User login
Which clinical features and lab findings increase the likelihood of temporal arteritis?
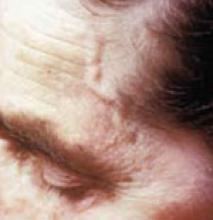
Jaw claudication, diplopia, or a temporal artery abnormality on physical exam increase the likelihood of temporal arteritis. A finding of thrombocytosis in a patient with suspected temporal arteritis moderately increases the likelihood of this diagnosis (strength of recommendation: B, based on systematic reviews of retrospective cohort studies).
Patients with temporal arteritis frequently complain of headaches, and often have mildly abnormal erythrocyte sedimentation rates (ESR), but neither of these findings helps in the diagnosis.
You may forgo biopsy if clinical probability is sufficiently high
Derek Wright, MD
Idaho state university Family Medicine, Pocatello
Because treatment for temporal arteritis involves at least several months of glucocorticoids, most clinicians prefer to confirm the diagnosis with a temporal artery biopsy. However, a unilateral biopsy has a sensitivity of only 86%; thus, a negative biopsy does not always exclude the diagnosis. As a result, many patients will be treated for temporal arteritis even after a negative biopsy because of a high clinical suspicion of the diagnosis.
It is therefore reasonable to forgo biopsy if the clinical probability of temporal arteritis is sufficiently high that one would treat for the disease even if the biopsy result were negative. Given the variable and often nonspecific nature of symptoms and findings, it is helpful to know which clinical features increase the likelihood of the disease.
Evidence summary
The prevalence of temporal arteritis (also called giant cell arteritis) increases significantly with age. For those under 50 years of age, this condition is extremely rare; the prevalence increases exponentially with age.1
Jaw claudication quadruples likelihood of temporal arteritis
A 2002 systematic review2 and a 2005 decision analysis3 examined validating cohort studies to determine the likelihood ratios of symptoms, signs, and blood tests (TABLE). These cohort studies are subject to verification bias, as most cohorts represent a selected sample of patients who had a positive temporal artery biopsy. The authors of the 2005 decision analysis note that unilateral temporal artery biopsy has a mean sensitivity of 86.9% (95% confidence interval [CI], 83.1%–90.6%) when compared with a gold standard derived from bilateral artery biopsy, American College of Rheumatology criteria, or clinical diagnosis.3
A headache—even a temporal headache—has a low positive likelihood ratio. Diplopia doubles and jaw claudication quadruples the likelihood of temporal arteritis, but the presence of other symptoms (such as anorexia, weight loss, arthralgia, fatigue, fever, polymyalgia rheumatica, vertigo, and unilateral visual loss) does not significantly increase the probability of temporal arteritis. An abnormal temporal artery on physical examination doubles the likelihood of temporal arteritis.2
TABLE
Jaw claudication and thrombocytosis increase likelihood of temporal arteritis2,3
| SYMPTOMS AND SIGNS | LR+ (95% CI) | LR– (95% CI) | SENSITIVITY (95% CI) |
|---|---|---|---|
| Diplopia | 2.0 (1.3–3.1) | 1.0 (0.9–1.0) | 0.09 (0.07–0.13) |
| Headache | 1.2 (1.0–1.4) | 0.7 (0.6–1.0) | 0.76 (0.72–0.79) |
| Headache, temporal | 1.5 (0.8–3.0) | 0.8 (0.6–1.0) | 0.52 (0.36–0.67) |
| Jaw claudication | 4.0 (2.4–6.8) | 0.8 (0.7–0.9) | 0.34 (0.29–0.41) |
| Temporal artery abnormality, any | 2.0 (1.4–3.0) | 0.5 (0.4–0.8) | 0.65 (0.54–0.74) |
| TESTS | |||
| ESR <50 | 0.6 (0.2–1.3) | 1.6 (0.8–3.3) | Not available |
| ESR 50–100 | 1.1 (0.6–2.0) | 1.0 (0.6–1.6) | Not available |
| ESR >100 | 2.5 (0.7–8.3) | 0.8 (0.5–1.1) | 0.39 (0.29–0.50) |
| Platelets >375,000 | 6.0 (1.4–24) | 0.6 (0.4–0.9) | Not available |
| LR, likelihood ratio; ESR, erythrocyte sedimentation rate; CI, confidence interval. | |||
Testing for thrombocytosis more helpful than ESR
Lab tests using ESR have been examined in various studies. A high ESR (>100 mm/hour) may only slightly increase the chance of temporal arteritis (TABLE). Five studies3 have documented that thrombocytosis (platelets >375,000/mm3) is more helpful for ruling in temporal arteritis than an elevated ESR.4 Conversely, normal platelets are more accurate for ruling out temporal arteritis than a normal ESR.
Recommendations from others
According to the American College of Rheumatology, a patient is said to have temporal arteritis when 3 of the following 5 criteria are met:
1. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998;41:778-799.
2. Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA 2002;287:92-101.
3. Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis. A decision-analytic approach. Ophthalmology 2005;112:744-756.
4. Foroozan R, Danesh-Meyer H, Savino PJ, Gamble G, Mekari-Sabbagh ON, Sergott RC. Thrombocytosis in patients with biopsy-proven giant cell arteritis. Ophthalmology 2002;109:1267-1271.
5. Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122-1128.
6. Hunder GG. Classification/diagnostic criteria for GCA/PMR. Clin Exp Rheumatol 2000;18(4 Suppl 20):S4-S5.
Jaw claudication, diplopia, or a temporal artery abnormality on physical exam increase the likelihood of temporal arteritis. A finding of thrombocytosis in a patient with suspected temporal arteritis moderately increases the likelihood of this diagnosis (strength of recommendation: B, based on systematic reviews of retrospective cohort studies).
Patients with temporal arteritis frequently complain of headaches, and often have mildly abnormal erythrocyte sedimentation rates (ESR), but neither of these findings helps in the diagnosis.
You may forgo biopsy if clinical probability is sufficiently high
Derek Wright, MD
Idaho state university Family Medicine, Pocatello
Because treatment for temporal arteritis involves at least several months of glucocorticoids, most clinicians prefer to confirm the diagnosis with a temporal artery biopsy. However, a unilateral biopsy has a sensitivity of only 86%; thus, a negative biopsy does not always exclude the diagnosis. As a result, many patients will be treated for temporal arteritis even after a negative biopsy because of a high clinical suspicion of the diagnosis.
It is therefore reasonable to forgo biopsy if the clinical probability of temporal arteritis is sufficiently high that one would treat for the disease even if the biopsy result were negative. Given the variable and often nonspecific nature of symptoms and findings, it is helpful to know which clinical features increase the likelihood of the disease.
Evidence summary
The prevalence of temporal arteritis (also called giant cell arteritis) increases significantly with age. For those under 50 years of age, this condition is extremely rare; the prevalence increases exponentially with age.1
Jaw claudication quadruples likelihood of temporal arteritis
A 2002 systematic review2 and a 2005 decision analysis3 examined validating cohort studies to determine the likelihood ratios of symptoms, signs, and blood tests (TABLE). These cohort studies are subject to verification bias, as most cohorts represent a selected sample of patients who had a positive temporal artery biopsy. The authors of the 2005 decision analysis note that unilateral temporal artery biopsy has a mean sensitivity of 86.9% (95% confidence interval [CI], 83.1%–90.6%) when compared with a gold standard derived from bilateral artery biopsy, American College of Rheumatology criteria, or clinical diagnosis.3
A headache—even a temporal headache—has a low positive likelihood ratio. Diplopia doubles and jaw claudication quadruples the likelihood of temporal arteritis, but the presence of other symptoms (such as anorexia, weight loss, arthralgia, fatigue, fever, polymyalgia rheumatica, vertigo, and unilateral visual loss) does not significantly increase the probability of temporal arteritis. An abnormal temporal artery on physical examination doubles the likelihood of temporal arteritis.2
TABLE
Jaw claudication and thrombocytosis increase likelihood of temporal arteritis2,3
| SYMPTOMS AND SIGNS | LR+ (95% CI) | LR– (95% CI) | SENSITIVITY (95% CI) |
|---|---|---|---|
| Diplopia | 2.0 (1.3–3.1) | 1.0 (0.9–1.0) | 0.09 (0.07–0.13) |
| Headache | 1.2 (1.0–1.4) | 0.7 (0.6–1.0) | 0.76 (0.72–0.79) |
| Headache, temporal | 1.5 (0.8–3.0) | 0.8 (0.6–1.0) | 0.52 (0.36–0.67) |
| Jaw claudication | 4.0 (2.4–6.8) | 0.8 (0.7–0.9) | 0.34 (0.29–0.41) |
| Temporal artery abnormality, any | 2.0 (1.4–3.0) | 0.5 (0.4–0.8) | 0.65 (0.54–0.74) |
| TESTS | |||
| ESR <50 | 0.6 (0.2–1.3) | 1.6 (0.8–3.3) | Not available |
| ESR 50–100 | 1.1 (0.6–2.0) | 1.0 (0.6–1.6) | Not available |
| ESR >100 | 2.5 (0.7–8.3) | 0.8 (0.5–1.1) | 0.39 (0.29–0.50) |
| Platelets >375,000 | 6.0 (1.4–24) | 0.6 (0.4–0.9) | Not available |
| LR, likelihood ratio; ESR, erythrocyte sedimentation rate; CI, confidence interval. | |||
Testing for thrombocytosis more helpful than ESR
Lab tests using ESR have been examined in various studies. A high ESR (>100 mm/hour) may only slightly increase the chance of temporal arteritis (TABLE). Five studies3 have documented that thrombocytosis (platelets >375,000/mm3) is more helpful for ruling in temporal arteritis than an elevated ESR.4 Conversely, normal platelets are more accurate for ruling out temporal arteritis than a normal ESR.
