User login
Why our brains wear out at the end of the day
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
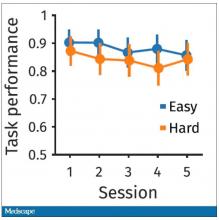
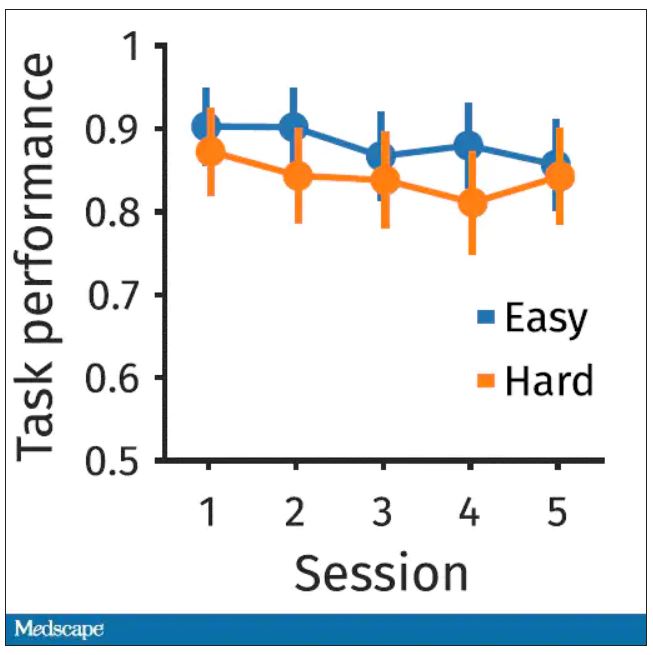
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
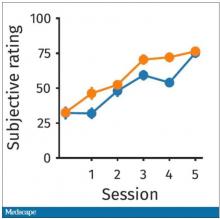
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
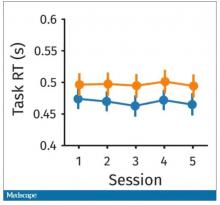
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
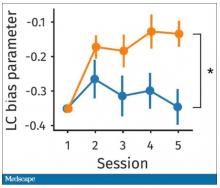
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
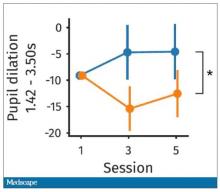
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
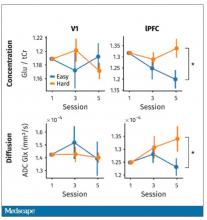
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
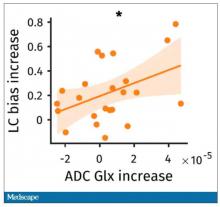
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Could a common cold virus be causing severe hepatitis in kids?
This is a transcript of a video that first appeared on Medscape.com. It has been edited for clarity.
On April 21, 2022, the Centers for Disease Control and Prevention released a Health Alert Network advisory regarding a cluster of nine cases of acute hepatitis in children in Alabama over a 5-month period from October 2021 to February 2022 – a rate substantially higher than what would be expected, given the relative rarity of hepatitis in children.
Standard workup was negative for the common causative agents – hepatitis A, B, and C – and no toxic exposures were identified. But there was one common thread among all these kids: They all tested positive for adenovirus.
And that is really strange.
There are about 100 circulating adenoviruses in the world that we know of, and around 50 of them infect humans. If you are an adult, it’s a virtual certainty that you have been infected with an adenovirus in the past. Most strains cause symptoms we would describe as the common cold: runny nose, sore throat. Some strains cause conjunctivitis (pink eye). Some cause gastrointestinal illness – the stomach bugs that kids get.
It’s the banality of adenovirus that makes this hepatitis finding so surprising.
The United States is not alone in reporting this new hepatitis syndrome. As of April 21, 169 cases have been reported across the world, including 114 in the United Kingdom.
Of the 169 cases reported worldwide, 74 had evidence of adenovirus infection. On molecular testing, 18 of those were adenovirus 41.
