User login
Implementing the VA/DoD Type 2 Diabetes Mellitus Clinical Practice Guideline (FULL)
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management to make a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
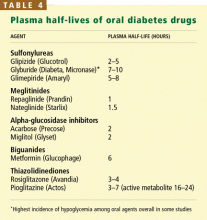
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and high-density lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management to make a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and high-density lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management to make a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and high-density lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Implementing the 2017 VA/DoD Diabetes Clinical Practice Guideline (FULL)
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Evaluation of E-Consults in the VHA: Provider Perspectives
Electronic consultations (e-consults), also called e-referrals, are an alternative method of obtaining general patient information through the electronic health record (EHR) shared by primary care providers (PCPs) and specialists in the VHA. In the e-consult system, test results, medication lists, and other pertinent data are available.1 Many PCPs are willing to use new technologies to maximize practice efficiency and patient convenience.2 In the VHA’s hub-and-spoke model of care, e-consults have the potential to make delivery of specialty care more efficient by prearranging or completing necessary diagnostic testing and redirecting inappropriate referrals to the correct specialists.1
Some early studies of e-consults report better communication, improved referral appropriateness, and greater access to specialty care as well as better continuity of care and information transfer between patients and PCPs.3-5 Researchers at the VA Boston Healthcare System in Massachusetts found that 61% of specialists surveyed agreed that e-consults improve quality of care and found the approach beneficial to help initiate diagnostic testing prior to a face-to-face visit.6 However, researchers at the Michael E. DeBakey VAMC in Houston, Texas, found no improvement in care coordination.7 To date, there have been no large-scale evaluations of e-consult programs or assessments of implementation of e-consult programs.
Related: HHS Grants Fund Health IT in Communities
In early 2011, the VHA Office of Specialty Care Services (OSCS), Office of Specialty Care Transformation launched a national e-consult pilot as part of a broader effort to improve the delivery of patient-centered specialty care. This initiative was based on core concepts advanced by the American College of Physicians, which highlighted the importance of specialty care within a patient- centered medical home and provided a framework for collaboration.8,9 The goals of the e-consult program were to improve access to specialty care for veterans and their PCPs, to enhance the collaborative relationship between PCPs and specialists, and to augment PCP education.
The OSCS created an Electronic Consultation Implementation Guide to help sites develop and implement each of their e-consult programs.10 The Implementation Guide established operating rules, strategies for engaging key stakeholders, and recommendations for provider education and training.
As with face-to-face referrals, e-consults are organized in a hub-and-spoke model, where community-based outpatient clinics (CBOCs) are linked to a central VAMC. An e-consult can be accessed by any CBOC, VAMC, medical center-based primary care clinic or specialist, and between medical centers that share the same EHR. There were 217,014 completed e-consults between May 2011 and December 2013 across VHA.11
Some programs created an e-consult template to aid in the transition to electronic referrals (Figure). Although not mandatory, the template helped organize needed information to expedite the e-consult.
The objective of this evaluation is to describe the implementation of e-consults from the perspectives of PCPs, specialists, and other key staff involved in the pilot. Key findings were related to: (1) how the e-consult pilot was implemented; (2) how implementation of the e-consult pilot affected providers; and (3) to what extent the e-consult pilot achieved programmatic objectives from the provider’s perspective.
Methods
The authors conducted a key informant analysis with 2 waves of interviews at 8 e-consult pilot sites across the U.S., selected for variation on early progress in implementation. The sites cannot be identified based on an agreement with the VA Office of Labor-Management Relations.
Setting
The e-consult pilot involved 15 VAMCs in 2 cohorts: alpha sites, which began using e-consults in May 2011, and beta sites, which began using e-consults in July 2011. The alpha sites included 10 VAMCs in 12 medical specialties, with a total of 21 facility-specialty combinations. For the evaluation, sites were defined based on specialty, regardless of location within the same medical center (eg, cardiology and diabetes at the same VAMC would be 2 sites). Beta sites included 5 VAMCs with 6 medical specialties for a total of 6 sites. For 1 year, alpha sites received $175,000 and beta sites received $150,000 to support start-up activities.
Initial specialties included diabetes, hepatitis C, geriatrics, cardiology, liver transplant, dementia, gastrointestinal disease, pulmonary medicine, rheumatology, pain management, neurosurgery, infectious diseases, hematology/oncology, and vascular surgery. Facilities could add additional e-consult specialties but did not receive further funding.
Sample
Study participants were selected from 8 of the 15 pilot sites (geographic site/specialty combinations). Site selection was based on 2 measures of baseline e-consult implementation: (1) overall e-consult implementation rates, measured as the ratio of e-consults to all consults for the specialties of interest; and (2) CBOC participation, measured as the ratio of e-consults for patients from CBOCs vs e-consults for patients from primary care clinics located within the 152 VAMCs. Participation with CBOCs was important for ensuring that implementation factors that influenced uptake of e-consults within tertiary medical centers and between VAMCs and CBOCs could be identified. Two e-consult sites were randomly selected from each of the 4 resulting categories (VAMC high volume, VAMC low volume, CBOC high volume, and CBOC low volume). Volume data of e-consults were obtained from the VA Corporate Data Warehouse and assessed from the beginning of the pilot period to initial site selection, May 2011 to February 2012.
Respondents were identified using a modified snowball sampling process. Snowball sampling is a qualitative sampling technique that identifies study participants, who then identify other potential participants to participate in the study. The researchers started with the local e-consult initiative lead and then contacted the directors of primary care and specialty care services for help identifying PCPs, specialists, and support staff (nurse practitioners, pharmacists, program managers, informatics staff, and medical support personnel) engaged in the initiative. The goal for follow-up interviews was to interview at least 2 of the following respondents at each site: e-consult project manager, PCP, and/or specialist. Due to turnover and changes in clinic roles, some follow-up interviews were conducted with different individuals from the baseline interviews.
Data Collection
Interviews followed semistructured interview guidelines and included open-ended questions designed to elicit rich responses to a variety of aspects related to e-consult implementation, including patient needs, communication, leadership, resources, priorities, knowledge about the program, and unintended consequences. Follow-up interviews addressed how e-consults impacted the quality of specialty care; the impact of e-consults on Patient Aligned Care Teams (PACTs), the VHA patient-centered medical home initiative for primary care; and how e-consults have been used, eg, whether patients were involved in the decision to seek an e-consult.
Two interviewers who had participated in a 1-day, in-person training covering both data collection and analyzing key informant data conducted the 40 to 60 minute telephone interviews. One team member conducted the interview while the other took field notes. Interviews were also recorded. Follow-up probes were used to elicit specific examples and ensure sufficiently rich data. Following each interview, the notetaker reviewed the audio recording and filled in details in the field notes. The interview team debriefed and reviewed the augmented field notes and audio recordings, which became the primary data sources for the study.
Analysis
This was a qualitative descriptive analysis.12 Interview data were analyzed using an iterative, inductive content analysis method using an open coding approach (ie, a priori codes were not defined for this portion of the analysis).13 Two members of the research team used audio recordings and summary transcripts simultaneously to code data. Summary transcripts were compared with the recorded interviews to assure fidelity.
The researchers used Atlas.ti (Berlin, Germany) qualitative data analysis software to organize the coding process. Emergent codes were iteratively added throughout the analysis to reflect quotations that did not adequately fit previously developed codes. Codes were combined weekly to biweekly. After the combinations were completed, the analytics team met to review meanings of codes to ensure consistency of coding and interpretations.
To create categories, broad themes were identified from interview responses and grouped under high- order headings that described distinct aspects of participant experience. The analysis was intentionally kept close to the original data to reflect and describe the participant’s experience as accurately as possible. In support of analytical rigor, members of the multidisciplinary research team, composed of clinicians, implementation scientists, and mixed methodologists, reviewed findings to assess their thoroughness, comprehensiveness, and representativeness across roles and participating sites.14
Results
The e-consult evaluation period was from November 1, 2011, to July 31, 2013. Key conclusions were drawn from both alpha and beta sites (Table). Baseline interviews were conducted with 37 participants at 8 sites from April 10, 2012, to August 6, 2012. Follow-up interviews were conducted with 21 of the 37 participants at the 8 sites. Follow-up interviews with either a PCP or specialist could not be scheduled at 1 site. Follow-up interviews were conducted from April 16, 2013, to June 18, 2013. Open coding continued until saturation (the point at which subsequent data failed to produce new findings).15 This occurred after analysis of 22 baseline interviews (12 PCPs, 6 specialists, 1 pharmacist, and 4 other staff members) and 17 follow-up interviews (10 PCPs, 4 specialists, 1 pharmacist, and 2 other staff members).
Implementation
The e-consults provided a programmatic structure to the more informal practice of obtaining diagnostic or therapeutic advice from a specialist. Several of the specialists interviewed described having previously used existing informal consult processes that were “like e-consult.” These specialists reported that their practice patterns did not change significantly since implementing e-consults, because they have been “using the Computerized Patient Record System (CPRS) in an e-consult way for many years.” In these cases, the primary change resulting from the initiative was that national VHA workload policy was revised so that e-consults were assigned a CPT (Current Procedural Terminology) code and specialists began receiving workload credit for completing e-consults.
At sites where an informal e-consult practice was already in place, the initiative was consistently described as flexible. Many specialists reported that this degree of flexibility allowed them to make a relatively easy transition to e-consults by adopting new mechanisms to support existing processes. The e-consult initiative also allowed specialists to formally document this work and to increase the efficiency of specialty care.
Specialists drove the implementation process across sites. The e-consults were envisioned as a collaborative process; however, during initial interviews, few specialists mentioned PCPs when describing the development and implementation of the e-consult program. Primary care providers also reported having little awareness of or input into how the initiative was implemented, although this had little consequence on the use of e-consults.
In a rare case, a PCP reported that poorly designed, lengthy e-consult templates were a major barrier to using e-consults for specific specialties. The PCP said, “E-consults have created an elaborate but extraordinarily cumbersome tool that is difficult for PCPs to actually accomplish, because you have a consult menu that requires a lot of data to be entered—a lot of history from the chart, a lot of exam findings, a lot of previous cognitive testing scores; neurologic findings—lab and imaging tests.”
Still, many other PCPs described receiving detailed information and guidance from e-consults. “E-consults help me to be more accurate. Many providers don’t have a comfort with pain management. To get guidance and education and to really hold our hand, this is how to do this…this has been a big change. If they give you a great response, then [for] the next patient [with that condition], you go back to that note and then follow what was said there,” said one PCP.
In follow-up interviews, providers and other key staff stated there were more data available on the patient as a result of the e-consult and, consequently, even when specialists determined that a patient needed an in-person visit, the data obtained in the e-consult improved the quality of the in-person consultation.
Enhanced Communication and Collaboration
Neither the PCPs nor the specialists were aware of the collaborative intent of the initiative. They focused, instead, on other key aims, such as increasing accessibility and minimizing unnecessary patient travel. Most participants were generally positive about e-consults during baseline interviews, and this perception increased over time.
Both the PCPs and the specialists reported improved communication following the launch of e-consults. In follow-up interviews, some PCPs reported that before e-consults, they had trouble getting timely responses from specialists unless they knew them personally. “You had to know the person in the old days,” one respondent said. “After e-consults, responses improved…e-consult is available to have the resources to tap that knowledge base, and the team is answering the question. I think it opens up access and information and knowledge to everybody.”
Many PCPs spoke positively about this new communication tool as an opportunity to learn from specialists and said they valued the input they received. They felt the increased interaction between the 2 groups positively benefited patient care. One example cited that collaborative communication improved care coordination for veterans: “We are able to step in with e-consults to coordinate services, and this has been huge in improving care.”
Furthermore, follow-up interviews found that all participating PCPs and specialists were communicating more frequently and effectively. “Services that have embraced e-consult give a lot of great information flowing back; it’s closer to a real-time conversation,” said one respondent.
Related: Home-Based Video Telehealth for Veterans With Dementia
In baseline interviews, some specialists described how e-consults went against their belief that patient care is synonymous with face-to-face medical treatment and voiced dissatisfaction with e-consults as “sitting in front of a computer” rather than “seeing patients.” Others were concerned that medical center administration would not recognize the time it takes to conduct an e-consult and therefore not add necessary specialists staff. “E-consults take work and time, just like seeing a patient. I worry that won’t be seen,” one specialist said.
In order to successfully implement the e-consult initiative, providers and staff needed to incorporate new processes into their daily workflow.
Most sites did not develop a mechanism in which specialists received feedback regarding the outcome of their consultations. This lack of response created anxiety for some specialists in the absence of the face-to-face encounter, leaving some wondering whether they or the PCP had missed anything. According to one specialist, “That’s always in the back of your head: ‘Have I [the specialist] missed something?’”
In follow-up interviews, none of these concerns were raised. Primary care providers tended to speak of the care provided by specialists through e-consults in very positive terms, except in those instances where PCPs felt the e-consult template was difficult to use and required too much time to complete. “I was worried in the beginning about patients thinking less of me, but we ask for help all the time. We’re asking for help and not inconveniencing the patient; they seem to like it very much,” one PCP said.
The e-consults also complement PACTs. Initially, a few participants described soliciting patient input regarding the choice to have an e-consult or a face-to-face visit. During follow-up interviews, participants highlighted how well e-consults fit in to the PACT philosophy. One participant said, “The PACT team seeks to improve quality of care. E-consult fits very well with this, because answers to questions can come quickly, and the veteran may not need to come back to the clinic to be seen, even though things are still getting accomplished. E-consult works very well. E-consults were credited with improving access to specialty care as a tool for PACT.”
Achieving Program Objectives
Based on interviews, support for the e-consult program has increased over time as providers have gained experience with the program and have seen its benefits. Respondents at all sites consistently supported the concept of e-consults and expressed their belief in the importance and value of e-consults in improving patient-c entered care, primarily by reducing the need for patients to travel to see specialists, reducing the time to obtain feedback from specialists, and maintaining the provision of high quality care.
“Last year we only had 2 clinics categorized as e-consults. As of now we have 14 e-consults available for our providers. I think the numbers are growing. They are realizing the value of e-consults as far as the provider’s needs being met,” said one respondent.
The e-consults were credited with improving access to specialty care for veterans. Several participants stated that e-consults improved access to specialty care services and decreased travel for veterans. “It’s another way of getting care to the patient when the patient needs it without having to wait,” said one respondent.
Many PCPs described how difficult it was for patients to get to specialty appointments—particularly for their elderly, disabled, and rural patients—before the implementation of e-consults. “I like the fact that patients who live very far don’t have to come back. A lot of our patients are older…diabetic, see me Monday and back on Thursday. Now, they are able to stay home and follow the recommendations I write,” said one PCP.
Most providers were of the opinion that patients liked the program. “I think e-consults are helping patients...It’s been very successful regarding decreasing travel…Quicker response time for specialty care,” said a PCP. Several providers also stated in follow-up interviews that there was a greater degree of patient participation in the e-consult process and that “patients are definitely informed.”
Discussion
Most PCPs reported that the e-consults were an effective means of consultation and contained the information they needed to provide high-quality coordinated care. Most also found e-consult templates easy to complete. A majority of PCPs felt sufficient control over the choice of whether to use e-consults or an in-person visit, and a minority of patients were involved in the decision to receive an e-consult. Although the OSCS outlined guiding principles and operational rules in the Implementation Guide to help sites implement the e-consult program, its contribution was limited. Few examples were found that engaged PCPs in development of the e-consult program locally; involving patients in the decision to obtain a specialty consult electronically or in person; and PCPs feeding back results to specialists.
Implementing e-consults posed a number of challenges, including lack of resources to respond to referral requests, lack of referral policies and standardized procedures, and confusion related to roles and responsibilities. This is consistent with findings from another VHA research project of e-consults in 2 VHA health systems that was conducted prior to this national level e-consult pilot.7
Related: Using Facilitative Coaching to Support Patient Aligned Care Teams
Communication by OSCS of key aspects of the e-consult initiative will be critical as more sites implement e-consults. Since initiation of this pilot, workload specifications and credit have changed from 1 code to 3 codes, to more accurately reflect the amount of time a specialist consultant spends reviewing the EHR and responding to the consult. Without seeing the patient directly, specialists are more reliant on the PCP to describe the problem and provide adequate information in the e-consult request in order to provide recommendations back to the PCP.
Primary care physicians need to know that e-consults are available and determine when they are appropriate. A template or other guidance may be helpful to ensure adequate information is provided in the e-consult request; and the information provided by the specialist in response to the e-consult has to be sufficient for the PCP to provide care. VHA continues to expand the use of e-consults throughout the system, as this pilot found that the electronic option was often more timely than were face-to-face consultations. The result of this evaluation has informed national implementation of this effort.
Limitations
There are 3 main limitations to this study. First, because there was no practical way to preidentify participants who participated in implementing e-consults, a modified snowball sampling was used. However, this limited the degree to which the group was representative of the pilot participants. Second, the authors reported findings from a real-world initiative, not an experimental study. As such, not all participants in the first wave of key informant interviews were available for follow-up interview, which may have introduced bias. Third, the VHA is unlike most of the rest of the U.S. health care system in that it is a fully integrated system with salaried PCPs and specialists and an EHR.
