User login
2015 Update on cancer
As the proportion of the elderly in the US population continues to increase, with life expectancy trending upward, we can expect to see more gynecologic cancers in our patients.1,2 At present, the most effective approach to these cancers commonly includes aggressive surgical resection with chemotherapy and, in some cases, radiation. It remains unclear whether elderly patients should be managed the same as younger patients, with minimal data to guide physicians. Some evidence suggests an increased risk of surgical complications in older adults.3
To optimize surgical care in our elderly patients, we need to understand the risks of perioperative mortality and morbidity in this population. For example, the current standard of care for advanced epithelial ovarian cancer is aggressive cytoreductive surgery followed by adjuvant chemotherapy,4 although neoadjuvant chemotherapy is gaining utility and popularity in certain circumstances. During pretreatment counseling, it is imperative that we communicate patient-specific outcomes so that patients and their families can make educated decisions in line with their goals. What should we know about age-dependent outcomes when counseling our patients?
To optimize surgical care in this population, we also need to develop and use new methods of surgical decision making. Although some data suggest that age is an independent risk factor for postoperative complications, not all elderly patients are the same in terms of comorbidities and functional status. In order to truly assess risks, we need to identify additional preoperative risk factors. Are there accurate scoring tools or predictors of outcomes available to help us assess the risks of postoperative mortality and morbidity?
In this article, we highlight recent developments in surgical treatment of the elderly, focusing on:
- postoperative mortality and morbidity in patients older than 80 years
- adjuncts to preoperative assessment for oncogeriatric surgical patients.
Risks rise sharply in older patients undergoing treatment for ovarian Ca
Moore KN, Reid MS, Fong DN, et al. Ovarian cancer in the octogenarian: does the paradigm of aggressive cytoreductive surgery and chemotherapy still apply? Gynecol Oncol. 2008;110(2):133–139.
Mahdi H, Wiechert A, Lockhart D, Rose PG. Impact of age on 30-day mortality and morbidity in patients undergoing surgery for ovarian cancer. Int J Gynecol Cancer. 2015;25(7):1216–1223.
The cornerstone of optimal survival from certain gynecologic cancers, such as advanced ovarian cancer, is aggressive debulking surgery. However, older adults are classically under-represented in clinical trials that guide this standard of care.
To determine whether patients aged 80 years or older respond differently from younger patients to conventional ovarian cancer management, Moore and colleagues retrospectively reviewed their institutional experience. They found that postoperative mortality increased from 5.4% in patients aged 80 to 84 years to 9.1% in those aged 85 to 89 and 14.4% in those older than 90. The rates for younger patients were 0.6% for patients younger than 60 years, 2.8% for those aged 60 to 69 years, and 2.5% for those aged 70 to 79 years (P<.001).
Notably, 13% of patients aged 80 years or older who underwent primary surgery died during their primary hospitalization. Of those who survived, 50% were discharged to skilled nursing facilities. Of patients who underwent cytoreductive surgery, 13% were unable to undergo any intended adjuvant therapy, and only 57% completed more than 3 cycles of chemotherapy, either due to demise or toxicities. Two-month survival for patients 80 years or older was comparable between patients who underwent primary surgery and those who had primary chemotherapy (20% and 26%, respectively).
With a similar objective, Mahdi and colleagues identified 2,087 patients with ovarian cancer who underwent surgery. After adjusting for confounders with multivariable analyses, they found that octogenarians whose initial management was surgery were 9 times more likely than younger patients to die and 70% more likely to develop complications within 30 days. Among patients who underwent neoadjuvant chemotherapy, there were no significant differences between older and younger patients in 30-day postoperative mortality or morbidity.
When evaluating elderly patients for surgery, the use of multiple risk-assessment strategies may improve accuracy
Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: a multicenter prospective cohort study. Eur J Surg Oncol. 2015;41(7):844–851.
Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
The National Comprehensive Cancer Network recommends that clinicians determine baseline life expectancy for older adults with cancer to aid in management decision making. The use of tools such as www.eprognosis.com, developed to determine anticipated life expectancy independent of cancer, can prove useful in determining a patient’s risk of dying or suffering from their cancer before dying of another cause.5
When it comes to the determination of risk related to a patient’s cancer diagnosis and selection of potential management options, many argue that the subgroup of elderly patients is not homogenous and that the use of age alone to guide management decisions may be unfair. Preoperative evaluation ideally should incorporate a global assessment of predictive risk factors.
Three assessment tools are especially useful
Huisman and colleagues set out to identify accurate preoperative assessment methods in elderly patients undergoing oncologic surgery. They prospectively recruited 328 patients aged 70 years or older and evaluated patients preoperatively using 11 well-known geriatric screening tools. They compared these evaluations with outcomes to determine which tools best predict the occurrence of major postoperative complications. They found the strongest correlation with outcomes when combining gender and type of surgery with the following 3 assessment tools:
- Timed Up and Go (TUG)—a walking test to measure functional status
- American Society of Anesthesiologists scale—a scoring system that quantifies preoperative physical status and estimates anesthetic risk
- Nutritional Risk Screening—an assessment of nutritional risk based on recent weight loss, overall condition, and reduction of food intake.
All 3 are simple and short screening tools. When used together, they can provide clinicians with accurate risk estimations.
The findings of Huisman and colleagues reinforce the importance of a global assessment of the patient’s comorbidities, functional status, and nutritional status when determining candidacy for oncologic surgery.
Functional index predicts need for postoperative ICU care and risk of death
Uppal and colleagues set out to quantify the predictive value of the modified Functional Index (mFI) in assessing the need for postoperative critical care support and/or the risk of death within 30 days after gynecologic cancer surgery. The mFI can be calculated by adding 1 point for each variable listed in the TABLE, with a score of 4 or higher representing a high-frailty cohort.
Of 6,551 patients who underwent gynecologic surgery, 188 were admitted to the intensive care unit (ICU) or died within 30 days after surgery. The mFI was calculated, with multivariate analyses of additional variables. An mFI score of 3 or higher was predictive of the need for critical care support and the risk of 30-day mortality and was associated with a significantly higher number of complications (P<.001).
Predictors significant for postoperative critical care support or death were:
- preoperative albumin level less than 3 g/dL (odds ratio [OR] = 6.5)
- operative time (OR = 1.003 per minute of increase)
- nonlaparoscopic surgery (OR = 3.3)
- mFI score, with a score of 0 serving as the reference (OR for a score of 1 = 1.26; score of 2 = 1.9; score of 3 = 2.33; and score of 4 or higher = 12.5).
When they combined the mFI and albumin scores—both readily available in the preoperative setting—Uppal and colleagues were able to develop an algorithm to determine patients who were at “low risk” versus “high risk” for ICU admission and/or death postoperatively (FIGURE).
[[{"fid":"79834","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"","field_file_image_title_text[und][0][value]":"","field_file_image_caption[und][0][value]":"Postoperative risk stratification based on preoperative albumin level
and modified Functional Index","field_file_image_credit[und][0][value]":"6"},"type":"media","attributes":{"height":"316","width":"665","class":"media-element file-medstat-image-full-text"}}]]
Bottom line
Older patients are more commonly affected by multiple medical comorbidities, as well as functional, cognitive, and nutritional deficiencies, which contribute to their increased risk of morbidity and mortality after surgery. The elderly experience greater morbidity with noncardiac surgery in general.
Clearly, the decision to operate on an elderly patient should be approached with caution, and a critical assessment of the patient’s risk factors should be performed to inform counseling about the patient’s management options. Future randomized prospective data will help us better understand the relationship between age and surgical outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- US Census Bureau. Population Projections: Projections of the Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- US Census Bureau. Population Projections: Percent Distribution of the Projected Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- Aletti G, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced stage ovarian cancer. Obstet Gynecol. 2006;107(1):77–85.
- National Comprehensive Cancer Network. NCCN Guidelines for Age-Related Recommendations: Older Adult Oncology. . Published 2015. Accessed August 31, 2015.
- Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
As the proportion of the elderly in the US population continues to increase, with life expectancy trending upward, we can expect to see more gynecologic cancers in our patients.1,2 At present, the most effective approach to these cancers commonly includes aggressive surgical resection with chemotherapy and, in some cases, radiation. It remains unclear whether elderly patients should be managed the same as younger patients, with minimal data to guide physicians. Some evidence suggests an increased risk of surgical complications in older adults.3
To optimize surgical care in our elderly patients, we need to understand the risks of perioperative mortality and morbidity in this population. For example, the current standard of care for advanced epithelial ovarian cancer is aggressive cytoreductive surgery followed by adjuvant chemotherapy,4 although neoadjuvant chemotherapy is gaining utility and popularity in certain circumstances. During pretreatment counseling, it is imperative that we communicate patient-specific outcomes so that patients and their families can make educated decisions in line with their goals. What should we know about age-dependent outcomes when counseling our patients?
To optimize surgical care in this population, we also need to develop and use new methods of surgical decision making. Although some data suggest that age is an independent risk factor for postoperative complications, not all elderly patients are the same in terms of comorbidities and functional status. In order to truly assess risks, we need to identify additional preoperative risk factors. Are there accurate scoring tools or predictors of outcomes available to help us assess the risks of postoperative mortality and morbidity?
In this article, we highlight recent developments in surgical treatment of the elderly, focusing on:
- postoperative mortality and morbidity in patients older than 80 years
- adjuncts to preoperative assessment for oncogeriatric surgical patients.
Risks rise sharply in older patients undergoing treatment for ovarian Ca
Moore KN, Reid MS, Fong DN, et al. Ovarian cancer in the octogenarian: does the paradigm of aggressive cytoreductive surgery and chemotherapy still apply? Gynecol Oncol. 2008;110(2):133–139.
Mahdi H, Wiechert A, Lockhart D, Rose PG. Impact of age on 30-day mortality and morbidity in patients undergoing surgery for ovarian cancer. Int J Gynecol Cancer. 2015;25(7):1216–1223.
The cornerstone of optimal survival from certain gynecologic cancers, such as advanced ovarian cancer, is aggressive debulking surgery. However, older adults are classically under-represented in clinical trials that guide this standard of care.
To determine whether patients aged 80 years or older respond differently from younger patients to conventional ovarian cancer management, Moore and colleagues retrospectively reviewed their institutional experience. They found that postoperative mortality increased from 5.4% in patients aged 80 to 84 years to 9.1% in those aged 85 to 89 and 14.4% in those older than 90. The rates for younger patients were 0.6% for patients younger than 60 years, 2.8% for those aged 60 to 69 years, and 2.5% for those aged 70 to 79 years (P<.001).
Notably, 13% of patients aged 80 years or older who underwent primary surgery died during their primary hospitalization. Of those who survived, 50% were discharged to skilled nursing facilities. Of patients who underwent cytoreductive surgery, 13% were unable to undergo any intended adjuvant therapy, and only 57% completed more than 3 cycles of chemotherapy, either due to demise or toxicities. Two-month survival for patients 80 years or older was comparable between patients who underwent primary surgery and those who had primary chemotherapy (20% and 26%, respectively).
With a similar objective, Mahdi and colleagues identified 2,087 patients with ovarian cancer who underwent surgery. After adjusting for confounders with multivariable analyses, they found that octogenarians whose initial management was surgery were 9 times more likely than younger patients to die and 70% more likely to develop complications within 30 days. Among patients who underwent neoadjuvant chemotherapy, there were no significant differences between older and younger patients in 30-day postoperative mortality or morbidity.
When evaluating elderly patients for surgery, the use of multiple risk-assessment strategies may improve accuracy
Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: a multicenter prospective cohort study. Eur J Surg Oncol. 2015;41(7):844–851.
Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
The National Comprehensive Cancer Network recommends that clinicians determine baseline life expectancy for older adults with cancer to aid in management decision making. The use of tools such as www.eprognosis.com, developed to determine anticipated life expectancy independent of cancer, can prove useful in determining a patient’s risk of dying or suffering from their cancer before dying of another cause.5
When it comes to the determination of risk related to a patient’s cancer diagnosis and selection of potential management options, many argue that the subgroup of elderly patients is not homogenous and that the use of age alone to guide management decisions may be unfair. Preoperative evaluation ideally should incorporate a global assessment of predictive risk factors.
Three assessment tools are especially useful
Huisman and colleagues set out to identify accurate preoperative assessment methods in elderly patients undergoing oncologic surgery. They prospectively recruited 328 patients aged 70 years or older and evaluated patients preoperatively using 11 well-known geriatric screening tools. They compared these evaluations with outcomes to determine which tools best predict the occurrence of major postoperative complications. They found the strongest correlation with outcomes when combining gender and type of surgery with the following 3 assessment tools:
- Timed Up and Go (TUG)—a walking test to measure functional status
- American Society of Anesthesiologists scale—a scoring system that quantifies preoperative physical status and estimates anesthetic risk
- Nutritional Risk Screening—an assessment of nutritional risk based on recent weight loss, overall condition, and reduction of food intake.
All 3 are simple and short screening tools. When used together, they can provide clinicians with accurate risk estimations.
The findings of Huisman and colleagues reinforce the importance of a global assessment of the patient’s comorbidities, functional status, and nutritional status when determining candidacy for oncologic surgery.
Functional index predicts need for postoperative ICU care and risk of death
Uppal and colleagues set out to quantify the predictive value of the modified Functional Index (mFI) in assessing the need for postoperative critical care support and/or the risk of death within 30 days after gynecologic cancer surgery. The mFI can be calculated by adding 1 point for each variable listed in the TABLE, with a score of 4 or higher representing a high-frailty cohort.
Of 6,551 patients who underwent gynecologic surgery, 188 were admitted to the intensive care unit (ICU) or died within 30 days after surgery. The mFI was calculated, with multivariate analyses of additional variables. An mFI score of 3 or higher was predictive of the need for critical care support and the risk of 30-day mortality and was associated with a significantly higher number of complications (P<.001).
Predictors significant for postoperative critical care support or death were:
- preoperative albumin level less than 3 g/dL (odds ratio [OR] = 6.5)
- operative time (OR = 1.003 per minute of increase)
- nonlaparoscopic surgery (OR = 3.3)
- mFI score, with a score of 0 serving as the reference (OR for a score of 1 = 1.26; score of 2 = 1.9; score of 3 = 2.33; and score of 4 or higher = 12.5).
When they combined the mFI and albumin scores—both readily available in the preoperative setting—Uppal and colleagues were able to develop an algorithm to determine patients who were at “low risk” versus “high risk” for ICU admission and/or death postoperatively (FIGURE).
[[{"fid":"79834","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"","field_file_image_title_text[und][0][value]":"","field_file_image_caption[und][0][value]":"Postoperative risk stratification based on preoperative albumin level
and modified Functional Index","field_file_image_credit[und][0][value]":"6"},"type":"media","attributes":{"height":"316","width":"665","class":"media-element file-medstat-image-full-text"}}]]
Bottom line
Older patients are more commonly affected by multiple medical comorbidities, as well as functional, cognitive, and nutritional deficiencies, which contribute to their increased risk of morbidity and mortality after surgery. The elderly experience greater morbidity with noncardiac surgery in general.
Clearly, the decision to operate on an elderly patient should be approached with caution, and a critical assessment of the patient’s risk factors should be performed to inform counseling about the patient’s management options. Future randomized prospective data will help us better understand the relationship between age and surgical outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
As the proportion of the elderly in the US population continues to increase, with life expectancy trending upward, we can expect to see more gynecologic cancers in our patients.1,2 At present, the most effective approach to these cancers commonly includes aggressive surgical resection with chemotherapy and, in some cases, radiation. It remains unclear whether elderly patients should be managed the same as younger patients, with minimal data to guide physicians. Some evidence suggests an increased risk of surgical complications in older adults.3
To optimize surgical care in our elderly patients, we need to understand the risks of perioperative mortality and morbidity in this population. For example, the current standard of care for advanced epithelial ovarian cancer is aggressive cytoreductive surgery followed by adjuvant chemotherapy,4 although neoadjuvant chemotherapy is gaining utility and popularity in certain circumstances. During pretreatment counseling, it is imperative that we communicate patient-specific outcomes so that patients and their families can make educated decisions in line with their goals. What should we know about age-dependent outcomes when counseling our patients?
To optimize surgical care in this population, we also need to develop and use new methods of surgical decision making. Although some data suggest that age is an independent risk factor for postoperative complications, not all elderly patients are the same in terms of comorbidities and functional status. In order to truly assess risks, we need to identify additional preoperative risk factors. Are there accurate scoring tools or predictors of outcomes available to help us assess the risks of postoperative mortality and morbidity?
In this article, we highlight recent developments in surgical treatment of the elderly, focusing on:
- postoperative mortality and morbidity in patients older than 80 years
- adjuncts to preoperative assessment for oncogeriatric surgical patients.
Risks rise sharply in older patients undergoing treatment for ovarian Ca
Moore KN, Reid MS, Fong DN, et al. Ovarian cancer in the octogenarian: does the paradigm of aggressive cytoreductive surgery and chemotherapy still apply? Gynecol Oncol. 2008;110(2):133–139.
Mahdi H, Wiechert A, Lockhart D, Rose PG. Impact of age on 30-day mortality and morbidity in patients undergoing surgery for ovarian cancer. Int J Gynecol Cancer. 2015;25(7):1216–1223.
The cornerstone of optimal survival from certain gynecologic cancers, such as advanced ovarian cancer, is aggressive debulking surgery. However, older adults are classically under-represented in clinical trials that guide this standard of care.
To determine whether patients aged 80 years or older respond differently from younger patients to conventional ovarian cancer management, Moore and colleagues retrospectively reviewed their institutional experience. They found that postoperative mortality increased from 5.4% in patients aged 80 to 84 years to 9.1% in those aged 85 to 89 and 14.4% in those older than 90. The rates for younger patients were 0.6% for patients younger than 60 years, 2.8% for those aged 60 to 69 years, and 2.5% for those aged 70 to 79 years (P<.001).
Notably, 13% of patients aged 80 years or older who underwent primary surgery died during their primary hospitalization. Of those who survived, 50% were discharged to skilled nursing facilities. Of patients who underwent cytoreductive surgery, 13% were unable to undergo any intended adjuvant therapy, and only 57% completed more than 3 cycles of chemotherapy, either due to demise or toxicities. Two-month survival for patients 80 years or older was comparable between patients who underwent primary surgery and those who had primary chemotherapy (20% and 26%, respectively).
With a similar objective, Mahdi and colleagues identified 2,087 patients with ovarian cancer who underwent surgery. After adjusting for confounders with multivariable analyses, they found that octogenarians whose initial management was surgery were 9 times more likely than younger patients to die and 70% more likely to develop complications within 30 days. Among patients who underwent neoadjuvant chemotherapy, there were no significant differences between older and younger patients in 30-day postoperative mortality or morbidity.
When evaluating elderly patients for surgery, the use of multiple risk-assessment strategies may improve accuracy
Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: a multicenter prospective cohort study. Eur J Surg Oncol. 2015;41(7):844–851.
Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
The National Comprehensive Cancer Network recommends that clinicians determine baseline life expectancy for older adults with cancer to aid in management decision making. The use of tools such as www.eprognosis.com, developed to determine anticipated life expectancy independent of cancer, can prove useful in determining a patient’s risk of dying or suffering from their cancer before dying of another cause.5
When it comes to the determination of risk related to a patient’s cancer diagnosis and selection of potential management options, many argue that the subgroup of elderly patients is not homogenous and that the use of age alone to guide management decisions may be unfair. Preoperative evaluation ideally should incorporate a global assessment of predictive risk factors.
Three assessment tools are especially useful
Huisman and colleagues set out to identify accurate preoperative assessment methods in elderly patients undergoing oncologic surgery. They prospectively recruited 328 patients aged 70 years or older and evaluated patients preoperatively using 11 well-known geriatric screening tools. They compared these evaluations with outcomes to determine which tools best predict the occurrence of major postoperative complications. They found the strongest correlation with outcomes when combining gender and type of surgery with the following 3 assessment tools:
- Timed Up and Go (TUG)—a walking test to measure functional status
- American Society of Anesthesiologists scale—a scoring system that quantifies preoperative physical status and estimates anesthetic risk
- Nutritional Risk Screening—an assessment of nutritional risk based on recent weight loss, overall condition, and reduction of food intake.
All 3 are simple and short screening tools. When used together, they can provide clinicians with accurate risk estimations.
The findings of Huisman and colleagues reinforce the importance of a global assessment of the patient’s comorbidities, functional status, and nutritional status when determining candidacy for oncologic surgery.
Functional index predicts need for postoperative ICU care and risk of death
Uppal and colleagues set out to quantify the predictive value of the modified Functional Index (mFI) in assessing the need for postoperative critical care support and/or the risk of death within 30 days after gynecologic cancer surgery. The mFI can be calculated by adding 1 point for each variable listed in the TABLE, with a score of 4 or higher representing a high-frailty cohort.
Of 6,551 patients who underwent gynecologic surgery, 188 were admitted to the intensive care unit (ICU) or died within 30 days after surgery. The mFI was calculated, with multivariate analyses of additional variables. An mFI score of 3 or higher was predictive of the need for critical care support and the risk of 30-day mortality and was associated with a significantly higher number of complications (P<.001).
Predictors significant for postoperative critical care support or death were:
- preoperative albumin level less than 3 g/dL (odds ratio [OR] = 6.5)
- operative time (OR = 1.003 per minute of increase)
- nonlaparoscopic surgery (OR = 3.3)
- mFI score, with a score of 0 serving as the reference (OR for a score of 1 = 1.26; score of 2 = 1.9; score of 3 = 2.33; and score of 4 or higher = 12.5).
When they combined the mFI and albumin scores—both readily available in the preoperative setting—Uppal and colleagues were able to develop an algorithm to determine patients who were at “low risk” versus “high risk” for ICU admission and/or death postoperatively (FIGURE).
[[{"fid":"79834","view_mode":"medstat_image_full_text","fields":{"format":"medstat_image_full_text","field_file_image_alt_text[und][0][value]":"","field_file_image_title_text[und][0][value]":"","field_file_image_caption[und][0][value]":"Postoperative risk stratification based on preoperative albumin level
and modified Functional Index","field_file_image_credit[und][0][value]":"6"},"type":"media","attributes":{"height":"316","width":"665","class":"media-element file-medstat-image-full-text"}}]]
Bottom line
Older patients are more commonly affected by multiple medical comorbidities, as well as functional, cognitive, and nutritional deficiencies, which contribute to their increased risk of morbidity and mortality after surgery. The elderly experience greater morbidity with noncardiac surgery in general.
Clearly, the decision to operate on an elderly patient should be approached with caution, and a critical assessment of the patient’s risk factors should be performed to inform counseling about the patient’s management options. Future randomized prospective data will help us better understand the relationship between age and surgical outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- US Census Bureau. Population Projections: Projections of the Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- US Census Bureau. Population Projections: Percent Distribution of the Projected Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- Aletti G, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced stage ovarian cancer. Obstet Gynecol. 2006;107(1):77–85.
- National Comprehensive Cancer Network. NCCN Guidelines for Age-Related Recommendations: Older Adult Oncology. . Published 2015. Accessed August 31, 2015.
- Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
- US Census Bureau. Population Projections: Projections of the Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- US Census Bureau. Population Projections: Percent Distribution of the Projected Population by Sex and Selected Age Groups for the United States: 2015 to 2060. https://www.census.gov/population/projections/data/national/2014/summarytables.html. Published December 2014. Accessed August 31, 2015.
- Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- Aletti G, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced stage ovarian cancer. Obstet Gynecol. 2006;107(1):77–85.
- National Comprehensive Cancer Network. NCCN Guidelines for Age-Related Recommendations: Older Adult Oncology. . Published 2015. Accessed August 31, 2015.
- Uppal S, Igwe E, Rice L, Spencer R, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
IN THIS ARTICLE
- Preoperative risk-assessment strategies
- The 11-item modified Functional Index
- Using the Functional Index in practice
2014 Update on ovarian cancer
Ovarian cancer remains the deadliest gynecologic malignancy in the United States, with more than 22,000 women newly diagnosed and more than 14,000 deaths each year. We have made slow progress in terms of survival with new drugs and applications, such as intraperitoneal chemotherapy combined with more aggressive cytoreductive efforts. Five-year survival rates have increased—from 36% to 44%—since the late 1970s.1 To make the leap from molecular genetics to successful screening, early diagnosis, and targeted treatment, we must first:
- Enhance our understanding of the changes that lead to ovarian cancer. Currently, malignant transformation of the fallopian tube epithelium is thought to result in high-grade papillary serous cancer.2 If this is indeed the pathologic origin of ovarian cancers, then early detection or even detection in the premalignant phase may be possible using tests of vaginal fluid. Are early detection, and even screening, possible and how would it effect treatment and survival?
- Develop new and powerful tools to detect molecular changes that might impact treatment and survival. Just a few years ago, initial sequencing of the human genome cost more than $100 million, but DNA sequencing technologies have evolved rapidly, with current estimates at less than a few thousand dollars per genome.3 Knowing the mutations responsible for an individual’s cancer would allow for targeted, individualized treatment plans. Would one patient benefit from neoadjuvant therapy while another needs primary surgical debulking?
In this article, we highlight the historical basis and recent developments in the field of ovarian cancer, focusing on:
- etiologic heterogeneity and molecular biology detection of small numbers of cancer cells in vaginal secretions and the blood stream.
- detection of small numbers of cancer cells in vaginal secretions and the blood stream.
What mutations are we looking for?
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609−615.
In last year’s Update, we discussed the role of The Cancer Genome Atlas (TCGA) project in endometrial cancer.4 For ovarian cancer, TCGA analyzed messenger RNA expression, microRNA expression, promoter methylation, and DNA copy number in 489 high-grade serous ovarian adenocarcinomas and the DNA sequences of exons from coding genes in 316 of these tumors.
Almost all tumors (96%) were characterized by mutations of the gene encoding TP53 in addition to statistically recurrent mutations in nine other loci, including NF1, BRCA1, BRCA2, RB1, and CDK12, although these were of low prevalence. Analyses also brought new insight regarding the survival impact of tumors containing BRCA1 or BRCA2 and CCNE1 mutations. Findings included NOTCH and FOXM1 signaling involvement in serous ovarian cancer pathophysiology as well as defective homologous recombination in approximately half of the tumors studied.
What this evidence means for practiceWith these mutations as our targets, we can screen vaginal secretions as well as blood for markers of ovarian cancer.
Ovarian and endometrial cancer cells detected in the vagina
Kinde I, Bettegowda C, Wang Y, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5(167):167ra4.
Erickson BK, Kinde I, Dobbin AC, et al. Detection of somatic TP53 mutations in tampons of patients with high-grade serous ovarian cancer [published online ahead of print October 2014]. Obstet Gynecol. 2014;124(5).
Ruth Graham, Papanicolaou’s cytology technician in the 1940s, first described ovarian cancer cells detected in vaginal/cervical cytology obtained from vaginal secretions.5 Current studies now demonstrate that we have technology capable of more than simple cytologic detection. We can isolate and evaluate these cancer cells in very small numbers.
Ovarian and endometrial cancer DNA identified in Pap specimenKinde and colleagues assembled a catalog of common mutations previously found in ovarian cancer as well as new data on 22 endometrial tumors. They tested 24 endometrial and 22 ovarian samples from patients with endometrial or ovarian cancers and confirmed that all 46 harbored at least some component of the common genetic changes in their catalog. Hypothesizing that the cancers likely shed cells from their surfaces, they sought to determine whether they could detect these cells among the cervical cells in a Pap smear.
These investigators used massively parallel sequencing to test DNA collected in modern liquid-based cytologic specimens for the same mutations found in the cancer cells. They found that 100% of the endometrial cancers and 41% of ovarian cancers were detectable by this method.
TP53 mutations in ovarian cancer cells detected in vaginally placed tamponWith similar technology, but a different collection method, Erickson and colleagues sought to detect tumor cells in the vagina of women with serous ovarian cancer by TP53 analysis of DNA samples collected via vaginal tampon.
Thirty-three women with pelvic masses suspicious for malignancy and scheduled to undergo diagnostic or therapeutic surgery were enrolled. Of the 25 patients who placed the tampon 8 to 12 hours prior to surgery; 13 had benign disease; three had nonovarian malignancies; and nine had serous adenocarcinoma of ovarian, tubal, or primary peritoneal origin. DNA from tumor specimens of eight patients with serous carcinoma and adequate DNA samples were analyzed for TP53 mutations. The corresponding DNA extracted from the tampon was then probed for the mutation identified in the tumor.
Mutational analysis of the tampon specimen DNA revealed no mutations in the tampon DNA of the three patients who had previously undergone tubal ligation, while mutations were observed in three of the five patients with intact tubes—producing a sensitivity of 60%. The fraction of mutant alleles in the tampon DNA was extremely low at 0.01% to 0.07%, requiring ultra-deep sequencing and increasing the importance of paired primary tumor specimens.
What this evidence means for practiceWhile sensitivity in a population of high-risk patients with intact tubes was found to be 60%, it is unclear what it would be in patients with less advanced disease. The ability of the test to detect mutations at exceptionally low limits is impressive; however, it increases the risk that a variant represents a sequencing error or a sample-to-sample contamination. This study is novel in its approach to diagnosis of ovarian cancer and is a stride toward screening, providing an opportunity to further validate the technology prior to widespread use and clinical application.
Circulating tumor cells—the future of cancer management?
Obermayr E, Castillo-Tong DC, Pils D, et al. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance: a study of the OVCAD consortium. Gynecol Oncol. 2013;128(1):15−21.
Similar in concept to noninvasive prenatal testing for fetal aneuploidy, high circulating tumor cell (CTC) numbers have been correlated with aggressive disease, increased metastasis, and decreased time to relapse. As with cancer cells in vaginal secretions, CTCs also may provide an opportunity for early detection and targeted treatment.6
While many CTC studies have used epithelial cell adhesion molecule (EpCAM)−based CTC detection, results have been found to be highly variable between tumor subtypes and phase of disease.7 Therefore, Obermayer and colleagues sought to identify novel markers for CTCs in patients with epithelial ovarian cancer and elucidate their impact on outcome.
Details of the studyMatched ovarian cancer tissues and peripheral blood leukocytes of 35 patients underwent microarray analysis to identify novel CTC markers. Gene expression of the novel markers as well as EpCAM were analyzed using blood samples taken from 39 healthy females and from 216 patients with ovarian cancer before primary treatment and 6 months after adjuvant chemotherapy. Overexpression of at least one gene, compared with the healthy control group, was considered CTC positivity.
CTCs were detected in 24.5% of the baseline and 20.4% of the follow-up samples, of which two-thirds showed overexpression of the cyclophilin C gene (PPIC), and just a few by EpCAM overexpression. PPIC-positive CTCs during follow-up were detected significantly more often in the platinum resistant group, and indicated poor outcome even when controlling for classical prognostic parameters.
What this evidence means for practiceThe study authors found that molecular characterization of CTC is superior to CTC enumeration. Ultimately, CTC diagnostics may lead to earlier detection and more personalized treatment of ovarian cancer.
Therefore, this technology could have great impact on screening for and the survival of a large subset of patients with ovarian cancer. In addition, the cells obtained preoperatively could help assess the risk of malignancy in an ovarian mass prior to surgery, or even help in treatment planning, as we enter an era in which we have the ability to assess cancers for prognosis and features of treatment response.
Share your thoughts on this article! Send your Letter to the Editor to [email protected].
1. Siegel R, Ma J, Zou Z, et al. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
2. Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195(4):451–456.
3. Wetterstrand KA. DNA Sequencing Costs: Data from the NHGRI Genome Sequencing Program (GSP). http://www.genome.gov/sequencingcosts. Updated July 18, 2014. Accessed September 21, 2014.
4. Kandoth C, Schultz N, Cherniack AD, et al; Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
5. Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. Am J Obstet Gynecol. 1941;42:193–206.
6. Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumor cells. Science. 2013;341(6151):1186–1188.
7. Sieuwerts AM, Kraan J, Bolt J, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009;101(1):61–66.
Ovarian cancer remains the deadliest gynecologic malignancy in the United States, with more than 22,000 women newly diagnosed and more than 14,000 deaths each year. We have made slow progress in terms of survival with new drugs and applications, such as intraperitoneal chemotherapy combined with more aggressive cytoreductive efforts. Five-year survival rates have increased—from 36% to 44%—since the late 1970s.1 To make the leap from molecular genetics to successful screening, early diagnosis, and targeted treatment, we must first:
- Enhance our understanding of the changes that lead to ovarian cancer. Currently, malignant transformation of the fallopian tube epithelium is thought to result in high-grade papillary serous cancer.2 If this is indeed the pathologic origin of ovarian cancers, then early detection or even detection in the premalignant phase may be possible using tests of vaginal fluid. Are early detection, and even screening, possible and how would it effect treatment and survival?
- Develop new and powerful tools to detect molecular changes that might impact treatment and survival. Just a few years ago, initial sequencing of the human genome cost more than $100 million, but DNA sequencing technologies have evolved rapidly, with current estimates at less than a few thousand dollars per genome.3 Knowing the mutations responsible for an individual’s cancer would allow for targeted, individualized treatment plans. Would one patient benefit from neoadjuvant therapy while another needs primary surgical debulking?
In this article, we highlight the historical basis and recent developments in the field of ovarian cancer, focusing on:
- etiologic heterogeneity and molecular biology detection of small numbers of cancer cells in vaginal secretions and the blood stream.
- detection of small numbers of cancer cells in vaginal secretions and the blood stream.
What mutations are we looking for?
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609−615.