What I wanted to do today was go through the various hypotheses for what could be going on with these hepatitis cases, one by one, and highlight the evidence supporting them. We won’t reach a conclusion, but hopefully by the end, the path forward will be more clear. OK, let’s get started.
Hypothesis 1: Nothing is happening.
It’s worth noting that “clusters” of disease occur all the time, even when no relevant epidemiologic process has occurred. If there is some baseline rate of hepatitis, every once in a while, through bad luck alone, you’d see a group of cases all at once. This is known as the clustering illusion. And I’m quite confident in saying that this is not the case here.
For one, this phenomenon is worldwide, as we know from the World Health Organization report. In fact, the CDC didn’t provide the most detailed data about the nine (now 12) cases in the United States. This study from Scotland is the first to give a detailed accounting of cases, reporting on 13 cases of acute hepatitis of unknown cause in kids at a single hospital from January to April. Typically, the hospital sees fewer than four cases of hepatitis per year. Five of these 13 kids tested positive for adenovirus. So let’s take the clustering illusion off the list.
Hypothesis 2: It’s adenovirus.
The major evidence supporting adenovirus as the causative agent here is that a lot of these kids had adenovirus, and adenovirus 41 – a gut-tropic strain – in particular. This is important, because stool testing might be necessary for diagnosis and lots of kids with this condition didn’t get that. In other words, we have hard evidence of adenovirus infection in about 40% of the cases so far, but the true number might be substantially higher.
That said, adenovirus is seasonal, and we are in adenovirus season. Granted, 40% seems quite a bit higher than the background infection rate, but we have to be careful not to assume that correlation means causation.
The evidence against adenovirus, even adenovirus 41, is that this acute hepatitis syndrome is new, and adenovirus 41 is not. To be fair, we know adenoviruses can cause acute hepatitis, but the vast majority of reports are in immunocompromised individuals – organ transplant recipients and those with HIV. I was able to find just a handful of cases of immunocompetent kids developing hepatitis from adenovirus prior to this current outbreak.
The current outbreak would exceed the published literature by nearly two orders of magnitude. It feels like something else has to be going on.
Hypothesis 3: It’s coronavirus.
SARS-CoV-2 is a strange virus, both in its acute presentation and its long-term outcomes. It was clear early in the pandemic that some children infected by the coronavirus would develop MIS-C – multisystem inflammatory syndrome in children. MIS-C is associated with hepatitis in about 10% of children, according to this New England Journal of Medicine
But the presentation of these kids is quite different from MIS-C; fever is rare, for example. The WHO reports that of the 169 identified cases so far, 20 had active COVID infection. The Scotland cohort suggests that a similar proportion had past COVID infections. In other times, we might consider this a smoking gun, but at this point a history of COVID is not remarkable – after the Omicron wave, it’s about as common to have a history of COVID as it is not to have a history of COVID.
A brief aside here. This is not because of coronavirus vaccination. Of the more than 100 cases reported in the United Kingdom, none of these kids were vaccinated. So let’s put aside the possibility that this is a vaccine effect – there’s no real evidence to support that.
Which brings us to …
Hypothesis 4: It’s coronavirus and adenovirus.
This is sort of intriguing and can work a few different ways, via a direct and indirect path.
In the direct path, we posit that COVID infection does something to kids’ immune systems – something we don’t yet understand that limits their ability to fight off adenovirus. There is some support for this idea. This study in Immunity found that COVID infection can functionally impair dendritic cells and T-cells, including natural killer cells. These cells are important components of our innate antiviral immunity.
There’s an indirect path as well. COVID has led to lockdowns, distancing, masking – stuff that prevents kids from being exposed to germs from other kids. Could a lack of exposure to adenovirus or other viruses because of distancing increase the risk for severe disease when restrictions are lifted? Also possible – the severity of respiratory syncytial virus (RSV) infections this year is substantially higher than what we’ve seen in the past, for example.
And finally, hypothesis 5: This is something new.