Generalizability of the study may be limited, as a modified snowball sampling approach is not entirely random and has potential for community bias, because initial participants influence subsequent sampling. Additionally, though the sample size (n = 37) was sufficient for qualitative, in-depth analysis, it may be too small for confident generalization of findings. However, as health care moves toward an accountable care organization system, the authors’ analysis may provide insights.
Issues include revision of reimbursement policy for e-consults and developing or coordinating informational technology infrastructures to permit e-consults. It is also important to note that this evaluation reports solely on the extent of implementation of e-consults and the effects of e-consult implementation from the perspectives of staff, including specialists and PCPs.
Evaluating the effectiveness of the program in improving access, care coordination, and patient satisfaction was beyond the scope of the study. Further research is needed, because findings on those outcomes are critical for drawing inferences about this study’s implementation results.
Conclusion
The assessment of the e-consult system by providers and staff was based on a perception that e-consults are a valuable tool in providing greater access to quality care. Currently, e-consults have been expanded across VHA in medical and surgical specialties. VHA policymakers have drafted field guidance and a communication plan to support these efforts.
Acknowledgement
This material is based on work supported by the VA Office of Specialty Care Transformation, the office overseeing the e-consult initiative, and the Office of Research and Development Quality Enhancement Research Initiative.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Chen AH, Murphy EJ, Yee HF Jr. eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450-2453.
2. Hanna L, May C, Fairhurst K. The place of information and communication technology-mediated consultations in primary care: GPs’ perspectives. Fam Pract. 2012;29(3):361-366.
3. Kim-Hwang JE, Chen AH, Bell DS, Guzman D, Yee HF Jr, Kushel MB. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25(10):1123-1128.
4. Straus SG, Chen AH, Yee HF Jr, Kushel MB, Bell DS. Implementation of an electronic referral system for outpatient specialty care. AMIA Annu Symp Proc. 2011;2011:1337-1346.
5. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
6. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014;31(5):26-31.
7. Hysong SJ, Esquivel A, Sittig DF, et al. Towards successful coordination of electronic health record based-referrals: a qualitative analysis. Implement Sci. 2011;6:84.
8. American College of Physicians. The Patient- Centered Medical Home Neighbor: The Interface of the Patient-Centered Medical Home with Specialty/Subspecialty Practices. Philadelphia, PA: American College of Physicians; 2010. Policy paper.
9. Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med. 2008;359(12): 1202-1205.
10. Department of Veterans Affairs. Electronic Consultation (E-Consult) Implementation Guide, Version 1.2. Washington, DC: Department of Veterans Affairs, Office of Specialty Care Services, Specialty Care Transformation. 2013.
11. Kirsh S, Cary E, Aron DC et al. Results of a national pilot project for specialty care e-consultation in primary care medical homes: the impact of specialty e-consultation on access. Am J Manag Care. In press.
12. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340.
13. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115.
14. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 2000;284(3):357-362.
15. Sandelowski M. The problem of rigor in qualitative research. ANS Adv Nurs Sci. 1986;8(3):27-37.
Electronic consultations (e-consults), also called e-referrals, are an alternative method of obtaining general patient information through the electronic health record (EHR) shared by primary care providers (PCPs) and specialists in the VHA. In the e-consult system, test results, medication lists, and other pertinent data are available.1 Many PCPs are willing to use new technologies to maximize practice efficiency and patient convenience.2 In the VHA’s hub-and-spoke model of care, e-consults have the potential to make delivery of specialty care more efficient by prearranging or completing necessary diagnostic testing and redirecting inappropriate referrals to the correct specialists.1
Some early studies of e-consults report better communication, improved referral appropriateness, and greater access to specialty care as well as better continuity of care and information transfer between patients and PCPs.3-5 Researchers at the VA Boston Healthcare System in Massachusetts found that 61% of specialists surveyed agreed that e-consults improve quality of care and found the approach beneficial to help initiate diagnostic testing prior to a face-to-face visit.6 However, researchers at the Michael E. DeBakey VAMC in Houston, Texas, found no improvement in care coordination.7 To date, there have been no large-scale evaluations of e-consult programs or assessments of implementation of e-consult programs.
Related: HHS Grants Fund Health IT in Communities
In early 2011, the VHA Office of Specialty Care Services (OSCS), Office of Specialty Care Transformation launched a national e-consult pilot as part of a broader effort to improve the delivery of patient-centered specialty care. This initiative was based on core concepts advanced by the American College of Physicians, which highlighted the importance of specialty care within a patient- centered medical home and provided a framework for collaboration.8,9 The goals of the e-consult program were to improve access to specialty care for veterans and their PCPs, to enhance the collaborative relationship between PCPs and specialists, and to augment PCP education.
The OSCS created an Electronic Consultation Implementation Guide to help sites develop and implement each of their e-consult programs.10 The Implementation Guide established operating rules, strategies for engaging key stakeholders, and recommendations for provider education and training.
As with face-to-face referrals, e-consults are organized in a hub-and-spoke model, where community-based outpatient clinics (CBOCs) are linked to a central VAMC. An e-consult can be accessed by any CBOC, VAMC, medical center-based primary care clinic or specialist, and between medical centers that share the same EHR. There were 217,014 completed e-consults between May 2011 and December 2013 across VHA.11
Some programs created an e-consult template to aid in the transition to electronic referrals (Figure). Although not mandatory, the template helped organize needed information to expedite the e-consult.
The objective of this evaluation is to describe the implementation of e-consults from the perspectives of PCPs, specialists, and other key staff involved in the pilot. Key findings were related to: (1) how the e-consult pilot was implemented; (2) how implementation of the e-consult pilot affected providers; and (3) to what extent the e-consult pilot achieved programmatic objectives from the provider’s perspective.
Methods
The authors conducted a key informant analysis with 2 waves of interviews at 8 e-consult pilot sites across the U.S., selected for variation on early progress in implementation. The sites cannot be identified based on an agreement with the VA Office of Labor-Management Relations.
Setting
The e-consult pilot involved 15 VAMCs in 2 cohorts: alpha sites, which began using e-consults in May 2011, and beta sites, which began using e-consults in July 2011. The alpha sites included 10 VAMCs in 12 medical specialties, with a total of 21 facility-specialty combinations. For the evaluation, sites were defined based on specialty, regardless of location within the same medical center (eg, cardiology and diabetes at the same VAMC would be 2 sites). Beta sites included 5 VAMCs with 6 medical specialties for a total of 6 sites. For 1 year, alpha sites received $175,000 and beta sites received $150,000 to support start-up activities.
Initial specialties included diabetes, hepatitis C, geriatrics, cardiology, liver transplant, dementia, gastrointestinal disease, pulmonary medicine, rheumatology, pain management, neurosurgery, infectious diseases, hematology/oncology, and vascular surgery. Facilities could add additional e-consult specialties but did not receive further funding.
Sample
Study participants were selected from 8 of the 15 pilot sites (geographic site/specialty combinations). Site selection was based on 2 measures of baseline e-consult implementation: (1) overall e-consult implementation rates, measured as the ratio of e-consults to all consults for the specialties of interest; and (2) CBOC participation, measured as the ratio of e-consults for patients from CBOCs vs e-consults for patients from primary care clinics located within the 152 VAMCs. Participation with CBOCs was important for ensuring that implementation factors that influenced uptake of e-consults within tertiary medical centers and between VAMCs and CBOCs could be identified. Two e-consult sites were randomly selected from each of the 4 resulting categories (VAMC high volume, VAMC low volume, CBOC high volume, and CBOC low volume). Volume data of e-consults were obtained from the VA Corporate Data Warehouse and assessed from the beginning of the pilot period to initial site selection, May 2011 to February 2012.
Respondents were identified using a modified snowball sampling process. Snowball sampling is a qualitative sampling technique that identifies study participants, who then identify other potential participants to participate in the study. The researchers started with the local e-consult initiative lead and then contacted the directors of primary care and specialty care services for help identifying PCPs, specialists, and support staff (nurse practitioners, pharmacists, program managers, informatics staff, and medical support personnel) engaged in the initiative. The goal for follow-up interviews was to interview at least 2 of the following respondents at each site: e-consult project manager, PCP, and/or specialist. Due to turnover and changes in clinic roles, some follow-up interviews were conducted with different individuals from the baseline interviews.
Data Collection
Interviews followed semistructured interview guidelines and included open-ended questions designed to elicit rich responses to a variety of aspects related to e-consult implementation, including patient needs, communication, leadership, resources, priorities, knowledge about the program, and unintended consequences. Follow-up interviews addressed how e-consults impacted the quality of specialty care; the impact of e-consults on Patient Aligned Care Teams (PACTs), the VHA patient-centered medical home initiative for primary care; and how e-consults have been used, eg, whether patients were involved in the decision to seek an e-consult.
Two interviewers who had participated in a 1-day, in-person training covering both data collection and analyzing key informant data conducted the 40 to 60 minute telephone interviews. One team member conducted the interview while the other took field notes. Interviews were also recorded. Follow-up probes were used to elicit specific examples and ensure sufficiently rich data. Following each interview, the notetaker reviewed the audio recording and filled in details in the field notes. The interview team debriefed and reviewed the augmented field notes and audio recordings, which became the primary data sources for the study.
Analysis
This was a qualitative descriptive analysis.12 Interview data were analyzed using an iterative, inductive content analysis method using an open coding approach (ie, a priori codes were not defined for this portion of the analysis).13 Two members of the research team used audio recordings and summary transcripts simultaneously to code data. Summary transcripts were compared with the recorded interviews to assure fidelity.
The researchers used Atlas.ti (Berlin, Germany) qualitative data analysis software to organize the coding process. Emergent codes were iteratively added throughout the analysis to reflect quotations that did not adequately fit previously developed codes. Codes were combined weekly to biweekly. After the combinations were completed, the analytics team met to review meanings of codes to ensure consistency of coding and interpretations.
To create categories, broad themes were identified from interview responses and grouped under high- order headings that described distinct aspects of participant experience. The analysis was intentionally kept close to the original data to reflect and describe the participant’s experience as accurately as possible. In support of analytical rigor, members of the multidisciplinary research team, composed of clinicians, implementation scientists, and mixed methodologists, reviewed findings to assess their thoroughness, comprehensiveness, and representativeness across roles and participating sites.14
Results
The e-consult evaluation period was from November 1, 2011, to July 31, 2013. Key conclusions were drawn from both alpha and beta sites (Table). Baseline interviews were conducted with 37 participants at 8 sites from April 10, 2012, to August 6, 2012. Follow-up interviews were conducted with 21 of the 37 participants at the 8 sites. Follow-up interviews with either a PCP or specialist could not be scheduled at 1 site. Follow-up interviews were conducted from April 16, 2013, to June 18, 2013. Open coding continued until saturation (the point at which subsequent data failed to produce new findings).15 This occurred after analysis of 22 baseline interviews (12 PCPs, 6 specialists, 1 pharmacist, and 4 other staff members) and 17 follow-up interviews (10 PCPs, 4 specialists, 1 pharmacist, and 2 other staff members).
Implementation
The e-consults provided a programmatic structure to the more informal practice of obtaining diagnostic or therapeutic advice from a specialist. Several of the specialists interviewed described having previously used existing informal consult processes that were “like e-consult.” These specialists reported that their practice patterns did not change significantly since implementing e-consults, because they have been “using the Computerized Patient Record System (CPRS) in an e-consult way for many years.” In these cases, the primary change resulting from the initiative was that national VHA workload policy was revised so that e-consults were assigned a CPT (Current Procedural Terminology) code and specialists began receiving workload credit for completing e-consults.
At sites where an informal e-consult practice was already in place, the initiative was consistently described as flexible. Many specialists reported that this degree of flexibility allowed them to make a relatively easy transition to e-consults by adopting new mechanisms to support existing processes. The e-consult initiative also allowed specialists to formally document this work and to increase the efficiency of specialty care.
Specialists drove the implementation process across sites. The e-consults were envisioned as a collaborative process; however, during initial interviews, few specialists mentioned PCPs when describing the development and implementation of the e-consult program. Primary care providers also reported having little awareness of or input into how the initiative was implemented, although this had little consequence on the use of e-consults.
In a rare case, a PCP reported that poorly designed, lengthy e-consult templates were a major barrier to using e-consults for specific specialties. The PCP said, “E-consults have created an elaborate but extraordinarily cumbersome tool that is difficult for PCPs to actually accomplish, because you have a consult menu that requires a lot of data to be entered—a lot of history from the chart, a lot of exam findings, a lot of previous cognitive testing scores; neurologic findings—lab and imaging tests.”
Still, many other PCPs described receiving detailed information and guidance from e-consults. “E-consults help me to be more accurate. Many providers don’t have a comfort with pain management. To get guidance and education and to really hold our hand, this is how to do this…this has been a big change. If they give you a great response, then [for] the next patient [with that condition], you go back to that note and then follow what was said there,” said one PCP.
In follow-up interviews, providers and other key staff stated there were more data available on the patient as a result of the e-consult and, consequently, even when specialists determined that a patient needed an in-person visit, the data obtained in the e-consult improved the quality of the in-person consultation.
Enhanced Communication and Collaboration
Neither the PCPs nor the specialists were aware of the collaborative intent of the initiative. They focused, instead, on other key aims, such as increasing accessibility and minimizing unnecessary patient travel. Most participants were generally positive about e-consults during baseline interviews, and this perception increased over time.
Both the PCPs and the specialists reported improved communication following the launch of e-consults. In follow-up interviews, some PCPs reported that before e-consults, they had trouble getting timely responses from specialists unless they knew them personally. “You had to know the person in the old days,” one respondent said. “After e-consults, responses improved…e-consult is available to have the resources to tap that knowledge base, and the team is answering the question. I think it opens up access and information and knowledge to everybody.”
Many PCPs spoke positively about this new communication tool as an opportunity to learn from specialists and said they valued the input they received. They felt the increased interaction between the 2 groups positively benefited patient care. One example cited that collaborative communication improved care coordination for veterans: “We are able to step in with e-consults to coordinate services, and this has been huge in improving care.”
Furthermore, follow-up interviews found that all participating PCPs and specialists were communicating more frequently and effectively. “Services that have embraced e-consult give a lot of great information flowing back; it’s closer to a real-time conversation,” said one respondent.
Related: Home-Based Video Telehealth for Veterans With Dementia
In baseline interviews, some specialists described how e-consults went against their belief that patient care is synonymous with face-to-face medical treatment and voiced dissatisfaction with e-consults as “sitting in front of a computer” rather than “seeing patients.” Others were concerned that medical center administration would not recognize the time it takes to conduct an e-consult and therefore not add necessary specialists staff. “E-consults take work and time, just like seeing a patient. I worry that won’t be seen,” one specialist said.
In order to successfully implement the e-consult initiative, providers and staff needed to incorporate new processes into their daily workflow.
Most sites did not develop a mechanism in which specialists received feedback regarding the outcome of their consultations. This lack of response created anxiety for some specialists in the absence of the face-to-face encounter, leaving some wondering whether they or the PCP had missed anything. According to one specialist, “That’s always in the back of your head: ‘Have I [the specialist] missed something?’”
In follow-up interviews, none of these concerns were raised. Primary care providers tended to speak of the care provided by specialists through e-consults in very positive terms, except in those instances where PCPs felt the e-consult template was difficult to use and required too much time to complete. “I was worried in the beginning about patients thinking less of me, but we ask for help all the time. We’re asking for help and not inconveniencing the patient; they seem to like it very much,” one PCP said.
The e-consults also complement PACTs. Initially, a few participants described soliciting patient input regarding the choice to have an e-consult or a face-to-face visit. During follow-up interviews, participants highlighted how well e-consults fit in to the PACT philosophy. One participant said, “The PACT team seeks to improve quality of care. E-consult fits very well with this, because answers to questions can come quickly, and the veteran may not need to come back to the clinic to be seen, even though things are still getting accomplished. E-consult works very well. E-consults were credited with improving access to specialty care as a tool for PACT.”
Achieving Program Objectives
Based on interviews, support for the e-consult program has increased over time as providers have gained experience with the program and have seen its benefits. Respondents at all sites consistently supported the concept of e-consults and expressed their belief in the importance and value of e-consults in improving patient-c entered care, primarily by reducing the need for patients to travel to see specialists, reducing the time to obtain feedback from specialists, and maintaining the provision of high quality care.
“Last year we only had 2 clinics categorized as e-consults. As of now we have 14 e-consults available for our providers. I think the numbers are growing. They are realizing the value of e-consults as far as the provider’s needs being met,” said one respondent.
The e-consults were credited with improving access to specialty care for veterans. Several participants stated that e-consults improved access to specialty care services and decreased travel for veterans. “It’s another way of getting care to the patient when the patient needs it without having to wait,” said one respondent.
Many PCPs described how difficult it was for patients to get to specialty appointments—particularly for their elderly, disabled, and rural patients—before the implementation of e-consults. “I like the fact that patients who live very far don’t have to come back. A lot of our patients are older…diabetic, see me Monday and back on Thursday. Now, they are able to stay home and follow the recommendations I write,” said one PCP.