Recommendations from others
According to the American College of Rheumatology, a patient is said to have temporal arteritis when 3 of the following 5 criteria are met:
Jaw claudication, diplopia, or a temporal artery abnormality on physical exam increase the likelihood of temporal arteritis. A finding of thrombocytosis in a patient with suspected temporal arteritis moderately increases the likelihood of this diagnosis (strength of recommendation: B, based on systematic reviews of retrospective cohort studies).
Patients with temporal arteritis frequently complain of headaches, and often have mildly abnormal erythrocyte sedimentation rates (ESR), but neither of these findings helps in the diagnosis.
You may forgo biopsy if clinical probability is sufficiently high
Derek Wright, MD
Idaho state university Family Medicine, Pocatello
Because treatment for temporal arteritis involves at least several months of glucocorticoids, most clinicians prefer to confirm the diagnosis with a temporal artery biopsy. However, a unilateral biopsy has a sensitivity of only 86%; thus, a negative biopsy does not always exclude the diagnosis. As a result, many patients will be treated for temporal arteritis even after a negative biopsy because of a high clinical suspicion of the diagnosis.
It is therefore reasonable to forgo biopsy if the clinical probability of temporal arteritis is sufficiently high that one would treat for the disease even if the biopsy result were negative. Given the variable and often nonspecific nature of symptoms and findings, it is helpful to know which clinical features increase the likelihood of the disease.
Evidence summary
The prevalence of temporal arteritis (also called giant cell arteritis) increases significantly with age. For those under 50 years of age, this condition is extremely rare; the prevalence increases exponentially with age.1
Jaw claudication quadruples likelihood of temporal arteritis
A 2002 systematic review2 and a 2005 decision analysis3 examined validating cohort studies to determine the likelihood ratios of symptoms, signs, and blood tests (TABLE). These cohort studies are subject to verification bias, as most cohorts represent a selected sample of patients who had a positive temporal artery biopsy. The authors of the 2005 decision analysis note that unilateral temporal artery biopsy has a mean sensitivity of 86.9% (95% confidence interval [CI], 83.1%–90.6%) when compared with a gold standard derived from bilateral artery biopsy, American College of Rheumatology criteria, or clinical diagnosis.3
A headache—even a temporal headache—has a low positive likelihood ratio. Diplopia doubles and jaw claudication quadruples the likelihood of temporal arteritis, but the presence of other symptoms (such as anorexia, weight loss, arthralgia, fatigue, fever, polymyalgia rheumatica, vertigo, and unilateral visual loss) does not significantly increase the probability of temporal arteritis. An abnormal temporal artery on physical examination doubles the likelihood of temporal arteritis.2
TABLE
Jaw claudication and thrombocytosis increase likelihood of temporal arteritis2,3
| SYMPTOMS AND SIGNS | LR+ (95% CI) | LR– (95% CI) | SENSITIVITY (95% CI) |
|---|---|---|---|
| Diplopia | 2.0 (1.3–3.1) | 1.0 (0.9–1.0) | 0.09 (0.07–0.13) |
| Headache | 1.2 (1.0–1.4) | 0.7 (0.6–1.0) | 0.76 (0.72–0.79) |
| Headache, temporal | 1.5 (0.8–3.0) | 0.8 (0.6–1.0) | 0.52 (0.36–0.67) |
| Jaw claudication | 4.0 (2.4–6.8) | 0.8 (0.7–0.9) | 0.34 (0.29–0.41) |
| Temporal artery abnormality, any | 2.0 (1.4–3.0) | 0.5 (0.4–0.8) | 0.65 (0.54–0.74) |
| TESTS | |||
| ESR <50 | 0.6 (0.2–1.3) | 1.6 (0.8–3.3) | Not available |
| ESR 50–100 | 1.1 (0.6–2.0) | 1.0 (0.6–1.6) | Not available |
| ESR >100 | 2.5 (0.7–8.3) | 0.8 (0.5–1.1) | 0.39 (0.29–0.50) |
| Platelets >375,000 | 6.0 (1.4–24) | 0.6 (0.4–0.9) | Not available |
| LR, likelihood ratio; ESR, erythrocyte sedimentation rate; CI, confidence interval. | |||
Testing for thrombocytosis more helpful than ESR
Lab tests using ESR have been examined in various studies. A high ESR (>100 mm/hour) may only slightly increase the chance of temporal arteritis (TABLE). Five studies3 have documented that thrombocytosis (platelets >375,000/mm3) is more helpful for ruling in temporal arteritis than an elevated ESR.4 Conversely, normal platelets are more accurate for ruling out temporal arteritis than a normal ESR.
Recommendations from others
According to the American College of Rheumatology, a patient is said to have temporal arteritis when 3 of the following 5 criteria are met:
1. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998;41:778-799.
2. Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA 2002;287:92-101.
3. Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis. A decision-analytic approach. Ophthalmology 2005;112:744-756.
4. Foroozan R, Danesh-Meyer H, Savino PJ, Gamble G, Mekari-Sabbagh ON, Sergott RC. Thrombocytosis in patients with biopsy-proven giant cell arteritis. Ophthalmology 2002;109:1267-1271.
5. Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122-1128.
6. Hunder GG. Classification/diagnostic criteria for GCA/PMR. Clin Exp Rheumatol 2000;18(4 Suppl 20):S4-S5.
1. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998;41:778-799.
2. Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA 2002;287:92-101.
3. Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis. A decision-analytic approach. Ophthalmology 2005;112:744-756.
4. Foroozan R, Danesh-Meyer H, Savino PJ, Gamble G, Mekari-Sabbagh ON, Sergott RC. Thrombocytosis in patients with biopsy-proven giant cell arteritis. Ophthalmology 2002;109:1267-1271.
5. Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122-1128.
6. Hunder GG. Classification/diagnostic criteria for GCA/PMR. Clin Exp Rheumatol 2000;18(4 Suppl 20):S4-S5.
Evidence-based answers from the Family Physicians Inquiries Network
Are major bleeding events from falls more likely in patients on warfarin?
There is no evidence of increased risk for major bleeding as a result of falls in hospitalized patients taking warfarin (strength of recommendation [SOR]: B, based on retrospective cohort studies). In the average patient taking warfarin for atrial fibrillation, the risk of intracranial hemorrhage from a fall is much smaller than the benefit gained from reducing risk of stroke (SOR: A, based on decision analysis of systematic reviews with sensitivity analysis).
Major bleeding infrequent in fall patients with a therapeutic INR; more common with higher INR
Paul Crawford, MD
Eglin Air Force Base Family Practice Residency, Eglin AFB, Fla
Decisions to initiate or withhold anticoagulation can be difficult to make, but this Clinical Inquiry should simplify matters. Clearly, for patients with atrial fibrillation, the risk of stroke while not taking warfarin is greater than the risk of major bleeding from a fall while on it. Also, major bleeding from a fall occurs infrequently in patients with a therapeutic internal normalized ratio (INR). However, bleeding is more common in patients with a supratherapeutic INR, so remain alert to possible uncontrolled anticoagulation either from medication interactions or from impaired cognition.
This Clinical Inquiry should also help physicians considering an inferior vena cava filter instead of warfarin. Complications with inferior vena cava filters include death (0.82%), filter migration (3%–69%), and penetration (9%–24%) or obstruction (6%–30%) of the inferior vena cava.6
Evidence summary
Increased risk of falling is often given as a reason for not recommending anticoagulation for atrial fibrillation in frail or elderly patients. However, no studies directly address the risk for major bleeding in anticoagulated patients who fall.
One retrospective study of 2633 falls in 1861 hospital inpatients compared the rate of major hemorrhage between those taking anticoagulation therapy with those not taking it.1 Major hemorrhage was defined as bruising or cuts requiring immediate attention from a physician. The rate of major hemorrhage was 6.2% for patients taking warfarin and 11.3% for patients receiving no therapy. Patients with INR=2–3 had a major hemorrhage rate of 6.9% compared with 10.1% for those with INR <1.3. Criteria for using warfarin were not reported; there may have been selection bias in favor of prescribing warfarin for patients judged less likely to fall.
A smaller study of 400 consecutive falls among 264 post-stroke patients in a rehab hospital found no difference in minor injury rates (19% vs 18%, NS); no major hemorrhagic complications were seen following 131 falls in the anticoagulation group (93 patients) and 269 falls in the group not on anticoagulation (175 patients).2 Patients on anticoagulation had an average protime of 16.1 seconds (INR was not reported). The calculated risk of major hemorrhage in an anticoagulated patient from a single fall was 2.3% or less. The study was limited because most falls were from a seated position or partially controlled by an attendant; few patients fell from a standing position.
Another study presented a Markov decision analysis (comparison of risk estimates in separate disease states) evaluating whether risk from falls should influence choice of anticoagulation therapy in elderly patients with atrial fibrillation.3 Risk of intracranial bleeding from falls was calculated from prospective cohort studies and retrospective case series from anticoagulation clinics, and stroke reduction benefit from anticoagulation was taken from a meta-analysis of 5 randomized controlled trials. Sensitivity analyses were performed to test the results of the decision analysis. The calculated risk of subdural hematoma from falling was such that a patient with a 5% annual stroke risk from atrial fibrillation would need to fall 295 times in a year for the fall risk to outweigh the stroke reduction benefit of warfarin.