We can’t ignore the possibility that this is simply a new disease-causing agent. Toxicology studies so far have been negative, and we wouldn’t expect hepatitis from a chemical toxin to appear in multiple countries around the world; this is almost certainly a biological phenomenon. It is possible that this is a new strain of adenovirus 41, or that adenovirus is a red herring altogether. Remember, we knew about “non-A/non-B viral hepatitis” for more than 2 decades before hepatitis C was discovered.
The pace of science is faster now, fortunately, and information is coming out quickly. As we learn more, we’ll share it with you.
Dr. Wilson, MD, MSCE, is an associate professor of medicine at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com. Dr. Wilson has disclosed no relevant financial relationships.
This is a transcript of a video that first appeared on Medscape.com. It has been edited for clarity.
On April 21, 2022, the Centers for Disease Control and Prevention released a Health Alert Network advisory regarding a cluster of nine cases of acute hepatitis in children in Alabama over a 5-month period from October 2021 to February 2022 – a rate substantially higher than what would be expected, given the relative rarity of hepatitis in children.
Standard workup was negative for the common causative agents – hepatitis A, B, and C – and no toxic exposures were identified. But there was one common thread among all these kids: They all tested positive for adenovirus.
And that is really strange.
There are about 100 circulating adenoviruses in the world that we know of, and around 50 of them infect humans. If you are an adult, it’s a virtual certainty that you have been infected with an adenovirus in the past. Most strains cause symptoms we would describe as the common cold: runny nose, sore throat. Some strains cause conjunctivitis (pink eye). Some cause gastrointestinal illness – the stomach bugs that kids get.
It’s the banality of adenovirus that makes this hepatitis finding so surprising.
The United States is not alone in reporting this new hepatitis syndrome. As of April 21, 169 cases have been reported across the world, including 114 in the United Kingdom.
Of the 169 cases reported worldwide, 74 had evidence of adenovirus infection. On molecular testing, 18 of those were adenovirus 41.
What I wanted to do today was go through the various hypotheses for what could be going on with these hepatitis cases, one by one, and highlight the evidence supporting them. We won’t reach a conclusion, but hopefully by the end, the path forward will be more clear. OK, let’s get started.
Hypothesis 1: Nothing is happening.
It’s worth noting that “clusters” of disease occur all the time, even when no relevant epidemiologic process has occurred. If there is some baseline rate of hepatitis, every once in a while, through bad luck alone, you’d see a group of cases all at once. This is known as the clustering illusion. And I’m quite confident in saying that this is not the case here.
For one, this phenomenon is worldwide, as we know from the World Health Organization report. In fact, the CDC didn’t provide the most detailed data about the nine (now 12) cases in the United States. This study from Scotland is the first to give a detailed accounting of cases, reporting on 13 cases of acute hepatitis of unknown cause in kids at a single hospital from January to April. Typically, the hospital sees fewer than four cases of hepatitis per year. Five of these 13 kids tested positive for adenovirus. So let’s take the clustering illusion off the list.
Hypothesis 2: It’s adenovirus.
The major evidence supporting adenovirus as the causative agent here is that a lot of these kids had adenovirus, and adenovirus 41 – a gut-tropic strain – in particular. This is important, because stool testing might be necessary for diagnosis and lots of kids with this condition didn’t get that. In other words, we have hard evidence of adenovirus infection in about 40% of the cases so far, but the true number might be substantially higher.
That said, adenovirus is seasonal, and we are in adenovirus season. Granted, 40% seems quite a bit higher than the background infection rate, but we have to be careful not to assume that correlation means causation.
The evidence against adenovirus, even adenovirus 41, is that this acute hepatitis syndrome is new, and adenovirus 41 is not. To be fair, we know adenoviruses can cause acute hepatitis, but the vast majority of reports are in immunocompromised individuals – organ transplant recipients and those with HIV. I was able to find just a handful of cases of immunocompetent kids developing hepatitis from adenovirus prior to this current outbreak.
The current outbreak would exceed the published literature by nearly two orders of magnitude. It feels like something else has to be going on.
Hypothesis 3: It’s coronavirus.