Most providers were of the opinion that patients liked the program. “I think e-consults are helping patients...It’s been very successful regarding decreasing travel…Quicker response time for specialty care,” said a PCP. Several providers also stated in follow-up interviews that there was a greater degree of patient participation in the e-consult process and that “patients are definitely informed.”
Discussion
Most PCPs reported that the e-consults were an effective means of consultation and contained the information they needed to provide high-quality coordinated care. Most also found e-consult templates easy to complete. A majority of PCPs felt sufficient control over the choice of whether to use e-consults or an in-person visit, and a minority of patients were involved in the decision to receive an e-consult. Although the OSCS outlined guiding principles and operational rules in the Implementation Guide to help sites implement the e-consult program, its contribution was limited. Few examples were found that engaged PCPs in development of the e-consult program locally; involving patients in the decision to obtain a specialty consult electronically or in person; and PCPs feeding back results to specialists.
Implementing e-consults posed a number of challenges, including lack of resources to respond to referral requests, lack of referral policies and standardized procedures, and confusion related to roles and responsibilities. This is consistent with findings from another VHA research project of e-consults in 2 VHA health systems that was conducted prior to this national level e-consult pilot.7
Related: Using Facilitative Coaching to Support Patient Aligned Care Teams
Communication by OSCS of key aspects of the e-consult initiative will be critical as more sites implement e-consults. Since initiation of this pilot, workload specifications and credit have changed from 1 code to 3 codes, to more accurately reflect the amount of time a specialist consultant spends reviewing the EHR and responding to the consult. Without seeing the patient directly, specialists are more reliant on the PCP to describe the problem and provide adequate information in the e-consult request in order to provide recommendations back to the PCP.
Primary care physicians need to know that e-consults are available and determine when they are appropriate. A template or other guidance may be helpful to ensure adequate information is provided in the e-consult request; and the information provided by the specialist in response to the e-consult has to be sufficient for the PCP to provide care. VHA continues to expand the use of e-consults throughout the system, as this pilot found that the electronic option was often more timely than were face-to-face consultations. The result of this evaluation has informed national implementation of this effort.
Limitations
There are 3 main limitations to this study. First, because there was no practical way to preidentify participants who participated in implementing e-consults, a modified snowball sampling was used. However, this limited the degree to which the group was representative of the pilot participants. Second, the authors reported findings from a real-world initiative, not an experimental study. As such, not all participants in the first wave of key informant interviews were available for follow-up interview, which may have introduced bias. Third, the VHA is unlike most of the rest of the U.S. health care system in that it is a fully integrated system with salaried PCPs and specialists and an EHR.
Generalizability of the study may be limited, as a modified snowball sampling approach is not entirely random and has potential for community bias, because initial participants influence subsequent sampling. Additionally, though the sample size (n = 37) was sufficient for qualitative, in-depth analysis, it may be too small for confident generalization of findings. However, as health care moves toward an accountable care organization system, the authors’ analysis may provide insights.
Issues include revision of reimbursement policy for e-consults and developing or coordinating informational technology infrastructures to permit e-consults. It is also important to note that this evaluation reports solely on the extent of implementation of e-consults and the effects of e-consult implementation from the perspectives of staff, including specialists and PCPs.
Evaluating the effectiveness of the program in improving access, care coordination, and patient satisfaction was beyond the scope of the study. Further research is needed, because findings on those outcomes are critical for drawing inferences about this study’s implementation results.
Conclusion
The assessment of the e-consult system by providers and staff was based on a perception that e-consults are a valuable tool in providing greater access to quality care. Currently, e-consults have been expanded across VHA in medical and surgical specialties. VHA policymakers have drafted field guidance and a communication plan to support these efforts.
Acknowledgement
This material is based on work supported by the VA Office of Specialty Care Transformation, the office overseeing the e-consult initiative, and the Office of Research and Development Quality Enhancement Research Initiative.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Electronic consultations (e-consults), also called e-referrals, are an alternative method of obtaining general patient information through the electronic health record (EHR) shared by primary care providers (PCPs) and specialists in the VHA. In the e-consult system, test results, medication lists, and other pertinent data are available.1 Many PCPs are willing to use new technologies to maximize practice efficiency and patient convenience.2 In the VHA’s hub-and-spoke model of care, e-consults have the potential to make delivery of specialty care more efficient by prearranging or completing necessary diagnostic testing and redirecting inappropriate referrals to the correct specialists.1
Some early studies of e-consults report better communication, improved referral appropriateness, and greater access to specialty care as well as better continuity of care and information transfer between patients and PCPs.3-5 Researchers at the VA Boston Healthcare System in Massachusetts found that 61% of specialists surveyed agreed that e-consults improve quality of care and found the approach beneficial to help initiate diagnostic testing prior to a face-to-face visit.6 However, researchers at the Michael E. DeBakey VAMC in Houston, Texas, found no improvement in care coordination.7 To date, there have been no large-scale evaluations of e-consult programs or assessments of implementation of e-consult programs.
Related: HHS Grants Fund Health IT in Communities
In early 2011, the VHA Office of Specialty Care Services (OSCS), Office of Specialty Care Transformation launched a national e-consult pilot as part of a broader effort to improve the delivery of patient-centered specialty care. This initiative was based on core concepts advanced by the American College of Physicians, which highlighted the importance of specialty care within a patient- centered medical home and provided a framework for collaboration.8,9 The goals of the e-consult program were to improve access to specialty care for veterans and their PCPs, to enhance the collaborative relationship between PCPs and specialists, and to augment PCP education.
The OSCS created an Electronic Consultation Implementation Guide to help sites develop and implement each of their e-consult programs.10 The Implementation Guide established operating rules, strategies for engaging key stakeholders, and recommendations for provider education and training.
As with face-to-face referrals, e-consults are organized in a hub-and-spoke model, where community-based outpatient clinics (CBOCs) are linked to a central VAMC. An e-consult can be accessed by any CBOC, VAMC, medical center-based primary care clinic or specialist, and between medical centers that share the same EHR. There were 217,014 completed e-consults between May 2011 and December 2013 across VHA.11
Some programs created an e-consult template to aid in the transition to electronic referrals (Figure). Although not mandatory, the template helped organize needed information to expedite the e-consult.
The objective of this evaluation is to describe the implementation of e-consults from the perspectives of PCPs, specialists, and other key staff involved in the pilot. Key findings were related to: (1) how the e-consult pilot was implemented; (2) how implementation of the e-consult pilot affected providers; and (3) to what extent the e-consult pilot achieved programmatic objectives from the provider’s perspective.
Methods
The authors conducted a key informant analysis with 2 waves of interviews at 8 e-consult pilot sites across the U.S., selected for variation on early progress in implementation. The sites cannot be identified based on an agreement with the VA Office of Labor-Management Relations.
Setting
The e-consult pilot involved 15 VAMCs in 2 cohorts: alpha sites, which began using e-consults in May 2011, and beta sites, which began using e-consults in July 2011. The alpha sites included 10 VAMCs in 12 medical specialties, with a total of 21 facility-specialty combinations. For the evaluation, sites were defined based on specialty, regardless of location within the same medical center (eg, cardiology and diabetes at the same VAMC would be 2 sites). Beta sites included 5 VAMCs with 6 medical specialties for a total of 6 sites. For 1 year, alpha sites received $175,000 and beta sites received $150,000 to support start-up activities.
Initial specialties included diabetes, hepatitis C, geriatrics, cardiology, liver transplant, dementia, gastrointestinal disease, pulmonary medicine, rheumatology, pain management, neurosurgery, infectious diseases, hematology/oncology, and vascular surgery. Facilities could add additional e-consult specialties but did not receive further funding.
Sample
Study participants were selected from 8 of the 15 pilot sites (geographic site/specialty combinations). Site selection was based on 2 measures of baseline e-consult implementation: (1) overall e-consult implementation rates, measured as the ratio of e-consults to all consults for the specialties of interest; and (2) CBOC participation, measured as the ratio of e-consults for patients from CBOCs vs e-consults for patients from primary care clinics located within the 152 VAMCs. Participation with CBOCs was important for ensuring that implementation factors that influenced uptake of e-consults within tertiary medical centers and between VAMCs and CBOCs could be identified. Two e-consult sites were randomly selected from each of the 4 resulting categories (VAMC high volume, VAMC low volume, CBOC high volume, and CBOC low volume). Volume data of e-consults were obtained from the VA Corporate Data Warehouse and assessed from the beginning of the pilot period to initial site selection, May 2011 to February 2012.
Respondents were identified using a modified snowball sampling process. Snowball sampling is a qualitative sampling technique that identifies study participants, who then identify other potential participants to participate in the study. The researchers started with the local e-consult initiative lead and then contacted the directors of primary care and specialty care services for help identifying PCPs, specialists, and support staff (nurse practitioners, pharmacists, program managers, informatics staff, and medical support personnel) engaged in the initiative. The goal for follow-up interviews was to interview at least 2 of the following respondents at each site: e-consult project manager, PCP, and/or specialist. Due to turnover and changes in clinic roles, some follow-up interviews were conducted with different individuals from the baseline interviews.
Data Collection
Interviews followed semistructured interview guidelines and included open-ended questions designed to elicit rich responses to a variety of aspects related to e-consult implementation, including patient needs, communication, leadership, resources, priorities, knowledge about the program, and unintended consequences. Follow-up interviews addressed how e-consults impacted the quality of specialty care; the impact of e-consults on Patient Aligned Care Teams (PACTs), the VHA patient-centered medical home initiative for primary care; and how e-consults have been used, eg, whether patients were involved in the decision to seek an e-consult.
Two interviewers who had participated in a 1-day, in-person training covering both data collection and analyzing key informant data conducted the 40 to 60 minute telephone interviews. One team member conducted the interview while the other took field notes. Interviews were also recorded. Follow-up probes were used to elicit specific examples and ensure sufficiently rich data. Following each interview, the notetaker reviewed the audio recording and filled in details in the field notes. The interview team debriefed and reviewed the augmented field notes and audio recordings, which became the primary data sources for the study.
Analysis
This was a qualitative descriptive analysis.12 Interview data were analyzed using an iterative, inductive content analysis method using an open coding approach (ie, a priori codes were not defined for this portion of the analysis).13 Two members of the research team used audio recordings and summary transcripts simultaneously to code data. Summary transcripts were compared with the recorded interviews to assure fidelity.
The researchers used Atlas.ti (Berlin, Germany) qualitative data analysis software to organize the coding process. Emergent codes were iteratively added throughout the analysis to reflect quotations that did not adequately fit previously developed codes. Codes were combined weekly to biweekly. After the combinations were completed, the analytics team met to review meanings of codes to ensure consistency of coding and interpretations.
To create categories, broad themes were identified from interview responses and grouped under high- order headings that described distinct aspects of participant experience. The analysis was intentionally kept close to the original data to reflect and describe the participant’s experience as accurately as possible. In support of analytical rigor, members of the multidisciplinary research team, composed of clinicians, implementation scientists, and mixed methodologists, reviewed findings to assess their thoroughness, comprehensiveness, and representativeness across roles and participating sites.14
Results
The e-consult evaluation period was from November 1, 2011, to July 31, 2013. Key conclusions were drawn from both alpha and beta sites (Table). Baseline interviews were conducted with 37 participants at 8 sites from April 10, 2012, to August 6, 2012. Follow-up interviews were conducted with 21 of the 37 participants at the 8 sites. Follow-up interviews with either a PCP or specialist could not be scheduled at 1 site. Follow-up interviews were conducted from April 16, 2013, to June 18, 2013. Open coding continued until saturation (the point at which subsequent data failed to produce new findings).15 This occurred after analysis of 22 baseline interviews (12 PCPs, 6 specialists, 1 pharmacist, and 4 other staff members) and 17 follow-up interviews (10 PCPs, 4 specialists, 1 pharmacist, and 2 other staff members).
Implementation
The e-consults provided a programmatic structure to the more informal practice of obtaining diagnostic or therapeutic advice from a specialist. Several of the specialists interviewed described having previously used existing informal consult processes that were “like e-consult.” These specialists reported that their practice patterns did not change significantly since implementing e-consults, because they have been “using the Computerized Patient Record System (CPRS) in an e-consult way for many years.” In these cases, the primary change resulting from the initiative was that national VHA workload policy was revised so that e-consults were assigned a CPT (Current Procedural Terminology) code and specialists began receiving workload credit for completing e-consults.
At sites where an informal e-consult practice was already in place, the initiative was consistently described as flexible. Many specialists reported that this degree of flexibility allowed them to make a relatively easy transition to e-consults by adopting new mechanisms to support existing processes. The e-consult initiative also allowed specialists to formally document this work and to increase the efficiency of specialty care.
Specialists drove the implementation process across sites. The e-consults were envisioned as a collaborative process; however, during initial interviews, few specialists mentioned PCPs when describing the development and implementation of the e-consult program. Primary care providers also reported having little awareness of or input into how the initiative was implemented, although this had little consequence on the use of e-consults.
In a rare case, a PCP reported that poorly designed, lengthy e-consult templates were a major barrier to using e-consults for specific specialties. The PCP said, “E-consults have created an elaborate but extraordinarily cumbersome tool that is difficult for PCPs to actually accomplish, because you have a consult menu that requires a lot of data to be entered—a lot of history from the chart, a lot of exam findings, a lot of previous cognitive testing scores; neurologic findings—lab and imaging tests.”
Still, many other PCPs described receiving detailed information and guidance from e-consults. “E-consults help me to be more accurate. Many providers don’t have a comfort with pain management. To get guidance and education and to really hold our hand, this is how to do this…this has been a big change. If they give you a great response, then [for] the next patient [with that condition], you go back to that note and then follow what was said there,” said one PCP.
In follow-up interviews, providers and other key staff stated there were more data available on the patient as a result of the e-consult and, consequently, even when specialists determined that a patient needed an in-person visit, the data obtained in the e-consult improved the quality of the in-person consultation.
Enhanced Communication and Collaboration
Neither the PCPs nor the specialists were aware of the collaborative intent of the initiative. They focused, instead, on other key aims, such as increasing accessibility and minimizing unnecessary patient travel. Most participants were generally positive about e-consults during baseline interviews, and this perception increased over time.
Both the PCPs and the specialists reported improved communication following the launch of e-consults. In follow-up interviews, some PCPs reported that before e-consults, they had trouble getting timely responses from specialists unless they knew them personally. “You had to know the person in the old days,” one respondent said. “After e-consults, responses improved…e-consult is available to have the resources to tap that knowledge base, and the team is answering the question. I think it opens up access and information and knowledge to everybody.”
Many PCPs spoke positively about this new communication tool as an opportunity to learn from specialists and said they valued the input they received. They felt the increased interaction between the 2 groups positively benefited patient care. One example cited that collaborative communication improved care coordination for veterans: “We are able to step in with e-consults to coordinate services, and this has been huge in improving care.”
Furthermore, follow-up interviews found that all participating PCPs and specialists were communicating more frequently and effectively. “Services that have embraced e-consult give a lot of great information flowing back; it’s closer to a real-time conversation,” said one respondent.
Related: Home-Based Video Telehealth for Veterans With Dementia
In baseline interviews, some specialists described how e-consults went against their belief that patient care is synonymous with face-to-face medical treatment and voiced dissatisfaction with e-consults as “sitting in front of a computer” rather than “seeing patients.” Others were concerned that medical center administration would not recognize the time it takes to conduct an e-consult and therefore not add necessary specialists staff. “E-consults take work and time, just like seeing a patient. I worry that won’t be seen,” one specialist said.
In order to successfully implement the e-consult initiative, providers and staff needed to incorporate new processes into their daily workflow.
Most sites did not develop a mechanism in which specialists received feedback regarding the outcome of their consultations. This lack of response created anxiety for some specialists in the absence of the face-to-face encounter, leaving some wondering whether they or the PCP had missed anything. According to one specialist, “That’s always in the back of your head: ‘Have I [the specialist] missed something?’”
In follow-up interviews, none of these concerns were raised. Primary care providers tended to speak of the care provided by specialists through e-consults in very positive terms, except in those instances where PCPs felt the e-consult template was difficult to use and required too much time to complete. “I was worried in the beginning about patients thinking less of me, but we ask for help all the time. We’re asking for help and not inconveniencing the patient; they seem to like it very much,” one PCP said.
The e-consults also complement PACTs. Initially, a few participants described soliciting patient input regarding the choice to have an e-consult or a face-to-face visit. During follow-up interviews, participants highlighted how well e-consults fit in to the PACT philosophy. One participant said, “The PACT team seeks to improve quality of care. E-consult fits very well with this, because answers to questions can come quickly, and the veteran may not need to come back to the clinic to be seen, even though things are still getting accomplished. E-consult works very well. E-consults were credited with improving access to specialty care as a tool for PACT.”