In last year’s Update, we discussed the role of The Cancer Genome Atlas (TCGA) project in endometrial cancer.4 For ovarian cancer, TCGA analyzed messenger RNA expression, microRNA expression, promoter methylation, and DNA copy number in 489 high-grade serous ovarian adenocarcinomas and the DNA sequences of exons from coding genes in 316 of these tumors.
Almost all tumors (96%) were characterized by mutations of the gene encoding TP53 in addition to statistically recurrent mutations in nine other loci, including NF1, BRCA1, BRCA2, RB1, and CDK12, although these were of low prevalence. Analyses also brought new insight regarding the survival impact of tumors containing BRCA1 or BRCA2 and CCNE1 mutations. Findings included NOTCH and FOXM1 signaling involvement in serous ovarian cancer pathophysiology as well as defective homologous recombination in approximately half of the tumors studied.
What this evidence means for practiceWith these mutations as our targets, we can screen vaginal secretions as well as blood for markers of ovarian cancer.
Ovarian and endometrial cancer cells detected in the vagina
Kinde I, Bettegowda C, Wang Y, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5(167):167ra4.
Erickson BK, Kinde I, Dobbin AC, et al. Detection of somatic TP53 mutations in tampons of patients with high-grade serous ovarian cancer [published online ahead of print October 2014]. Obstet Gynecol. 2014;124(5).
Ruth Graham, Papanicolaou’s cytology technician in the 1940s, first described ovarian cancer cells detected in vaginal/cervical cytology obtained from vaginal secretions.5 Current studies now demonstrate that we have technology capable of more than simple cytologic detection. We can isolate and evaluate these cancer cells in very small numbers.
Ovarian and endometrial cancer DNA identified in Pap specimenKinde and colleagues assembled a catalog of common mutations previously found in ovarian cancer as well as new data on 22 endometrial tumors. They tested 24 endometrial and 22 ovarian samples from patients with endometrial or ovarian cancers and confirmed that all 46 harbored at least some component of the common genetic changes in their catalog. Hypothesizing that the cancers likely shed cells from their surfaces, they sought to determine whether they could detect these cells among the cervical cells in a Pap smear.
These investigators used massively parallel sequencing to test DNA collected in modern liquid-based cytologic specimens for the same mutations found in the cancer cells. They found that 100% of the endometrial cancers and 41% of ovarian cancers were detectable by this method.
TP53 mutations in ovarian cancer cells detected in vaginally placed tamponWith similar technology, but a different collection method, Erickson and colleagues sought to detect tumor cells in the vagina of women with serous ovarian cancer by TP53 analysis of DNA samples collected via vaginal tampon.
Thirty-three women with pelvic masses suspicious for malignancy and scheduled to undergo diagnostic or therapeutic surgery were enrolled. Of the 25 patients who placed the tampon 8 to 12 hours prior to surgery; 13 had benign disease; three had nonovarian malignancies; and nine had serous adenocarcinoma of ovarian, tubal, or primary peritoneal origin. DNA from tumor specimens of eight patients with serous carcinoma and adequate DNA samples were analyzed for TP53 mutations. The corresponding DNA extracted from the tampon was then probed for the mutation identified in the tumor.
Mutational analysis of the tampon specimen DNA revealed no mutations in the tampon DNA of the three patients who had previously undergone tubal ligation, while mutations were observed in three of the five patients with intact tubes—producing a sensitivity of 60%. The fraction of mutant alleles in the tampon DNA was extremely low at 0.01% to 0.07%, requiring ultra-deep sequencing and increasing the importance of paired primary tumor specimens.
What this evidence means for practiceWhile sensitivity in a population of high-risk patients with intact tubes was found to be 60%, it is unclear what it would be in patients with less advanced disease. The ability of the test to detect mutations at exceptionally low limits is impressive; however, it increases the risk that a variant represents a sequencing error or a sample-to-sample contamination. This study is novel in its approach to diagnosis of ovarian cancer and is a stride toward screening, providing an opportunity to further validate the technology prior to widespread use and clinical application.
Circulating tumor cells—the future of cancer management?
Obermayr E, Castillo-Tong DC, Pils D, et al. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance: a study of the OVCAD consortium. Gynecol Oncol. 2013;128(1):15−21.
Similar in concept to noninvasive prenatal testing for fetal aneuploidy, high circulating tumor cell (CTC) numbers have been correlated with aggressive disease, increased metastasis, and decreased time to relapse. As with cancer cells in vaginal secretions, CTCs also may provide an opportunity for early detection and targeted treatment.6
While many CTC studies have used epithelial cell adhesion molecule (EpCAM)−based CTC detection, results have been found to be highly variable between tumor subtypes and phase of disease.7 Therefore, Obermayer and colleagues sought to identify novel markers for CTCs in patients with epithelial ovarian cancer and elucidate their impact on outcome.
Details of the studyMatched ovarian cancer tissues and peripheral blood leukocytes of 35 patients underwent microarray analysis to identify novel CTC markers. Gene expression of the novel markers as well as EpCAM were analyzed using blood samples taken from 39 healthy females and from 216 patients with ovarian cancer before primary treatment and 6 months after adjuvant chemotherapy. Overexpression of at least one gene, compared with the healthy control group, was considered CTC positivity.
CTCs were detected in 24.5% of the baseline and 20.4% of the follow-up samples, of which two-thirds showed overexpression of the cyclophilin C gene (PPIC), and just a few by EpCAM overexpression. PPIC-positive CTCs during follow-up were detected significantly more often in the platinum resistant group, and indicated poor outcome even when controlling for classical prognostic parameters.
What this evidence means for practiceThe study authors found that molecular characterization of CTC is superior to CTC enumeration. Ultimately, CTC diagnostics may lead to earlier detection and more personalized treatment of ovarian cancer.
Therefore, this technology could have great impact on screening for and the survival of a large subset of patients with ovarian cancer. In addition, the cells obtained preoperatively could help assess the risk of malignancy in an ovarian mass prior to surgery, or even help in treatment planning, as we enter an era in which we have the ability to assess cancers for prognosis and features of treatment response.
Share your thoughts on this article! Send your Letter to the Editor to [email protected].
Ovarian cancer remains the deadliest gynecologic malignancy in the United States, with more than 22,000 women newly diagnosed and more than 14,000 deaths each year. We have made slow progress in terms of survival with new drugs and applications, such as intraperitoneal chemotherapy combined with more aggressive cytoreductive efforts. Five-year survival rates have increased—from 36% to 44%—since the late 1970s.1 To make the leap from molecular genetics to successful screening, early diagnosis, and targeted treatment, we must first:
- Enhance our understanding of the changes that lead to ovarian cancer. Currently, malignant transformation of the fallopian tube epithelium is thought to result in high-grade papillary serous cancer.2 If this is indeed the pathologic origin of ovarian cancers, then early detection or even detection in the premalignant phase may be possible using tests of vaginal fluid. Are early detection, and even screening, possible and how would it effect treatment and survival?
- Develop new and powerful tools to detect molecular changes that might impact treatment and survival. Just a few years ago, initial sequencing of the human genome cost more than $100 million, but DNA sequencing technologies have evolved rapidly, with current estimates at less than a few thousand dollars per genome.3 Knowing the mutations responsible for an individual’s cancer would allow for targeted, individualized treatment plans. Would one patient benefit from neoadjuvant therapy while another needs primary surgical debulking?
In this article, we highlight the historical basis and recent developments in the field of ovarian cancer, focusing on:
- etiologic heterogeneity and molecular biology detection of small numbers of cancer cells in vaginal secretions and the blood stream.
- detection of small numbers of cancer cells in vaginal secretions and the blood stream.
What mutations are we looking for?
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609−615.
In last year’s Update, we discussed the role of The Cancer Genome Atlas (TCGA) project in endometrial cancer.4 For ovarian cancer, TCGA analyzed messenger RNA expression, microRNA expression, promoter methylation, and DNA copy number in 489 high-grade serous ovarian adenocarcinomas and the DNA sequences of exons from coding genes in 316 of these tumors.
Almost all tumors (96%) were characterized by mutations of the gene encoding TP53 in addition to statistically recurrent mutations in nine other loci, including NF1, BRCA1, BRCA2, RB1, and CDK12, although these were of low prevalence. Analyses also brought new insight regarding the survival impact of tumors containing BRCA1 or BRCA2 and CCNE1 mutations. Findings included NOTCH and FOXM1 signaling involvement in serous ovarian cancer pathophysiology as well as defective homologous recombination in approximately half of the tumors studied.
What this evidence means for practiceWith these mutations as our targets, we can screen vaginal secretions as well as blood for markers of ovarian cancer.
Ovarian and endometrial cancer cells detected in the vagina
Kinde I, Bettegowda C, Wang Y, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5(167):167ra4.
Erickson BK, Kinde I, Dobbin AC, et al. Detection of somatic TP53 mutations in tampons of patients with high-grade serous ovarian cancer [published online ahead of print October 2014]. Obstet Gynecol. 2014;124(5).
Ruth Graham, Papanicolaou’s cytology technician in the 1940s, first described ovarian cancer cells detected in vaginal/cervical cytology obtained from vaginal secretions.5 Current studies now demonstrate that we have technology capable of more than simple cytologic detection. We can isolate and evaluate these cancer cells in very small numbers.
Ovarian and endometrial cancer DNA identified in Pap specimenKinde and colleagues assembled a catalog of common mutations previously found in ovarian cancer as well as new data on 22 endometrial tumors. They tested 24 endometrial and 22 ovarian samples from patients with endometrial or ovarian cancers and confirmed that all 46 harbored at least some component of the common genetic changes in their catalog. Hypothesizing that the cancers likely shed cells from their surfaces, they sought to determine whether they could detect these cells among the cervical cells in a Pap smear.
These investigators used massively parallel sequencing to test DNA collected in modern liquid-based cytologic specimens for the same mutations found in the cancer cells. They found that 100% of the endometrial cancers and 41% of ovarian cancers were detectable by this method.
TP53 mutations in ovarian cancer cells detected in vaginally placed tamponWith similar technology, but a different collection method, Erickson and colleagues sought to detect tumor cells in the vagina of women with serous ovarian cancer by TP53 analysis of DNA samples collected via vaginal tampon.
Thirty-three women with pelvic masses suspicious for malignancy and scheduled to undergo diagnostic or therapeutic surgery were enrolled. Of the 25 patients who placed the tampon 8 to 12 hours prior to surgery; 13 had benign disease; three had nonovarian malignancies; and nine had serous adenocarcinoma of ovarian, tubal, or primary peritoneal origin. DNA from tumor specimens of eight patients with serous carcinoma and adequate DNA samples were analyzed for TP53 mutations. The corresponding DNA extracted from the tampon was then probed for the mutation identified in the tumor.
Mutational analysis of the tampon specimen DNA revealed no mutations in the tampon DNA of the three patients who had previously undergone tubal ligation, while mutations were observed in three of the five patients with intact tubes—producing a sensitivity of 60%. The fraction of mutant alleles in the tampon DNA was extremely low at 0.01% to 0.07%, requiring ultra-deep sequencing and increasing the importance of paired primary tumor specimens.
What this evidence means for practiceWhile sensitivity in a population of high-risk patients with intact tubes was found to be 60%, it is unclear what it would be in patients with less advanced disease. The ability of the test to detect mutations at exceptionally low limits is impressive; however, it increases the risk that a variant represents a sequencing error or a sample-to-sample contamination. This study is novel in its approach to diagnosis of ovarian cancer and is a stride toward screening, providing an opportunity to further validate the technology prior to widespread use and clinical application.
Circulating tumor cells—the future of cancer management?
Obermayr E, Castillo-Tong DC, Pils D, et al. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance: a study of the OVCAD consortium. Gynecol Oncol. 2013;128(1):15−21.
Similar in concept to noninvasive prenatal testing for fetal aneuploidy, high circulating tumor cell (CTC) numbers have been correlated with aggressive disease, increased metastasis, and decreased time to relapse. As with cancer cells in vaginal secretions, CTCs also may provide an opportunity for early detection and targeted treatment.6
While many CTC studies have used epithelial cell adhesion molecule (EpCAM)−based CTC detection, results have been found to be highly variable between tumor subtypes and phase of disease.7 Therefore, Obermayer and colleagues sought to identify novel markers for CTCs in patients with epithelial ovarian cancer and elucidate their impact on outcome.
Details of the studyMatched ovarian cancer tissues and peripheral blood leukocytes of 35 patients underwent microarray analysis to identify novel CTC markers. Gene expression of the novel markers as well as EpCAM were analyzed using blood samples taken from 39 healthy females and from 216 patients with ovarian cancer before primary treatment and 6 months after adjuvant chemotherapy. Overexpression of at least one gene, compared with the healthy control group, was considered CTC positivity.
CTCs were detected in 24.5% of the baseline and 20.4% of the follow-up samples, of which two-thirds showed overexpression of the cyclophilin C gene (PPIC), and just a few by EpCAM overexpression. PPIC-positive CTCs during follow-up were detected significantly more often in the platinum resistant group, and indicated poor outcome even when controlling for classical prognostic parameters.
What this evidence means for practiceThe study authors found that molecular characterization of CTC is superior to CTC enumeration. Ultimately, CTC diagnostics may lead to earlier detection and more personalized treatment of ovarian cancer.
Therefore, this technology could have great impact on screening for and the survival of a large subset of patients with ovarian cancer. In addition, the cells obtained preoperatively could help assess the risk of malignancy in an ovarian mass prior to surgery, or even help in treatment planning, as we enter an era in which we have the ability to assess cancers for prognosis and features of treatment response.
Share your thoughts on this article! Send your Letter to the Editor to [email protected].
1. Siegel R, Ma J, Zou Z, et al. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
2. Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195(4):451–456.
3. Wetterstrand KA. DNA Sequencing Costs: Data from the NHGRI Genome Sequencing Program (GSP). http://www.genome.gov/sequencingcosts. Updated July 18, 2014. Accessed September 21, 2014.
4. Kandoth C, Schultz N, Cherniack AD, et al; Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
5. Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. Am J Obstet Gynecol. 1941;42:193–206.
6. Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumor cells. Science. 2013;341(6151):1186–1188.
7. Sieuwerts AM, Kraan J, Bolt J, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009;101(1):61–66.
1. Siegel R, Ma J, Zou Z, et al. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
2. Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195(4):451–456.
3. Wetterstrand KA. DNA Sequencing Costs: Data from the NHGRI Genome Sequencing Program (GSP). http://www.genome.gov/sequencingcosts. Updated July 18, 2014. Accessed September 21, 2014.
4. Kandoth C, Schultz N, Cherniack AD, et al; Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
5. Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. Am J Obstet Gynecol. 1941;42:193–206.
6. Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumor cells. Science. 2013;341(6151):1186–1188.
7. Sieuwerts AM, Kraan J, Bolt J, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009;101(1):61–66.
In this article:
What mutations are we looking for?
Ovarian and endometrial cancer cells detected in the vagina
Circulating tumor cells—the future of cancer management?
Laparoscopic dual-port contained power morcellation: An offered solution
Minimally invasive surgery utilizing laparoscopy for hysterectomy and myomectomy has become more common in women with gynecologic pathology. The benefits of this approach compared with laparotomy include decreased hospital stay, shorter recovery and, in experienced hands, significantly decreased morbidity.1–3
Approximately 600,000 hysterectomies are performed annually in the United States—30% of which are performed laparoscopically.4 The primary indication for surgical intervention is uterine leiomyoma. This pathology accounts for 40% of procedures.5 During these surgeries, electromechanical morcellation (EMM), or open “power” morcellation, is commonly used to cut large tissue specimens into small pieces for removal and thereby avoid a larger incision. Concerns have been raised regarding the use of open power morcellation because of the risk of spreading an unrecognized malignancy.
Based on case reports and retrospective studies, the FDA issued a statement in April of this year discouraging the use of EMM for hysterectomy and myomectomy in women with uterine fibroids.6 The concern for inadvertent spread of an occult malignancy was the reasoning for the communication. Since that time, the FDA’s Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee held a public meeting in which the panel heard comments from patients, societies, and industry regarding their positions on the safety of laparoscopic power morcellation. The panel made several recommendations to the FDA but, at the time of this writing, the FDA has yet to issue a final decision.
Reaction to FDA’s action/inaction
The FDA’s “safety” communication was in response to the concern of a few who experienced a bad outcome believed to be secondary to open power morcellation of enlarged uteri or fibroid tumors. In its statement, the FDA estimated the risk of an occult sarcoma to be about 1 in 350 and stated that the risk of disseminating a sarcoma with morcellation is substantial. The FDA discouraged the use of the power morcellator during hysterectomy or myomectomy for uterine fibroids.
Many organizations, including the Society of Gynecologic Oncology, The American Association of Gynecologic Laparoscopists (AAGL), and the American College of Obstetricians and Gynecologists, issued less stringent statements regarding this technology.7–9 These organizations stated generally that there were too few data to make a statement at that time, advocated the collection of more data, and encouraged detailed informed consent to be given to patients undergoing these procedures.
However, the FDA’s statement, and lack of a timely follow-up to clarify the role of the laparoscopic power morcellator in gynecologic surgery, has effectively stopped the use of this technology in its current form. In fact, in response to the statement, Ethicon Endosurgery has discontinued the distribution and sales of its power morcellator and many institutions have severely or completely restricted the use of this technology. The reason for these restrictions is that the medicolegal consequences of an adverse outcome would be very difficult to defend given the current, albeit premature, recommendations of the FDA. This statement makes it difficult to defend any adverse outcome that may occur in association with the use of the laparoscopic power morcellator. Furthermore, this statement by the FDA has largely prevented the medical community at large from collecting additional useful information to allow for a data-driven determination.
Power morcellation is not without risks. In fact, we outline them in this article. However, we believe that minimally invasive surgery should be allowed to continue to advance. In that vein, here we describe a technique of dual-port contained EMM. This surgical approach is performed under direct visualization—which solves the problem of poor visualization that hinders other contained EMM techniques.
Risks of power morcellation
The potential for inadvertent spread of occult malignancy is not the only risk of open EMM. Reports of disseminated leiomyomatosis, adenomyosis, and endometriosis also have been described from inadvertent tissue dispersion during open EMM with resulting ectopic reperitonealization.10–12
The procedure itself is not without risks. A recent systematic review documented 55 major and minor complications from EMM.13 Multiple organ systems were injured including bowel, urinary, vascular, and others, resulting in six deaths from these complications. The investigators concluded that “laparoscopic morcellator–related injuries continue to increase and short- and long-term complications are emerging in both the medical literature and device-related databases. Surgeon inexperience is descriptively identified as one of the most common contributing factors.”
All of the above risks must be weighed against the known benefits of laparoscopic surgery and presented to each patient to assist in deciding which route of surgery should be performed.
Tissue extraction options for large specimens
Large specimen extraction options during gynecologic surgery include:
Vaginal coring. Delivery through the vagina or colpotomy during vaginal or laparoscopic hysterectomy uses the technique of coring, which has long been established in our field.
Manual morcellation through a single incision. Mini-laparotomy or laparoendoscopic single-site surgery (LESS) incisions provide another option of removal with manual morcellation after laparoscopic hysterectomy or myomectomy. One study revealed that specimens up to 22 weeks in size can be placed in a large EndoCatch bag and morcellated extracorporeally by circumferentially coring with a scalpel.14
Contained power morcellation through a single port. Finally, the technique of contained EMM was recently described.15 This technique uses a large containment bag placed through a LESS incision with EMM being performed in an artificially created pneumoperitoneum. This technique isolates the specimen so that it can be morcellated without risk of exposing the patient to any malignant cells that might be unrecognized within the specimen.
Each of these techniques allows many patients to consider a minimally invasive option for their surgery. However, the ability to safely morcellate a very large uterus or myoma may be limited by visualization, and the experience of the surgeon is often critical in the successful performance of these procedures.16
Therefore, at Washington Universitywe have developed a technique using dual ports, with isolation of the uterus or myomas to improve visualization and prevent spillage of malignant tumor or dispersion of other benign tissue.
Dual-port EMM: Technique, tips, and tricks
Our technique of dual-port contained EMM allows the removal of large fibroids or uteri much larger than 20 weeks in size safely under direct visualization through a 15-mm incision. The technique uses:
- Karl Storz Rotocut tissue morcellator with spacers (FIGURE 1)
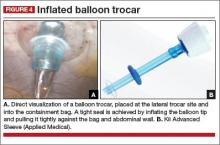
- 15-mm trocar
- 5-mm balloon trocar
- 20320-inch containment bag (FIGURE 2).
Containment bag placement
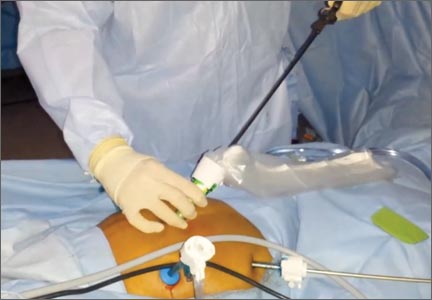
Once the specimen is free, we place it to the right or left side of the abdomen. The 15-mm trocar is placed through the umbilicus while visualizing from a lateral trocar site. We then fan-fold the containment bag and introduce it through the 15-mm trocar, keeping the bag oriented with the opening anterior (FIGURE 3). The bag is then grasped at the opening along the drawstring with an atraumatic grasper.
Tip: Care must be taken when introducing the bag in order to avoid tearing or making a small hole in it.
The leading edge is then introduced into the deepest part of the pelvis, and the remainder of the bag (left outside of the abdomen) is then fed cephalad into the abdomen.
Once the bag is completely in the abdomen, we orient the bag with the opening as wide as possible. This allows placement of a very large specimen. Once the specimen is within the containment bag, the drawstring is pulled tight and the mouth of the bag is removed through the 15-mm trocar site at the umbilicus.
The abdominal lateral gas port is opened to allow the intra-abdominal pneumoperitoneum to escape. A 5-mm trocar is placed into the bag through the opening at the umbilicus and the containment bag is insufflated with carbon dioxide and the insufflation pressure is set to 30 mm. The laparoscope placed through this trocar allows the artificial pneumoperitoneum being created to be observed (VIDEO).

Tip: The containment bag covers the entire abdominal cavity and should be fully distended. If it does not distend fully, a hole in the bag may be present and the bag must be replaced.
At this point, we place a balloon trocar at the lateral trocar site and into the bag under direct visualization. The balloon tip is inflated and pulled up tightly against the bag and abdominal wall (FIGURE 4). This allows a tight seal so there is no gas leak or spillage of the morcellated specimen. The laparoscope is placed through this trocar and the insufflation tubing is moved to this port.
Morcellator insertion
The morcellator is introduced through the umbilicus under direct visualization using the short morcellator blade in most instances. Spacers are used to set the length of the morcellator within the containment bag. The tip of the morcellator should be approximately 3 cm to 4 cm within the bag but well away from the retroperitoneum. Remember, any bag will be cut easily by the morcellator and should be thought of as peritoneum only and not a tough barrier. Serious injuries could otherwise develop.
At this point, place the patient flat or out of Trendelenburg position. Morcellation may now proceed.
Tip: Morcellation is best performed with the morcellator perpendicular to the abdomen under direct visualization using a 30° laparoscope to optimize the view. Morcellation in this position uses gravity to facilitate “peeling” of the specimen during morcellation and allows for faster removal.
Before removing the morcellator, inspect the containment bag for any large pieces that may have been dispersed during the morcellation process and remove them. Once there are only small fragments remaining, remove the morcellator, allowing the carbon dioxide to escape. Deflate the balloon tip on the trocar.
Now the containment bag with the remaining specimen may be removed through the umbilicus, while simultaneously removing the balloon-tip trocar from the bag.
A safe minimally invasive approach
This technique has allowed us to safely remove specimens larger than 1,500 g while keeping them in a contained environment with no spill of tissue within the abdomen.
Tracking and adaptation needed
The FDA safety communication has severely limited the practice of morcellation in the minimally invasive gynecologic surgical setting. Many hospitals around the country have reacted by placing significant restrictions on the use of EMM or banned it outright. This action may reverse the national trend of increasing rates of laparoscopic hysterectomy and force many practitioners to return to open surgery.
Currently, it is unclear what the true risk of tissue extraction is whether it is performed via EMM or manually. Large national databases including the BOLD database from the Surgical Review Corporation, as well as AAGL, must be utilized to track these cases and their outcomes to guide therapy. In the meantime, in order to continue to offer a minimally invasive approach to gynecologic surgery, new techniques and instrumentation in the operating room will need to be modified to adapt to these new guidelines. This is vital to maintain or even reduce the rates of open hysterectomy and associated morbidity while diminishing the potential risks of inadvertent benign as well as malignant tissue dispersion with tissue extraction.
Share your thoughts on this article! Send your Letter to the Editor to: [email protected]
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677. doi:10.1002/14651858.CD003677.pub4.
2. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
3. Wiser A, Holcroft CA, Tolandi T, Abenhaim HA. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10(2):117–122.
4. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
5. Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34.e1–e7.
6. U S Food and Drug Administration. Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids: Summary and key findings. Silver Spring, Maryland: FDA. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM393589.pdf. Published April 17, 2014. Accessed August 19, 2014.
7. Society of Gynecologic Oncology (SGO). SGO Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation. Published December 2013. Accessed March 1, 2014.
8. AAGL. Member Update: Disseminated leiomyosarcoma with power morcellation (Update #2). https://www.aagl.org/aaglnews/member-update-disseminated-leiomyosarcoma-with-power-morcellation-update-2/. Published July 11, 2014. Accessed August 19, 2014.
9. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery. http://www.acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Power_Morcellation_and_Occult_Malignancy_in_Gynecologic_Surgery. Published May 2014. Accessed August 19, 2014.
10. Sepilian V, Della Badia C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet Gynecol. 2003;102(5 Pt 2):1125–1127.
11. Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14(6):770–775.
12. Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007;14(2):156–160.
13. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21(3):486–491.
14. Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: an original technique that permits the safe and quick removal of a large uterus. Am J. Obstet Gynecol. 2011;204(6):566.e1–e2.
15. Shibley KA. Feasibilty of intra-abdominal tissue isolation and extraction with an artificially created pneumoperitoneum, at laparoscopy for gynecologic procedures. J Min Invasive Gynecol. 2012;19(6):S75.
Minimally invasive surgery utilizing laparoscopy for hysterectomy and myomectomy has become more common in women with gynecologic pathology. The benefits of this approach compared with laparotomy include decreased hospital stay, shorter recovery and, in experienced hands, significantly decreased morbidity.1–3
Approximately 600,000 hysterectomies are performed annually in the United States—30% of which are performed laparoscopically.4 The primary indication for surgical intervention is uterine leiomyoma. This pathology accounts for 40% of procedures.5 During these surgeries, electromechanical morcellation (EMM), or open “power” morcellation, is commonly used to cut large tissue specimens into small pieces for removal and thereby avoid a larger incision. Concerns have been raised regarding the use of open power morcellation because of the risk of spreading an unrecognized malignancy.
Based on case reports and retrospective studies, the FDA issued a statement in April of this year discouraging the use of EMM for hysterectomy and myomectomy in women with uterine fibroids.6 The concern for inadvertent spread of an occult malignancy was the reasoning for the communication. Since that time, the FDA’s Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee held a public meeting in which the panel heard comments from patients, societies, and industry regarding their positions on the safety of laparoscopic power morcellation. The panel made several recommendations to the FDA but, at the time of this writing, the FDA has yet to issue a final decision.
Reaction to FDA’s action/inaction
The FDA’s “safety” communication was in response to the concern of a few who experienced a bad outcome believed to be secondary to open power morcellation of enlarged uteri or fibroid tumors. In its statement, the FDA estimated the risk of an occult sarcoma to be about 1 in 350 and stated that the risk of disseminating a sarcoma with morcellation is substantial. The FDA discouraged the use of the power morcellator during hysterectomy or myomectomy for uterine fibroids.
Many organizations, including the Society of Gynecologic Oncology, The American Association of Gynecologic Laparoscopists (AAGL), and the American College of Obstetricians and Gynecologists, issued less stringent statements regarding this technology.7–9 These organizations stated generally that there were too few data to make a statement at that time, advocated the collection of more data, and encouraged detailed informed consent to be given to patients undergoing these procedures.
However, the FDA’s statement, and lack of a timely follow-up to clarify the role of the laparoscopic power morcellator in gynecologic surgery, has effectively stopped the use of this technology in its current form. In fact, in response to the statement, Ethicon Endosurgery has discontinued the distribution and sales of its power morcellator and many institutions have severely or completely restricted the use of this technology. The reason for these restrictions is that the medicolegal consequences of an adverse outcome would be very difficult to defend given the current, albeit premature, recommendations of the FDA. This statement makes it difficult to defend any adverse outcome that may occur in association with the use of the laparoscopic power morcellator. Furthermore, this statement by the FDA has largely prevented the medical community at large from collecting additional useful information to allow for a data-driven determination.
Power morcellation is not without risks. In fact, we outline them in this article. However, we believe that minimally invasive surgery should be allowed to continue to advance. In that vein, here we describe a technique of dual-port contained EMM. This surgical approach is performed under direct visualization—which solves the problem of poor visualization that hinders other contained EMM techniques.
Risks of power morcellation
The potential for inadvertent spread of occult malignancy is not the only risk of open EMM. Reports of disseminated leiomyomatosis, adenomyosis, and endometriosis also have been described from inadvertent tissue dispersion during open EMM with resulting ectopic reperitonealization.10–12
The procedure itself is not without risks. A recent systematic review documented 55 major and minor complications from EMM.13 Multiple organ systems were injured including bowel, urinary, vascular, and others, resulting in six deaths from these complications. The investigators concluded that “laparoscopic morcellator–related injuries continue to increase and short- and long-term complications are emerging in both the medical literature and device-related databases. Surgeon inexperience is descriptively identified as one of the most common contributing factors.”
All of the above risks must be weighed against the known benefits of laparoscopic surgery and presented to each patient to assist in deciding which route of surgery should be performed.
Tissue extraction options for large specimens
Large specimen extraction options during gynecologic surgery include:
Vaginal coring. Delivery through the vagina or colpotomy during vaginal or laparoscopic hysterectomy uses the technique of coring, which has long been established in our field.
Manual morcellation through a single incision. Mini-laparotomy or laparoendoscopic single-site surgery (LESS) incisions provide another option of removal with manual morcellation after laparoscopic hysterectomy or myomectomy. One study revealed that specimens up to 22 weeks in size can be placed in a large EndoCatch bag and morcellated extracorporeally by circumferentially coring with a scalpel.14
Contained power morcellation through a single port. Finally, the technique of contained EMM was recently described.15 This technique uses a large containment bag placed through a LESS incision with EMM being performed in an artificially created pneumoperitoneum. This technique isolates the specimen so that it can be morcellated without risk of exposing the patient to any malignant cells that might be unrecognized within the specimen.
Each of these techniques allows many patients to consider a minimally invasive option for their surgery. However, the ability to safely morcellate a very large uterus or myoma may be limited by visualization, and the experience of the surgeon is often critical in the successful performance of these procedures.16
Therefore, at Washington Universitywe have developed a technique using dual ports, with isolation of the uterus or myomas to improve visualization and prevent spillage of malignant tumor or dispersion of other benign tissue.
Dual-port EMM: Technique, tips, and tricks
Our technique of dual-port contained EMM allows the removal of large fibroids or uteri much larger than 20 weeks in size safely under direct visualization through a 15-mm incision. The technique uses:
- Karl Storz Rotocut tissue morcellator with spacers (FIGURE 1)
- 15-mm trocar
- 5-mm balloon trocar
- 20320-inch containment bag (FIGURE 2).
Containment bag placement
Once the specimen is free, we place it to the right or left side of the abdomen. The 15-mm trocar is placed through the umbilicus while visualizing from a lateral trocar site. We then fan-fold the containment bag and introduce it through the 15-mm trocar, keeping the bag oriented with the opening anterior (FIGURE 3). The bag is then grasped at the opening along the drawstring with an atraumatic grasper.
Tip: Care must be taken when introducing the bag in order to avoid tearing or making a small hole in it.
The leading edge is then introduced into the deepest part of the pelvis, and the remainder of the bag (left outside of the abdomen) is then fed cephalad into the abdomen.
Once the bag is completely in the abdomen, we orient the bag with the opening as wide as possible. This allows placement of a very large specimen. Once the specimen is within the containment bag, the drawstring is pulled tight and the mouth of the bag is removed through the 15-mm trocar site at the umbilicus.
The abdominal lateral gas port is opened to allow the intra-abdominal pneumoperitoneum to escape. A 5-mm trocar is placed into the bag through the opening at the umbilicus and the containment bag is insufflated with carbon dioxide and the insufflation pressure is set to 30 mm. The laparoscope placed through this trocar allows the artificial pneumoperitoneum being created to be observed (VIDEO).

Tip: The containment bag covers the entire abdominal cavity and should be fully distended. If it does not distend fully, a hole in the bag may be present and the bag must be replaced.
At this point, we place a balloon trocar at the lateral trocar site and into the bag under direct visualization. The balloon tip is inflated and pulled up tightly against the bag and abdominal wall (FIGURE 4). This allows a tight seal so there is no gas leak or spillage of the morcellated specimen. The laparoscope is placed through this trocar and the insufflation tubing is moved to this port.
Morcellator insertion
The morcellator is introduced through the umbilicus under direct visualization using the short morcellator blade in most instances. Spacers are used to set the length of the morcellator within the containment bag. The tip of the morcellator should be approximately 3 cm to 4 cm within the bag but well away from the retroperitoneum. Remember, any bag will be cut easily by the morcellator and should be thought of as peritoneum only and not a tough barrier. Serious injuries could otherwise develop.