SARS-CoV-2 is a strange virus, both in its acute presentation and its long-term outcomes. It was clear early in the pandemic that some children infected by the coronavirus would develop MIS-C – multisystem inflammatory syndrome in children. MIS-C is associated with hepatitis in about 10% of children, according to this New England Journal of Medicine
But the presentation of these kids is quite different from MIS-C; fever is rare, for example. The WHO reports that of the 169 identified cases so far, 20 had active COVID infection. The Scotland cohort suggests that a similar proportion had past COVID infections. In other times, we might consider this a smoking gun, but at this point a history of COVID is not remarkable – after the Omicron wave, it’s about as common to have a history of COVID as it is not to have a history of COVID.
A brief aside here. This is not because of coronavirus vaccination. Of the more than 100 cases reported in the United Kingdom, none of these kids were vaccinated. So let’s put aside the possibility that this is a vaccine effect – there’s no real evidence to support that.
Which brings us to …
Hypothesis 4: It’s coronavirus and adenovirus.
This is sort of intriguing and can work a few different ways, via a direct and indirect path.
In the direct path, we posit that COVID infection does something to kids’ immune systems – something we don’t yet understand that limits their ability to fight off adenovirus. There is some support for this idea. This study in Immunity found that COVID infection can functionally impair dendritic cells and T-cells, including natural killer cells. These cells are important components of our innate antiviral immunity.
There’s an indirect path as well. COVID has led to lockdowns, distancing, masking – stuff that prevents kids from being exposed to germs from other kids. Could a lack of exposure to adenovirus or other viruses because of distancing increase the risk for severe disease when restrictions are lifted? Also possible – the severity of respiratory syncytial virus (RSV) infections this year is substantially higher than what we’ve seen in the past, for example.
And finally, hypothesis 5: This is something new.
We can’t ignore the possibility that this is simply a new disease-causing agent. Toxicology studies so far have been negative, and we wouldn’t expect hepatitis from a chemical toxin to appear in multiple countries around the world; this is almost certainly a biological phenomenon. It is possible that this is a new strain of adenovirus 41, or that adenovirus is a red herring altogether. Remember, we knew about “non-A/non-B viral hepatitis” for more than 2 decades before hepatitis C was discovered.
The pace of science is faster now, fortunately, and information is coming out quickly. As we learn more, we’ll share it with you.
Dr. Wilson, MD, MSCE, is an associate professor of medicine at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com. Dr. Wilson has disclosed no relevant financial relationships.
This is a transcript of a video that first appeared on Medscape.com. It has been edited for clarity.
On April 21, 2022, the Centers for Disease Control and Prevention released a Health Alert Network advisory regarding a cluster of nine cases of acute hepatitis in children in Alabama over a 5-month period from October 2021 to February 2022 – a rate substantially higher than what would be expected, given the relative rarity of hepatitis in children.
Standard workup was negative for the common causative agents – hepatitis A, B, and C – and no toxic exposures were identified. But there was one common thread among all these kids: They all tested positive for adenovirus.
And that is really strange.
There are about 100 circulating adenoviruses in the world that we know of, and around 50 of them infect humans. If you are an adult, it’s a virtual certainty that you have been infected with an adenovirus in the past. Most strains cause symptoms we would describe as the common cold: runny nose, sore throat. Some strains cause conjunctivitis (pink eye). Some cause gastrointestinal illness – the stomach bugs that kids get.
It’s the banality of adenovirus that makes this hepatitis finding so surprising.
The United States is not alone in reporting this new hepatitis syndrome. As of April 21, 169 cases have been reported across the world, including 114 in the United Kingdom.
Of the 169 cases reported worldwide, 74 had evidence of adenovirus infection. On molecular testing, 18 of those were adenovirus 41.
What I wanted to do today was go through the various hypotheses for what could be going on with these hepatitis cases, one by one, and highlight the evidence supporting them. We won’t reach a conclusion, but hopefully by the end, the path forward will be more clear. OK, let’s get started.
Hypothesis 1: Nothing is happening.
It’s worth noting that “clusters” of disease occur all the time, even when no relevant epidemiologic process has occurred. If there is some baseline rate of hepatitis, every once in a while, through bad luck alone, you’d see a group of cases all at once. This is known as the clustering illusion. And I’m quite confident in saying that this is not the case here.
