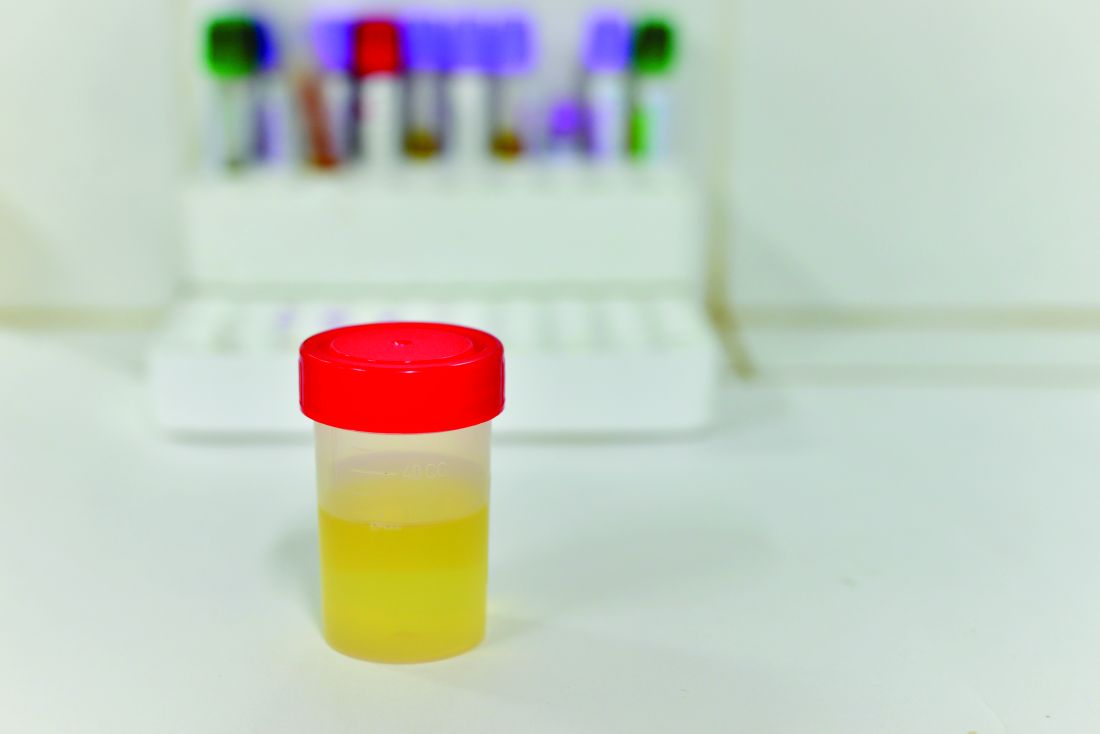
Researchers develop 30-min antibiotic susceptibility test for UTI
Researchers in Sweden have developed a 30-minute test capable of determining whether a bacterial urinary tract infection is susceptible or resistant to nine antibiotics. Their findings suggest that it is possible to develop a point-of-care test for patients with UTI.
Most phenotypic and genotypic antibiotic susceptibility tests are too slow to guide treatment, ranging from 2 days to 1 hour. The researchers at Uppsala (Sweden) University cut the testing time down to less than 30 minutes by using a microfluidic chip and direct single-cell imaging.
The chip traps the bacterial cells and allows growth media with different antibiotics (or none) to flow around them. “With this setup, we could detect the differential growth rate between treatment and reference populations in 3 min for ciprofloxacin, levofloxacin, mecillinam, nitrofurantoin, and trimethoprim-sulfamethoxazole; 7 min for amoxicillin-clavulanate and doripenem; 9 min for fosfomycin; and 11 min for ampicillin based on 99.9% confidence intervals,” wrote Özden Baltekin and his coauthors.
That test specifically used Escherichia coli cells; comparable speed and accuracy was replicated using Klebsiella pneumoniae and Staphylococcus saprophyticus. For the development of a point-of-care test for patients, the researchers said all that would be needed are about 100 bacteria cells.
“We have here focused on bacterial species and antibiotics related to UTIs, but it is likely that the same principles would work for sepsis, mastitis, or meningitis,” they suggested (Proc Natl Acad Sci. 2017 Aug 8. doi: 10.1073/pnas.1708558114).
Researchers in Sweden have developed a 30-minute test capable of determining whether a bacterial urinary tract infection is susceptible or resistant to nine antibiotics. Their findings suggest that it is possible to develop a point-of-care test for patients with UTI.
Most phenotypic and genotypic antibiotic susceptibility tests are too slow to guide treatment, ranging from 2 days to 1 hour. The researchers at Uppsala (Sweden) University cut the testing time down to less than 30 minutes by using a microfluidic chip and direct single-cell imaging.
The chip traps the bacterial cells and allows growth media with different antibiotics (or none) to flow around them. “With this setup, we could detect the differential growth rate between treatment and reference populations in 3 min for ciprofloxacin, levofloxacin, mecillinam, nitrofurantoin, and trimethoprim-sulfamethoxazole; 7 min for amoxicillin-clavulanate and doripenem; 9 min for fosfomycin; and 11 min for ampicillin based on 99.9% confidence intervals,” wrote Özden Baltekin and his coauthors.
That test specifically used Escherichia coli cells; comparable speed and accuracy was replicated using Klebsiella pneumoniae and Staphylococcus saprophyticus. For the development of a point-of-care test for patients, the researchers said all that would be needed are about 100 bacteria cells.