Achieving Program Objectives
Based on interviews, support for the e-consult program has increased over time as providers have gained experience with the program and have seen its benefits. Respondents at all sites consistently supported the concept of e-consults and expressed their belief in the importance and value of e-consults in improving patient-c entered care, primarily by reducing the need for patients to travel to see specialists, reducing the time to obtain feedback from specialists, and maintaining the provision of high quality care.
“Last year we only had 2 clinics categorized as e-consults. As of now we have 14 e-consults available for our providers. I think the numbers are growing. They are realizing the value of e-consults as far as the provider’s needs being met,” said one respondent.
The e-consults were credited with improving access to specialty care for veterans. Several participants stated that e-consults improved access to specialty care services and decreased travel for veterans. “It’s another way of getting care to the patient when the patient needs it without having to wait,” said one respondent.
Many PCPs described how difficult it was for patients to get to specialty appointments—particularly for their elderly, disabled, and rural patients—before the implementation of e-consults. “I like the fact that patients who live very far don’t have to come back. A lot of our patients are older…diabetic, see me Monday and back on Thursday. Now, they are able to stay home and follow the recommendations I write,” said one PCP.
Most providers were of the opinion that patients liked the program. “I think e-consults are helping patients...It’s been very successful regarding decreasing travel…Quicker response time for specialty care,” said a PCP. Several providers also stated in follow-up interviews that there was a greater degree of patient participation in the e-consult process and that “patients are definitely informed.”
Discussion
Most PCPs reported that the e-consults were an effective means of consultation and contained the information they needed to provide high-quality coordinated care. Most also found e-consult templates easy to complete. A majority of PCPs felt sufficient control over the choice of whether to use e-consults or an in-person visit, and a minority of patients were involved in the decision to receive an e-consult. Although the OSCS outlined guiding principles and operational rules in the Implementation Guide to help sites implement the e-consult program, its contribution was limited. Few examples were found that engaged PCPs in development of the e-consult program locally; involving patients in the decision to obtain a specialty consult electronically or in person; and PCPs feeding back results to specialists.
Implementing e-consults posed a number of challenges, including lack of resources to respond to referral requests, lack of referral policies and standardized procedures, and confusion related to roles and responsibilities. This is consistent with findings from another VHA research project of e-consults in 2 VHA health systems that was conducted prior to this national level e-consult pilot.7
Related: Using Facilitative Coaching to Support Patient Aligned Care Teams
Communication by OSCS of key aspects of the e-consult initiative will be critical as more sites implement e-consults. Since initiation of this pilot, workload specifications and credit have changed from 1 code to 3 codes, to more accurately reflect the amount of time a specialist consultant spends reviewing the EHR and responding to the consult. Without seeing the patient directly, specialists are more reliant on the PCP to describe the problem and provide adequate information in the e-consult request in order to provide recommendations back to the PCP.
Primary care physicians need to know that e-consults are available and determine when they are appropriate. A template or other guidance may be helpful to ensure adequate information is provided in the e-consult request; and the information provided by the specialist in response to the e-consult has to be sufficient for the PCP to provide care. VHA continues to expand the use of e-consults throughout the system, as this pilot found that the electronic option was often more timely than were face-to-face consultations. The result of this evaluation has informed national implementation of this effort.
Limitations
There are 3 main limitations to this study. First, because there was no practical way to preidentify participants who participated in implementing e-consults, a modified snowball sampling was used. However, this limited the degree to which the group was representative of the pilot participants. Second, the authors reported findings from a real-world initiative, not an experimental study. As such, not all participants in the first wave of key informant interviews were available for follow-up interview, which may have introduced bias. Third, the VHA is unlike most of the rest of the U.S. health care system in that it is a fully integrated system with salaried PCPs and specialists and an EHR.
Generalizability of the study may be limited, as a modified snowball sampling approach is not entirely random and has potential for community bias, because initial participants influence subsequent sampling. Additionally, though the sample size (n = 37) was sufficient for qualitative, in-depth analysis, it may be too small for confident generalization of findings. However, as health care moves toward an accountable care organization system, the authors’ analysis may provide insights.
Issues include revision of reimbursement policy for e-consults and developing or coordinating informational technology infrastructures to permit e-consults. It is also important to note that this evaluation reports solely on the extent of implementation of e-consults and the effects of e-consult implementation from the perspectives of staff, including specialists and PCPs.
Evaluating the effectiveness of the program in improving access, care coordination, and patient satisfaction was beyond the scope of the study. Further research is needed, because findings on those outcomes are critical for drawing inferences about this study’s implementation results.
Conclusion
The assessment of the e-consult system by providers and staff was based on a perception that e-consults are a valuable tool in providing greater access to quality care. Currently, e-consults have been expanded across VHA in medical and surgical specialties. VHA policymakers have drafted field guidance and a communication plan to support these efforts.
Acknowledgement
This material is based on work supported by the VA Office of Specialty Care Transformation, the office overseeing the e-consult initiative, and the Office of Research and Development Quality Enhancement Research Initiative.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Chen AH, Murphy EJ, Yee HF Jr. eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450-2453.
2. Hanna L, May C, Fairhurst K. The place of information and communication technology-mediated consultations in primary care: GPs’ perspectives. Fam Pract. 2012;29(3):361-366.
3. Kim-Hwang JE, Chen AH, Bell DS, Guzman D, Yee HF Jr, Kushel MB. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25(10):1123-1128.
4. Straus SG, Chen AH, Yee HF Jr, Kushel MB, Bell DS. Implementation of an electronic referral system for outpatient specialty care. AMIA Annu Symp Proc. 2011;2011:1337-1346.
5. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
6. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014;31(5):26-31.
7. Hysong SJ, Esquivel A, Sittig DF, et al. Towards successful coordination of electronic health record based-referrals: a qualitative analysis. Implement Sci. 2011;6:84.
8. American College of Physicians. The Patient- Centered Medical Home Neighbor: The Interface of the Patient-Centered Medical Home with Specialty/Subspecialty Practices. Philadelphia, PA: American College of Physicians; 2010. Policy paper.
9. Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med. 2008;359(12): 1202-1205.
10. Department of Veterans Affairs. Electronic Consultation (E-Consult) Implementation Guide, Version 1.2. Washington, DC: Department of Veterans Affairs, Office of Specialty Care Services, Specialty Care Transformation. 2013.
11. Kirsh S, Cary E, Aron DC et al. Results of a national pilot project for specialty care e-consultation in primary care medical homes: the impact of specialty e-consultation on access. Am J Manag Care. In press.
12. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340.
13. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115.
14. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 2000;284(3):357-362.
15. Sandelowski M. The problem of rigor in qualitative research. ANS Adv Nurs Sci. 1986;8(3):27-37.
1. Chen AH, Murphy EJ, Yee HF Jr. eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450-2453.
2. Hanna L, May C, Fairhurst K. The place of information and communication technology-mediated consultations in primary care: GPs’ perspectives. Fam Pract. 2012;29(3):361-366.
3. Kim-Hwang JE, Chen AH, Bell DS, Guzman D, Yee HF Jr, Kushel MB. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25(10):1123-1128.
4. Straus SG, Chen AH, Yee HF Jr, Kushel MB, Bell DS. Implementation of an electronic referral system for outpatient specialty care. AMIA Annu Symp Proc. 2011;2011:1337-1346.
5. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
6. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014;31(5):26-31.
7. Hysong SJ, Esquivel A, Sittig DF, et al. Towards successful coordination of electronic health record based-referrals: a qualitative analysis. Implement Sci. 2011;6:84.
8. American College of Physicians. The Patient- Centered Medical Home Neighbor: The Interface of the Patient-Centered Medical Home with Specialty/Subspecialty Practices. Philadelphia, PA: American College of Physicians; 2010. Policy paper.
9. Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med. 2008;359(12): 1202-1205.
10. Department of Veterans Affairs. Electronic Consultation (E-Consult) Implementation Guide, Version 1.2. Washington, DC: Department of Veterans Affairs, Office of Specialty Care Services, Specialty Care Transformation. 2013.
11. Kirsh S, Cary E, Aron DC et al. Results of a national pilot project for specialty care e-consultation in primary care medical homes: the impact of specialty e-consultation on access. Am J Manag Care. In press.
12. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340.
13. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115.
14. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 2000;284(3):357-362.
15. Sandelowski M. The problem of rigor in qualitative research. ANS Adv Nurs Sci. 1986;8(3):27-37.
Preventing and managing diabetic complications in elderly patients
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
KEY POINTS
- Compared with strict glycemic control, treating cardiovascular risk factors offers more benefit in a shorter time and should be a higher priority.
- Diabetic retinopathy is a leading cause of blindness. Yearly eye examinations are recommended.
- Elderly patients with diabetes are prone to rapidly progressive nephropathy, especially after receiving iodinated contrast agents. Good glycemic control and control of blood pressure, especially with angiotensin-converting enzyme inhibitors, reduce the risk and the rate of progression.
- Elderly patients with diabetes are at higher risk of cognitive decline, depression, and polypharmacy, all of which impede good diabetes management.
Managing diabetes in the elderly: Go easy, individualize
Guidelines for treating diabetes mellitus are mostly based on clinical studies in middle-aged people, and recommendations tend to be the same for everyone, whether young and strong or elderly and frail. But diabetes management should be individualized, especially in the elderly, taking into account each patient’s medical history, functional ability, home care situation, and life expectancy. Aggressive glycemic control is less important than avoiding hypoglycemia and achieving a good quality of life.
This article reviews the general principles for recognizing and managing diabetes in elderly patients, focusing on the management of blood sugar per se. In a future issue of this journal, we will discuss some of the many complications of diabetes in the elderly.
DIABETES DIFFERS IN ELDERLY PATIENTS
“The elderly” is a heterogeneous group with widely varying physiologic profiles, functional capabilities, and life expectancy (on average, about 88 years for men and 90 years for women in the United States). Although the elderly are sometimes classified as “young-old” (age 65–80) and “old-old” (80+), this distinction is too simplistic for clinical decision-making.
Diabetes mellitus in the elderly also is heterogeneous. One distinction is the age at which the disease developed.
Aging is associated with declining beta-cell function and lower blood insulin levels independent of insulin resistance, and with insulin resistance itself. The risk of developing type 2 diabetes mellitus increases with obesity, lack of physical activity, and loss of muscle mass, all of which often develop with aging.1
Middle-aged patients with diabetes have increased fasting hepatic glucose production, increased insulin resistance, and an abnormal insulin response to a glucose load. On the other hand, patients who develop diabetes at an older age tend to have normal hepatic glucose production. Older patients who are lean secrete markedly less insulin in response to a glucose load but have relatively less insulin resistance.2 Patients who develop type 2 diabetes in old age are more likely to have near-normal fasting blood glucose levels but significant postprandial hyperglycemia.3,4 Elderly patients who developed diabetes during middle age have metabolic abnormalities more typical of middle-aged patients with type 2 diabetes.
DIABETES IS COMMON, AND INCREASING IN PREVALENCE
By age 75, 40% of people in the United States have either glucose intolerance or diabetes mellitus.5 Metabolic syndrome, which is the constellation of insulin resistance (type 2 diabetes mellitus), hyperlipidemia, hypertension, and obesity, is more prevalent in people age 65 to 74 years than in younger and older people.3
The National Diabetes Surveillance System of the US Centers for Disease Control and Prevention estimated that the prevalence of diabetes mellitus in people 65 to 74 years old in 2005 was 18.5%, about 12 times the prevalence among those younger than 45years.6 The prevalence has been gradually increasing and has nearly doubled over the past 25 years, with certain groups—native Americans, Hispanics, and African-Americans—at particularly high risk of developing the disease.
Although the prevalence of diabetes in people older than 75 years is lower than among people in the 65-to-74-year range, the elderly segment of our population is increasing, and the impact of diabetes and its associated burden of death and disease from vascular complications is enormous.
SYMPTOMS ARE OFTEN NONSPECIFIC
Unfortunately, diabetes is underdiagnosed and frequently undertreated, resulting in even more disease and death.7–9
Diabetes is often missed in the elderly because its presenting symptoms may be nonspecific, eg, failure to thrive, low energy, falls, dizziness, confusion, nocturia (with or without incontinence), and urinary tract infection.The classic symptoms of frequent urination (often leading to worsening incontinence), thirst, and increased hunger usually occur only when plasma glucose levels are above 200 mg/dL. Weight loss, blurred vision, and dehydration may also be present with high blood glucose levels. With lesser degrees of hyperglycemia, patients may have no symptoms or present with weight loss or signs and symptoms of chronic infection, especially of the genitourinary tract, skin, or mouth.
Hyperglycemia in elderly patients is also associated with reduced cognitive function (which may improve with blood glucose control).10
The American Diabetes Association recommends screening by measuring the fasting plasma glucose level every 3 years beginning at 45 years.11 However, some experts believe that this method is inadequate for the elderly12; some suggest that screening should be done more often in those with risk factors for diabetes, including obesity, inactivity, hypertension, and dyslipidemia, all of which are common in the elderly. Targeted screening in patients with hypertension may be the most cost-effective strategy.13
Screening with hemoglobin A1c levels is not recommended because of lack of standardization among laboratories.14
INDIVIDUALIZED MANAGEMENT IS BEST
Despite disease differences, the general goals for diabetes care are the same for all ages:
- To control hyperglycemia and its symptoms
- To prevent, evaluate, and treat macrovascular and microvascular complications
- To teach patients to manage themselves
- To maintain or improve the patient’s general health status.
Unfortunately, most specific recommendations are based on studies in younger people. Guidelines should ideally reflect the complexities of a particular clinical situation, but most recommendations are applied to the young and old alike, as well as to the relatively healthy and the frail and ill.15–17 Consideration should be given to a patient’s health beliefs, severity of vascular complications and other medical problems, economic situation, life expectancy ,functional status, and availability of support services. In addition, some patients prefer aggressive treatment, while others would rather compromise some aspects of care in order to maintain a certain quality of life, to save money, or to avoid having caregivers provide treatment.
Age-related changes in pharmacokinetics as well as polypharmacy increase the risk of drug interactions and adverse effects, especially drug-induced hypoglycemia. In addition, age-associated changes in cognitive, visual, and physical function, dentition, and taste perception can reduce a patient’s ability to carry out treatment. Frequent hospitalizations also disrupt outpatient regimens.
Comorbidities make treatment more challenging, but some conditions—such as hypertension, renal insufficiency and eye disorders—make doctors more likely to control hyperglycemia more aggressively, fearing that the loss of a little more function in an impaired organ may lead to failure.
The benefits of tight glycemic control should be weighed against the risks and the realities of an individual situation. Priority should be given to achieving the best quality of life possible.17 Recent guidelines from the California Health Foundation and the American Geriatrics Association focused on the major health threats to older patients and prioritizing care for each person.15 The guidelines recommend screening for geriatric syndromes that are more prevalent inpatients with diabetes or are strongly affected by the disease or its treatment. Diabetes care should be examined in the setting of common geriatric problems: depression, polypharmacy, cognitive impairment, urinary incontinence, falls, and pain.
Heart risk trumps glycemic control
The expert panel15 concluded that rates of disease and death can be reduced more by targeting cardiovascular risk factors than by intensively managing hyperglycemia. One rationale is that it takes 8 years for aggressive glycemic control to reduce the risk of diabetic retinopathy or renal disease but only 2 years of treating hypertension and dyslipidemia to reduce the risk of cardiovascular disease.15,17–21 A recent Japanese study found normal mortality rates in elderly patients under long-term, intensive multifactorial diabetes control.22 High-functioning, motivated patients could benefit from therapy aimed at achieving most or all of the recommended goals, but frail patients may suffer from applying all therapies and may benefit from only some of them.
If appropriate goals cannot be met, it may help to refer patients to a geriatric specialist to evaluate possible barriers to adherence such as depression or poor cognition, physical functioning, or support.
MANAGEMENT STRATEGIES
Weight loss and exercise help prevent diabetes
The Diabetes Prevention Program23 randomized 3,234 people (mean age 51 years) with impaired glucose tolerance to receive either metformin (Fortamet, Glucophage) 850 mg twice daily or placebo or to undertake lifestyle modifications with goals of at least a 7% weight loss and at least 150 minutes of physical activity per week. Compared with the placebo group, the lifestyle modification group had a 58% lower incidence of diabetes while those in the metformin group had only a 31% lower incidence. Among those older than 60 years, the advantage of lifestyle modification over metformin was even greater.
Control blood glucose, avoid hypoglycemia
- Hemoglobin A1c levels < 7.0%
- Preprandial blood glucose levels 90–130 mg/dL
- Bedtime blood glucose levels 110–150 mg/dL.