For one, this phenomenon is worldwide, as we know from the World Health Organization report. In fact, the CDC didn’t provide the most detailed data about the nine (now 12) cases in the United States. This study from Scotland is the first to give a detailed accounting of cases, reporting on 13 cases of acute hepatitis of unknown cause in kids at a single hospital from January to April. Typically, the hospital sees fewer than four cases of hepatitis per year. Five of these 13 kids tested positive for adenovirus. So let’s take the clustering illusion off the list.
Hypothesis 2: It’s adenovirus.
The major evidence supporting adenovirus as the causative agent here is that a lot of these kids had adenovirus, and adenovirus 41 – a gut-tropic strain – in particular. This is important, because stool testing might be necessary for diagnosis and lots of kids with this condition didn’t get that. In other words, we have hard evidence of adenovirus infection in about 40% of the cases so far, but the true number might be substantially higher.
That said, adenovirus is seasonal, and we are in adenovirus season. Granted, 40% seems quite a bit higher than the background infection rate, but we have to be careful not to assume that correlation means causation.
The evidence against adenovirus, even adenovirus 41, is that this acute hepatitis syndrome is new, and adenovirus 41 is not. To be fair, we know adenoviruses can cause acute hepatitis, but the vast majority of reports are in immunocompromised individuals – organ transplant recipients and those with HIV. I was able to find just a handful of cases of immunocompetent kids developing hepatitis from adenovirus prior to this current outbreak.
The current outbreak would exceed the published literature by nearly two orders of magnitude. It feels like something else has to be going on.
Hypothesis 3: It’s coronavirus.
SARS-CoV-2 is a strange virus, both in its acute presentation and its long-term outcomes. It was clear early in the pandemic that some children infected by the coronavirus would develop MIS-C – multisystem inflammatory syndrome in children. MIS-C is associated with hepatitis in about 10% of children, according to this New England Journal of Medicine
But the presentation of these kids is quite different from MIS-C; fever is rare, for example. The WHO reports that of the 169 identified cases so far, 20 had active COVID infection. The Scotland cohort suggests that a similar proportion had past COVID infections. In other times, we might consider this a smoking gun, but at this point a history of COVID is not remarkable – after the Omicron wave, it’s about as common to have a history of COVID as it is not to have a history of COVID.
A brief aside here. This is not because of coronavirus vaccination. Of the more than 100 cases reported in the United Kingdom, none of these kids were vaccinated. So let’s put aside the possibility that this is a vaccine effect – there’s no real evidence to support that.
Which brings us to …
Hypothesis 4: It’s coronavirus and adenovirus.
This is sort of intriguing and can work a few different ways, via a direct and indirect path.
In the direct path, we posit that COVID infection does something to kids’ immune systems – something we don’t yet understand that limits their ability to fight off adenovirus. There is some support for this idea. This study in Immunity found that COVID infection can functionally impair dendritic cells and T-cells, including natural killer cells. These cells are important components of our innate antiviral immunity.
There’s an indirect path as well. COVID has led to lockdowns, distancing, masking – stuff that prevents kids from being exposed to germs from other kids. Could a lack of exposure to adenovirus or other viruses because of distancing increase the risk for severe disease when restrictions are lifted? Also possible – the severity of respiratory syncytial virus (RSV) infections this year is substantially higher than what we’ve seen in the past, for example.
And finally, hypothesis 5: This is something new.
We can’t ignore the possibility that this is simply a new disease-causing agent. Toxicology studies so far have been negative, and we wouldn’t expect hepatitis from a chemical toxin to appear in multiple countries around the world; this is almost certainly a biological phenomenon. It is possible that this is a new strain of adenovirus 41, or that adenovirus is a red herring altogether. Remember, we knew about “non-A/non-B viral hepatitis” for more than 2 decades before hepatitis C was discovered.
The pace of science is faster now, fortunately, and information is coming out quickly. As we learn more, we’ll share it with you.