At this point, place the patient flat or out of Trendelenburg position. Morcellation may now proceed.
Tip: Morcellation is best performed with the morcellator perpendicular to the abdomen under direct visualization using a 30° laparoscope to optimize the view. Morcellation in this position uses gravity to facilitate “peeling” of the specimen during morcellation and allows for faster removal.
Before removing the morcellator, inspect the containment bag for any large pieces that may have been dispersed during the morcellation process and remove them. Once there are only small fragments remaining, remove the morcellator, allowing the carbon dioxide to escape. Deflate the balloon tip on the trocar.
Now the containment bag with the remaining specimen may be removed through the umbilicus, while simultaneously removing the balloon-tip trocar from the bag.
A safe minimally invasive approach
This technique has allowed us to safely remove specimens larger than 1,500 g while keeping them in a contained environment with no spill of tissue within the abdomen.
Tracking and adaptation needed
The FDA safety communication has severely limited the practice of morcellation in the minimally invasive gynecologic surgical setting. Many hospitals around the country have reacted by placing significant restrictions on the use of EMM or banned it outright. This action may reverse the national trend of increasing rates of laparoscopic hysterectomy and force many practitioners to return to open surgery.
Currently, it is unclear what the true risk of tissue extraction is whether it is performed via EMM or manually. Large national databases including the BOLD database from the Surgical Review Corporation, as well as AAGL, must be utilized to track these cases and their outcomes to guide therapy. In the meantime, in order to continue to offer a minimally invasive approach to gynecologic surgery, new techniques and instrumentation in the operating room will need to be modified to adapt to these new guidelines. This is vital to maintain or even reduce the rates of open hysterectomy and associated morbidity while diminishing the potential risks of inadvertent benign as well as malignant tissue dispersion with tissue extraction.
Share your thoughts on this article! Send your Letter to the Editor to: [email protected]
Minimally invasive surgery utilizing laparoscopy for hysterectomy and myomectomy has become more common in women with gynecologic pathology. The benefits of this approach compared with laparotomy include decreased hospital stay, shorter recovery and, in experienced hands, significantly decreased morbidity.1–3
Approximately 600,000 hysterectomies are performed annually in the United States—30% of which are performed laparoscopically.4 The primary indication for surgical intervention is uterine leiomyoma. This pathology accounts for 40% of procedures.5 During these surgeries, electromechanical morcellation (EMM), or open “power” morcellation, is commonly used to cut large tissue specimens into small pieces for removal and thereby avoid a larger incision. Concerns have been raised regarding the use of open power morcellation because of the risk of spreading an unrecognized malignancy.
Based on case reports and retrospective studies, the FDA issued a statement in April of this year discouraging the use of EMM for hysterectomy and myomectomy in women with uterine fibroids.6 The concern for inadvertent spread of an occult malignancy was the reasoning for the communication. Since that time, the FDA’s Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee held a public meeting in which the panel heard comments from patients, societies, and industry regarding their positions on the safety of laparoscopic power morcellation. The panel made several recommendations to the FDA but, at the time of this writing, the FDA has yet to issue a final decision.
Reaction to FDA’s action/inaction
The FDA’s “safety” communication was in response to the concern of a few who experienced a bad outcome believed to be secondary to open power morcellation of enlarged uteri or fibroid tumors. In its statement, the FDA estimated the risk of an occult sarcoma to be about 1 in 350 and stated that the risk of disseminating a sarcoma with morcellation is substantial. The FDA discouraged the use of the power morcellator during hysterectomy or myomectomy for uterine fibroids.
Many organizations, including the Society of Gynecologic Oncology, The American Association of Gynecologic Laparoscopists (AAGL), and the American College of Obstetricians and Gynecologists, issued less stringent statements regarding this technology.7–9 These organizations stated generally that there were too few data to make a statement at that time, advocated the collection of more data, and encouraged detailed informed consent to be given to patients undergoing these procedures.
However, the FDA’s statement, and lack of a timely follow-up to clarify the role of the laparoscopic power morcellator in gynecologic surgery, has effectively stopped the use of this technology in its current form. In fact, in response to the statement, Ethicon Endosurgery has discontinued the distribution and sales of its power morcellator and many institutions have severely or completely restricted the use of this technology. The reason for these restrictions is that the medicolegal consequences of an adverse outcome would be very difficult to defend given the current, albeit premature, recommendations of the FDA. This statement makes it difficult to defend any adverse outcome that may occur in association with the use of the laparoscopic power morcellator. Furthermore, this statement by the FDA has largely prevented the medical community at large from collecting additional useful information to allow for a data-driven determination.
Power morcellation is not without risks. In fact, we outline them in this article. However, we believe that minimally invasive surgery should be allowed to continue to advance. In that vein, here we describe a technique of dual-port contained EMM. This surgical approach is performed under direct visualization—which solves the problem of poor visualization that hinders other contained EMM techniques.
Risks of power morcellation
The potential for inadvertent spread of occult malignancy is not the only risk of open EMM. Reports of disseminated leiomyomatosis, adenomyosis, and endometriosis also have been described from inadvertent tissue dispersion during open EMM with resulting ectopic reperitonealization.10–12
The procedure itself is not without risks. A recent systematic review documented 55 major and minor complications from EMM.13 Multiple organ systems were injured including bowel, urinary, vascular, and others, resulting in six deaths from these complications. The investigators concluded that “laparoscopic morcellator–related injuries continue to increase and short- and long-term complications are emerging in both the medical literature and device-related databases. Surgeon inexperience is descriptively identified as one of the most common contributing factors.”
All of the above risks must be weighed against the known benefits of laparoscopic surgery and presented to each patient to assist in deciding which route of surgery should be performed.
Tissue extraction options for large specimens
Large specimen extraction options during gynecologic surgery include:
Vaginal coring. Delivery through the vagina or colpotomy during vaginal or laparoscopic hysterectomy uses the technique of coring, which has long been established in our field.
Manual morcellation through a single incision. Mini-laparotomy or laparoendoscopic single-site surgery (LESS) incisions provide another option of removal with manual morcellation after laparoscopic hysterectomy or myomectomy. One study revealed that specimens up to 22 weeks in size can be placed in a large EndoCatch bag and morcellated extracorporeally by circumferentially coring with a scalpel.14
Contained power morcellation through a single port. Finally, the technique of contained EMM was recently described.15 This technique uses a large containment bag placed through a LESS incision with EMM being performed in an artificially created pneumoperitoneum. This technique isolates the specimen so that it can be morcellated without risk of exposing the patient to any malignant cells that might be unrecognized within the specimen.
Each of these techniques allows many patients to consider a minimally invasive option for their surgery. However, the ability to safely morcellate a very large uterus or myoma may be limited by visualization, and the experience of the surgeon is often critical in the successful performance of these procedures.16
Therefore, at Washington Universitywe have developed a technique using dual ports, with isolation of the uterus or myomas to improve visualization and prevent spillage of malignant tumor or dispersion of other benign tissue.
Dual-port EMM: Technique, tips, and tricks
Our technique of dual-port contained EMM allows the removal of large fibroids or uteri much larger than 20 weeks in size safely under direct visualization through a 15-mm incision. The technique uses:
- Karl Storz Rotocut tissue morcellator with spacers (FIGURE 1)
- 15-mm trocar
- 5-mm balloon trocar
- 20320-inch containment bag (FIGURE 2).
Containment bag placement
Once the specimen is free, we place it to the right or left side of the abdomen. The 15-mm trocar is placed through the umbilicus while visualizing from a lateral trocar site. We then fan-fold the containment bag and introduce it through the 15-mm trocar, keeping the bag oriented with the opening anterior (FIGURE 3). The bag is then grasped at the opening along the drawstring with an atraumatic grasper.
Tip: Care must be taken when introducing the bag in order to avoid tearing or making a small hole in it.
The leading edge is then introduced into the deepest part of the pelvis, and the remainder of the bag (left outside of the abdomen) is then fed cephalad into the abdomen.
Once the bag is completely in the abdomen, we orient the bag with the opening as wide as possible. This allows placement of a very large specimen. Once the specimen is within the containment bag, the drawstring is pulled tight and the mouth of the bag is removed through the 15-mm trocar site at the umbilicus.
The abdominal lateral gas port is opened to allow the intra-abdominal pneumoperitoneum to escape. A 5-mm trocar is placed into the bag through the opening at the umbilicus and the containment bag is insufflated with carbon dioxide and the insufflation pressure is set to 30 mm. The laparoscope placed through this trocar allows the artificial pneumoperitoneum being created to be observed (VIDEO).

Tip: The containment bag covers the entire abdominal cavity and should be fully distended. If it does not distend fully, a hole in the bag may be present and the bag must be replaced.
At this point, we place a balloon trocar at the lateral trocar site and into the bag under direct visualization. The balloon tip is inflated and pulled up tightly against the bag and abdominal wall (FIGURE 4). This allows a tight seal so there is no gas leak or spillage of the morcellated specimen. The laparoscope is placed through this trocar and the insufflation tubing is moved to this port.
Morcellator insertion
The morcellator is introduced through the umbilicus under direct visualization using the short morcellator blade in most instances. Spacers are used to set the length of the morcellator within the containment bag. The tip of the morcellator should be approximately 3 cm to 4 cm within the bag but well away from the retroperitoneum. Remember, any bag will be cut easily by the morcellator and should be thought of as peritoneum only and not a tough barrier. Serious injuries could otherwise develop.
At this point, place the patient flat or out of Trendelenburg position. Morcellation may now proceed.
Tip: Morcellation is best performed with the morcellator perpendicular to the abdomen under direct visualization using a 30° laparoscope to optimize the view. Morcellation in this position uses gravity to facilitate “peeling” of the specimen during morcellation and allows for faster removal.
Before removing the morcellator, inspect the containment bag for any large pieces that may have been dispersed during the morcellation process and remove them. Once there are only small fragments remaining, remove the morcellator, allowing the carbon dioxide to escape. Deflate the balloon tip on the trocar.
Now the containment bag with the remaining specimen may be removed through the umbilicus, while simultaneously removing the balloon-tip trocar from the bag.
A safe minimally invasive approach
This technique has allowed us to safely remove specimens larger than 1,500 g while keeping them in a contained environment with no spill of tissue within the abdomen.
Tracking and adaptation needed
The FDA safety communication has severely limited the practice of morcellation in the minimally invasive gynecologic surgical setting. Many hospitals around the country have reacted by placing significant restrictions on the use of EMM or banned it outright. This action may reverse the national trend of increasing rates of laparoscopic hysterectomy and force many practitioners to return to open surgery.
Currently, it is unclear what the true risk of tissue extraction is whether it is performed via EMM or manually. Large national databases including the BOLD database from the Surgical Review Corporation, as well as AAGL, must be utilized to track these cases and their outcomes to guide therapy. In the meantime, in order to continue to offer a minimally invasive approach to gynecologic surgery, new techniques and instrumentation in the operating room will need to be modified to adapt to these new guidelines. This is vital to maintain or even reduce the rates of open hysterectomy and associated morbidity while diminishing the potential risks of inadvertent benign as well as malignant tissue dispersion with tissue extraction.
Share your thoughts on this article! Send your Letter to the Editor to: [email protected]
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677. doi:10.1002/14651858.CD003677.pub4.
2. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
3. Wiser A, Holcroft CA, Tolandi T, Abenhaim HA. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10(2):117–122.
4. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
5. Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34.e1–e7.
6. U S Food and Drug Administration. Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids: Summary and key findings. Silver Spring, Maryland: FDA. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM393589.pdf. Published April 17, 2014. Accessed August 19, 2014.
7. Society of Gynecologic Oncology (SGO). SGO Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation. Published December 2013. Accessed March 1, 2014.
8. AAGL. Member Update: Disseminated leiomyosarcoma with power morcellation (Update #2). https://www.aagl.org/aaglnews/member-update-disseminated-leiomyosarcoma-with-power-morcellation-update-2/. Published July 11, 2014. Accessed August 19, 2014.
9. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery. http://www.acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Power_Morcellation_and_Occult_Malignancy_in_Gynecologic_Surgery. Published May 2014. Accessed August 19, 2014.
10. Sepilian V, Della Badia C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet Gynecol. 2003;102(5 Pt 2):1125–1127.
11. Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14(6):770–775.
12. Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007;14(2):156–160.
13. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21(3):486–491.
14. Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: an original technique that permits the safe and quick removal of a large uterus. Am J. Obstet Gynecol. 2011;204(6):566.e1–e2.
15. Shibley KA. Feasibilty of intra-abdominal tissue isolation and extraction with an artificially created pneumoperitoneum, at laparoscopy for gynecologic procedures. J Min Invasive Gynecol. 2012;19(6):S75.
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677. doi:10.1002/14651858.CD003677.pub4.
2. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
3. Wiser A, Holcroft CA, Tolandi T, Abenhaim HA. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10(2):117–122.
4. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
5. Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34.e1–e7.
6. U S Food and Drug Administration. Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids: Summary and key findings. Silver Spring, Maryland: FDA. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM393589.pdf. Published April 17, 2014. Accessed August 19, 2014.
7. Society of Gynecologic Oncology (SGO). SGO Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation. Published December 2013. Accessed March 1, 2014.
8. AAGL. Member Update: Disseminated leiomyosarcoma with power morcellation (Update #2). https://www.aagl.org/aaglnews/member-update-disseminated-leiomyosarcoma-with-power-morcellation-update-2/. Published July 11, 2014. Accessed August 19, 2014.
9. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery. http://www.acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Power_Morcellation_and_Occult_Malignancy_in_Gynecologic_Surgery. Published May 2014. Accessed August 19, 2014.
10. Sepilian V, Della Badia C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet Gynecol. 2003;102(5 Pt 2):1125–1127.
11. Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14(6):770–775.
12. Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007;14(2):156–160.
13. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21(3):486–491.
14. Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: an original technique that permits the safe and quick removal of a large uterus. Am J. Obstet Gynecol. 2011;204(6):566.e1–e2.
15. Shibley KA. Feasibilty of intra-abdominal tissue isolation and extraction with an artificially created pneumoperitoneum, at laparoscopy for gynecologic procedures. J Min Invasive Gynecol. 2012;19(6):S75.
Video: Laparoscopic dual-port contained power morcellation

Read Dr. Biest's and Dr Mutch's article, "Laparoscopic dual-port contained power morcellation: An offered solution" (September 2014)

Read Dr. Biest's and Dr Mutch's article, "Laparoscopic dual-port contained power morcellation: An offered solution" (September 2014)

Read Dr. Biest's and Dr Mutch's article, "Laparoscopic dual-port contained power morcellation: An offered solution" (September 2014)
Endometrial Cancer Update: The move toward personalized cancer care
Endometrial cancer is the most common malignancy of the female reproductive tract in the United States, and its incidence continues to rise, with an estimated 49,560 new cases predicted for 2013.1 If we are to successfully traverse the pathway from molecular cell genetics to the development of targeted therapies and personalized cancer care, we need to meet a few benchmarks:
- We need to enhance our understanding of the molecular changes that lead to endometrial cancer. Of particular interest are nonendometrioid tumors. Increased mortality from endometrial cancer appears to be related to the growing number of uterine papillary serous carcinomas and clear-cell cancers. Although these cancers constitute less than 10% of all endometrial cancers, they account for a disproportionately high number of recurrences and cancer-related deaths.2,3 Do recent studies validate the original classification of endometrial cancers as Type I (endometrioid) or Type II (serous and clear cell), or is there more heterogeneity than was originally thought? How do recent studies affect treatment options?
- We need to establish a genomic characterization of endometrial cancer to supplement clinical research data. The identification of novel mutations specific to each histologic type has the potential to improve adjuvant therapy. How close are we to performing a comprehensive genomic analysis of endometrial cancer?
- We need to develop new adjuvant treatment options for recurrent and advanced disease. When clinical symptoms of endometrial cancer are overt, as they often are, early diagnosis is possible, with a 5-year survival rate of 80% to 90%. The prognosis declines dramatically in women with advanced-stage disease or high-risk histologies, with a 5-year survival rate of 57% and 19% for Stage III and Stage IV disease, respectively.1 Adjuvant treatment options are limited in the setting of recurrent or advanced disease. Do any biologic agents increase survival?
In this article, we highlight the historical foundation and newest advances in the field of endometrial cancer, focusing on:
- histologic classification
- etiologic heterogeneity and molecular biology
- genome-guided clinical trials involving targeted therapy, with the ultimate goal of achieving individualized cancer care.
Should we reclassify endometrial cancers to reflect molecular characteristics of tumors?
Brinton LA, Felix AS, McMeekin DS, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129(2):277–284.
Cancer Genome Atlas Research Network, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
Uterine papillary serous carcinoma (UPSC) was first established as a distinct subtype of endometrial cancer in the early 1980s, when teams led by Lauchlan4 and Hendrickson5 described it as histologically similar to serous epithelial ovarian carcinoma. Shortly thereafter, Bokhman proposed two broad categories of endometrial carcinoma characterized by distinct microscopic appearance, epidemiology, and endocrine and metabolic functioning (TABLE, page 28).6
More recently, research has focused on expanding this histologic classification system to encompass molecular differences. Brinton and colleagues conducted a study within Gynecologic Oncology Group 210, investigating the etiologic heterogeneity of endometrial cancers by comparing risk factors for different histologies. They found that risk factors for aggressive endometrial cancers, including Grade 3 endometrioid and nonendometrioid tumors, appear to differ from those of lower-grade endometrioid carcinomas.
Details of the study by Brinton and colleagues
A total of 3,434 women were included, representing endometrioid (78%) and serous (9%) carcinomas. Grade 3 endometrioid tumors resembled Type II endometrial cancers more closely than did Grade 1–2 endometrioid tumors. Patients with Grade 3 endometrioid and Type II cancers were diagnosed at a significantly older age than patients with Grades 1–2 endometrioid cancers (eg, diagnosis of serous cancers: median age, 67.4 years; Grade 3 endometrioid cancers: median age, 61.9 years; Grade 1–2 endometrioid cancers: median age, 59.6 years). They also were more likely to be nonwhite than patients with Grades 1–2 endometrioid histology. Specifically, black patients were rarely diagnosed with Grades 1–2 endometrioid cancers (5% vs 9% for Grade 3 endometrioid cancers; 20% for serous cancers, 23% for carcinosarcomas, and 12% for clear-cell cancers).
After adjustments for age, enrollment year, and race, patients with Type II tumors (serous, carcinosarcomas, or clear-cell tumors) were much more likely to be multiparous or smokers or to have a history of breast cancer treated with tamoxifen, compared with women with Grade 1–2 endometrioid cancers. An adequately powered subanalysis of serous carcinomas and Grades 1–2 endometrioid cancers revealed that associations persisted between serous carcinomas and multiparity, body mass index, and a history of breast cancer treated with tamoxifen.
Related article: The future of the Pap test: Identifying endometrial and ovarian cancers (Janelle Yates, August 2013)
Overall, this study provides some of the strongest epidemiologic support we have that endometrial cancers are heterogeneous, with evidence to suggest that we might classify Grade 3 endometrioid carcinomas as Type II cancers. These findings paved the way for molecular profiling of endometrial cancers.
Details of the study by the Cancer Genome Atlas Research Network
The Cancer Genome Atlas Research Network recently published an integrated genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas using array- and sequencing-based technologies. The goal was to provide better insight into disease biology and tumor classification to help guide clinical trials and drug development, with the ultimate goal of achieving personalized cancer care.
The group classified endometrial cancers into four new categories:
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations (SCNA) low
- SCNA high.
Most endometrioid tumors had few SCNA or P53 mutations but frequent mutations in PTEN, CTNNB1, PIK3CA, ARID1A, and KRAS genes. Novel mutations also were discovered in the SWI/SNF chromatin remodeling complex gene ARID5B. About 10% of endometrioid tumors had markedly increased transversion mutations and newly identified mutations in POLE, a gene involved in nuclear DNA replication and repair.
As expected, serous tumors had significantly worse progression-free survival (PFS) than endometrioid tumors (P = .003, log-rank). A subset (25%) of high-grade endometrioid tumors had SCNAs and mutation spectra similar to those of uterine serous carcinomas, suggesting that patients with such tumors might benefit from treatment options that parallel those for serous tumors.
Other overlapping treatment paradigms existed between different organ systems. For example, some molecular features were similar in uterine serous carcinomas, basal-like breast carcinomas, and high-grade serous ovarian carcinomas. All three carcinomas displayed a high frequency of P53 mutations, very low frequency of PTEN mutations, similar focal SCNA patterns, and minimal DNA methylation changes. However, investigators also found several mutations that are unique to uterine serous carcinomas (eg, PIK3CA, FBXW7, PPP2R1A, and ARID1A), providing potential opportunities for targeted pharmacotherapy.
What this evidence means for practice
These studies highlight the etiologic heterogeneity of endometrial cancer. The histologic groundwork laid by Bokhman was not only correct but provided a foundation for molecular characterization of endometrial cancers to drive translational science into targeted therapeutics.
The similar molecular phenotypes of high-grade endometrioid carcinomas and serous endometrial carcinomas strengthens existing evidence that chemotherapy may be preferable to adjuvant radiotherapy for patients with highly mutated endometrioid cancers (eg, SCNA). Current chemotherapy regimens for serous endometrial cancers remain appropriate, given the compelling similarities between these cancers and serous ovarian and basal-like breast cancers. However, the identification of unique molecular features not shared by breast or ovarian cancer may expand standard options to include more targeted therapy.
Overall, this type of research, which encompasses proper histologic classification refined by genomics, has the potential to achieve personalized cancer care.
How we are achieving individualized cancer care: 3 genome-guided clinical trials
Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29(24):3278–3285.
Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265.
Alvarez EA, Brady WE, Walker JL, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129(1):22–27.
Women with locally recurrent, advanced, or metastatic endometrial cancer have limited options for treatment. Hormonal therapies have modest effects at best, with a median survival rate of 7 to 12 months.7–9 To address this lack of options, researchers have begun to focus on targeted therapies directed at molecular pathways of cellular proliferation. These therapies include but are not limited to inhibitors of:
- mammalian target of rapamycin (mTOR)
- human epidermal growth factor receptor 2
- epidermal growth factor receptor
- vascular endothelial growth factor (VEGF).
Several studies have produced promising findings in recent years. They involve investigations of temsirolimus and bevacizumab as single agents in two independent clinical trials, and a study of the drugs in combination in women with recurrent or persistent endometrial carcinoma.
mTOR inhibitors elicited a greater response in chemotherapy-naïve women
Phosphatase and tensin homolog (PTEN) is a tumor-suppressor gene more commonly associated with endometrioid endometrial cancers (26%–80%) than with Type II cancers.10 Loss of PTEN expression leads to deregulated signaling of the phosphatidylinositol-3 kinase (PI3K)/serine-threonine kinase (Akt)/mTOR pathway. Disruption of this pathway is thought to provide cells with a selective survival advantage by enhancing angiogenesis, protein translation, and cell-cycle progression.10
Temsirolimus is an mTOR inhibitor recently explored by Oza and colleagues. They performed a multicenter, Phase II study involving 62 patients with recurrent and/or metastatic endometrial cancer as part of the National Cancer Institute of Canada (NCIC) Clinical Trials Group. Patients were divided into two groups on the basis of their treatment history:
- chemotherapy-naïve women, with no more than one prior hormonal treatment (n = 33)
- chemotherapy-treated women (n = 27).
Temsirolimus was given weekly in 4-week cycles at an intravenous (IV) dose of 25 mg over 30 minutes.
The drug elicited a response regardless of the histologic type of cancer. That response was more pronounced in chemotherapy-naïve women and not limited to patients with PTEN loss. In the chemotherapy-naïve group, four women (14%) had a partial response, 20 (69%) had stable disease, and five (18%) had progressive disease. Median PFS was 7.33 months (95% confidence interval [CI], 3.61–9.86), compared with 3.25 months (95% CI, 1.97–3.84) in chemotherapy-treated women. Among chemotherapy-treated women, one (4%) had a partial response and 12 (48%) had stable disease.
An angiogenesis inhibitor alone was well tolerated
VEGF is the principal growth factor responsible for angiogenesis, initiating the process of neovascularization. Bevacizumab is a humanized monoclonal antibody that binds to circulating VEGF-A, stimulating clinical effects in multiple tumor types, including persistent or recurrent ovarian and cervical cancers.
Aghajanian and colleagues conducted a Phase II trial of single-agent bevacizumab in women with recurrent or persistent endometrial cancer to assess the drug’s activity and tolerability. Eligible patients had histologic confirmation by central pathology review, recurrent or persistent disease after one or two cytotoxic regimens, and a Gynecologic Oncology Group performance score of 2 or lower. They received IV bevacizumab 15 mg/kg every 3 weeks until the disease progressed or toxicity became prohibitive. Fifty-two women participated.
Seven women (13.5%) experienced a clinical response (one complete response and six partial responses), and 21 (40%) had PFS of at least 6 months. Median PFS and overall survival were 4.2 and 10.5 months, respectively.
Combined with temsirolimus, bevacizumab increased overall survival
Alvarez and colleagues conducted a Phase II trial of combination bevacizumab and temsirolimus in women with recurrent or persistent endometrial carcinoma. Forty-nine patients participated.
These women had undergone earlier treatment with one (82%) or two (18%) chemotherapy regimens and radiation (41%). Median PFS and overall survival were 5.6 and 16.9 months, respectively. Toxicity was significant, with 38.8% of women withdrawn from the study due to toxicity.
What this evidence means for practice
Given the findings regarding temsirolimus and bevacizumab as single agents, their use in combination was expected to produce a robust effect. The 6-month PFS rate is similar for all three regimens, but for overall survival, combination therapy had a 6.4-month advantage over bevacizumab alone.
Given the significant toxicity associated with the combination of bevacizumab and temsirolimus, further study is needed to develop biomarkers to predict response and toxicity. Other areas meriting future research include optimal timing of angiogenesis and mTOR pathway inhibitors, different combinations of agents, and the identification, through genomic analysis, of patient populations most likely to benefit from these drugs with minimal toxicity.
The studies presented here show promise in the area of genomics and represent the beginning of our movement toward personalized cancer care.
We want to hear from you! Tell us what you think.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- Boruta DM 2nd, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115(1):142–153.
- Ueda SM, Kapp DS, Cheung MK, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198(2):218.e1–e6.
- Lauchlan SC. Tubal (serous) carcinoma of the endometrium. Arch Pathol Lab Med. 1981;105(11):615–618.
- Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6(2):93–108.
- Bokhman JV. Two pathogenetic types of endometrial
carcinoma. Gynecol Oncol. 1983;15(1):10–17. - Bellone S, Shah HR, McKenney JK, Stone PJ, Santin AD. Recurrent endometrial carcinoma regression with the use of the aromatase inhibitor anastrozole. Am J Obstet Gynecol. 2008;199(3):e7–e10.
- Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee
RB. A phase II trial of anastrozole in advanced recurrent
or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78(2):212–216. - Thigpen T, Brady MF, Homesley HD, Soper JT, Bell J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2001;19(2):364–367.
- Kanamori Y, Kigawa J, Itamochi H, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001; 7(4):892–895.
Endometrial cancer is the most common malignancy of the female reproductive tract in the United States, and its incidence continues to rise, with an estimated 49,560 new cases predicted for 2013.1 If we are to successfully traverse the pathway from molecular cell genetics to the development of targeted therapies and personalized cancer care, we need to meet a few benchmarks:
- We need to enhance our understanding of the molecular changes that lead to endometrial cancer. Of particular interest are nonendometrioid tumors. Increased mortality from endometrial cancer appears to be related to the growing number of uterine papillary serous carcinomas and clear-cell cancers. Although these cancers constitute less than 10% of all endometrial cancers, they account for a disproportionately high number of recurrences and cancer-related deaths.2,3 Do recent studies validate the original classification of endometrial cancers as Type I (endometrioid) or Type II (serous and clear cell), or is there more heterogeneity than was originally thought? How do recent studies affect treatment options?
- We need to establish a genomic characterization of endometrial cancer to supplement clinical research data. The identification of novel mutations specific to each histologic type has the potential to improve adjuvant therapy. How close are we to performing a comprehensive genomic analysis of endometrial cancer?
- We need to develop new adjuvant treatment options for recurrent and advanced disease. When clinical symptoms of endometrial cancer are overt, as they often are, early diagnosis is possible, with a 5-year survival rate of 80% to 90%. The prognosis declines dramatically in women with advanced-stage disease or high-risk histologies, with a 5-year survival rate of 57% and 19% for Stage III and Stage IV disease, respectively.1 Adjuvant treatment options are limited in the setting of recurrent or advanced disease. Do any biologic agents increase survival?
In this article, we highlight the historical foundation and newest advances in the field of endometrial cancer, focusing on:
- histologic classification
- etiologic heterogeneity and molecular biology
- genome-guided clinical trials involving targeted therapy, with the ultimate goal of achieving individualized cancer care.
Should we reclassify endometrial cancers to reflect molecular characteristics of tumors?
Brinton LA, Felix AS, McMeekin DS, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129(2):277–284.
Cancer Genome Atlas Research Network, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
Uterine papillary serous carcinoma (UPSC) was first established as a distinct subtype of endometrial cancer in the early 1980s, when teams led by Lauchlan4 and Hendrickson5 described it as histologically similar to serous epithelial ovarian carcinoma. Shortly thereafter, Bokhman proposed two broad categories of endometrial carcinoma characterized by distinct microscopic appearance, epidemiology, and endocrine and metabolic functioning (TABLE, page 28).6
More recently, research has focused on expanding this histologic classification system to encompass molecular differences. Brinton and colleagues conducted a study within Gynecologic Oncology Group 210, investigating the etiologic heterogeneity of endometrial cancers by comparing risk factors for different histologies. They found that risk factors for aggressive endometrial cancers, including Grade 3 endometrioid and nonendometrioid tumors, appear to differ from those of lower-grade endometrioid carcinomas.
Details of the study by Brinton and colleagues
A total of 3,434 women were included, representing endometrioid (78%) and serous (9%) carcinomas. Grade 3 endometrioid tumors resembled Type II endometrial cancers more closely than did Grade 1–2 endometrioid tumors. Patients with Grade 3 endometrioid and Type II cancers were diagnosed at a significantly older age than patients with Grades 1–2 endometrioid cancers (eg, diagnosis of serous cancers: median age, 67.4 years; Grade 3 endometrioid cancers: median age, 61.9 years; Grade 1–2 endometrioid cancers: median age, 59.6 years). They also were more likely to be nonwhite than patients with Grades 1–2 endometrioid histology. Specifically, black patients were rarely diagnosed with Grades 1–2 endometrioid cancers (5% vs 9% for Grade 3 endometrioid cancers; 20% for serous cancers, 23% for carcinosarcomas, and 12% for clear-cell cancers).
After adjustments for age, enrollment year, and race, patients with Type II tumors (serous, carcinosarcomas, or clear-cell tumors) were much more likely to be multiparous or smokers or to have a history of breast cancer treated with tamoxifen, compared with women with Grade 1–2 endometrioid cancers. An adequately powered subanalysis of serous carcinomas and Grades 1–2 endometrioid cancers revealed that associations persisted between serous carcinomas and multiparity, body mass index, and a history of breast cancer treated with tamoxifen.
Related article: The future of the Pap test: Identifying endometrial and ovarian cancers (Janelle Yates, August 2013)
Overall, this study provides some of the strongest epidemiologic support we have that endometrial cancers are heterogeneous, with evidence to suggest that we might classify Grade 3 endometrioid carcinomas as Type II cancers. These findings paved the way for molecular profiling of endometrial cancers.
Details of the study by the Cancer Genome Atlas Research Network
The Cancer Genome Atlas Research Network recently published an integrated genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas using array- and sequencing-based technologies. The goal was to provide better insight into disease biology and tumor classification to help guide clinical trials and drug development, with the ultimate goal of achieving personalized cancer care.
The group classified endometrial cancers into four new categories:
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations (SCNA) low
- SCNA high.
Most endometrioid tumors had few SCNA or P53 mutations but frequent mutations in PTEN, CTNNB1, PIK3CA, ARID1A, and KRAS genes. Novel mutations also were discovered in the SWI/SNF chromatin remodeling complex gene ARID5B. About 10% of endometrioid tumors had markedly increased transversion mutations and newly identified mutations in POLE, a gene involved in nuclear DNA replication and repair.
As expected, serous tumors had significantly worse progression-free survival (PFS) than endometrioid tumors (P = .003, log-rank). A subset (25%) of high-grade endometrioid tumors had SCNAs and mutation spectra similar to those of uterine serous carcinomas, suggesting that patients with such tumors might benefit from treatment options that parallel those for serous tumors.
Other overlapping treatment paradigms existed between different organ systems. For example, some molecular features were similar in uterine serous carcinomas, basal-like breast carcinomas, and high-grade serous ovarian carcinomas. All three carcinomas displayed a high frequency of P53 mutations, very low frequency of PTEN mutations, similar focal SCNA patterns, and minimal DNA methylation changes. However, investigators also found several mutations that are unique to uterine serous carcinomas (eg, PIK3CA, FBXW7, PPP2R1A, and ARID1A), providing potential opportunities for targeted pharmacotherapy.
What this evidence means for practice
These studies highlight the etiologic heterogeneity of endometrial cancer. The histologic groundwork laid by Bokhman was not only correct but provided a foundation for molecular characterization of endometrial cancers to drive translational science into targeted therapeutics.