Guidelines from the Department of Veterans Affairs24 and the American Geriatrics Society15 are slightly different, and are based on randomized trials in younger patients, primarily the Diabetes Control and Complications Trial (DCCT)25 and the United Kingdom Prospective Diabetes Study(UKPDS).21,26 A recent position statement from the American College of Physicians, based on a review of all the major guidelines, recommends the following: “Statement 1: To prevent microvascular complication of diabetes, the goal for glycemic control should be as low as is feasible without undue risk for adverse events or an unacceptable burden on patients. Treatment goals should be based on a discussion of the benefits and harms of specific levels of glycemic control with the patient. A hemoglobin A1c level less than 7% based on individualized assessment is a reasonable goal for many but not all patients. Statement 2: The goal for hemoglobin A1c should be based on individualized assessment of risk for complication from diabetes, comorbidity, life expectancy, and patient preferences.”27
Although few data exist for elderly patients, these guidelines are the most current approach to treating diabetes in the elderly. Less stringent goals are appropriate for patients who have limited life expectancy, hypoglycemia unawareness (lack of autonomic warning symptoms of low blood sugar), seizures, dementia, psychiatric illness, or alcoholism. It is important to keep in mind the following as one strives for lower A1c levels: Although the relative risk reduction accomplished by lowering hemoglobin A1c is linear, the absolute risk reduction is log-linear—more benefit is gained by lowering hemoglobin A1c from 9% to 8% than from 8% to 7%.28
Hypoglycemia is a major limiting factor in glycemic control. Many risk factors for hypoglycemia are common in the elderly (Table 2). Hypoglycemia was a chief adverse event in both the DCCT and the UKPDS, with a twofold to threefold higher rate in patients who were intensively treated.29 Even mild hypoglycemia in the elderly can result in an injurious fall, which can lead to long-term functional decline. The rate of severe or fatal hypoglycemia—the major risk of tight glycemic treatment—increases exponentially with age.30–33
As people age, the mechanisms that regulate blood sugar are impaired: the glucagon response is diminished, which increases dependence on the epinephrine response to prevent hypoglycemia.34 Medications such as beta-blockers, which can suppress the symptoms of hypoglycemia, may further impair the response. Consequently, older patients may be less aware of hypoglycemia, and the symptoms may be less intense. Renal insufficiency may also exacerbate the problem by reducing clearance of oral agents. In addition, confused patients may take extra doses of medications.
Patients with type 2 diabetes treated with insulin, sulfonylureas, or meglitinides should be evaluated for symptoms of hypoglycemia. Older patients may have more neuroglycopenic symptoms (eg, dizziness, weakness, confusion, nightmares, violent behavior) than adrenergic symptoms (eg, sweating, palpitations, tremors), although both types should be asked about during an evaluation.2,32,33 Hypoglycemia may also present as transient hemiparesis, coma, or falls.35
We carefully evaluate the glycemic regimen and care environment of any elderly patient who presents with a blood glucose level below 100 mg/dL. The regimen should be altered for less strict control if the patient is cognitively impaired, is at risk of falling, or has an unstable care situation (eg, has irregular meals or needs assistance with daily activities and does not have a regular caregiver). Patients at significant risk of hypoglycemia should be encouraged to check their blood glucose level with a fingerstick before driving.
Tight control in the hospital is controversial
Glycemic control in the hospital has traditionally been designed primarily to maintain “safe” blood glucose levels, ie, to prevent hyperglycemia-induced dehydration and catabolism while avoiding hypoglycemia. Recent studies have suggested that tighter glycemic control may reduce the rates of complications and death perioperatively and in patients with myocardial infarction or who are seriously ill in the intensive care unit, although the evidence is mixed.36–38 Specific targets are controversial, and although studies have included some elderly patients, results cannot be generalized to this group.
DIABETES CARE TAKES A TEAM
Geriatric patients have complex problems. In the face of multiple comorbidities, difficult social situations, and polypharmacy, the physician can best address the drug therapy and lifestyle changes that diabetes management requires by working with a certified diabetes educator, dietitian, social worker, and pharmacist.
Nonpharmacologic therapy
The first step in therapy for glycemic control is diet and exercise, although such measures are often limited in the elderly.
Diet. Carbohydrate control can maintain euglycemia in some patients with type 2 diabetes. But for the elderly, especially those living in long-term health care facilities, malnutrition may be of more concern than obesity, making dietary restrictions harmful. Patients in danger of malnutrition should be given unrestricted menus with consistent amounts of carbohydrate at meals and snacks. Medications should be adjusted to control blood glucose levels if necessary.39
For patients living in the community, dietary therapy should be individualized by a dietitian. Medicare covers up to 10 hours of diabetes education with a certified diabetes educator or registered dietitian within a 12-month period if at least one of the following criteria are met: the patient is newly diagnosed with diabetes, the hemoglobin A1c level is higher than 8.5%, medication has been recently started, or the risk of complications is high.
Supplementation of vitamins and minerals is prudent. Supplemental magnesium, zinc, and vitamins C and E may improve glycemic control.40–44
Exercise reduces insulin resistance, weight, and blood pressure; increases muscle mass; and improves lipid levels. Both aerobic and nonaerobic activity are beneficial.45–47 The best time to exercise is 1 to 2 hours after a meal, when glucose levels tend to be highest. Either hypoglycemia or hyperglycemia may occur up to 24 hours following exercise, and medications may need to be adjusted.
Oral medications
Insulin
Insulin therapy is necessary if oral combination therapy proves insufficient. Insulin is generally required for patients with moderate or severe hyperglycemia, especially for those with renal or hepatic insufficiency.52,53 Before prescribing insulin therapy to elderly patients, we need to consider their visual acuity, manual dexterity and sensation, cognitive function, family support, and financial situation.However, several studies showed that quality of life improves in the year after starting insulin for patients whose blood sugar was previously poorly controlled with oral agents.54,55
An evening dose of neutral protamine Hagedorn (NPH) insulin is a good way to start. More complex regimens may be necessary, depending on glycemic goals.
A number of premixed preparations of various types of insulin with different durations of action are available. They may improve accuracy, acceptability, and ease of insulin administration, although glycemic control and the risk of hypoglycemia may not change or in fact may be worse.56 Some patients may not achieve adequate glucose control with fixed-dose regimens.57,58
Frequent, small, titrated doses of short-acting agents control hyperglycemia better, particularly postprandial hyperglycemia,resulting in less hypoglycemia. However, these regimens may be too complex for many elderly patients; a patient’s support system must be evaluated before recommending this type of therapy.
Most insulins are available in vials and in pens, the latter of which are quick and easy to use, provide precise doses, and can be managed by many elderly patients. Pens require the user to attach a needle, set the dose by a dial, and depress the plunger to inject the dose. Some are prefilled and disposable, others have refillable cartridges. Studies in patients older than 60 years have shown the pen systems to be more acceptable, safer, and more effective than conventional syringes.
If conventional syringes are used, low-dose syringes (30-unit or 50-unit), which have more visible unit markings, should be prescribed whenever possible rather than the 100-unit sizes. Magnifying devices that attach to a syringe are also available.
Studies have also shown that continuous subcutaneous insulin infusion is safe for selected elderly patients.
Incretin mimetics: Possibly well-suited
Incretins, such as glucagon-like peptide-1, are hormones released from the gastrointestinal tract in response to eating. They stimulate insulin secretion by non-glucose-related pathways.
Exenatide (Byetta), a 39-amino-acid peptide incretin mimetic, is a synthetic version of exendin-4, an incretin isolated from the saliva of the Gila monster. Recently approved for treating type 2 diabetes, it is given subcutaneously.59,60 Oral dipeptidyl peptidase-4 inhibitors (sitagliptin and vildagliptin) decrease the degradation of endogenous incretin and thus prolong its action.61 Because a decline in glucose-mediated beta-cell insulin secretion is a major contributor to the development of diabetes in the elderly, the drug may be especially helpful for this population.However, further clinical research and experience is needed before specific recommendations for elderly patients can be made.
SELF-MANAGEMENT IS IMPORTANT
Patient education is critical
Patient education is a cornerstone of diabetes self-management,62–66 and is especially important for patients who are cognitively impaired or who have limited language proficiency.Patient education is covered under Medicare Part B. Ample resources are available in print and electronic formats. Community resources can also be important.
Home glucose monitoring is simpler now
A patient’s insulin regimen should ideally be tailored according to home blood glucose level monitoring before and after meals and at bedtime. Medicare reimburses for once daily testing for patients who are not taking insulin and for three-times-daily testing for those taking insulin.
Elderly patients can be taught to reliably monitor their own blood glucose levels without diminishing their quality of life. Monitoring is now easier with new glucometers and test strips that use small amounts of blood. Testing can now also be performed on blood taken from the forearm, upper arm, thigh, or calf with the FreeStyle (TheraSense), One Touch Ultra (LifeScan) and Soft Tac (MediSense) meters. The Soft Tac meter lances skin and automatically transfers blood to the test strip, making use even easier. Talking glucometers are available for blind patients.
Coordination counts
A variety of models of chronic care delivery have been proposed. Regardless of which model is chosen, the complexities of management call for a multidisciplinary team approach, and coordination of care in order to ensure appropriate information flow becomes critical.
Editor’s note: In next month’s issue of this journal, Drs. Hornick and Aron will discuss the management of diabetic complications in the elderly, including coronary artery disease, neuropathy, and kidney disease.
- Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997; 46:701–710.
- Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol A Biol Sci Med Sci 2001; 56:M5–M13.
- Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore Longitudinal Study of Aging. Metabolism Clin Exper 2005; 54:542–547.
- Crandall J, Barzilai N. Treatment of diabetes mellitus in older people: oral therapy options. J Am Geriatr Soc 2003; 51:272–274.
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998; 21:518–524.
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. Diabetes Public Health Resource. National Diabetes Surveillance System. www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm.
- Franse LV, Di Bari M, Shorr RI, et al; Health, Aging, and Body Composition Study Group. Type 2 diabetes in older well-functioning people: who is underdiagnosed? Data from the Health, Aging, and Body Composition study. Diabetes Care 2001; 24:2065–2070. Erratum in: Diabetes Care 2002; 25:413.
- Shorr RI, Franse LV, Resnick HE, Di Bari M, Johnson KC, Pahor M. Glycemic control of older adults with type 2 diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Geriatr Soc 2000; 48:264–267.
- Smith NL, Savage PJ, Heckbert SR, et al. Glucose, blood pressure, and lipid control in older people with and without diabetes mellitus: the Cardiovascular Health Study. J Am Geriatr Soc 2002; 50:416–423.
- Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol 1993; 48:M117–M121.
- American Diabetes Association. Standards of medical care in diabetes.Diabetes Care 2005; 28:S4–S36.
- Motta M, Bennati E, Ferlito L, Malaguarnera M. Diabetes mellitus in the elderly: diagnostic features. Arch Gerontol Geriatr 2006; 42:101–106.
- Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost effective analysis. Ann Intern Med 2004; 140:689–699.
- Harris RP, Lux LJ, Bunton AJ, et al. Screening for type 2 diabetes mellitus. Systematic Evidence Review Number 19. February 4, 2003.US Department of Health and Human Services, Agency for Healthcare Research and Quality. Rockville, MD. www.ahrq.gov/downloads/pub/prevent/pdfser/diabser.pdf.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA; California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA 2006; 295:1935–1940.
- Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA 1996; 276:1886–1892. Erratum in: JAMA 1997; 277:1356.
- Goddijn PP, Bilo HJ, Feskens EJ, Groeniert KH, van der Zee KI, Meyboom-de Jong B. Longitudinal study on glycaemic control and quality of life in patients with Type 2 diabetes mellitus referred for intensified control. Diabet Med 1999; 16:23–30.
- Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997; 20:614–620. Erratum in: Diabetes Care 1997; 20:1048.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Katakura M, Naka M, Kondo T, et al, and the Nagano Elderly Diabetes Study Group. Normal mortality in the elderly with diabetes under strict glycemic and blood pressure control: outcome of 6-year prospective study. Diabetes Res Clin Practice 2007; 78:108–114.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Pogach LM, Brietzke SA, Cowan CL Jr, Conlin P, Walder DJ, Sawin CT; VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK for the Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians. Ann Intern Med 2007; 147:417–422.
- Pogach L, Engelgau M, Aron D. Measuring progress toward achieving hemoglobin A1c goals in diabetes care: pass/fail or partial credit. JAMA 2007; 297:520–523.
- Egger M, Davey Smith G, Stettler C, Diem P. Risk of adverse effects of intensified treatment in insulin-dependent diabetes mellitus: ameta-analysis. Diabet Med 1997; 14:919–928.
- Burge MR, Schmitz-Fiorentino K, Fischette C, Qualls CR, Schade DS. A prospective trial of risk factors for sulfonylurea-induced hypoglycemia in type 2 diabetes mellitus. JAMA 1998; 279:137–143.
- Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med 1999; 159:281–284.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996; 44:751–755.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med 1997; 157:1681–1685.
- Burge MR, Kamin JR, Timm CT, Qualls CR, Schade DS. Low-dose epinephrine supports plasma glucose in fasted elderly patients with type 2 diabetes. Metabolism 2000; 49:195–202.
- Alagiakrishnan K, Lechelt K, McCracken P, Torrible S, Sclater A.Atypical presentation of silent nocturnal hypoglycemia in an olderperson. J Am Geriatr Soc 2001; 49:1577–1578.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999; 99:2626–2632.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Metchick LN, Petit WA Jr, Inzucchi SE. Inpatient management of diabetes mellitus. Am J Med 2002; 113:317–323.
- Coulston AM, Mandelbaum D, Reaven GM. Dietary management of nursing home residents with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1990; 51:67–71.
- Song MK, Rosenthal MJ, Naliboff BD, Phanumas L, Kang KW. Effects of bovine prostate powder on zinc, glucose and insulin metabolism in old patients with non-insulin-dependent diabetes mellitus. Metabolism 1998; 47:39–43.
- Paolisso G, D’Amore A, Galzerano D, et al. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care 1993; 16:1433–1437.
- Paolisso G, Scheen A, Cozzolino D, et al. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J Clin Endocrinol Metab 1994; 78:1510–1514.
- Paolisso G, Passariello N, Pizza G, et al. Dietary magnesium supplements improve B-cell response to glucose and arginine in elderly non-insulin dependent diabetic subjects. Acta Endocrinol (Copenh) 1989; 121:16–20.
- Paolisso G, D’Amore A, Balbi V, et al. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. Am J Physiol 1994; 266:E261–E268. Erratum in: Am J Physiol 1994; 267:section E following table of contents.
- Agurs-Collins TD, Kumanyika SK, Ten Have TR, Adams-Campbell LL. A randomized controlled trail of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care 1997; 200:1503–1511.
- Skarfors ET, Wegener TA, Lithell H, Selinus I. Physical training as treatment for type 2 (non-insulin-dependent) diabetes in elderly men. A feasibility study over 2 years. Diabetologia 1987; 30:930–933.
- Raz I, Hauser E, Bursztyn M. Moderate exercise improves glucose metabolism in uncontrolled elderly patients with non-insulin-dependent diabetes mellitus. Isr J Med Sci 1994; 30:766–770.
- Brodows RG. Benefits and risks with glyburide and glipizide in elderly NIDDM patients. Diabetes Care 1992; 15:75–80.
- Landgraf R. Meglitinide analogues in the treatment of type 2 diabetes mellitus. Drugs Aging 2000; 17:411–425.
- Ron Y, Wainstein J, Leibovitz A, et al. The effect of acarbose on the colonic transit time of elderly long-term care patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 2002; 57:M111–M114.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Gerstein HC, Haynes RB, eds. Evidence-Based Diabetes Management. Hamilton, Ont.; London: B.C. Dekker; 2001.
- Tovi J, Engfeldt P. Well being and symptoms in elderly type 2 diabetes patients with poor metabolic control: effect of insulin treatment. Practical Diabetes Int 1998; 15:73–77.
- Reza M, Taylor CD, Towse K, Ward JD, Hendra TJ. Insulin improves well-being for selected elderly type 2 diabetic subjects. Diabetes Res Clin Pract 2002; 55:201–207.
- Janka HU, Plewe G, Busch K. Combination of oral antidiabetic agents with basal insulin versus premixed insulin alone in randomized elderly patients with type 2 diabetes mellitus. J Am Geriatrics Soc 2007; 55:182–188.
- Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care 1992; 15:1628–1630.
- Rolla AR, Rakel RE. Practical approaches to insulin therapy for type 2 diabetes mellitus with premixed insulin analogues. Clin Ther 2005; 27:1113–1125.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Mathieu C, Bollaerts K. Antihyperglycaemic therapy in elderly patients with type 2 diabetes: potential role of incretin mimetics and DPP-4 inhibitors. Int J Clin Pract 2007; 61(suppl 154):29–37.
- Bernbaum M, Albert SG, McGinnis J, Brusca S, Mooradian AD. The reliability of self blood glucose monitoring in elderly diabetic patients. J Am Geriatr Soc 1994; 42:779–781.
- Gilden JL, Hendryx M, Casia C, Singh SP. The effectiveness of diabetes education programs for older patients and their spouses. J Am Geriatr Soc 1989; 37:1023–1030.
- Glasgow RE, Toobert DJ, Hampson SE, Brown JE, Lewinsohn PM, Donnelly J. Improving self-care among older patients with type II diabetes: the ‘Sixty Something…’ Study. Patient Educ Couns 1992; 19:61–74.
- Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc 2005; 53:306–311.
- Langa KM, Vijan S, Hayward RA, et al. Informal caregiving for diabetes and diabetic complications among elderly Americans. J Gerontol B Psychol Sci Soc Sci 2002; 57:S177–S186.