Recommendations from others
Guidelines from the American Heart Association and the American College of Chest Physicians do not include fall risk in the decision to use anticoagulation.4
Guidelines from the Institute for Clinical Systems Improvement note that patients with 3 falls in the previous year or with recurrent, injurious falls were excluded from trials evaluating efficacy and safety of anticoagulation in patients with nonvalvular atrial fibrillation.5
1. Bond AJ, Molnar FJ, Li M, Mackey M, Man-Son-Hing M. The risk of hemorrhagic complications in hospital in-patients who fall while receiving antithrombotic therapy. Thromb J 2005;3:1-6.
2. Stein J, Viramontes BS, Kerrigan DC. Fall-related injuries in anticoagulated stroke patients during inpatient rehabilitation. Arch Phys Med Rehabil 1995;76:840-843.
3. Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999;159:677-685.
4. Institute for Clinical Systems Improvement. Anticoagulation therapy supplement. April 2005. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=151t. Accessed on January 9, 2006.
5. Singer D, Albers G. Antithrombotic therapy in atrial fibrillation—the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:429s-456s.
6. Kinney TB. Update on inferior vena cava filters. J Vasc Inter Rad 2003;14:425.
There is no evidence of increased risk for major bleeding as a result of falls in hospitalized patients taking warfarin (strength of recommendation [SOR]: B, based on retrospective cohort studies). In the average patient taking warfarin for atrial fibrillation, the risk of intracranial hemorrhage from a fall is much smaller than the benefit gained from reducing risk of stroke (SOR: A, based on decision analysis of systematic reviews with sensitivity analysis).
Major bleeding infrequent in fall patients with a therapeutic INR; more common with higher INR
Paul Crawford, MD
Eglin Air Force Base Family Practice Residency, Eglin AFB, Fla
Decisions to initiate or withhold anticoagulation can be difficult to make, but this Clinical Inquiry should simplify matters. Clearly, for patients with atrial fibrillation, the risk of stroke while not taking warfarin is greater than the risk of major bleeding from a fall while on it. Also, major bleeding from a fall occurs infrequently in patients with a therapeutic internal normalized ratio (INR). However, bleeding is more common in patients with a supratherapeutic INR, so remain alert to possible uncontrolled anticoagulation either from medication interactions or from impaired cognition.
This Clinical Inquiry should also help physicians considering an inferior vena cava filter instead of warfarin. Complications with inferior vena cava filters include death (0.82%), filter migration (3%–69%), and penetration (9%–24%) or obstruction (6%–30%) of the inferior vena cava.6
Evidence summary
Increased risk of falling is often given as a reason for not recommending anticoagulation for atrial fibrillation in frail or elderly patients. However, no studies directly address the risk for major bleeding in anticoagulated patients who fall.
One retrospective study of 2633 falls in 1861 hospital inpatients compared the rate of major hemorrhage between those taking anticoagulation therapy with those not taking it.1 Major hemorrhage was defined as bruising or cuts requiring immediate attention from a physician. The rate of major hemorrhage was 6.2% for patients taking warfarin and 11.3% for patients receiving no therapy. Patients with INR=2–3 had a major hemorrhage rate of 6.9% compared with 10.1% for those with INR <1.3. Criteria for using warfarin were not reported; there may have been selection bias in favor of prescribing warfarin for patients judged less likely to fall.
A smaller study of 400 consecutive falls among 264 post-stroke patients in a rehab hospital found no difference in minor injury rates (19% vs 18%, NS); no major hemorrhagic complications were seen following 131 falls in the anticoagulation group (93 patients) and 269 falls in the group not on anticoagulation (175 patients).2 Patients on anticoagulation had an average protime of 16.1 seconds (INR was not reported). The calculated risk of major hemorrhage in an anticoagulated patient from a single fall was 2.3% or less. The study was limited because most falls were from a seated position or partially controlled by an attendant; few patients fell from a standing position.
Another study presented a Markov decision analysis (comparison of risk estimates in separate disease states) evaluating whether risk from falls should influence choice of anticoagulation therapy in elderly patients with atrial fibrillation.3 Risk of intracranial bleeding from falls was calculated from prospective cohort studies and retrospective case series from anticoagulation clinics, and stroke reduction benefit from anticoagulation was taken from a meta-analysis of 5 randomized controlled trials. Sensitivity analyses were performed to test the results of the decision analysis. The calculated risk of subdural hematoma from falling was such that a patient with a 5% annual stroke risk from atrial fibrillation would need to fall 295 times in a year for the fall risk to outweigh the stroke reduction benefit of warfarin.
Recommendations from others
Guidelines from the American Heart Association and the American College of Chest Physicians do not include fall risk in the decision to use anticoagulation.4
Guidelines from the Institute for Clinical Systems Improvement note that patients with 3 falls in the previous year or with recurrent, injurious falls were excluded from trials evaluating efficacy and safety of anticoagulation in patients with nonvalvular atrial fibrillation.5
There is no evidence of increased risk for major bleeding as a result of falls in hospitalized patients taking warfarin (strength of recommendation [SOR]: B, based on retrospective cohort studies). In the average patient taking warfarin for atrial fibrillation, the risk of intracranial hemorrhage from a fall is much smaller than the benefit gained from reducing risk of stroke (SOR: A, based on decision analysis of systematic reviews with sensitivity analysis).
Major bleeding infrequent in fall patients with a therapeutic INR; more common with higher INR
Paul Crawford, MD
Eglin Air Force Base Family Practice Residency, Eglin AFB, Fla
Decisions to initiate or withhold anticoagulation can be difficult to make, but this Clinical Inquiry should simplify matters. Clearly, for patients with atrial fibrillation, the risk of stroke while not taking warfarin is greater than the risk of major bleeding from a fall while on it. Also, major bleeding from a fall occurs infrequently in patients with a therapeutic internal normalized ratio (INR). However, bleeding is more common in patients with a supratherapeutic INR, so remain alert to possible uncontrolled anticoagulation either from medication interactions or from impaired cognition.
This Clinical Inquiry should also help physicians considering an inferior vena cava filter instead of warfarin. Complications with inferior vena cava filters include death (0.82%), filter migration (3%–69%), and penetration (9%–24%) or obstruction (6%–30%) of the inferior vena cava.6
Evidence summary
Increased risk of falling is often given as a reason for not recommending anticoagulation for atrial fibrillation in frail or elderly patients. However, no studies directly address the risk for major bleeding in anticoagulated patients who fall.
One retrospective study of 2633 falls in 1861 hospital inpatients compared the rate of major hemorrhage between those taking anticoagulation therapy with those not taking it.1 Major hemorrhage was defined as bruising or cuts requiring immediate attention from a physician. The rate of major hemorrhage was 6.2% for patients taking warfarin and 11.3% for patients receiving no therapy. Patients with INR=2–3 had a major hemorrhage rate of 6.9% compared with 10.1% for those with INR <1.3. Criteria for using warfarin were not reported; there may have been selection bias in favor of prescribing warfarin for patients judged less likely to fall.
A smaller study of 400 consecutive falls among 264 post-stroke patients in a rehab hospital found no difference in minor injury rates (19% vs 18%, NS); no major hemorrhagic complications were seen following 131 falls in the anticoagulation group (93 patients) and 269 falls in the group not on anticoagulation (175 patients).2 Patients on anticoagulation had an average protime of 16.1 seconds (INR was not reported). The calculated risk of major hemorrhage in an anticoagulated patient from a single fall was 2.3% or less. The study was limited because most falls were from a seated position or partially controlled by an attendant; few patients fell from a standing position.
Another study presented a Markov decision analysis (comparison of risk estimates in separate disease states) evaluating whether risk from falls should influence choice of anticoagulation therapy in elderly patients with atrial fibrillation.3 Risk of intracranial bleeding from falls was calculated from prospective cohort studies and retrospective case series from anticoagulation clinics, and stroke reduction benefit from anticoagulation was taken from a meta-analysis of 5 randomized controlled trials. Sensitivity analyses were performed to test the results of the decision analysis. The calculated risk of subdural hematoma from falling was such that a patient with a 5% annual stroke risk from atrial fibrillation would need to fall 295 times in a year for the fall risk to outweigh the stroke reduction benefit of warfarin.
Recommendations from others
Guidelines from the American Heart Association and the American College of Chest Physicians do not include fall risk in the decision to use anticoagulation.4
Guidelines from the Institute for Clinical Systems Improvement note that patients with 3 falls in the previous year or with recurrent, injurious falls were excluded from trials evaluating efficacy and safety of anticoagulation in patients with nonvalvular atrial fibrillation.5
1. Bond AJ, Molnar FJ, Li M, Mackey M, Man-Son-Hing M. The risk of hemorrhagic complications in hospital in-patients who fall while receiving antithrombotic therapy. Thromb J 2005;3:1-6.
2. Stein J, Viramontes BS, Kerrigan DC. Fall-related injuries in anticoagulated stroke patients during inpatient rehabilitation. Arch Phys Med Rehabil 1995;76:840-843.
3. Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999;159:677-685.
4. Institute for Clinical Systems Improvement. Anticoagulation therapy supplement. April 2005. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=151t. Accessed on January 9, 2006.
5. Singer D, Albers G. Antithrombotic therapy in atrial fibrillation—the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:429s-456s.
6. Kinney TB. Update on inferior vena cava filters. J Vasc Inter Rad 2003;14:425.
1. Bond AJ, Molnar FJ, Li M, Mackey M, Man-Son-Hing M. The risk of hemorrhagic complications in hospital in-patients who fall while receiving antithrombotic therapy. Thromb J 2005;3:1-6.