The similar molecular phenotypes of high-grade endometrioid carcinomas and serous endometrial carcinomas strengthens existing evidence that chemotherapy may be preferable to adjuvant radiotherapy for patients with highly mutated endometrioid cancers (eg, SCNA). Current chemotherapy regimens for serous endometrial cancers remain appropriate, given the compelling similarities between these cancers and serous ovarian and basal-like breast cancers. However, the identification of unique molecular features not shared by breast or ovarian cancer may expand standard options to include more targeted therapy.
Overall, this type of research, which encompasses proper histologic classification refined by genomics, has the potential to achieve personalized cancer care.
How we are achieving individualized cancer care: 3 genome-guided clinical trials
Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29(24):3278–3285.
Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265.
Alvarez EA, Brady WE, Walker JL, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129(1):22–27.
Women with locally recurrent, advanced, or metastatic endometrial cancer have limited options for treatment. Hormonal therapies have modest effects at best, with a median survival rate of 7 to 12 months.7–9 To address this lack of options, researchers have begun to focus on targeted therapies directed at molecular pathways of cellular proliferation. These therapies include but are not limited to inhibitors of:
- mammalian target of rapamycin (mTOR)
- human epidermal growth factor receptor 2
- epidermal growth factor receptor
- vascular endothelial growth factor (VEGF).
Several studies have produced promising findings in recent years. They involve investigations of temsirolimus and bevacizumab as single agents in two independent clinical trials, and a study of the drugs in combination in women with recurrent or persistent endometrial carcinoma.
mTOR inhibitors elicited a greater response in chemotherapy-naïve women
Phosphatase and tensin homolog (PTEN) is a tumor-suppressor gene more commonly associated with endometrioid endometrial cancers (26%–80%) than with Type II cancers.10 Loss of PTEN expression leads to deregulated signaling of the phosphatidylinositol-3 kinase (PI3K)/serine-threonine kinase (Akt)/mTOR pathway. Disruption of this pathway is thought to provide cells with a selective survival advantage by enhancing angiogenesis, protein translation, and cell-cycle progression.10
Temsirolimus is an mTOR inhibitor recently explored by Oza and colleagues. They performed a multicenter, Phase II study involving 62 patients with recurrent and/or metastatic endometrial cancer as part of the National Cancer Institute of Canada (NCIC) Clinical Trials Group. Patients were divided into two groups on the basis of their treatment history:
- chemotherapy-naïve women, with no more than one prior hormonal treatment (n = 33)
- chemotherapy-treated women (n = 27).
Temsirolimus was given weekly in 4-week cycles at an intravenous (IV) dose of 25 mg over 30 minutes.
The drug elicited a response regardless of the histologic type of cancer. That response was more pronounced in chemotherapy-naïve women and not limited to patients with PTEN loss. In the chemotherapy-naïve group, four women (14%) had a partial response, 20 (69%) had stable disease, and five (18%) had progressive disease. Median PFS was 7.33 months (95% confidence interval [CI], 3.61–9.86), compared with 3.25 months (95% CI, 1.97–3.84) in chemotherapy-treated women. Among chemotherapy-treated women, one (4%) had a partial response and 12 (48%) had stable disease.
An angiogenesis inhibitor alone was well tolerated
VEGF is the principal growth factor responsible for angiogenesis, initiating the process of neovascularization. Bevacizumab is a humanized monoclonal antibody that binds to circulating VEGF-A, stimulating clinical effects in multiple tumor types, including persistent or recurrent ovarian and cervical cancers.
Aghajanian and colleagues conducted a Phase II trial of single-agent bevacizumab in women with recurrent or persistent endometrial cancer to assess the drug’s activity and tolerability. Eligible patients had histologic confirmation by central pathology review, recurrent or persistent disease after one or two cytotoxic regimens, and a Gynecologic Oncology Group performance score of 2 or lower. They received IV bevacizumab 15 mg/kg every 3 weeks until the disease progressed or toxicity became prohibitive. Fifty-two women participated.
Seven women (13.5%) experienced a clinical response (one complete response and six partial responses), and 21 (40%) had PFS of at least 6 months. Median PFS and overall survival were 4.2 and 10.5 months, respectively.
Combined with temsirolimus, bevacizumab increased overall survival
Alvarez and colleagues conducted a Phase II trial of combination bevacizumab and temsirolimus in women with recurrent or persistent endometrial carcinoma. Forty-nine patients participated.
These women had undergone earlier treatment with one (82%) or two (18%) chemotherapy regimens and radiation (41%). Median PFS and overall survival were 5.6 and 16.9 months, respectively. Toxicity was significant, with 38.8% of women withdrawn from the study due to toxicity.
What this evidence means for practice
Given the findings regarding temsirolimus and bevacizumab as single agents, their use in combination was expected to produce a robust effect. The 6-month PFS rate is similar for all three regimens, but for overall survival, combination therapy had a 6.4-month advantage over bevacizumab alone.
Given the significant toxicity associated with the combination of bevacizumab and temsirolimus, further study is needed to develop biomarkers to predict response and toxicity. Other areas meriting future research include optimal timing of angiogenesis and mTOR pathway inhibitors, different combinations of agents, and the identification, through genomic analysis, of patient populations most likely to benefit from these drugs with minimal toxicity.
The studies presented here show promise in the area of genomics and represent the beginning of our movement toward personalized cancer care.
We want to hear from you! Tell us what you think.
Endometrial cancer is the most common malignancy of the female reproductive tract in the United States, and its incidence continues to rise, with an estimated 49,560 new cases predicted for 2013.1 If we are to successfully traverse the pathway from molecular cell genetics to the development of targeted therapies and personalized cancer care, we need to meet a few benchmarks:
- We need to enhance our understanding of the molecular changes that lead to endometrial cancer. Of particular interest are nonendometrioid tumors. Increased mortality from endometrial cancer appears to be related to the growing number of uterine papillary serous carcinomas and clear-cell cancers. Although these cancers constitute less than 10% of all endometrial cancers, they account for a disproportionately high number of recurrences and cancer-related deaths.2,3 Do recent studies validate the original classification of endometrial cancers as Type I (endometrioid) or Type II (serous and clear cell), or is there more heterogeneity than was originally thought? How do recent studies affect treatment options?
- We need to establish a genomic characterization of endometrial cancer to supplement clinical research data. The identification of novel mutations specific to each histologic type has the potential to improve adjuvant therapy. How close are we to performing a comprehensive genomic analysis of endometrial cancer?
- We need to develop new adjuvant treatment options for recurrent and advanced disease. When clinical symptoms of endometrial cancer are overt, as they often are, early diagnosis is possible, with a 5-year survival rate of 80% to 90%. The prognosis declines dramatically in women with advanced-stage disease or high-risk histologies, with a 5-year survival rate of 57% and 19% for Stage III and Stage IV disease, respectively.1 Adjuvant treatment options are limited in the setting of recurrent or advanced disease. Do any biologic agents increase survival?
In this article, we highlight the historical foundation and newest advances in the field of endometrial cancer, focusing on:
- histologic classification
- etiologic heterogeneity and molecular biology
- genome-guided clinical trials involving targeted therapy, with the ultimate goal of achieving individualized cancer care.
Should we reclassify endometrial cancers to reflect molecular characteristics of tumors?
Brinton LA, Felix AS, McMeekin DS, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129(2):277–284.
Cancer Genome Atlas Research Network, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
Uterine papillary serous carcinoma (UPSC) was first established as a distinct subtype of endometrial cancer in the early 1980s, when teams led by Lauchlan4 and Hendrickson5 described it as histologically similar to serous epithelial ovarian carcinoma. Shortly thereafter, Bokhman proposed two broad categories of endometrial carcinoma characterized by distinct microscopic appearance, epidemiology, and endocrine and metabolic functioning (TABLE, page 28).6
More recently, research has focused on expanding this histologic classification system to encompass molecular differences. Brinton and colleagues conducted a study within Gynecologic Oncology Group 210, investigating the etiologic heterogeneity of endometrial cancers by comparing risk factors for different histologies. They found that risk factors for aggressive endometrial cancers, including Grade 3 endometrioid and nonendometrioid tumors, appear to differ from those of lower-grade endometrioid carcinomas.
Details of the study by Brinton and colleagues
A total of 3,434 women were included, representing endometrioid (78%) and serous (9%) carcinomas. Grade 3 endometrioid tumors resembled Type II endometrial cancers more closely than did Grade 1–2 endometrioid tumors. Patients with Grade 3 endometrioid and Type II cancers were diagnosed at a significantly older age than patients with Grades 1–2 endometrioid cancers (eg, diagnosis of serous cancers: median age, 67.4 years; Grade 3 endometrioid cancers: median age, 61.9 years; Grade 1–2 endometrioid cancers: median age, 59.6 years). They also were more likely to be nonwhite than patients with Grades 1–2 endometrioid histology. Specifically, black patients were rarely diagnosed with Grades 1–2 endometrioid cancers (5% vs 9% for Grade 3 endometrioid cancers; 20% for serous cancers, 23% for carcinosarcomas, and 12% for clear-cell cancers).
After adjustments for age, enrollment year, and race, patients with Type II tumors (serous, carcinosarcomas, or clear-cell tumors) were much more likely to be multiparous or smokers or to have a history of breast cancer treated with tamoxifen, compared with women with Grade 1–2 endometrioid cancers. An adequately powered subanalysis of serous carcinomas and Grades 1–2 endometrioid cancers revealed that associations persisted between serous carcinomas and multiparity, body mass index, and a history of breast cancer treated with tamoxifen.
Related article: The future of the Pap test: Identifying endometrial and ovarian cancers (Janelle Yates, August 2013)
Overall, this study provides some of the strongest epidemiologic support we have that endometrial cancers are heterogeneous, with evidence to suggest that we might classify Grade 3 endometrioid carcinomas as Type II cancers. These findings paved the way for molecular profiling of endometrial cancers.
Details of the study by the Cancer Genome Atlas Research Network
The Cancer Genome Atlas Research Network recently published an integrated genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas using array- and sequencing-based technologies. The goal was to provide better insight into disease biology and tumor classification to help guide clinical trials and drug development, with the ultimate goal of achieving personalized cancer care.
The group classified endometrial cancers into four new categories:
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations (SCNA) low
- SCNA high.
Most endometrioid tumors had few SCNA or P53 mutations but frequent mutations in PTEN, CTNNB1, PIK3CA, ARID1A, and KRAS genes. Novel mutations also were discovered in the SWI/SNF chromatin remodeling complex gene ARID5B. About 10% of endometrioid tumors had markedly increased transversion mutations and newly identified mutations in POLE, a gene involved in nuclear DNA replication and repair.
As expected, serous tumors had significantly worse progression-free survival (PFS) than endometrioid tumors (P = .003, log-rank). A subset (25%) of high-grade endometrioid tumors had SCNAs and mutation spectra similar to those of uterine serous carcinomas, suggesting that patients with such tumors might benefit from treatment options that parallel those for serous tumors.
Other overlapping treatment paradigms existed between different organ systems. For example, some molecular features were similar in uterine serous carcinomas, basal-like breast carcinomas, and high-grade serous ovarian carcinomas. All three carcinomas displayed a high frequency of P53 mutations, very low frequency of PTEN mutations, similar focal SCNA patterns, and minimal DNA methylation changes. However, investigators also found several mutations that are unique to uterine serous carcinomas (eg, PIK3CA, FBXW7, PPP2R1A, and ARID1A), providing potential opportunities for targeted pharmacotherapy.
What this evidence means for practice
These studies highlight the etiologic heterogeneity of endometrial cancer. The histologic groundwork laid by Bokhman was not only correct but provided a foundation for molecular characterization of endometrial cancers to drive translational science into targeted therapeutics.
The similar molecular phenotypes of high-grade endometrioid carcinomas and serous endometrial carcinomas strengthens existing evidence that chemotherapy may be preferable to adjuvant radiotherapy for patients with highly mutated endometrioid cancers (eg, SCNA). Current chemotherapy regimens for serous endometrial cancers remain appropriate, given the compelling similarities between these cancers and serous ovarian and basal-like breast cancers. However, the identification of unique molecular features not shared by breast or ovarian cancer may expand standard options to include more targeted therapy.
Overall, this type of research, which encompasses proper histologic classification refined by genomics, has the potential to achieve personalized cancer care.
How we are achieving individualized cancer care: 3 genome-guided clinical trials
Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29(24):3278–3285.
Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265.
Alvarez EA, Brady WE, Walker JL, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129(1):22–27.
Women with locally recurrent, advanced, or metastatic endometrial cancer have limited options for treatment. Hormonal therapies have modest effects at best, with a median survival rate of 7 to 12 months.7–9 To address this lack of options, researchers have begun to focus on targeted therapies directed at molecular pathways of cellular proliferation. These therapies include but are not limited to inhibitors of:
- mammalian target of rapamycin (mTOR)
- human epidermal growth factor receptor 2
- epidermal growth factor receptor
- vascular endothelial growth factor (VEGF).
Several studies have produced promising findings in recent years. They involve investigations of temsirolimus and bevacizumab as single agents in two independent clinical trials, and a study of the drugs in combination in women with recurrent or persistent endometrial carcinoma.
mTOR inhibitors elicited a greater response in chemotherapy-naïve women
Phosphatase and tensin homolog (PTEN) is a tumor-suppressor gene more commonly associated with endometrioid endometrial cancers (26%–80%) than with Type II cancers.10 Loss of PTEN expression leads to deregulated signaling of the phosphatidylinositol-3 kinase (PI3K)/serine-threonine kinase (Akt)/mTOR pathway. Disruption of this pathway is thought to provide cells with a selective survival advantage by enhancing angiogenesis, protein translation, and cell-cycle progression.10
Temsirolimus is an mTOR inhibitor recently explored by Oza and colleagues. They performed a multicenter, Phase II study involving 62 patients with recurrent and/or metastatic endometrial cancer as part of the National Cancer Institute of Canada (NCIC) Clinical Trials Group. Patients were divided into two groups on the basis of their treatment history:
- chemotherapy-naïve women, with no more than one prior hormonal treatment (n = 33)
- chemotherapy-treated women (n = 27).
Temsirolimus was given weekly in 4-week cycles at an intravenous (IV) dose of 25 mg over 30 minutes.
The drug elicited a response regardless of the histologic type of cancer. That response was more pronounced in chemotherapy-naïve women and not limited to patients with PTEN loss. In the chemotherapy-naïve group, four women (14%) had a partial response, 20 (69%) had stable disease, and five (18%) had progressive disease. Median PFS was 7.33 months (95% confidence interval [CI], 3.61–9.86), compared with 3.25 months (95% CI, 1.97–3.84) in chemotherapy-treated women. Among chemotherapy-treated women, one (4%) had a partial response and 12 (48%) had stable disease.
An angiogenesis inhibitor alone was well tolerated
VEGF is the principal growth factor responsible for angiogenesis, initiating the process of neovascularization. Bevacizumab is a humanized monoclonal antibody that binds to circulating VEGF-A, stimulating clinical effects in multiple tumor types, including persistent or recurrent ovarian and cervical cancers.
Aghajanian and colleagues conducted a Phase II trial of single-agent bevacizumab in women with recurrent or persistent endometrial cancer to assess the drug’s activity and tolerability. Eligible patients had histologic confirmation by central pathology review, recurrent or persistent disease after one or two cytotoxic regimens, and a Gynecologic Oncology Group performance score of 2 or lower. They received IV bevacizumab 15 mg/kg every 3 weeks until the disease progressed or toxicity became prohibitive. Fifty-two women participated.
Seven women (13.5%) experienced a clinical response (one complete response and six partial responses), and 21 (40%) had PFS of at least 6 months. Median PFS and overall survival were 4.2 and 10.5 months, respectively.
Combined with temsirolimus, bevacizumab increased overall survival
Alvarez and colleagues conducted a Phase II trial of combination bevacizumab and temsirolimus in women with recurrent or persistent endometrial carcinoma. Forty-nine patients participated.
These women had undergone earlier treatment with one (82%) or two (18%) chemotherapy regimens and radiation (41%). Median PFS and overall survival were 5.6 and 16.9 months, respectively. Toxicity was significant, with 38.8% of women withdrawn from the study due to toxicity.
What this evidence means for practice
Given the findings regarding temsirolimus and bevacizumab as single agents, their use in combination was expected to produce a robust effect. The 6-month PFS rate is similar for all three regimens, but for overall survival, combination therapy had a 6.4-month advantage over bevacizumab alone.
Given the significant toxicity associated with the combination of bevacizumab and temsirolimus, further study is needed to develop biomarkers to predict response and toxicity. Other areas meriting future research include optimal timing of angiogenesis and mTOR pathway inhibitors, different combinations of agents, and the identification, through genomic analysis, of patient populations most likely to benefit from these drugs with minimal toxicity.
The studies presented here show promise in the area of genomics and represent the beginning of our movement toward personalized cancer care.
We want to hear from you! Tell us what you think.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- Boruta DM 2nd, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115(1):142–153.
- Ueda SM, Kapp DS, Cheung MK, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198(2):218.e1–e6.
- Lauchlan SC. Tubal (serous) carcinoma of the endometrium. Arch Pathol Lab Med. 1981;105(11):615–618.
- Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6(2):93–108.
- Bokhman JV. Two pathogenetic types of endometrial
carcinoma. Gynecol Oncol. 1983;15(1):10–17. - Bellone S, Shah HR, McKenney JK, Stone PJ, Santin AD. Recurrent endometrial carcinoma regression with the use of the aromatase inhibitor anastrozole. Am J Obstet Gynecol. 2008;199(3):e7–e10.
- Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee
RB. A phase II trial of anastrozole in advanced recurrent
or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78(2):212–216. - Thigpen T, Brady MF, Homesley HD, Soper JT, Bell J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2001;19(2):364–367.
- Kanamori Y, Kigawa J, Itamochi H, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001; 7(4):892–895.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- Boruta DM 2nd, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115(1):142–153.
- Ueda SM, Kapp DS, Cheung MK, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198(2):218.e1–e6.
- Lauchlan SC. Tubal (serous) carcinoma of the endometrium. Arch Pathol Lab Med. 1981;105(11):615–618.
- Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6(2):93–108.
- Bokhman JV. Two pathogenetic types of endometrial
carcinoma. Gynecol Oncol. 1983;15(1):10–17. - Bellone S, Shah HR, McKenney JK, Stone PJ, Santin AD. Recurrent endometrial carcinoma regression with the use of the aromatase inhibitor anastrozole. Am J Obstet Gynecol. 2008;199(3):e7–e10.
- Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee
RB. A phase II trial of anastrozole in advanced recurrent
or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78(2):212–216. - Thigpen T, Brady MF, Homesley HD, Soper JT, Bell J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2001;19(2):364–367.
- Kanamori Y, Kigawa J, Itamochi H, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001; 7(4):892–895.
UPDATE ON OVARIAN CANCER
- Update on ovarian cancer screening
David G. Mutch, MD; Nora Kizer, MD (July 2010)
A majority of ovarian cancers are diagnosed at an advanced stage, requiring extensive surgical cytoreductive procedures.1 Because the presence of residual macroscopic disease correlates highly with decreased survival,2 these procedures can be lengthy, complicated, and risky for the patient. Many patients who undergo cytoreduction will be left with a suboptimal result despite surgery.
Better identification and improved treatment of patients who are at high risk of a suboptimal result are clearly needed. One treatment option is neoadjuvant chemotherapy, the administration of chemotherapy prior to the main treatment. Although early data suggested that it was associated with worse outcomes, recent studies have yielded new information:
- Neoadjuvant chemotherapy followed by interval debulking surgery is not inferior to primary debulking surgery followed by chemotherapy for patients who have bulky stage III or IV ovarian cancer
- In patients who have advanced ovarian cancer, neoadjuvant chemotherapy followed by surgical cytoreduction is associated with improved perioperative outcomes
- Postoperative intraperitoneal chemotherapy after neoadjuvant chemotherapy has not yet proved to be associated with improved survival.
Several questions prompted by these findings include:
- Will neoadjuvant chemotherapy improve surgical outcomes in patients who have advanced ovarian cancer and, thus, improve survival?
- Is neoadjuvant chemotherapy a better strategy for all patients?
- Will neoadjuvant chemotherapy reduce the surgical effort necessary to achieve an optimal result?
- What is the role of intraperitoneal chemotherapy in patients who undergo neoadjuvant chemotherapy?
Further national (or international) data are needed to confirm a survival advantage for patients who receive neoadjuvant chemotherapy, compared with those who undergo primary surgery before the administration of chemotherapy.
Neoadjuvant chemotherapy is an acceptable alternative to primary surgical cytoreduction
Vergote I, Tropé CG, Amant F, et al; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
Historically, the standard of care in ovarian cancer treatment has been surgical cytoreduction followed by chemotherapy.3-6 However, data from prospective randomized trials to support this practice are limited. Neoadjuvant chemotherapy is an alternative strategy that has been explored as a way to improve outcomes from interval surgical debulking in patients who have ovarian cancer in whom suboptimal cytoreduction is otherwise expected. Vergote and coworkers attempted to determine which strategy is better through a randomized trial of 632 patients.
Participants had to have biopsy-proven stage IIIc or IV ovarian, fallopian tube, or primary peritoneal cancer. The two treatment arms were:
- primary debulking surgery followed by at least 6 cycles of platinum-based chemotherapy
- 3 cycles of platinum-based neoadjuvant chemotherapy followed by interval debulking surgery in responders and those who had stable disease. These patients then received an additional 3 cycles of platinum-based chemotherapy post-operatively.
All surgical procedures were completed by qualified gynecologic oncologists, and all patients were evaluated for eligibility before randomization, with no additional selection criteria.
Postoperative death occurred in 2.5% of patients in the primary-surgery group, compared with 0.7% of patients in the neoadjuvant-chemotherapy group. Grade 3 or 4 hemorrhage occurred in 7.4% of patients after primary debulking, compared with 4.1% of patients after interval debulking. Patients who received neoadjuvant chemotherapy experienced a lower rate of infection (1.7% versus 8.1%) and venous complications (0% versus 2.6%).
Overall and progression-free survival rates were similar between the two groups. After multivariate analysis, the strongest predictors of survival were absence of residual disease after surgery (P<.001), small tumor size before randomization (P=.001), and endometrioid histology (P=.001)
Neoadjuvant chemotherapy is a preferred treatment strategy for patients who are expected to have a suboptimal result after surgery. Because neoadjuvant chemotherapy has a survival outcome similar to that of primary surgery followed by chemotherapy, it may be considered for all patients who have bulky stage IIIc or IV disease.
Although neoadjuvant chemotherapy improves the rate of optimal surgical cytoreduction, data are lacking to demonstrate that this improvement boosts survival.
Administration of neoadjuvant chemotherapy in these patients may improve perioperative morbidity and mortality, although no formal analysis was conducted in this study.
Neoadjuvant chemotherapy improves perioperative outcomes
Milam MR, Tao X, Coleman RL, et al. Neoadjuvant chemotherapy is associated with prolonged primary treatment intervals in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21(1):66–71.
Milam and coworkers investigated chemotherapy-associated morbidity and timing in two groups of patients who had advanced epithelial ovarian cancer:
- those undergoing neoadjuvant chemotherapy followed by maximal cytoreductive surgery
- those undergoing primary surgery followed by chemotherapy.
Their retrospective study involved 263 consecutive patients who were treated at MD Anderson Cancer Center from 1993 to 2005. In this cohort, 47 women (18%) received neoadjuvant chemotherapy. These patients experienced less blood loss (400 mL versus 750 mL) and a shorter hospital stay (6 versus 8 days). Time to the initiation of chemotherapy from the date of diagnosis did not differ between groups, and the amount of residual disease and rate of survival were also similar between arms. However, patients who received neoadjuvant chemotherapy underwent more cycles of chemotherapy over a longer treatment period.
Although neoadjuvant chemotherapy does not appear to offer a survival advantage, it is equivalent to primary surgery followed by adjuvant chemotherapy and may be associated with improved perioperative outcomes.
The results of the trial by Vergote and colleagues (page 25), should discourage oncologists from prescribing more than 6 cycles of chemotherapy in the neoadjuvant setting; patients from their study in the neoadjuvant group received a total of 6 cycles and had survival outcomes equivalent to those of women in the primary surgery group.
In the pipeline: Data on intraperitoneal chemotherapy after neoadjuvant chemotherapy
Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol. 2011;121(3):451–454.
Although several studies have demonstrated that intraperitoneal (IP) chemotherapy provides a survival advantage, compared with intravenous (IV) chemotherapy, after primary surgical debulking, it remains unclear whether IP chemotherapy would provide a similar superior survival outcome following neoadjuvant chemotherapy (FIGURE).
Intraperitoneal chemotherapy: How efficacious?
The jury is still out on whether intraperitoneal chemotherapy improves survival after neoadjuvant chemotherapy and interval debulking in stages III and IV ovarian cancer.The authors of this paper attempted to answer this question through a retrospective review of 71 patients. All patients were treated with neoadjuvant chemotherapy followed by interval debulking and either IP or IV chemotherapy. Overall, 17 patients (24%) received IP chemotherapy, and 54 patients (76%) received IV chemotherapy. The median number of cycles given prior to and after surgery was the same for both groups (3 for both neoadjuvant chemotherapy and chemotherapy following surgery).
Although patients who received IP chemotherapy had a higher overall response rate (82% versus 67%), there were no differences between groups in terms of progression-free (P=.42) and overall survival (P=.72).
One important limitation of this study was its small sample size and lack of statistical power. In addition, more patients in the IP group had macroscopic residual disease than in the IV group (71% versus 52%; P=.17).
A phase II/III study is under way to evaluate the use of IP chemotherapy following neoadjuvant chemotherapy in ovarian cancer patients.7 The two-stage randomized trial will compare IV chemotherapy with platinum-based IP chemotherapy in women who have undergone optimal surgical debulking (>1 cm) after 3 to 4 cycles of platinum-based neoadjuvant chemotherapy. This study is led by the US National Cancer Institute in collaboration with the Society of Gynecologic Oncologists of Canada, the UK National Cancer Research Institute, the Spanish Ovarian Cancer Research Group, and the US Southwest Oncology Group.
Data are limited on the use of intraperitoneal (IP) chemotherapy following neoadjuvant chemotherapy and interval surgical cytoreduction. We await the results of larger prospective studies to definitively determine whether there is a role for IP chemotherapy in this setting. For now, patients who receive neoadjuvant chemotherapy are limited to IV chemotherapy following surgery.
We want to hear from you! Tell us what you think.
1. Howlader N, Noone AM, Krapcho M, et all. eds. SEER Cancer Statistics Review 1975-2008. National Cancer Institute. http://seer.cancer.gov/csr/1975_2008. Published April 15, 2011. Accessed June 10, 2011.
2. du Bois A, Ruess A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials; by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234-1244.
3. Meigs JV. Tumors of the pelvic organs. New York: Macmillan: 1934.
4. Aure JC, Hoeg K, Kolstad P. Clinical and histologic studies of ovarian carcinoma. Long-term follow-up of 990 cases. Obstet Gynecol. 1971;37(1):1-9.
5. Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131-142.
6. du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(suppl 8):viii7-viii12.
7. Mackay HJ, Provencheur D, Heywood M, et al. Phase II/III study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21. Curr Oncol. 2011;18(2):84-90.
- Update on ovarian cancer screening
David G. Mutch, MD; Nora Kizer, MD (July 2010)
A majority of ovarian cancers are diagnosed at an advanced stage, requiring extensive surgical cytoreductive procedures.1 Because the presence of residual macroscopic disease correlates highly with decreased survival,2 these procedures can be lengthy, complicated, and risky for the patient. Many patients who undergo cytoreduction will be left with a suboptimal result despite surgery.
Better identification and improved treatment of patients who are at high risk of a suboptimal result are clearly needed. One treatment option is neoadjuvant chemotherapy, the administration of chemotherapy prior to the main treatment. Although early data suggested that it was associated with worse outcomes, recent studies have yielded new information:
- Neoadjuvant chemotherapy followed by interval debulking surgery is not inferior to primary debulking surgery followed by chemotherapy for patients who have bulky stage III or IV ovarian cancer
- In patients who have advanced ovarian cancer, neoadjuvant chemotherapy followed by surgical cytoreduction is associated with improved perioperative outcomes
- Postoperative intraperitoneal chemotherapy after neoadjuvant chemotherapy has not yet proved to be associated with improved survival.
Several questions prompted by these findings include:
- Will neoadjuvant chemotherapy improve surgical outcomes in patients who have advanced ovarian cancer and, thus, improve survival?
- Is neoadjuvant chemotherapy a better strategy for all patients?
- Will neoadjuvant chemotherapy reduce the surgical effort necessary to achieve an optimal result?
- What is the role of intraperitoneal chemotherapy in patients who undergo neoadjuvant chemotherapy?
Further national (or international) data are needed to confirm a survival advantage for patients who receive neoadjuvant chemotherapy, compared with those who undergo primary surgery before the administration of chemotherapy.
Neoadjuvant chemotherapy is an acceptable alternative to primary surgical cytoreduction
Vergote I, Tropé CG, Amant F, et al; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
Historically, the standard of care in ovarian cancer treatment has been surgical cytoreduction followed by chemotherapy.3-6 However, data from prospective randomized trials to support this practice are limited. Neoadjuvant chemotherapy is an alternative strategy that has been explored as a way to improve outcomes from interval surgical debulking in patients who have ovarian cancer in whom suboptimal cytoreduction is otherwise expected. Vergote and coworkers attempted to determine which strategy is better through a randomized trial of 632 patients.
Participants had to have biopsy-proven stage IIIc or IV ovarian, fallopian tube, or primary peritoneal cancer. The two treatment arms were:
- primary debulking surgery followed by at least 6 cycles of platinum-based chemotherapy
- 3 cycles of platinum-based neoadjuvant chemotherapy followed by interval debulking surgery in responders and those who had stable disease. These patients then received an additional 3 cycles of platinum-based chemotherapy post-operatively.
All surgical procedures were completed by qualified gynecologic oncologists, and all patients were evaluated for eligibility before randomization, with no additional selection criteria.
Postoperative death occurred in 2.5% of patients in the primary-surgery group, compared with 0.7% of patients in the neoadjuvant-chemotherapy group. Grade 3 or 4 hemorrhage occurred in 7.4% of patients after primary debulking, compared with 4.1% of patients after interval debulking. Patients who received neoadjuvant chemotherapy experienced a lower rate of infection (1.7% versus 8.1%) and venous complications (0% versus 2.6%).
Overall and progression-free survival rates were similar between the two groups. After multivariate analysis, the strongest predictors of survival were absence of residual disease after surgery (P<.001), small tumor size before randomization (P=.001), and endometrioid histology (P=.001)
Neoadjuvant chemotherapy is a preferred treatment strategy for patients who are expected to have a suboptimal result after surgery. Because neoadjuvant chemotherapy has a survival outcome similar to that of primary surgery followed by chemotherapy, it may be considered for all patients who have bulky stage IIIc or IV disease.
Although neoadjuvant chemotherapy improves the rate of optimal surgical cytoreduction, data are lacking to demonstrate that this improvement boosts survival.
Administration of neoadjuvant chemotherapy in these patients may improve perioperative morbidity and mortality, although no formal analysis was conducted in this study.
Neoadjuvant chemotherapy improves perioperative outcomes
Milam MR, Tao X, Coleman RL, et al. Neoadjuvant chemotherapy is associated with prolonged primary treatment intervals in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21(1):66–71.
Milam and coworkers investigated chemotherapy-associated morbidity and timing in two groups of patients who had advanced epithelial ovarian cancer:
- those undergoing neoadjuvant chemotherapy followed by maximal cytoreductive surgery
- those undergoing primary surgery followed by chemotherapy.
Their retrospective study involved 263 consecutive patients who were treated at MD Anderson Cancer Center from 1993 to 2005. In this cohort, 47 women (18%) received neoadjuvant chemotherapy. These patients experienced less blood loss (400 mL versus 750 mL) and a shorter hospital stay (6 versus 8 days). Time to the initiation of chemotherapy from the date of diagnosis did not differ between groups, and the amount of residual disease and rate of survival were also similar between arms. However, patients who received neoadjuvant chemotherapy underwent more cycles of chemotherapy over a longer treatment period.
Although neoadjuvant chemotherapy does not appear to offer a survival advantage, it is equivalent to primary surgery followed by adjuvant chemotherapy and may be associated with improved perioperative outcomes.
The results of the trial by Vergote and colleagues (page 25), should discourage oncologists from prescribing more than 6 cycles of chemotherapy in the neoadjuvant setting; patients from their study in the neoadjuvant group received a total of 6 cycles and had survival outcomes equivalent to those of women in the primary surgery group.
In the pipeline: Data on intraperitoneal chemotherapy after neoadjuvant chemotherapy
Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol. 2011;121(3):451–454.
Although several studies have demonstrated that intraperitoneal (IP) chemotherapy provides a survival advantage, compared with intravenous (IV) chemotherapy, after primary surgical debulking, it remains unclear whether IP chemotherapy would provide a similar superior survival outcome following neoadjuvant chemotherapy (FIGURE).

Intraperitoneal chemotherapy: How efficacious?
The jury is still out on whether intraperitoneal chemotherapy improves survival after neoadjuvant chemotherapy and interval debulking in stages III and IV ovarian cancer.The authors of this paper attempted to answer this question through a retrospective review of 71 patients. All patients were treated with neoadjuvant chemotherapy followed by interval debulking and either IP or IV chemotherapy. Overall, 17 patients (24%) received IP chemotherapy, and 54 patients (76%) received IV chemotherapy. The median number of cycles given prior to and after surgery was the same for both groups (3 for both neoadjuvant chemotherapy and chemotherapy following surgery).