Guidelines for treating diabetes mellitus are mostly based on clinical studies in middle-aged people, and recommendations tend to be the same for everyone, whether young and strong or elderly and frail. But diabetes management should be individualized, especially in the elderly, taking into account each patient’s medical history, functional ability, home care situation, and life expectancy. Aggressive glycemic control is less important than avoiding hypoglycemia and achieving a good quality of life.
This article reviews the general principles for recognizing and managing diabetes in elderly patients, focusing on the management of blood sugar per se. In a future issue of this journal, we will discuss some of the many complications of diabetes in the elderly.
DIABETES DIFFERS IN ELDERLY PATIENTS
“The elderly” is a heterogeneous group with widely varying physiologic profiles, functional capabilities, and life expectancy (on average, about 88 years for men and 90 years for women in the United States). Although the elderly are sometimes classified as “young-old” (age 65–80) and “old-old” (80+), this distinction is too simplistic for clinical decision-making.
Diabetes mellitus in the elderly also is heterogeneous. One distinction is the age at which the disease developed.
Aging is associated with declining beta-cell function and lower blood insulin levels independent of insulin resistance, and with insulin resistance itself. The risk of developing type 2 diabetes mellitus increases with obesity, lack of physical activity, and loss of muscle mass, all of which often develop with aging.1
Middle-aged patients with diabetes have increased fasting hepatic glucose production, increased insulin resistance, and an abnormal insulin response to a glucose load. On the other hand, patients who develop diabetes at an older age tend to have normal hepatic glucose production. Older patients who are lean secrete markedly less insulin in response to a glucose load but have relatively less insulin resistance.2 Patients who develop type 2 diabetes in old age are more likely to have near-normal fasting blood glucose levels but significant postprandial hyperglycemia.3,4 Elderly patients who developed diabetes during middle age have metabolic abnormalities more typical of middle-aged patients with type 2 diabetes.
DIABETES IS COMMON, AND INCREASING IN PREVALENCE
By age 75, 40% of people in the United States have either glucose intolerance or diabetes mellitus.5 Metabolic syndrome, which is the constellation of insulin resistance (type 2 diabetes mellitus), hyperlipidemia, hypertension, and obesity, is more prevalent in people age 65 to 74 years than in younger and older people.3
The National Diabetes Surveillance System of the US Centers for Disease Control and Prevention estimated that the prevalence of diabetes mellitus in people 65 to 74 years old in 2005 was 18.5%, about 12 times the prevalence among those younger than 45years.6 The prevalence has been gradually increasing and has nearly doubled over the past 25 years, with certain groups—native Americans, Hispanics, and African-Americans—at particularly high risk of developing the disease.
Although the prevalence of diabetes in people older than 75 years is lower than among people in the 65-to-74-year range, the elderly segment of our population is increasing, and the impact of diabetes and its associated burden of death and disease from vascular complications is enormous.
SYMPTOMS ARE OFTEN NONSPECIFIC
Unfortunately, diabetes is underdiagnosed and frequently undertreated, resulting in even more disease and death.7–9
Diabetes is often missed in the elderly because its presenting symptoms may be nonspecific, eg, failure to thrive, low energy, falls, dizziness, confusion, nocturia (with or without incontinence), and urinary tract infection.The classic symptoms of frequent urination (often leading to worsening incontinence), thirst, and increased hunger usually occur only when plasma glucose levels are above 200 mg/dL. Weight loss, blurred vision, and dehydration may also be present with high blood glucose levels. With lesser degrees of hyperglycemia, patients may have no symptoms or present with weight loss or signs and symptoms of chronic infection, especially of the genitourinary tract, skin, or mouth.
Hyperglycemia in elderly patients is also associated with reduced cognitive function (which may improve with blood glucose control).10
The American Diabetes Association recommends screening by measuring the fasting plasma glucose level every 3 years beginning at 45 years.11 However, some experts believe that this method is inadequate for the elderly12; some suggest that screening should be done more often in those with risk factors for diabetes, including obesity, inactivity, hypertension, and dyslipidemia, all of which are common in the elderly. Targeted screening in patients with hypertension may be the most cost-effective strategy.13
Screening with hemoglobin A1c levels is not recommended because of lack of standardization among laboratories.14
INDIVIDUALIZED MANAGEMENT IS BEST
Despite disease differences, the general goals for diabetes care are the same for all ages:
- To control hyperglycemia and its symptoms
- To prevent, evaluate, and treat macrovascular and microvascular complications
- To teach patients to manage themselves
- To maintain or improve the patient’s general health status.
Unfortunately, most specific recommendations are based on studies in younger people. Guidelines should ideally reflect the complexities of a particular clinical situation, but most recommendations are applied to the young and old alike, as well as to the relatively healthy and the frail and ill.15–17 Consideration should be given to a patient’s health beliefs, severity of vascular complications and other medical problems, economic situation, life expectancy ,functional status, and availability of support services. In addition, some patients prefer aggressive treatment, while others would rather compromise some aspects of care in order to maintain a certain quality of life, to save money, or to avoid having caregivers provide treatment.
Age-related changes in pharmacokinetics as well as polypharmacy increase the risk of drug interactions and adverse effects, especially drug-induced hypoglycemia. In addition, age-associated changes in cognitive, visual, and physical function, dentition, and taste perception can reduce a patient’s ability to carry out treatment. Frequent hospitalizations also disrupt outpatient regimens.
Comorbidities make treatment more challenging, but some conditions—such as hypertension, renal insufficiency and eye disorders—make doctors more likely to control hyperglycemia more aggressively, fearing that the loss of a little more function in an impaired organ may lead to failure.
The benefits of tight glycemic control should be weighed against the risks and the realities of an individual situation. Priority should be given to achieving the best quality of life possible.17 Recent guidelines from the California Health Foundation and the American Geriatrics Association focused on the major health threats to older patients and prioritizing care for each person.15 The guidelines recommend screening for geriatric syndromes that are more prevalent inpatients with diabetes or are strongly affected by the disease or its treatment. Diabetes care should be examined in the setting of common geriatric problems: depression, polypharmacy, cognitive impairment, urinary incontinence, falls, and pain.
Heart risk trumps glycemic control
The expert panel15 concluded that rates of disease and death can be reduced more by targeting cardiovascular risk factors than by intensively managing hyperglycemia. One rationale is that it takes 8 years for aggressive glycemic control to reduce the risk of diabetic retinopathy or renal disease but only 2 years of treating hypertension and dyslipidemia to reduce the risk of cardiovascular disease.15,17–21 A recent Japanese study found normal mortality rates in elderly patients under long-term, intensive multifactorial diabetes control.22 High-functioning, motivated patients could benefit from therapy aimed at achieving most or all of the recommended goals, but frail patients may suffer from applying all therapies and may benefit from only some of them.
If appropriate goals cannot be met, it may help to refer patients to a geriatric specialist to evaluate possible barriers to adherence such as depression or poor cognition, physical functioning, or support.
MANAGEMENT STRATEGIES
Weight loss and exercise help prevent diabetes
The Diabetes Prevention Program23 randomized 3,234 people (mean age 51 years) with impaired glucose tolerance to receive either metformin (Fortamet, Glucophage) 850 mg twice daily or placebo or to undertake lifestyle modifications with goals of at least a 7% weight loss and at least 150 minutes of physical activity per week. Compared with the placebo group, the lifestyle modification group had a 58% lower incidence of diabetes while those in the metformin group had only a 31% lower incidence. Among those older than 60 years, the advantage of lifestyle modification over metformin was even greater.
Control blood glucose, avoid hypoglycemia
- Hemoglobin A1c levels < 7.0%
- Preprandial blood glucose levels 90–130 mg/dL
- Bedtime blood glucose levels 110–150 mg/dL.
Guidelines from the Department of Veterans Affairs24 and the American Geriatrics Society15 are slightly different, and are based on randomized trials in younger patients, primarily the Diabetes Control and Complications Trial (DCCT)25 and the United Kingdom Prospective Diabetes Study(UKPDS).21,26 A recent position statement from the American College of Physicians, based on a review of all the major guidelines, recommends the following: “Statement 1: To prevent microvascular complication of diabetes, the goal for glycemic control should be as low as is feasible without undue risk for adverse events or an unacceptable burden on patients. Treatment goals should be based on a discussion of the benefits and harms of specific levels of glycemic control with the patient. A hemoglobin A1c level less than 7% based on individualized assessment is a reasonable goal for many but not all patients. Statement 2: The goal for hemoglobin A1c should be based on individualized assessment of risk for complication from diabetes, comorbidity, life expectancy, and patient preferences.”27
Although few data exist for elderly patients, these guidelines are the most current approach to treating diabetes in the elderly. Less stringent goals are appropriate for patients who have limited life expectancy, hypoglycemia unawareness (lack of autonomic warning symptoms of low blood sugar), seizures, dementia, psychiatric illness, or alcoholism. It is important to keep in mind the following as one strives for lower A1c levels: Although the relative risk reduction accomplished by lowering hemoglobin A1c is linear, the absolute risk reduction is log-linear—more benefit is gained by lowering hemoglobin A1c from 9% to 8% than from 8% to 7%.28
Hypoglycemia is a major limiting factor in glycemic control. Many risk factors for hypoglycemia are common in the elderly (Table 2). Hypoglycemia was a chief adverse event in both the DCCT and the UKPDS, with a twofold to threefold higher rate in patients who were intensively treated.29 Even mild hypoglycemia in the elderly can result in an injurious fall, which can lead to long-term functional decline. The rate of severe or fatal hypoglycemia—the major risk of tight glycemic treatment—increases exponentially with age.30–33
As people age, the mechanisms that regulate blood sugar are impaired: the glucagon response is diminished, which increases dependence on the epinephrine response to prevent hypoglycemia.34 Medications such as beta-blockers, which can suppress the symptoms of hypoglycemia, may further impair the response. Consequently, older patients may be less aware of hypoglycemia, and the symptoms may be less intense. Renal insufficiency may also exacerbate the problem by reducing clearance of oral agents. In addition, confused patients may take extra doses of medications.
Patients with type 2 diabetes treated with insulin, sulfonylureas, or meglitinides should be evaluated for symptoms of hypoglycemia. Older patients may have more neuroglycopenic symptoms (eg, dizziness, weakness, confusion, nightmares, violent behavior) than adrenergic symptoms (eg, sweating, palpitations, tremors), although both types should be asked about during an evaluation.2,32,33 Hypoglycemia may also present as transient hemiparesis, coma, or falls.35
We carefully evaluate the glycemic regimen and care environment of any elderly patient who presents with a blood glucose level below 100 mg/dL. The regimen should be altered for less strict control if the patient is cognitively impaired, is at risk of falling, or has an unstable care situation (eg, has irregular meals or needs assistance with daily activities and does not have a regular caregiver). Patients at significant risk of hypoglycemia should be encouraged to check their blood glucose level with a fingerstick before driving.
Tight control in the hospital is controversial
Glycemic control in the hospital has traditionally been designed primarily to maintain “safe” blood glucose levels, ie, to prevent hyperglycemia-induced dehydration and catabolism while avoiding hypoglycemia. Recent studies have suggested that tighter glycemic control may reduce the rates of complications and death perioperatively and in patients with myocardial infarction or who are seriously ill in the intensive care unit, although the evidence is mixed.36–38 Specific targets are controversial, and although studies have included some elderly patients, results cannot be generalized to this group.
DIABETES CARE TAKES A TEAM
Geriatric patients have complex problems. In the face of multiple comorbidities, difficult social situations, and polypharmacy, the physician can best address the drug therapy and lifestyle changes that diabetes management requires by working with a certified diabetes educator, dietitian, social worker, and pharmacist.
Nonpharmacologic therapy
The first step in therapy for glycemic control is diet and exercise, although such measures are often limited in the elderly.
Diet. Carbohydrate control can maintain euglycemia in some patients with type 2 diabetes. But for the elderly, especially those living in long-term health care facilities, malnutrition may be of more concern than obesity, making dietary restrictions harmful. Patients in danger of malnutrition should be given unrestricted menus with consistent amounts of carbohydrate at meals and snacks. Medications should be adjusted to control blood glucose levels if necessary.39
For patients living in the community, dietary therapy should be individualized by a dietitian. Medicare covers up to 10 hours of diabetes education with a certified diabetes educator or registered dietitian within a 12-month period if at least one of the following criteria are met: the patient is newly diagnosed with diabetes, the hemoglobin A1c level is higher than 8.5%, medication has been recently started, or the risk of complications is high.
Supplementation of vitamins and minerals is prudent. Supplemental magnesium, zinc, and vitamins C and E may improve glycemic control.40–44
Exercise reduces insulin resistance, weight, and blood pressure; increases muscle mass; and improves lipid levels. Both aerobic and nonaerobic activity are beneficial.45–47 The best time to exercise is 1 to 2 hours after a meal, when glucose levels tend to be highest. Either hypoglycemia or hyperglycemia may occur up to 24 hours following exercise, and medications may need to be adjusted.
Oral medications
Insulin
Insulin therapy is necessary if oral combination therapy proves insufficient. Insulin is generally required for patients with moderate or severe hyperglycemia, especially for those with renal or hepatic insufficiency.52,53 Before prescribing insulin therapy to elderly patients, we need to consider their visual acuity, manual dexterity and sensation, cognitive function, family support, and financial situation.However, several studies showed that quality of life improves in the year after starting insulin for patients whose blood sugar was previously poorly controlled with oral agents.54,55
An evening dose of neutral protamine Hagedorn (NPH) insulin is a good way to start. More complex regimens may be necessary, depending on glycemic goals.
A number of premixed preparations of various types of insulin with different durations of action are available. They may improve accuracy, acceptability, and ease of insulin administration, although glycemic control and the risk of hypoglycemia may not change or in fact may be worse.56 Some patients may not achieve adequate glucose control with fixed-dose regimens.57,58
Frequent, small, titrated doses of short-acting agents control hyperglycemia better, particularly postprandial hyperglycemia,resulting in less hypoglycemia. However, these regimens may be too complex for many elderly patients; a patient’s support system must be evaluated before recommending this type of therapy.
Most insulins are available in vials and in pens, the latter of which are quick and easy to use, provide precise doses, and can be managed by many elderly patients. Pens require the user to attach a needle, set the dose by a dial, and depress the plunger to inject the dose. Some are prefilled and disposable, others have refillable cartridges. Studies in patients older than 60 years have shown the pen systems to be more acceptable, safer, and more effective than conventional syringes.
If conventional syringes are used, low-dose syringes (30-unit or 50-unit), which have more visible unit markings, should be prescribed whenever possible rather than the 100-unit sizes. Magnifying devices that attach to a syringe are also available.
Studies have also shown that continuous subcutaneous insulin infusion is safe for selected elderly patients.
Incretin mimetics: Possibly well-suited
Incretins, such as glucagon-like peptide-1, are hormones released from the gastrointestinal tract in response to eating. They stimulate insulin secretion by non-glucose-related pathways.
Exenatide (Byetta), a 39-amino-acid peptide incretin mimetic, is a synthetic version of exendin-4, an incretin isolated from the saliva of the Gila monster. Recently approved for treating type 2 diabetes, it is given subcutaneously.59,60 Oral dipeptidyl peptidase-4 inhibitors (sitagliptin and vildagliptin) decrease the degradation of endogenous incretin and thus prolong its action.61 Because a decline in glucose-mediated beta-cell insulin secretion is a major contributor to the development of diabetes in the elderly, the drug may be especially helpful for this population.However, further clinical research and experience is needed before specific recommendations for elderly patients can be made.
SELF-MANAGEMENT IS IMPORTANT
Patient education is critical
Patient education is a cornerstone of diabetes self-management,62–66 and is especially important for patients who are cognitively impaired or who have limited language proficiency.Patient education is covered under Medicare Part B. Ample resources are available in print and electronic formats. Community resources can also be important.
Home glucose monitoring is simpler now
A patient’s insulin regimen should ideally be tailored according to home blood glucose level monitoring before and after meals and at bedtime. Medicare reimburses for once daily testing for patients who are not taking insulin and for three-times-daily testing for those taking insulin.
Elderly patients can be taught to reliably monitor their own blood glucose levels without diminishing their quality of life. Monitoring is now easier with new glucometers and test strips that use small amounts of blood. Testing can now also be performed on blood taken from the forearm, upper arm, thigh, or calf with the FreeStyle (TheraSense), One Touch Ultra (LifeScan) and Soft Tac (MediSense) meters. The Soft Tac meter lances skin and automatically transfers blood to the test strip, making use even easier. Talking glucometers are available for blind patients.
Coordination counts
A variety of models of chronic care delivery have been proposed. Regardless of which model is chosen, the complexities of management call for a multidisciplinary team approach, and coordination of care in order to ensure appropriate information flow becomes critical.
Editor’s note: In next month’s issue of this journal, Drs. Hornick and Aron will discuss the management of diabetic complications in the elderly, including coronary artery disease, neuropathy, and kidney disease.