2. Stein J, Viramontes BS, Kerrigan DC. Fall-related injuries in anticoagulated stroke patients during inpatient rehabilitation. Arch Phys Med Rehabil 1995;76:840-843.
3. Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999;159:677-685.
4. Institute for Clinical Systems Improvement. Anticoagulation therapy supplement. April 2005. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=151t. Accessed on January 9, 2006.
5. Singer D, Albers G. Antithrombotic therapy in atrial fibrillation—the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:429s-456s.
6. Kinney TB. Update on inferior vena cava filters. J Vasc Inter Rad 2003;14:425.
Evidence-based answers from the Family Physicians Inquiries Network
When is neuroimaging warranted for headache?
Neuroimaging is warranted to evaluate headaches when patients present to an emergency department with signs or symptoms of an intracranial lesion. These signs or symptoms include abrupt onset of headache, focal neurological abnormalities (strength of recommendation [SOR]: B, based on a validating cohort study), decreased level of consciousness (SOR: B, based on a retrospective, nonconsecutive case study), occipitonuchal location, multiple associated symptoms, and age older than 55 years (SOR: B, based on a case-control study).
Neuroimaging is also recommended in the ambulatory setting for patients with headaches of migraine type and abnormal findings on neurological exam; that are accompanied by signs or symptoms of increased intracranial pressure; or that are new for a patient who is HIV-positive (SOR: C, based on expert opinion).
There are no studies or consistent opinions on the need for neuroimaging with headaches of tension type, described as the “worst ever,” increasing in frequency, that awaken the patient, or are associated with nausea, dizziness, or syncope.
Careful clinical judgment is important in decision for neuroimaging
Zahida Siddiqi, MD
Baylor College of Medicine, Houston, Tex
Determining the utility of neuroimaging for headache is a taxing question for clinicians working in the emergency room or an outpatient clinic. In the county health system where I work, I find it increasingly difficult to get neuroimaging studies done within an appropriate time frame. Thus I must rely heavily on clinical judgment to determine how urgently they must be done. I also feel an ethical obligation to avoid unnecessary demands on this limited resource.
I have found the criteria recommended in this Clinical Inquiry to be most helpful in prioritizing the need for neuroimaging. These include focal neurological deficit, alteration in the character of headache, persistence of headache despite analgesics, abrupt onset, and increasing frequency and intensity of headache. In addition, I have found the persistence of the patient in returning for reevaluation to be a helpful indicator of pathology.
Evidence summary
A validating cohort study looked at 5 clinical warning criteria (TABLE) for patients seen in an emergency department for headache; 70 adults with acute headache as the chief complaint were included. All patients received computed tomography (CT) scanning as part of their evaluation. Abrupt onset and focal neurologic findings most strongly predicted intracranial lesions. Overall, 36% of the patients (25/70) had significant pathology.1
A retrospective study reviewed records of 111 patients seen in an emergency department with headache and who had undergone neuroimaging (CT or magnetic resonance imaging [MRI]). Three symptoms predicted a lesion: decreased level of consciousness (sensitivity=23%; positive likelihood ratio [LR+]=3.8), paralysis (sensitivity=25%; LR+=3.5), and papilledema (numbers not reported). In this study, 35% (39/111) of those receiving neuroimaging had intracranial pathology.2
A case-control study reviewed hospital records of 468 patients evaluated in the emergency department for nontraumatic headache. Neuroimaging (CT scan or cerebral angiogram) was performed for 160 of these patients. Final diagnosis and outcome was obtained at 6 months. The symptoms and their ability to predict intracranial pathology are as follows: abnormal neurologic examination (sensitivity=39%; LR+=19.5), location of headache (sensitivity=78%; LR+=4.87), age of patient (sensitivity=61%; LR+=2.26), multiple associated symptoms (sensitivity=61%; LR+=2.26), mode of onset of headache (sensitivity=78%; LR+=2.23), and presence of associated symptoms (sensitivity=89%; LR+=1.41). Again, abnormal neurologic examination was the most significant indicator for imaging. This study did not define associated symptoms nor did it specify what determined which patients were imaged.3
Information concerning the workup of headache in the ambulatory setting is limited. In actual practice, only about 3% of patients who present with a new headache in the office setting have neuroimaging ordered.4 When neuroimaging is performed, about 4% of CT scans find a significant and treatable lesion (in one sample of 293 CT scans, there were 12 true-positive scans and 2 false-positive scans).5 Expert guidelines regarding headaches among ambulatory patients recommend neuroimaging for migraine patients only in the presence of persistent focal abnormal neurological findings. They note insufficient evidence for recommendations concerning neuroimaging for patients with tension-type headaches. They also note insufficient evidence for or against neuroimaging when headache occurs in the presence or absence of nonfocal symptoms: dizziness, syncope, nausea, lack of coordination, the “worst headache ever,” headache that awakens the patient from sleep, and increasing frequency of headaches.6
TABLE
Five clinical warning criteria for headache
| CLINICAL FEATURE | SENSITIVITY | SPECIFICITY | LR+ | LR– |
|---|---|---|---|---|
| FOR INTRACRANICAL PATHOLOGY | ||||
| Presence of focal neurological symptoms or findings | 1.0 | 0.76 | 4.21 | 0 |
| Abrupt onset | 0.55 | 0.79 | 2.5 | 0.57 |
| Alteration of characteristics | 0.67 | 0.67 | 2.0 | 0.49 |
| Increased intensity and frequency | 0.39 | 0.73 | 1.44 | 0.83 |
| Persistence despite analgesics | 0.60 | 0.56 | 1.36 | 0.71 |
| LR+, positive likelihood ratio; LR–, negative likelihood ratio. | ||||
| Source: Aygun and Bildik, Eur J Neurol 2003.1 | ||||
Recommendations from others
Rosen’s Emergency Medicine and Mettler: Essentials of Radiology add the following indications for imaging in headache: signs and symptoms of elevated intracranial pressure (eg, papilledema); meningismus; partial seizure; nocturnal headaches that awaken the patient from sleep; increase in pain with coughing, sneezing or change in body position; sudden onset headaches that reach maximum intensity in 2 to 3 minutes; headache associated with mental status changes or decreased alertness; any new headache in an HIV-positive patient.7,8
1. Aygun D, Bildik F. Clinical warning criteria in evaluation by computed tomography the secondary neurological headaches in adults. Eur J Neurol 2003;10:437-442.
2. Sobri M, Lamont AC, Alias NA, Win MN. Red flags in patients presenting with headache: clinical indications for neuroimaging. Br J Radiol 2003;76:532-535.
3. Ramirez-Lassepas M, Espinosa C, Cicero JJ, Johnston KL, Cipolle RJ, Barber DL. Predictors of intracranial pathologic findings in patients who see emergency care because of headache. Arch Neurol 1997;54:1506-1509.
4. Becker L, Iverson DC, Reed FM, Calonge N, Miller RS, Freeman WL. Patients with a new headache in primary care: a report from ASPN. J Fam Pract 1988;27:41-47.
5. Becker LA, Green LA, Beaufait D, Kirk J, Froom J, Freeman WL. Use of CT scans for the investigation of headache: a report from ASPN, part 1. J Fam Pract 1993;37:129-134.
6. Morey SS. Headache Consortium releases guidelines for use of CT or MRI in migraine work-up. Am Fam Physician 2000;62:1699-1701.
7. Mettler FA, Jr. Essentials of Radiology. 2nd ed. Philadelphia, Pa: Saunders; 2005.
8. Marx JA, Hockberger RS, Walls JM. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 5th ed. St. Louis, Mo: Mosby; 2002.
Neuroimaging is warranted to evaluate headaches when patients present to an emergency department with signs or symptoms of an intracranial lesion. These signs or symptoms include abrupt onset of headache, focal neurological abnormalities (strength of recommendation [SOR]: B, based on a validating cohort study), decreased level of consciousness (SOR: B, based on a retrospective, nonconsecutive case study), occipitonuchal location, multiple associated symptoms, and age older than 55 years (SOR: B, based on a case-control study).
Neuroimaging is also recommended in the ambulatory setting for patients with headaches of migraine type and abnormal findings on neurological exam; that are accompanied by signs or symptoms of increased intracranial pressure; or that are new for a patient who is HIV-positive (SOR: C, based on expert opinion).
There are no studies or consistent opinions on the need for neuroimaging with headaches of tension type, described as the “worst ever,” increasing in frequency, that awaken the patient, or are associated with nausea, dizziness, or syncope.
Careful clinical judgment is important in decision for neuroimaging
Zahida Siddiqi, MD
Baylor College of Medicine, Houston, Tex
Determining the utility of neuroimaging for headache is a taxing question for clinicians working in the emergency room or an outpatient clinic. In the county health system where I work, I find it increasingly difficult to get neuroimaging studies done within an appropriate time frame. Thus I must rely heavily on clinical judgment to determine how urgently they must be done. I also feel an ethical obligation to avoid unnecessary demands on this limited resource.
I have found the criteria recommended in this Clinical Inquiry to be most helpful in prioritizing the need for neuroimaging. These include focal neurological deficit, alteration in the character of headache, persistence of headache despite analgesics, abrupt onset, and increasing frequency and intensity of headache. In addition, I have found the persistence of the patient in returning for reevaluation to be a helpful indicator of pathology.