Dr. Wilson, MD, MSCE, is an associate professor of medicine at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com. Dr. Wilson has disclosed no relevant financial relationships.
Don’t drink calories: Artificial sweeteners beat sugar in new analysis
This transcript of Impact Factor with F. Perry Wilson has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
When I counsel patients who are trying to lose weight, there is something I always discuss: “Don’t drink calories.” The idea is that it is so easy to consume sweetened beverages (and alcoholic ones, for that matter) and we don’t really get a sense of how many calories we’re taking in.
Some patients balk at the idea, saying they can’t stand the taste of water or just can’t bring themselves to drink it. While, as a nephrologist, this pains me deeply to hear, I often suggest going for low- or zero-calorie flavored drinks instead of the sugary stuff.
And yet ... I need to admit that recently I’ve been more nervous about that advice. A very nice study in Nature, for example, found that artificial sweeteners induce glucose intolerance and weight gain – in mice.
Several observational studies have suggested that the use of nonnutritive sweeteners – sucralose, aspartame, and so on – are associated with higher body weight and type 2 diabetes. Of course,
Randomized trials, as ever, are the key to deeper understanding, but most trials in this space are relatively small. That makes a good case for this study, appearing in JAMA Network Open, which combines data from 17 randomized trials to determine what effects substituting sugary drinks with low- and zero-calorie drinks truly has.
So, what’s the bottom line? Should I ditch the Splenda in my morning coffee and drop in some sugar cubes?
It turns out that the effects of drinking low- or zero-calorie drinks instead of sugary ones is modest, but overall beneficial, depending on the outcome you’re trying to achieve.
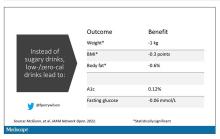
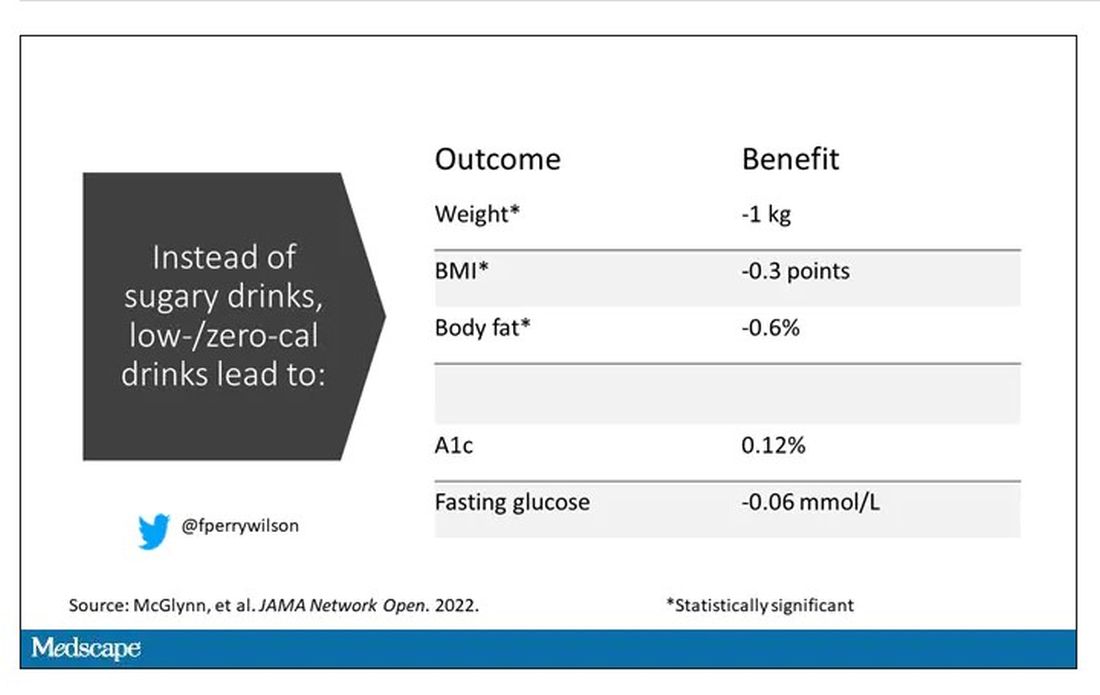
Randomized trials show that switching to low-cal drinks reduces body weight by about a kilogram, and BMI by 0.3 points. It also reduces body fat by about half a percent.