“We have here focused on bacterial species and antibiotics related to UTIs, but it is likely that the same principles would work for sepsis, mastitis, or meningitis,” they suggested (Proc Natl Acad Sci. 2017 Aug 8. doi: 10.1073/pnas.1708558114).
Researchers in Sweden have developed a 30-minute test capable of determining whether a bacterial urinary tract infection is susceptible or resistant to nine antibiotics. Their findings suggest that it is possible to develop a point-of-care test for patients with UTI.
Most phenotypic and genotypic antibiotic susceptibility tests are too slow to guide treatment, ranging from 2 days to 1 hour. The researchers at Uppsala (Sweden) University cut the testing time down to less than 30 minutes by using a microfluidic chip and direct single-cell imaging.
The chip traps the bacterial cells and allows growth media with different antibiotics (or none) to flow around them. “With this setup, we could detect the differential growth rate between treatment and reference populations in 3 min for ciprofloxacin, levofloxacin, mecillinam, nitrofurantoin, and trimethoprim-sulfamethoxazole; 7 min for amoxicillin-clavulanate and doripenem; 9 min for fosfomycin; and 11 min for ampicillin based on 99.9% confidence intervals,” wrote Özden Baltekin and his coauthors.
That test specifically used Escherichia coli cells; comparable speed and accuracy was replicated using Klebsiella pneumoniae and Staphylococcus saprophyticus. For the development of a point-of-care test for patients, the researchers said all that would be needed are about 100 bacteria cells.
“We have here focused on bacterial species and antibiotics related to UTIs, but it is likely that the same principles would work for sepsis, mastitis, or meningitis,” they suggested (Proc Natl Acad Sci. 2017 Aug 8. doi: 10.1073/pnas.1708558114).
FROM PNAS
South African child has suppressed HIV without ART since infancy
A 9-year-old South African child treated with antiretroviral therapy (ART) for HIV infection for 40 weeks as an infant has been living with an undetectable level of the virus ever since without ART, researchers reported on July 24.
“To our knowledge, this is the first reported case of sustained control of HIV in a child enrolled in a randomized trial of ART interruption following treatment early in infancy,” said Avy Violari, MD, at the International AIDS Society Conference on HIV Pathogenesis and Treatment in Paris.
Starting with a high viral load at 9 weeks of age, the child’s treatment brought the viral load to undetectable levels and was halted after 40 weeks. Follow-up examinations and blood sampling over the next 8.5 years showed the child in good health, with only a small reservoir of virus in a tiny portion of immune cells, but a completely undetectable viral load by standard assays. The child’s immune system is healthy, and there are no symptoms of HIV infection.
“Further study is needed to learn how to induce long-term HIV remission in infected babies,” Anthony S. Fauci, MD, director of NIAID, said in an NIH news release about the case. “However, this new case strengthens our hope that by treating HIV-infected children for a brief period beginning in infancy, we may be able to spare them the burden of lifelong therapy and the health consequences of long-term immune activation typically associated with HIV disease.”
A 9-year-old South African child treated with antiretroviral therapy (ART) for HIV infection for 40 weeks as an infant has been living with an undetectable level of the virus ever since without ART, researchers reported on July 24.
“To our knowledge, this is the first reported case of sustained control of HIV in a child enrolled in a randomized trial of ART interruption following treatment early in infancy,” said Avy Violari, MD, at the International AIDS Society Conference on HIV Pathogenesis and Treatment in Paris.
Starting with a high viral load at 9 weeks of age, the child’s treatment brought the viral load to undetectable levels and was halted after 40 weeks. Follow-up examinations and blood sampling over the next 8.5 years showed the child in good health, with only a small reservoir of virus in a tiny portion of immune cells, but a completely undetectable viral load by standard assays. The child’s immune system is healthy, and there are no symptoms of HIV infection.
“Further study is needed to learn how to induce long-term HIV remission in infected babies,” Anthony S. Fauci, MD, director of NIAID, said in an NIH news release about the case. “However, this new case strengthens our hope that by treating HIV-infected children for a brief period beginning in infancy, we may be able to spare them the burden of lifelong therapy and the health consequences of long-term immune activation typically associated with HIV disease.”
A 9-year-old South African child treated with antiretroviral therapy (ART) for HIV infection for 40 weeks as an infant has been living with an undetectable level of the virus ever since without ART, researchers reported on July 24.
“To our knowledge, this is the first reported case of sustained control of HIV in a child enrolled in a randomized trial of ART interruption following treatment early in infancy,” said Avy Violari, MD, at the International AIDS Society Conference on HIV Pathogenesis and Treatment in Paris.
Starting with a high viral load at 9 weeks of age, the child’s treatment brought the viral load to undetectable levels and was halted after 40 weeks. Follow-up examinations and blood sampling over the next 8.5 years showed the child in good health, with only a small reservoir of virus in a tiny portion of immune cells, but a completely undetectable viral load by standard assays. The child’s immune system is healthy, and there are no symptoms of HIV infection.
“Further study is needed to learn how to induce long-term HIV remission in infected babies,” Anthony S. Fauci, MD, director of NIAID, said in an NIH news release about the case. “However, this new case strengthens our hope that by treating HIV-infected children for a brief period beginning in infancy, we may be able to spare them the burden of lifelong therapy and the health consequences of long-term immune activation typically associated with HIV disease.”
FROM IAS 2017