Guidelines for treating diabetes mellitus are mostly based on clinical studies in middle-aged people, and recommendations tend to be the same for everyone, whether young and strong or elderly and frail. But diabetes management should be individualized, especially in the elderly, taking into account each patient’s medical history, functional ability, home care situation, and life expectancy. Aggressive glycemic control is less important than avoiding hypoglycemia and achieving a good quality of life.
This article reviews the general principles for recognizing and managing diabetes in elderly patients, focusing on the management of blood sugar per se. In a future issue of this journal, we will discuss some of the many complications of diabetes in the elderly.
DIABETES DIFFERS IN ELDERLY PATIENTS
“The elderly” is a heterogeneous group with widely varying physiologic profiles, functional capabilities, and life expectancy (on average, about 88 years for men and 90 years for women in the United States). Although the elderly are sometimes classified as “young-old” (age 65–80) and “old-old” (80+), this distinction is too simplistic for clinical decision-making.
Diabetes mellitus in the elderly also is heterogeneous. One distinction is the age at which the disease developed.
Aging is associated with declining beta-cell function and lower blood insulin levels independent of insulin resistance, and with insulin resistance itself. The risk of developing type 2 diabetes mellitus increases with obesity, lack of physical activity, and loss of muscle mass, all of which often develop with aging.1
Middle-aged patients with diabetes have increased fasting hepatic glucose production, increased insulin resistance, and an abnormal insulin response to a glucose load. On the other hand, patients who develop diabetes at an older age tend to have normal hepatic glucose production. Older patients who are lean secrete markedly less insulin in response to a glucose load but have relatively less insulin resistance.2 Patients who develop type 2 diabetes in old age are more likely to have near-normal fasting blood glucose levels but significant postprandial hyperglycemia.3,4 Elderly patients who developed diabetes during middle age have metabolic abnormalities more typical of middle-aged patients with type 2 diabetes.
DIABETES IS COMMON, AND INCREASING IN PREVALENCE
By age 75, 40% of people in the United States have either glucose intolerance or diabetes mellitus.5 Metabolic syndrome, which is the constellation of insulin resistance (type 2 diabetes mellitus), hyperlipidemia, hypertension, and obesity, is more prevalent in people age 65 to 74 years than in younger and older people.3
The National Diabetes Surveillance System of the US Centers for Disease Control and Prevention estimated that the prevalence of diabetes mellitus in people 65 to 74 years old in 2005 was 18.5%, about 12 times the prevalence among those younger than 45years.6 The prevalence has been gradually increasing and has nearly doubled over the past 25 years, with certain groups—native Americans, Hispanics, and African-Americans—at particularly high risk of developing the disease.
Although the prevalence of diabetes in people older than 75 years is lower than among people in the 65-to-74-year range, the elderly segment of our population is increasing, and the impact of diabetes and its associated burden of death and disease from vascular complications is enormous.
SYMPTOMS ARE OFTEN NONSPECIFIC
Unfortunately, diabetes is underdiagnosed and frequently undertreated, resulting in even more disease and death.7–9
Diabetes is often missed in the elderly because its presenting symptoms may be nonspecific, eg, failure to thrive, low energy, falls, dizziness, confusion, nocturia (with or without incontinence), and urinary tract infection.The classic symptoms of frequent urination (often leading to worsening incontinence), thirst, and increased hunger usually occur only when plasma glucose levels are above 200 mg/dL. Weight loss, blurred vision, and dehydration may also be present with high blood glucose levels. With lesser degrees of hyperglycemia, patients may have no symptoms or present with weight loss or signs and symptoms of chronic infection, especially of the genitourinary tract, skin, or mouth.
Hyperglycemia in elderly patients is also associated with reduced cognitive function (which may improve with blood glucose control).10
The American Diabetes Association recommends screening by measuring the fasting plasma glucose level every 3 years beginning at 45 years.11 However, some experts believe that this method is inadequate for the elderly12; some suggest that screening should be done more often in those with risk factors for diabetes, including obesity, inactivity, hypertension, and dyslipidemia, all of which are common in the elderly. Targeted screening in patients with hypertension may be the most cost-effective strategy.13
Screening with hemoglobin A1c levels is not recommended because of lack of standardization among laboratories.14
INDIVIDUALIZED MANAGEMENT IS BEST
Despite disease differences, the general goals for diabetes care are the same for all ages:
- To control hyperglycemia and its symptoms
- To prevent, evaluate, and treat macrovascular and microvascular complications
- To teach patients to manage themselves
- To maintain or improve the patient’s general health status.
Unfortunately, most specific recommendations are based on studies in younger people. Guidelines should ideally reflect the complexities of a particular clinical situation, but most recommendations are applied to the young and old alike, as well as to the relatively healthy and the frail and ill.15–17 Consideration should be given to a patient’s health beliefs, severity of vascular complications and other medical problems, economic situation, life expectancy ,functional status, and availability of support services. In addition, some patients prefer aggressive treatment, while others would rather compromise some aspects of care in order to maintain a certain quality of life, to save money, or to avoid having caregivers provide treatment.
Age-related changes in pharmacokinetics as well as polypharmacy increase the risk of drug interactions and adverse effects, especially drug-induced hypoglycemia. In addition, age-associated changes in cognitive, visual, and physical function, dentition, and taste perception can reduce a patient’s ability to carry out treatment. Frequent hospitalizations also disrupt outpatient regimens.
Comorbidities make treatment more challenging, but some conditions—such as hypertension, renal insufficiency and eye disorders—make doctors more likely to control hyperglycemia more aggressively, fearing that the loss of a little more function in an impaired organ may lead to failure.
The benefits of tight glycemic control should be weighed against the risks and the realities of an individual situation. Priority should be given to achieving the best quality of life possible.17 Recent guidelines from the California Health Foundation and the American Geriatrics Association focused on the major health threats to older patients and prioritizing care for each person.15 The guidelines recommend screening for geriatric syndromes that are more prevalent inpatients with diabetes or are strongly affected by the disease or its treatment. Diabetes care should be examined in the setting of common geriatric problems: depression, polypharmacy, cognitive impairment, urinary incontinence, falls, and pain.
Heart risk trumps glycemic control
The expert panel15 concluded that rates of disease and death can be reduced more by targeting cardiovascular risk factors than by intensively managing hyperglycemia. One rationale is that it takes 8 years for aggressive glycemic control to reduce the risk of diabetic retinopathy or renal disease but only 2 years of treating hypertension and dyslipidemia to reduce the risk of cardiovascular disease.15,17–21 A recent Japanese study found normal mortality rates in elderly patients under long-term, intensive multifactorial diabetes control.22 High-functioning, motivated patients could benefit from therapy aimed at achieving most or all of the recommended goals, but frail patients may suffer from applying all therapies and may benefit from only some of them.
If appropriate goals cannot be met, it may help to refer patients to a geriatric specialist to evaluate possible barriers to adherence such as depression or poor cognition, physical functioning, or support.
MANAGEMENT STRATEGIES
Weight loss and exercise help prevent diabetes
The Diabetes Prevention Program23 randomized 3,234 people (mean age 51 years) with impaired glucose tolerance to receive either metformin (Fortamet, Glucophage) 850 mg twice daily or placebo or to undertake lifestyle modifications with goals of at least a 7% weight loss and at least 150 minutes of physical activity per week. Compared with the placebo group, the lifestyle modification group had a 58% lower incidence of diabetes while those in the metformin group had only a 31% lower incidence. Among those older than 60 years, the advantage of lifestyle modification over metformin was even greater.
Control blood glucose, avoid hypoglycemia
- Hemoglobin A1c levels < 7.0%
- Preprandial blood glucose levels 90–130 mg/dL
- Bedtime blood glucose levels 110–150 mg/dL.
Guidelines from the Department of Veterans Affairs24 and the American Geriatrics Society15 are slightly different, and are based on randomized trials in younger patients, primarily the Diabetes Control and Complications Trial (DCCT)25 and the United Kingdom Prospective Diabetes Study(UKPDS).21,26 A recent position statement from the American College of Physicians, based on a review of all the major guidelines, recommends the following: “Statement 1: To prevent microvascular complication of diabetes, the goal for glycemic control should be as low as is feasible without undue risk for adverse events or an unacceptable burden on patients. Treatment goals should be based on a discussion of the benefits and harms of specific levels of glycemic control with the patient. A hemoglobin A1c level less than 7% based on individualized assessment is a reasonable goal for many but not all patients. Statement 2: The goal for hemoglobin A1c should be based on individualized assessment of risk for complication from diabetes, comorbidity, life expectancy, and patient preferences.”27
Although few data exist for elderly patients, these guidelines are the most current approach to treating diabetes in the elderly. Less stringent goals are appropriate for patients who have limited life expectancy, hypoglycemia unawareness (lack of autonomic warning symptoms of low blood sugar), seizures, dementia, psychiatric illness, or alcoholism. It is important to keep in mind the following as one strives for lower A1c levels: Although the relative risk reduction accomplished by lowering hemoglobin A1c is linear, the absolute risk reduction is log-linear—more benefit is gained by lowering hemoglobin A1c from 9% to 8% than from 8% to 7%.28
Hypoglycemia is a major limiting factor in glycemic control. Many risk factors for hypoglycemia are common in the elderly (Table 2). Hypoglycemia was a chief adverse event in both the DCCT and the UKPDS, with a twofold to threefold higher rate in patients who were intensively treated.29 Even mild hypoglycemia in the elderly can result in an injurious fall, which can lead to long-term functional decline. The rate of severe or fatal hypoglycemia—the major risk of tight glycemic treatment—increases exponentially with age.30–33
As people age, the mechanisms that regulate blood sugar are impaired: the glucagon response is diminished, which increases dependence on the epinephrine response to prevent hypoglycemia.34 Medications such as beta-blockers, which can suppress the symptoms of hypoglycemia, may further impair the response. Consequently, older patients may be less aware of hypoglycemia, and the symptoms may be less intense. Renal insufficiency may also exacerbate the problem by reducing clearance of oral agents. In addition, confused patients may take extra doses of medications.
Patients with type 2 diabetes treated with insulin, sulfonylureas, or meglitinides should be evaluated for symptoms of hypoglycemia. Older patients may have more neuroglycopenic symptoms (eg, dizziness, weakness, confusion, nightmares, violent behavior) than adrenergic symptoms (eg, sweating, palpitations, tremors), although both types should be asked about during an evaluation.2,32,33 Hypoglycemia may also present as transient hemiparesis, coma, or falls.35
We carefully evaluate the glycemic regimen and care environment of any elderly patient who presents with a blood glucose level below 100 mg/dL. The regimen should be altered for less strict control if the patient is cognitively impaired, is at risk of falling, or has an unstable care situation (eg, has irregular meals or needs assistance with daily activities and does not have a regular caregiver). Patients at significant risk of hypoglycemia should be encouraged to check their blood glucose level with a fingerstick before driving.
Tight control in the hospital is controversial
Glycemic control in the hospital has traditionally been designed primarily to maintain “safe” blood glucose levels, ie, to prevent hyperglycemia-induced dehydration and catabolism while avoiding hypoglycemia. Recent studies have suggested that tighter glycemic control may reduce the rates of complications and death perioperatively and in patients with myocardial infarction or who are seriously ill in the intensive care unit, although the evidence is mixed.36–38 Specific targets are controversial, and although studies have included some elderly patients, results cannot be generalized to this group.
DIABETES CARE TAKES A TEAM
Geriatric patients have complex problems. In the face of multiple comorbidities, difficult social situations, and polypharmacy, the physician can best address the drug therapy and lifestyle changes that diabetes management requires by working with a certified diabetes educator, dietitian, social worker, and pharmacist.
Nonpharmacologic therapy
The first step in therapy for glycemic control is diet and exercise, although such measures are often limited in the elderly.
Diet. Carbohydrate control can maintain euglycemia in some patients with type 2 diabetes. But for the elderly, especially those living in long-term health care facilities, malnutrition may be of more concern than obesity, making dietary restrictions harmful. Patients in danger of malnutrition should be given unrestricted menus with consistent amounts of carbohydrate at meals and snacks. Medications should be adjusted to control blood glucose levels if necessary.39
For patients living in the community, dietary therapy should be individualized by a dietitian. Medicare covers up to 10 hours of diabetes education with a certified diabetes educator or registered dietitian within a 12-month period if at least one of the following criteria are met: the patient is newly diagnosed with diabetes, the hemoglobin A1c level is higher than 8.5%, medication has been recently started, or the risk of complications is high.
Supplementation of vitamins and minerals is prudent. Supplemental magnesium, zinc, and vitamins C and E may improve glycemic control.40–44
Exercise reduces insulin resistance, weight, and blood pressure; increases muscle mass; and improves lipid levels. Both aerobic and nonaerobic activity are beneficial.45–47 The best time to exercise is 1 to 2 hours after a meal, when glucose levels tend to be highest. Either hypoglycemia or hyperglycemia may occur up to 24 hours following exercise, and medications may need to be adjusted.
Oral medications
Insulin
Insulin therapy is necessary if oral combination therapy proves insufficient. Insulin is generally required for patients with moderate or severe hyperglycemia, especially for those with renal or hepatic insufficiency.52,53 Before prescribing insulin therapy to elderly patients, we need to consider their visual acuity, manual dexterity and sensation, cognitive function, family support, and financial situation.However, several studies showed that quality of life improves in the year after starting insulin for patients whose blood sugar was previously poorly controlled with oral agents.54,55
An evening dose of neutral protamine Hagedorn (NPH) insulin is a good way to start. More complex regimens may be necessary, depending on glycemic goals.
A number of premixed preparations of various types of insulin with different durations of action are available. They may improve accuracy, acceptability, and ease of insulin administration, although glycemic control and the risk of hypoglycemia may not change or in fact may be worse.56 Some patients may not achieve adequate glucose control with fixed-dose regimens.57,58
Frequent, small, titrated doses of short-acting agents control hyperglycemia better, particularly postprandial hyperglycemia,resulting in less hypoglycemia. However, these regimens may be too complex for many elderly patients; a patient’s support system must be evaluated before recommending this type of therapy.
Most insulins are available in vials and in pens, the latter of which are quick and easy to use, provide precise doses, and can be managed by many elderly patients. Pens require the user to attach a needle, set the dose by a dial, and depress the plunger to inject the dose. Some are prefilled and disposable, others have refillable cartridges. Studies in patients older than 60 years have shown the pen systems to be more acceptable, safer, and more effective than conventional syringes.
If conventional syringes are used, low-dose syringes (30-unit or 50-unit), which have more visible unit markings, should be prescribed whenever possible rather than the 100-unit sizes. Magnifying devices that attach to a syringe are also available.
Studies have also shown that continuous subcutaneous insulin infusion is safe for selected elderly patients.
Incretin mimetics: Possibly well-suited
Incretins, such as glucagon-like peptide-1, are hormones released from the gastrointestinal tract in response to eating. They stimulate insulin secretion by non-glucose-related pathways.
Exenatide (Byetta), a 39-amino-acid peptide incretin mimetic, is a synthetic version of exendin-4, an incretin isolated from the saliva of the Gila monster. Recently approved for treating type 2 diabetes, it is given subcutaneously.59,60 Oral dipeptidyl peptidase-4 inhibitors (sitagliptin and vildagliptin) decrease the degradation of endogenous incretin and thus prolong its action.61 Because a decline in glucose-mediated beta-cell insulin secretion is a major contributor to the development of diabetes in the elderly, the drug may be especially helpful for this population.However, further clinical research and experience is needed before specific recommendations for elderly patients can be made.
SELF-MANAGEMENT IS IMPORTANT
Patient education is critical
Patient education is a cornerstone of diabetes self-management,62–66 and is especially important for patients who are cognitively impaired or who have limited language proficiency.Patient education is covered under Medicare Part B. Ample resources are available in print and electronic formats. Community resources can also be important.
Home glucose monitoring is simpler now
A patient’s insulin regimen should ideally be tailored according to home blood glucose level monitoring before and after meals and at bedtime. Medicare reimburses for once daily testing for patients who are not taking insulin and for three-times-daily testing for those taking insulin.
Elderly patients can be taught to reliably monitor their own blood glucose levels without diminishing their quality of life. Monitoring is now easier with new glucometers and test strips that use small amounts of blood. Testing can now also be performed on blood taken from the forearm, upper arm, thigh, or calf with the FreeStyle (TheraSense), One Touch Ultra (LifeScan) and Soft Tac (MediSense) meters. The Soft Tac meter lances skin and automatically transfers blood to the test strip, making use even easier. Talking glucometers are available for blind patients.
Coordination counts
A variety of models of chronic care delivery have been proposed. Regardless of which model is chosen, the complexities of management call for a multidisciplinary team approach, and coordination of care in order to ensure appropriate information flow becomes critical.
Editor’s note: In next month’s issue of this journal, Drs. Hornick and Aron will discuss the management of diabetic complications in the elderly, including coronary artery disease, neuropathy, and kidney disease.
- Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997; 46:701–710.
- Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol A Biol Sci Med Sci 2001; 56:M5–M13.
- Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore Longitudinal Study of Aging. Metabolism Clin Exper 2005; 54:542–547.
- Crandall J, Barzilai N. Treatment of diabetes mellitus in older people: oral therapy options. J Am Geriatr Soc 2003; 51:272–274.
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998; 21:518–524.
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. Diabetes Public Health Resource. National Diabetes Surveillance System. www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm.
- Franse LV, Di Bari M, Shorr RI, et al; Health, Aging, and Body Composition Study Group. Type 2 diabetes in older well-functioning people: who is underdiagnosed? Data from the Health, Aging, and Body Composition study. Diabetes Care 2001; 24:2065–2070. Erratum in: Diabetes Care 2002; 25:413.
- Shorr RI, Franse LV, Resnick HE, Di Bari M, Johnson KC, Pahor M. Glycemic control of older adults with type 2 diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Geriatr Soc 2000; 48:264–267.
- Smith NL, Savage PJ, Heckbert SR, et al. Glucose, blood pressure, and lipid control in older people with and without diabetes mellitus: the Cardiovascular Health Study. J Am Geriatr Soc 2002; 50:416–423.
- Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol 1993; 48:M117–M121.
- American Diabetes Association. Standards of medical care in diabetes.Diabetes Care 2005; 28:S4–S36.
- Motta M, Bennati E, Ferlito L, Malaguarnera M. Diabetes mellitus in the elderly: diagnostic features. Arch Gerontol Geriatr 2006; 42:101–106.
- Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost effective analysis. Ann Intern Med 2004; 140:689–699.
- Harris RP, Lux LJ, Bunton AJ, et al. Screening for type 2 diabetes mellitus. Systematic Evidence Review Number 19. February 4, 2003.US Department of Health and Human Services, Agency for Healthcare Research and Quality. Rockville, MD. www.ahrq.gov/downloads/pub/prevent/pdfser/diabser.pdf.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA; California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA 2006; 295:1935–1940.
- Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA 1996; 276:1886–1892. Erratum in: JAMA 1997; 277:1356.
- Goddijn PP, Bilo HJ, Feskens EJ, Groeniert KH, van der Zee KI, Meyboom-de Jong B. Longitudinal study on glycaemic control and quality of life in patients with Type 2 diabetes mellitus referred for intensified control. Diabet Med 1999; 16:23–30.
- Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997; 20:614–620. Erratum in: Diabetes Care 1997; 20:1048.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Katakura M, Naka M, Kondo T, et al, and the Nagano Elderly Diabetes Study Group. Normal mortality in the elderly with diabetes under strict glycemic and blood pressure control: outcome of 6-year prospective study. Diabetes Res Clin Practice 2007; 78:108–114.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Pogach LM, Brietzke SA, Cowan CL Jr, Conlin P, Walder DJ, Sawin CT; VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK for the Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians. Ann Intern Med 2007; 147:417–422.
- Pogach L, Engelgau M, Aron D. Measuring progress toward achieving hemoglobin A1c goals in diabetes care: pass/fail or partial credit. JAMA 2007; 297:520–523.
- Egger M, Davey Smith G, Stettler C, Diem P. Risk of adverse effects of intensified treatment in insulin-dependent diabetes mellitus: ameta-analysis. Diabet Med 1997; 14:919–928.
- Burge MR, Schmitz-Fiorentino K, Fischette C, Qualls CR, Schade DS. A prospective trial of risk factors for sulfonylurea-induced hypoglycemia in type 2 diabetes mellitus. JAMA 1998; 279:137–143.
- Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med 1999; 159:281–284.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996; 44:751–755.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med 1997; 157:1681–1685.
- Burge MR, Kamin JR, Timm CT, Qualls CR, Schade DS. Low-dose epinephrine supports plasma glucose in fasted elderly patients with type 2 diabetes. Metabolism 2000; 49:195–202.
- Alagiakrishnan K, Lechelt K, McCracken P, Torrible S, Sclater A.Atypical presentation of silent nocturnal hypoglycemia in an olderperson. J Am Geriatr Soc 2001; 49:1577–1578.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999; 99:2626–2632.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Metchick LN, Petit WA Jr, Inzucchi SE. Inpatient management of diabetes mellitus. Am J Med 2002; 113:317–323.
- Coulston AM, Mandelbaum D, Reaven GM. Dietary management of nursing home residents with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1990; 51:67–71.
- Song MK, Rosenthal MJ, Naliboff BD, Phanumas L, Kang KW. Effects of bovine prostate powder on zinc, glucose and insulin metabolism in old patients with non-insulin-dependent diabetes mellitus. Metabolism 1998; 47:39–43.
- Paolisso G, D’Amore A, Galzerano D, et al. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care 1993; 16:1433–1437.
- Paolisso G, Scheen A, Cozzolino D, et al. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J Clin Endocrinol Metab 1994; 78:1510–1514.
- Paolisso G, Passariello N, Pizza G, et al. Dietary magnesium supplements improve B-cell response to glucose and arginine in elderly non-insulin dependent diabetic subjects. Acta Endocrinol (Copenh) 1989; 121:16–20.
- Paolisso G, D’Amore A, Balbi V, et al. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. Am J Physiol 1994; 266:E261–E268. Erratum in: Am J Physiol 1994; 267:section E following table of contents.
- Agurs-Collins TD, Kumanyika SK, Ten Have TR, Adams-Campbell LL. A randomized controlled trail of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care 1997; 200:1503–1511.
- Skarfors ET, Wegener TA, Lithell H, Selinus I. Physical training as treatment for type 2 (non-insulin-dependent) diabetes in elderly men. A feasibility study over 2 years. Diabetologia 1987; 30:930–933.
- Raz I, Hauser E, Bursztyn M. Moderate exercise improves glucose metabolism in uncontrolled elderly patients with non-insulin-dependent diabetes mellitus. Isr J Med Sci 1994; 30:766–770.
- Brodows RG. Benefits and risks with glyburide and glipizide in elderly NIDDM patients. Diabetes Care 1992; 15:75–80.
- Landgraf R. Meglitinide analogues in the treatment of type 2 diabetes mellitus. Drugs Aging 2000; 17:411–425.
- Ron Y, Wainstein J, Leibovitz A, et al. The effect of acarbose on the colonic transit time of elderly long-term care patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 2002; 57:M111–M114.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Gerstein HC, Haynes RB, eds. Evidence-Based Diabetes Management. Hamilton, Ont.; London: B.C. Dekker; 2001.
- Tovi J, Engfeldt P. Well being and symptoms in elderly type 2 diabetes patients with poor metabolic control: effect of insulin treatment. Practical Diabetes Int 1998; 15:73–77.
- Reza M, Taylor CD, Towse K, Ward JD, Hendra TJ. Insulin improves well-being for selected elderly type 2 diabetic subjects. Diabetes Res Clin Pract 2002; 55:201–207.
- Janka HU, Plewe G, Busch K. Combination of oral antidiabetic agents with basal insulin versus premixed insulin alone in randomized elderly patients with type 2 diabetes mellitus. J Am Geriatrics Soc 2007; 55:182–188.
- Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care 1992; 15:1628–1630.
- Rolla AR, Rakel RE. Practical approaches to insulin therapy for type 2 diabetes mellitus with premixed insulin analogues. Clin Ther 2005; 27:1113–1125.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Mathieu C, Bollaerts K. Antihyperglycaemic therapy in elderly patients with type 2 diabetes: potential role of incretin mimetics and DPP-4 inhibitors. Int J Clin Pract 2007; 61(suppl 154):29–37.
- Bernbaum M, Albert SG, McGinnis J, Brusca S, Mooradian AD. The reliability of self blood glucose monitoring in elderly diabetic patients. J Am Geriatr Soc 1994; 42:779–781.
- Gilden JL, Hendryx M, Casia C, Singh SP. The effectiveness of diabetes education programs for older patients and their spouses. J Am Geriatr Soc 1989; 37:1023–1030.
- Glasgow RE, Toobert DJ, Hampson SE, Brown JE, Lewinsohn PM, Donnelly J. Improving self-care among older patients with type II diabetes: the ‘Sixty Something…’ Study. Patient Educ Couns 1992; 19:61–74.
- Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc 2005; 53:306–311.
- Langa KM, Vijan S, Hayward RA, et al. Informal caregiving for diabetes and diabetic complications among elderly Americans. J Gerontol B Psychol Sci Soc Sci 2002; 57:S177–S186.
- Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997; 46:701–710.
- Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol A Biol Sci Med Sci 2001; 56:M5–M13.
- Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore Longitudinal Study of Aging. Metabolism Clin Exper 2005; 54:542–547.
- Crandall J, Barzilai N. Treatment of diabetes mellitus in older people: oral therapy options. J Am Geriatr Soc 2003; 51:272–274.
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998; 21:518–524.
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. Diabetes Public Health Resource. National Diabetes Surveillance System. www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm.
- Franse LV, Di Bari M, Shorr RI, et al; Health, Aging, and Body Composition Study Group. Type 2 diabetes in older well-functioning people: who is underdiagnosed? Data from the Health, Aging, and Body Composition study. Diabetes Care 2001; 24:2065–2070. Erratum in: Diabetes Care 2002; 25:413.
- Shorr RI, Franse LV, Resnick HE, Di Bari M, Johnson KC, Pahor M. Glycemic control of older adults with type 2 diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Geriatr Soc 2000; 48:264–267.
- Smith NL, Savage PJ, Heckbert SR, et al. Glucose, blood pressure, and lipid control in older people with and without diabetes mellitus: the Cardiovascular Health Study. J Am Geriatr Soc 2002; 50:416–423.
- Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol 1993; 48:M117–M121.
- American Diabetes Association. Standards of medical care in diabetes.Diabetes Care 2005; 28:S4–S36.
- Motta M, Bennati E, Ferlito L, Malaguarnera M. Diabetes mellitus in the elderly: diagnostic features. Arch Gerontol Geriatr 2006; 42:101–106.
- Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost effective analysis. Ann Intern Med 2004; 140:689–699.
- Harris RP, Lux LJ, Bunton AJ, et al. Screening for type 2 diabetes mellitus. Systematic Evidence Review Number 19. February 4, 2003.US Department of Health and Human Services, Agency for Healthcare Research and Quality. Rockville, MD. www.ahrq.gov/downloads/pub/prevent/pdfser/diabser.pdf.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA; California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA 2006; 295:1935–1940.
- Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA 1996; 276:1886–1892. Erratum in: JAMA 1997; 277:1356.
- Goddijn PP, Bilo HJ, Feskens EJ, Groeniert KH, van der Zee KI, Meyboom-de Jong B. Longitudinal study on glycaemic control and quality of life in patients with Type 2 diabetes mellitus referred for intensified control. Diabet Med 1999; 16:23–30.
- Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997; 20:614–620. Erratum in: Diabetes Care 1997; 20:1048.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Katakura M, Naka M, Kondo T, et al, and the Nagano Elderly Diabetes Study Group. Normal mortality in the elderly with diabetes under strict glycemic and blood pressure control: outcome of 6-year prospective study. Diabetes Res Clin Practice 2007; 78:108–114.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Pogach LM, Brietzke SA, Cowan CL Jr, Conlin P, Walder DJ, Sawin CT; VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK for the Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians. Ann Intern Med 2007; 147:417–422.
- Pogach L, Engelgau M, Aron D. Measuring progress toward achieving hemoglobin A1c goals in diabetes care: pass/fail or partial credit. JAMA 2007; 297:520–523.
- Egger M, Davey Smith G, Stettler C, Diem P. Risk of adverse effects of intensified treatment in insulin-dependent diabetes mellitus: ameta-analysis. Diabet Med 1997; 14:919–928.
- Burge MR, Schmitz-Fiorentino K, Fischette C, Qualls CR, Schade DS. A prospective trial of risk factors for sulfonylurea-induced hypoglycemia in type 2 diabetes mellitus. JAMA 1998; 279:137–143.
- Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med 1999; 159:281–284.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996; 44:751–755.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med 1997; 157:1681–1685.
- Burge MR, Kamin JR, Timm CT, Qualls CR, Schade DS. Low-dose epinephrine supports plasma glucose in fasted elderly patients with type 2 diabetes. Metabolism 2000; 49:195–202.
- Alagiakrishnan K, Lechelt K, McCracken P, Torrible S, Sclater A.Atypical presentation of silent nocturnal hypoglycemia in an olderperson. J Am Geriatr Soc 2001; 49:1577–1578.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999; 99:2626–2632.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Metchick LN, Petit WA Jr, Inzucchi SE. Inpatient management of diabetes mellitus. Am J Med 2002; 113:317–323.
- Coulston AM, Mandelbaum D, Reaven GM. Dietary management of nursing home residents with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1990; 51:67–71.
- Song MK, Rosenthal MJ, Naliboff BD, Phanumas L, Kang KW. Effects of bovine prostate powder on zinc, glucose and insulin metabolism in old patients with non-insulin-dependent diabetes mellitus. Metabolism 1998; 47:39–43.
- Paolisso G, D’Amore A, Galzerano D, et al. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care 1993; 16:1433–1437.
- Paolisso G, Scheen A, Cozzolino D, et al. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J Clin Endocrinol Metab 1994; 78:1510–1514.
- Paolisso G, Passariello N, Pizza G, et al. Dietary magnesium supplements improve B-cell response to glucose and arginine in elderly non-insulin dependent diabetic subjects. Acta Endocrinol (Copenh) 1989; 121:16–20.
- Paolisso G, D’Amore A, Balbi V, et al. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. Am J Physiol 1994; 266:E261–E268. Erratum in: Am J Physiol 1994; 267:section E following table of contents.
- Agurs-Collins TD, Kumanyika SK, Ten Have TR, Adams-Campbell LL. A randomized controlled trail of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care 1997; 200:1503–1511.
- Skarfors ET, Wegener TA, Lithell H, Selinus I. Physical training as treatment for type 2 (non-insulin-dependent) diabetes in elderly men. A feasibility study over 2 years. Diabetologia 1987; 30:930–933.
- Raz I, Hauser E, Bursztyn M. Moderate exercise improves glucose metabolism in uncontrolled elderly patients with non-insulin-dependent diabetes mellitus. Isr J Med Sci 1994; 30:766–770.
- Brodows RG. Benefits and risks with glyburide and glipizide in elderly NIDDM patients. Diabetes Care 1992; 15:75–80.
- Landgraf R. Meglitinide analogues in the treatment of type 2 diabetes mellitus. Drugs Aging 2000; 17:411–425.
- Ron Y, Wainstein J, Leibovitz A, et al. The effect of acarbose on the colonic transit time of elderly long-term care patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 2002; 57:M111–M114.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Gerstein HC, Haynes RB, eds. Evidence-Based Diabetes Management. Hamilton, Ont.; London: B.C. Dekker; 2001.
- Tovi J, Engfeldt P. Well being and symptoms in elderly type 2 diabetes patients with poor metabolic control: effect of insulin treatment. Practical Diabetes Int 1998; 15:73–77.
- Reza M, Taylor CD, Towse K, Ward JD, Hendra TJ. Insulin improves well-being for selected elderly type 2 diabetic subjects. Diabetes Res Clin Pract 2002; 55:201–207.
- Janka HU, Plewe G, Busch K. Combination of oral antidiabetic agents with basal insulin versus premixed insulin alone in randomized elderly patients with type 2 diabetes mellitus. J Am Geriatrics Soc 2007; 55:182–188.
- Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care 1992; 15:1628–1630.
- Rolla AR, Rakel RE. Practical approaches to insulin therapy for type 2 diabetes mellitus with premixed insulin analogues. Clin Ther 2005; 27:1113–1125.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Mathieu C, Bollaerts K. Antihyperglycaemic therapy in elderly patients with type 2 diabetes: potential role of incretin mimetics and DPP-4 inhibitors. Int J Clin Pract 2007; 61(suppl 154):29–37.
- Bernbaum M, Albert SG, McGinnis J, Brusca S, Mooradian AD. The reliability of self blood glucose monitoring in elderly diabetic patients. J Am Geriatr Soc 1994; 42:779–781.
- Gilden JL, Hendryx M, Casia C, Singh SP. The effectiveness of diabetes education programs for older patients and their spouses. J Am Geriatr Soc 1989; 37:1023–1030.
- Glasgow RE, Toobert DJ, Hampson SE, Brown JE, Lewinsohn PM, Donnelly J. Improving self-care among older patients with type II diabetes: the ‘Sixty Something…’ Study. Patient Educ Couns 1992; 19:61–74.
- Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc 2005; 53:306–311.
- Langa KM, Vijan S, Hayward RA, et al. Informal caregiving for diabetes and diabetic complications among elderly Americans. J Gerontol B Psychol Sci Soc Sci 2002; 57:S177–S186.
KEY POINTS
- The diagnosis of diabetes in the elderly is often missed because its symptoms, such as dizziness, confusion, and nocturia, are often common and nonspecific.
- Elderly people at risk of malnutrition should have unrestricted meals and snacks; medications should be adjusted as necessary to control blood glucose levels.
- Tight control of blood glucose reduces the risk of death and diabetes-related complications but poses the risk of hypoglycemia.