Evidence summary
A validating cohort study looked at 5 clinical warning criteria (TABLE) for patients seen in an emergency department for headache; 70 adults with acute headache as the chief complaint were included. All patients received computed tomography (CT) scanning as part of their evaluation. Abrupt onset and focal neurologic findings most strongly predicted intracranial lesions. Overall, 36% of the patients (25/70) had significant pathology.1
A retrospective study reviewed records of 111 patients seen in an emergency department with headache and who had undergone neuroimaging (CT or magnetic resonance imaging [MRI]). Three symptoms predicted a lesion: decreased level of consciousness (sensitivity=23%; positive likelihood ratio [LR+]=3.8), paralysis (sensitivity=25%; LR+=3.5), and papilledema (numbers not reported). In this study, 35% (39/111) of those receiving neuroimaging had intracranial pathology.2
A case-control study reviewed hospital records of 468 patients evaluated in the emergency department for nontraumatic headache. Neuroimaging (CT scan or cerebral angiogram) was performed for 160 of these patients. Final diagnosis and outcome was obtained at 6 months. The symptoms and their ability to predict intracranial pathology are as follows: abnormal neurologic examination (sensitivity=39%; LR+=19.5), location of headache (sensitivity=78%; LR+=4.87), age of patient (sensitivity=61%; LR+=2.26), multiple associated symptoms (sensitivity=61%; LR+=2.26), mode of onset of headache (sensitivity=78%; LR+=2.23), and presence of associated symptoms (sensitivity=89%; LR+=1.41). Again, abnormal neurologic examination was the most significant indicator for imaging. This study did not define associated symptoms nor did it specify what determined which patients were imaged.3
Information concerning the workup of headache in the ambulatory setting is limited. In actual practice, only about 3% of patients who present with a new headache in the office setting have neuroimaging ordered.4 When neuroimaging is performed, about 4% of CT scans find a significant and treatable lesion (in one sample of 293 CT scans, there were 12 true-positive scans and 2 false-positive scans).5 Expert guidelines regarding headaches among ambulatory patients recommend neuroimaging for migraine patients only in the presence of persistent focal abnormal neurological findings. They note insufficient evidence for recommendations concerning neuroimaging for patients with tension-type headaches. They also note insufficient evidence for or against neuroimaging when headache occurs in the presence or absence of nonfocal symptoms: dizziness, syncope, nausea, lack of coordination, the “worst headache ever,” headache that awakens the patient from sleep, and increasing frequency of headaches.6
TABLE
Five clinical warning criteria for headache
| CLINICAL FEATURE | SENSITIVITY | SPECIFICITY | LR+ | LR– |
|---|---|---|---|---|
| FOR INTRACRANICAL PATHOLOGY | ||||
| Presence of focal neurological symptoms or findings | 1.0 | 0.76 | 4.21 | 0 |
| Abrupt onset | 0.55 | 0.79 | 2.5 | 0.57 |
| Alteration of characteristics | 0.67 | 0.67 | 2.0 | 0.49 |
| Increased intensity and frequency | 0.39 | 0.73 | 1.44 | 0.83 |
| Persistence despite analgesics | 0.60 | 0.56 | 1.36 | 0.71 |
| LR+, positive likelihood ratio; LR–, negative likelihood ratio. | ||||
| Source: Aygun and Bildik, Eur J Neurol 2003.1 | ||||
Recommendations from others
Rosen’s Emergency Medicine and Mettler: Essentials of Radiology add the following indications for imaging in headache: signs and symptoms of elevated intracranial pressure (eg, papilledema); meningismus; partial seizure; nocturnal headaches that awaken the patient from sleep; increase in pain with coughing, sneezing or change in body position; sudden onset headaches that reach maximum intensity in 2 to 3 minutes; headache associated with mental status changes or decreased alertness; any new headache in an HIV-positive patient.7,8
Neuroimaging is warranted to evaluate headaches when patients present to an emergency department with signs or symptoms of an intracranial lesion. These signs or symptoms include abrupt onset of headache, focal neurological abnormalities (strength of recommendation [SOR]: B, based on a validating cohort study), decreased level of consciousness (SOR: B, based on a retrospective, nonconsecutive case study), occipitonuchal location, multiple associated symptoms, and age older than 55 years (SOR: B, based on a case-control study).
Neuroimaging is also recommended in the ambulatory setting for patients with headaches of migraine type and abnormal findings on neurological exam; that are accompanied by signs or symptoms of increased intracranial pressure; or that are new for a patient who is HIV-positive (SOR: C, based on expert opinion).
There are no studies or consistent opinions on the need for neuroimaging with headaches of tension type, described as the “worst ever,” increasing in frequency, that awaken the patient, or are associated with nausea, dizziness, or syncope.
Careful clinical judgment is important in decision for neuroimaging
Zahida Siddiqi, MD
Baylor College of Medicine, Houston, Tex
Determining the utility of neuroimaging for headache is a taxing question for clinicians working in the emergency room or an outpatient clinic. In the county health system where I work, I find it increasingly difficult to get neuroimaging studies done within an appropriate time frame. Thus I must rely heavily on clinical judgment to determine how urgently they must be done. I also feel an ethical obligation to avoid unnecessary demands on this limited resource.
I have found the criteria recommended in this Clinical Inquiry to be most helpful in prioritizing the need for neuroimaging. These include focal neurological deficit, alteration in the character of headache, persistence of headache despite analgesics, abrupt onset, and increasing frequency and intensity of headache. In addition, I have found the persistence of the patient in returning for reevaluation to be a helpful indicator of pathology.
Evidence summary
A validating cohort study looked at 5 clinical warning criteria (TABLE) for patients seen in an emergency department for headache; 70 adults with acute headache as the chief complaint were included. All patients received computed tomography (CT) scanning as part of their evaluation. Abrupt onset and focal neurologic findings most strongly predicted intracranial lesions. Overall, 36% of the patients (25/70) had significant pathology.1
A retrospective study reviewed records of 111 patients seen in an emergency department with headache and who had undergone neuroimaging (CT or magnetic resonance imaging [MRI]). Three symptoms predicted a lesion: decreased level of consciousness (sensitivity=23%; positive likelihood ratio [LR+]=3.8), paralysis (sensitivity=25%; LR+=3.5), and papilledema (numbers not reported). In this study, 35% (39/111) of those receiving neuroimaging had intracranial pathology.2
A case-control study reviewed hospital records of 468 patients evaluated in the emergency department for nontraumatic headache. Neuroimaging (CT scan or cerebral angiogram) was performed for 160 of these patients. Final diagnosis and outcome was obtained at 6 months. The symptoms and their ability to predict intracranial pathology are as follows: abnormal neurologic examination (sensitivity=39%; LR+=19.5), location of headache (sensitivity=78%; LR+=4.87), age of patient (sensitivity=61%; LR+=2.26), multiple associated symptoms (sensitivity=61%; LR+=2.26), mode of onset of headache (sensitivity=78%; LR+=2.23), and presence of associated symptoms (sensitivity=89%; LR+=1.41). Again, abnormal neurologic examination was the most significant indicator for imaging. This study did not define associated symptoms nor did it specify what determined which patients were imaged.3
Information concerning the workup of headache in the ambulatory setting is limited. In actual practice, only about 3% of patients who present with a new headache in the office setting have neuroimaging ordered.4 When neuroimaging is performed, about 4% of CT scans find a significant and treatable lesion (in one sample of 293 CT scans, there were 12 true-positive scans and 2 false-positive scans).5 Expert guidelines regarding headaches among ambulatory patients recommend neuroimaging for migraine patients only in the presence of persistent focal abnormal neurological findings. They note insufficient evidence for recommendations concerning neuroimaging for patients with tension-type headaches. They also note insufficient evidence for or against neuroimaging when headache occurs in the presence or absence of nonfocal symptoms: dizziness, syncope, nausea, lack of coordination, the “worst headache ever,” headache that awakens the patient from sleep, and increasing frequency of headaches.6
TABLE
Five clinical warning criteria for headache
| CLINICAL FEATURE | SENSITIVITY | SPECIFICITY | LR+ | LR– |
|---|---|---|---|---|
| FOR INTRACRANICAL PATHOLOGY | ||||
| Presence of focal neurological symptoms or findings | 1.0 | 0.76 | 4.21 | 0 |
| Abrupt onset | 0.55 | 0.79 | 2.5 | 0.57 |
| Alteration of characteristics | 0.67 | 0.67 | 2.0 | 0.49 |
| Increased intensity and frequency | 0.39 | 0.73 | 1.44 | 0.83 |
| Persistence despite analgesics | 0.60 | 0.56 | 1.36 | 0.71 |
| LR+, positive likelihood ratio; LR–, negative likelihood ratio. | ||||
| Source: Aygun and Bildik, Eur J Neurol 2003.1 | ||||
Recommendations from others
Rosen’s Emergency Medicine and Mettler: Essentials of Radiology add the following indications for imaging in headache: signs and symptoms of elevated intracranial pressure (eg, papilledema); meningismus; partial seizure; nocturnal headaches that awaken the patient from sleep; increase in pain with coughing, sneezing or change in body position; sudden onset headaches that reach maximum intensity in 2 to 3 minutes; headache associated with mental status changes or decreased alertness; any new headache in an HIV-positive patient.7,8
1. Aygun D, Bildik F. Clinical warning criteria in evaluation by computed tomography the secondary neurological headaches in adults. Eur J Neurol 2003;10:437-442.
2. Sobri M, Lamont AC, Alias NA, Win MN. Red flags in patients presenting with headache: clinical indications for neuroimaging. Br J Radiol 2003;76:532-535.