Although patients who received IP chemotherapy had a higher overall response rate (82% versus 67%), there were no differences between groups in terms of progression-free (P=.42) and overall survival (P=.72).
One important limitation of this study was its small sample size and lack of statistical power. In addition, more patients in the IP group had macroscopic residual disease than in the IV group (71% versus 52%; P=.17).
A phase II/III study is under way to evaluate the use of IP chemotherapy following neoadjuvant chemotherapy in ovarian cancer patients.7 The two-stage randomized trial will compare IV chemotherapy with platinum-based IP chemotherapy in women who have undergone optimal surgical debulking (>1 cm) after 3 to 4 cycles of platinum-based neoadjuvant chemotherapy. This study is led by the US National Cancer Institute in collaboration with the Society of Gynecologic Oncologists of Canada, the UK National Cancer Research Institute, the Spanish Ovarian Cancer Research Group, and the US Southwest Oncology Group.
Data are limited on the use of intraperitoneal (IP) chemotherapy following neoadjuvant chemotherapy and interval surgical cytoreduction. We await the results of larger prospective studies to definitively determine whether there is a role for IP chemotherapy in this setting. For now, patients who receive neoadjuvant chemotherapy are limited to IV chemotherapy following surgery.
We want to hear from you! Tell us what you think.
- Update on ovarian cancer screening
David G. Mutch, MD; Nora Kizer, MD (July 2010)
A majority of ovarian cancers are diagnosed at an advanced stage, requiring extensive surgical cytoreductive procedures.1 Because the presence of residual macroscopic disease correlates highly with decreased survival,2 these procedures can be lengthy, complicated, and risky for the patient. Many patients who undergo cytoreduction will be left with a suboptimal result despite surgery.
Better identification and improved treatment of patients who are at high risk of a suboptimal result are clearly needed. One treatment option is neoadjuvant chemotherapy, the administration of chemotherapy prior to the main treatment. Although early data suggested that it was associated with worse outcomes, recent studies have yielded new information:
- Neoadjuvant chemotherapy followed by interval debulking surgery is not inferior to primary debulking surgery followed by chemotherapy for patients who have bulky stage III or IV ovarian cancer
- In patients who have advanced ovarian cancer, neoadjuvant chemotherapy followed by surgical cytoreduction is associated with improved perioperative outcomes
- Postoperative intraperitoneal chemotherapy after neoadjuvant chemotherapy has not yet proved to be associated with improved survival.
Several questions prompted by these findings include:
- Will neoadjuvant chemotherapy improve surgical outcomes in patients who have advanced ovarian cancer and, thus, improve survival?
- Is neoadjuvant chemotherapy a better strategy for all patients?
- Will neoadjuvant chemotherapy reduce the surgical effort necessary to achieve an optimal result?
- What is the role of intraperitoneal chemotherapy in patients who undergo neoadjuvant chemotherapy?
Further national (or international) data are needed to confirm a survival advantage for patients who receive neoadjuvant chemotherapy, compared with those who undergo primary surgery before the administration of chemotherapy.
Neoadjuvant chemotherapy is an acceptable alternative to primary surgical cytoreduction
Vergote I, Tropé CG, Amant F, et al; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
Historically, the standard of care in ovarian cancer treatment has been surgical cytoreduction followed by chemotherapy.3-6 However, data from prospective randomized trials to support this practice are limited. Neoadjuvant chemotherapy is an alternative strategy that has been explored as a way to improve outcomes from interval surgical debulking in patients who have ovarian cancer in whom suboptimal cytoreduction is otherwise expected. Vergote and coworkers attempted to determine which strategy is better through a randomized trial of 632 patients.
Participants had to have biopsy-proven stage IIIc or IV ovarian, fallopian tube, or primary peritoneal cancer. The two treatment arms were:
- primary debulking surgery followed by at least 6 cycles of platinum-based chemotherapy
- 3 cycles of platinum-based neoadjuvant chemotherapy followed by interval debulking surgery in responders and those who had stable disease. These patients then received an additional 3 cycles of platinum-based chemotherapy post-operatively.
All surgical procedures were completed by qualified gynecologic oncologists, and all patients were evaluated for eligibility before randomization, with no additional selection criteria.
Postoperative death occurred in 2.5% of patients in the primary-surgery group, compared with 0.7% of patients in the neoadjuvant-chemotherapy group. Grade 3 or 4 hemorrhage occurred in 7.4% of patients after primary debulking, compared with 4.1% of patients after interval debulking. Patients who received neoadjuvant chemotherapy experienced a lower rate of infection (1.7% versus 8.1%) and venous complications (0% versus 2.6%).
Overall and progression-free survival rates were similar between the two groups. After multivariate analysis, the strongest predictors of survival were absence of residual disease after surgery (P<.001), small tumor size before randomization (P=.001), and endometrioid histology (P=.001)
Neoadjuvant chemotherapy is a preferred treatment strategy for patients who are expected to have a suboptimal result after surgery. Because neoadjuvant chemotherapy has a survival outcome similar to that of primary surgery followed by chemotherapy, it may be considered for all patients who have bulky stage IIIc or IV disease.
Although neoadjuvant chemotherapy improves the rate of optimal surgical cytoreduction, data are lacking to demonstrate that this improvement boosts survival.
Administration of neoadjuvant chemotherapy in these patients may improve perioperative morbidity and mortality, although no formal analysis was conducted in this study.
Neoadjuvant chemotherapy improves perioperative outcomes
Milam MR, Tao X, Coleman RL, et al. Neoadjuvant chemotherapy is associated with prolonged primary treatment intervals in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21(1):66–71.
Milam and coworkers investigated chemotherapy-associated morbidity and timing in two groups of patients who had advanced epithelial ovarian cancer:
- those undergoing neoadjuvant chemotherapy followed by maximal cytoreductive surgery
- those undergoing primary surgery followed by chemotherapy.
Their retrospective study involved 263 consecutive patients who were treated at MD Anderson Cancer Center from 1993 to 2005. In this cohort, 47 women (18%) received neoadjuvant chemotherapy. These patients experienced less blood loss (400 mL versus 750 mL) and a shorter hospital stay (6 versus 8 days). Time to the initiation of chemotherapy from the date of diagnosis did not differ between groups, and the amount of residual disease and rate of survival were also similar between arms. However, patients who received neoadjuvant chemotherapy underwent more cycles of chemotherapy over a longer treatment period.
Although neoadjuvant chemotherapy does not appear to offer a survival advantage, it is equivalent to primary surgery followed by adjuvant chemotherapy and may be associated with improved perioperative outcomes.
The results of the trial by Vergote and colleagues (page 25), should discourage oncologists from prescribing more than 6 cycles of chemotherapy in the neoadjuvant setting; patients from their study in the neoadjuvant group received a total of 6 cycles and had survival outcomes equivalent to those of women in the primary surgery group.
In the pipeline: Data on intraperitoneal chemotherapy after neoadjuvant chemotherapy
Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol. 2011;121(3):451–454.
Although several studies have demonstrated that intraperitoneal (IP) chemotherapy provides a survival advantage, compared with intravenous (IV) chemotherapy, after primary surgical debulking, it remains unclear whether IP chemotherapy would provide a similar superior survival outcome following neoadjuvant chemotherapy (FIGURE).

Intraperitoneal chemotherapy: How efficacious?
The jury is still out on whether intraperitoneal chemotherapy improves survival after neoadjuvant chemotherapy and interval debulking in stages III and IV ovarian cancer.The authors of this paper attempted to answer this question through a retrospective review of 71 patients. All patients were treated with neoadjuvant chemotherapy followed by interval debulking and either IP or IV chemotherapy. Overall, 17 patients (24%) received IP chemotherapy, and 54 patients (76%) received IV chemotherapy. The median number of cycles given prior to and after surgery was the same for both groups (3 for both neoadjuvant chemotherapy and chemotherapy following surgery).
Although patients who received IP chemotherapy had a higher overall response rate (82% versus 67%), there were no differences between groups in terms of progression-free (P=.42) and overall survival (P=.72).
One important limitation of this study was its small sample size and lack of statistical power. In addition, more patients in the IP group had macroscopic residual disease than in the IV group (71% versus 52%; P=.17).
A phase II/III study is under way to evaluate the use of IP chemotherapy following neoadjuvant chemotherapy in ovarian cancer patients.7 The two-stage randomized trial will compare IV chemotherapy with platinum-based IP chemotherapy in women who have undergone optimal surgical debulking (>1 cm) after 3 to 4 cycles of platinum-based neoadjuvant chemotherapy. This study is led by the US National Cancer Institute in collaboration with the Society of Gynecologic Oncologists of Canada, the UK National Cancer Research Institute, the Spanish Ovarian Cancer Research Group, and the US Southwest Oncology Group.
Data are limited on the use of intraperitoneal (IP) chemotherapy following neoadjuvant chemotherapy and interval surgical cytoreduction. We await the results of larger prospective studies to definitively determine whether there is a role for IP chemotherapy in this setting. For now, patients who receive neoadjuvant chemotherapy are limited to IV chemotherapy following surgery.
We want to hear from you! Tell us what you think.
1. Howlader N, Noone AM, Krapcho M, et all. eds. SEER Cancer Statistics Review 1975-2008. National Cancer Institute. http://seer.cancer.gov/csr/1975_2008. Published April 15, 2011. Accessed June 10, 2011.
2. du Bois A, Ruess A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials; by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234-1244.
3. Meigs JV. Tumors of the pelvic organs. New York: Macmillan: 1934.
4. Aure JC, Hoeg K, Kolstad P. Clinical and histologic studies of ovarian carcinoma. Long-term follow-up of 990 cases. Obstet Gynecol. 1971;37(1):1-9.
5. Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131-142.
6. du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(suppl 8):viii7-viii12.
7. Mackay HJ, Provencheur D, Heywood M, et al. Phase II/III study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21. Curr Oncol. 2011;18(2):84-90.
1. Howlader N, Noone AM, Krapcho M, et all. eds. SEER Cancer Statistics Review 1975-2008. National Cancer Institute. http://seer.cancer.gov/csr/1975_2008. Published April 15, 2011. Accessed June 10, 2011.
2. du Bois A, Ruess A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials; by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234-1244.
3. Meigs JV. Tumors of the pelvic organs. New York: Macmillan: 1934.
4. Aure JC, Hoeg K, Kolstad P. Clinical and histologic studies of ovarian carcinoma. Long-term follow-up of 990 cases. Obstet Gynecol. 1971;37(1):1-9.
5. Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131-142.
6. du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(suppl 8):viii7-viii12.
7. Mackay HJ, Provencheur D, Heywood M, et al. Phase II/III study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21. Curr Oncol. 2011;18(2):84-90.
UPDATE: OVARIAN CANCER
Because ovarian cancer is usually diagnosed at an advanced stage—when prognosis is much worse than earlier in its course—a great deal of effort has been directed toward developing strategies to detect it early. These strategies include screening by a woman’s primary gynecologist with 1) a test of the serum CA-125 level and 2) transvaginal ultrasonography (TVU).
But how useful are the results of those screening tests? How should they be interpreted?
The answers aren’t clear.
Recent studies have yielded new information about ovarian cancer screening and detection. We discuss them in this Update:
- Screening with serial testing of the CA-125 level and TVU still is not recommended by the US Preventive Services Task Force or by ACOG
- Initial preliminary data from a prevalence screen of more than 50,000 subjects in the United Kingdom are encouraging, and show that new screening strategies may be feasible
- Using a patient’s report of her symptoms to trigger medical evaluation for ovarian cancer is not an effective screening tool
- Women who have an adnexal mass and a serum CA-125 level >35 U/mL and abnormal sonographic findings have an increased likelihood of ovarian cancer. They should be referred directly to a specialist.
The effect of screening on ovarian cancer mortality remains unknown
Partridge E, Greenlee RT, Xu J-L, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113(4):775–782.
The potential benefit of an effective screening program for ovarian cancer is great; the disease is the most lethal of all common gynecologic malignancies and carries significant individual and societal costs.1 Furthermore, diagnosis at an early stage is associated with improved survival.
To date, however, studies have not shed light on whether screening with CA-125 testing or TVU has an impact on morbidity or mortality from ovarian cancer. In a large, multicenter trial of more than 30,000 women, Partridge and colleagues attempted to answer this question.
Investigators sought to determine whether this cohort of healthy women, ranging in age from 55 to 74 years, experienced a reduction in mortality from ovarian cancer when subjects were screened annually with a combination of CA-125 testing and TVU. The study was part of a larger trial (the Prostate, Lung, Colorectal and Ovarian [PLCO] Cancer Screening Trial2).
Subjects were randomized 1:1 to 1) the screening arm or 2) their customary gynecologic care without screening. The regimen in the screening arm comprised:
- annual measurement of the CA-125 in Years 1 through 6
- annual TVU in Years 1 through 4
- evaluation and follow-up of positive screening tests at the discretion of each subject’s treating physician.
Distribution of staging was unaffected. Overall, the positive predictive value of the screening regimen was relatively constant—and quite low—across the screening years (1.1% in Year 1 [95% confidence interval (CI), 0.6–1.6]; 1.0% in Year 2 [95% CI, 0.4–1.5]; 1.1% in Year 3 [95% CI, 0.5–1.7]; and 1.3% in Year 4 [95% CI, 0.6–2.0]).
Of 3,388 women who had at least one positive result on either screening test, 1,170 (34.5%) underwent biopsy at some point. Of those, 60 (5.1%) had invasive cancer—yielding a surgery-to-detected-cancer ratio of 19.5:1.
Approximately 70% of cancers detected by screening were a Stage-III or -IV tumor. As such, the screening effort did not change the expected distribution of staging from what would be expected in an unscreened population.
Do not yet screen your general patient population for ovarian cancer with combined annual CA-125 testing and transvaginal ultrasonography. Determination of whether screening with this strategy will reduce mortality from ovarian cancer must await the final results of the larger PLCO trial.
Is it feasible to screen for ovarian cancer on a large scale?
Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009;10(4):327–340.
The low prevalence of ovarian cancer presents a significant challenge to anyone hoping to devise a useful screening program: A screening test designed to detect a low-prevalence disease must have exceptionally high sensitivity and specificity to achieve a clinically useful positive predictive value—especially when the intervention is relatively risky (surgical removal of the ovaries) and has known harmful health implications.
With that requirement in mind, researchers have refined ovarian cancer screening methods. One of these refinements is a risk-of-ovarian-cancer algorithm by which clinicians would be able to interpret serial CA-125 results.3,4
Interim results from a prevalence screen. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) evaluated these new screening methods in a multicenter, randomized, controlled trial in an effort that assessed not only mortality but also cost, acceptance by patients, and the physical and psychosocial morbidities associated with screening. At this point, investigators are reporting the results of a prevalence screen.
The team evaluated more than 200,000 low-risk women who were randomized to either 1) no screening, 2) multimodal screening (MMS), or 3) annual TVU screening, in, respectively, a 2:1:1 ratio. Multimodal screening comprised:
- annual CA-125 testing (interpreted using a risk-of-ovarian-cancer algorithm) and
- TVU as a second-line test.
Follow-up algorithms for women in both groups were determined a priori, based on a risk score (“normal,” “intermediate,” and “high”) from initial screening results. An initial, basic level-1 sonogram was performed on all women; a subsequent, more focused level-2 sonogram was performed only if indicated.
Among women assigned to screening, the following was noted:
- fewer women in the MMS group (0.3%) required clinical evaluation than in the TVU group (3.9%)
- fewer women in the MMS group (0.2%) required surgery than in the TVU group (1.8%)
- a similar number of cancers was detected in the two groups (MMS, 42; TVU, 45)
- more borderline tumors were detected in the TVU group than in the MMS group.
MMS had higher specificity and positive predictive value than TVU (respectively: 99.8% and 98.2%; 43.3% and 5.35%). Almost 50% of cancers detected on the initial screen were Stage I or II.
Again, do not screen for ovarian cancer with combined annual CA-125 testing and TVU. This study suggests, however, that large-scale screening strategies are feasible, and that they may provide useful guidance. We await the results of the researchers’ ongoing screening trial to determine what effects such screening might have on mortality from ovarian cancer.
Symptoms are not predictive of the risk of ovarian cancer
Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst. 2010;102(4):222–229.
Evaluation of symptoms has been suggested as a way of identifying women who may be at risk of ovarian cancer. In a 2007 consensus statement on the topic, contributors note that certain symptoms—bloating, pelvic or abdominal pain, difficulty eating, early satiety—are more common in women who have ovarian cancer than they are in the general population.5 They recommend that women who have these symptoms consult their physician for prompt evaluation.
But concerns have been raised about the true utility of these symptoms as a tool for detecting ovarian cancer at an earlier stage and, therefore, improving survival.
Linking symptom onset to time of diagnosis. Using a population-based registry that is part of the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, the investigators conducted a large, population-based study to examine the occurrence and timing of symptoms in 1) women who have ovarian cancer and 2) controls. They identified women in a 13-county area of western Washington State, ranging from 35 to 74 years old, who were given a diagnosis of epithelial ovarian cancer or had a borderline epithelial ovarian tumor over a 3-year period.
Of 1,058 eligible women who had ovarian cancer, the team interviewed 812. An additional 1,313 controls (providing a 69% response rate) were interviewed.
Results showed that most case patients who had symptoms often associated with ovarian cancer experienced those symptoms only within 5 months before their initial diagnosis. Symptoms were also less likely to occur in early-stage ovarian cancer than in late-stage disease.
The positive predictive value for the symptom index was extremely low (<0.5% in early-stage disease and 0.6%–1.1% in late-stage disease).
Proceed cautiously with use of any symptom index to trigger referral to a subspecialist, because it will detect ovarian cancer in only 1 of every 100 women in the general population whose presentation includes such symptoms. Data suggest that it will have limited utility for detecting early-stage cancer. Symptoms should not be completely ignored, however, because they do manifest more often in women who have ovarian cancer than in the general population.
Ultrasonography in combination with serum CA-125 can facilitate early referral to a subspecialist
McDonald JM, Doran S, DeSimone CP, et al. Predicting risk of malignancy in adnexal masses. Obstet Gynecol. 2010;115(4):687–694.
The finding of an adnexal mass is a common clinical scenario in gynecology. Any number of benign causes may be responsible, but it is important to identify which of those masses present a high likelihood of malignancy because complete surgical resection, along with adjuvant therapy administered in a timely manner, will maximize survival.
For that reason, an individualized risk profile in patients who have an adnexal mass confirmed by ultrasonography (US) would assist clinicians in making early referral to a cancer specialty care center.
Researchers evaluated 399 women who had been referred because of an adnexal mass on pelvic examination. Their objective was to estimate the accuracy of the following combination in predicting the risk of malignancy:
- patient demographics
- tumor morphology on US
- the serum CA-125 level.
The serum CA-125 level correlated directly with risk of malignancy in women who had an adnexal mass: Only 7.7% of women whose serum CA-125 level was within the normal range had ovarian cancer, compared with 34.2% women whose CA-125 level was 35–59 U/mL, and 86.8% whose level was 60–120 U/mL (P < .001). Multivariate analysis revealed that the most accurate significant predictor of a high risk of malignancy in patients who have an adnexal mass with complex or solid morphology is a serum CA-125 level >35 U/mL. This cutoff yielded a sensitivity of 77.3% for early stage ovarian cancer and 98.6% for advanced stage disease.
In summary
To repeat: As we await results of the UKCTOCS and the PLCO trial, do not screen patients routinely for ovarian cancer. Women who have an adnexal mass, an elevated CA-125 level, and troubling US findings should be referred—early—to a specialist.
Refer women who have a complex or solid adnexal mass and a CA-125 level >35 U/mL to a specialist. Early referral is important: Studies have shown a survival advantage as high as 24% among patients who have early-stage ovarian cancer and are treated by a gynecologic oncologist.6,7
The only benign histologic finding consistently associated with an elevated serum CA-125 level is ovarian endometriosis. In patients who have a history of endometriosis or other symptoms consistent with endometriosis, and an elevated CA-125 level, ovarian cancer is much less likely.
Also be aware that all 54 patients in this study who had ascites on US had invasive epithelial ovarian cancer, giving that finding a positive predictive value of 100%.
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
2. Prorok PC, Andriole Gl, Bresalier RS, et al. For Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Design of the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(suppl 6):273s-309s.
3. Skates SJ, Pauler D, Jacobs IJ. Screening based on the risk of cancer calculation from Bayesian hierarchical change point and mixture models of longitudinal markers. J Am Stat Assoc. 2001;96(454):429-439.
4. Skates SJ, Menon U, MacDonald N, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol. 2003;21(suppl 10):206s-210s.
5. Twombly R. Cancer killer may be “silent” no more. J Natl Cancer Inst. 2007;99(18):1359-1361.
6. Le T, Adolph A, Krepart G, et al. The benefits of comprehensive surgical staging in the management of early-stage epithelial ovarian carcinoma. Gynecol Oncol. 1992;47:223-7.
7. Suh-Burgmann E. Long-term outcomes following conservative surgery for borderline tumor of the ovary: a large population based study. Gynecol Oncol. 2006;103(3):841-847.
Because ovarian cancer is usually diagnosed at an advanced stage—when prognosis is much worse than earlier in its course—a great deal of effort has been directed toward developing strategies to detect it early. These strategies include screening by a woman’s primary gynecologist with 1) a test of the serum CA-125 level and 2) transvaginal ultrasonography (TVU).
But how useful are the results of those screening tests? How should they be interpreted?
The answers aren’t clear.
Recent studies have yielded new information about ovarian cancer screening and detection. We discuss them in this Update:
- Screening with serial testing of the CA-125 level and TVU still is not recommended by the US Preventive Services Task Force or by ACOG
- Initial preliminary data from a prevalence screen of more than 50,000 subjects in the United Kingdom are encouraging, and show that new screening strategies may be feasible
- Using a patient’s report of her symptoms to trigger medical evaluation for ovarian cancer is not an effective screening tool
- Women who have an adnexal mass and a serum CA-125 level >35 U/mL and abnormal sonographic findings have an increased likelihood of ovarian cancer. They should be referred directly to a specialist.
The effect of screening on ovarian cancer mortality remains unknown
Partridge E, Greenlee RT, Xu J-L, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113(4):775–782.
The potential benefit of an effective screening program for ovarian cancer is great; the disease is the most lethal of all common gynecologic malignancies and carries significant individual and societal costs.1 Furthermore, diagnosis at an early stage is associated with improved survival.
To date, however, studies have not shed light on whether screening with CA-125 testing or TVU has an impact on morbidity or mortality from ovarian cancer. In a large, multicenter trial of more than 30,000 women, Partridge and colleagues attempted to answer this question.
Investigators sought to determine whether this cohort of healthy women, ranging in age from 55 to 74 years, experienced a reduction in mortality from ovarian cancer when subjects were screened annually with a combination of CA-125 testing and TVU. The study was part of a larger trial (the Prostate, Lung, Colorectal and Ovarian [PLCO] Cancer Screening Trial2).
Subjects were randomized 1:1 to 1) the screening arm or 2) their customary gynecologic care without screening. The regimen in the screening arm comprised:
- annual measurement of the CA-125 in Years 1 through 6
- annual TVU in Years 1 through 4
- evaluation and follow-up of positive screening tests at the discretion of each subject’s treating physician.
Distribution of staging was unaffected. Overall, the positive predictive value of the screening regimen was relatively constant—and quite low—across the screening years (1.1% in Year 1 [95% confidence interval (CI), 0.6–1.6]; 1.0% in Year 2 [95% CI, 0.4–1.5]; 1.1% in Year 3 [95% CI, 0.5–1.7]; and 1.3% in Year 4 [95% CI, 0.6–2.0]).
Of 3,388 women who had at least one positive result on either screening test, 1,170 (34.5%) underwent biopsy at some point. Of those, 60 (5.1%) had invasive cancer—yielding a surgery-to-detected-cancer ratio of 19.5:1.
Approximately 70% of cancers detected by screening were a Stage-III or -IV tumor. As such, the screening effort did not change the expected distribution of staging from what would be expected in an unscreened population.
Do not yet screen your general patient population for ovarian cancer with combined annual CA-125 testing and transvaginal ultrasonography. Determination of whether screening with this strategy will reduce mortality from ovarian cancer must await the final results of the larger PLCO trial.
Is it feasible to screen for ovarian cancer on a large scale?
Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009;10(4):327–340.
The low prevalence of ovarian cancer presents a significant challenge to anyone hoping to devise a useful screening program: A screening test designed to detect a low-prevalence disease must have exceptionally high sensitivity and specificity to achieve a clinically useful positive predictive value—especially when the intervention is relatively risky (surgical removal of the ovaries) and has known harmful health implications.
With that requirement in mind, researchers have refined ovarian cancer screening methods. One of these refinements is a risk-of-ovarian-cancer algorithm by which clinicians would be able to interpret serial CA-125 results.3,4
Interim results from a prevalence screen. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) evaluated these new screening methods in a multicenter, randomized, controlled trial in an effort that assessed not only mortality but also cost, acceptance by patients, and the physical and psychosocial morbidities associated with screening. At this point, investigators are reporting the results of a prevalence screen.
The team evaluated more than 200,000 low-risk women who were randomized to either 1) no screening, 2) multimodal screening (MMS), or 3) annual TVU screening, in, respectively, a 2:1:1 ratio. Multimodal screening comprised:
- annual CA-125 testing (interpreted using a risk-of-ovarian-cancer algorithm) and
- TVU as a second-line test.
Follow-up algorithms for women in both groups were determined a priori, based on a risk score (“normal,” “intermediate,” and “high”) from initial screening results. An initial, basic level-1 sonogram was performed on all women; a subsequent, more focused level-2 sonogram was performed only if indicated.
Among women assigned to screening, the following was noted:
- fewer women in the MMS group (0.3%) required clinical evaluation than in the TVU group (3.9%)
- fewer women in the MMS group (0.2%) required surgery than in the TVU group (1.8%)
- a similar number of cancers was detected in the two groups (MMS, 42; TVU, 45)
- more borderline tumors were detected in the TVU group than in the MMS group.
MMS had higher specificity and positive predictive value than TVU (respectively: 99.8% and 98.2%; 43.3% and 5.35%). Almost 50% of cancers detected on the initial screen were Stage I or II.
Again, do not screen for ovarian cancer with combined annual CA-125 testing and TVU. This study suggests, however, that large-scale screening strategies are feasible, and that they may provide useful guidance. We await the results of the researchers’ ongoing screening trial to determine what effects such screening might have on mortality from ovarian cancer.
Symptoms are not predictive of the risk of ovarian cancer
Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst. 2010;102(4):222–229.
Evaluation of symptoms has been suggested as a way of identifying women who may be at risk of ovarian cancer. In a 2007 consensus statement on the topic, contributors note that certain symptoms—bloating, pelvic or abdominal pain, difficulty eating, early satiety—are more common in women who have ovarian cancer than they are in the general population.5 They recommend that women who have these symptoms consult their physician for prompt evaluation.
But concerns have been raised about the true utility of these symptoms as a tool for detecting ovarian cancer at an earlier stage and, therefore, improving survival.
Linking symptom onset to time of diagnosis. Using a population-based registry that is part of the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, the investigators conducted a large, population-based study to examine the occurrence and timing of symptoms in 1) women who have ovarian cancer and 2) controls. They identified women in a 13-county area of western Washington State, ranging from 35 to 74 years old, who were given a diagnosis of epithelial ovarian cancer or had a borderline epithelial ovarian tumor over a 3-year period.
Of 1,058 eligible women who had ovarian cancer, the team interviewed 812. An additional 1,313 controls (providing a 69% response rate) were interviewed.
Results showed that most case patients who had symptoms often associated with ovarian cancer experienced those symptoms only within 5 months before their initial diagnosis. Symptoms were also less likely to occur in early-stage ovarian cancer than in late-stage disease.
The positive predictive value for the symptom index was extremely low (<0.5% in early-stage disease and 0.6%–1.1% in late-stage disease).
Proceed cautiously with use of any symptom index to trigger referral to a subspecialist, because it will detect ovarian cancer in only 1 of every 100 women in the general population whose presentation includes such symptoms. Data suggest that it will have limited utility for detecting early-stage cancer. Symptoms should not be completely ignored, however, because they do manifest more often in women who have ovarian cancer than in the general population.
Ultrasonography in combination with serum CA-125 can facilitate early referral to a subspecialist
McDonald JM, Doran S, DeSimone CP, et al. Predicting risk of malignancy in adnexal masses. Obstet Gynecol. 2010;115(4):687–694.
The finding of an adnexal mass is a common clinical scenario in gynecology. Any number of benign causes may be responsible, but it is important to identify which of those masses present a high likelihood of malignancy because complete surgical resection, along with adjuvant therapy administered in a timely manner, will maximize survival.
For that reason, an individualized risk profile in patients who have an adnexal mass confirmed by ultrasonography (US) would assist clinicians in making early referral to a cancer specialty care center.
Researchers evaluated 399 women who had been referred because of an adnexal mass on pelvic examination. Their objective was to estimate the accuracy of the following combination in predicting the risk of malignancy:
- patient demographics
- tumor morphology on US
- the serum CA-125 level.
The serum CA-125 level correlated directly with risk of malignancy in women who had an adnexal mass: Only 7.7% of women whose serum CA-125 level was within the normal range had ovarian cancer, compared with 34.2% women whose CA-125 level was 35–59 U/mL, and 86.8% whose level was 60–120 U/mL (P < .001). Multivariate analysis revealed that the most accurate significant predictor of a high risk of malignancy in patients who have an adnexal mass with complex or solid morphology is a serum CA-125 level >35 U/mL. This cutoff yielded a sensitivity of 77.3% for early stage ovarian cancer and 98.6% for advanced stage disease.
In summary
To repeat: As we await results of the UKCTOCS and the PLCO trial, do not screen patients routinely for ovarian cancer. Women who have an adnexal mass, an elevated CA-125 level, and troubling US findings should be referred—early—to a specialist.
Refer women who have a complex or solid adnexal mass and a CA-125 level >35 U/mL to a specialist. Early referral is important: Studies have shown a survival advantage as high as 24% among patients who have early-stage ovarian cancer and are treated by a gynecologic oncologist.6,7
The only benign histologic finding consistently associated with an elevated serum CA-125 level is ovarian endometriosis. In patients who have a history of endometriosis or other symptoms consistent with endometriosis, and an elevated CA-125 level, ovarian cancer is much less likely.
Also be aware that all 54 patients in this study who had ascites on US had invasive epithelial ovarian cancer, giving that finding a positive predictive value of 100%.
Because ovarian cancer is usually diagnosed at an advanced stage—when prognosis is much worse than earlier in its course—a great deal of effort has been directed toward developing strategies to detect it early. These strategies include screening by a woman’s primary gynecologist with 1) a test of the serum CA-125 level and 2) transvaginal ultrasonography (TVU).
But how useful are the results of those screening tests? How should they be interpreted?
The answers aren’t clear.
Recent studies have yielded new information about ovarian cancer screening and detection. We discuss them in this Update:
- Screening with serial testing of the CA-125 level and TVU still is not recommended by the US Preventive Services Task Force or by ACOG
- Initial preliminary data from a prevalence screen of more than 50,000 subjects in the United Kingdom are encouraging, and show that new screening strategies may be feasible
- Using a patient’s report of her symptoms to trigger medical evaluation for ovarian cancer is not an effective screening tool
- Women who have an adnexal mass and a serum CA-125 level >35 U/mL and abnormal sonographic findings have an increased likelihood of ovarian cancer. They should be referred directly to a specialist.
The effect of screening on ovarian cancer mortality remains unknown
Partridge E, Greenlee RT, Xu J-L, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113(4):775–782.
The potential benefit of an effective screening program for ovarian cancer is great; the disease is the most lethal of all common gynecologic malignancies and carries significant individual and societal costs.1 Furthermore, diagnosis at an early stage is associated with improved survival.
To date, however, studies have not shed light on whether screening with CA-125 testing or TVU has an impact on morbidity or mortality from ovarian cancer. In a large, multicenter trial of more than 30,000 women, Partridge and colleagues attempted to answer this question.
Investigators sought to determine whether this cohort of healthy women, ranging in age from 55 to 74 years, experienced a reduction in mortality from ovarian cancer when subjects were screened annually with a combination of CA-125 testing and TVU. The study was part of a larger trial (the Prostate, Lung, Colorectal and Ovarian [PLCO] Cancer Screening Trial2).
Subjects were randomized 1:1 to 1) the screening arm or 2) their customary gynecologic care without screening. The regimen in the screening arm comprised:
- annual measurement of the CA-125 in Years 1 through 6
- annual TVU in Years 1 through 4
- evaluation and follow-up of positive screening tests at the discretion of each subject’s treating physician.
Distribution of staging was unaffected. Overall, the positive predictive value of the screening regimen was relatively constant—and quite low—across the screening years (1.1% in Year 1 [95% confidence interval (CI), 0.6–1.6]; 1.0% in Year 2 [95% CI, 0.4–1.5]; 1.1% in Year 3 [95% CI, 0.5–1.7]; and 1.3% in Year 4 [95% CI, 0.6–2.0]).
Of 3,388 women who had at least one positive result on either screening test, 1,170 (34.5%) underwent biopsy at some point. Of those, 60 (5.1%) had invasive cancer—yielding a surgery-to-detected-cancer ratio of 19.5:1.
Approximately 70% of cancers detected by screening were a Stage-III or -IV tumor. As such, the screening effort did not change the expected distribution of staging from what would be expected in an unscreened population.