Effects on glucose homeostasis – hemoglobin A1c level and fasting glucose – were not that impressive, though.
The authors also compared sugar-sweetened beverages with plain old water. I expected this analysis to show more dramatic benefits. After all, we’re all just ugly, giant bags of mostly water. Interestingly, the effects of switching to water were not as dramatic and largely nonsignificant with respect to most outcomes evaluated.
So, what do we make of this? If someone is a habitual drinker of sugar-sweetened beverages, is it preferable to switch to a zero-calorie flavored drink, compared with plain water?
One possibility is that in the trials where people are randomized to switch to water, they aren’t as adherent. Just because we ask someone to drink water doesn’t mean they do it, and so there may be a tendency to “cheat” with sugar-sweetened beverages. However, if told that low- or zero-calorie flavored drinks are okay, maybe it’s easier to stick to the plan? This is essentially the argument you get from people who say that vaping is a good way to quit smoking. It may or may not be true.
It could also be that we just don’t have enough rigorous data to make a firm conclusion. Of the 17 trials examined, only three of them used water substitution as an intervention.
All in all, these data provide some reassurance that the zero-calorie sweeteners aren’t secretly exacerbating the obesity epidemic. I’d certainly rather my patients drink Diet Coke than regular Coke. That said, these studies are necessarily short term; the longer-term effects of sugar substitutes, while perhaps not as bad as the long-term effects of sugar, must necessarily be worse than the long-term effects of drinking water. Maybe this is the nephrologist in me talking again, but I doubt that there could possibly be a fluid better for the human body than good old H2O. Except coffee, of course.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript of Impact Factor with F. Perry Wilson has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
When I counsel patients who are trying to lose weight, there is something I always discuss: “Don’t drink calories.” The idea is that it is so easy to consume sweetened beverages (and alcoholic ones, for that matter) and we don’t really get a sense of how many calories we’re taking in.
Some patients balk at the idea, saying they can’t stand the taste of water or just can’t bring themselves to drink it. While, as a nephrologist, this pains me deeply to hear, I often suggest going for low- or zero-calorie flavored drinks instead of the sugary stuff.
And yet ... I need to admit that recently I’ve been more nervous about that advice. A very nice study in Nature, for example, found that artificial sweeteners induce glucose intolerance and weight gain – in mice.
Several observational studies have suggested that the use of nonnutritive sweeteners – sucralose, aspartame, and so on – are associated with higher body weight and type 2 diabetes. Of course,
Randomized trials, as ever, are the key to deeper understanding, but most trials in this space are relatively small. That makes a good case for this study, appearing in JAMA Network Open, which combines data from 17 randomized trials to determine what effects substituting sugary drinks with low- and zero-calorie drinks truly has.
So, what’s the bottom line? Should I ditch the Splenda in my morning coffee and drop in some sugar cubes?
It turns out that the effects of drinking low- or zero-calorie drinks instead of sugary ones is modest, but overall beneficial, depending on the outcome you’re trying to achieve.
Randomized trials show that switching to low-cal drinks reduces body weight by about a kilogram, and BMI by 0.3 points. It also reduces body fat by about half a percent.
Effects on glucose homeostasis – hemoglobin A1c level and fasting glucose – were not that impressive, though.
The authors also compared sugar-sweetened beverages with plain old water. I expected this analysis to show more dramatic benefits. After all, we’re all just ugly, giant bags of mostly water. Interestingly, the effects of switching to water were not as dramatic and largely nonsignificant with respect to most outcomes evaluated.
So, what do we make of this? If someone is a habitual drinker of sugar-sweetened beverages, is it preferable to switch to a zero-calorie flavored drink, compared with plain water?