3. Ramirez-Lassepas M, Espinosa C, Cicero JJ, Johnston KL, Cipolle RJ, Barber DL. Predictors of intracranial pathologic findings in patients who see emergency care because of headache. Arch Neurol 1997;54:1506-1509.
4. Becker L, Iverson DC, Reed FM, Calonge N, Miller RS, Freeman WL. Patients with a new headache in primary care: a report from ASPN. J Fam Pract 1988;27:41-47.
5. Becker LA, Green LA, Beaufait D, Kirk J, Froom J, Freeman WL. Use of CT scans for the investigation of headache: a report from ASPN, part 1. J Fam Pract 1993;37:129-134.
6. Morey SS. Headache Consortium releases guidelines for use of CT or MRI in migraine work-up. Am Fam Physician 2000;62:1699-1701.
7. Mettler FA, Jr. Essentials of Radiology. 2nd ed. Philadelphia, Pa: Saunders; 2005.
8. Marx JA, Hockberger RS, Walls JM. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 5th ed. St. Louis, Mo: Mosby; 2002.
1. Aygun D, Bildik F. Clinical warning criteria in evaluation by computed tomography the secondary neurological headaches in adults. Eur J Neurol 2003;10:437-442.
2. Sobri M, Lamont AC, Alias NA, Win MN. Red flags in patients presenting with headache: clinical indications for neuroimaging. Br J Radiol 2003;76:532-535.
3. Ramirez-Lassepas M, Espinosa C, Cicero JJ, Johnston KL, Cipolle RJ, Barber DL. Predictors of intracranial pathologic findings in patients who see emergency care because of headache. Arch Neurol 1997;54:1506-1509.
4. Becker L, Iverson DC, Reed FM, Calonge N, Miller RS, Freeman WL. Patients with a new headache in primary care: a report from ASPN. J Fam Pract 1988;27:41-47.
5. Becker LA, Green LA, Beaufait D, Kirk J, Froom J, Freeman WL. Use of CT scans for the investigation of headache: a report from ASPN, part 1. J Fam Pract 1993;37:129-134.
6. Morey SS. Headache Consortium releases guidelines for use of CT or MRI in migraine work-up. Am Fam Physician 2000;62:1699-1701.
7. Mettler FA, Jr. Essentials of Radiology. 2nd ed. Philadelphia, Pa: Saunders; 2005.
8. Marx JA, Hockberger RS, Walls JM. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 5th ed. St. Louis, Mo: Mosby; 2002.
Evidence-based answers from the Family Physicians Inquiries Network
Are liver function tests required for patients taking isoniazid for latent TB?
Routine liver function test monitoring is not required for all patients on isoniazid therapy for latent tuberculosis (TB) infection (strength of recommendation: B, based on case series). No clinical trials have studied the potential risks and benefits of routinely monitoring liver function tests for all patients taking isoniazid for latent TB infection. Data from 2 case series suggest that routine liver function test monitoring leads to withdrawal of isoniazid prophylaxis from about 6% of patients because of abnormal lab results.1,2 This is 10 to 60 times the hepatitis rate found in case series using a symptom-based monitoring strategy.3,6 Data are insufficient, however, to conclude that routine liver function test monitoring leads to a lower rate of fatal isoniazid hepatitis compared with a strategy of symptom-based screening. Given that complete recovery from nonfatal hepatitis is the rule, and that patients withdrawn from isoniazid prophylaxis remain at risk for developing active tuberculosis, current evidence does not support routine liver function test monitoring for all patients.
Evidence summary
Several large population-based case series have tried to define the incidence of isoniazid-induced hepatitis and fatal hepatitis. Because these series differed in patient selection, diagnostic criteria for hepatitis, and toxicity monitoring strategies, and because their data span decades, they provide limited insight. Data from 6 large case series1,3-7 and 1 pooled compilation of published and unpublished reports8 are summarized in the Table.
Two studies1,2 that defined hepatitis as asymptomatic liver function test elevation (>5 times normal) on monthly screening found a 6% to 6.4% incidence of hepatitis, a rate 10 to 60 times higher than 4 case series3-6 that relied on symptom-based monitoring. A pooled analysis of more than 200,000 patients receiving isoniazid prophylaxis and monitored according to 1983 American Thoracic Society guidelines reported an intermediate hepatitis rate (1.2%) and only 2 deaths.8 Mortality from isoniazid hepatitis is rare, whichever monitoring strategy is selected. Some deaths attributed to isoniazid prophylaxis may also have had other contributing causes, such as unrecognized hepatitis C; most cases and deaths reported in these large series occurred before testing for hepatitis C became available in 1991.
Symptom-based monitoring strategies require stopping isoniazid promptly if symptoms of hepatotoxicity develop. In a series of 62 fatal cases of probable or possible isoniazid hepatitis, 42% had been monitored at least monthly for symptoms, and 38% stopped isoniazid within 1 week of symptom onset.9 Seven of the 8 patients receiving a liver transplant following the development of fulminant, isoniazid-related hepatitis continued to take the drug for a least 10 days after onset of symptoms of hepatotoxicity.10
Several series report increasing hepatitis risk with advancing age.1,3,5,6 In 1 series,3 rates were 3/1000 in those aged 20 to 34 years, 12/1000 in those aged 35 to 49 years, 23/1000 in those aged 50 to 64 years, and 8/1000 after age 65.
TABLE
INH hepatitis incidence and mortality rates: summary of the largest case series
| Study | Time period | Monitoring strategy | Hepatitis definition | No. of patients | No. of hepatitis cases | No. of fatal cases mortality rate |
|---|---|---|---|---|---|---|
| Byrd1 | ~early/mid 1970s | Monthly symptom and LFT screening | AST >5x normal, with or without symptoms | 1000 | 64 (6.4%) | 0 |
| Salpeter8 | 1983-early 1990s | Presumed to follow 1983 ATS guidelinesa | Not defined | 202,497 | 2,459 (1.2%) | 2 (0.001%) |
| Kopanoff3 | July 1971 to Nov. 1972 | Monthly symptom-based screening | AST ≥250 Karmen units or ALT>AST, and no other cause | 13,838 | 92 (0.66%) | 8 (0.06%) |
| IUATCP4 | mid-1970s | Every-4-week symptom-based screening | Not defined | 20,840 | 95 (0.5%) | 3 (0.014%) |
| Dash4 | Jan. 1973 to June 1977 | Monthly symptom based screening | Jaundice, scleral icterus, or “hepatitis” notation | 5300 | 15 (0.37%)b | 1 (.019%) |
| Nolan6 | Jan. 1989 to 1 December 1995 | Monthly symptom-based screening | AST >5x normal with symptoms, and no other cause | 11,141 | 11 (0.1%) | 0 |
| LoBue7 | July 1999 to Nov. 2002 | Monthly clinical monitoring, routine LFTs for patients >34 before 2000 | LFTs >3x normal with symptoms, or LFTs >5x normal without symptoms | 3,788 | 10 (0.3%) | 0 |
| a Withhold treatment in presence of active liver disease, limit prophylaxis of patients aged >35 to those at highest risk of developing active disease, baseline and periodic LFTs for those over 35, discontinue isoniazid if transaminases exceed 3 to 5 times normal. | ||||||
| b Calculation based on life-table analysis, because of high dropout rate during treatment LFT, liver function test; AST, aspartate transaminase; ALT, alanine transaminase; IUSTCP, International Union Against Tuberculosis Committee on Prophylaxis | ||||||
Recommendations From Others
The Centers for Disease Control and Prevention (CDC) and the American Thoracic Society joint guidelines for the treatment of latent TB infection state that baseline laboratory testing is not routinely indicated, even for persons aged >35 years, but may be considered for patients who are taking other hepatotoxic medications or have chronic medical conditions.11
Baseline measurements of bilirubin and aspartate transaminase (AST) or alanine transaminase (ALT) along with monthly liver function test monitoring are recommended for patients with pre-existing liver disease, patients at risk for chronic liver disease, patients with HIV infection, pregnant or postpartum women, and regular users of alcohol. All patients should be evaluated at least monthly for symptoms of hepatitis, and liver function tests should also be obtained for patients with symptoms compatible with hepatotoxicity. The guideline suggests that isoniazid be stopped if liver function tests exceed 5 times the upper limits of normal, or 3 times the upper limits of normal if the patient is symptomatic. The Canadian Tuberculosis Standards (5th ed, 2000) recommend baseline AST before isoniazid preventive therapy is started, and regular monitoring in those with pre-existing liver disease, a history of ethanol abuse, or age ≥35 years.12
Patients need to understand risks and benefits of TB treatment
Lauren DeAlleaume, MD
University of Colorado Health Sciences Center, Denver
As the number of immigrants increases, FPs will see more patients at high risk for TB. Patients whose risk of developing active TB exceeds the risk of isoniazid toxicity should be tested (targeted testing). It is challenging to ensure an asymptomatic patient completes a 9-month course of therapy while undergoing monthly monitoring for symptoms of isoniazid toxicity. Overall, only 60% of patients complete a full course of isoniazid. Clinical and public health systems that make it easier for patients to follow-up can enhance compliance.
Patients need to understand the benefits of treatment and the symptoms of isoniazid toxicity. The CDC recommends clinical monitoring without routine blood testing for patients of any age without additional risk factors for isoniazid hepatitis. Excessive monitoring can lead to premature discontinuation of therapy because 10%–20% of patients develop some liver function test elevation. The CDC has an excellent course on the basics of latent TB testing and treatment ( at www.phppo.cdc.gov/phtn/tbmodules/Default.htm). Patient education materials and risk assessment and monitoring forms can be obtained from state health departments.