Do not yet screen your general patient population for ovarian cancer with combined annual CA-125 testing and transvaginal ultrasonography. Determination of whether screening with this strategy will reduce mortality from ovarian cancer must await the final results of the larger PLCO trial.
Is it feasible to screen for ovarian cancer on a large scale?
Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009;10(4):327–340.
The low prevalence of ovarian cancer presents a significant challenge to anyone hoping to devise a useful screening program: A screening test designed to detect a low-prevalence disease must have exceptionally high sensitivity and specificity to achieve a clinically useful positive predictive value—especially when the intervention is relatively risky (surgical removal of the ovaries) and has known harmful health implications.
With that requirement in mind, researchers have refined ovarian cancer screening methods. One of these refinements is a risk-of-ovarian-cancer algorithm by which clinicians would be able to interpret serial CA-125 results.3,4
Interim results from a prevalence screen. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) evaluated these new screening methods in a multicenter, randomized, controlled trial in an effort that assessed not only mortality but also cost, acceptance by patients, and the physical and psychosocial morbidities associated with screening. At this point, investigators are reporting the results of a prevalence screen.
The team evaluated more than 200,000 low-risk women who were randomized to either 1) no screening, 2) multimodal screening (MMS), or 3) annual TVU screening, in, respectively, a 2:1:1 ratio. Multimodal screening comprised:
- annual CA-125 testing (interpreted using a risk-of-ovarian-cancer algorithm) and
- TVU as a second-line test.
Follow-up algorithms for women in both groups were determined a priori, based on a risk score (“normal,” “intermediate,” and “high”) from initial screening results. An initial, basic level-1 sonogram was performed on all women; a subsequent, more focused level-2 sonogram was performed only if indicated.
Among women assigned to screening, the following was noted:
- fewer women in the MMS group (0.3%) required clinical evaluation than in the TVU group (3.9%)
- fewer women in the MMS group (0.2%) required surgery than in the TVU group (1.8%)
- a similar number of cancers was detected in the two groups (MMS, 42; TVU, 45)
- more borderline tumors were detected in the TVU group than in the MMS group.
MMS had higher specificity and positive predictive value than TVU (respectively: 99.8% and 98.2%; 43.3% and 5.35%). Almost 50% of cancers detected on the initial screen were Stage I or II.
Again, do not screen for ovarian cancer with combined annual CA-125 testing and TVU. This study suggests, however, that large-scale screening strategies are feasible, and that they may provide useful guidance. We await the results of the researchers’ ongoing screening trial to determine what effects such screening might have on mortality from ovarian cancer.
Symptoms are not predictive of the risk of ovarian cancer
Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst. 2010;102(4):222–229.
Evaluation of symptoms has been suggested as a way of identifying women who may be at risk of ovarian cancer. In a 2007 consensus statement on the topic, contributors note that certain symptoms—bloating, pelvic or abdominal pain, difficulty eating, early satiety—are more common in women who have ovarian cancer than they are in the general population.5 They recommend that women who have these symptoms consult their physician for prompt evaluation.
But concerns have been raised about the true utility of these symptoms as a tool for detecting ovarian cancer at an earlier stage and, therefore, improving survival.
Linking symptom onset to time of diagnosis. Using a population-based registry that is part of the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, the investigators conducted a large, population-based study to examine the occurrence and timing of symptoms in 1) women who have ovarian cancer and 2) controls. They identified women in a 13-county area of western Washington State, ranging from 35 to 74 years old, who were given a diagnosis of epithelial ovarian cancer or had a borderline epithelial ovarian tumor over a 3-year period.
Of 1,058 eligible women who had ovarian cancer, the team interviewed 812. An additional 1,313 controls (providing a 69% response rate) were interviewed.
Results showed that most case patients who had symptoms often associated with ovarian cancer experienced those symptoms only within 5 months before their initial diagnosis. Symptoms were also less likely to occur in early-stage ovarian cancer than in late-stage disease.
The positive predictive value for the symptom index was extremely low (<0.5% in early-stage disease and 0.6%–1.1% in late-stage disease).
Proceed cautiously with use of any symptom index to trigger referral to a subspecialist, because it will detect ovarian cancer in only 1 of every 100 women in the general population whose presentation includes such symptoms. Data suggest that it will have limited utility for detecting early-stage cancer. Symptoms should not be completely ignored, however, because they do manifest more often in women who have ovarian cancer than in the general population.
Ultrasonography in combination with serum CA-125 can facilitate early referral to a subspecialist
McDonald JM, Doran S, DeSimone CP, et al. Predicting risk of malignancy in adnexal masses. Obstet Gynecol. 2010;115(4):687–694.
The finding of an adnexal mass is a common clinical scenario in gynecology. Any number of benign causes may be responsible, but it is important to identify which of those masses present a high likelihood of malignancy because complete surgical resection, along with adjuvant therapy administered in a timely manner, will maximize survival.
For that reason, an individualized risk profile in patients who have an adnexal mass confirmed by ultrasonography (US) would assist clinicians in making early referral to a cancer specialty care center.
Researchers evaluated 399 women who had been referred because of an adnexal mass on pelvic examination. Their objective was to estimate the accuracy of the following combination in predicting the risk of malignancy:
- patient demographics
- tumor morphology on US
- the serum CA-125 level.
The serum CA-125 level correlated directly with risk of malignancy in women who had an adnexal mass: Only 7.7% of women whose serum CA-125 level was within the normal range had ovarian cancer, compared with 34.2% women whose CA-125 level was 35–59 U/mL, and 86.8% whose level was 60–120 U/mL (P < .001). Multivariate analysis revealed that the most accurate significant predictor of a high risk of malignancy in patients who have an adnexal mass with complex or solid morphology is a serum CA-125 level >35 U/mL. This cutoff yielded a sensitivity of 77.3% for early stage ovarian cancer and 98.6% for advanced stage disease.
In summary
To repeat: As we await results of the UKCTOCS and the PLCO trial, do not screen patients routinely for ovarian cancer. Women who have an adnexal mass, an elevated CA-125 level, and troubling US findings should be referred—early—to a specialist.
Refer women who have a complex or solid adnexal mass and a CA-125 level >35 U/mL to a specialist. Early referral is important: Studies have shown a survival advantage as high as 24% among patients who have early-stage ovarian cancer and are treated by a gynecologic oncologist.6,7
The only benign histologic finding consistently associated with an elevated serum CA-125 level is ovarian endometriosis. In patients who have a history of endometriosis or other symptoms consistent with endometriosis, and an elevated CA-125 level, ovarian cancer is much less likely.
Also be aware that all 54 patients in this study who had ascites on US had invasive epithelial ovarian cancer, giving that finding a positive predictive value of 100%.
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
2. Prorok PC, Andriole Gl, Bresalier RS, et al. For Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Design of the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(suppl 6):273s-309s.
3. Skates SJ, Pauler D, Jacobs IJ. Screening based on the risk of cancer calculation from Bayesian hierarchical change point and mixture models of longitudinal markers. J Am Stat Assoc. 2001;96(454):429-439.
4. Skates SJ, Menon U, MacDonald N, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol. 2003;21(suppl 10):206s-210s.
5. Twombly R. Cancer killer may be “silent” no more. J Natl Cancer Inst. 2007;99(18):1359-1361.
6. Le T, Adolph A, Krepart G, et al. The benefits of comprehensive surgical staging in the management of early-stage epithelial ovarian carcinoma. Gynecol Oncol. 1992;47:223-7.
7. Suh-Burgmann E. Long-term outcomes following conservative surgery for borderline tumor of the ovary: a large population based study. Gynecol Oncol. 2006;103(3):841-847.
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
2. Prorok PC, Andriole Gl, Bresalier RS, et al. For Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project Team. Design of the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(suppl 6):273s-309s.
3. Skates SJ, Pauler D, Jacobs IJ. Screening based on the risk of cancer calculation from Bayesian hierarchical change point and mixture models of longitudinal markers. J Am Stat Assoc. 2001;96(454):429-439.
4. Skates SJ, Menon U, MacDonald N, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol. 2003;21(suppl 10):206s-210s.
5. Twombly R. Cancer killer may be “silent” no more. J Natl Cancer Inst. 2007;99(18):1359-1361.
6. Le T, Adolph A, Krepart G, et al. The benefits of comprehensive surgical staging in the management of early-stage epithelial ovarian carcinoma. Gynecol Oncol. 1992;47:223-7.
7. Suh-Burgmann E. Long-term outcomes following conservative surgery for borderline tumor of the ovary: a large population based study. Gynecol Oncol. 2006;103(3):841-847.
UPDATE: ENDOMETRIAL CANCER
Dr. Mutch reports that he has received grant or research support from Lilly and Genentech. He serves as a speaker for GSK, Lilly, and Merck. Dr. Rimel reports no financial relationships relevant to this article.
Endometrial cancer is a great concern in industrialized nations, where it is the most common gynecologic cancer—with incidence increasing every year. Survival is generally very good for women who have low-grade disease confined to the uterus. However, for patients who have high-grade disease, an aggressive histologic type, or other features that suggest a poor prognosis, the cure rate approaches 75%.1
Primary surgery is the mainstay of initial treatment and basis of FIGO staging ( TABLE ), which requires:
- total hysterectomy
- bilateral salpingo-oophorectomy
- complete examination of the abdomen
- pelvic washings
- lymph-adenectomy (anatomic boundaries and node counts aren’t specified).
Controversy clouds our understanding of the optimal type of surgery, utility of pelvic lymphadenectomy, and possible benefit of adjuvant radiation therapy. During the past year, fuel has been added to this debate:
- Two randomized, controlled trials of surgery with and without pelvic lymphadenectomy in early-stage patients demonstrated no survival benefit. Earlier studies investigating the benefits of lymphadenectomy in endometrial cancer have been largely retrospective, and results have varied.
- A concurrent randomized, controlled trial of external-beam radiotherapy for women who have intermediate- or high-risk disease showed no improvement in overall survival, although local control increased by 3%.
TABLE
FIGO surgical staging for endometrial cancer
| Stage | Description |
|---|---|
| I | Tumor is confined to uterine fundus |
| IA | Tumor is limited to endometrium |
| IB | Tumor invades less than half of the myometrial thickness |
| IC | Tumor invades more than half of the myometrial thickness |
| II | Tumor extends to cervix |
| IIA | Cervical extension is limited to endocervical glands |
| IIB | Tumor invades cervical stroma |
| III | There is regional tumor spread |
| IIIA | Tumor invades uterine serosa or adnexa, or cells in the peritoneum show signs of cancer |
| IIIB | Vaginal metastases are present |
| IIIC | Tumor has spread to lymph nodes near the uterus |
| IV | There is bulky pelvic disease or distant spread |
| IVA | Tumor has spread to bladder or rectum |
| IVB | Distant metastases are present |
No survival advantage to pelvic lymphadenectomy—but it has other benefits
ASTEC study group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136.
Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716.
Among the arguments for lymphadenectomy in endometrial cancer staging are:
- It aids in the selection of women for radiation or other adjuvant treatment
- It may have a direct survival benefit, as suggested by retrospective studies.
But lymphadenectomy is time-consuming, requires a specialized gynecologic surgeon, and is associated with some increase in the risk of morbidity—namely, lymphedema, lymphocyst formation, deep-vein thrombosis (DVT), and blood loss.
The much-anticipated report of the 85-center, multinational ASTEC trial [ A S urgical T rial of E ndometrial C ancer], published earlier this year, offers further insight into the practice of lymphadenectomy. ASTEC involved two randomizations: The first, to pelvic lymphadenectomy; the second, to radiation therapy.
The ASTEC trial enrolled 1,408 women who had histologically confirmed endometrial carcinoma that was believed to be confined to the uterus. How this determination was made was not specified. Patients who had enlarged lymph nodes corroborated by computed tomography or magnetic resonance imaging were not excluded.
Participants were randomized to either of the following treatment groups:
- traditional surgery with total hysterectomy and bilateral salpingo-oophorectomy, pelvic washings, and palpation of para-aortic nodes
- the same surgery plus systematic lymphadenectomy of the iliac and obturator nodes.
If any para-aortic nodes were suspicious, biopsy or lymphadenectomy was performed at the discretion of the surgeon ( FIGURE ).
FIGURE Nodes reveal when cancer has spread
Women in the ASTEC trial were randomized to traditional surgery (total hysterectomy and bilateral salpingo-oophorectomy), pelvic washings, and palpation of para-aortic nodes or to the same surgery plus lymphadenectomy of the iliac and obturator nodes.
Operative findings determined a patient’s level of risk
After surgery, patients were categorized as having one of the following:
- low-risk, early-stage disease. This group included patients who had disease classified as stage IA or IB, grade 1 or 2. They were deemed to have a suitably low risk of recurrence to be offered further treatment according to their physician’s standard practice.
- intermediate- or high-risk, early-stage disease. These patients were randomized to the ASTEC radiation-therapy trial, which compared external-beam radiotherapy with no external-beam radiotherapy. The authors assert that this second randomization was necessary to prevent over- or undertreatment of patients who had unknown node status, which might alter survival outcomes.
- advanced disease. These patients were referred to their physician for further treatment.
In both surgical groups (with and without lymphadenectomy), approximately 80% of patients had disease confined to the uterus. Nodes were harvested in 91% of the patients in the lymphadenectomy group, compared with 5% of patients in the traditional-surgery group. Nine percent of women in the lymphadenectomy group had positive nodes.
The authors observe that more women had deeply invasive disease and adverse histologic types in the group that underwent lymphadenectomy. There were no differences between the two groups in overall survival; disease-specific survival; recurrence-free survival; or recurrence-free, disease-specific survival, after adjustment for baseline differences. Subgroup analysis for low-risk, high-risk, and advanced disease also failed to demonstrate differences in overall survival and recurrence-free survival.
Study from Italy produces similar findings
An independent randomized, controlled trial examining survival outcomes for endometrial cancer patients with and without lymphadenectomy was released by the Italian group in late 2008. In this study, 537 patients who had histologically confirmed endometrial carcinoma believed to be confined to the uterus were randomized to total abdominal hysterectomy and bilateral salpingo-oophorectomy with or without pelvic lymphadenectomy.
Anatomic boundaries of the pelvic lymph-node dissection were clearly defined, and a minimum lymph-node count of 20 was specified for inclusion. Intraoperative frozen section was utilized to exclude patients who had grade-1 disease that was less than 50% invasive. The option of para-aortic lymph-node dissection or sampling was left to the discretion of the surgeon. If pelvic nodes were larger than 1 cm, they were removed or sampled regardless of randomization.
Unlike the ASTEC trial, this study did not attempt to control adjuvant treatment. Patients were treated according to the discretion of the physician. Most patients received no further therapy; only 20% underwent radiation therapy, and 7% received chemotherapy.
Given the findings of these two, large, multi-institutional trials with strikingly similar results but major problems, what is a gynecologist to do? Can lymphadenectomy be avoided in patients whose disease is believed to be confined to the uterus?
For now, the answer is a tentative “No.”
There appears to be no survival advantage to removal of lymph nodes when disease is confined to the uterus, but that is not to say there is no benefit to systematic lymphadenectomy—just that there is no survival benefit afforded by the procedure. Benefits of lymphadenectomy, which include more precise definition of the extent of disease, minimization of over- or undertreatment, and a reduction in overall treatment and cost, still remain. The concept of surgical debulking put forward by Bristow and coworkers still has merit, and any gross disease should be removed, if feasible.2
Lymphadenectomy in endometrial cancer remains controversial and complex, especially as we lack a precise method for determining which patients will have nodal disease. Our practice remains to remove the lymph nodes whenever possible to better tailor any adjuvant treatment.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Women in the lymphadenectomy group were more likely to have stage-IIIC disease, which is directly attributable to histologic evaluation of the lymph nodes in this group. The authors point out that these patients had more accurate assessment of their prognosis, allowing for the tailoring of adjuvant treatment.
The overall survival and disease-free survival curves for the two experimental groups were similar, consistent with data from the ASTEC trial. This proved to be true for both the intention-to-treat and according-to-protocol groups. The authors note that their results are similar to those of the ASTEC trial, despite the significant difference in the number of nodes removed in each trial.
Some aspects of the trials hamper interpretation and comparison
Outcomes are improved when surgery is performed by a trained gynecologic oncologist. In the ASTEC trial, each lymphadenectomy was performed by a specialized gynecologic surgeon who was “skilled in the procedure.” In the Italian study, the type of surgeon was not specified, but the specific anatomic boundaries of the dissection and the minimum node count were. More specific data are needed before any conclusions can be drawn about the effect of surgical skill on outcome in these trials.
In the ASTEC trial, 9% of patients in the lymphadenectomy group had no nodes removed, and more than 60% of patients would not have met criteria for inclusion in the lymphadenectomy arm of the Italian study—suggesting that the majority of women in the ASTEC trial had inadequate lymphadenectomy. Para-aortic lymphadenectomy was left to the discretion of the attending surgeon, and some patients did have resection of these nodes. The data do not include information about whether these patients were treated in the para-aortic region based on the histology of these nodes.
Randomization in a prospective study is supposed to equalize the risks between groups. In the ASTEC trial, despite randomization, there were 10% more patients who had deeply invasive disease in the lymphadenectomy group, along with 3% more adverse histologies and high-grade (grade-3) tumors. Given the higher incidence of positive nodes and poorer outcome in these cases, this difference may have had a significant impact on the evaluation of the groups for overall or disease-specific survival.
External-beam radiotherapy reduces local recurrence of endometrial Ca but does not improve survival
ASTEC/EN.5 Study Group, Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–146.
Radiation therapy has been a standard treatment for endometrial cancer when there is high risk of recurrence. This report combines two independent randomized, controlled trials investigating the benefit of postoperative adjuvant pelvic radiation in women who had early-stage disease and who met histologic criteria for high risk of recurrence and death. The trials are the EN.5 trial from Canada, and the radiation-therapy randomization of the ASTEC trial). Neither found a benefit in terms of overall survival, disease-specific survival, or recurrence-free survival, although local recurrence was reduced by 2.9% The authors also provide a review of the literature and a meta-analysis of other randomized, controlled trials on this subject.
Details of the EN.5 and ASTEC radiation-therapy trials
Criteria for enrollment were similar for the two trials, which focused on women who had histologically confirmed endometrial cancer and an intermediate or high risk of recurrence. This included women who had FIGO stage IA or stage IB (grade 3), stage IC (all grades), or papillary serous or clear-cell histology (all stages).
Survival is the primary goal of cancer treatment. External-beam radiotherapy does not improve survival, but does provide a small but real increase in local control. Regrettably, this improvement in local control comes at a cost: 3% of patients experience acute severe or life-threatening toxicity from treatment. The absolute difference in local recurrence between women who received external-beam radiotherapy and those who did not was only 2.9%. Local recurrences are largely salvageable in women who have not been irradiated.
Therefore, external-beam radiotherapy, as delivered in this trial, regardless of node status, should not be the standard of care. Improvement in technology with intensity-modulated radiotherapy, and the further evaluation of vaginal brachytherapy alone, may provide new ways to apply this kind of treatment in endometrial cancer.
This aspect of endometrial cancer treatment clearly needs further investigation. Trials are under way that may determine the role of radiation therapy in women who have endometrial cancer.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Lymphadenectomy was not required for patients enrolled in EN.5, but was part of the surgical randomization for ASTEC. This distinction could confound the results of the combined trials, as the investigators were trying to answer two questions within one patient population.
In both the EN.5 and ASTEC trials, women were randomized to observation or external-beam radiotherapy, with these parameters:
- Radiation therapy was to begin no later than 12 weeks after surgery (most patients began radiation therapy 6 to 8 weeks after surgery)
- For ASTEC, the target dosage was 40–46 Gy in 20–26 daily fractions to the pelvis, with treatment five times each week. For EN.5, the dosage and timing were very similar: 45 Gy, 25 daily fractions, five times weekly
- In both trials, vaginal brachytherapy was allowed if it was the local practice or the center’s policy
- Women were classified as being at intermediate risk or high risk, based on the likelihood of distant recurrence, as defined by GOG99 and PORTEC1 studies. Intermediate risk included all patients who had stage-IA or -IB (grade-3) or stage-IC or -IIA (grade-1 or -2) disease. Women who had papillary serous or clear-cell histology, stage-IC or -IIA (grade-3) disease, or any stage-IIB disease were considered at high risk.
The primary outcome evaluated for both trials was overall survival. Secondary endpoints were:
- disease-specific survival
- recurrence-free survival
- locoregional recurrence
- treatment toxicity.
A total of 905 women were enrolled in the ASTEC and EN.5 trials, with most patients having endometrial histology (83%) and being categorized as at intermediate risk (75%). Approximately half the patients in both trials received brachytherapy, which was allowed according to local practice. Only 47% of the observation group actually received no treatment.
Findings were remarkably similar in EN.5 and ASTEC
Here are the main findings:
- no difference between groups in overall survival, disease-specific survival, and recurrence-free survival
- significantly fewer isolated vaginal or pelvic initial recurrences in the external-beam radiotherapy group, with an absolute difference of 2.9%. (Only 35% of all recurrences were isolated recurrences)
- no significant difference between groups in distant or local and distant recurrences
- as expected, higher toxicity in the group receiving external-beam radiotherapy, including life-threatening toxicity (acute toxicity, 3% vs <1%; late toxicity, 1% vs 0%).
Subgroup analysis comparing overall survival in intermediate- and high-risk patients demonstrated no improvement with external-beam radiotherapy. Nor was overall survival altered by lymphadenectomy. The authors performed a meta-analysis using data from GOG99, PORTEC1, and this combined trial, and found no significant difference in overall survival or disease-specific survival, regardless of histologic risk group.
Trial has notable strengths and weaknesses
This large prospective trial has significant strengths: its size and its multi-institutional nature. The authors also evaluated their data in combination with other randomized, controlled trials to further investigate the effect of external-beam radiotherapy on survival. However, allowing brachytherapy somewhat confounds the true effect of external-beam radiotherapy on local recurrence. (There were few local recurrences, and the authors did not evaluate whether women who had an isolated vaginal recurrence received vaginal brachytherapy.) Moreover, 15% of women who were randomized to external-beam radiotherapy did not complete it.
In addition, secondary randomization of patients in the intermediate-risk and high-risk categories to external-beam radiotherapy versus no treatment may have significantly confounded the results of the entire ASTEC trial. Because women were, or were not, randomized to treatment regardless of node status, some patients who had positive nodes failed to receive adjuvant treatment. This may have had a significant effect on overall survival, as positive lymph nodes are a negative prognostic factor.
1. Keys HM, Roberts JA, Brunetto VL, et al. Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744-751.
2. Bristow RE, Zahurak ML, Alexander CJ, Zellars RC, Montz FJ. FIGO stage IIIC endometrial carcinoma: resection of macroscopic nodal disease and other determinants of survival. Int J Gynecol Cancer. 2003;13:664-672.
Dr. Mutch reports that he has received grant or research support from Lilly and Genentech. He serves as a speaker for GSK, Lilly, and Merck. Dr. Rimel reports no financial relationships relevant to this article.
Endometrial cancer is a great concern in industrialized nations, where it is the most common gynecologic cancer—with incidence increasing every year. Survival is generally very good for women who have low-grade disease confined to the uterus. However, for patients who have high-grade disease, an aggressive histologic type, or other features that suggest a poor prognosis, the cure rate approaches 75%.1
Primary surgery is the mainstay of initial treatment and basis of FIGO staging ( TABLE ), which requires:
- total hysterectomy
- bilateral salpingo-oophorectomy
- complete examination of the abdomen
- pelvic washings
- lymph-adenectomy (anatomic boundaries and node counts aren’t specified).
Controversy clouds our understanding of the optimal type of surgery, utility of pelvic lymphadenectomy, and possible benefit of adjuvant radiation therapy. During the past year, fuel has been added to this debate:
- Two randomized, controlled trials of surgery with and without pelvic lymphadenectomy in early-stage patients demonstrated no survival benefit. Earlier studies investigating the benefits of lymphadenectomy in endometrial cancer have been largely retrospective, and results have varied.
- A concurrent randomized, controlled trial of external-beam radiotherapy for women who have intermediate- or high-risk disease showed no improvement in overall survival, although local control increased by 3%.
TABLE
FIGO surgical staging for endometrial cancer
| Stage | Description |
|---|---|
| I | Tumor is confined to uterine fundus |
| IA | Tumor is limited to endometrium |
| IB | Tumor invades less than half of the myometrial thickness |
| IC | Tumor invades more than half of the myometrial thickness |
| II | Tumor extends to cervix |
| IIA | Cervical extension is limited to endocervical glands |
| IIB | Tumor invades cervical stroma |
| III | There is regional tumor spread |
| IIIA | Tumor invades uterine serosa or adnexa, or cells in the peritoneum show signs of cancer |
| IIIB | Vaginal metastases are present |
| IIIC | Tumor has spread to lymph nodes near the uterus |
| IV | There is bulky pelvic disease or distant spread |
| IVA | Tumor has spread to bladder or rectum |
| IVB | Distant metastases are present |
No survival advantage to pelvic lymphadenectomy—but it has other benefits
ASTEC study group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136.
Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716.
Among the arguments for lymphadenectomy in endometrial cancer staging are:
- It aids in the selection of women for radiation or other adjuvant treatment
- It may have a direct survival benefit, as suggested by retrospective studies.
But lymphadenectomy is time-consuming, requires a specialized gynecologic surgeon, and is associated with some increase in the risk of morbidity—namely, lymphedema, lymphocyst formation, deep-vein thrombosis (DVT), and blood loss.
The much-anticipated report of the 85-center, multinational ASTEC trial [ A S urgical T rial of E ndometrial C ancer], published earlier this year, offers further insight into the practice of lymphadenectomy. ASTEC involved two randomizations: The first, to pelvic lymphadenectomy; the second, to radiation therapy.
The ASTEC trial enrolled 1,408 women who had histologically confirmed endometrial carcinoma that was believed to be confined to the uterus. How this determination was made was not specified. Patients who had enlarged lymph nodes corroborated by computed tomography or magnetic resonance imaging were not excluded.
Participants were randomized to either of the following treatment groups:
- traditional surgery with total hysterectomy and bilateral salpingo-oophorectomy, pelvic washings, and palpation of para-aortic nodes
- the same surgery plus systematic lymphadenectomy of the iliac and obturator nodes.
If any para-aortic nodes were suspicious, biopsy or lymphadenectomy was performed at the discretion of the surgeon ( FIGURE ).
FIGURE Nodes reveal when cancer has spread
Women in the ASTEC trial were randomized to traditional surgery (total hysterectomy and bilateral salpingo-oophorectomy), pelvic washings, and palpation of para-aortic nodes or to the same surgery plus lymphadenectomy of the iliac and obturator nodes.
Operative findings determined a patient’s level of risk
After surgery, patients were categorized as having one of the following:
- low-risk, early-stage disease. This group included patients who had disease classified as stage IA or IB, grade 1 or 2. They were deemed to have a suitably low risk of recurrence to be offered further treatment according to their physician’s standard practice.
- intermediate- or high-risk, early-stage disease. These patients were randomized to the ASTEC radiation-therapy trial, which compared external-beam radiotherapy with no external-beam radiotherapy. The authors assert that this second randomization was necessary to prevent over- or undertreatment of patients who had unknown node status, which might alter survival outcomes.
- advanced disease. These patients were referred to their physician for further treatment.
In both surgical groups (with and without lymphadenectomy), approximately 80% of patients had disease confined to the uterus. Nodes were harvested in 91% of the patients in the lymphadenectomy group, compared with 5% of patients in the traditional-surgery group. Nine percent of women in the lymphadenectomy group had positive nodes.
The authors observe that more women had deeply invasive disease and adverse histologic types in the group that underwent lymphadenectomy. There were no differences between the two groups in overall survival; disease-specific survival; recurrence-free survival; or recurrence-free, disease-specific survival, after adjustment for baseline differences. Subgroup analysis for low-risk, high-risk, and advanced disease also failed to demonstrate differences in overall survival and recurrence-free survival.
Study from Italy produces similar findings
An independent randomized, controlled trial examining survival outcomes for endometrial cancer patients with and without lymphadenectomy was released by the Italian group in late 2008. In this study, 537 patients who had histologically confirmed endometrial carcinoma believed to be confined to the uterus were randomized to total abdominal hysterectomy and bilateral salpingo-oophorectomy with or without pelvic lymphadenectomy.
Anatomic boundaries of the pelvic lymph-node dissection were clearly defined, and a minimum lymph-node count of 20 was specified for inclusion. Intraoperative frozen section was utilized to exclude patients who had grade-1 disease that was less than 50% invasive. The option of para-aortic lymph-node dissection or sampling was left to the discretion of the surgeon. If pelvic nodes were larger than 1 cm, they were removed or sampled regardless of randomization.
Unlike the ASTEC trial, this study did not attempt to control adjuvant treatment. Patients were treated according to the discretion of the physician. Most patients received no further therapy; only 20% underwent radiation therapy, and 7% received chemotherapy.
Given the findings of these two, large, multi-institutional trials with strikingly similar results but major problems, what is a gynecologist to do? Can lymphadenectomy be avoided in patients whose disease is believed to be confined to the uterus?
For now, the answer is a tentative “No.”
There appears to be no survival advantage to removal of lymph nodes when disease is confined to the uterus, but that is not to say there is no benefit to systematic lymphadenectomy—just that there is no survival benefit afforded by the procedure. Benefits of lymphadenectomy, which include more precise definition of the extent of disease, minimization of over- or undertreatment, and a reduction in overall treatment and cost, still remain. The concept of surgical debulking put forward by Bristow and coworkers still has merit, and any gross disease should be removed, if feasible.2
Lymphadenectomy in endometrial cancer remains controversial and complex, especially as we lack a precise method for determining which patients will have nodal disease. Our practice remains to remove the lymph nodes whenever possible to better tailor any adjuvant treatment.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Women in the lymphadenectomy group were more likely to have stage-IIIC disease, which is directly attributable to histologic evaluation of the lymph nodes in this group. The authors point out that these patients had more accurate assessment of their prognosis, allowing for the tailoring of adjuvant treatment.
The overall survival and disease-free survival curves for the two experimental groups were similar, consistent with data from the ASTEC trial. This proved to be true for both the intention-to-treat and according-to-protocol groups. The authors note that their results are similar to those of the ASTEC trial, despite the significant difference in the number of nodes removed in each trial.
Some aspects of the trials hamper interpretation and comparison
Outcomes are improved when surgery is performed by a trained gynecologic oncologist. In the ASTEC trial, each lymphadenectomy was performed by a specialized gynecologic surgeon who was “skilled in the procedure.” In the Italian study, the type of surgeon was not specified, but the specific anatomic boundaries of the dissection and the minimum node count were. More specific data are needed before any conclusions can be drawn about the effect of surgical skill on outcome in these trials.
In the ASTEC trial, 9% of patients in the lymphadenectomy group had no nodes removed, and more than 60% of patients would not have met criteria for inclusion in the lymphadenectomy arm of the Italian study—suggesting that the majority of women in the ASTEC trial had inadequate lymphadenectomy. Para-aortic lymphadenectomy was left to the discretion of the attending surgeon, and some patients did have resection of these nodes. The data do not include information about whether these patients were treated in the para-aortic region based on the histology of these nodes.
Randomization in a prospective study is supposed to equalize the risks between groups. In the ASTEC trial, despite randomization, there were 10% more patients who had deeply invasive disease in the lymphadenectomy group, along with 3% more adverse histologies and high-grade (grade-3) tumors. Given the higher incidence of positive nodes and poorer outcome in these cases, this difference may have had a significant impact on the evaluation of the groups for overall or disease-specific survival.
External-beam radiotherapy reduces local recurrence of endometrial Ca but does not improve survival
ASTEC/EN.5 Study Group, Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–146.
Radiation therapy has been a standard treatment for endometrial cancer when there is high risk of recurrence. This report combines two independent randomized, controlled trials investigating the benefit of postoperative adjuvant pelvic radiation in women who had early-stage disease and who met histologic criteria for high risk of recurrence and death. The trials are the EN.5 trial from Canada, and the radiation-therapy randomization of the ASTEC trial). Neither found a benefit in terms of overall survival, disease-specific survival, or recurrence-free survival, although local recurrence was reduced by 2.9% The authors also provide a review of the literature and a meta-analysis of other randomized, controlled trials on this subject.
Details of the EN.5 and ASTEC radiation-therapy trials
Criteria for enrollment were similar for the two trials, which focused on women who had histologically confirmed endometrial cancer and an intermediate or high risk of recurrence. This included women who had FIGO stage IA or stage IB (grade 3), stage IC (all grades), or papillary serous or clear-cell histology (all stages).
Survival is the primary goal of cancer treatment. External-beam radiotherapy does not improve survival, but does provide a small but real increase in local control. Regrettably, this improvement in local control comes at a cost: 3% of patients experience acute severe or life-threatening toxicity from treatment. The absolute difference in local recurrence between women who received external-beam radiotherapy and those who did not was only 2.9%. Local recurrences are largely salvageable in women who have not been irradiated.
Therefore, external-beam radiotherapy, as delivered in this trial, regardless of node status, should not be the standard of care. Improvement in technology with intensity-modulated radiotherapy, and the further evaluation of vaginal brachytherapy alone, may provide new ways to apply this kind of treatment in endometrial cancer.
This aspect of endometrial cancer treatment clearly needs further investigation. Trials are under way that may determine the role of radiation therapy in women who have endometrial cancer.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Lymphadenectomy was not required for patients enrolled in EN.5, but was part of the surgical randomization for ASTEC. This distinction could confound the results of the combined trials, as the investigators were trying to answer two questions within one patient population.