One possibility is that in the trials where people are randomized to switch to water, they aren’t as adherent. Just because we ask someone to drink water doesn’t mean they do it, and so there may be a tendency to “cheat” with sugar-sweetened beverages. However, if told that low- or zero-calorie flavored drinks are okay, maybe it’s easier to stick to the plan? This is essentially the argument you get from people who say that vaping is a good way to quit smoking. It may or may not be true.
It could also be that we just don’t have enough rigorous data to make a firm conclusion. Of the 17 trials examined, only three of them used water substitution as an intervention.
All in all, these data provide some reassurance that the zero-calorie sweeteners aren’t secretly exacerbating the obesity epidemic. I’d certainly rather my patients drink Diet Coke than regular Coke. That said, these studies are necessarily short term; the longer-term effects of sugar substitutes, while perhaps not as bad as the long-term effects of sugar, must necessarily be worse than the long-term effects of drinking water. Maybe this is the nephrologist in me talking again, but I doubt that there could possibly be a fluid better for the human body than good old H2O. Except coffee, of course.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript of Impact Factor with F. Perry Wilson has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
When I counsel patients who are trying to lose weight, there is something I always discuss: “Don’t drink calories.” The idea is that it is so easy to consume sweetened beverages (and alcoholic ones, for that matter) and we don’t really get a sense of how many calories we’re taking in.
Some patients balk at the idea, saying they can’t stand the taste of water or just can’t bring themselves to drink it. While, as a nephrologist, this pains me deeply to hear, I often suggest going for low- or zero-calorie flavored drinks instead of the sugary stuff.
And yet ... I need to admit that recently I’ve been more nervous about that advice. A very nice study in Nature, for example, found that artificial sweeteners induce glucose intolerance and weight gain – in mice.
Several observational studies have suggested that the use of nonnutritive sweeteners – sucralose, aspartame, and so on – are associated with higher body weight and type 2 diabetes. Of course,
Randomized trials, as ever, are the key to deeper understanding, but most trials in this space are relatively small. That makes a good case for this study, appearing in JAMA Network Open, which combines data from 17 randomized trials to determine what effects substituting sugary drinks with low- and zero-calorie drinks truly has.
So, what’s the bottom line? Should I ditch the Splenda in my morning coffee and drop in some sugar cubes?
It turns out that the effects of drinking low- or zero-calorie drinks instead of sugary ones is modest, but overall beneficial, depending on the outcome you’re trying to achieve.
Randomized trials show that switching to low-cal drinks reduces body weight by about a kilogram, and BMI by 0.3 points. It also reduces body fat by about half a percent.
Effects on glucose homeostasis – hemoglobin A1c level and fasting glucose – were not that impressive, though.
The authors also compared sugar-sweetened beverages with plain old water. I expected this analysis to show more dramatic benefits. After all, we’re all just ugly, giant bags of mostly water. Interestingly, the effects of switching to water were not as dramatic and largely nonsignificant with respect to most outcomes evaluated.
So, what do we make of this? If someone is a habitual drinker of sugar-sweetened beverages, is it preferable to switch to a zero-calorie flavored drink, compared with plain water?
One possibility is that in the trials where people are randomized to switch to water, they aren’t as adherent. Just because we ask someone to drink water doesn’t mean they do it, and so there may be a tendency to “cheat” with sugar-sweetened beverages. However, if told that low- or zero-calorie flavored drinks are okay, maybe it’s easier to stick to the plan? This is essentially the argument you get from people who say that vaping is a good way to quit smoking. It may or may not be true.
It could also be that we just don’t have enough rigorous data to make a firm conclusion. Of the 17 trials examined, only three of them used water substitution as an intervention.
All in all, these data provide some reassurance that the zero-calorie sweeteners aren’t secretly exacerbating the obesity epidemic. I’d certainly rather my patients drink Diet Coke than regular Coke. That said, these studies are necessarily short term; the longer-term effects of sugar substitutes, while perhaps not as bad as the long-term effects of sugar, must necessarily be worse than the long-term effects of drinking water. Maybe this is the nephrologist in me talking again, but I doubt that there could possibly be a fluid better for the human body than good old H2O. Except coffee, of course.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.