1. Byrd RB, Horn BR, Solomon DA, Griggs GA. Toxic effects of isoniazid in tuberculosis chemoprophylaxis. Role of biochemical monitoring in 1,000 patients. JAMA 1979;241:1239-1241.
2. Stuart RL, Wilson J, Grayson ML. Isoniazid toxicity in health care workers. Clin Infect Dis 1999;28:895-897.
3. Kopanoff DE, Snider DE, Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis 1978;117:991-1001.
4. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ 1982;60:555-564.
5. Dash LA, Comstock GW, Flynn JP. Isoniazid preventive therapy: retrospect and prospect. Am Rev Respir Dis 1980;121:1039-1044.
6. Dash CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy; a 7-year survey from a public health tuberculosis clinic. JAMA 1999;281:1014-1018.
7. LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med 2003;168:443-447.
8. Salpeter SR. Fatal isoniazid-induced hepatitis. Its risk during chemoprophylaxis. West J Med 1993;159:560-564.
9. Millard PS, Wilcosky TC, Reade-Christopher SJ, Weber DJ. Isoniazid-related fatal hepatitis. West J Med 1996;164:486-491.
10. Centers for Disease Control and Prevention. Severe isoniazid-associated hepatitis—New York, 1991–1993. MMWR Morb Mortal Wkly Rep 42:545-547.
11. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep 2000;49(RR-6):1-51.
12. Canadian Tuberculosis Standards 2000. 5th ed. Available at: www.lung.ca/tb/TBStandards_Eng.pdf. Accessed on November 28, 2003.
Routine liver function test monitoring is not required for all patients on isoniazid therapy for latent tuberculosis (TB) infection (strength of recommendation: B, based on case series). No clinical trials have studied the potential risks and benefits of routinely monitoring liver function tests for all patients taking isoniazid for latent TB infection. Data from 2 case series suggest that routine liver function test monitoring leads to withdrawal of isoniazid prophylaxis from about 6% of patients because of abnormal lab results.1,2 This is 10 to 60 times the hepatitis rate found in case series using a symptom-based monitoring strategy.3,6 Data are insufficient, however, to conclude that routine liver function test monitoring leads to a lower rate of fatal isoniazid hepatitis compared with a strategy of symptom-based screening. Given that complete recovery from nonfatal hepatitis is the rule, and that patients withdrawn from isoniazid prophylaxis remain at risk for developing active tuberculosis, current evidence does not support routine liver function test monitoring for all patients.
Evidence summary
Several large population-based case series have tried to define the incidence of isoniazid-induced hepatitis and fatal hepatitis. Because these series differed in patient selection, diagnostic criteria for hepatitis, and toxicity monitoring strategies, and because their data span decades, they provide limited insight. Data from 6 large case series1,3-7 and 1 pooled compilation of published and unpublished reports8 are summarized in the Table.
Two studies1,2 that defined hepatitis as asymptomatic liver function test elevation (>5 times normal) on monthly screening found a 6% to 6.4% incidence of hepatitis, a rate 10 to 60 times higher than 4 case series3-6 that relied on symptom-based monitoring. A pooled analysis of more than 200,000 patients receiving isoniazid prophylaxis and monitored according to 1983 American Thoracic Society guidelines reported an intermediate hepatitis rate (1.2%) and only 2 deaths.8 Mortality from isoniazid hepatitis is rare, whichever monitoring strategy is selected. Some deaths attributed to isoniazid prophylaxis may also have had other contributing causes, such as unrecognized hepatitis C; most cases and deaths reported in these large series occurred before testing for hepatitis C became available in 1991.
Symptom-based monitoring strategies require stopping isoniazid promptly if symptoms of hepatotoxicity develop. In a series of 62 fatal cases of probable or possible isoniazid hepatitis, 42% had been monitored at least monthly for symptoms, and 38% stopped isoniazid within 1 week of symptom onset.9 Seven of the 8 patients receiving a liver transplant following the development of fulminant, isoniazid-related hepatitis continued to take the drug for a least 10 days after onset of symptoms of hepatotoxicity.10
Several series report increasing hepatitis risk with advancing age.1,3,5,6 In 1 series,3 rates were 3/1000 in those aged 20 to 34 years, 12/1000 in those aged 35 to 49 years, 23/1000 in those aged 50 to 64 years, and 8/1000 after age 65.
TABLE
INH hepatitis incidence and mortality rates: summary of the largest case series
| Study | Time period | Monitoring strategy | Hepatitis definition | No. of patients | No. of hepatitis cases | No. of fatal cases mortality rate |
|---|---|---|---|---|---|---|
| Byrd1 | ~early/mid 1970s | Monthly symptom and LFT screening | AST >5x normal, with or without symptoms | 1000 | 64 (6.4%) | 0 |
| Salpeter8 | 1983-early 1990s | Presumed to follow 1983 ATS guidelinesa | Not defined | 202,497 | 2,459 (1.2%) | 2 (0.001%) |
| Kopanoff3 | July 1971 to Nov. 1972 | Monthly symptom-based screening | AST ≥250 Karmen units or ALT>AST, and no other cause | 13,838 | 92 (0.66%) | 8 (0.06%) |
| IUATCP4 | mid-1970s | Every-4-week symptom-based screening | Not defined | 20,840 | 95 (0.5%) | 3 (0.014%) |
| Dash4 | Jan. 1973 to June 1977 | Monthly symptom based screening | Jaundice, scleral icterus, or “hepatitis” notation | 5300 | 15 (0.37%)b | 1 (.019%) |
| Nolan6 | Jan. 1989 to 1 December 1995 | Monthly symptom-based screening | AST >5x normal with symptoms, and no other cause | 11,141 | 11 (0.1%) | 0 |
| LoBue7 | July 1999 to Nov. 2002 | Monthly clinical monitoring, routine LFTs for patients >34 before 2000 | LFTs >3x normal with symptoms, or LFTs >5x normal without symptoms | 3,788 | 10 (0.3%) | 0 |
| a Withhold treatment in presence of active liver disease, limit prophylaxis of patients aged >35 to those at highest risk of developing active disease, baseline and periodic LFTs for those over 35, discontinue isoniazid if transaminases exceed 3 to 5 times normal. | ||||||
| b Calculation based on life-table analysis, because of high dropout rate during treatment LFT, liver function test; AST, aspartate transaminase; ALT, alanine transaminase; IUSTCP, International Union Against Tuberculosis Committee on Prophylaxis | ||||||
Recommendations From Others
The Centers for Disease Control and Prevention (CDC) and the American Thoracic Society joint guidelines for the treatment of latent TB infection state that baseline laboratory testing is not routinely indicated, even for persons aged >35 years, but may be considered for patients who are taking other hepatotoxic medications or have chronic medical conditions.11
Baseline measurements of bilirubin and aspartate transaminase (AST) or alanine transaminase (ALT) along with monthly liver function test monitoring are recommended for patients with pre-existing liver disease, patients at risk for chronic liver disease, patients with HIV infection, pregnant or postpartum women, and regular users of alcohol. All patients should be evaluated at least monthly for symptoms of hepatitis, and liver function tests should also be obtained for patients with symptoms compatible with hepatotoxicity. The guideline suggests that isoniazid be stopped if liver function tests exceed 5 times the upper limits of normal, or 3 times the upper limits of normal if the patient is symptomatic. The Canadian Tuberculosis Standards (5th ed, 2000) recommend baseline AST before isoniazid preventive therapy is started, and regular monitoring in those with pre-existing liver disease, a history of ethanol abuse, or age ≥35 years.12
Patients need to understand risks and benefits of TB treatment
Lauren DeAlleaume, MD
University of Colorado Health Sciences Center, Denver
As the number of immigrants increases, FPs will see more patients at high risk for TB. Patients whose risk of developing active TB exceeds the risk of isoniazid toxicity should be tested (targeted testing). It is challenging to ensure an asymptomatic patient completes a 9-month course of therapy while undergoing monthly monitoring for symptoms of isoniazid toxicity. Overall, only 60% of patients complete a full course of isoniazid. Clinical and public health systems that make it easier for patients to follow-up can enhance compliance.
Patients need to understand the benefits of treatment and the symptoms of isoniazid toxicity. The CDC recommends clinical monitoring without routine blood testing for patients of any age without additional risk factors for isoniazid hepatitis. Excessive monitoring can lead to premature discontinuation of therapy because 10%–20% of patients develop some liver function test elevation. The CDC has an excellent course on the basics of latent TB testing and treatment ( at www.phppo.cdc.gov/phtn/tbmodules/Default.htm). Patient education materials and risk assessment and monitoring forms can be obtained from state health departments.
Routine liver function test monitoring is not required for all patients on isoniazid therapy for latent tuberculosis (TB) infection (strength of recommendation: B, based on case series). No clinical trials have studied the potential risks and benefits of routinely monitoring liver function tests for all patients taking isoniazid for latent TB infection. Data from 2 case series suggest that routine liver function test monitoring leads to withdrawal of isoniazid prophylaxis from about 6% of patients because of abnormal lab results.1,2 This is 10 to 60 times the hepatitis rate found in case series using a symptom-based monitoring strategy.3,6 Data are insufficient, however, to conclude that routine liver function test monitoring leads to a lower rate of fatal isoniazid hepatitis compared with a strategy of symptom-based screening. Given that complete recovery from nonfatal hepatitis is the rule, and that patients withdrawn from isoniazid prophylaxis remain at risk for developing active tuberculosis, current evidence does not support routine liver function test monitoring for all patients.