In both the EN.5 and ASTEC trials, women were randomized to observation or external-beam radiotherapy, with these parameters:
- Radiation therapy was to begin no later than 12 weeks after surgery (most patients began radiation therapy 6 to 8 weeks after surgery)
- For ASTEC, the target dosage was 40–46 Gy in 20–26 daily fractions to the pelvis, with treatment five times each week. For EN.5, the dosage and timing were very similar: 45 Gy, 25 daily fractions, five times weekly
- In both trials, vaginal brachytherapy was allowed if it was the local practice or the center’s policy
- Women were classified as being at intermediate risk or high risk, based on the likelihood of distant recurrence, as defined by GOG99 and PORTEC1 studies. Intermediate risk included all patients who had stage-IA or -IB (grade-3) or stage-IC or -IIA (grade-1 or -2) disease. Women who had papillary serous or clear-cell histology, stage-IC or -IIA (grade-3) disease, or any stage-IIB disease were considered at high risk.
The primary outcome evaluated for both trials was overall survival. Secondary endpoints were:
- disease-specific survival
- recurrence-free survival
- locoregional recurrence
- treatment toxicity.
A total of 905 women were enrolled in the ASTEC and EN.5 trials, with most patients having endometrial histology (83%) and being categorized as at intermediate risk (75%). Approximately half the patients in both trials received brachytherapy, which was allowed according to local practice. Only 47% of the observation group actually received no treatment.
Findings were remarkably similar in EN.5 and ASTEC
Here are the main findings:
- no difference between groups in overall survival, disease-specific survival, and recurrence-free survival
- significantly fewer isolated vaginal or pelvic initial recurrences in the external-beam radiotherapy group, with an absolute difference of 2.9%. (Only 35% of all recurrences were isolated recurrences)
- no significant difference between groups in distant or local and distant recurrences
- as expected, higher toxicity in the group receiving external-beam radiotherapy, including life-threatening toxicity (acute toxicity, 3% vs <1%; late toxicity, 1% vs 0%).
Subgroup analysis comparing overall survival in intermediate- and high-risk patients demonstrated no improvement with external-beam radiotherapy. Nor was overall survival altered by lymphadenectomy. The authors performed a meta-analysis using data from GOG99, PORTEC1, and this combined trial, and found no significant difference in overall survival or disease-specific survival, regardless of histologic risk group.
Trial has notable strengths and weaknesses
This large prospective trial has significant strengths: its size and its multi-institutional nature. The authors also evaluated their data in combination with other randomized, controlled trials to further investigate the effect of external-beam radiotherapy on survival. However, allowing brachytherapy somewhat confounds the true effect of external-beam radiotherapy on local recurrence. (There were few local recurrences, and the authors did not evaluate whether women who had an isolated vaginal recurrence received vaginal brachytherapy.) Moreover, 15% of women who were randomized to external-beam radiotherapy did not complete it.
In addition, secondary randomization of patients in the intermediate-risk and high-risk categories to external-beam radiotherapy versus no treatment may have significantly confounded the results of the entire ASTEC trial. Because women were, or were not, randomized to treatment regardless of node status, some patients who had positive nodes failed to receive adjuvant treatment. This may have had a significant effect on overall survival, as positive lymph nodes are a negative prognostic factor.
Dr. Mutch reports that he has received grant or research support from Lilly and Genentech. He serves as a speaker for GSK, Lilly, and Merck. Dr. Rimel reports no financial relationships relevant to this article.
Endometrial cancer is a great concern in industrialized nations, where it is the most common gynecologic cancer—with incidence increasing every year. Survival is generally very good for women who have low-grade disease confined to the uterus. However, for patients who have high-grade disease, an aggressive histologic type, or other features that suggest a poor prognosis, the cure rate approaches 75%.1
Primary surgery is the mainstay of initial treatment and basis of FIGO staging ( TABLE ), which requires:
- total hysterectomy
- bilateral salpingo-oophorectomy
- complete examination of the abdomen
- pelvic washings
- lymph-adenectomy (anatomic boundaries and node counts aren’t specified).
Controversy clouds our understanding of the optimal type of surgery, utility of pelvic lymphadenectomy, and possible benefit of adjuvant radiation therapy. During the past year, fuel has been added to this debate:
- Two randomized, controlled trials of surgery with and without pelvic lymphadenectomy in early-stage patients demonstrated no survival benefit. Earlier studies investigating the benefits of lymphadenectomy in endometrial cancer have been largely retrospective, and results have varied.
- A concurrent randomized, controlled trial of external-beam radiotherapy for women who have intermediate- or high-risk disease showed no improvement in overall survival, although local control increased by 3%.
TABLE
FIGO surgical staging for endometrial cancer
| Stage | Description |
|---|---|
| I | Tumor is confined to uterine fundus |
| IA | Tumor is limited to endometrium |
| IB | Tumor invades less than half of the myometrial thickness |
| IC | Tumor invades more than half of the myometrial thickness |
| II | Tumor extends to cervix |
| IIA | Cervical extension is limited to endocervical glands |
| IIB | Tumor invades cervical stroma |
| III | There is regional tumor spread |
| IIIA | Tumor invades uterine serosa or adnexa, or cells in the peritoneum show signs of cancer |
| IIIB | Vaginal metastases are present |
| IIIC | Tumor has spread to lymph nodes near the uterus |
| IV | There is bulky pelvic disease or distant spread |
| IVA | Tumor has spread to bladder or rectum |
| IVB | Distant metastases are present |
No survival advantage to pelvic lymphadenectomy—but it has other benefits
ASTEC study group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136.
Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716.
Among the arguments for lymphadenectomy in endometrial cancer staging are:
- It aids in the selection of women for radiation or other adjuvant treatment
- It may have a direct survival benefit, as suggested by retrospective studies.
But lymphadenectomy is time-consuming, requires a specialized gynecologic surgeon, and is associated with some increase in the risk of morbidity—namely, lymphedema, lymphocyst formation, deep-vein thrombosis (DVT), and blood loss.
The much-anticipated report of the 85-center, multinational ASTEC trial [ A S urgical T rial of E ndometrial C ancer], published earlier this year, offers further insight into the practice of lymphadenectomy. ASTEC involved two randomizations: The first, to pelvic lymphadenectomy; the second, to radiation therapy.
The ASTEC trial enrolled 1,408 women who had histologically confirmed endometrial carcinoma that was believed to be confined to the uterus. How this determination was made was not specified. Patients who had enlarged lymph nodes corroborated by computed tomography or magnetic resonance imaging were not excluded.
Participants were randomized to either of the following treatment groups:
- traditional surgery with total hysterectomy and bilateral salpingo-oophorectomy, pelvic washings, and palpation of para-aortic nodes
- the same surgery plus systematic lymphadenectomy of the iliac and obturator nodes.
If any para-aortic nodes were suspicious, biopsy or lymphadenectomy was performed at the discretion of the surgeon ( FIGURE ).
FIGURE Nodes reveal when cancer has spread
Women in the ASTEC trial were randomized to traditional surgery (total hysterectomy and bilateral salpingo-oophorectomy), pelvic washings, and palpation of para-aortic nodes or to the same surgery plus lymphadenectomy of the iliac and obturator nodes.
Operative findings determined a patient’s level of risk
After surgery, patients were categorized as having one of the following:
- low-risk, early-stage disease. This group included patients who had disease classified as stage IA or IB, grade 1 or 2. They were deemed to have a suitably low risk of recurrence to be offered further treatment according to their physician’s standard practice.
- intermediate- or high-risk, early-stage disease. These patients were randomized to the ASTEC radiation-therapy trial, which compared external-beam radiotherapy with no external-beam radiotherapy. The authors assert that this second randomization was necessary to prevent over- or undertreatment of patients who had unknown node status, which might alter survival outcomes.
- advanced disease. These patients were referred to their physician for further treatment.
In both surgical groups (with and without lymphadenectomy), approximately 80% of patients had disease confined to the uterus. Nodes were harvested in 91% of the patients in the lymphadenectomy group, compared with 5% of patients in the traditional-surgery group. Nine percent of women in the lymphadenectomy group had positive nodes.
The authors observe that more women had deeply invasive disease and adverse histologic types in the group that underwent lymphadenectomy. There were no differences between the two groups in overall survival; disease-specific survival; recurrence-free survival; or recurrence-free, disease-specific survival, after adjustment for baseline differences. Subgroup analysis for low-risk, high-risk, and advanced disease also failed to demonstrate differences in overall survival and recurrence-free survival.
Study from Italy produces similar findings
An independent randomized, controlled trial examining survival outcomes for endometrial cancer patients with and without lymphadenectomy was released by the Italian group in late 2008. In this study, 537 patients who had histologically confirmed endometrial carcinoma believed to be confined to the uterus were randomized to total abdominal hysterectomy and bilateral salpingo-oophorectomy with or without pelvic lymphadenectomy.
Anatomic boundaries of the pelvic lymph-node dissection were clearly defined, and a minimum lymph-node count of 20 was specified for inclusion. Intraoperative frozen section was utilized to exclude patients who had grade-1 disease that was less than 50% invasive. The option of para-aortic lymph-node dissection or sampling was left to the discretion of the surgeon. If pelvic nodes were larger than 1 cm, they were removed or sampled regardless of randomization.
Unlike the ASTEC trial, this study did not attempt to control adjuvant treatment. Patients were treated according to the discretion of the physician. Most patients received no further therapy; only 20% underwent radiation therapy, and 7% received chemotherapy.
Given the findings of these two, large, multi-institutional trials with strikingly similar results but major problems, what is a gynecologist to do? Can lymphadenectomy be avoided in patients whose disease is believed to be confined to the uterus?
For now, the answer is a tentative “No.”
There appears to be no survival advantage to removal of lymph nodes when disease is confined to the uterus, but that is not to say there is no benefit to systematic lymphadenectomy—just that there is no survival benefit afforded by the procedure. Benefits of lymphadenectomy, which include more precise definition of the extent of disease, minimization of over- or undertreatment, and a reduction in overall treatment and cost, still remain. The concept of surgical debulking put forward by Bristow and coworkers still has merit, and any gross disease should be removed, if feasible.2
Lymphadenectomy in endometrial cancer remains controversial and complex, especially as we lack a precise method for determining which patients will have nodal disease. Our practice remains to remove the lymph nodes whenever possible to better tailor any adjuvant treatment.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Women in the lymphadenectomy group were more likely to have stage-IIIC disease, which is directly attributable to histologic evaluation of the lymph nodes in this group. The authors point out that these patients had more accurate assessment of their prognosis, allowing for the tailoring of adjuvant treatment.
The overall survival and disease-free survival curves for the two experimental groups were similar, consistent with data from the ASTEC trial. This proved to be true for both the intention-to-treat and according-to-protocol groups. The authors note that their results are similar to those of the ASTEC trial, despite the significant difference in the number of nodes removed in each trial.
Some aspects of the trials hamper interpretation and comparison
Outcomes are improved when surgery is performed by a trained gynecologic oncologist. In the ASTEC trial, each lymphadenectomy was performed by a specialized gynecologic surgeon who was “skilled in the procedure.” In the Italian study, the type of surgeon was not specified, but the specific anatomic boundaries of the dissection and the minimum node count were. More specific data are needed before any conclusions can be drawn about the effect of surgical skill on outcome in these trials.
In the ASTEC trial, 9% of patients in the lymphadenectomy group had no nodes removed, and more than 60% of patients would not have met criteria for inclusion in the lymphadenectomy arm of the Italian study—suggesting that the majority of women in the ASTEC trial had inadequate lymphadenectomy. Para-aortic lymphadenectomy was left to the discretion of the attending surgeon, and some patients did have resection of these nodes. The data do not include information about whether these patients were treated in the para-aortic region based on the histology of these nodes.
Randomization in a prospective study is supposed to equalize the risks between groups. In the ASTEC trial, despite randomization, there were 10% more patients who had deeply invasive disease in the lymphadenectomy group, along with 3% more adverse histologies and high-grade (grade-3) tumors. Given the higher incidence of positive nodes and poorer outcome in these cases, this difference may have had a significant impact on the evaluation of the groups for overall or disease-specific survival.
External-beam radiotherapy reduces local recurrence of endometrial Ca but does not improve survival
ASTEC/EN.5 Study Group, Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–146.
Radiation therapy has been a standard treatment for endometrial cancer when there is high risk of recurrence. This report combines two independent randomized, controlled trials investigating the benefit of postoperative adjuvant pelvic radiation in women who had early-stage disease and who met histologic criteria for high risk of recurrence and death. The trials are the EN.5 trial from Canada, and the radiation-therapy randomization of the ASTEC trial). Neither found a benefit in terms of overall survival, disease-specific survival, or recurrence-free survival, although local recurrence was reduced by 2.9% The authors also provide a review of the literature and a meta-analysis of other randomized, controlled trials on this subject.
Details of the EN.5 and ASTEC radiation-therapy trials
Criteria for enrollment were similar for the two trials, which focused on women who had histologically confirmed endometrial cancer and an intermediate or high risk of recurrence. This included women who had FIGO stage IA or stage IB (grade 3), stage IC (all grades), or papillary serous or clear-cell histology (all stages).
Survival is the primary goal of cancer treatment. External-beam radiotherapy does not improve survival, but does provide a small but real increase in local control. Regrettably, this improvement in local control comes at a cost: 3% of patients experience acute severe or life-threatening toxicity from treatment. The absolute difference in local recurrence between women who received external-beam radiotherapy and those who did not was only 2.9%. Local recurrences are largely salvageable in women who have not been irradiated.
Therefore, external-beam radiotherapy, as delivered in this trial, regardless of node status, should not be the standard of care. Improvement in technology with intensity-modulated radiotherapy, and the further evaluation of vaginal brachytherapy alone, may provide new ways to apply this kind of treatment in endometrial cancer.
This aspect of endometrial cancer treatment clearly needs further investigation. Trials are under way that may determine the role of radiation therapy in women who have endometrial cancer.—DAVID G. MUTCH, MD; B. J. RIMEL, MD
Lymphadenectomy was not required for patients enrolled in EN.5, but was part of the surgical randomization for ASTEC. This distinction could confound the results of the combined trials, as the investigators were trying to answer two questions within one patient population.
In both the EN.5 and ASTEC trials, women were randomized to observation or external-beam radiotherapy, with these parameters:
- Radiation therapy was to begin no later than 12 weeks after surgery (most patients began radiation therapy 6 to 8 weeks after surgery)
- For ASTEC, the target dosage was 40–46 Gy in 20–26 daily fractions to the pelvis, with treatment five times each week. For EN.5, the dosage and timing were very similar: 45 Gy, 25 daily fractions, five times weekly
- In both trials, vaginal brachytherapy was allowed if it was the local practice or the center’s policy
- Women were classified as being at intermediate risk or high risk, based on the likelihood of distant recurrence, as defined by GOG99 and PORTEC1 studies. Intermediate risk included all patients who had stage-IA or -IB (grade-3) or stage-IC or -IIA (grade-1 or -2) disease. Women who had papillary serous or clear-cell histology, stage-IC or -IIA (grade-3) disease, or any stage-IIB disease were considered at high risk.
The primary outcome evaluated for both trials was overall survival. Secondary endpoints were:
- disease-specific survival
- recurrence-free survival
- locoregional recurrence
- treatment toxicity.
A total of 905 women were enrolled in the ASTEC and EN.5 trials, with most patients having endometrial histology (83%) and being categorized as at intermediate risk (75%). Approximately half the patients in both trials received brachytherapy, which was allowed according to local practice. Only 47% of the observation group actually received no treatment.
Findings were remarkably similar in EN.5 and ASTEC
Here are the main findings:
- no difference between groups in overall survival, disease-specific survival, and recurrence-free survival
- significantly fewer isolated vaginal or pelvic initial recurrences in the external-beam radiotherapy group, with an absolute difference of 2.9%. (Only 35% of all recurrences were isolated recurrences)
- no significant difference between groups in distant or local and distant recurrences
- as expected, higher toxicity in the group receiving external-beam radiotherapy, including life-threatening toxicity (acute toxicity, 3% vs <1%; late toxicity, 1% vs 0%).
Subgroup analysis comparing overall survival in intermediate- and high-risk patients demonstrated no improvement with external-beam radiotherapy. Nor was overall survival altered by lymphadenectomy. The authors performed a meta-analysis using data from GOG99, PORTEC1, and this combined trial, and found no significant difference in overall survival or disease-specific survival, regardless of histologic risk group.
Trial has notable strengths and weaknesses
This large prospective trial has significant strengths: its size and its multi-institutional nature. The authors also evaluated their data in combination with other randomized, controlled trials to further investigate the effect of external-beam radiotherapy on survival. However, allowing brachytherapy somewhat confounds the true effect of external-beam radiotherapy on local recurrence. (There were few local recurrences, and the authors did not evaluate whether women who had an isolated vaginal recurrence received vaginal brachytherapy.) Moreover, 15% of women who were randomized to external-beam radiotherapy did not complete it.
In addition, secondary randomization of patients in the intermediate-risk and high-risk categories to external-beam radiotherapy versus no treatment may have significantly confounded the results of the entire ASTEC trial. Because women were, or were not, randomized to treatment regardless of node status, some patients who had positive nodes failed to receive adjuvant treatment. This may have had a significant effect on overall survival, as positive lymph nodes are a negative prognostic factor.
1. Keys HM, Roberts JA, Brunetto VL, et al. Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744-751.
2. Bristow RE, Zahurak ML, Alexander CJ, Zellars RC, Montz FJ. FIGO stage IIIC endometrial carcinoma: resection of macroscopic nodal disease and other determinants of survival. Int J Gynecol Cancer. 2003;13:664-672.
1. Keys HM, Roberts JA, Brunetto VL, et al. Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744-751.
2. Bristow RE, Zahurak ML, Alexander CJ, Zellars RC, Montz FJ. FIGO stage IIIC endometrial carcinoma: resection of macroscopic nodal disease and other determinants of survival. Int J Gynecol Cancer. 2003;13:664-672.
Do some women with CIN 3 test negative for high-risk HPV?
EXPERT COMMENTARY
When it comes to cervical cancer prevention, screening and diagnostic tests have limits to their accuracy. Cancer-prevention strategies work because we assess patients over time and because we accept a small number of false positives as necessary to minimize the loss of sensitivity; in that way, we also minimize cancer-related morbidity.1
Link between high-risk HPV and cancer is a given
Cervical cancer is invariably linked to high-risk HPV. Women who are not truly infected with high-risk HPV are believed to have no risk for cervical cancer.2 Assay-based endpoints such as HPV DNA testing have good accuracy indices, whereas cytologic and histologic endpoints are subject to greater human error.
A CIN 3 lesion should never occur in a woman who is negative for high-risk HPV DNA. When the combination is found, which is rare, one of two mechanisms is involved:
- a falsely negative HPV test
- a falsely positive diagnosis of CIN 3.
In ALTS, falsely positive histology was probably to blame
The CIN 3 detected in women who tested negative for high-risk HPV DNA (i.e., after borderline cytology rather than because of a high-grade squamous intraepithelial lesion [HSIL]) was probably associated with falsely positive histology rather than falsely negative HPV testing.
CIN 3 in women who tested negative for high-risk HPV DNA was more likely to be:
- diagnosed at exit
- from a center where CIN 3 diagnoses were not confirmed by the Pathology
and less likely to be:
- associated with a referral Pap test classified as LSIL than as ASCUS
- associated with an enrollment Pap test classified as HSIL
- symptomatic
- associated with high-grade Cervigrams.
These women also were likely to test negative for HPV DNA using Linear Array.
In reviewing cases of CIN 3 in the 33 women who tested negative for high-risk HPV DNA, investigators found only 8 cases attributable to falsely-negative HPV testing, whereas 12 were related to incident HPV infection. Eight cases were caused by histologic overcall; 5 represented non–high-risk HPV that was unlikely to progress to cancer.
HPV testing has good, though not perfect, sensitivity for CIN 3. These findings certainly do not suggest that HPV testing is insufficiently accurate for clinical use.
Counsel women who have borderline cytology and who test negative for high-risk HPV to undergo continued testing according to the guidelines of the American Society for Colposcopy and Cervical Pathology.—DAVID G. MUTCH, MD
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical management guidelines for obstetricians-gynecologists. Number 61, April 2005. Human papillomavirus. Obstet Gynecol. 2005;105:905-918.
2. Kjaer S, Høgdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630-10636.
3. Safaeian M, Solomon D, Wacholder S, Schiffman M, Castle P. Risk of precancer and follow-up management strategies for women with human papillomavirus-negative atypical squamous cells of undetermined significance. Obstet Gynecol. 2007;109:1325-1331.
4. Schiffman M, Wheeler CM, Dasgupta A, Solomon D, Castle PE. for The ALTS Group. A comparison of a prototype PCR assay and hybrid capture 2 for detection of carcinogenic human papillomavirus DNA in women with equivocal or mildly abnormal Papanicolaou smears. Am J Clin Pathol. 2005;124:722-732.
5. Eltoum IA, Chhieng DC, Crowe DR, Roberson J, Jin G, Broker TR. Significance and possible causes of false-negative results of reflex human papillomavirus infection testing. Cancer. 2007;111:154-159.
EXPERT COMMENTARY
When it comes to cervical cancer prevention, screening and diagnostic tests have limits to their accuracy. Cancer-prevention strategies work because we assess patients over time and because we accept a small number of false positives as necessary to minimize the loss of sensitivity; in that way, we also minimize cancer-related morbidity.1
Link between high-risk HPV and cancer is a given
Cervical cancer is invariably linked to high-risk HPV. Women who are not truly infected with high-risk HPV are believed to have no risk for cervical cancer.2 Assay-based endpoints such as HPV DNA testing have good accuracy indices, whereas cytologic and histologic endpoints are subject to greater human error.
A CIN 3 lesion should never occur in a woman who is negative for high-risk HPV DNA. When the combination is found, which is rare, one of two mechanisms is involved:
- a falsely negative HPV test
- a falsely positive diagnosis of CIN 3.
In ALTS, falsely positive histology was probably to blame
The CIN 3 detected in women who tested negative for high-risk HPV DNA (i.e., after borderline cytology rather than because of a high-grade squamous intraepithelial lesion [HSIL]) was probably associated with falsely positive histology rather than falsely negative HPV testing.
CIN 3 in women who tested negative for high-risk HPV DNA was more likely to be:
- diagnosed at exit
- from a center where CIN 3 diagnoses were not confirmed by the Pathology
and less likely to be:
- associated with a referral Pap test classified as LSIL than as ASCUS
- associated with an enrollment Pap test classified as HSIL
- symptomatic
- associated with high-grade Cervigrams.
These women also were likely to test negative for HPV DNA using Linear Array.
In reviewing cases of CIN 3 in the 33 women who tested negative for high-risk HPV DNA, investigators found only 8 cases attributable to falsely-negative HPV testing, whereas 12 were related to incident HPV infection. Eight cases were caused by histologic overcall; 5 represented non–high-risk HPV that was unlikely to progress to cancer.
HPV testing has good, though not perfect, sensitivity for CIN 3. These findings certainly do not suggest that HPV testing is insufficiently accurate for clinical use.
Counsel women who have borderline cytology and who test negative for high-risk HPV to undergo continued testing according to the guidelines of the American Society for Colposcopy and Cervical Pathology.—DAVID G. MUTCH, MD
EXPERT COMMENTARY
When it comes to cervical cancer prevention, screening and diagnostic tests have limits to their accuracy. Cancer-prevention strategies work because we assess patients over time and because we accept a small number of false positives as necessary to minimize the loss of sensitivity; in that way, we also minimize cancer-related morbidity.1
Link between high-risk HPV and cancer is a given
Cervical cancer is invariably linked to high-risk HPV. Women who are not truly infected with high-risk HPV are believed to have no risk for cervical cancer.2 Assay-based endpoints such as HPV DNA testing have good accuracy indices, whereas cytologic and histologic endpoints are subject to greater human error.
A CIN 3 lesion should never occur in a woman who is negative for high-risk HPV DNA. When the combination is found, which is rare, one of two mechanisms is involved:
- a falsely negative HPV test
- a falsely positive diagnosis of CIN 3.
In ALTS, falsely positive histology was probably to blame
The CIN 3 detected in women who tested negative for high-risk HPV DNA (i.e., after borderline cytology rather than because of a high-grade squamous intraepithelial lesion [HSIL]) was probably associated with falsely positive histology rather than falsely negative HPV testing.
CIN 3 in women who tested negative for high-risk HPV DNA was more likely to be:
- diagnosed at exit
- from a center where CIN 3 diagnoses were not confirmed by the Pathology
and less likely to be:
- associated with a referral Pap test classified as LSIL than as ASCUS
- associated with an enrollment Pap test classified as HSIL
- symptomatic
- associated with high-grade Cervigrams.
These women also were likely to test negative for HPV DNA using Linear Array.
In reviewing cases of CIN 3 in the 33 women who tested negative for high-risk HPV DNA, investigators found only 8 cases attributable to falsely-negative HPV testing, whereas 12 were related to incident HPV infection. Eight cases were caused by histologic overcall; 5 represented non–high-risk HPV that was unlikely to progress to cancer.
HPV testing has good, though not perfect, sensitivity for CIN 3. These findings certainly do not suggest that HPV testing is insufficiently accurate for clinical use.
Counsel women who have borderline cytology and who test negative for high-risk HPV to undergo continued testing according to the guidelines of the American Society for Colposcopy and Cervical Pathology.—DAVID G. MUTCH, MD
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical management guidelines for obstetricians-gynecologists. Number 61, April 2005. Human papillomavirus. Obstet Gynecol. 2005;105:905-918.
2. Kjaer S, Høgdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630-10636.
3. Safaeian M, Solomon D, Wacholder S, Schiffman M, Castle P. Risk of precancer and follow-up management strategies for women with human papillomavirus-negative atypical squamous cells of undetermined significance. Obstet Gynecol. 2007;109:1325-1331.
4. Schiffman M, Wheeler CM, Dasgupta A, Solomon D, Castle PE. for The ALTS Group. A comparison of a prototype PCR assay and hybrid capture 2 for detection of carcinogenic human papillomavirus DNA in women with equivocal or mildly abnormal Papanicolaou smears. Am J Clin Pathol. 2005;124:722-732.
5. Eltoum IA, Chhieng DC, Crowe DR, Roberson J, Jin G, Broker TR. Significance and possible causes of false-negative results of reflex human papillomavirus infection testing. Cancer. 2007;111:154-159.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical management guidelines for obstetricians-gynecologists. Number 61, April 2005. Human papillomavirus. Obstet Gynecol. 2005;105:905-918.
2. Kjaer S, Høgdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630-10636.
3. Safaeian M, Solomon D, Wacholder S, Schiffman M, Castle P. Risk of precancer and follow-up management strategies for women with human papillomavirus-negative atypical squamous cells of undetermined significance. Obstet Gynecol. 2007;109:1325-1331.
4. Schiffman M, Wheeler CM, Dasgupta A, Solomon D, Castle PE. for The ALTS Group. A comparison of a prototype PCR assay and hybrid capture 2 for detection of carcinogenic human papillomavirus DNA in women with equivocal or mildly abnormal Papanicolaou smears. Am J Clin Pathol. 2005;124:722-732.
5. Eltoum IA, Chhieng DC, Crowe DR, Roberson J, Jin G, Broker TR. Significance and possible causes of false-negative results of reflex human papillomavirus infection testing. Cancer. 2007;111:154-159.
Endometrial Cancer
The authors report no financial relationships relevant to this article.
Postmenopausal bleeding is a symptom evaluated often by general gynecologists. It necessitates assessment of the endometrium, most often by tissue sampling. When endometrial cancer is confirmed by biopsy, management becomes complex. Should the patient be referred to a gynecologic oncologist? What kind of surgery does she need? What kind of adjuvant treatment will be offered? Could the diagnosis be part of a genetic cancer syndrome?
Recent studies have yielded new information:
- Preoperative, intraoperative, and postoperative care by a gynecologic oncologist significantly lowers the cost of health care
- Lymphadenectomy for endometrial cancer remains controversial, and may be unnecessary in low-risk patients
- Chemotherapy plays an expanding role in the treatment of endometrial cancer. Adjuvant therapy with doxorubicin, cisplatin, and paclitaxel is the treatment of choice for patients who have advanced-stage disease
- Nine percent of women who are given a diagnosis of endometrial cancer before 50 years of age have a germ-line Lynch syndrome-associated mutation, which demonstrates that heredity is an important aspect of endometrial cancer and should be considered at all times.
It’s good economics to refer patients to gyn oncology sooner, not later
Hoekstra A, Singh DK, Garb M, Arekapudi S, Rademaker A, Lurain JR. Participation of the general gynecologist in the surgical staging of endometrial cancer: analysis of cost and perioperative outcomes. Gynecol Oncol. 2006;103:897–901.
Early-stage endometrial cancer is often curable with surgery alone because a full 88% of endometrial cancers present as clinical stage I.1 The role of the general gynecologist in surgical management of these cases is controversial; at some institutions, the practice is to call in the gynecologic oncologist for lymph-node sampling or when gross disease is identified; at others, the standard is to refer the patient to gynecologic oncology as soon as malignancy is diagnosed by endometrial biopsy. Hoekstra and colleagues have attempted to shed light on this issue with a retrospective chart review of 121 patients who were treated at one institution from 1998 to 2000.
Costs of early treatment by a gynecologic oncologist were lower than without referral
The authors performed a retrospective analysis of a group of women with clinical stage-I endometrial cancer who were treated surgically at Prentice Women’s Hospital in Chicago.
The cohort was divided in two:
- Group 1 comprised patients who underwent surgery with a general gynecologist, who consulted a gynecologic oncologist intraoperatively
- Group 2 comprised patients who were referred to a gynecologic oncologist before surgery and underwent the procedure with a gynecologic oncologist.
Overall, subjects in both groups were similar in age, distribution of surgical stage, need for lymphadenectomy, and length of follow-up.
Group 2 had a significantly shorter operative time overall, and shorter total time in the operating room. Cost per procedure was also significantly lower in this group, in terms of cost to the payer and the physician’s charge. Perioperative costs were also lower in Group 2.
No difference was observed in postoperative outcome. Total hospital costs and lengths of stay were also similar.
Recommendation for practice
With health-care costs rising, be aware of referral strategies that promote cost containment. Women who have endometrial cancer may benefit from the early involvement of a gynecologic oncologist.
Is lymphadenectomy necessary when risk of metastasis is low?
Mariani A, Dowdy S, Cliby W, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18.
The need for surgical staging of endometrial cancer has been recognized since surgical staging criteria were adopted by the International Federation of Gynecology and Obstetrics (FIGO) in 1988. Staging includes hysterectomy, bilateral salpingo-oophorectomy, and biopsy of any gross disease. Clear guidelines on the assessment of lymphatic dissemination and the anatomic extent of this assessment are, however, still lacking.
Proponents of systematic pelvic and para-aortic lymph-node dissection for patients with endometrial cancer cite:
- the 15% risk of lymph-node metastasis in women who have tumors 2 cm or larger in diameter2
- poor correlation between frozen-section grade and myometrial invasion with final pathology3
- the potential therapeutic benefit of the procedure.4
Opponents of such lymph-node dissection argue that women who have grade-1, stage-I disease will be overtreated if standardized lymphadenectomy is adopted.
Several retrospective studies have explored this question, with varying results. A large, prospective, randomized trial evaluating lymphadenectomy in clinical stage-I patients (ASTEC trial) has been completed, but is yet to be published.
When lymphadenectomy may (or may not) be necessary
After prospectively studying more than 300 endometrial cancer patients treated at the Mayo Clinic between 1984 and 1996,5 Mariani and colleagues launched a new study to assess a novel pattern of surgical management that aims to reduce the number of low-risk patients receiving lymphadenectomy. According to this pattern, the following types of women were able to bypass lymphadenectomy:
- those who had type-I, grades-1 and -2 tumors
- those with myometrial invasion ≤50%
- those with a primary tumor ≤2 cm in diameter.
Women who had endometrial cancer that did not meet these criteria underwent complete lymphadenectomy to the level of the renal vessels. Histologic assessment of the uterus to determine grade, depth of invasion, and primary tumor diameter was performed by frozen-section analysis in all cases.
The study included 422 women from January 2004 to December 2006. According to the guidelines of the study, 112 patients did not require lymphadenectomy. However, 22 (20%) women in this group did undergo the procedure because of palpable lymphadenopathy, initiation of dissection before the frozen-section report was received, or physician preference. All nodes were negative in these patients.
Of the women who met criteria for lymphadenectomy, 29 (9%) did not undergo dissection; among the reasons were disseminated disease, comorbid conditions, and advanced age. Of the women defined as at-risk who did undergo lymphadenectomy, 22% had lymph-node metastases.
Most positive para-aortic nodes lay above the inferior mesenteric artery
Information regarding the anatomic location of para-aortic nodal metastases was available for a small subset of women in the study. Seventy-seven percent of these women had para-aortic nodal metastasis above the inferior mesenteric artery. In addition, 71% of these patients had ipsilateral pelvic nodes that were free of disease. However, these patients had a poorer prognosis, and many would have received adjuvant therapy based on their hysterectomy specimen alone.
Recommendation for practice
This study suggests that there is a subset of patients who have endometrial cancer that is very low in risk and, because of this, they may forego lymph-node dissection without harm. In addition, a significant number of periaortic nodal metastases occur above the inferior mesenteric artery and in the absence of pelvic node involvement.
One of the limitations of this study is the need for intraoperative uterine assessment by frozen section by an expert pathologist—a service that is not widely available.
Taken together, these data suggest that, if the uterus can be assessed by frozen section at the time of surgery, a subset of clinical stage-I patients can be spared lymphadenectomy and its attendant risks.
Patients undergoing lymphatic assessment should undergo full systematic lymphnode dissection, not sampling. The dissection should include the region above the inferior mesenteric artery.
Removal of lymph nodes in endometrial cancer remains complex and controversial, a fact that strengthens the argument that an experienced gynecologic oncologist should be involved in the care of patients who have this disease.
Chemotherapy is warranted in advanced or recurrent disease
Homesley H, Filiaci V, Gibbons S, et al. Randomized phase III trial in advanced endometrial carcinoma of surgery and volume-directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Abstract presented at the Society of Gynecologic Oncologists, March 2008.
Adjuvant therapy for advanced-stage endometrial cancer has varied considerably over the years, and treatment of these patients remains somewhat controversial. Clinical trials comparing chemotherapy with radiation, and chemotherapy regimens with each other, have led to an era in which chemotherapy is used to treat more women than ever before.
In 2006, the Gynecologic Oncology Group (GOG) published the results of a prospective randomized study (GOG 122) that compared whole-abdomen radiation to doxorubicin and cisplatin in stage-III or -IV endometrial cancer. The investigators determined that chemotherapy was superior to whole-abdomen radiation in this trial.6
This finding was quickly followed by another trial (GOG 177) in which doxorubicin plus cisplatin was compared with a regimen of doxorubicin, cisplatin, and paclitaxel. Women in this trial had stage-III or -IV or recurrent disease. A history of radiation treatment did not disqualify patients from the study, and the treatment groups were well balanced in randomization. The doxorubicin–cisplatin–paclitaxel arm improved progression-free and overall survival, making this combination the preferred treatment.7
After volume-directed radiation, paclitaxel does not add benefit
Several retrospective analyses of radiation versus chemotherapy have shown improvement with radiation or combination therapy.8 Most recently, the GOG released data from a trial comparing chemotherapy regimens after radiation treatment. GOG 184 evaluated surgically debulked, stage-III or -IV patients who had received volume-directed radiation; subjects were randomized into either of two chemotherapy regimens:
- doxorubicin plus cisplatin
- doxorubicin, cisplatin, and paclitaxel.
In these patients, there was no improvement in survival when paclitaxel was added to the chemotherapeutic regimen, compared with doxorubicin plus cisplatin. Morbidity increased, however, with the addition of paclitaxel.
Patients in this trial underwent surgical resection of all gross disease, with no residual tumor larger than 2 cm. The role of optimal cytoreduction in endometrial cancer has been debated, however. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter (FIGURE).9-11 GOG 184 inclusion criteria required surgical resection of gross disease to ≤2 cm in diameter. It is possible that the therapeutic benefit of surgical debulking may have improved outcome in these patients—to the extent that the addition of paclitaxel did not provide appreciable benefit.
FIGURE Debulk gross disease?
The role of optimal cytoreduction in endometrial cancer has been debated. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter.
Recommendation for practice
Data on adjuvant therapy for endometrial cancer remains conflicting. Women who have advanced-stage or recurrent endometrial cancer should receive chemotherapy—either with doxorubicin, cisplatin, and paclitaxel, or a platinum taxane regimen. The addition of paclitaxel in surgically debulked patients who have undergone radiation treatment does not appear to improve survival.
There is, however, a clear recommendation for paclitaxel in radiation-naïve patients and those who have gross residual disease. Further studies are needed to elucidate the role of radiation therapy in an era of volume-directed radiation.
Look for Lynch syndrome in young women with endometrial cancer
Lu K, Schorge J, Rodabaugh K, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164.
Endometrial cancer is part of the spectrum of Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). Patients with this autosomal-dominant hereditary cancer susceptibility syndrome may present with colorectal cancer, endometrial cancer, or, more rarely, ovarian cancer. Lynch syndrome derives from germline mutations in DNA mismatch repair genes, most often MLH1, MSH2, and MSH6.12 Genetic testing for all three genes is available for clinical use.
In the past, screening for Lynch syndrome focused on colorectal cancer, but it is now clear that women who have this disorder have a lifetime risk of developing endometrial cancer that exceeds 40%.13
In Lynch syndrome, gynecologic cancer often precedes colon cancer
Women with Lynch syndrome-associated endometrial cancer typically present at a younger age than their syndrome-free counterparts (48 compared with 60 years).14 Previous retrospective studies demonstrated that 50% of women with Lynch syndrome-associated colon and gynecologic cancers had gynecologic cancer preceding the colon cancer. The average was 11 years earlier for endometrial cancer, which suggests that, if these women could be identified at the time they are given their diagnosis of endometrial cancer, more intensive screening for colon cancer could then be initiated.15
9% of women who develop endometrial cancer before age 50 have Lynch syndrome
One of the screening criteria for Lynch syndrome-associated colon cancer is age <50 years. In this recent prospective, multicenter study involving 100 women who were diagnosed with endometrial cancer at less than 50 years of age, germline Lynch syndrome mutations were identified in 9% of patients. (In this study, germline mutation testing was performed for MLH1, MSH2, and MSH6 genes by full sequencing, and immunohistochemistry was performed for all three genes. Microsatellite analysis was performed on 95 patients, with five women having insufficient tumor for DNA extraction.)
All women who had a germline mutation had a first-degree relative with Lynch syndrome-associated cancer. The combination of a negative family history for Lynch syndrome and a body mass index greater than 30 was highly predictive of having no Lynch syndrome mutation, with a negative predictive value of 96%.
Recommendation for practice
Patients who have an hereditary cancer syndrome such as Lynch syndrome can begin cancer-prevention screening—and be engaged in that screening—when the syndrome is recognized early. Because women who have endometrial cancer that was diagnosed before they were 50 years old are at significant risk of a germline mutation, they should be offered genetic counseling and testing.
1. Fanning J, Hoffman ML, Andrews SJ, Harrah AW, Feldmeier JJ. Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: postoperative brachytherapy vs. observation. Gynecol Oncol. 2004;93:632-636.
2. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor size in endometrial cancer. Cancer. 1991;67:2791-2794.
3. Case AS, Rocconi RP, Straughn JM Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375-1379.
4. Horowitz NS, Peters WA, 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235-240.
5. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506-1519.
6. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
7. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159-2166.
8. Alvarez Secord A, Havrilesky LJ, Bac-Jump V, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285-291.
9. Bristow RE, Zerbe MJ, Rosenshein NB, Grumbine FC, Montz FJ. Stage IVB endometrial carcinoma: the role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85-91.
10. Bristow RE, Santillan A, Zahurak ML, Gardner GJ, Giuntoli RL, 2nd, Armstrong DK. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol Oncol. 2006;103:281-287.
11. Lambrou NC, Gómez-Marín O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: a study of morbidity and survival. Gynecol Oncol. 2004;93:653-658.
12. Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborate study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146-1158.
13. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
14. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on HNPCC. Anticancer Res. 1994;14:1661-1664.
15. Lu KH, Broaddus RR. Gynecological tumors in hereditary nonpolyposis colorectal cancer: we know they are common—now what? Gynecol Oncol. 2001;82:221-222.
The authors report no financial relationships relevant to this article.
Postmenopausal bleeding is a symptom evaluated often by general gynecologists. It necessitates assessment of the endometrium, most often by tissue sampling. When endometrial cancer is confirmed by biopsy, management becomes complex. Should the patient be referred to a gynecologic oncologist? What kind of surgery does she need? What kind of adjuvant treatment will be offered? Could the diagnosis be part of a genetic cancer syndrome?
Recent studies have yielded new information:
- Preoperative, intraoperative, and postoperative care by a gynecologic oncologist significantly lowers the cost of health care
- Lymphadenectomy for endometrial cancer remains controversial, and may be unnecessary in low-risk patients
- Chemotherapy plays an expanding role in the treatment of endometrial cancer. Adjuvant therapy with doxorubicin, cisplatin, and paclitaxel is the treatment of choice for patients who have advanced-stage disease
- Nine percent of women who are given a diagnosis of endometrial cancer before 50 years of age have a germ-line Lynch syndrome-associated mutation, which demonstrates that heredity is an important aspect of endometrial cancer and should be considered at all times.
It’s good economics to refer patients to gyn oncology sooner, not later
Hoekstra A, Singh DK, Garb M, Arekapudi S, Rademaker A, Lurain JR. Participation of the general gynecologist in the surgical staging of endometrial cancer: analysis of cost and perioperative outcomes. Gynecol Oncol. 2006;103:897–901.
Early-stage endometrial cancer is often curable with surgery alone because a full 88% of endometrial cancers present as clinical stage I.1 The role of the general gynecologist in surgical management of these cases is controversial; at some institutions, the practice is to call in the gynecologic oncologist for lymph-node sampling or when gross disease is identified; at others, the standard is to refer the patient to gynecologic oncology as soon as malignancy is diagnosed by endometrial biopsy. Hoekstra and colleagues have attempted to shed light on this issue with a retrospective chart review of 121 patients who were treated at one institution from 1998 to 2000.
Costs of early treatment by a gynecologic oncologist were lower than without referral
The authors performed a retrospective analysis of a group of women with clinical stage-I endometrial cancer who were treated surgically at Prentice Women’s Hospital in Chicago.
The cohort was divided in two:
- Group 1 comprised patients who underwent surgery with a general gynecologist, who consulted a gynecologic oncologist intraoperatively
- Group 2 comprised patients who were referred to a gynecologic oncologist before surgery and underwent the procedure with a gynecologic oncologist.
Overall, subjects in both groups were similar in age, distribution of surgical stage, need for lymphadenectomy, and length of follow-up.
Group 2 had a significantly shorter operative time overall, and shorter total time in the operating room. Cost per procedure was also significantly lower in this group, in terms of cost to the payer and the physician’s charge. Perioperative costs were also lower in Group 2.
No difference was observed in postoperative outcome. Total hospital costs and lengths of stay were also similar.
Recommendation for practice
With health-care costs rising, be aware of referral strategies that promote cost containment. Women who have endometrial cancer may benefit from the early involvement of a gynecologic oncologist.
Is lymphadenectomy necessary when risk of metastasis is low?
Mariani A, Dowdy S, Cliby W, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18.
The need for surgical staging of endometrial cancer has been recognized since surgical staging criteria were adopted by the International Federation of Gynecology and Obstetrics (FIGO) in 1988. Staging includes hysterectomy, bilateral salpingo-oophorectomy, and biopsy of any gross disease. Clear guidelines on the assessment of lymphatic dissemination and the anatomic extent of this assessment are, however, still lacking.
Proponents of systematic pelvic and para-aortic lymph-node dissection for patients with endometrial cancer cite:
- the 15% risk of lymph-node metastasis in women who have tumors 2 cm or larger in diameter2
- poor correlation between frozen-section grade and myometrial invasion with final pathology3
- the potential therapeutic benefit of the procedure.4
Opponents of such lymph-node dissection argue that women who have grade-1, stage-I disease will be overtreated if standardized lymphadenectomy is adopted.
Several retrospective studies have explored this question, with varying results. A large, prospective, randomized trial evaluating lymphadenectomy in clinical stage-I patients (ASTEC trial) has been completed, but is yet to be published.
When lymphadenectomy may (or may not) be necessary
After prospectively studying more than 300 endometrial cancer patients treated at the Mayo Clinic between 1984 and 1996,5 Mariani and colleagues launched a new study to assess a novel pattern of surgical management that aims to reduce the number of low-risk patients receiving lymphadenectomy. According to this pattern, the following types of women were able to bypass lymphadenectomy:
- those who had type-I, grades-1 and -2 tumors
- those with myometrial invasion ≤50%
- those with a primary tumor ≤2 cm in diameter.
Women who had endometrial cancer that did not meet these criteria underwent complete lymphadenectomy to the level of the renal vessels. Histologic assessment of the uterus to determine grade, depth of invasion, and primary tumor diameter was performed by frozen-section analysis in all cases.
The study included 422 women from January 2004 to December 2006. According to the guidelines of the study, 112 patients did not require lymphadenectomy. However, 22 (20%) women in this group did undergo the procedure because of palpable lymphadenopathy, initiation of dissection before the frozen-section report was received, or physician preference. All nodes were negative in these patients.
Of the women who met criteria for lymphadenectomy, 29 (9%) did not undergo dissection; among the reasons were disseminated disease, comorbid conditions, and advanced age. Of the women defined as at-risk who did undergo lymphadenectomy, 22% had lymph-node metastases.
Most positive para-aortic nodes lay above the inferior mesenteric artery
Information regarding the anatomic location of para-aortic nodal metastases was available for a small subset of women in the study. Seventy-seven percent of these women had para-aortic nodal metastasis above the inferior mesenteric artery. In addition, 71% of these patients had ipsilateral pelvic nodes that were free of disease. However, these patients had a poorer prognosis, and many would have received adjuvant therapy based on their hysterectomy specimen alone.
Recommendation for practice
This study suggests that there is a subset of patients who have endometrial cancer that is very low in risk and, because of this, they may forego lymph-node dissection without harm. In addition, a significant number of periaortic nodal metastases occur above the inferior mesenteric artery and in the absence of pelvic node involvement.
One of the limitations of this study is the need for intraoperative uterine assessment by frozen section by an expert pathologist—a service that is not widely available.
Taken together, these data suggest that, if the uterus can be assessed by frozen section at the time of surgery, a subset of clinical stage-I patients can be spared lymphadenectomy and its attendant risks.
Patients undergoing lymphatic assessment should undergo full systematic lymphnode dissection, not sampling. The dissection should include the region above the inferior mesenteric artery.
Removal of lymph nodes in endometrial cancer remains complex and controversial, a fact that strengthens the argument that an experienced gynecologic oncologist should be involved in the care of patients who have this disease.
Chemotherapy is warranted in advanced or recurrent disease
Homesley H, Filiaci V, Gibbons S, et al. Randomized phase III trial in advanced endometrial carcinoma of surgery and volume-directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Abstract presented at the Society of Gynecologic Oncologists, March 2008.
Adjuvant therapy for advanced-stage endometrial cancer has varied considerably over the years, and treatment of these patients remains somewhat controversial. Clinical trials comparing chemotherapy with radiation, and chemotherapy regimens with each other, have led to an era in which chemotherapy is used to treat more women than ever before.
In 2006, the Gynecologic Oncology Group (GOG) published the results of a prospective randomized study (GOG 122) that compared whole-abdomen radiation to doxorubicin and cisplatin in stage-III or -IV endometrial cancer. The investigators determined that chemotherapy was superior to whole-abdomen radiation in this trial.6
This finding was quickly followed by another trial (GOG 177) in which doxorubicin plus cisplatin was compared with a regimen of doxorubicin, cisplatin, and paclitaxel. Women in this trial had stage-III or -IV or recurrent disease. A history of radiation treatment did not disqualify patients from the study, and the treatment groups were well balanced in randomization. The doxorubicin–cisplatin–paclitaxel arm improved progression-free and overall survival, making this combination the preferred treatment.7
After volume-directed radiation, paclitaxel does not add benefit
Several retrospective analyses of radiation versus chemotherapy have shown improvement with radiation or combination therapy.8 Most recently, the GOG released data from a trial comparing chemotherapy regimens after radiation treatment. GOG 184 evaluated surgically debulked, stage-III or -IV patients who had received volume-directed radiation; subjects were randomized into either of two chemotherapy regimens:
- doxorubicin plus cisplatin
- doxorubicin, cisplatin, and paclitaxel.
In these patients, there was no improvement in survival when paclitaxel was added to the chemotherapeutic regimen, compared with doxorubicin plus cisplatin. Morbidity increased, however, with the addition of paclitaxel.
Patients in this trial underwent surgical resection of all gross disease, with no residual tumor larger than 2 cm. The role of optimal cytoreduction in endometrial cancer has been debated, however. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter (FIGURE).9-11 GOG 184 inclusion criteria required surgical resection of gross disease to ≤2 cm in diameter. It is possible that the therapeutic benefit of surgical debulking may have improved outcome in these patients—to the extent that the addition of paclitaxel did not provide appreciable benefit.
FIGURE Debulk gross disease?
The role of optimal cytoreduction in endometrial cancer has been debated. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter.
Recommendation for practice
Data on adjuvant therapy for endometrial cancer remains conflicting. Women who have advanced-stage or recurrent endometrial cancer should receive chemotherapy—either with doxorubicin, cisplatin, and paclitaxel, or a platinum taxane regimen. The addition of paclitaxel in surgically debulked patients who have undergone radiation treatment does not appear to improve survival.
There is, however, a clear recommendation for paclitaxel in radiation-naïve patients and those who have gross residual disease. Further studies are needed to elucidate the role of radiation therapy in an era of volume-directed radiation.
Look for Lynch syndrome in young women with endometrial cancer
Lu K, Schorge J, Rodabaugh K, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164.
Endometrial cancer is part of the spectrum of Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). Patients with this autosomal-dominant hereditary cancer susceptibility syndrome may present with colorectal cancer, endometrial cancer, or, more rarely, ovarian cancer. Lynch syndrome derives from germline mutations in DNA mismatch repair genes, most often MLH1, MSH2, and MSH6.12 Genetic testing for all three genes is available for clinical use.
In the past, screening for Lynch syndrome focused on colorectal cancer, but it is now clear that women who have this disorder have a lifetime risk of developing endometrial cancer that exceeds 40%.13
In Lynch syndrome, gynecologic cancer often precedes colon cancer
Women with Lynch syndrome-associated endometrial cancer typically present at a younger age than their syndrome-free counterparts (48 compared with 60 years).14 Previous retrospective studies demonstrated that 50% of women with Lynch syndrome-associated colon and gynecologic cancers had gynecologic cancer preceding the colon cancer. The average was 11 years earlier for endometrial cancer, which suggests that, if these women could be identified at the time they are given their diagnosis of endometrial cancer, more intensive screening for colon cancer could then be initiated.15
9% of women who develop endometrial cancer before age 50 have Lynch syndrome
One of the screening criteria for Lynch syndrome-associated colon cancer is age <50 years. In this recent prospective, multicenter study involving 100 women who were diagnosed with endometrial cancer at less than 50 years of age, germline Lynch syndrome mutations were identified in 9% of patients. (In this study, germline mutation testing was performed for MLH1, MSH2, and MSH6 genes by full sequencing, and immunohistochemistry was performed for all three genes. Microsatellite analysis was performed on 95 patients, with five women having insufficient tumor for DNA extraction.)
All women who had a germline mutation had a first-degree relative with Lynch syndrome-associated cancer. The combination of a negative family history for Lynch syndrome and a body mass index greater than 30 was highly predictive of having no Lynch syndrome mutation, with a negative predictive value of 96%.
Recommendation for practice
Patients who have an hereditary cancer syndrome such as Lynch syndrome can begin cancer-prevention screening—and be engaged in that screening—when the syndrome is recognized early. Because women who have endometrial cancer that was diagnosed before they were 50 years old are at significant risk of a germline mutation, they should be offered genetic counseling and testing.
The authors report no financial relationships relevant to this article.
Postmenopausal bleeding is a symptom evaluated often by general gynecologists. It necessitates assessment of the endometrium, most often by tissue sampling. When endometrial cancer is confirmed by biopsy, management becomes complex. Should the patient be referred to a gynecologic oncologist? What kind of surgery does she need? What kind of adjuvant treatment will be offered? Could the diagnosis be part of a genetic cancer syndrome?
Recent studies have yielded new information:
- Preoperative, intraoperative, and postoperative care by a gynecologic oncologist significantly lowers the cost of health care
- Lymphadenectomy for endometrial cancer remains controversial, and may be unnecessary in low-risk patients
- Chemotherapy plays an expanding role in the treatment of endometrial cancer. Adjuvant therapy with doxorubicin, cisplatin, and paclitaxel is the treatment of choice for patients who have advanced-stage disease
- Nine percent of women who are given a diagnosis of endometrial cancer before 50 years of age have a germ-line Lynch syndrome-associated mutation, which demonstrates that heredity is an important aspect of endometrial cancer and should be considered at all times.
It’s good economics to refer patients to gyn oncology sooner, not later
Hoekstra A, Singh DK, Garb M, Arekapudi S, Rademaker A, Lurain JR. Participation of the general gynecologist in the surgical staging of endometrial cancer: analysis of cost and perioperative outcomes. Gynecol Oncol. 2006;103:897–901.
Early-stage endometrial cancer is often curable with surgery alone because a full 88% of endometrial cancers present as clinical stage I.1 The role of the general gynecologist in surgical management of these cases is controversial; at some institutions, the practice is to call in the gynecologic oncologist for lymph-node sampling or when gross disease is identified; at others, the standard is to refer the patient to gynecologic oncology as soon as malignancy is diagnosed by endometrial biopsy. Hoekstra and colleagues have attempted to shed light on this issue with a retrospective chart review of 121 patients who were treated at one institution from 1998 to 2000.
Costs of early treatment by a gynecologic oncologist were lower than without referral
The authors performed a retrospective analysis of a group of women with clinical stage-I endometrial cancer who were treated surgically at Prentice Women’s Hospital in Chicago.
The cohort was divided in two:
- Group 1 comprised patients who underwent surgery with a general gynecologist, who consulted a gynecologic oncologist intraoperatively
- Group 2 comprised patients who were referred to a gynecologic oncologist before surgery and underwent the procedure with a gynecologic oncologist.
Overall, subjects in both groups were similar in age, distribution of surgical stage, need for lymphadenectomy, and length of follow-up.
Group 2 had a significantly shorter operative time overall, and shorter total time in the operating room. Cost per procedure was also significantly lower in this group, in terms of cost to the payer and the physician’s charge. Perioperative costs were also lower in Group 2.
No difference was observed in postoperative outcome. Total hospital costs and lengths of stay were also similar.
Recommendation for practice
With health-care costs rising, be aware of referral strategies that promote cost containment. Women who have endometrial cancer may benefit from the early involvement of a gynecologic oncologist.
Is lymphadenectomy necessary when risk of metastasis is low?
Mariani A, Dowdy S, Cliby W, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18.
The need for surgical staging of endometrial cancer has been recognized since surgical staging criteria were adopted by the International Federation of Gynecology and Obstetrics (FIGO) in 1988. Staging includes hysterectomy, bilateral salpingo-oophorectomy, and biopsy of any gross disease. Clear guidelines on the assessment of lymphatic dissemination and the anatomic extent of this assessment are, however, still lacking.
Proponents of systematic pelvic and para-aortic lymph-node dissection for patients with endometrial cancer cite:
- the 15% risk of lymph-node metastasis in women who have tumors 2 cm or larger in diameter2
- poor correlation between frozen-section grade and myometrial invasion with final pathology3
- the potential therapeutic benefit of the procedure.4
Opponents of such lymph-node dissection argue that women who have grade-1, stage-I disease will be overtreated if standardized lymphadenectomy is adopted.
Several retrospective studies have explored this question, with varying results. A large, prospective, randomized trial evaluating lymphadenectomy in clinical stage-I patients (ASTEC trial) has been completed, but is yet to be published.
When lymphadenectomy may (or may not) be necessary
After prospectively studying more than 300 endometrial cancer patients treated at the Mayo Clinic between 1984 and 1996,5 Mariani and colleagues launched a new study to assess a novel pattern of surgical management that aims to reduce the number of low-risk patients receiving lymphadenectomy. According to this pattern, the following types of women were able to bypass lymphadenectomy:
- those who had type-I, grades-1 and -2 tumors
- those with myometrial invasion ≤50%
- those with a primary tumor ≤2 cm in diameter.
Women who had endometrial cancer that did not meet these criteria underwent complete lymphadenectomy to the level of the renal vessels. Histologic assessment of the uterus to determine grade, depth of invasion, and primary tumor diameter was performed by frozen-section analysis in all cases.
The study included 422 women from January 2004 to December 2006. According to the guidelines of the study, 112 patients did not require lymphadenectomy. However, 22 (20%) women in this group did undergo the procedure because of palpable lymphadenopathy, initiation of dissection before the frozen-section report was received, or physician preference. All nodes were negative in these patients.
Of the women who met criteria for lymphadenectomy, 29 (9%) did not undergo dissection; among the reasons were disseminated disease, comorbid conditions, and advanced age. Of the women defined as at-risk who did undergo lymphadenectomy, 22% had lymph-node metastases.
Most positive para-aortic nodes lay above the inferior mesenteric artery
Information regarding the anatomic location of para-aortic nodal metastases was available for a small subset of women in the study. Seventy-seven percent of these women had para-aortic nodal metastasis above the inferior mesenteric artery. In addition, 71% of these patients had ipsilateral pelvic nodes that were free of disease. However, these patients had a poorer prognosis, and many would have received adjuvant therapy based on their hysterectomy specimen alone.
Recommendation for practice
This study suggests that there is a subset of patients who have endometrial cancer that is very low in risk and, because of this, they may forego lymph-node dissection without harm. In addition, a significant number of periaortic nodal metastases occur above the inferior mesenteric artery and in the absence of pelvic node involvement.
One of the limitations of this study is the need for intraoperative uterine assessment by frozen section by an expert pathologist—a service that is not widely available.
Taken together, these data suggest that, if the uterus can be assessed by frozen section at the time of surgery, a subset of clinical stage-I patients can be spared lymphadenectomy and its attendant risks.
Patients undergoing lymphatic assessment should undergo full systematic lymphnode dissection, not sampling. The dissection should include the region above the inferior mesenteric artery.
Removal of lymph nodes in endometrial cancer remains complex and controversial, a fact that strengthens the argument that an experienced gynecologic oncologist should be involved in the care of patients who have this disease.
Chemotherapy is warranted in advanced or recurrent disease
Homesley H, Filiaci V, Gibbons S, et al. Randomized phase III trial in advanced endometrial carcinoma of surgery and volume-directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Abstract presented at the Society of Gynecologic Oncologists, March 2008.
Adjuvant therapy for advanced-stage endometrial cancer has varied considerably over the years, and treatment of these patients remains somewhat controversial. Clinical trials comparing chemotherapy with radiation, and chemotherapy regimens with each other, have led to an era in which chemotherapy is used to treat more women than ever before.
In 2006, the Gynecologic Oncology Group (GOG) published the results of a prospective randomized study (GOG 122) that compared whole-abdomen radiation to doxorubicin and cisplatin in stage-III or -IV endometrial cancer. The investigators determined that chemotherapy was superior to whole-abdomen radiation in this trial.6
This finding was quickly followed by another trial (GOG 177) in which doxorubicin plus cisplatin was compared with a regimen of doxorubicin, cisplatin, and paclitaxel. Women in this trial had stage-III or -IV or recurrent disease. A history of radiation treatment did not disqualify patients from the study, and the treatment groups were well balanced in randomization. The doxorubicin–cisplatin–paclitaxel arm improved progression-free and overall survival, making this combination the preferred treatment.7
After volume-directed radiation, paclitaxel does not add benefit
Several retrospective analyses of radiation versus chemotherapy have shown improvement with radiation or combination therapy.8 Most recently, the GOG released data from a trial comparing chemotherapy regimens after radiation treatment. GOG 184 evaluated surgically debulked, stage-III or -IV patients who had received volume-directed radiation; subjects were randomized into either of two chemotherapy regimens:
- doxorubicin plus cisplatin
- doxorubicin, cisplatin, and paclitaxel.
In these patients, there was no improvement in survival when paclitaxel was added to the chemotherapeutic regimen, compared with doxorubicin plus cisplatin. Morbidity increased, however, with the addition of paclitaxel.
Patients in this trial underwent surgical resection of all gross disease, with no residual tumor larger than 2 cm. The role of optimal cytoreduction in endometrial cancer has been debated, however. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter (FIGURE).9-11 GOG 184 inclusion criteria required surgical resection of gross disease to ≤2 cm in diameter. It is possible that the therapeutic benefit of surgical debulking may have improved outcome in these patients—to the extent that the addition of paclitaxel did not provide appreciable benefit.
FIGURE Debulk gross disease?
The role of optimal cytoreduction in endometrial cancer has been debated. Several studies have pointed to improved survival in women after removal of visible disease to less than 1 to 2 cm in diameter.
Recommendation for practice
Data on adjuvant therapy for endometrial cancer remains conflicting. Women who have advanced-stage or recurrent endometrial cancer should receive chemotherapy—either with doxorubicin, cisplatin, and paclitaxel, or a platinum taxane regimen. The addition of paclitaxel in surgically debulked patients who have undergone radiation treatment does not appear to improve survival.
There is, however, a clear recommendation for paclitaxel in radiation-naïve patients and those who have gross residual disease. Further studies are needed to elucidate the role of radiation therapy in an era of volume-directed radiation.
Look for Lynch syndrome in young women with endometrial cancer
Lu K, Schorge J, Rodabaugh K, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164.
Endometrial cancer is part of the spectrum of Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). Patients with this autosomal-dominant hereditary cancer susceptibility syndrome may present with colorectal cancer, endometrial cancer, or, more rarely, ovarian cancer. Lynch syndrome derives from germline mutations in DNA mismatch repair genes, most often MLH1, MSH2, and MSH6.12 Genetic testing for all three genes is available for clinical use.
In the past, screening for Lynch syndrome focused on colorectal cancer, but it is now clear that women who have this disorder have a lifetime risk of developing endometrial cancer that exceeds 40%.13
In Lynch syndrome, gynecologic cancer often precedes colon cancer
Women with Lynch syndrome-associated endometrial cancer typically present at a younger age than their syndrome-free counterparts (48 compared with 60 years).14 Previous retrospective studies demonstrated that 50% of women with Lynch syndrome-associated colon and gynecologic cancers had gynecologic cancer preceding the colon cancer. The average was 11 years earlier for endometrial cancer, which suggests that, if these women could be identified at the time they are given their diagnosis of endometrial cancer, more intensive screening for colon cancer could then be initiated.15
9% of women who develop endometrial cancer before age 50 have Lynch syndrome
One of the screening criteria for Lynch syndrome-associated colon cancer is age <50 years. In this recent prospective, multicenter study involving 100 women who were diagnosed with endometrial cancer at less than 50 years of age, germline Lynch syndrome mutations were identified in 9% of patients. (In this study, germline mutation testing was performed for MLH1, MSH2, and MSH6 genes by full sequencing, and immunohistochemistry was performed for all three genes. Microsatellite analysis was performed on 95 patients, with five women having insufficient tumor for DNA extraction.)
All women who had a germline mutation had a first-degree relative with Lynch syndrome-associated cancer. The combination of a negative family history for Lynch syndrome and a body mass index greater than 30 was highly predictive of having no Lynch syndrome mutation, with a negative predictive value of 96%.
Recommendation for practice
Patients who have an hereditary cancer syndrome such as Lynch syndrome can begin cancer-prevention screening—and be engaged in that screening—when the syndrome is recognized early. Because women who have endometrial cancer that was diagnosed before they were 50 years old are at significant risk of a germline mutation, they should be offered genetic counseling and testing.
1. Fanning J, Hoffman ML, Andrews SJ, Harrah AW, Feldmeier JJ. Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: postoperative brachytherapy vs. observation. Gynecol Oncol. 2004;93:632-636.
2. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor size in endometrial cancer. Cancer. 1991;67:2791-2794.
3. Case AS, Rocconi RP, Straughn JM Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375-1379.
4. Horowitz NS, Peters WA, 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235-240.
5. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506-1519.
6. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
7. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159-2166.
8. Alvarez Secord A, Havrilesky LJ, Bac-Jump V, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285-291.
9. Bristow RE, Zerbe MJ, Rosenshein NB, Grumbine FC, Montz FJ. Stage IVB endometrial carcinoma: the role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85-91.
10. Bristow RE, Santillan A, Zahurak ML, Gardner GJ, Giuntoli RL, 2nd, Armstrong DK. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol Oncol. 2006;103:281-287.
11. Lambrou NC, Gómez-Marín O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: a study of morbidity and survival. Gynecol Oncol. 2004;93:653-658.
12. Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborate study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146-1158.
13. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
14. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on HNPCC. Anticancer Res. 1994;14:1661-1664.
15. Lu KH, Broaddus RR. Gynecological tumors in hereditary nonpolyposis colorectal cancer: we know they are common—now what? Gynecol Oncol. 2001;82:221-222.
1. Fanning J, Hoffman ML, Andrews SJ, Harrah AW, Feldmeier JJ. Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: postoperative brachytherapy vs. observation. Gynecol Oncol. 2004;93:632-636.
2. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor size in endometrial cancer. Cancer. 1991;67:2791-2794.
3. Case AS, Rocconi RP, Straughn JM Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375-1379.
4. Horowitz NS, Peters WA, 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235-240.
5. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506-1519.
6. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
7. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159-2166.
8. Alvarez Secord A, Havrilesky LJ, Bac-Jump V, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007;107:285-291.
9. Bristow RE, Zerbe MJ, Rosenshein NB, Grumbine FC, Montz FJ. Stage IVB endometrial carcinoma: the role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85-91.
10. Bristow RE, Santillan A, Zahurak ML, Gardner GJ, Giuntoli RL, 2nd, Armstrong DK. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol Oncol. 2006;103:281-287.
11. Lambrou NC, Gómez-Marín O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with Stage III and Stage IV endometrial carcinoma: a study of morbidity and survival. Gynecol Oncol. 2004;93:653-658.
12. Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborate study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146-1158.
13. Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110.
14. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on HNPCC. Anticancer Res. 1994;14:1661-1664.
15. Lu KH, Broaddus RR. Gynecological tumors in hereditary nonpolyposis colorectal cancer: we know they are common—now what? Gynecol Oncol. 2001;82:221-222.