Evidence summary
Several large population-based case series have tried to define the incidence of isoniazid-induced hepatitis and fatal hepatitis. Because these series differed in patient selection, diagnostic criteria for hepatitis, and toxicity monitoring strategies, and because their data span decades, they provide limited insight. Data from 6 large case series1,3-7 and 1 pooled compilation of published and unpublished reports8 are summarized in the Table.
Two studies1,2 that defined hepatitis as asymptomatic liver function test elevation (>5 times normal) on monthly screening found a 6% to 6.4% incidence of hepatitis, a rate 10 to 60 times higher than 4 case series3-6 that relied on symptom-based monitoring. A pooled analysis of more than 200,000 patients receiving isoniazid prophylaxis and monitored according to 1983 American Thoracic Society guidelines reported an intermediate hepatitis rate (1.2%) and only 2 deaths.8 Mortality from isoniazid hepatitis is rare, whichever monitoring strategy is selected. Some deaths attributed to isoniazid prophylaxis may also have had other contributing causes, such as unrecognized hepatitis C; most cases and deaths reported in these large series occurred before testing for hepatitis C became available in 1991.
Symptom-based monitoring strategies require stopping isoniazid promptly if symptoms of hepatotoxicity develop. In a series of 62 fatal cases of probable or possible isoniazid hepatitis, 42% had been monitored at least monthly for symptoms, and 38% stopped isoniazid within 1 week of symptom onset.9 Seven of the 8 patients receiving a liver transplant following the development of fulminant, isoniazid-related hepatitis continued to take the drug for a least 10 days after onset of symptoms of hepatotoxicity.10
Several series report increasing hepatitis risk with advancing age.1,3,5,6 In 1 series,3 rates were 3/1000 in those aged 20 to 34 years, 12/1000 in those aged 35 to 49 years, 23/1000 in those aged 50 to 64 years, and 8/1000 after age 65.
TABLE
INH hepatitis incidence and mortality rates: summary of the largest case series
| Study | Time period | Monitoring strategy | Hepatitis definition | No. of patients | No. of hepatitis cases | No. of fatal cases mortality rate |
|---|---|---|---|---|---|---|
| Byrd1 | ~early/mid 1970s | Monthly symptom and LFT screening | AST >5x normal, with or without symptoms | 1000 | 64 (6.4%) | 0 |
| Salpeter8 | 1983-early 1990s | Presumed to follow 1983 ATS guidelinesa | Not defined | 202,497 | 2,459 (1.2%) | 2 (0.001%) |
| Kopanoff3 | July 1971 to Nov. 1972 | Monthly symptom-based screening | AST ≥250 Karmen units or ALT>AST, and no other cause | 13,838 | 92 (0.66%) | 8 (0.06%) |
| IUATCP4 | mid-1970s | Every-4-week symptom-based screening | Not defined | 20,840 | 95 (0.5%) | 3 (0.014%) |
| Dash4 | Jan. 1973 to June 1977 | Monthly symptom based screening | Jaundice, scleral icterus, or “hepatitis” notation | 5300 | 15 (0.37%)b | 1 (.019%) |
| Nolan6 | Jan. 1989 to 1 December 1995 | Monthly symptom-based screening | AST >5x normal with symptoms, and no other cause | 11,141 | 11 (0.1%) | 0 |
| LoBue7 | July 1999 to Nov. 2002 | Monthly clinical monitoring, routine LFTs for patients >34 before 2000 | LFTs >3x normal with symptoms, or LFTs >5x normal without symptoms | 3,788 | 10 (0.3%) | 0 |
| a Withhold treatment in presence of active liver disease, limit prophylaxis of patients aged >35 to those at highest risk of developing active disease, baseline and periodic LFTs for those over 35, discontinue isoniazid if transaminases exceed 3 to 5 times normal. | ||||||
| b Calculation based on life-table analysis, because of high dropout rate during treatment LFT, liver function test; AST, aspartate transaminase; ALT, alanine transaminase; IUSTCP, International Union Against Tuberculosis Committee on Prophylaxis | ||||||
Recommendations From Others
The Centers for Disease Control and Prevention (CDC) and the American Thoracic Society joint guidelines for the treatment of latent TB infection state that baseline laboratory testing is not routinely indicated, even for persons aged >35 years, but may be considered for patients who are taking other hepatotoxic medications or have chronic medical conditions.11
Baseline measurements of bilirubin and aspartate transaminase (AST) or alanine transaminase (ALT) along with monthly liver function test monitoring are recommended for patients with pre-existing liver disease, patients at risk for chronic liver disease, patients with HIV infection, pregnant or postpartum women, and regular users of alcohol. All patients should be evaluated at least monthly for symptoms of hepatitis, and liver function tests should also be obtained for patients with symptoms compatible with hepatotoxicity. The guideline suggests that isoniazid be stopped if liver function tests exceed 5 times the upper limits of normal, or 3 times the upper limits of normal if the patient is symptomatic. The Canadian Tuberculosis Standards (5th ed, 2000) recommend baseline AST before isoniazid preventive therapy is started, and regular monitoring in those with pre-existing liver disease, a history of ethanol abuse, or age ≥35 years.12
Patients need to understand risks and benefits of TB treatment
Lauren DeAlleaume, MD
University of Colorado Health Sciences Center, Denver
As the number of immigrants increases, FPs will see more patients at high risk for TB. Patients whose risk of developing active TB exceeds the risk of isoniazid toxicity should be tested (targeted testing). It is challenging to ensure an asymptomatic patient completes a 9-month course of therapy while undergoing monthly monitoring for symptoms of isoniazid toxicity. Overall, only 60% of patients complete a full course of isoniazid. Clinical and public health systems that make it easier for patients to follow-up can enhance compliance.
Patients need to understand the benefits of treatment and the symptoms of isoniazid toxicity. The CDC recommends clinical monitoring without routine blood testing for patients of any age without additional risk factors for isoniazid hepatitis. Excessive monitoring can lead to premature discontinuation of therapy because 10%–20% of patients develop some liver function test elevation. The CDC has an excellent course on the basics of latent TB testing and treatment ( at www.phppo.cdc.gov/phtn/tbmodules/Default.htm). Patient education materials and risk assessment and monitoring forms can be obtained from state health departments.
1. Byrd RB, Horn BR, Solomon DA, Griggs GA. Toxic effects of isoniazid in tuberculosis chemoprophylaxis. Role of biochemical monitoring in 1,000 patients. JAMA 1979;241:1239-1241.
2. Stuart RL, Wilson J, Grayson ML. Isoniazid toxicity in health care workers. Clin Infect Dis 1999;28:895-897.
3. Kopanoff DE, Snider DE, Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis 1978;117:991-1001.
4. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ 1982;60:555-564.
5. Dash LA, Comstock GW, Flynn JP. Isoniazid preventive therapy: retrospect and prospect. Am Rev Respir Dis 1980;121:1039-1044.
6. Dash CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy; a 7-year survey from a public health tuberculosis clinic. JAMA 1999;281:1014-1018.
7. LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med 2003;168:443-447.
8. Salpeter SR. Fatal isoniazid-induced hepatitis. Its risk during chemoprophylaxis. West J Med 1993;159:560-564.
9. Millard PS, Wilcosky TC, Reade-Christopher SJ, Weber DJ. Isoniazid-related fatal hepatitis. West J Med 1996;164:486-491.
10. Centers for Disease Control and Prevention. Severe isoniazid-associated hepatitis—New York, 1991–1993. MMWR Morb Mortal Wkly Rep 42:545-547.
11. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep 2000;49(RR-6):1-51.
12. Canadian Tuberculosis Standards 2000. 5th ed. Available at: www.lung.ca/tb/TBStandards_Eng.pdf. Accessed on November 28, 2003.
1. Byrd RB, Horn BR, Solomon DA, Griggs GA. Toxic effects of isoniazid in tuberculosis chemoprophylaxis. Role of biochemical monitoring in 1,000 patients. JAMA 1979;241:1239-1241.
2. Stuart RL, Wilson J, Grayson ML. Isoniazid toxicity in health care workers. Clin Infect Dis 1999;28:895-897.
3. Kopanoff DE, Snider DE, Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis 1978;117:991-1001.
4. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ 1982;60:555-564.
5. Dash LA, Comstock GW, Flynn JP. Isoniazid preventive therapy: retrospect and prospect. Am Rev Respir Dis 1980;121:1039-1044.
6. Dash CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy; a 7-year survey from a public health tuberculosis clinic. JAMA 1999;281:1014-1018.
7. LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med 2003;168:443-447.
8. Salpeter SR. Fatal isoniazid-induced hepatitis. Its risk during chemoprophylaxis. West J Med 1993;159:560-564.
9. Millard PS, Wilcosky TC, Reade-Christopher SJ, Weber DJ. Isoniazid-related fatal hepatitis. West J Med 1996;164:486-491.
10. Centers for Disease Control and Prevention. Severe isoniazid-associated hepatitis—New York, 1991–1993. MMWR Morb Mortal Wkly Rep 42:545-547.
11. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep 2000;49(RR-6):1-51.
12. Canadian Tuberculosis Standards 2000. 5th ed. Available at: www.lung.ca/tb/TBStandards_Eng.pdf. Accessed on November 28, 2003.
Evidence-based answers from the Family Physicians Inquiries Network